Method and kit for adopting colloidal gold chromatographic technique for detecting mycoplasma pneumoniae nucleic acid
A technology of Mycoplasma pneumoniae and detection kit, applied in the field of medical biology, can solve the problems of cumbersome operation, lack of versatility, etc., and achieve the effects of low technical requirements, reduced requirements, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] [Example 1] Universal nucleic acid probe labeling colloidal gold particles
[0063] 1. After the design of Universal Probe 1 is completed, carry out thiol modification at its 5' end.
[0064] 2. Add 20 μl of the synthesized universal probe (final concentration 0.1 mM) to 10 μl TCEP-HCl (final concentration 100 mM), and use ddH 2 Make up to 100 μl with O, and reduce the sulfhydrylated DNA universal probe.
[0065] 3. Add the treated universal probe to 500ml of colloidal gold solution with 30nm diameter particles, and incubate overnight at room temperature.
[0066] 4. Add 2% SDS solution to make the final concentration 0.01%, incubate at room temperature for 30min.
[0067] 5. Add 2M NaCl dropwise to the solution until the final concentration is 0.15M.
[0068] 6. Centrifugal purification of gold-labeled nucleic acid probes: Centrifuge at 15,000rpm for 15min, wash with washing solution (0.15MNaCl,
[0069] 0.01% SDS) and washed four times, the colloidal gold precipit...
Embodiment 2
[0070] [Example 2] Preparation of nucleic acid detection test strips
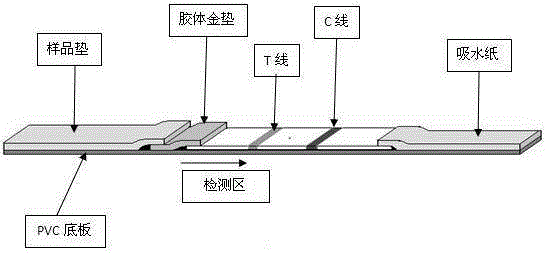
[0071] The main raw materials needed in the preparation of nucleic acid detection test strips: glass fiber membrane, nitrocellulose membrane (NC membrane), sample pad, PVC bottom plate, etc.
[0072] 1. Preparation of colloidal gold pads: cut the glass fiber membrane into small modules of 0.5×1 cm square, and evenly drop 10 μl of gold-labeled nucleic acid probe solution on each module, let it dry at room temperature, and seal it for future use.
[0073] 2. Spray film (coated with NC film):
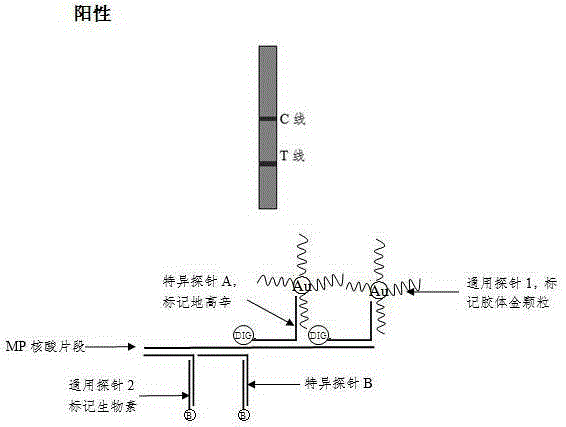
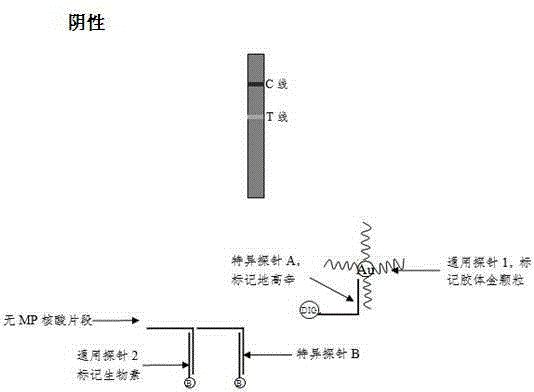
[0074] Detection line (T line): avidin (about 0.5~1.0mg / ml), spray film volume: 1.5~3μl / cm;
[0075] Quality control line (C line): anti-digoxigenin antibody (about 0.5~1.0mg / ml), spray film volume: 1.5~3μl / cm;
[0076] After spraying the film, put the film strip in a clean incubator at 37°C to dry for 3 to 4 hours, and store it in a dry environment for later use.
[0077] 3. Assembly of test strips:
[0078] Cut ou...
Embodiment 3
[0079] [Example 3] Detection of Mycoplasma pneumoniae (MP) NASBA amplification products
[0080] a. Amplify Mycoplasma pneumoniae nucleic acid:
[0081] components Volume (μl) Mycoplasma pneumoniae nucleic acid (or lysate) 2 Amplification reaction solution: containing dNTPs, NTPs, primer R&F and various salt ions 17 total 19
[0082] 95°C, 2min, 42°C, 2min, add 1μl of amplification enzyme mixture (AMV, T7 polymerase and RNaseH), react at 42°C for 45min, and wait for detection.
[0083] b. Detect the specific amplification product of Mycoplasma pneumoniae obtained in a:
[0084] Amplified product: 10μl
[0085] Specific probe A1 (5μM): 1μl
[0086] Specific probe A2 (5μM): 1μl
[0087] Specific probe B1 (5 μM): 1 μl
[0088] Specific probe B2 (5 μM): 1 μl
[0089] Universal Probe 2 (1 μM): 1 μl
[0090] Chromatography solution (4×SSC containing 5% formamide) to a total volume of 100 μl
[0091] After 10 minutes at 42°C, point on the test ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com