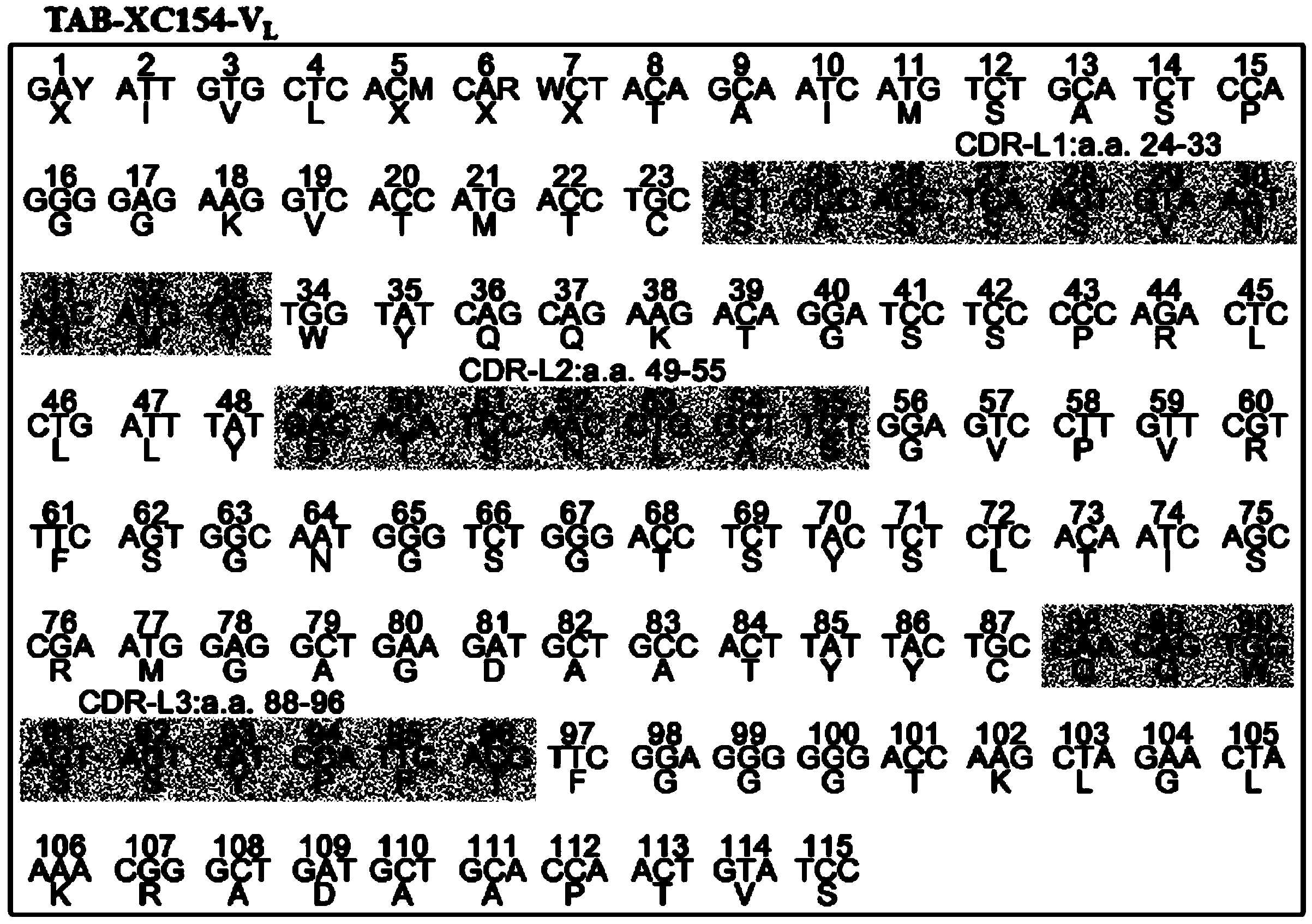
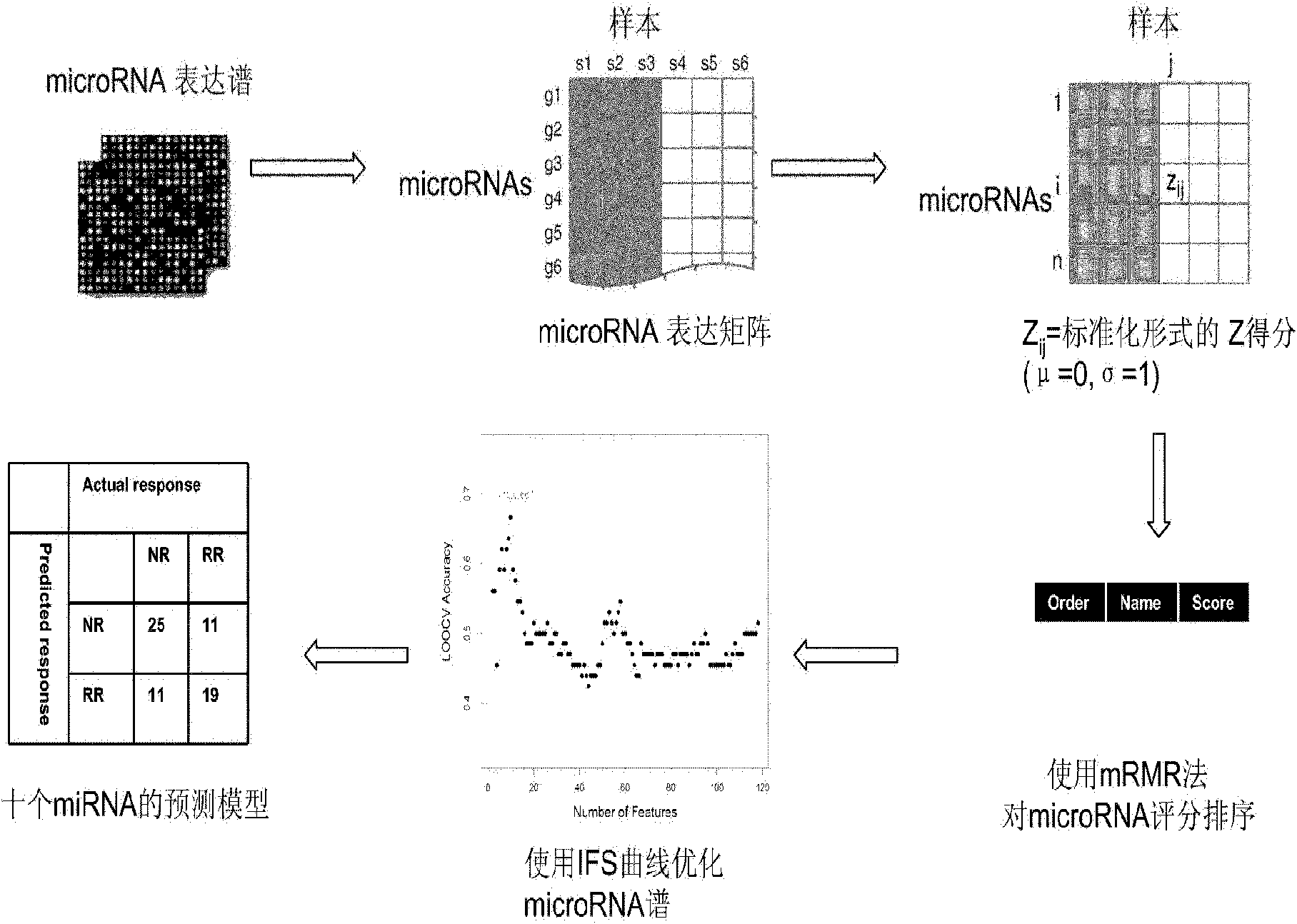
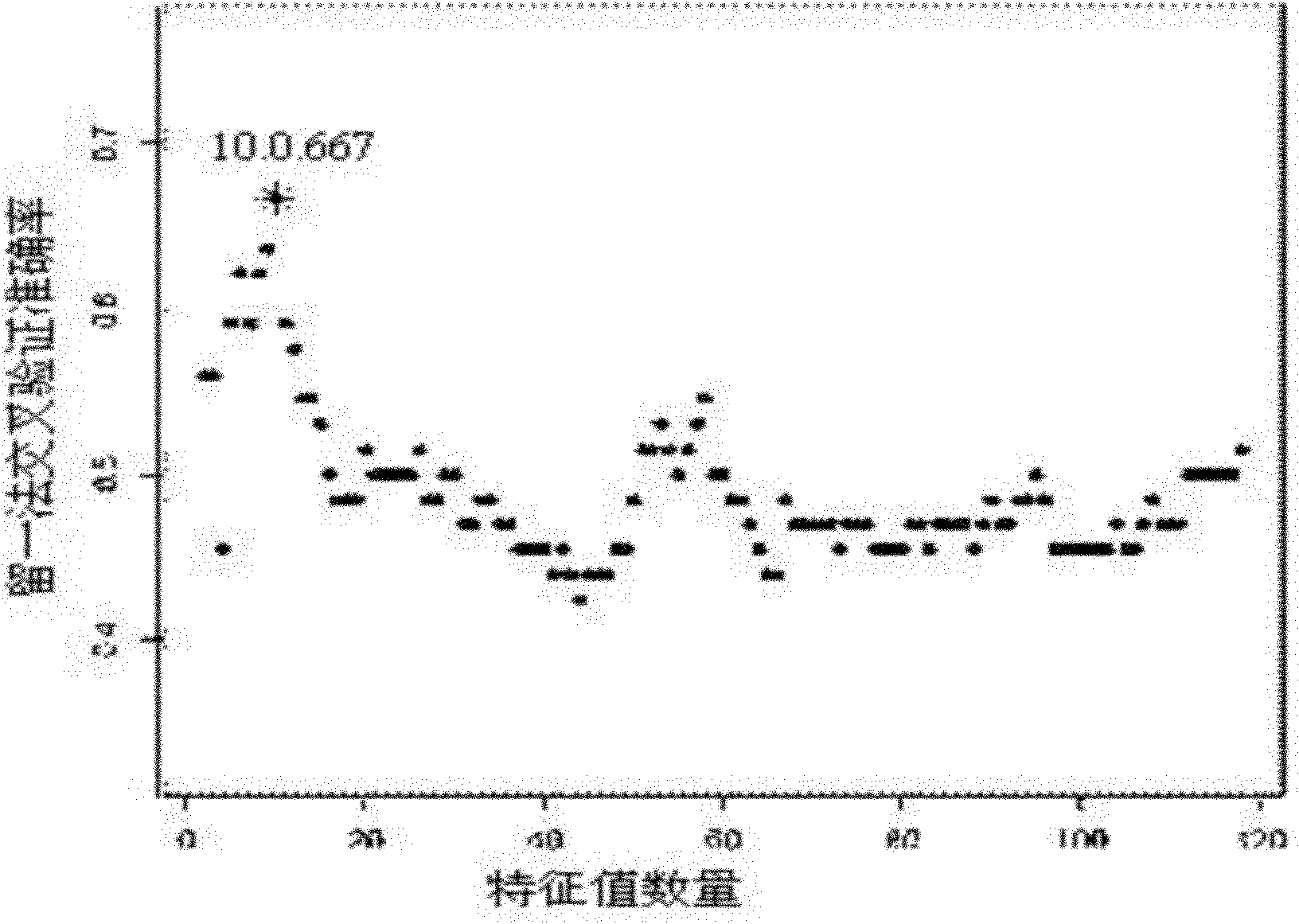
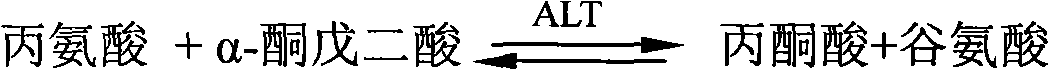Patents
Literature
48 results about "Plasma serum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
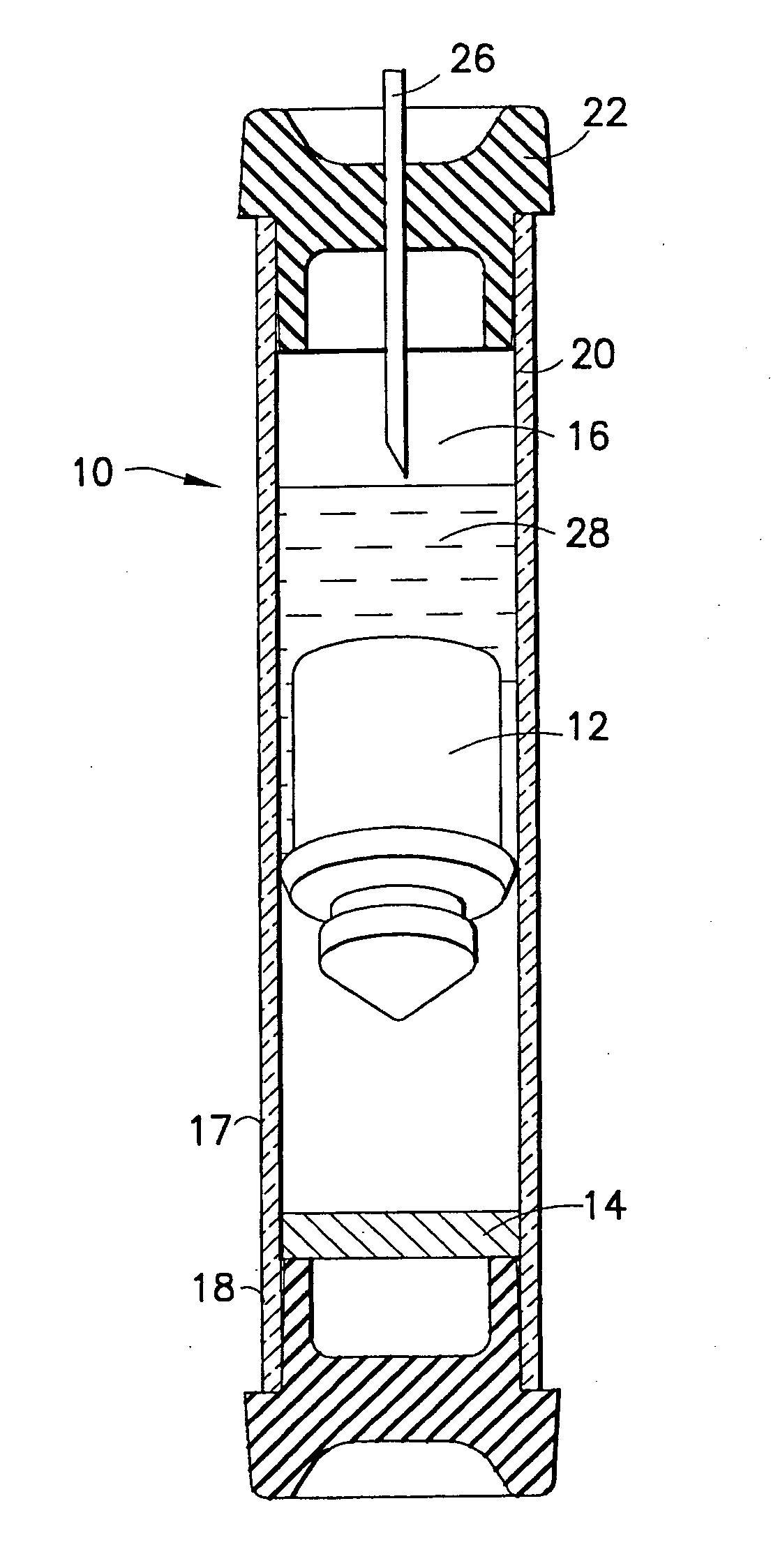
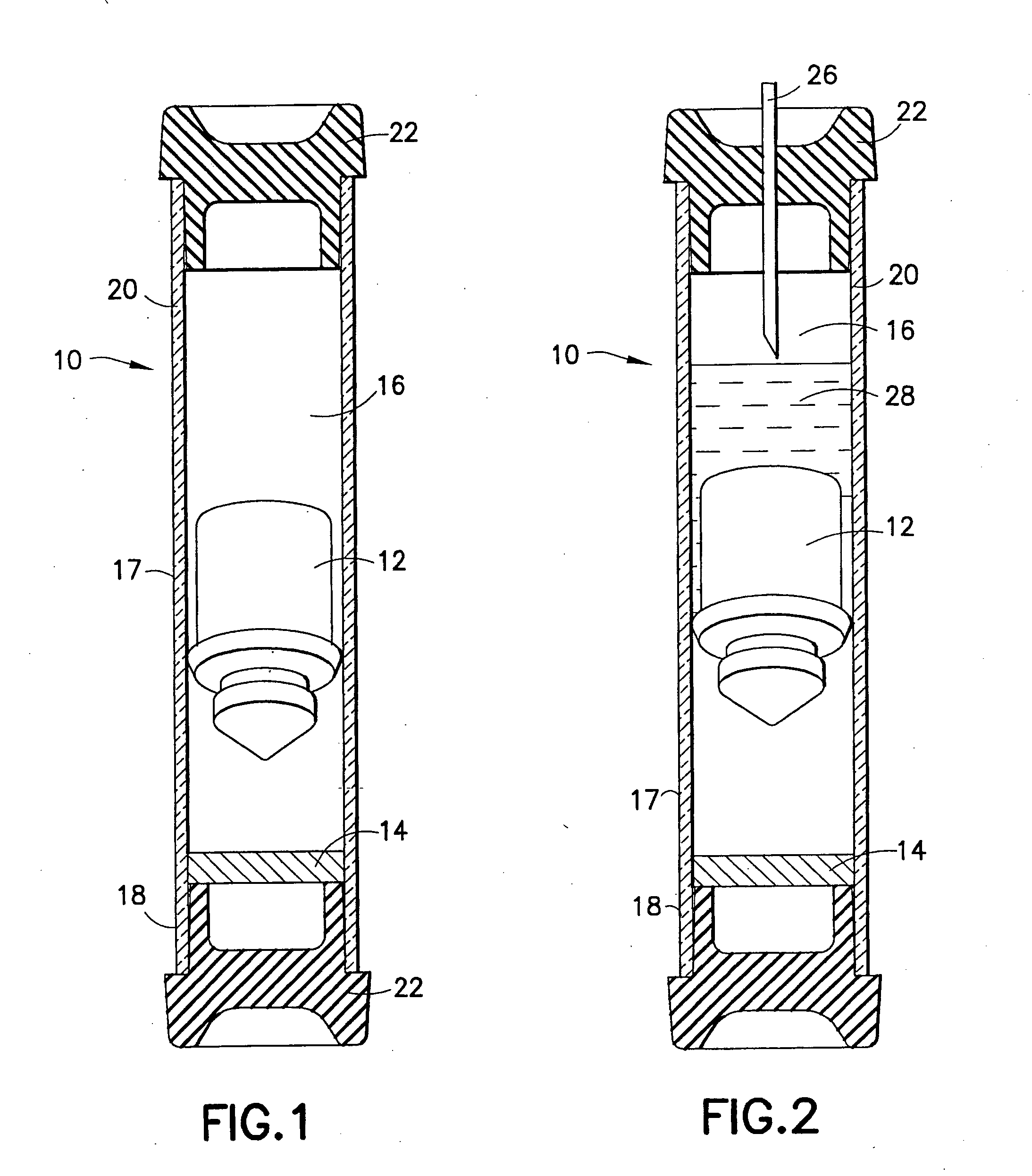
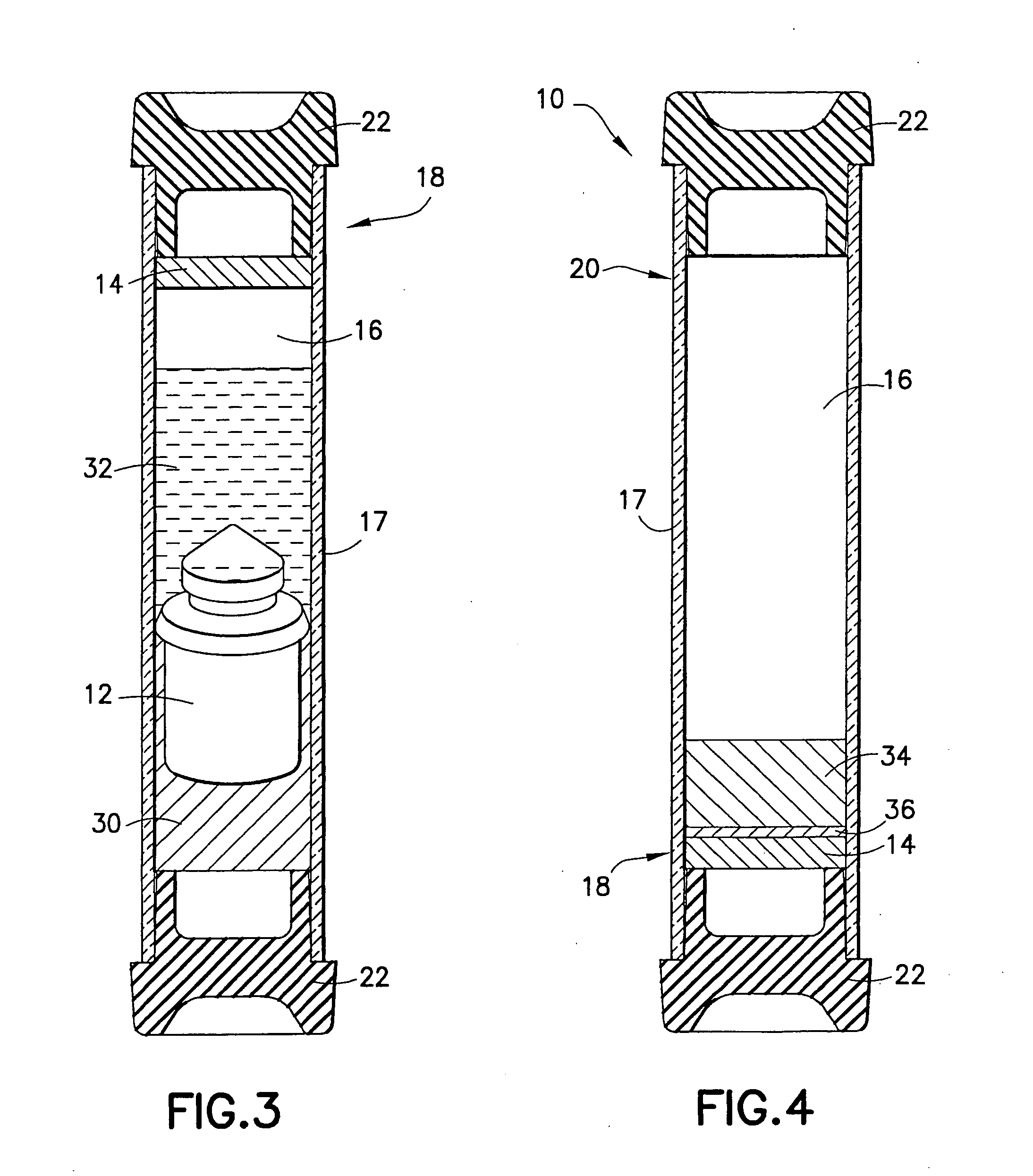
Device and methods for collection of biological fluid sample and treatment of selected components
A collection device and a method for collecting a biological sample, particularly whole blood, includes a separating member to separate the whole blood into its components, and at least reagent positioned to selectively interact with a component of the separated sample. The reagent is able to selectively interact with the plasma / serum, and is prevented from contacting or interacting with the whole blood.
Owner:BECTON DICKINSON & CO
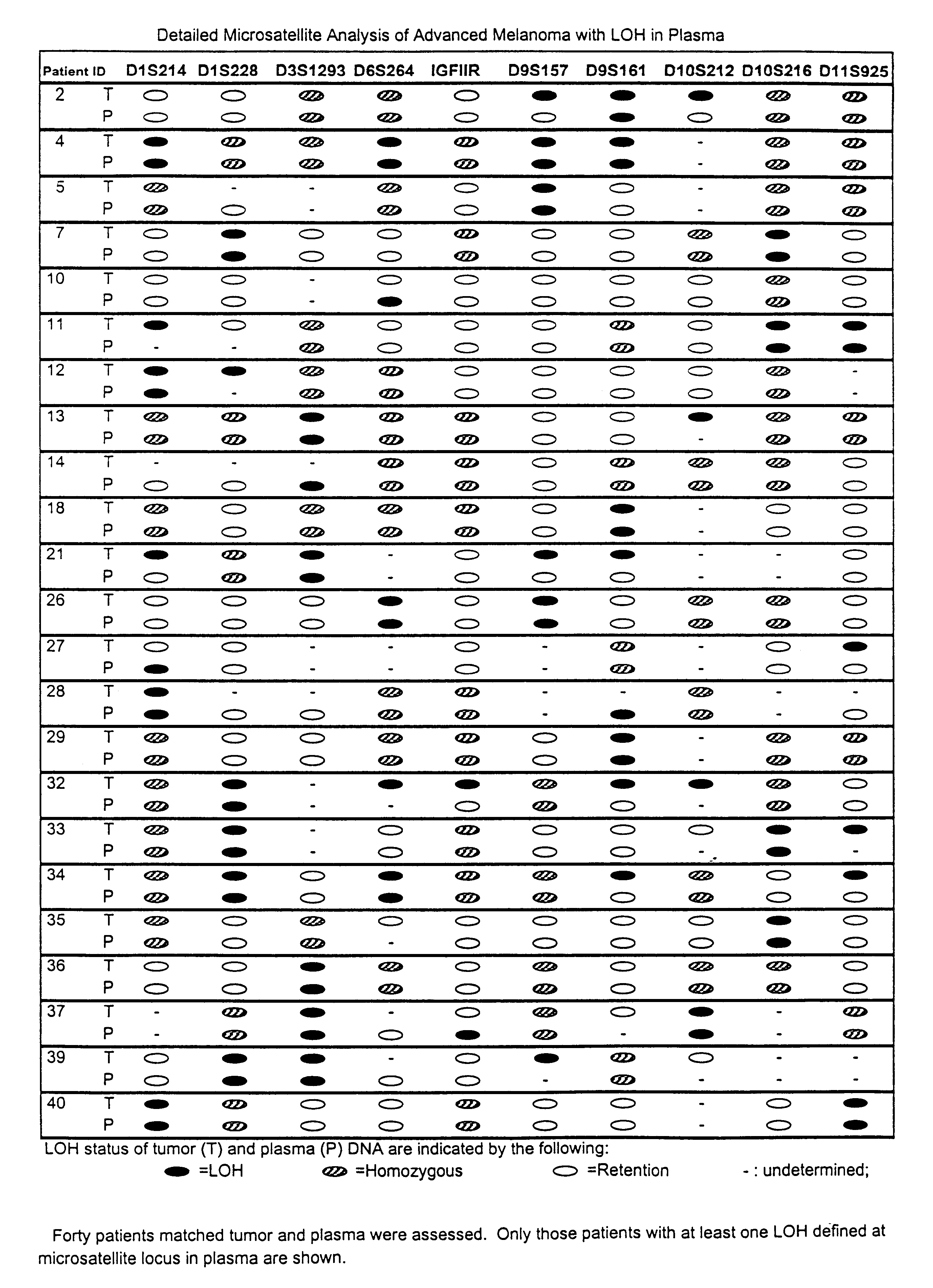
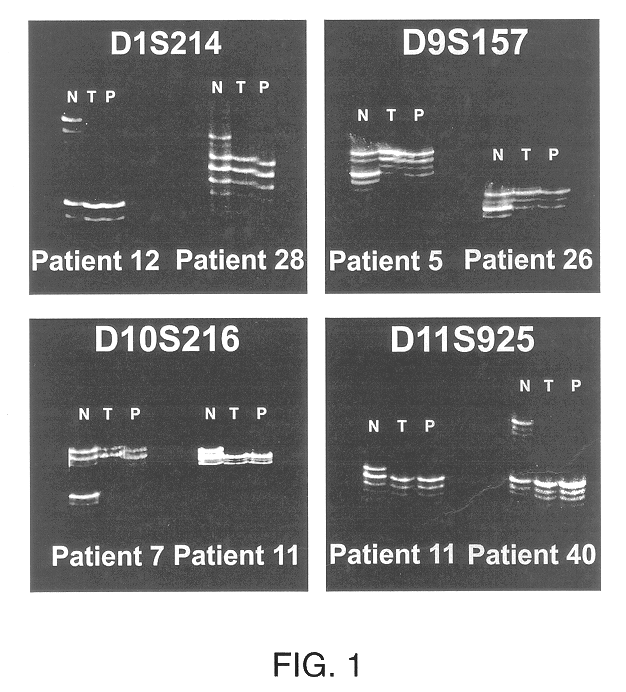
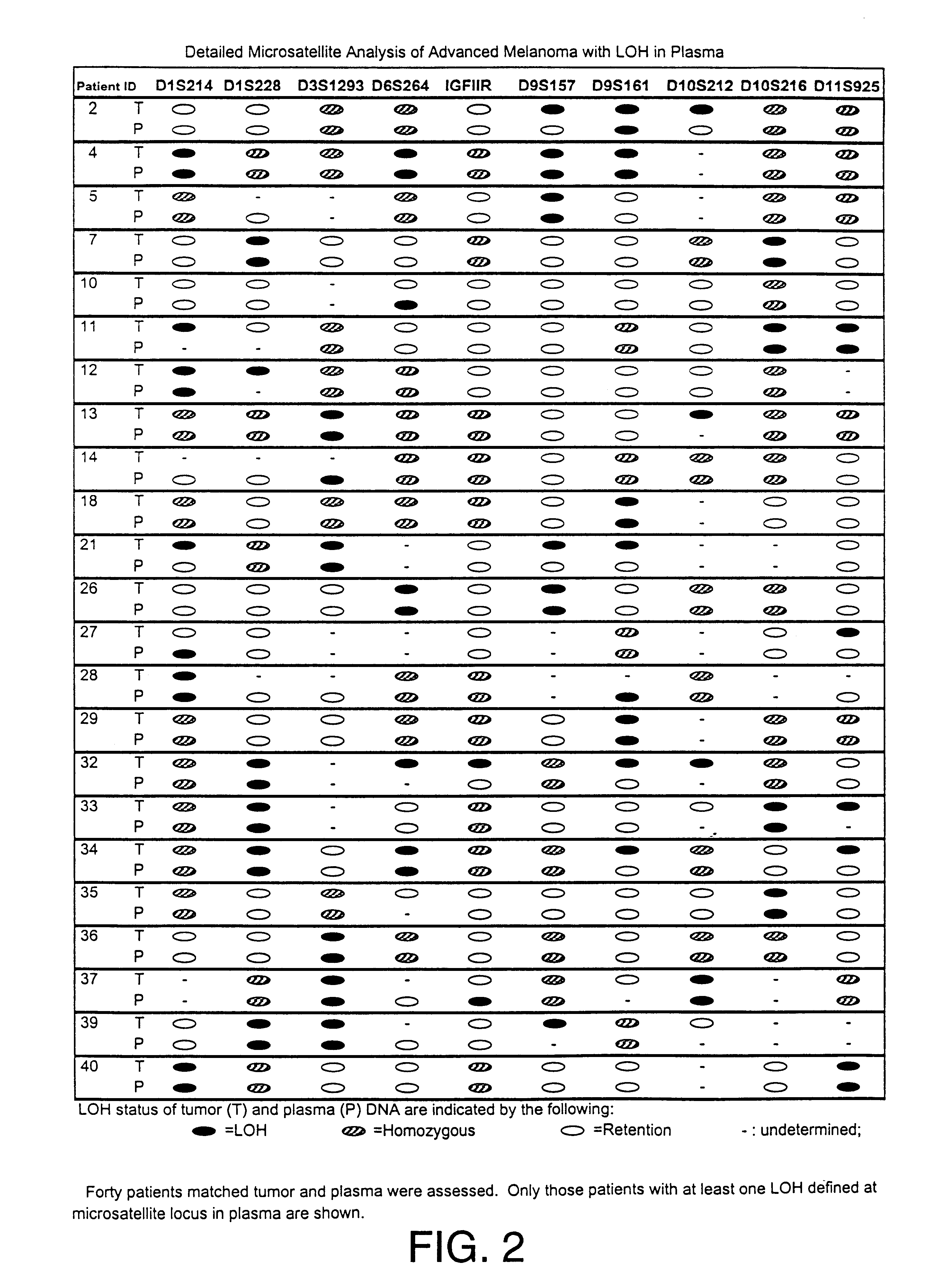
Detection of loss of heterozygosity in tumor and serum of melanoma patients
A method is provided for assessing allelic losses on specific chromosomal regions in melanoma patents. The method relies on the evidence that free DNA may be released in the plasma / serum of cancer patients allowing the detection of DNA with LOH in the plasma / serum of cancer patients by analysis for microsatellite markers. The amount of and specific allelic loss allows a prognosis to be made regarding tumor diagnosis and progression, tumor metastasis, tumor recurrence, and mortality.
Owner:JOHN WAYNE CANCER INST
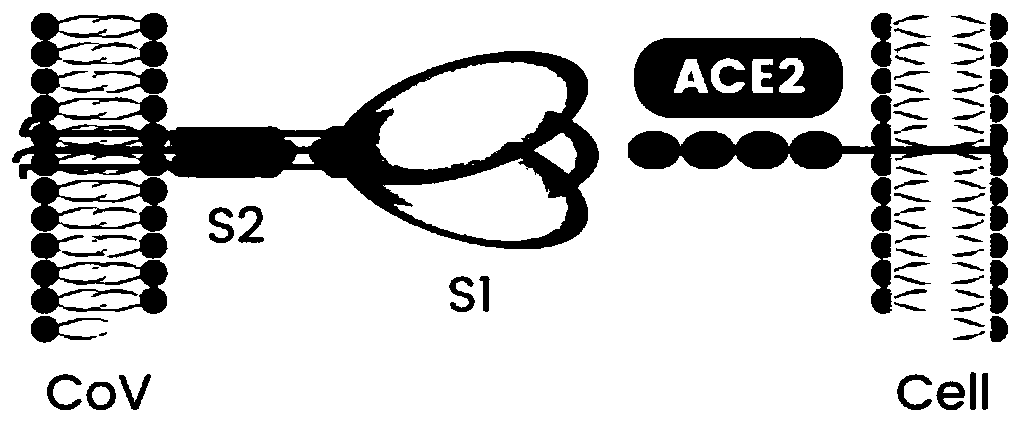
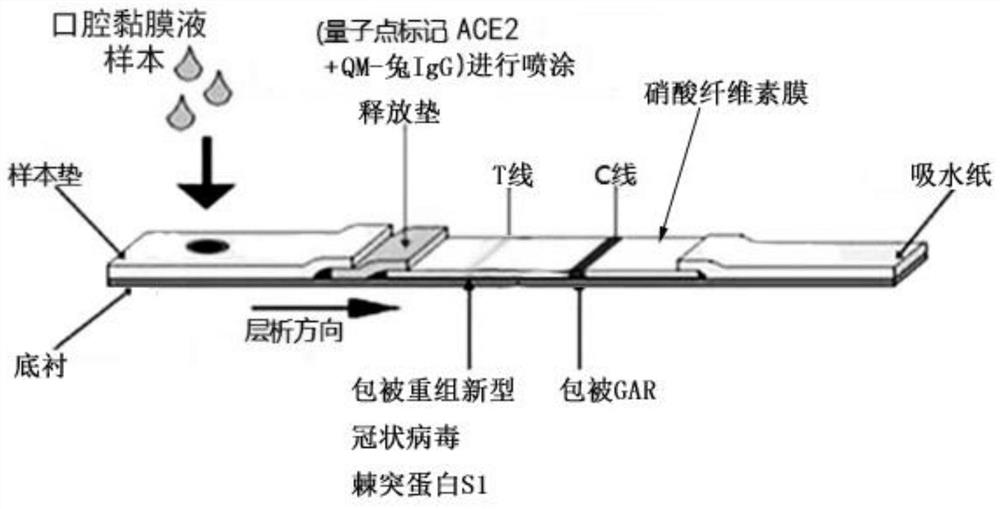
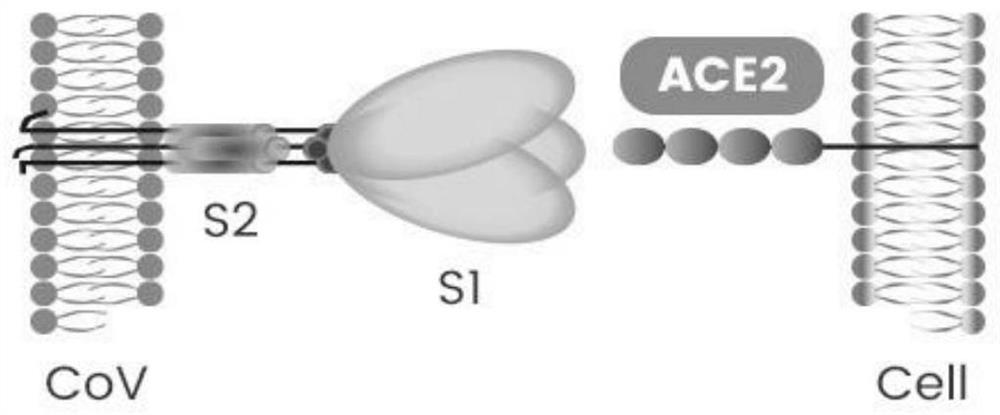
Coronavirus rapid detection kit based on S protein ligand and ACE2 receptor competitive chromatography
ActiveCN111273016AImmunochromatographic fastEasy immunochromatographyCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
LncRNA combination for detecting prognosis condition of stomach cancer and kit containing combination
InactiveCN107488740AAvoid detection errorsImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationMissed diagnosisOncology
The invention discloses an LncRNA combination for detecting a prognosis condition of stomach cancer and a kit containing the combination. According to the invention, a fluorescent quantitation PCR or digital PCR technique is adopted to identify the difference change of a set of specific LncRNA expression quantity in a stomach cancer patient sample (tissue, plasma, serum, gastric juice, and the like) and a corresponding paracancerous sample or normal sample and to early and accurately evaluate the risk of stomach cancer relapse or transfer. The kit disclosed by the invention provides a biomarker LncRNA combination for detecting the prognosis condition of stomach cancer, a primer for detecting the LncRNA contained in the combination and a related reagent, so that the kit is capable of effectively increasing the detection efficiency and accuracy for the stomach cancer prognosis relapse or transfer. The invention adopts a set of prognosis transfer related LncRNA combination for avoiding the defect of great increasing of the fault diagnosis rate and missed diagnosis rate of the stomach cancer diagnosis caused by lower sensitivity and specificity resulted from a detection index LncRNA served as a tumor marker kit.
Owner:NANYANG NORMAL UNIV
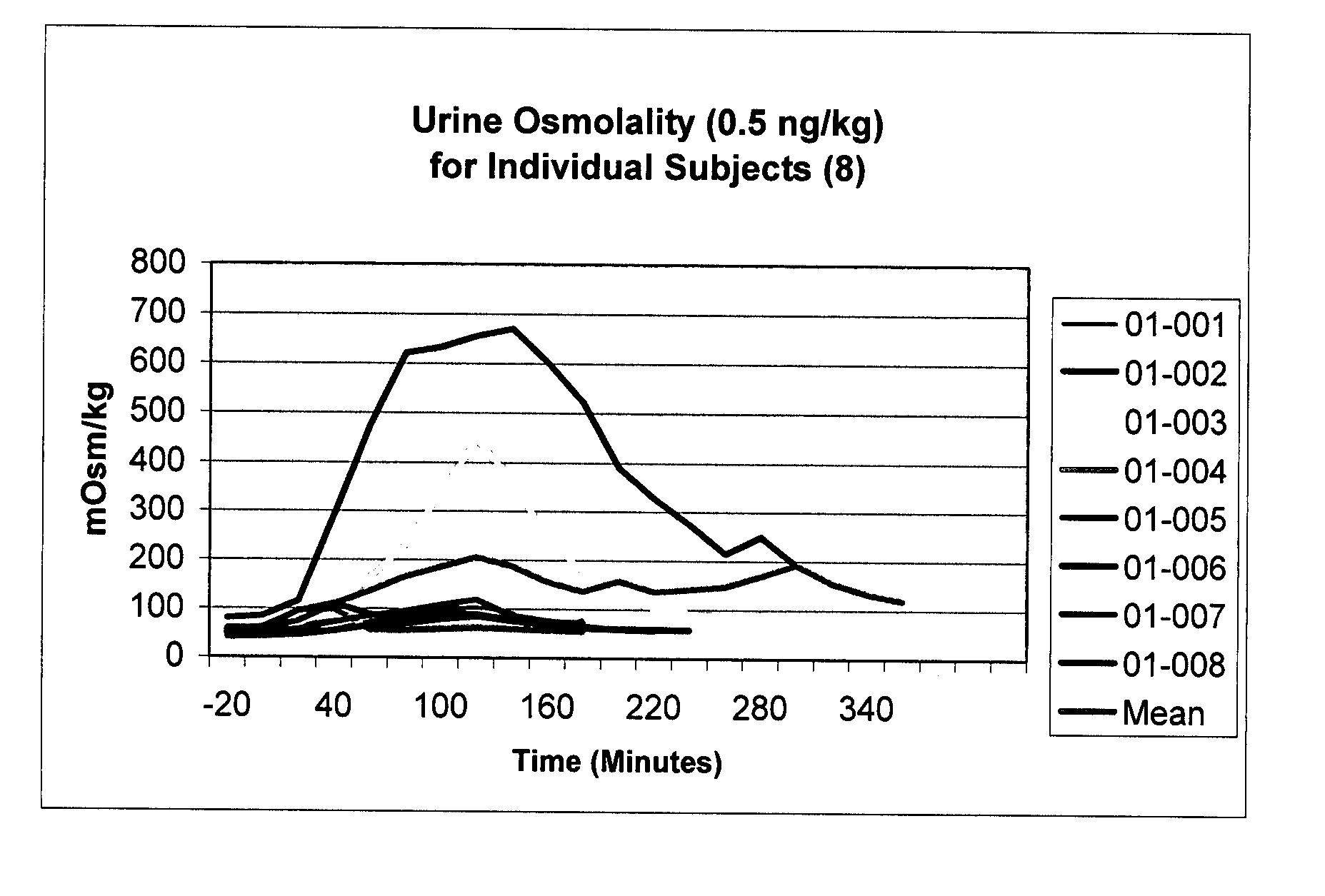
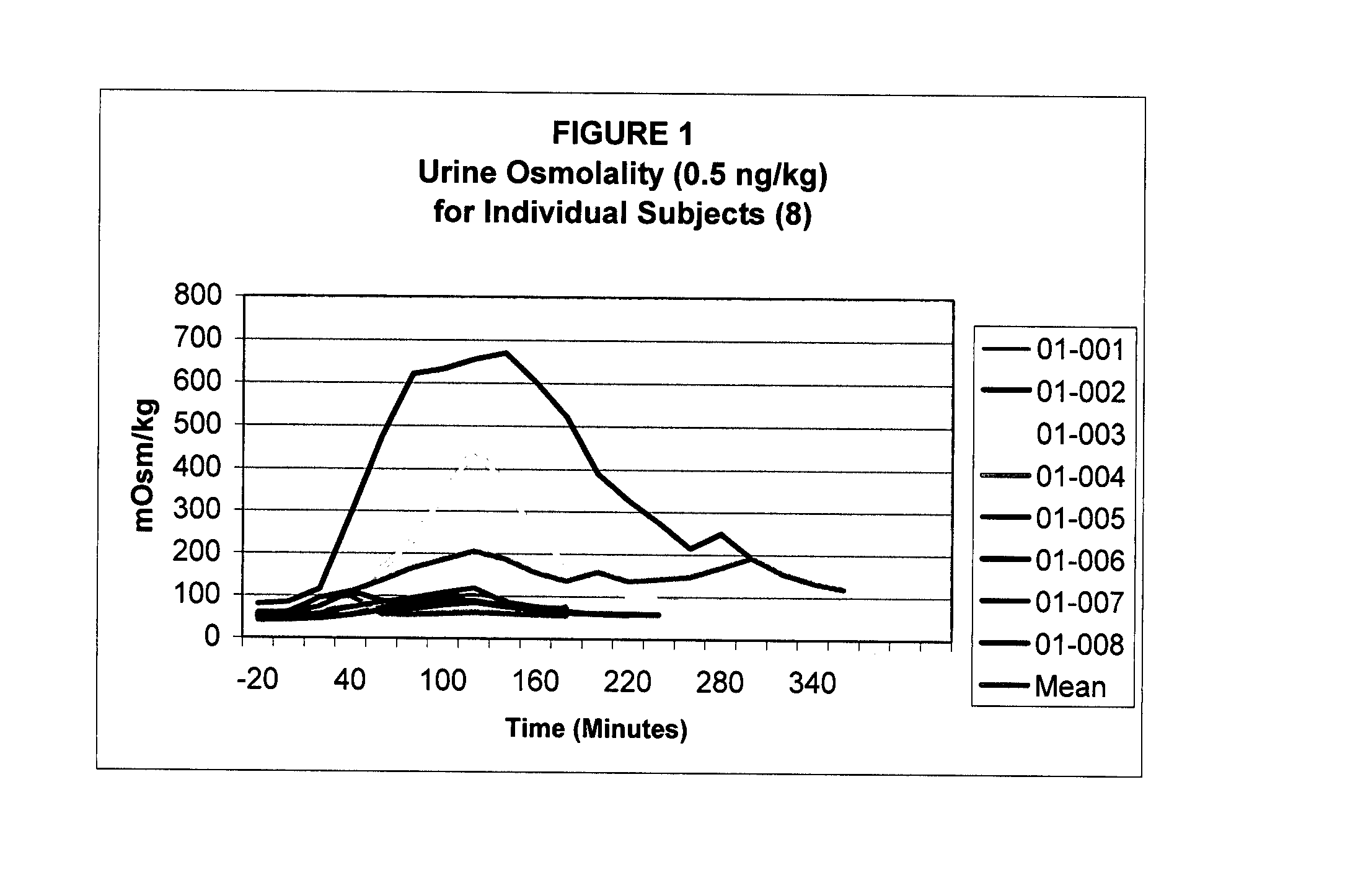
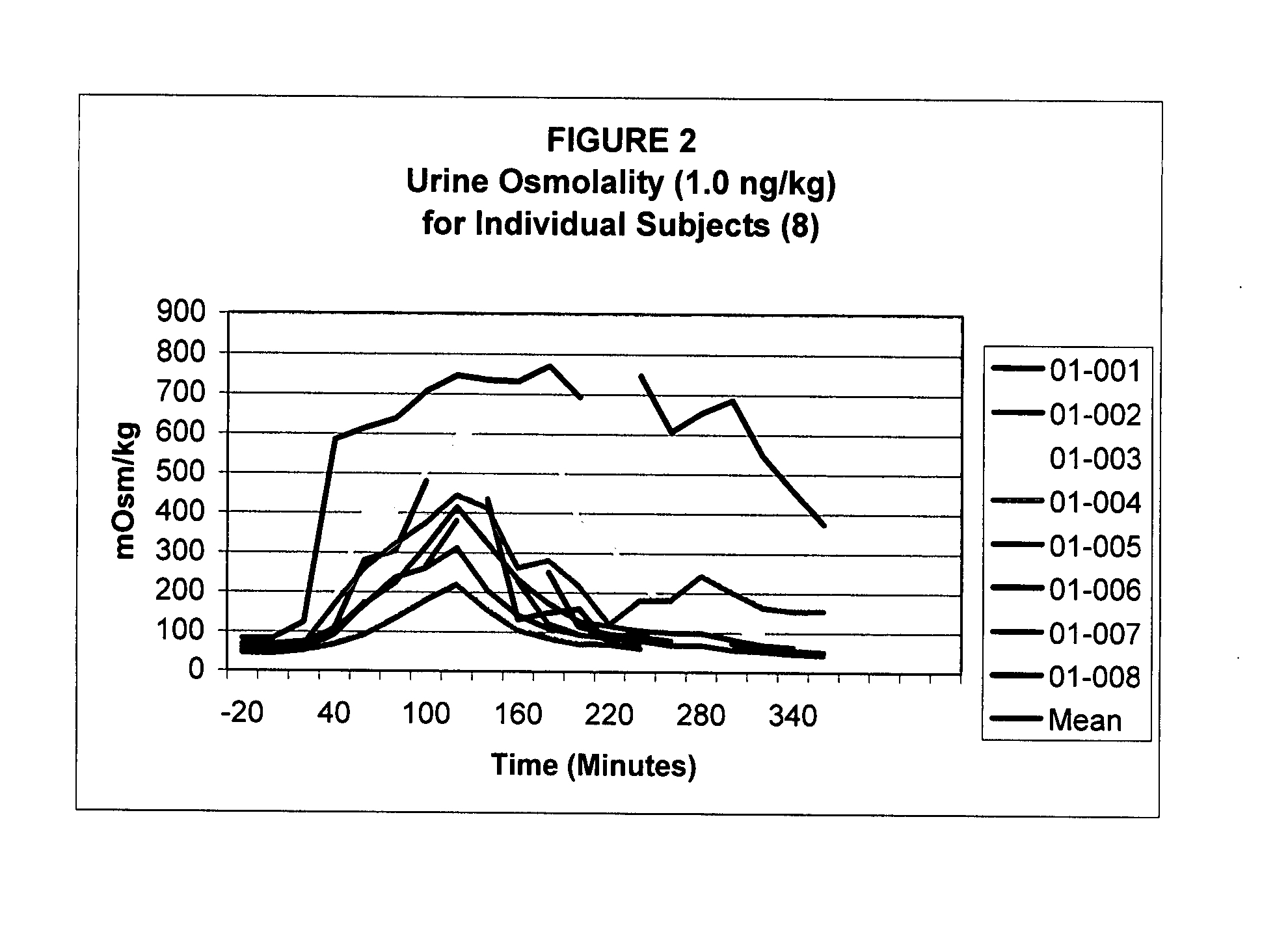
Pharmaceutical Compositions Including Low Dosages of Desmopressin
The present invention is directed to a pharmaceutical composition comprising 0.5 ng to 20 μg desmopressin and a pharmaceutically acceptable carrier. The present invention is also directed to a pharmaceutical composition comprising desmopressin and a pharmaceutically acceptable carrier, wherein the pharmaceutical composition is effective to establish a steady plasma / serum desmopressin concentration in the range of from about 0.1 picograms desmopressin per mL plasma / serum to about 10.0 picogram desmopressin per mL plasma / serum. Articles of manufacture and methods of using the above invention are also disclosed.
Owner:ACERUS PHARM USA LLC
Multifunctional dry plate test board and preparation method and application thereof
InactiveCN101354399ASmall sample sizeImprove detection efficiencyMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementChemistryBlood plasma
The invention provides a multifunctional dry plate checkout console, and a preparation method and application thereof, belonging to the field of medical instrument. The multifunctional dry plate checkout console comprises a solid reaction carrier film (1) used for adsorbing biochemical reagent, a carbon fibre bar frame (2), a supporting pad (5) with the upper layer attached by thin fibreglass paper (3), a hard bracket (7) added by whole blood filter paper (4), and an elastic pad (6). The product of the invention is a disposable medical detection consumable belonging to quick micro-detection, can quantitatively / qualitatively and quickly detect a plurality of biochemical indexes in human body blood samples (whole blood, blood plasma and serum), with the detection process completed within 5 minutes; the preparation method and the application thereof have important significance for critical quick quantitative detection and disease diagnosis.
Owner:吕炜锋
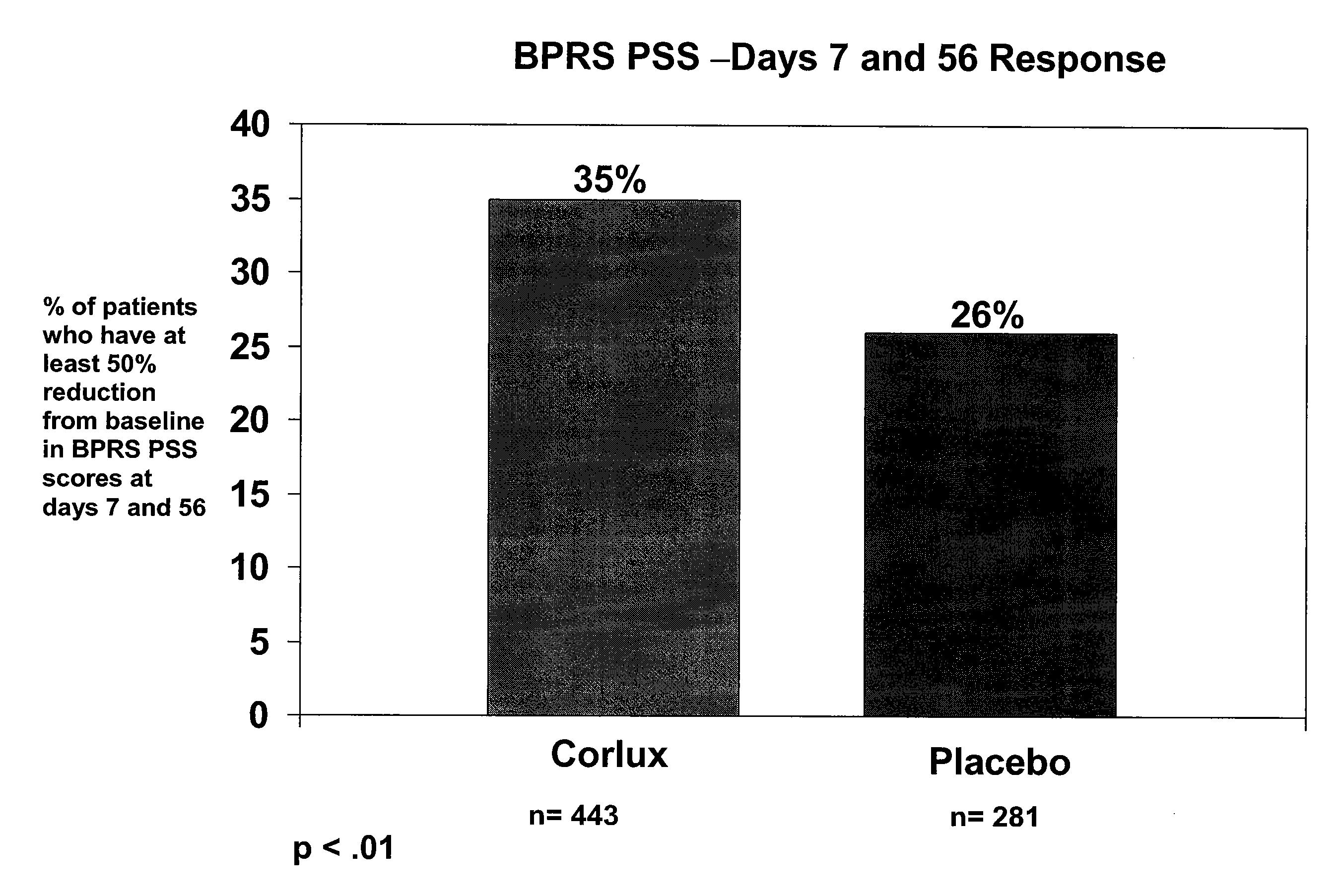
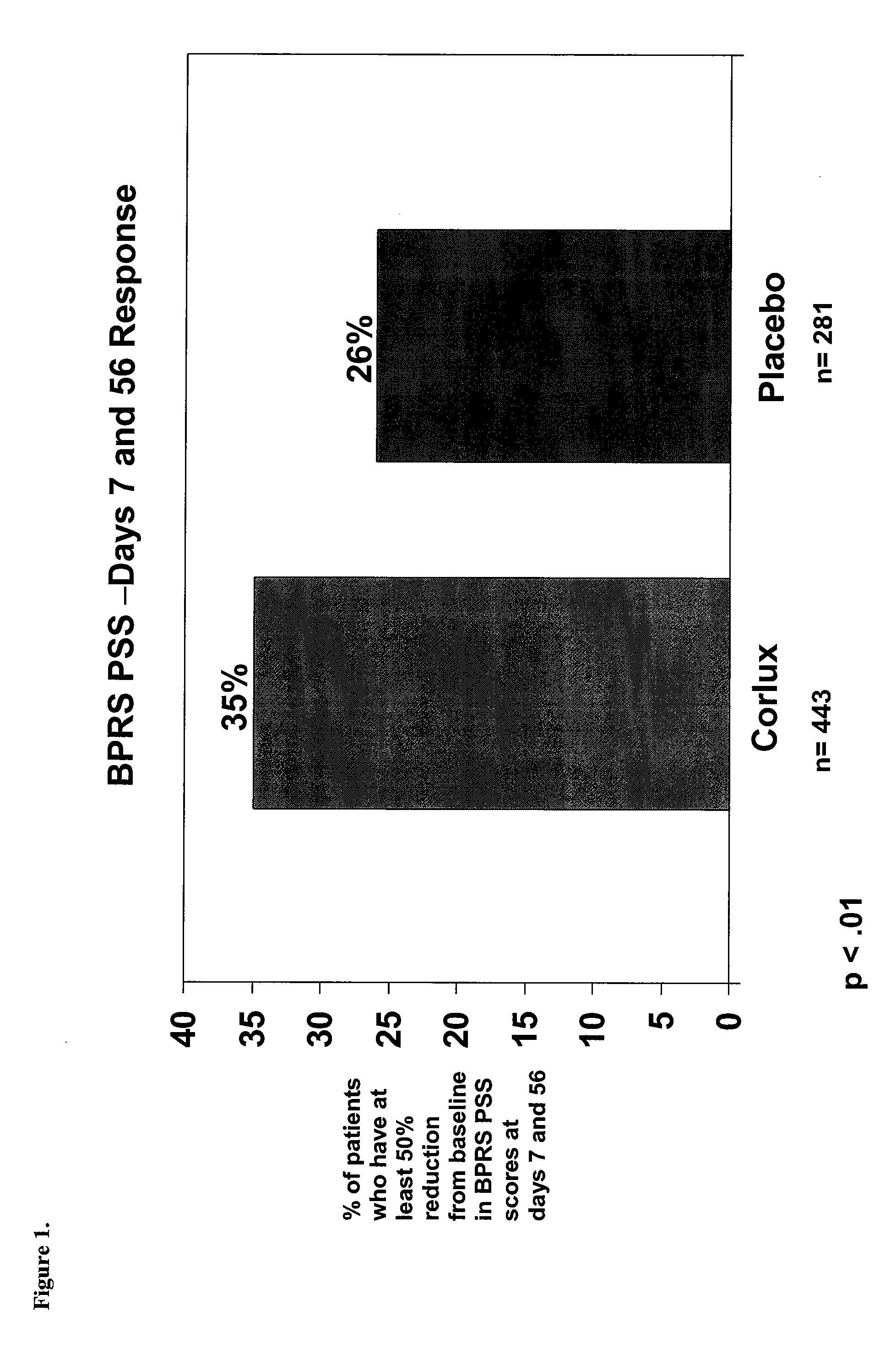
Optimizing mifepristone levels in plasma serum of patients suffering from mental disorders treatable with glucocorticoid receptor antagonists
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from a mental disorder amenable to treatment by mifepristone. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1300 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1300 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
Apolipoprotein fingerprinting technique and methods related thereto
InactiveUS8389222B2Facilitate detection and measurementConfidenceBiological material analysisBiological testingAntiendomysial antibodiesA lipoprotein
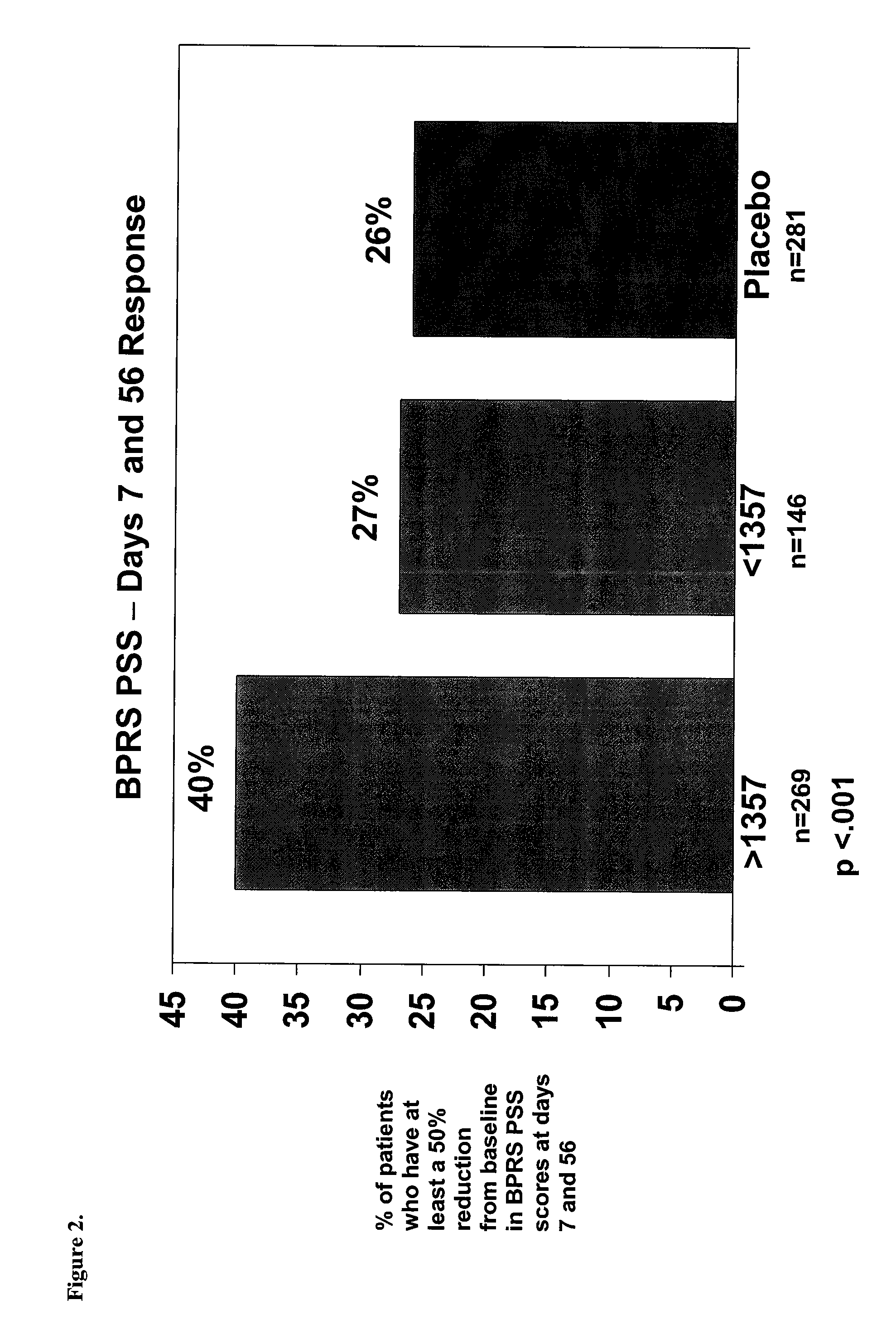
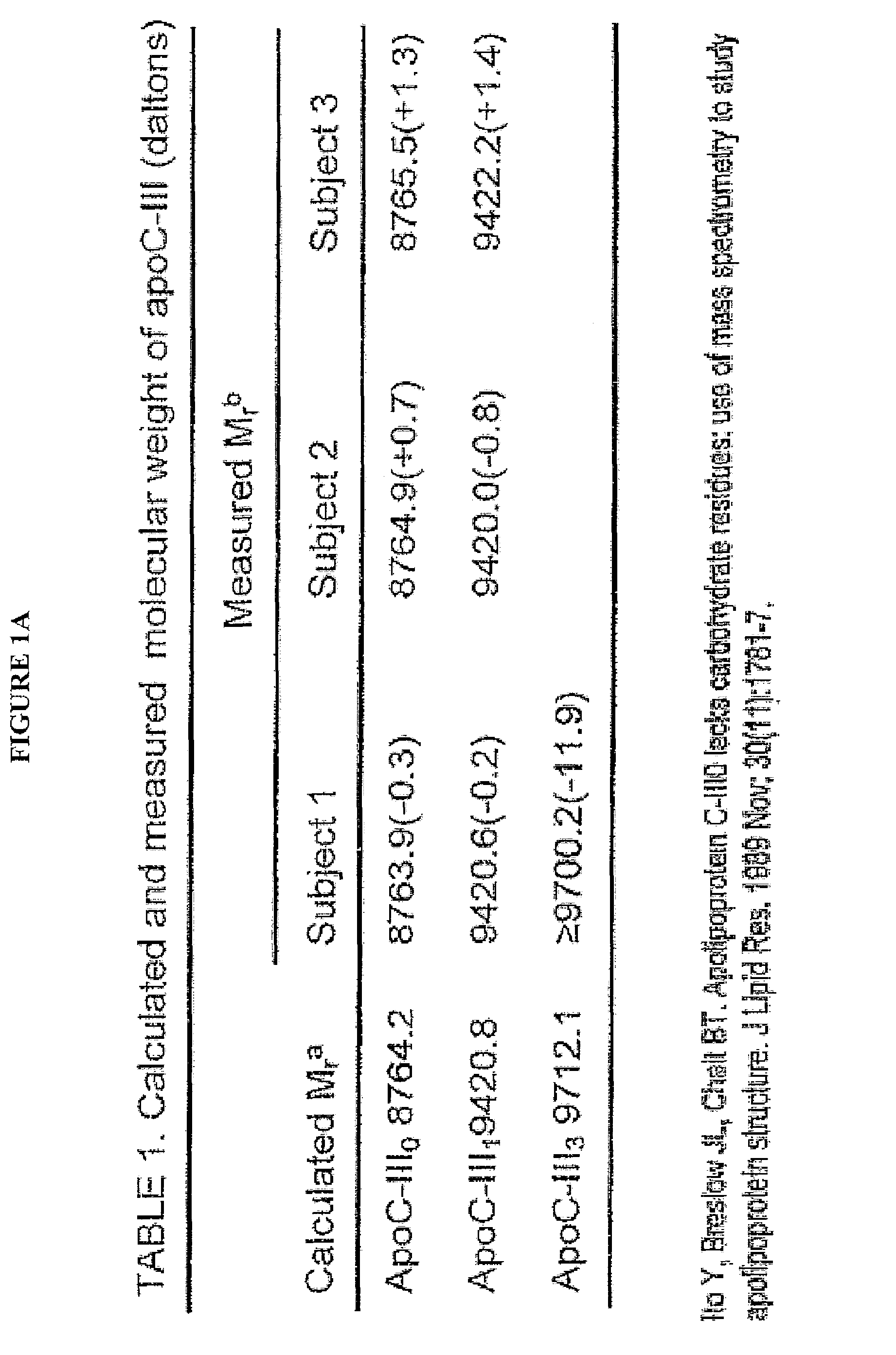
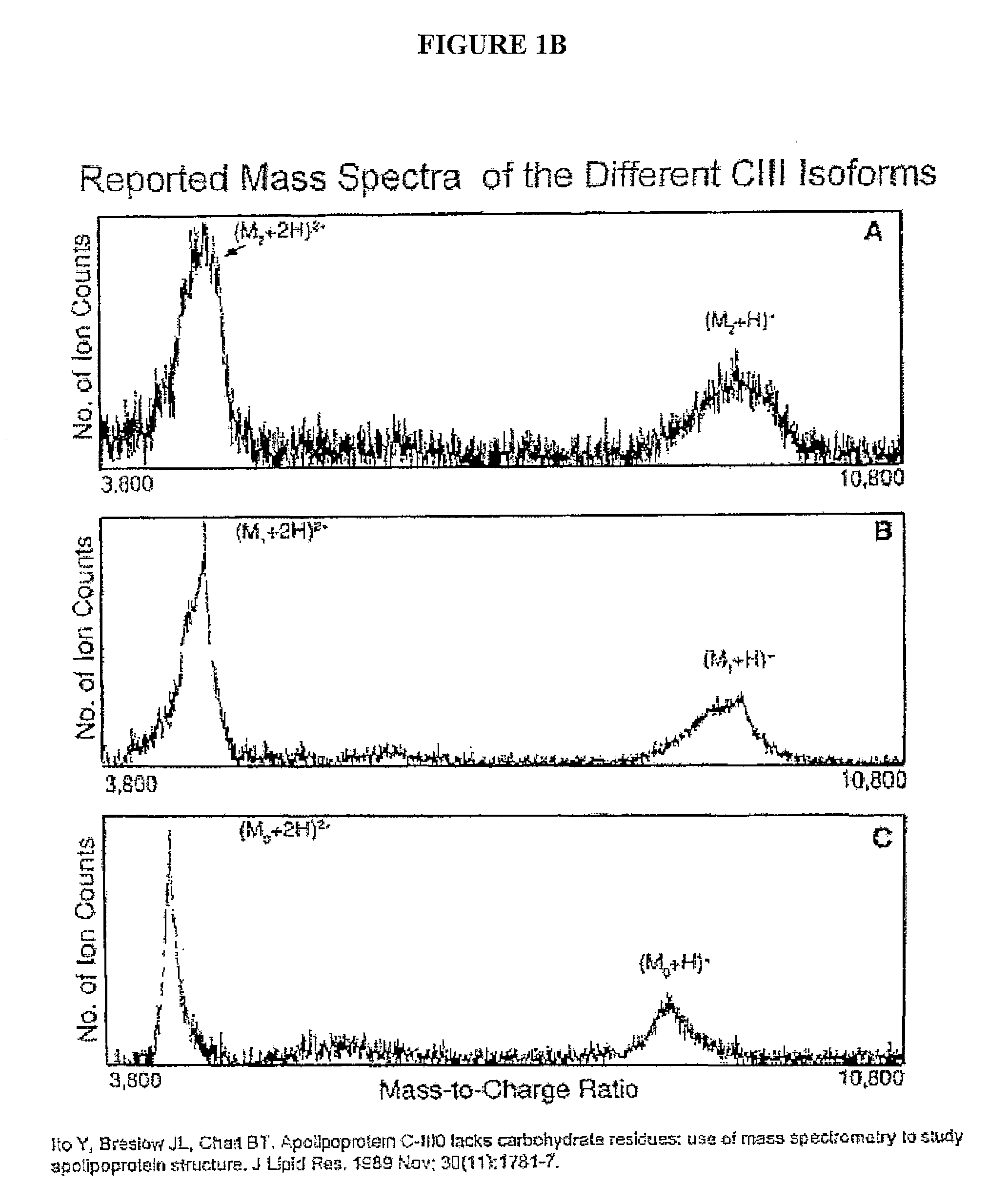
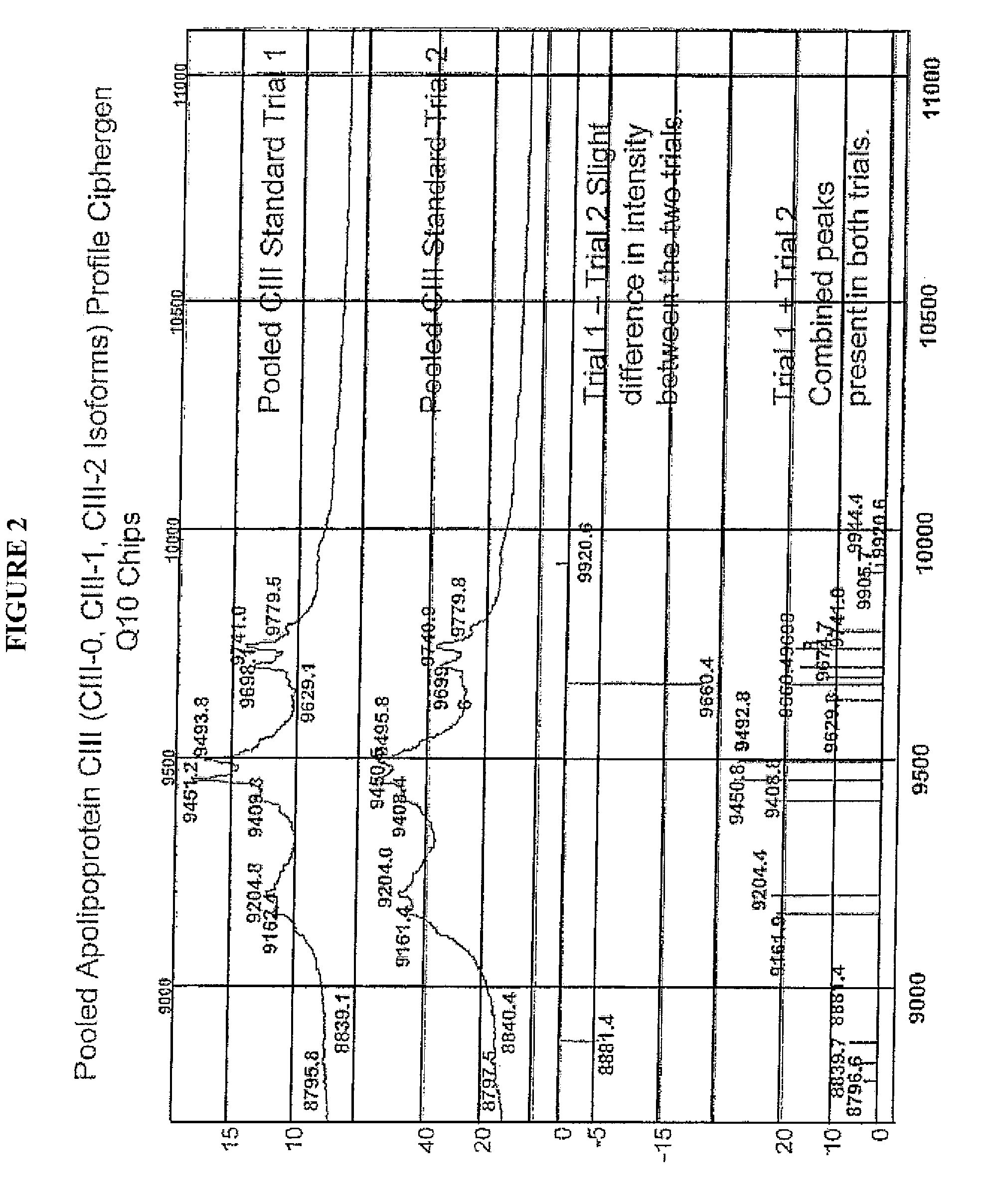
A method for determining the concentration and modifications of apolipoprotein in biological samples including plasma, serum, and lipoprotein fractions, by obtaining a sample from a patient, adding a specific volume of an internal standard to the sample, applying the sample to a surface-enhanced, Protein G-coated, antibody-bound chip and removing unbound sample components, analyzing the sample by mass spectrometry, determining the concentration of the apolipoprotein using values of internal standards, and evaluating the concentration of the apolipoprotein, its isoforms, amino acid substitutions and modifications for use as a tool for diagnosing cancer, diabetes, stroke, stress, Alzheimer's disease, inflammation, neurological disease and cardiovascular diseases.
Owner:DE GUZMAN BREYER EMELITA +1
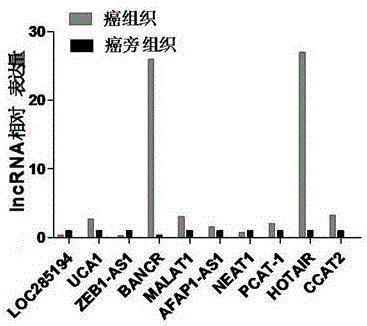
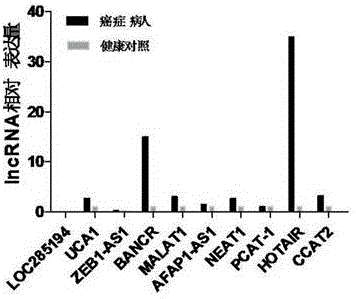
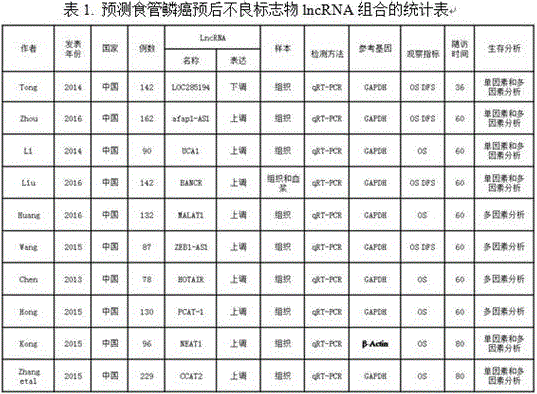
LncRNA composition for detecting prognosis of early esophageal cancer and kit comprising LncRNA composition
InactiveCN107523647APredict risk of poor prognosisImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationReference sampleCvd risk
The invention discloses an LncRNA composition for detecting prognosis of an early esophageal cancer and a kit comprising the LncRNA composition. By fluorescent quantitative PCR, a sample (which can be tissues / plasma serum and the like) of a patient with an esophageal cancer and difference change of the expression amount of a group of LncRNA in a corresponding para-carcinoma tissue / reference sample are identified, and the risk of esophageal cancerrelapse or transfer is accurately evaluated early. The kit can be used for accurately detecting early esophageal cancer transfer potential, then the transfer or relapse risk of a postoperative esophageal cancer patient can be accurately predicted and evaluated, the patient with high risk is intensively monitored and intervened effectively, relapse or transfer of the esophageal cancer is reduced, and prognosis of the patient is improved further. The poor prognosis related lncRNA composition is adopted, and the shortcomings that sensitivity and specificity are low due to the fact that a kit only uses single lncRNA as a tumor marker, and therefore, rate of fault diagnosis and rate of missed diagnosis on the esophageal cancer are greatly increased are overcome.
Owner:NANYANG NORMAL UNIV
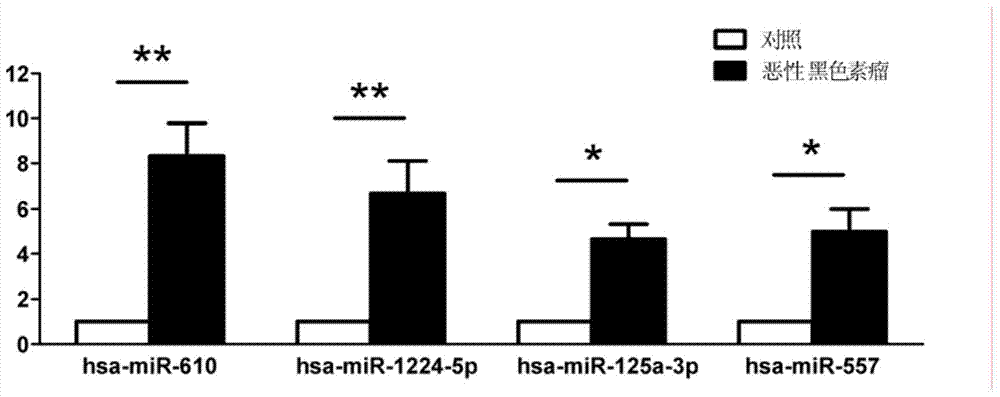
Plasma/serum circulation microRNA marker related to mlignnt melnom and application of marker
ActiveCN103642914AEasy to detectQuantitatively accurateMicrobiological testing/measurementDNA/RNA fragmentationMir 125aBlood plasma
The invention relates to a plasma / serum circulation microRNA marker related to mlignnt melnom and application of the marker. The plasma / serum circulation microRNA marker related to mlignnt melnom is combination of miR-610, miR-1224-5p, miR-125a-3p and miR-557. The invention provides the plasma / serum circulation microRNA marker which has the capability of early diagnosis and mlignnt melnom screening and is related to mlignnt melnom, and the application of the marker.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
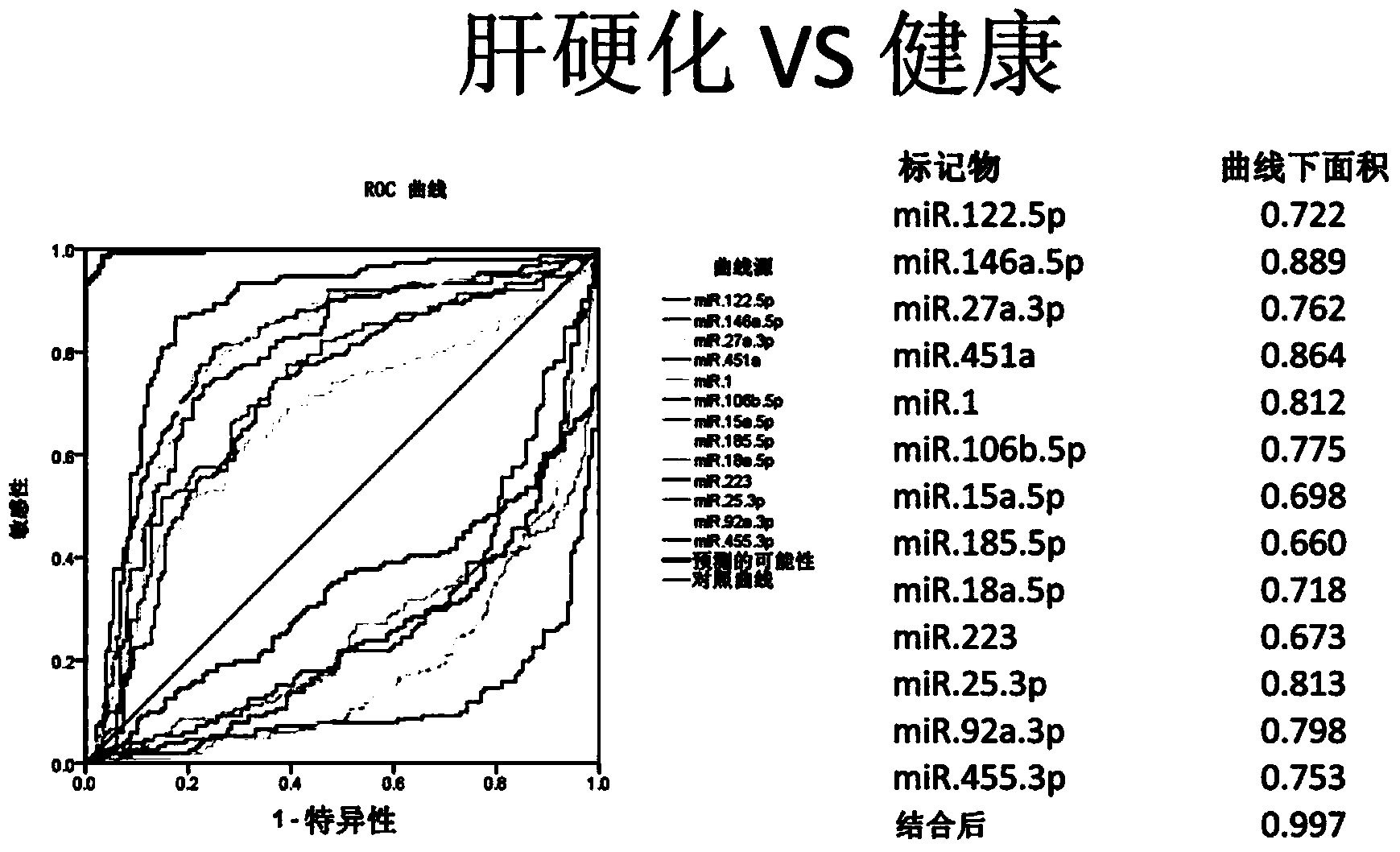
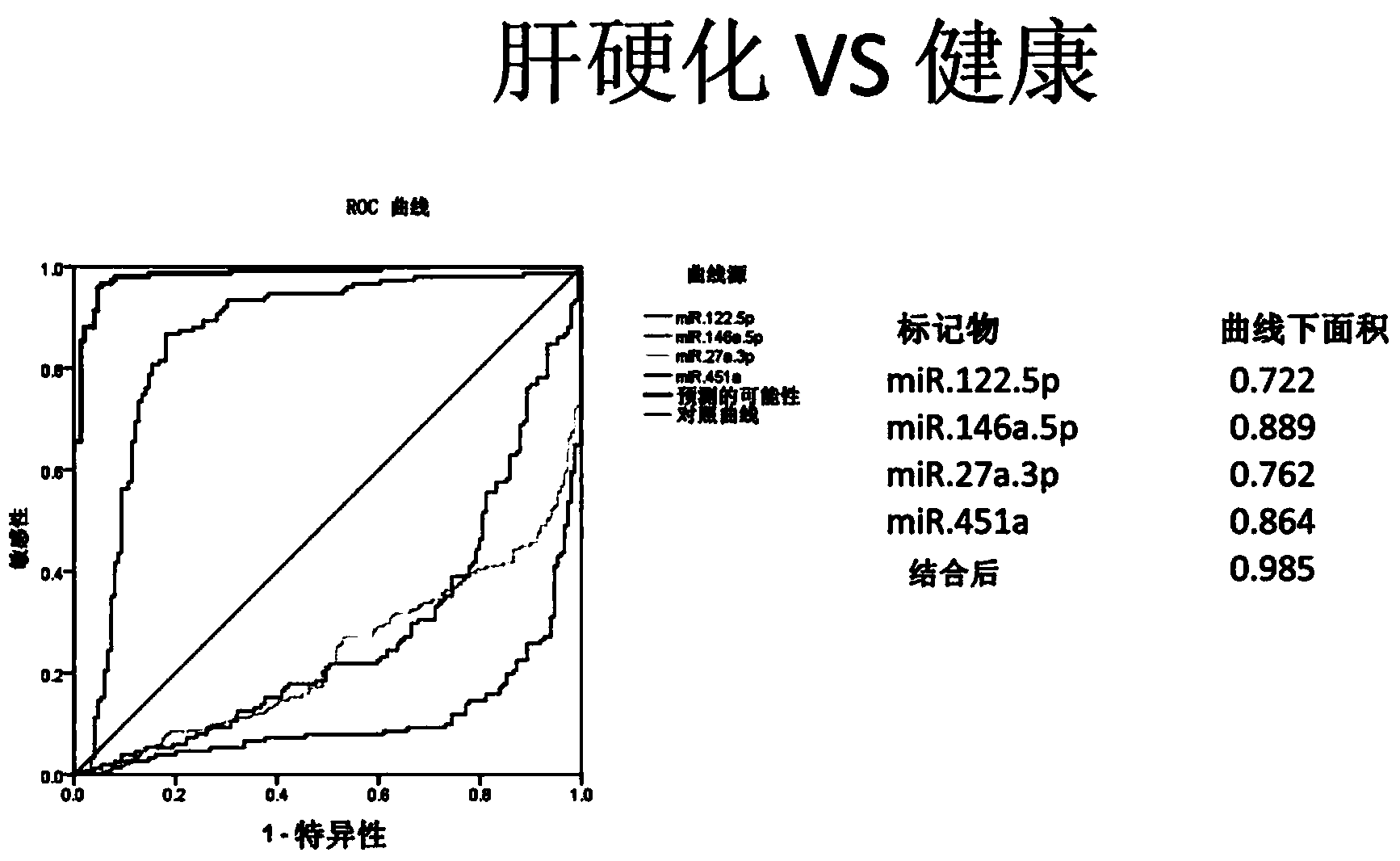
Cirrhosis microRNA molecular marker composition and application thereof
InactiveCN104232638AEasy to operateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationUse medicationMedicine
The invention discloses a cirrhosis microRNA molecular marker composition, with sequence No. ranging from 1 to 13, as well as application of the molecular marker composition in preparing a cirrhosis personalized medicine diagnostic reagent. Due to a significant difference between content of a microRNA molecular marker of a cirrhosis patient in plasma / serum and content in plasma / serum in health control, cirrhosis patient can be effectively distinguished from people of the health control; in a process of applying medicines to the cirrhosis patient, administration can be stopped by guiding and assistance, when cirrhosis outcome microRNA molecular marker is adjusted to a normal level. Furthermore, the invention discloses a diagnostic kit for guiding personalized administration of the cirrhosis. The liver cirrhosis outcome microRNA molecular marker disclosed by the invention, when being used for guiding personalized administration of cirrhosis patients, has characteristics of being simple to operate, safe and noninvasive, high in specificity, high in sensitivity and easy in massive screening.
Owner:北京旷博生物技术股份有限公司
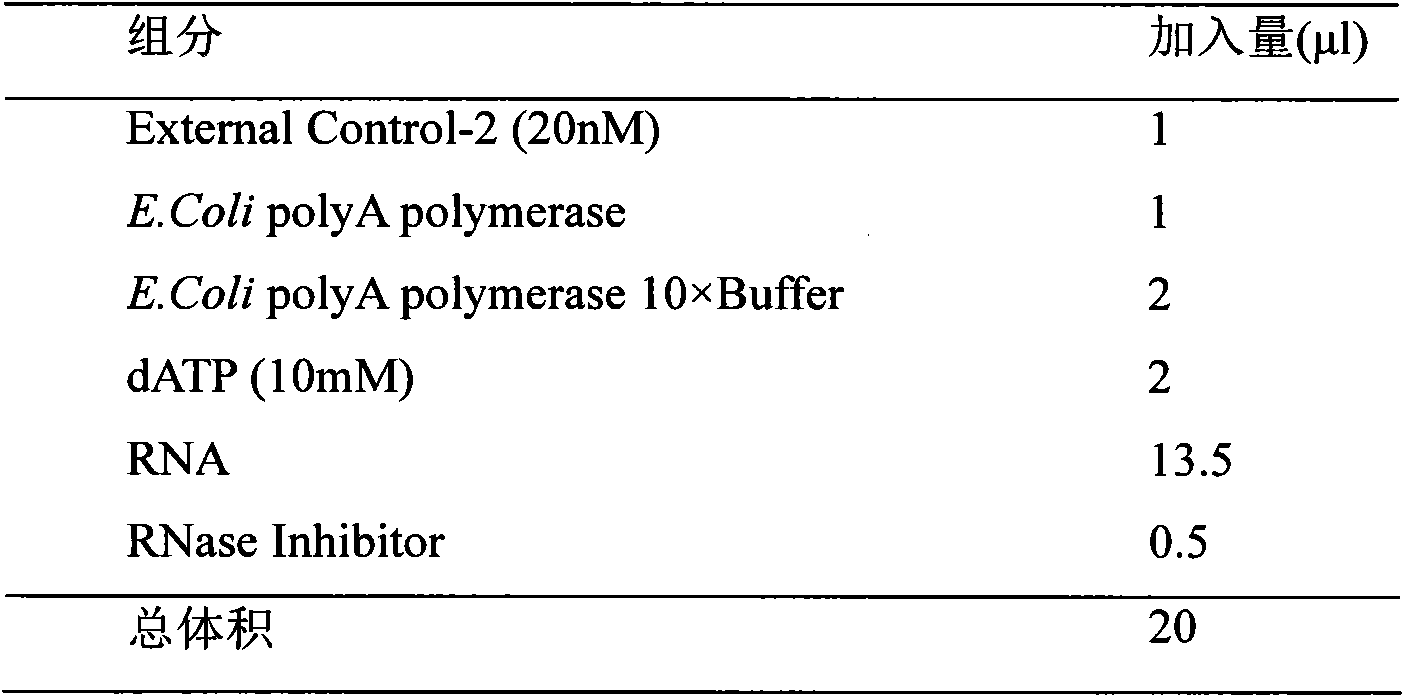
Plasma miRNA (micro Ribonucleic Acid) profile and assay kit for predicting curative effect of interferon on treatment of chronic hepatitis B
InactiveCN102643803AImprove developmentLow cost of treatmentMicrobiological testing/measurementDNA/RNA fragmentationInterferon therapyAlanine aminotransferase
The invention belongs to the fields of molecular biology and medicine, and provides a plasma miRNA (micro Ribonucleic Acid) profile and an assay kit for predicting the curative effect of interferon on treatment of chronic hepatitis B. The miRNA profile comprises one or more miRNAs in SEQ ID NO1-11. Meanwhile, the invention provides corresponding high-efficiency and low-cost kit, assay chip and assay method. According to the plasma miRNA profile and the assay kit, the miRNA level in plasma and serum is assayed; and the plasma miRNA profile and the assay kit are suitable for being applied to clinically judging the effectiveness of interferon treatment. Compared with other known curative effect related factors, the expression profile is an independent prediction factor. Higher prediction accuracy can be achieved by combining the prediction profile with the ALT (Alanine Aminotransferase) level of a patient before treatment. The development of a personalized treatment plan is facilitated by combining the expression profile with HBV (Hepatitis B Virus) medicine resistance mutation analysis; and finally, the treatment cost is reduced and the proportion of complete response is increased.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
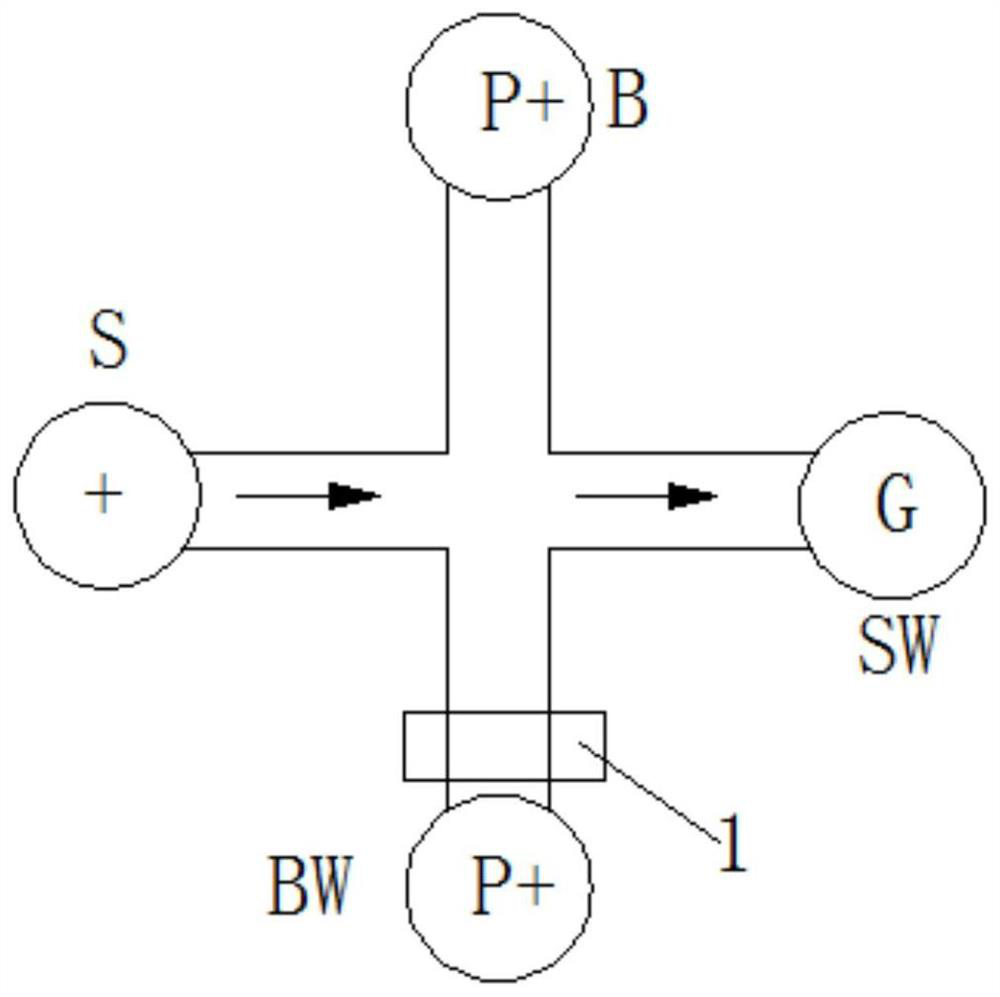
Micro-fluidic chip and whole blood separation method based on micro-fluidic chip
InactiveCN110918144AAchieving Whole Blood SeparationGreat application potentialLaboratory glasswaresWhole blood unitsBlood plasma
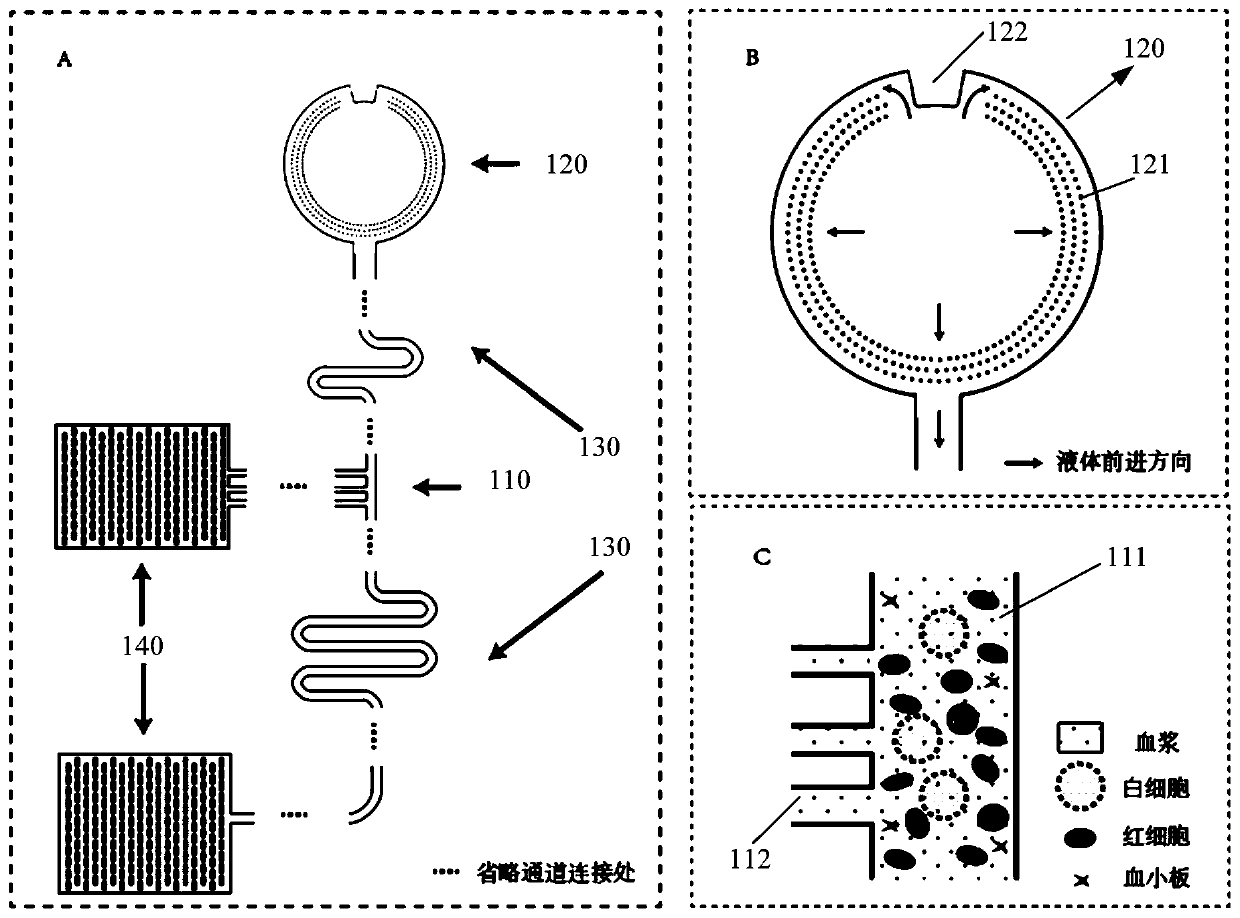
The invention provides a micro-fluidic chip. When total blood flow passes through a bifurcated branch channel port, blood cells are subjected to asymmetric shearing force on two sides, the blood cellsselect a main channel with the large flow speed and the small flow resistance to continue to advance, and part of blood plasma can enter a bifurcated branch channel. According to the micro-fluidic chip, the micro-fluidic chip constructed through a bifurcated multi-branch structure can achieve whole blood separation without exogenous power. When the micro-fluidic chip performs flow resistance adjustment through a flow resistance adjusting unit 130 located in the bifurcated branch channel, the collection efficiency of the blood plasma is adjusted. According to the micro-fluidic chip, capillarydriving force is provided through a capillary pump unit 140 located at a tail end, the functions of quantifying and collecting plasma / serum are achieved, and finally on-chip self-driven whole blood separation is achieved. The micro-fluidic chip provided by the invention shows huge application potential in the field of integrated and micro-miniaturized bedside instant detection.
Owner:SHENZHEN INST OF ADVANCED TECH
Hepatitis b/hepatic cirrhosis microRNA molecular marker combination and purpose thereof
PendingCN106337051AEasy to operateStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseChronic hepatitis
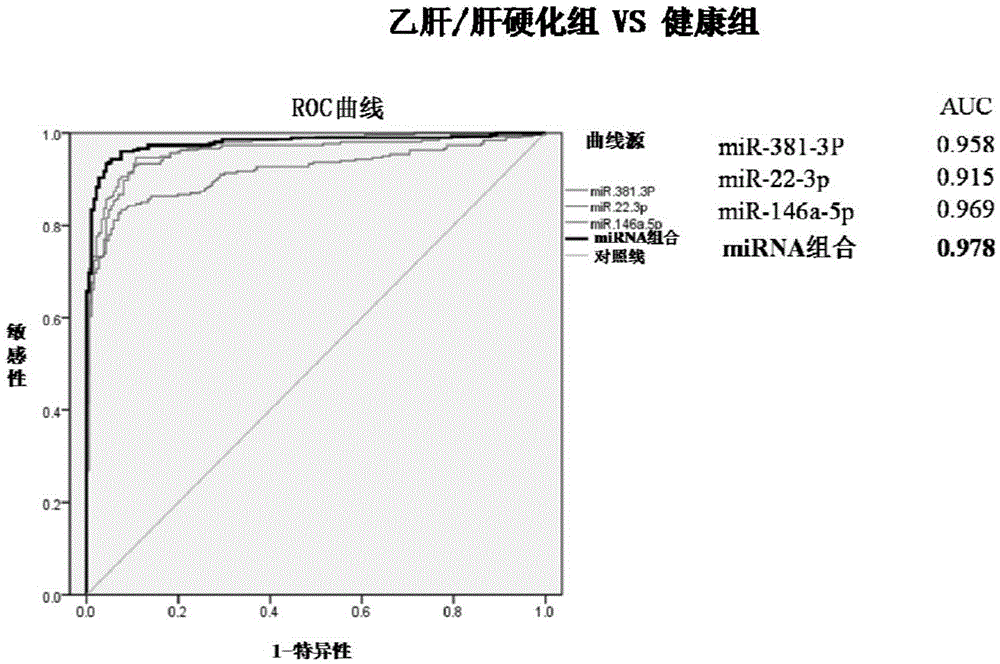
The invention provides a hepatitis b / hepatic cirrhosis microRNA molecular marker combination, which comprises more than one micro RNA nucleic acid molecules selected from mir.146a-5p, miR-22-3P and miR-381.3P, and a purpose of the marker combination for preparing hepatitis b / hepatic cirrhosis infection diagnosis and / or prognosis assessment kit. Due to a significant difference between content of a microRNA molecular marker of a hepatitis b / hepatic cirrhosis patient in plasma / serum and content in plasma / serum in health control, the hepatitis b / hepatic cirrhosis patient can be effectively distinguished from people of the health control; a chronic hepatitis b / hepatic cirrhosis disease state can be comprehensively analyzed, which can be used for guiding the patient treatment and usage of medication. The invention also provides a diagnostic kit for guiding the hepatitis b / hepatic cirrhosis infection diagnosis and / or prognosis assessment. By using the hepatitis b / hepatic cirrhosis microRNA molecular marker and the diagnostic kit, infection diagnosis and / or prognosis assessment of the hepatitis b / hepatic cirrhosis patient can be guided, and the combination has the characteristics of simple operation, safety and no wound, high specificity, high sensitivity and easy massive screening.
Owner:BEIJING YOUAN HOSPITAL CAPITAL MEDICAL UNIV +1
LncRNA gene marker and kit for ovarian cancer clinical diagnosis
InactiveCN112239786AImprove accuracyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBenign Ovarian CystOncology
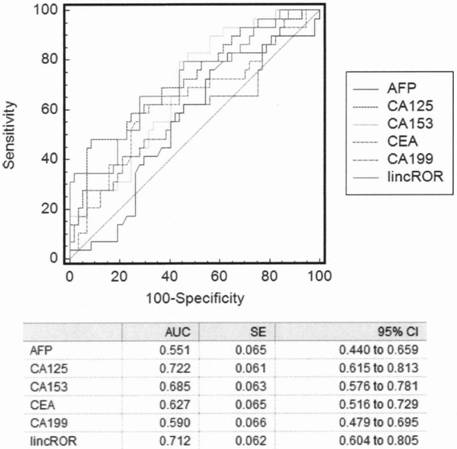
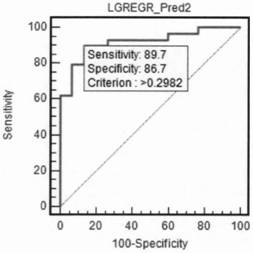
The invention discloses application of a long-chain non-coding RNA gene marker LincROR in early diagnosis, prognosis and chemotherapy sensitivity judgment of ovarian cancer. The blood plasma and bloodserum LincROR provided by the invention have excellent diagnostic performance for diagnosing ovarian cancer and non-ovarian cancer (including benign ovarian cyst and health control), can improve theaccuracy, sensitivity and specificity of judgment in ovarian cancer clinical diagnosis and chemotherapy sensitivity by combining with application of the existing tumor marker CA125, and can be developed into a diagnostic kit for early diagnosis of ovarian cancer.
Owner:NANJING FIRST HOSPITAL
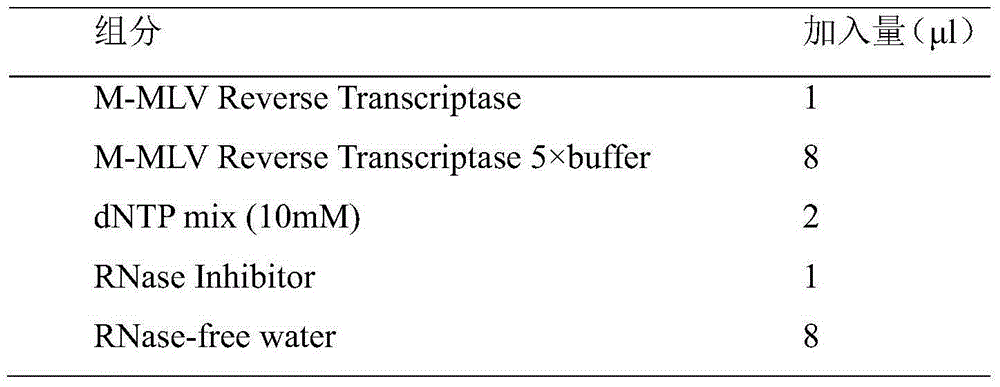
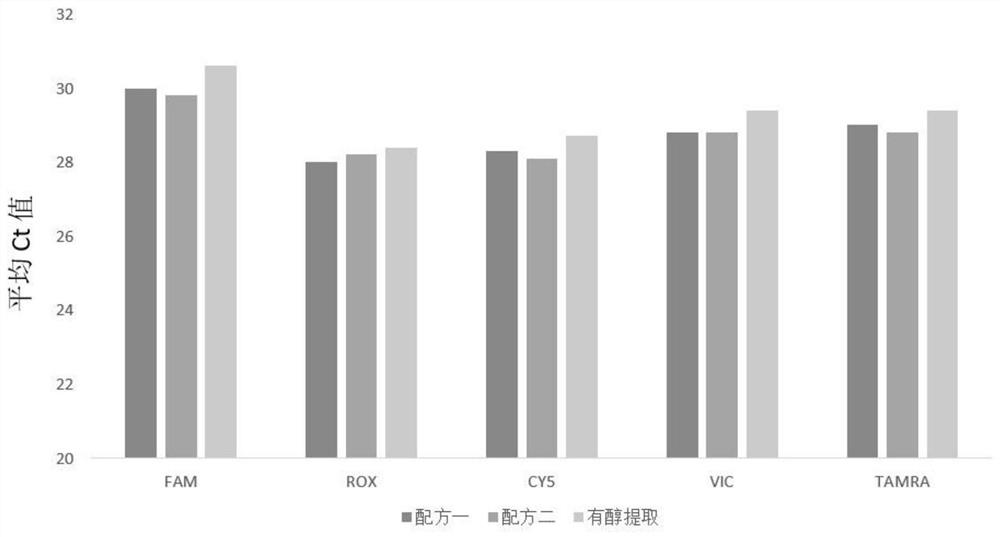
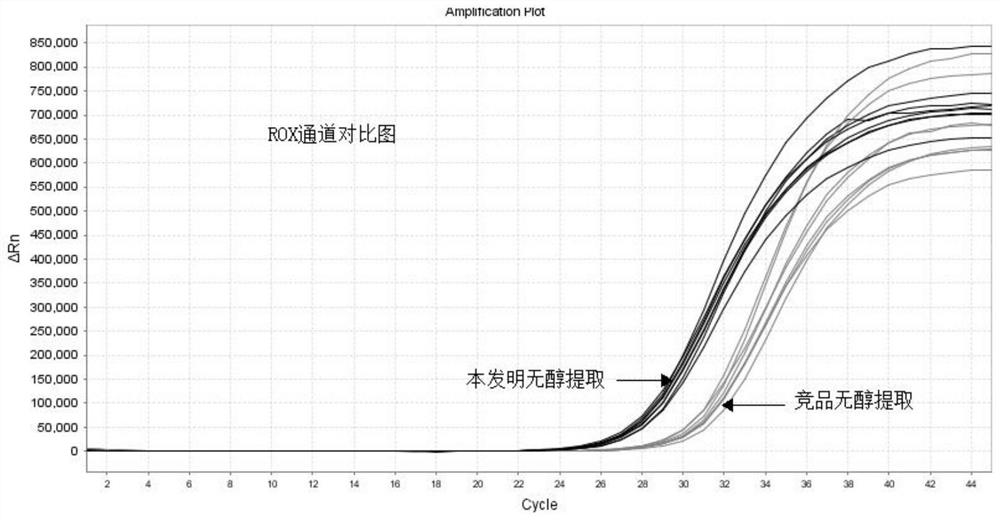
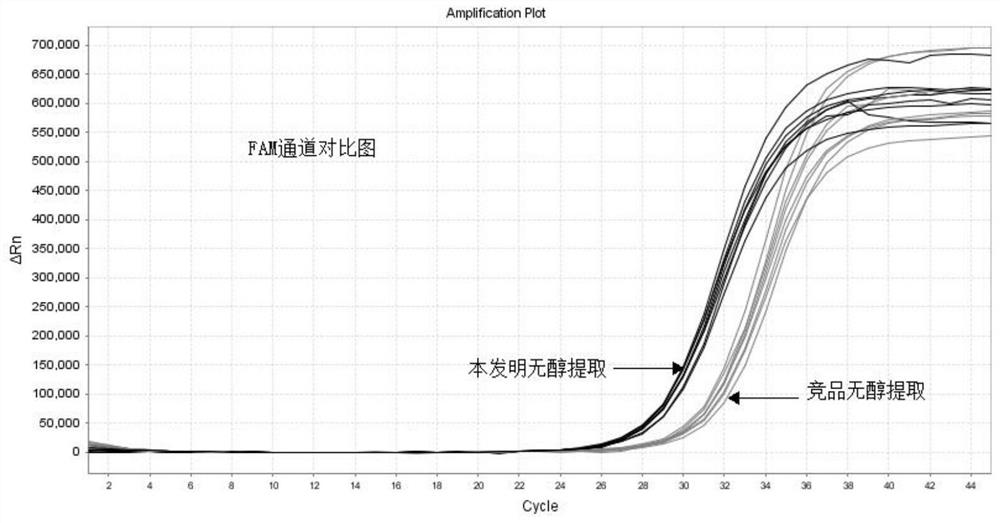
Alcohol-free cleaning solution suitable for nucleic acid extraction of multiple samples and nucleic acid extraction kit
The invention discloses an alcohol-free cleaning solution suitable for nucleic acid extraction of various samples and a nucleic acid extraction kit. The alcohol-free cleaning solution comprises a cleaning solution A and a cleaning solution B, the cleaning solution A comprises 30 mM to 50 mM of Tris-HCl, 15% to 30% of PEG and 2 to 3 M of sodium salt, and the cleaning solution B comprises 30 mM to 50 mM of Tris-HCl, 50 mg / mL to 100 mg / mL of silicon-based magnetic beads, 10% to 15% of PEG and 1.5 M to 2.5 M of sodium salt. The nucleic acid extraction kit provided by the invention comprises the alcohol-free cleaning solution, a lysis solution, protease K and an eluent. The nucleic acid extraction kit can be used for extracting various types of samples (oropharynx swab samples, nasopharynx swab samples, plasma, serum, sputum and saliva). By adopting the nucleic acid extraction kit, the influence of ethanol residue on downstream experiments can be reduced, the nucleic acid purity is improved, and the detection accuracy is further improved.
Owner:3D BIOMEDICINE SCI & TECH CO LTD
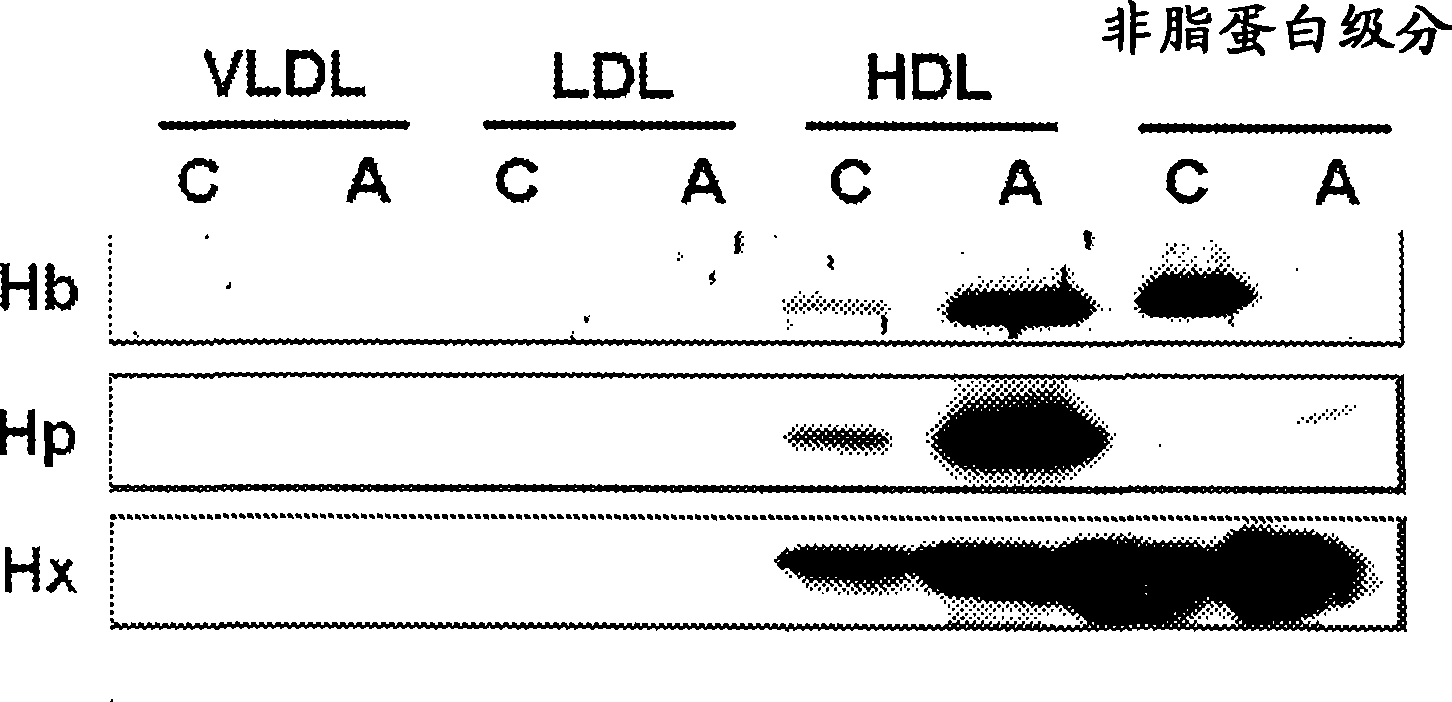
Assays to predict atherosclerosis and dysfunctional high-density lipoprotein
This invention provides novel assays for the detection of dysfunctional HDL. The assays are good diagnostics and / or prognostics for atherosclerosis or other pathologies characterized by an inflammatory response. In certain embodiments the methods involve measurements of heme-related HDL-associated proteins (e.g., haptoglobin, hemopexin, etc.), and / or measurements of the relative distribution of HDL-associated proteins between HDL and the non-lipoprotein fractions of plasma / serum, and / or measurements of the ability of pro-inflammatory HDL to consume nitric oxide, and / or measurement of the ability of HDL to inhibit LDL aggregation.
Owner:RGT UNIV OF CALIFORNIA
Preparation method of prp serum for repairing sport injury
PendingCN114099543APromote repairEasy to degradeSerum immunoglobulinsAerosol deliveryAthletic injuryPlatelets blood
The invention provides a preparation method of prp serum for repairing sport injury, and relates to the technical field of serum preparation. The preparation method of the prp serum for sports injury repair comprises the following steps: conveying anticoagulant blood formed by mixing whole blood and an anticoagulant, carrying out secondary nanofiltration and centrifugation on the anticoagulant blood to obtain plasma serums with different concentrations, mixing platelet-rich plasma serums prepared in advance with the plasma serums with different concentrations to obtain prp serums with different concentrations, and refrigerating to obtain the prp serum for sports injury repair. The prp serum can be mixed with sodium hyaluronate gel to be injected into the sport injury part, the environment of natural cartilage is fully simulated by means of the gel with high water content, and the prp serum is easy to degrade and non-toxic, so that the curative effect research of the prp serum for repairing the sport injury is facilitated, the optimal treatment concentration scheme is obtained, and the quick repair of the sport injury part is promoted.
Owner:南京国青血液净化科技有限公司
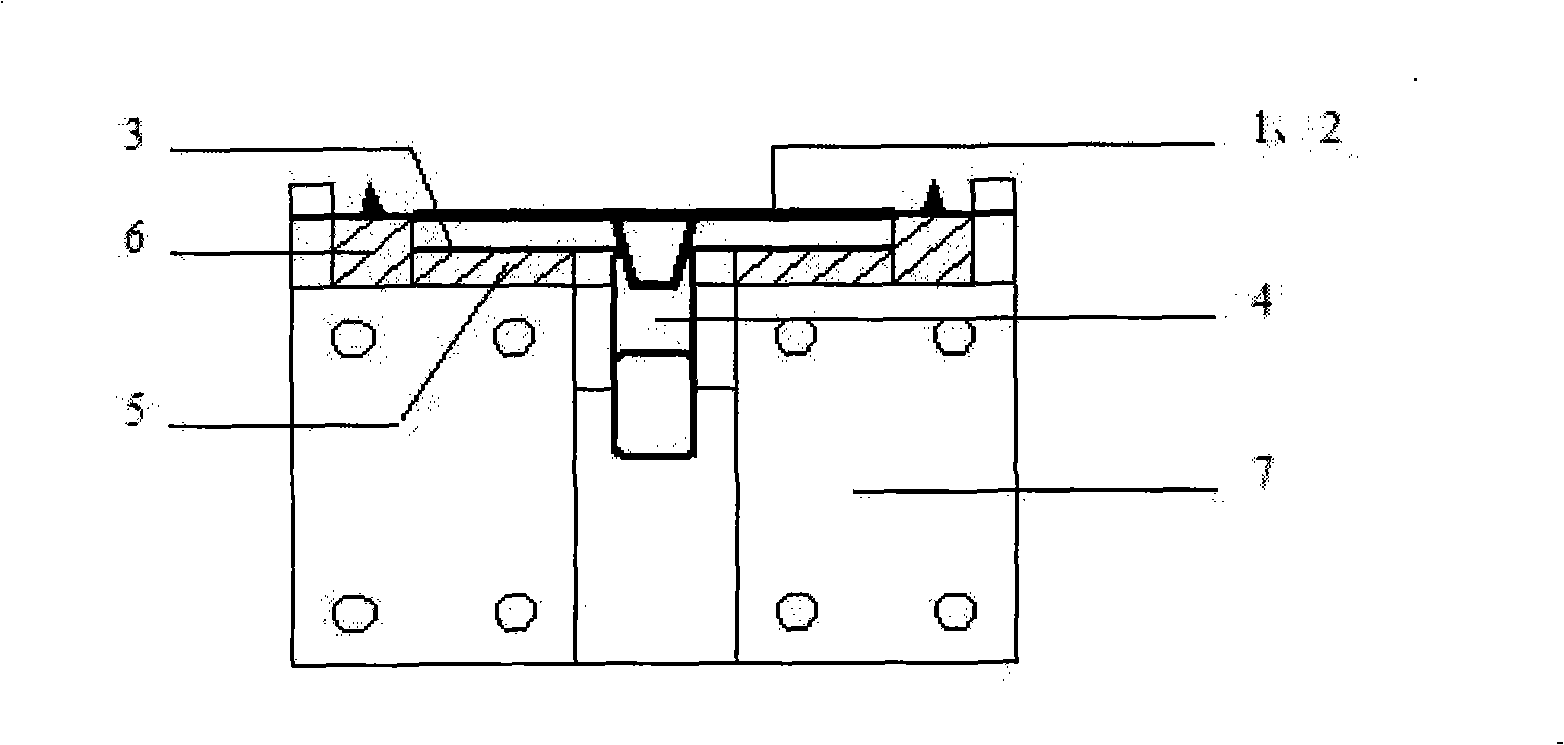
Device for cross flow filtration
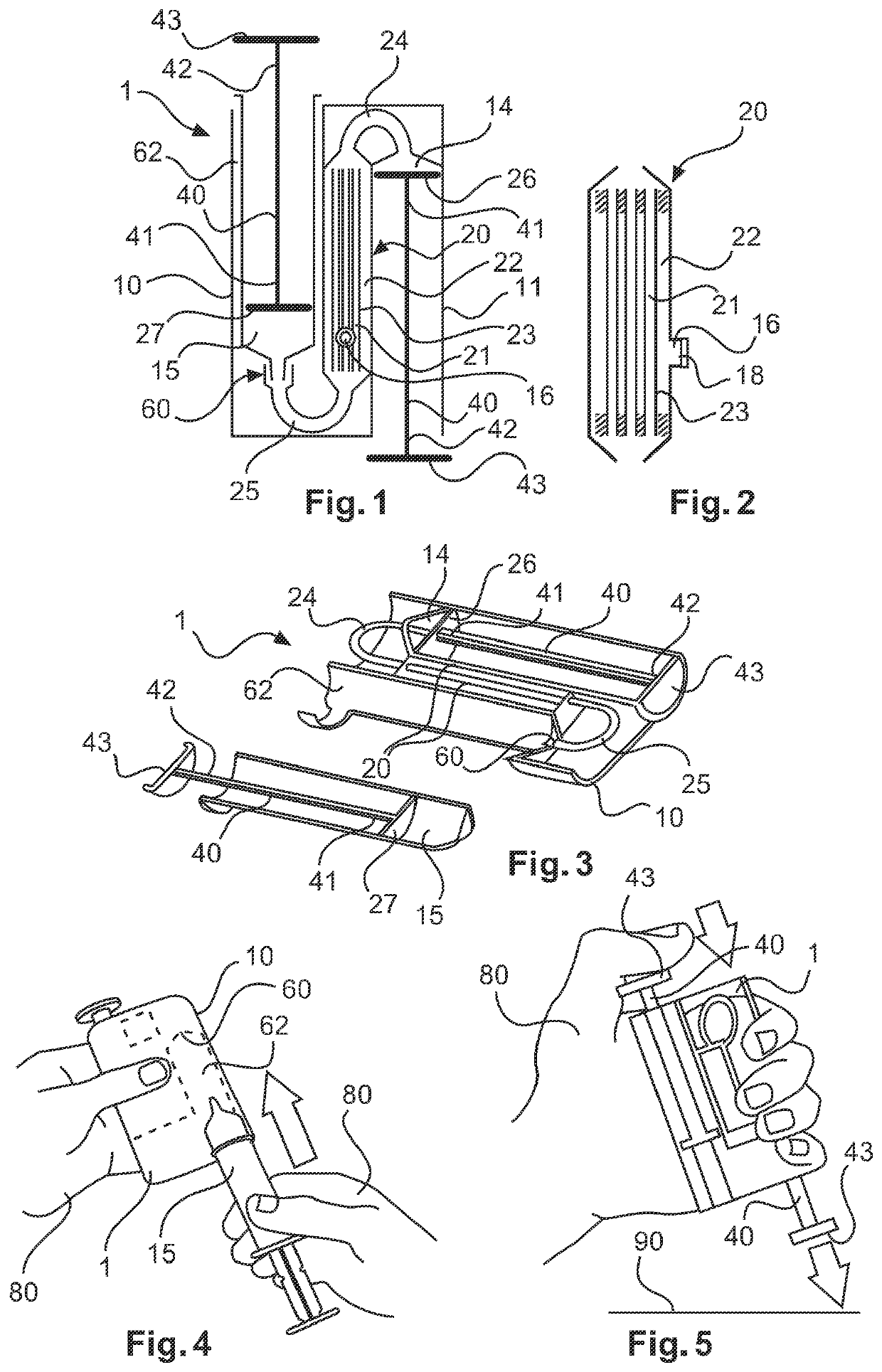
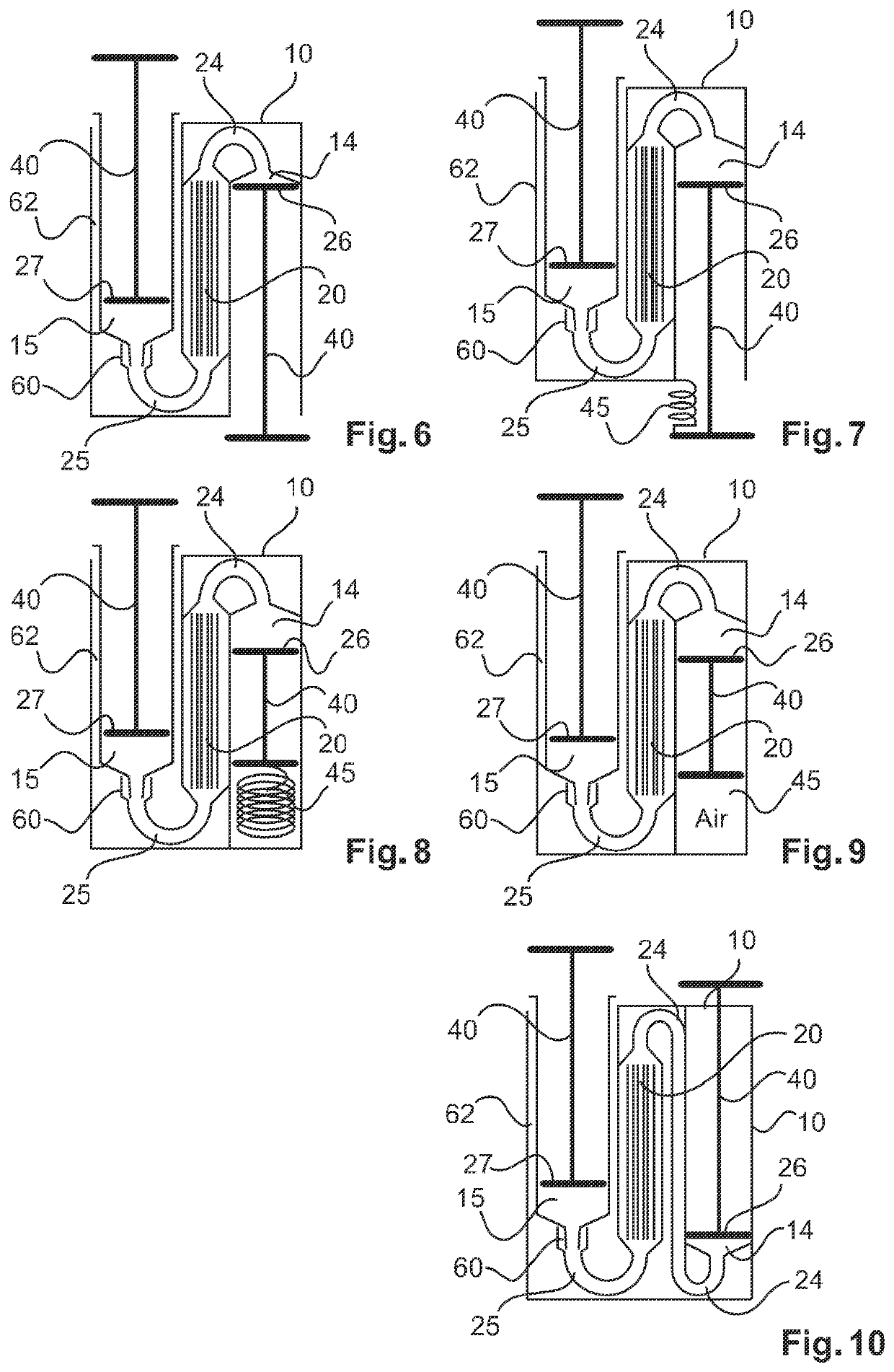
ActiveUS10687750B2Simple configurationQuick filterOther blood circulation devicesHaemofiltrationWhole blood unitsCross-flow filtration
A whole blood filtration device is provided with a filter membrane separating a feeding volume and a clean side of the filter membrane from each other. The feeding volume communicates with a first feeding side opening and with a second feeding side opening. The filter membrane has pores with a pore size that ensures permeability of the filter membrane to blood plasma / serum and that retains blood cells. The first feeding side opening can be coupled to a first blood pump for feeding blood from the first feeding side opening into the feeding volume so that blood plasma / serum permeates the filter membrane and blood cells, retained by the filter membrane, exit from the feeding volume through the second feeding side opening.
Owner:MANN HUMMEL GMBH
Marker for diagnosing liver cancer containing anti-ATIC autoantibody, and composition for diagnosing liver cancer containing antigen thereof
ActiveCN103732627AEffective developmentEasy diagnosisImmunoglobulins against cell receptors/antigens/surface-determinantsFused cellsNon invasiveLiver cancer
The present invention relates to a fragment comprising an autoantibody which specifically binds to ATIC or an antigen-binding site (i.e. paratope) of the autoantibody, a composition for diagnosing liver cancer containing an agent which measures the expression level of the fragment, a hybridoma cell line producing the autoantibody, a diagnostic kit for liver cancer containing the composition, a method for diagnosing liver using the composition, and a method for screening a liver cancer therapeutic agent using the autoantibody. Using the anti-ATIC specific autoantibody of the present invention as a marker for diagnosing liver cancer, the occurrence of liver cancer can be diagnosed using a non-invasive biological sample such as blood, plasma, serum, lymph tissue, and the like, with a sensitivity of about 87% and a specificity of about 88%, and as liver cancer can be easily diagnosed using an amino acid sequence identified in the present invention, the autoantibody is effective for developing a diagnostic kit for liver cancer.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Blood plasma miRNA spectrum and detection kit used for predicting chronic hepatitis b curative effect of interferon
InactiveCN102399852ALow cost of treatmentLow costMicrobiological testing/measurementDNA/RNA fragmentationInterferon therapyIndividualized treatment
The invention belongs to the fields of molecular biology and medical science, and provides a blood plasma miRNA spectrum used for predicting the chronic hepatitis b curative effect of an interferon. The miRNA spectrum comprises one or a plurality of miRNA in SEQ ID No.1-10. The invention also provides a corresponding high-efficiency and low-cost kit, a detection chip and a detection method. The method and the kit provided by the invention are used for detecting miRNA levels in blood plasma and blood serum. The method and the kit are suitable to be used clinically for determining the effectiveness of an interferon. Compared to other known related factors, the expression spectrum is an independent prediction factor. The combination of the expression spectrum and HBV drug-resistance mutation analysis assists in the practice of individualized treatment schemes, and finally in reducing treatment costs and improving complete response proportions.
Owner:SHANGHAI PUBLIC HEALTH CLINICAL CENT
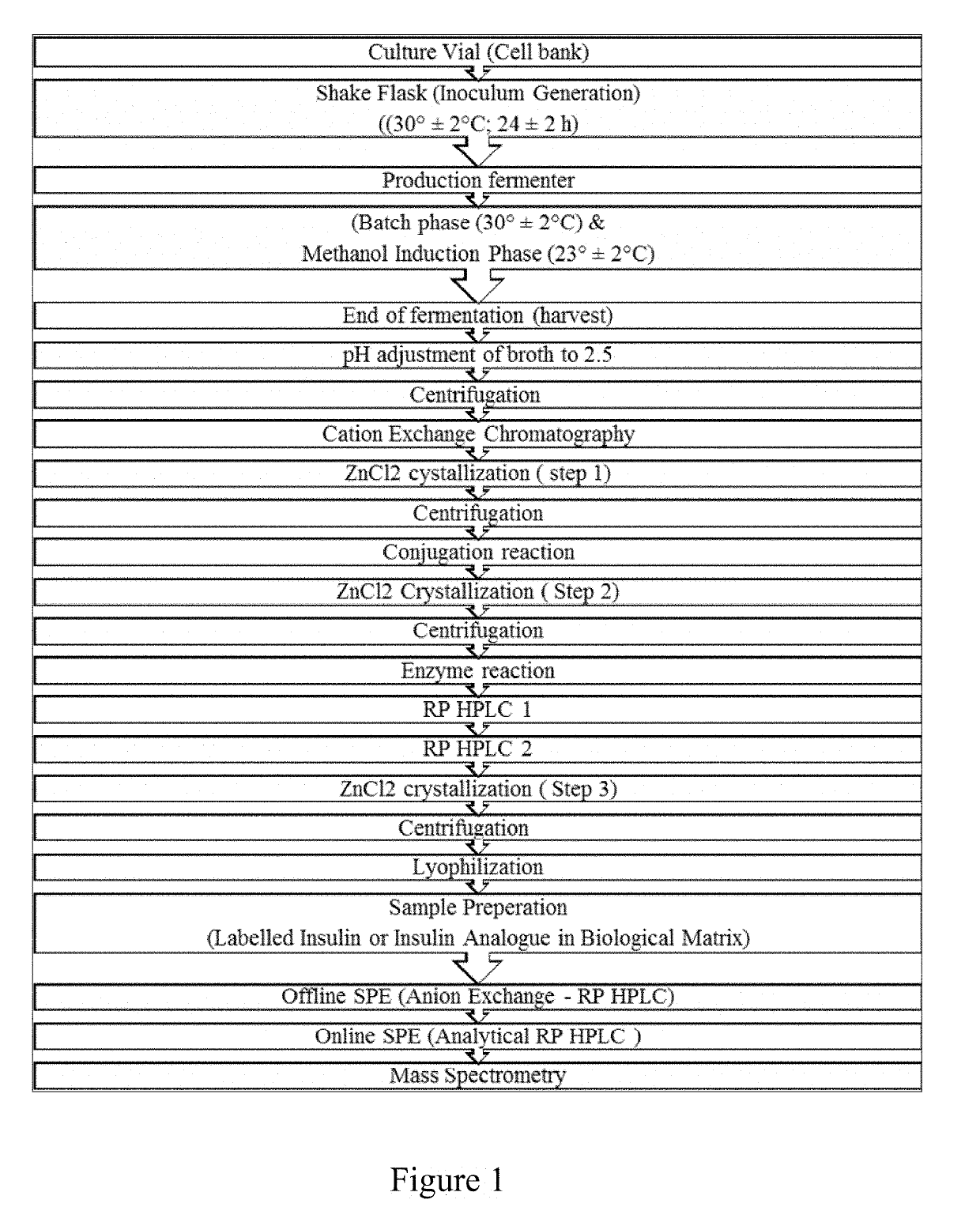
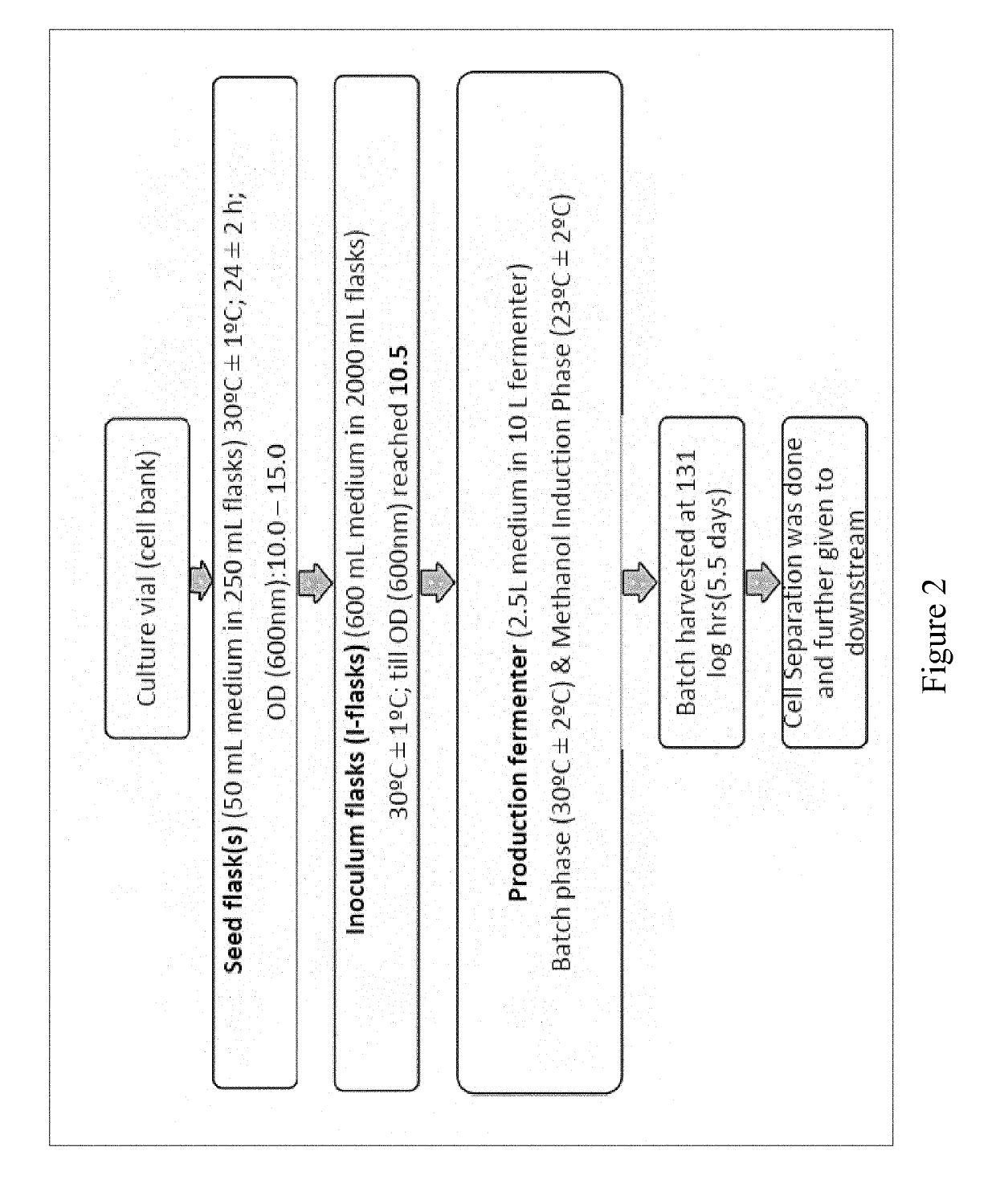
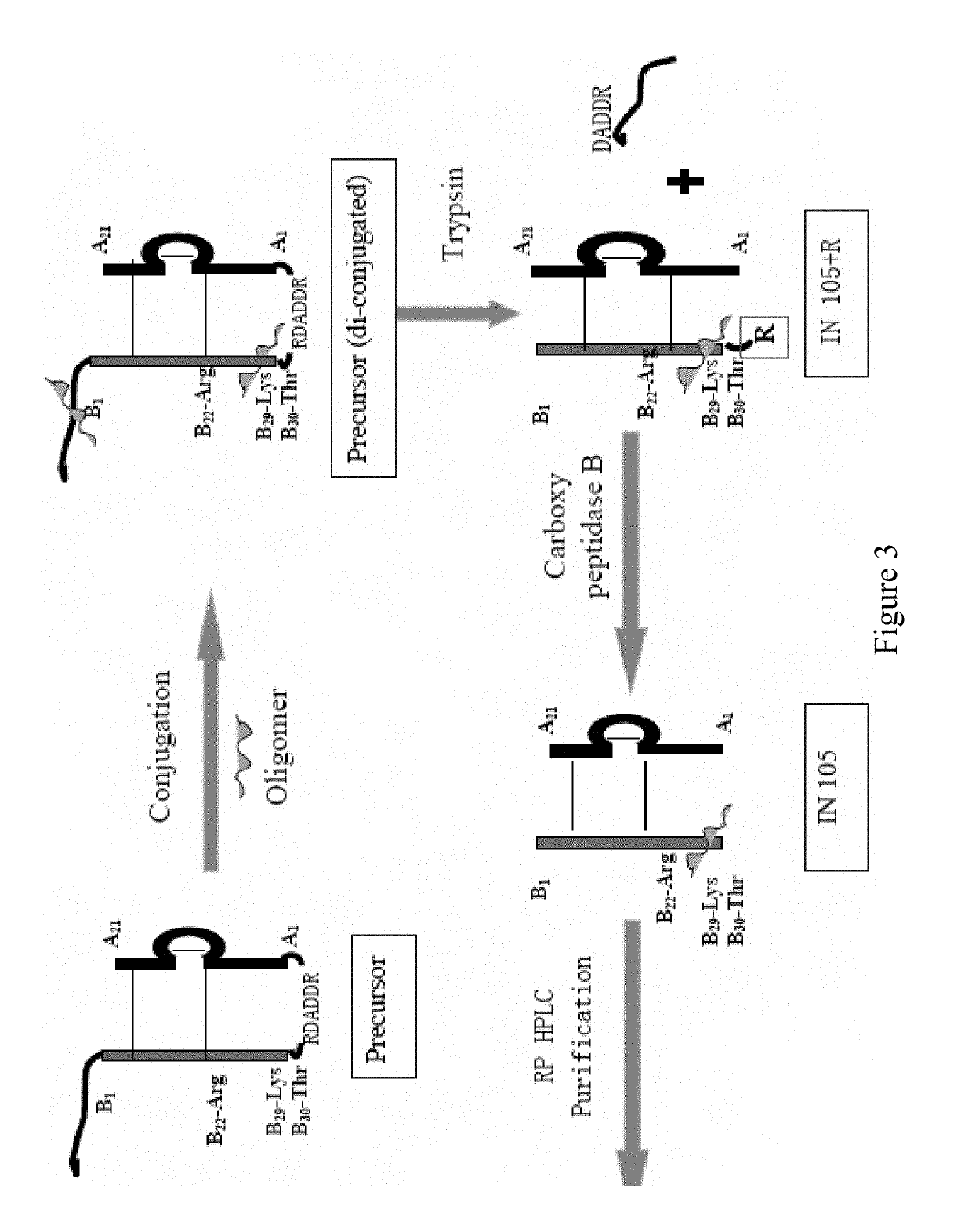
Bio-analytical method for insulin analogues
The present invention provides for a specific and sensitive bio-analytical method for detection of insulin or insulin analogues in plasma, serum or any other biological fluid, wherein the insulin or insulin analogues are labelled with a stable isotopic nitrogen for detection by the use of solid phase extraction and liquid chromatography with tandem mass spectrometric detection.
Owner:BIOCON LTD
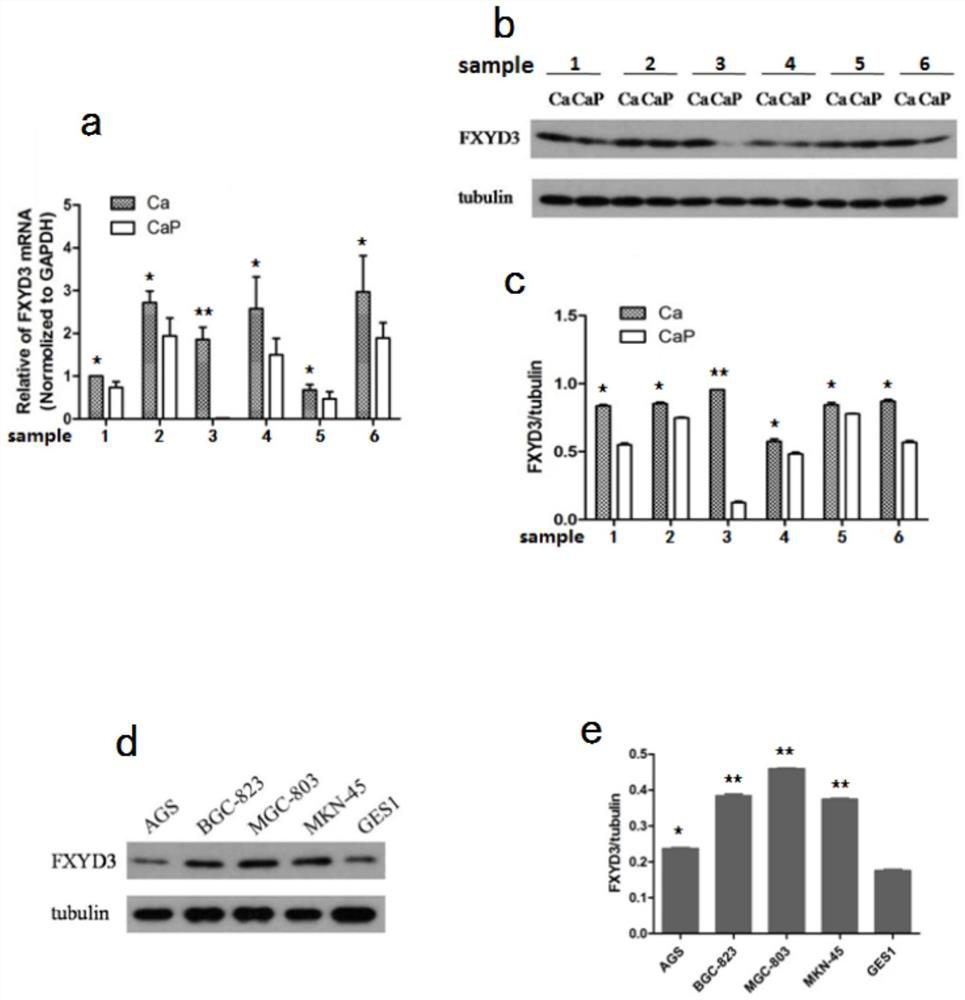
Application of FXYD3 as gastric cancer diagnosis marker and treatment target
ActiveCN112410429AReduce proliferationReduce invasionInorganic active ingredientsMicrobiological testing/measurementCancers diagnosisTreatment targets
The invention discloses application of FXYD3 as a gastric cancer diagnosis marker and a treatment target, and relates to the technical field of medical biological detection. According to the invention, a specific detection primer is constructed, which can be used for gastric cancer diagnostic reagent or kit, and can be used for detecting tissue specimens or blood samples such as blood plasma, blood serum and blood platelets. The invention also comprises application of the FXYD3 in preparation of a pharmaceutical composition for preventing or treating gastric cancer, and the drug can inhibit expression of the FXYD3. The FXYD3 has an important function in the occurrence and progress of gastric cancer, which can be used as a target for diagnosis and treatment, and has a good clinical application value.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
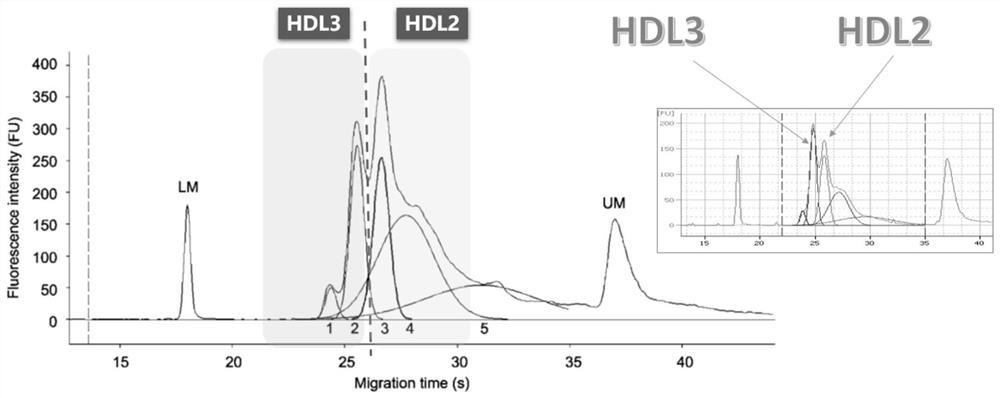
Method for detecting high-density lipoprotein subcomponent in preeclampsia judgment process
InactiveCN111879840AImprove measurement accuracyMaterial analysis by electric/magnetic meansDisease diagnosisA lipoproteinMicrochip Analysis
The invention discloses a method for detecting a high-density lipoprotein subcomponent in a preeclampsia judgment process, which comprises the following steps: presetting a high-density lipoprotein HDL subcomponent analysis kit, obtaining a blood sample, and detecting the to-be-detected blood sample by using the high-density lipoprotein HDL subcomponent analysis kit; carrying out standing centrifugal treatment on the blood sample, separating out plasma / serum, and adding the plasma / serum sample into a sample buffer solution containing lipophilic fluorescent dye, wherein the dye can be specifically combined with lipoprotein; injecting a gel dye into a microfluidic electrophoresis chip, adding a to-be-detected blood sample, performing detecting on a microfluidic electrophoresis chip analyzer,sending the sample into a separation channel under the action of an external electric field and electroosmotic flow, and separating HDL in the to-be-detected sample into a plurality of sub-componentsunder the action of a molecular sieve and charges. According to the structure, the judgment accuracy of preeclampsia is improved.
Owner:广东国盛医学科技有限公司
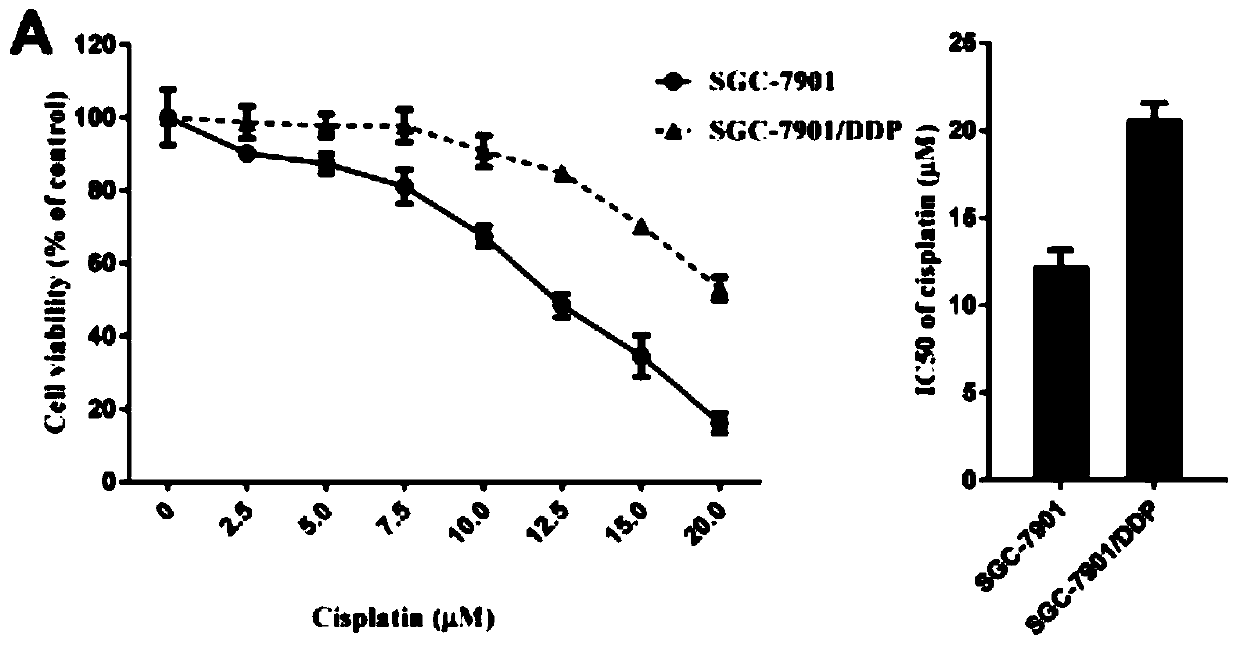
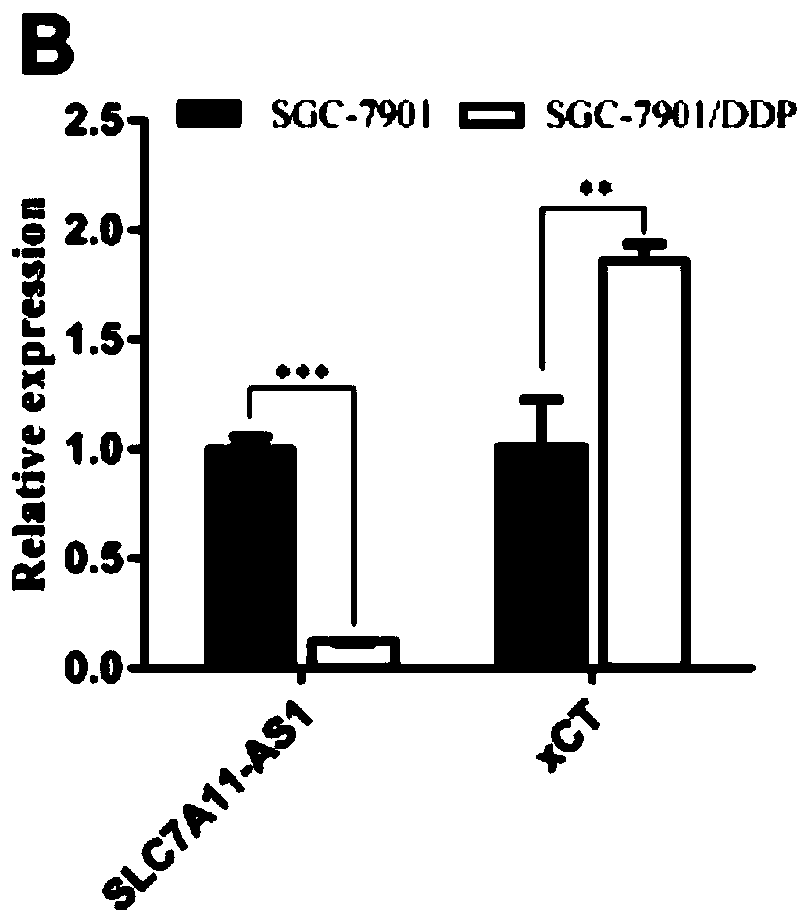
Application of lncRNA SLC7A11-AS1 as stomach cancer drug-resistance diagnosis marker
ActiveCN110607372AGood specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesBlood plasmaStomach cancer
The invention discloses application of lncRNA SLC7A11-AS1 as a stomach cancer drug-resistance diagnosis marker and relates to the technical field of medical biological detection. The invention provides application of the lncRNA SLC7A11-AS1 as the stomach cancer drug-resistance diagnosis marker. The invention also constructs a stomach cancer drug-resistance diagnosis marker or kit containing a pairof specific detection primers to detect tissue specimens or blood samples such as plasma, serum and blood platelets. The lncRNA SLC7A11-AS1 has good clinical application values and has better detection effects on stomach cancer drug-resistance diagnosis.
Owner:成都医学院第一附属医院
Blood filtering device
ActiveUS10561781B2Easy to handleReliable and effective processMembranesOther blood circulation devicesBlood flowFilter media
Owner:MANN HUMMEL GMBH
Assay for JC virus antibodies
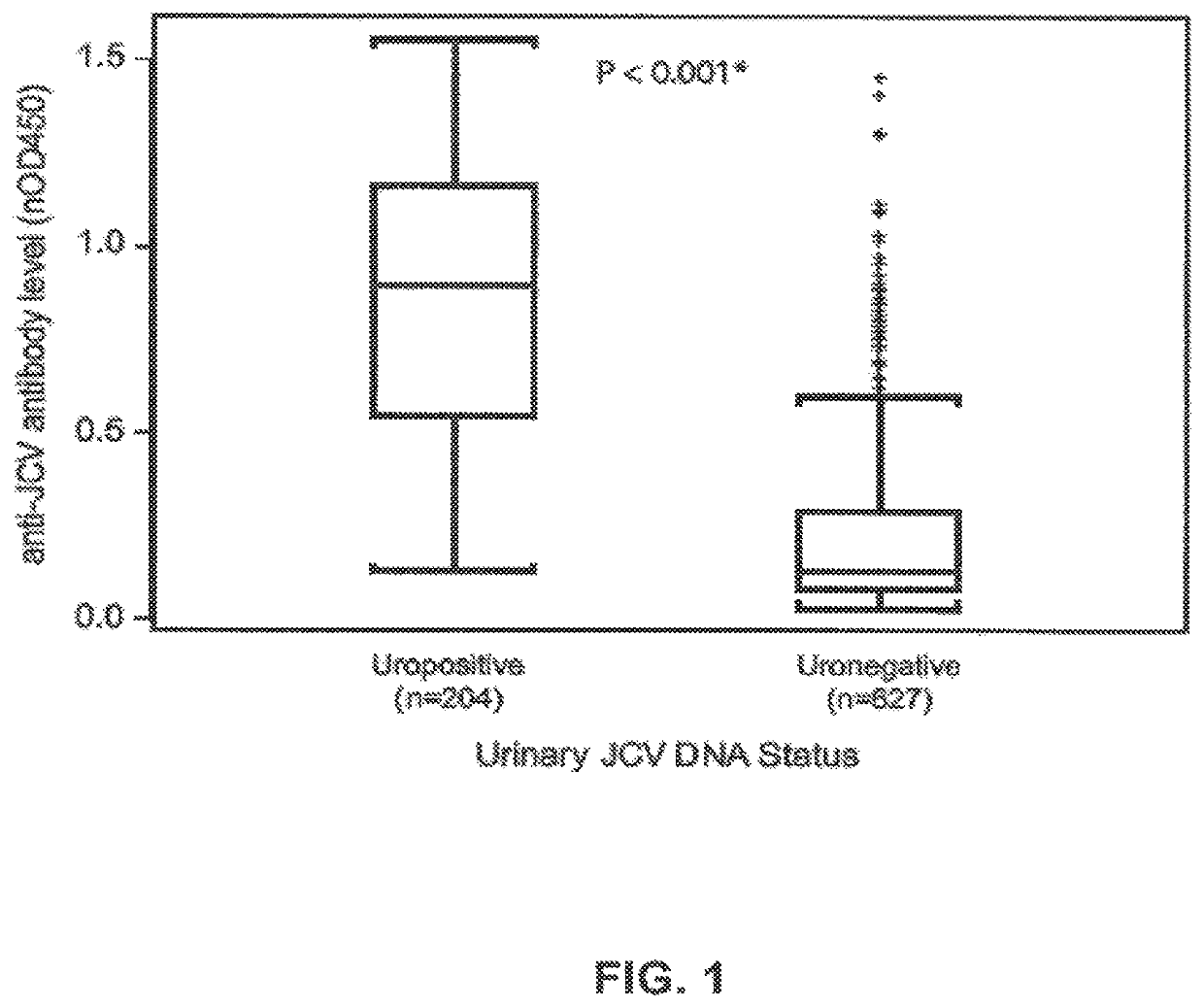
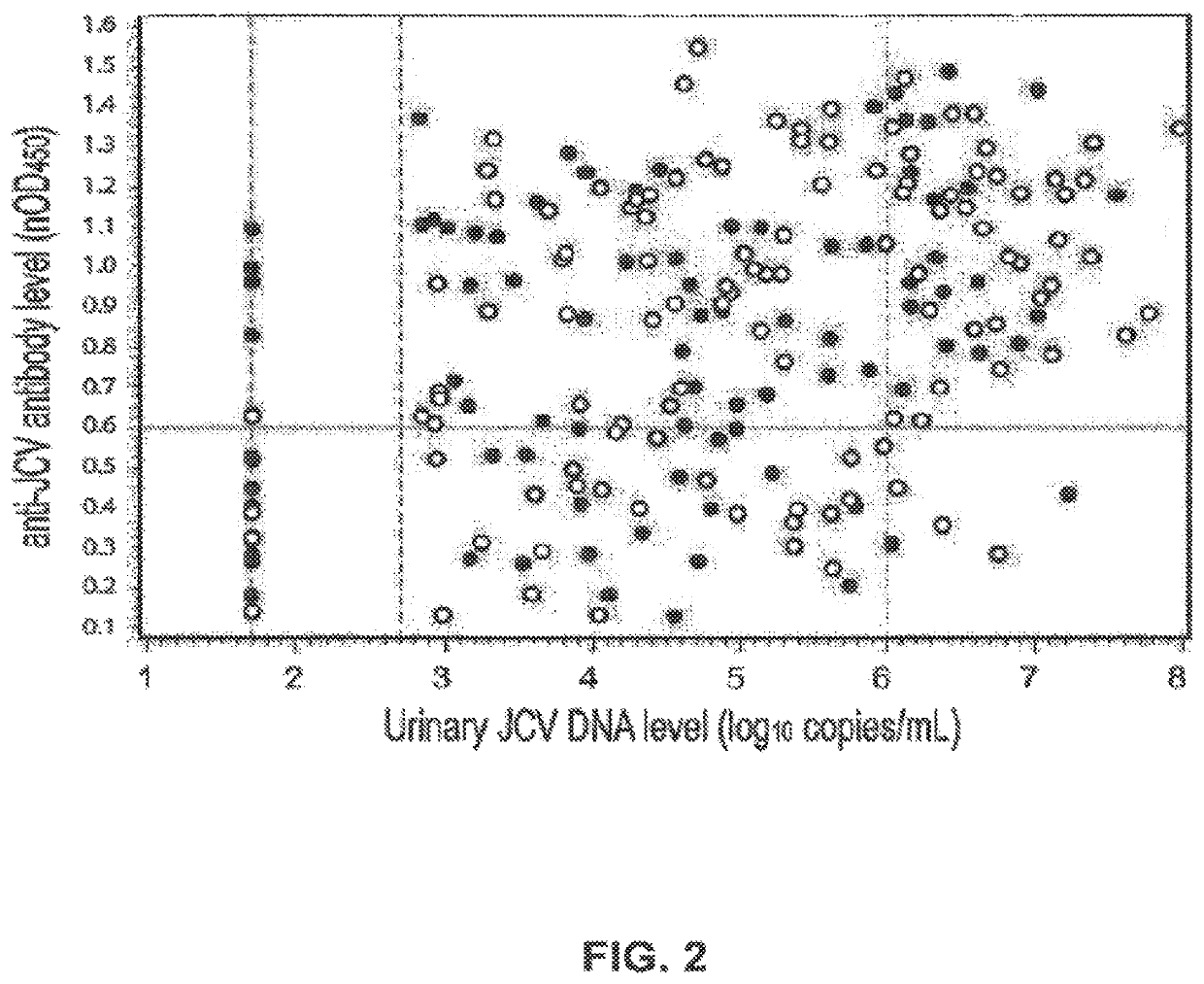
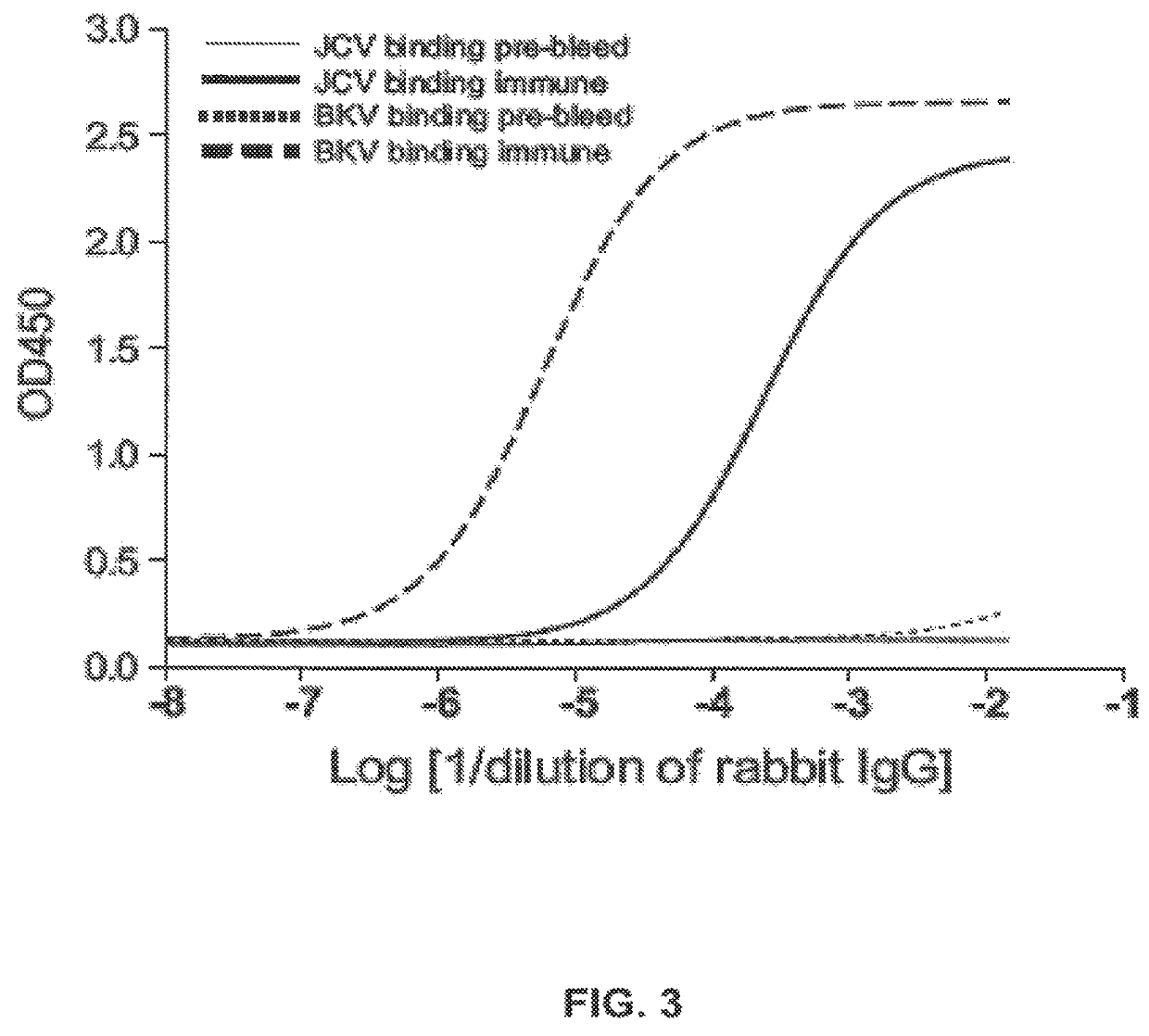
The disclosure relates to methods and reagents for analyzing samples for the presence of JC virus antibodies. Disclosed is a method that includes obtaining a biological sample from a subject (e.g., plasma, serum, blood, urine, or cerebrospinal fluid), contacting the sample with highly purified viral-like particles (HPVLPs) under conditions suitable for binding of a JCV antibody in the sample to an HPVLP, and detecting the level of JCV antibody binding in the sample to HPVLP. In one embodiment, determining the level of anti-JCV antibodies in the subject sample provides a method of identifying PML risk in a subject.
Owner:BIOGEN MA INC
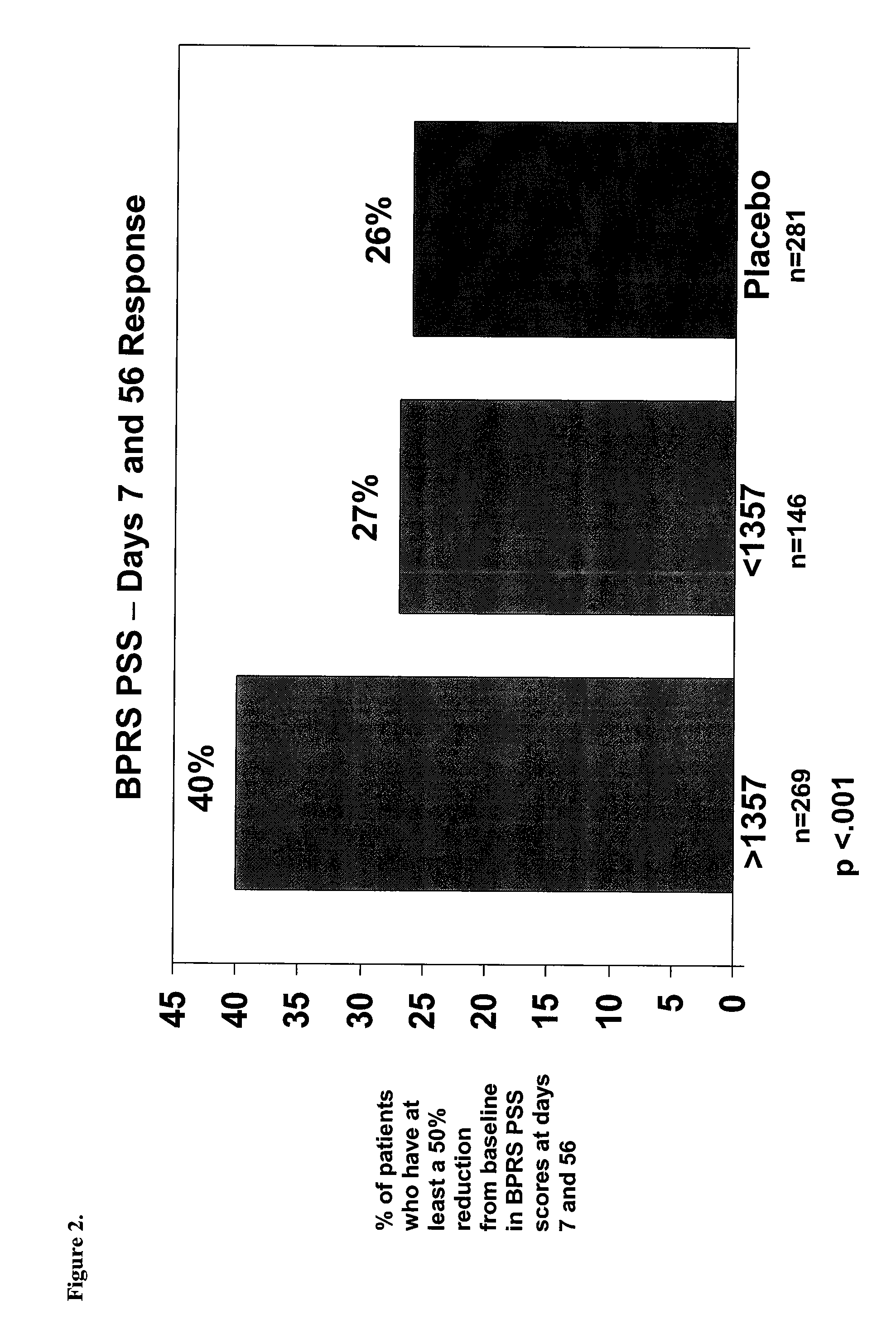
Optimizing mifepristone levels in plasma serum of patients suffering from mental disorders treatable with glucocorticoid receptor antagonists
ActiveUS20090062248A1Level of optimizationOrganic active ingredientsNervous disorderSerum igeBlood level
The present invention provides a method for optimizing levels of mifepristone in a patient suffering from a mental disorder amenable to treatment by mifepristone. The method comprises the steps of treating the patient with seven or more daily doses of mifepristone over a period of seven or more days; testing the serum levels of the patient to determine whether the blood levels of mifepristone are greater than 1300 ng / mL; and adjusting the daily dose of the patient to achieve mifepristone blood levels greater than 1300 ng / mL.
Owner:CORCEPT THERAPEUTICS INC
A kit for rapid detection of coronavirus based on s protein ligand and ace2 receptor competition chromatography
ActiveCN111273016BHigh detection sensitivityAvoid the development cycleCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsReceptorBlood plasma
Owner:浙江诺迦生物科技有限公司 +1
Application of a lncrna SGOL1-AS1 as a diagnostic marker for gastric cancer
ActiveCN108034722BHas clinical application valueMicrobiological testing/measurementDNA/RNA fragmentationBlood plasmaCancer research
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com