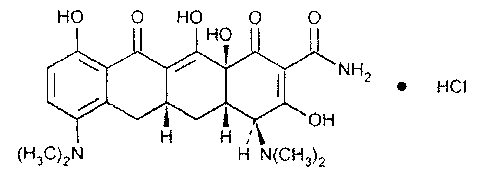
Minocycline hydrochloride sustained-release capsule and preparation method thereof
A minocycline hydrochloride and sustained-release capsule technology, which is applied in the field of medicine, can solve the problem of unsatisfactory pharmacokinetic parameters such as bioavailability in vivo, large differences in pharmacokinetic parameters of in vitro release, and poor release stability. problems, achieve stable and uniform release performance, facilitate long-term treatment, and improve compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
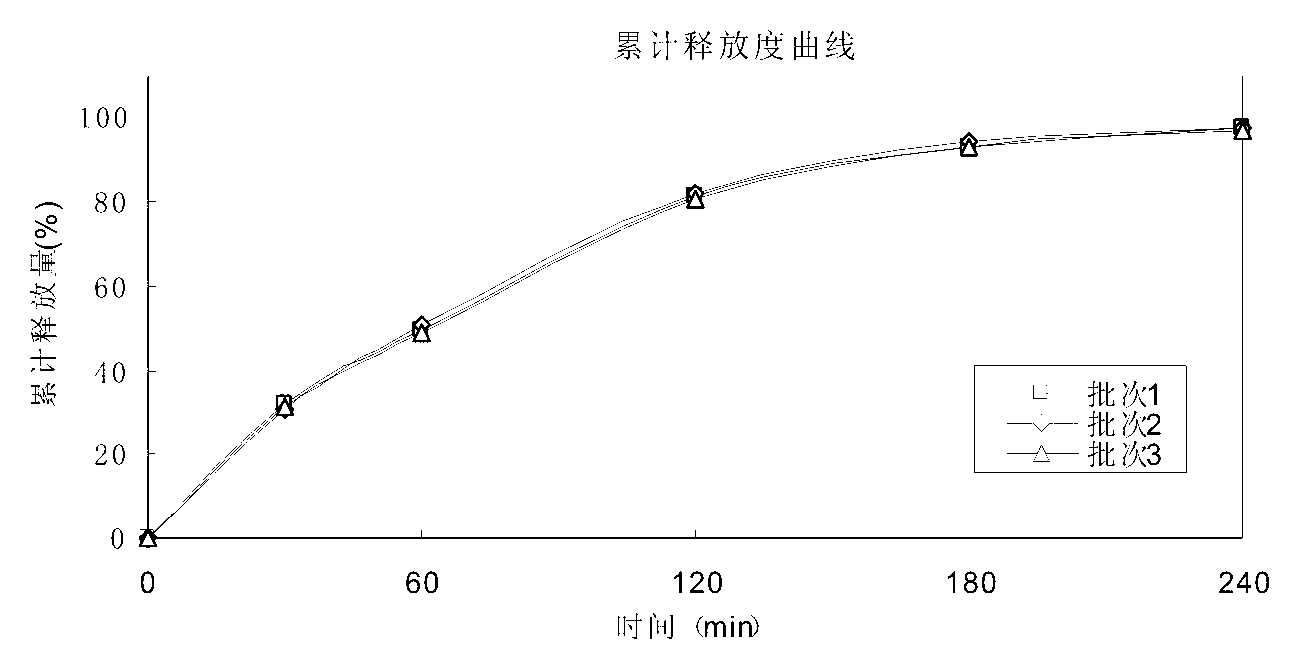
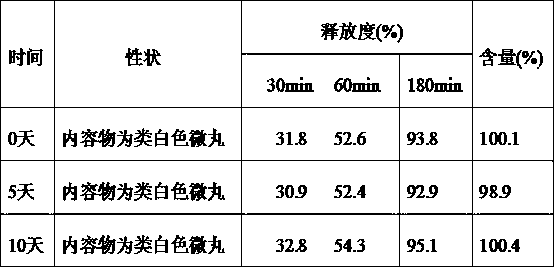
[0057] A minocycline hydrochloride sustained-release capsule, the content of which is sustained-release pellets. The sustained-release pellets are prepared from the following raw and auxiliary materials in weight percentages. The raw and auxiliary materials are as follows: Minocycline hydrochloride 39.2 %, filler microcrystalline cellulose 21.9%, filler lactose 13.2%, binder hypromellose 3.8%, isolation layer film-forming material hypromellose 3.9%, opacifying agent titanium dioxide 0.8%, slow-release material Ethyl cellulose 10.4%, plasticizer B diethyl phthalate 1.6%, porogen hypromellose 5.2%.
Embodiment 2
[0059] A minocycline hydrochloride sustained-release capsule, the content of which is sustained-release pellets, and the sustained-release pellets are prepared from the following raw and auxiliary materials in percentage by weight. The raw and auxiliary materials are as follows: Minocycline hydrochloride 37.3 %, filler microcrystalline cellulose 35%, binder hypromellose 5%, isolation layer film-forming material hypromellose 3%, opacifying agent titanium dioxide 1.2%, slow-release material ethyl cellulose 10.5% , plasticizer B diethyl phthalate 2%, porogen hypromellose 6%.
Embodiment 3
[0061] A minocycline hydrochloride sustained-release capsule, the contents of which are sustained-release pellets, and the sustained-release pellets are prepared from the following raw and auxiliary materials in percentage by weight, and the preparation of the raw and auxiliary materials is as follows: Minocycline hydrochloride 42 %, filler lactose 34.5%, binder hypromellose 3.5%, isolation layer film-forming material hypromellose 3.5%, opacifier titanium dioxide 2%, slow-release material ethyl cellulose 9%, plasticizer Agent B diethyl phthalate 1.5%, porogen hypromellose 4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com