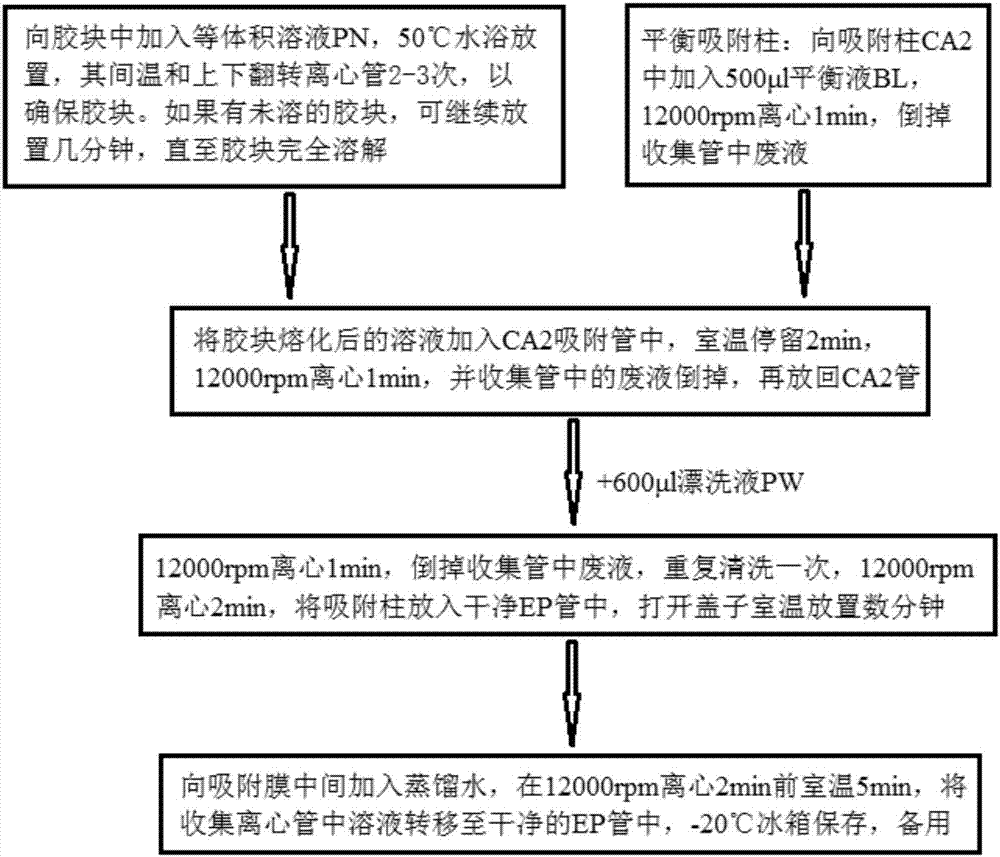
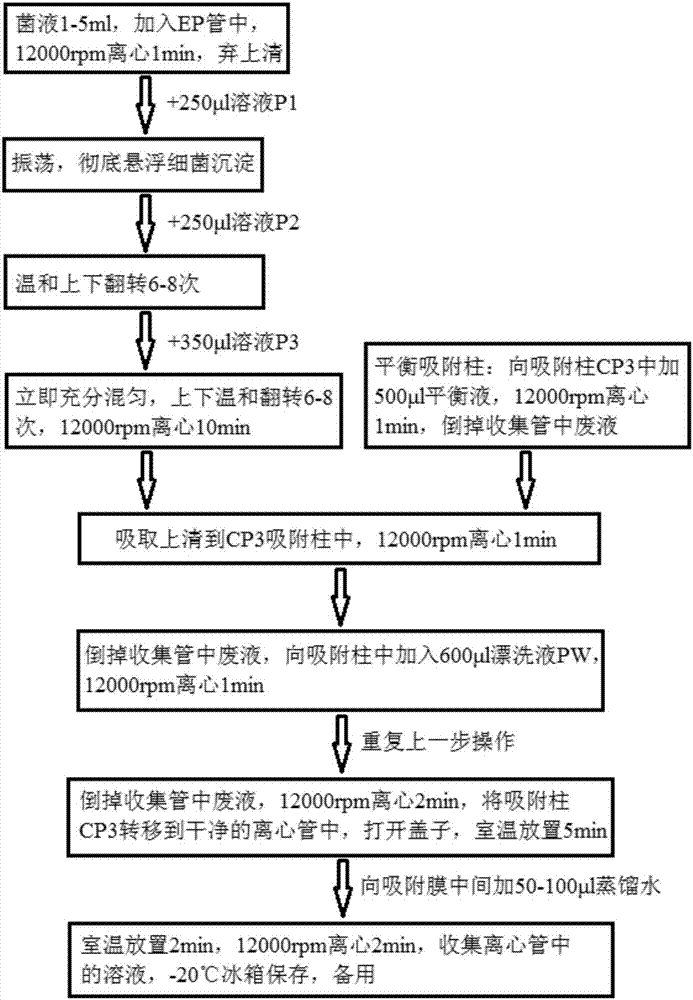
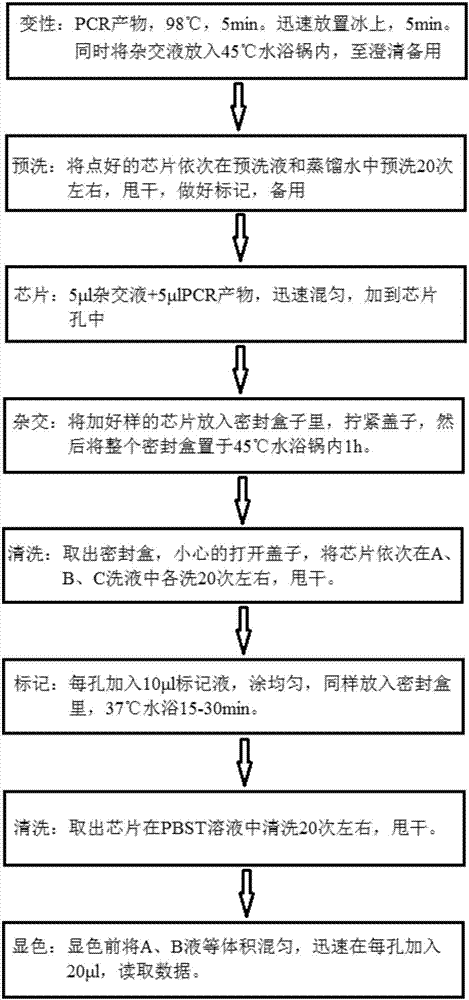
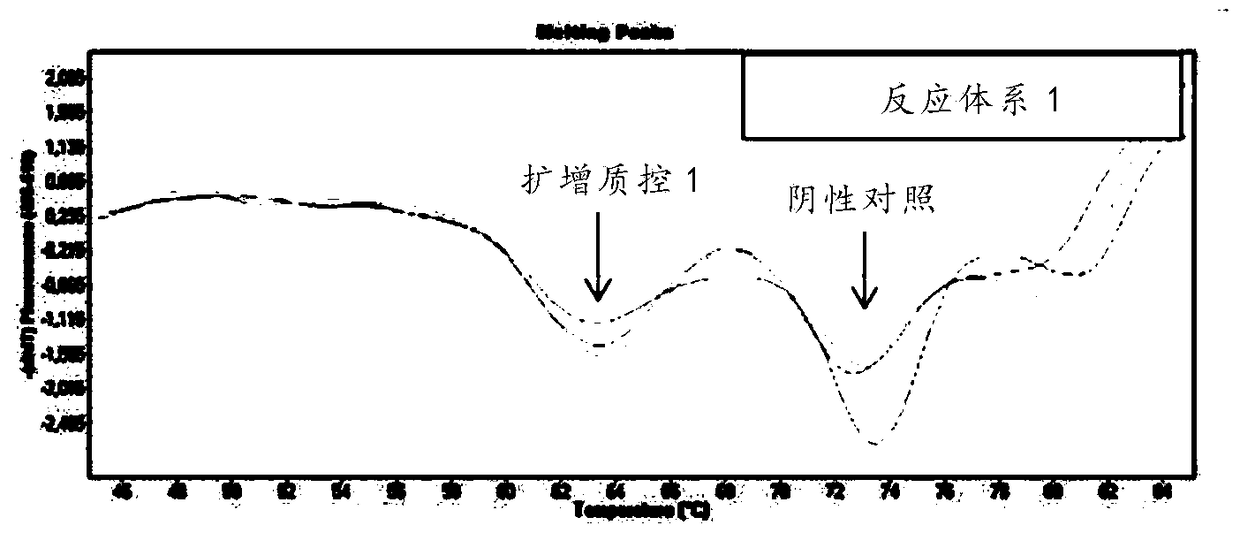
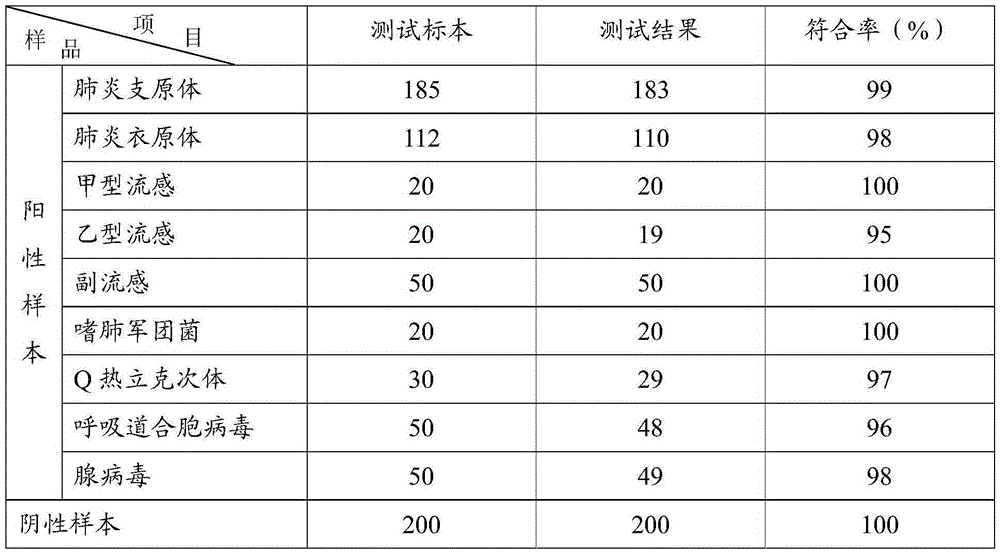
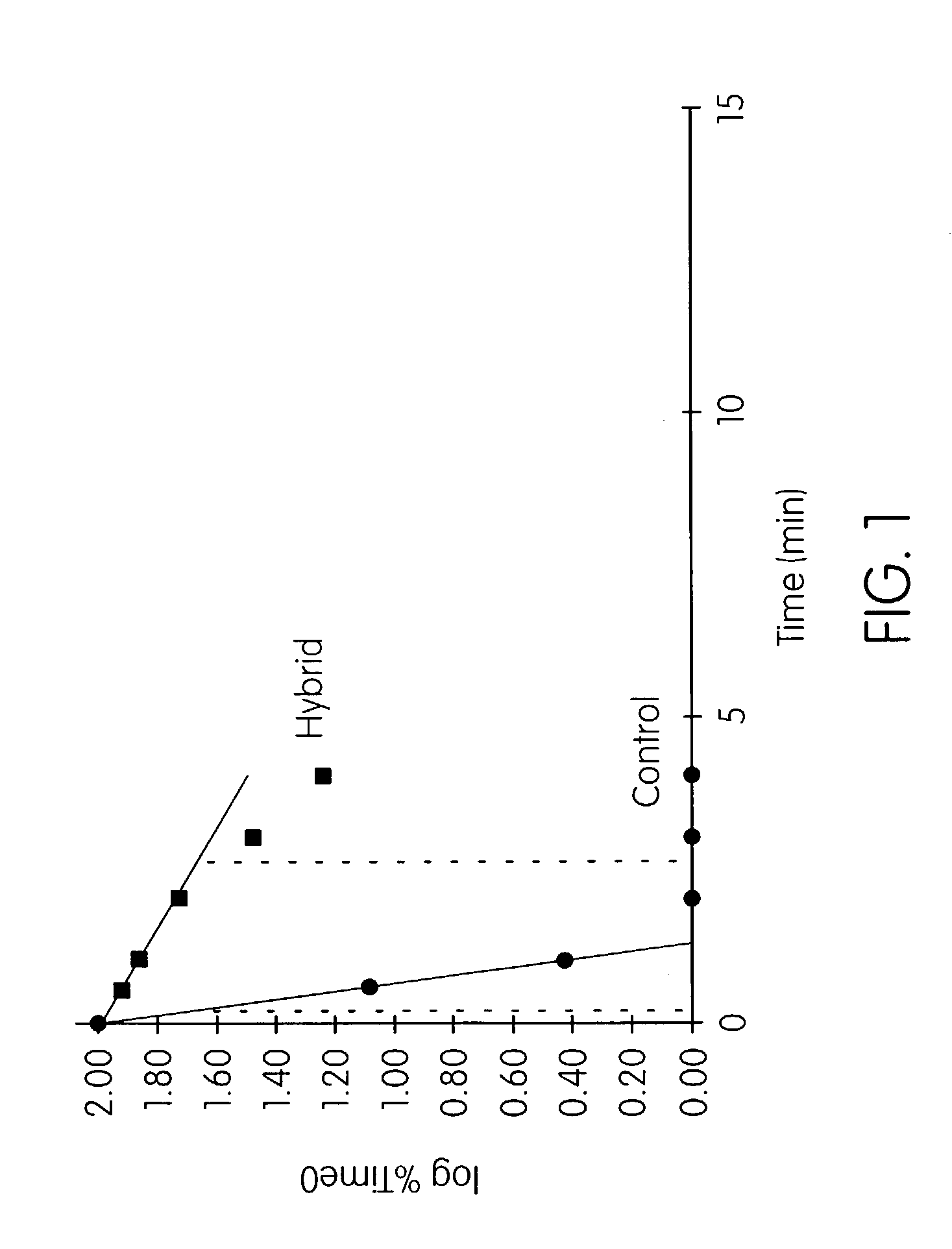
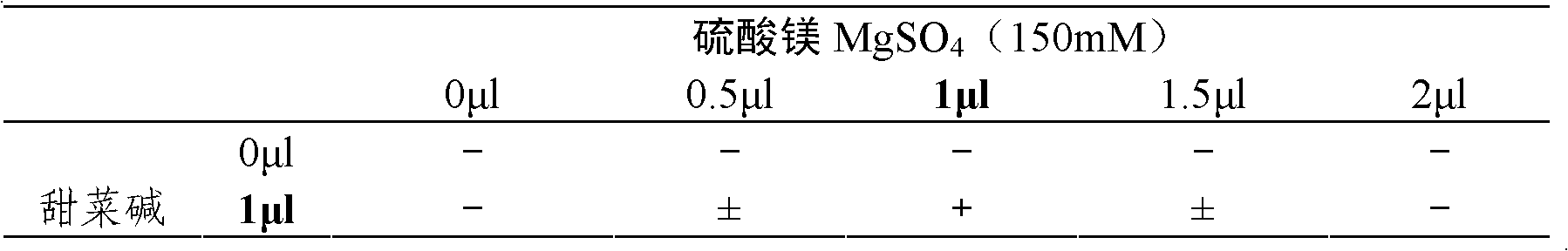
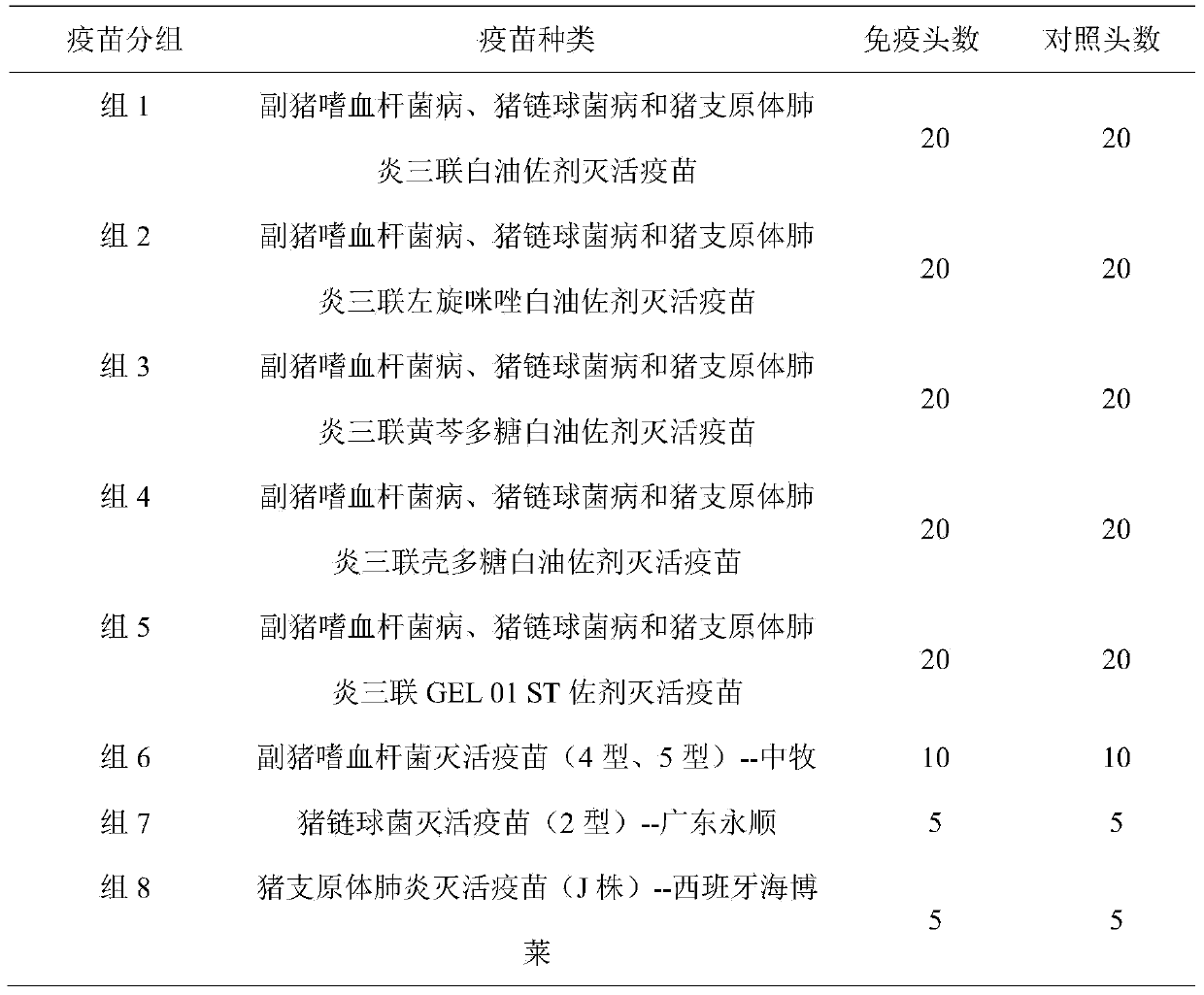
Patents
Literature
242 results about "Mycoplasma pneumoniae" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mycoplasma pneumoniae is a very small bacterium in the class Mollicutes. It is a human pathogen that causes the disease mycoplasma pneumonia, a form of atypical bacterial pneumonia related to cold agglutinin disease. M. pneumoniae is characterized by the absence of a peptidoglycan cell wall and resulting resistance to many antibacterial agents. The persistence of M. pneumoniae infections even after treatment is associated with its ability to mimic host cell surface composition.
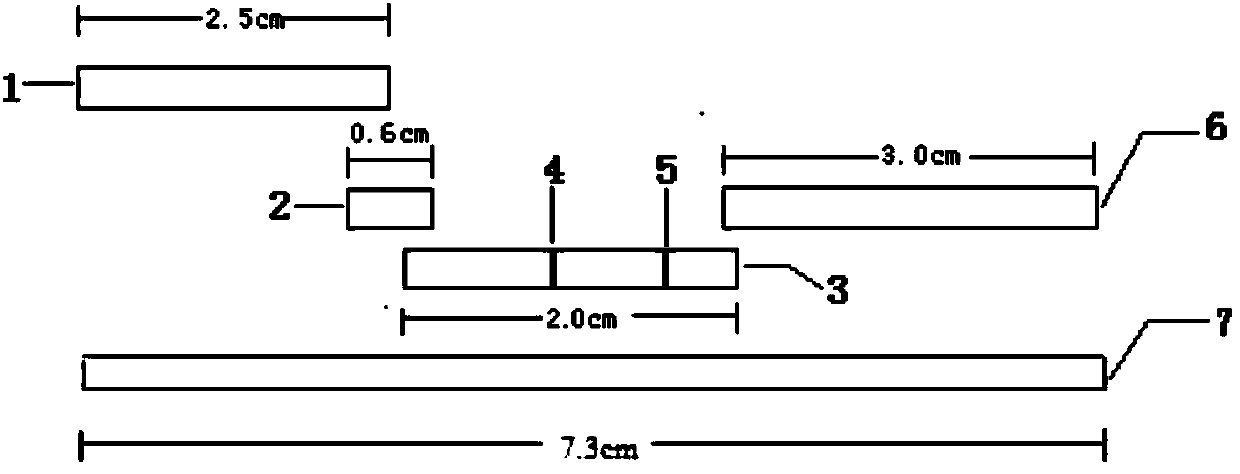
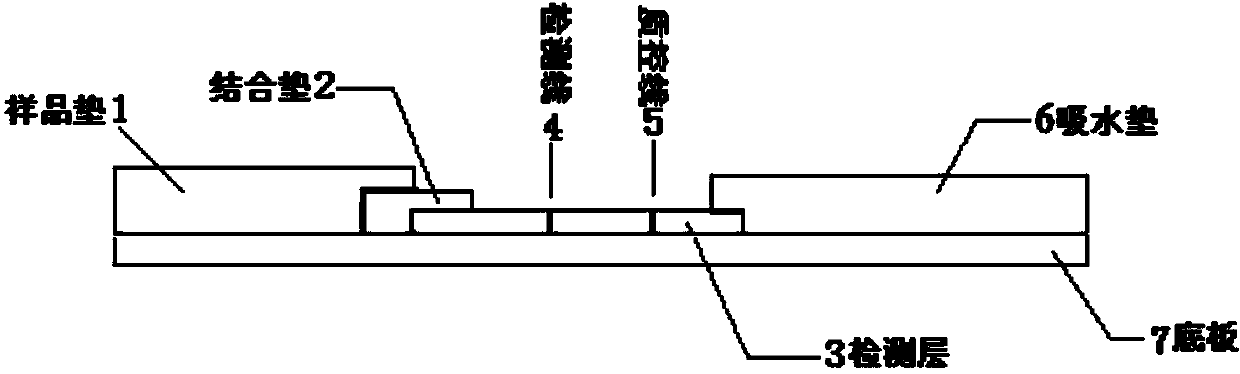
Human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and preparation method and application thereof
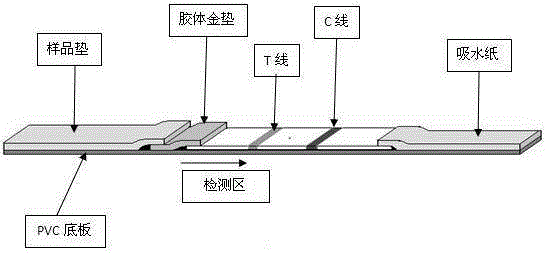
The invention provides a human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit and a preparation method and application thereof. The assay kit comprises a detection card and a silver-stained sensitivity-enhanced pad, wherein the detection card is composed of a bottom plate, a sample pad, an absorbent pad, a conjugate pad and a detection layer; the conjugate pad is coated with a colloidal gold-marked polyclonal antibody mixture of colloidal gold marked rabbit anti-human mycoplasma pneumoniae P1 protein and P30 protein; the detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line; the detection layer is bonded on the bottom plate, the conjugate pad and the absorbent pad are partially overlapped with the detection layer respectively and are bonded with the detection layer and the bottom plate respectively; the sample pad and the conjugate pad are partially overlapped to be bonded with the conjugate pad and the bottom plate respectively; and the silver-stained sensitivity-enhanced pad consists of a AgNO3 pad and a restoring pad. The human mycoplasma pneumoniae gold-marked silver-stained immunochromatographic assay kit can effectively improve the detection sensitivity of the human mycoplasma pneumoniae, has the strong specificity and has the high application value in the aspects of clinical diagnosis of human mycoplasma pneumoniae, etiology identification, epidemiological investigation and the like.
Owner:HUBEI UNIV OF TECH +1
Mycoplasma Hyopneumoniae Avirulent Adjuvanted Live Vaccine
ActiveUS20090117152A1Preventing and minimize severityElicit immune responseAntibacterial agentsBacterial antigen ingredientsViral antigensImmunogenicity
Provided are immunogenic and vaccine compositions and methods for their preparation and use, which compositions are effective in protecting against, minimizing the severity of, preventing, and / or ameliorating M. hyopneumoniae infection. Administration to an animal of one or two doses of an adjuvanted live avirulent M. hyopneumoniae composition disclosed herein is effective in providing immunity to the animal and protection from infection with a virulent strain of M. hyopneumoniae thereby reducing the severity of and / or preventing disease caused by one or more virulent strain of M. hyopneumoniae. Also provided are compositions, which further comprise one or more antigen such as, for example, one or more live bacteria, bacterin, toxoid, and / or virus and / or viral antigen. Exemplified are immunogenic compositions, comprising an adjuvanted live avirulent M. hyopneumoniae and compositions, comprising Porcine Circovirus Type 1-Type 2 chimera modified live vaccine (cPCV1-2) in further combination with an adjuvanted live avirulent M. hyopneumoniae.
Owner:ZOETIS SERVICE LLC
Kit for quickly detecting 15 pneumonia pathogenic bacteria
ActiveCN107338315AMicrobiological testing/measurementMicroorganism based processesBacteroidesStaphylococcus aureus
The invention discloses a kit for quickly detecting 15 pneumonia pathogenic bacteria. The kit can detect streptococcus pneumoniae, staphylococcus aureus, haemophilus influenzae, mycoplasma pneumoniae, pseudomonas aeruginosa, baumanii, enterococcus faecalis, enterococcus faecium, klebsiella pneumoniae, escherichia coli, enterobacter cloacae, stenotrophomonas maltophilia, burkholderia cepacia, legionella pneumophila and chlamydia pneumoniae which cover clinically common pneumonia pathogenic bacteria difficult to culture. 16S rDNA and specific gene sequences corresponding to the pneumonia pathogenic bacteria are detected by combining gene chips with multiple asymmetric PCR reactions, and the categories of the bacteria in a to-be-detected sample are identified in genus and species. The kit makes up for the defect that current clinical detection of pneumonia pathogenic bacteria is not in time or comprehensive and a novel detection means for early diagnosis and early treatment of patients suffering from pneumonia is provided.
Owner:GENERAL HOSPITAL OF PLA +1
Respiratory pathogen multi-detection reagent kit
InactiveCN109355437AHigh detection sensitivityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoronavirus 229EFluorescence
The invention discloses a respiratory pathogen multi-detection reagent kit. The respiratory pathogen multi-detection reagent kit has the advantages that the respiratory pathogen multi-detection reagent kit is based on multi-PCR (polymerase chain reaction) technologies, detection results can be determined by the aid of fluorescence resonance energy transfer via the melting temperature ranges, the respiratory pathogen multi-detection reagent kit can be used for qualitatively simultaneously detecting 16 types of respiratory pathogens, the 16 types of respiratory pathogens include 12 types of RNA(ribonucleic acid) viruses (influenza A viruses, influenza B viruses, H1N1 influenza A viruses, type A and type B respiratory syncytial viruses, type -1 / -2 / -3 parainfluenza viruses, type OC43 coronaviruses, type 229E coronaviruses, rhinoviruses and human metapneumovirus), 2 types of DNA (deoxyribonucleic acid) viruses (adenoviruses and bocavirus) and 2 types of bacteria (mycoplasma pneumoniae andbordetella pertussis), the respiratory pathogen multi-detection reagent kit is high in detection sensitivity, and the sensitivity even can reach 1 copy / reaction; the multi-detection reagent kit is good in specificity, and negative results of pathogens which have identical sampling sites and similar pathogenic mechanisms and are not in the detection range of the respiratory pathogen multi-detectionreagent kit can be obtained; the respiratory pathogen multi-detection reagent kit is short in operation time and easy to operate and can be used for quickly detecting the 16 types of respiratory pathogens in a single tube of a reaction system, the results are clear and are easy to interpret, and the like.
Owner:上海捷诺生物科技股份有限公司
Kit for jointly detecting respiratory tract pathogen through multiple fluorescent PCR method
ActiveCN107058622ANo further action requiredShorten the course of the diseaseMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlFluorescence
The invention provides a kit for jointly detecting respiratory tract pathogen through a multiple fluorescent PCR method. The kit comprises six components: reaction liquid A, reaction liquid B, reaction liquid C, enzyme mixed liquid, positive control and negative control, and comprises 11 common respiratory tract pathogen detections (general type of influenza virus A, influenza virus B, respiratory syncytial virus, 1 / 2 / 3 type of human parainfluenza virus, adenovirus, mycoplasma pneumoniae, chlamydia pneumonia, legionella pneumophila, streptococcus pneumonia, haemophilus influenza, A streptococcal); the amplification is performed through three reaction buffers, and each reaction buffer contains four fluorescent channels, 90% pathogen infection on the clinic can be checked.
Owner:DEBIQI BIOTECH XIAMEN
LAMP primer composite for detecting respiratory pathogens and kit of LAMP primer composite
InactiveCN107099619ADoes not affect amplificationMeet quality control requirementsMicrobiological testing/measurementMicroorganism based processesColor changesBiology
The invention relates to a primer composite for detecting respiratory pathogens. The primer composite comprises at least one group of a mycoplasma pneumoniae group, a chlamydia pneumoniae primer group, an influenza A / B virus primer group, a parainfluenza virus primer group, an adenovirus primer group and a respiratory syncytial virus primer group. The invention further relates to a kit comprising the primer composite. The kit further comprises a micro-fluidic chip wrapping primers, and a macroscopic indicator. The invention further relates to a detection method adopting the primer composite. The detection method comprises the steps of primer composite coating, to-be-detected sample nucleic acid extraction, LAMP reaction and visual result interpretation. The kit and the method are applied to the micro-fluidic chip for visual judgment, instant detection of the seven respiratory pathogens is rapidly and accurately realized, and a result is judged by a naked eye by color change, so that the kit and the method are simpler, more convenient and quicker in practical applications, are easy to operate, and are suitable for site operation.
Owner:SHANGHAI IGENETEC DIAGNOSTICS CO LTD
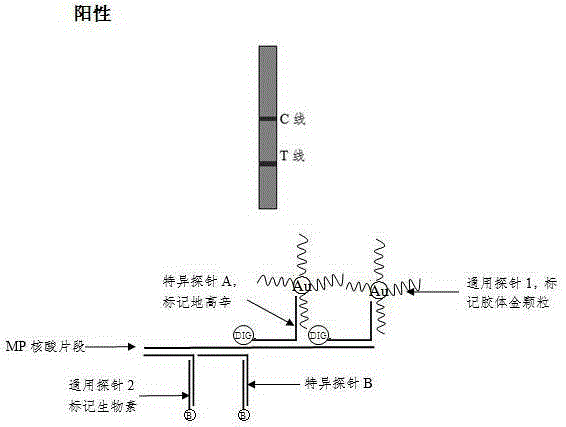
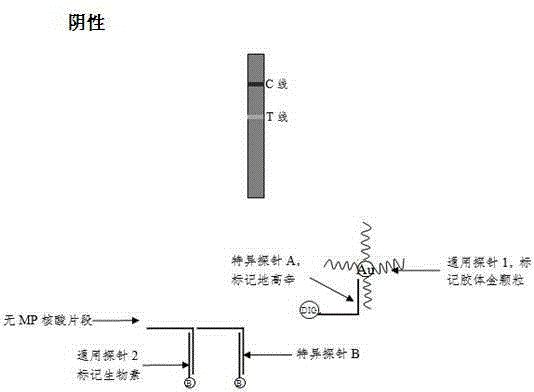
Method and kit for adopting colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid
The invention discloses a method and a kit for adopting a colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid and belongs to the technical field of medical biochemistry. According to the method, a colloidal gold grain is directly marked on a nucleic acid probe; the sequence for the marked nucleic acid probe is designed as a universal sequence; the nucleic acid probe also can be used for detecting other pathogens. During the design process for the kit provided by the invention, the introduced special probe A and special probe B have the functions of bridge molecular components and a gold marked probe and an MP (Mycoplasma Pneumoniae) nucleic acid amplified fragment are successively combined with each other in series by the two probes, so that the special detection for the MP nucleic acid fragment is realized. More than two probes can be designed in each set of probes; such a design is beneficial to the increasing of the sensitivity of the test strip; the advantages of the amplification technique for the depending nucleic acid sequence of MP and the colloidal gold marked detection for the products after amplification are integrated; the technical demand on the experimenter is low; no special instrument is required; the popularization of the MP nucleic acid detection in basic and faraway rural medical institutions is easily realized.
Owner:武汉中帜生物科技股份有限公司
Colloidal gold test strip and test strip card for detecting IgM antibody, and preparation and detection method
InactiveCN105259345AThe detection process is fastImprove efficiencyBiological testingParainfluenza virus antigenIgm antibody
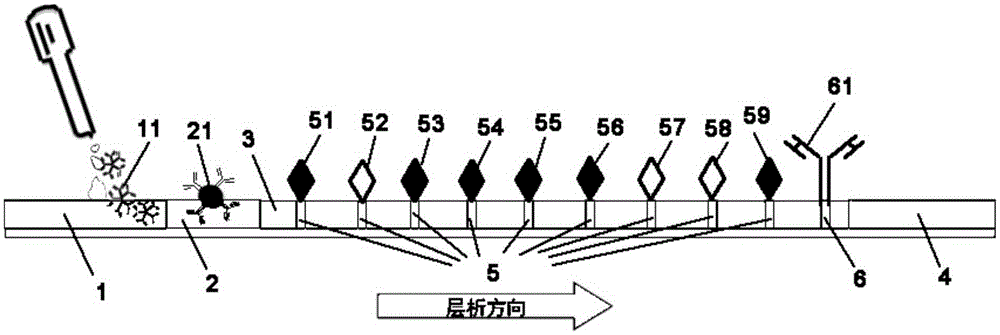
The invention provides a colloidal gold test strip for detecting an IgM antibody. The IgM antibody is a specific IgM antibody for nine respiratory tract infection pathogens, and the colloidal gold test strip comprises a sample pad, a conjugate pad, a nitrocellulose film and a water absorption pad which are attached to a polyvinyl chloride base plate in sequence; the conjugate pad is a glass fiber film wrapped with a rabbit-anti-human IgM antibody-colloidal gold conjugate; the nitrocellulose film is wrapped with 9 detection lines and 1 quality control line in sequence; the 9 detection lines are respectively a mycoplasma pneumoniae recombined antigen, a chlamydia pneumoniae recombined antigen, an influenza a virus antigen, an influenza B virus antigen, a sendai virus antigen, a legionella pneumophila antigen, a Coxiella burnetii antigen, a respiratory syncytial virus antigen and an adenovirus antigen, and the quality control line is a second antibody. The invention further provides a colloidal gold test strip card comprising the colloidal gold test strip and a colloid gold kit, a preparation method of the colloidal gold test strip card, and a method for realizing detection by adopting the colloidal gold test strip, the test strip card or the kit.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Primer probe combination and kit for combined inspection of 15 kinds of respiratory tract infection pathogens
ActiveCN107937578ARapid combined detectionGuaranteed matchMicrobiological testing/measurementMicroorganism based processesStreptococcus pyogenesStaphylococcus aureus
The invention provides a pathogen inspection reagent, and concretely discloses a primer probe combination and a kit for combined inspection of 15 kinds of respiratory tract infection pathogens. By aiming at specific target sequences of the gene sequence conserved region of klebsiella pneumoniae, haemophilus influenzae, streptococcus pyogenes, staphylococcus aureus, escherichia coli, chlamydia pneumoniae, mycobacterium tuberculosis, stenotrophomonas maltophilia, baumanii, mycoplasma pneumoniae, enterococcus faecalis, ligionella pneumohpillia, streptococcus pneumoniae, bacillus pyocyaneus and mycobacterium abscessus, primers and probes which do not have mutual crossed reaction are designed, so that the problem that detection probes of different pathogens can easily generate mutual influenceor interference is solved; the combination and matching of different primers and probes are ensured; the goal of simultaneously performing efficient specific inspection at the same temperature can also be achieved.
Owner:西安九安生物技术有限公司
Target sequence used for detecting mycoplasma pnoumoniae and reagent box
ActiveCN1724686AEfficient detectionEffective distinctionMicrobiological testing/measurementNucleotideOligonucleotide primers
The invention supplies a method to rapidly test the genetic marker of Mycoplasma pneumoniae. It supplies the nucleotide sequence of Pl CytadhesinGene and primer and probe that is designed based on the sequence. The invention also supplies the method to rapidly testing the Mycoplasma pneumoniae by using the primer.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD
Method for detecting resistant mutant of mycoplasma pneumoniae
InactiveCN102002522AQuick checkoutSimple and fast operationMicrobiological testing/measurementFluorescence/phosphorescenceResistant strainThermal cycler
The invention belongs to the fields of biology and medicines and particularly relates to a method for detecting a resistant mutant of mycoplasma pneumoniae. By adopting Real-time PCR (Polymerase Chain Reaction) and a cycling probe technology for distinguishing the difference of monobases and searching a specific primer aiming at the mycoplasma pneumoniae in an upstream and a downstream sequence of 2063 bit / 2064 bit of the mycoplasma pneumoniae 23SrRNA gene, specific cycling probes aiming at mutation-free sensitive strains, 2063-bit mutant drug-resistant strains and 2064-bit mutant drug-resistant strains can be respectively designed according to the difference of the bases at the 2063 bit / 2064 bit of the mutation-free sensitive strains and mutant drug-resistant 23SrRNA gene of the mycoplasma pneumoniae; the PCR amplification can be carried out by using the specific primer of the mycoplasma pneumoniae; and the fluorescence intensity change can be read by a Real-time PCR thermal cycler in real time to determine the mutant types of samples to be detected. The invention can correctly distinguish the mutation-free sensitive strains from the mutant drug-resistant strains of clinical separation strains of the mycoplasma pneumoniae. The specificity is 100 percent and the sensitivity can reach 102copy / PCR reaction.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Composition for detecting and typing pathogens causing respiratory tract infection, kit, method and application
ActiveCN111321251AReduce wasteReduce psychological burdenMicrobiological testing/measurementMicroorganism based processesRespiratory syncytial virus (RSV)Pneumonitis
The invention relates to the field of molecular biology detection, and particularly relates to detection of novel coronavirus 2019- nCoV, influenza A virus, influenza B virus, respiratory adenovirus,respiratory syncytial virus and mycoplasma pneumoniae. The invention provides a composition for detecting the pathogens. Meanwhile, the invention further provides a kit containing the composition, application of the composition and a method for detecting and typing the pathogens causing respiratory tract infection. The composition is combined with a fluorescent probe method, six pathogens causingrespiratory tract infection can be detected and typed at the same time in two tubes, and the advantages that the cost is low, the flux is high, the influence of interference substances is avoided, operation is easy and convenient, and false positive and environmental pollution caused by sample crossing are avoided are achieved.
Owner:SANSURE BIOTECH INC
Colloidal gold method detection test strip and reagent kit for IgG antibody of respiratory disease and preparation method of reagent kit
InactiveCN102928589AEasy to detectQuick checkMaterial analysisBovine respiratory diseasePrimary screening
The invention discloses a colloidal gold method detection test strip and a reagent kit for an IgG antibody of a respiratory disease and a preparation method of the reagent kit. The test strip determines the IgG antibody by using a principle of an immunocapture method; respiratory herpes viruses, adenoviruses, influenzaviruses and an IgG antibody of mycoplasma pneumoniae can be detected jointly by one operation; the operation process is simplified; the test strip is simple, convenient, rapid and accurate in detection, suitable for mass detection and applicable to primary screening and epidemiological survey; and a result is distinct and easy to distinguish.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Construction and application of porcine circovirus type II-porcine mycoplasma pneumoniae expressing strains
ActiveCN102080074ALow costGood immune securityBacterial antigen ingredientsBacteriaCatabolite activator proteinProkaryotic expression
The invention relates to construction and application of porcine circovirus type II-porcine mycoplasma pneumoniae expressing strains and belongs to the technical field of biology. By a method provided by the invention, prokaryotic expression engineering strains of expressing porcine circovirus type II-porcine mycoplasma pneumoniae major antigenic protein are constructed. The strains can simultaneously express the major antigenic protein R1 of porcine mycoplasma pneumoniae and a major antigenic protein catabolite activator protein (CAP) of porcine circovirus, purified recombinant protein can stimulate organisms to produce a protective immune response which resists attacks of porcine circovirus type II and the porcine mycoplasma pneumoniae, and the infection of the porcine circovirus and the porcine mycoplasma pneumoniae can be effectively prevented.
Owner:兆丰华生物科技(南京)有限公司 +2
Probes, compositions and kits for determining the presence of Mycoplasma pneuomoniae in a test sample
The present invention relates to oligonucleotides useful for determining the presence of Mycoplasma pneumoniae and / or Mycoplasma genitalium in a test sample. The oligonucleotides of the present invention may be incorporated into hybridization assay probes, capture probes and amplification primers, and used in various combinations thereof.
Owner:GEN PROBE INC
Method for detecting infectious disease pathogens and kit
ActiveCN101545010AThe detection process is fastImprove efficiencyMicrobiological testing/measurementMicroorganism based processesOrganismStreptococcus constellatus
The invention discloses a method for detecting infectious disease pathogens possibly existing in a biological sample. The infectious disease pathogens comprise chlamydia pneumoniae, haemophilus influenzae, mycoplasma pneumoniae, pneumoniae streptococcus and legionella pneumophila. The method comprises the following steps: expanding nucleic acid fragments of the biological sample, and detecting the nucleic acid fragments by using a probe. The invention also provides primers used for expanding and the probe used for detection. The invention also provides a kit comprising the primers. The method has the advantages of high sensitivity, strong specificity, simple operation and wide sample range, can simultaneously detect various infectious disease pathogens, and is suitable for early diagnosis of respiratory infectious diseases.
Owner:HAI KANG LIFE
Loop-mediated isotherm amplification (LAMP) kit for detecting mycoplasma pneumoniae (Mp)
ActiveCN102618655AEasy to operateEasy to observeMicrobiological testing/measurementMicroorganism based processesDisease monitoringMycoplasma
The invention relates to the technical field of biology, in particular to a loop-mediated isotherm amplification (LAMP) kit for detecting mycoplasma pneumoniae (Mp). The kit contains 4 LAMP primers, the LAMP primers and an LAMP reaction solution form a detection system together, and the nucleotide sequences of the 4 LAMP primers are shown as SEQ ID No. 1-4. The kit can be used for quickly and sensitively detecting the Mp, and the lowest detection limit is 100 copies. The kit is easy to use and low in cost, the reaction result is easy to observe, and the kit has good specificity, is very suitable for disease monitoring, field emergency and detection of clinical specimens and facilitates large-range popularization and application.
Owner:ICDC CHINA CDC
Double-fluorescent PCR detection primer, probe, reaction liquid and kit capable of detecting pathogens of respiratory tract
InactiveCN105463129ASave testing timeSave testing costMicrobiological testing/measurementMicroorganism based processesEnterovirusCoronavirus 229E
The invention discloses a double-fluorescent PCR detection primer, a probe, reaction liquid and a kit capable of rapidly detecting 16 pathogens of the respiratory tract. The kit comprises pre-subpackaged PCR reaction liquid, RT-PCR enzyme, a positive quality control product and a negative quality control product, wherein the pre-subpackaged PCR reaction liquid is provided with a primer and a TaqMan probe for detecting at least two of the following pathogens: respiratory syncytial virus, enterovirus, coronavirus NL63, coronavirus HKU1, coronavirus 229E, coronavirus OC43, parainfluenza virus type I, parainfluenza virus type II, rhinovirus, parainfluenza virus type III, human bocavirus, human metapneumovirus, mycoplasma pneumoniae, chlamydia pneumoniae, adenovirus and legionella pneumophila. The kit is convenient to operate, and can be used for at most screening 16 syndrome pathogens of the respiratory tract within 2 hours, so that the detection time and cost are greatly saved. The kit has the greatest advantages of simplicity in operation and strong practicability, and can be easily popularized in laboratories of the ports.
Owner:SHENZHEN INT TRAVEL HEALTHCARE CENT +1
Respiratory tract infection pathogen nucleic acid combined detection kit
PendingCN112280897AHigh detection throughputAvoid false negative resultsMicrobiological testing/measurementAgainst vector-borne diseasesNucleic acidChlamydophila Pneumonia
The invention discloses a respiratory tract infection pathogen nucleic acid combined detection kit. The invention develops a primer and probe combination for detecting various respiratory tract infection pathogens such as novel corona virus, influenza A virus, influenza B virus, respiratory syncytial virus, human parainfluenza virus, adenovirus, mycoplasma pneumoniae and chlamydia pneumoniae by combining a multiple fluorescent quantitative PCR technology and a diversion hybridization gene chip technology. The nucleotide sequences of the primer and probe combination are shown in SEQ ID NO: 1-36in sequence. The respiratory tract infection pathogen nucleic acid combined detection kit is constructed. Synchronous joint detection of eight respiratory tract infection pathogens can be realized, the detection accuracy is good, the specificity is strong, the sensitivity is high, the repeatability is good, false negative and false positive are low, the detection time is short, the cost is low, apatient can be comprehensively detected, the pathogens can be accurately positioned, timely treatment is carried out or corresponding isolation measures are carried out, and the kit has important significance for effectively controlling respiratory tract infection to prevent related infectious infection outbreak.
Owner:SHANGHAI CITY PUDONG NEW DISTRICT ZHOUPU HOSPITAL +2
Preparation technology of mycoplasma ovipneumoniae inactivated vaccine
InactiveCN103110583ASimple preparation processInfection controlAntibacterial agentsBacterial antigen ingredientsTiterTGE VACCINE
The invention discloses a preparation technology of a mycoplasma ovipneumoniae inactivated vaccine, which is based on the improvement on the preparation technology of the mycoplasma ovipneumoniae inactivated vaccine, and provides a preparation method of the mycoplasma ovipneumoniae inactivated vaccine. Through the application of the method provided by the invention, an ideal efficient mycoplasma ovipneumoniae inactivated vaccine adjuvant is screened, the content of toxin in antigen is greatly reduced, and the vaccine safety is improved. After the vaccine is applied to sheep, the antibody is generated early, the titer is high, the duration is long, and the mycoplasma ovipneumoniae infection can be effectively prevented and controlled.
Owner:SOUTHWEST UNIVERSITY FOR NATIONALITIES
Biological fermentation pig forage and preparation method thereof
InactiveCN105285335AReduce the incidence of asthmaReduce the incidence of coldsFood processingAnimal feeding stuffVitamin E AcetateLactobacillus fermentum
A biological fermentation pig forage is prepared from the following raw materials in parts by weight: 45-53 parts of corn flour, 8-10 parts of herb of common nipplewort, 3-4 parts of konjac glucomannan, 3-4 parts of glucosamine sulfate, 35-40 parts of soybean meal, 35-40 parts of barley vinasse, 17-25 parts of wheat bran, 10-13 parts of euglena, 3-4 parts of spora lygodii, 1-1.5 parts of lactobacillus fermentum, 1-1.5 parts of aspergillus niger, 1.5-2 parts of lactobacillus plantaum, 1.5-2 parts of lactobacillus johnsonii, 0.2-0.3 part of sodium selenosulfate, 3-4 parts of vitamin e acetate, and 10-12 parts of illite powder. The biological fermentation pig forage is improved in digestion rate, and is capable of increasing digestion capability of animals on the forage and improving animal growth performance, especially is capable of enhancing immunity, possesses protrude effects on reducing pig cold and mycoplasma pneumoniae of swine, and is capable of improving pork tenderness and reducing muscle shearing force.
Owner:乔生艮
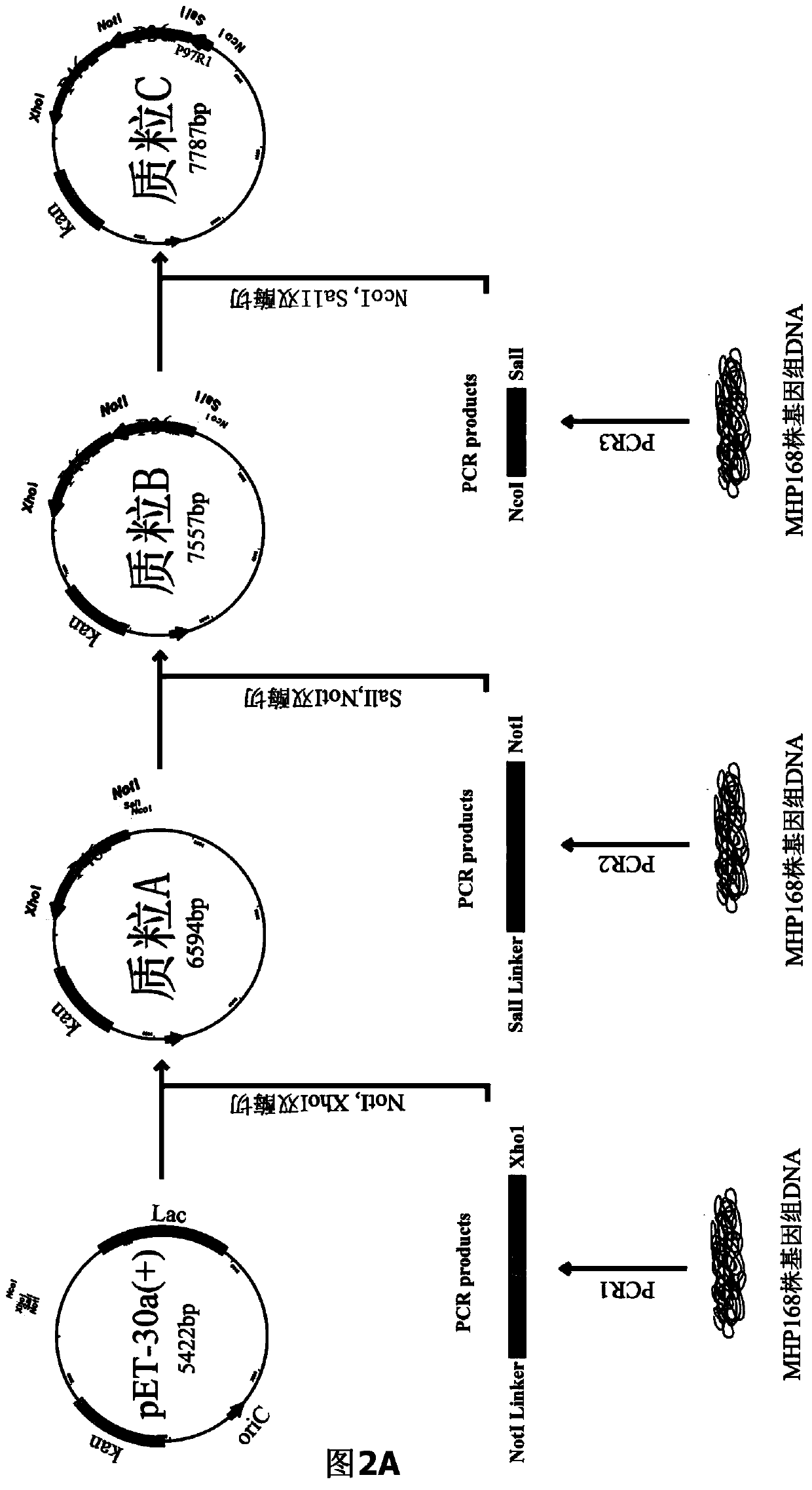
Mycoplasma hyopneumoniae fusion gene and application
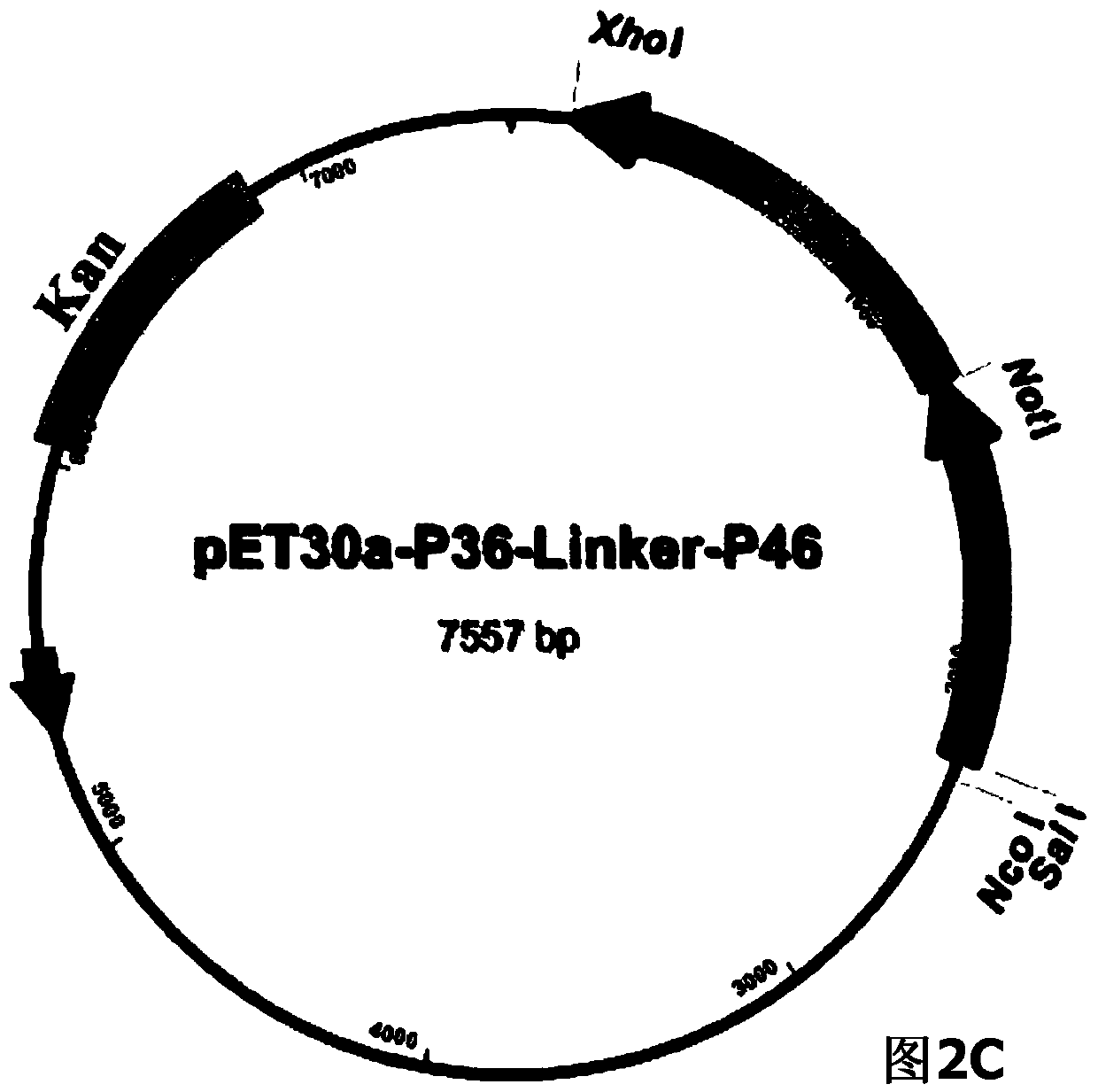
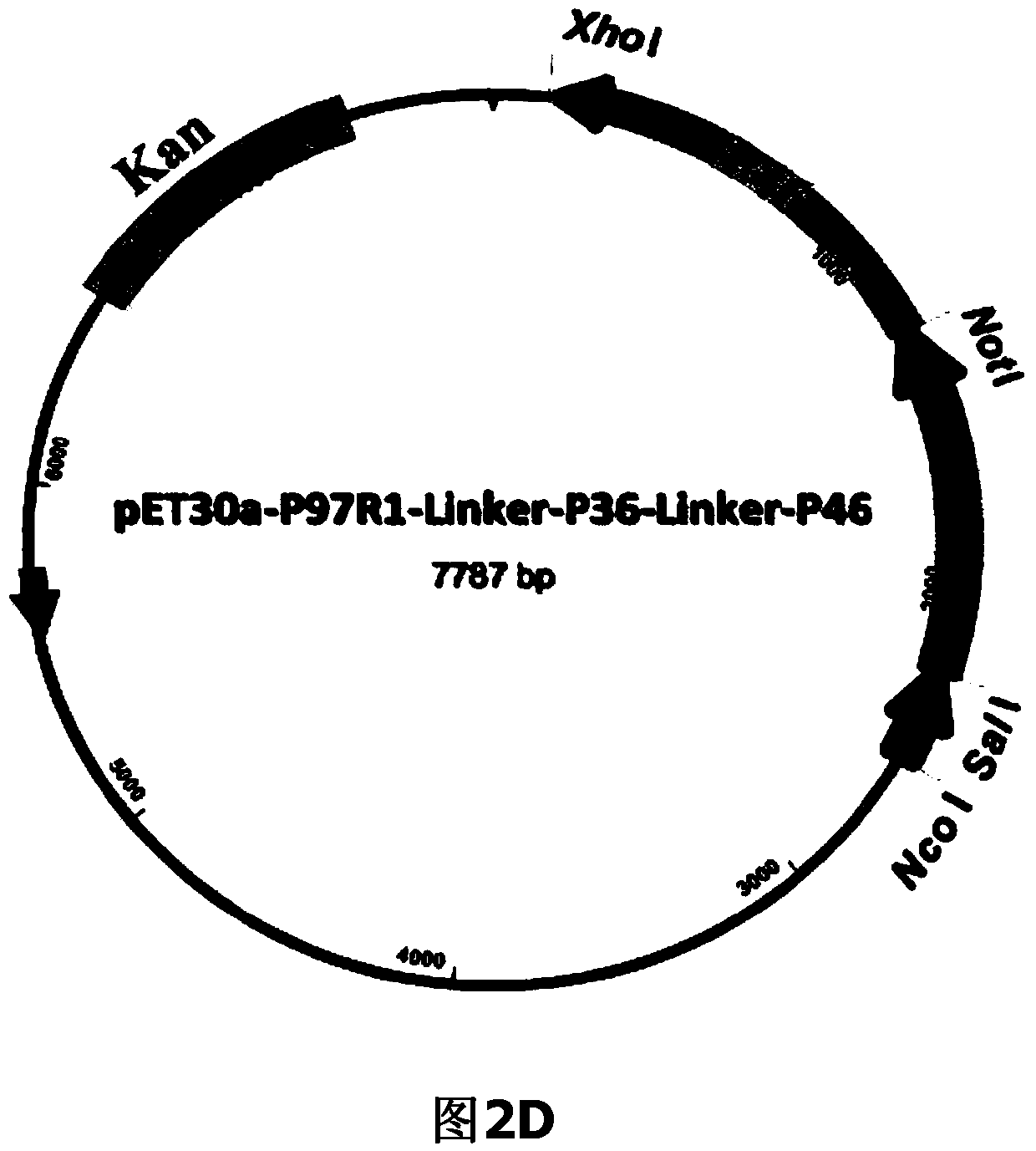
ActiveCN104293816AImproving immunogenicityHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliNucleotide
The invention belongs to the field of animal gene engineering, and in particular relates to a synthetic fusion gene for expressing mycoplasma hyopneumoniae and application. The fusion gene is characterized in that a mycoplasma hyopneumoniae P97R1 gene is connected in series with a P36 gene through a Linker, the termination codon of the P36 gene is deleted, the P46 gene with signal peptide removed is fused at the C end of the P36 gene through Linker, and then the fusion gene P97R1-P36-P46 is obtained, wherein the nucleotide sequence of the fusion gene is shown as SEQ ID NO: 1. The fusion gene is included in a prokaryotic expression plasmid, and escherichia coli BL21 / pET30a-P97R1-Linker-P36-Linker-P46 containing the plasmid is collected in CCTCC with the collection number CCTCC NO: M2014269. The invention further discloses immune efficacy evaluation of the fusion gene and application of the fusion gene in a novel vaccine.
Owner:HUAZHONG AGRI UNIV
Mycoplasma pneumoniae rapid detection kit and use method thereof
InactiveCN101665827AGuaranteed reliabilityEasy to detectMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementPolymerase LLoop-mediated isothermal amplification
The invention relates to a mycoplasma pneumoniae rapid detection kit and a use method thereof, wherein the kit holds a loop-mediated isothermal amplification reaction tube and BstDNA polymerase; the reaction tube holds reaction buffer, dNTP, magnesium sulfate, a primer 1:5-ACCAATGCCATCAACCCG-3, a primer 2:5-TACCGGCGTAACGCAAAG-3, a primer 3:5-ATTTTCACCCGTGAGGGGGAGTTTTCGCTTAACCCCGTGAACG-3, a primer4:5-ACAGCGCTAAGGGCATCACTGTTTTTCAAAGCCGCTTCGGTTC-3, lycine, manganese chloride and calcein. The method for detecting mycoplasma pneumoniae comprises the following steps: extraction of a sample to be detected or bacterium DNA to be detected, loop-mediated isothermal amplification reaction of mycoplasma pneumoniae and colour development detection. The invention has the characteristics of accurate detection, high sensitivity, strong specificity, simpleness, convenience and rapidness.
Owner:ZHUHAI ENCODE MEDICAL ENG
Kit for loop-mediated isothermal amplification detection of Mycoplasma ovipneumoniae and preparation and usage methods thereof
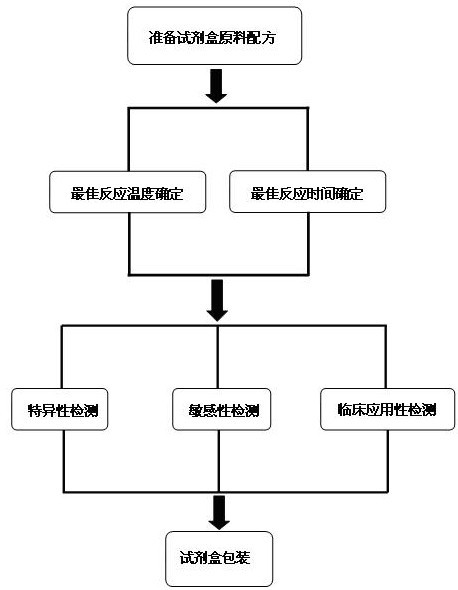
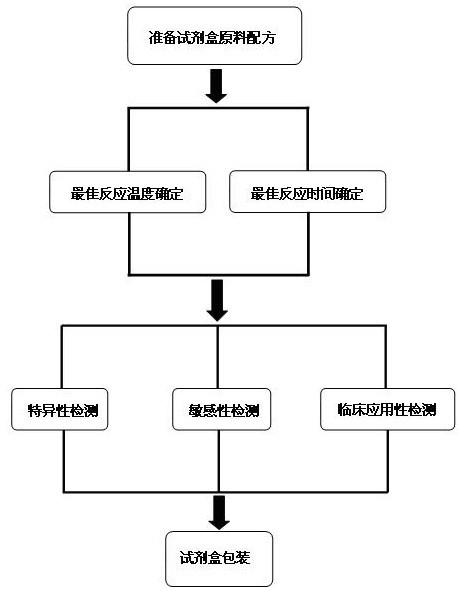
ActiveCN102634602AQuick checkReduce high costMicrobiological testing/measurementMicroorganism based processesPositive controlSpecific detection
The invention discloses a raw material composition of a kit for loop-mediated isothermal amplification (LAMP) detection of Mycoplasma ovipneumoniae and a preparation method and a usage method of the kit. The raw material composition comprises 1000-2000muL of reaction solution, 100-200muL of Bst (Bacillus stearothermophilus) DNA polymerase, 50-100muL of positive control, 50-100muL of negative control, 1-2mL of liquid paraffin and 1-2mL of ultrapure water. The reaction solution comprises an inner primer mixture solution, an outer primer mixture solution, an LAMP reaction buffer, Mg<2+> and dNTPs (deoxyribonucleotide triphosphates), wherein the volume ratio of the five liquids in the reaction solution is 2:2:2.5:2:1. The preparation method comprises the following steps: 1) determination of an optimum reaction temperature and an optimum reaction time; 2) specific detection, sensibility test and clinical application detection and 3) kit packaging. The kit disclosed by the invention has rapid, simple and accurate characteristics for pathogen detection of goat suspected cases of mycoplasma ovipneumoniae infection in goat farms.
Owner:GUIZHOU UNIV
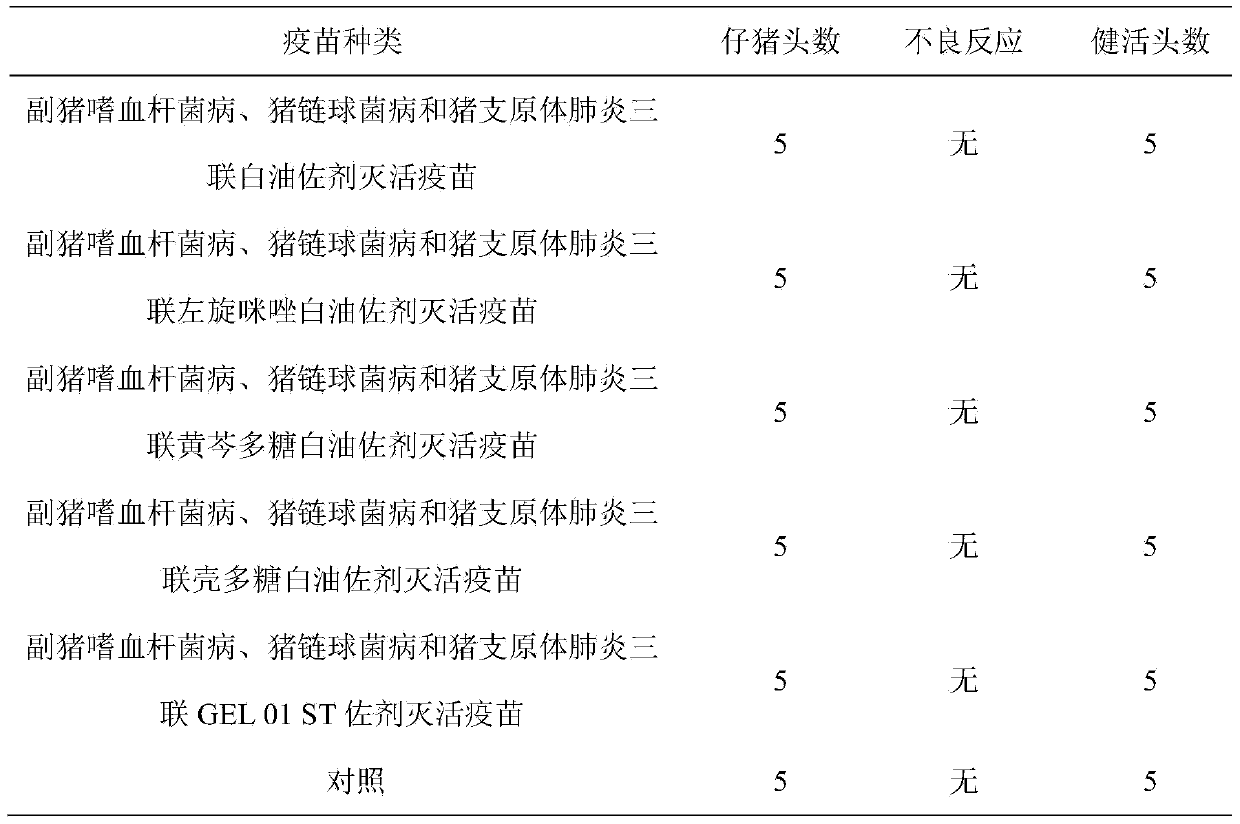
Method for preparing triple inactivated vaccine
InactiveCN104208667AReduce stressLow costAntibacterial agentsBacterial antigen ingredientsDiseaseHaemophilus
The invention provides a method for preparing a triple inactivated vaccine. The triple inactivated vaccine has a relatively good immunizing effect on haemophilus parasuis, swine streptococcosis and mycoplasma pneumoniae of swine, and the aims of preventing multiple diseases by one injection, reducing cost and reducing swine stress.
Owner:TIANJIN RINGPU BIO TECH
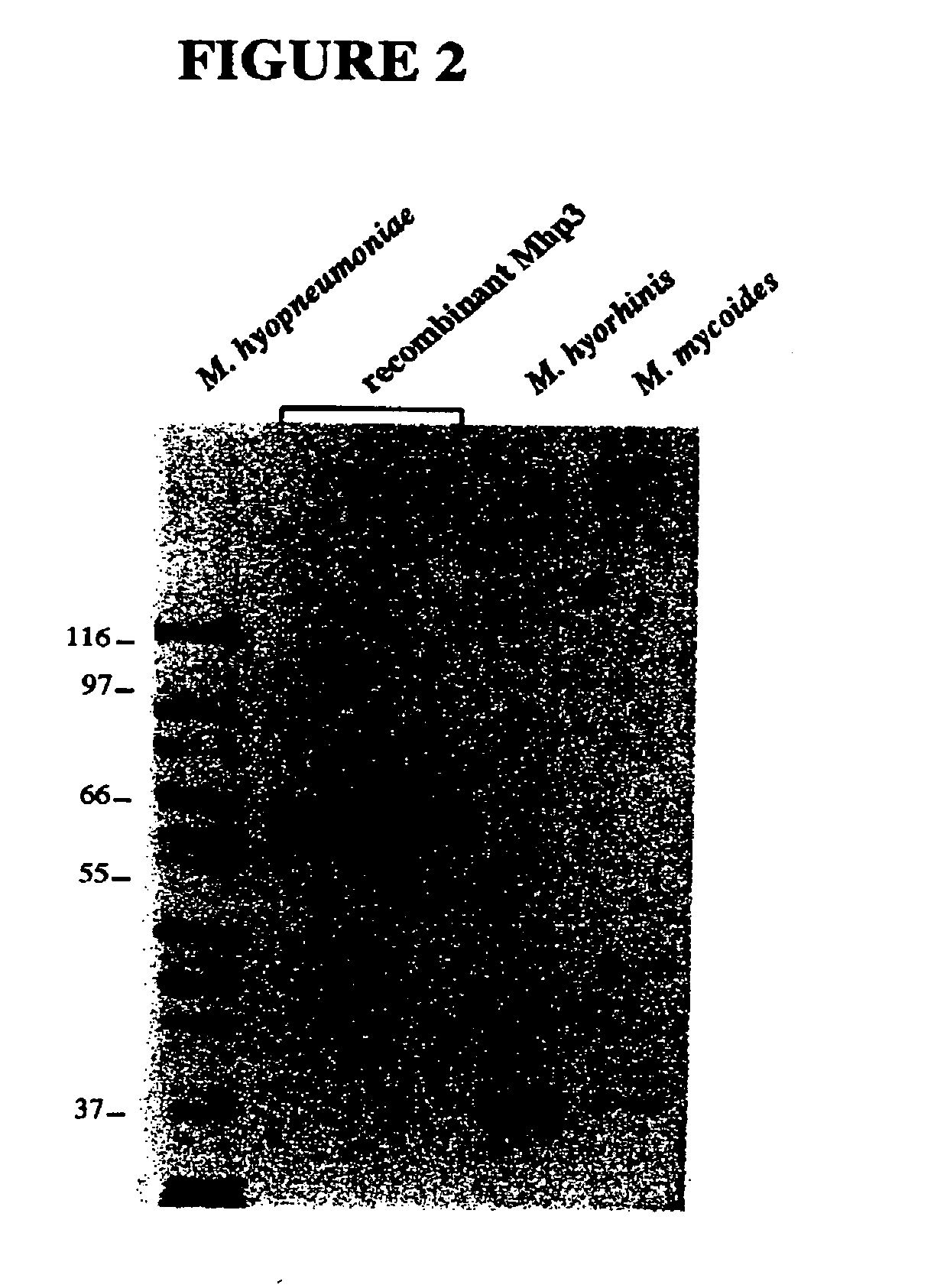
Nucleic acids and proteins of the mycoplasma hyopneumoniae mhp3 gene and uses thereof
InactiveUS20060233823A1Increase stringencyAntibacterial agentsBacteriaAntigenMycoplasma hyopneumoniae
The present invention relates to mhp3 nucleic acids and proteins encoded by the foregoing. The present invention further relates to novel apoprotein antigens encoded by mhp3 for use in vaccines to prevent and treat diseases caused by infection with Mycoplasma hyopneumoniae. The invention further relates to methods for the recombinant production of such antigens.
Owner:KING KENDALL +2
LAMP (loop-mediated isothermal amplification) kit for detection of mycoplasma pneumoniae and special LAMP primer for detection of mycoplasma pneumoniae
InactiveCN105238860AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesLoop-mediated isothermal amplificationPneumonitis
The invention discloses a LAMP (loop-mediated isothermal amplification) kit for detection of mycoplasma pneumoniae and a special LAMP primer for detection of mycoplasma pneumoniae. The special LAMP primer for detection of mycoplasma pneumoniae is designed according to a specificity conservative target sequence of a mycoplasma pneumoniae P1 gene (GenBank number: CP002077.1). The LAMP primer is formed by six primers including outer primers MP-16F3 and MP-16B3, inner primers MP-16FIP and MP-16BIP and loop primers MP-16LF and MP-16LB. By the aid of the LAMP kit and the special LAMP primer for detection of mycoplasma pneumoniae, quickness, convenience, high efficiency, high specificity and high sensitivity in qualitative detection of the mycoplasma pneumoniae in samples of pure bacteria, sputum, bronchoalveolar lavage fluid, throat swabs and the like can be realized without complicated instruments, and a new technical platform is provided for detection of the mycoplasma pneumoniae.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION +1
Chinese medicinal composition for treating ovine mycoplasma pneumoniae
InactiveCN102228611AHeat-clearing and detoxifyingWith cough and phlegmAntibacterial agentsRespiratory disorderMedicinal herbsSyndrome differentiation
The invention relates to a Chinese medicinal composition for treating ovine mycoplasma pneumoniae, which comprises the following components in part by weight: 15 to 25 parts of Vietnamese sophora root, 15 to 25 parts of indigowoad root, 15 to 25 parts of indigowoad leaf, 5 to 15 parts of baical skullcap root, 15 to 20 parts of barbed skullcap herb, 5 to 15 parts of caladium, 15 to 20 parts of Indian buead, 20 to 30 parts of astragalus, 15 to 20 parts of szechuan tangshen root, 10 to 20 parts of Szechuan lovage rhizome and 5 to 15 parts of liquoric root. In the Chinese medicinal composition, a pure Chinese medicinal herb preparation is developed by combining the research achievements of the pharmacology of modern Chinese veterinary medicines, formulating prescriptions elaborately and screening strictly according to the treatment based on syndrome differentiation and holism concept in Chinese veterinary science. The Chinese medicinal composition consists of the following 11 Chinese medicinal herbs of the Vietnamese sophora root, the indigowoad root, the baical skullcap root, the barbed skullcap herb, the caladium, the Indian buead, the astragalus, the Chinese angelica, the szechuan tangshen root, the Szechuan lovage rhizome, the liquoric root and the like, and has the effects of clearing heat, detoxicating, relieving cough, reducing sputum, tonifying qi and promoting blood circulation.
Owner:SRICK TIANJIN BIO TECH
Multi-item respiratory tract antigen detection card and kit
ActiveCN112362869AAdequate responseHigh sensitivityBiological testingImmunoassaysAntibody conjugateAntigen testing
The invention relates to a multi-item respiratory tract antigen detection card which comprises an influenza A virus antigen test strip, an influenza B virus antigen test strip, a respiratory tract adenovirus antigen test strip, a respiratory tract syncytial virus antigen test strip and a mycoplasma pneumoniae antigen test strip, wherein each of the influenza A virus antigen test strip, the influenza B virus antigen test strip, the respiratory tract adenovirus antigen test strip, the respiratory tract syncytial virus antigen test strip and the mycoplasma pneumoniae antigen test strip comprisesa colloidal gold conjugate pad, and each colloidal gold conjugate pad comprises a streptavidin conjugate pad and a double-nanoparticle double-labeled antibody conjugate pad; the test strip in the detection card enables biological raw materials to react fully, improves the sensitivity of antigen detection, effectively reduces missing detection, and meanwhile, the blocking agent is added into the sample pad to improve the specificity, so that influenza A virus, influenza B virus, respiratory adenovirus, respiratory syncytial virus and mycoplasma pneumoniae antigen can be detected at the same time.
Owner:山东康华生物医疗科技股份有限公司
Probes, compositions and kits for determining the presence of Mycoplasma genitalium in a test sample
The present invention relates to oligonucleotides useful for determining the presence of Mycoplasma pneumoniae and / or Mycoplasma genitalium in a test sample. The oligonucleotides of the present invention may be incorporated into hybridization assay probes, capture probes and amplification primers, and used in various combinations thereof.
Owner:GEN PROBE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com