Quadruple inactivated vaccine of mycoplasma pneumoniae and haemophilus parasuis, streptococcus suis, and actinobacillus pleuropneumoniae and application thereof
A technology of Mycoplasma hyopneumoniae and Haemophilus suis, applied in vaccines, multivalent vaccines, veterinary vaccines, etc., can solve the problems of lack of immune cross-protection, achieve good immunogenicity, reduce costs, and reduce stress effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, the separation and identification of Mycoplasma hyopneumoniae
[0052] 1.1 Collection of disease data
[0053] The disease material came from the lungs of a 2-month-old nursery pig that had severe pneumonia and dyspnea in a large pig farm in Puyang City, Henan Province in April 2017.
[0054] 1.2 Preparation of medium
[0055] PPLO liquid medium: 10.5 g of PPLO broth powder, 2.5 g of glucose, and 2.5 g of yeast powder, dissolved in 440 mL of ultrapure water, sterilized at 115°C for 15 min, added with 5 mL of MEM medium, 50 mL of horse serum, penicillin 80,000 units, 10 mL of sterile 10% arginine and 500 μL of 1% (w / v) phenol red. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use;
[0056] PPLO solid medium: PPLO liquid medium with 1.5% (w / v) agar powder added. After the culture was sterilized at 115°C for 15 minutes, it was stored at 4°C for later use.
[0057] 1.3 Isolation and culture of mycoplasma
[0058...
Embodiment 2
[0070] Example 2: Isolation and identification of strain HNHPS1
[0071] 2.1 Collection of disease data
[0072] The disease material came from a 2-month-old nursery pig that died of severe pneumonia, dyspnea and neurological symptoms in a large pig farm in Ruyang County, Henan Province in November 2016.
[0073] 2.2 Preparation of medium
[0074] TSB liquid medium: Dissolve 30 g of TSB broth powder (Tryptic Soy broth, tryptone soybean broth) in 1000 mL of ultrapure water, sterilize at 115 °C for 15 min, add 50 mL of fetal bovine serum, sterile 1% NAD (Nicotinamide adeninedinucleotide, nicotinamide adenine dinucleotide, coenzyme Ⅰ) 1 mL;
[0075] TSA solid medium: Dissolve 40g of TSA agar powder (Tryptic Soy Agar, tryptone soybean agar) in 1000mL ultrapure water, sterilize at 115°C for 15min, add 50mL of fetal bovine serum, 1mL of sterile 1% NAD, in 4 Store at ℃ for later use.
[0076] 2.3 Isolation and culture of bacteria
[0077] Aseptically collect the brain tissue of ...
Embodiment 3
[0097] Example 3: Isolation and Identification of Bacterial Strain HN1570
[0098] 3.1 Culture medium preparation
[0099] TSB liquid medium and TSA solid medium were prepared according to the method in Example 2.
[0100] 3.2 Isolation and cultivation of strain HN1570
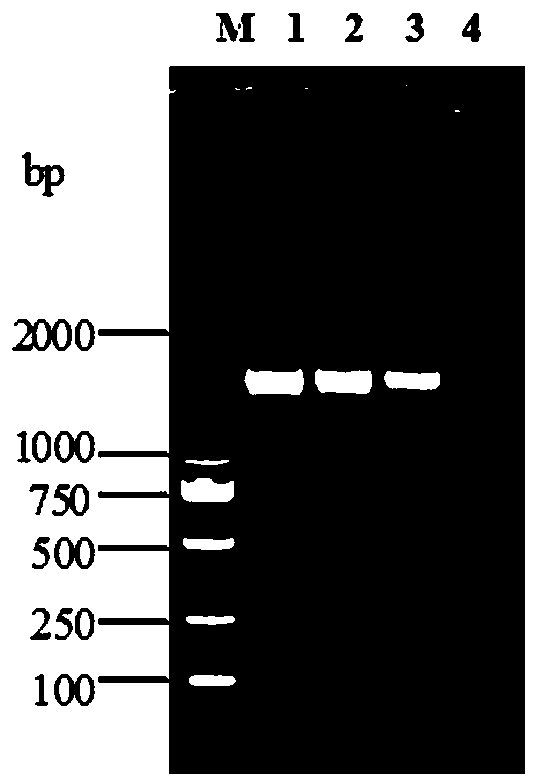
[0101] In 2017, the applicant collected the heart blood of nursery pigs with typical polyserositis under aseptic conditions, inoculated them on TSA solid medium containing NAD, cultured them in a constant temperature incubator at 37°C for 36 hours, and picked suspected colonies for passage Purify and culture for another 24-48 hours to observe the colony morphology of Haemophilus parasuis, and grow consistent needle-shaped, colorless, transparent, smooth, moist colonies with a diameter of 1-2 mm (see Figure 7 ); while purified and inoculated on NAD-free TSA solid medium, it cannot grow on NAD-free TSA medium;
[0102] Pick a single purified colony for Gram staining and microscopic examination, and observe t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com