Synthesis method of minocycline hydrochloride
A technology of minocycline hydrochloride and a synthesis method, applied in the field of drug synthesis, can solve the problems of complicated process operation, difficult purification operation, low purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0160] The invention provides a method for synthesizing 7-amino-6-demethyltetracycline. The 7-amino-6-demethyltetracycline prepared by the method has the advantages of high purity and high yield.
[0161] In a preferred example of the present invention, the synthetic method of described 7-amino-6-desmethyltetracycline comprises:
[0162] (1) In the first organic solvent, in the presence of a palladium metal complex, demethylaureomycin reacts with an aminating reagent to obtain 7-amino-6-demethyltetracycline;
[0163]
[0164] Preferably, the desmethylautetracycline is free desmethylautetracycline or a desmethylautetetracycline salt.
[0165] Preferably, the desmethylautetracycline salt is desmethylautetracycline hydrochloride.
[0166] Preferably, in the step (1), the first organic solvent includes (but not limited to): triethylamine, DMF (N,N-dimethylformamide), DMA (N,N-dimethyl Acetamide), NMP (N-methylpyrrolidone), or a combination thereof.
[0167] Preferably, in th...
Embodiment 1
[0319] The synthesis of embodiment 1 minocycline hydrochloride
[0320] (1) Synthesis of 7-amino-6-desmethyltetracycline
[0321]
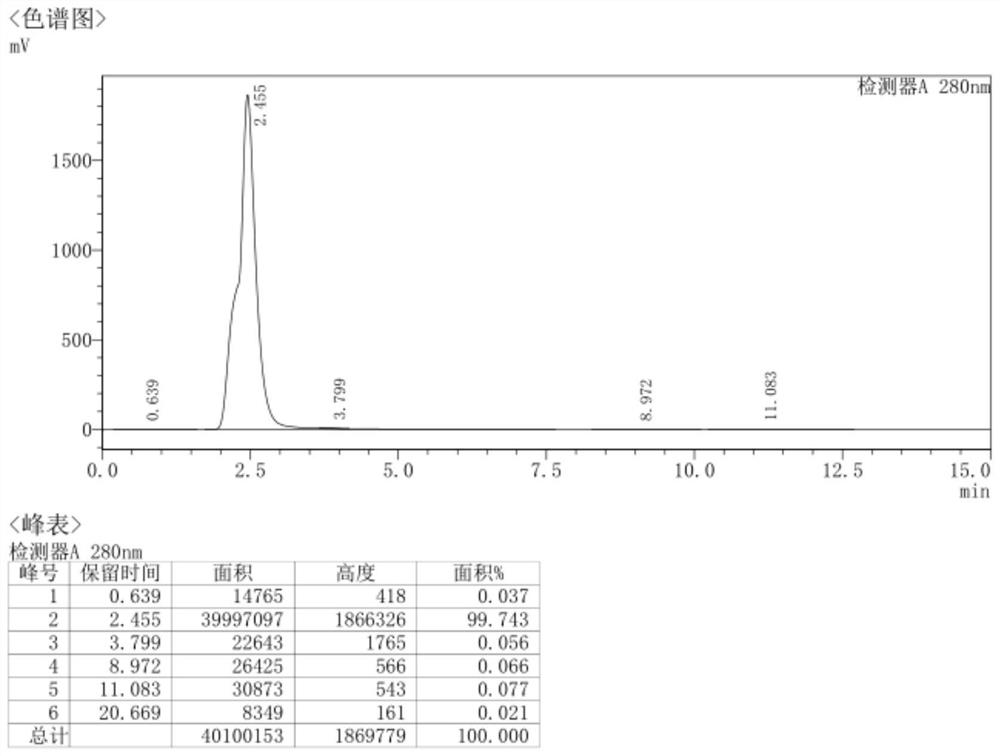
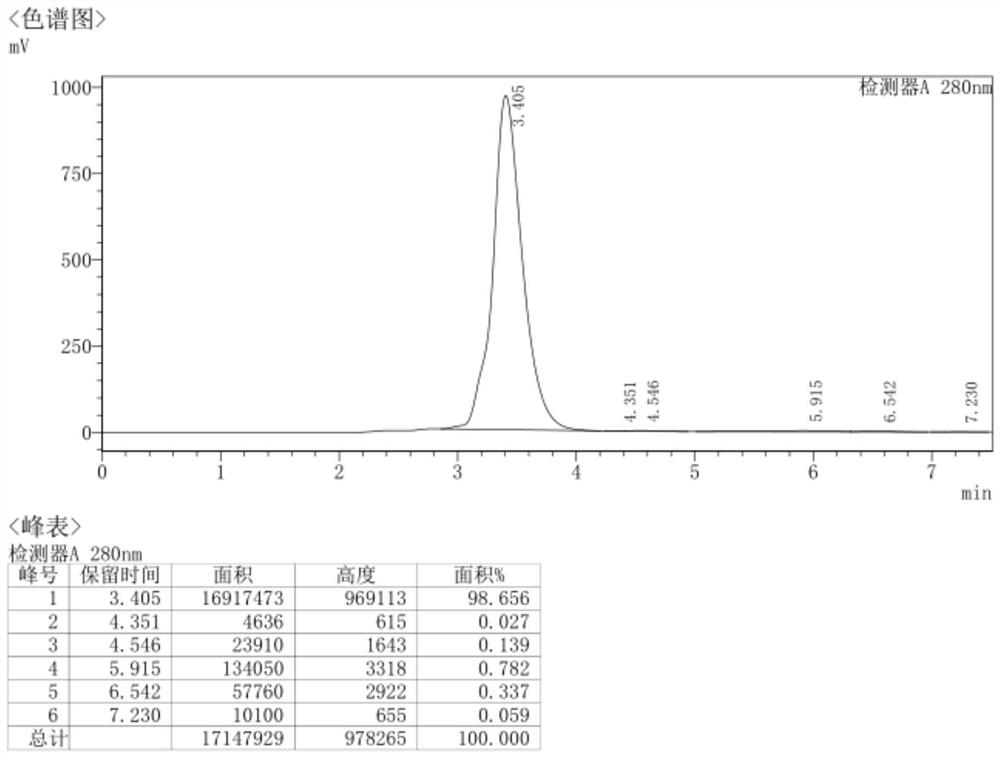
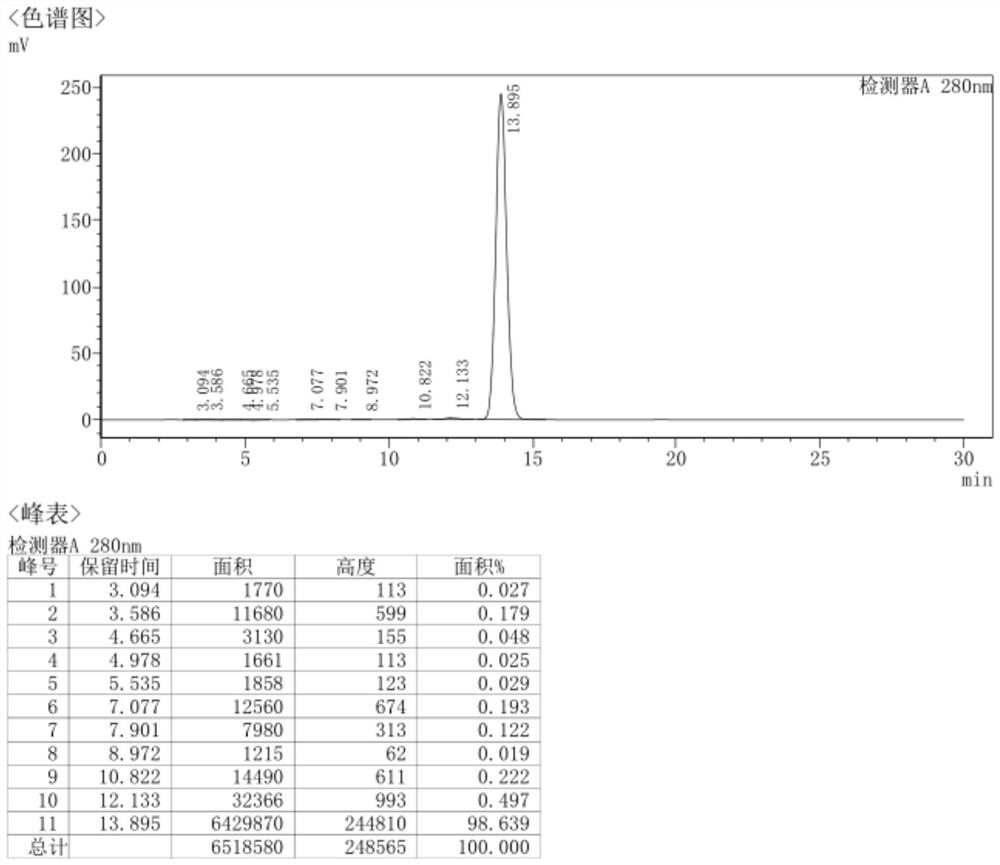
[0322] In a 500ml autoclave, put 50g of demethylaureomycin hydrochloride, 300ml of DMF (N,N-dimethylformamide), 2.5g of bis(triphenylphosphine)palladium dichloride, 15g of 27% ammonia water, and replace with nitrogen After three times in the autoclave, evacuate the nitrogen, then replace it with hydrogen and fill it with hydrogen until the pressure of the hydrogen gas in the autoclave is 3MPa, react at 45°C for 8h to obtain the reaction solution; pour the reaction solution into 500ml of isopropanol, stir The solid was precipitated, then cooled to -5°C to crystallize, crystallized for 2.5h, filtered, and the filter cake was dried under reduced pressure at 40°C to obtain 42.4g of 7-amino-6-desmethyltetracycline with a yield of 95.4%, HPLC Measure purity 99.7%, the HPLC collection of illustrative plates of the prepared 7-amino-6-demethyltetracycl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com