Therapeutical effect of atabrine and substitutes thereof on hepatitis B
A hepatitis B, peaceful technology, applied in the field of biomedical research, can solve the problems of expensive research, decline, and clearance of HBV
Inactive Publication Date: 2010-10-13
蔡荣 +3
View PDF0 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Immunomodulatory anti-HBV drugs such as IFN and nucleoside analogs can only partially inhibit HBV replication but cannot completely eliminate HBV in the human body, and the ability to induce IFN after HBV infection is significantly reduced, and the expression of HLA21 antigens in target cells is also reduced. Therefore, Higher recurrence rate after drug discontinuation
[0003] The current clinical treatment methods against HBV infection are far from ideal, and biomacromolecular drugs are pushed by the international communi
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
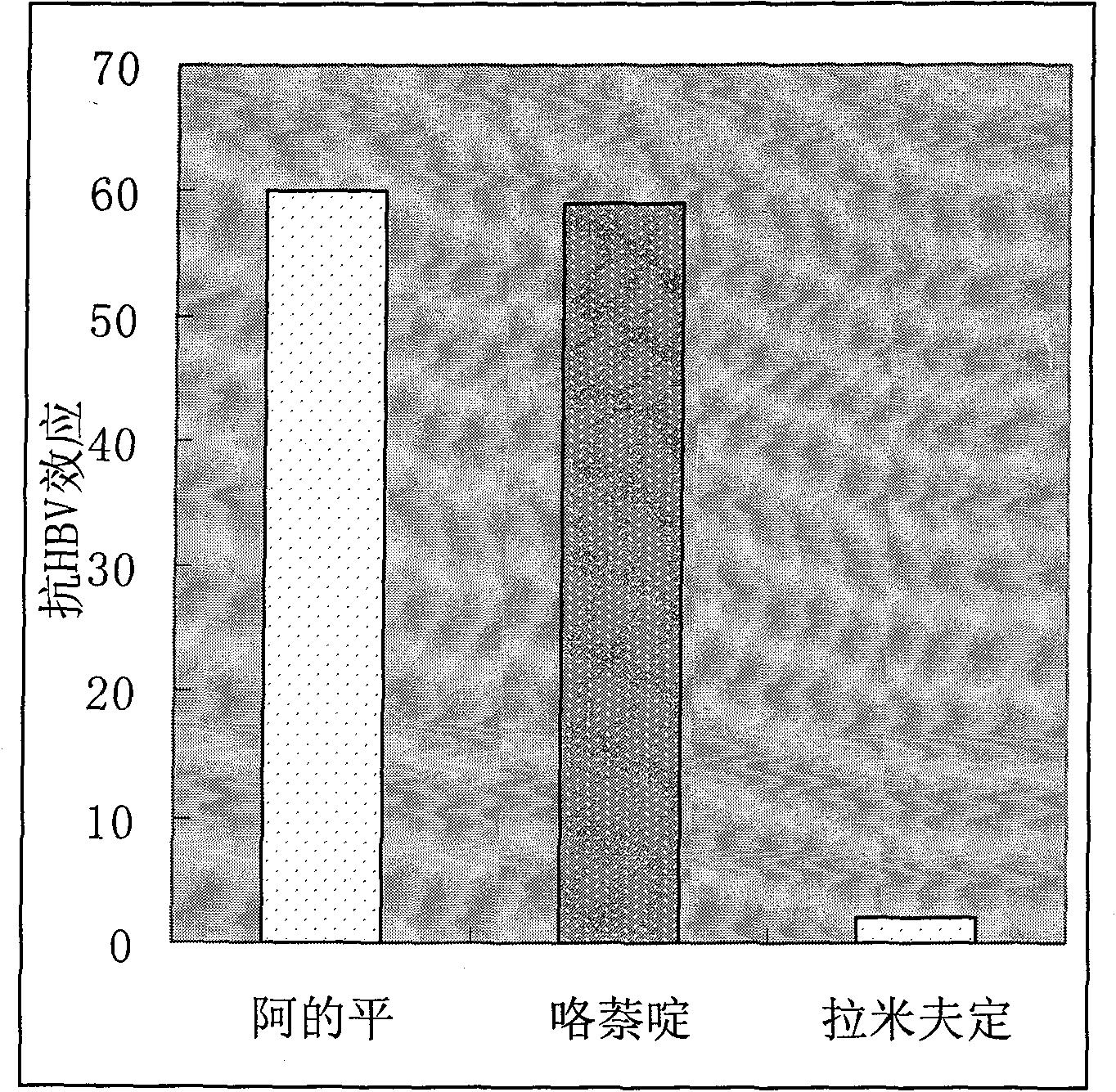
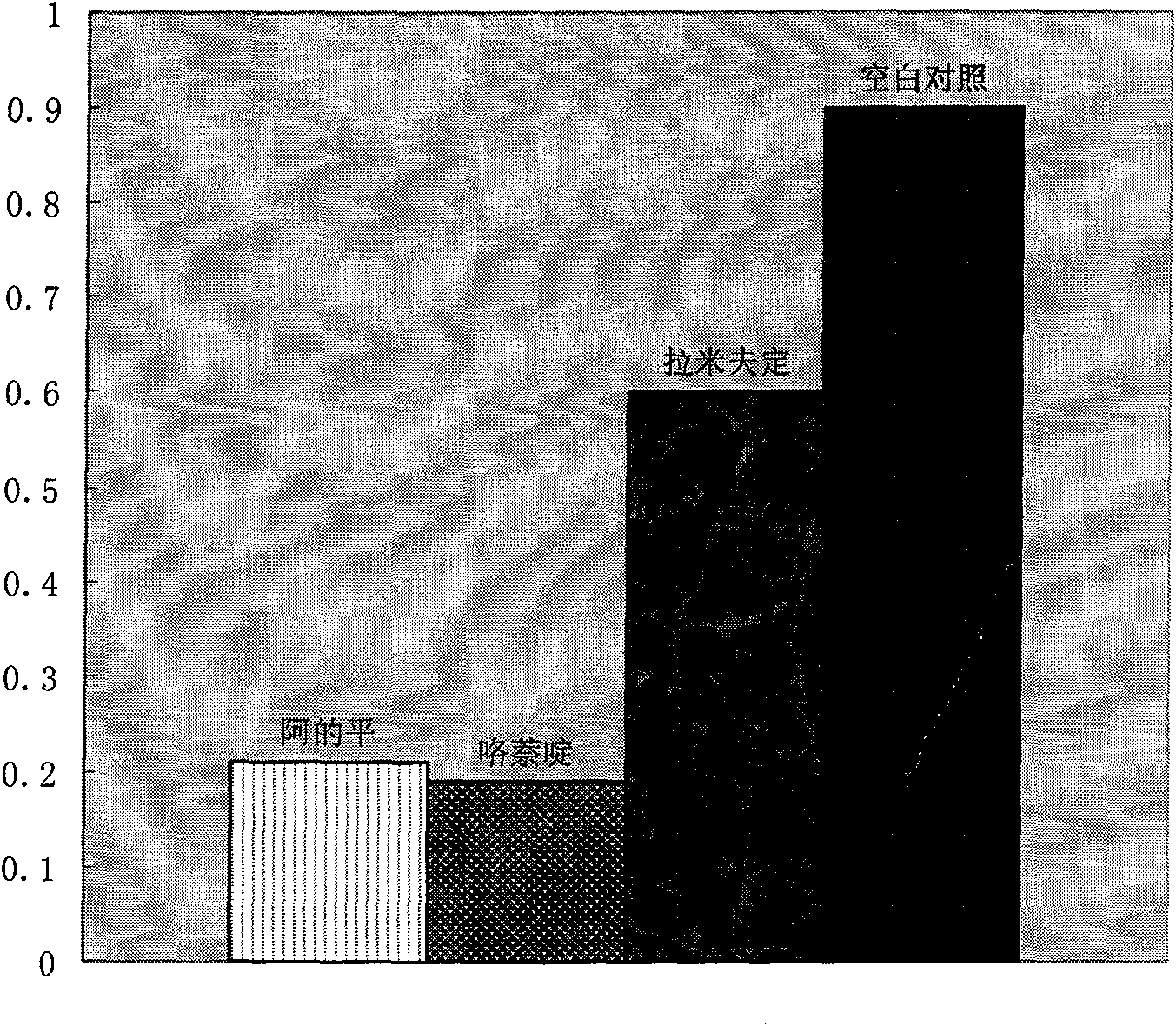
By adopting methods of fluorescent quantitation PCR, fluorescent quantitation RT-PCR, HBV DNA quantitation, HBsAg quantitation, HBeAg quantitation, cccDNA quantitation, Northern blot, Southern blot, Western blot, immunohistochemistry, and the like in the research and using the maintenance dose within the treating dose range for a long time (30-60 days), supernatant HBV DNA HBsAg HBeAg cultured by HepG-2.2.15 cells can completely disappear, HBsAg, HBeAg, HBcAg, and the like in the cells are completely turned to be negative, HBV DNA is in a high inhibited state, HBV cccDNA is completely negative, fluorescent quantitation RT-PCR detection finds that the mRNA for expressing HBsAg and HBcAg antigens in the cells is completely negative, and in addition, the curative effect of the atabrine is 30 times that of lamivudine as a first-line drug for resisting HBV in current clinic. In order to illustrate the action mechanism of the atabrine and the pyronaridine as a drug belonging to the same kind with the atabrine, an HBV genome is divided into 3 segments which are respectively inserted into an Xb1 position on a luciferase report carrier PGL3, a multifunctional microplate reader detects and finds that the light production value of the luciferase is remarkably reduced, which shows that the molecules of the atabrine and the pyronaridine as a drug belonging to the same kind with the atabrine can be nonspecifically combined with the HBV DNA in the cells, thereby inhibiting the copying of the virus and ensuring that the HBV DNA content of the virus in cells copied by the filial generation is reduced till to disappear. The patent requires protecting the application of 3 linked benzyl structures (named as ethyleneimine) and radicals such as CH3O-,-NH-, CL-, and the like for resisting HBV virus in clinic.
Description
technical field [0001] The findings fall within the field of biomedical research. Clinical research and development of chemotherapeutic drugs for anti-hepatitis B virus (HBV) treatment. The study found another indication for aldipine and its substitute pyronaridine, which can effectively inhibit and kill hepatitis B virus. Background technique [0002] Hepatitis B virus is one of the major global infectious diseases that threaten human health. According to the report of the World Health Organization, about 2 billion people in the world have been infected with HBV, of which 350 million to 400 million are chronic HBV carriers, and there are about 1 million people every year. He died of major diseases such as liver cirrhosis, primary hepatocellular carcinoma, and liver failure caused by HBV infection. China is a high-prevalence area of HBV infection. There are about 100 million HBV-infected people, accounting for one-third of the world's total and ranking first in the world...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K31/4745A61K31/473A61P31/20
Inventor 蔡荣蔡立翔韩叶余鸿鳦
Owner 蔡荣
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com