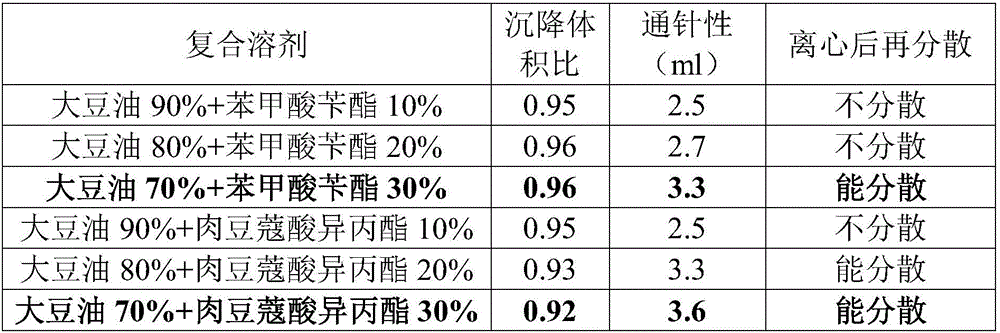
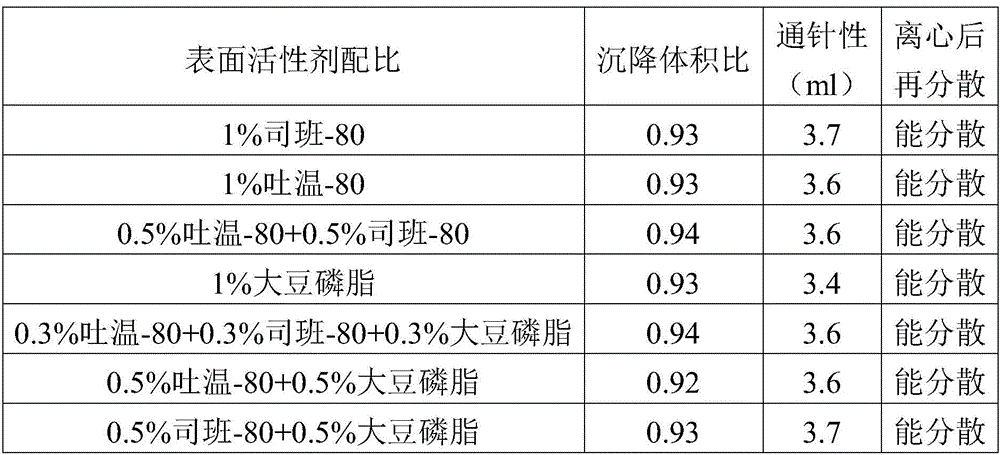
Patents
Literature
57results about How to "Good acupuncture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
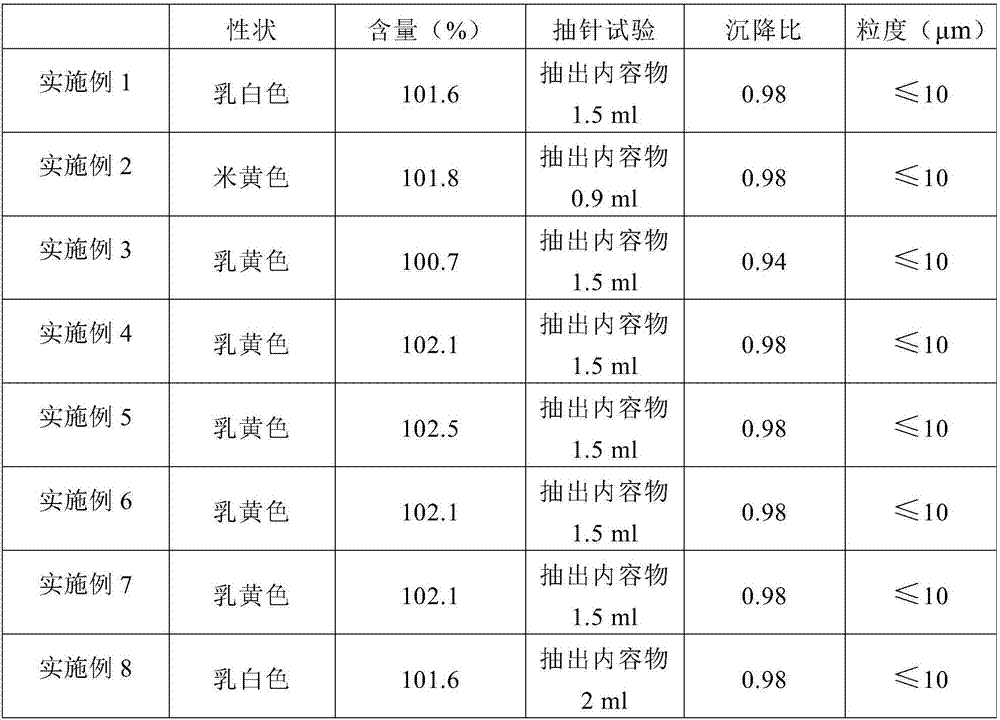
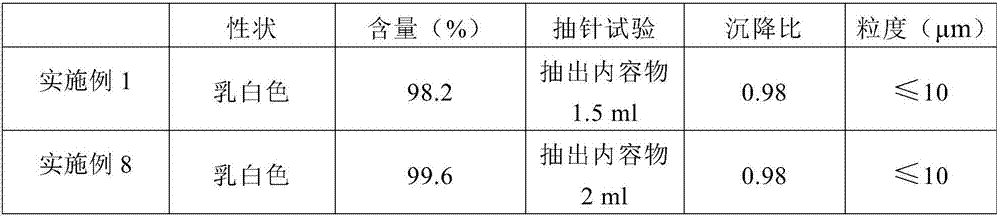
Insoluble medicine gel composition and preparation method for same
InactiveCN103054794AReduce dosageEliminate hidden dangers such as allergiesAerosol deliveryOintment deliveryRelease timePharmaceutical formulation
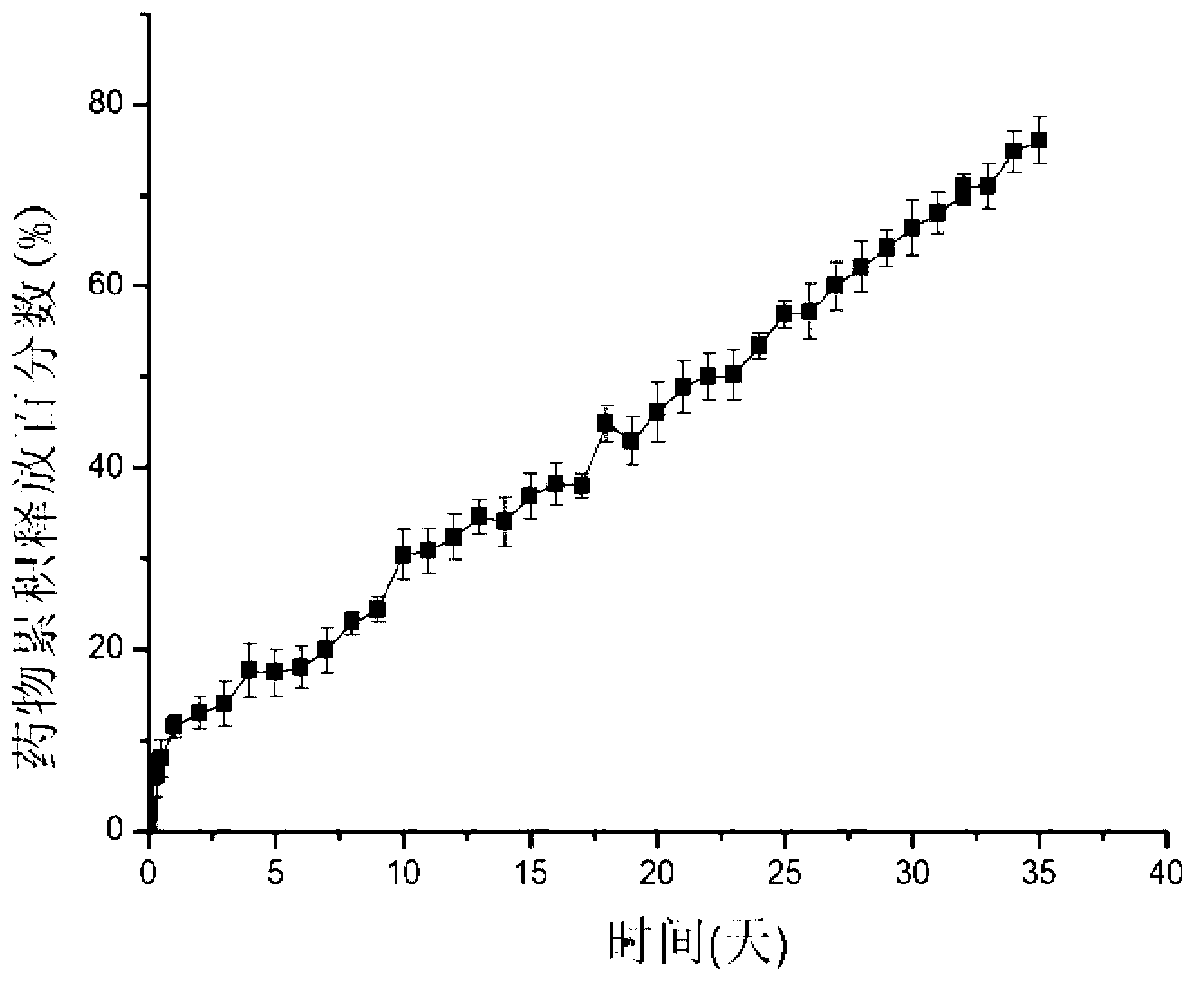
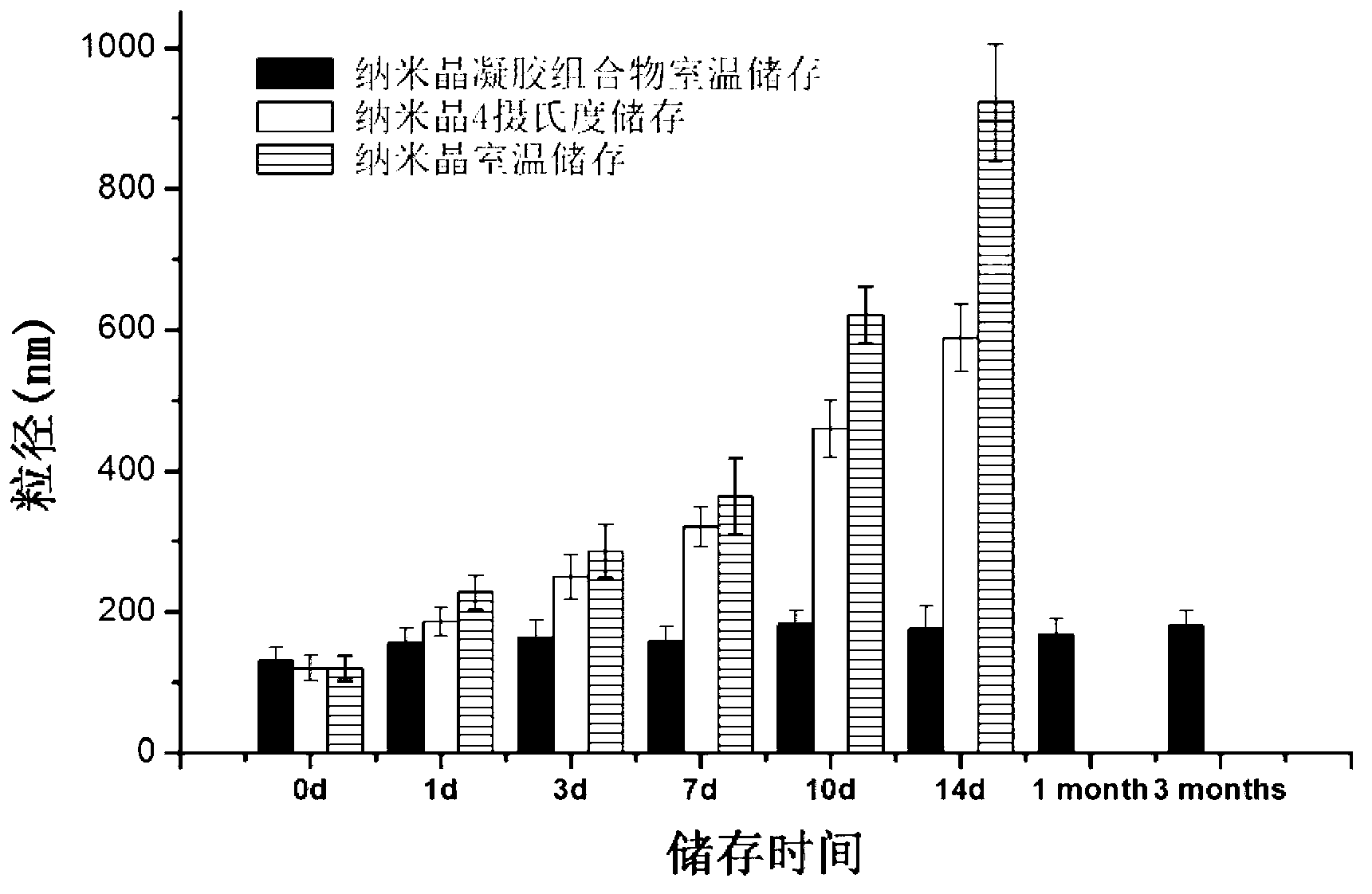
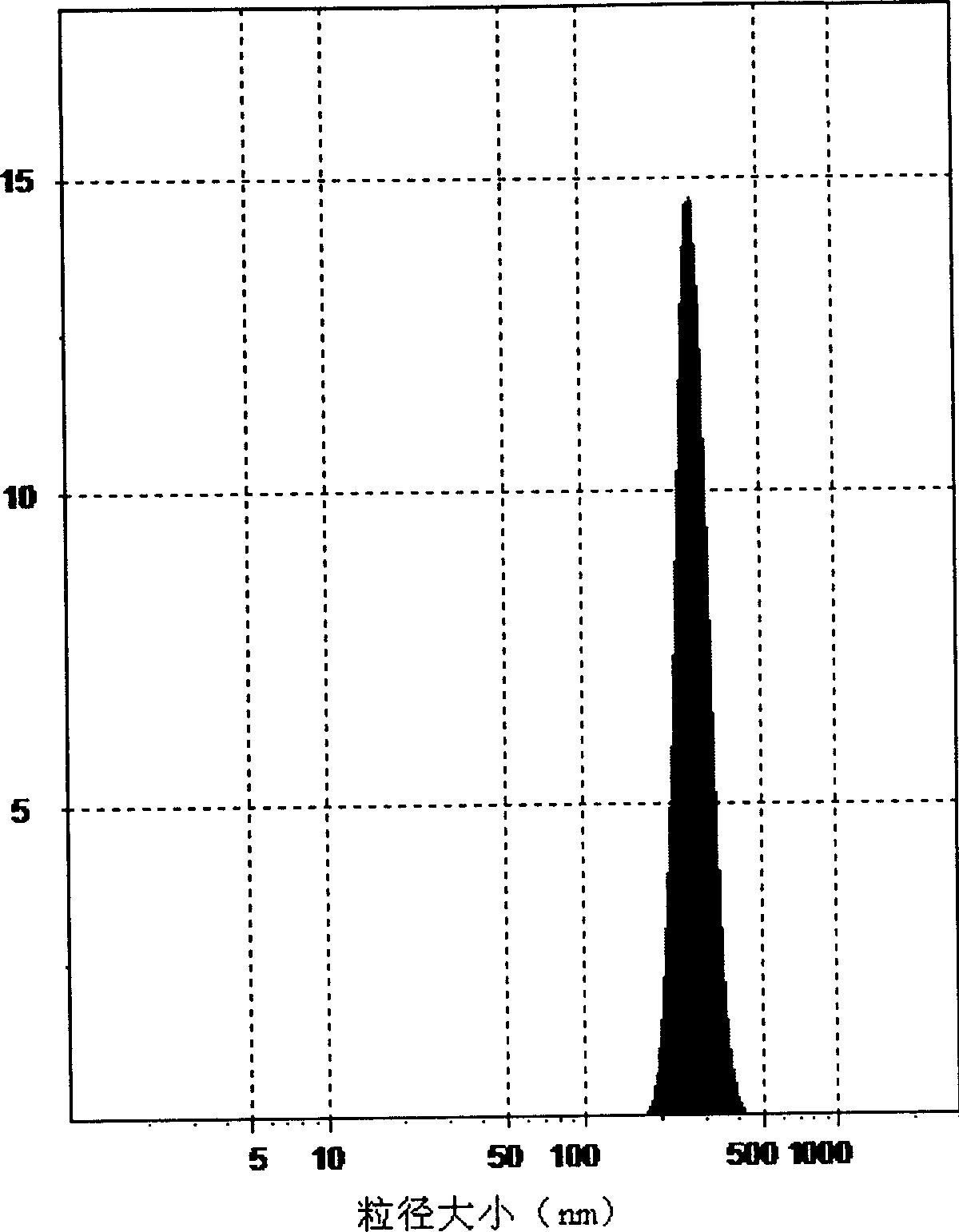
The invention discloses an insoluble medicine gel composition and a preparation method for the same, and particularly relates to a temperature-sensitive gel composition containing insoluble medicine nanocrystallines and a preparation method for the same. The medicine nanocrystallines are uniformly dispersed in a temperature-sensitive gel, and belongs to the field of medicinal preparation. The preparation process comprises the following steps of: preparing a medicine nanocrystalline suspension at first, and then directly adding a temperature-sensitive material into the medicine nanocrystalline suspension and preparing the medicine nanocrystalline gel composition; or preparing medicine nanocrystallines and a blank temperature-sensitive gel respectively, and then uniformly dispersing the medicine nanocrystallines in the prepared temperature-sensitive gel. The insoluble medicine gel composition disclosed by the invention is simple in preparation method, and capable of improving the stability of common medicine nanocrystallines, increasing the medicine-loading capacity of the insoluble medicine gel, and obviously prolonging the release time of the medicine.
Owner:PEKING UNIV
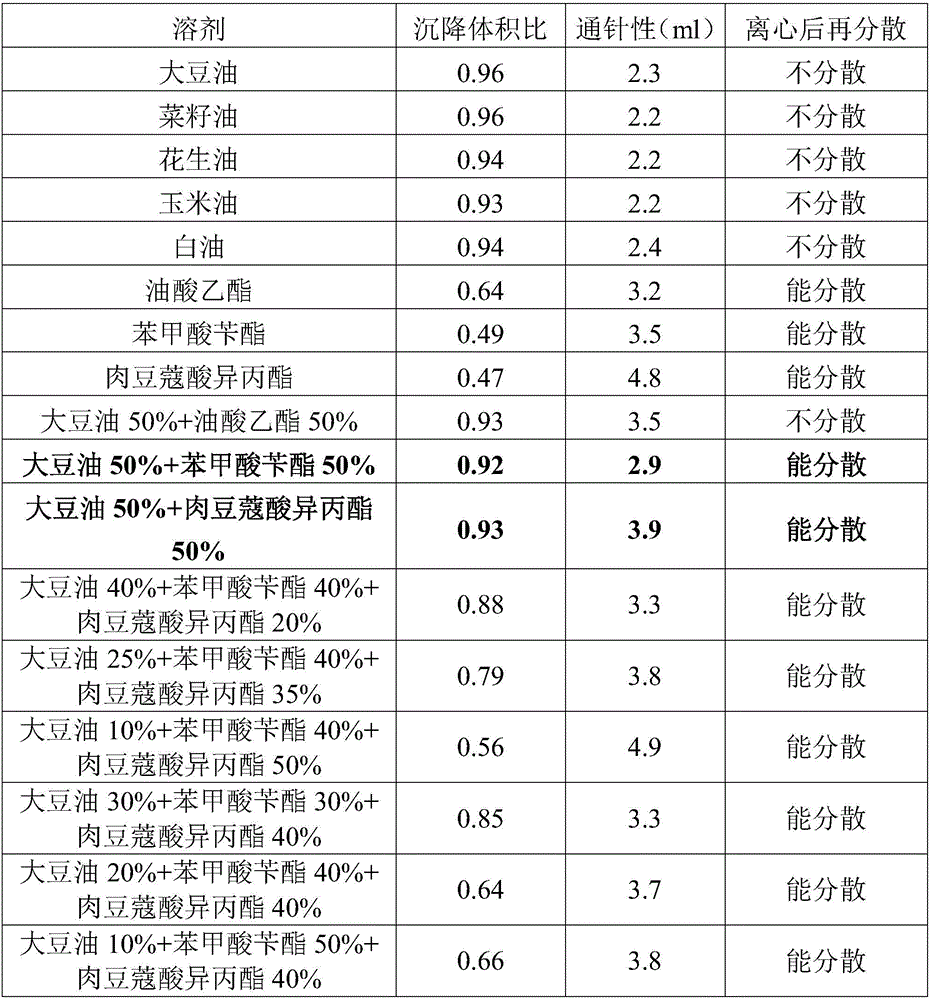
Precursor suspension of lyotropic liquid crystal and preparation method thereof
ActiveCN103040741AHigh viscosityHigh strengthSolution deliveryEmulsion deliveryOrganic solventUltimate tensile strength
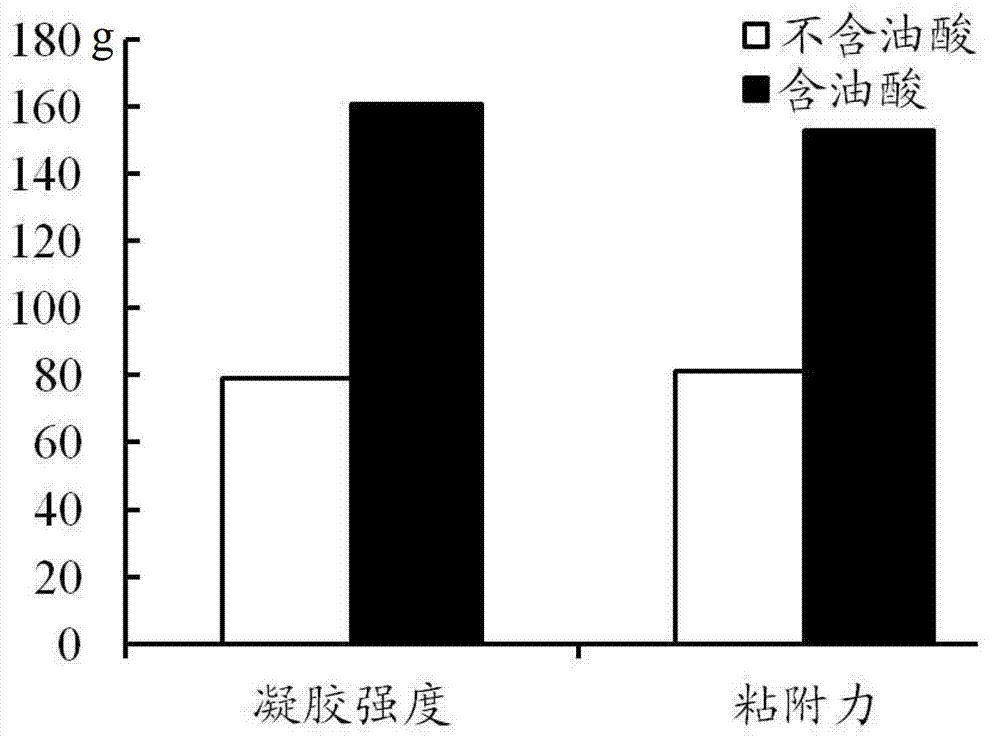
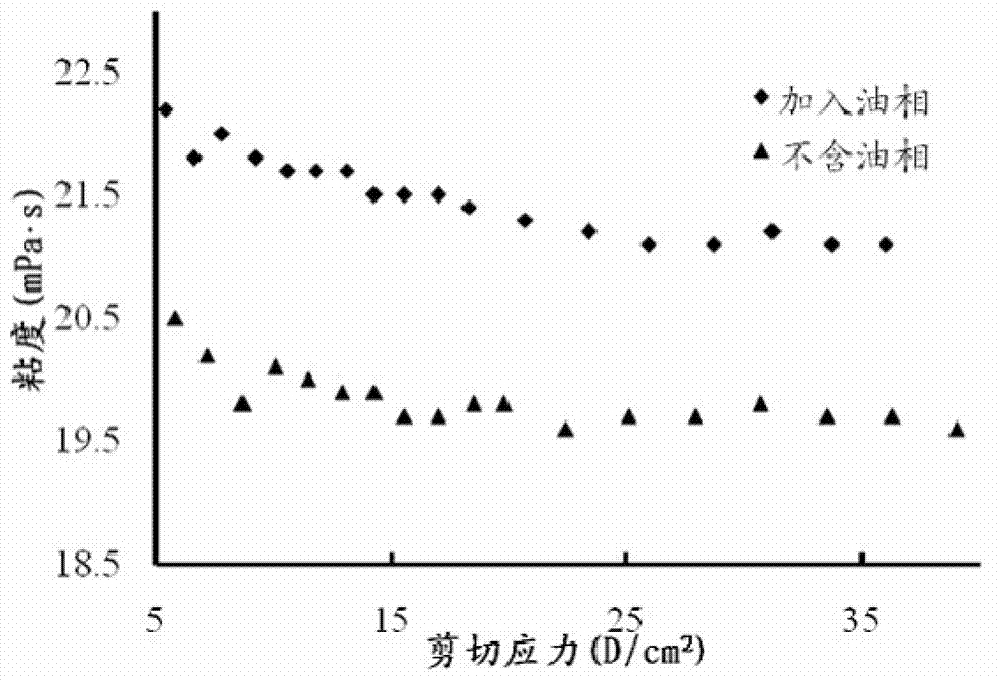
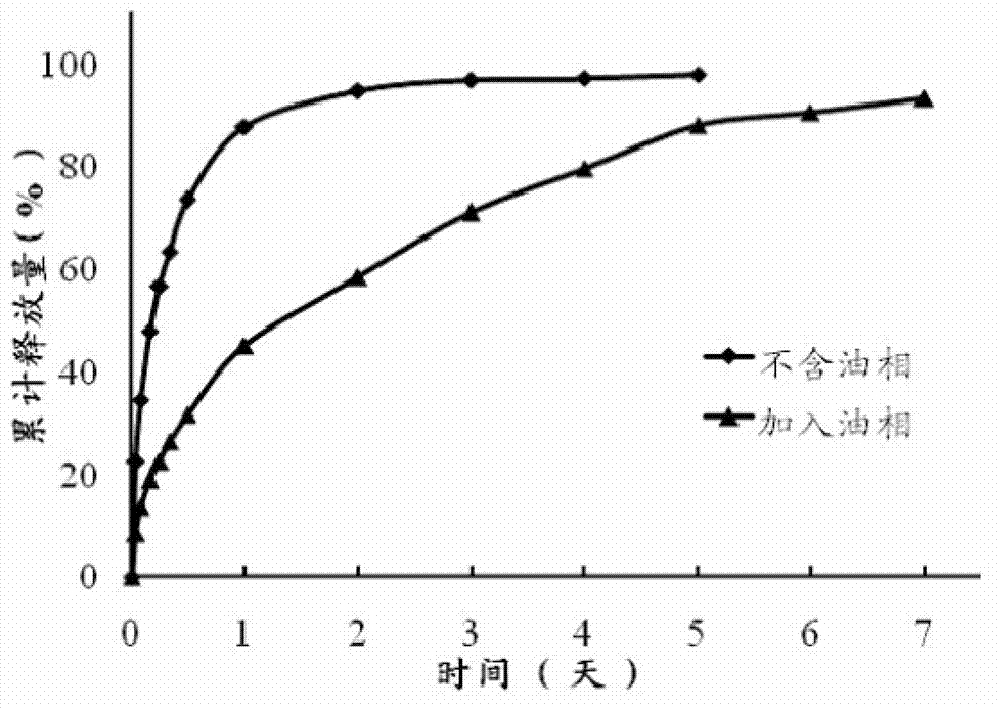
The invention discloses a precursor suspension of a lyotropic liquid crystal. The precursor suspension comprises lyotropic liquid crystal material, organic solvent, oil phase and a drug, wherein the weight percentage of the oil phase in the precursor suspension is 2-50 percent, the weight percentage of the drug in the precursor suspension is 1-30 percent, and the weight ratio of the lyotropic liquid crystal material and the organic solvent in the precursor suspension is 2-9:1. According to the invention, through the adding of the oil phase into the precursor suspension, the stability of the suspension is improved, the sedimentation rate is reduced, and the strength and the adhesive force of the gel formed are enhanced at the same time; the gel formed in the body is more liable to stay at a lesion location and less liable to be relocated and the shape is less liable to be damaged by the mechanical motion of the body, so that the drug therapy can be located effectively; and the preparation technology is simple and the precursor suspension of the lyotropic liquid crystal is a partial slow-release drug delivering system provided with a favorable perspective.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
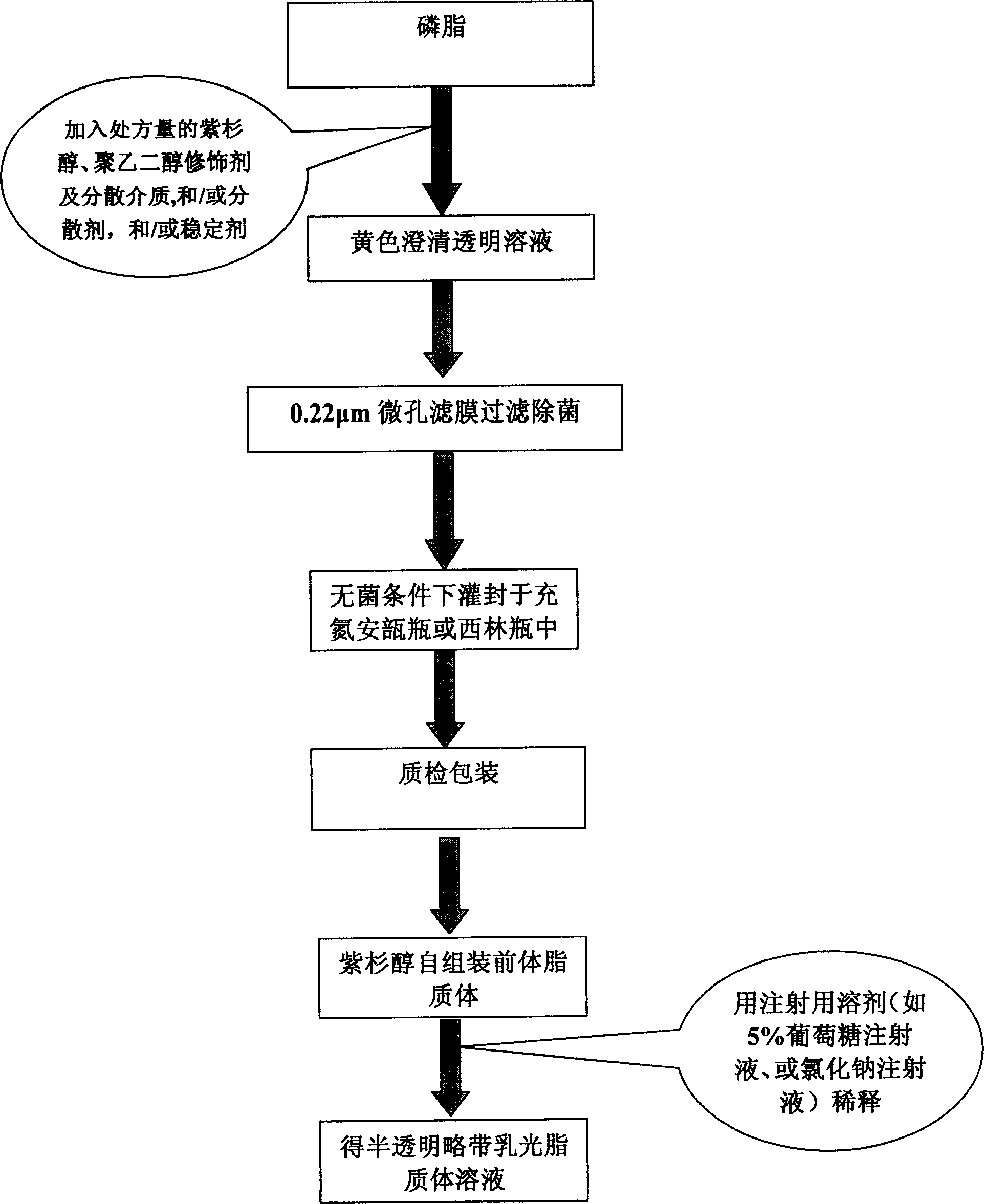
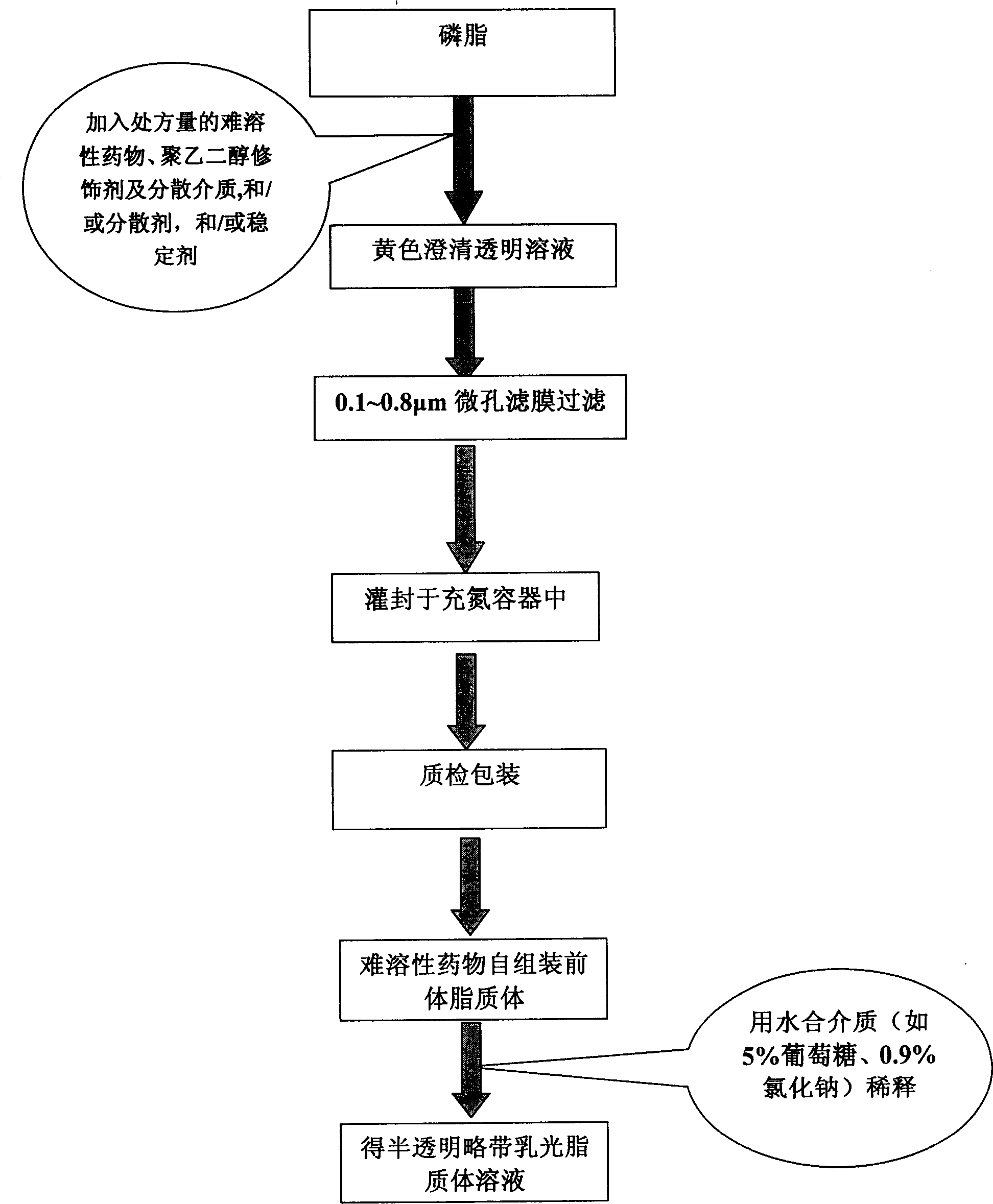
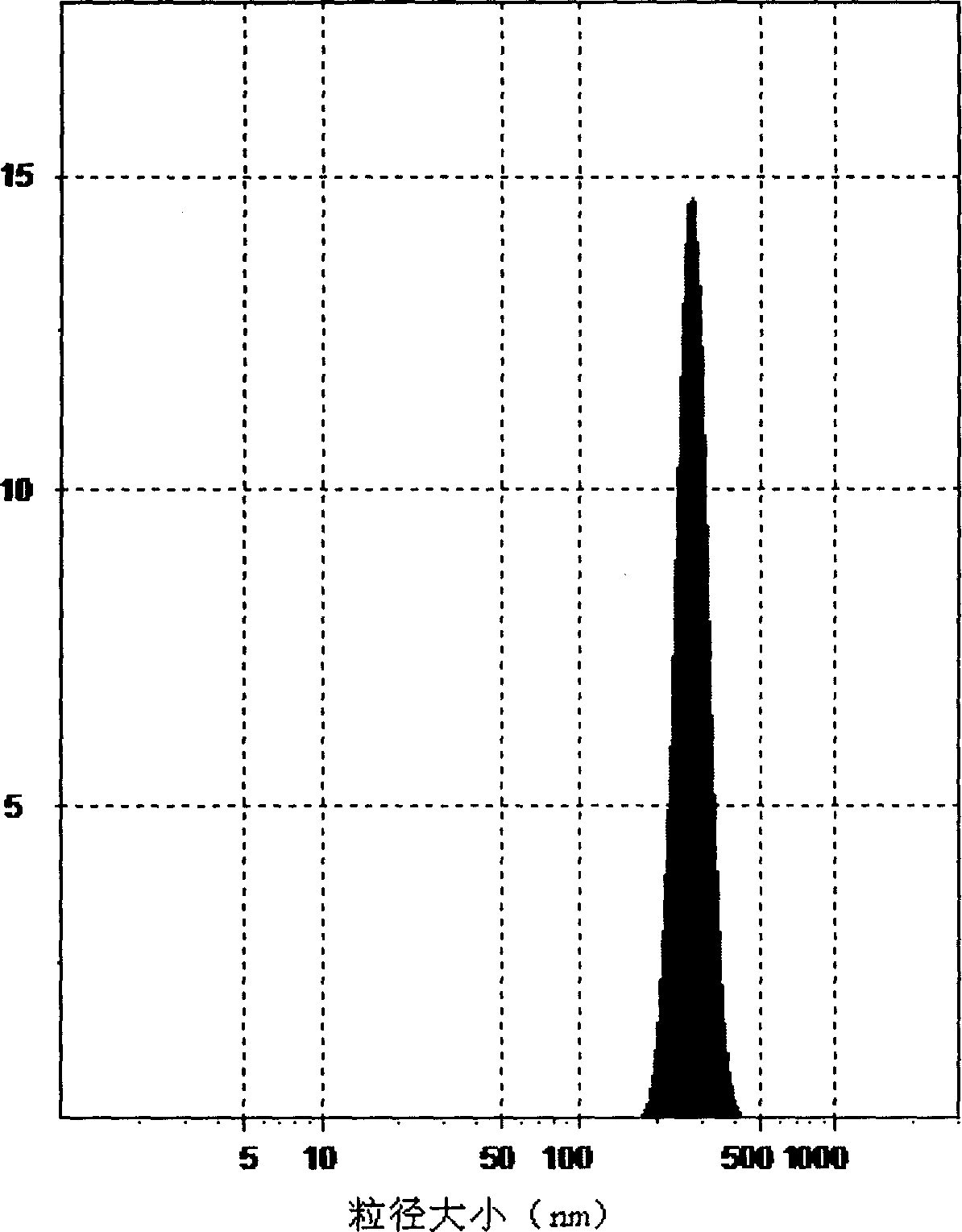
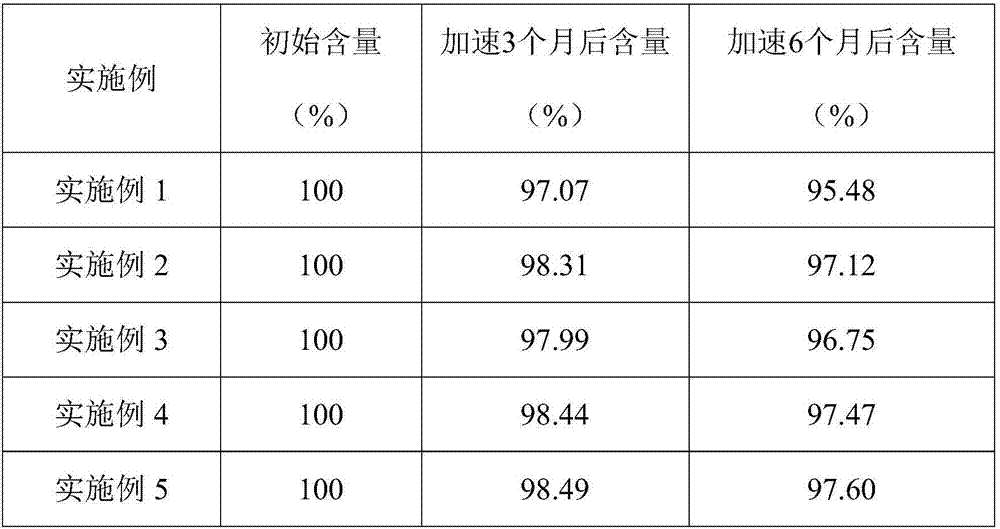
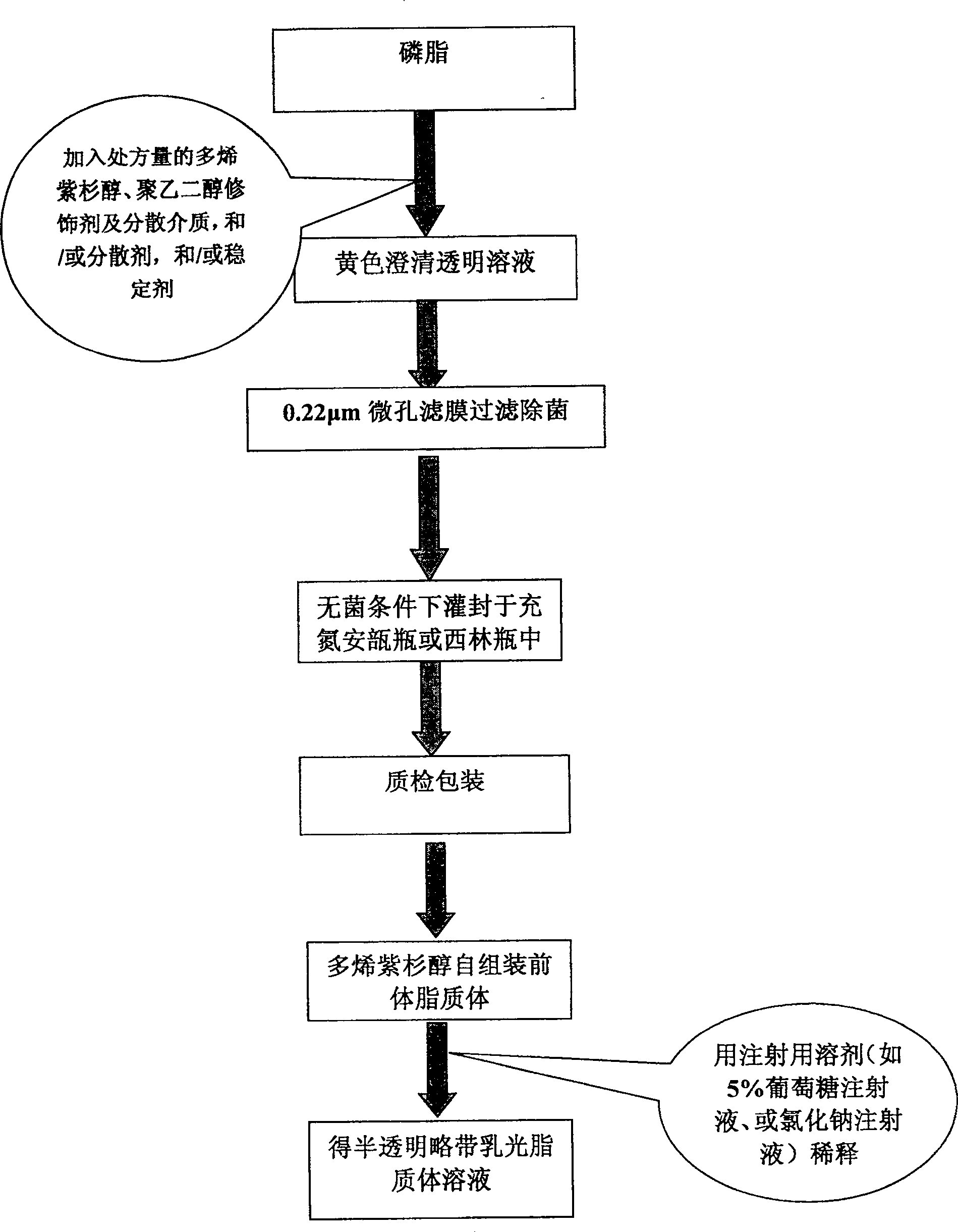
Poly olefinic taxadol self assembled precusor liposome and its preparation method
ActiveCN1823732AImprove product qualityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsPhospholipinPolymer science
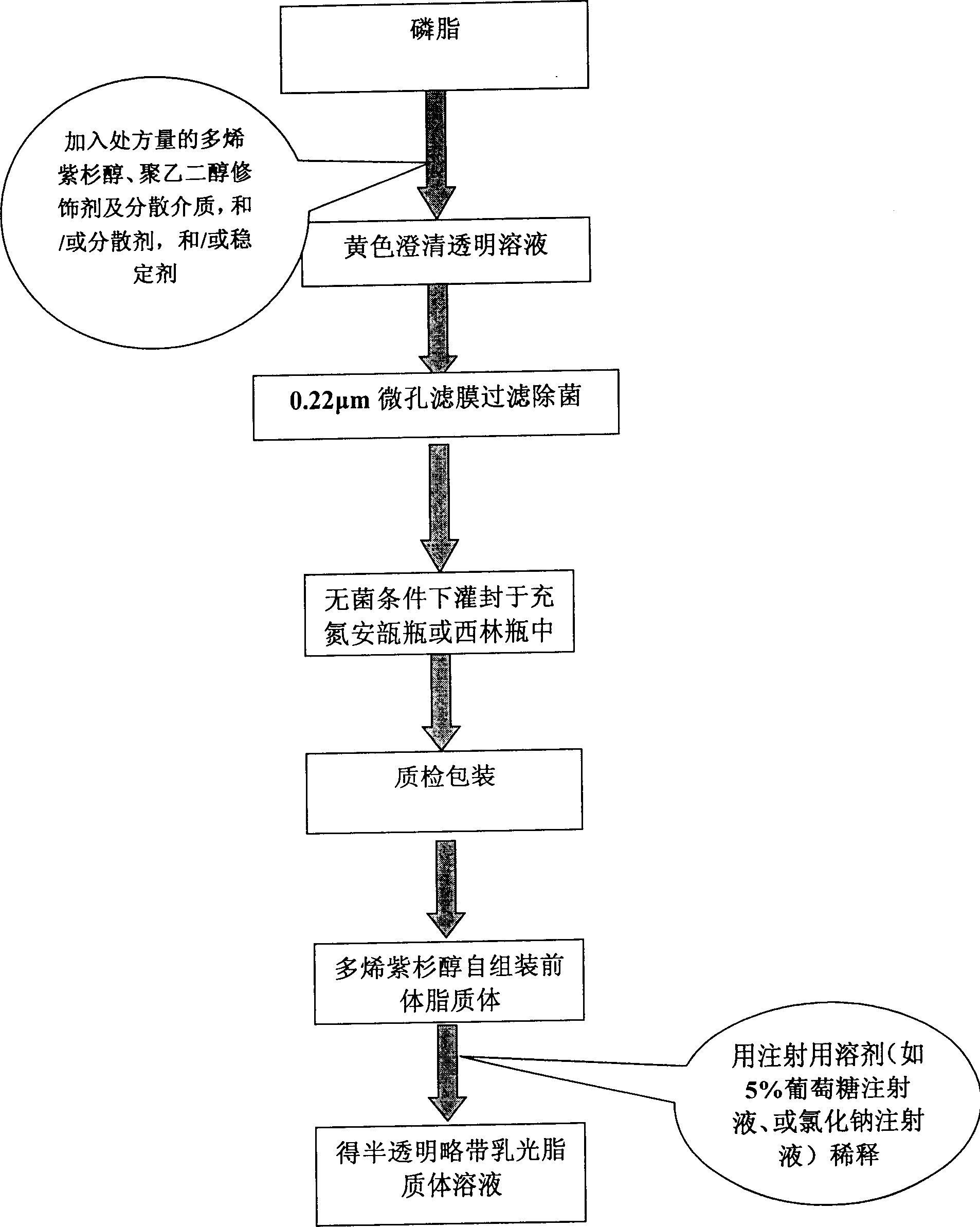
A self-assembled precursor liposome of polyenic taxusol is proportionally prepared from polyenic taxusol, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press-filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV +1
Intravenous nanometer suspension injection contg. oxaliplatin platinum phospholipid compound
InactiveCN1868455AHigh drug loadingImprove stabilityAntineoplastic agentsOil/fats/waxes non-active ingredientsFreeze-dryingPhospholipid complex
A nano-class suspension injection of oxaliplatin-phoshpatide composition for intravenous injection is proportionally prepared from oxaliplatin, surfactant, pH regulator, isotonic agent, antioxidant, water for injection, and optional excipient (for the freeze-dried powder injection).
Owner:SHENYANG PHARMA UNIVERSITY
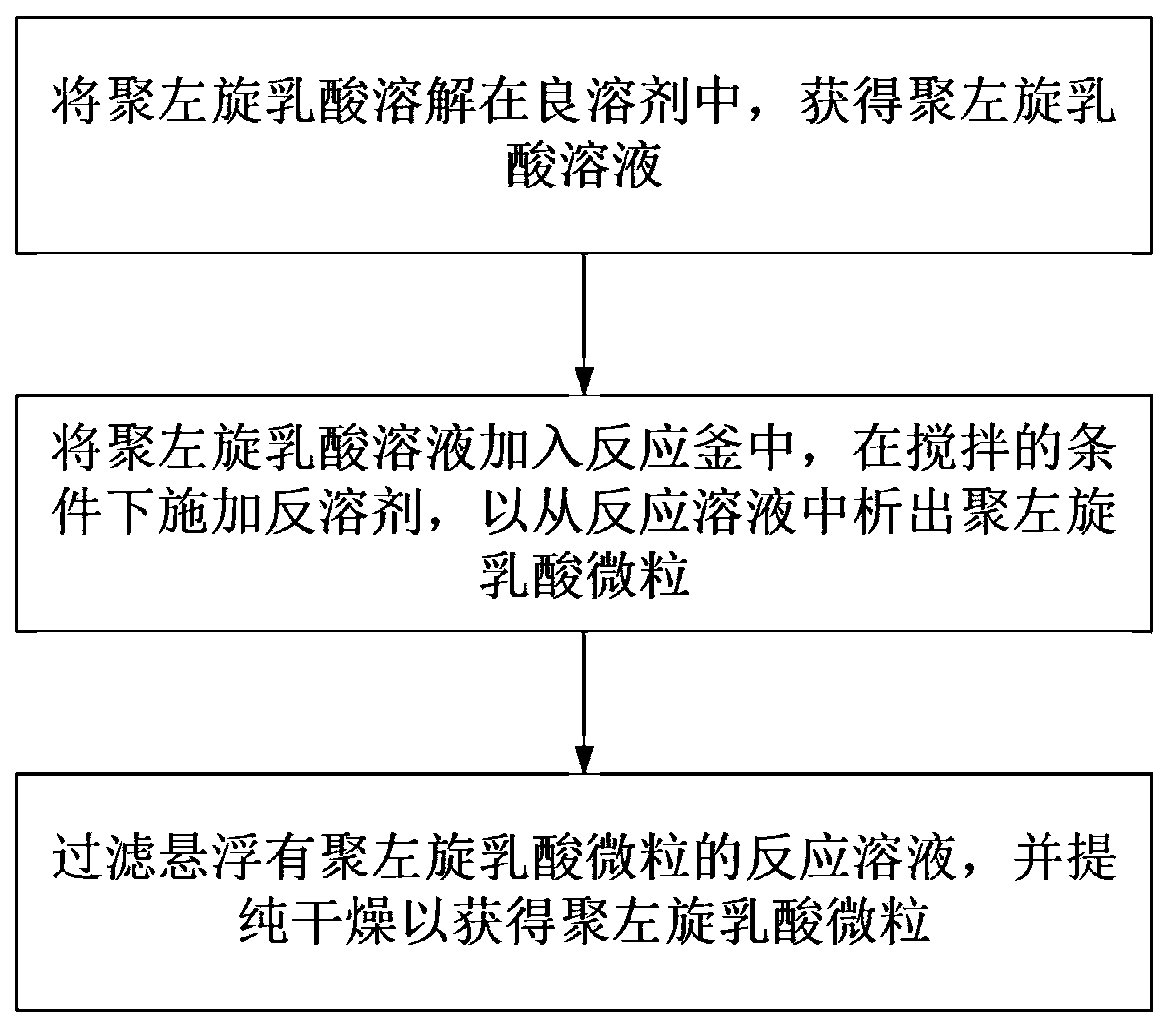
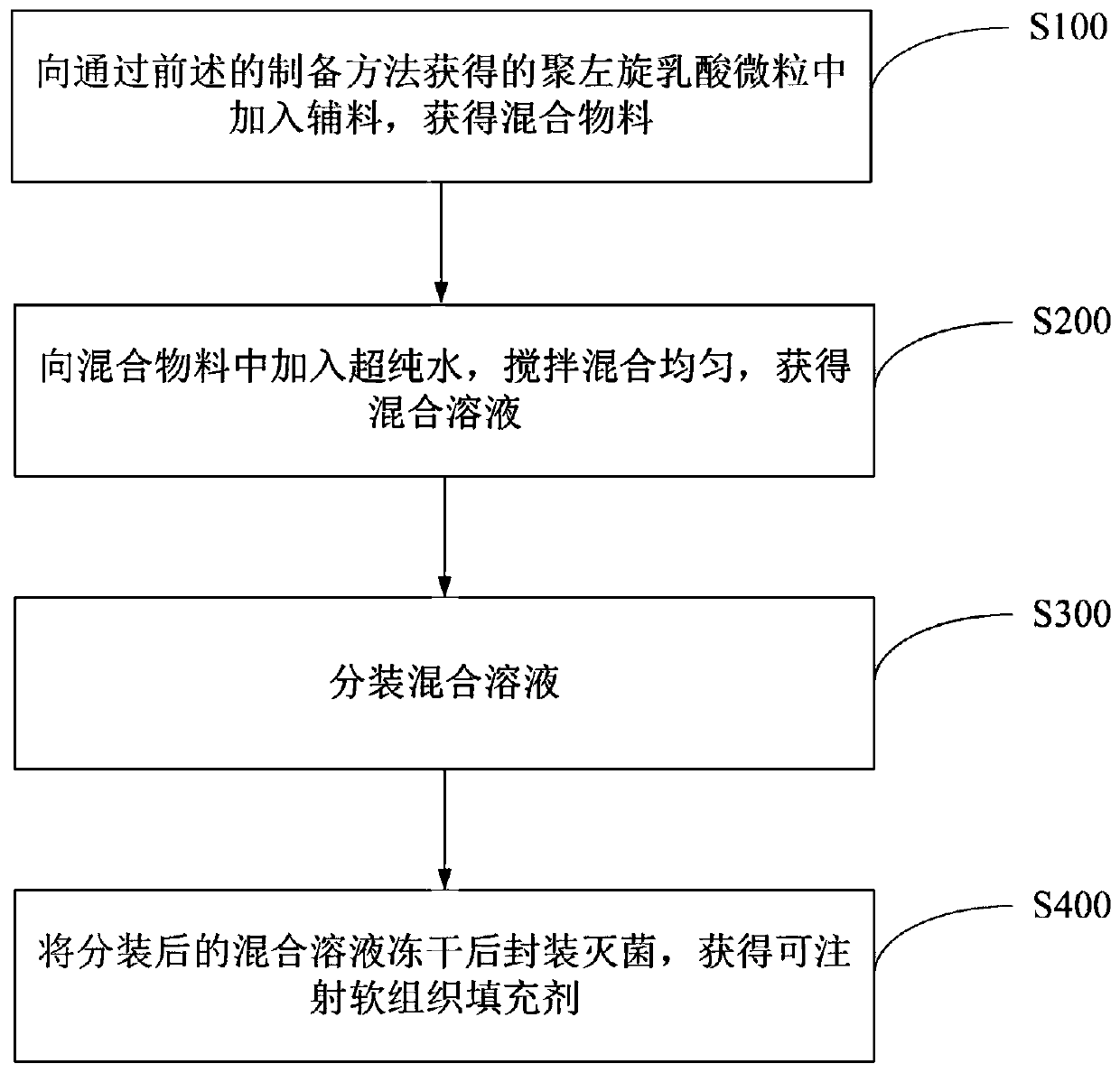
Preparation methods of poly-l-lactide particles and injectable soft tissue filler
InactiveCN110841108AEasy to operateReduce manufacturing costPharmaceutical delivery mechanismTissue regenerationLactideOrganic chemistry
The invention provides a preparation method of poly-l-lactide particles and a preparation method of an injectable soft tissue filler. The preparation method of the poly-l-lactide particles includes the steps: first, dissolving poly-l-lactide in good solvents to obtain poly-l-lactide solution; second, adding the poly-l-lactide solution into a reactor and adding anti-solvents under the condition ofstirring to separate out the poly-l-lactide particles from reaction solution; third, filtering the reaction solution with suspending poly-l-lactide particles, purifying and drying the reaction solution to obtain the poly-l-lactide particles. The poly-l-lactide particles are prepared by a soap-free good solvent-anti-solvent precipitation method, addition of any emulsifiers and dispersing agents isomitted in the preparation process of the particles, a technological process is simple in operation, and the preparation method is low in production cost, high in production efficiency and suitable for industrial amplified production.
Owner:南京思元医疗技术有限公司
Taxdol self assembled precusor liposome and its preparation method
ActiveCN1823734ASolve the "bottleneck" problem that is difficult for efficient industrial productionImprove product qualityOrganic active ingredientsOil/fats/waxes non-active ingredientsDispersed mediaPolyethylene glycol
A self-assembled precursor liposome of taxusol is proportionally prepared from tausol, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press-filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV +1
Antibiotic oil-water double suspension type injection emulsion for livestock and preparation method thereof
ActiveCN102119923AAvoid drug resistanceLess irritatingAntibacterial agentsImmunological disordersIntramuscular injectionInjection emulsion
The invention discloses an antibiotic oil-water double suspension type injection emulsion for livestock. The effective antibiotic components of the antibiotic oil-water suspension type injection emulsion are respectively suspended in an oil phase and a water phase, the water phase is in the outer layer and the oil phase is enveloped therein. In the invention, based on the metabolic characteristics of the effective antibiotic components in a target animal body, the effective antibiotic components of the end products of the new preparation are distributed in an equivalent or nonequivalent mode in different oil and water carriers to form the double suspension type emulsion. After the double suspension type emulsion is administered by intramuscular injection, the antibiotics present a split type secondary release characteristic, thus achieving quick acting and long acting combination. The invention has the characteristics that: (1) the preparation process is relatively simple, the materials can be acquired easily, and the preparation process is particularly suitable for large-scale production; (2) medicaments with different amounts of carriers can be designed according to the pharmacokinetic characteristics of different target animals on different antibiotics, thus achieving quick acting and long acting combination and the effect on treating infection, and the medicament is particularly suitable for clinical application; and (3) the carriers of the double suspension type emulsion can carry health raw materials for animals for preparing compound health products for animals.
Owner:HUNAN AGRICULTURAL UNIV +2
Florfenicol suspension injection and preparation procedure thereof
InactiveCN102920655AGood acupunctureImprove stabilityAntibacterial agentsOrganic active ingredientsButylated hydroxytolueneAntibacterial activity
The invention discloses a florfenicol suspension injection and a preparation procedure thereof, and relates to a medicine and a preparation procedure. The drug substance of the florfenicol suspension injection provided by the invention comprises the following components in part by weight: 40-100 parts of fat soluble florfenicol, 1.5-5 parts of suspending agent, 2-4 parts of wetting agent, 0.8-1.2 parts of citric acid, 0.3-0.5 parts of sodium citrate, 0.5-1 part of butylated hydroxytoluene and 5-40 parts of benzyl alcohol. The preparation procedure comprises the following steps: water for injection is used to dissolve the prescribed suspending agent into rubber paste to get solution 1; fat soluble florfenicol is evenly mixed with a wetting agent and water is added into the mixture of the fat soluble florfenicol and the wetting agent to form suspension to get solution 2; solution 1 is slowly added to solution 2 while stirring; citric acid, sodium citrate, butylated hydroxytoluene and benzyl alcohol is added, and then water is added to get 1000 ml of suspension; and the suspension is split and packed after stirring for 30-40 minutes to get florfenicol suspension injection. The florfenicol suspension injection has the advantages of broad antibacterial effect, strong antibacterial activity, low dosing frequency, little stress response and low price.
Owner:HARBIN WILLHOPE ANIMAL HEALTH CARE PROD CO LTD
A formula of an antimicrobial oil emulsion microcapsule for veterinary use
InactiveCN103054833ASimple processLow costAntibacterial agentsTetracycline active ingredientsAnti bacterialOral medication
The invention discloses a formula of an antimicrobial oil emulsion microcapsule for veterinary use, wherein per 1000g of the antimicrobial oil emulsion microcapsule contains 50-100g of an antimicrobial agent, 250-500ml of oil for injection, 250-500ml of water for injection, 1-5g of PEG, 5-10ml of sorbitan fatty acid ester, 5-10ml of polyoxyethylene sorbitan fatty acid ester, 250-500g of soluble starch, and 50-100g of methyl cellulose. According to the present invention, the oil emulsion is developed to be microcapsules which can be used for oral administration, thereby maintaining the long-acting and sustained-release effects of the oil emulsion, while in a large extent preventing disadvantages and side reactions in the production and application of the oil emulsion. The preparation process of the microcapsules is relatively simple, reducing the use of organic solvents and thereby reducing the cost of the product.
Owner:HUNAN AGRICULTURAL UNIV
Tilmicosin inclusion compound chitosan temperature-sensitive gel and preparation method thereof
InactiveCN106177989AImprove solubilityGood acupunctureAntibacterial agentsOrganic active ingredientsChemical synthesisSodium glycerophosphate
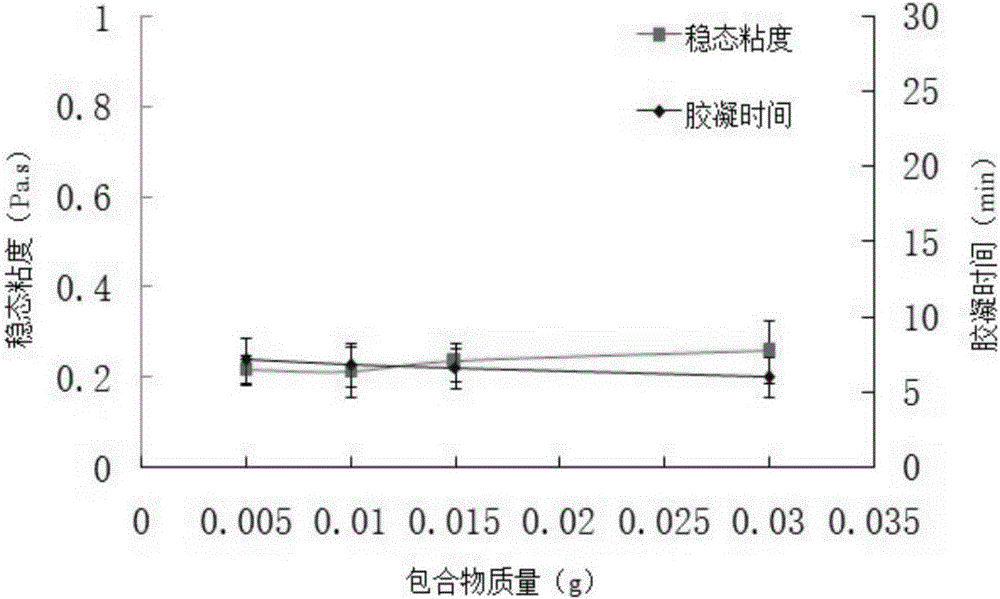
The invention belongs to the field of chemical synthesis, and in particular relates to a tilmicosin inclusion complex chitosan temperature-sensitive gel and a preparation method thereof. The preparation method comprises the following steps: dissolving chitosan in 0.1mol / L hydrochloric acid by weighing and fully stirring until Dissolve the sodium glycerophosphate in deionized water, add the sodium glycerophosphate solution dropwise into the chitosan solution, adjust the pH value of the mixed solution, and stir the resulting mixed solution at 4°C to obtain the chitosan thermosensitive gel, which is called Take a tilmicosin inclusion compound in a conical flask, add an appropriate amount of chitosan thermosensitive gel into the conical flask, and stir evenly with a magnetic stirrer at room temperature to obtain a tilmicosin inclusion complex thermosensitive gel. The invention has the advantages of low toxicity, high bioavailability and low injection irritation.
Owner:QINGDAO AGRI UNIV
Memantine insoluble salt sustained-release injection and preparation method thereof
InactiveCN108969478AReduce solubilityExtended release timePowder deliveryNervous disorderSolubilityMemantine Hydrochloride
The invention discloses a memantine insoluble salt sustained-release injection and a preparation method thereof. The memantine insoluble salt sustained-release injection is characterized by being prepared from the following materials by mass percentage: 1% to 30% of memantine insoluble salt as an active ingredient, 0.1% to 10% of stabilizer , 0% to 1% of pH adjuster, 0.1% to 5% of isotonic regulator and 60% to 98% of injection water; the memantine insoluble salt includes one of memantine-oleate, memantine-linoleate, memantine-linolenate, memantine-arachidonate, memantine-stearate, memantine-laurate, memantine-tannate, memantine palmitate, memantine-pamoate, and memantine-furanate. An insoluble salt technology is adopted to prepare the memantine insoluble salt, and the drug release time isprolonged by reducing the solubility of the drug. Compared with memantine hydrochloride, the solubility of the memantine insoluble salt is reduced by 40 to 600 times, and the memantine insoluble saltcan be slowly released for several days to several weeks.
Owner:JIANGNAN UNIV
Amoxicillin and colistin sulfate oil suspension and preparation method thereof
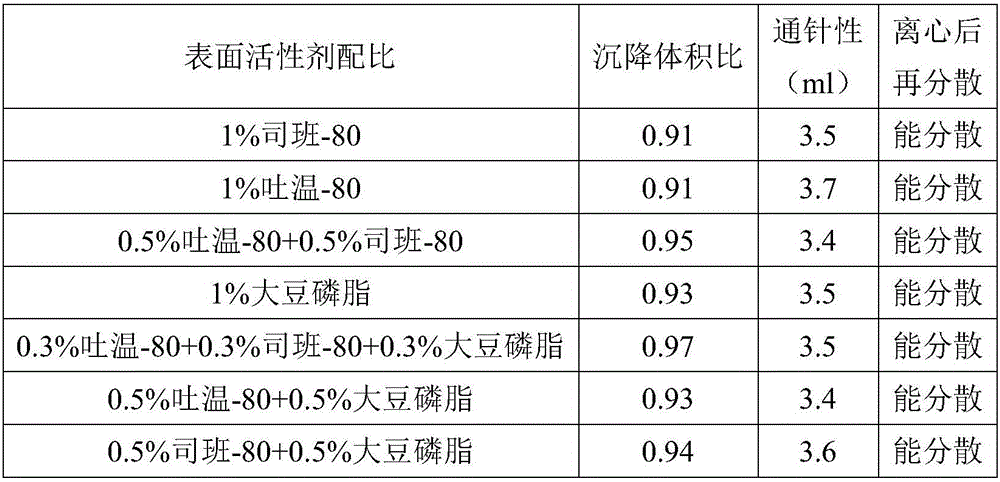
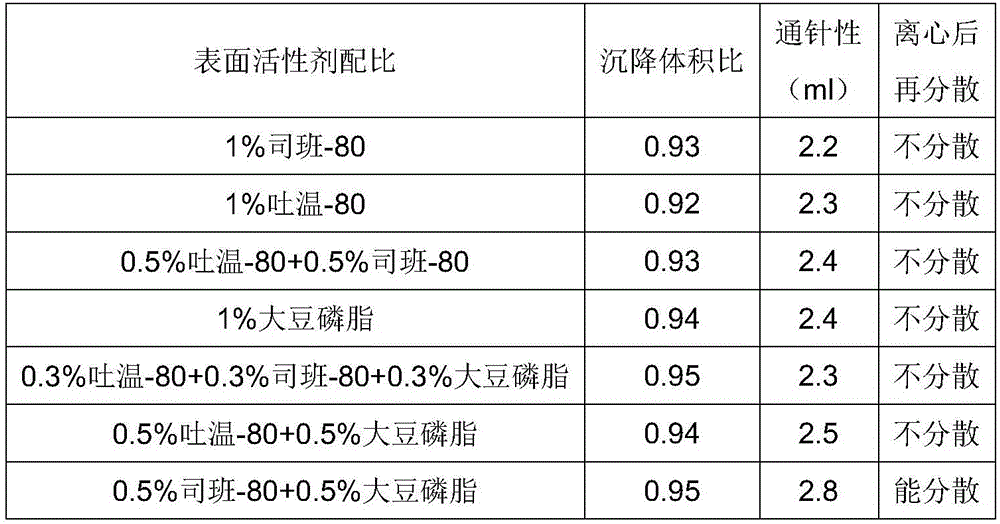
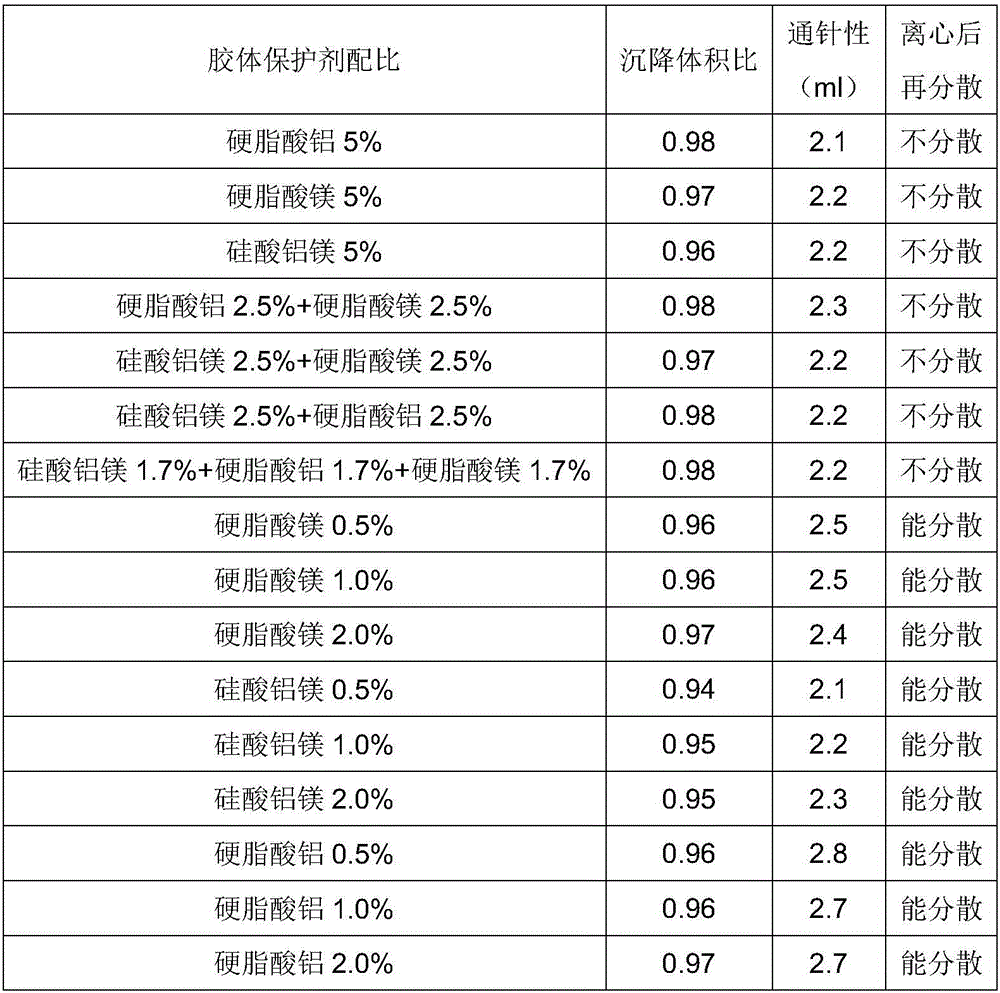
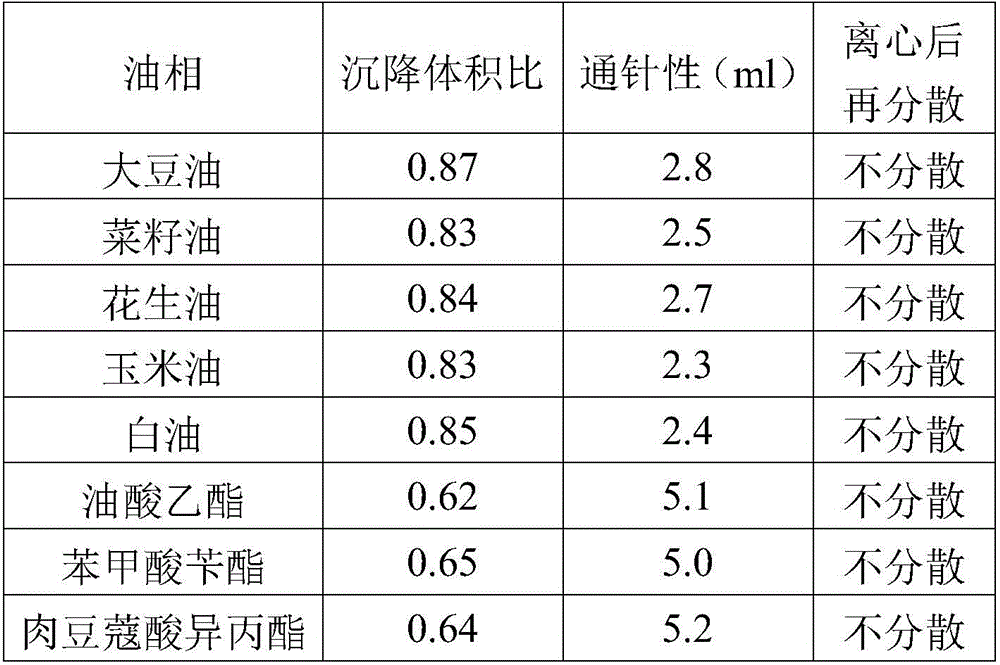
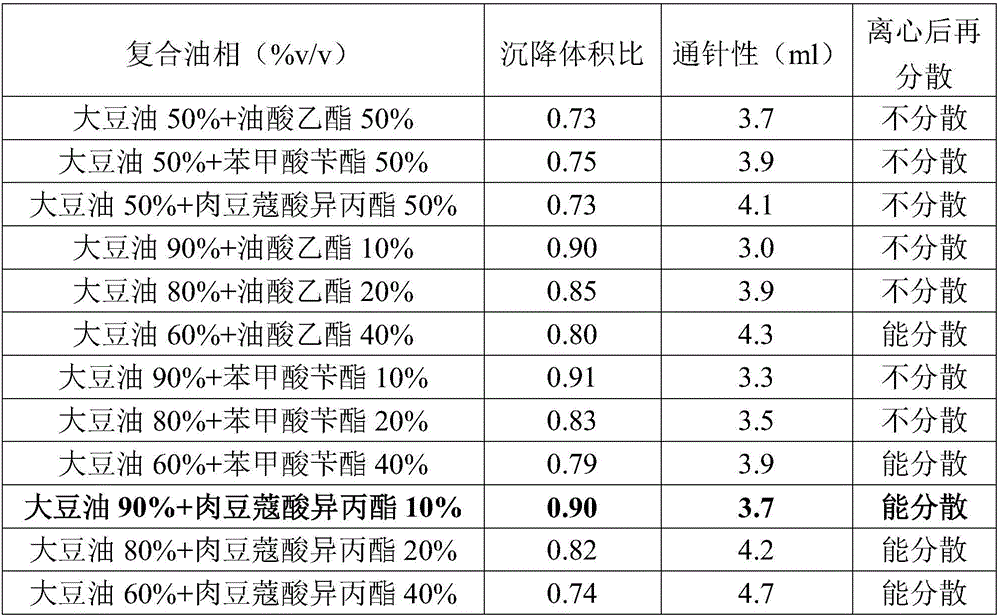
ActiveCN106265506AImprove physical stabilityGood acupunctureAntibacterial agentsSolution deliveryDispersityColistin Sulfate
The invention discloses an oil suspension. Every 100 mL of the oil suspension contains 10 g of amoxicillin, 1 g of colistin sulfate, 0.9-1.0 g of surfactant, 1.0-4.0 g of colloid protective agent, 3.0-15.0 g of suspending agent, 0.075-0.1 g of antioxidant and the balance oil phases. Under the condition of adopting specific auxiliary types and dosage ratios, the prepared suspension is good in physical stability, permeability and re-dispersity; the mass of the suspension is not reduced after the suspension is stored in a 4-60 DEG C environment for three months, which indicates that the mass of the suspension is stable.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Performance-improved injection implant
InactiveCN107335098ALong-lasting clinical (filling) resultsLow incidence of adverse reactionsMicrocapsulesProsthesisSide effectMicrosphere
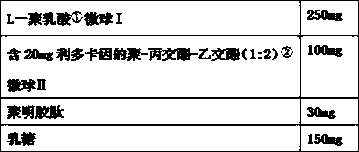
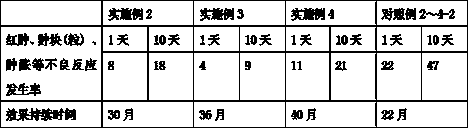
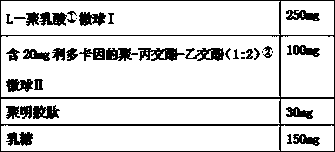
The invention discloses a performance-improved injection implant. The injection implant consists of a polylactic acid (1) microsphere I which is basically free from lidocaine, a polylactic-co-glycolic acid (1: 2) (2) microsphere II which contains lidocaine granules and can achieve continuous release for 10-14 days basically at 0 level and polygeline. The injection implant (filled) is more lasting in effect and lower in side effects, and relatively high needle passing performances and redissolving (reconstruction) performances can be guaranteed.
Owner:江苏西宏生物医药有限公司
Soft tissue filling hydrogel for medical cosmetology and preparation method of soft tissue filling hydrogel
PendingCN112354018APromote regenerationGood water solubilityPharmaceutical delivery mechanismTissue regenerationPolyethylene glycolEngineering
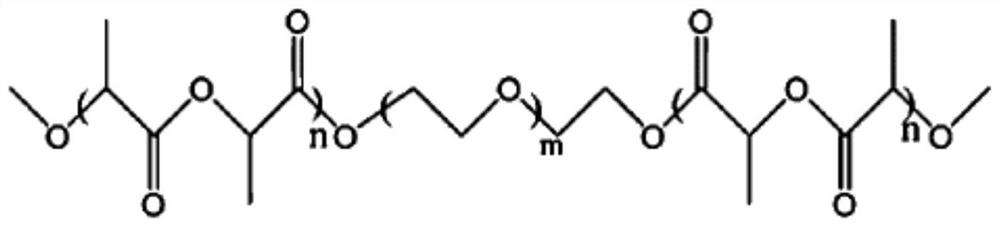
The invention provides soft tissue filling hydrogel for medical cosmetology and a preparation method of the soft tissue filling hydrogel. The soft tissue filling hydrogel comprises a poly-L-lactic acid-polyethylene glycol-poly-L-lactic acid copolymer or a poly-racemic lactic acid-polyethylene glycol-poly-racemic lactic acid copolymer. According to the scheme of the embodiment of the invention, thePLLA-PEG-PLLA copolymer or the PDLLA-PEG-PDLLA copolymer is used as a main substance of the soft tissue filling hydrogel, so that the soft tissue filling hydrogel has excellent water solubility, biocompatibility and biodegradability, and has the advantages of being good in needle passing property, quick in effect, outstanding in filling effect, low in toxic and side effects, capable of promotingregeneration of fibroblasts and collagen and the like; and the soft tissue filling hydrogel is suitable for soft tissue filling in plastic surgery.
Owner:南京思元医疗技术有限公司
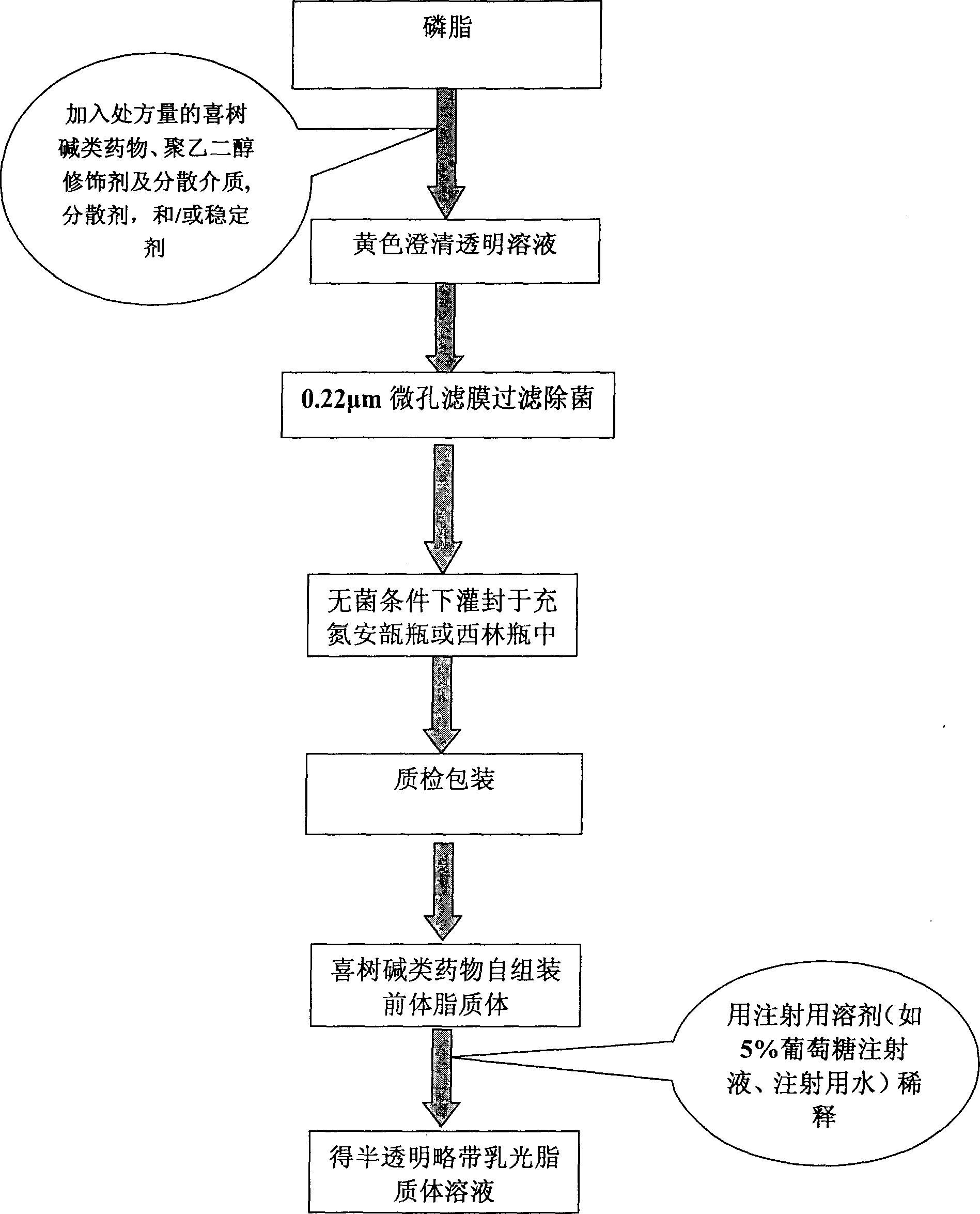
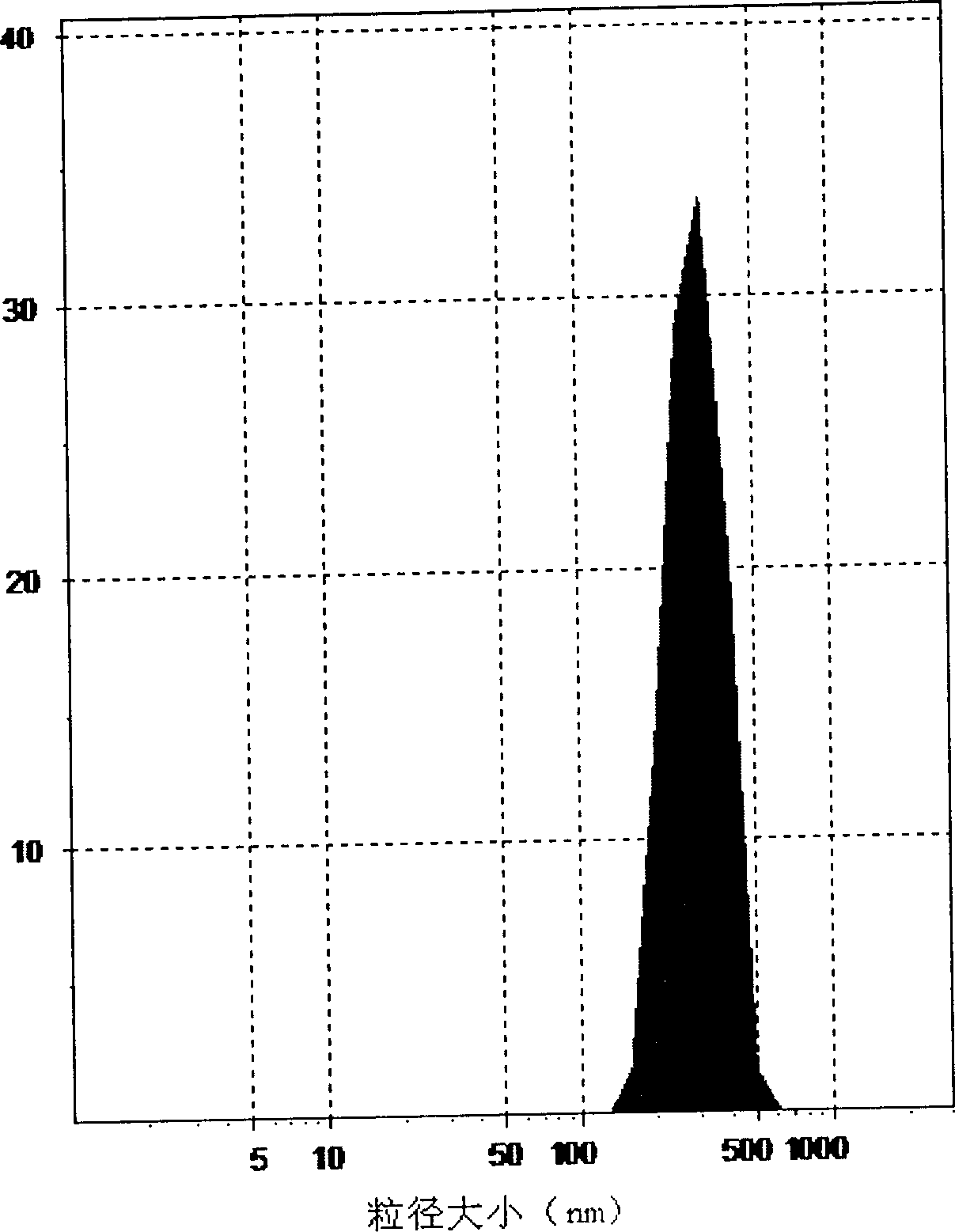
Self asembled precusor liposome containing camptothecin kind medicine and its preparation method
InactiveCN1823733ASolve the "bottleneck" problem that is difficult for efficient industrial productionRapid self-assemblyOrganic active ingredientsAntineoplastic agentsDispersed mediaMedicine
A self-assembled precursor liposome of camptothecine contained medicine is proportionally prepared from camptothecine contained medicine, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV +1
Cefquinome sulfate oil suspension and preparation method thereof
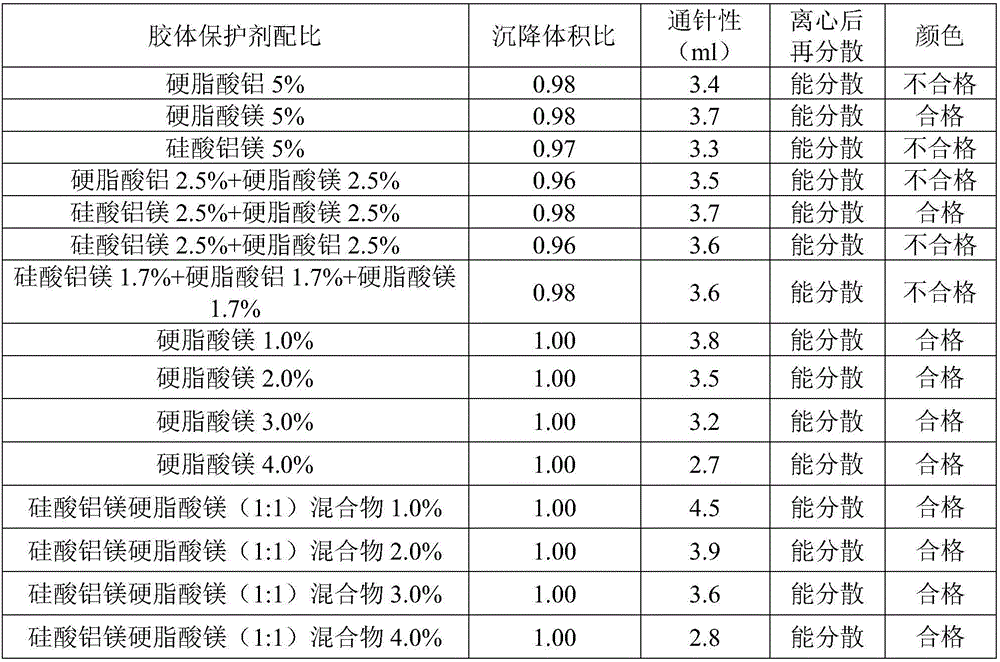
ActiveCN106344509AImprove qualityReduce volumeAntibacterial agentsOrganic active ingredientsDispersityDrug content
The invention discloses a cefquinome sulfate oil suspension. Every 100mL of the suspension contains the following raw and auxiliary materials: 2-15g of cefquinome sulfate, 0.9-1.0g of surfactant, 5.0-5.1g of colloid protective agent, 2.0-8.0g of suspending agent, 0-0.1g of antioxidant and the balance of oil phase. The high-quality cefquinome sulfate oil suspension with the main drug content of 2-15% is obtained by screening the formula. The used auxiliary materials lower the drug sedimentation volume and sedimentation rate. The product has the advantages of low flowability, low sedimentation rate, high sedimentation volume, high needle penetration property and favorable dispersity after centrifugation. In addition, the inspection on the stability under different temperature conditions further proves the high quality of the suspension.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Tumor targeted drug nanocrystal delivery system
PendingCN112426535AGood water dispersibilityGood acupunctureOrganic active ingredientsPowder deliveryPolythylene glycolSuccinic acid
The invention belongs to the field of pharmaceutical preparations, and relates to a tumor targeted drug nanocrystal delivery system and a preparation method and application thereof in pharmacy. The tumor targeted drug nanocrystal delivery system is composed of an anti-tumor drug nanocrystal core and a coating material; one or more biocompatible phospholipids, vitamin E polyethylene glycol 1000 succinate, folic acid-polyethylene glycol-phosphatidyl ethanolamine and methoxy polyethylene glycol-phosphatidyl ethanolamine are mixed according to a certain ratio, and insoluble anti-tumor drug nanocrystals are coated with the obtained mixture; the obtained tumor targeted drug nanocrystal drug delivery system can be stably dispersed in an aqueous environment, and experiments prove that the tumor targeted drug nanocrystal drug delivery system can effectively inhibit the growth of tumor cells and can effectively reduce the tumor volume after being subjected to peritumoral injection for an animalmodel. The invention provides a new preparation form capable of peritumoral injection for preoperative adjuvant chemotherapy of a solid tumor.
Owner:FUDAN UNIV
Duck circovirus and adenovirus bivalent inactivated vaccine and preparation method of yolk antibody thereof
InactiveCN111420042ASolving Manufacturing ChallengesBest ratioEgg immunoglobulinsViral antigen ingredientsAntigenAdjuvant
The invention relates to a duck circovirus and adenovirus bivalent inactivated vaccine and a preparation method of a yolk antibody thereof. A duck circovirus antigen is obtained by infecting 1-day-oldhealthy cherry valley ducklings and collecting infected duck livers at the age of 25 days to obtain antigen tissues, and an adenovirus antigen is obtained by infecting SPF chickens of 20 days old to30 days old and collecting livers of dead chickens; an antigen of the vaccine consists of liver tissues infected with the duck circovirus and liver tissues infected with the adenovirus in a mass ratioof (1-5):1, and an antigen solution for the vaccine is prepared; and a pine pollen polysaccharide with a concentration of 5-40mg / mL is added into the antigen solution to serve as an immunopotentiator, and the antigen solution is emulsified with a conventional white oil adjuvant to obtain the vaccine. The yolk antibody is obtained by immunizing laying hens with the vaccine, and then performing extracting and purifying on egg yolks of high-immunity laying hens, and the yolk antibody simultaneously contains two antibodies against the duck circovirus and the adenovirus. The vaccine and yolk antibody provided by the invention have the advantages of simple preparation process, low cost, good action effect, stable dosage form and easy storage, and has a wide application prospect.
Owner:山东百瑞凯来生物科技有限公司
Cefalexin oil suspension and preparation method thereof
InactiveCN106309365AGood penetration and dispersibilityStableAntibacterial agentsOrganic active ingredientsPhysical stabilityAntioxidant
The invention discloses a cefalexin oil suspension. Every 100mL of the suspension contains the following raw and auxiliary materials: 2-30g of cefalexin, 0.9-1.0g of surfactant, 1.0-5.0g of colloid protective agent, 7.5-12.5g of suspending agent, 0-0.1% of antioxidant and the balance of oil phase. Under the condition of adopting specific auxiliary material varieties and proportions, the prepared suspension has the advantages of favorable physical stability, favorable needle penetrability and favorable redispersibility, and does not have the condition of quality reduction after being stored in a 4-60-DEG C environment for 3 months, which indicates stable quality.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Self assembled precusor liposome containing hard soluble medicine and its preparation method
InactiveCN1823735AImprove product qualityHigh encapsulation efficiencyPharmaceutical non-active ingredientsLiposomal deliveryDispersed mediaPolyethylene glycol
A self-assembled precursor liposome of medicine difficult to dissolve is proportionally prepared from medicine difficult to dissolve, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV
Ceftiofur hydrochloride injection for dairy cattle and preparation method thereof
ActiveCN107049943AImprove dispersion stabilityGood acupunctureAntibacterial agentsOrganic active ingredientsDispersion stabilityAntioxidant
The invention provides ceftiofur hydrochloride injection for dairy cattle, comprising, by weight, 5-10 parts of ceftiofur hydrochloride, 0.5-3 parts of a suspending agent, 0.05-5 parts of a flocculant, 0.01-2 parts of an emulsifier, 0.1-2 parts of an antioxidant, and 55-100 parts of a lipid solvent. The ceftiofur hydrochloride injection has good dispersion stability and good needle passage, milk withdrawal period of dairy cattle injected with the ceftiofur hydrochloride injection is short, and the ceftiofur hydrochloride injection has a promising application prospect. A preparation method of the ceftiofur hydrochloride injection for dairy cattle is also provided; the preparation method is simple to perform and applicable to industrial application.
Owner:ZHENGZHOU BARY ANIMAL PHARMA
Preparation method of ceftiofur microspheres
ActiveCN112353765AImprove stabilityReduce dispersionAntibacterial agentsOrganic active ingredientsMicrospherePolythylene glycol
The invention discloses a preparation method of ceftiofur microspheres, and relates to the field of veterinary drugs, and the preparation method comprises the following steps: 1, dissolving a polylactic acid-polyethylene glycol segmented copolymer as a carrier material in an organic solvent; 2, adding ceftiofur, and stirring and mixing to prepare a dispersion phase; 3, adding the dispersion phaseinto water, adding an emulsifier, and magnetically stirring and emulsifying to obtain an emulsion; 4, rapidly stirring the emulsion for a period of time T1, supplementing water, and continuously stirring for a period of time T2 until the organic solvent is completely volatilized; and 5, centrifuging, washing, collecting and drying in vacuum to obtain the ceftiofur microsphere. According to the ceftiofur sustained-release microsphere disclosed by the invention, a drug is more uniformly dispersed in a carrier material, the drug loading capacity is high, a dispersion system of drug particles is reduced by virtue of a nanoscale microsphere technology, and the ceftiofur sustained-release microsphere has better needle permeability, so that the drug has a stronger sustained-release effect and a longer sustained drug effect.
Owner:山东华辰制药有限公司
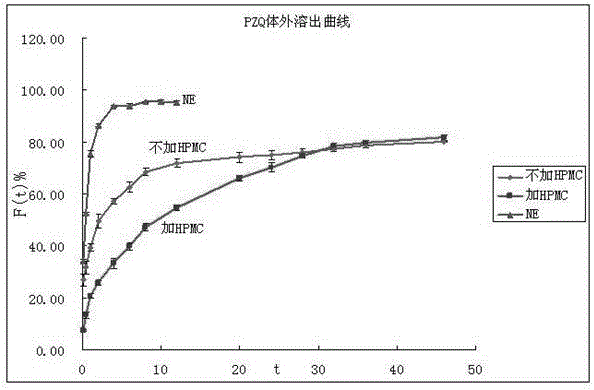
Praziquantel nanoemulsion in situ gel for preventing and treating bilharziasis and preparation method and application thereof
ActiveCN105816421AImprove stabilityGood acupunctureOrganic active ingredientsAerosol deliveryDrugIrritation
The invention discloses praziquantel nanoemulsion in situ gel for preventing and treating bilharziasis and a preparation method and application thereof. The modern nano preparation technology is applied, firstly, hydrophobic praziquantel is prepared into O / W type nanoemulsion which can be mixed with water in any proportion, secondly, the O / W type nanoemulsion is highly dispersed into hydrophilic reverse gel, and water-based injection which is uniform, transparent, good in stability and nozzle cleaning performance and appropriate in viscosity is prepared. The praziquantel nanoemulsion in situ gel has the advantages that in vivo experiments show that the provided praziquantel nanoemulsion in situ gel has an obvious controlled-release character, the function of preventing and treating bilharziasis can be prolonged, medicinal degradable materials are adopted in the medicine composition, and the problems of irritation and injection site lesions do not exist.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Amoxicillin and potassium clavulafiate oil suspension and preparation method thereof
InactiveCN106265505AImprove physical stabilityGood acupunctureSolution deliveryEmulsion deliveryPotassiumOil phase
The invention discloses an oil suspension. Per 100mL of the oil suspension is prepared from the following raw auxiliary materials: 14g of amoxicillin, 3.5g of potassium clavulafiate, 0.9-1.0g of a surfactant, 1.0-4.0g of a colloid protective agent, 3.0-15.0g of a suspending agent and the balance of an oil phase. Under the conditions of specific varieties and dosage proportion of auxiliary materials, the suspension prepared by the preparation method is favorable in physical stability, warming promotion property and redispersion property, and has no quality reduction situation even if being stored in an environment at a temperature of 4-60 DEG C for three months, which indicates that the quality is stable.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
ActiveCN103071151BStrengthen cellsImprove the effectiveness of anti-virus protectionAntibacterial agentsBacterial antigen ingredientsCholesterolLevamisole
The special diluent for swine mycoplasma pneumonia vaccine of the present invention mainly contains recombinant P97R1 protein immunostimulatory complex (ISCOM-P97R1), and may also contain other aqueous adjuvant components, including levamisole, or / and astragalus polysaccharide, or / and carboxy bohm. Each ml solution of the diluent contains 0.1-1 mg of QuilA, 0.02-0.2 mg of cholesterol, 0.02-0.2 mg of phosphatidylcholine, 0.4-4 mg of P97R1 antigen, and may further contain 2-20 mg of levamisole , or / and astragalus polysaccharide 20-100 mg, or / and carbomer 3-10 mg, and the rest is phosphate buffer. This diluent is specially used for dissolving or diluting Mycoplasma pneumoniae vaccine, including live vaccine and inactivated vaccine. It has the dual effects of supplementing the protective spectrum of vaccine antigens and enhancing the immune stimulating ability of vaccine cells and humoral, which can significantly improve the protective efficacy of vaccines against viruses. At the same time, due to the use of the water solvent system, it also has the advantages of simple operation, good needle penetration, and no obvious toxic and side effects.
Owner:JIANGSU ACAD OF AGRI SCI
Oil suspension with procaine benzylpenicillin and method for preparing oil suspension
InactiveCN106420608ASedimentation volume ratio is highGood acupunctureAntibacterial agentsAntimycoticsProcaineAntioxidant
The invention provides oil suspension with procaine benzylpenicillin. Every 100 mL of oil suspension contains raw and auxiliary materials including 5-45 g of procaine benzylpenicillin, 0.5-2 g of surfactants, 0.5-2 g of colloid protective agents, 2-5 g of suspending agents, 0-0.1 g of antioxidants and the balance oil phases. The oil suspension has the advantages that the oil suspension is high in sedimentation volume ratio and excellent in syringeability and re-dispersibility; deterioration of the quality of the oil suspension is unseen after the oil suspension is stored in environments at the temperatures of 4-60 DEG C for 3 months, and accordingly the oil suspension is stable in quality and has an excellent clinical application prospect.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Amoxicillin enrofloxacin oil suspension and preparation method thereof
InactiveCN106727567AImprove physical stabilityGood acupunctureAntibacterial agentsInorganic non-active ingredientsAntioxidantSuspending Agents
The invention discloses an oil suspension. The oil suspension contains the following raw materials(per 100mL): 10g of amoxicillin, 10g of enrofloxacin or salts thereof, 0.9-1.0g of a surfactant, 5.0-5.1g of a colloid protectant, 4.0-7.0g of a suspending agent, 0.075-0.1g of an antioxidant, and the balance of an oil phase. Under the condition of adopting specific auxiliary materials and compounding ratio, the oil suspension prepared by the invention has good physical stability, syringeability and redispersion. The quality of the oil suspension is not lowered after the suspension is stored in a 60 DEG C environment for 10 days, so that the quality is stable.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Long-acting cefquinome sulfate injection and preparation method thereof
InactiveCN107260666ARestore the animal's staminaImprove stabilityAntibacterial agentsOrganic active ingredientsChemistryInjection solution
The invention relates to a long-acting cefquinome sulfate injection and a preparation method thereof. The injection solution comprises, by weight, 2 to 3 parts of cefquinome sulfate, 2 to 3 parts of a suspending aid, 0. 5 to 1 part of a stabilizer, 0.2 to 0.4 parts of an antioxidant, 0.2 to 0.4 parts of a polylactic acid-glycolic acid copolymer, 0.3 to 0.7 parts of a crosslinking agent, 1 to 1.5 parts of a pH stabilizer and 80 to 120 parts of a dispersion medium. In preparation, the suspending aid, stabilizer, antioxidant, polylactic acid-glycolic acid copolymer, crosslinking agent and pH stabilizer are added into the dispersion medium, the mixture is cooled, then cefquinome sulfate is added into the cooled mixture and the mixture is ground. Compared with the prior art, the preparation method improves injection stability and dispersibility and prolongs pharmacodynamic metabolism time. The injection can be slowly released after intramuscular injection, realize a stable drug concentration in the blood, realize the effects the same to those of the multiple interval injection processes of the same type of the conventional dosage form only through one injection process, reduce drug use frequency, reduce a drug use cost and a labor cost and prevent stress response and can be easily stored.
Owner:JIANGXI AOXIN BIOTECH CO LTD +1
Slow-release florfenicol suspension injection and preparation process thereof
PendingCN113827561ALittle side effectsLow toxicityAntibacterial agentsOrganic active ingredientsActive agentAntibacterial activity
The invention discloses a florfenicol suspension injection. The florfenicol suspension injection is prepared from the following components in parts by weight of 10 to 30 parts of florfenicol, 60 to 80 parts of ethyl oleate, 1 to 5 parts of a surfactant, 0.1 to 0.9 part of polyoxyethylene ether (40) hydrogenated castor oil, 0.5 to 1.2 parts of span 80, 0.2 to 0.9 part of Tween 80, 0.1 to 0.3 part of poloxamer and 0.1 to 0.9 part of an antioxidant. The preparation process comprises the following steps of firstly, putting ethyl oleate into a round-bottom flask, and heating at 150 DEG C for 2 hours; cooling to 70 DEG C, and separately adding the antioxidant, the surfactant and a wetting agent while stirring; after uniformly stirring, adding florfenicol; and finally, stirring at a high speed for 30 minutes, adding all the dissolved materials into a colloid mill, carrying out colloid milling, sub-packaging the solution, and sealing to obtain a product which is wide in antibacterial range, high in antibacterial activity, small in administration frequency, small in stress, long in slow release time and low in price.
Owner:杭州艾贝德生命科技研究院有限公司
Poly olefinic taxadol self assembled precusor liposome and its preparation method
ActiveCN100486569CImprove product qualityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsDispersed mediaAlfaxalone
A self-assembled precursor liposome of polyenic taxusol is proportionally prepared from polyenic taxusol, phosphatide, polyethylene glycol and dispersing medium through mixing, dispersing, press-filtering by millipore film, and pouring it in a container full of N2.
Owner:CHINA PHARM UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com