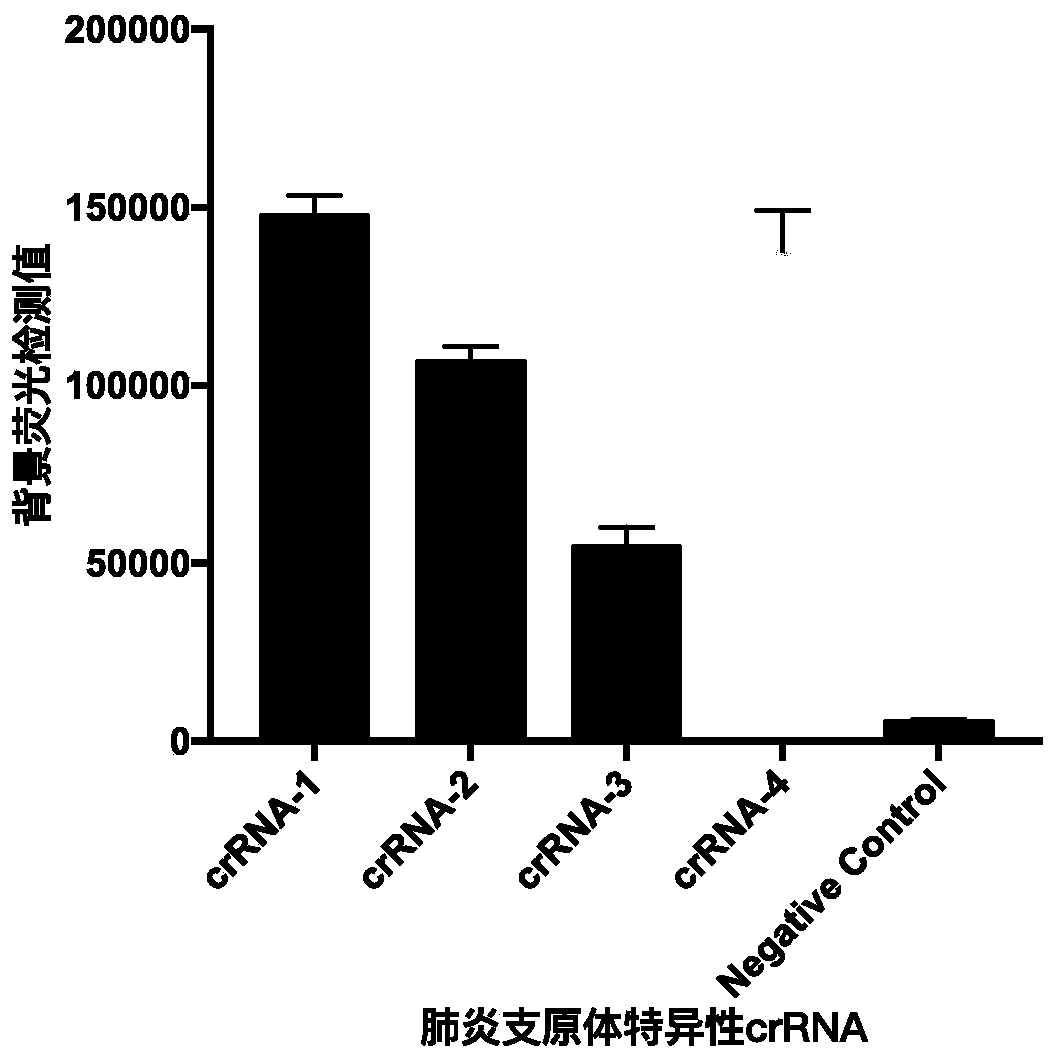
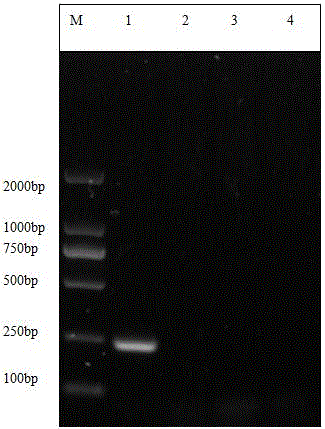
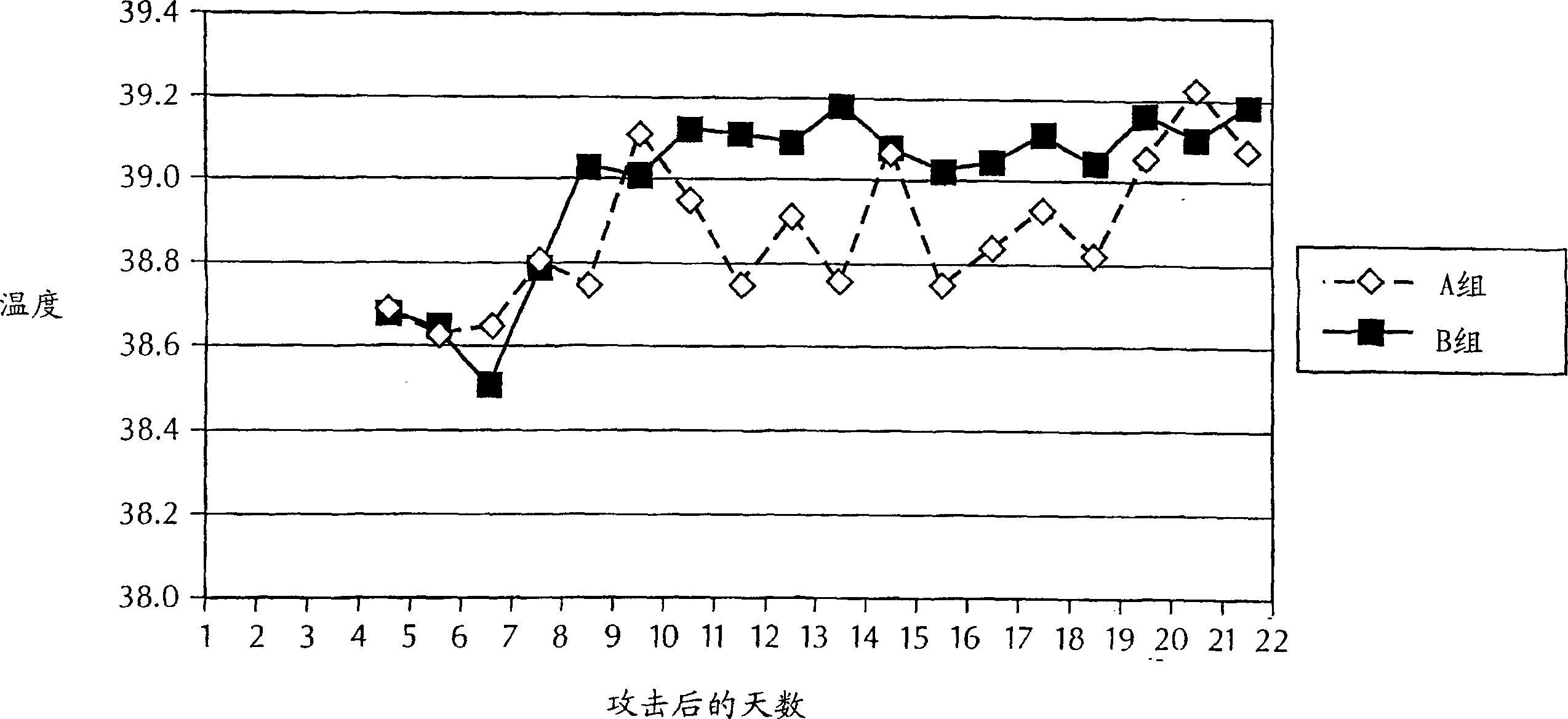
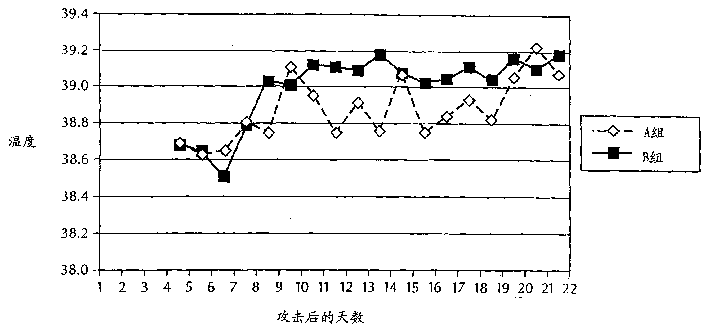
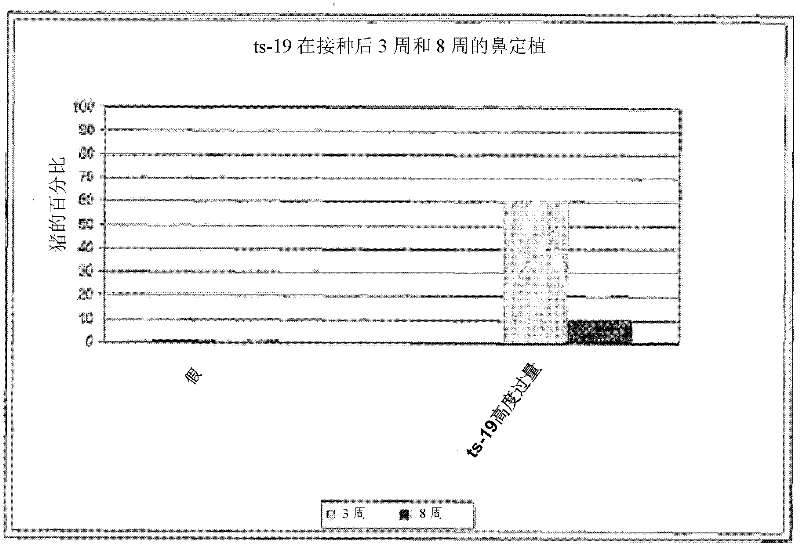
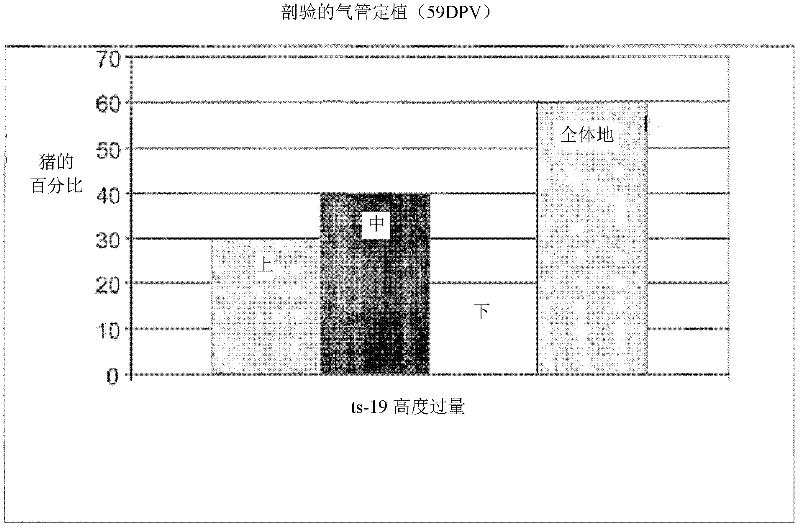
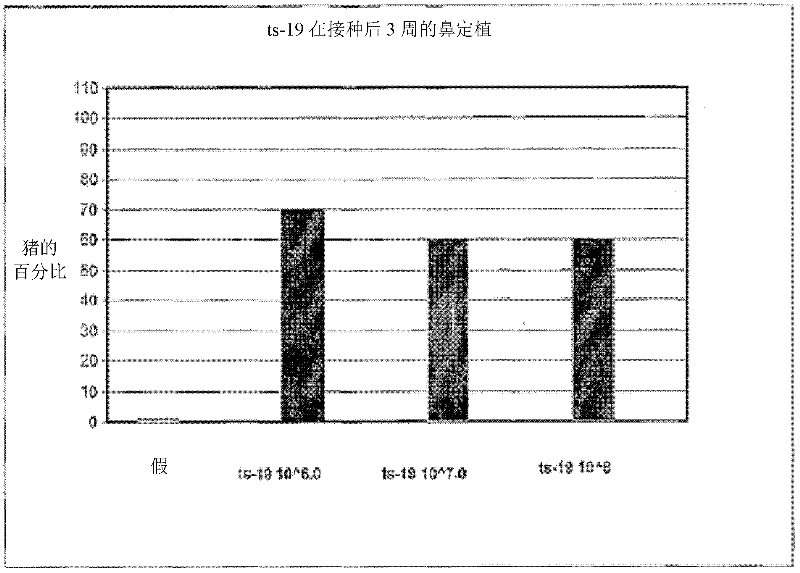
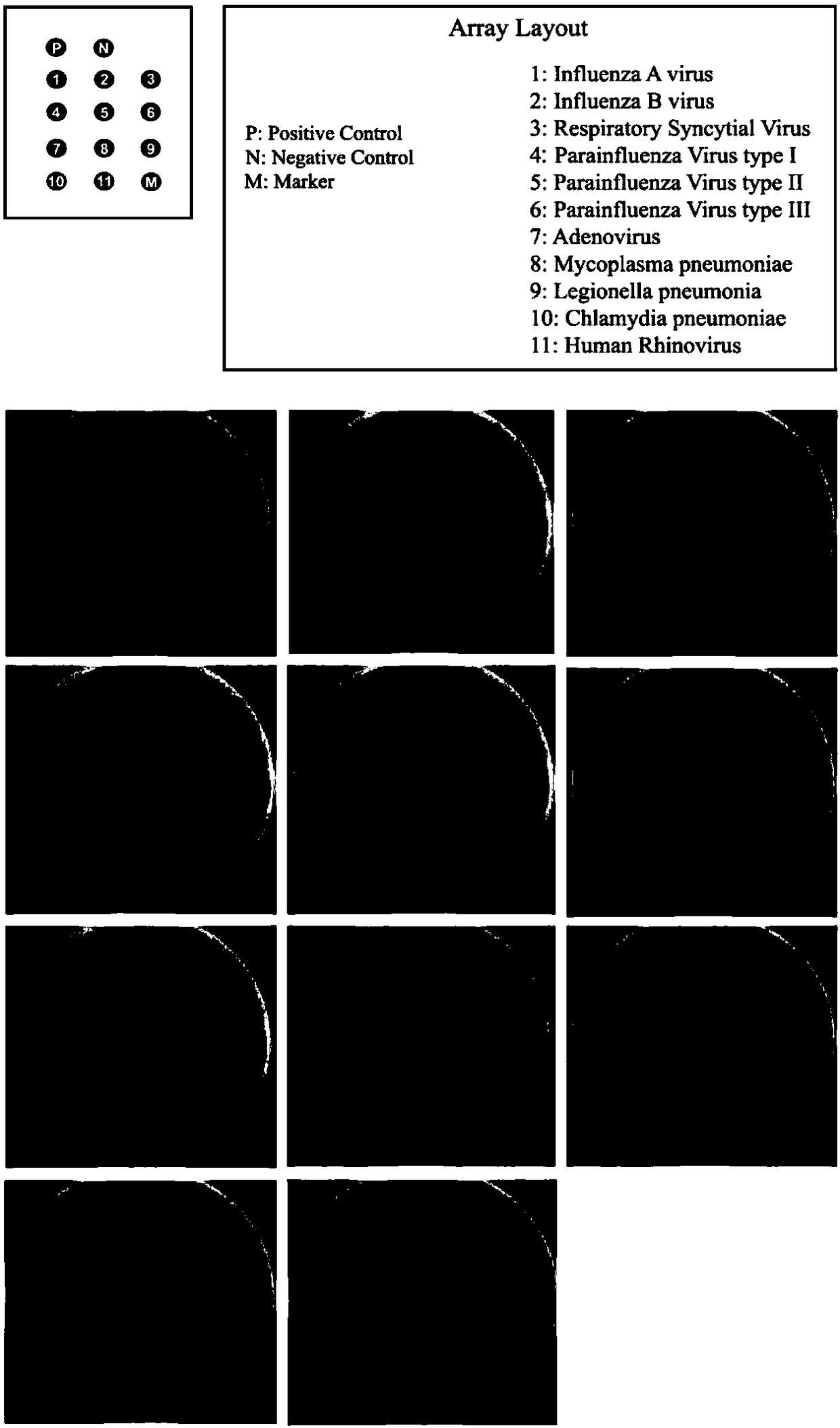
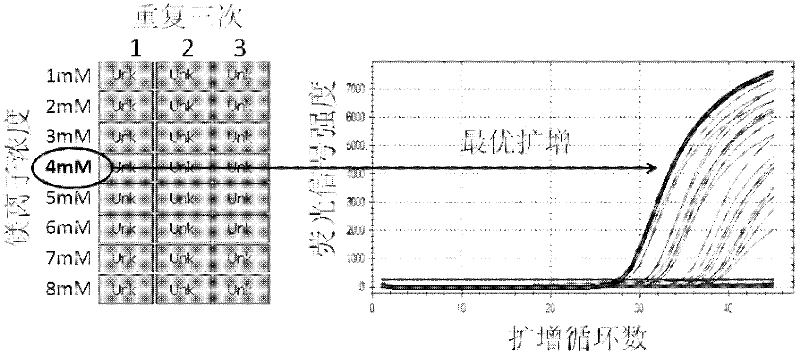
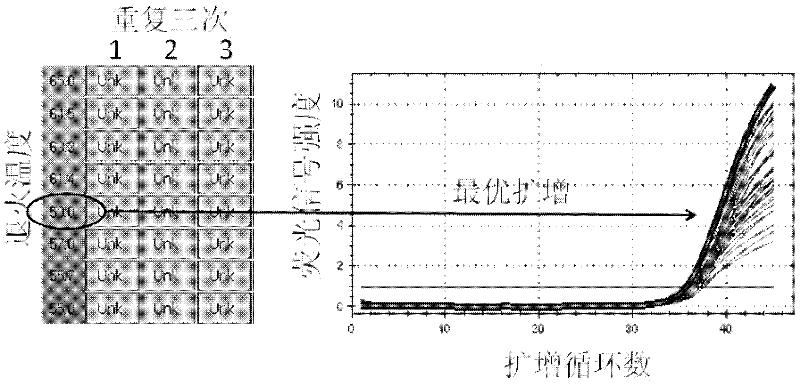
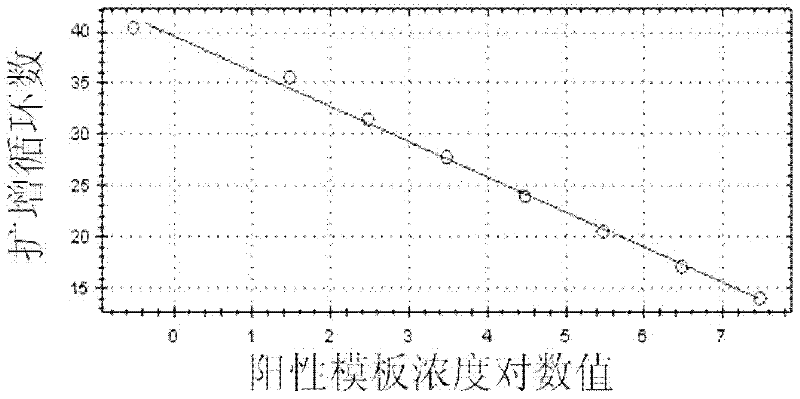
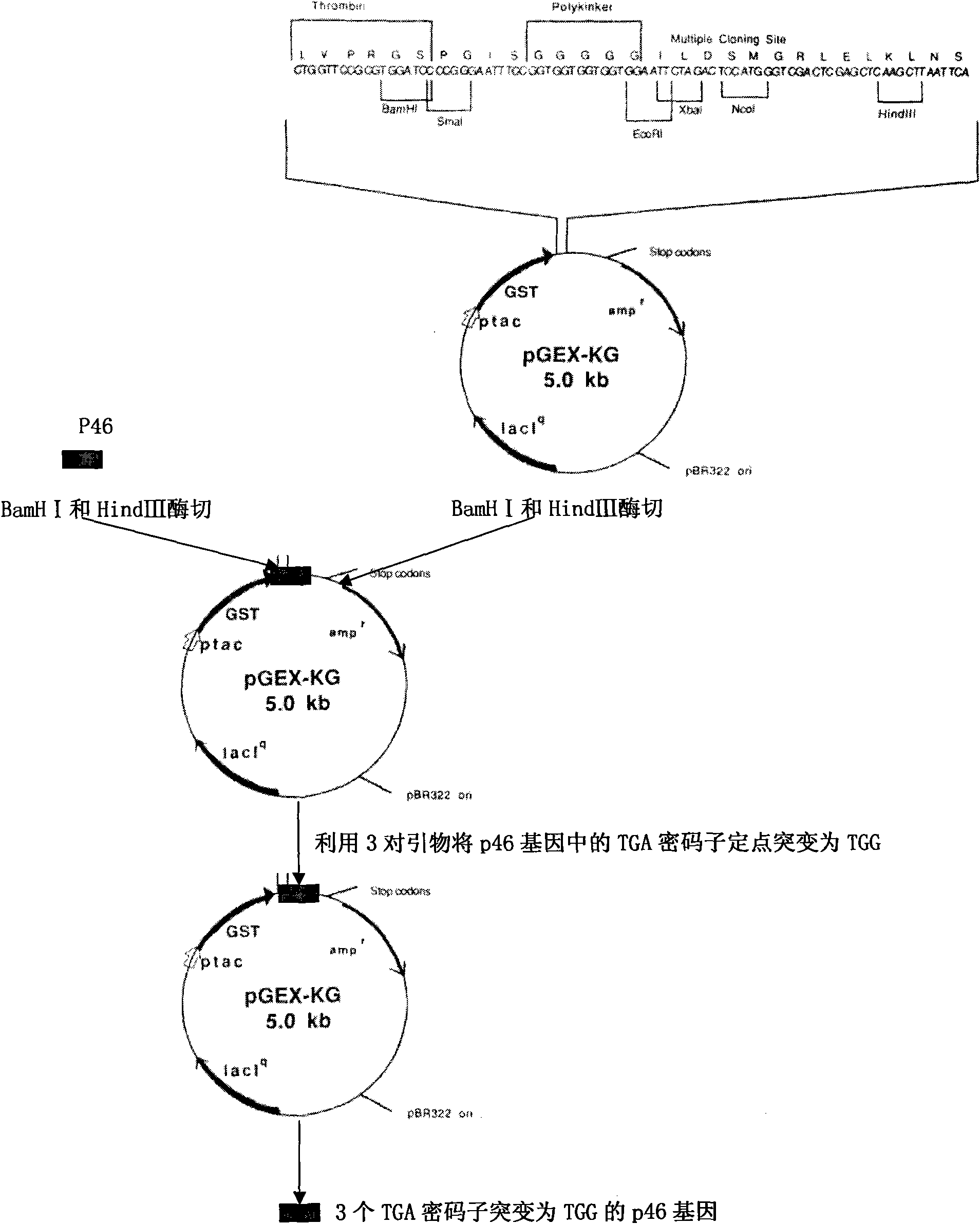
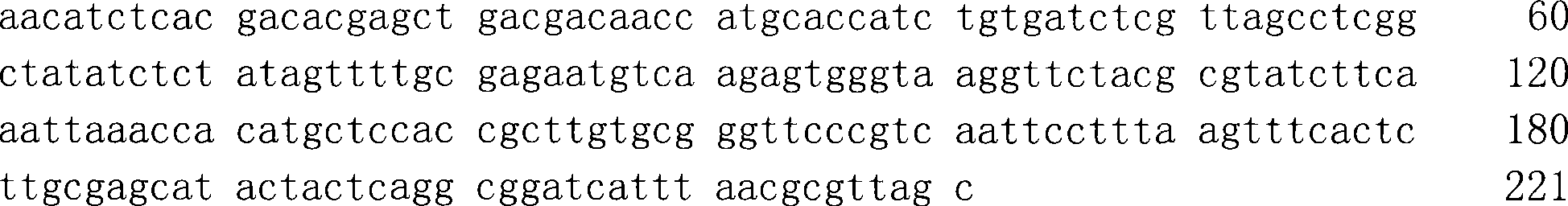Patents
Literature
187 results about "Mycoplasma pneumonia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma pneumonia (also known as "walking pneumonia" because it can spread bilaterally (“walk”) from one lung to the other) is a form of bacterial pneumonia caused by the bacterial species Mycoplasma pneumoniae.
One dose vaccination with Mycoplasma hyopneumoniae
InactiveUS6846477B2Preventing and reducing lung lesionMaintaining immunityAntibacterial agentsBiocideDiseaseVaccines Administered
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma hyopneumoniae (M. hyo) by administering to the animal at approximately three (3) to ten (10) days of age, a single dose of an effective amount of a M. hyo vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:ZOETIS SERVICE LLC +1
Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
ActiveCN103031258AEffective preventionEffective therapeuticAntibacterial agentsBacterial antigen ingredientsCircovirusPneumonia mrsa
The invention provides a mycoplasma hyopneumoniae strain HN0613 which is isolated and identified to have better immunogenicity, and further provides a mycoplasma hyopneumoniae antigen prepared from the mycoplasma hyopneumoniae strain HN0613 and mycoplasma pneumonia pneumovax containing the mycoplasma hyopneumoniae antigen. The invention further provides a bivalent combined vaccine of porcine circovirus II and mycoplasma hyopneumoniae. The duplex combination vaccine comprises PCV (Porcine Circovirus) antigen II (inactivated porcine circovirus antigen II or PCV20 RF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant. The combination vaccine can achieve the purpose of preventing the porcine circovirus disease and the mycoplasma pneumonia swine by injection once, and has a protective effect of preventing mycoplasma hyopneumoniae infection.
Owner:PU LIKE BIO ENG
Goat TLR4 gene knock-out vector and construction method thereof
InactiveCN106755097AThe method is simple and fastHigh knockout efficiencyNucleic acid vectorVector-based foreign material introductionEscherichia coliCompetent cell
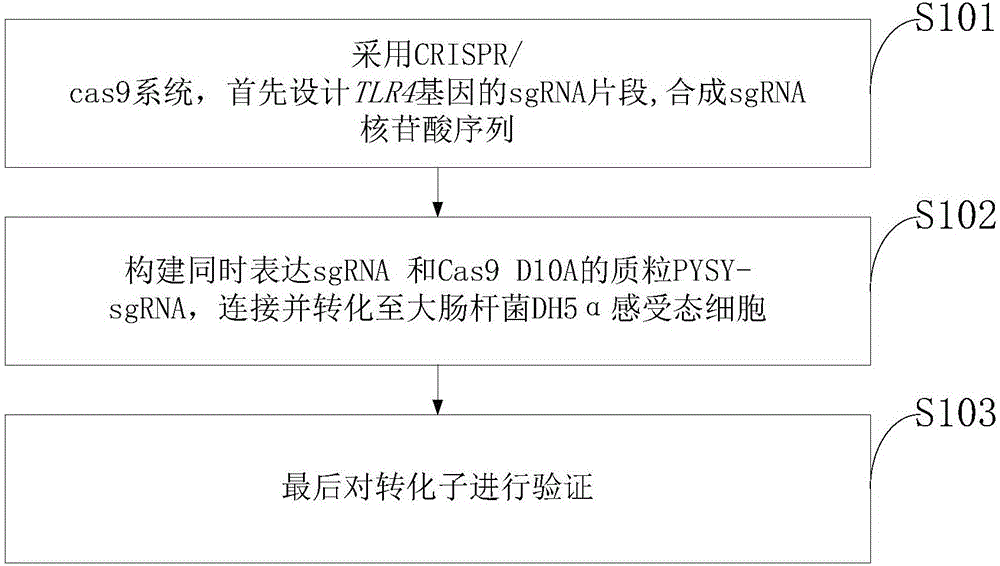
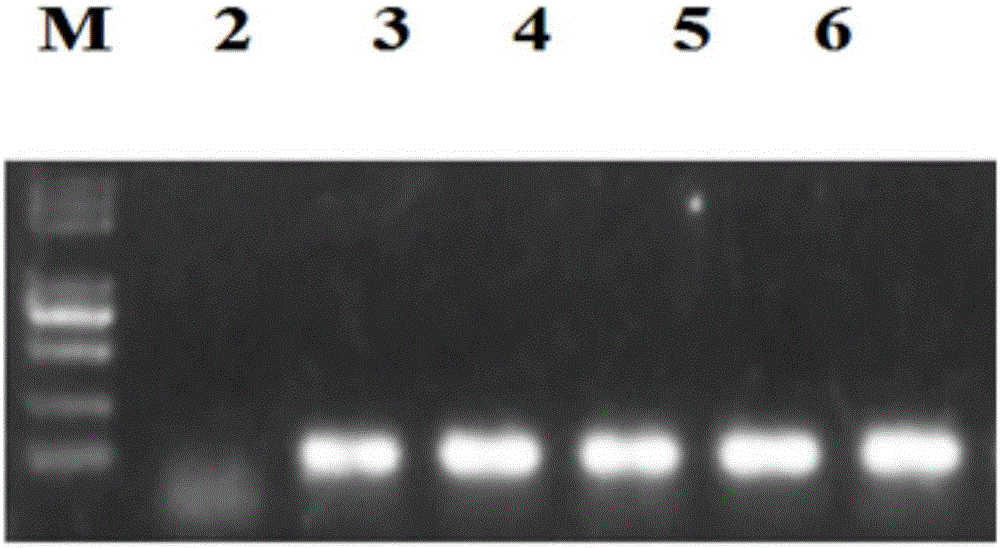
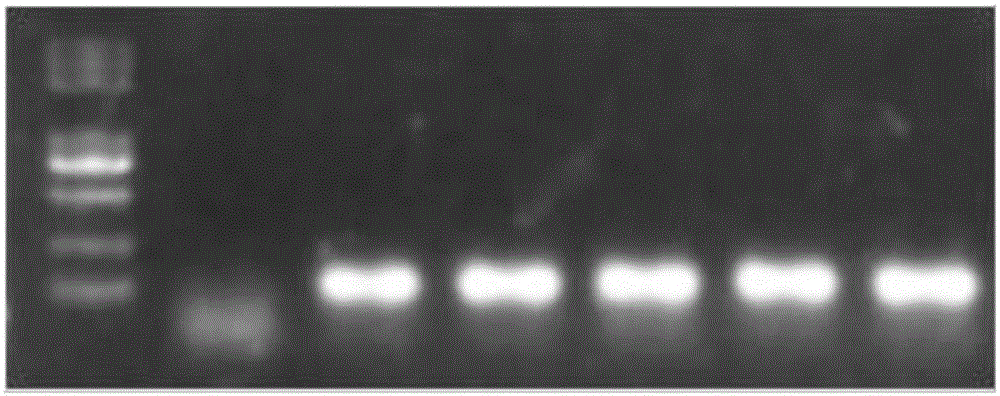
The invention discloses a goat TLR4 gene knock-out vector and a construction method thereof. The construction method comprises the following steps: firstly, designing an sgRNA fragment of the TLR4 gene by adopting a CRISPR / cas9 system, synthesizing an sgRNA nucleotide sequence, constructing and simultaneously expressing the sgRNA and plasmid PYSY-sgRNA of Cas9 D10A, connecting and transforming to an Escherichia coli DH5 alpha competent cell, and verifying the transformant; and judging and proving by enzyme digestion and sequencing that the TLR4 gene knock-out vector is constructed correctly. The invention adopts the CRISPR / cas9 for constructing the vector, and provides a theoretical basis for subsequently acquiring a goat TLR4 gene deletion type alveolar epithelial cell system, and studying the immune response molecular mechanism of mycoplasma pneumonia infection.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE ANHUI ACAD OF AGRI SCI
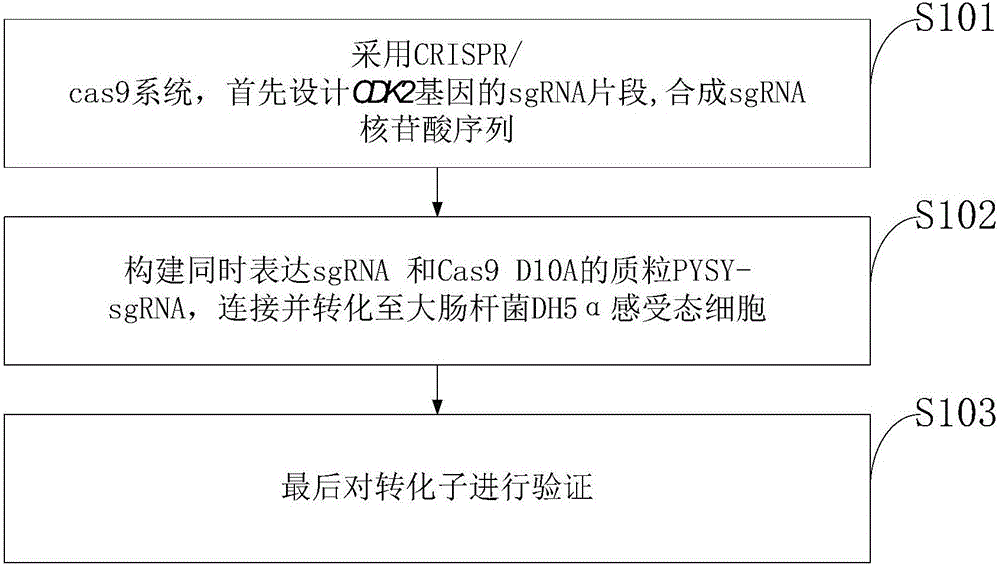
Goat CDK2 (Cyclin-dependent kinases 2) gene knockout vector and construction method thereof
InactiveCN106834347AThe method is simple and fastHigh knockout efficiencyNucleic acid vectorVector-based foreign material introductionEscherichia coliRestriction enzyme digestion
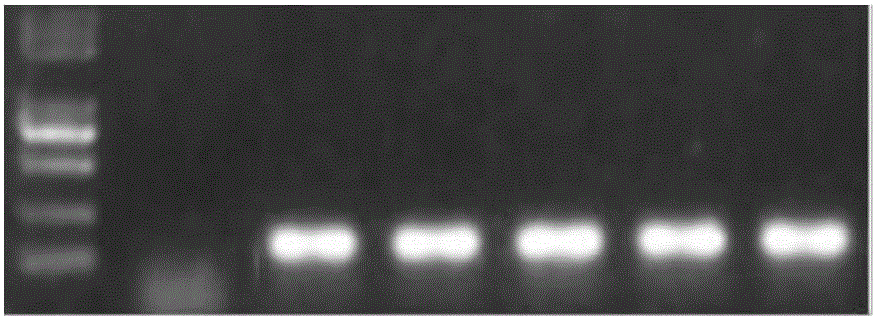
The invention discloses a goat CDK2 (Cyclin-dependent kinases 2) gene knockout vector and a construction method thereof. A CRISPR / Cas9 system is adopted; an SgRNA segment of a CDK2 gene is designed at first and an SgRNA nucleotide sequence is synthesized; a plasmid PYSY-sgRNA for expressing SgRNA and Cas9D10A at the same time is constructed; the plasmid PYSY-sgRNA is connected and transformed to an escherichia coli DH5alpha competent cell; finally, a transformant is verified; restriction enzyme digestion and sequencing identification prove that the construction of the CDK2 gene knockout vector is accurate. The vector constructed by CRISPR / Cas9 is adopted, and a theoretical basis is provided for subsequently obtaining a goat CDK2 gene deletion type cell line and researching a molecular mechanism of cell apoptosis molecules triggered by mycoplasma pneumonia infection.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE ANHUI ACAD OF AGRI SCI
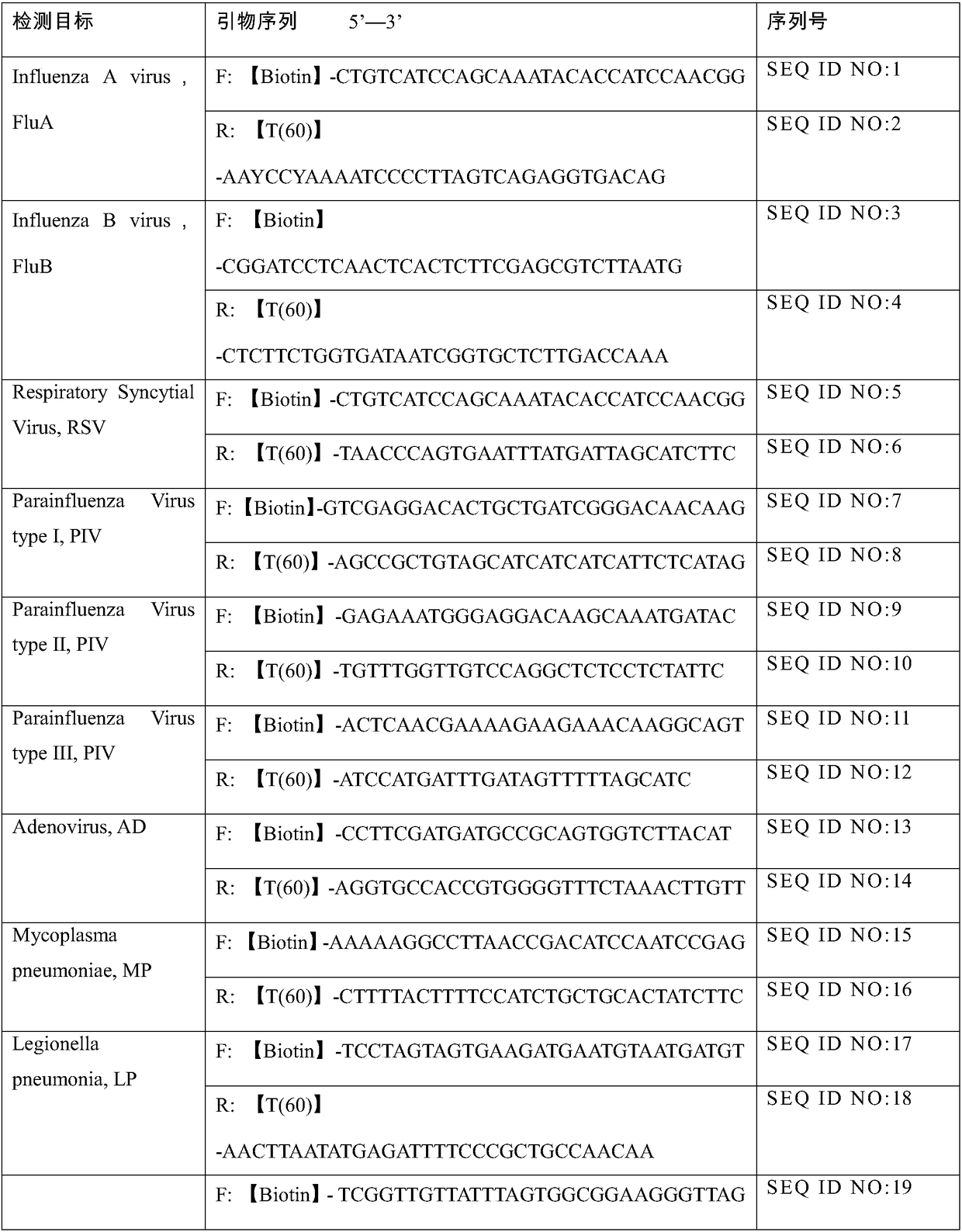
Kit for detecting 10 respiratory tract infection pathogens and application method thereof
ActiveCN107365876AStrong specificityReduce pollution interferenceMicrobiological testing/measurementAgainst vector-borne diseasesRespiratory tract infectionsPathogen
The invention provides a kit for detecting 10 respiratory tract infection pathogens and an application method thereof. The kit comprises primers and a TaqMan probe, wherein the primers are used for amplifying the 10 respiratory tract infection pathogens which include mycoplasma pneumoniae, chlamydia pneumoniae, legionella pneumophila, bordetella pertussis, rhinovirus, respiratory tract adenovirus, influenza A virus, influenza B virus, respiratory syncytial virus and parainfluenza virus. The application method includes: mixing 2 microliters of sample DNA / RNA co-extraction template, 20 microliters of RT-PCR buffer, 1 microliter of mixed enzyme liquid and 2 microliter of primer-probe mixed liquid to perform PCR amplification reaction. The kit uses the Taqman probe fluorescent PCR technology to detect the 10 common respiratory tract infection pathogens, a detection result can be obtained within 2 hours, the kit is high in specificity, and the sensitivity of the kit can reach 100copies / microliter.
Owner:NANJING LANSION BIOTECH CO LTD
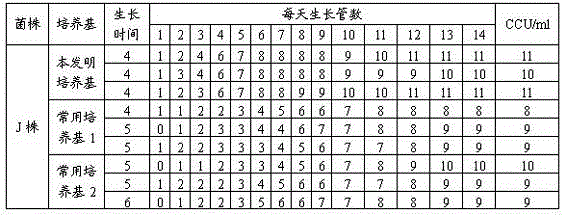
Mycoplasma hyopneumoniae culture medium and preparation method thereof
ActiveCN102154167ARich in nutrientsIncrease the titer of live bacteriaBacteriaMicroorganism based processesPenicillinMycoplasma culture
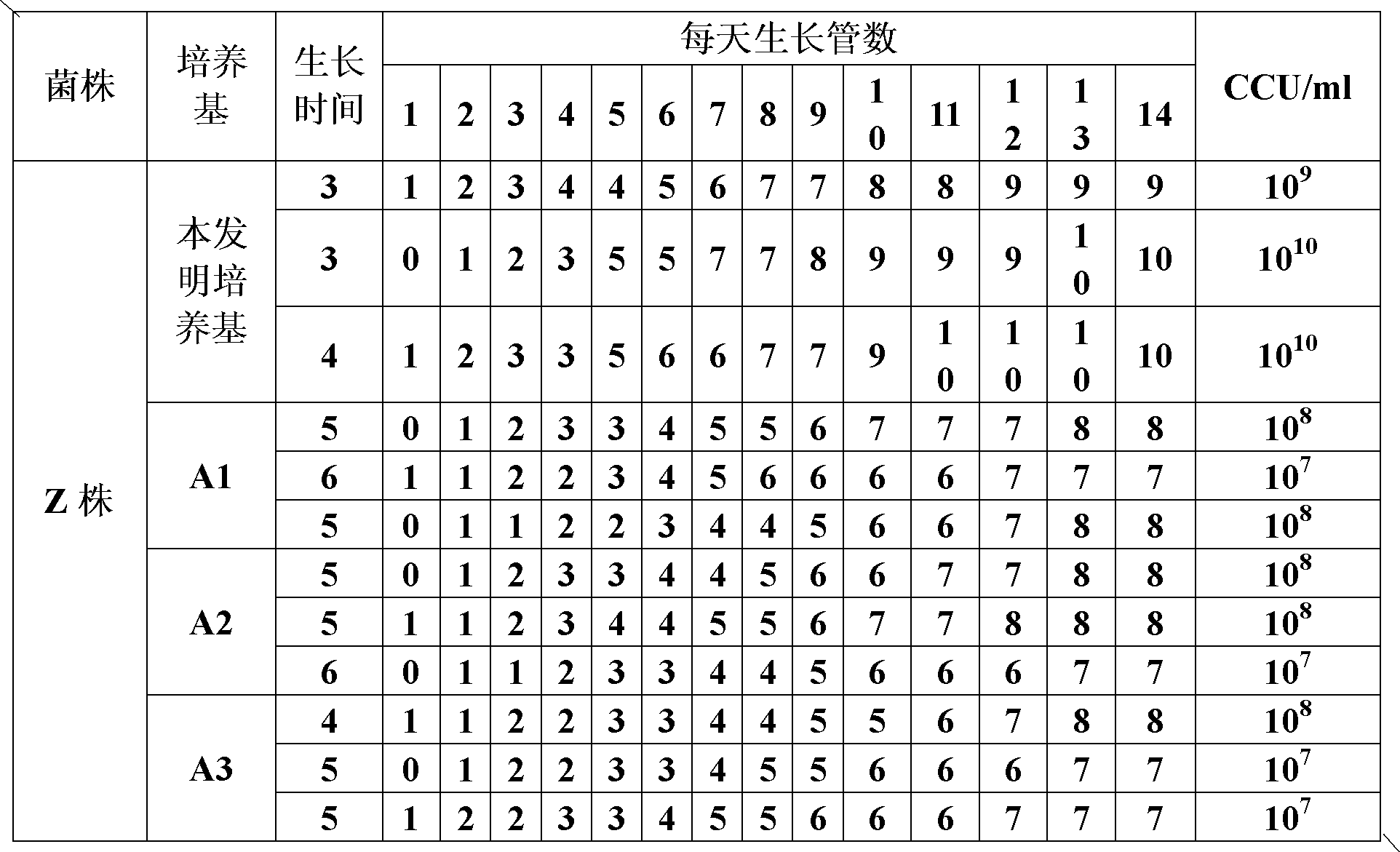
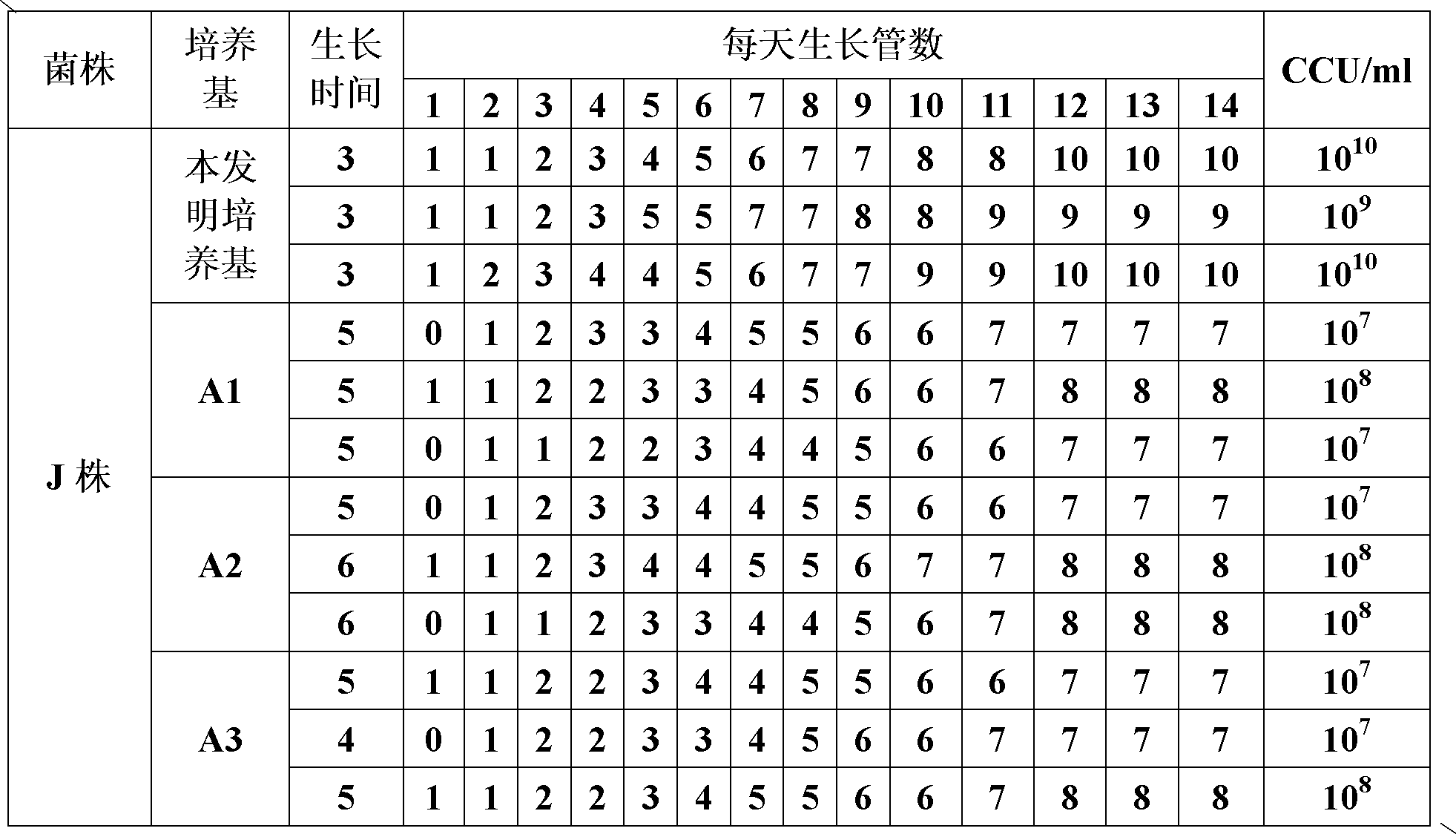
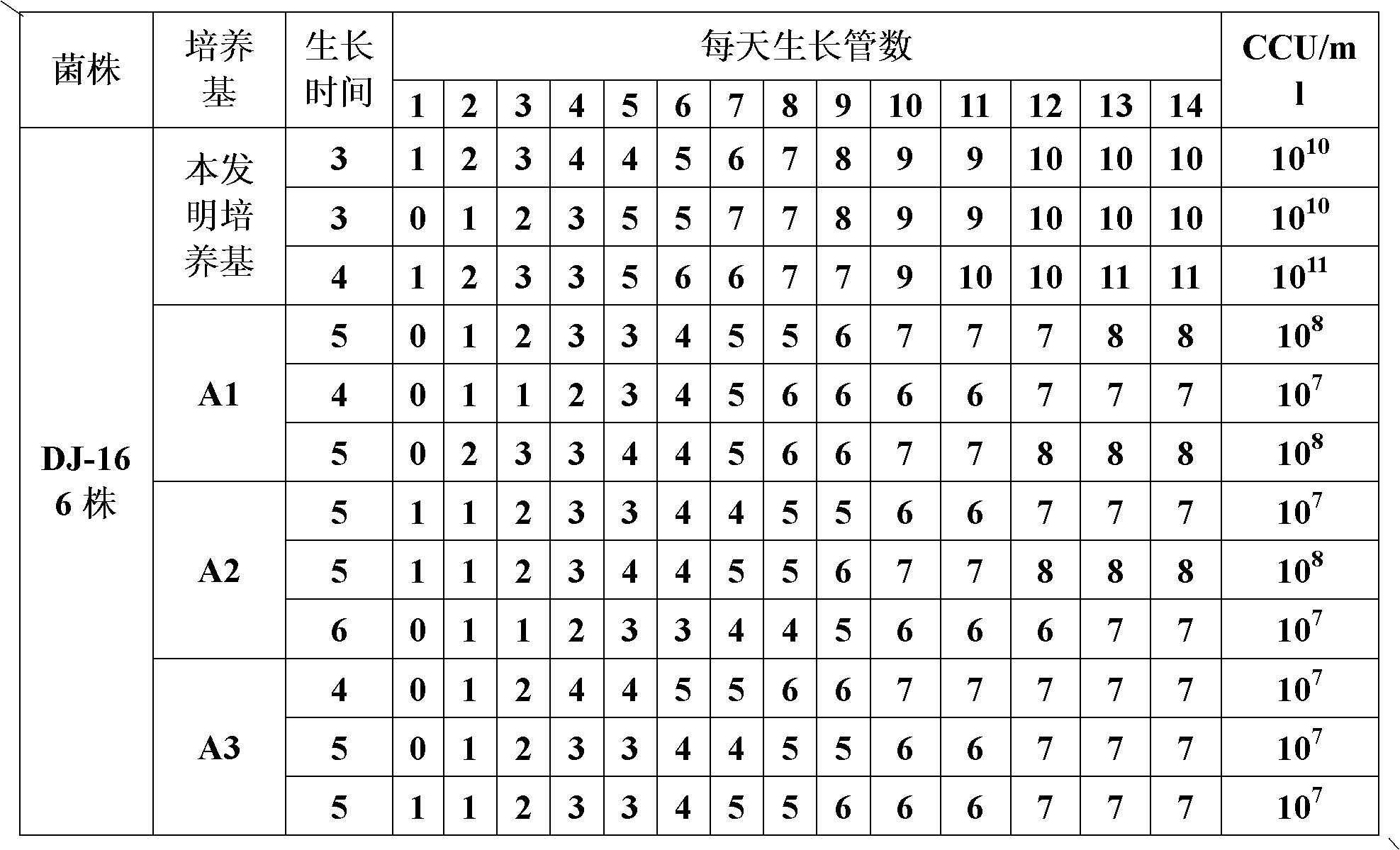
The invention provides a mycoplasma hyopneumoniae culture medium and a preparation method thereof, belonging to the technical field of veterinary biology. The mycoplasma hyopneumoniae liquid culture medium comprises the components as follows: brain heart infusion, lactalbumin hydrolysate, PPLO (pleuropneumonia-like organism) broth, yeast extract powder, proteose peptone, sodium thiosulfate, Hank's liquid, sodium pyruvate, 0.1% phenol red solution, penicillin and deionized water. The preparation method comprises the following steps of: adding health horse serum before using, and adding agar into the liquid culture medium to obtain a solid culture medium of mycoplasma hyopneumoniae. The viable bacteria titer of the mycoplasma hyopneumoniae culture medium can reach 1*109CCU / ml-1*1010CCU / ml; the viable bacteria titer and the separation sensibility are far higher than those of the existing culture medium, and the mycoplasma hyopneumoniae is fast in growth speed and high in the separation sensibility; and the preparation method of the culture medium is simple in technology, strong in operability, and suitable for industrial large-scale production.
Owner:兆丰华生物科技(南京)有限公司 +1
Vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection and preparation method thereof
ActiveCN103083655ASimplified immunization programReduce manufacturing costAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a vaccine composition for preventing and treating porcine circovirus type 2, haemophilus parasuis and mycoplasma hyopneumoniae infection. The vaccine composition comprises an inactivated porcine circovirus type 2 antigen, inactivated haemophilus parasuis, inactivated mycoplasma hyopneumoniae and a vaccine adjuvant. The vaccine composition disclosed by the invention can realize the aim of preventing three diseases including a porcine circovirus disease, mycoplasma pneumonia, a haemophilus parasuis disease by one injection of the vaccine; the content of antigen is 1 / 2 of the content of a common single-vaccine antigen when the vaccine composition disclosed by the invention is prepared by mixing the three antigens; and compared with the existing condition that three injections of single vaccine are injected to prevent three infectious diseases, the technical scheme disclosed by the invention is economical and practical, reduces the production cost, simplifies an immune procedure and reduces the epidemic prevention cost.
Owner:PU LIKE BIO ENG
Nucleic acid combined testing kit of respiratory tract infection pathogens
InactiveCN111378789AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementMicroorganism based processesDiseaseNucleotide
The invention discloses a nucleic acid combined testing kit of respiratory tract infection pathogens. The invention develops a set of primer-probe combinations which can detect multiple types of respiratory tract infection pathogens such as novel coronavirus, influenza virus a, influenza virus b, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumonia and chlamydiapneumonia through combination of a multiple fluorescence quantitative PCR technology and a flow-through hybridization and gene chip technology, wherein nucleotide sequences thereof are shown by SEQ ID NO:1-36 respectively. The nucleic acid combined testing kit of the respiratory tract infection pathogens is established. The kid can realize synchronous combined testing of the 8 respiratory tract infection pathogens, is high in detection accuracy, specificity and sensitivity, good in repeatability, low in false negativity and false positivity, short in detection time and low in cost, can realize comprehensive detection of a patient, can locate a disease source accurately, can realize treatment in time or make corresponding quarantine measures and is of important significance to effective control of respiratory tract infection and subsequent prevention of outbreak of relevant contagion and infection.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Pig mycoplasma pneumoniae recombination antigen ELISA detection reagent kit
The invention discloses a pig mycoplasma pneumonia recombination antigen ELISA detection Kit. The Kit is provided with an antibody detection plate, enzyme conjugate treatment fluid, a positive control, a negative control, sample diluent, 10x condensed cleaning solution, developing solution A, developing solution B and termination solution. The detection plate of the Kit is a detachable 96-pore enzyme label plate enveloped by the mutational pig mycoplasma pneumonia membrane protein P46 gene protein antigen, the enzyme conjugate treatment fluid is a rabbit anti-pig antibody labeled by horse radish peroxidase, the positive control serum is taken from a pig which is detected positive through indirect hemagglutination and the ELISA Kit of IDEXX and has obvious pig mycoplasma pneumonia lesions in the lungs after anatomy, and the negative control serum is taken from a pig which is detected negative through indirect hemagglutination and the ELISA Kit of IDEXX and has no pig mycoplasma pneumonia lesions in the lungs after anatomy. The pig mycoplasma pneumonia recombination antigen ELISA detection Kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale generation and application, and broad market prospect.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Mycoplasma hyopneumoniae and Mycoplasma hyorhinosus dual inactivated vaccine and preparation method thereof
ActiveCN102258776AStrong specificityGood immune effectAntibacterial agentsBacterial antigen ingredientsDiseaseImmune effects
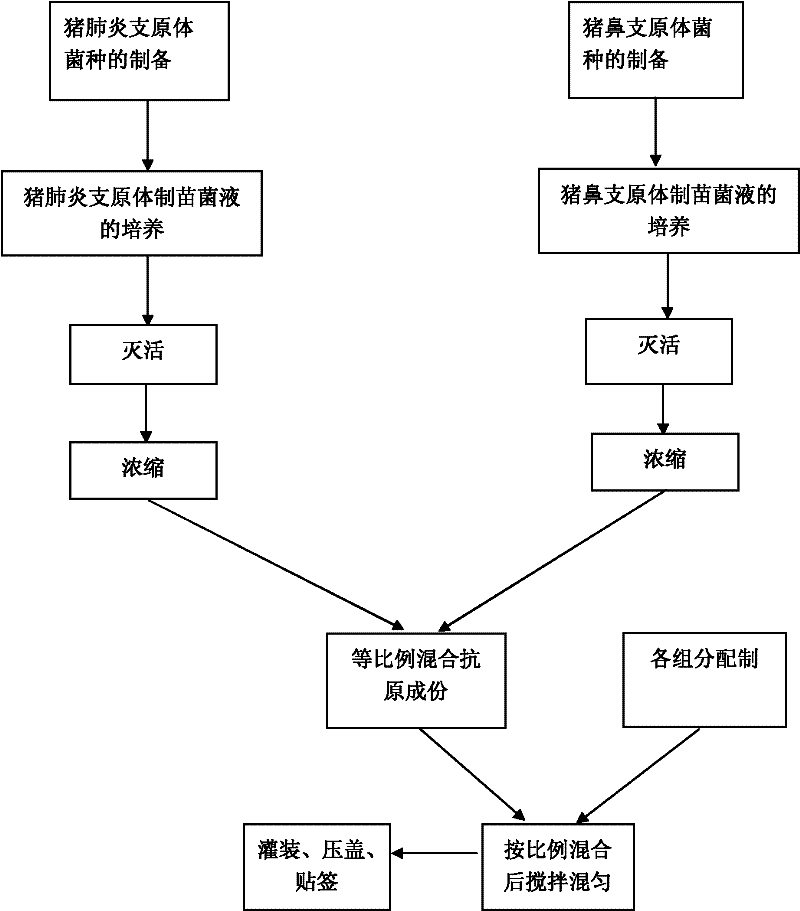
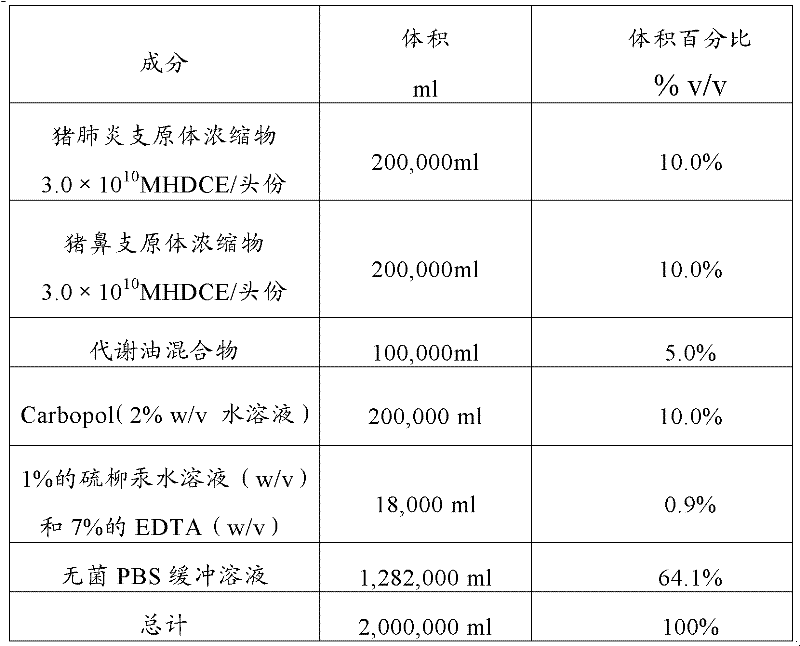
The invention provides a combined inactivated vaccine against mycoplasma hyopneumoniae (MHP) and mycoplasma hyorhinis. The combined inactivated vaccine contains the MHP and the mycoplasma hyorhinis with preferred content as well as a Carbomer adjuvant with concentration of 10% (V / V). The invention further provides a preparation method of the combined inactivated vaccine against the MHP and the mycoplasma hyorhinis. The preparation method comprises the following steps: preparing production strains; preparing bacterium solution for producing seedlings; and inactivating, concentrating and blending to obtain the vaccine. The combined inactivated vaccine has the advantages of strong specificity and good immunity, thus solving the problem of specific infection caused by the MHP in the current domestic breeding farm and obtaining the mycoplasma hyorhinis vaccine under a blank state at home and abroad at present. The combined inactivated vaccine has the beneficial effects that the step of vaccine inoculation is simplified, trouble caused by a plurality of inoculation and easily produced side effects are avoided, and vaccine cost is saved, thus being especially applicable to preventing andtreating mixed infection diseases in the breeding farms at home and abroad and the like; and compared with the existing single vaccine, application range is widened and immune effect is enhanced.
Owner:PU LIKE BIO ENG
Colloidal gold method detection test strip and reagent kit for IgM and IgG antibodies of mycoplasma pneumoniae and preparation method of reagent kit
The invention discloses a colloidal gold method detection test strip and a reagent kit for IgM and IgG antibodies of mycoplasma pneumonia (MP) and a preparation method of the reagent kit. The test strip determines the IgM antibody and the IgG antibody by using a principle of an immunocapture method; the specific IgM and IgG antibodies of the MP can be detected jointly by one operation; the operation process is simplified; and the reagent kit is simple, convenient, rapid and accurate in detection, is particularly applicable to primary screening and epidemiological survey, and has an auxiliary diagnosis effect on early and interim mycoplasma pneumoniae infection.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Mycoplasma pneumoniae recombinant antigen, and preparation method and application of mycoplasma pneumoniae recombinant antigen
ActiveCN103275196AStrong specificityHigh sensitivityDepsipeptidesBiological testingEpitopeMycoplasma pneumonia
The invention relates to the technical field of biology and discloses a mycoplasma pneumoniae (MP) recombinant antigen, and a preparation method and an application of the mycoplasma pneumoniae recombinant antigen. An amino acid sequence of the mycoplasma pneumonia recombinant antigen is shown as SEQ ID No: 1. According to the antigen, a section of amino acid is taken as connecting oligopeptide, and an MP specific antigen P1 proteantigen dominant epitope and a P30 antigen dominant epitope form a recombinant protein. Tests prove that the recombinant protein has higher specificity and sensitivity in comparison with the existing MP antigen, and can be used for preparing MP antibody testing products.
Owner:WUHAN CHANGLI BIOLOGICAL TECH CO LTD
Pig mycoplasma pneumonia live attenuated vaccine and application thereof
ActiveCN103740625AAvoid infectionSimple and fast operationAntibacterial agentsBacterial antigen ingredientsAdjuvantPneumonia mrsa
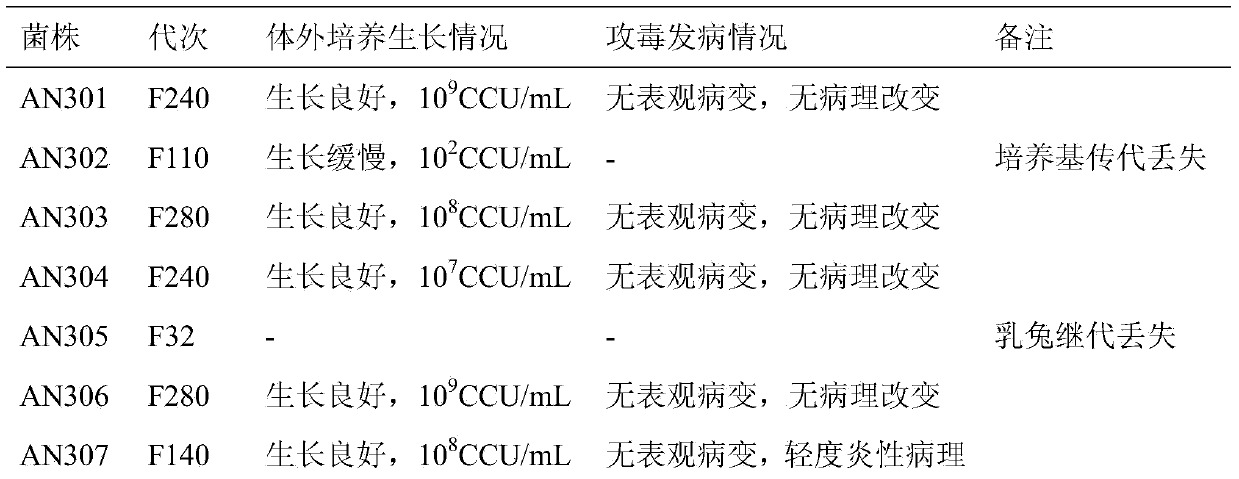
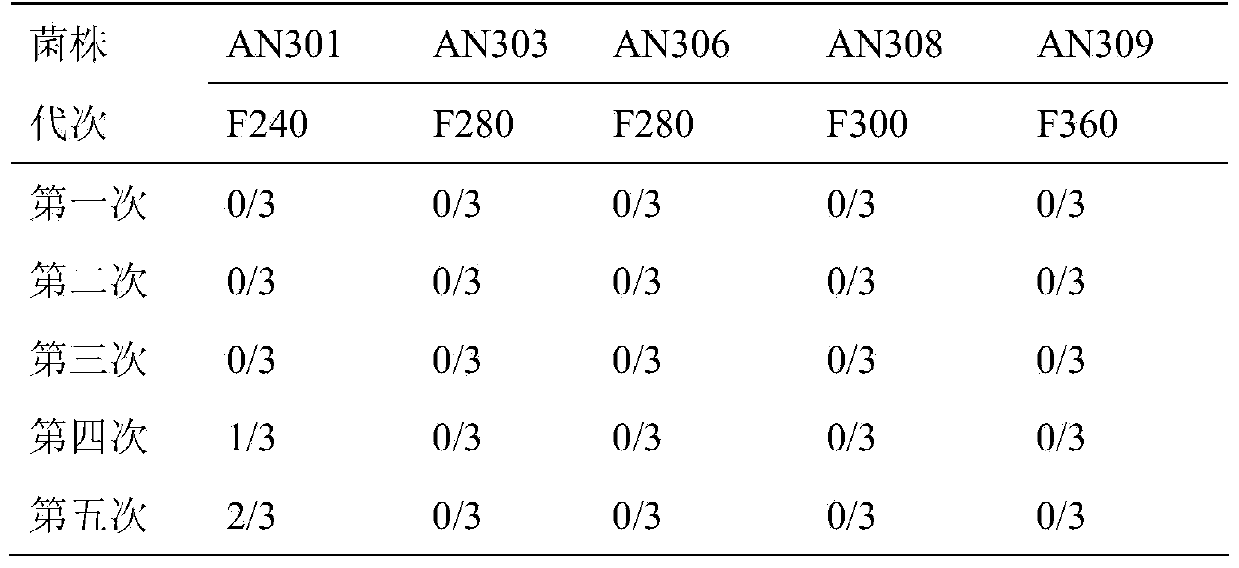
The invention discloses a pig mycoplasma pneumonia live attenuated vaccine and application thereof. A pig lung disease material infected by typical pig mycoplasma hyopneumoniae and not infected by other pathogens is screened and then is subcultured to the 100th generation through the lung of a baby rabbit, then pig mycoplasma hyopneumoniae strains are separated and are continuously subcultured through a culture medium; meanwhile, the mycoplasma hyopneumoniae AN306 is obtained through screening of a plurality of strains, and the preservation number of the mycoplasma hyopneumoniae AN306 is CCTCC M2012431. The invention also relates to a pig mycoplasma pneumonia live vaccine preparation based on the preparation of the attenuated vaccine strain. The pig mycoplasma pneumonia live vaccine preparation comprises a live attenuated vaccine strain, a pharmaceutically acceotable carrier or auxiliary ingredient and an adjuvant, and further comprises immunogens of other pathogens. The pig mycoplasma pneumonia live vaccine disclosed by the invention can be immunized in multiple ways and can enable animals to gain protection ability for resisting pig mycoplasma hyopneumoniae infection.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
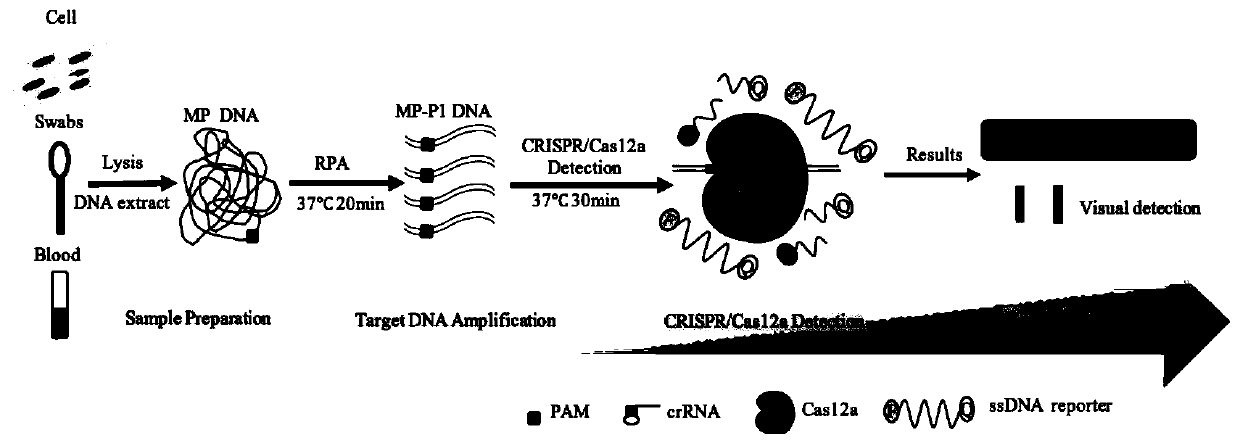
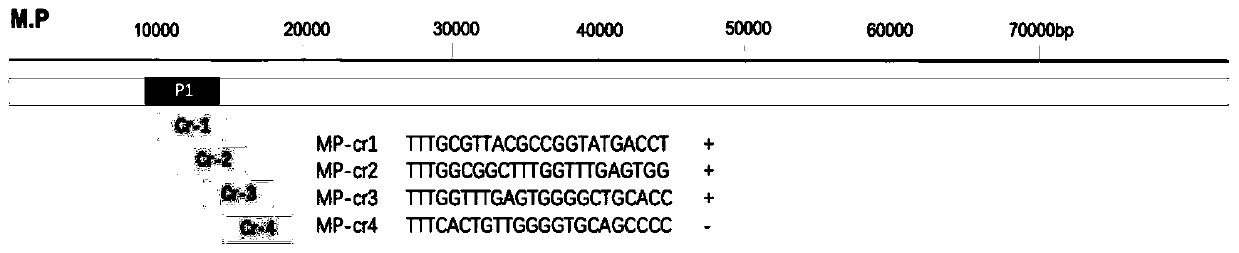
Kit and detection method for rapid detection of nucleic acid of mycoplasma pneumonia on basis of CRISPR/Cas12a
InactiveCN111187804AHigh sensitivityHighly conservativeMicrobiological testing/measurementMicroorganism based processesSingle strandNucleic acid sequencing
Owner:国家卫生健康委科学技术研究所
Primers for triple PCR of three types of sheep pathogenic mycoplasmas and detection method
InactiveCN106434919AOvercome limitationsQuick checkMicrobiological testing/measurementDNA/RNA fragmentationEpidemiologic surveyMycoplasma pneumonia
The invention provides primers for triple PCR of three types of sheep pathogenic mycoplasmas and a detection method. The three pairs of special primers MO, MCC and MCCP are synthesized respectively according to the designs of sheep mycoplasma pneumoniae, a goat mycoplasma mycoide subspecies and a goat mycoplasma pneumonia subspecies, and a triple PCR optimum reaction system and reaction conditions for the three types of sheep pathogenic mycoplasmas are provided. The simultaneous pathogenic detection of the sheep mycoplasma pneumoniae, the goat mycoplasma mycoide subspecies and the goat mycoplasma pneumonia subspecies can be rapidly carried out without cloning, sequencing and sequence comparison, and the detection method has the advantages of being rapid, accurate, strong in specificity, good in repeatability and the like, suitable for rapid detection of the sheep mycoplasma pneumoniae, the goat mycoplasma mycoide subspecies and the goat mycoplasma pneumonia subspecies and large-scale epidemiological investigation and has great economic and social benefits.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
Mycoplasma hyopneumoniae vaccine and methods for reducing mycoplasma bovis pneumonia in cattle
The present invention relates to methods for treating or preventing a disease or disorder in an animal caused by infection by Mycoplasma bovis (M. bovis) by administering to the animal an effective amount of a Mycoplasma hyopneumoniae (M. hyo) vaccine. The M. hyo vaccine can be a whole or partial cell inactivated or modified live preparation, a subunit vaccine, or a nucleic acid or DNA vaccine. The M. hyo vaccine administered in accordance with the present invention can be synthesized or recombinantly produced.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
A temperature sensitive vaccine strain of mycoplasma hyopneumoniae and uses thereof
ActiveCN102458462AAntibacterial agentsBacterial antigen ingredientsGene listMycoplasma hyopneumoniae
The present invention relates to a Mycoplasma hyopneumoniae vaccine strain comprising a mutation in at least one of the genes listed or as deposited with the National Measurements Institute (Australia) under accession number NM04 / 41259, which strain is temperature sensitive and attenuated, a vaccine comprising such strains and methods and uses thereof.
Owner:BIOPROPERTIES +1
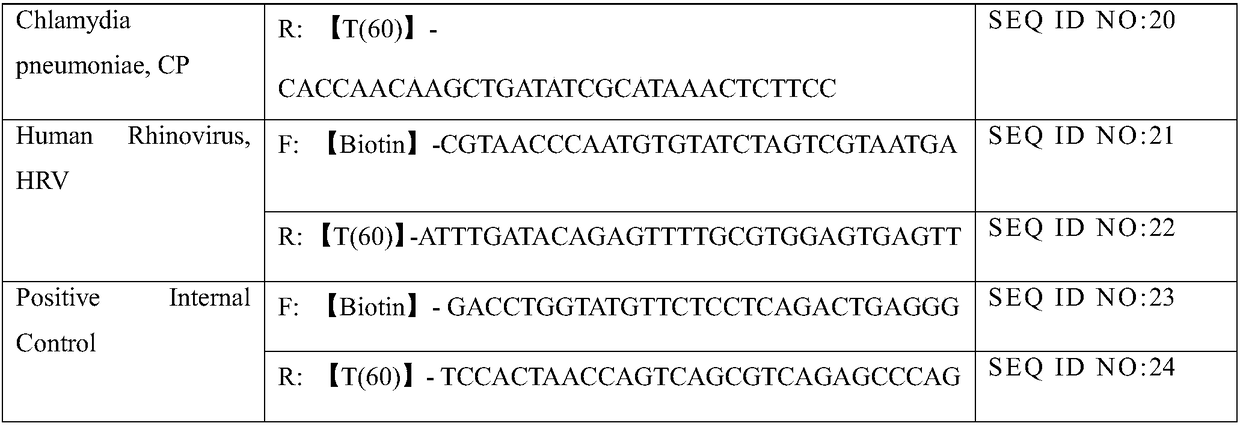
Primer set for respiratory tract infection pathogen detection, rapid diagnostic kit and detection method
InactiveCN108300803AImmediate rapid diagnosisMultiplexingMicrobiological testing/measurementAgainst vector-borne diseasesPolymerase LRecombinase
Belonging to the molecular biology field, the invention relates to a primer set for respiratory tract infection pathogen detection, a rapid diagnostic kit and a detection method. The primer set can achieve one-time detection of the following 9 respiratory tract infection pathogens: influenza A virus, influenza B virus, respiratory syncytial virus, parainfluenza virus type I, parainfluenza virus type II and parainfluenza virus type III, adenovirus, mycoplasma pneumonia, legionella pneumonia, chlamydia pneumonia and human rhinovirus. The invention adopts solid phase recombinase-polymerase constant temperature gene amplification method to detect respiratory multiple infection pathogens. The primer set adopted by the invention for respiratory tract infection pathogen detection has strong specificity and high amplification efficiency, can effectively and rapidly detect the 9 pathogens simultaneously, and realizes multiplex detection.
Owner:博迪泰(厦门)生物科技有限公司
Mycoplasma hyopneumoniae culture medium and preparation method thereof
ActiveCN103555641AImprove enduranceReduce apoptosis rateBacteriaMicroorganism based processesBacteroidesMycoplasma culture
The invention discloses a mycoplasma hyopneumoniae culture medium and a preparation method thereof, which belong to the technical field of veterinary biological products. Each 1000 ml of phosphate buffer contains 20-50 g of PPLO (pleuropneumonia-like organism), 2-10 g of yeast powder, 3-15 g of glucose, 0.5-4 ml of 1% phenol red, 2-10 g of amino acid composition, 15-30 g of compound traditional Chinese medicine polysaccharide and 50-100 ml of pig serum. When culturing the mycoplasma hyopneumoniae, the mycoplasma hyopneumoniae culture medium disclosed by the invention can improve the tolerance of the mycoplasma hyopneumoniae and reduce the apoptosis rate of the mycoplasma hyopneumoniae; the mycoplasma hyopneumoniae culture medium disclosed by the invention can increase the amount of culture bacteria of the mycoplasma hyopneumoniae, the concentration of the culture bacteria liquid reaches 10<10-11> / ml, which is increased by more than 5-10 times compared with that of a common culture medium; the mycoplasma hyopneumoniae culture medium disclosed by the invention can inhibit the growth of other bacteria when culturing the mycoplasma hyopneumoniae so as to reduce the culture pollution risk of culturing the mycoplasma hyopneumoniae.
Owner:浙江美保龙生物技术有限公司
Anti-swine SC protein monoclonal antibody and application of monoclonal antibody in preparing mycoplasma hyopneumoniae SIgA antibody ELISA detection kit
ActiveCN104877027AAvoid cross interferenceAvoid interferenceTissue cultureImmunoglobulinsImmune effectsProtein.monoclonal
The invention provides an anti-swine SC protein monoclonal antibody and an application of the monoclonal antibody in preparing a mycoplasma hyopneumoniae SIgA antibody ELISA detection kit, and relates to the technical field of animal virological and epizootiological detection. The anti-swine SC protein monoclonal antibody is secreted by a hybridoma cell strain 4H11 and the collection number of the anti-swine SC protein monoclonal antibody is CCTCC NO: C201526. The invention also discloses the monoclonal antibody and the application of the monoclonal antibody in preparing the mycoplasma hyopneumoniae SIgA antibody ELISA detection kit. The kit has high specificity, high stability and high sensitivity; a detection sample can be sampled conveniently; and the kit is capable of distinguishing porcine mycoplasma pneumonia inactivated vaccine immunization and natural infection, and can be applied to the early diagnosis of pneumonic porcine mycoplasma infection and the evaluation of the immune effect after attenuated live vaccine immunization.
Owner:JIANGSU ACAD OF AGRI SCI
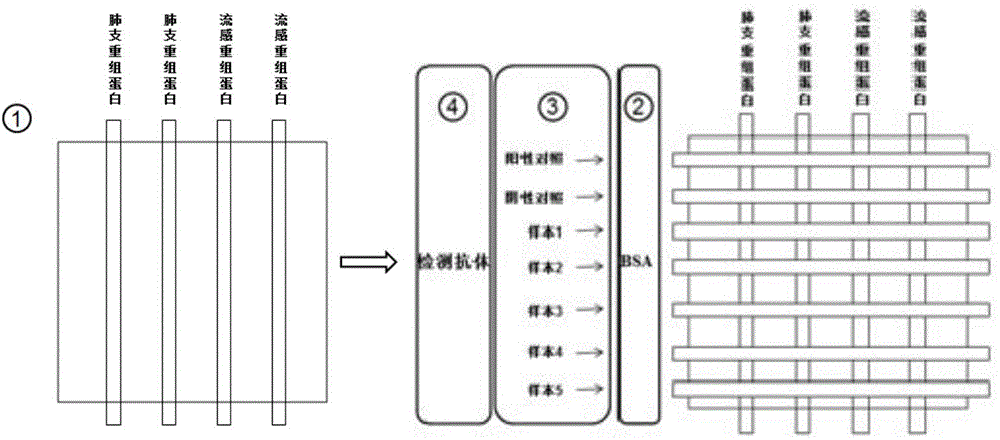
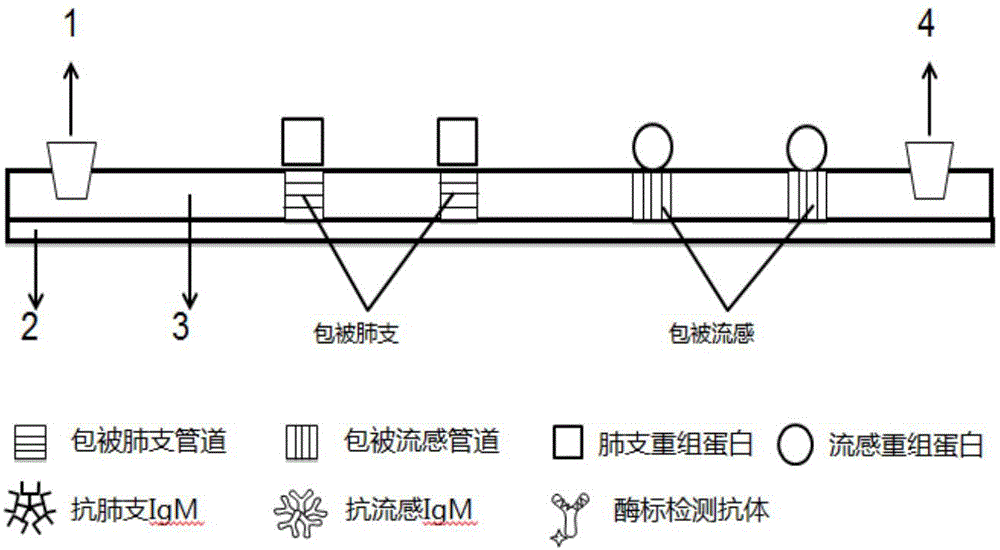
Detection method for specific antibodies IgM of mycoplasma pneumonia and influenza viruses based on micro-fluidic chip
ActiveCN104360060AAvoid non-specific reactionsMeet testing needsChemiluminescene/bioluminescenceBiological material analysisSmall sampleSpecific antibody
The invention relates to a detection method for specific antibodies IgM of mycoplasma pneumonia and influenza viruses based on a micro-fluidic chip and application of the detection method. The method is characterized by taking the micro-fluidic chip with a plurality of pipelines as a reaction platform and detecting by using a chemical luminescent signal so as to independently or simultaneously detect the serum specific antibodies IgM of mycoplasma pneumonia and influenza viruses. As the micro-fluidic chip is combined with chemiluminiscence, the detection method integrates the advantages of rapidness, high efficiency and small sample amount of the micro-fluidic chip and high specificity of chemiluminiscence, also has the advantages of simplicity in operation, low cost, rapidness and the like, is a multi-target, real-time and high-sensitivity detection method and can be applied to rapid diagnosis of pneumonia and influenza.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
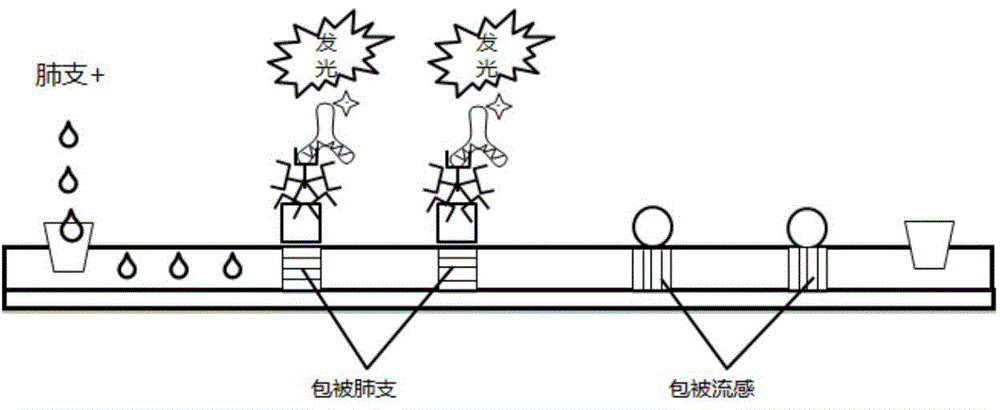
Fusion protein of mycoplasma pneumonia protein epitope and preparation and application thereof
ActiveCN105884902AEasy to purifyRefolding is not requiredAntibacterial agentsBacterial antigen ingredientsEpitopeGlycine
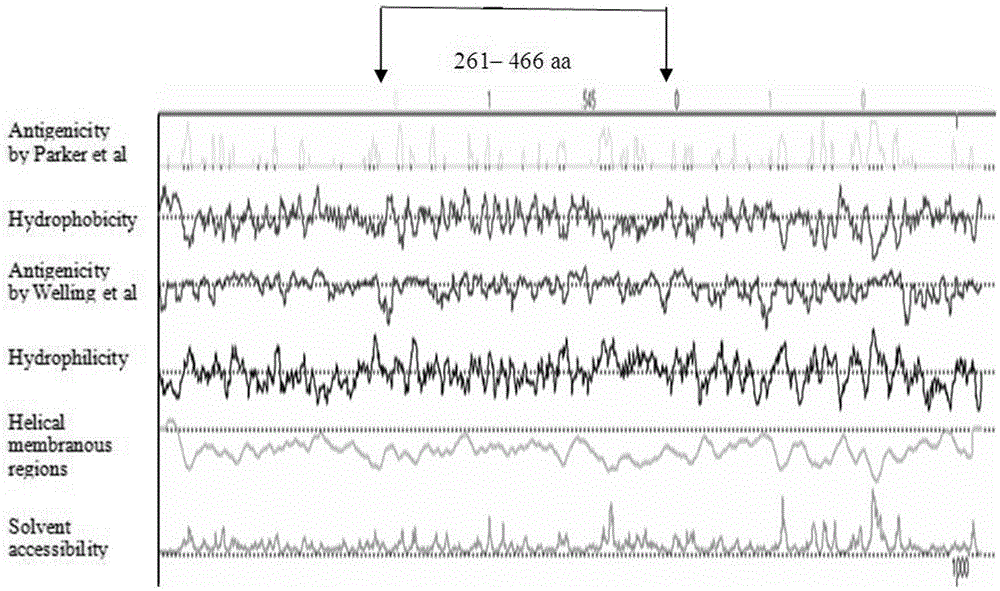
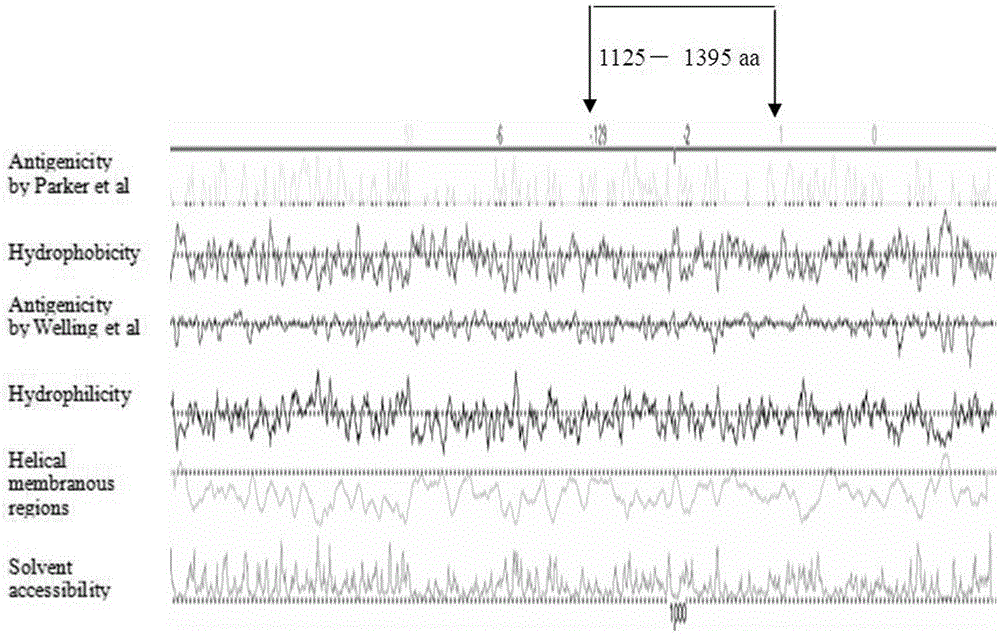
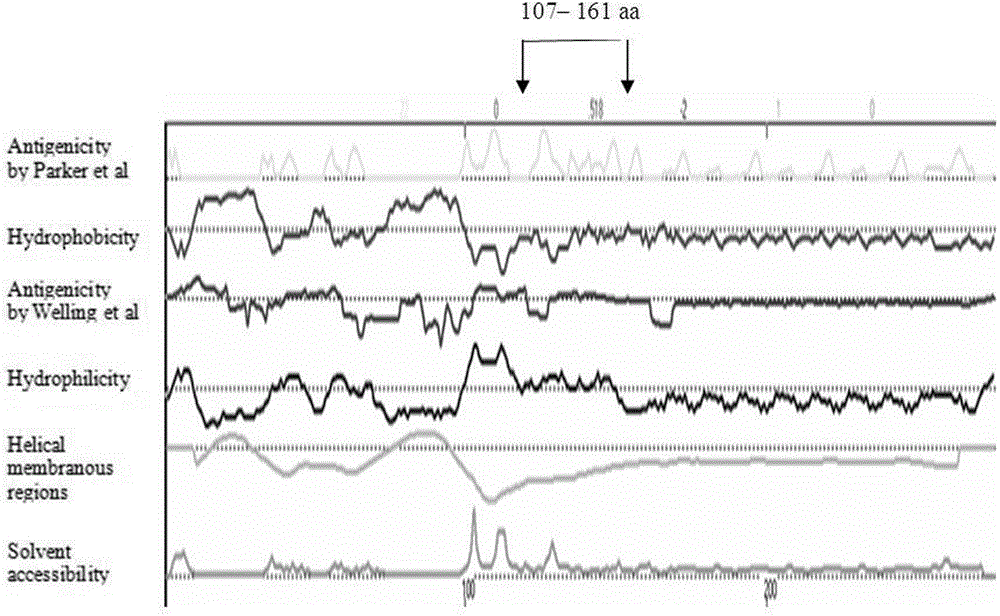
The invention discloses a fusion protein of a mycoplasma pneumonia protein epitope and preparation and application thereof, and relates to the fields of genetic engineering technologies, vaccines and diagnosis reagents. Amino acid sequences of a mycoplasma pneumoniae P116 protein, a P1 protein and a P30 protein are analyzed through a computer, mycoplasma pneumoniae P116 protein fragments, namely from the 261st amino acid to the 466th amino acid containing the strong epitope, mycoplasma pneumoniae P1 protein fragments, namely from the 1125th amino acid to the 1395th amino acid containing the strong epitope and mycoplasma pneumoniae P30 protein fragments, namely from the 107th amino acid to the 161st amino acid containing the strong epitope are screened out. Series connection gene sequences of novel mycoplasma pneumoniae P116 protein fragments, mycoplasma pneumoniae P1 protein fragments and mycoplasma pneumoniae P30 protein fragments are synthesized chemically, three gene fragments are connected in series, the fusion protein of P116 protein fragments, P1 protein fragments and P30 protein fragments is expressed by means of the genetic engineering technology, the three protein fragments are connected through glycine-glycine-serine, and 538 amino acids are formed in the overall length of the fusion protein.
Owner:李越希
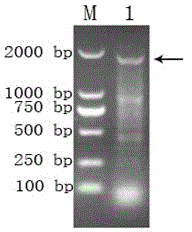
Method for rapidly and qualitatively detecting mycoplasma pneumoniae of sheep
InactiveCN103103273AGood qualitySimple Qualitative TestMicrobiological testing/measurementHsp70Mycoplasma pneumonia
The invention discloses a method for rapidly and qualitatively detecting mycoplasma pneumoniae of sheep. The method comprises the following steps of: carrying out species specificity polymerase chain reaction (PCR) by employing a self-designed species specificity primer of a sheep mycoplasma pneumoniae heat shock protein 70 (HSP70) gene sequence, and establishing a simple, rapid and accurate qualitative detection method for the sheep mycoplasma pneumoniae through agarose gel electrophoresis atlas analysis. The method can be used for fast diagnosis on the sheep mycoplasma pneumonia, and has the advantages of being strong in specificity, good in repeatability, and high in sensitivity, and can facilitate the standardization.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Mycopasma hyopneumoniae strain
InactiveCN103484414AReduce generation costImproving immunogenicityBacteriaMicroorganism based processesMicroorganismMycoplasma hyopneumoniae
The invention discloses a mycoplasma hyopneumoniae strain which is preserved in the Ordinary Microorganism Center of the Chinese Microorganism Culture Collection and Management Committee which is located in #3, No.1 Yard, Beichen Road, Chaoyang District, Beijing, the preservation number is CGMCC No.8096, and the preservation date is August 15th, 2013. The name of the strain is mycoplasma hyopneumoniae HDZK-Mhp57. According to the mycoplasma hyopneumoniae HDZK-Mhp57, the viable bacteria titer of a prepared acellular culture medium is as high as 1012CCU / mL, the immunogenicity is good, the pneumonia lesion is reduced by more than 90%, the generation cost of an animal biological product is greatly lowered, the stain can be prepared into preventing animal biological products or veterinary medicines, and a foundation for researching and preparing mycoplasma hyopneumoniae inactivated vaccines, genetic engineering vaccines and mycoplasma hyopneumoniae diagnosis kits is laid.
Owner:HEILONGJIANG UNIV
Mycoplasma pneumonia mosaic antigen, antigen detection reagent, and preparation method of both
InactiveCN107573417AStrong specificityEasy to culture and purifyBiological testingHybrid peptidesAntigenMycoplasma pneumonia
The invention provides a mycoplasma pneumonia mosaic antigen amino acid sequence containing an amino acid sequence as shown in SEQ ID NO:1 as well as a full-gene synthesized mycoplasma pneumonia mosaic antigen full-gene sequence containing an amino acid sequence as shown in SEQ ID NO:2. The invention also provides a method for constructing the two gene sequences. The invention also provides a preparation method of the mycoplasma pneumonia mosaic antigen containing full-gene synthesis, a mycoplasma pneumonia detection kit and a preparation method thereof. Mp recombinant mosaic antigen is selected as a mark material and is applied to a gold immunochromatography system, and the detection system is directly marked and captured, so the sensitivity is greatly improved, the specificity of the antigen is high, the antigen is easy in cultivation and purification, and cost is reduced; and a new method for detecting mycoplasma pneumoniae IgG rapidly and accurately is provided for clinical use, and a good market prospect is achieved.
Owner:HANGZHOU CLONGENE BIOTECH
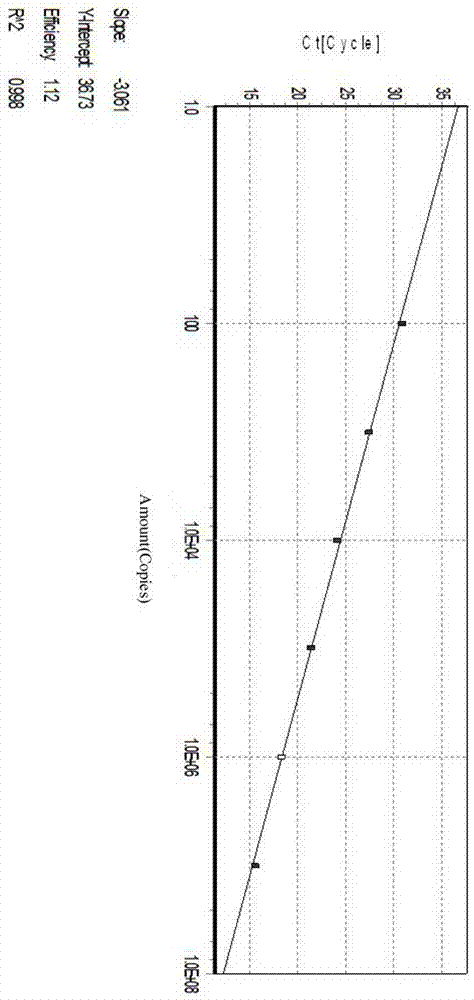
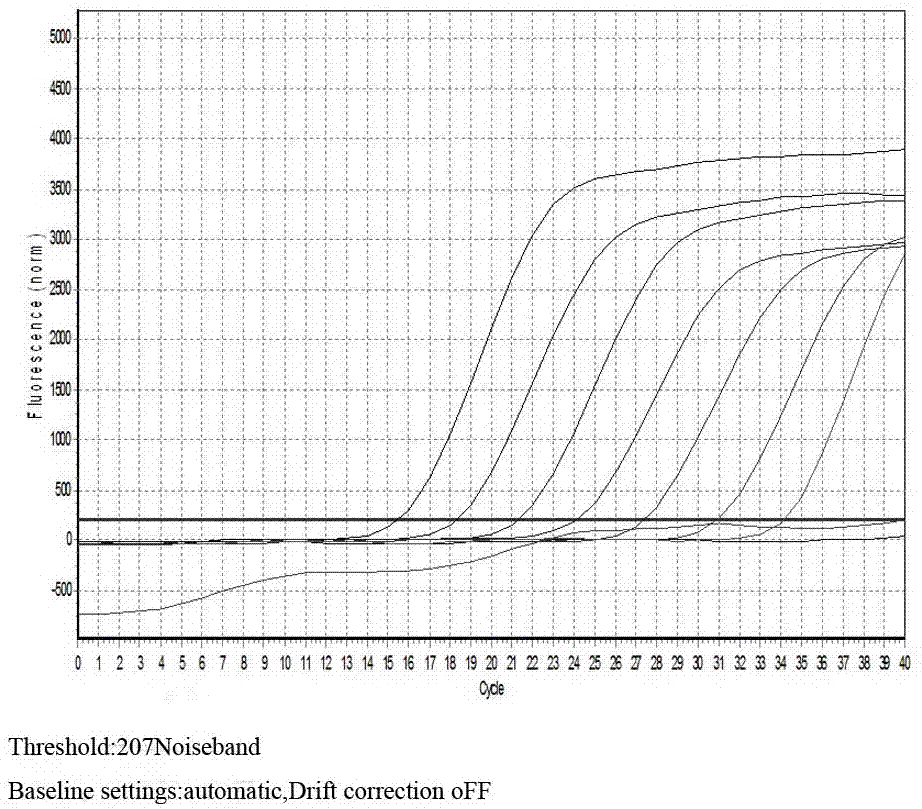
Target sequence, primer, probe and kit for detecting Mycoplasma pneumonia
ActiveCN102230013ASimplify testing proceduresShort detection cycleMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNucleotide sequencing
The invention provides a genetic marker, real-time fluorescence quantitative PCR (polymerase chain reaction) primers and probe for detecting Mycoplasma pneumonia, by sequencing and comparing of Mycoplasma pneumonia genes, which have the nucleotide sequences shown in SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4 respectively. The invention also provides a method and kit for quantitatively detecting Mycoplasma pneumonia. The detection method has the advantages of accuracy in detection, high sensitivity and strong specificity, is simple and rapid in operation, and is superior in sample detection capacity.
Owner:ICDC CHINA CDC
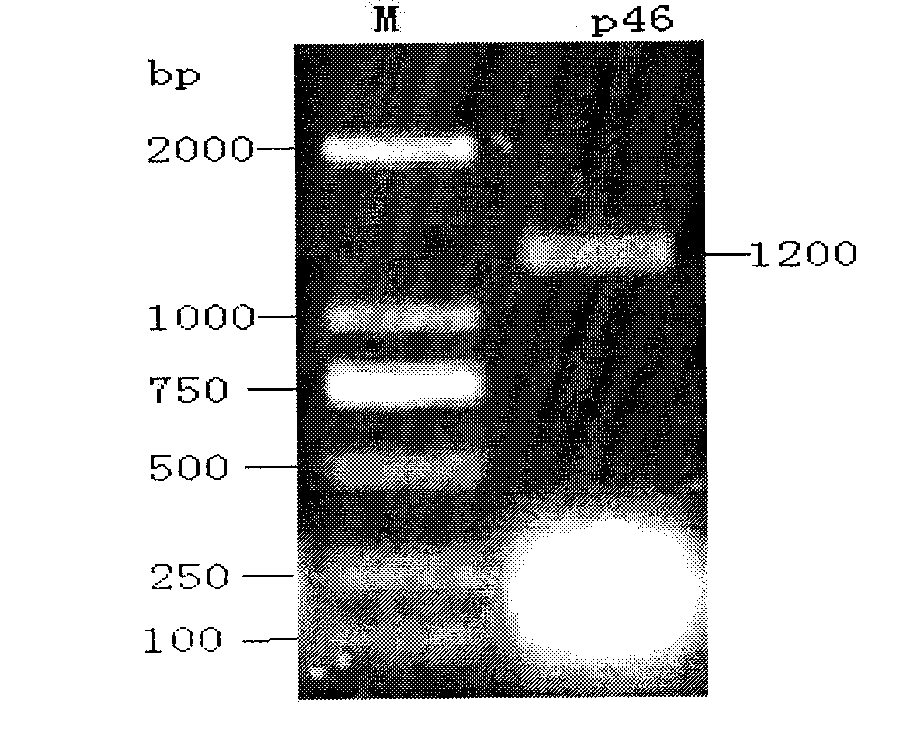
Recombinant salmonella choleraesuis expressing mycoplasma hyopneumoniae p46 protein, preparation method and application thereof
InactiveCN102732473AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesGenetic engineering
The invention belongs to the genetic engineering technology field of animal bacteria, especially relates to construction, vaccine preparation and application of recombinant salmonella choleraesuis expressing a main immunogenic membrane protein of mycoplasma hyopneumoniae and the expression contains no resistance markers. A strain of salmonella choleraesuis C500 (pYA-46) expressing mycoplasma hyopneumoniae p46 protein from the expression which contains no resistance markers is provided by the invention, and the preservation number is: CCTCC NO: M2011106. The recombinant strain lacks of an asd gene which is essential for the growth of samonella choleraesuis, and contains plasmid which can express asd in the strain and mycoplasma hyopneumoniae p46 protein. The invention also discloses the preparation method and the application of salmonella choleraesuis and pig mycoplasma pneumonia vaccine prepared by the recombination stain. The bivalent vaccine prepared in the invention can stimulate porcine to produce an immune reaction to protect porcine from the salmonella choleraesuis and the porcine pneumonia mycoplasma and can effectively prevent from being infected by the salmonella choleraesuis and the porcine pneumonia mycoplasma.
Owner:HUAZHONG AGRI UNIV +1
Primer for detecting sheep mycoplasma pneumoniae
PendingCN107502676AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationFluorescencePcr method
The invention provides a primer for detecting sheep mycoplasma pneumonia. The sequence of the primer comprises an upstream primer: 5'-GGGACTTCGGGACTTATTGGA-3', and a downstream primer: 5'-CACGAGATGCAAACTGATTTACTTG-3'. According to a p80 gene sequence of KM435069.1 logged in GenBank, a specific primer is designed, a real-time fluorescence quantification PCR (polymerase chain reaction) method is established for a p80 gene of sheep mycoplasma pneumonia for a first time, and the method is good in specificity, high in sensitivity and good in repeatability and can be applied to diagnosis and epidemiological investigation on sheep mycoplasma pneumonia.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI
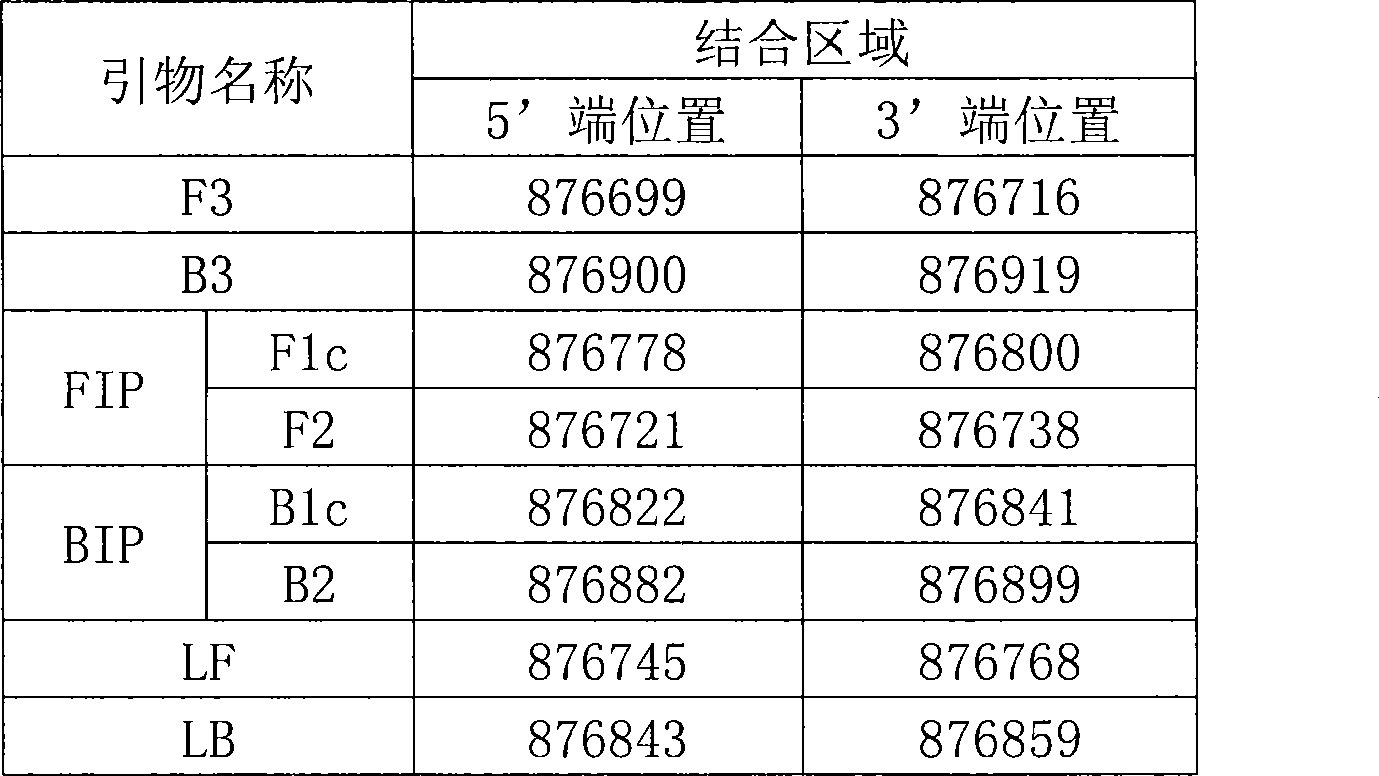
Detection kit for Mycoplasma hyopneumoniae and use thereof
InactiveCN101481742AEasy to understandMicrobiological testing/measurementMicroorganism based processesMycoplasma hyopneumoniaeMycoplasma pneumonia
The invention discloses an Mycoplasma hyopneumoniae detection kit and the application thereof, and provides a special primer for detecting Mycoplasma hyopneumoniae. The special primer consists of following three pairs of primers used for loop-mediated isothermal amplification: one pair of primers is a medial primer pair combined with a 16S ribosomal RNA gene in Mycoplasma hyopneumoniae (GenBank Accession Number NC-006360), one pair of primers is a lateral primer pair combined with the 16S ribosomal RNA gene in Mycoplasma hyopneumoniae (GenBank Accession Number NC-006360), and one pair of primers is a ring primer pair combined with the 16S ribosomal RNA gene in Mycoplasma hyopneumoniae (GenBank Accession Number NC-006360). The detection kit provided by the invention is a kit including the special primer. The detection kit of the invention has the advantages of high detection sensitivity and simple and convenient operation; only ten copies are needed to detect a target DNA; and the detection kit is particularly suitable for detection of clinical medicine conducted in the base field and detection of Mycoplasma hyopneumoniae possibly polluted in food.
Owner:CHINA AGRI UNIV
Mycoplasma pneumonia P1-RFLP gene typing and detecting primer and method
InactiveCN103031373AMicrobiological testing/measurementDNA/RNA fragmentationRflp typingEnzyme digestion
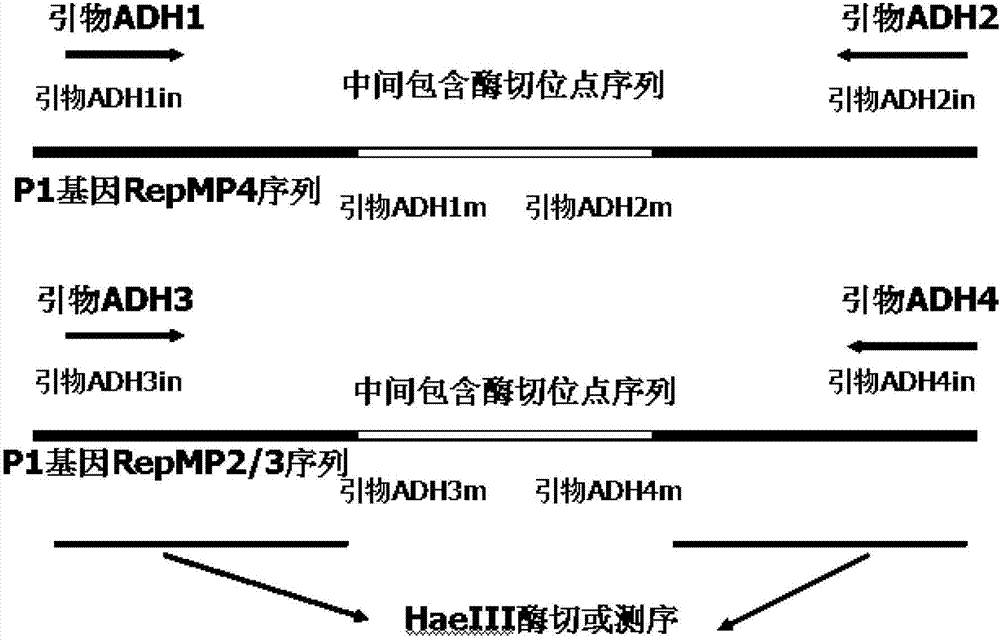
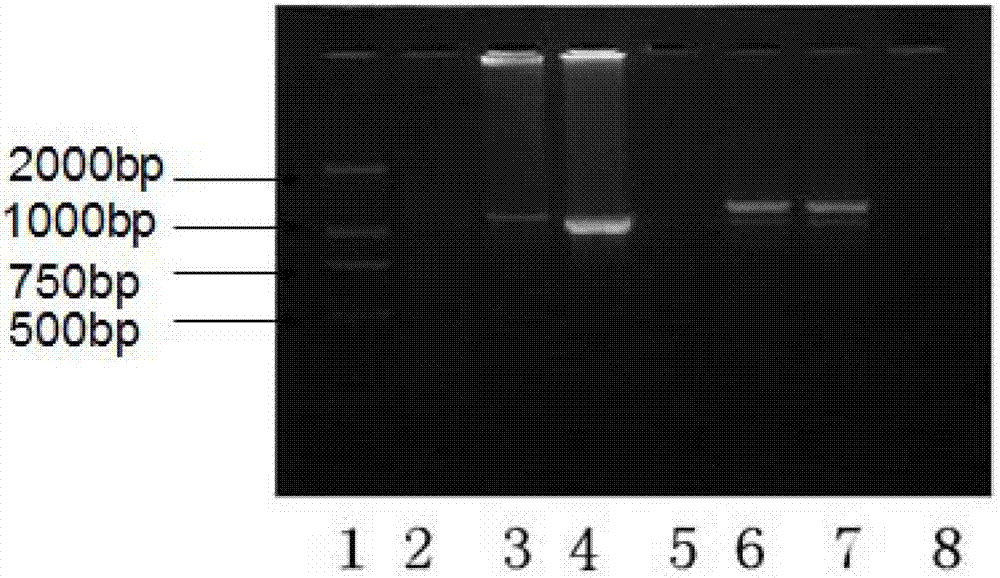
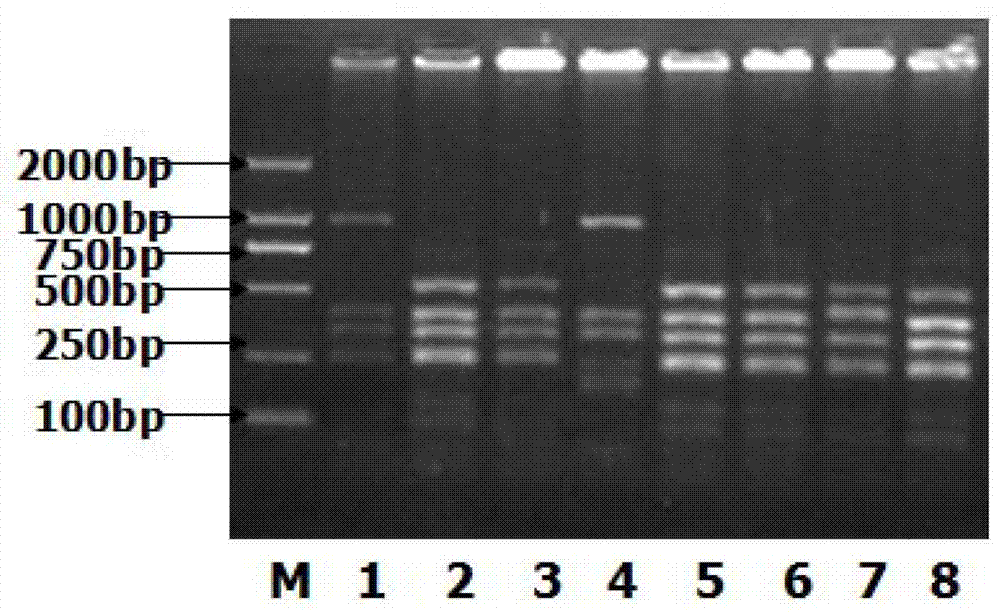
The invention discloses a mycoplasma pneumonia P1-RFLP gene typing and detecting primer and method. The method comprises the following steps of: analyzing the region where a HaeIII enzyme digestion site is arranged according to the RepMP4 and RepMP2 / 3 sequences of two known Mp natural standard strain P1 genes; designing adjacent inner nested primers in middle parts of the sequences and regions containing the enzyme digestion site, and respectively carrying out specific amplification towards two ends; then, respectively mixing the inner nested amplification products, and then carrying out enzyme digestion by using the HaeIII incision enzyme; and judging different types according to different enzyme-digested product atlases. The problems that the typed and amplified fragment of a mycoplasma pneumonia P1-RFLP gene is longer and not easy to amplify, time and effort are wasted, and large-scale clinical detection can not be carried out are solved.
Owner:首都儿科研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com