Kit for quickly detecting 15 pneumonia pathogenic bacteria
A technology for pathogenic bacteria and kits, applied in the field of genetic technology, can solve the problems of aggravating drug-resistant strains, treatment can only rely on empirical treatment, and the sensitivity and specificity are not ideal.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
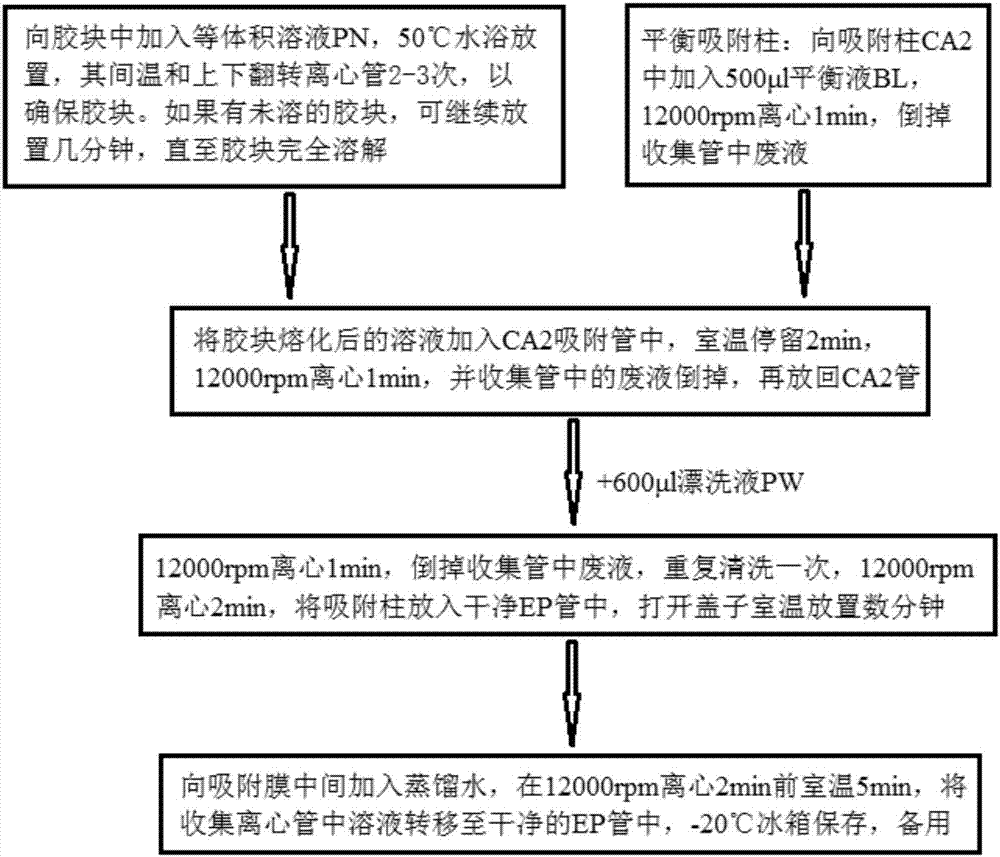
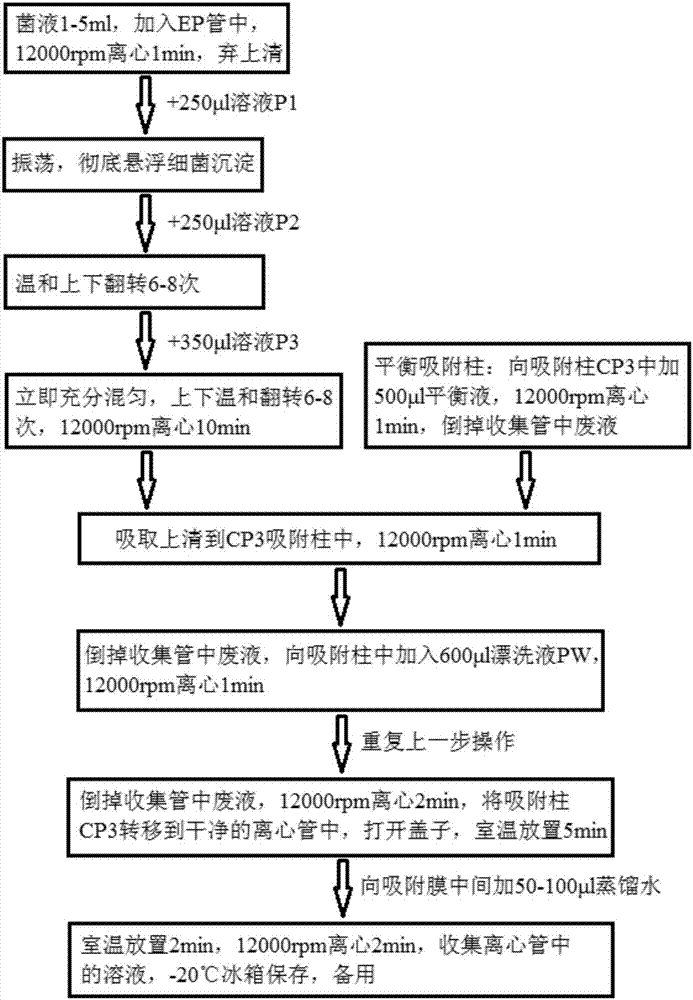
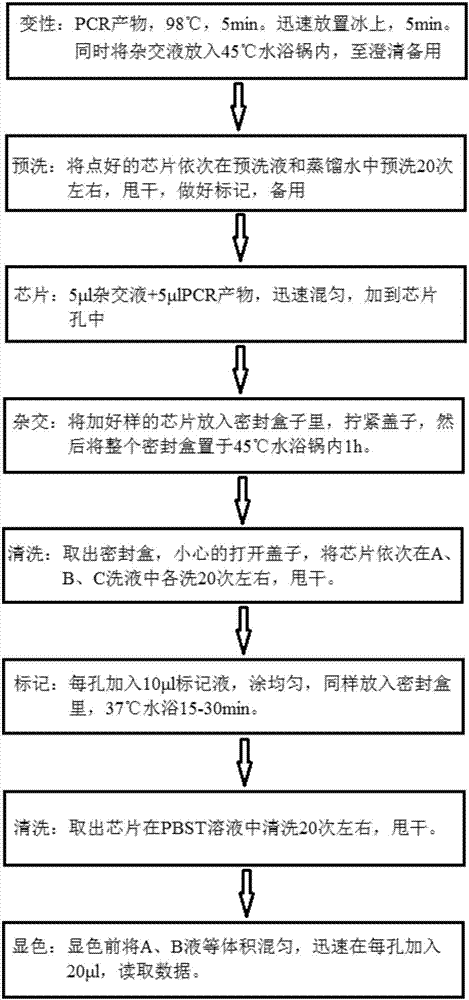
[0082]Hereinafter, the gene chip for rapid identification of pneumonia pathogenic bacteria of the present invention will be described in detail in conjunction with the accompanying drawings.
[0083] Materials and Methods
[0084] 1. Experimental materials
[0085] 1. Standard strain
[0086] Standard strains used in this experiment: Streptococcus pneumoniae, Stenotrophomonas maltophilia, Burkholderia cepacia, Staphylococcus aureus, Haemophilus influenzae, Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Mycoplasma pneumoniae, Enterococcus faecalis, Enterococcus faecium, Enterobacter cloacae, etc. were collected by the Institute of Radiation and Radiation Medicine of the Academy of Military Medical Sciences of the Chinese People's Liberation Army, and the General Microorganisms Committee of the Chinese Microbiological Culture Collection Management Committee. Center (CGMCC) and China Food and Drug Control Institute to provide or purcha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com