Patents
Literature
317 results about "Streptococcus suis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Streptococcus suis is a peanut-shaped, Gram-positive bacterium, and an important pathogen of pigs. Endemic in nearly all countries with an extensive pig industry, S. suis is also a zoonotic disease, capable of transmission to humans from pigs.
Streptococcus suis vaccines and diagnostic tests
InactiveUS7125548B2ImmunogenicityImproving immunogenicityAntibacterial agentsBacteriaAntigenSpecific detection
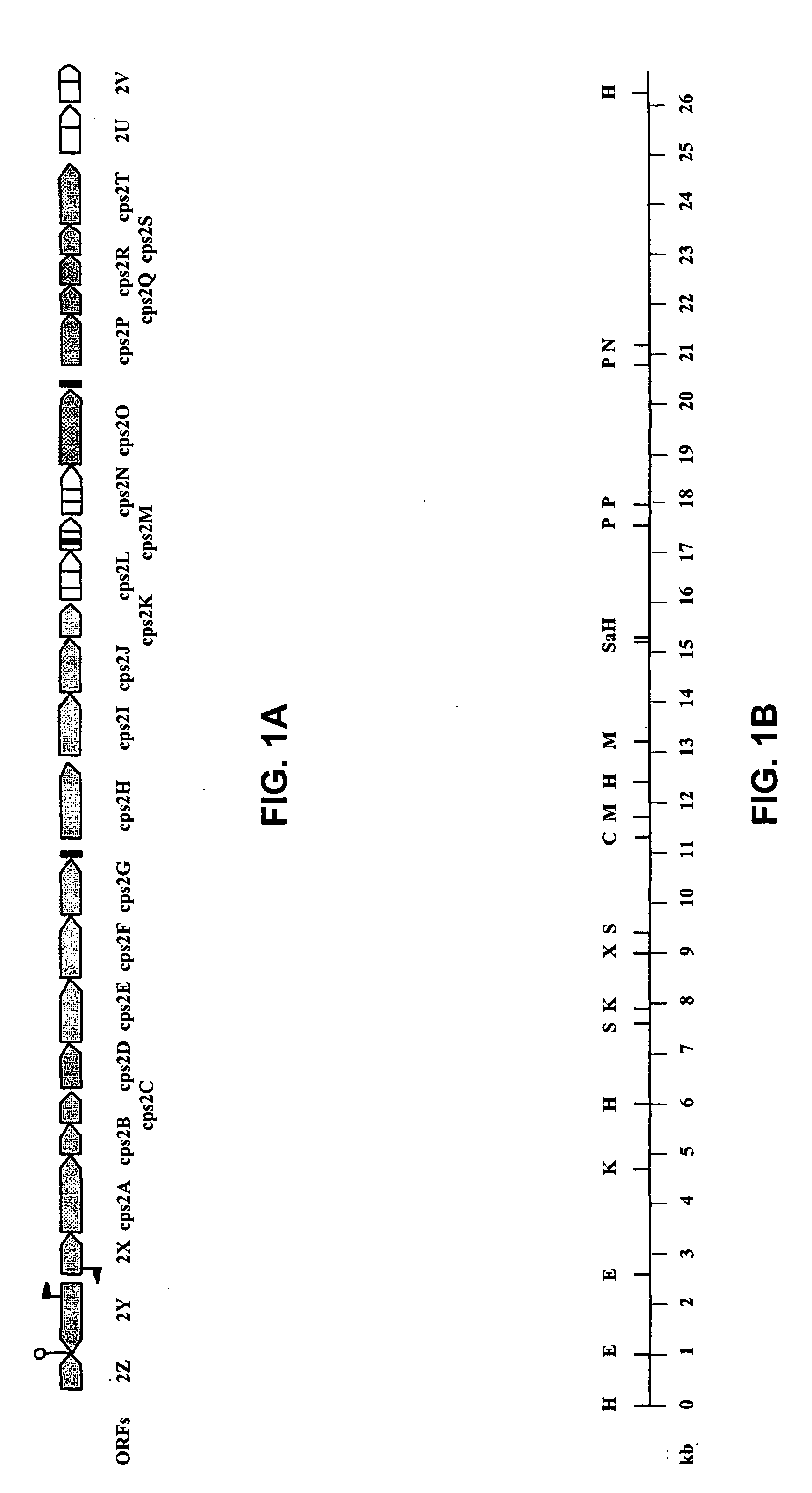
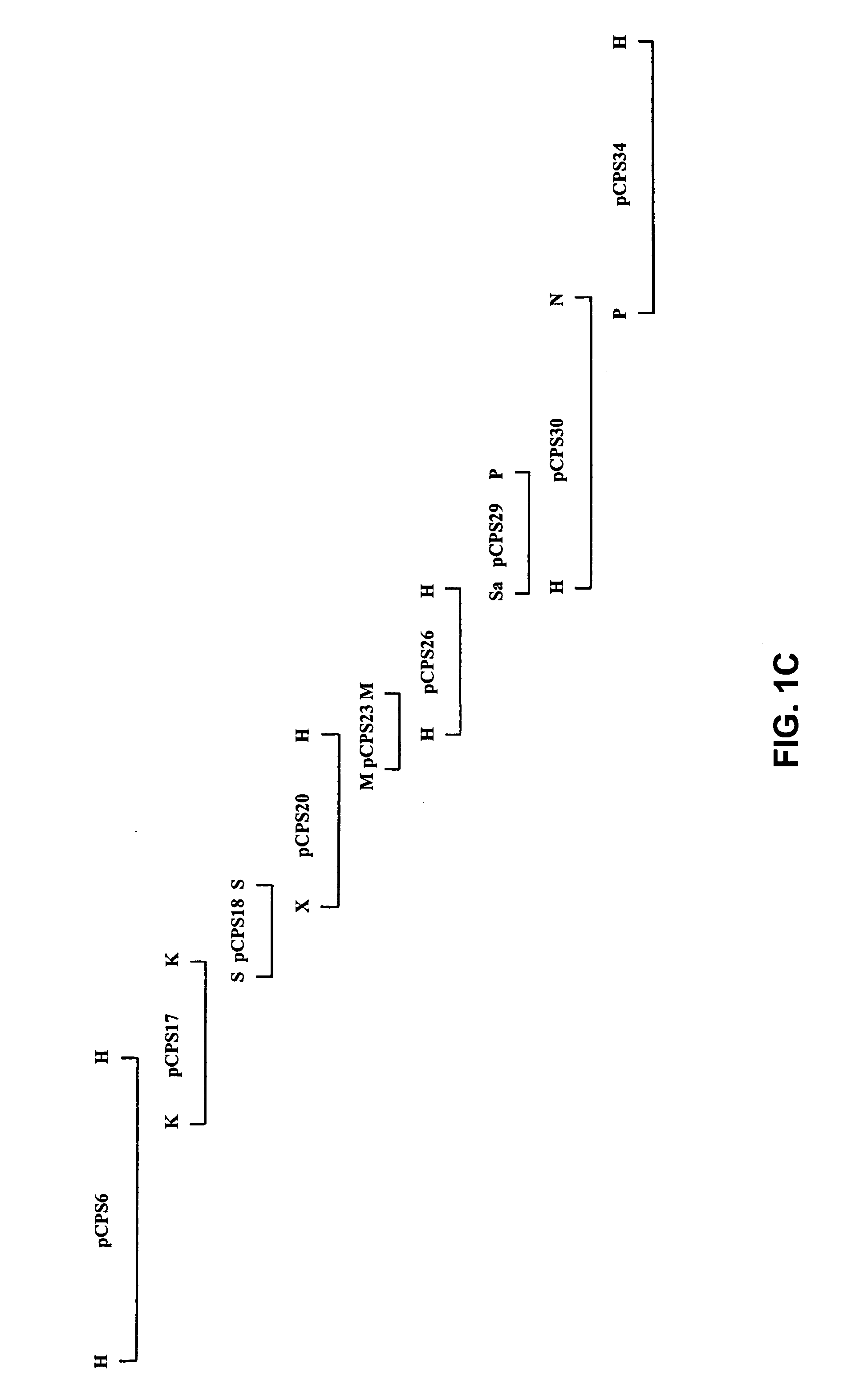
The invention relates to Streptococcus suis infection in pigs, vaccines directed against those infections and tests for diagnosing Streptococcus suis infections. The invention provides an isolated or recombinant nucleic acid encoding a capsular gene cluster of Streptococcus suis or a gene or gene fragment derivated thereof. The invention further provides a nucleic acid probe or primer allowing species or serotype-specific detection of Streptococcus suis. The invention also provides a Streptococcus suis antigen and vaccine derived thereof.
Owner:STICHTING DIENST LANBOUWKUNDIG ONDERZOEK
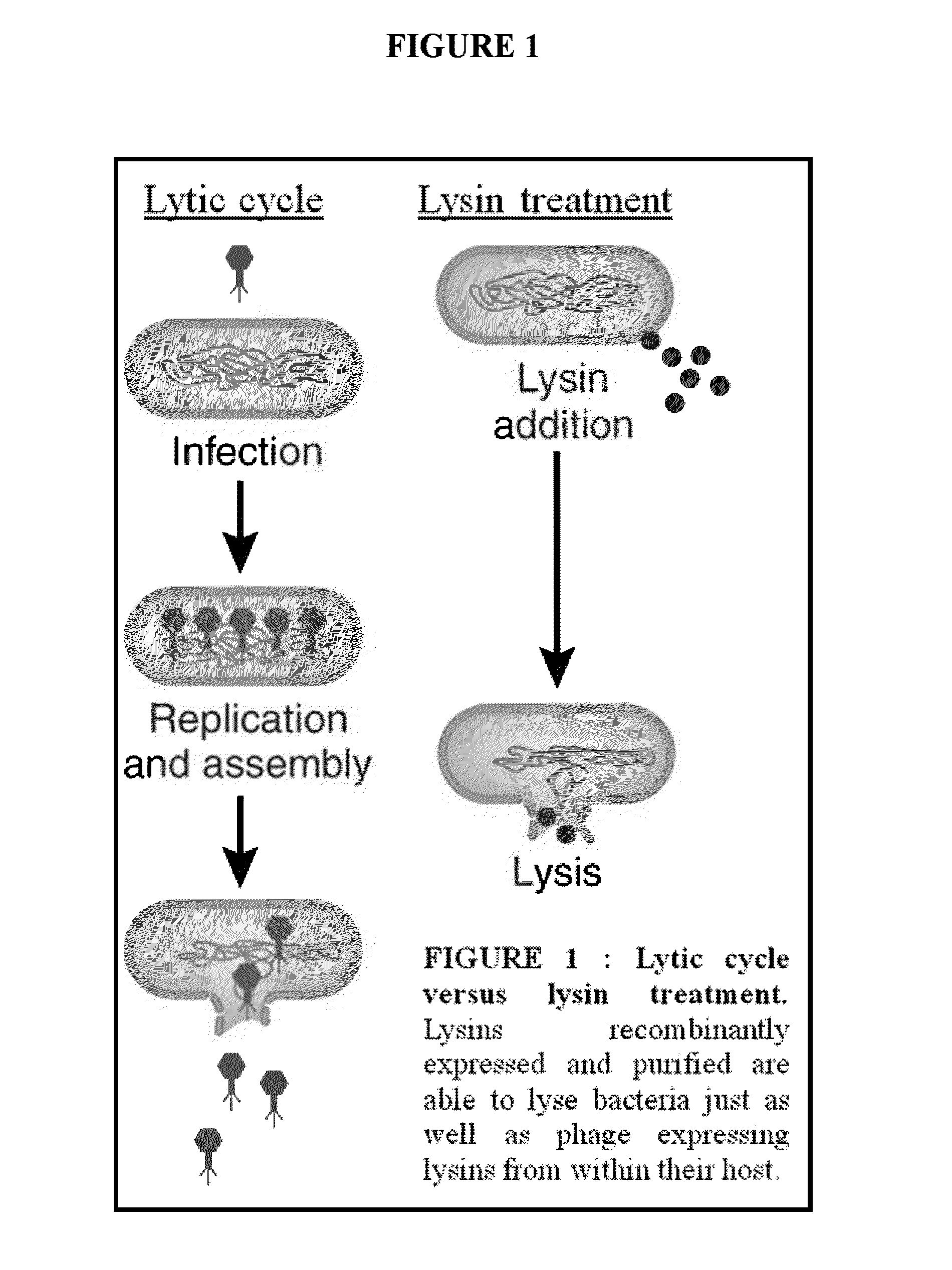
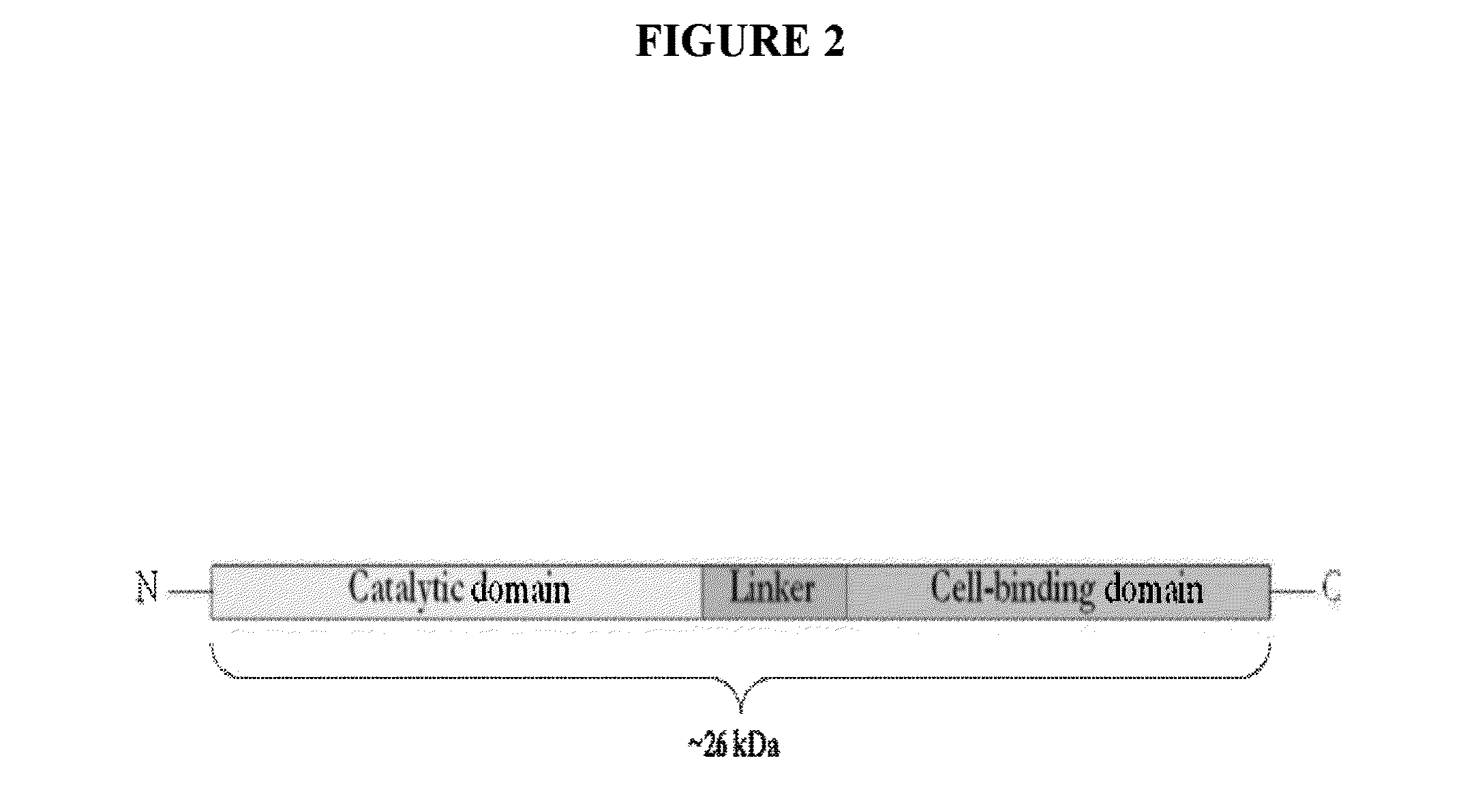
Streptococcus bacteriophage lysins for detection and treatment of gram positive bacteria
ActiveUS9034322B2Efficient killingReduce in quantityAntibacterial agentsPowder deliveryStreptococcus constellatusStaphylococcus xylosus
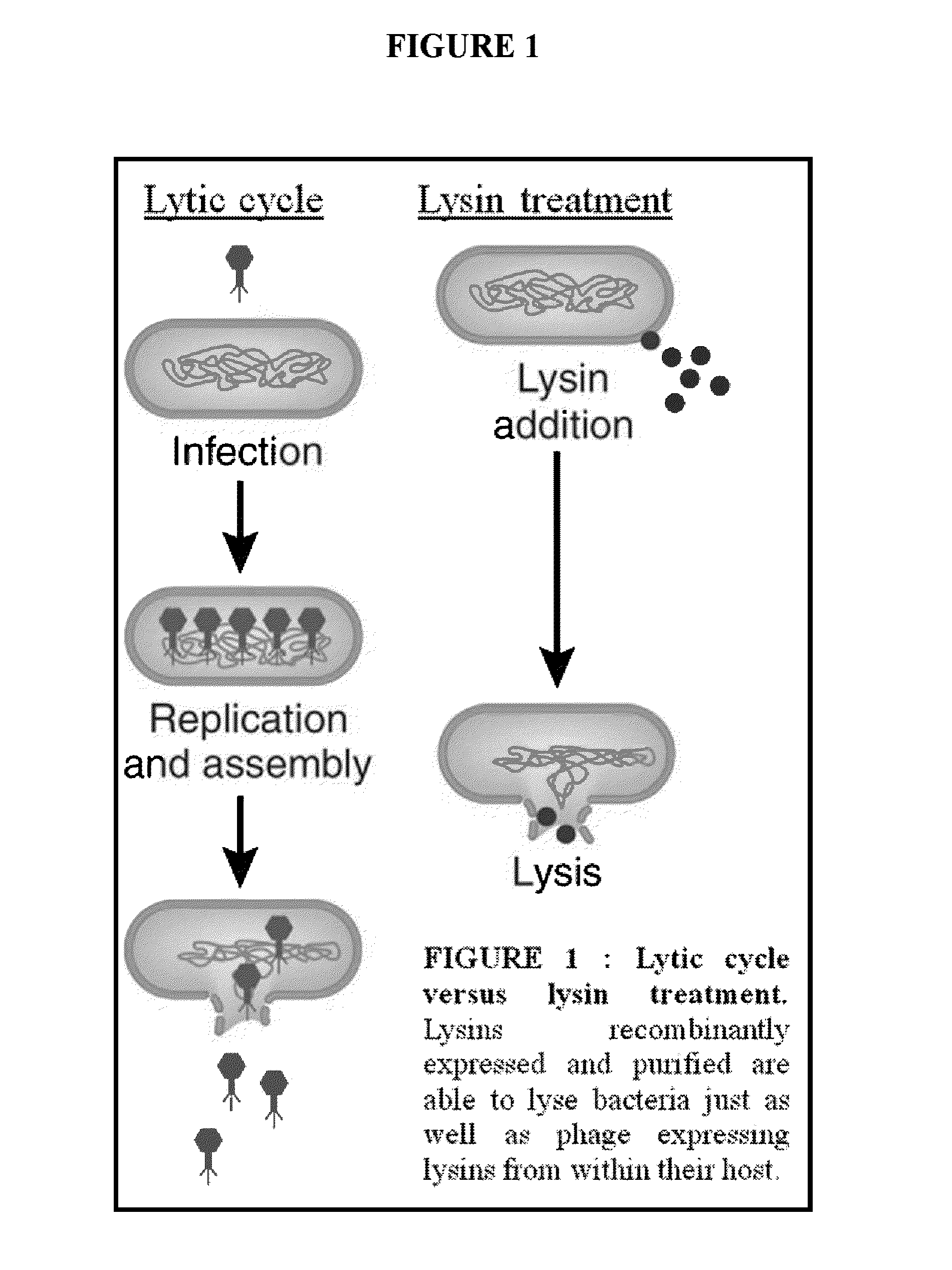
The present invention provides methods, compositions and articles of manufacture useful for the prophylactic and therapeutic amelioration and treatment of gram-positive bacteria, including Streptococcus and Staphylococcus, and related conditions. The invention provides compositions and methods incorporating and utilizing Streptococcus suis derived bacteriophage lysins, particularly PlySs2 and / or PlySs1 lytic enzymes and variants thereof, including truncations thereof. Methods for treatment of humans are provided.
Owner:THE ROCKEFELLER UNIV
Taqman-MGB fluorescent quantitative PCR kit and method for detecting 12 common viruses and bacteria of pig at same time
ActiveCN105624330AQuick checkSensitive detectionMicrobiological testing/measurementPorcine reproductive and respiratory syndrome virusPorcine circovirus
The invention provides a Taqman-MGB fluorescent quantitative PCR kit and a method for detecting 12 common viruses and bacteria of pigs at the same time. The kit comprises PCR reaction liquids A / B / C, wherein the PCR liquids comprise primer pairs and Taqman probes for porcine parvovirus (PPV), type-II streptococcus suis (SS-II), a porcine pseudorabies virus (PRV), type-II porcine circovirus (PCV-2), a hog cholera virus (CSFV), a pig foot and mouth disease virus (FMDV), a porcine reproductive and respiratory syndrome virus (PRRSV), a high pathogenicity porcine reproductive and respiratory syndrome virus strain (Hp-PRRSV), a transmissible gastroenteritis virus (TGEV), an epidemic diarrhea virus (PEDV), rotavirus (PRTV) and a swine influenza virus (SIV) respectively. 12 pathogens of pigs can be detected rapidly and effectively at the same time, the detection method is high in accuracy, specificity and sensitivity and is good in stability, and rapid diagnosis and effective detection on pathogens to be detected can be achieved.
Owner:BEIJING YISEN BIOTECH
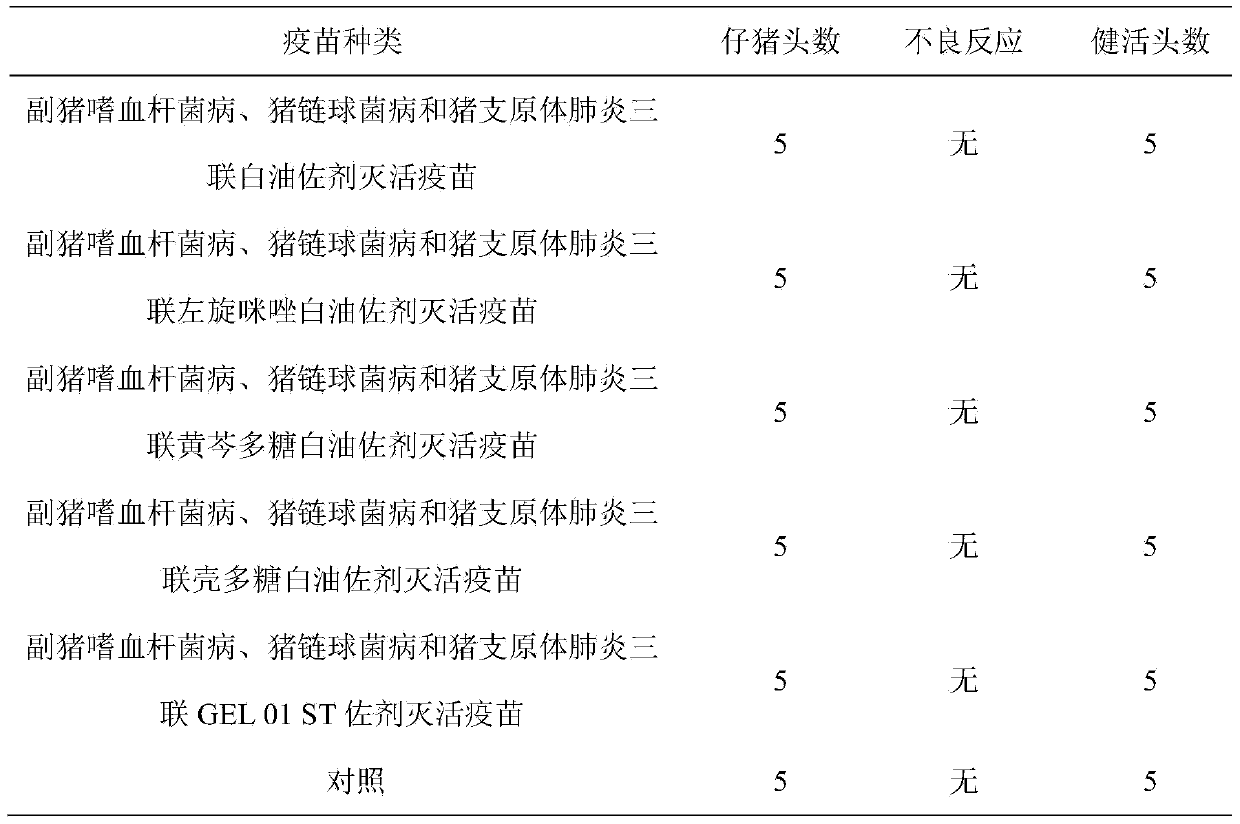
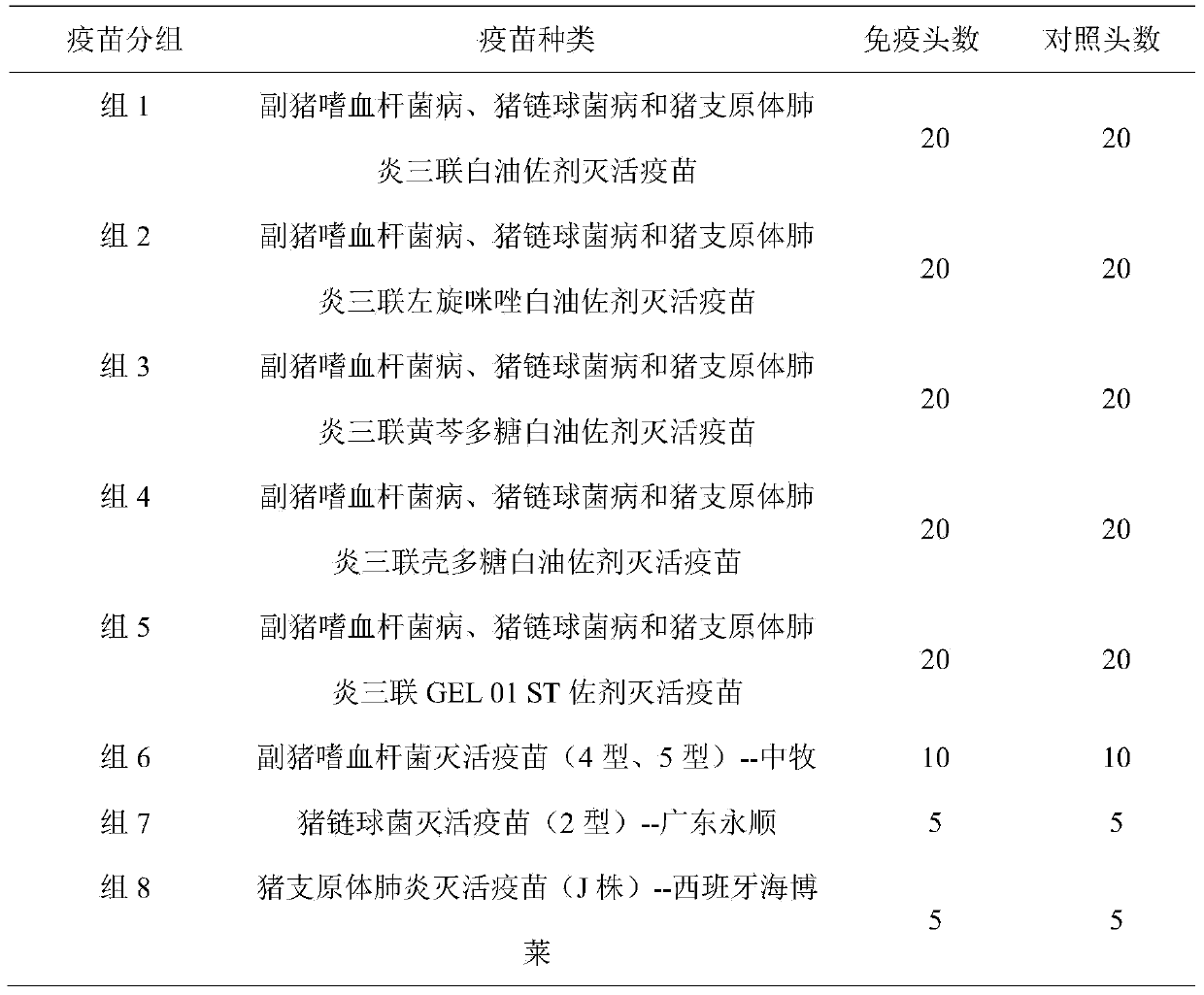
Porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and preparation method thereof
ActiveCN102329746AReduce stressEasy to useAntibacterial agentsBacterial antigen ingredientsHaemophilusAluminium stearate
The invention discloses a porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine and a preparation method thereof. The preparation method comprises the following steps of: a, respectively carrying out enrichment culture on a porcine streptococcus strain, a haemophilus parasuis strain and a haemophilus parasuis strain to obtain a porcine streptococcus strain bacterial solution, a haemophilus parasuis strain bacterial solution and a haemophilus parasuis strain bacterial solution; b, respectively adding a formaldehyde solution into the porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, and inactivating; c, mixing the collected porcine streptococcus strain bacterial solution, the haemophilus parasuis strain bacterial solution and the haemophilus parasuis strain bacterial solution, adding Tween-80 for preparing a water phase, preparing white oil, Span-80 and aluminium stearate into an oil phase, mixing the water phase with the oil phase to prepare a uniform emulsion, i.e. an oil emulsion inactivating vaccine; and 4, sub-packaging the oil emulsion inactivating vaccine. The porcine streptococcus disease and haemophilus parasuis disease combined inactivate vaccine can effectively prevent the porcine streptococcus disease and haemophilus parasuis disease, does not have hidden danger of scattering viruses and is safe and reliable; and the immunization is realized by one vaccine, thus the cost is reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Virulence of streptococci
InactiveUS7670835B2Increase virulenceMinimal effectAntibacterial agentsBacterial antigen ingredientsGenomic SegmentVaccination
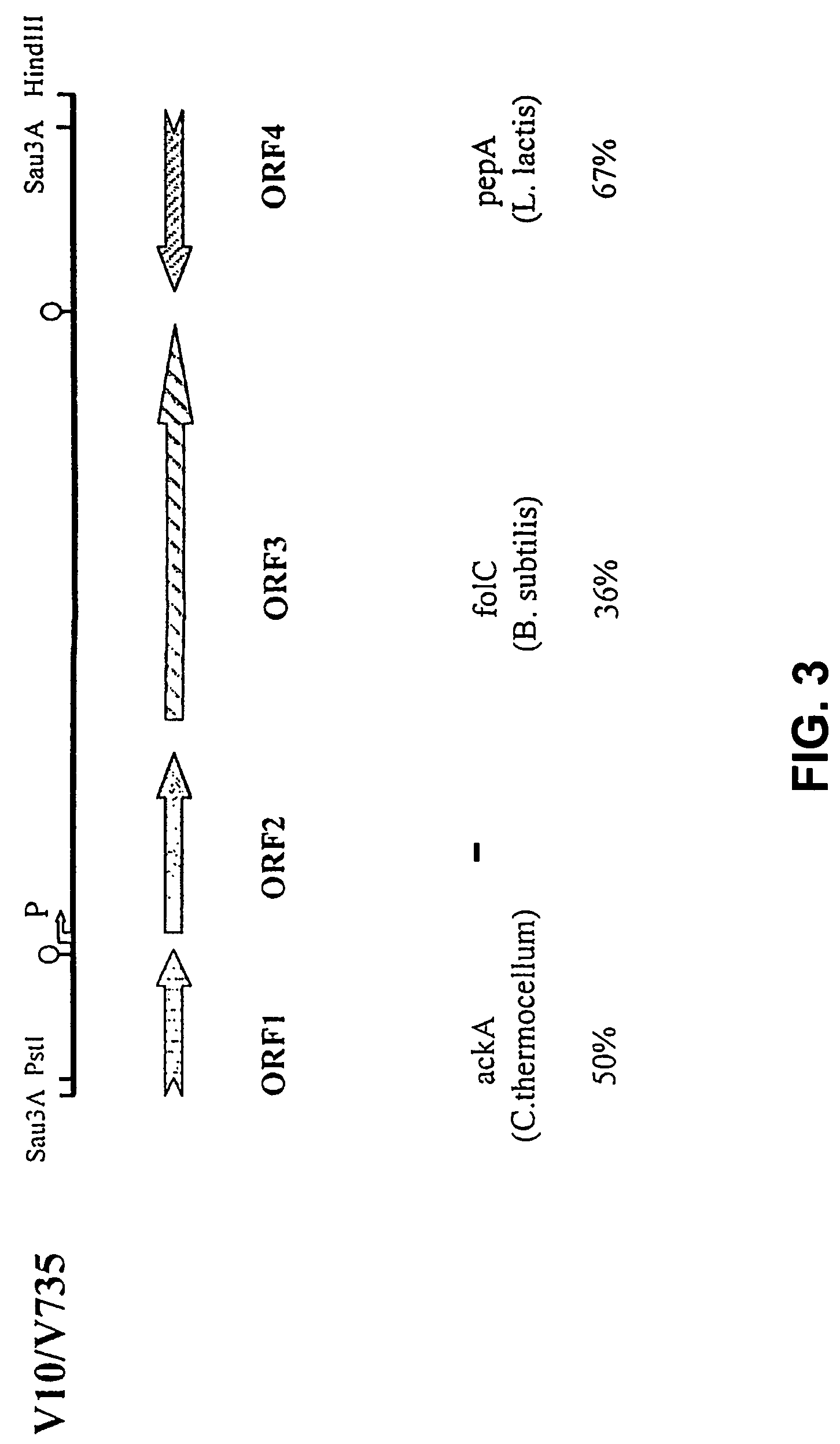
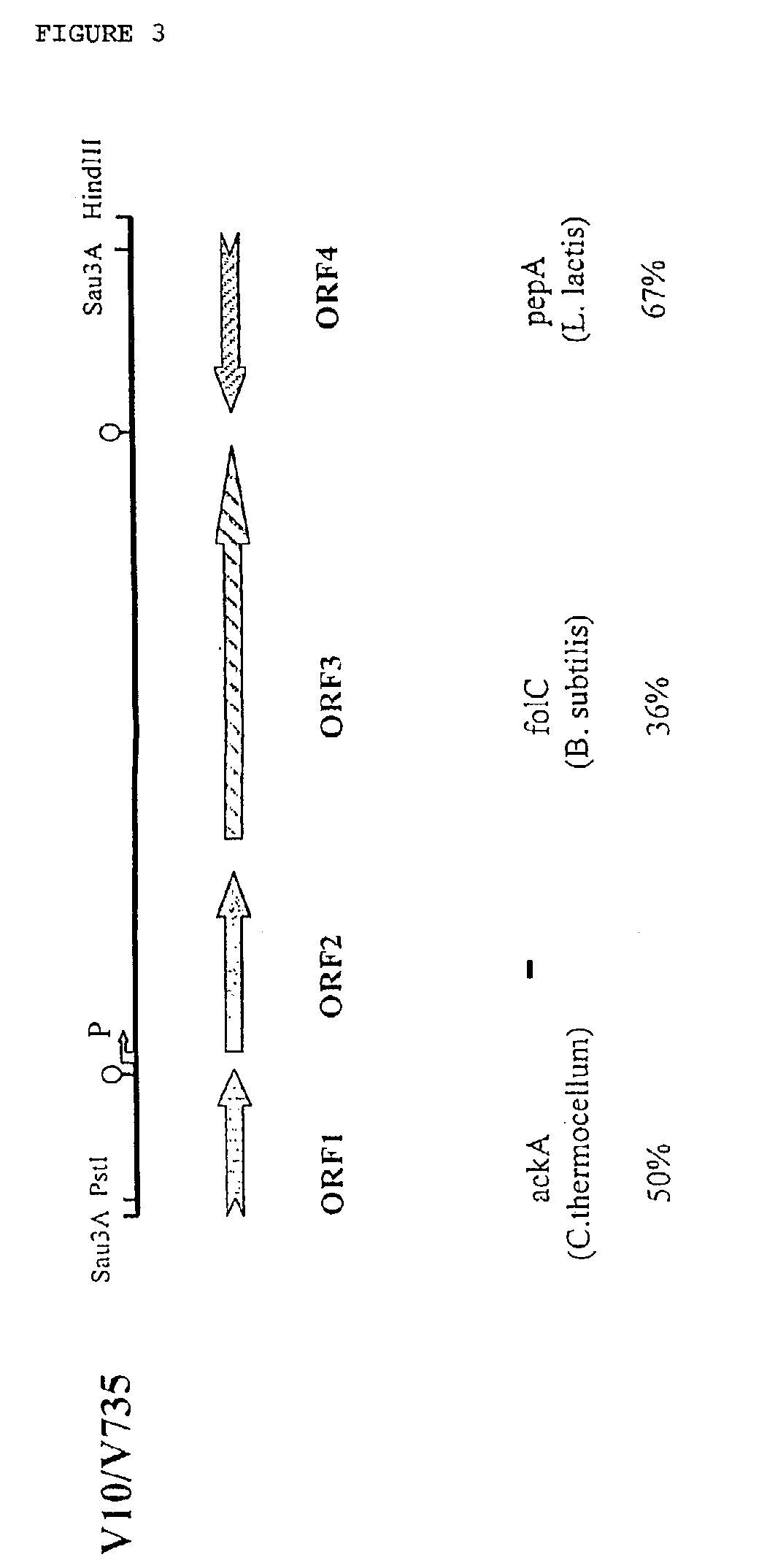
The invention relates to the field of diagnosis of and vaccination against Streptococcal infections and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus, the method comprising modifying a genomic fragment of Streptococcus wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof as shown in FIG. 5.
Owner:STICHTING WAGENINGEN RES
Streptococcus bacteriophage lysins for detection and treatment of gram positive bacteria
Owner:THE ROCKEFELLER UNIV
Immunomagnetic bead purification kit and method for 12 kinds of swine common viruses and germs
InactiveCN105779398AEfficient separationSimple separation and purificationBacteriaMicrobiological testing/measurementBiotinStreptomycin
The invention provides an immunomagnetic bead purification kit and method for 12 kinds of swine common viruses and germs. The kit comprises a reagent A, a reagent B and a reagent C, wherein the reagent A is streptomycin immunomagnetic suspension; the reagent B is a 12-biotinylation monoclonal antibody mixed solution; and the reagent C is a 10* immunomagnetic bead separation and purification system buffer solution. The method comprises the following steps of (1) performing a pathogen suspension preparation process; (2) performing a biotin antibody preparation process; and (3) performing a pathogen immunological purification process. The immunomagnetic bead purification kit and method for 12 kinds of swine common viruses and germs provided by the invention have the advantages that 12 kinds of viruses and germs such as PPV (Porcine Parvovirus), SS-II (Streptococcus Suis Type 2), PRV (Porcine Pseudorabies Virus) and the like in a sample can be simultaneously, fast and effectively purified. The detection method has the advantages of high accuracy, high specificity, high sensitivity and high stability, and the subsequent fast diagnosis and effective detection work is facilitated.
Owner:BEIJING YISEN BIOTECH
Expression method of lactococcus lactis of porcine streptococcus phage catenase
InactiveCN102181420AFermentationVector-based foreign material introductionStreptococcus PhageBiology
The invention discloses an expression method of lactococcus lactis of porcine streptococcus phage catenase in the technical field of biology. A pyrolyzed gene of porcine streptococcus virulent phage SMP is augmented by utilizing polymerase chain reaction (PCR) to obtain a target fragment which is connected with a pNZ8148 vector so as to obtain a recombined expression plasmid; lactococcus lactis L9000 expression is adopted so s to obtain a fusion protein the molecular weight of which is 52kDa, so as to obtain activated catenase which has a pyrolyzed actioin on multiple clinically separated porcine streptococcus in vitro.
Owner:SHANGHAI JIAO TONG UNIV
Kit for detecting streptococcus suis type 2 and application of kit
ActiveCN103409546AAccurate detectionHigh sensitivityMicrobiological testing/measurementMicroorganism based processesElectrophoresisPolymerase L
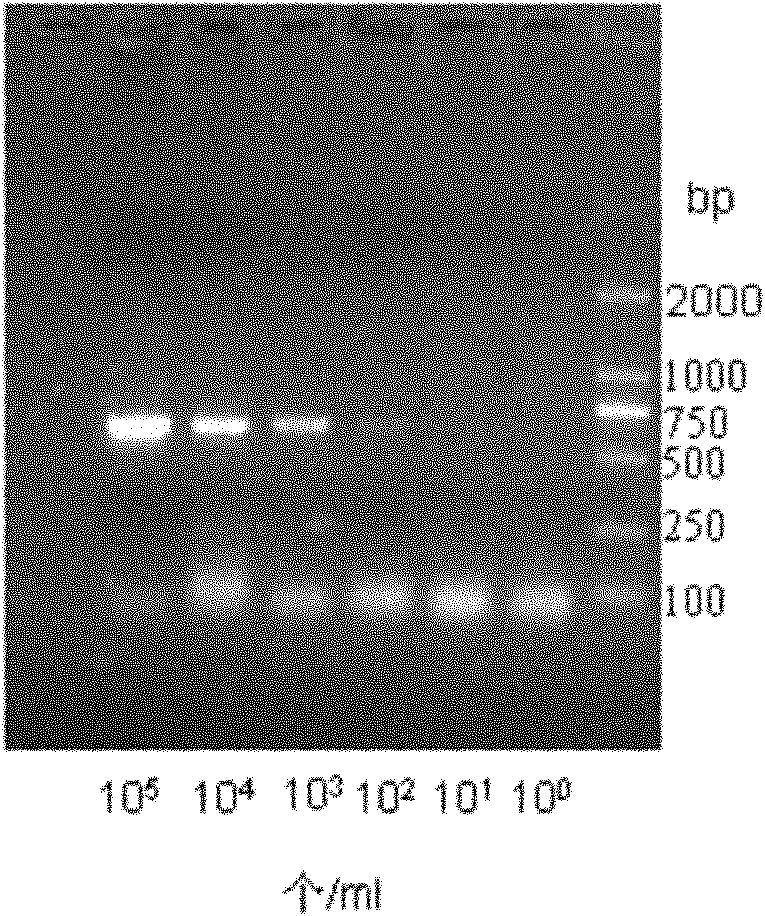
The invention relates to a kit for detecting streptococcus suis type 2 nucleic acid by an isothermal nucleic acid amplification method and application of the kit to detection of streptococcus suis type 2 nucleic acid. The kit comprises amplification reaction liquid, primer reaction liquid, DNA (deoxyribonucleic acid) polymerase, nucleic acid dye, an S.suis type 2 positive standard product and a negative control product. A method for applying the kit to S.suis type 2 positive detection comprises five steps of extracting DNA of to-be-tested specimen bacteria, performing loop-mediated isothermal amplification (LAMP) on S.suis type 2 CPS2J gene, performing electrophoretic detection on an amplification product, performing chromogenic reaction of the LAMP amplification product and judging positive of S.suis type 2 streptococcus. The kit has the advantages of quickness, convenience, strong specificity and high sensitivity when being applied to positive detection of the S.suis type 2 streptococcus, and is very suitable for quick detection of the S.suis type 2 streptococcus in medical or food industry.
Owner:长沙市疾病预防控制中心
Hemophilus parasuis disease, swine streptococcosis bivalent inactivated vaccine and preparation method thereof
ActiveCN103157100ALittle side effectsFulfil requirementsAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
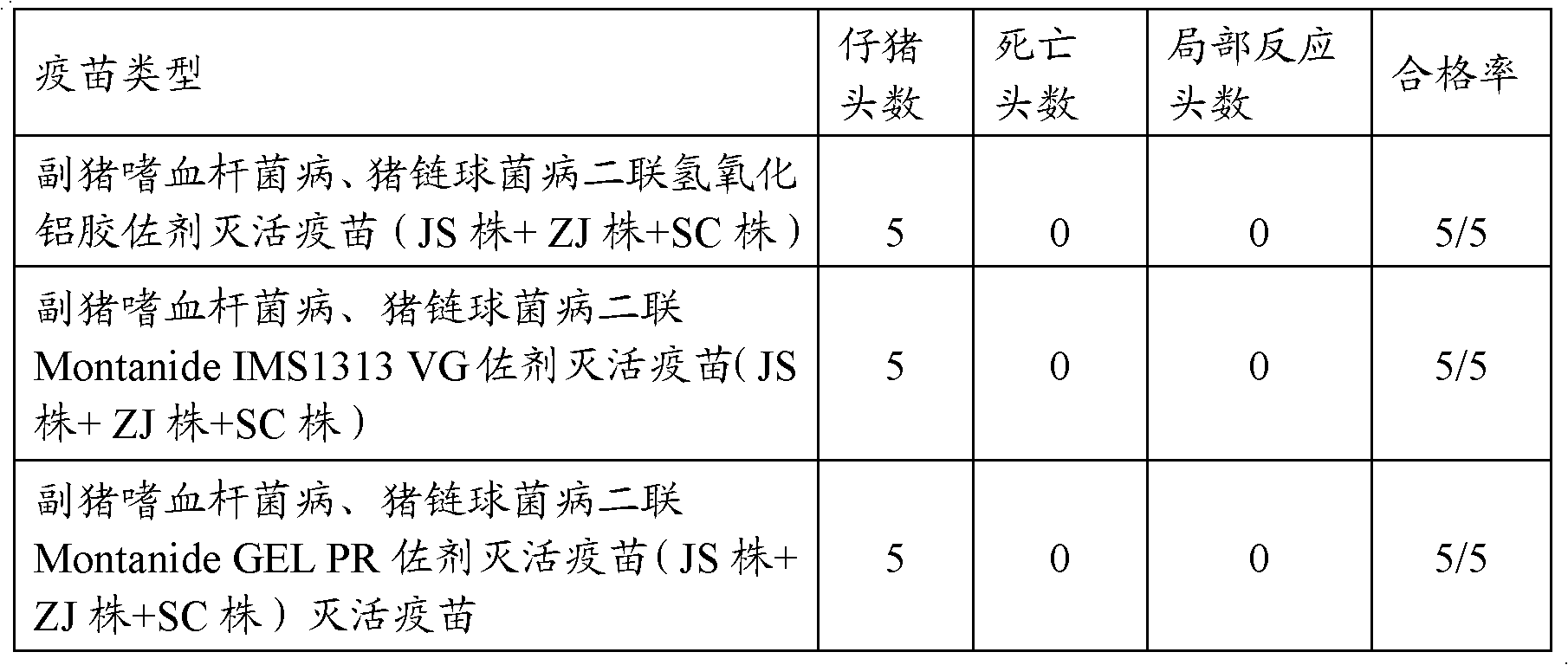
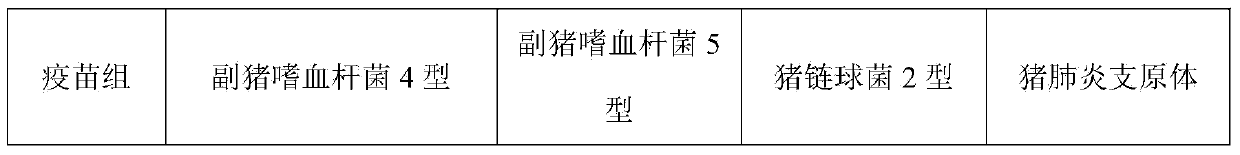
The present invention relates to a hemophilus parasuis disease, a swine streptococcosis bivalent inactivated vaccine and a preparation method thereof. The vaccine is prepared by adopting a hemophilus parasuis serotype 4 JS strain, a hemophilus parasuis serotype 5 ZJ strain and a streptococcus suis type 2 SC strain as antigens, and adopting a veterinarily acceptable adjuvant, concurrently provides immunoprophylaxis for hemophilus parasuis diseases caused by hemophilus parasuis, swine streptococcosis caused by streptococcus suis, and mixed infection of the hemophilus parasuis disease and the swine streptococcosis, has effects of low side effect, no endotoxin, no impurity protein in serum, good immunization safety, multi-prevention effect with one needle in the clinic, and cost reducing, and can meet different requirements of different users.
Owner:PU LIKE BIO ENG
Trigeminy inactivated vaccine for porcine circovirus disease, porcine streptococcus suis disease and porcine haemophilus parasuis disease, preparation method of the vaccine and applications of the vaccine
InactiveCN103409374APromote mass replicationImprove reproductive performanceAntibacterial agentsAntiviralsHaemophilusCircovirus
The invention discloses a trigeminy inactivated vaccine for porcine circovirus disease, porcine streptococcus suis disease and porcine haemophilus parasuis disease, a preparation method of the vaccine and applications of the vaccine. Porcine circovirus 2-WH has an accession number of CCTCC NO: V20133 and has strong virulence, good immunogenicity, a high antigen titer, and a high virulence reaching 10<7.4>TCID[50] / mL. The trigeminy inactivated vaccine, which is prepared by mixing the porcine circovirus 2-WH, porcine streptococcus suis 2-LT, and porcine haemophilus parasuis 4-MD0322 and porcine haemophilus parasuis 5-SH0165, can simultaneously prevent the porcine circovirus disease and diseases caused by porcine streptococcus suis 2-type, porcine haemophilus parasuis 4-type and porcine haemophilus parasuis 5-type, the immune protective effect on the same serotype reaches over 80%.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
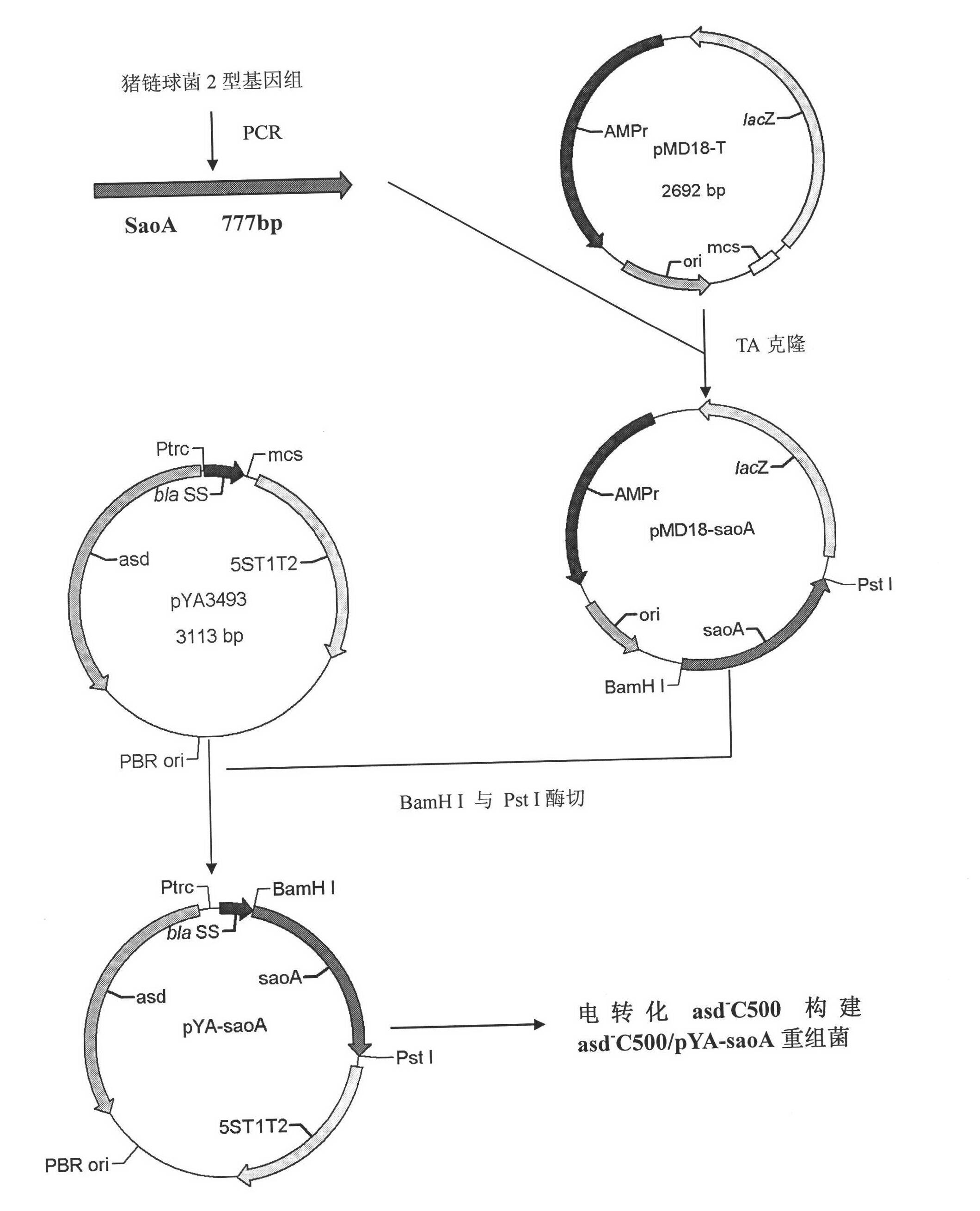
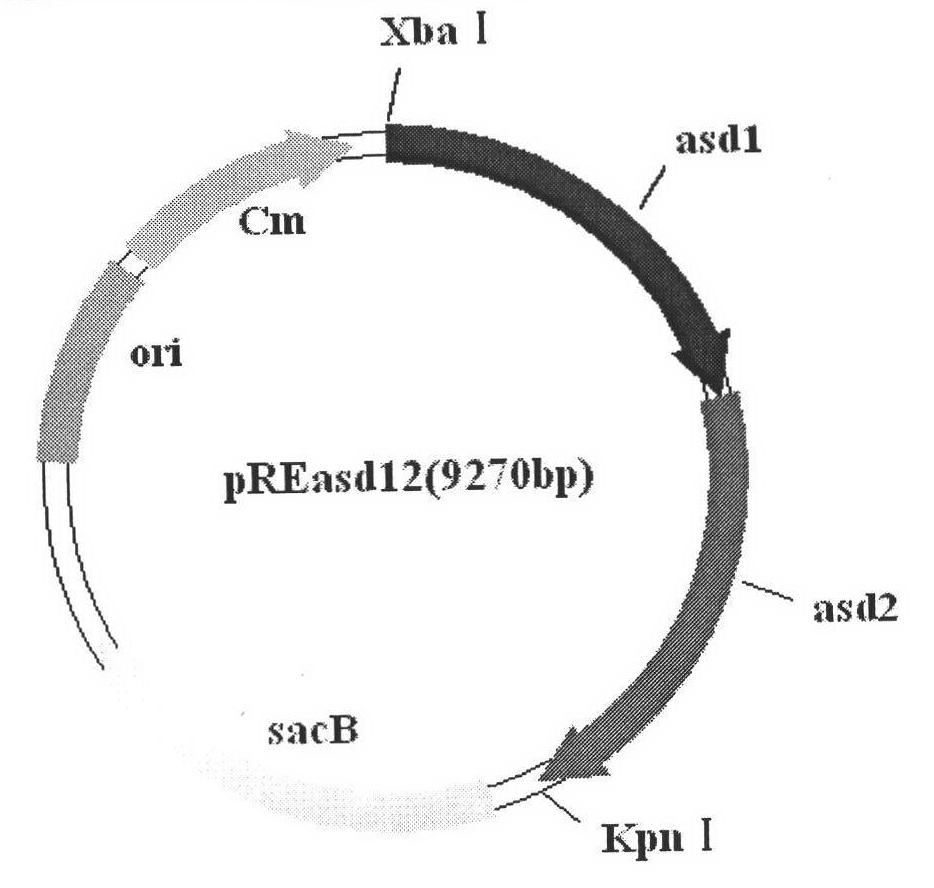
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
Multiple PCR detection kit for virulence factors of streptococcus suis and detection method thereof
InactiveCN101812518AStrong specificityStable and reliableMicrobiological testing/measurementMicroorganism based processesSurvey researchVirulent characteristics
The invention discloses a multiple PCR detection kit for virulence factors of streptococcus suis and a detection method thereof, and belongs to the technical field of biology. The multiple PCR detection kit comprises nucleic acid shown by base sequences, such as SEQ ID NO:1 to SEQ ID NO:20. The detection method for the multiple PCR detection kit comprises the following steps of: 1, providing DNA of samples to be detected; 2, taking the kit, amplifying the DNA of the samples to be detected by adopting the conventional PCR method, detecting an amplified result by adopting an agarose gel electrophoresis method, and judging according to the result; and by using positive DNA of streptococcus suis 2 type as contrast, if the contrast proves that not all the strips are amplified, re-detecting, and if the contrast proves that all the strips are amplified, judging the result. The multiple PCR detection method established by the invention is specific and sensitive, and has the advantages of simpleness, convenience and quickness. The multiple PCR detection kit has reliable stability, and can be used for rapid detection of clinical samples and survey research on molecular epidemiology for the streptococcus suis.
Owner:SHANGHAI JIAO TONG UNIV
Virulence of Streptococci
InactiveUS7109006B2Increase virulenceMinimal effectAntibacterial agentsBacteriaGenomic SegmentVirulent characteristics
The invention relates to the field of diagnosis of and vaccination against Streptococcal infections and to the detection of virulence markers of Streptococci. The invention discloses a method for modulating virulence of a Streptococcus comprising modifying a genomic fragment of Streptococcus wherein the genomic fragment comprises at least a functional part of a fragment identifiable by hybridization in Streptococcus suis to a nucleic acid or fragment thereof as shown in FIG. 5.
Owner:STICHTING WAGENINGEN RES
Kit and method for detecting streptococcus suis type 2
InactiveCN102605046AIncreased sensitivityStrong specificityMicrobiological testing/measurementBiotinMicrobiology
The invention provides a kit and a method for detecting streptococcus suis type 2. The kit comprises an upstream primer, a downstream primer and a probe; the 5' end of the upstream primer and / or 5' end of the downstream primer are / is modified by a biotin; the 5' end and / or the 3' end of the of the probe are / is modified by digoxin; the upstream primer and the downstream primer can amplify the DNA molecule D or the fragment of the DNA molecule D through polymerase chain reaction to obtain a double-stranded DNA amplification product; the base sequence of the DNA molecule D is shown as in the sequence table 1; and the probe can hybridize flexibly with a biotin modified single-stranded DNA in the double-stranded DNA amplification product. The kit and the method have the advantages of high sensitivity, high specificity and low detection cost.
Owner:BIOLOGICAL TECH INST OF FUJIAN ACADEMY OF AGRI SCI
Method for preparing triple inactivated vaccine
InactiveCN104208667AReduce stressLow costAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
The invention provides a method for preparing a triple inactivated vaccine. The triple inactivated vaccine has a relatively good immunizing effect on haemophilus parasuis, swine streptococcosis and mycoplasma pneumoniae of swine, and the aims of preventing multiple diseases by one injection, reducing cost and reducing swine stress.
Owner:TIANJIN RINGPU BIO TECH
Inactivated vaccine for streptococcus suis and pasteurella multocida diseases and preparation method thereof
ActiveCN101745105AAvoid infectionAchieve the effect of multiple defenses with one injectionAntibacterial agentsBacterial antigen ingredientsDiseaseSerum ige
The invention provides an inactivated vaccine for streptococcus suis and pasteurella multocida diseases and a preparation method thereof, which can prevent streptococcus suis infection caused by a plurality of different sero-group streptococcus and infection caused by a plurality of different capsular serotype pasteurella multocida, achieves multiple preventions with one injection clinically, and is lowered in cost, free from the hidden hazard of virus dispersion, safe and reliable. The invention further provides a method for preparing the inactivated vaccine for streptococcus suis and pasteurella multocida diseases.
Owner:广东永顺生物制药股份有限公司
Chain coccus recombination subunit vaccine and prepn. thereof
InactiveCN1833723AEasy to useImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsStreptococcus mitisProtein Fragment
A streptococci recombined subunit vaccine for preventing the streptococcicosis of pig includes the fusion protein of the horseí»s Streptococcus equi subsp zooepidemicus type M protein fragment and the pigí»s Streptococussuis 2 type lysozyme releasing protein fragment. Said fusion protein has high immunogenicity. The gene for coding said fusion protein is also disclosed.
Owner:范红结 +2
Streptococcus suis type 2 10-gene-deleted strain and application
ActiveCN106085936AWith cross protectionAvoid side effectsAntibacterial agentsBacterial antigen ingredientsMucosal Immune ResponsesOral medication
The invention discloses a streptococcus suis type 2 10 gene-deleted strain and application. The strain is obtained by knocking out 10 genes on the basis of a streptococcus suis type 2 virulent strain, and the preservation serial number is CCTCC NO: M 2015800. The virulence of the strain is significantly reduced compared with that of the parent strain, safety is high, immunogenicity is high, and immunized mice can be protected against the attack of the streptococcus suis type 2 virulent strain after being immunized in an injection or nasal drip or oral administration mode, which shows that the vaccine can effectively activate the mucosal immune system and systemic immunology of a host; meanwhile, a cross protection function on streptococcus suis type 2 virulent strains of different ST types can also be achieved. The vaccine is simple in preparation method and suitable for industrial production; meanwhile, the immunogenicity of the mutant strain is similar to that of a wild virulent strain, and thus the mutant strain can be applied to the development of rapid reagent kits as a standard substance or a whole-cell antigen.
Owner:HUAZHONG AGRI UNIV
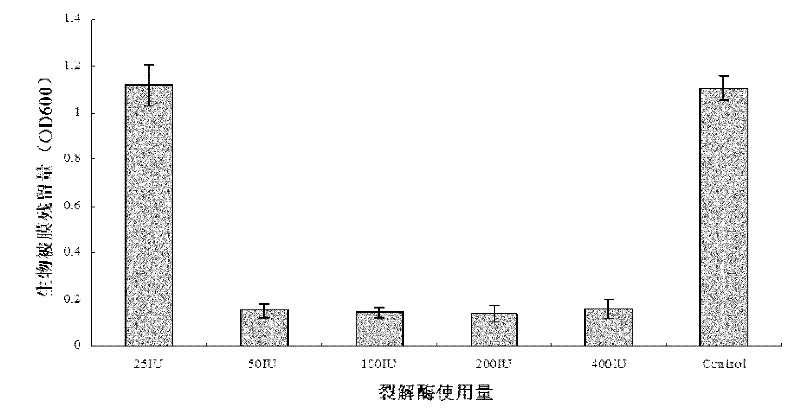
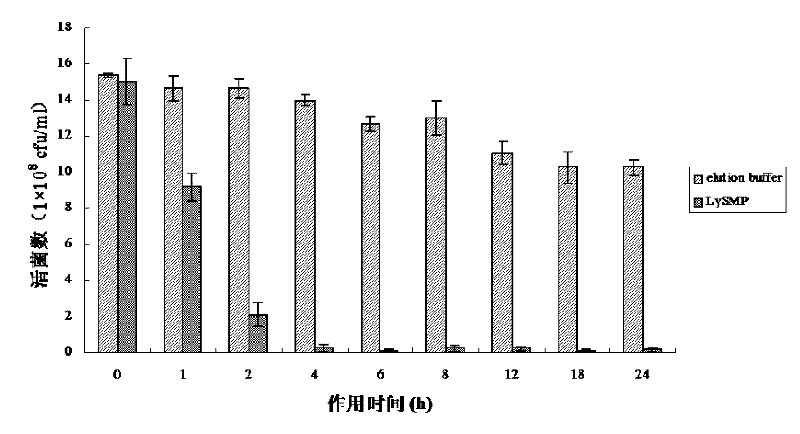
Method for degrading streptococcus suis biofilm by applying phage lyase
ActiveCN102198265AEfficient lysisClean up thoroughlyAntibacterial agentsPeptide/protein ingredientsBiofilmLyase
The invention provides a method for degrading a streptococcus suis biofilm by applying phage lyase, belonging to the field of biotechnology. The method comprises the following steps of: expressing lyase LySMP of which the molecular weight is 55kDa by adopting expression bacteria BL21-lys, purifying with a Ni column to obtain lyase, and degrading the streptococcus suis biofilm obtained by culture on a cell culture plate by using the lyase in vitro. The method provided by the invention can sterilize streptococcus suis SS2-4 and SS2-H and can simultaneously destroy the structure of the biofilm to achieve the effect on thoroughly clearing the biofilm.
Owner:SHANGHAI JIAO TONG UNIV
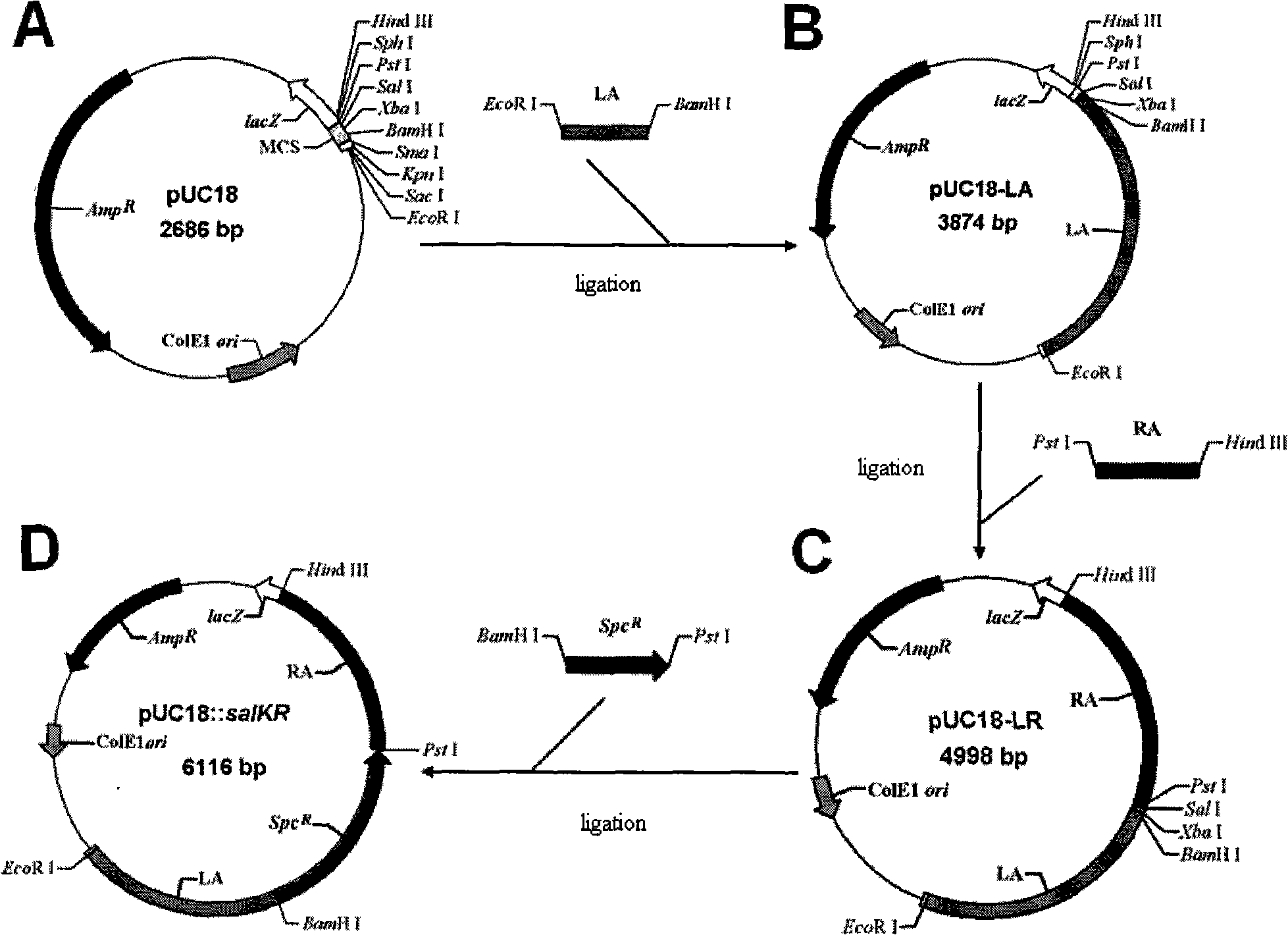
Type II streptococcus suis sa1KR gene knockout mutant strain, preparation method and application thereof
InactiveCN101294144AHigh similaritySuitable for researchAntibacterial agentsBacterial antigen ingredientsAgricultural sciencePathogenicity island
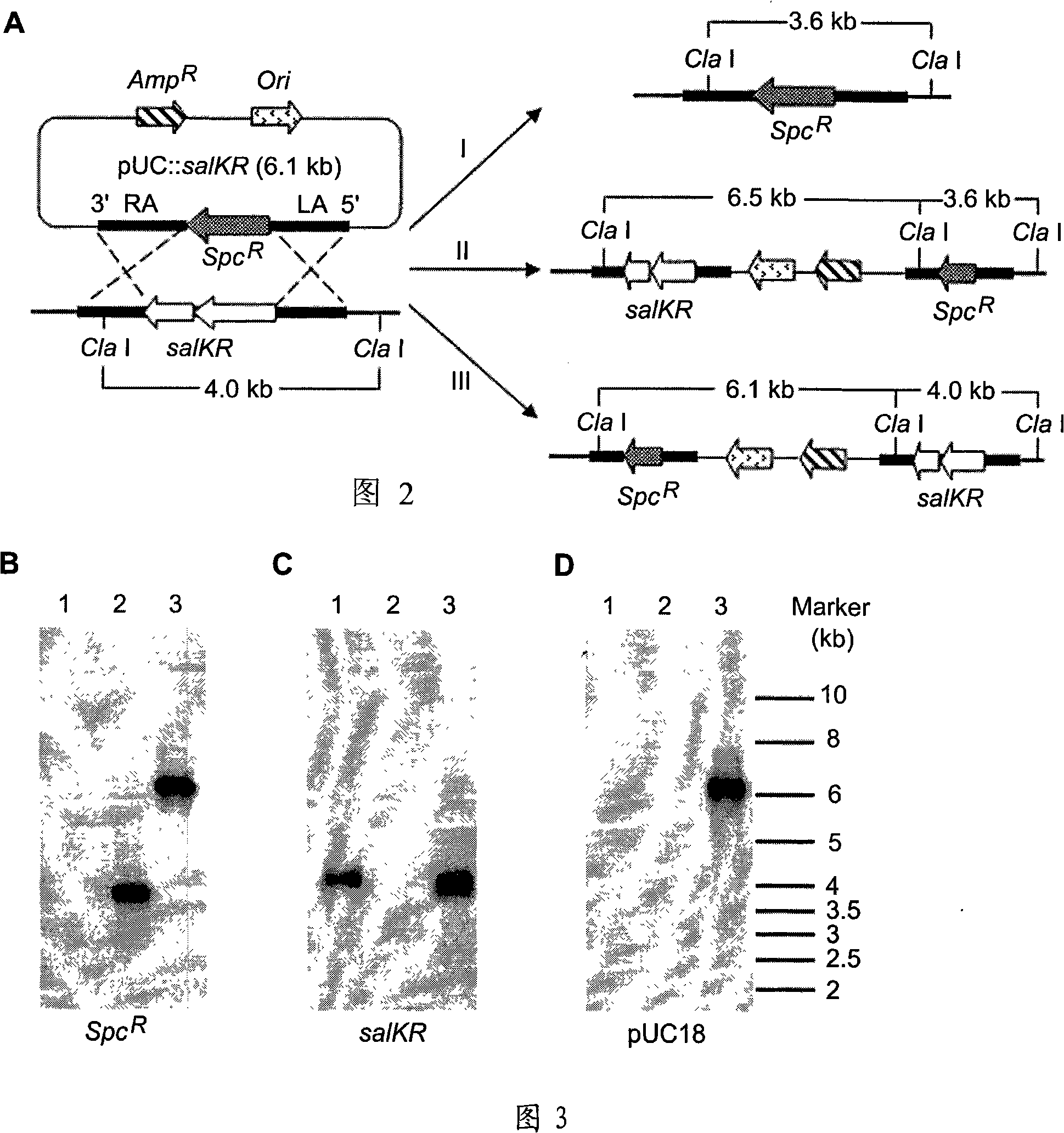
The invention relates to a gene knock-out mutant of Streptococcus suis type 2 salKR and the preparation method and the application thereof. The mutant 05ZYH33 delta salKR has the preservation number of CGMCC No.2259. The preparation method comprises a step of subjecting a dual signal transduction system in PAI89K pathogenicity island on chromosome 05ZYH33 of wild S.suis 2 strain to gene knock-out by homologous recombination technology. Verified by PCR identification and Southern blotting, the obtained stain is gene knock-out mutant named as 05ZYH33 delta salKR. Animal test shows that the mutant has no harm to animals, and the whole genome sequence of the mutant has large similarity to that of the wild strain, so that the mutant is suitable for research and production of vaccines.
Owner:ARMY MEDICAL UNIV
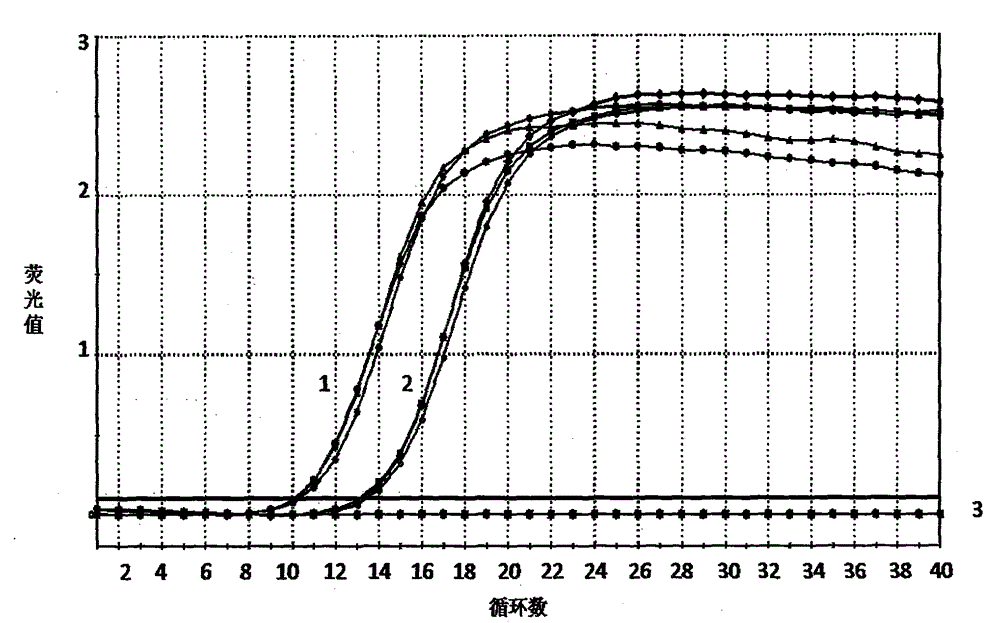
Fast inspection reagent kit for streptococcus suis 2-type triple PCR
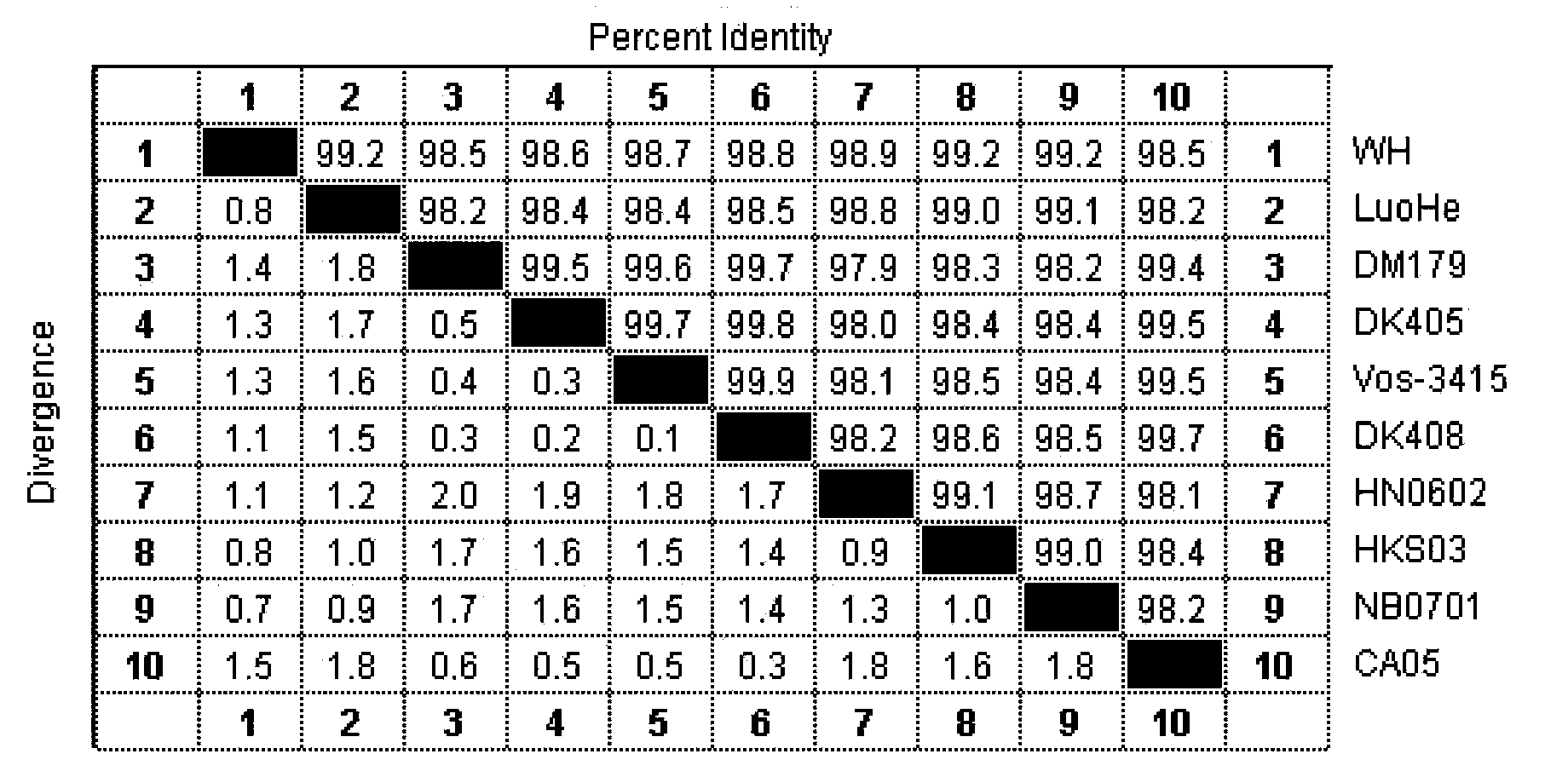
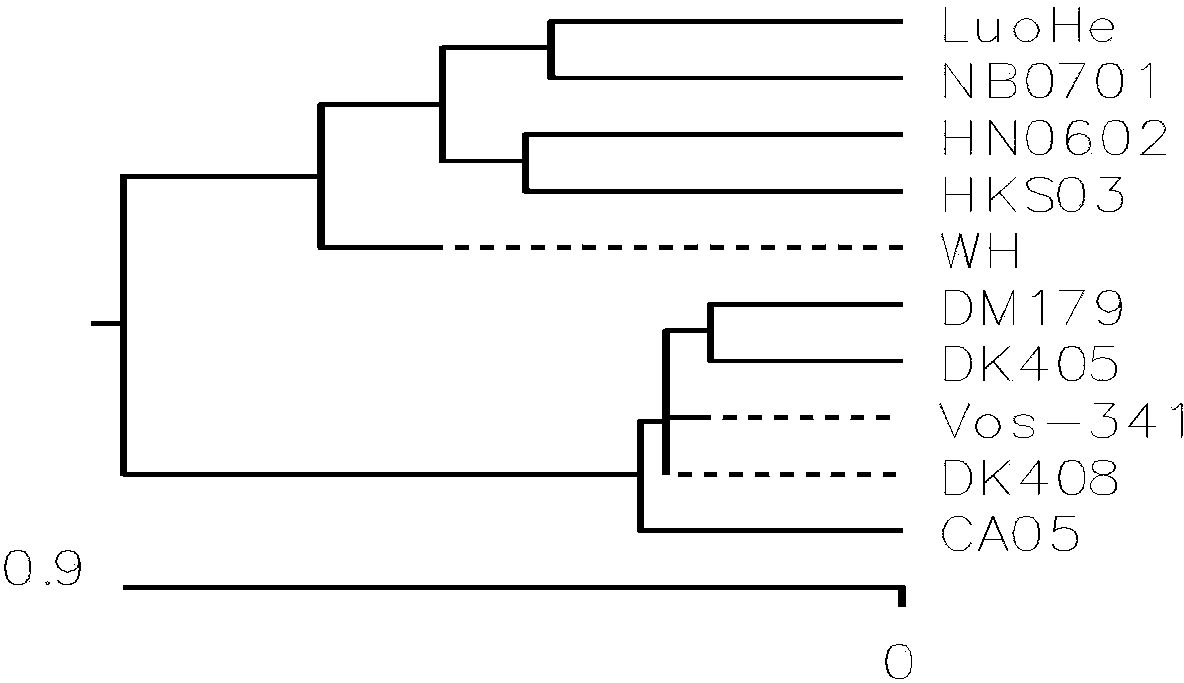
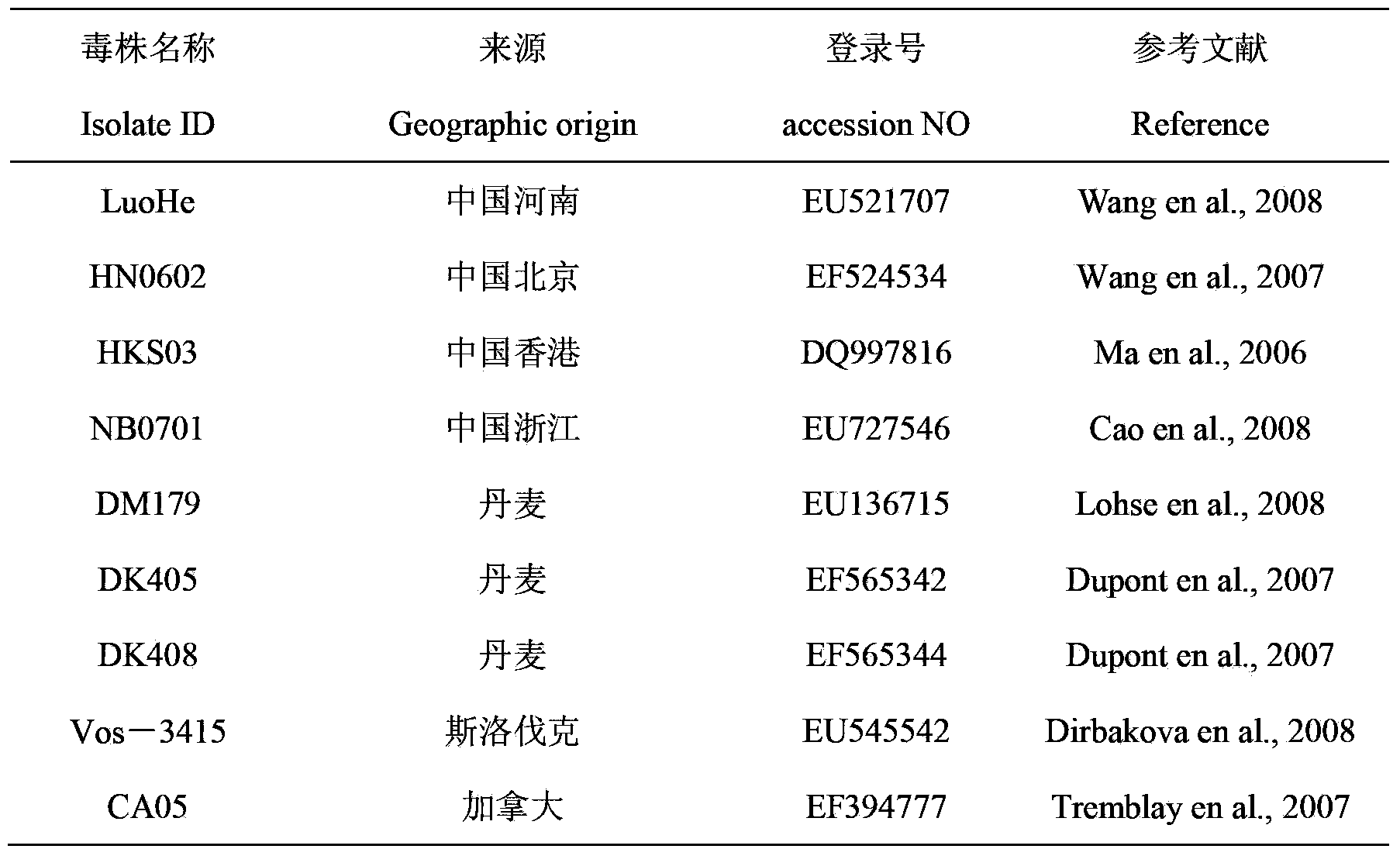
The triplex PCR-based rapid detection kit for Streptococcus suis Type 2 which is composed of four cryopreservation tubes containing different components, 100 PCR reaction tubes and the brochure of the detection steps for the cryopreservation tubes. The four cryopreservation tubes contain mixture of 10*buffer, dNTP, primer gdh-1, gdh-2, mrp-1, mrp-2,cps2J-1, cps2J-2 and Taq DNA polymerase; lysis buffer; positive control solution; diluent respectively. The triplex PCR process established in the present invention can amplify gene gdh, mrp and cps2J of SS-2 at the same time and has high specificity. The homology of sequencing is always between 98.84% and 100% exhibiting high accuracy and specificity.
Owner:ZHEJIANG UNIV
Streptococcus suis type 2 three-component subunit vaccine and use
ActiveCN101412984AImproving immunogenicityImprove the immunityAntibacterial agentsBacterial antigen ingredientsEscherichia coliRecombinant escherichia coli
The present invention belongs to the technical field of murrain vaccine preparation, and in particular relates to a Streptococcus suis type 2 trimaceral subunit vaccine and a preparation method thereof. The key technology is that recombinant Escherichia coli such as Escherichia coli BL21 / pET-28a-1036N, Escherichia coli BL21 / pET-28a-0197 and Escherichia coli BL21 / pET-28a-enolase is prepared, which can excrete and express Streptococcus suis type 2 antigen albumen 1036N,0197 and enolase, and which is preserved as CCTCC NO M208147, CCTCC NO M208146 and CCTCC NO M208148 in China Center for Type Culture Collection respectively. The present invention also discloses a preparation method and a use suitable for the trimaceral subunit vaccine of Streptococcus suis type 2.
Owner:HUAZHONG AGRI UNIV
Electrochemical immunosensor making method and Streptococcus suis detection method using electrochemical immunosensor
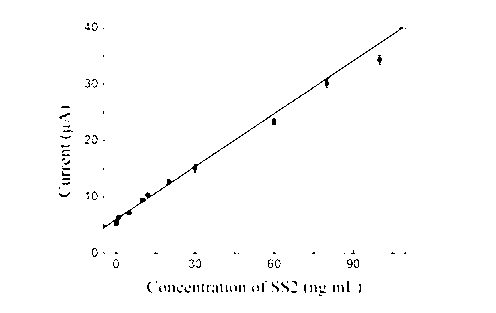
InactiveCN103207218AEasy to prepareReduce manufacturing costMaterial electrochemical variablesBovine serum albuminElectrochemistry
An electrochemical immunosensor making method is characterized in that the making method comprises the following steps: modifying a glassy carbon electrode by nano-gold, washing with a buffer solution, airing, coating the surface of the glassy carbon electrode by one droplet of a Streptococcus suis type 2 antibody, staying at 4DEG C overnight, and immersing the obtained glassy carbon tube in a bovine serum albumin solution to obtain an electrochemical immunosensor. The electrochemical immunosensor has a simple making method and is convenient to use, and the electrochemical immunosensor type 2 detection method using the electrochemical immunosensor has the advantages of simplicity, easy implementation, high sensitivity, short response time, low detection cost, accurate and reliable detection result, and creation of important conditions for the diagnosis and treatment of cardiovascular diseases.
Owner:SOUTHWEST UNIVERSITY
Haemophilus parasuis detection kit and detection method thereof
ActiveCN104711359AHigh sensitivityAccurate distinctionMicrobiological testing/measurementMicroorganism based processesPasteurellaBacilli
The invention relates to a haemophilus parasuis detection kit and a detection method thereof, and belongs to the technical field of molecular biology. The detection kit comprises a primer pair, PCR Mix, a position control and dd H2O. The haemophilus parasuis detection kit disclosed by the invention has the primer pair designed according to an mviN gene sequence in a high conserved domain, is good in specificity, and can accurately distinguish the haemophilus parasuis strain LC from haemophilus paragallinarum, actinobacillus pleuropneumoniae, pasteurella muhocida, arcanobacterium pyogenes, staphylococcus aureus and streptococcus suis; the detection kit and the detection method provided by the invention are high in sensitivity, short in consumed time, accurate in detection, and important in significance of monitoring haemophilus parasuis reproduction, disease occurrence and prevalence as well as timely control of the disease.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Novel tetravalent inactivated vaccine for streptococcus suis disease
ActiveCN101703769AOvercoming deficiencies in infectionAchieve the effect of multiple defenses with one injectionAntibacterial agentsBacterial antigen ingredientsDiseaseAntigen
The invention relates to a novel tetravalent inactivated vaccine for streptococcus suis disease, which is prepared from a Lancefield group C streptococcus equi zooepidemicus C55138 antigen, a Lancefield group D streptococcus C55914 antigen, a Lancefield group E streptococcus C55949 antigen and a streptococcus suis type 2 HA9801 antigen by the steps of strain reproduction, bacterial liquid cultivation, pure inspection, live bacteria counting, inactivation, inactivation inspection, sedimentation and concentration, toxin removal, vaccine preparation by mixing, asepsis inspection of the vaccine, potency inspection and subpackaging. The novel tetravalent inactivated vaccine for the streptococcus suis disease can take precautions against four streptococcus suis diseases caused by Lancefield group C streptococcus, Lancefield group D streptococcus, Lancefield group E streptococcus and streptococcus suis type 2 through one-time vaccination, and has wide application prospect.
Owner:广东永顺生物制药股份有限公司
Preparation method of triple inactivated vaccine for pigs
InactiveCN104998256AImprove immunityHighlight immune functionAntibacterial agentsBacterial antigen ingredientsAntigenProtective antigen
The invention provides a preparation method of a triple inactivated vaccine for pigs. The method determines an antigen composition with excellent immunization effects by selection of the antigen. The prepared polyvalent vaccine has outstanding immunization effects. The prepared vaccine contains a PCP immunization protective antigen exotoxin (Aps), has cross immunization protection effects better than those of a whole cell inactivated vaccine, greatly reduces side reaction, and can simultaneously prevent haemophilus parasuis, swine streptococcosis and actinobacillus pleuropneumonia by combined immunization with inactivated haemophilus parasuis and streptococcus suis. Compared with haemophilus parasuis and streptococcus suis inactivated vaccines sold on the market, the triple inactivated vaccine has the same corresponding pathogen immune protection force. Compared with the actinobacillus pleuropneumonia inactivated vaccine sold on the market, the triple inactivated vaccine has a cross immunization protecting force on diseased pigs with different serotypes and realizes multiple protection purposes.
Owner:TIANJIN RINGPU BIO TECH
Natural antimicrobial peptides and application thereof
InactiveCN107857803AImprove the bactericidal effectHigh antibacterial activityAntibacterial agentsCosmetic preparationsPichia pastorisFood additive
The invention discloses a group of natural antimicrobial peptides derived from fungi. Amino acid sequences of the natural antimicrobial peptides are separately shown in SEQ ID NO: 3-4. Mature peptidesequences of the antimicrobial peptides are obtained through retrieving a fungal protein database and carrying out screening analysis; proven by comparative analysis, the amino acid sequences of the natural antimicrobial peptides are obviously different from those of all currently-known antimicrobial peptides, and the natural antimicrobial peptides belong to novel antimicrobial peptides. The antimicrobial peptides are optimized through codon, and then, the recombinant expression of the antimicrobial peptides can be achieved in pichia pastoris. Proven by antimicrobial experiments, the antimicrobial peptides disclosed by the invention have remarkable bacteriostasis activity to gram-positive bacteria, particularly Staphylococcus aureus ATCC43300, Streptococcus suis CVCC 3928 and CVCC 606, canbe applied to the fields of antibacterials, food additives, cosmetics, feed additives, preservatives and the like and have broad application values and market prospects.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Novel lactococcus garvieae bacteriophage lac-gap-1 and use thereof in suppressing proliferation of lactococcus garvieae bacteria
ActiveUS20180000125A1Strong specificityLess side effectsAnimal feeding stuffViral/bacteriophage medical ingredientsAnaplasma phagocytophilumNucleotide
The present invention relates to a Myoviridae bacteriophage Lac-GAP-1 that is isolated from the nature and can kill specifically Lactococcus garvieae cells, which has a genome represented by the nucleotide sequence of SEQ. ID. NO: 1 (Accession NO: KCTC 12686BP), and a method for preventing and treating the infections of Lactococcus garvieae using the composition comprising said bacteriophage as an active ingredient.
Owner:INTRON BIOTECHNOLOGY INC
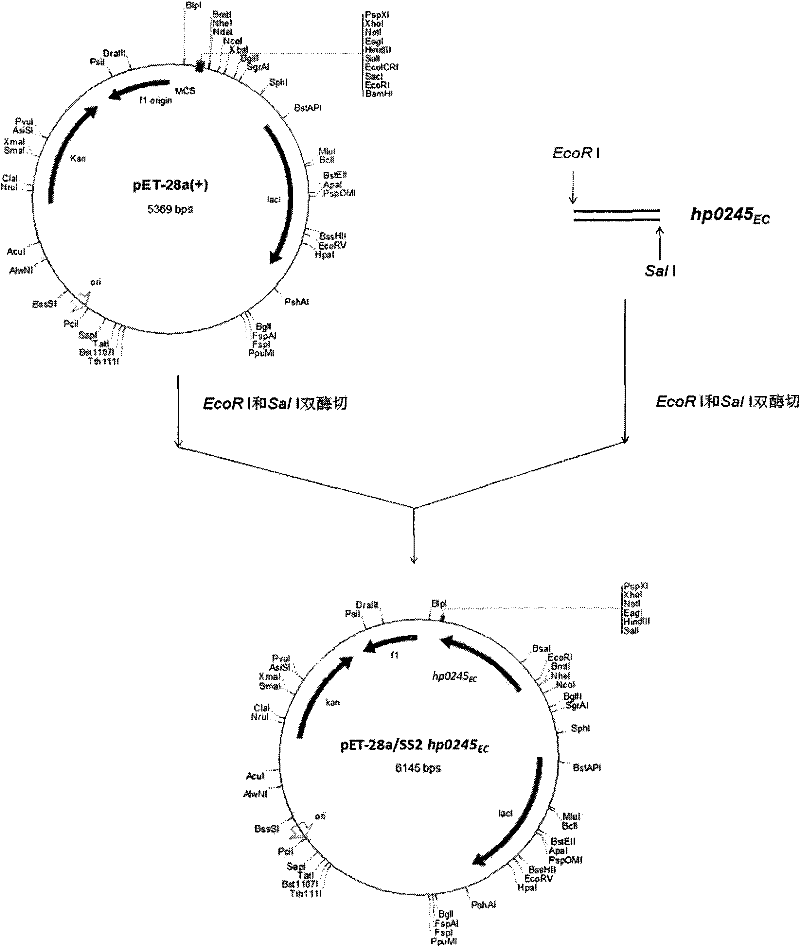
Application of using streptococcus suis type-2 hy0245 gene encoded proteins as protective antigens
InactiveCN102443053AImproving immunogenicityGood immune protectionAntibacterial agentsBacterial antigen ingredientsEscherichia coliProtective antigen
The invention belongs to the technical fields of veterinary microbiology and zoonotic diseases, and in particular relates to application of immunogenic protein genes of streptococcus suis type-2 for separating, cloning and expressing exocellular peptides and recombinant proteins of the encoded proteins in vaccine and diagnosis. In the invention, a new immunogenic protein gene hp0245 is separated from SC-19 bacterial strains of streptococcus suis type-2 virulent strains, wherein DNA (hp0245EC) for encoding the exocellular peptides has nucleotide sequences represented in the sequence table SEQ ID No:1 and is encoded with 261 amino acids. The recombinant protein HP0245EC remains the immunogenic feature of the primitive protein, can provide effective immunological protection to the SC-19 bacterial strain of the streptococcus suis type-2 infected in mice and has potential application value of vaccine. The invention further comprises a composition and preparation method for a streptococcus suis type-2 HP0245-ELISA diagnostic reagent kit, and a preparation of hp0245EC for cloning hp0245EC escherichia coli; the recombinant escherichia coli has been preserved in the China Center for Type Culture Collection (CCTCC) and the preservation number is CCTCC No: M2010258.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com