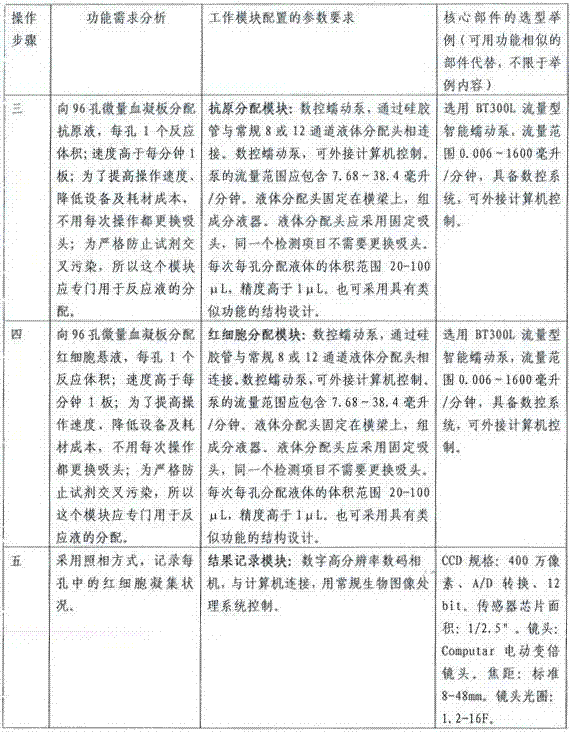
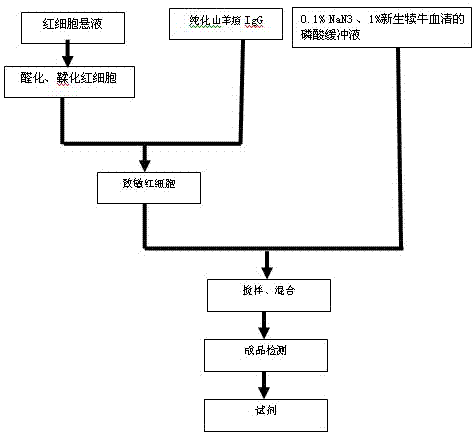
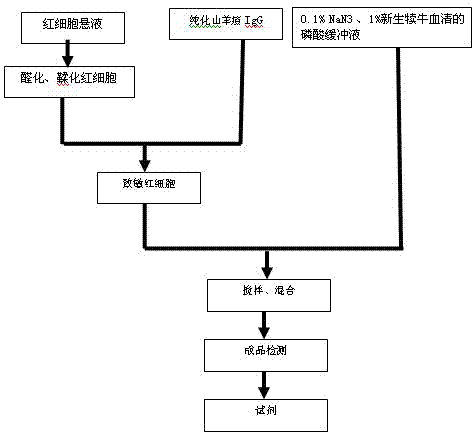
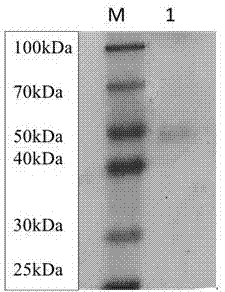
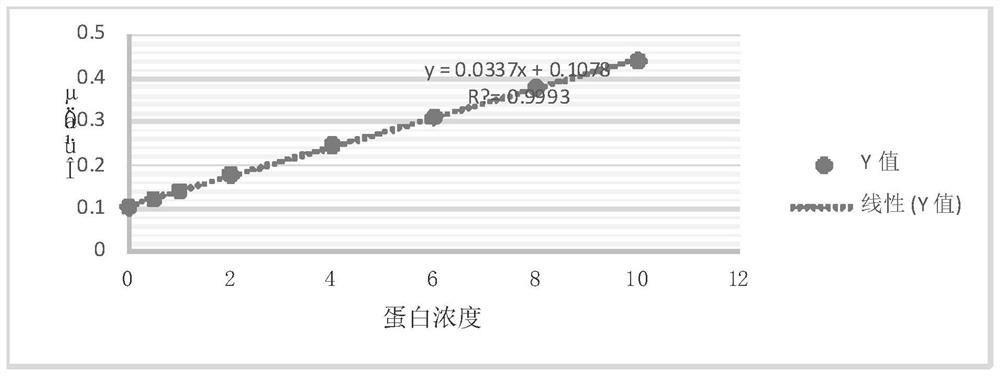
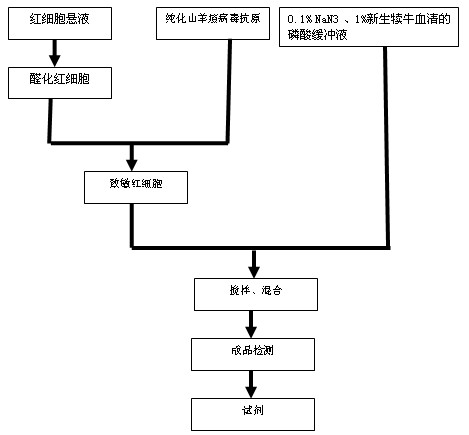
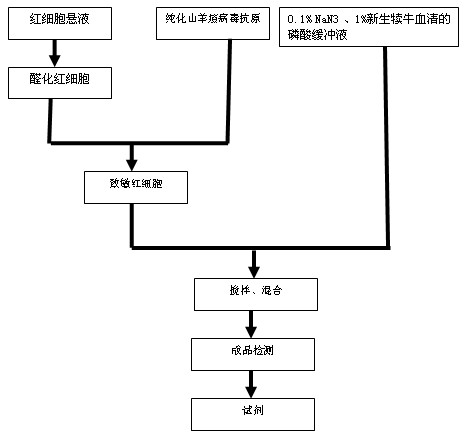
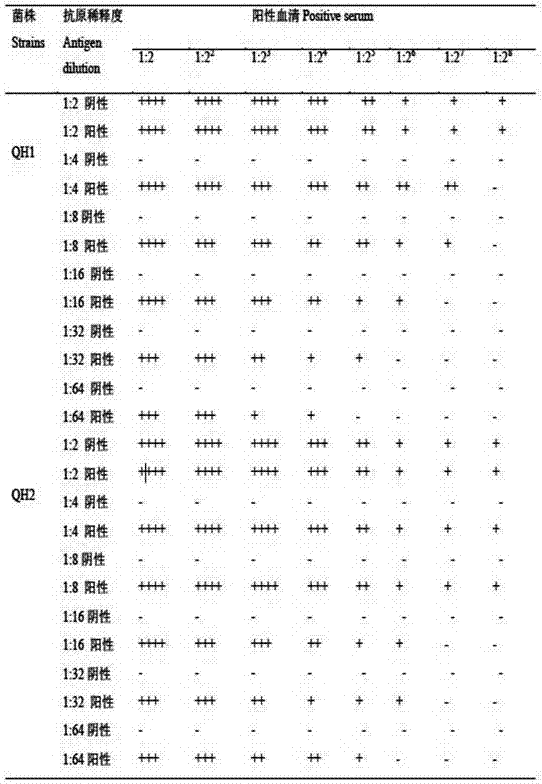
Patents
Literature
32 results about "Indirect hemagglutination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
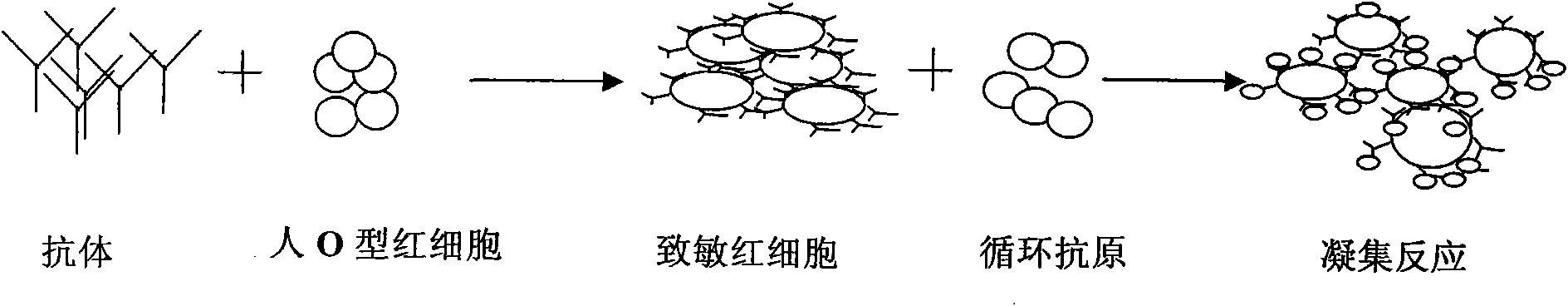
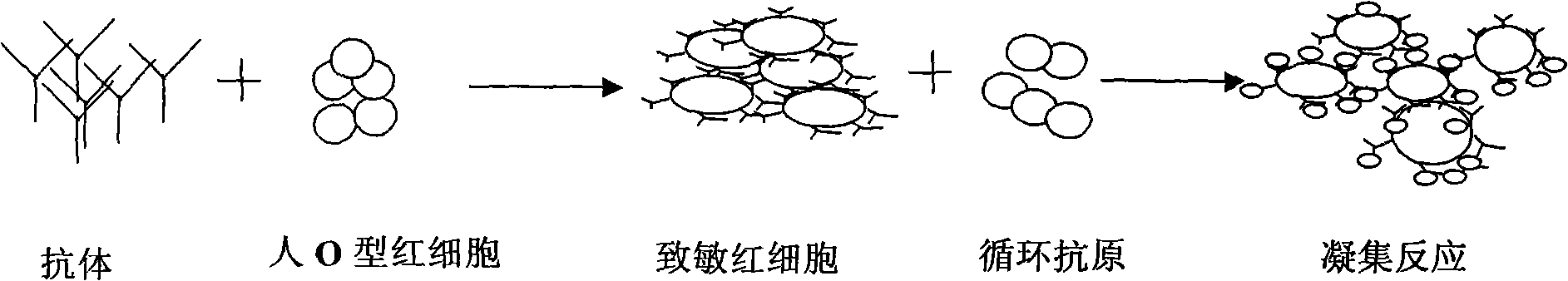
A kind of passive agglutination in which erythrocytes, usually modified by mild treatment with tannic acid or other chemicals, are used to adsorb soluble antigen onto their surface, and which then agglutinate in the presence of antiserum specific for the adsorbed antigen. Synonym(s): indirect hemagglutination test.
Pig mycoplasma pneumoniae recombination antigen ELISA detection reagent kit
The invention discloses a pig mycoplasma pneumonia recombination antigen ELISA detection Kit. The Kit is provided with an antibody detection plate, enzyme conjugate treatment fluid, a positive control, a negative control, sample diluent, 10x condensed cleaning solution, developing solution A, developing solution B and termination solution. The detection plate of the Kit is a detachable 96-pore enzyme label plate enveloped by the mutational pig mycoplasma pneumonia membrane protein P46 gene protein antigen, the enzyme conjugate treatment fluid is a rabbit anti-pig antibody labeled by horse radish peroxidase, the positive control serum is taken from a pig which is detected positive through indirect hemagglutination and the ELISA Kit of IDEXX and has obvious pig mycoplasma pneumonia lesions in the lungs after anatomy, and the negative control serum is taken from a pig which is detected negative through indirect hemagglutination and the ELISA Kit of IDEXX and has no pig mycoplasma pneumonia lesions in the lungs after anatomy. The pig mycoplasma pneumonia recombination antigen ELISA detection Kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale generation and application, and broad market prospect.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Kit for detecting circulating antigen indirect hemagglutination of schistosomiasis and manufacturing method thereof
InactiveCN101893628AHigh utility valueSimple and fast operationBiological testingFreeze-dryingDiluent
The invention provides a kit for detecting circulating antigen indirect hemagglutination of schistosomiasis and a manufacturing method thereof. The kit is composed of freeze-drying anti-SEA-IgY sensitized erythrocyte, sensitized erythrocyte diluent, sample diluents, positive comparison products and negative comparison products. The method for manufacturing the kit for detecting circulating antigen indirect hemagglutination of schistosomiasis comprises the steps of preparing a schistosoma japonica soluble antigen, performing antigen immunization and collecting eggs, extracting and purifying specific anti-SEA-IgY, preparing hydroformylated red cells, preparing tanned red cells, preparing a specific anti-SEA-IgY antibody sensitized erythrocyte, and preparing freeze-drying red cells. The kit in the invention has the advantages of simple and convenient operation, rapidness, high sensibility, strong specificity and good repeatability.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Preparation of duck hepatitis I type virus indirect hemagglutination diagnostic antigen and kit
InactiveCN101423548AQuick responseEasy to useVirus peptidesMaterial analysisDuck hepatitis A virusType antigen
The invention relates to a method for preparing Duck hepatitis virus I type indirect blood coagulation diagnosing antigen, in particular to a method for preparing indirect blood coagulation diagnosing antigen by Duck hepatitis virus I type antigen sensibilization double hydroformylation mutton red cell. The Duck hepatitis virus I type indirect blood coagulation diagnosing antigen produced by the method has long storage time up to half a year; moreover, the diagnosing time is shortened and only half an hour is needed, and the use is convenient and simple; therefore, the method is suitable to be popularized and applied to the practical production.
Owner:陶海静
Hog cholera indirect hemagglutination detection kit and preparation
InactiveCN101487017AAccurate responseStrong specificityVirus peptidesMicroorganism based processesEscherichia coliNucleotide
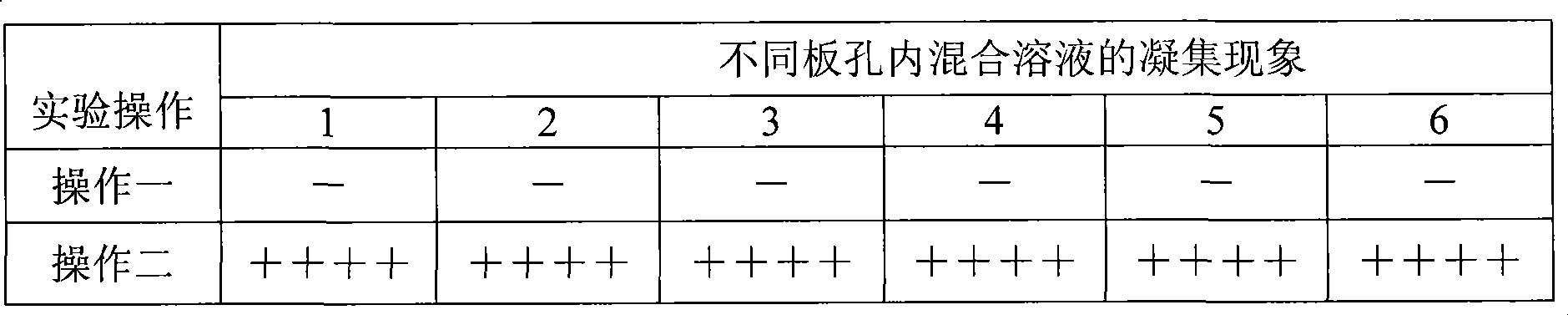
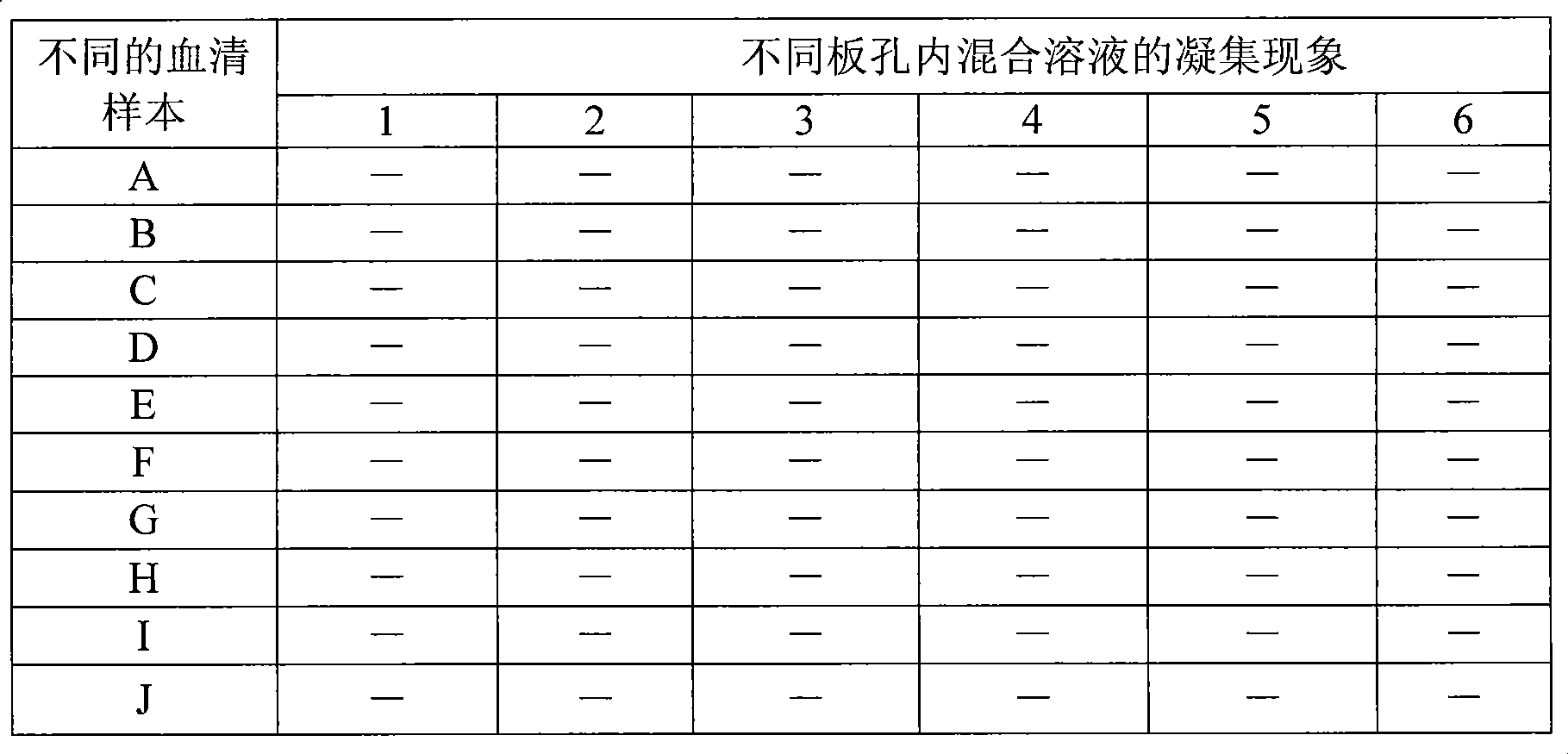
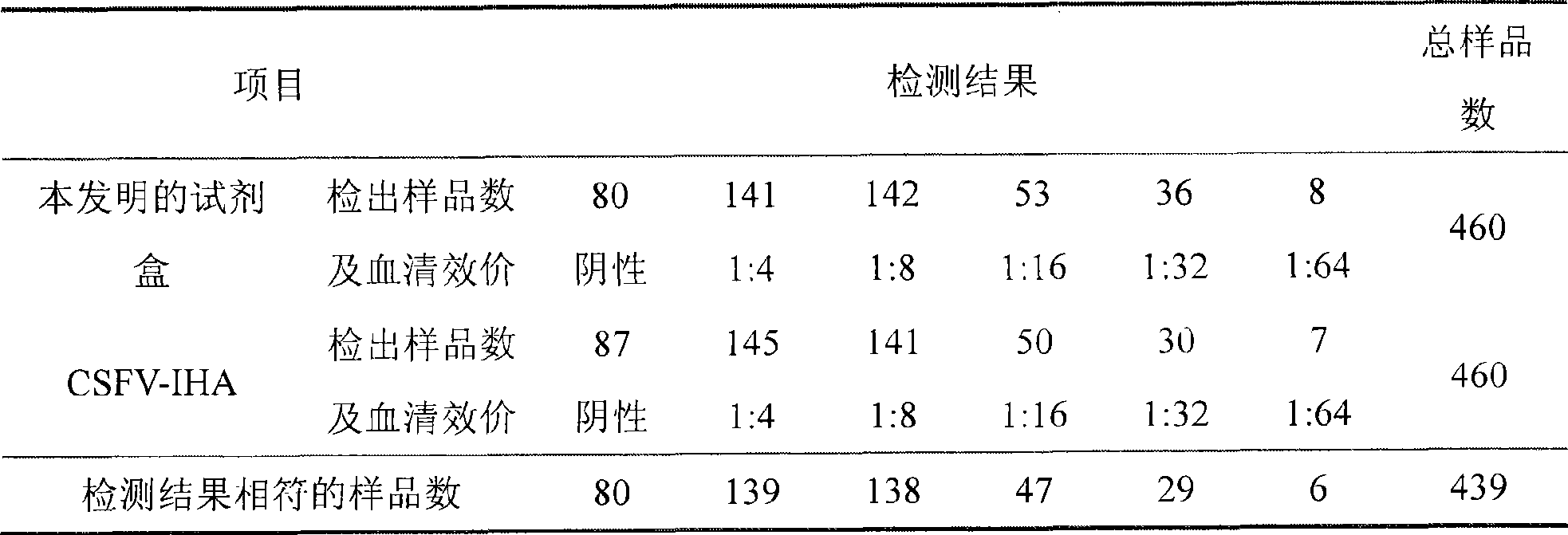
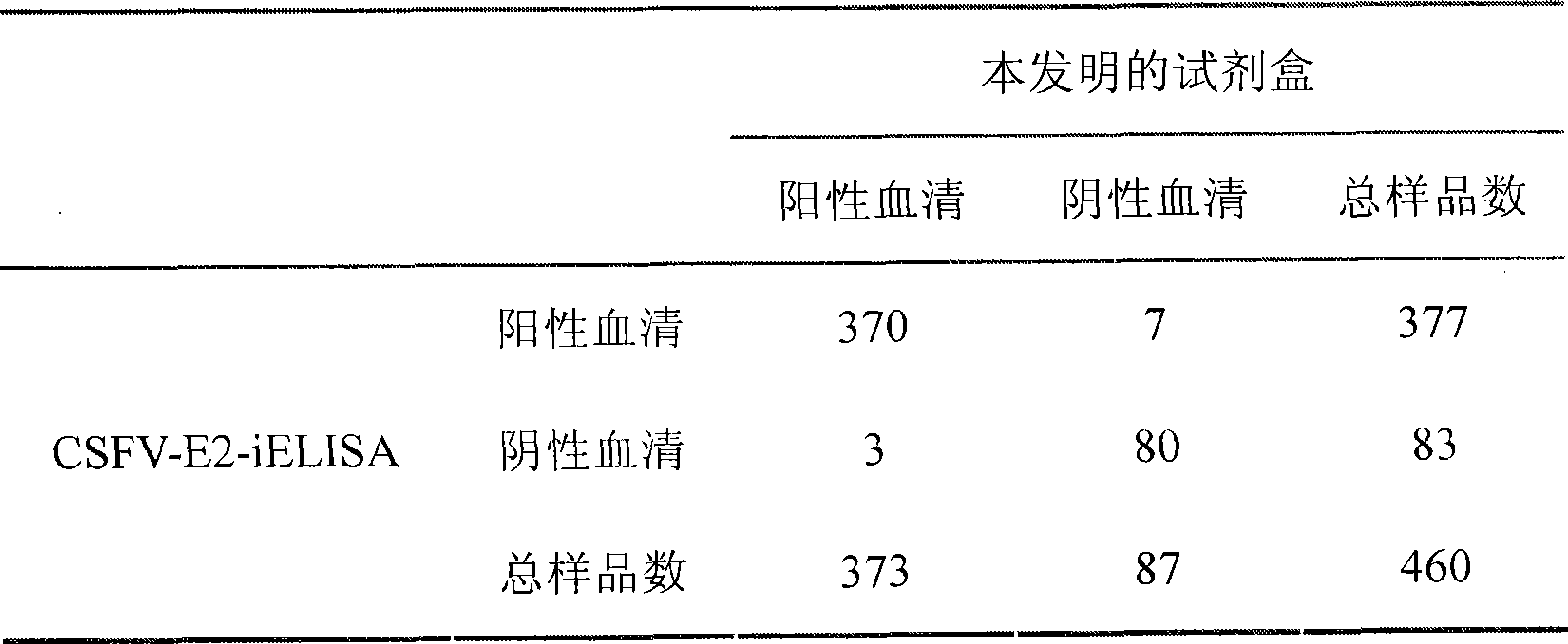
The invention relates to a preparation method of a recombinant protein, an indirect classical swine fever hemagglutination test kit that is prepared by the protein, and a preparation method of the test kit. The preparation method of the protein comprises following steps: a segment with the gene homeology degree comparing with a bovine viral diarrhea-mucosal disease virus (BVDV) that is lower than 35 percent is selected from a conservative region of a classical swine fever virus (CSFV) gene, thus obtaining a nucleotide sequence S1 that is corresponding to an amino acid A1; a forth amino acid in the nucleotide sequence S1 is modified to an hydrophilic amino acid, thus obtaining an amino acid A2; a corresponding nucleotide sequence S2 of A2 is modified, thus respectively obtaining three new nucleotide sequences; the three new nucleotide sequences are orderly and serially connected by connecting nucleotides, thus obtaining a sequence S9 that is corresponding to an amino acid sequence A3; and a nucleotide sequence S10 is obtained by processing S9 correspondingly, and after artificially synthesizing and digesting S10, connecting S10 with a carrier pGEX-4T-1 and inducing the recombinant carrier into colon bacillus for induction expression, the recombinant protein is obtained by separating and purifying an expression product.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation method of sensitized chicken erythrocyte as well as IBV (Infectious Bronchitis Virus) detection kit
ActiveCN103543257AStrong specificityImprove complianceBiological testingImmunoassaysInfectious bronchitis virusAssay
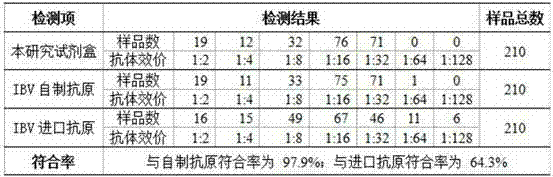
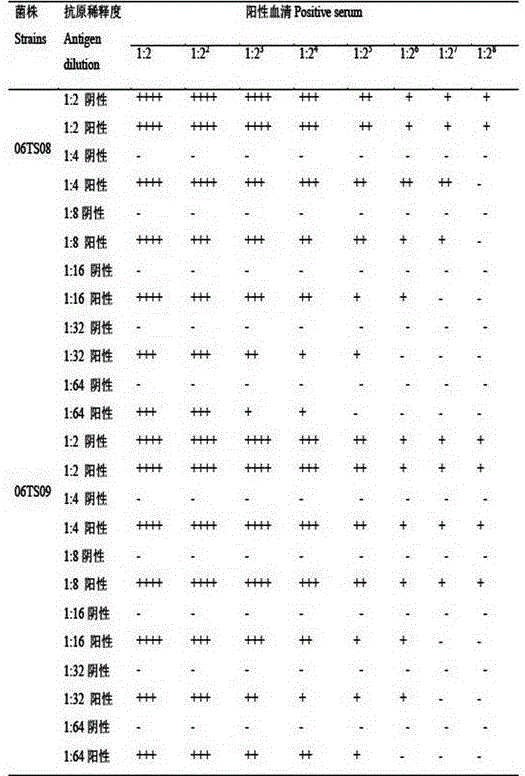
The invention discloses a preparation method of sensitized chicken erythrocyte as well as an IBV (Infectious Bronchitis Virus) detection kit. The preparation method of the sensitized chicken erythrocyte comprises the following steps of cell curing, wherein fresh chicken erythrocyte is washed by using buffer liquor, diluted by adding aldehyde liquor, oscillated for reaction and washed to obtain cured chicken erythrocyte; cell tanning, wherein the cured chicken erythrocyte is diluted, and added with tannic acid to obtain tanned chick erythrocyte; and cell sensitization, wherein the tanned chicken erythrocyte is diluted, added with IBV liquor for mixing and acting to obtain the sensitized chicken erythrocyte. The sensitized chicken erythrocyte disclosed by the invention has very high specificity, and a detection result has very good conformance. An IHA (Indirect Hemagglutination Assay) agent prepared and obtained by the sensitized chicken erythrocyte has low cost and is easy to operate, so that the result can be observed within 25 minutes and detection time is greatly shortened.
Owner:GUANGZHOU SOUTH CHINA BIOLOGICAL MEDICINE
Toxoplasma indirect hemagg lutination diagnostic reagent and producing process thereof
The insect indirect blood diagnosis regent and its making method are made by the antigen secreted by the bow form insect. It inoculates the 1*10-5-1*10-6 bow form insect into the small rat belly cavity, then to soak in the alcohol to disinfect the body surface after making the small white rat death to collect a belly cavity liquid by the centrifugation; the red cell treated by the secreted antigen which is the bow insect indirect blood diagnosis antigen; the 5% red cell treated by the secreted antigen are suspended in the water bath box, then to centrifugate and wash for 3-5 times; then to wash by the 2% NRS of 0.15M PBS with the pH7.2 for 2-3 times. the sediments is to matched to the 1% sensitized red cell suspension with the 0.15M PBS of pH7.2 containing 2% NRS. It is dried by the freeze drier and vacuumize to get the indirect blood diagnosis regent with the IHA secreted by the bow form insect. The invention can be used for examining the bow form insect disease and the census aspect of bow form insect disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
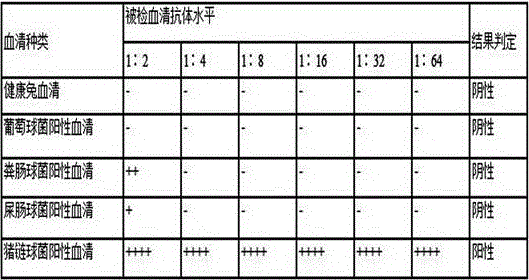
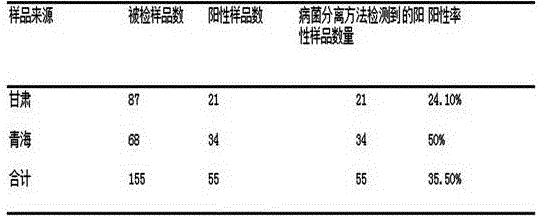
Kit for detecting indirect hemagglutination of streptococcus suis and application thereof
ActiveCN106093439AAvoid timeAvoid the disadvantage of low sensitization potencyBiological testingMicrobiologyStreptococcus mitis
The invention provides a kit for detecting indirect hemagglutination of streptococcus suis. The kit comprises indirect hemagglutination antigen of streptococcus suis, and the indirect hemagglutination antigen of streptococcus suis is composed of whole bacterial protein of streptococcus suis CCTCC M 2016028 and aldehydated-tanned sheep red blood cell. The kit has the advantages that the operation is simple, convenient and rapid, no special equipment and apparatus is required, a whole course can be completed in 2-3 hours, the kit is suitable for basic popularization and application, and can be widely used for investigation and research of epidemiology of swine streptococcosis. The results have the advantages of good repeatability, stabilization, and reliablility, and can accurately detect the antibody of streptococcus suis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Polymerase chain reaction (PCR) detection method of porcine eperythrozoon
InactiveCN103865992AAccurate detectionDetection accuracy and reliabilityMicrobiological testing/measurementSorbentEpidemiologic survey
The invention discloses a polymerase chain reaction (PCR) detection method of porcine eperythrozoon, and the method successively comprises the steps of pathogen separation and purification, porcine eperythrozoon DNA extraction, primer design and synthesis, ordinary PCR detection, positive plasmid standard substance preparation and real-time fluorescent quantitative PCR detection. Compared with IHA (indirect hemagglutination assay), ELISA (enzyme-linked immuno sorbent assay) and other detection methods, the method has the advantages of high specificity, high sensitivity, high accuracy and reliability, can fast, simply, reliably and accurately detect eperythrozoon in a sample, and can also be used for epidemiological investigation and curative effect detection of eperythrozoonosis.
Owner:QINGDAO ZHONGREN PHARMA
Method for indirect immunofluorescence assay (IFA) detection of eperythrozoonosis by use of monoclonal antibody
InactiveCN103869071AReliable detectionQuick checkDisease diagnosisFluorescence/phosphorescenceAntigenPositive control
The invention discloses a method for indirect immunofluorescence assay (IFA) detection of eperythrozoonosis by use of a monoclonal antibody. The method includes establishment of a standard positive control and a standard negative control, antigen coating, fluorescence second antibody dilution, IFA determination and other steps. An antibody used in the method is a monoclonal antibody against porcine eperythrozoon, and compared with general antibodies used in IHA (indirect hemagglutination assay), ELISA (enzyme-linked immuno sorbent assay) and other detection methods, the antibody used in the method has the advantages of high specificity, high sensitivity, high accuracy and reliability, can fast, simply, reliably and accurately detect eperythrozoon in a sample, and can also be used for epidemiological investigation and curative effect detection of the eperythrozoonosis.
Owner:QINGDAO ZHONGREN PHARMA
Establishing method and application of pasteurella multocida indirect haemagglutination assay
ActiveCN103513031ALow costEasy to operateDisease diagnosisBiological testingHemagglutination testsP. multocida
The invention relates to an establishing method for pasteurella multocida indirect haemagglutination assay and application of the pasteurella multocida indirect haemagglutination assay in pasteurella multocida vaccine potency testing. According to the method, the stable pasteurella multocida indirect haemagglutination assay with good sensitivity and repeatability is established on the basis of polysaccharide content in the capsular antigen of pasteurella multocida through screening the optimal dilution of antigens in indirect hemagglutination test; the assay is used to detect the indirect hemagglutination titer of the serum of vaccinated animals and explore a parallel relationship between the indirect hemagglutination titer of the serum and a virulent virus attack protection rate after immunization and is applied to potency testing of pasteurella multocida vaccines.
Owner:PU LIKE BIO ENG
Mycoplasma hyopneumoniae antibody detection method and application
PendingCN113092783AExpand the scope ofIncreased sensitivityDepsipeptidesBiological testingEscherichia coliRed blood cell
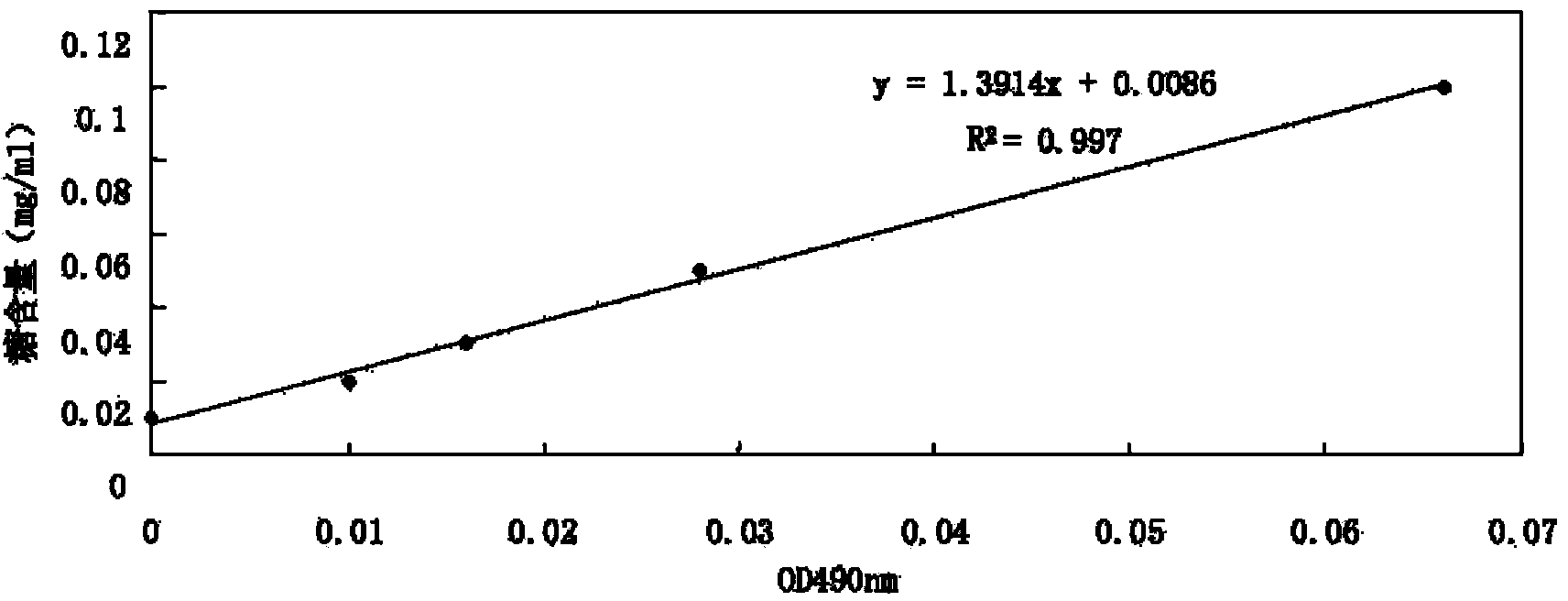
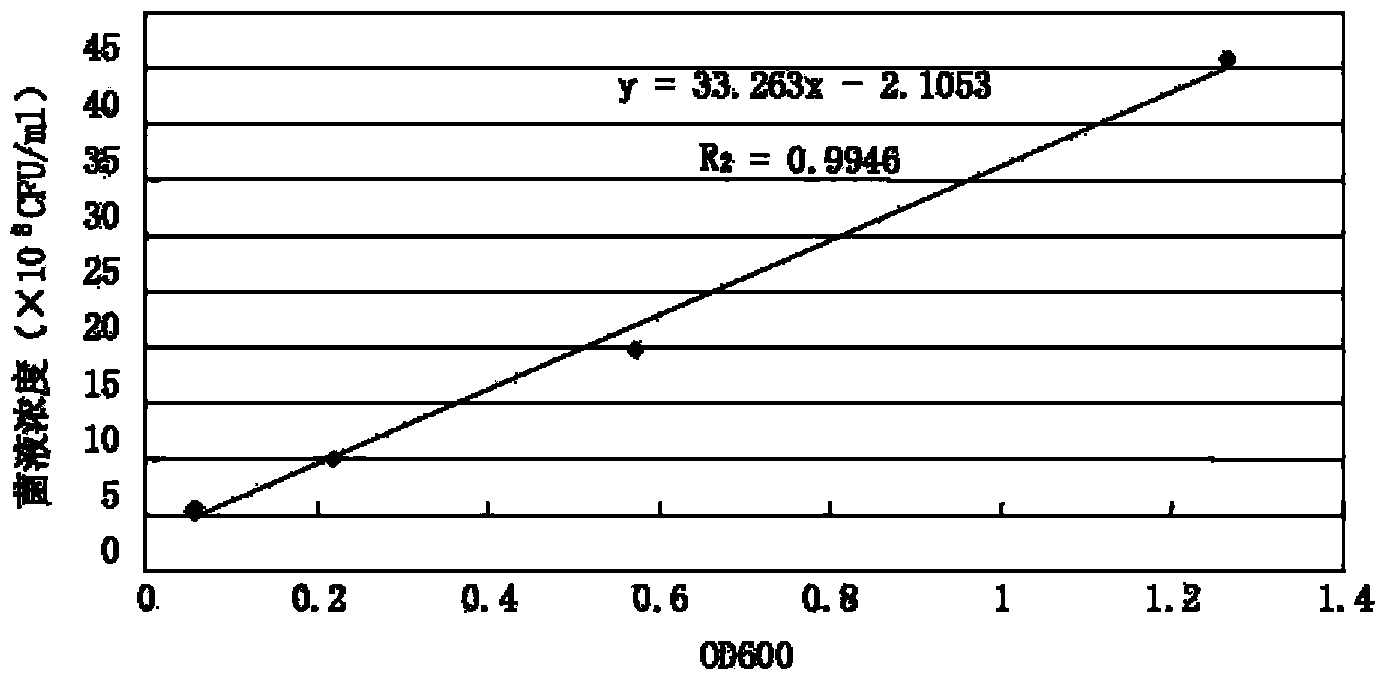
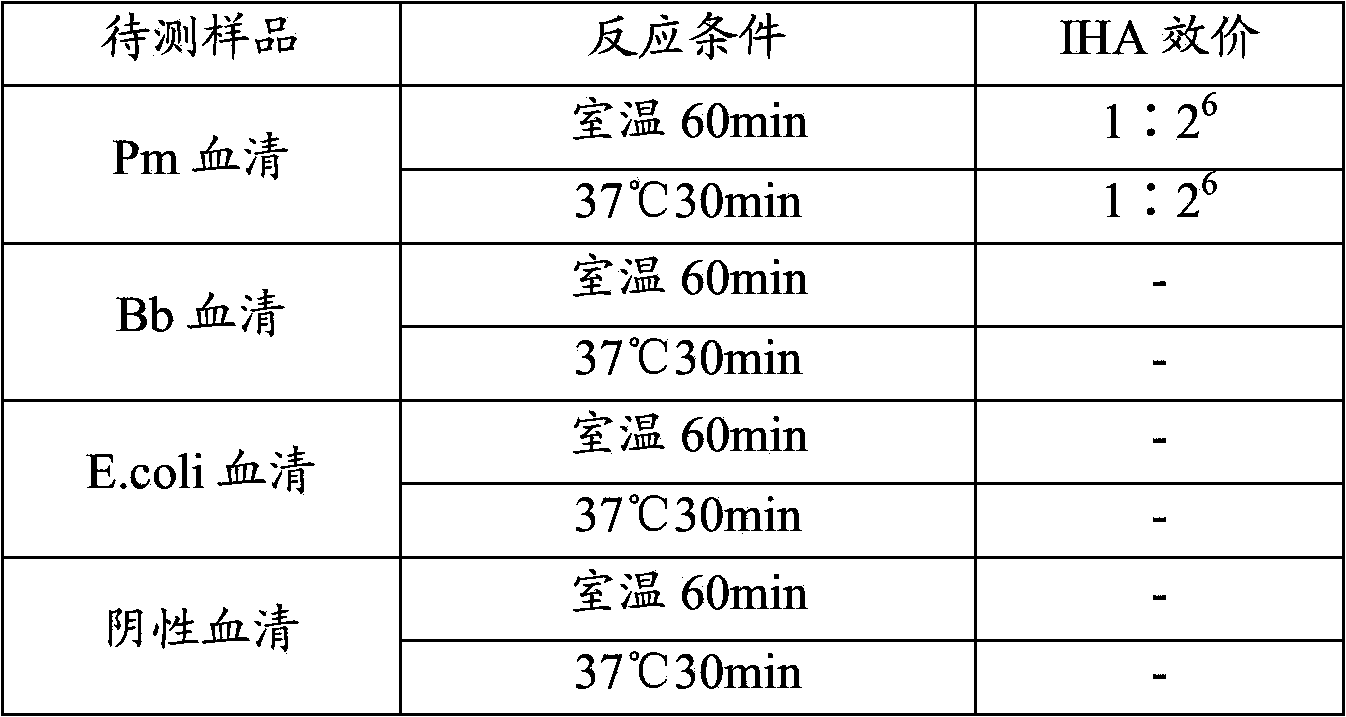
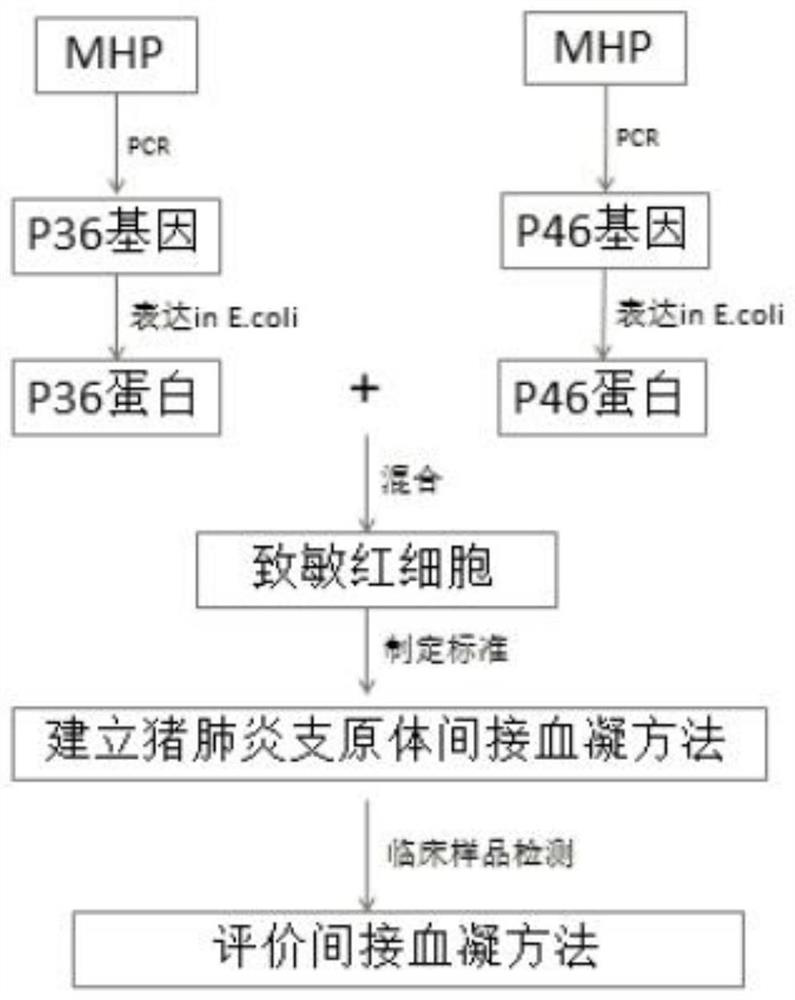
The invention discloses a detection method and application of a mycoplasma hyopneumoniae antibody. The mycoplasma hyopneumoniae antibody is detected by mixing P36 and P46 proteins expressed by escherichia coli with sensitized red blood cells. According to the method, mycoplasma hyopneumoniae specific proteins P36 and P46 are specifically selected, the indirect hemagglutination sensitization antigen prepared through escherichia coli expression and Ni column purification reacts with a mycoplasma hyopneumoniae antibody to the maximum extent, the reaction range and sensitivity are greatly increased, cross reaction with other mycoplasmas is avoided, escherichia coli expresses the sensitization protein antigen, the growth period is greatly shortened, and production cost is reduced. The method is advantaged in that the detection reagent is good in stability, high in sensitivity and good in repeatability, has no obvious cross reaction on other pathogenic antibodies, is simple, convenient and rapid to operate, and can be used for clinical sample detection, immune level monitoring and epidemiological investigation.
Owner:中农华大(武汉)检测科技有限公司
Total design scheme of automatic hemagglutination inhibition experiment workstation and application of total design scheme
PendingCN107091935AReduce manual operationsReduce labor intensityBiological testingDiseaseExperimental laboratory
The invention discloses a total design scheme of an automatic workstation for a large scale of hemagglutination inhibition (HI) experiments, and application of the design scheme in development of hemagglutination inhibition experiment special instruments and detection of the hemagglutination inhibition experiments. The workstation is a special instrument designed for specific steps of the HI experiments, and no similar products exist before. Compared with a PCR and an ELISA workstation, the workstation can meet the specific requirements of the HI experiments and can conduct HI detection on a large scale; compared with ordinary manual operation HI experiments, detection efficiency can be improved, labor cost can be saved, and working strength can be reduced. The workstation can detect the HI antibody titer of human and animal avian influenza, Newcastle disease, adenovirus and the like, can also be used for detecting the indirect hemagglutination (IHA) antibody titer of the foot-and-mouth disease, swine fever and the like and has good application prospects in all stages of laboratories.
Owner:陈凡
Preparation method and detection method of goat pox reverse indirect hemagglutination diagnostic reagent
InactiveCN102288756AThe detection process is fastIncreased sensitivityMaterial analysisRed blood cellReaction temperature
The invention discloses a preparation method and a detection method of a goat pox reverse indirect hemagglutination diagnostic reagent capable of detecting goat pox antigen. The preparation method is: mix glutaraldehyde and sheep red blood cells to prepare red blood cell suspension, mix with the same amount of purified goat pox IgG, stir, centrifuge, wash, and use PBS containing 1% newborn calf serum to make 1% sensitization Red blood cells, this is the diagnostic reagent for goat pox reverse indirect hemagglutination. The optimized preparation conditions are: use glutaraldehyde monoformylation method to fix red blood cells, the concentration of sensitized red blood cells is 0.8%-1.0%, and the IgG content is controlled at 1.5-1.55mg.ml-1, which can achieve a better labeling effect; The reagent is used to detect goat pox virus antigen according to the conventional reverse indirect hemagglutination test method, the reaction temperature is 20-25°C, and the reaction time is 50min. The method has good specificity, sensitivity and repeatability, and can be used for rapid detection of goat pox antigen in veterinary clinic.
Owner:GUIZHOU UNIV
Colloidal gold immunochromatography test paper for detecting cat toxoplasma gondii infection and preparation method thereof
The invention discloses a colloidal gold immunochromatographic test paper for detecting toxoplasma gondii infection in cats, and provides the same and a preparation method for serum detection of toxoplasma gondii infection in cats. The prepared colloidal gold test paper for toxoplasma gondii infection in cats has simple, Fast, no special equipment and other characteristics. The test results are easy to interpret and are suitable for rapid clinical diagnosis and large-scale epidemiological investigation. The indirect colloidal gold detection method is used, wherein the purified recombinant protein SAG3 ensures the specificity of the present invention. Use Staphylococcus aureus (SPA) for labeling, which is readily available and inexpensive. Compared with the traditional indirect hemagglutination test and indirect immunofluorescence test, the detection speed is faster, avoiding contact with live insects, and ensuring the safety of testing personnel.
Owner:JILIN UNIV
Goat pox indirect hemagglutination diagnosis reagent as well as preparation method and detecting method thereof
InactiveCN102313803AQuick checkAccurate detectionMaterial analysisRed blood cellIndirect hemagglutination
The invention discloses a goat pox indirect hemagglutination diagnosis reagent as well as a preparation method and a detecting method thereof. The hemagglutination diagnosis reagent comprises the following components by weight percent: 0.6-1.2% of goat pox virus antigen sensitized red blood cells, and 20-200 mu g / ml of purified goat pox virus antigen. Compared with the commonly used agar diffusion experiment, the goat pox indirect hemagglutination diagnosis reagent is prepared from purified goat pox virus antigen sensitized hydroformylated sheep red blood cells in the invention, thus, the goat pox virus antibody can be detected fast, accurately and simply, the result can be determined in 50 minutes, and the antibody with low valence can be detected; moreover, the goat pox indirect hemagglutination diagnosis reagent has good specificity, sensitivity and repeatability, and can be widely used for fast and substantive detection of goat pox virus antibodies in clinical vet, and can provide the basis for prevention, diagnosis and prevention and control of a disease.
Owner:GUIZHOU UNIV
Hybridoma cell strain CMOMP (chlamydophila major outer membrane protein)-5D7, monoclonal antibody secreted by same and application of monoclonal antibody
ActiveCN110129278AImprove expression levelImprove purification efficiencyImmunoglobulins against bacteriaMicroorganism based processesElisa methodCell Membrane Proteins
The invention provides a hybridoma cell strain CMOMP (chlamydophila major outer membrane protein)-5D7 and a monoclonal antibody secreted by the same. The preservation number of the hybridoma cell strain CMOMP-5D7 is CCTCC NO: C2012141. The monoclonal antibody is high in immunological competence, high in titer which reaches to 1x105 and high in specificity. Shown by test results of a blocking ELISA(enzyme-linked immunosorbent assay) detection kit established by the aid of the monoclonal antibody and a method, the obtained monoclonal antibody can detect MOMP (major outer membrane protein) of Chlamydophila abortus, C.abortus, so that the monoclonal antibody is high in specificity, high in within-run repeatability and between-run repeatability and high in stability. Compared with a normal ELISA method, the method has the advantages that requirements on recombinant protein purification are lowered, and specificity in detection is improved. Compared with an indirect hemagglutination assay method, the method has the advantages that sensitivity in detection is improved, judging results are more objective, and the method can be used for detecting samples in large batches.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Hog cholera indirect hemagglutination detection kit and preparation
InactiveCN101487017BAccurate responseStrong specificityVirus peptidesMicroorganism based processesEscherichia coliNucleotide
The invention relates to a preparation method of a recombinant protein, an indirect classical swine fever hemagglutination test kit that is prepared by the protein, and a preparation method of the test kit. The preparation method of the protein comprises following steps: a segment with the gene homeology degree comparing with a bovine viral diarrhea-mucosal disease virus (BVDV) that is lower than35 percent is selected from a conservative region of a classical swine fever virus (CSFV) gene, thus obtaining a nucleotide sequence S1 that is corresponding to an amino acid A1; a forth amino acid in the nucleotide sequence S1 is modified to an hydrophilic amino acid, thus obtaining an amino acid A2; a corresponding nucleotide sequence S2 of A2 is modified, thus respectively obtaining three new nucleotide sequences; the three new nucleotide sequences are orderly and serially connected by connecting nucleotides, thus obtaining a sequence S9 that is corresponding to an amino acid sequence A3; and a nucleotide sequence S10 is obtained by processing S9 correspondingly, and after artificially synthesizing and digesting S10, connecting S10 with a carrier pGEX-4T-1 and inducing the recombinant carrier into colon bacillus for induction expression, the recombinant protein is obtained by separating and purifying an expression product.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Detection method of actinobacillus pleuropneumoniae in pig serums
InactiveCN103267847AEasy to breedEnhance disease preventionMaterial analysisSerum igeActinobacillus pleuropneumoniae
The invention relates to a detection method of actinobacillus pleuropneumoniae in pig serums. The detection method comprises the steps as follows: randomly selecting serum samples of piglets and finishing pigs in a scale farm, firstly carrying out IHA (indirect hemagglutination test) method detection on the collected serum samples, and secondly carrying out APPELISA detection on the collected serum samples to detect the actinobacillus pleuropneumoniae in the pig serums. According to the detection method, the APP indirect hemagglutination test and the APPELISA test are jointly adopted, so that the detection result of the actinobacillus pleuropneumoniae is accurate, reliable, quick, flexible and stable without error, and the detection method is low in cost, simple in method, suitable for basic level, and brings great convenience for cultivation, disease prevention and disease treatment of pigs.
Owner:TIANJIN TIANZE LIVESTOCK & POULTRY BREEDING SPECIALIZED COOP
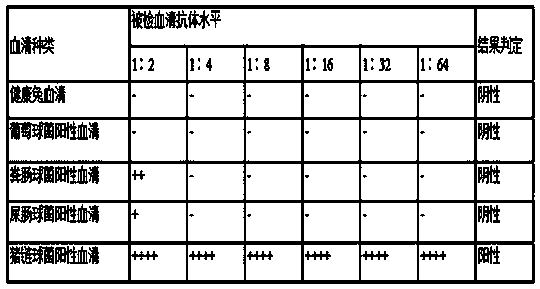
Detection kit for indirect coagulation antibody against Staphylococcus saprophyticus and preparation method thereof
The invention discloses an indirect coagulation kit for detecting a serum antibody against Staphylococcus saprophyticus, and methods for preparing and using the kit. The kit comprises (1) the indirecthemagglutination antigen of Staphylococcus saprophyticus, (2) standard positive serum, (3) standard negative serum, and (4) a diluent. The invention also provides methods for preparing and using thekit. The invention provides the detection kit for the indirect coagulation antibody against Staphylococcus saprophyticus and application thereof. The kit is simple and convenient to operate, and is extensively applicable to various veterinary-related units and farms; the kit in the invention only needs simple instrument consumables during detection of samples, and the whole detection process can be completed in 2-3 hours; results are intuitive and reproducible; and the kit can realize fast, stable and accurate detection of antibodies against Staphylococcus saprophyticus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Detection method of actinobacillus pleuropneumoniae in porcine serum
InactiveCN105588938AThe test result is accurateReliable test resultsMaterial analysisPig breedingHemagglutination tests
The invention relates to a detection method of actinobacillus pleuropneumoniae in porcine serum. The steps include: conducting random selection of scale farm piglets and fattening pig serum samples, firstly conducting IHA (indirect hemagglutination test) detection on the collected serum samples, then carrying out APP ELISA detection on the collected serum samples so as to be able to detect the actinobacillus pleuropneumoniae in porcine serum. The detection method provided by the invention employs combined use of APP indirect hemagglutination test and APP ELISA test, makes the actinobacillus pleuropneumoniae detection result faster and more accurate, reliable and sensitive, the detection result is stable and has no error. Also the detection method has the advantages of low cost and simplicity, is suitable for grass-roots application, and brings about great convenience to pig breeding, disease prevention and resisting.
Owner:天津康源生物技术有限公司
A kind of antigen for porcine infectious pleuropneumonia antibody detection and preparation method
ActiveCN104502581BStrong specificityStrong characteristicBiological material analysisColor/spectral properties measurementsAntigenNormal concentration
The invention provides an antigen for detecting a porcine infectious pleuropneumonia antibody of swinery and a preparation method of the antigen. The antigen is a polysaccharide antigen which is extracted from type 1-15 strain cultures of porcine infectious pleuropneumonia actinobacillus; the polysaccharide antigen is a bacterial excreted polysaccharide which has very strong specificity and sensibility and is capable of truly detecting the porcine infectious pleuropneumonia antibody; the determination of a normal concentration critical value of the antigen provides guarantee for the detection of enzyme linked immunosorbent assay and the detection of indirect hemagglutination test; the preparation method of the antigen is simple to operate, convenient and quick, and suitable for popularization and application at the basic level, and does not need any special equipment and instrument.
Owner:YEBIO BIOENG OF QINGDAO
An indirect hemagglutination antibody detection kit for Riemerella anatipestifer and its application
ActiveCN104007269BEasy to operateLow costBiological material analysisBiological testingRiemerella anatipestiferIndirect hemagglutination
The invention discloses a riemerella anatipestifer indirect coagulation antibody detection kit and application thereof. The kit comprises a riemerella anatipestifer indirect coagulation antigen, standard positive serum, standard negative serum and a diluent. The riemerella anatipestifer indirect coagulation antibody detection kit and the application thereof have the beneficial effects that the kit is simple and convenient to operate, can be widely popularized and used in related grassroots veterinarian departments and farms; the kit only needs a few simple consumable materials and incubators when being used detecting samples without any other device, and can be used even in duck farms with relatively simple and rough conditions; the result can be determined intuitively, and can be observed by eyes; professional knowledge is not required for operators, special training is not required, and the detection can be performed only referring to a specification; the kit is low in cost, is economical and practical, and can be accepted by most of users.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A Mycoplasma hyopneumoniae recombinant antigen ELISA detection kit
Owner:CHINA INST OF VETERINARY DRUG CONTROL
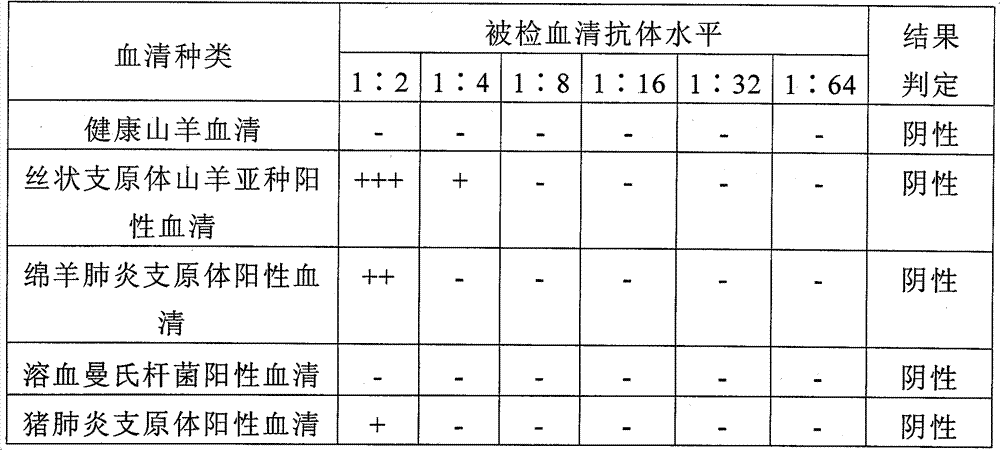
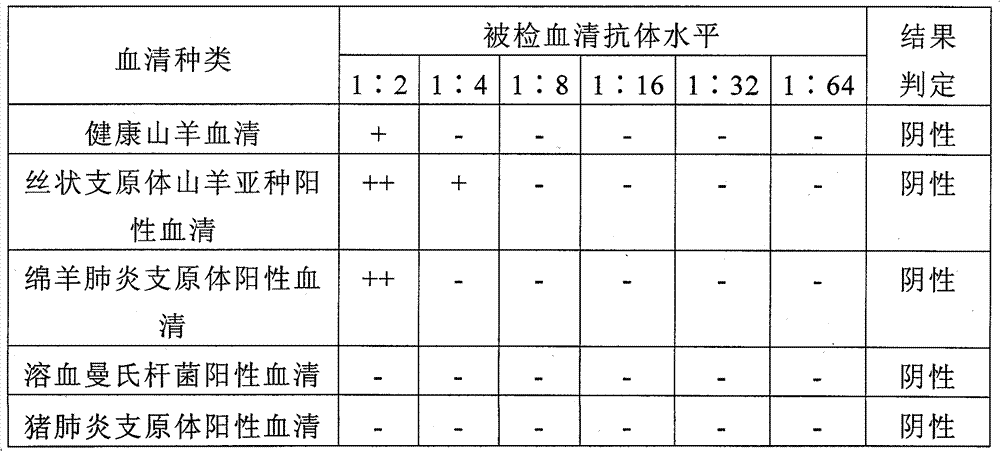
Mycoplasma capricolum subsp. pneumonia antigen of goats and preparation method thereof
ActiveCN101712971BStrong specificityEasy to storeBiological testingFermentationRed blood cellSubspecies
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A kind of Streptococcus suis indirect hemagglutination detection kit and its application
ActiveCN106093439BNo pollution in the processGood repeatabilityBiological testingMicrobiologyStreptococcus mitis
The invention provides a kit for detecting indirect hemagglutination of streptococcus suis. The kit comprises indirect hemagglutination antigen of streptococcus suis, and the indirect hemagglutination antigen of streptococcus suis is composed of whole bacterial protein of streptococcus suis CCTCC M 2016028 and aldehydated-tanned sheep red blood cell. The kit has the advantages that the operation is simple, convenient and rapid, no special equipment and apparatus is required, a whole course can be completed in 2-3 hours, the kit is suitable for basic popularization and application, and can be widely used for investigation and research of epidemiology of swine streptococcosis. The results have the advantages of good repeatability, stabilization, and reliablility, and can accurately detect the antibody of streptococcus suis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A kind of Enterococcus faecium indirect hemagglutination detection kit and its application
The invention provides a detection kit for enterococcus faecium indirect blood clotting. The detection kit is characterized by comprising an enterococcus faecium indirect blood clotting antigen, wherein the enterococcus faecium indirect blood clotting antigen is composed of enterococcus faecium CCTCC M 2016047 whole bacterial proteins and hydroformylation-tannin sheep red blood cells. The kit provided by the invention is simple, convenient and quick in operation; special equipment and instrument are not required; the whole process can be completed within 2-3 hours; the detection kit is fit for popularization and application in basic level; the detection kit can be widely applied to the investigation of enterococcus faecium epidemiology; the repeatability of the result is excellent; the result is stable and reliable; the antibody of the enterococcus faecium can be accurately detected.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
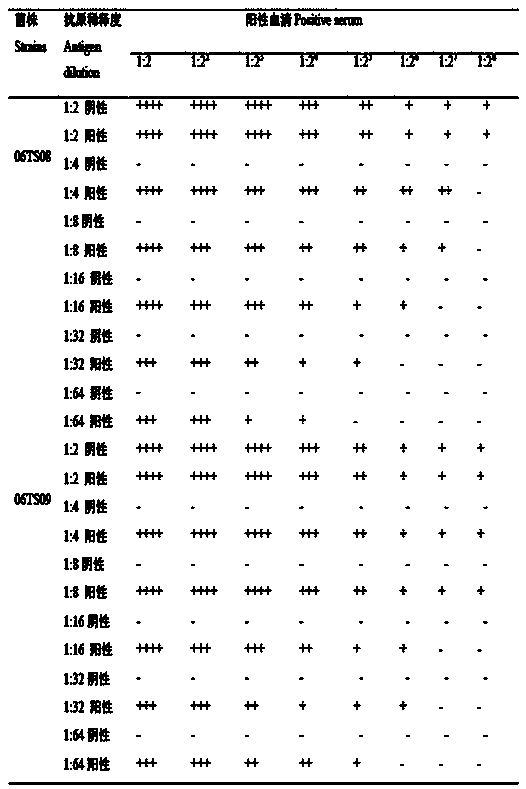
Establishment method of mycoplasma ovipneumoniae indirect hemagglutination detection method
The invention discloses an establishment method of a mycoplasma ovipneumoniae indirect hemagglutination detection method. According to the method, recombinant protein obtained by cloning and expressing a C terminal of a mycoplasma ovipneumoniae adhesin gene P113 is used as an antigen, healthy sheep erythrocytes are sensitized by the recombinant protein antigen, the optimal sensitization concentration and the optimal reaction conditions are screened for IHA titers of the same batch of serum, and the mycoplasma ovipneumoniae indirect hemagglutination detection method which is high in specificity, sensitivity and coincidence rate and easy and convenient to operate is established. The detection method can be applied to rapid detection of mycoplasma ovipneumoniae in the veterinary field, and provides a powerful tool for disease prevention, diagnosis, prevention and control.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Preparation method and detection method of goat pox positive indirect hemagglutination diagnostic reagent
InactiveCN102253198AThe detection process is fastHigh speedMaterial analysisRed Blood Cell FolateReaction temperature
The invention discloses a goat pox positive indirect hemagglutination diagnostic reagent capable of detecting goat pox antibody and a preparation method and detection method thereof. The preparation method comprises the following steps: preparing red blood cell suspension by mixing glutaraldehyde and sheep red blood cells; mixing the red blood cell suspension with equivalent purified goat poxvirus, and stirring, centrifuging and washing; and preparing 1% sensitized red blood cells from PBS containing 1% of newly born calf serum to obtain the goat pox positive indirect hemagglutination diagnostic reagent. The optimized preparation conditions are that: the red blood cells are fixed by a glutaraldehyde single-hydroformylation method so that the concentration of sensitized red blood cells is 0.8-1.0%, and the antigen content is controlled between 20 ug.mL<-1> and 200 ug.mL<-1> so as to achieve relatively good sensitization effect; and the detection method is performed according to a conventional positive indirect hemagglutination test method, the reaction temperature is 20-25 DEG C, and the reaction time is 50 minutes. The method has relatively good specificity, sensitivity and repeatability, and can be applied to veterinarian clinical rapid detection of goat pox antibody.
Owner:GUIZHOU UNIV
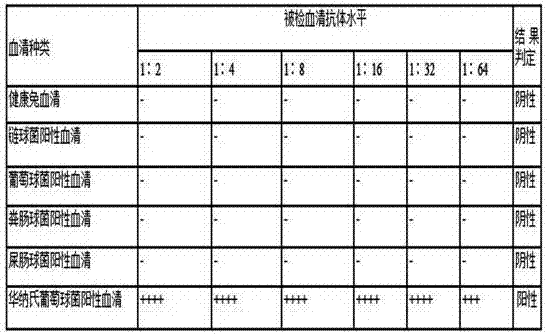
A kind of staphylococcus warnerii indirect hemagglutination detection kit and its application
ActiveCN105866436BDetect trueEasy to detectBiological testingDisease epidemiologyIndirect hemagglutination
The invention provides a staphylococcus Warner indirect blood coagulation detection kit and application thereof. The kit is characterized by comprising a staphylococcus Warner indirect blood coagulation antigen, wherein the staphylococcus Warner indirect blood coagulation antigen is composed of whole bacterial protein of staphylococcus Warner CCTCC M2016048 and hydroformylated-tanned sheep red blood cells. The kit provided by the invention is simple to operate and is convenient and rapid, and special equipment and instruments are not needed; and a whole process is finished within 2-3 hours, so that the kit is suitable for being popularized and applied to a basic level and can be widely applied to investigation and study of staphylococcus Warner disease epidemiology. A detection result has good stability and is stable and reliable, and an antibody of staphylococcus Warner can be accurately detected.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Enterococcus hirae indirect hemagglutination antibody detection kit and preparation method thereof
The invention disclose an indirect hemagglutination kit for detecting enterococcus hirae serum antibodies and a preparation method for preparing and using the kit. The kit comprises (1), enterococcushirae indirect hemagglutination antigens; (2), standard positive serums; (3), standard negative serums; (4), a diluting solution. The invention further provides a preparation and application method ofthe kit. The invention provides the enterococcus hirae indirect hemagglutination antibody detection kit and the application therefore. The kit has the advantages that the operation of the kit is simple and convenient, and the kit is extensively popularized and used in each base-level veterinarian related units and farms; the kit only needs simple instrument and consumable materials when a sampleis detected, the whole process can be completed within 2-3 hours, the result judgement is visualized, the repeatability is good, and the enterococcus hirae antibodies can be rapidly, stably and accurately detected.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com