Application of human fetal lung fibroblast line in preparation of hepatitis A vaccines
A technology of fibroblasts and human embryonic lungs, applied in the field of vaccines, can solve the problems of limiting the increase and further use of virus production, and the use of cells without promoting the use of cell generations, so as to avoid cell protein residues, good immune effect, and safety high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of embodiment 1 Walvax-2 cell strain
[0025] 1.1. Acquisition of experimental materials: After obtaining the approval of the national health management department and the ethics committee, investigate the age, occupation and health status of the couples who donate pregnant women, as well as the three generations of parents of the fetus. Fetuses with a family history of genetic defects. After obtaining the consent of the parents of the fetus and their relatives, sign the "Donor Consent".
[0026] 1.2. Primary culture: embryos sent to the laboratory in time after induction of water sac, the lung tissue was taken out under sterile conditions, the membrane on the surface of the lung tissue was torn off, and cut into 1-2 mm 3 The tissue pieces were washed with PBS for 3 to 4 times; the tissue pieces were transferred into the Erlenmeyer flask; digested with trypsin for 30 minutes, neutralized with serum, centrifuged at 1000rpm for 5 minutes to discard the trypsi...
Embodiment 2
[0032] Embodiment 2 prepares hepatitis A inactivated vaccine with Walvax-2 cell line
[0033] 2.1 Cell factory preparation of Walvax-2 cells
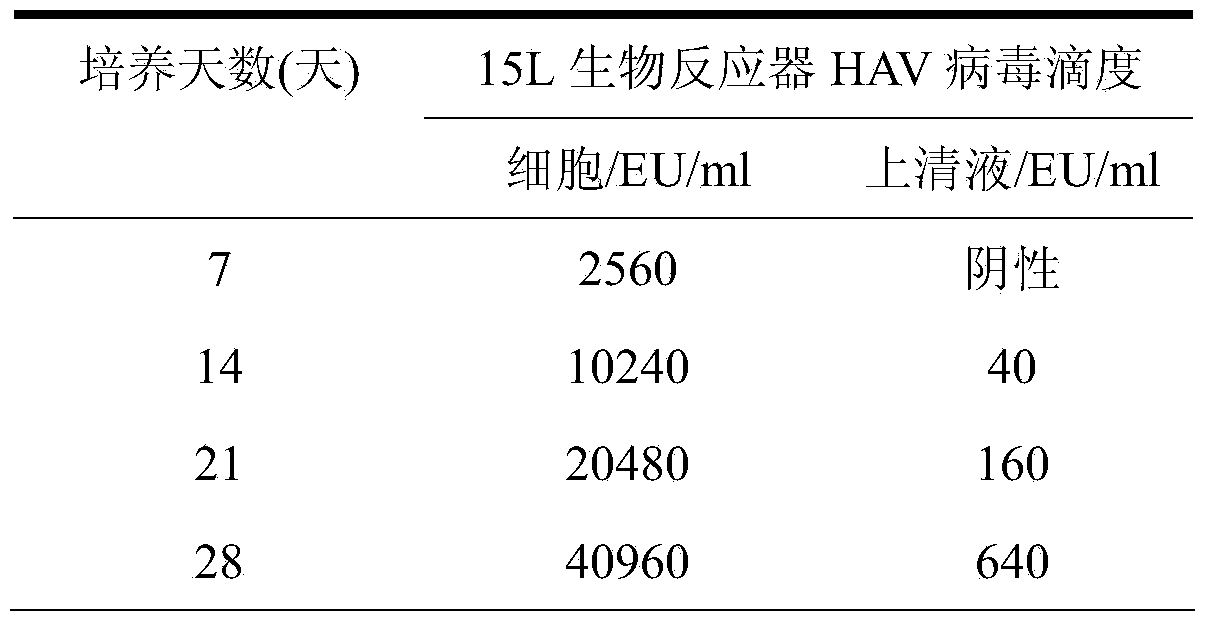
[0034] Walvax-2 cells were resuscitated in T75 flasks for 20 passages, the cell viability rate was above 99%, and cultured in a closed manner at 37°C±0.5°C until the cells covered the growth surface, digested with 0.25% trypsin, and passaged at a split ratio of 1:2 Cultivated to a cell mass that can be used for large-scale production in bioreactors. After the cells were digested, they were collected into Erlenmeyer flasks and waited to be inoculated with the virus. The cell growth medium in the cell culture medium is MEM nutrient solution containing 10% newborn bovine serum by volume.
[0035] 2.2 Walvax-2 cells inoculated with HAV virus
[0036] The collected Walvax-2 cells were sampled and counted, the cell concentration was adjusted, and the hepatitis A virus YN5 strain CCTCC NO:V200104 was inoculated. The MOI of infection was 0.5...
Embodiment 3
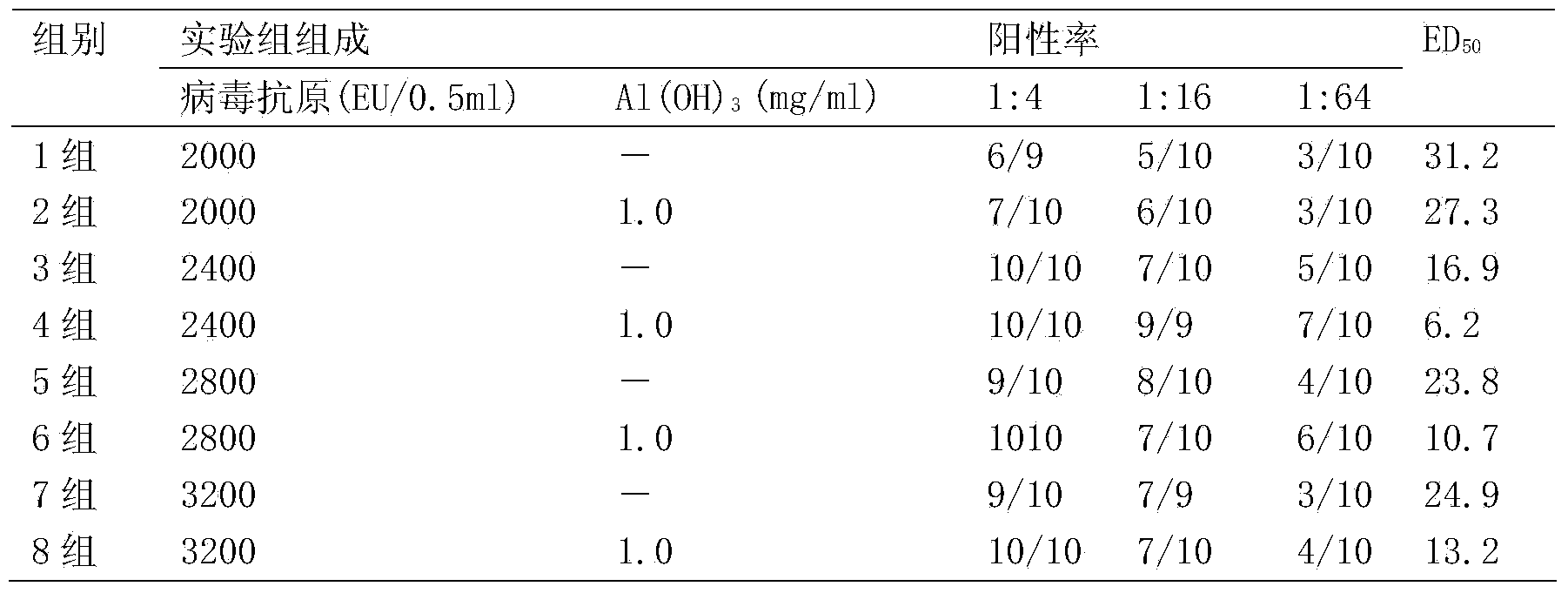
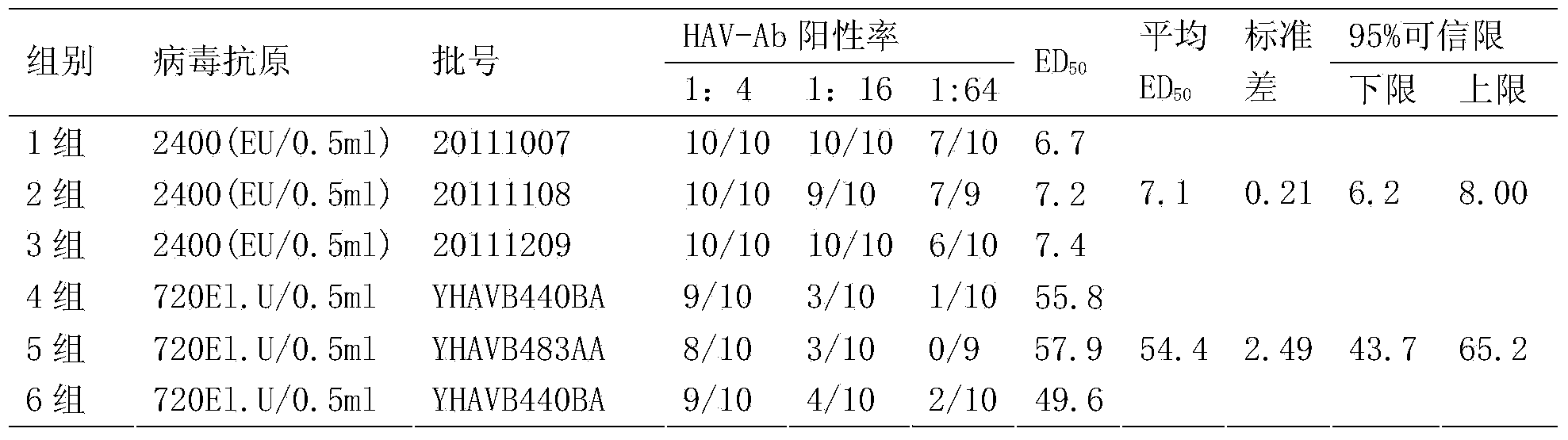
[0048] The immunogenicity detection of embodiment 3 different concentrations hepatitis A inactivated vaccines
[0049] The immunogenicity of inactivated hepatitis A vaccine was tested by in vivo potency in mice. ED50 is the half effective dose, which refers to the dose of the vaccine that causes half of the animal-specific antibody positive reactions in a group of animals. For the same animal in different vaccines or different contents of the same vaccine, the lower the ED50 value, the higher the ability of an animal to produce specific antibodies.
[0050] Adopt the production method of the present invention to prepare a batch of hepatitis A inactivated vaccines, the batch number is 20110403, the virus titer is 40960EU / ml, the adjusted hepatitis A antigen content is respectively 2000EU / 0.5ml, 2400EU / 0.5ml, 2800EU / 0.5ml and 3200EU / 0.5 ml. 260 ICR mice (SFP grade) aged 4-6 weeks were selected as mice. Hepatitis A inactivated vaccines of each antigen concentration (with Al(OH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com