Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
A technology of Mycoplasma suis pneumonia and diluent, which is applied in the field of diluent, can solve the problems of massive expression, lack of antigen immunity, inability of pigs to produce P97R1 antibody, etc., and achieves simple operation, good acupuncture, and enhancement of cellular and humoral immune stimulation capabilities. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
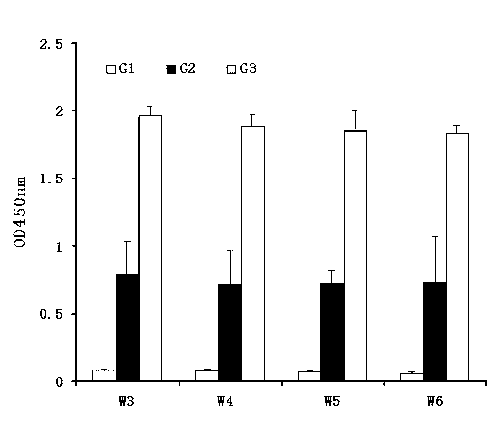
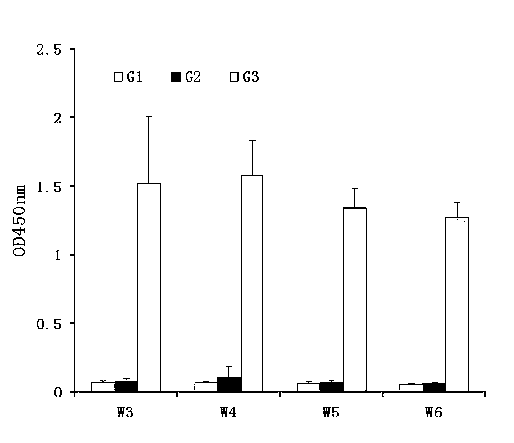
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of recombinant Mycoplasma hyopneumoniae P97R1 protein
[0039] According to the genome sequence of Mycoplasma hyopneumoniae published by GenBank, primers were designed to amplify the P97R1 fragment. Upstream primer: 5'-CCGAATTCTTTACCTCAGCCGCCAGCAG-3' (SEQ ID No.3); Downstream primer: 5'-GCGAAGCTTAAGCCATTGGGAAATAGTCT-3' (SEQ ID No.4). The upstream and downstream primers contain EcoR I site and Hind III site. Using 168 F344 strains of Mycoplasma hyopneumoniae genomes as templates, the P97R1 gene fragment was amplified by PCR. EcoR I and Hind After Ⅲ double enzyme digestion, it was inserted into the pET-32a(+) vector to obtain the prokaryotic plasmid vector pET-32a(+)-P97R1 expressing the recombinant P97R1 protein. Escherichia coli BL-21 (DE3) was transformed, positive clones were screened and verified by DNA sequencing, and recombinant genetically engineered bacteria expressing recombinant P97R1 protein were obtained.
[0040] The gene se...
Embodiment 2
[0046] Example 2 Preparation of Immunostimulatory Complex ISCOM-P97R1 Containing Recombinant P97R1 Protein
[0047] (1) Preparation of 20% Mega-10 stock solution
[0048] Dissolve 0.4 g of Mega-10 (also known as "N-quinoyl-N-methylglucamine") dry powder in 1.6 ml of distilled water, dissolve at 37°C and dilute to 2 ml.
[0049] (2) Preparation of lipid mixture stock solution
[0050]Add 100 mg phosphatidylcholine and 100 mg cholesterol to 10 ml 20% Mega-10, heat slightly (30-60°C) and stir slowly to dissolve completely, filter and aliquot, store at -20°C for later use.
[0051] (3) Preparation of Quil A solution
[0052] Dissolve 25 mg of Quil A in 4.5 ml of phosphate buffered saline, pH 7.2-7.4.
[0053] (4) Mycoplasma hyopneumoniae P97R1 protein solution
[0054] The recombinant P97R1 protein was prepared and purified by the method described in Example 1, dissolved in phosphate buffer, the protein concentration was measured and diluted to 10 mg / ml, and stored at -20°...
Embodiment 3
[0057] Embodiment 3 Preparation of special diluent for swine mycoplasma pneumonia vaccine
[0058] When preparing the special diluent for swine mycoplasma pneumonia vaccine containing only ISCOM-P97R1 components, the preparation method is as follows: take an appropriate amount of ISCOM-P97R1 stock solution with a Quil A concentration of 1.67 mg / ml, and dilute it with an appropriate amount of phosphate buffer to obtain Each ml solution contains: Quil A 0.1-1 mg, cholesterol 0.02-0.2 mg, phosphatidylcholine 0.02-0.2 mg, P97R1 antigen 0.4-4 mg, ISCOM-P97R1 vaccine special diluent.
[0059] When preparing the special diluent for mycoplasma pneumonia vaccine containing ISCOM-P97R1 and other water adjuvant components, the preparation method is as follows: first prepare ISCOM-P97R1 mother solution, levamisole mother solution, astragalus polysaccharide mother solution, and carbomer mother solution respectively, Then dilute to the required concentration with phosphate buffer solution t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com