Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
A technology of Mycoplasma hyopneumoniae and vaccine composition, applied in the directions of bacteria, microorganisms, antibacterial drugs, etc., can solve problems such as poor clinical prevention and treatment effects, improve immune efficacy, do not affect weight gain, and reduce lung disease loss. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
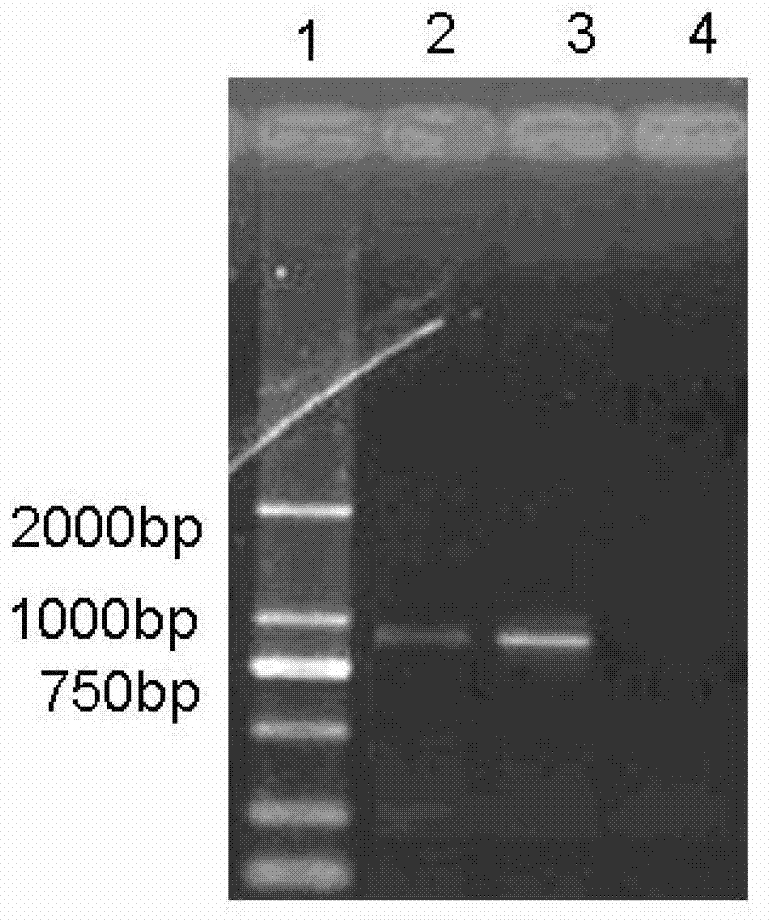
Image
Examples
Embodiment 1
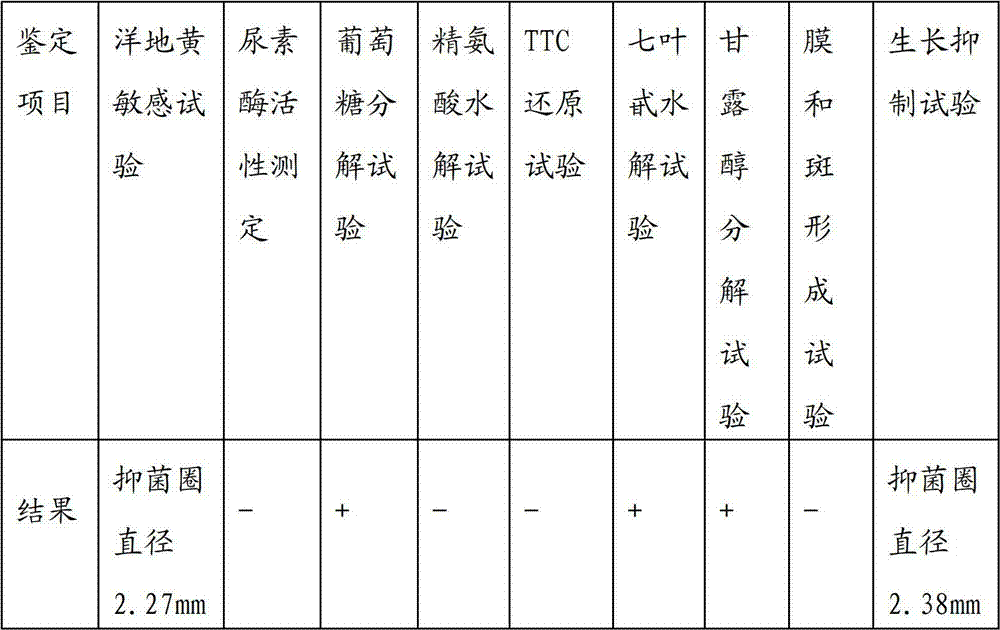
[0040] Embodiment 1, isolation and identification of Mycoplasma hyopneumoniae HN0613 strain
[0041] 1. Materials and methods
[0042] 1.1 Materials
[0043] 1.1.1 Source of disease materials In 2006, 16 lungs suspected of being caused by mycoplasma swine pneumonia were collected from various places in Henan Province.
[0044] 1.1.2 Medium Mycoplasma hyopneumoniae Friis medium was purchased from BD Company; pig serum was purchased from GIBCO Company; phenol red indicator was purchased from American AMRESCO Company.
[0045] 1.1.3 Biochemical reagents Chemical reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. Bacterial Genomic DNA Extraction Kit was purchased from TIANGEN Company, and Dieter's Staining Kit was purchased from Chongqing Pangtong Medical Instrument Co., Ltd. Biochemical identification reagents were purchased from Hangzhou Tianhe Microbiological Reagent Co., Ltd.
[0046] 1.1.4 Negative and positive serum Rabbit anti-Mycoplasma hyopneumoniae lip...
Embodiment 2
[0083] The preparation of the combination vaccine antigen of embodiment 2 mycoplasma pneumonia vaccine composition and porcine circovirus type 2, mycoplasma hyopneumoniae
[0084] 1.1 Preparation of bacteria (virus) species for production
[0085] Preparation of porcine circovirus type 2 SH: the virus seed PCV2SH strain was diluted 1:9 with MEM liquid medium (prepared with MEM dry powder medium purchased from Invitrogen Company of the United States according to the instructions), and then divided into 5% of the volume of cell culture medium. % inoculated in PK15 (ATCC, preservation number CCL-33) monolayer cells, adsorbed at 37°C for 30 minutes, added cell maintenance solution (4% calf serum and 2mmol / L D-glucosamine hydrochloride were added to MEM liquid medium) ), cultivated at 37°C for 4 days, freeze-thawed 2-3 times, harvested the virus, and the virus titer was 10 6.5 TCID 50 / ml.
[0086] Preparation of Mycoplasma hyopneumoniae strains: After unsealing the freeze-dried...
Embodiment 3
[0123] Embodiment 3, the preparation of composition vaccine:
[0124] In order to compare the immune effect of the vaccine, Montanide is selected when preparing the composition vaccine TM Gel adjuvant (produced by Sepic SEPPIC, France) was used to prepare porcine circovirus disease (whole virus antigen or ORF2 protein), dual inactivated vaccine against mycoplasma pneumoniae and single vaccine against mycoplasma pneumoniae. The specific configuration is as follows:
[0125] Preparation method: formula is as table 5. Get the PCV2 antigen and Mycoplasma hyopneumoniae antigen prepared in Example 1. The concentrated antigen solution was prepared into a mixed antigen solution or directly prepared into an antigen solution according to the final antigen content of the combined vaccine and the single vaccine, and then the antigen solution was mixed with Gel 01 adjuvant (produced by SEPPIC, France) at 90: 10 (V / V) mix, supplement the volume with PBS solution with pH 7.2 and stir at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com