O-type foot-and-mouth disease virus antibody chemiluminescence detection kit
A technology for chemiluminescence detection and foot-and-mouth disease virus, which is applied in chemiluminescence/bioluminescence, analysis through chemical reactions of materials, and measurement devices, etc., to achieve strong specificity, low carcinogenicity and harm, and stable markers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A chemiluminescent detection kit for O-type foot-and-mouth disease virus antibody, comprising polystyrene plate coated with antigen, monoclonal antibody, standard, luminescent substrate A solution and luminescent substrate B solution;
[0038] The coated antigen polystyrene plate is an opaque polystyrene plate coated with O-type foot-and-mouth disease virus RE2 recombinant protein. The volume ratio of the buffer solution is 1:5000, and the coated antigen is diluted; the diluted coated antigen is added to a 48-well or 96-well opaque white microwell plate with a sample volume of 100 μl / well. Coating at 4°C for 20 hours, washing with washing solution 2-3 times, then adding 150 µl / well of blocking solution, blocking at 4°C for 16 hours, then throwing off the liquid, drying at 37°C for 3 hours, and sealing in bags;
[0039] The monoclonal antibody is the O-type foot-and-mouth disease virus monoclonal antibody labeled with horseradish peroxidase, and the working concentration...
Embodiment 2
[0060] Preparation of a kind of O-type foot-and-mouth disease virus antibody chemiluminescent detection kit:
[0061] 1. The foot-and-mouth disease O-type recombinant protein was used as an antigen to immunize Balb / c mice, and the optimal antigen coating concentration (2.5mg / L) was determined by square array titration indirect ELISA method to detect the mouse serum, and the titer reached 1:4.096 ×10 4 , the splenocytes were fused with Sp2 / 0 myeloma cells to prepare FMD type O monoclonal antibody. Positive hybridoma cell lines were screened by limited dilution method and established indirect ELISA method, and finally the specific hybridoma cell line that can stably secrete monoclonal antibody was obtained from the cell line with the highest positive value after 4 times of subcloning screening . The hybridoma cells have good stability through the in vitro passage test, continuous cryopreservation recovery test, and ascites continuous passage test; after the specific type detec...
experiment example
[0070] 1. Specificity test
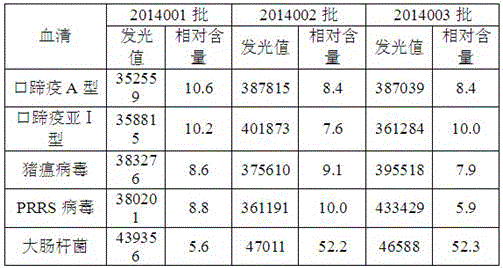
[0071] The positive sera of FMD A, Asia-1, swine fever, porcine reproductive and respiratory syndrome virus and Escherichia coli were detected with 3 batches of trial-produced FMDV antibody chemiluminescent detection kits.
[0072]
[0073] 2. Sensitivity test
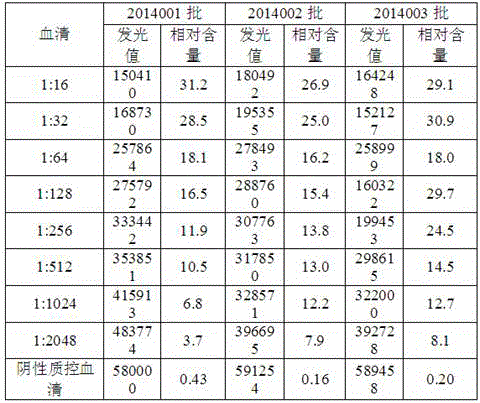
[0074] Dilute the sensitive positive quality control serum at 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, 1:2048, from the 3 batches of test kits 1 kit was randomly selected from each batch for detection to determine the sensitivity of the O-FMDV antibody chemiluminescence detection kit.
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com