Patents
Literature
566 results about "Recombinant antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A recombinant antigen on a drug-resistant bacterium is created when multiple proteins on the bacterium's surface bind together in a way that makes certain antibiotics unable to destroy the bacterium. This resistance allows the organism to multiply and spread more recombinant antigens around its host.
Formyl methionyl peptide vaccine adjuvant
InactiveUS6017537AConvenient easy to formulateStimulate immune responseBiocideNanotechPeptide vaccinePeptide
The present invention relates to immunological adjuvants comprised of the N-formyl methionyl peptide fMLP. FMLP, when used as an adjuvant in accordance with the present invention, provides for an immune response to suboptimal doses of recombinant antigens.
Owner:VIROGENETICS
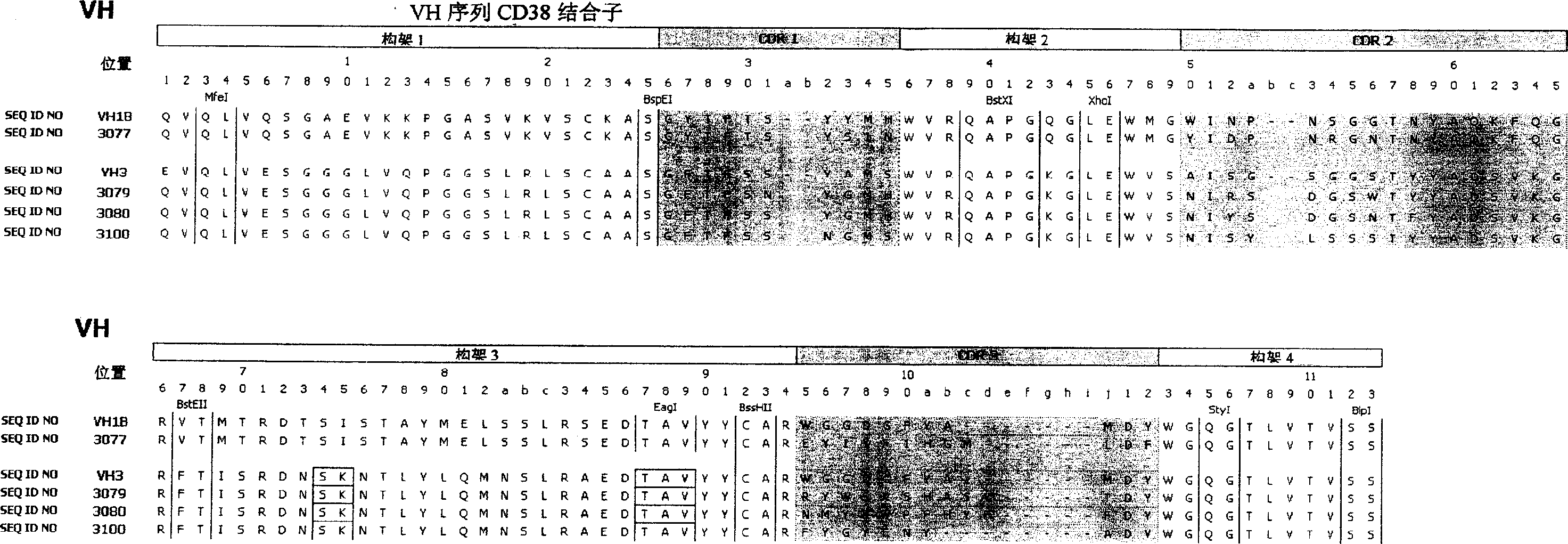
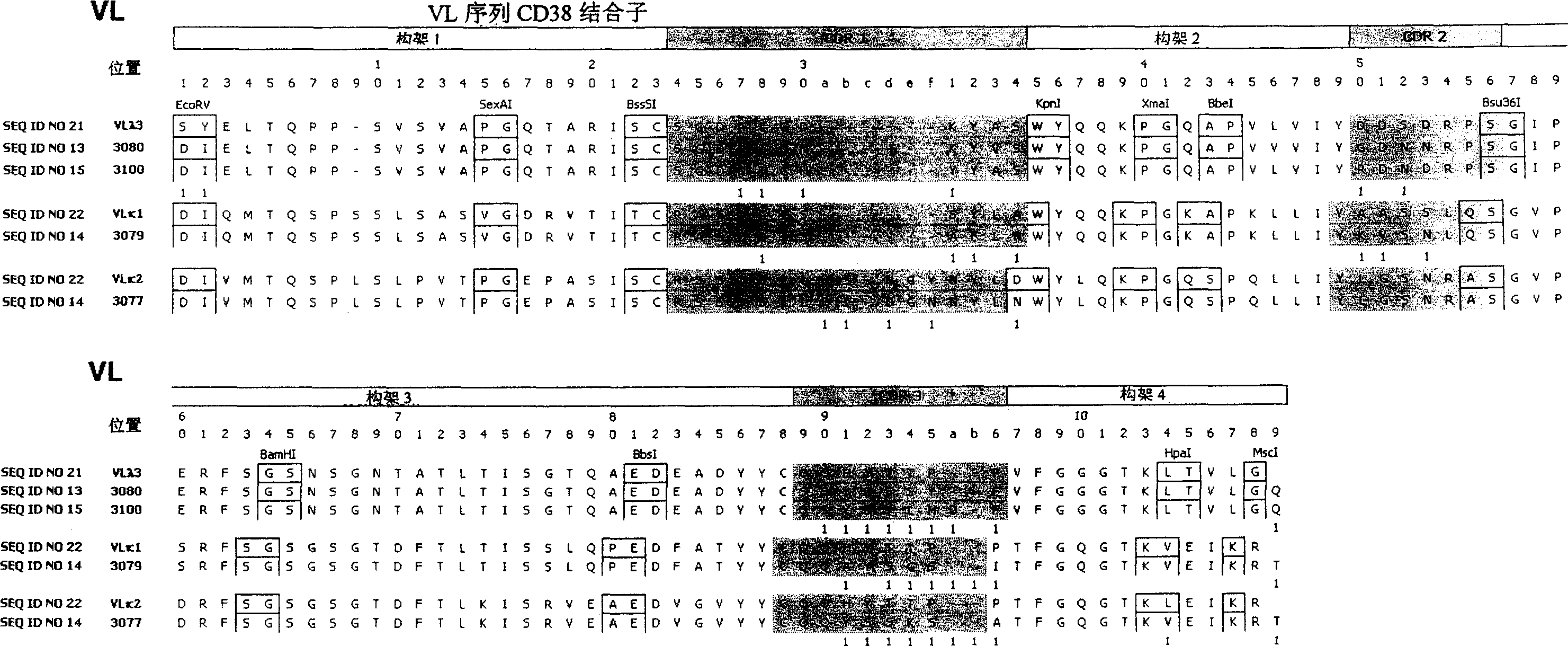
Anti-CD38 human antibodies and uses thereof
ActiveUS20100285004A1Effectively mediate killing of CD38-overexpressingBacteriaPeptide/protein ingredientsAntiendomysial antibodiesAntigen binding
The present invention provides recombinant antigen-binding regions and antibodies and functional fragments containing such antigen-binding regions that are specific for CD38, which plays an integral role in various disorders or conditions. These antibodies, accordingly, can be used to treat, for example, hematological malignancies such as multiple myeloma. Antibodies of the invention also can be used in the diagnostics field, as well as for investigating the role of CD38 in the progression of disorders associated with malignancies. The invention also provides nucleic acid sequences encoding the foregoing antibodies, vectors containing the same, pharmaceutical compositions and kits with instructions for use. The invention also provides isolated novel epitopes of CD38 and methods of use therefore
Owner:MORFOZIS AG
Detection kit of IgM/IgG antibodies of novel coronavirus (SARS-CoV-2)
ActiveCN111187354AImprove response accuracyOvercoming cumbersome detection operationsSsRNA viruses positive-senseAntibody mimetics/scaffoldsEpitopeCoronavirus antibody
The invention provides a detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), and relates to the technical field of biology. According to the detection kit of IgM / IgG antibodies of novel coronavirus (SARS-CoV-2), the IgM / IgG antibodies of novel coronavirus (SARS-CoV-2) are detected through adoption of a colloidal gold capture method, a colloidal gold indirect method and a colloidal gold double antigen sandwich method, wherein adopted antigens are highly active recombinant antigens which are obtained through fusion expression of N protein fragments and S protein dominant epitope fragments, and colloidal gold is labeled indirectly, so that steric hindrance is reduced, and the activity, reaction consistency and accuracy of the antigens are increased. Since a sample pad is pretreated, the interference of complex components in a blood sample to a reaction can be reduced greatly, the detection kit has no requirements for instruments during detection, simple and flexible operation, direct and manual interpretation of results and a short test time, the results can be interpreted in only 15 minutes, and the test results are accurate.
Owner:北京新创生物工程有限公司
Method for rapid detection of lymphatic filariasis
InactiveUS20100021926A1The process is simple and fastRapid and simple diagnosisBiological testingAntibody conjugateFilarial Elephantiasis
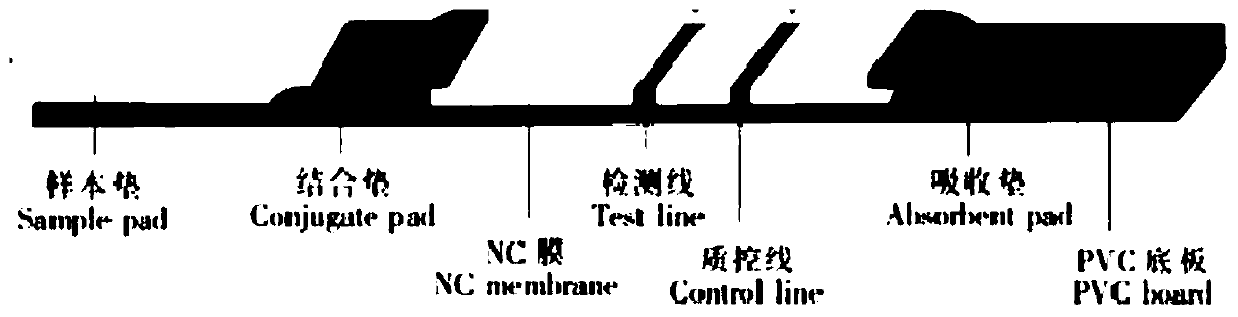
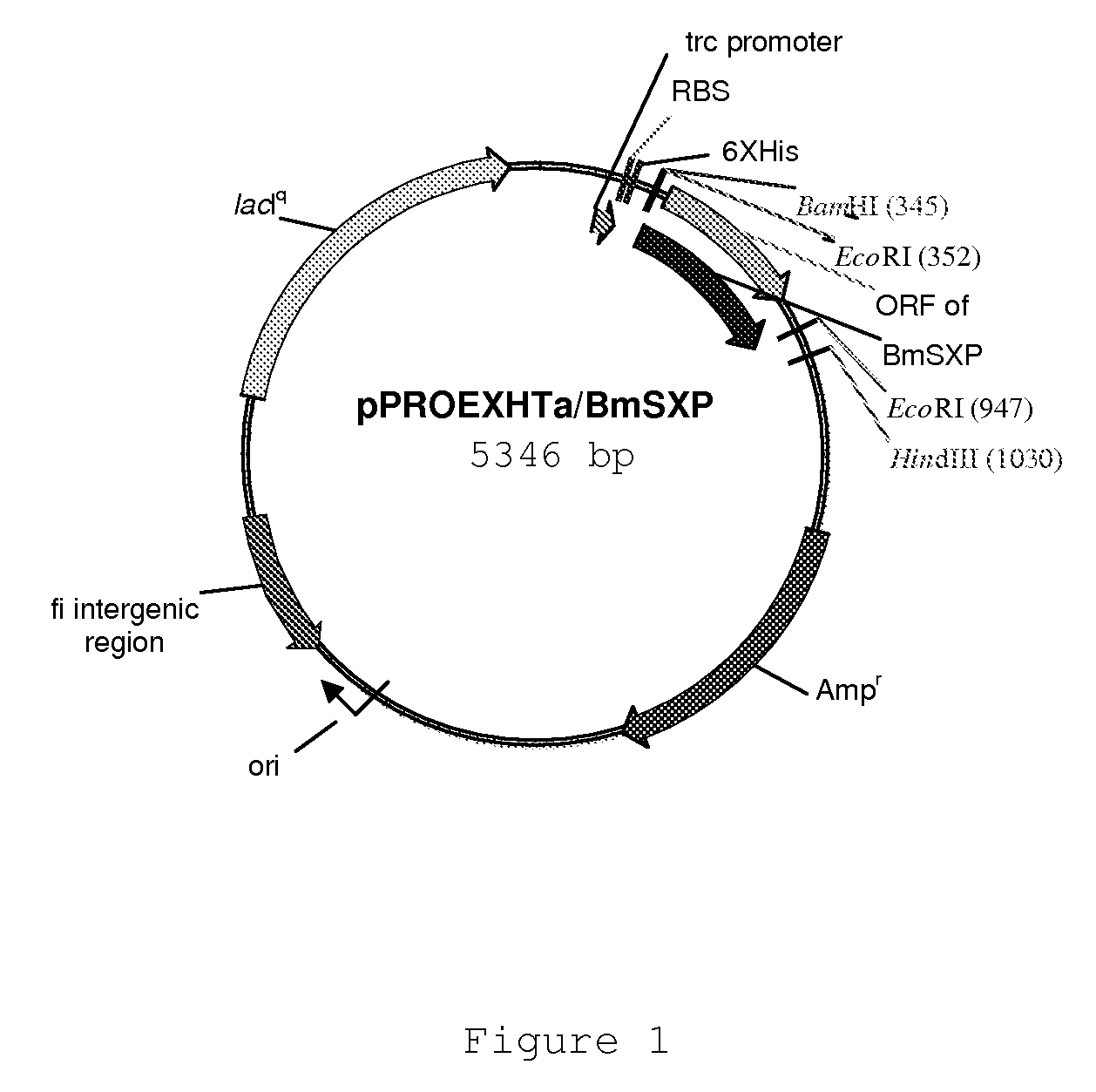
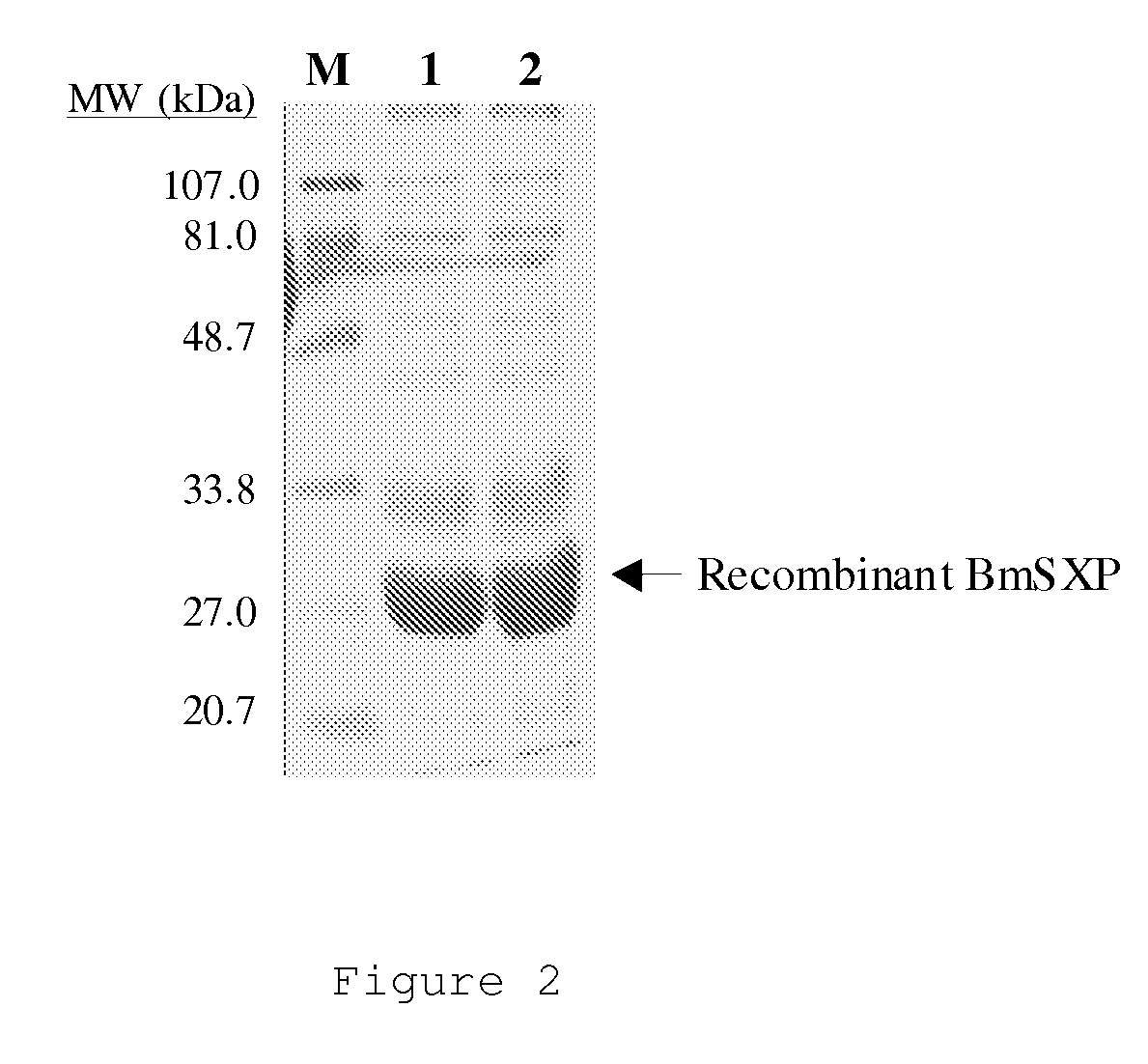
There is provided by this invention a specific and sensitive diagnostic method for rapid detection of lymphatic filariasis. The method employs a combination of SXP / SXP-recombinant antigen, mouse monoclonal anti-human IgG4 antibody conjugated to a detection reagent and the technique of immunochromatography.
Owner:UNIVERSITI SAINS MALAYSIA
Anti-Gm-Csf Antibodies and Uses Therefor
The present invention provides recombinant antigen-binding regions, antibodies and functional fragments thereof that are specific for GM-CSF, which plays an integral role in various disorders or conditions. These antibodies, accordingly, can be used to treat, for example, inflammatory diseases such as rheumatoid arthritis. Antibodies of the invention also can be used in the diagnostics field, as well as for further investigating the role of GM-CSF in the progression of various disorders. The invention also provides nucleic acid sequences encoding the foregoing antibodies, vectors containing the same, pharmaceutical compositions and kits with instructions for use.
Owner:MORFOZIS AG
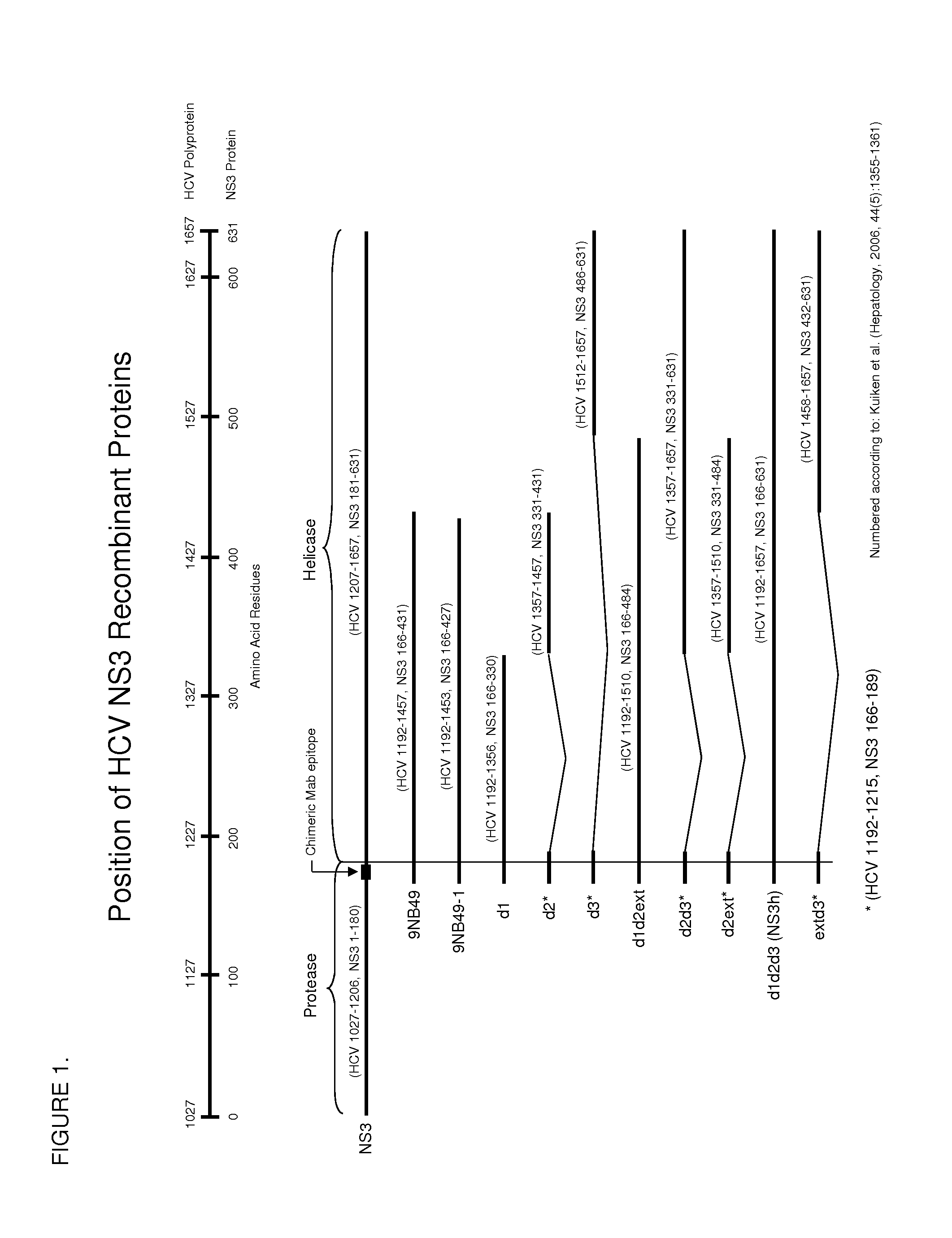
Hcv ns3 recombinant antigens and mutants thereof for improved antibody detection
ActiveUS20140272932A1Excellent redox stabilityImprove stabilitySsRNA viruses positive-senseBacteriaAnti hcv antibodyHCV Antibody
Owner:ABBOTT LAB INC
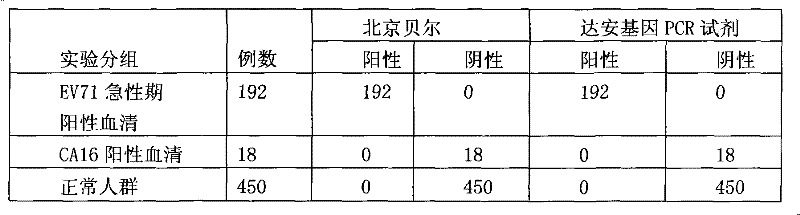
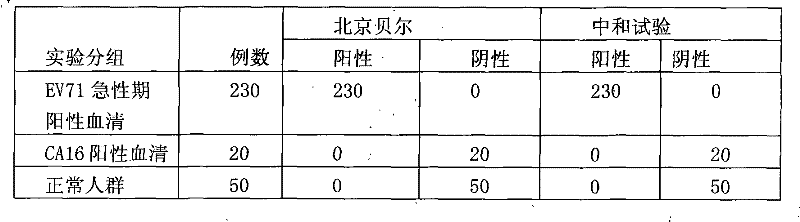
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
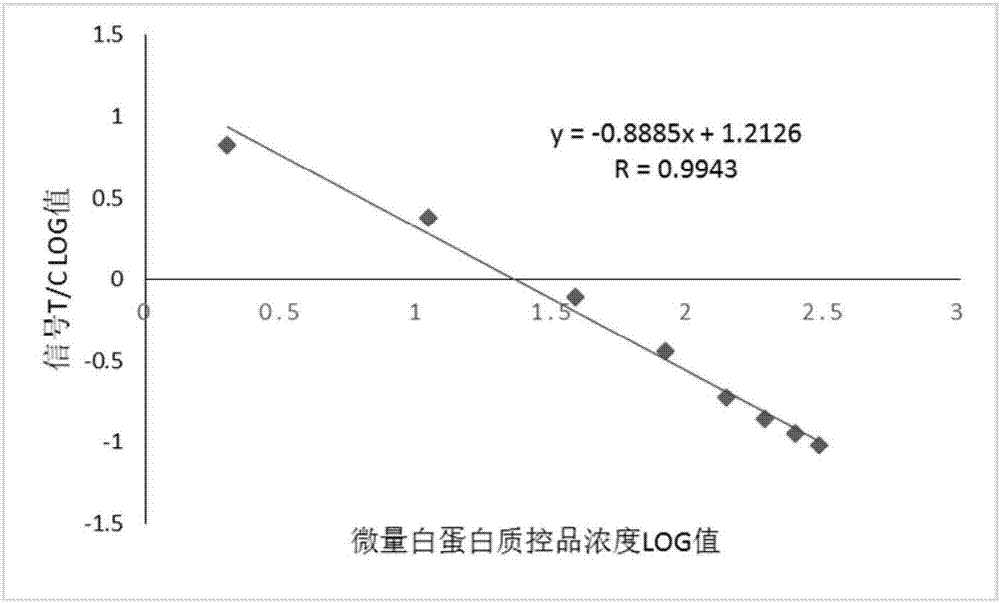
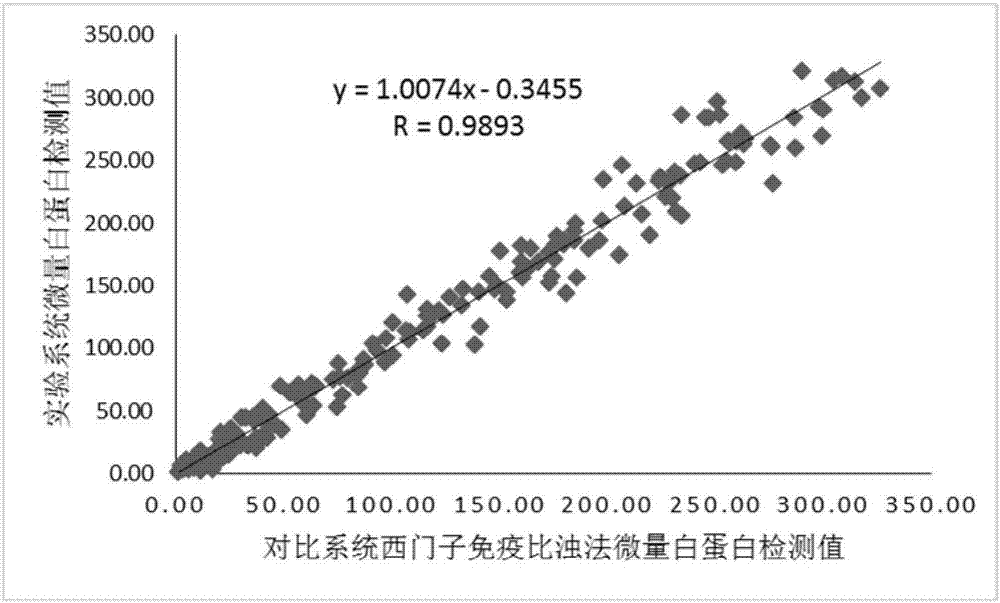
Kit and method for quantitatively detecting urine microalbumin through time-resolved fluorescence
ActiveCN106872420AAvoid fluorescence interferenceMark stableFluorescence/phosphorescenceFluorescenceMicrosphere
The invention relates to a kit and a method for quantitatively detecting urine microalbumin through time-resolved fluorescence. The kit comprises a time-resolved fluorescence test paper card, sample diluent and a DS card containing a calibration curve, wherein the time-resolved fluorescence test paper card comprises a detection test paper strip which consists of a bottom liner, and a sample pad, a nitrocellulose membrane and absorbent paper which are pasted on the bottom liner; the nitrocellulose membrane is successively provided with a microsphere line, a detection line and a quality control line; the microsphere line consists of time-resolved fluorescence microspheres marked with human seroalbumin monoclonal antibodies; the detection line is coated with human seroalbumin recombinant antigens; the quality control line is coated with goat anti-mouse IgG. The kit provided by the invention can accurately and quantitatively detect the content of microalbumin in human urine; markers are used for connecting the microspheres with antibodies through covalent bonds; and the marked products are stable. The kit has the characteristics of wide detection range (2 to 300mg / ml), high sensitivity (the detection limit is 2300mg / ml), high accuracy, fast and easy detection and the like.
Owner:厦门奥德生物科技有限公司
Anti-GM-CSF antibodies and uses therefor
Owner:MORFOZIS AG
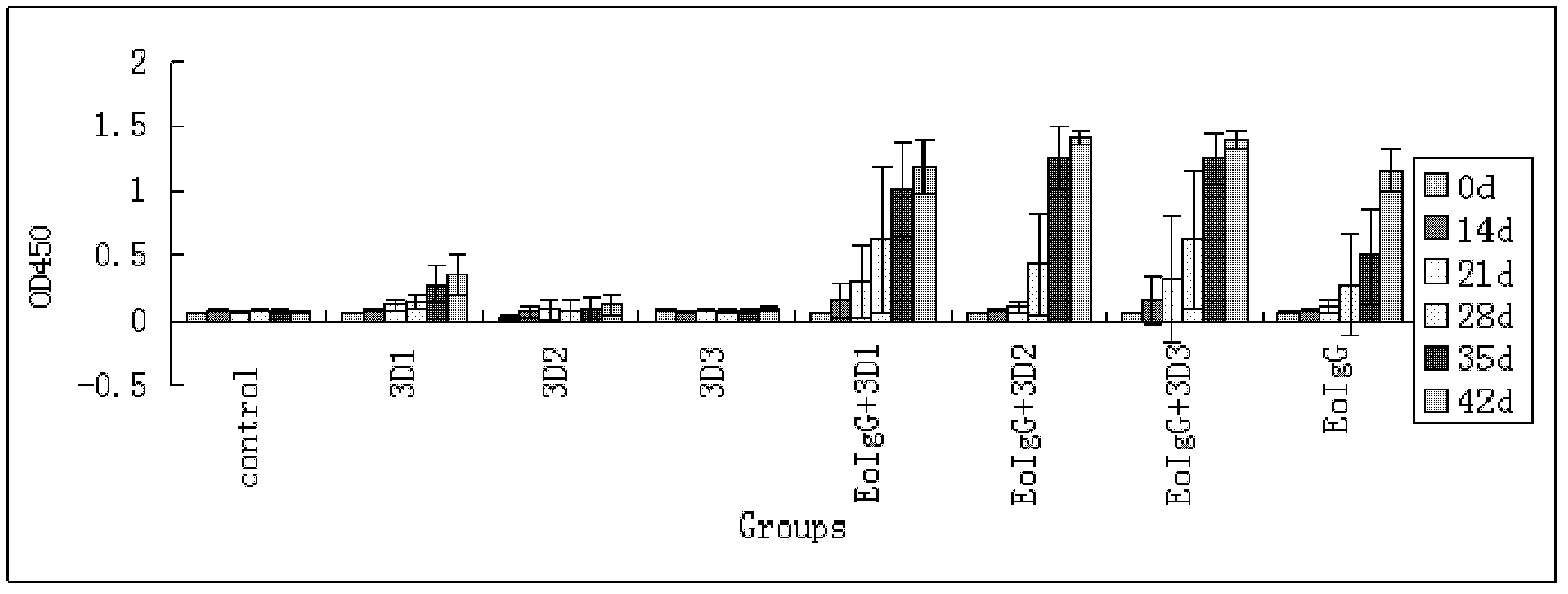
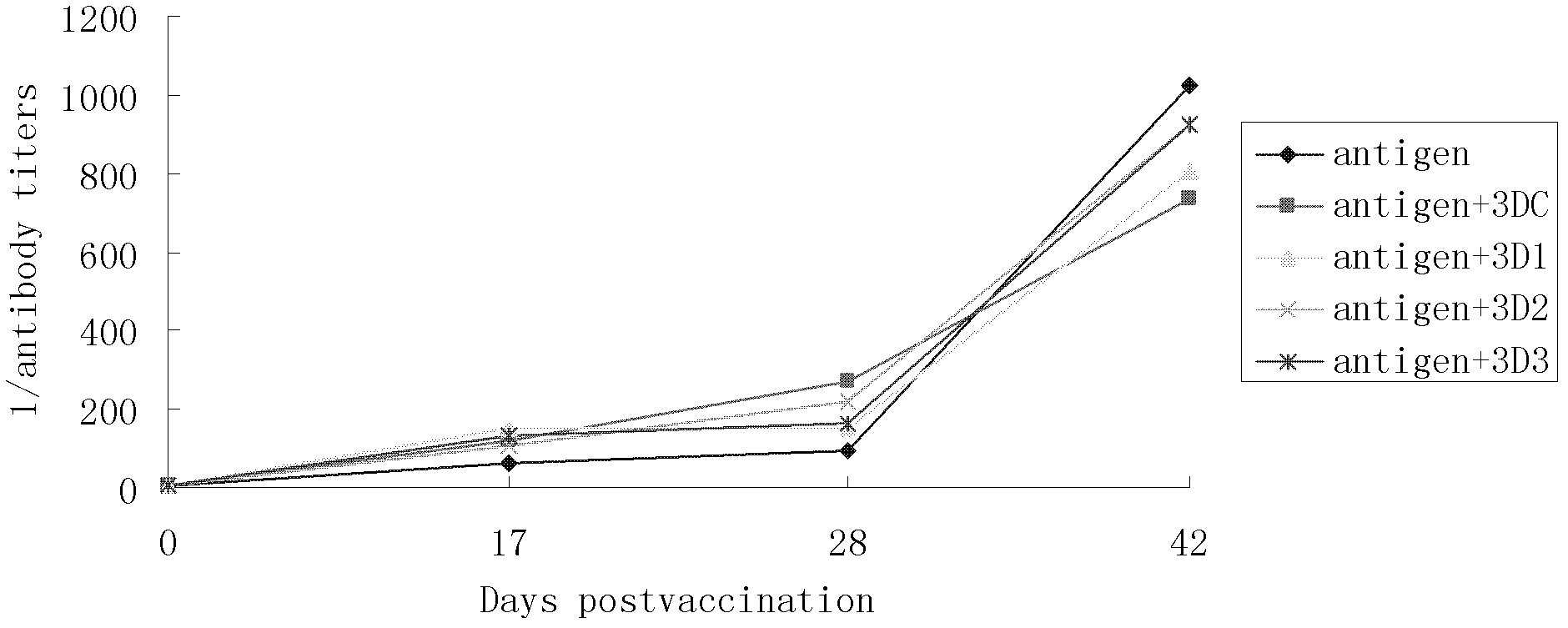
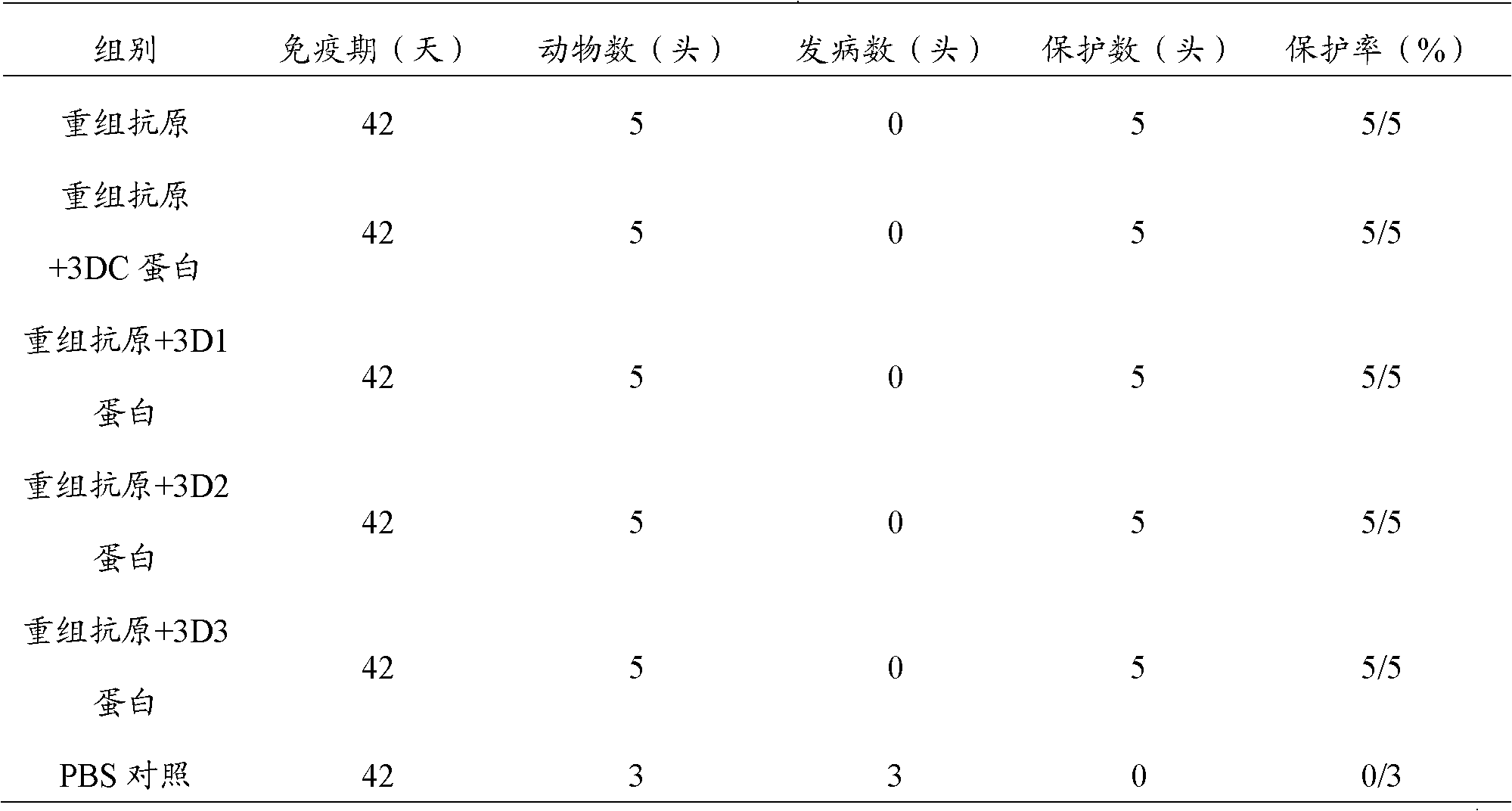
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
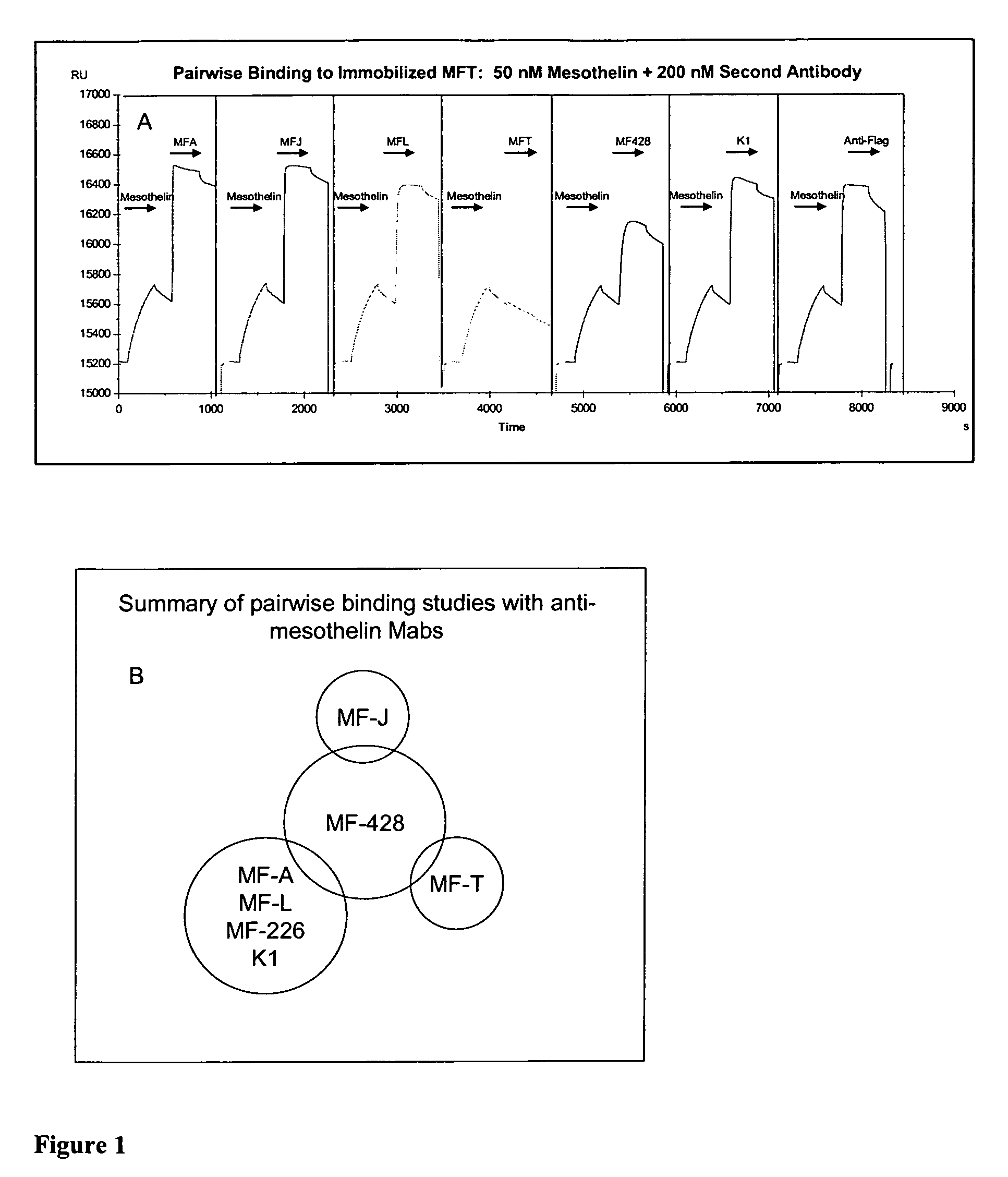
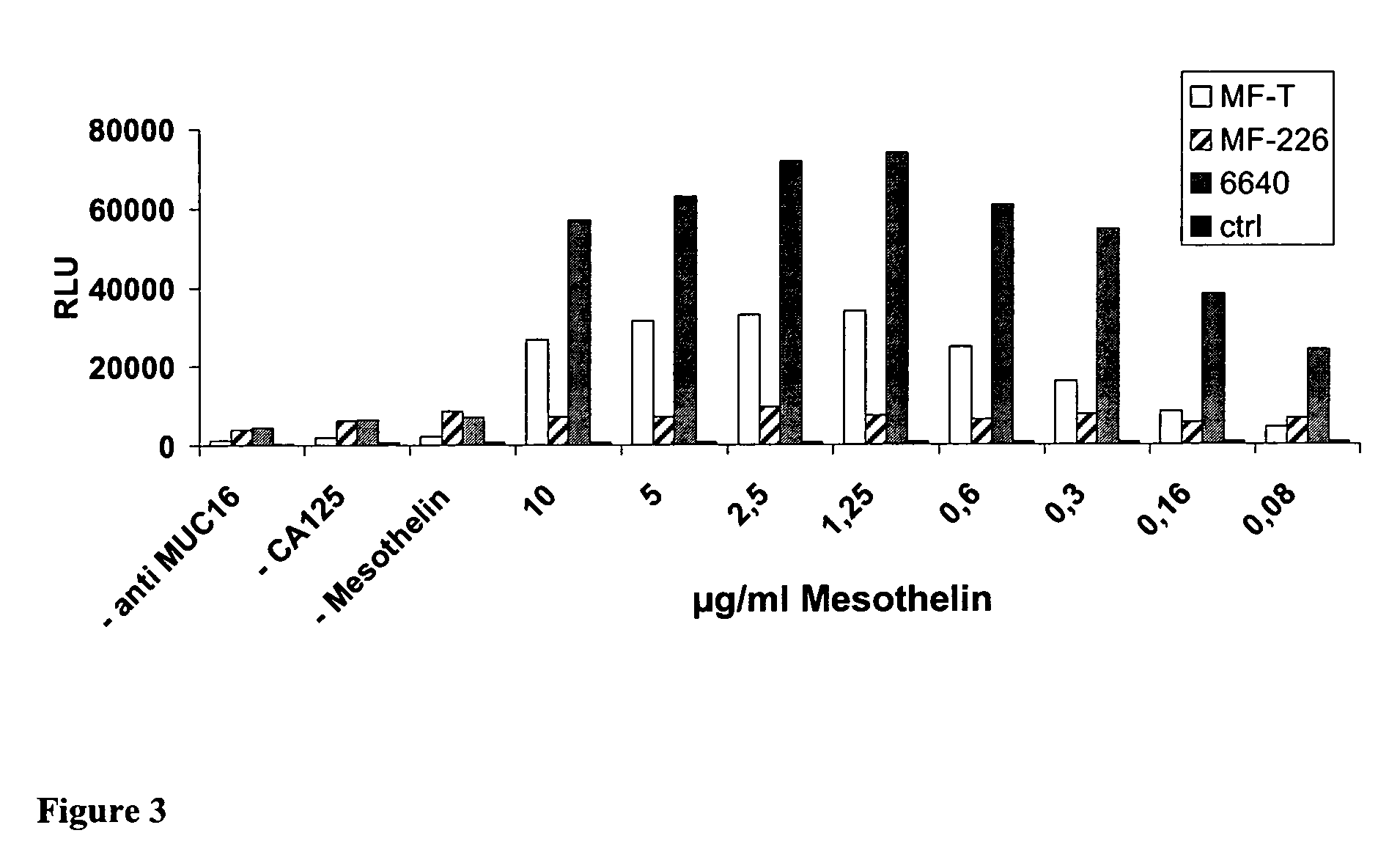
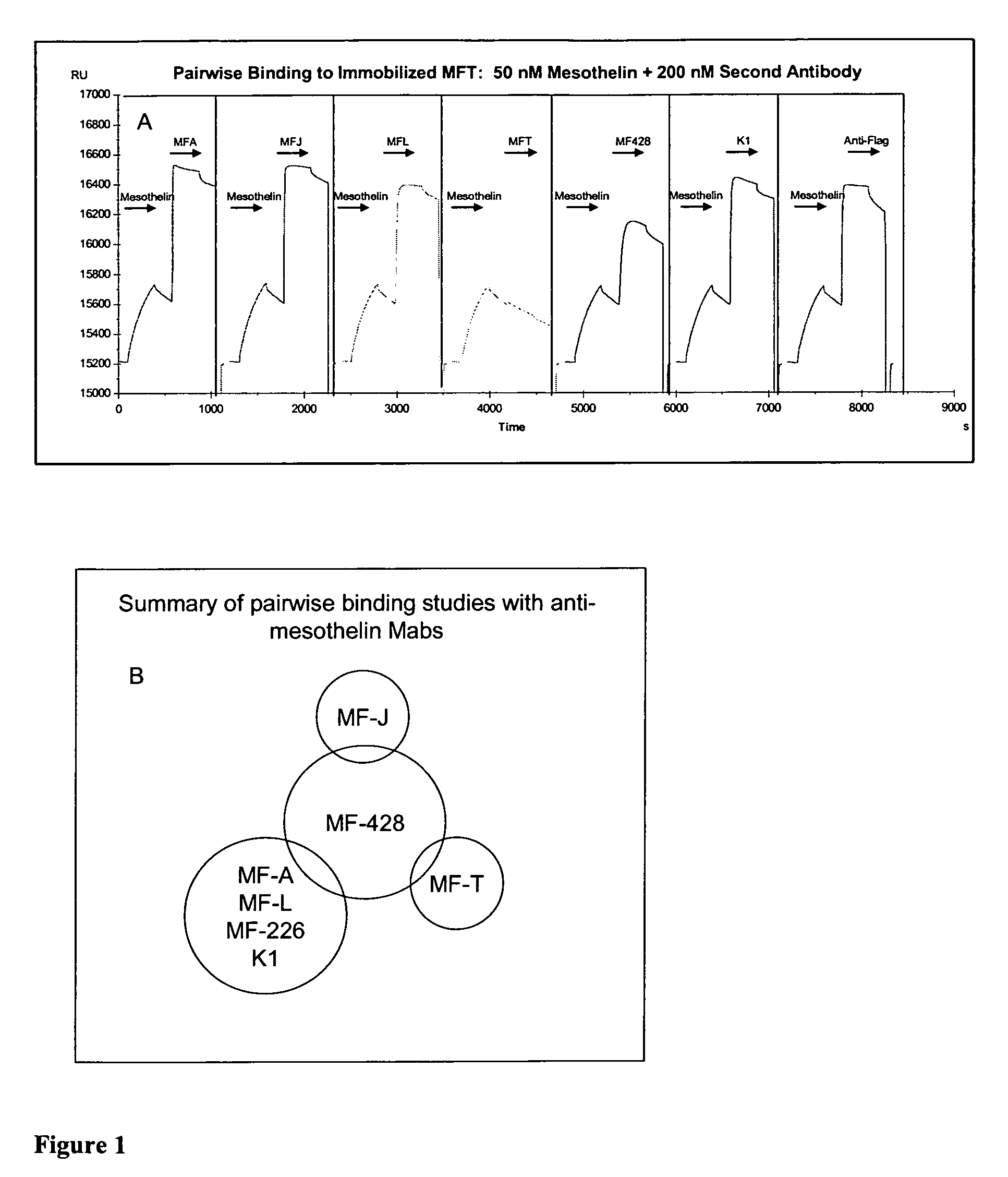
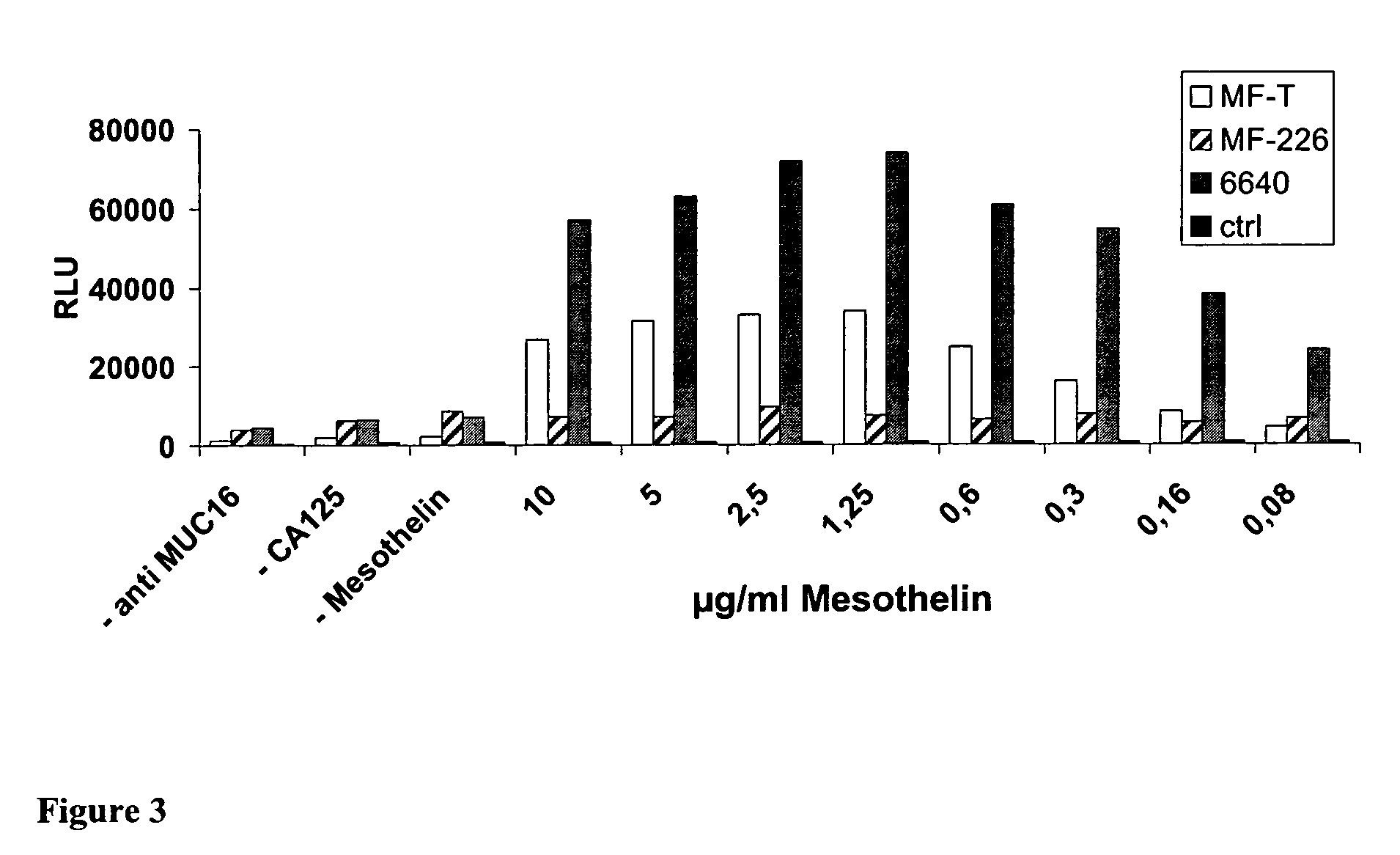
Anti-mesothelin antibodies and uses thereof
ActiveUS9023351B2BacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAnti-Mesothelin Antibody
The present invention provides recombinant antigen-binding regions and antibodies and functional fragments containing such antigen-binding regions that are specific for the membrane-anchored, 4O.kDa mesothelin polypeptide, which is overexpressed in several tumors, such as pancreatic and ovarian tumors, mesothelioma and lung cancer cells. These antibodies, accordingly, can be used to treat these and other disorders and conditions. Antibodies of the invention also can be used in the diagnostics field, as well as for further investigating the role of mesothelin in the progression of disorders associated with cancer. The invention also provides nucleic acid sequences encoding the foregoing antibodies, vectors containing the same, pharmaceutical compositions and kits with instructions for use.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Test strip for detecting HIV antibodies in spittle and preparation method thereof
The invention discloses a test strip for detecting HIV antibodies in spittle and a preparation method thereof. The test strip comprises a sample pad, a fiberglass film, a nitrocellulose film and absorbent paper, wherein the fiberglass film is tightly connected with one end of the sample pad and contains colloidal gold particle markers; the nitrocellulose film is tightly connected with the other end of the fiberglass film; the absorbent paper is tightly connected with the other end of the nitrocellulose film; the sample pad, the fiberglass film, the nitrocellulose film and the absorbent paper are all arranged on a base plate; the nitrocellulose film comprises a detection area coated with HIV recombinant antigens and a control area coated with goat anti rabbit antibodies; and the colloidal gold particle marker comprises a colloidal gold particle-avidin-biotin-antihuman IgG antibody composite micro-signal amplification system, and colloidal gold marking rabbie IgG antibodies. Due to the adoption of the avidin-biotin micro-signal amplification system, the test strip amplifies signals of the target antibodies, improves detection sensitivity and avoids false negative or detection omission caused by too weak signals.
Owner:GUANGZHOU WONDFO BIOTECH
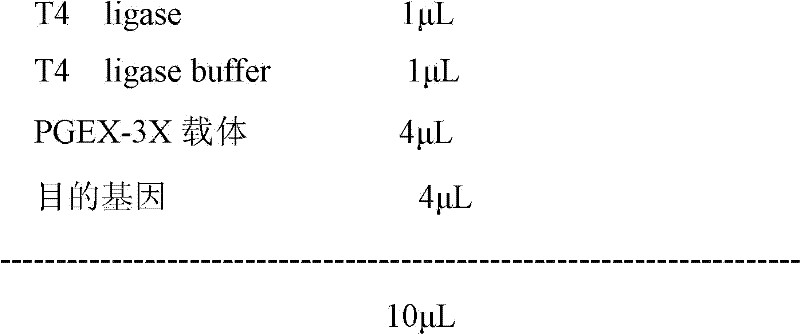
Recombinant antigenic protein for diagnosing echinococcosis granulosus, preparation method thereof and use thereof
ActiveCN101948521AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingEscherichia coliCystic echinococcosis
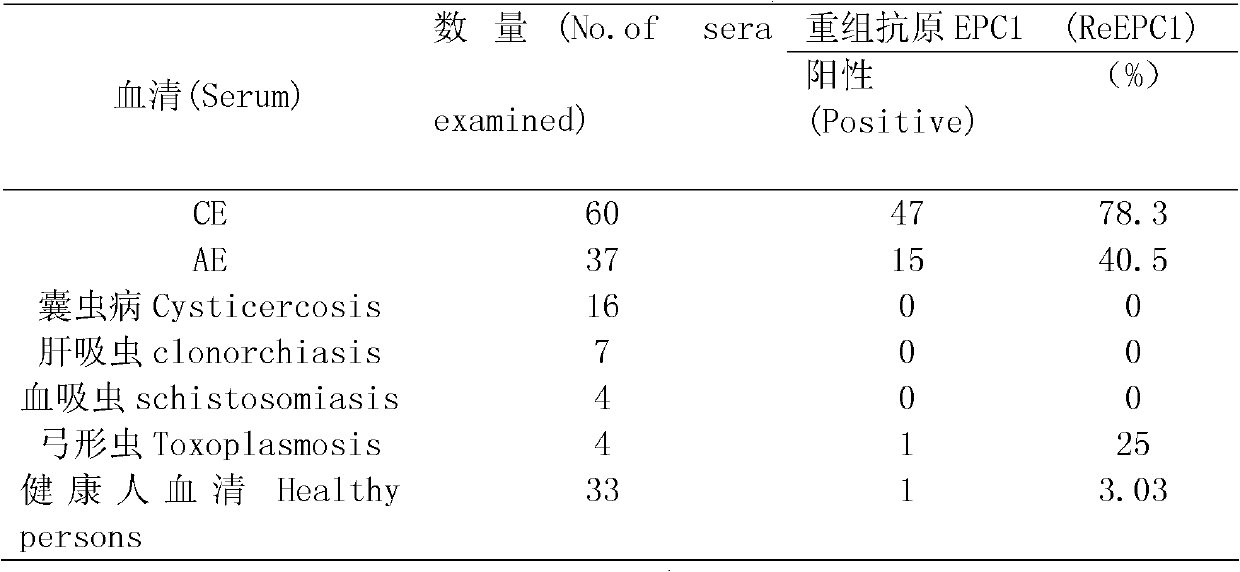
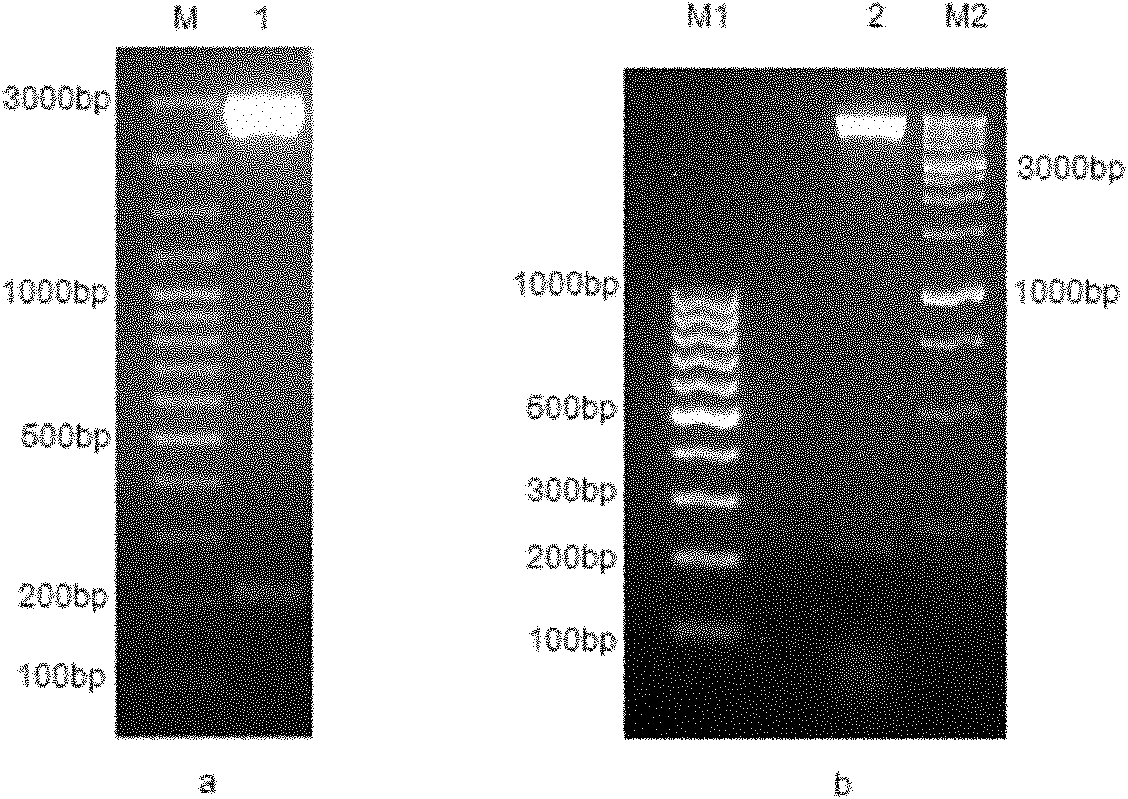
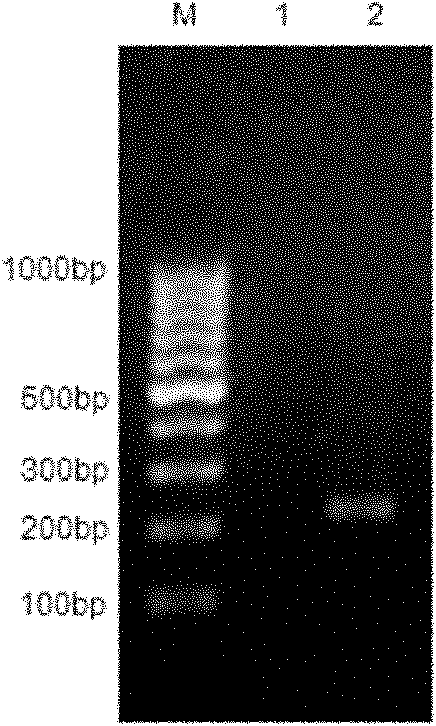
The invention discloses a recombinant antigenic protein for diagnosing echinococcosis granulosus (having an amino acid sequence represented by SEQIDNo.1). In addition, the invention also discloses the preparation method of the recombinant antigenic protein, which comprises: amplifying an EgEPC1 gene by using RT-PCR; cloning the EgEPC1 gene in a pGEM-T vector; connecting the EgEPC1 gene with an expression vector PET28a(+) to form a recombinant plasmid PET28a-EgEPC1; transforming the recombinant plasmid PET28a-EgEPC1 to Escherichia coli BL21(DE3) and expressing the recombinant protein through IPTG induction; and identifying a purified recombinant antigen by using SDS-PAGE and Western blotting. In addition, the invention also discloses the diagnosis use of the recombinant antigenic protein. Experiments show that the recombinant antigenic protein of the invention has the advantages of high sensibility and specificity for the diagnosis of echinococcosis granulosus, and has a promising application prospect in the diagnosis of the echinococcosis granulosus.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Dengue virus IgG/IgM antibody detection test strip, kit and preparation method thereof
InactiveCN110007096ASmall sample sizeHigh detection sensitivityBiological testingAgainst vector-borne diseasesIgm antibodyDisease course
The invention discloses a dengue virus IgG / IgM antibody detection test strip, kit and preparation method thereof, and relates to the technical field of dengue virus detection. The dengue virus IgG / IgMantibody detection test strip of the invention comprises a sample pad, an immune combination pad, a nitrocellulose membrane and absorbent paper, wherein the sample pad is sequentially stuck to the bottom of polyvinyl chloride; the immune combination pad is coated with dengue virus recombinant antigen marked by colloidal gold and chicken IgY polyclonal antibody; the nitrocellulose membrane is coated with dengue virus anti-human IgM antibody, a detection line of IgG antibody and a quality control line of anti-chicken IgY polyclonal antibody. The test strip can detect whether dengue virus IgG / IgM antibody exists in a sample to be detected through a method for detecting a marker. The test strip and the detection card comprising the test strip can be used as supplement for antigen detection onthe early acute infection of the dengue virus, covering the middle and later stages of the disease course and reducing the risk of missed detection.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Infectious bursal disease virus subunit antigen-containing vaccine composition, preparation method and application thereof
ActiveCN103849631AReduce formationIncreased expression of solubleViral antigen ingredientsVirus peptidesAntigenAdjuvant
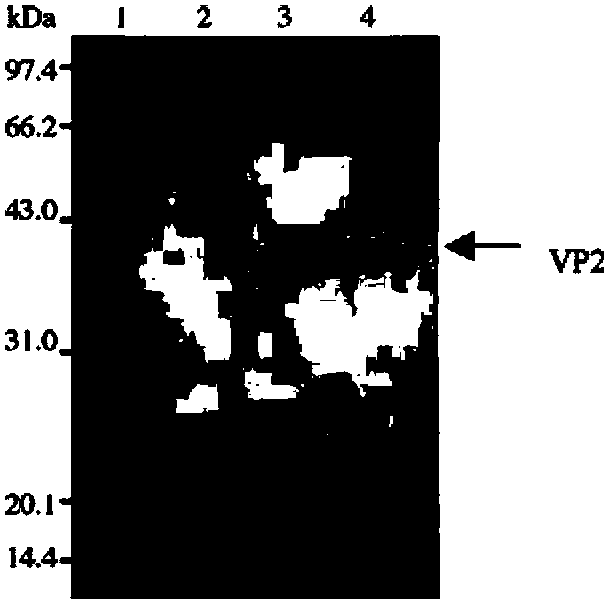
The present invention provides an infectious bursal disease virus (IBDV) subunit antigen-containing vaccine composition, which contains an immune amount of recombinant VP2 protein and an adjuvant. According to the present invention, with the subunit gene engineering vaccine composition, the infectious bursal disease can be effectively prevented and controlled; and with the purification technology adopted by the recombinant antigen protein, the endotoxin content in the vaccine composition is significantly reduced, the side reaction of the chicken individuals is significantly reduced, and the safety of the vaccine is substantially increased.
Owner:PU LIKE BIO ENG
Mycoplasma pneumoniae recombinant antigen, and preparation method and application of mycoplasma pneumoniae recombinant antigen
ActiveCN103275196AStrong specificityHigh sensitivityDepsipeptidesBiological testingEpitopeMycoplasma pneumonia
The invention relates to the technical field of biology and discloses a mycoplasma pneumoniae (MP) recombinant antigen, and a preparation method and an application of the mycoplasma pneumoniae recombinant antigen. An amino acid sequence of the mycoplasma pneumonia recombinant antigen is shown as SEQ ID No: 1. According to the antigen, a section of amino acid is taken as connecting oligopeptide, and an MP specific antigen P1 proteantigen dominant epitope and a P30 antigen dominant epitope form a recombinant protein. Tests prove that the recombinant protein has higher specificity and sensitivity in comparison with the existing MP antigen, and can be used for preparing MP antibody testing products.
Owner:WUHAN CHANGLI BIOLOGICAL TECH CO LTD
Recombinant cell surface capture proteins
ActiveUS20140134719A1DifferentiateImprove the level ofAnimal cellsSugar derivativesHeterologousAmino acid substitution
Recombinant cell surface capture proteins and detection molecules that are useful for isolating and detecting cells that produce a secreted heterodimeric protein of interest (POI) that has an immunoglobulin CH3 domain and / or substituted CH3 domain are provided. Recombinant cell surface capture proteins and detection molecules that isolate and detect bispecific antibodies are also provided. The invention also provides recombinant antigen-binding proteins that are capable of recognizing and binding to proteins of interest that contain a CH3 domain and / or a modified CH3 domain, such as a CH3 domain with or without amino acid substitutions at H95 and Y96 (IMGT).
Owner:REGENERON PHARM INC
Broad spectrum antibody spray for SARS-CoV-2 and SARS-CoV
InactiveCN111228483AFast preparationLow costEgg immunoglobulinsPharmaceutical delivery mechanismAntigenImmunologic preparation
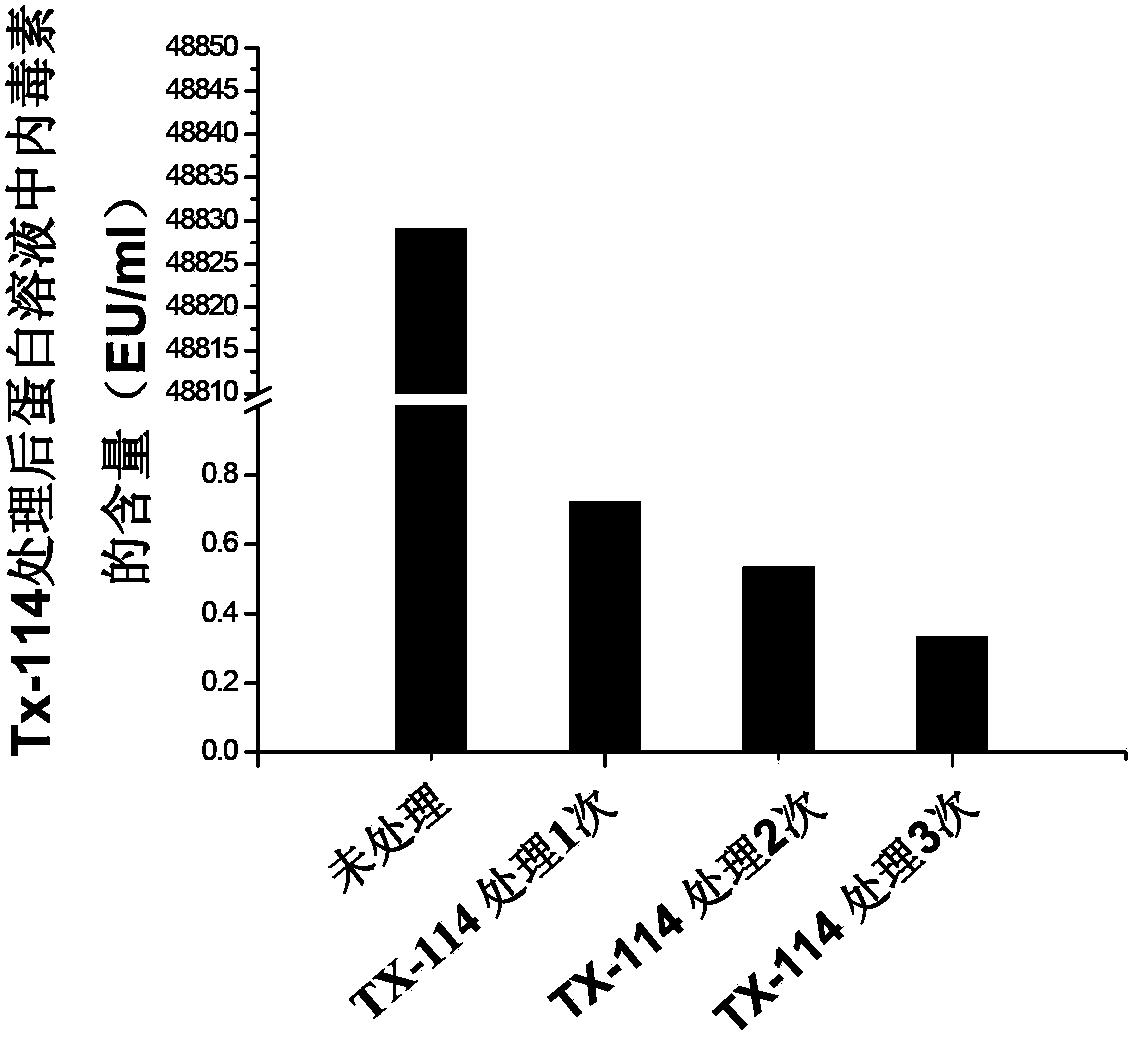
The invention discloses a composite IgY antibody for resisting COVID-19 and SARS. The antibody is prepared by the following method: constructing a recombinant antigen of SARS-CoV-2 and SARS-CoV; preparing an immune preparation with the recombinant antigen to immunize egg-producing birds, and obtaining the specific composite IgY antibody from the immunized eggs. A spray prepared by the antibody canbe used for broad-spectrum killing of SARS-CoV-2 and SARS-CoV, and is suitable for spraying on nasal cavity, oral cavity, skin and other parts. The antibody preparation has a specific virus neutralizing effect, can effectively control infection sources, protect susceptible people, disinfect and protect first-line medical staff and prevent spread of SARS-CoV-2.
Owner:SICHUAN UNIV
Western blot kit for detecting antibody of autoimmune disease and preparation method thereof
ActiveCN103105489AOvercome the cumbersome operation of individual detection one by oneImprove accuracyMaterial analysisAntigenAnti-mitochondrial antibody
The invention provides a western blot kit for detecting the antibody of autoimmune disease and a preparation method of the western blot kit, and relates to a western blot kit for detecting related antibodies of various autoimmune diseases, aiming at overcoming the technical defect that a western blot product is unavailable for testing and screening various autoimmune diseases in the prior art. The nitrocellulose membrane or the nylon membrane contains at least two parallel detection lines coated by at least two of ten natural antigens or recombinant antigens, i.e. dsDNA (deoxyribonucleic acid), Sm / RNP (ribonucleoprotein), CCP (critical compression pressure), SSA (sulfosalicylic acid), SSB (single-strand binding protein), GAD (glutamic acid decarboxylase), ICA (islet cell antibody), IA-2A (islet cell), TG (triglyceride) and AMA-M2 (anti-mitochondrial antibody), a high-concentration quality control band, a median-concentration quality control band and a low-concentration quality control band. The deficiency of the detection sensitivity and the specificity of the single autoantibody can be overcome, the operating complexity for independently detecting the related autoantibody of various diseases one by one can be overcome, and the detection efficiency and the result judging accuracy degree can be greatly improved.
Owner:SHENZHEN YHLO BIOTECH
Anti-mesothelin antibodies and uses thereof
ActiveUS20110027268A1Large indexBacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAnti-Mesothelin Antibody
The present invention provides recombinant antigen-binding regions and antibodies and functional fragments containing such antigen-binding regions that are specific for the membrane-anchored, 4O.kDa mesothelin polypeptide, which is overexpressed in several tumors, such as pancreatic and ovarian tumors, mesothelioma and lung cancer cells. These antibodies, accordingly, can be used to treat these and other disorders and conditions. Antibodies of the invention also can be used in the diagnostics field, as well as for further investigating the role of mesothelin in the progression of disorders associated with cancer. The invention also provides nucleic acid sequences encoding the foregoing antibodies, vectors containing the same, pharmaceutical compositions and kits with instructions for use.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
Test paper bar for testing colloidal gold of antibody of dengue fever virus
InactiveCN1963515ASimple methodThe result is clear and easy to distinguishMaterial analysisAgainst vector-borne diseasesDengue virus antibodyDengue virus Antigen
This invention provides one glue gold test bar to test dengue fever virus IgG and IgM antibody, which separately covers IgM antibody and dengue virus antigen and antigen multiple clone antibody on NC film and combines glue gold label dengue virus reestablish antigen and applies immune film analysis technique and tests dengue virus IgG and IgM antibody.
Owner:BEIJING ZHUANGDI HAOHE BIOMEDICINE SCI & TECH
Novel coronavirus recombinant antigen coating solution and pretreatment method and application thereof and product
Owner:ZHUHAI LIVZON DIAGNOSTICS
Hepatitis virus type C immune body chemiluminescence method diagnostic reagent kit and its producing method
ActiveCN101196518AWide applicabilityLow costChemiluminescene/bioluminescenceBiological testingAntigenChemiluminescence
The invention relates to a diagnostic reagent kit for testing the hepatitis c virus (HCV) and the preparation and test method, which is to add the HCV recombinant antigen used for peridium into the buffer solution, blend it, move into the luminous microplate, make incubation for 18 hours under 4DEG.C, wash the luminous microplate, add into the confining liquid, leave the liquid after incubation and fully dry the luminous microplate to complete the preparation of the pre-peridium luminous microplate; combine the anti-human IgG used for marking and the horse radish peroxidase by improving the sodium periodate to complete the preparation of the enzyme marker; prepare the chemical luminous substrate solution A with luminal, Tween20 and luminous intensifier and prepare the chemical luminous substrate solution B with the hydrogen peroxide. The reagent kit also comprises the sample diluent and concentrated scrub solution. The negative corresponds to the normal human serum while the positive corresponds to the people with serum of pooled serum with HCV antibody. The reagent kit provided in the invention has much higher detection sensitivity than the ELISA, which is safe and reliable, easy to operate with low cost, and without any expensive full-automatic chemical luminous measuring apparatus required.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
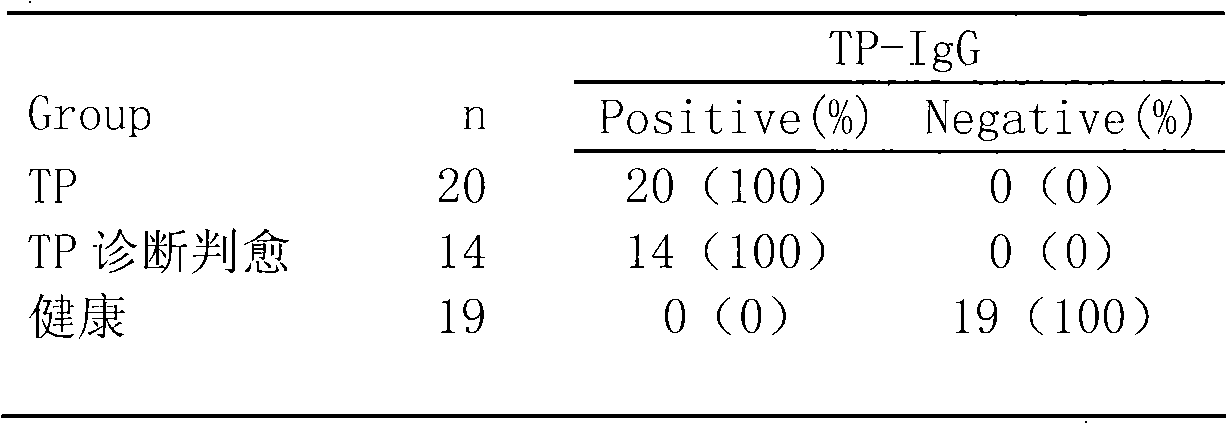
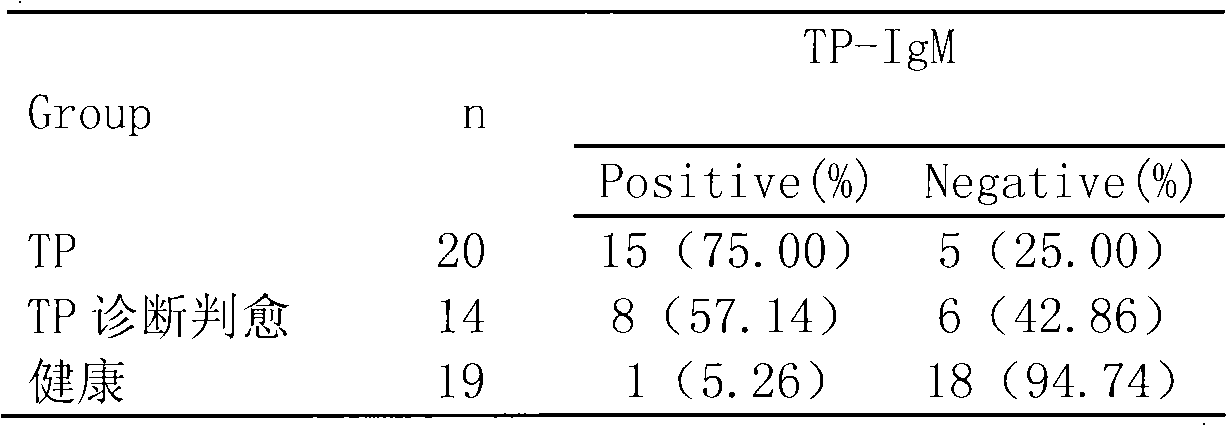
Treponema pallidum antibody test kit and preparation method and detection method thereof
The invention relates to a treponema pallidum (TP) antibody test kit based on the flow microspheric carrier technology and a preparation method and detection method thereof, belonging to the technical field of immunoassay medical diagnosis. The preparation method comprises the following steps: using TP recombinant antigen to coat heavy polymer microsphere, using bovine serum albumin to seal empty binding site, preparing specific TP probe-heavy polymer microsphere; performing coculture with a sample to be tested to capture TP antibody, washing and centrifuging to remove the unbound TP antibody, then adding fluorescently-labeled anti-human IgG or IgM antibody; and using a flow cytometry to detect the fluorescence intensity of the microsphere, and performing qualitative or quantitative analysis to the tested antibody. The method has the advantages of high sensitivity and specificity and good stability and can be used to perform microanalysis or multivalent analysis to the sample.
Owner:NANJING UNIV OF TECH
Recombinant antigen protein for diagnosing echinococcosis granulosa as well as preparation method and application thereof
InactiveCN102863524AIncreased sensitivityImprove featuresMicrobiological testing/measurementBiological testingAntigenThiogalactosides
The invention discloses a recombinant antigen protein (of which the amino acid sequence is shown as SEQ ID NO:1) for diagnosing echinococcosis granulose. Moreover, the invention further discloses a preparation method of the recombinant antigen protein. The method comprises the following steps of: amplifying an EgENO gene by adopting RT-PCR (Reverse Transcription-Polymerase Chain Reaction); cloning the EgENO gene into an expression vector PET28a (+) for constructing a recombined plasmid PET28a-EgENO; converting into escherichia coli BL21(DE3); inducing the expression of a recombinant protein through IPTG (Isopropyl-beta-d-Thiogalactoside); and identifying a purified recombinant antigen by using SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and Western blotting. In addition, the invention further discloses a diagnosis application of the recombinant antigen protein. As proved by an experiment, the recombinant antigen protein has the advantages of high sensitivity, high specificity and the like for the diagnosis of the echinococcosis granulosa, and has a wide application prospect on the aspect of diagnosis of the echinococcosis granulosa.
Owner:STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
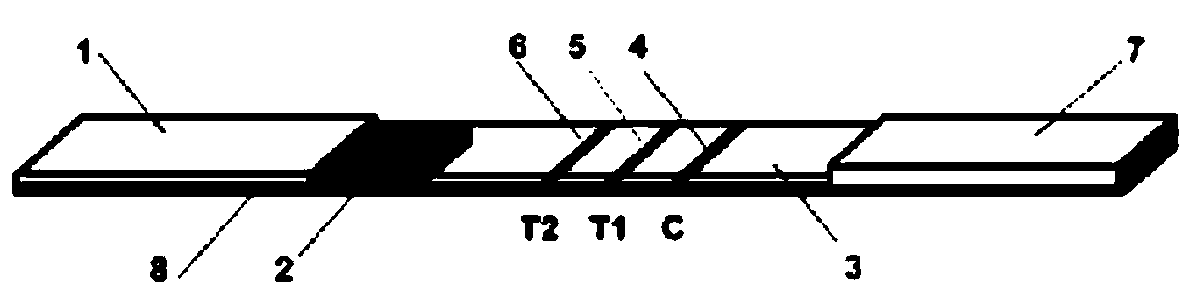
Immune chromatography test strip for detecting cystic echinococcosis and alveolar echinococcosis and preparation method
The invention belongs to the field of bioengineering and discloses an immune chromatography test strip for detecting cystic echinococcosis and alveolar echinococcosis, which comprises a back plate. A sample cushion is arranged at one end of the upper side of the back plate, and a water absorption cushion is arranged at the other end of the upper side of the back plate, a cellulose membrane is arranged between the sample cushion and the water absorption cushion, and a gold-labeled cushion is arranged between the sample cushion and the cellulose membrane. The cellulose membrane is provided with a first detecting line, a second detecting line and a quality control line. The first detecting line contains a purified thick antigen specific to the cystic echinococcosis, the second detecting line contains a recombinant antigen specific to the alveolar echinococcosis, and the quality control line contains antibodies or antiantibodies capable of being in specificity combination with a colloid gold-labeled probe. The invention further provides a preparation method of the immune chromatography test strip for detecting the cystic echinococcosis and the alveolar echinococcosis. The immune chromatography test strip has the advantages of simplicity, sensitivity, specificity and rapidity and being suitable for clinic and on-site use.
Owner:SHANGHAI NEW JIEER CLEANING PRODS
Anti-CD38 human antibodies and uses therefor.
ActiveCN1976950AImmunoglobulins against cell receptors/antigens/surface-determinantsImmunological disordersEpitopeAntigen binding
The present invention provides recombinant antigen-binding regions and antibodies and functional fragments containing such antigen-binding regions that are specific for CD38, which plays an integral role in various disorders or conditions. These antibodies, accordingly, can be used to treat, for example, hematological malignancies such as multiple myeloma. Antibodies of the invention also can be used in the diagnostics field, as well as for investigating the role of CD38 in the progression of disorders associated with malignancies. The invention also provides nucleic acid sequences encoding the foregoing antibodies, vectors containing the same, pharmaceutical compositions and kits with instructions for use. The invention also provides isolated novel epitopes of CD38 and methods of use therefore.
Owner:MORFOZIS AG
Novel detection reagent card for coronavirus antibody detection and preparation method of detection reagent card
PendingCN111190005AThe result is accurateHigh detection sensitivityBiological material analysisAntiendomysial antibodiesCoronavirus antibody
The invention relates to a novel detection reagent card for coronavirus antibody detection. The reagent card comprises a sample pad, a quantum pad, a nitrocellulose membrane, absorbent paper and a lining plate, the quantum pad is coated with a quantum dot microsphere labeled anti-human IgM antibody or IgG antibody, a detection line arranged on the nitrocellulose membrane is coated with an SARS-COV2 recombinant antigen, and a quality control line is coated with goat anti-mouse IgG. According to the detection reagent card for detecting the novel coronavirus (SARS-COV2) IgM antibody or IgG antibody provided by the invention, a result can be rapidly and accurately obtained, and auxiliary diagnosis is provided for clinical COVID-19.
Owner:重庆新赛亚生物科技有限公司
Method for preparing porcine circovirus type 2 colloidal gold antibody fast test strip
InactiveCN101881770AMicroorganism based processesFermentationGenetic engineeringEnzyme linked immunoassay
The invention relates to a method for preparing a porcine circovirus type 2 colloidal gold antibody fast test strip. In the method, a porcine circovirus type 2 ORF2 protein is expressed by utilizing genetic engineering, and the test strip is prepared by the principle of enzyme-linked immunoassay and membrane chromatography to fast test antibodies in the blood or serum of pigs. The test strip can be widely used for clinically testing porcine circovirus diseases, has no cross reaction with other viruses, and obtains test results 98.63 percent and 95.83 percent consistent with those obtained by the two methods of the ELISA and neutralization test. Compared with the ELISA, a recombinant antigen immune colloidal gold has the remarkable advantages of high security, no need of culturing the viruses per se, the avoidance of virus spread caused by the operation of the viruses, large-batch preparation, simple process, low production cost, stable and homogeneous antigen components, simple, convenient and labor-saving operation, no apparatus, high detection result specificity, high repeatability, the short time of 15 minutes for the whole test, simple, convenient, fast and accurate operation, high sensitivity, intuition and easy result judgment.
Owner:QINGDAO AGRI UNIV +2
Preparation method for African swine fever virus antibody detection colloidal gold immunochromatography test paper strip
InactiveCN103293306AEasy to manufactureShorten detection timeMaterial analysisAfrican swine fever virus AntibodyTrue positive rate
The invention relates to a preparation method for an African swine fever virus (ASFV) antibody detection colloidal gold immunochromatography test paper strip, belonging to the field of bioengineering and animal epidemic disease diagnosis. The preparation method comprises the following steps of: expressing an ASFV recombinant antigen p54, purifying, preparing an antibody, preparing a colloidal gold immunochromatography test paper strip, and detecting the sensitivity and specificity of the ASFV antibody. Compared with the other conventional serological methods, the colloidal gold immunochromatography test paper strip prepared by the method has the advantages of convenience, quickness, sensitivity, specificity and the like, can be used for obtaining a definite diagnosis result within 5 minutes and directly detecting a suspicious swine serum (or anticoagulation) virus antibody, and is particularly suitable for field ASFV serological diagnosis, epidemiological survey and swine international trade quarantine and inspection.
Owner:YANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com