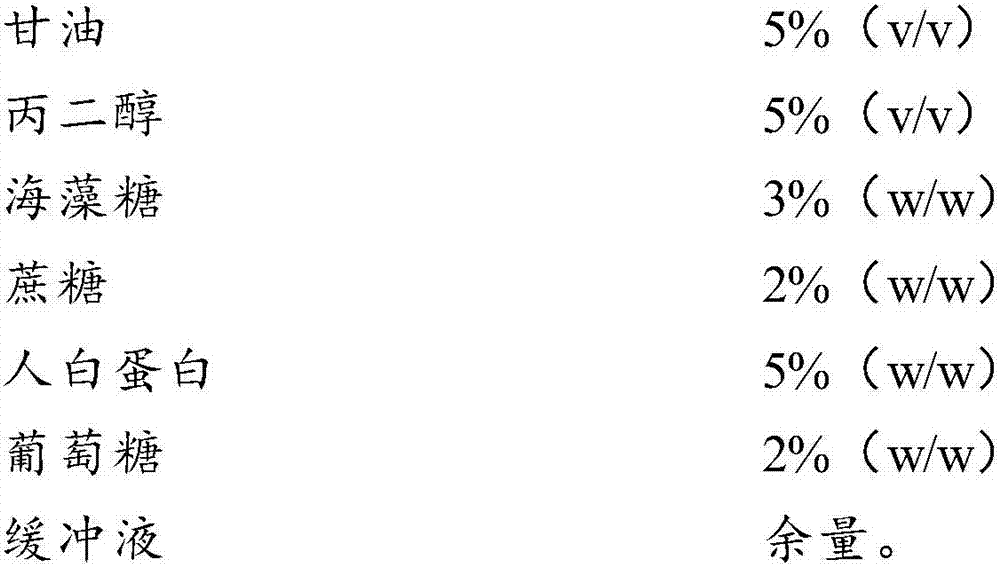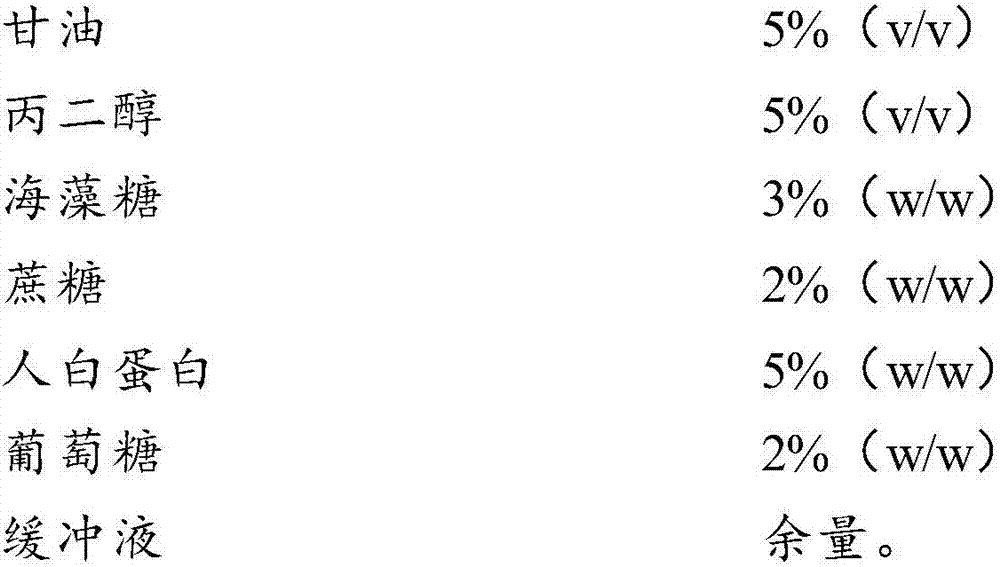Patents
Literature
320results about How to "Small batch-to-batch variance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of pellet-type formula granules
InactiveCN107744510ASimple preparation processImprove compliancePharmaceutical product form changeCoatingsMedicineMoisture absorption
The invention discloses a preparation method of pellet-type formula granules. The method comprises the following steps: (1) selecting at least one of the traditional Chinese medicinal materials; (2) respectively extracting the medicinal materials selected in step (1) for obtaining extracts, or respectively grinding the medicinal materials, or respectively carrying out partial extraction and partial grinding, thus obtaining pretreated materials; and (3) preparing the pretreated materials obtained in step (2) into pellets, thus obtaining the pellet-type formula granules. According to the preparation method provided by the invention, on the basis of the prior art, the preparation process of the formula granules is innovated, the formula granules are innovated into pellets, the fluidity and moisture absorption resistance are good, the friability is small, the shape is round and uniform as well as regular, the content uniformity is small, and the divided dose is accurate.
Owner:GUANGDONG LUOFUSHAN SINOPHARM
Method for detecting, identifying and/ or quantifying compound using adapter type reagent
InactiveCN101144814AImprove stabilityGood repeatabilityBiological testingNucleotide sequencingRepeatability
The present invention relates to a method using adapter type reagent to detect, identify, and / or ration the compound. The present invention adopts the SELEX technology, uses various biologic molecules possessing the diagnosing value including the nucleotide sequence, the protein, the aminophenol, the polypeptide chain, the glucide, or the complete thalli as the target matters, and filters the corresponding high specificity adapter from which to obtain the target matters. The present invention has the advantages that the repeatability and the stability of the test result can be improved greatly, lots difference of the test can be reduced, and the reliability of the test result can be fully ensured. The present invention can be used to diagnose the clinic target molecular quickly and simply, and can provide favorable basis for the lab diagnosis. The present invention has short test time, low reagent cost, short research and development period, stable quality, etc.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Process for cold extrusion forming of one-way device spline sleeve
InactiveCN104148888AEliminates drilling/boring stepsDrilling/boring steps meetEngineeringUltimate tensile strength
The invention provides a process for cold extrusion forming of a one-way device spline sleeve. The process comprises the steps of utilizing hollow pipes to serve as raw materials, performing blanking according to specified dimensions, sequentially performing annealing, head flattening, shot blasting, phosphatization and saponification treatment, cold extrusion shaping, cold extrusion spline forming, machining, quenching, tempering, shot blasting, cleaning and grinding machine processing, and obtaining finished product one-way device spline sleeves. The one-way device spline sleeves obtained through the process are high in precision, small in individual differences, good in strength performance and long in service lives.
Owner:宁波惠山汽配制造有限公司
Screening method of safety and efficacy of skin antioxidants through use of a plurality of normal human skin cells
InactiveCN103320490AStrong ability to divide and proliferateHigh degree of standardizationMicrobiological testing/measurementAntioxidantUltraviolet lights
The invention discloses a screening method of safety and efficacy of skin antioxidants through use of a plurality of normal human skin cells. The screening method includes the following steps: (1) separation, culture and identification of normal human primary cells; (2) toxicity test of substances for testing; (3) preparation of the substances for testing and concentration determination of the substances for testing; (4) determination of ultraviolet-light induced radiation dose; and (5) verification of antioxidant effects of the substances for testing. The normal human skin cells are prepared through standardized culture in vitro, so that the normal human skin cells has strong division propagation capability, high degree of standardization, less difference among batches, and the same activity and functions as that in vivo. Results which are obtained through use of the normal healthy human skin cells are more reliable than the results obtained through use of animal or human cell lines. Through use of the screening method, toxic effects and antioxidant efficacy of the substances for testing can be evaluated in two aspects including a qualitative aspect and a quantitative aspect. The method, through replacement of the living animal and human skin, can be directly used for toxicity and efficacy tests of antioxidant substances in products including chemicals, cosmetics, pharmaceuticals and the like.
Owner:GUANGZHOU HUADAI BIOLOGICAL TECH CO LTD
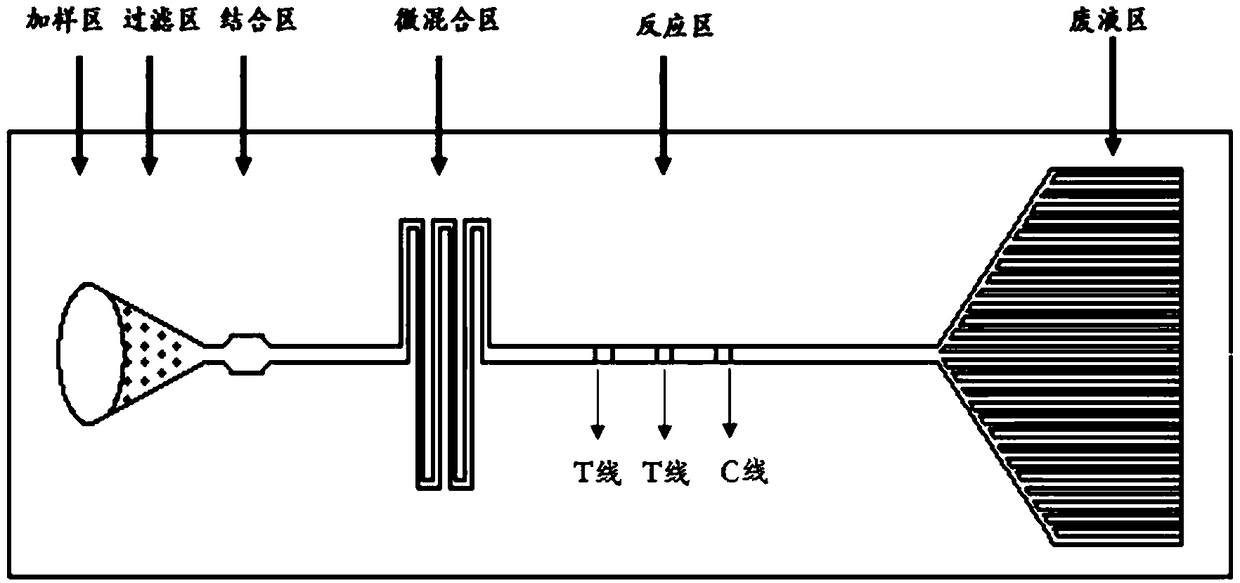
Micro-fluidic chip according to time-resolved fluorescent technique, preparation method of micro-fluidic chip and application of micro-fluidic chip
InactiveCN108080042AHigh precisionHigh sensitivityLaboratory glasswaresMaterial analysisAntigenSingle sample
The invention belongs to the field of immunology, and particularly relates to a micro-fluidic chip according to a time-resolved fluorescent technique, a preparation method of the micro-fluidic chip and an application of the micro-fluidic chip. The micro-fluidic chip according to the time-resolved fluorescent technique comprises a sample adding area, a filtering area, a time-resolved fluorescent micro-sphere antibody or antibody compound binding area, a micro-mixer, a reaction area and a waste liquid area. According to the micro-fluidic chip, antigens or antibodies marked by time-resolved fluorescent nano-particles, light stability is strong, sensitivity is high, and interference of samples can be effectively avoided. The antigens or antibodies of a plurality of items to be detected can besimultaneously marked on the micro-fluidic chip, a plurality of items of a single sample can be simultaneously detected, efficiency is improved, and sample cost and time cost are saved. The preparation method of the micro-fluidic chip is simple, detection accuracy is high, low difference between batches and high stability are ensured, and full quantitative detection of the samples can be realized.
Owner:成都微康生物科技有限公司
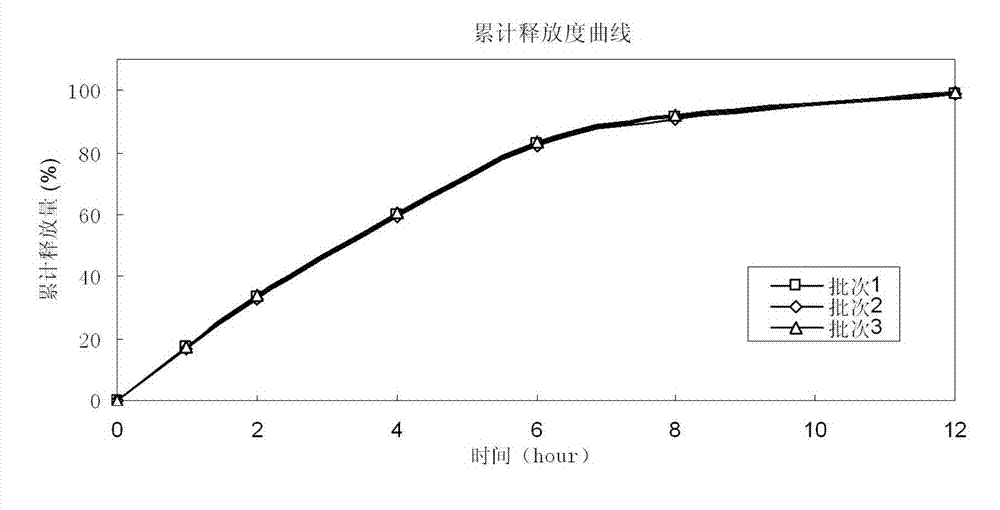
Guanidine hydrochloride sustained release preparation and preparation method thereof
ActiveCN102579381AImprove stabilitySimple manufacturing processOrganic active ingredientsGranular deliveryBlood drug concentrationTableting
The invention discloses guanidine hydrochloride sustained release preparation and a preparation method thereof. The guanidine hydrochloride sustained release preparation is mainly prepared by guanidine hydrochloride, a filling agent, sustained release materials and potential of hydrogen (pH) sensitive materials. The preparation method comprises the steps of firstly preparing all raw materials according to proportion, evenly mixing the guanidine hydrochloride, the filling agent, the sustained release materials and the pH sensitive materials at high speed, granulating and drying the evenly mixed powder, finally adding a flow agent, a lubrication agent and an adhesion agent, tableting according to a general method, and achieving guanidine hydrochloride sustained release tablets. The guanidine hydrochloride sustained release preparation is small in side effect, obviously reduces difference of preparation of different batches, improves stability of samples, is convenient long term curing of patients and improves compliance of medicine. A patient can take the guanidine hydrochloride sustained release tablets once a day, so that effective drug concentration in bodies can be guaranteed for 24 hours, and nervous centralis side reactions caused by the fact that a blood concentration peak value is high due to general preparation is reduced.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
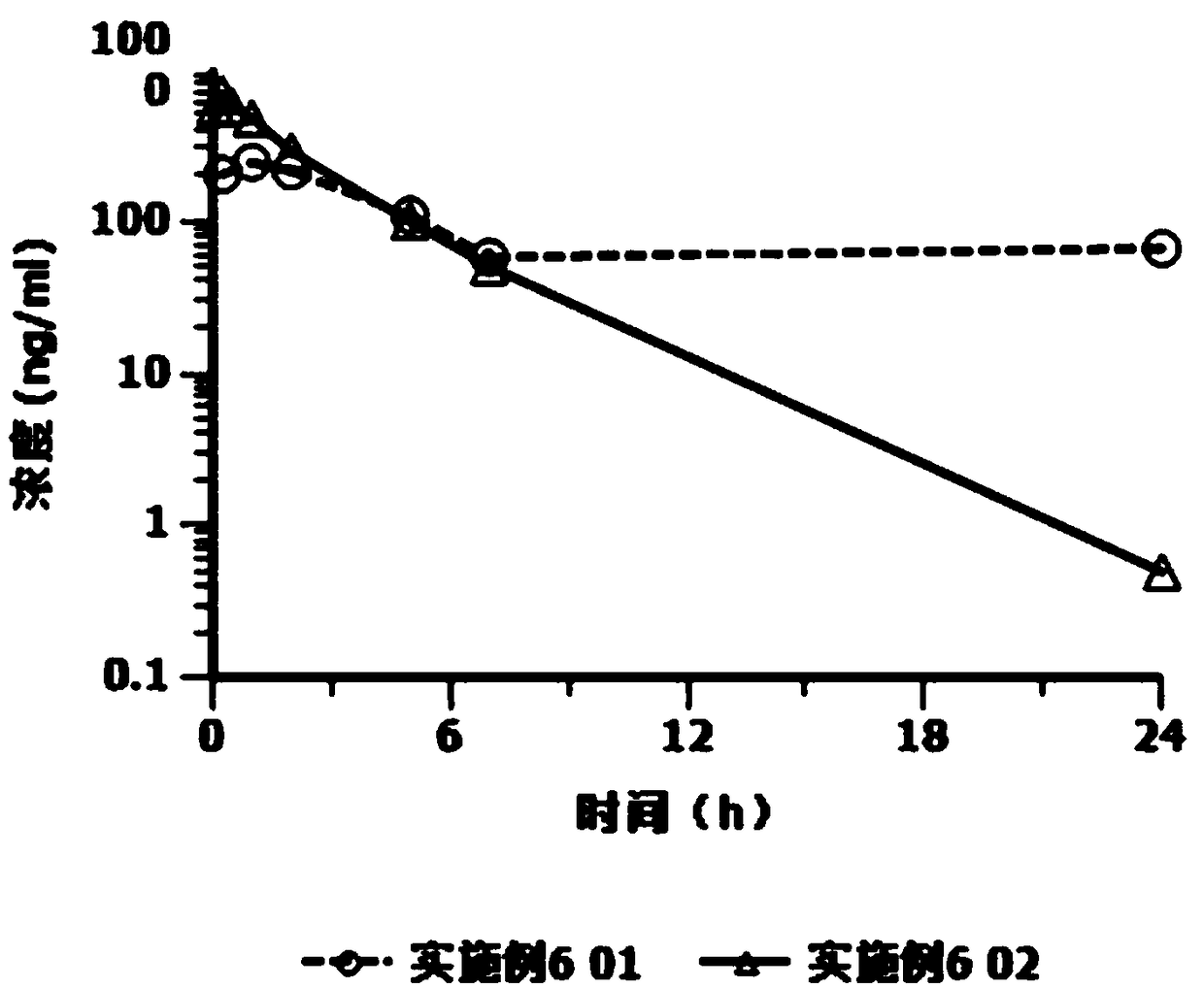
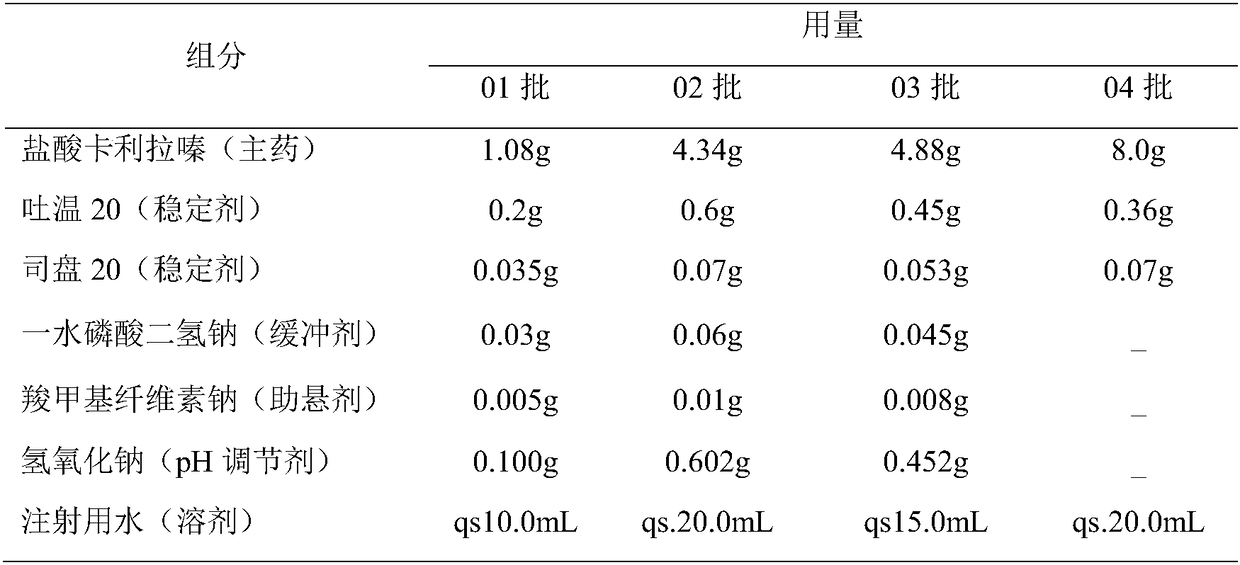
Cariprazine hydrochloride injection preparation, and preparation method and use thereof
ActiveCN108261394AImprove drug stabilitySmall batch-to-batch varianceOrganic active ingredientsPowder deliveryHigh concentrationInjection volume
The invention provides a cariprazine hydrochloride injection preparation, and a preparation method and a use thereof. The injection preparation is an aqueous suspension when used, cariprazine hydrochloride has a high concentration and a good stability, a high dosage can be obtained within a limited injection volume, the dosage can be flexibly adjusted according to the long-acting administration time, the particle size distribution and the injection dosage are controlled to achieve the long-acting effect, the cariprazine hydrochloride is continuously released in at least one week after the preparation is injected, and the preparation is administrated every one week or more to increase the compliance of patients. The invention also provides the preparation method of the cariprazine hydrochloride injection preparation. The injection preparation prepared by the method has the advantages of good stability and high safety, and the method is simple, is easy to implement, and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Protein A immuo adsorption material and preparing method
ActiveCN101069751AOvercome toxicityOvercome instabilityChemicalsMolecular materialsCombinatorial chemistry
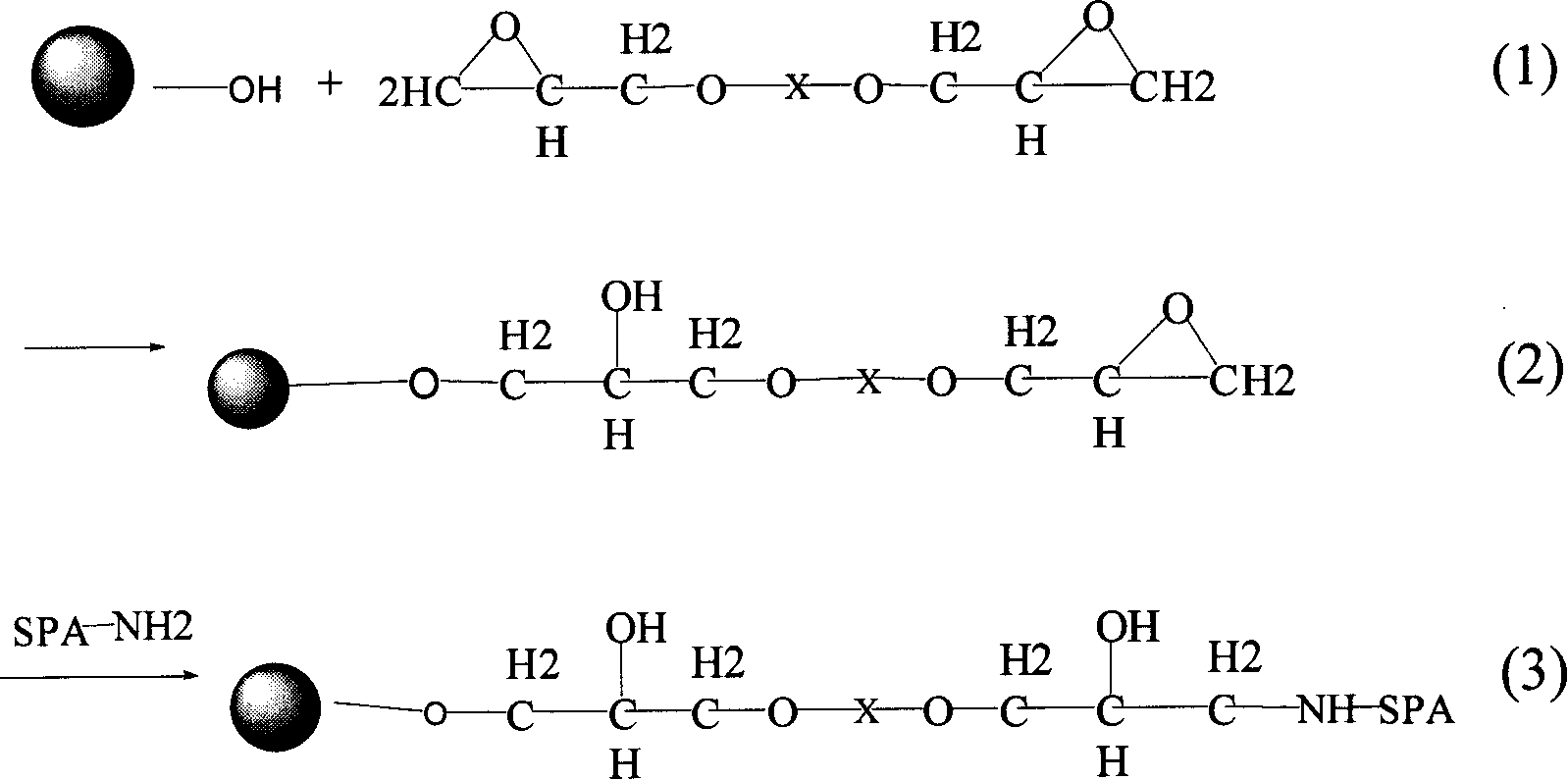
The present invention relates to a protein A immunoadsorption material for purifying blood and its preparation method. Said protein A immunoadsorption material is a high-molecular material which is obtained by using agarose gel as carrier matrix, making said agarose gel be reacted with diglycidyl ether coupling reagent to obtain active carrier, then making said active carrier and protein A implement coupling reaction. Said invention can be used for making clinical immunoadsorption therapy.
Owner:GUANGZHOU KONCEN BIOSCI +1
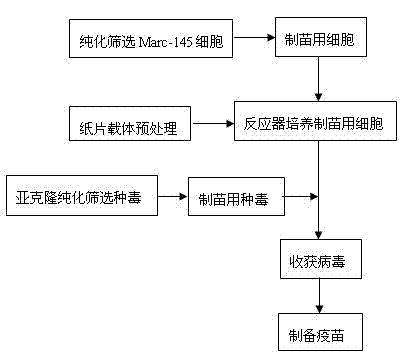
Method for producing pseudorabies living vaccines by using subculture cell source and product thereof
ActiveCN101695573AImprove securityImprove immune efficiencyAntiviralsViruses/bacteriophagesPig kidneyAntibiotic Y
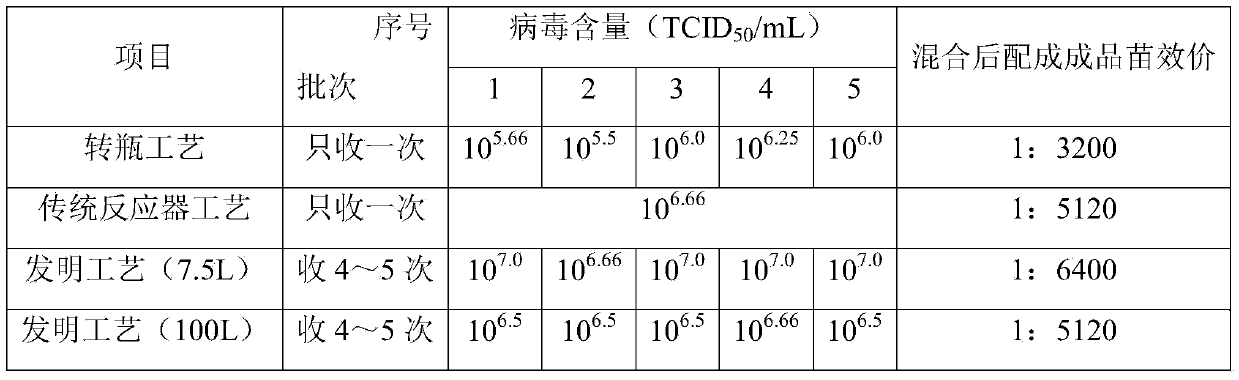
The invention provides a method for producing pseudorabies living vaccines by using a subculture cell source and a pseudorabies living vaccine product thereof. The method comprises the following steps: culturing pseudorabies virus low-virulent strains by using subculture cells; harvesting the strains to obtain cell culture venom; and then adding a stabilizing agent and an antibiotic into the cellculture venom, and freezing and vacuum-drying the mixture to obtain the pseudorabies living vaccines of the subculture cell source. The subculture cells are subculture cells ST of pig testicle or subculture cells PK15 or IBRS-2 of pig kidney. The method for producing the pseudorabies living vaccines by using the subculture cell source has the advantages of simple and stable production process, easy operation, high virus content, little batch difference and controllable quality, can remarkably improve the yield and quality of the vaccines and reduce the anaphylactic reaction and the like. The pseudorabies living vaccines obtained by using the production method of the invention have good safety and high immune efficacy, and have better immune protection effect on pseudorabies virulent attack.
Owner:广东永顺生物制药股份有限公司
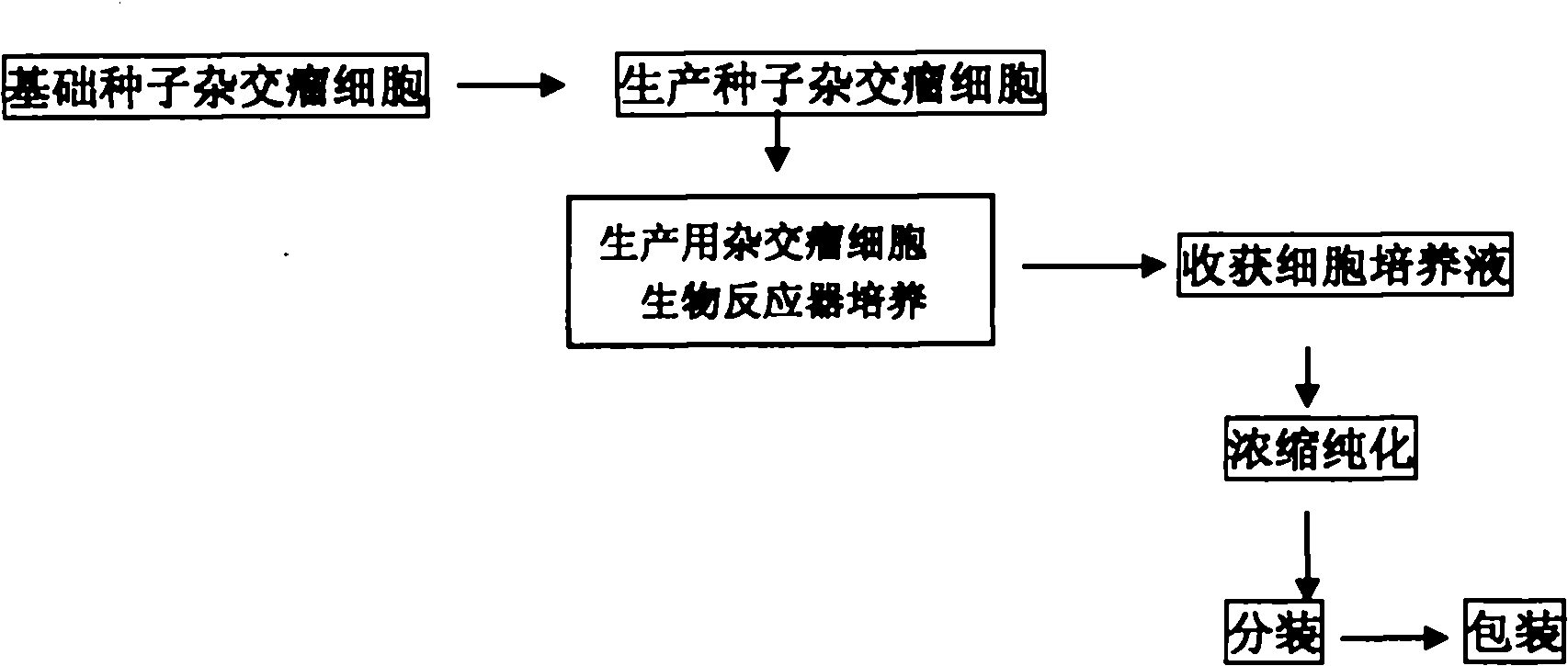
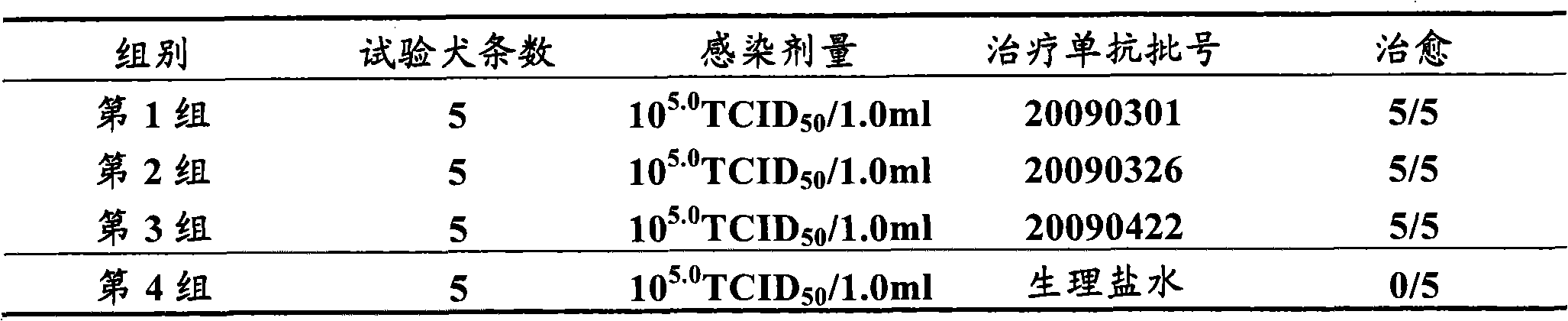
Method for producing curative canine parvovirus virus monoclonal antibody by using bioreactor
ActiveCN102120768AGuaranteed to be pureEnsure safetyImmunoglobulins against animals/humansTissue cultureMonoclonal antibodyCanine parvovirus
The invention discloses a method for producing a curative canine parvovirus virus monoclonal antibody by using a bioreactor.
Owner:北京世纪元亨动物防疫技术有限公司
Preparation method of porcine reproductive and respiratory syndrome vaccines by utilizing bioreactor
ActiveCN102552896AUniform stateSmall batch-to-batch varianceViral antigen ingredientsAntiviralsImmune effectsTGE VACCINE
A preparation method of porcine reproductive and respiratory syndrome vaccines by utilizing a bioreactor utilizes a stream perfusion type bioreactor as a cultivation tool. The preparation method comprises the steps of a cultivating cells for preparing the vaccines; b vaccinating and cultivating viruses; c harvesting virus liquor; d preparing the vaccines and the like. The preparation method has the advantages that the preparation method is simple and convenient in operation, low in contamination probability and capable of remarkably reducing preparation cost, the vaccines are large in yield, uniform and stable in quality and good in immune effect, and the like. The whole preparation process does not involve other problems of biological safety and public hygiene so that the preparation method is suitable for large-scale preparation of the porcine reproductive and respiratory syndrome vaccines.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
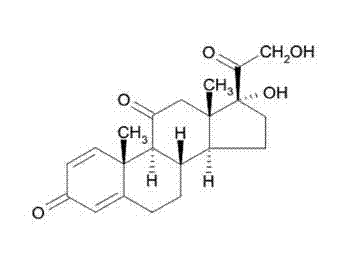
Oral prednisone time-selecting release preparation and preparation method thereof
InactiveCN103690545AGive full play to the therapeutic effectImprove balanceOrganic active ingredientsAntipyreticCelluloseFormulary
The invention discloses an oral prednisone time-selecting release preparation and a preparation method thereof. The oral prednisone time-selecting release preparation provided by the invention mainly consists of 0.3-5 parts of prednisone and derivatives thereof, 10-50 parts of glyceryl behenate and 3-30 parts of hydroxypropyl cellulose, and can further contain a disintegrating agent and other pharmaceutically acceptable excipients. The preparation method is as below: extruding tablet cores or granules containing the drug according to the formula by a tablet press or a dry granulator; and coating the tablet cores or particles containing the drug by a coating pan or a fluidized bed to attach the coating film to the tablet cores or particles containing the drug, so as to obtain the oral prednisone time-selecting release preparation. The oral prednisone time-selecting release preparation provided by the invention can achieve a good balance between the biological rhythm of the patients and the curative effects, and is safer, more convenient and effective compared with a traditional preparation. The oral prednisone time-selecting release preparation is prepared by an extrusion-coating process, which is simple for operation, and the obtained time-selecting release preparation has the advantages of drug stability and high reproducibility.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Cell freezing medium and application thereof
InactiveCN107996558AImprove survival rateSmall batch-to-batch varianceDead animal preservationSucroseFrost
The invention relates to the field of cells, in particular to a cell freezing medium and application thereof. The cell freezing medium is prepared by dissolving glycerol, propanediol, trehalose, sucrose, human albumin, glucose and the like in PBS buffer solution, wherein permeable protection fluid comprises the glycerol and the propanediol, and impermeable protection fluid comprises the trehalose,the sucrose and the human albumin, meanwhile the glucose is used for proving energy to cells, and the PBS buffer solution is used for maintaining pH stability of the cell freezing medium. According to the cell freezing medium, components are fixed, batch-to-batch variation is small, and pollutant sources such as germs and viruses are not introduced, the permeable and impermeable liquids are usedsimultaneously to provide frost resisting capability for the cells to improve survival rate of the cells, the glucose is further added to provide energy for the cells to further improve the survival rate of the cells, and finally the PBS buffer solution is used for maintaining the pH stability of the cell freezing medium, so that environment influence on the cells is reduced to improve the survival rate of the cells.
Owner:湖南丰晖生物科技有限公司
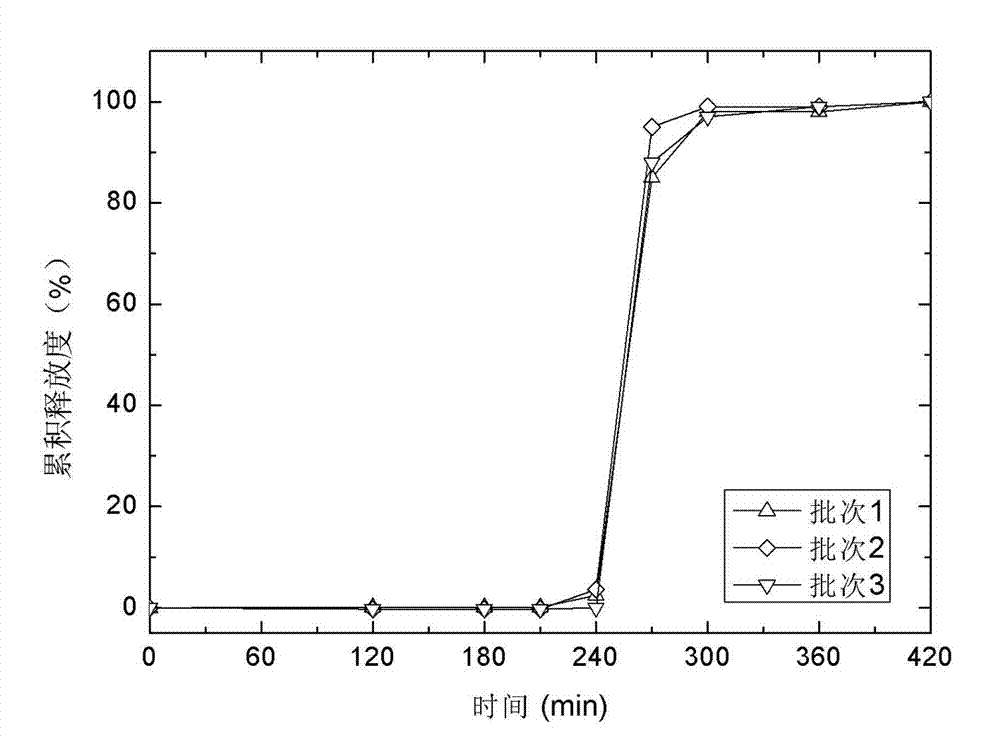
Minocycline hydrochloride sustained-release capsule and preparation method thereof
ActiveCN103054832AImprove stabilitySimple manufacturing processAntibacterial agentsTetracycline active ingredientsSustained release pelletsAdhesive
The invention relates to a minocycline hydrochloride sustained-release capsule and a preparation method thereof. A substance in the capsule is a sustained-release pellet which is prepared from the following raw and accessory material by weight: minocycline hydrochloride, a filler, an adhesive, an isolating layer film forming material, a plasticizer A, an opacifying agent, a sustained-release material, a plasticizer B, an antiadherent and a pore forming agent. The preparation method comprises the following steps: preparing a drug-loaded pellet core according to a formula; preparing an isolating layer pellet from the pellet core; preparing the sustained-release pellet from the isolating layer pellet; and filling the sustained-release pellet into a capsule so as to obtain the minocycline hydrochloride sustained-release capsule. According to the invention, the opacifying agent is added into an isolating layer, so stability of the photosensitive drug minocycline is improved; a sustained-release layer is prepared by using an optimized prescription, so usage amount of a coating material is substantially reduced; and the method provided by the invention has good reproducibility and is applicable to industrial production.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Oligomeric fucosylated glycosaminoglycan and preparation method thereof
ActiveCN101735336BSmall batch-to-batch varianceOrganic active ingredientsBlood disorderDepolymerizationGlycan
The invention discloses a method for preparing oligomeric fucosylated glycosaminoglycan which is prepared by depolymerizing fucosylated glycosaminoglycan by a depolymerization method of peroxide catalyzed by a 4th period transition metal ion in an aqueous medium, and the preparation method has mild reaction condition, good reproducibility and stability, high pyrolysis selectivity and uniform and controllable product quality. The polysaccharide molecule number of the obtained oligomeric fucosylated glycosaminoglycan using GalNAc as a reducing end is not less than 80 percent, the weight averagemolecular weight is about 6, 000-20, 000Da, and the protein disulfide isomerase (PDI) is 1.0-2.0.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Cefdinir capsule and preparation method thereof
ActiveCN102935075AGood dissolution effectImprove liquidityAntibacterial agentsOrganic active ingredientsMedicineQuality control
The invention provides a cefdinir capsule and a preparation method of the cefdinir capsule. The content of the cefdinir capsule comprises the necessary components based on parts by weight: 70-90 parts of cefdinir, 0.01-5 parts of polyoxyl (40) ester stearate, 0.2-5 parts of silicon dioxide, 0.1-5 parts of magnesium stearate and 5-30 parts of hydroxypropyl cellulose. The medicine prepared by cefdinir capsule prescription is good in dissolution effect and fluidity, and stable in medicine effect; the provided preparation method is simple in technology; the product is less in introduced impurity, easy in quality control and good in reproducibility and uniformity; and the preparation method saves the energy and reduces the consumption, thus easily meeting the demand of mass production.
Owner:海南三叶美好制药有限公司
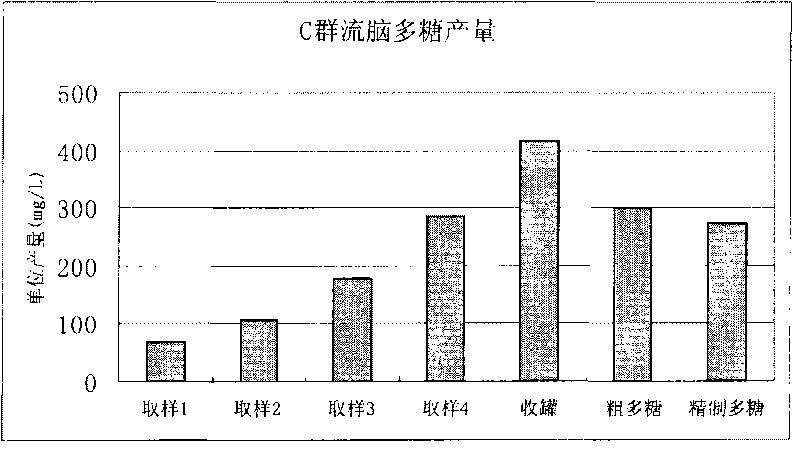
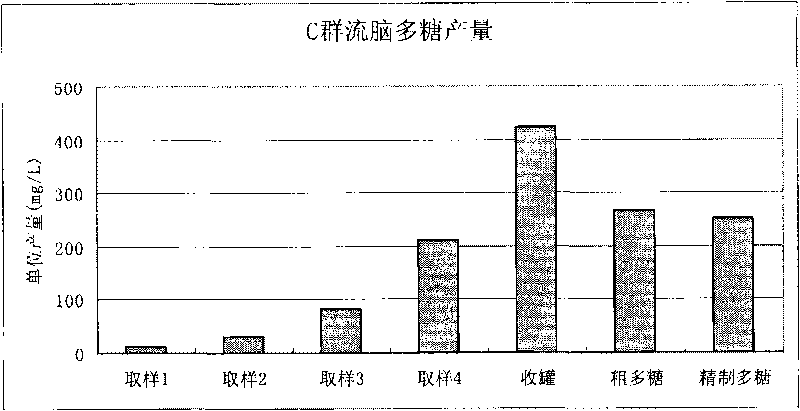
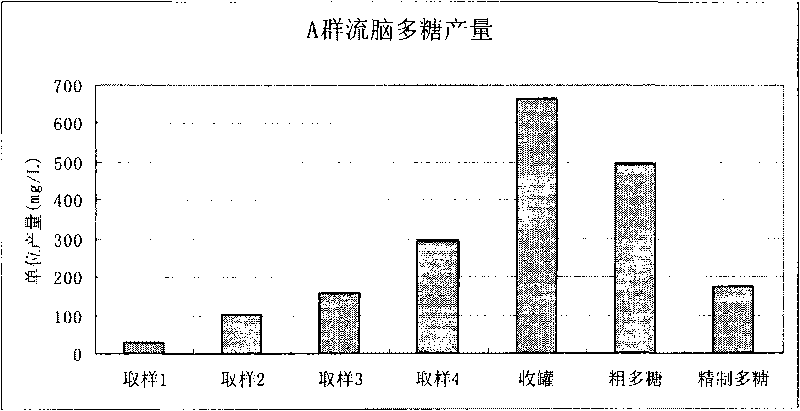
Method for purifying capsular polysaccharide
ActiveCN101724085AAvoid damageProtection securityAntibacterial agentsOrganic active ingredientsAlcoholChloroform
The invention relates to a treatment method for purifying capsular polysaccharide, comprising the following steps of: (a), adding a lower alcohol with low final concentration into a cell capsular polysaccharide precipitate, and dissolving the capsular polysaccharide to form a mixed liquid; (b), carrying out solid-liquid separation on the mixed liquid and collecting a supernate; (c), adding a lower alcohol with high final concentration into the obtained supernate to form a rough precipitate containing the cell capsular polysaccharide; (d) adding the lower alcohol with the low final concentration into the rough precipitate so that the polysaccharide in the rough precipitate is in a dissolving state; (e) adding the lower alcohol with the high final concentration into the supernate so as to form the capsular polysaccharide precipitate; and (f) collecting the obtained capsular polysaccharide precipitate to obtain a purified cell capsular polysaccharide. The method conveniently, controllably and effectively improves recycling rates of the capsular polysaccharide and improves the security without using toxic reagents of phenol or chloroform.
Owner:SHANGHAI INST OF BIOLOGICAL PROD CO LTD
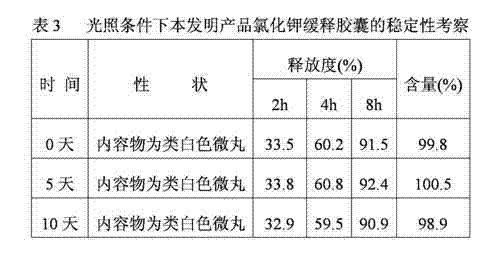
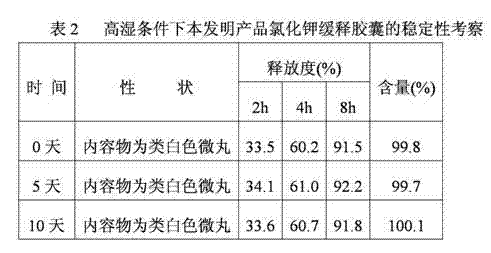
Potassium chloride slow release capsule
ActiveCN102961363ASimple manufacturing processSolve problems such as complianceMetabolism disorderGranular deliveryPlasticizerLong term treatments
The invention discloses a potassium chloride slow release capsule. A content of the potassium chloride slow release capsule is a slow release micro-pill; and the slow release micro-pill consists of 70-97 percent by weight of the potassium chloride used as a raw material, 0-10 percent by weight of forming materials, 2-20 percent of slow release materials, 0.5-5 percent of plasticizers and 0-10 percent of antisticking agents. The potassium chloride and the forming materials are uniformly mixed; a medicine carrier micro-pill is prepared into a dry type granulator; the slow release materials, the plasticizers and the antisticking agents are mixed to prepare slow release layer coating solution; the medicine carrier micro-pill is put into a fluidized bed; the prepared slow release layer coating solution is ejected into the fluidized bed; the medicine carrier micro-pill is coated according to the conventional method so as to prepare the slow release micro-pill; and the slow release micro-pill is filled into the capsule so as to obtain the potassium chloride slow release capsule. The prepared potassium chloride slow release capsule is low in toxin side effects, convenient to treat patients for a long time, improves the medicine safety and improves the compliance of the medicines.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
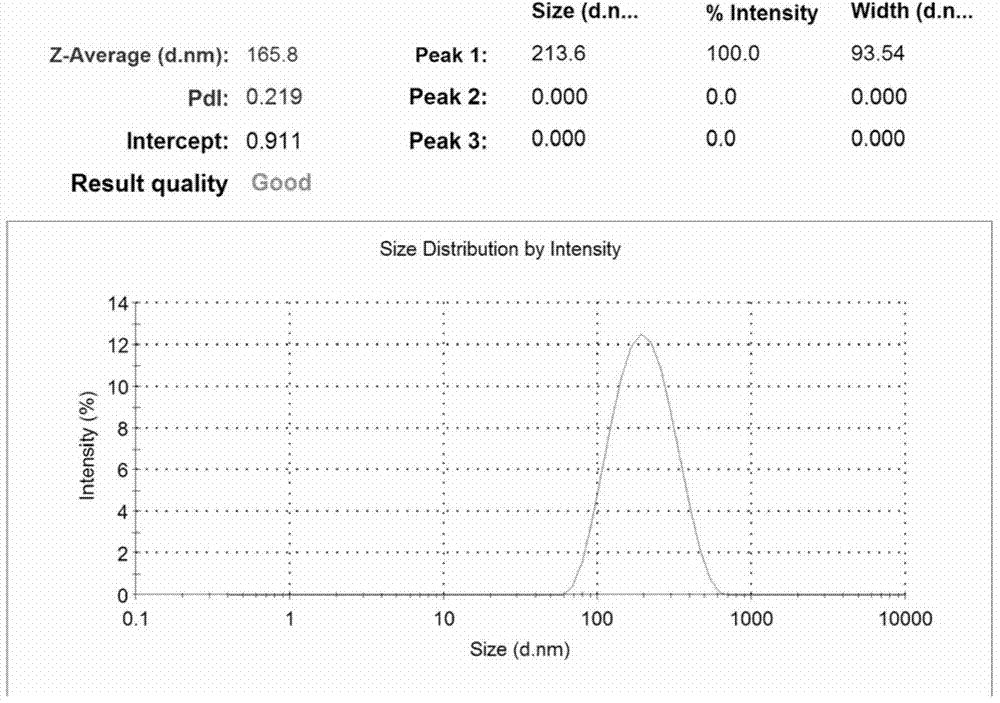
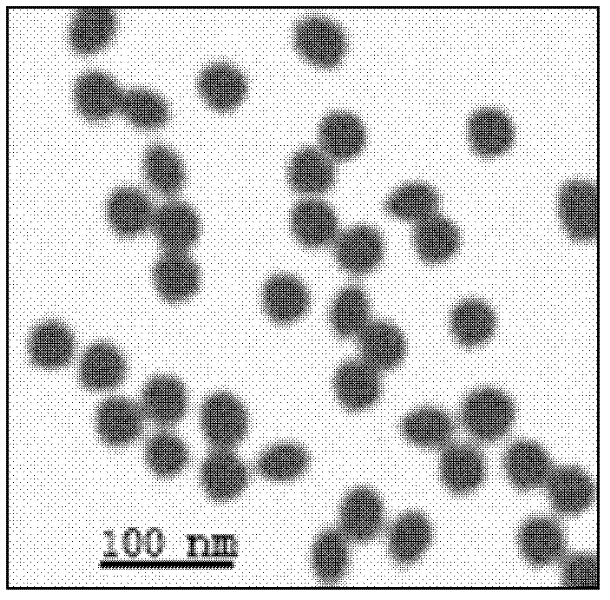
Preparation method of calcium alginate nanometer microspheres
InactiveCN104710631AMild conditionsSmall batch-to-batch variancePharmaceutical non-active ingredientsOrganic solventMicrosphere
The invention relates to calcium alginate nanometer microspheres and a preparation method thereof. The preparation method of the nanometer microspheres comprises the following specific preparation steps: using a natural polysaccharide material sodium alginate as a raw material, adopting high-pressure homogenization method to be combined with an external gelation method, and preparing the calcium alginate nanometer gel microspheres. The particle size of the nanometer gel microspheres prepared by applying the method is in a range of 50-1000 nm, and the particle size distribution is uniform. Compared with a traditional emulsification-external gelation method, the nanometer microspheres prepared by the high-pressure homogenization method has uniform particle size distribution and high yield; during the preparation process, no organic solvent is required to be added, the amount of an emulsifier is greatly reduced, and the preparation method is green, environmentally friendly, safe and nontoxic; and the calcium alginate nanometer microspheres are simple and convenient to prepare and can be prepared on large scale.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
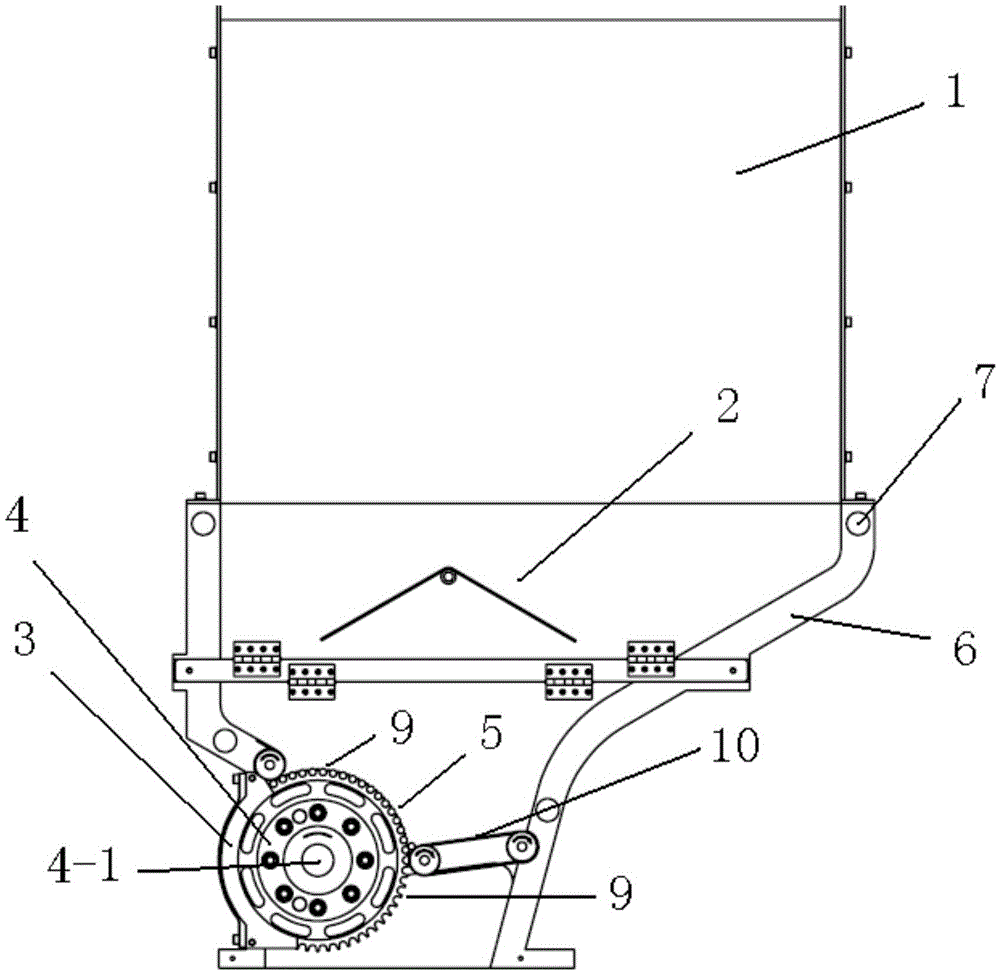
Automatic rotatable filter stick machining device
ActiveCN105533801AGood lookingHigh hardnessTobacco smoke filtersMetal working apparatusVertical planeAnnular cutter
The invention relates to an automatic rotatable filter stick machining device which comprises a machine frame (6), a drum (4) and a filter stick annular cutter (8). The machine frame (6) is provided with a conveying belt (10) for conveying filter sticks. The drum (4) is arranged on the machine frame (6) and can rotate around a horizontal rotating shaft (4-1) in the vertical plane, a plurality of grooves (9) which are parallel to one another are formed in the periphery of the drum or an annular groove belt (5) on the periphery of the drum, each groove is used for containing one filter stick, the bottom of each groove is provided with an air sucking opening, a pair of arc-shaped fixed stop pieces (3) are arranged on the circumference of the side face of the drum (4), an air sucking device communicated with an arc-shaped air flow section between the starting ends of the arc-shaped fixed stop pieces and the discharge point of the conveying belt is arranged in the drum (4), the filter stick annular cutter (8) is provided with a rotating shaft (8-1) parallel to the horizontal rotating shaft (4-1) of the drum (4) and can be driven by an annular cutting motor (8-2) to rotate, and the rotating shaft is provided with at least one annular blade (8-3).
Owner:CHINA TOBACCO YUNNAN IND
Method for preparing norfloxacin tablets
ActiveCN102940612AImprove liquidityEasy to compressAntibacterial agentsOrganic active ingredientsGranularityHeight difference
The invention discloses a method for preparing norfloxacin tablets. The method comprises the following steps of: sieving norfloxacin with a sieve of 40 to 100 meshes, sieving a disintegrating agent with a sieve of 60 to 100 meshes, sieving a lubricating agent and a flow aid with a sieve of 80 to 120 meshes, mixing the norfloxacin with a large part of disintegrating agent uniformly, mixing less than 2 percent of disintegrating agent, the lubricating agent and the flow aid uniformly, adding into the mixed powder, mixing uniformly, tabletting, and thus obtaining the norfloxacin tablets. The norfloxacin tablets comprise the following raw materials in percentage by weight: 20 to 80 percent of norfloxacin, 5 to 79 percent of disintegrating agent, and 1 to 8 percent of lubricating agent and flow aid. 5 to 70 percent of filling agent can also be added. The mixed materials obtain high flowability, excellent compression property and adhesive property, high attachment and low sensitivity to the lubricating agent through a large quantity of tests, advantage complementation of auxiliary materials, proper formula proportioning, reasonable granularity distribution and accurate powder mixing sequence. The method overcomes the defects of common material non-layering, non-uniform content, high tablet height difference, tablet powder fall, slow disintegration and the like in the direct tabletting production process, and solves the problems of color change of tablet cores, low dissolubility and the like of the wet granulation process.
Owner:YUNNAN PHYTOPHARML
Repaglinide troche and preparation method thereof
ActiveCN103610677AAvoid stickingGood dispersionOrganic active ingredientsMetabolism disorderMedicineDissolution
The invention relates to an oral troche which contains repaglinide or pharmaceutically acceptable derivatives of repaglinide as well as a preparation method of the oral troche. According to the preparation method, powder of repaglinide or pharmaceutically acceptable derivatives of repaglinide is directly pressed into troche, so that the production cost is remarkably lowered, and the disintegration and the dissolution rate are greatly improved. The bioavailability and the stability of the medicine can be improved, and the problem of low content uniformity of existing small-dose medicines formed by the direct pressing method is overcome, so that the quality of the troche is better guaranteed.
Owner:华益泰康药业股份有限公司
Combined live vaccine against porcine reproductive and respiratory syndrome and pseudorabies, and preparation method thereof
ActiveCN102727884AImprove immune efficiencyIncrease productionViral antigen ingredientsGenetic material ingredientsPseudorabiesImmunosuppression
The invention provides a combined live vaccine for preventing porcine reproductive and respiratory syndrome and pseudorabies, and a preparation method and application thereof. According to the invention, no immunosuppression occurs between two vaccines of the combined live vaccine; compared with each single vaccine, the combined live vaccine has no obvious difference in security, immunogenicity, immunity duration and immuno-protective effects and has remarkable immuno-protective effects on preventing porcine reproductive and respiratory syndrome and pseudorabies.
Owner:华威特(江苏)生物制药有限公司
Method for detecting ochratoxin A based on near-infrared up-conversion luminescence marking and magnetic separation
InactiveCN106053790AImprove stabilityImprove accuracyBiological material analysisAptamerSingle strand
A method for detecting ochratoxin A based on near-infrared up-conversion luminescence marking and magnetic separation is used for detecting ochratoxin A (OTA) content in wheat and its products and the like. By connecting near-infrared up-conversion luminescent material with NaYF4:Yb 0.2 and Tm 0.02 with ochratoxin A aptamer to form a signal probe, the signal probe and aptamer complementary oligonucleotide single strand modified Fe3O4 magnetic nanomaterial form a nano composite, and at the moment up-conversion luminescence signal is maximum; when OTA is present in a detection system, OTA specifically binds with OTA aptamer so that double strands are unlinked, and by monitoring up-conversion luminescence signal strength 804 nm from a near-infrared zone, it is possible to quantitatively detect OTA, with a linear range of 0.01-100 ng / mL and a detection limit of 0.005 ng / mL. The method for OTA detection has the advantages of high sensitivity, high speed and good simplicity and is applied to detecting beer samples, with accurate and reliable results.
Owner:JIANGNAN UNIV
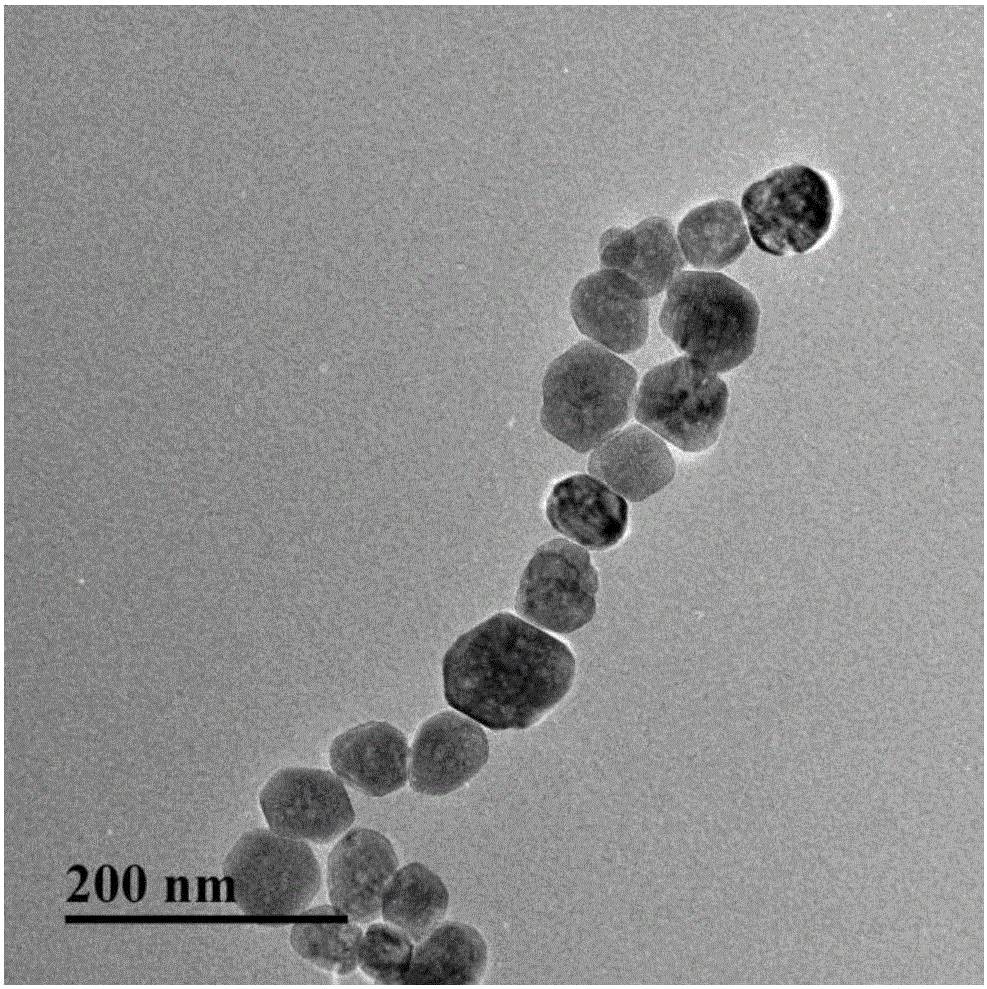
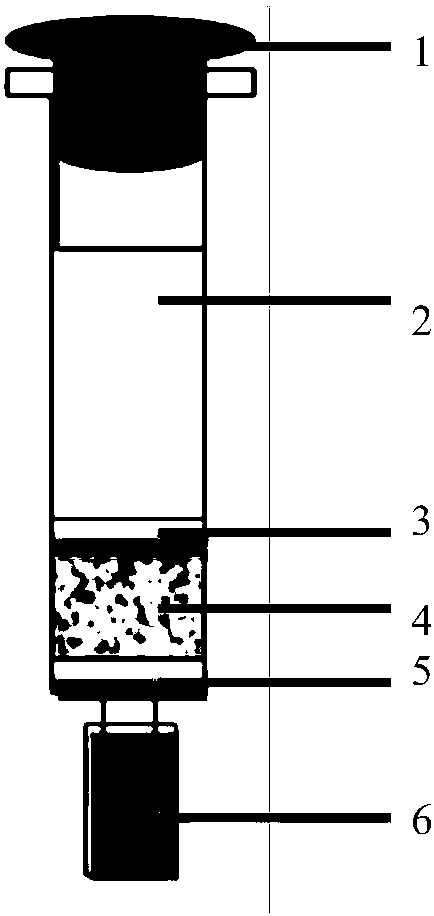
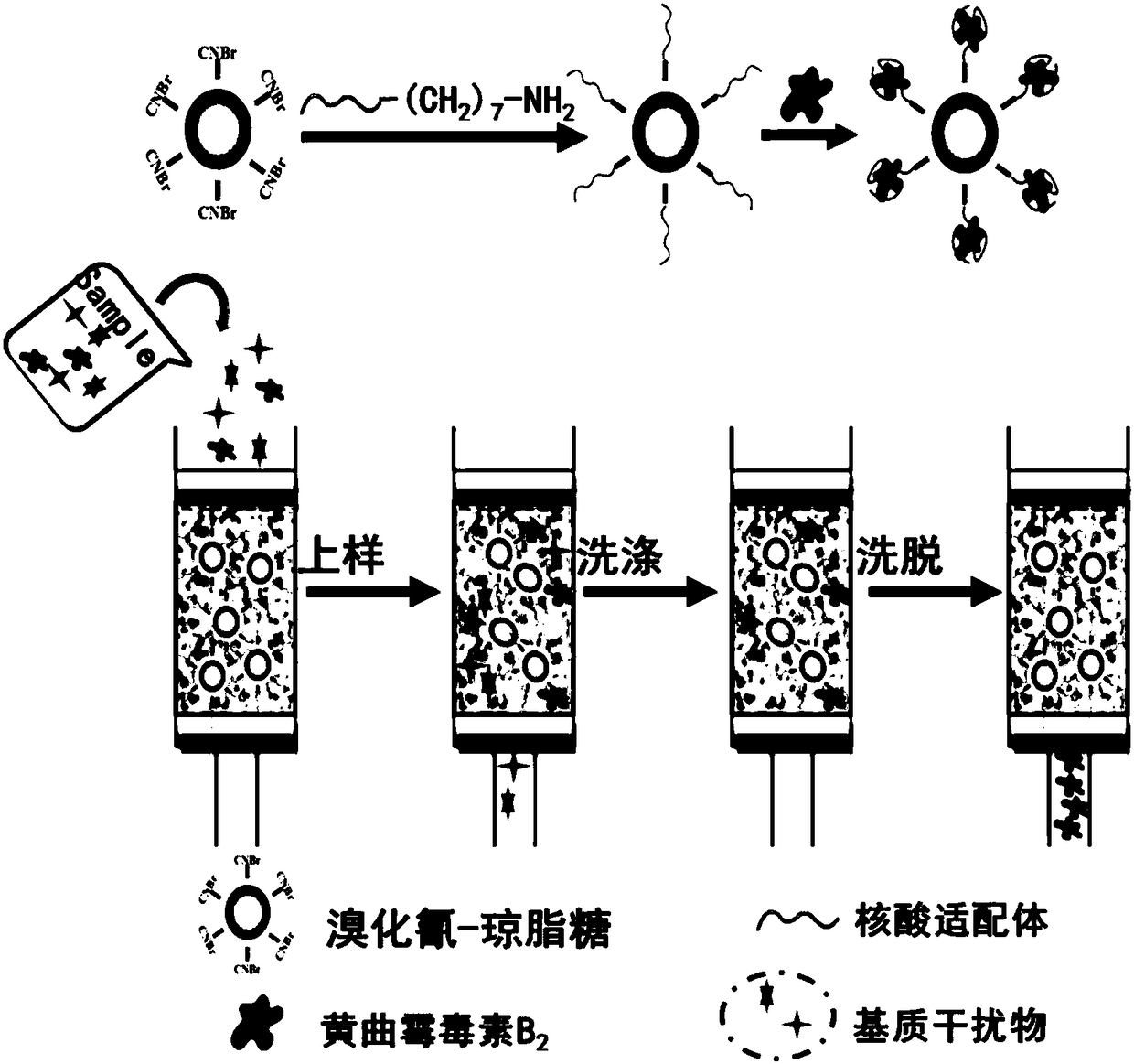
Aflatoxin B2 aptamer affinity column and preparation method and application thereof
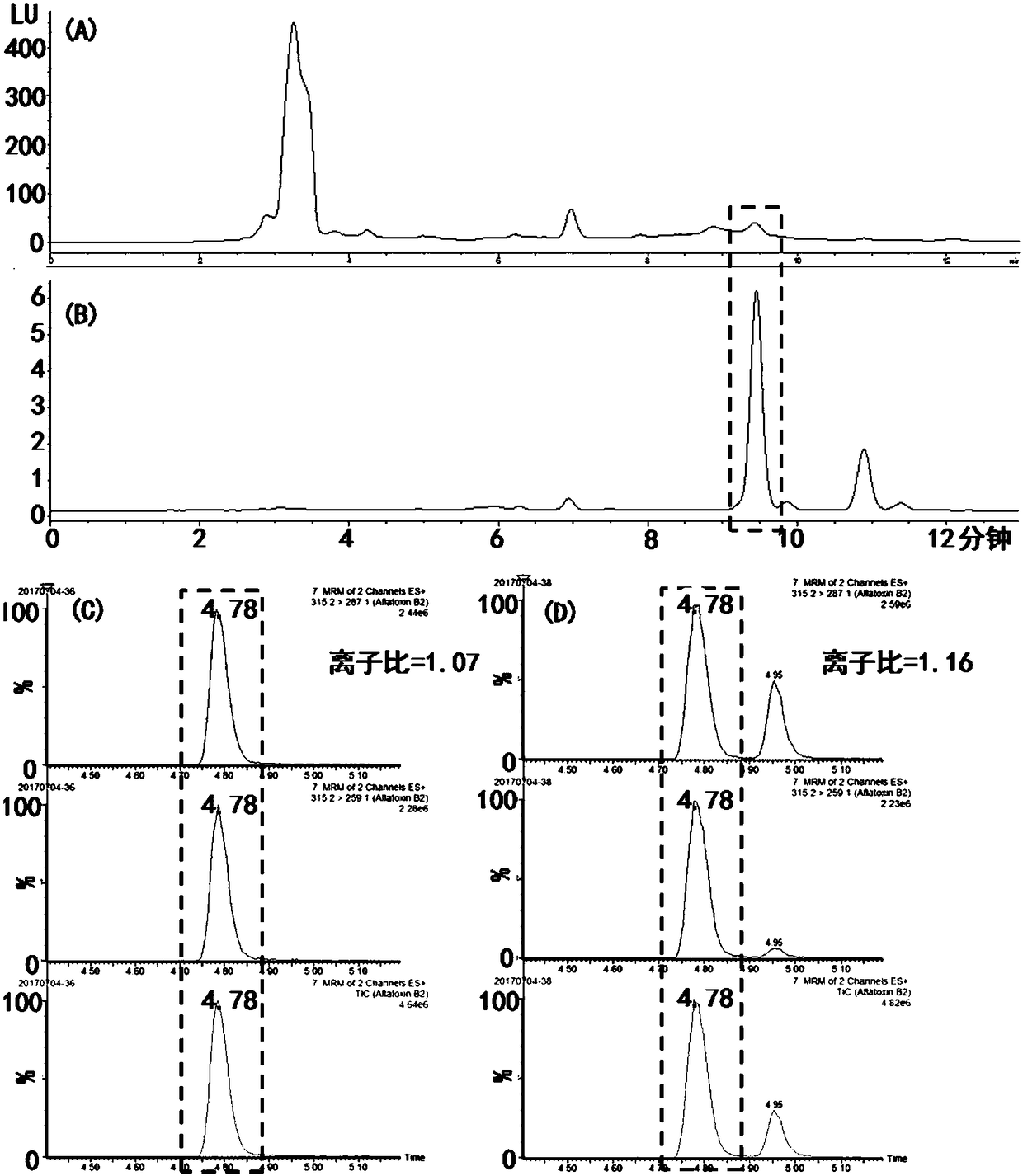
ActiveCN108251427ASimple and efficient operationLow priceOrganic chemistryOther chemical processesFluorescenceChemistry
The invention provides an aflatoxin B2 aptamer affinity column and a preparation method thereof, wherein the affinity column uses cyanogens-bromide-modified agarose as a vector, a nucleic acid aptamercapable of recognizing aflatoxin B2 with high affinity and high specificity is covalently coupled to the vector, and the coupled aflatoxin B2 aptamer complex vector is loaded into the affinity column. The affinity column is mainly used for purification and cleaning of the aflatoxin B2 in food, feeds, milk, blood samples, traditional Chinese medicines and other various samples so as to facilitatehigh performance liquid chromatography and fluorescence detection of the aflatoxin B2 in the samples.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Method for mass production of pseudorabies virus vaccine
InactiveCN101804203ASolve the problems of low production output, high labor intensity and high costExpand production scaleAntiviralsTissue/virus culture apparatusFiberPolyester
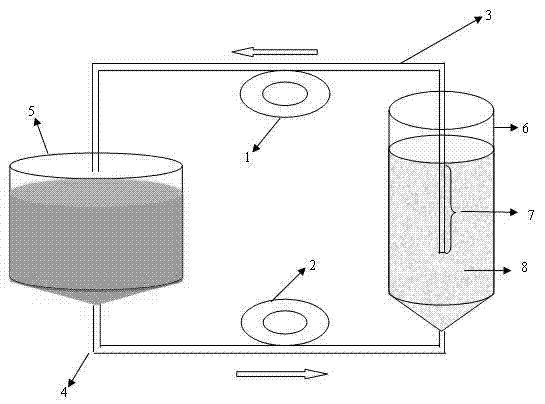
The invention discloses a method for mass production of pseudorabies virus vaccine, comprising the following steps: (a) adding netty polyester fiber which serves as a carrier into a bioreactor provided with a tide type micro-carrier suspension culture system and inoculating cells for producing vaccine; (b) inoculating pseudorabies virus vaccine when the culture cell grows to a certain intensity, so that the cells are infected by the pseudorabies virus vaccine; (c) reproducing the virus in great numbers under appropriate conditions; (d) harvesting the virus when cytopathic rate reaches above 70%; (e) carrying out freeze thawing on the harvested virus for once or twice to lead the cells to completely come off and disperse and then adding freeze-drying protective agent, evenly mixing the mixture, packaging the mixture in fixed volume and freeze-drying.The method of the invention has the advantages of good stability, explicit process control indicators, good controllability, easy operation, large process scale and the like.
Owner:PU LIKE BIO ENG
Method for preparing chloroethylene-n-butyl acrylate copolymer resin with suspension process
InactiveCN106279490AImprove product qualityCopolymerization effect is obviousApparent densityCopolymer
The invention provides a preparation method of chloroethylene-n-butyl acrylate copolymer resin with a suspension process and belongs to the technical field of chemical polymer synthesis. The suspension polymerization process is adopted, and a polymerization formula is adjusted and optimized, and the n-butyl acrylate copolymer adding technology is controlled, therefore, the prepared chloroethylene-n-butyl acrylate copolymer resin product has the advantages of regular shape, concentrated size distribution, higher apparent density and lower oil absorption rate, is transparent and presents remarkable internal plasticity and impact resistance.
Owner:YIBIN HAIFENG HERUI
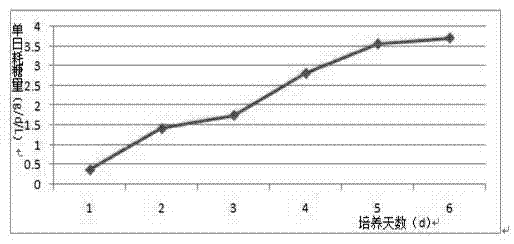
Method for producing porcine circovirus type 2 antigens in large scale with high density
ActiveCN104004720AIncrease productionQuality improvementViral antigen ingredientsAntiviralsAntigenHigh density
The invention relates to a method for producing porcine circovirus type 2 antigens in large scale with high density. A bioreactor microcarrier suspension culture technology used for replacing an existing spinner bottle culture technology for producing the porcine circovirus type 2 antigens. The method can greatly reduce the production cost and improve the yield. Compared with the spinner bottle technology, the unit antigen cost is reduced by 80% to 90%, the production period is reduced by 7 days, and the yield is improved by 3 times to 10 times; due to the fact that the antigens can be obtained repeatedly, compared with a traditional reactor culture technology, the unit antigen cost is reduced by 40% to 50%, and the yield is improved by 3 times to 5 times. The antigens produced through the method has no serum residues, the produced vaccine is higher in safety and small in batch difference, the quality is stable and easy to control, and the yield and quality of the produced vaccine can be obviously improved.
Owner:JIANGSU NANNONG HI TECH
Detection kit and preparation method thereof for drugs of benzodiazepines
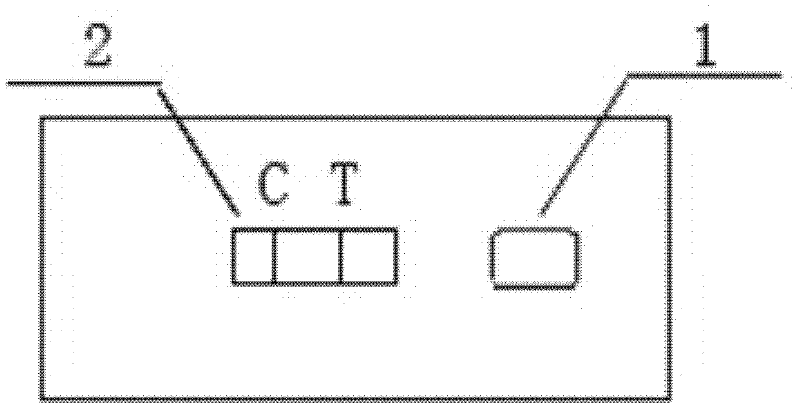
ActiveCN102565409AEnsure controllabilitySmall batch-to-batch varianceMaterial analysisReagent stripBenzodiazepine
The invention discloses a detection kit and a preparation method thereof for drugs of benzodiazepines. The detection kit provided by the invention includes a reagent strip, wherein the reagent strip includes sample filtering paper, immune colloid gold paper, an immune cellulose nitrate membrane and water absorbent paper which are arranged sequentially from the sample feeding end, wherein the immune colloid gold paper is a fiber glass gasket coated by an anti-benzodiazepine monoclonal antibody labeled by colloid gold; the immune cellulose nitrate membrane is a cellulose nitrate membrane provided with a detection line and a quality control line; the detection line is coated with a benzodiazepine-bovine serum albumin compound; and the quality control line is coated with goat-anti-mouse IgG. The invention further provides a preparation method of the detection kit for drugs of benzodiazepines. Through adopting the detection kit and the preparation method provided by the invention, the consumption of monoclonal antibodies can be reduced, and the manufacturing cost is saved.
Owner:SHANGHAI CHEMTRON BIOTECH
Method for preparing heat-resisting attenuated virus live vaccine for goatpox by using BHK21-C13 passage cell
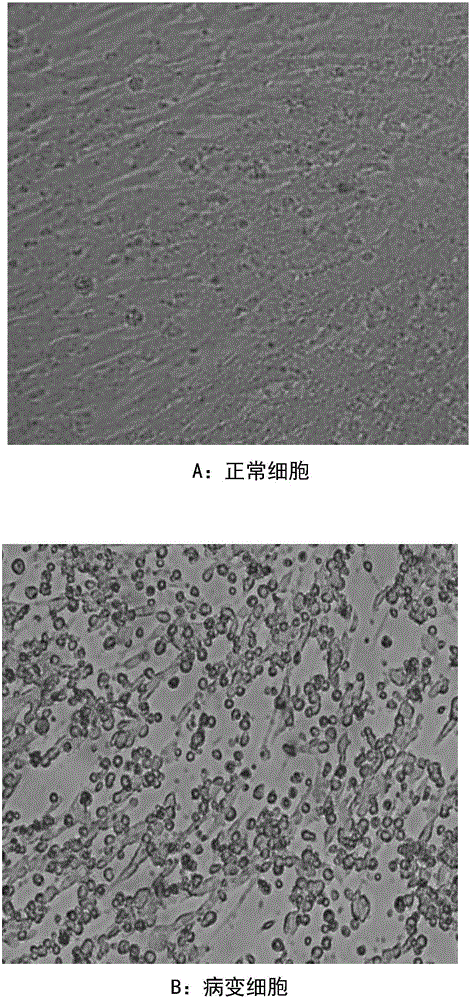
InactiveCN106282129AAvoid pollutionStrong process controllabilityPowder deliveryViral antigen ingredientsHeterologousImmunogenicity
The invention discloses a method for preparing a heat-resisting attenuated virus live vaccine for goatpox by using a BHK21-C13 passage cell. Based on the existing attenuated virus AV41 for goatpox, which has excellent immunogenicity, the method comprises the following steps: culturing 25-28th generations of virus solution with a heterologous BHK21 cell, and then adding an appropriate heat-resisting freeze-drying protective additive to obtain the heat-resisting attenuated virus live vaccine for goatpox. The passage cell vaccine used by the method is superior to the primary cell vaccine in the aspects of virus yield, homogeneity, purity and the like, not only guarantees the effective level of the vaccine and has a better heat-resisting protection effect, but also can ensure the safety of a purebred pregnant goat. As the existing BHK21 cell adopts a mature suspension culture process, the exploration of various parameters of adherent culture lays a solid foundation for the next promotion of suspension process.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com