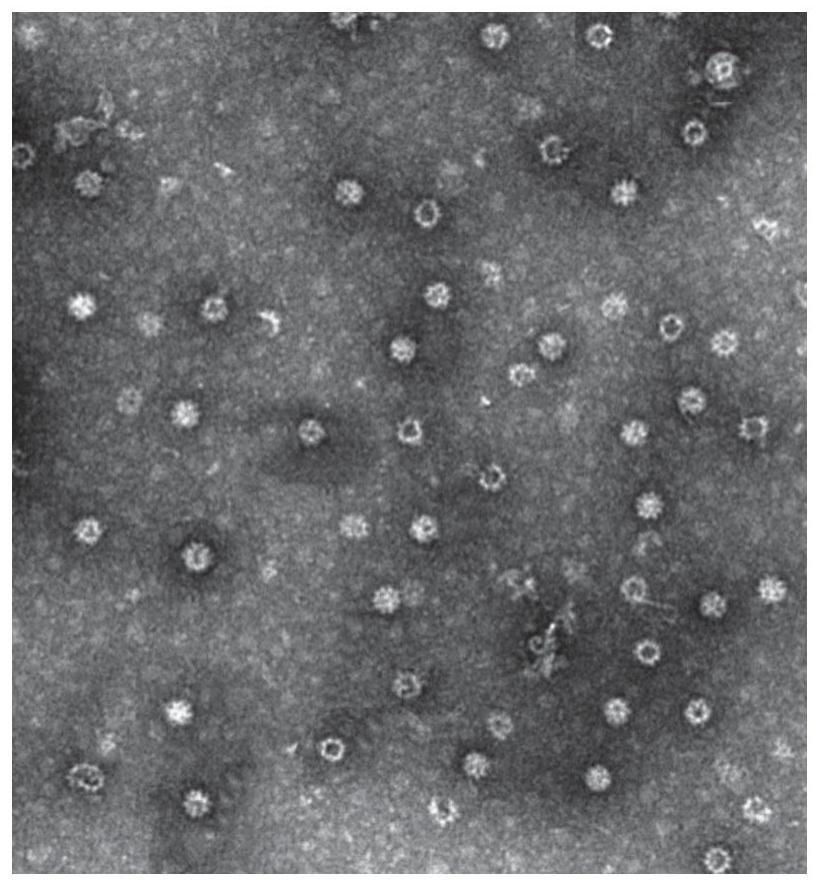
Patents
Literature
246 results about "Post immunization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
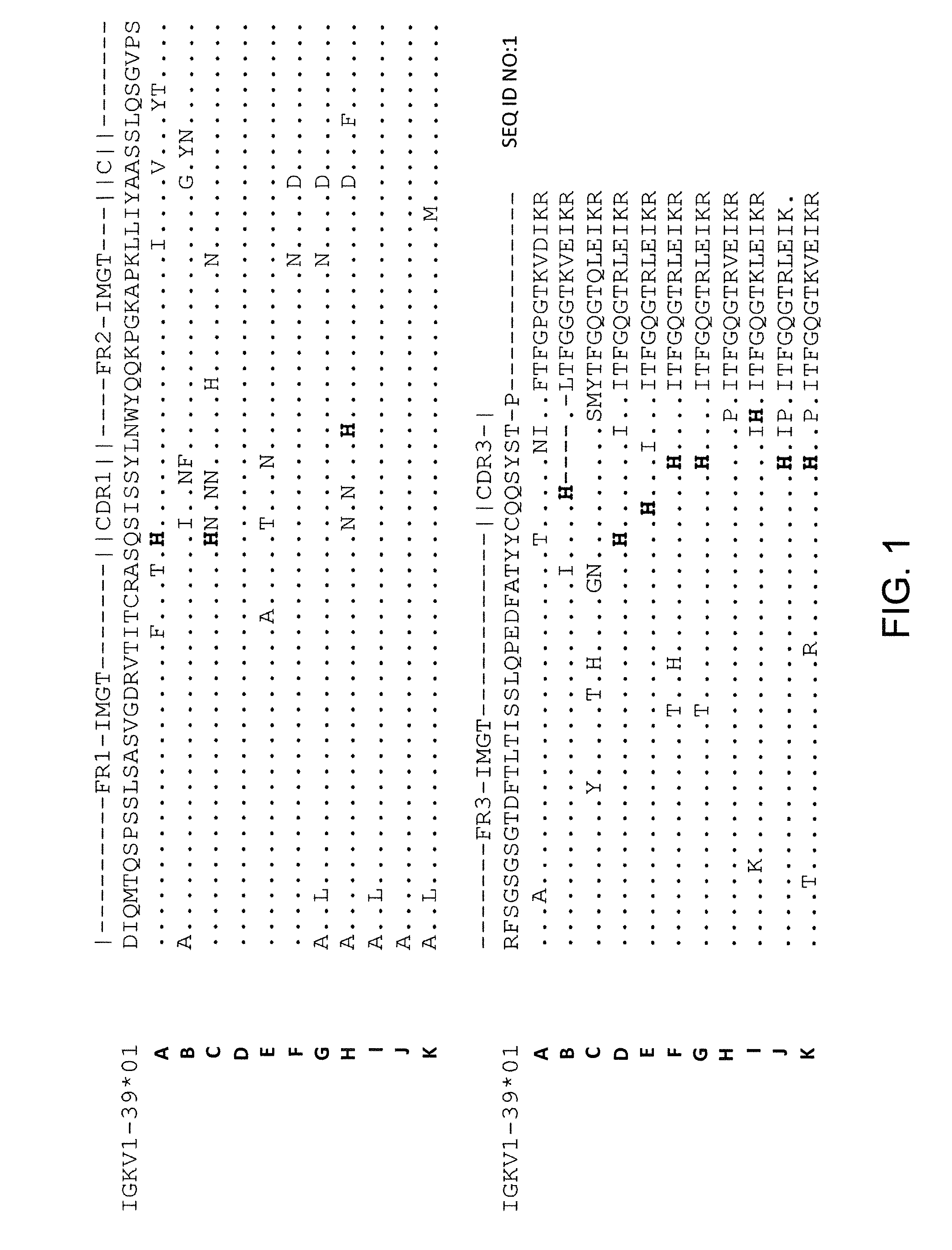
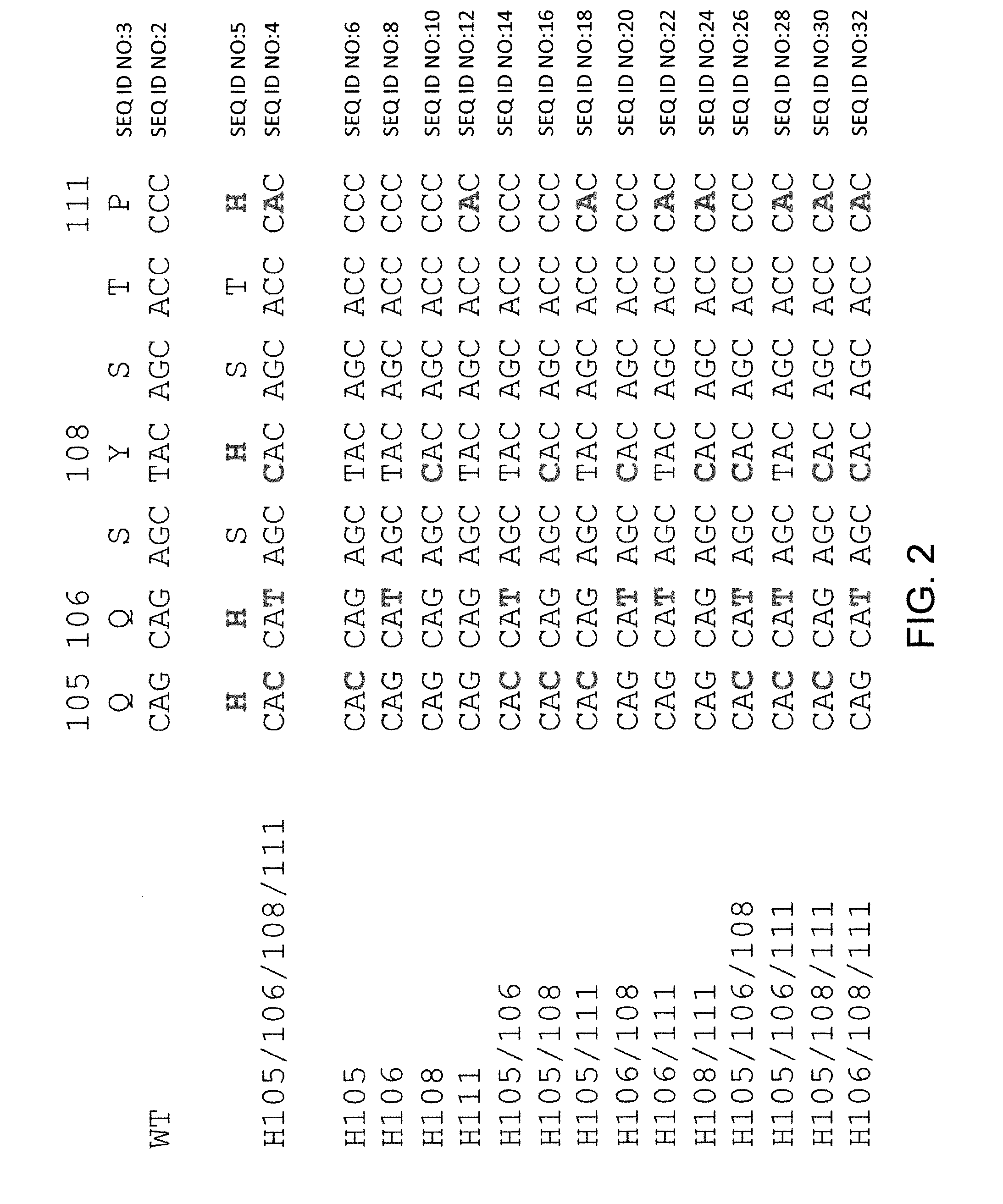
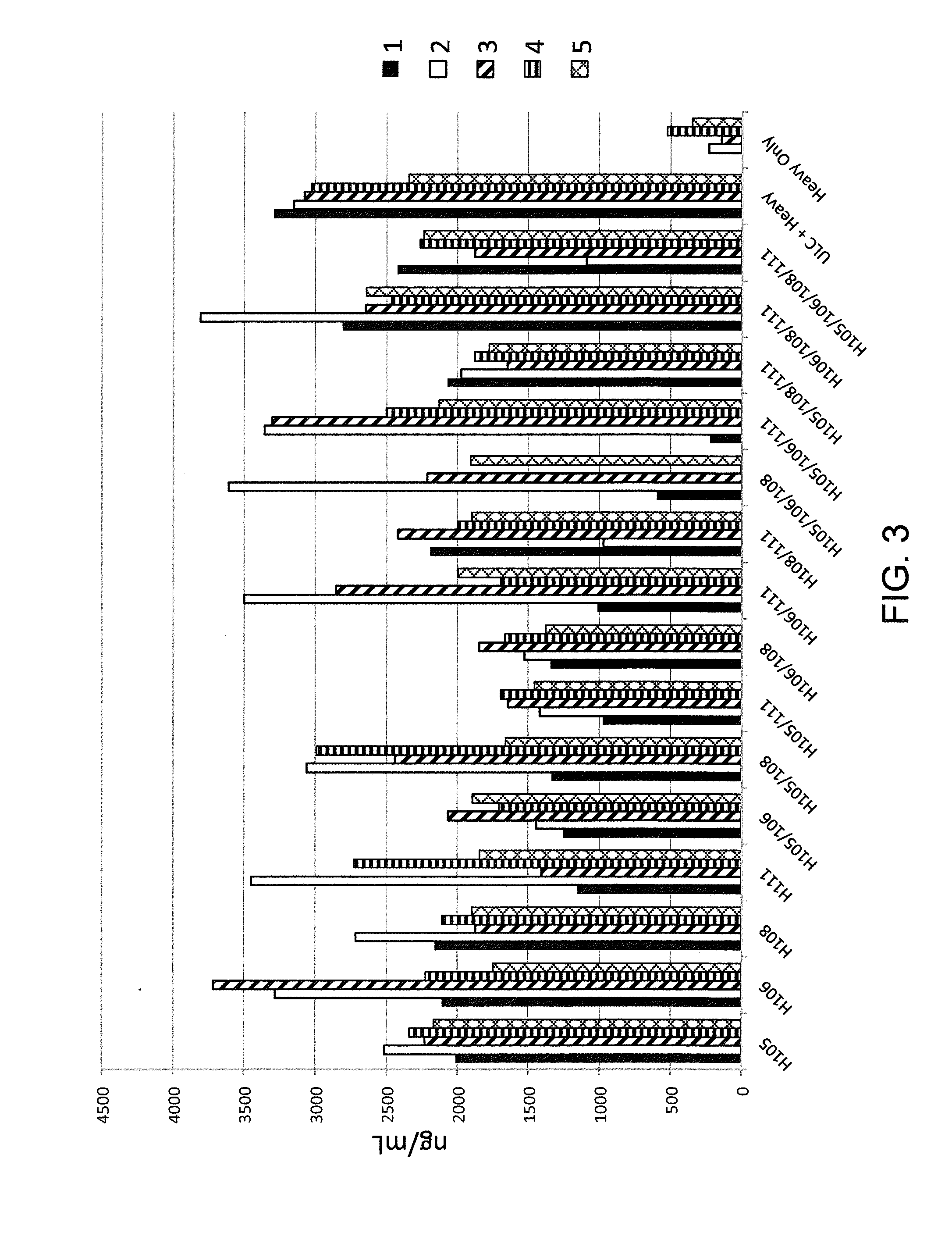
Histidine Engineered Light Chain Antibodies and Genetically Modified Non-Human Animals for Generating the Same
ActiveUS20130247234A1Reduce the binding forceImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHuman animalVariable domain
A genetically modified non-human animal is provided, wherein the non-human animal expresses an antibody repertoire capable of pH dependent binding to antigens upon immunization. A genetically modified non-human animal is provided that expresses a single light chain variable domain derived from a single rearranged light chain variable region gene in the germline of the non-human animal, wherein the single rearranged light chain variable region gene comprises a substitution of at least one non-histidine encoding codon with a histidine encoding codon. Methods of making non-human animals that express antibodies comprising a histidine-containing universal light chain are provided.
Owner:REGENERON PHARM INC
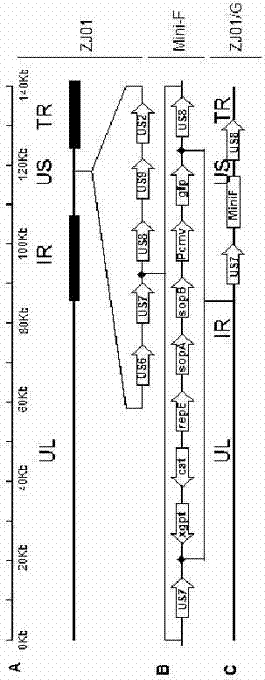
gE- and gI-deleted porcine pseudorabies virus variant strain and use thereof
The invention relates to the technical field of porcine pseudorabies viruses and especially relates to a gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G and a use thereof. The gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G has the accession number of CGMCC No.7957. The invention discloses the use of the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G in vaccine preparation. After the New Zealand big white rabbit is inoculated with the 106.0TCID50 recombinant viruses, clinical symptoms such as pruritus are not caused. An oil-in-water inactivated vaccine prepared from the gE- and gI-deleted porcine pseudorabies virus variant strain PRV-ZJ011G is injected into a piglet and after four weeks, the BELISA antibody is produced but the gE antibody does not exist, and the immunization protection efficiency is 100%. After immunization on sows, the piglets produced by the sows get immunization protection and the efficiency of PRV variant virus and traditional virus immunization protection is 100%. It is proved that the ZJ011G recombinant virus has good immunogenicity and can be used for vaccine preparation.
Owner:JIANGSU NANNONG HI TECH
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic polypeptide
InactiveCN103509107AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansPenicillinKeyhole-limpet haemocyanin
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic polypeptide. The method comprises the following steps: synthesizing a "CGAGAGSGAGAGS" polypeptide sequence by utilizing an Fmoc method, coupling the polypeptide with keyhole limpet hemocyanin (KLH) through the cysteine on the N terminus of the polypeptide so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain a primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then adding streptomycin and penicillin to carry out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antibody titer in the rabbit blood sample reaches 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate.
Owner:ZHEJIANG UNIV +1
Rapid generation of t cell-independent antibody responses to t cell-dependent antigens
InactiveUS20090104221A1Easy to demonstrateLess immunogenicMicrobiological testing/measurementDrug screeningEndemic diseasesGerminal center
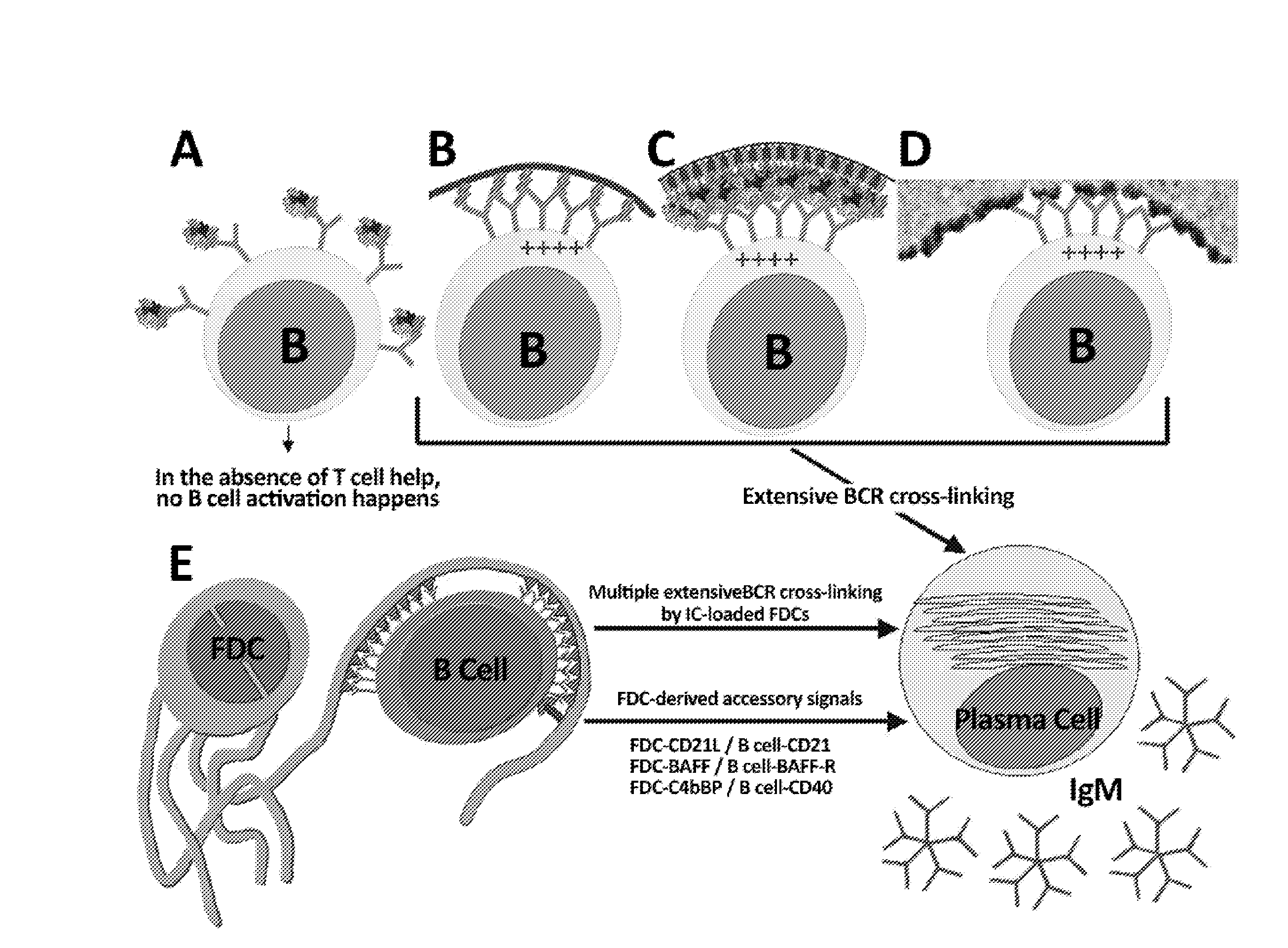
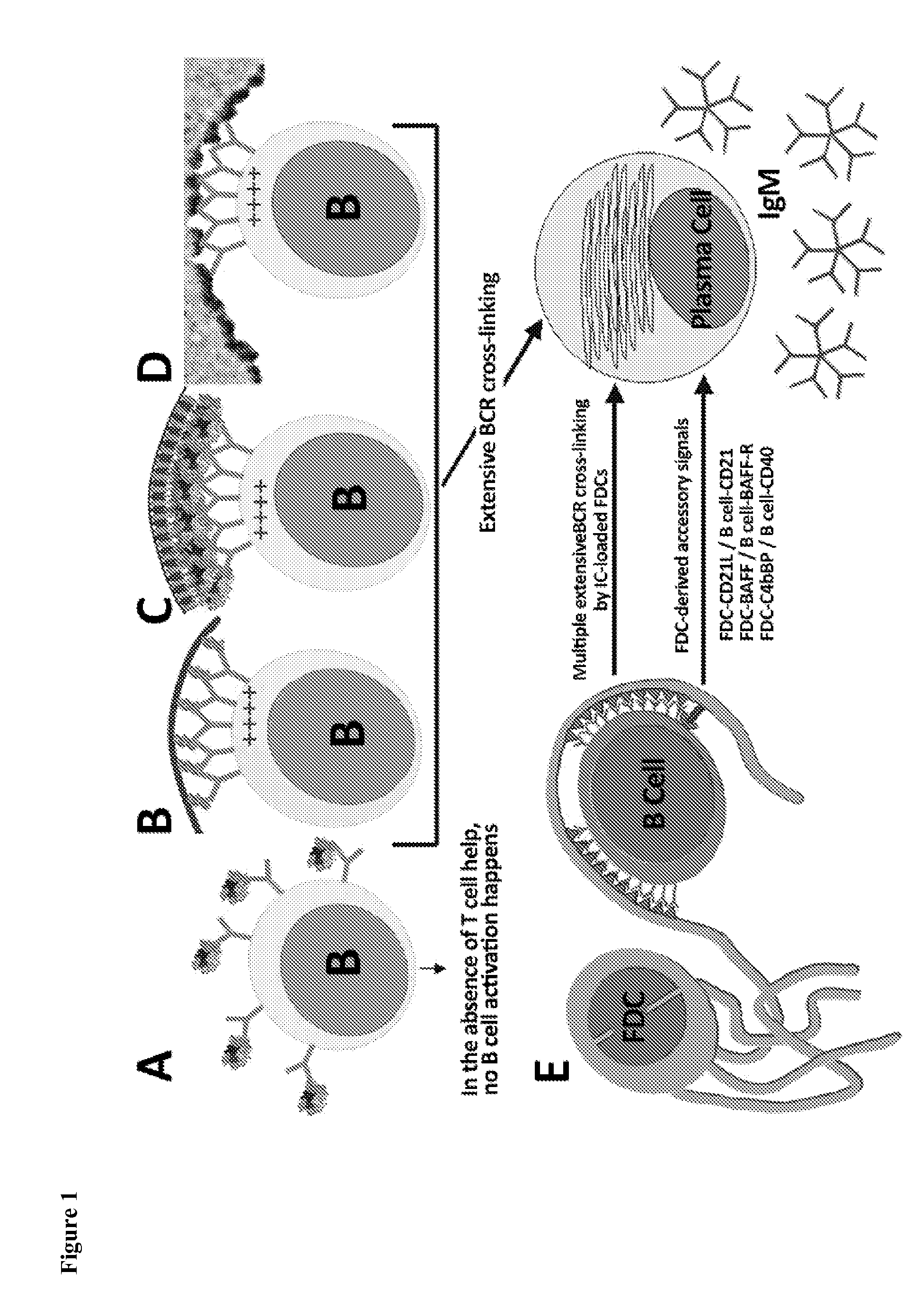
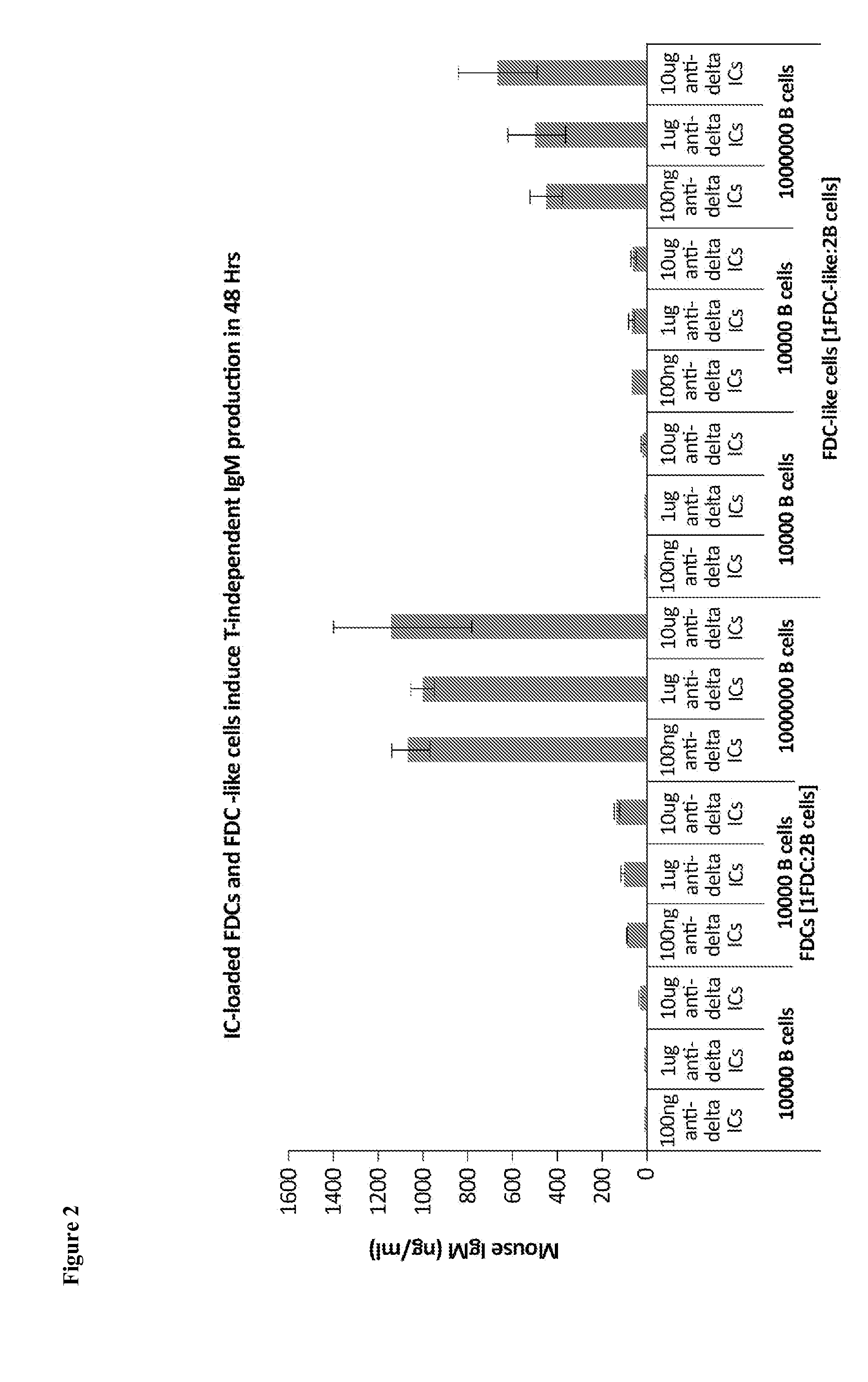
The present invention comprises the use of follicular dendritic cells (FDCs) or FDC-like cells to generate FDC-dependent, but T cell-independent, B cell responses to T cell-dependent antigens, with antigen-specific and polyclonal antibody production in ˜48 h. In another embodiment, a germinal center (GC) lymphoid tissue equivalent (LTE) was used to generate antigen-specific IgM, followed by switching to IgG. The GC LTE model can be used in vaccine assessment. Dual forms of immunogen were used in the GC LTE and in vivo. Dual immunogens resulted in rapid, specific IgM responses and enhanced IgG responses. This vaccine design approach can be used, for example, to provide rapid IgM protection (˜24-48 h) and high-affinity IgG more quickly in people moving to areas with endemic disease, or in people with T cell insufficiencies, who can be immunized to rapidly generate protective IgM.
Owner:SANOFI PASTEUR VAX DESIGN +1
Peptides which elicit a high neutralizing antibody titer, cytotoxic T lymphocyte response and T helper cell response in a broad range of MHC type recipients
InactiveUS7094405B1High titerHigh titer of neutralizing antibodyPeptide/protein ingredientsAntibody mimetics/scaffoldsV3 loopT helper cell
Peptide constructs comprised of multideterminant T helper peptides from the envelope glycoprotein of HIV previously identified to induce proliferative responses in four different haplotypes of mice and IL-2 responses in 52-73% of HIV positive, flu positive patients (cluster peptides), were co-linearly synthesized with the peptide 18 of the V3 loop of HIV-1 gp 160, corresponding to the principal neutralizing determinant of HIV-IIIB and also shown to contain a dominant CTL epitope. Cognate help for peptide 18 antibody was elicited following a single immunization in all strains of mice which had previously responded to a T cell epitope encompassed by the peptides. In two strains of mice, the level of neutralizing antibody achieved was comparable to levels adequate for protection from homologous viral challenge in chimpanzees. After a single boost, much higher antibody titers for 90% neutralization in the range of 1:1000 to 1:16,000 were achieved. Spleen cells from mice of three distinct MHC haplotypes sharing the Dd class I MHC molecule but with different class II molecules, immunized with the compound peptides, exhibited enhanced gp160-specific CTL activity.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
African swine fever B and T cell tandem epitope fusion vaccine
InactiveCN111018995AGood immune effectAvoid the risk of accelerated viral infectionAntibody mimetics/scaffoldsViral antigen ingredientsAfrican swine feverTGE VACCINE
The invention, which belongs to the technical field of vaccines, particularly relates to an African swine fever B and T cell tandem epitope fusion vaccine. The main component of the African swine fever B and T cell tandem epitope fusion vaccine is African swine fever tandem epitope fusion protein. The African swine fever tandem epitope fusion protein comprises a B cell neutralizing epitope peptidefragment and a T cell epitope; and the B cell neutralizing epitope peptide comprises the following fragments: at least one neutralizing epitope peptide of each of p72, p54, p30 proteins. When the African swine fever tandem epitope fusion protein is used as a vaccine, the immune effect is good; and the antibody level significantly higher than that of a control group can be detected after one immunization. Since the non-neutralizing epitope is reduced as much as possible in the fusion protein, the risk of accelerating virus infection (ADE effect and antibody dependence enhancement effect) by anon-neutralizing antibody after immunization can be avoided.
Owner:河南省生物工程技术研究中心 +1
Method of preparing bombyx mori silk fibroin specific antibody by utilizing characteristic dodecapeptide
InactiveCN103509108AStrong specificityStrong immune responseSerum immunoglobulinsImmunoglobulins against animals/humansKeyhole-limpet haemocyaninPrimary immunization
The invention discloses a method of preparing a bombyx mori silk fibroin specific antibody by utilizing a characteristic dodecapeptide. The method comprises the following steps: synthesizing a polypeptide with a "CGYGAGAGAGYGA" sequence, coupling the polypeptide with keyhole limpet hemocyanin (KLH) so as to obtain a complete antigen; diluting the complete antigen with normal saline, mixing the diluted complete antigen with a complete Freund's adjuvant, carrying out an emulsion treatment so as to obtain primary immunized antigen emulsion, subjecting a rabbit to a primary immunization by using the primary immunized antigen emulsion, then subjecting the rabbit to a strengthened immunization, wherein the strengthened immunization uses a strengthened immunized antigen emulsion, which is prepared by the following steps: mixing the diluted complete antigen with an incomplete Freund's adjuvant, and then carrying out an emulsion treatment so as to obtain the target product; collecting the blood of the immunized rabbit, when the antiserum titer of rabbit arrives at 1 / 10000; making the blood blocks fully contract to completely separate out the antiserum, then collecting the antiserum, and subjecting the antiserum to a centrifugation treatment so as to obtain a supernate. The antibody prepared by the invention has a strong specificity, and can be used for detection and analysis of silk fibroin in textile, and the like.
Owner:ZHEJIANG UNIV +1
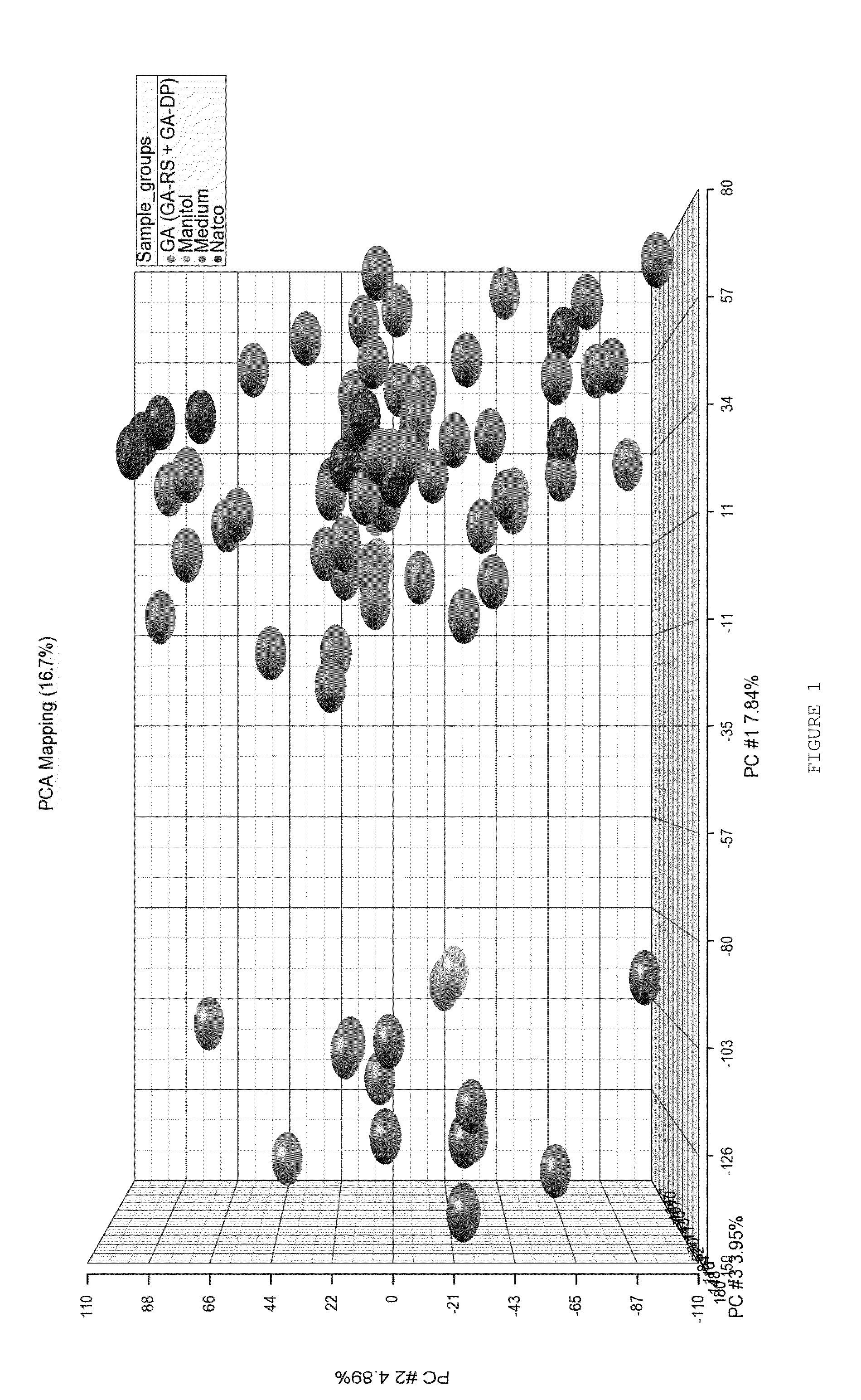
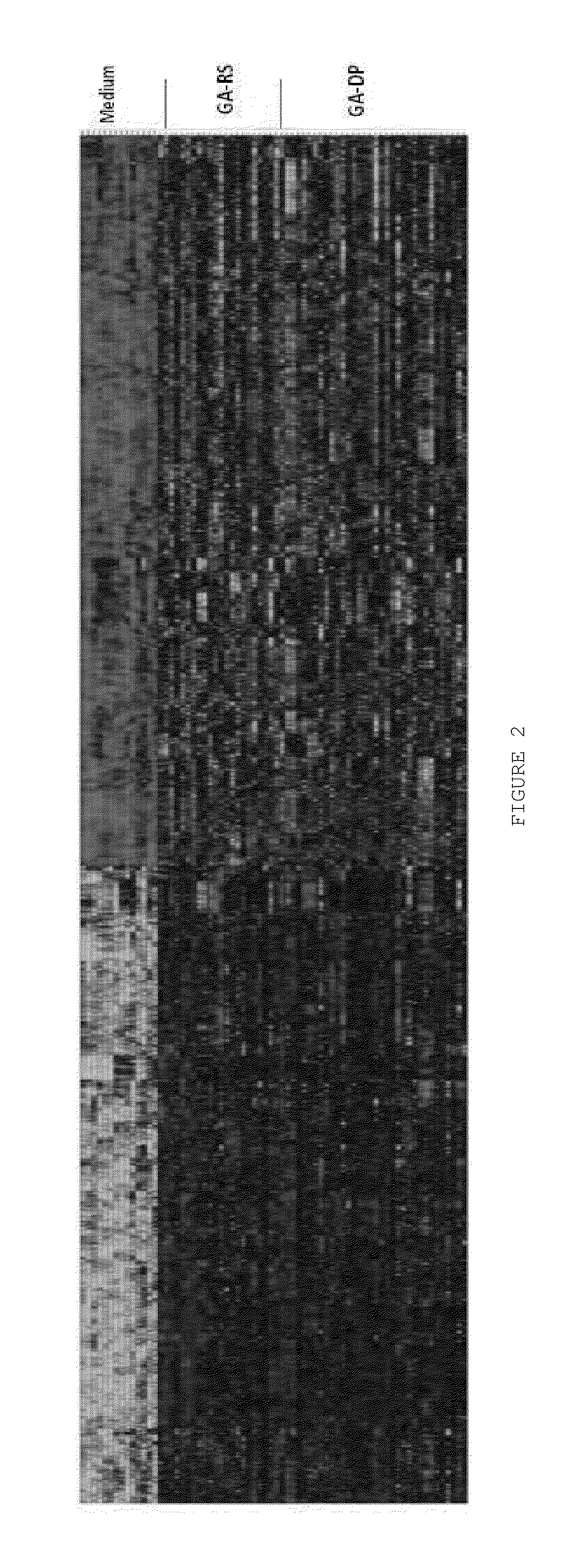
Characterizing a glatiramer acetate related drug product
InactiveUS20140193827A1Safe and effectiveReduce recurrenceMicrobiological testing/measurementBiological testingCombinatorial chemistryDrug product
The present invention provides a process for characterizing a glatiramer acetate related drug substance or drug product comprising the steps of:a) obtaining a batch of the glatiramer acetate related drug substance or drug product;b) immunizing a mammal with a predetermined amount of a glatiramer acetate related drug substance or drug product;c) preparing a culture of cells from the mammal of step b) at a predetermined time after immunization;d) incubating cells from the culture of step c) with a predetermined amount of the glatiramer acetate drug related substance or drug product of step a); ande) determining the level of expression of at least one gene disclosed herein or determining the level of biological activity of the cells of step c) as disclosed herein,thereby characterizing the glatiramer acetate related drug substance or drug product of step a).
Owner:TEVA PHARMA IND LTD
Immunity enhancing agent, inactivated vaccine, and preparation method thereof
InactiveCN103083663AImprove immunityEnhance immune responseViral antigen ingredientsAntiviralsDipeptideOil phase
The invention provides an immunity enhancing agent, an inactivated vaccine, and a preparation method thereof. The invention relates to the field of biopharmaceutical. The immunity enhancing agent comprises 0.1-21mg / mL of monophosphoryl lipid A, 1.5-125mg / mL of muramyl dipeptide, and 0.7-4.5mg / mL of beta-glucan. The invention also provides the inactivated vaccine comprising the immunity enhancing agent, and a preparation method of the inactivated vaccine. According to the invention, the immunity enhancing agent is mixed with an inactivated antigen solution, such that a water-phase solution is obtained; and the water-phase solution is mixed with an oil-phase solution, such that the inactivated vaccine is obtained. According to the immunity enhancing agent provided by the invention, with a synergetic effect of the components, body immunity level can be improved, and immune response to antigen can be improved, such that antibody level after immunization can be increased, immune window period can be shortened, and vaccine immunization effect can be enhanced. According to the inactivated vaccine comprising the immunity enhancing agent, antibody level after immunization is high, a protection period is long, and immunization window period is short.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES +1
Haemophilus parasuis LC strain and application thereof
ActiveCN102399724AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacteriaHeterologousDisease
The invention relates to the field of haemophilus parasuis vaccines in veterinary biological products, in particular to a haemophilus parasuis LC strain. The collection number of the strain is CGMCC (China General Microbiological Culture Collection Center) No.5257. The invention also relates to application of the haemophilus parasuis LC strain to preparation of haemophilus parasuis inactivated vaccines. The haemophilus parasuis LC strain has stronger pathogenicity to pigs and has better immunogenicity; an inactivated alumina gel vaccine prepared by the strain is safe and reliable; not only a homologous attacking protection is provided, but also a better cross protection to blood serums type 4, type 5, type 10, type 12, type 14 and type 15 HPS (Hantavirus Pulmonary Syndrome) heterologous attacking can be provided; after the pigs are immunized, a stronger immunity can be generated and the morbidity and the mortality of the inoculated pigs are obviously reduced; the immune effect achieves or is better than the traditional commercialized vaccines in the market; the vaccine has the advantages to compete with like products at home and abroad and is capable of effectively preventing the epidemic and the transmission of a haemophilus parasuis disease and reducing the economic losses caused by the disease, so that the application range is wide.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Rabbit triple inactivated vaccine, preparation method and application thereof
InactiveCN101708332AImprove immune efficiencyImprove securityAntibacterial agentsBacterial antigen ingredientsDiseaseSide effect
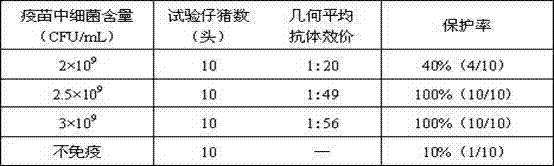
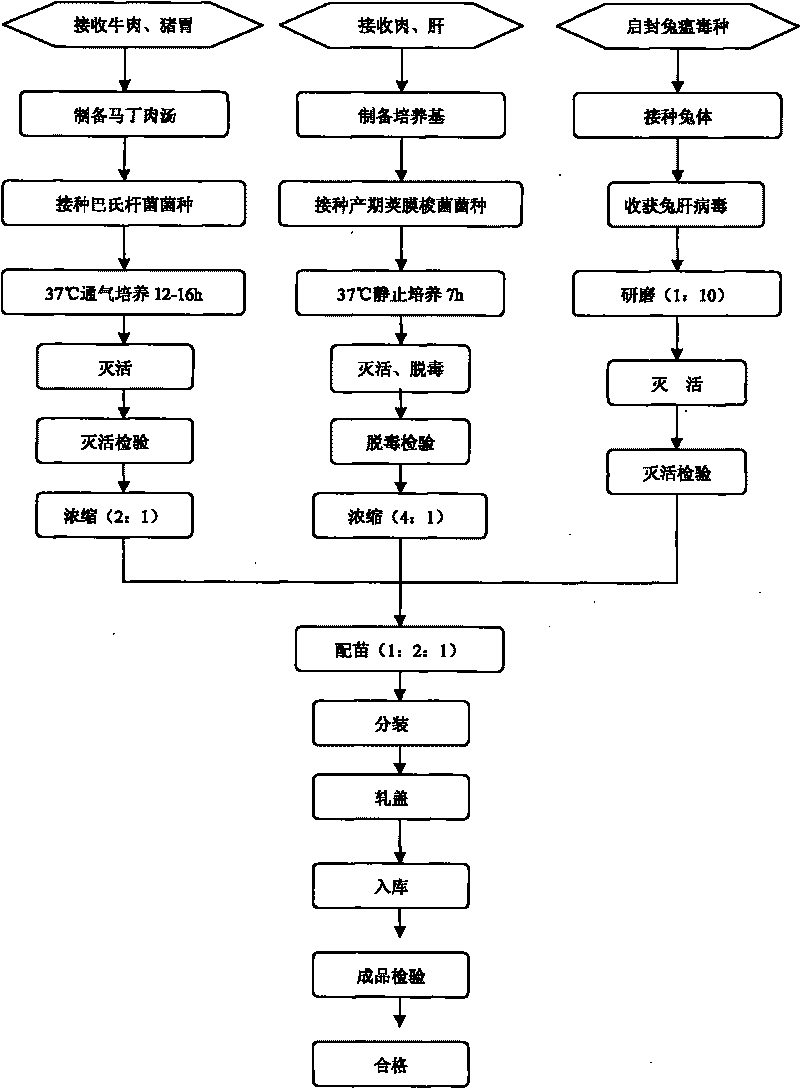
The invention discloses a rabbit triple inactivated vaccine, a preparation method and the application thereof; the rabbit triple inactivated vaccine is formed mainly by rabbit hemorrhagic disease virus liquid which is inactivated and detoxified, rabbit pasteurella multocida liquid and rapid A type clostridium perfringens liquid which have 1:1:2 volume ratio; in the invention, by optimizing culture technology, concentration technology and immunizing dose and other measures, the rabbit triple inactivated vaccine which has good immunity effect and safety is obtained and can effectively control the rabbit hemorrhagic disease, the pasteurella multocida disease and the pasteurella multocida disease (A type) and reduce vaccine injection times of farmers, so as to reduce the stress reaction of animals and improve the production performance. The clinical application proves that the immunization effect of the rabbit triple inactivated vaccine is ideal, each rabbit is vaccinated with 2ml and has immunity after 5-7 days, the immune period is at least 180 days, and the immune safety is good without toxic and side effect.
Owner:哈药集团生物疫苗有限公司
Method for refining chicken egg yolk antibodies against Escherichia coli
InactiveCN101948538AHigh potencyEgg immunoglobulinsImmunoglobulins against bacteriaBiotechnologyAdjuvant
The invention discloses a method for refining chicken egg yolk antibodies against Escherichia coli, which comprises the following steps of: culturing by adopting formaldehyde, thiomersalate and a white oil adjuvant to prepare antigen solution; immunizing healthy chickens by using the antigen solution, and collecting immune eggs; extracting egg yolk antibodies from the immune eggs; and purifying by adopting ammonium sulfate to obtain the chicken egg yolk antibodies against the Escherichia coli. For the chicken egg yolk antibodies against the Escherichia coli, pili are collected first, and then Escherichia coli antibodies in the chicken egg yolk are collected after organism immunization of chickens, wherein when the Escherichia coli antibodies in the chicken egg yolk are collected, ammonium sulfate solution is adopted for purification. Experiments prove that the potency of the chicken egg yolk antibodies against the Escherichia coli is obviously improved.
Owner:ZHENGZHOU HOUYI PHARMA
Preparation method of porcine epidemic diarrhea recombinant adenovirus vaccine
InactiveCN102512693AImprove abilitiesThe production is effectiveGenetic material ingredientsAntiviralsEnzyme digestionA-DNA
The invention discloses a preparation method of a porcine epidemic diarrhea recombinant adenovirus vaccine. The preparation method provided by the invention comprises the following steps of inserting a DNA sequence of a zone S1 of a porcine epidemic diarrhea virus (PEDV) into an adenovirus shuttle plasmid pShuttle-CMV to obtain pShuttle-CMV-S1, carrying out linearization of the pShuttle-CMV-S1, transforming the linear pShuttle-CMV-S1 into a BJ5183 competent cell containing pAdEasy-1, carrying out homologous recombination, carrying out enzyme digestion, carrying out AD-293 cell transfection, carrying out packaging to obtain a recombinant adenovirus rAd-S1, and carrying out purification, amplification and sub-packaging. After oral immunization, the porcine epidemic diarrhea recombinant adenovirus vaccine can induce generation of mucosal immunity thereby preventing porcine epidemic diarrhea (PED) well.
Owner:GENIFARM LAB INC
Polynucleotide herpes virus vaccine
InactiveUS7094767B2Avoid infectionAmeliorate HSV-related diseaseSugar derivativesGenetic material ingredientsMammalSeroconversion
Genes encoding herpes simplex virus type 2 (HSV-2) proteins were cloned into eukaryotic expression vectors to express the encoded proteins in mammalian muscle cells in vivo. Animals were immunized by injection of these DNA constructs, termed polynucleotide vaccines or PNV, into their muscles. In a DNA titration, it was found that a single immunization of ≧0.5 μg of (one) PNV, gave >90% seroconversion by ten weeks post immunization. Immune antisera neutralized both HSV-2 and HSV-1 in cell culture. When animals were challenged with HSV-2, significant (p<0.001) protection from lethal infection was achieved following PNV vaccination. DNA constructs may be full-length, truncated and / or mutated forms and may be delivered along or in combination in order to optimize immunization and protection from HSV infection.
Owner:MERCK SHARP & DOHME CORP
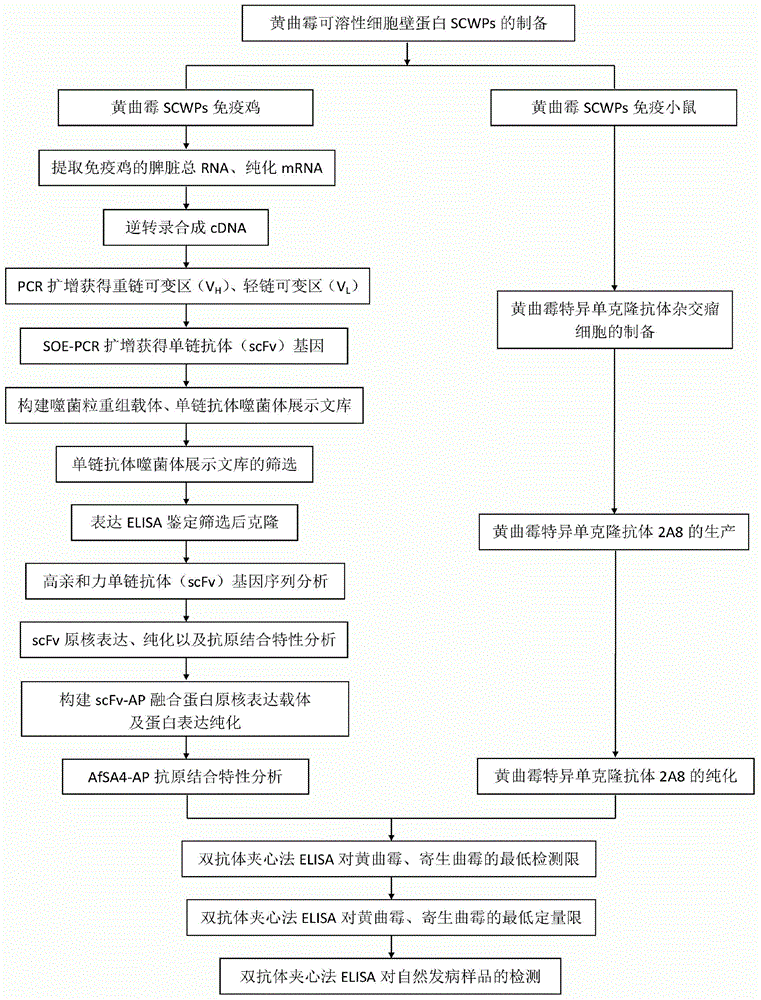
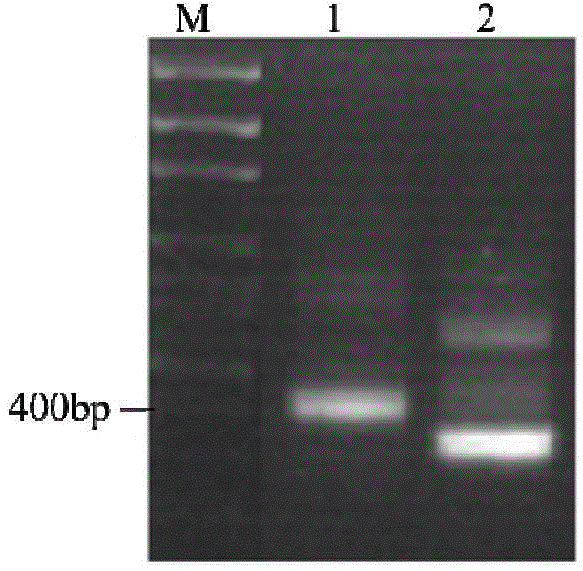
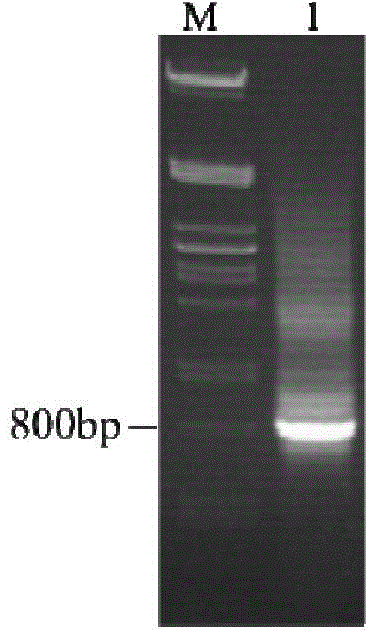
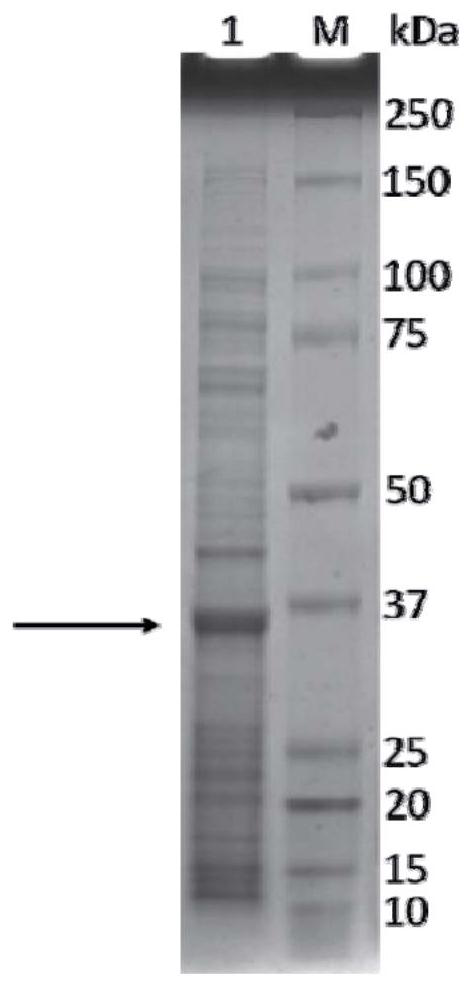
Preparation method and application of aspergillus flavus specific single-chain antibody
InactiveCN103555732AHigh affinityEasy to operateMicrobiological testing/measurementMicroorganism based processesEscherichia coliSingle-Chain Antibodies
The invention belongs to the field of detection of harmful biological molecules in food, and relates to a preparation method and application of an Aspergillus flavus specific single-chain antibody. The cell wall proteins of the Aspergillus flavus hypha are used for immunizing chickens and mice respectively; messenger RNA of chicken splenic lymphocytes after immunization are extracted; a chicken source single-chain antibody library is constructed; a phage display technology is employed to screen the library to obtain an Aspergillus specific single-chain antibody gene with high affinity to Aspergillus flavus. The antibody gene is subcloned into an expression vector of an alkaline phosphatase protein, and expressed and purified in Escherichia coli, so as to obtain a fusion protein and a hybridoma cell 2A8 secreting Aspergillus flavus specific monoclonal antibody. The monoclonal antibody as a capture antibody and AfSA4-AP fusion protein as a detection antibody are employed to establish a SandWich ELISA immunological detection system for detecting Aspergillus flavus contamination in crops and stored plant-derived products.
Owner:HUAZHONG AGRI UNIV
Novel coronavirus vaccine based on chimeric virus-like particles
PendingCN111620952AEnhance immune responseGood immune protectionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationReceptor
Owner:SUZHOU MIDI BIOTECH CO LTD
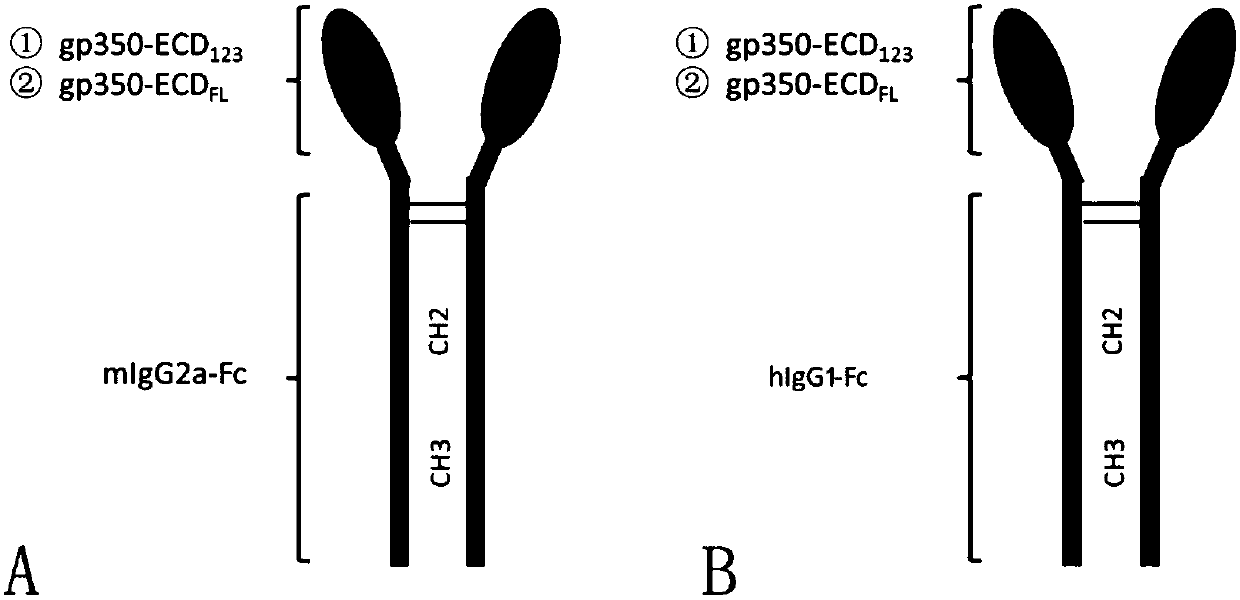
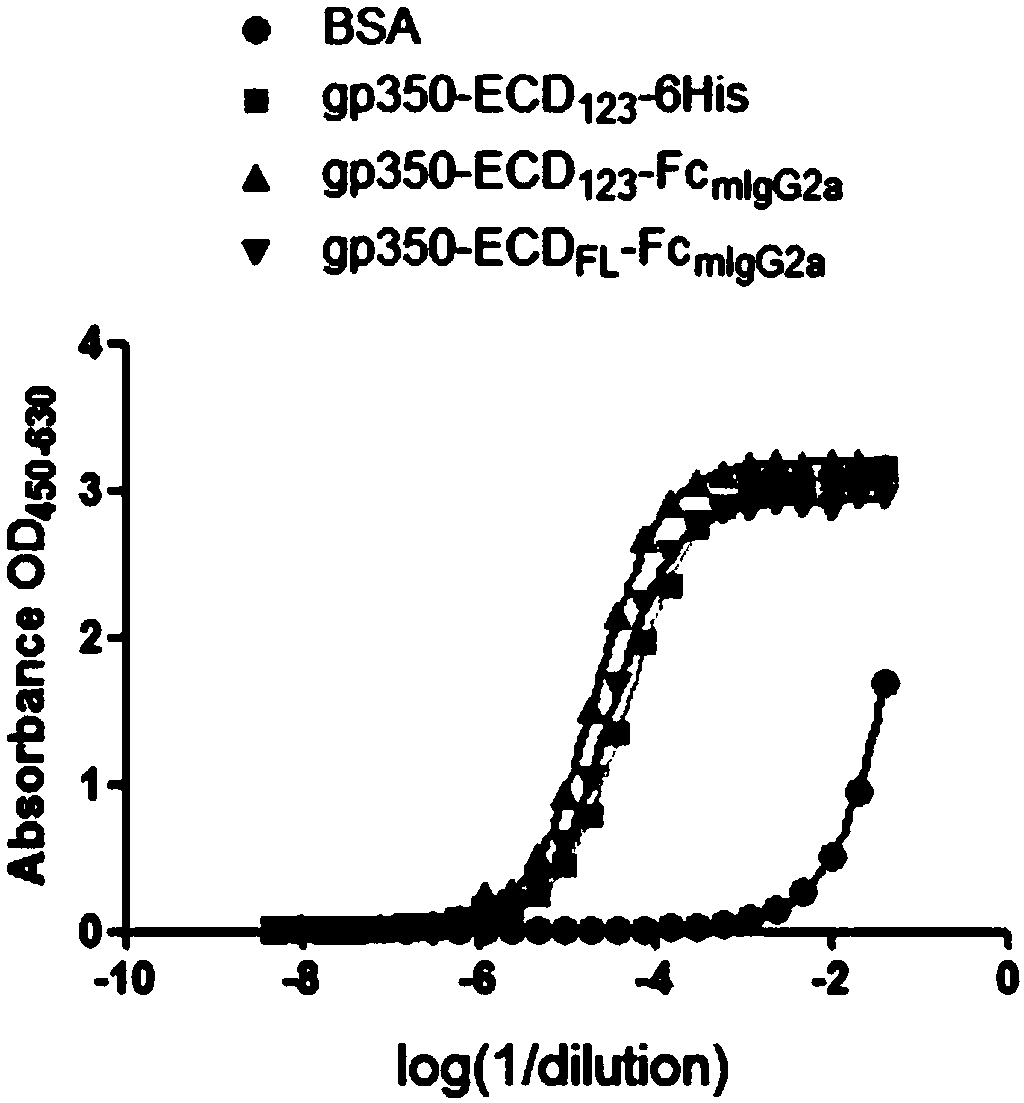
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
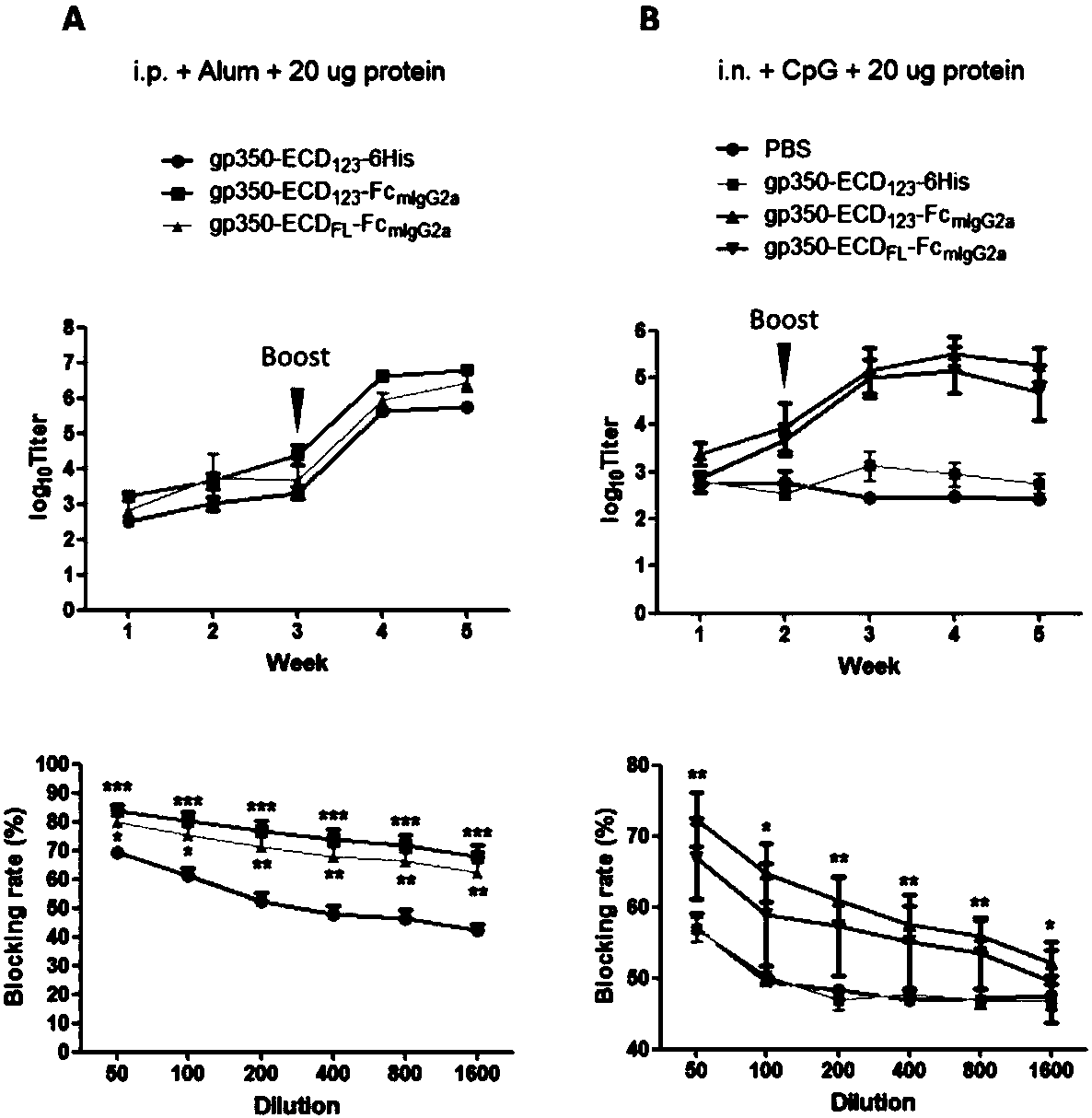
ActiveCN109824779AImproving immunogenicityHigh infection blocking efficiencyAntiviralsAntibody ingredientsDiseaseImmunogenicity
The invention discloses a fusion protein comprising an Fc domain of IgG and an extracellular domain of an EB virus envelope glycoprotein. The fusion protein is represented by the following formula: P-E-F, wherein P represents a secretion signal peptide, E represents an amino acid sequence of the extracellular domain of the EB virus envelope glycoprotein gp350, and F represents the amino acid sequence of the Fc domain of the IgG. It is found for the first time that after the fusion of the Fc domain of the immunoglobulin IgG with the envelope glycoprotein gp350 from the surface of the EB virus,the immunogenicity in vivo is significantly improved. After immunization with the fusion protein, the total serum titer an immunized animal, the serum specific neutralizing antibody titer and the serum in vitro viral infection blocking efficiency are significantly higher than that of a non-fused control protein. The fusion protein using the Fc domain of the immunoglobulin IgG and the EB virus membrane glycoprotein is adopted as an evidence for the efficacy of an EB virus vaccine and has important practical and theoretical significance and application prospects for prevention and treatment of EB virus-related diseases.
Owner:SUN YAT SEN UNIV +1
Monoclonal antibody for detecting imidacloprid pesticide residue
InactiveCN101880325AHigh analytical capacityLarge analysis capacitySerum albuminMicroorganism based processesBALB/cPesticide residue
The invention relates to an imidacloprid pesticide against monoclonal antibody and a preparation method thereof, and belongs to the technical field of biology. The preparation method comprises the following steps of: immunizing a BALB / c mouse by using a coupling substance of immune hapten 1-[6-(2-carboxyethylsulfenyl-3-pyridine) methyl]-N-nitro-2-imidazoline imine and bovine serum albumin, preparing hybrid tumor cells from spleen cells and myeloma cells Sp2 / 0 of the immunized mouse by the hybrid tumor technology, and obtaining hybrid tumor strains 2F11 / A9 capable of stably secreting the imidacloprid pesticide against monoclonal antibody. By effectiveness verification, the antibody can be used for sensitive and quick detection of imidacloprid residues in agricultural production environments and agricultural products. The preparation technique for the imidacloprid pesticide against monoclonal antibody is simple and feasible, does not need special instruments and equipment in the whole preparation process of the antibody, and is easy for scale production in factories.
Owner:NANJING AGRICULTURAL UNIVERSITY
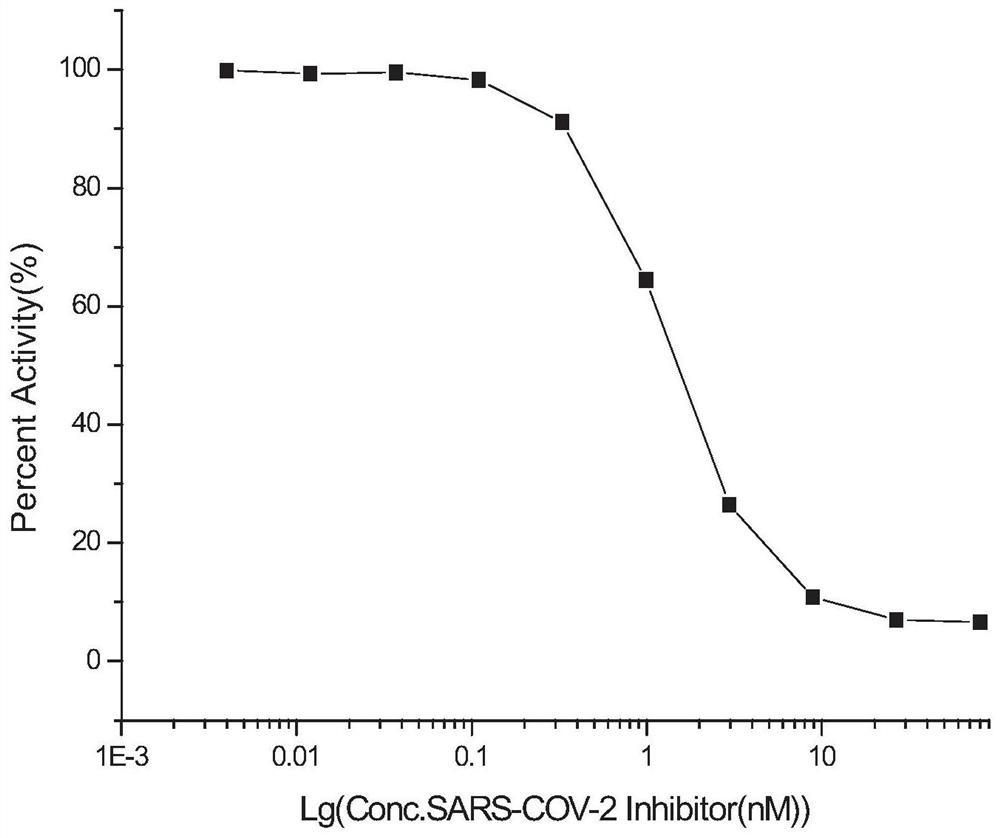
Novel coronavirus SARS-CoV-2 mRNA vaccines and preparation method and application thereof
ActiveCN113151312AProlong half-lifeEasy to getSsRNA viruses positive-senseViral antigen ingredientsCoronavirus vaccinationSpecific igg
The invention provides novel coronavirus SARS-CoV-2mRNA vaccines and a preparation method and application thereof. The invention provides three mRNA vaccines, namely RBD, S1 and S vaccines. The RBD vaccine disclosed by the invention can induce a high-titer antigen-specific IgG antibody and a virus neutralization antibody after immunization with one dose, the high-titer neutralization antibody can be maintained for at least 26 weeks, and remarkable immune protection can be provided for human ACE2 transgenic mice in serum adoptive transfer protection experiments. The RBD and S vaccine disclosed by the invention can induce immune protection capable of completely resisting SARS-CoV-2 virus infection in the human ACE2 transgenic mice after immunization with two doses. A large number of experimental results show that the mRNA vaccine provided by the invention has good immunogenicity, forms powerful immune protection after immunizing an organism, and has a huge development potential.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Porcine pseudorabies virus, and vaccine composition and applications thereof
ActiveCN102952785ARaise antibody levelsLong durationMicroorganism based processesAntiviralsPig farmsAdjuvant
The invention provides a p porcine pseudorabies virus and vaccine composition and applications thereof, belonging to the field of biotechnology. The microbial preservation number of the porcine pseudorabies virus PRV-JS strain is CGMCCNO.6604. The invention further provides a vaccine composition which comprises an inactivated porcine pseudorabies virus PRV-JS strain and adjuvant acceptable on veteriary pharmacy. The vaccine composition further comprises a carrier acceptable on the veterinary pharmacy. The porcine pseudorabies virus PRV-JS strain is screened from porcine pseudorabies prevalent strains separated from all pig farms and has good immunogenicity, and can be used as inactivated vaccine production virus seeds or virus seeds for testing. After being immunized by the vaccine composition, pigs have higher produced antibody level and the lasting period is long. The vaccine composition prepared by adopting the porcine pseudorabies virus PRV-JS strain can be used for preventing the sow abortionbreeding difficulty and mortality syndromeboar infertility caused by the porcine pseudorabies virus, boar infertility and pseudorabies of other pigs.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Streptococcus protective antigen C5a and preparation method thereof
InactiveCN102746388AStrong immune responseSignificant passive immune protectionAntibacterial agentsBacterial antigen ingredientsForward primerProtective antigen
The present invention relates to a streptococcus antigen C5a and a preparation method thereof. The streptococcus protective antigen C5a is an SEZC5a recombinant protein, which consists of 571 amino acids and has a molecular weight of 60.3kDa; a forward primer has one BamHI restriction enzyme cutting site, and a reverse primer has one EcoRI restriction enzyme cutting site. According to the preparation method of the streptococcus protective antigen C5a, the SEZ C5a recombinant protein is treated with cloning, expression and purification; and a series of biological engineering technologies and experiments on mice are applied to conduct system analysis on an rSCPZ. After vaccination, the rSCPZ can provide high protective efficacy; an anti-rSCPZ mice double-immunized serum has significant passive immune protection on mice; and the mice immunized by the rSCPZ show high level of antibody titer in serum. The anti-rSCPZ antibody can induce high level of bactericidal capability; an scpZ gene has a transcription level in SEZ (Streptococcus zooepidemicus) infected mice higher than that of culture in vitro; and the rSCPZ can adhere to hep-2 cells and inhibit cell infection ability of SEZ.
Owner:广东艾佩克科技有限公司
Attenuated Salmonella pullorum and application thereof
InactiveCN102154184ALow toxicityImproving immunogenicityAntibacterial agentsBacteriaHorizontal transmissionMicroorganism
The invention discloses an attenuated Salmonella pullorum and application thereof. By using gene knockout technology, the in vivo colonization gene and the main virulence gene of Salmonella pullorum are deleted, thus obtaining an dual-gene-deleted attenuated Salmonella pullorum SM091-DED strain; and the microbiological preservation number of the strain is CGMCC NO.4604. The virulence of the attenuated Salmonella pullorum disclosed by the invention is obviously reduced, and the in vivo colonization time is short after the attenuated Salmonella pullorum is inoculated into a host; the herd infection test shows that the attenuated Salmonella pullorum has no horizontal transmission capability; the attenuated Salmonella pullorum provides full cross protection for homotype and allotype high-virulence Salmonella pullorum; and after SPF (Specific Pathogen Free) chicks are immunized, the infection of the high-virulence strain can be effectively eliminated, and the herd infection can not be caused. Thus, the invention provides a safe, fine-immunogenicity and low-virulence Salmonellosis avium attenuated live vaccine for preventing Salmonellosis avium.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Application of inhibin recombinant fusion protein to preparing medicines for promoting oestrus and hybridization of sows
InactiveCN102166348ANo toxicityNon-pathogenicPeptide/protein ingredientsRecombinant DNA-technologyMedicinePharmaceutical drug
The invention discloses an application of an inhibin recombinant fusion protein to preparing medicines for promoting oestrus and hybridization of sows. When inhibin is used for the sows: 0.5-1mg of the inhibin recombinant protein is used for initial immunization; and after 15-21 days, 0.25-0.5mg of the inhibin recombinant protein is used for second immunization. The immune inhibin can increase the estrus rate and the litter sizes of the sows. The invention has obvious effects on overcoming anestrus of the sows, caused by heat stress in summer, promoting the breeding activities of breeding sows, and particularly on promoting oestrus of breeding sows and back-up sows and increasing litter sizes. Thus, the non-productive time of the breeding sows is shortened, the breeding performance of thebreeding sows is improved, and the economic benefit of sow raising is increased.
Owner:SOUTH CHINA AGRI UNIV
A screening method for grouper iridovirus protective antigen
InactiveCN102277361AShorten the timeCutting costsViral antigen ingredientsGenetic material ingredientsProtective antigenTGE VACCINE
The present invention relates to a method for screening protective antigens of grouper iridescent virus (SGIV), specifically the identification of protective antigen genes from grouper iridescent virus with vaccine development prospects, including bioinformatics analysis and DNA vaccine identification. Protective antigens were screened out by challenge after preparation and immunization. The present invention provides three protective antigen genes screened out from SGIV162 virus-encoded genes, and provides a method for analyzing and screening potential vaccine candidate antigens in virus-encoded genes, which is beneficial to the further development of new grouper iridescent The virus vaccine can effectively prevent and control grouper iridescent virus disease, and improve the survival rate and breeding efficiency of grouper.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Methods for preparing monoclonal antibody and hybridoma cell strain thereof by multiple antigens in immune high-flux manner
InactiveCN103255128AIncrease productivitySave time and costImmunoglobulins against animals/humansTissue cultureEx vivoMonoclonal
The invention discloses methods for preparing a monoclonal antibody and a hybridoma cell strain thereof by multiple antigens in an immune high-flux manner. The method for preparing a hybridoma cell strain for secreting a specific monoclonal antibody by multiple antigens in the immune high-flux manner comprises the following steps of: 1) mixing n antigen proteins serving as immune antigens for immunizing a mice, thereby obtaining an immunized mice; 2) combining in-vitro spleen cells and SP2 / 0 myeloma cell of the immunized mice to obtain combined cells; 3) performing primary enzyme-linked immuno sorbent assay (ELISA) selection on the combined cells, and selecting hybridoma cells generating fos-like immunoreactivity; and 4) performing secondary ELISA selection on the hybridoma cells generating the fos-like immunoreactivity, and selecting the hybridoma cells aiming at single-antigen fos-like immunoreactivity. A high-flux technical method for obtaining multiple protein monoclonal antibodies by once cell combination is constructed; the time and the labor cost are saved for the preparation of the monoclonal antibodies; and contributions is made to improvement of the production efficiency of the monoclonal antibodies.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Preparation methods for duck hepatitis virus immunogen and hyperimmune serum and application of duck hepatitis virus hyperimmune serum
InactiveCN104926939AThe preparation method requires low conditionsEasy to operateSerum immunoglobulinsImmunoglobulins against virusesDuck hepatitis A virusSerum ige
The invention provides preparation methods for duck hepatitis virus immunogen and hyperimmune serum and application of the duck hepatitis virus hyperimmune serum. According to the preparation methods and the application of the duck hepatitis virus hyperimmune serum, the duck hepatitis virus immunogen is obtained through inoculating a serum 1 type duck hepatitis virus CH60 strain DHAV-1 (Duck Hepatitis A Virus type 1) or a serum 3 type duck hepatitis virus CH1 strain DHAV-3 (Duck Hepatitis A Virus type 3) to an allantoic cavity of a chick embryo of 9-10 days old or a duck embryo of 10-12 days old and carrying out proliferation and treatment, and the hyperimmune serum is obtained through mixing the duck hepatitis virus immunogen with a Freund's complete adjuvant or Freund's incomplete adjuvant to prepare solutions of different concentrations, carrying out repeated immunization on immune animals and then sampling and collecting blood and can be applied to the diagnosis and detection on a duck hepatitis virus. The preparation methods provided by the invention have the advantages that the conditional requirements are low, the operation is simple, and the obtained immunogen can meet the requirements on the preparation of specific antiserum.
Owner:SICHUAN AGRI UNIV
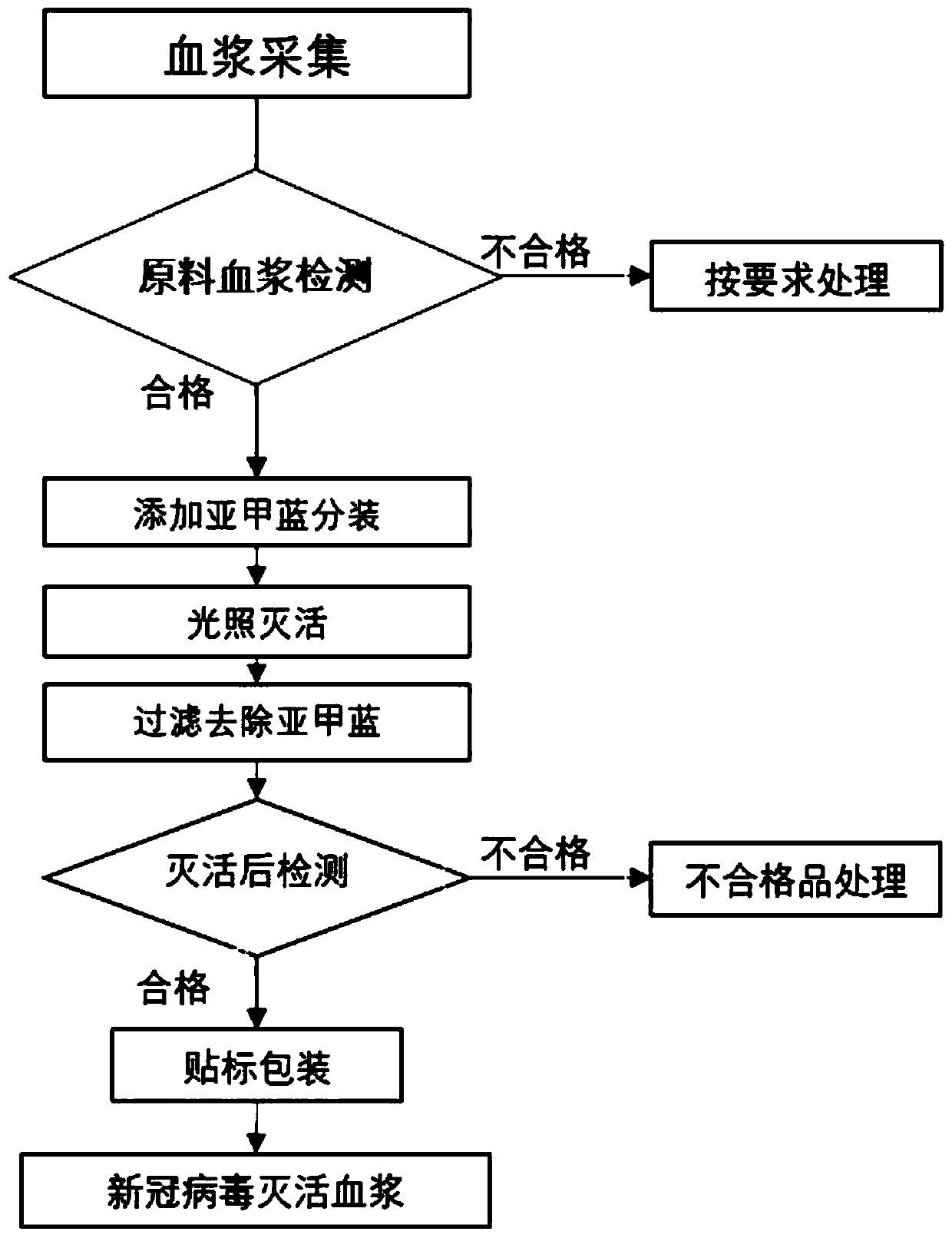
Production method of virus-inactivated plasma for treating COVID-19
InactiveCN111346108AQuick Preparation and AcquisitionHigh cure rateMammal material medical ingredientsAntiviralsVirus inactivationCoronavirus vaccination
The invention relates to a production method of virus-inactivated plasma for treating COVID-19. The method comprises the following steps: (1) collecting convalescent plasma of COVID-19 survivors or plasma immune to a Severe Acute Respiratory Syndrome Coronavirus 2 vaccine (SARS-CoV-2 vaccine), and conducting pretreatment; (2) adding methylene blue, and conducting photo-inactivation on the plasma;and (3) conducting labeling and packaging after the plasma passes tests.
Owner:国药集团武汉血液制品有限公司
Method for preparing aspergillus fumigatus galactomannan polyclonal antibody
InactiveCN106220730AHigh potencyStrong specificitySerum immunoglobulinsDisease diagnosisAspergillus fumigatusSerum ige
The invention provides an aspergillus fumigatus galactomannan antigen resisting polyclonal antibody and a preparation method thereof. The preparation method of the polyclonal antibody includes: adopting aspergillus fumigatus galactomannan prepared by affinity chromatography to perform animal immunization, taking blood from immunized animals to obtain serum, and purifying the serum by a saturated ammonium sulfate salting-out method and immunoaffinity chromatography to obtain the polyclonal antibody. The polyclonal antibody prepared according to the method is high in titer and purity, is the first aspergillus fumigatus galactomannan antigen resisting polyclonal antibody prepared by immunoaffinity chromatography in China and has a promising application prospect in the field of medical and scientific research.
Owner:DYNAMIKER BIOTECH TIANJIN
Infectious bursal disease antigen-antibody complex as well as preparation and preparation method thereof
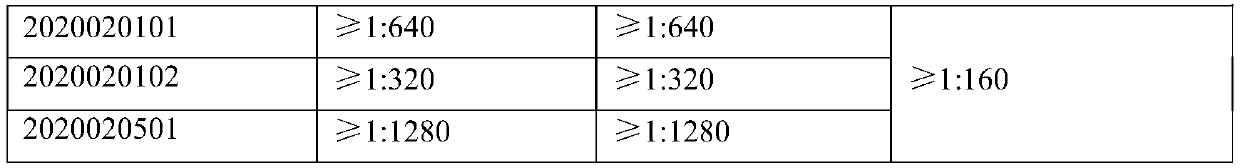
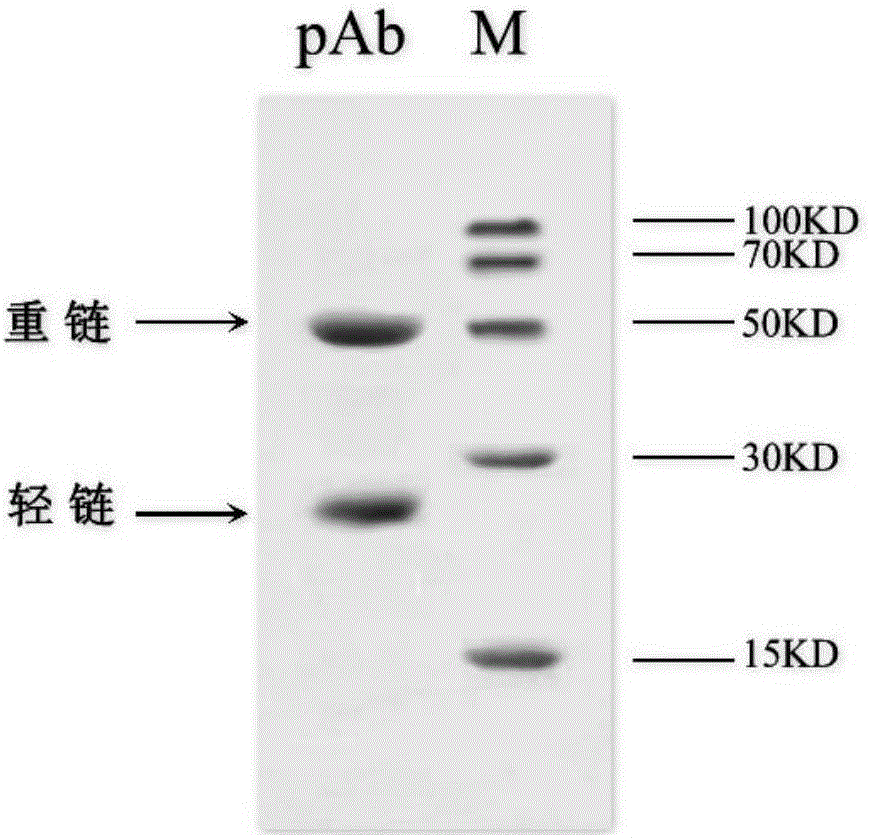
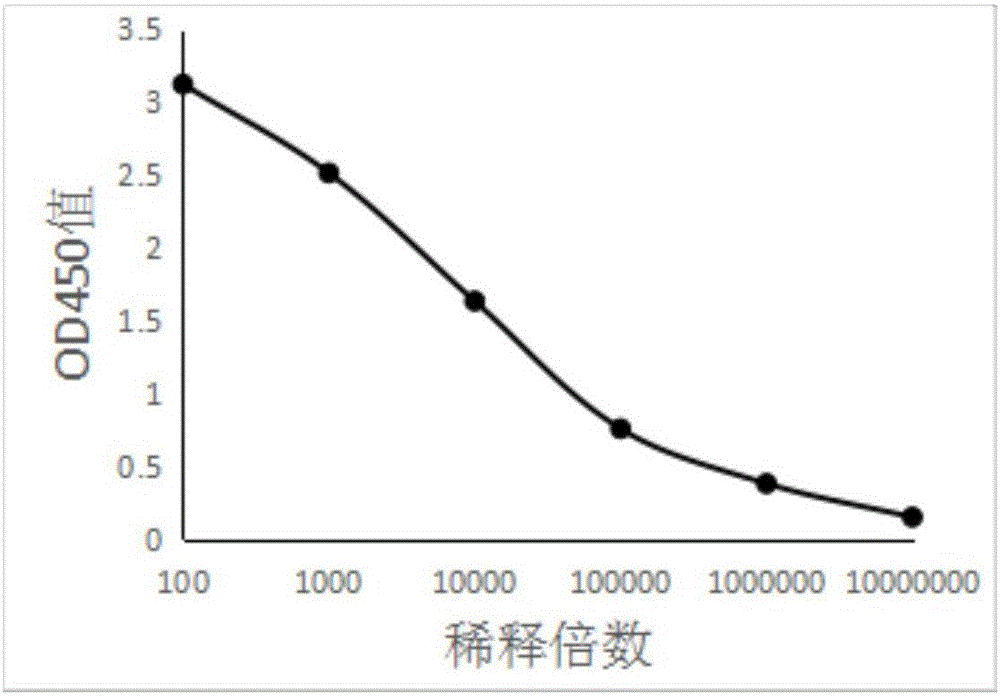
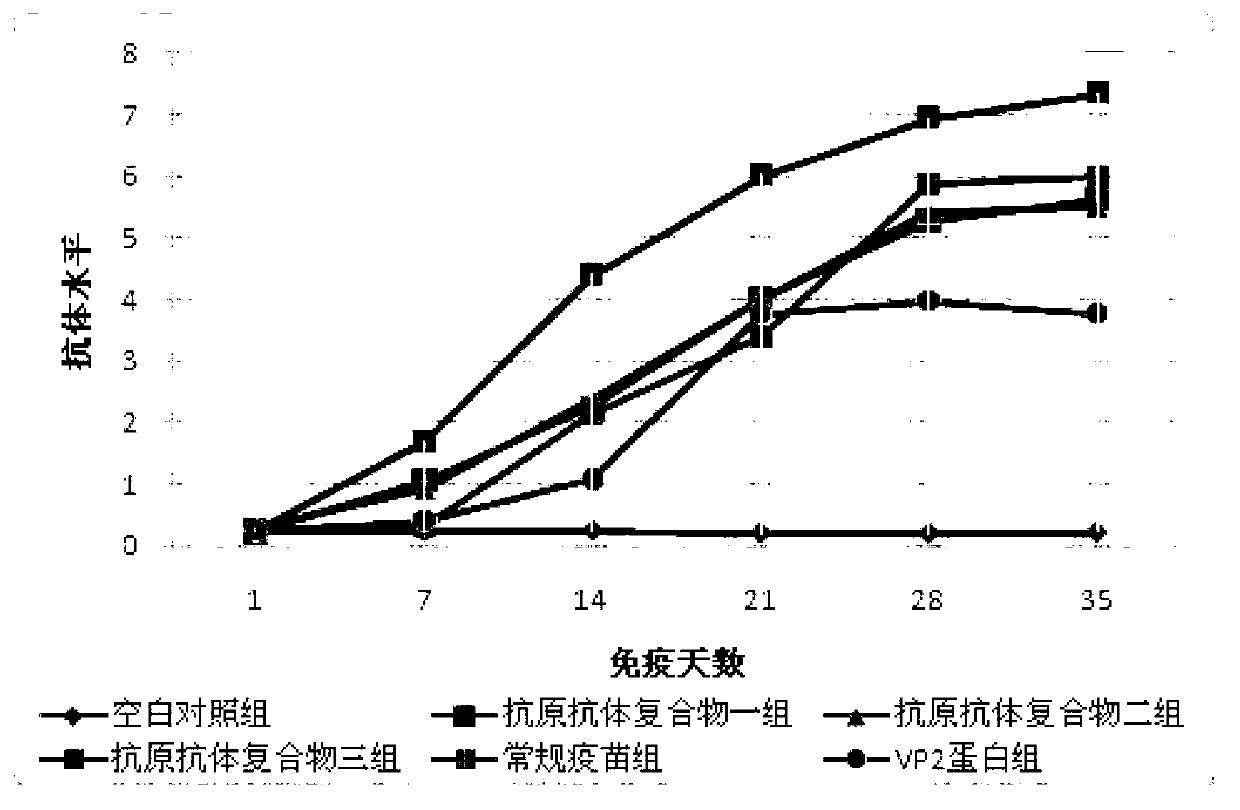
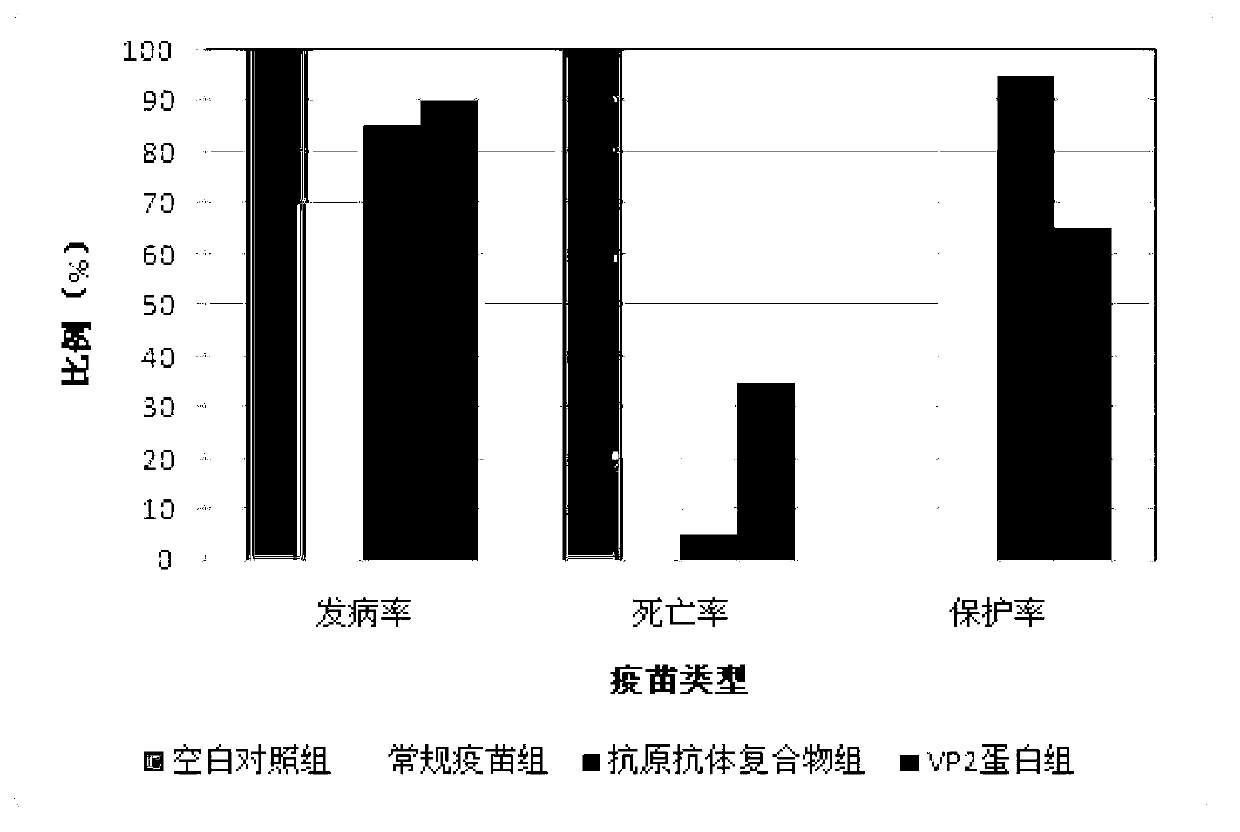
ActiveCN103341164AImprove performanceImprove securityViral antigen ingredientsAntiviralsOil emulsionInfectious bursitis
The invention discloses an infectious bursal disease antigen-antibody complex as well as a preparation and a preparation method thereof. The preparation method of the antigen-antibody complex comprises the following steps of: (1) preparing a VP2 protein as an antigen; (2) preparing an egg yolk antibody from an infectious bursal disease virus inactivated vaccine as an antibody; and (3) mixing the antigen and the antibody according to the prescription proportion, and performing sterilization treatment. The antigen-antibody complex preparation mainly refers to a common liquid preparation, an oil emulsion and a solid preparation prepared from the infectious bursal disease antigen-antibody complex. The antigen-antibody complex disclosed by the invention has the characteristics of stable performance, good safety, fast immunization effect after animal immunization, strong immunity and high protection rate; and the prepared complex preparation is simple and quick to prepare and safe and convenient to use.
Owner:TIANJIN RINGPU BIO TECH
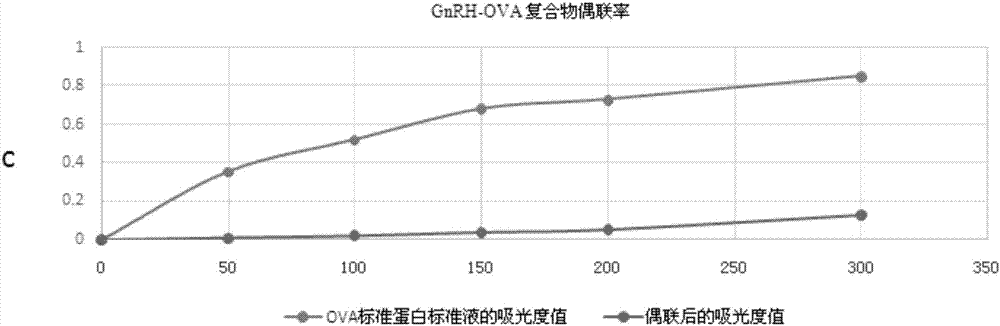
GnRH antigen and application thereof in active immunization affecting castration effect and meat quality of oxen
ActiveCN106986923AImproving immunogenicityEnhance antigen immunogenicityVertebrate antigen ingredientsLuteinising hormone-releasing hormoneActive immunizationMale rats
The invention discloses a GnRH antigen and application thereof in active immunization affecting the castration effect and meat quality of oxen. The invention provides a GnRH derivative which is obtained by inserting oligopeptides, which can form alpha helixes, among multiple serially-connected single GnRH antigens, and each single GnRH antigen is a polypeptide obtained by replacing the sixth-site glycine in the amino acid sequence of gonadotropin releasing hormone GnRH with D type lysine. When a GnRH two-string alpha-helix vaccine screened by experiments is used to actively immunize male rats and the oxen, the vaccine can allow the biological activity of testosterone to be partially or completely lost, an immunocastration effect is achieved, a good immunization enhancing effect is achieved, and the carcass quality of immunized animals can be increased to a certain degree.
Owner:XINJIANG ACADEMY OF AGRI & RECLAMATION SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com