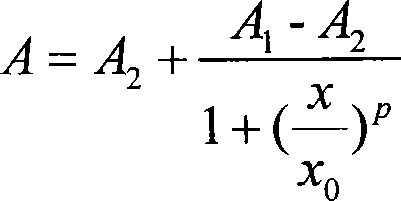Patents
Literature
49 results about "A antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
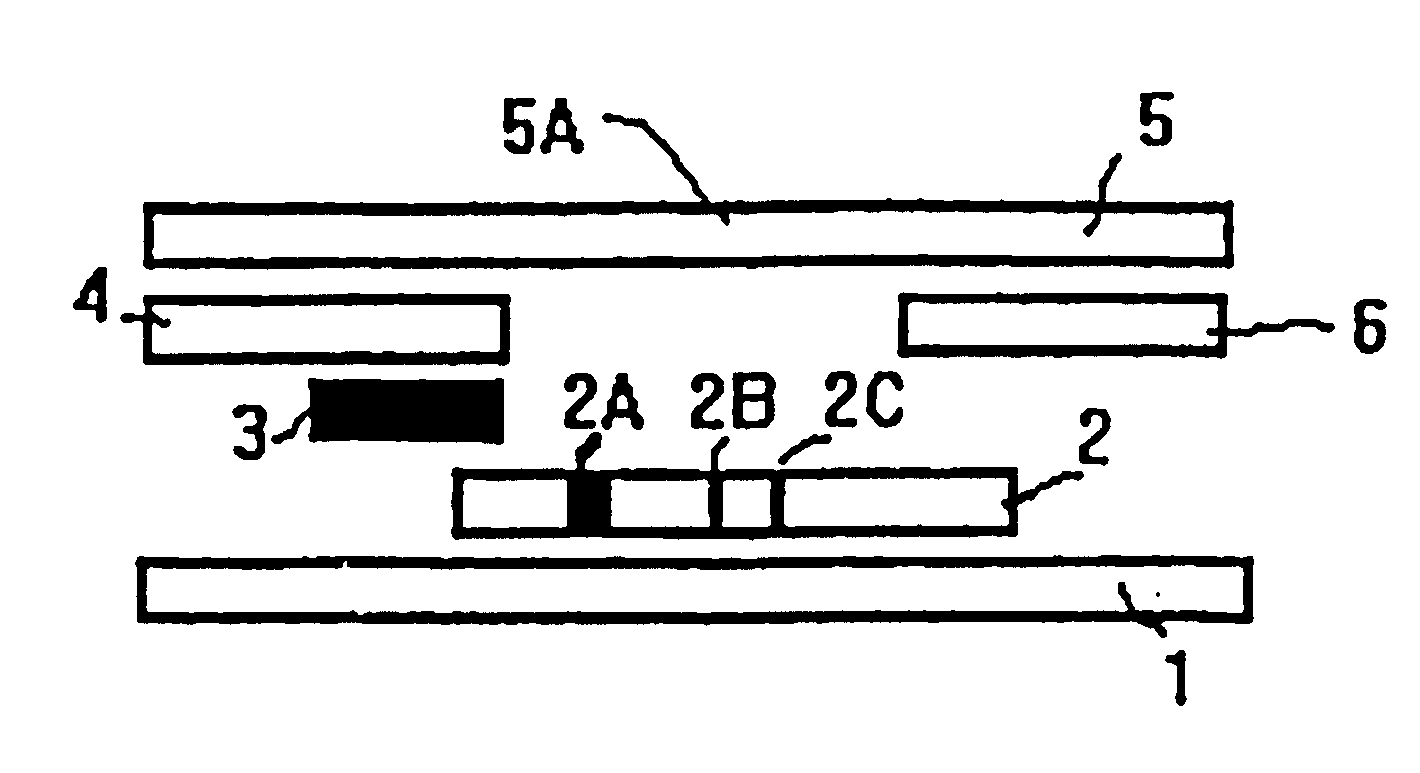
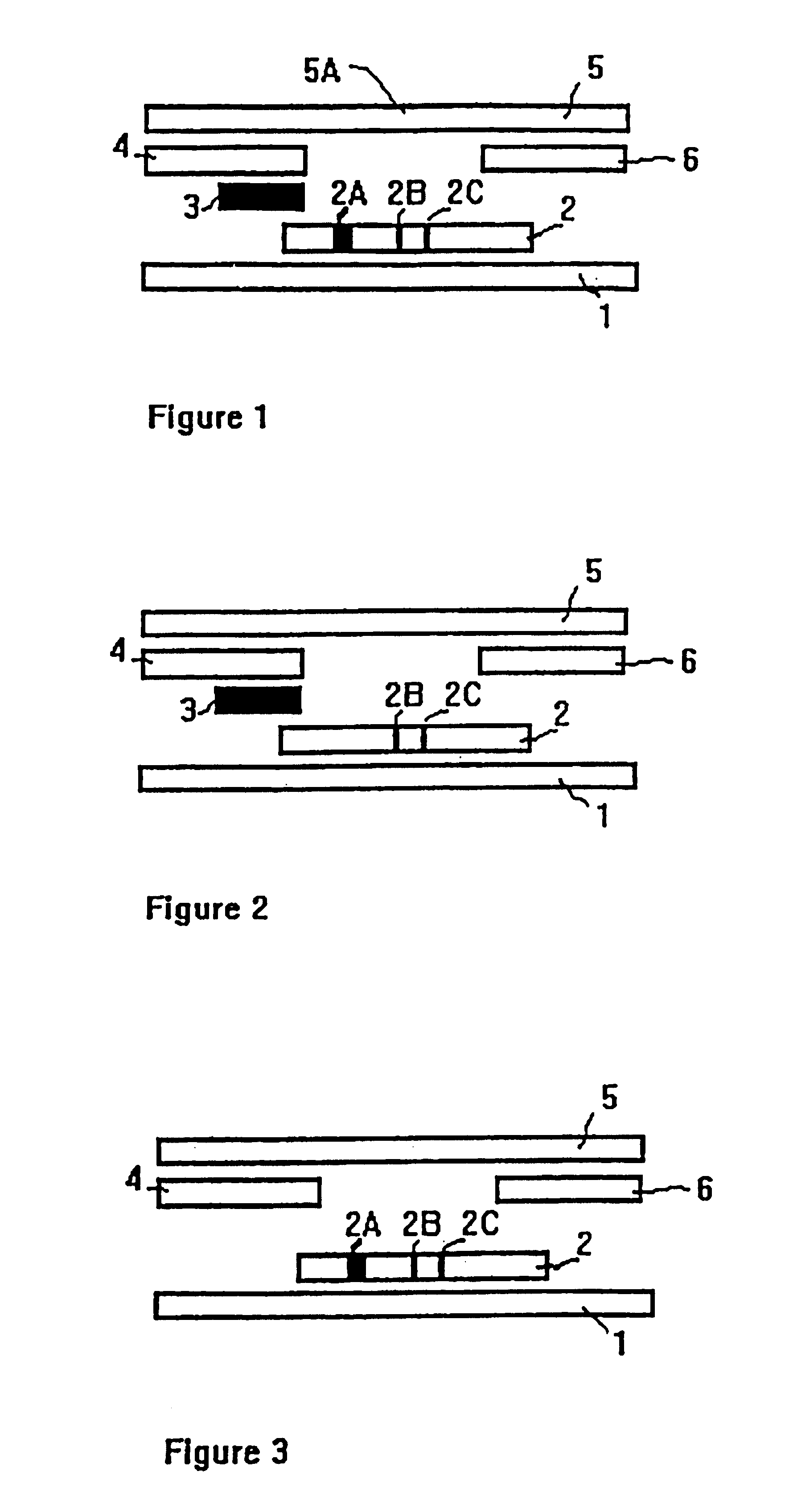
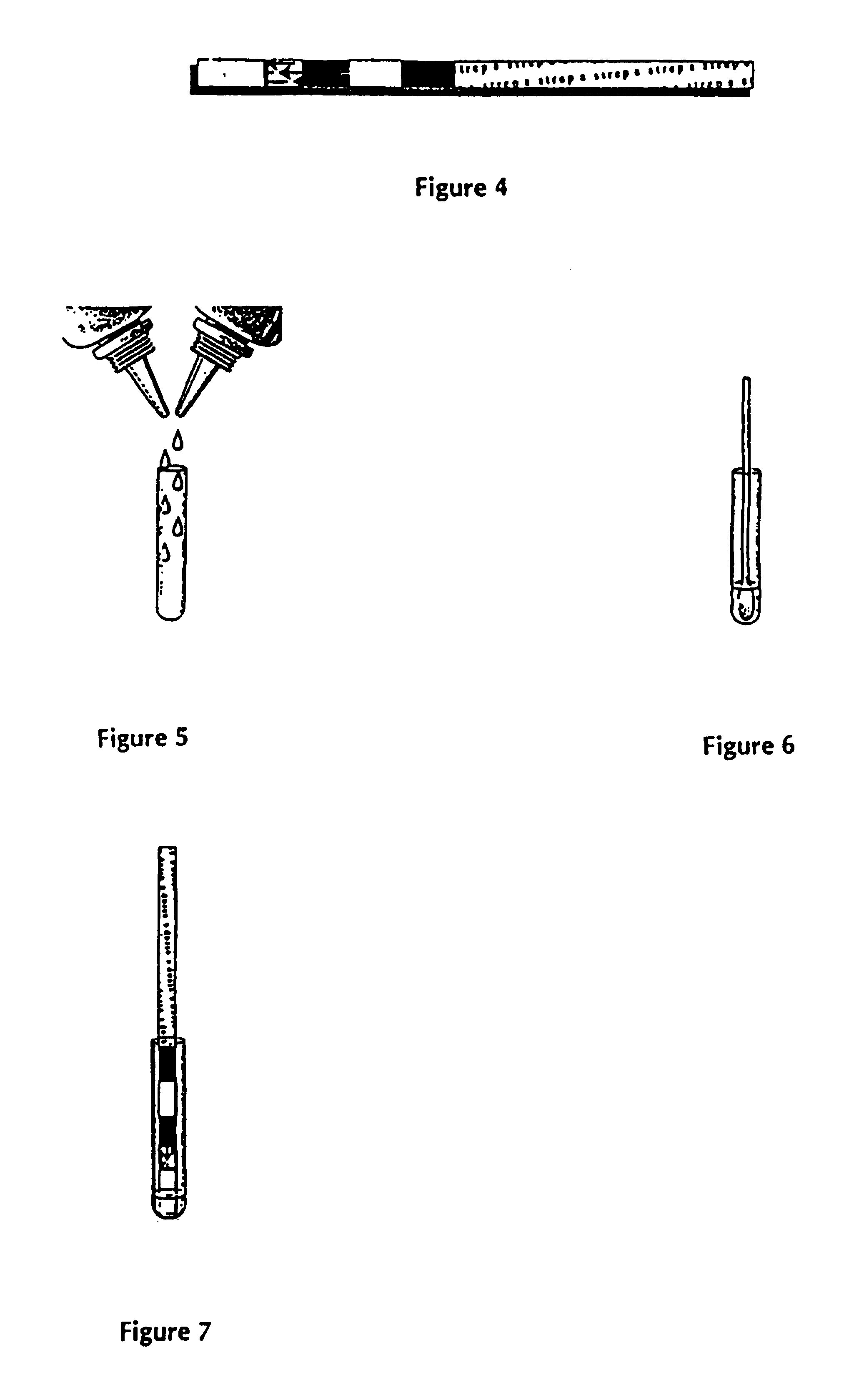
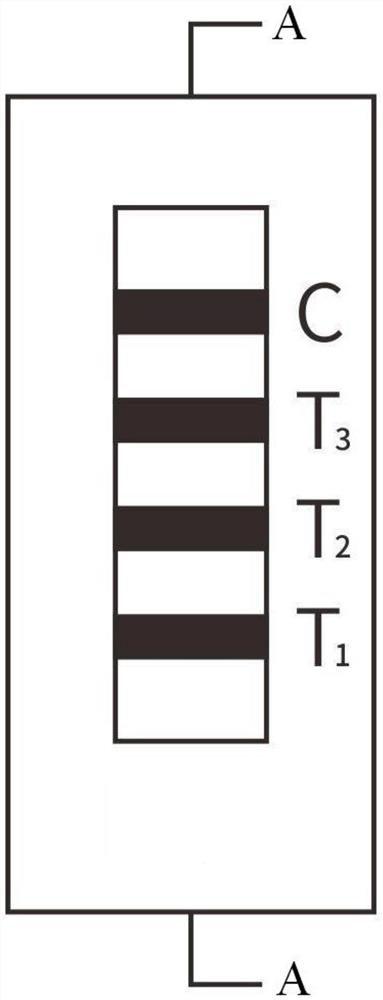
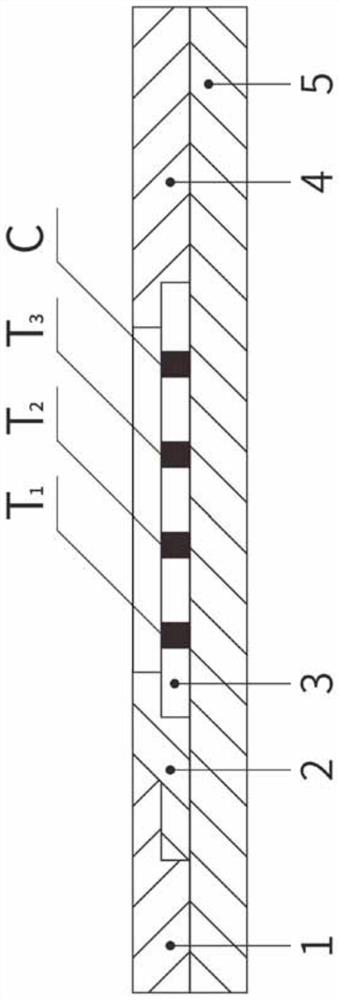
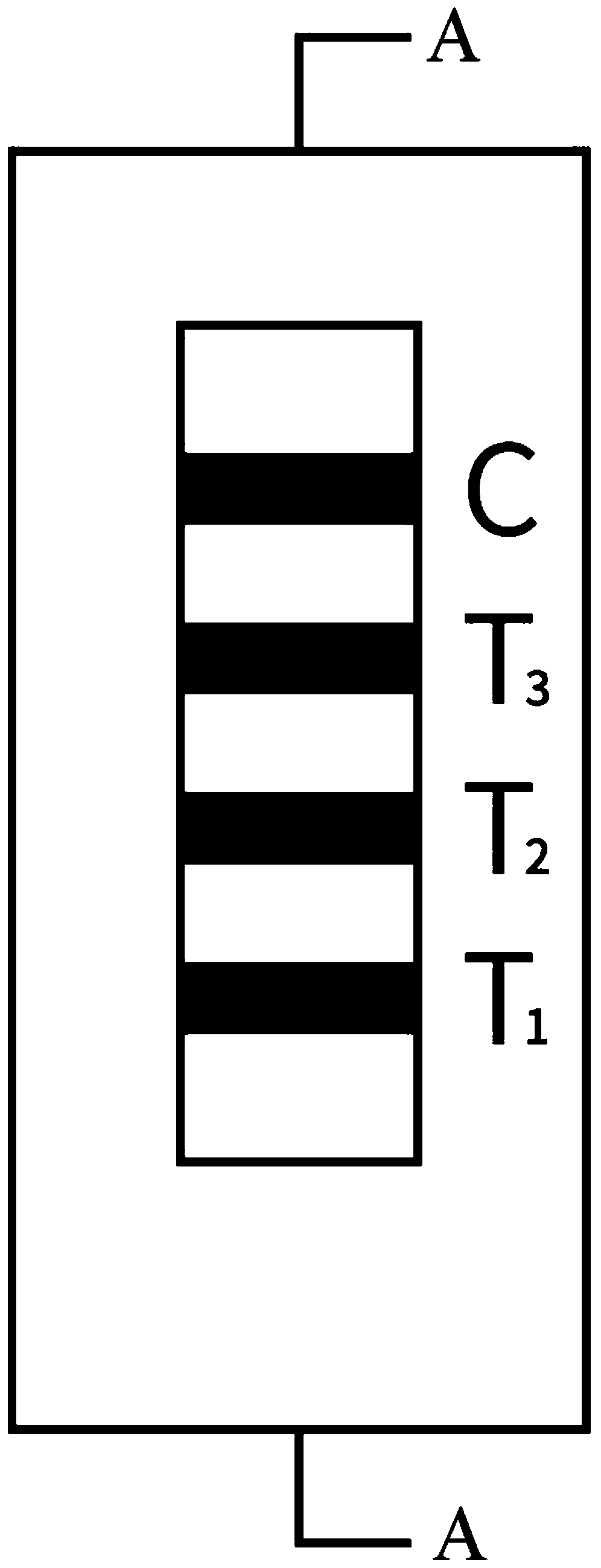
Methods of use of one step immunochromatographic device for Streptococcus A antigen
InactiveUS6979576B1Reduce the numberLess manipulationBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenReagent
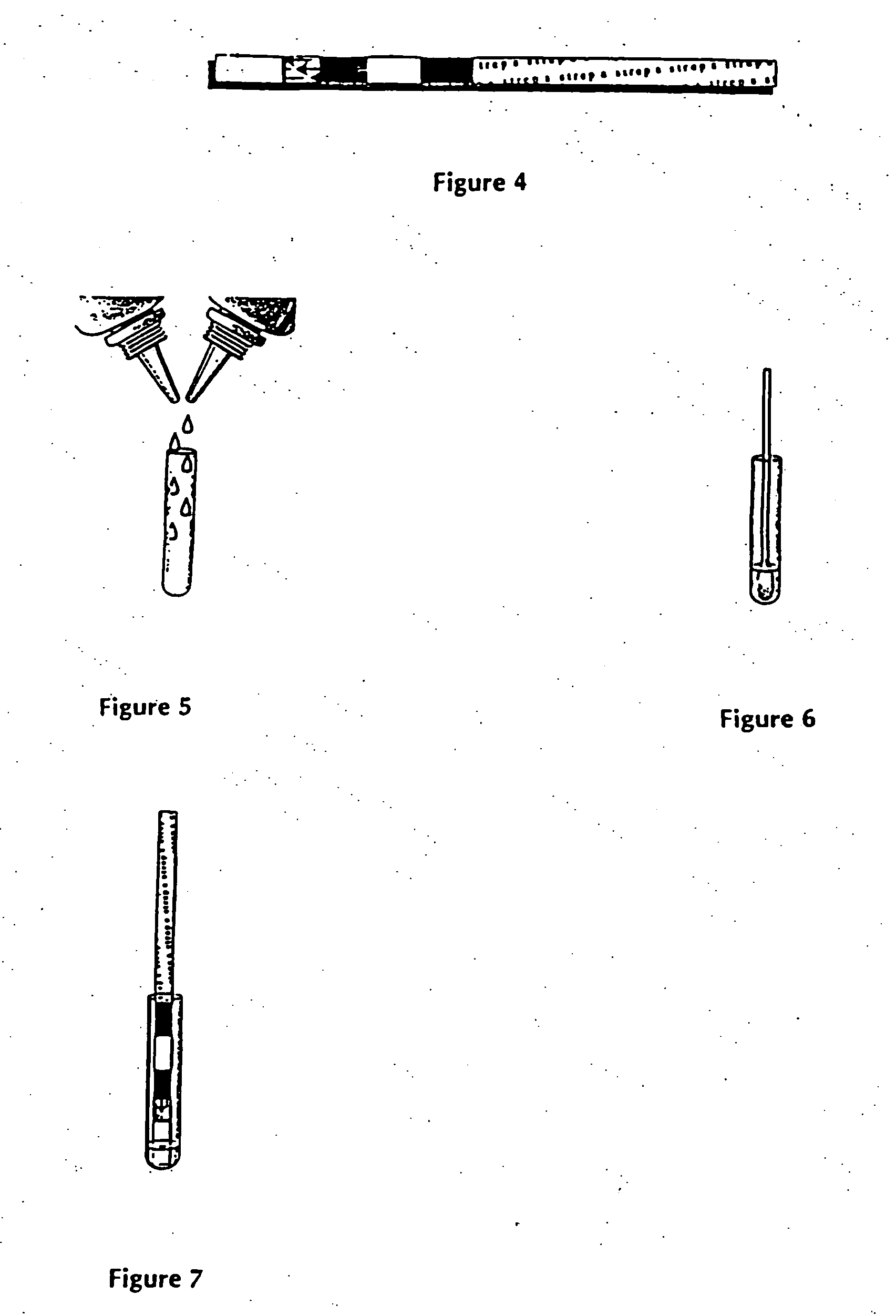
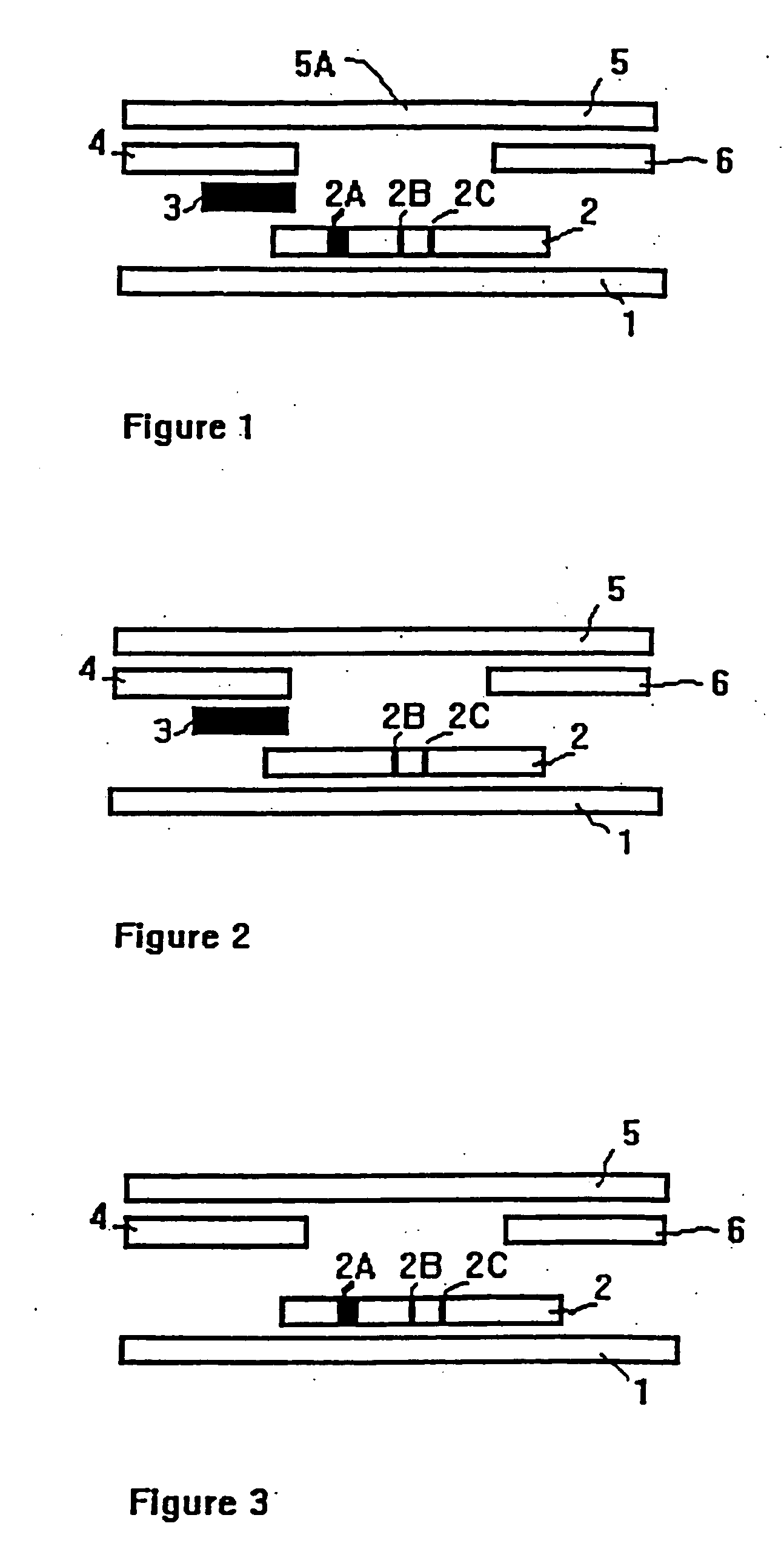
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:SEKISUI DIAGNOSTICS
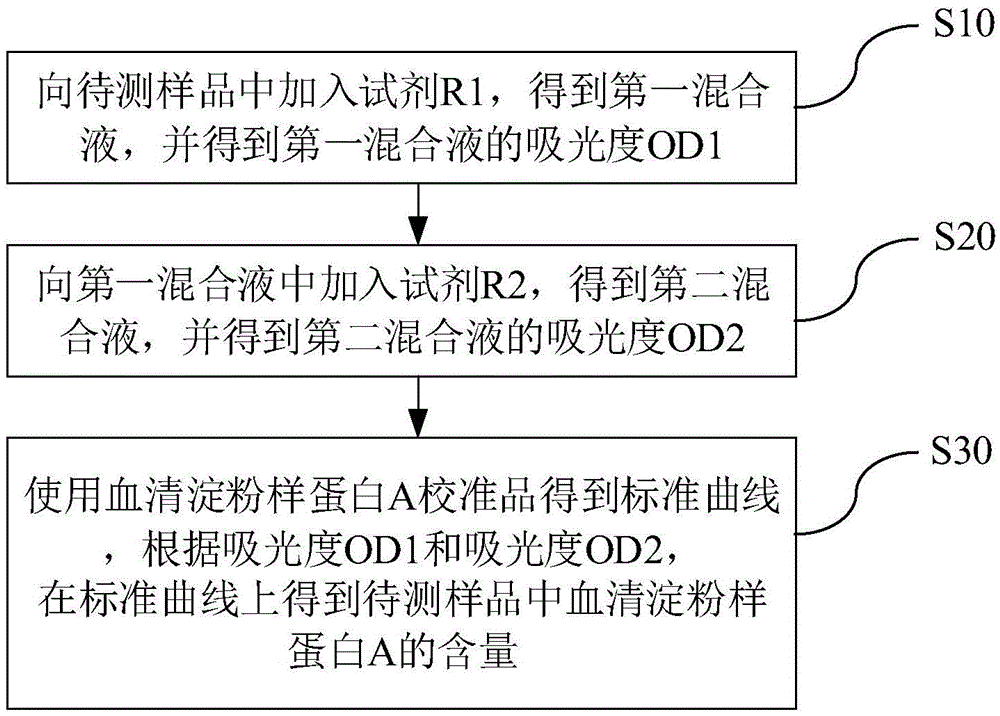
Kit and method for detecting content of serum amyloid protein A and application
InactiveCN105548571AEasy to useDetection is simple and fastBiological material analysisBiological testingPreservativeAmyloid A Protein
The invention discloses a kit for detecting the content of a serum amyloid protein A. The kit comprises a reagent R1, a reagent R2 and a serum amyloid protein A calibrator, wherein the reagent R1 comprises a first buffer solution, a first electrolyte, a surfactant, a first stabilizer, a high-molecular accelerant and a first preservative; the reagent R2 comprises a second buffer solution, anti-human serum amyloid protein A antibody coated latex particles, a second electrolyte, a second stabilizer and a second preservative; the serum amyloid protein A calibrator comprises a third buffer solution, a third stabilizer, a third preservative, an antioxidant and an anti-human serum amyloid protein A antigen. The invention further discloses an application of the kit for detecting the content of the serum amyloid protein A to the detection of the content of the serum amyloid protein A and a method for detecting the content of the serum amyloid protein A by adopting the kit for detecting the content of the serum amyloid protein A. By adopting the kit and the method disclosed by the invention, the content of the serum amyloid protein A can be detected easily and rapidly.
Owner:潍坊三维生物工程集团有限公司
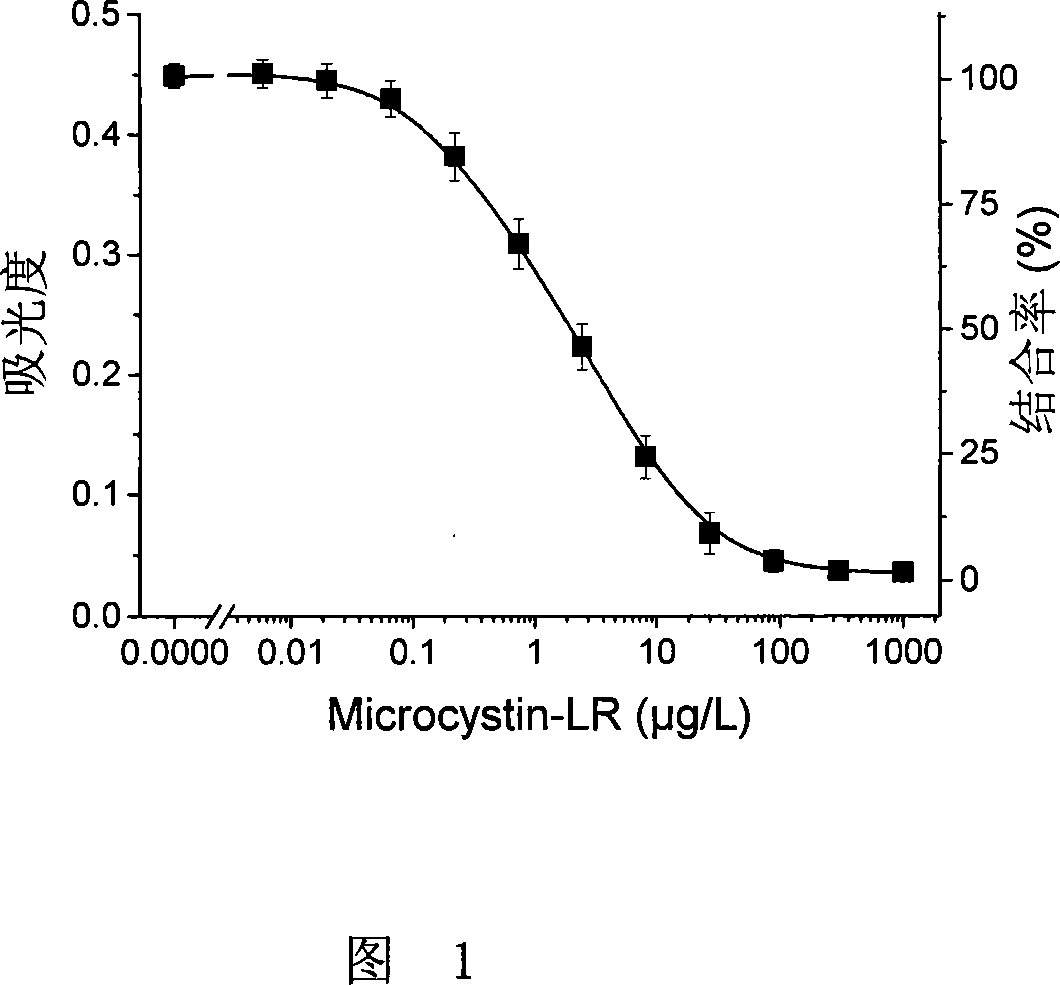
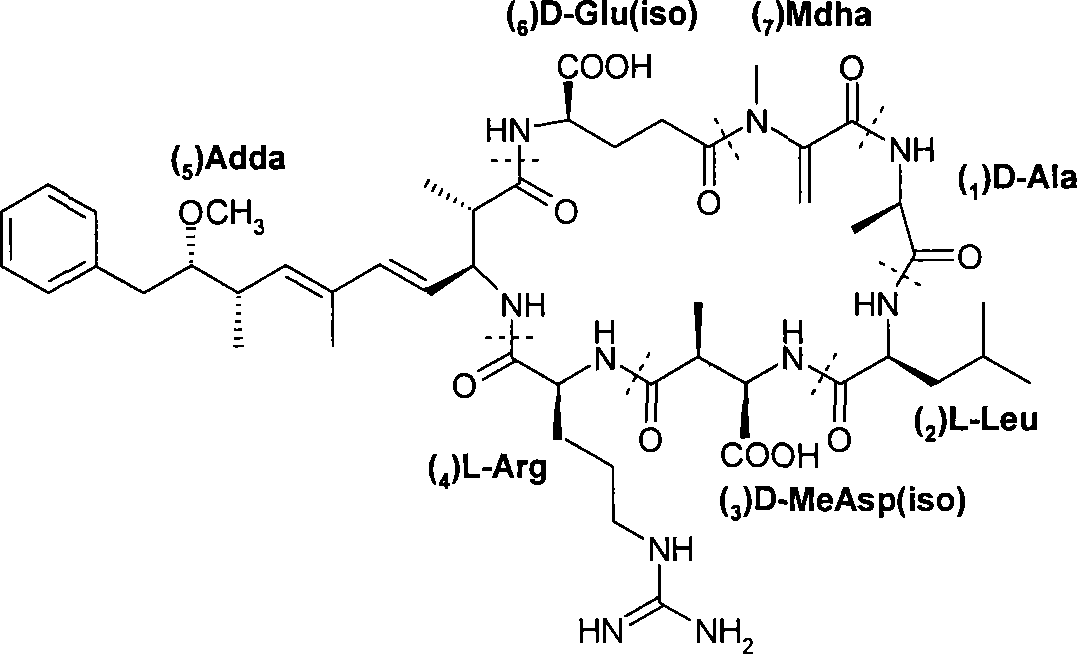
Microcystin monoclonal antibody and its preparation method and application
ActiveCN101125889AUniform textureImprove stabilityImmunoglobulins against animals/humansTissue cultureMicrocystinSpleen cell
The invention discloses a microcystlin monoclonal antibody and the preparation method and the application thereof. The microcystlin monoclonal antibody is a microcystlin-LR monoclonal antibody, and the preparation method thereof is that: 1) an amino group is introduced to a seventh amino acid residue N-methyl dehydroalanine on microcystlin-LR, thus obtaining amino modified microcystlin-LR polypeptide; the amino modified microcystlin-LR polypeptide is coupled with carrier protein to obtain complete A antigen; 2) the immunity mouse of complete A antigen obtained in step 1) to get spleen cell of immunity mouse, which then is fused with myeloma cell of mouse to screen out positive hybrid cancer cell strain; the positive hybrid cancer cell strain is cultured or is injected in sygeneous mouse abdominal cavity to induce ascites to get the microcystlin-LR monoclonal antibody. The monoclonal antibody can be used for examining microcystlin-LR and immunity affinity purification thereof.
Owner:TSINGHUA UNIV
SYNTHESIS OF HUMAN SECRETORY IgA AND IgM AND THE FORMATION OF A MEDICAMENT THEREFROM
A composition for treating a subject is provided. The composition includes antigen specific dimeric secretory IgA and pentameric IgM therapeutic. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the modification of antigen specific dimeric secretory IgA and pentameric IgM with secretory component to form a antigen specific dimeric secretory IgA and pentameric secretory IgM therapeutic. The antigen specific dimeric secretory IgA and the pentameric secretory IgM therapeutic is then mixed with formulating agents to create a capsule, tablet, liquid or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R +2
Repertoire of allo-restricted peptide-specific t cell receptor sequences and use thereof
InactiveUS20120128704A1Effectively minimize immune selectionEasy to useOrganic active ingredientsBiocideDiseaseTyrosinase
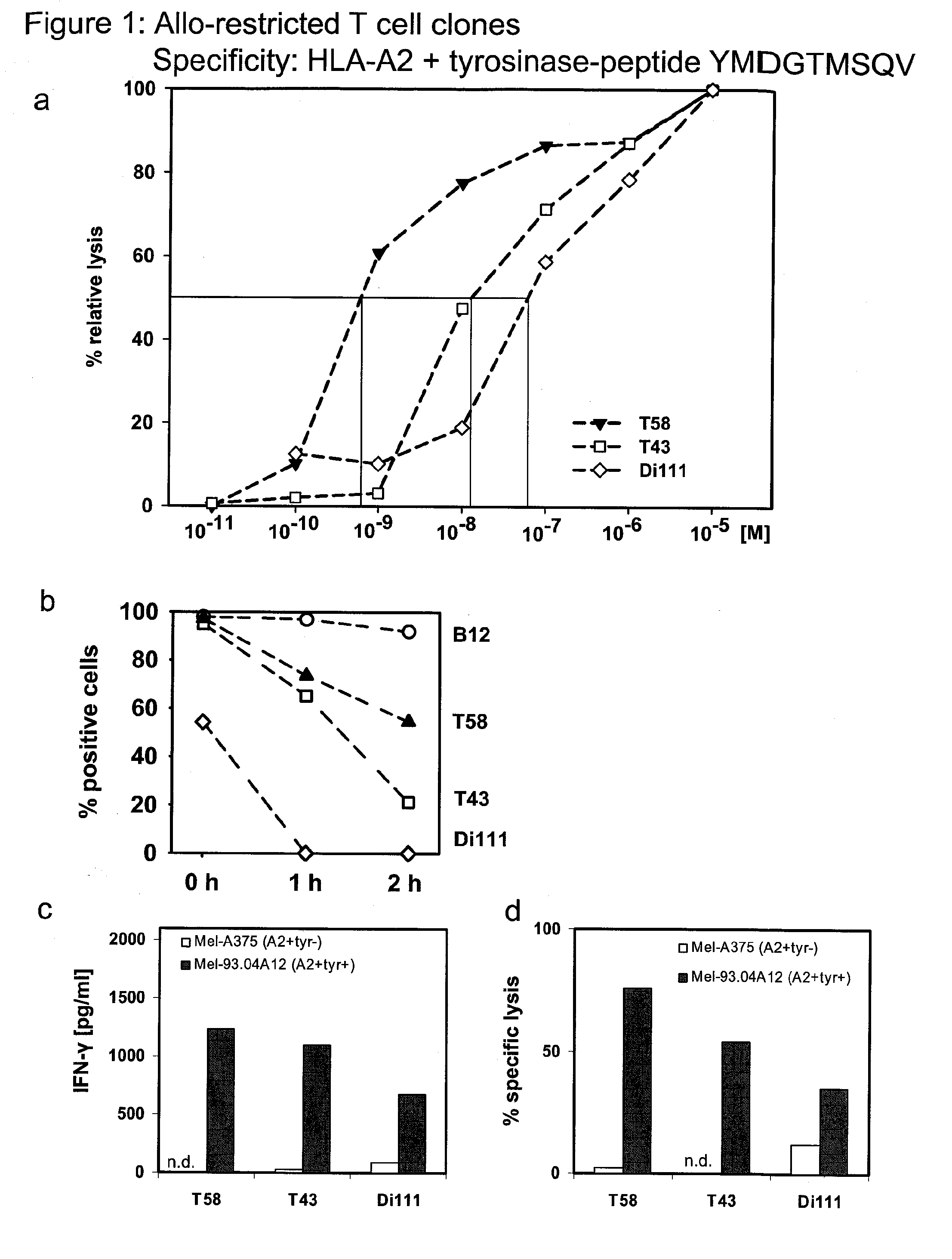
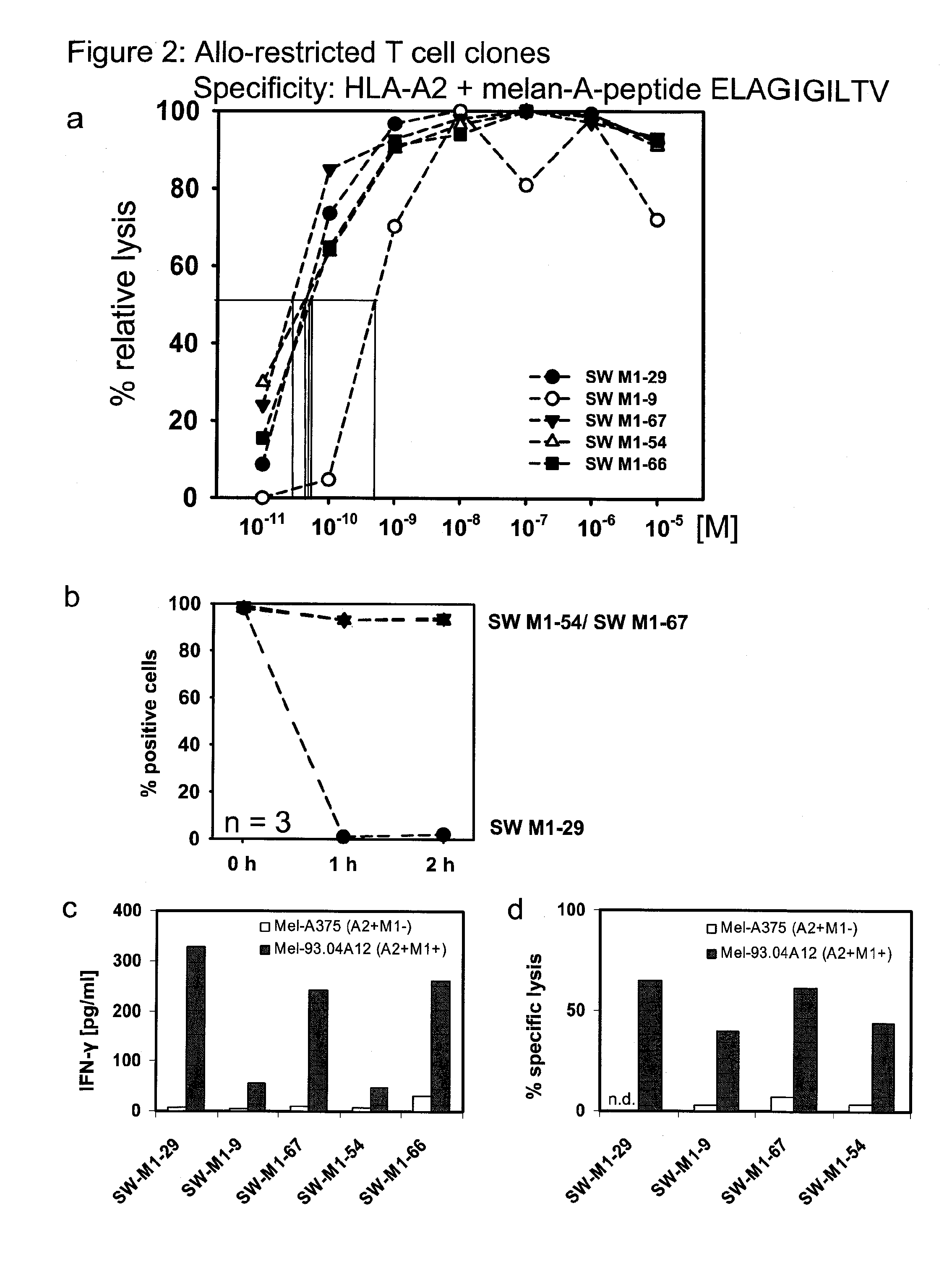
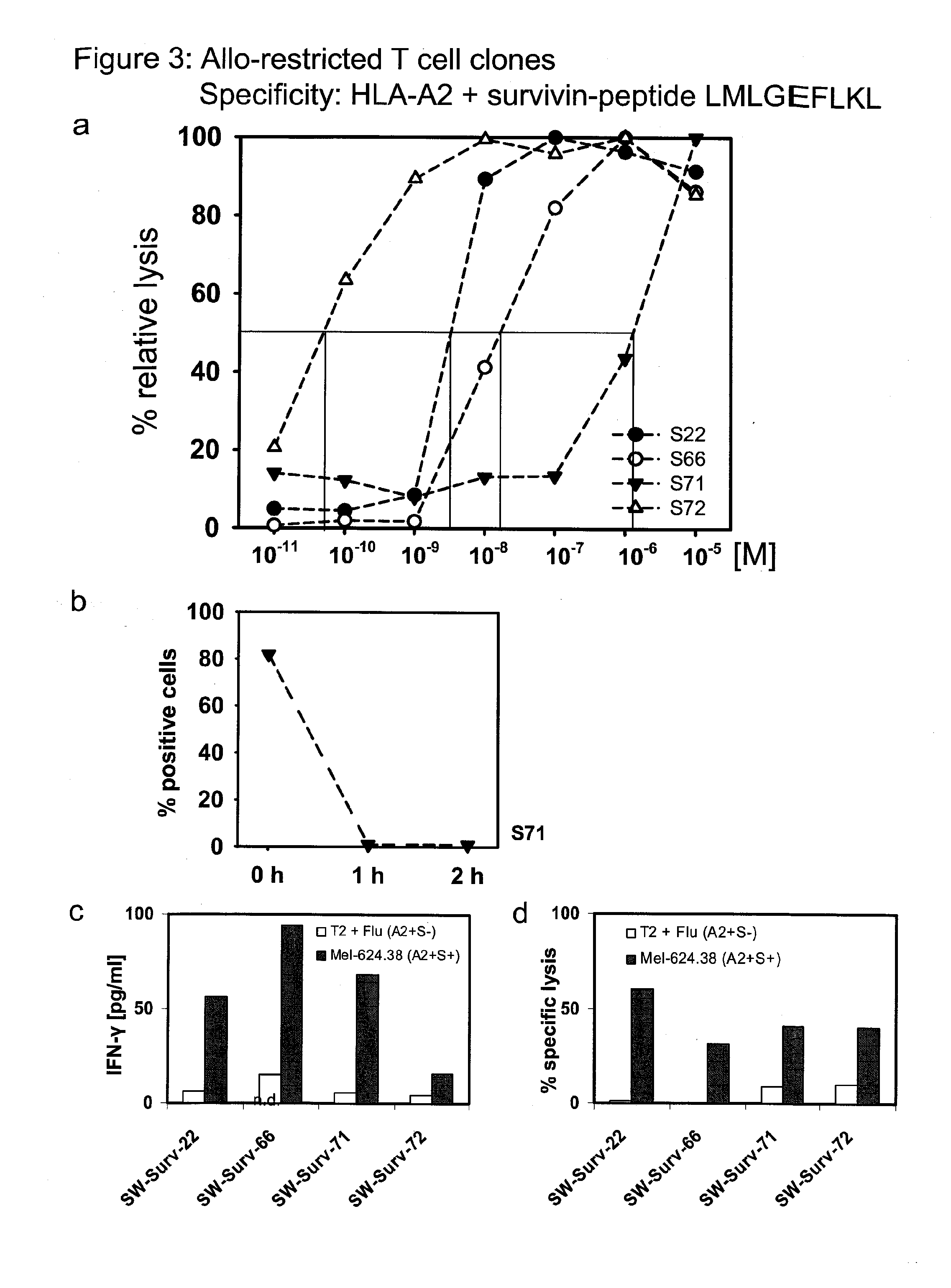
The present invention is directed to a kit-of-parts or composition containing nucleic acid sequences coding for high-avidity, allo-restricted TCR, wherein the TCR are independently directed against the tyrosinase antigen, the melan-A antigen and the survivin antigen. The invention is further directed to a kit-of-parts or composition containing at least three groups of transgenic lymphocytes transformed with vectors coding for TCR against said antigens. Furthermore, the present invention provides a pharmaceutical composition and its use in the treatment of diseases involving malignant cells expressing said tumor-associated antigens. The invention further relates to a nucleic acid molecule coding for a TCR that recognizes the survivin antigen, a TCR encoded thereby and a T cell expressing said TCR. Further, the invention discloses a vector, a cell and a pharmaceutical composition encoding / containing same and their use in the treatment of diseases involving malignant cells expressing survivin.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT +1
Synthesis of human secretory IgA and IgM and the formation of a medicament therefrom
A composition for treating a subject is provided. The composition includes antigen specific dimeric secretory IgA and pentameric IgM therapeutic. A process for manufacturing a medicament for the treatment of C. difficile associated disease in a human is also provided that the modification of antigen specific dimeric secretory IgA and pentameric IgM with secretory component to form a antigen specific dimeric secretory IgA and pentameric secretory IgM therapeutic. The antigen specific dimeric secretory IgA and the pentameric secretory IgM therapeutic is then mixed with formulating agents to create a capsule, tablet, liquid or suppository dosing form. The therapeutic is amenable to enrobement directly through microencapsulation or the dosing form is coated with an enteric coating. A method of C. difficile treatment with the therapeutic is also provided that is amenable to supplementation with concurrent or prior antibiotic administration.
Owner:SIMON MICHAEL R +2
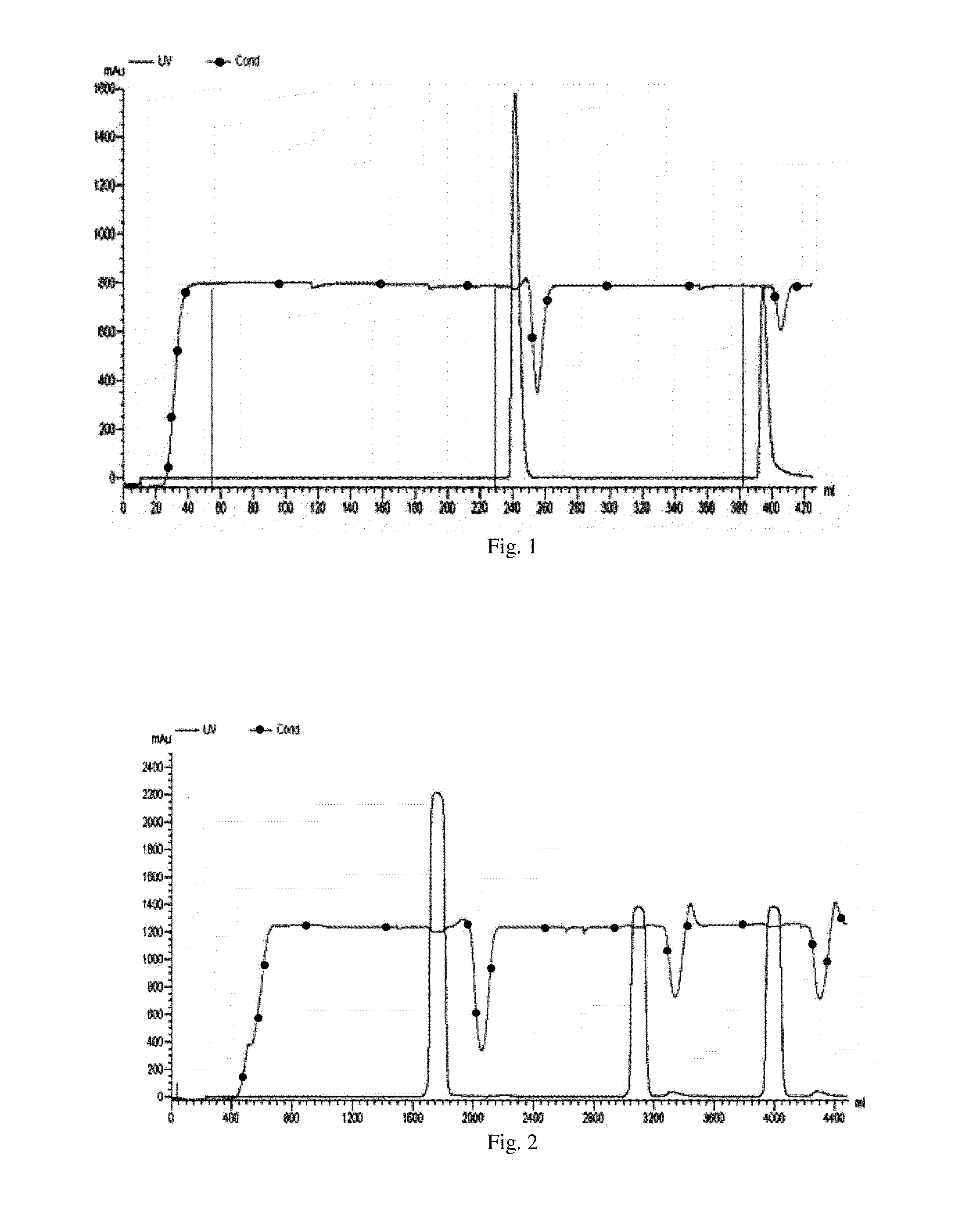
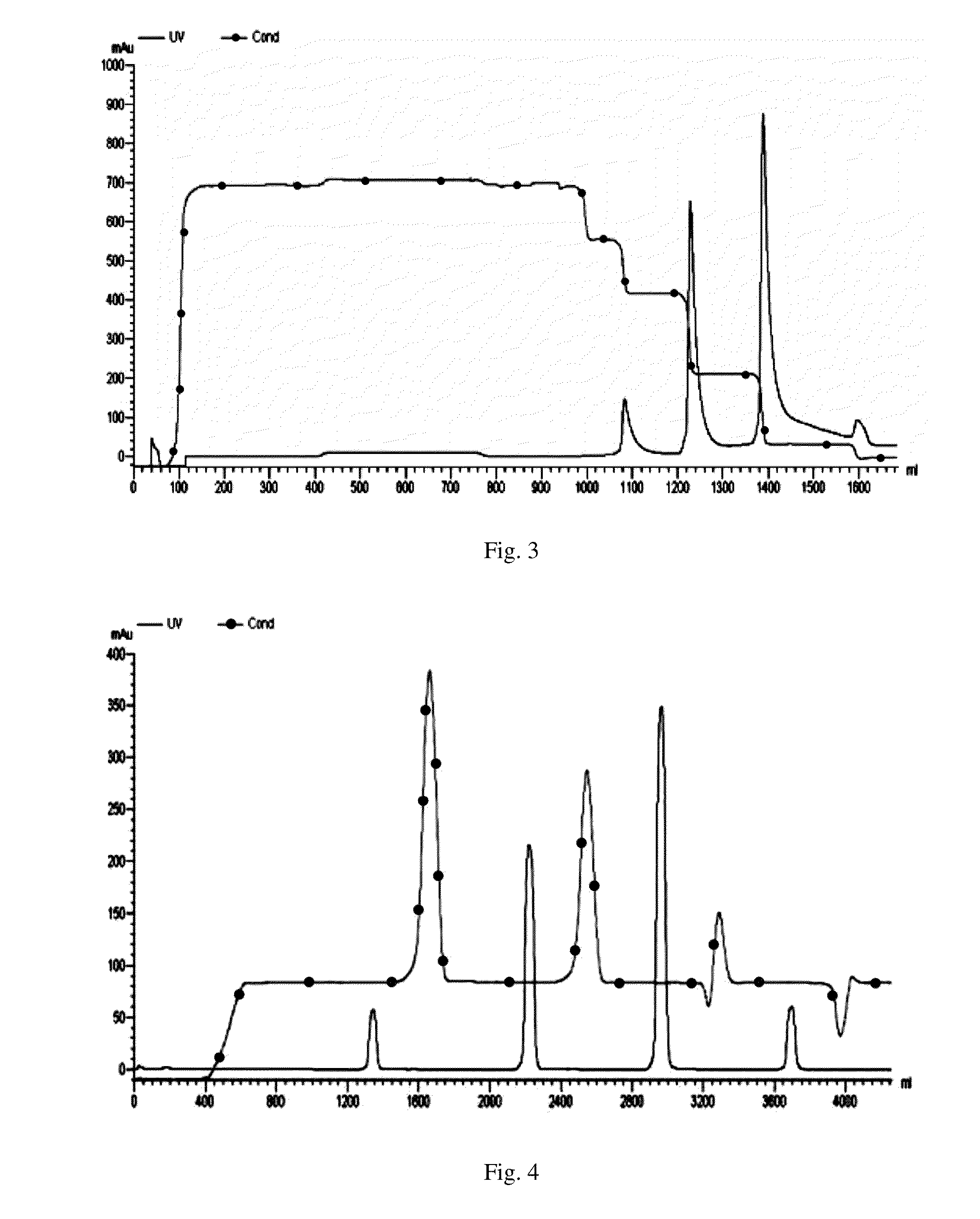
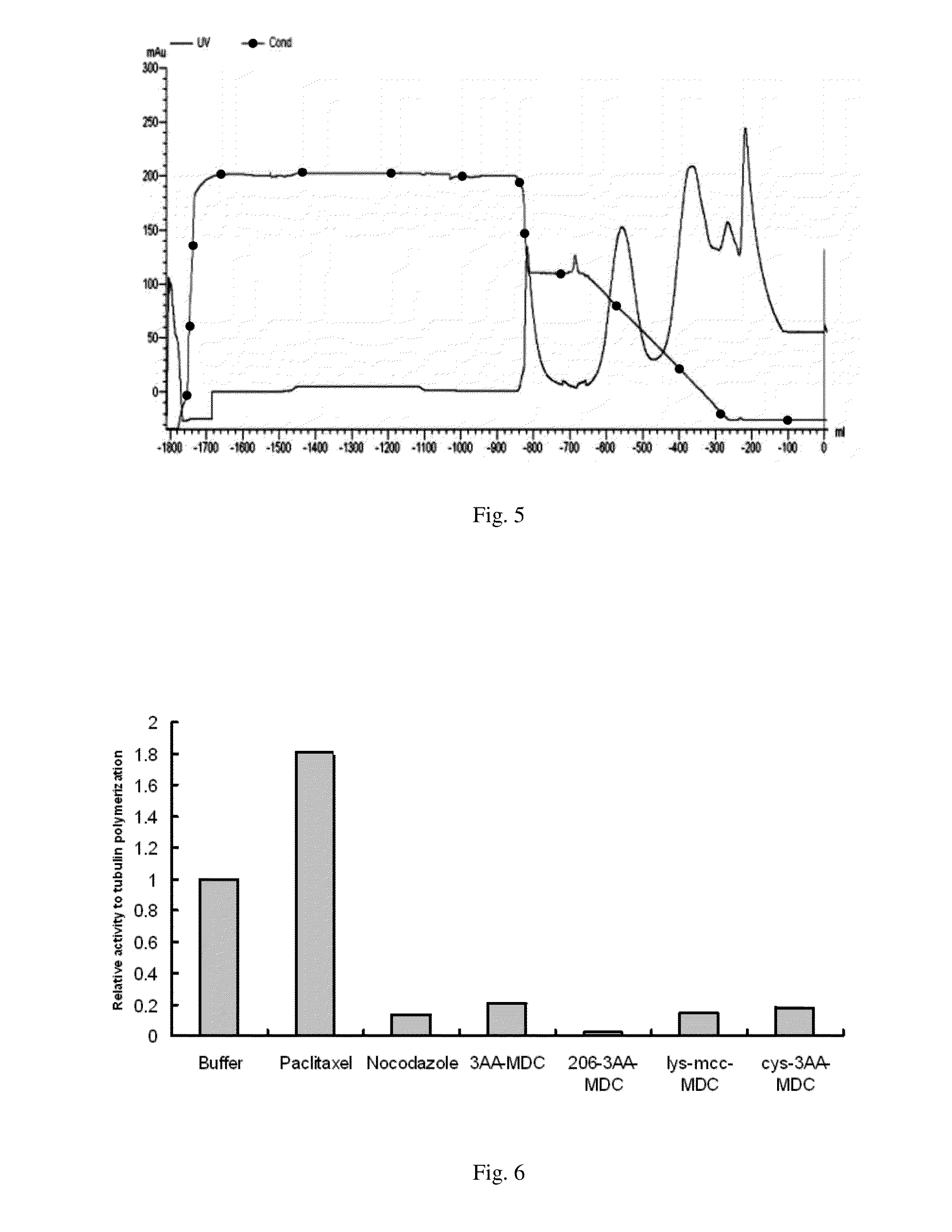
Maytansinoid derivatives
ActiveUS8877706B2Prevent relapseUndesirable side-effectOrganic active ingredientsPeptide/protein ingredientsDiseaseMedicine
Disclosed herein are maytansinoid drug linker derivatives which can be linked to a antigen binding unit (Abu), and maytansinoid drugs linked with an antigen binding unit (Drug-Linker-Antigen binding Unit: D-L-Abu), for targeted delivery to disease tissues. D-L-Abu, D-L-Abu derivatives, and methods relating to the use of such drug conjugates to treat antigen positive cells in cancers and immunological disorders are provided.
Owner:BIO THERA SOLUTIONS LTD
Method of use of one step immunochromatographic device for streptococcus a antigen
InactiveUS20060154315A1Reduce the numberLess manipulationBiological testingImmunoassaysReagentSTREPTOCOCCUS ANTIGEN
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:GENZYME CORP
Methods of use of one step immunochromatographic device for streptococcus a antigen
InactiveUS20050277163A1Avoid lateral flowReduce the numberBiological testingImmunoassaysReagentSTREPTOCOCCUS ANTIGEN
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:GENZYME CORP
Method and kit for typing feline blood
InactiveUS6830895B2The method is simple and fastReduce needAnalysis using chemical indicatorsImmunoglobulins against blood group antigensMonoclonal antibodyGroup A - blood
Owner:KANSAS STATE UNIV RES FOUND
Senile dementia recombinant adenovirus gene vaccine and preparation method thereof
ActiveCN101357227AHigh molecular weightAvoid spatially rigid structuresNervous disorderPeptide/protein ingredientsChemical synthesisAmyloid
The invention discloses senile dementia Ad-GFP gene vaccine and a preparation method theerof and is Ad-GFP vaccine of flexible foldable multivalence Beta- amyloid 1-15 (ABeta1-15) of secretion co-expression and genetic adjuvant, which a flexible connecting peptide is used for connecting multivalence. The invention selects foldable multivalence ABeta1-15 as immunogen, which avoids the space rigid structure of multiple copy ABetaB cell epitopes, also ensures that ABeta15 epitopes are more easily exposed, thus enhancing immunogenicity and also increasing immunogenic molecular weight and reducing the possibility of degradation; a antigen delivery system that takes adenovirus as a carrier is selected, which avoids the defects of high challenge and high cost of chemosynthesis.
Owner:LIVZON PHARM GRP INC
Method for the production of antibodies
InactiveUS20070266448A1Immunoglobulins against animals/humansBlood/immune system cellsHuman animalLiver Stem Cell
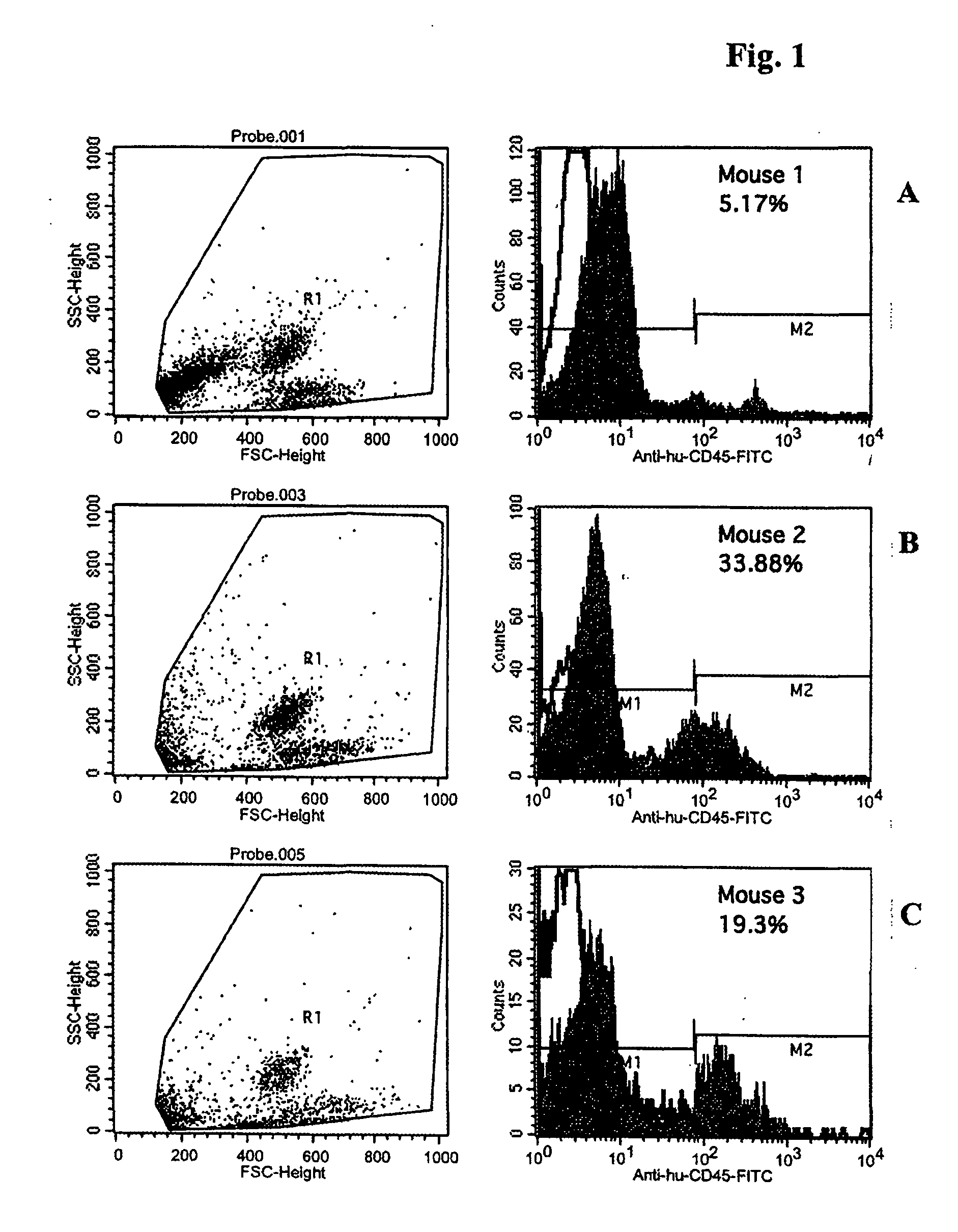
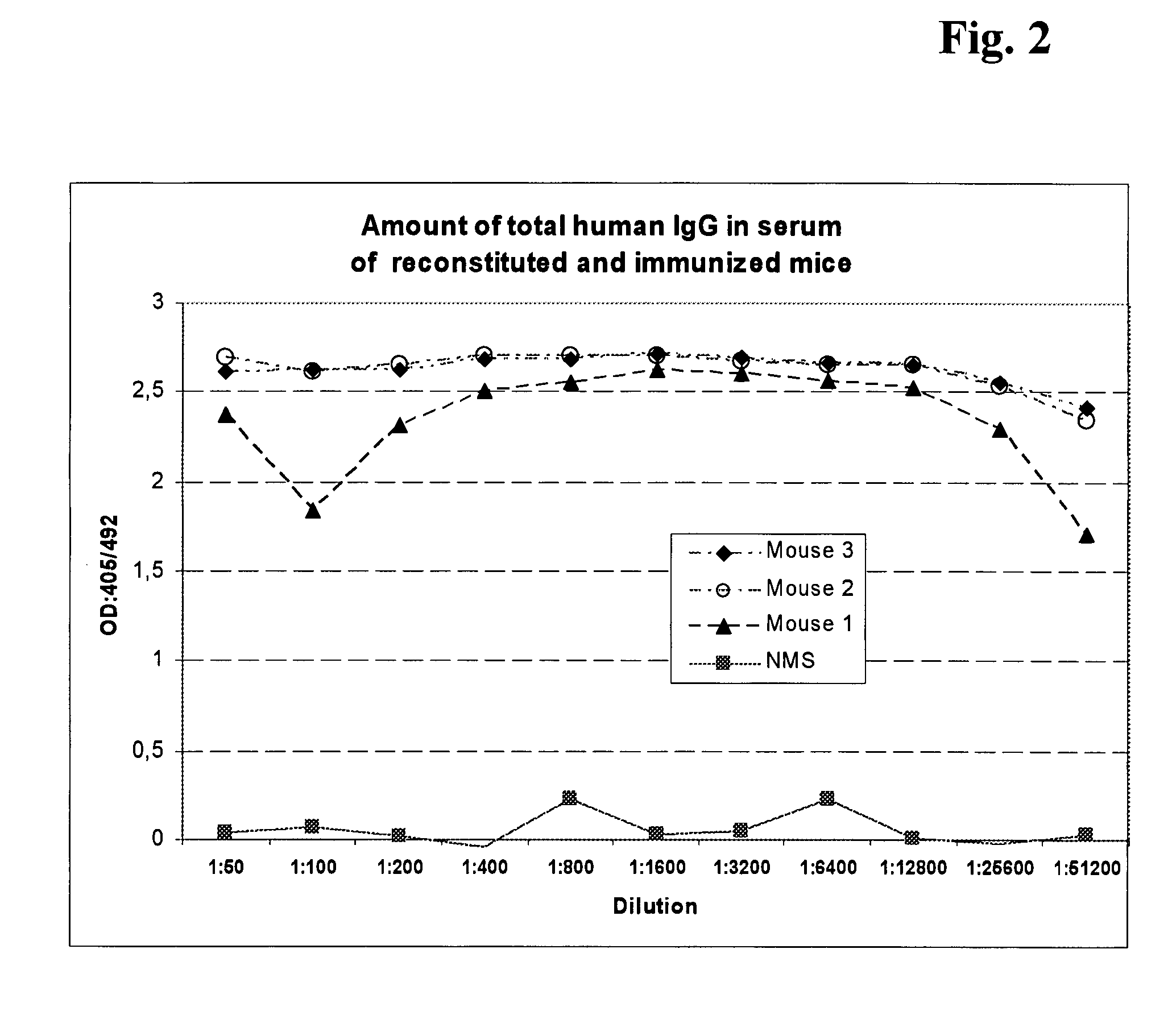
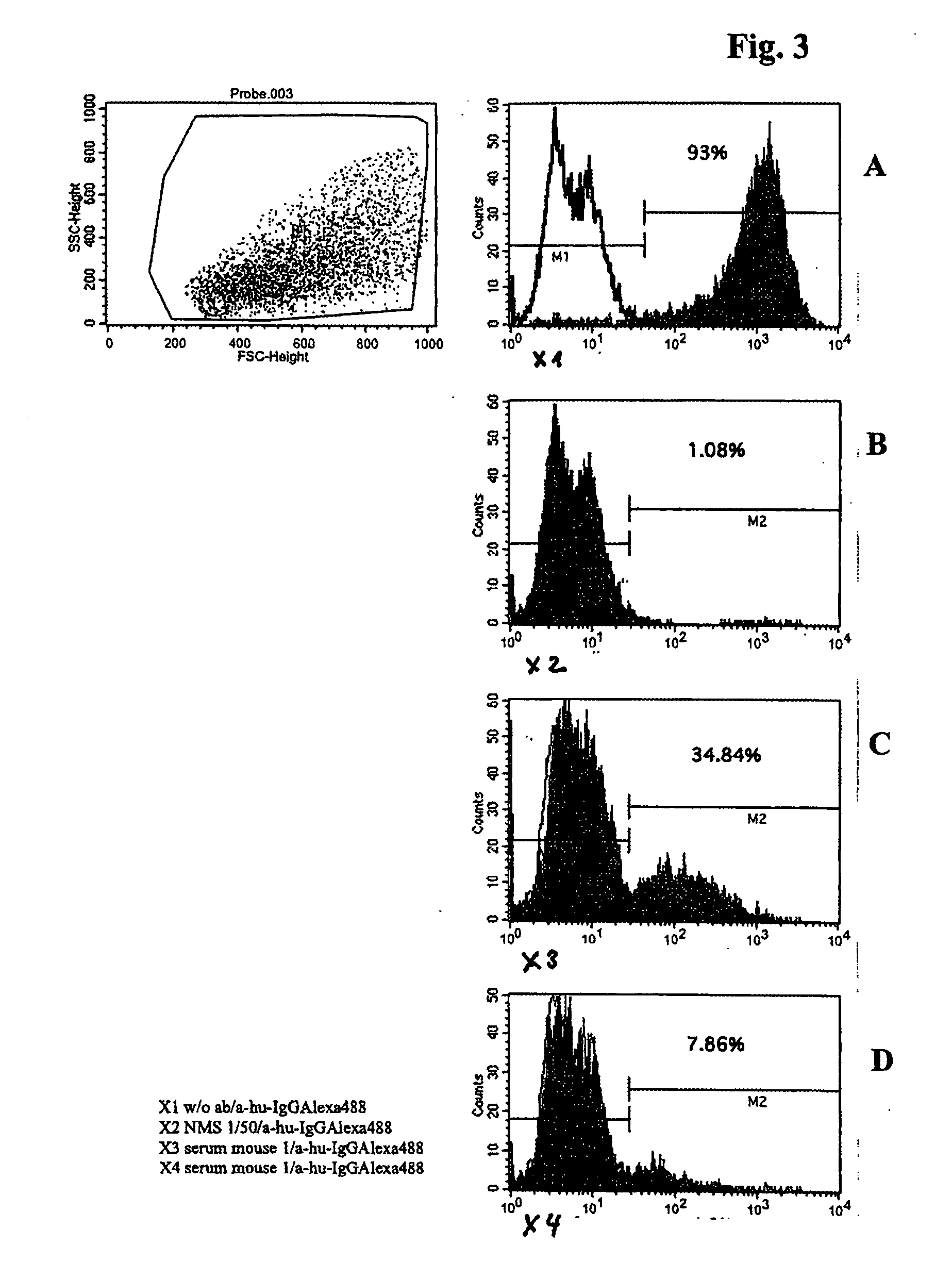
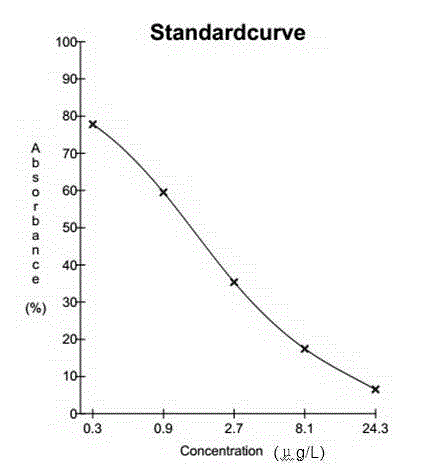
The current invention is related to a method for the production of a human monoclonal antibody from a immundeficient non-human animal, said method comprising contacting a new borne immunodeficient non-human animal with a human fetal liver stem cell (FL cell) to generate an immune transplanted non-human animal (reconstituted animal), subsequently contacting said reconstituted animal with a antigen, collecting from said reconstituted animal a human cell producing human antibody against said antigen, and isolating said antibody from said antibody producing cell.
Owner:F HOFFMANN LA ROCHE & CO AG
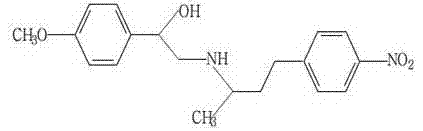
Phenylethanolamine A hapten, antigen as well as preparation method and application thereof
InactiveCN105272866AHigh yieldThe preparation method is simple and feasibleOrganic compound preparationOvalbuminElisa kitCarrier protein
The invention discloses a phenylethanolamine A hapten, a related artificial antigen and a monoclonal antibody; the invention simultaneously discloses preparation methods and applications of the phenylethanolamine A hapten, the related artificial antigen and the monoclonal antibody. The phenylethanolamine A hapten is the product shown in the formula 1, and the product shown in the formula 1 and a carrier protein can be connected to obtain a phenylethanolamine A antigen. The phenylethanolamine A antigen can be used for preparing a phenylethanolamine A specific antibody. The preparation methods have the advantages of simple and practicable operation, low cost and high yield of the hapten. The phenylethanolamine A artificial antigen can be used for producing the specific antibody aiming at the phenylethanolamine A by immune animals, and can be used for preparing an ELISA kit for detecting phenylethanolamine A residuals; the kit has the advantages of simple and rapid operation, large sample treatment amount, high sensitivity, good specificity, etc.
Owner:北京维德维康生物技术有限公司
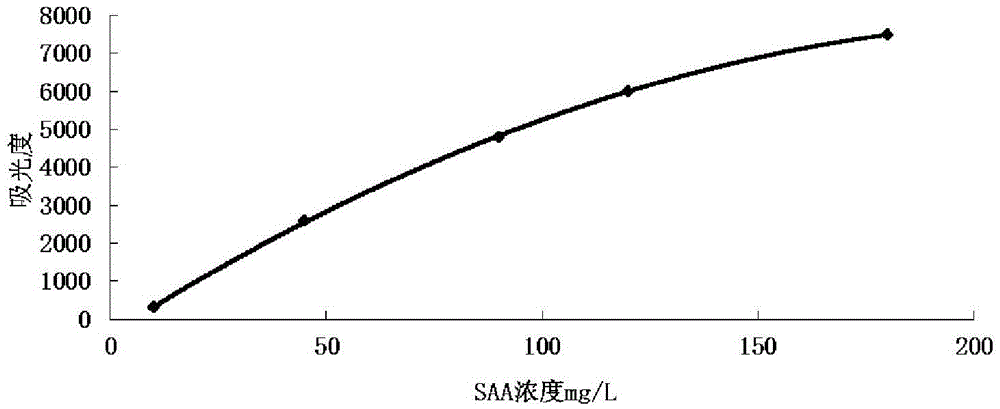
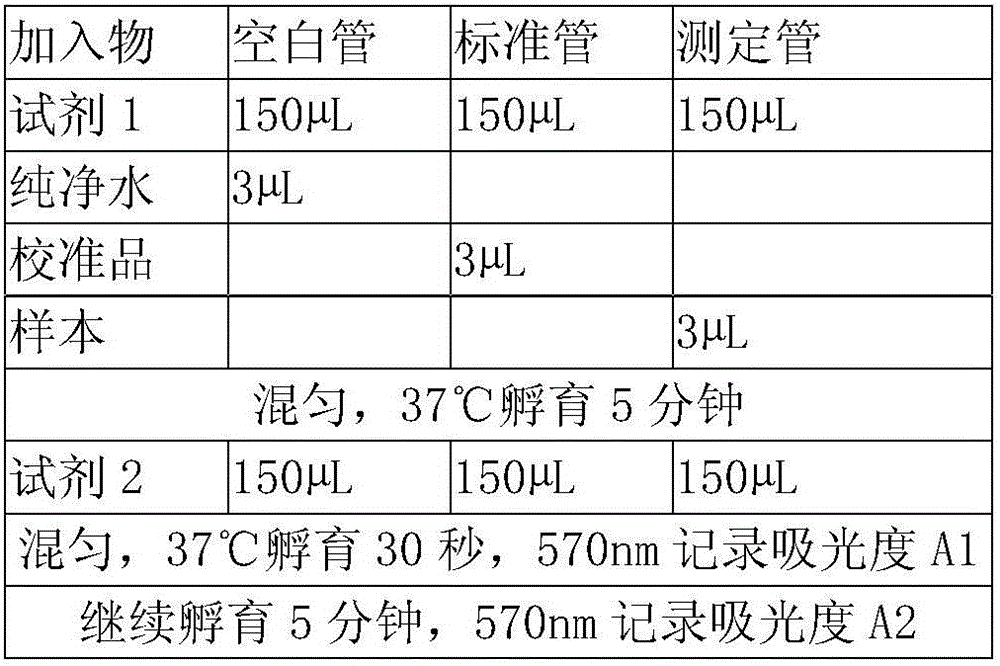
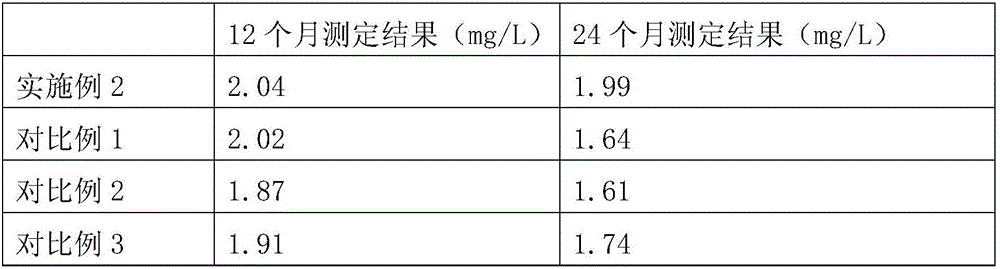
Serum amyloid a (saa) assay kit
ActiveCN106053862BThe measurement result is accurateHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingAssayA Antibody
The invention relates to a serum amyloid protein A (SAA) measuring kit, and a measuring method thereof, and belongs to the technical field of medicine biological detection. The kit comprises a reagent (1), a reagent (2), a calibration material, and a quality control material. The reagent (1) comprises Tris-HCl, sodium azide, and PEG-6000. The reagent (2) comprises Tris-HCl, emulsion particles with an embedded anti-human serum amyloid protein A antibody, sodium azide, octyl phenol polyoxyethylene, and methyl paraben. The calibration material comprises a serum amyloid protein A antigen, and sodium azide. The quality control material comprises a serum amyloid protein A antigen, and sodium azide. The provided serum amyloid protein A (SAA) measuring kit and measuring method thereof have the advantages that compared with the latex immunological turbidimetry technology, the analysis sensitivity is high, the results are accurate, the operation is simple, and the cost is proper, and thus the method is convenient for popularization.
Owner:河北艾驰生物科技有限公司
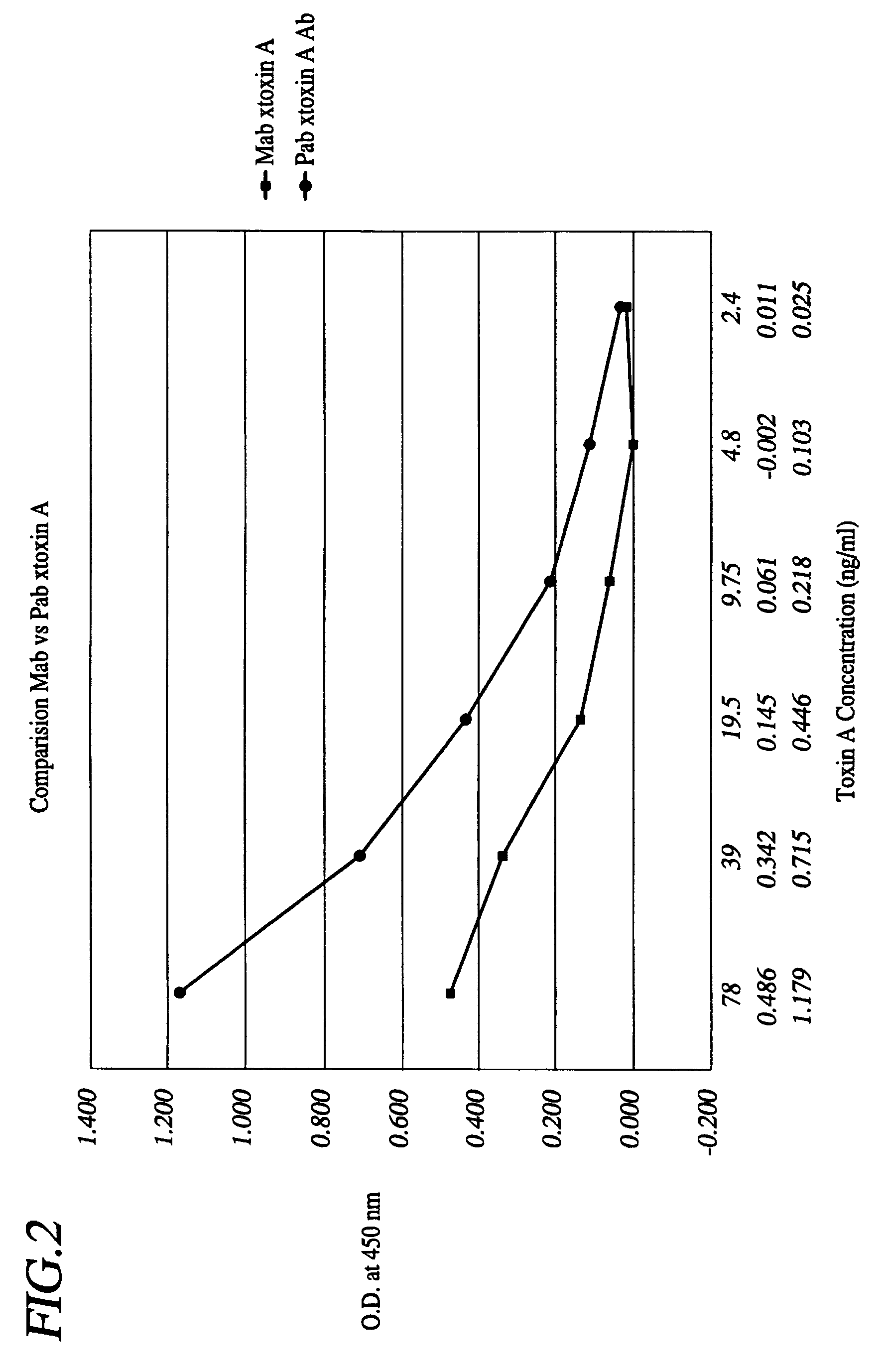
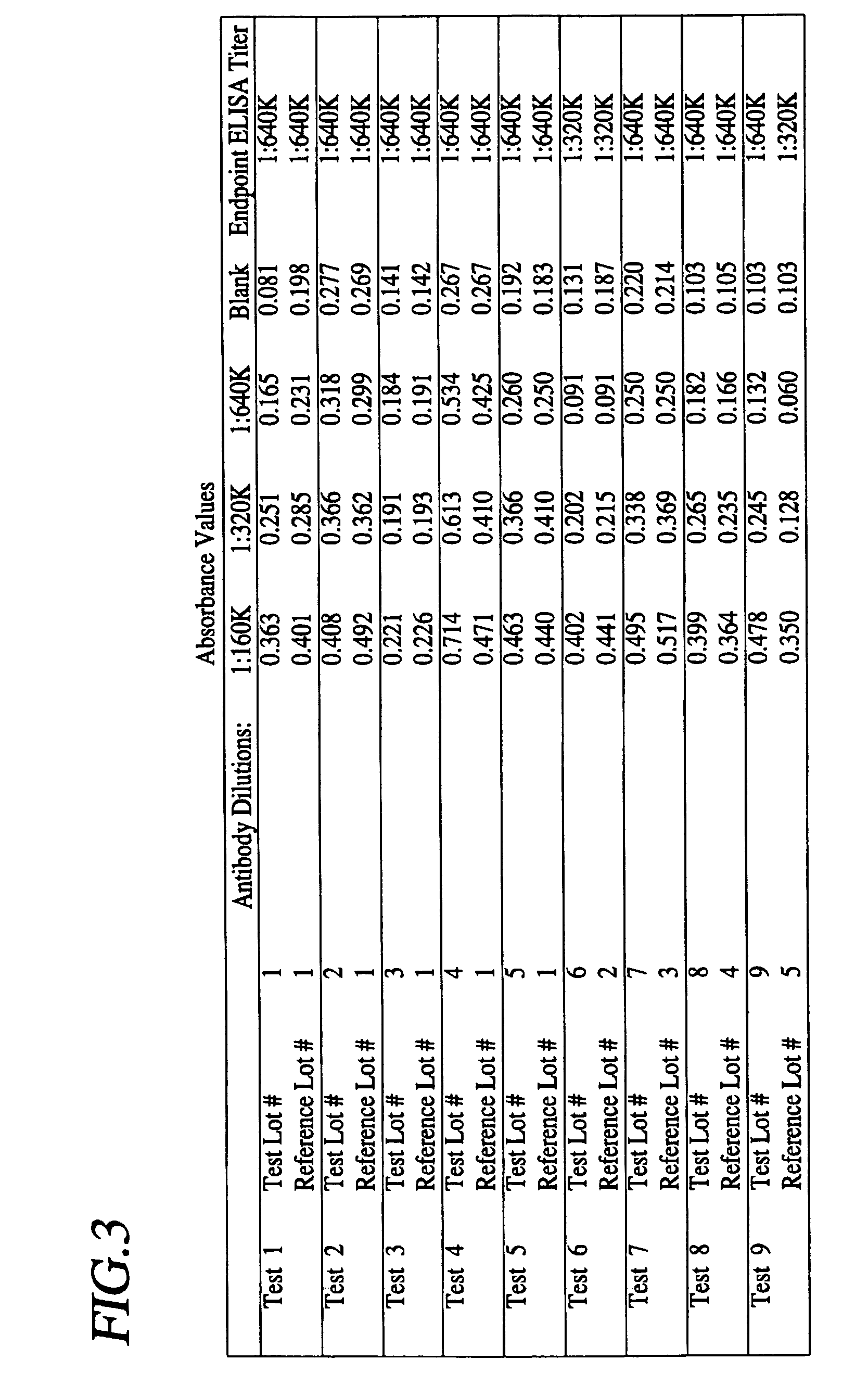
Mono-specific polyclonal antibodies and methods for detecting Clostridium difficile Toxin A
InactiveUS7179611B2High sensitivityProduct can be usedImmunoglobulins against bacteriaPeptide preparation methodsClostridial toxinImmobilized Antibodies
A method is provided for the purification of Clostridium difficile Toxin A antigen comprising reacting impure Toxin A with immobilized mono-specific polyclonal antibodies. The polyclonal antibodies are coupled to a hydrazide group containing matrix such as hydrazide activated agarose gel. The immobilized antibody is specific for Toxin A and will greatly purify Toxin A from a Toxin A containing solution. Antibodies raised to Toxin A purified according to the method are of higher activity than antibodies produced from prior art purified Toxin A.
Owner:BECTON DICKINSON LEE LAB
Serum amyloid protein A (SAA) measuring kit
ActiveCN106053862AThe measurement result is accurateHigh detection sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingTurbidimetrySerum amyloid protein
The invention relates to a serum amyloid protein A (SAA) measuring kit, and a measuring method thereof, and belongs to the technical field of medicine biological detection. The kit comprises a reagent (1), a reagent (2), a calibration material, and a quality control material. The reagent (1) comprises Tris-HCl, sodium azide, and PEG-6000. The reagent (2) comprises Tris-HCl, emulsion particles with an embedded anti-human serum amyloid protein A antibody, sodium azide, octyl phenol polyoxyethylene, and methyl paraben. The calibration material comprises a serum amyloid protein A antigen, and sodium azide. The quality control material comprises a serum amyloid protein A antigen, and sodium azide. The provided serum amyloid protein A (SAA) measuring kit and measuring method thereof have the advantages that compared with the latex immunological turbidimetry technology, the analysis sensitivity is high, the results are accurate, the operation is simple, and the cost is proper, and thus the method is convenient for popularization.
Owner:河北艾驰生物科技有限公司
Enzyme-linked immunoabsorbent kit for detecting serum amyloid protein A as well as preparation method and application of kit
InactiveCN106771257ARapid determinationQuantitative determinationBiological testingAbzymeAntiendomysial antibodies
The invention discloses an enzyme-linked immunoabsorbent kit for detecting serum amyloid protein A as well as a preparation method and an application of the kit and belongs to the field of analytical test. The kit comprises an ELISA plate coated by a monoclonal antibody of a cow serum amyloid protein A, a cow serum amyloid protein A antigen standard substance, a cow serum amyloid protein A monoclonal antibody, an enzyme labeled goat anti-rat antibody, a diluent, a scrubbing solution, a developing solution and a stop buffer. The content of the serum amyloid protein A in milk is measured by a double-antibody sandwiched indirect enzyme-linked immunosorbent assay method so as to diagnose bovine subclinical mastitis. The kit disclosed by the invention is easy and convenient to operate, has the characteristics of high specificity, high sensitivity and high reproducibility and provides a novel testing tool for detection of the bovine subclinical mastitis.
Owner:BEIJING MEIZHENG BIOTECHNOLOGY CO LTD
Method and kit for typing feline blood
InactiveUS20030092088A1Easy to produceExpand the populationAnalysis using chemical indicatorsImmunoglobulins against blood group antigensMonoclonal antibodyGroup A - blood
The present invention relates to a kit for determining feline blood type, wherein the kit includes a mixture comprised of a first monoclonal antibody and a second monoclonal antibody, wherein both antibodies recognize feline blood group specific A antigens. The present invention also relates to a method for determining feline blood type, wherein the method utilizes two distinct monoclonal antibodies, which recognize feline blood group specific A antigens.
Owner:KANSAS STATE UNIV RES FOUND
Serum amyloid protein A (SAA) antigen epitope and predication and verification method thereof as well as preparation method of monoclonal antibody
InactiveCN109678945AOvercoming the problem of low prediction accuracySolve unknown problemsImmunoglobulins against animals/humansTissue cultureLinear epitopeAmyloid A Protein
The invention relates to a predication and verification method of a serum amyloid protein A (SAA) antigen epitope and a preparation method of a monoclonal antibody. The predication and verification method comprises the following steps: predicating surface accessibility, an flexibility sequence region, a beta turn, antigenicity, hydrophilicity, a linear epitope and a long-fragment epitope of a serum amyloid protein A antigen to obtain a plurality of epitope sequences respectively; removing repeated epitopes in the plurality of epitope sequences and other sequences contained in other relativelylong epitopes to obtain a plurality of non-redundant predication epitopes; and verifying whether the plurality of non-redundant predication epitopes are identification epitopes of the antibody or notby adopting ELISA (Enzyme Linked Immunosorbent Assay). According to the invention, the problem that the predication accuracy of a single method is low is overcome; through experimental verification, the problem that the SAA antigen epitope is unknown is solved; and the monoclonal antibody is prepared by applying synthesized antigen epitope polypeptide, so that the problem that SAA is difficult topurify and obtain is overcome and the cost is reduced by one time or more.
Owner:迪亚莱博(张家港)生物科技有限公司
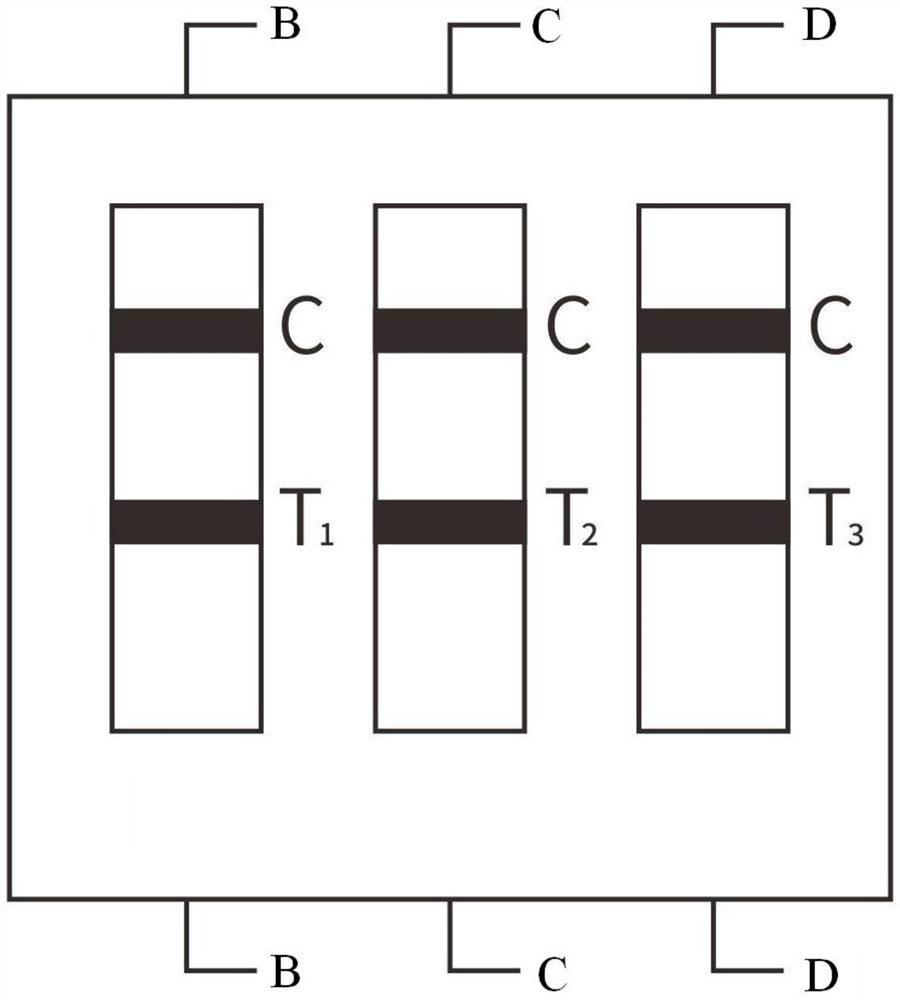
Influenza virus and 2019 new coronavirus antibody combined detection device and preparation method thereof
The invention relates to an influenza virus and 2019 novel coronavirus antibody combined detection device and a preparation method thereof and belongs to the field of medical detection equipment. Thekit is prepared by adhering a nitrocellulose film, a glass fiber film, a sample pad, absorbent paper and other auxiliary materials, wherein the nitrocellulose film contains an influenza virus A antigen, an influenza virus B antigen, a novel coronavirus S antigen and a goat-anti-mouse IgG polyclonal antibody in a solid phase, and the glass fiber film absorbs a high-specificity anti-human IgM antibody. On the basis of ensuring complete release of the immune colloidal gold, the reaction sensitivity is effectively improved, under the same threshold value, the dosage of the immune colloidal gold can be reduced, cost is saved, the influenza virus A, the influenza virus B and novel coronavirus IgM antibodies in a specimen can be detected at the same time, and complexity of production operation isnot increased. The test paper is high in sensitivity, strong in specificity, simple and convenient to operate, time-saving and strong in practicability.
Owner:杨小军
Influenza virus and 2019 novel coronavirus antibody combined detection device and preparation method thereof
ActiveCN111579792AImprove reasonable comprehensive judgmentSolve the adsorption problemBiological testingImmunoassaysCelluloseIgm antibody
The invention relates to an influenza virus and 2019 novel coronavirus antibody combined detection device and a preparation method thereof and belongs to the field of medical detection equipment. Thekit is prepared by adhering a nitrocellulose film, a glass fiber film, a sample pad, absorbent paper and other auxiliary materials, wherein the nitrocellulose film contains an influenza virus A antigen, an influenza virus B antigen, a novel coronavirus S antigen and a goat-anti-mouse IgG polyclonal antibody in a solid phase, and the glass fiber film absorbs a high-specificity anti-human IgM antibody. On the basis of ensuring complete release of the immune colloidal gold, the reaction sensitivity is effectively improved, under the same threshold value, the dosage of the immune colloidal gold can be reduced, cost is saved, the influenza virus A, the influenza virus B and novel coronavirus IgM antibodies in a specimen can be detected at the same time, and complexity of production operation isnot increased. The test paper is high in sensitivity, strong in specificity, simple and convenient to operate, time-saving and strong in practicability.
Owner:杨小军
Combined device and detection method for synchronously detecting influenza A virus, influenza B virus, chlamydia pneumoniae IgM antibody and mycoplasma IgM antibody
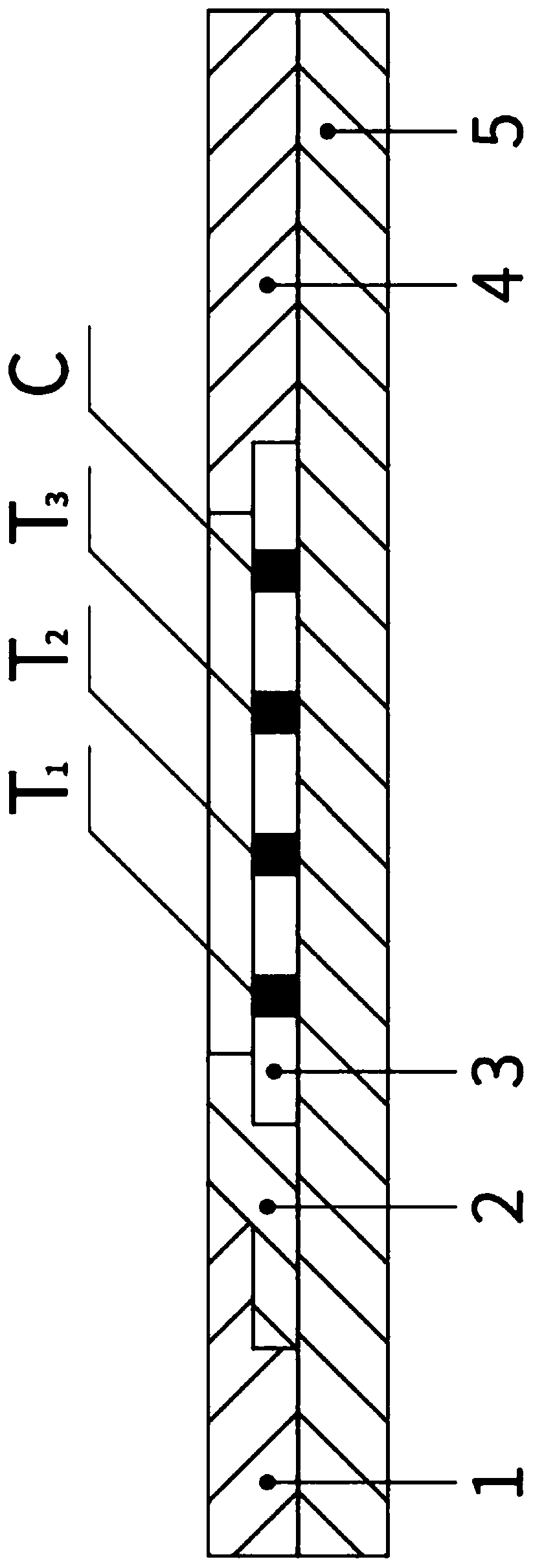
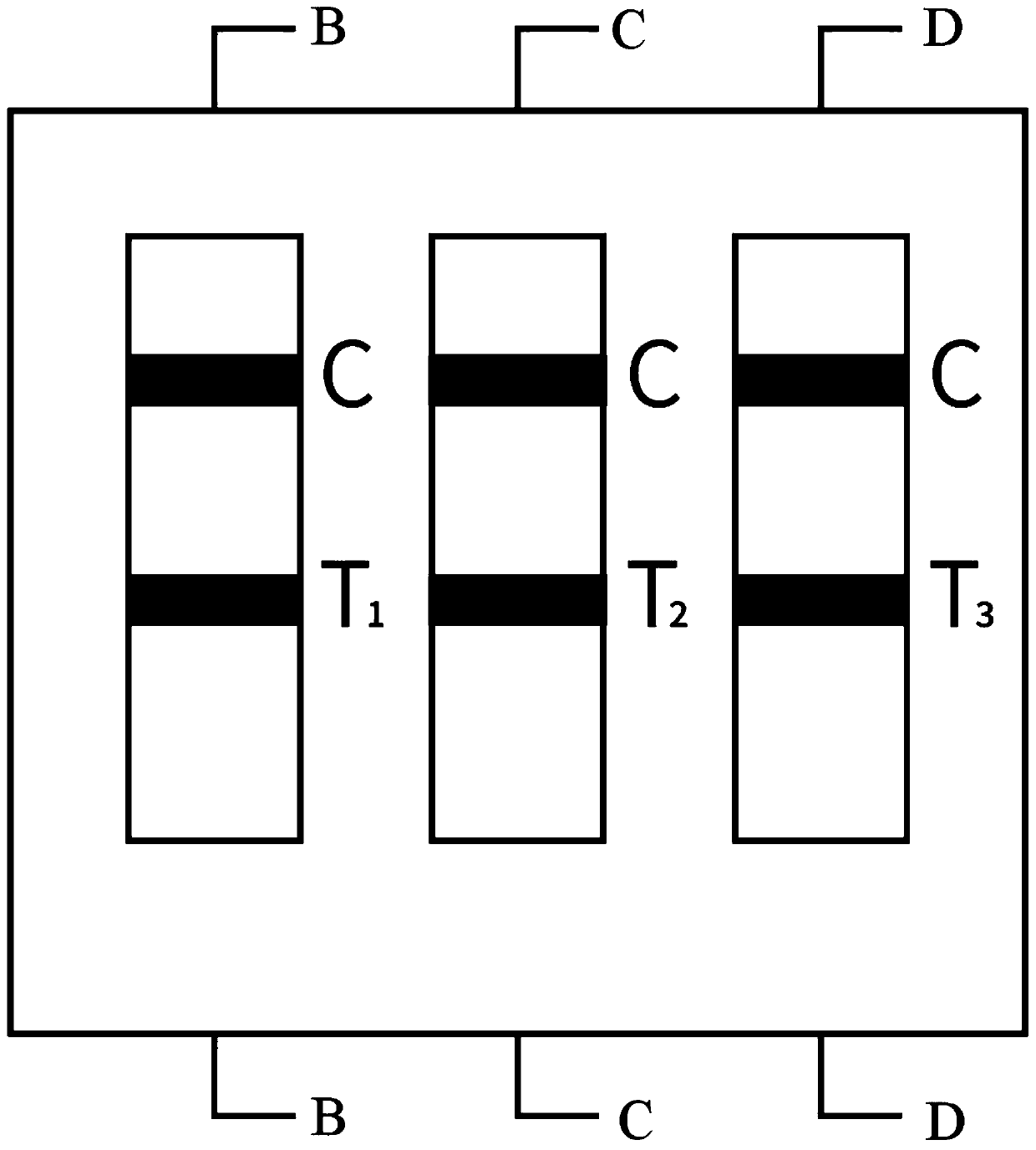
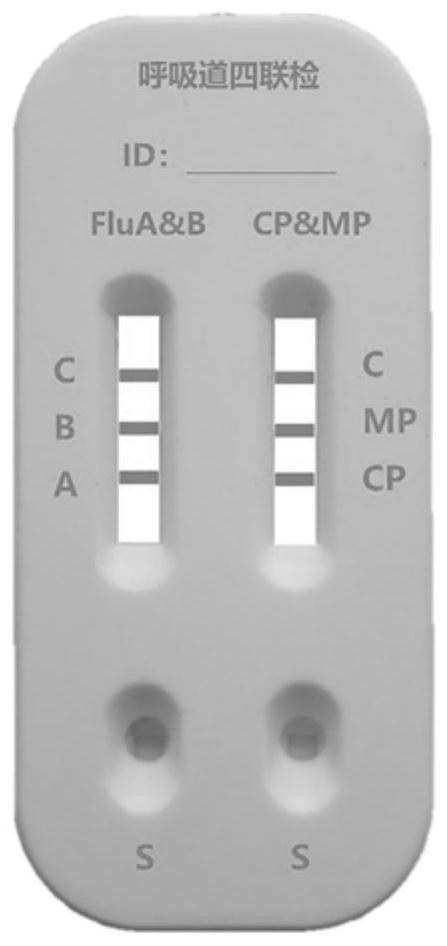
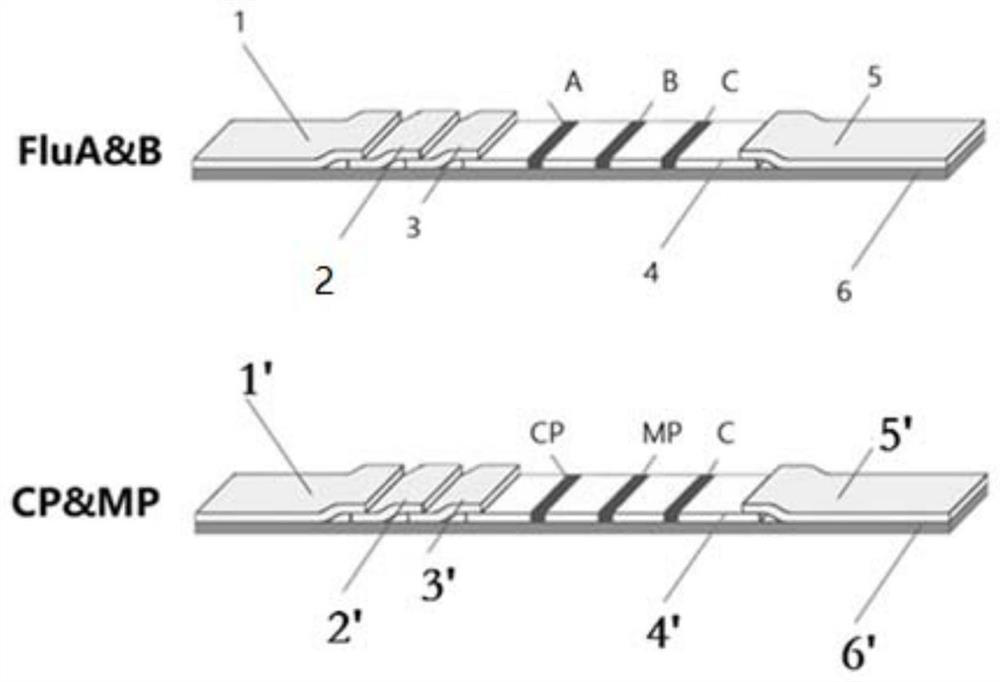
PendingCN111610329ASynchronize independent test resultsIndependent Testing ProcessMaterial analysisReceptorIgm antibody
The invention belongs to the field of medical detection equipment, and provides a combined device for synchronously detecting IgM antibodies of influenza A and B viruses and chlamydia pneumoniae and mycoplasma pneumoniae. The device comprises a double-channel clamping shell, a test strip FluA & B and a test strip CP & MP which are arranged in the double-channel clamping shell in parallel; influenza virus A detection lines arranged on a nitrocellulose membrane of the test strip FluA & B at intervals are coated with high-specificity influenza virus A antigens, influenza virus B detection linesare coated with high-specificity influenza virus B antigens, and first quality control lines are coated with quality control line coating receptors; chlamydia pneumoniae detection lines (CP) arranged on a nitrocellulose membrane of the test strip CP & MP at intervals are coated with high-specificity chlamydia pneumoniae antigens, mycoplasma pneumoniae detection lines (MP) are coated with high-specificity mycoplasma pneumoniae antigens, and second quality control lines are coated with quality control line coating receptors.
Owner:北京柏兆嘉业科技有限公司
Enzyme linked immuno kit for detecting residual phenylethanolamine A and application method thereof
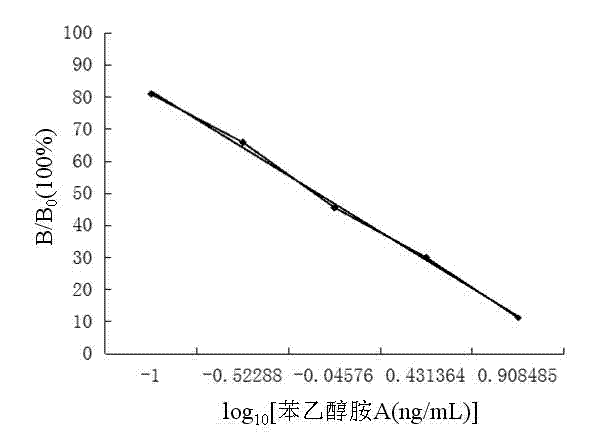
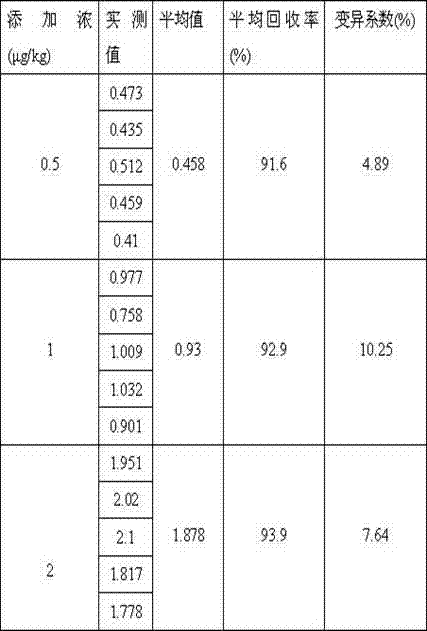
The invention discloses an enzyme linked immuno kit for detecting residual phenylethanolamine A and an application method thereof and belongs to the technical field of enzyme-linked immunosorbent assay methods. The enzyme linked immuno kit comprises an elisa plate coated by a benzene ethanolamine A antigen, phenylethanolamine A standard substance working solution, phenylethanolamine A antibody working solution, phenylethanolamine A IgG-HRP working solution, substrate solution, stop solution, concentrated washing solution and concentrated sample diluent. The enzyme linked immuno kit adopts an indirect competition enzyme-linked immunosorbent assay (ELISA) method. Residual phenylethanolamine A on the standard substance or the sample to be detected and an antigen pre-coated on the elisa plate contend for the phenylethanolamine A antibodies together. The application method can be used for directly detecting the phenylethanolamine A in animal derived food, urine samples, animal tissues and serum samples. The enzyme linked immuno kit has the advantages of being convenient, fast, sensitive and the like, and is suitable for detection of large-volume samples. In addition, the sensitivity of the enzyme linked immuno kit is 0.1ng / mL.
Owner:JIANGSU WISE SCI & TECH DEV
Monoclonal antibody for resisting foot and mouth disease virus type A and application thereof
The invention relates to monoclonal antibodies 2E11 and 3B4 of a foot and mouth disease virus type A. The kit comprises an effective amount of the monoclonal antibody 2E11, an effective amount of a foot and mouth disease virus type A antigen, an effective amount of an enzyme-labeled monoclonal antibody 3B4, a detection reagent for detecting the antigen-antibody reaction of the foot and mouth disease virus type A, and a support medium, wherein the monoclonal antibody 2E11 is coated on a support medium. The solid-phase competitive ELISA detection kit provided by the invention solves the problems of large batch-to-batch difference and poor stability of the existing liquid-phase blocking ELISA kit, the detection titer reaches 1: 128, and the sensitivity is high.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Incompatible blood group antigen for cancer detection and treatment
InactiveUS20200209263A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyDiseaseEpitope
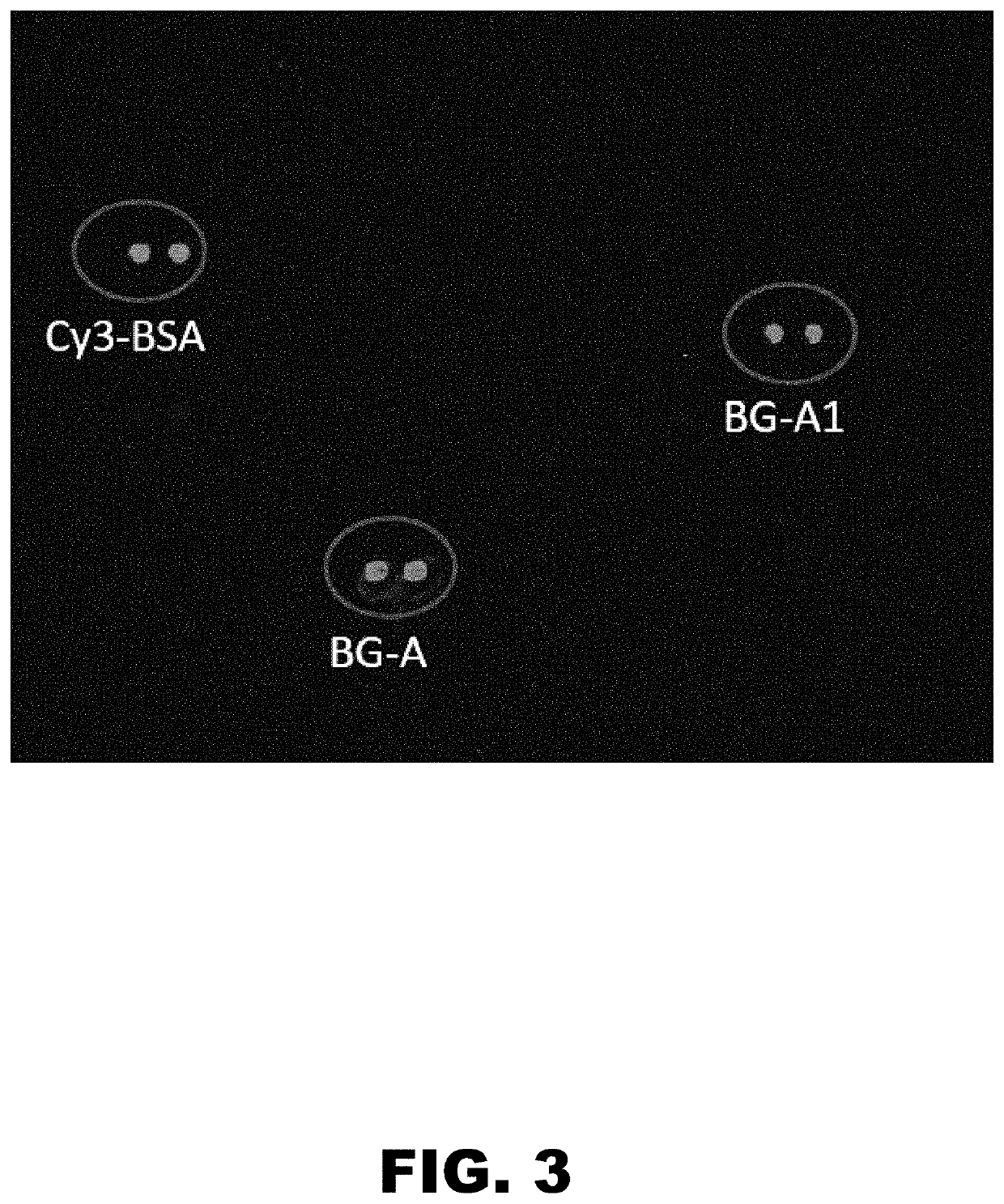
The present invention provides compositions and methods directed to incompatible blood group antigens. In particular, the present invention relates to anti-incompatible BG-A antibody molecules targeting a novel immune epitope in the incompatible BG-A antigen, such as the epitope bound by CRC-A1. The invention also relates to nucleic acids encoding such antibody molecules; to host cells expressing or capable of expressing such antibody molecules; to compositions comprising such antibody molecules or fragments thereof; and to uses of such antibody molecules or such compositions, in particular for therapeutic and detection purposes in the field of cancer diseases.
Owner:WASHINGTON UNIV IN SAINT LOUIS
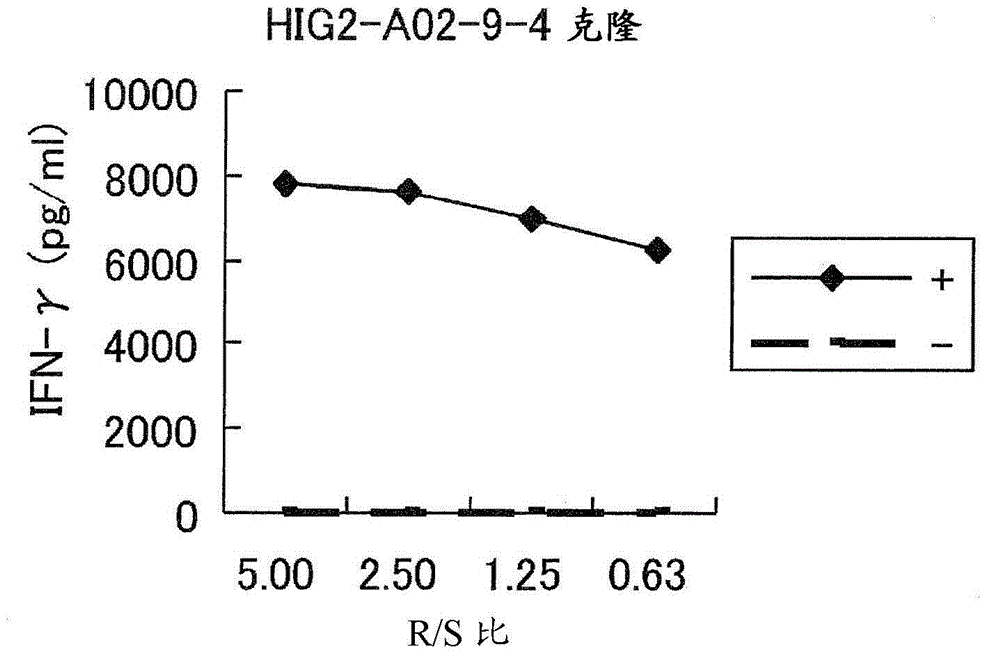
HIG2 and URLC10 epitope peptide and vaccines containing the same
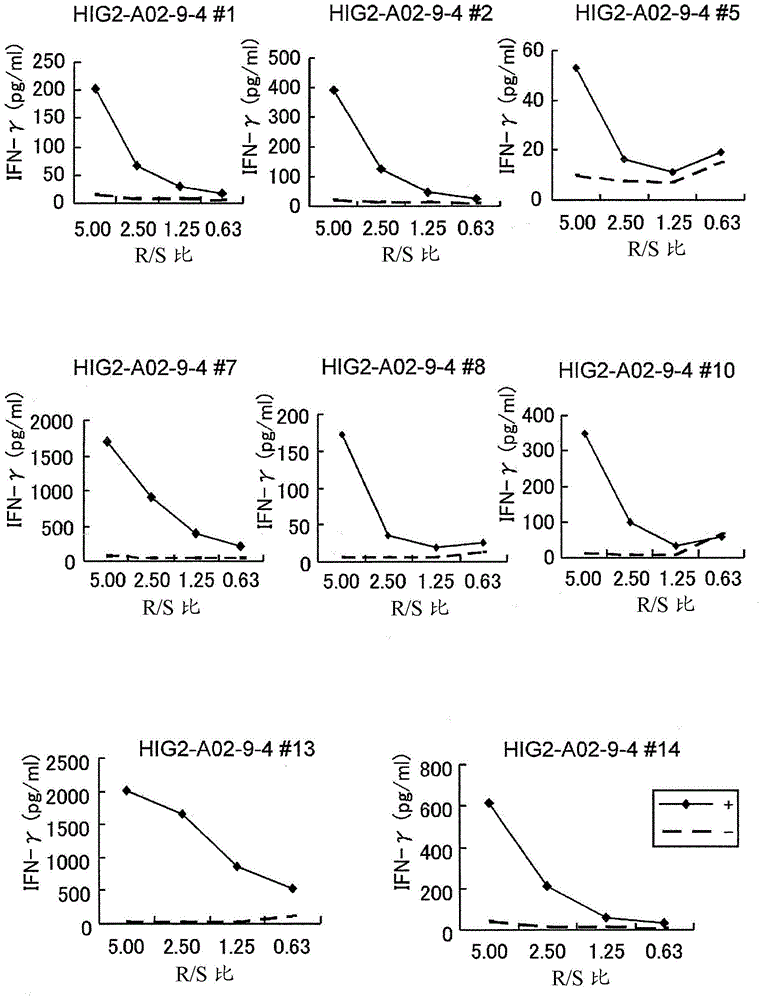
The present invention provides a pharmaceutical agent or composition containing one or more peptides having the amino acid sequence of SEQ ID NO: 1 or 2, or one or more polynucleotides encoding such a peptide formulated for the treatment and / or prevention of cancer in a subject whose HLA-A antigen is HLA-A0206. Furthermore, the present invention provides a method of inducing CTL and antigen-presenting cells using such peptides, polynucleotides or pharmaceutical agents.
Owner:ONCOTHERAPY SCI INC
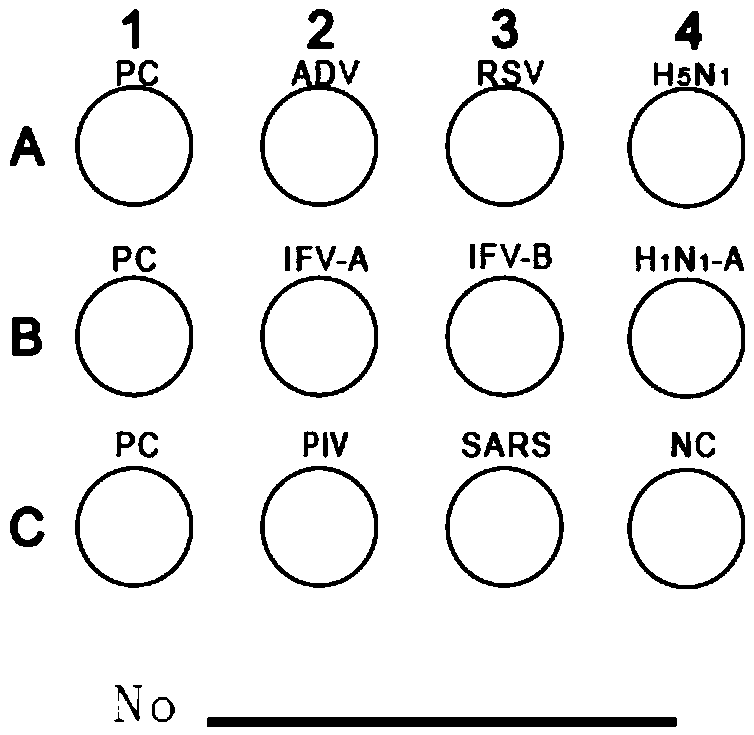
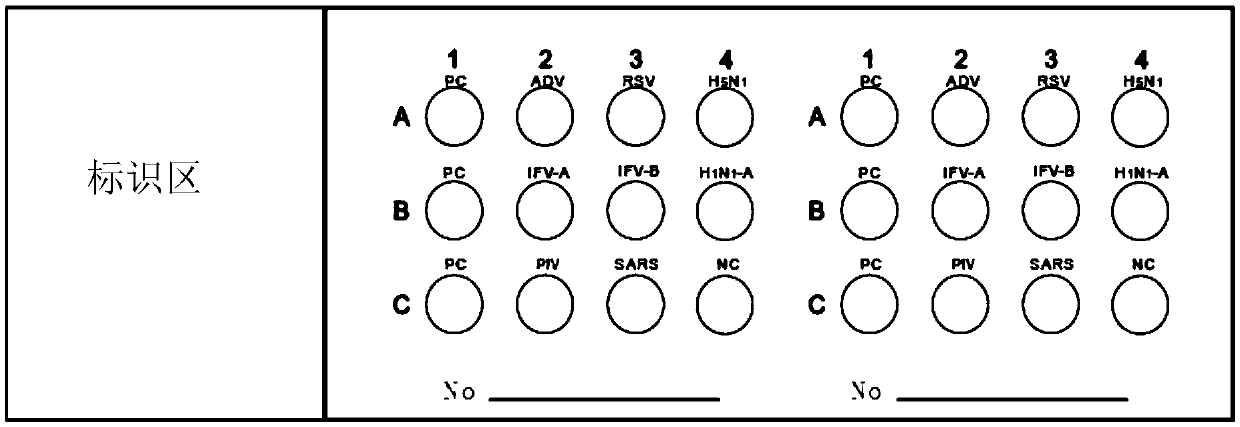
Protein chip for detecting respiratory virus antigens as well as kit and preparation method thereof
InactiveCN105510596ATo achieve the purpose of quantitative diagnosisImprove throughputBiological material analysisBiological testingParainfluenza virus antigenInfectious Disorder
The invention provides a protein chip for detecting respiratory virus antigens as well as a kit and a preparation method thereof. The protein chip for detecting the respiratory virus antigens comprises a glass matrix vector and a quality control protein probe fixed in a protein quality comparison hole in the glass matrix vector; quality control protein comprises an adenovirus antigen, a respiratory syncytial virus antigen, influenza A and B antigens, a parainfluenza virus antigen, a SARS virus antigen, an H5N1 antigen and an H1N1-a antigen. Through adoption of the technical scheme, the diagnosis of simultaneous detection of eight types or more types of viral infection can be realized through one experiment, so that the efficiency in diagnosis of respiratory viral infectious diseases is improved, anti-bioterrorism screening is assisted, and a purpose of efficient, time-saving and high-flux diagnosis is realized.
Owner:深圳市赛尔生物技术有限公司
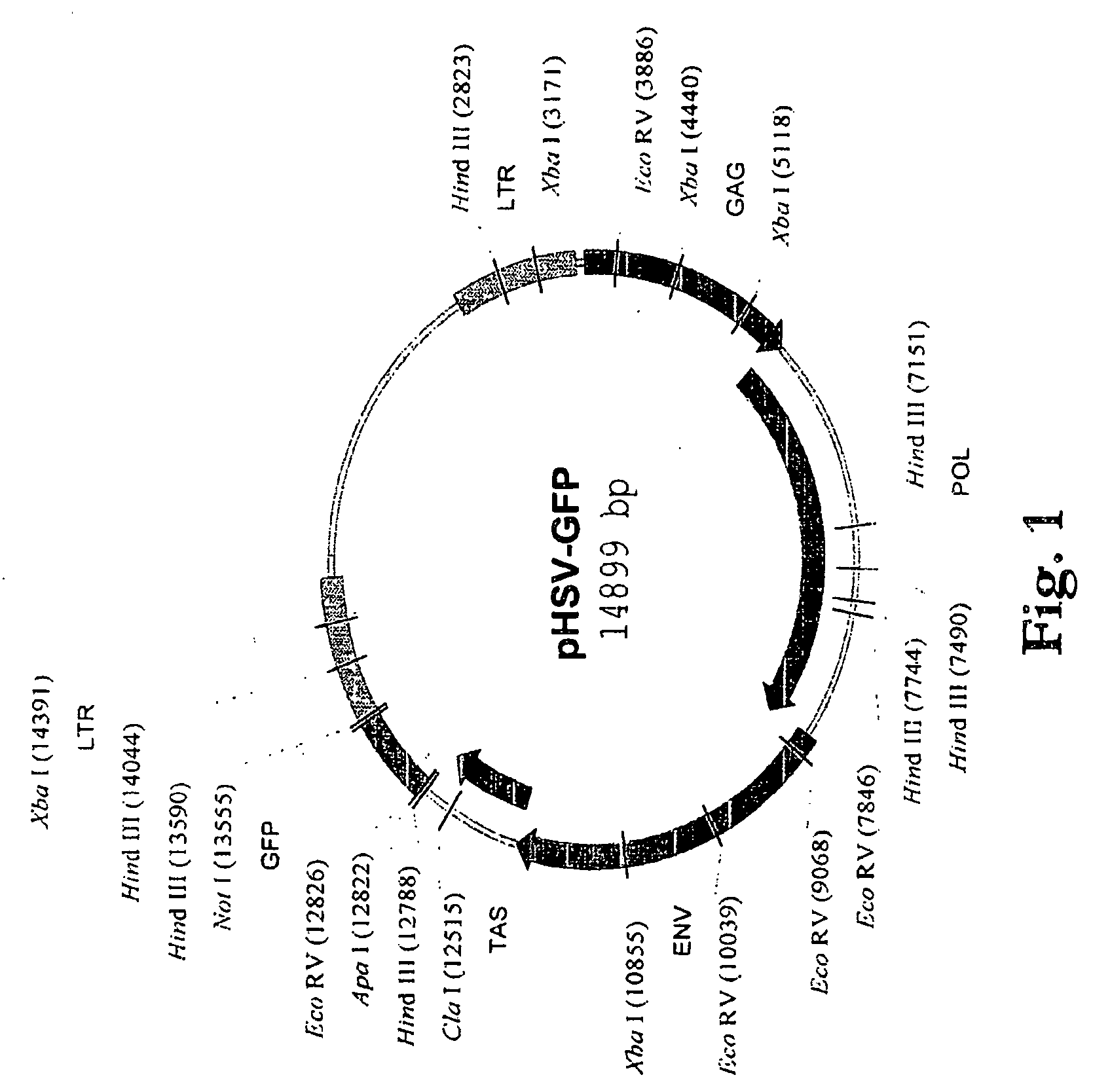
Live replicating spumavirus vector
The present invention provides a vector or vector containing composition comprising a spumavirus backbone and a antigen-encoding nucleic acid. The present invention also provides methods of treating or preventing a condition resulting from a vital, bacterial, or parasitic infection in a subject comprising administering to the subject an effective amount of the vector or vector containing composition comprising a spumavirus backbone and an antigen-encoding nucleic acid. Also provided in the present invention are methods of treating a condition resulting from a cancer in a subject comprising administering to the subject an effective amount of the vector or vector containing composition comprising a spumavirus backbone and an antigen-encoding nucleic acid.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SE CRETARY DEPT OF HEALTH & HUMAN SERVICES CENTS FOR DISEASE CONT +1
Maytansinoid derivatives
ActiveUS20140178414A1Minimizing undesirable side effectPrevent relapseOrganic active ingredientsAntipyreticDiseaseMedicine
Disclosed herein are maytansinoid drug linker derivatives which can be linked to a antigen binding unit (Abu), and maytansinoid drugs linked with an antigen binding unit (Drug-Linker-Antigen binding Unit: D-L-Abu), for targeted delivery to disease tissues. D-L-Abu, D-L-Abu derivatives, and methods relating to the use of such drug conjugates to treat antigen positive cells in cancers and immunological disorders are provided.
Owner:BIO THERA SOLUTIONS
Foot-and-mouth disease virus type a antigen polypeptide, fusion antigen polypeptide and vaccine
ActiveCN105418738BImproving immunogenicityBroad spectrum immunitySsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseVirus type
The invention discloses a foot-and-mouth disease virus type A antigen polypeptide, a fusion antigen polypeptide and a vaccine, specifically a foot-and-mouth disease virus type A antigen polypeptide, a fusion antigen polypeptide, and a foot-and-mouth disease virus vaccine containing the above-mentioned antigen polypeptide and / or fusion antigen polypeptide. The invention also provides the preparation method of the antigen polypeptide, fusion antigen polypeptide and vaccine. The present invention further provides the application of the antigen polypeptide, fusion antigen polypeptide and vaccine in preventing and controlling foot-and-mouth disease virus infection. The foot-and-mouth disease virus type A antigen polypeptide, the fusion antigen polypeptide and the vaccine have broad-spectrum immunogenicity, and can produce good immunogenicity to different foot-and-mouth disease viruses and variant strains thereof.
Owner:SHANGHAI SHEN LIAN BIOMEDICAL CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com