Patents
Literature
674 results about "Mycoplasma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma is a mollicute genus of bacteria that lack a cell wall around their cell membranes. This characteristic makes them naturally resistant to antibiotics that target cell wall synthesis (like the beta-lactam antibiotics). They can be parasitic or saprotrophic. Several species are pathogenic in humans, including M. pneumoniae, which is an important cause of "walking" pneumonia and other respiratory disorders, and M. genitalium, which is believed to be involved in pelvic inflammatory diseases. Mycoplasma species are the smallest bacterial cells yet discovered, can survive without oxygen, and come in various shapes. For example, M. genitalium is flask-shaped (about 300 x 600 nm), while M. pneumoniae is more elongated (about 100 x 1000 nm). Hundreds of mycoplasma species infect animals.
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Mycoplasma hyopneumoniae bacterin vaccine
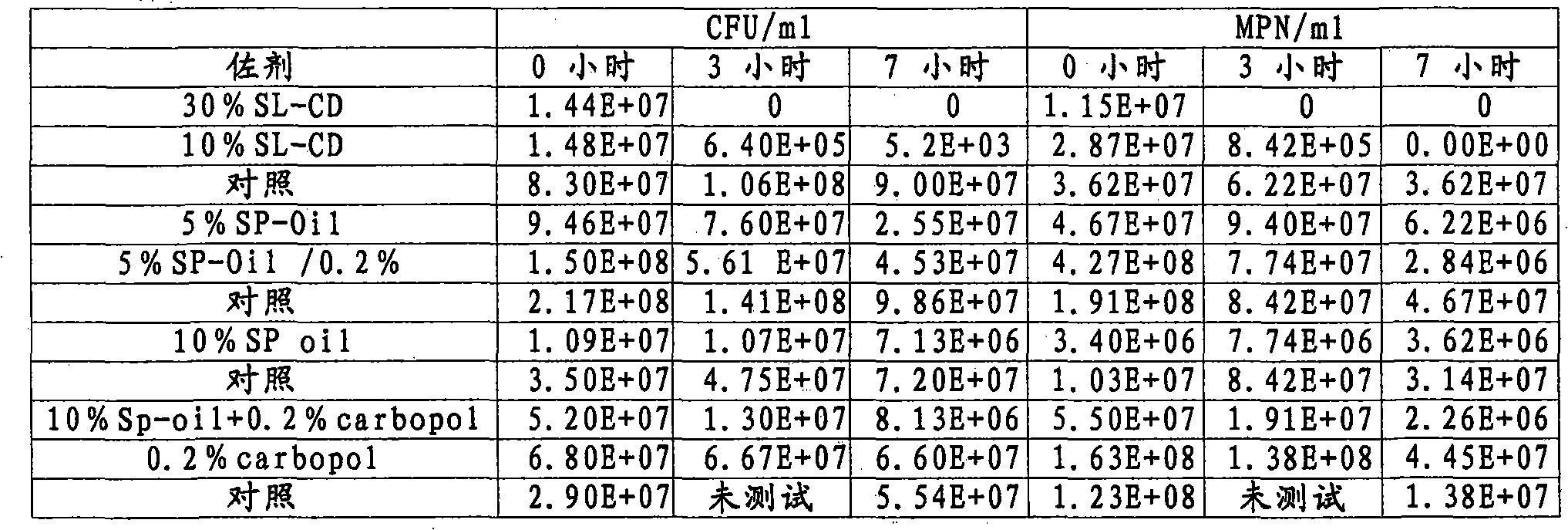
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably CARBOPOL®, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polyoxypropylene block copolymer such as PLURONIC®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another embodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
Vaccines against cancer and infectious diseases
InactiveUS6440416B1Promote antibody productionPromote productionOrganic active ingredientsImmunoglobulins against animals/humansProtozoaPrimate
A method of stimulating an immune response in a human against malignant cells or an infectious agent comprises the step of administering to the human an immunogenic amount of a primate anti-idiotype antibody or antibody fragment that acts as an immunogenic functional mimic of an antigen produced by or associated with a malignant cell or an infectious agent. Sub-human primate anti-idiotype antisera, especially from baboons, are preferred. Such anti-idiotype antibodies are used to make vaccines for inducing preventive immunity or a therapeutic immune response against tumors, viruses, bacteria, rickettsia, mycoplasma, protozoa, fungi and multicellular parasites.
Owner:IMMUNOMEDICS INC
Pcv2 mycoplasma hyopneumoniae immunogenic compositions and methods of producing such compositions
InactiveUS20090317423A1Reduce incidenceEffective immunityViral antigen ingredientsAntiinfectivesDiseaseActive component
Multivalent combination vaccines are provided which include an immunological agent effective for reducing the incidence of or lessening the severity of M. hyo infection, preferably M. hyo bacterin, or an immunogenic composition comprising M. hyo bacterin, and at least one immunogenic active component of another disease-causing organism in swine, preferably PCV2 wherein the preferred PCV2 antigen for such a multivalent vaccine is PCV2 ORF 2 protein.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
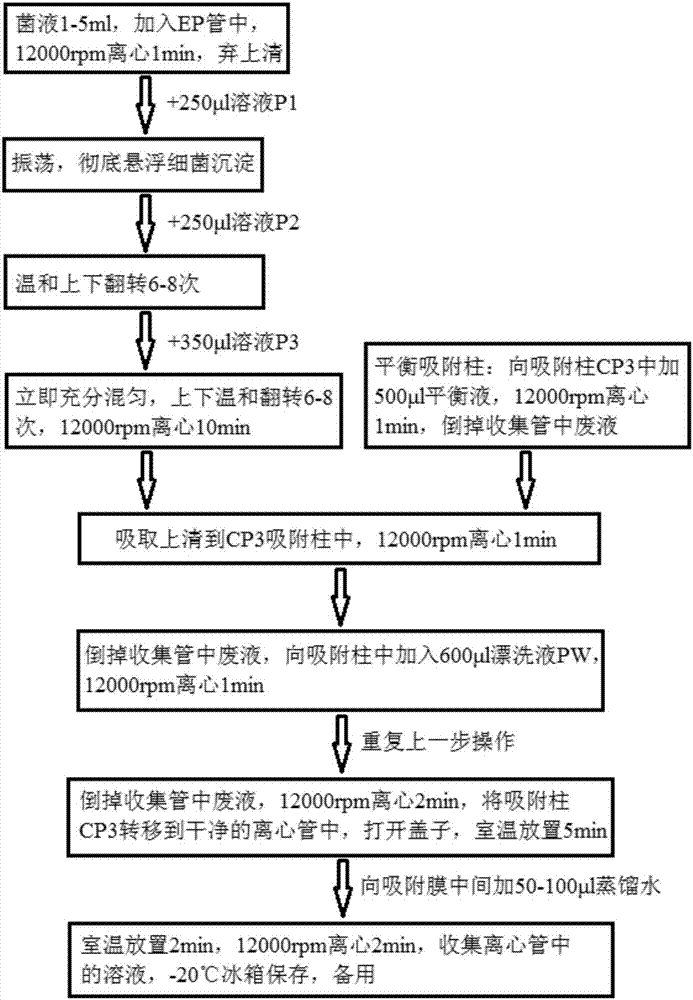
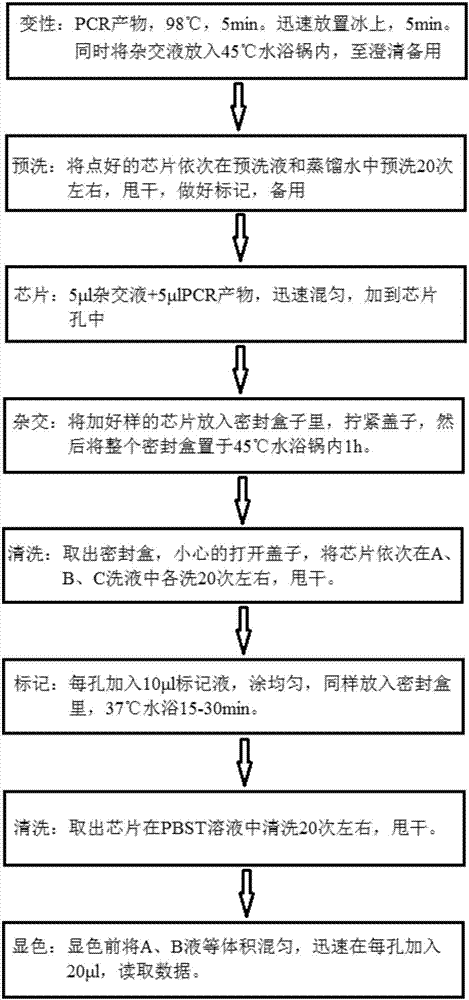
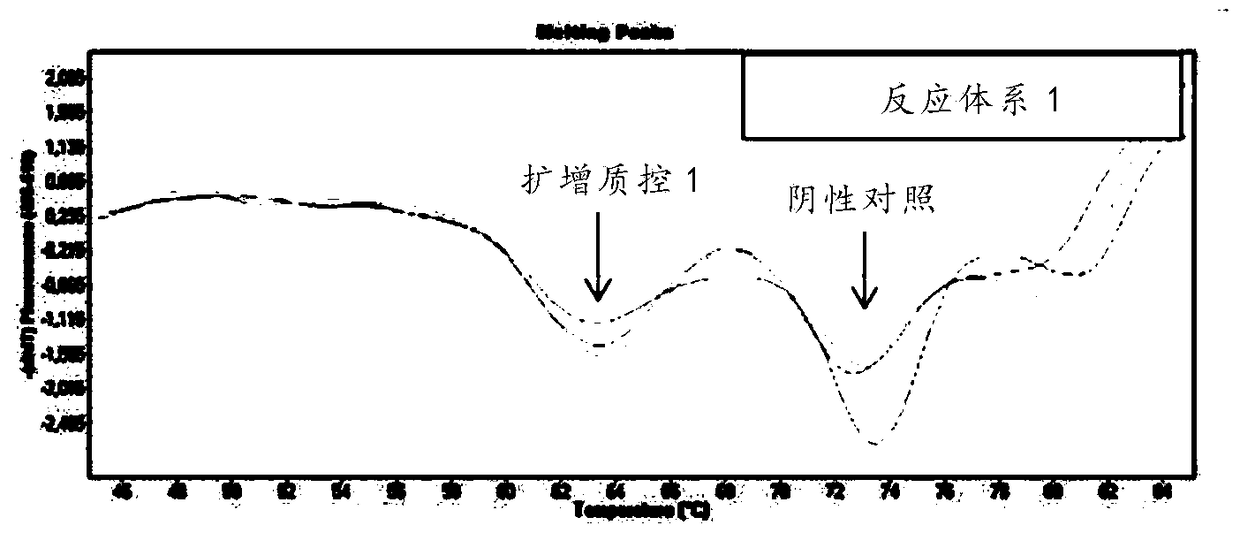
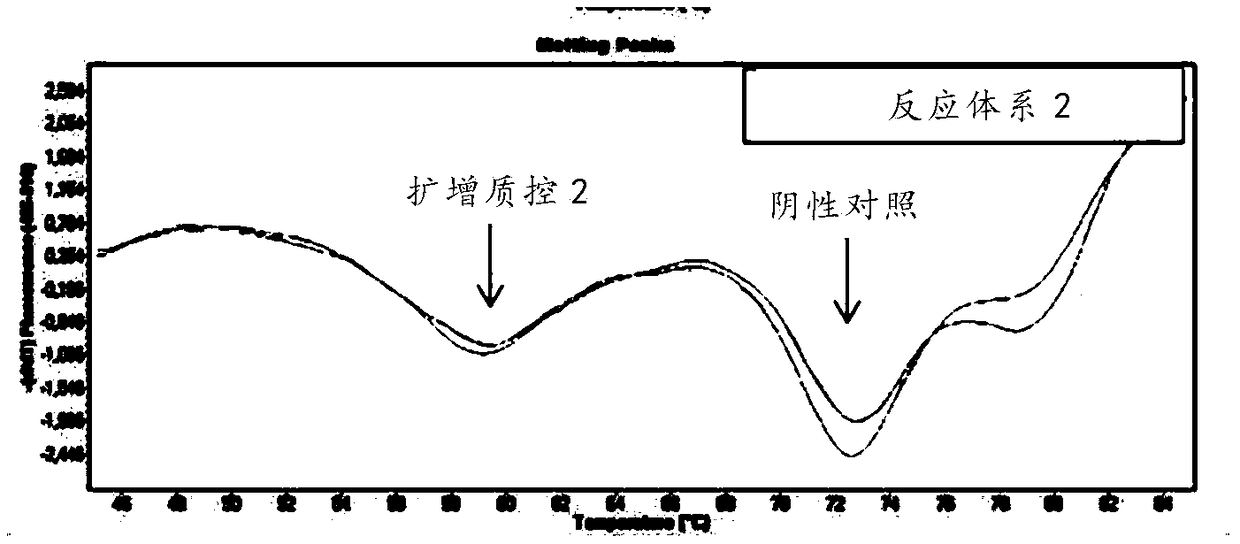
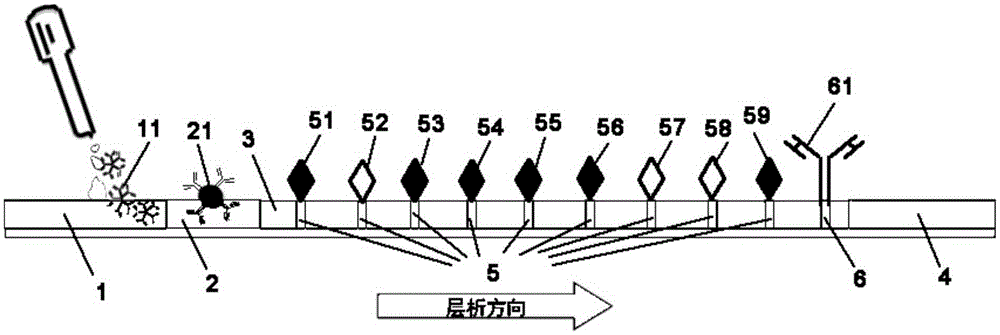
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
Immunogenic Mycoplasma hyopneumoniae polypeptides
Mycoplasma hyopneumoniae polypeptides and nucleic acids, as well as nucleic acid expression vectors and host cells containing nucleic acid vectors are provided. In addition, compositions containing M. hyopneumoniae polypeptides and nucleic acids are provided for use in methods of treating swine to prevent enzootic pneumonia. Furthermore, the invention provides diagnostic tests for the detecting of M. hyopneumoniae infection in swine herds.
Owner:IOWA STATE UNIV RES FOUND +1
Mycoplasma hyopneumoniae bacterin vaccine
The invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration. The composition comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity. In a preferred embodiment, the adjuvant mixture comprises an acrylic acid polymer, most preferably Carbopol, and a mixture of a metabolizable oil such as one or more unsaturated terpene hydrocarbons, preferably squalene or squalane, and a polyoxyethylene-polypropylene block copolymer such as Pluronic®. The vaccine composition may optionally include a preservative, preferably thimerosol and / or EDTA. In another emodiment, the invention provides an improved Mycoplasma hyopneumoniae bacterin vaccine composition, which advantageously provides immunity from infection after a single administration, and comprises an inactivated Mycoplasma hyopneumoniae bacterin and an adjuvant or adjuvant mixture, which, in combination, provide immunity from Mycoplasma hyopneumoniae infection after a single administration, and elicit an immune response specific to Mycoplasma hyopneumoniae bacterin and including cell-mediated immunity and local (secretory IgA) immunity, in combination with other vaccine components.
Owner:ZOETIS SERVICE LLC
Respiratory pathogen multi-detection reagent kit
InactiveCN109355437AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoronavirus 229EFluorescence
The invention discloses a respiratory pathogen multi-detection reagent kit. The respiratory pathogen multi-detection reagent kit has the advantages that the respiratory pathogen multi-detection reagent kit is based on multi-PCR (polymerase chain reaction) technologies, detection results can be determined by the aid of fluorescence resonance energy transfer via the melting temperature ranges, the respiratory pathogen multi-detection reagent kit can be used for qualitatively simultaneously detecting 16 types of respiratory pathogens, the 16 types of respiratory pathogens include 12 types of RNA(ribonucleic acid) viruses (influenza A viruses, influenza B viruses, H1N1 influenza A viruses, type A and type B respiratory syncytial viruses, type -1 / -2 / -3 parainfluenza viruses, type OC43 coronaviruses, type 229E coronaviruses, rhinoviruses and human metapneumovirus), 2 types of DNA (deoxyribonucleic acid) viruses (adenoviruses and bocavirus) and 2 types of bacteria (mycoplasma pneumoniae andbordetella pertussis), the respiratory pathogen multi-detection reagent kit is high in detection sensitivity, and the sensitivity even can reach 1 copy / reaction; the multi-detection reagent kit is good in specificity, and negative results of pathogens which have identical sampling sites and similar pathogenic mechanisms and are not in the detection range of the respiratory pathogen multi-detectionreagent kit can be obtained; the respiratory pathogen multi-detection reagent kit is short in operation time and easy to operate and can be used for quickly detecting the 16 types of respiratory pathogens in a single tube of a reaction system, the results are clear and are easy to interpret, and the like.
Owner:上海捷诺生物科技股份有限公司
Method for preparing human umbilical cord mesenchymal stem cell exosomes
PendingCN109880797AHigh purityClear sourceSkeletal/connective tissue cellsMesenchymal stem cellMycoplasma contamination
The invention discloses a method for preparing human umbilical cord mesenchymal stem cell exosomes. The method uses a newborn umbilical cord as a source of umbilical cord mesenchymal stem cells, usesa medium prepared by a fetal calf serum or a serum substitute in which exosome carried by itself is removedfor culturing, collects a supernatant, and further separates and extracts the exosomesby differential centrifugation, the purity is high, and the source of the umbilical cord mesenchymal stem cell exosomes is ensured; the exosomes is prepared by the differential centrifugation, the operationis simple, and the method is suitable for large-scale production; during the whole process, sterility is guaranteed, the contamination ofmycoplasma is prevented, and the product safety is higher.
Owner:JINAN PANSHENG BIOTECH
One dose vaccination against mycoplasma infections of pigs
The present invention provides a one phase, aqueous vaccine composition for immunizing an animal against infection by Mycoplasma hyopneumoniae, comprising: an immunizing amount of a Mycoplasma hyopneumoniae bacterin, an acrylic acid polymer in the concentration range between 0.8 and 1.2 mg / ml, and a pharmaceutically acceptable carrier, and substantially no oil. It is especially useful for immunizing a pig against infection by Mycoplasma hyopneumoniae for at least 20 weeks after a single administration, which effective immunity is reached within 4 weeks after vaccination.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
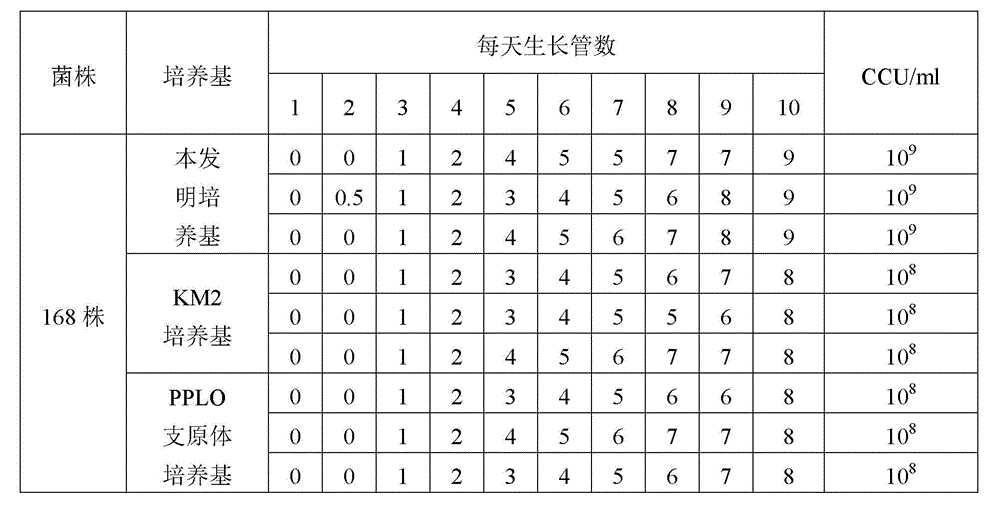
Low serum efficiency mycoplasma gallisepticam attenuated strain culture medium and preparation method thereof
ActiveCN103074246AIncrease the titer of live bacteriaReduce allergic reactionsBacteriaMicroorganism based processesAntigenCulture mediums
The present invention relates to a low serum efficiency mycoplasma gallisepticam attenuated strain culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The culture medium comprises: (1) a base culture medium; and (2) an auxiliary culture medium, wherein the auxiliary culture medium mainly comprises MEM, yeast extract powder, tryptone, glucose, an inorganic salt, and the like, and growth, high titer and stability of the semi-finished product can be ensured with the auxiliary culture medium. According to the present invention, a titer of the semi-finished product bacterial liquid prepared by using the preparation method is up to 10<11> CCU / ml; and the culture medium adopts reduced pig serum to culture mycoplasma gallisepticam so as to reduce allergic stress reactions on chicken by heterologous pig serum, consider animal biosafety, improve antigen titer, and reduce production cost.
Owner:兆丰华生物科技(南京)有限公司
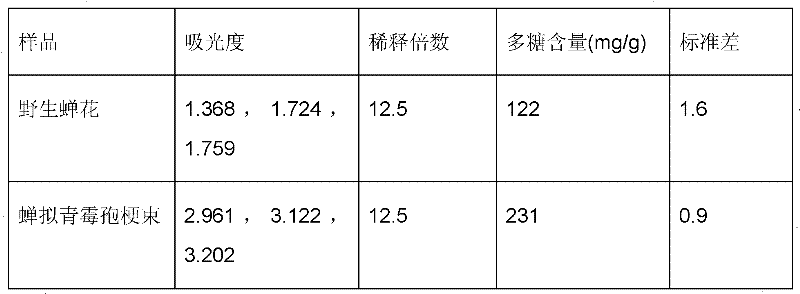
Cordyceps cicadae wine and preparation method thereof
ActiveCN102242044AIntegrity guaranteedGuaranteed extraction efficiencyDigestive systemAlcoholic beverage preparationBiotechnologyAdditive ingredient
The invention discloses cordyceps cicadae wine and a preparation method thereof. The cordyceps cicadae wine is obtained by dipping coremium of paecilomyces cicadae and / or mycoplasma, as raw materials, in base wine, or obtained by mixing an extract of the coremium of paecilomyces cicadae and / or an extract of mycoplasma, as raw materials, with drinking wine. In the cordyceps cicadae wine, the raw materials are fully utilized, activated secondary metabolism components in the mycoplasma are effectively kept, the integrity of active components in the cordyceps cicadae wine is ensured, the product quality is stable and the production cost is low.
Owner:ZHEJIANG BIOASIA PHARMA CO LTD
Low-serum culture medium for efficiently culturing mycoplasma hyopneumoniae and preparation method thereof
ActiveCN103060220AIncrease the titer of live bacteriaReduce allergic reactionsAntibacterial agentsBacterial antigen ingredientsMycoplasma cultureOrganism
The invention relates to an efficient mycoplasma hyopneumoniae culture medium and a preparation method thereof, and belongs to the technical field of veterinary biology. The efficient mycoplasma hyopneumoniae culture medium comprises an A liquid and a B liquid mainly consisting of MEM, yeast leaching powder, tryptone, glucose, inorganic salt and the like. The prepared culture medium of the invention has the main advantages of low serum content which is only 10%-15%, while the serum content in common culture medium is 20% even more. The culture medium prepared by the low serum relieves the pig allergic to the stress reaction, meanwhile gives consideration to the biosafety of animals. Besides the valence of the semi-finished bacterial solution prepared by the method is up to 109CCU / ml, which is much higher than the culture medium prepared by the common technology.
Owner:兆丰华生物科技(南京)有限公司 +1
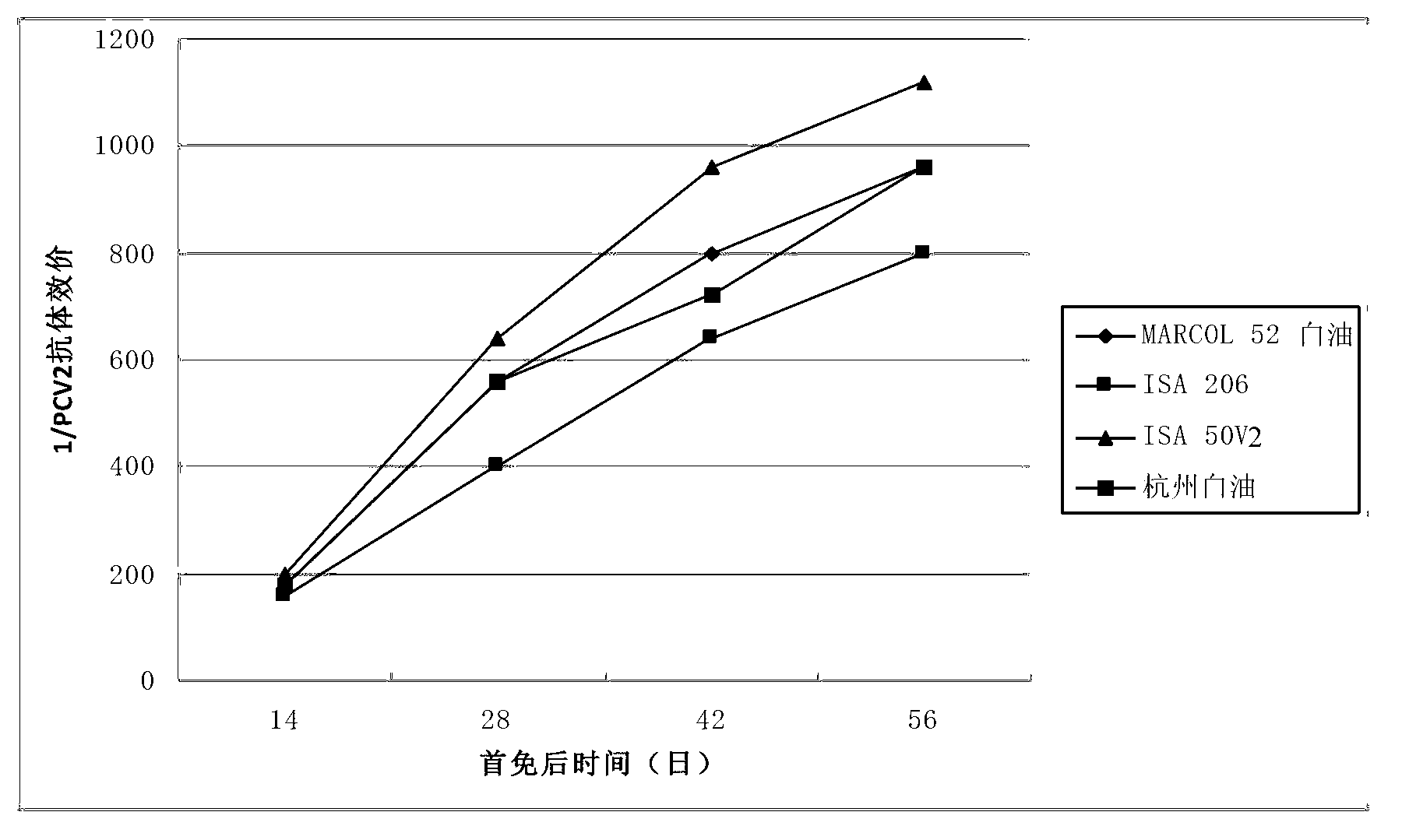
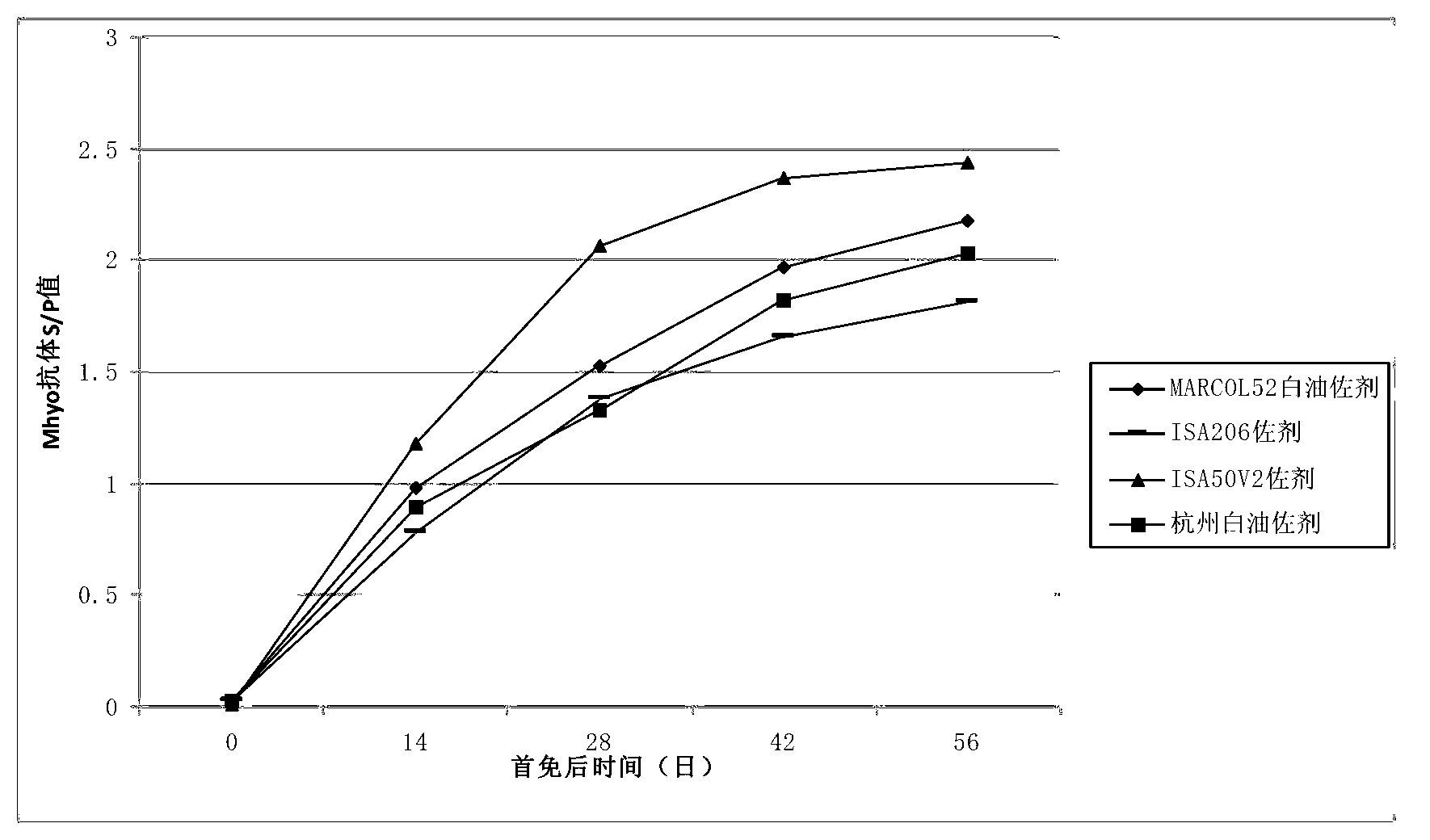
Duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae and preparation method of duplex inactivated vaccine
ActiveCN103263666AImprove protectionEffective protectionAntibacterial agentsBacteriaOil adjuvantMental state
The invention discloses a duplex inactivated vaccine of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The duplex inactivated vaccine comprises an inactivated porcine circovirus type 2 antigen, inactivated mycoplasma hyopneumoniae and an oil adjuvant, wherein the mycoplasma hyopneumoniae is of DJ-166 strains and has the preservation number No.4545 in China general microbiological culture collection center. The porcine duplex inactivated vaccine has an obvious technical effect on prevention of porcine circovirus type 2 and porcine mycoplasma hyopneumoniae. The safety test shows that the single dosage of the vaccine, the repetition of the single dosage and an overdosing amount of inoculation against test animals are safe, the test animals have normal body temperature and mental states, and the clinical symptoms are avoided; and the efficacy test shows that the duplex inactivated vaccine has a good protection function of virulently attacking the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae, so that the porcine circovirus type 2 and porcine mycoplasma hyopneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +3
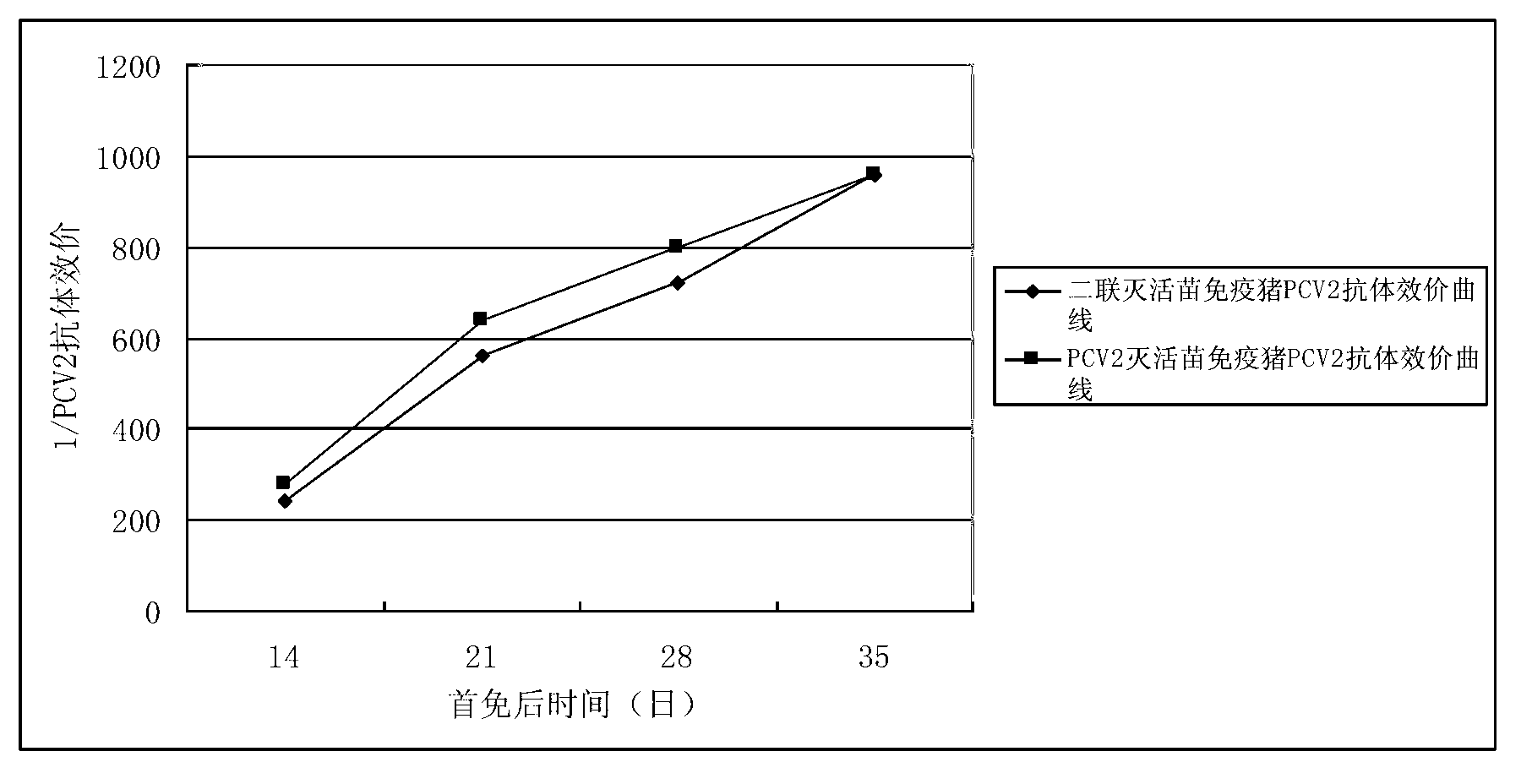
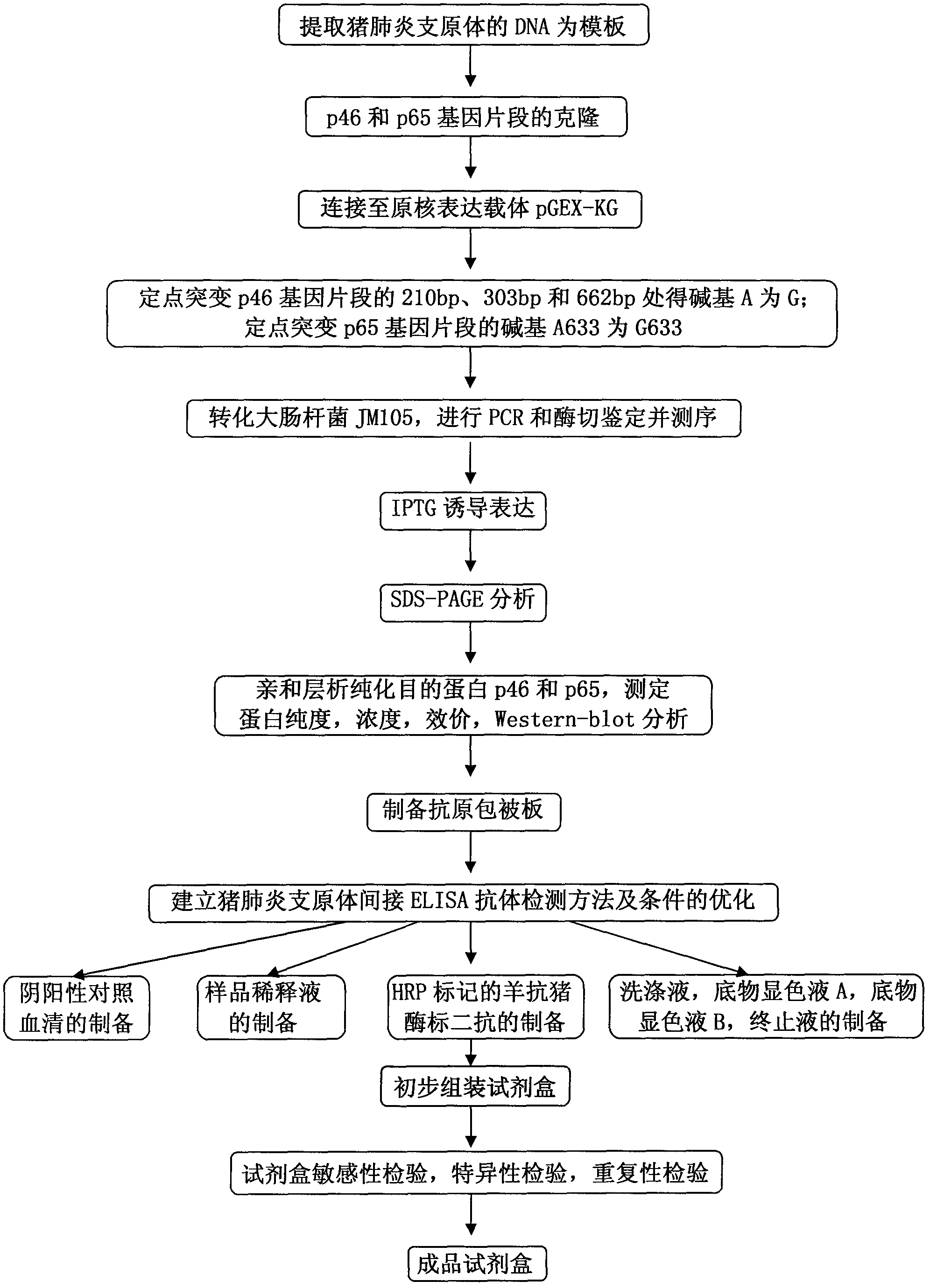
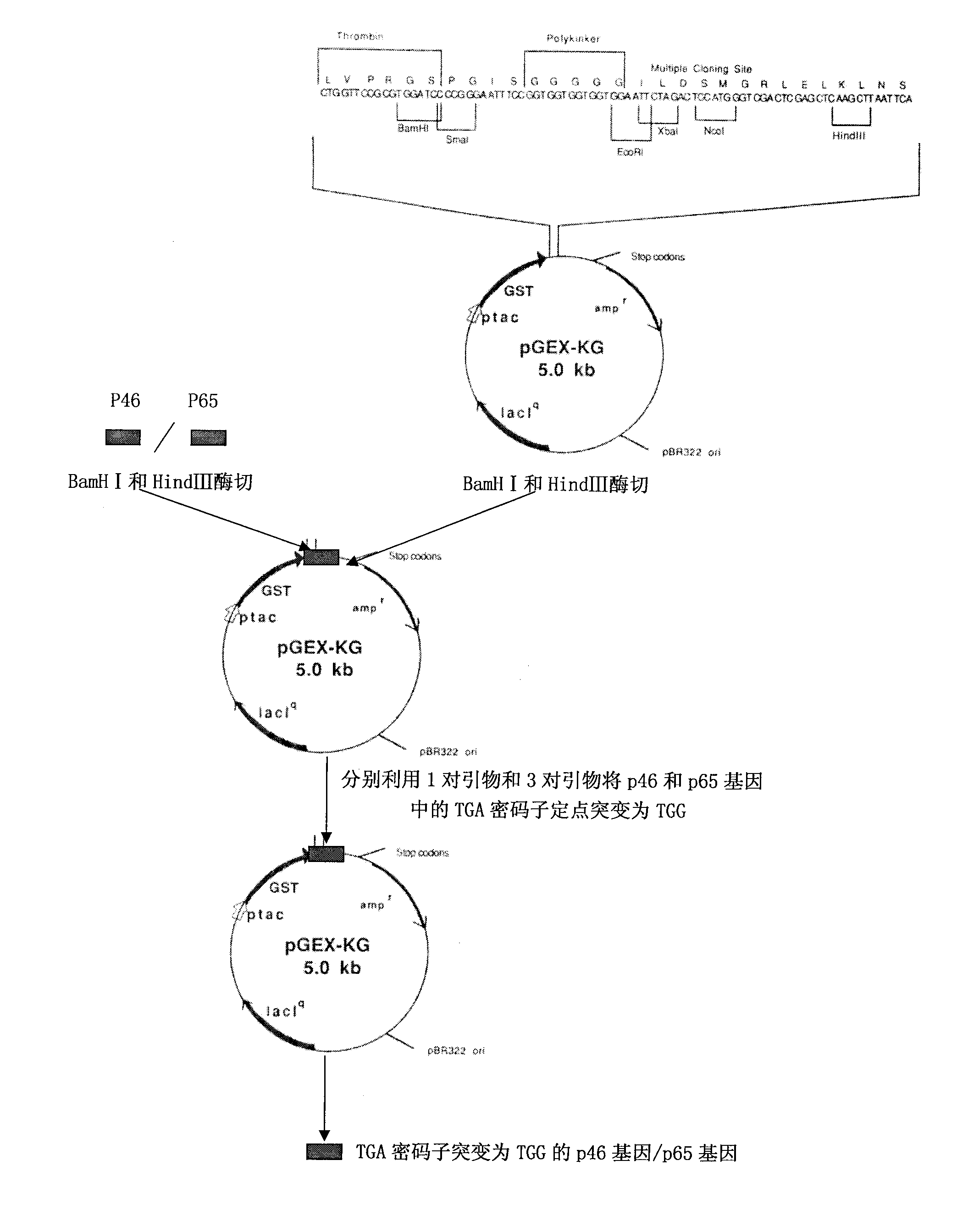
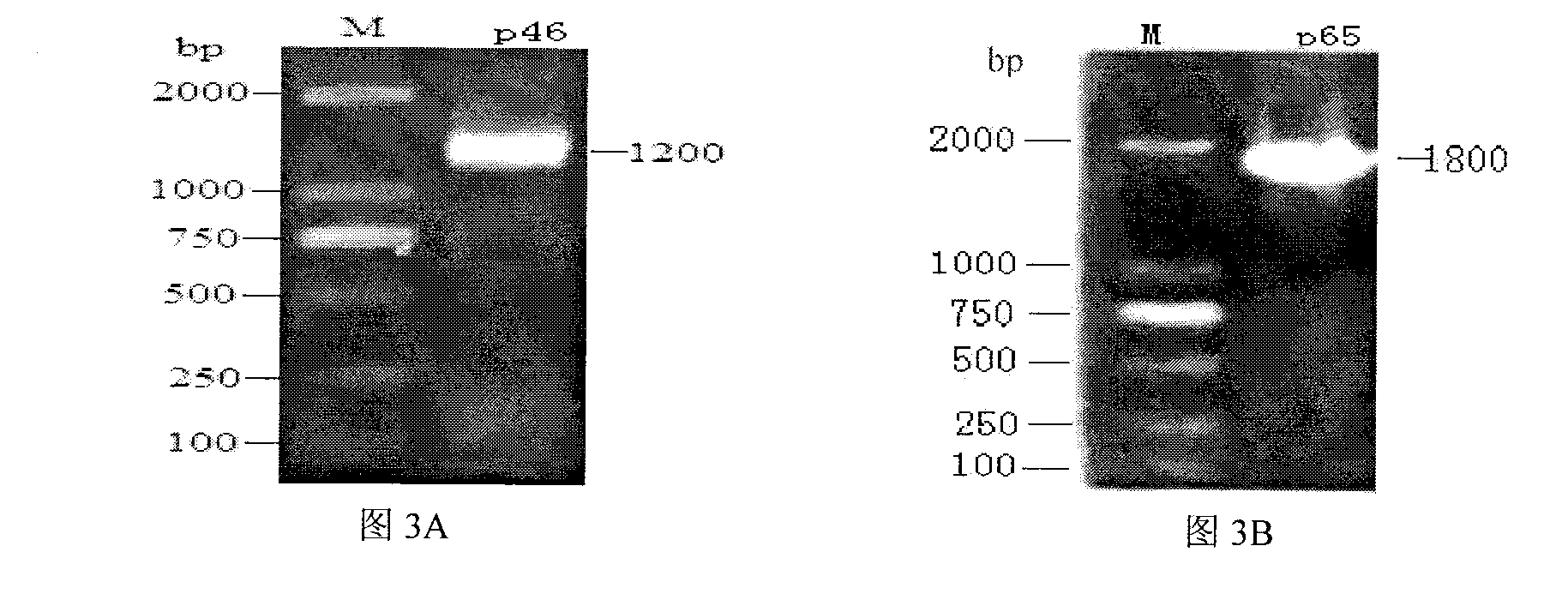
Mycoplasma hyopneumoniae indirect ELISA antibody detection kit and application
ActiveCN103018442AStrong specificityHigh sensitivityMaterial analysisEscherichia coliPurification methods
The invention belongs to the technical field of animal virology and animal infectious diseases detection, and concretely relates to a Mycoplasma hyopneumoniae indirect ELISA antibody detection kit and an application. According to the invention, two strains of recombinant E. coli pGEX-KG-46 and pGEX-KG-65 expressing M. hyopneumoniae p46 protein and p65 protein are obtained through a gene engineering recombinant technology. The kit provided by the invention comprises an ELISA plate using expression proteins of mutant P46 gene and P65 gene of the Mycoplasma hyopneumoniae membrane proteins as common antigen coating, and other core reagents. The invention discloses clone of the P46 gene and the P65 gene of the Mycoplasma hyopneumoniae membrane proteins, site-directed mutagenesis, and expression and purification methods of the p46 protein and the p65 protein, and further discloses a Mycoplasma hyopneumoniae indirect ELISA antibody detection method. The indirect ELISA antibody detection kit provided by the invention can be used for a large-scale clinical testing and an epidemiological investigation of the Mycoplasma hyopneumoniae antibody, and has a wide market prospect.
Owner:HUAZHONG AGRI UNIV +1
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151AStrengthen cellsEnhance humoral immune stimulationAntibacterial agentsBacterial antigen ingredientsCholesterolVaccine antigen
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
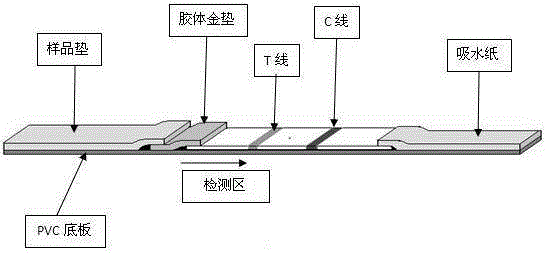
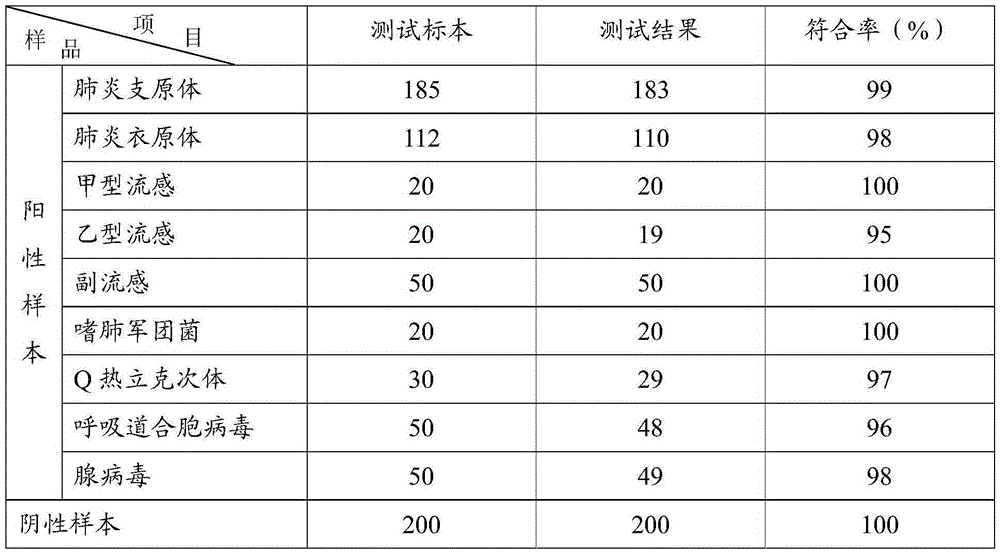
Method and kit for adopting colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid
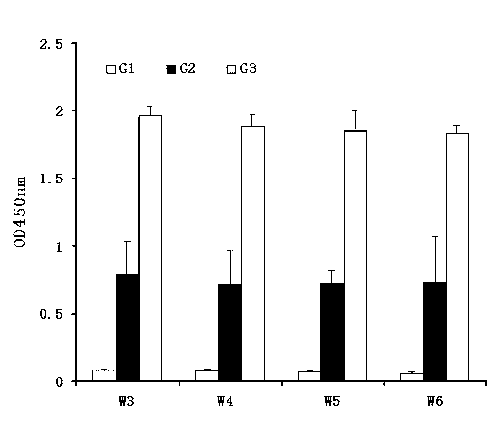
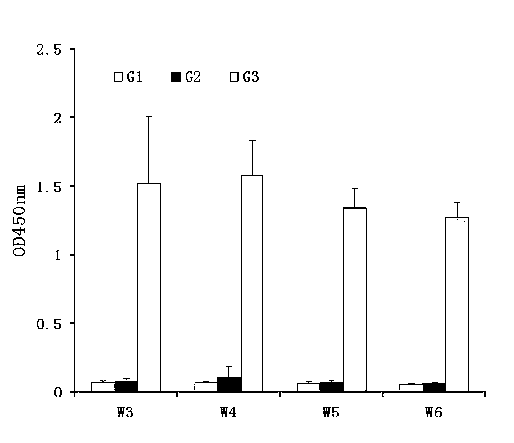
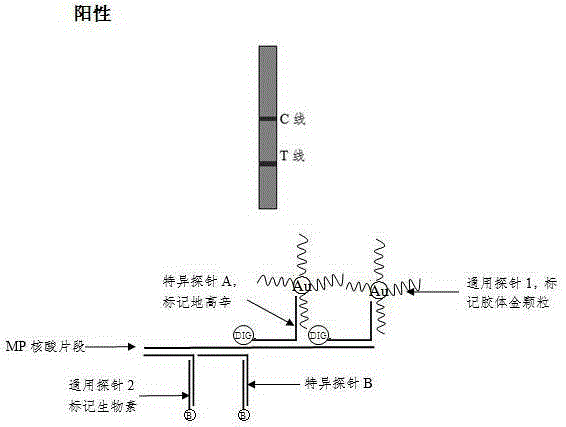
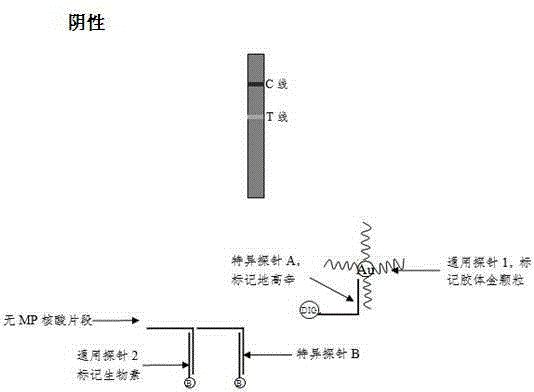
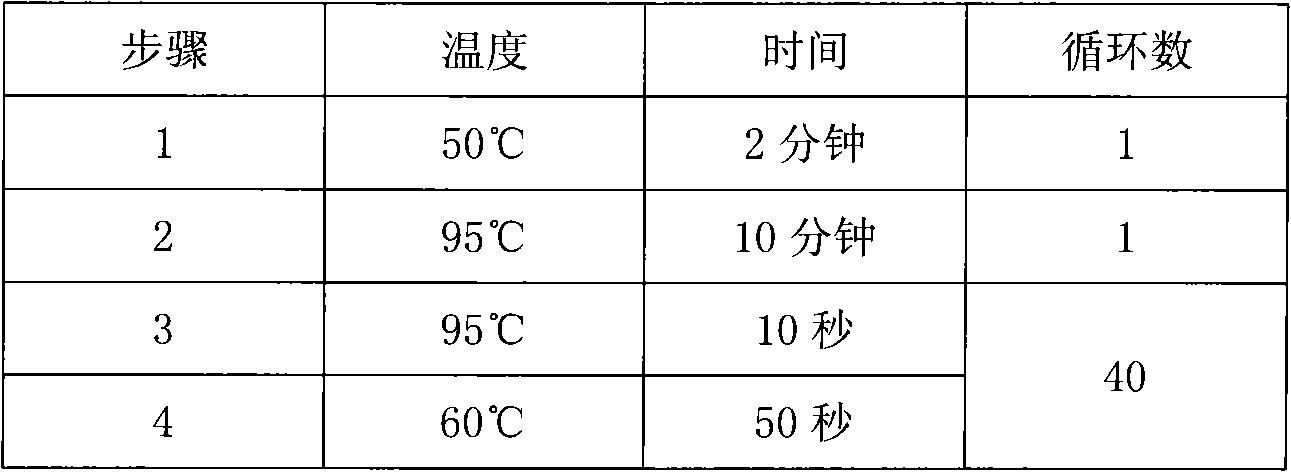
The invention discloses a method and a kit for adopting a colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid and belongs to the technical field of medical biochemistry. According to the method, a colloidal gold grain is directly marked on a nucleic acid probe; the sequence for the marked nucleic acid probe is designed as a universal sequence; the nucleic acid probe also can be used for detecting other pathogens. During the design process for the kit provided by the invention, the introduced special probe A and special probe B have the functions of bridge molecular components and a gold marked probe and an MP (Mycoplasma Pneumoniae) nucleic acid amplified fragment are successively combined with each other in series by the two probes, so that the special detection for the MP nucleic acid fragment is realized. More than two probes can be designed in each set of probes; such a design is beneficial to the increasing of the sensitivity of the test strip; the advantages of the amplification technique for the depending nucleic acid sequence of MP and the colloidal gold marked detection for the products after amplification are integrated; the technical demand on the experimenter is low; no special instrument is required; the popularization of the MP nucleic acid detection in basic and faraway rural medical institutions is easily realized.
Owner:武汉中帜生物科技股份有限公司
Mycoplasma hyopneumoniae avirulent adjuvanted live vaccine
InactiveCN101883581AAntibacterial agentsBacterial antigen ingredientsDiseaseVirulent characteristics
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS W LLC
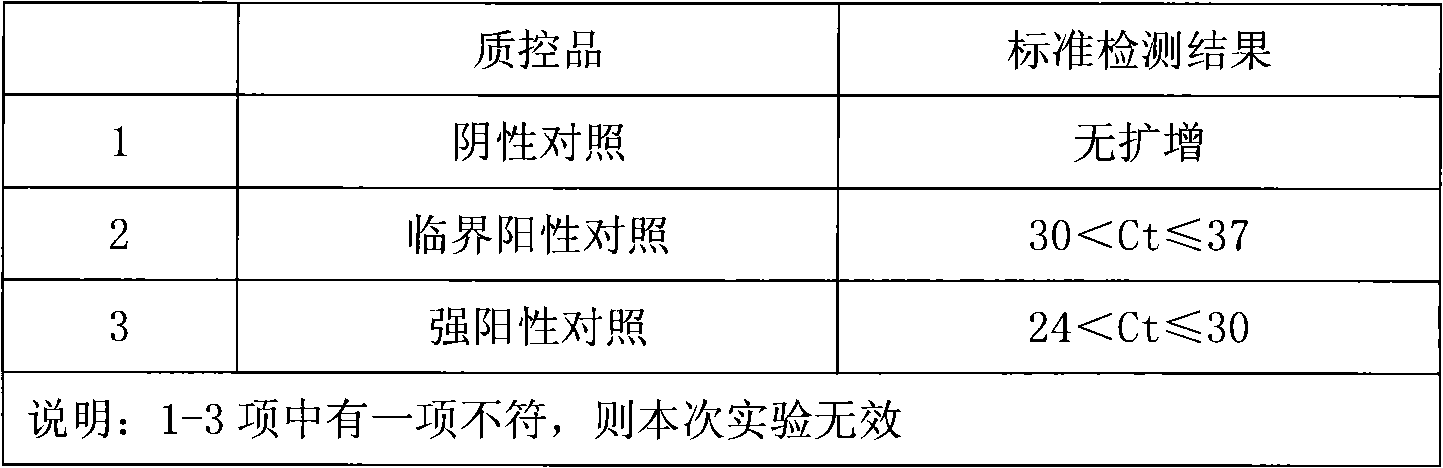
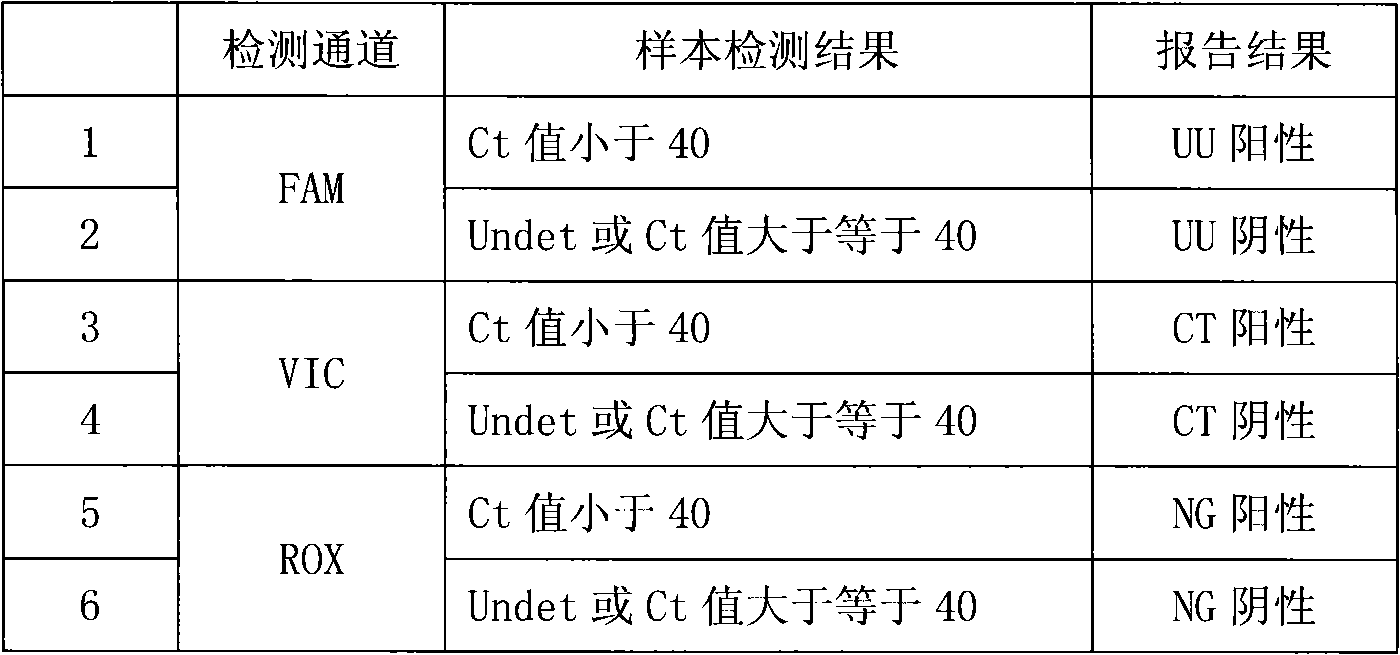
Fluorescence PCR method for diagnosing infection of Chlamydia trachomatis, neisseria gonorrhoeae and ureaplasma urealyticum
ActiveCN101613763AMicrobiological testing/measurementMicroorganism based processesForward primerFluorescence
The invention relates to a fluorescence PCR (polymerase chain reaction) detection method for diagnosing infection of Chlamydia trachomatis (CT), neisseria gonorrhoeae (NG) and ureaplasma urealyticum (UU), which belongs to the field of nucleic acid in vitro diagnosis. The method comprises a polymerase chain reaction (PCR) system based on fluorescence PCR technology, contains a forward primer and areverse primer aiming at the CT / the NG / the UU and a fluorescent probe, and can detects DNA of three pathogens such as the CT, the NG, the UU and the like simultaneously in a reaction tub under suitable PCR condition. The method can diagnosing the infection of the CT / the NG / the UU in a clinical sample simply, conveniently and rapidly, has high sensitivity and specificity, and has important clinical value to early control and prevention of relevant genitourinary tract infections, blocking of an infection sources, and infection reduction of related pathogen.
Owner:CITY UNIVERSITY OF HONG KONG
Mycoplasma hyopneumoniae DJ-166 strain and application thereof
ActiveCN103184171AReduce manufacturing costImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsMicroorganismCell free
The invention belongs to the field of veterinary microbial technology, and particularly relates to a mycoplasma hyopneumoniae DJ-166 strain and an application thereof. The mycoplasma hyopneumoniae can be used for preparing veterinary biological products or veterinary drugs for prevention. Experiments demonstrate that the mycoplasma hyopneumoniae DJ-166 strain has high active bacterium titer reaching 10<10-11> CCU / mL in a prepared cell-free culture medium, can greatly reduce production cost, and has good immunogenicity with an average pneumonia pathology reduction ratio reaching over 80%.
Owner:兆丰华生物科技(南京)有限公司 +1
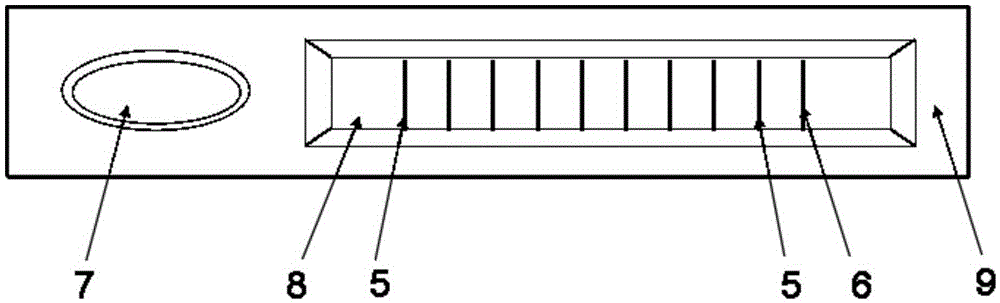
Colloidal gold test strip and test strip card for detecting IgM antibody, and preparation and detection method
InactiveCN105259345AThe detection process is fastImprove efficiencyBiological testingParainfluenza virus antigenIgm antibody
The invention provides a colloidal gold test strip for detecting an IgM antibody. The IgM antibody is a specific IgM antibody for nine respiratory tract infection pathogens, and the colloidal gold test strip comprises a sample pad, a conjugate pad, a nitrocellulose film and a water absorption pad which are attached to a polyvinyl chloride base plate in sequence; the conjugate pad is a glass fiber film wrapped with a rabbit-anti-human IgM antibody-colloidal gold conjugate; the nitrocellulose film is wrapped with 9 detection lines and 1 quality control line in sequence; the 9 detection lines are respectively a mycoplasma pneumoniae recombined antigen, a chlamydia pneumoniae recombined antigen, an influenza a virus antigen, an influenza B virus antigen, a sendai virus antigen, a legionella pneumophila antigen, a Coxiella burnetii antigen, a respiratory syncytial virus antigen and an adenovirus antigen, and the quality control line is a second antibody. The invention further provides a colloidal gold test strip card comprising the colloidal gold test strip and a colloid gold kit, a preparation method of the colloidal gold test strip card, and a method for realizing detection by adopting the colloidal gold test strip, the test strip card or the kit.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Soluble powder for treating respiratory illness of livestock and poultry
InactiveCN101468032ASolve the problem that easily makes the body develop drug resistancePromote oral absorptionPowder deliveryOrganic active ingredientsDiseaseTrimethoprim
The invention relates to soluble powder for treating respiratory diseases of poultry, comprising tylosin tartrate, trimethoprim and auxiliary materials. As an antibiotic medicine, the inventive medicament is mainly used to prevent and treat various respiratory tract infections and chronic respiratory diseases of poultry. The tylosin tartrate and the trimethoprim have compatibility when used together. The soluble powder simultaneously acts on different sensitive organisms and mycoplasma, solves the problem of medicament tolerance caused by solely using tylosin to treat, has good absorption and relatively high safety when orally taken.
Owner:TIANJIN RINGPU BIO TECH
Compounded medicine for anti pathogeny and immunoenhancement
InactiveCN1539441AImprove autoimmunityControl intrusionAntiviralsUnknown materialsDiseaseTherapy HIV
A Chinese medicine in the form of decoction, tincture, powder, capsule, or pill for softening blood vessel, improving immunity and treating the diseases caused by virus, chlamydia, or mycoplasma, such as AIDS and SARS is disclosed. Its advantages are high curative effect, broad spectrum and no toxic by-effect.
Owner:黄益新
Polycyclic agents for the treatment of respiratory syncytial virus infections
Owner:BIOTA SCI MANAGEMENT PTI LTD
Primer probe combination and kit for combined inspection of 15 kinds of respiratory tract infection pathogens
ActiveCN107937578ARapid combined detectionGuaranteed matchMicrobiological testing/measurementMicroorganism based processesStreptococcus pyogenesStaphylococcus aureus
The invention provides a pathogen inspection reagent, and concretely discloses a primer probe combination and a kit for combined inspection of 15 kinds of respiratory tract infection pathogens. By aiming at specific target sequences of the gene sequence conserved region of klebsiella pneumoniae, haemophilus influenzae, streptococcus pyogenes, staphylococcus aureus, escherichia coli, chlamydia pneumoniae, mycobacterium tuberculosis, stenotrophomonas maltophilia, baumanii, mycoplasma pneumoniae, enterococcus faecalis, ligionella pneumohpillia, streptococcus pneumoniae, bacillus pyocyaneus and mycobacterium abscessus, primers and probes which do not have mutual crossed reaction are designed, so that the problem that detection probes of different pathogens can easily generate mutual influenceor interference is solved; the combination and matching of different primers and probes are ensured; the goal of simultaneously performing efficient specific inspection at the same temperature can also be achieved.
Owner:西安九安生物技术有限公司
Target sequence used for detecting mycoplasma pnoumoniae and reagent box
ActiveCN1724686AEfficient detectionEffective distinctionMicrobiological testing/measurementNucleotideOligonucleotide primers
The invention supplies a method to rapidly test the genetic marker of Mycoplasma pneumoniae. It supplies the nucleotide sequence of Pl CytadhesinGene and primer and probe that is designed based on the sequence. The invention also supplies the method to rapidly testing the Mycoplasma pneumoniae by using the primer.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD
Traditional Chinese medicine for treating mycoplasma infection
InactiveCN101690784AImprove the immunitySignificant effectHeavy metal active ingredientsAnthropod material medical ingredientsMedicinal herbsAdditive ingredient
The invention relates to a traditional Chinese medicine, in particular to a traditional Chinese medicine for treating mycoplasma infection. The traditional Chinese medicine is made from the following raw Chinese medicinal herbs by weight portion: 8-15 portions of blackberry lily, 20-25 portions of ephedra, 20-25 portions of almond, 50-60 portions of gypsum, 20-25 portions of aster, 20-25 portions of common coltsfoot flower, 8-15 portions of shizandra, 8-16 portions of pinellia ternate, 3-5 portions of asarum, 35-40 portions of dried tangerine or orange peel; 8-15 portions of rhubarb, 7-12 portions of radix scutellariae, 50-60 portions of cornu saiga tatarica, 30-40 portions of artificial bezoar; 18-25 portions of lapis chlorite, 45-55 portions of Sichuan fritillary bulb, 2-5 portions of cinnabar, 8-15 portions of ballonflower root; 50-60 portion of honeysuckle, 8-15 portions of fructus forsythiae, 8-17 portions of kudzu vine root, 10-20 portions of cicada slough, 10-20 portions of mulberry bark, 8-17 portions of radix stemonae, 10-15 portions of tuber of dwarf lilyturf, 16-22 portions of hogfennel root and 15-22 portions of liquorice. The traditional Chinese medicine has obvious efficacy of treating fever, cough, yellow phlegm coughing, heavy night wheezing and inappetence which are caused by the mycoplasma infectionin in short time without toxic and side effect and dependence. The traditional Chinese medicine is pure Chinese herbal preparation which contains no western medicine component and has the efficacy of regulating the vital energy, tonifying the spleen, eliminating the dampness, relieving the cough and reducing the sputum.
Owner:马增惠
Wild jujube seeds extract and preparation method and use thereof
ActiveCN101455736AMake up for the loss caused by poor prevention effectMake up for the lossAntibacterial agentsAntiviralsDiseaseEscherichia coli
The invention relates to a wild jujube seed extract which is prepared by the following method: crushing wild jujube seeds, screening, soaking with ethanol to extract, after removing alcohol, adding water, uniformly stirring, leaching with aqueous n-butylalcohol, lastly concentrating and airing. The wild jujube seed extract of the invention can be prepared into oral liquid, granules, injection or freeze-drying powder injection through routine techniques. The wild jujube seed extract can be used prepare medicament compositions preventing poultry mycoplasma, Escherichia coli, brusal disease virus, avian H9 influenza virus disease, makes up for loss caused by poor preventing effect of existing bacterin in our country, substitutes bacteriophage to prevent and treat poultry resistant virus disease to control prevalence of infectious disease and solve a problem of bacteriophage residue in foods, thus guaranteeing food security and promoting export competitive ability of animal products.
Owner:CHINA AGRI UNIV
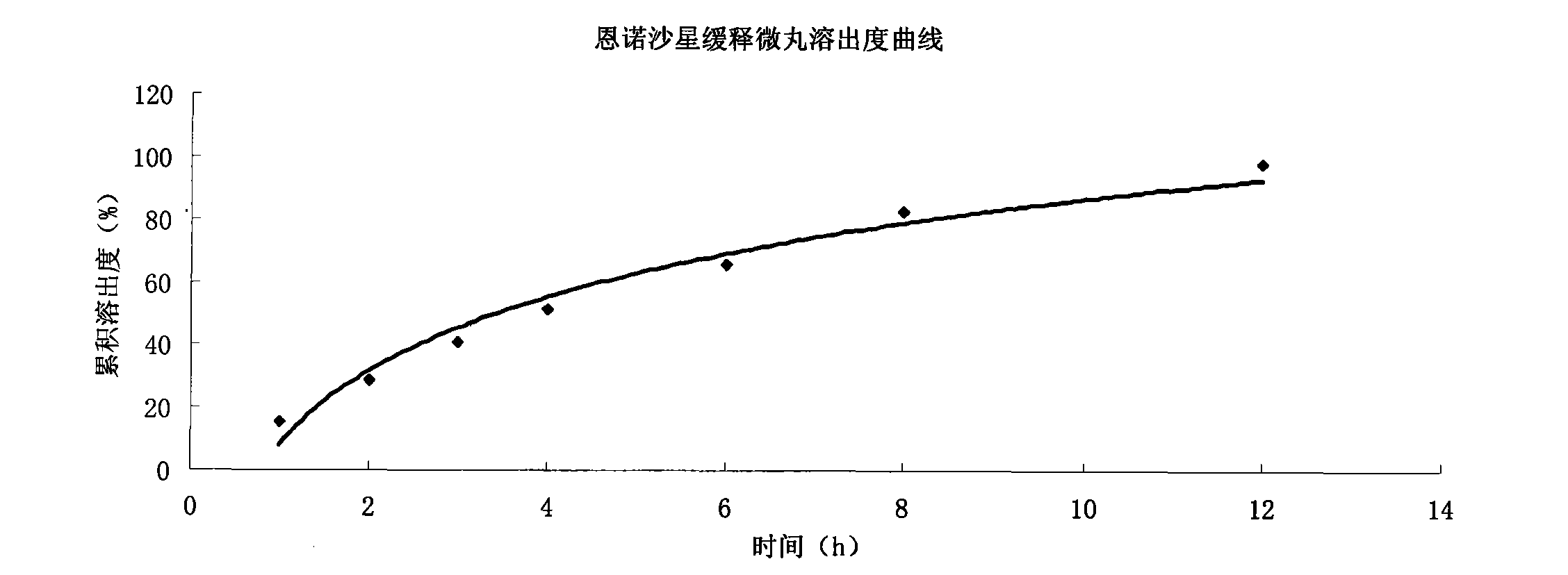
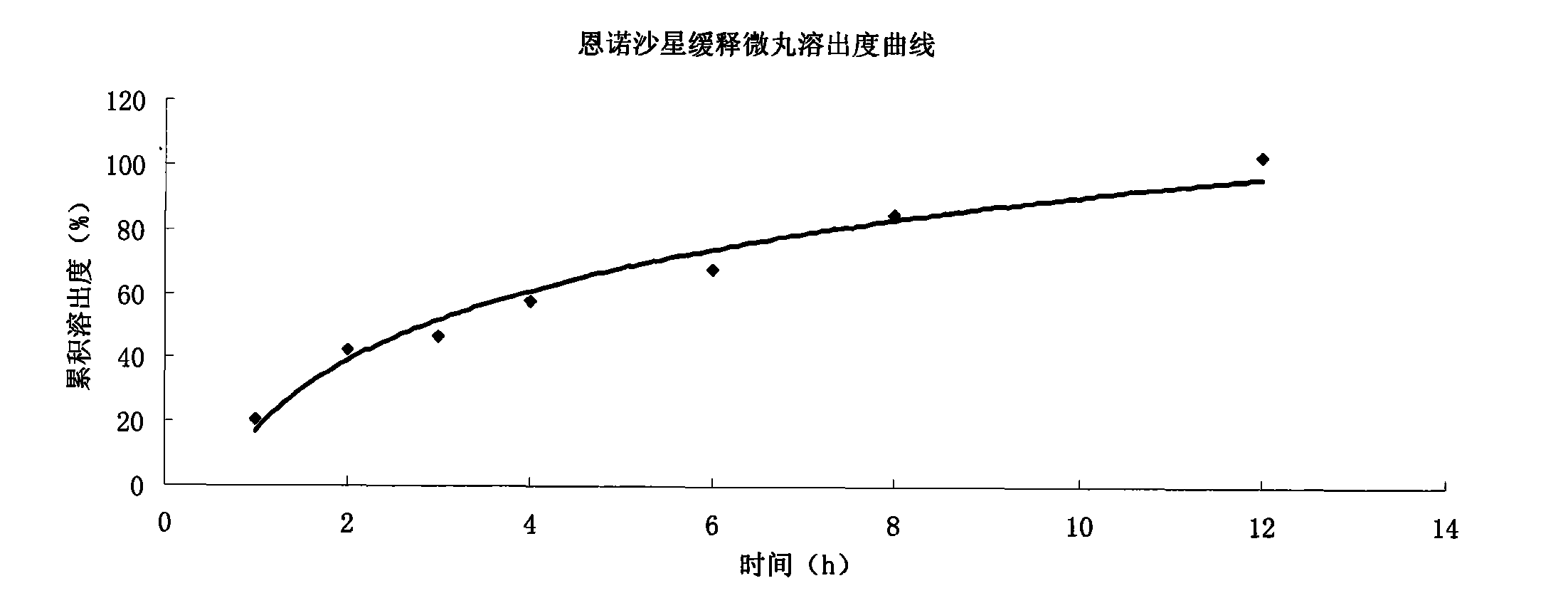
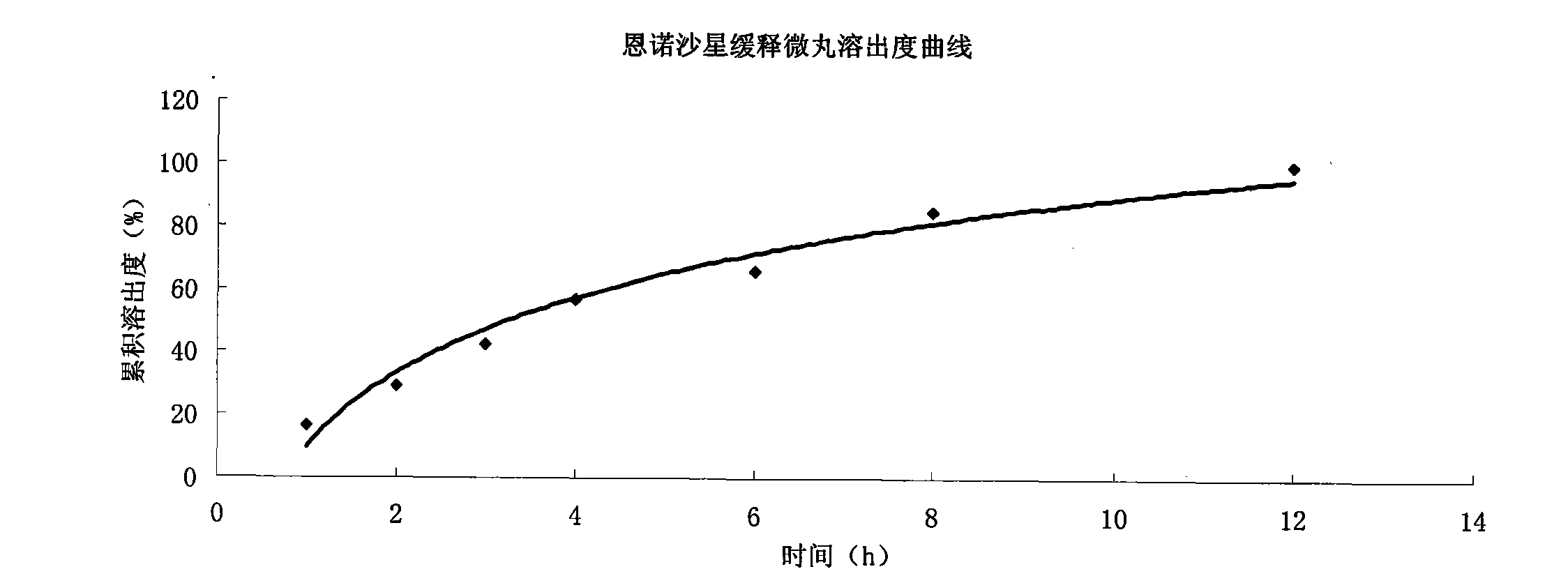
Enrofloxacin slow-release micropill for livestocks, and preparation method of same
InactiveCN102648896AExpand the scope of clinical applicationImprove the characteristics of easy color change when exposed to lightAntibacterial agentsOrganic active ingredientsPharmaceutical formulationSodium carboxymethyl starch
The invention relates to the field of pharmaceutical preparations and particularly relates to a slow-release micropill preparation containing enrofloxacin and a preparation method of the preparation. The slow-release micropill provided by the invention is formed by coating an enrofloxacin micropill; the enrofloxacin micropill comprises enrofloxacin and auxiliary material and is formed through extruding and rounding; the auxiliary material is any one or more of microcrystalline cellulose, starch, cane sugar, artificial gum, lactose and sodium carboxymethyl starch; according to weight percent, the auxiliary material in the micropill accounts for 70% to 95%; and coating is made of high-molecular enteric material, film forming material, opaquer and the like. The slow-release micropill preparation has the characteristics of slow release and high bioavailability, can be used for treating bacteria and mycoplasma infection of livestocks, and has better curative effect on chronic respiratory diseases, colibacillosis and salmonellosis, and the frequency of medicine taking can be reduced; and in addition, the slow-release micropill provided by the invention has the advantages that the stability of medicine is improved, and peculiar bitter of enrofloxacin can be covered completely, so that feeding intake of the animals is not influenced, and the recovery rate is improved.
Owner:ZHENGZHOU FUYUAN ANIMAL PHARMA
Sophora fungus mycoplasma extract identification and detection method
ActiveCN102621260AControl contentRaise quality standardsComponent separationMonosaccharide compositionAnion-exchange chromatography
The invention relates to a sophora fungus mycoplasma extract identification and detection method. The identification method is characterized in that an anion exchange chromatography method is used for establishing chromatographic fingerprint composed by monosaccharide of proteoglycan protein in the sophora fungus mycoplasma extract, and the quality is controlled by comparing the similarity of a sample fingerprint spectrum and a fingerprint spectrum of a standard extract. The detection method is characterized in that the anion exchange chromatography method is used for detecting the monosaccharide composition and content of proteoglycan protein of the sophora fungus mycoplasma extract, thereby the content limit can be made. The fingerprint spectrum of the anion exchange chromatography method combines the content determination method to realize the effective control of quality of the sophora fungus mycoplasma extract and the relative products as well as distinguish with other products. The method of the invention has the advantages of rapidity, convenience and strong specificity.
Owner:QIDONG GAITIANLI MEDICINES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com