Special diluent for swine mycoplasmal pneumonia vaccines and preparation method of special diluent
A technology of Mycoplasma porcine pneumonia and diluents, applied in the field of diluents, can solve the problems of pigs’ inability to produce P97R1 antibodies, large-scale expression, lack of antigen immunity, etc., to enhance the ability of cell and humoral immune stimulation, easy to operate, and good needle penetration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
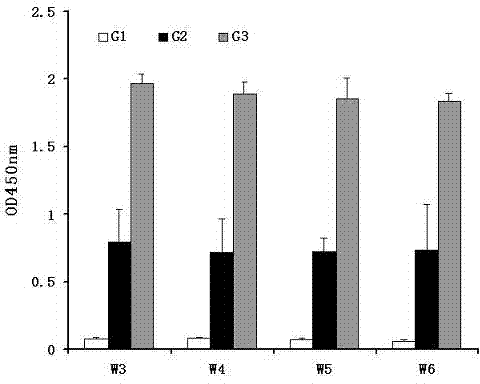
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of recombinant Mycoplasma hyopneumoniae P97R1 protein
[0039] According to the genome sequence of Mycoplasma hyopneumoniae published by GenBank, primers were designed to amplify the P97R1 fragment. Upstream primer: 5'-CCGAATTCTTTACCTCAGCCGCCAGCAG-3' (SEQ ID No.3); Downstream primer: 5'-GCGAAGCTTAAGCCATTGGGAAATAGTCT-3' (SEQ ID No.4). The upstream and downstream primers contain EcoR I site and Hind III site. Using 168 F344 strains of Mycoplasma hyopneumoniae genomes as templates, the P97R1 gene fragment was amplified by PCR. EcoR I and Hind After Ⅲ double enzyme digestion, it was inserted into the pET-32a(+) vector to obtain the prokaryotic plasmid vector pET-32a(+)-P97R1 expressing the recombinant P97R1 protein. Escherichia coli BL-21 (DE3) was transformed, positive clones were screened and verified by DNA sequencing, and recombinant genetically engineered bacteria expressing recombinant P97R1 protein were obtained.
[0040] The gene se...
Embodiment 2
[0045] Example 2 Preparation of Immunostimulatory Complex ISCOM-P97R1 Containing Recombinant P97R1 Protein
[0046] (1) Preparation of 20% Mega-10 stock solution
[0047] Dissolve 0.4 g of Mega-10 (also known as "N-quinoyl-N-methylglucamine") dry powder in 1.6 ml of distilled water, dissolve at 37°C and dilute to 2 ml.
[0048] (2) Preparation of lipid mixture stock solution
[0049] Add 100 mg phosphatidylcholine and 100 mg cholesterol to 10 ml 20% Mega-10, heat slightly (30-60°C) and stir slowly to dissolve completely, filter and aliquot, store at -20°C for later use.
[0050] (3) Preparation of Quil A solution
[0051] Dissolve 25 mg of Quil A in 4.5 ml of phosphate buffered saline, pH 7.2-7.4.
[0052] (4) Mycoplasma hyopneumoniae P97R1 protein solution
[0053] The recombinant P97R1 protein was prepared and purified by the method described in Example 1, dissolved in phosphate buffer, the protein concentration was measured and diluted to 10 mg / ml, and stored at -20°C...
Embodiment 3
[0056] Embodiment 3 Preparation of special diluent for swine mycoplasma pneumonia vaccine
[0057] When preparing the special diluent for swine mycoplasma pneumonia vaccine containing only ISCOM-P97R1 components, the preparation method is as follows: take an appropriate amount of ISCOM-P97R1 stock solution with a Quil A concentration of 1.67 mg / ml, and dilute it with an appropriate amount of phosphate buffer to obtain Each ml solution contains: Quil A 0.1-1 mg, cholesterol 0.02-0.2 mg, phosphatidylcholine 0.02-0.2 mg, P97R1 antigen 0.4-4 mg, ISCOM-P97R1 vaccine special diluent.
[0058] When preparing the special diluent for mycoplasma pneumonia vaccine containing ISCOM-P97R1 and other water adjuvant components, the preparation method is as follows: first prepare ISCOM-P97R1 mother solution, levamisole mother solution, astragalus polysaccharide mother solution, and carbomer mother solution respectively, Then dilute to the required concentration with phosphate buffer solution t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com