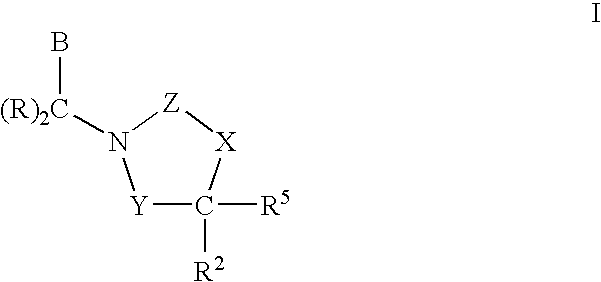
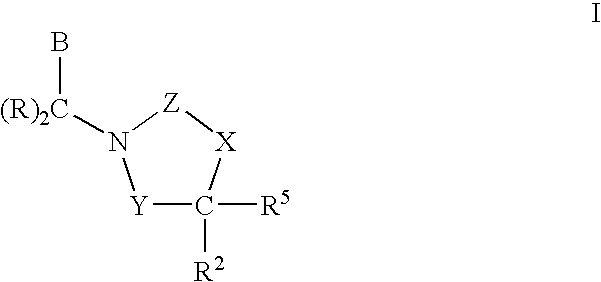
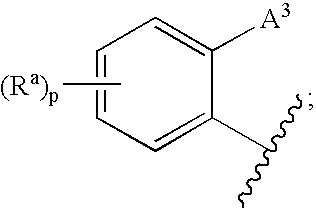
Cetp Inhibitors
a technology of hdl-c and inhibitors, applied in the field of chemical compounds, can solve the problems of chd risk, high risk of statins, and true burden on health care systems, and achieve the effect of only reducing the risk of approximately one-third
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0227]
2-Amino-5-(trifluoromethyl)benzonitrile
[0228]A 2-liter flask was charged with 100 g (0.348 mol) of 4-amino-3-iodobenzotrifluoride, 40 g of CuCN and 750 mL of DMF. The mixture was heated to and then maintained at reflux for 1 hour. The reaction was cooled and poured into 3 L of water containing 300 mL of concentrated ammonium hydroxide. To the mixture was added 1 L CH2Cl2. The mixture was then filtered through celite. The layers were separated and the aqueous layer was back extracted with CH2Cl2. The organic extracts were combined and the solvent removed under reduced pressure. The residue was dissolved in 1.5 L of ether and the resulting solution was washed with 1N ammonium hydroxide, aqueous sodium bisulfite, 1N aqueous HCl and brine. The solution was dried over anhydrous MgSO4 and filtered through a silica gel plug containing a layer of MgSO4 on top. The plug was washed with 0.5 L ether. The ether solutions were combined and concentrated to 750 mL and let stand at room tempe...
example 2
[0229]
2-Iodo-5-(trifluoromethyl)benzonitrile
[0230]To a solution of 2-amino-5-(trifluoromethyl)benzonitrile (15.1 g) and diiodomethane (24 mL) in acetonitrile (150 mL) at 35° C. was added t-butyl nitrite (21 mL) dropwise. The reaction was maintained at 30-35° C. during the addition. The reaction was aged for 30 min and then was heated to 60° C. for 30 minutes. The reaction mixture was cooled, diluted with ether and washed 2× with water, 2× with aqueous sodium bisulfite, water and then brine. The solution was dried over anhydrous MgSO4, filtered through a silica gel plug and then concentrated giving 100 g of a red oil. The product was purified by silica gel chromatography eluting sequentially with hexanes, 3:1 hexanes / CH2Cl2 and 1:1 hexanes / CH2Cl2 to afford 2-iodo-5-(trifluoromethyl)benzonitrile. 1H NMR (CDCl3, 500 MHz) δ 8.10 (d, J=8.5 Hz, 1H), 7.85 (d, J=1.8 Hz, 1H), 7.52 (dd, J=8.5, 1.8 Hz, 1H).
example 3
[0231]
5′-Isopropyl-2′-methoxy-4-(trifluoromethyl)biphenyl-2-carbonitrile
[0232]To a solution of 2-iodo-5-(trifluoromethyl)benzonitrile (2.0 g, 6.7 mmol) and (5-isopropyl-2-methoxyphenyl)boronic acid (1.6 g, 8.4 mmol) in dimethyl ethylene glycol (30.4 mL) was added 2M Na2CO3 (6.8 mL), ethanol (9.6 mL), and water (10 mL). The solution was degassed with nitrogen for 2 minutes. Pd(PPh3)4 (774 mg, 0.67 mmol) was added and the solution was degassed with nitrogen again for 2 minutes. The solution was divided equally into two 40 mL microwave tubes. Each tube was degassed with nitrogen for 1 minute, sealed, and placed in a microwave reactor. The wattage was set for 200 W until the temperature reached 150° C. and then the temperature was held at 150° C. for ten minutes. The tubes were then cooled to room temperature, combined, poured into H2O (50 mL), and extracted with EtOAc (100 mL). The organic layer was washed with brine (50 mL), dried over Na2SO4, filtered, and concentrated. Purification ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com