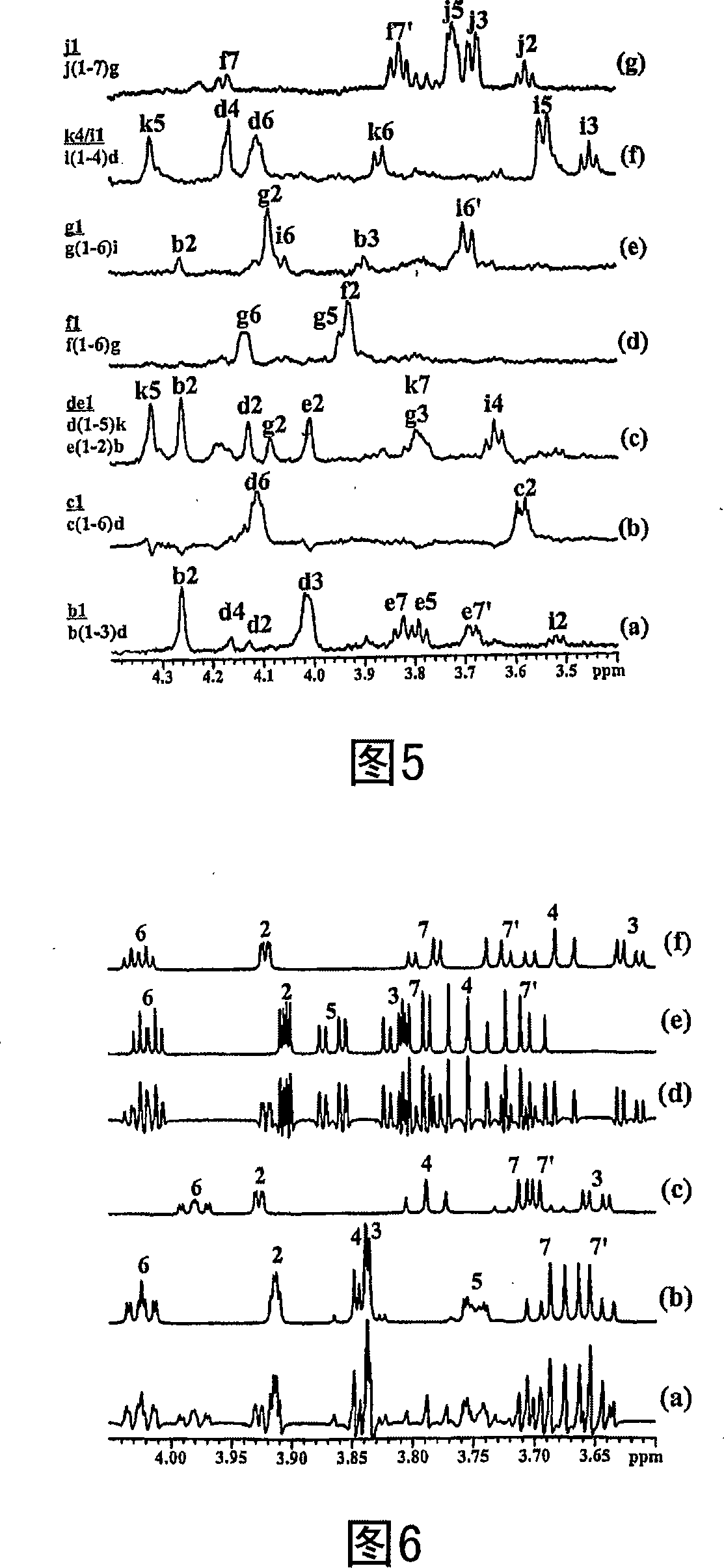
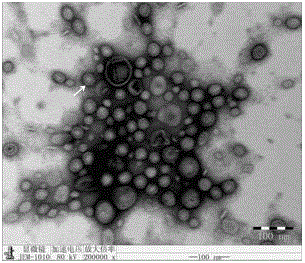
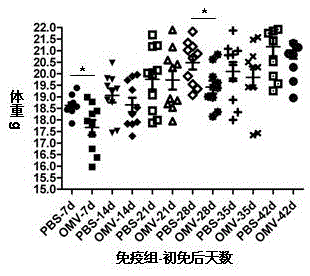
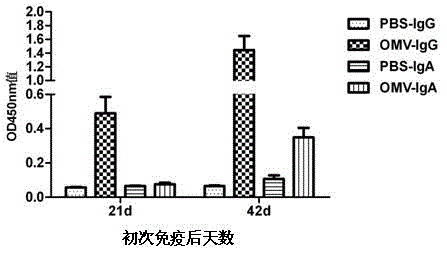
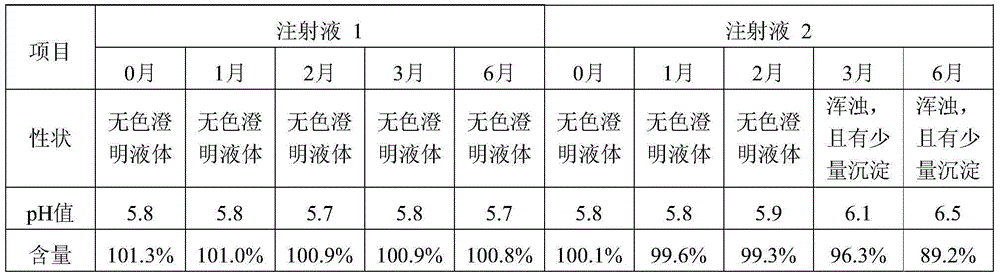
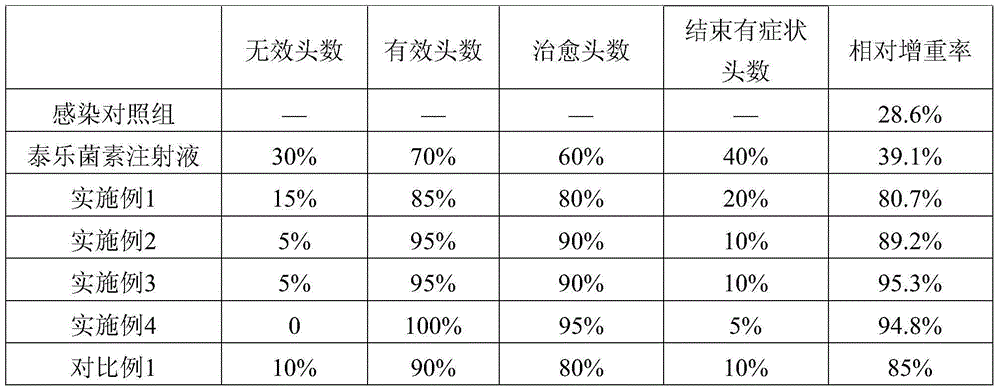
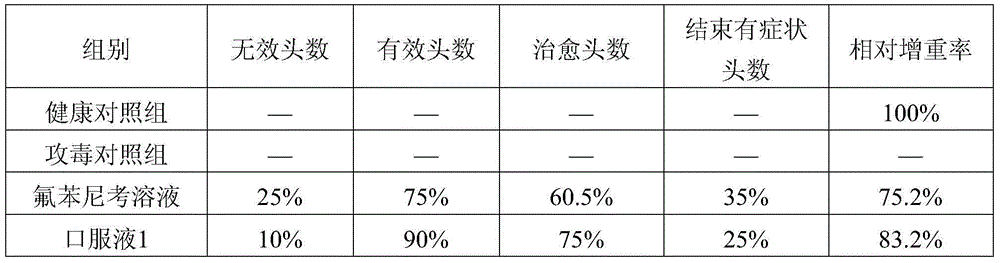
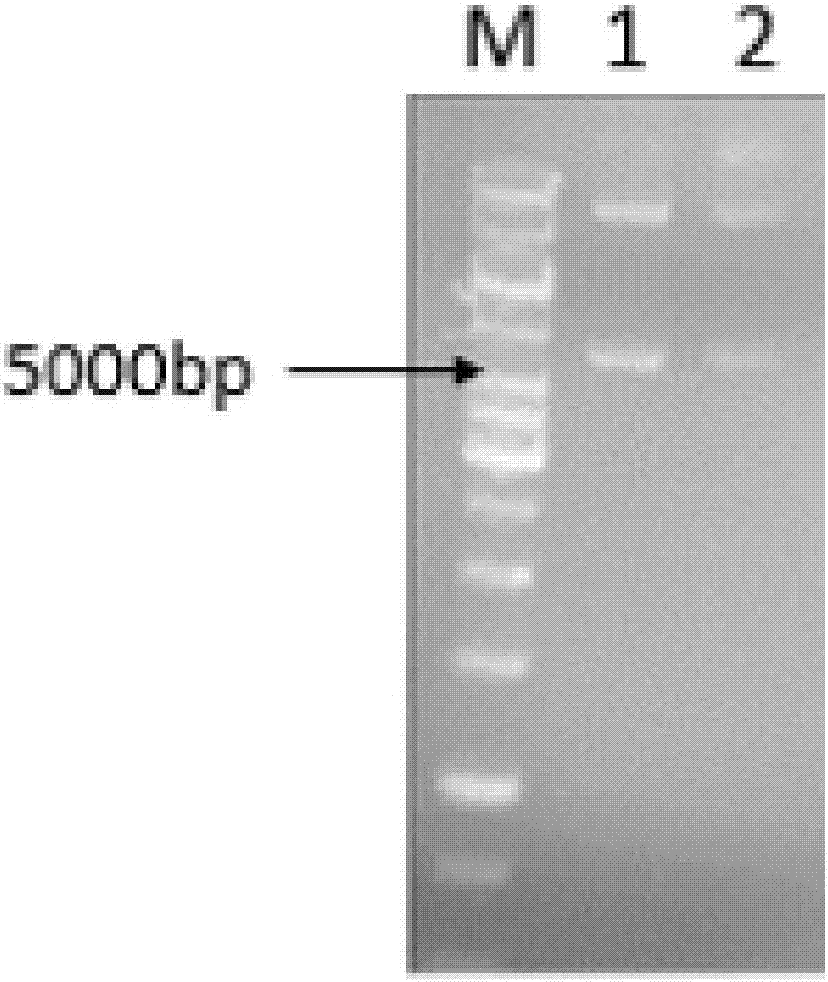
Patents
Literature
80 results about "Actinobacillus pleuropneumoniae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Actinobacillus pleuropneumoniae (previously Haemophilus pleuropneumoniae), is a Gram-negative, facultative anaerobic, respiratory pathogen found in pigs. It was first reported in 1957, and was formally declared to be the causative agent of porcine pleuropneumonia in 1964. It was reclassified in 1983 after DNA studies showed it was more closely related to A. lignieresii.
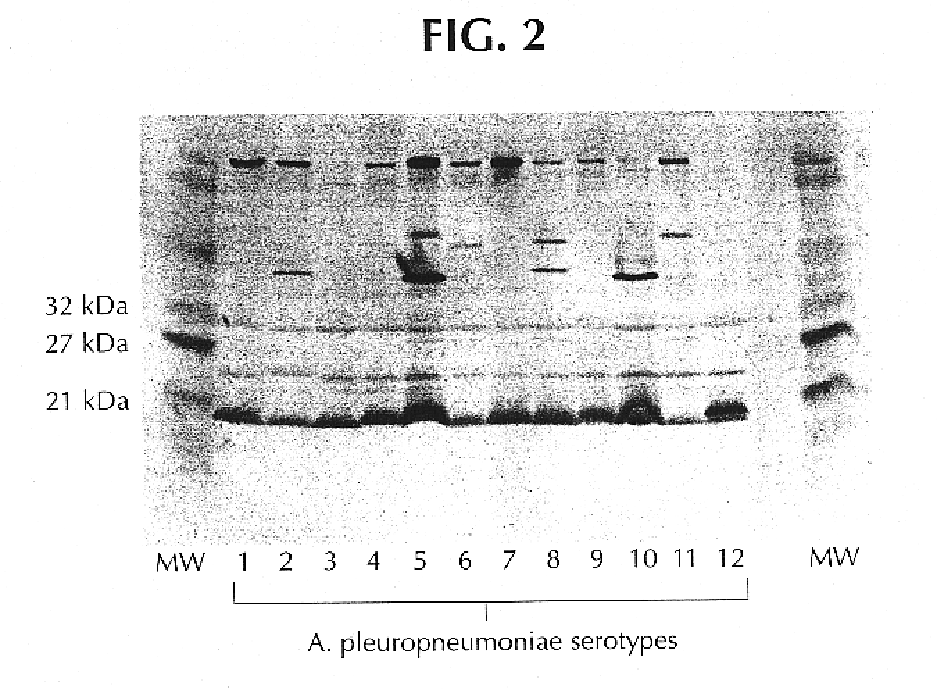
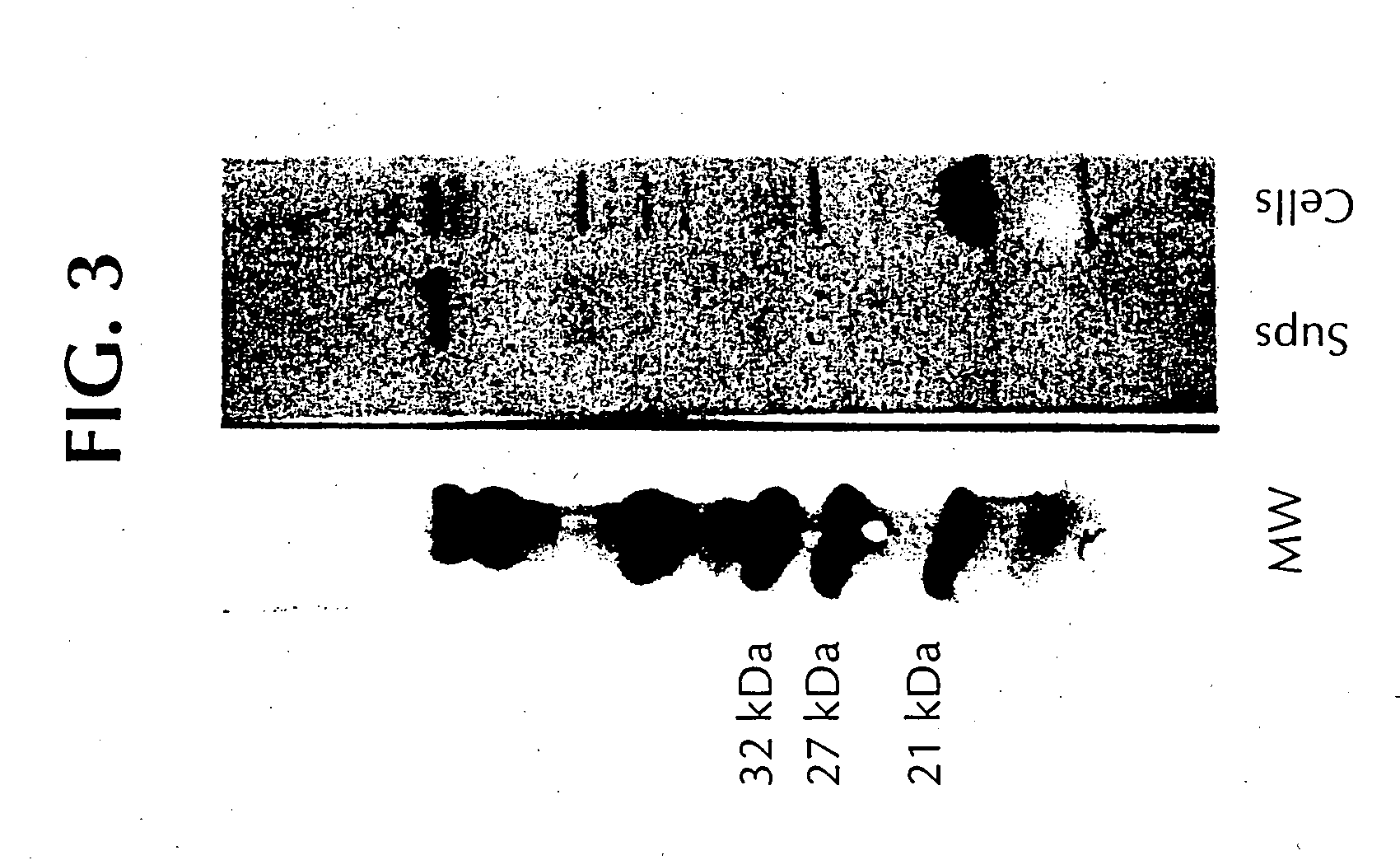
Proteins from actinobacillus pleuropneumoniae
InactiveUS6713071B1Bacterial antigen ingredientsPeptide preparation methodsActinobacillus pleuropneumoniaeProtection sex
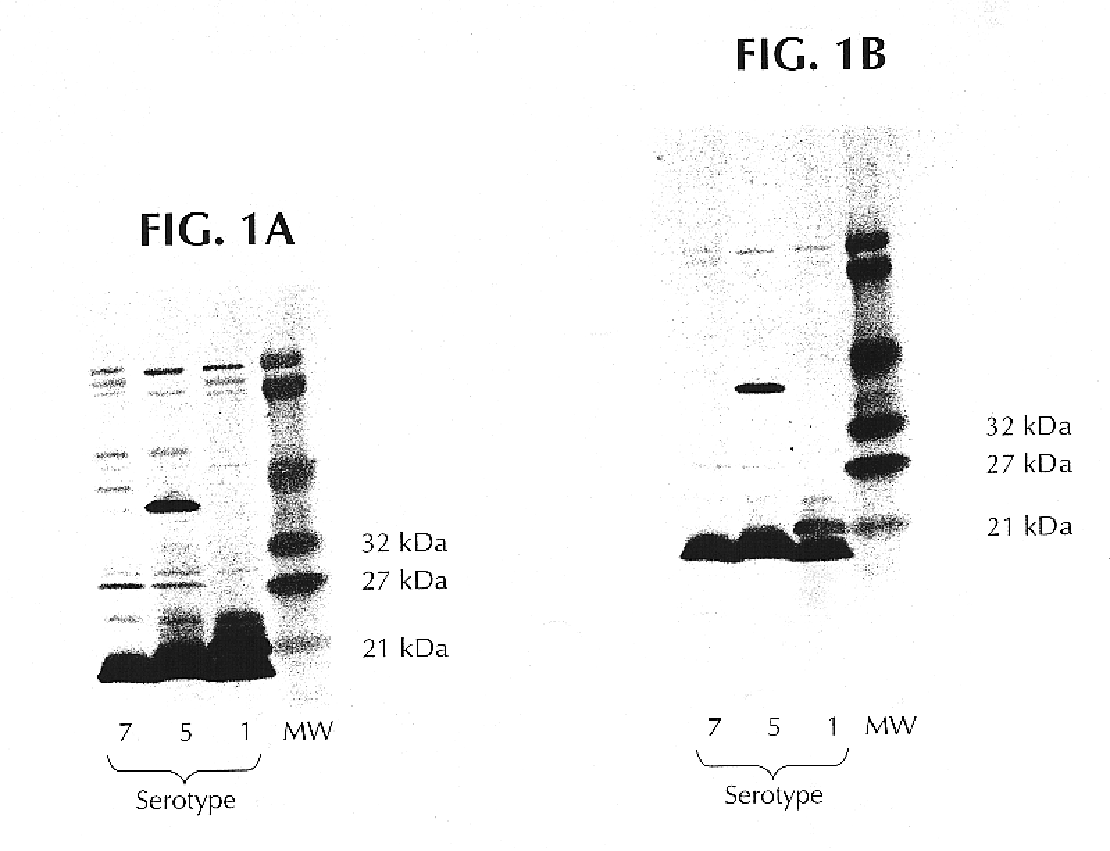
The present invention is directed to five novel, low molecular weight proteins from Actinobacillus pleuropneumoniae (APP), which are capable of inducing, or contributing to the induction of, a protective immune response in swine against APP. The present invention is further directed to polynucleotide molecules having nucleotide sequences that encode the proteins, as well as vaccines comprising the proteins or polynucleotide molecules, and methods of making and using the same.
Owner:PFIZER INC +1
Recombination outer membrane protein, coding gene and expression vector of porcine actinobacillus pleuropneumoniae (APP) and preparation method thereof
InactiveCN101691396AHigh yieldGood antigenicityMicroorganism based processesBacteria peptidesNucleotideA-DNA
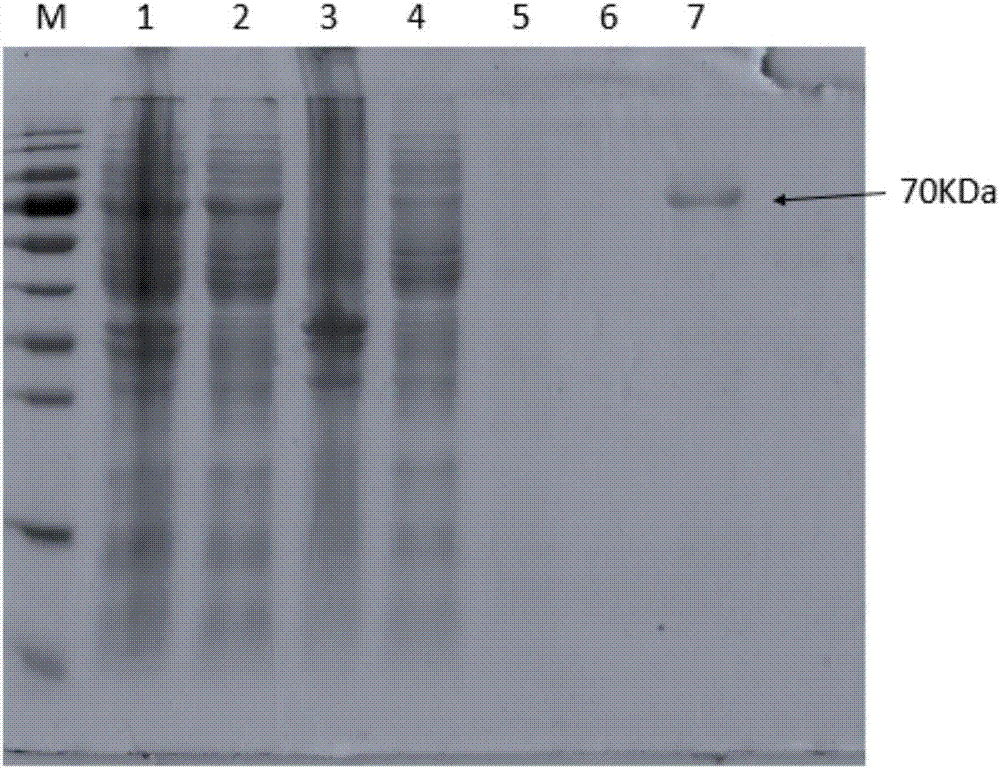
The invention discloses a recombination outer membrane protein, a coding gene and an expression vector of porcine actinobacillus pleuropneumoniae (APP) and a preparation method thereof. The porcine actinobacillus pleuropneumoniae (APP) comprises recombination outer membrane protein (a) or (b), wherein (a) is a protein with amino acid sequence shown as SEQ NO.2, and (b) is a protein with immunological competence, which is derived from (a) and is obtained by replacing, deleting or adding one or a plurality of amino acids in the amino acid sequence of (a). The coding gene of the porcine actinobacillus pleuropneumoniae (APP) is one of the nucleotide sequences: (1) a DNA sequence shown as SEQ NO.1; and (2) a DNA sequence which has more than 90% of homology with the DNA sequence defined by SEQ NO.1 and codes proteins with the same function. By utilizing the gene provided by the invention, high expression product yield can be obtained, 13mg of recombination protein can be obtained in every 100mlLB culture medium, and the recombination protein has better antigenicity.
Owner:TIANJIN AGRICULTURE COLLEGE
ApNGT gene of actinobacillus pleuropneumoniae and application of ApNGT gene
InactiveCN105505959ABasement efficiency improvementHigh stereoselectivityTransferasesPeptide preparation methodsEscherichia coliActinobacillus pleuropneumoniae
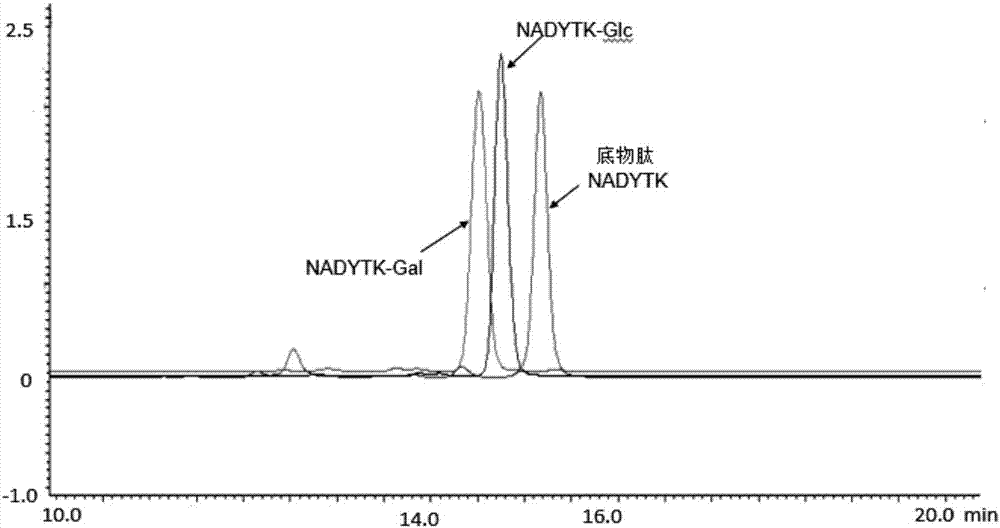
The invention relates to specificity mutation and application of nucleotide at the 1405th bit and the 1406th bit of the ApNGT gene (NCBI:ACCESSION A3N2T3) of actinobacillus pleuropneumoniae. The nucleotide is the nucleotide shown in SEQ ID NO:1 or the nucleotide shown in SEQ ID NO:2. ApNGT is glycosylation transferase coming from actinobacillus pleuropneumoniae, can recognize the N-X-S / T sequence in polypeptide or protein, and can transfer Glucose (Glc) to an Asn residue from an activated donor UDP-Glc. After mutated ApNGT is expressed in actinobacillus pleuropneumoniae, the glycosylation efficiency at the polypeptide level is improved by 160 or more times compared with wild ApNGT, great convenience is provided for galactosylated modification of polypeptide and protein type biological products. Stability of mutated ApNGT is good, and ApNGT can be used as a tool enzyme, can be easily produced in a commercialized mode and has wide application prospects.
Owner:NANKAI UNIV
Conserved inner core lipopolysaccharide epitopes as multi-species vaccine candidates
InactiveCN101014698AEsterified saccharide compoundsAntibacterial agentsDiseaseMANNHEIMIA HAEMOLYTICA
A conserved inner-core oligosaccharide epitope expressed on the lipopolysaccharide (LPS) of a range of disease causing pathogenic bacterial isolates, including Actinobacillus pleuropneumoniae (Ap), Mannheimia haemolytica (Mh) and Pasteurella multocida (Pm), is disclosed. Construction of a mutant bacterial strain exclusively expressing the conserved inner core OS epitope as a terminally exposed structure has allowed the identification, production and isolation of an inner core LPS which is common to all three organisms. Further provided are associated vaccines, antibodies raised against the conserved LPS inner core and glycoconjugates comprising the LPS inner core linked to an immunogenic carrier.
Owner:NAT RES COUNCIL OF CANADA
Preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and vaccine of APP OMVs
ActiveCN105420161AExperimental evaluation of immunostimulatory effectsAssessing immunostimulatory effectsAntibacterial agentsBacterial antigen ingredientsIMMUNE STIMULANTSFiltration
The invention relates to a preparation method of APP (Actinobacillus Pleuropneumoniae) OMVs (Outer Membrane Vesicles) and a vaccine of the APP OMVs, and belongs to the technical field of biology. The preparation method comprises the steps of culturing APP shope strains in vitro by using a culture medium which is in iron ion limit, obtaining acellular cultural supernatant after carrying out centrifugation and 0.22-[mu]m filtration treatment, and preparing the OMVs released by germs through ultracentrifugation, wherein the observation through a transmission electron microscope shows that the diameter of most OMVs is 50 to 100 nm, the OMVs are used as subunit vaccines to carry out secondary intranasal immunization on a mouse, the weight of the OMV immune mouse is increased for a long time, and the visual forms of lungs have no obvious difference from a PBS (Phosphate Buffer Saline) immune group. Meanwhile, an experiment shows that the OMVs are efficient immune stimulants, not only can high-level IgG (Immunoglobulin G) be stimulated to be generated by mouse sera, but also high-level IgA (Immunoglobulin A) can be generated in mouse lungs, mucosal immunity of the mouse lungs is effectively stimulated, and a better application prospect of using the OMVs as the subunit vaccines is expressed.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Haemophilus parasuis detection kit and detection method thereof
ActiveCN104711359AHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesPasteurellaBacilli
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
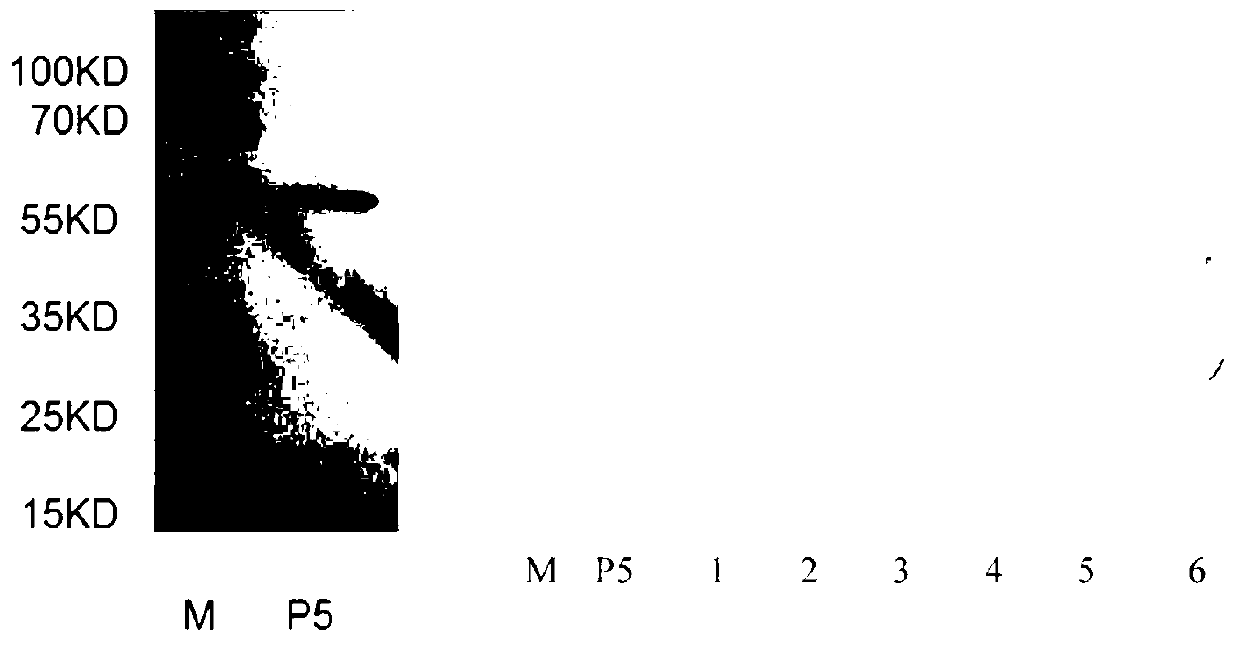
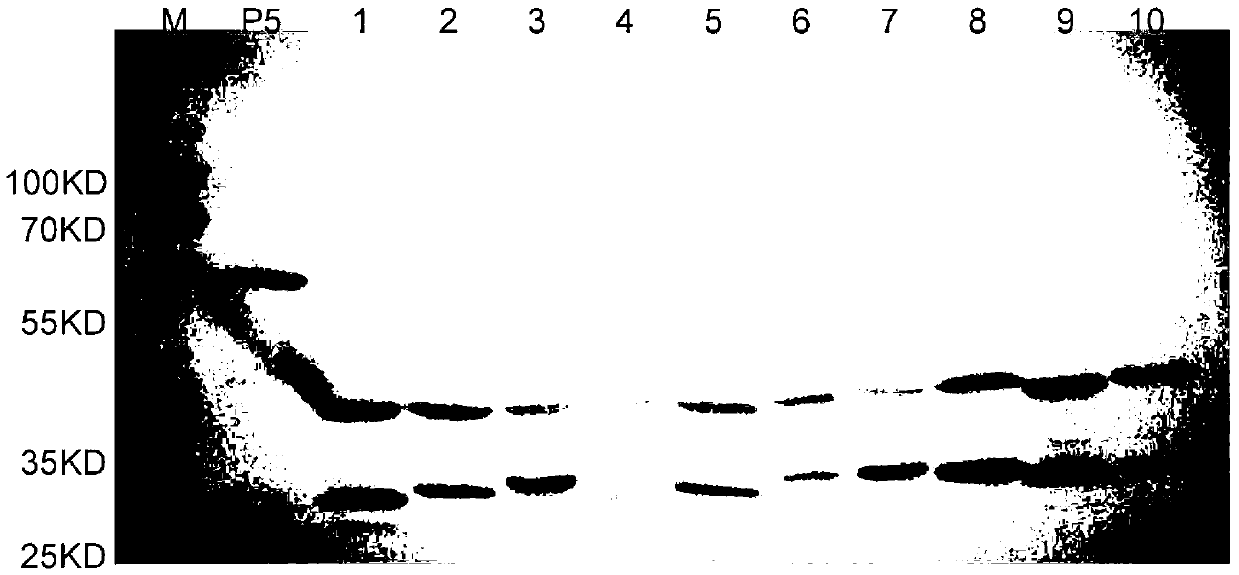
Haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, hybridoma cell strain and application
InactiveCN102876635ANo cross reactionStrong specificityImmunoglobulins against bacteriaMicroorganism based processesEscherichia coliBordetella
The invention discloses a haemophilus parasuis outer membrane protein P5 (OMP5) resistant monoclonal antibody, a hybridoma cell strain and an application. The hybridoma cell strain is preserved in the China center for type culture collection (CCTCC), and the preservation serial number is CCTCCC2012135. The monoclonal antibody prepared by the hybridoma cell strain is good in specificity, high in valence, high in generality, free from cross reaction with swine Escherichia coli, swine pasteurella, swine pleuropneumonia actinobacillus, streptococcus suis and swine bordetella bacilli, capable of detecting haemophilus parasuis with different serotypes and widely applicable to etiology diagnosis, serology detection and immunology detection and prevention of haemophilus parasuis diseases, and the enzyme-linked immuno sorbent assay (ELISA) antibody valence can reach 1:204800 after purification.
Owner:广东省农业科学院兽医研究所
Compound doxycycline-hydrochloride florfenicol sustained-release microsphere suspension injection for veterinary use
ActiveCN105287607AImprove clinical efficacyReduce clinical dosageAntibacterial agentsTetracycline active ingredientsSuspending AgentsVeterinary Drugs
The invention belongs to the technical field of veterinary drug preparation, and relates to a compound doxycycline-hydrochloride florfenicol sustained-release microsphere suspension injection for veterinary use. The suspension injection is produced through a preparation technology combining an inclusion technology, a microcapsule technology and a high-pressure homogenization technology. The suspension injection comprises the following ingredients according to W / V: 10-30% of an inclusion material, 5-20% of doxycycline hydrochloride, 5-20% of florfenicol, 2.5-7.5% of a high-molecular capsule material, 0.2-1% of a suspending agent, 0.25-1% of an anti-oxidant, 0.05-0.2% of a metal chelator, 0.1-0.4% of an antiseptic, and the balance injection water. The active ingredients in the injection possess synergic antibacterial effects and obvious sustained release effect, clinic dosing frequency is reduced, the injection does not contain organic solvents, does not stimulate target animals, is small in toxic and side effects, and is capable of controlling respiratory diseases caused by streptococcus suis, actinobacillus pleuropneumoniae, pasteurella multocida, haemophilus parasuis, mycoplasma and the like.
Owner:HUAZHONG AGRI UNIV
Bacterin for pleuropneumonia actinobacillus serotype 1 double-gene deletion mutant without resistance marker
InactiveCN101037664ANo longer cytotoxicNo longer activeAntibacterial agentsBacterial antigen ingredientsDeletion mutantWild strain
The invention belongs to animal bacterium gene engineering field, relates to form stem of Actinobacillus pleuropneumoniae APP-1-mut01 digene deletion mutants without fastness label, preparing vaccine and application thereof. The invention gets a stem of Actinobacillus pleuropneumoniae APP-1-mut01 (storing number is CCTCC NO; M207005). The stem absents two main toxin gene activity factors apxIC and apxIIC of Actinobacillus pleuropneumoniae APP-1-mut01, produces toxin albumen ApxIA and ApxIIA without toxin, but these two toxin albumen also have immunity originality. The invention also discloses producing vaccine of Actinobacillus pleuropneumoniae APP-1-mut01 using the digene deletion mutants stem and application thereof. The digene deletion vaccine can irritate pig generate protective immunity reaction to resist isogeny and nonhomology serum wild strain of actinobacillus pleuropneumoniae to prevent infection thereof.
Owner:HUAZHONG AGRI UNIV
Tildipirosin-containing medicinal composition
ActiveCN104906121AGood treatment effectExtended half-lifeAntibacterial agentsOrganic active ingredientsRespiratory tract diseaseCross-resistance
The invention provides a tildipirosin-containing medicinal composition. The dosage form of the medicinal composition is an injection, the composition comprises, by mass / volume, 1-20% of tildipirosin, 10-25% of polyvinylpyrrolidone, an acid, and the balance of water, and the use amount of the acid makes the pH value of the composition be 5.5-6.5; and the tildipirosin is tildipirosin or a pharmaceutically acceptable salt thereof. Tildipirosin in above raw materials is a special antibiotic for livestock and poultry, does not bring a cross drug resistance problem for people, is a first selected medicine for treating animal respiratory tract diseases, and has a very good treatment effect on Streptococcus suis, Pasteurella multocida, Haemophilus parasuis, Actinobacillus pleuropneumoniae and mycoplasma.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
N glycosyl transferase AaNGT and application thereof
InactiveCN107034202ATransferasesPeptide preparation methodsActinobacillus pleuropneumoniaeGlycopeptide
The invention discloses a N glycosyl transferase AaNGT. An amino acid sequence thereof is shown as SEQ ID NO.1. The invention also discloses an application of the N glycosyl transferase AaNGT in polypeptide galactosylated modification. AaNGT is capable of supplying a simple and convenient method for the forming of glycopeptide; the capacity of AaNGT for utilizing UDP-Glc is obviously higher than that of the reported N glycosyl transferase ApNGT which is sourced from Actinobacillus pleuropneumoniae; the AaNGT also can transfer the special glycosyl donor UDP-GlcNH2 to the polypeptide containing N glycosylation sites, and then the other glycosyl transferases can be utilized to convert the GlcNH2 into the GlcNAc so as to form natural N glycan linking, so that a new method is supplied for the development of the glycoprotein vaccine. The invention is beneficial to the AaNGT becoming a protein modified tool enzyme.
Owner:SHANDONG UNIV
Infectious actinobacillus pleuropneumoniae and application thereof
ActiveCN107201326AHigh proliferative titerStrong pathogenicityAntibacterial agentsBacteriaDiseaseBacteroides
The invention discloses an infectious actinobacillus pleuropneumoniae and application thereof. The infectious actinobacillus pleuropneumoniae is an actinobacillus pleuropneumoniae HNAPP1 with a preservation number of CGMCC NO:13333 and preserved on November 22, 2016 in the China General Microbiological Culture Collection Center (3#, 1# Yard, West Beichen road, Chaoyang District, Beijing). The strain HNAPP1 provided by the invention is separated from a heart of an acute-death fattening pig with typical dyspnea, no other bacteria grow but only the infectious actinobacillus pleuropneumoniae grows during separation on a TSA solid culture medium, and the proliferation titer in a TSB liquid culture medium is high and reaches 109 CFU / mL or above. The strain HNAPP1 in the invention has strong pathogenicity for nursery pigs to cause diseases and death to the nursery pigs, and has high immunogenicity.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Pair of specific primers for detecting Bergeyella, kit and PCR detection method
PendingCN109055588ARapid identificationEasy to identifyMicrobiological testing/measurementAgainst vector-borne diseasesP. multocidaSpecific primers
The invention discloses a pair of specific primers for detecting Bergeyella, a kit and a PCR detection method. The primers comprise an upstream primer Bergeyella-F: 5'-TTGAAAGCTCCGGCGGATAG-3' and a downstream primer Bergeyella-R: 5'-ACCCTCACGAGAGTAGGTTT-3'. According to the specific primers, the kit and the PCR detection method, streptococcus, bacillus erysipelatos-suis, pasteurella multocida, salmonella, haemophilus parasuis, infectious actinobacillus pleuropneumoniae, stenotrophomonas maltophilia, pig Bergeyella and a Bergeyella zoohelcum 16S rRNA gene sequence are downloaded from a GenBankdatabase; sequence comparison is carried out through MEGA 5.05 software to search a Bergeyella 16S rRNA specific sequence; and Primer-BLAST design is utilized to amplify a pair of specific primers ofBergeyella; and a Bergeyella PCR rapid detection method is established to identify Bergeyella. The method has the advantages of being rapid, convenient, high in sensitivity and strong in specificity.
Owner:HENAN UNIV OF ANIMAL HUSBANDRY & ECONOMY
LAMP detection kit for actinobacillus pleuropneumoniae
PendingCN111575394ASimple and fast operationMild conditionsMicrobiological testing/measurementMicroorganism based processesActinobacillus pleuropneumoniaeBiochemistry
The invention discloses an LAMP detection kit for actinobacillus pleuropneumoniae. The LAMP detection kit is provided with a detection primer group and an internal standard primer group, wherein the detection primer group comprises a detection outer primer pair, a detection inner primer pair and a detection loop primer pair, and the internal standard primer group comprises an internal standard outer primer pair, an internal standard inner primer pair and an internal standard loop primer pair. The LAMP detection kit has the beneficial effects of rapidness, high efficiency, simplicity and convenience in operation, high specificity, high sensitivity, low cost, no need of expensive instruments, suitability for field detection and the like, and more importantly, a use method improves the detection accuracy, false negative results in detection can be discovered in time, and various accidents caused by the false negative detection result can be effectively prevented.
Owner:XIAMEN YINXIANG GROUP
Porcine actinobacillus pleuropneumoniae attenuated strain and porcine pleuropneumonia-preventing product prepared from same
InactiveCN104726387AReduced toxicityLow toxicityAntibacterial agentsBacterial antigen ingredientsBiotechnologyImmunogenicity
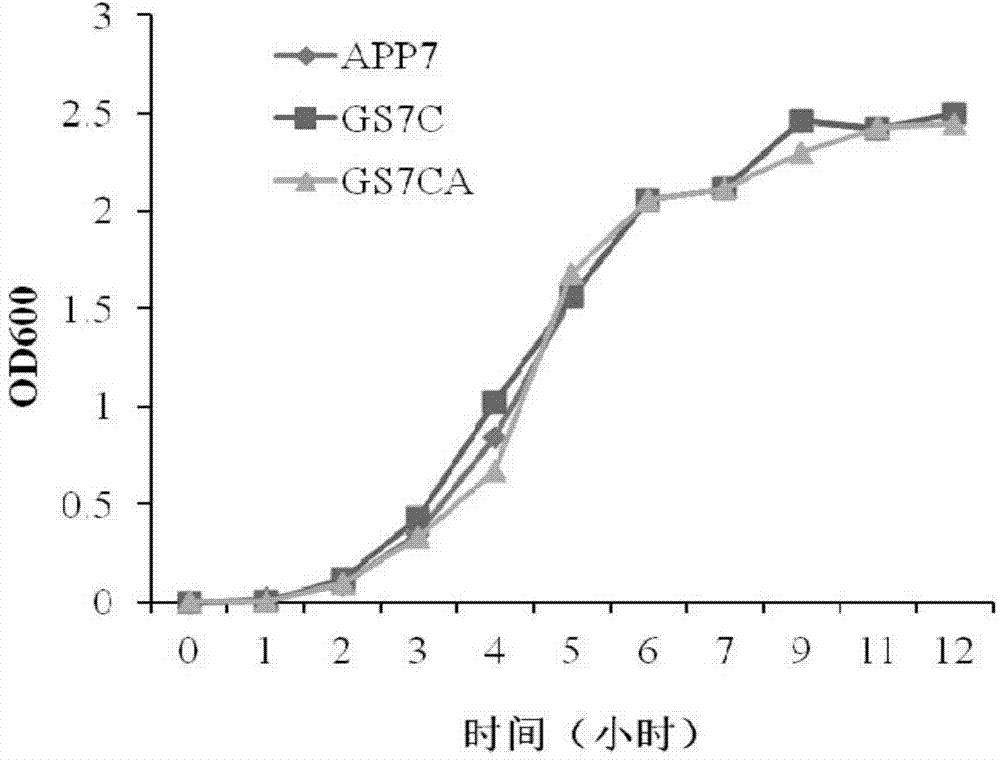
The invention discloses a porcine actinobacillus pleuropneumoniae attenuated strain and a porcine pleuropneumonia-preventing product prepared from the same, and relates to the fields of microorganisms and immunology. According to the porcine actinobacillus pleuropneumoniae attenuated strain and the porcine pleuropneumonia-preventing product prepared from the same disclosed by the invention, on the basis of a single mutant strain GS7C, a ureC gene segment in a genome is further deleted, and an apxIII-N gene segment with immunogenicity is inserted, and then the double mutant strains (GS7CA) of apxIIC- / apxIA+ and ureC- / apxIII+ which successfully express ApxIII-N proteins are screened by virtue of a sacB negative gene screening system, and the collection number is CGMCC NO. 10016. Experimental data indicates that, the hemolytic activity and urease activity of the double mutant strains are completely lost, the toxicity is greatly reduced, and stable inheritance can be realized; the attenuated live vaccine prepared by the porcine actinobacillus pleuropneumoniae attenuated strain disclosed by the invention has low toxicity, cross protection activity, high bio-safety and stable quality.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Novel proteins from Actinobacillus pleuropneumoniae
InactiveUS20040198954A1Peptide preparation methodsDepsipeptidesActinobacillus pleuropneumoniaeNucleotide
The present invention is directed to five novel, low molecular weight proteins from Actinobacillus pleuropneumoniae (APP), which are capable of inducing, or contributing to the induction of, a protective immune response in swine against APP. The present invention is further directed to polynucleotide molecules having nucleotide sequences that encode the proteins, as well as vaccines comprising the proteins or polynucleotide molecules, and methods of making and using the same.
Owner:ANKENBAUER ROBERT G +6
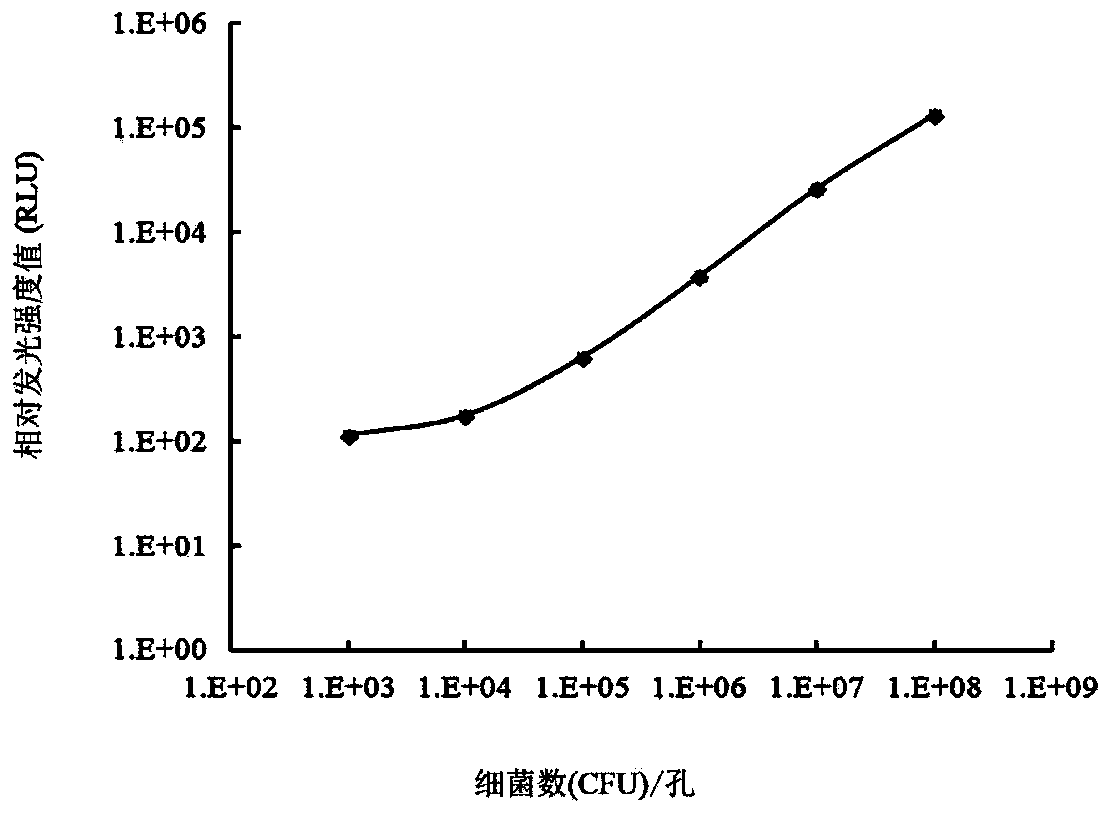
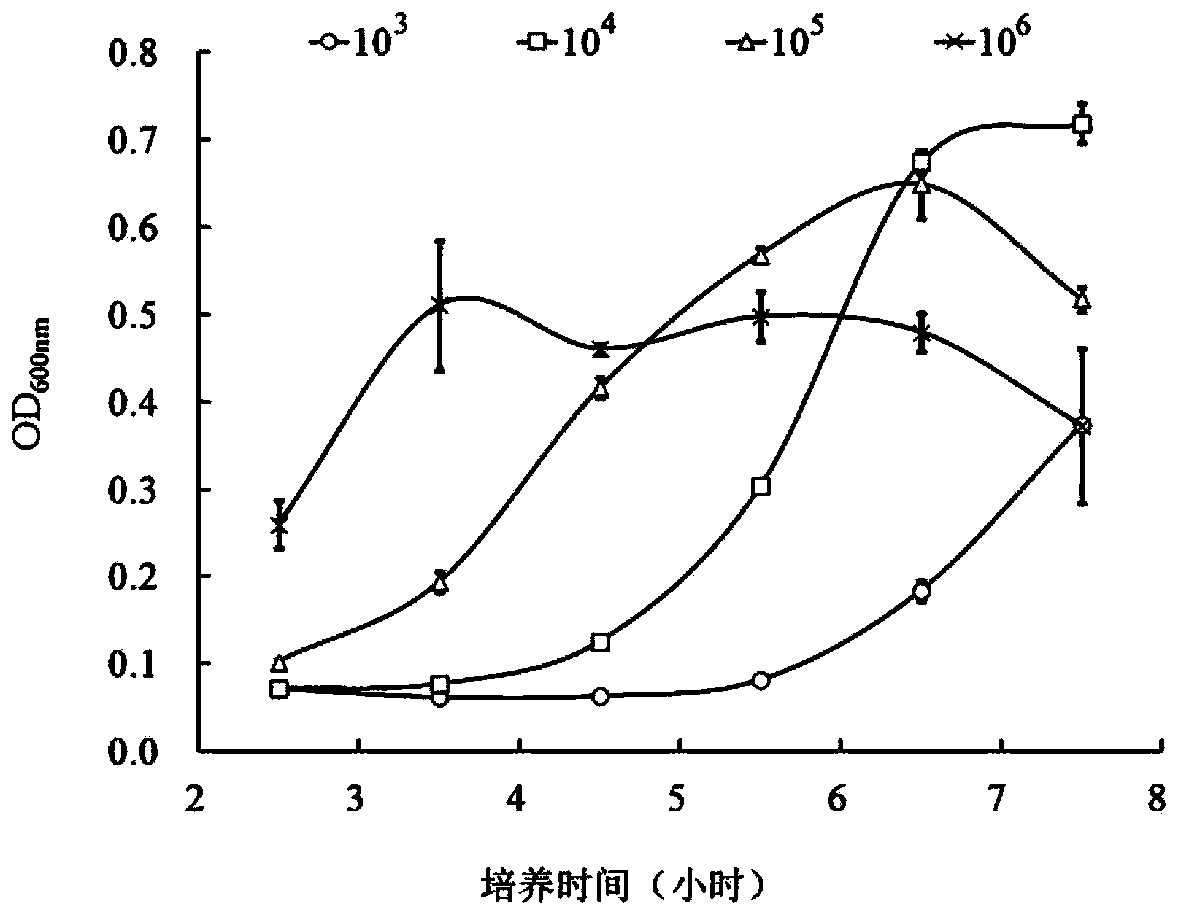
High throughput screening method for antimicrobial compounds and application of high throughput screening method
InactiveCN103421876AHigh sensitivityShort detection cycleMicrobiological testing/measurementMicroorganism based processesEscherichia coliBacteroides
The invention belongs to the technical field of veterinary microbiology and veterinary pharmacy and particularly relates to a high throughput screening method for antimicrobial compounds and the application of the high throughput screening method. Actinobacillus pleuropneumoniae isolates HB0503 resisting six antibiotics are taken as target bacteria; tetracycline is taken as positive control; an opaque 96-well plate is taken as a screening carrier; the number of live bacteria is measured through the chemiluminescence; the antibacterial effect of the antibacterial compound is identified. According to the quality determination criterion of the high throughput screening method, the bacterial turbidimetry is compared with the chemiluminescence, and the chemiluminescence is determined as the optimal solution. Eight compounds are indentified to have inhibitory activity to drug-resistant actinobacillus pleuropneumoniae strains, wherein four of the eight compounds has relatively good bacteriostatic activity to haemophilus parasuis, escherichia coli, streptococcus suis type 2and staphylococcus aureus.
Owner:HUAZHONG AGRI UNIV
Haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae triple inactivated vaccine and application thereof
PendingCN110075289AStrong pathogenicityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention relates to the technical field of animal biological products and specifically relates to a haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae triple inactivatedvaccine. The inactivated vaccine includes an inactivated haemophilus parasuis HNHPS1 serotype antigen, an inactivated haemophilus parasuis strain HN1570 serotype antigen, an inactivated streptococcussuis strain HNSS1 serotype antigen and an inactivated actinobacillus pleuropneumoniae strain HNAPP1 antigen; the four strains all have excellent immunogenicity; when the four strain inactivated antigens are used for preparing the vaccine, the infection of clinic haemophilus parasuis, streptococcus suis and actinobacillus pleuropneumoniae can be effectively prevented; the vaccine is characterizedby simple preparation process, high safety, excellent immune effect and long immunity period and meanwhile is capable of achieving the purposes of preventing various diseases with one injection, reducing cost and reducing stress.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
Multiple PCR detection method for porcine bacteria
InactiveCN101984073AQuick checkAccurate detectionMicrobiological testing/measurementP. multocidaMultiplex pcrs
The invention provides a multiple PCR detection method for porcine bacteria. Streptococcus suis type 2, Actinobacillus pleuropneumoniae and pasteurella multocida positive samples are detected by extracting the DNA of test samples, carrying out PCR amplification with PCR testing kit and analyzing the amplified products.The method of the invention can rapidly and accurately detect the samples infected with one or more of the above three bacteria. The template DNA preparation step of the method is simple and the cost is low. The method can exclude the interferences of bacteria and impurity particles, thus greatly improving diagnostic accuracy and reducing false positive rate. The method can also be used for the molecular epidemiological investigation and the efficacy monitoring of Streptococcus suis type 2, Actinobacillus pleuropneumoniae and pasteurella multocida.
Owner:ZHEJIANG UNIV
Pichia anomala as well as fermentation culture and application thereof
ActiveCN107937286AResistant to gastric acidPromote growthAntibacterial agentsFungiEscherichia coliPhytase
The invention belongs to the technical field of biology, and discloses pichia anomala as well as a fermentation culture and application thereof. The pichia anomala is pichia anomala AR2016, CCTCC NO:M2017594. The fermentation culture medium provided by the invention is used for liquid state fermentation; the biomass of the fermentation liquid is 15g / L; the yield of the Killer toxin is 5.86mg / L; the yield of beta-1,3-glucanase is 964 U / L; the yield of phytase is 1675 U / L. The pichia anomala provided by the invention has the effects of inhibiting dysentery colibacillus, salmonella pullorum, sensitive C type candida albicans, actinobacillus pleuropneumoniae, sarcina lutea and staphylococcus aureus and resisting gastric acid and bile salts. Therefore the pichia anomala provided by the invention can be used as feed additives for livestock, poultry and aquatic animals; wide application prospects are realized.
Owner:SOUTHWEST UNIV
Bacteriophage having bactericidal activity with respect to actinobacillus pleuropneumoniae
ActiveUS20140377842A1Little side effectsWeakened immunityAntibacterial agentsViral/bacteriophage medical ingredientsActinobacillus pleuropneumoniaeBacteroides
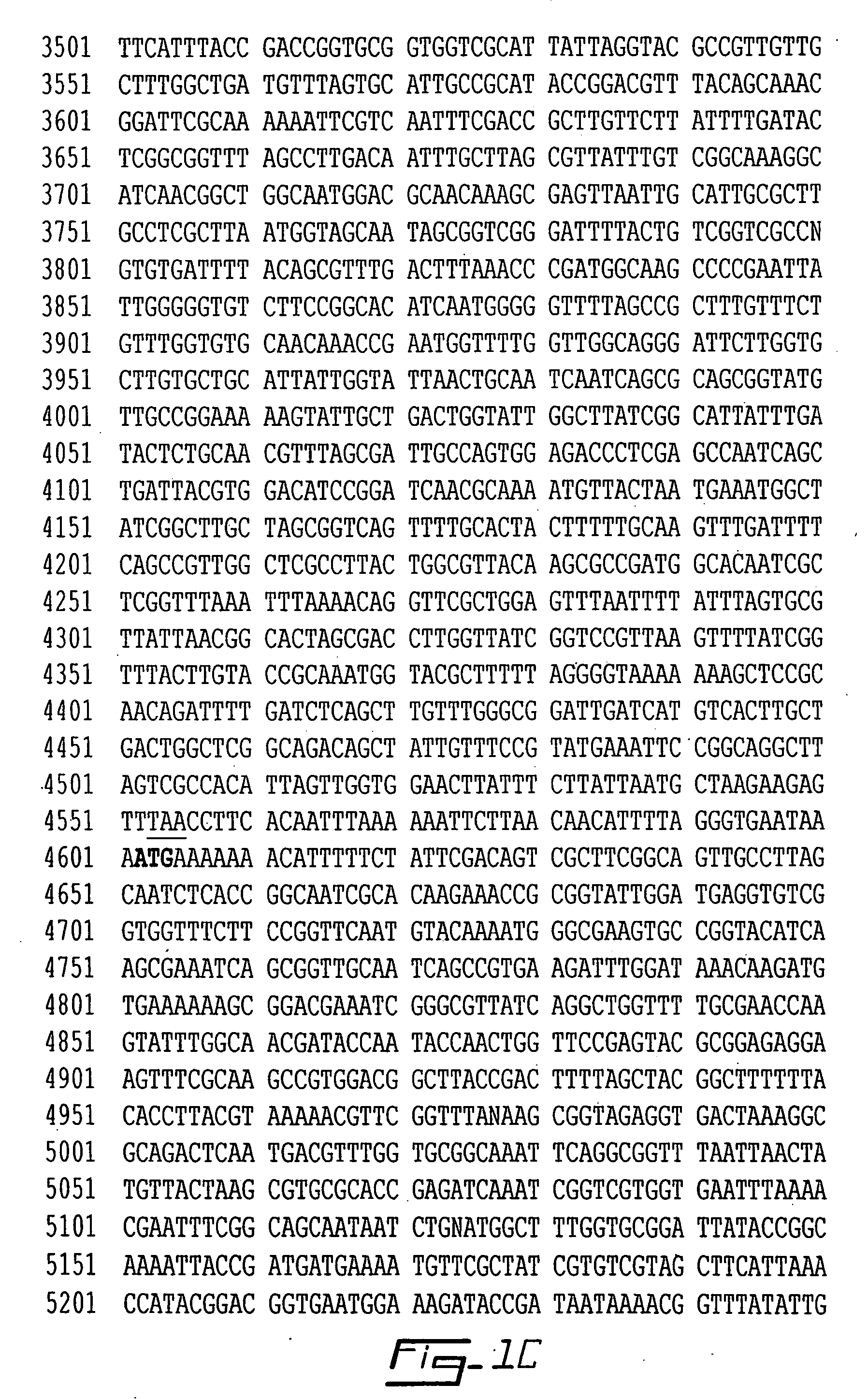
The present invention relates to a bacteriophage having bactericidal activity against Actinobacillus pleuropneumoniae. Bacteriophage PA1Φ can infect Actinobacillus pleuropneumoniae and kill the same bacteria and is characterized by the genome of 34,553 by represented by SEQ ID NO: 1.
Owner:INTRON BIOTECHNOLOGY INC
Antigens for actinobacillus pleuropneumoniae and methods thereof
The present invention is related to a DNA sequence of outer membrane proteins of Actinobacillus pleuropneumoniae expressed in a subject suffering from pleuropneumonia, the sequence coding for the receptor for the uptake of ferric hydroxamate FhuA as set forth in SEQ ID NO: 1 in Actinobacillus pleuropneumoniae, or any functional fragment thereof. The present invention also relates to a DNA sequence of outer membrane proteins of Actinobacillus pleuropneumoniae expressed in a subject suffering from pleuropneumonia, the sequence coding for a hemoglobin-binding protein as set forth in SEQ ID NO: 4 in Actinobacillus pleuropneumoniae, or any functional fragment thereof. The present invention further relates to composition, diagnostic kit and methods using the DNA sequences of the present invention.
Owner:JACQUES MARIO +4
Kit of actinobacillus pleuropneumoniae and use thereof
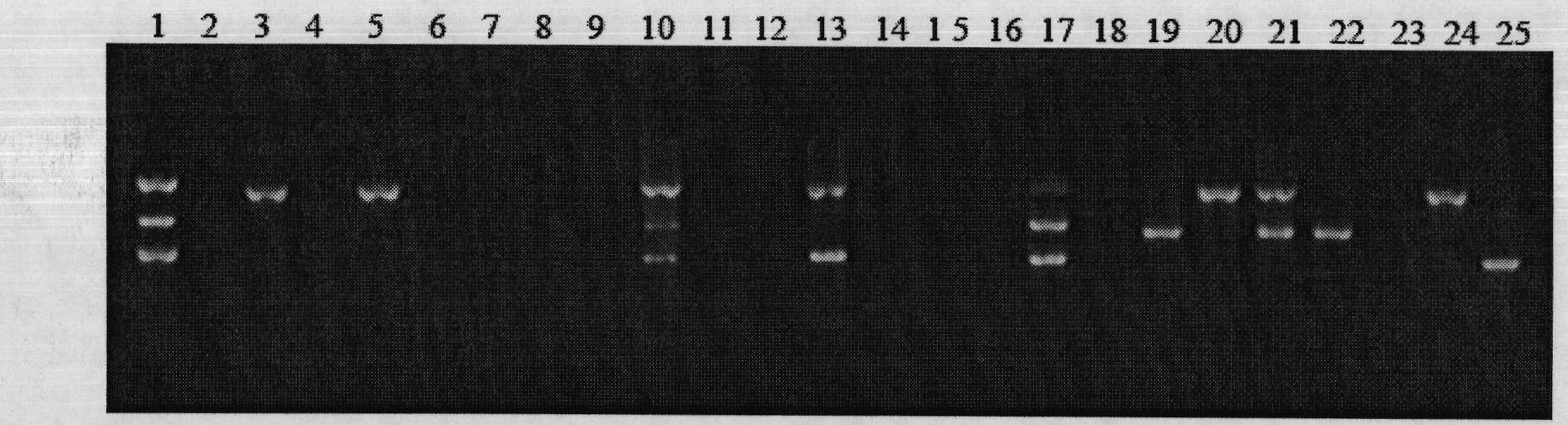
InactiveCN100567504CStrong specificityHigh sensitivityMicrobiological testing/measurementActinobacillus pleuropneumoniaePositive control
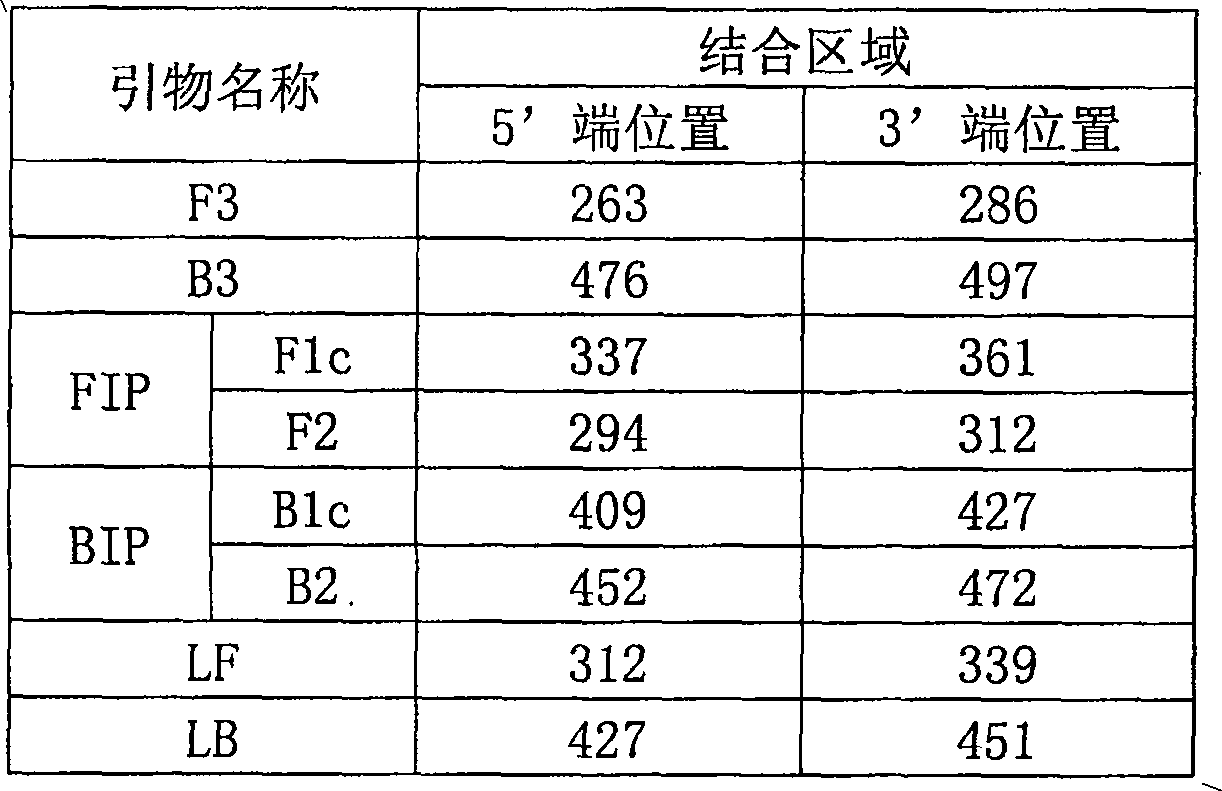
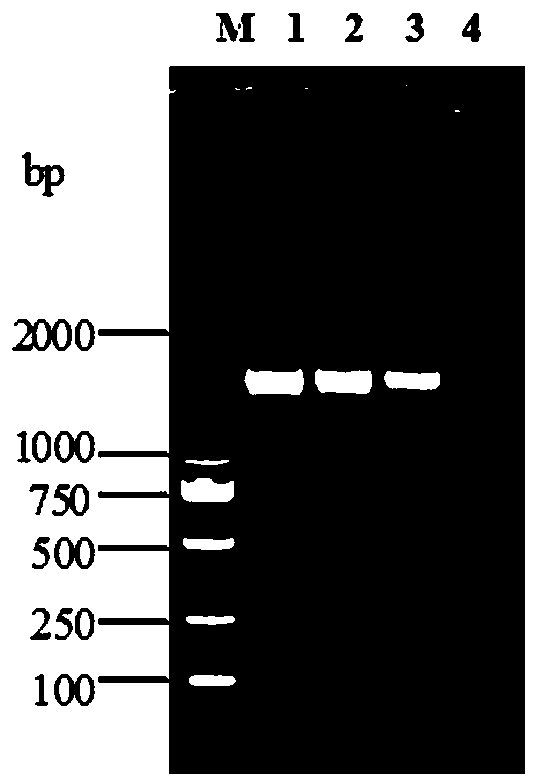
The invention discloses a detection kit for Actinobacillus pleuropneumoniae and its application. The kit provided by the present invention includes three pairs of primers, namely an inner primer pair, an outer primer pair and a circular primer pair combined with the 3' end 1000bp gene in the Actinobacillus pleuropneumoniae Gen Bank Accession Number AF021919 sequence. The kit also includes a loop-mediated isothermal amplification reagent, a positive control, a negative control and a fluorescent chromogenic reagent, and the positive control is the DNA of Actinobacillus pleuropneumoniae. The invention also provides a method for detecting whether the animal carries the Actinobacillus pleuropneumoniae by using the kit of the invention. The kit of the present invention has high detection sensitivity, 6-10 copies can detect the target DNA, is simple and convenient to operate, and is especially suitable for the detection of pathogens carried out at the grassroots level and the detection of Actinobacillus pleuropneumoniae in possibly contaminated animal food .
Owner:CHINA AGRI UNIV
Medicine formula for eliminating in-vivo enolotoxin
ActiveCN104800220AEffective treatmentInhibition of reproductionAntibacterial agentsAntipyreticEscherichia coliFLUNIXIN MEGLUMINE
The invention relates to a medicine formula for eliminating in-vivo enolotoxin. The medicine formula comprises a formula A and a formula B, wherein the formula A comprises raw materials in parts by weight as follows: 1-4 parts of flunixin meglumine and 1-6 parts of vitamin C; the formula B comprises raw materials in parts by weight as follows: 2-8 parts of ceftiofur sodium and 1.5-5 parts of enrofloxacin, wherein flunixin meglumine is an injection with a specification of 0.05g / ml, the vitamin C is an injection with a specification of 0.1g / ml, enrofloxacin is an injection with a specification of 0.05g / ml, and ceftiofur sodium is a freeze-dried powder injection. The medicine formula can effectively eliminate enolotoxin causing diseases to animals, has high sensitivity to pathogenic bacteria such as escherichia coli, infectious actinobacillus pleuropneumoniae, haemophilus parasuis and the like, can be used for effectively treating diseases such as hydropsy, infectious pleuropneumonia, fevering, loss of appetite and the like caused by the pathogenic bacteria and has remarkable curative effects.
Owner:SICHUAN CHENGKANG ANIMAL PHARMA
Conserved Inner Core Lipopolysaccharide Epitopes as Multi-Species Vaccine Candidates
InactiveUS20080008723A1Poorly immunogenicImproving immunogenicityAntibacterial agentsCosmetic preparationsBacteroidesDisease
A conserved inner-core oligosaccharide epitope expressed on the lipopolysaccharide (LPS) of a range of disease causing pathogenic bacterial isolates, including Actinobacillus pleuropneumoniae (Ap), Mannheimia haemolytica (Mh) and Pasteurella multocida (Pm), is disclosed. Construction of a mutant bacterial strain exclusively expressing the conserved inner core OS epitope as a terminally exposed structure has allowed the identification, production and isolation of an inner core LPS which is common to all three organisms. Further provided are associated vaccines, antibodies raised against the conserved LPS inner core and glycoconjugates comprising the LPS inner core linked to an immunogenic carrier.
Owner:NAT RES COUNCIL OF CANADA
Conserved inner core lipopolysaccharide epitopes as multi-species vaccine candidates
InactiveUS7759070B2Wide coveragePreventing human diseaseAntibacterial agentsOrganic active ingredientsDiseaseMANNHEIMIA HAEMOLYTICA
A conserved inner-core oligosaccharide epitope expressed on the lipopolysaccharide (LPS) of a range of disease causing pathogenic bacterial isolates, including Actinobacillus pleuropneumoniae (Ap), Mannheimia haemolytica (Mh) and Pasteurella multocida (Pm), is disclosed. Construction of a mutant bacterial strain exclusively expressing the conserved inner core OS epitope as a terminally exposed structure has allowed the identification, production and isolation of an inner core LPS which is common to all three organisms. Further provided are associated vaccines, antibodies raised against the conserved LPS inner core and glycoconjugates comprising the LPS inner core linked to an immunogenic carrier.
Owner:NAT RES COUNCIL OF CANADA
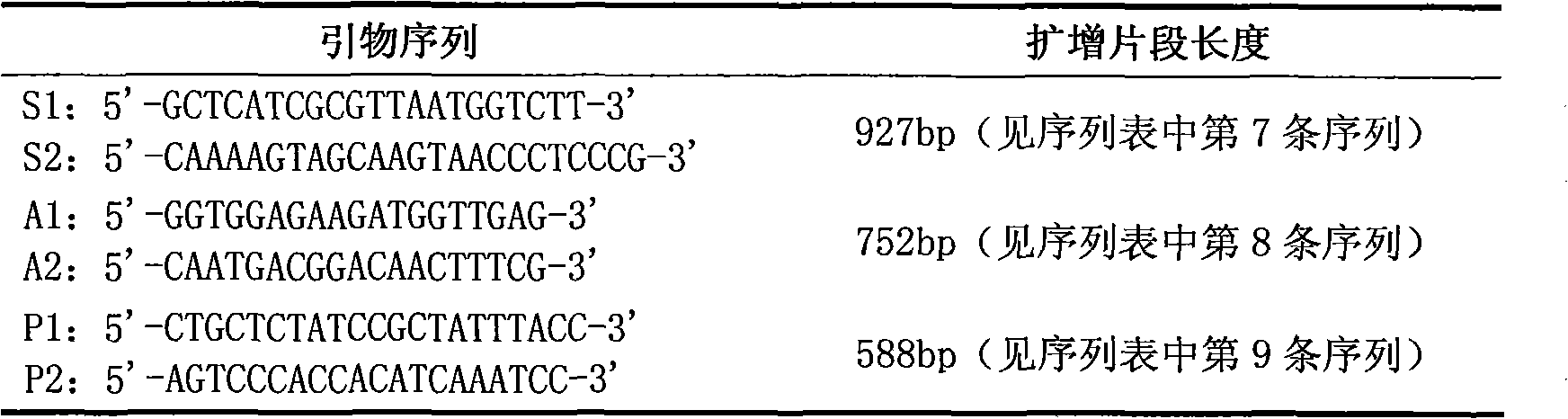
Compound PCR typing kit for distinguishing serotypes of eight pig actinobacillus pleuropneumoniae and application thereof
ActiveCN108676900AImprove accuracyHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPositive controlNucleotide
The invention belongs to the technical field of biotechnology, and particularly relates to a composite polymerase chain reaction (PCR) typing kit for distinguishing serotypes of eight pig actinobacillus pleuropneumoniae and application thereof. The composite PCR typing kit comprises a PCR test tube, a PCR mixed enzyme, five pairs of specific primers, a positive control, a negative control and ddH2O, wherein the nucleotide sequences of the five pairs of specific primers are shown by SEQ ID NO:1-10. The composite PCR typing kit is used for distinguishing serotypes of eight pig pleuropneumonia actinobacillus of type 1, type 3, type 4, type 5, type 6, type 10, type 12 and type 14, and has high accuracy, good specificity and sensitivity up to a pg level. Sources of samples for identification include isolated strain resources or animal tissues, and a tissue washing liquid or a single colony and a bacterial solution can be directly cracked by boiling water as a template, thus eliminating tedious extraction of bacterial genomes and greatly saving time, manpower and cost.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Monoclonal antibody of hemophilus parasuis resistant OMP5 (outer membrane protein), hybridoma cell strain HPS1E2 and application
InactiveCN102876636ANo cross reactionStrong specificityImmunoglobulins against bacteriaTissue cultureEscherichia coliBordetella
The invention discloses a monoclonal antibody of hemophilus parasuis resistant OMP5 (outer membrane protein), a hybridoma cell strain HPS1E2 and application. The hybridoma cell strain is preserved in a Chinese typical culture preservation center, and the preservation number is CCTCCC2012138. The monoclonal antibody prepared with the hybridoma cell strain is fine in specificity, high in valence and high in universality, is not in cross reaction with swine Escherichia coli, swine pasteurella, actinobcillus pleuropneumoniae, streptococcus suis and swine bordetella, the valence of the purified ELISA (enzyme-linked immuno sorbent assay) antibody can reach 1:409600, hemophilus parasuis with different serotypes can be detected, and the monoclonal antibody can be widely used for etiology diagnosis, serological test, immunological test, prevention and treatment and the like of hemophflussuis.
Owner:广东省农业科学院兽医研究所
Quadruple inactivated vaccine of mycoplasma pneumoniae and haemophilus parasuis, streptococcus suis, and actinobacillus pleuropneumoniae and application thereof
ActiveCN110124022ATypical asthma symptomsStrong pathogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseImmunogenicity
The invention relates to the technical field of veterinary biological products, in particular to a quadruple inactivated vaccine of mycoplasma pneumoniae and haemophilus parasuis, streptococcus suis,and actinobacillus pleuropneumoniae. The quadruple inactivated vaccine comprises inactivated mycoplasma hyopneumoniae strain HNMhy1 serotype antigen, inactivated haemophilus parasuis strain HNHPS1 serotype antigen, inactivated haemophilus parasuis strain HN1570 serotype antigen, inactivated streptococcus suis strain HNSS1 serotype antigen, inactivated actinobacillus pleuropneumoniae strain HNAPP1serotype antigen, and an immune adjuvant, the five strains have good immunogenicity and can prevent the infection of clinical mycoplasma pneumoniae and haemophilus parasuis, streptococcus suis, and actinobacillus pleuropneumoniae, the quadruple inactivated vaccine has good safety, good immune effect and long immunization period, can achieve a purpose of preventing various diseases by one-time injection, and reduces costs.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY MEDICINE HENAN ACAD OF AGRI SCI
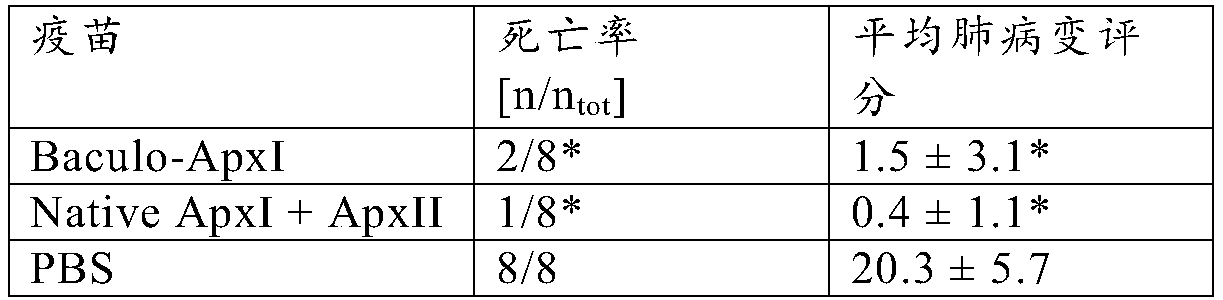
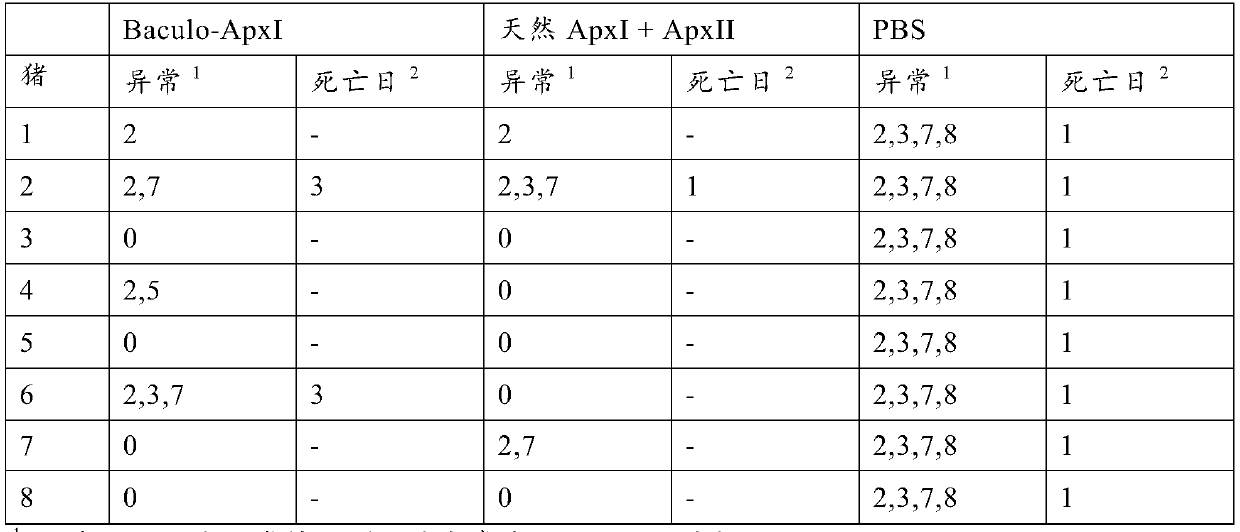
Vaccine to protect a pig against actinobacillus pleuropneumoniae
Owner:INTERVET INT BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com