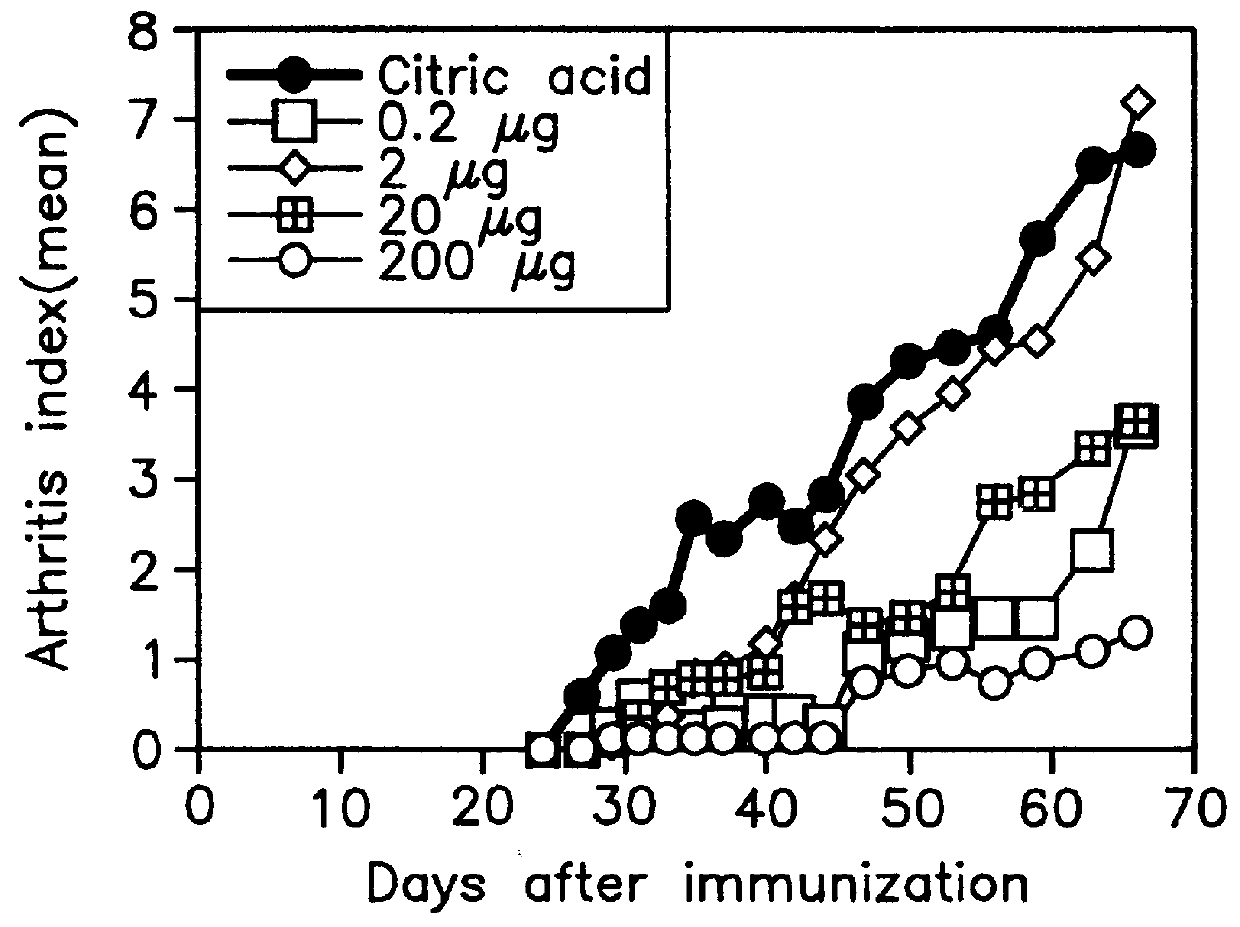
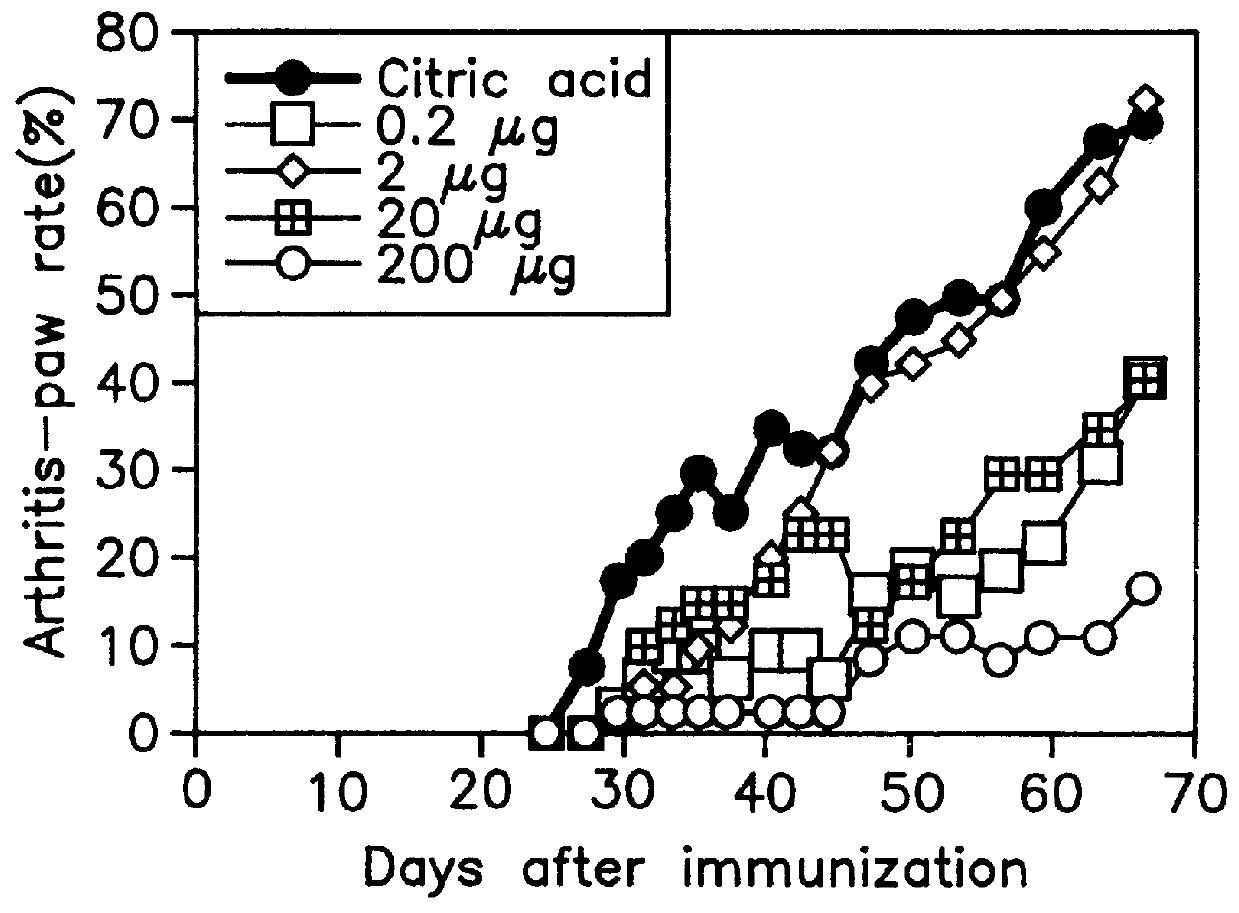
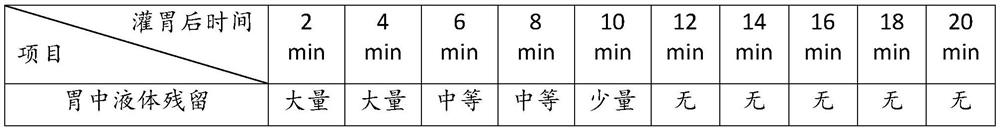
Patents
Literature
55 results about "Oral immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An oral vaccine is one that is taken by mouth, instead of being injected directly into the blood. The goal of all vaccines, whether administered by injection or as an oral vaccine, is to trigger the body’s immune system without the person actually having the disease. It is a way of immunizing, or protecting, the body from getting the disease.
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
InactiveCN102512693AImprove abilitiesThe production is effectiveGenetic material ingredientsAntiviralsEnzyme digestionA-DNA
The invention discloses a preparation method of a porcine epidemic diarrhea recombinant adenovirus vaccine. The preparation method provided by the invention comprises the following steps of inserting a DNA sequence of a zone S1 of a porcine epidemic diarrhea virus (PEDV) into an adenovirus shuttle plasmid pShuttle-CMV to obtain pShuttle-CMV-S1, carrying out linearization of the pShuttle-CMV-S1, transforming the linear pShuttle-CMV-S1 into a BJ5183 competent cell containing pAdEasy-1, carrying out homologous recombination, carrying out enzyme digestion, carrying out AD-293 cell transfection, carrying out packaging to obtain a recombinant adenovirus rAd-S1, and carrying out purification, amplification and sub-packaging. After oral immunization, the porcine epidemic diarrhea recombinant adenovirus vaccine can induce generation of mucosal immunity thereby preventing porcine epidemic diarrhea (PED) well.
Owner:GENIFARM LAB INC
Oral remedy for rheumatoid arthritis and functional food
InactiveUS6010722AHighly effective oral immune toleranceAvoid immune responsePeptide/protein ingredientsAntipyreticDiseaseOral medication
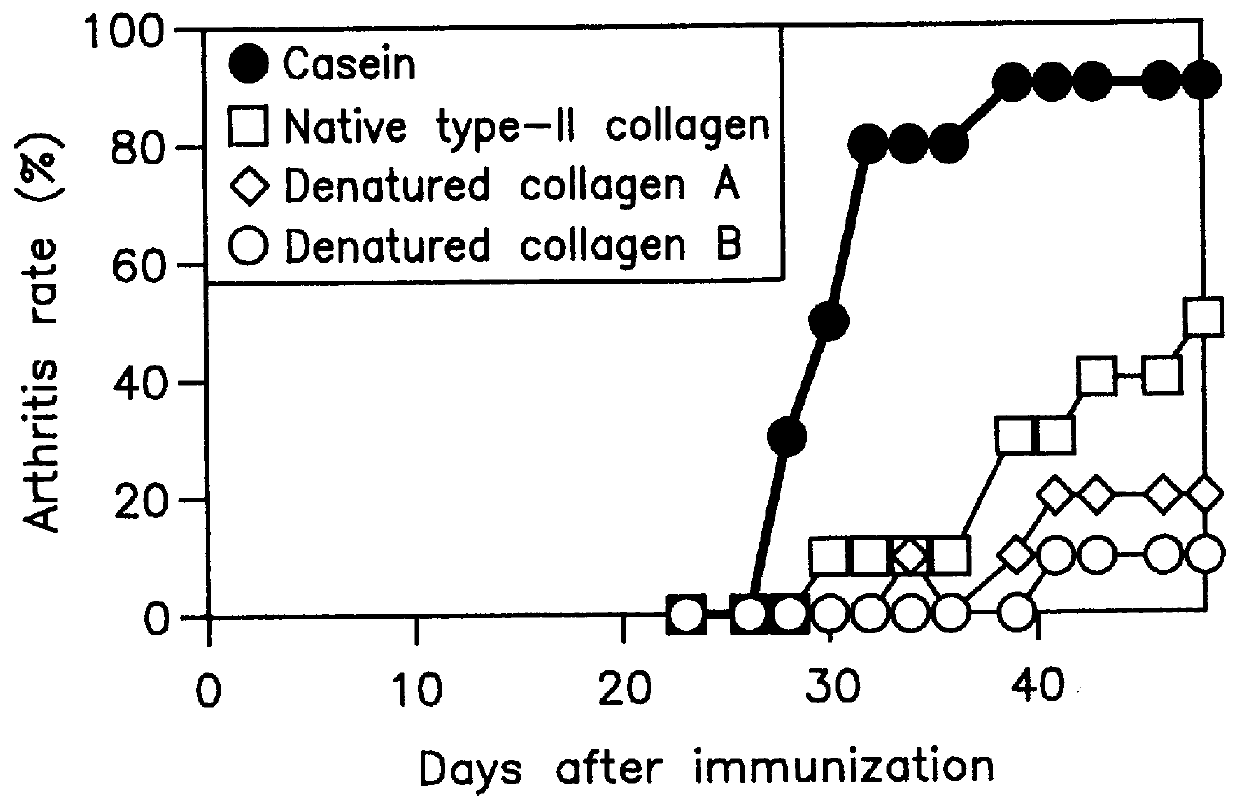
PCT No. PCT / JP96 / 01623 Sec. 371 Date May 18, 1998 Sec. 102(e) Date May 18, 1998 PCT Filed Jun. 13, 1996 PCT Pub. No. WO96 / 41644 PCT Pub. Date Dec. 27, 1996Oral drugs and functional foods of the present invention contain type-II collagen denatured (fragmented) under specific conditions. Rheumatoid arthritis (RA) has been considered to be an autoimmune disease against type-II collagen as an antigen. Since denatured type-II collagen of the invention induces high oral immune tolerance and inhibits immune responses against type-II collagen, it can prevent and treat RA. Particularly, RA can effectively be prevented and treated by simple oral administration of type-II collagen.
Owner:NIPPON HAM
Preparation method and application of recombinant lactococcus lactis
ActiveCN107653260AImprove infection abilityAntibacterial agentsBacterial antigen ingredientsVector vaccineSerotype
The invention relates to a preparation method and application of a recombinant lactococcus lactis. The invention is characterized in that the surface immunogenic protein (Sip) of tilapia-source streptococcus agalactiae is intracellularly or secretorily expressed in lactococcus lactis cells, vectors which are used for the recombinant expression of the Sip are pNZ8124 and pNZ8148 respectively, signal peptide segments are removed from Sip gene segments inserted into the vectors, and histidine sequence tags are added to the Sip gene segments, the recombinant protein is induced to be expressed by Nisin, the optimum induction condition is 4h of induction by 100ng / mL of Nisin, the optimum oral immunization concentration is 2.24*10<10>CFU / mL, and the oral dosage is 100 mu L. The recombinant lactococcus lactis disclosed by the invention which is applied in lactobacillus living vector vaccines against tilapia streptococcus agalactiae has the advantages of wide serotype coverage, direct oral administration, high safety, easiness in operation, easy large-scale herd immunity, good immune effect and the like.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Swine fever oral attenuated freezing-dry vaccine and preparation method thereof and freeze-drying protective agent
InactiveCN106310250APrevent atrophyAvoid excessively large internal aperturesAntiviralsAntibody medical ingredientsGlycineFreeze-drying
The invention discloses a swine fever oral attenuated freezing-dry vaccine and a preparation method thereof and a freeze-drying protective agent, the swine fever oral attenuated freezing-dry vaccine includes antigen swine fever virus, a mucosal adjuvant and the freeze-drying protective agent, the freeze-drying protective agent includes 1-3% of gelatin, 1-3% of glycine and balance of water, the swine fever oral attenuated freezing-dry vaccine can well protect the activity of CSFV (Classical Swine Fever Virus), reduces live virus loss, can induce higher levels of IgG and IgA antibodies in oral immunization tests of mice, can promote expression of type I interferon in thymus and spleen, has the effect of protecting antigen activity and improving oral swine fever antigen immune efficiency, and can be used as a swine fever oral vaccine protective agent for industry development.
Owner:SHANGHAI ACAD OF AGRI SCI
Preparation method of porcine epidemic diarrhea virus genetic engineering subunit oral vaccine
ActiveCN102988971AAvoid stressAvoid absorptionAntiviralsAntibody medical ingredientsStaphylococcus lactisLocal immunity
The invention discloses a preparation method of a porcine epidemic diarrhea virus genetic engineering subunit oral vaccine. The method comprises the following steps of: 1) synthesizing an M gene; 2) constructing an expression vector; 3) performing protein induction expression; 4) carrying out recombinant strain preservation experiment; and 5) preparing the vaccine. The oral immunization has the advantage of effectively stimulating local immune cells of the intestinal tract to generate secretion type IgA, is especially applicable to the infectious disease of the intestinal mucosa, and avoids stress and vaccine absorption problems resulting from conventional vaccine injection; and as a safe and non-toxic live vector system capable of living on the intestinal mucosa, the lactococcus lactis is the most suitable vector system. The oral vaccine performs antigen presentation through the gastrointestinal mucosa, and produces immune reaction similar to conventional injection through different immune pathways.
Owner:北京信得威特科技有限公司
Use of immunological stimulant compound(ISCOMs)in fish immunity by oral administration and dipping bath method
InactiveCN1879880AImprove the ability of proliferation and transformationHigh antibody titerImmunological disordersAntibody medical ingredientsStimulantT lymphocyte
The invention relates to a bath or oral application of immune activate compound in the fish immunity, wherein the invention has the advantages that: said compound ISCOMs is used to bath immunity to improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection; when in the same dosage, the antibody level is higher than non-adjuvant group and the ISM1312 group; and the ISCOMs used in oral can improve the antibody level and improve the increment transformation ability of T-lymphocyte, to induce the immune protection.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +3
Mink parvovirus virus-like particle as well as preparation method and application thereof
ActiveCN104403006AImprove protectionPromote safe productionDigestive systemInactivation/attenuationTransfer vectorImmunogenicity
The invention provides a mink parvovirus virus-like particle which is characterized in that VP2 gene of mink parvovirus is optimized according to insect cell optimal codons, the 5' end of the optimized VP2 gene is directly connected with the nucleotide sequence of polyhedron of a coding part and then is cloned into a transfer vector, and the mink parvovirus virus-like particle can be produced through a baculovirus / insect cell expression system. The mink parvovirus virus-like particle can be produced through the expression system in a safe, efficient and large-scale manner, and the obtained virus-like particle is high in titer and good in immunogenicity; both muscle immunization and oral immunization can induce a mink body to produce high-level specific antibody which can resist attack of virulent virus and well protect a mink, and a foundation is laid for the preparation of mink viral enteritis vaccine.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Transformant for blocking effect of porcine somatostatin by oral immunization and application thereof
InactiveCN101892189AImprove securityEnhance immune responseBacteriaBacteria material medical ingredientsCell wallImmunoenhancing factor
The invention discloses transformant. The transformant comprises a recipient bacterium and a recombinant vector transferred to the recipient bacterium, wherein the recipient bacterium is lactococcus lactis NZ9800; the original vector of the recombinant vector is pNZ8112; and the exogenous target gene of the recombinant vector comprises a porcine somatostatin gene, a cholera toxin B subunit gene and a cell wall anchor stator M6 gene. The transformant can enter the porcine intestinal tract by oral administration; compared with injection immunization, the oral immunization mode reduces the toxicity caused by direct contact with a circulatory system and increases the safety of vaccines; and the immune response of organisms is enhanced and the immune effect is improved by combined immunization of the transformant, an antigen gene and an immune enhancing factor.
Owner:ZHEJIANG UNIV
Attenuated strain of grass carp reovirus II and application of attenuated strain
ActiveCN111593027ANon-pathogenicReduce morbidityViral antigen ingredientsMicroorganism based processesBiotechnologyViral type
The invention belongs to the technical field of vaccine preparation, and particularly relates to an attenuated strain of a grass carp reovirus II and an application of the attenuated strain. The preservation number of the attenuated strain is CCTCC NO:V201867. A live vaccine prepared from the attenuated strain can prevent diseases caused by the grass carp reovirus II; the immune protection effectfor the same genotype reaches 95% or above during injection use, reaches 65% or above during soaking immunization and reaches 40% or above during oral immunization; no stress reaction is generated toa body; the risk of virulence return is avoided; the vaccine can be applied to prevention of hemorrhagic diseases, especially viral hemorrhagic diseases of grass carp and black carp caused by the grass carp reovirus II; the morbidity of the hemorrhagic diseases of the grass carp and the black carp is reduced; and the survival rate of the grass carp and the black carp is increased.
Owner:广东富伦德生物科技有限公司
Recombinant bacillus subtilis for expressing S protein of transmissible gastroenteritis of swine virus
InactiveCN105886523ABacteriaMicroorganism based processesSwine Transmissible GastroenteritisSpecific immunity
The invention relates to recombinant bacillus subtilis for expressing S protein of transmissible gastroenteritis of swine virus (TGEV), and belongs to the fields of biotechnology and genetic engineering. According to the recombinant bacillus subtilis disclosed by the invention, a TGEV S protein recombinant bacillus subtilis integrated expression plasmid pIncotGSR is successfully constructed, and the expression plasmid is successfully transformed into bacillus subtilis WB800 through electric shock. Based upon Western-blot verification, the TGEV S protein can be successfully expressed in the recombinant bacillus subtilis. An effective TGEV specific immune response level can effectively generate in the body of a one-month-old piglet through stimulation of oral immunization. The recombinant bacillus subtilis is expected to be developed as a genetic engineering oral vaccine for preventing transmissible gastroenteritis of swine.
Owner:NANJING AGRICULTURAL UNIVERSITY
Vibrio anguillarum O3 serotype bacterial strain and application thereof
InactiveCN106047763AReduce abuseReduce the risk of contaminationAntibacterial agentsBacteriaBacteroidesAdjuvant
The invention relates to a vibrio anguillarum O3 serotype bacterial strain and an application method thereof. The vibrio anguillarum O3 serotype bacterial strain is separated from the body of scophthalmus maximu, is a wild bacterial strain with strong toxicity, and has the preservation number of CCTCC M 2016261. An antigen preparation method of the vibrio anguillarum O3 serotype bacterial strain comprises any one or more of inactivated bacteria, bacterial ghost ingredients, attenuated bacterial strains, antigen subunits and antigen gene expression products. The produced vaccine can be a single component of an antigen prepared by the bacterial strain, or a combined vaccine is produced by mixing of the antigen prepared by the bacterial strain and antigens of other bacteria, or a vaccine is produced by the antigen of the prepared single or combined vaccine and an adjuvant. The vaccination mode during vaccine application can adopt injection immunization, bath immunization or oral immunization.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
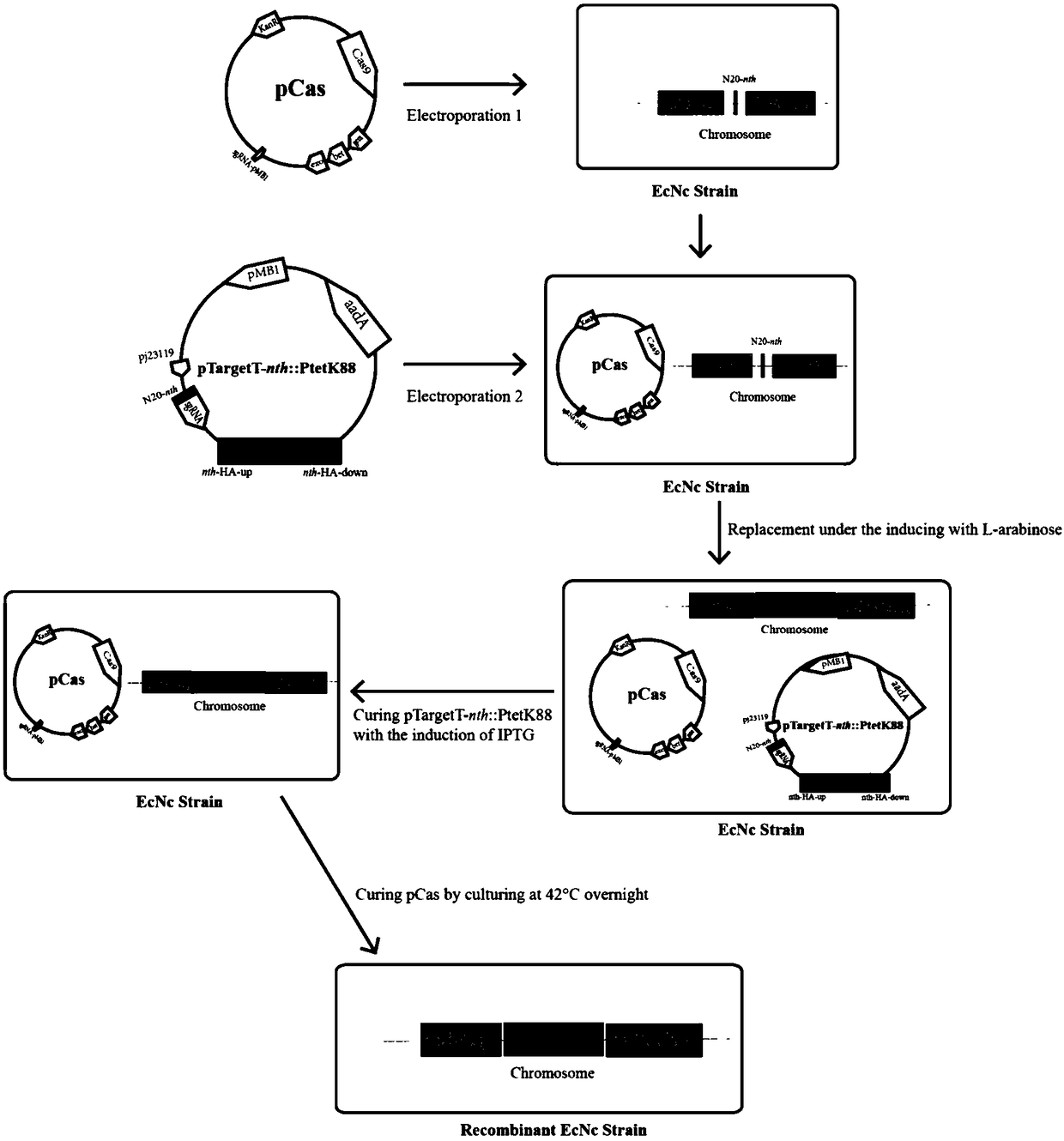
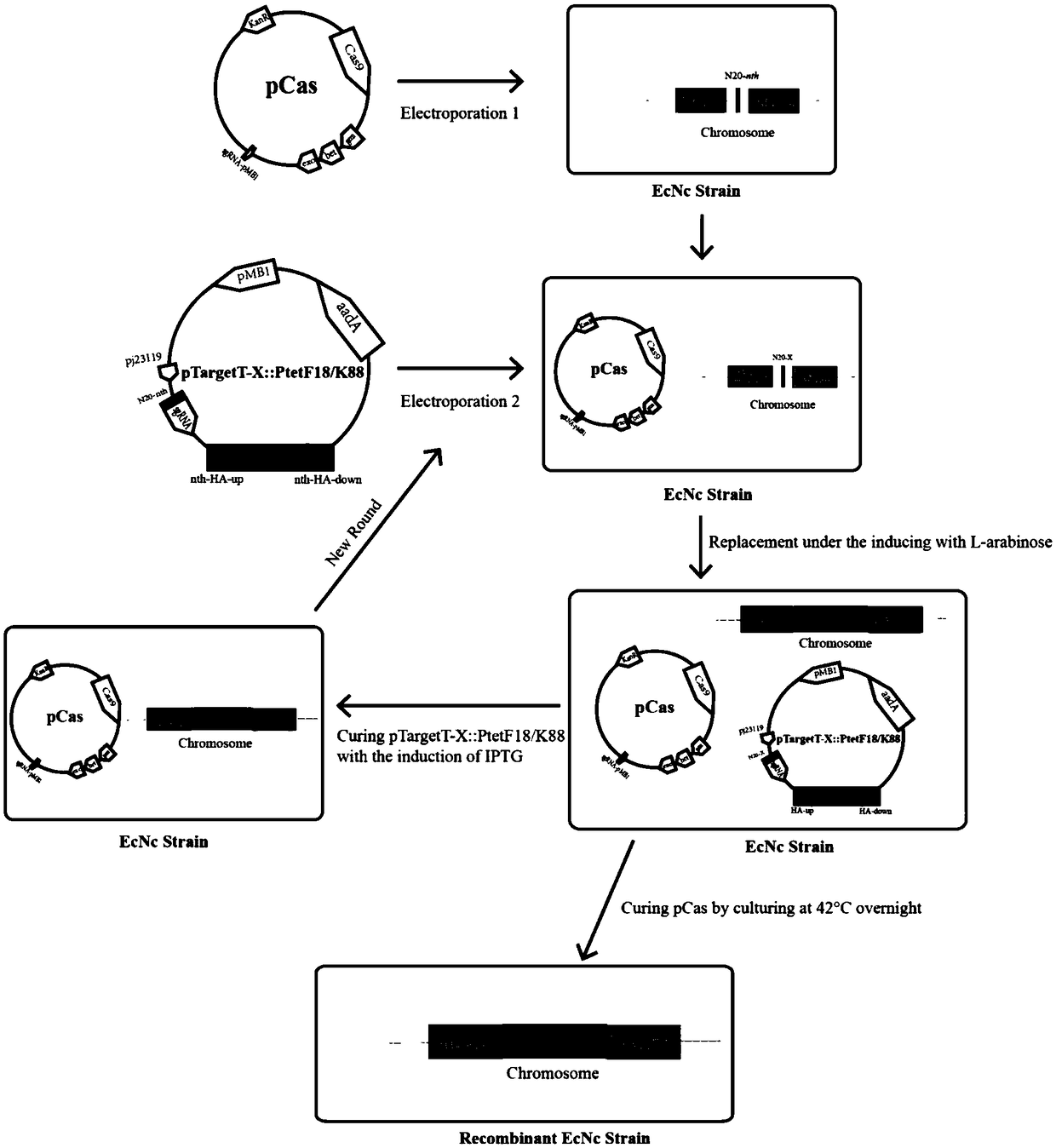
Probiotics cloned strain of integrated single copy functional F4 pili operon gene, construction method and application
ActiveCN109468255AEfficient editingDoes not affect its own biological traitsAntibacterial agentsBacteriaSerum igeMicrobiology
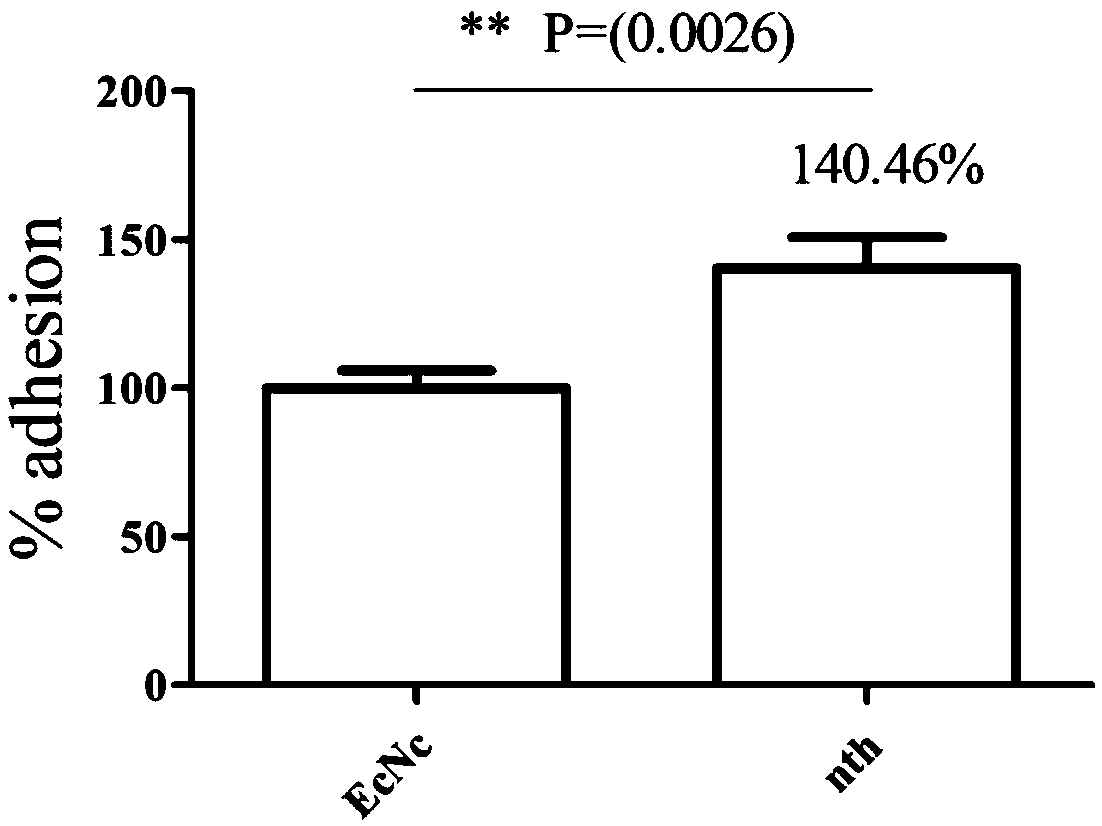
The invention relates to the technical field of biology, in particular to probiotics cloned strain of integrated single copy functional F4 pili operon gene, a construction method and application. Themethod comprises the following steps: constructing pTargetT-nth / tppB::PtetF4 recombinant plasmid; and obtaining nonresistant single copy F4 pili gene integration cloned strain through construction ofthe probiotics cloned strain of the integrated single copy functional F4 pili operon gene. The probiotics cloned strain serves as probiotics live vaccine candidate strain and drug for the treatment ofpost-weaning diarrhea and hydropsy. Compared with the prior art, the probiotics cloned strain has the benefits that Nissle1917 probiotics original plasmid is removed, and resistance gene does not exist; recombinant bacteria can effectively improve the adhesion property to pig intestinal passage epithelial cell; mice subjected to oral immunization can produce immune serum namely anti-F4 pili IgG antibody; and serum of the mice subjected to oral immunization can obviously reduce adhesion to pig intestinal passage cell line by F4+ strain.
Owner:YANGZHOU UNIV
Rabbit coccidiosis vaccine and application thereof
PendingCN108619498ANo pollution in the processWill not polluteProtozoa material medical ingredientsPeptidesEmoia loyaltiensisCoccidiosis vaccine
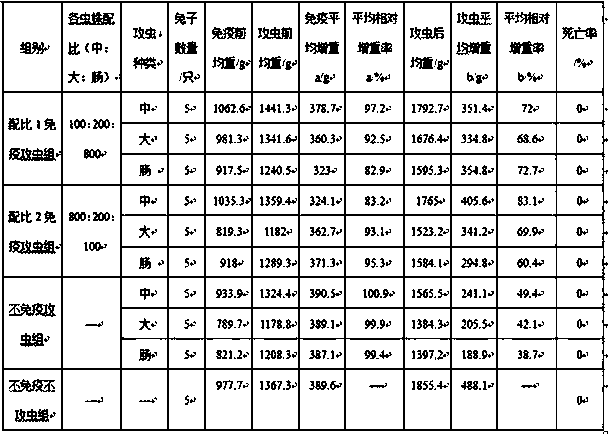
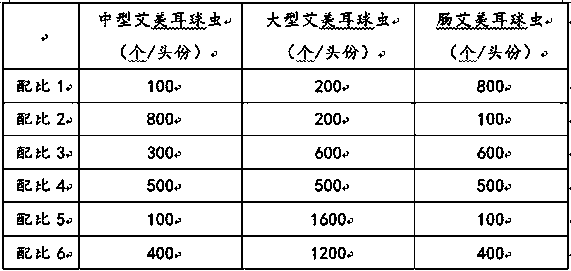
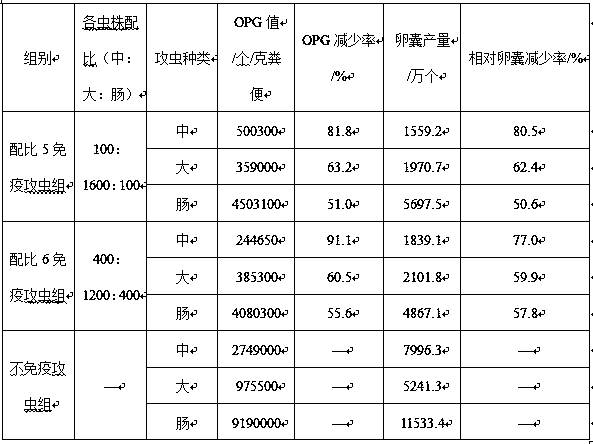
The invention discloses a rabbit coccidiosis vaccine and application thereof. The rabbit coccidiosis vaccine comprises 100-800 pieces of medium eimeria, 200-1600 pieces of large eimeria and 100-800 pieces of eimeria intestinalis. The vaccine is scientific and reasonable in composition, low in cost, free of drug residue after use, drug tolerance and environment pollution, high in immunogenicity andsafe to use; after oral immunization, rabbits can effectively resist infection of 1x105 medium eimeria, 5x104 large eimeria and 3x103 eimeria intestinalis, and the vaccine can be applied in preparingdrug preparation for preventing rabbit coccidiosis.
Owner:FOSHAN STANDARD BIO TECH
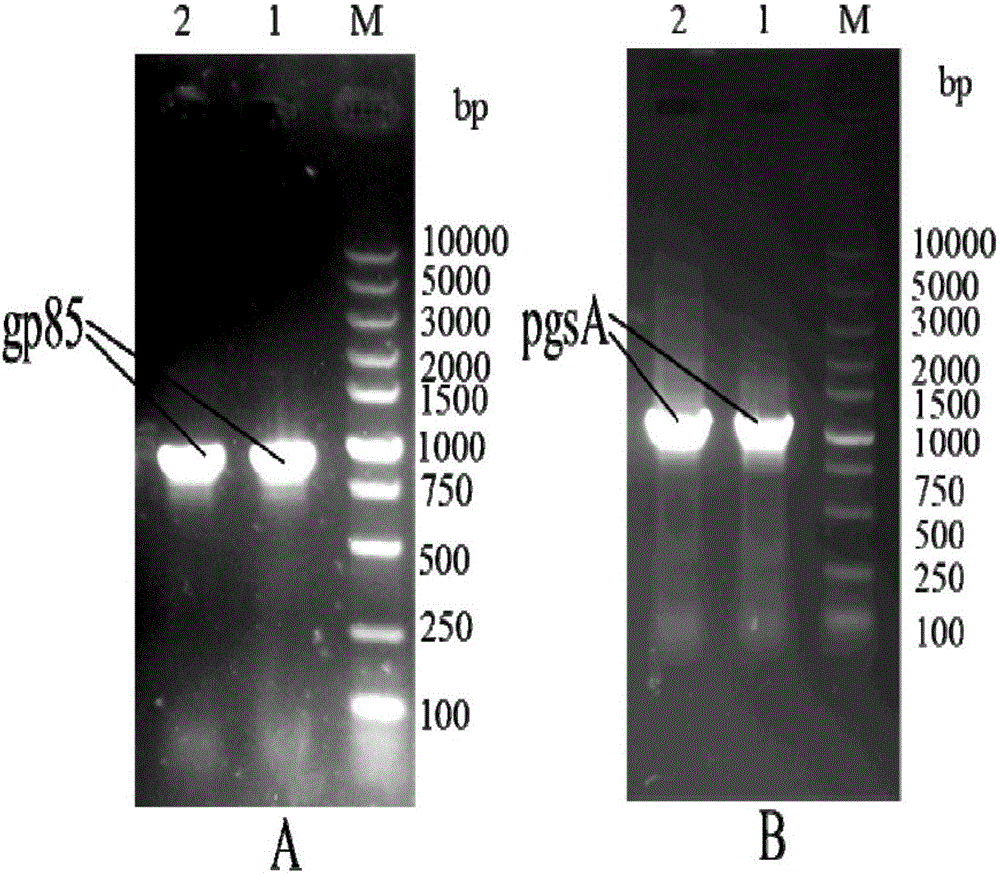
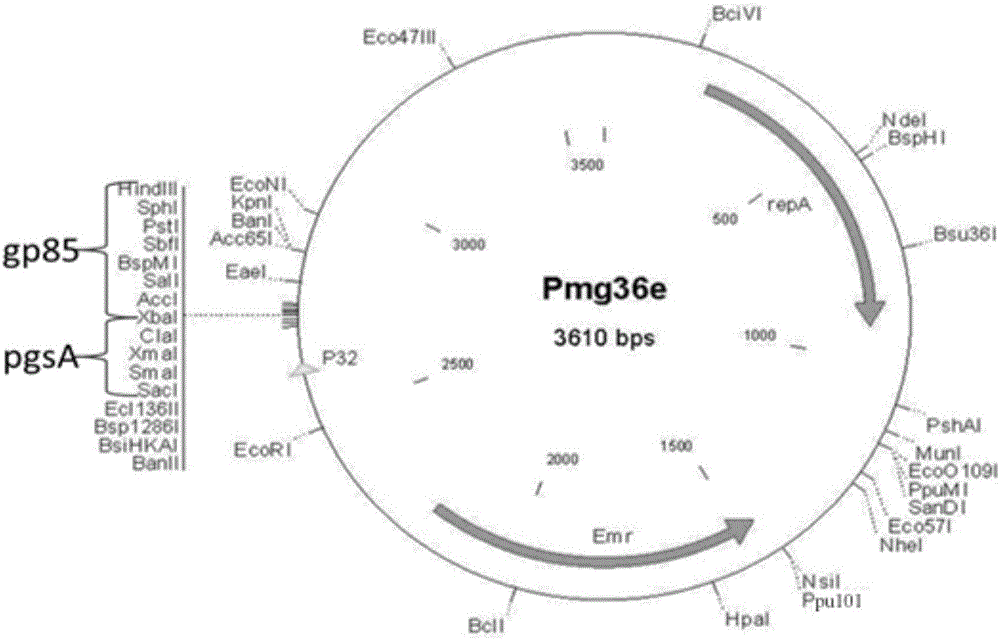
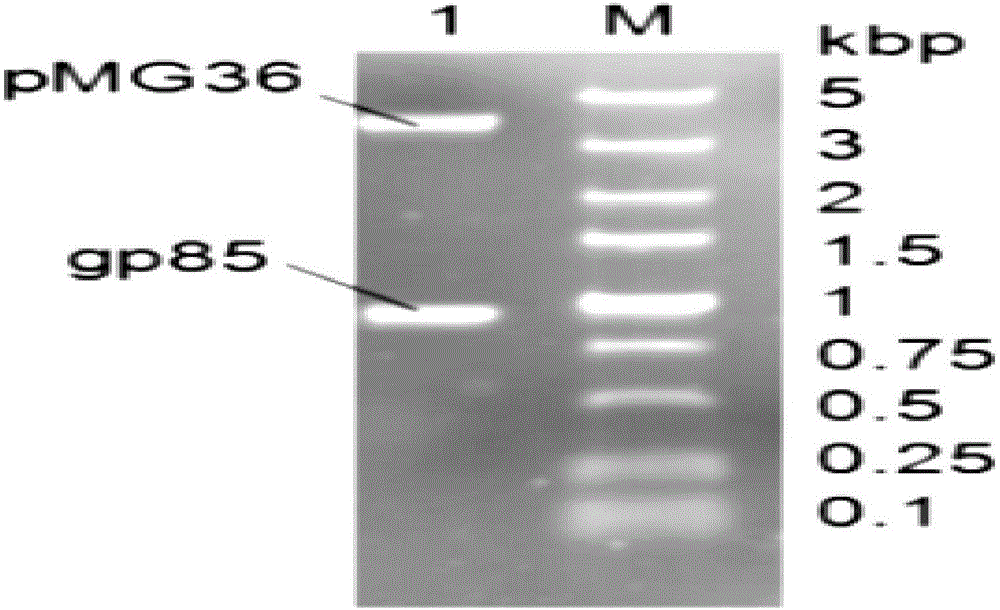
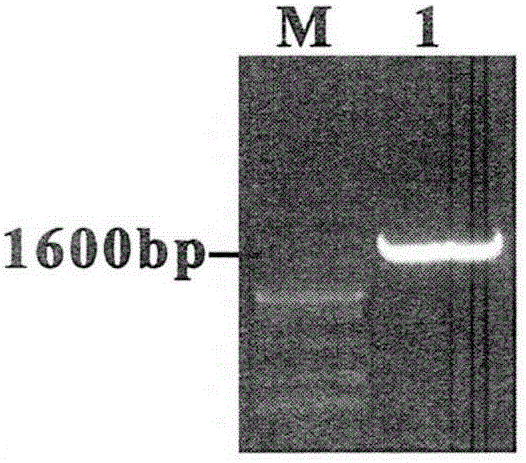
pMG36e pgsA gp85 recombinant plasmid-containing genetically engineered bacterium
InactiveCN107523531AIncrease daily weight gainImprove the ability to resist ALV-J infectionBacteriaViral antigen ingredientsEscherichia coliAvian leukosis viruses
The invention provides a pMG36e pgsA gp85 recombinant plasmid-containing genetically engineered bacterium. A lactobacillus plantarum engineering bacterial strain which can express fusion protein pgsA gp85 is constructed by inserting a gene gp85 and a gene pgsa into Escherichia coli-lactobacillus shuttle type expression vector pMG36e to obtain a recombinant vector pMG36e-pgsA gp85, and then electrically converting the recombinant vector pMG36e-pgsA gp85 into the lactobacillus plantarum engineering bacterial strain, wherein the bacterial strain can express to obtain corresponding pgsA gp85 fusion protein; the fusion protein can be widely applied to oral immunization, the average daily weight gain of chickens in each period can be greatly improved, and moreover, the resistance of the chickens to ALV-J (Avian Leukosis Virus subgroup) infection can be improved, so that the culture benefit is beneficially increased, and the ALV-J infecting risk of chicken bodies is beneficially reduced.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Recombinational bacillus subtilis of expressing highly pathogenic avian influenza H5N1 hemagglutinin HA protein
ActiveCN106434728AImprove developmentBoosts specific immune response levelsSsRNA viruses negative-senseBacteriaHemagglutininWestern blot
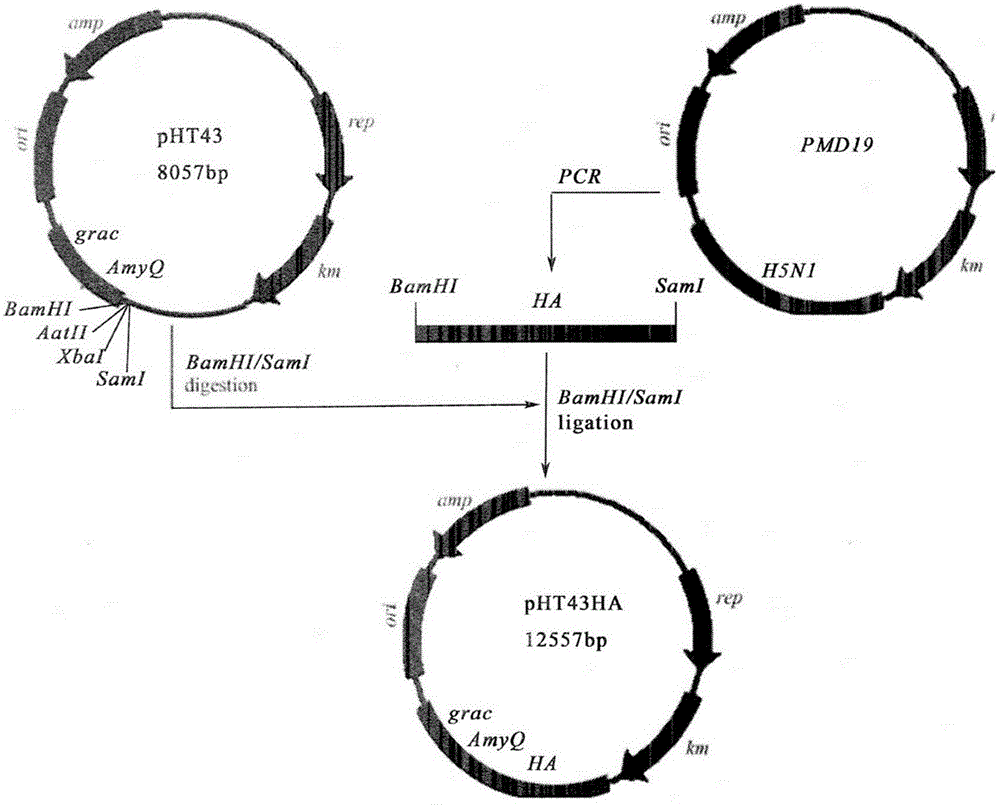
The invention relates to recombinational bacillus subtilis(B.S.-HA) of expressing highly pathogenic avian influenza(AIV) HSN1 hemagglutinin HA protein, and belongs to the field of biotechnology genetic engineering. The recombinational bacillus subtilis(B.S.-HA) of expressing the highly pathogenic avian influenza(AIV) HSN1 hemagglutinin HA protein successfully establishes recombinational bacillus subtilis expression plasmid pHT43 of the highly pathogenic avian influenza hemagglutinin HA protein, and successfully conducts elec-transformation of the recombinational bacillus subtilis expression plasmid pHT43 of the highly pathogenic avian influenza hemagglutinin HA protein into bacillus subtilis WB800N bacterial strain (B.S.). As verified by Western-blot, the highly pathogenic avian influenza hemagglutinin HA protein can be successfully expressed in the recombinational bacillus subtilis. Oral immunization can stimulate chicken body of 10-day old chicken to generate an effective AIV specific immune response level. The recombinational bacillus subtilis(B.S.-HA) is hopeful to be developed to genetic engineering oral vaccine in preventing the highly pathogenic avian influenza.
Owner:NANJING AGRICULTURAL UNIVERSITY
Probiotics cloned strain of integrated four-cope F18 pili operon gene and two-copy F4 pili operon gene and construction method
ActiveCN109468256AImprove adhesionReduce adhesionAntibacterial agentsBacteriaBiotechnologyAntiendomysial antibodies
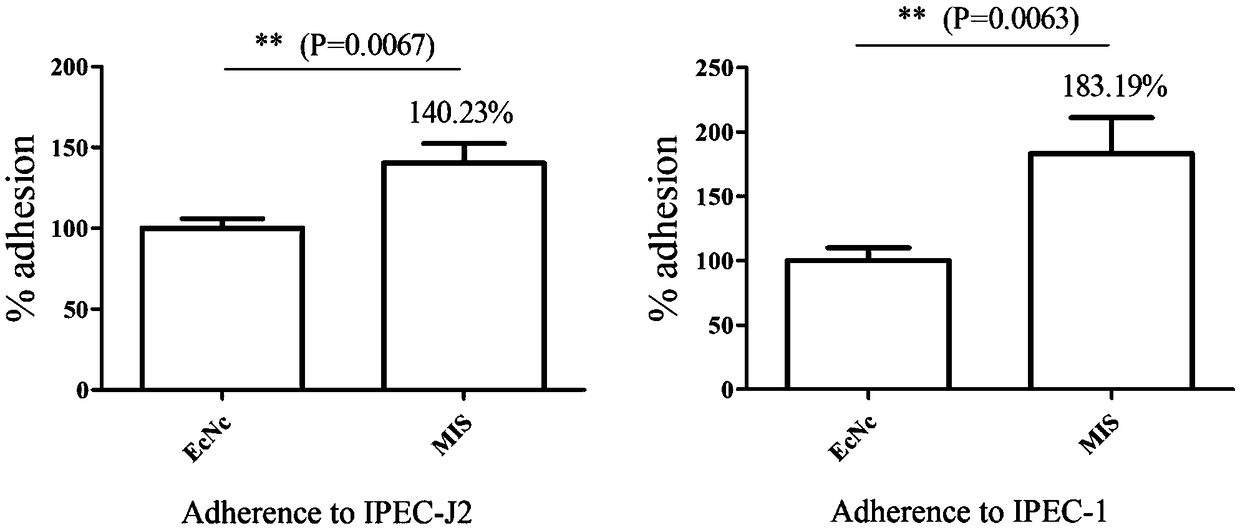
The invention relates to the technical field of biology, in particular to probiotics cloned strain of integrated four-cope F18 pili operon gene and two-copy F4 pili operon gene and a construction method. The method comprises the following steps: constructing pTargetT-X::PtetF18 and pTargetT-X::PtetF4 recombinant plasmid; and obtaining nonresistant four-cope F18 pili gene and two-copy F4 pili geneintegration cloned strain through construction of the probiotics cloned strain of the four-copy functional F18 pili operon gene and the two-copy F4 pili operon gene. The probiotics cloned strain has the benefits that recombinant bacteria can effectively improve the adhesion property to pig intestinal passage epithelial cell; mice subjected to oral immunization can produce immune serum, namely anti-F18 pili IgG antibody and anti-F4 pili IgG antibody; and serum of the mice subjected to oral immunization can obviously reduce adhesion to pig intestinal passage cell line IPEC-J2 by F18+ strain andF4+ strain.
Owner:YANGZHOU UNIV
Flavobacterium columnare transgenic engineering oral vaccine and using method and application thereof
InactiveCN110755605AEasy to useLabor savingAntibacterial agentsBacterial antigen ingredientsProtective antigenShuttle plasmid
The invention relates to a flavobacterium columnare transgenic engineering oral vaccine. Preparation steps are as follows: (1) analysis of a mature peptide fragment sequence of a protective antigenicgene, namely lip, of flavobacterium columnare; (2) construction of shuttle plasmids; (3) screening of recombinant strains; (4) culture of the recombinant strains; and (5) preparation of the oral vaccine. The vaccine is an oral vaccine and is inoculated in an oral immunization mode, the oral vaccine is convenient to use compared with injection immunization and immersion immunization vaccines, morelabor force is saved, and the flavobacterium columnare transgenic engineering oral vaccine has the advantage of being not limited by the size of fish bodies.
Owner:天津市水产研究所
Porcine parvovirus oral vaccine composition, preparation method and application thereof
PendingCN108096573AImproving immunogenicityHigh titerDigestive systemAntiviralsImmunogenicityPorcine parvovirus
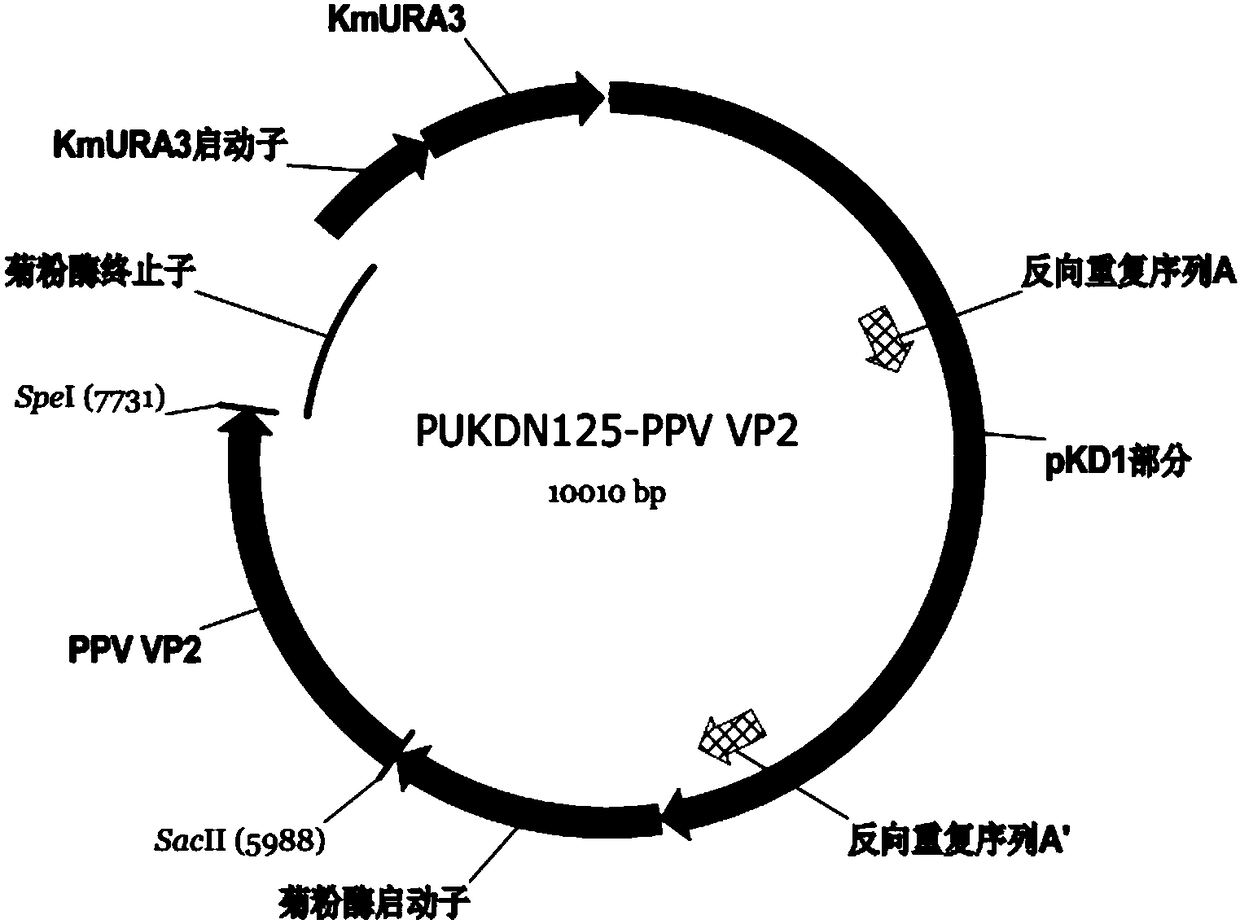
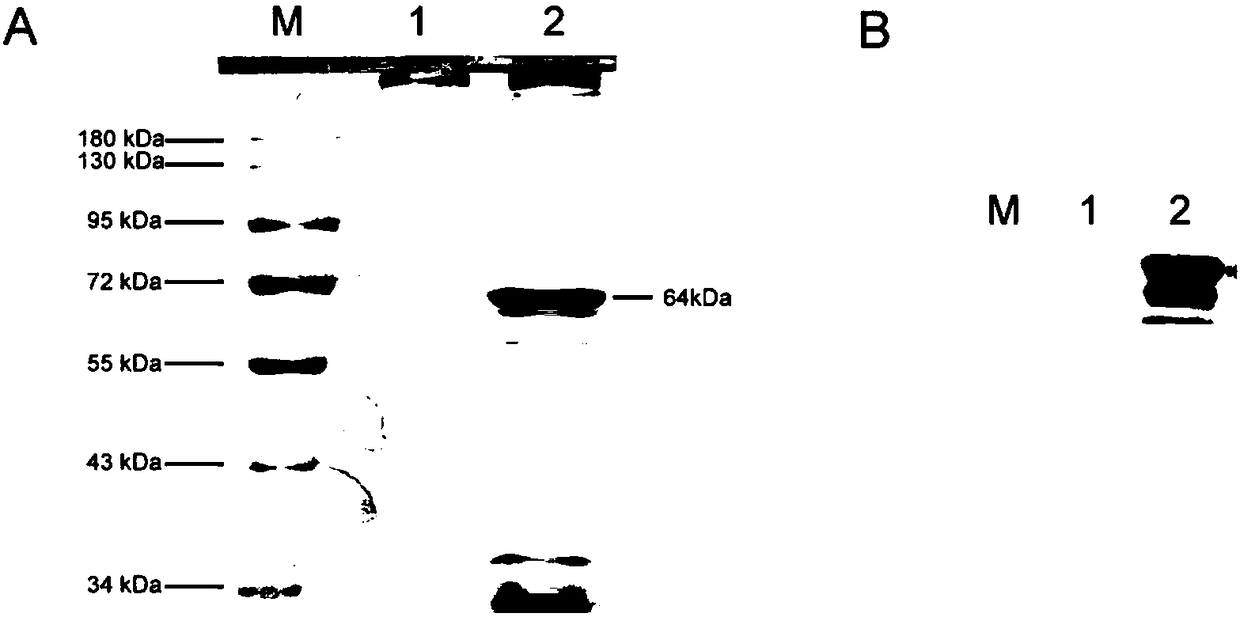
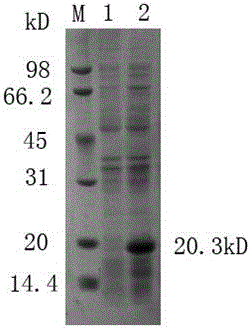
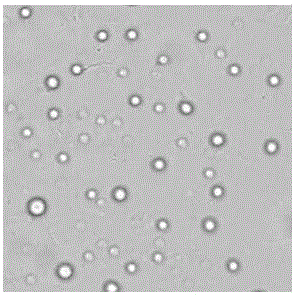
The invention discloses a porcine parvovirus oral vaccine composition which includes the components a) and b), is basically composed of the a) and b), or is composed of the a) and b), wherein the component a) is viroid particles formed by self-assembling porcine parvovirus VP2 protein expressed by Kluyveromyces marxianus recombinant engineering bacteria; b) pharmaceutically or veterinarily acceptably auxiliary material for oral-taking preparations. The invention also discloses a preparation method and an application of the porcine parvovirus oral vaccine composition. The porcine parvovirus oral vaccine composition has excellent immunogenicity. An orally immunized mouse can generate protective antibodies which have high titer. The oral vaccine composition has high safety and low productioncost, can be produced to an amplified scale and has simple operation, and can be used for preventing and alleviating clinical symptoms related to PPV infection.
Owner:FUDAN UNIV
Haemophilus parasuis subunit vaccine and preparation method thereof
InactiveCN106177934AEnsure safetyNo side effectsAntibacterial agentsAntibody medical ingredientsDiseaseHaemophilus
The invention discloses a subunit vaccine for preventing Glasser's disease and a preparation method thereof. According to a prokaryotic expression method, an outer membrane protein Omp16 with immunogenicity is expressed by cloning a haemophilus parasuis (Haemophilus parasuis, H. parasuis) strain (culture collection number: CGMCC NO. 11145, patent application number for invention: CN105524857A). The haemophilus parasuis subunit microsphere vaccine is prepared by taking the protein Omp16 as an antigen and coating the protein Omp16 by taking chitosan, olive oil and sodium alga acid as materials. For mice subjected to injection and oral immunization by using the vaccine, the immune protective rates of H. parasuis serum type 5 attack are respectively 80% and 60%.
Owner:SOUTH CHINA AGRI UNIV +1
Oral immunology using plant product containing a non-enteric pathogen antigen
InactiveUS7572466B1Lower immune responseEnhance immune responseBiocideVirus peptidesAdjuvantHepatitis B Surface Antigens
A method for obtaining an immune response to a non-enteric pathogen antigen (NEPA) such as hepatitis B surface antigen (HBsAg) by feeding the antigen in a plant material to an animal that is immunoreceptive to the NEPA. It has now been discovered that the animal may be made immunoreceptive to the NEPA such as HBsAg by administering the plant material containing the NEPA in conjunction with a suitable adjuvant. The plant material is a substance comprising a physiologically acceptable plant material, especially potatoes, containing the NEPA, e.g. hepatitis B surface antigen (HBsAg). The NEPA, e.g. HBsAg in the plant results from expression by the plant of the NEPA due to genetic alteration.
Owner:HEALTH RES INC +1
Porcine epidemic diarrhea virus genetic engineering subunit oral vaccine
InactiveCN102989010AAvoid stressAvoid absorptionGenetic material ingredientsAntiviralsLocal immunityInfectious Disorder
The invention discloses a porcine epidemic diarrhea virus genetic engineering subunit oral vaccine which is collected in China Center for Type Culture Collection (CCTCC) and the collection number thereof is lactococcus lactis MG1363 / pMG36e-M CCTCC M 2012354. The oral immunization has the advantage of effectively stimulating local immune cells of the intestinal tract to generate secretion type IgA, is especially applicable to the infectious disease of the intestinal mucosa, and avoids stress and vaccine absorption problems resulting from conventional vaccine injection; and as a safe and non-toxic live vector system capable of living on the intestinal mucosa, the lactococcus lactis is the most suitable vector system. The oral vaccine performs antigen presentation through the gastrointestinal mucosa, and produces immune reaction similar to conventional injection through different immune pathways.
Owner:SHANDONG SINDER TECH
Medicament for improving efficacy of oral helicobacter pylori vaccine and use thereof
The invention discloses a medicament for improving efficacy of an oral helicobacter pylori vaccine and use thereof, and belongs to the technical field of vaccine development. The oral helicobacter pylori vaccine comprises an A1 protein, sodium chloride, sodium carbonate, sodium bicarbonate, glycerol and water. When a proton pump inhibitor is used before the oral helicobacter pylori vaccine is used, a pH value of gastric juice of a mouse can be neutral, and the helicobacter pylori vaccine can be effectively protected from being damaged by gastric acid. The medicament breaks through a bottleneck that a positive conversion rate of oral immunization saliva titer detection is zero and lays a foundation for the helicobacter pylori vaccine to exert an immune effect in intestinal tracts.
Owner:CHENGDU OLYMVAX BIOPHARM
Helicobacter pylori HefC recombinant protein and application thereof
ActiveCN113150086AResist colonizationEasy to purifyAntibacterial agentsBacterial antigen ingredientsMucosal adjuvantMicrobiology
The invention discloses a helicobacter pylori HefC recombinant protein and application thereof, and belongs to the technical field of genetic engineering. The invention discloses the application of the helicobacter pylori HefC recombinant protein in preparation of a helicobacter pylori vaccine. The helicobacter pylori HefC recombinant protein is cloned and expressed by adopting a genetic engineering technology, the supernatant expression quantity is high, the separation and purification steps are simple and convenient, the immune titer is high, and the helicobacter pylori HefC recombinant protein has protectiveness. The HefC recombinant protein can be directly matched with a mucosal adjuvant LT(B)5 for use, and is suitable for oral immunization.
Owner:CHENGDU OLYMVAX BIOPHARM
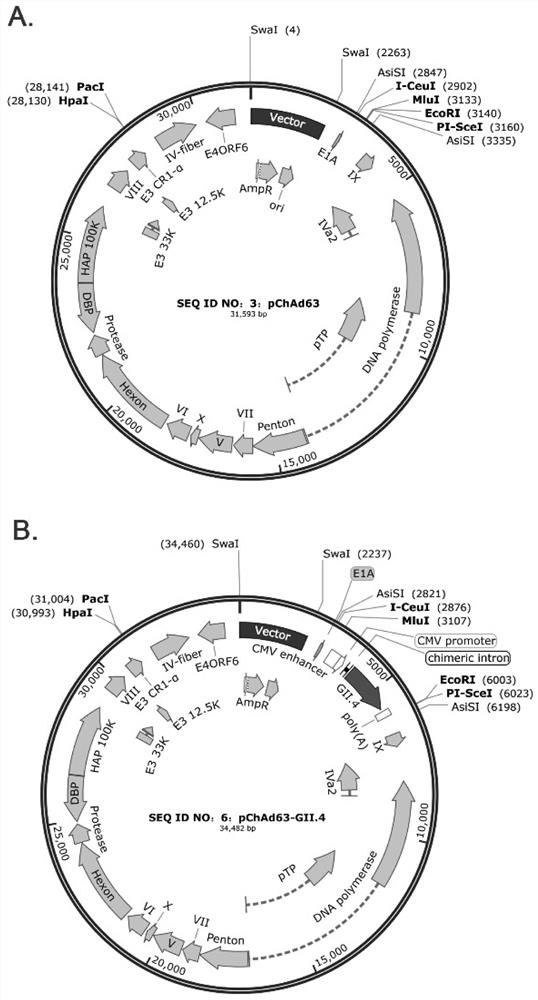
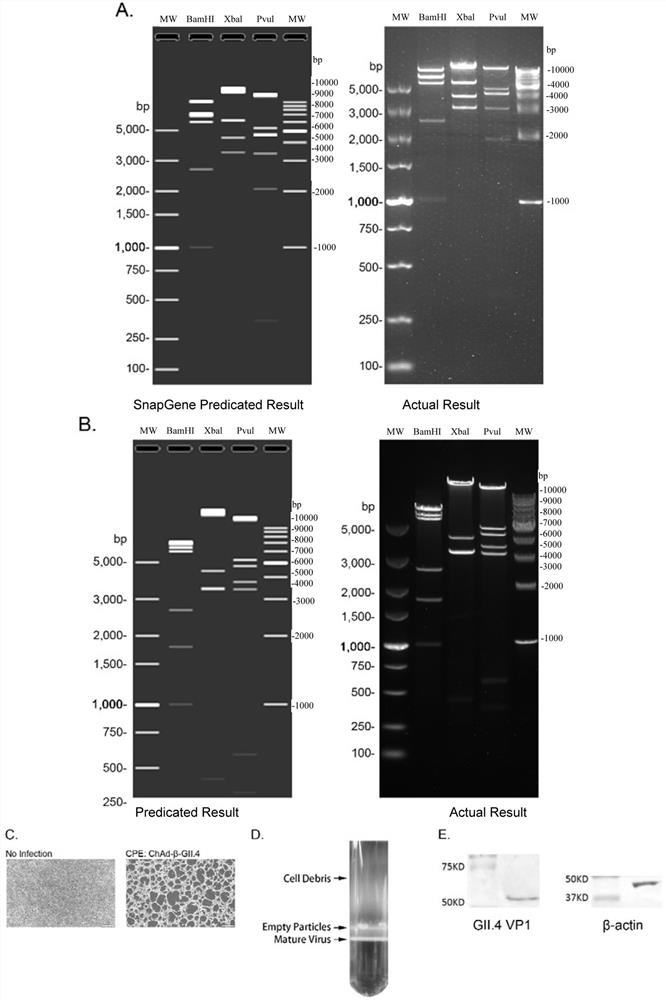
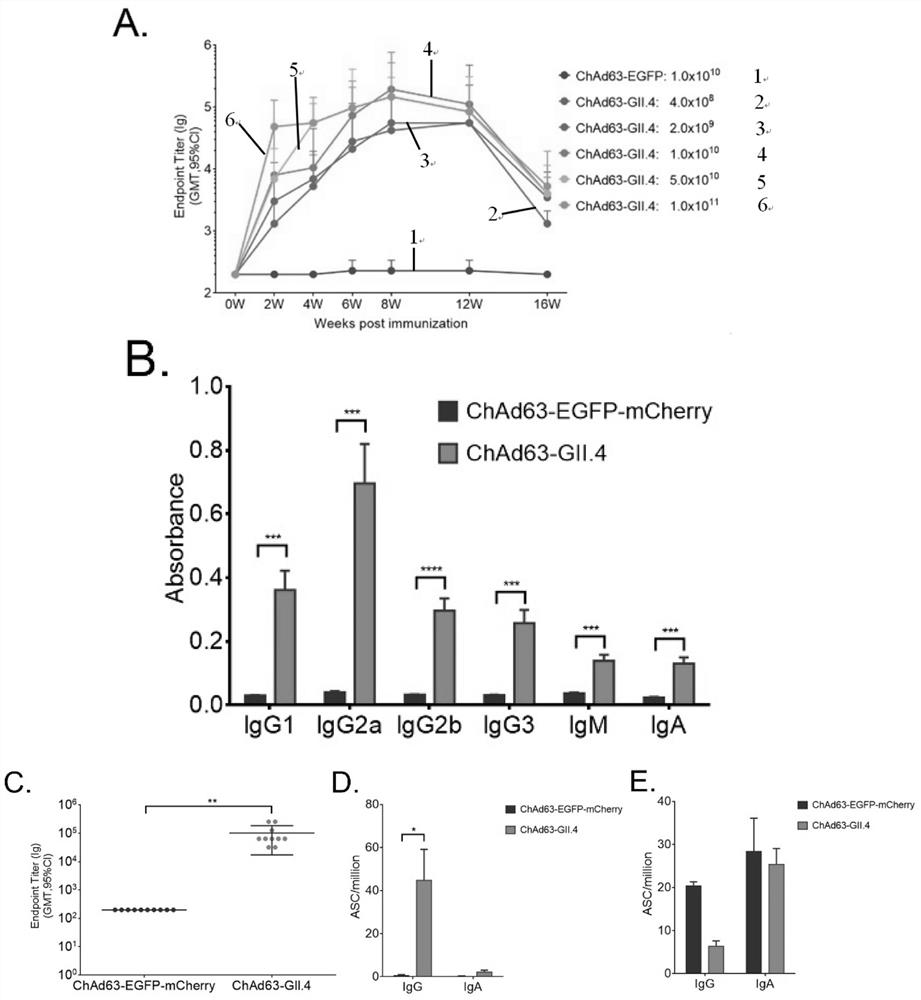
Norovirus vaccine based on chimpanzee adenovirus vector and preparation method and application thereof
ActiveCN112156178AEasy to makeImprove securitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantNucleotide
The invention discloses a norovirus vaccine based on a chimpanzee adenovirus vector and a preparation method and application thereof. The norovirus vaccine is prepared by the following steps that a nucleotide sequence of a norovirus target protein is inserted into a recombinant chimpanzee adenovirus vector plasmid to construct a recombinant plasmid, and then packaging and processing are carried out to obtain the norovirus recombinant adenovirus vaccine with immunogenicity. According to the norovirus vaccine, the norovirus vaccine is developed for the first time based on the chimpanzee adenovirus vector, and the vaccine cannot be influenced by a pre-stored adenovirus antibody in a human body; the adenovirus vector vaccine is simple to prepare and good in safety, an adjuvant does not need tobe added, and immunization routes such as muscle immunization or oral immunization can be adopted; and the norovirus preventive vaccine is prepared on the basis of the replication-defective chimpanzee adenovirus vector, and a vaccine product which is easy to prepare, low in cost, good in safety, free of adjuvant and wide in application prospect is expected to be obtained.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
Preparation method of porcine epidemic diarrhea virus genetic engineering subunit oral vaccine
ActiveCN102988971BAvoid stressAvoid absorptionAntibody medical ingredientsStaphylococcus lactisLocal immunity
The invention discloses a preparation method of a porcine epidemic diarrhea virus genetic engineering subunit oral vaccine. The method comprises the following steps of: 1) synthesizing an M gene; 2) constructing an expression vector; 3) performing protein induction expression; 4) carrying out recombinant strain preservation experiment; and 5) preparing the vaccine. The oral immunization has the advantage of effectively stimulating local immune cells of the intestinal tract to generate secretion type IgA, is especially applicable to the infectious disease of the intestinal mucosa, and avoids stress and vaccine absorption problems resulting from conventional vaccine injection; and as a safe and non-toxic live vector system capable of living on the intestinal mucosa, the lactococcus lactis is the most suitable vector system. The oral vaccine performs antigen presentation through the gastrointestinal mucosa, and produces immune reaction similar to conventional injection through different immune pathways.
Owner:北京信得威特科技有限公司
Animal oral immunization vaccine vector and preparation method thereof
InactiveCN108837155AReduce labor intensityAvoid direct contactPharmaceutical non-active ingredientsImmunological disordersBiotechnologyNutrition
The invention discloses a method for preparing an animal oral immunization vaccine vector. The preparation method comprises the following steps: taking green vegetables as a vaccine vector, and injecting vaccine liquid in a first vaccine dose on the vaccine vector; sterilizing the vaccine vector, naturally airing, refrigerating and preserving, thereby obtaining the product. The animal oral immunization vaccine vector prepared according to the preparation method disclosed in the invention is prepared by enabling the green vegetables that are often fed to animals to carry the immunized vaccine liquid. Therefore, when the green vegetables are broken by teeth of the animals, the stem chambers containing the vaccine liquid are broken in animal mouths, the vaccine liquid flows out, the vaccine liquid is in sufficient contact with the animal mouths by virtue of repeated chewing of the animals, and the expected mucosal immunity is achieved. After the residues of the green vegetables are directly eaten, poisoning to the outside is avoided, and nutrition needed by the animals is supplemented.
Owner:灵格技术有限公司
A kind of Haemophilus parasuis subunit vaccine and its preparation method
InactiveCN106177934BImproving immunogenicityEnsure safetyAntibacterial agentsAntibody medical ingredientsDiseaseSerotype
The invention discloses a subunit vaccine for preventing Glasser's disease and a preparation method thereof. According to a prokaryotic expression method, an outer membrane protein Omp16 with immunogenicity is expressed by cloning a haemophilus parasuis (Haemophilus parasuis, H. parasuis) strain (culture collection number: CGMCC NO. 11145, patent application number for invention: CN105524857A). The haemophilus parasuis subunit microsphere vaccine is prepared by taking the protein Omp16 as an antigen and coating the protein Omp16 by taking chitosan, olive oil and sodium alga acid as materials. For mice subjected to injection and oral immunization by using the vaccine, the immune protective rates of H. parasuis serum type 5 attack are respectively 80% and 60%.
Owner:SOUTH CHINA AGRI UNIV +1
Bivalent nucleic acid vaccine against bronchitis and preparation method and application thereof
InactiveCN111053899AIncrease production capacityReduce purificationSsRNA viruses positive-senseViral antigen ingredientsS typhimuriumPeptide expression
The invention provides a bivalent nucleic acid vaccine against bronchitis and a preparation method and application thereof. According to the invention, the core expression region of the bivalent nucleic acid vaccine is only 1491 bp; a secretory signal peptide is introduced before an expression sequence; a Kozak sequence is introduced into the expression sequence; plasmids containing avian promotersequences are selected; hydrophobic regions which are not easy to become antigens are removed; M41 and H120 characteristic proteins are spliced, and a linker sequence is arranged between and connected with the M41 and H120 characteristic proteins, so generation of neutralizing antibodies aiming at the two toxic strains can be stimulated, and the defect of poor crossover of immunity to infectiousbronchitis viruses is overcome; Salmonella typhimurium is selected as a vector, and Salmonella typhimurium has no hidden infection dangers to a host, but can support long-term expression of nucleic acid vaccines to achieve a long-term immune effect; and an oral immunization mode is adopted for the vaccine, and the vaccine can be added into a feed, and is convenient to use and superior to a nasal dripping or injection immunization mode. During preparation of the vaccine, a culture collected after fermentation is directly used as a vaccine, so the steps of protein purification, endotoxin removaland the like are reduced, cost is saved, and large-scale production is facilitated.
Owner:徐晓芳
Application of immunological stimulant compound in preparing fish immunity preparation
InactiveCN100569285CImprove the ability of proliferation and transformationHigh antibody titerImmunological disordersAntibody medical ingredientsImmunologic preparationImmune complex deposition
The invention discloses an application of an immunostimulating compound in preparing fish immune preparation, and the immune preparation is an oral immune preparation or a bath immune preparation. The advantage of the present invention is that: the oral immune preparation or bath immune preparation made of the immune stimulating compound ISCOMs can not only increase the antibody level, but also improve the proliferation and transformation ability of T lymphocytes and induce immune protection. When immunized with the same dose, the antibody titer of ISCOMs group was significantly higher than that of no adjuvant group and ISM1312 adjuvant group. Similarly, oral immunization with ISCOMs can not only increase the antibody level, but also improve the proliferation and transformation ability of T lymphocytes and induce immune protection.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI +3
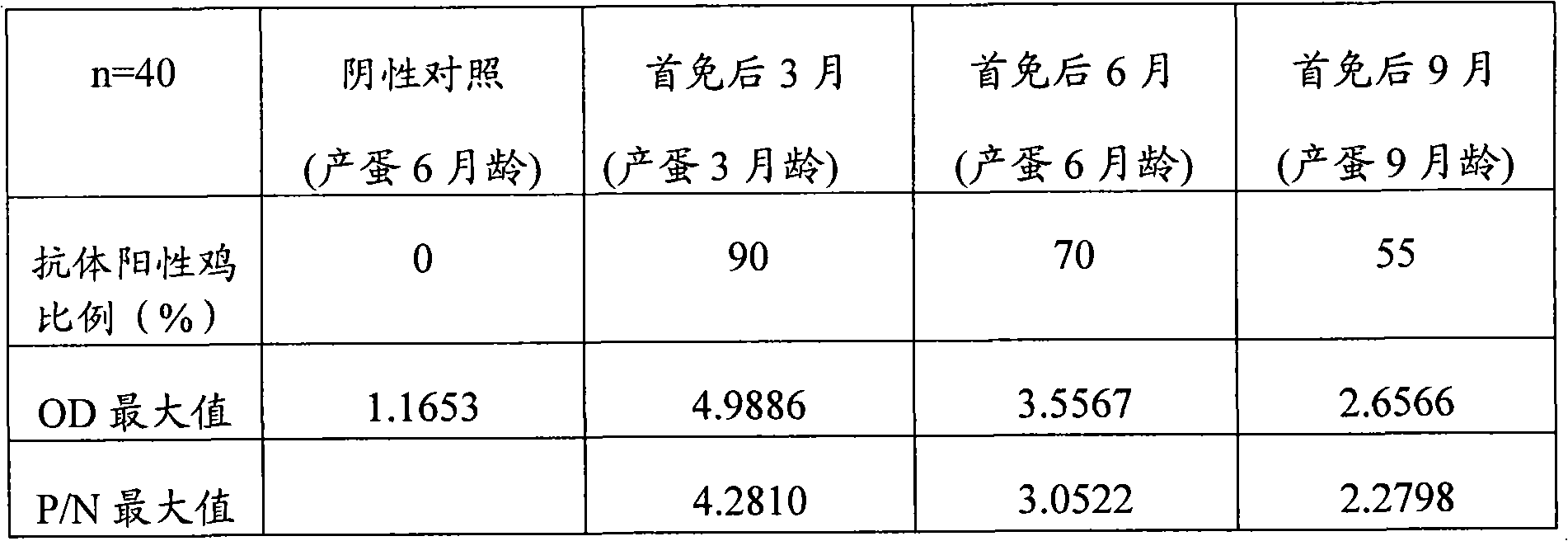
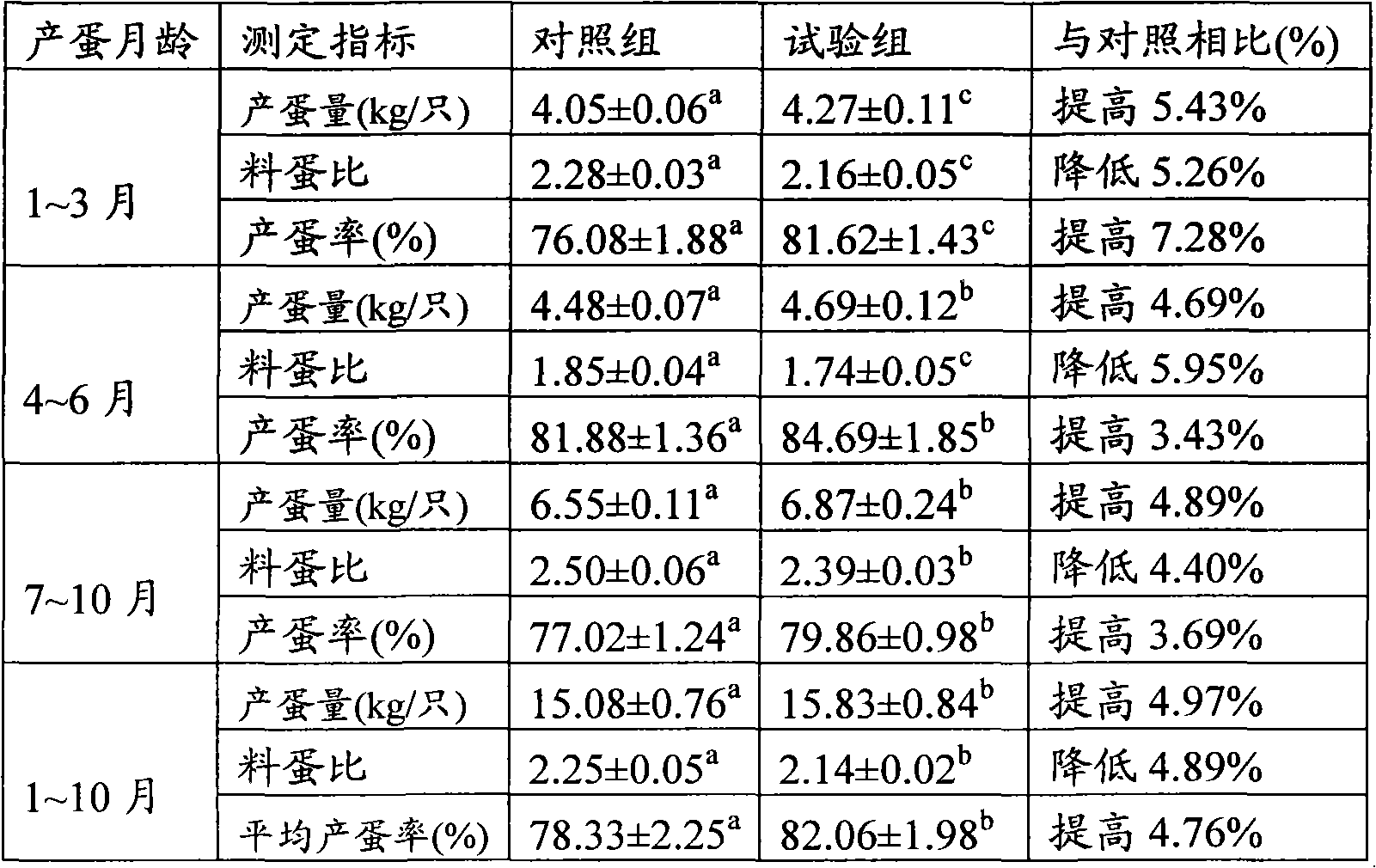
Oral transformant for immunizing and blocking chicken inhibin and application thereof
ActiveCN101921726AImprove securityEnhance immune responseBacteriaBacteria material medical ingredientsInhibin AlphaCell wall
The invention discloses a transformant which comprises a recipient bacterium and a recombinant vector inversed into the recipient bacterium, wherein the recipient bacterium is lactococcus lactis NZ9800, and the original vector of the recombinant vector is pNZ8112; and exogenous target genes of the recombinant vector comprise a chicken inhibin alpha sub-gene, a cholera toxin B sub-gene and a cell wall tie stator M6 gene. The transformant can be fed into chicken intestinal canals in an oral mode, and compared with injection immunization, the invention reduces the toxicity caused by directly contacting the circulation system and increases the safety of vaccines. In the invention, the combined immunization of antigen genes / immunization enhancement factors is used, thereby the organism immune response is enhanced, and the immunization effect is improved.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com