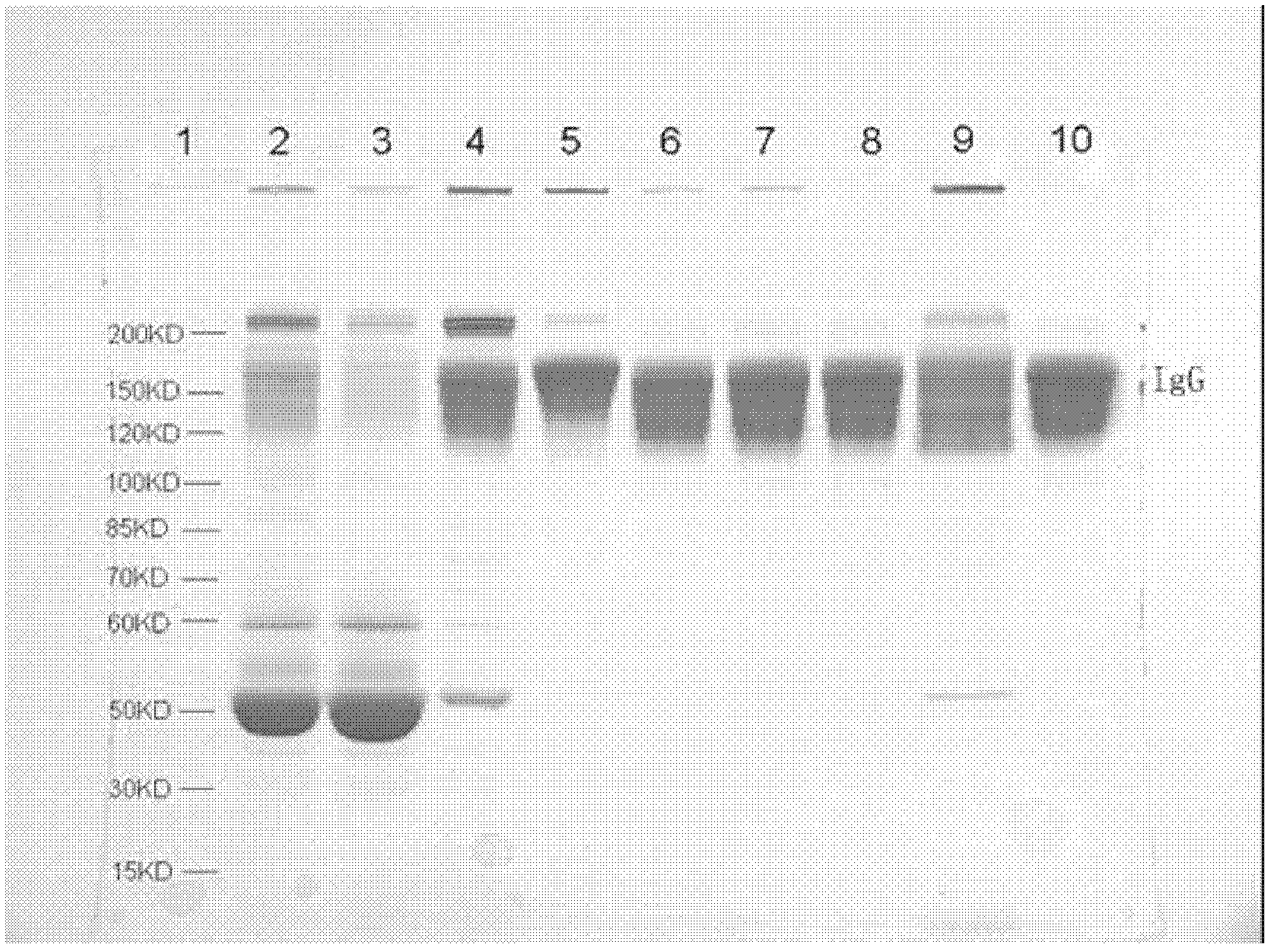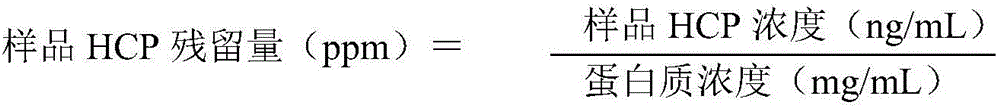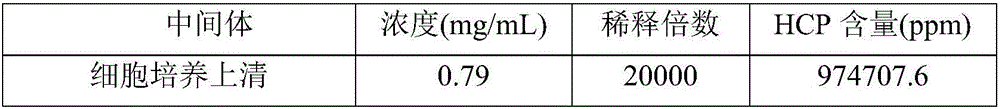Patents
Literature
531 results about "Virus inactivation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virus Inactivation. The inactivation of virus contaminants can be achieved by subjecting the bioprocess fluid to conditions that denature the virus protein but not the active ingredient. In the production of biologic therapies, the two most commonly employed inactivation methods are the use of low pH or addition of detergents.
Viral inactivated platelet extract, use and preparation thereof
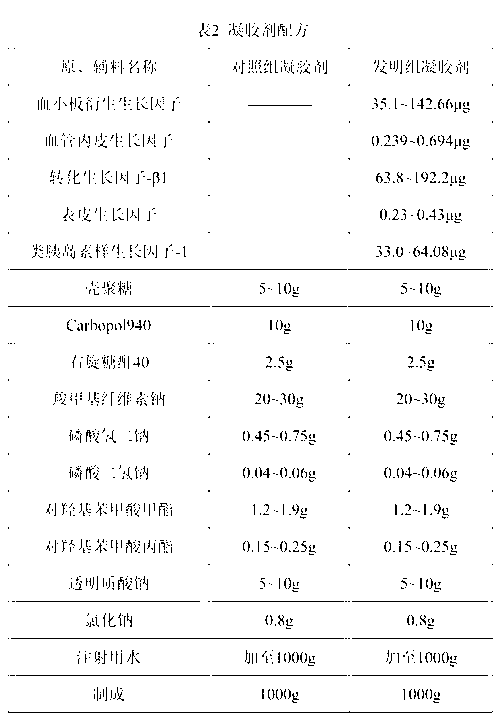
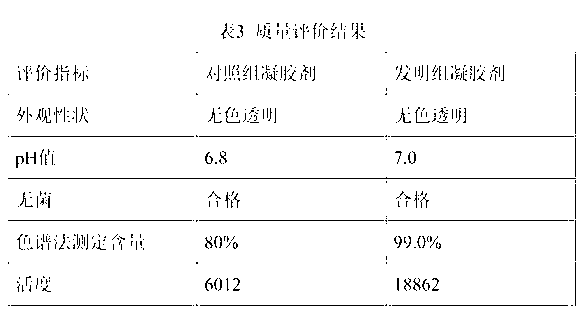
ActiveUS20120156306A1Need longerImprove performancePeptide/protein ingredientsAntipyreticVirus inactivationBiological organism
Owner:OMRIX BIOPHARM
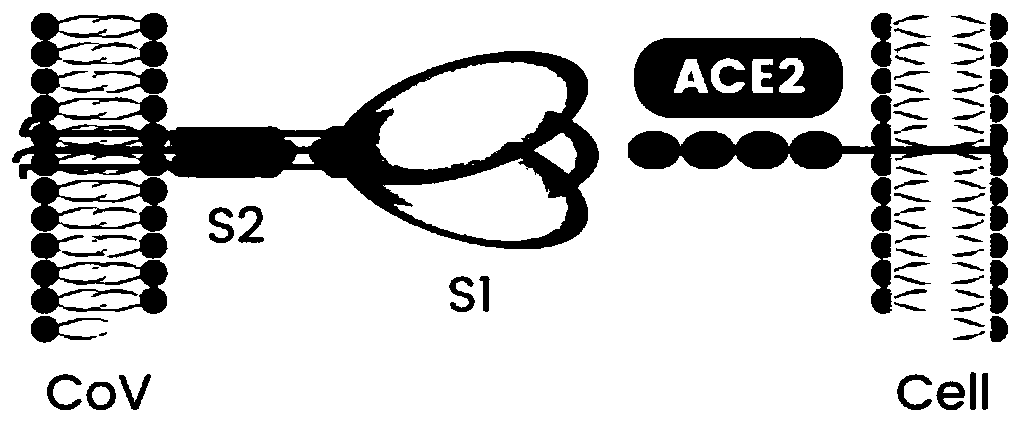
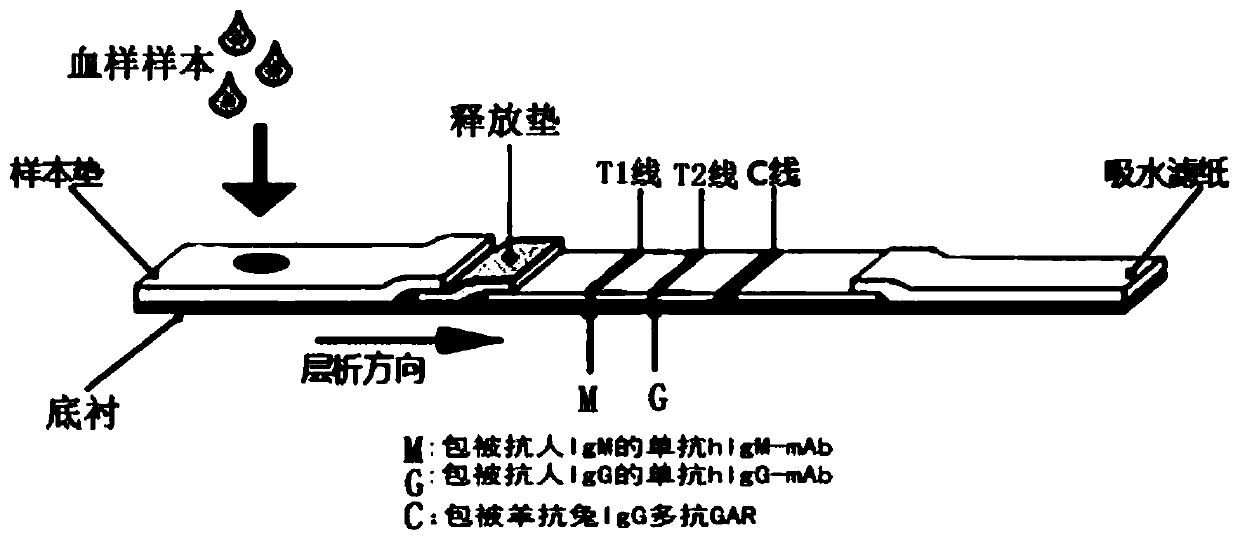
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
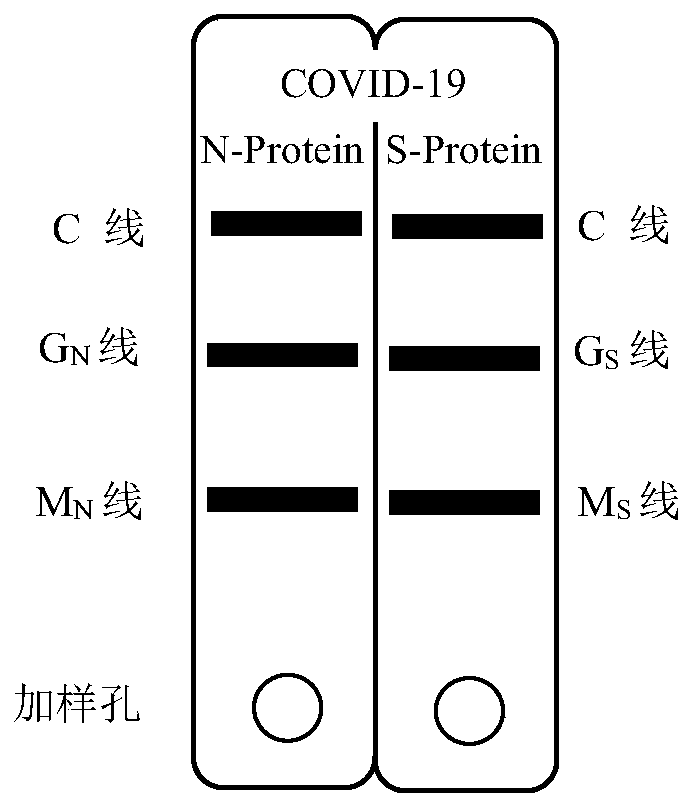
Novel coronavirus pneumonia (COVID-19) serological diagnosis kit
ActiveCN111239392AHigh detection sensitivityImprove detection accuracySsRNA viruses positive-senseVirus peptidesSerodiagnosesAntigen
The invention discloses a novel coronavirus pneumonia (COVID-19) serological diagnosis kit. The kit comprises an S-IgM / IgG test strip and an N-IgM / IgG test strip, the double-antigen quadruple detection kit can be used for simultaneously detecting four indexes of an IgM / IgG antibody for resisting novel coronavirus spinous process protein S and an IgM / IgG antibody for resisting novel coronavirus nucleocapsid protein N in serum of a patient suffering from novel coronavirus pneumonia COVID-19. According to the kit, the detection sensitivity is improved through quantum dot fluorescence labeling andmultistage coupling amplification signals, the detection accuracy is improved through double-antigen quadruple detection, and the biological safety in the detection process is guaranteed by establishing a virus inactivation system. The kit is suitable for whole blood, plasma and serum detection, and can be applied to novel COVID-19 serological diagnosis.
Owner:浙江诺迦生物科技有限公司 +1
Preparation method and application of feed
ActiveCN103222541AImprove immunityImprove survival rateAnimal feeding stuffBiotechnologyAnimal science
The invention belongs to the technical field of feeds, and particularly relates to a preparation method of a feed. The feed which can be directly fed is obtained by taking kitchen wastes as raw materials, feeding cockroaches, catching and killing the cockroaches and carrying out sterilization and / or virus inactivation treatment. The method comprises the following steps of: collecting kitchen swill; feeding the kitchen swill to the cockroaches; and catching and killing the cockroaches, and sterilizing and disinfecting the cockroaches to obtain the feed which can be directly fed. According to the invention, the kitchen wastes are taken as the raw materials to feed the cockroaches, after the cockroaches are grown to adults, the cockroaches are killed and / or subjected to the sterilization treatment to obtain the feed with high protein content, and the feed is used as the feed directly used for breeding; and the feed is dried and grinded into powder and then fed to poultry or other animals, or the feed is dried and grinded into the powder and then mixed with various other vegetable proteins, starches, or fibers, and the like to be fed to the poultry or other animals, so that the problem of protein homology is prevented, the problem of potential safety hazard of foods is solved, the law of a food chain is followed, and the problems of resource and harmlessness utilization of the kitchen wastes are solved.
Owner:山东鲲鹏农业发展有限公司
Method for preparing animal derived implantable medical biomaterial
The invention provides a method for preparing an animal derived implantable medical biomaterial. The method comprises the following steps of: pretreatment, separation and primary cleaning of an animal tissue material; virus inactivation; decellularization; sodium chloride treatment; formation; packing and sterilization. According to the animal derived decellularized extracellular cell matrix (ECM) material prepared by using the method, animal derived cell components and DNA components are completely removed, meanwhile, the components and the three-dimensional structure of the natural ECM are completely kept, active growth factors for promoting tissue regeneration can be induced, residues of endotoxin, organic solvent and toxic solvent are avoided, and products with different sizes, thicknesses and mechanical strengths can be formed according to different indications.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Method for purifying anti-HER2 or/and anti-HER3 antibody proteins
ActiveCN102492040ASpeed up filteringReduce manufacturing costImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsVirus inactivationPurification methods
The invention belongs to the biochemistry technical field, concretely relates to a method for purifying anti-HER2 or / and anti-HER3 antibody proteins. The method of the invention comprises the following the steps: clarifying a broth, carrying out affinity chromatography, carrying out inactivation on virus by acidic pH value, carrying out cation exchange chromatography, carrying out anion exchange chromatography; and filtering virus; wherein, the step of virus inactivation by acidic pH value and the step of virus filtration can be inserted at any position of the step after the affinity chromatography. The method of the invention comprises the following advantages that: 1) the chromatography step can be reduced to three steps, thereby the production efficiency can be enhanced and the production cost can be minimized. 2) the step of virus filtration is added, thereby the security can be raised.
Owner:GENOR BIOPHARMA +1
Method for purifying human immunoglobulin from separated component I+III of blood plasma
ActiveCN102250240ARealize comprehensive utilizationAvoid pollutionPeptide preparation methodsImmunoglobulinsBiotechnologyUltrafiltration
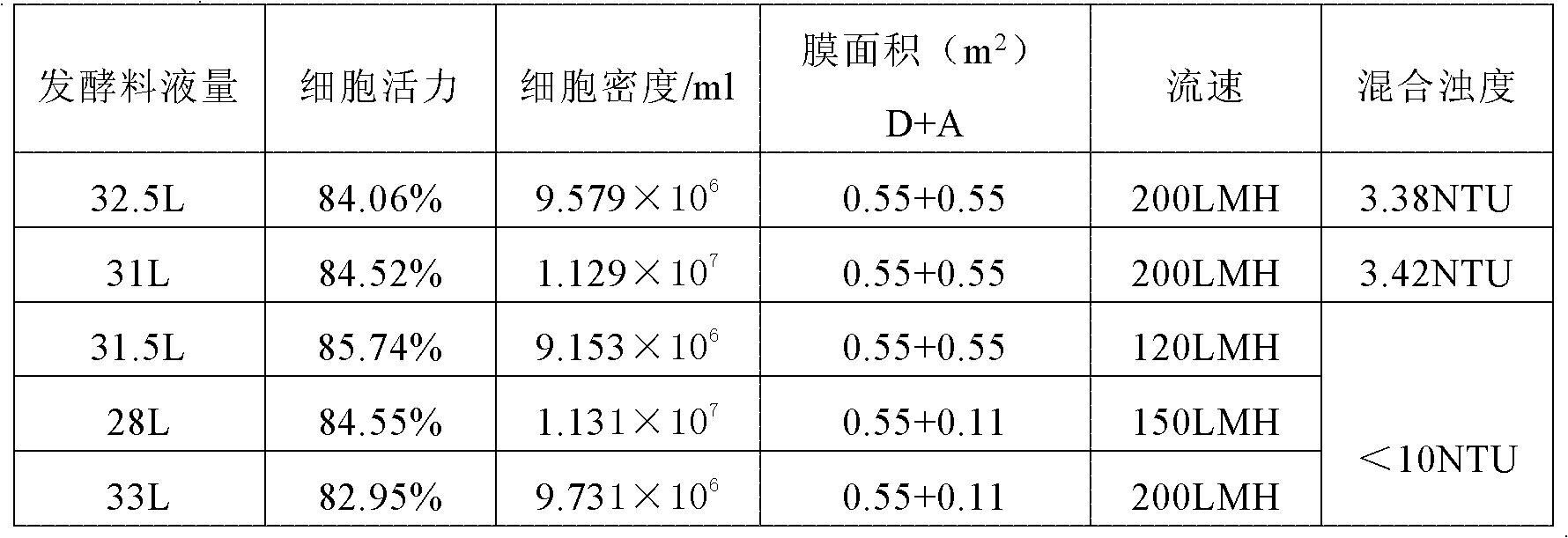
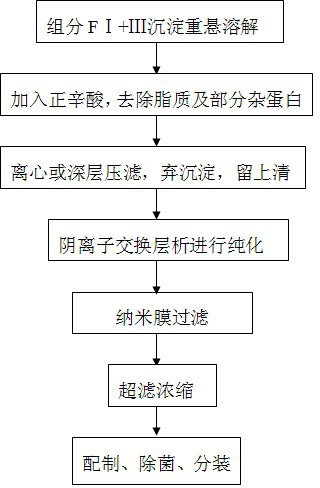
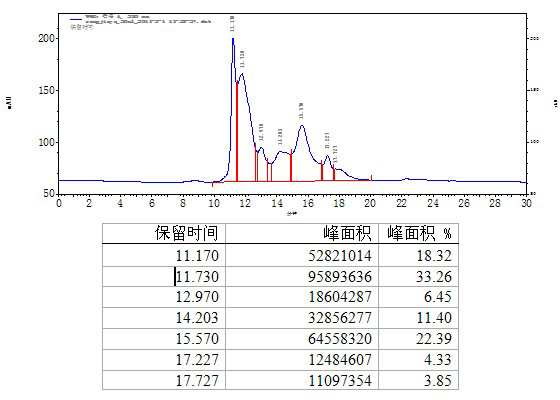
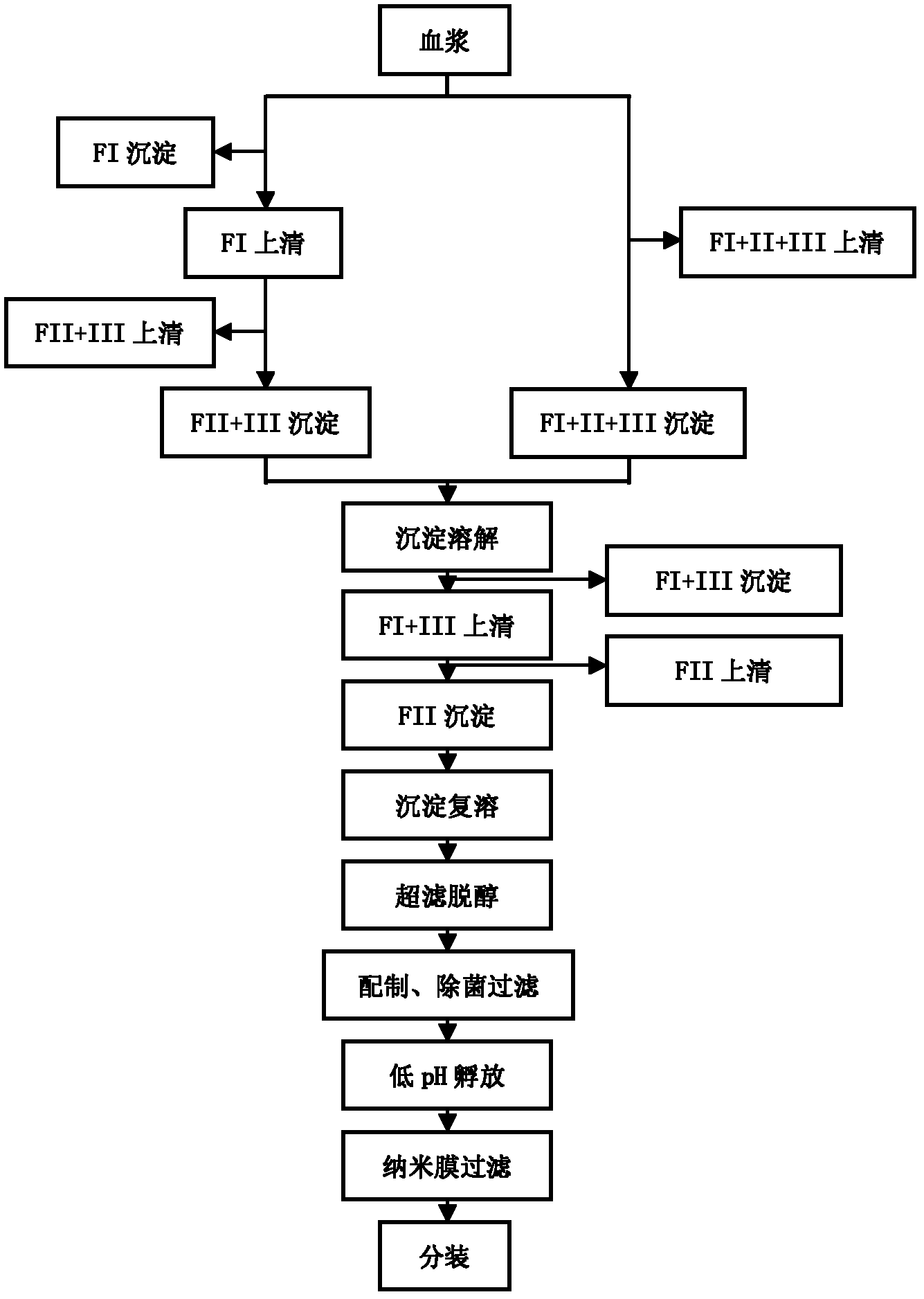
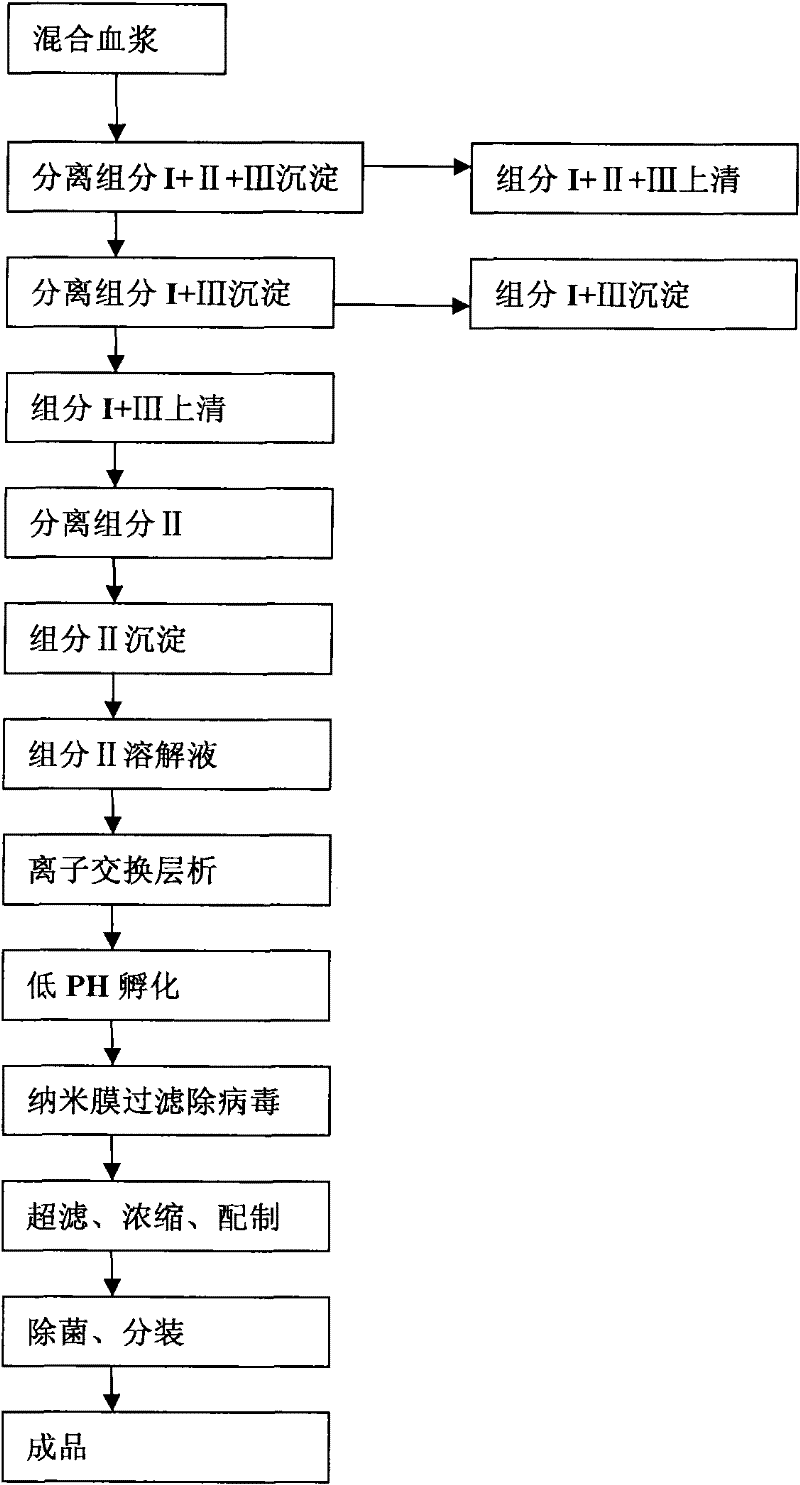
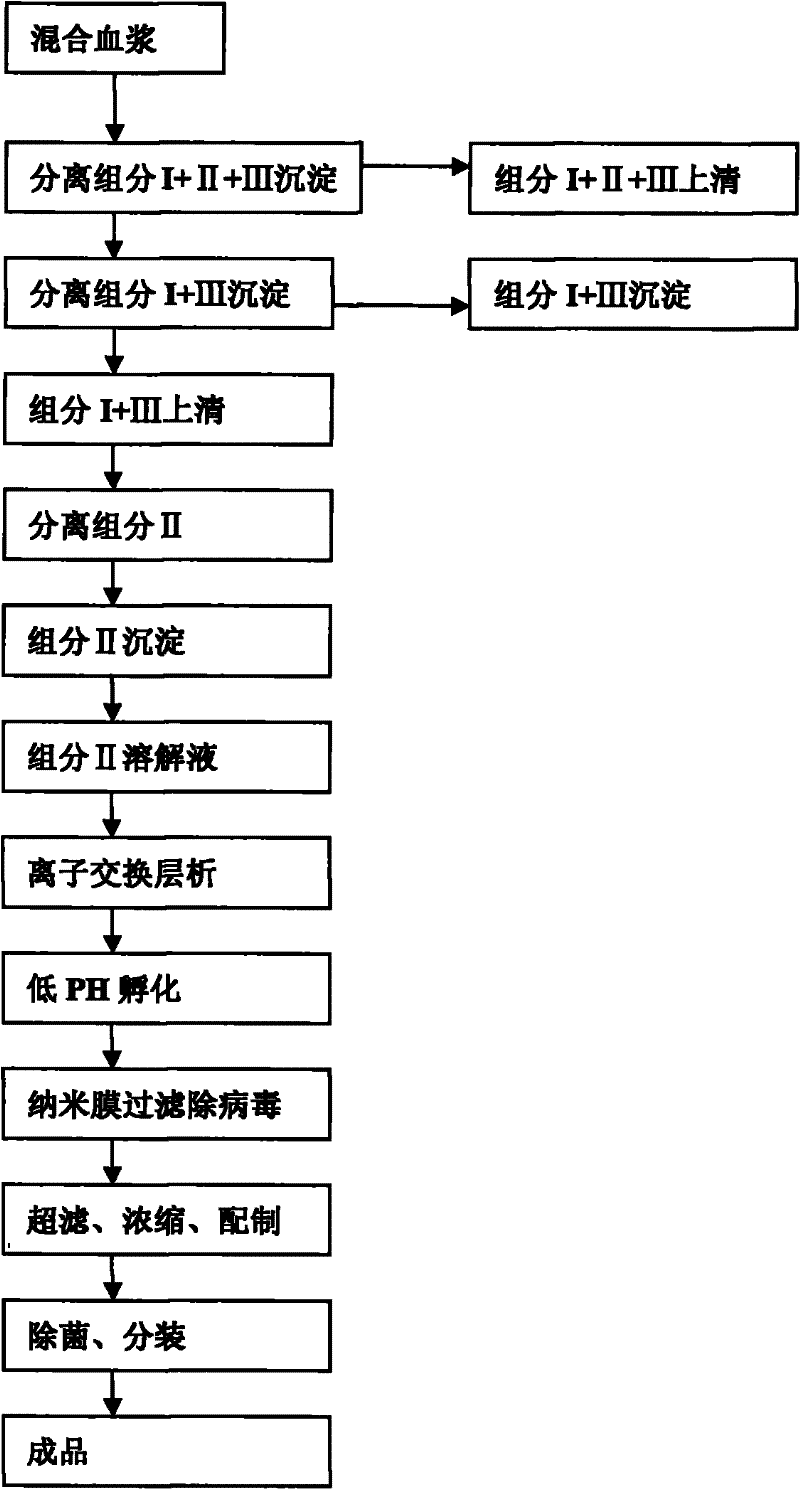
The invention relates to a method for separating and purifying human immunoglobulin from a component I+III of blood plasma, and aims to provide a high-efficiency method for recovering high-purity human immunoglobulin. According to the technical scheme provided by the invention, the method comprises the following steps of: a, fully dissolving component I+III precipitate; b, precipitating with octylic acid and removing lipid and a part of impurity protein to prepare IgG (Immunoglobulin G); c, purifying through anion exchange column chromatography; and d, collecting flow-through liquid, performing membrane nanofiltration, ultrafiltration and concentration, preparing the human immunoglobulin, sterilizing and packaging. The method has the beneficial effects of capability of being operated at the room temperature, simple and short steps, high yield, low energy consumption and high output and is suitable for mass production; comprehensive utilization of the blood plasma is fully realized; the time of the entire production process is shortened; the cost is reduced; extremely considerable economic benefit can be produced; the safety of a product is guaranteed by using two virus inactivation / elimination methods of different mechanisms; the environmental pollution is avoided; and the method has high economic and social values.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Intravenous injection of cytomegalovirus human immunoglobulin and its preparation method
ActiveCN102286099ASteps to reduce precipitationKeep aliveImmunoglobulins against virusesAntiviralsEthanol precipitationAnion-exchange chromatography
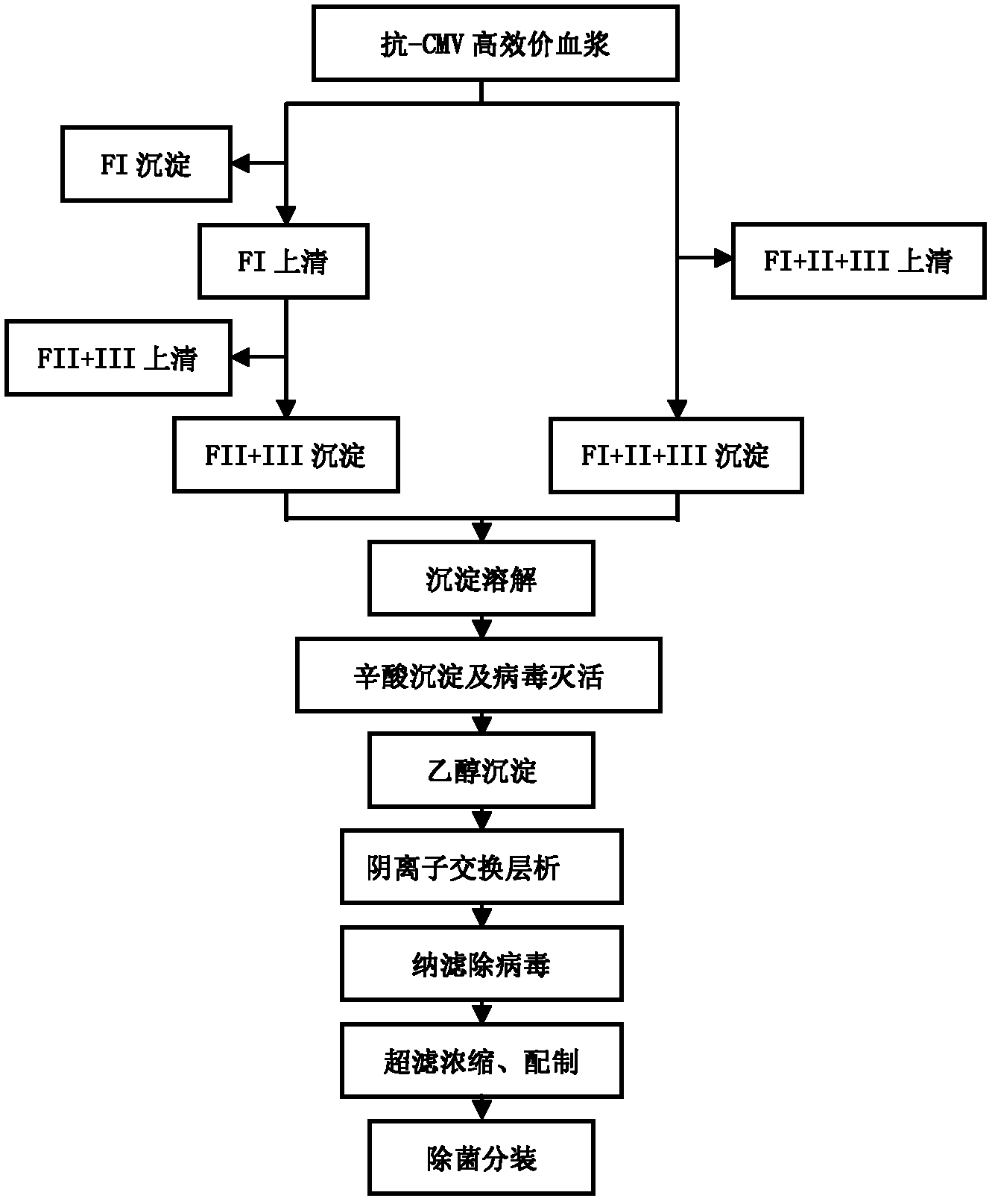
The invention discloses a human cytomegalovirus immunoglobulin for intravenous injection and a preparation method thereof, and aims to improve the purity, yield and safety of the product. In the invention, the specific activity of the human cytomegalovirus immunoglobulin for intravenous injection is not less than 2.5 PEI-U / mg, the anti-CMV titer is not less than 100 PEI-U / ml, the purity is greater than 98.2%, and the protein content is 51-55 mg / ml. Caprylic acid precipitation and anion exchange chromatography are used instead of the partial ethanol precipitation step in the traditional low-temperature ethanol method, thereby keeping IgG in the supernate all the time so as to keep the IgG activity; and processes of caprylic acid virus inactivation and nano film virus removal are used, thereby effectively ensuring the safety of the product. Researches show that the preparation method disclosed by the invention improves the purity, yield and safety of the product, saves the energy and reduces the production cost.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
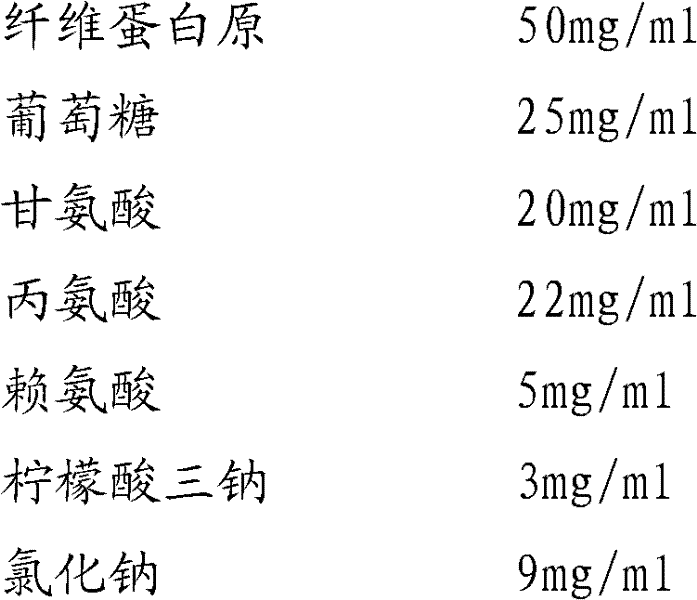
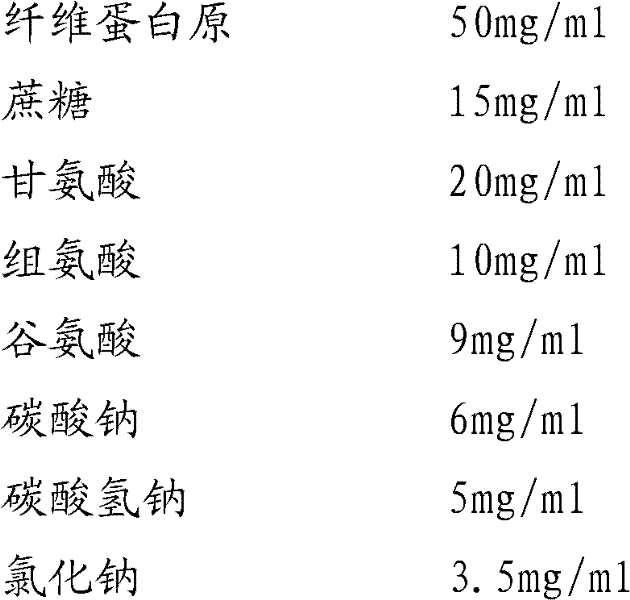
Instant lyophilized fibrinogen and fibrin ferment formulation composition, preparation method and use thereof
ActiveCN101371921ASolve the shortcoming of short storage timeImprove solubilityPeptide/protein ingredientsBlood disorderArginineIrritation
The invention provides a composition of instant freeze-dried fibrinogen and thrombin preparation, a preparation method and a use thereof, and the composition comprises composition 1 composed of 35-70mg / ml of fibrinogen, 9-15mg / ml of sodium citrate, 7-12mg / ml of sodium chloride, 0.3-0.6mg / ml of polysorbate-80, 10-15mg / ml of mannitol, 4 -8mg / ml of arginine and 3.5-5.5mg / ml of glutamic acid by mg / ml and composition 2 composed of 700-1,200 mg / ml of thrombin, 3-6mg / ml of dextran 20, 15-25mg / ml of glycine, 5-7mg / ml of sodium chloride and 4-7mg / ml of calcium chloride by mg / ml. A sealant avoids the risk of spreading of AIDS virus, and the like, increases the drug stability, reduces the degeneration during the virus inactivation process by dry-heat method, protects biological activity, avoids local liquid storage of the using part caused by high permeability and the irritation of a large amount of inorganic salts to tissues, is conductive to the healing of trauma sites and ensures product safety and independent package to the maximum extent, thereby increasing storage time, facilitating use and meeting the clinical and field first-aid needs.
Owner:HANBANG MEDICAL SCI & TECH HARBIN CITY
Preparation method of fibrinogen
ActiveCN102286095AHigh purityImprove extraction efficiencyFibrinogenPeptide preparation methodsSolubilityFreeze-drying
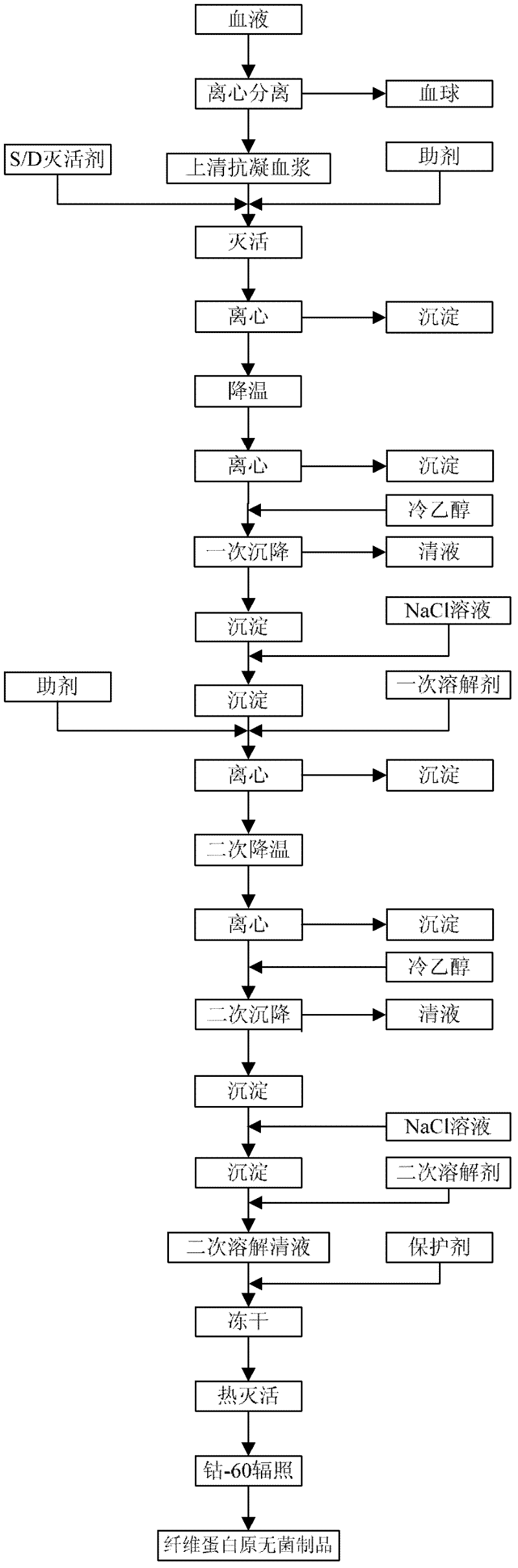
The invention provides a preparation method for fibrinogen with the advantages of high dissolution rate in the production process, short production period, fast solubility in clinical use, good biological activity, high safety in clinical use and high yield. An aid is added into supernate anticoagulant plasma. Compared with the prior art, the preparation method has the advantages that: 1, the preparation method provided by the invention is high in extraction efficiency; 2, the aid adopted in the method can improve the quality of the fibrinogen product, and plays a role in protecting the biological activities of the fibrinogen and blood coagulation factors XIII in virus inactivation and cold ethanol settlement processes; 3, in the process, the process solubility time of the fibrinogen is shortened to about 10 minutes; and 4, the solubility time of the freeze dried product of the fibrinogen is within 30 seconds, so precious time is won for rescuing patients in time in clinic.
Owner:DATIAN HUACAN BIO TECH
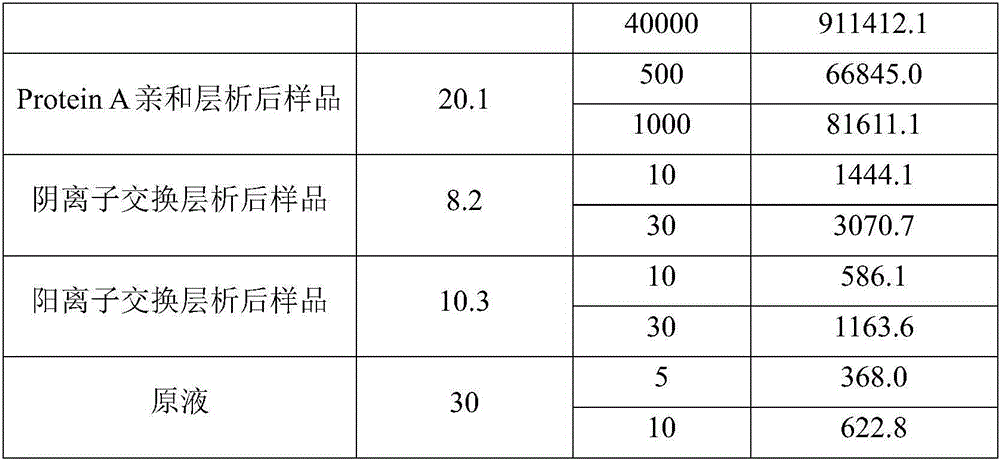
Method for effectively removing host protein in monoclonal antibody downstream purification process
ActiveCN106749660AImmunoglobulins against growth factorsPeptide preparation methodsVirus inactivationFiltration
The invention relates to a method for effectively removing a host protein in a monoclonal antibody downstream purification process. The method comprises the following steps: (1) performing deep filtration on cell culture fluid, and collecting the filtrate; (2) performing Protein A affinity chromatography on the filtrate obtained in the step (1); (3) performing virus inactivation on the sample obtained in the step (2), performing deep filtering on the sample subjected to virus inactivation, thereby effectively removing the host protein in the sample containing the monoclonal antibody. According to the method disclosed by the invention, the deep filtering after virus inactivation is adopted, the deep filtering process after Protein A affinity chromatography and virus inactivation is optimized, the content of HCP in the culture fluid supernatant collected by deep filtering can be reduced to 2.3ppm by virtue of two purification steps, namely the Protein A affinity chromatography and deep filtering after virus inactivation, and the content of the HCP can be reduced to 0.4ppm by virtue of further anion and cation exchange chromatography.
Owner:GENOR BIOPHARMA
Method for stabilizing a cryoprecipitate of plasmatic proteins for being subjected to a viral inactivation thermal treatment
ActiveUS20060247426A1Shorten the timeImprove protectionPowder deliveryPeptide/protein ingredientsCryoprecipitateMedicine
The invention relates to a method for obtaining cryoprecipitatable proteins, comprising a viral inactivation step by thermally treating a lyophilisate of these proteins, comprising, before rendering the proteins in the form of a lyophilisate, an initial addition step, to these proteins, of a stabilizing and solubilizing formulation containing a mixture consisting of arginine, at least one hydrophobic amino acid and of tribasic sodium citrate. The invention also relates to a concentrate consisting of at least one cryoprecipitable protein containing the stabilizing and solubilizing formulation introduced according to the method and being suited for therapeutic use.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Accellular epimatrix and preparation method and application thereof
InactiveCN108261564AGood flexibilityGood biocompatibilityTissue regenerationProsthesisVirus inactivationCollagen fiber
The invention discloses a preparation method of an accellular epimatrix, the accellular epimatrix prepared by the preparation method and application of the accellular epimatrix in the field of preparation of medical instruments. The preparation method of the accellular epimatrix comprises the following steps: carrying out pretreatment on tissues and organs of mammals to obtain an analogy tissue precursor; separately carrying out virus inactivation treatment, DNA eliminating treatment, accellular treatment and defatting treatment on the analogy tissue precursor to obtain an accellular epimatrixprecursor; and successively carrying out gradient dewatering treatment and crosslinking treatment on the accellular epimatrix precursor, and finally shaping and drying to obtain the accellular epimatrix. By the gradient dewatering treatment, the accellular epimatrix precursor is soaked in organic matter solutions of which the concentrations are successively increased successively. According to the preparation method of the accellular epimatrix, the tissue precursor is treated through gradient dewatering, and thus, the orderly structure of collagenous fibers of the finally prepared accellularepimatrix is not damaged.
Owner:SHENZHEN LANDO BIOMATERIALS
Acellular dermal matrix and preparing method of acellular dermal matrix
The invention discloses an acellular dermal matrix and a preparing method of the acellular dermal matrix. The preparing method of the acellular dermal matrix comprises the following steps of: performing pre-processing on the skin of a mammal to obtain a skin corium flap; respectively performing degreasing processing, virus inactivation processing and immunogenicity removal processing on the skin corium flap to obtain an acellular dermal matrix precursor (the immunogenicity removal processing operation comprises the step of alternately soaking the skin corium flap into a prolease solution and a surfactant solution); and sequentially performing dewatering, freeze-drying and shaping on the acellular dermal matrix precursor to obtain the acellular dermal matrix. The preparing method of the acellular dermal matrix has the advantages that the skin corium flap is alternately soaked in the prolease solution and the surfactant solution, so that the immunogenicity of the acellular dermal matrix can be reduced to the maximum degree; the normal collagen structure of the acellular dermal matrix is also remained; the prepared acellular dermal matrix has the proper degradation speed, good biocompatibility and good bone induction capability.
Owner:SHENZHEN LANDO BIOMATERIALS
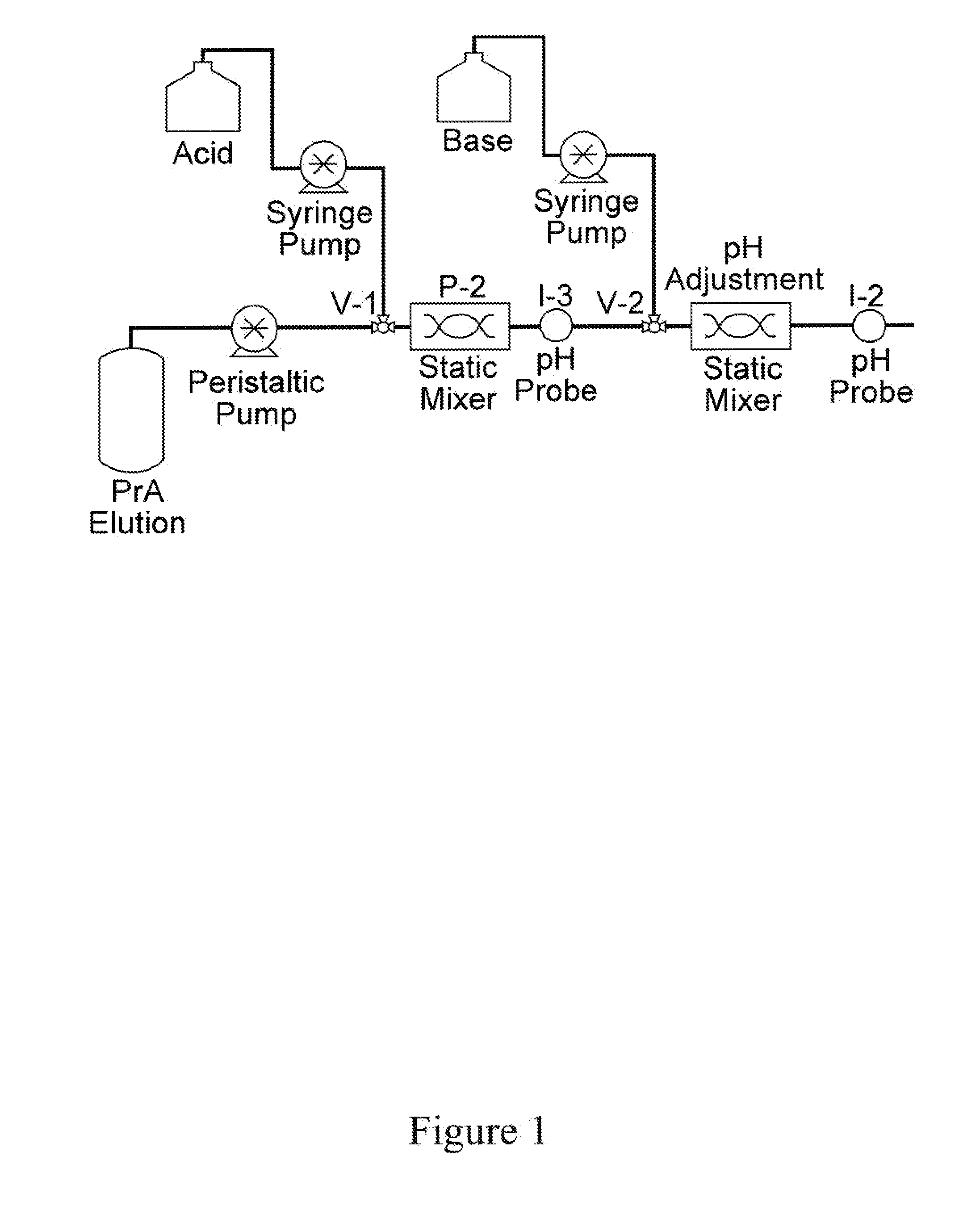
Methods for Inactivating Viruses During a Protein Purification Process
ActiveUS20150064769A1Shorten the timeReduce spacingFlow mixersTransportation and packagingVirus inactivationImproved method
Owner:MILLIPORE CORP
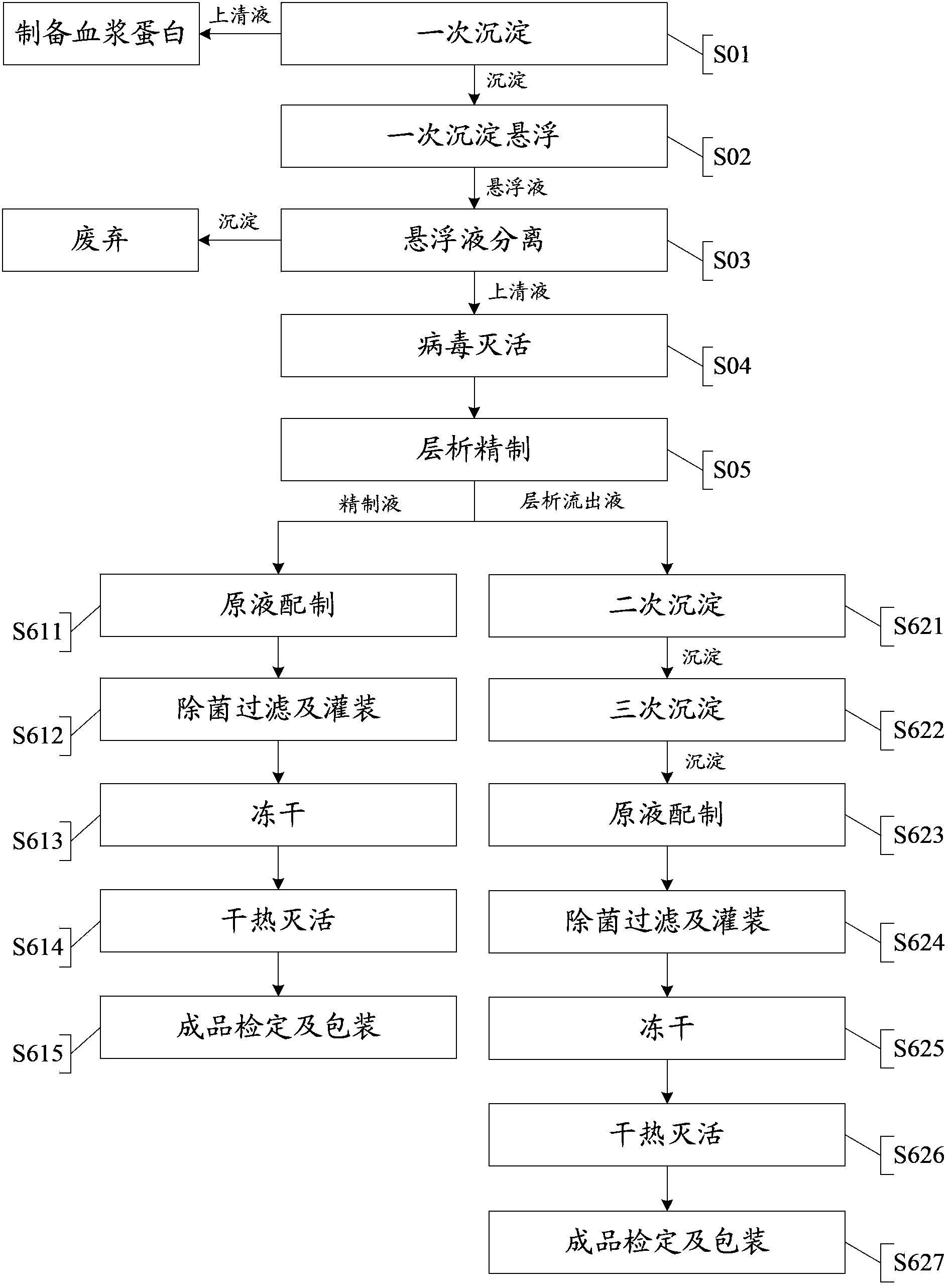
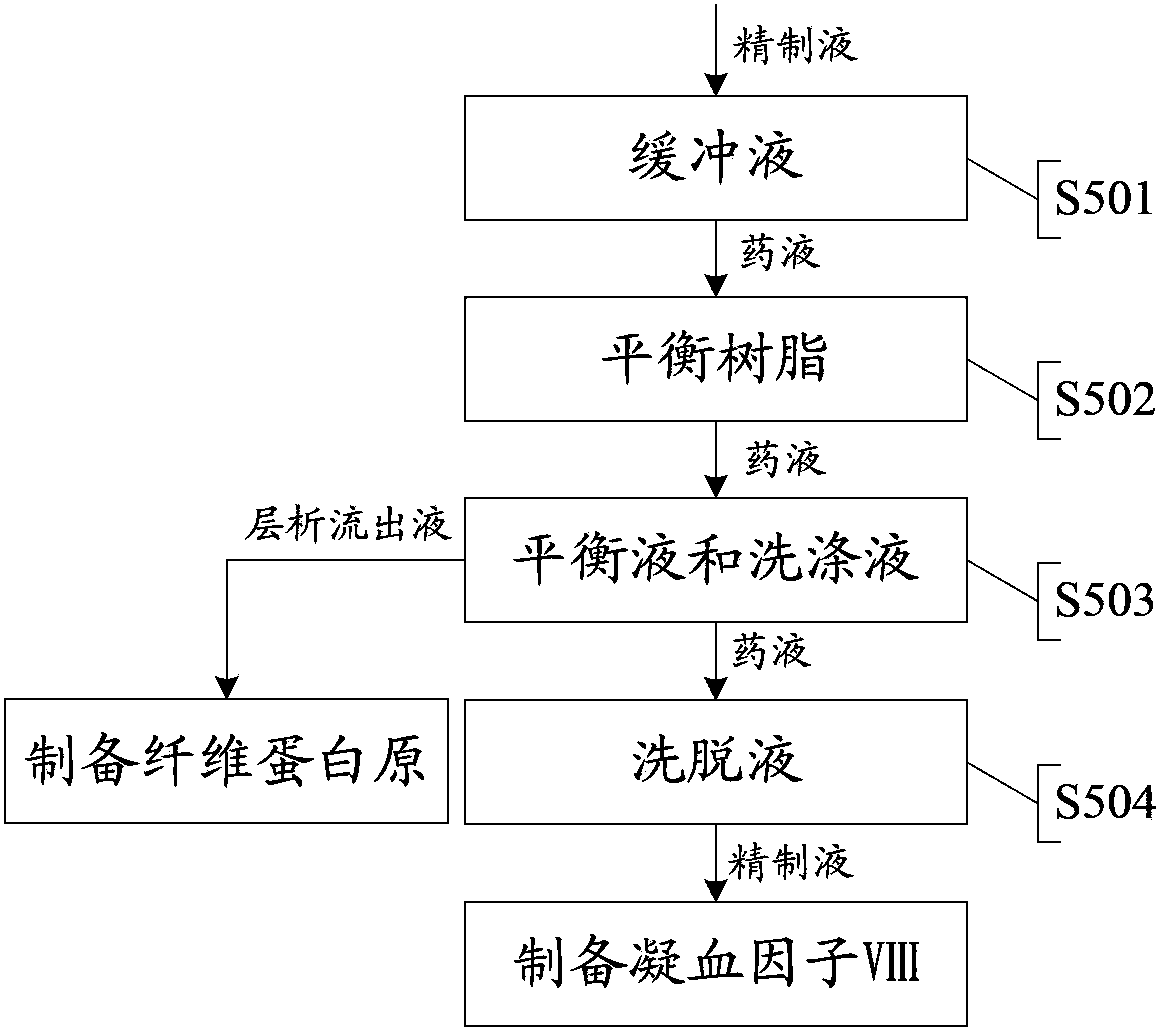
Technology for extracting human blood coagulation factor VIII and human fibrinogen from plasma constituent precipitation
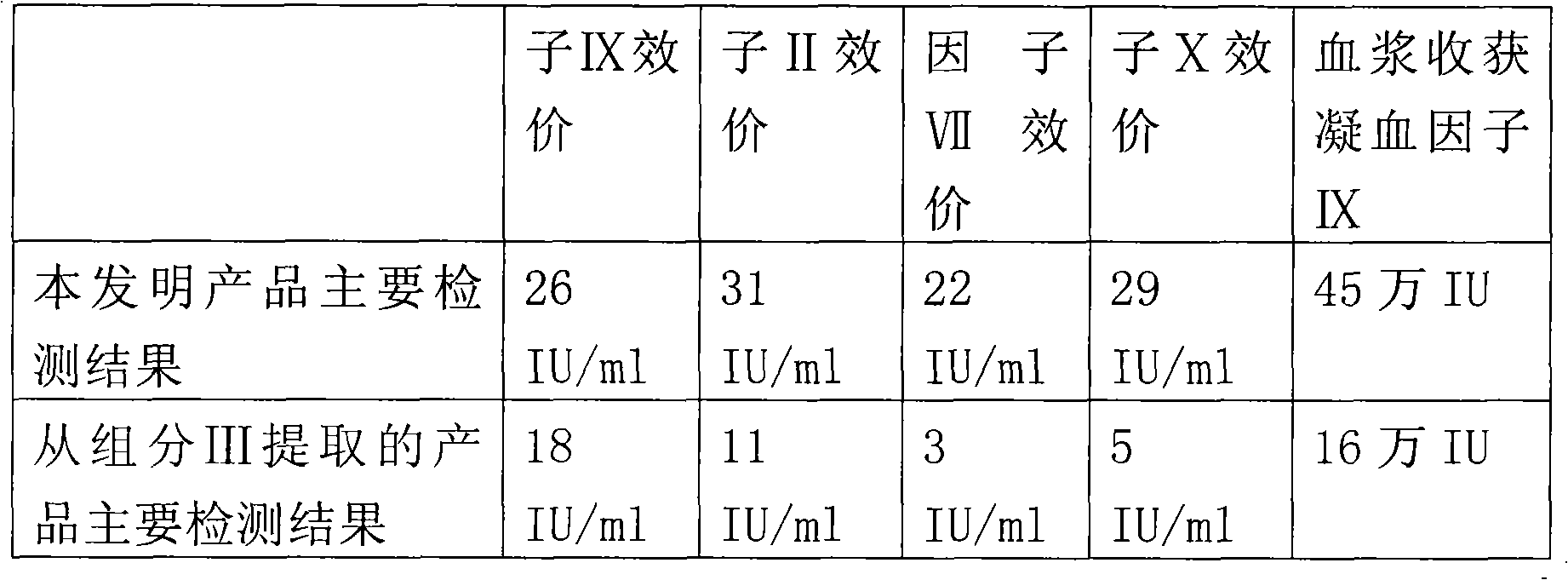
The invention provides a technology for extracting human blood coagulation factor VIII and human fibrinogen from plasma constituent precipitation. The preparation technology comprises the following steps: fresh plasma is subjected to primary sedimentation, so that blood coagulation factor VIII and fibrinogen precipitation can be jointly precipitated from the plasma; the primary precipitation is subjected to suspension; the suspension liquid is subjected to centrifugal separation to obtain supernatant; the centrifugally separated supernatant is subjected to virus inactivation and chromatography refining to respectively obtain human blood coagulation factor VIII refined liquid used for preparing human blood coagulation factor VIII products and chromatography effluent used for preparing human fibrinogen products. According to the invention, the human blood coagulation factor VIII and the human fibrinogen are precipitated through the one-step plasma constituent precipitation, and an ion-exchange column chromatography technology is adopted to perform purification preparation of the human blood coagulation factor VIII and the human fibrinogen, so that the deficiency that the human fibrinogen cannot be normally produced as the human blood coagulation factor VIII is prepared through cryoprecipitation is overcome.
Owner:WUHAN ZHONGYUAN RUIDE BIOLOGICAL PROD CO LTD
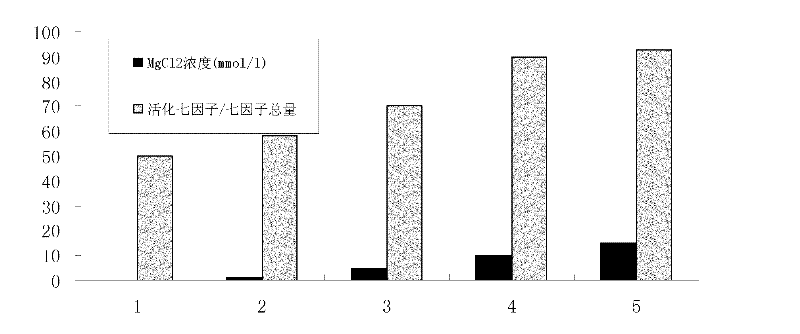
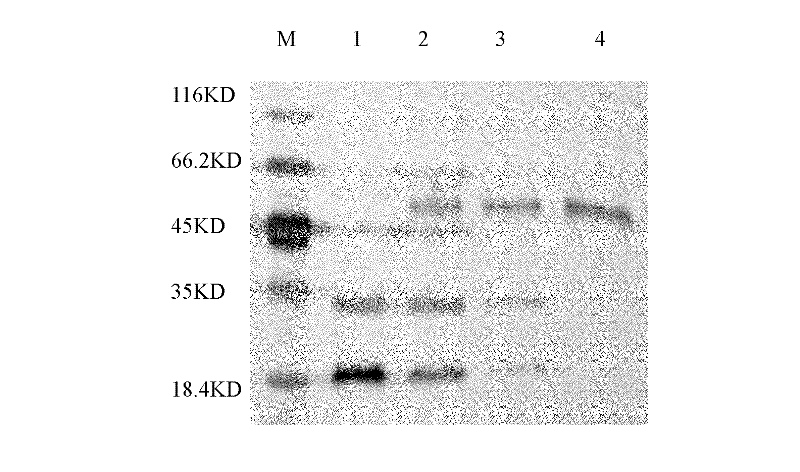
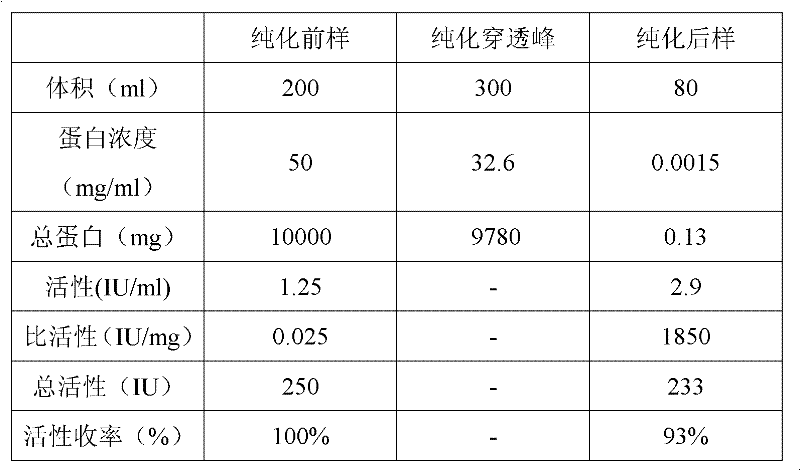
Method for separating and purifying high-purity activated clotting seventh factors from cell culture solution or plasma components
InactiveCN102161701AThe activation process is simpleIncrease profitPeptide preparation methodsBlood coagulation/fibrinolysis factorsVirus inactivationBlood plasma
The invention discloses a method for separating and purifying high-purity activated clotting seventh factors from a cell culture solution or plasma components, successively comprising the following steps: carrying out affinity chromatography purification on raw materials; collecting an eluent containing a clotting seventh factor; adding thrombin and magnesium ions to activate the clotting seventh factor; and finally, condensing an activated product, and carrying out virus inactivation to obtain a final product. The clotting seventh factor can be purified in one step with the affinity chromatography method, the thrombin activation process is simple and efficient, the seventh factor can be purified and separated from the cell culture solution or plasma components, the multipurpose utilization ratio of the plasma is greatly improved, and a safe and efficient high-purity seventh factor protein drug can be provided.
Owner:SYNDEGEN SHANGHAI BIOTECH
Bioprotein sponge and preparation method thereof
InactiveCN102357259AStrong ability to absorb wound exudateReduce volumeSurgical adhesivesPeptide/protein ingredientsSide effectFreeze-drying
The invention discloses bioprotein sponge and a preparation method thereof. According to the characteristics that internal cells of a human body and an animal body adapt to cell growth environments, the bioprotein is freezed into a dry sponge shape through freeze drying technology by utilizing intercellular substances as controlled-release carrieres of growth factors and acts on the trauma of the human body or the animal body in controlled-release medicament feeding mode. The preparation method of the bioprotein sponge changes the traditional preparation process, adopts S / D virus inactivation, and adds protective agent to reduce probability of virus cross infection of people and livestock simultaneously. The CM cation chromatographic purification process improves purity of bioprotein, promotes stanching and tissue union effect of the bioprotein sponge and reduces clinical side effect. An ultrafiltration process is adopted to replace a dialysis process, so that preparation period is shortened, activity of the bioprotein is protected, and production cost is reduced. A method of heating inactivation virus replaces the method of cobalt60 radiation sterilization, so that the fact that radiation rays bring damage to products is avoided, the activity of the bioprotein is protected, and safe and reliable use of products in clinical application is achieved.
Owner:王珊珊
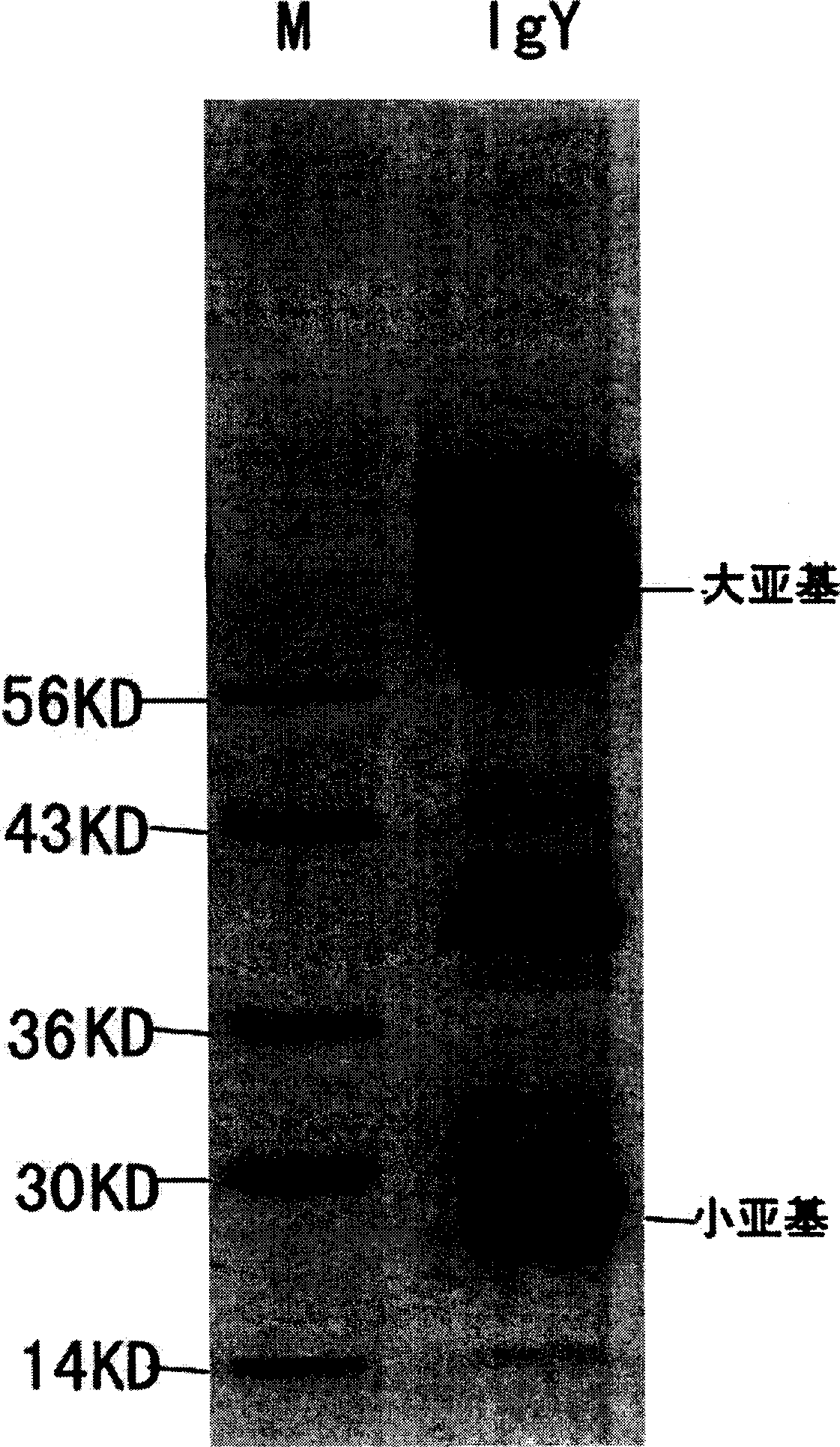
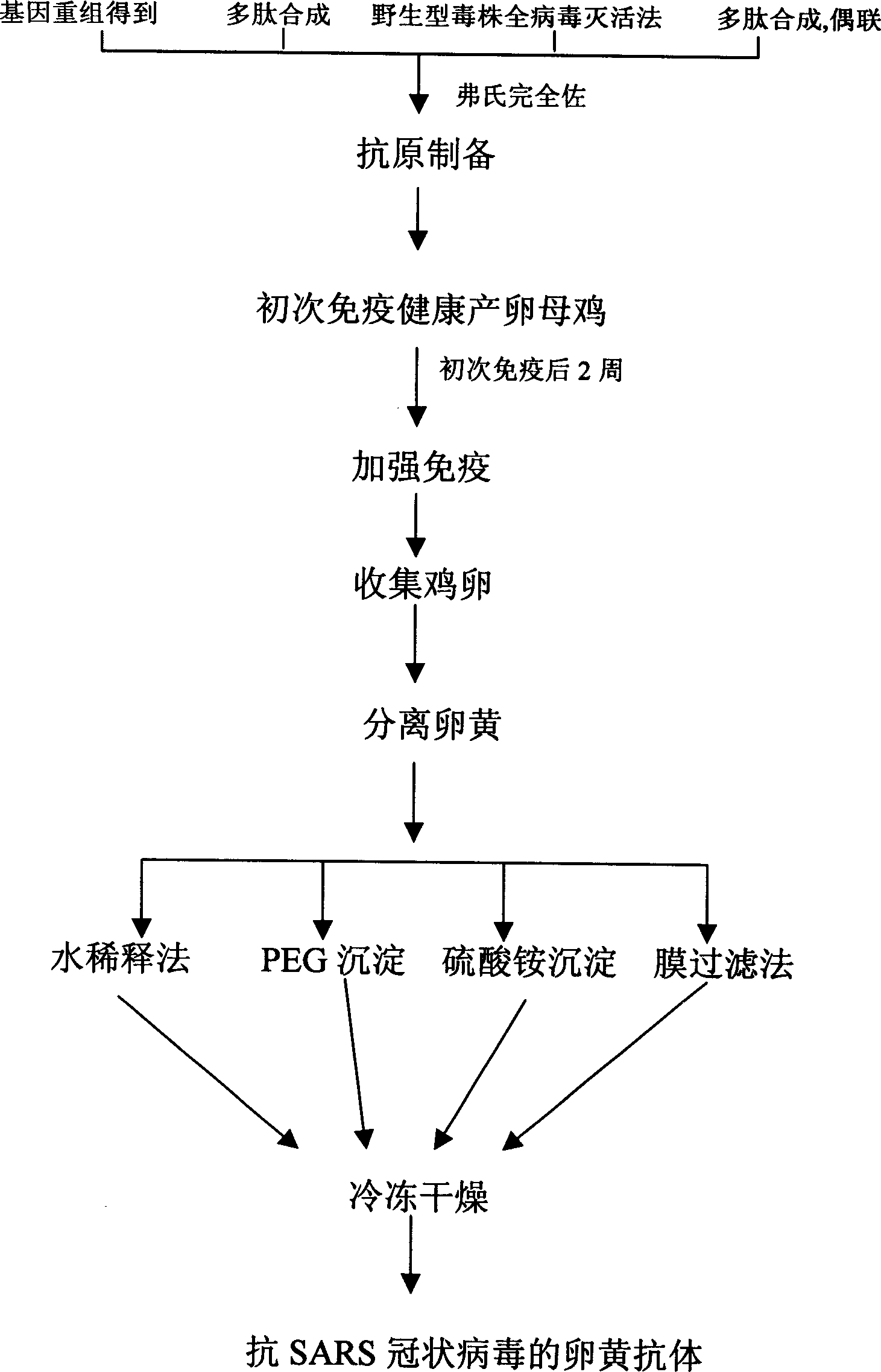
Yolk antibody of anti SARS coronavirus and its preparation method and liquid preparation
InactiveCN1556113AEasy to prepareFast preparation methodEgg immunoglobulinsImmunoglobulins against virusesYolkEpitope
A yolk antibody for SARS coronavirus is prepared through injecting the antigen, which may be recombinant genetic protein S, M, or E,or the antigen epitope for said protein able to represent SARS coronavirus, or the synthetic polypeptide of said protein, etc, in health hen for primary immunizing, booster immunizing, collecting its egg, extracting yolk, and extracting the yolk antibody from it. It can also be prepared to become liquid preparation. It can be used to prevent SARS.
Owner:BIOINFORBODY
Biological amnion and preparation method thereof
ActiveCN103520780AEasy to prepareWide variety of sourcesLayered productsSurgeryFreeze-dryingDrug biological activity
The invention relates to a biological amnion and a preparation method thereof. The biological amnion has three layers including a slow release layer, an amnion layer and a collagen layer from top to bottom, wherein the slow release layer consists of collagen and biological active factors, the amnion layer consists of an amnion subjected to decellularization treatment, and the collagen layer is formed by freeze-drying and compounding collagen. The biological amnion is prepared through the steps of raw material pretreatment, virus inactivation, decellularization treatm. The biological amnion prepared by the invention has the characteristics of an effect of slowly releasing the active factors, low antigenicity on removing epithelial cells, convenience in product operation, difficulty in curling, good adhesiveness with surrounding tissues, difficulty in sliding and the like. Meanwhile, by using a process for performing the decellularization treatment on the amnion, disclosed by the invention, a natural compact collagen structure in the amnion can be effectively retained, the biological amnion can fully achieve a physical barrier effect after being applied to tenorrhaphy, and an animal experiment proves that the biological amnion can effectively achieve an effect of preventing tissue adhesion.
Owner:SHAANXI RUISHENG BIOTECH
Preparation process of human prothrombin compound
ActiveCN101974070AImprove securitySimplify production stepsPeptide preparation methodsPharmacyVirus inactivation
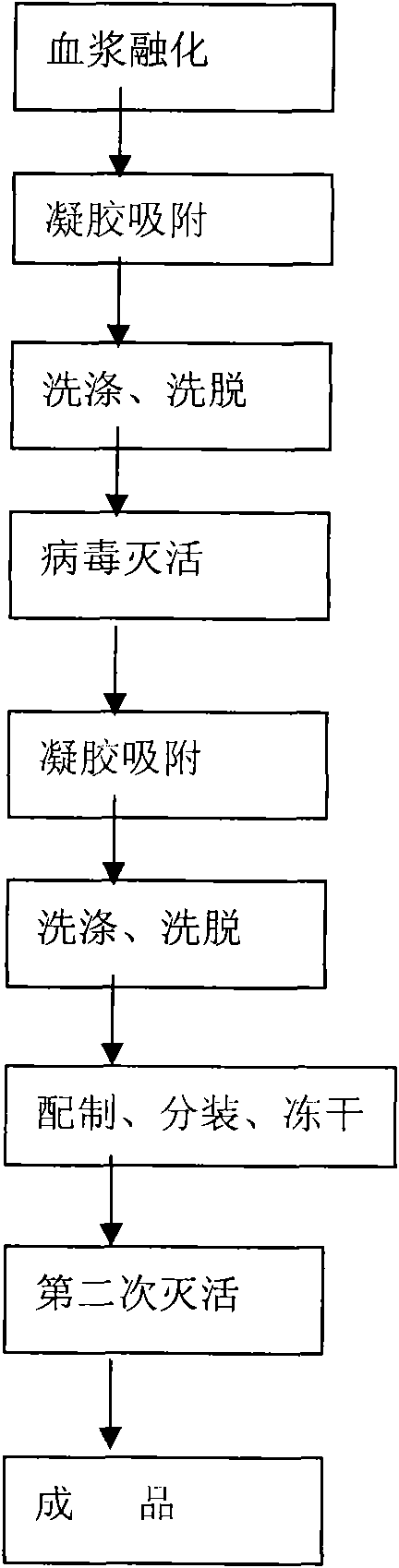
The invention relates to a preparation process of a human prothrombin compound, belonging to the field of biological pharmacy. The preparation process comprises the following steps of: absorbing blood plasma, washing, eluting and clarifying by filtration; inactivating viruses by using the S / D (Solvent / Detergent) method; purifying by absorption; subpackaging; freeze-drying; and inactivating viruses by the dry heat method. The human prothrombin compound is directly absorbed from blood plasma by using gel, only the gel chromatography technology is used in the entire extraction process, so that the production steps are simplified, the pollution of various factors on the production process of the product is reduced, meanwhile, the yield of the product is increased by 25-30%. In addition, the S / D method is used for removing lipid-enveloped viruses and the dry heat method is used for removing non lipid-enveloped viruses in the production process, and the safety of clinic medication is obviously increased through the two virus inactivation steps.
Owner:华润博雅生物制药集团股份有限公司
Antibacterial/antiviral composition, antibacterial/antiviral agent, photocatalyst, and bacteria/virus inactivation method
InactiveCN106470550AExcellent antibacterial and antiviral activityBiocidePhysical/chemical process catalystsVirus inactivationCopper
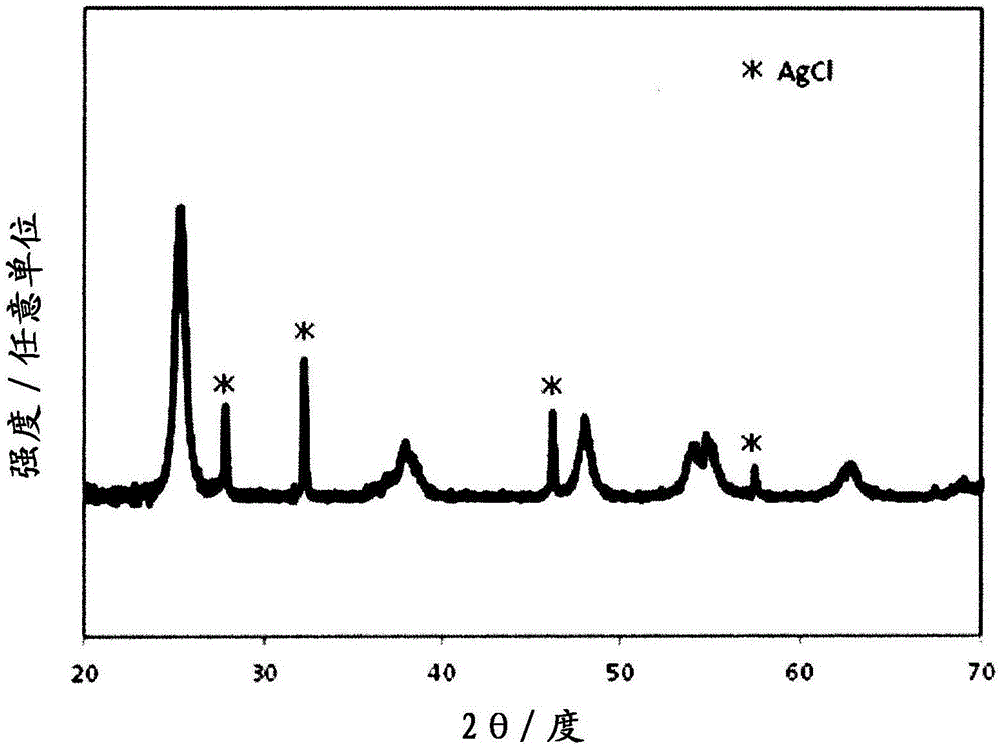
The present invention provides: an antibacterial / antiviral composition which has excellent antibacterial / antiviral activity under visible light irradiation; an antibacterial / antiviral agent; a photocatalyst; and a bacteriairus inactivation method. An antibacterial / antiviral composition according to the present invention contains titanium oxide on which both a divalent copper compound and a silver compound are loaded. An antibacterial / antiviral agent according to the present invention and a photocatalyst according to the present invention contain an antibacterial / antiviral composition according to the present invention. A bacteriairus inactivation method according to the present invention inactivates bacteria and a virus with use of an antibacterial / antiviral composition according to the present invention, an antibacterial / antiviral agent according to the present invention or a photocatalyst according to the present invention.
Owner:SHOWA DENKO KK
Method for preparing human immunoglobulin
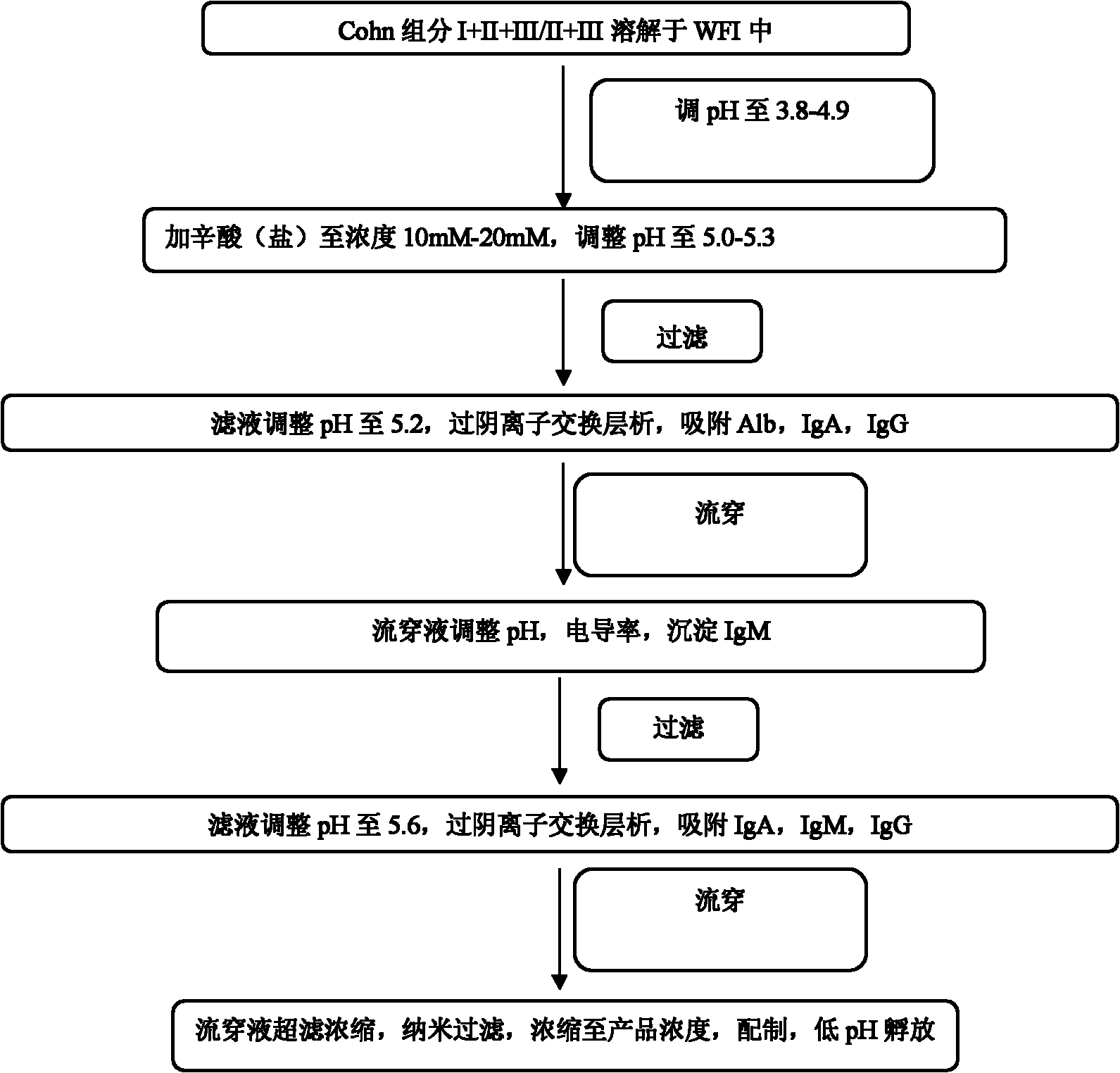
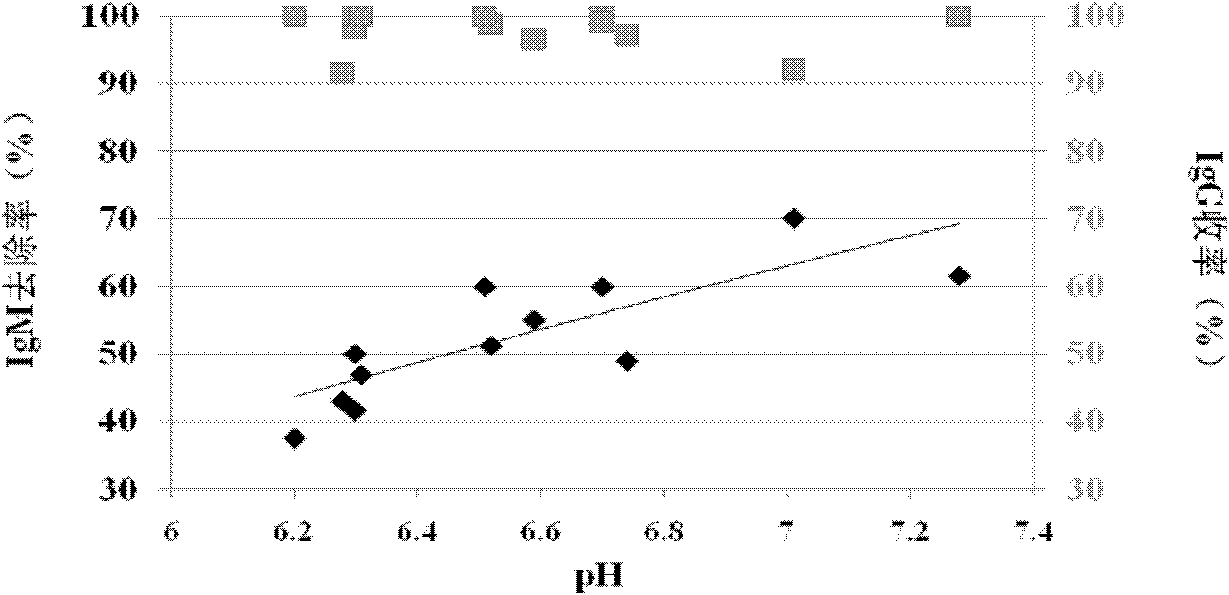
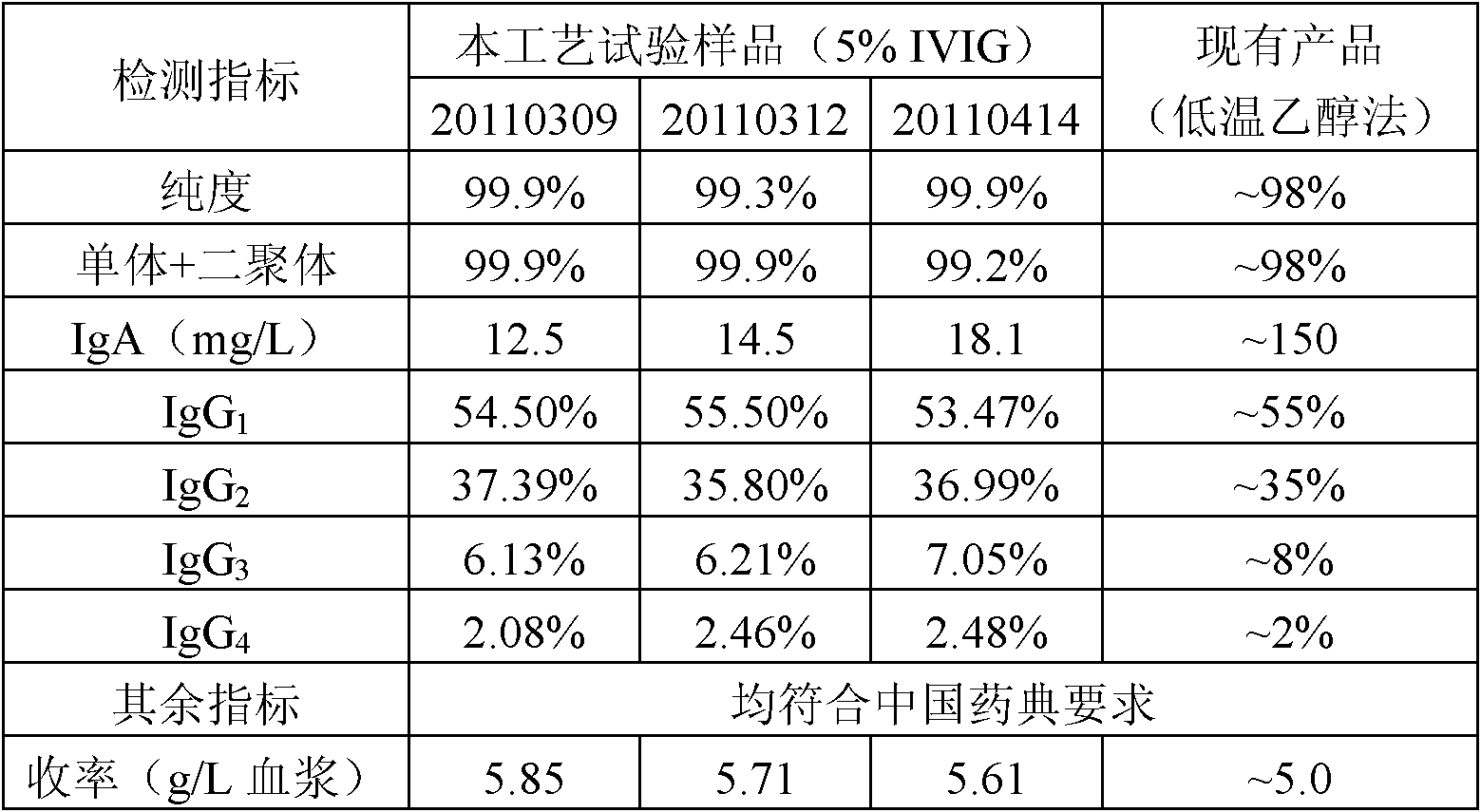
ActiveCN102532307ASmall volumeReduce manufacturing costSerum immunoglobulinsPeptide preparation methodsOctanoic AcidsVirus inactivation
The invention provides a method for preparing human immunoglobulin. The method comprises the following steps of: dissolving Cohn components I, II and III and Cohn components II and III, precipitating by using octanoic acid, performing anion-exchange chromatography for the first time, precipitating IgM, performing anion-exchange chromatography for the second time, performing ultrafiltration or dialyzing, preparing, inactivating virus and the like to obtain the high-purity human immunoglobulin. The invention also provides the human immunoglobulin prepared by the method and a medicinal composition. The method for preparing the human immunoglobulin is simple, and low in cost and has good industrial application prospect.
Owner:CHENGDU RONGSHENG PHARMA
Method for producing human prothrombin complex
ActiveCN102151289AInhibition of activationHigh yieldPeptide/protein ingredientsMammal material medical ingredientsActivation methodVirus inactivation
The invention relates to a method for producing a human prothrombin complex. The method is characterized in that the following steps of direct separation and extraction from blood plasma, virus inactivation, refining and secondary virus inactivation are adopted to obtain finished human prothrombin complex. As the method adopts the step of direct separation and extraction from the blood plasma, the separation condition is mild, the batch-to-batch difference of the products is small, the blood coagulation factor activity is stable, the yield rate is high, and the activation phenomenon basicallydoes not exist. The virus inactivation process adopts a method of combining an S / D (organic solvent / detergent) method and a dry and thermal activation method and fully ensures that the virus safety of the human prothrombin complex.
Owner:哈尔滨派斯菲科生物制药有限公司
Biological repair tablet for herniae and preparation method thereof
The invention provides a biological repair tablet for herniae and a preparation method thereof. The repair tablet uses small intestine submucosa tissues of inbred line animals without cell and DNA (deoxyribonucleic acid) components as the raw material, completely reserves the extracellular matrix component and structure, and has a micropore structure. The preparation method of the repair tablet comprises the following operation steps: determination of animal source, pretreatment and rough cleaning of small intestine tissues, virus inactivation, cell removal, DNA removal treatment, formation, packaging and sterilization. The biological repair tablet for herniae prepared by the method uses an inbred line animal as an animal source, and thus, the hereditary features are pure, stable and uniform, thereby radically ensuring the stability and uniformity of different batches of products; and the biological repair tablet for herniae has fewer animal source DNA residues, completely reserves the three-dimensional structure of natural ECM, and has the advantages of low immune source property and high infection resistance.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
Compound growth factor as well as preparation method and application thereof
InactiveCN102988964AOvercome high pricesOvercome purification difficultiesOrganic active ingredientsPeptide/protein ingredientsMicrosphereOrganosolv
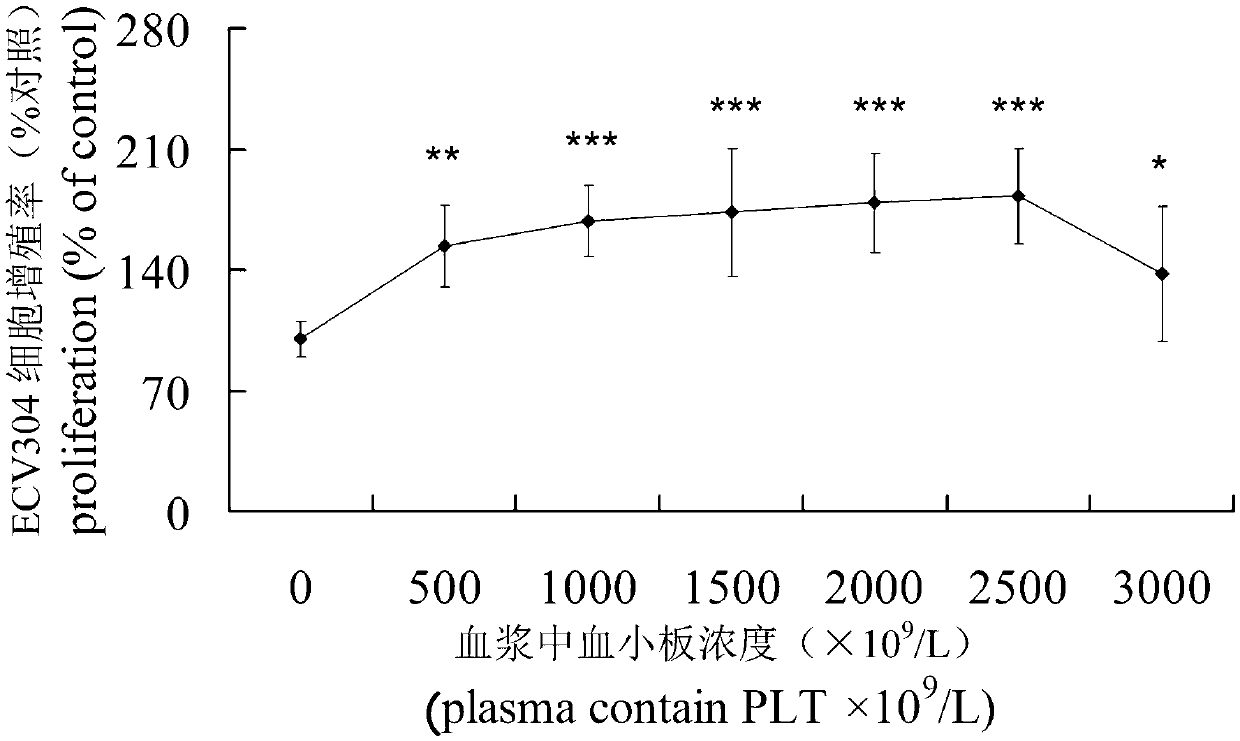
The invention discloses a compound growth factor of blood platelet sources as well as a preparation method and application of the compound growth factor. According to the compound growth factor as well as the preparation method and the application of the compound growth factor, platelet-rich plasma as the raw material is firstly subjected to virus inactivation through an S / D (Solvent / Detergent) method to remove such reagents as organic solvents and eradicators in the platelet-rich plasma and then activated by adding calcium or / and thrombin, supernatant fluid after activation is extracted to obtain the compound growth factor fitting for the natural proportion of the human body; and the problems that single recombinant growth factors in clinic are high in price, and difficult to purify, is involved into gene recombination biological safety and on the like are overcome. The invention further discloses a compound growth factor gel obtained based on the compound growth factor by adding chitosan microspheres carriers, which can slowly release growth factors during use. The compound growth factor has remarkable treatment effects for acute wounds, burns, cosmetic treatment, and chronic hard-healing wounds, is convenient to use, and has high safety.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Preparing method of human serum albumin
ActiveCN105037487AReduce yield lossReduce processing timePeptide preparation methodsWhole blood productUltrafiltration
The invention relates to the technical field of biological product and blood product production, and mainly relates to a separation and purification method of human serum albumin in the blood product production, in particular to a preparing method of human serum albumin. According to the method, healthy plasma supernatant is used as raw materials; a Kistler-Nitchmann low-temperature ethanol method is adopted for precipitating and separating ingredients FI+II+III and ingredients FIV; supernatant after the ingredient FIV separation is subjected to dealcoholization treatment; then, one-step ion exchange chromatography and ultrafiltration are further performed; a proper amount of sodium caprylate is added as a stabilizer; after the Pasteur virus inactivation treatment is performed, the human serum albumin finished product is obtained. Through efficient liquid chromatography detection, the purity of the human serum albumin prepared by the process is higher than 99 percent; the polymer content is lower than or equal to 1 percent; the yield of the plasma reaches 28 to 30g / L. The purity of the product is higher; the impurity protein content is lower, so that the clinical medication is safer.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
Method for extracting human TIG (Tetanus Immune Globulin) based on chromatography
ActiveCN102178952AHigh purityHigh yieldAntibacterial agentsImmunoglobulins against bacteriaWhole blood productActive matter
The invention belongs to the field of blood products in biological products, in particular relates to a method for extracting human TIG (Tetanus Immune Globulin) based on chromatography. The method comprises the following three steps: 1, primary separation; 2, purification by chromatography; and 3, virus inactivation. When the chromatography process provided by the invention is used for further purifying products, the purity of the products is improved greatly, the purity of human TIC in the products can be improved to above 98%, which is not only far higher than the standard specified in Chinese Pharmacopoeia version 2010, but also higher than the standard specified in European Pharmacopoeia; in addition, the contents of other impurities causing anaphylactic reaction are reduced greatly, for instance, two major anaphylactic substances, namely anticomplement active substance (ACA) and depressor substance (PKA), are both reduced remarkably. Two different virus inactivation processes, namely low-pH incubation and nano-film filtering, are adopted in the invention and can be used for effectively inactivating and removing both lipid-enveloped viruses and non lipid-enveloped viruses.
Owner:哈尔滨派斯菲科生物制药有限公司
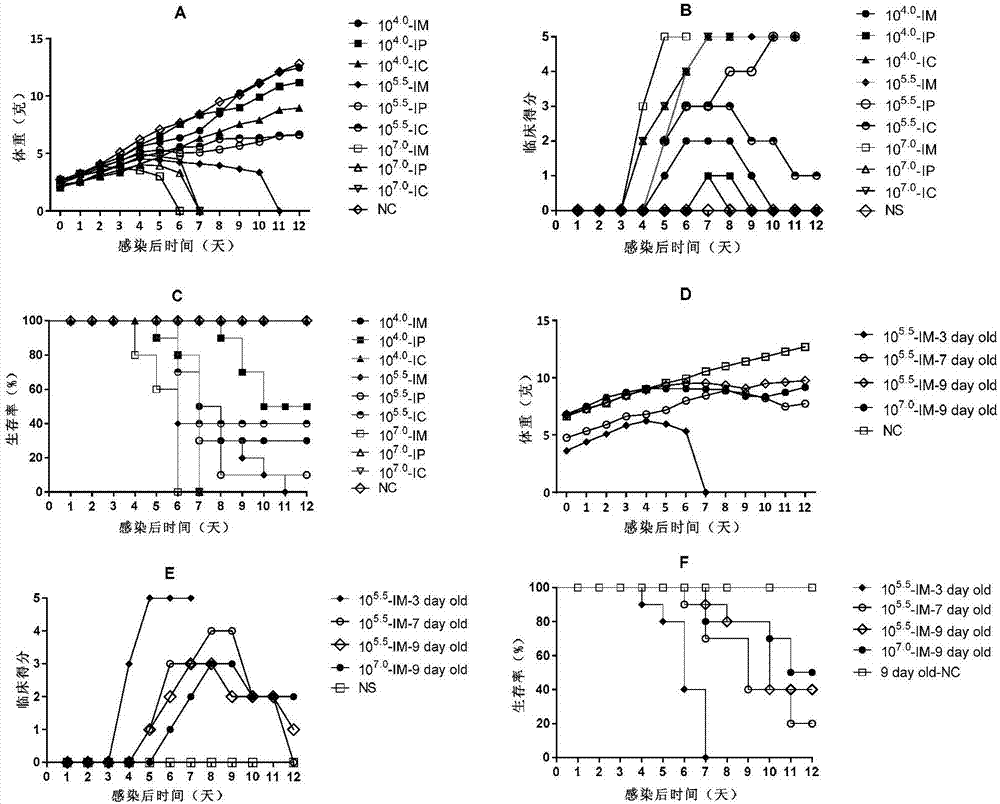
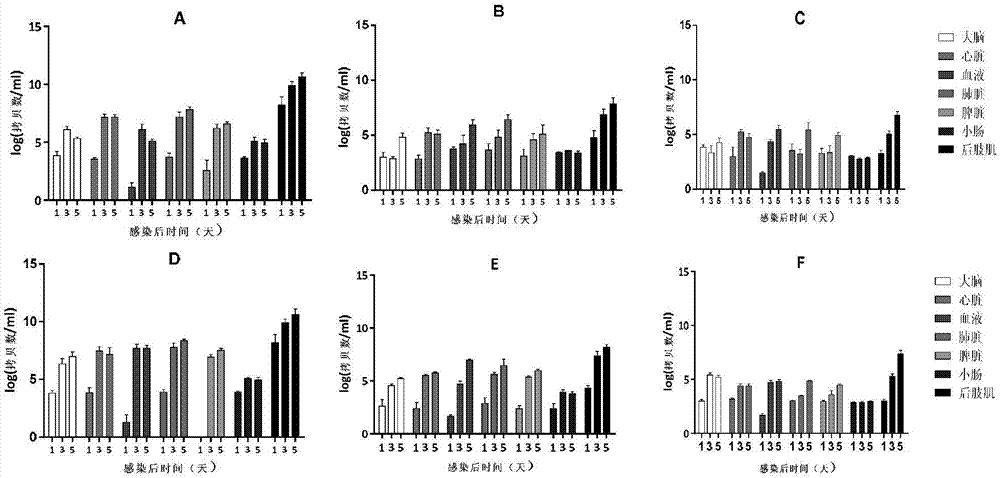
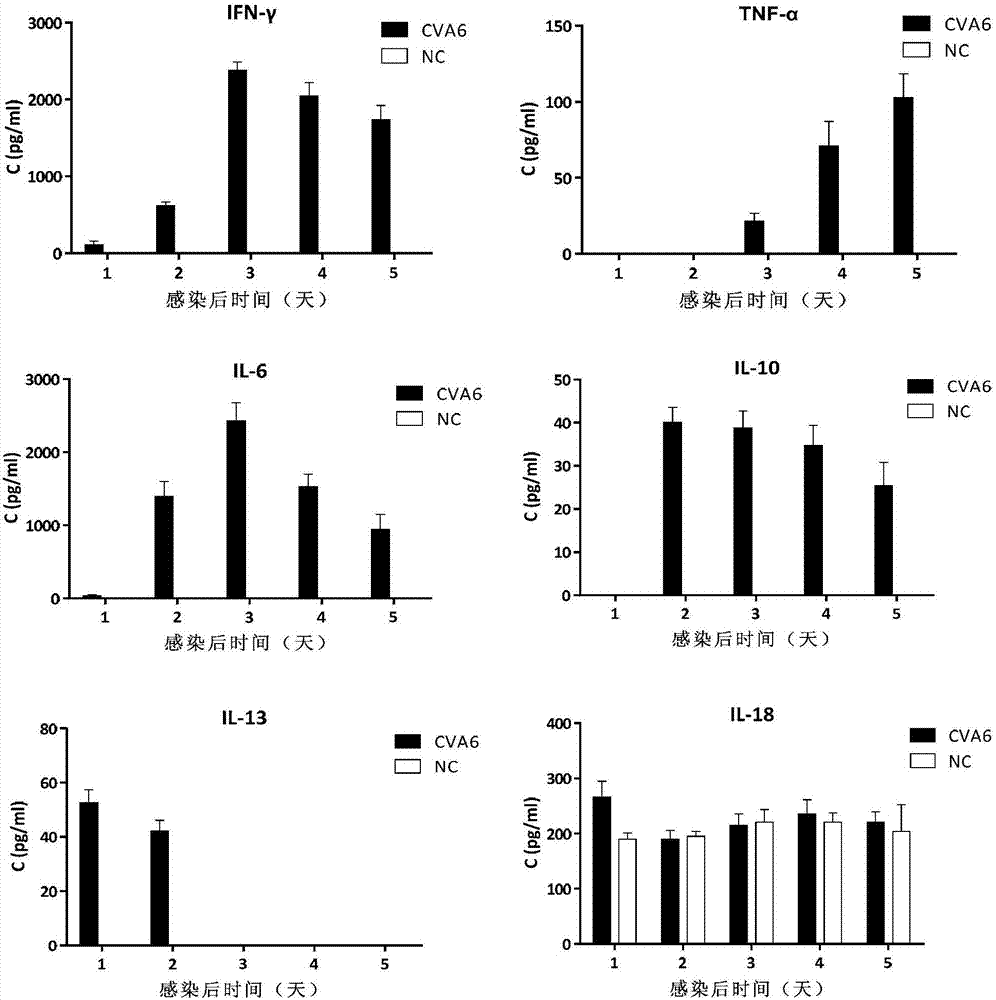
Coxsackievirus A6 type infected animal model as well as preparation method and application thereof
InactiveCN106867974AGood repeatabilitySsRNA viruses positive-senseMicroorganism based processesMicroorganismViral Vaccine
The invention discloses a coxsackievirus A6 type infected animal model as well as a preparation method and an application thereof. The preparation method of the coxsackievirus A6 type infected animal model provided by the invention comprises a step of infecting an animal with an inactivated coxsackievirus A6 type strain, so that the coxsackievirus A6 type infected animal model is obtained, wherein the coxsackievirus A6 type strain is preserved in China General Microbiological Culture Collection Center with preservation number of CGMCC No.13393. The coxsackievirus A6 type infected animal model, which is stable and is good in repeatability, is constructed on the basis of the CVA6 strain WF057R; and with the application of the model, a research tool is provided for next medicine antiviral therapy and assessment of an immune protective effect of an inactivated viral vaccine.
Owner:TAISHAN MEDICAL UNIV
Preparing method of heterogeneous acellular dermal matrix substrate with good biocompatibility
ActiveCN105079880AProtect physical propertiesGuaranteed biocompatibilityProsthesisVirus inactivationBiocompatibility
The invention relates to a preparing method of a heterogeneous acellular dermal matrix substrate with good biocompatibility. The method concretely comprises the following steps of (1) split thickness skin graft preparation; (2) epidermis and dermis separation; (3) acellular processing; (4) cross linking; (5) virus inactivation; (6) refining; (7) Co-60 irradiation sterilization. The ecellular tissue engineering materials obtained by the method provided by the invention has the advantages that the toxicity is stable, and the cell compatibility is better.
Owner:HEBEI AINENG BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com