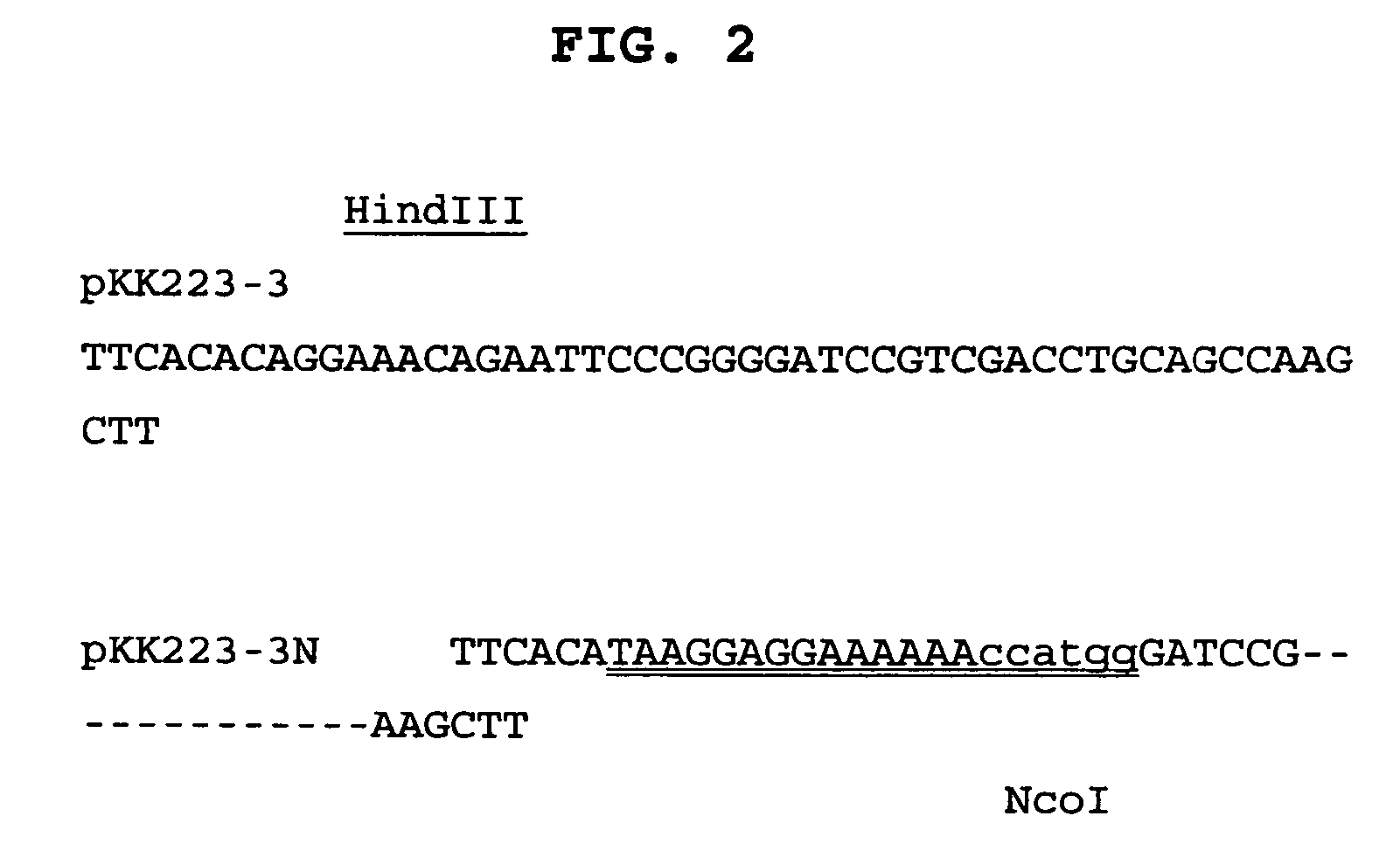
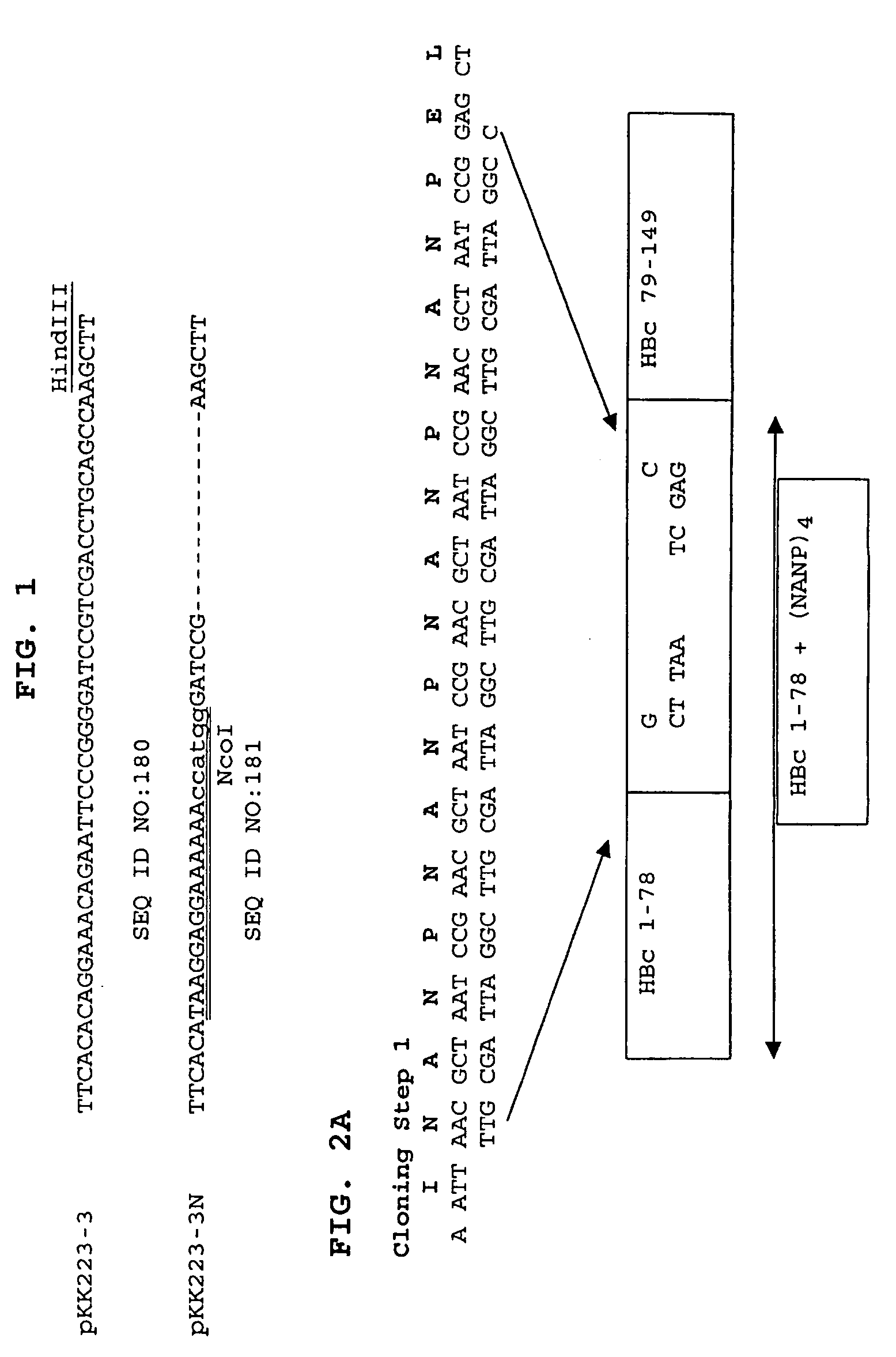
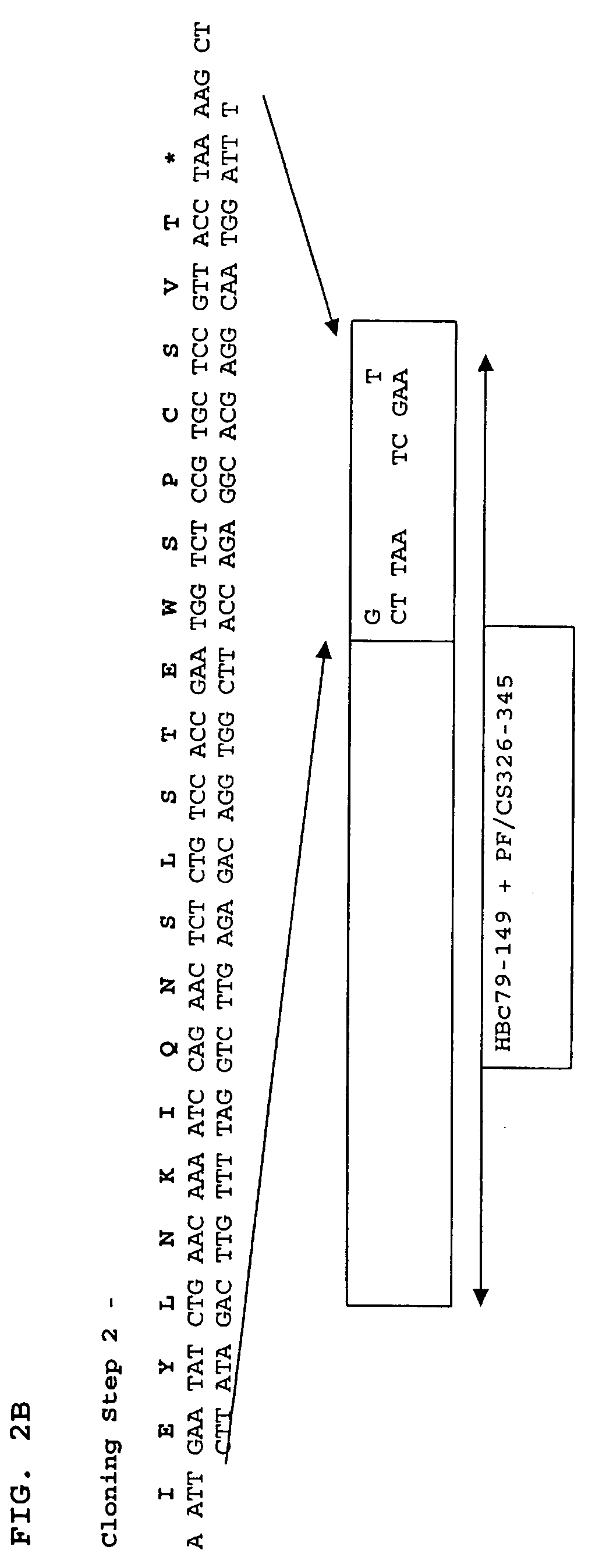
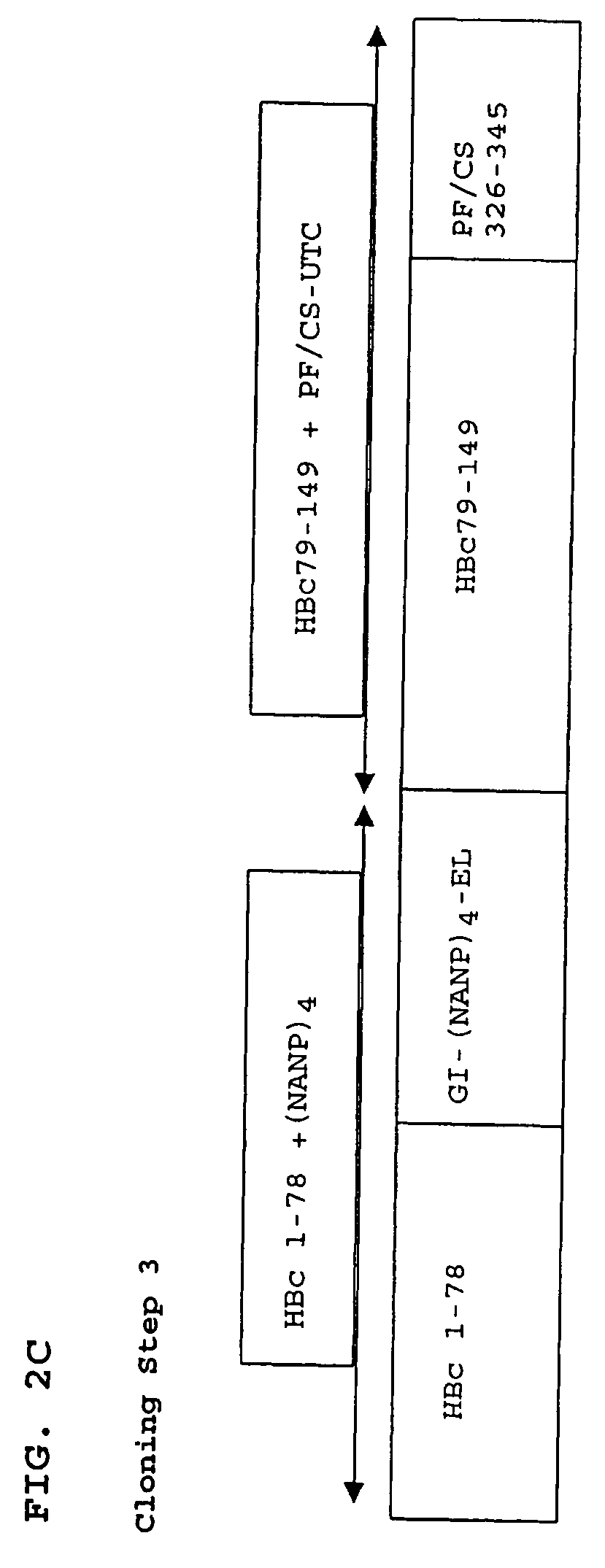
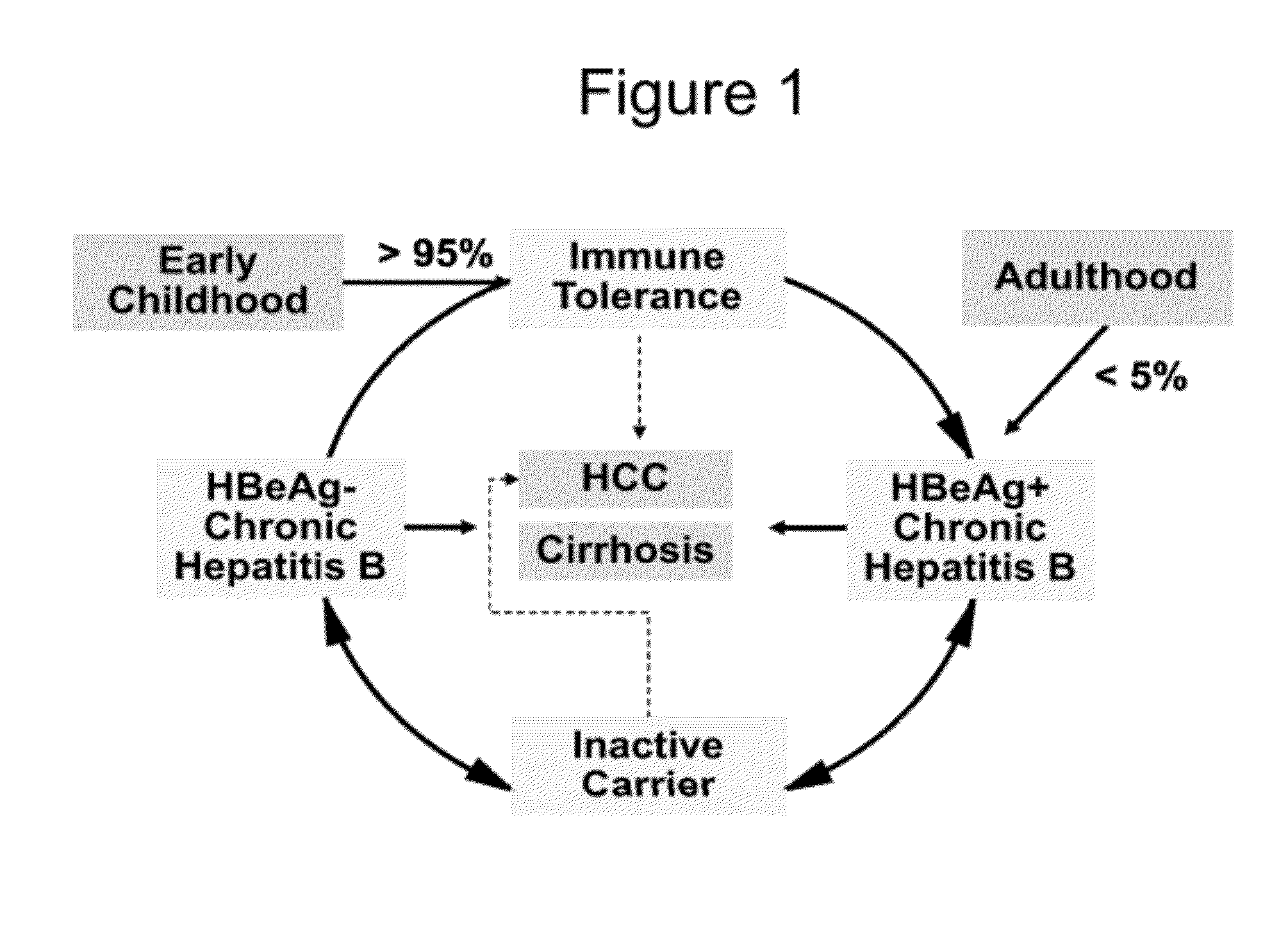
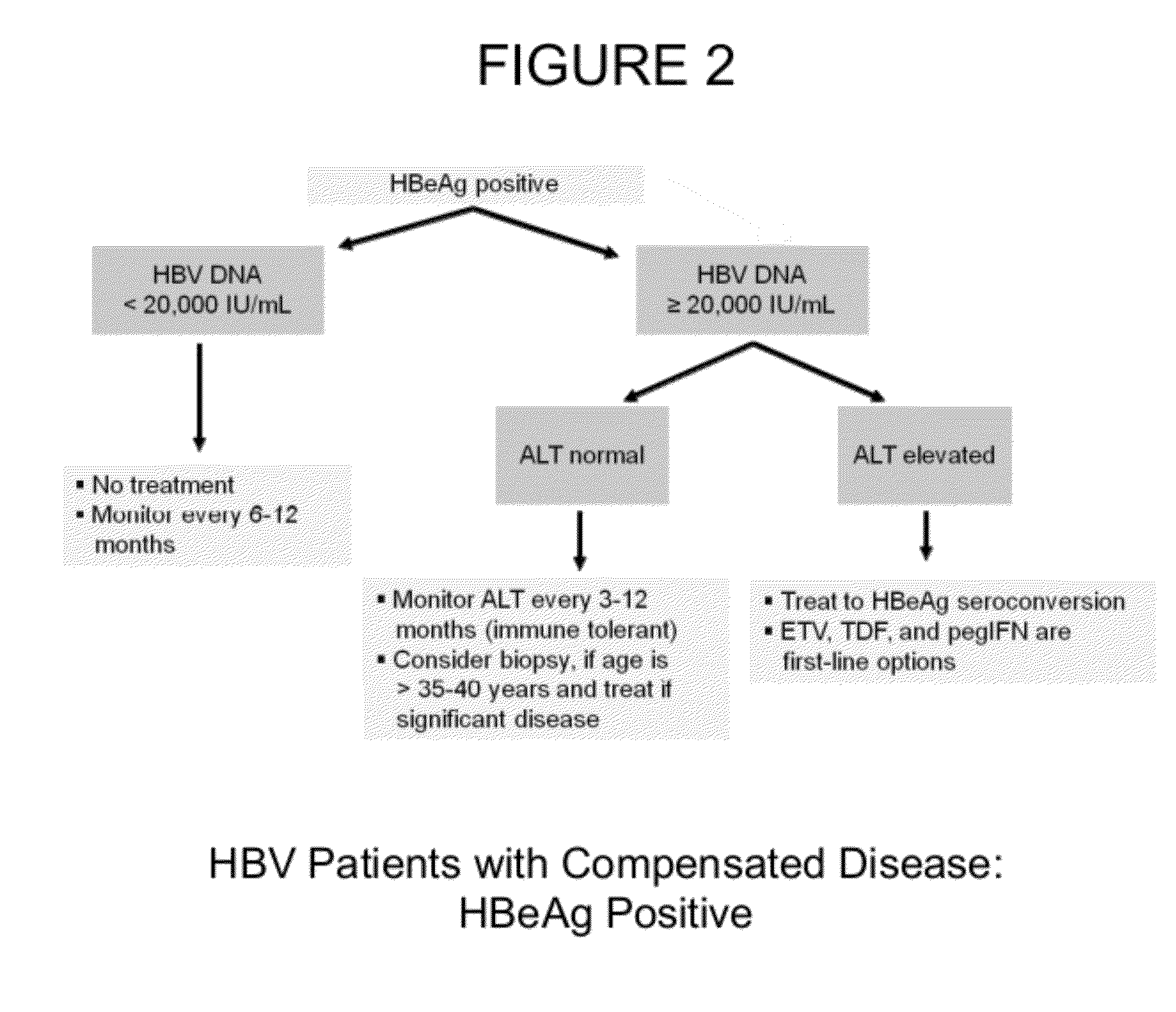
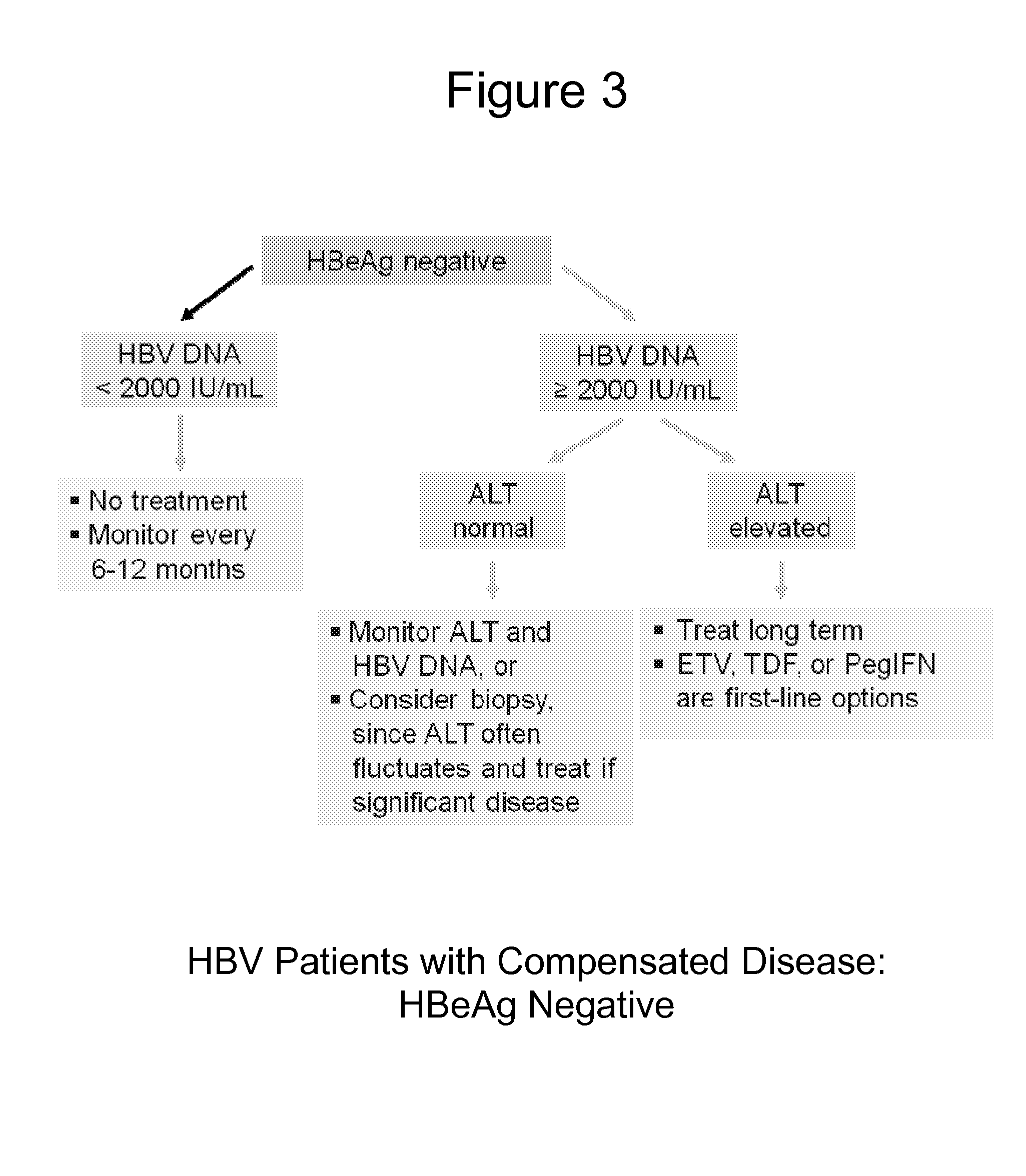
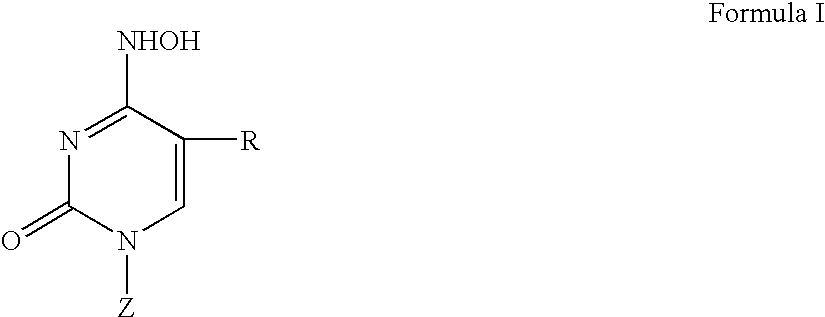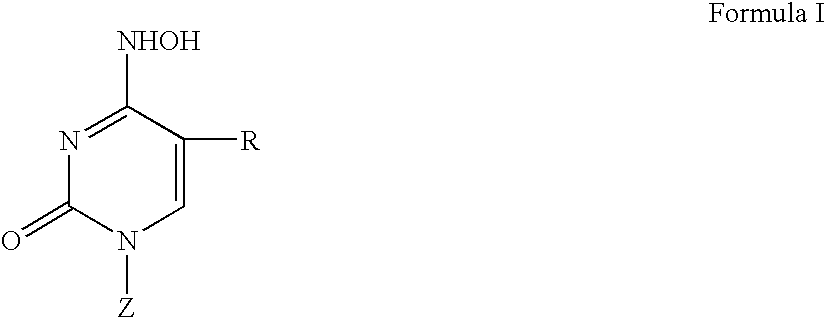Patents
Literature
666 results about "Hepatitis B immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hepatitis B vaccine is an injectable vaccine containing the outer cell wall of the hepatitis B virus. This induces the production of the antibodies that attack the outer cell wall without risking infecting the patient with the disease.
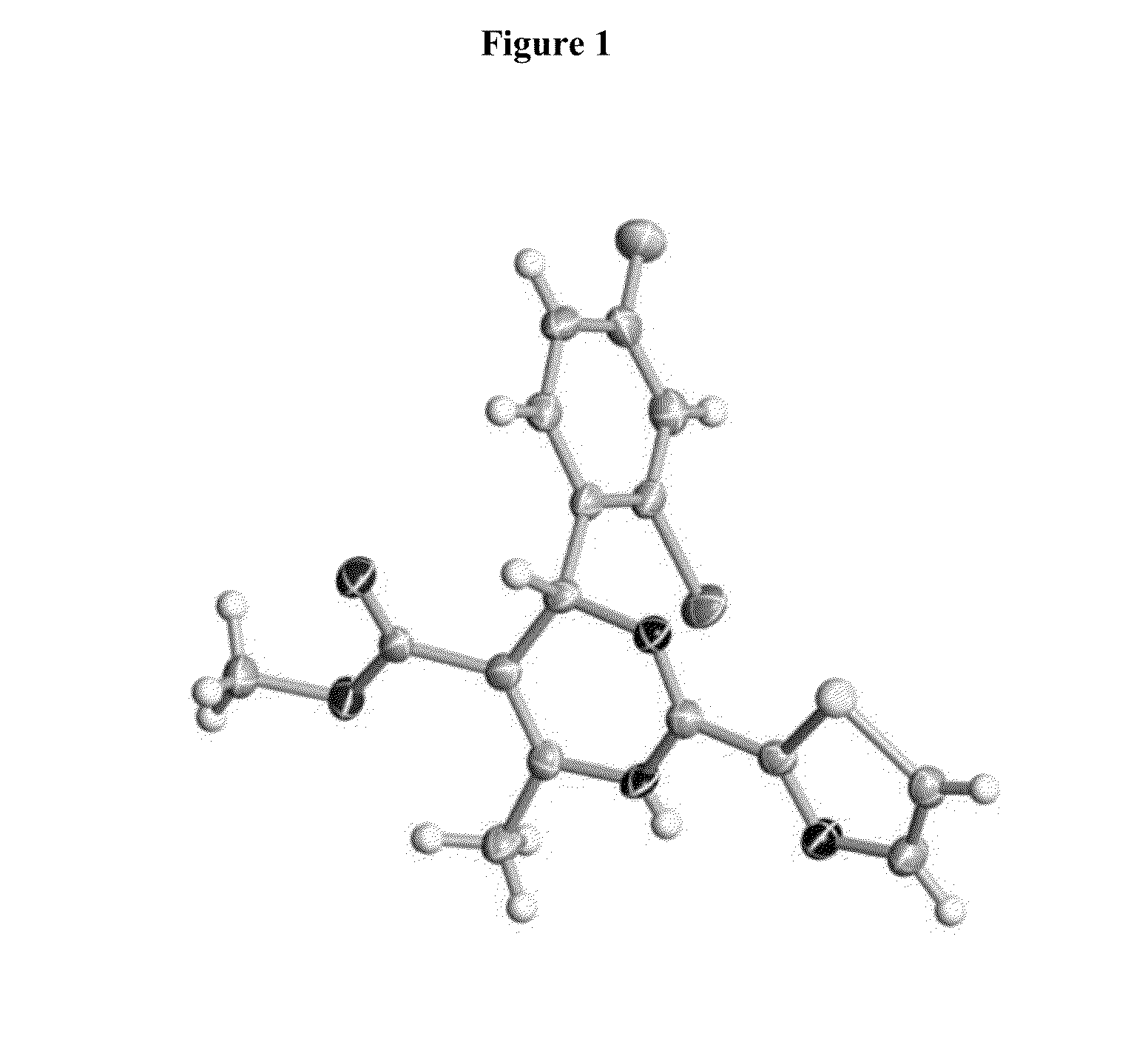
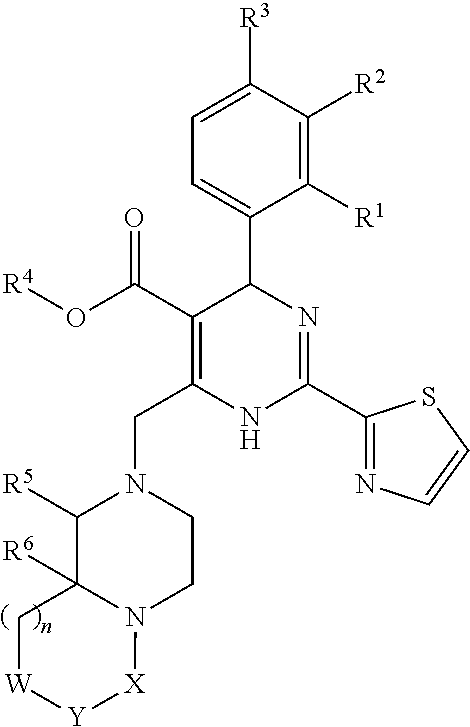
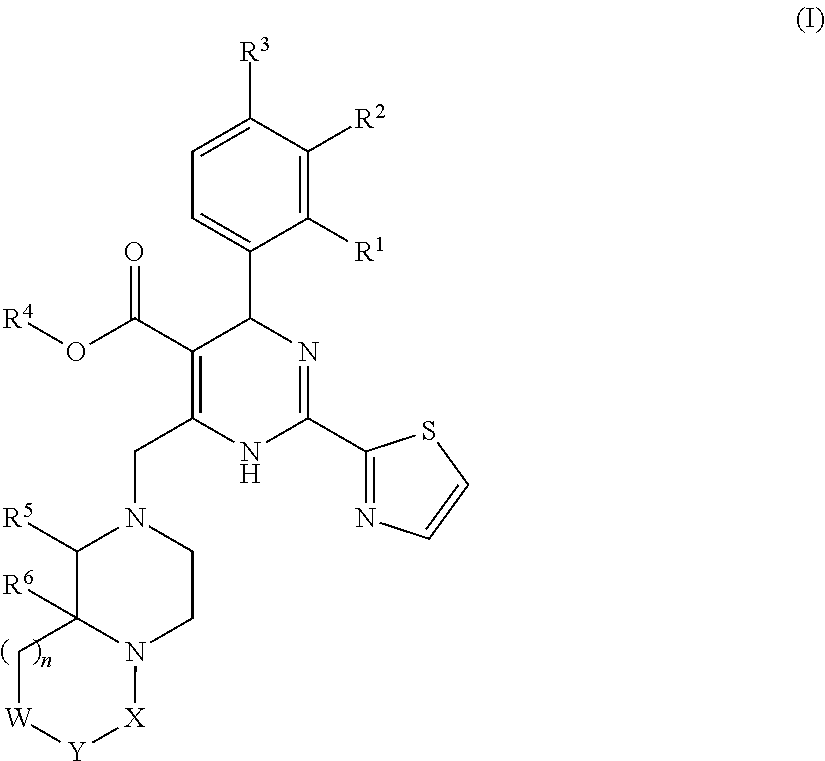
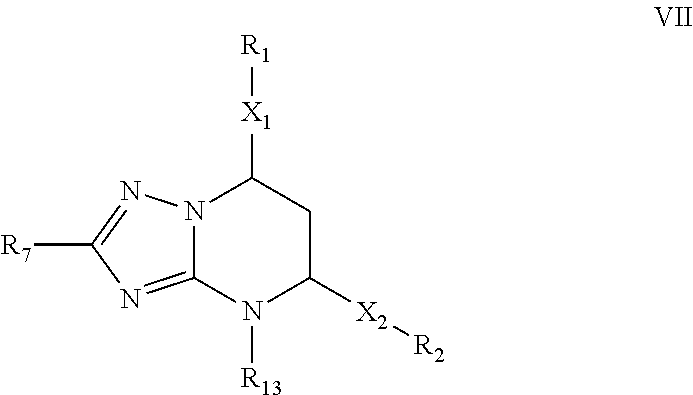
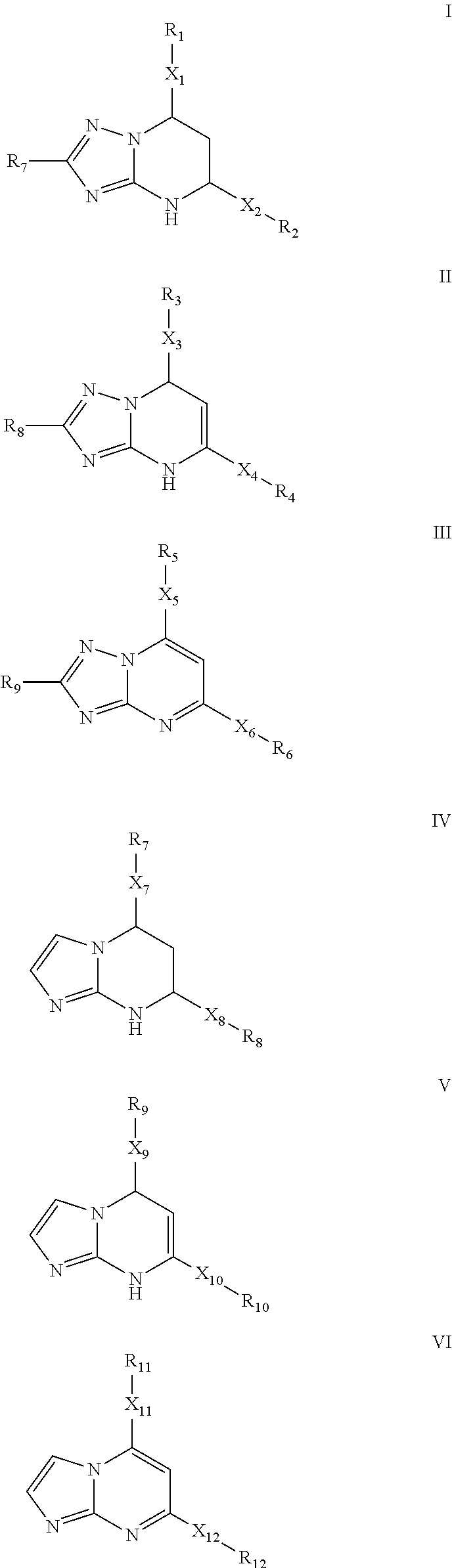
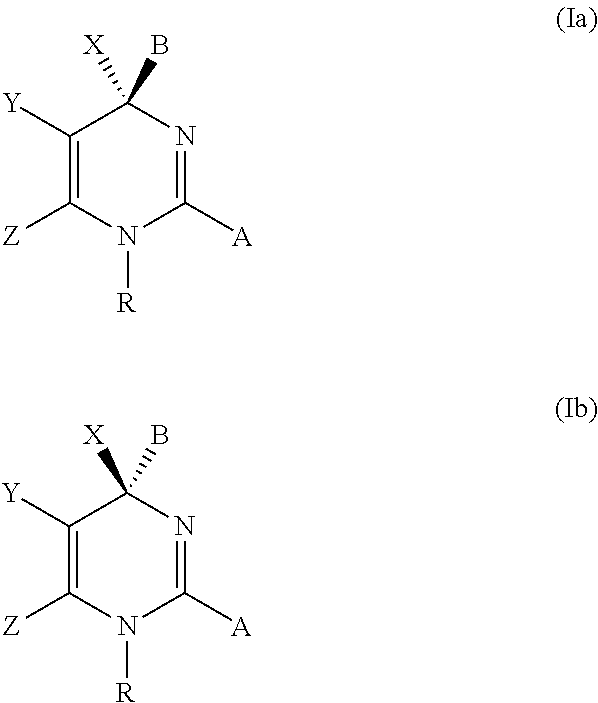
Novel 6-fused heteroaryldihydropyrimidines for the treatment and prophylaxis of hepatitis B virus infection
Owner:F HOFFMANN LA ROCHE INC
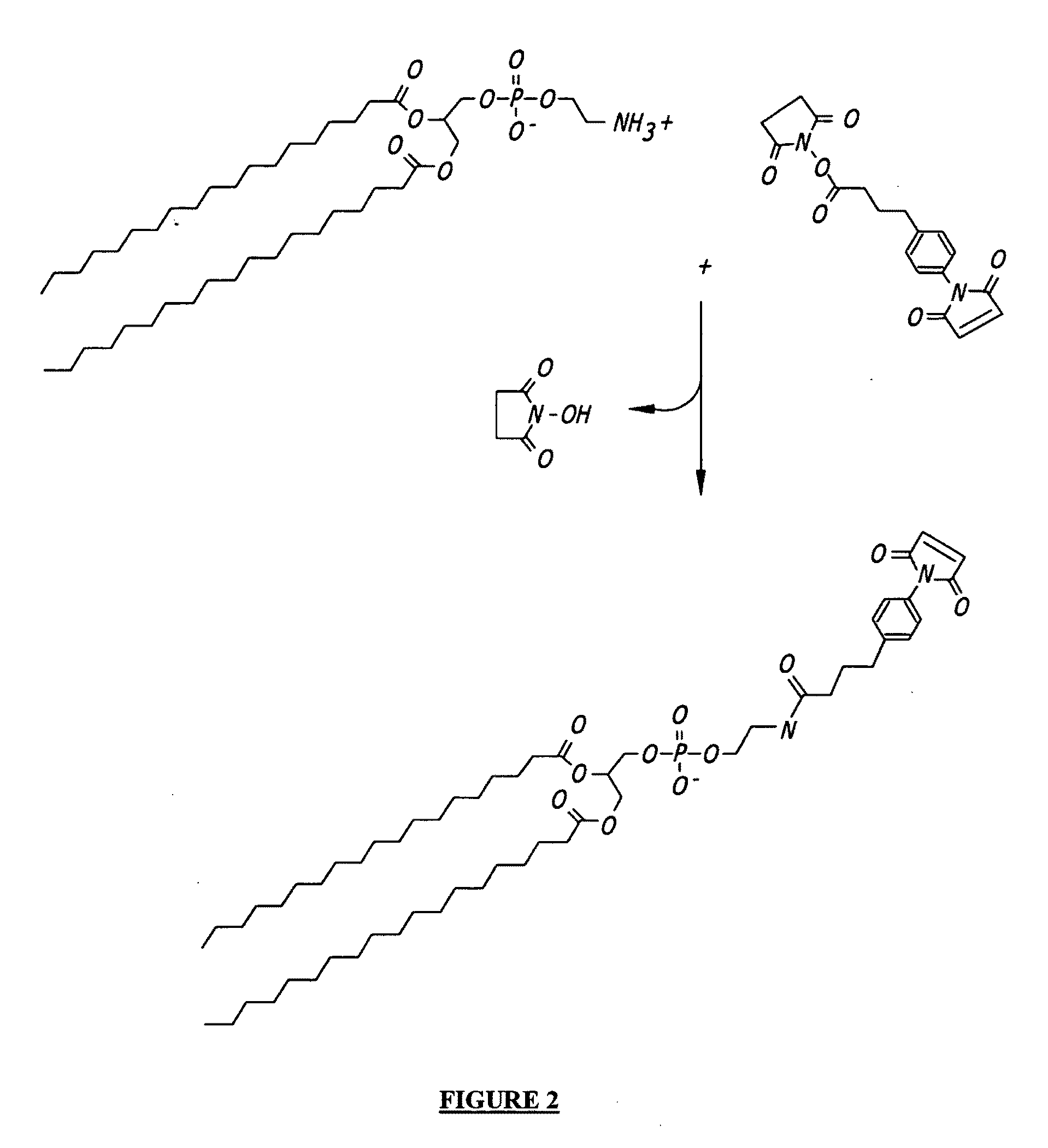
Self-assembling nanoparticle drug delivery system
InactiveUS20090226525A1High binding affinityHigh affinityPowder deliveryMicroencapsulation basedLipid formationMedicine
A self-assembling nanoparticle drug delivery system for the delivery of various bioactive agents including peptides, proteins, nucleic acids or synthetic chemical drugs is provided. The self-assembling nanoparticle drug delivery system described herein includes viral capsid proteins, such as Hepatitis B Virus core protein, encapsulating the bioactive agent, a lipid layer or lipid / cholesterol layer coat and targeting or facilitating molecules anchored in the lipid layer. A method for construction of the self-assembling nanoparticle drug delivery system is also provided.
Owner:CHIMEROS
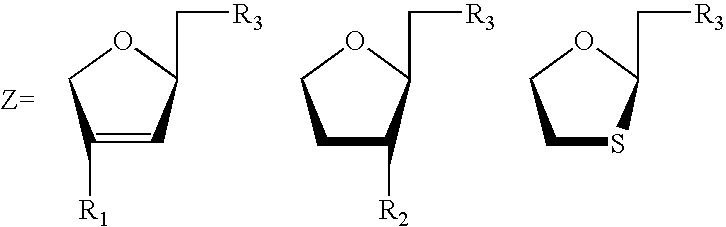
3′-or 2′-hydroxymethyl substituted nucleoside derivatives for treatment of hepatitis virus infections
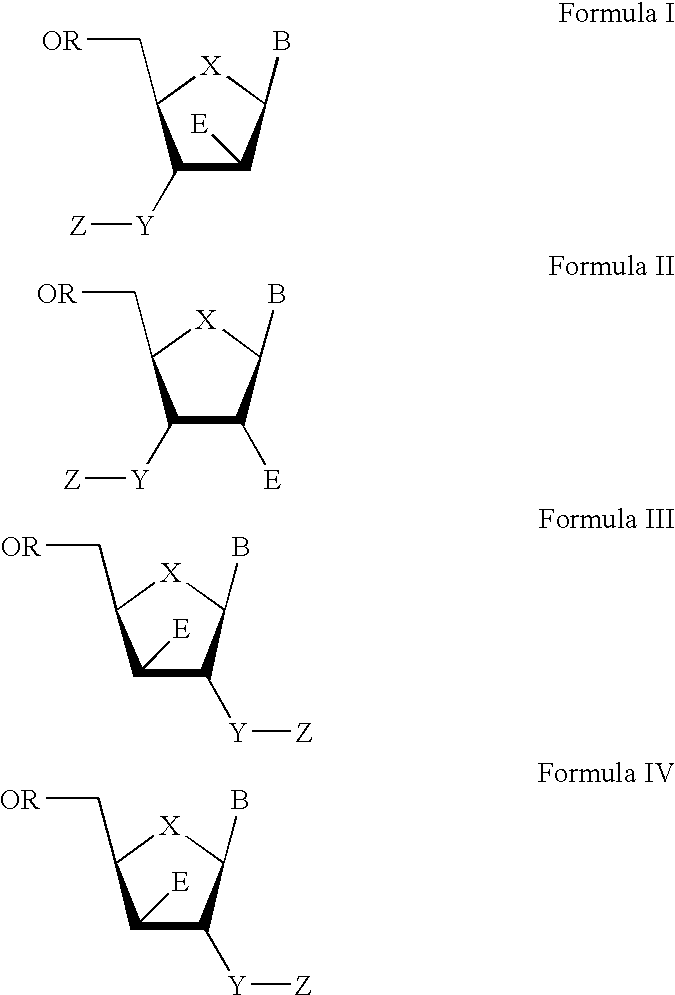
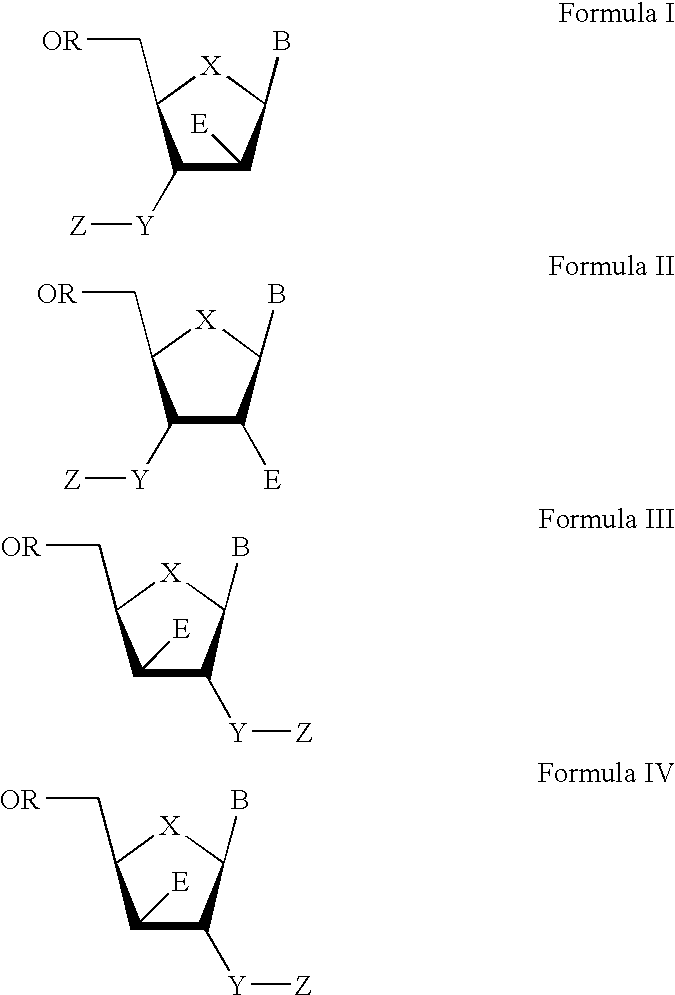
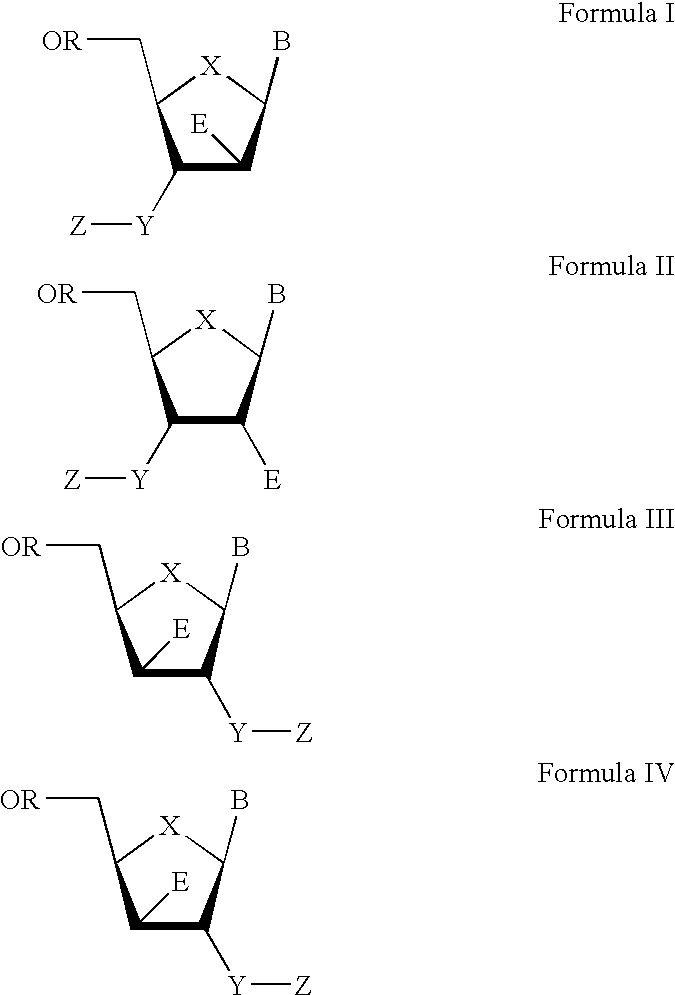
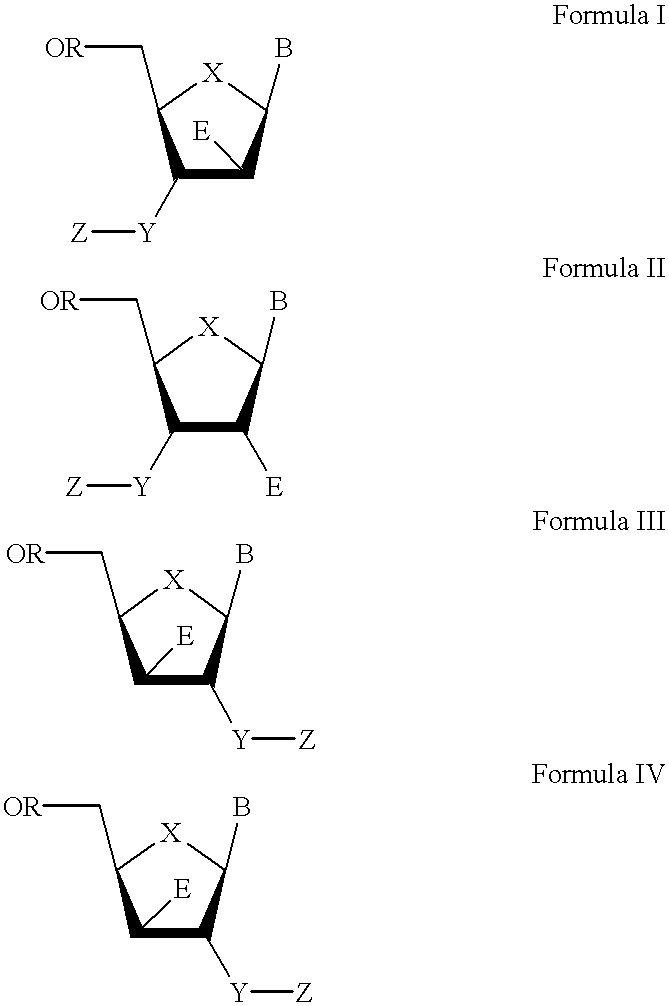
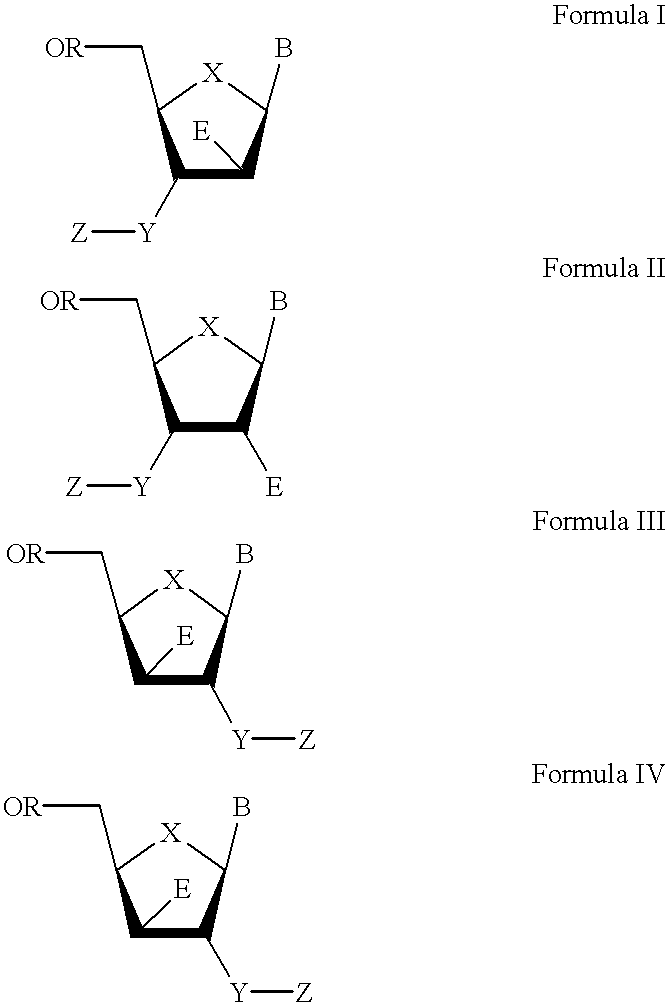
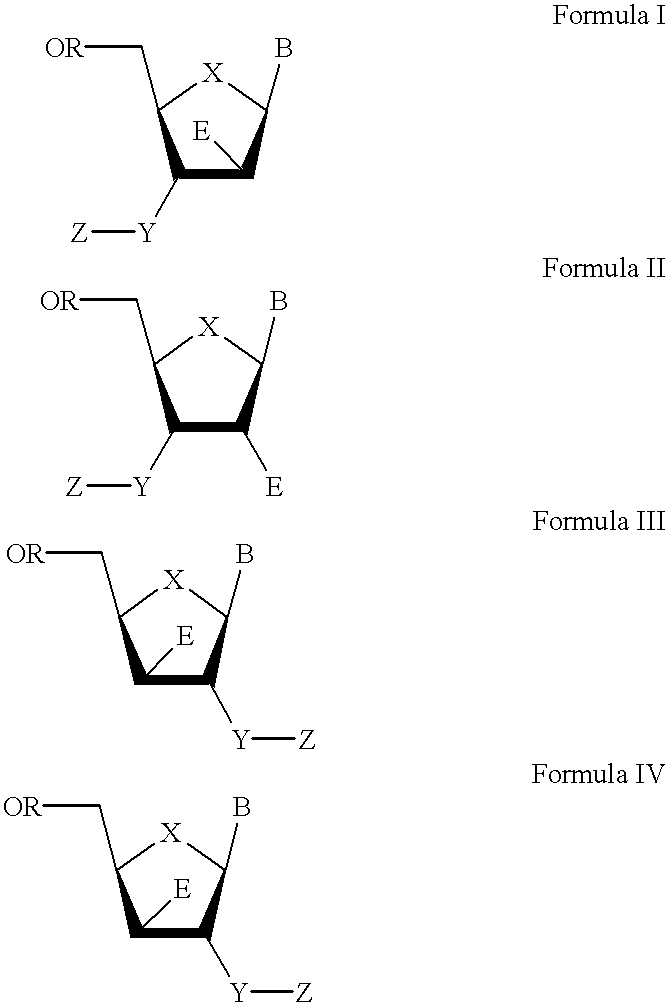
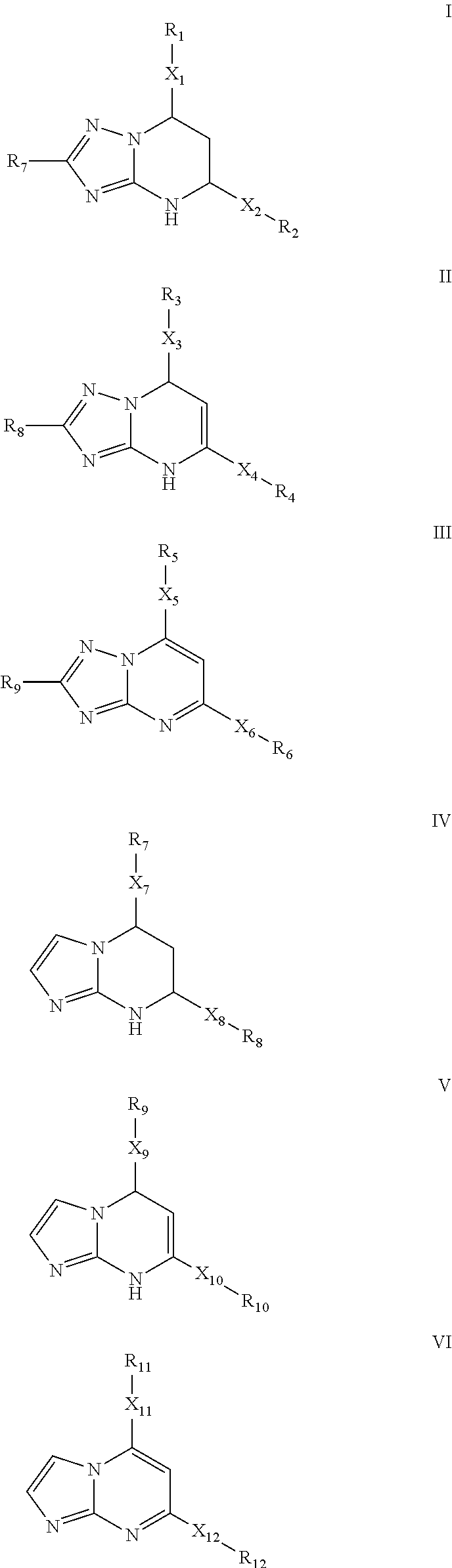
The present invention relates to a composition for and a method of treating hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hepatitis D virus (HDV) infection or a proliferative disorder in a patient using an effective amount of a compound selected from the group consisting of formulas [I]–[IV] below and mixtures of two or more thereof:wherein the substituents are as defined herein. Pharmaceutical compositions comprising these compounds in combination with other HBV, HCV, or HDV agents is also disclosed.
Owner:PHARMASSET
Pharmaceutical use of ent-eudesmane alcohol type sesquiterpene for inhibiting hepatitis virus
InactiveCN1935762APrevention and treatment of viral hepatitis BHBsAg reductionSugar derivativesHydroxy compound active ingredientsDiseaseSolvent
The invention relates to an enantiomorphic amine alkyl sesquiterpene alcohol and glucoside and the medicated salt or solvent thereof, as well as the effect and activity of the composed medicine combination, mainly relating to the medical use in reducing HBV-DNA replication activity. And it has considerably strong inhibiting effect on HBsAG screted by HepG2.2.15 and HBV-DNA replication as compared with positive contrast Lamivudine; and it has obvious inhibition activity to HBV-DNA replication at large dosage (100 mug / mL) and medium dosage(20 mug / mL) as contrasted with Lamivudine, and can be expected to apply to preparing medicines for curing HB virus infection disease.
Owner:赵昱
3'-or 2'-hydroxymethyl substituted nucleoside derivatives for treatment of hepatites virus infections
InactiveUS20020055483A1Good effectEasy to modifyBiocideSugar derivativesDiseaseHepatitis B immunization
The present invention relates to a composition for and a method of treating hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hepatitis D virus (HDV) infection or a proliferative disorder in a patient using an effective amount of a compound selected from the group consisting of formulas [I]- [IV] below and mixtures of two or more thereof: wherein the substituents are as defined herein. Pharmaceutical compositions comprising these compounds in combination with other HBV, HCV, or HDV agents is also disclosed.
Owner:PHARMASSET
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Influenza immunogen and vaccine
InactiveUS20060115489A1High antibody titerEasy to prepareSsRNA viruses negative-senseAntibody mimetics/scaffoldsHepatitis B immunizationHepatitis B virus
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (HBc) protein is disclosed that contains an immunogen for inducing the production of antibodies to the influenza M2 protein. An immunogenic influenza sequence in two to four copies is preferably expressed at or near the N-terminus or in the HBc immunogenic loop sequence. The HBc chimer preferably contains an influenza-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:SANOFI PASTEUR BIOLOGICS CO +1
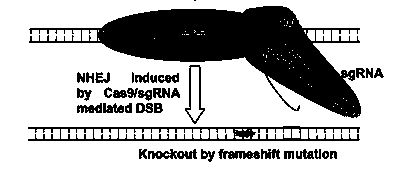
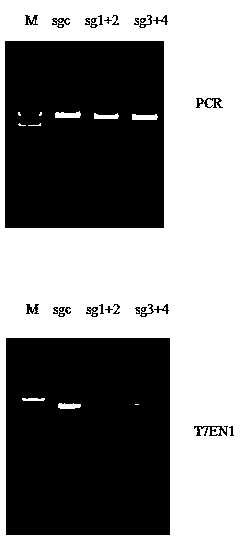
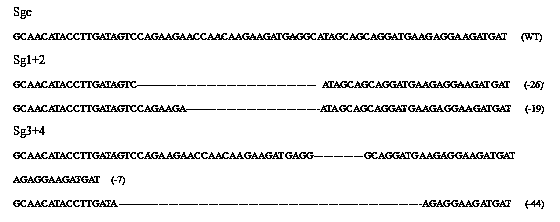
CRISPR-Cas9 targeted knockout hepatitis b virus cccDNA and specific sgRNA thereof
The invention belongs to the field of genetic engineering, and particularly relates to a method for specifically knocking out hepatitis b virus cccDNA by using CRISPR-Cas9 and sgRNA for specifically targeting the hepatitis b virus cccDNA. The invention provides a method for specifically knocking out hepatitis b virus cccDNA by using CRISPR-Cas9 and sgRNA for specifically targeting the hepatitis b virus cccDNA. The sgRNA of specific targeted hepatitis b virus cccDNA prepared according to the invention can precisely target hepatitis b virus cccDNA and realize gene knockout. A preparation method is simple in steps and good in sgRNA targeting, and the knockout efficiency of a CRISPR-Cas9 system is high.
Owner:AOMIAO BIOTECH GUANGZHOU CO LTD
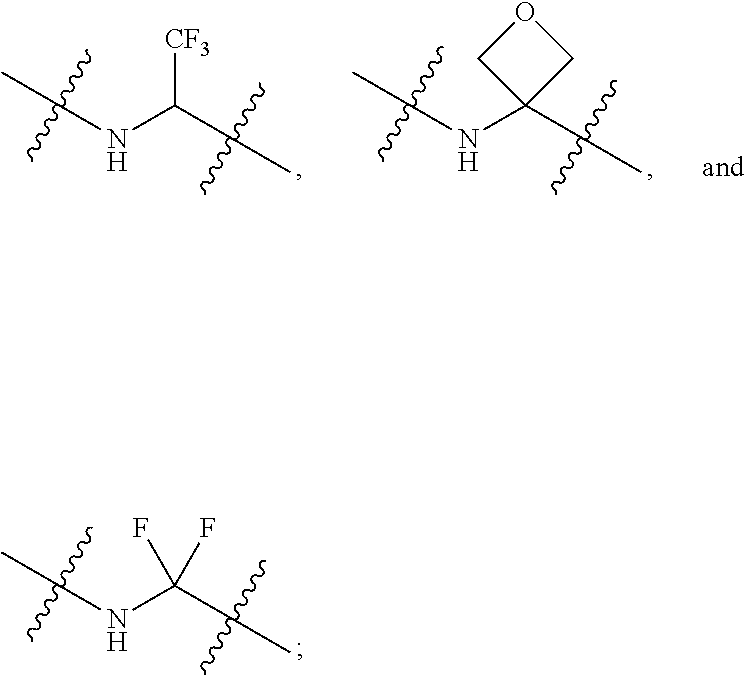
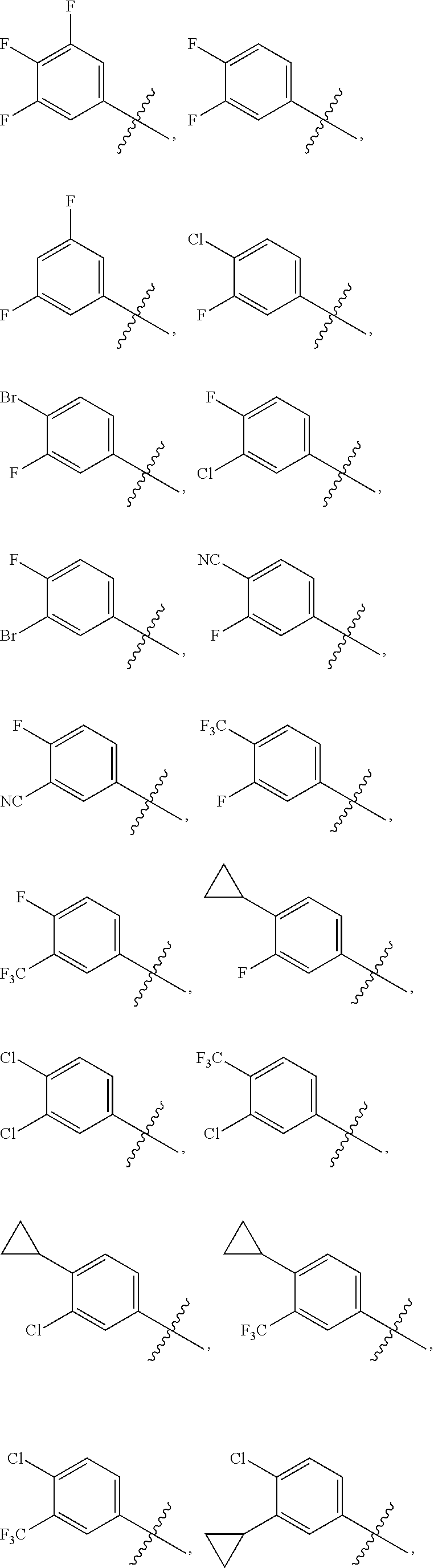
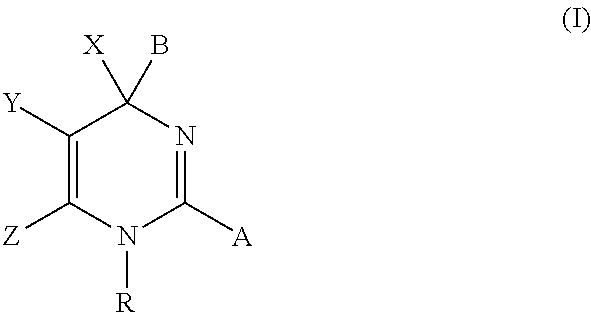
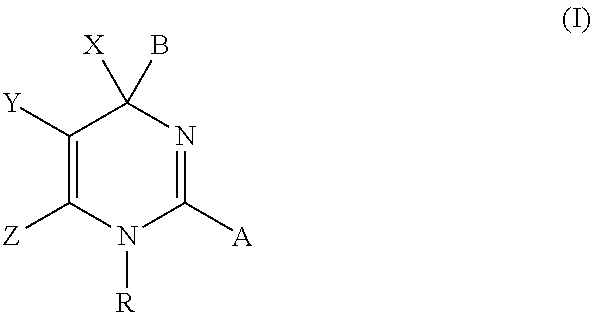
Hepatitis b antiviral agents
ActiveUS20170253609A1AntiviralsAmide active ingredientsHepatitis B immunizationPharmaceutical medicine
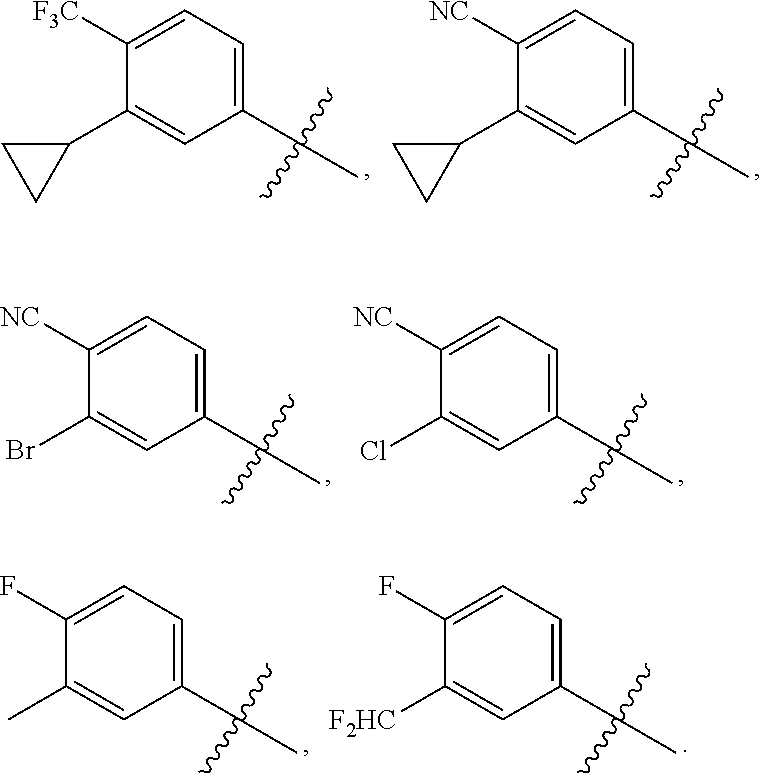
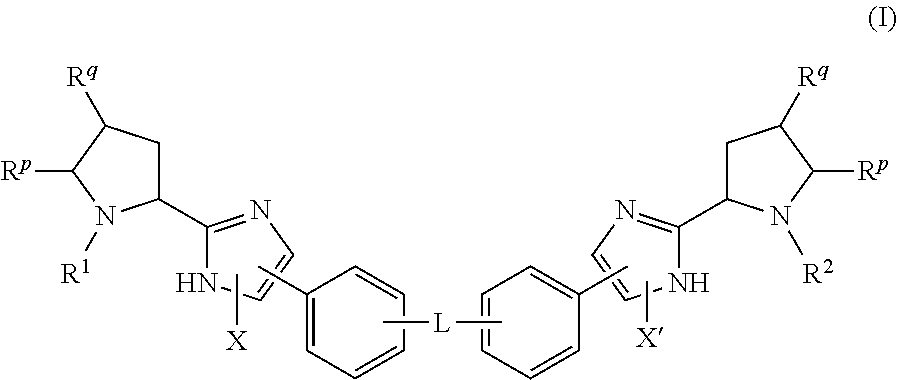
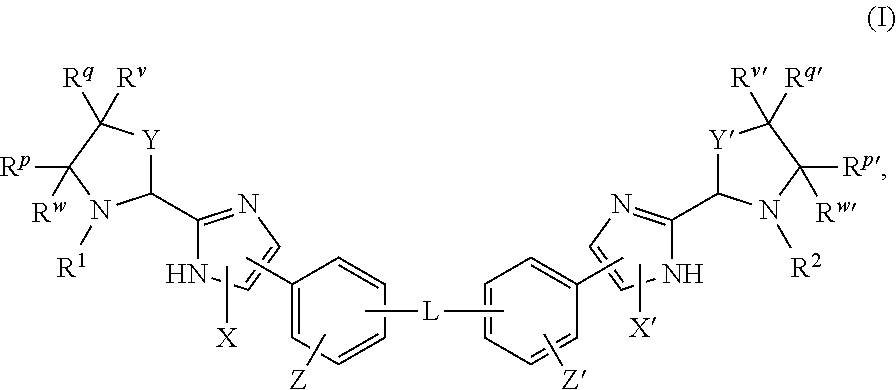
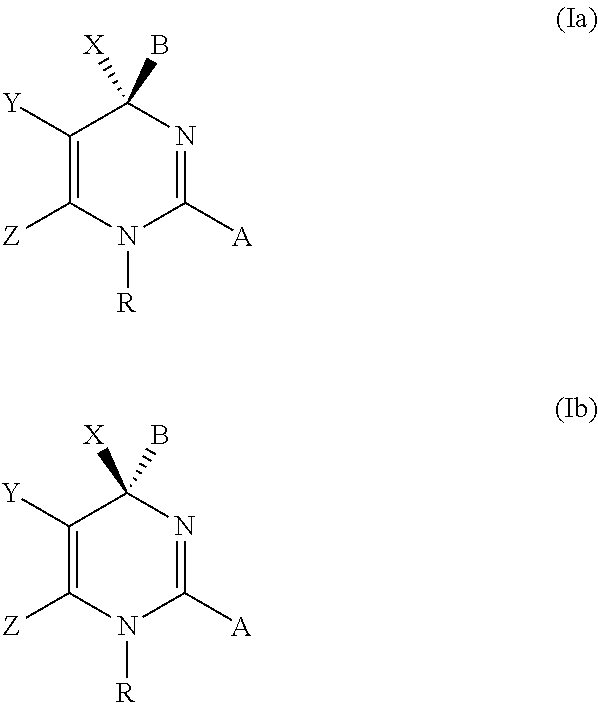
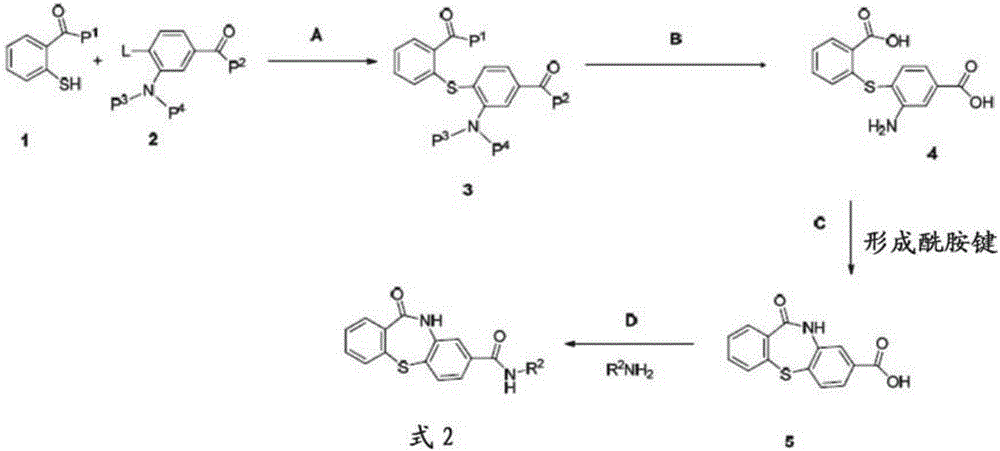
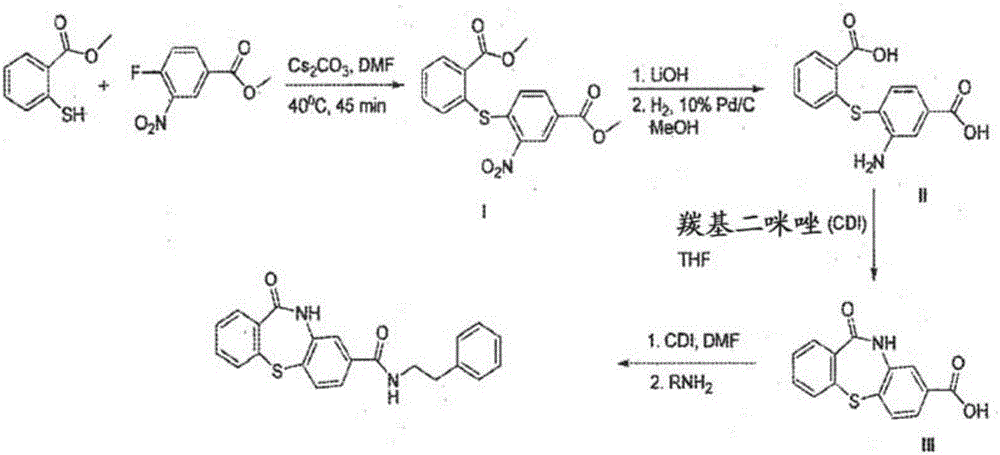
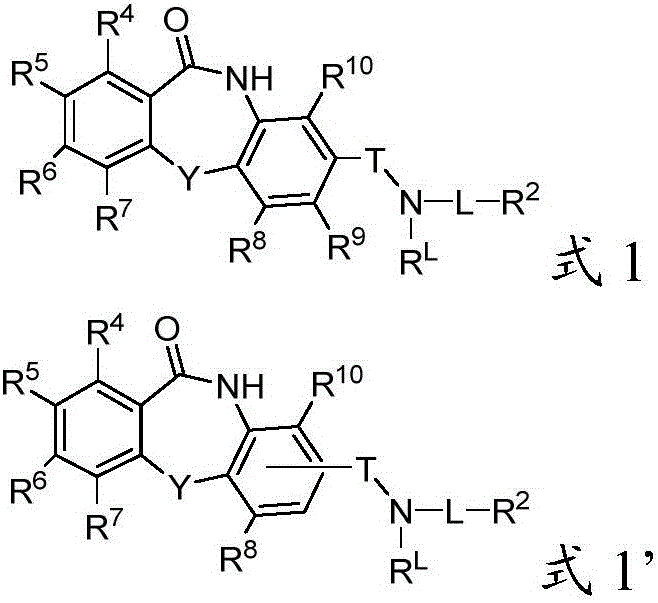
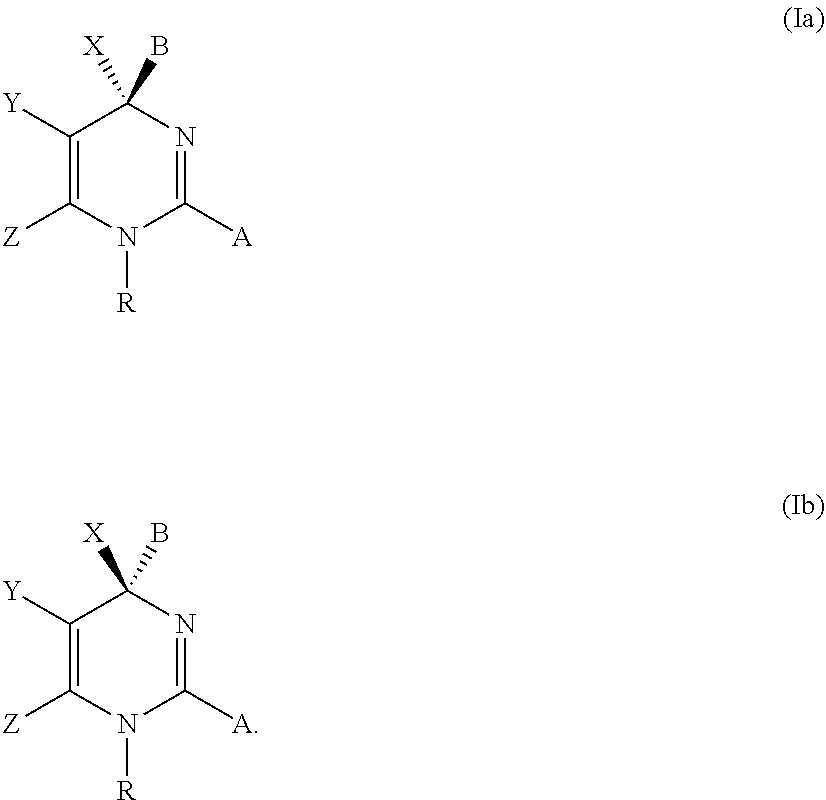
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:X-A-Y-L-R (I)which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Hepatitis C Virus Inhibitors
This disclosure concerns novel compounds of Formula (I) as defined in the specification and compositions comprising such novel compounds. These compounds are useful antiviral agents, especially in inhibiting the function of the NS5A protein encoded by Hepatitis C virus (HCV). Thus, the disclosure also concerns a method of treating HCV related diseases or conditions by use of these novel compounds or a composition comprising such novel compounds.
Owner:BRISTOL MYERS SQUIBB CO
Malaria immunogen and vaccine
InactiveUS6942866B2Easily preparedGreat stabilityHydrolasesAntibody mimetics/scaffoldsImmunogenicityPHA granule
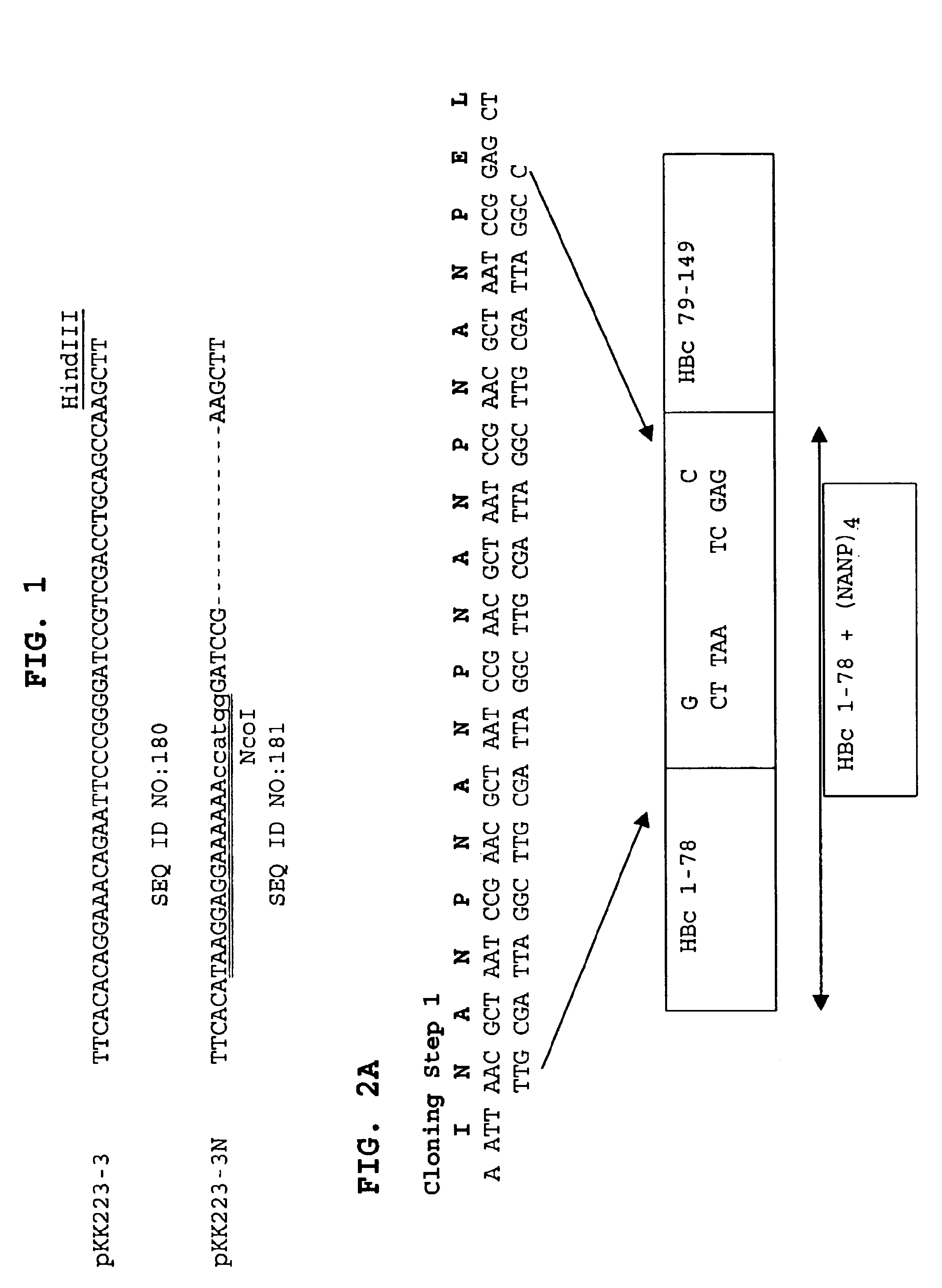
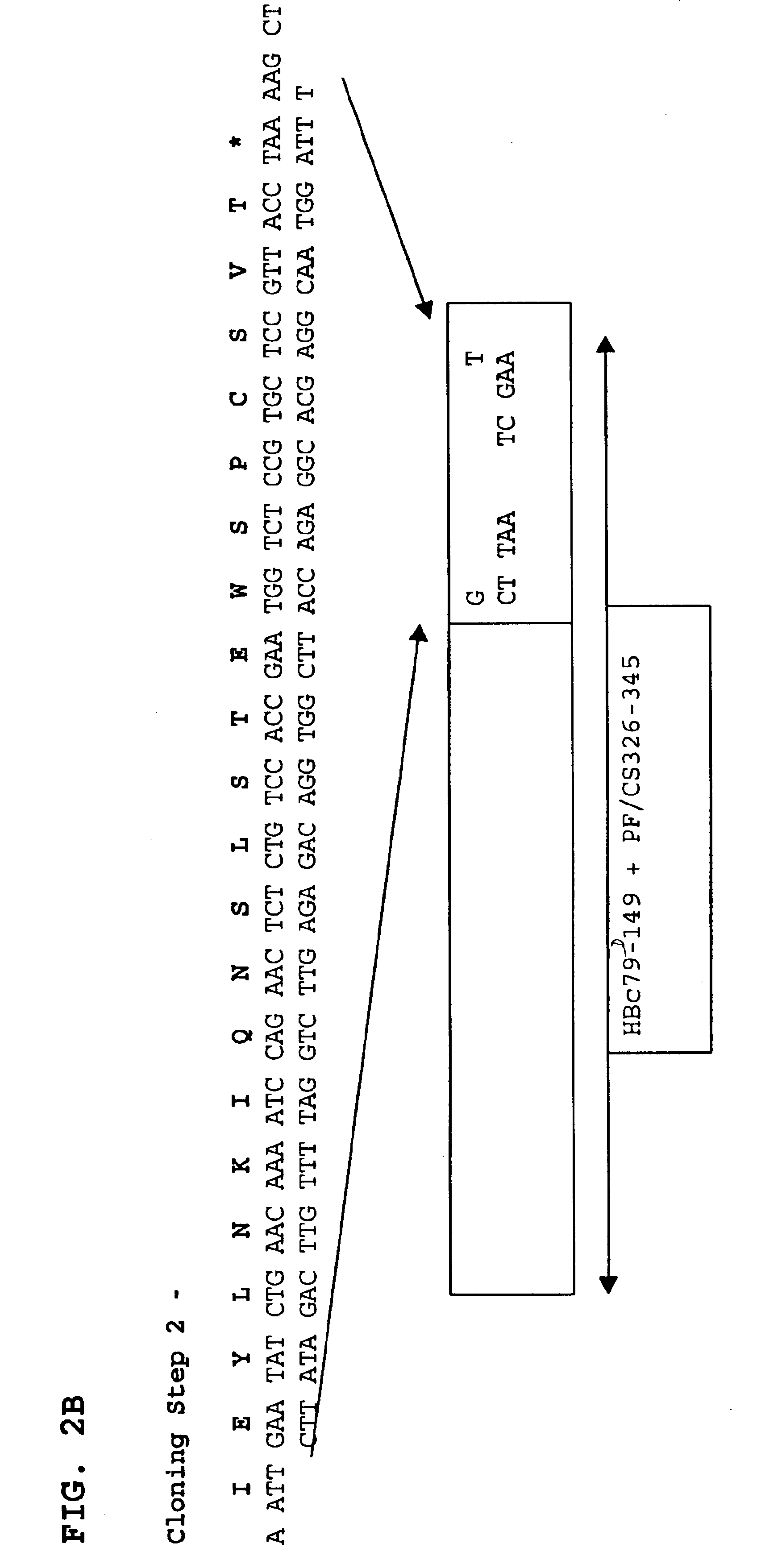
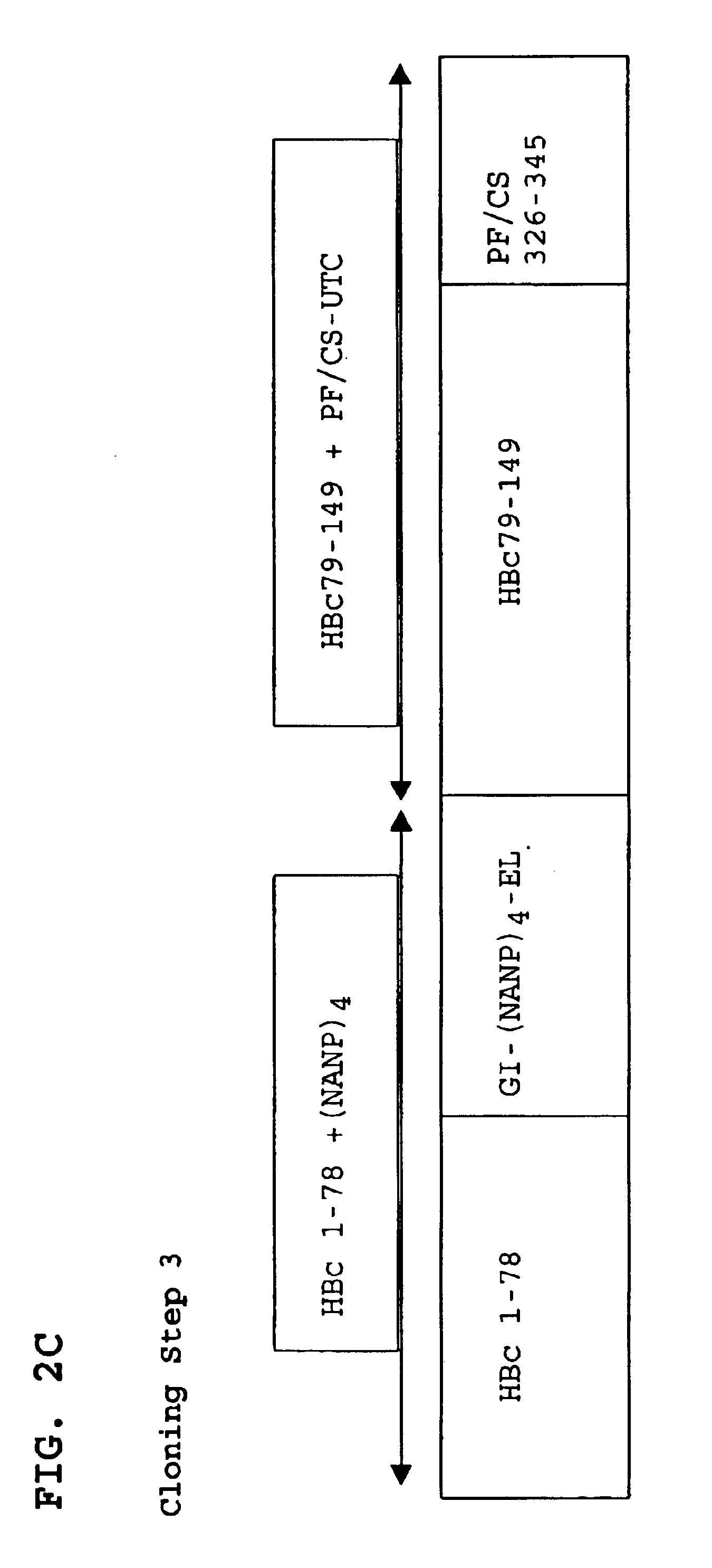
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid protein (HBc) is disclosed that contains an immunogen for inducing the production of antibodies to malarial proteins. An immunogenic malarial epitope is expressed between residues 78 and 79 of the HBc immunogenic loop sequence. The chimer preferably contains a malaria-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:APOVIA INC
Inhibitors of secretion of hepatitis B virus antigens
InactiveUS8921381B2Low serum levelsBiocideOrganic active ingredientsHepatitis B Virus AntigenHepatitis B virus
Owner:DREXEL UNIV +2
Hepatitis b antiviral agents
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, thereof:which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
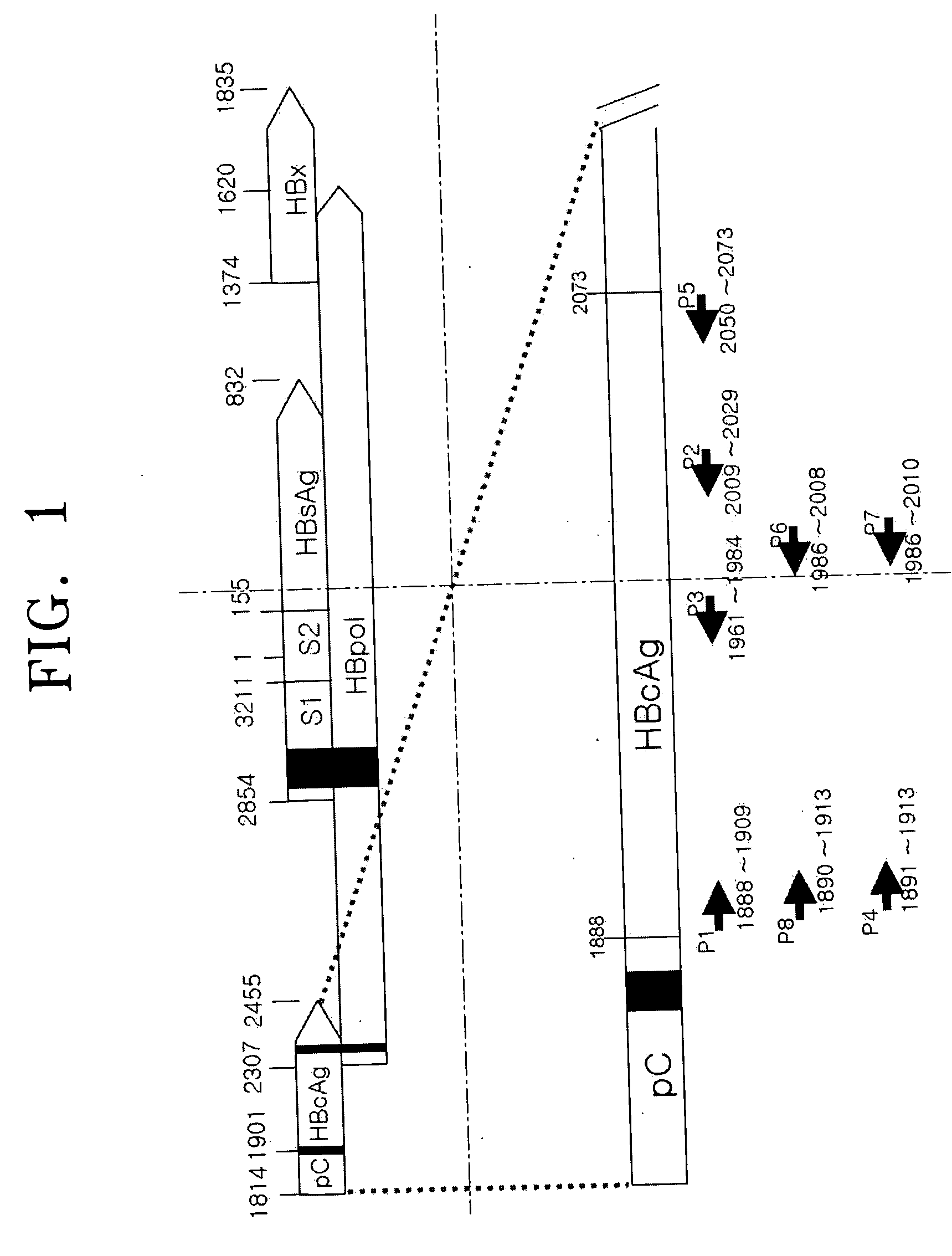
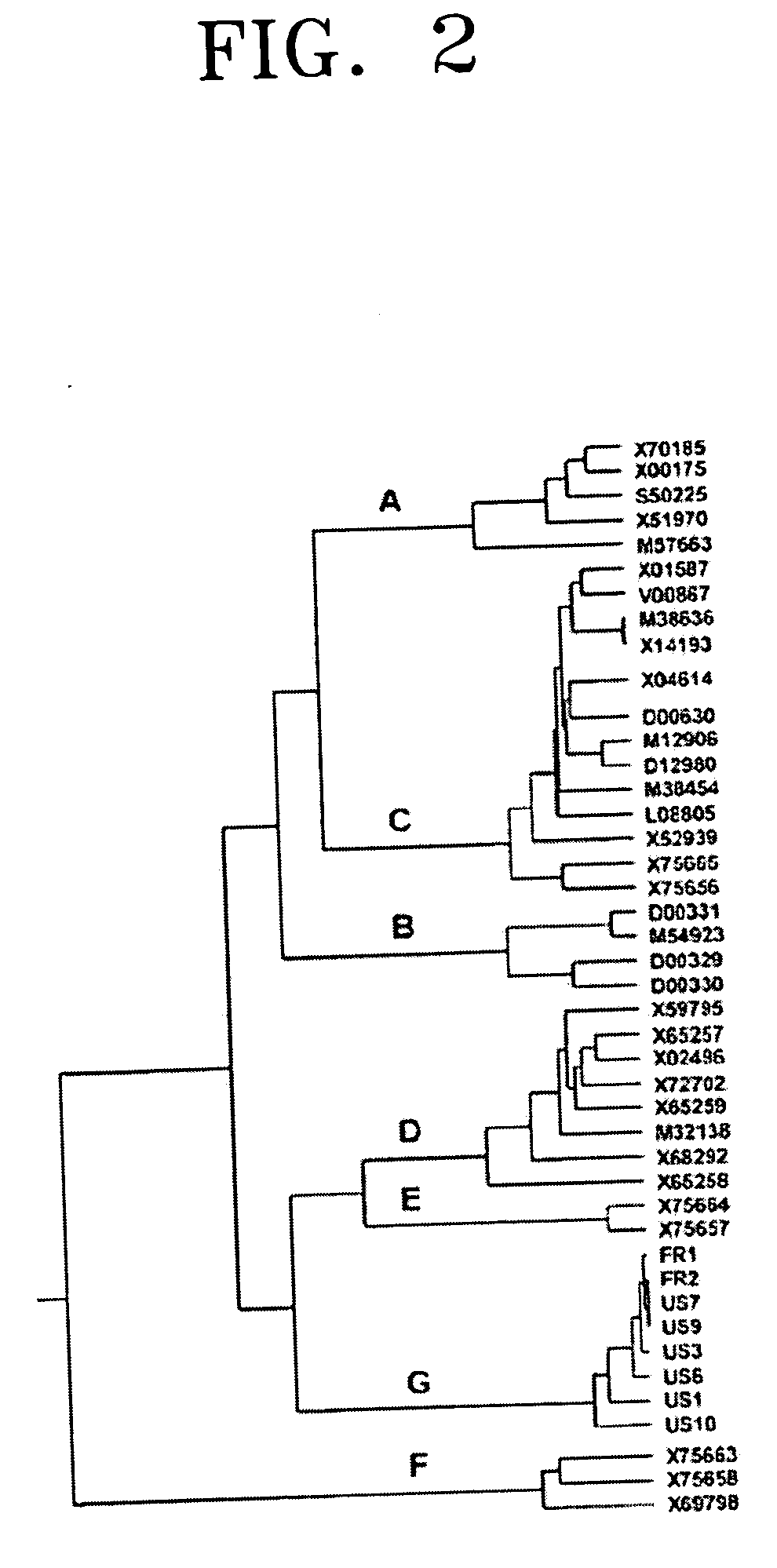
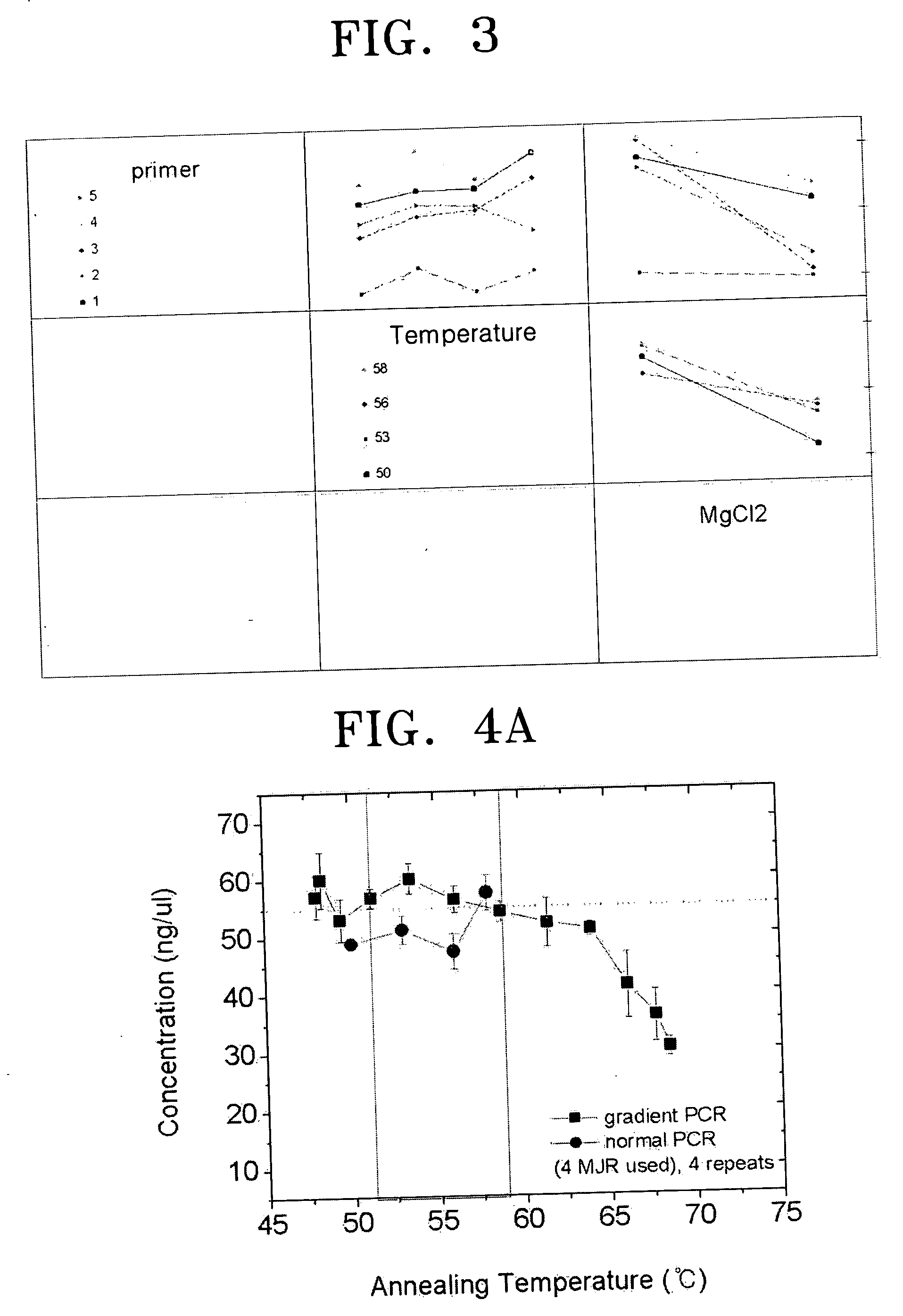
PCR primer set for detecting hepatitis B virus, method for detecting hepatitis B using the primer set, and hepatitis B virus detection kit including the primer set
ActiveUS20050037414A1Microbiological testing/measurementRecombinant DNA-technologyNucleotide sequencingHepatitis B virus
Provided are a primer set selected from the group consisting of primers having nucleotide sequences as set forth in SEQ ID NOS: 1-40, a method for detecting hepatitis B virus by polymerase chain reaction (PCR) using the primer set, and a hepatitis B virus detection kit including the primer set.
Owner:SAMSUNG ELECTRONICS CO LTD
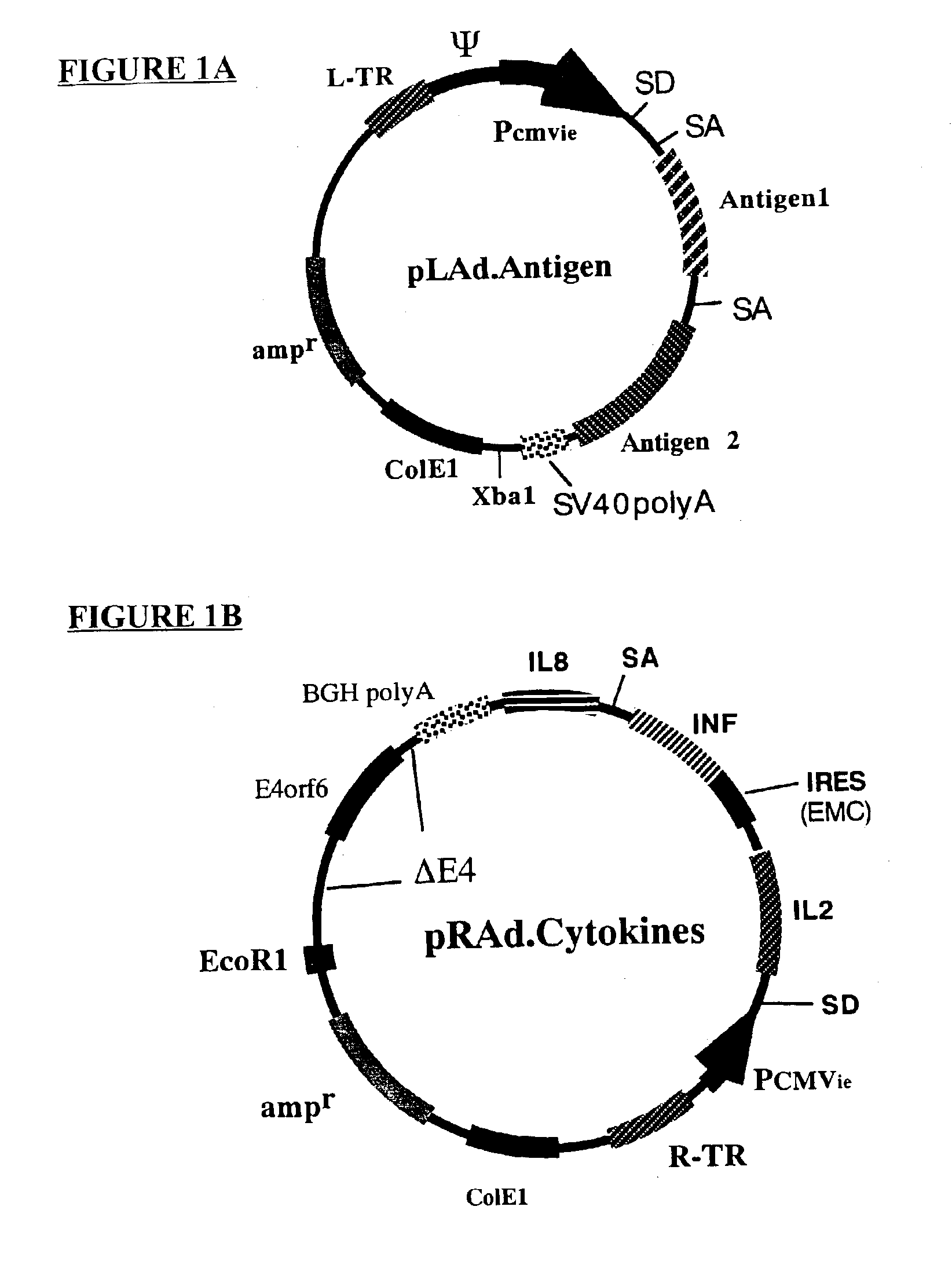
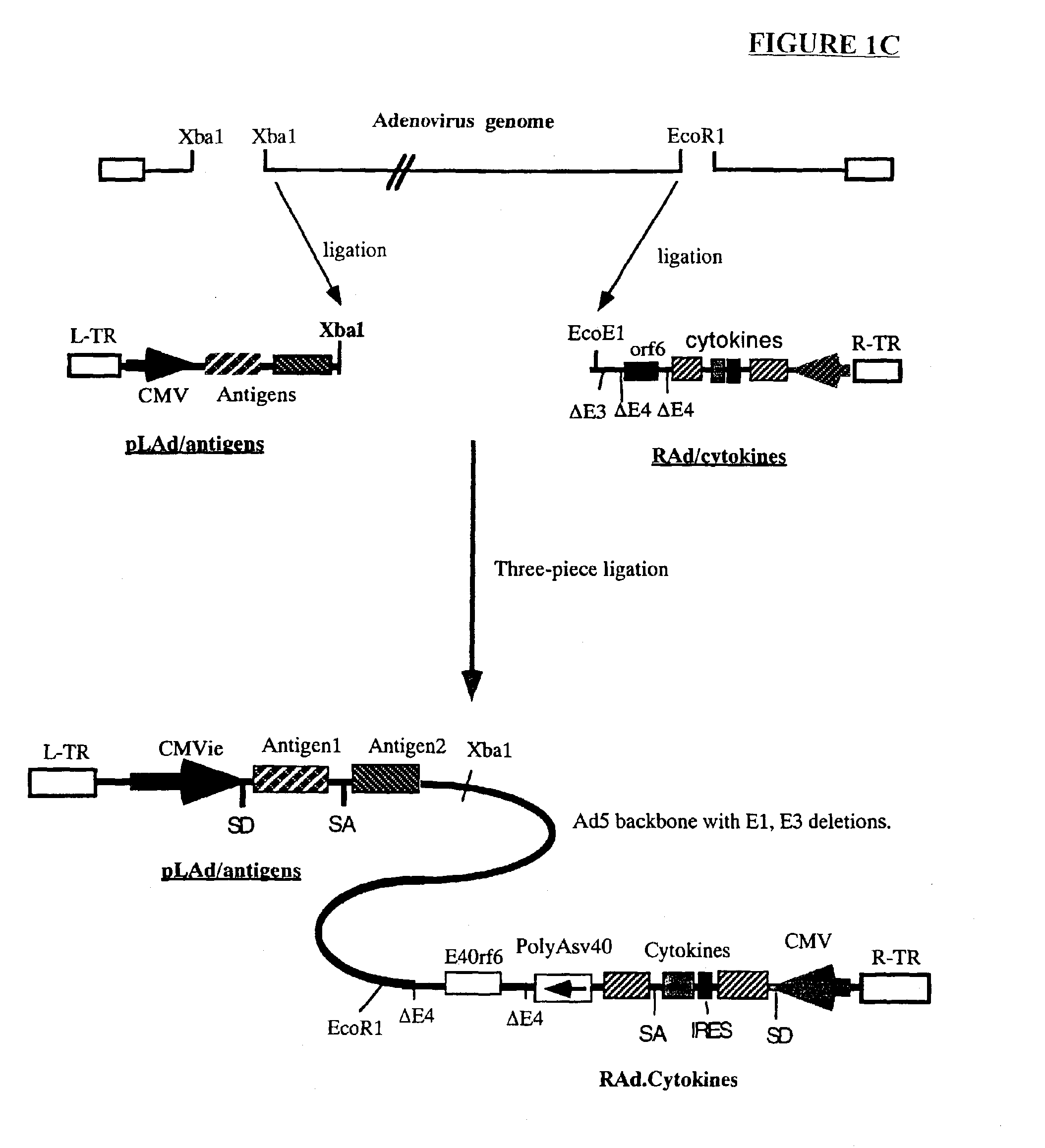
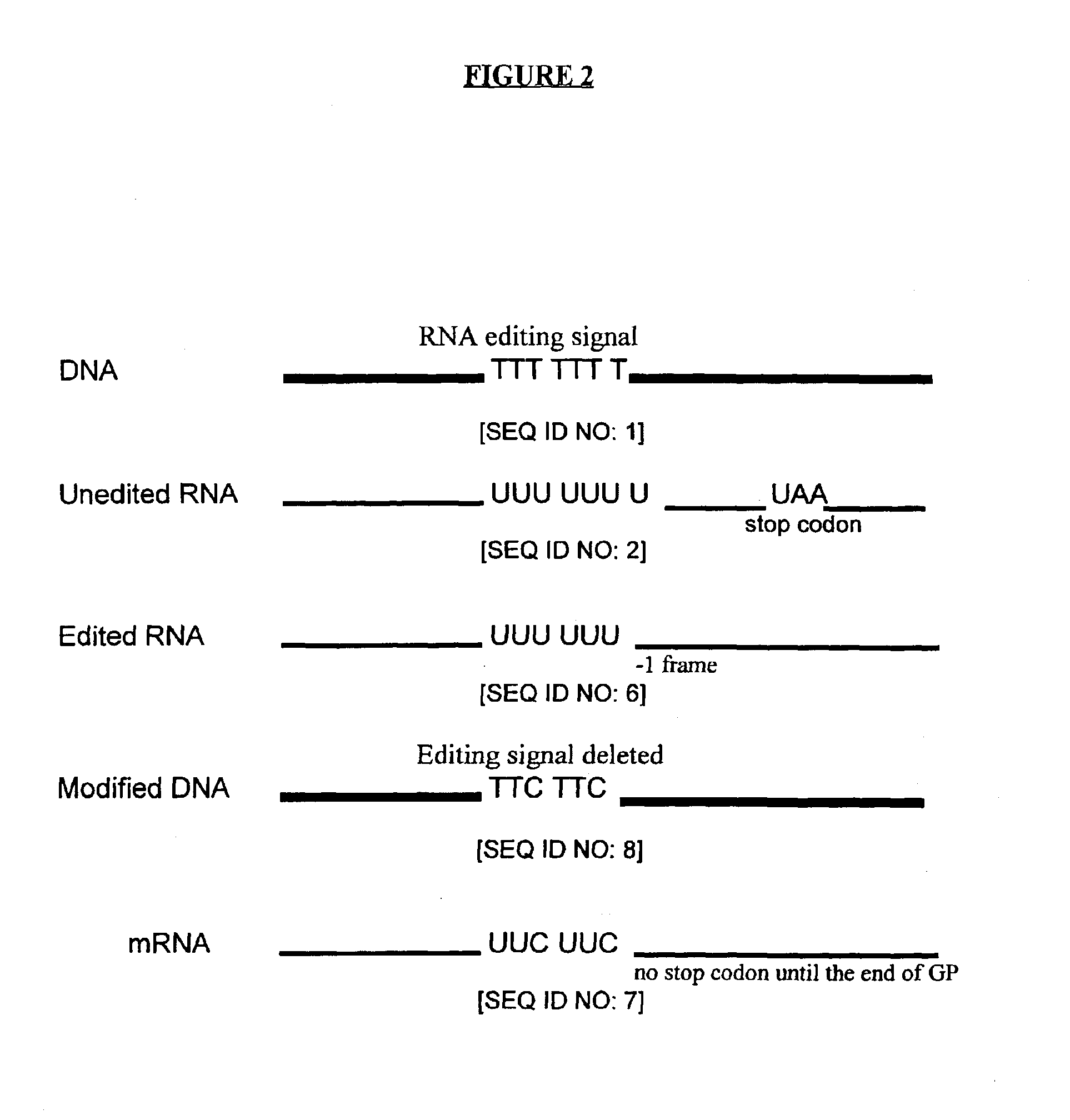
Composition and method for stimulating immune response to pathogen using complex adenoviral vector
InactiveUS6964762B2Improving immunogenicityStrong immune responseSsRNA viruses negative-senseAntibacterial agentsHeterologousProgenitor
Genetic vaccines and methods are provided for enhancing the immunity of a host such as a human to one or more pathogens. In one aspect, a method of enhancing the immunity of a host to a pathogen is provided. The method comprises administering to the host a recombinant virus comprising an antigen sequence that is heterologous to a native progenitor of the recombinant adenovirus and encodes a viral antigen from a pathogenic virus, expression of which is under the transcriptional control of a first promoter; and a cytokine sequence that is heterologous to the native progenitor of the recombinant adenovirus and encodes a cytokine, expression of which is under the transcriptional control of a second promoter. Expression of the antigen and cytokine sequences elicits an immune response directed against the viral antigen upon infection of the host by the recombinant virus. The method can be used for immunizing a host against a wide variety of pathogen viruses, such as HIV, Ebola virus, Marburg virus, hepatitis B virus, hepatitis C virus, influenza virus, human simplex virus, human papilloma virus and respiratory syncytial virus.
Owner:GENPHAR INC
Hepatitis b antiviral agents
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:X-A1-Y-A2-Z-L-R (I)which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Universal multi-variant detection system
InactiveUS7348164B2Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementParvovirusHepatitis C
The present invention provides a method to diagnostically detect the variants of a given pathogen, such as HIV, hepatitis C, hepatitis B (HBV), Parvovirus B19, etc., with the use of a single detection probe.
Owner:NEW YORK BLOOD CENT
Stabilized HBc chimer particles as immunogens for chronic hepatitis
InactiveUS7351413B2Easy to prepareImprove stabilityAntibody mimetics/scaffoldsAntipyreticProtein moleculesChronic hepatitis
A method of treating chronic hepatitis B is disclosed that comprises administering a T cell-stimulating amount of a vaccine to a patient. The vaccine comprises an immunogenic amount of chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid (core) protein (HBc) that is engineered for both enhanced stability of self-assembled particles and the substantial absence of nucleic acid binding by those particles. The chimeric protein molecule can include one or more immunogenic epitopes peptide-bonded to one or more of the N-terminus, the immunogenic loop or the C-terminus of HBc. The enhanced stability of self-assembled particles is obtained by the presence of at least one heterologous cysteine residue near one or both of the amino-terminus and carboxy-terminus of the chimer molecule.
Owner:LORANTIS
Malaria immunogen and vaccine
InactiveUS20050208068A1High antibody titersEasy to prepareAntibody mimetics/scaffoldsVirus peptidesHepatitis B immunizationMalaria
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid protein (HBc) is disclosed that contains an immunogen for inducing the production of antibodies to malarial proteins. An immunogenic malarial epitope is expressed between residues 78 and 79 of the HBc immunogenic loop sequence. The chimer preferably contains a malaria-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:MILICH DAVID +1
HBV antisense inhibitors
ActiveUS8598334B2Promoting seroconversionReduce the amount requiredOrganic active ingredientsSugar derivativesOligomerMethylene bridge
Antisense oligomers useful for modulating hepatitis B virus infections, and for the treatment of hepatitis B virus (HBV) and hepatitis B virus-related conditions in animals including humans. More particularly, antisense oligomers with modified nucleotides for treatment of HBV in animals, more particularly antisense oligomers comprising 2′O-4′C-methylene-bridged sugars, or nucleotides with other 2′O-4′C bridged sugars, also known as locked nucleic acids (LNA), for treatment of HBV in animals, and more particularly for treatment of HBV in humans.
Owner:GLAXO GRP LTD
Beta-L-N4-Hydroxycytosine Deoxynucleosides and their use as Pharmaceutical Agents in the Prophylaxis or Therapy of Viral Diseases
The invention relates to β-L-N4-hydroxycytosine nucleo-sides, pharmaceutical agents comprising same, and to the use of said β-L-N4-hydroxycytosine nucleosides and pharmaceutical agents in the prophylaxis or therapy of an infection caused by hepatitis B virus (HBV) or human immunodeficiency virus (HIV). The invention also relates to a method for the preparation of said β-L-nucleoside analogs.
Owner:MAX DELBRUECK CENT FUER MOLEKULARE MEDIZIN
Hepatitis B antiviral agents
ActiveUS10189846B2Organic active ingredientsOrganic chemistryHepatitis B immunizationPharmaceutical drug
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, thereof:which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
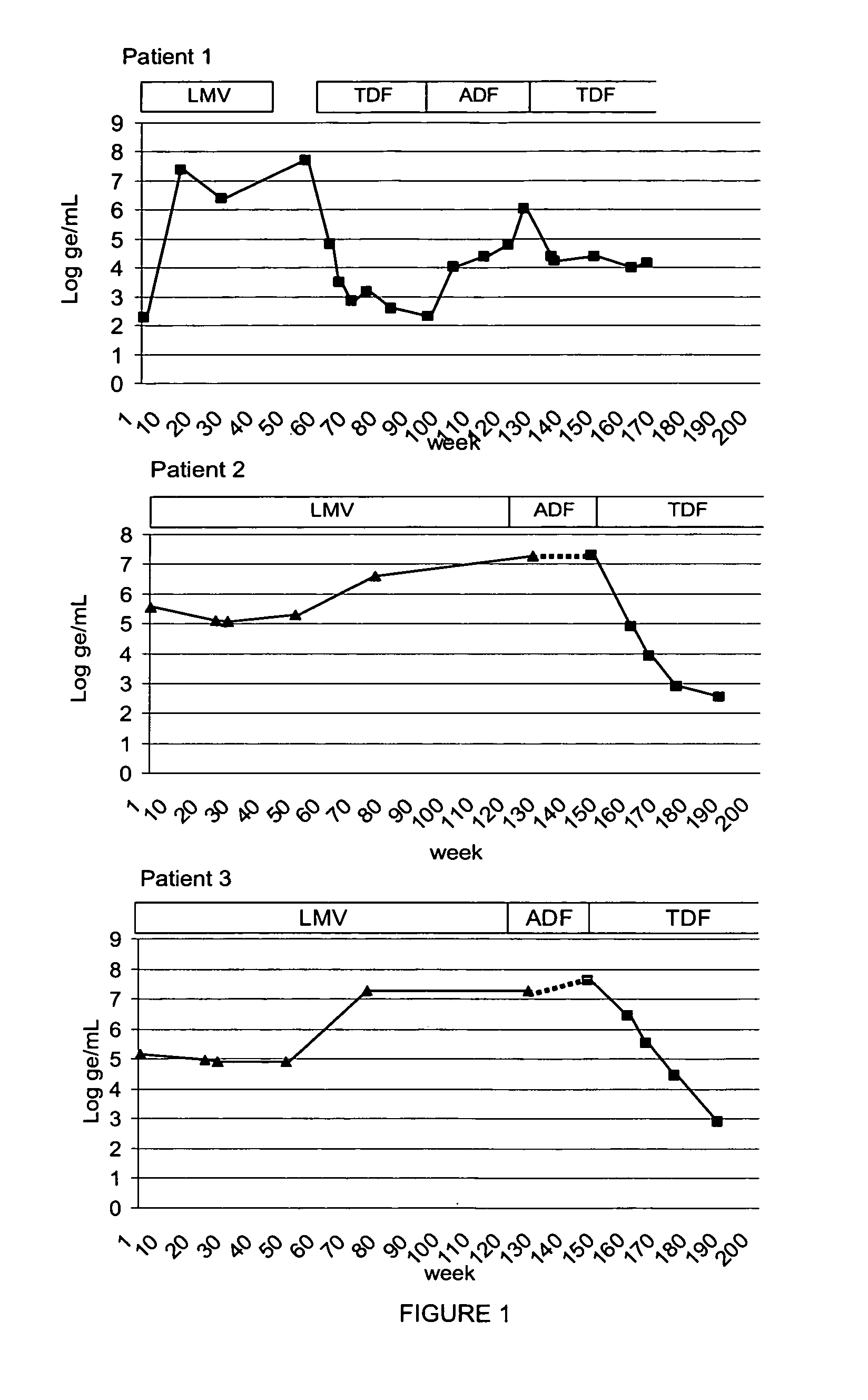
Variants of hepatitis B virus resistant against some nucleoside analogues, but sensitive to others, and uses thereof
ActiveUS20070042356A1Reduce sensitivityRapid and reliable detectionBiocideVirus peptidesHepatitis B immunizationReverse transcriptase
The present invention relates generally to the field of Hepatitis B variants exhibiting a reduced sensitivity to nucleoside analogues both in vivo and in vitro. More in particular, reverse transcriptase mutant rt I233V is provided. Present invention provides assays and methods for detecting such variant, which assays are useful in monitoring anti-viral therapeutic regimes and adjusting patient therapy. A diagnostic kit for detecting the presence of an HBV variant in a biological sample has also been described. Finally, the use of a farmaceutical composition to cure a subject suffering from a HBV infection, which HBV is resistant to lamuvidine and / or adefovir has been provided, which farmaceutical composition comprises the nucleoside analogue tenofovir.
Owner:UNIVERSITY OF BONN +2
Hepatitis b antiviral agents
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, thereof:which inhibit the protein(s) encoded by hepatitis B virus (HBV) or interfere with the function of the HBV life cycle of the hepatitis B virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HBV infection. The invention also relates to methods of treating an HBV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Solid of tenofovir disoproxil, and preparation method and application thereof
ActiveCN103626803AEasy to prepareCrystal form controllableOrganic active ingredientsGroup 5/15 element organic compoundsMedicineHepatitis B virus
The invention relates to a solid of tenofovir disoproxil. The solid is (1) a tenofovir disoproxil compound represented by a formula IV or (2) a tenofovir disoproxil cocrystal or salt represented by a formula V. The invention further relates to a preparation method for the solid of tenofovir disoproxil, a pharmaceutical composition containing the solid and application of the solid in preparation of drugs used for preventing and / or treating virus infection, especially hepatitis b virus (HBV) and / or human immunodeficiency virus (HIV) infection.
Owner:SICHUAN HAISCO PHARMA CO LTD
Conserved hbv and hcv sequences useful for gene silencing
ActiveUS20120035240A1Inhibit expressionOrganic active ingredientsSsRNA viruses positive-senseHepatitis c viralHepatitis B immunization
Conserved consensus sequences from known hepatitis B virus strains and known hepatitis C virus strains, which are useful in inhibiting the expression of the viruses in mammalian cells, are provided. These sequences are useful to silence the genes of HBV and HCV, thereby providing therapeutic utility against HBV and HCV viral infection in humans.
Owner:ALNYLAM PHARMA INC
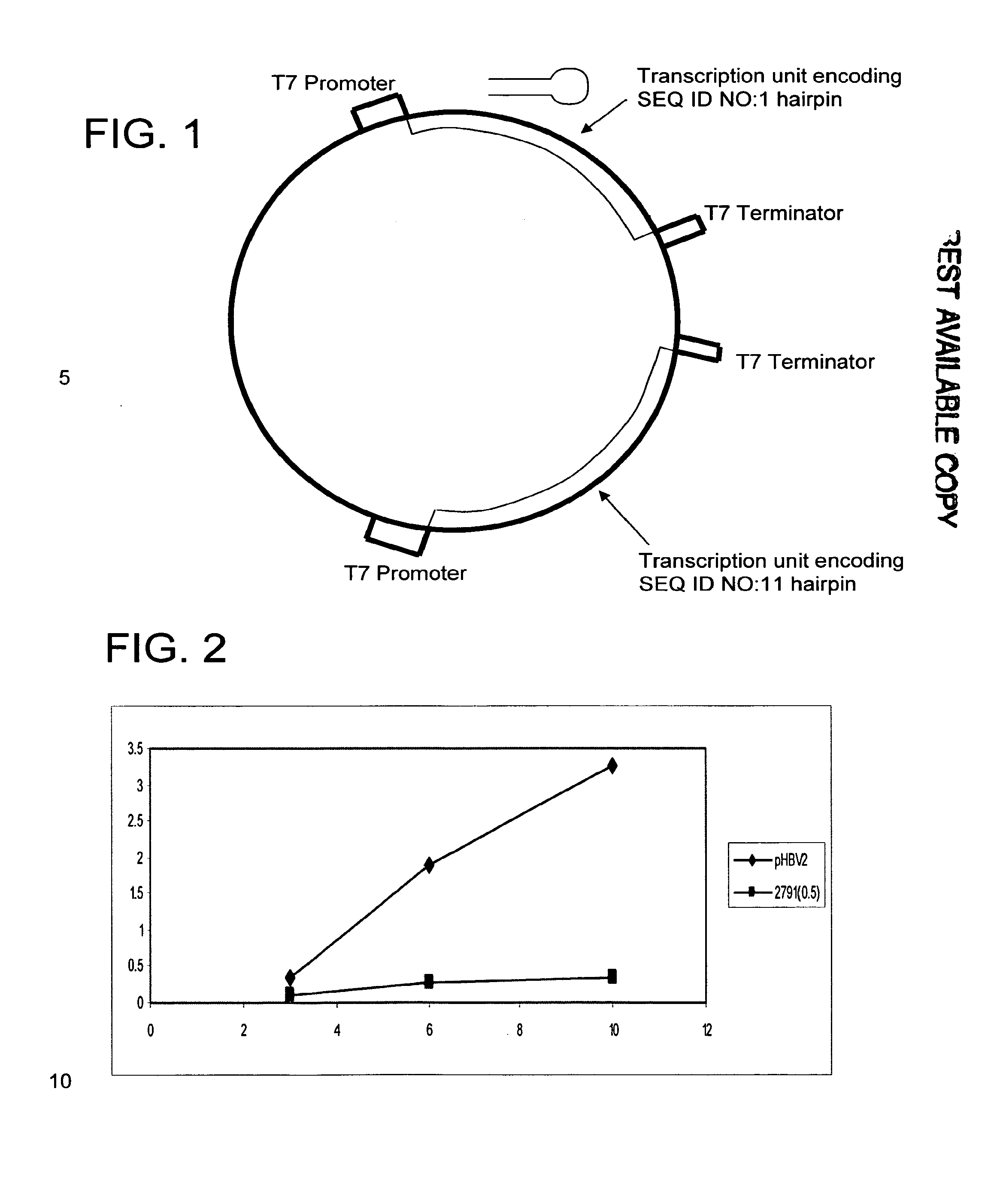
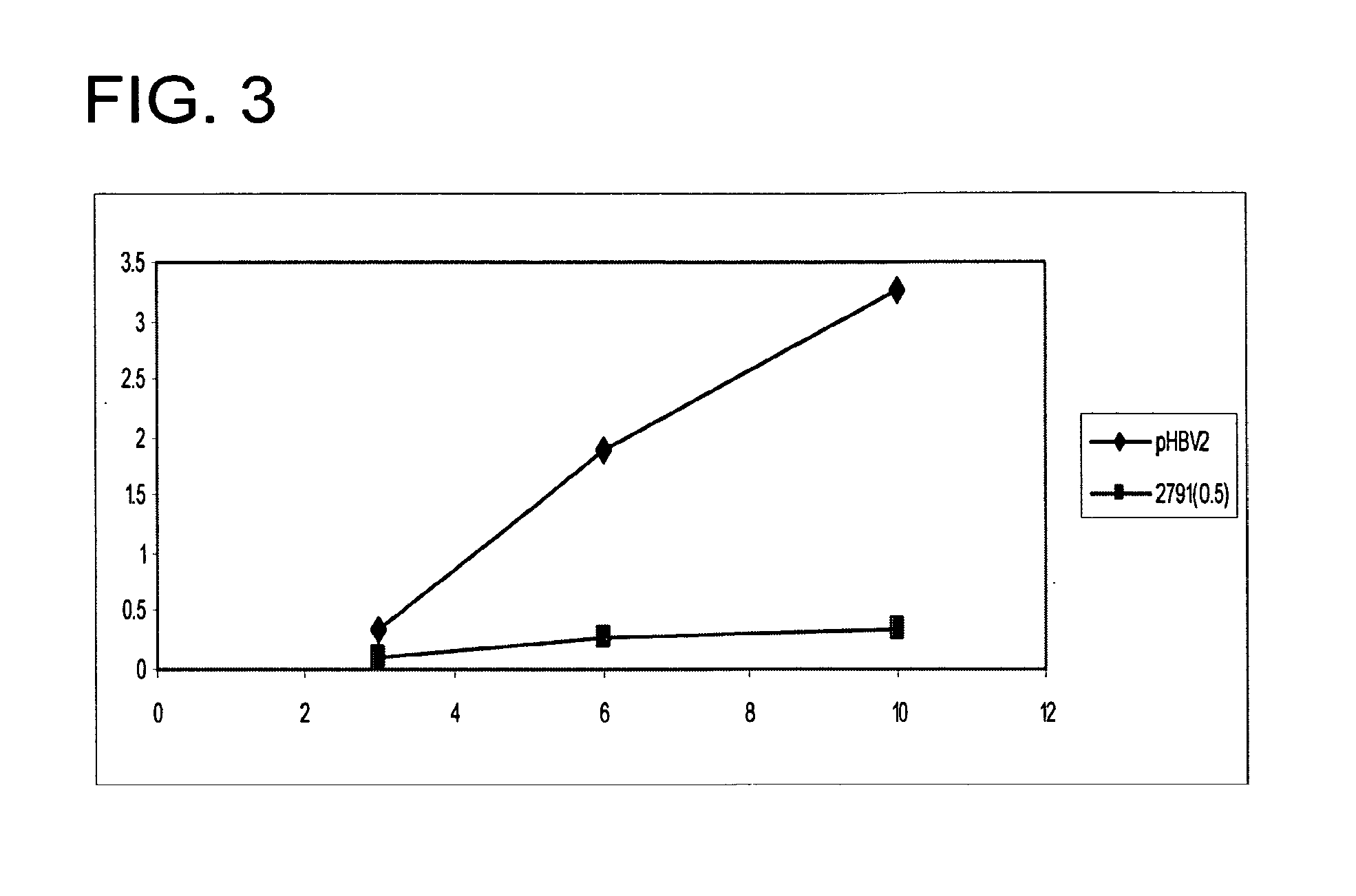
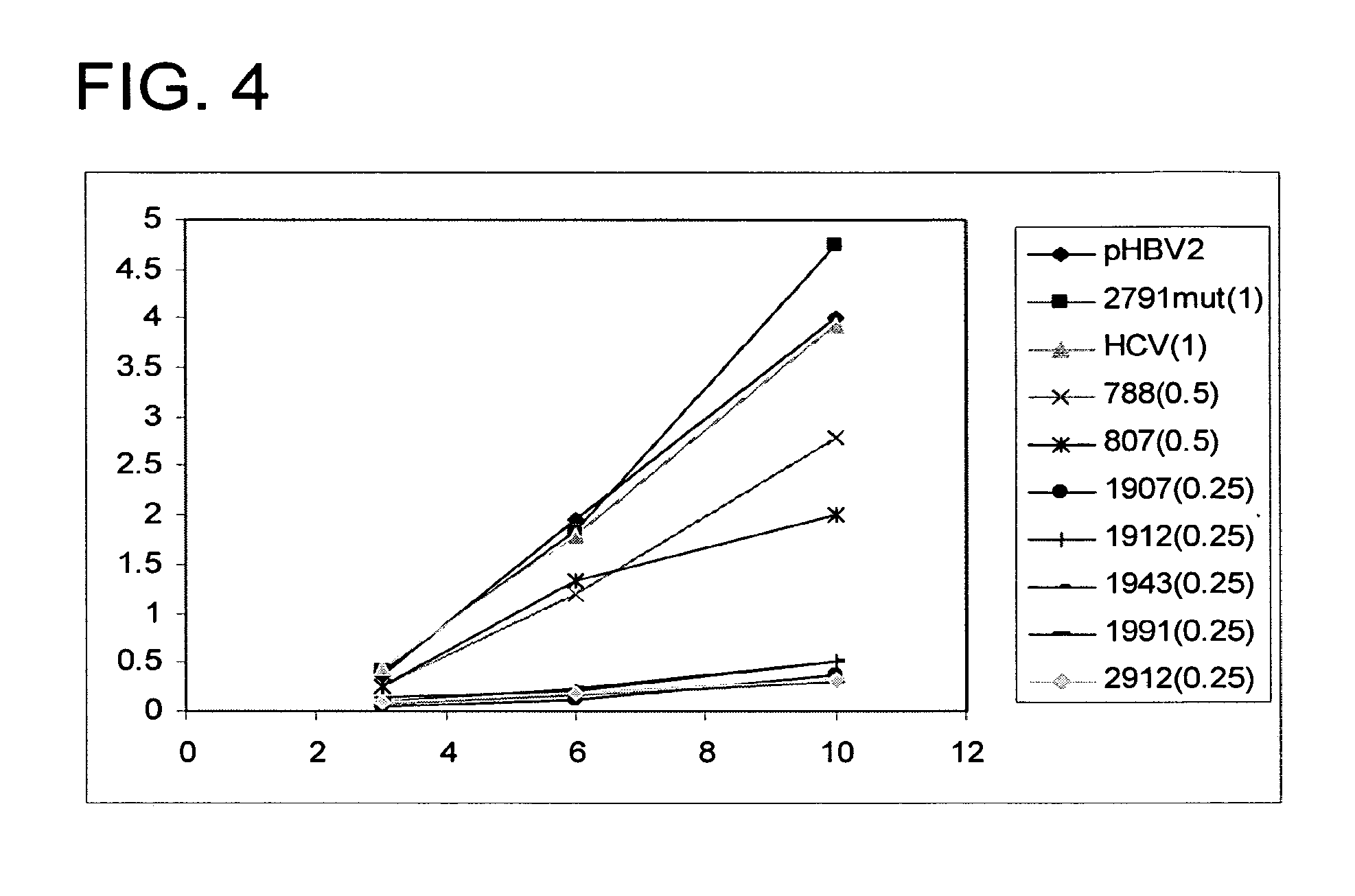
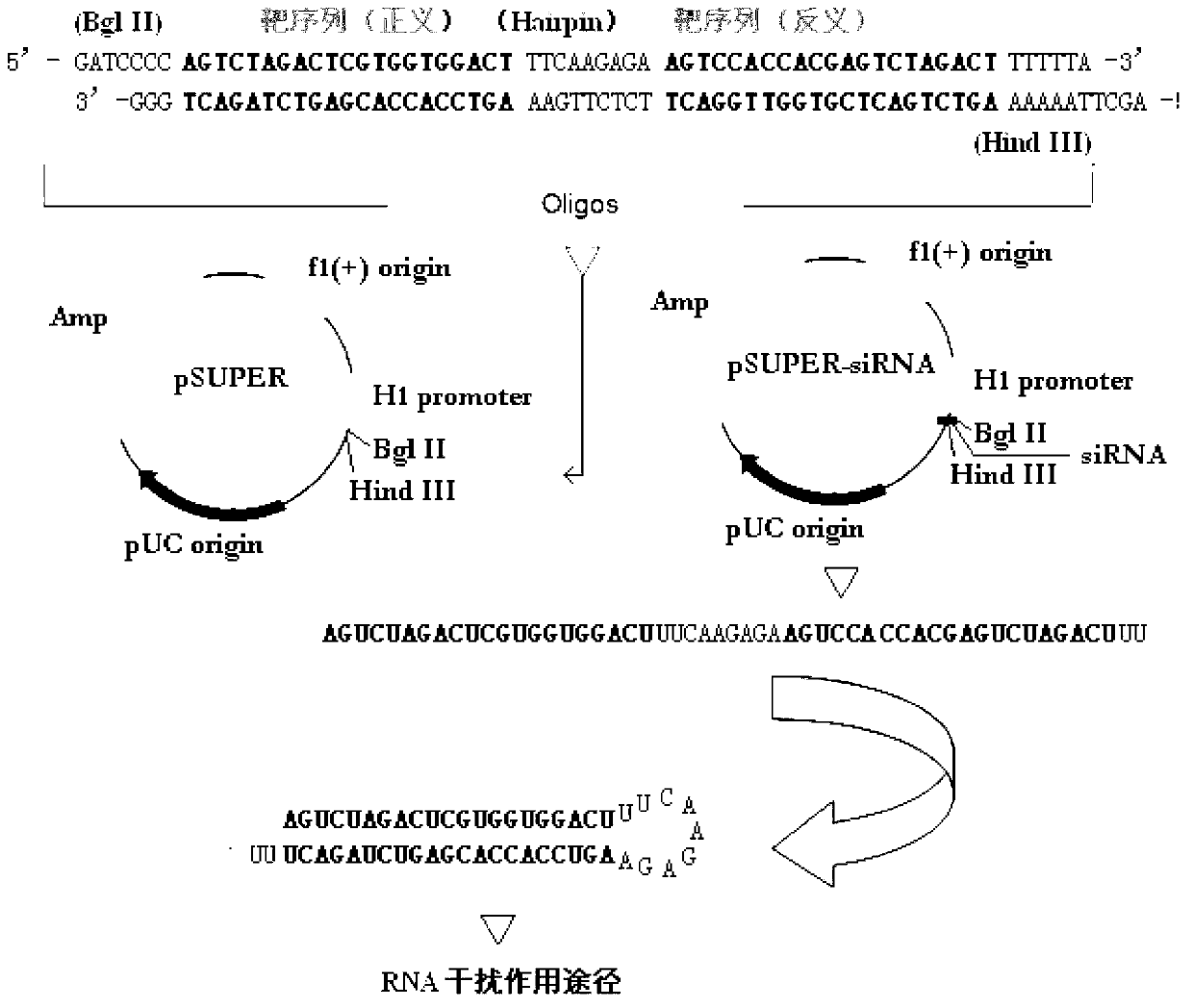
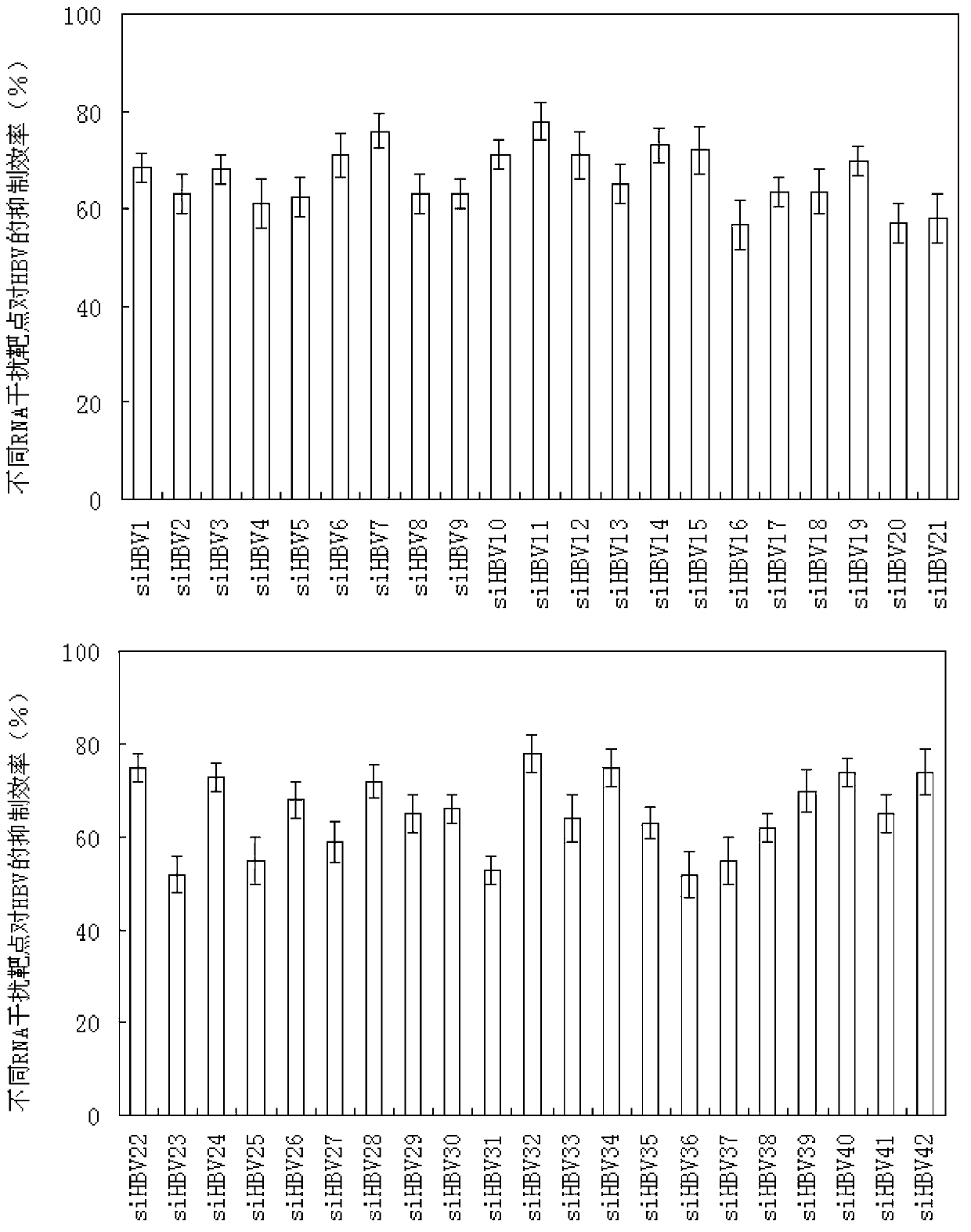
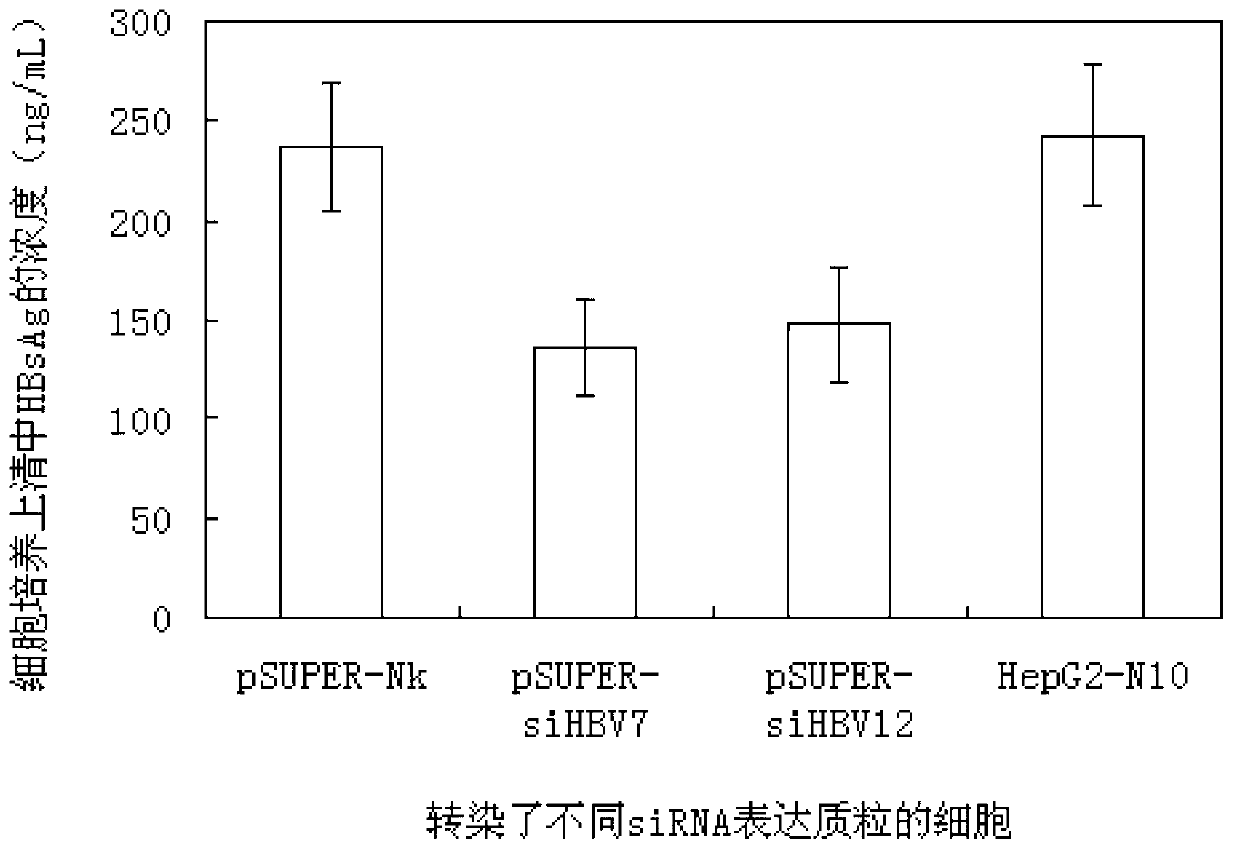
RNA interference targets for hepatitis b virus (HBV) infection treatment
The invention relates to 42 different hepatitis b virus (HBV)-targeting RNA interference targets for HBV infection treatment. The RNA interference targets can be used for preparation of a drug for HBV infection treatment. The invention provides recombinant expression vectors for expression of HBV-targeting siRNA and / or miRNA and / or ribozyme and / or antisense oligonucleotide. The invention relates to cells which can inhibit HBV gene expression and can express and / or be introduced with the siRNA and / or the miRNA and / or the ribozyme and / or the antisense oligonucleotide and / or a drug obtained according to the RNA interference targets.
Owner:XIAMEN UNIV +1
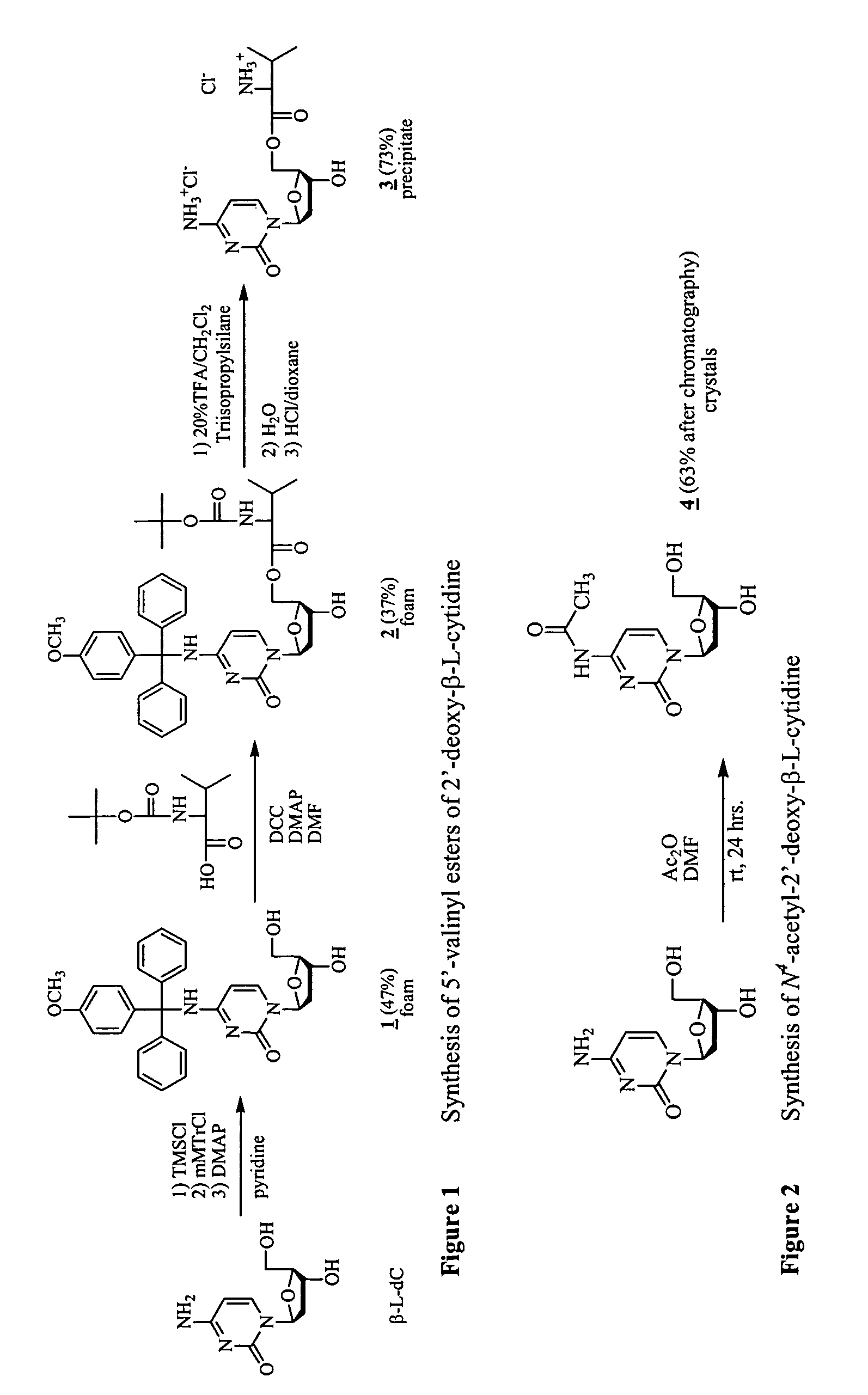
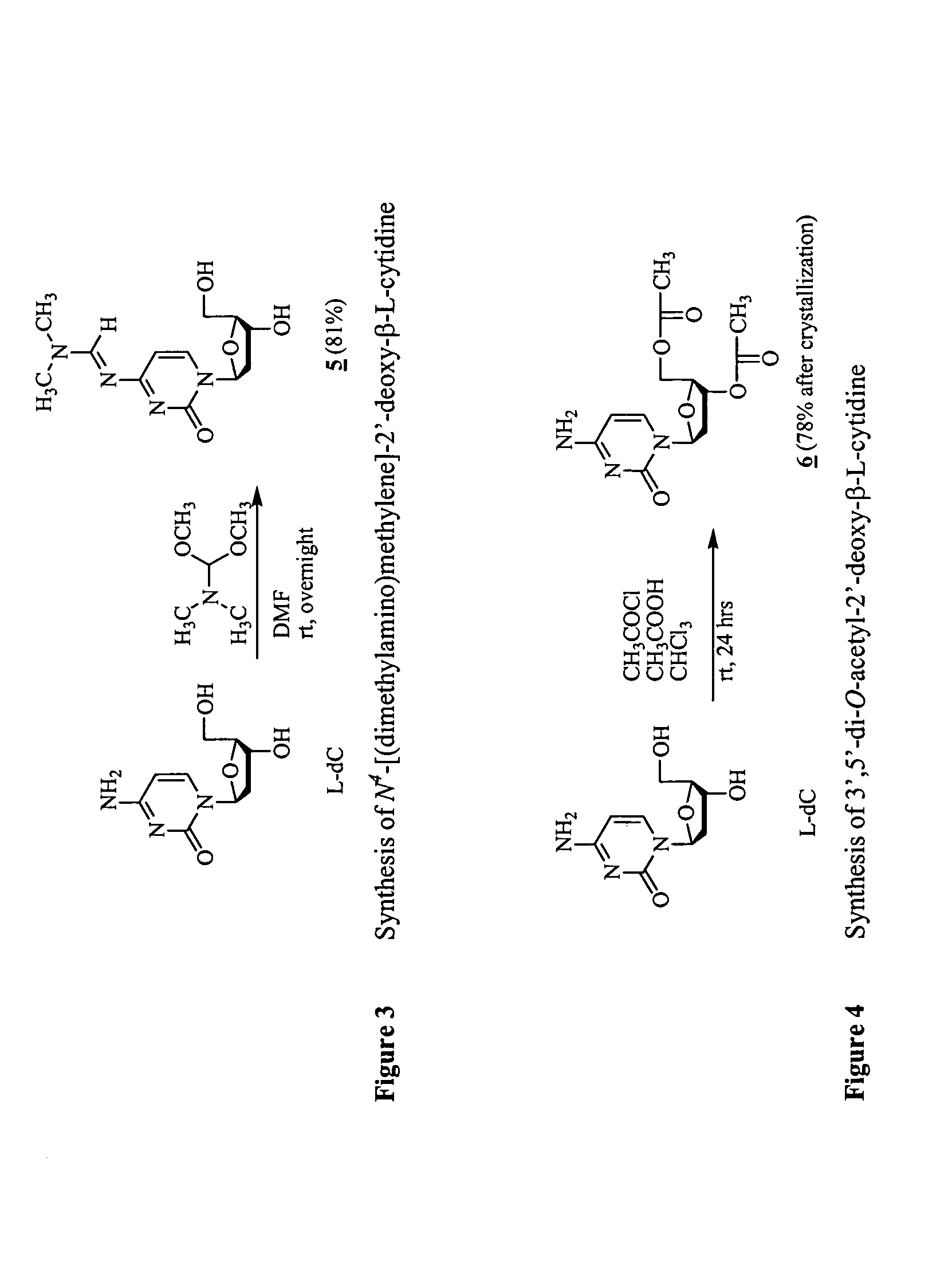
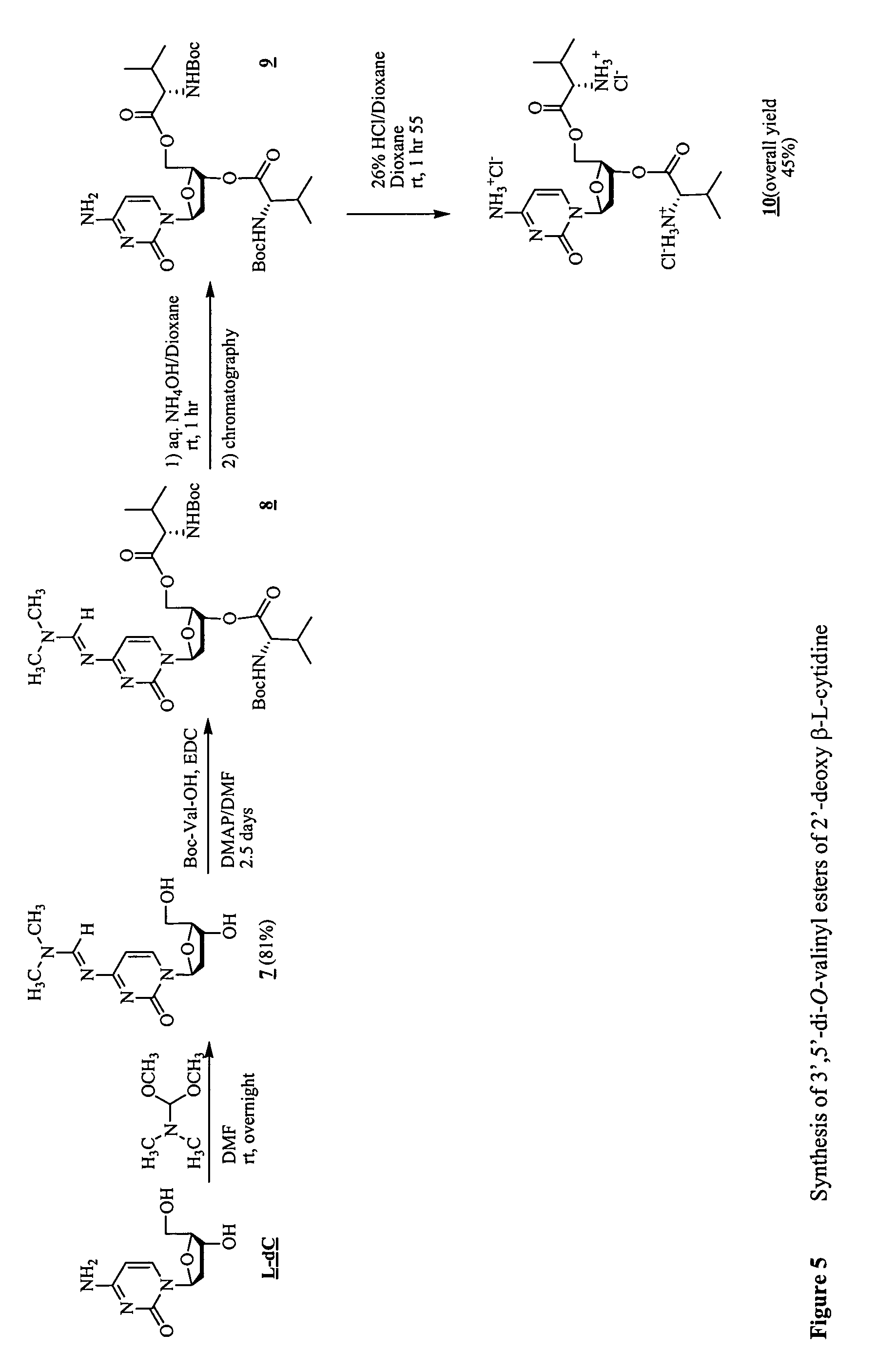
β-L-2′-deoxynucleosides for the treatment of resistant HBV strains and combination therapies
ActiveUS7186700B2Preventing and suppressing emergenceBiocidePeptide/protein ingredientsMedicineLamivudine resistance
It has been discovered that β-L-2′-deoxynucleosides are active against drug-resistant hepatitis B virus with mutations. A method for treating lamivudine resistant HBV (M552V) in a host is provided that includes administering a β-L-2′-deoxynucleoside or its pharmaceutically acceptable salt, ester or prodrug. In addition, a method for preventing lamivudine resistant HBV (M552V) mutation from occurring in a naïve host is provided that includes administering a β-L-2′-deoxynucleoside or its pharmaceutically acceptable salt, ester or prodrug. A method for preventing and / or suppressing the emergence of the HBV double mutant (L528M / M552V) in a host is also provided that includes administering a β-L-2′-deoxynucleoside or its pharmaceutically acceptable salt, ester or prodrug.
Owner:NOVARTIS AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com