Mycoplasma pneumoniae recombinant antigen, and preparation method and application of mycoplasma pneumoniae recombinant antigen
A technology of Mycoplasma pneumoniae and recombinant antigens, applied in the biological field, can solve the problems of poor antigen sensitivity, missed treatment of patients, false negatives, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
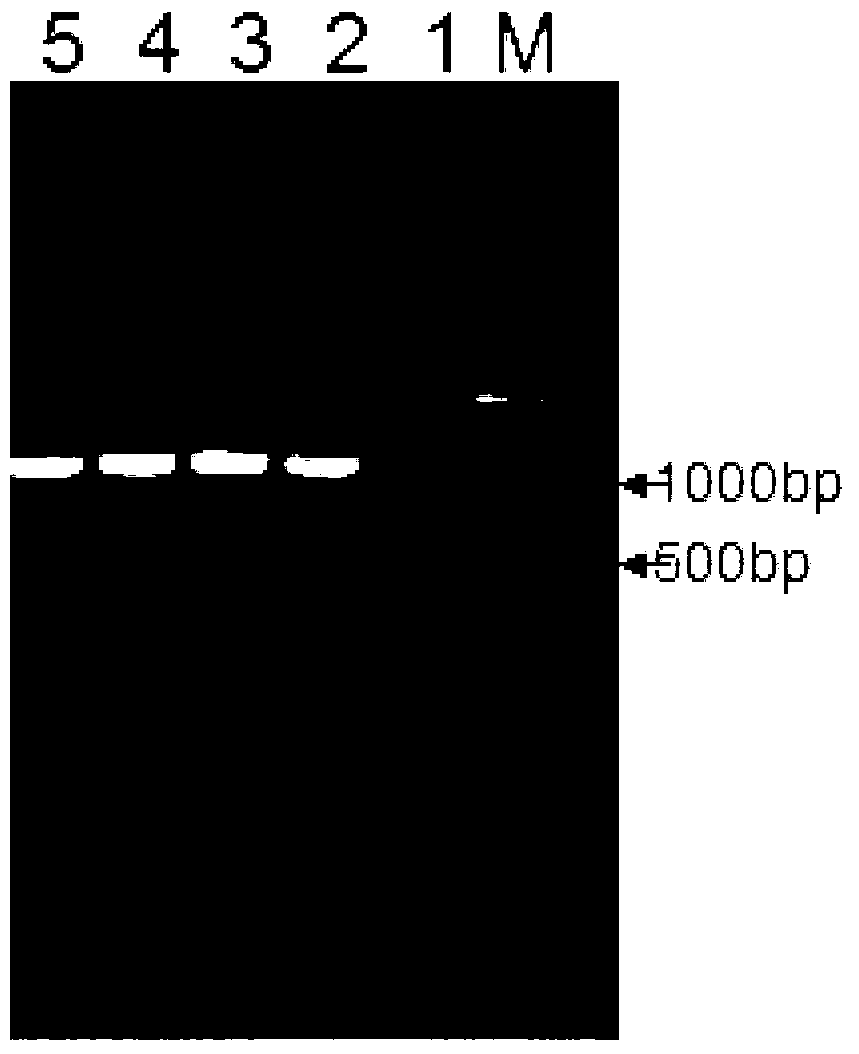
[0042] Example 1: Amplification of the target gene of the present invention and construction of a recombinant vector
[0043] 1. Amplify the target band
[0044] The nucleotide sequence shown in SEQ ID NO: 2 is synthesized by the whole gene as an amplification template.
[0045] Design primers for PCR amplification, the primer sequence is as follows:
[0046] lan-MP-501: 5'-GGATCCGATGACGACGATAAAG-3' (SEQ ID NO: 3)
[0047] lan-MP-304: 5'-GAATTCCTAGCGTTTCGGCGGAAAACCTG-3' (SEQ ID NO: 4)
[0048] PCR amplification system:
[0049]
[0050] PCR amplification conditions:
[0051] Pre-denaturation, 94℃, 5min;
[0052] A total of 30 cycles of denaturation, annealing and extension, 94℃, 60s; 58℃, 60s; 72℃, 60s;
[0053] Keep warm, 72°C, 6min.
[0054] 2. Recombinant vector construction
[0055] The amplified target band was constructed into the pET-30a (purchased from Novagen) prokaryotic expression vector, and the restriction enzymes BamHI (purchased from NEB) and EcoRI (purchased from NEB) were used...
Embodiment 2
[0063] Example 2: Induced expression, purification, dialysis and concentration of the MP recombinant antigen of the present invention
[0064] Transform the recombinant plasmid into E. coli BL-21, and induce 0.4mM-1.0mM IPTG at 37°C for 2-4h;
[0065] Collect E. coli BL-21 cells by centrifugation, suspend the cells with 20mM-50mM, pH8.0 tris, sonicate them, and collect the supernatant by centrifugation;
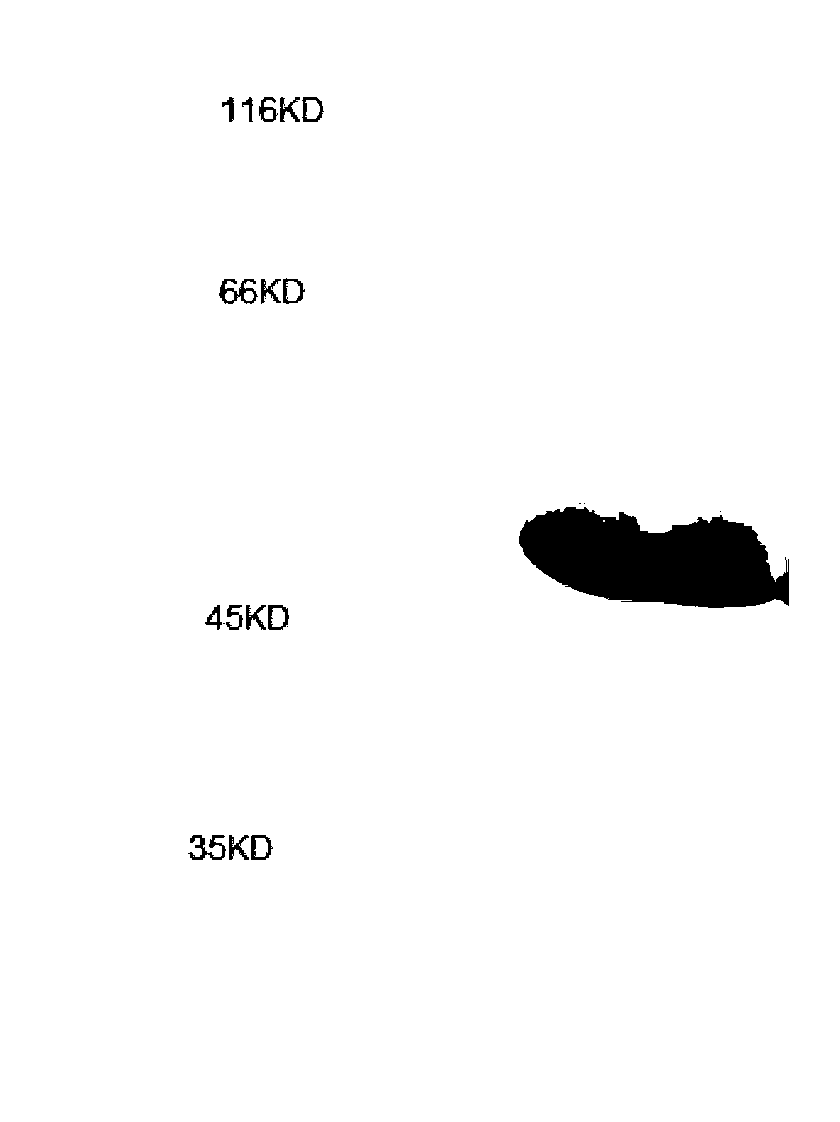
[0066] Purify the supernatant on a Ni column. Wash the column with a binding buffer solution containing 20mM imidazole, and then use a binding buffer solution to gradient elution of the MP recombinant antigen protein. The binding buffer solution is pH8.0 with 20mM-50mM tris and 200-500mM The solution composed of NaCl; the purified protein was detected by polyacrylamide gel electrophoresis, the result is figure 2 , Plus the label of the carrier itself, about 50KD, consistent with the strip position in the figure;
[0067] Put the purified MP recombinant antigen protein into a dialys...
Embodiment 3
[0068] Example 3: Elisa identification of the MP recombinant antigen of the present invention and the existing MP antigen (RP14 protein)
[0069] Coating: Dilute the antigen with PBS (pH7.4) buffer to a protein content of 10μg / mL. Add 0.1 mL to the reaction well of each polystyrene plate at 4°C overnight. Discard the solution in the well and wash 5 times with washing buffer for 2 minutes each (referred to as washing, the same below);
[0070] Blocking: Use 5% bovine serum albumin as a blocking agent, add 0.1 mL to each well, incubate at 37°C for 2 hours, discard the solution in the well, and wash 5 times with washing buffer;
[0071] Add sample: add 0.1 mL of diluted positive serum and negative serum (1:100-1:400) to the above-mentioned coated reaction wells, and incubate at room temperature for 1 hour. Then wash 5 times with washing buffer;
[0072] Add enzyme-labeled antibody: Add 0.1 mL of freshly diluted enzyme-labeled antibody (goat anti-human HRP1:2000) to each reaction well. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com