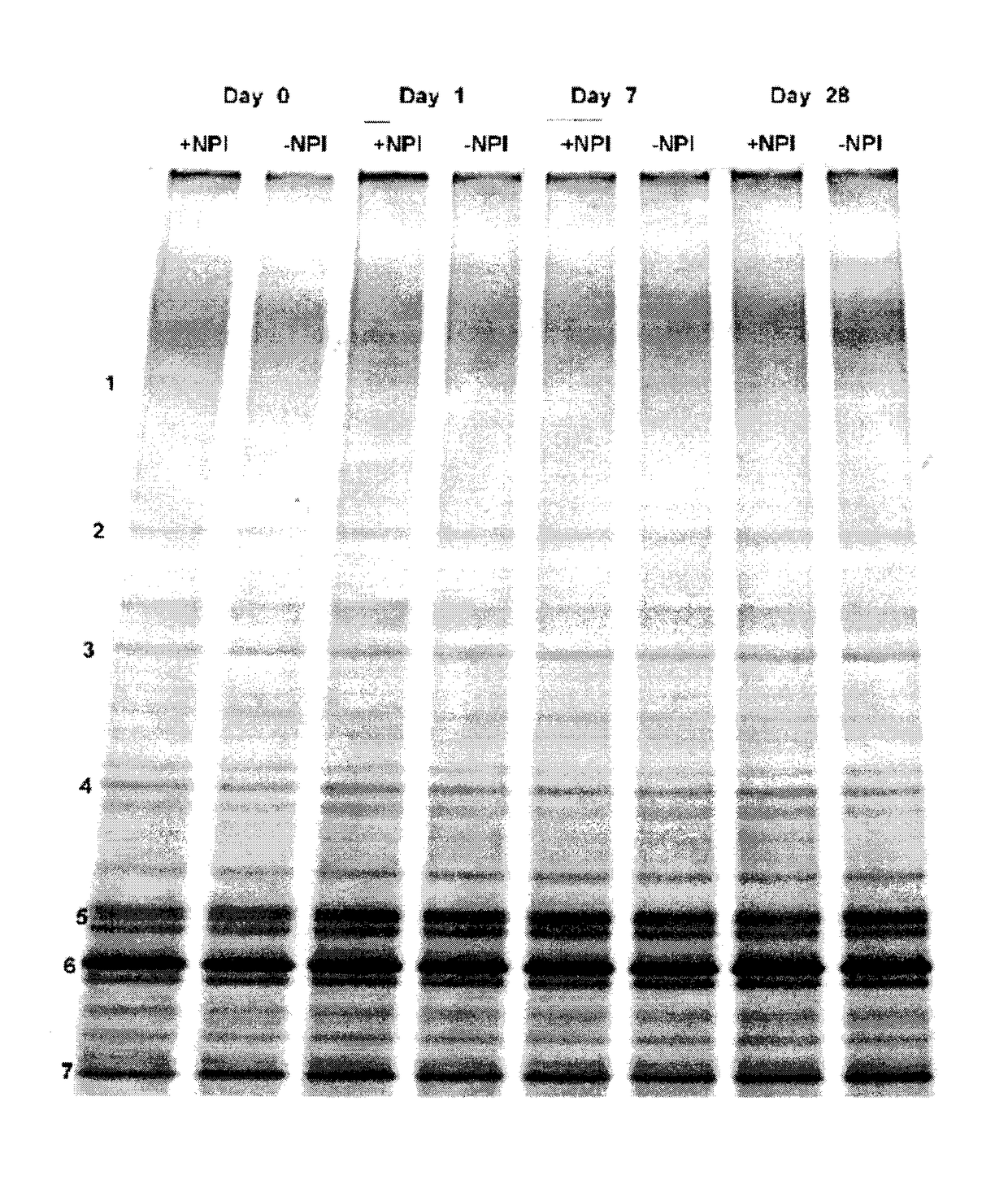
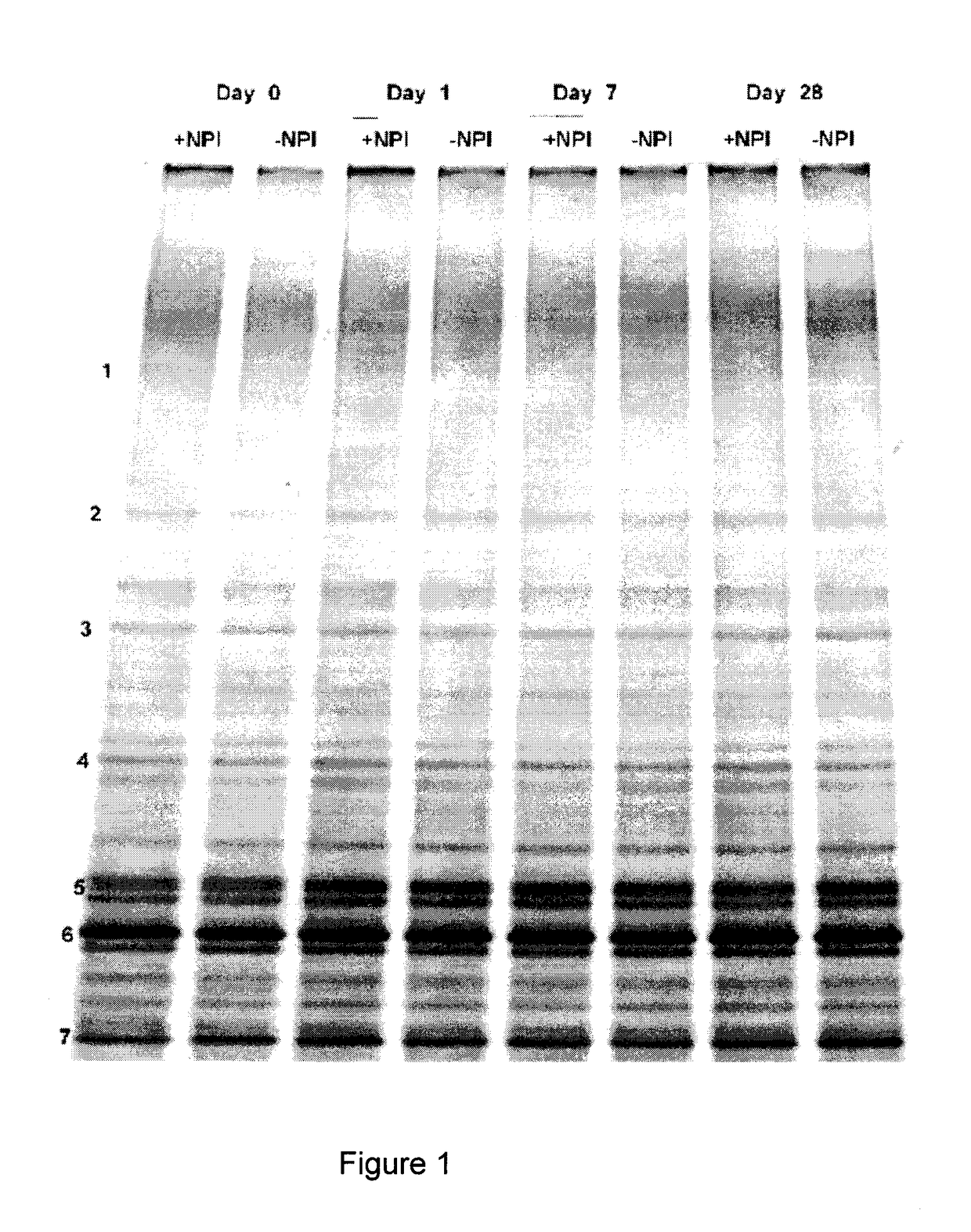
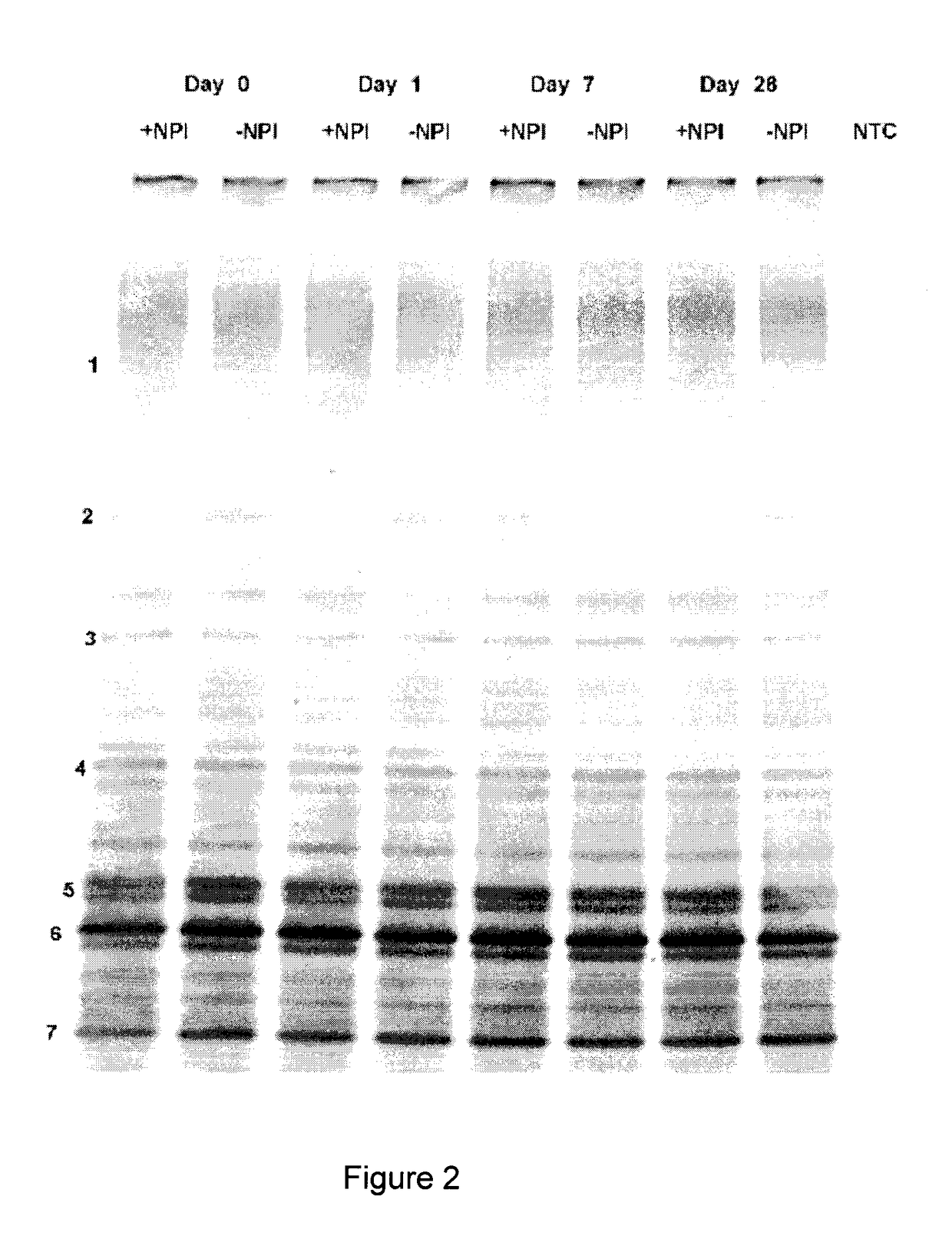
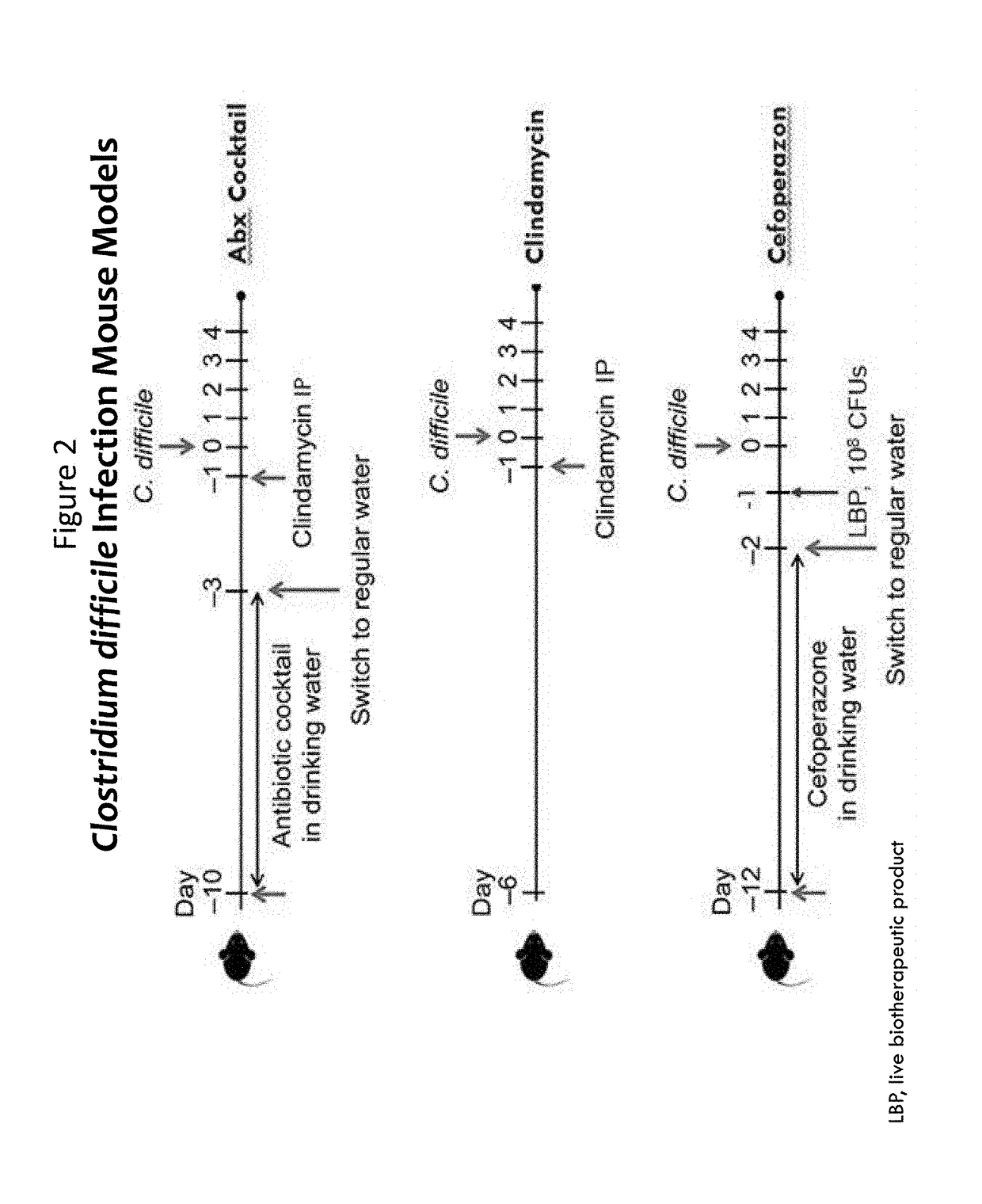
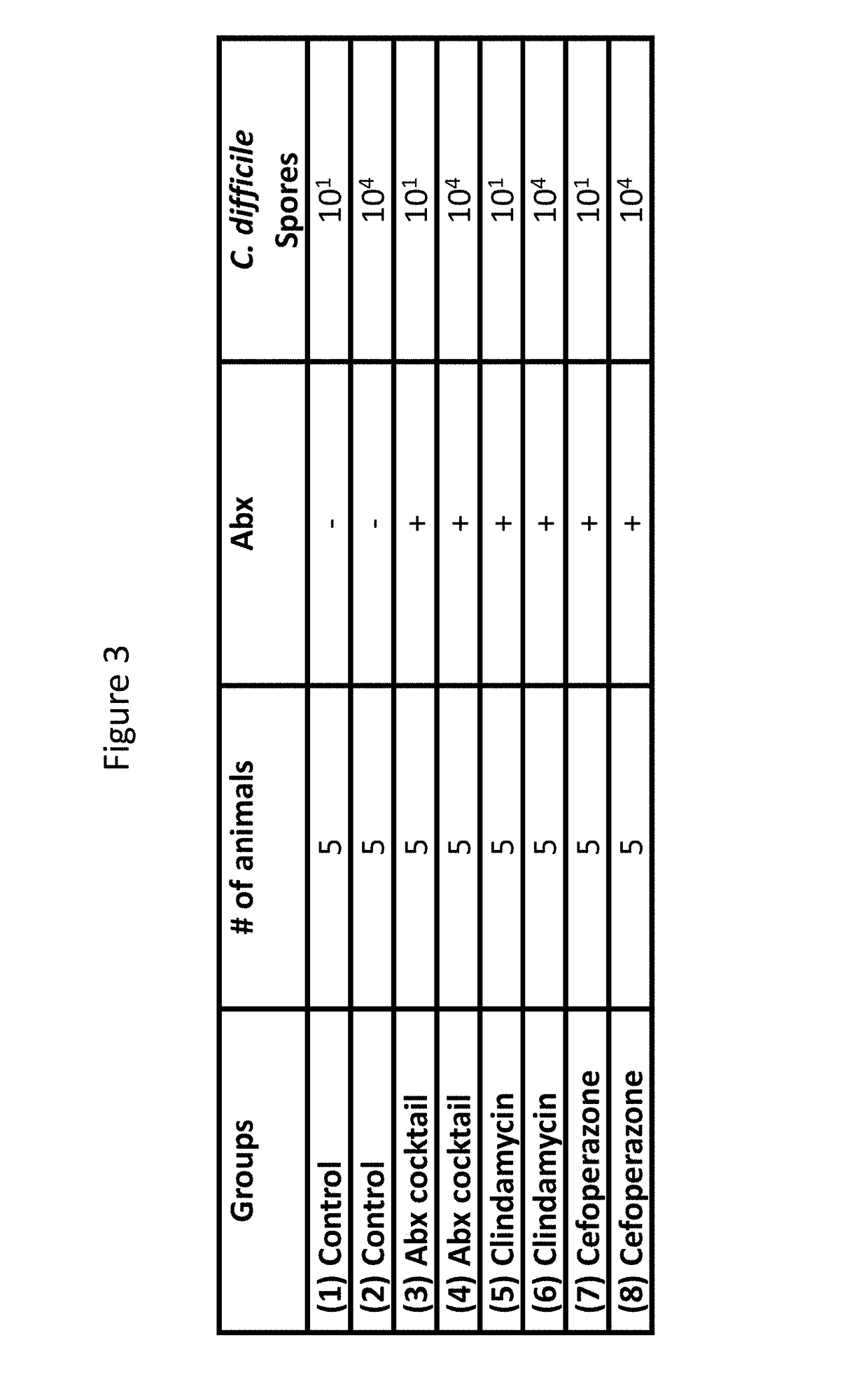
Patents
Literature
199 results about "Clostridium difficile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
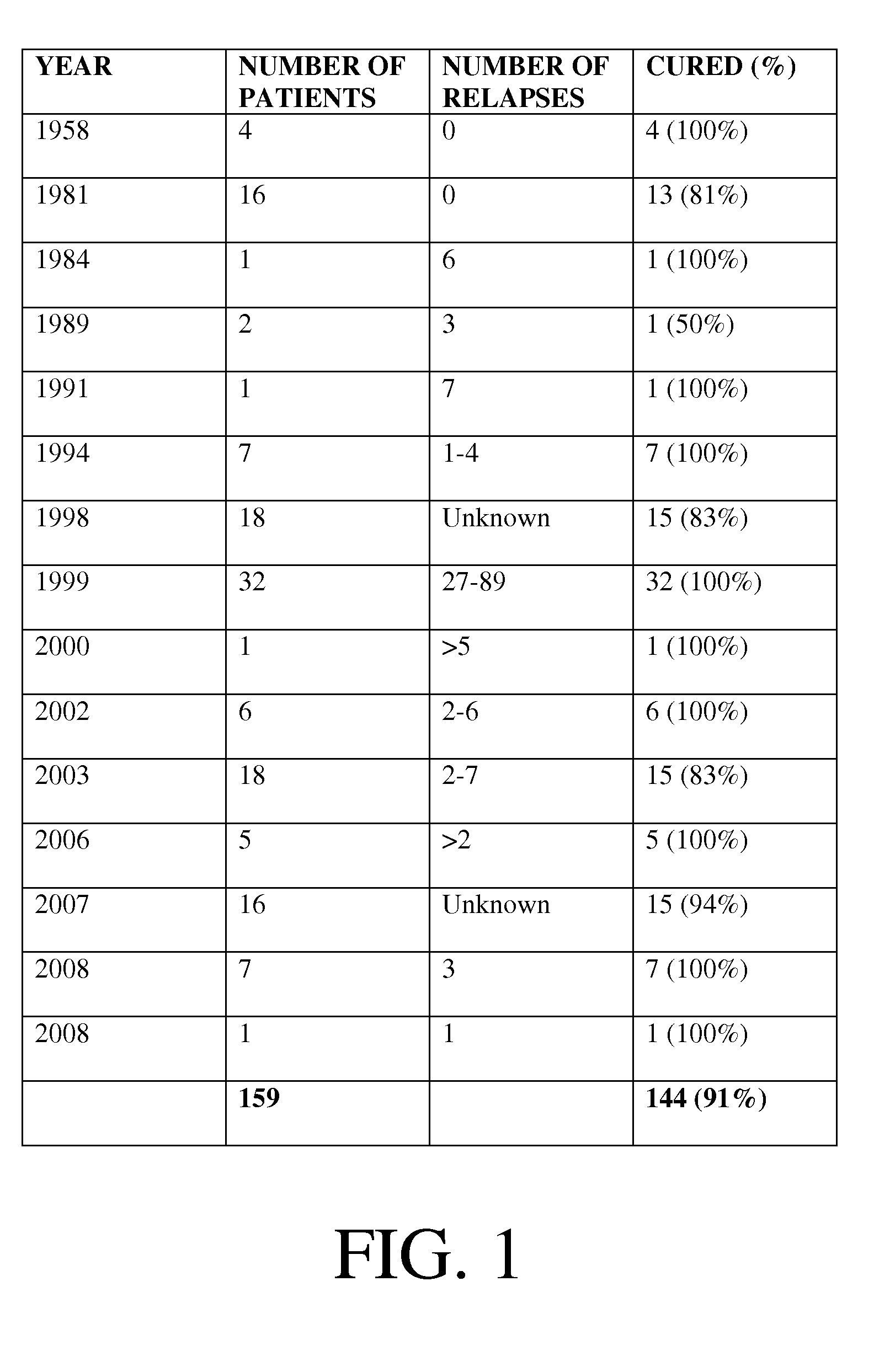
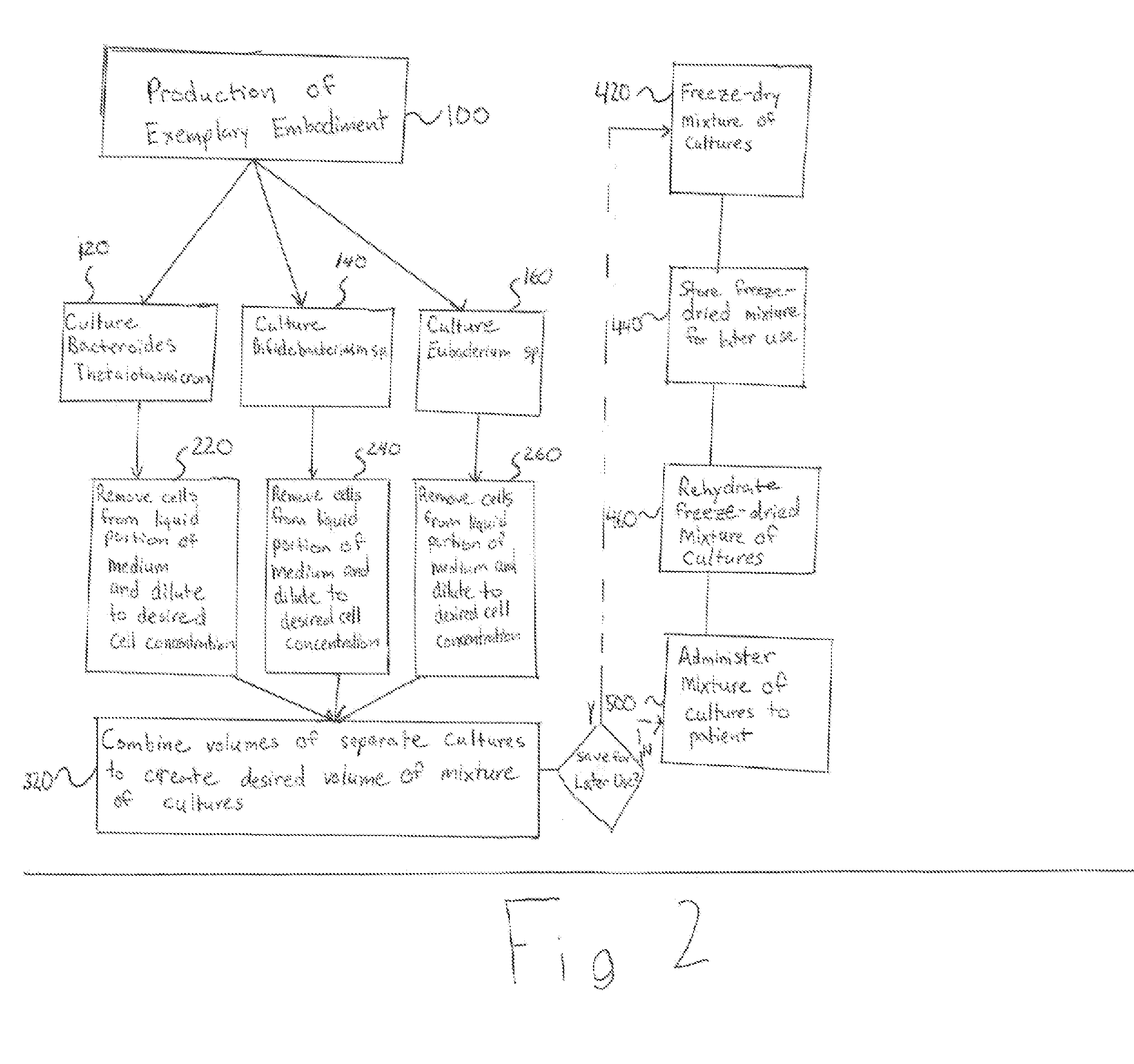
Systems and methods of replacing intestinal flora
InactiveUS20130022575A1Reduce the risk of infectionEliminate needBiocideBacteriaBacteroidesClostridium difficile
The invention relates to one or more systems and methods for creating a mixture of cultures used to replace intestinal flora. Specifically, the invention relates to methods and systems of treating diseases including Clostridium difficile and Crohn's disease by introducing a mixture of pure cultures of viable bacteria into the gastrointestinal tract.
Owner:MICROBIAL RX
Compositions and methods for characterizing and restoring gastrointestinal, skin, and nasal microbiota
ActiveUS20100074872A1Growth inhibitionFacilitate calorie uptakeBiocideMetabolism disorderBacteroidesDisease
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
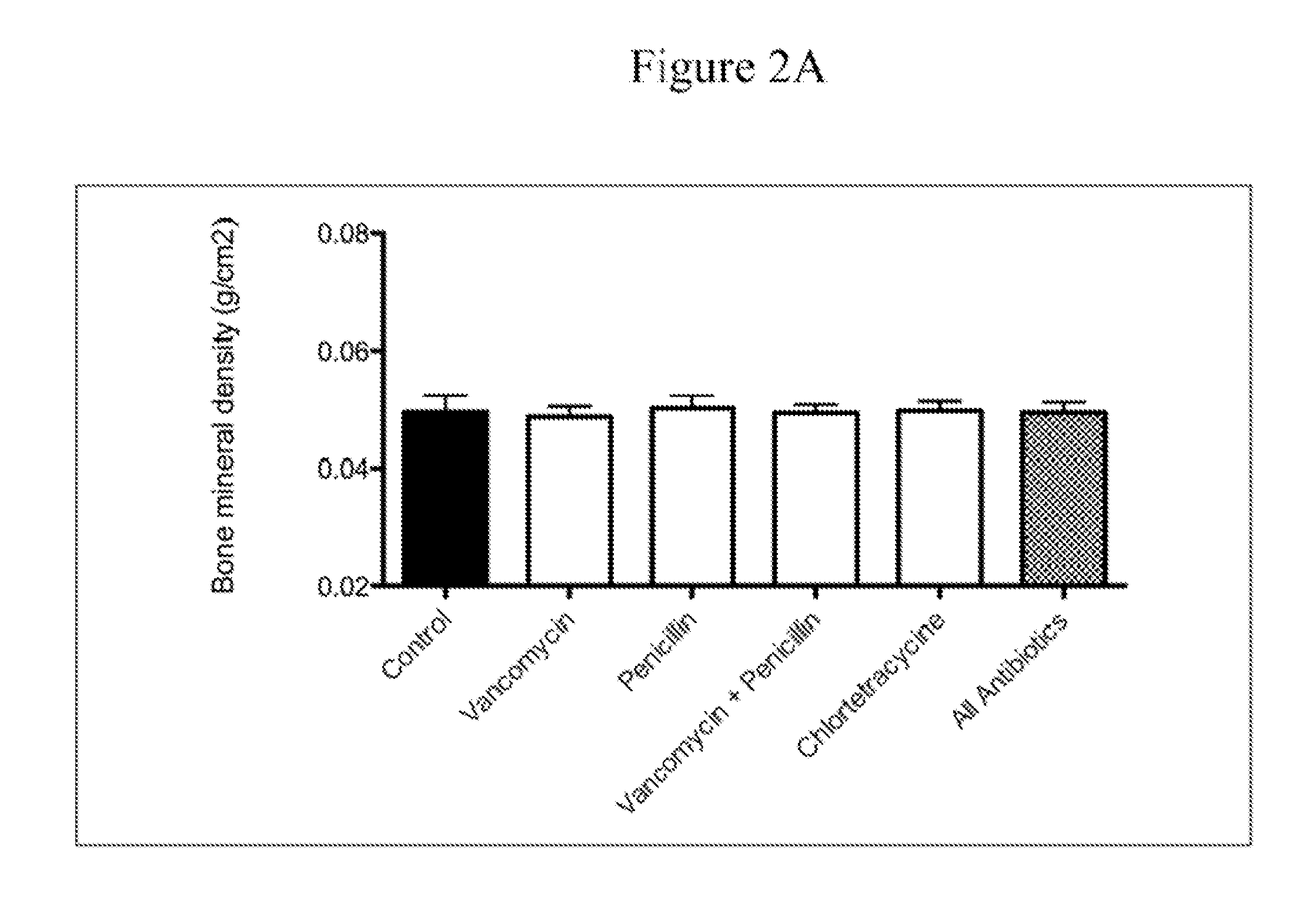
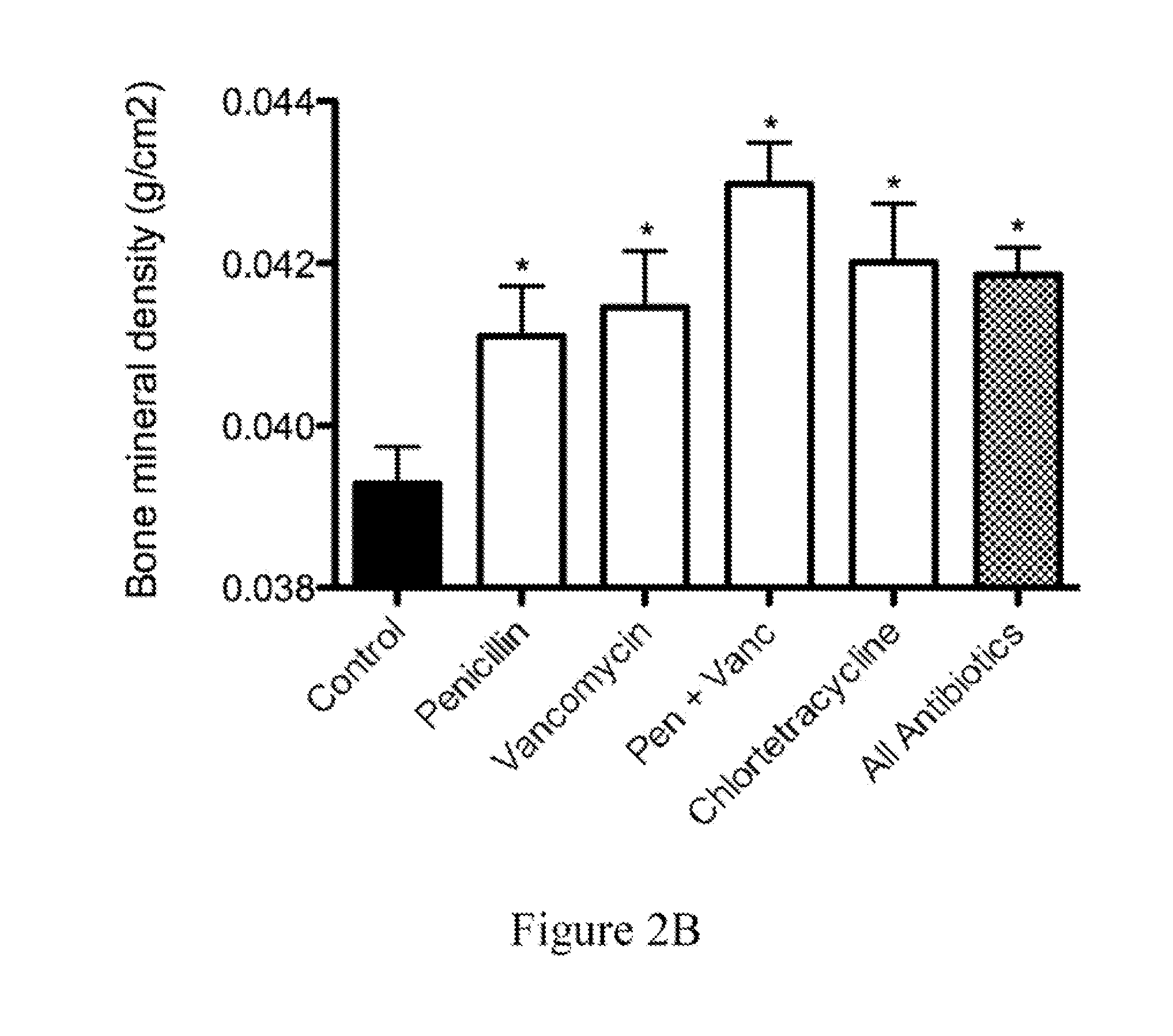
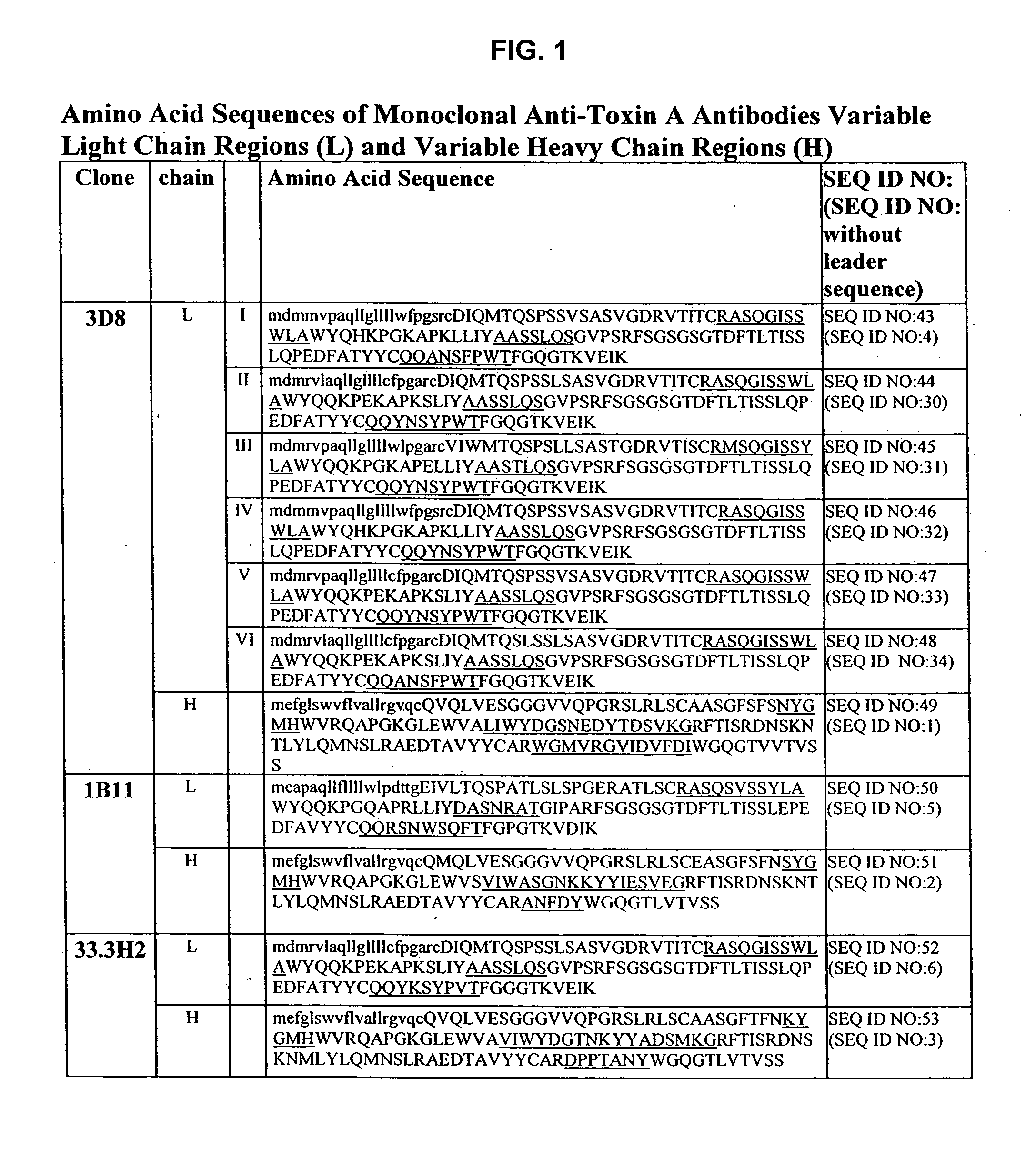
Antibodies against Clostridium difficile toxins and uses thereof
ActiveUS20050287150A1High affinityLess immunogenicAntibacterial agentsBacteriaClostridium difficileClostridium difficile (bacteria)
Owner:MASSACHUSETTS UNIV OF +2
Affinity purified human polyclonal antibodies and methods of making and using them
The present invention describes a method for treating, removing or preventing a bacterial infection, which method comprises administering to a human suffering, suspected of suffering or at risk of suffering from Staphylococcus aureus (S. aureus) infection, a Streptococcus infection, Escherichia coli (E. coli) infection, Pseudomonas aeruginosa (P. aeruginosa) infection, Acinetobacter baumannii (A. baumannii) infection, Enterococcus faecium (E. faecium) infection and / or Clostridium difficile (C. difficile) infection, an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from bacterial cells selected from S. aureus, a Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, and optionally, wherein said affinity purified human polyclonal antibodies are purified (e.g., as made more concentrated as compared to the starting or unpurified material) relative to the same human polyclonal antibodies in the unpurified or non-affinity-purified human blood sample, e.g., intravenous immunoglobulin (IVIG) sample, and / or also optionally, wherein said affinity purified human polyclonal antibodies are specific for the bacterial antigens used in the affinity purification, and / or further optionally wherein the affinity purified human polyclonal antibodies are substantially free of human antibodies that specifically bind to non-bacterial antigens in the human blood sample. Pharmaceutical compositions for treating bacterial infections, comprising an effective amount of human polyclonal antibodies affinity purified from a human blood sample with an antigenic preparation comprising cellular and / or secreted antigen(s) from S. aureus, Streptococcus, E. coli, P. aeruginosa, A. baumannii, E. faecium, C. difficile or a combination thereof, are also provided.
Owner:SCANTIBODIES LAB
Compositions relating to a mutant clostridium difficile toxin and methods thereof
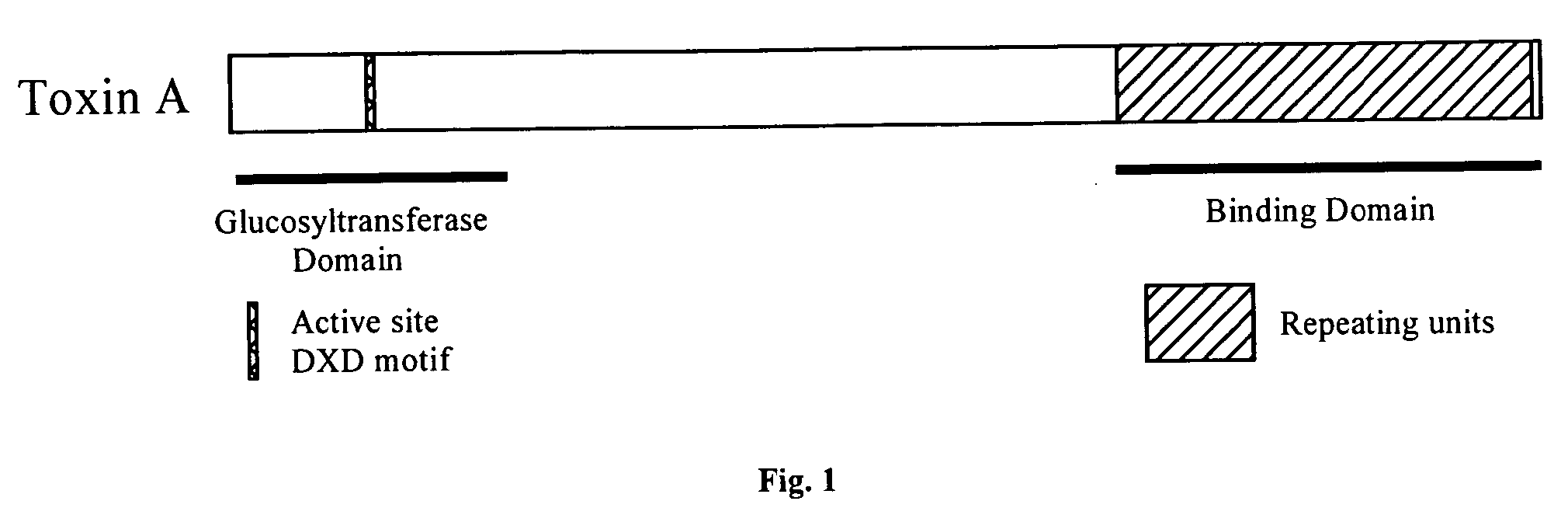
ActiveUS20120269841A1Antibacterial agentsBacterial antigen ingredientsClostridium difficile toxin BNucleotide
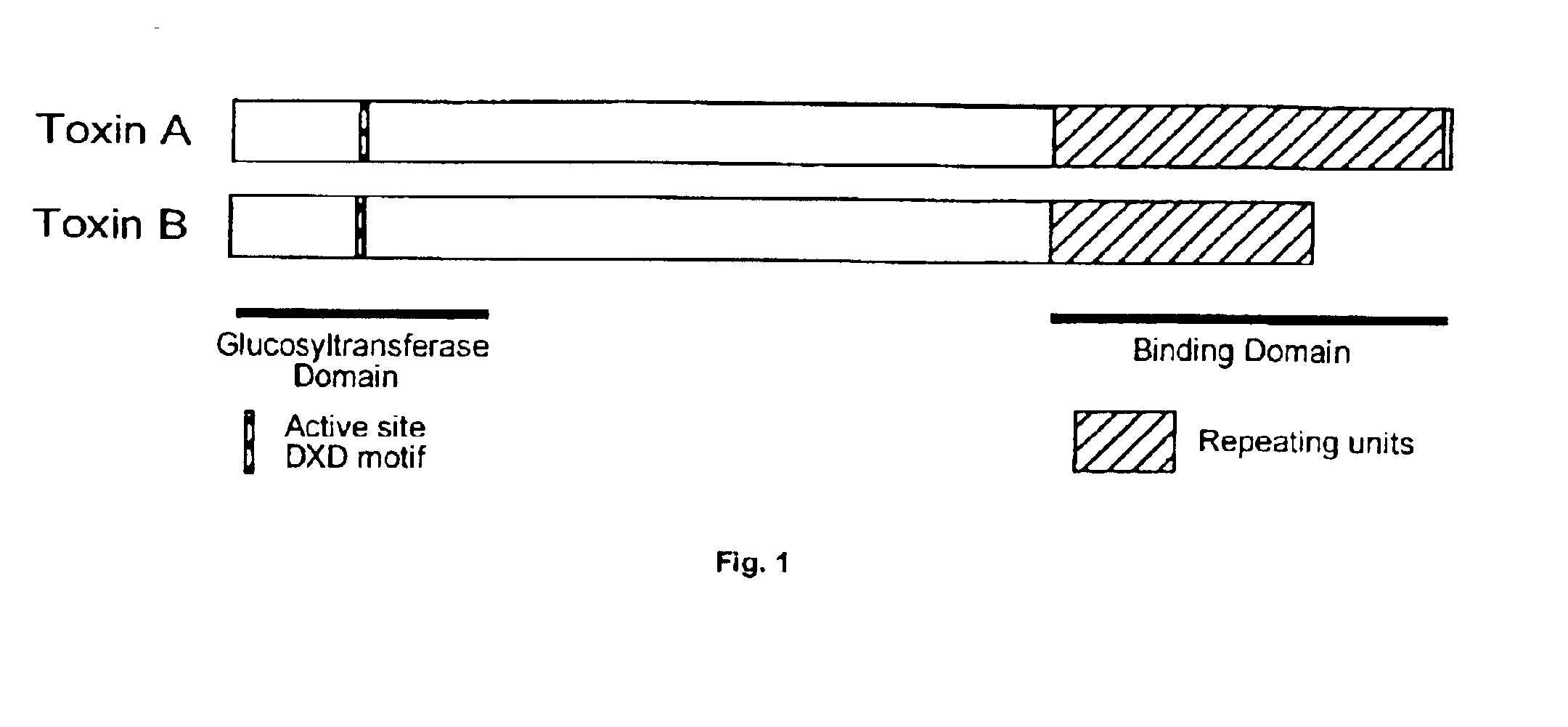
In one aspect, the invention relates to an immunogenic composition that includes a mutant Clostridium difficile toxin A and / or a mutant Clostridium difficile toxin B. Each mutant toxin includes a glucosyltransferase domain having at least one mutation and a cysteine protease domain having at least one mutation, relative to the corresponding wild-type C. difficile toxin. The mutant toxins may further include at least one amino acid that is chemically crosslinked. In another aspect, the invention relates to antibodies or binding fragments thereof that binds to said immunogenic compositions. In further aspects, the invention relates to isolated nucleotide sequences that encode any of the foregoing, and methods of use of any of the foregoing compositions.
Owner:WYETH LLC
HUMAN SECRETORY IgA FOR THE TREATMENT OF CLOSTRIDIUM DIFFICILE ASSOCIATED DISEASES
A composition for treating a subject is provided. The composition includes dimeric or polymeric IgA therapeutic. Formulating agents are mixed with the dimeric or polymeric IgA to yield a dosing form of a capsule, tablet, and a suppository. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the sequential modification of monomeric IgA with J chain and secretory component to form a dimeric or polymeric IgA therapeutic. The dimeric or polymeric IgA therapeutic is then mixed with formulating agents to create a capsule, tablet, or suppository dosing form. The therapeutic is amenable to enrobement directly through microeneapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R
Sporicidal composition for clostridium difficile spores
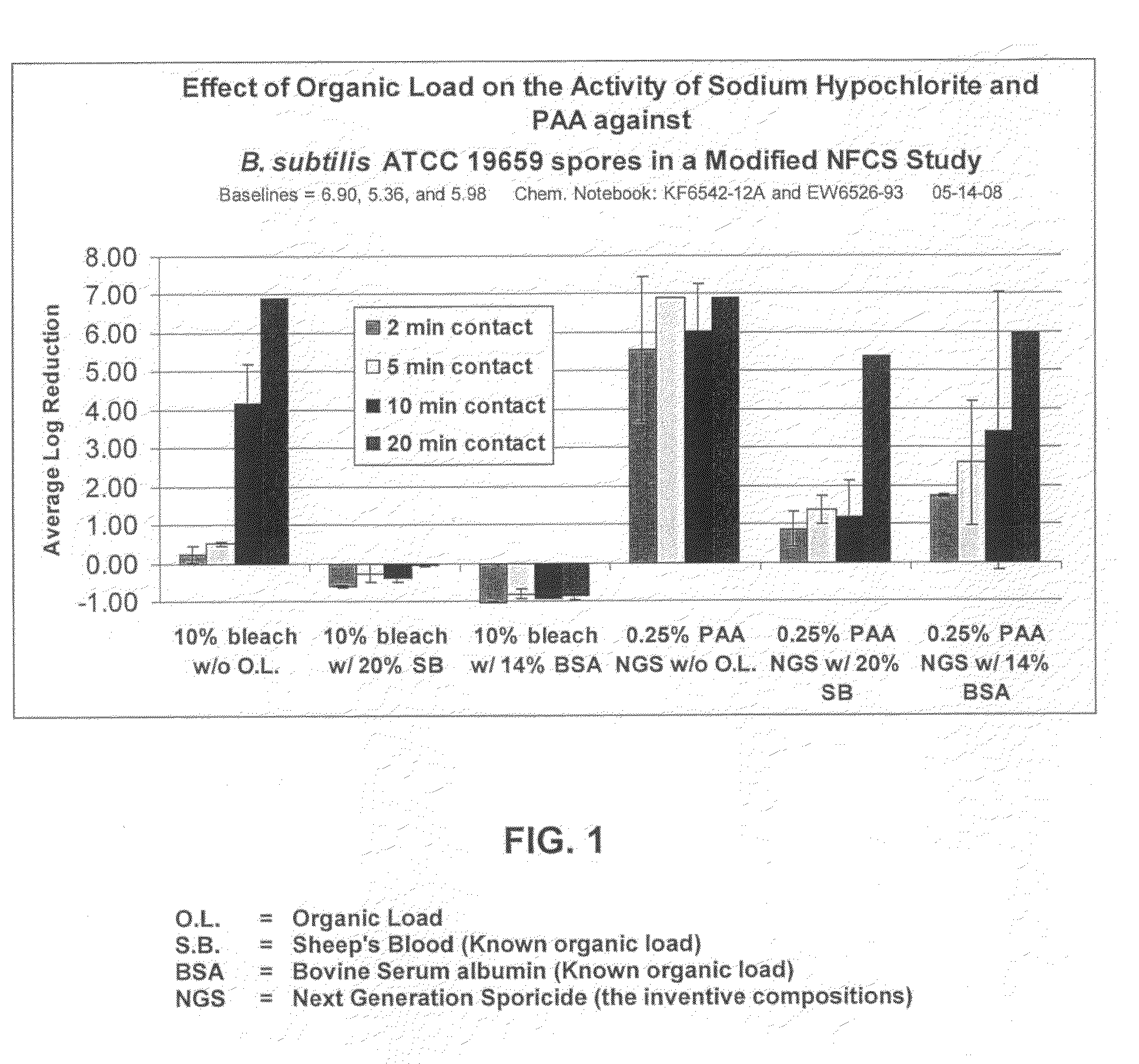
A cleaning medium or formulation that contains a sporicidal composition is described. The composition includes about 0.1-20% weight / weight of a germinant agent, about 0.01-75% w / w of an antimicrobial agent, in terms of dry or wet total weight, and which is admixed with water to generate a solution with a pH of 3.5-9.5. The composition can help trigger the germination of spores, in particular C. difficile, and subsequently deactivate or kill the spores. A means of applying the cleaning formulation in a medium and process for cleaning are also described.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Antibacterial compounds
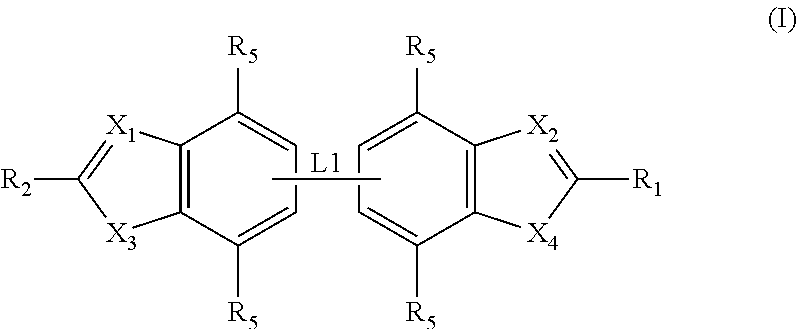
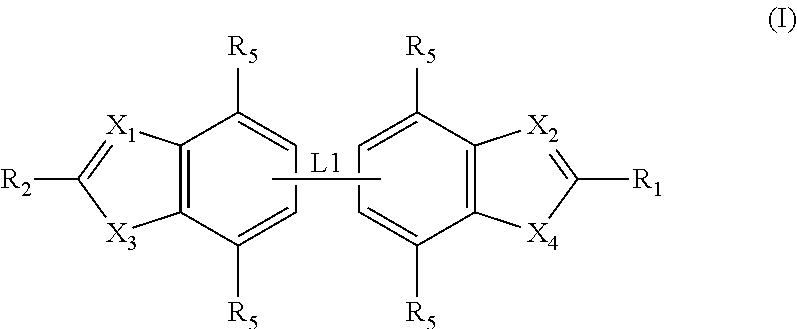
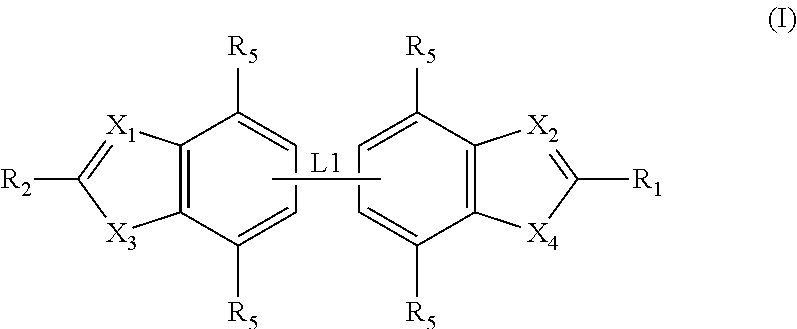
ActiveUS20120020950A1Augment other biological activityHigh activityAntibacterial agentsBiocideClostridium difficileBacterial Disorder
Disclosed are compounds of formula (I), which are of use in the treatment of bacterial diseases and infections, to compositions containing those compounds and to methods of treating bacterial diseases and infections using the compounds. In particular, the compounds are useful for the treatment of infection with, and diseases caused by, Clostridium difficile.
Owner:SUMMIT OXFORD
Low odor, hard surface sporicide
ActiveUS20100196503A1Safer and easy to handleSafer and easy to and transportBiocidePeroxide active ingredientsClostridium difficile toxin BNuclear chemistry
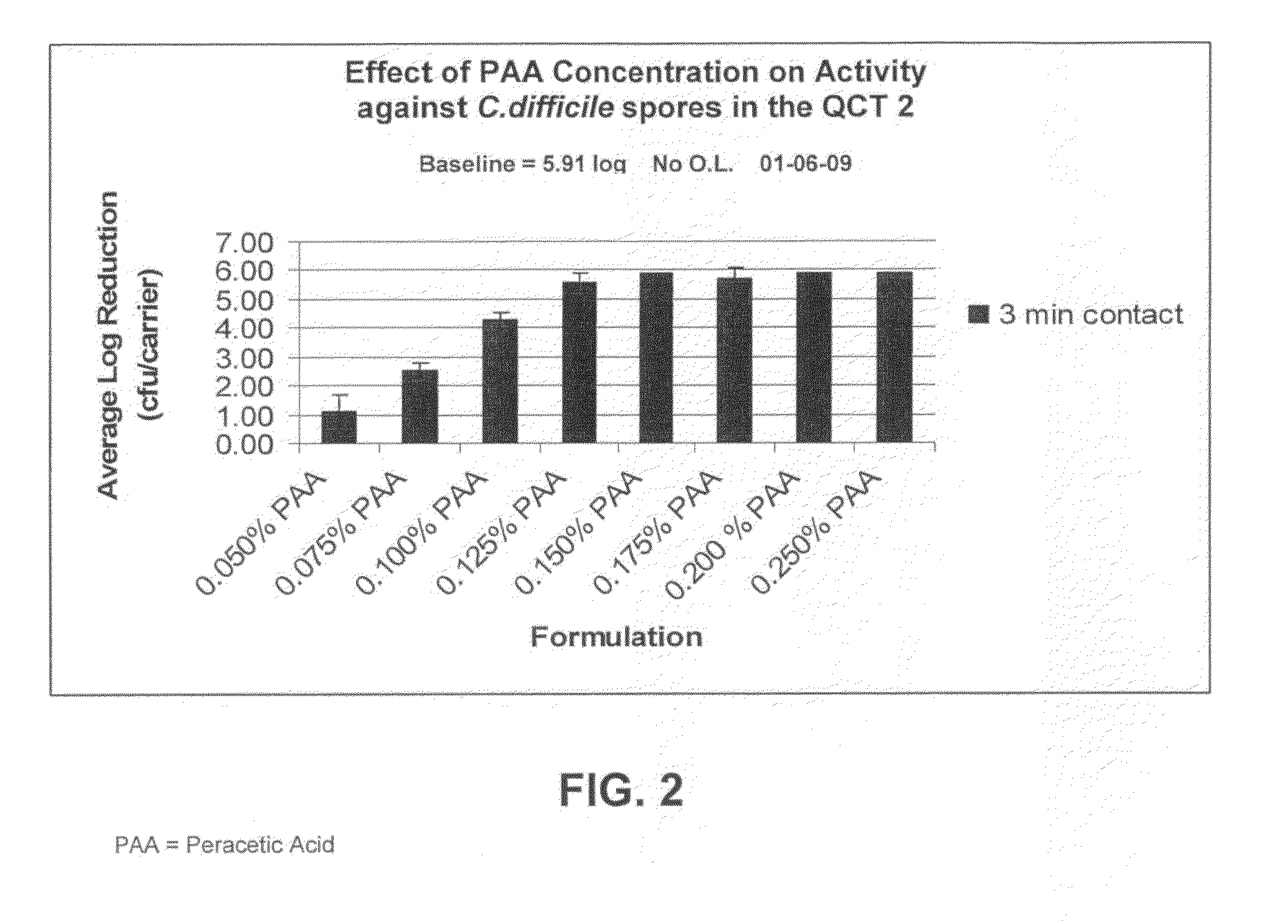
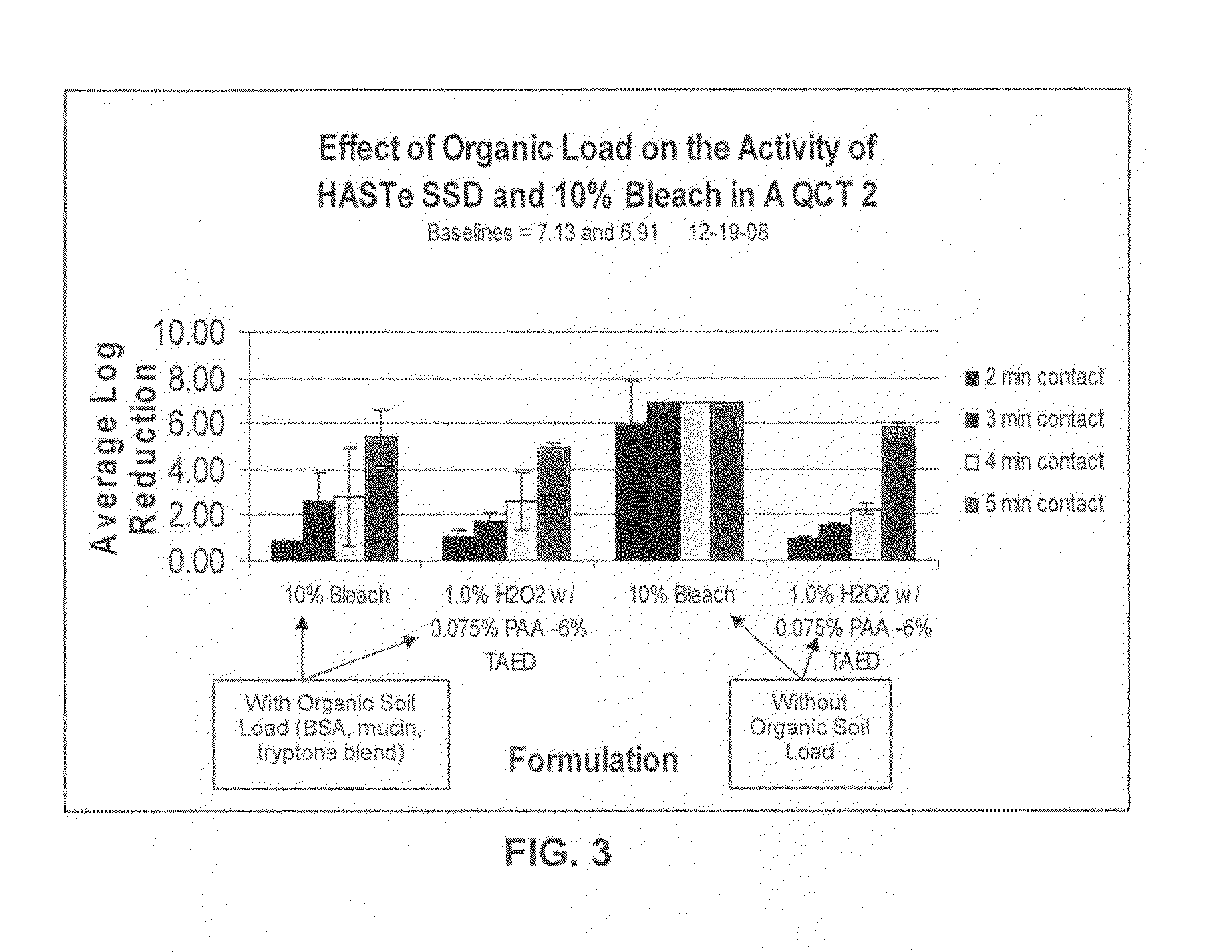
A low odor, liquid disinfectant composition comprising multiple components, which, upon mixing, provide an aqueous solution comprising low levels of peracetic acid for use in decontaminating articles and surfaces contaminated with bacteria, viruses, fungi and other biological contaminants such as spores, including, but not limited to, Clostridium difficile (C.diff). The disinfectant composition is prepared just prior to use by combining two or more separately packaged components.
Owner:AMERICAN STERILIZER CO
Detection of toxigenic strains of clostridium difficile
ActiveUS20090208948A1Sugar derivativesMicrobiological testing/measurementClostridium difficileClostridium difficile (bacteria)
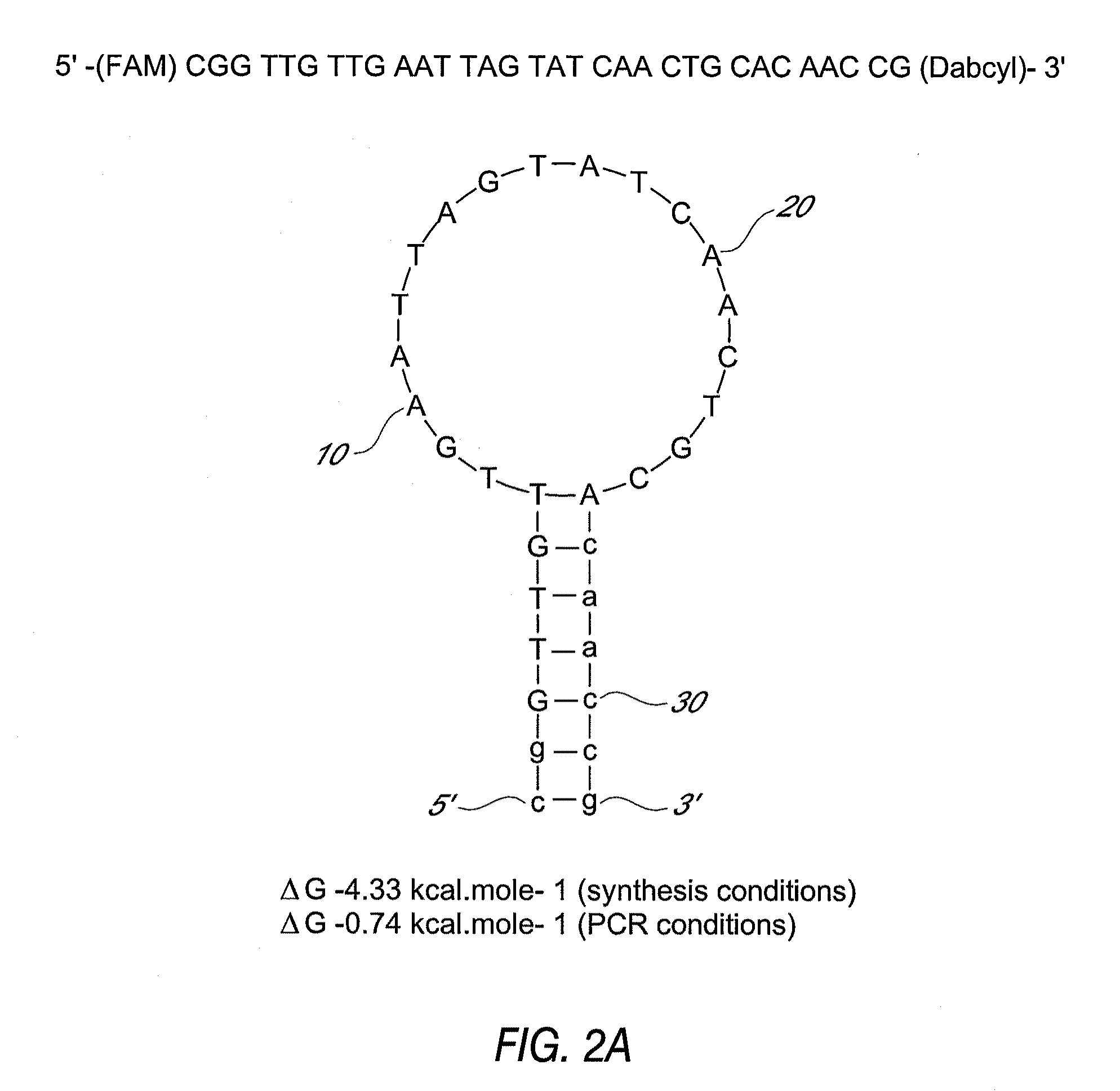
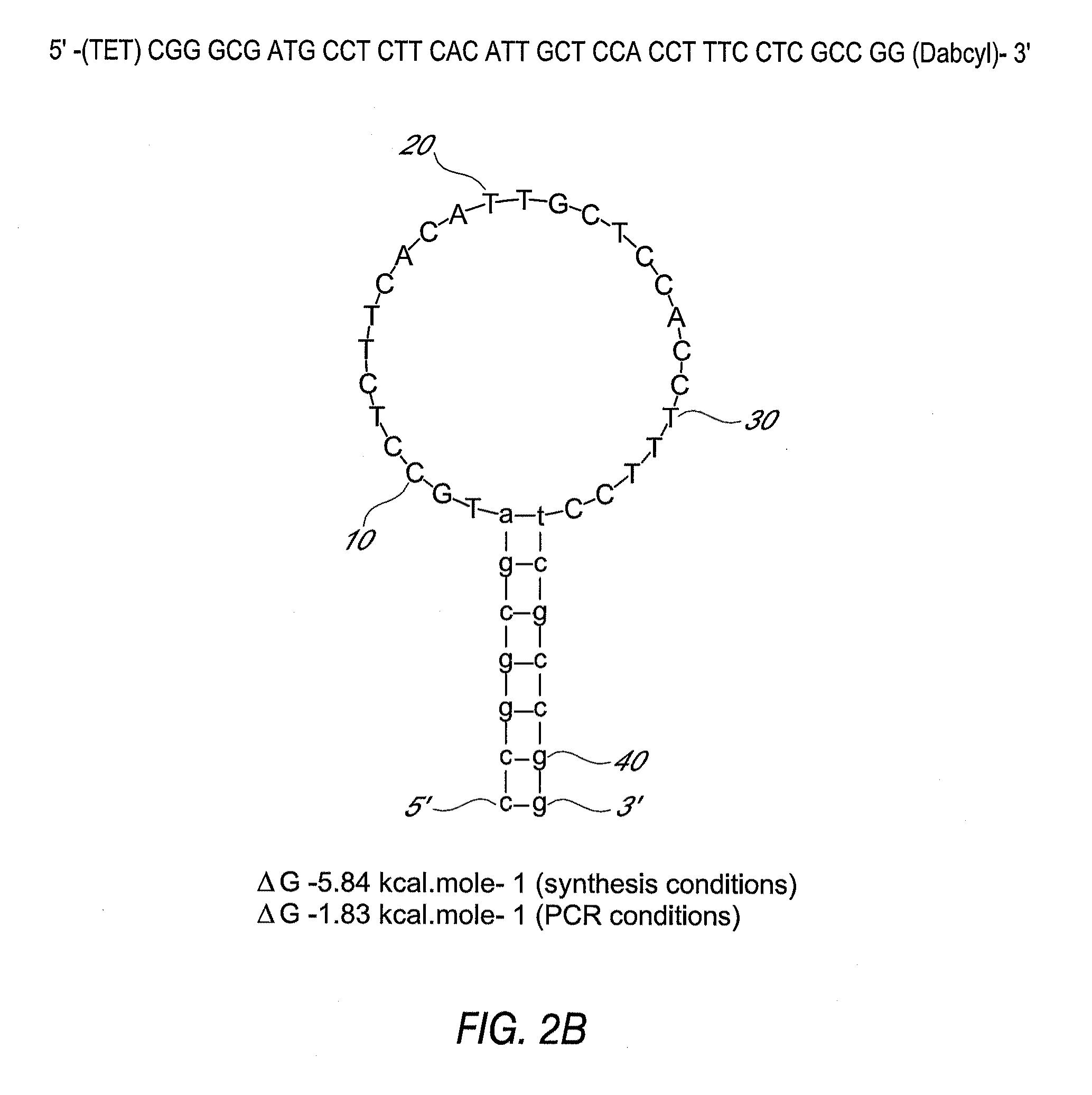
Primers and probes for detection of toxin-producing (toxigenic) strains of Clostridium difficile, and to methods of detecting toxigenic strains using these primers and probes. Toxigenic strains of C. difficile are detected by nucleic acid-based amplification methods using particular primers and probes that bind to the toxin B (TcdB) gene. These primers and probes are used to amplify C. difficile nucleic acids in clinical samples to determine the presence of these toxigenic strains.
Owner:GENEOHM SCI CANADA
Methods to produce high levels of C. difficile toxins
InactiveUS6939548B2Improve the level ofProvide protectionPeptide/protein ingredientsAntibody mimetics/scaffoldsClostridium difficileToxin
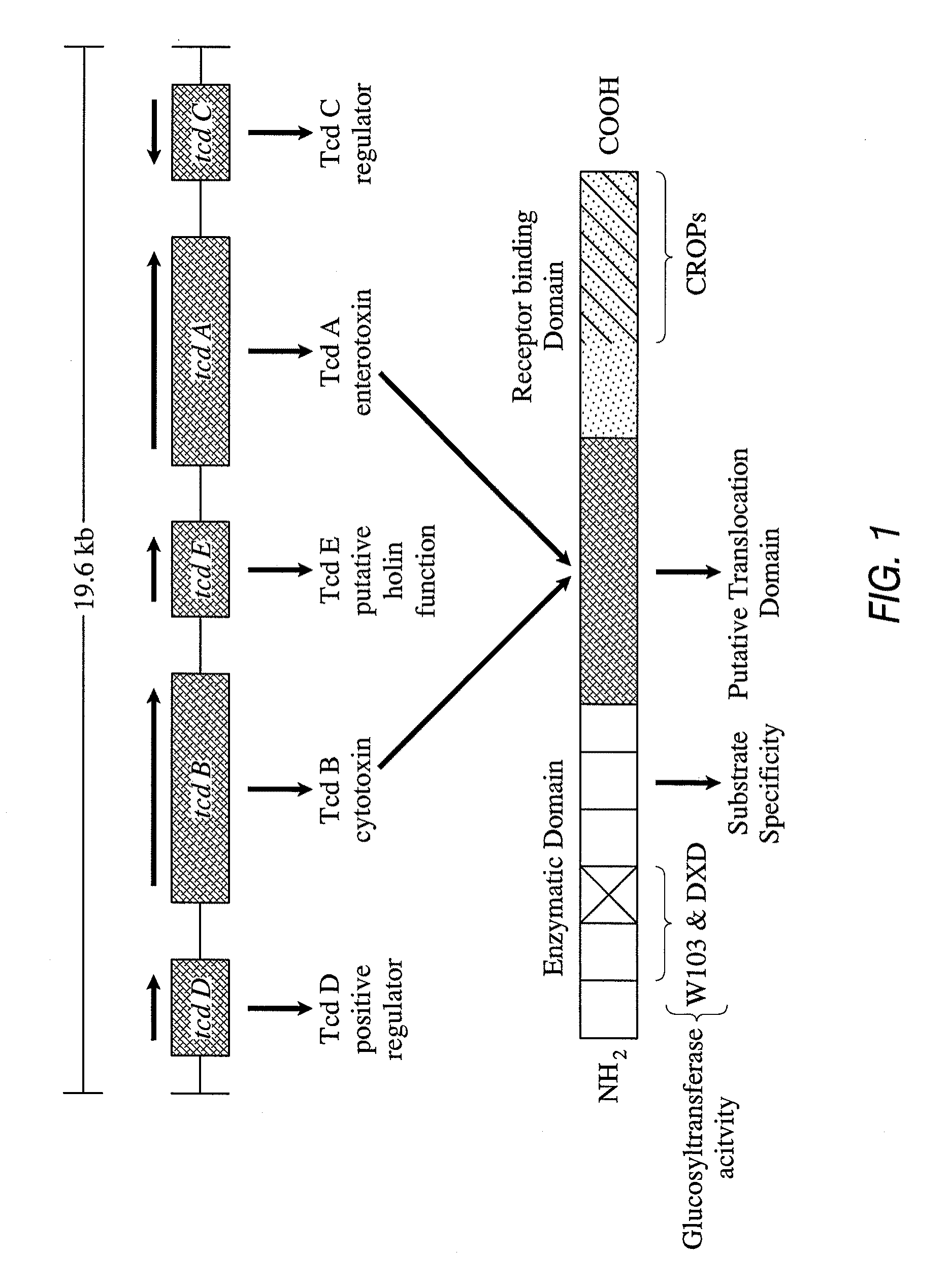
The present invention relates to the field of medical immunology and further to pharmaceutical compositions, methods of making and methods of use of vaccines. More specifically this invention relates to recombinant proteins derived from the genes encoding Clostridium difficile toxin A and toxin B, and their use in an active vaccine against C. difficile.
Owner:INTERCELL USA
Synthesis of human secretory IgM and the treatment of clostridium difficile associated diseases herewith
Owner:SECRETORY IGA INC
Compositions and methods for restoring gastrointestinal microbiota following antibiotic treatment
ActiveUS9603876B2Increase the number ofGrowth inhibitionBiocideMetabolism disorderDiseaseIntestinal microorganisms
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
Multiplex PCR based primer pair and kit for detecting multiple intestinal pathogens
ActiveCN104531898AQuick checkMultiple testing sites at one timeMicrobiological testing/measurementMicroorganism based processesEnteroinvasive E. coliEscherichia coli
The invention relates to a multiplex PCR based primer pair and kit for detecting intestinal pathogens, particularly relates to a multiplex PCR based primer pair and kit for detecting 14 intestinal pathogens, and belongs to the technical field of PCR application. The 14 intestinal pathogens comprise vibrio cholerae (group O1 phage, group O139 phage and group non-O1 / O139 phage), listeria monocytogenes, enteropathogenic escherichia coli (EPEC), enterohemorrhagic escherichia coli (EHEC), enterotoxigenic escherichia coli (ETEC), enteroinvasive escherichia coli (EIEC), enteroaggregative escherichia coli (EAEC), shigella, intestinal virus EV71, enterohemorrhagic escherichia coli O157:H7, clostridium difficile, vibrio parahaemolyticus, salmonella enteritidis and salmonella typhimurium. The multiplex PCR based primer pair and kit can change the situation that only a few intestinal pathogens can be detected in one time and can be used for detecting 14 intestinal pathogens and 26 genes simultaneously.
Owner:AGCU SCIENTECH
Composition And Method For Stabilizing And Maintaining The Viability Of Hardy Microorganisms
PendingUS20170226469A1Stabilizing and maintaining viabilityBacteriaMicrobiological testing/measurementMicroorganismBacteroides
The present application is to provide a composition and method for stabilizing and maintaining the viability of hardy microorganisms from sample collection to downstream analysis. In particular, there is a method for preserving viable hardy bacteria, such as Mycobacteria, Bacillus anthracis, or Clostridium difficile, in a biological sample, comprising contacting the biological sample with a stabilization composition, wherein the stabilization composition comprises a chelating agent, a denaturing, a salt and has a pH between about 6 and about 11.
Owner:DNA GENOTEK
Colonic delivery of antimicrobial agents
InactiveUS20100239682A1Provides for selectionMinimizes problemAntibacterial agentsPowder deliveryBacteroidesActive agent
Antimicrobial compositions for oral delivery, and administration to the colon, distal ileum, or other portion of the gastrointestinal tract other than the stomach, of bacteriophage, phage proteins, antimicrobial peptides, or antimicrobial aptamers, are disclosed. In one embodiment, the active agent is capable of lysing the bacterial cell wall. In another embodiment, the active agent is capable of interacting with a receptor or enzyme in the bacteria. In some embodiments, the active agents selectively act on one or more harmful bacteria, such as Clostridium difficile, and either do not act, or act to a lesser extent, on helpful bacteria, such as bifidobacteria. When the agents are not delivered directly to the colon, they active agents ultimately enter the colon and affect the bacteria that are present in the colon. The compositions can include beads of pectin in the form of a cationic salt enclosing the active agent, or other types of drug delivery systems designed for targeted delivery to the desired portion of the gastrointestinal tract.
Owner:ASSISTANCE PUBLIQUE HOPITAUX DE PARIS +2
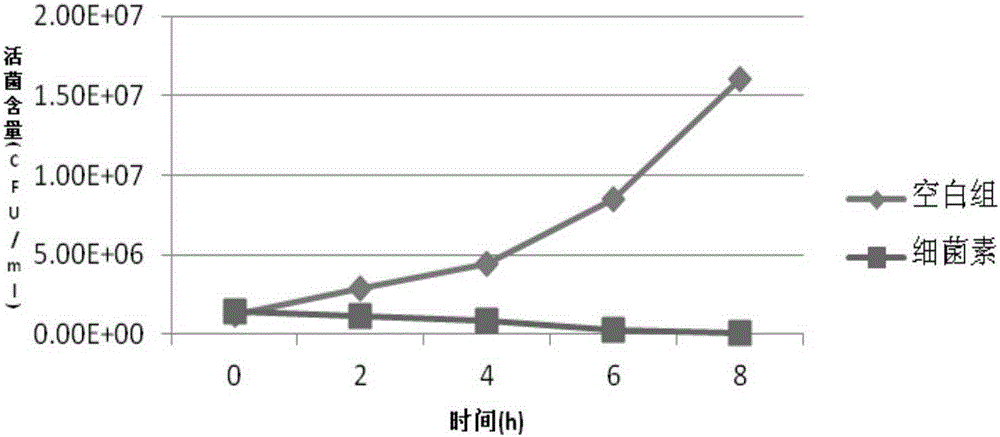
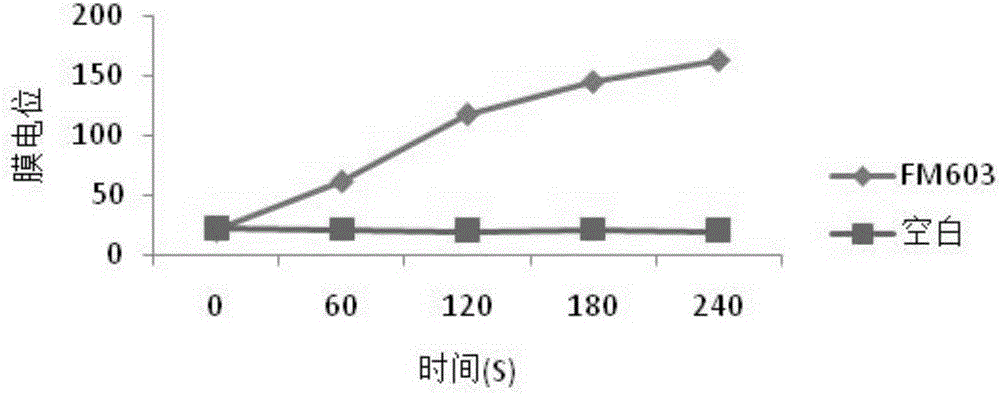
Bacillus coagulans FM 603 and application thereof
ActiveCN105176874AReduce or substituteIncrease production capacityBacteriaMicroorganism based processesFeed conversion ratioAnimal testing
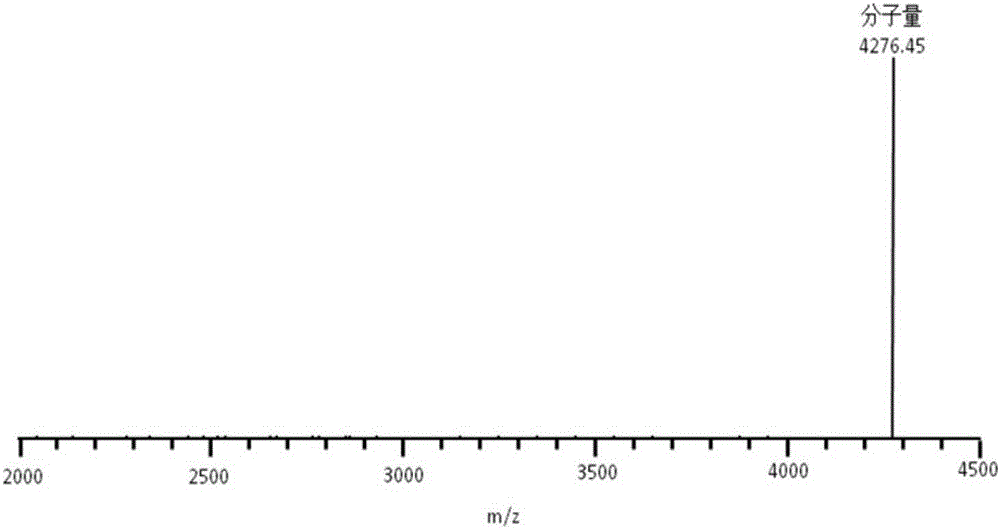
The invention provides a new strain FM 603 of bacillus coagulans, and the preservation number of the strain is CGMCC NO.10221. The strain can generate bacteriocin antibacterial substances and achieves antibacterial activity for Gram-positive pathogenic bacteria such as Listeria monocytogenes, staphylococcus aureus, methicillin-resistant staphylococcus aureus, clostridium perfringens and clostridium difficile. The molecular weight of bacteriocin is 4276.45 Da, the partial amino acid sequence is Ala-Gly-His-Dhb-Phe-Val-Dhb-Gly-Pro, and the bacteriocin is stable under treatment of heat, acid, and pepsase or trypsin and can be degraded easily by pronase to be inactivated. The invention further provides an FM603 fermentation product which can be used as a microbiological feed additive. Animal test results show that the fermentation product can increase the laying rate of laying hens, reduce the feed-egg ratio and improve egg quality; the feed intake and daily gain of piglets are increased, and the feed conversion ratio is reduced; the daily gain and the content of lysozyme in serum of broiler chickens are increased, and the feed conversion ratio and the death rate are reduced.
Owner:沈阳丰美生物技术有限公司
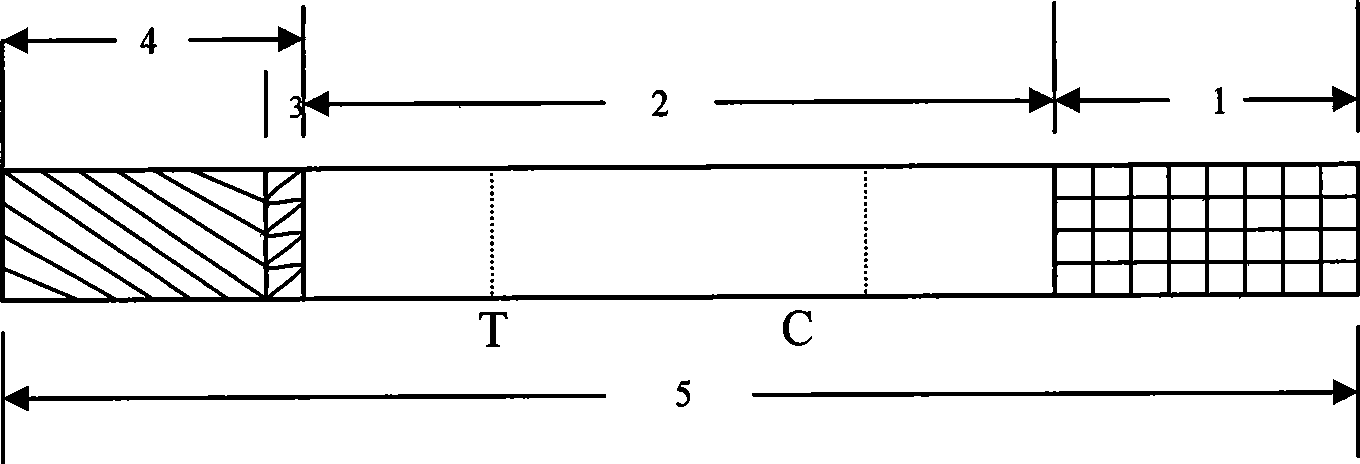
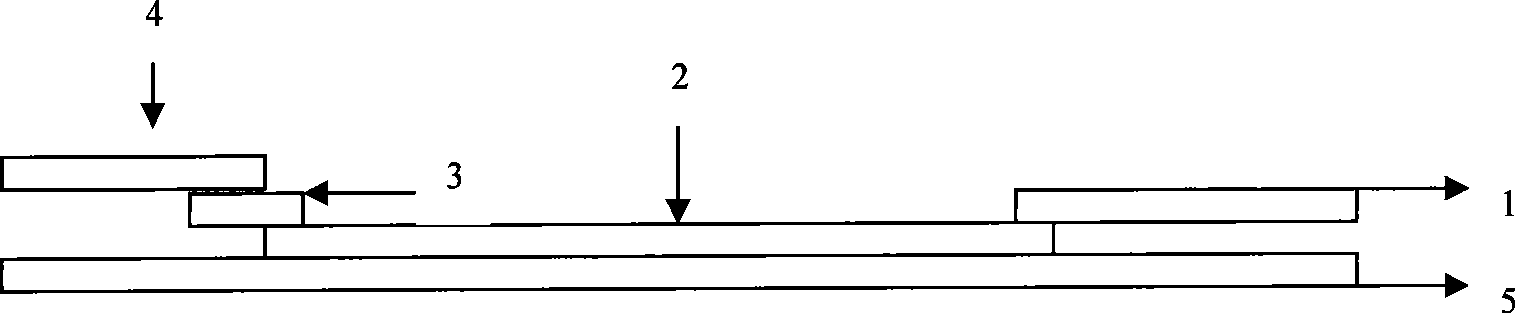
Test paper strip for detecting clostridium difficile toxin A and toxin B colloidal gold, method for making same and applications
ActiveCN101363867ASave manpower and material resourcesThe result is clear and easy to distinguishMaterial analysisCelluloseClostridium difficile infections
The invention provides a test strip for rapid detection of clostridium difficile toxins A and B. A monoclonal antibody or polyclonal antibody of the clostridium difficile toxin A, a monoclonal antibody or polyclonal antibody of the clostridium difficile toxin B, and a quality control double-antibody IgG coat a nitrate cellulose film (NC film), and a membrane chromatography double antibody sandwich method is adopted to detect the clostridium difficile toxins A and B in a specimen in combination with a monoclonal antibody of colloidal gold labeled clostridium difficile toxins A and B. The test strip is simple in operation, convenient, and fast, and has the advantages of no requirements of special instruments and special training, clear and identified result, and easy popularization. The test strip is suitable for base course, site detection and epidemiological investigation, and has auxiliary effect on the diagnosis of clostridium difficile toxin infection.
Owner:辽宁迪浩生物科技有限公司
Reducing Risk of Contracting Clostridium-Difficile Associated Disease
ActiveUS20140045808A1Reduce development riskOrganic active ingredientsBiocideClostridium difficileCvd risk
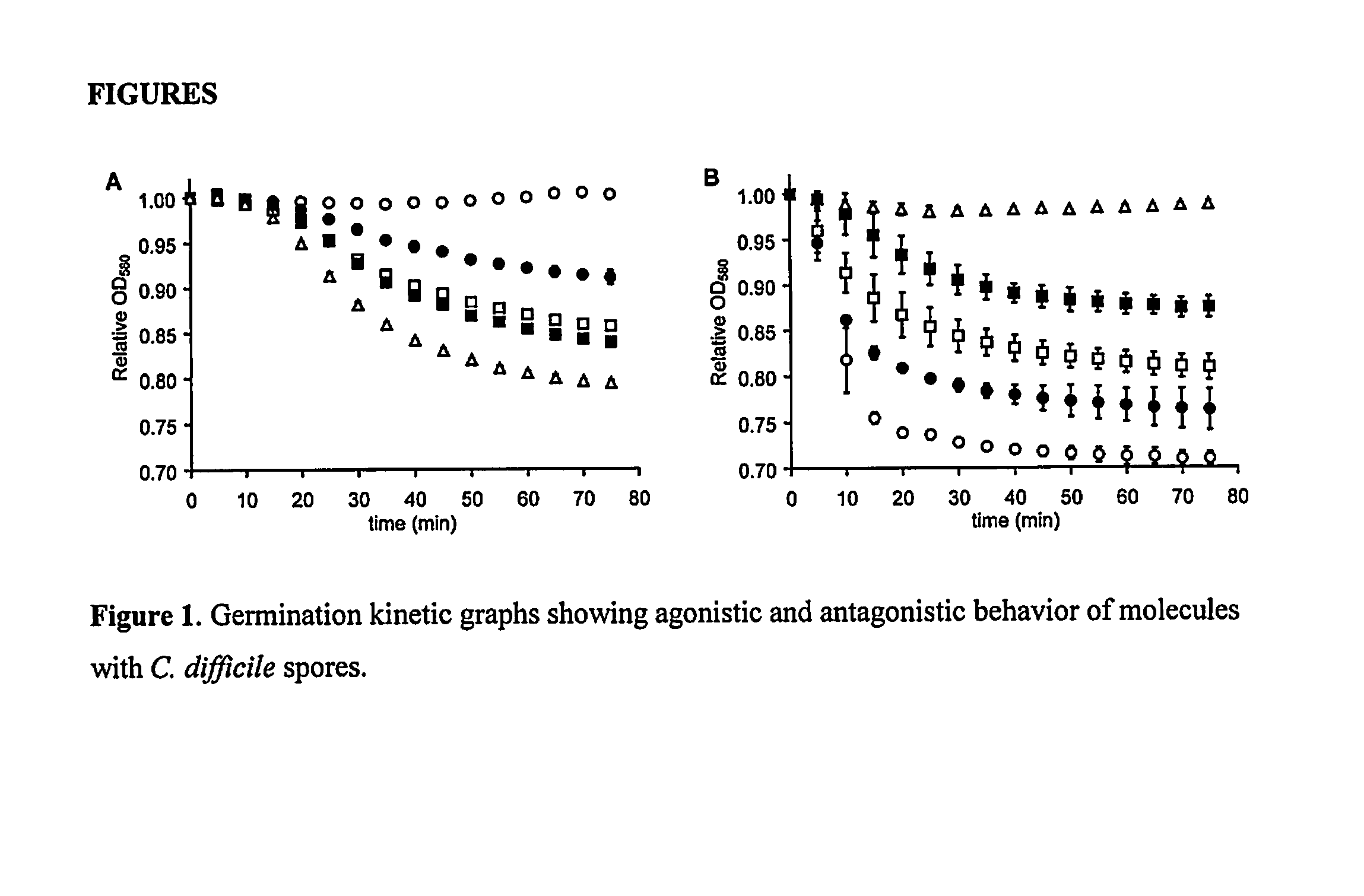
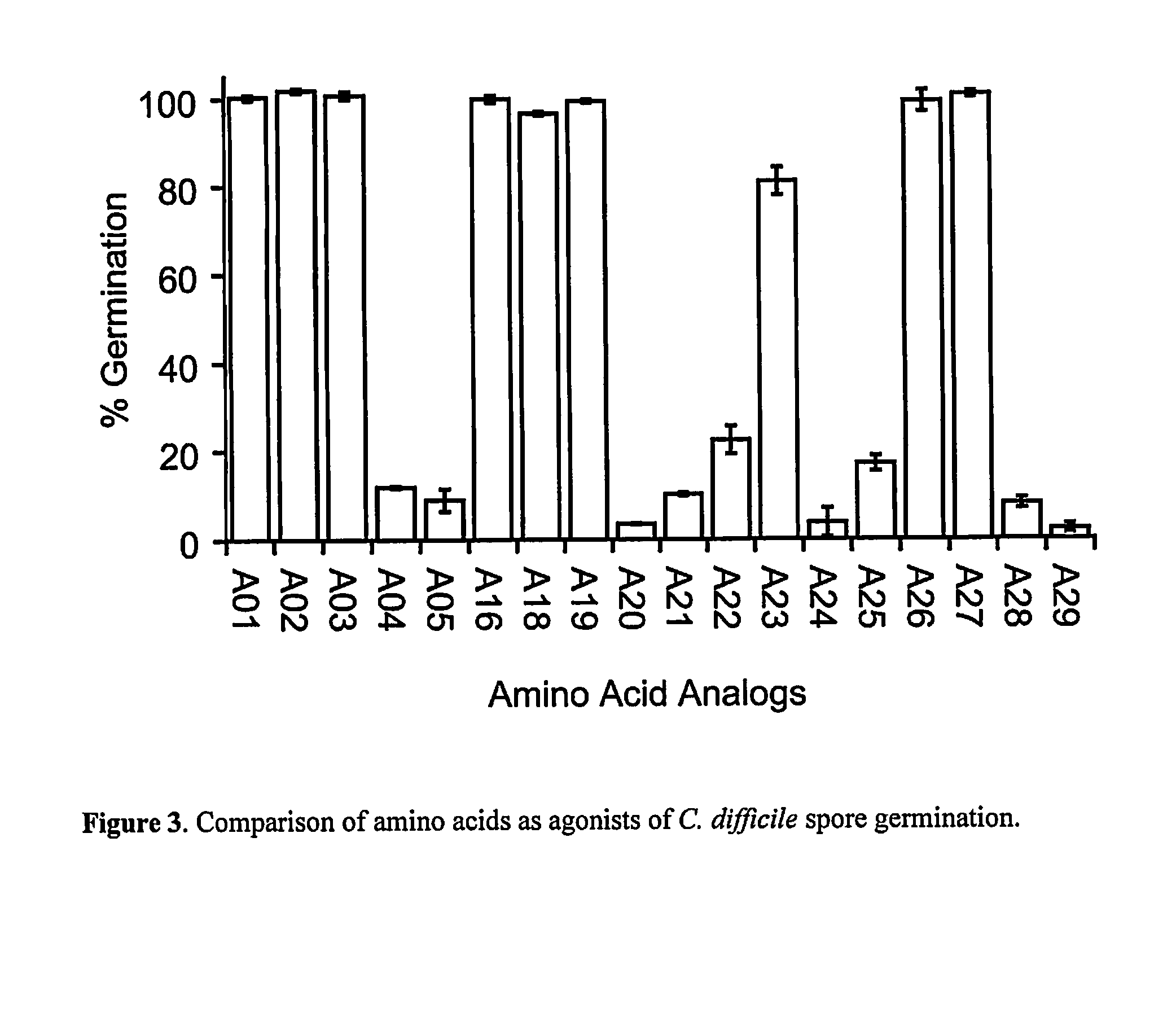
A method of treating a patient to reduce risk of developing Clostridium difficile-associated disease or reducing existing Clostridium difficile-associated disease in a mammalian subject involves administering to a mammalian subject an effective amount of a germination-inhibiting compound derived from taurocholate. Novel compounds of this class are also provided.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
Novel Polypeptides Having Endolysin Activity and Uses Thereof
InactiveUS20100310522A1Reduce sensitivityExtended half-lifeAntibacterial agentsOrganic active ingredientsDiseaseLysin
The present invention provides isolated polypeptides comprising the amino acid sequence of SEQ ID NO:1, or a fragment, variant, derivative or fusion thereof which is capable of binding specifically to and / or lysing cells of Clostridium difficile, and means for producing the same, with the proviso that the fragment, variant, derivative or fusion is not a naturally occurring lysin of a bacteriophage of Clostridium difficile. The invention further provides methods for killing bacterial cells, such as cells of Clostridium difficile, and for diagnosing, treating and preventing diseases and conditions associated with infection of the same. The invention also provides diagnostic kits for use in such methods.
Owner:PLANT BIOSCI LTD
Synthesis of human secretory IgA and IgM and the formation of a medicament therefrom
A composition for treating a subject is provided. The composition includes antigen specific dimeric secretory IgA and pentameric IgM therapeutic. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the modification of antigen specific dimeric secretory IgA and pentameric IgM with secretory component to form a antigen specific dimeric secretory IgA and pentameric secretory IgM therapeutic. The antigen specific dimeric secretory IgA and the pentameric secretory IgM therapeutic is then mixed with formulating agents to create a capsule, tablet, liquid or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R +2
Recombinant toxin A and toxin B protein carrier for polysaccharide conjugate vaccines
InactiveUS20050202042A1Provide protectionConstant stableBiocideOrganic active ingredientsConjugate vaccineMicroorganism
The present invention provides for immunogenic compositions and their methods of use as vaccines and their method of preparation. These immunogenic compositions comprise a recombinant protein of toxin A or toxin B of Clostridium difficile conjugated to a polysaccharide of a microbial pathogen. The immunogenic compositions may include only a truncated portion of toxin A or toxin B, particularly the repeating units (rARU or rBRU), that is associated with a microbial pathogen polysaccharide. Such compositions are effective in eliciting T-cell dependent and antibody responses. These compositions are therefore effective as vaccines for humans, particularly children, and animals in affording protection against one or more microbial pathogens.
Owner:TECHLAB THERAPEUTICS
SYNTHESIS OF HUMAN SECRETORY IgM AND THE TREATMENT OF COLSTRIDIUM DIFFICILE ASSOCIATED DISEASES HEREWITH
A composition for treating a subject is provided. The composition includes dimeric or polymeric secretory IgM therapeutic. Formulating agents are mixed with the dimeric or polymeric secretory IgM to yield a dosing form of a capsule, tablet, and a suppository. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the modification of pentameric or hexameric IgM with secretory component to form a pentameric or hexameric secretory IgM therapeutic. The pentameric or hexameric secretory IgM therapeutic is then mixed with formulating agents to create a capsule, tablet, or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SECRETORY IGA INC
Healthcare facility disinfecting system
ActiveUS20120100037A1Effective but inexpensive systemReduce the amount requiredLavatory sanitoryDeodrantsEscherichia coliClostridium difficile toxin B
A system and process for disinfecting rooms such as health care facility rooms with an oxygen / ozone mixture is described, which is effective to combat “superbugs” such as Clostridium difficile (C. difficile); E. coli; Pseudomonas aeruginosa; methicillin-resistant Staphylococcus aureus (MRSA); and vancomycin-resistant Enterococcus (VRE). In preferred embodiments, hydrogen peroxide is additionally used. The system and process is effective to destroy bacteria deposited on surfaces as biofilm, and, accompanied by physical agitation such as jet nozzle outlets, is effective to disinfect carpet, drapery and similar absorbent and porous surfaces.
Owner:DD STEROZONE LLC +1
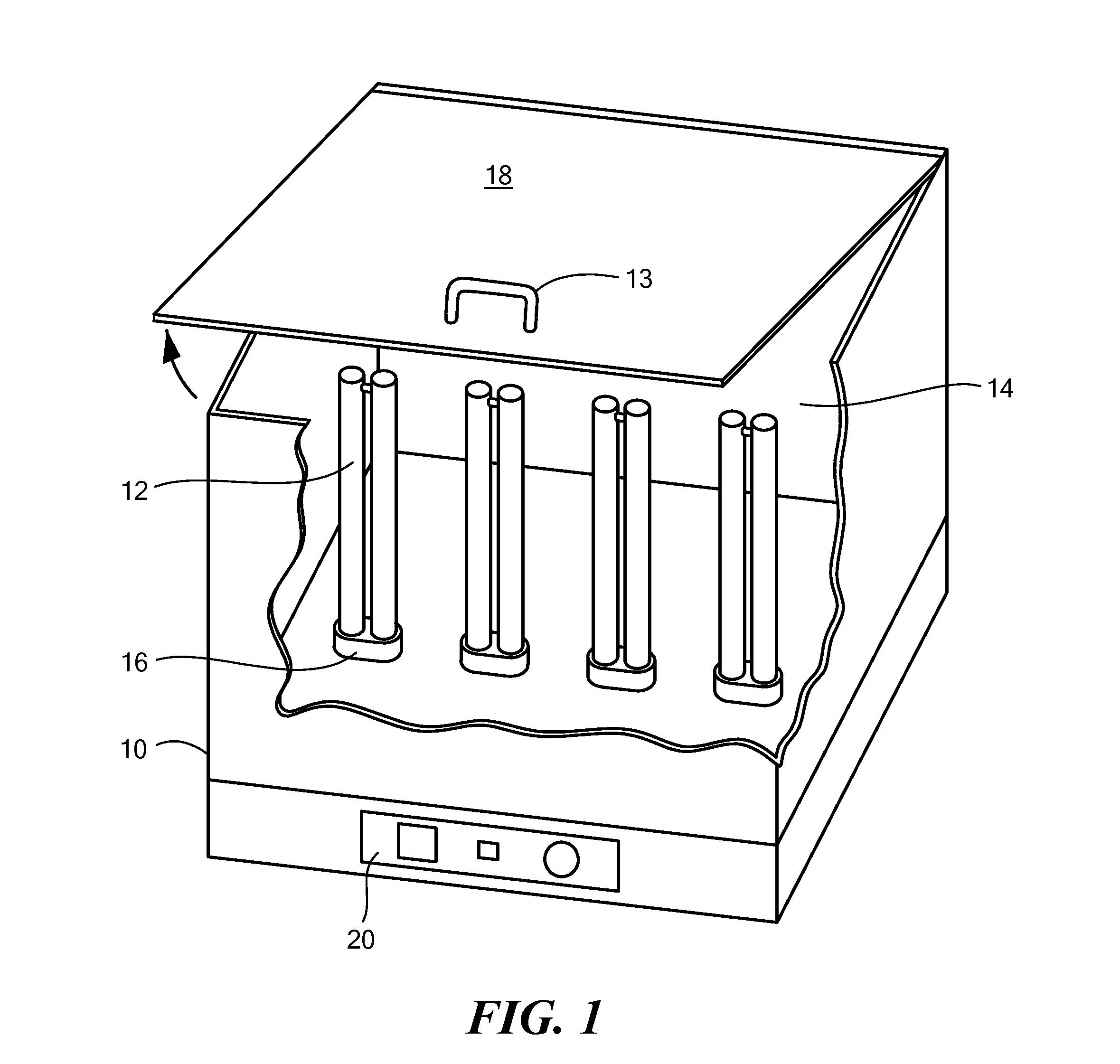
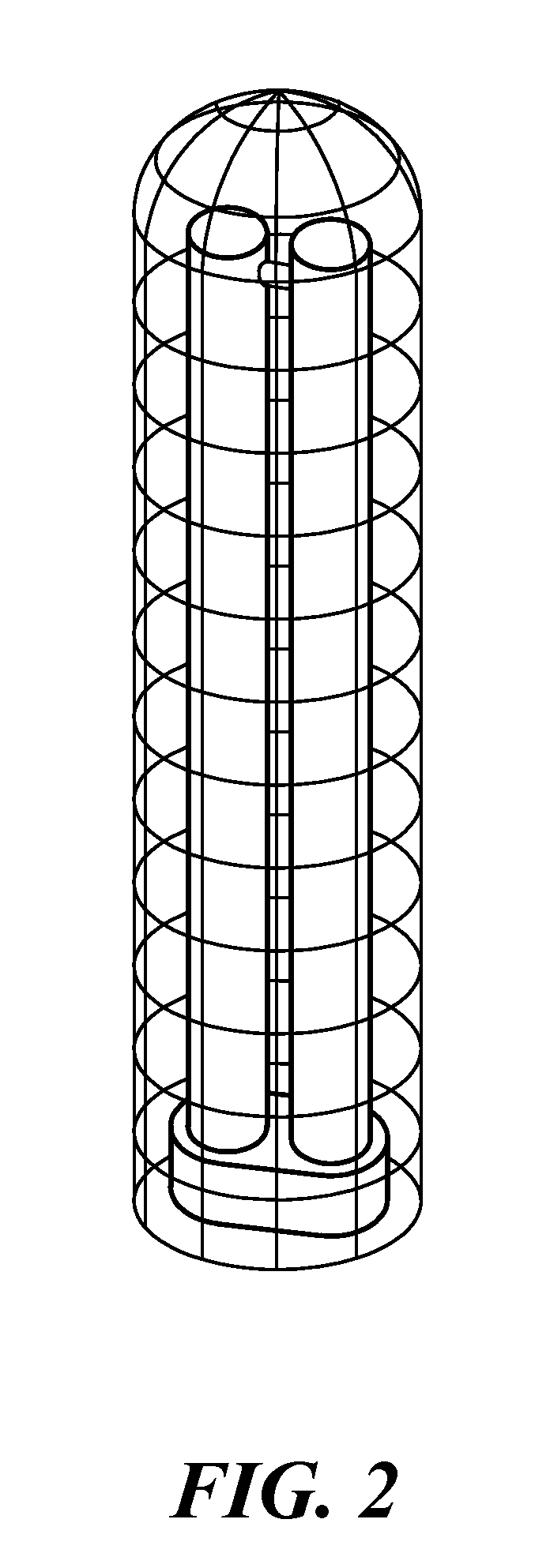
Ultraviolet disinfection system for athletic items
InactiveUS20160339126A1Easy to replaceEffect performanceLavatory sanitoryRadiationClostridium difficileUVC Radiation
An ultraviolet disinfection system to generate UVC radiation inside athletic clothing, gear and other items to be decontaminated. The system includes a plurality of UVC lamps vertically disposed in a housing and having UVC transmissive lamp enclosures over which items to be decontaminated are placed. The system has a microprocessor based controller and can kill pathogens including Clostridium difficile in 30 seconds or less.
Owner:LICHTBLAU GEORGE J
SYNTHESIS OF HUMAN SECRETORY IgA FOR THE TREATMENT OF CLOSTRIDIUM DIFFICILE ASSOCIATED DISEASES
A composition for treating a subject is provided. The composition includes dimeric or polymeric secretory IgA therapeutic. Formulating agents are mixed with the dimeric or polymeric secretory IgA to yield a dosing form of a capsule, tablet, and a suppository. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the modification of dimeric or polymeric IgA with secretory component to form a dimeric or polymeric secretory IgA therapeutic. The dimeric or polymeric secretory IgA therapeutic is then mixed with formulating agents to create a capsule, tablet, or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R
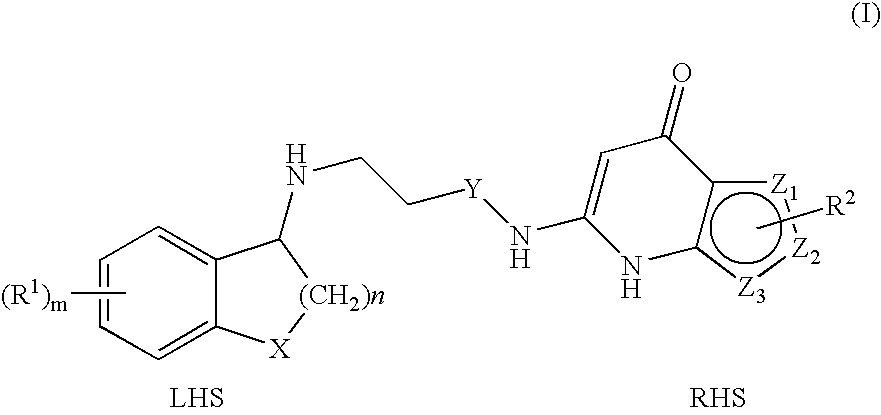
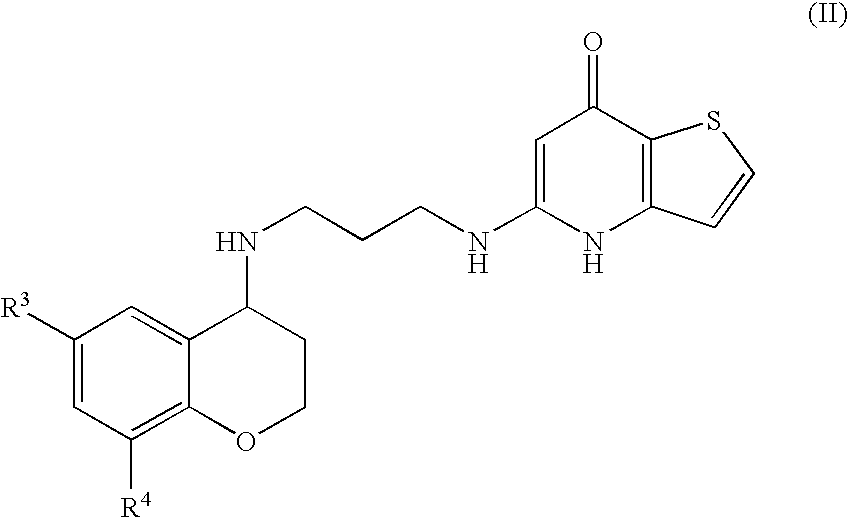
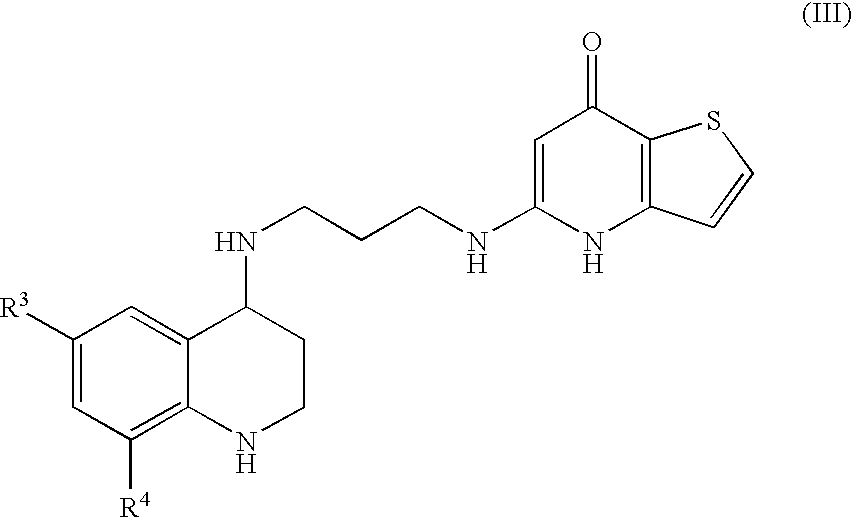
Substituted Thienopyridone Compounds With Antibacterial Activity
Novel bicyclic heteroaromatic compounds are provided that are inhibitors of bacterial methionyl tRNA synthetase (MetRS). Compounds of the invention generally have a left hand side chroman group or left hand side tetrahydroquinoline group and a right hand side thienopyridone group. Also disclosed are methods for their preparation and their use in therapy as antibacterial agents, particularly as anti-Clostridium difficile agents.
Owner:REPLIDYNE
Treatment of gluten intolerance and related conditions
ActiveUS20140186330A1High affinityDifferent cleavage affinityAntibacterial agentsPeptide/protein ingredientsClostridium difficileGluten intolerance
Owner:CODEXIS INC
Clostridium difficile toxin-based vaccine
ActiveUS20150132333A1Promote cloningEasy to purifyAntibacterial agentsBacterial antigen ingredientsAntigenDisease
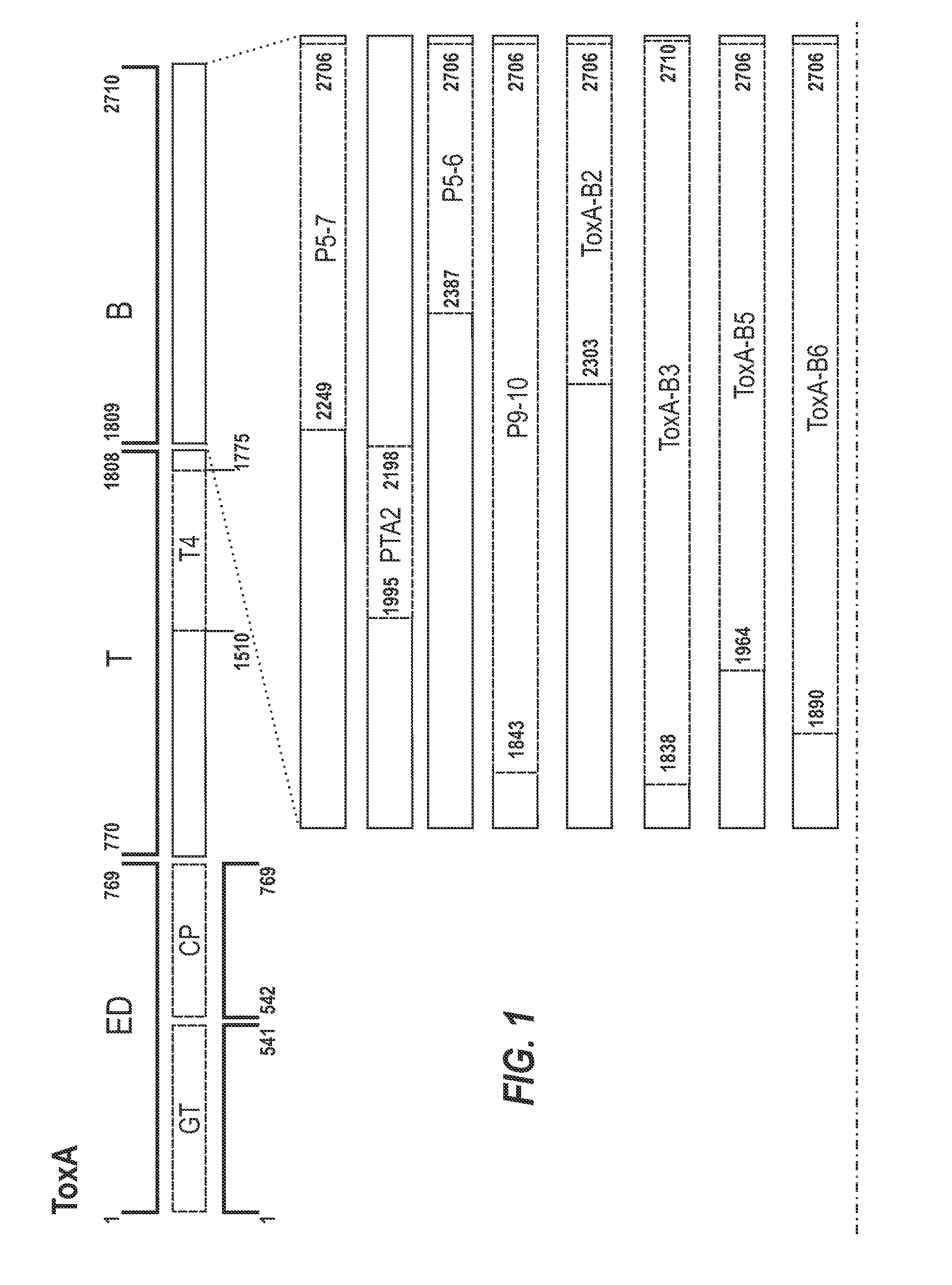
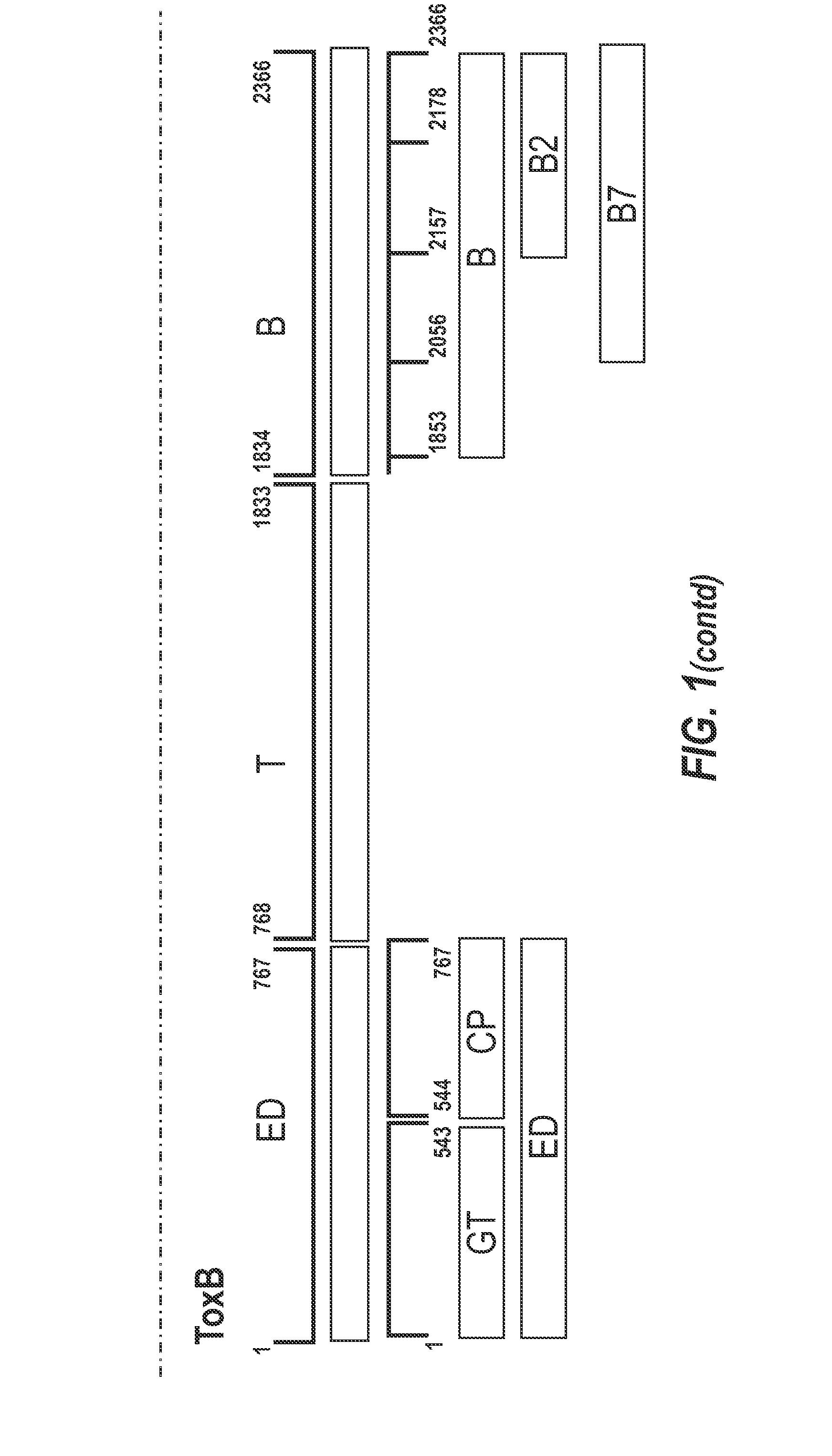
The present invention relates to recombinant fragments of C. difficile TcdA and TcdB that may be used in the development of vaccines against C. difficile associated disease. More particularly it relates to combinations comprising a ToxB-GT antigen and a TcdA antigen or a ToxA-GT antigen and a TcdB antigen.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
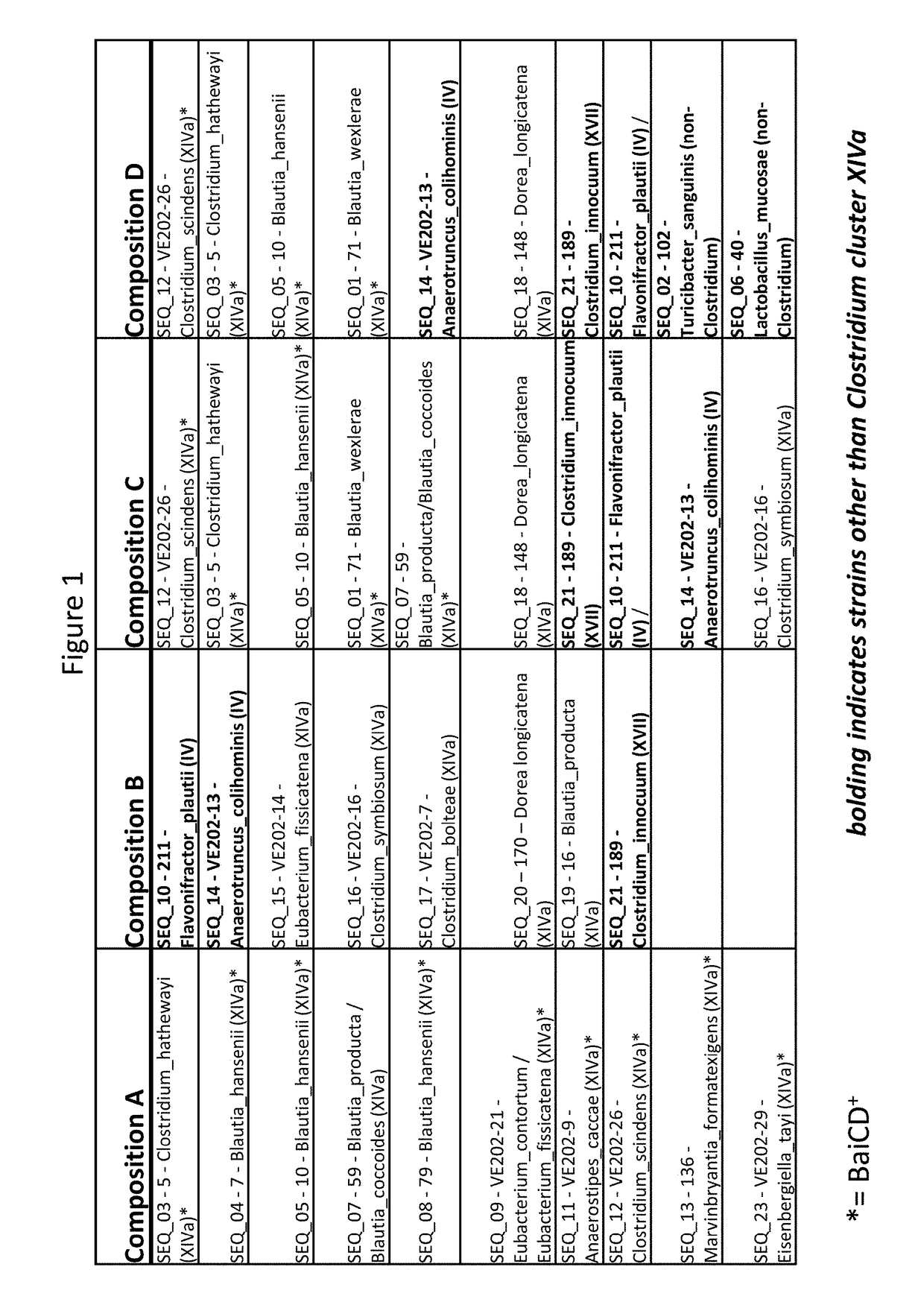
Treatment of Clostridium difficile infection
Owner:VEDANTA BIOSCIENCES INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com