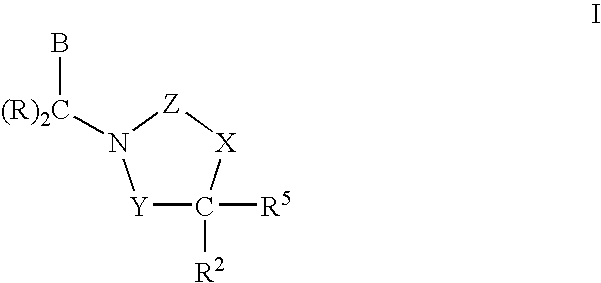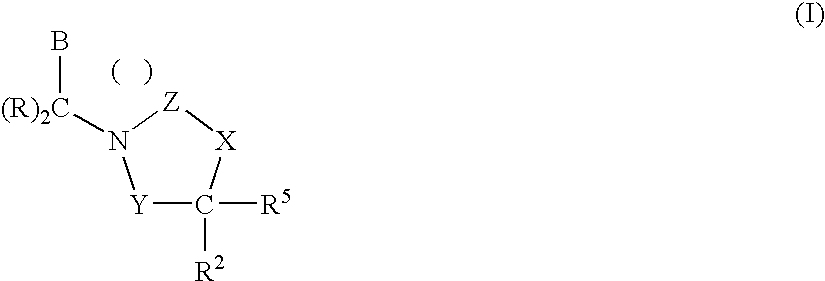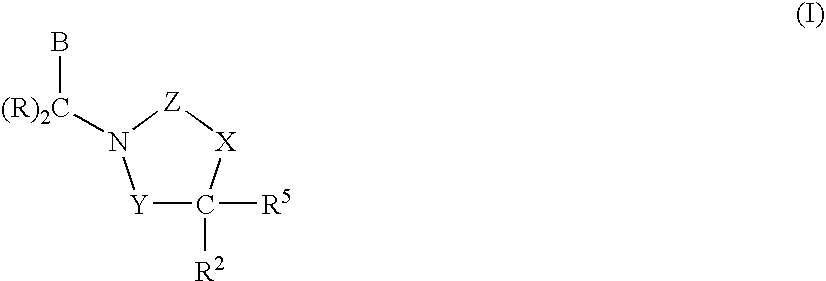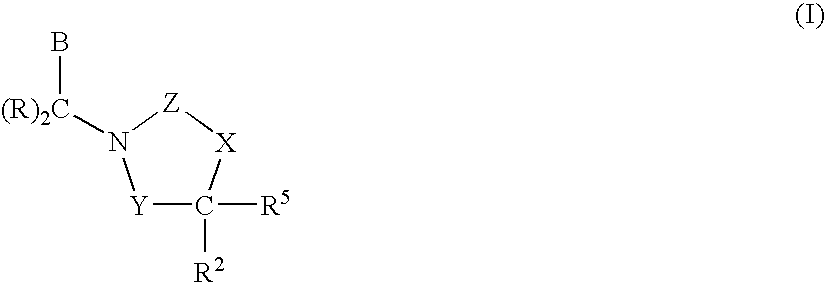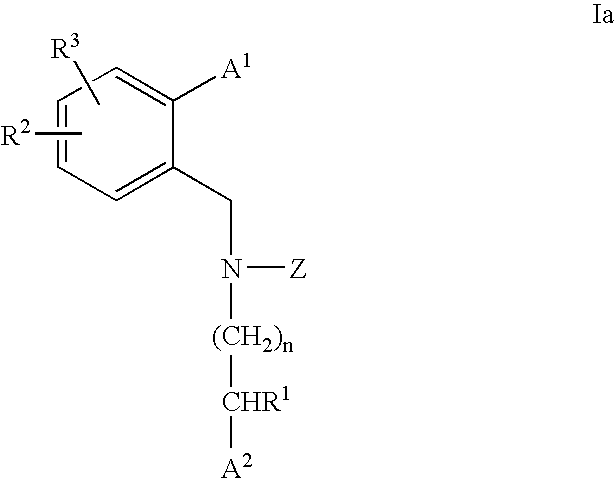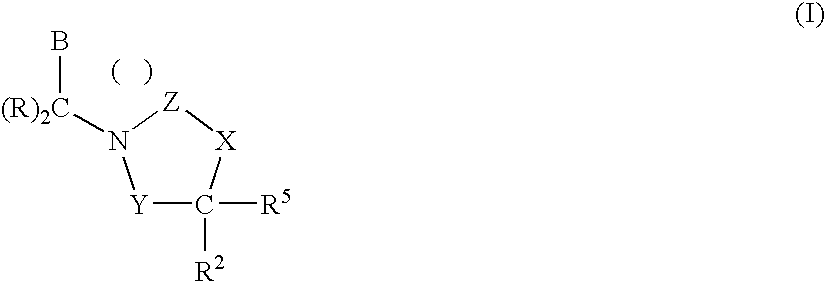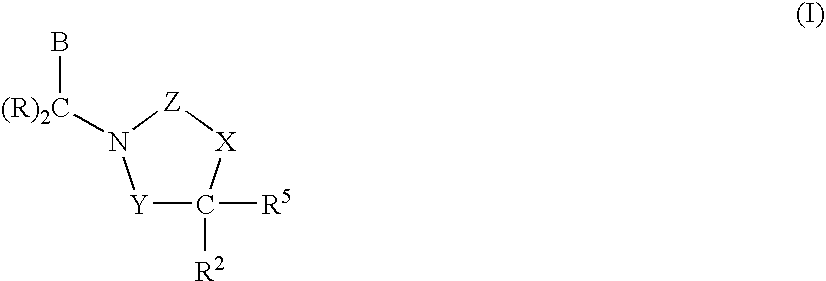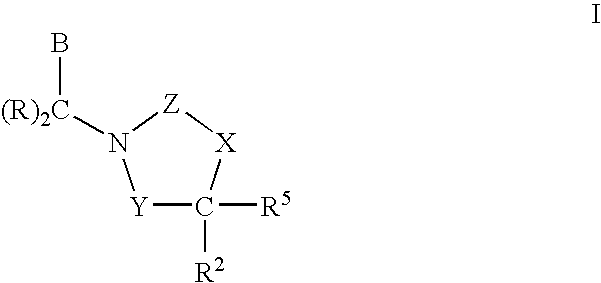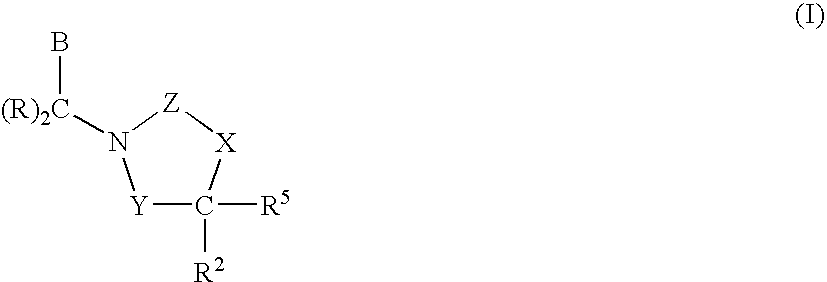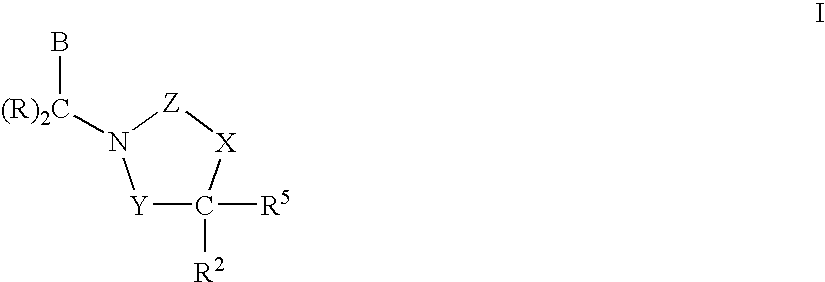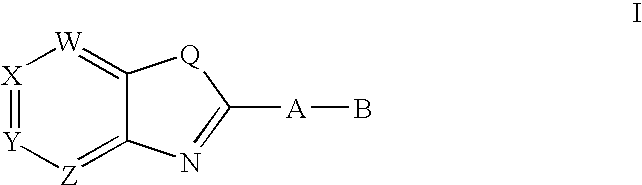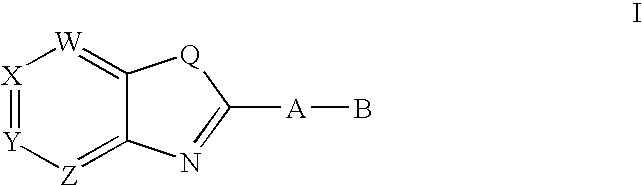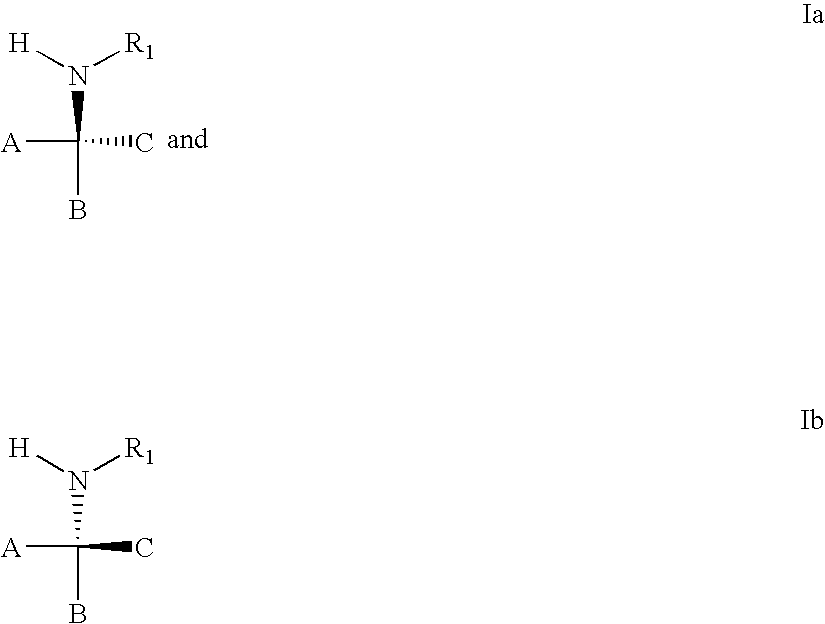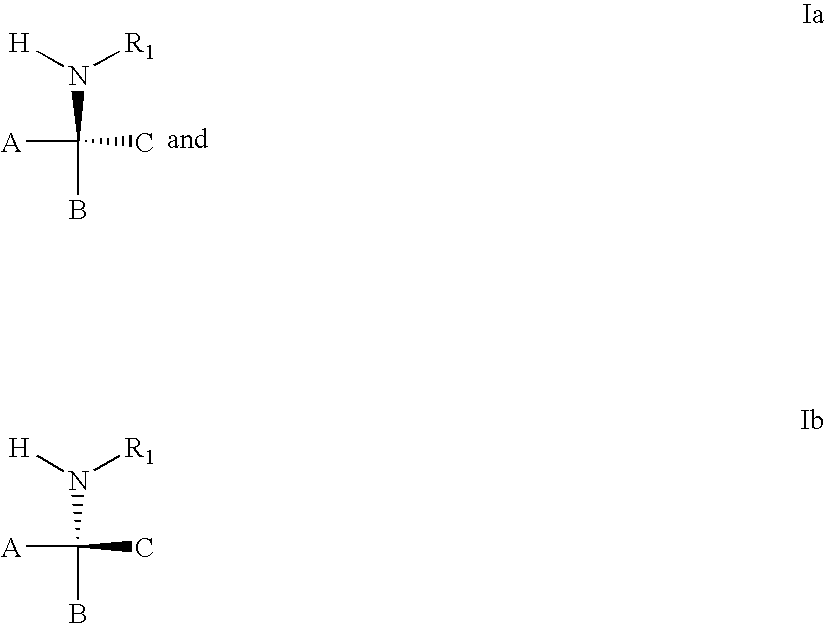Patents
Literature
105 results about "CETP inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A CETP inhibitor is a member of a class of drugs that inhibit cholesterylester transfer protein (CETP). They are intended to reduce the risk of atherosclerosis (a cardiovascular disease) by improving blood lipid levels. At least three medications within this class have failed to result in benefits however.
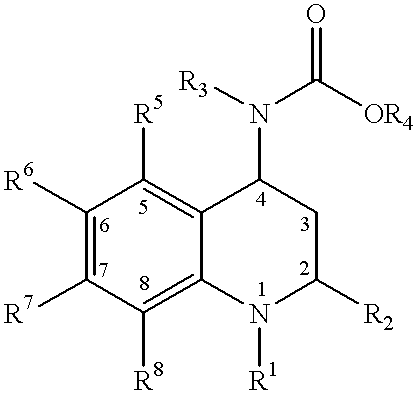
Method for making 4-carboxyamino-2-substituted-1,2,3,4-tetrahydroquinoline
InactiveUS6313142B1Improve isolationEasy to purifyBiocideMetabolism disorderStereochemistryCETP inhibitor
Owner:PFIZER INC
CETP inhibitors
Compounds having the structures of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis: In the compounds of Formula I, B or R2 is a phenyl group which has an ortho aryl, heterocyclic, benzoheterocyclic or benzocycloalkyl substituent, and one other position on the 5-membered ring has an aromatic, heterocyclic, cycloalkyl, benzoheterocyclic or benzocycloalkyl substituent connected directly to the ring or attached to the ring through a —CH2—.
Owner:MERCK SHARP & DOHME LLC
Cholesteryl Ester Transfer Protein Inhibitors
Compounds of Formula (I), including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In the compounds of Formula (I), A1 is a cyclic group, and B is a cyclic group which is attached to the heterocyclic ring directly or through a methylene group.
Owner:MERCK SHARP & DOHME LLC
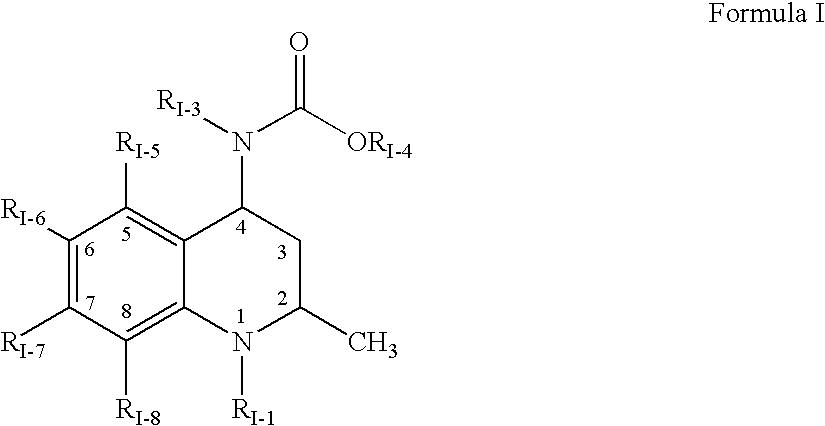
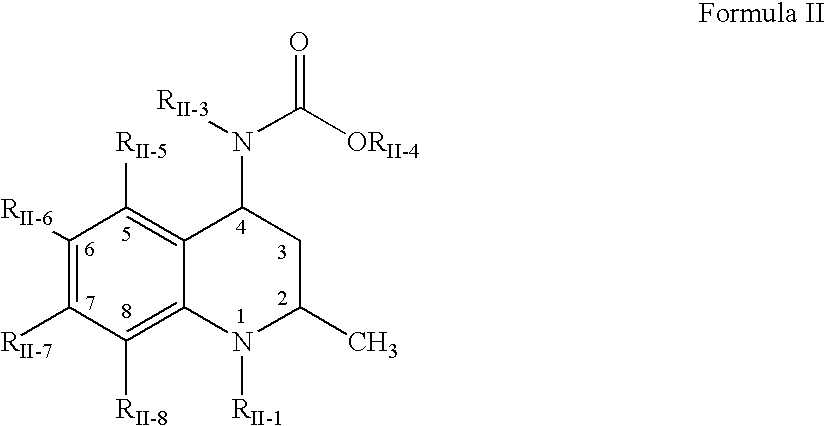
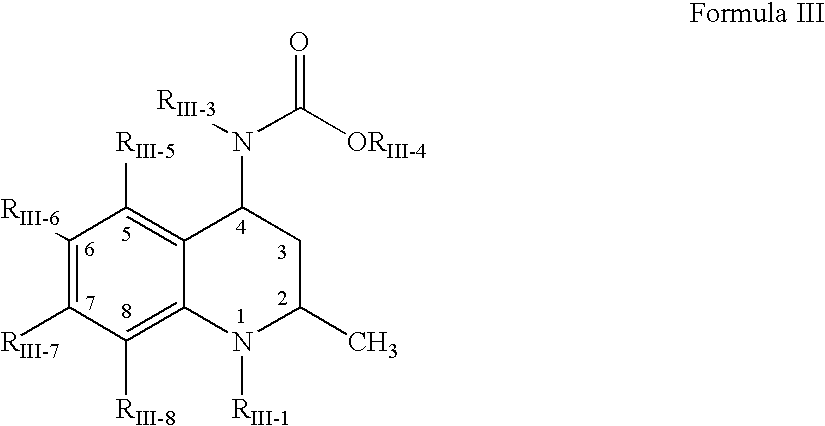
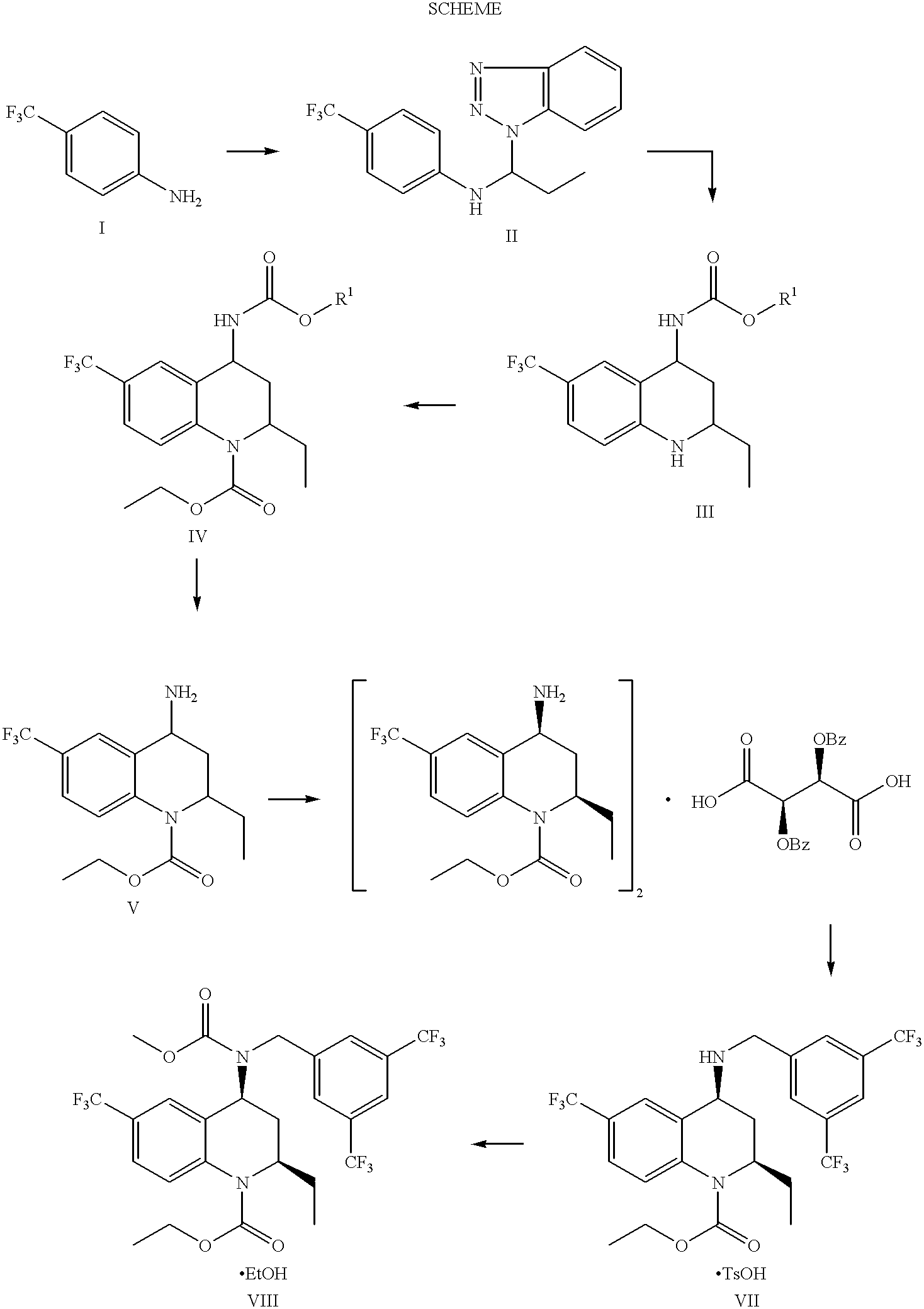
1,2,3,4-tetrahydro-quinoline derivatives as CETP inhibitors
Owner:JANSSEN PHARMA NV
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
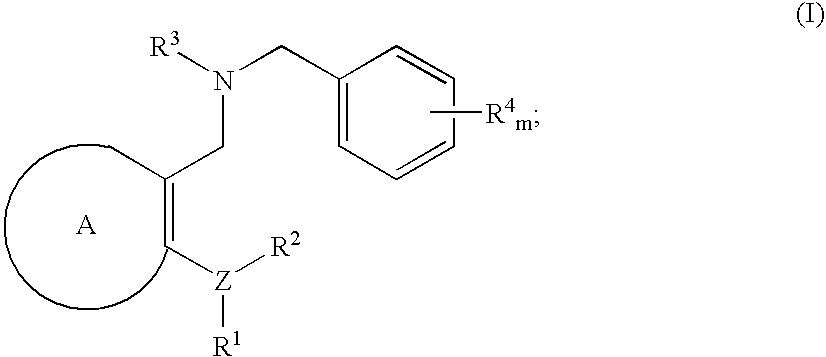
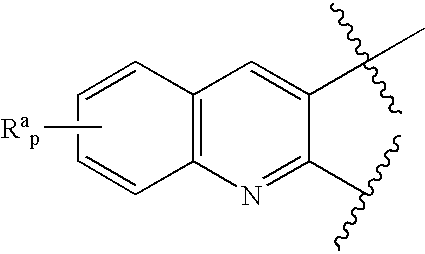
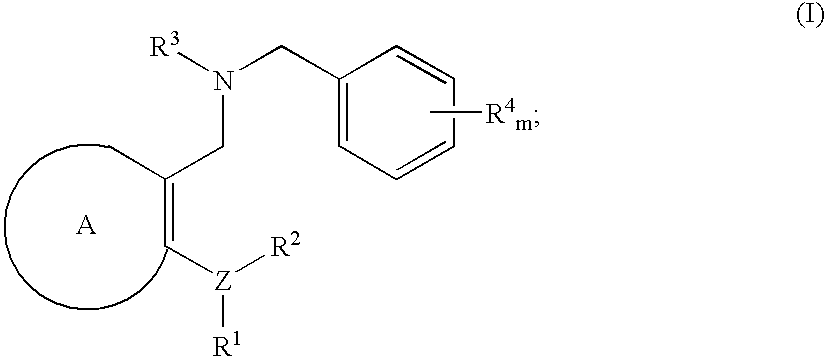
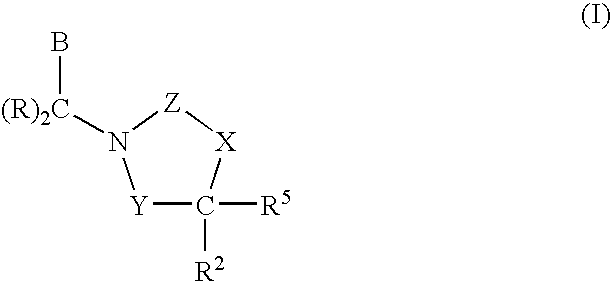
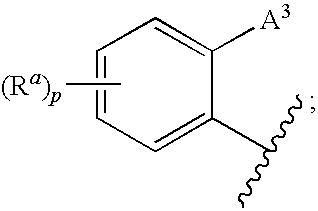
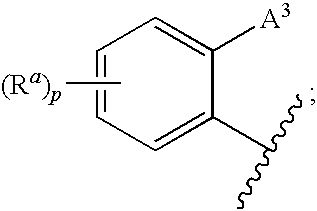
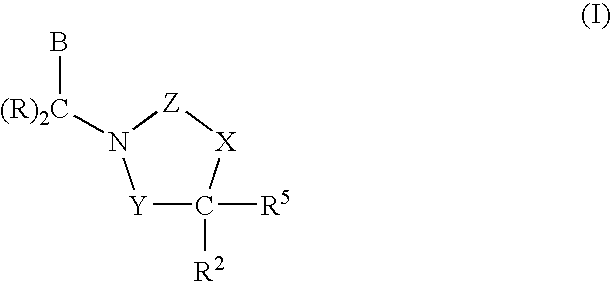
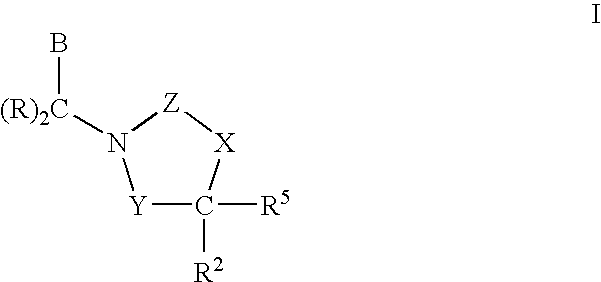
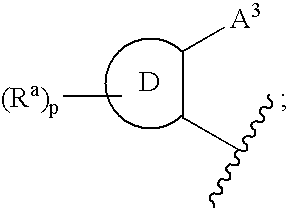
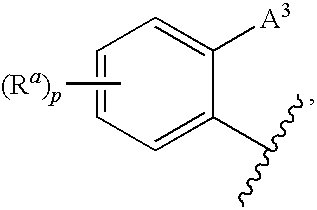
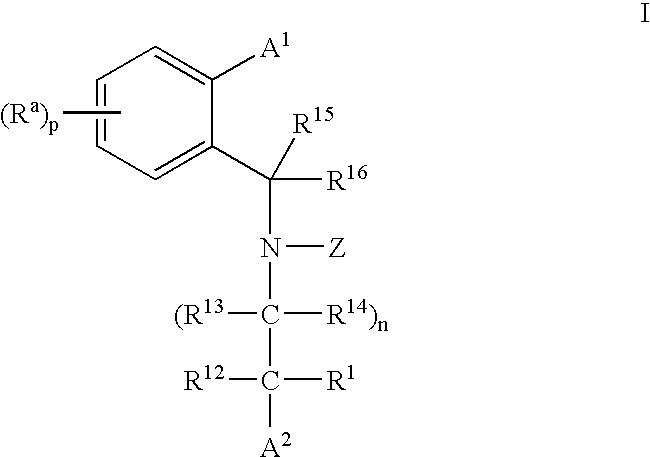
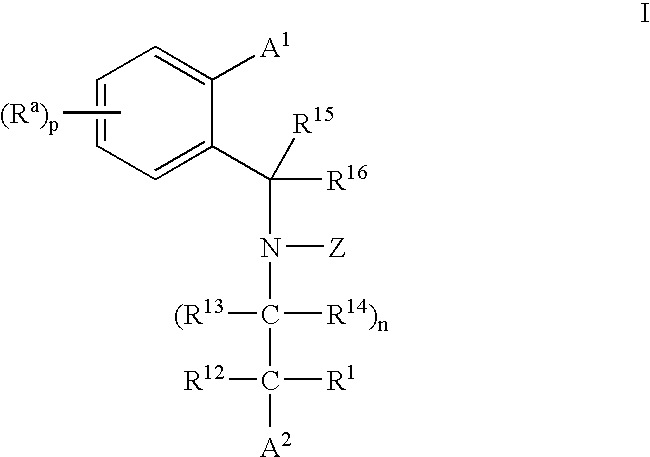
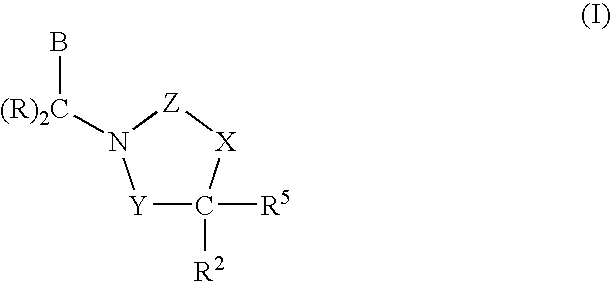
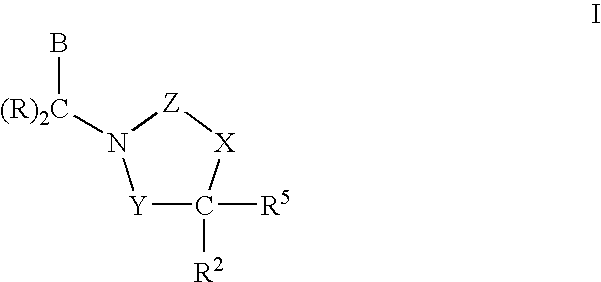
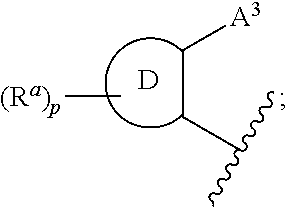
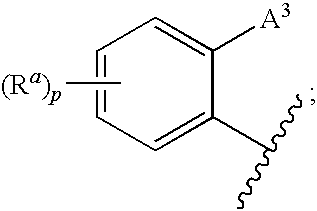
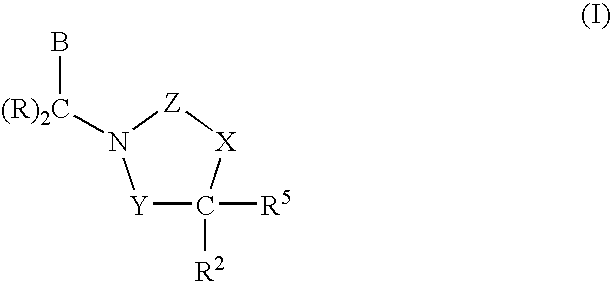
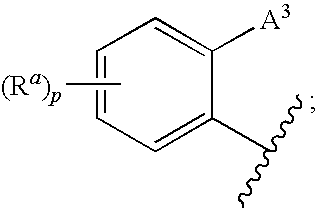
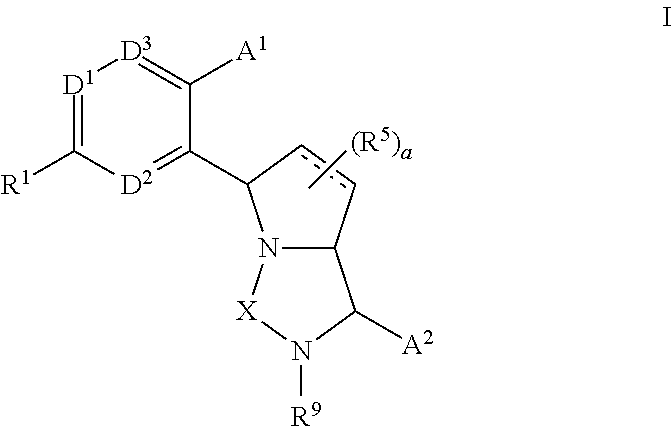
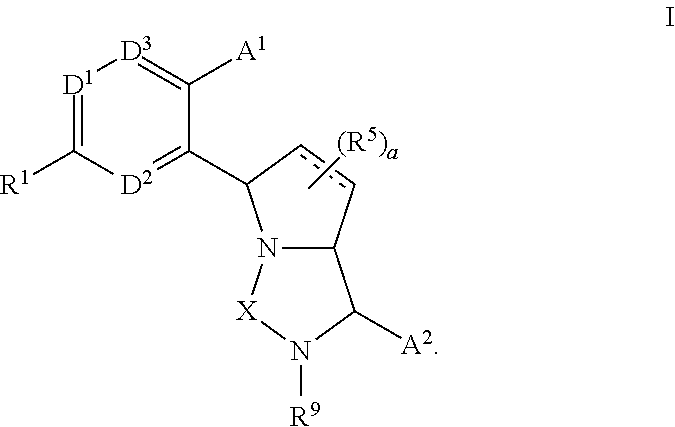
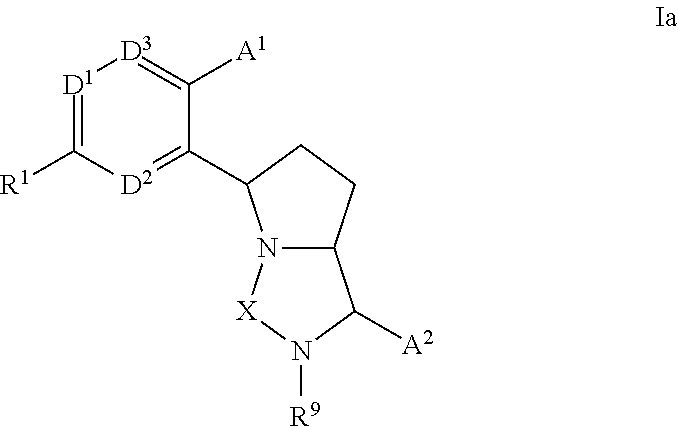
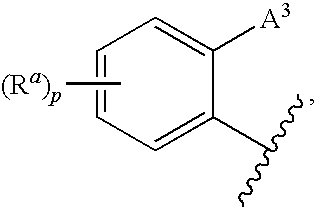
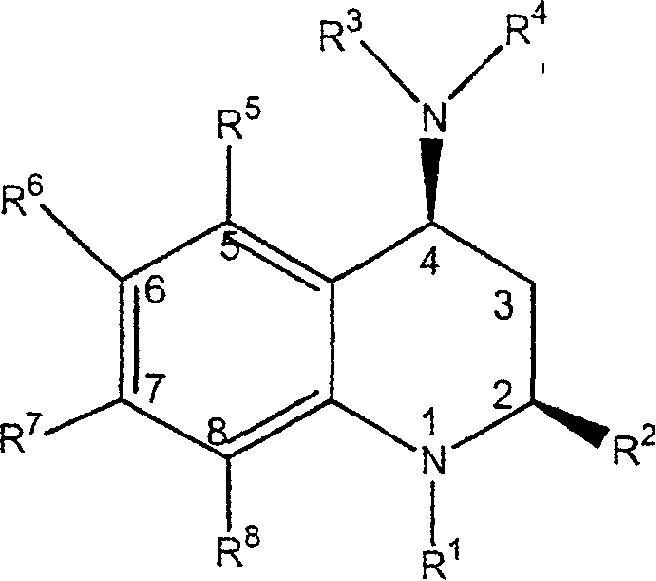
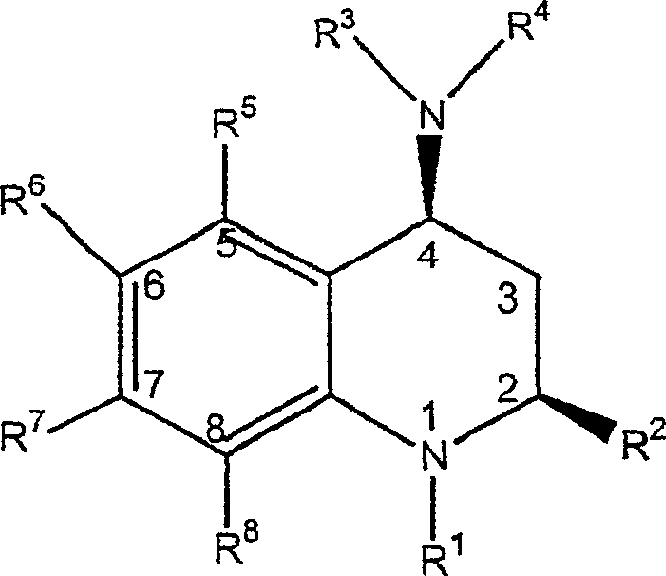
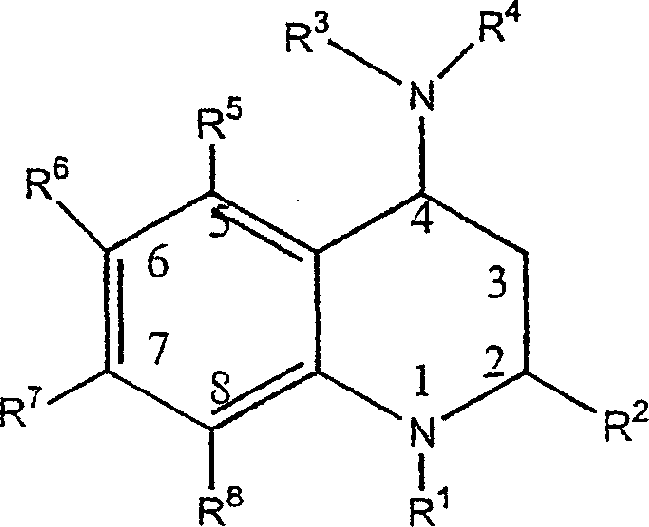
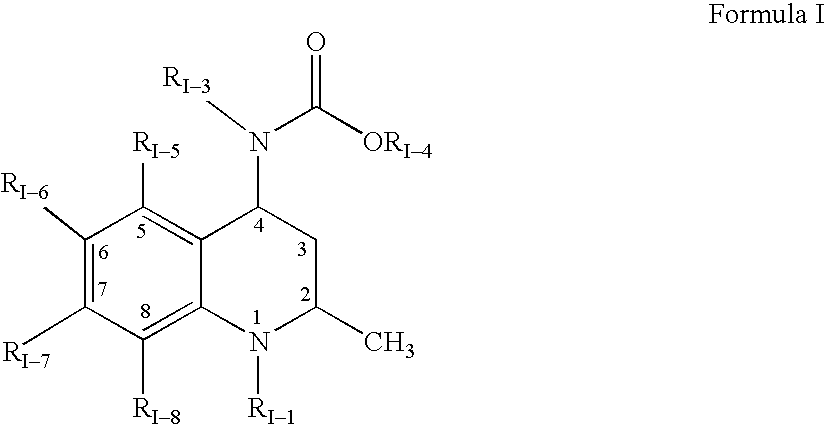
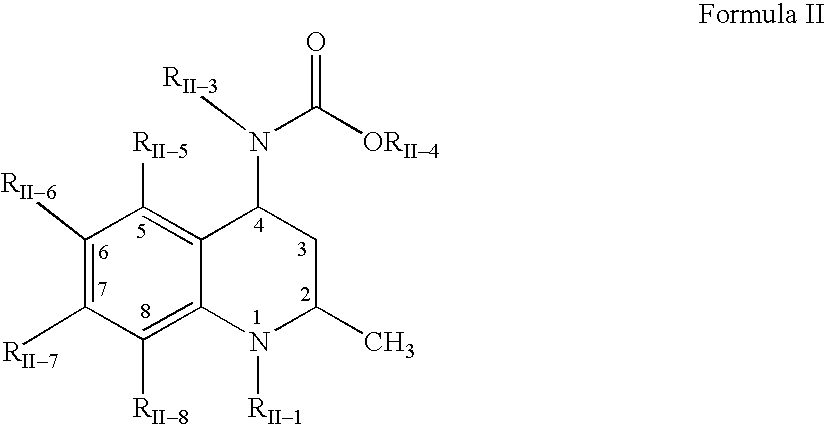
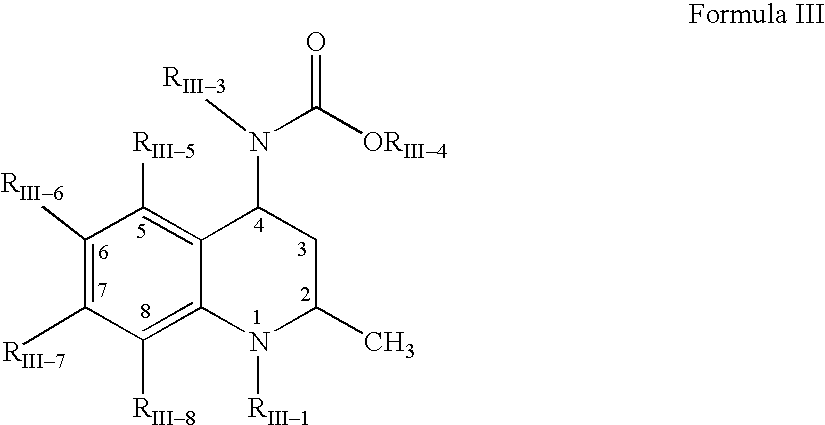
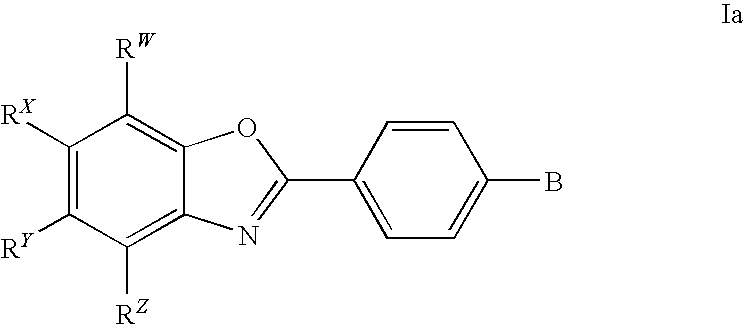
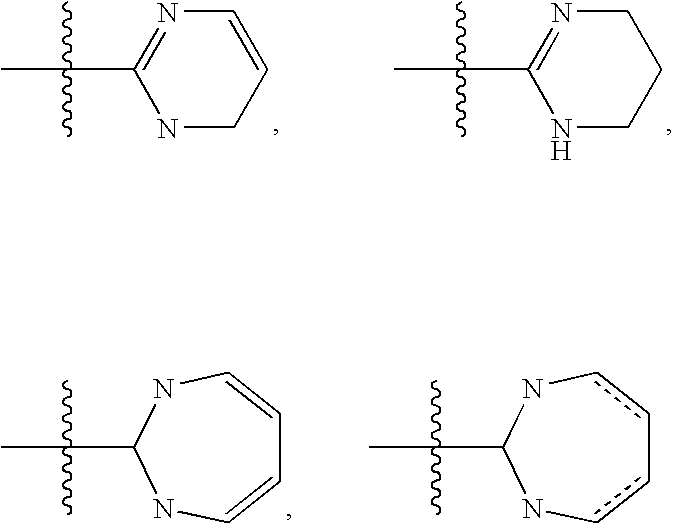
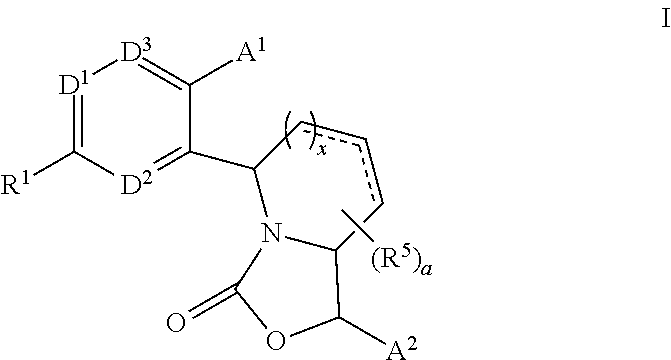
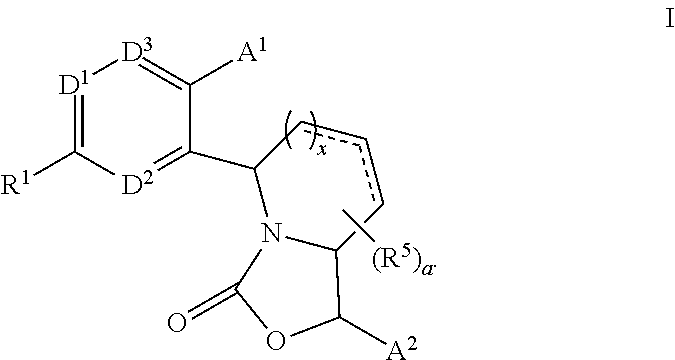
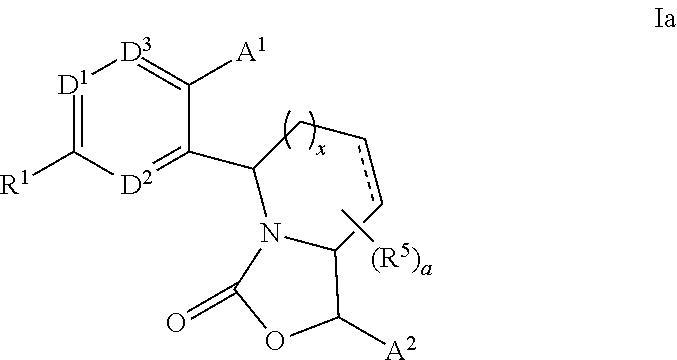
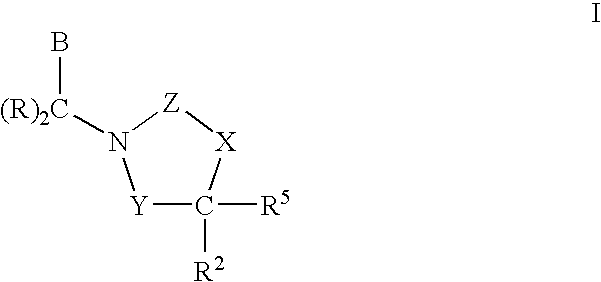
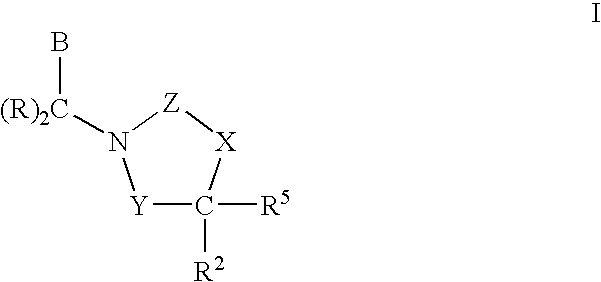
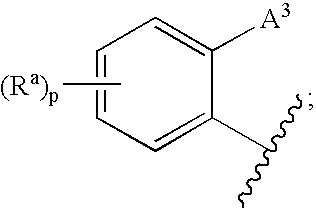
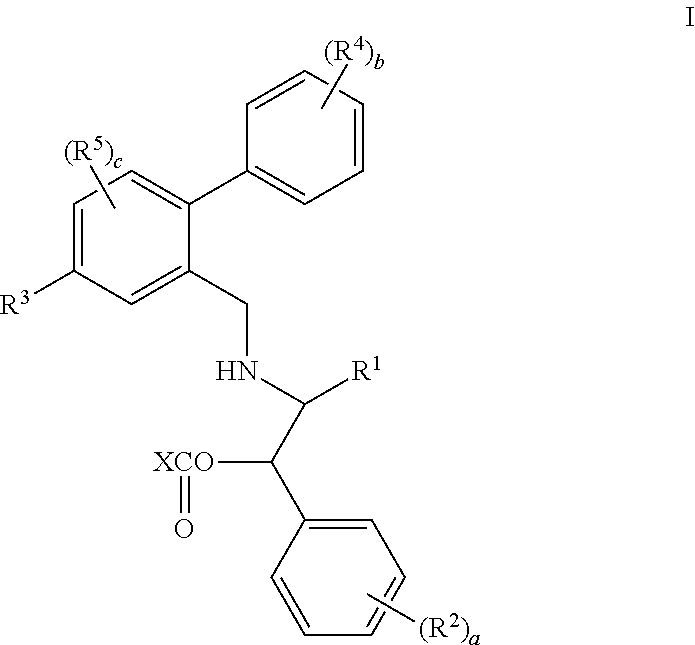
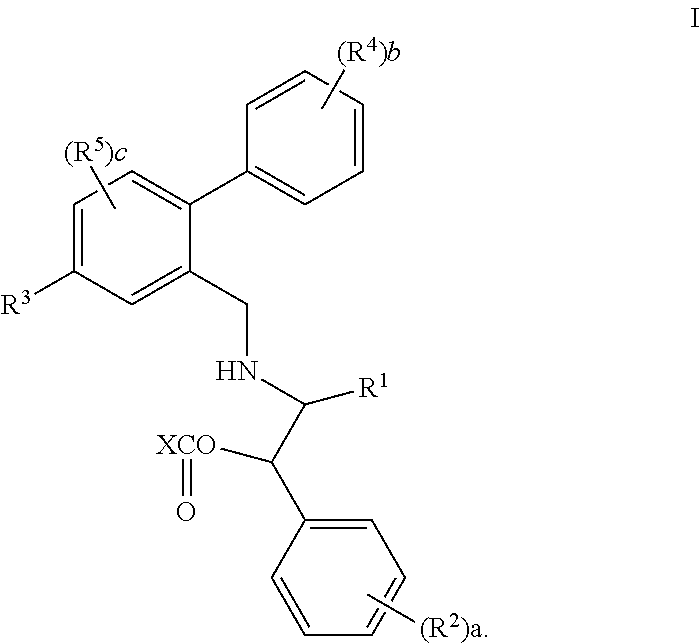
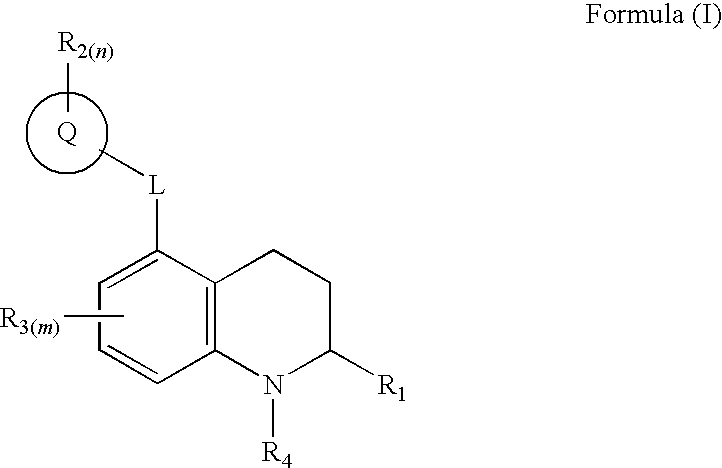
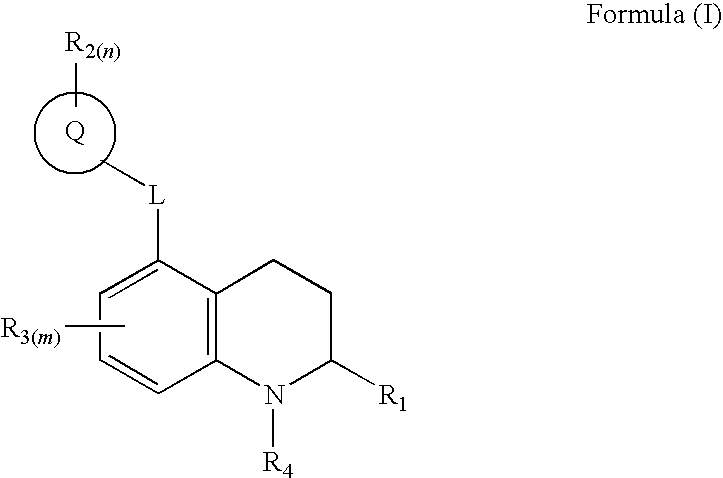
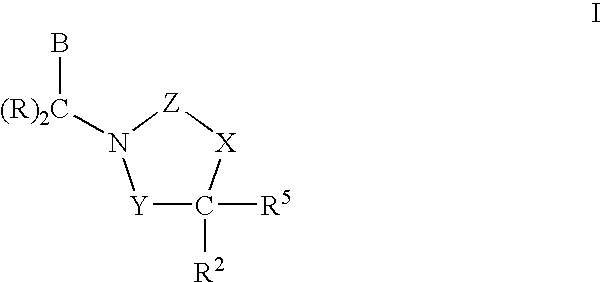
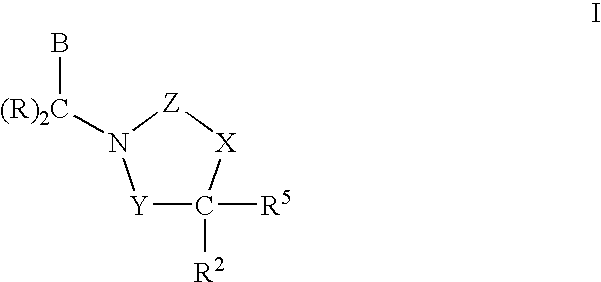
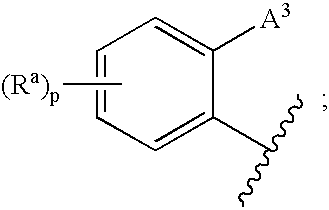
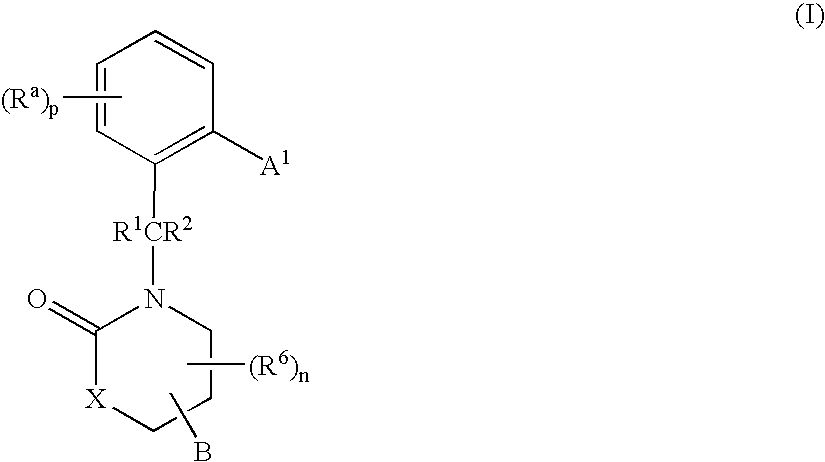
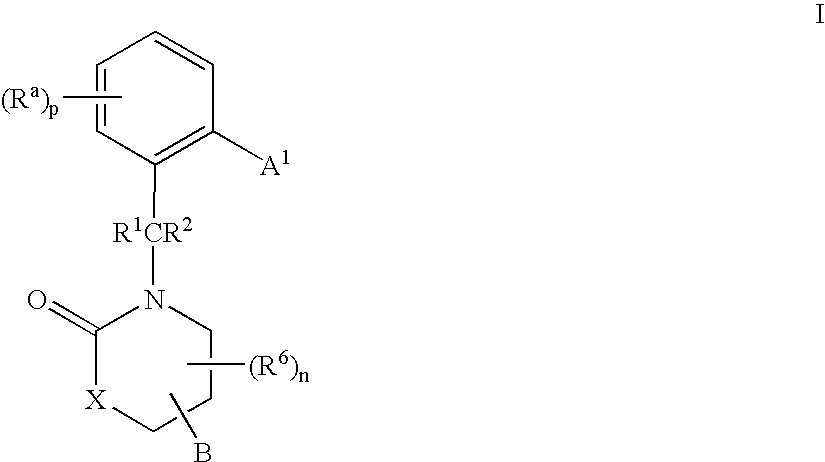
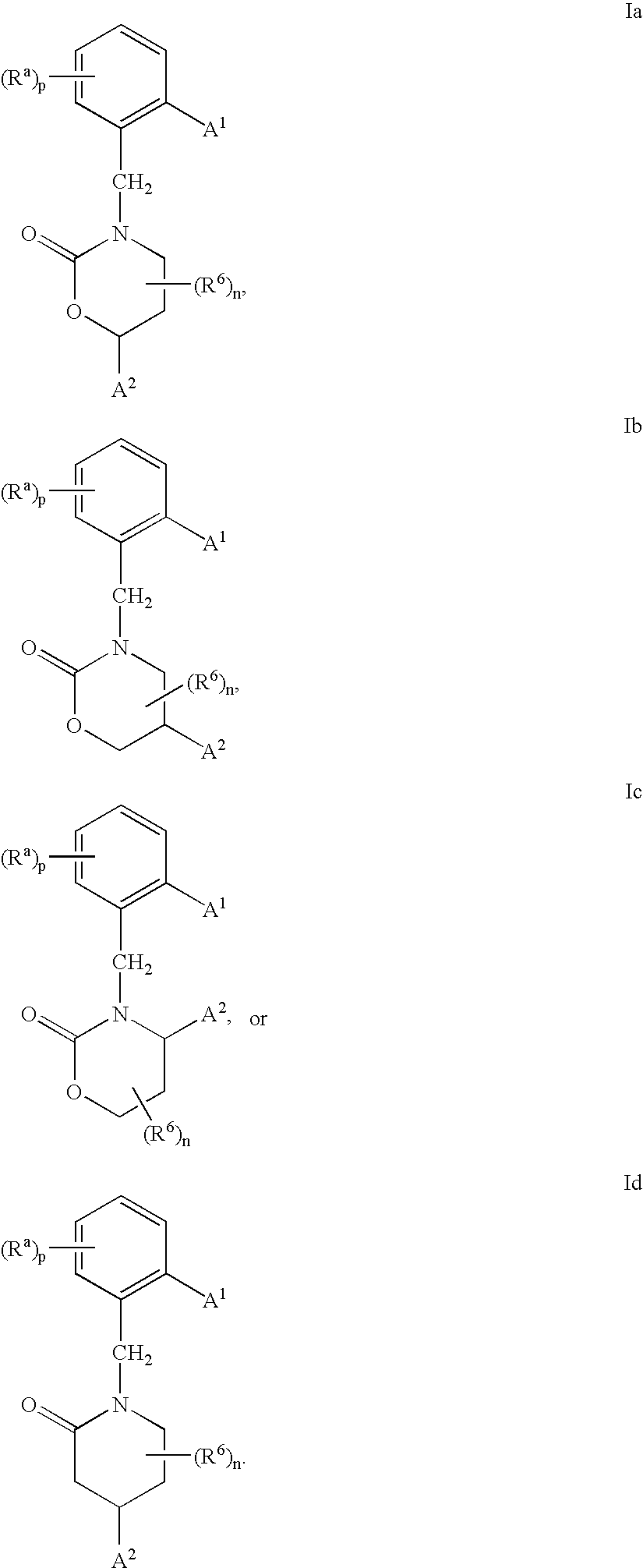
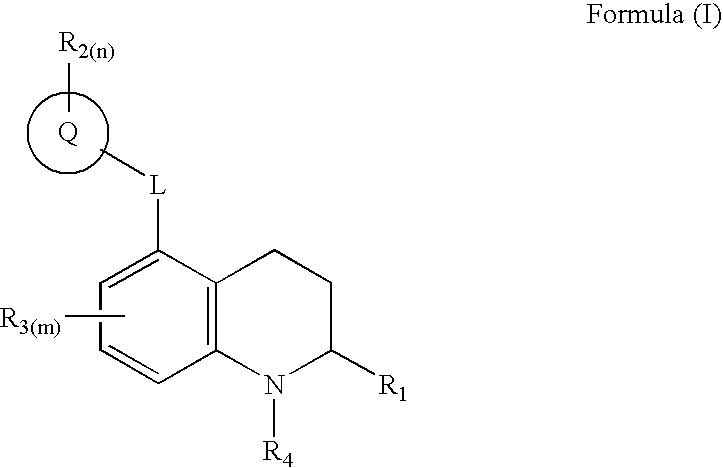
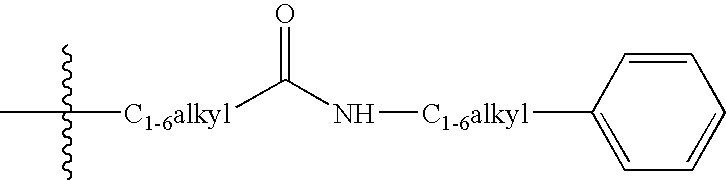
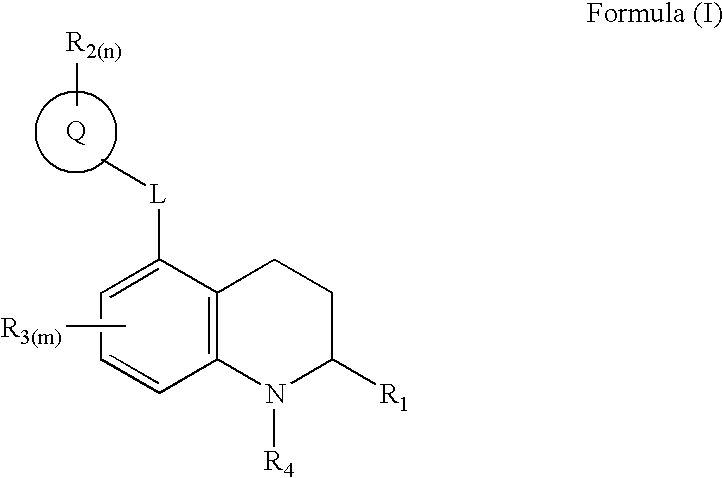
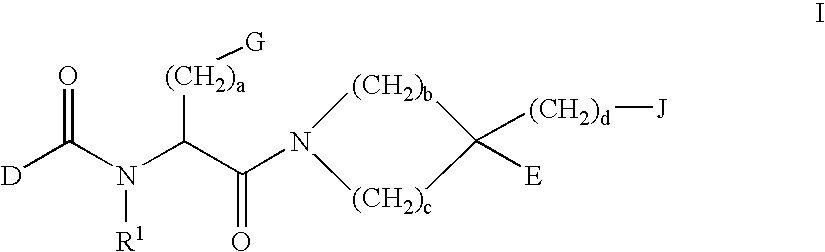
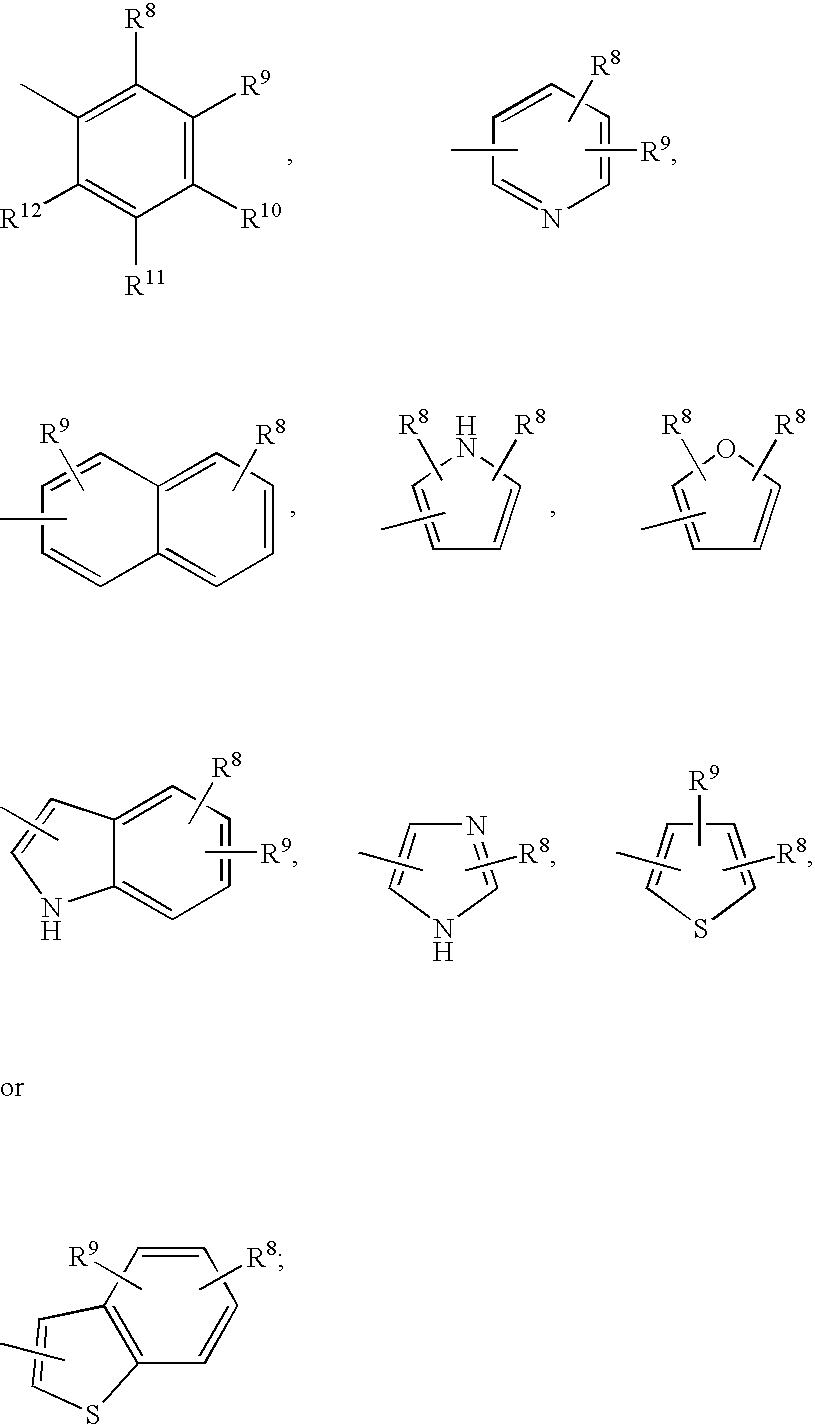
3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors
Owner:JANSSEN PHARMA NV
Novel benzylamine derivatives as CETP inhibitors
The present invention provides, among other things, new benzylamine compounds, compositions comprising benzylamine compounds, methods of making benzylamine compounds, and methods of using benzylamine compounds for treating or preventing a variety of conditions or diseases associated with lipoprotein metabolism.
Owner:DR REDDYS LAB LTD
Polymer Formulations of CETP Inhibitors
ActiveUS20100227903A1Improve bioavailabilityGood dispersionHalogenated hydrocarbon active ingredientsBiocideChemical compoundSURFACTANT BLEND
A pharmaceutical composition comprises (a) a CETP inhibiting compound, or a pharmaceutically acceptable salt thereof; (b) a concentration-enhancing polymer, and (c) optionally one or more surfactants; wherein the compound has the structure shown as Formula I below. The composition raises HDL-cholesterol and lowers LDL-cholesterol.
Owner:MERCK SHARP & DOHME LLC
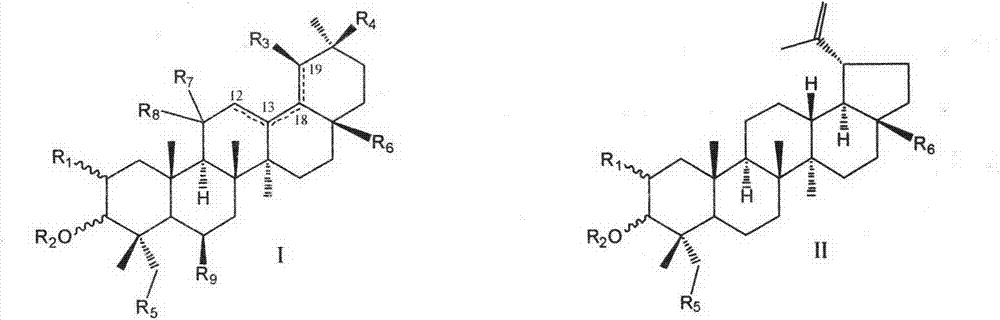
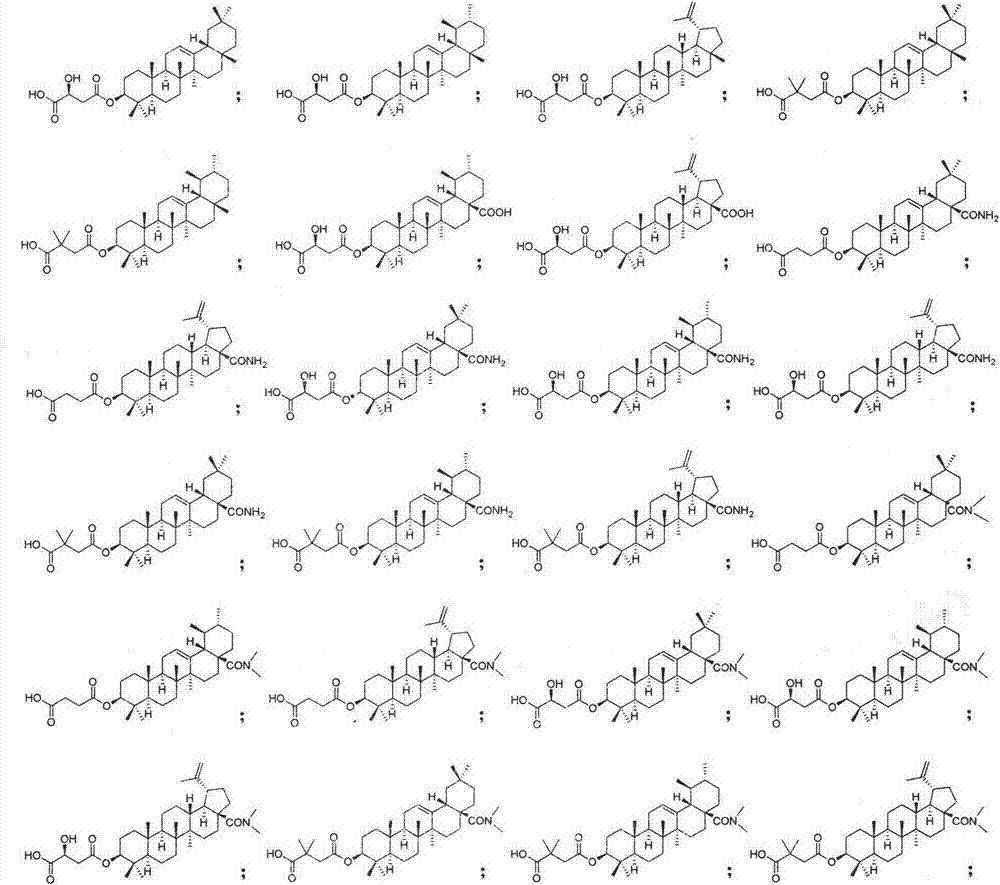
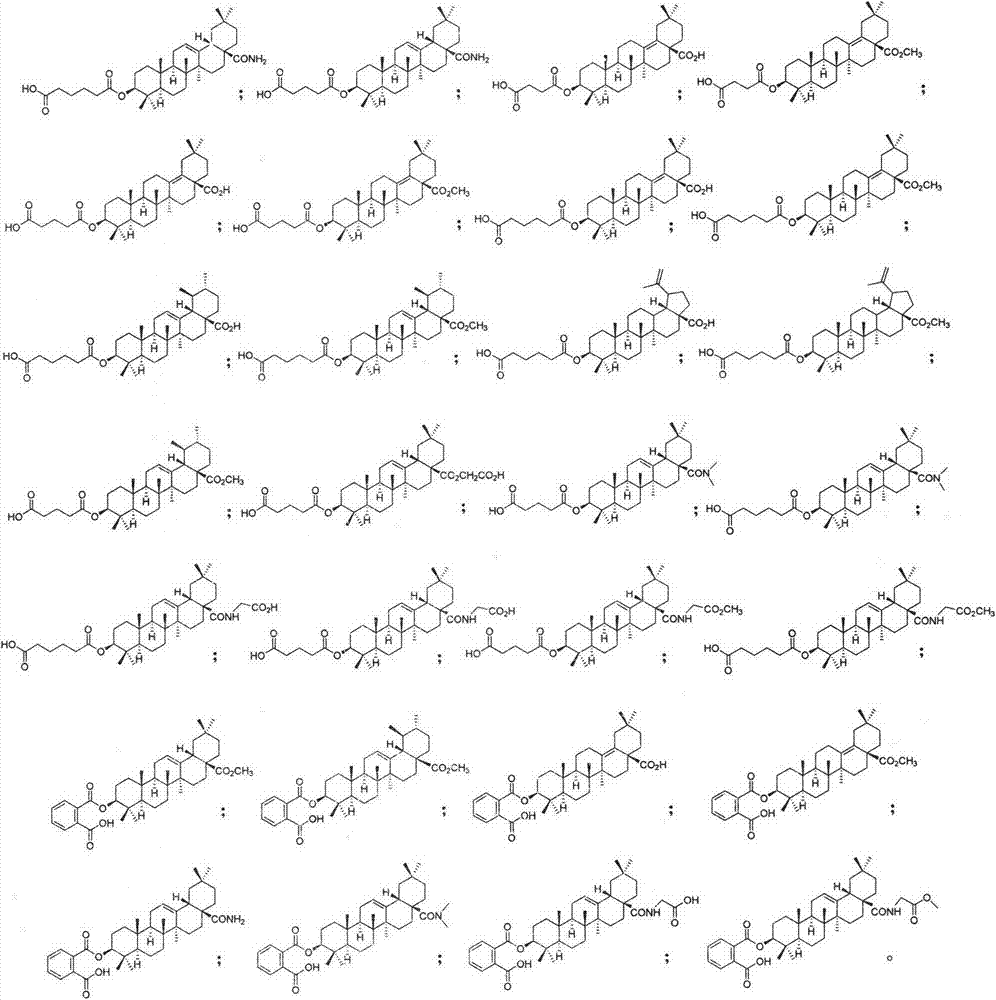
Pentacyclic triterpenoid cholesterol ester transfer protein (CETP) inhibitor, pharmaceutical composition thereof and medical application
The invention relates to the pharmaceutical field, in particular to application of a series of pentacyclic triterpenoid compounds as a cholesterol ester transfer protein (CETP) inhibitor, in particular to application in factors of preparing medicines for treating cardiovascular and cerebrovascular diseases, atherosclerosis diseases and hyperlipemia. The invention further discloses a series of novel pentacyclic triterpenoid compounds, application of the compounds as the CETP inhibitor and a pharmaceutical composition.
Owner:CHINA PHARM UNIV
Compositions of choleseteryl ester transfer protein inhibitors and HMG-CoA reductase inhibitors
InactiveUS20040132771A1Overcomes drawbackImprove concentrationPowder deliveryBiocideHMG-CoA reductaseBioavailability
A composition comprises (1) a solid amorphous adsorbate comprising a cholesteryl ester transfer protein (CETP) inhibitor and a substrate; and (2) an HMG-CoA reductase inhibitor. The solid amorphous adsorbate provides concentration enhancement of the CETP inhibitor relative to a control composition consisting essentially of the unadsorbed CETP inhibitor alone, resulting in improved bioavailability.
Owner:BEND RES
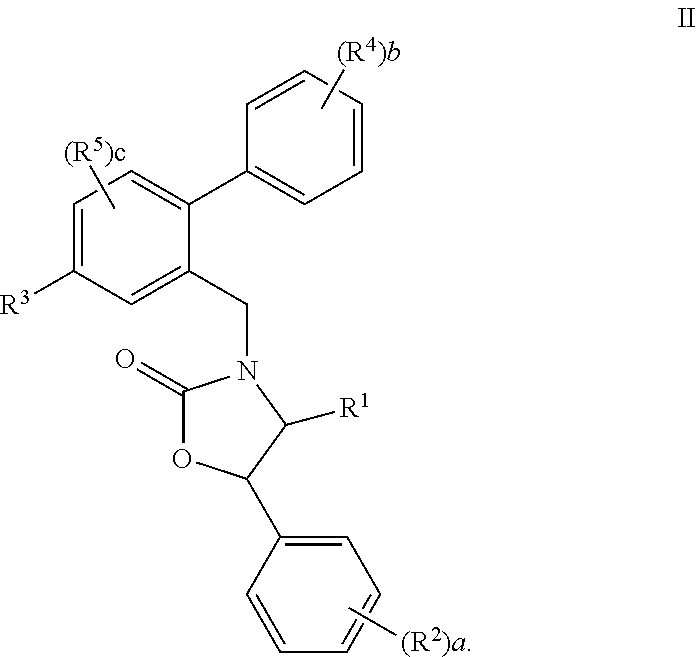
1,3-Oxazolidin-2-One Derivatives Useful as Cetp Inhibitors
Compounds having the structure of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. The compounds have 3 cyclic groups connected by single bonds, as for example triphenyl, which are attached directly to the ring of formula I or attached at the position B.
Owner:MERCK SHARP & DOHME LLC
Cetp Inhibitors
Compounds having the structure of Formula (I), including pharmaceutically acceptable salts of the compounds, are CETP inhibitors and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In the compounds of Formula (I), B is a cyclic group other than phenyl, and B has a cyclic substituent at a position that is ortho to the position at which B is connected to the remainder of the structure of Formula (I). The 5-membered ring of Formula (I) has a second cyclic substituent in addition to B.
Owner:MERCK SHARP & DOHME LLC
CETP inhibitors
Compounds having the structure of Formula (I), including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In the compounds of Formula (I), B or R2 is a phenyl group which has an ortho amine or aminomethyl substituent which is further substituted, and the other of B or R2 is also a cyclic group.
Owner:MERCK SHARP & DOHME LLC
CETP inhibitors
Compounds of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In the compounds of Formula 1, A1 and A2 are each an aromatic ring, a 5-6-membered heterocyclic ring, an aromatic ring fused to a heterocyclic ring, a phenyl ring fused to a heterocyclic ring, or a cycloalkyl ring.
Owner:MERCK SHARP & DOHME LLC
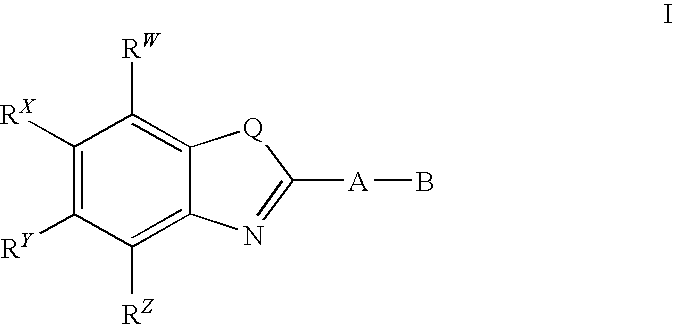
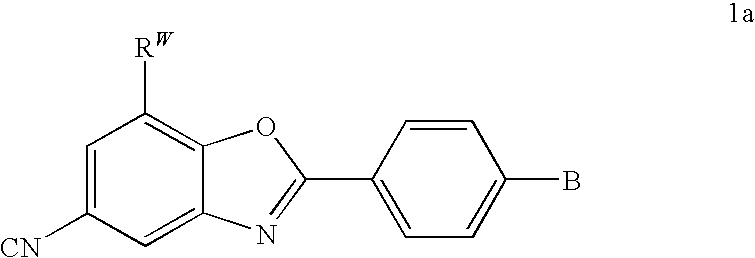
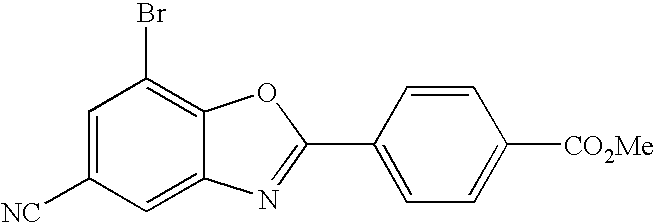
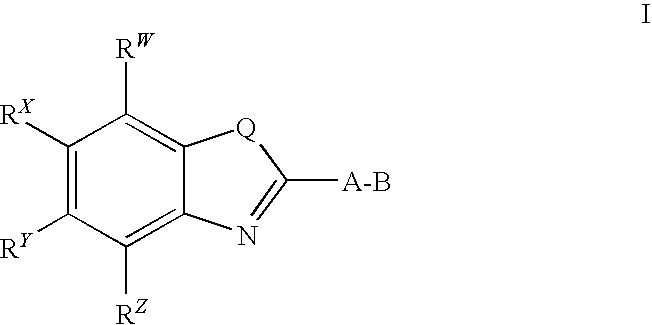
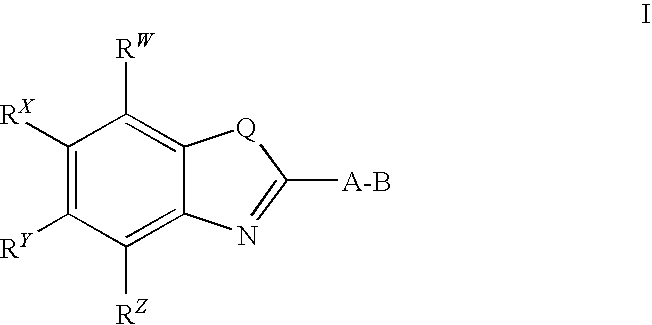
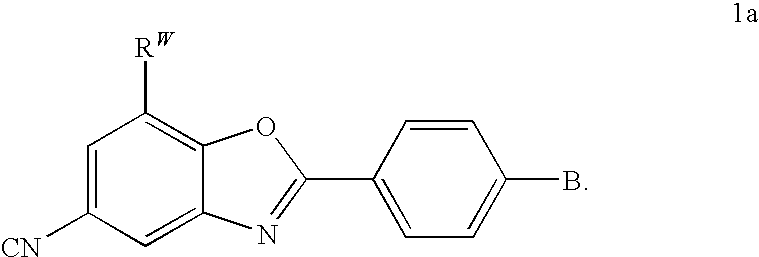
CETP inhibitors derived from benzoxazole arylamides
ActiveUS8445480B2Increasing HDL-cholesterolLowering LDL-cholesterolBiocideOrganic chemistryVascular diseaseBenzoxazole
Owner:MERCK SHARP & DOHME LLC
CETP inhibitors derived from benzoxazole arylamides
ActiveUS8436028B2Increasing HDL-cholesterolLowering LDL-cholesterolBiocideSilicon organic compoundsBenzoxazoleDecreasing ldl
Compounds having the structure of Formula I, including pharmaceutically acceptable salts of the compounds, are potent CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In formula I, A-B is an arylamide moiety.
Owner:MERCK SHARP & DOHME LLC
CETP inhibitors
Compounds having the structure of Formula (I), including pharmaceutically acceptable salts of the compounds, are CETP inhibitors and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In the compounds of Formula (I), B is a cyclic group other than phenyl, and B has a cyclic substituent at a position that is ortho to the position at which B is connected to the remainder of the structure of Formula (I). The 5-membered ring of Formula (I) has a second cyclic substituent in addition to B.
Owner:MERCK SHARP & DOHME LLC
1,3-oxazolidin-2-one derivatives useful as CETP inhibitors
Compounds having the structure of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. The compounds have 3 cyclic groups connected by single bonds, as for example triphenyl, which are attached directly to the ring of formula I or attached at the position B.
Owner:MERCK SHARP & DOHME LLC
Polymer formulations of CETP inhibitors
InactiveUS8030359B2Improve bioavailabilityGood dispersionHalogenated hydrocarbon active ingredientsBiocideCompound (substance)SURFACTANT BLEND
A pharmaceutical composition comprises (a) a CETP inhibiting compound, or a pharmaceutically acceptable salt thereof; (b) a concentration-enhancing polymer, and (c) optionally one or more surfactants; wherein the compound has the structure shown as Formula I below. The composition raises HDL-cholesterol and lowers LDL-cholesterol.
Owner:MERCK SHARP & DOHME LLC
Bicyclic ureas and thiadiazolidine-1, 1-dioxides as CETP inhibitors
Compounds having the structure of Formula I, including pharmaceutically acceptable salts of the compounds, wherein X is —C(═O) or —S(O)2—, are CETP inhibitors and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis.
Owner:MERCK SHARP & DOHME LLC
CETP Inhibitors
Compounds having the structure of Formula (I), including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis. In the compounds of Formula (I), B or R2 is a phenyl group which has an ortho amine or aminomethyl substituent which is further substituted, and the other of B or R2 is also a cyclic group.
Owner:MERCK SHARP & DOHME LLC
Use of CETP inhibitors and optionally HMG COA reductable inhibitors and/or antihypertensive agents
The present invention relates to cholesteryl ester transfer protein (CETP) inhibitors, pharmaceutical compositions containing such inhibitors and the use of such inhibitors optionally in combination with certain therapeutic agents, such as antihypertensive agents, in the treatment of certain diseases / disorders Applications.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
Self-emulsifying formulations of cholesteryl ester transfer protein inhibitors
InactiveUS20060014788A1Reduce food effectGood miscibilityBiocideOrganic chemistrySolventCholesteryl ester
CETP Inhibitors have improved solubility and bioavailability in a lipophilic vehicle comprising a digestible oil, a lipophilic solvent, or a surfactant. Preferred such compositions are self-emulsifying or self-microemulsifying, and comprise 1. a CETP inhibitor; 2. a cosolvent; 3. a surfactant having an HLB of 1 to 8; 4. a surfactant having an HLB of over 8 to 20; and 5. optionally, a digestible oil.
Owner:PFIZER INC
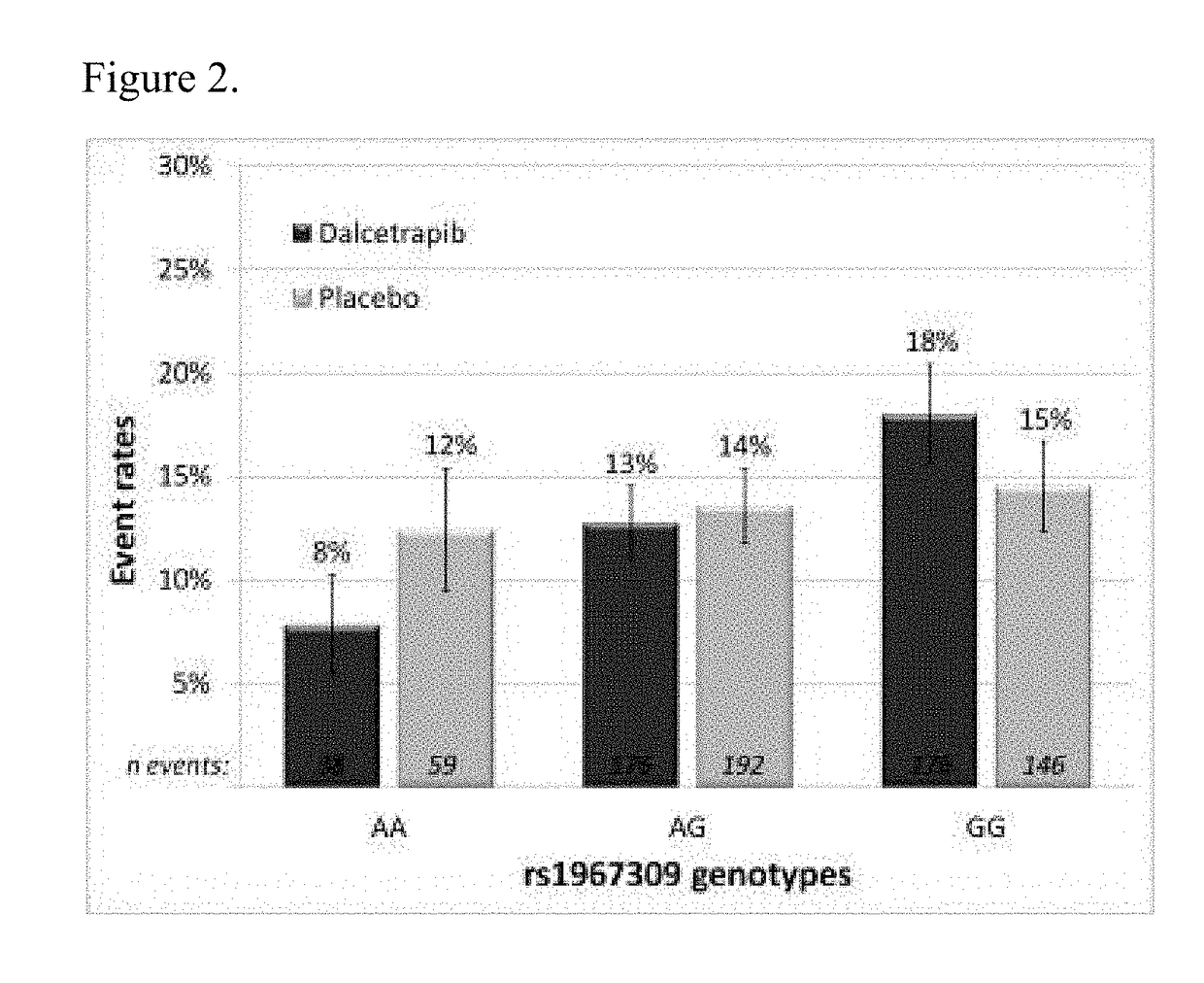
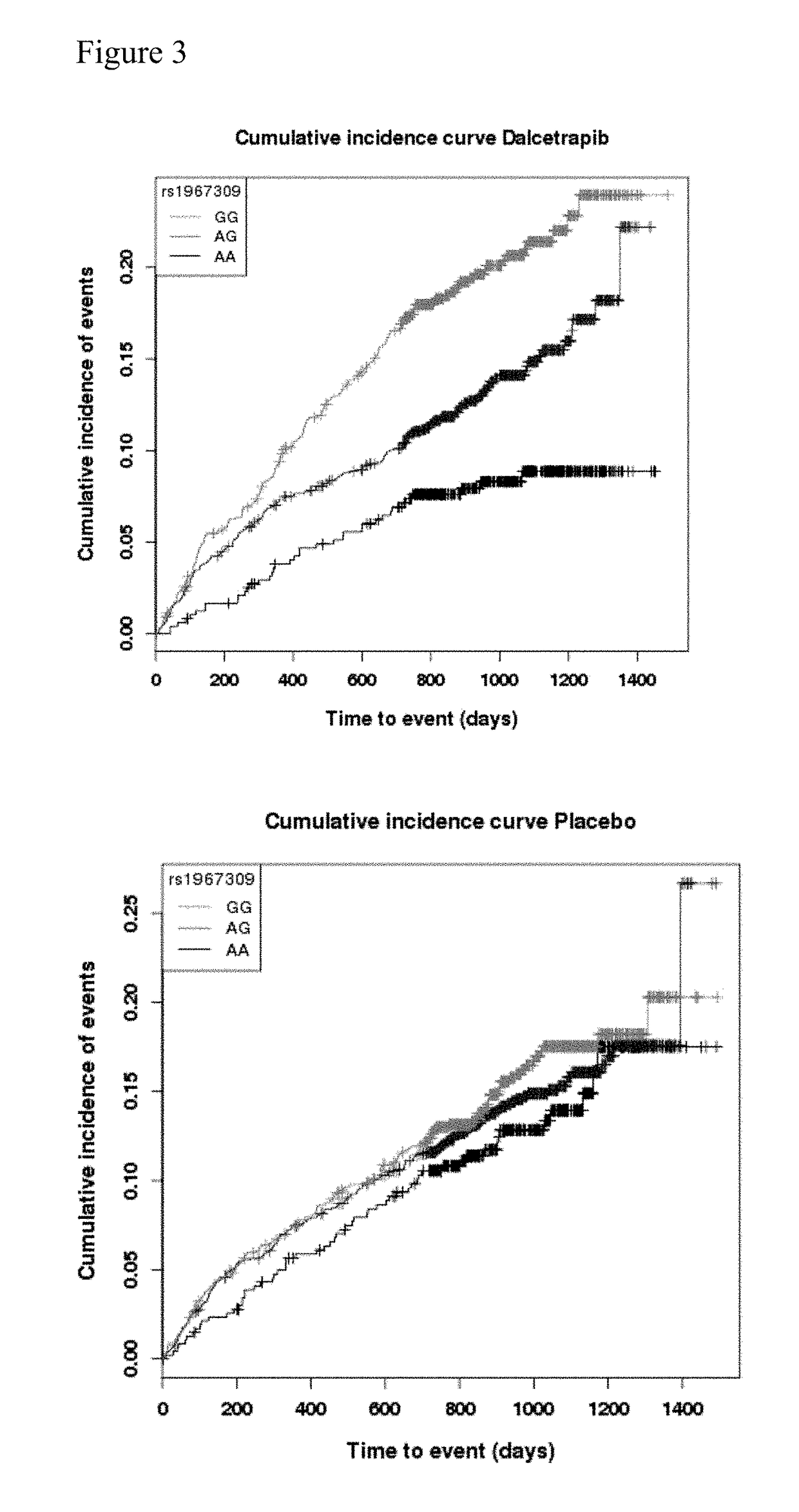
Genetic markers for predicting responsiveness to therapy with hdl-raising or hdl mimicking agent
Genotyping methods and compositions for selecting patients with cardiovascular disease who will benefit from treatment with HDL-raising or HDL mimicking agent, in particular with a CETP inhibitor / modulator
Owner:F HOFFMANN LA ROCHE & CO AG
CETP inhibitors
Compounds of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis.
Owner:MERCK SHARP & DOHME LLC
Heterocyclic CETP inhibitors
Owner:BRISTOL MYERS SQUIBB CO
Fused bicyclic oxazolidinone CETP inhibitor
Compounds having the structure of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis.
Owner:MERCK SHARP & DOHME LLC
Cetp Inhibitors
Compounds having the structures of Formula I, including pharmaceutically acceptable salts of the compounds, are CETP inhibitors, and are useful for raising HDL-cholesterol, reducing LDL-cholesterol, and for treating or preventing atherosclerosis:In the compounds of Formula I, B or R2 is a phenyl group which has an ortho aryl, heterocyclic, benzoheterocyclic or benzocycloalkyl substituent, and one other position on the 5-membered ring has an aromatic, heterocyclic, cycloalkyl, benzoheterocyclic or benzocycloalkyl substituent connected directly to the ring or attached to the ring through a —CH2—.
Owner:ALI AMJAD +12
Prodrugs of oxazolidinone CETP inhibitors
ActiveUS20110218177A1Easy to convertBiocideOrganic compound preparationStereochemistryCETP inhibitor
The compounds of Formula I are pro-drugs of CETP inhibitors having a central oxazolidinone ring. The compounds cyclize by the elimination of HX to form an oxazolidinone ring after administration to a patient.
Owner:MERCK SHARP & DOHME LLC
1,2,3,4-tetrahydro-quinoline derivatives as CETP inhibitors
Owner:JANSSEN PHARMA NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors 3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors](https://images-eureka.patsnap.com/patent_img/e909f95f-0ce5-414d-b397-a3a54c263a0f/US20070265252A1-20071115-C00001.png)
![3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors 3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors](https://images-eureka.patsnap.com/patent_img/e909f95f-0ce5-414d-b397-a3a54c263a0f/US20070265252A1-20071115-C00002.png)
![3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors 3,4-dihydro-2h-benzo[1,4]oxazine and thiazine derivatives as CETP inhibitors](https://images-eureka.patsnap.com/patent_img/e909f95f-0ce5-414d-b397-a3a54c263a0f/US20070265252A1-20071115-C00003.png)