Vaccine composition and application thereof
A technology of vaccine composition and protein, which is applied in the direction of drug combination, medical preparations containing active ingredients, bacterial antigen components, etc., and can solve problems such as poor immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 mycoplasma hyopneumoniae whole bacterium antigen
[0049] Dilute the freeze-dried strain of Mycoplasma hyopneumoniae HN0613 strain with liquid medium, inoculate it on a solid medium plate by streaking, culture at 37°C for 7 days, select well-growing colonies, inoculate on the slope of solid medium, and culture at 37°C 7 days, as a primary seed.
[0050] Take a small amount of liquid medium to wash the slant culture of the primary seed, inoculate it in a large tube of liquid medium, culture it at 37°C for 7 days, and use it as the secondary seed after being tested to be pure.
[0051] The secondary seed liquid cultured in the liquid medium is inoculated in the liquid medium at a volume ratio of 10% (V / V). Cultivate at 37°C for 3-6 days, the color will turn yellow, and the bacterial liquid will be harvested when the pH value drops to 6.8-7.0. After passing the pure inspection, expand the culture in the same way (the number of subcultures s...
Embodiment 2
[0054] Example 2 Expression, Identification and Purification of Mycoplasma Hyopneumoniae Immunogenic Protein
[0055] According to the XylF protein nucleoside in the Mycoplasma hyopneumoniae J strain (accession number: AE017243.1) and 7448 strain (accession number: AE017244.1) reported in NCBI (http: / / www.ncbi.nlm.nih.gov) acid sequence, and the P78 protein nucleotide sequence in strain J (accession number: AE017243.1) and strain 232 (accession number: AE017332.1), which were synthesized by Shanghai Bioengineering Co., Ltd. by artificial synthesis. The full lengths of the gene fragments are XylF-I 1341bp, XylF-II 1341bp, P78-I 2076bp, P78-II 2076bp. On the basis of artificially synthesizing XylF-I, XylF-II, P78-I, and P78-II gene fragments, templates for XylF-I, XylF-II, P78-I, and P78-II proteins were prepared respectively.
[0056] The gene fragments synthesized in the previous step were used as templates for PCR reactions respectively, and primers for XylF-I, XylF-II, P78-...
Embodiment 3
[0059] The preparation of embodiment 3 swine mycoplasma pneumonia vaccine composition
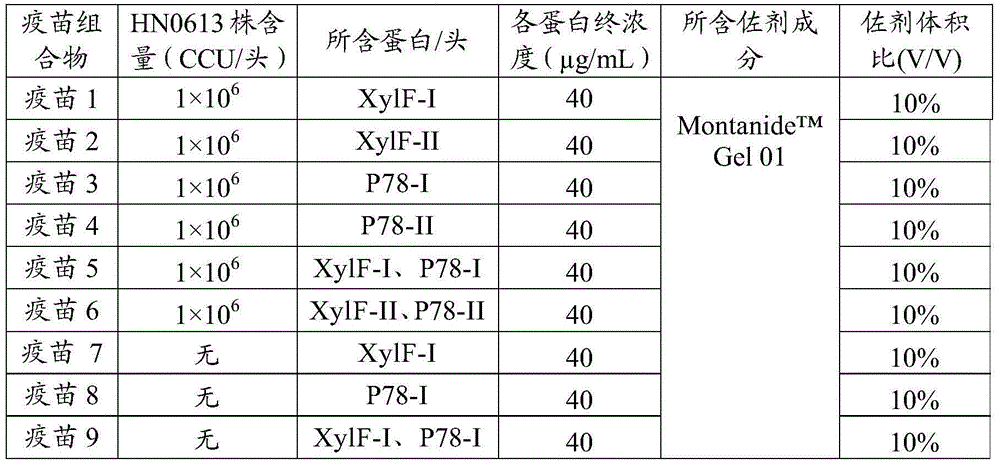
[0060] The mycoplasma hyopneumoniae whole bacterium antigen prepared in embodiment 1, the mycoplasma hyopneumoniae immunogenic protein prepared in embodiment 2 are diluted with pH7.2 PBS solution, and each protein solution after dilution is mixed with Montanide TM Gel 01 adjuvant is mixed together by the components and ratio contained in the Mycoplasma pneumoniae vaccine composition in Table 1, stirred at a speed of 500-800r / min for 10-15min, and added 1% (volume ratio) thimerosal solution before terminating the stirring , so that the final concentration does not exceed 1 / 10,000, fully shake and mix, and store at 2-8°C after aliquoting.
[0061] Table 1 Components and ratios contained in the swine mycoplasma pneumonia vaccine composition
[0062]
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com