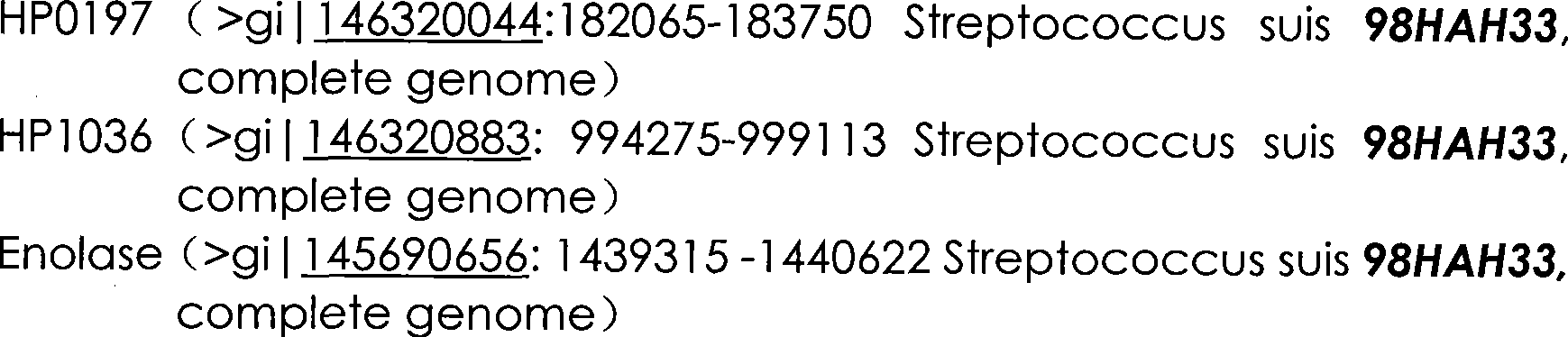Streptococcus suis type 2 three-component subunit vaccine and use
A subunit vaccine, Streptococcus suis technology, applied in the field of three-component subunit vaccine of Streptococcus suis type 2 and its preparation, can solve the problem of inability to clear bacteria of recessive infection, inability to clear type 2 Streptococcus suis, and the inability to exist anti-bacteria Drug particles and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Cloning and expression of three kinds of Streptococcus suis type 2 antigenic proteins
[0030] one material
[0031] 1) Plasmids and strains
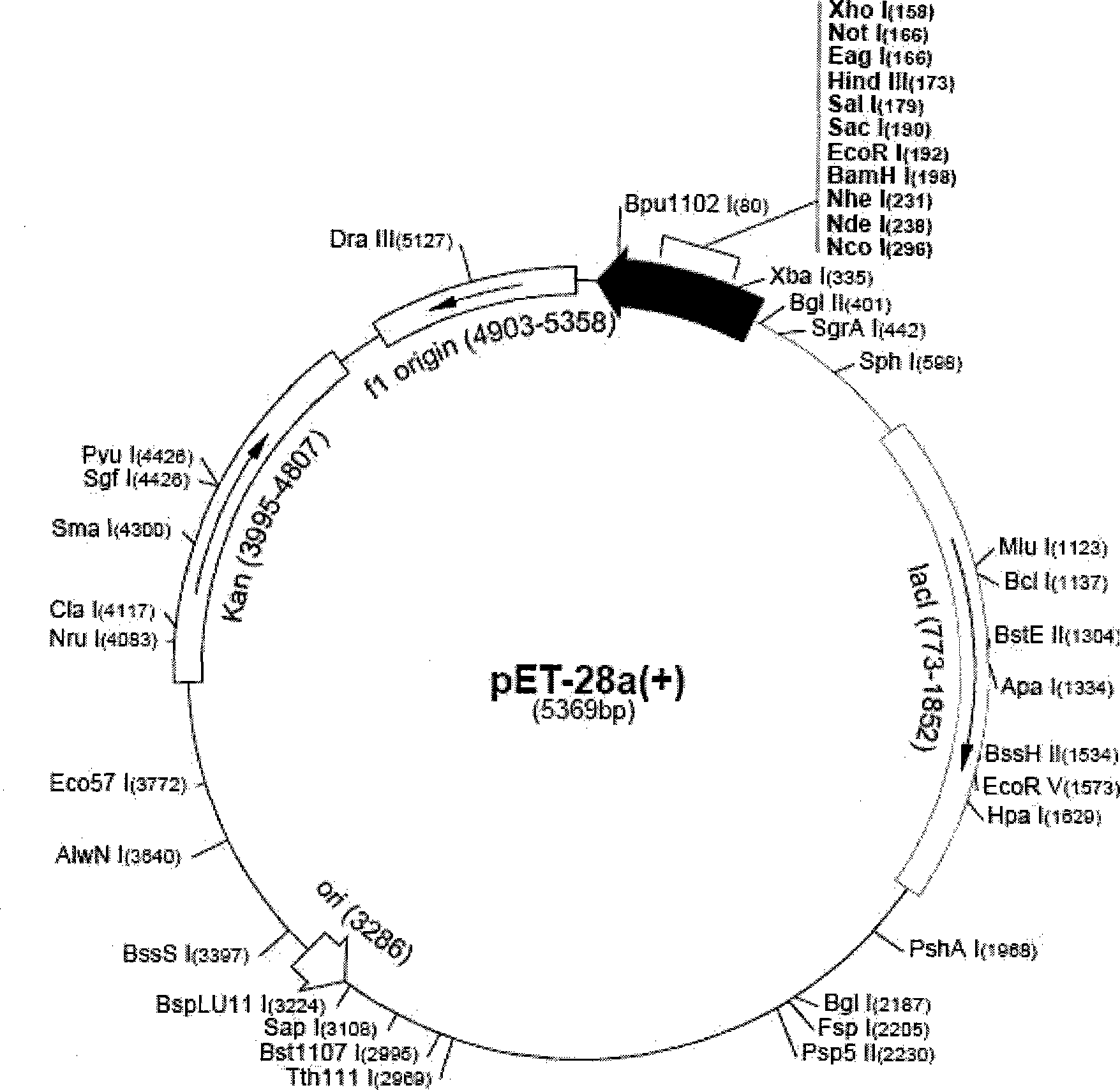
[0032] The plasmid pET-28a (+) that the present invention adopts is purchased from Noagen company (this pET-28a (+) plasmid map is as follows figure 1 Shown) Competent Escherichia coli BL21 (DE3) was purchased from Wuhan Life Technology Co., Ltd., Hubei Province, China.
[0033] The bacterial strain used in the present invention is the Streptococcus suis type 2 CVCC606 bacterial strain purchased from the China Veterinary Drug Control Institute in Beijing, China, and belongs to a commercial bacterial strain.
[0034] 2) The accession number of the gene derived from Streptococcus suis type 2, the position of the gene sequence in the genome of Streptococcus strains can be found in the appendix of the instructions figure 1 mentioned.
[0035] 3) Main reagents and buffer (Buffer)
Embodiment 2
[0124] Example 2: Characteristic Analysis of Recombinant Antigen Protein
[0125] 1. Western-blot analysis of the antigenicity of the recombinant protein
[0126] The above-mentioned purified recombinant antigen proteins 0197, 1036N and enolase were subjected to SDS-PAGE electrophoresis respectively. Proceed as follows:
[0127] 1) Transfer: Cut out 6 pieces of Whatman 3M filter paper and 1 piece of nitrocellulose membrane (NC membrane). The size of the filter paper and membrane should be exactly the same as the size of the gel or slightly smaller than the size of the gel. Mark the corner of the filter membrane with a pencil. Ensure the relative direction of the membrane and gel after transfer; soak the nitrocellulose membrane in purified water for 5 minutes; add a small amount of transfer buffer to another shallow tray, and soak 6 pieces of Whatman 3M filter paper in it. Then install the transfer electrophoresis tank as follows: lay the base (anode) of the graphite electrod...
Embodiment 3
[0143] Embodiment 3: Recombinant antigenic protein immune protection test
[0144] Expression and purification of recombinant antigens
[0145] The expression strains containing pET-28a-0197, pET-28a-1036N, and pET-28a-enolase plasmids were inoculated in 3 mL LB liquid medium containing 0.25 μg / ml, respectively, and cultured on a shaker at 37°C. Take 100 μL of the cultured bacterial liquid and inoculate it into 10 mL of fresh LB liquid medium containing 2.5 μg kanamycin, culture it with shaking at 37°C for about 3 hours, until OD 600 When it reaches 0.6-1.0, add IPTG to a final concentration of 0.8mmol / L, continue to cultivate for 3h, and then collect the bacteria.
[0146] The recombinant antigen-expressing cells collected above were resuspended with Binding buffer (20mM Tris-HCl pH7.9, 5mMlmidazole, 0.5MNaCl), ultrasonically disrupted, centrifuged at 12,000g at 4°C for 15min, and the supernatant was taken for loading. Use Binding buffer and Washing buffer (20mM Tris-HCl pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com