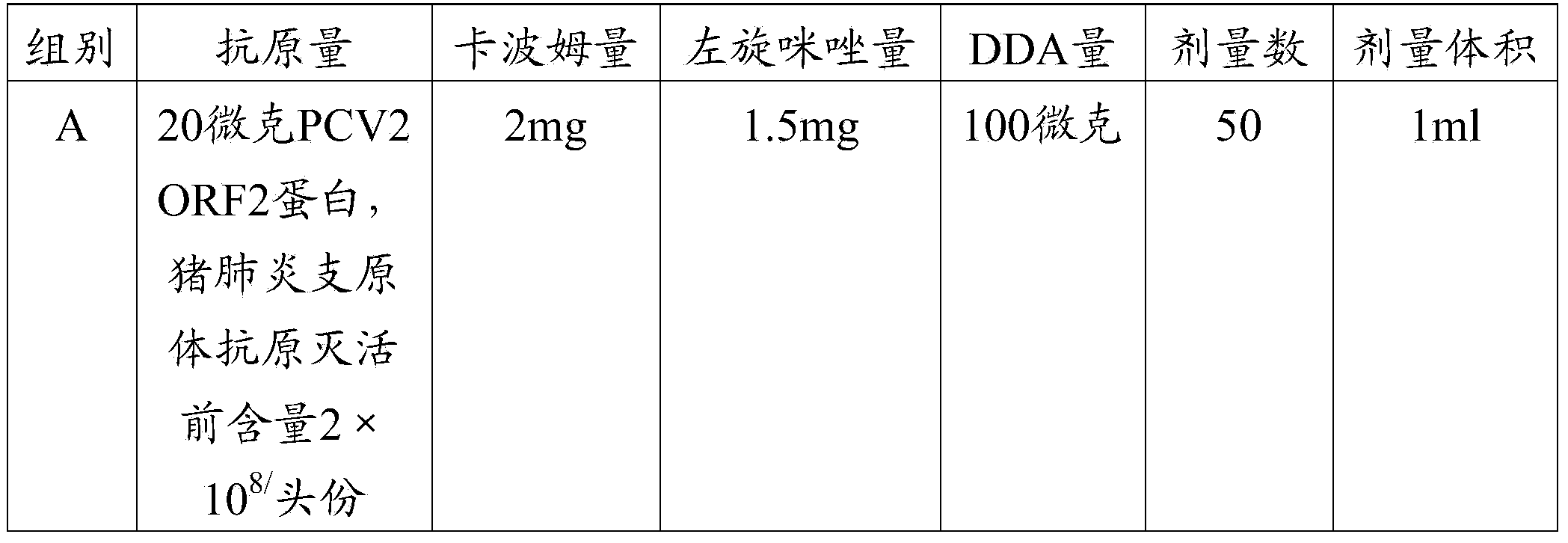
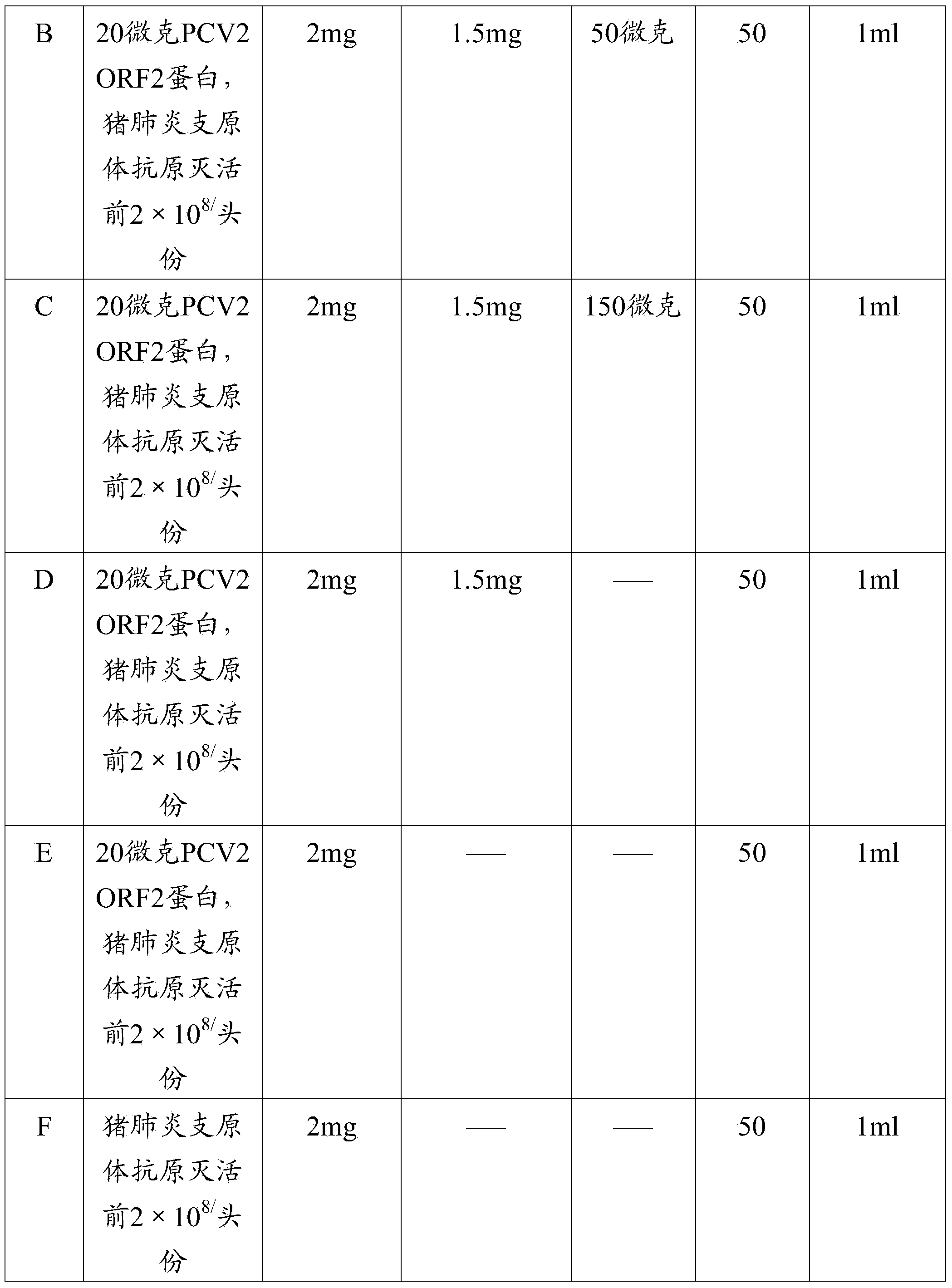
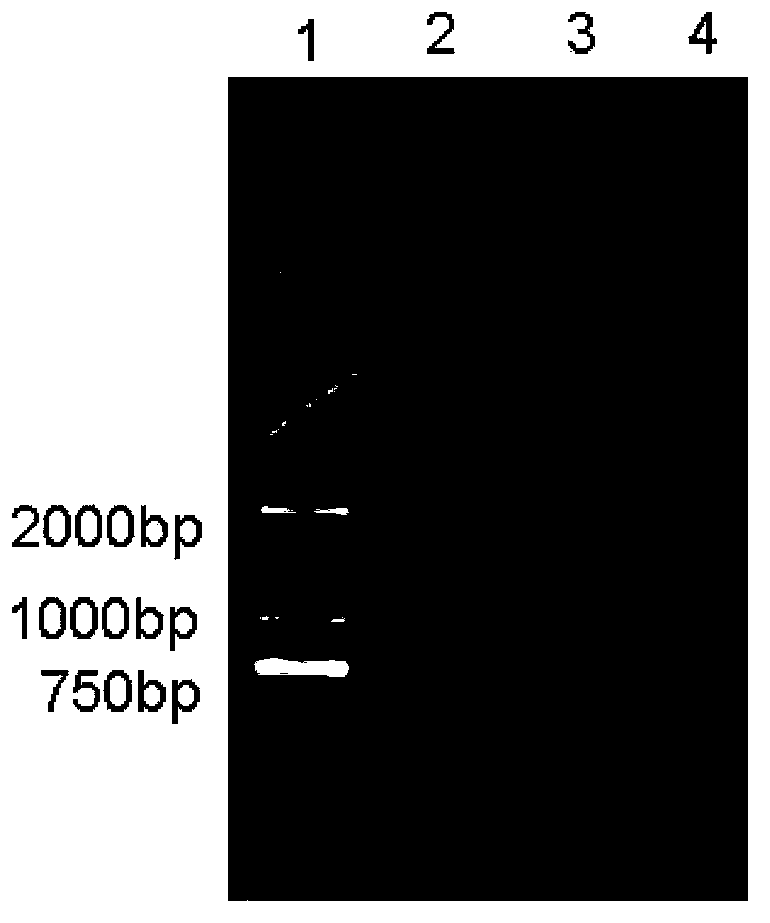
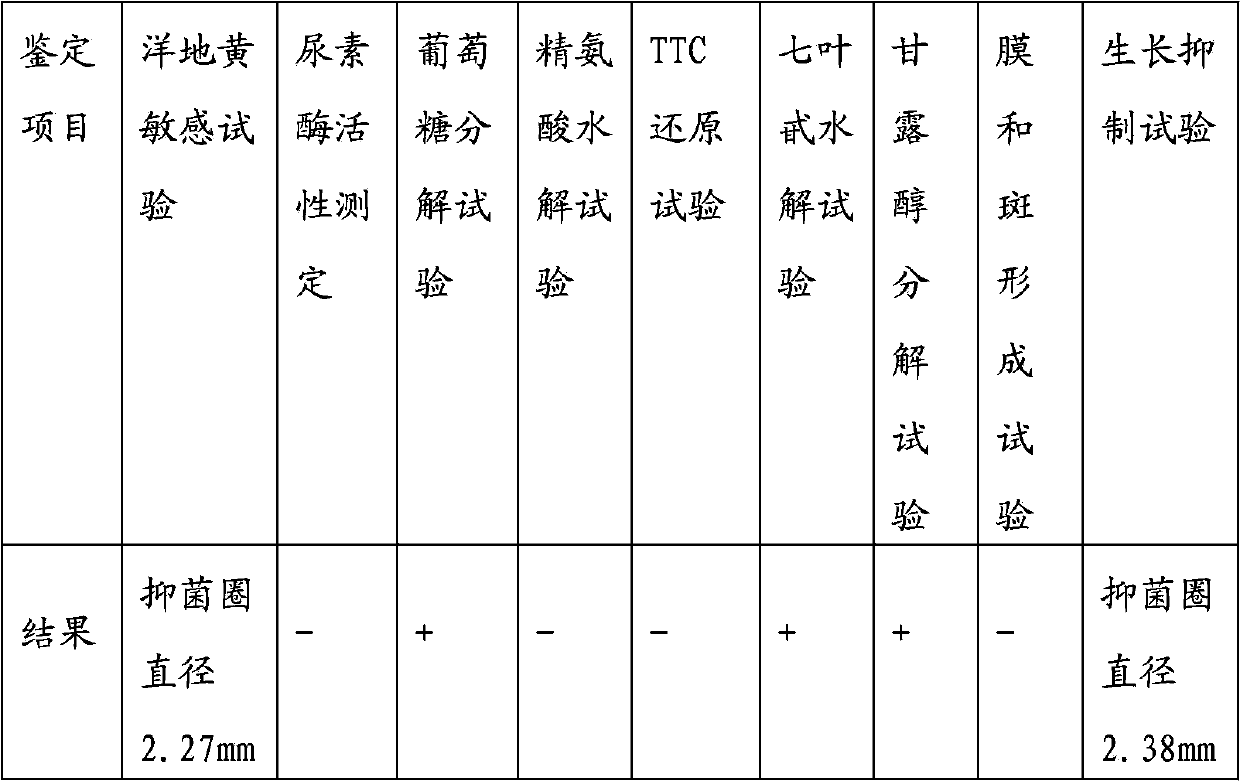
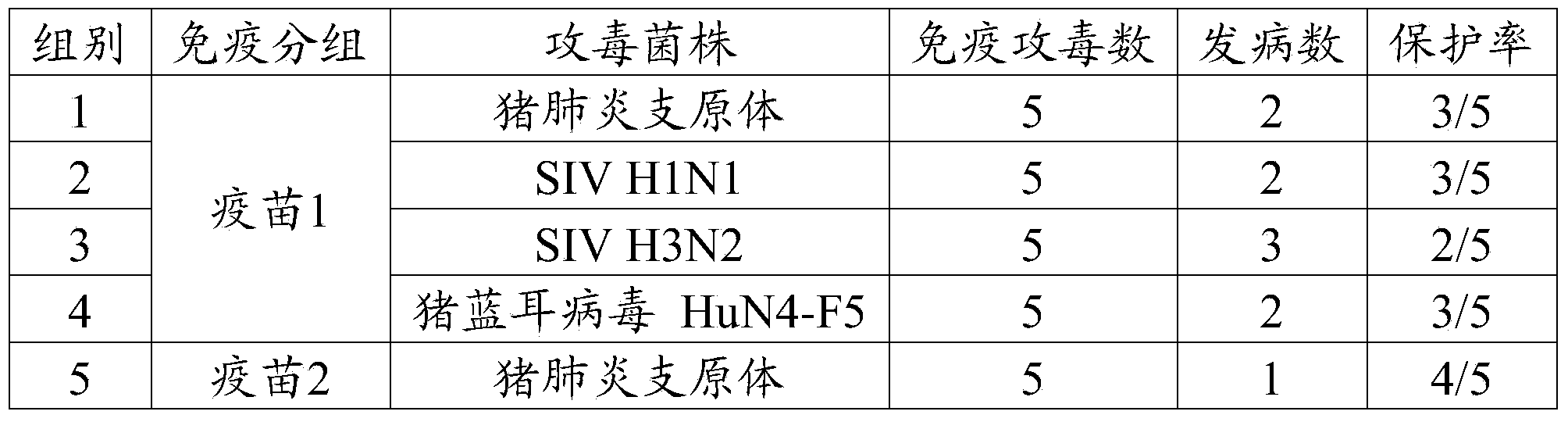
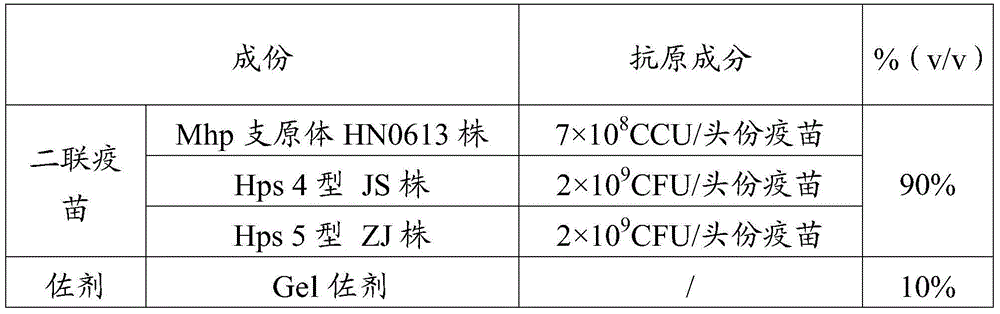
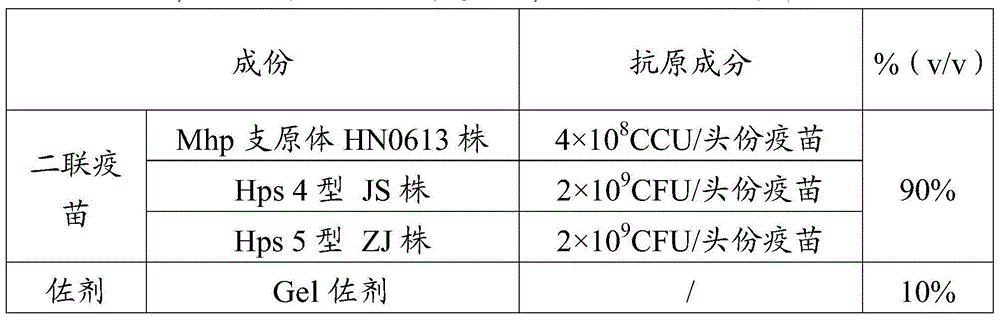
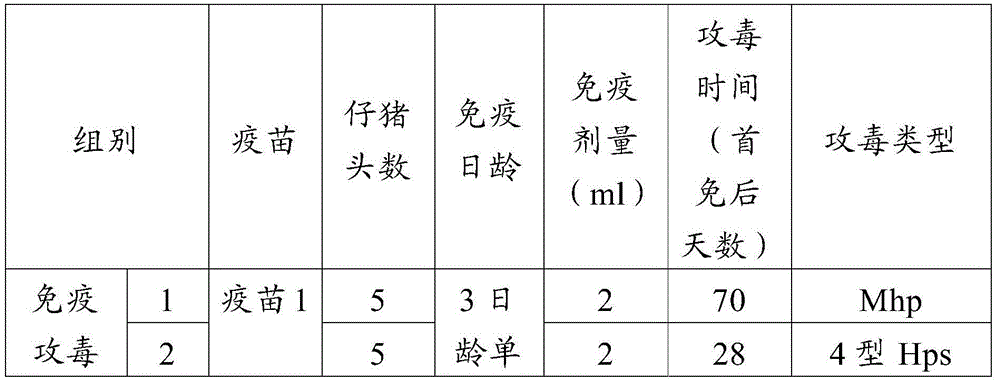
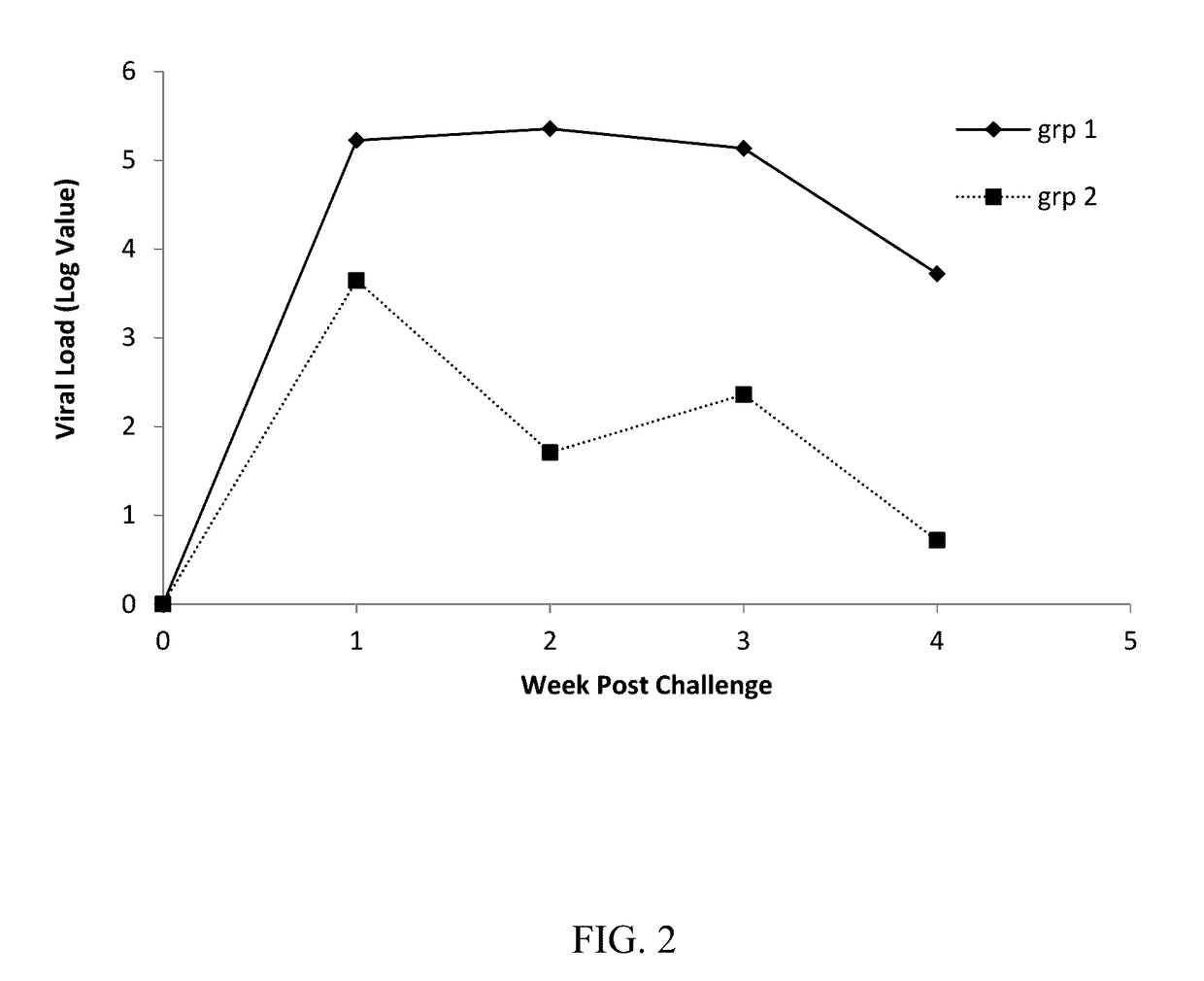
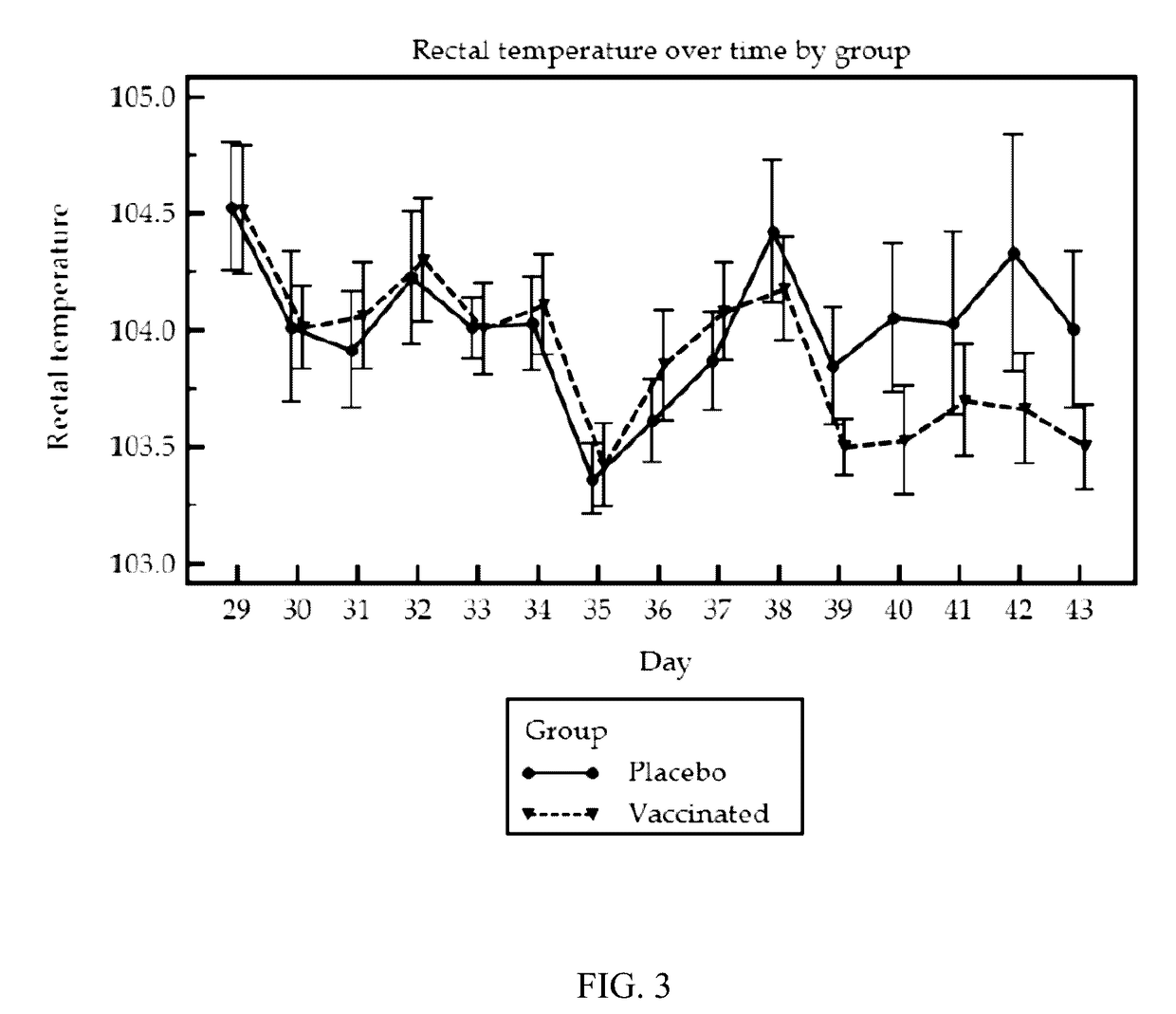
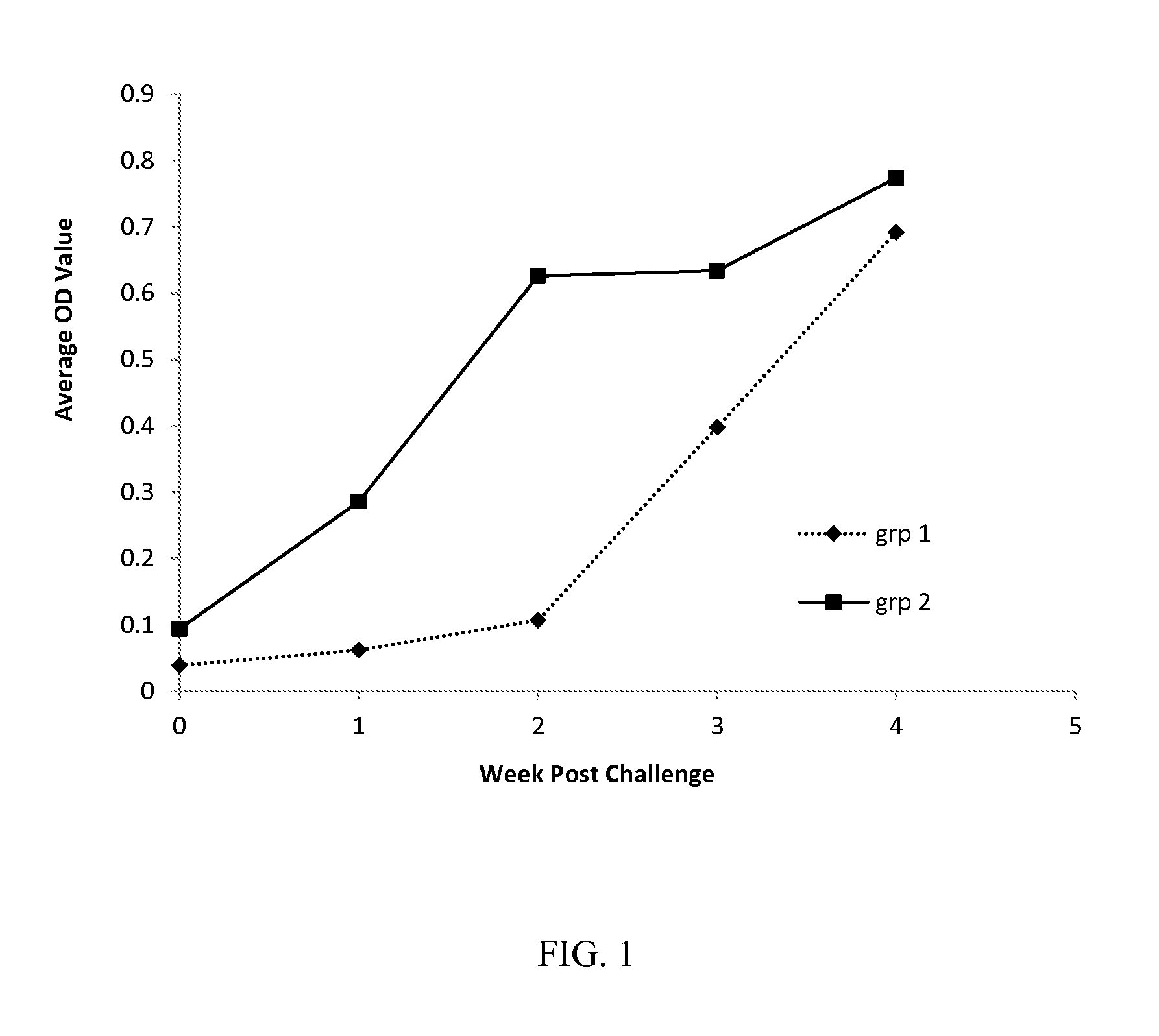
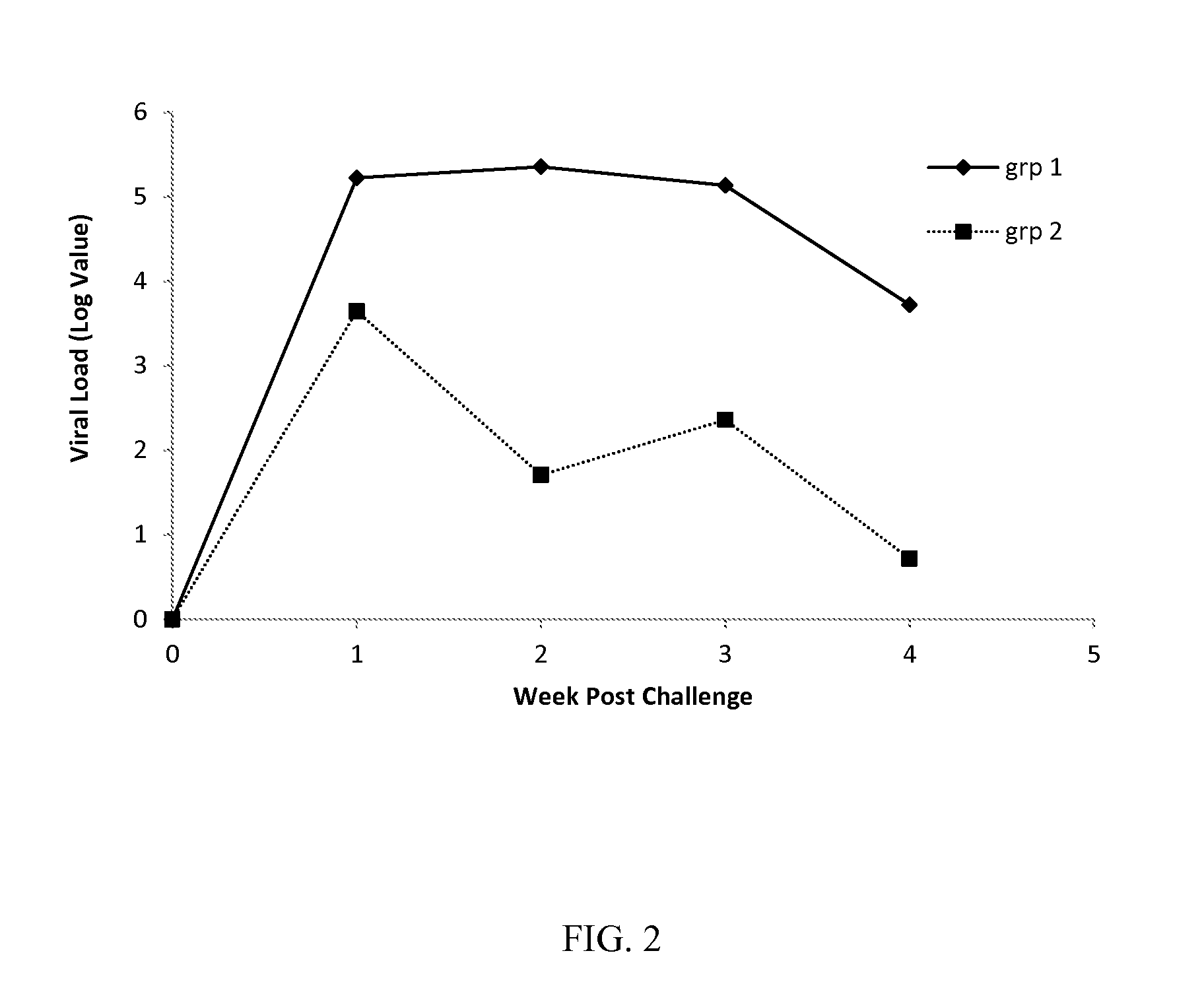
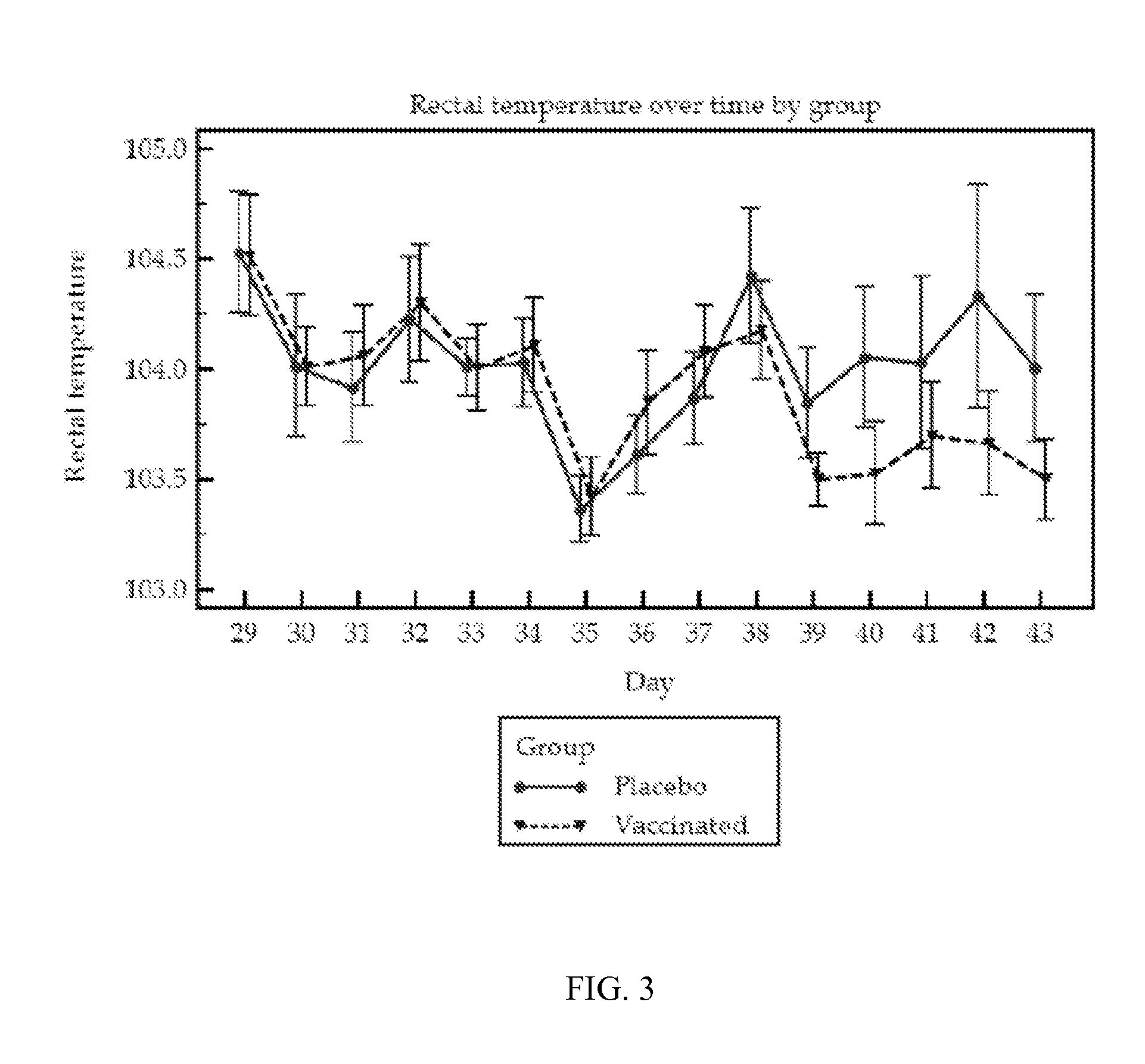
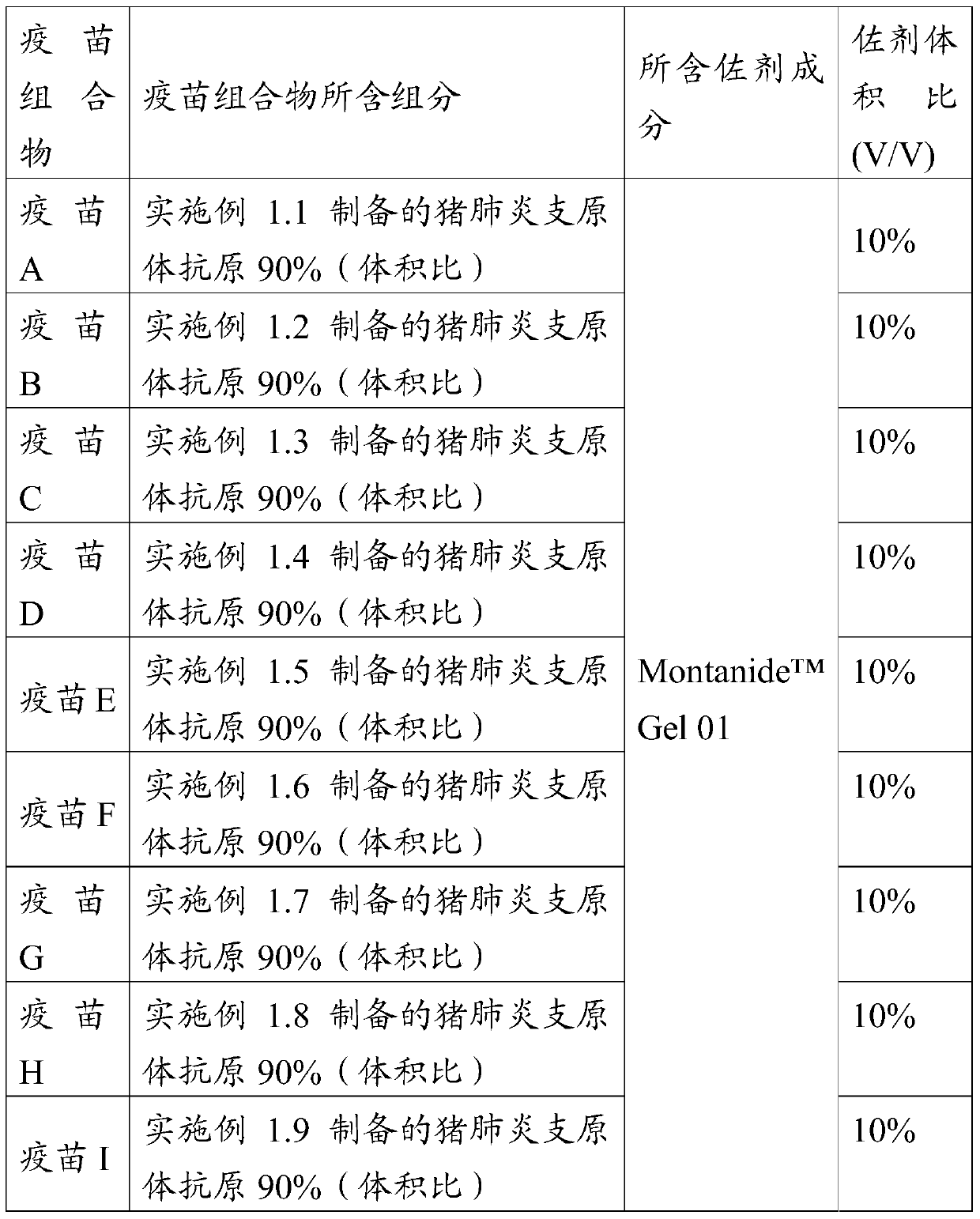
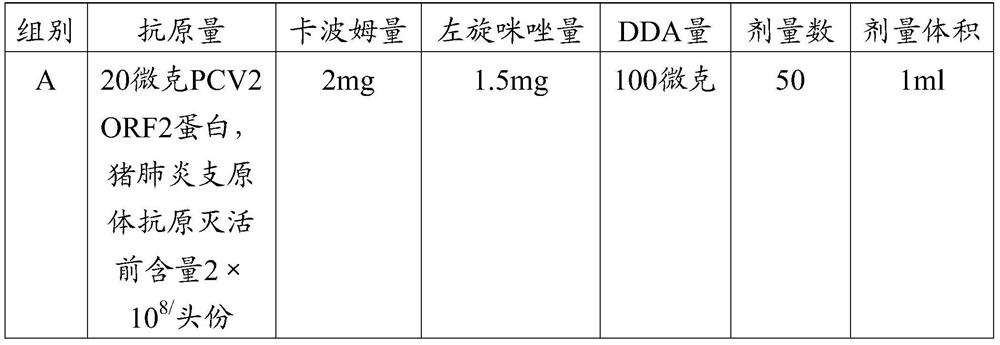
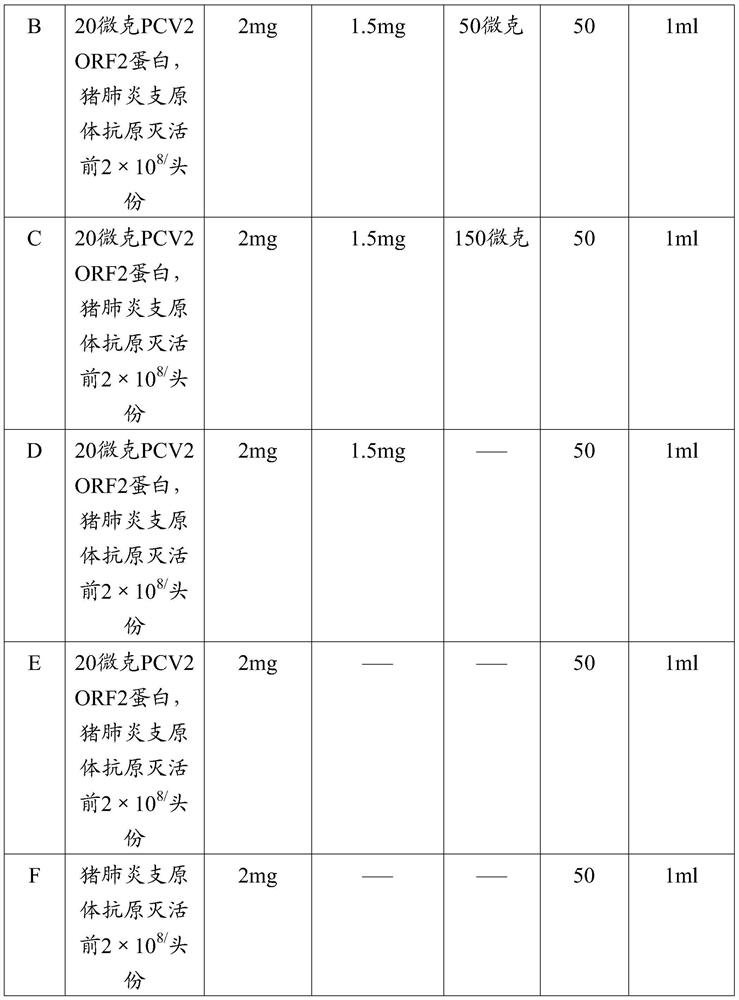
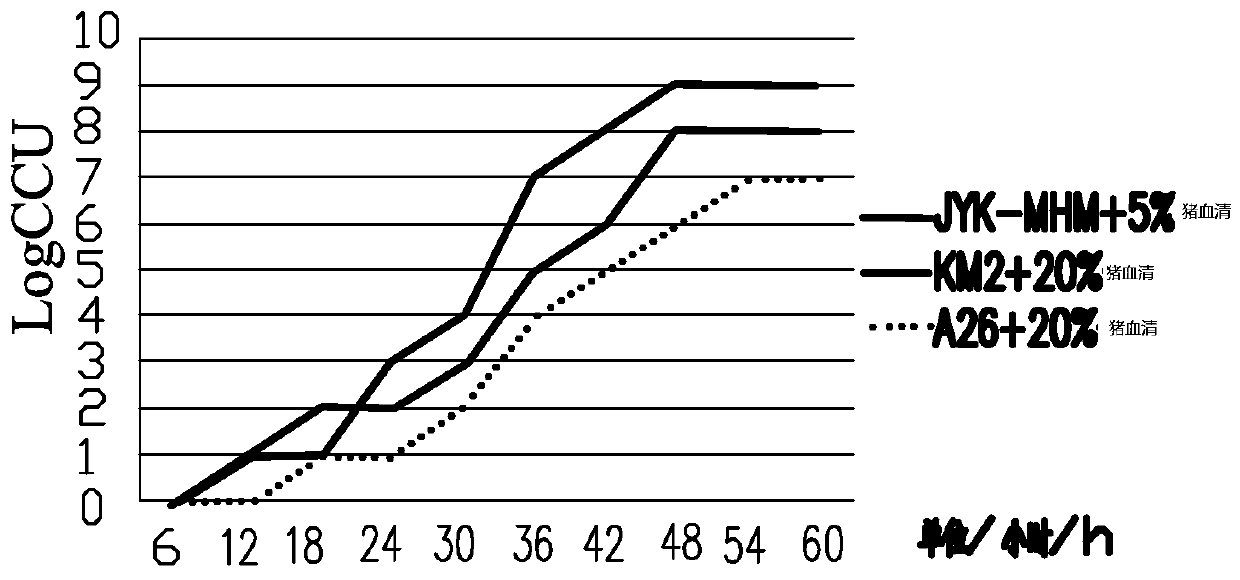
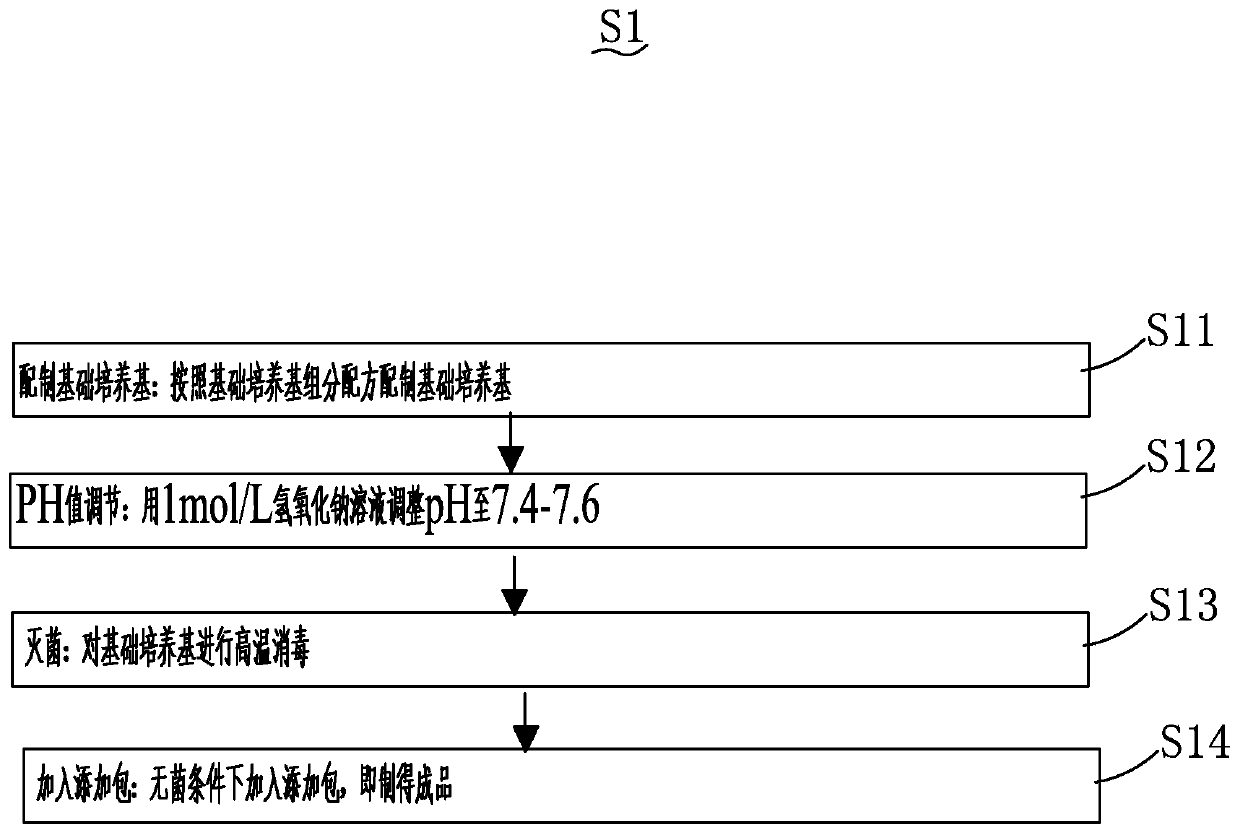
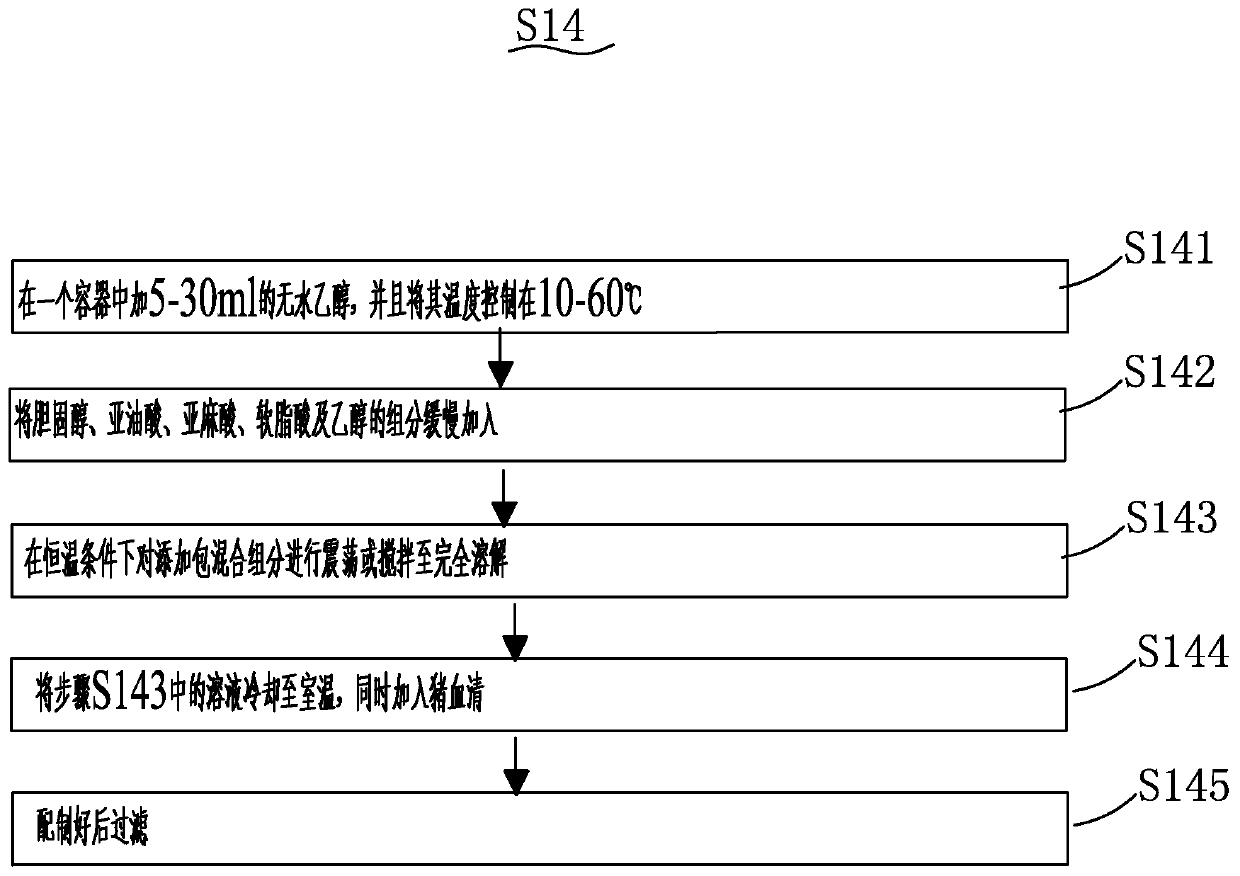
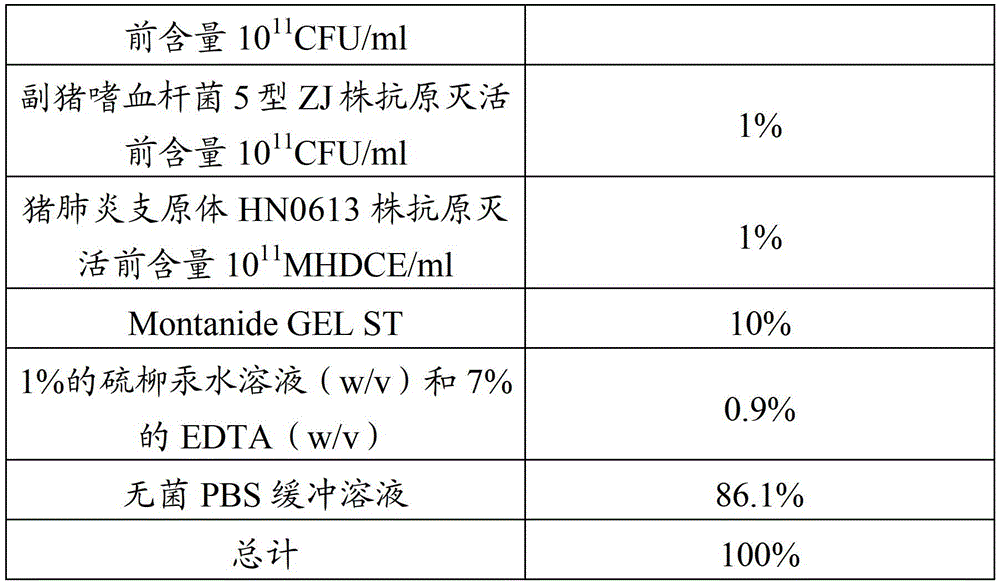
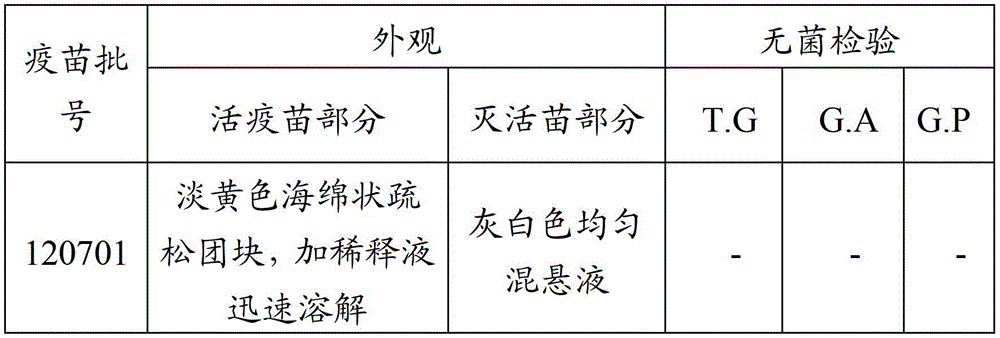
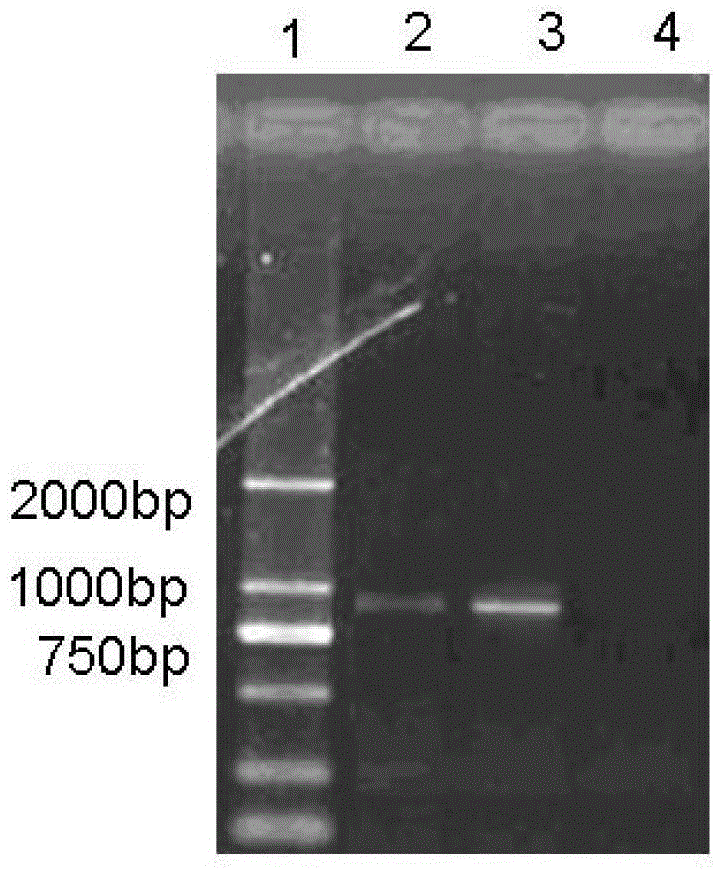
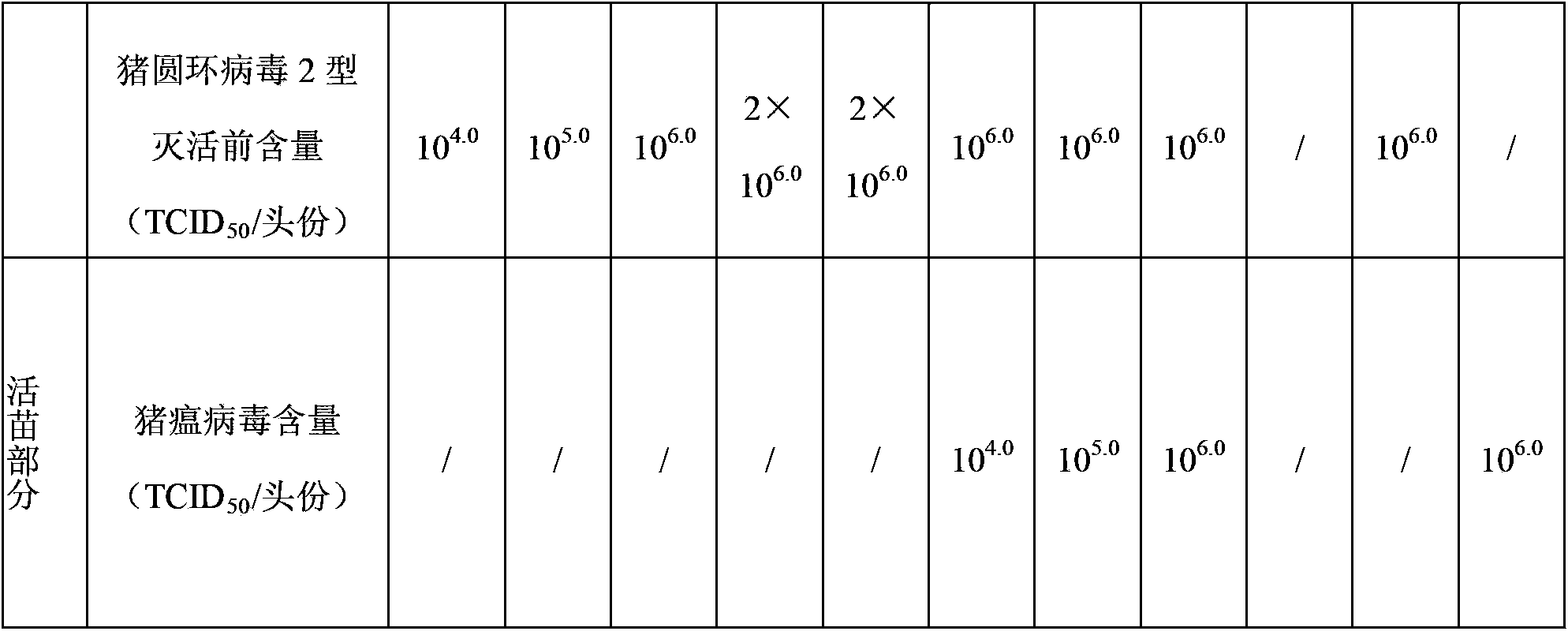Patents
Literature
37 results about "Mycoplasma hyopneumoniae Antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
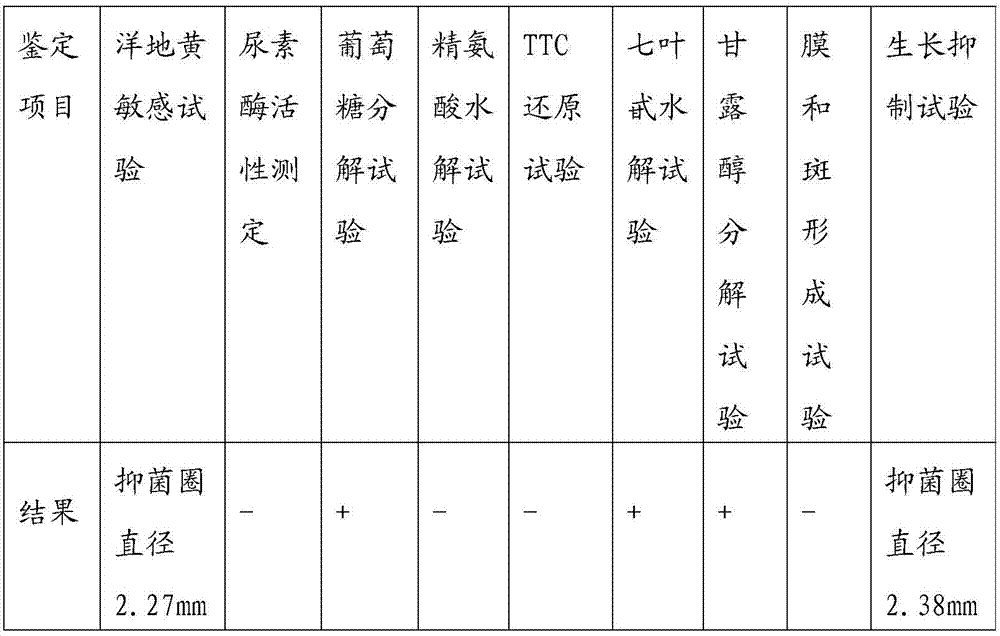
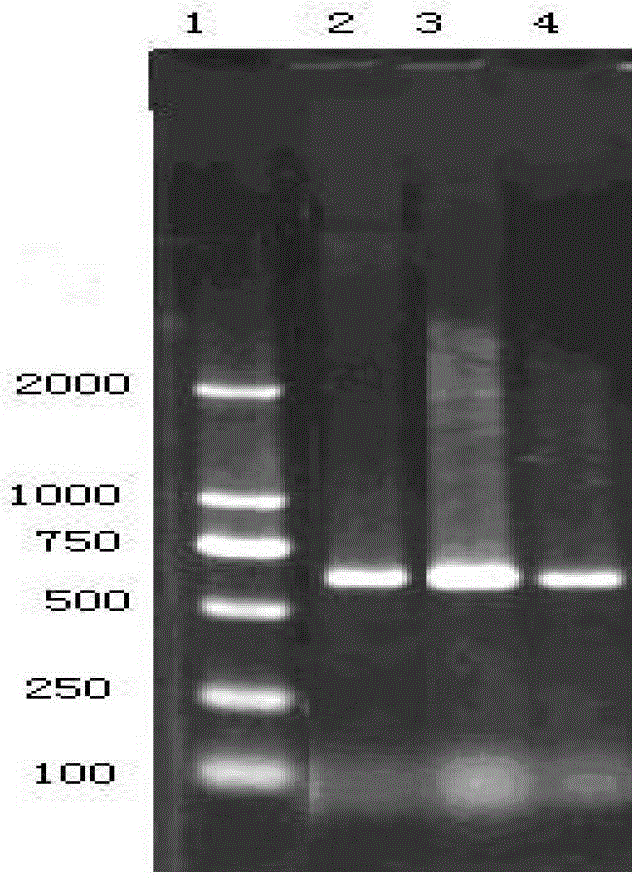
Methods of preparation of Mycoplasma hyopneumoniae antigens for the enzyme-linked immunosorbent assay to detect specific antibody, and properties of the antigens, are described. ... Nicolet J, Paroz P, Bruggmann S. Tween 20 soluble proteins of Mycoplasma hyopneumoniae as antigen for an enzyme linked immunosorbent assay.
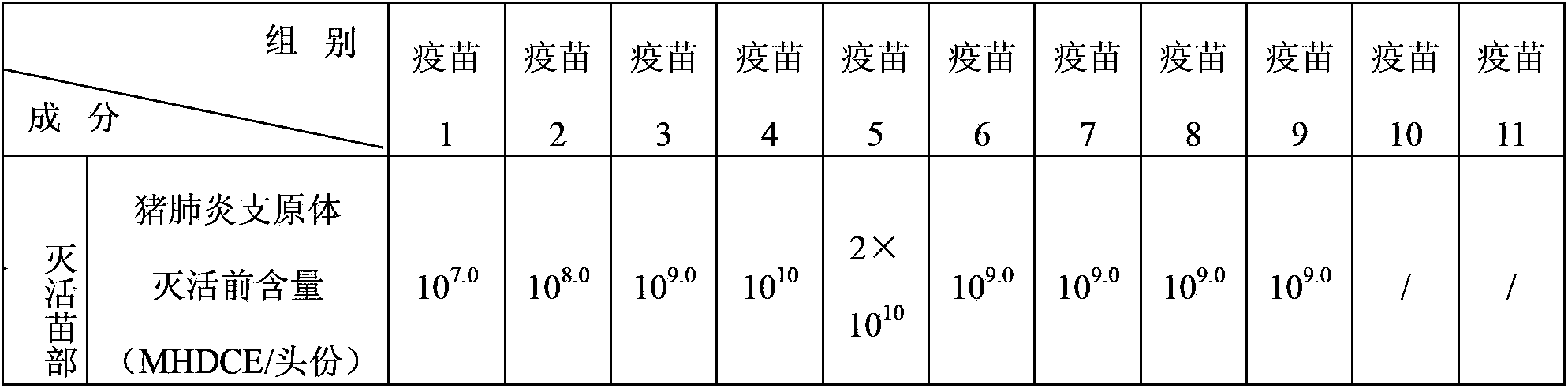
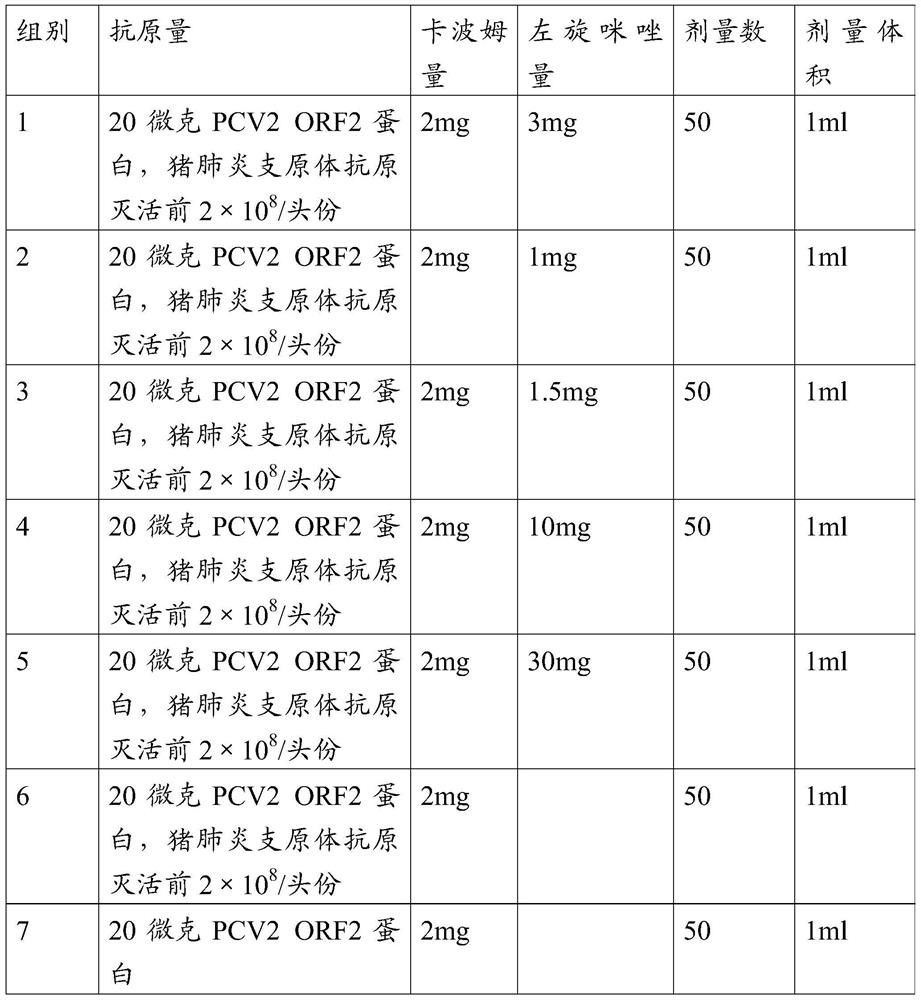
Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
ActiveCN103031258AEffective preventionEffective therapeuticAntibacterial agentsBacterial antigen ingredientsCircovirusPneumonia mrsa
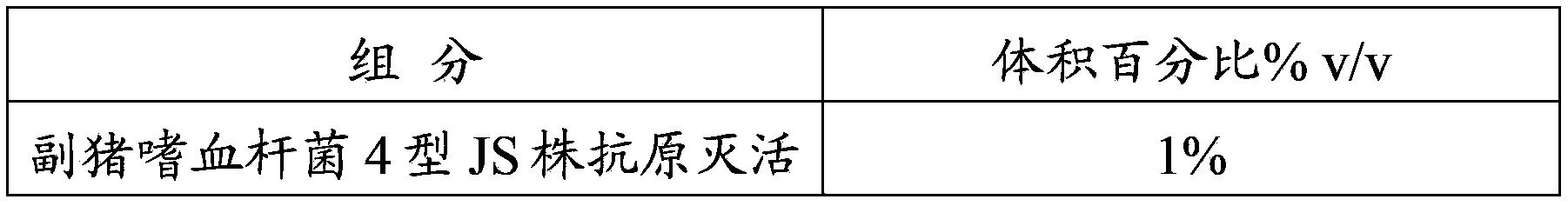
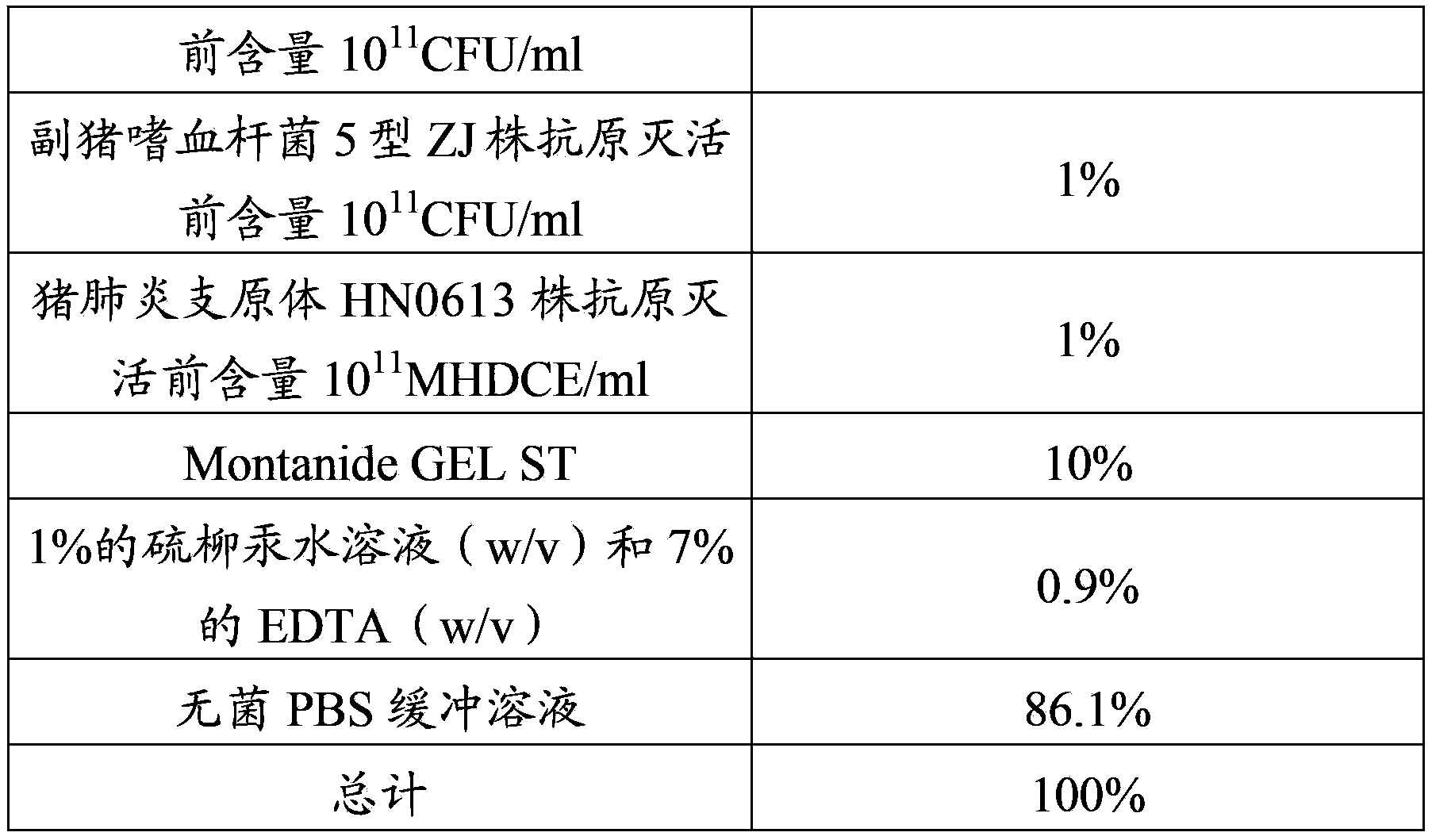
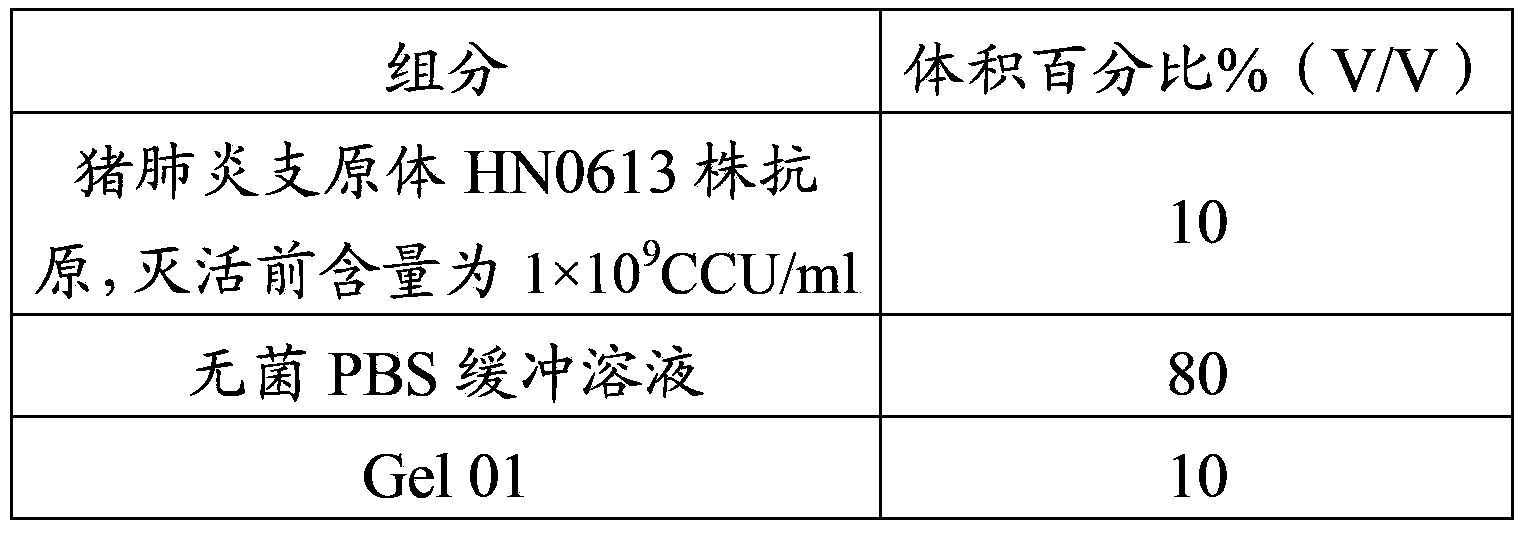
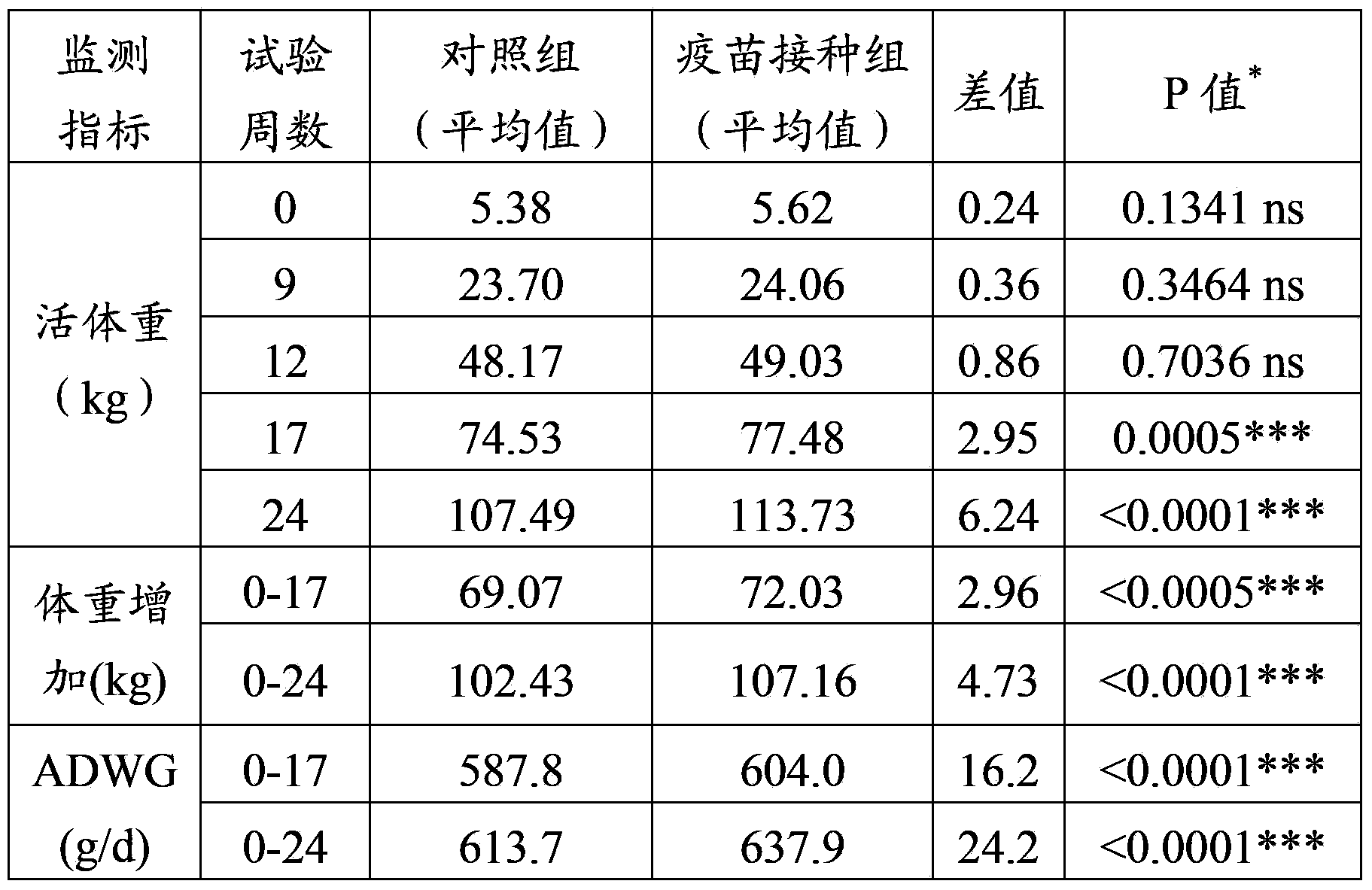
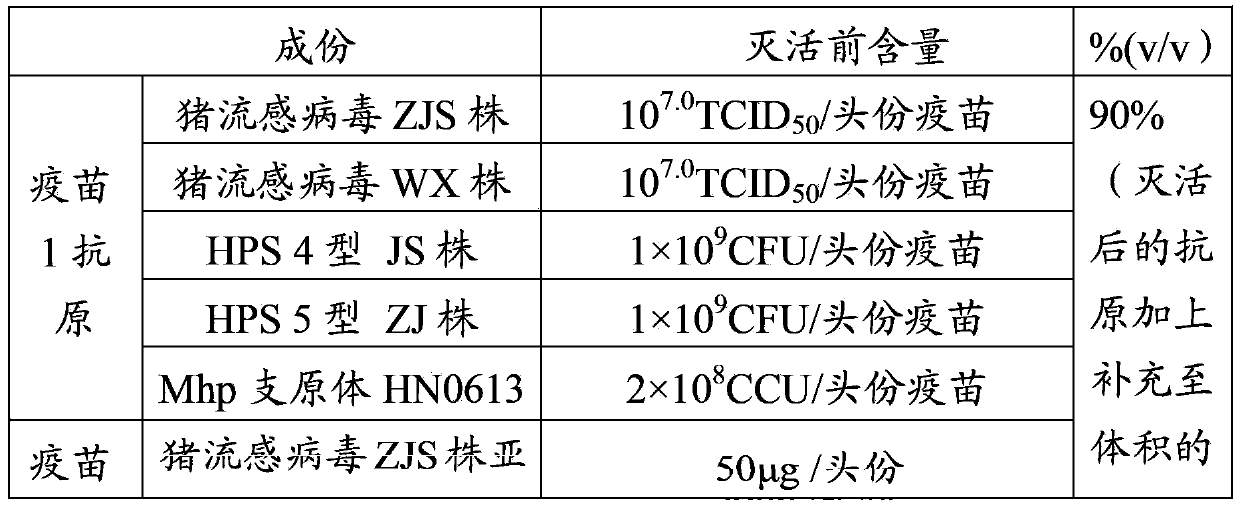
The invention provides a mycoplasma hyopneumoniae strain HN0613 which is isolated and identified to have better immunogenicity, and further provides a mycoplasma hyopneumoniae antigen prepared from the mycoplasma hyopneumoniae strain HN0613 and mycoplasma pneumonia pneumovax containing the mycoplasma hyopneumoniae antigen. The invention further provides a bivalent combined vaccine of porcine circovirus II and mycoplasma hyopneumoniae. The duplex combination vaccine comprises PCV (Porcine Circovirus) antigen II (inactivated porcine circovirus antigen II or PCV20 RF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant. The combination vaccine can achieve the purpose of preventing the porcine circovirus disease and the mycoplasma pneumonia swine by injection once, and has a protective effect of preventing mycoplasma hyopneumoniae infection.
Owner:PU LIKE BIO ENG
Vaccine composition, preparation method and application thereof
ActiveCN104248760APlay a role in immune enhancementMaintain stabilityAntiviralsAntibody medical ingredientsLevamisolePolymer
The invention provides a vaccine composition, which comprises an immune amount of a porcine circovirus antigen, an immune amount of a mycoplasma hyopneumoniae antigen, and levamisole or its derivative, and a polymer. The vaccine composition not only can have an immunological enhancement effect on two antigens at the same time, but also has stability, and can be used for dilution of porcine reproductive and respiratory syndrome live vaccines.
Owner:PU LIKE BIO ENG
New mycoplasma hyopneumoniae strain and vaccine composite of new mycoplasma hyopneumoniae
ActiveCN104450559AImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsPneumonia mrsaMycoplasma
The invention provides a mycoplasma hyopneumoniae strain HN0613 which has better immunogenicity proved through isolation and identification, also provides a mycoplasma hyopneumoniae antigen prepared by using the mycoplasma hyopneumoniae strain HN0613 and a mycoplasma hyopneumoniae vaccine containing the mycoplasma hyopneumoniae antigen. The invention further provides a porcine circovirus type 2 / mycoplasma hyopneumoniae bicombinant vaccine, containing a PCV2 antigen (inactivated PCV2 antigen or PCV2ORF2 protein), an inactivated mycoplasma hyopneumoniae and vaccine adjuvants. Through injection of the bicombinant vaccine, the aim of preventing PCV and mycoplasma hyopneumoniae can be achieved and the effect of prevention and protection from mycoplasma hyopneumoniae infection can be achieved further.
Owner:PU LIKE BIO ENG +1
Vaccine composition, preparation method and application thereof
ActiveCN103908665AReduce the number of vaccinationsIncrease costAntibacterial agentsBacterial antigen ingredientsDiluentWater soluble
The invention relates to a vaccine composition, which contains classical swine fever virus antigen, porcine circovirus type 2 antigen, mycoplasma hyopneumoniae antigen and a vaccine adjuvant. The invention also provides a method for preparing the vaccine composition, a water-soluble vaccine adjuvant is used for preparing a mixture of the porcine circovirus type 2 antigen and mycoplasma hyopneumoniae antigen, and the mixture is taken as a diluent for diluting classical swine fever virus antigen. The vaccine composition has the advantages of simple preparation method, high titer content of the vaccine, and convenient and fast immunization, one time immunization can simultaneously preventing or treating swine fever, porcine circovirus type 2 antigen and mycoplasma hyopneumoniae, immunization cost is reduced, the immunization program is saved, and the application is more economic and reliable.
Owner:PU LIKE BIO ENG
Porcine circovirus culture method and application
ActiveCN104250636AProliferation titerLow costBacteriaViral antigen ingredientsCircovirusMycoplasma hyopneumoniae Antigen
The invention provides a porcine circovirus culture method and application and a mycoplasma hyopneumoniae culture method. The porcine circovirus culture method can simultaneously culture porcine circovirus and mycoplasma hyopneumoniae, simultaneously proliferate the titer of the porcine circovirus and the mycoplasma hyopneumoniae, and obtain high titer porcine circovirus. A vaccine combination prepared by adding mycoplasma hyopneumoniae antigens into the porcine circovirus which is prepared by the porcine circovirus culture method can be used for the prevention and / or treatment of diseases caused by the porcine circovirus and the mycoplasma hyopneumoniae.
Owner:PU LIKE BIO ENG
Preparation method and application of fusion protein and vaccine composition containing same
ActiveCN104277115AShort timeEase of mass productionAntibacterial agentsBacterial antigen ingredientsPseudomonas aeruginosa exotoxin AMycoplasmal pneumonia
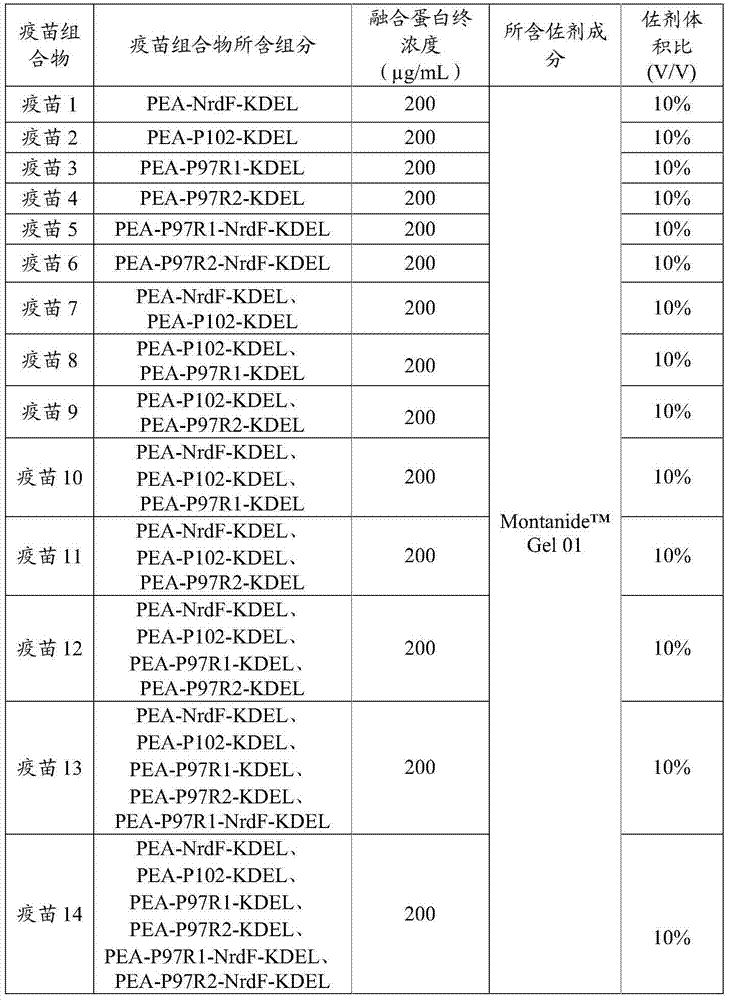
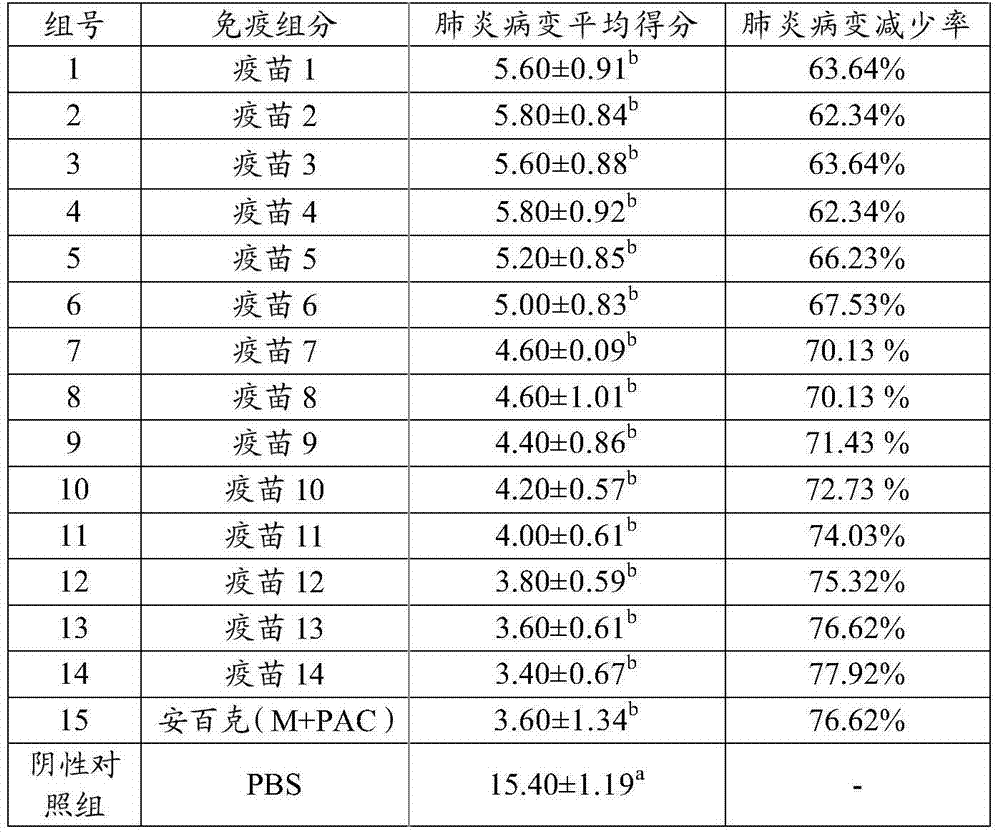
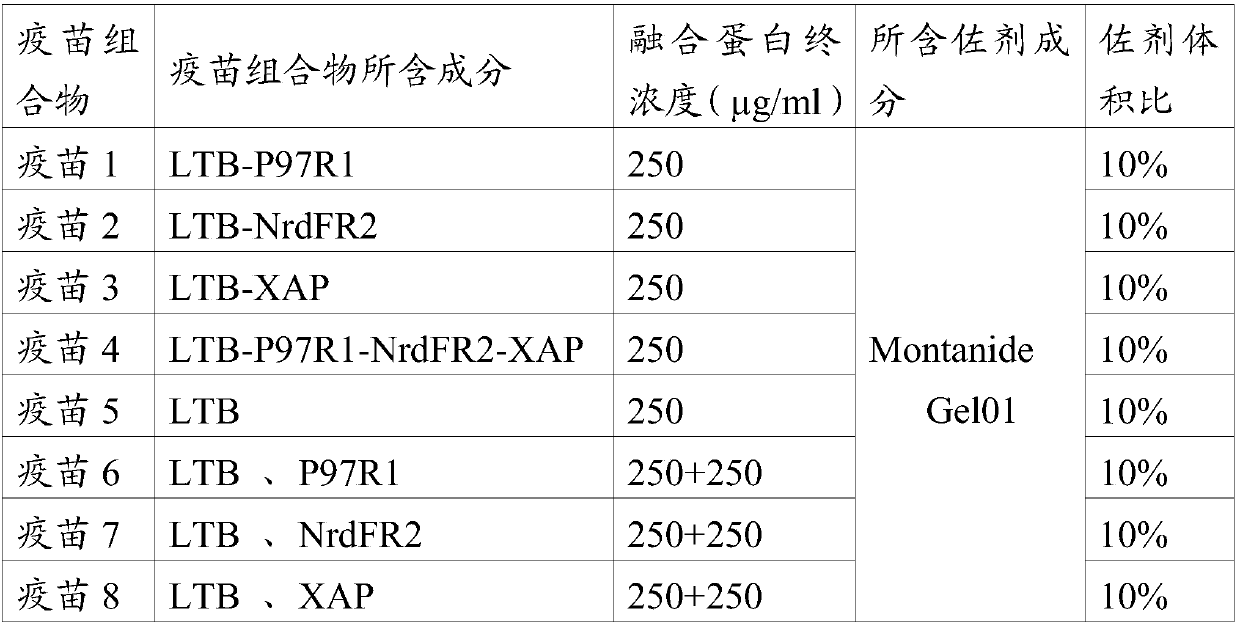
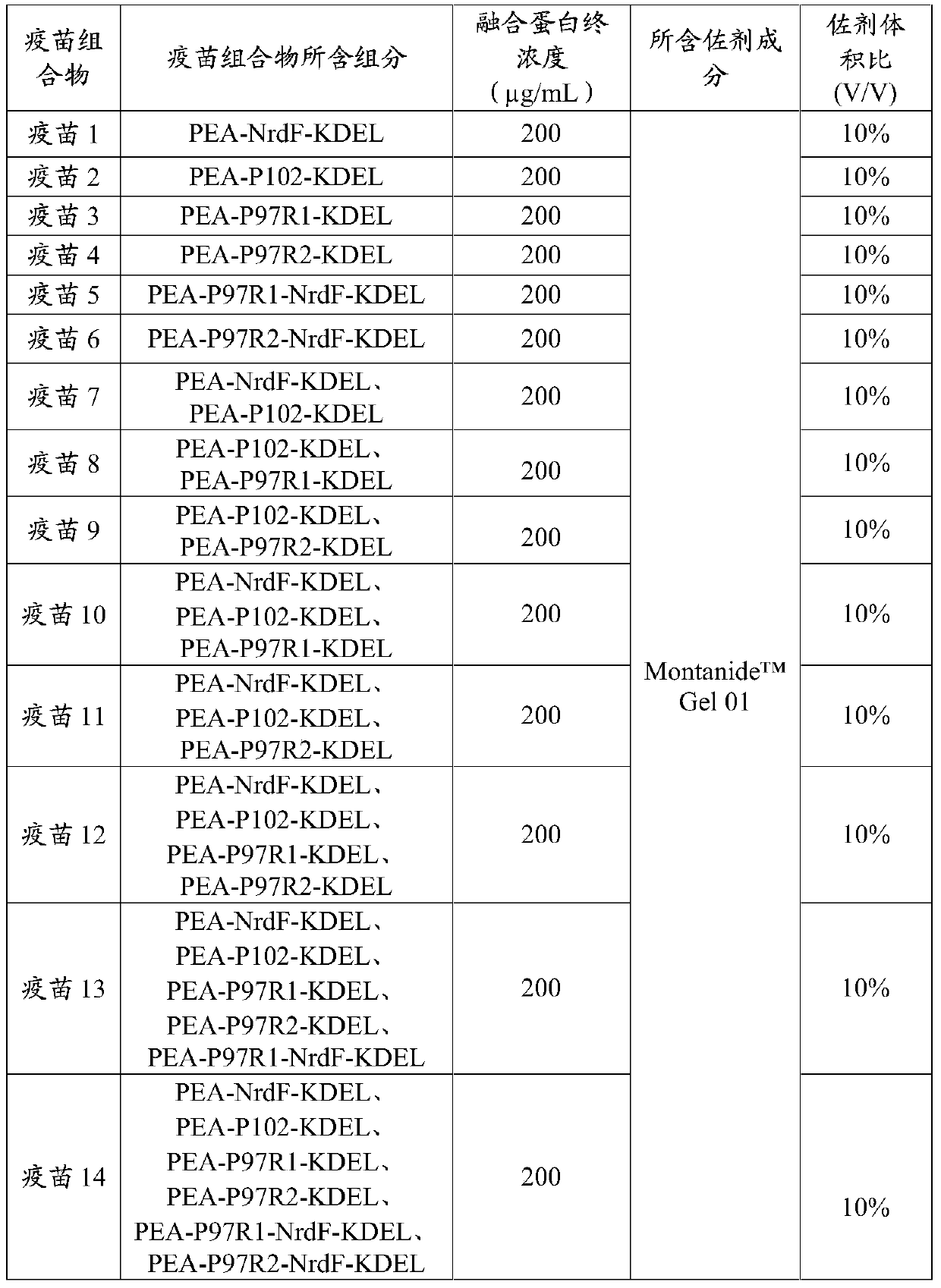
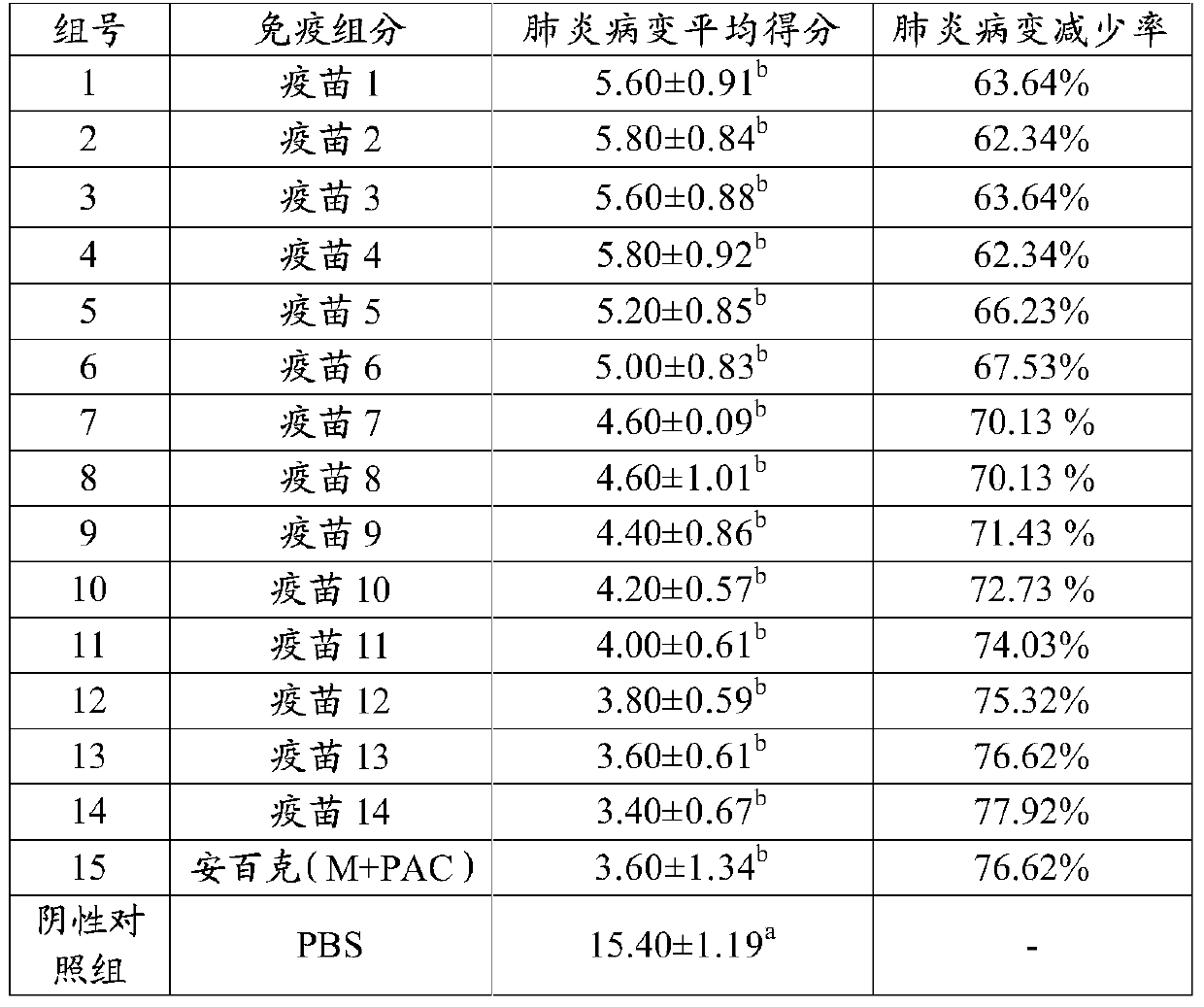
The invention provides a fusion protein, a fusion protein-containing vaccine composition for mycoplasmal pneumonia of swine (MPS) and an application of the vaccine composition. The fusion protein comprises pseudomonas exotoxin A structural domains I and II, an antigenic protein for MPS and a carboxyl terminal part, wherein the antigenic protein for MPS comprises any one of an NrdF protein, a P102 protein, a P97R1 protein, a P97R2 protein, a P97R1-NrdF protein and a P97R2-NrdF protein. The invention also discloses the vaccine composition containing immune dosage of fusion protein and the application of the vaccine composition to preparation of drugs for preventing and / or treating MPS. The vaccine composition has the advantages that recombinant expression can be carried out on the components of the vaccine composition in quantity by means of genetic engineering, thus not only consuming short time but also facilitating large-scale production; the vaccine composition can generate humoral immunity and cellular immunity and can gain better immune effects.
Owner:PU LIKE BIO ENG
Vaccine composition, and preparation method and application thereof
ActiveCN104096222ANo mutual interferenceSatisfied with the immune effectBiological testingRespiratory disorderHaemophilusRespiratory syndrome virus
The invention provides a vaccine composition. The vaccine composition comprises an immune dosage of a mycoplasma hyopneumoniae antigen, an immune dosage of a haemophilus parasuis antigen and an immune dosage of a porcine reproductive and respiratory syndrome virus antigen. The vaccine composition can protect more than 80% of pigs infected with the porcine reproductive and respiratory syndrome virus, mycoplasma hyopneumoniae and mycoplasma hyopneumoniae in 14 days after the pigs have received secondary immunization by the vaccine composition, mutual interference among the antigens does not occur, and a satisfactory immunization effect is obtained.
Owner:PU LIKE BIO ENG
Applications of porcine reproductive and respiratory syndrome virus (PRRSV), mycoplasma hyopneumoniae and PCV-2 antigen in preparation of vaccine
InactiveCN104043118AAntibacterial agentsBacterial antigen ingredientsImmune effectsPorcine reproductive and respiratory syndrome virus
The invention provides applications of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in immune dose, a mycoplasma hyopneumoniae antigen in immune dose and a PCV-2 antigen in immune dose in preparation of a vaccine which is used for preventing and treating pigs being polluted by mycoplasma hyopneumoniae or pigs being threatened by the mycoplasma hyopneumoniae. On the premise of not influencing an immune effect on the mycoplasma hyopneumoniae, the applications, aiming at pigs being polluted by mycoplasma hyopneumoniae or pigs being threatened by the mycoplasma hyopneumoniae, can enable immune time of the PCV-2 and the PRRSV to be brought forward and can control the three diseases: the mycoplasma hyopneumoniae, the PPRSV and the PCV-2 at the same time.
Owner:PU LIKE BIO ENG
Vaccine adjuvant composition for treatment or prevention of swine infectious diseases
ActiveCN103083658AGood immune protectionImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsSide effectAdditive ingredient
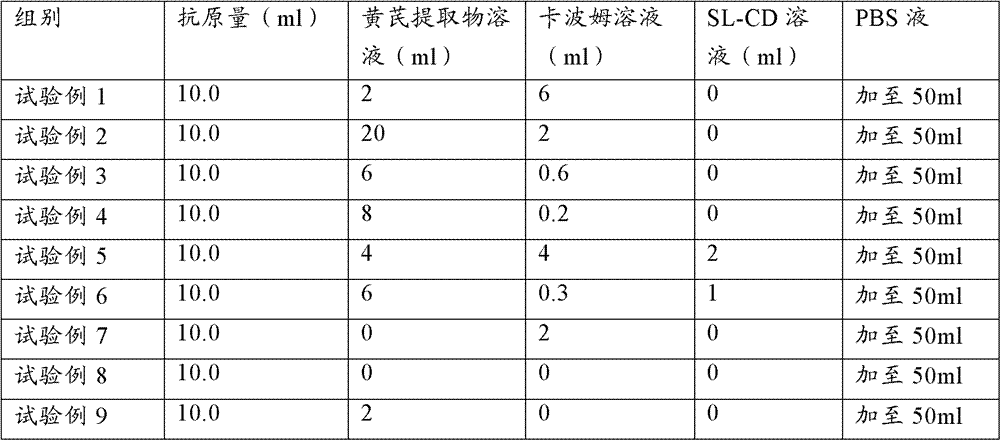
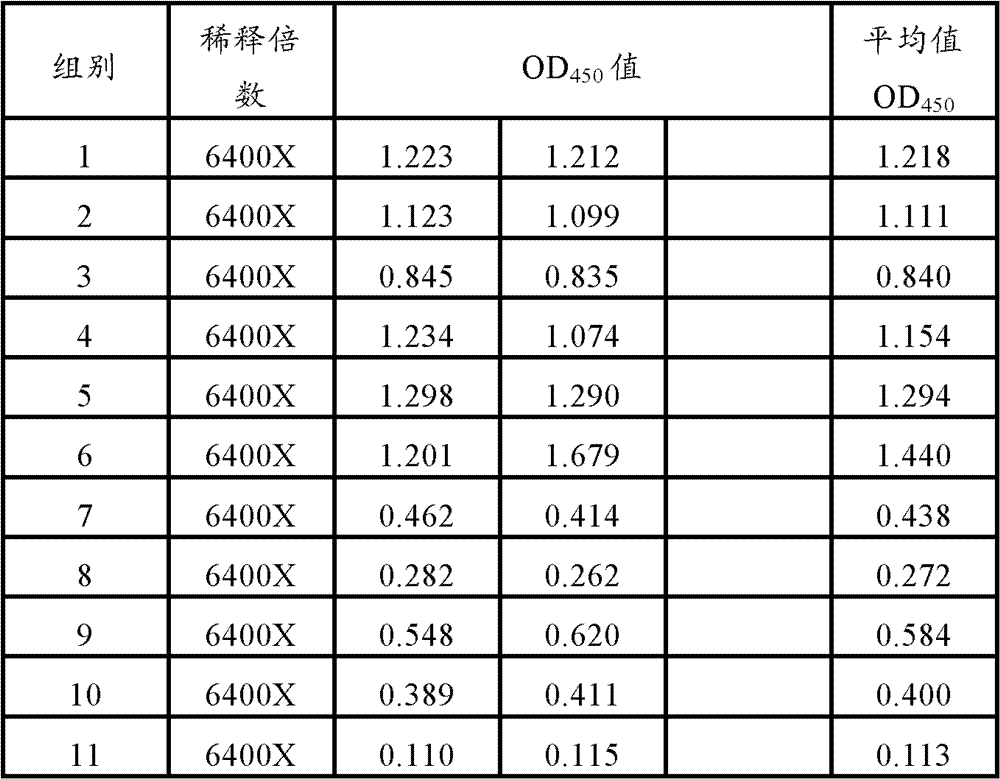
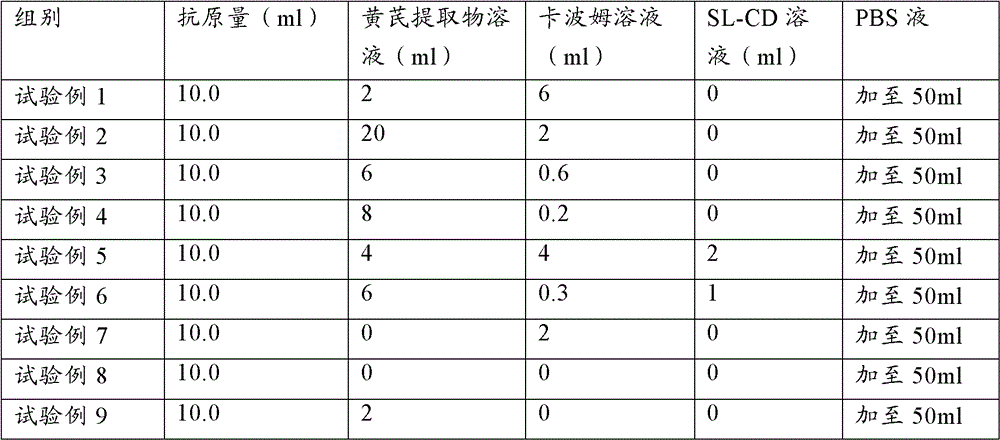
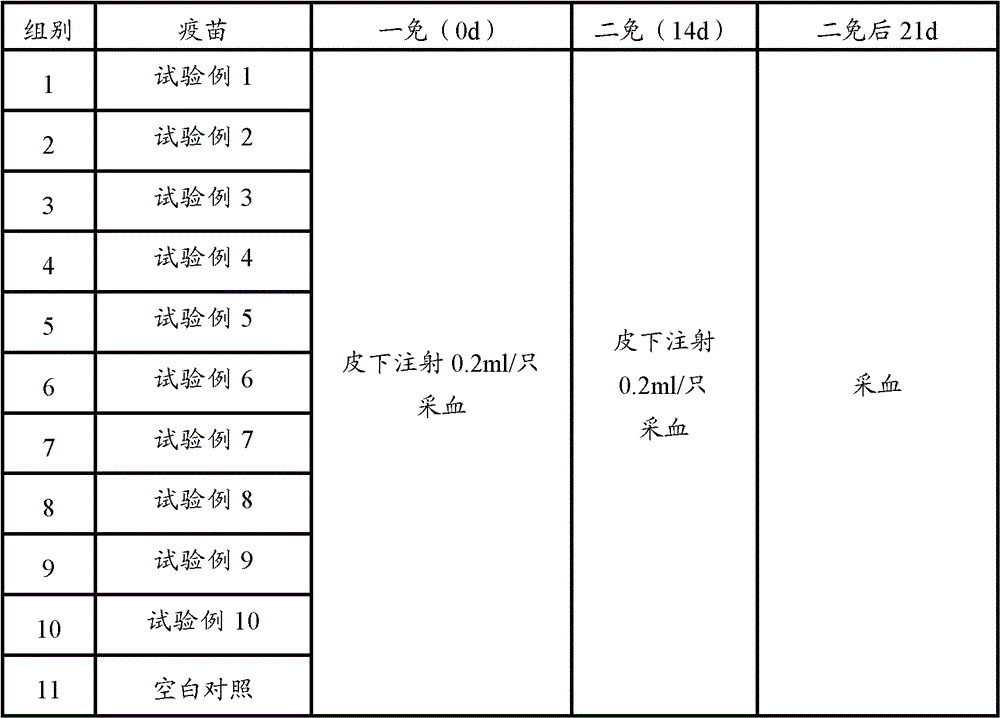
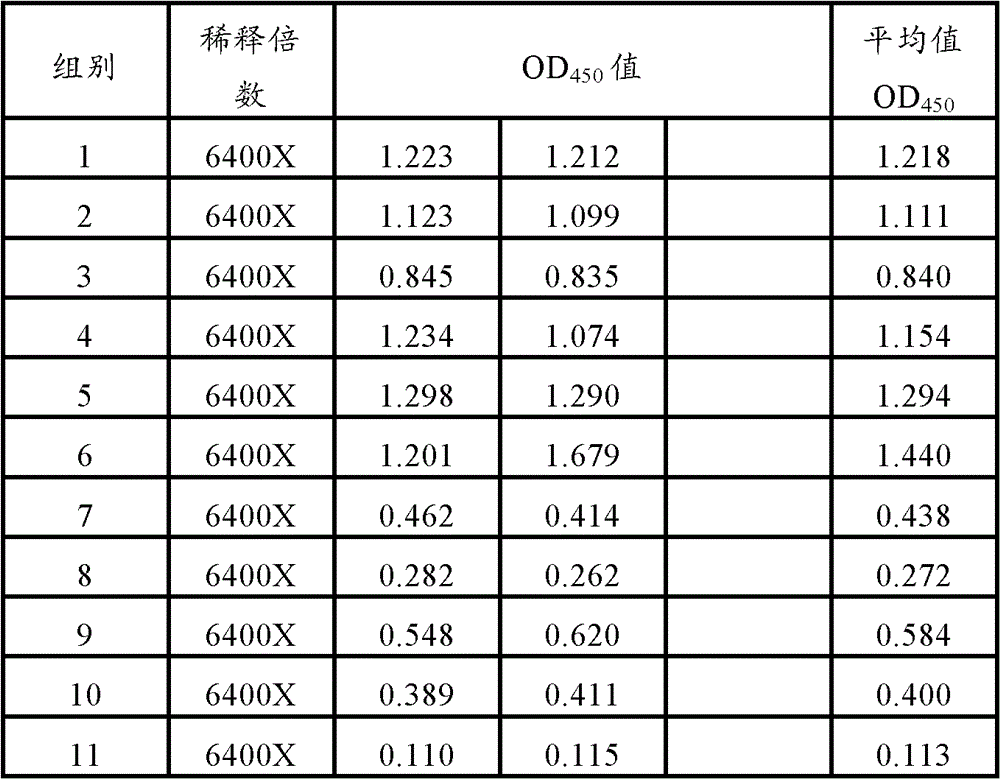
The present invention provides a vaccine adjuvant composition for treatment or prevention of swine infectious diseases. The vaccine adjuvant composition contains a milkvetch root extract, a polymer and an antigen component, or contains a milkvetch root extract, a polymer, cyclodextrin and an antigen component. The vaccine adjuvant can be combined with porcine circovirus antigen or mycoplasma hyopneumoniae antigen so as to substantially improve vaccine immune efficacy, wherein the original immune efficacy can be achieved after reducing the amount of the vaccine to the half of the amount of the original antigen; and test results of the vaccine adjuvant with a plurality of doses show that: the vaccine adjuvant does not have a significant side effect, and provides significantly reduced side effect compared with the commonly used oil phase adjuvant.
Owner:PU LIKE BIO ENG +2
Mycoplasma hyopneumoniae solid-phase competition ELISA (Enzyme Linked Immunosorbent Assay) kit, and preparation method and application thereof
InactiveCN110045117AImprove welfareShort detection cycleBiological testingReference sampleVaccine Production
The invention discloses a mycoplasma hyopneumoniae solid-phase competition ELISA (Enzyme Linked Immunosorbent Assay) kit, and a preparation method and application thereof. The kit comprises an ELISA plate coated with mycoplasma hyopneumoniae antibody protein, a reference sample, rabbit-anti mycoplasma hyopneumoniae positive serum and rabbit-anti mycoplasma hyopneumoniae negative serum. The kit disclosed by the invention can measure the relative content / efficacy of the mycoplasma hyopneumoniae antigen and has good specificity and repeatability. In addition, the provided solid-phase competitionELISA kit is simple in operation, consumes short time, does not need high-end instrument equipment, is easy in promotion and is suitable for applying to vaccine production inspection and laboratory research.
Owner:JIANGSU NANNONG HI TECH
Vaccine composition and application thereof
InactiveCN106139137AReduce contentImprove immunityAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
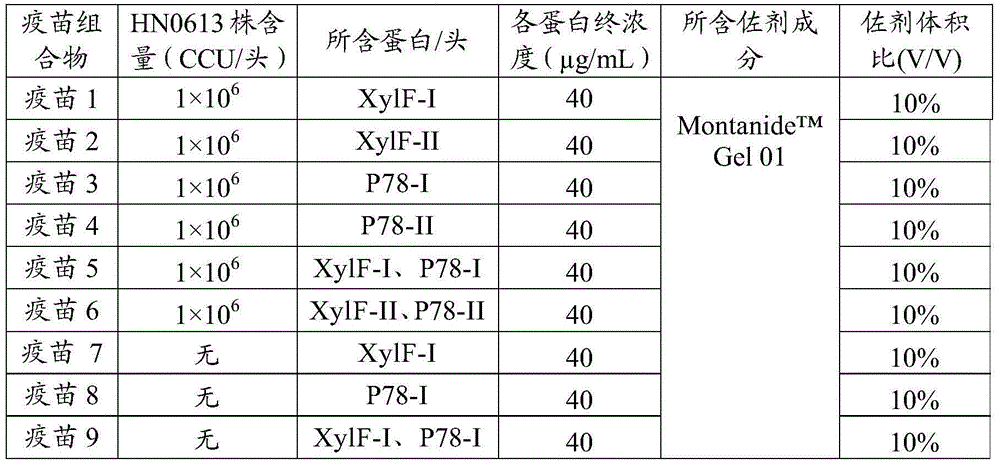
The invention provides a swine mycoplasma pneumonia vaccine composition, the vaccine composition includes swine pneumonia mycoplasma whole cell antigen and an immunogenic protein, and also comprises a veterinary-medicine-acceptable carrier, wherein the immunogenic protein comprises at least one of XylF and P78 proteins. The present invention also discloses the application of the vaccine composition in preparing of medicines for preventing and / or treating swine mycoplasma pneumonia. The content of the whole cell antigen and the immunogenic protein of the vaccine composition is decreased significantly compared with that of single use of a whole cell vaccine or a subunit vaccine for immunization, and immune effect is enhanced; protein part can be recombinationally expressed by means of genetic engineering in quantity, short time is taken, and the swine mycoplasma pneumonia vaccine composition can also be convenient for mass production. In addition, when an animal is immunized with the vaccine composition, the immune effects of the vaccine composition on the swine mycoplasma pneumonia antigen contained in the vaccine composition and other antigens are not affected.
Owner:PU LIKE BIO ENG
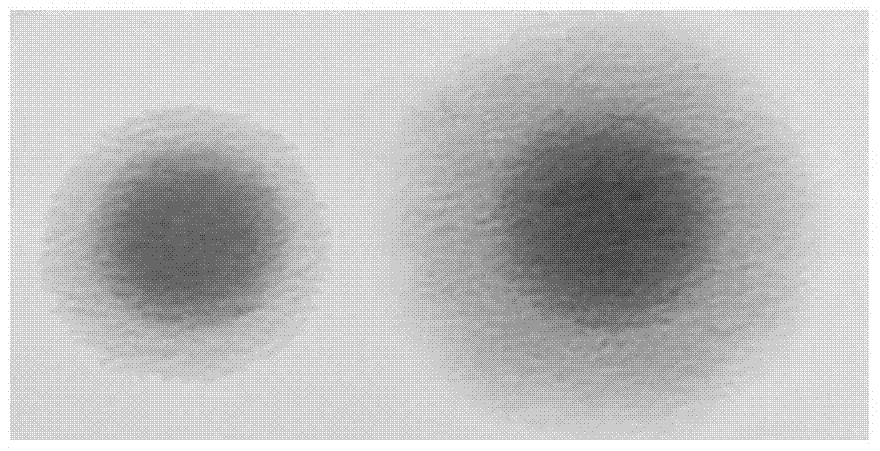
Application of mycoplasma hyopneumoniae antigen in prevention and treatment of porcine respiratory disease complex
InactiveCN104338125APromote growthReduced feed efficiencyAntibacterial agentsBacterial antigen ingredientsDiseaseVaccination
The invention provides application of a mycoplasma hyopneumoniae antigen or an immunogenic composition containing the antigen in prevention and treatment of PRDC (porcine respiratory disease complex) and diseases with PRDC relevant clinical evidence. Specifically, the application includes immunization of pigs with an immune dosage of the mycoplasma hyopneumoniae antigen or the immunogenic composition containing the mycoplasma hyopneumoniae antigen. Vaccination with vaccines containing the mycoplasma hyopneumoniae antigen on animals can significantly alleviate and reduce the severity and duration of PRDC relevant clinical evidence.
Owner:PU LIKE BIO ENG
Fusion protein containing mycoplasma hyopneumoniae antigen, vaccine composition and application
ActiveCN107868130AImprove protectionImprove purification effectAntibacterial agentsBacterial antigen ingredientsImmune effectsMycoplasma
The invention relates to a fusion protein. The fusion protein comprises enterotoxigenic escherichia coil heat-labile enterotoxin B subunit and mycoplasma hyopneumoniae antigen protein in a sequence from an N end to a C end. The invention also relates to a vaccine composition prepared from the fusion protein and an application thereof. The vaccine composition provided by the invention can achieve the immune effect of the commercial inactivated vaccine or better immune effect after one-time immunization.
Owner:PU LIKE BIO ENG
Novel mycoplasma hyopneumoniae bacterial strain and vaccine composition thereof
ActiveCN103031258BImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsDiseaseCircovirus
The invention provides a mycoplasma hyopneumoniae strain HN0613 which is isolated and identified to have better immunogenicity, and further provides a mycoplasma hyopneumoniae antigen prepared from the mycoplasma hyopneumoniae strain HN0613 and mycoplasma pneumonia pneumovax containing the mycoplasma hyopneumoniae antigen. The invention further provides a bivalent combined vaccine of porcine circovirus II and mycoplasma hyopneumoniae. The duplex combination vaccine comprises PCV (Porcine Circovirus) antigen II (inactivated porcine circovirus antigen II or PCV20 RF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant. The combination vaccine can achieve the purpose of preventing the porcine circovirus disease and the mycoplasma pneumonia swine by injection once, and has a protective effect of preventing mycoplasma hyopneumoniae infection.
Owner:PU LIKE BIO ENG
Vaccine composition, preparation method and application thereof
ActiveCN104248759AImprove immunityReduce stress responseAntibacterial agentsBacterial antigen ingredientsImmune effectsAdditive ingredient
The invention provides a vaccine composition, which includes an immune amount of a mycoplasma hyopneumoniae antigen, an immune amount of a swine influenza virus antigen and an immune amount of a porcine reproductive and respiratory syndrome virus antigen. All antigens of the vaccine composition not only do not generate mutual interference or influence of antigen components, but have the effect of mutually enhancing the immune effect instead. One immunization can achieve the immune effect and the antibody continuous level of single antigen twice immunization. Also, the vaccine composition has the advantages of good security, simple preparation method, convenient and fast immunization, and reduction of the immunization cost, etc.
Owner:PU LIKE BIO ENG
Vaccine composition, and preparation method and applications thereof
ActiveCN104857508AAnti-interferenceEffective treatmentAntibacterial agentsBacterial antigen ingredientsMaternal antibodyHaemophilus
The invention provides a vaccine composition comprising mycoplasma hyopneumoniae antigen of an immunizing dose, haemophilus parasuis antigen of an immunizing dose, and a pharmaceutically acceptable carrier. The vaccine composition is capable of eliminating requirements on multiple vaccines and multiple-dose immunizing; immune interference of the mycoplasma hyopneumoniae antigen with the haemophilus parasuis antigen is not induced, synergistic effect is improved, maternal antibody interference can be eliminated by immunizing with the vaccine composition, and effect is more stable; and in addition, animal stress response and side effect caused by single dose immunizing of the vaccine composition are little, so that immunizing procedure is simplified, production cost, transportation cost, and prevention cost are reduced, and practicability is excellent.
Owner:PU LIKE BIO ENG
Mycoplasma hyopneumoniae and haemophilus parasuis combined inactivated vaccine and application thereof
ActiveCN109745555ATypical asthma symptomsStrong pathogenicityAntibacterial agentsBacterial antigen ingredientsHaemophilusSerotype
The invention belongs to the technical field of animal biological products, and particularly relates to a mycoplasma hyopneumoniae and haemophilus parasuis serotype-2, serotype-5, serotype-7 combinedinactivated vaccine. The vaccine is prepared from a mycoplasma hyopneumoniae antigen, three haemophilus parasuis serotype antigens and an immunologic adjuvant, wherein the mycoplasma hyopneumoniae antigen is the inactivated mycoplasma hyopneumoniae HNMhy1 antigen, the preservation number of the mycoplasma hyopneumoniae HNMhy1 antigen is CGMCC NO:13858, and the preservation date of the mycoplasma hyopneumoniae HNMhy1 antigen is May 26 th, 2017. The three haemophilus parasuis serotype antigens are inactivated haemophilus parasuis type-2 HN1553, type-5 HN1570 and type-7 HN1565 antigens respectively, wherein the preservation number of the haemophilus parasuis type-2 HN1553 strain is CGMCC NO:16801, the preservation number of the haemophilus parasuis serotype-5 HN1570 strain is CGMCC NO:16802,and the preservation number of haemophilus parasuis serotype-7 HN1565 strain is CGMCC NO:16803. The immunologic adjuvant is an aluminum hydroxide gel adjuvant.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and preparation method
ActiveCN103623400AReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsTGE VACCINEMycoplasma pneumonia
The invention relates to a vaccine composition for resisting pig mycoplasma pneumonia and infectious pleuropneumonia and a preparation method. The vaccine composition comprises pig mycoplasma hyopneumoniae antigens and pig actinobacillus pleuropneumoniae antigens, is simple in immunization procedure, not only is capable of generating antibodies, but also has the functions of toxicity attack protection and effectively controlling pig mycoplasma pneumonia and infectious pleuropneumonia. The vaccine composition has the immunization effect same to an independently injected vaccine, is small in side reaction, long in immunization period, less in consumed time, less in consumed labor, small in encroaching on a pig. The vaccine composition is simple in production technology, low in immunization cost and strong in practicality.
Owner:PU LIKE BIO ENG
Novel mycoplasma hyopneumoniae strain and its vaccine composition
ActiveCN104450559BImprove immunityDoes not affect weight gainAntibacterial agentsBacterial antigen ingredientsCircovirusImmunogenicity
The present invention provides a Mycoplasma hyopneumoniae strain HN0613 strain which is isolated and identified as having better immunogenicity, and also provides the Mycoplasma hyopneumoniae antigen prepared by the above-mentioned Mycoplasma hyopneumoniae HN0613 strain and the Mycoplasma hyopneumoniae pneumonia vaccine containing the Mycoplasma hyopneumoniae antigen . The present invention also provides a dual combination vaccine of porcine circovirus type 2 and mycoplasma hyopneumoniae, comprising PCV2 antigen (inactivated porcine circovirus type 2 antigen or PCV2 ORF2 protein), inactivated mycoplasma hyopneumoniae and vaccine adjuvant agent. The combination vaccine of the present invention can prevent porcine circovirus disease and swine mycoplasma pneumonia with one shot of the vaccine, and has a preventive and protective effect on mycoplasma hyopneumoniae infection.
Owner:PULIKE BIOLOGICAL ENG INC +1
Vaccine for PCV2 and mycoplasma
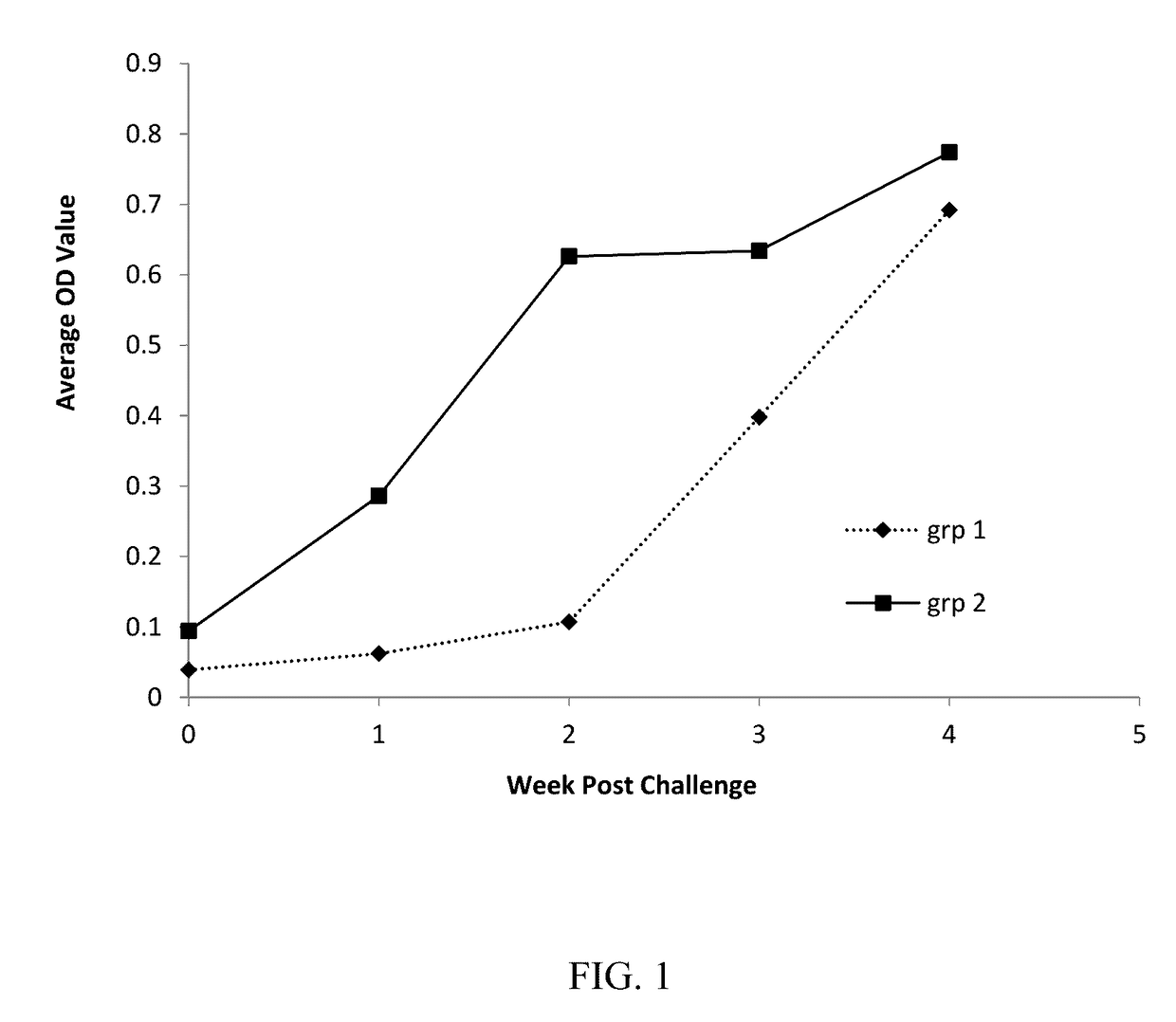
ActiveUS9649370B2Reduction of growing weightFacilitate entryBacterial antigen ingredientsViral antigen ingredientsMultivalent VaccineReady to use
A premixed multivalent vaccine in ready-to-use form comprising PCV2 ORF2 capsid antigen and M. hyopneumoniae antigen that reduces or prevents PCV2 infection and / or M. hyopneumoniae infection in pigs after a single dose administration of the vaccine is disclosed.
Owner:PROTATEK INT
Vaccine for pcv2 and mycoplasma
ActiveUS20160082095A1Reduces of conditionImprove severityBacterial antigen ingredientsViral antigen ingredientsMultivalent VaccineReady to use
A premixed multivalent vaccine in ready-to-use form comprising PCV2 ORF2 capsid antigen and M. hyopneumoniae antigen that reduces or prevents PCV2 infection and / or M. hyopneumoniae infection in pigs after a single dose administration of the vaccine is disclosed.
Owner:PROTATEK INT
Preparation and application of a fusion protein and its vaccine composition
ActiveCN104277115BShort timeEase of mass productionAntibacterial agentsBacterial antigen ingredientsPseudomonasHumoral immune reaction
Owner:PULIKE BIOLOGICAL ENG INC
Vaccine composition for preventing and treating porcine respiratory syndrome, and preparation method and application thereof
ActiveCN103961695ALow costReduce the number of vaccinationsAntibacterial agentsAntiviralsHaemophilusMedicine
A provided vaccine composition for preventing and treating porcine respiratory syndrome comprises immunization amount of porcine influenza virus antigen, immunization amount of porcine pneumonia mycoplasma antigen, immunization amount of haemophilus parasuis antigen, and an adjuvant. The vaccine composition is capable of effectively preventing and controlling porcine respiratory syndrome caused by mixed infection of the three kinds of pathogeny, and also is capable of reducing vaccine inoculating frequency and avoiding unavailable full-access immunization caused by missed inoculation.
Owner:PU LIKE BIO ENG
A kind of dual inactivated vaccine of Mycoplasma hyopneumoniae and Haemophilus parasuis and its application
ActiveCN109745555BTypical asthma symptomsStrong pathogenicityAntibacterial agentsBacterial antigen ingredientsType antigenSerotype
The invention belongs to the technical field of veterinary biological products, in particular to a dual inactivated vaccine of Mycoplasma hyopneumoniae and Haemophilus parasuis serotypes 2, 5 and 7. The vaccine is made of Mycoplasma hyopneumoniae antigen, three Haemophilus parasuis serotype antigens and immune adjuvants, wherein the Mycoplasma hyopneumoniae antigen is the inactivated Mycoplasma hyopneumoniae HNMhy1 strain antigen, and the Mycoplasma hyopneumoniae HNMhy1 strain , the preservation number is CGMCC NO: 13858, and the preservation date is May 26, 2017; the three serotype antigens of Haemophilus parasuis are respectively inactivated Haemophilus parasuis type 2 HN1553 strain and type 5 HN1570 strain and type 7 HN1565 strain antigen, wherein the Haemophilus parasuis type 2 HN1553 strain has a preservation number of CGMCC NO: 16801; the Haemophilus parasuis serotype 5 HN1570 strain has a preservation number of CGMCC NO: 16802; The Haemophilus parasuis serotype 7 HN1565 strain has a preservation number of CGMCC NO: 16803; the immune adjuvant is aluminum hydroxide gel adjuvant.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Vaccine composition and its preparation method and application
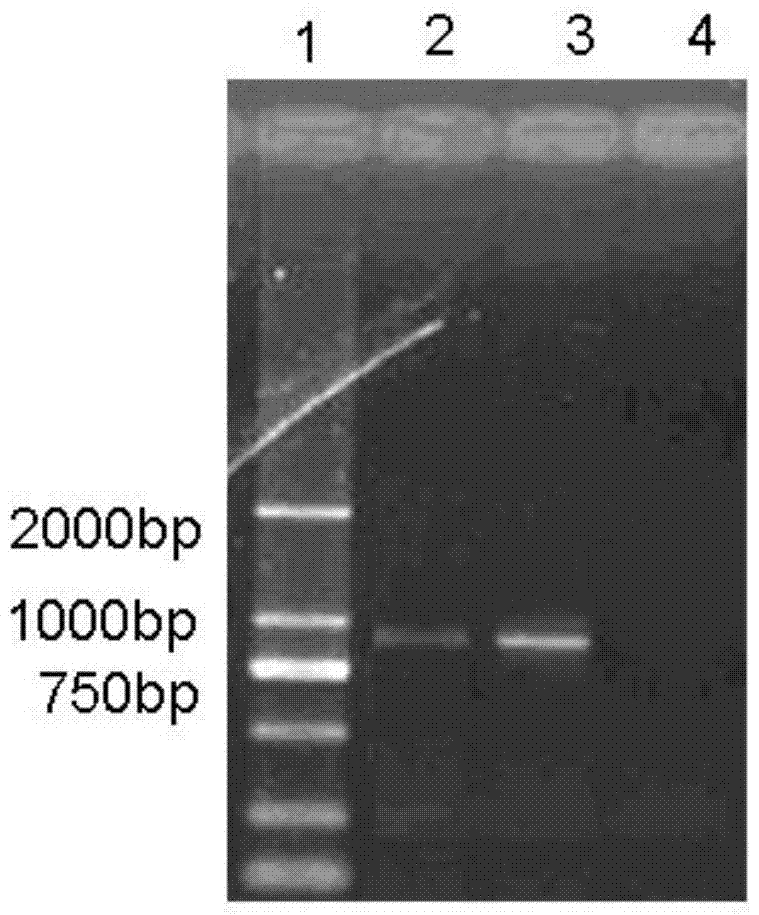
ActiveCN104324370BAntigen purification method is simplePreserve immune active ingredientsAntibacterial agentsBacterial antigen ingredientsMedicineActive component
The present invention provides a method for preparing a vaccine composition containing Mycoplasma hyopneumoniae antigen, wherein the method comprises: (1) culturing and inactivating Mycoplasma hyopneumoniae; (2) treating step (1) with a nonionic surfactant inactivated Mycoplasma hyopneumoniae; and (3) separating the non-ionic surfactant-insoluble antigen components after treatment with the non-ionic surfactant, and adding an adjuvant. The present invention also provides that the vaccine composition containing Mycoplasma hyopneumoniae antigen prepared by the preparation method removes the immunosuppressive components, and at the same time retains the immune active components to the greatest extent; It has a good protective effect on the lung disease of pigs.
Owner:PU LIKE BIO ENG
A kind of vaccine composition and its preparation method and application
ActiveCN104248760BPlay a role in immune enhancementMaintain stabilityAntiviralsAntibody medical ingredientsLevamisoleMicrobiology
The invention provides a vaccine composition, which comprises porcine circovirus antigen of immune amount, mycoplasma hyopneumoniae antigen of immune amount, and levamisole or its derivatives and polymers. The vaccine composition not only can simultaneously play an immune enhancing effect on two antigens, but also has stability, and can be used for diluting porcine reproduction and respiratory syndrome live vaccine.
Owner:PU LIKE BIO ENG
Low-serum high-efficiency culture medium for culturing Mycoplasma hyopneumoniae and preparation method thereof
ActiveCN106497826BAffect performanceEffect on performanceBacteriaMicroorganism based processesBiotechnologyLipid formation
The invention provides a low-serum efficient culture medium for culturing mycoplasma hyopneumoniae and a preparation method thereof. The low-serum efficient culture medium comprises a base medium and an addition bag. The base medium comprises M199, brain extract powder, lactoalbumin hydrolysate, BSA, glucose, fresh yeast extract, Hepes, iron citrate, calcium chloride, 2H2O, phenol red and dd H2O. The addition bag comprises cholesterol, linoleic acid, linolenic acid, palmitic acid, ethanol and porcine serum. Through use of some hydrolyzates and lipid, a serum addition ratio is less than 5% so that a cost is reduced, the quality of mycoplasma is improved, and mycoplasma hyopneumoniae antigen titer is improved.
Owner:内蒙古金源康生物工程股份有限公司
A kind of vaccine composition and its preparation method and application
ActiveCN104096222BNo mutual interferenceSatisfied with the immune effectBiological testingRespiratory disorderHaemophilusRespiratory syndrome virus
Owner:PULIKE BIOLOGICAL ENG INC
Vaccine composition and preparation method against swine mycoplasma pneumonia and infectious pleuropneumonia
ActiveCN103623400BReduce harmSimplified immunization programAntibacterial agentsBacterial antigen ingredientsSingle injectionSwine Mycoplasma Pneumonia
The invention relates to a vaccine composition against swine mycoplasma pneumonia and infectious pleuropneumonia and a preparation method thereof. The vaccine composition contains Mycoplasma hyopneumoniae antigen and Actinobacillus pleuropneumoniae antigen, has simple immunization procedures, can produce antibodies, and has protection against viruses at the same time, and can effectively prevent and treat Mycoplasma pneumoniae pneumonia and porcine infectious pleuropneumonia. The immune effect of the vaccine composition is at least equal to the effect of separate injection of single vaccine, less side effects, high serum antibody titer, long immunization period, less time-consuming, less labor, and less damage to pigs. The vaccine composition has simple production process, low immunization cost and strong practicability.
Owner:PU LIKE BIO ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com