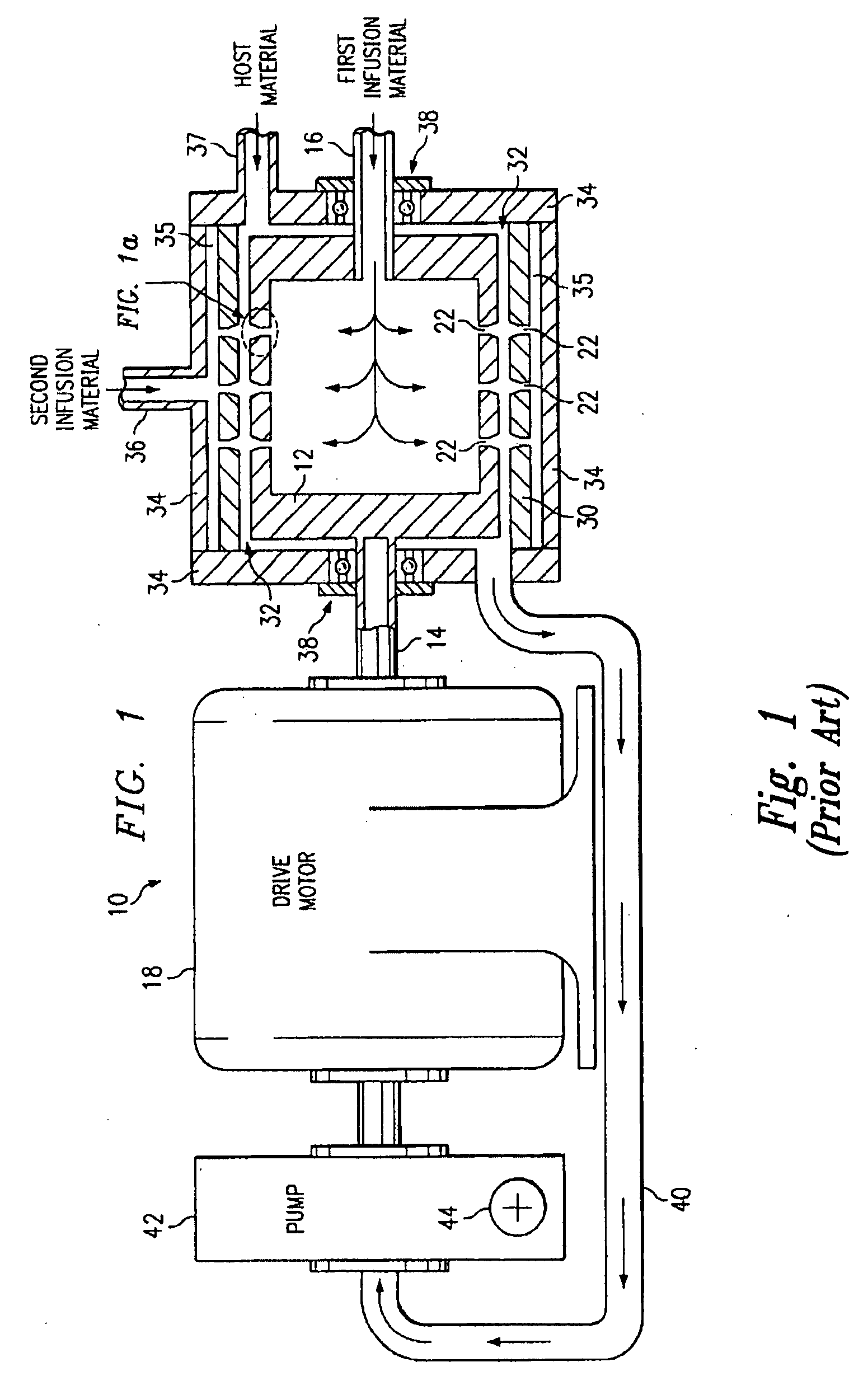
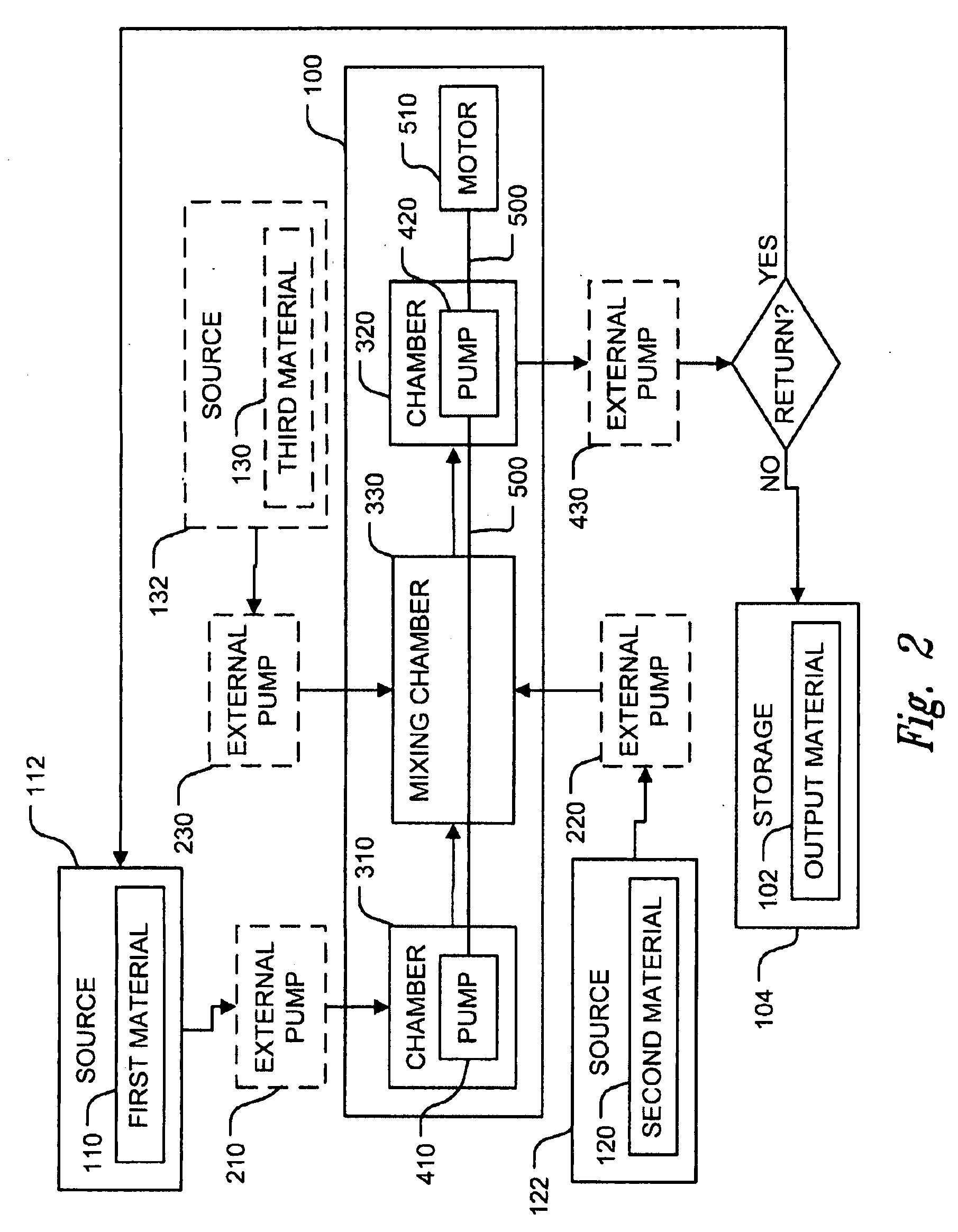
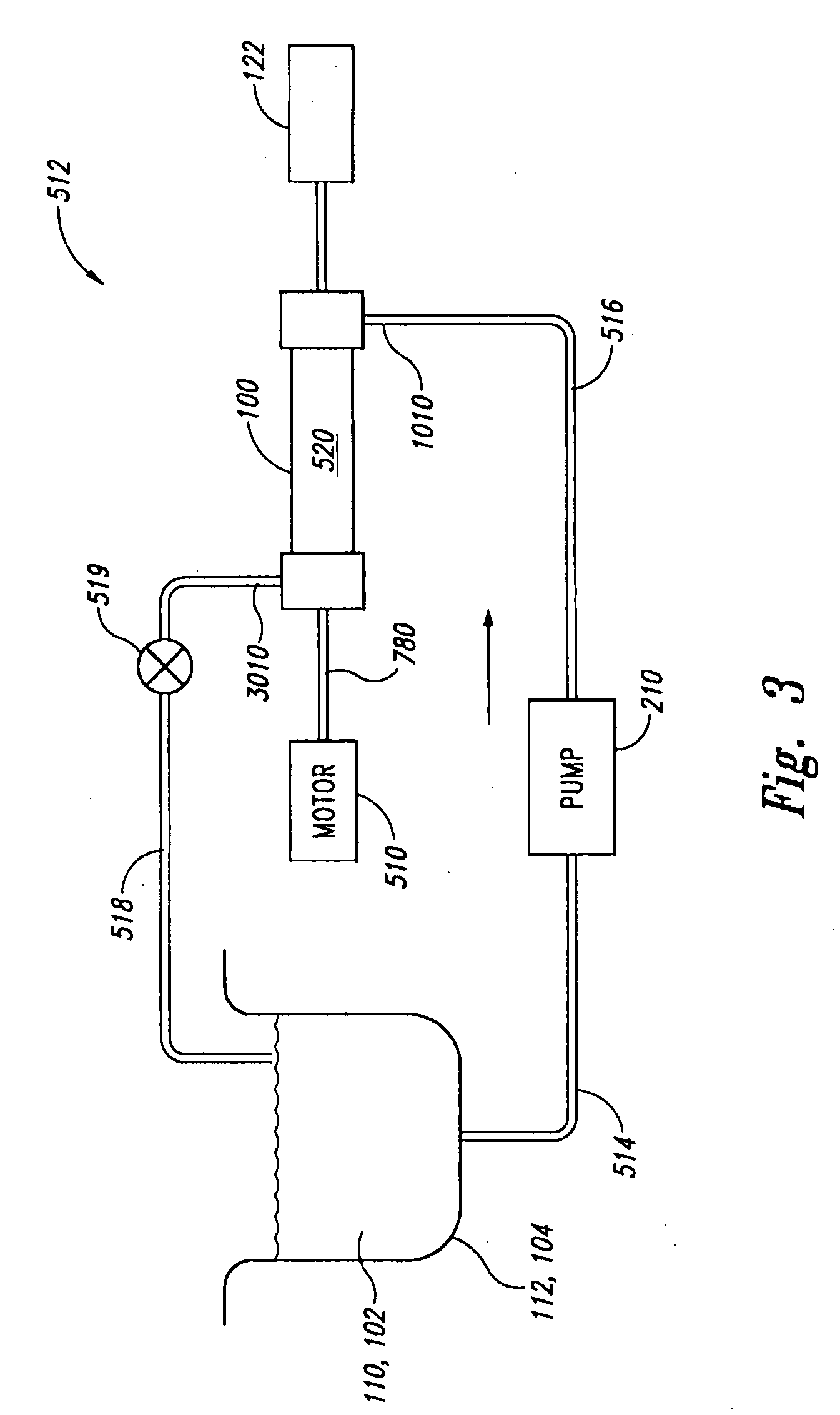
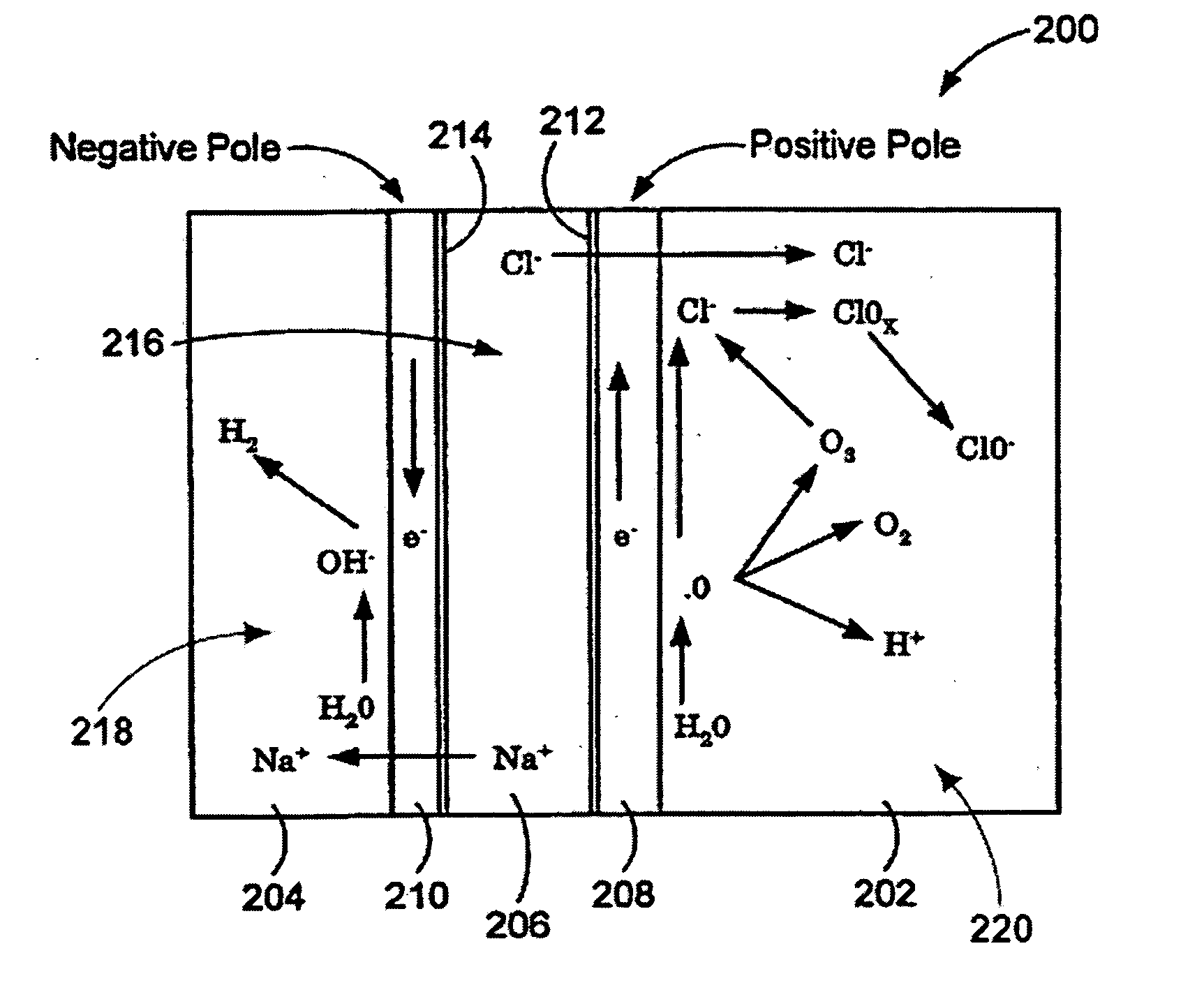
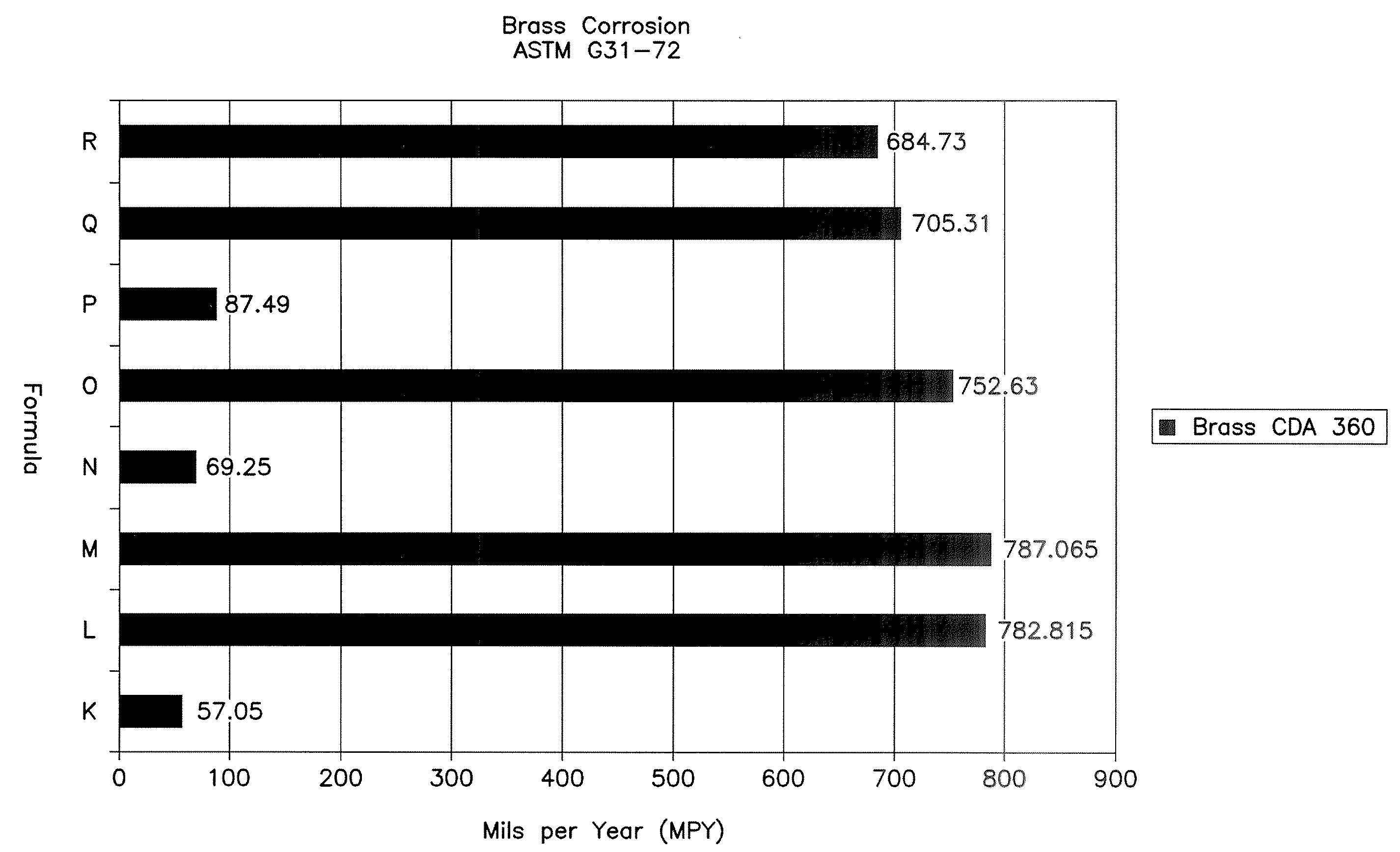
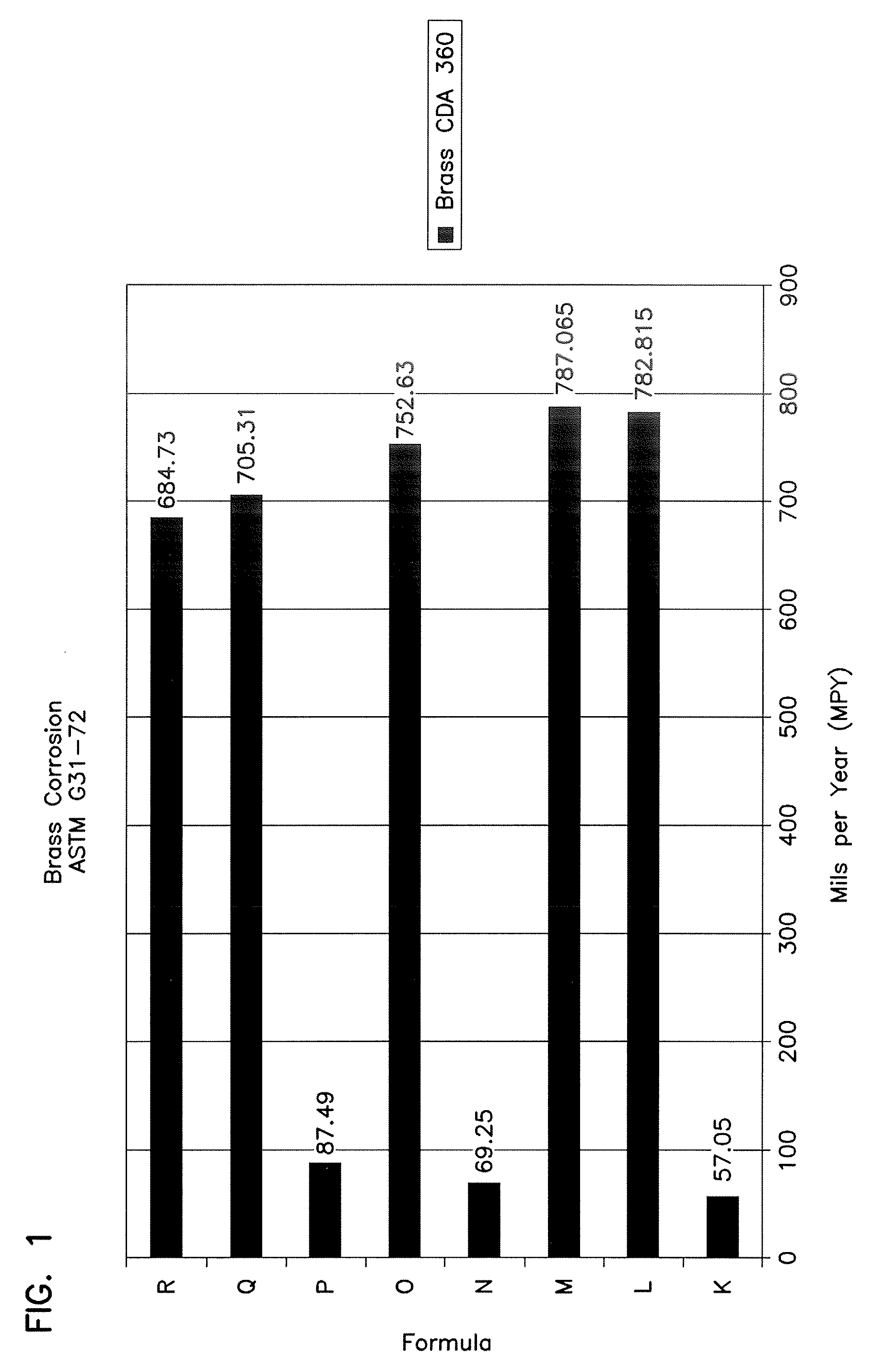
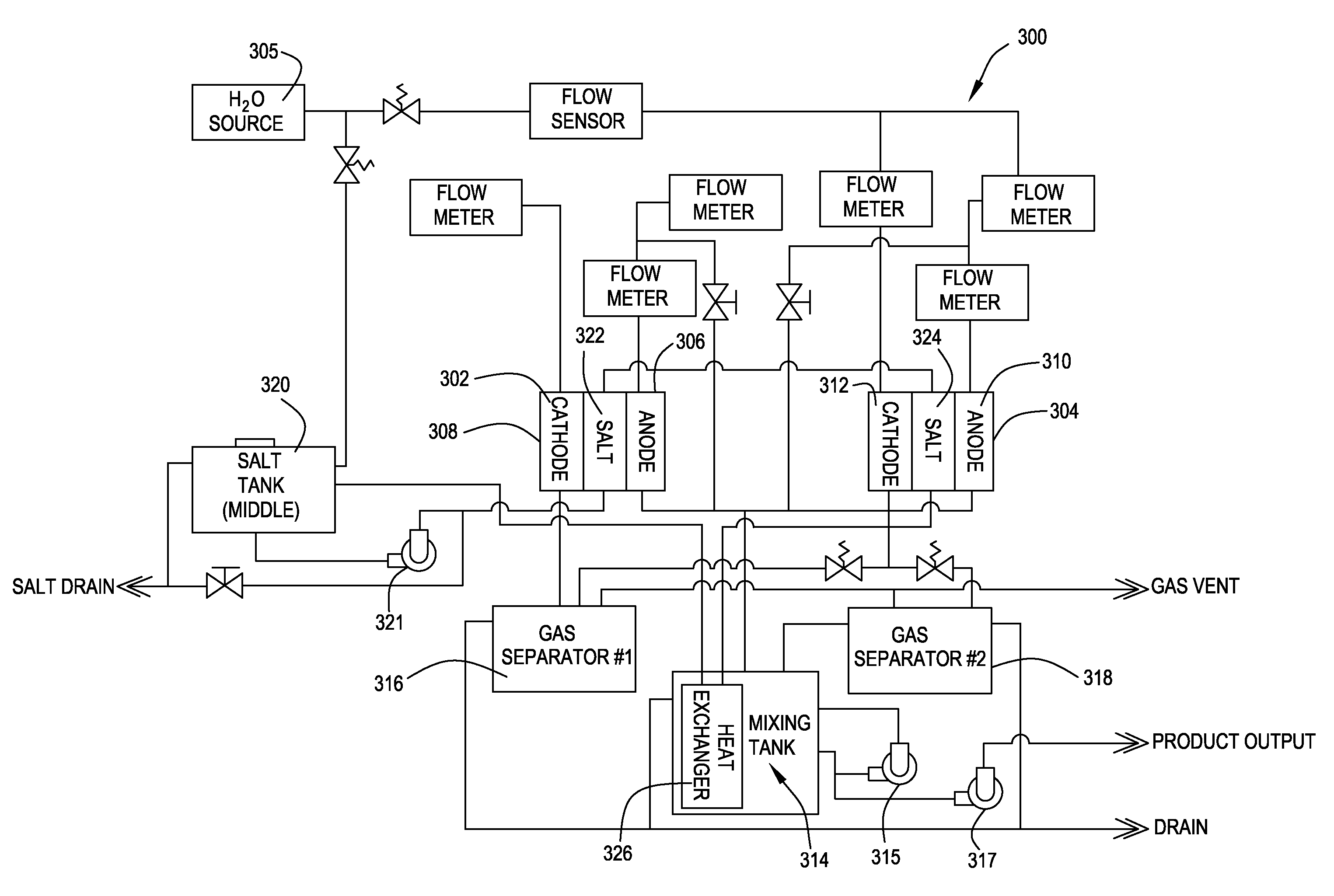
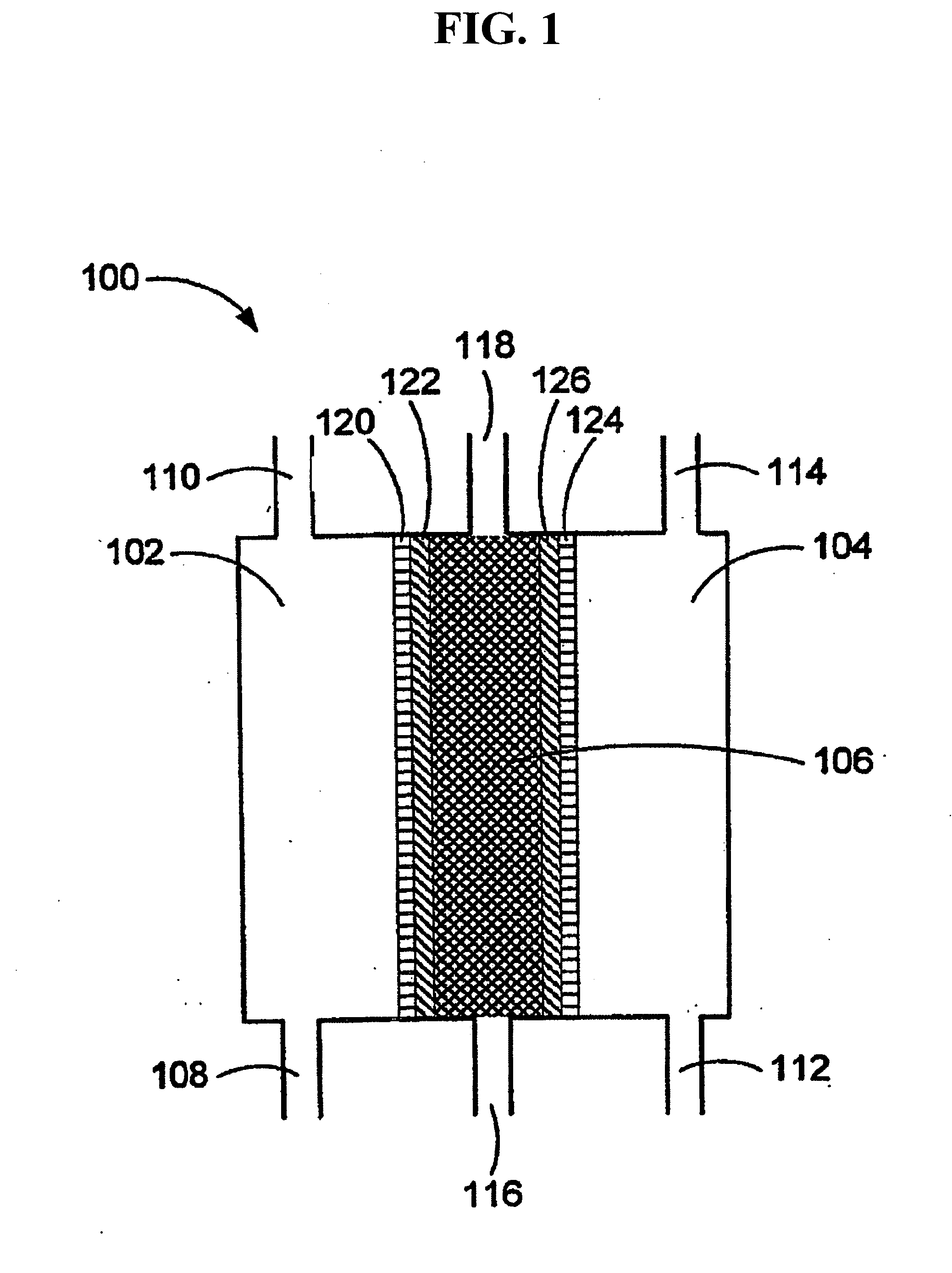
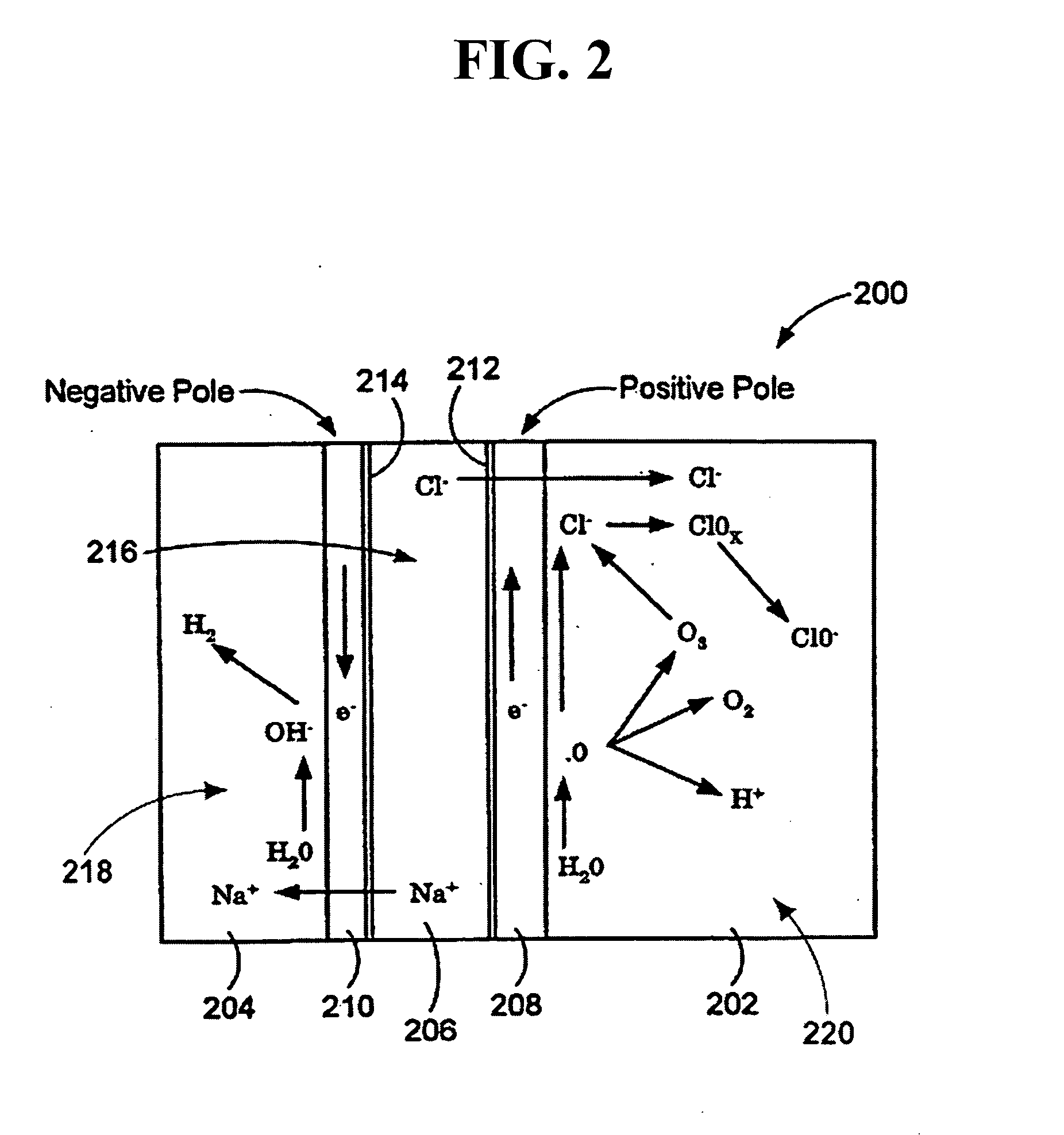
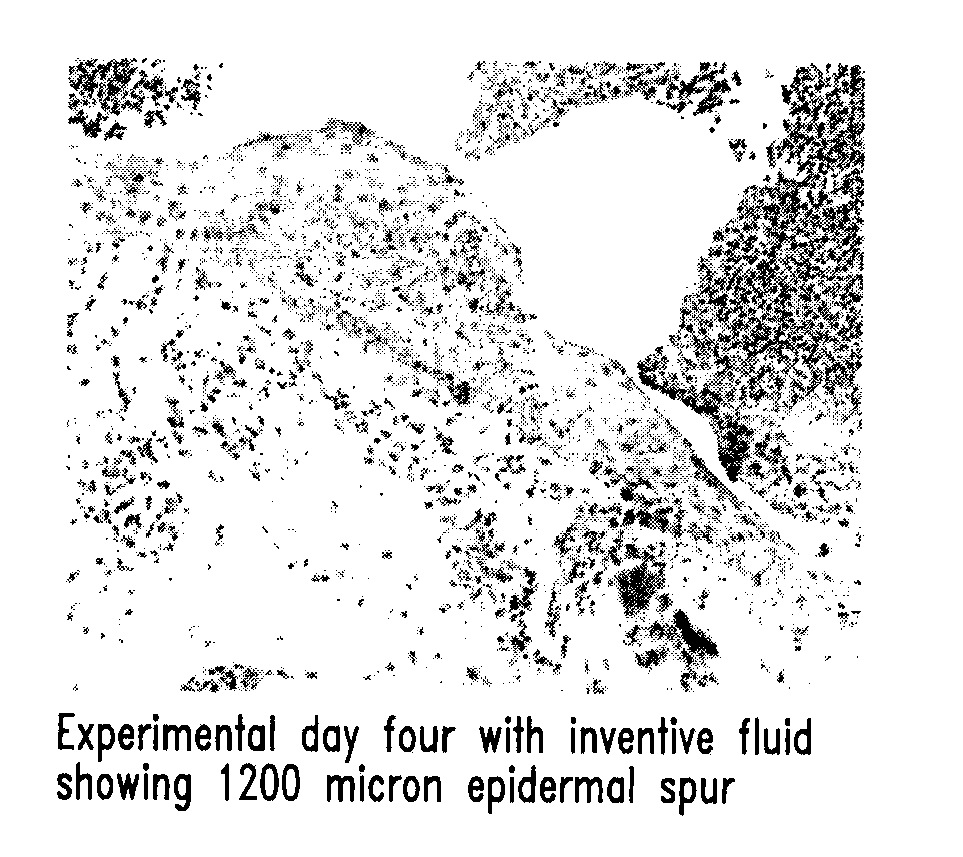
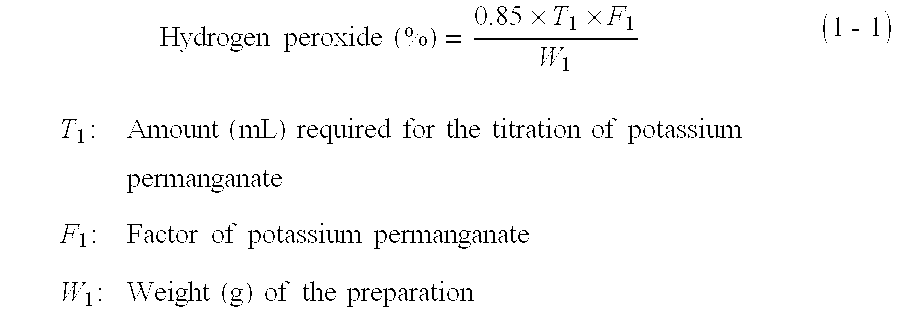Patents
Literature
1159results about "Peroxide active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
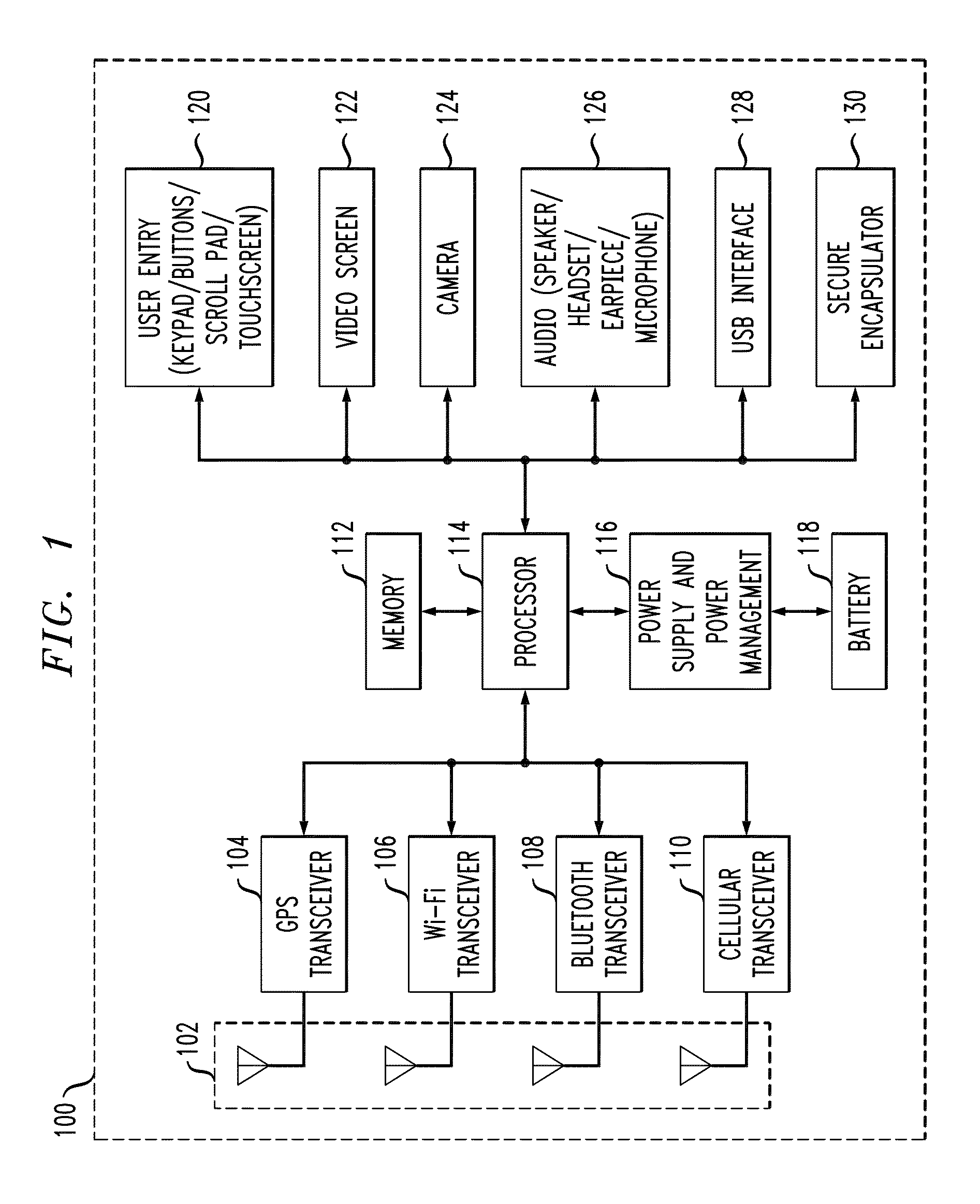
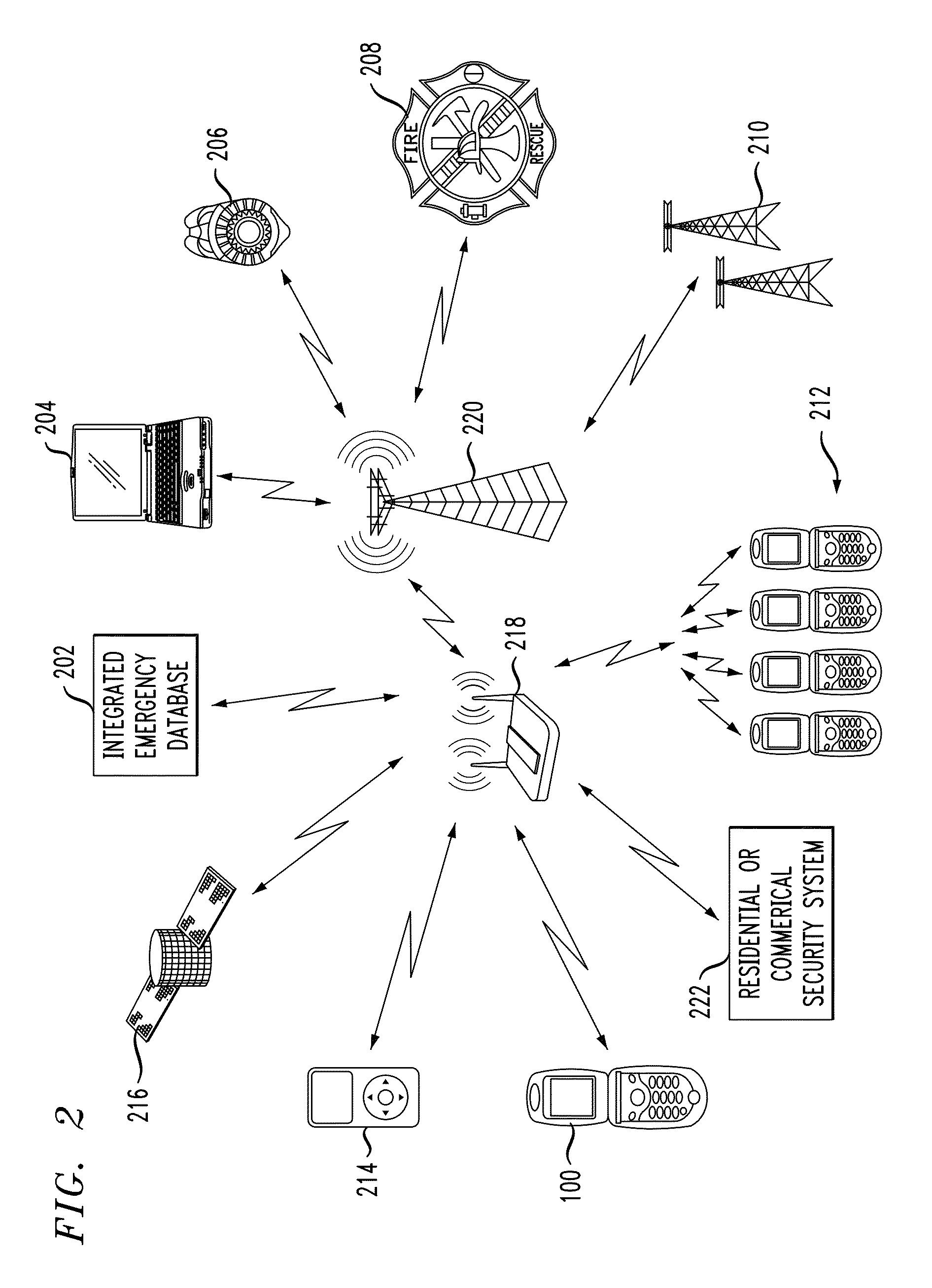
Personal safety mobile notification system
ActiveUS20130183924A1Well formedBiocideHydroxy compound active ingredientsGlobal Positioning SystemMobile device
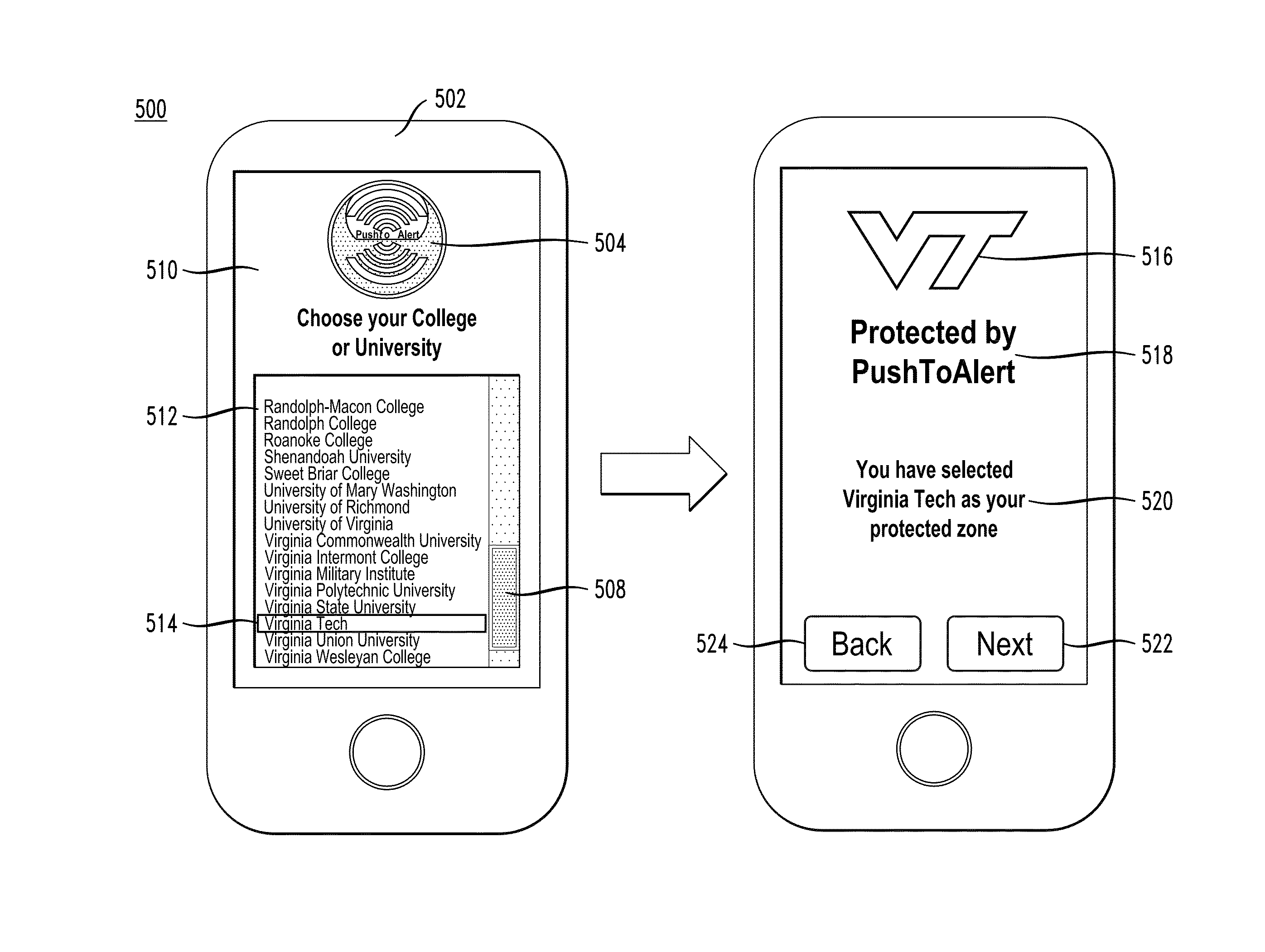
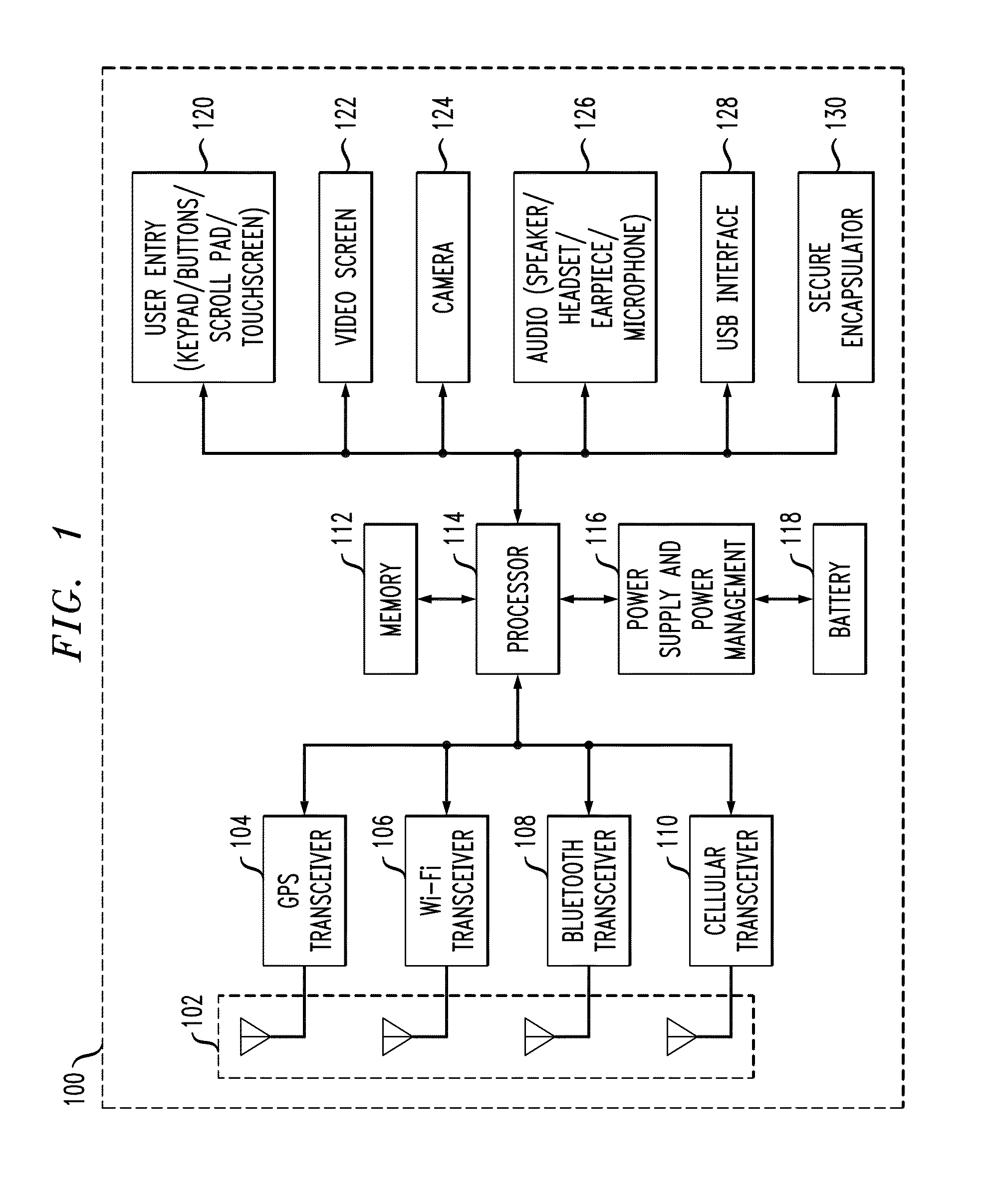
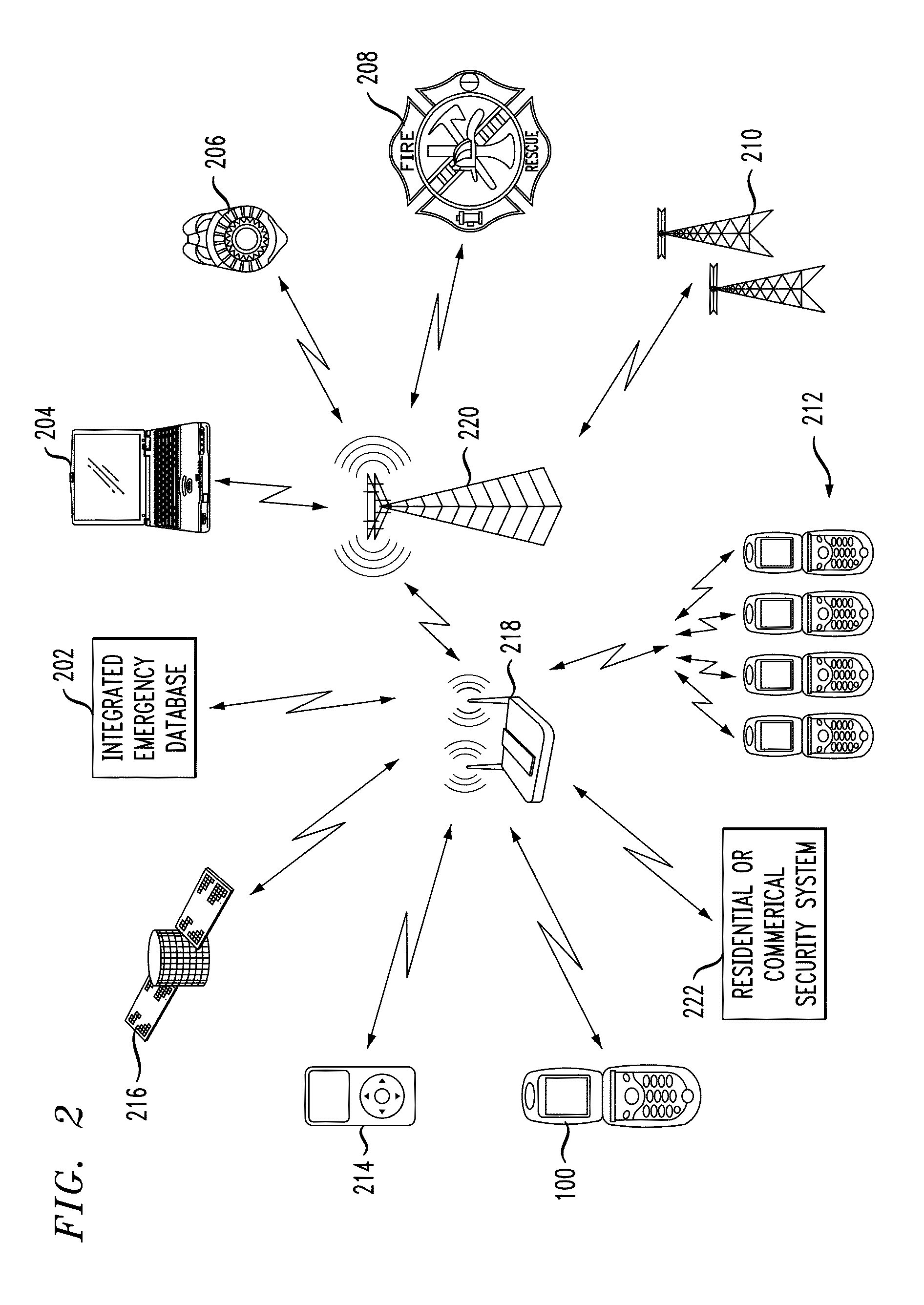
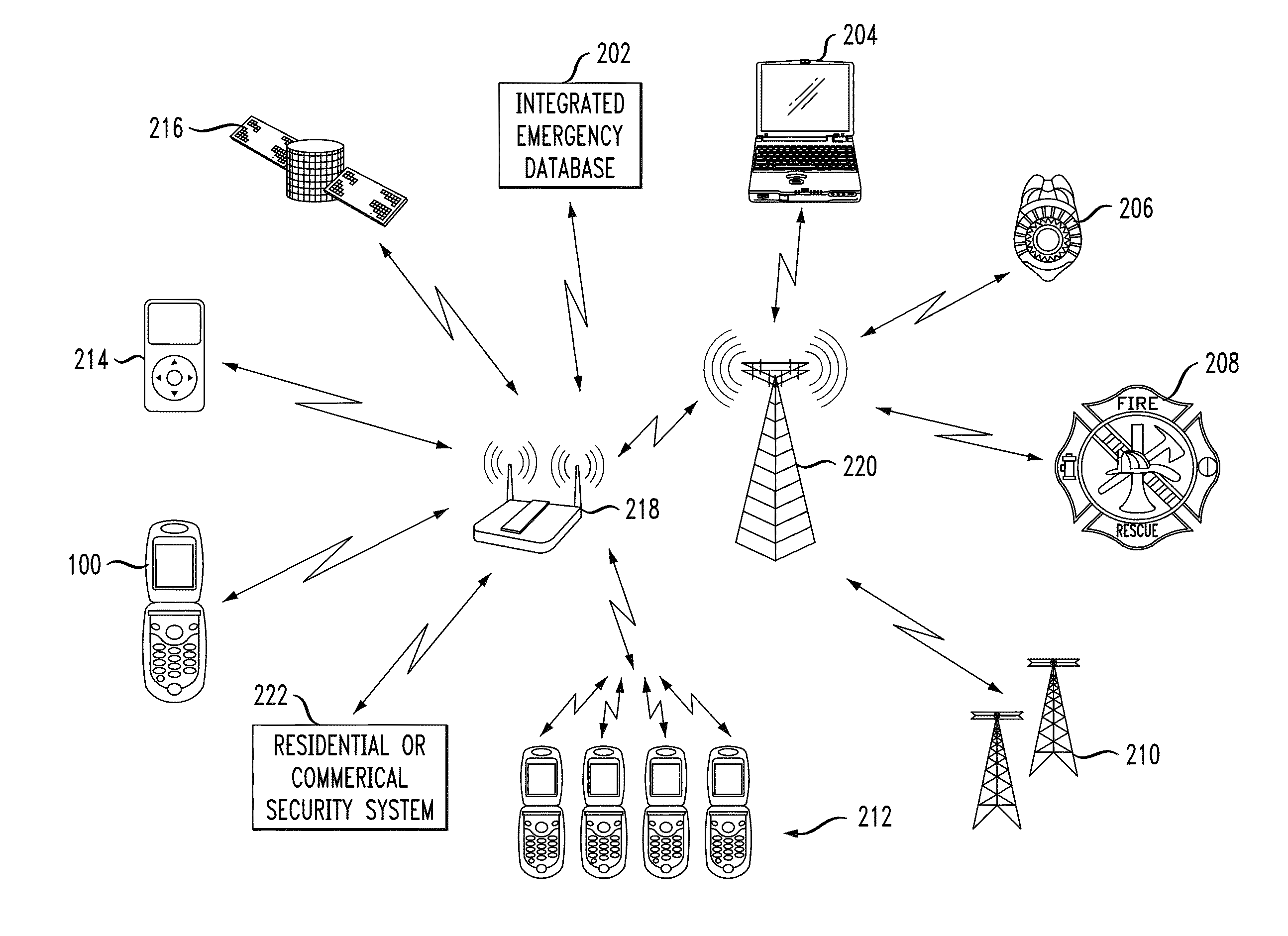
In described embodiments, a system establishes a perimeter around an area, and mobile devices within the established perimeter communicate with a server that provides and collects personal and asset safety information. The provided information might enable users associated with the mobile devices to plan actions or take routes based on a given criteria, such as a safest route, through display on the mobile device. The collected information from the mobile device might be location, emergency event, environmental factors, sensor information and the like, which might then be communicated to users and / or administrators of the system. Location information, such as through global positioning system (GPS), might provide tracking of mobile devices and users or assets associated with each mobile device. GPS functionality associates latitude, longitude and elevation (X-Y-Z coordinate axis) data with the collected and provided information.
Owner:LIQUID RARITY EXCHANGE LLC
Personal safety mobile notification system
In described embodiments, a system establishes a perimeter around an area, and mobile devices within the established perimeter communicate with a server that provides and collects personal and asset safety information. The provided information might enable users associated with the mobile devices to plan actions or take routes based on a given criteria, such as a safest route, through display on the mobile device. The collected information from the mobile device might be location, emergency event, environmental factors, sensor information and the like, which might then be communicated to users and / or administrators of the system. Location information, such as through global positioning system (GPS), might provide tracking of mobile devices and users or assets associated with each mobile device. GPS functionality associates latitude, longitude and elevation (X-Y-Z coordinate axis) data with the collected and provided information.
Owner:LIQUID RARITY EXCHANGE LLC
Combination Wound Therapy
InactiveUS20100150991A1Promote wound healingOrganic active ingredientsBiocideWound therapyAtmospheric pressure
A device for providing improved wound healing is described. The device includes a vacuum system for applying a sub-atmospheric pressure to the wound, a gas supply system for applying a gaseous wound healing agent to the wound, and a controller connected with the vacuum system and the gas supply system that controls the applications of the sub-atmospheric pressure and the application of the gaseous wound healing agent to the wound. A method of using the device for improved wound healing is also described.
Owner:BERNSTEIN BRENT H
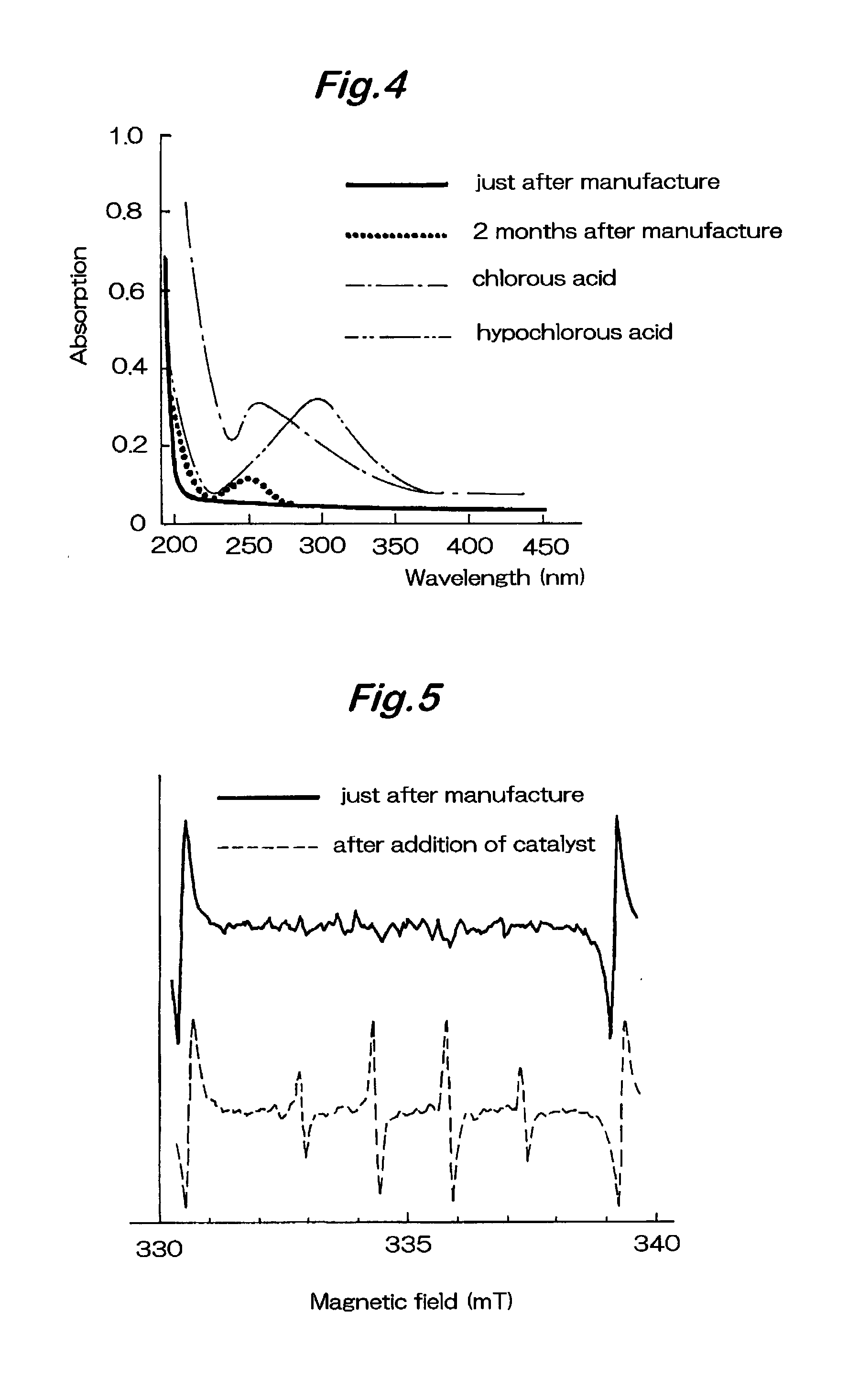
Oxidative reductive potential water solution and methods of using the same
Provided is an oxidative reduction potential (ORP) water solution that is stable for at least twenty-four hours and methods of using the solution. The present invention provides a method of preventing or treating a condition in a patient, which method comprises administering a therapeutically effective amount of the ORP water solution. Additionally provided is a method of treating impaired or damaged tissue, which method comprises contacting the tissue with a therapeutically effective amount of the ORP water solution. Further provided is a method of disinfecting a surface, which method comprises contacting the surface with an anti-infective amount of the ORP water solution.
Owner:SONOMA PHARMA INC
Materials, methods, and devices for treatment of arthropathies and spondylopathies
Novel modalities are introduced to treat joint and cartilage ischemia and related pathologies to improve outcome in the treatment of arthropathies and spondylopathies. The invention includes compositions, materials or devices which will improve oxygen, substrate and nutrient delivery to joint tissues and modalities to decrease the degradation of joint tissues by inflammatory and other destructive processes.
Owner:LEVIN BRUCE
Enamel-safe tooth bleach and method for use
InactiveUS6500408B2High viscositySignificant interferenceBiocideCosmetic preparationsBleachTeeth Bleaching
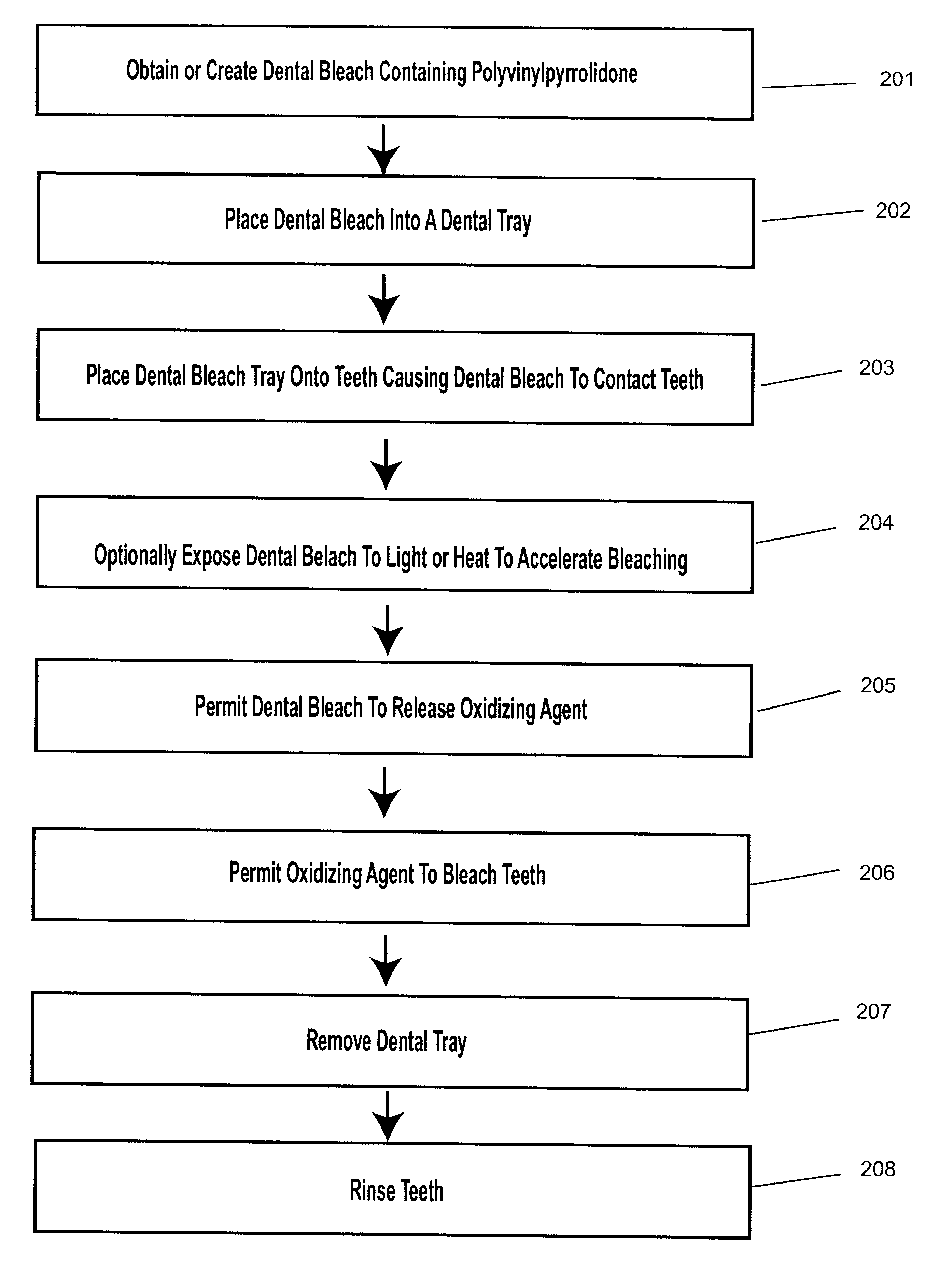
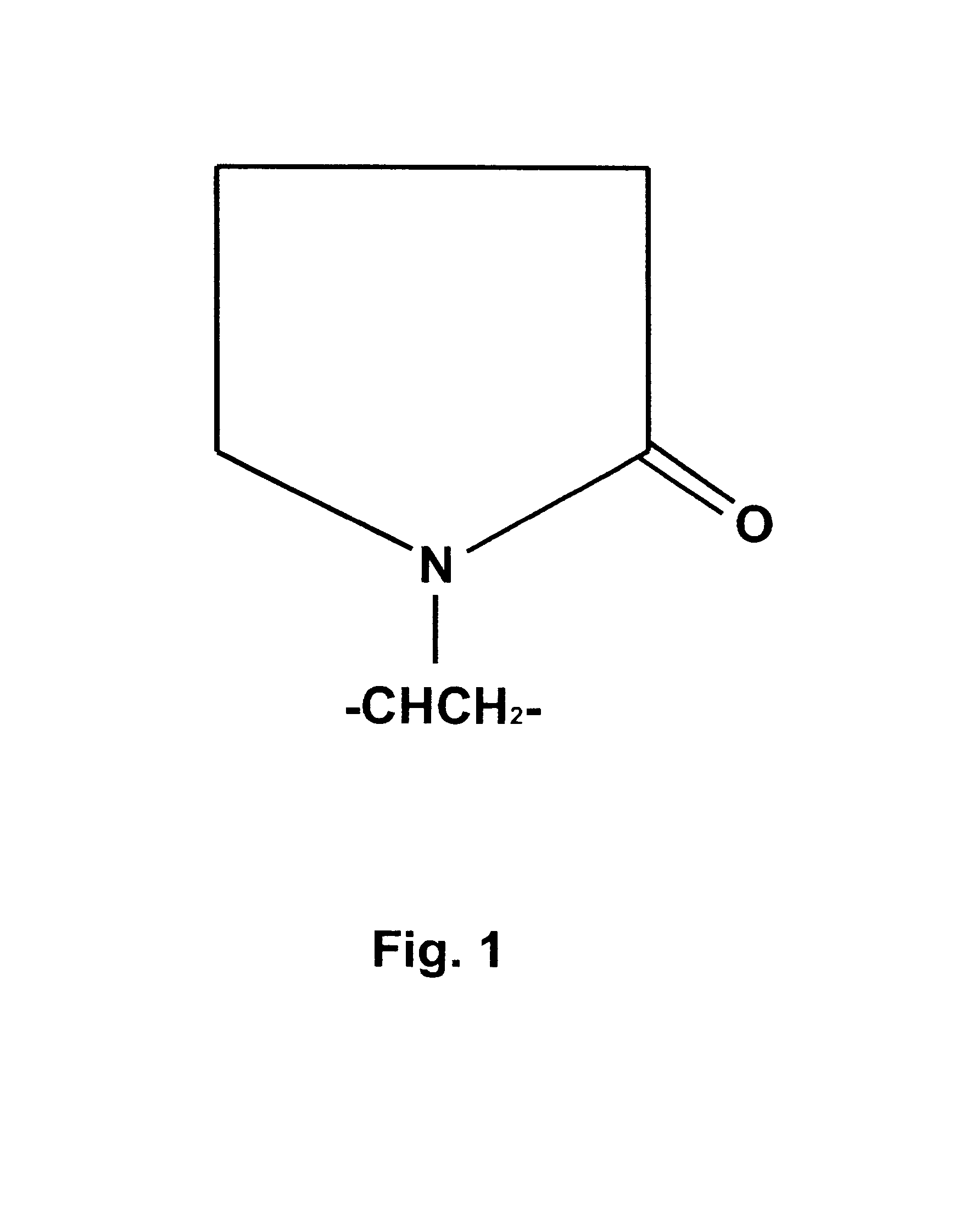
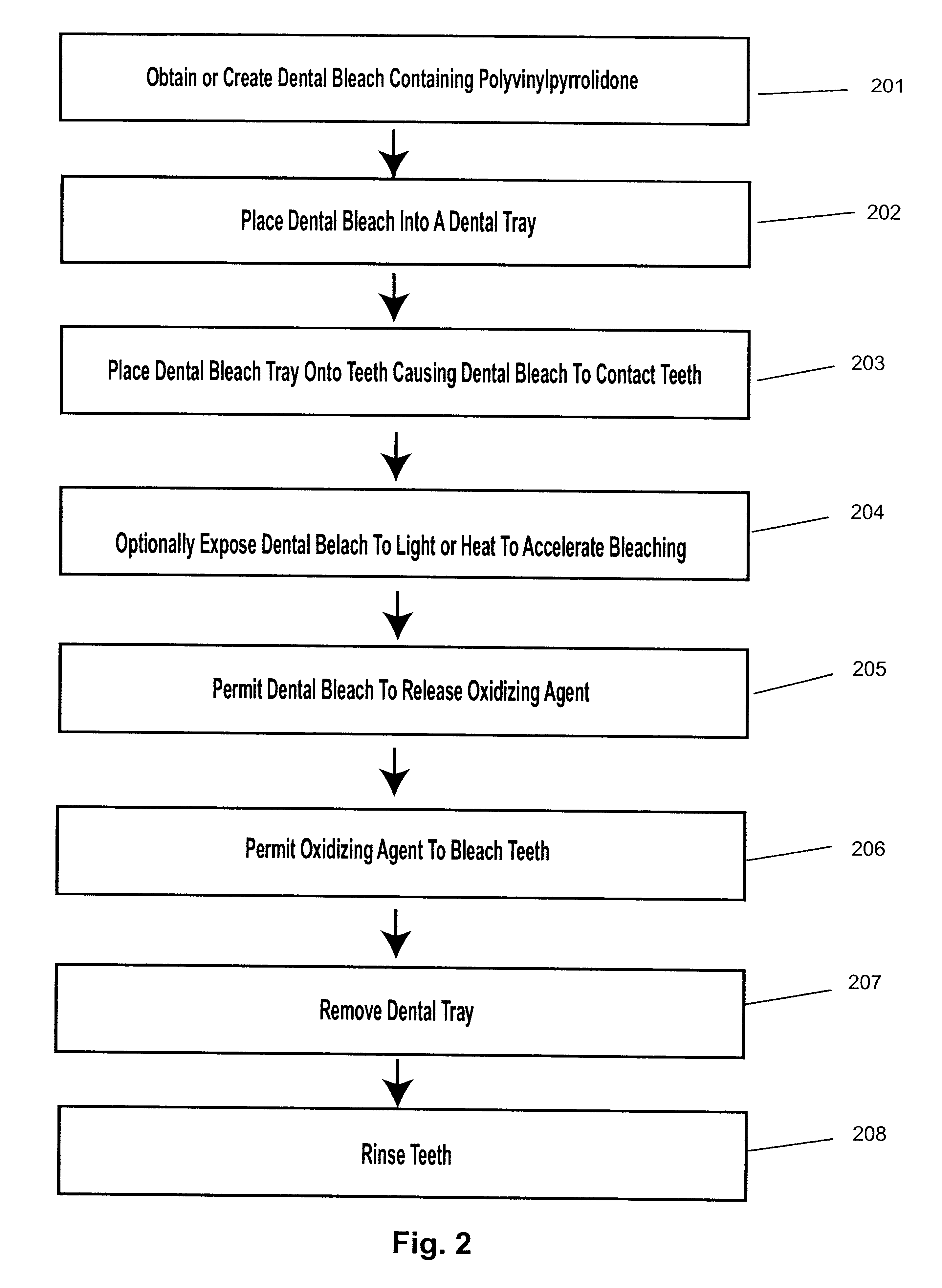
A dental bleach that includes a bleaching agent and a thickening agent. The bleaching agent is typically a peroxide and the thickening agent is polyvinylpyrrolidone. A viscous or sticky dental bleach results. Optionally, a basic agent, light or heat may be added to accelerate bleaching. Bleaching may take place with the use of a dental tray. Bleach may be placed into the dental tray from a single barrel, double barrel or multi-barrel syringe containing the components of the bleach. Bleach may be placed against a flexible strip which is placed onto teeth to be bleached.
Owner:ULTRADENT PROD INC
Matrix for oxygen delivery to compromised tissues
The present invention comprises methods and compositions for delivery devices. More particularly, the present invention comprises methods and compositions for devices comprising a matrix comprising a polymer network and a non-gellable polysaccharide having oxygen and optionally active agents incorporated therein. The matrix may be formed into any desired shape for treatment of compromised tissue or for delivery of oxygen to a localized environment.
Owner:VIRCHOW BIOTECH INC +1
Critical fluid antimicrobial compositions and their use and generation
The present invention relates to antimicrobial compositions including a critical, near critical, or supercritical (densified) fluid and an antimicrobial agent, to methods of forming these compositions, and to methods employing these compositions. An antimicrobial agent can be generated in the presence of a densified fluid, for example, by reacting an oxidizing agent with a precursor to the antimicrobial agent.
Owner:ECOLAB USA INC
Methods and compositions for antimicrobial surfaces
The present invention comprises methods and compositions for treating solid surfaces having antimicrobial and biocidal properties. Such surfaces are capable of controlling or killing a broad spectrum of biological agents, including viruses, bacteria and other microbial agents in solids, liquids or gases that subsequently contact the treated surface.
Owner:SISHIELD TECH
Concentrated aqueous bromine solutions and their preparation
Described is a process of producing a concentrated liquid biocide formulation. Mixed together are (a) bromine chloride or bromine and (b) an aqueous solution of alkali metal salt of sulfamic acid having a pH of at least about 7, in amounts such that (i) the active bromine content of the solution is at least about 100,000 ppm (wt / wt), and (ii) the atom ratio of nitrogen to active bromine from (a) and (b) is greater than 1 when bromine is used and is greater than 0.93 when bromine chloride is used. Use of bromine chloride as the source of the active bromine in the process is preferred because in the resulting aqueous compositions, all of the bromine of the bromine chloride is made available as active bromine in solution. In other words, the chlorine of the bromine chloride is converted in the process to dissolved alkali metal chloride salt, thereby liberating all of the bromine as the active bromine content of the biocidal composition.
Owner:ALBEMARLE CORP
Methods of treating or preventing peritonitis with oxidative reductive potential water solution
ActiveUS20070173755A1Inhibition of secretionReduce bacterial loadAntibacterial agentsBiocideMedicinePeritoneal Hemorrhage
Provided is a method for treating or preventing peritonitis by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers and can be administered in conjunction with one or more additional therapeutic agents. Further provided is a method for preventing peritoneal hemorrhage, adhesions and abscesses.
Owner:SONOMA PHARMA INC
Film coating composition for whitening teeth
PCT No. PCT / CN97 / 00004 Sec. 371 Date Sep. 17, 1998 Sec. 102(e) Date Sep. 17, 1998 PCT Filed Jan. 20, 1997 PCT Pub. No. WO97 / 25968 PCT Pub. Date Jul. 24, 1997The invention relates to a tooth-whitening varnish composition, comprising 6-20% of carbamide peroxide, 2-9% of film forming agent and 77-88% of volatile organic solvent, based on the total weight of the composition. The volatile organic solvent is selected from ether, ethylacetate, ethyl alcohol, or acetone. The film forming agent is artificial or natural material selected from cellulose, polyvinyl, butyral, coumarone resin or shellac. The composition can rapidly form films on dry tooth surfaces, and a remarkable tooth-whitening effect can be obtained.
Owner:IVOCLAR VIVADENT INC
Methods of wound care and treatment
InactiveUS20100021464A1Reduce scarsImprove the level ofBiocideSenses disorderCell membraneWound care
Provided are electrokinetically-altered fluids (e.g., gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating a wound to a surface tissue or a symptom thereof. The electrokinetically-altered fluids or therapeutic compositions and methods include electrokinetically-altered ionic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with said inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
Methods of wound care and treatment
InactiveUS20080139674A1Accelerates epidermalAccelerates dermal layeringAntibacterial agentsBiocideDiseaseWound healing
Particular embodiments disclosed herein relate to gas-enriched fluids, methods of making the same, systems for making the same and / or methods of treatment utilizing the gas-enriched fluids for wound care related conditions and / or diseases. In certain embodiments, the gas-enriched fluid is oxygen-enriched water. Certain embodiments relate to cosmetic and / or therapeutic fluids and / or methods of treatment utilizing the fluids in order to treat a cosmetic and / or therapeutic symptom of wound care and / or increase proper wound healing.
Owner:REVALESIO CORP
Methods of treating or preventing inflammation and hypersensitivity with oxidative reductive potential water solution
InactiveUS20070196357A1Potent anti-inflammatory activityFree them from any toxicityAntibacterial agentsBiocideMedicineReduction potential
Provided is a method for preventing or treating inflammation and associated states (e.g. infection, hypersensitivity, pain) by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers and can be administered in conjunction with one or more additional therapeutic agents.
Owner:SONOMA PHARMA INC
Hydrogen peroxide disinfectant with increased activity
InactiveUS6346279B1High activityReduced activityBiocideInorganic phosphorous active ingredientsDisinfectantPhosphoric acid
An acidic aqueous hydrogen peroxid solution is provided, with improved disinfectant activity. Concentrated solutions preferably contain up to about 8% and as-used concentrations contain about 0.5% peroxide. The solution also contains from 0.1 to 5.0% of at least one acid compound, e.g. phosphoric and / or a phosphonate with from 1 to 5 phosphonic acid groups, and from 0.02 to 5% of at least one anionic surfactant. The surfactant is selected from C8 to C16-alkyl aryl sulphonic acids, sulphonated C12 to C22 carboxylic acids, C8 to C22-alkyl diphenyl oxide sulphonic acids, naphthalene sulphonic acids, C8 to C22 alkyl sulphonic acids, and alkali metal and ammonium salts thereof, and alkali metal C8 to C18 alkyl sulphates, and mixtures thereof. Most preferably the solution has an emulsifier, e.g. a salt of an alkylated diphenyl oxide. The solution may also contain corrosion inhibitors and / or lower alcohols.
Owner:VIROX TECH
Method of treating skin ulcers using oxidative reductive potential water solution
ActiveUS20060235350A1Reduce inflammatory processReduce microbial loadAntibacterial agentsBiocideMedicineSkin ulcerations
Provided is a method of treating skin ulcers and related complications in patients by administering an oxidative reduction potential (ORP) water solution that is stable for at least twenty-four hours.
Owner:SONOMA PHARMA INC
Topical formulations and delivery systems
InactiveUS7060253B1Improves effervescenceGood removal effectBiocideAerosol deliveryFoaming agentActive agent
A system for delivering a chemical agent in the form of a spray or foam, which in a preferred embodiment involves the use of an aerosol dispenser to deliver a formulation containing both an anionic surface active agent such as sodium lauryl sulfate as a foaming agent and a chemical agent such as either hydrogen peroxide as a disinfecting chemical agent or natural sea water.
Owner:PHYLOMED CORP
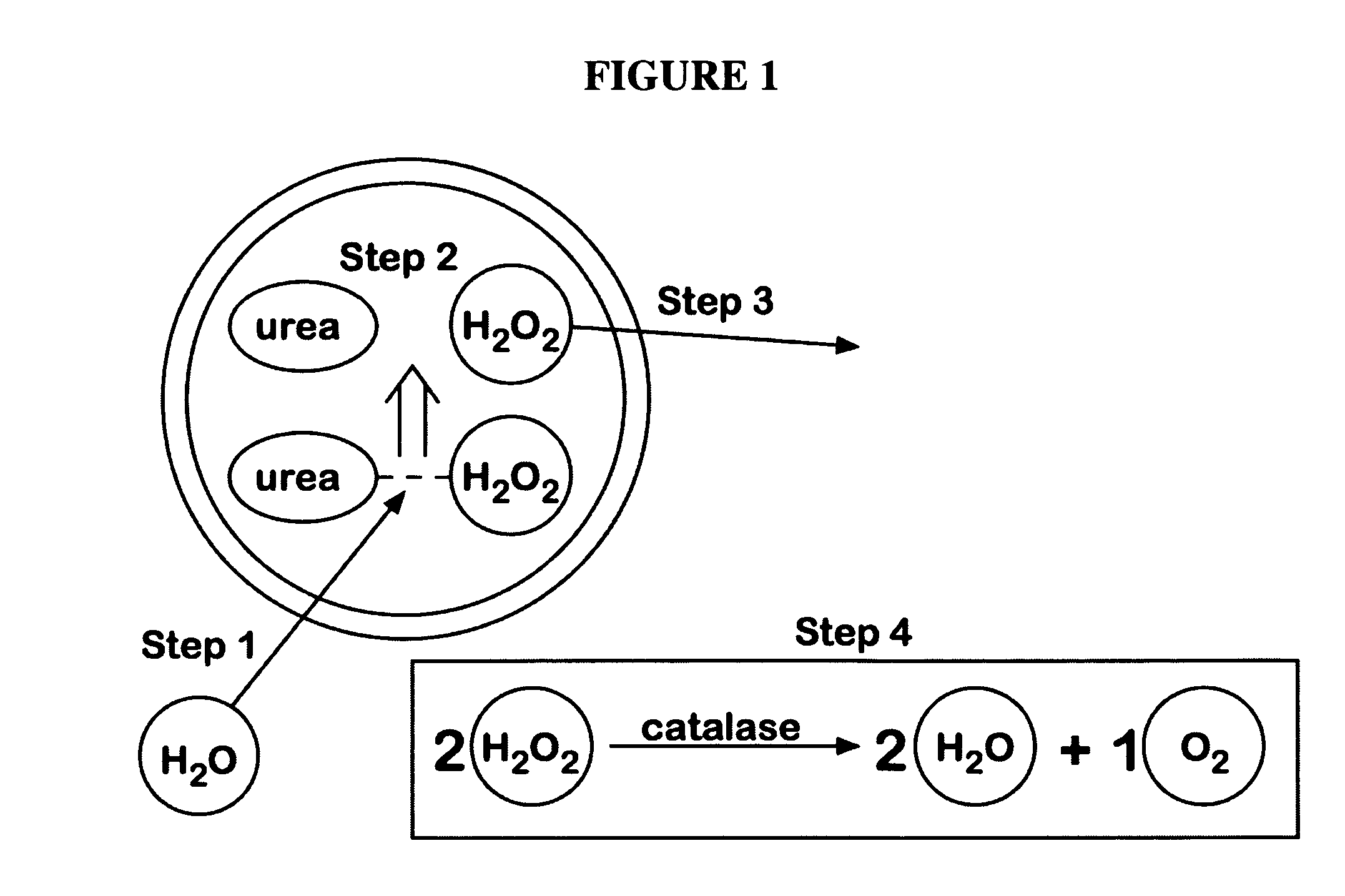
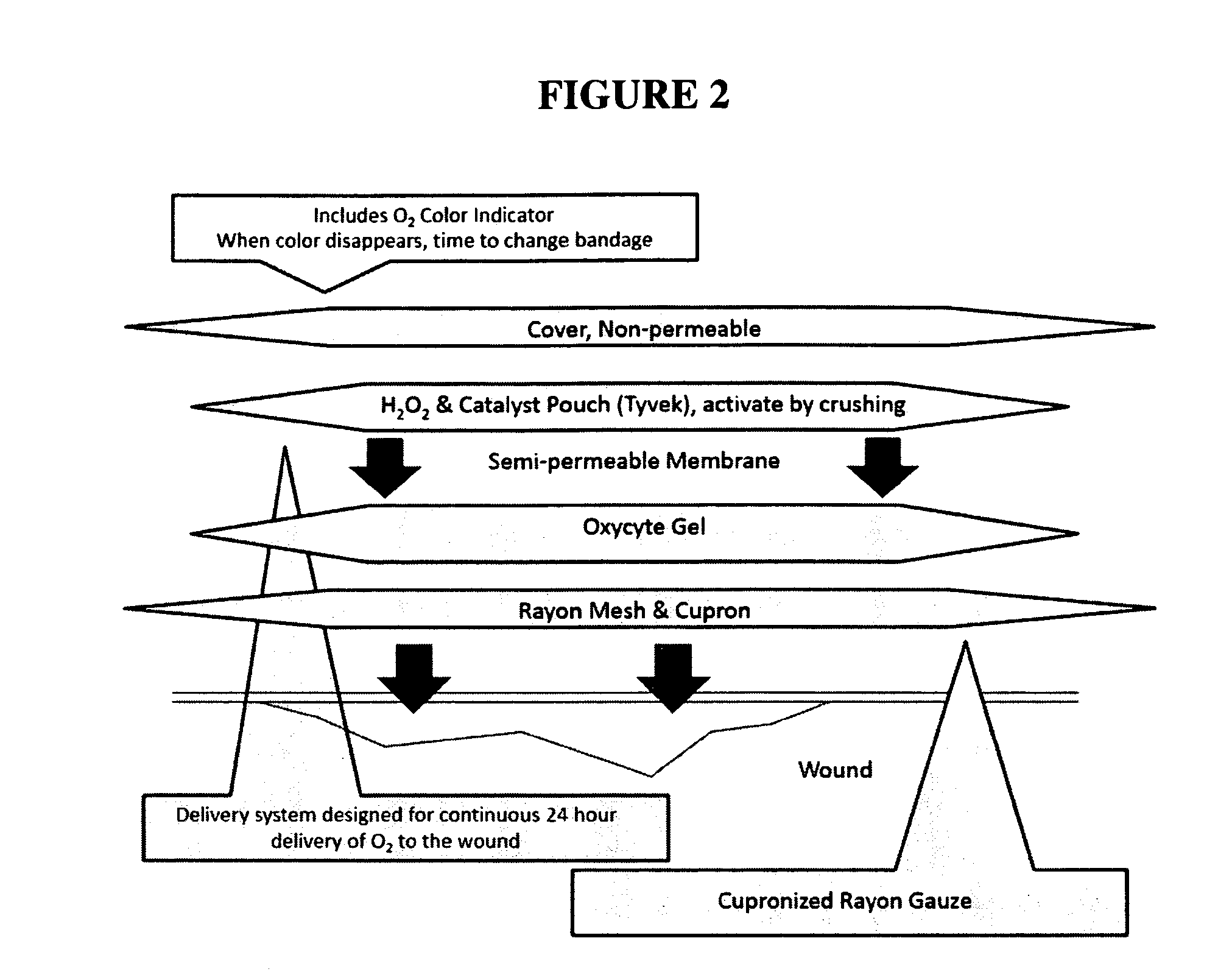
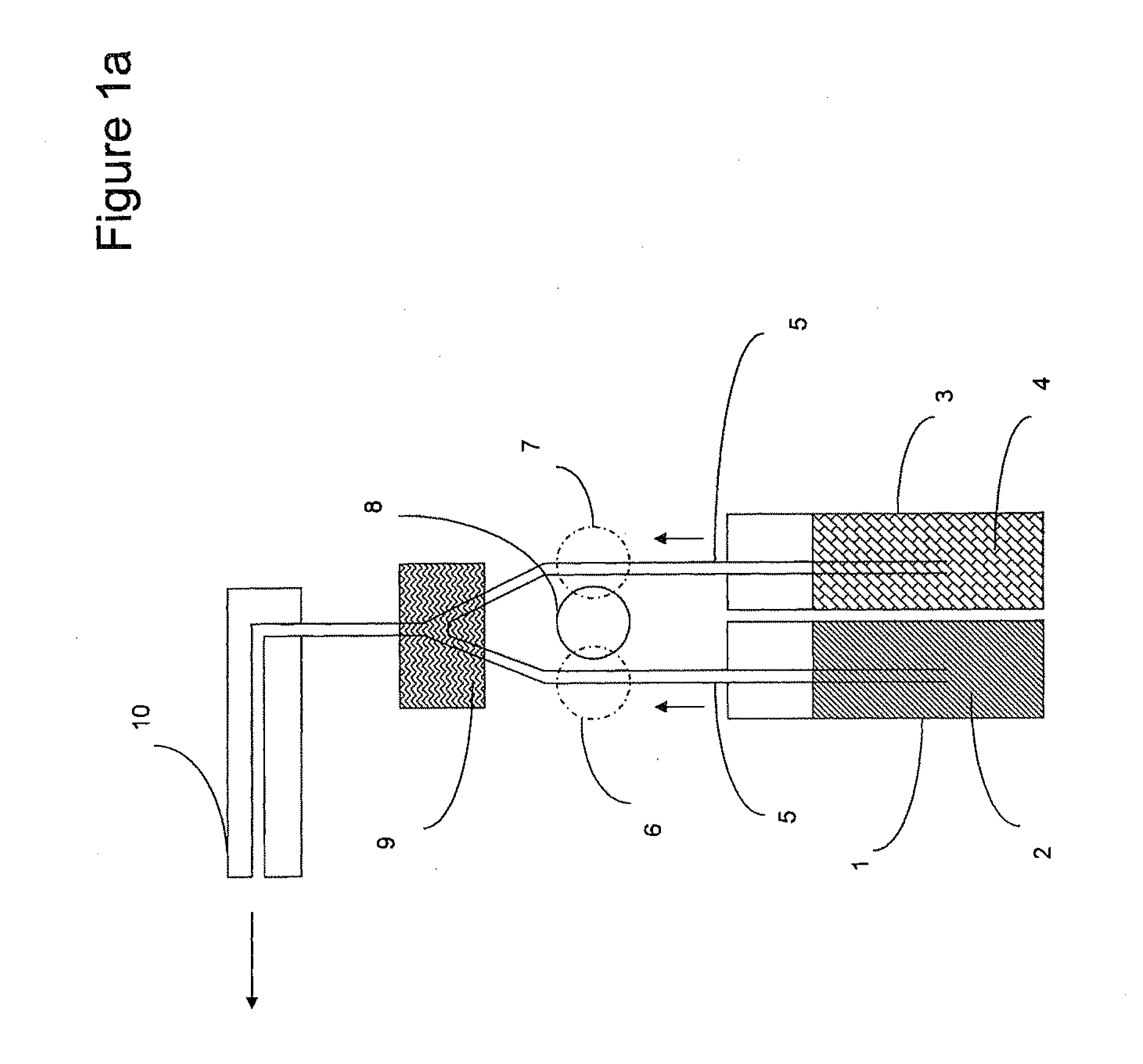
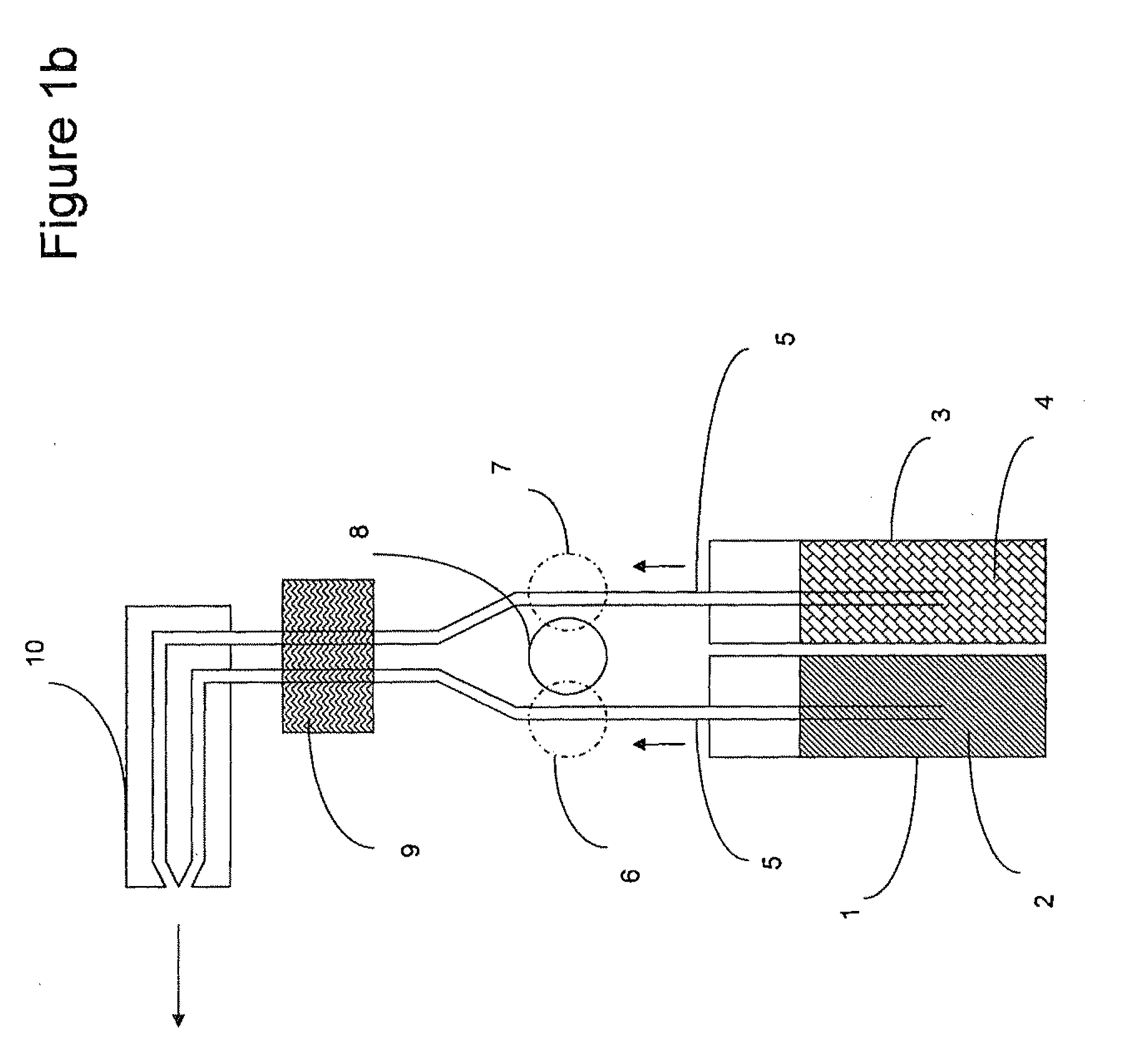
Physiologically balanced, ionized, acidic solution and methodology for use in wound healing
Described herein is a physiologically-balanced, acidic solution. Typically the solution is prepared by a chemical reactions or by the electrolysis of a solution comprising a mixture of an inorganic salt to form a physiologically balanced solution. This invention also relates to methods for use of the solutions, including a specialized bandage which may be used in combination with the solutions, or optionally with other topically applied materials. A mixture of inorganic salts and, optionally minerals, is used in order to mimic the electrolyte concentration and mixture of body fluid in an isotonic state. The solution typically comprises of one halide salt of lithium, sodium, potassium, calcium, and other cations. Typically the halide is fluoride, chloride, bromide, or iodide, and most typically chloride. A typical electrolyzed solution of the present invention has a pH within the range of about 2 to about 5, an oxidation reduction potential within the range of about +600 mV to about +1200 mV, and hypohalous acid concentration in the range of about 10 ppm to about 200 ppm. The solution has bactericidal, fungicidal, and sporicidal properties. The composition of the invention is nontoxic and has antibacterial properties, and is useful in any application in which antimicrobial properties are desirable.
Owner:NOVABAY PHARM INC
Wound and ulcer treatment with super-oxidized water
Super-oxidized water based on hypochlorous acid, such as is obtained by the electrochemical treatment of a saline solution, may be used in the treatment of leg ulcers or other open wounds. Preferably, the pH of the super-oxidized water is in a range of 4 to 7, and the water has a redox potential of >950 mV. Medicaments based on the super-oxidized water may be in liquid or gel form. The super-oxidized water is able to control the microbial population within the wound and at the same time permit cell proliferation.
Owner:STERILOX TECH INT +1
Methods Of Providing Antioxidants To Implantable Medical Devices
Methods of incorporating an antioxidant into a medical device including a polymer are described, and methods of packaging medical devices.
Owner:ABBOTT CARDIOVASCULAR
Aqueous composition containing H2O2, acids and Ag, preparation method therefor and use thereof for disinfection, hygiene and/or pollution control
The present invention relates to an aqueous decontaminating composition comprising(A) an amount of H2O2 less than or equal to 60% by weight, based on the total weight of said composition;(B) an RCO3H / RCO2H mixture, where R is methyl or ethyl, as indicated above, said mixture being present in an amount such that the weight ratio of said mixture to the hydrogen peroxide is between 0.15 / 1 and 0.85 / 1;(C) a silver component as a source of Ag ions, selected from the group consisting of silver salts and complexes, said silver component being present in an amount such that the weight ratio of said silver component to the hydrogen peroxide is between 0.0005 / 1 and 0.015 / 1;(D) a stabilizer present in an amount such that the weight ratio of said stabilizer to the hydrogen peroxide is between 0.0005 / 1 and 0.025 / 1; andwater to make up to 100% by weight. It further relates to the method of preparation and to the use of said composition.
Owner:SODIFRA
Solution for promoting growth of tissue cells at wound sites and production process therefor
InactiveUS20020160053A1Promote growthWound can be promotedBiocidePhotography auxillary processesGrowth promotingNeutrophil granulocyte
A tissue cell growth-promoting solution produced by this invention comprising water containing at least 1 to 500 ppm of active oxygen, when applied to a wound, supplies active oxygen originating from outside the biobody to supplement the active oxygen produced by the biobody's own protective system cells such as neutrophils and macrophages which gather at the wound site, thus increasing the concentration of active oxygen at the site of the wound, mimicking a state in which a large quantity of such bio-signals is secreted by the biobody itself, to promote the reconstruction of tissues, the action corresponding to the last of the four main steps involved in wound healing biochemical processes of "blood vessel reaction", "blood vessel coagulation", "inflammation", "reconstruction of tissues" and which would otherwise have to rely on the natural healing power of the biobody itself.
Owner:SONOMA PHARMA INC
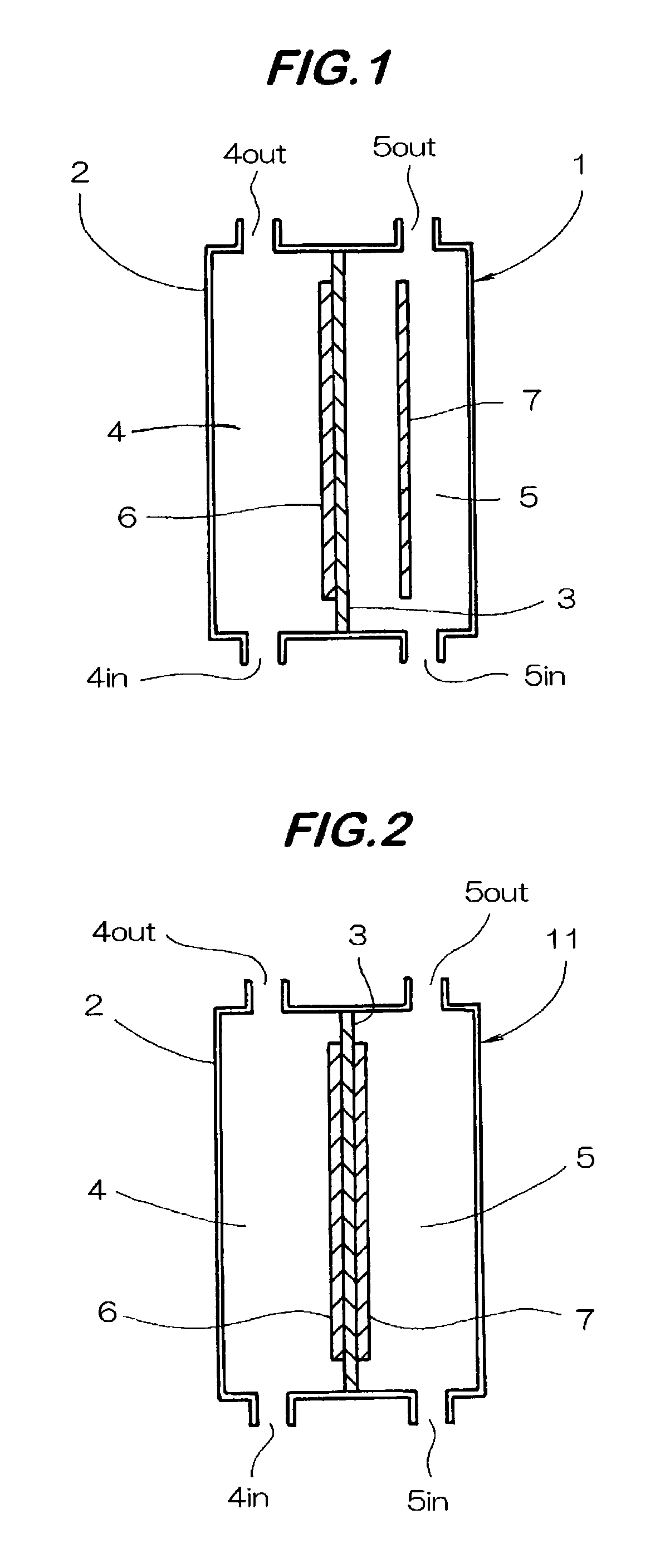
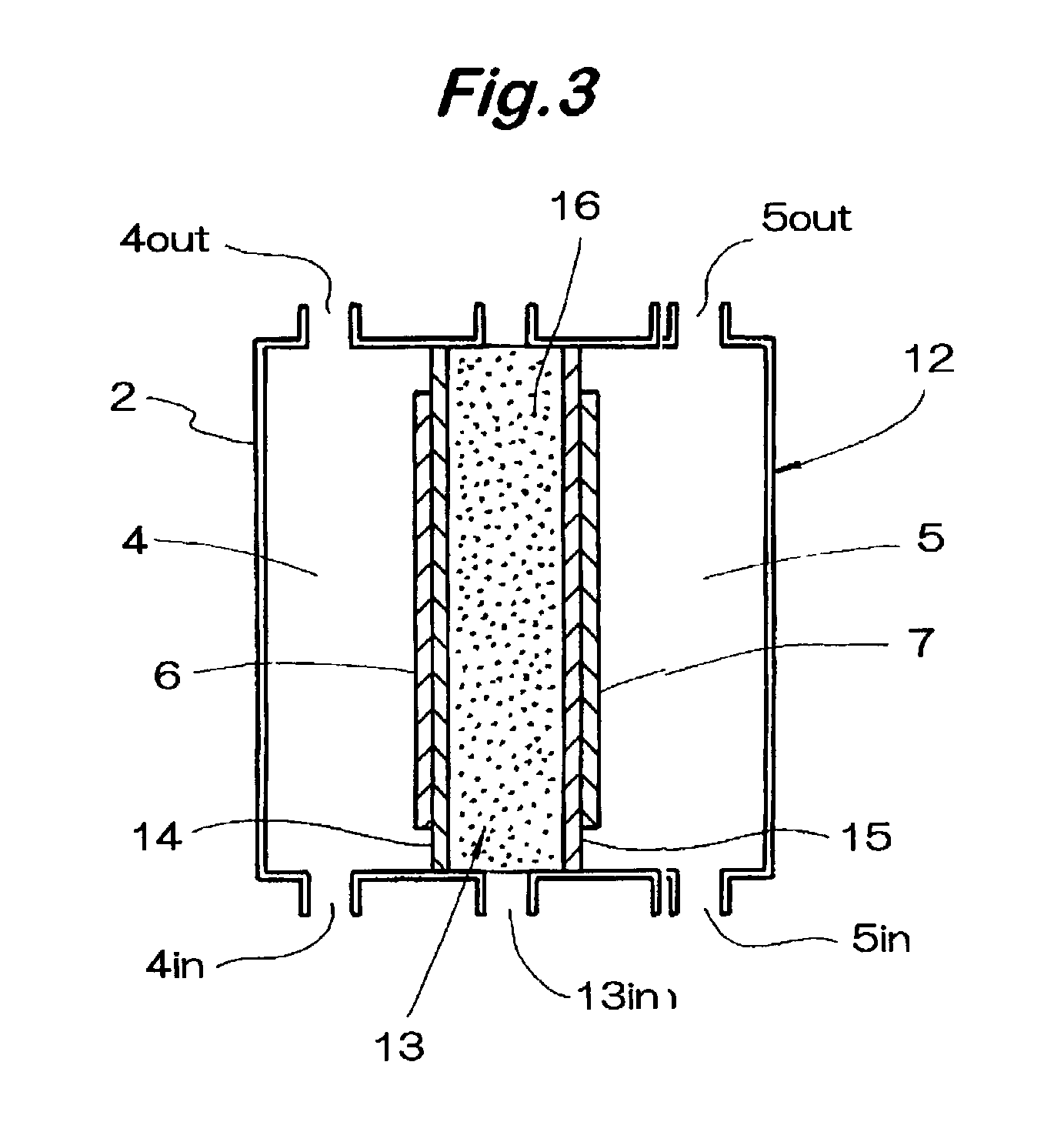
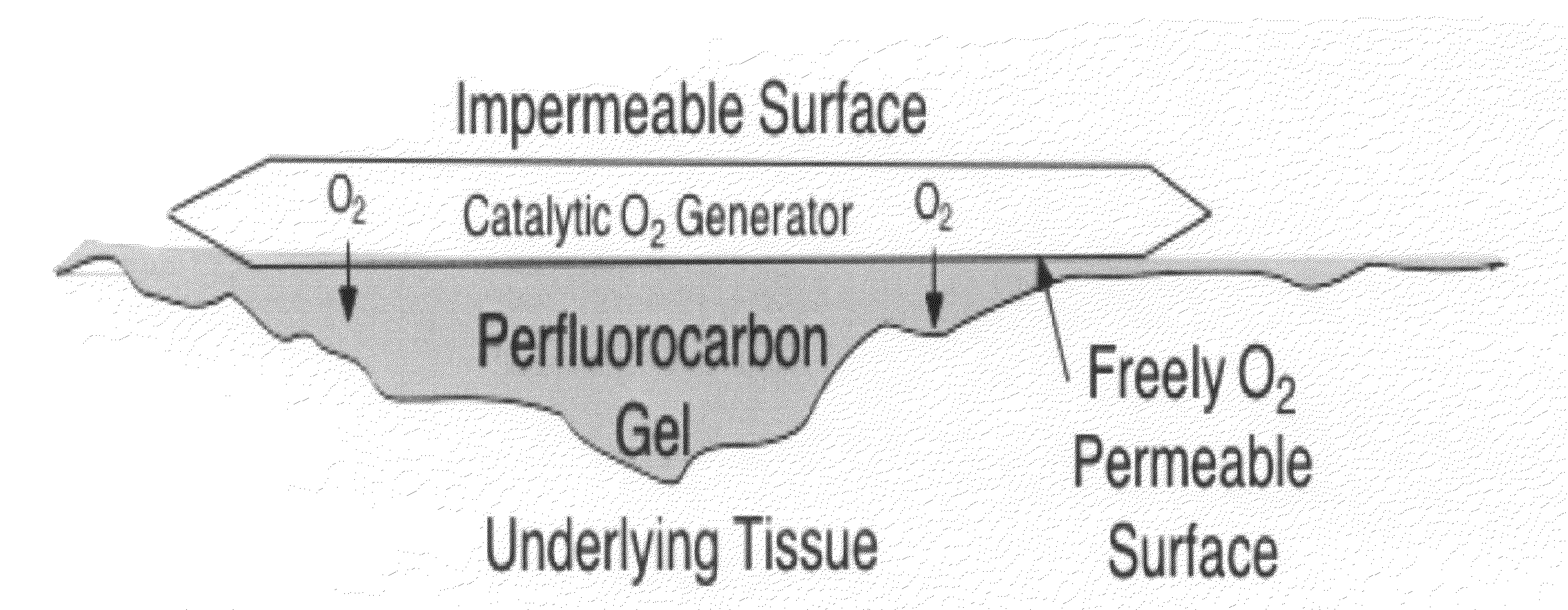
Gas based wound and tissue therapeutics
This invention provides articles of manufacture and bandages comprising compartments and layers comprising oxygen and other therapeutic gas storage forms and perfluorocarbons. This invention also provides for methods of delivering oxygen and other therapeutic gases to a tissue in a subject comprising a administering to the tissue a composition comprising a perfluorocarbon and a oxygen or therapeutic gas storage form, so as to thereby deliver oxygen or the therapeutic gas to the tissue.
Owner:VIRGINIA COMMONWEALTH UNIV +1
Multi-component peracid generation system
InactiveUS20100086621A1Good storage stabilityGood mixing propertiesInorganic/elemental detergent compounding agentsBiocideDisinfectantCarboxylic acid
Disclosed herein are multi-component peroxycarboxylic acid generation systems for enzymatically producing aqueous formulations of peroxycarboxylic acids suitable for use in, e.g., disinfectant and / or bleaching applications. The multi-component peroxycarboxylic acid generation systems comprise at least one carbohydrate esterase family 7 enzyme having perhydrolytic activity.
Owner:EI DU PONT DE NEMOURS & CO
Composition and Method for the Prevention of Oral Disease
InactiveUS20090016973A1Slow and prevent re-colonizationReduce productionAntibacterial agentsCosmetic preparationsOral diseaseDisease
The discovery of use of stabilized chlorine dioxide at a concentration range of about 0.5% to about 0.9% (w / v) as a composition for the prevention of oral disease through bactericidal and bacteriostatic properties within a microbial environment is disclosed. The bactericidal properties of stabilized chlorine dioxide are further expanded to include kill of an increased number of anaerobic / aerobic / facultative gram-negative and gram-positive oral bacteria occurring in mixed microbial communities. The further discovery of the bacteriostatic properties of stabilized chlorine dioxide present within a microbial environment which inhibit the re-growth rate of oral bacteria after exposure resulting in significant reduction of plaque and oral malodor production is disclosed. The stabilized chlorine dioxide may be in the form of an oral wash or rinse in solution.
Owner:MICROPURE
Shelf stable, reduced corrosion, ready to use peroxycarboxylic acid antimicrobial compositions
ActiveUS20090061017A1Advantageous sporicidal activityBiocidePeroxide active ingredientsSporeProtonation
The present invention relates to shelf stable and / or less corrosive peroxycarboxylic acid antimicrobial compositions, including ready-to-use compositions. Shelf stable compositions can include defined ratios of hydrogen peroxide to peroxycarboxylic acid and / or hydrogen peroxide to protonated carboxylic acid, but need not include strong acid. Reduced corrosion compositions can include carboxylic acid and corrosion inhibitor at acid pH. Compositions of the invention can have advantageous activity against spores.
Owner:ECOLAB USA INC
Methods of preventing or treating sinusitis with oxidative reductive potential water solution
InactiveUS20070196434A1Treating and preventing sinusitisAntibacterial agentsBiocideSinusitisMedicine
Provided is a method for preventing or treating sinusitis by administering a therapeutically effective amount of an oxidative reduction potential (ORP) water solution that is stable for at least about twenty-four hours. The ORP water solution administered in accordance with the invention can be combined with one or more suitable carriers. The ORP water solution can be administered alone or, e.g., in combination with one or more additional therapeutic agents.
Owner:SONOMA PHARMA INC
Virucide composition and sporicide composition
InactiveUS6506416B1Avoid it happening againImprove the immunityBiocidePeroxide active ingredientsAlkaline earth metalTetraacetylethylenediamine
The present invention provides a virucide composition and / or sporicide composition having a high virucidal effect and sporicidal effect and being excellent in safety and workability. That is, the present invention provides a virucide composition and / or sporicide composition comprising (a) an inorganic peroxide, (b) tetraacetylethylenediamine and (c) at least one selected from a salt of an alkaline metal salt with an inorganic acid and a salt of an alkaline earth metal with an inorganic acid in a specific ratio.
Owner:KAO CORP
Methods of therapeutic treatment of eyes and other human tissues using an oxygen-enriched solution
Particular embodiments disclosed herein relate to electrokinetically altered gas-enriched fluids, methods of making the same, systems for making the same and / or methods of treatment utilizing the gas-enriched fluids for eye related conditions and / or diseases. In certain embodiments, the electrokinetically altered gas-enriched fluid is oxygen-enriched water. Certain embodiments relate to cosmetic and / or therapeutic fluids and / or methods of treatment utilizing the fluids to treat a cosmetic and / or therapeutic symptom related to eye conditions and / or diseases.
Owner:REVALESIO CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com