Production method of virus-inactivated plasma for treating COVID-19
A COVID-19, plasma technology, applied in the field of medicine, can solve the problems of missed detection during the detection technology window period, and achieve the effect of reducing mortality and improving cure rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
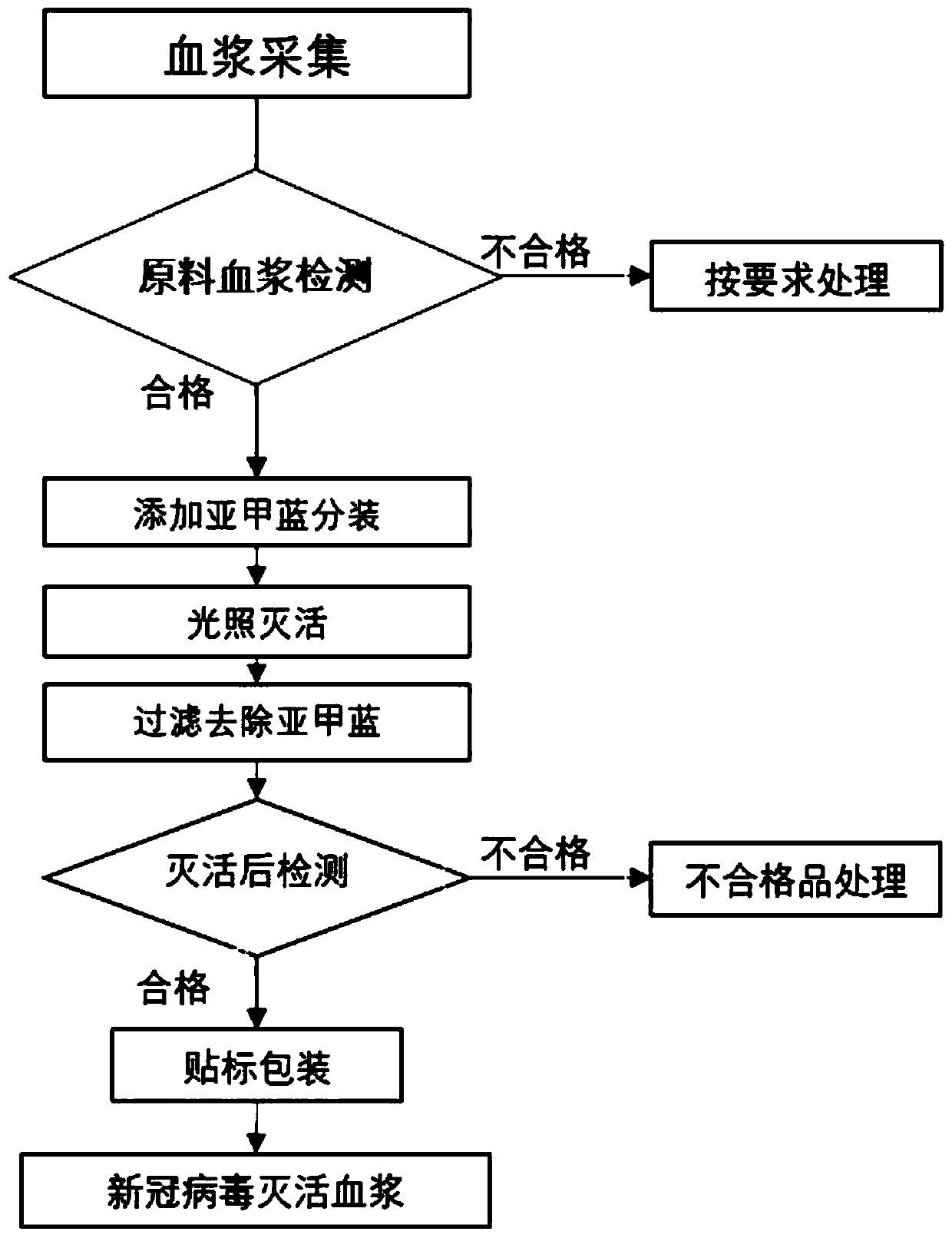
[0022] Example 1. Plasma collection of convalescent patients with COVID-19
[0023] 1. Plasma collection standards,
[0024] Rehabilitation patients who donate plasma should meet the following conditions at the same time:
[0025] ①The recovery from the new crown is not less than 3 weeks from the first symptom;
[0026] ②Recovered patients meet the criteria for release from isolation and discharge in the latest version of the COVID-19 diagnosis and treatment plan issued by the National Health and Medical Commission;
[0027] ③The age should be at least 18 years old and not more than 55 years old;
[0028] ④The weight of men is not less than 50 kg, and that of women is not less than 45 kg;
[0029] ⑤ No history of menstrual blood-borne diseases;
[0030] ⑥Those who can donate plasma after comprehensive patient treatment and other related evaluation by clinicians.
[0031] 2. Collection steps:
[0032] After signing the informed consent form, plasma collection is performed...
Embodiment 2
[0042] Embodiment 2, the operation process of methylene blue inactivated plasma
[0043] This method requires aseptic technique throughout.
[0044] 1. Add methylene blue to the raw material plasma: the plasma flows through the methylene blue (MB) adding element, flows into the light bag, and the methylene blue content in the blood plasma flowing into the light bag is 0.9-2.6 μmol / L, preferably 0.9-1.3 μmol / L.
[0045] 2. After the addition of methylene blue, heat seal (seal by heating) the plasma inlet tube, put the plasma-containing light bag and the adsorption filter component connected to it into the medical plasma virus inactivation cabinet, and light inactivate it. The light intensity is 30000-40000lx (30W fluorescent lamp, wavelength 600-700nm), the swing range is 50mm±10mm, the swing frequency is 60 times / minute±5 times / minute, and the effective light time is not less than 30min.
[0046]3. After the light inactivation is completed, place the light bag at a high pla...
Embodiment 3
[0050] Embodiment 3, inactivated plasma preparation example 1
[0051] 1. Melt 3 bags of plasma from patients who have recovered from COVID-19, and follow the steps below for each bag of plasma;
[0052] 2. Add methylene blue (MB): Flow a small amount of plasma through the "MB adding element", and then close the stop clamp. When the plasma stays in the MB adding element for about 1-2 minutes, the sub The content of formazan blue is 0.9-1.3μmol / L, and then open the stop clip to make the plasma completely flow into the light bag;
[0053] 2. After the addition of methylene blue, heat-seal the inlet tube, put the plasma-containing light bag and adsorption filter parts into the medical plasma virus inactivation cabinet, and light inactivate the plasma virus. The light intensity is 30000-40000lx, the swing range is 50mm±10mm, the swing frequency is 60 times / minute±5 times / minute, and the effective light time is 30min;
[0054] 3. After the light inactivation is completed, place t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com