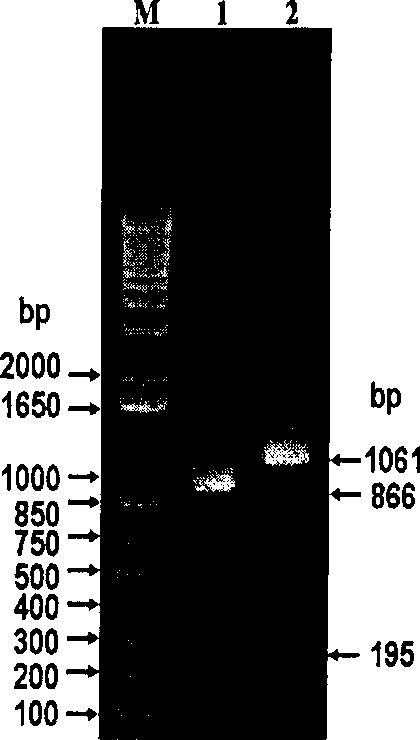
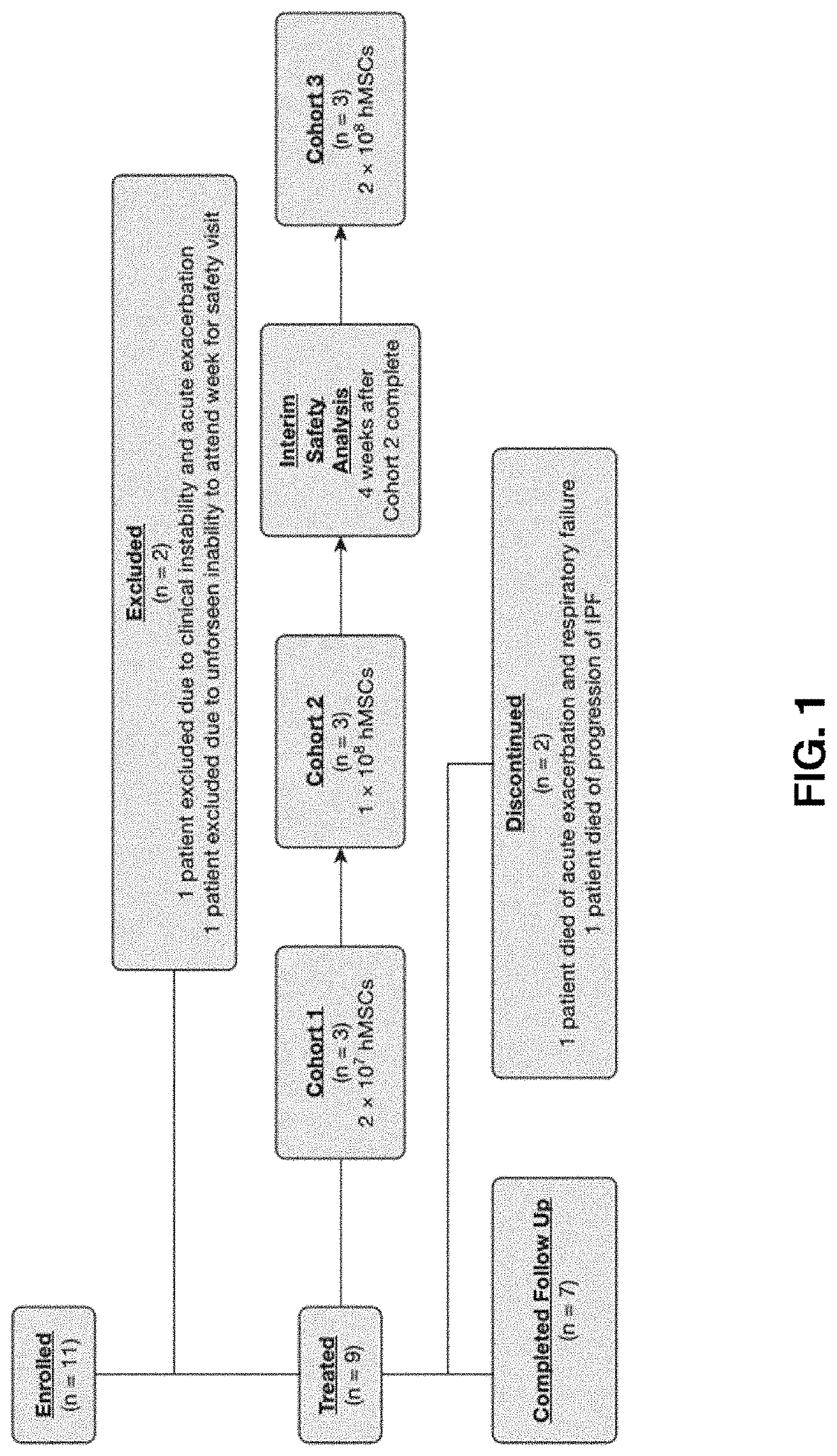
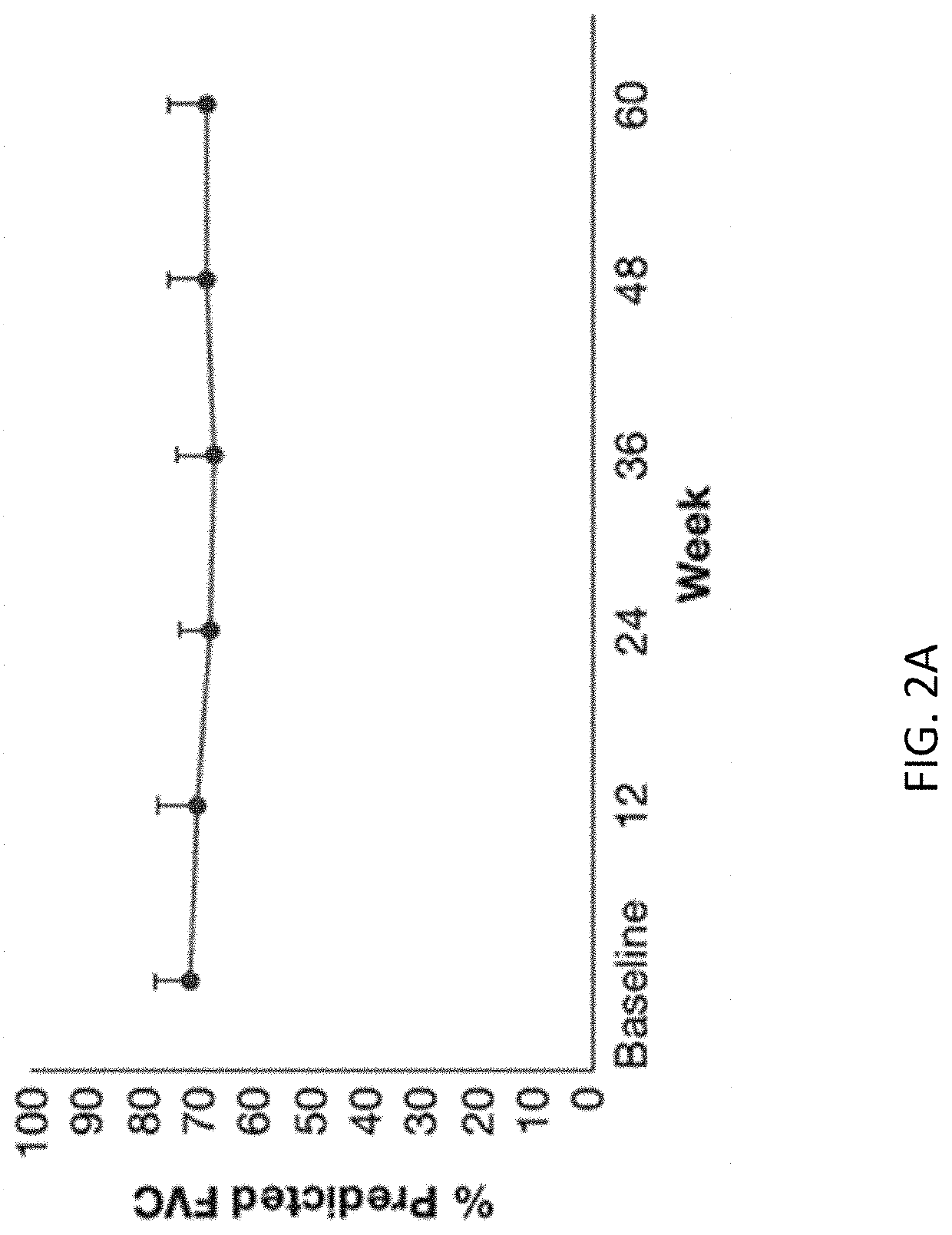
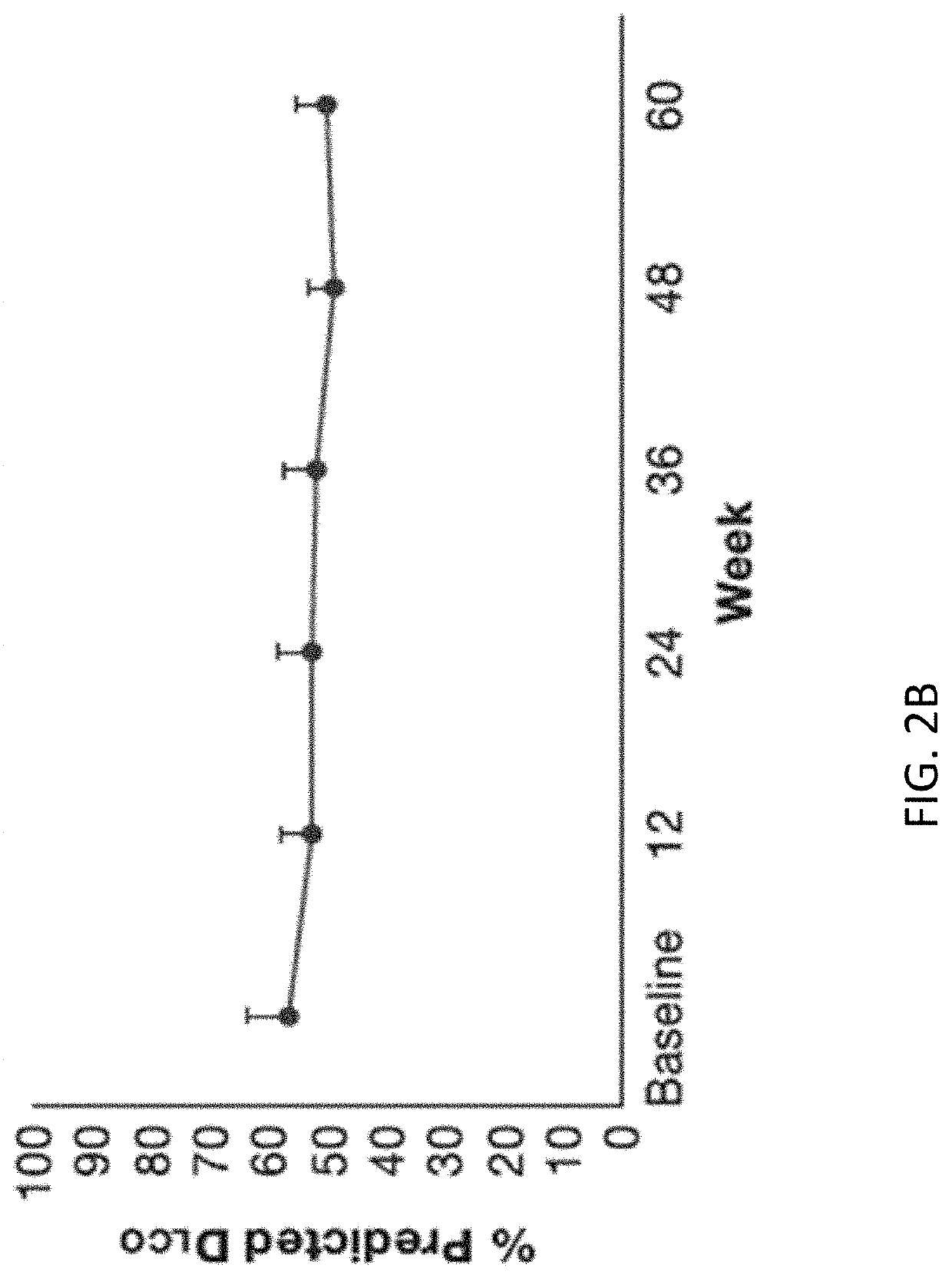
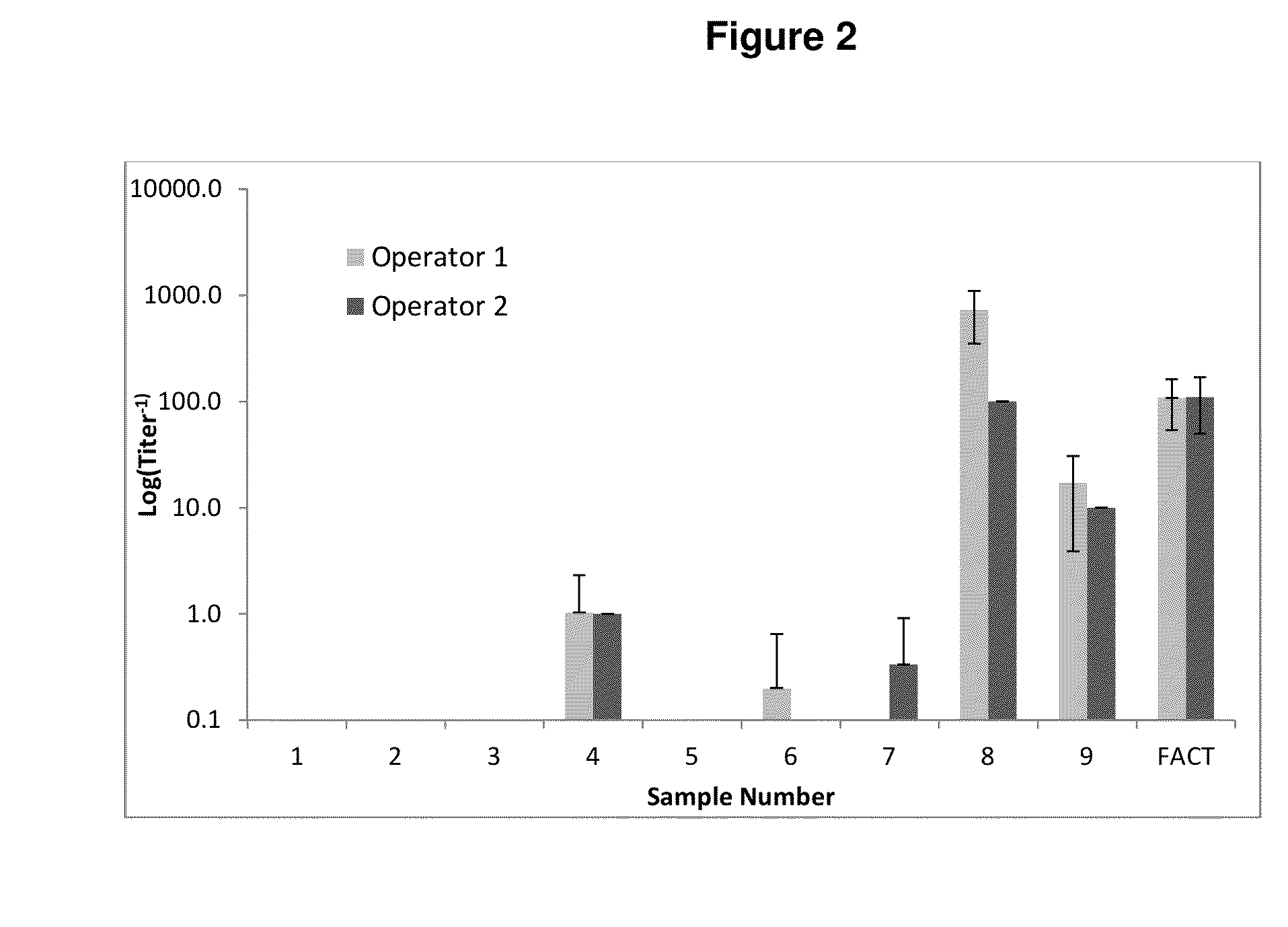
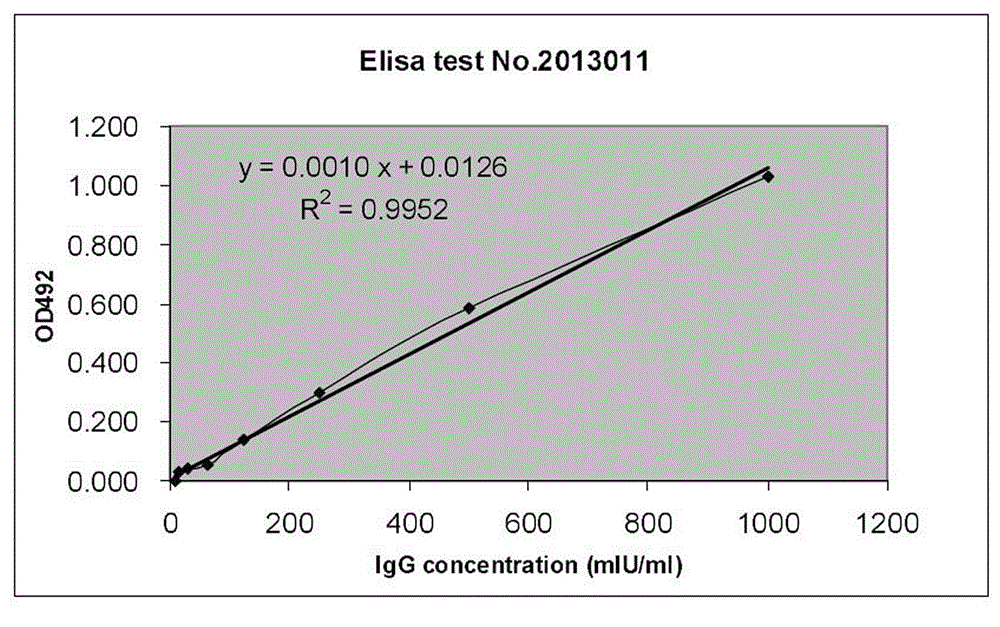
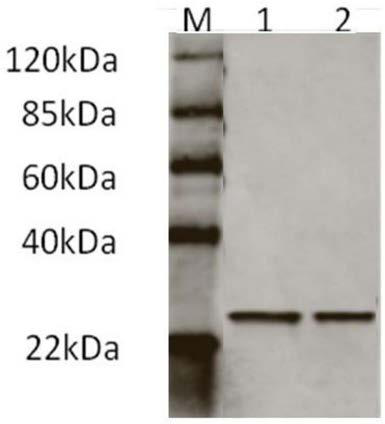
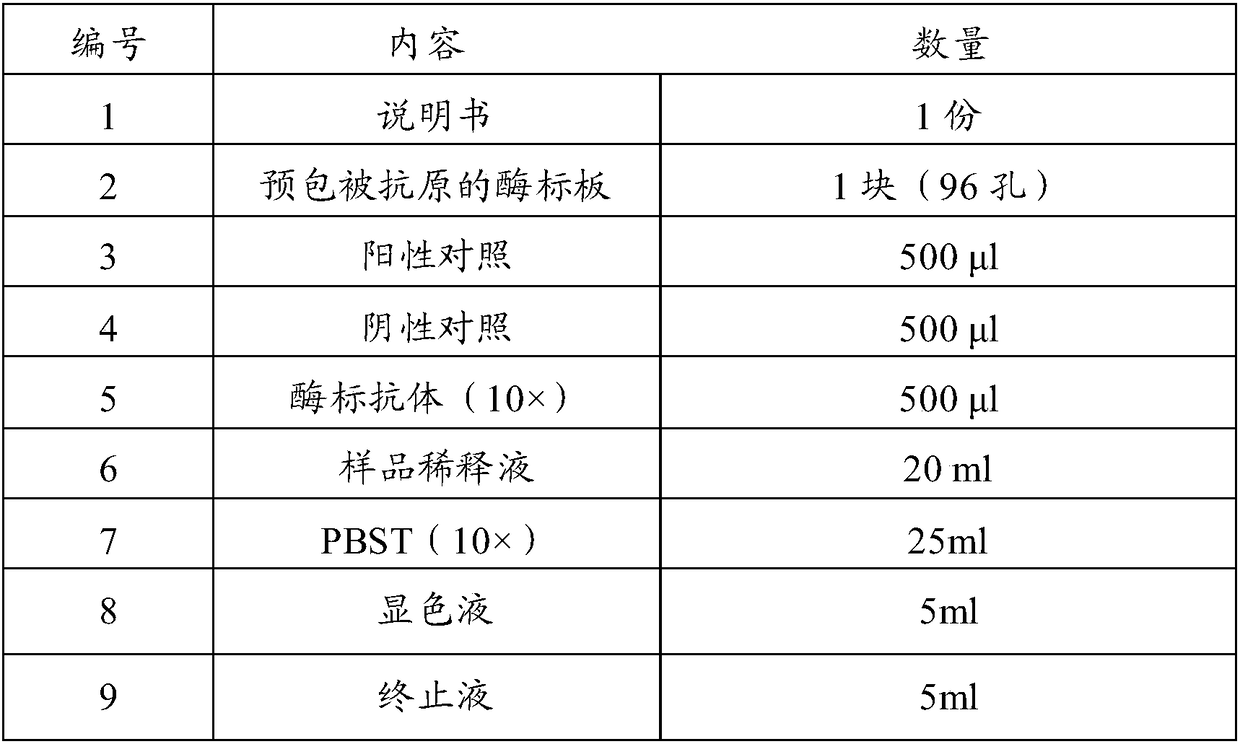
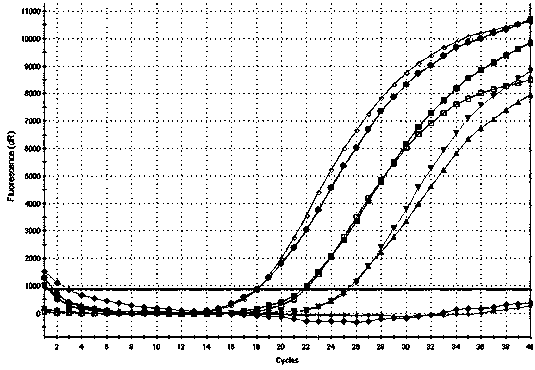
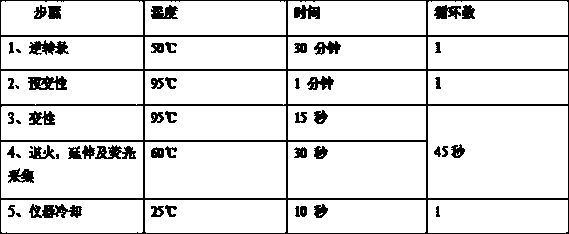
Patents
Literature
263 results about "Viral antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
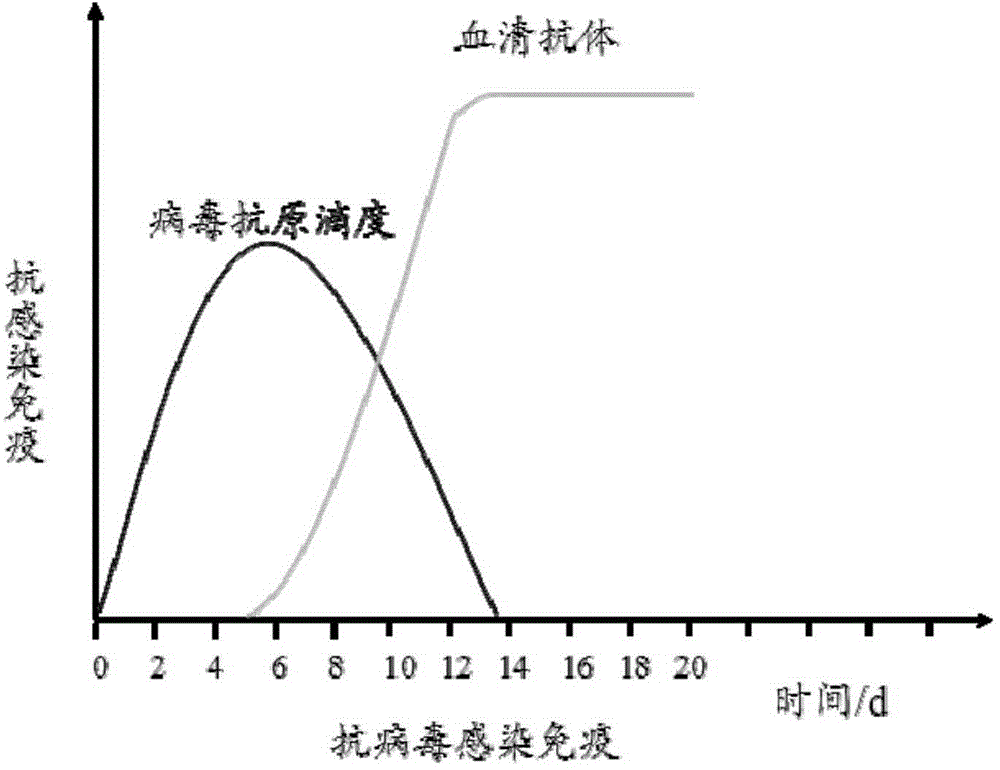
Virus Antibodies. Viruses are small infectious agents that exist in the gray area between "living" and "nonliving" entities. In contrast to most bacteria, fungi, or parasites, viruses are completely dependent on the host cell for their replication, hijacking the cell's biochemical machinery through the actions of viral genome-encoded factors.
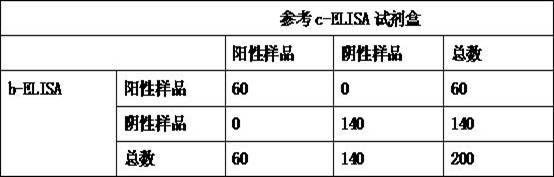
Kit for detecting antibody against Peste des petits ruminants virus b-ELISA and preparation method thereof
InactiveCN102419369AReduce economic costsLow costMaterial analysisViral antibodyEpidemiologic survey
The invention relates to the technical field of biology, particularly the field of viral antibody detection. A kit for detecting the antibody against Peste des petits ruminants virus b-ELISA comprises the following ingredients which are arranged respectively: Peste des petits ruminants nucleoprotein antigen, Peste des petits ruminants monoclonal antibody, diluent, strong positive serum, weak positive serum, negative serum, HRP sheep anti-mouse secondary antibody, 20 times the concentration of washing liquid, substrate liquid, stopping solution and enzyme-linked immunosorbent plate. The optimum proportion of each ingredient in the kit is determined by experiments. The kit can be used for rapid diagnosis and detection of animal Peste des petits ruminants virus antibody, especially for the antibody detection of a lot of samples in the epidemiological survey of Peste des petits ruminants. The detection method of Peste des petits ruminants virus b-ELISA has different detection principle and experiment operating procedures and the like from those of a c-ELISA detection method in a BIRAD laboratory. The Peste des petits ruminants nucleoprotein antigen and Peste des petits ruminants monoclonal antibody in the kit are self-developed. The detection sensitivity, singularity and other indexes of the kit are the same with those of the c-ELISA detection method in the internationally recognized BIRAD laboratory.
Owner:CHECKOUT & QUARANTINE TECH CENT YUNNAN ENTRY &EXIT CHECKOUT & QUARANTINE BUR +1
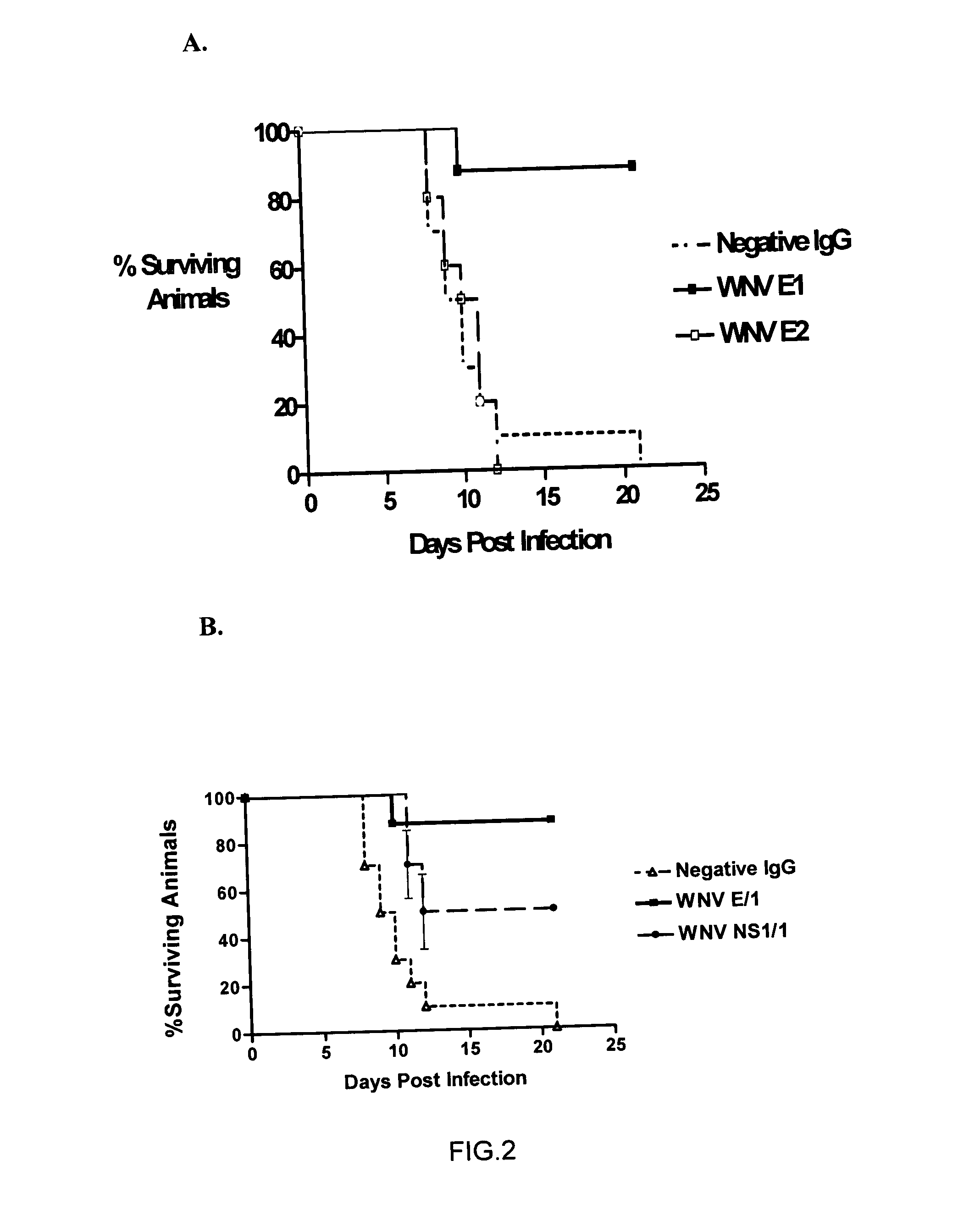
Antibodies against West Nile Virus and therapeutic and prophylactic uses thereof
ActiveUS20060067940A1Reduce morbidityLimit mortalityImmunoglobulins against virusesAntiviralsAntigenAnti-West Nile virus IgG
The present invention relates to compositions comprising antibodies or fragments thereof that immunospecifically bind to one or more antigens of a flavivirus, particularly of West Nile Virus (WNV), and methods for preventing, treating or ameliorating symptoms associated with a flavivirus, particularly of West Nile Virus (WNV), infection utilizing said compositions. In particular, the present invention relates to methods for preventing, treating or ameliorating symptoms associated with WNV infection, said methods comprising administering to a human subject an effective amount of one or more antibodies or fragments thereof that immunospecifically bind to a WNV antigen. The present invention also relates to detectable or diagnostic compositions comprising antibodies or fragments thereof that immunospecifically bind to a WNV antigen and methods for detecting or diagnosing WNV infection utilizing said compositions.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Methods for detection or measurement of viruses
InactiveUS20110262892A1Improve automationMicrobiological testing/measurementBiological material analysisViral antibodyActive agent
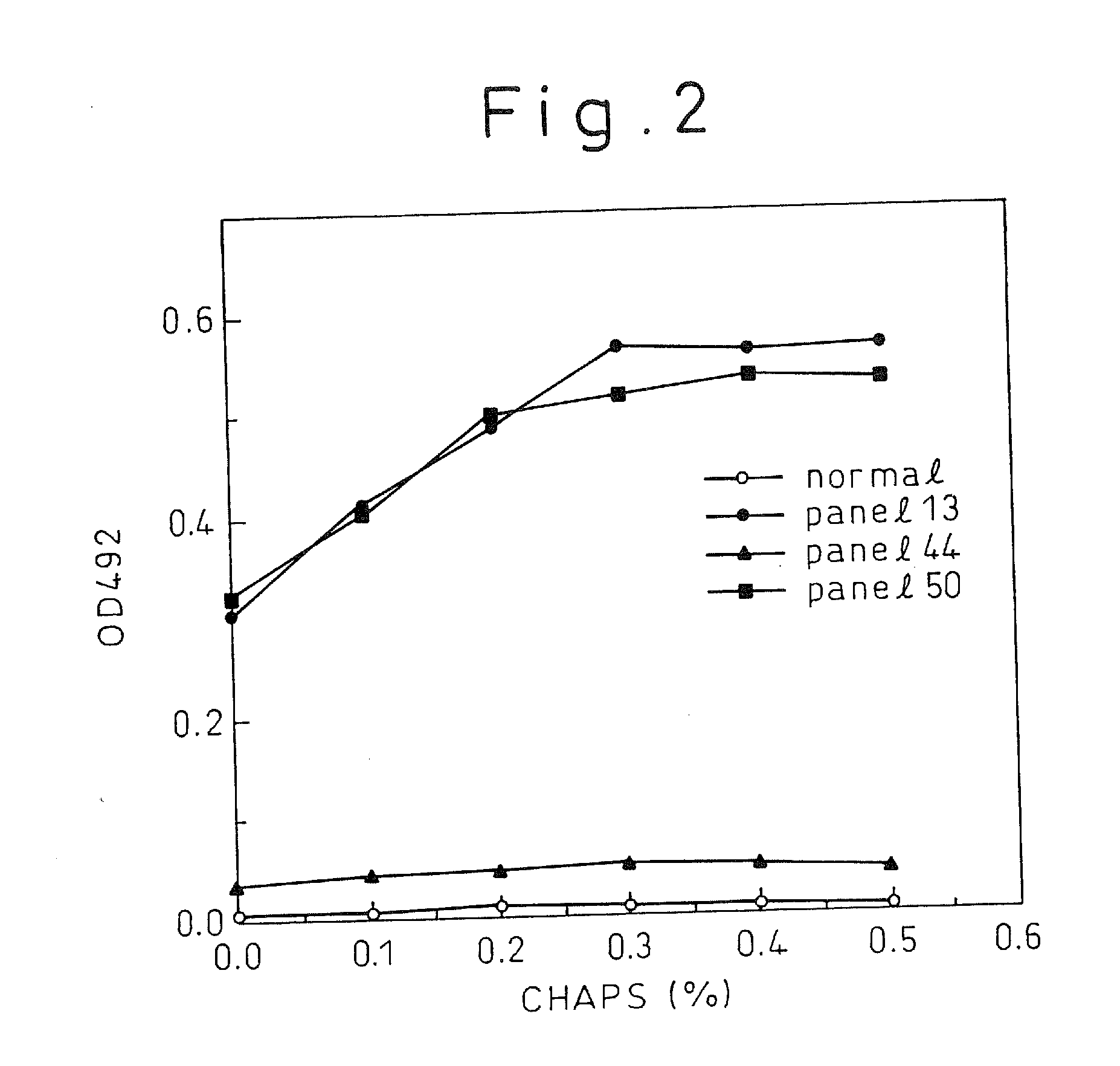
A method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) an anionic surfactant and (2) an amphoteric surfactant, nonionic surfactant or protein denaturant; a virus assay method using said treating method; a method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) a chaotropic ion and (2) an acidifying agent; a virus assay method using said treating method; a virus assay method, characterized in that a virus antigen and a virus antibody are measured based on their binding to their probe in the presence of a surfactant with an alkyl group of 10 or more carbon atoms and a secondary, tertiary or quaternary amine, or a nonionic surfactant, or of both of them; and a monoclonal antibody and a hybridoma producing the same for carrying out said method.
Owner:AOYAGI KATSUMI +4
Hybridoma cell line of monoclonal antibody against African swine fever virus and secreted monoclonal antibody thereof
InactiveCN101831407AHigh utility valueMicroorganism based processesImmunoglobulins against virusesBALB/cPurification methods
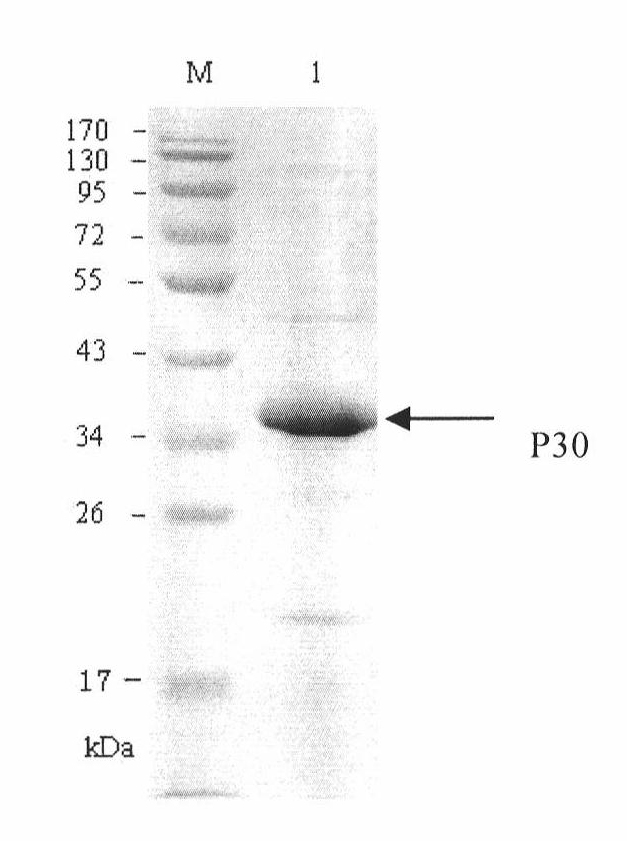
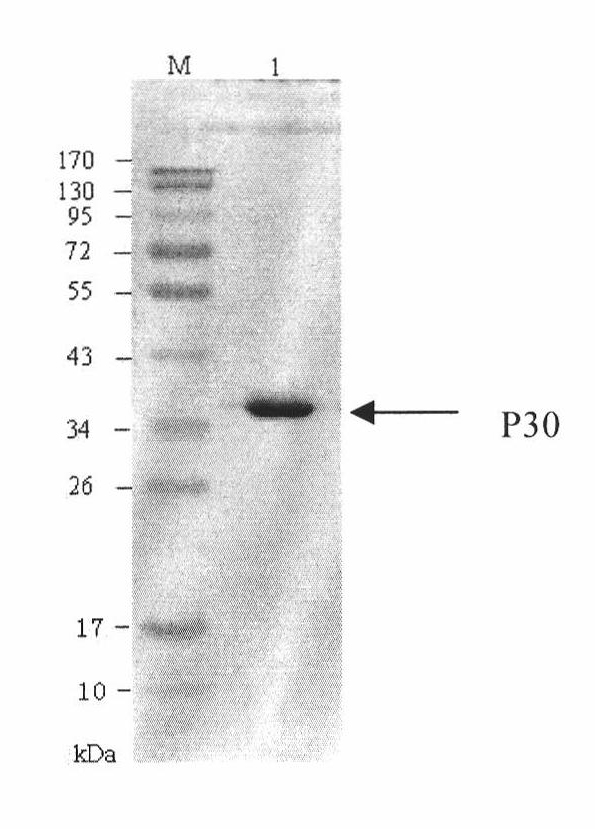
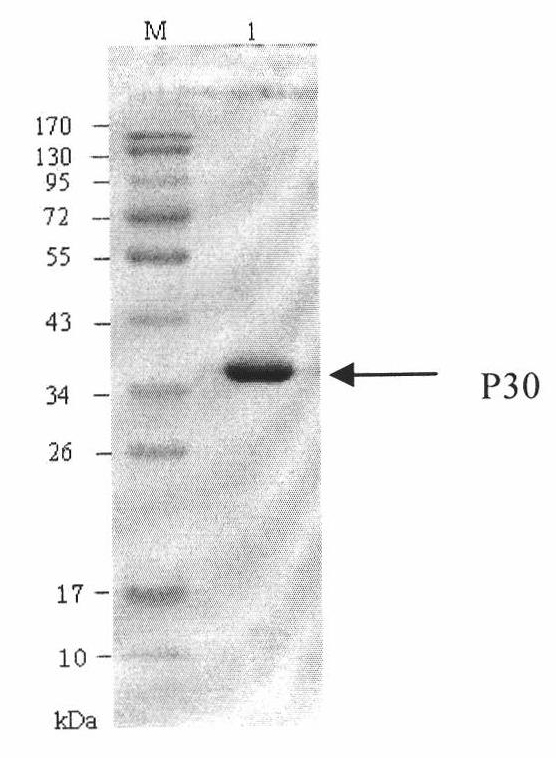
The invention discloses a hybridoma cell line of a monoclonal antibody against African swine fever virus and the secreted monoclonal antibody thereof. The preparation method of the invention comprises the following steps: preparing a recombined P30 soluble antigen by prokaryotic expression; immunizing a BALB / c mouse; and finally fusing, screening and cloning by a hybridoma technology to obtain the hybridoma cell line which can stably secrete the monoclonal antibody against African swine fever virus P30 protein. The invention further discloses a method for preparing the monoclonal antibody with the cell line, an antibody purification method and a labeling method for horseradish peroxidase of the antibody. The monoclonal antibody can be used in detecting the African swine fever viral antibody in pig serum.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit
InactiveCN102127531ASolve the difficulty of purificationImprove securityRecombinant DNA-technologyFermentationDuck hepatitis A virusViral antibody
The invention discloses a Korean novel duck hepatitis viral antibody ELISA (Enzyme-Linked Immunosorbent Assay) detection kit. The detection kit contains an ELISA board coated by Korean novel duck hepatitis VP1 (Phenotypic Variance1) recombination protein, a sample diluent, concentrated washing liquid, an enzyme conjugate working solution, a chromogenic reagent (A), a chromogenic reagent (B), a stopping solution, a positive contrast solution and a negative contrast solution. The VP1 recombination protein is obtained by using the following method: using Korean novel duck hepatitis viruses as a material, augmenting and cloning the VP1 gene through an RT-PCR (Reverse Transcriptase-Polymerase Chain Reaction) method to obtain recombinant expression plasmid pMD (physical medium dependent)-VP1; then, directionally inserting to an expression vector pET-32a (+) and screening to obtain recombinant expression plasmid pET-32a(+)-VP1; and inducing, expressing and purifying by ITPG (Isopropyl beta-D-Thiogalactopyranoside) to obtain VP1 recombination protein. The detection kit is used for detecting the Korean novel duck hepatitis and has strong specificity, high sensitivity, simplicity of operation, easiness of popularization and application in a large-area range and wide market prospects.
Owner:POULTRY INST SHANDONG ACADEMY OF AGRI SCI
Method for immobilizing viral glycoproteins for use in solid-phase immunoassays
InactiveUS6165710ABioreactor/fermenter combinationsBiological substance pretreatmentsAntigenViral antibody
A process for selectively immobilizing viral glycoproteins on lectin-coated surfaces for use in solid phase immunoassays is disclosed. This method does not require that the virus or antigen be purified prior to immobilization. This method provides an inexpensive and effective immunoassay method to screen fluids for the presence of viral antibodies.
Owner:ROBINSON JAMES E
Remedy for prion disease and method of producing the same
InactiveUS20070160585A1Good effectSymptoms improvedBiocideNervous disorderAntiendomysial antibodiesViral antibody
It is intended to provide a drug which is efficacious in treating a prion disease and has a high safety. A remedy for a prion disease which contains a mesenchymal stem cell as the active ingredient and a method of producing the same. A remedy for a prion disease which contains a mesenchymal stem cell, in particular, a mesenchymal stem cell having an anti-prion antibody gene transferred thereinto as the active ingredient and a method of producing the same. These remedies can not only prevent the progress of a prion disease but also contribute to the recovery of nerve dysfunction caused by the disease.
Owner:RENOMEDIX INST
Porcine circovirus I type infectious clone and virus rescued thereby and application thereof
InactiveCN101423836ASimple and fast operationInfectious cloneViruses/bacteriophagesFermentationViral antibodyPorcine circovirus type 1
The invention discloses infective clone for porcine circovirus type 1 and rescued virus and application thereof. Two genomes of the porcine circovirus type 1 are connected in cis and inserted into a vector to construct and obtain an infective molecular clone. A Sal I enzyme cutting site as a molecular target is inserted in the infective molecular clone. A rescued recombinant virus (PCV1 / G strains) carries a molecular marker, and the preservation number of the rescued recombinant virus is CGMCC NO.2658. An antigen of the recombinant virus is only reacted with a univalent specific antibody of PCV1 / Cap protein, but has no cross with an antibody against PCV2 / Cap protein. The recombinant virus and a parental virus are identified by combination of PCR and RFLP. The virus strain cultured in vitro has stable propagation property and high virus titer, and the rescued virus strain can be used for detecting the antibody of the virus. The invention lays a foundation for research such as genesis and evolution, genetic variation rule, molecular differential diagnosis, and the like for porcine circovirus in future.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Methods for attenuating viral infection and for treating lung injury
PendingUS20200384034A1Reduce developmentReduce expressionPowder deliverySpray deliveryViral antibodyAntiendomysial antibodies
The described invention provides compositions and methods for treating a susceptible subject at risk of pulmonary complications of an acute lung injury caused by a severe infection with a respiratory virus and for restoring lung function to donor lungs. The methods include administering a therapeutic amount of a pharmaceutical composition comprising extracellular vesicles (EVs) comprising one or more miRNAs and a pharmaceutically acceptable carrier. The population of EVs can be derived from a patient who has recovered from an infection with the respiratory virus or has been exposed to anti-viral antibodies through treatment, can be derived from MSCs of a normal healthy individual, can be modified by a viral vector, or can be synthetic.
Owner:SPIRITUS THERAPEUTICS INC
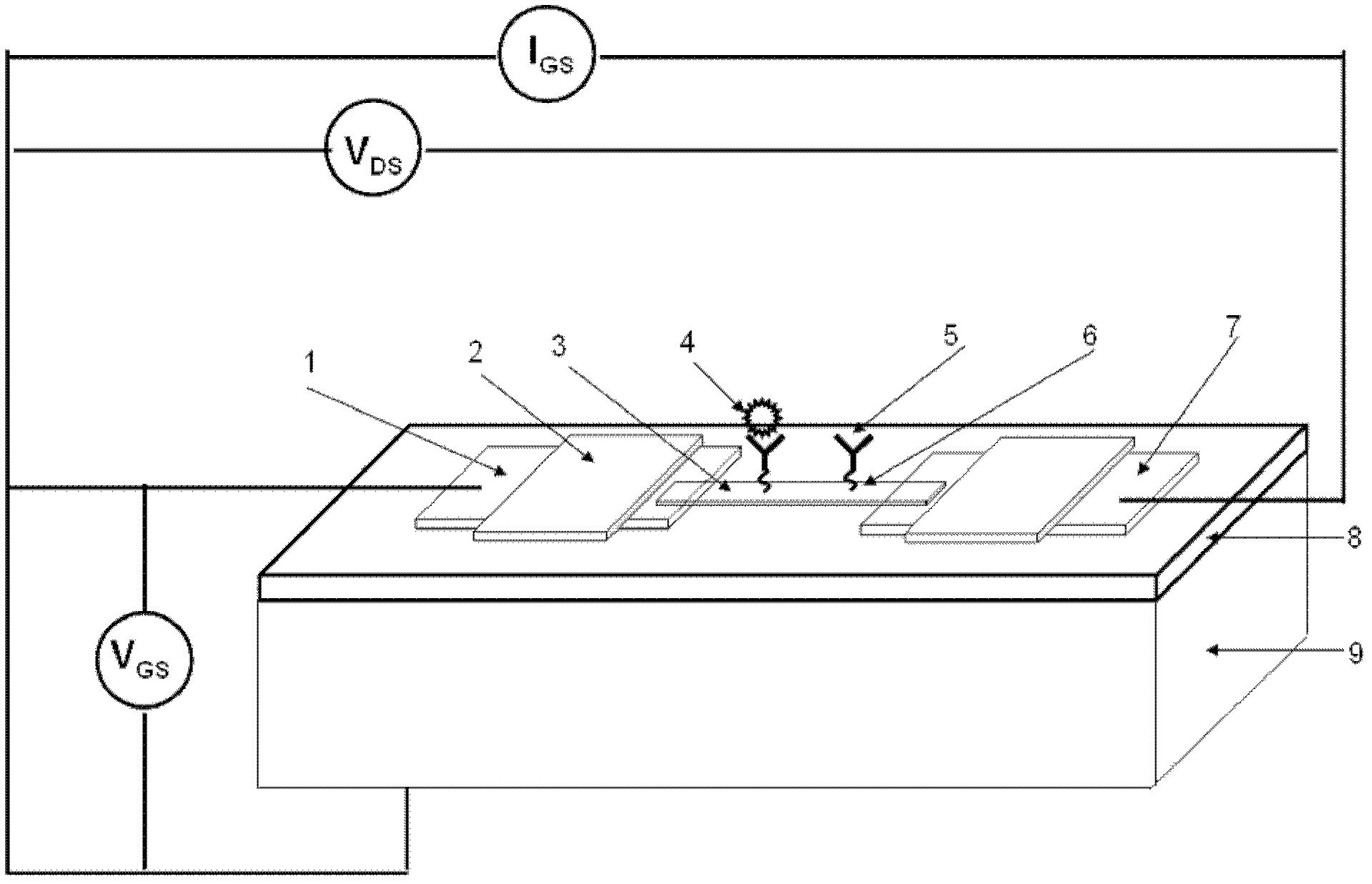
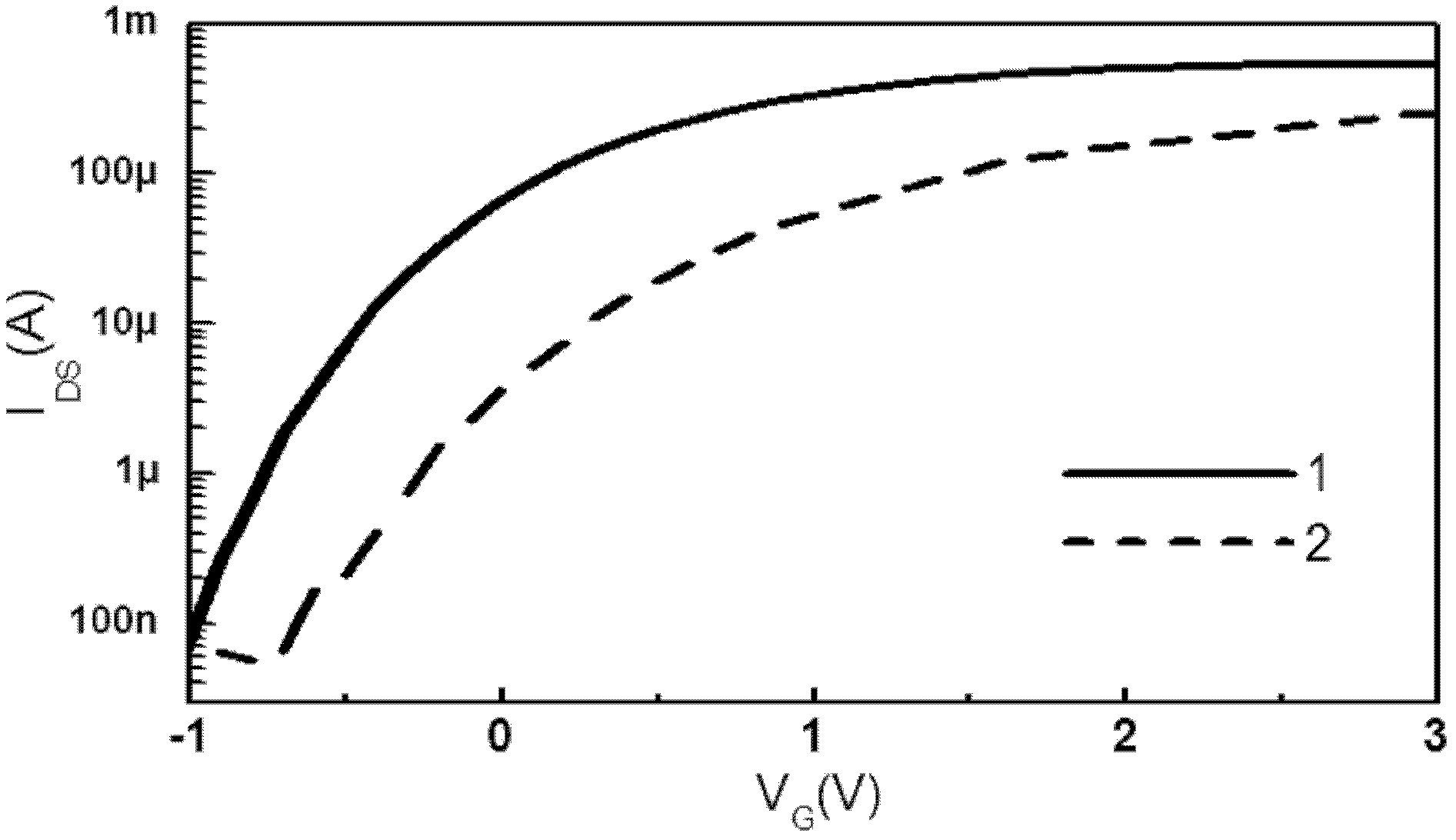
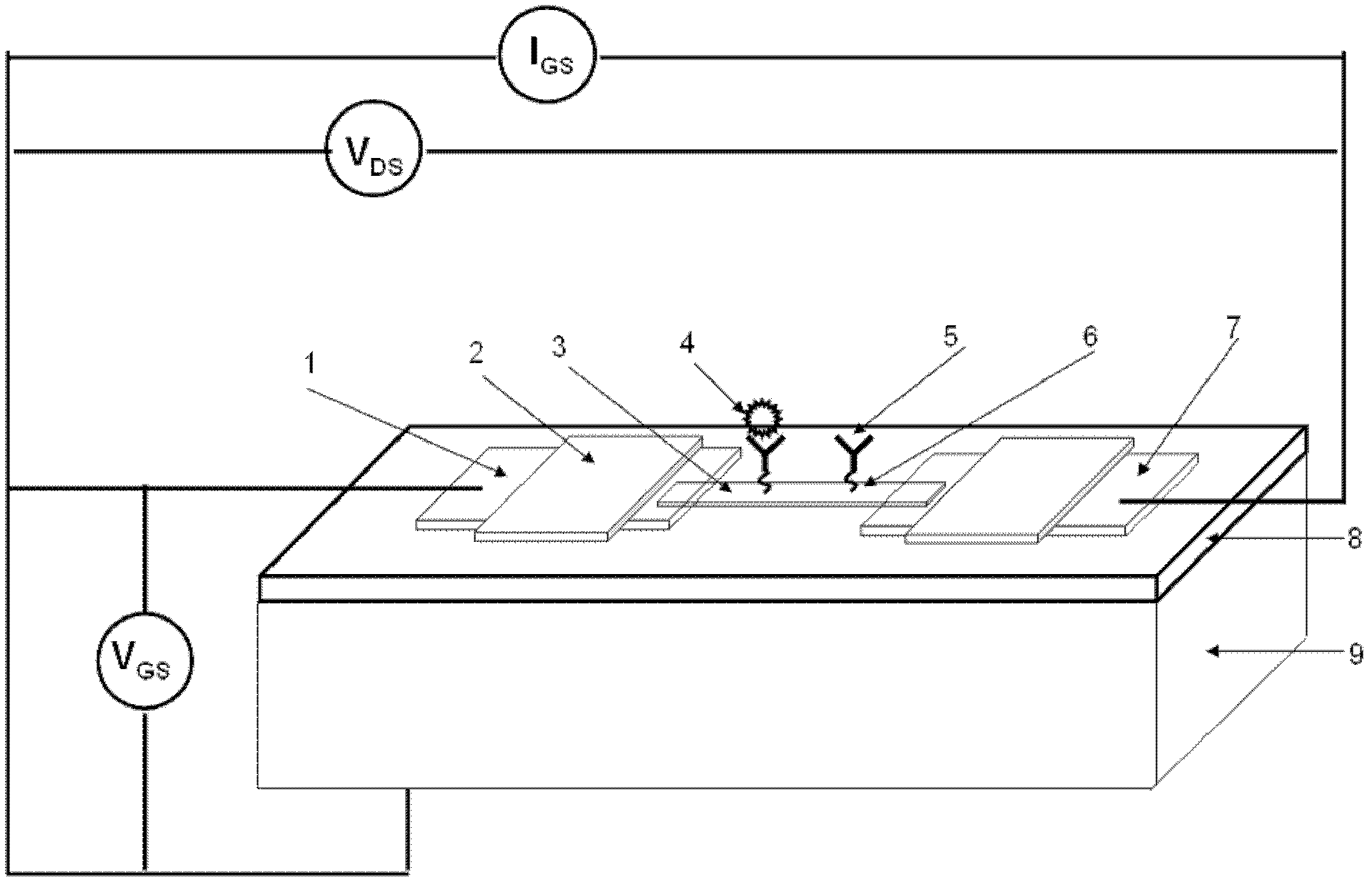
Viral disease diagnosis device and method based on field effect transistor
InactiveCN102435654AMiniaturizationEasy to integrateMaterial analysis by electric/magnetic meansViral antibodySilanes
The invention discloses a quick diagnosis device for a viral communicable disease based on a field effect transistor, which is composed of three parts, i.e., a field effect transistor biological chip, a microfluid channel sample introduction encapsulation system and an electronic detection system. A source electrode, a drain electrode and a semiconductor channel between the source electrode and the drain electrode are constructed on a silicon substrate by utilizing a semiconductor manufacturing process; and a covalent modification silane reagent on the channel is taken as a connection molecule for connecting viral antibodies; then a microfluid channel made from polydimethylsiloxane is used to be encapsulated in tight fit with the silicon substrate; finally the source electrode and the drain electrode are connected with a set of a signal detection system, so that a person or an animal can be diagnosed whether to be infected with the viral disease quickly, accurately and sensitively. The quick diagnosis device is a portable epidemic communicable disease detection instrument.
Owner:HUNAN UNIV
SARS virus antibody detecting method, rapid diagnosis kit and preparation method
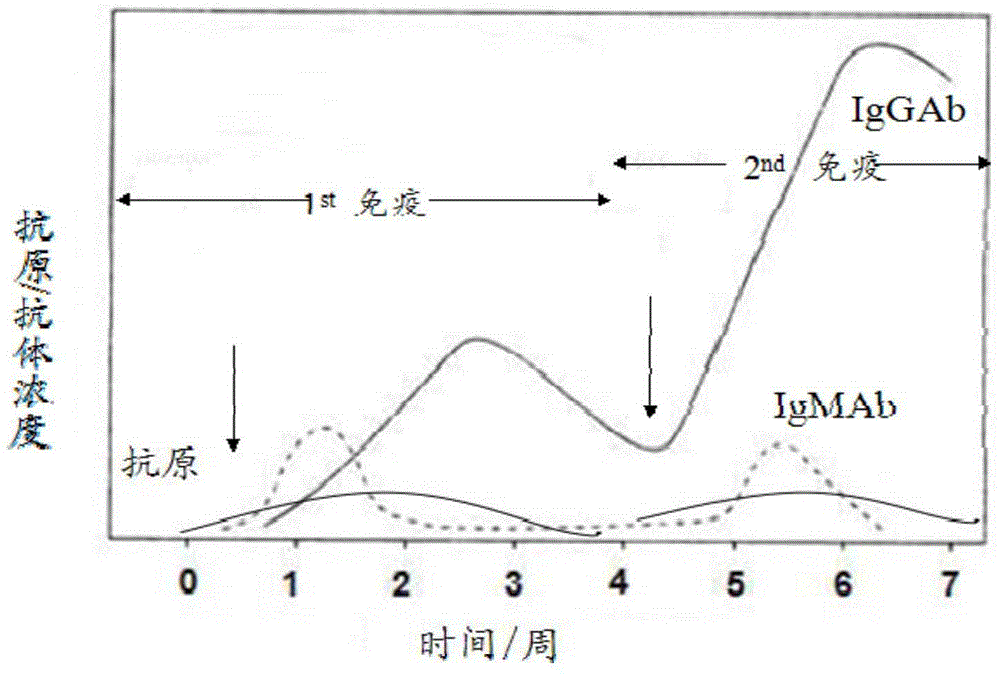
InactiveCN1570638AImprove featuresIncreased sensitivityMicrobiological testing/measurementBiological testingSerum igeSARS coronavirus
This invention relates to a detection mode of SARS virus antibody and a preparing method of the box for rapid diagnosis reagent. The checking method includes that (1) putting antihuman Ig antibody marked by colloidal gold in the carrier, and coating detecting thread made of variant antigen of SARS coronavirus and controlling thread made of antipest IgG antibody in the detecting carrier connecting with the marked one. (2) putting human serum in the marked carrier. The box for rapid diagnosis reagent includes checkerboard marked by variant antibody of SARS coronavirus, which comprises water-absorbing layer of adding-kind edge, detecting layer and water-absorbing layer of water-uptake edge. There is antihuman Ig antibody layer between detecting layer and water-absorbing layer of water-uptake edge, and in the detecting layer there is detecting thread made of variant antigen of SARS coronavirus and controlling thread made of antipest IgG antibody coating.
Owner:LANZHOU YAHUA BIOTECH
Biosensor, preparation method and virus detection system and method
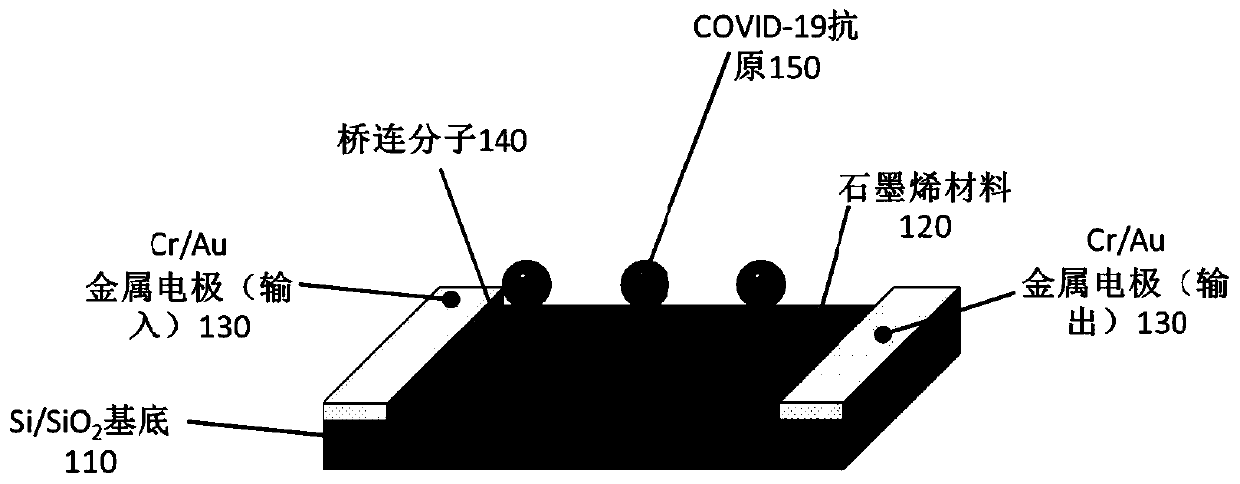
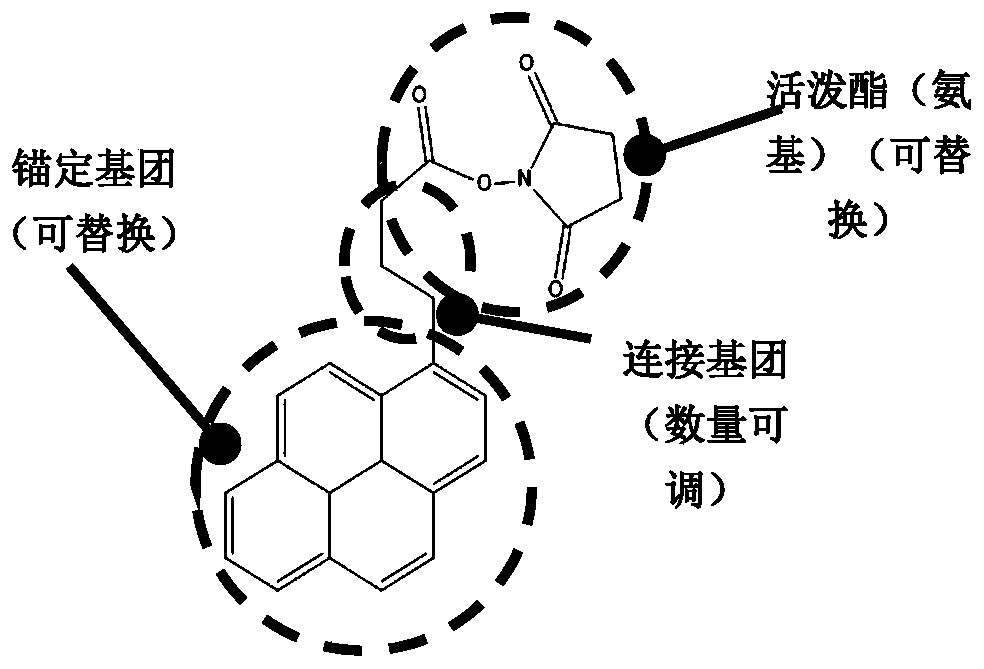
ActiveCN111474365ARealize detectionMicrobiological testing/measurementBiological testingViral antibodyNucleic Acid Probes
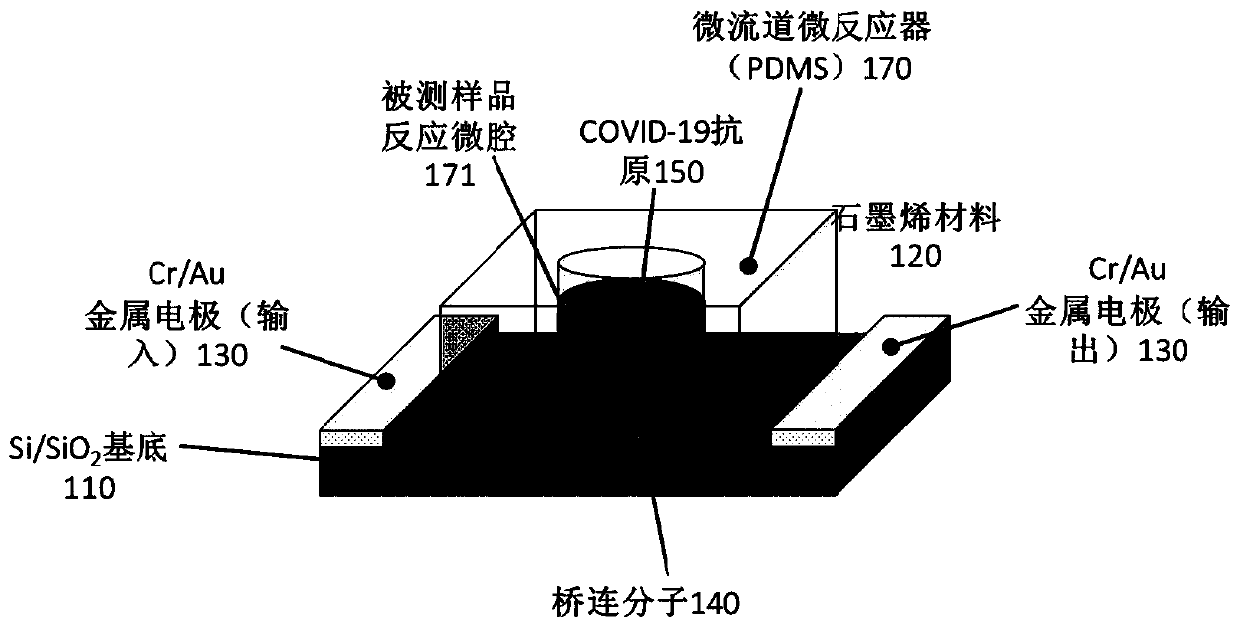
The embodiment of the invention provides a biosensor, a preparation method, a virus detection system and method. The method comprises the following steps: modifying a virus antigen or a virus antibodyor a nucleic acid probe for detecting viruses on a biosensor through bridging molecules capable of connecting various biomacromolecules, inputting a to-be-detected sample into the biosensor, and analyzing current signals before and after reaction, so as to detect whether viruses exist in the to-be-detected sample or not. The bridging molecule of the biosensor provided by the embodiment of the invention can be connected with various biomacromolecules, so that different viruses can be detected.
Owner:PEKING UNIV
Polypeptide chip and application thereof in virus detection
The invention provides a polypeptide chip, which comprises a substrate and n polypeptides distributed on the substrate in an array, each polypeptide sequence has 10-20 amino acids, a group consistingof sequences of first to nth polypeptides covers at least 95% of a viral protein sequence, the adjacent polypeptides have an overlap of 3-8 amino acids, and n is 810-1370. According to the method, proteomics and system biology strategies are adopted, all coded protein sequences of COVID-19 are extracted from NCBI data, an SARS-CoV-2 virus proteome polypeptide chip is designed and prepared, and panoramic scanning of all SARS-CoV-2 virus antibodies in blood of a novel pneumonia virus infected patient is achieved.
Owner:ACADEMY OF MILITARY MEDICAL SCI
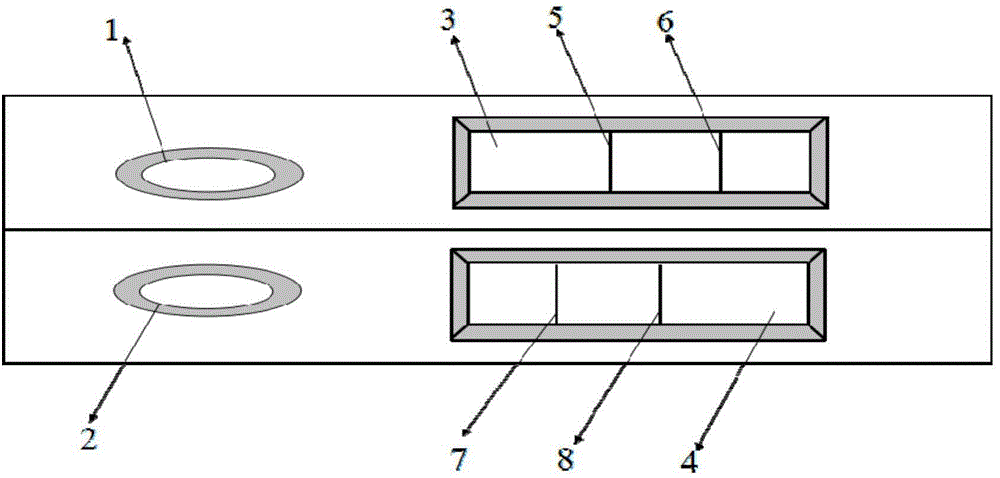
Colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies
The invention relates to a colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies. The test strip comprises a first glue strip and a second glue strip which are arranged in parallel, wherein the first glue strip comprises a first sample pad, a first conjugate pad, a first nitrocellulose membrane and first absorbent paper, which are sequentially lapped with one another, the first conjugate pad is coated with colloidal gold marked by the viral antigens, the first nitrocellulose membrane is coated with viral antibodies and antibodies, the viral antibodies are taken as a first detection line, and the antibodies are used for resisting the viral antigens on the colloidal gold and are taken as a quality control line; the second glue strip comprises a second sample pad, a second conjugate pad, a second nitrocellulose membrane and second absorbent paper, which are sequentially lapped with one another, the second conjugate pad is coated with the colloidal gold marked by the viral antigens, the second nitrocellulose membrane is coated with anti-human IgG antibodies and anti-human IgM antibodies, the anti-human IgG antibodies are taken as a second detection line, and the anti-human IgM antibodies are taken as a third detection line. The colloidal gold immunochromatography test strip can be used for simultaneously detecting the viral antigens as well as the IgG antibodies and IgM antibodies of the viral antigens.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
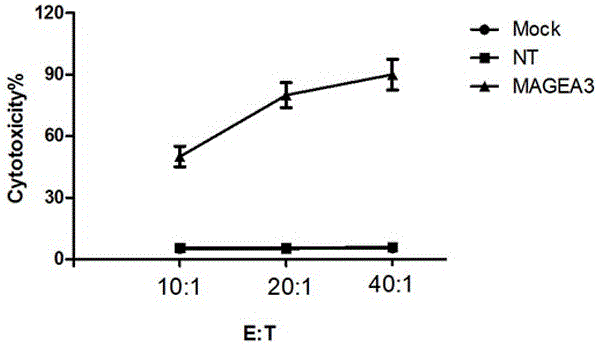
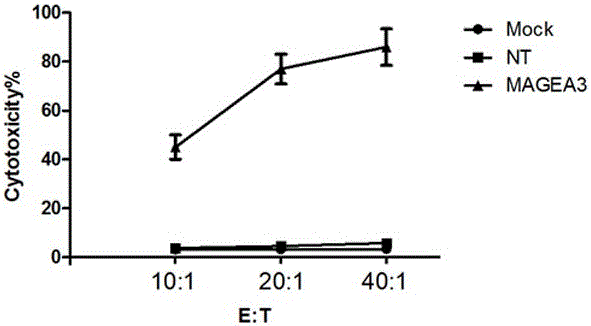
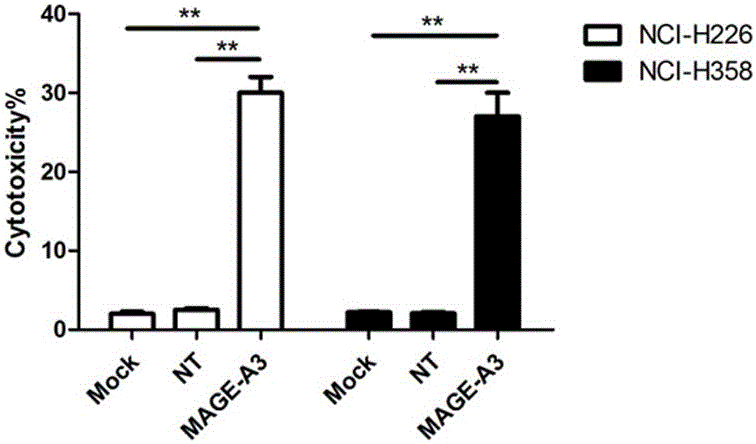
Novel viral vaccine for treating non-small cell lung cancer and preparation method thereof
InactiveCN105039269ALittle side effectsEnhanced ability to clear tumor cellsCarrier-bound antigen/hapten ingredientsViruses/bacteriophagesViral antibodyViral Vaccine
The invention provides a novel viral vaccine for treating a non-small cell lung cancer and a preparation method thereof. The preparation method of the viral vaccine comprises the first step of constructing a carrier of an MVA virus and the second step of obtaining specificity presenting MAGE-3 antigen DC cells. According to the viral vaccine prepared through the method, the biological stability is high, adverse effects on human bodies are not produced, and pathogenic dangers do not exist; a virus antibody can obtain a large number of viral particles with high purity easily; MAGE-A3 expressed by the viral vaccine has better immunogenicity, more epitopes are achieved, and drug resistance is not prone to being caused; the viral load needed in inoculation of the viral vaccine is smaller than other viruses.
Owner:BEIJING DCTY BIOTECH CO LTD
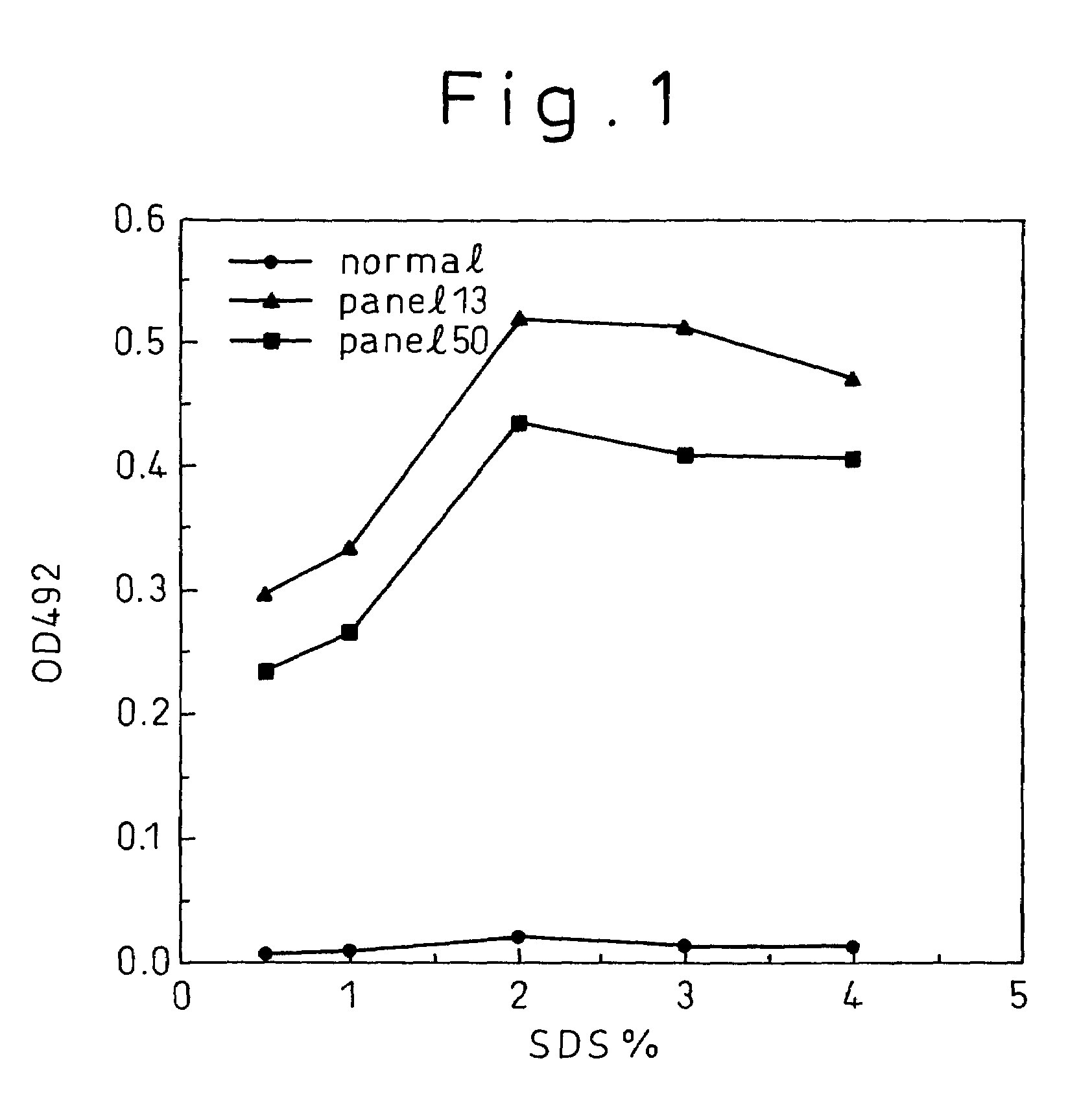
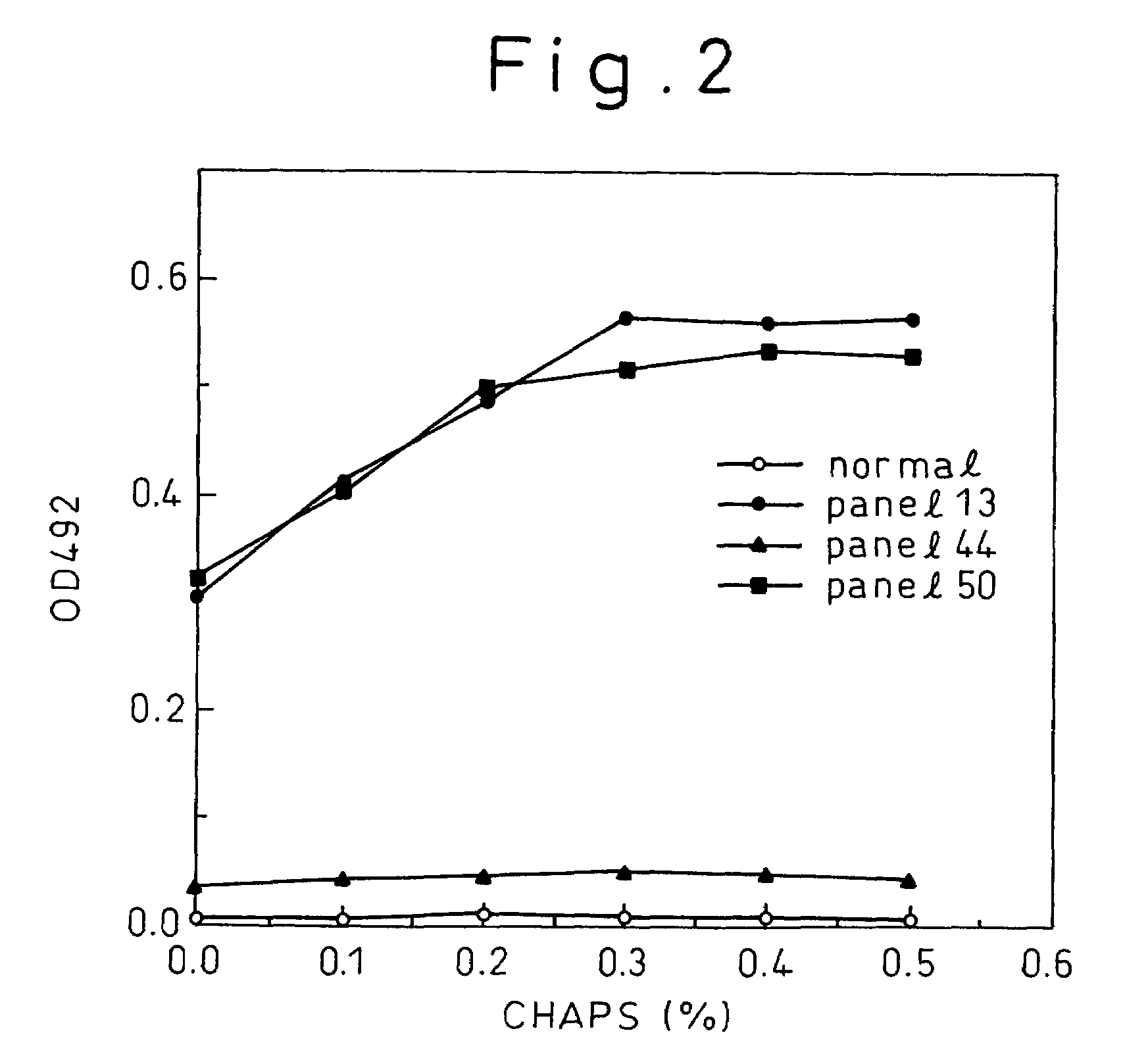
Methods for the detection of hepatitis B and C viruses
InactiveUS7776542B1Simple and sensitive detectionSimple and sensitive and quantitationMicrobiological testing/measurementBiological material analysisViral antibodyMonoclonal antibody
A method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) an anionic surfactant and (2) an amphoteric surfactant, nonionic surfactant or protein denaturant; a virus assay method using said treating method; a method for treating a virus-containing sample, characterized by treatment of a virus-containing sample with a treatment solution containing (1) a chaotropic ion and (2) an acidifying agent; a virus assay method using said treating method; a virus assay method, characterized in that a virus antigen and a virus antibody are measured based on their binding to their probe in the presence of a surfactant with an alkyl group of 10 or more carbon atoms and a secondary, tertiary or quaternary amine, or a nonionic surfactant, or of both of them; and a monoclonal antibody and a hybridoma producing the same for carrying out said method.
Owner:ADVANCED LIFE SCI INST
O-type foot-and-mouth disease virus antibody chemiluminescence detection kit
InactiveCN106226519AStrong specificityCarcinogenicity is smallChemiluminescene/bioluminescenceAntigenViral antibody
The invention relates to an O-type foot-and-mouth disease virus antibody chemiluminescence detection kit. The kit includes an polystyrene board coated by antigen, a monoclonal antibody, a standard substance, a luminescence substrate solution A and a luminescence substrate solution B. The polystyrene board coated by antigen is an opaque polystyrene plate coated by an O-type foot-and-mouth disease virus RE2 recombinant protein; the monoclonal antibody is a horseradish peroxidase labeled O-type foot-and-mouth virus monoclonal antibody; the luminescence substrate solution A comprises a Lumino, hydroxycoumarin, gallic acid, a Tris-Hcl buffer and water; the standard substance is foot-and-mouth virus O-type antibody respectively diluted buy a calibration diluent, wherein the dilution is 0NU / mL, 2NU / mL, 5NU / mL, 10NU / mL, 30NU / mL and 60NU / mL respectively. The kit of the invention can be used for detecting O-type foot-and-mouth disease virus antibody, has the advantages of high sensitivity and wide detection range, and realizes the quantitative detection of foot-and-mouth disease antibody.
Owner:洛阳现代生物技术研究院有限公司
SARS-CoV-2 antigen polypeptides and application thereof
ActiveCN111978378AHigh sensitivityReduce testing costsSsRNA viruses positive-senseVirus peptidesAntigen epitopeAntigen
The invention discloses SARS-CoV-2 antigen polypeptides and application thereof. The invention relates to four SARS-CoV-2 polypeptide antigens and application thereof. The amino acid sequences of thefour SARS-CoV-2 polypeptide antigens are SEQ ID NO. 1, SEQ ID NO. 2, SEQ ID NO. 3, SEQ ID NO. 4 or derivative sequences thereof. The epitopes screened out in the invention can be used for detecting anSARS-CoV-2 virus antibody, are high in specificity and accuracy, have an application value in clinical diagnosis, and play a positive and effective role in preventing and controlling SARS-CoV-2 virusinfection.
Owner:WUHAN UNIV
Recombinant protein and test strip for detection of antibodies of 2019 novel coronavirus through double-antigen sandwich method and preparation method and application of recombinant protein and test strip
ActiveCN111303297AQuick checkAccurate detectionSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeViral antibody
The invention relates to a recombinant protein and test strip for detection of antibodies of 2019 novel coronavirus through a double-antigen sandwich method and a preparation method and application ofthe recombinant protein and test strip, and belongs to the technical field of virus detection. The amino acid sequence of the recombinant protein for detection of the antibodies of the 2019 novel coronavirus through the double-antigen sandwich method is shown in SEQ ID NO. 1. The recombinant protein is a fusion protein of multiple dominant epitopes of the 2019-nCoV, a reagent for detection of theantibodies of the 2019-nCoV through the double-antigen sandwich method can be prepared, storage at room temperature, fast and single detection with high sensitivity, high throughput and low instrument cost can be achieved at any time, the operation is simple, and convenience of clinical use can be improved greatly.
Owner:GENERAL HOSPITAL OF PLA +1
Dot immunogold filter kit for detecting IBR (infectious bovine rhinotracheitis) virus antibody and detection method thereof
The invention discloses a dot immunogold filter kit for detecting an IBR (infectious bovine rhinotracheitis) virus antibody and a detection method thereof. The kit comprises a) an infectious bovine rhinotracheitis virus antigen, b) a gold marked goat anti-bovine antibody, c) a cleaning solution, and d) a confining liquid. The method comprises the following steps: dotting the infectious bovine rhinotracheitis virus antigen on a nitrocellulose film; closing, and adding a serum sample to be detected; cleaning, and detecting the infectious bovine rhinotracheitis virus antibody by using the gold marked goat anti-bovine antibody as colloidal gold marked protein. Detection of the infectious bovine rhinotracheitis virus antibody by adopting the kit disclosed by the invention has the advantages of specificity, sensitivity, quickness, reliability, intuitive effect, easily determined result and the like, special equipment is not required, and the detection result can be preserved for inspection.
Owner:INSPECTION & QUARANTINE TECH CENT OF FUJIAN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Aav vector and assay for Anti-aav (adeno-associated virus) neutralizing antibodies
ActiveUS20160123990A1Microbiological testing/measurementGenetic material ingredientsAnti virusViral antibody
Virus vectors, virus particles, and methods and uses of screening for, detecting, analyzing and determining amounts of virus antibody, or neutralizing antibody activity of samples are provided. Such virus vectors, virus particles, and methods and uses are applicable to a broad range of virus types, such as lentiviruses, adenovirus, and adeno-associated virus (AAV) serotypes. Methods and uses include virus antibody screening, such as anti-virus immunoglobulins screened for, detected, analyzed and amounts determined
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Senecavirus ELISA antibody detection kit and preparation method and application
InactiveCN108761074AQuick checkIncreased sensitivityBiological material analysisEscherichia coliSerum ige
The invention discloses a Senecavirus ELISA antibody detection kit and a preparation method and application. The Senecavirus ELISA antibody detection kit comprises a Senecavirus VP1 protein-precoatedELISA plate, a blocking solution, a diluted sample solution, an enzyme conjugate, a concentrated washing solution, an enzyme substrate solution and a stopping solution. Senecavirus VP1 protein is usedas a coating antigen for the first time, a kit capable of detecting Senecavirus is established, and the antibody level of the Senecavirus in serum can be rapidly detected; the detection kit is high in sensitivity, good in specificity, good in repeatability and stable in result; the detection kit can be applied to monitoring of the antibody level of the Senecavirus so as to understand the status of the Senecavirus antibody in a whole pig herd; in addition, the detection kit uses an Escherichia coli expression system, and has the advantages of economy and cheapness.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
ELISA (enzyme linked immunosorbent assay) antibody detection kit for O type foot-and-mouth disease virus and detection method adopting ELISA antibody detection kit
The invention relates to an ELISA (enzyme linked immunosorbent assay) antibody detection kit for an O type foot-and-mouth disease virus and a detection method adopting the ELISA antibody detection kit. The kit comprises components as follows: (1) a recombinant RE2 protein antigen coated Elisa plate, (2) a horseradish peroxidase marked monoclonal antibody, (3) negative control, (4) positive control, (5) a washing solution, (6) a sample diluent, (7) a developing solution A and a developing solution B and (8) a stop solution. The detection kit combines a monoclonal antibody technology and a solid-phase ELISA detection technology, has the characteristics of rapidness, accuracy, stability and the like, and can be used for immune effect monitoring of a livestock farm as well as rapid and batch antibody detection of epidemic diseases of an inspection and quarantine department, an export port and the like.
Owner:LUOYANG LAIPSON INFORMATION TECH
Competitive Alpha LISA (linked immuno sorbent assay) detection kit for classical swine fever virus (CSFV) antibody and detection method thereof
InactiveCN103499693AStrong specificityHigh sensitivityBiological testingImmunoassaysAntigenSerum ige
The invention discloses a competitive Alpha LISA (linked immuno sorbent assay) detection kit for a classical swine fever virus (CSFV) antibody and a detection method thereof. The detection kit comprises donor microspheres, receptor microspheres, a swine fever virus E2 protein monoclonal antibody and an E2 protein antigen with a His label. A competitive Alpha LISA detection method for the CSFV antibody is created by optimizing test reaction conditions such as the donor microspheres, the receptor microspheres, the monoclonal antibody, the antigen and serum. The kit for detecting the CSFV antibody is good in specificity, high in sensitivity, low in usage amount of the serum, low in detection cost and short in detection time, does not need to be washed and can not be influenced by hemolysis.
Owner:INSPECTION & QUARANTINE TECH CENT OF CHONGQING ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Rabies virus antibody (IgG) enzyme-linked immunoassay kit and detection method thereof
The invention provides a rabies virus antibody (IgG) enzyme-linked immunoassay kit and a detection method thereof. The detection kit is composed of a purified rabies virus antigen coated microporous plate, enzyme labeled SPA and other reagents. The detection method adopts an indirect method principle to detect the rabies virus IgG antibody in human or animal serum or blood plasma, and is suitable for rabies vaccine immunized serology effect evaluation and epidemiology investigation. An enzyme labeled antibody applied in the invention is a Staphylococal protein A (SPA), and the SPA can be combined with an Fc fragment in IgG molecules in human or mammal serum, so the kit provided by the invention has all the characteristics of an ELISA kit, can be used for human rabies virus antibody detection, and can also be used for detecting the immune effect of various species of animals.
Owner:成大生物(本溪)有限公司
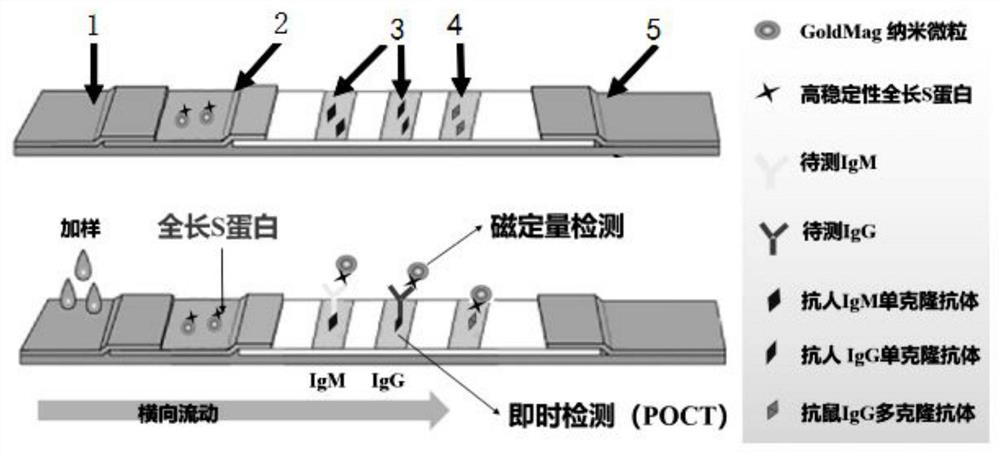
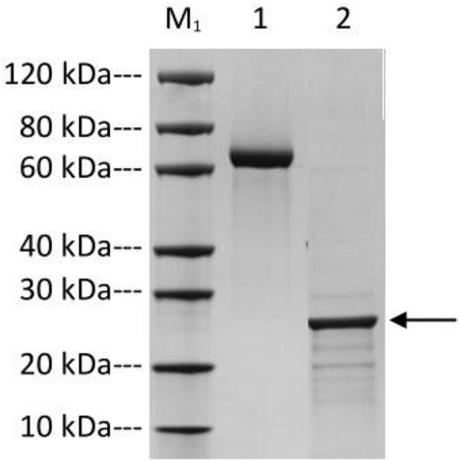
High-stability novel coronavirus spike protein, related biological material, application of related biological material, detection test paper and detection kit
ActiveCN112079906AEasy to controlImprove detection efficiencySsRNA viruses positive-senseVirus peptidesProtein trimerAntigen epitope
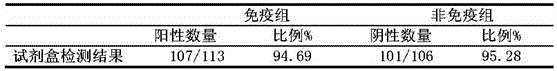
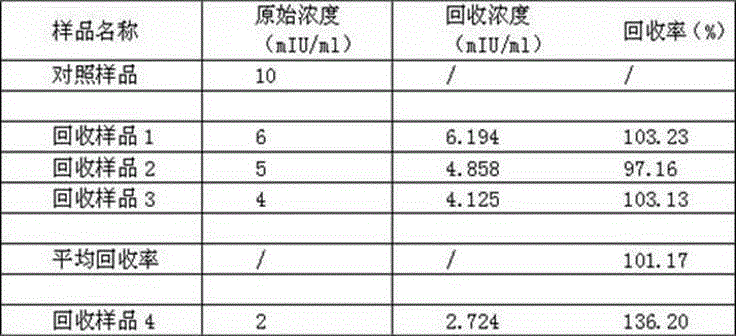
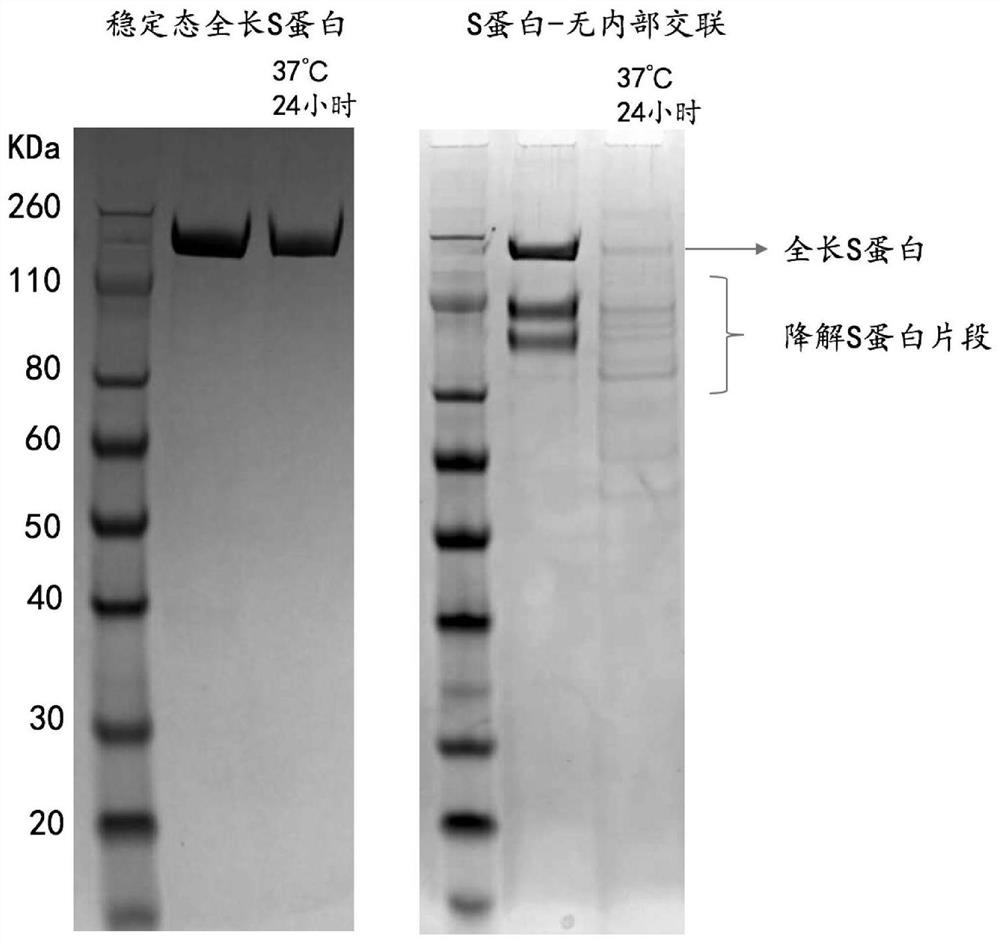
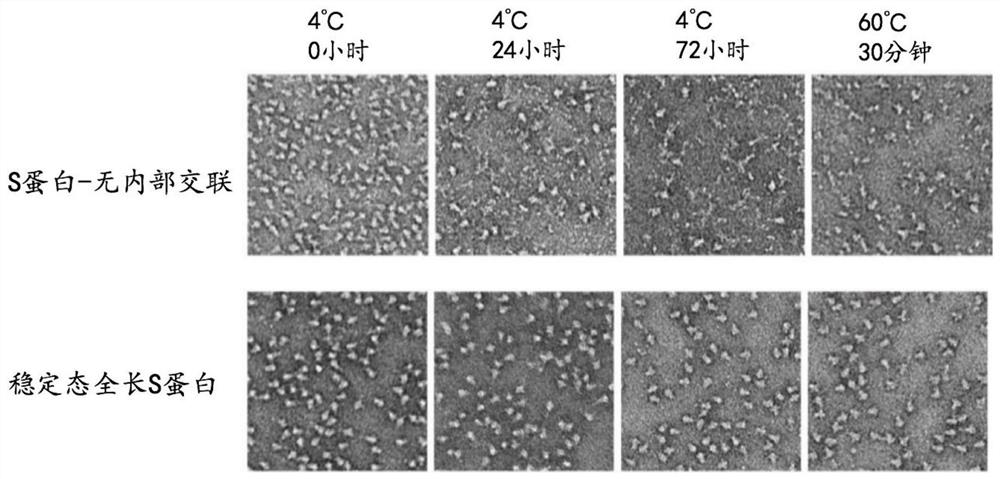
The invention discloses a high-stability novel coronavirus spike protein, a related biological material, application of the related biological material, detection test paper and a detection kit, relates to the field of biotechnology and biomedicine. The bottleneck of the industry is broken through through internal crosslinking modification on the optimal antigen spike protein, namely S protein, onthe surface of novel coronavirus, The high-stability full-length S protein trimer is obtained through expression and purification in mammals, all antigen epitopes of the new coronavirus surface spikeprotein are reserved to the maximum extent, the stable S protein serves as an antigen to capture a new coronavirus antibody in a patient sample, and a novel coronavirus antibody instant detection kitwith high accuracy, high sensitivity and high stability is produced.
Owner:深圳粒影生物科技有限公司
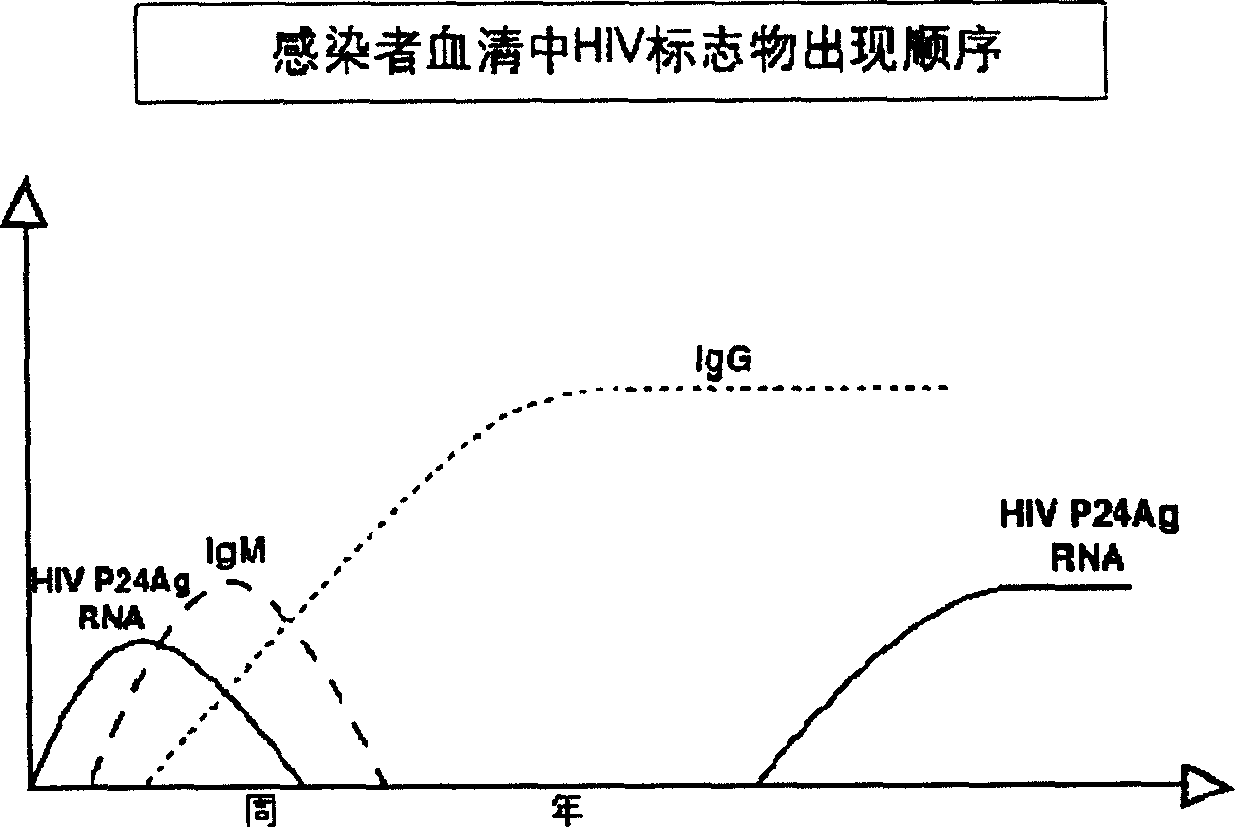
HIV viral antibody/antigen diagnostic reagent kit and preparing method thereof and detecting method
InactiveCN1673749AReduce the chance of infectionHigh sensitivityMaterial analysis by observing effect on chemical indicatorViral antibodyPhosphate
The present invention is HIV virus antibody / antigen diagnosis kit and its preparation process and detection method. The kit includes pre-coated enzyme-linked board with coated HIV antibody and P24 monoclonal antibody, rabbit anti-P24 polyclonal antibody labeled with biotin, conjugate of horseradish peroxidase labeled avidin and HIV antigen, detergent solution of phosphate buffer containing Tween, developing solution A of citrate buffer containing hydrogen peroxide, developing solution B of citrate buffer containing tetramethyl benzidine, terminating solution containing sulfuric acid solution, sample diluting solution containing phosphate buffer, normal human serum as positive contrast, HIV antibody serum as positive contrast, and P24 antigen serum as positive contrast. The present invention can detect HIV antibody and P24 antigen simultaneously in raised specificity and sensitivity and is suitable for diagnosis of human immune deficiency virus antibody.
Owner:北京科卫临床诊断试剂有限公司
Novel severe acute respiratory syndrome coronavirus 2 N proteantigen variant and application thereof to detection of novel severe acute respiratory syndrome coronavirus 2 antibody
PendingCN112028977AEfficient captureReduce false positive problemsSsRNA viruses positive-senseVirus peptidesViral antibodySevere acute respiratory syndrome
The invention provides a novel severe acute respiratory syndrome coronavirus 2 N proteantigen variant and an application thereof to detection of the novel severe acute respiratory syndrome coronavirus2 antibody, and relates to variants of N protein important epitope polypeptide. The variants reduce the non-specific reaction of the novel severe acute respiratory syndrome coronavirus 2 protein as acapture antigen with other coronavirus antibodies such as SARS and human influenza coronavirus antibodies. The polypeptide has coronavirus N protein epitope peptide activity, and cysteine residues are added to the C end of the polypeptide. Amino acid residue sites of the mutation are selected from: lysine at the 342rd site, lysine at the 347 site, lysine at the 387 site and lysine atthe 388 site are mutated into amino acids with relatively low homology. The sequence of the N protein 340-419 polypeptide is shown as SEQ ID NO 7. The invention further discloses an antigen composition, use and method of the variant.
Owner:SHANGHAI NAT ENG RES CENT FORNANOTECH
ELISA detection kit for foot-and-mouth disease O-type virus sIgA antibody and application thereof
ActiveCN109232720AQuick checkAccurate detectionSsRNA viruses positive-senseVirus peptidesPositive controlSIgA antibody
The invention discloses an ELISA detection kit for a foot-and-mouth disease O-type virus sIgA antibody and an application thereof. The kit comprises a foot-and-mouth disease O-type virus broad spectrum multi-epitope recombinant antigen coated enzyme labeled reaction plate, a 100*concentrated enzyme labeled antibody, an enzyme labeled antibody diluent, a sample diluent, a concentrated cleaning solution, a developing solution, a stop solution, a positive control sample and a negative control sample. The foot-and-mouth disease O-type virus broad spectrum multi-epitope recombinant antigen is composed of main neutral epitopes of Cathay, PanAsia and Burmese 98 three pedigrees of foot-and-mouth disease O-type virus typical strains, so that the sensitivity and specificity of kit detection can be promoted and the kit is suitable for detection of different foot-and-mouth disease O-type virus infections. The kit provided by the invention is suitable for detection of sIgA antibodies in easily infected animal mucosal secretion of pig, cattle and sheep and has a great significance in preventing and controlling the spreading and infection of foot-and-mouth disease O-type virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
One-step rapid and efficient viral nucleic acid extraction method
PendingCN111187769AHigh recovery rateHigh specific adsorption rateMicrobiological testing/measurementDNA preparationViral antibodyLysis
The invention discloses a one-step rapid and efficient viral nucleic acid extraction method. The one-step rapid and efficient viral nucleic acid extraction method comprises the following steps: (1) allowing binding of a virus antibody or receptor to hydroxyl group, carboxyl group, amino group or epoxy group on surface of magnetic microsphere (MNP) so as to form a stable magnetic microsphere-antibody complex (MNP-Ab); (2) uniformly mixing the MNP-Ab with nucleic acid extraction magnetic beads according to a certain proportion so as to obtain a magnetic bead mixture; (3) adding the magnetic beadmixture into a sample so as to allow virus in the sample to be tested with the MNP-Ab so as to obtain enriched virus; (4) causing lysis on the enriched virus by using a lysis solution so as to have nucleic acids released, and allowing combination of the nucleic acids with the nucleic acid extraction magnetic beads in the magnetic bead mixture; and (5) eluting the nucleic acids by using an eluant.The one-step rapid and efficient viral nucleic acid extraction method truly realizes the ''one-step one-tube method'' that immuno-magnetic beads and nucleic acid extraction magnetic beads are contained in the same container, so that neither of the magnetic beads are required to be removed in the process of operation, so that the one-step rapid and efficient viral nucleic acid extraction method iseasy to operate; and moreover, the immuno-magnetic beads are high in specificity and adsorption rate, high in virus recovery rate, and strong in repeatability.
Owner:CHANGZHOU SMART LIFESCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com