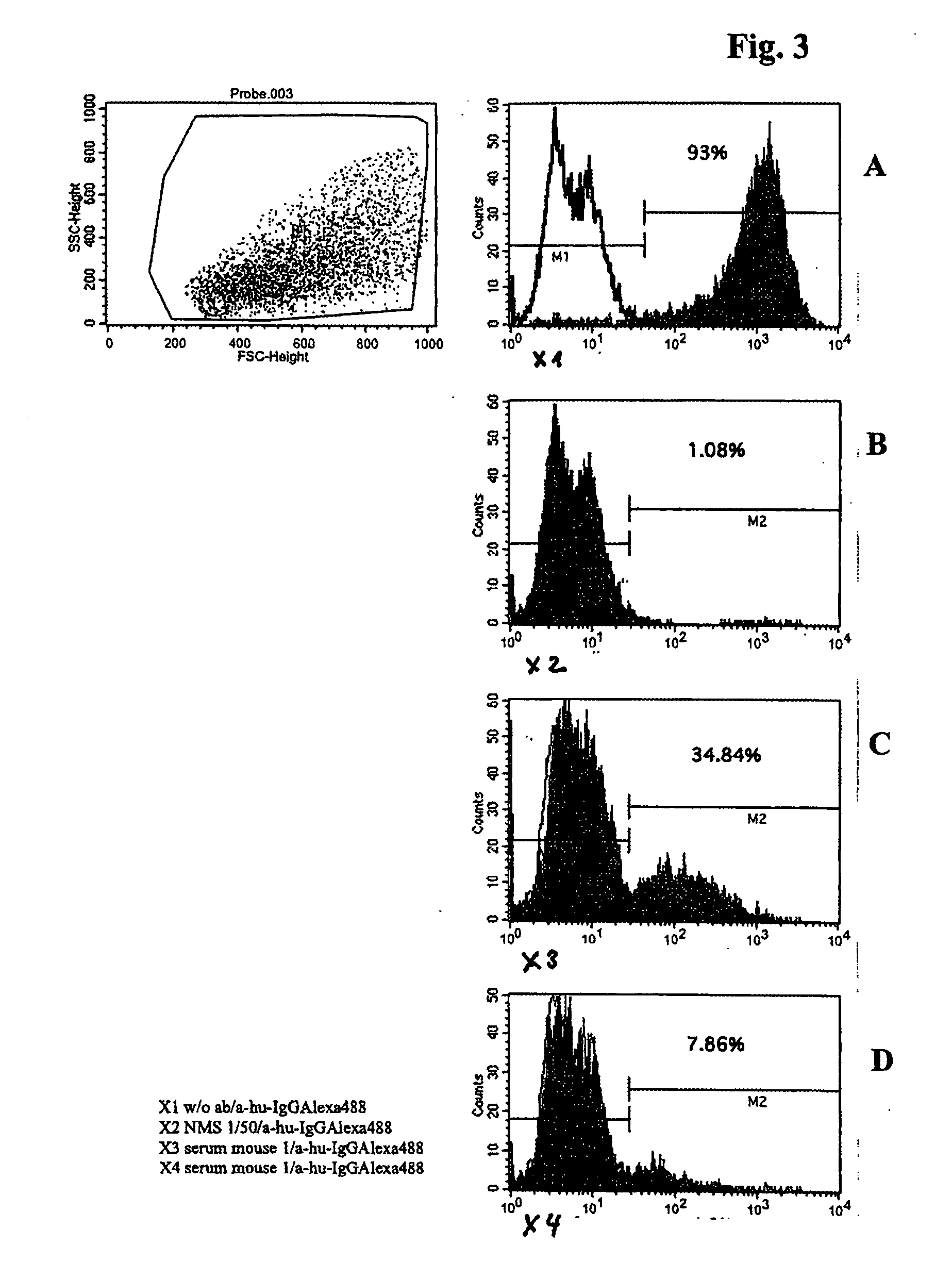
Patents
Literature
67 results about "Human fetal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
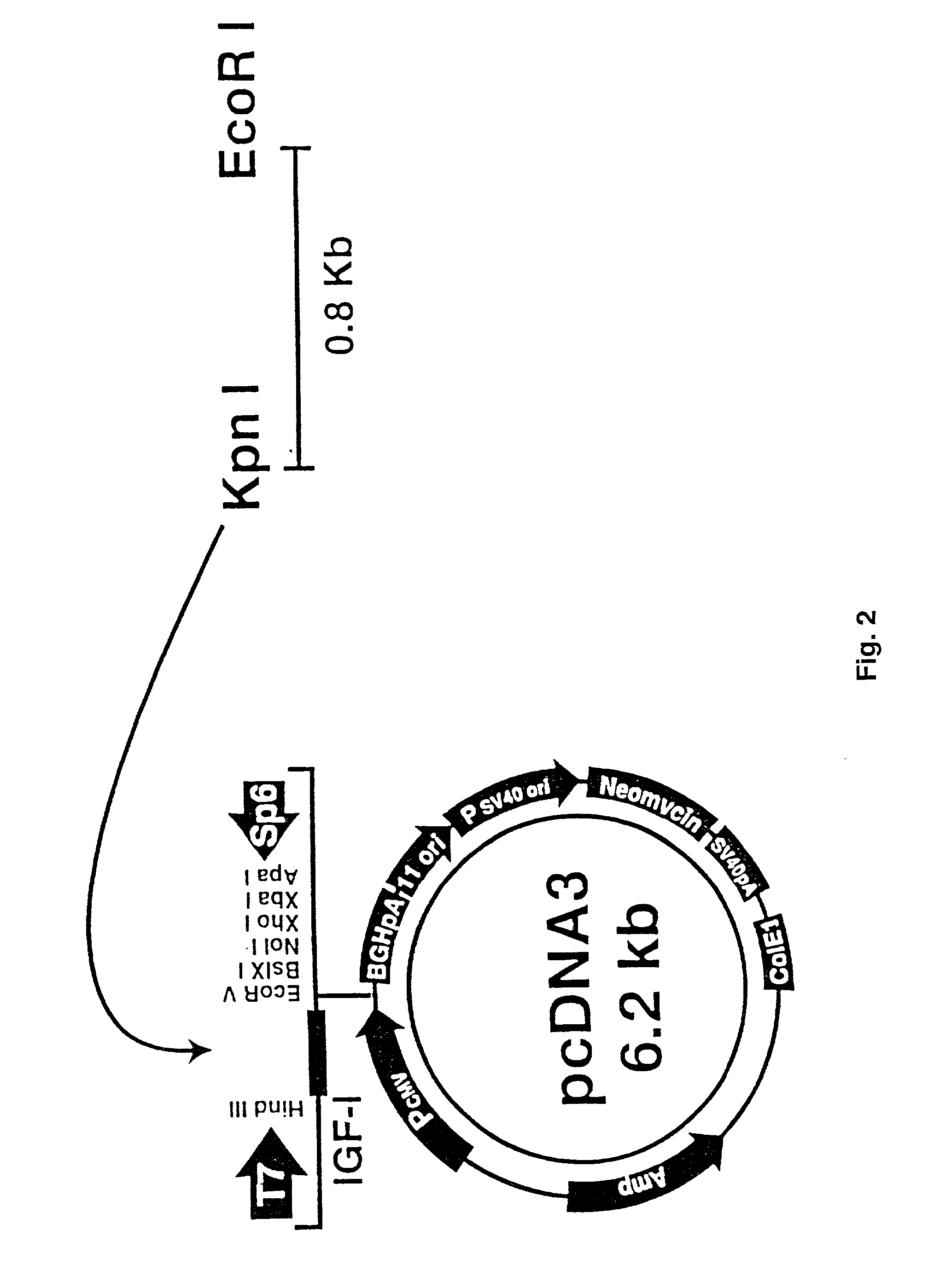
Methods of producing human rpe cells and pharmaceutical preparations of human rpe cells
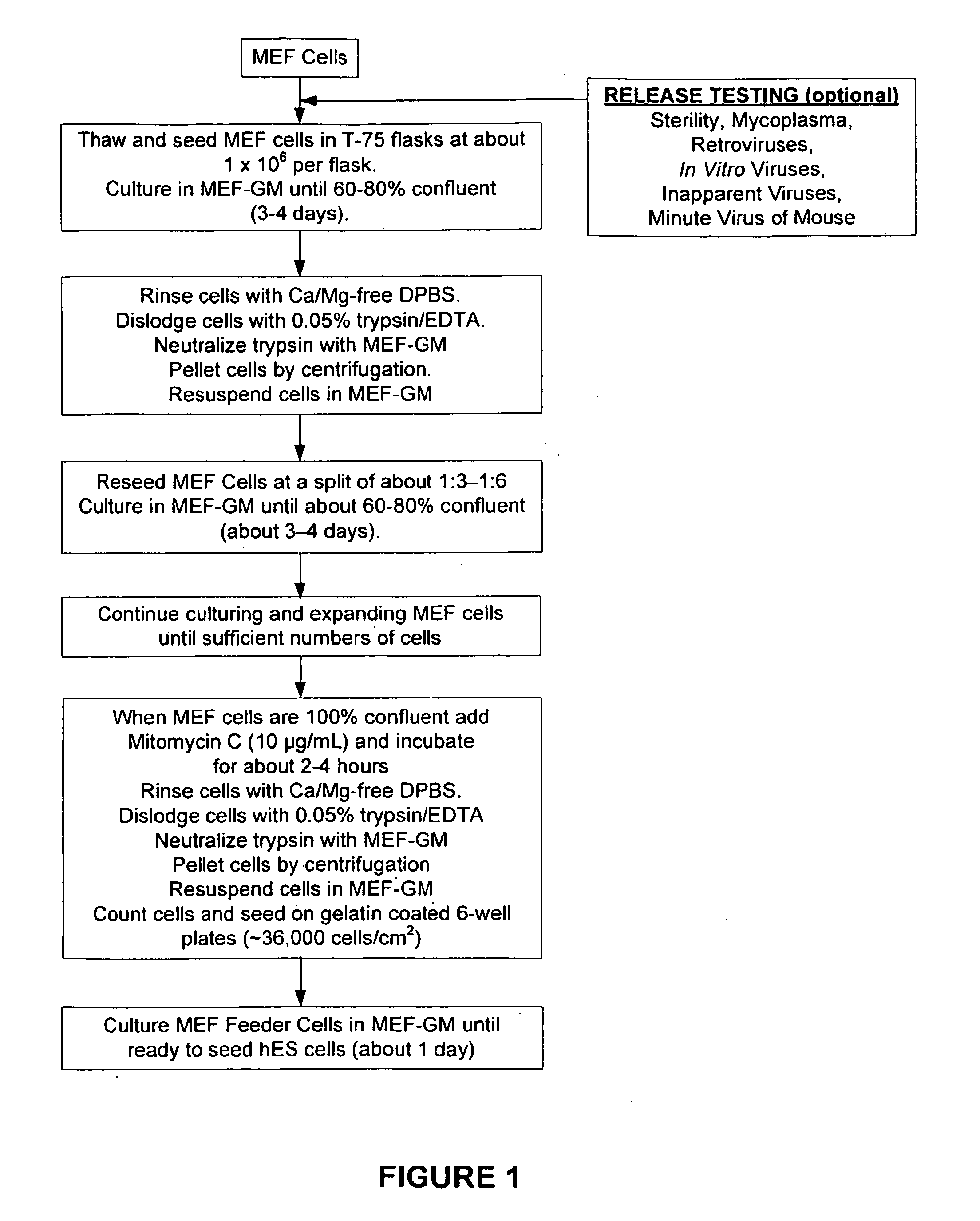
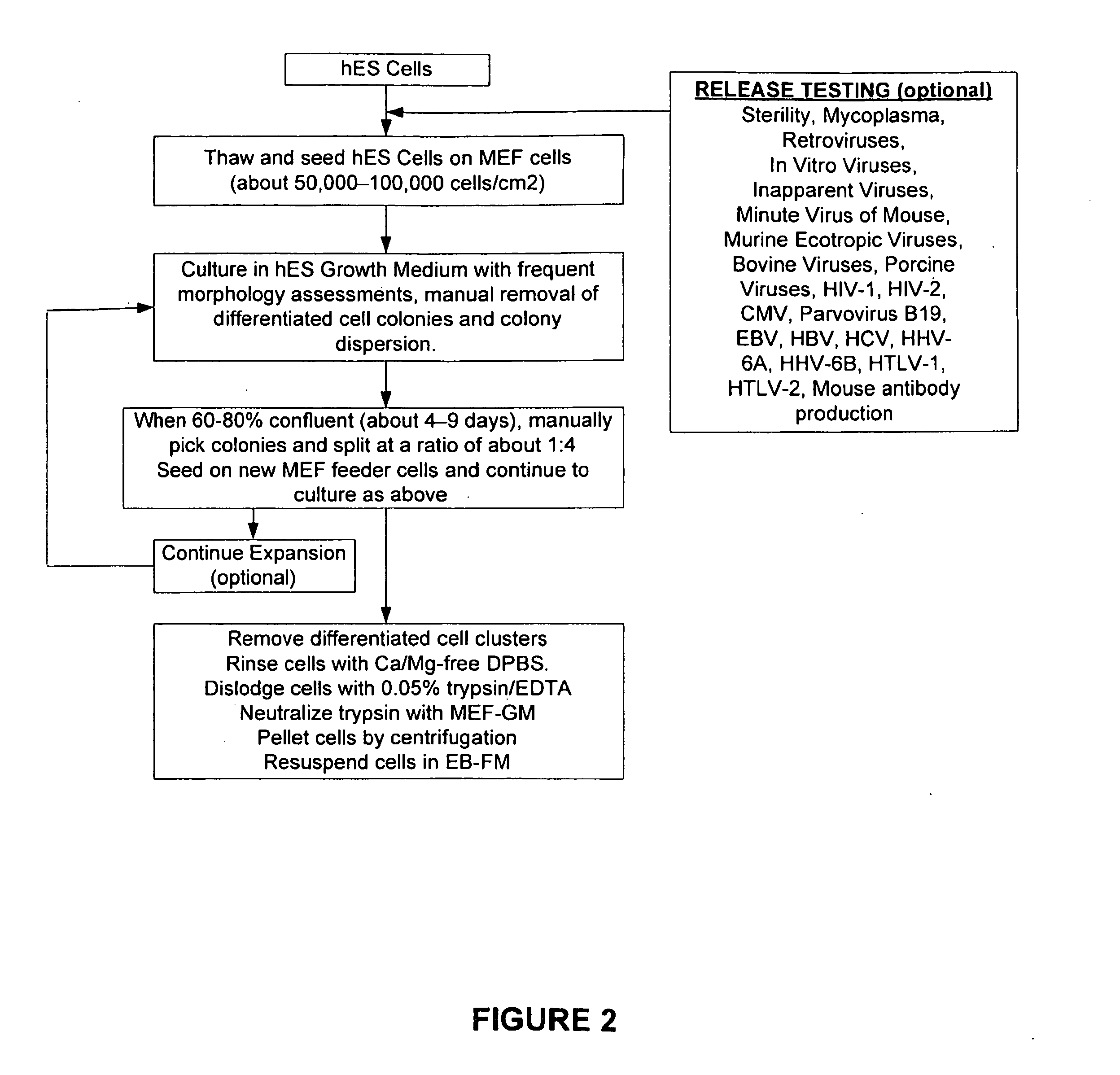
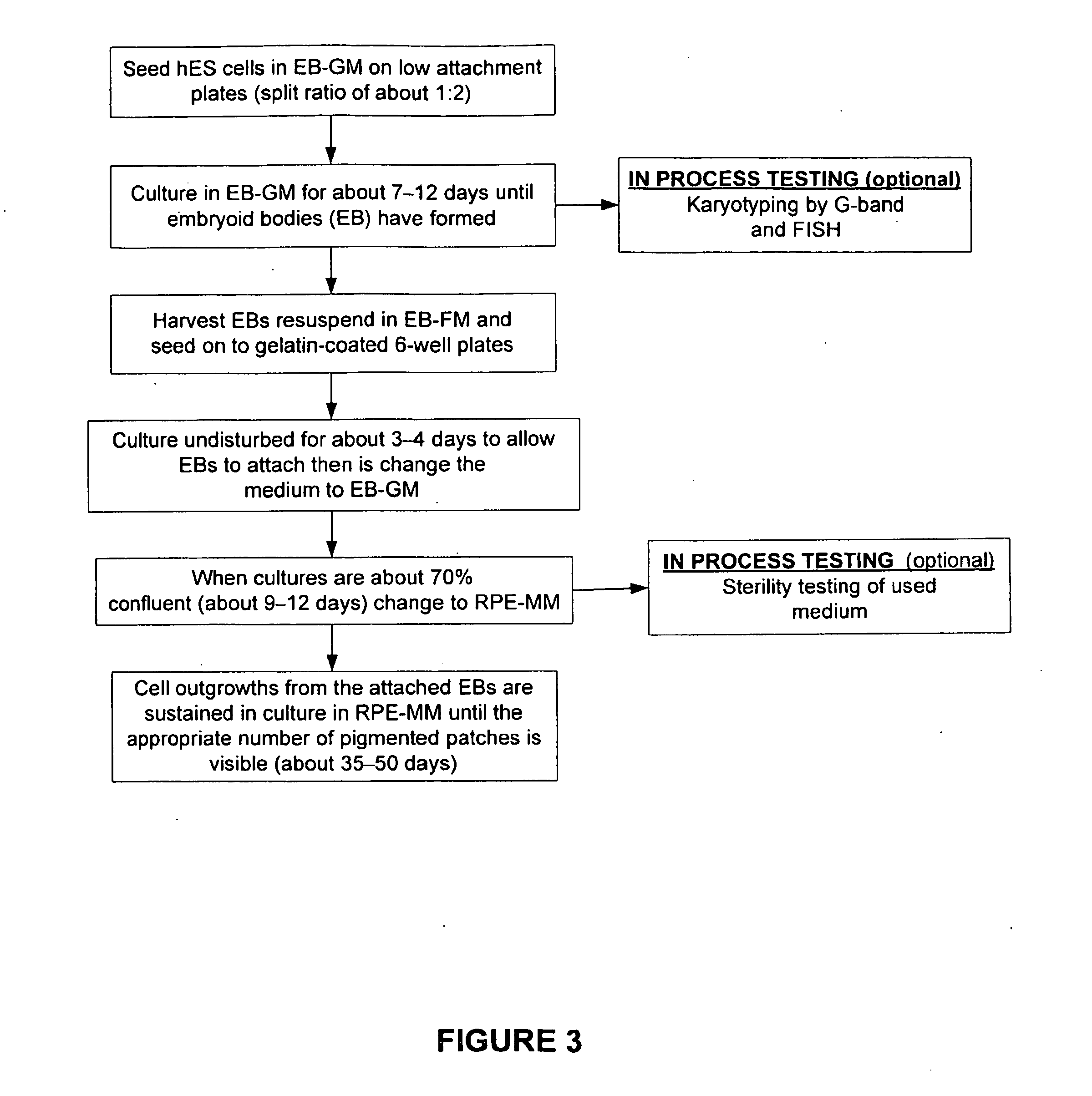
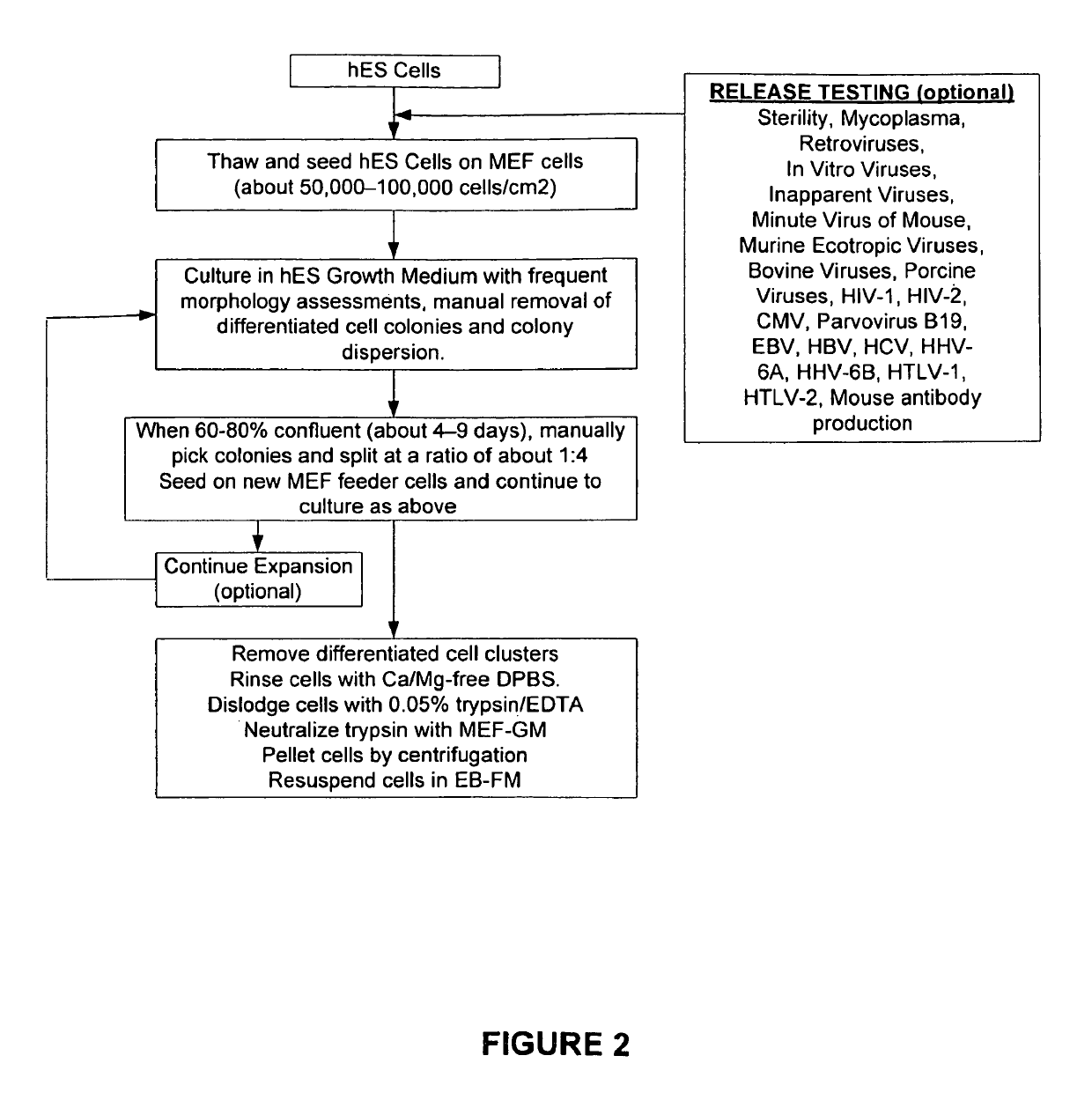
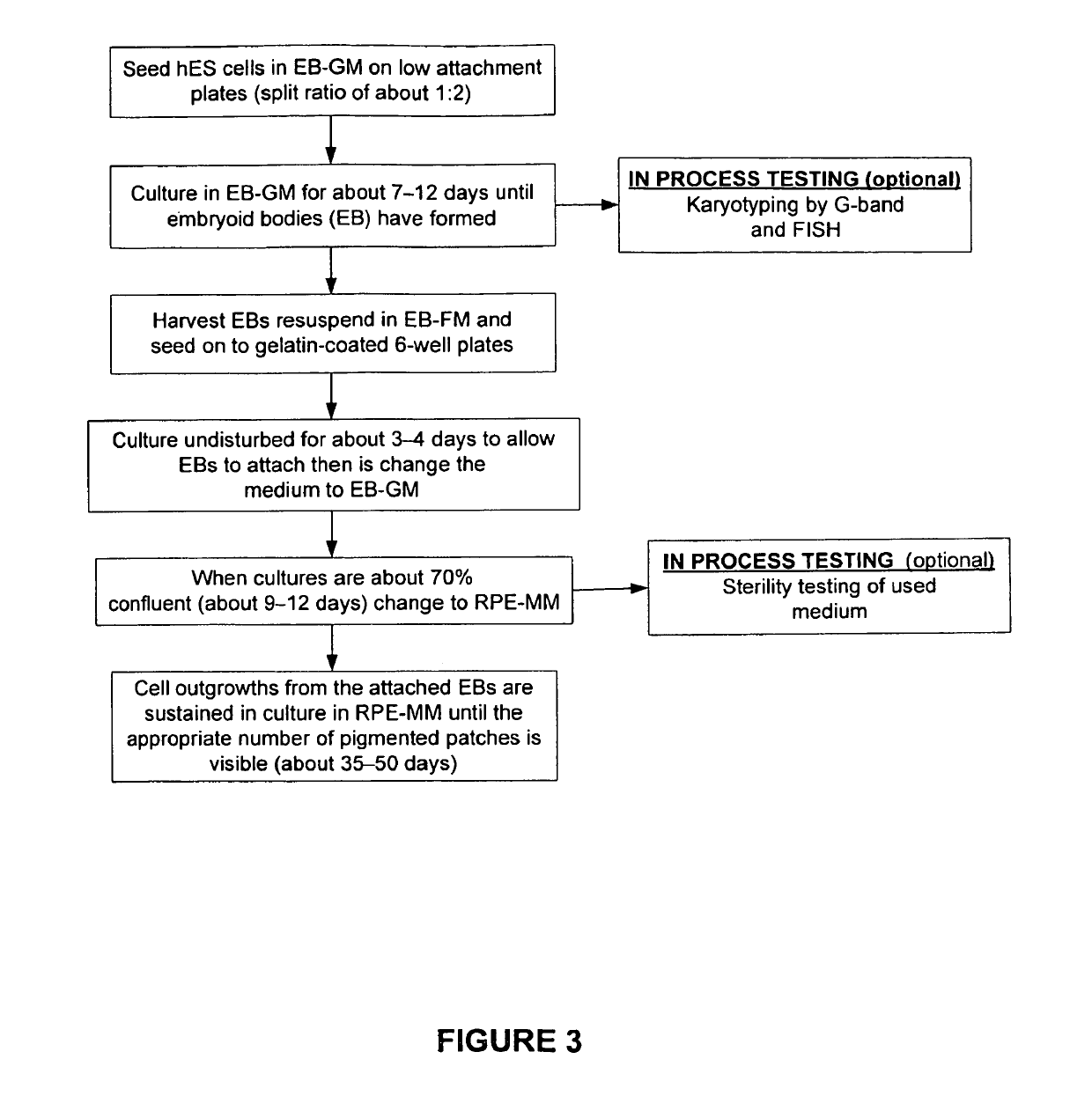
The present invention provides improved methods for producing retinal pigmented epithelial (RPE) cells from human embryonic stem cells, human induced pluripotent stem (iPS), human adult stem cells, human hematopoietic stem cells, human fetal stem cells, human mesenchymal stem cells, human postpartum stem cells, human multipotent stem cells, or human embryonic germ cells. The RPE cells derived from embryonic stem cells are molecularly distinct from adult and fetal-derived RPE cells, and are also distinct from embryonic stem cells. The RPE cells described herein are useful for treating retinal degenerative conditions including retinal detachment and macular degeneration.
Owner:ADVANCED CELL TECH INC
Cell culture media
Methods are taught for culturing mammalian cells, preferably human cells, to improve production of proteins, recombinant or endogenous. Methods are also provided for the growth and long-term survival of cell lines, particularly cell lines established from primary culture. Cell culture media are also provided, contained varying levels of selected amino acids, supplemented with various growth factors and trace elements. In additionally, the media are optionally serum-free, and preferably use an energy source other than glucose. The media are particularly suitable for the primary culture and long-term culture of human fetal cells.
Owner:RAVEN BIOTECHNOLOGIES INC +1
Stem cell growth factor-like polypeptides
The invention provides novel polynucleotides and polypeptides encoded by such polynucleotides and mutants or variants thereof that correspond to a novel human and mouse secreted stem cell growth factor-like polypeptide. These polynucleotides comprise nucleic acid sequences isolated from cDNA libraries prepared from mouse bone marrow and human fetal liver spleen, ovary, adult brain, lung tumor, spinal cord, cervix, ovary, endothelial cells, umbilical cord, placental, lymphocyte, lung fibroblast, fetal brain, and testis. Other aspects of the invention include vectors containing processes for producing novel human secreted stem cell growth factor-like polypeptides, and antibodies specific for such polypeptides.
Owner:KIRIN PHARMA +1
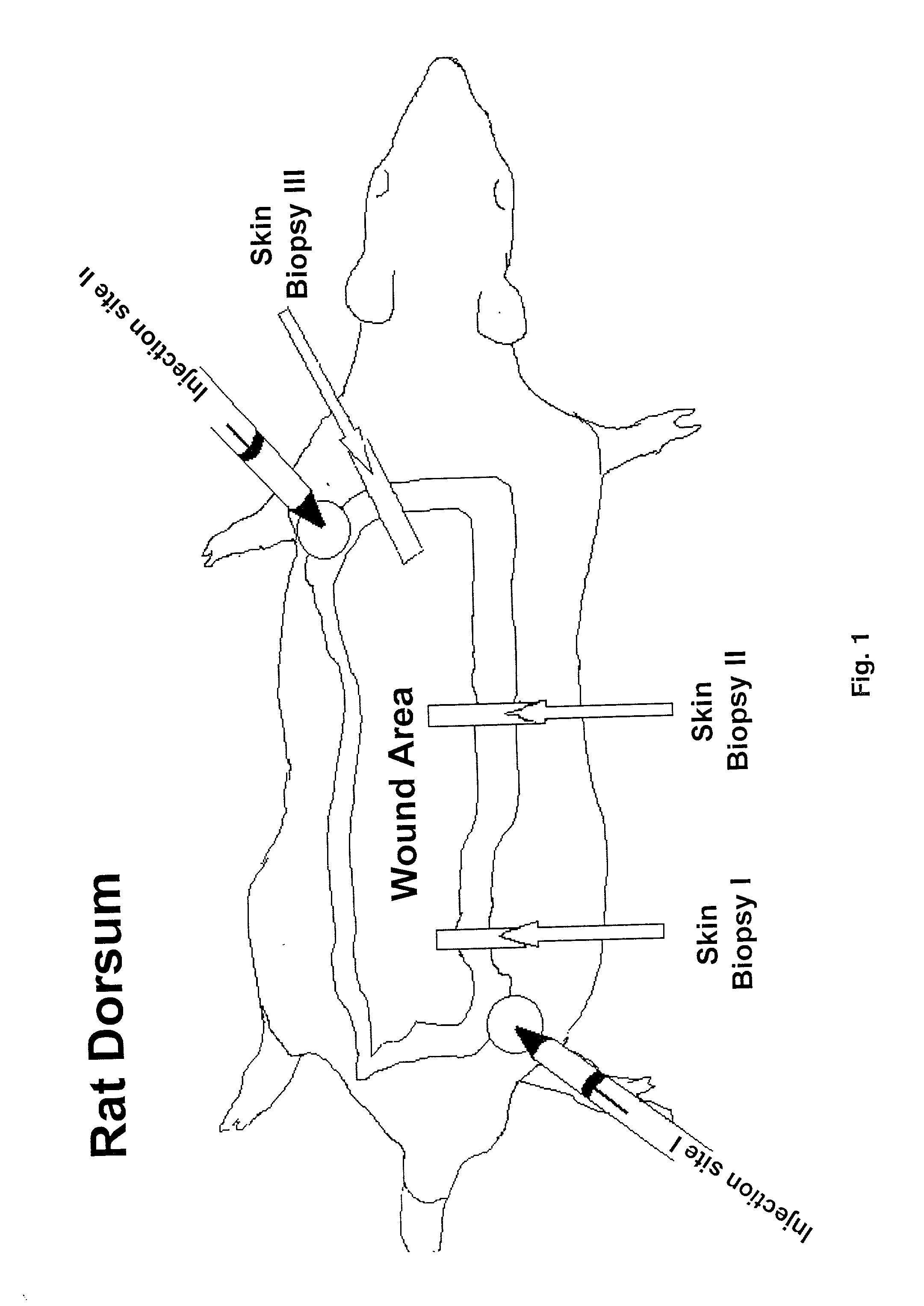
Methods to enhance wound healing and enhanced wound coverage material
InactiveUS20020128222A1Promote wound healingDecrease hypermetabolic responsePeptide/protein ingredientsGenetic material ingredientsWound healingBurn injury
The present invention describes the incorporation of liposomal gene constructs directly into a wound to further improve wound repair, or into wound coverage and / or closure materials to enhance the functionality of the material. The present invention further describes the use of human fetal membranes (e.g., amnion) enhanced with the liposomal gene therapy as a wound coverage material in full-thickness wound repair. The enhanced fetal membranes or enhanced cadaver skin have advantages over currently used materials lacking the liposomal gene construct and are an efficient and safe approach to improve clinical outcome in patients with burn injuries.
Owner:RES DEVMENT FOUND
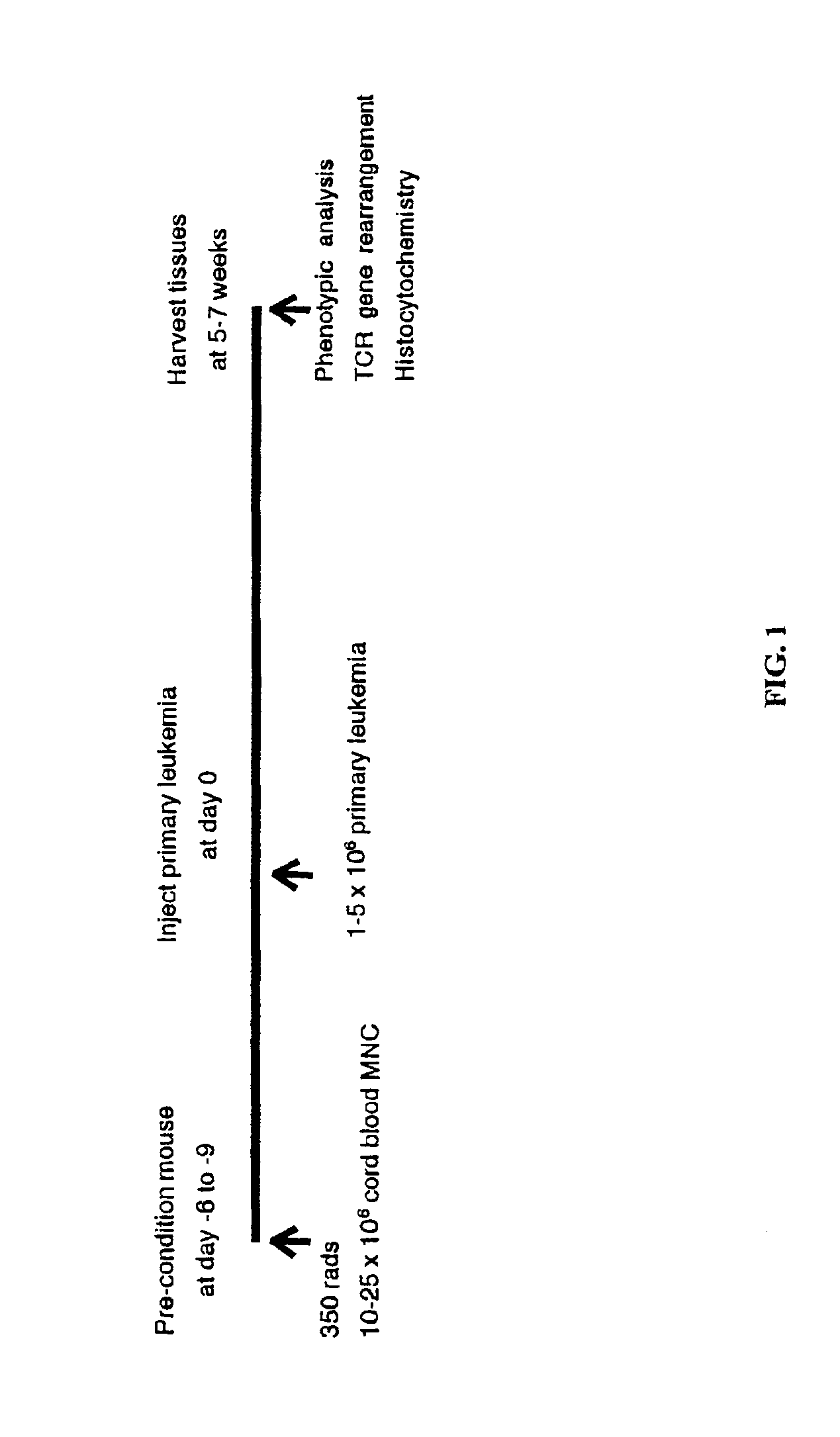
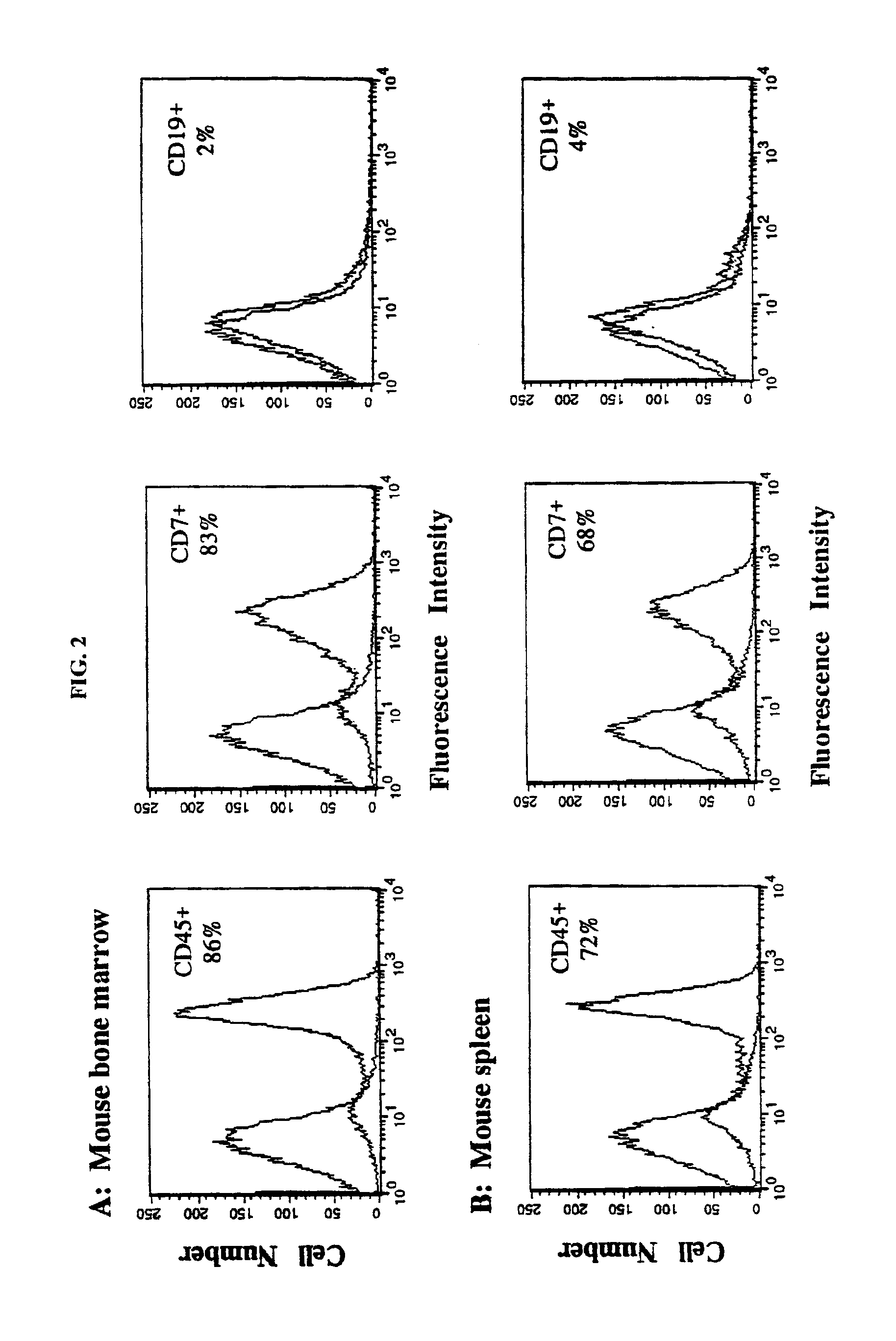
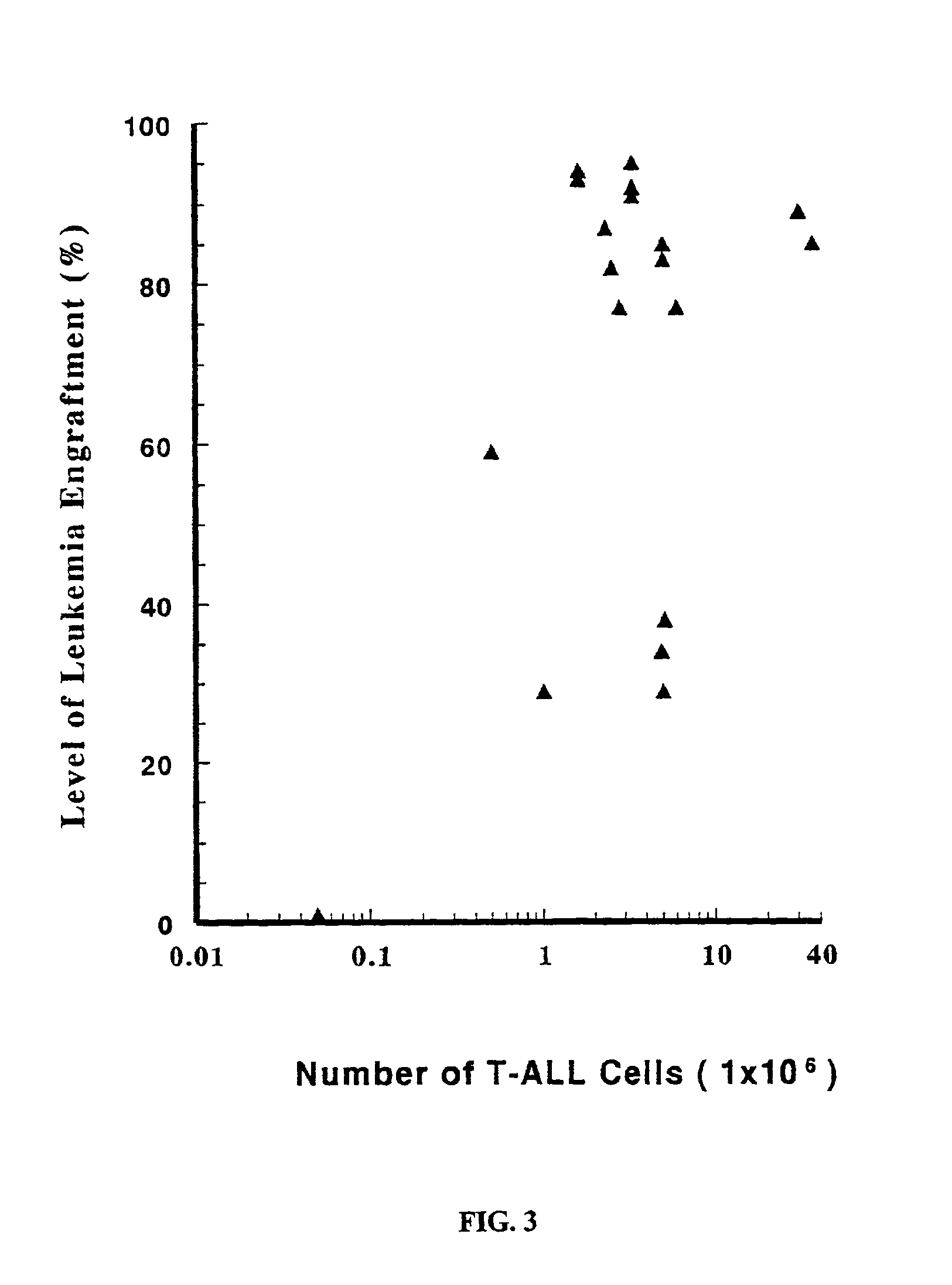
In vivo animal model of human leukemia
InactiveUS6930222B2Improve the level ofIncrease the number ofAnimal husbandryCord blood stem cellHuman leukemia
The present invention provides a process for making an in vivo model of human leukemia. The process includes the steps of: pre-conditioning an immunodeficient rodent by administering to the rodent a sub-lethal dose of irradiation and injecting the rodent with an effective pre-conditioning amount of human fetal cord blood mononuclear cells; maintaining the rodent for from about 5 to 10 days; and injecting the rodent with an effective engrafting amount of primary human leukemia cells. An in vivo and in vitro model of human leukemia are also provided.
Owner:THE SCRIPPS RES INST
Syringeability cardiac muscle tissue engineering products based on thermo-sensitive chitosan hydrogel
InactiveCN101288779APromote regenerationImprove retentionProsthesisExtracorporeal circulationLiquid temperature
The invention discloses an injective myocardial tissue engineering product basing on temperature responsive chitosan hydrogel and more particularly relates to liquid temperature responsive chitosan hydrogel which is used as stent material that is combined with seed cells from different sources, such as embryonic stem cells, mesenchymal stem cells, human fetal cadiacmyocytes, etc., and is injected and transplanted into the specific region of an animal myocardial infarction model for observing the condition of repairing the myocardial infarction region. Constructed by the stent material, the injective myocardial tissue engineering product can improve the retention rate and the survival rate of the seed cells, can promote the regeneration of myocardial tissues, can increase the wall thickness of the infarction region and can remold the shape of an original ventricular and improve the heart function. The product is provided with the injective character and is convenient for the treatment operation, thereby avoiding risks brought by the operations of cardioplegia arrest, extracorporeal circulation, etc. The injective myocardial tissue engineering product basing on the temperature responsive chitosan hydrogel has the advantages of simple operation process and mild implementation condition, provides a new product for the myocardial tissue engineering and has great significance for the clinical development of the tissue engineering myocardial treatment on heart diseases.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Cell culture media
Methods are taught for culturing mammalian cells, preferably human cells, to improve production of proteins, recombinant or endogenous. Methods are also provided for the growth and long-term survival of cell lines, particularly cell lines established from primary culture. Cell culture media are also provided, contained varying levels of selected amino acids, supplemented with various growth factors and trace elements. In additionally, the media are optionally serum-free, and preferably use an energy source other than glucose. The media are particularly suitable for the primary culture and long-term culture of human fetal cells.
Owner:RAVEN BIOTECHNOLOGIES INC +1
Methods and materials relating to CD39-like polypeptides
The invention provides novel polynucleotides isolated from cDNA libraries of human fetal liver-spleen and macrophage as well as polypeptides encoded by these polynucleotides and mutants or variants thereof. The polypeptides correspond to a novel human CD39-like protein. Other aspects of the invention include vectors containing polynucleotides of the invention and related host cells as well a processes for producing novel CD39-like polypeptides, and antibodies specific for such polypeptides.
Owner:NUVELO INC
Methods and materials relating to CD39-like polypeptides
InactiveUS6476211B1Enhance and inhibit activityCell receptors/surface-antigens/surface-determinantsSugar derivativesCDNA libraryHuman fetal
The invention provides polynucleotides isolated from cDNA libraries of human fetal liver-spleen and macrophage as well as polypeptides encoded by these polynucleotides and mutants or variants thereof. The polypeptides correspond to a human CD39-like protein. Other aspects of the invention include vectors containing polynucleotides of the invention and related host cells as well a processes for producing CD39-like polypeptides, and antibodies specific for such polypeptides.
Owner:NUVELO INC
Methods and materials relating to novel CD39-like polypeptides
InactiveUS6387645B1Enhance and inhibit activityCell receptors/surface-antigens/surface-determinantsHydrolasesCDNA libraryNucleotide
The invention provides novel polynucleotides isolated from cDNA libraries of human fetal liver-spleen and macrophage as well as polypeptides encoded by these polynucleotides and mutants or variants thereof. The polypeptides correspond to a novel human CD39-like protein. Other aspects of the invention include vectors containing polynucleotides of the invention and related host cells as well a processes for producing novel CD39-like polypeptides, and antibodies specific for such polypeptides.
Owner:NUVELO INC +1
Human fetal bladder-derived epithelial cells
This invention discloses a substantially pure population of human urinary bladder-derived epithelial cells and methods of isolating and culturing the urinary bladder-derived epithelial cells. In addition, several uses of human urinary bladder-derived epithelial cells and cells differentiating therefrom are disclosed herein.
Owner:RAVEN BIOTECHNOLOGIES INC +1
Fetal RNA in amniotic fluid to determine gene expression in the developing fetus
InactiveUS20060003342A1Sugar derivativesMicrobiological testing/measurementDiseasePrenatal diagnosis
The present invention provides improved methods of prenatal diagnosis, monitoring, screening and / or testing. The invention is based, at least in part, on the discovery that amniotic fluid is a rich source of cell-free fetal RNA. Methods of isolation and analysis of fetal RNA are described, that can lead to information about fetal gene expression that is not available by other techniques. The inventive systems allow for a more comprehensive determination of a living human fetus' health, growth and development and for the prenatal diagnosis of a variety of diseases and conditions.
Owner:NEW ENGLAND MEDICAL CENT HOSPITALS
Neural stem cell injection for treating brain damage disease
InactiveCN106619722AImprove drynessGood differentiation potentialNervous disorderCulture processDiseasePrimary cell
The invention relates to a neural stem cell injection, which is derived from a human fetal cerebral cortex source and is used for treating various brain damages. The neural stem cell injection at least comprises 1*10<6> neural stem cells, wherein the neural stem cells are obtained by separately culturing waste fetal cerebral cortex tissues; the neural stem cell are cultured by tissue separation and primary cell culture, purification and amplification culture of the neural stem cells, building of a neural stem cell library and preparation of the neural stem cell injection. The cells can be amplified by 5000 times in a serum-free culture system. According to the use condition of the cells, P5-generation neural stem cells of seed cells are recovered and counted, and undergo suspension culture in a serum-free culture medium and digestion passage, till P9-generation neural stem cells are obtain by repeating the previous steps. The neural stem cell injection is 95 percent in purity, and can be applied to treatment of various brain damage diseases such as cerebral apoplexy, hypoxic-ischemic brain damage and infantile cerebral palsy.
Owner:SHANGHAI ANGECON BIOTECH
Methods and materials relating to stem cell growth factor-like polypeptides and polynucleotides
InactiveUS20030022825A1Minimize side effectsPeptide/protein ingredientsEnzymologyCDNA librarySpinal cord
The invention provides novel polynucleotides and polypeptides encoded by such polynucleotides and mutants or variants thereof that correspond to a novel human and mouse secreted stem cell growth factor-like polypeptide. These polynucleotides comprise nucleic acid sequences isolated from cDNA libraries prepared from mouse bone marrow and human fetal liver spleen, ovary, adult brain, lung tumor, spinal cord, cervix, ovary, endothelial cells, umbilical cord, placental, lymphocyte, lung fibroblast, fetal brain, and testis. Other aspects of the invention include vectors containing processes for producing novel human secreted stem cell growth factor-like polypeptides, and antibodies specific for such polypeptides.
Owner:KIRIN PHARMA +1
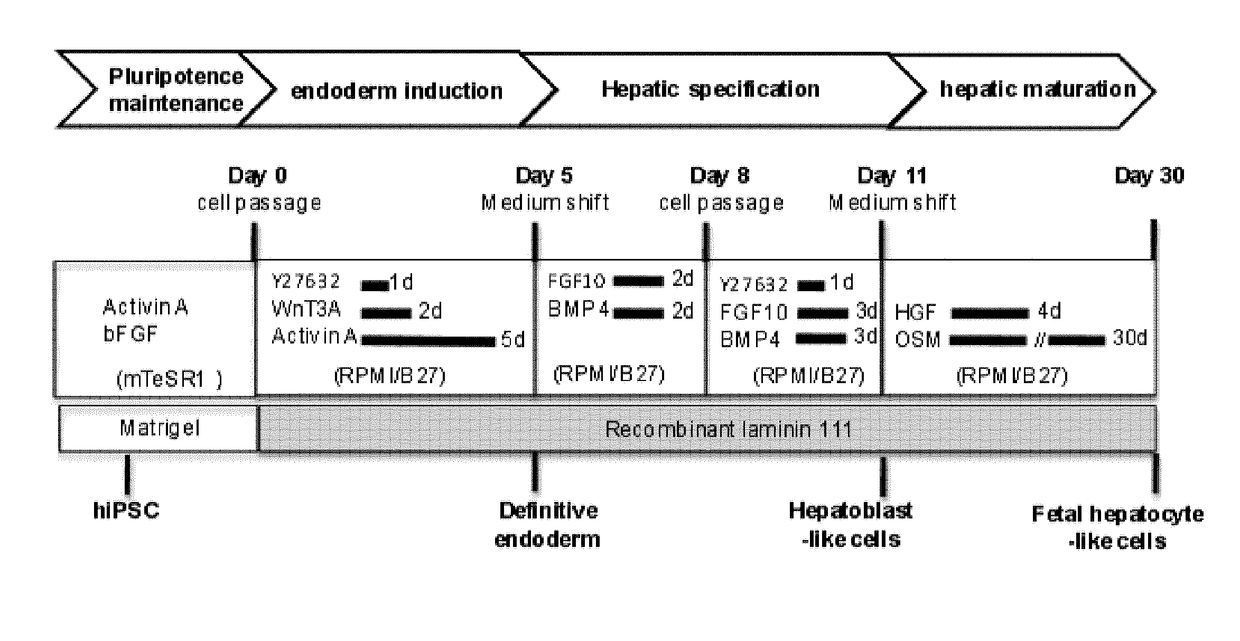
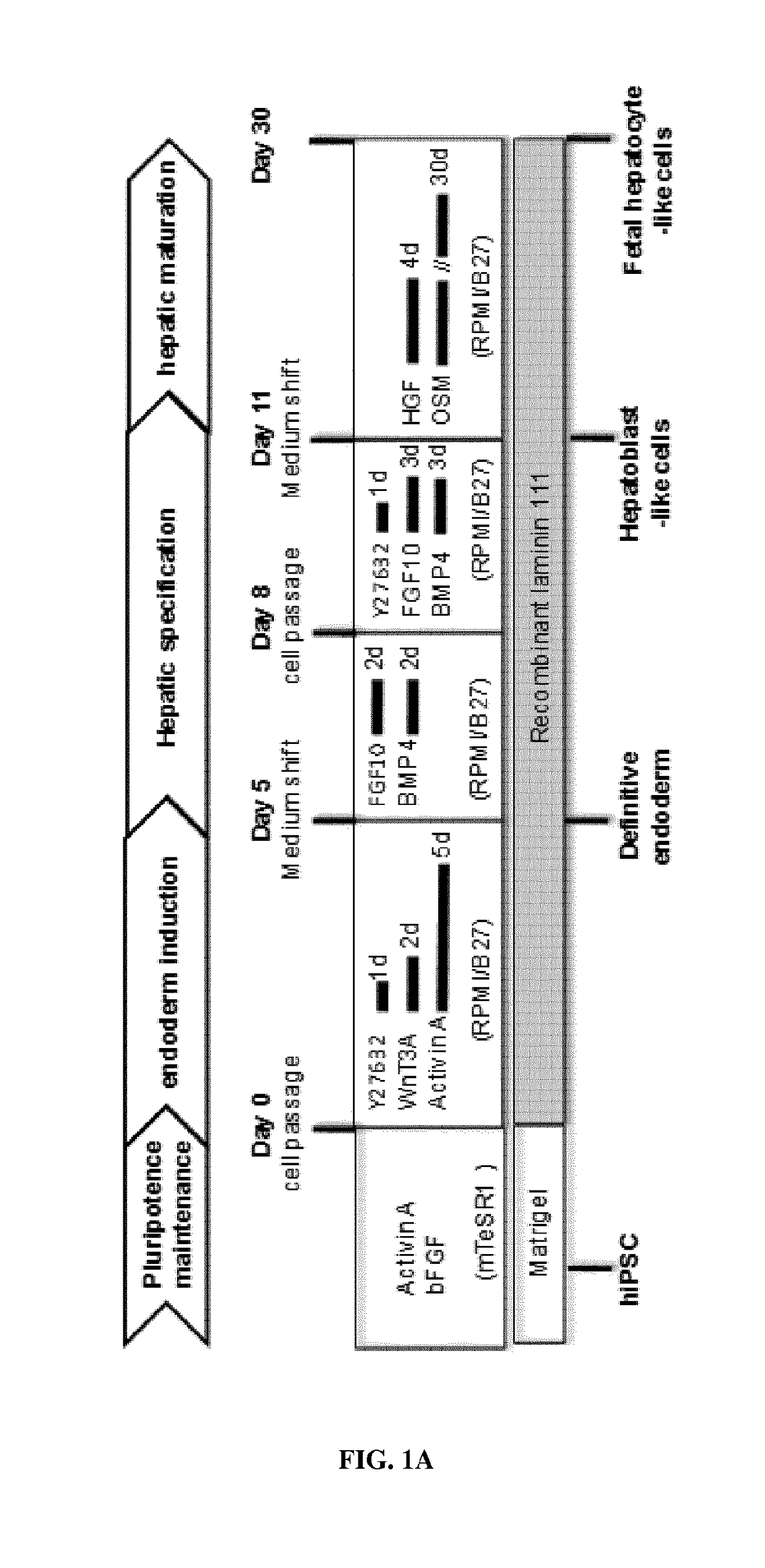
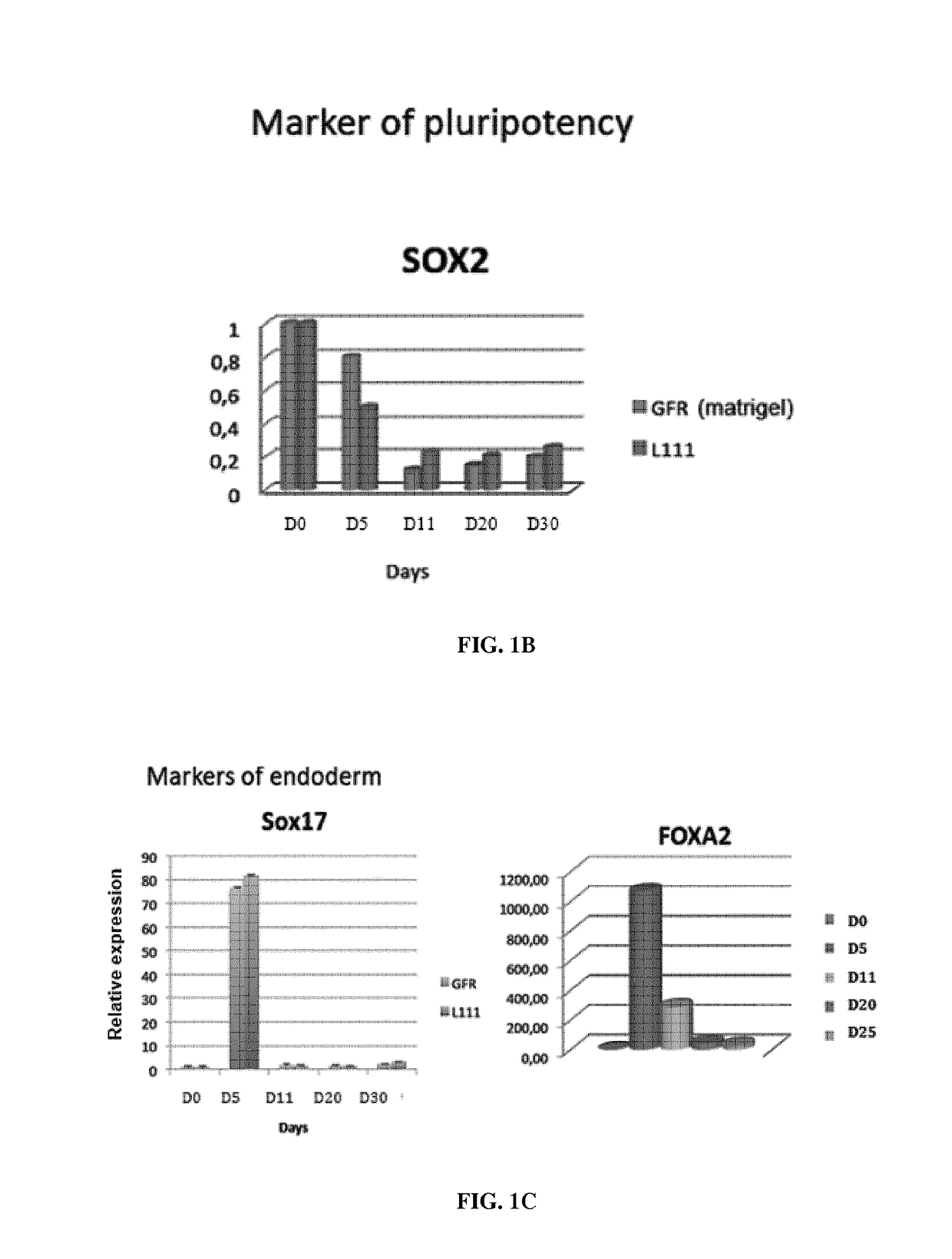
Use of a laminin for differentiating pluripotent cells into hepatocyte lineage cells
The invention relates to the use of a laminin (LN) as a matrix for hepatic differentiation. The invention also relates to a method for inducing hepatic differentiation comprising the steps of: (i) providing a population of human pluripotent cells, (ii) culturing the population on a support coated with a laminin in a endoderm induction medium to produce a population of human DE cells, (iii) culturing said population of human DE cells on a support coated with a laminin in a hepatic induction medium to produce a population of human hepatoblasts-like cells, and (iv) optionally culturing said population of human hepatoblasts-like cells on a support coated with a laminin in a hepatic maturation medium to produce a population of human hepatocyte-like cells. The invention further relates to a population of human hepatoblasts-like cells or human fetal hepatocyte-like cells obtained by the method of the invention. The invention further relates to a population of human hepatoblasts-like cells expressing HNF4α and expressing substantially AFP for use in a method of treatment of the human body.
Owner:UNIV DE NANTES +1
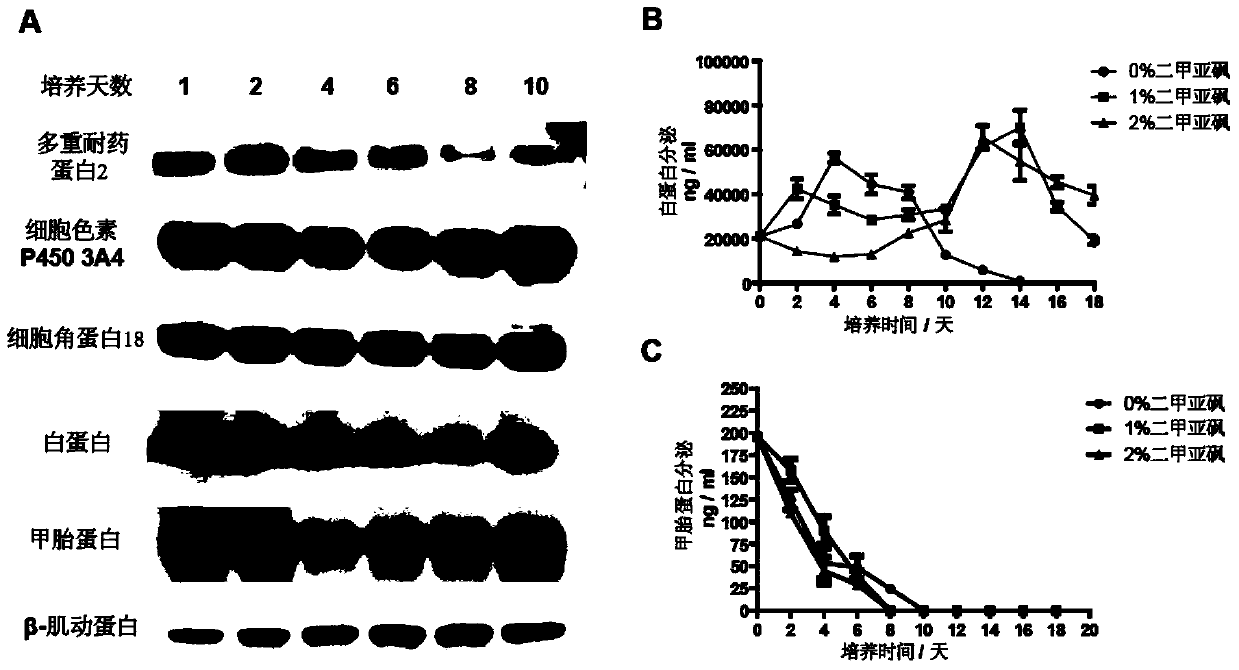
Method for separating, freezing and resuscitating human fetal hepatocytes susceptible to hepatitis B virus and application of method
InactiveCN103740637AObvious morphological featuresIncrease proliferative activityMicrobiological testing/measurementDead animal preservationHepatitis B immunizationPetri dish
The invention discloses a method for separating, freezing and resuscitating human fetal hepatocytes susceptible to hepatitis B virus and an application of the method. The method comprises the following steps of thoroughly rinsing fresh hepatic tissues by utilizing a filling solution through an exposed blood vessel until complete digestion; placing the fresh separated hepatic cells into a culture dish containing cell washing liquid, and filtering the cells by utilizing a cell sieve to obtain single cell suspended liquid; precipitating washed single cells, re-suspending the cells, and storing the precipitated single cells to liquid nitrogen by utilizing a gradual freezing method; and establishing compact cell single-layer culture by optimizing planking density of high-vitality fetal hepatic cells, purifying the low-vitality hepatic cells through a Percoll centrifugal medium, subsequentially culturing the cells so as to resuscitate the fetal hepatic cells. By adopting the method, a great amount of fetal hepatic cells can be separated, stored and high-efficiently resuscitated, and the resuscitated fetal hepatic cells can maintain good characteristics of the hepatic cells, good differentiation state and good susceptibility to the hepatitis B virus, so that the problem that the primary hepatic cells are in shortage can be solved, and a stable hepatitis B virus medicinal sieve platform can be established.
Owner:康珞生物科技(武汉)有限公司
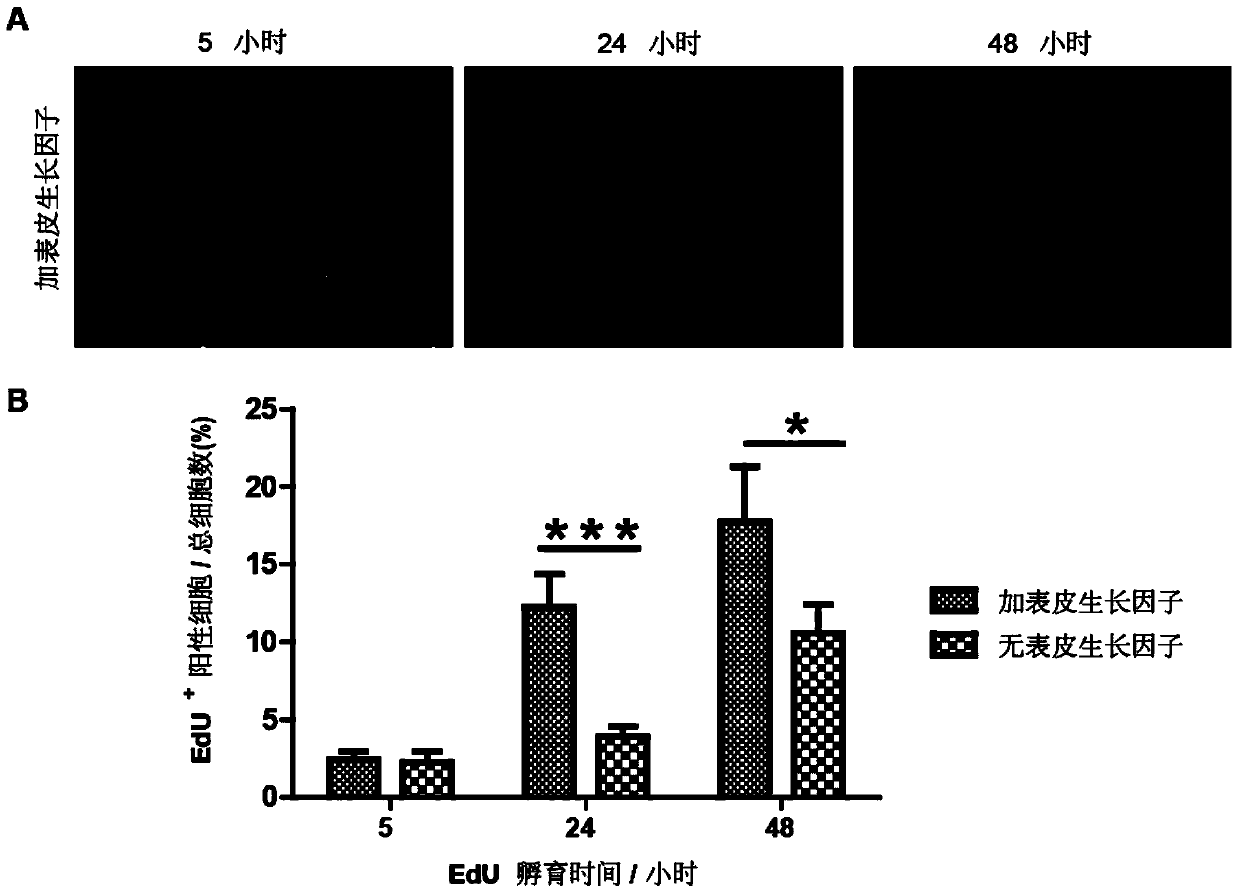
Production of typed human cells, tissues and organs
InactiveUS20020100065A1High yieldNew breed animal cellsArtificial cell constructsCord blood stem cellMammal
A method of obtaining a high yield of differentiated human cells and organs includes the steps of providing typed human bone marrow or cord blood stem cells, providing pre-immune non-human mammalian fetuses, implanting the cells into the fetuses, permitting the fetuses to grow for a sufficient time to produce differentiated cells in hybrid organs, and harvesting the differentiated cells from the mammals. A method is presented to produce hybrid functioning human-animal solid organs for clinical transplantations. The method includes obtaining bone marrow mononuclear cells (BMNC) from the patient, obtaining enriched populations of HSC from the BMNC, and transplanting the enriched cells into Preimmune fetal sheep or pigs intraperitoneally to produce functioning donor (patient)-animal hybrid organs. A method is also presented in which enriched HSC isolated from pre-HLA typed normal human fetal liver / bone marrow, cord blood, or bone marrow will be transplanted into preimmune fetal sheep or pigs in order to create functioning human-animal hybrid organs that can be transplanted into compatible patients. Methods are also presented to obtain high yield of different types (e.g. hepatocytes) of donor (patient or HLA-typed normal donors) cells from the human-animal hybrid organs that can be used either for transplant into patients and / or treatment of the patient. Also disclosed is a method of producing purified human proteins that includes providing a non-human, pre-immune mammal into which human bone marrow or cord blood cells has been implanted into the mammal at the pre-immune state, obtaining blood from the non-human mammal, and isolating the human proteins from the mammalian blood.
Owner:ZANJANI ESMAIL D
Human derived immortalized liver cell line
InactiveUS7186553B1Genetically modified cellsMicrobiological testing/measurementLiver functionNormal cell
The present invention relates to a new immortalized hepatocyte culture of human (preferably human fetal) normal cell origin. The immortalized hepatocyte culture of human normal cell origin of the present invention is useful in, for example, screening for compounds or salts thereof having therapeutic / preventive effects on hepatic insufficiency.
Owner:TAKEDA PHARMA CO LTD
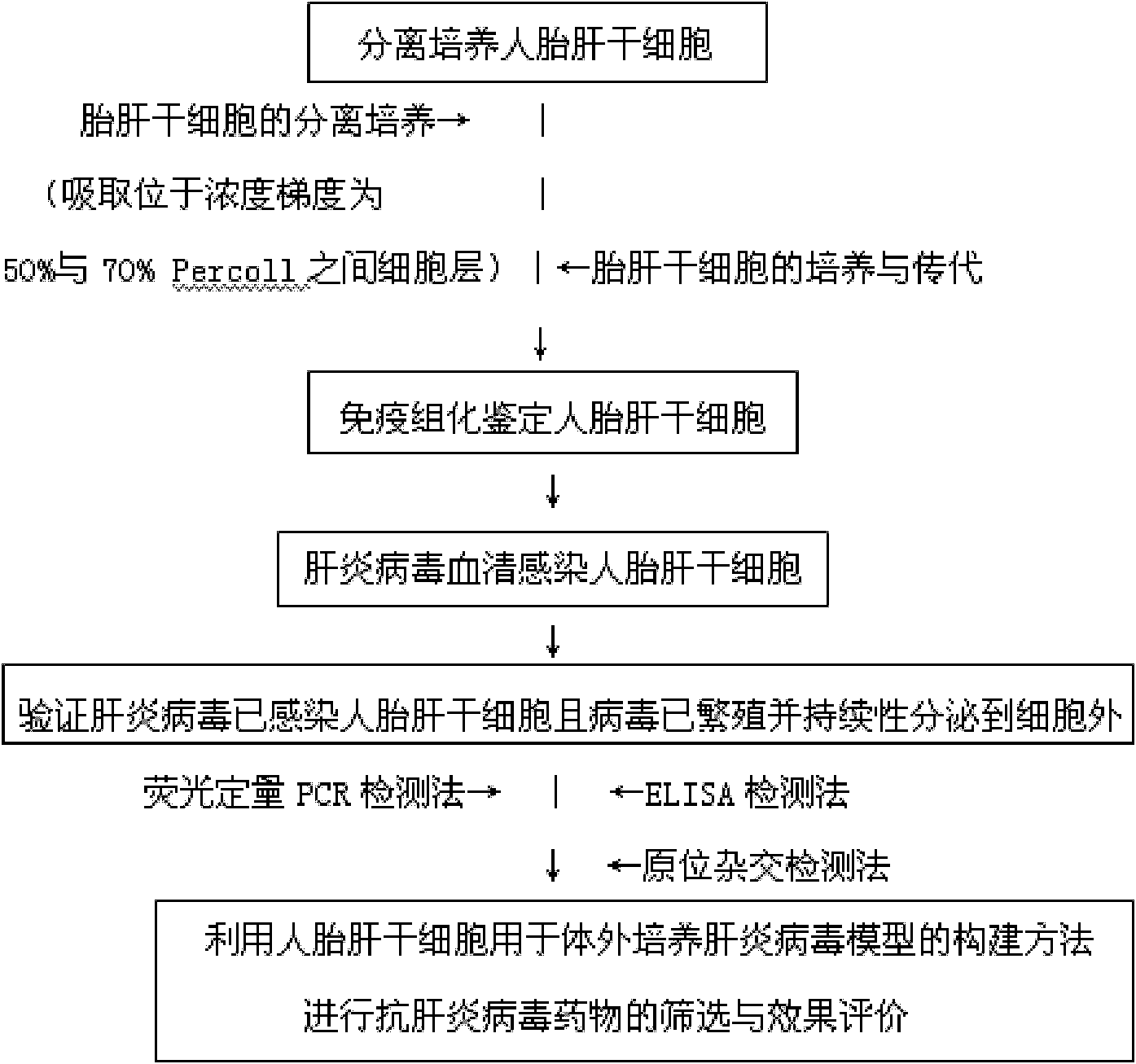
Construction method and use of model of hepatitis B virus infection in vivo
InactiveCN101696396ASimple methodGood repeatabilitySsRNA viruses positive-senseMicrobiological testing/measurementIn vivoHepatitis B virus
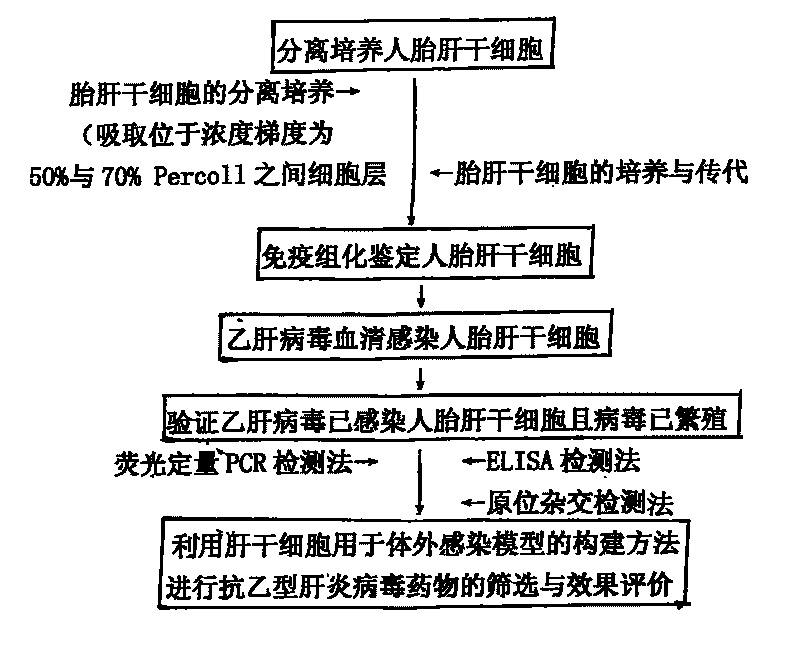
The invention discloses a construction method and use of a model of hepatitis B virus infection in vivo. The construction method of the model of hepatitis B virus infection in vivo, which uses stem cells of the human fetal liver in anti-hepatitis B virus medicament screening, comprises the following steps: separation and cultivation of the stem cells of the human fetal liver; immunohistochemical detection of the stem cells of the human fetal liver; hepatitis B virus serum infection of the stem cells of the human fetal liver; and verification of the infection of the stem cells of the human fetal liver by the hepatitis B viruses and the propagation of the viruses. The construction method, which uses the stem cells of the human fetal liver in the model of the infection in vivo, is used to screen and evaluate anti-hepatitis B virus medicaments. The method has the advantages that: the stem cells of the human fetal liver can be infected by the anti-hepatitis B viruses and constantly secrete anti-hepatitis B viruses; the stem cells of the human fetal liver can be passed and cultured; the method is simple and convenient; and the repeatability is desirable.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
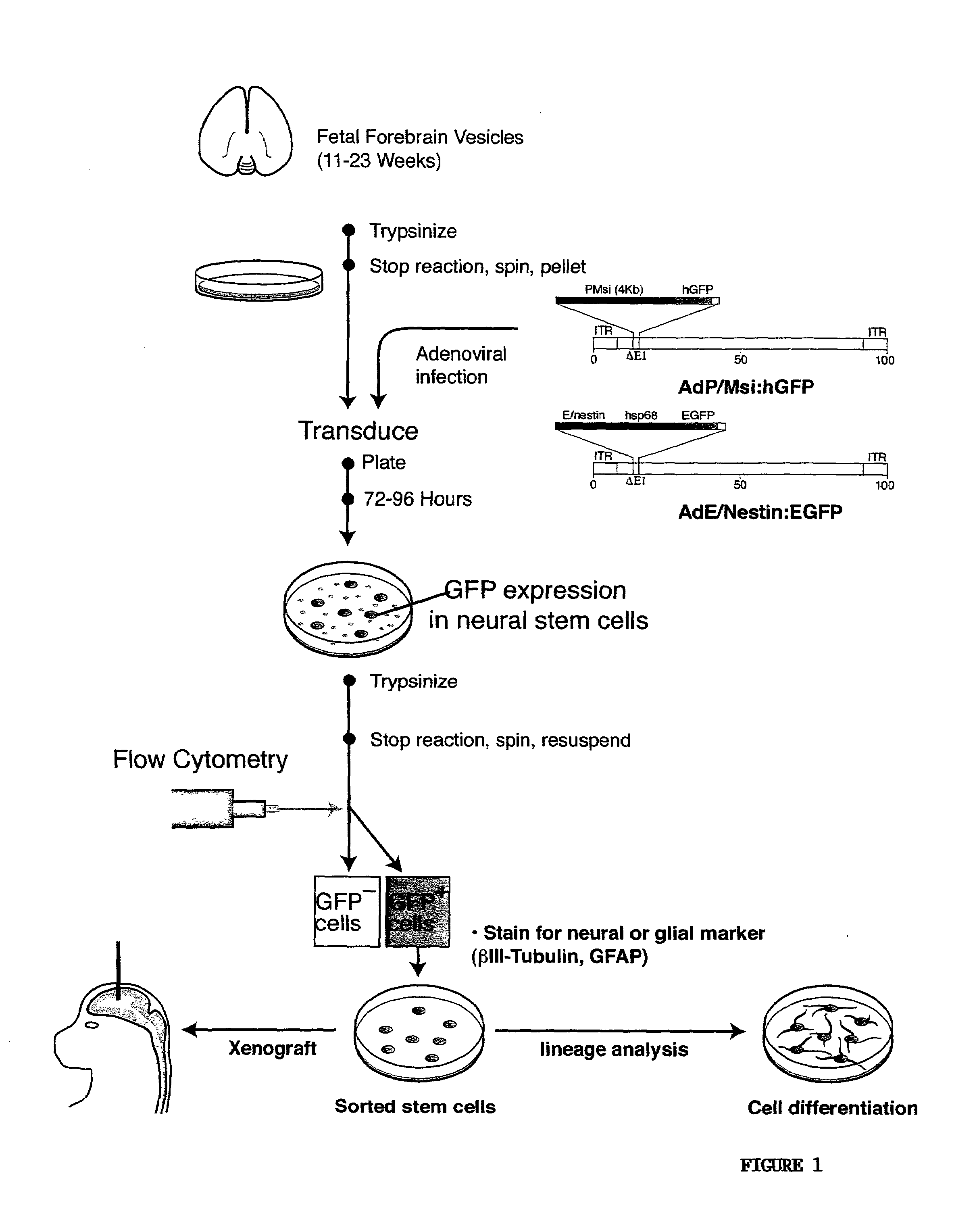
Enriched preparation of human fetal multipotential neural stem cells
The present invention relates to a method of separating multipotential neural progenitor cells from a mixed population of cell types. This method includes selecting a promoter which functions selectively in the neural progenitor cells, introducing a nucleic acid molecule encoding a fluorescent protein under control of said promoter into all cell types of the mixed population of cell types, allowing only the neural progenitor cells, but not other cell types, within the mixed population to express said fluorescent protein, identifying cells of the mixed population of cell types that are fluorescent, which are restricted to the neural progenitor cells, and separating the fluorescent cells from the mixed population of cell types, wherein the separated cells are restricted to the neural progenitor cells. The present invention also relates to an isolated human musashi promoter and an enriched preparation of isolated multipotential neural progenitor cells.
Owner:CORNELL RES FOUNDATION INC +1
Hepatitis virus in-vitro culture model as well as construction method and application thereof
InactiveCN101914489ASimple methodGood repeatabilitySsRNA viruses positive-senseMicrobiological testing/measurementLiver Stem CellHuman fetal
The invention relates to a hepatitis virus in-vitro culture model as well as a construction method and application thereof. The hepatitis virus in-vitro culture model is constructed by adopting human fetal liver stem cells and comprises the steps of carrying out separate culture on human fetal liver stem cell, immunohistochemically identifying human fetal liver stem cell and infecting human fetal liver stem cell with hepatitis virus by blood serum and verifying whether hepatitis virus infects the human fetal liver stem cells or not and the virus propagate and continuously secrete outside the cell or not; the model can be used for the screening and effect evaluation of an in-vitro hepatitis virus resistant medicament; the human fetal liver stem cells used in the hepatitis virus in-vitro culture model can be infected with hepatitis virus and can continuously secrete hepatitis virus; the human fetal liver stem cells can be subcultured; and the invention has simple and convenient method and high repeatability.
Owner:INST OF HYGIENE & ENVIRONMENTAL MEDICINE PLA ACAD OF MILITARY MEDICAL
Separation culture method of human fetal retinal pigment epithelial cells
The invention provides a separation culture method of human fetal retinal pigment epithelial cells, relating to a method for separating fRPE (fetal retinal pigment epithelial) cells by utilizing a mechanical method and performing in-vitro culture. The separation culture method of the human fetal retinal pigment epithelial cells, provided by the invention, provides a new source for in-vitro culture, amplification of RPE (retinal pigment epithelial) cells and treatment of AMD (age-related macular degeneration) and other diseases with RPE injuries.
Owner:SUZHOU RUIQI BIO PHARMA CO LTD
Methods and materials relating to stem cell growth factor-like polypeptides and polynucleotides
InactiveUS20030211987A1Bioreactor/fermenter combinationsBiological substance pretreatmentsCDNA librarySpinal cord
The invention provides novel polynucleotides and polypeptides encoded by such polynucleotides and mutants or variants thereof that correspond to a novel human secreted stem cell growth factor-like polypeptide. These polynucleotides comprise nucleic acid sequences isolated from cDNA libraries prepared from human fetal liver spleen, ovary, adult brain, lung tumor, spinal cord, cervix, ovary, endothelial cells, umbilical cord, lymphocyte, lung fibroblast, fetal brain, and testis. Other aspects of the invention include vectors containing processes for producing novel human secreted stem cell growth factor-like polypeptides, and antibodies specific for such polypeptides.
Owner:KIRIN PHARMA
Polypeptide, cDNA encoding the same, and use of them
A polypeptide prepared from mouse ES cell strains by the SST method and a homologous polypeptide obtained from mouse kidney, human uterus, mouse fetus, human fetal liver and pancreas libraries; a cDNA encoding the polypeptide; a fragment selectively hybridizing with the sequence of the cDNA; a replication or expression plasmid containing the cDNA integrated thereinto; a host cell transformed with plasmid; an antibody against the polypeptide; and a pharmaceutical composition containing the polypeptide or the antibody.
Owner:ONO PHARMA CO LTD
Cell culture method and application thereof
ActiveUS20100015103A1Maintain activityGuaranteed functionBiocidePeptide/protein ingredientsMesenchymal stem cellHuman fetal
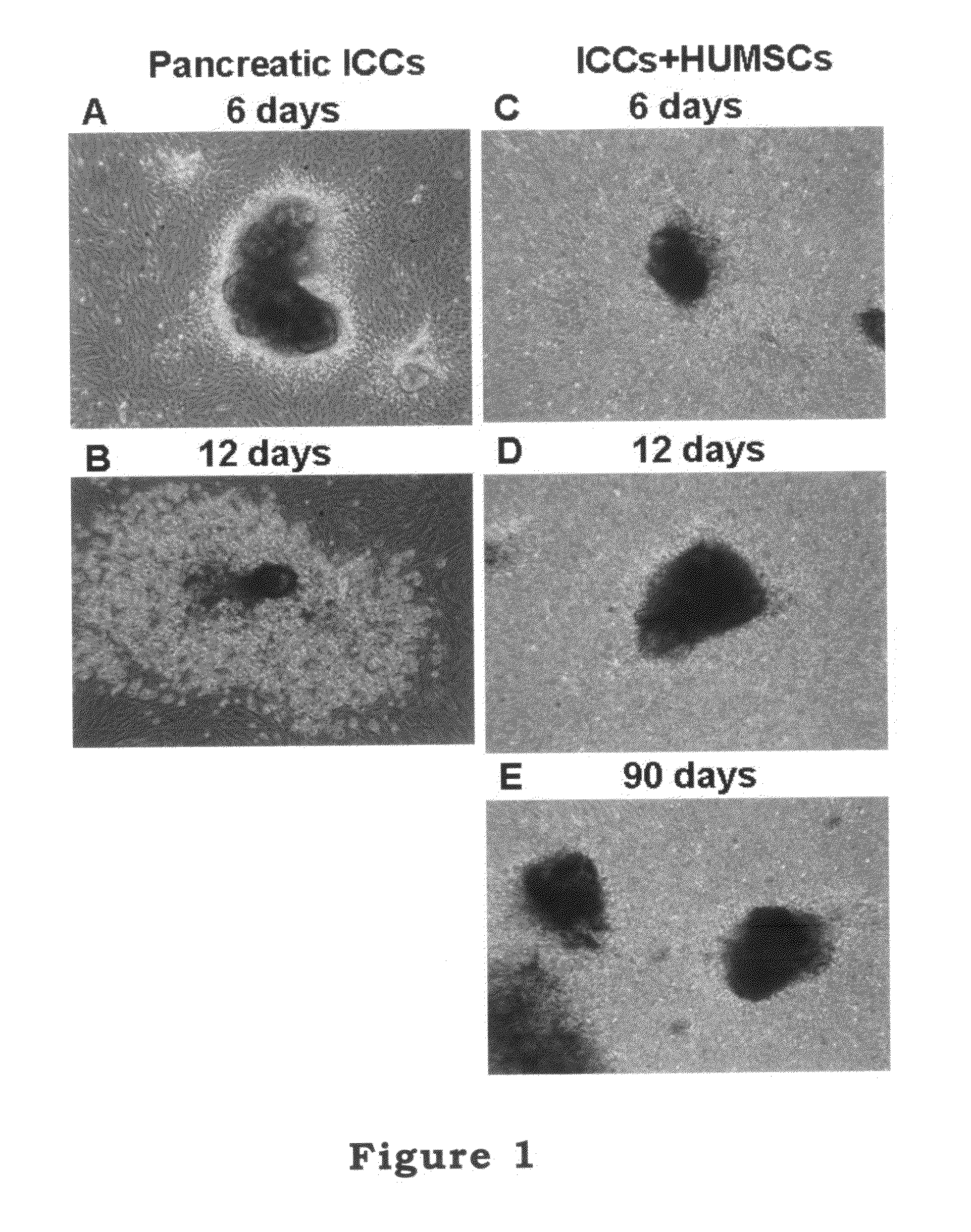
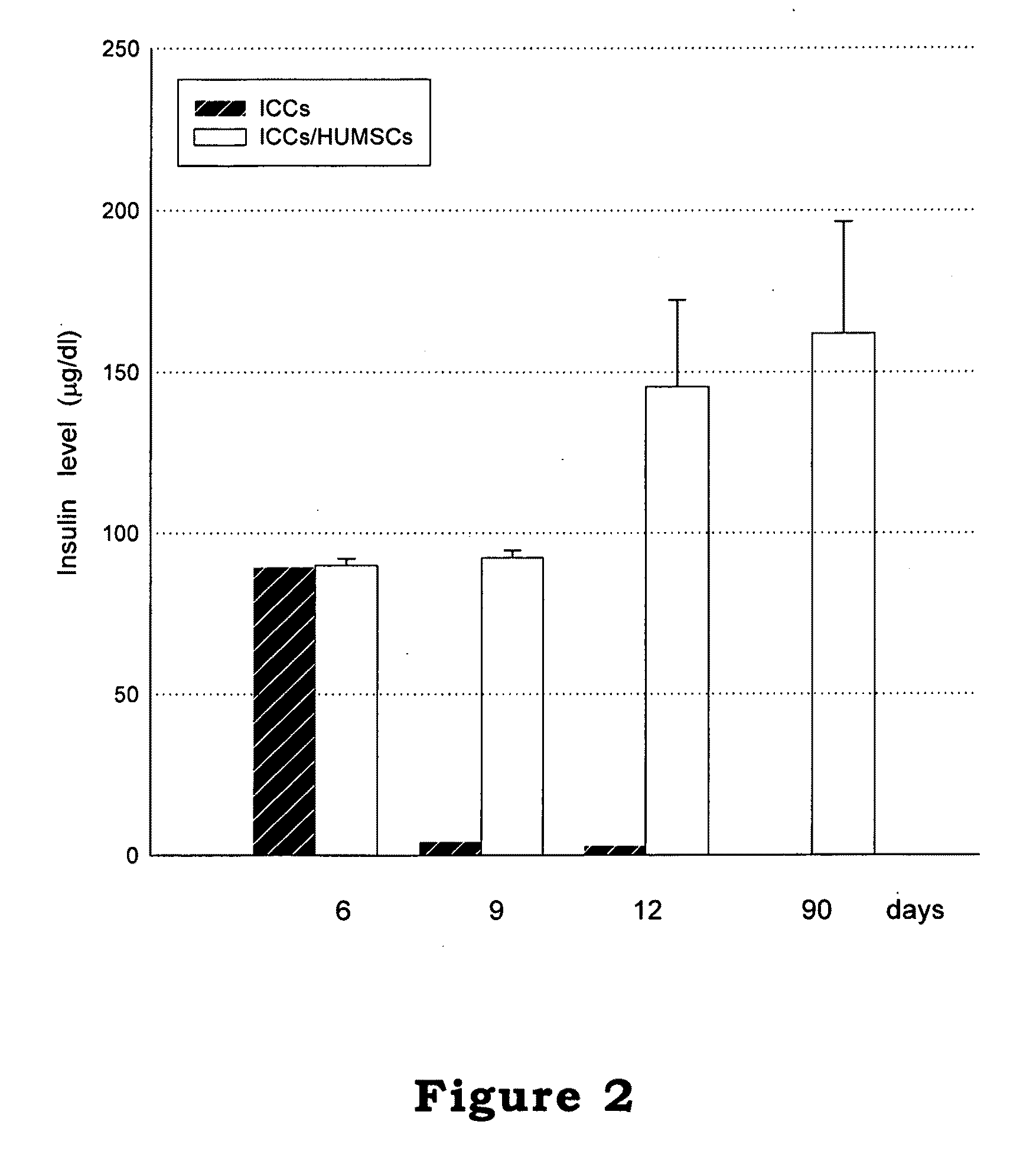
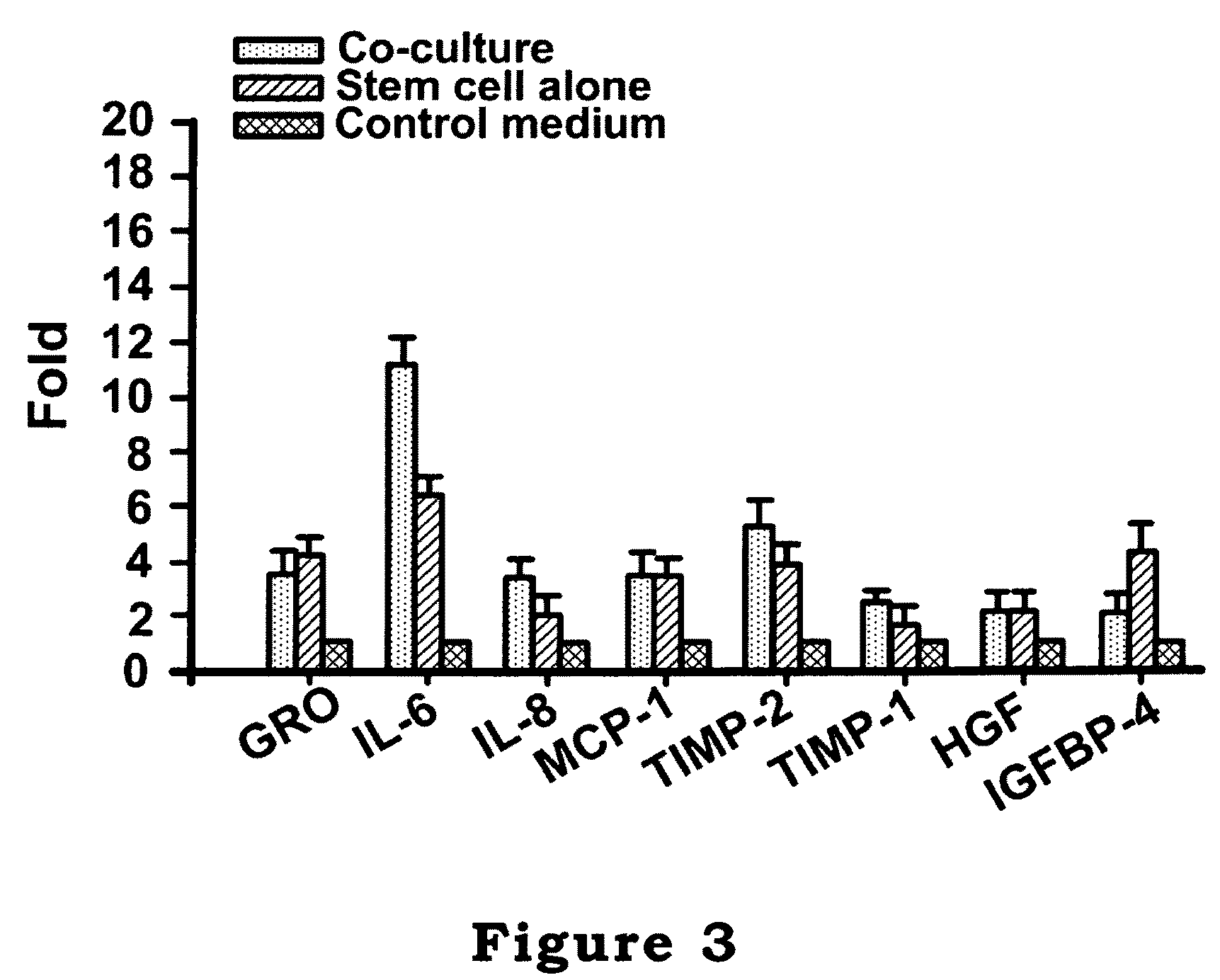
The invention relates to a cell culture method, particularly to a co-culture method for human mesenchymal stem cells and target animal cells, in order to solve the problem that animal cells are not easy to survive alone upon culturing. The invention also provides a method for using a stem cell conditioned medium to culture animal cells. The invention also provides a method to induce the transformation of human fetal islet-like cell clusters from human stem cells and its application thereof.
Owner:NAT TAIWAN UNIV
Method for inducing differentiation of human embryo mesenchymal stem cells into pancreatic islet beta-like cell
InactiveCN101186901AImprove proliferative abilityImprove plasticityVertebrate cellsArtificial cell constructsSocial benefitsMesenchymal stem cell
The invention provides a process for inducing mescenchymal stem cell of human fetal bone marrow to differentiate to be islet beta cells. The process provided by the invention comprises the following steps including separating, purifying and augmenting mescenchymal stem cell of human fetal bone marrow, and then inducing mescenchymal stem cell of human fetal bone marrow to differentiate to islet beta cells, and beta cell mass is achieved after six to twelve days of inducement. The process provided by the invention induces mescenchymal stem cell of human fetal bone marrow to be islet beta cells which can secrete insulin in vitro. The achieved islet beta cells can be further used for preparing medicament which is used for treating diabetes. The study of the field has wide clinical application prospect and can bring relatively great economic and social benefit.
Owner:JINAN UNIVERSITY
Method for the production of antibodies
InactiveUS20070266448A1Immunoglobulins against animals/humansBlood/immune system cellsHuman animalLiver Stem Cell
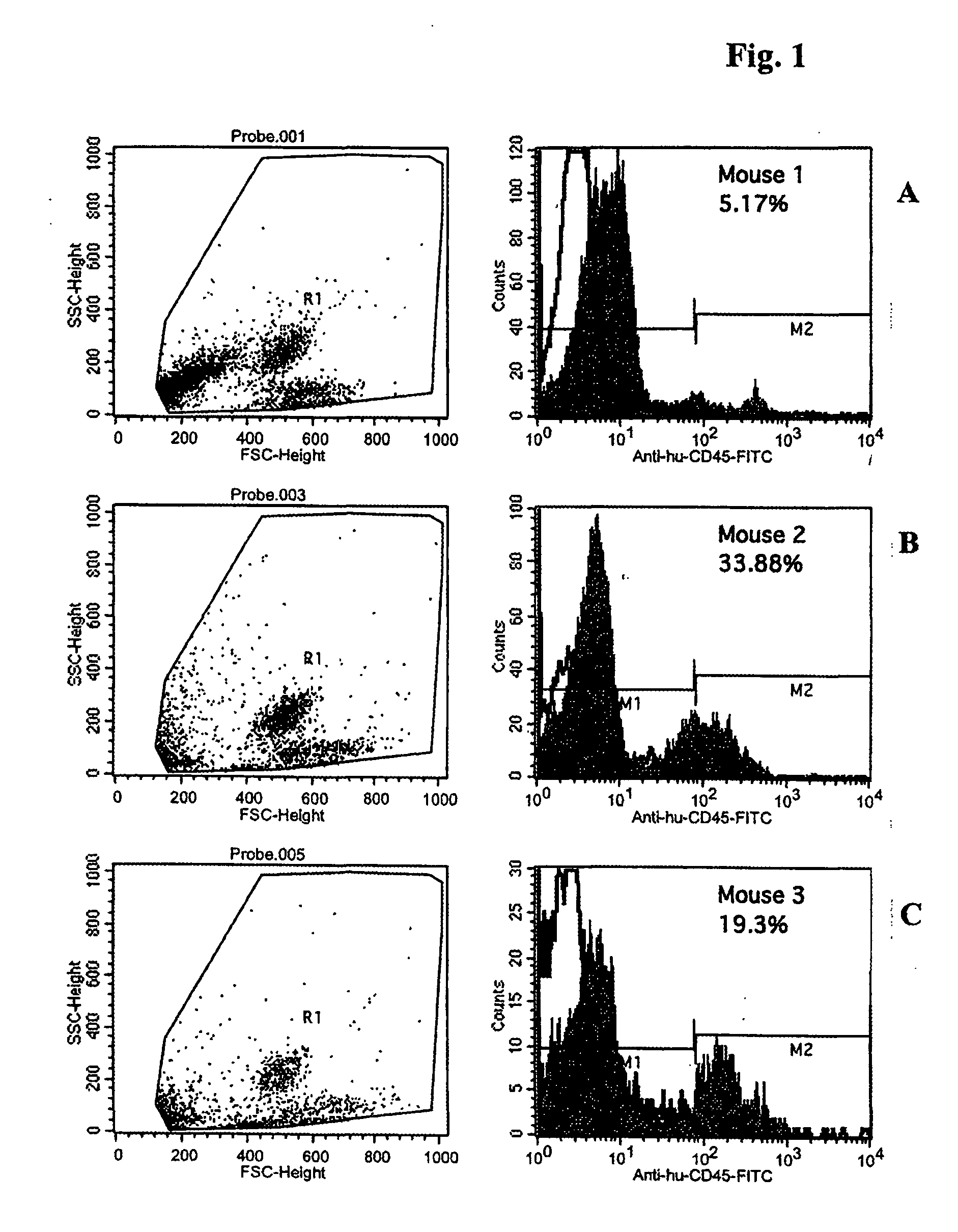
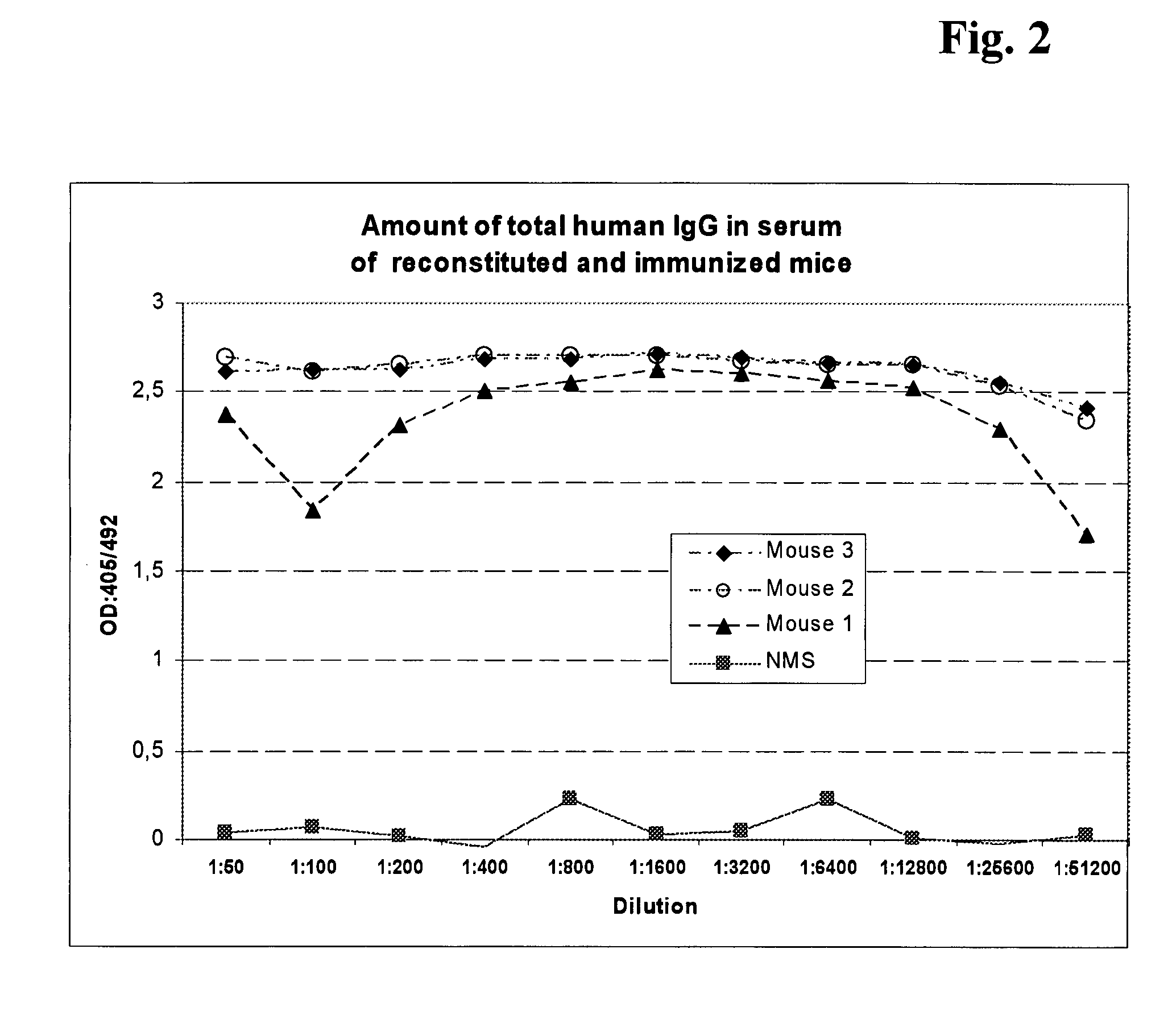
The current invention is related to a method for the production of a human monoclonal antibody from a immundeficient non-human animal, said method comprising contacting a new borne immunodeficient non-human animal with a human fetal liver stem cell (FL cell) to generate an immune transplanted non-human animal (reconstituted animal), subsequently contacting said reconstituted animal with a antigen, collecting from said reconstituted animal a human cell producing human antibody against said antigen, and isolating said antibody from said antibody producing cell.
Owner:F HOFFMANN LA ROCHE & CO AG
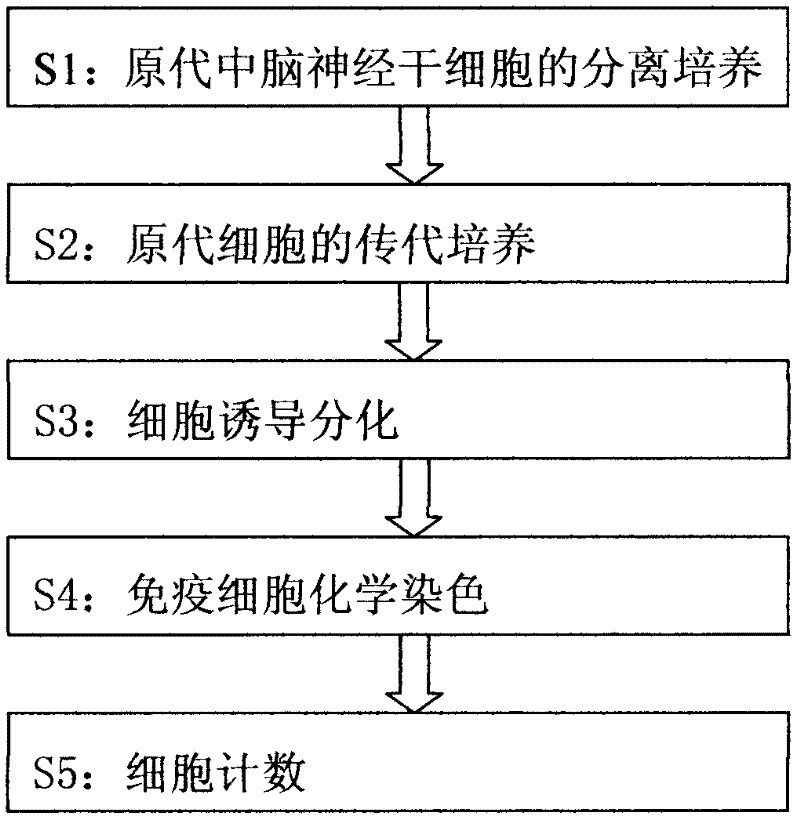
In-vitro separation culture and differentiation method for human fetal midbrain nerve stem cells
The invention provides an in-vitro separation culture and differentiation method for human fetal midbrain nerve stem cells, which is used for separation culture and identification of nerve stem cells from the human fetal midbrain and comprises the steps of: original generation midbrain nerve stem cell separation culture, original generation cell subculture, cell induction differentiation, immune cell chemical dyeing and cell counting. In the method, the serum-free culture and the single-cell clone technology is adopted for separating the cells with the single-cell clone capability in the embryonic cortex, in addition, the culture and the transfer culture are carried out, the wall pasting differentiation observation is carried out, and the indirect immunofluorescence is adopted for detecting the expression of the Nestin antigen of the clone cells and the antigen of the differentiated specific mature nerve cells.
Owner:江苏迈健生物科技发展股份有限公司
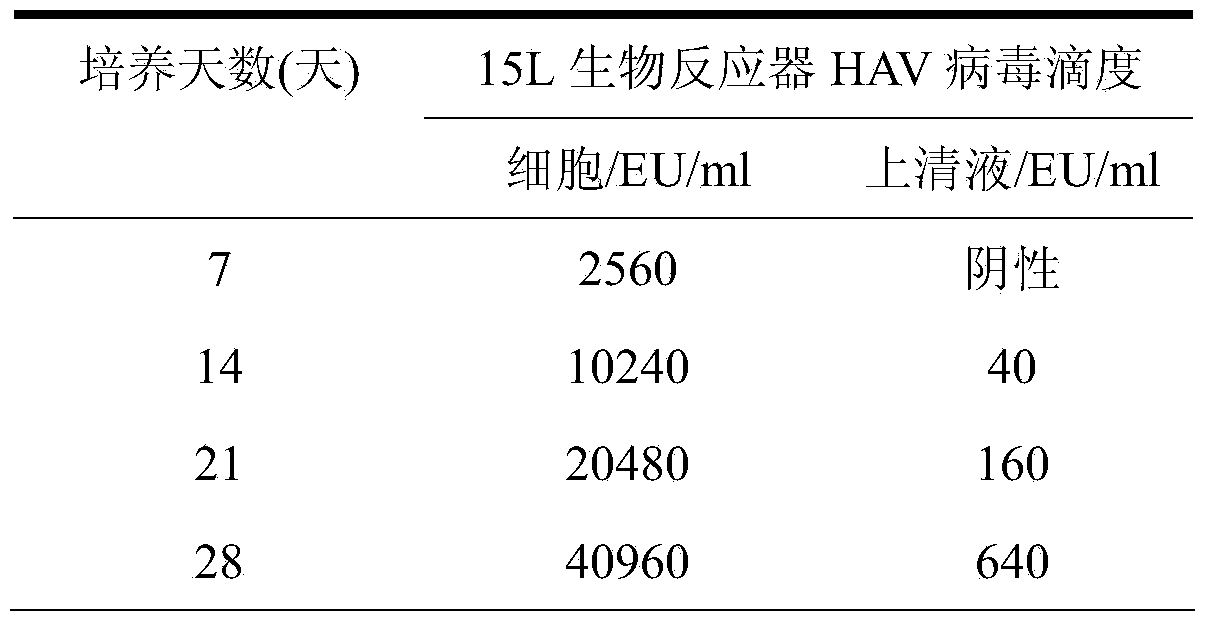
Application of human fetal lung fibroblast line in preparation of hepatitis A vaccines
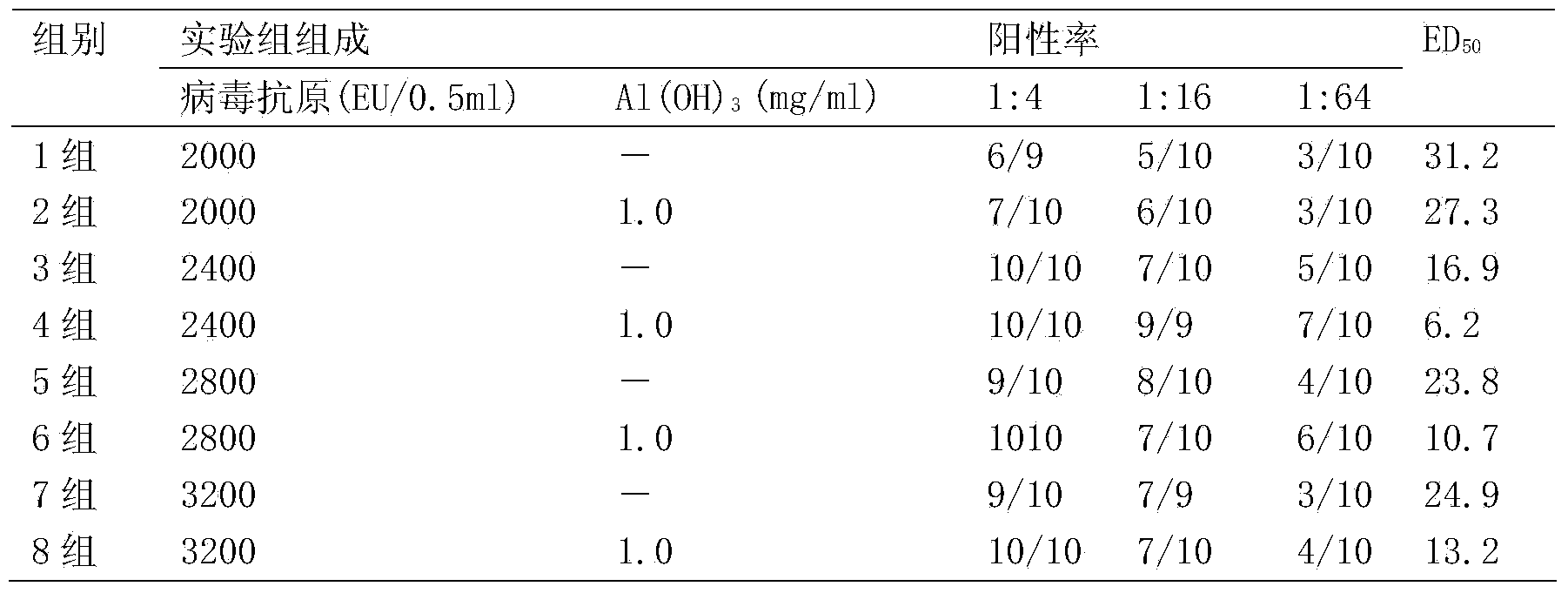
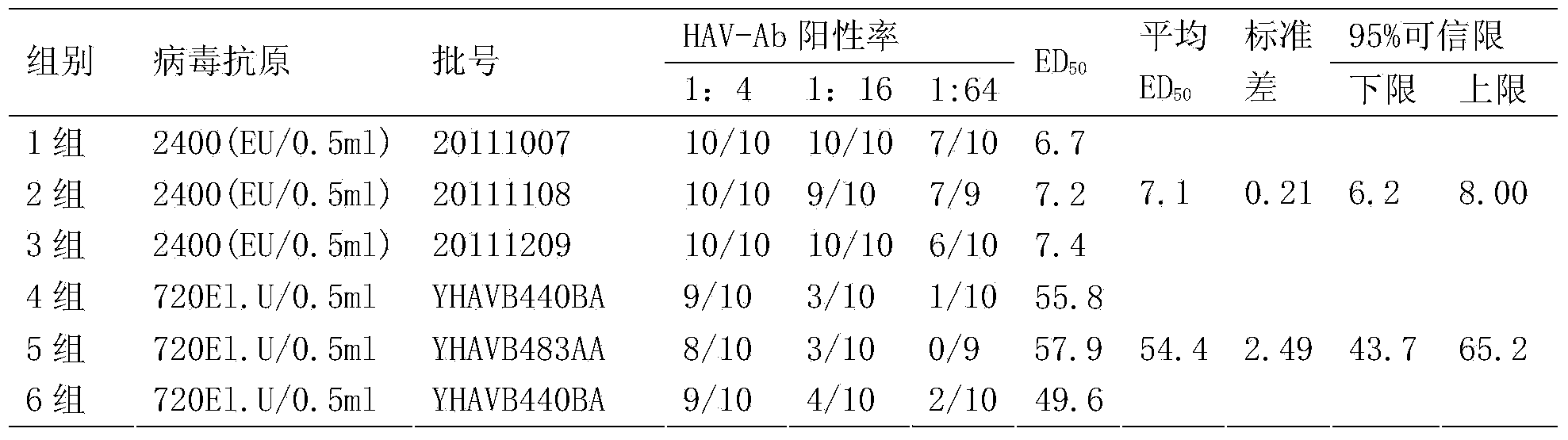
ActiveCN103525770AIncrease productionImproving immunogenicityMicroorganism based processesAntiviralsAntigenHepatitis A vaccine
The invention discloses an application of a human diploid fibroblast line Walvax-2 in the preparation of hepatitis A vaccines. The human diploid fibroblast line is susceptible to hepatitis A viruses. A human diploid cell hepatitis A virus purifying vaccine having the advantages of high antigen purity, good immunization effect, high security and low price can be obtained by adopting the Walvax-2 to produce the hepatitis A vaccines.
Owner:云南沃森生物技术股份有限公司
Methods of producing human RPE cells and pharmaceutical preparations of human RPE cells
ActiveUS10485829B2Reduced HLA antigen complexityImprove acuitySenses disorderNervous system cellsDiseaseEmbryo
The present invention provides improved methods for producing retinal pigmented epithelial (RPE) cells from human embryonic stem cells, human induced pluripotent stem (iPS), human adult stem cells, human hematopoietic stem cells, human fetal stem cells, human mesenchymal stem cells, human postpartum stem cells, human multipotent stem cells, or human embryonic germ cells. The RPE cells derived from embryonic stem cells are molecularly distinct from adult and fetal-derived RPE cells, and are also distinct from embryonic stem cells. The RPE cells described herein are useful for treating retinal degenerative conditions including retinal detachment and macular degeneration.
Owner:ADVANCED CELL TECH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com