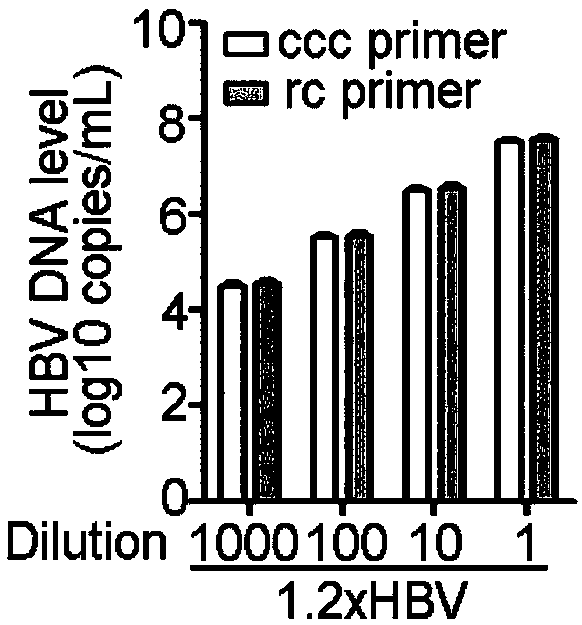
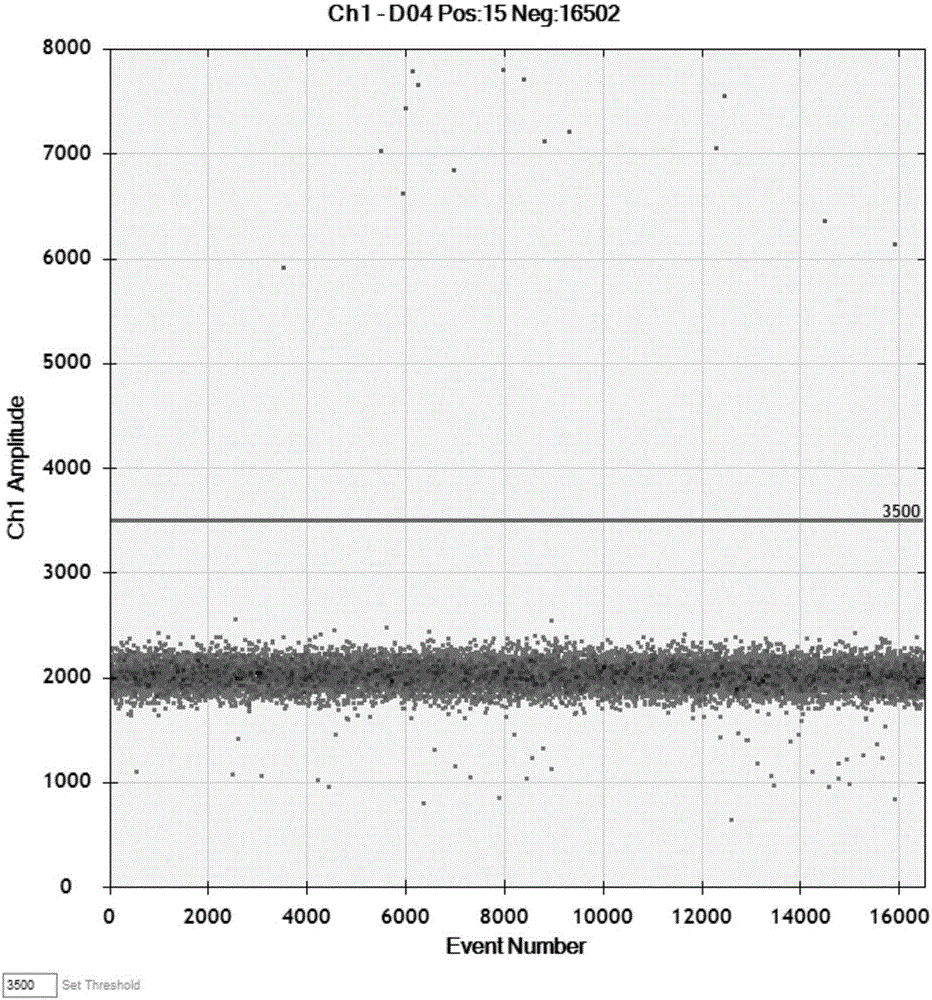
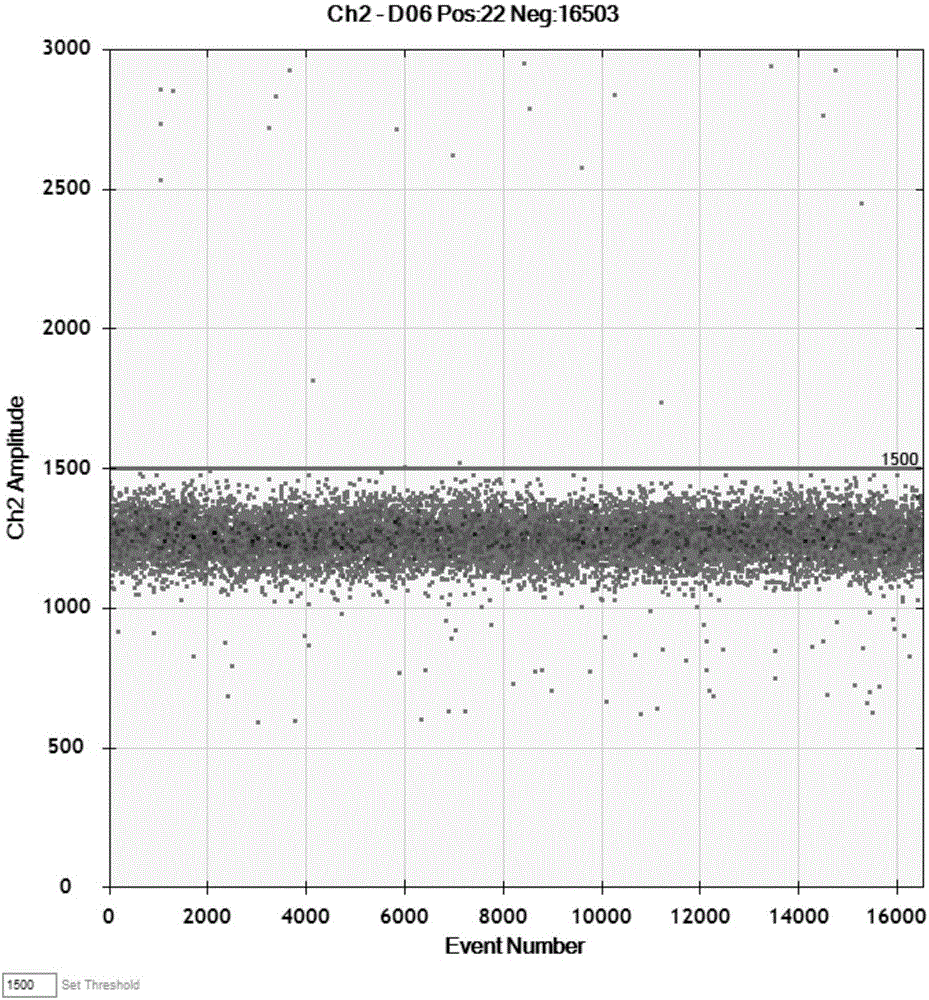
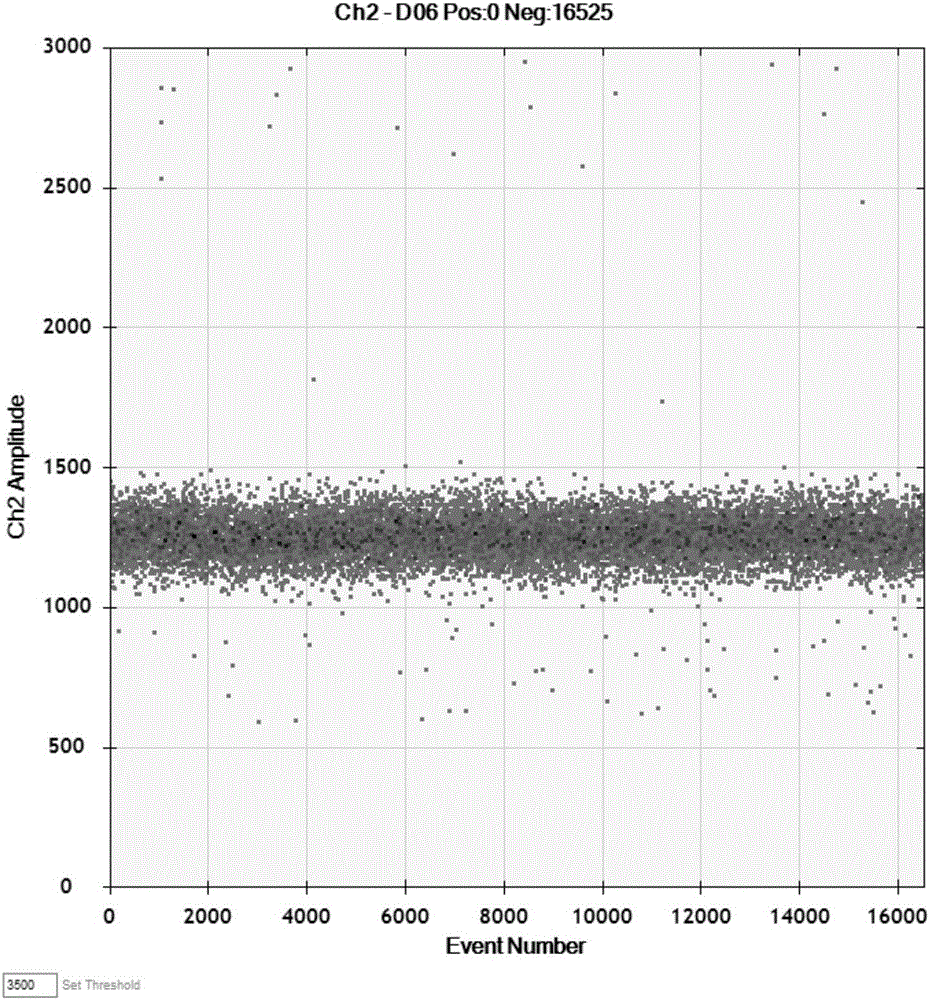
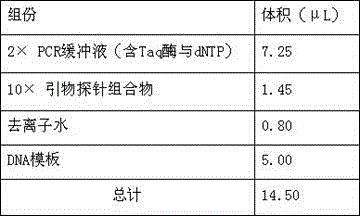
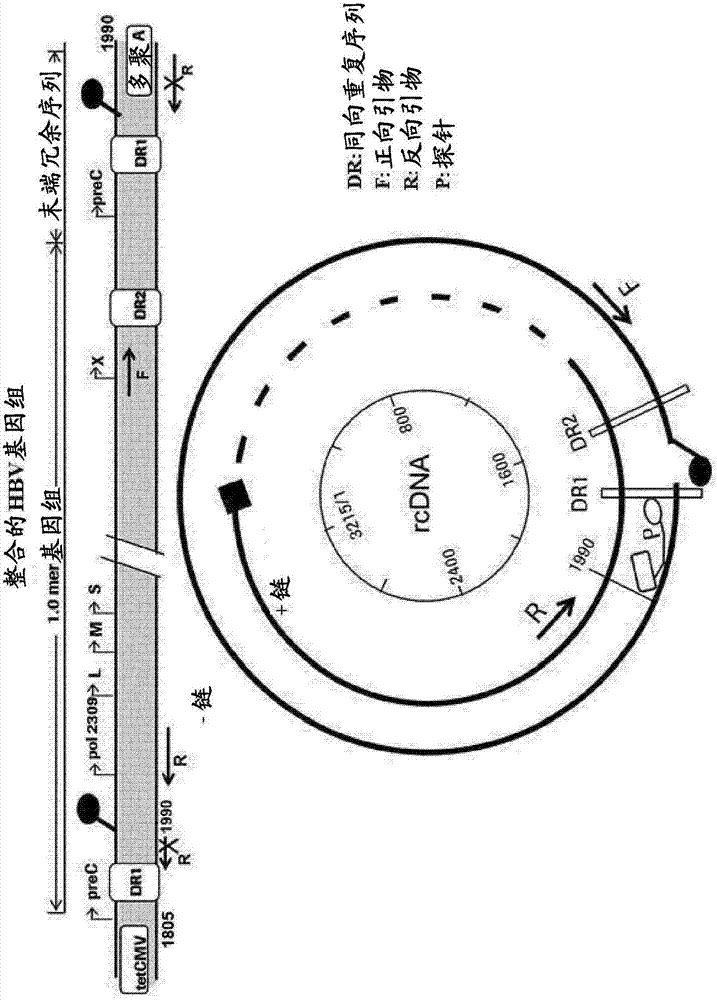
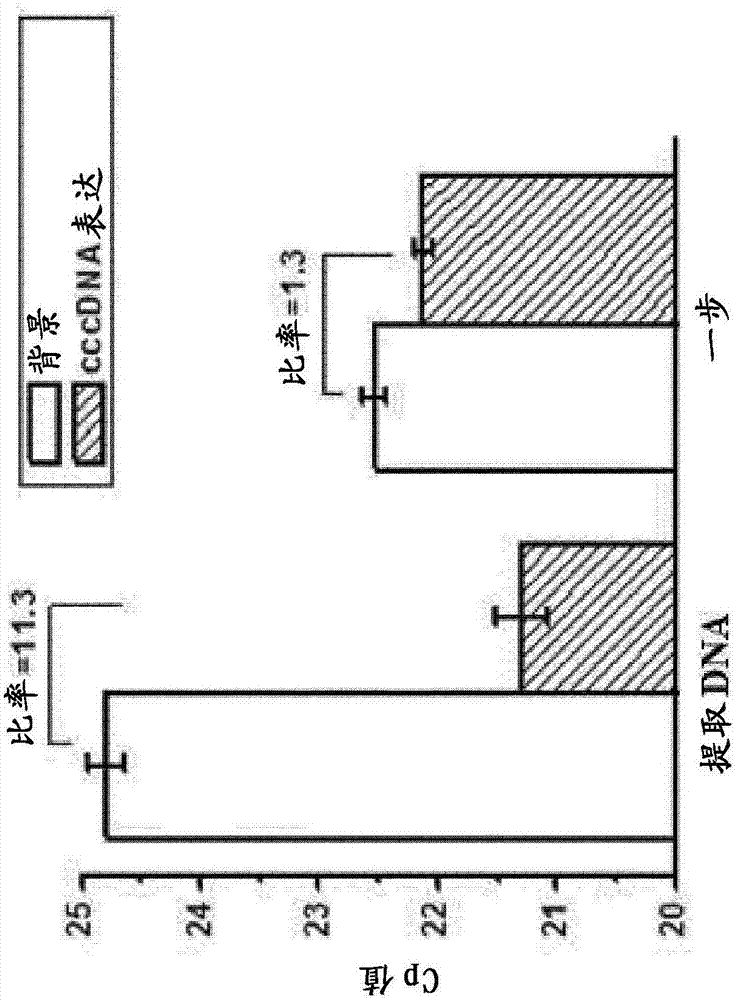
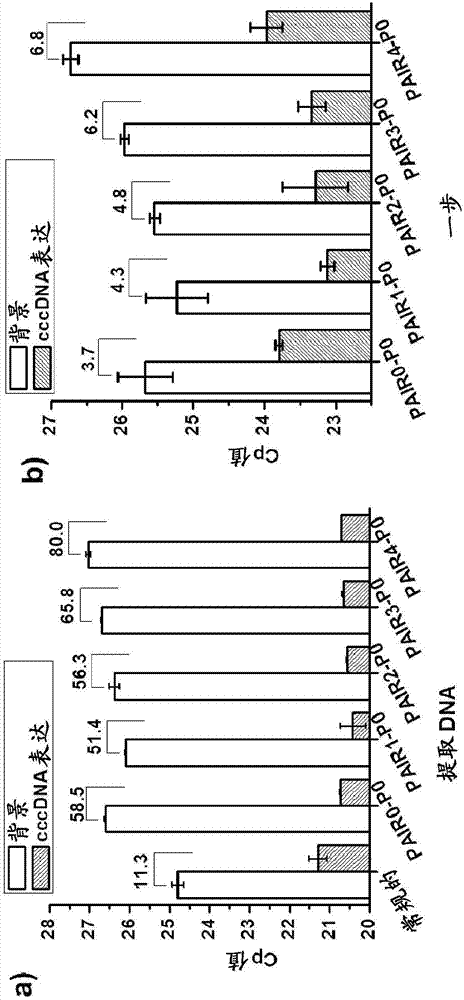
Patents
Literature
88 results about "CccDNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
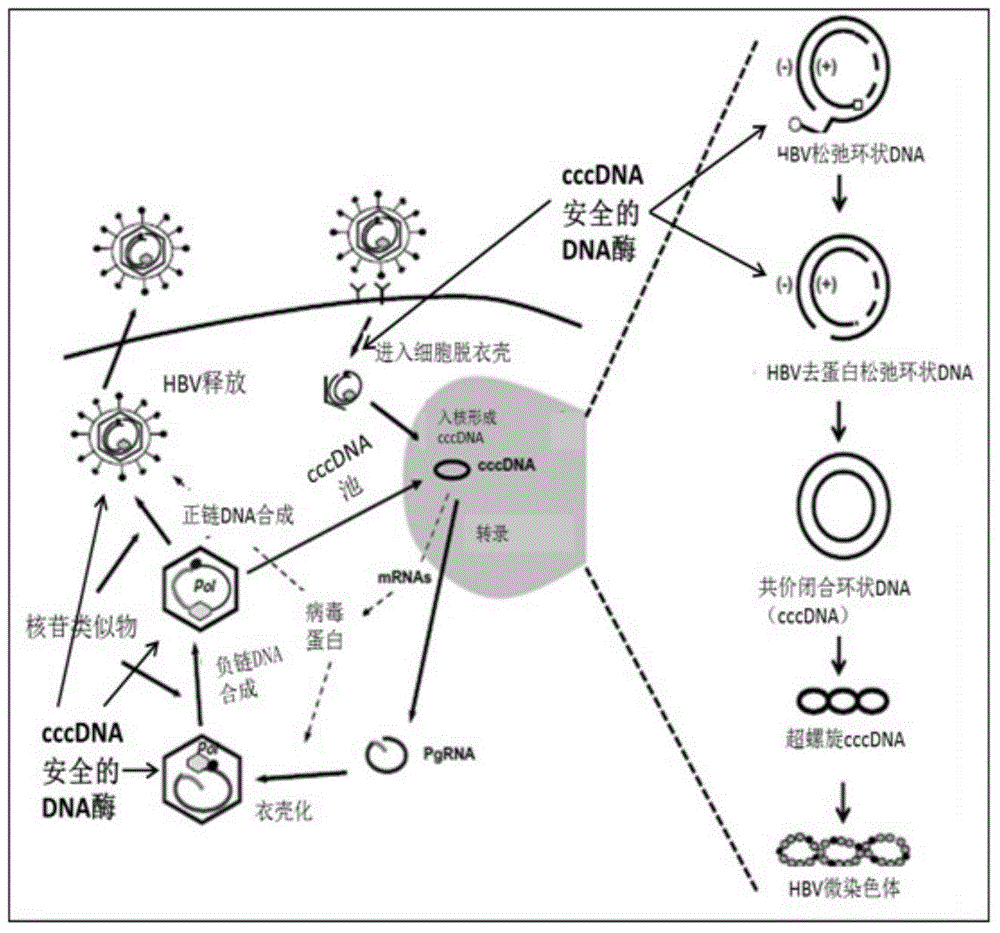
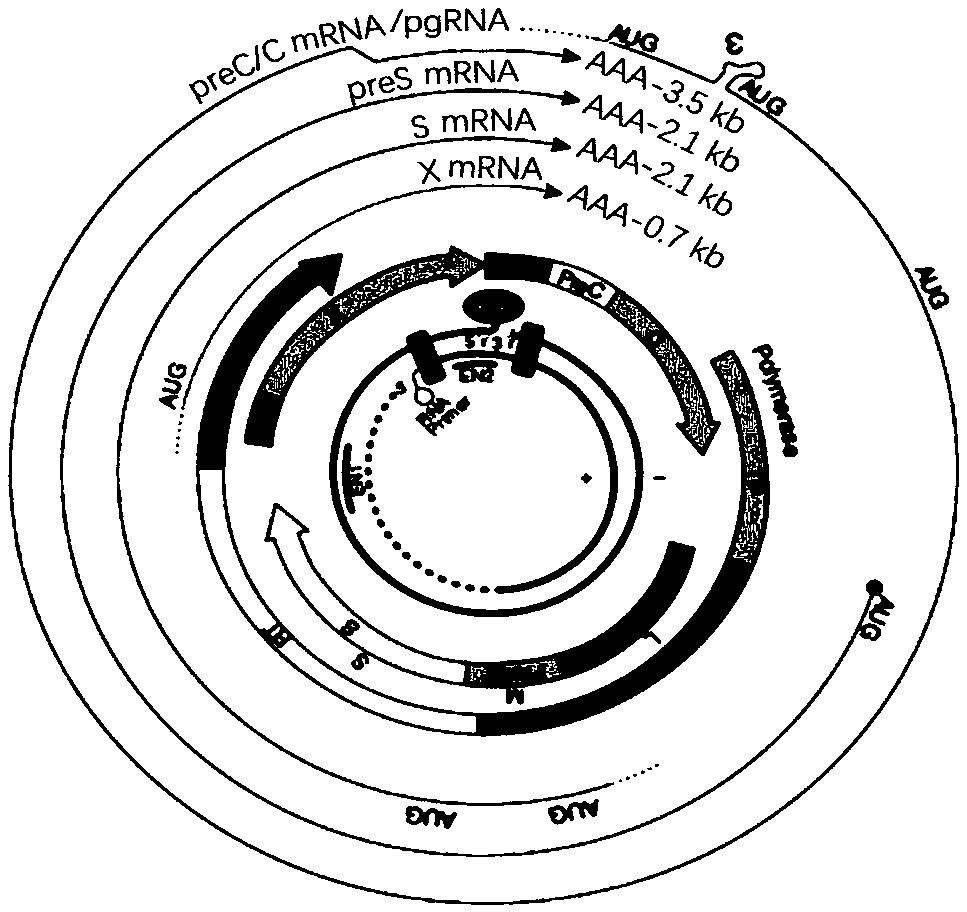
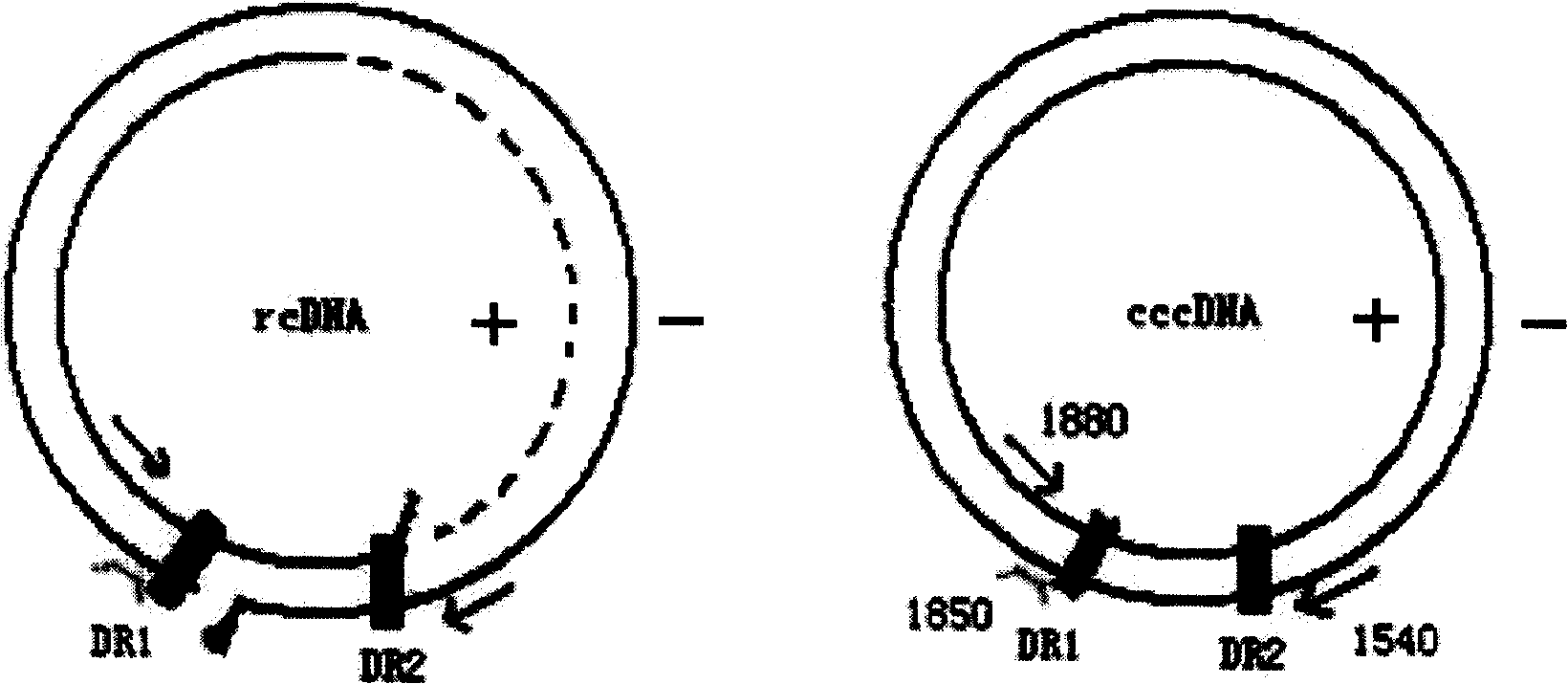
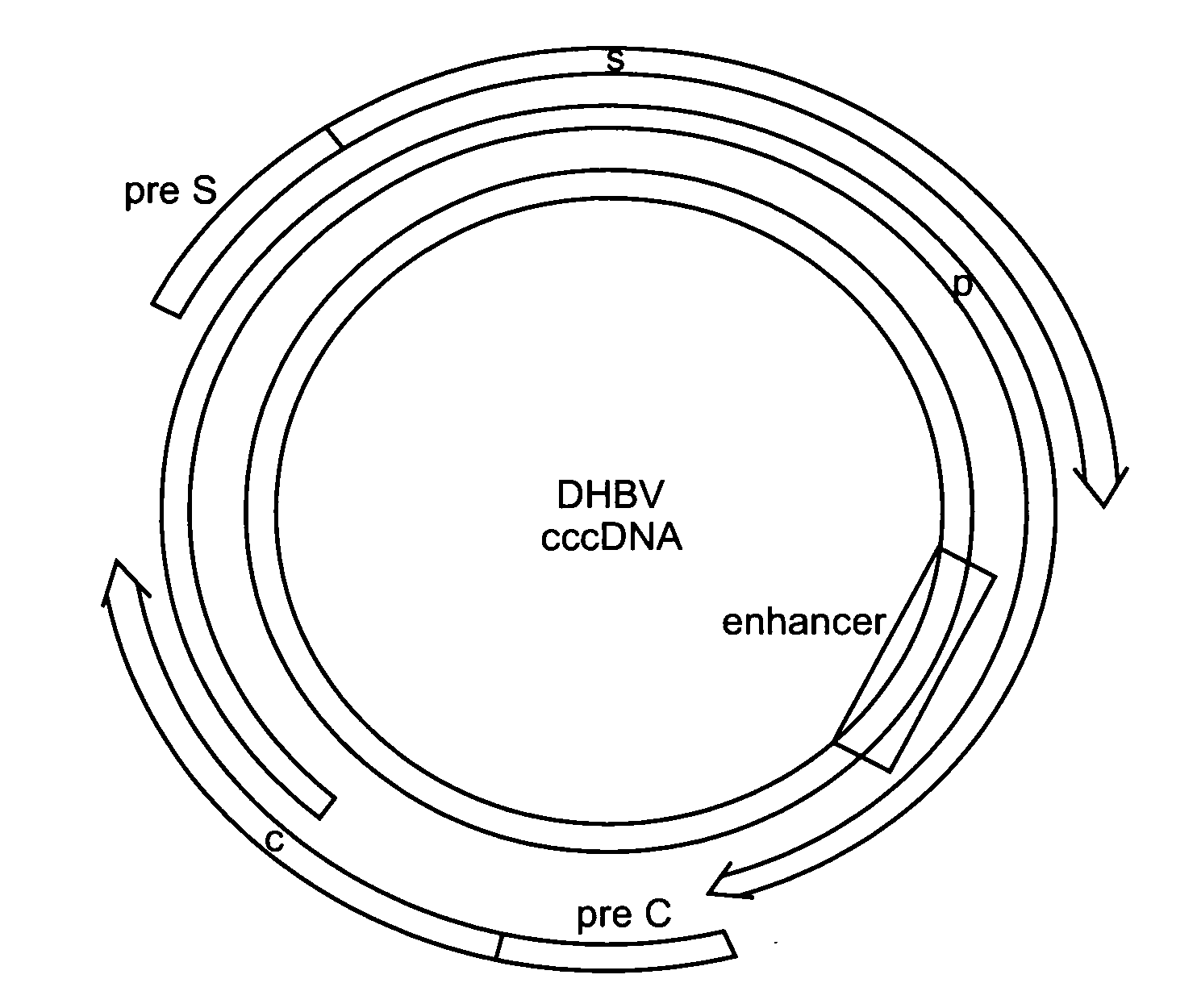
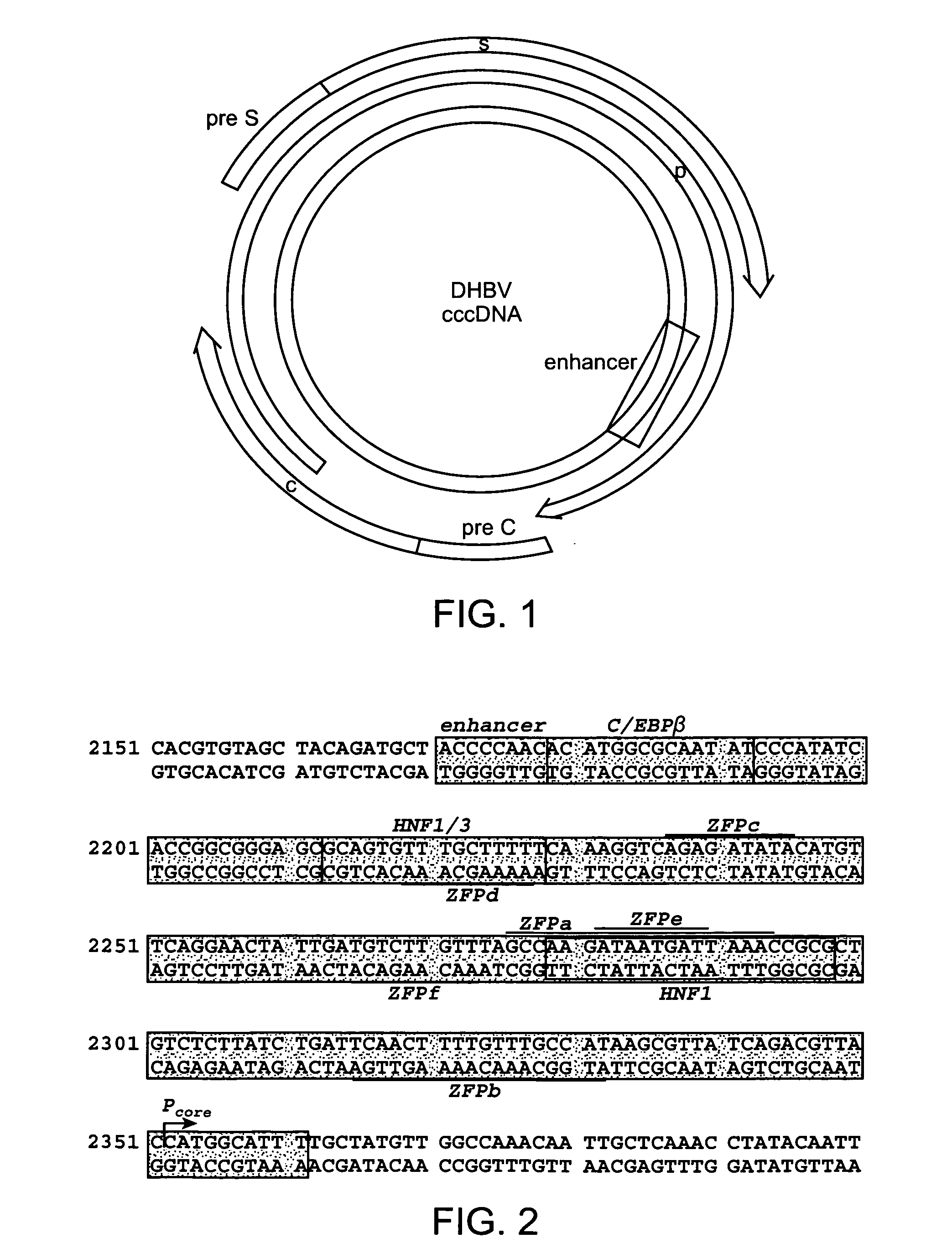
CccDNA (covalently closed circular DNA) is a special DNA structure that arises during the propagation of some viruses in the cell nucleus and may remain permanently there. It is a double-stranded DNA that originates in a linear form that is ligated by means of DNA ligase to a covalently closed ring. In most cases, transcription of viral DNA can occur from the circular form only. The cccDNA of viruses is also known as episomal DNA or occasionally as a minichromosome.
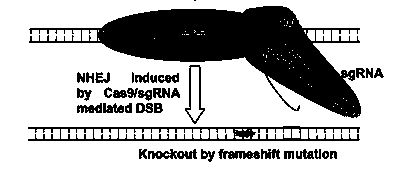
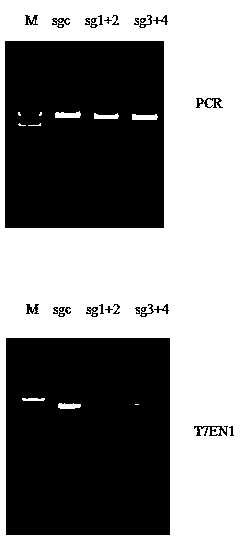
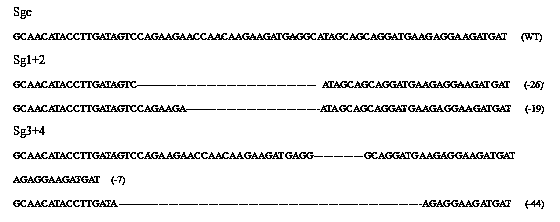
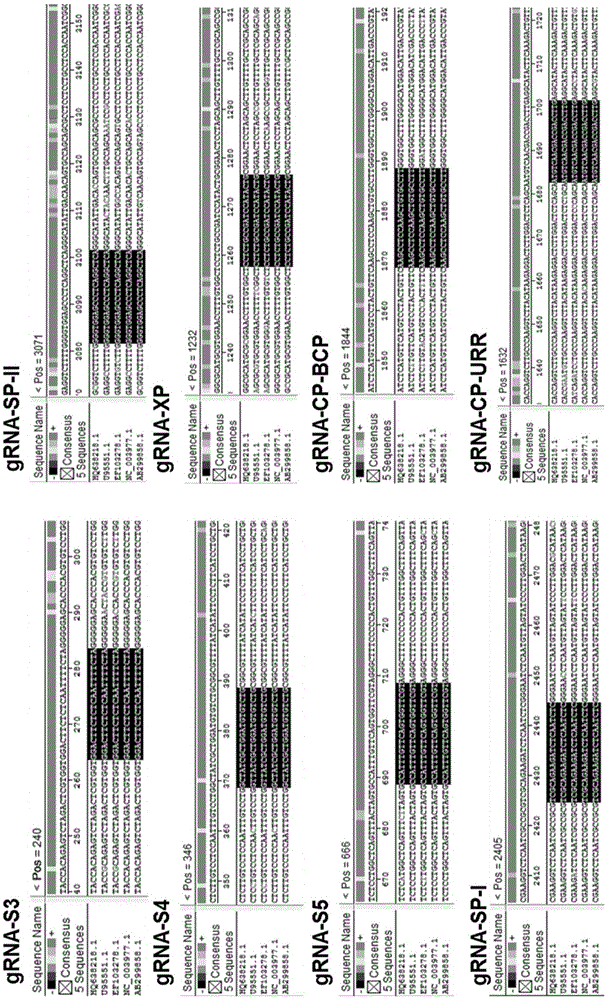
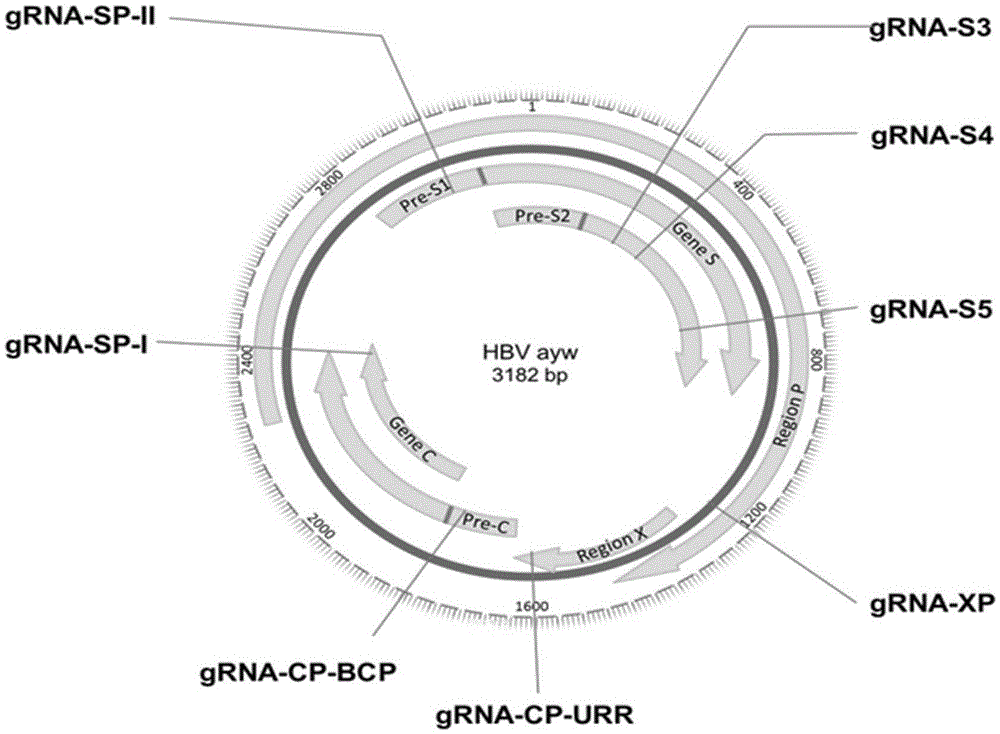
CRISPR-Cas9 targeted knockout hepatitis b virus cccDNA and specific sgRNA thereof
The invention belongs to the field of genetic engineering, and particularly relates to a method for specifically knocking out hepatitis b virus cccDNA by using CRISPR-Cas9 and sgRNA for specifically targeting the hepatitis b virus cccDNA. The invention provides a method for specifically knocking out hepatitis b virus cccDNA by using CRISPR-Cas9 and sgRNA for specifically targeting the hepatitis b virus cccDNA. The sgRNA of specific targeted hepatitis b virus cccDNA prepared according to the invention can precisely target hepatitis b virus cccDNA and realize gene knockout. A preparation method is simple in steps and good in sgRNA targeting, and the knockout efficiency of a CRISPR-Cas9 system is high.
Owner:AOMIAO BIOTECH GUANGZHOU CO LTD
Application of CRISPR-Cas9 system based on new gRNA (guide ribonucleic acid) sequence in preparing drugs for treating hepatitis B
PendingCN105647922AInhibition of replicationInhibit expressionOrganic active ingredientsVectorsHepatitis B virusHbv replication
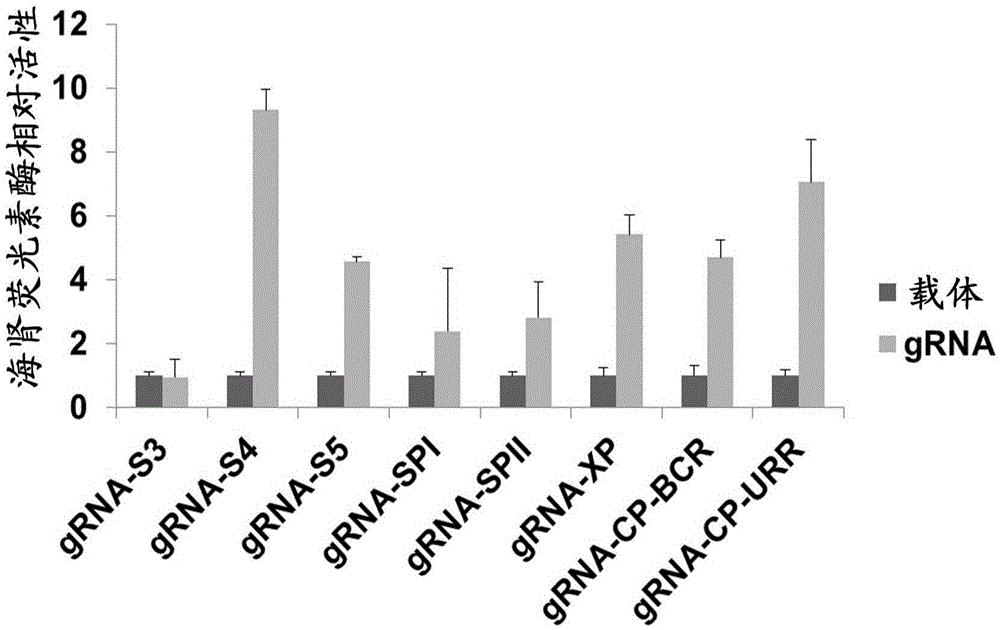
The invention discloses a gRNA (guide ribonucleic acid) sequence. The sequence can be used for DNA (deoxyribonucleic acid) sequence edition by using a hepatitis B virus genome S gene conserved region site as a target sequence, can successfully destroy the HBV (hepatitis B virus) cccDNA and integrated-state HBV DNA in a HBV stable cell model and an HBV hydrodynamic mouse model, and obtains obvious effects on inhibiting HBV replication and expression. The invention also discloses application of the CRISPR-Cas9 system containing the gRNA sequence in preparing drugs for treating hepatitis B.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Nest type-real time quantitative PCR method for detecting hepatitis B virus cccDNA
InactiveCN101104867AImprove featuresHigh sensitivityMicrobiological testing/measurementNegative strandFluorescence
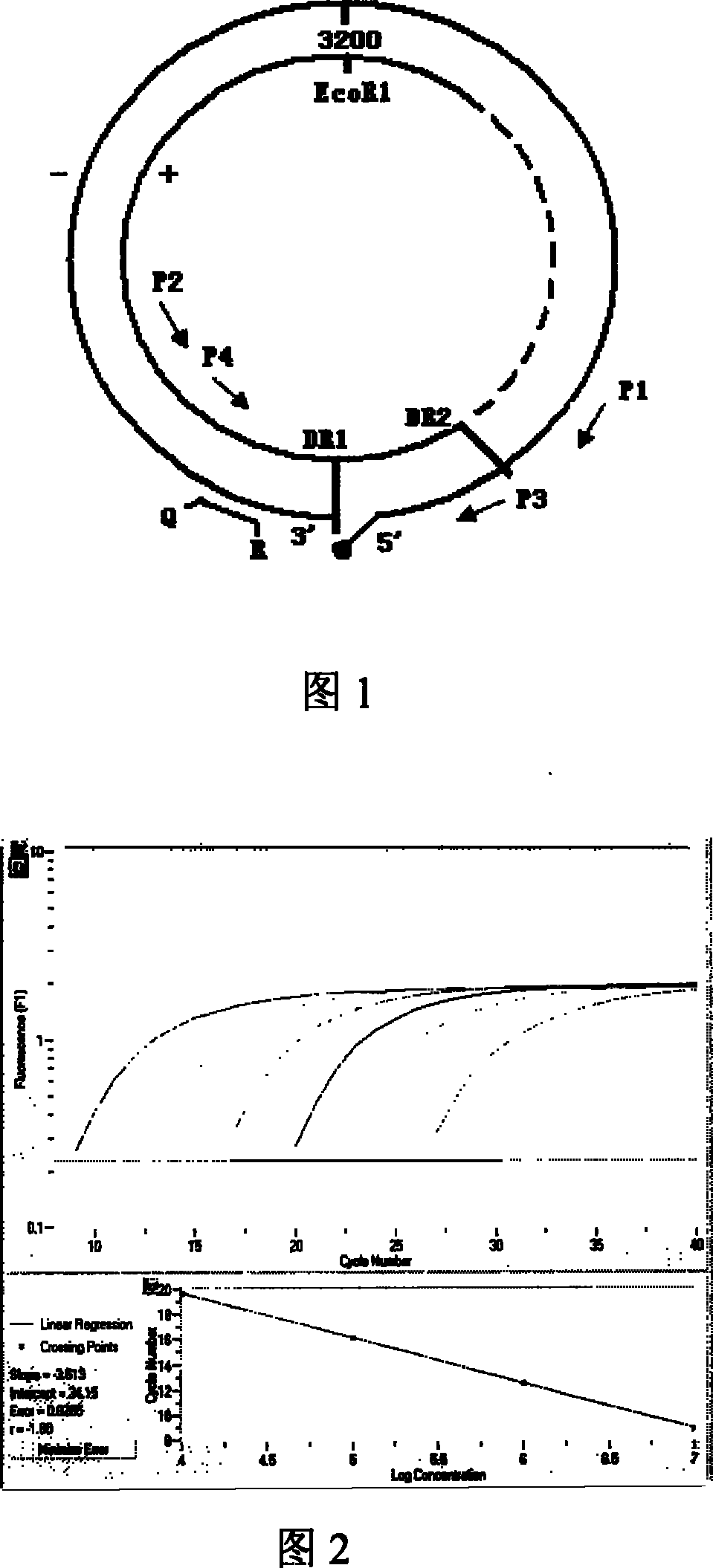
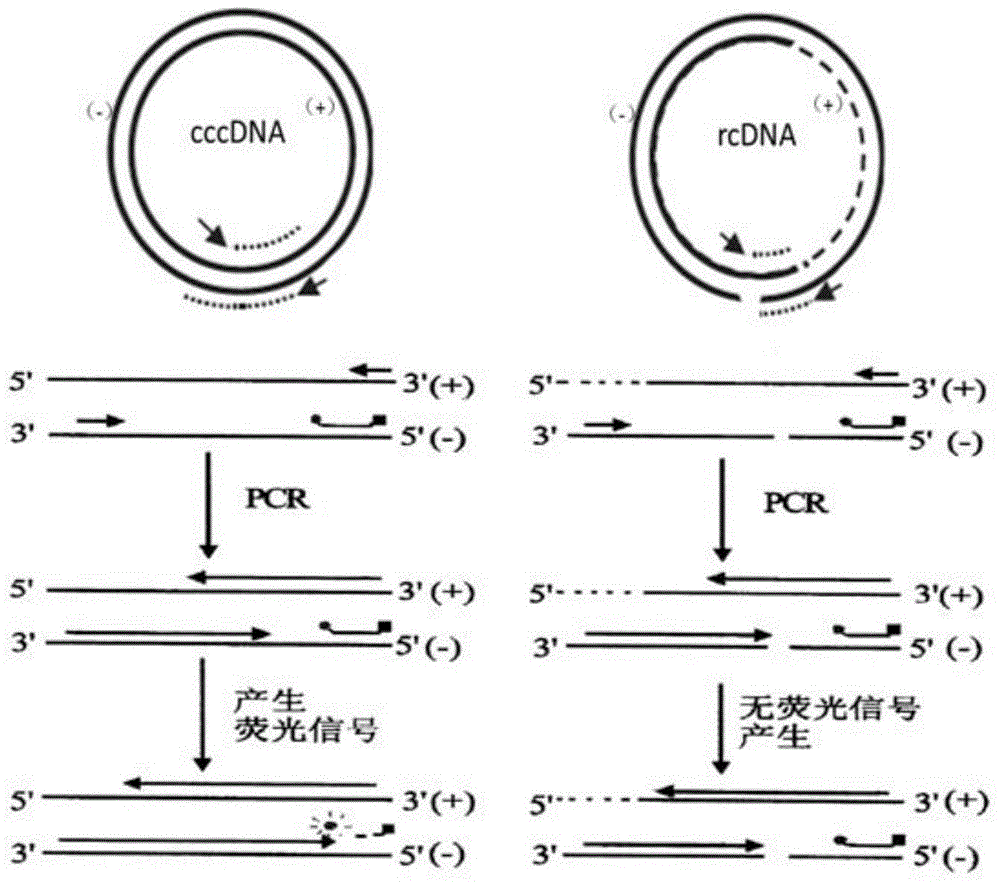
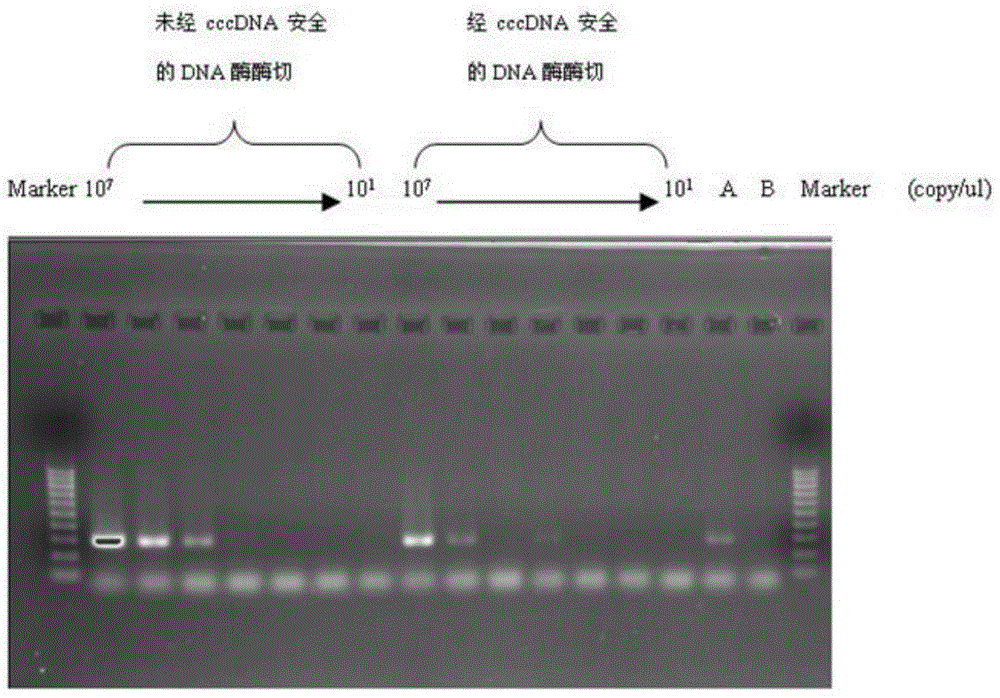
The invention relates to a nested quantitative PCR detection method for virus covalently closed circular DNA (ccc DNA) of hepatitis b virus. The particular detection steps are: 1) extraction DNA of hepatitis b virus; 2) design and synthesis primers and probe: design an internal and an external primer at conservative region of both sides of negative chain gap of HVB DNA, with Taqman fluorescence probe arranged at the lower part of the negative chain gap; 3) Plasmid-SafeTM ATP-Dependent DNase restriction enzyme purification; 4) nested real time quantitative PCR amplification, comprising: common PCR-template with external primer amplification purification and real time quantitative PCR-fluorescence quantitative amplification to common PCR production with internal primer and fluorescence probe. The invention can improve the specificity and sensitivity of detection reactive to make the operation process and result analysis much more accurate, simple and time saving.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Fluorescent quantitative PCR (Polymerase Chain Reaction) testing method for cccDNA (covalently closed circular deoxyribonucleic acid) of hepatitis B virus and kit thereof
InactiveCN102329888AMicrobiological testing/measurementFluorescence/phosphorescenceConserved sequenceFluorescence
The invention relates to a fluorescent quantitative PCR testing method for the cccDNA of the hepatitis B virus and a kit thereof. Firstly, the complete genomic sequence of the hepatitis B virus of the human body is retrieved, a pair of primers and a TaqMvan fluorescent dye-marked probe are designed for the conserved sequence of the hepatitis B virus, and the real-time fluorescent quantitative PCR method is adopted to amplify the target gene. The invention adopts Plasmid-Safe ATP-dependent Dnase (PSBD) enzyme to carry out restriction digestion on the DNA sample, thus increasing the specificity and sensitivity of tests.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
Digital PCR quantitative detecting kit for HBV (hepatitis B virus) cccDNA and application of digital PCR quantitative detecting kit for HBV cccDNA
ActiveCN104388598AAvoid non-HBV cccDNA signalsAssay design is smartMicrobiological testing/measurementMicroorganism based processesPcr chipNucleic acid detection
The invention belongs to the technical field of virus in-vitro nucleic acid detection and particularly relates to a digital PCR quantitative detecting kit for HBV (hepatitis B virus) cccDNA and an application of the digital PCR quantitative detecting kit for HBV cccDNA. A digital PCR quantitative detecting probe composition of the HBV cccDNA is firstly designed, and the kit comprises a container for detecting the probe composition and a container for a purifying reagent containing liver puncture tissue DNA. Using of the kit comprise the following steps of extracting the DNA in the liver puncture tissue, and adopting PSAD (prostate specific antigen density) enzyme to digest the HBV cccDNA; by taking a digital PCR detecting chip as a reaction container and adopting a complex fluorescent multiple PCR primer, amplifying target DNA in detecting micro pores of the digital PCR chip; and obtaining the quantitative results of the HBV cccDNA in each cell according to a fluorescence signal in each micro pore of the digital PCR detecting chip. The digital PCR quantitative detecting kit for HBV cccDNA is strong in specificity, high in sensitivity, simple and convenient and quick. HBV cccDNA and HBV rcDNA are not needed to be separated and extracted, and the defects in the conventional HBV cccDNA detection are overcome, and therefore, the digital PCR quantitative detecting kit is suitable for large-scale popularization and application.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
Recombinant hbv cccdna, the method to generate thereof and the use thereof
The present invention relates to a recombinant HBV cccDNA comprising HBV genome or the fragment or variant thereof and a site-hybrid insert, a method to generate said recombinant HBV cccDNA, a method for establishment of an in vitro or in vivo cccDNA based model for persistently hepatitis B virus replication by using the recombinant HBV cccDNA of the present invention, and a method for anti-HBV drug evaluation.
Owner:F HOFFMANN LA ROCHE & CO AG
Reagent and method for preparation of HBV persistently infected animal model
ActiveCN104278055AFermentationVector-based foreign material introductionPersistently infectedHepatitis B virus
The invention relates to a reagent and method for preparation of an HBV (hepatitis B virus) persistently infected animal model. According to the invention, a construct able to conditionally induce HBV covalently closed circular DNA (cccDNA) molecules in the liver of mice is constructed, and the HBV persistently infected animal model is successfully established.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Replicative HBV (Hepatitis B Virus) vector carrying foreign gene and recombinant HBV generated after transfection and corresponding preparation method and application
ActiveCN103173492AGuaranteed high level of replicationPreserve the ability to reinfectMicroorganism based processesViruses/bacteriophagesHepatitis B virusVirus strain
The invention discloses a replicative HBV (Hepatitis B Virus) vector carrying a foreign gene and a recombinant HBV generated after transfection and a corresponding preparation method and an application. The vector separates overlaying genes C and P on an HBV genome by a molecular cloning technique based on originally expressed HBV plasmid to respectively form an integral opened reading frame where a protein translation starting sequence or a protease enzyme cutting site is inserted to respectively guide expression of foreign gene and gene P. The replicative HBV vector carrying the foreign gene transiently transfecting hepatoma carcinoma cell secretes the recombinant HBV. The recombinant HBV prepared from the HBV vector transfection cells provided by the invention can express the foreign gene and maintain the replicative and infecting capacity. The invention is suitable for constructing an HBV chronic infection animal model, an HBV cell model with cccDNA stably and automatically replicated and a traceable HBV strain, researching a molecular mechanism of HBV infection, replication, packaging and the like, and screening anti-HBV novel medicines.
Owner:BEIQIUEN INT PEACE HOSPITAL P L A
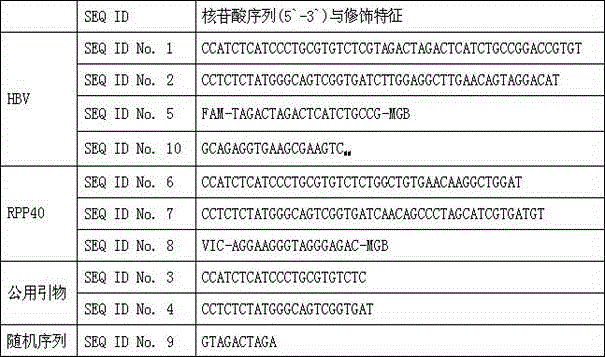
Qualitative and absolute quantification kit for detecting hepatitis B virus cccDNA
InactiveCN104630386ALow costStrong specificityMicrobiological testing/measurementMicroorganism based processesHepatitis B immunizationA-DNA
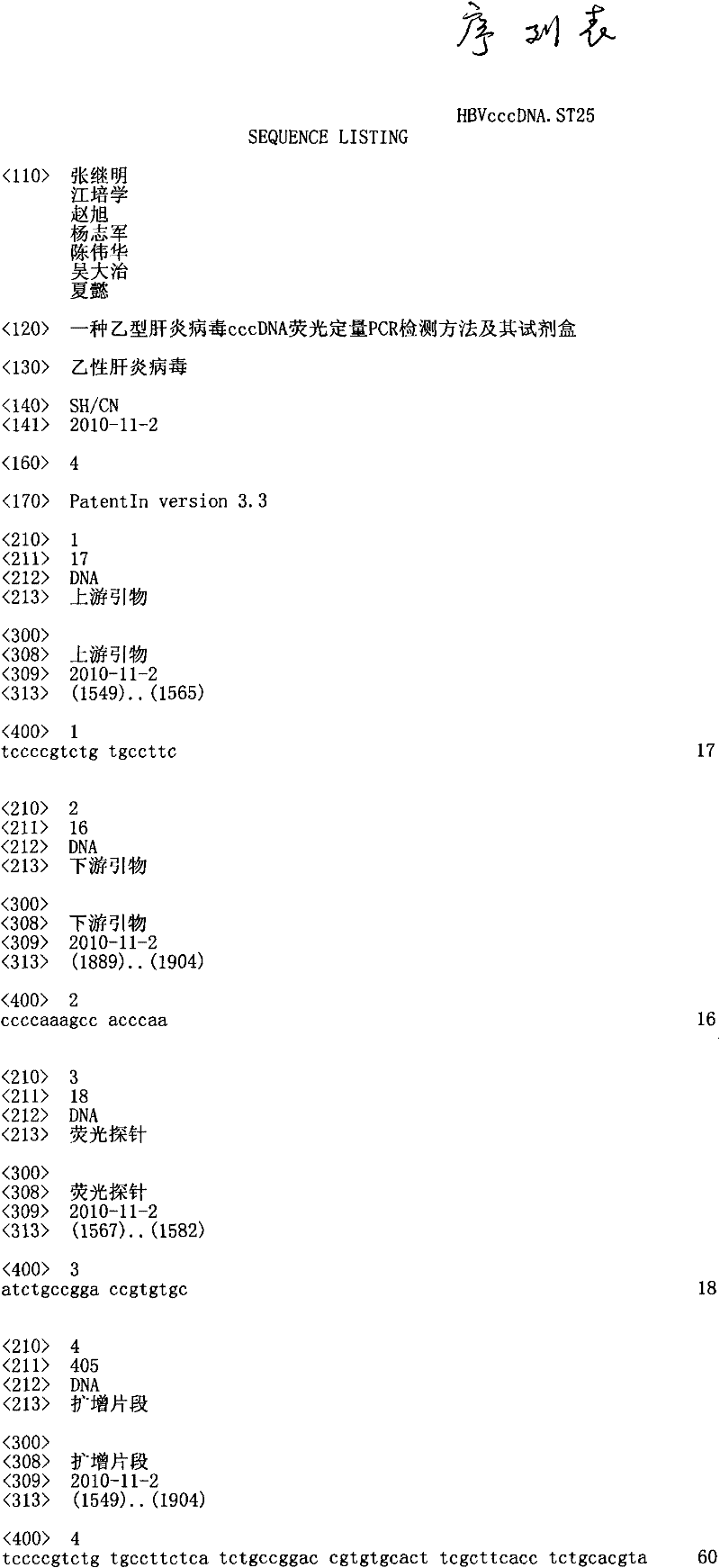
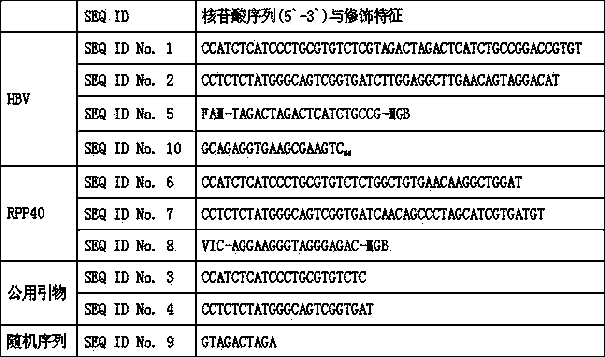
The invention discloses a qualitative and absolute quantification kit for detecting hepatitis B virus cccDNA. The kit comprises an HBV DNA extraction reagent, Plasmid-Safe TMATP-Dependent DNase, an upsteam primer with a DNA sequence of SEQ ID NO.1, a downstream primer with a DNA sequence of SEQ ID NO.2, and a Taqman probe with a DNA sequence of SEQ ID NO.3. The kit also comprises EvaGreen fluorescent dye, common PCR DNA polymerase and digital PCR DNA polymerase. According to the kit, the cccDNA can be rapidly subjected to qualitative and quantitative detection, and the kit can be used for clinically and rapidly judging whether cccDNA is completely eliminated after drug treatment.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV +1
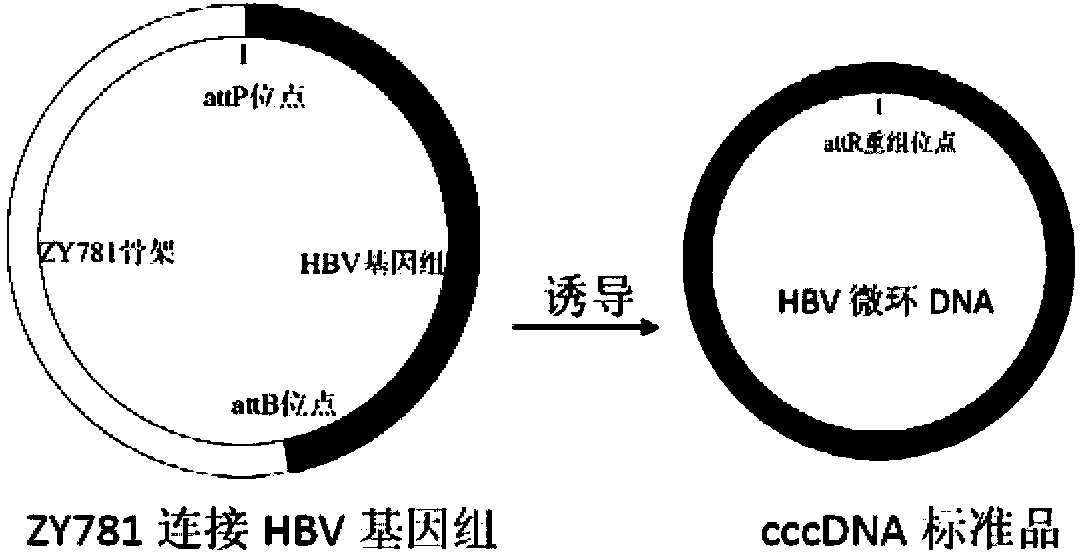
cccDNA standard substance, preparation method thereof, and method and kit for quantitatively detecting cccDNA of hepatitis b virus
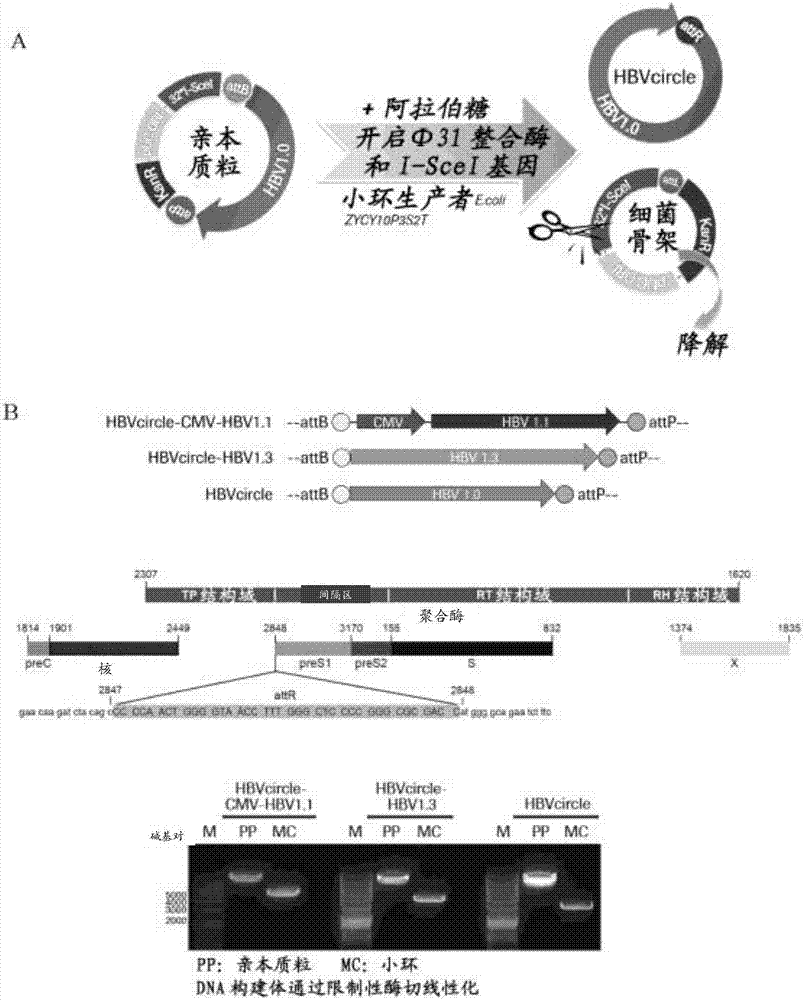
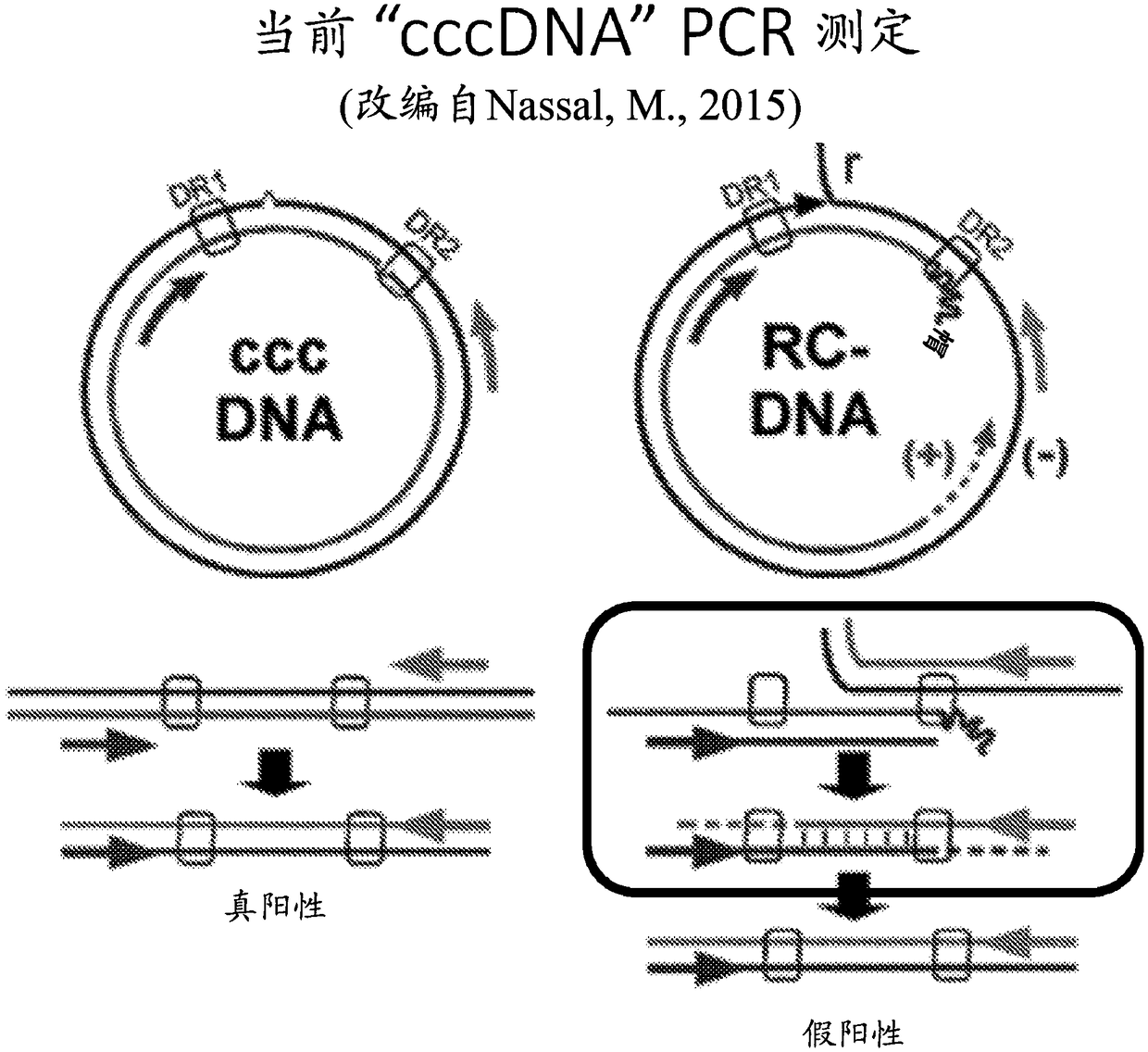
ActiveCN103898100ASolve the problem of detecting false positives of cccDNAQuantitatively accurateMicrobiological testing/measurementDNA preparationFluorescenceMung Bean Nuclease
The invention discloses a cccDNA standard substance and a preparation method thereof. The cccDNA standard substance comprises a unit of DNA of HBV genome and a recombination site attR. The invention also discloses a method and kit for quantitatively detecting the cccDNA of hepatitis B by using the cccDNA standard substance. The method utilizes mung-bean nuclease to eliminate the rcDNA interference, at the same time HBV mini-circle DNA is prepared as the cccDNA standard substance, and then a PCR technology is combined with a fluorescence labeled probe hybrid method so as to carry out quantitative detection on the cccDNA of hepatitis B virus. Compared to the conventional realtime fluorescent quantitative PCR detection method, the method has more precise quantification.
Owner:SHENZHEN INST OF ADVANCED TECH
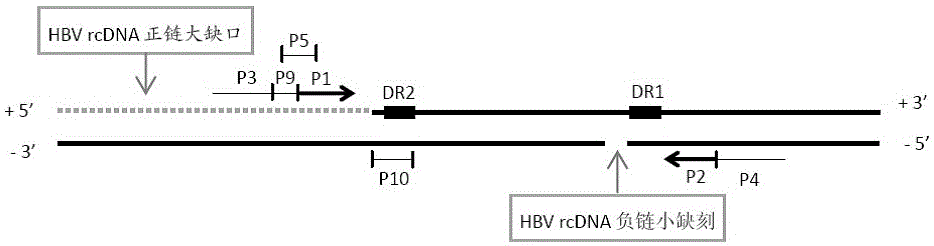
Oligonucleotide composition and method for detecting hepatitis B virus (HBV) rcDNA (Relaxed Circular deoxyribonucleic acid) and/ or cccDNA (Covalently Closed Circular DNA), kit and application of oligonucleotide composition
ActiveCN109321679AMicrobiological testing/measurementDNA/RNA fragmentationDirect repeatHepatitis B virus
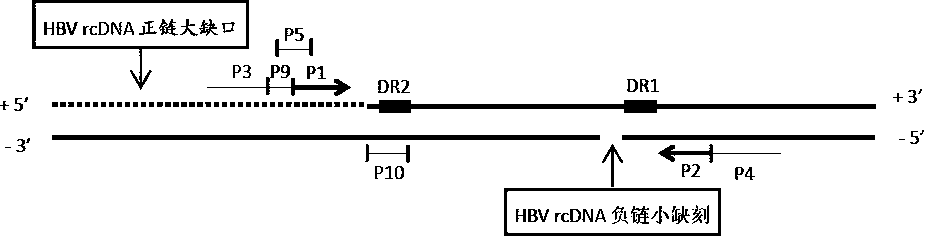
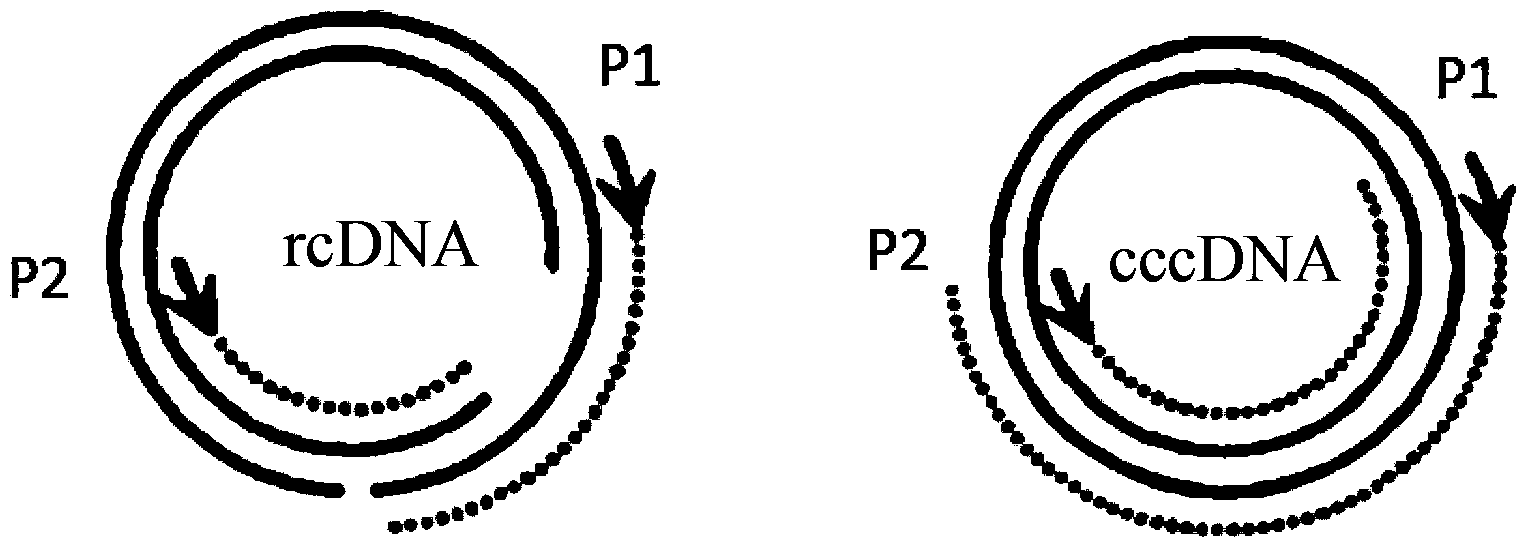
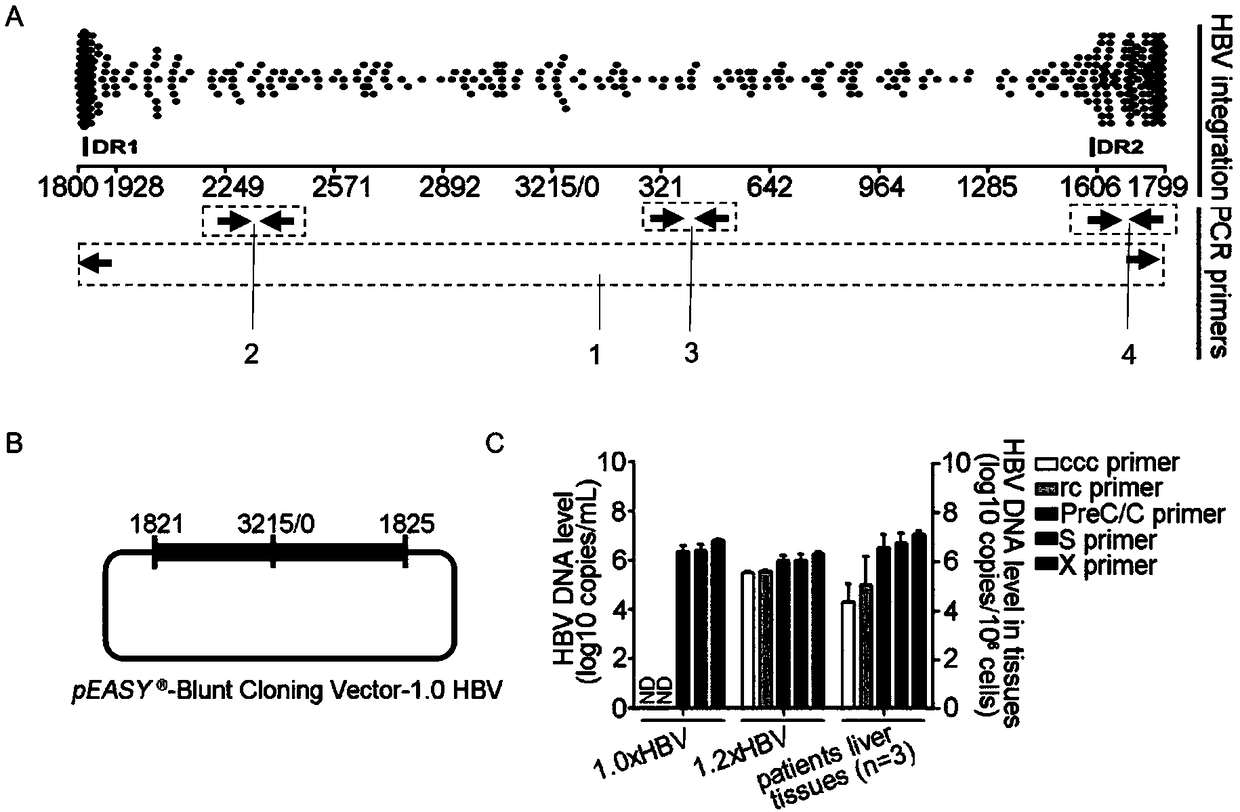
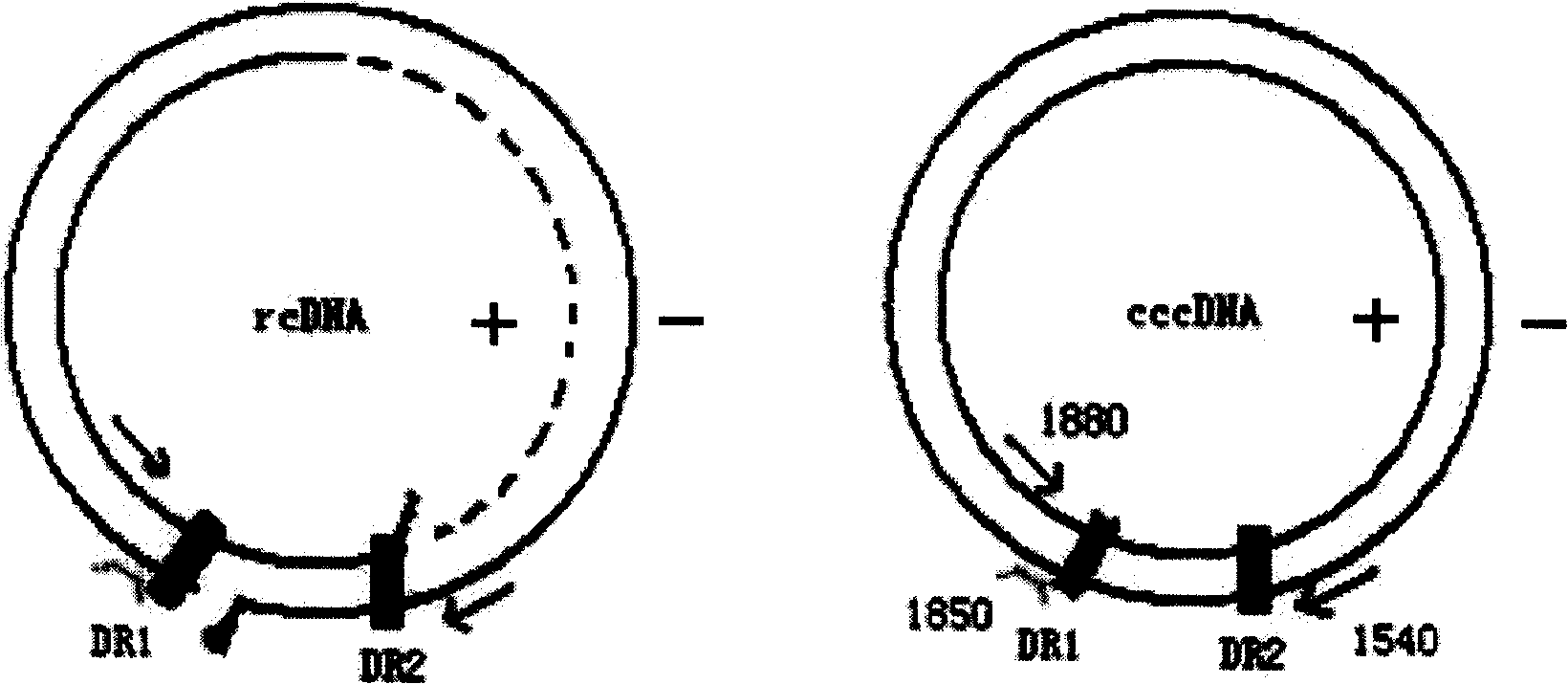
The invention discloses an oligonucleotide composition and a method for detecting hepatitis B virus (HBV) rcDNA (Relaxed Circular deoxyribonucleic acid) and / or cccDNA (Covalently Closed Circular DNA), a kit and application of the oligonucleotide composition. The oligonucleotide composition comprises first oligonucleotide, second oligonucleotide, or oligonucleotide complementary with the sequenceof the first oligonucleotide and oligonucleotide complementary with the sequence of the second oligonucleotide; the first oligonucleotide is specifically combined with at least one part of continuoussequence of the first sequence, and the second oligonucleotide is specifically combined with at least one part of continuous sequence of the second sequence; in addition, a distance between two continuous sequences is 30-350 nt; the first sequence is a sequence between DR (Direct Repeat) 2-DR1 of the HBV DR sequence of a cross-notch area which contains cccDNA or rcDNA, and the second sequence is asequence after the fifth basic group of the DR1. According to the oligonucleotide composition, the level of HBV rcDNA and / or cccDNA can be effectively detected without being affected by integrated HBV DNA.
Owner:北京旌准医疗科技有限公司
Precise quantification method of HBV (hepatitis B virus) cccDNA (covalent closed circular DNA)
InactiveCN106636466ASolve the problem of false positive interferenceRealize closed tube operationMicrobiological testing/measurementMicroorganism based processesPositive controlMicroparticle
The invention relates to a precise quantification method of HBV (hepatitis B virus) cccDNA (covalent closed circular DNA). The precise quantification method of the HBV cccDNA comprises steps as follows: the copy number of a sample is quantitatively controlled within 10,000 copes through nucleic acid; primers and probes of cccDNA and rcDNA are designed and detected with a double-gap method; digital PCR (polymerase chain reaction) amplification is performed; analysis parameters are adjusted in digital PCR instrument analysis software, 1 / 2-1 / 3 of the average fluorescence intensity of positive particles in positive control holes is set as a positive threshold, the to-be-detected sample is analyzed and the copy number of cccDNA in the sample is calculated using software of the digital PCR instrument, and false positive caused by non-specific amplification is eliminated. The method solves the false positive interference problem of double-gap method and the PCR method for rcDNA; compared with other digital PCR detection methods for cccDNA, the method has the advantages that specific DNA enzyme is not required to treat the sample, closed pipe operation is realized, the cross-pollution risk is reduced, and the operation process is simplified.
Owner:NANFANG HOSPITAL OF SOUTHERN MEDICAL UNIV
Kit for detecting hepatitis B virus cccDNA (Deoxyribonucleic Acid) through fluorescent quantification PCR (Polymerase Chain Reaction) of rolling cycle augmentation spanned notch
InactiveCN101948932AHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceFluorescent pcrHepatitis B virus
The invention relates to a method for quantitatively detecting hepatitis B virus cccDNA (Deoxyribonucleic Acid) by adopting a real-time fluorescent PCR (Polymerase Chain Reaction) technology of a rolling cycle augmentation spanned notch, and a novel detection kit developed by the method. The invention can simultaneously improve the specificity and the sensitivity of a detection reaction.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
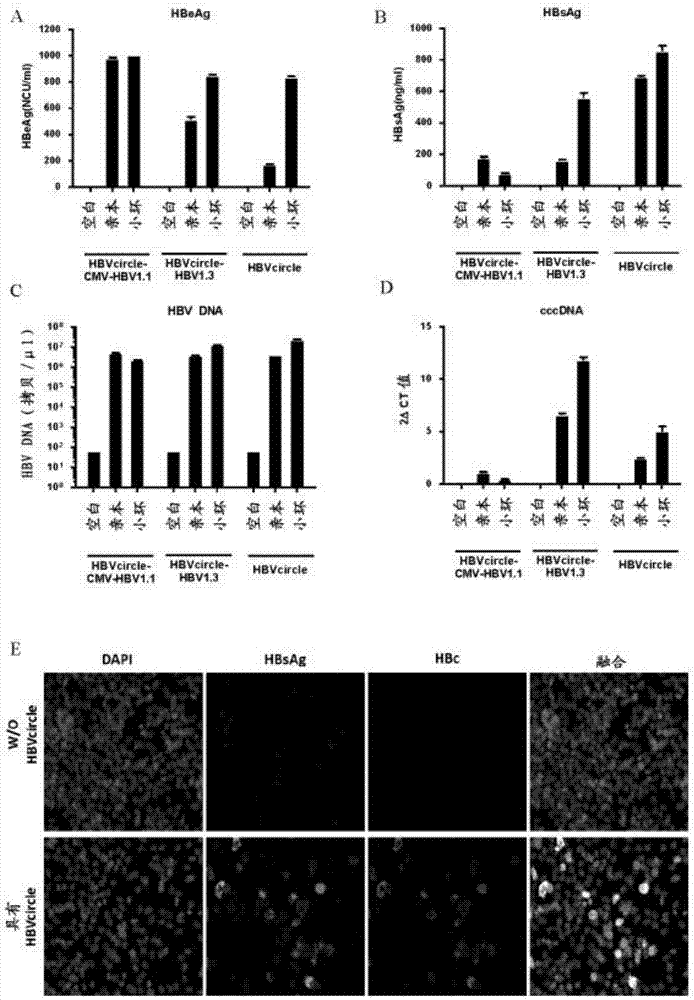
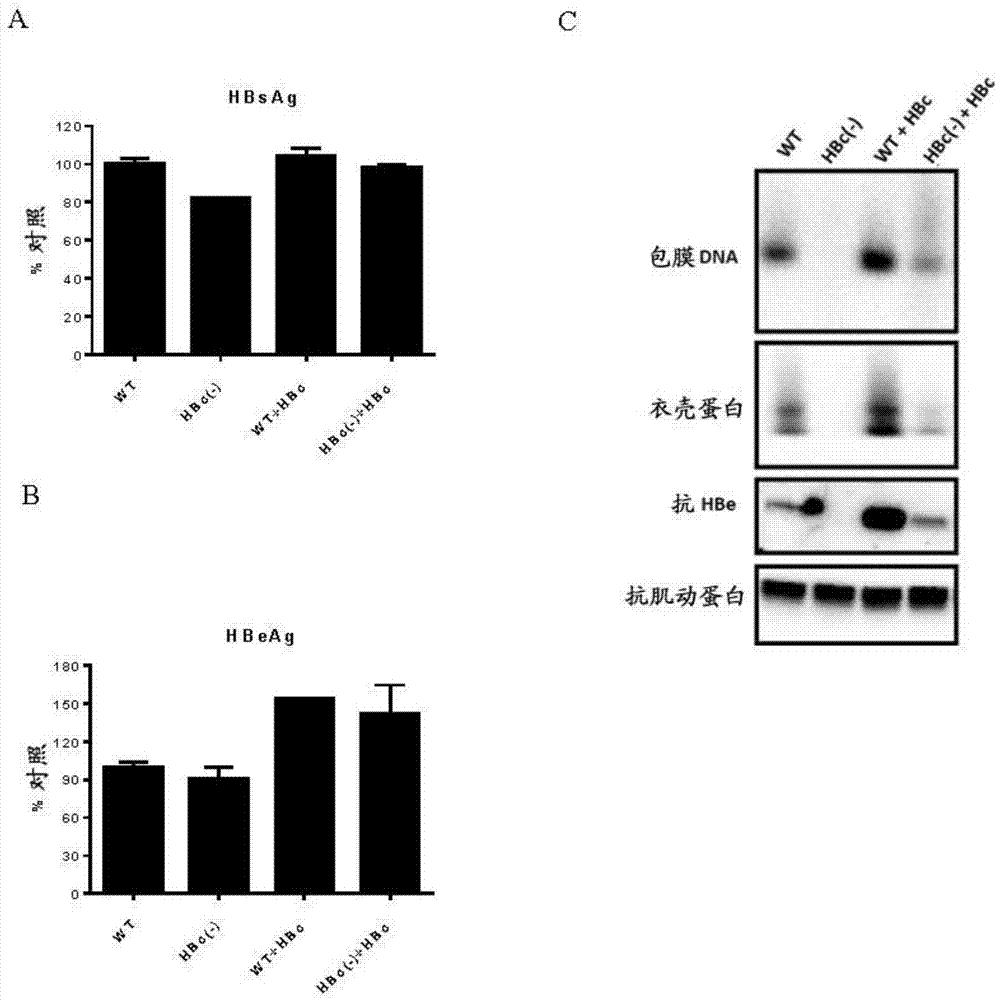
Cell system for simply and efficiently generating hepatitis B virus (HBV) recombinant cccDNA
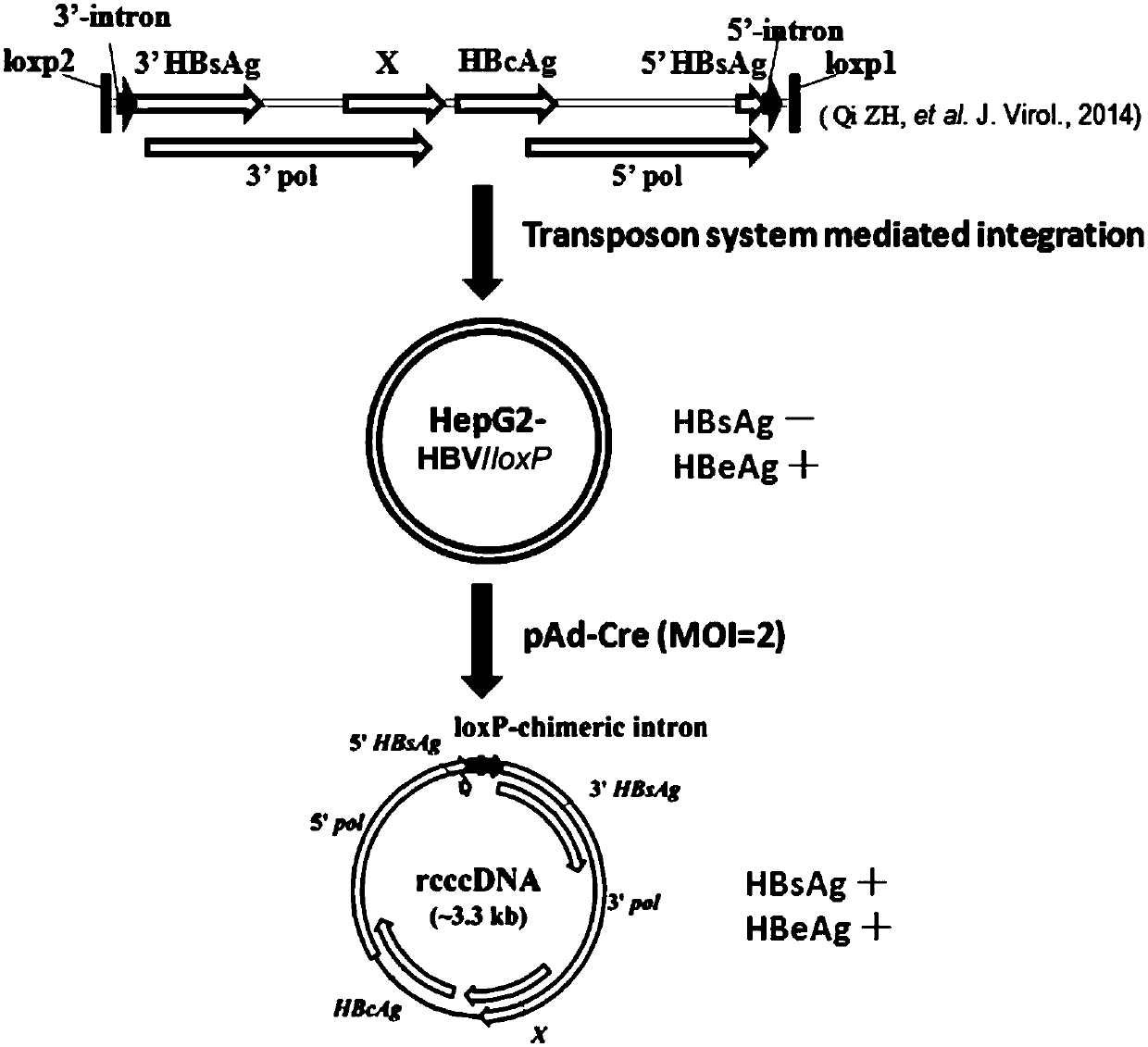
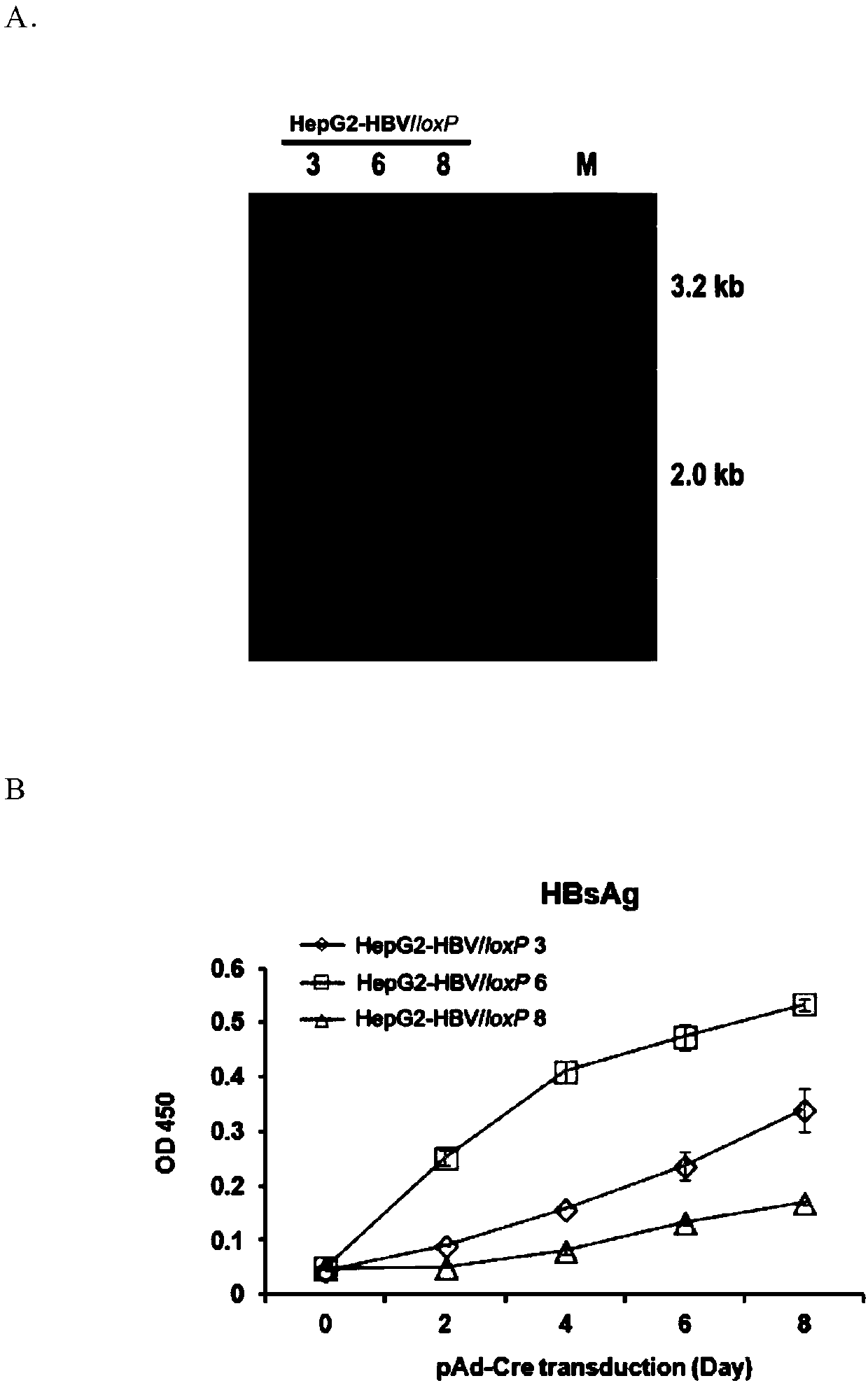
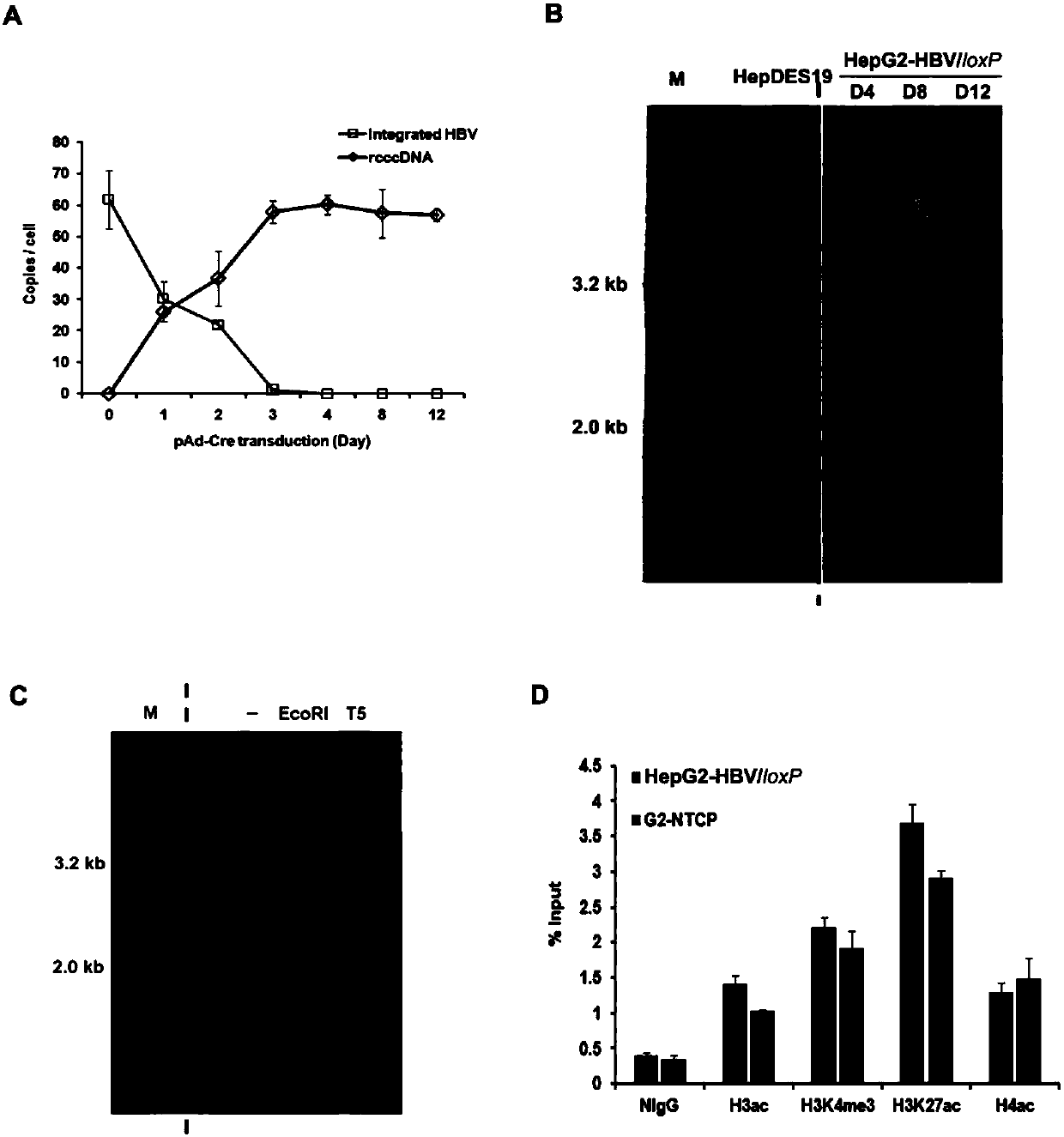
The invention belongs to the field of microorganism animal cell lines, and relates to a cell system for generating hepatitis B virus (HBV) recombinant cccDNA. Through a transposon system, HBV single-copy genomes with two ends provided with loxP sites are integrated into HepG2 cells, a clone HepG2-HBV / loxP cell line integrated with the different-copy-number HBV genomes is obtained, the cell line does not express HBsAg, after cyclization recombination enzyme (Cre) is introduced through adenovirus transduction, the rcccDNA is generated and the HBsAg is expressed, and expression of the HBsAg is closely relevant to the generation and level of the rcccDNA; the rcccDNA is rapidly and stably generated, the structure feature of the rcccDNA is similar to that of real HBV cccDNA, and transcription, copy and protein expression of viruses can be supported; the rcccDNA can be quantitatively detected through quantitative PCR by designing a specific primer, and Southern Blot detection can be carried out by utilizing a digoxin marking system. The cell system can be used for establishing a platform for HBV cccDNA relevant biological research and screening and evaluation of anti-HBV medicines.
Owner:FUDAN UNIV +1
Construction method for hepatitis B virus (HBV) non-human animal model and application of HBV non-human animal model
InactiveCN111705080AEasy to detectVirus peptidesMicroinjection basedSite-specific recombinationHepatitis B virus
The invention relates to a construction method for a hepatitis B virus (HBV) non-human animal model and application of the HBV non-human animal model. The construction method comprises the step: constructing a transgenic animal into which a first fusion gene segment and a second fusion gene segment are transferred, wherein the first fusion gene segment includes an inducible response element, a promoter, a nuclear localization signal and site-specific recombinase; the inducible response element can make the site-specific recombinase expressed under the condition that an inducer is present; thesecond fusion gene fragment includes an HBV gene and a reporter gene; the reporter gene is divided into two segments, and the two segments are fused with an N terminal and C terminal of the HBV gene respectively; two ends of the second fusion gene fragment are each further provided with a recognition site for the site-specific recombinase; under the action of the site-specific recombinase, the second fusion gene fragment can be cyclized, and the two segments of the reporter gene are fused into a complete active segment; and when the transgenic animal is injected with the inducer, HBV cccDNA can be generated.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
Fluorescence quantification detection method of hepatitis B virus cccDNA and kit
InactiveCN101285105ABroad representationStrong specificityMicrobiological testing/measurementFluorescenceHepatitis B virus
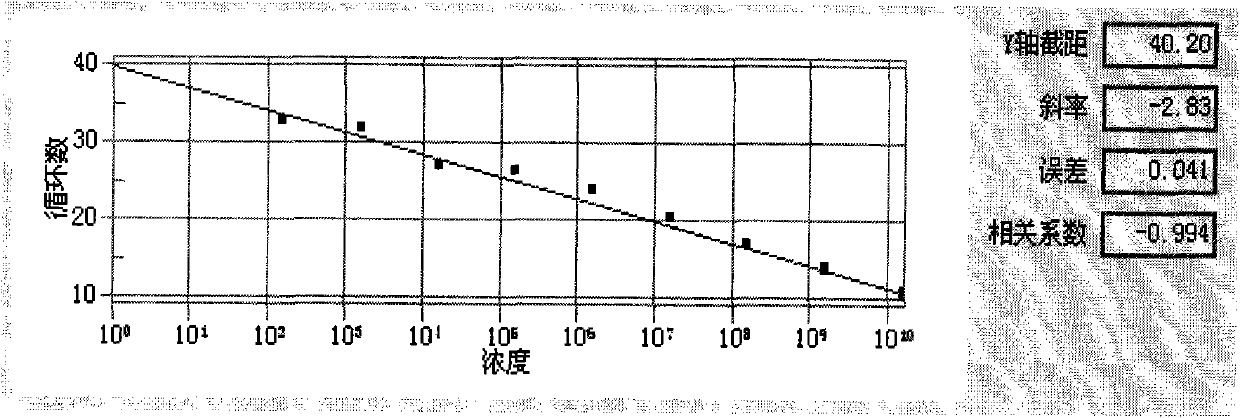
The invention relates to a fluorescent quantitative detection method and a reagent kit for a hepatitis B virus cccDNA, which belong to the genetic engineering field. The fluorescent quantitative detection method for the hepatitis B virus cccDNA comprises a PCR augmentation reaction system and is characterized in that: the PCR system comprises an upstream primer, a downstream primer and a probe, wherein, the upstream primer is positioned at a HBV genome nucleotide sequence 1520-1580, the downstream primer is positioned at a HBV genome nucleotide sequence 1920-2000; and the probe is positioned at a HBV genome nucleotide sequence 1850-1900, and both ends of the probe are respectively connected with a fluorescent group and a quenching group. The method has high sensitivity and a broad linear detection range, and can save the consumption of reagent.
Owner:NANJING ZHONGXIN TRANSLATIONAL MEDICINE INSTCO
Kit for detecting hepatitis B virus (HBV) cccDNA (Covalently Closed Circular DNA) of paraffin embedded hepatic tissue
InactiveCN101948933AEasy to acceptResolve detectionMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceHepatitis B virus
The invention relates to a kit for quantitatively detecting hepatitis B virus (HBV) cccDNA (Covalently Closed Circular DNA) of paraffin embedded hepatic tissue, which is characterized by comprising the following steps of: carrying out paraffin embedding on hepatic tissue; then respectively adding extracted total DNA into an HBV rolling loop primer and a notch crossing primer; and carrying out rolling and notch crossing fluorescent quantitative amplification. The kit has the advantages of no need of fresh hepatic tissue as a detecting sample, high detecting specificity and high sensitivity.
Owner:302 MILITARY HOSPITAL OF CHINA
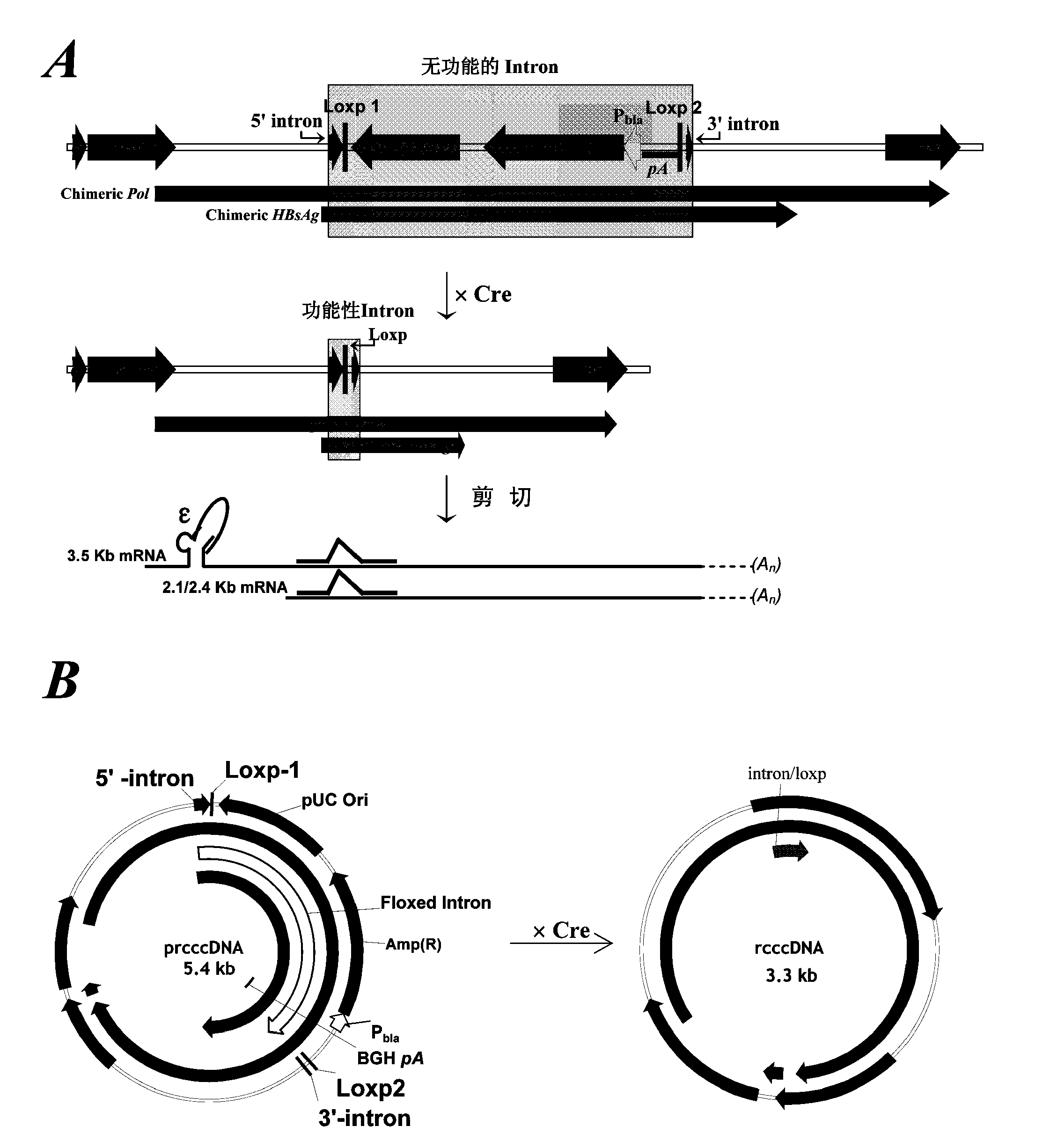
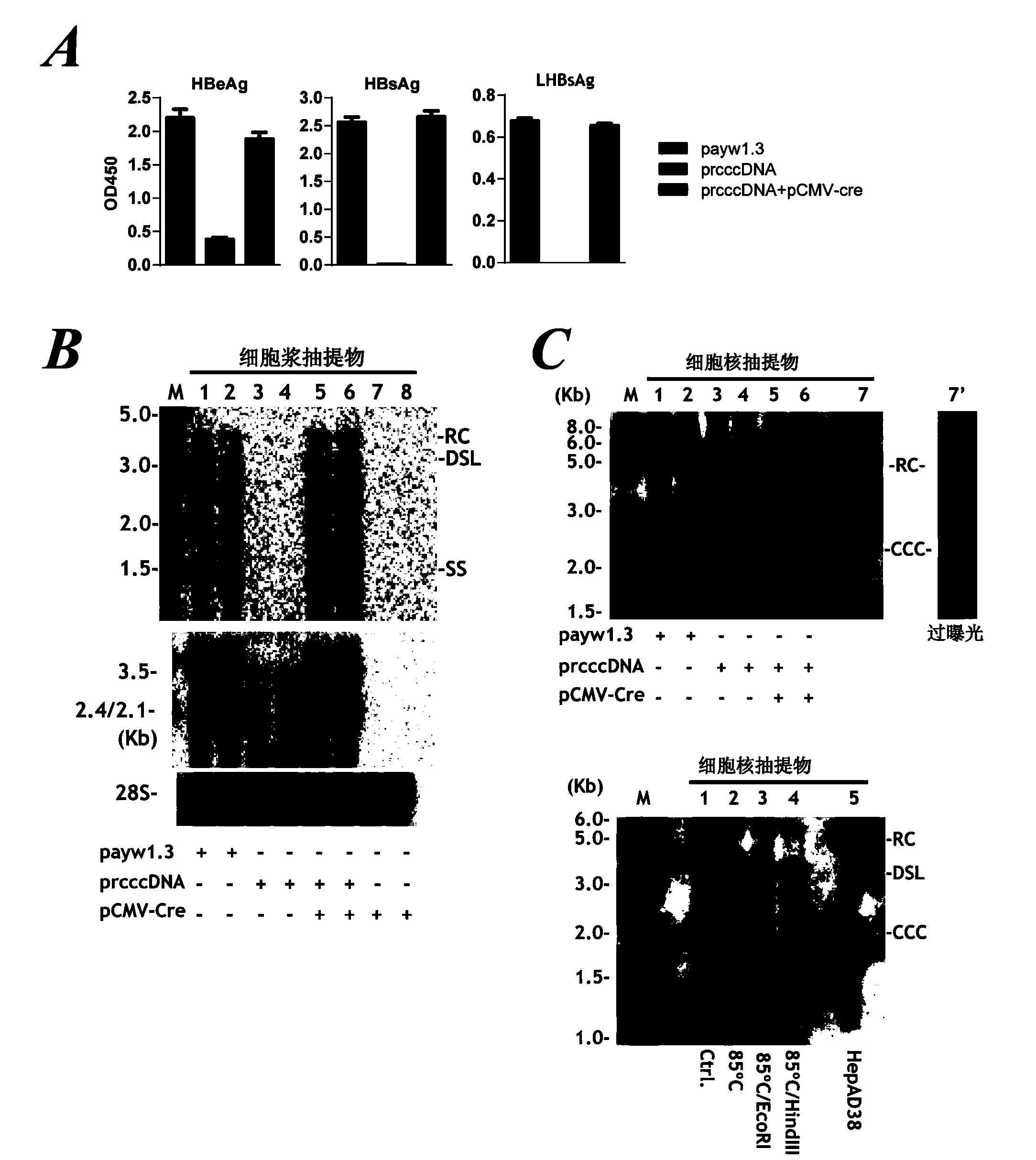
Construction and application of model for researching interaction between HBV cccDNA and host
ActiveCN110592026AReliable detection methodInhibition of replicationMicrobiological testing/measurementDigestive systemInteinTreatment targets
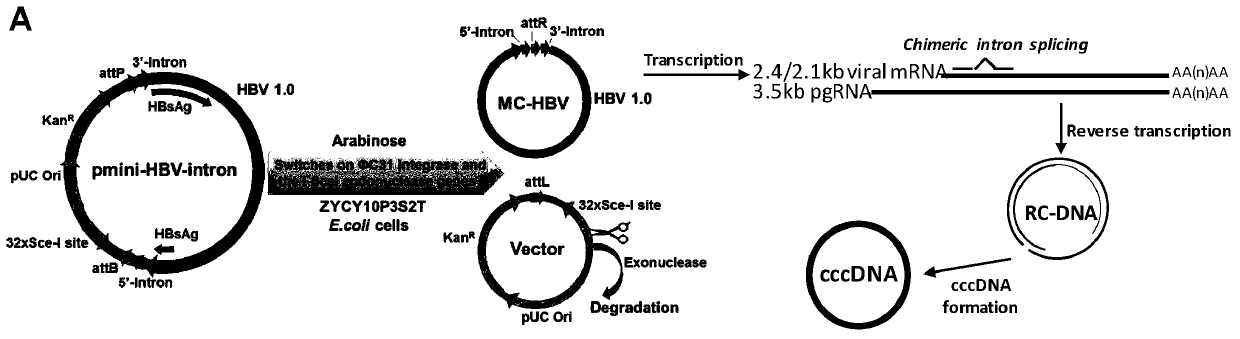
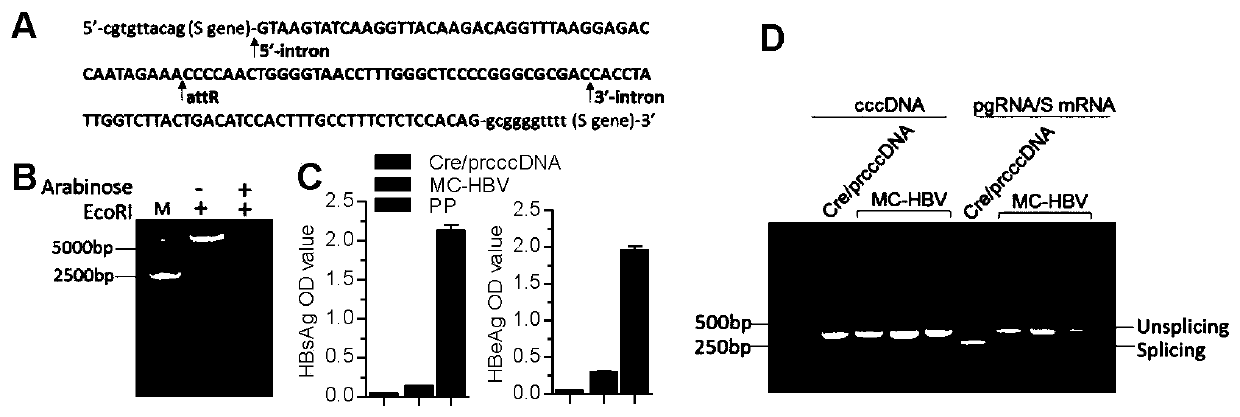
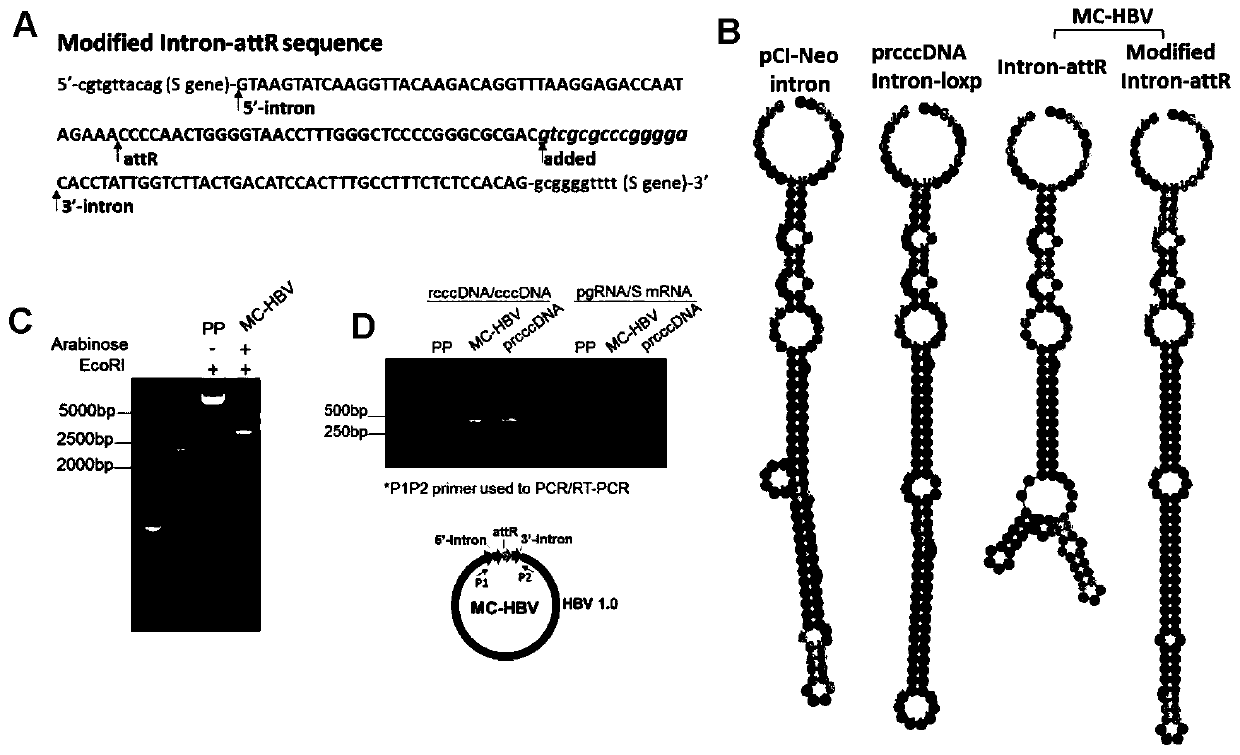
The invention belongs to the technical field of bioengineering, and particularly relates to construction and application of a model for researching interaction between HBV cccDNA and a host. The invention provides the construction and the application of the minicircle DNA model highly simulating hepatitis B virus genome cccDNA. By constructing a pmini-MC-HBV-Intron plasmid, a high-purity MC-HBV minicircle plasmid is obtained in vitro; and by optimizing a chimeric intron sequence and a secondary structure of RNA, efficient and variable splicing of the chimeric intron on the RNA level is achieved, and the MC-HBV capable of being copied at a high level in vitro is obtained. The MC-HBV can highly simulate the physiological activity between an HBV virus and host cells. The invention further discloses an in-vitro marker MC-HBV model and a detection technology, and provides the excellent model for revealing the interaction relationship between the HBV cccDNA and the host, and a function regulation mechanism, and exploring a new HBV treatment target.
Owner:SHANDONG UNIV
System for inducing formation of HBV cccDNA and construction method thereof
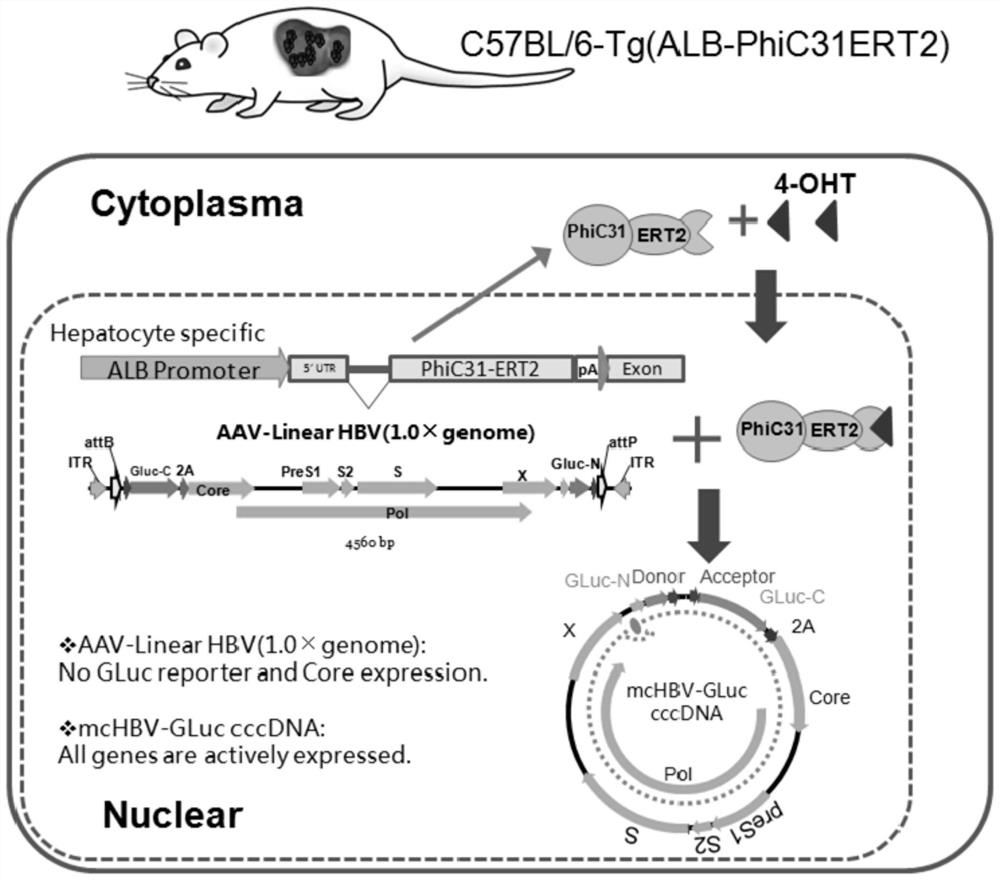
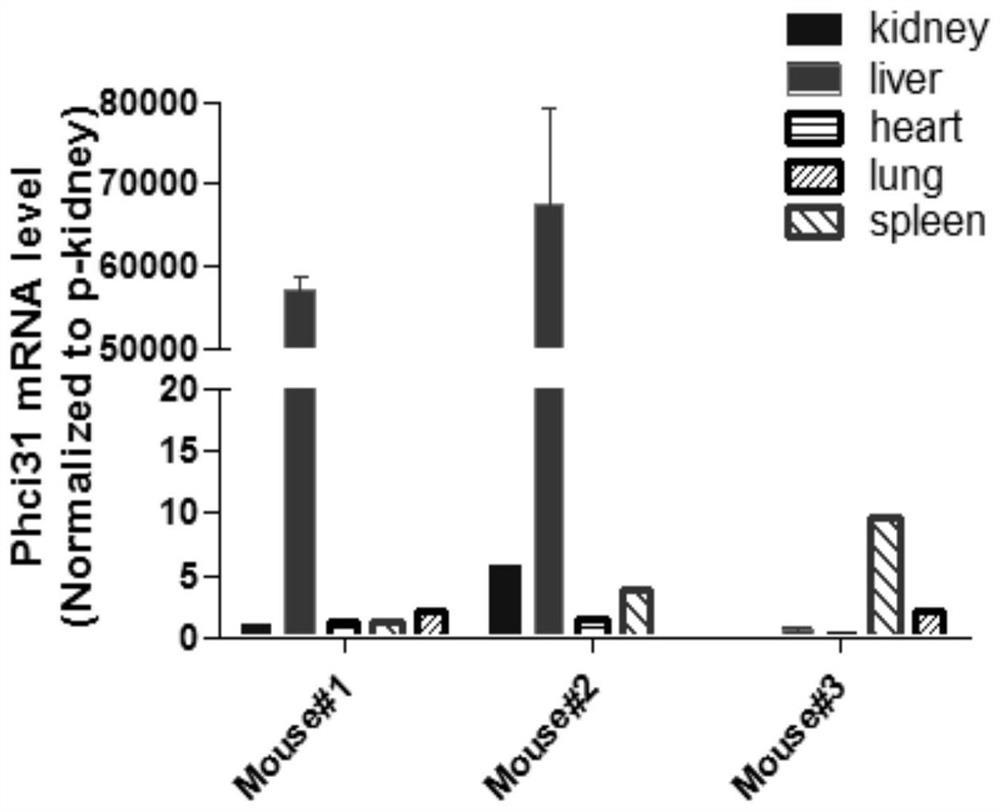
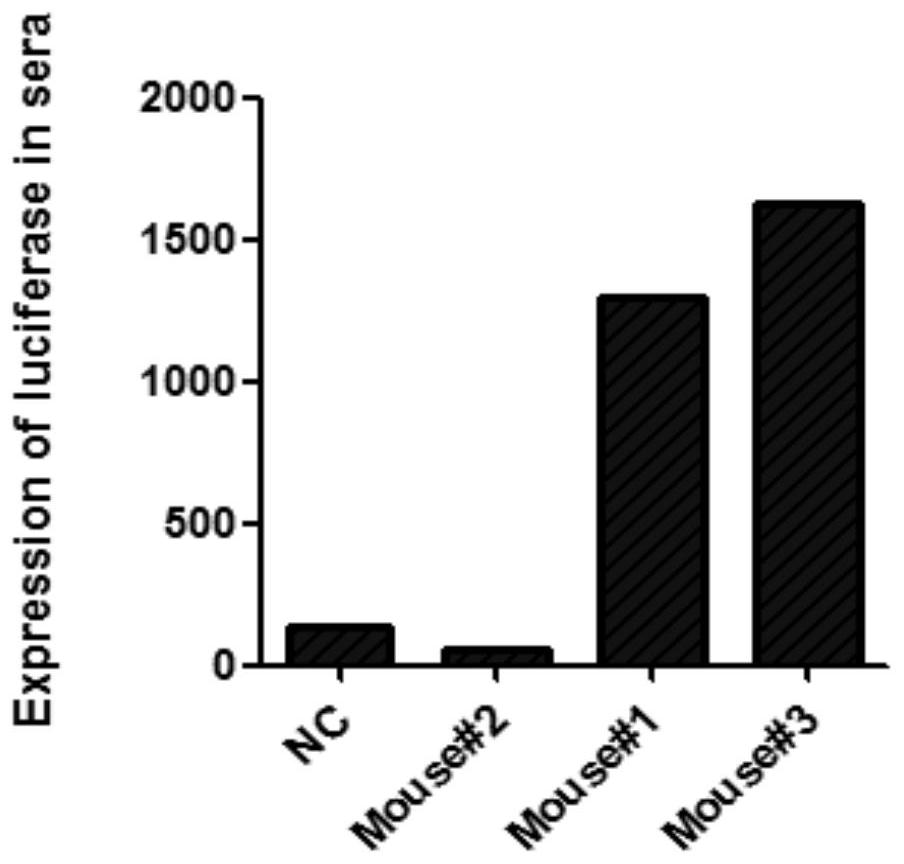
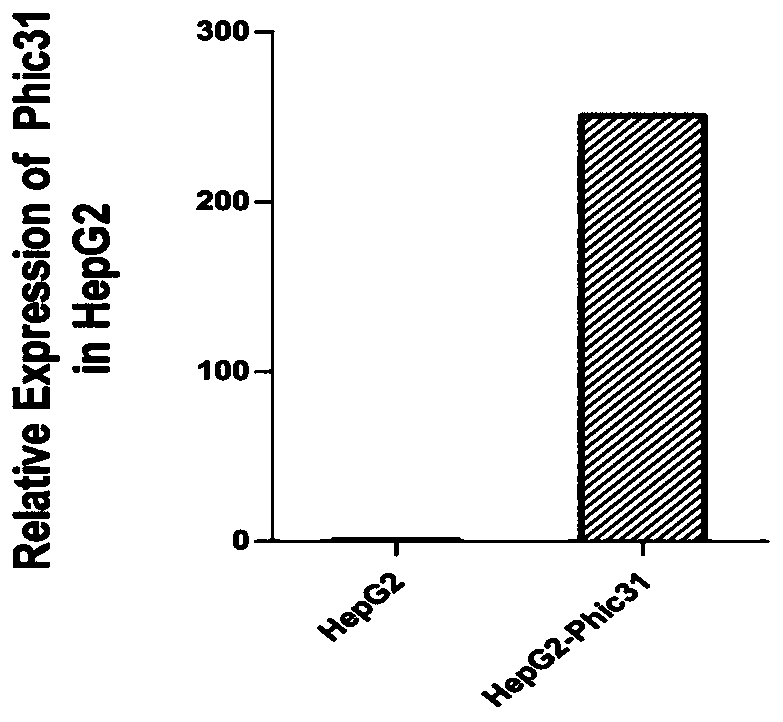
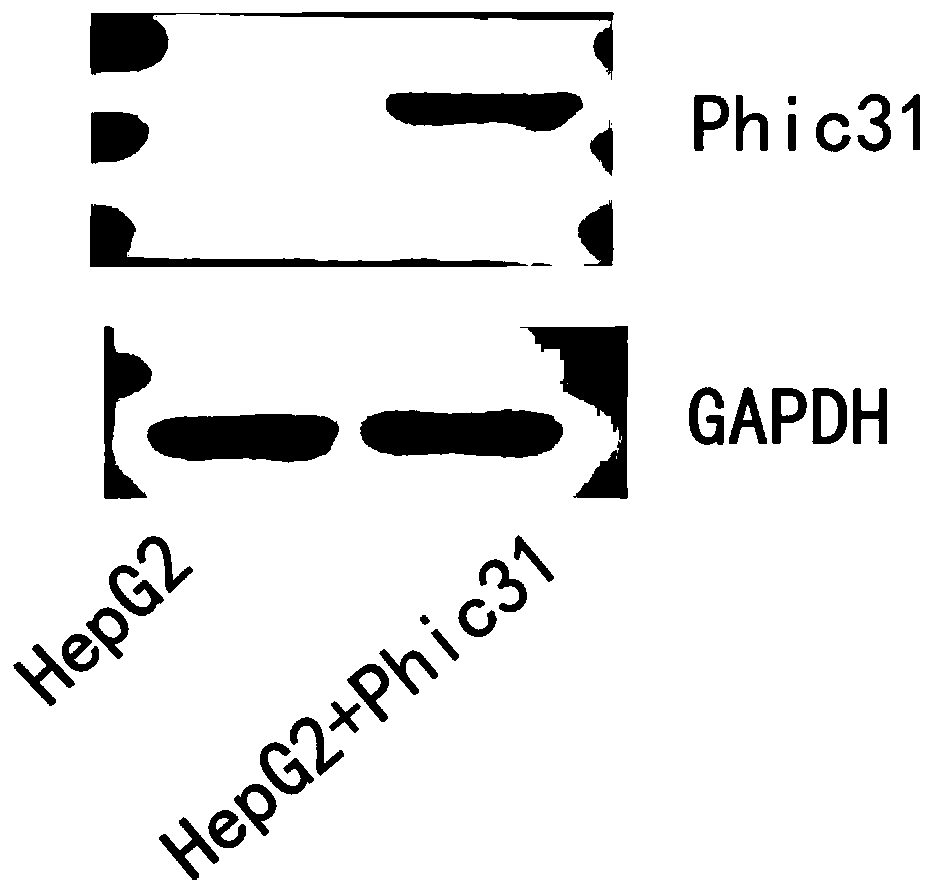
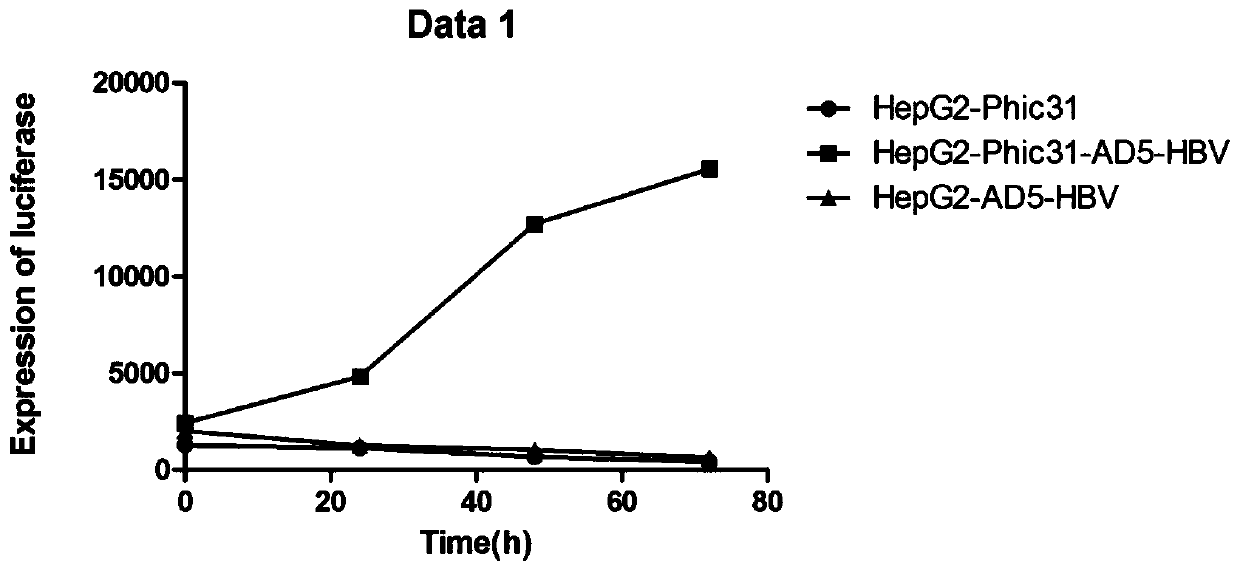
PendingCN110669735AAvoid influenceSimple biological backgroundCompound screeningApoptosis detectionEnzyme GeneLuciferase Gene
The invention discloses a system for inducing formation of HBV cccDNA and a construction method thereof. According to the invention, a stable cell line is constructed by integrating a Phic31 recombinase gene; the 5'end and the 3'end of the linear HBV are provided with complementary luciferase gene segments and specific sites recognized by the Phic31 recombinase, under the action of the Phic31 recombinase, cccDNA is formed through cyclization, and meanwhile, the complementary luciferase is also expressed; the luciferase provides a quick way for quickly detecting the active HBV cccDNA, and is suitable for high-throughput screening.
Owner:GUANGZHOU EIGHTH PEOPLES HOSPITAL
Fluorescence quantification detection kit of hepatitis B virus cccDNA
InactiveCN101285105BBroad representationStrong specificityMicrobiological testing/measurementHepatitis B immunizationHepatitis B virus
The invention relates to a fluorescent quantitative detection reagent kit for a hepatitis B virus cccDNA, which belongs to the genetic engineering field. The fluorescent quantitative detection reagent kit for the hepatitis B virus cccDNA comprises a PCR augmentation reaction system and is characterized in that: the PCR augmentation reaction system comprises a specific upstream primer, a downstream primer and a probe, wherein, a primer sequence is as follow: the upstream primer is 5'-cacctctctttacgcggtct-3', the downstream primer is 5'-atacgggtcaatgtccatgc-3'; the primer sequences is FAM TTCAAGCCTCCAAGCTGT 5NFQ. The reagent kit has wide linear detection range and can save reagent, and suit for the hepatitis B.
Owner:NANJING ZHONGXIN TRANSLATIONAL MEDICINE INSTCO
Hepatitis b virus-binding polypeptides and methods of use thereof
The present invention provides non-naturally occurring polypeptides that specifically bind hepatitis B virus (HBV) DNA; and polynucleotides encoding the polypeptides. The present invention further provides methods of detecting HBV DNA; methods of detecting a covalently closed circular DNA (cccDNA) form of HBV; and methods for treating HBV infection.
Owner:THE GOVERNORS OF THE UNIV OF ALBERTA
Hepatitis B covalently closed circular DNA magnetic trapping technique
ActiveCN107699562AEffective specific captureEfficient and fast enrichment and separationMicrobiological testing/measurementNanomedicineTrappingMicrosphere
The invention discloses a hepatitis B covalently closed circular DNA magnetic trapping technique. A method for preparing HBV cccDNA specific DNA molecular probe magnetic nano-microspheres comprises the following steps: (1) preparing Fe3O4 nanoparticles by virtue of a chemical coprecipitation method; (2) preparing silicon-shell magnetic nanoparticles by virtue of a reverse emulsion method; (3) conducting avidin modification on the silicon-shell magnetic nanoparticles; and (4) coupling an ssDNA probe for a cccDNA specific sequence. The invention also discloses the probe magnetic nano-microspheres prepared by the method, and a method for enriching HBV cccDNA by virtue of the microspheres. With the application of the HBV cccDNA specific DNA molecular probe magnetic nano-microspheres prepared by the method provided by the invention, the effective specific trapping of HBV cccDNA can be achieved, and the effective and rapid enriching and separation of the HBV cccDNA can be implemented; and the separating method is simple and has a good application prospect.
Owner:THE AFFILIATED HOSPITAL OF TRADITIONAL CHINESE MEDICAL TO SOUTHWEST MEDICAL UNIV
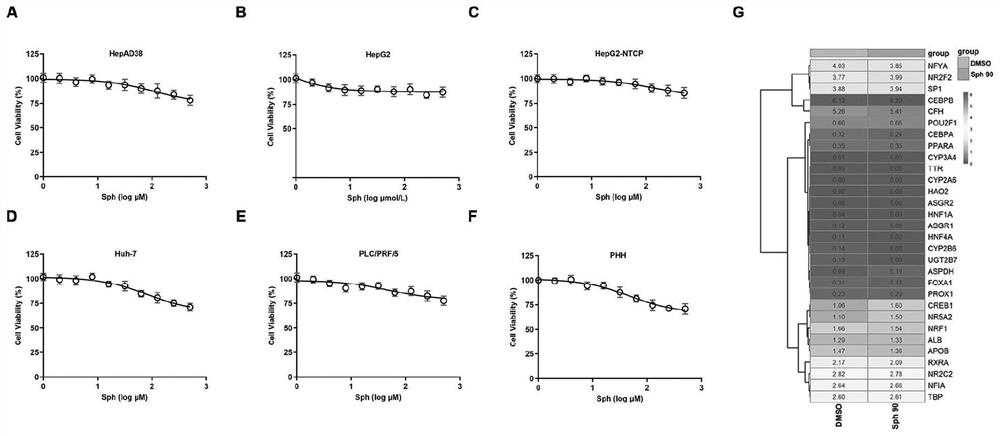
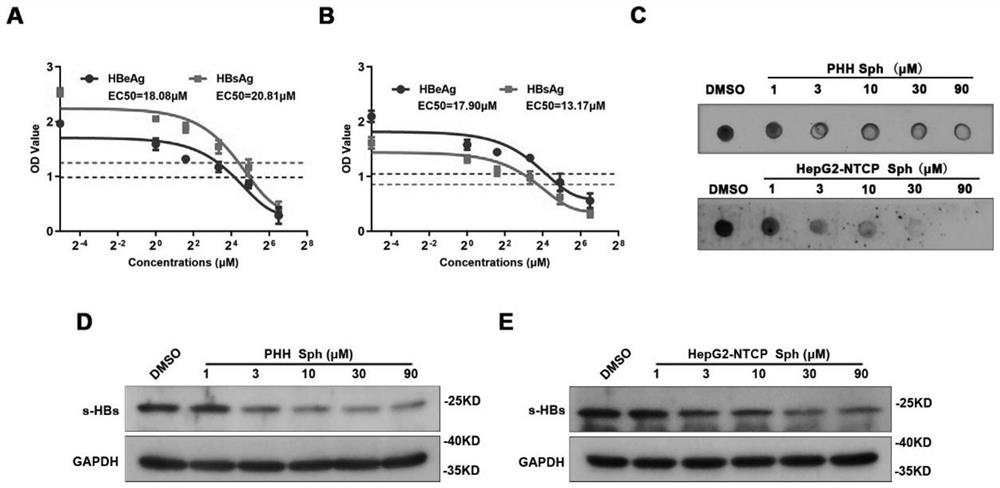
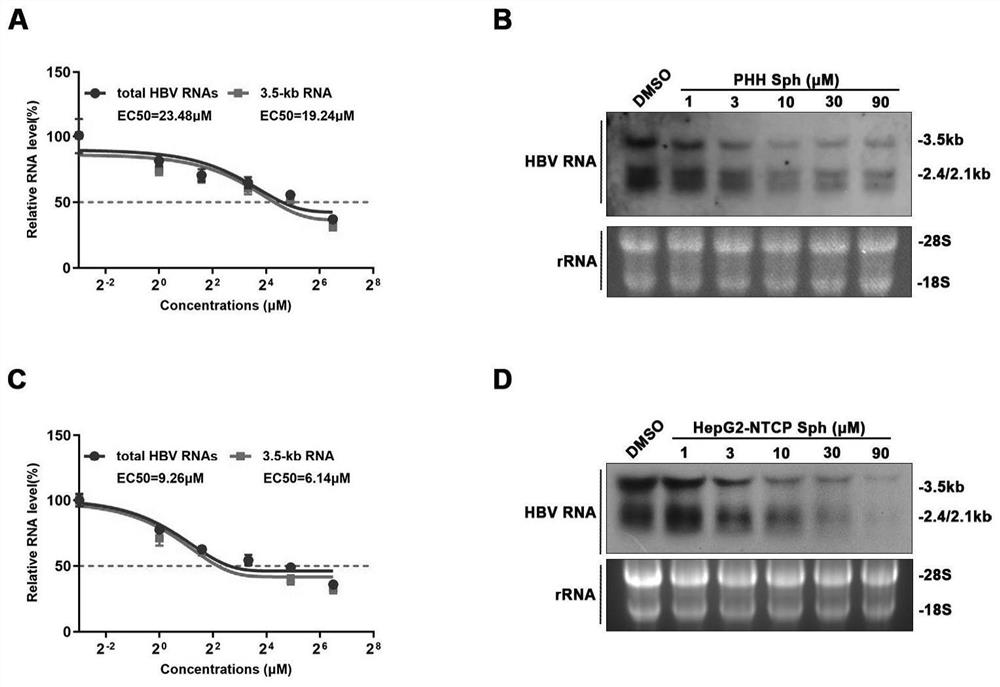
Application of Sphondin as effective component in preparation of drugs for treating hepatitis B
InactiveCN113069445AInhibition of secretionRepress transcription levelDigestive systemAntiviralsLiver tissuePharmaceutical Substances
Owner:CHONGQING MEDICAL UNIVERSITY
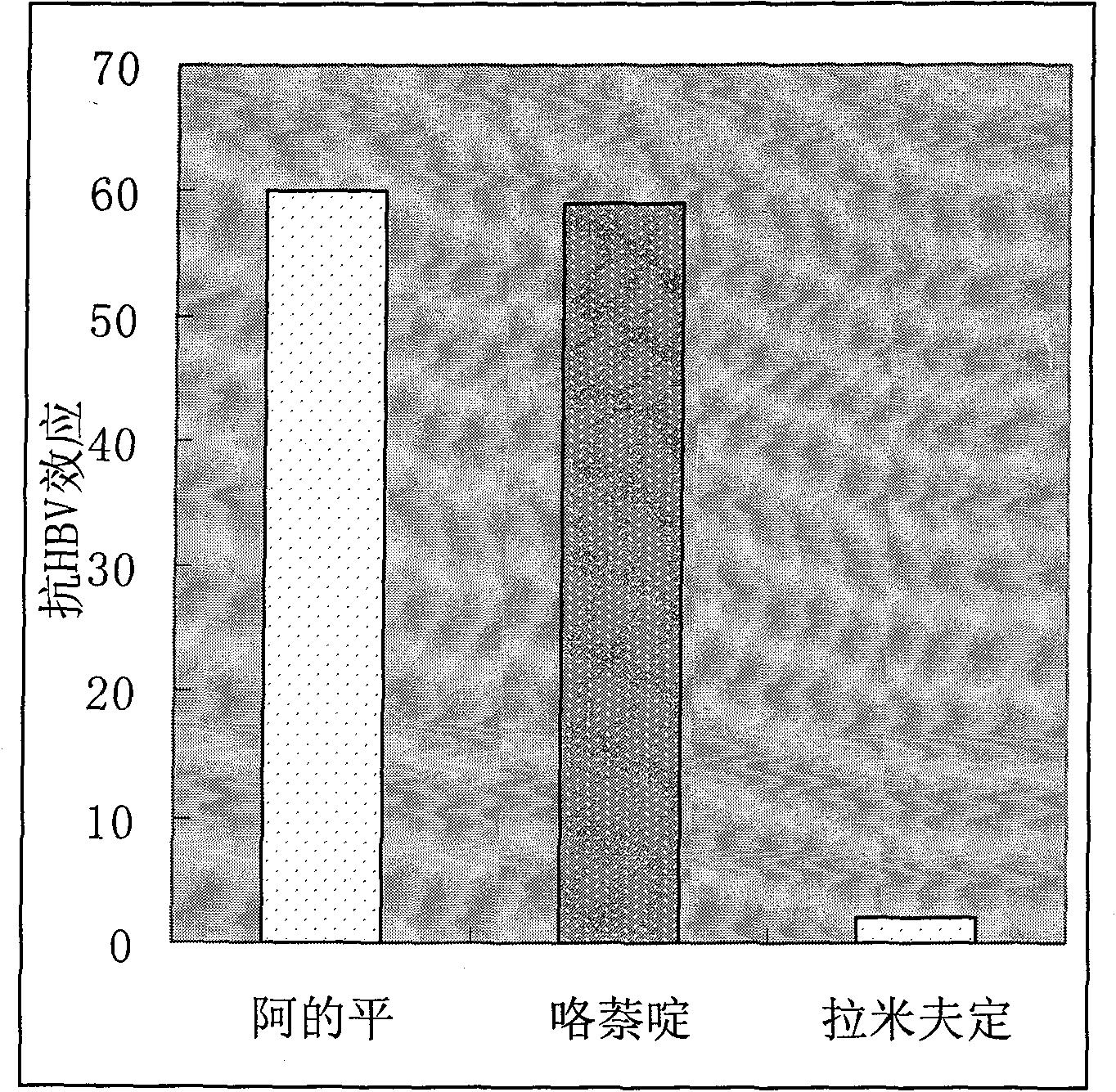
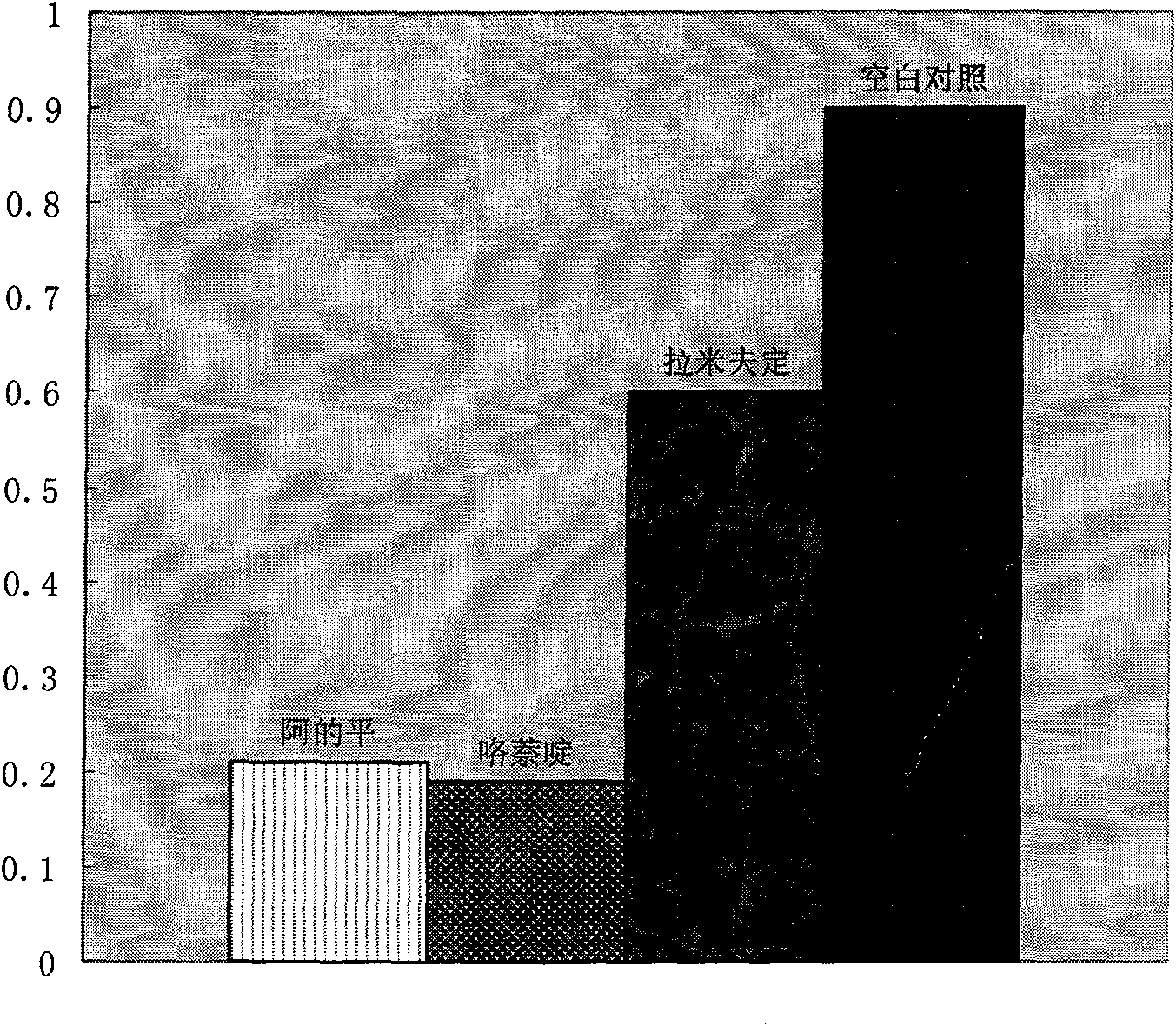
Therapeutical effect of atabrine and substitutes thereof on hepatitis B
InactiveCN101856355ALower titerInhibition killOrganic active ingredientsAntiviralsAntigenNorthern blot
Owner:蔡荣 +3
A hbv cccDNA digital PCR quantitative detection kit and its application
ActiveCN104388598BAvoid non-HBVcccDNA signalsStrong specificityMicrobiological testing/measurementMicroorganism based processesFluorescenceHepatitis B virus
Owner:SHANGHAI CHROMYSKY MEDICAL RES
A novel high-throughput method for quantification of HBV cccDNA from cell lysate by real-time PCR
InactiveCN107109497AMicrobiological testing/measurementMicroorganism based processesHepatitis B immunizationTherapeutic effect
The present invention provides (1) a set of novel DNA primers that can be used for high efficient real-time qPCR of Hepatitis B virus (HBV) circular DNA (cccDNA) and (2) a novel high throughput method for quantification of HBV cccDNA from cells using this set of novel DNA Primers by real-time qPCR. It also provides a use of this method in monitoring cccDNA level in experimentally infected liver cells and evaluation of therapeutic effect on HBV.
Owner:F HOFFMANN LA ROCHE & CO AG
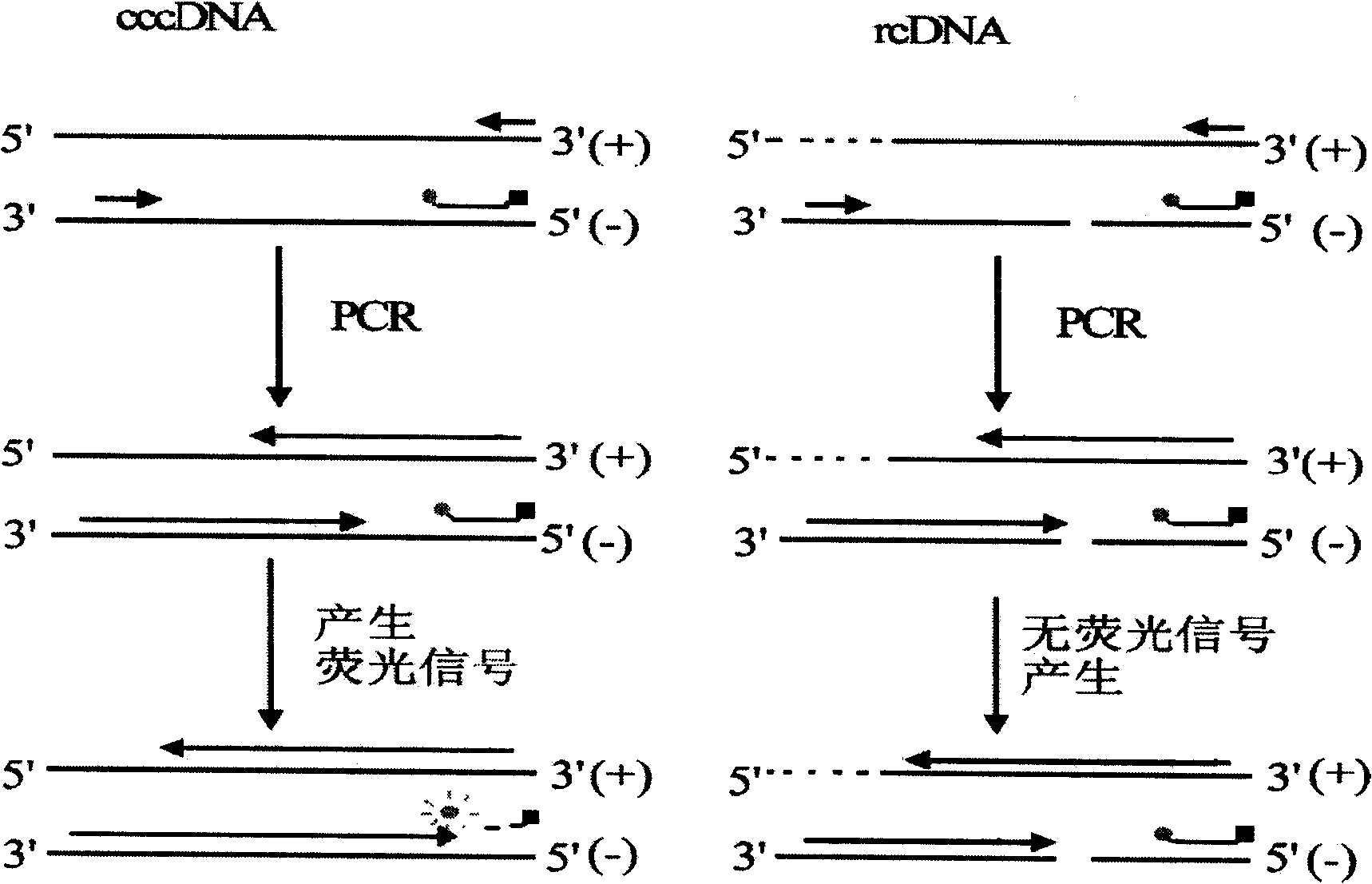
Quantitative measurement of hepatitis b virus cccdna
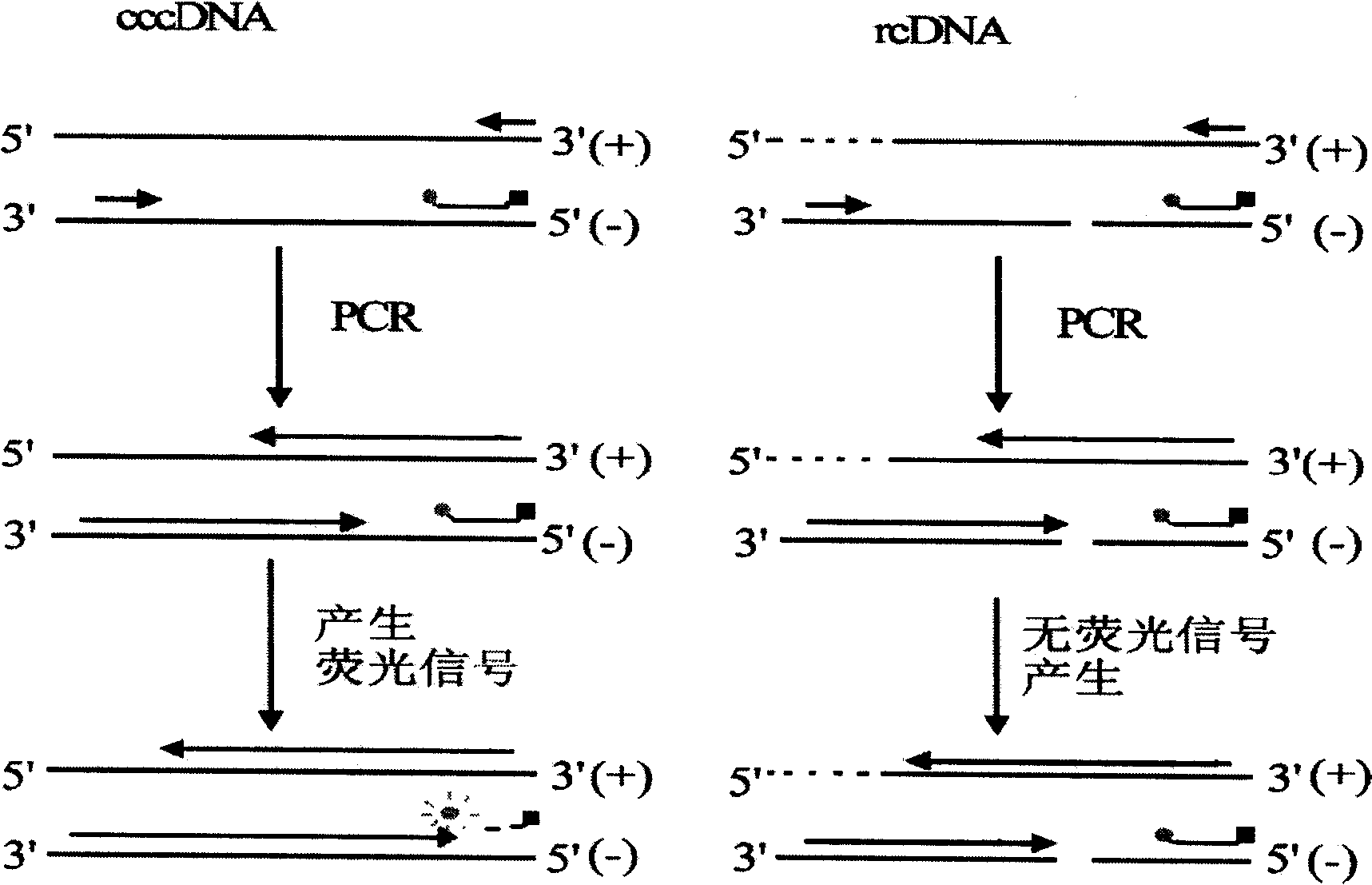
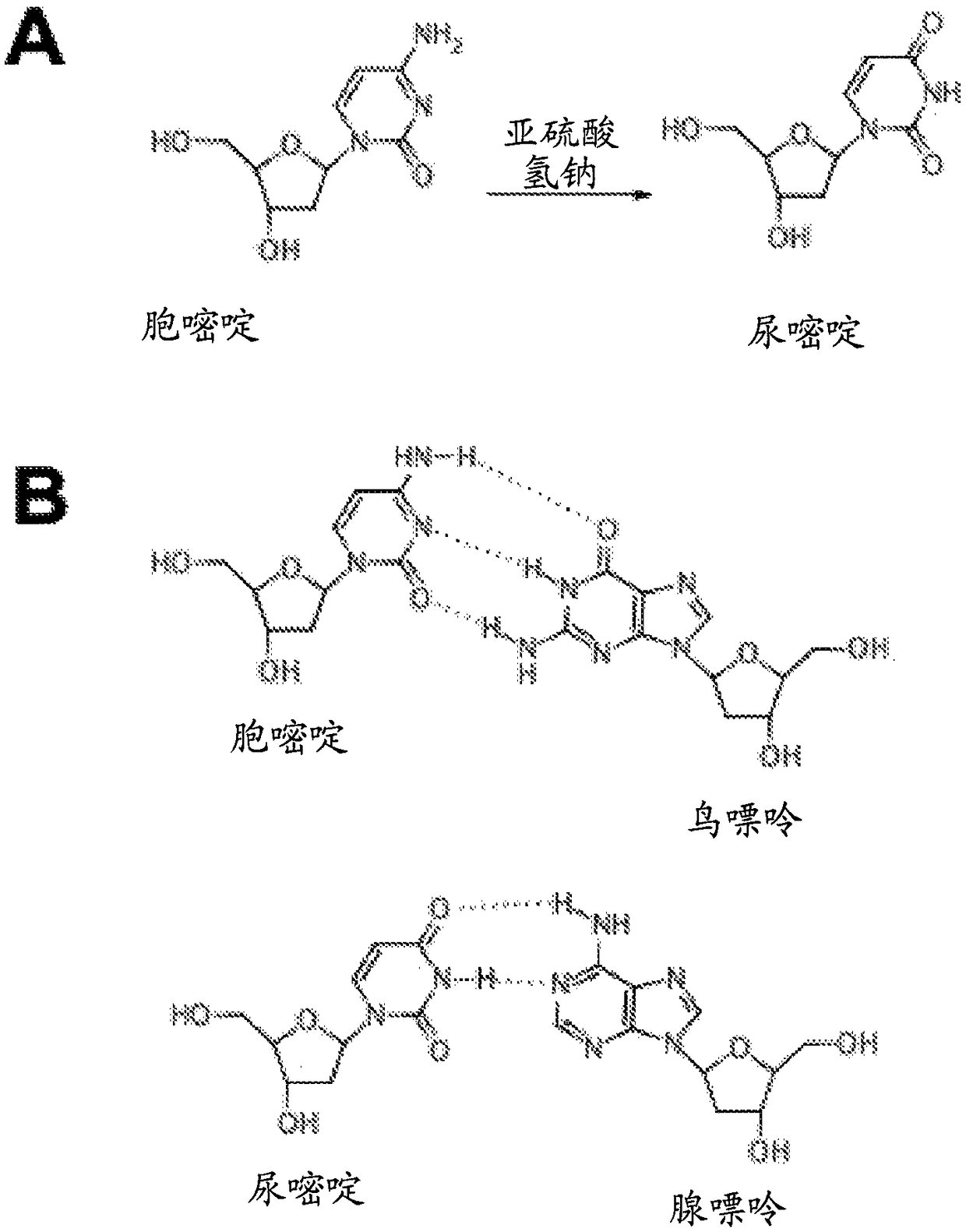
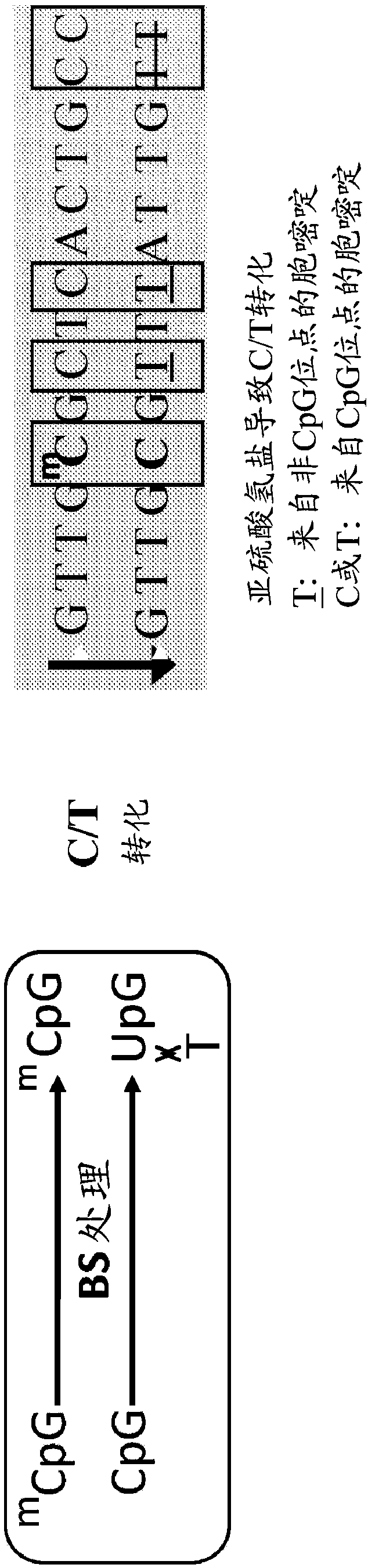
Hepatitis B Virus (HBV) infection results in the entry of viral genomic DNA into host liver cells. This viral relaxed circular DNA (rcDNA) is transported into the nucleus and converted into covalent closed-circular DNA (cccDNA), which serves as a template for viral transcription. Elimination of cccDNA is needed to cure HBV infection, which remains a major therapeutic challenge. A robust and sensitive method to measure cccDNA described here is useful to facilitate drug development and to monitor efficacy of therapy. A set of primers were designed in combination with sodium bisulfite treatment of viral DNA, allowing specific amplification of cccDNA without interfering amplification of rcDNA. This method can be used to further guide therapeutic development, and to provide a non-invasive alternative to monitoring of HBV-infected patients undergoing antiviral treatments.
Owner:JBS SCI
CpG methylated oligodeoxynucleotide for inhibiting replication of hepatitis b virus (HBV)
InactiveCN101613699AImprove stabilityIncrease production capacityMicroorganism based processesAntiviralsHepatitis B virusFhit gene
The invention relates to CpG methylated oligodeoxynucleotide for inhibiting the replication of hepatitis b virus (HBV) in a cell nuclear. A viral relaxed circular DNA (rcDNA) of the HBV after transfecting a host cell enters the cell nuclear to form a covalently closed circular DNA (cccDNA) under the action of a host enzyme, and the cccDNA is combined with biological molecules, such as histone, and the like in a liver cell nuclear to form a viral micro chromosome which is used as a template for the transcription and the replication of a HBV gene. In the invention, a CpG island 2 in a HBV cccDNA sequence is used as a target spot to design and combine into the CpG methylated oligodeoxynucleotide which has 15-30 bases and can inhibite the replication of the HBV. The invention can improve the methylation degree of the complementary HBVcccDNA sequence, can inhibite the replication of HBV DNA, has the advantage of high stability, is easy to product and store and can be used for treatments of resisting HBV infection.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
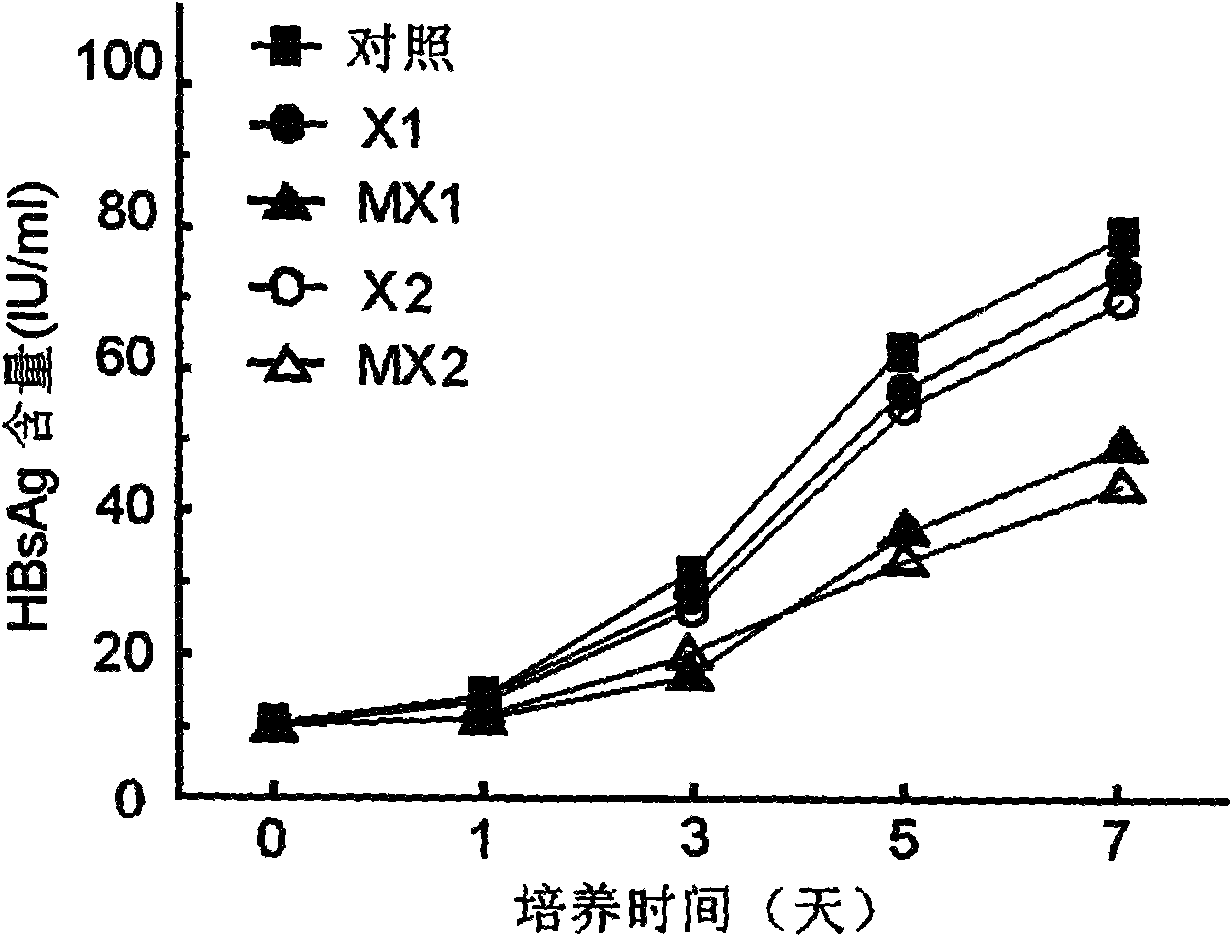
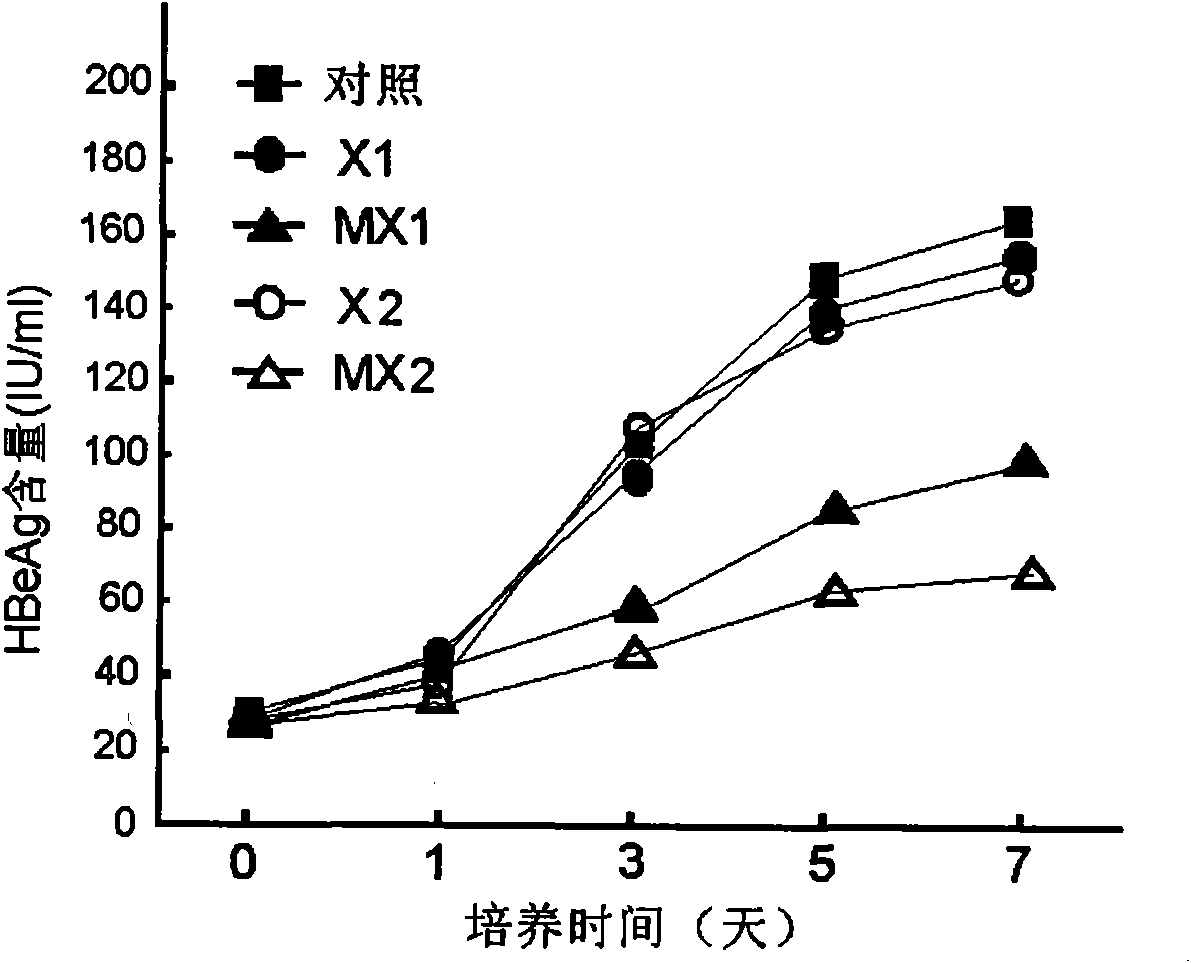
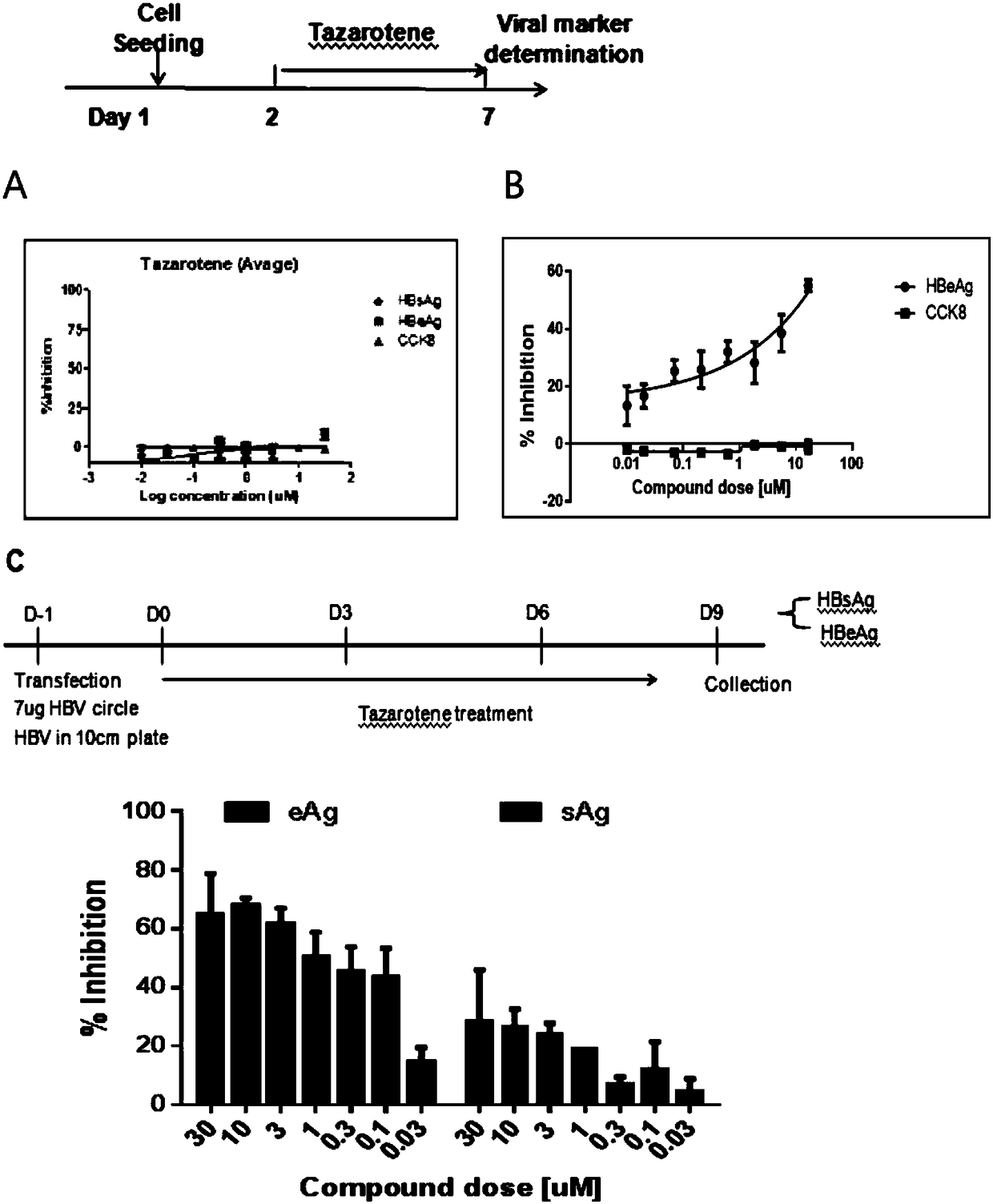
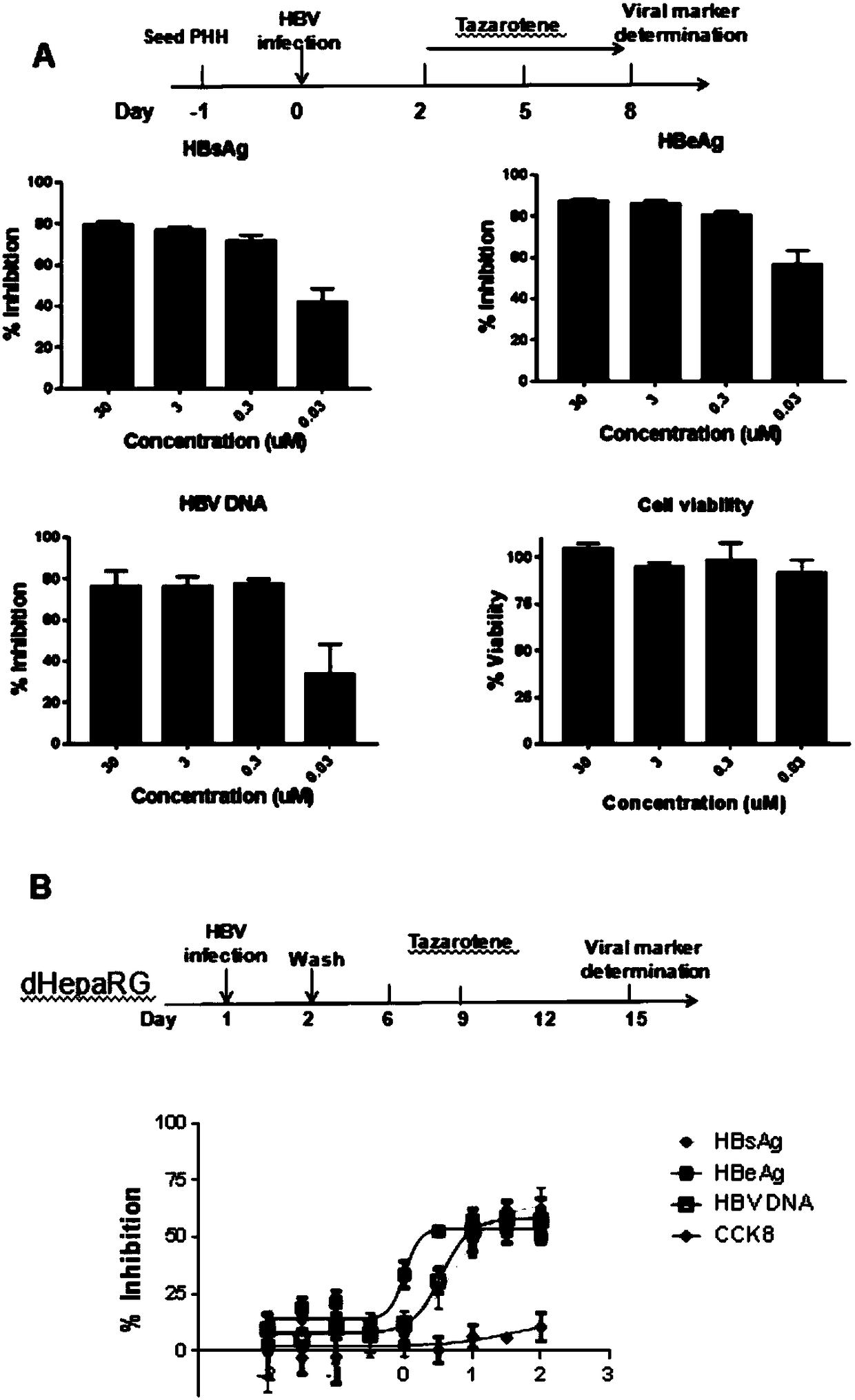
Application of tazarotene to preparation of drugs used for treating hepatitis B virus infection
InactiveCN109381463ASignificant anti-HBV activityOrganic active ingredientsAntiviralsHepatitis B virusTransfection
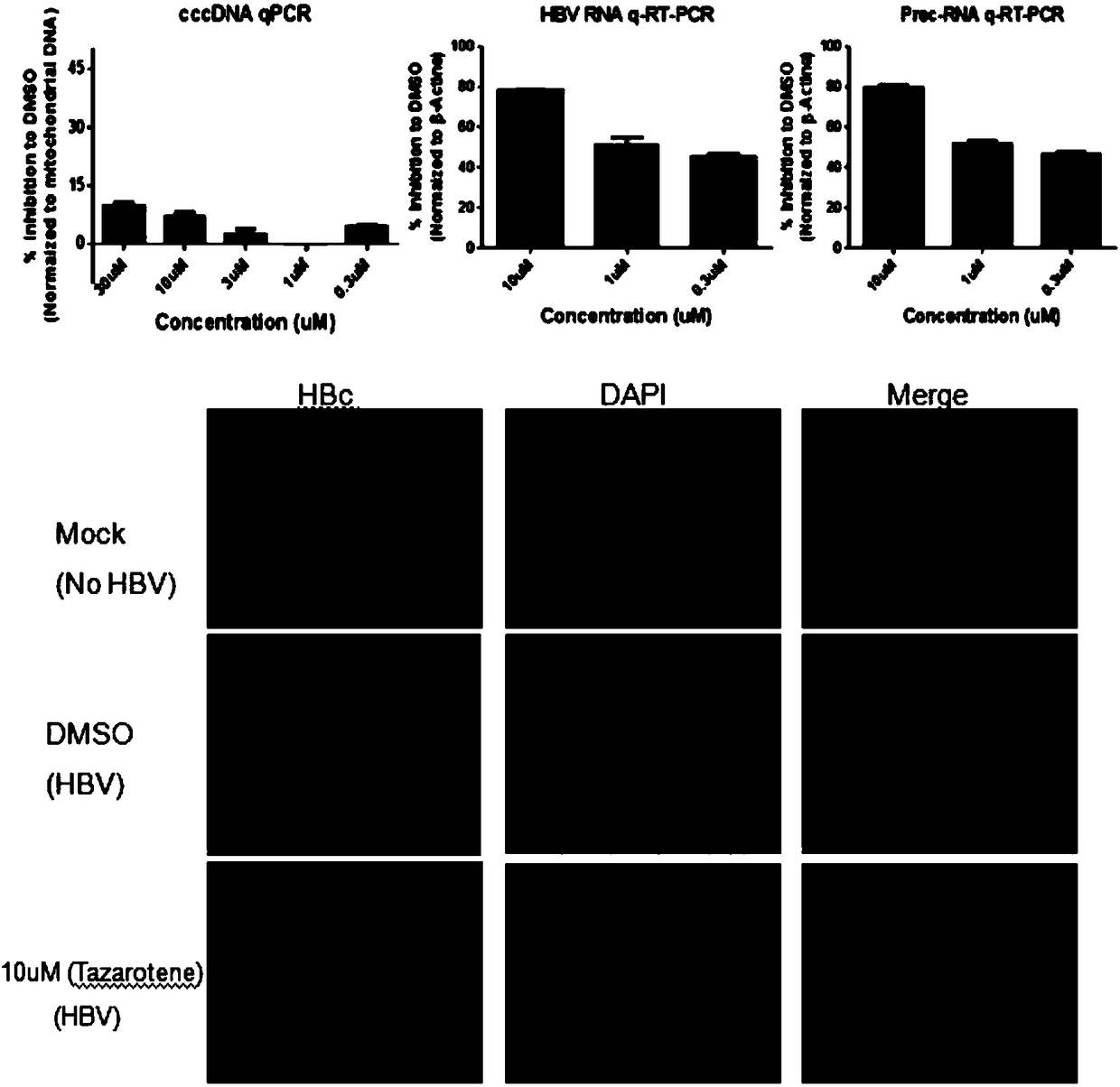
The invention relates to the application of tazarotene to preparation of drugs used for treating hepatitis B virus infection, belonging to the technical field of medicine. The identification results of virus transfection and infection cell model tests prove that tazarotene, a tretinoin drug, has strong anti-hepatitis B virus activity, presents long-lasting virus inhibition ability after withdrawal, and is free of cytotoxic effect at corresponding antiviral working concentrations. The invention also discloses that tazarotene has relatively specific activity in interfering with the stability ofcccDNA and inhibiting the transcription of cccDNA, and discloses the anti-hepatitis B virus activity of adapalene which also belongs to tretinoin drugs and is an analogue of tazarotene. The inventionprovides a technical and theoretical foundation for the application of tazarotene and its analogues to clinical anti-hepatitis B virus intervention and for the preparation of novel anti-hepatitis B virus drugs based on tazarotene and its analogues and derivatives.
Owner:FUDAN UNIV
Method for constructing experimental animal model carrying hepatitis B virus for a long time
InactiveCN109652452AFermentationVector-based foreign material introductionEukaryotic plasmidsHepatitis b e antigen negative
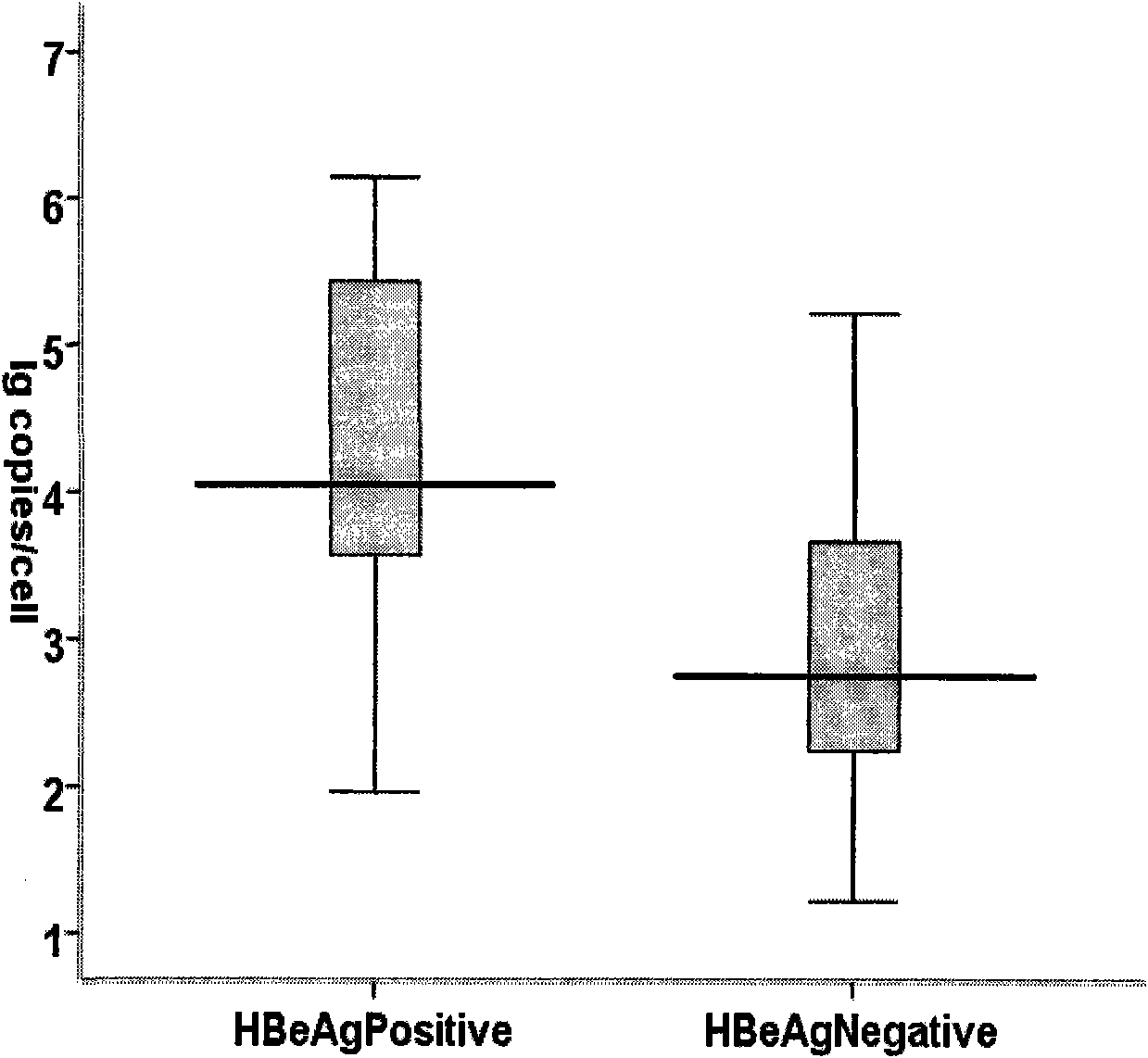
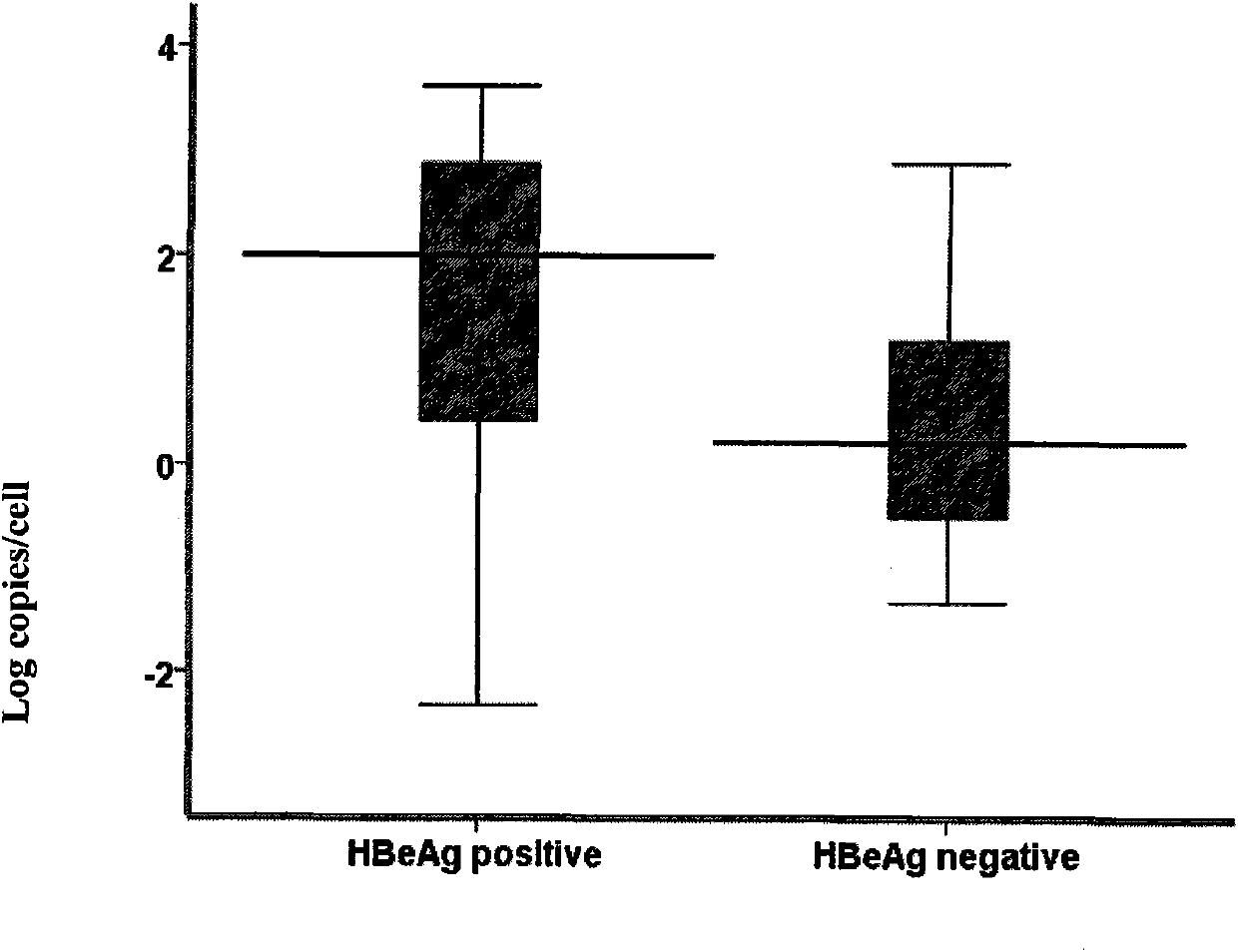
The invention discloses a method for constructing an experimental animal model carrying hepatitis B virus for a long term, which comprises the following steps of: 1) extracting serum virus DNA of a hepatitis B patient, and performing PCR amplification on HBVF / HBVR by using a primer pair; 2) cloning a PCR product into a pEASY-HBV vector, and selecting a positive clone; 3) extracting plasmid of thepositive clone, cutting the extracted plasmid with BspQI endonuclease, recovering enzyme-cut sections and purifying, cyclizing with T4 ligase and purifying to obtain HBV cccDNA; 4) injecting the cccDNA into a mouse to construct the hepatitis B e antigen negative mouse model. The method successfully constructs the CBA / CaJ mouse model with immune activity which can effectively simulate HBV chronic infection through a simple, rapid and convenient method, and the expression of HBsAg, HBeAg and HBcAg can be up to 26 weeks.
Owner:CHONGQING ACADEMY OF SCI & TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com