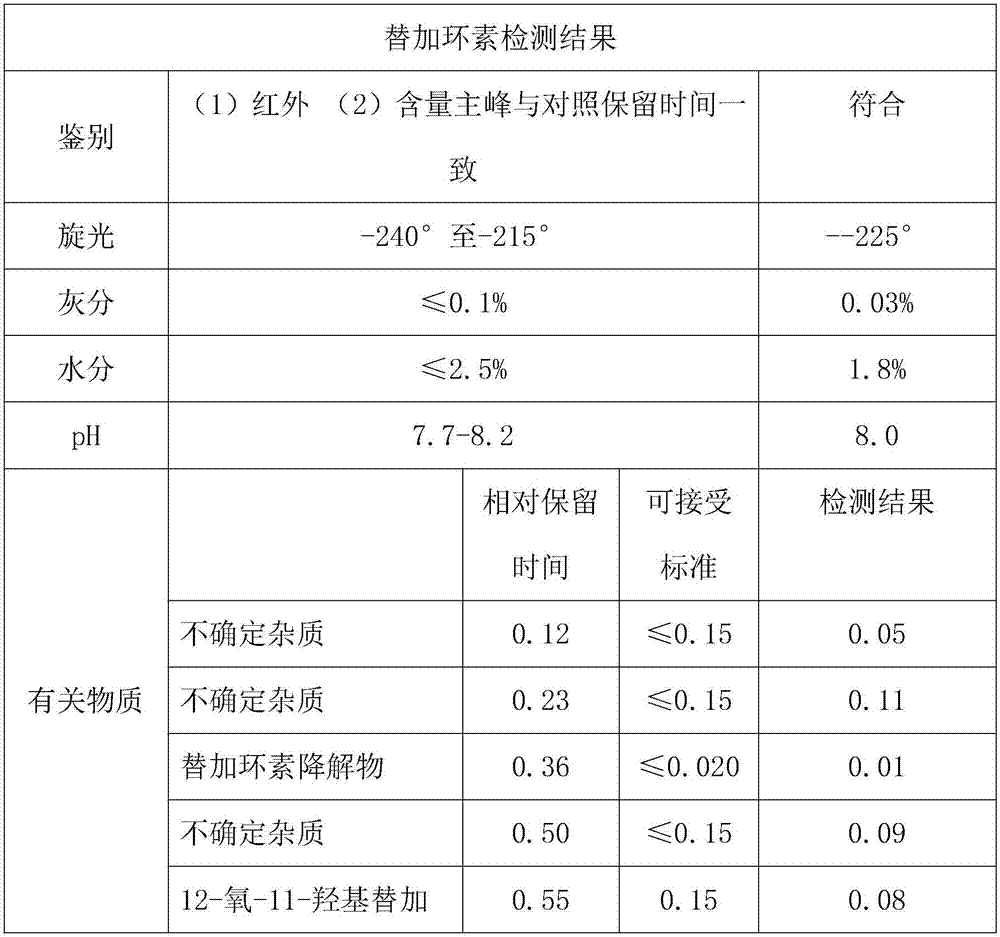
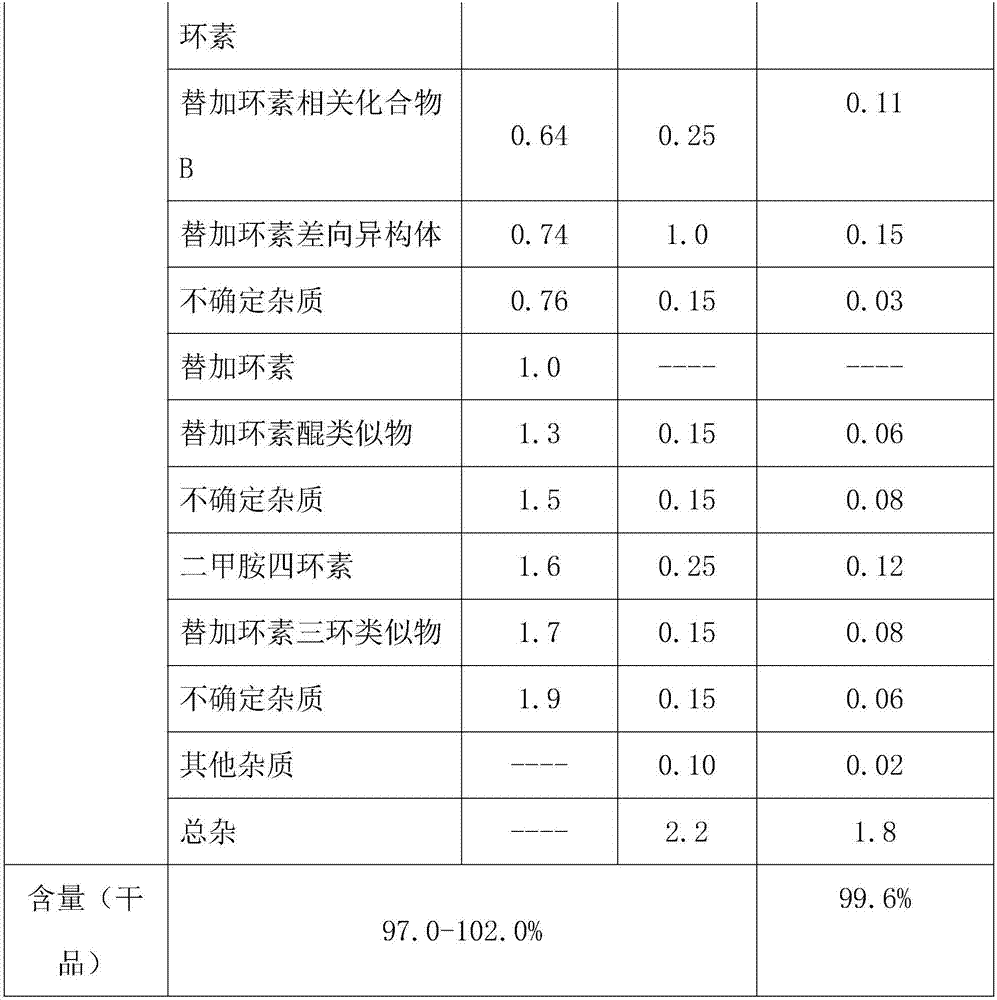
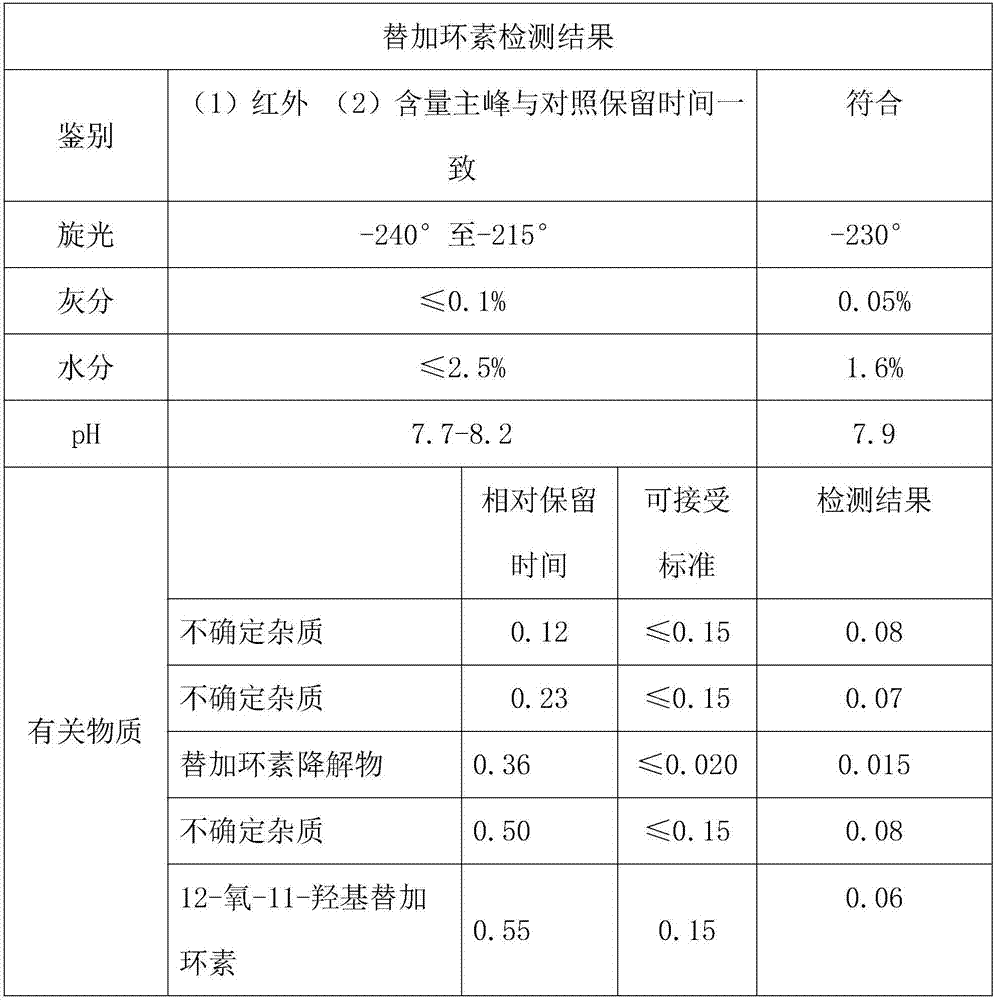
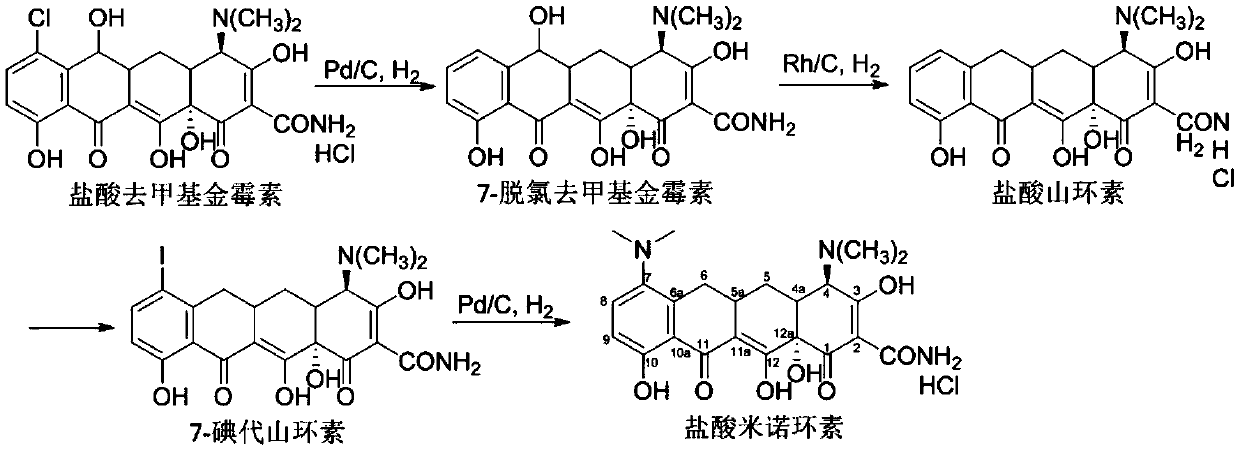
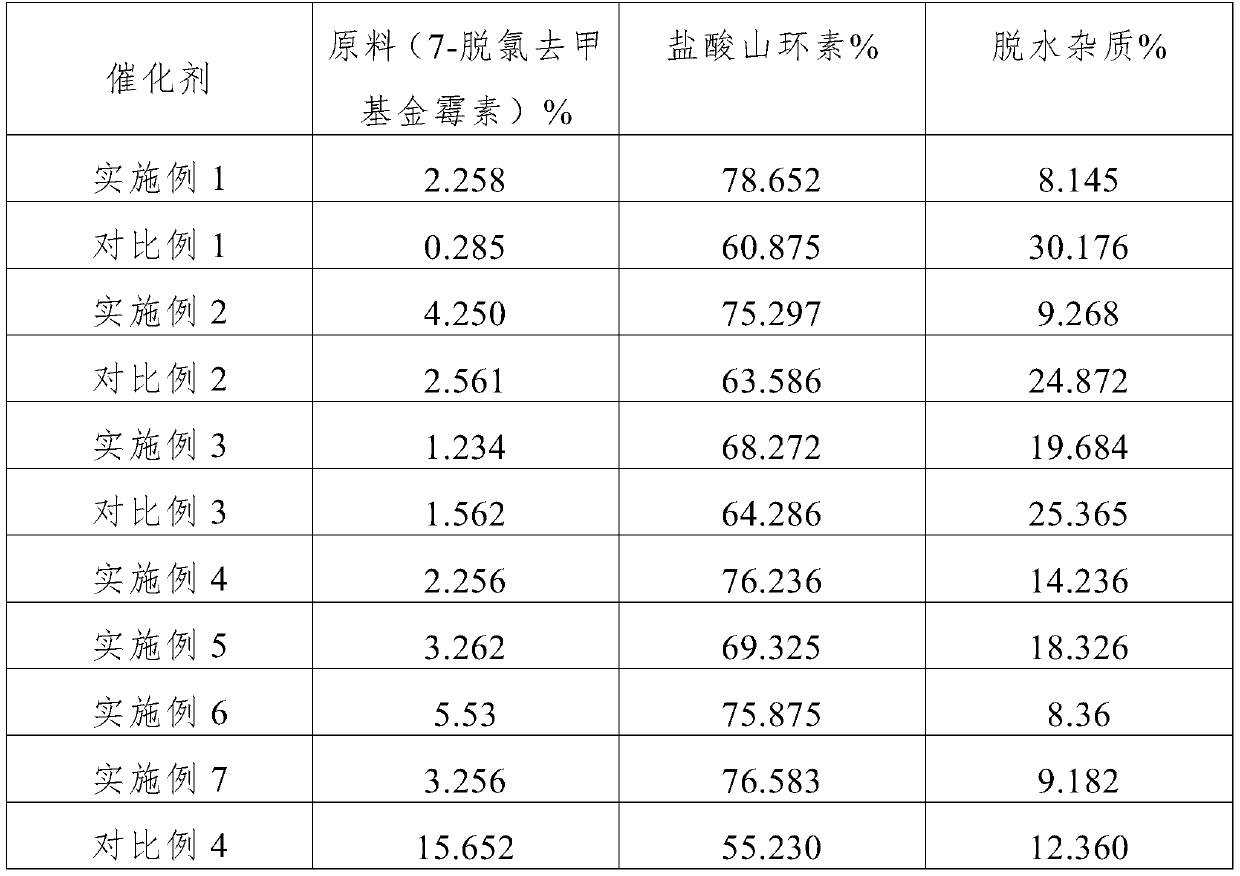
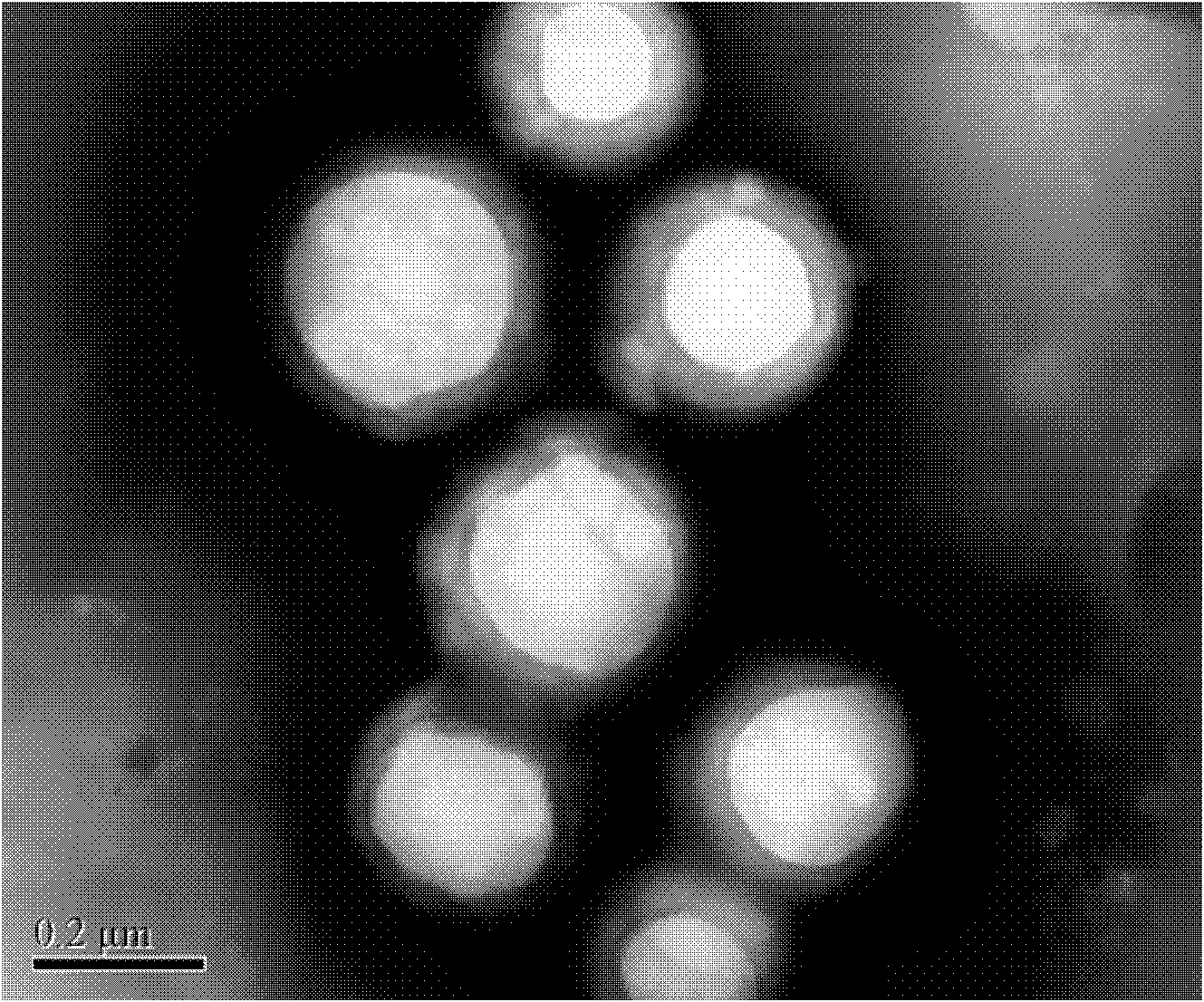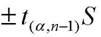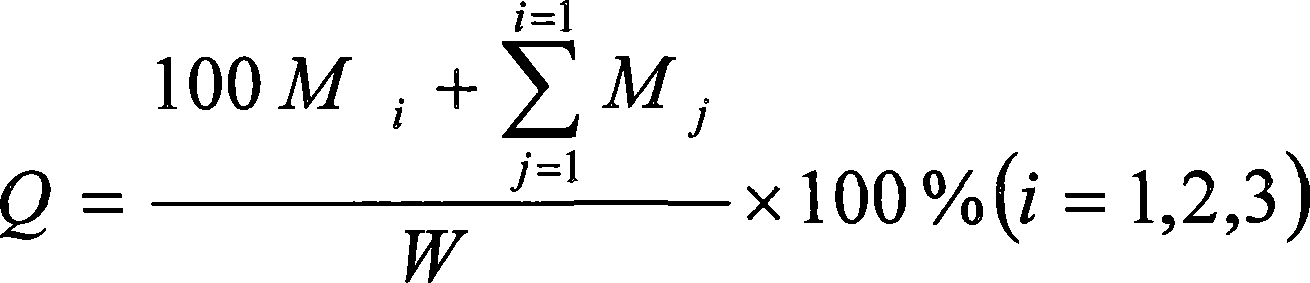Patents
Literature
53 results about "Minocycline Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrochloride salt of minocycline, a broad spectrum long-acting derivative of the antibiotic tetracycline, with antibacterial and anti-inflammatory activities. Minocycline binds to the bacterial 30S ribosomal subunit and interferes with the binding of tRNA to the ribosomal complex, thereby inhibiting protein translation in bacteria. In addition, minocycline inhibits the inflammatory enzyme 5-lipoxygenase (5LOX) and may impede T cell-microglia interactions; both activities may contribute to minocycline's neuroprotective effects. 5LOX catalyzes the synthesis of inflammatory mediators such as prostaglandins and leukotrienes.
Remedy for sarcoidosis and method of treating the same
InactiveUS20070111956A1Formation was suppressedAntibacterial agentsBiocideGranulomatous diseaseActive component
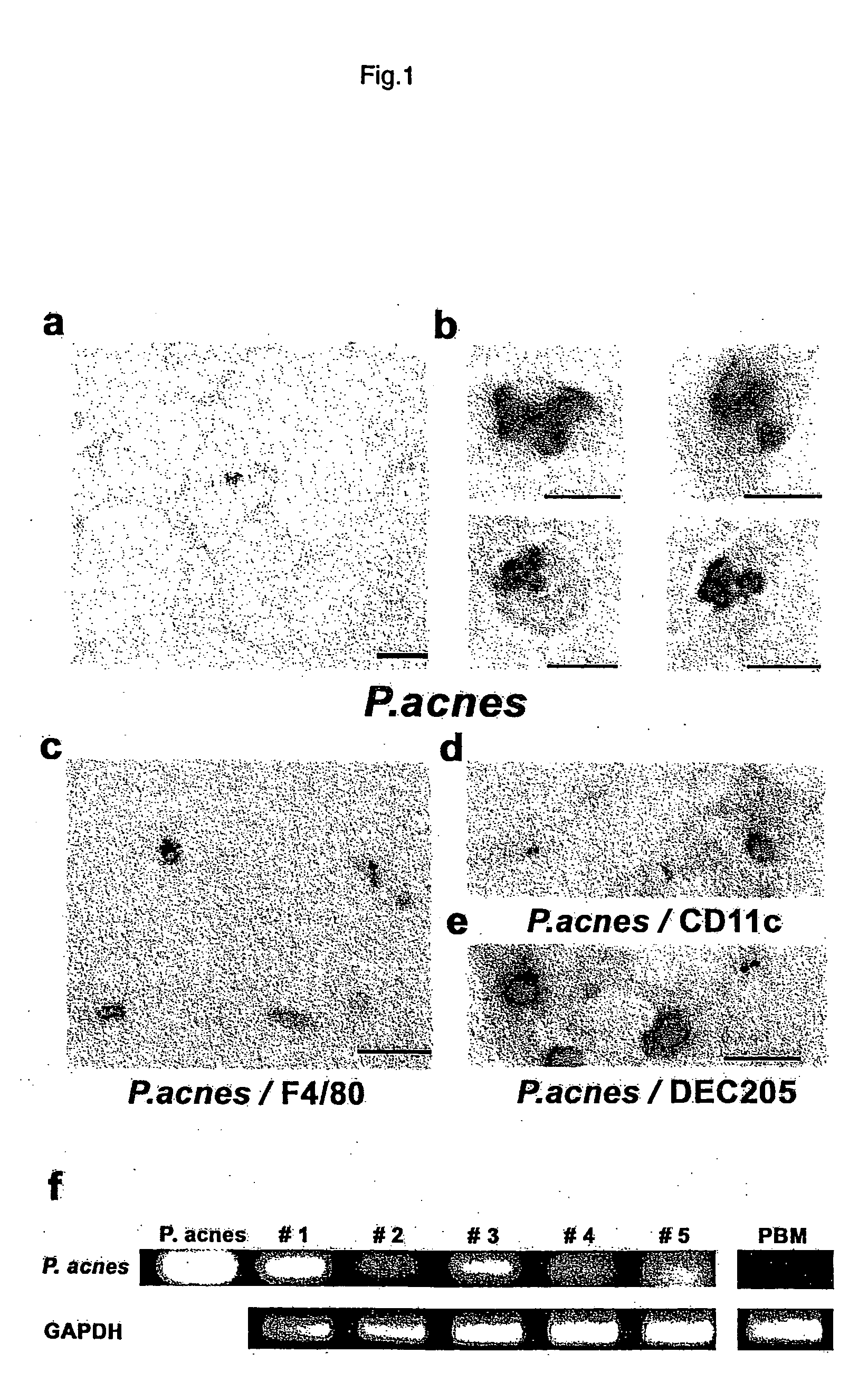
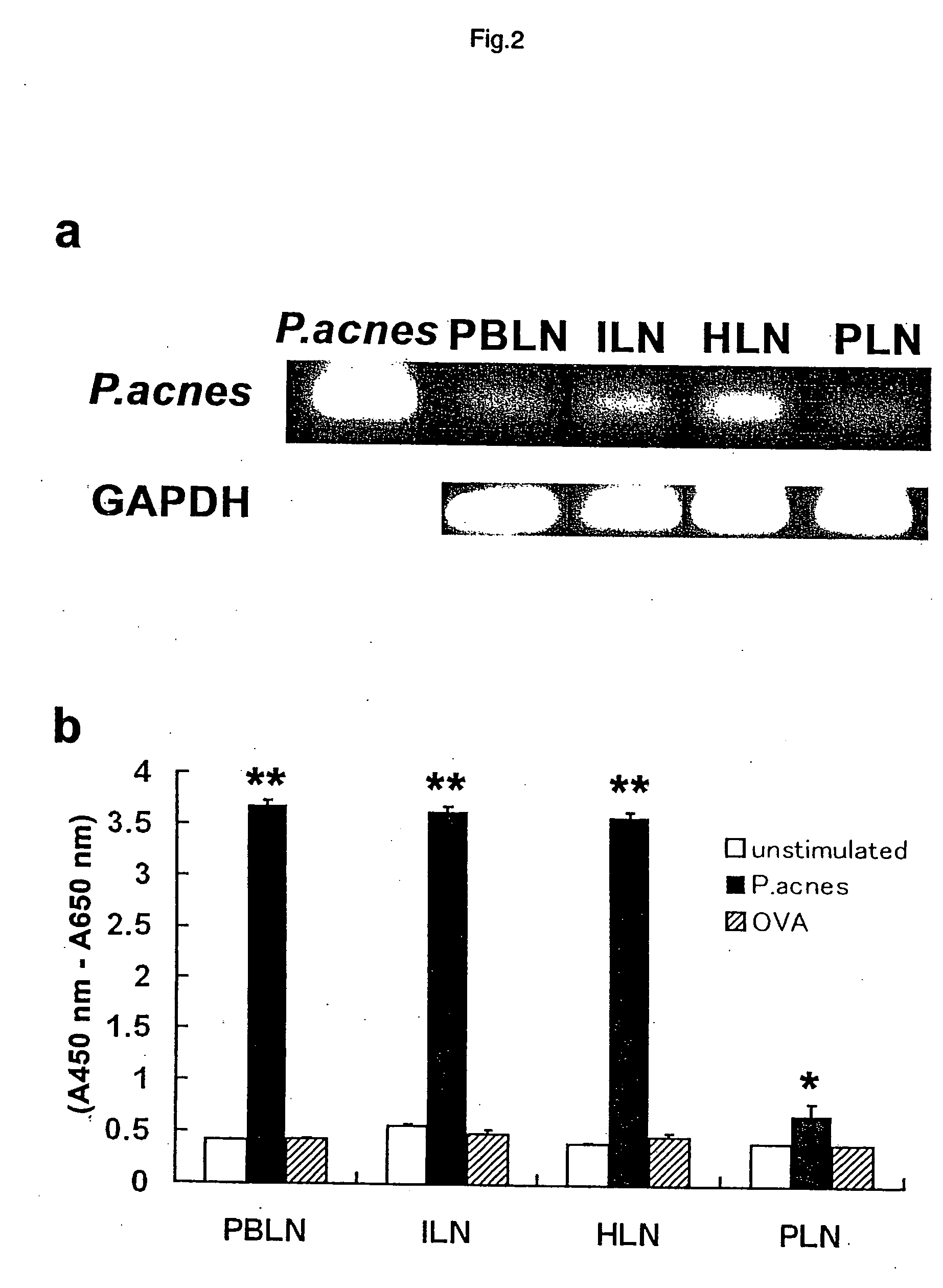
The present invention provides a remedy for sarcoidosis, one of systemic granulomatous diseases, and a method for treating sarcoidosis. A remedy for sarcoidosis containing a Propionibacterium acnes-targeting antibiotic such as minocycline hydrochloride and clindamycin as an active component is prepared. Further, sarcoidosis is treated by administering this remedy for sarcoidosis to sarcoidosis patients.
Owner:JAPAN SCI & TECH CORP
Freeze-dried minocycline hydrochloride powder injection and its preparing process
A freeze dried minocycline hydrochloride powder as antibacterial injection is prepared from minocycline hydrochloride (0.05-10 wt.portions), freeze dried power supporting agent (10-100) and pH regulator. Its advantages are broad antibacterial sprectrum, high stability to light, heat, oxygen and water, and no pollution.
Owner:于航
Minocycline hydrochloride microballoons and preparation method and application in pharmacy thereof
InactiveCN101288673AAvoid side effectsReduce the burden onTetracycline active ingredientsDigestive systemSide effectMicrosphere
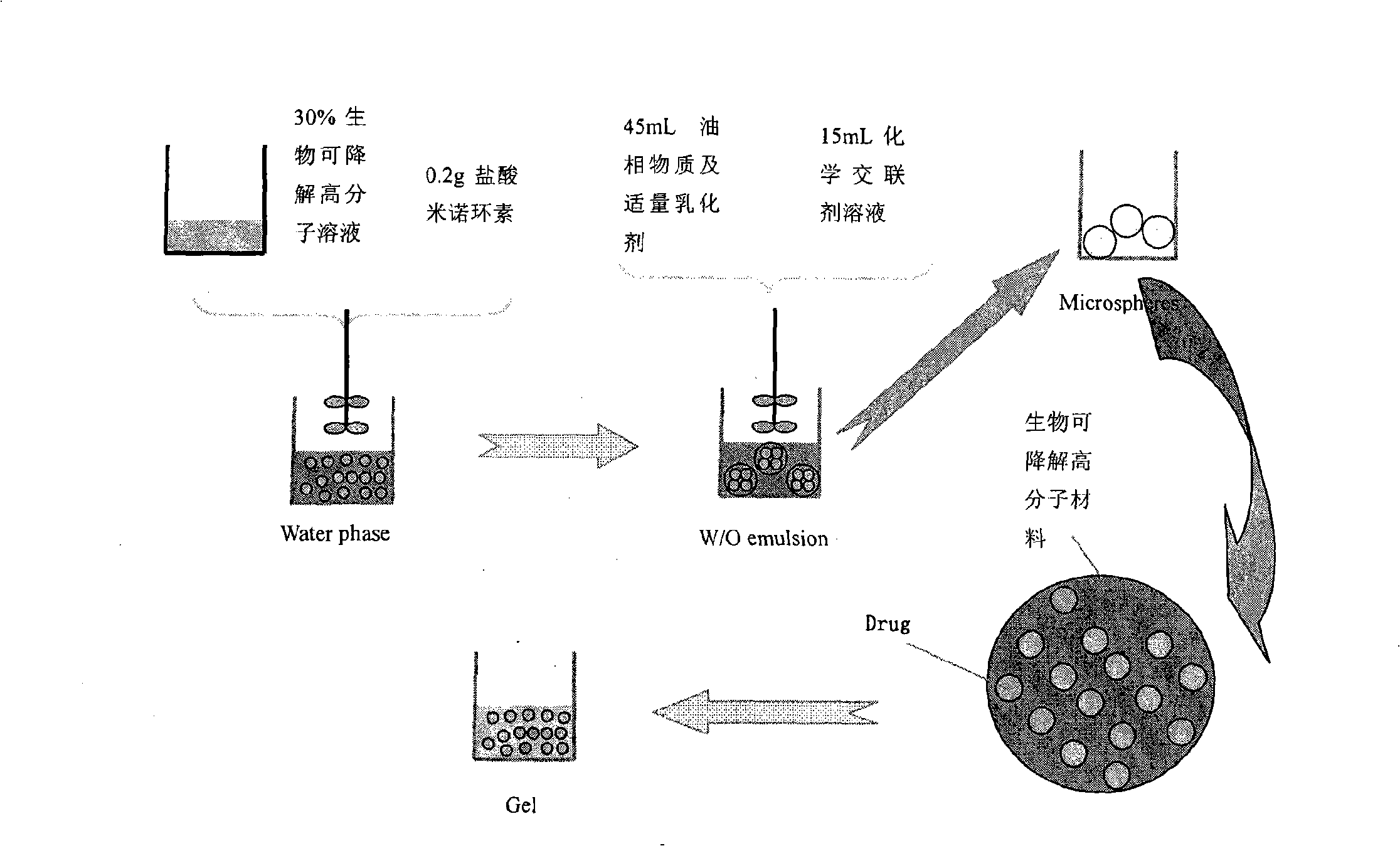
The invention relates to a minocycline hydrochloride microsphere, a preparation method thereof and the application thereof in medicine preparation; the invention is technically characterized in that the components comprise minocycline hydrochloride drug, biodegradable polymer material, emulsifier and chemical cross-linking reagent; the weight percentage of the components is 10 percent to 20 percent (W / W) of minocycline hydrochloride, 15 percent to 40 percent (W / W) of biodegradable polymer material; the ratio between biodegradable polymer material and chemical cross-linking reagent is 1g : 5mL to 1g : 20mL; the volume ratio between aqueous phase and fat phase is 1 : 10 to 1 : 20 (V / V). The minocycline hydrochloride microsphere and the related preparation thereof (such as jellies) prepared by the invention not only has the remarkable advantages of avoiding the toxic and side effects resulted from systemic antibiotics use, being able to reach the depth of periodontal pocket, sustained-release of the medicine at the bottom (one dose can result in continuous release for 7 days), etc., but also greatly reduces the marketing price owing to the cost reduction resulted from totally using home-made excipients, which is beneficial for reducing the patient burden.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Preparation method of tissue suitable type composite material dental implant
InactiveCN103611188AImplant healing period is shortSolve for bond strengthSurface reaction electrolytic coatingProsthesisAcid etchingTitanium alloy
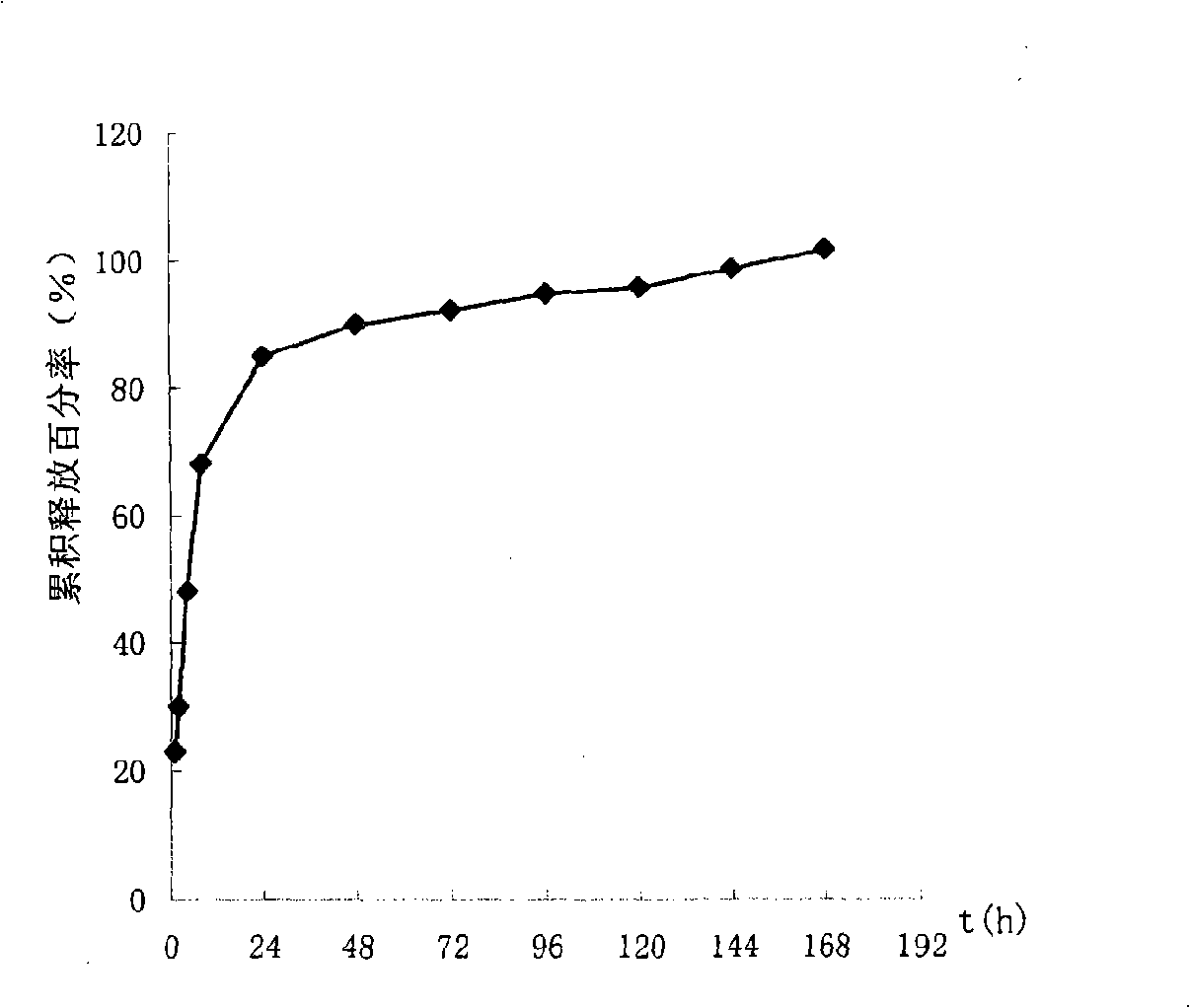
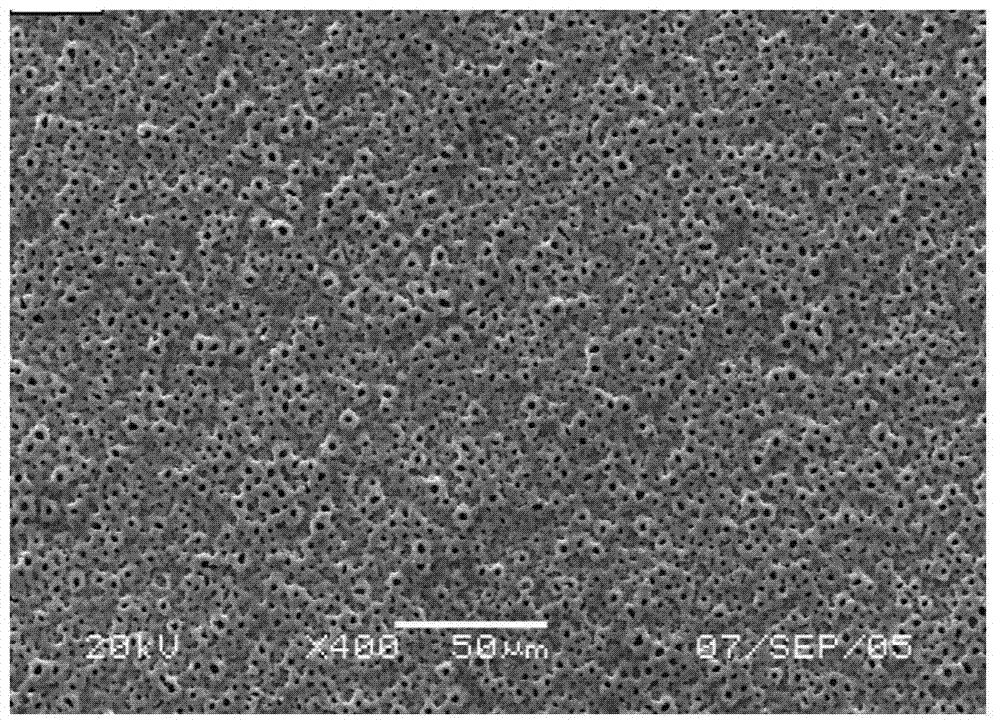
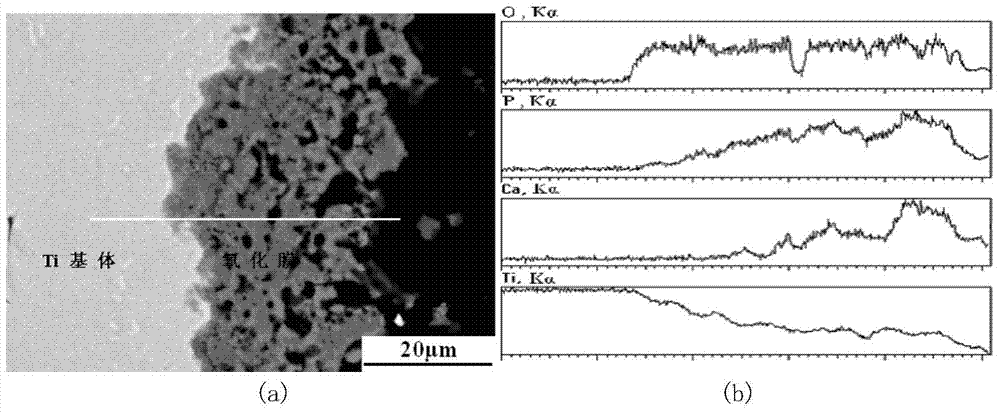
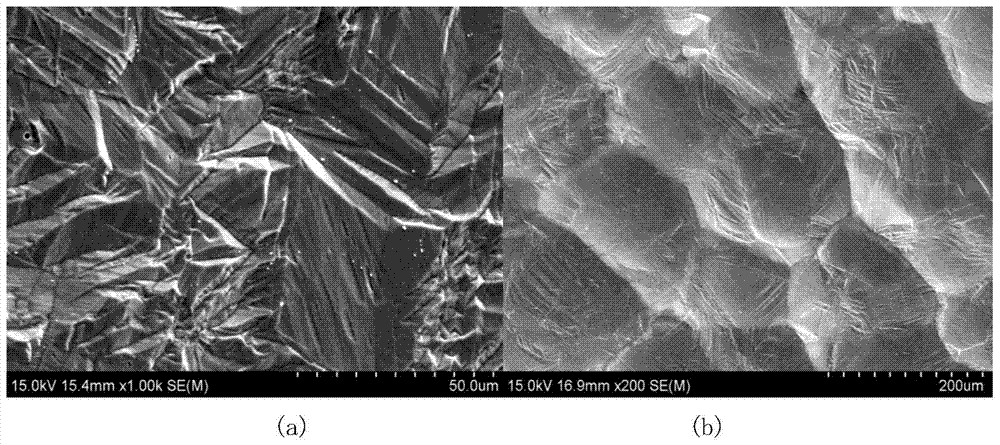
The invention relates to a preparation method of a tissue suitable type composite material dental implant. The preparation method comprises the steps of performing micro-arc oxidation treatment on a surface part, in contact with a bone tissue, of a titanium or titanium alloy implant to generate a layer of porous composite ceramic membrane which contains calcium phosphate and has biological activity; performing surface etching treatment on a surface part, in contact with a soft tissue, of the implant in a mixed acid etching agent consisting of hydrofluoric acid and hydrochloric acid; soaking the dental implant subjected to the micro-arc oxidation and surface etching treatment into composite simulated body fluid containing minocycline hydrochloride and bone morphogenetic protein to ensure that an anti-inflammatory drug namely minocycline hydrochloride and the bone morphogenetic protein are simultaneously loaded in surface holes of the implant. The bonding strength between the ceramic membrane on the surface of the implant and a titanium or titanium alloy matrix is improved to 20-30MPa from 10-20Mpa; the bone interface maturation period is shortened to be within 3 months from 3-6 months of the current titanium dental implant; the biological properties of the composite material dental implant accord with relevant regulations of ISO (International Standardization Organization), Chinese Pharmacopoeia and United States Pharmacopoeia.
Owner:SHANDONG UNIV
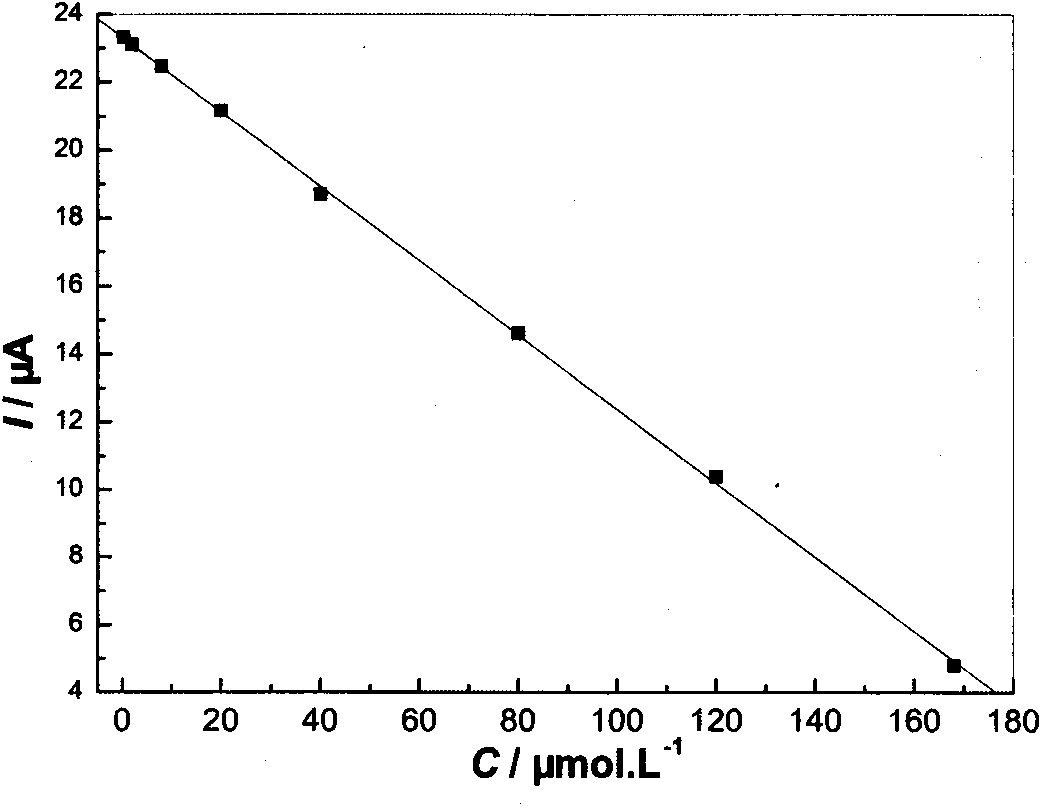
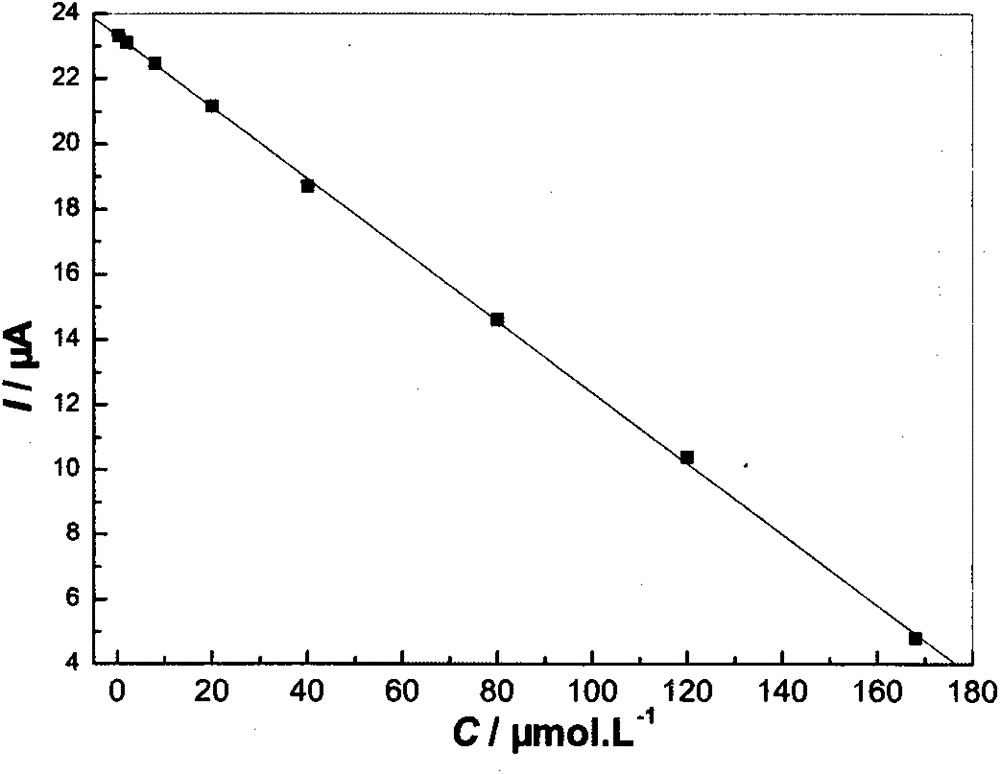
Nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity and preparation method thereof
InactiveCN103926287AEasy to manufactureMaterial electrochemical variablesCross-linkFunctional monomer
The invention discloses a nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity and a preparation method of the nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor. The minocycline hydrochloride is taken as a template molecule, 20(s)-O-3beta-acetoxyl-5-androstene-17beta-acyl camptothecin is taken as a functional monomer, azodiisobutyronitrile is taken as an initiator, nano cobaltous oxide is taken as a dopant, and maleic rosin ethylene glycol acrylate synthesized from rosin as a raw material is taken as a cross-linking agent, so as to prepare the nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity. The analysis method is simple and practical, and the defects that the traditional analysis method is complicated, expensive in equipment, and low in sensitivity are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Composite drug carried microsphere, minocycline hydrochloride nano controlled-release composite drug carried microsphere system and preparation method thereof
InactiveCN101836961ALow toxicityGood slow releaseAntibacterial agentsTetracycline active ingredientsMicrosphereCholesterol
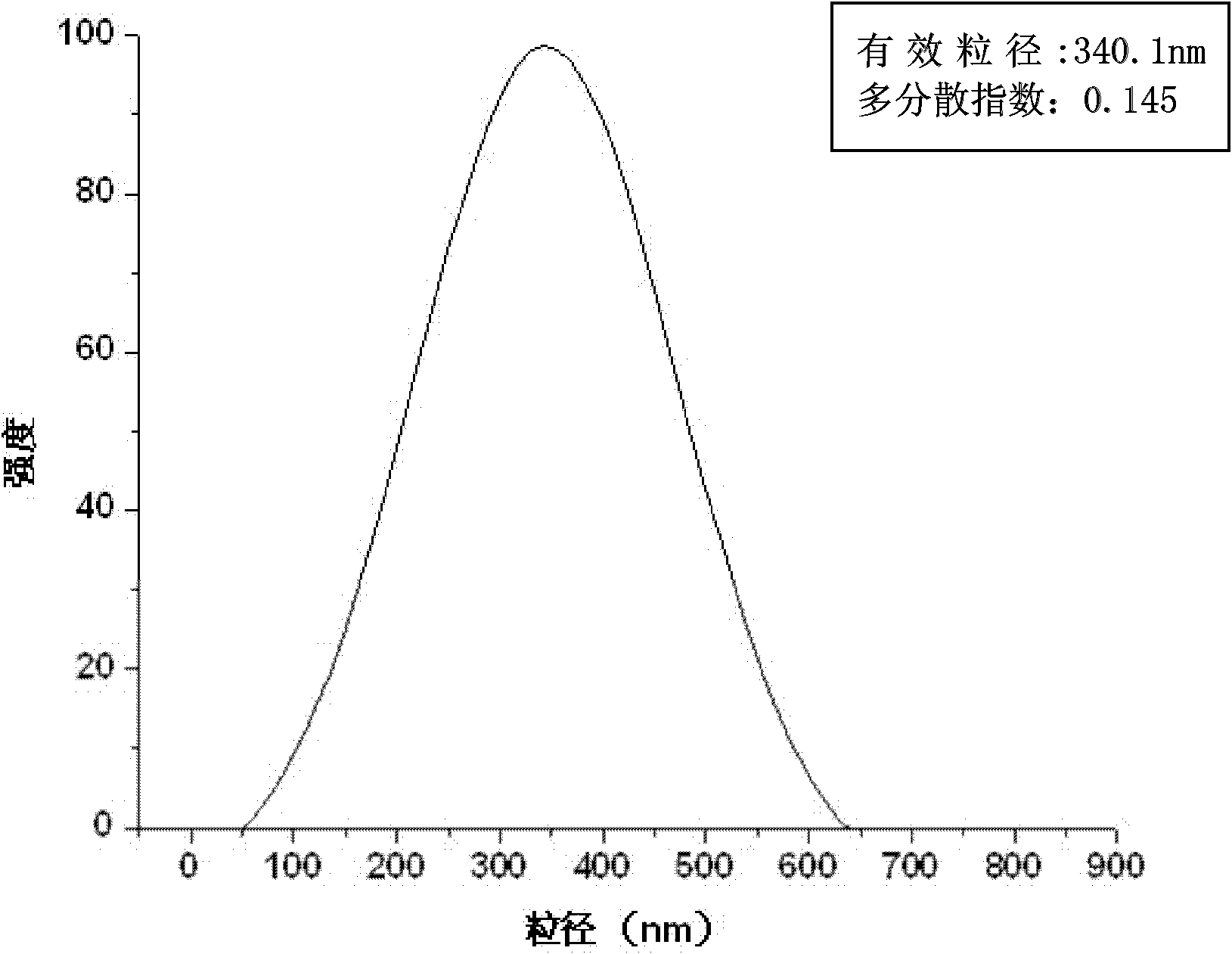
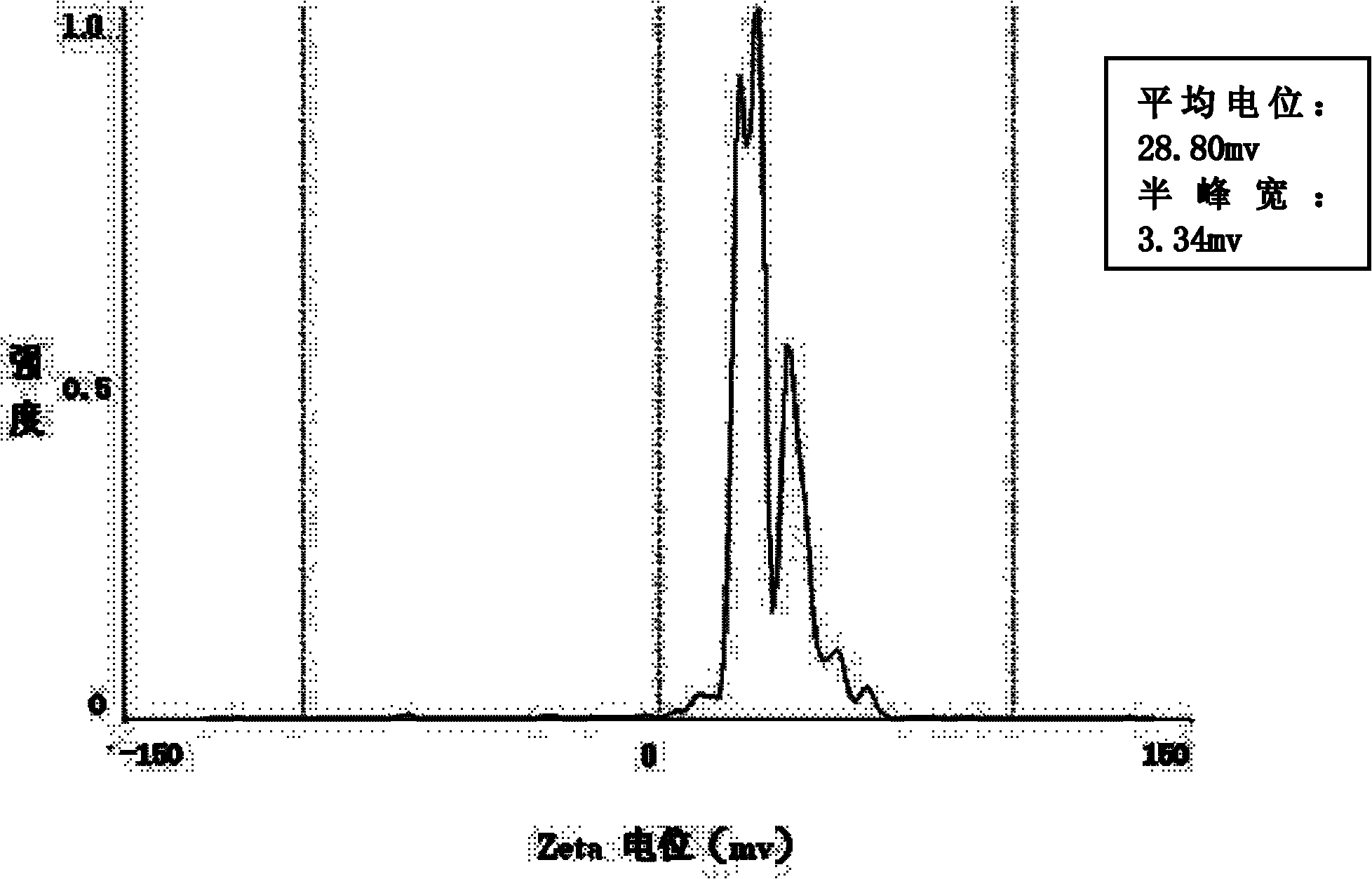
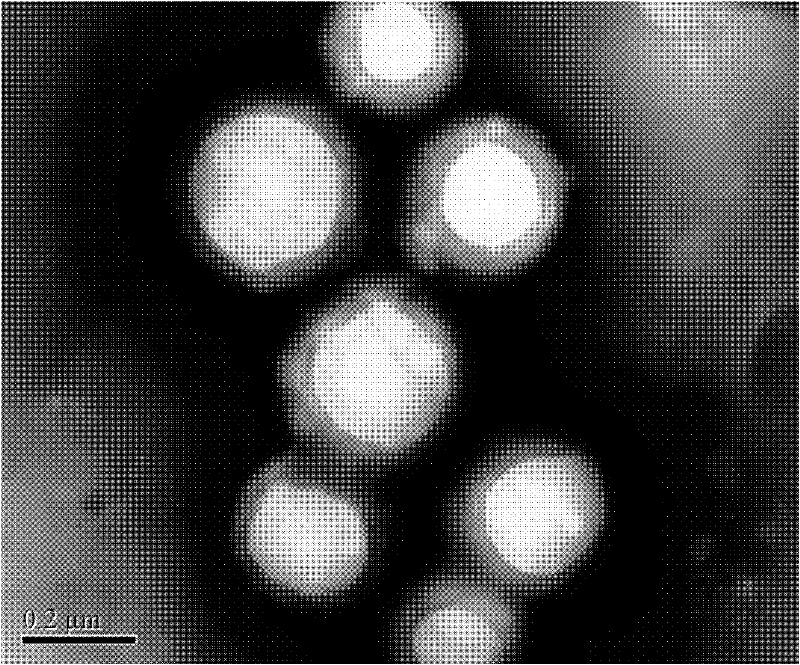
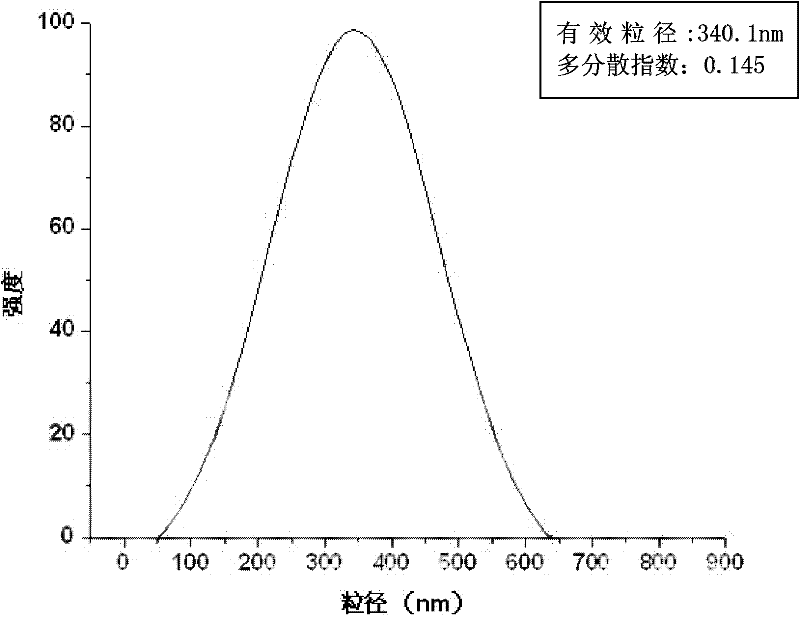
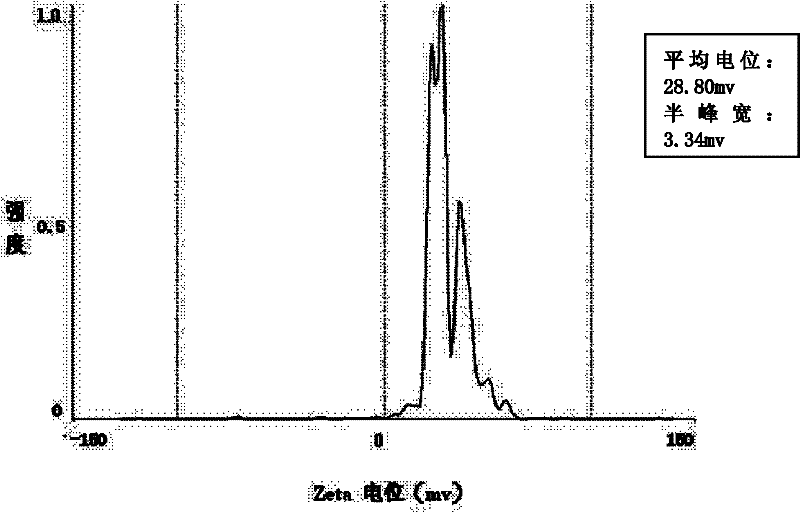
The invention relates to a composite drug carried microsphere, a minocycline hydrochloride nano controlled-release composite drug carried microsphere system and a preparation method thereof. A drug carried system with a nuclear shell structure is formed by embedding minocycline hydrochloride inside a poly D,L-lactide-co-glycolic acid polymer microsphere and covering a cationic polymeric liposome prepared from O-QACMC modified by polyethylene glycol, O-QACMC and cholesterol outside the poly D, L-lactide-co-glycolic acid polymer microsphere; and the composite drug carried microsphere system covered and carried with the minocycline hydrochloride has the grain diameter ranging from 340 nm to 400 nm and positive surface Zeta electric potential. The composite drug carried microsphere system can be remained in a water solution for at least 2 months, has high entrapment rate reaching larger than 90 percent on drugs and strong drug carrying capacity reaching 9 percent. The minocycline hydrochloride nano controlled-release composite drug carried microsphere system has the characteristics of uniform and controllable grain diameter, good preparation stability, simple preparation process, high drug carrying rate, favorable controlled release function, and the like, and is suitable for batch production.
Owner:TIANJIN UNIV
Minocycline hydrochloride sustained release tablet and preparation method thereof
InactiveCN102772384AReduce rateReduce doseTetracycline active ingredientsPharmaceutical non-active ingredientsSustained Release TabletMedicine
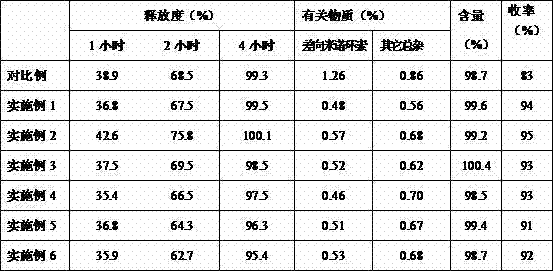
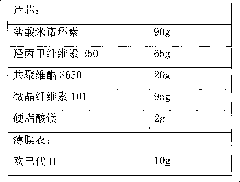
The invention discloses a minocycline hydrochloride sustained release tablet which is prepared from the following components in parts by weight: 40-90 parts of hydrochloride sustained release tablet, 80-120 parts of hydroxypropyl methylcellulose, 200-260 parts of lactose, 3-8 parts of silicon dioxide, 2-6 parts of magnesium stearate and 4-10 parts of coating materials. The invention also discloses a preparation method of the sustained release tablet. According to the minocycline hydrochloride sustained release tablet disclosed by the invention, the known unhealthy acute vestibule events caused by minocyline can be reduced, the tablet is released stably; and the preparation method has the advantages that the concept is ingenious, the flow is simple, the process is stable, the operation is simple and convenient, the production period is short, the product yield and the product stability are increased, and the production cost is lowered.
Owner:SICHUAN BAILI PHARM CO LTD
Minocycline hydrochloride sustained release tablet and preparation method thereof
InactiveCN101822650AReduce peak and valley fluctuationsEffectively control adverse reactionsAntibacterial agentsTetracycline active ingredientsSustained Release TabletBlood concentration
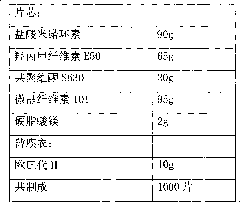
The invention relates to a minocycline hydrochloride sustained release tablet and a preparation method thereof. In the technical scheme, the minocycline hydrochloride sustained release tablet is composed of a tablet core and a film coat, the tablet core is composed of minocycline hydrochloride, a sustained release material, a bonding agent, a filling agent and a lubricating agent, the film coat is composed of a film forming material, in the preparation method, the granule is manufactured by means of the high shear wet method and obtained through the drying of a boiling bed, the temperature of the material dried by a fluidized bed is controlled to be 25-35 DEG C, and the moisture content of the granule is controlled to be 2.0-4.0 percent. The invention belongs to the technical field of pharmaceutical preparation, which aims to provide an available preparation process for the minocycline hydrochloride sustained release tablet and greatly reduce the relevant materials introduced druing the preparation by controlling the key parameters in the preparation process. The preparation method of the minocycline hydrochloride sustained release tablet and the method for controlling of the relevant materials aim to develop a sustained release formulation of minocycline hydrochloride, and various specifications can be issued based on the body weight of a patient, thereby reducing the peak valley fluctuation of the blood concentration, effectively controlling the adverse drug reaction, and improving the drug bioavailability.
Owner:HUZHOU R & D CENT FOR NUTRITION & HEALTH SHANGHAI INST FOR BIOLOGICAL SCI CHINESE ACADEMY OF SCI +1
Method for preparing standard substance for detecting antibiotics in cosmetics
ActiveCN101904806AAccurate detectionCosmetic preparationsToilet preparationsCosmetic creamChlortetracycline Hydrochloride
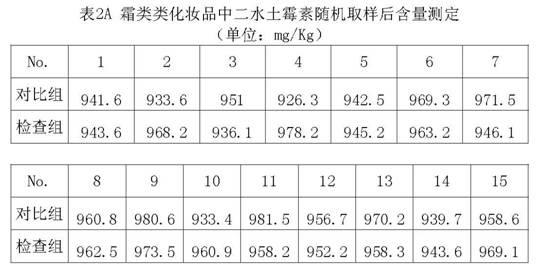
The invention relates to a method for preparing a standard substance for detecting antibiotics in cosmetics. The standard substance is formed by mixing aqueous solution of the antibiotics or solution of propylene glycol and cosmetic cream, wherein the cosmetic cream comprises the following components in percentage by weight: 73 to 78 percent of de-ionized water, 3 to 3.5 percent of propylene glycol and 0.2 to 0.25 percent of carbomer in phase A, 4.5 to 5 percent of stearate, 3 to 4 percent of cetyl alcohol and stearyl alcohol and 10 to 14 percent of 26# white oil in phase B and 1.2 to 1.25 percent of phenoxyethanol and ethylhexylglycerin in phase C; and the concentration of the antibiotics in the standard substance reaches 0.1g / 100mL. The method has the advantages that: the antibiotics and the cosmetic cream are mixed to form the standard substance for detecting the antibiotics such as minocycline hydrochloride, oxytetracycline dehydrate, tetracycline hydrochloride, chlortetracycline hydrochloride, doxycycline hydrochloride and chloramphenicol in the cosmetic; and the standard substance is the same as a sample of the cosmetic containing the antibiotics in the actual use; thus the detection of the antibiotics in the cosmetic conforms to the state of the actual application better and is more accurate.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH +1
Treatment for reactive arthritis or bursitis
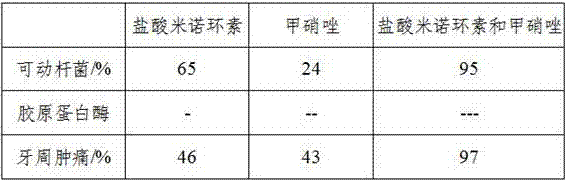
A treatment for conditions in human beings associated with either or both reactive arthritis or bursitis comprising a combination of valacyclovir hydrochloride, minocycline hydrochloride, and metronidazole.
Owner:BONNER ERNEST L JR +1
Preparation prepared from active fractions of baikal skullcap root and fortune's drynaria rhizome for treating periodontitis
ActiveCN103893261ASmall prescriptionEasy to prepareOrganic active ingredientsAntipyreticNaringinSide effect
Owner:XI AN JIAOTONG UNIV
Controlled ointment compound stroma and the preparing method
The invention relates to a method for preparing control-release paste medium, comprising cyclodextrin, drug, macromolecule and elasticizer. The invention uses package and macromolecule mixing technique to prepare the control-release paste medium with needed rigidity and rheological property. The invention is characterized in that it uses insoluble cellulose ethyl ether and low-permeability acrylic resin mixture medium as the skeleton control-release material, and uses cyclodextrin and ealsticizer to adjust the rheological property and drug release speed of the paste, uses glycerin and drug grind method to pack the drug. Via external release degree test, the paste which contains Minocycline Hydrochloride, polycyclic acid or the like, via slurry method (at 37Deg. C, 100rpm) can reach 20-40% in 1h, 40-60% in 3h, 50-80% in 7h, and 80-90% in 12h. The inventive paste can be used to treat mouth and vaginal diseases.
Owner:北京恩泽美科技有限公司
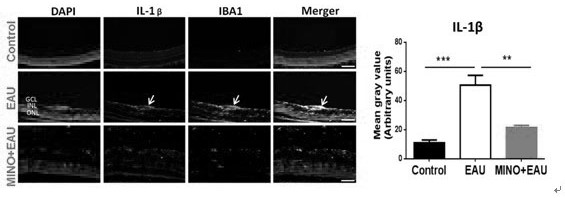
Application of minocycline hydrochloride to preparation of medicine for treating autoimmune uveitis and treatment method of autoimmune uveitis
InactiveCN111870607AInhibitionInhibition of developmentSenses disorderTetracycline active ingredientsMicroglial cell activationCellular infiltration
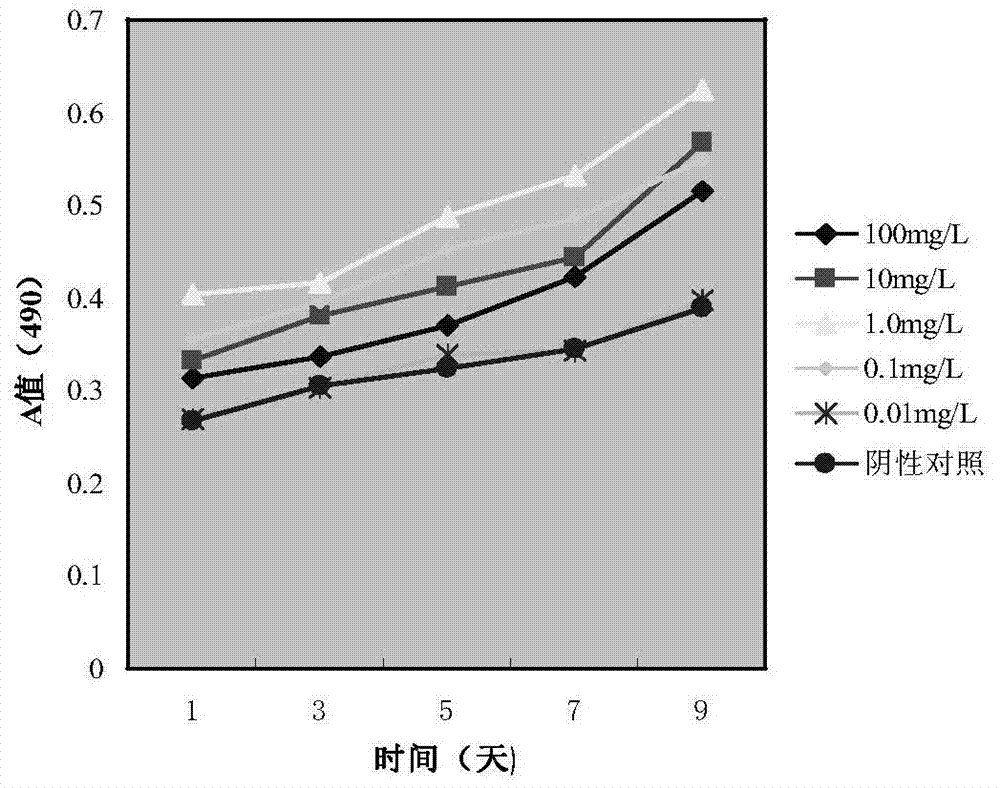
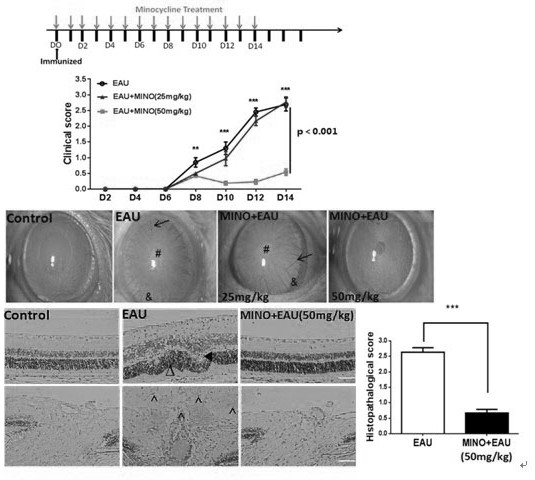
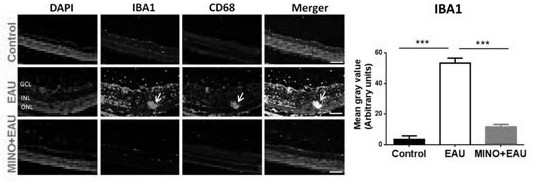
The invention discloses an application of minocycline hydrochloride to preparation of a medicine for treating autoimmune uveitis and a treatment method of the autoimmune uveitis. The inventor finds that the minocycline hydrochloride can be used as an early intervention medicine by exploring the treatment effect of minocycline with different administration dosages on the autoimmune uveitis. By inhibiting microglial cell activation of retina tissue, reducing immune cell infiltration and reconstructing a steady state in gastrointestinal tract flora, generation and development of the uveitis are effectively inhibited, a new application is provided for application of the minocycline hydrochloride, and besides, a new strategy and method are provided for early prevention and intervention of the autoimmune uveitis.
Owner:WENZHOU MEDICAL UNIV
Preparation method of high-purity tigecycline
InactiveCN103044281AReduce dosageHydrogenation pressure is reasonableOrganic compound preparationCarboxylic acid amides preparationNausea sicknessTigecycline
The invention relates to a preparation method of high-purity tigecycline, which comprises the steps of taking minocycline hydrochloride as an initial raw material, and conducting nitration, hydrogenation, acid conversion, amidation and purification. According to the preparation method, the hydrogenation pressure is reasonable; time used for a purification technology is shorter; the use amount of a solvent is reduced by more than 30%; when the prepared high-purity tigecycline with the purity of over 99.5% is used for a complicated skin and skin texture infected patient, compared with the existing tigecycline, the incidences of nausea and vomit are decreased to 15.1% and 10.2% respectively; and when tigecycline is used for treating a complicated intra-abdominal infected patient, the incidences of the nausea and the vomit are decreased to 15.4% and 11.3% respectively.
Owner:HUNAN SAILONG PHARMA
Composite drug carried microsphere, minocycline hydrochloride nano controlled-release composite drug carried microsphere system and preparation method thereof
InactiveCN101836961BLow toxicityGood slow releaseAntibacterial agentsTetracycline active ingredientsMicrosphereCholesterol
The invention relates to a composite drug carried microsphere, a minocycline hydrochloride nano controlled-release composite drug carried microsphere system and a preparation method thereof. A drug carried system with a nuclear shell structure is formed by embedding minocycline hydrochloride inside a poly D,L-lactide-co-glycolic acid polymer microsphere and covering a cationic polymeric liposome prepared from O-QACMC modified by polyethylene glycol, O-QACMC and cholesterol outside the poly D, L-lactide-co-glycolic acid polymer microsphere; and the composite drug carried microsphere system covered and carried with the minocycline hydrochloride has the grain diameter ranging from 340 nm to 400 nm and positive surface Zeta electric potential. The composite drug carried microsphere system canbe remained in a water solution for at least 2 months, has high entrapment rate reaching larger than 90 percent on drugs and strong drug carrying capacity reaching 9 percent. The minocycline hydrochloride nano controlled-release composite drug carried microsphere system has the characteristics of uniform and controllable grain diameter, good preparation stability, simple preparation process, highdrug carrying rate, favorable controlled release function, and the like, and is suitable for batch production.
Owner:TIANJIN UNIV
Anti-infection and anti-coagulation coating for central venous catheter and preparation method of anti-infection and anti-coagulation coating
InactiveCN111870743AImprove smoothnessReduce coefficient of frictionPharmaceutical containersMedical packagingPyrrolidinonesSurgery
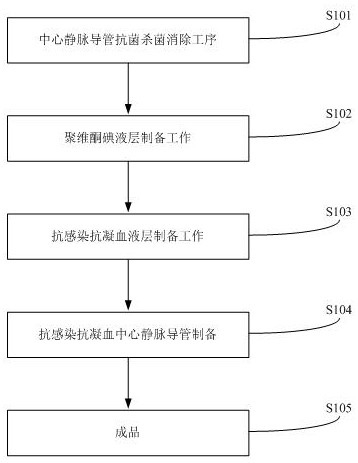
The invention provides an anti-infection and anti-coagulation coating for a central venous catheter. The anti-infection and anti-coagulation coating comprises the following raw materials in parts by weight: 0.1-1% of polyvinylpyrrolidone, 0.2-2% of minocycline hydrochloride, 0.7-1% of a KH560 silane coupling agent, 2-3% of povidone iodine and 90-93% of medical disinfectant fluid. The preparation method of the anti-infection and anti-coagulation coating for the central venous catheter comprises the following steps: a central venous catheter antibacterial sterilization elimination procedure, povidone iodine liquid layer preparation work, anti-infection and anti-coagulation blood layer preparation work, anti-infection and anti-coagulation central venous catheter preparation and finished product obtaining. The anti-infection and anti-coagulation coating is cured to the surface of the central venous catheter at high temperature, dip-coating processing is conducted in a multiple dip-coatingmode, the smoothness of the central venous catheter can be improved, the friction coefficient of the central venous catheter is reduced, and convenience of medical use and operation is guaranteed; andthe process is simple, the used raw materials are easy to obtain, the medical effect is obvious, the antibacterial and anti-infection rate is high, medical accidents can be reduced, and the medical operation safety is guaranteed.
Owner:SHANDONG ZHUSHI PHARMA GRP CO LTD
Minocycline hydrochloride nano calcium carbonate preparation and preparation method thereof
ActiveCN102525938AParticle size controllableUniform particle sizeAntibacterial agentsPowder deliveryEmulsionChloride
The invention relates to a minocycline hydrochloride nano calcium carbonate preparation and a preparation method thereof. The preparation method comprises the following steps: adding minocycline hydrochloride into calcium chloride aqueous solution, wherein the molar ratio of the calcium chloride to the minocycline hydrochloride is 5:1-1:1; after mixture is uniform by ultrasonic dispersion, addingthe calcium chloride aqueous solution containing medicine into an organic phase, wherein the volume ratio of the calcium chloride aqueous solution to the organic phase is 1:50-1:200; carrying out ultrasonic uniformity to form emulsion A; adding ammonium carbonate aqueous solution which has the same volume with the calcium chloride aqueous solution containing medicine in step 2 into the organic phase, and carrying out ultrasonic uniformity to form emulsion B; under the magneton stirring condition, slowly adding the emulsion B into the emulsion A; after adding, stirring for 0.5-2h; adding mixedsolution into ethanol for demulsification; then, carrying out centrifugal washing; and finally, naturally drying a sample. The preparation method has the advantages of simple and quick preparation process, short preparation period and high yield. The particle diameter of the prepared minocycline hydrochloride nano calcium carbonate is controllable within 40-150 nanometers and is even, and the medicine carrying ratio is 20-40%.
Owner:天津渤化讯创科技有限公司
Preparation utilizing compatibility prescription of effective parts of rhizoma coptidis and rhizoma drynariae in treatment of periodontitis
ActiveCN104083466ASmall prescriptionEasy to prepareOrganic active ingredientsAntipyreticSide effectWhole body
The invention relates to a preparation utilizing a compatibility prescription of the effective parts of rhizoma coptidis and rhizoma drynariae in treatment of periodontitis. The preparation comprises the following components by mass: 10-15 parts of a rhizoma coptidis effective part's water extract with a berberine content of more than 80%; and a rhizoma drynariae effective part's water extract with a naringin content of more than 30.71%. The preparation can be prepared into a ''rhizoma coptidis-rhizoma drynariae tooth strengthening capsule'' for systemic administration, or can be added with hydroxyethyl cellulose, concentrated glycerol, polyacrylic resin and other auxiliary materials to make a novel topical Chinese medicinal long-acting compound ointment ''rhizoma coptidis-rhizoma drynariae tooth strengthening ointment'', and a syringe administration mode is easy for operation at local parts of a periodontal pocket. With a good therapeutical effect on periodontitis, the preparation provided by the invention has the characteristics of small prescription, significant curative effect, no toxic or side effect. Especially in local application, after long-term use, the anti-inflammatory effect is similar to that of minocycline hydrochloride ointment (Periocline), but the osteogenic effect is obviously superior to that of Periocline. Thus, the preparation is a novel oral Chinese patent medicine with long-term efficacy and usability similar to western medicines.
Owner:XI AN JIAOTONG UNIV
Drug-containing medical catheter coating and synthetic method and application thereof
The invention belongs to the field of drug-containing medical apparatus and instruments, and particularly relates to a drug-containing medical catheter coating and a synthetic method and application thereof. The coating comprises drugs and a drug carrier, drugs are rifampin and minocycline hydrochloride in the mass ratio of 1:1-3, the drug carrier is a polyurethane coating reagent, and the polyurethane coating reagent comprises, by mass, 50-54% of butanone, 25-30.6% of ethyl lactate, 10-12% of tetrahydrofuran, 2.4-2.8% of N-methyl pyrrolidone, 0.8-1% of polyurethane polyvinylpyrrolidone and 3-8% of isocyanate group polyurethane prepolymer. The coating has the biggest advantages that after the coatign is applied to medical catheters, friction coefficient of the catheters is lowered by 97%, bacteriostasis rate reaches 99%, sustained drug release effect reaches one month, and the coating has functions of hydrophilic lubrication, infection resistance, blood congestion resistance, sustained drug release and the like.
Owner:苏州乐术智慧医学科技有限公司
Tigecycline purification method
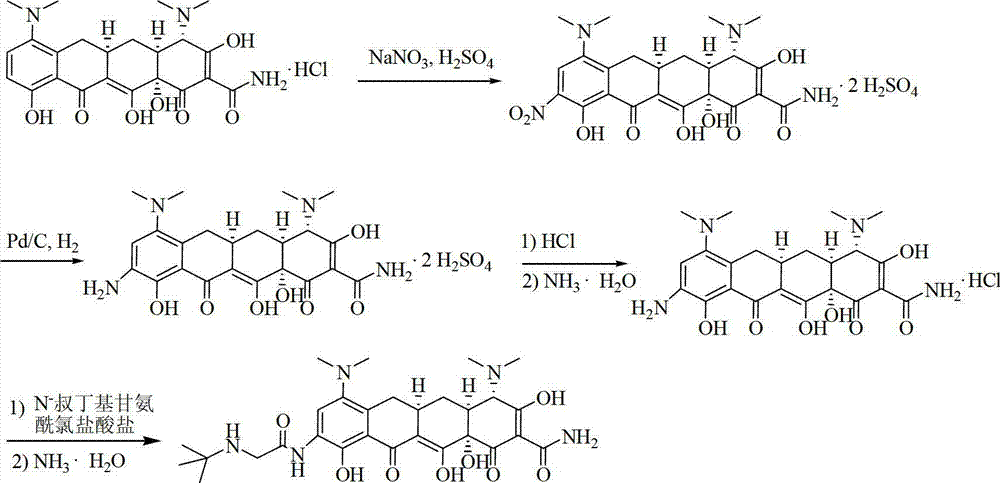
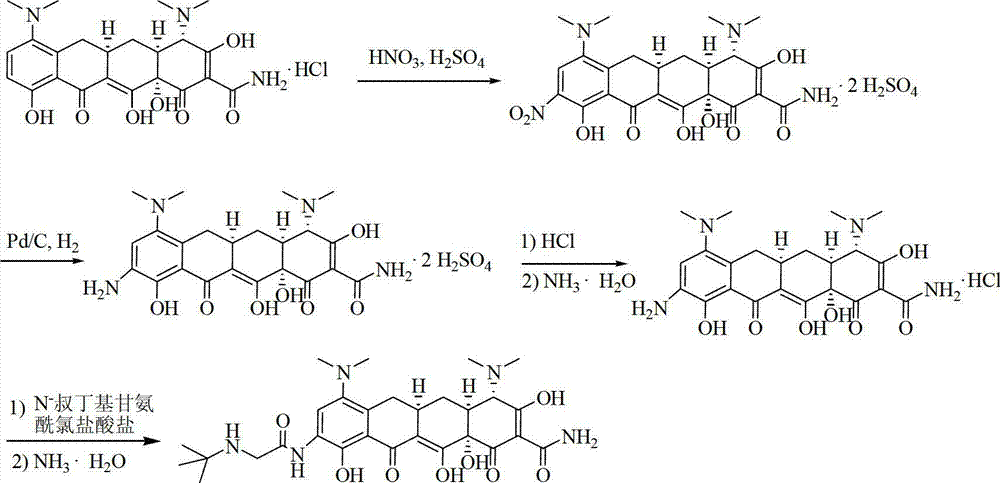
InactiveCN107118120AEasy to shapeReduce contentOrganic compound preparationOrganic chemistry methodsGlycinePurification methods
The invention discloses a tigecycline purification method in the technical field of pharmaceutical chemistry. The tigecycline purification method specifically comprises the following steps: S1. adding amino minocycline hydrochloride and tertbutyl-amino glycine chloride hydrochloride, and stirring for reacting; S2. after the reaction is complete, adding 14-20% ammonia water for regulating the pH to be 7.2; S3. combining an organic phase; S4. combining filtrate and washing liquor, and performing vacuum drying to obtain brown solid crude tigecycline; S5. filtering the crude tigecycline; and S6. crystalizing the filtered filter cakes with 2-butanol, and performing decompressed drying to obtain golden yellow solid refined tigecycline. The tigecycline purification method has the advantages of being high in yield, simple in technology and easy to control, the tigecycline meeting the quality standard can be obtained by simply concentrating, filtering and drying the washing liquor.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Oral liquid for treating heat toxin type periodontitis
InactiveCN107334847AGood treatment effectReduced activityDispersion deliveryTetracycline active ingredientsToxin typesTherapeutic effect
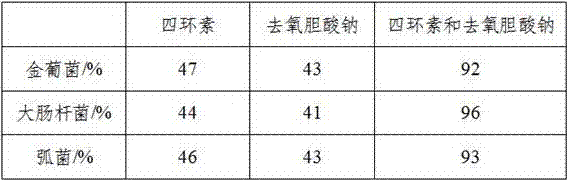
The invention relates to an oral liquid for treating pyretic periodontitis. The oral liquid is made of the following raw materials in parts by weight: 4-13 parts of sodium deoxycholate, 2-9 parts of tetracycline, and 5-11 parts of metronidazole powder 3-7 parts of minocycline hydrochloride, 6-10 parts of mountain bean root, 3-14 parts of nutmeg, 1-6 parts of Bupleurum, 1-4 parts of gypsum, 4-10 parts of tangerine peel, 3-3 catechu 12 parts, appropriate amount of water; the oral liquid prepared by the present invention makes up for the inconspicuous antibacterial effect and limited effect of treating periodontitis in the existing traditional Chinese medicine composition for treating periodontitis.
Owner:张桂英
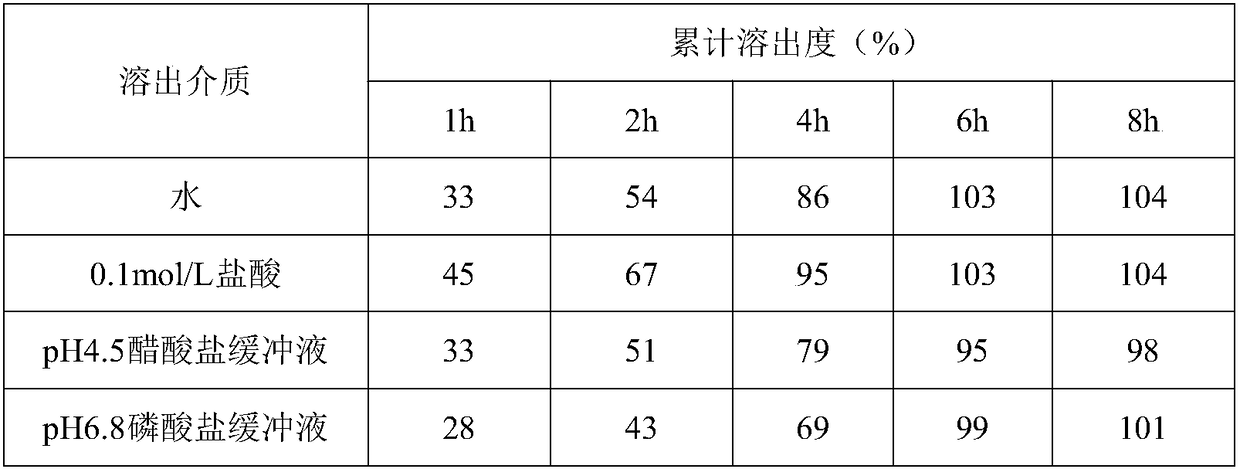
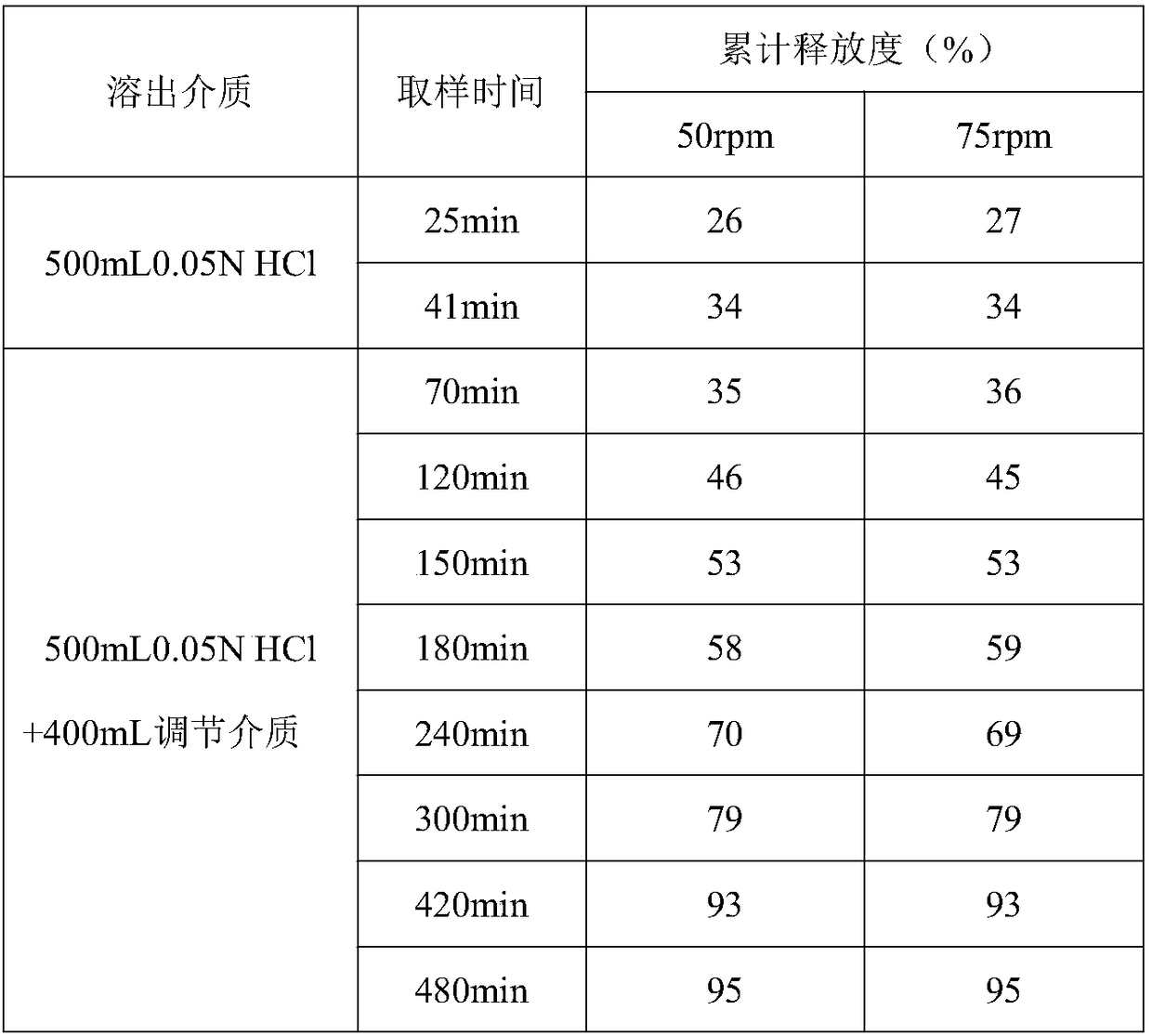
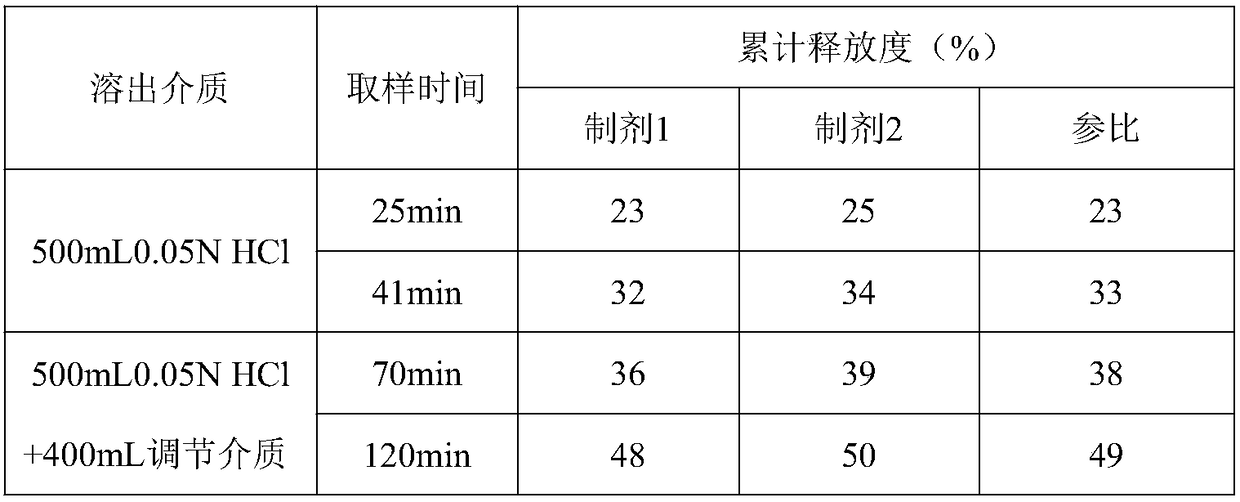
Method for in vitro dissolution of minocycline hydrochloride sustained-release tablet
The invention provides a method for in vitro dissolution of minocycline hydrochloride sustained-release tablets. The method for the vitro dissolution of the minocycline hydrochloride sustained-releasetablets includes the following steps of: (1) preparation of dissolution medium, wherein a solution 1 is HCl (hydrochloric acid) of 500 milliliters and 0.05 mol per liter, an adjusting solution is a mixed solution of trisodium phosphate dodecahydrate and anhydrous sodium acetate, and a solution 2 is the final solution of 900 milliliters mixed with 500 millititers and 0.05 mol per liter of HCl and400 milliliters of the adjusting solution; (2) preparation of a test solution; (3) preparation of a reference solution, which includes the steps of taking the minocycline hydrochloride sustained-release tablets as control substances to prepare to obtain the control solution with the final solution diluted and containing 12-17 micrograms of the minocycline hydrochloride sustained-release tablets per 1 milliliter; (4) determination, which includes the steps of taking the above control and test solution to respectively conduct absorbance measurement at a wavelength of 348 nanometres according toultraviolet spectrophotometry in two appendix IVA, Chinese pharmacopoeia 2015 edition, and calculating dissolution amount of the tablets according to the external standard method for determination.
Owner:浙江美华鼎昌医药科技有限公司
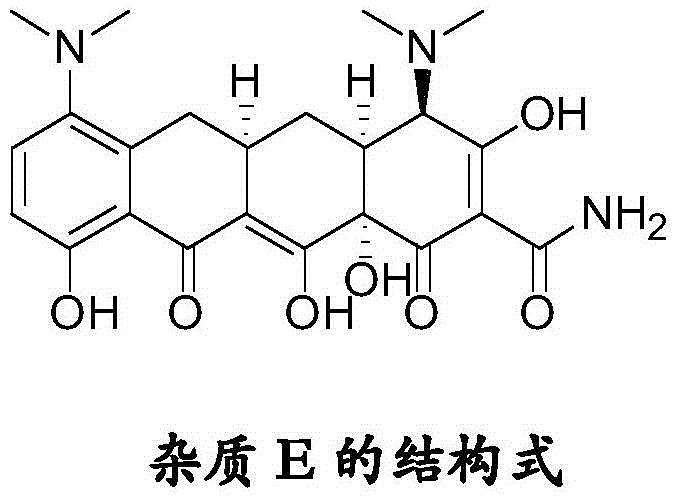
Tigecycline impurity stereoselective preparation method
ActiveCN104151186ADoes not affect configuration changesQuality improvementOrganic compound preparationCarboxylic acid amides preparationTigecyclineSolvent
The invention discloses a tigecycline impurity stereoselective preparation method. According to the invention, minocycline hydrochloride is adopted as a raw material, and is stirred in lower alkanol and liquid acid; the temperature is increased to 35-45 DEG C; the temperature is maintained and a reaction is allowed for 2-5h; the temperature is reduced, and the pH value of the mixed liquid is regulated to 6.5-8.5, such that solid is precipitated; a reaction is carried out for 2h under controlled temperature; the pH value is retested and the pH value is not changed; the material is filtered; a lower alkanol solvent is used for washing a filter cake; and drying is carried out, such that tigecycline impurity E is obtained. The purity can be higher than 98.0%, and a yield is higher than 50%.
Owner:福安药业集团重庆博圣制药有限公司 +1
Minocycline hydrochloride sustained release pellet and preparation method thereof
InactiveCN105663087AStable rateGood treatment effectAntibacterial agentsTetracycline active ingredientsSustained release pelletsPolyethylene glycol
The invention discloses a minocycline hydrochloride sustained release pellet and a preparation method thereof. The minocycline hydrochloride sustained release pellet consists of a coating layer and a drug-containing pellet body, wherein the coating layer is composed of Eudragit NE30D, talcum powder and lauryl sodium sulfate or polyethylene glycol; and the drug-containing pellet is composed of minocycline hydrochloride, a blank pellet core, a filling agent, a lubricating agent and a binding agent. The minocycline hydrochloride sustained release pellet disclosed by the invention makes use of a relatively novel sustained release preparation and a relatively novel pellet preparation; sustained release is to delay the release rate of drugs from the dosage form (the sustained release preparation) and to reduce the absorption rate of the drugs entering a body, so that a more stable therapeutic effect is achieved; by virtue of the pellet, the distribution area of the drugs on the surface of gastrointestinal tract is increased, irritation is relieved and bioavailability is enhanced, and meanwhile, influence of a gastric emptying factor is avoided and the drugs are uniformly absorbed in vivo and are low in individual difference; and with the simultaneous application of two advanced technologies, the technological superiority of the sustained release pellet is more enhanced. Compared with oral liquid, the sustained release pellet has the characteristics of being good in drug stability, convenient in packaging, transportation and storage, and the like; and the preparation method is simple and easy to implement and is applicable to industrial production.
Owner:HARBIN SHENGJI PHARMA
Preparation method of rhodium-carbon catalyst for synthesis of minocycline hydrochloride
PendingCN110841634AHigh selectivityReduced activityOrganic compound preparationOrganic chemistry methodsActivated carbonPtru catalyst
The invention discloses a preparation method of a rhodium-carbon catalyst for synthesis of minocycline hydrochloride. The rhodium-carbon catalyst comprises a carrier and rhodium nanoparticles dispersed on the carrier, the carrier is powdered activated carbon treated with a sodium hydroxide aqueous solution under microwave conditions, and the mass percentage of rhodium in the rhodium-carbon catalyst is 5%. The rhodium-carbon catalyst is prepared by adopting one-pot method, the activity of the rhodium-carbon catalyst is improved by introducing a chelating agent, the activity of the rhodium-carbon catalyst is properly reduced by microwave heating, and the selectivity of the rhodium-carbon catalyst is improved with ensured activity of the rhodium-carbon catalyst. The prepared rhodium-carbon catalyst is applied to a dehydroxylation step in minocycline hydrochloride synthesis, is high in selectivity, can effectively reduce the generation of side reaction dehydration impurities and improve the yield of target product sancycline hydrochloride, and is of great significance on reduction of production cost of minocycline hydrochloride and the improvement of the product quality.
Owner:XIAN CATALYST NEW MATERIALS CO LTD
Preparation method of minocycline hydrochloride chitosan microsphere and application thereof
The invention discloses a Minocycline hydrochloride chitosan microsphere and the process for preparing the microsphere. The prepared microsphere can be used for the treatment of oral diseases such as periodontitis.
Owner:北京华禧联合科技发展有限公司
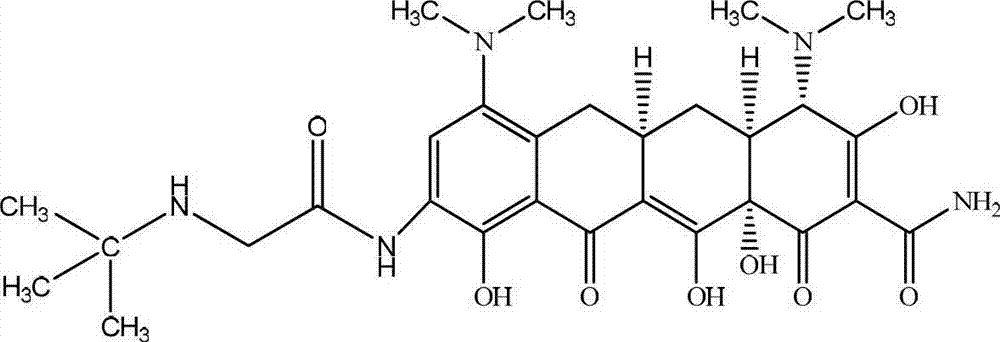
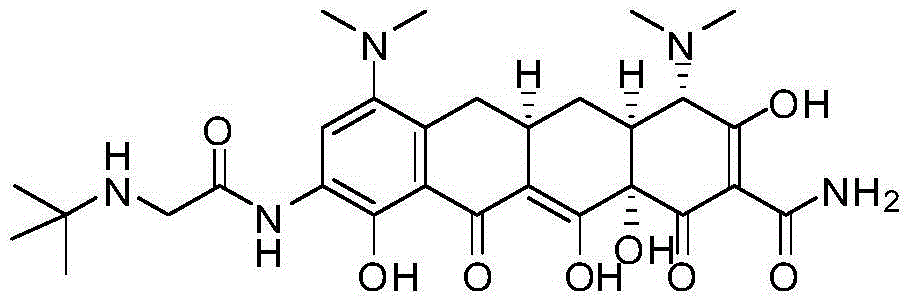
Synthesis method of minocycline hydrochloride
InactiveCN112961067AHigh purityHigh yieldOrganic compound preparationCarboxylic acid amides preparationPtru catalystOrganosolv
The invention relates to a synthesis method of minocycline hydrochloride, which comprises the following steps of: (1) reacting demeclocycline with an amination reagent in a first organic solvent in the presence of a palladium metal complex to obtain 7-amino-6-demethyltetracycline; (2) carrying out a dehydroxylation reaction on the 7-amino-6-demethyltetracycline in a second organic solvent in the presence of a first acid reagent and a first catalyst, so as to obtain 7-amino-6-demethyl-6-deoxytetracycline; and (3) in a third organic solvent, in the presence of a second acid reagent and a second catalyst, carrying out alkylation reaction on the 7-amino-6-demethyl-6-deoxytetracycline and an alkylation reagent to obtain minocycline hydrochloride. The minocycline hydrochloride prepared by the method disclosed by the invention has the advantages of simple synthesis process, high yield, high purity and easiness in large-scale production.
Owner:台州达辰药业有限公司
Xerostomia gel film and preparation method thereof
InactiveCN108926548AGood conditionCause toxic side effectsTetracycline active ingredientsDigestive systemDiseaseHigh concentration
The invention relates to a xerostomia gel film and a preparation method thereof, and belongs to the technical field of medicinal materials. By the combination of traditional Chinese medicines and minocycline hydrochloride and with the compatibility of medicines such as radix scrophulariae, radix rehmanniae, dark plum, polyghace seche, Radix Ophiopogonis and the like, the good effects of cooling the blood and clearing heat, promoting the production of body fluid to quench thirst and tonifying Qi and nourishing yin can be achieved, patient's condition is effectively improved, the amount of saliva secretion is increased, and pathogenic bacteria are reduced. Thereby, loss of tooth and gum diseases are prevented, natural infection resistance of the body can be enhanced, and special pathogenic bacteria can be specifically targeted to be killed. The chitosan can help protect mouth mucosa and teeth and reduce acidity of tooth surface, help keep a good pH value, assist the natural healing mechanism of the oral cavity, relieve xerostomia symptom and affect and inhibit bad breath, tooth decay and oral diseases, form high concentration in local parts to play the antibacterial action, achieve the effects of diminishing inflammation, promoting tissue regeneration and promoting dental care and raise the clinical efficacy.
Owner:FOSHAN SENANG BIO TECH CO LTD
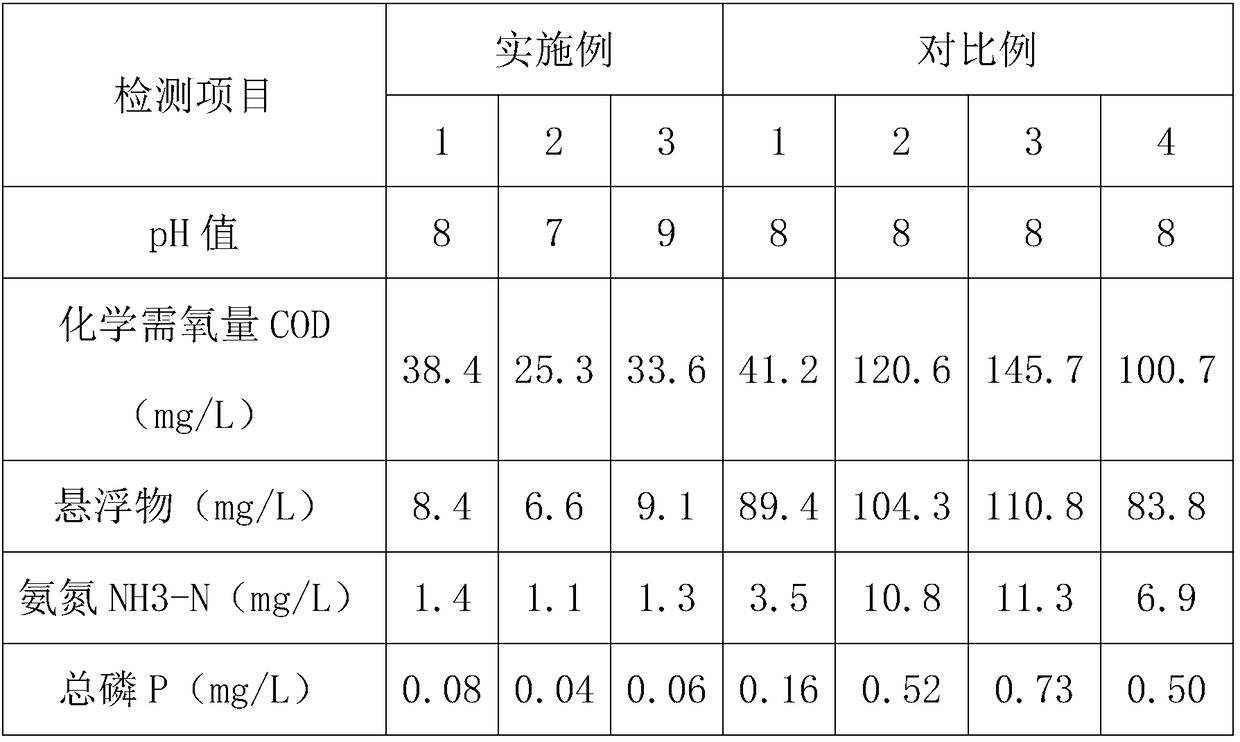
Sewage treatment technology of chemical engineering plant
InactiveCN109231567ASolve the costSolve efficiency problemsWater contaminantsMultistage water/sewage treatmentResin microsphereCarrageenan
The invention provides a sewage treatment technology of a chemical engineering plant. The sewage treatment technology comprises the following steps of S1, primary adjusting of the pH (potential of hydrogen) value of the sewage; S2, primary settling of the sewage: adding polyferric sulfate and polystyrenedivinylbenzene resin microspheres to settle, transferring the clean water into a nitrogen and phosphor removal tank, and discharging the sludge; S3, nitrogen and phosphor removal of the sludge: adding a nitrogen and phosphor removal agent, wherein the nitrogen and phosphor removal agent comprises the following raw materials of polyaluminum ferric silicate, aluminum potassium dodecahydrate, ceramic powder, hydroxyethyl-beta-cyclodextrin, minocycline hydrochloride, and carrageenan selenide; stirring and aerating, adding polyaluminum ferric silicate, settling, transferring the clean water into a pH final conditioning tank, and transferring the sludge into a sludge re-processing collectingtank; S4, post-treatment of the clean water: adjusting the pH value of the clean water, discoloring, filtering and detecting, and draining after the clean water is qualified in detection, so as to complete the sewage treatment technology of the chemical engineering plant. The sewage treatment technology has the advantages that the treatment cost is low, the operation is simple, the nitrogen and phosphor removal effect is good, and the utilization rate of resources is high.
Owner:陈志
A highly sensitive nano-cobalt oxide-doped minocycline hydrochloride molecularly imprinted electrochemical sensor and its preparation method
InactiveCN103926287BEasy to manufactureMaterial electrochemical variablesCross-linkFunctional monomer
The invention discloses a nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity and a preparation method of the nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor. The minocycline hydrochloride is taken as a template molecule, 20(s)-O-3beta-acetoxyl-5-androstene-17beta-acyl camptothecin is taken as a functional monomer, azodiisobutyronitrile is taken as an initiator, nano cobaltous oxide is taken as a dopant, and maleic rosin ethylene glycol acrylate synthesized from rosin as a raw material is taken as a cross-linking agent, so as to prepare the nano cobaltous oxide-doped minocycline hydrochloride molecular imprinting electrochemical sensor with high sensitivity. The analysis method is simple and practical, and the defects that the traditional analysis method is complicated, expensive in equipment, and low in sensitivity are overcome.
Owner:GUANGXI UNIV FOR NATITIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com