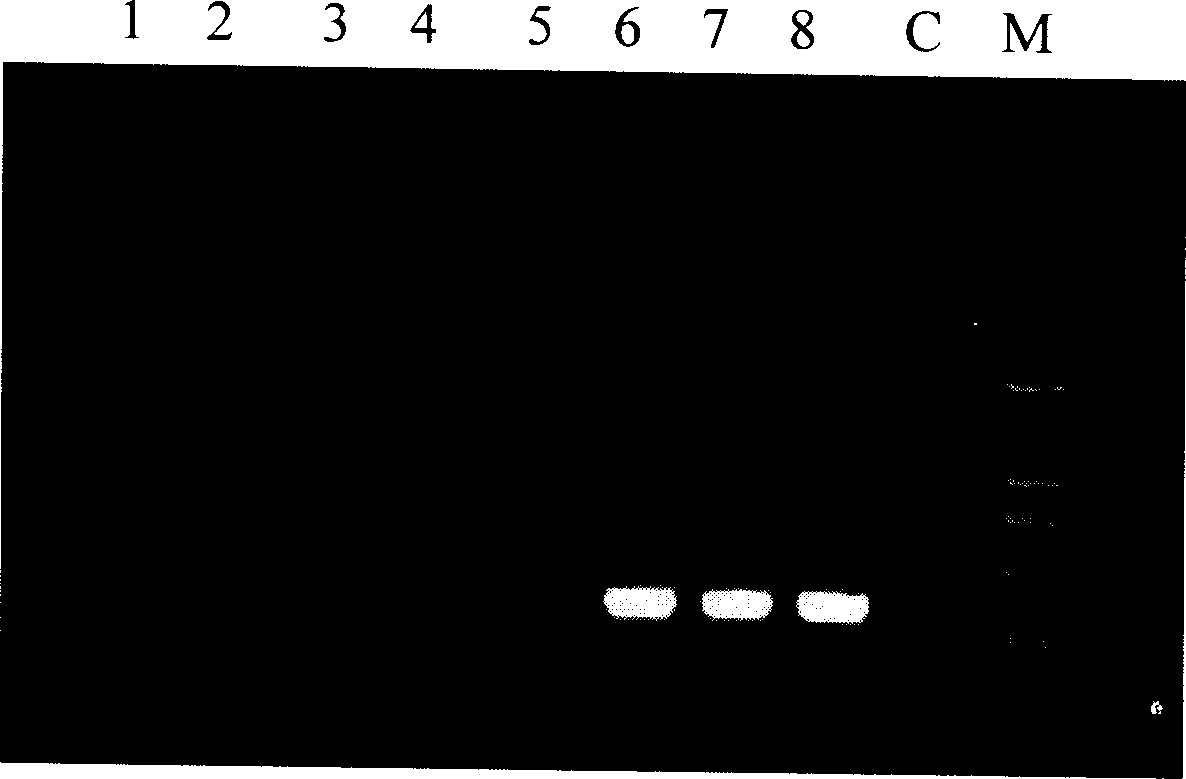
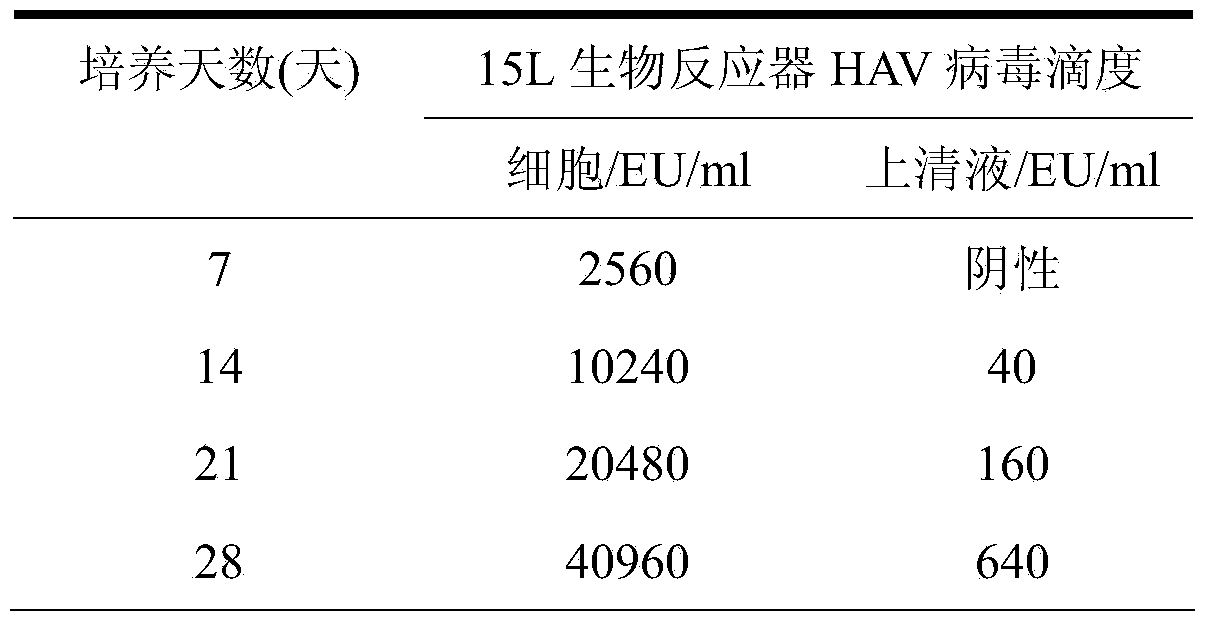
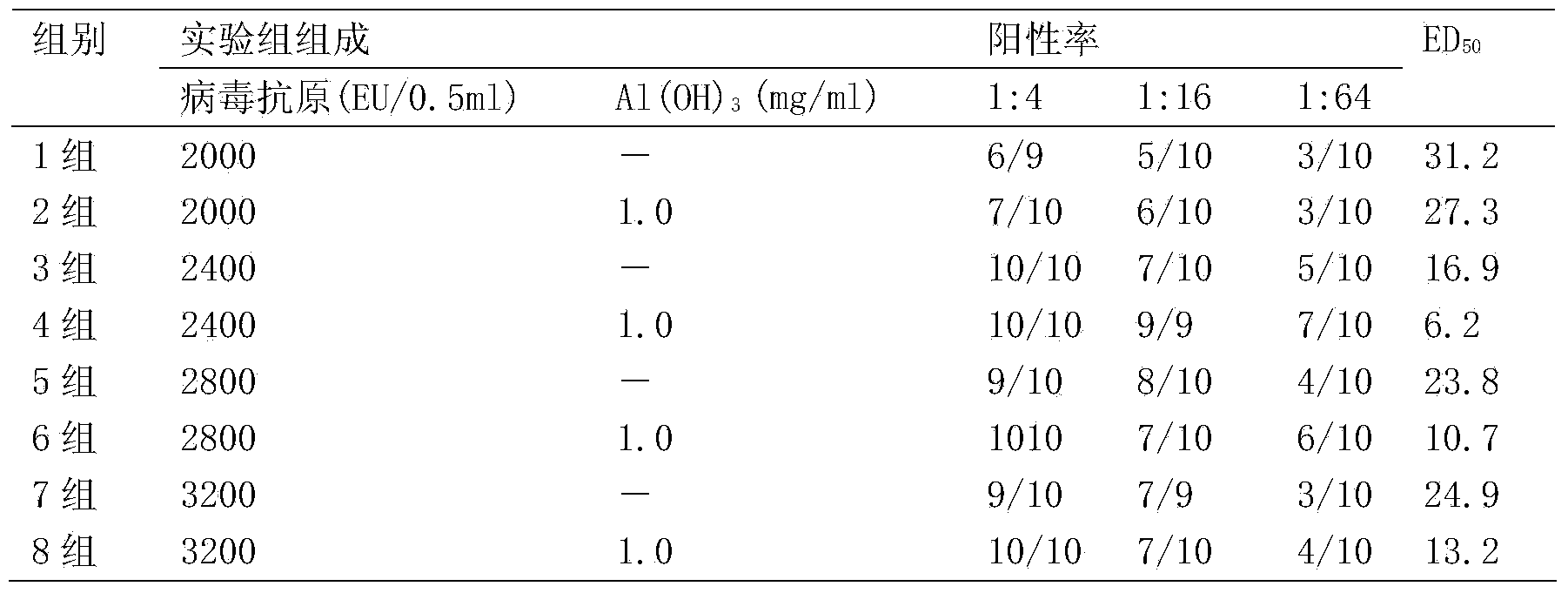
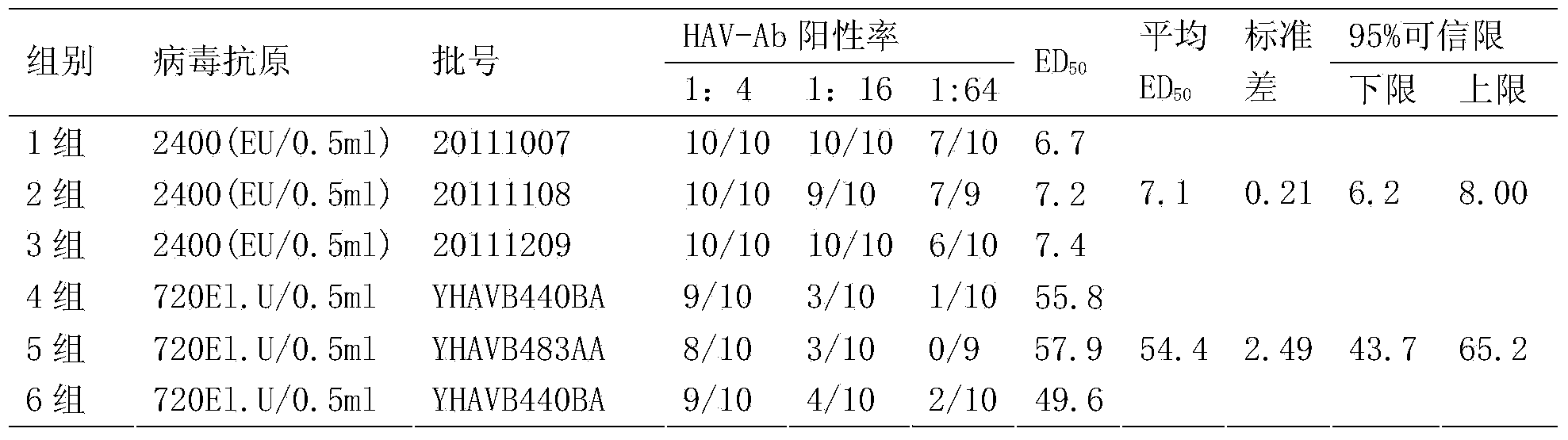
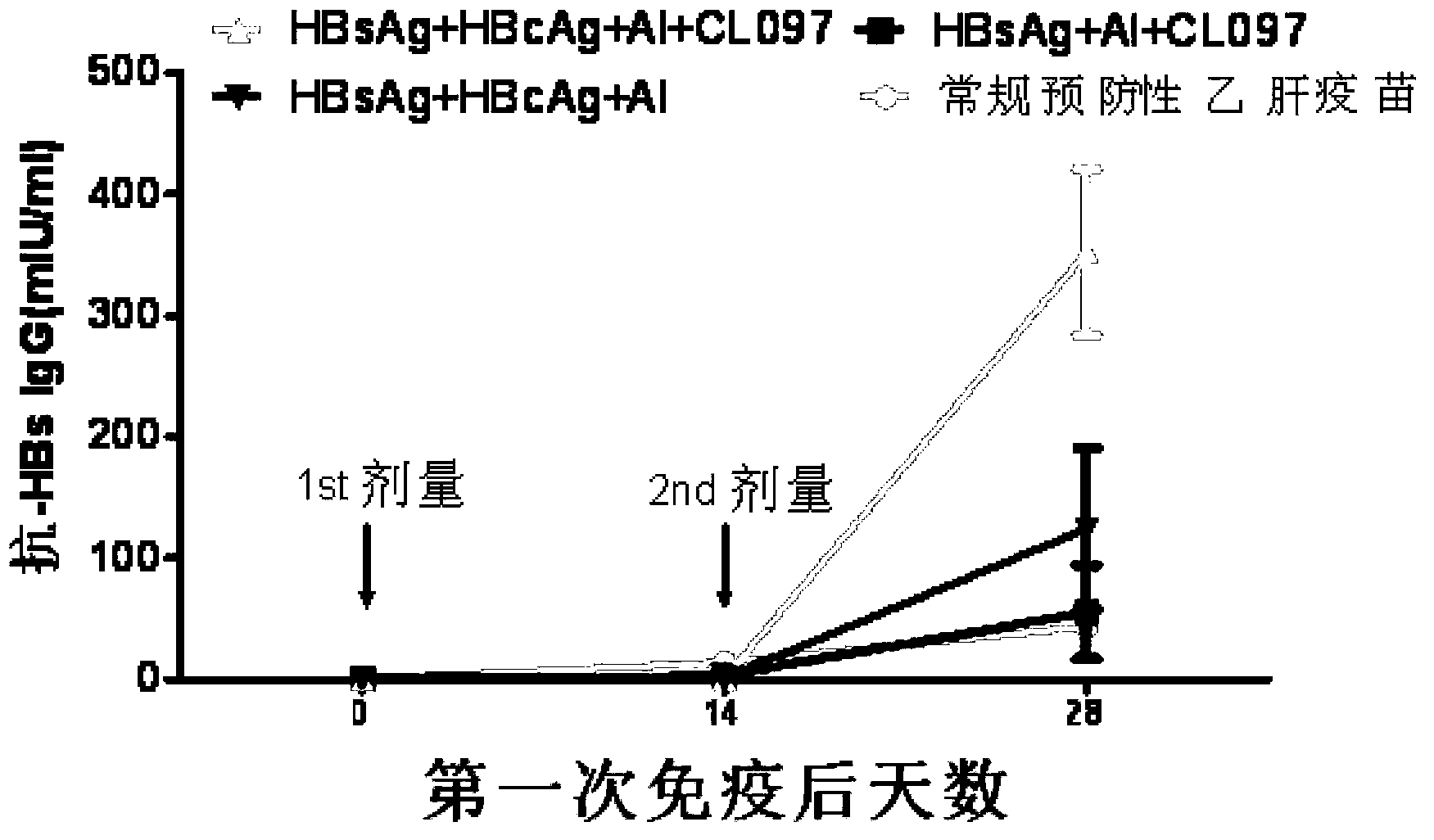
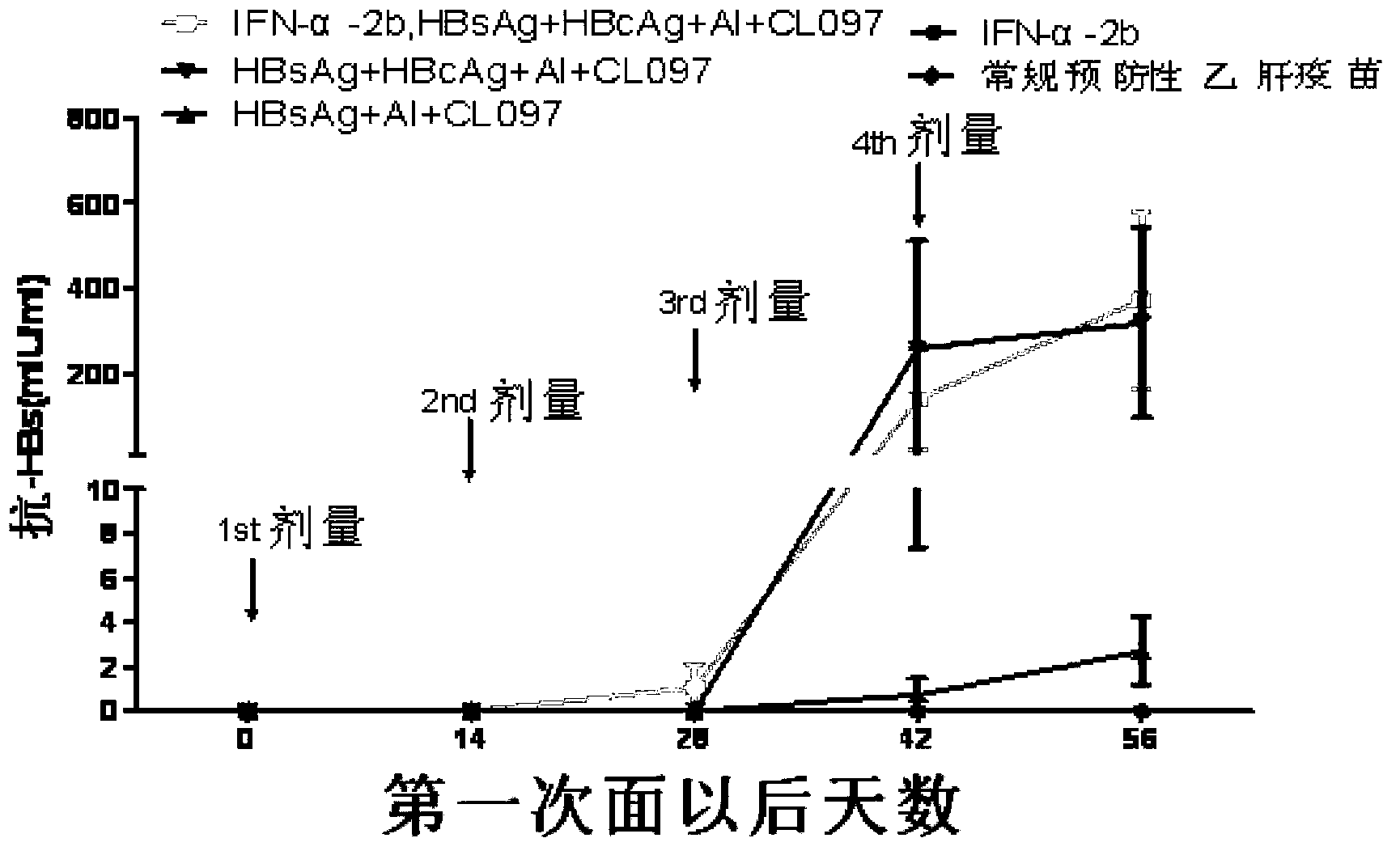
Patents
Literature
47 results about "Hepatitis A vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This vaccine is used to help prevent infection from the hepatitis A virus.
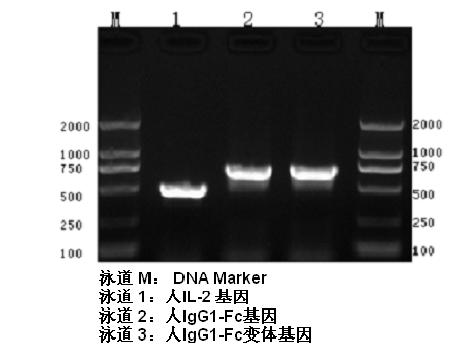
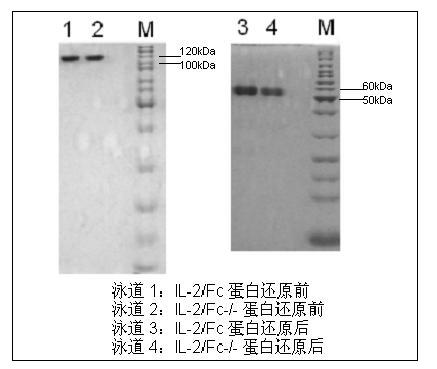
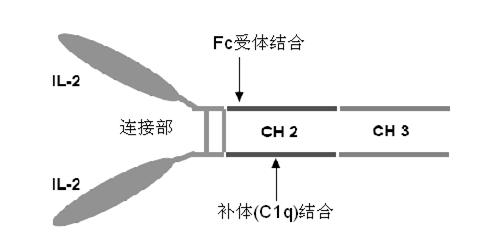
Human interleukin-2 (IL-2)/Fc fusion protein
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
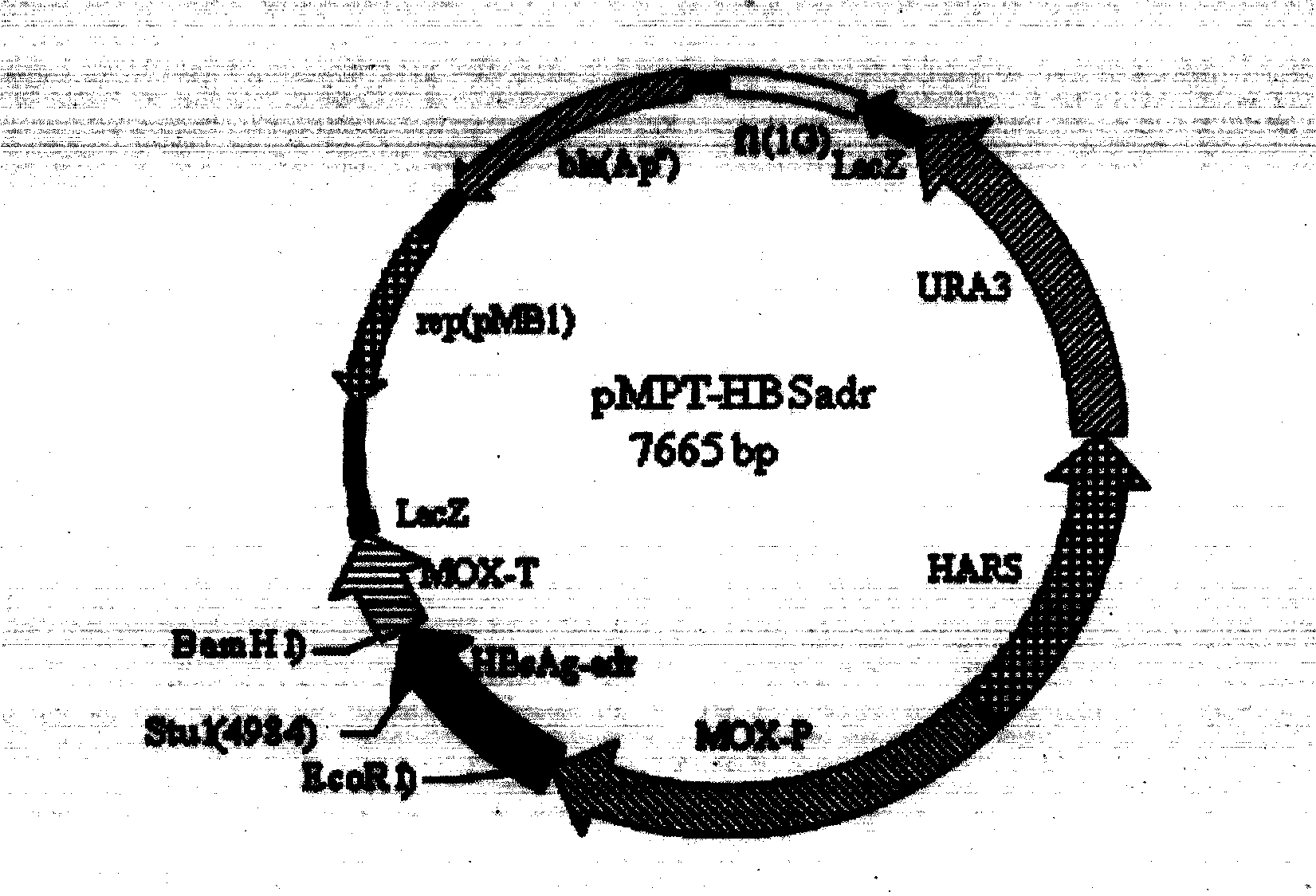
Recombinant DNA sequence, hansenula polymorpha, preparation method for hepatitis B surface antigen, and hepatitis B vaccine
InactiveCN104232661AImprove expression levelSplit genetic stability is highFungiMicroorganism based processesChemical synthesisHigh cell
The invention provides a recombinant DNA sequence, hansenula polymorpha, a preparation method for hepatitis B surface antigen, and a hepatitis B vaccine. The recombinant DNA sequence is obtained by codon optimization of coding genes of the hepatitis B virus surface antigen according to codon usage frequency of the hansenula polymorpha. The invention also provides the hansenula polymorpha comprising the recombinant DNA sequence, a method for preparing adr sub-type hepatitis B surface antigen by using the recombinant DNA sequence, and the hepatitis B vaccine. The adr sub-type hepatitis B surface antigen has high expression level of the recombinant DNA sequence. The recombinant hansenula polymorpha is fast in growth speed, has high HBsAg yield, can be fermented in high cell density by using a cheap chemically synthetic medium, has low fermentation contamination rate and is beneficial to large-scale production; and HBsAg adr vaccine provided by the invention has high trend of Th1 and Th2 type cellullar immunologic response.
Owner:北京天坛生物制品股份有限公司
Freeze-dried hepatitis A attenuated live vaccine and its stabilizer
InactiveUS6884422B1Improve abilitiesIncreased thermo-resistance and storage stabilitySsRNA viruses negative-sensePowder deliveryMedicineHepatitis A vaccine
The present invention relates to hepatitis A vaccine, especially to a lyophilized attenuated hepatitis A vaccine which can be stored at ambient temperature for extended periods of time, and to a method for producing the same. The present invention further relates to a stabilizer for lyophilized live vaccine and its use in improving thermostability of lyophilized live vaccine during lyophilization processing and storage period after lyophilization.
Owner:CHANGCHUN INST OF BIOLOGICAL PRODS
Chitosan nanoparticles oral preparations of hepatitis vaccine
InactiveCN101036785ARound appearanceNarrow particle size distributionPharmaceutical delivery mechanismPharmaceutical non-active ingredientsChitosan nanoparticlesHepatitis A vaccine
The invention relates to medicine technique domain. At present, the hepatitis vaccine is in common use of protective inoculation of hepatitis. The current formulation is injection, with the shortcomings of high cost, poor tolerance and need specialized technical personnel administrating. The chitosan nanoparticles preparation is formed by ionic crosslinking-high pressure homogeneization technique through hepatitis vaccine model and chitosan as carrier material, furthermore the chitosan nanoparticles are surface modified by lectin to obtain nanoparticles preparation suitable for oral inoculation. The said nanoparticles preparation vaccine provides a new route of oral inoculation to prevent hepatitis B, with high operability and low cost.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis B treating vaccine prepn and its prepn process and use
InactiveCN1548156AIncrease concentrationEnhance immune functionDigestive systemAntiviralsBALB/cYeast
The hepatitis B treating vaccine preparation includes recombinant hepatitis B vaccine 40-80 microgram / ml and aluminum 0.5 mg / ml. The preparation process, use and composition of the hepatitis B treating vaccine are also disclosed. The preparation can induce cell immune response for body to eliminate HBV, increase the cell immunity of Balb / c mouse, promote the high level expression of Th1 cell factor, raise T proliferation level and raise specific CTL activity.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Method for preparing lyophilized hepatitis A attenuated live vaccine using cell factory
The invention discloses a method for preparing a freeze-dried live attenuated hepatitis A vaccine by utilizing a cell factory. The method has good process controllability, is uneasy to pollute enviroment, is suitable for industrialized and mass production, improves the utilization rate of a culture space by more than 3 times, ensures that the growth density of cells reaches between 4.0x10<6> and 5.0x10<6> cells per ml, assures the multiplication space of viruses, and also effectively solves the problem of cell residue after the cell factory digests through methods such as sterile washing, centrifugal treatment and so on, so as to continuously use the cell factory for cell culture and improve the reuse rate of the cell factory.
Owner:长春生物制品研究所有限责任公司
Freeze-drying hepatitis A attenuated live vaccine and its preparation method
The invention relates to a freeze-dried live attenuated hepatitis A vaccine and its preparation, which comprises live attenuated hepatitis A vaccine virus liquid and freeze-drying protective agent by the volumetric ratio of 1:0.7-1.2, and the freeze-drying protective agent comprises the following constituents: trehalose 2-10%, gelatin 0.1-1%, dextroglucose glycoside 0.5-1.5%, glycine 0.5-1.5%.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Breeding of hepatitis A virus Chinese strain and attenuated strain and its complementary DNA sequence
InactiveCN1337463AMicrobiological testing/measurementInactivation/attenuationUltrasound attenuationHepatitis A vaccine
The present invention relates to hepatitis A virus Chinese strain and attenuated strain separation and cultivation and their complementary DNA sequence, in particular, it relates to separation and purification of hepatitis A virus Chinese strain, cultivation of series attenuated strain and its unique cDNA sequence. Said series cDNA series can provide basic material for further understanding genetic property and genetic stability of hepatitis A virus strain and expounding molecular mechanism of HAV attenuation, and can create condition for developing novel hepatitis A vaccine which can be quickly grown and possesses high titre and high antigenicity and can provide scientific basis for developing gene engineering polypeptide vaccine.
Owner:ZHEJIANG PUKANG BIOTECH
Fast and sensitive method of detecting virus infection titer of attenuated live hepatitis A vaccine
ActiveCN1772920ACalculation of infectivity titersReduce usageMicrobiological testing/measurementAgainst vector-borne diseasesHepatitis A vaccinePcr method
The present invention provides one kind of fast and sensitive method for detecting virus infection titer of attenuated live hepatitis A vaccine. The method determines the existence of the virus via detecting the negative brand RNA intermediate of marker appearing during duplicating hepatitis A virus, and is superior to available inverse transcription PCR method and cell culturing method. The method has culture period shortened to 8 days, short detection period, no need of replacing maintaining liquid, less contamination possibility, less cell post-treating steps, high work efficiency and capacity of providing virus heredity information via sequencing the PCR product.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
B-type hepatitis vaccine
InactiveCN1404875ABright future for treatmentImprove humoral immunity/cellular immunityDigestive systemAntiviralsAdjuvantChronic hepatitis
The present invention relates to a hepatitis B vaccine, the main composition of said vaccine comprises gene engineering hepatitis B virus surface antigen, muramyl dipeptide (MDP) and aluminium adjuvant. Said hepatitis B vaccine can be used for immunity of adult, renal transplanted patient and patient with renal dialysis therapy and for preventing infection of hepatitis B virus, and said hepatitis B vaccine also can be used for immunotherapy of chronic hepatitis B patient, and the immunogenicity of said vaccine is superior to that of existent aluminium adjuvant hepatitis B vaccine.
Owner:BEIJING LUZHU BIOTECH
Application of hepatitis B surface antigen-antibody complexes in preparing prophylaxis product with no response or low response to hepatitis B vaccine
ActiveCN1919341AEffective infectionProtection from infectionDigestive systemAntiviralsSerum igeHepatitis A vaccine
The invention discloses a new utility of hepatitis B surface antigen-antibody compound in the hepatitis B vaccine non-respond or low-respond prevention agent, which is characterized by the following: purifying recombined HBsAG or inactivated serum HBsAG in the mammal cell, adopting hepatitis B with antibody serum or vaccine globulin as compound, improving anti-HBs ability for non-respond or low-respond mouse.
Owner:FUDAN UNIV +1
Application of human fetal lung fibroblast line in preparation of hepatitis A vaccines
ActiveCN103525770AIncrease productionImproving immunogenicityMicroorganism based processesAntiviralsAntigenHepatitis A vaccine
The invention discloses an application of a human diploid fibroblast line Walvax-2 in the preparation of hepatitis A vaccines. The human diploid fibroblast line is susceptible to hepatitis A viruses. A human diploid cell hepatitis A virus purifying vaccine having the advantages of high antigen purity, good immunization effect, high security and low price can be obtained by adopting the Walvax-2 to produce the hepatitis A vaccines.
Owner:云南沃森生物技术股份有限公司
Hepatitis B nucleic acid vaccine and construction method thereof
InactiveCN101954093AImprove protectionGood immune effectGenetic material ingredientsDigestive systemTreatment effectHepatitis B virus DNA
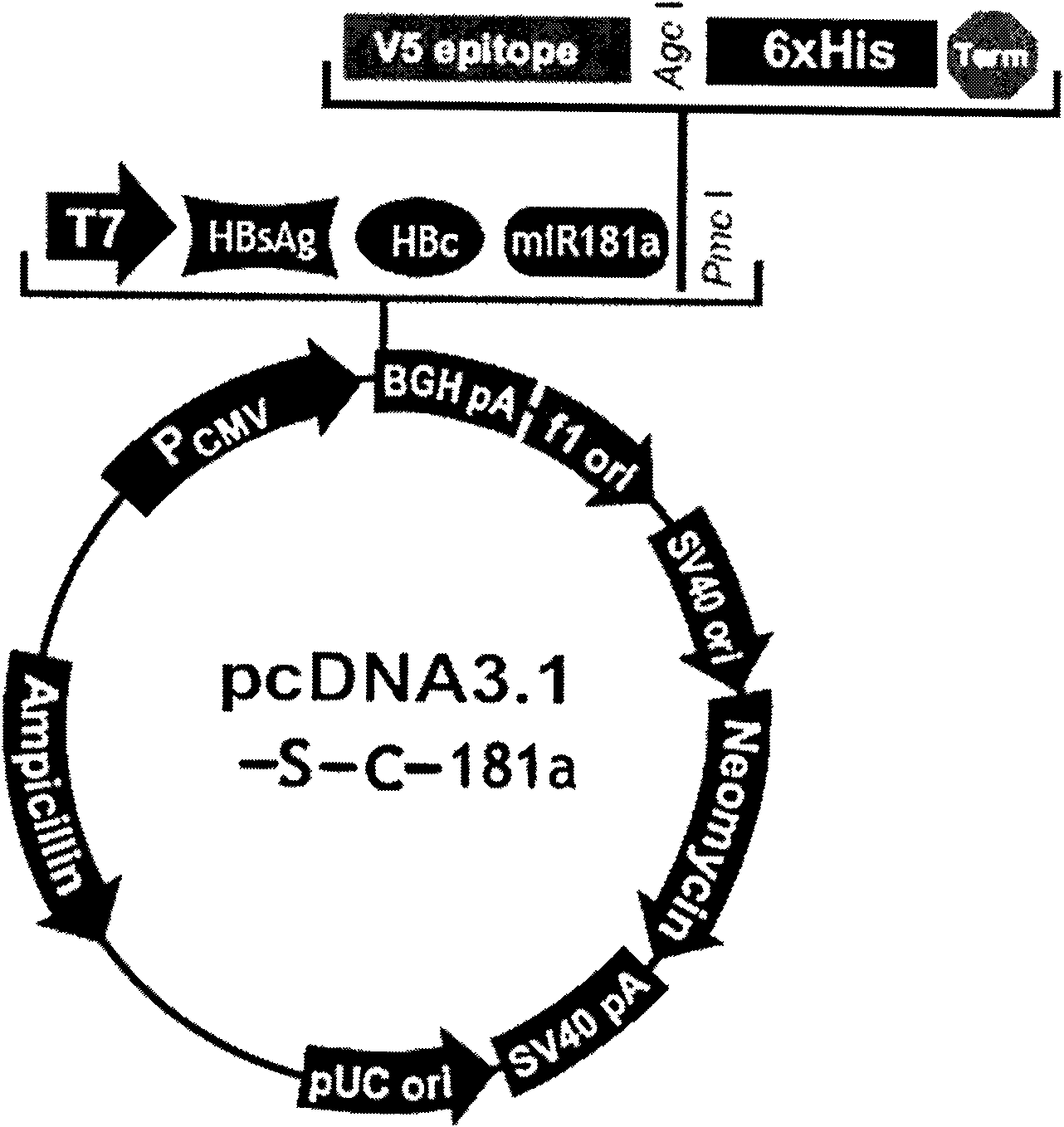
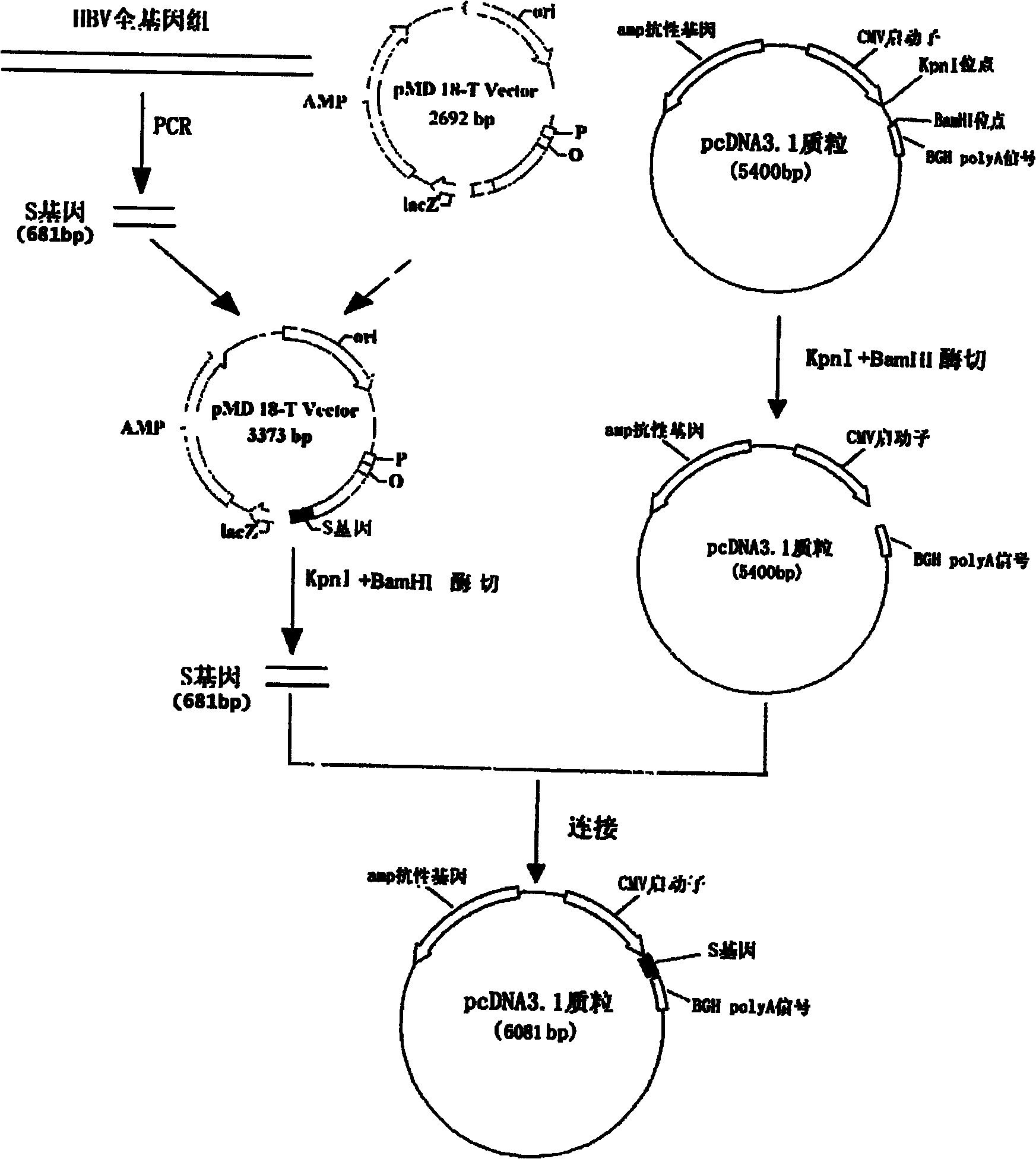
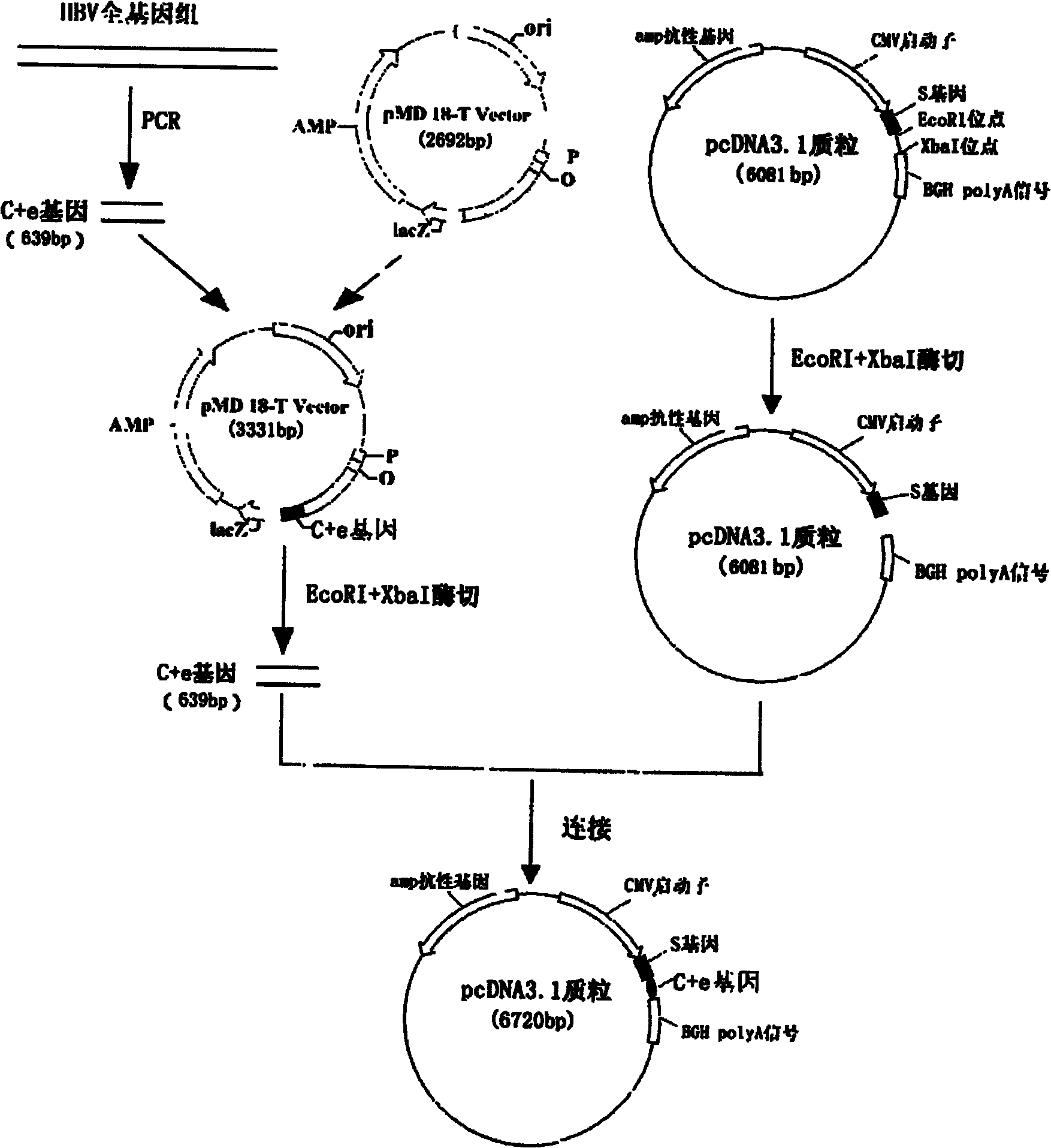
The invention relates to the technical field of biomedicines. The currently reported hepatitis B virus vaccine mainly comprises one or several of HBsAg, preS 1, preS2 and HBcAg, some cell factor genes with immunological enhancement function or lymphocyte epitope genes and the like, however, the immunoprophylaxis effect and treatment effect of the vaccine are always unsatisfactory. The invention provides a hepatitis B vaccine which comprises hepatitis B virus surface antigen gene HBsAg, core protein gene HBc and e-antigen gene, also comprises a human microRNA181a precursor gene sequence, and can assist stimulating the body immunity pathway. The construction process of the vaccine relates to PCR, enzyme cutting, connection, conversion and other molecularly biological operating means. The hepatitis B vaccine has the advantages of effectively activating the body to produce a specific antibody against the hepatitis B virus, stimulating body cell immunity and secreting various cell factors. Therefore, the aim of treating chronic hepatitis B is fulfilled.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis B vaccine for inducing organism to generate specific immunity in state of chronic hepatitis B virus infection
The invention discloses a hepatitis B vaccine, and in particular discloses a hepatitis B vaccine which contains an immunopotentiator and can induce an organism to generate specific immunity in a state of chronic hepatitis B virus infection. The hepatitis B vaccine disclosed by the invention contains a Toll-like receptor agonist serving as the immunopotentiator.
Owner:李金秋
Hepatitis A virus strain SH and diploid cell adaptation method thereof
ActiveCN102174477AShorten the growth cycleImprove reproductive efficiencySsRNA viruses positive-senseViral antigen ingredientsAntigenEarly generation
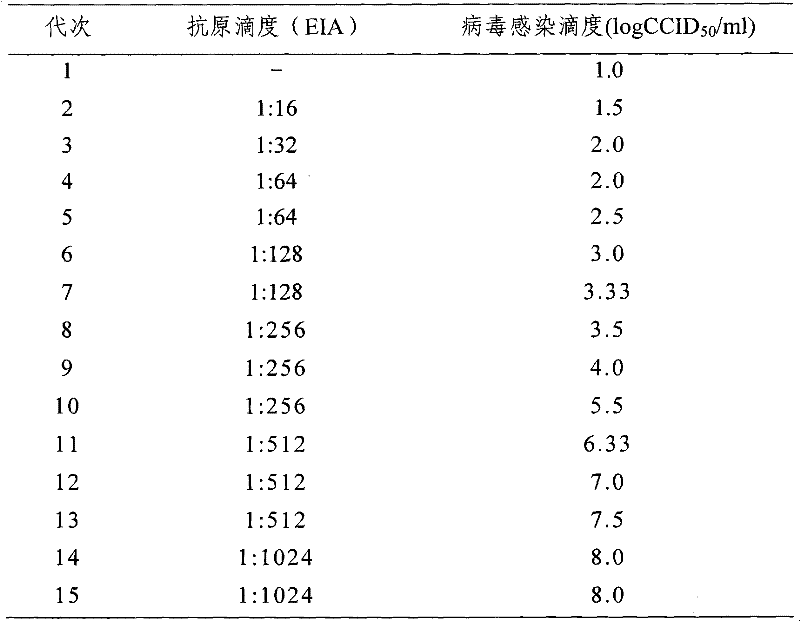
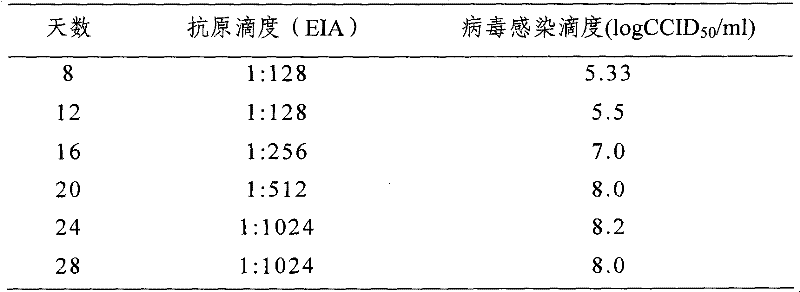
The invention provides a new hepatitis A vaccine virus strain SH and a separation method as well as an MRC-5 cell adaptation method thereof. The virus is separated from excrement of a hepatitis A acute infection patient and is transferred to a diploid cell MRC-5 for adaptation culture. The virus strain SH is proved to be a hepatitis A virus through methods such as gene sequencing, neutralization test and the like; and during adaptation, early-generation sub-culturing period is 35 days, and culturing period is shortened to be 24 days after the strain is sub-cultured for 8 generations. After the strain is continuously cultured for 8 generations, antigen titer can reach (1:512)-(1:1,024), and the virus infection titer can reach 7.0 to 8.01gCCID50 / ml. Immunogenicity tests and cross protection tests show that the strain has a good immunogenicity protection effect during production of a hepatitis A inactivated vaccine, is suitable for industrially producing the hepatitis A inactivated vaccine strain on a large scale and is an ideal strain for producing the hepatitis A inactivated vaccine.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen, production method of hepatitis B surface antigen, hepatitis B vaccine and production method of hepatitis B vaccine
ActiveCN103333938AComply with GMP requirementsHigh degree of automationDigestive systemVirus peptidesAntigenUltrafiltration
The invention discloses a production method of a recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen. The method comprises the steps of: circularly removing triton from an extracted antigen by using XAD-4 column, so as to obtain triton-removed antigen sample liquid; loading macroporous silica gel with the pore size of 1,000 A and the particle size of 35-70 microns into a chromatographic column, and balancing by using phosphoric acid buffer solution with the pH of 7.6+ / -0.2; adjusting the pH of the triton-removed antigen sample liquid to be 7.6+ / -0.2 by using NaOH, then, loading a sample to the macroporous silica gel column; and cleaning impurities by using phosphoric acid buffer solution with the pH of 7.2+ / -0.2, carrying out eluting treatment on the impurity-cleaned macroporous silica gel column by using boric acid buffer solution with the pH of 8.7+ / -0.2 at the temperature of 47-49 DEG C, collecting eluate, and carrying out ultrafiltration and concentration on the eluate, thereby obtaining a clarified antigen. The invention further discloses the corresponding recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen, a hepatitis B vaccine and a production method of the hepatitis B vaccine. The production methods have the advantages that the probability of product contamination is reduced greatly, the labor intensity for laborers is reduced, the equipment investment and repairing cost are reduced, the space occupied by equipment is reduced, and the production time is shortened.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
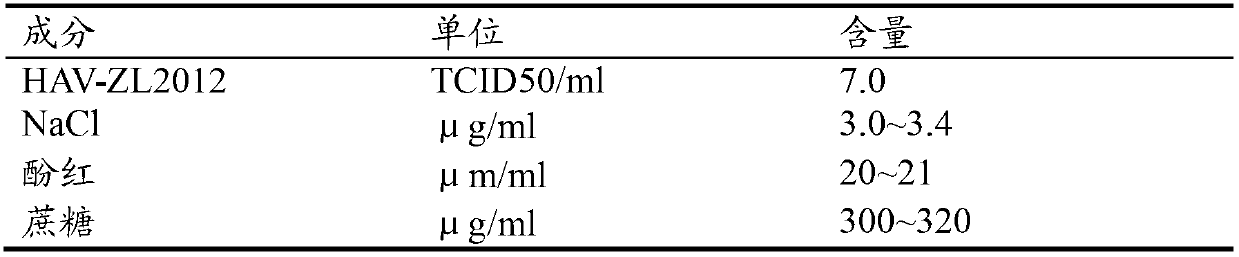
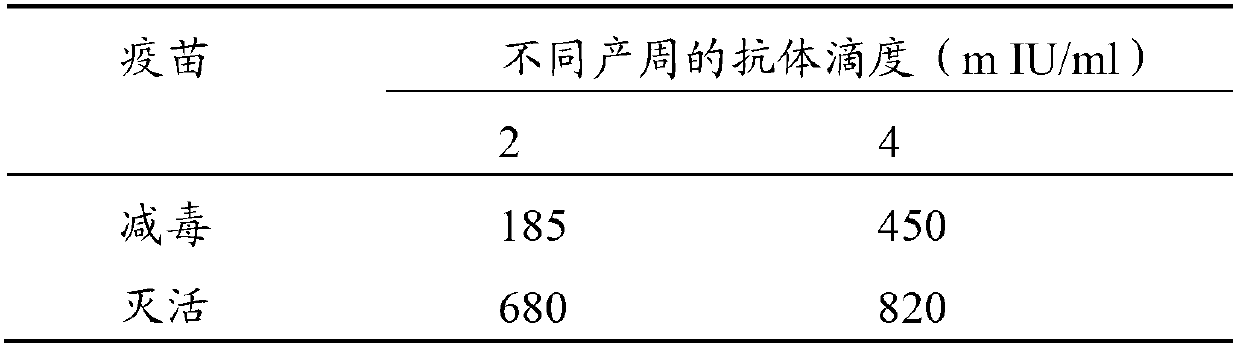
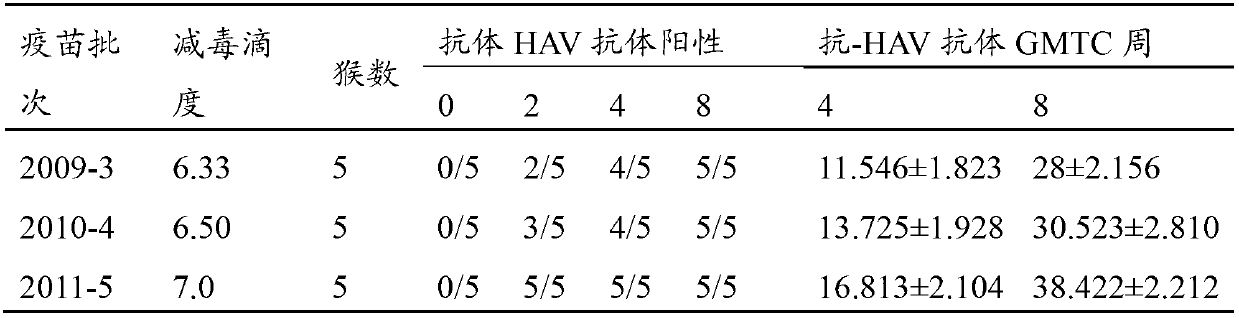
Hepatitis A virus strain HAV-ZL2012, vaccine prepared by using same and application thereof
ActiveCN102757941ALow toxicityReduced ability to replicateDigestive systemInactivation/attenuationUltrasound attenuationHepatitis A vaccine
The invention discloses a hepatitis A virus strain HAV-ZL2012, a vaccine prepared by using same and application thereof. According to the invention, through the adoption of a reverse molecular biology technology and a novel subculturing method, the adaption and attenuation of a virus strain obtained through primary isolation are realized and rapidly facilitated; and growth, immunity and safety studies show that the virus strain HAV-ZL2012 can be used for preparing both live attenuated vaccines and inactivated vaccines. According to the invention, through the adoption of a rapid attenuation and adaption method of the hepatitis A virus, the growth period of the hepatitis A virus is reduced from 100 days to 14 days. The generation order of subculture adaption on a diploid cell is reduced from 40 generations to 10 generations; the generation order of subculture adaption on a subculture cell is reduced to 10-15 generations; the production period and cost are greatly reduced; and a great promoting effect on the development of a hepatitis A vaccine is obtained.
Owner:内蒙古必威安泰生物科技有限公司
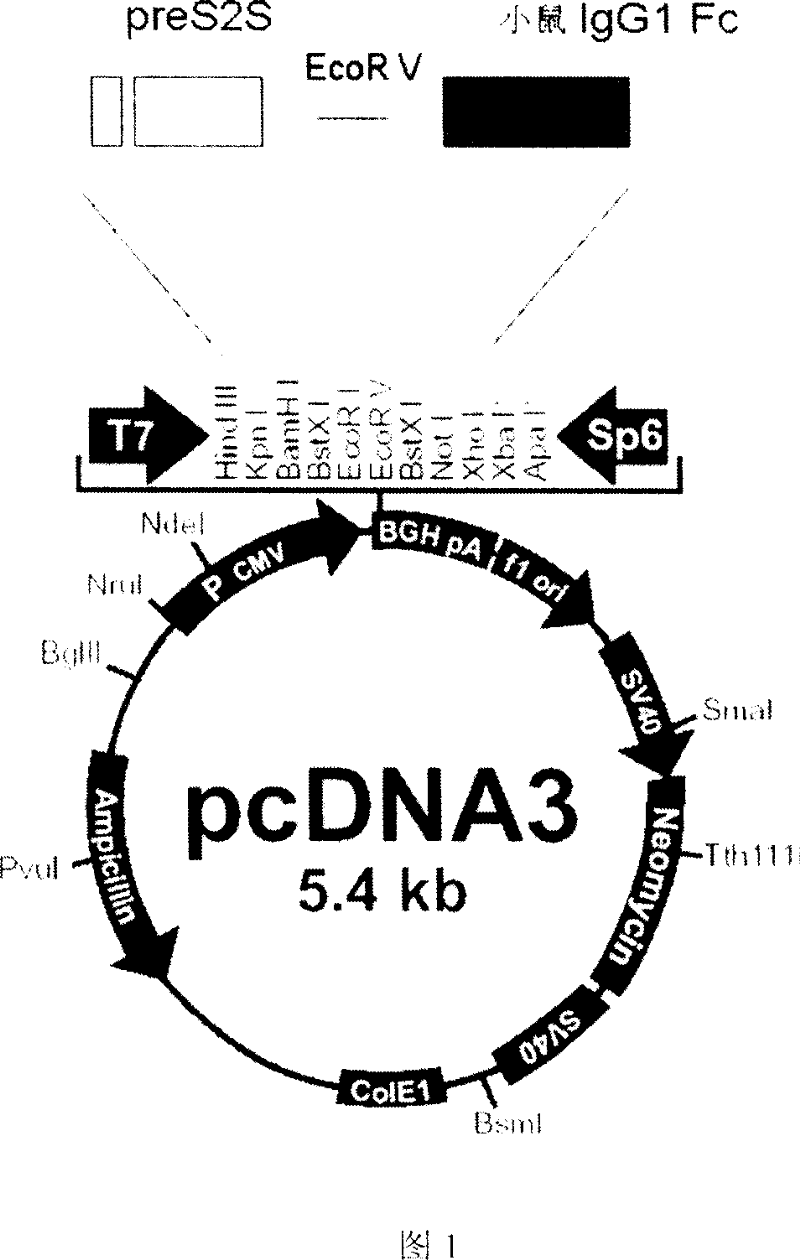
Fusion protein for preparing hepatitis B vaccine and its carrier
InactiveCN101037476APeptide/protein ingredientsAntibody medical ingredientsAntibody hepatitisBio engineering
The invention discloses a fusion protein of antibody Hepatitis B vaccine belonging to the bio-engineering field. It has two functional domains which composed of preS2 of Hepatitis B surface antigen in N-terminal and S protein, and IgG1 Fc in rats. The invention also discloses the vector containing the gene coding the said fusion protein. The invention also discloses a antibody Hepatitis B vaccine. The constructed antibody Hepatitis B vaccine may induce a strong humoral-mediated immune response and a cell-mediated immune response which has a better effect than the traditional vaccine.
Owner:FUDAN UNIV
Production method for hepatitis A virus for vaccine production
InactiveCN102492659ARealize high-density large-scale cultivationIncrease productionMicroorganism based processesViruses/bacteriophagesInfected cellBiotechnology
The invention relates to the field of biotechnology, in particular to a production method for a hepatitis A virus for vaccine production. The production method comprises the following steps sequentially: reviving, passaging and amplifying a cell; vaccinating a hepatitis A virus seed; keeping culturing the cell infected with hepatitis A virus; detecting the reproduction condition of viral infection; stopping culturing, discarding the maintenance medium, and reaping the cultured infected cell; collecting cell suspension; and extracting and purifying the cell suspension. The production method has the advantages that the operation is simple; the production intensity is low; the high-density large-scale cultivation of stromal cells is realized; the yield and the quality stability of hepatitis A vaccine are improved; and pollution caused by manual operation in the conventional manufacturing technology is avoided.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
HBsAg, technology for preparing HBsAg through expression by virtue of recombinant saccharymyces cerevisiae, separation and purification technology for HBsAg as well as hepatitis B vaccine
The invention discloses a technology for preparing HBsAg through expression by virtue of recombinant saccharymyces cerevisiae and a separation and purification technology of the HBsAg. The separation and purification technology of the HBsAg comprises the following steps: breaking cells, namely feeding cerevisiae cells extracted after fermentation into a high pressure homogenizer and breaking the cerevisiae cells at a pressure of 15000+ / -1500PSIG; heating feed liquid after breaking, namely heating the feed liquid to 50-80 DEG C and then cooling the feed liquid to 2-8 DEG C; and purifying, namely purifying the feed liquid and preparing HBsAg raw liquid. The invention also discloses a corresponding hepatitis B vaccine and HBsAg. The technology for preparing the HBsAg through expression by virtue of the recombinant saccharymyces cerevisiae has the advantages that protease can be inactivated without adding a protease inhibitor before the cells are broken, so that secondary pollution caused by the protease inhibitor is avoided; meanwhile, the yield of the raw liquid obtained through purification subsequently can be obviously increased, and quality indexes such as 'specific activity' and 'Sub-p24' of the raw liquid are greatly improved, so that the technology for preparing the HBsAg through expression by virtue of the recombinant saccharymyces cerevisiae has high economic benefit and social benefit.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Application of PreS1 in preparation of hepatitis B vaccine and treatment of chronic hepatitis B
The invention belongs to the technical field of biomedicine, relates to a hepatitis B virus (HBV) vaccine and a preparation method thereof and further relates to application of the vaccine in prevention of hepatitis B virus infection and treatment of chronic hepatitis B (CHB) infection and application of the vaccine in cooperation with an HBsAg vaccine in treatment of CHB. The vaccine comprises a hepatitis B virus envelope protein preS1 region, can effectively prevent HBV from infecting a host, has a treatment effect on chronic hepatitis virus infection, and achieves a more remarkable effect than that of a traditional HBV vaccine in the prior art.
Owner:PRESONE WUHAN BIOTECHNOLOGY CO LTD
Hepatitis E subunit vaccine
ActiveCN110013549AHigh antigen stabilityHigh puritySsRNA viruses positive-senseViral antigen ingredientsAntigenHepatitis E vaccine
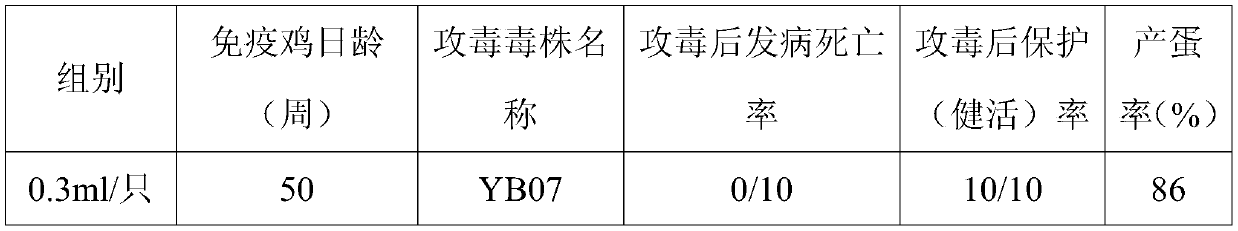
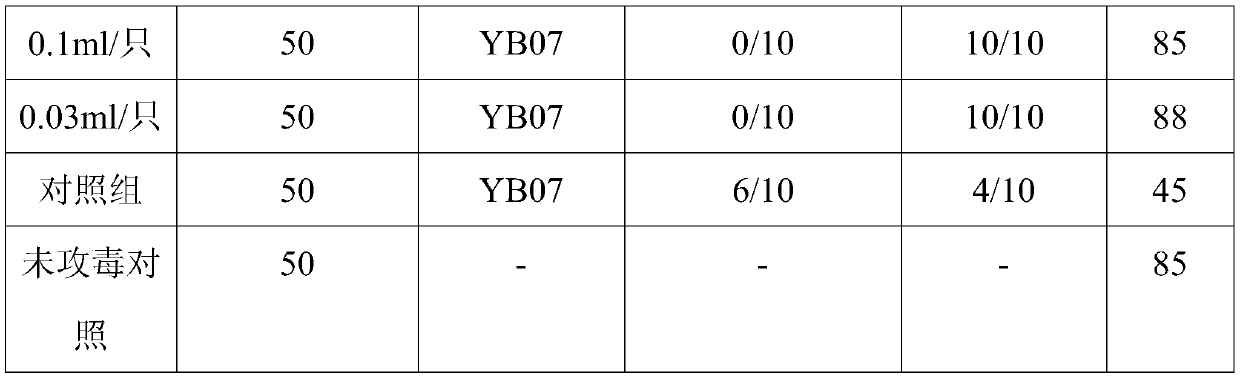
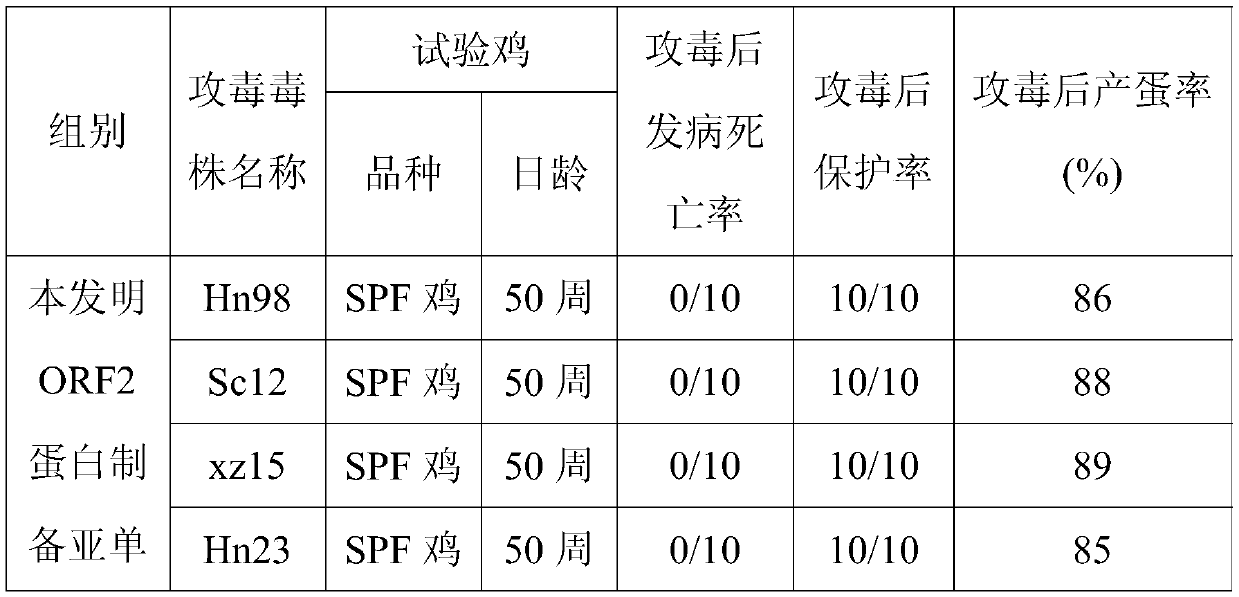
The invention provides a hepatitis E subunit vaccine. The antigen protein of the hepatitis E subunit vaccine is hepatitis E virus ORF2 protein of which the amino acid sequence is SEQID NO:2 or SEQID NO:4. The subunit vaccine prepared from the hepatitis E ORF2 protein has the characteristics of being high in antigen stability, high in purity, high in specificity and convenient and accurate in detection method, and other uncorrelated antibodies are not produced, and a firm foundation is established for industrialized production of a hepatitis E vaccine and a diagnosis reagent.
Owner:YEBIO BIOENG OF QINGDAO
Application of lettuces as host to expression of Hepatitis B vaccines
The invention relates to the technical field of biology, in particular to expression of a human hepatitis B vaccine (namely hepatitis B virus (Hepatitis B virus, HBV) surface antigen protein) throughplants. The plants such as lettuces are used as an effective expression platform for producing recombinant protein, and the human Hepatitis B vaccine is expressed by a simple and effective agrobacterium tumefaciens mediated vacuum osmotic method. The expression system determines that plant foreign protein can be collected after agrobacterium tumefaciens is subjected to infection for 4d. By an SDS-PAGE method, successful expression of the recombinant human Hepatitis B vaccine protein is determined. After expressed recombinant protein is prepared into virus like particles through self-assembly,New Zealand white rabbits can be immunized, and obtained serum can prove that the human Hepatitis B vaccine expressed by the plants has biology activity by an enzyme-linked immunoadsorbent assay method (ELISA) and a pseudovirion granule neutralization test.
Owner:王跃驹
Method for culturing chicken pox-herpes zoster vaccine virus with high-level human diploid cell
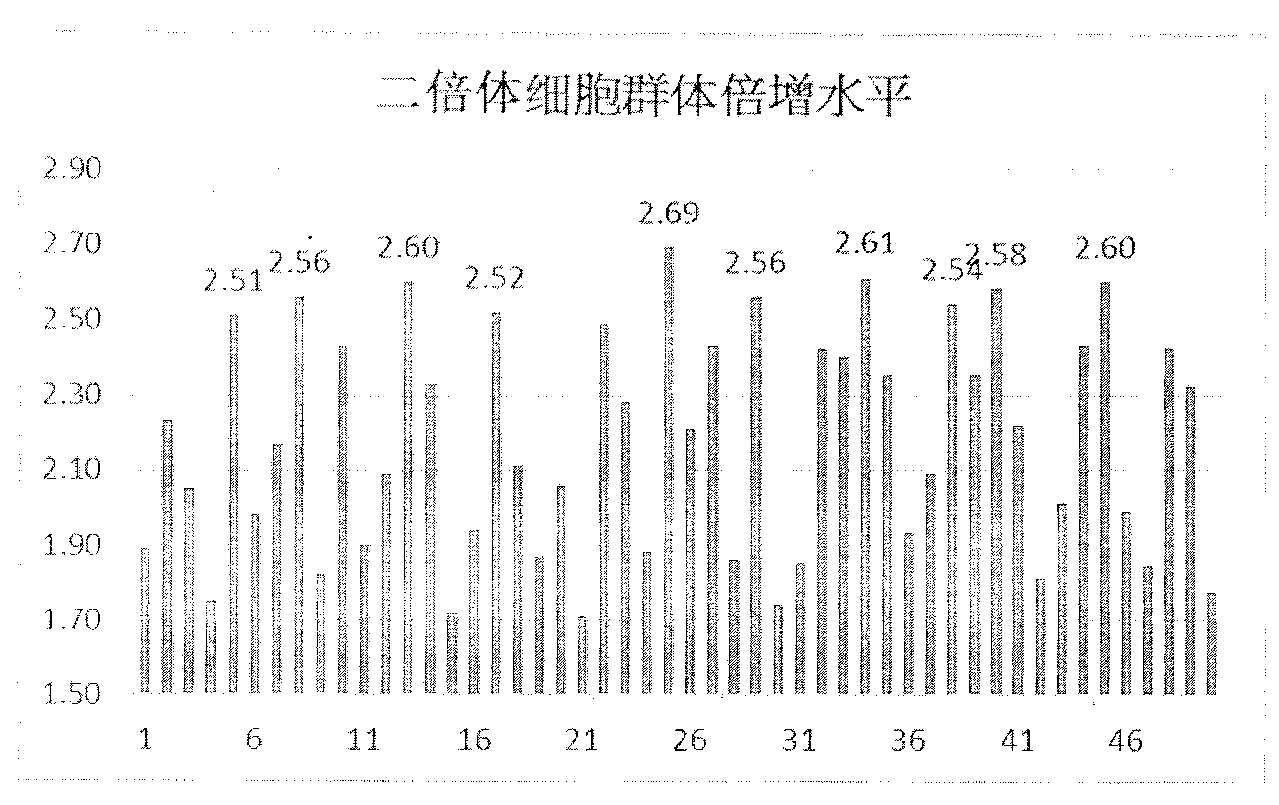
ActiveCN103074304AIncrease productionEasy to prepare in large quantitiesMicroorganism based processesViruses/bacteriophagesPopulation doublingHerpes zoster virus
The invention discloses a method for culturing a chicken pox-herpes zoster vaccine virus with a high-level human diploid cell. The method comprises the steps of establishing a main cell bank: taking an ATCC (American Type Culture Collection) (CCL171) cell strains at a 17th generation at the beginning according to United States FDA (Food and Drug Administration) standards, subculturing for totally 3-5 times in approximately 20 days, storing collected cells in liquid nitrogen with a cryovial, conducting working cell bank cell culture, horizontally synchronously monitoring diploid cell population doubling, and producing a vaccine with the cell strains having diploid cell population doubling levels above 2.5. With the adoption of the method, the yield of the attenuated chicken pox-herpes zoster virus vaccine is higher, and the method facilitates large-scale preparation of the herpes zoster vaccine and is applicable to scale production. The method can be used for industrial production of other live vaccines such as a hepatitis A vaccine, a rabies vaccine, a measles vaccine and a smallpox vaccine.
Owner:JIANGSU JIANAN BIOLOGICAL TECH
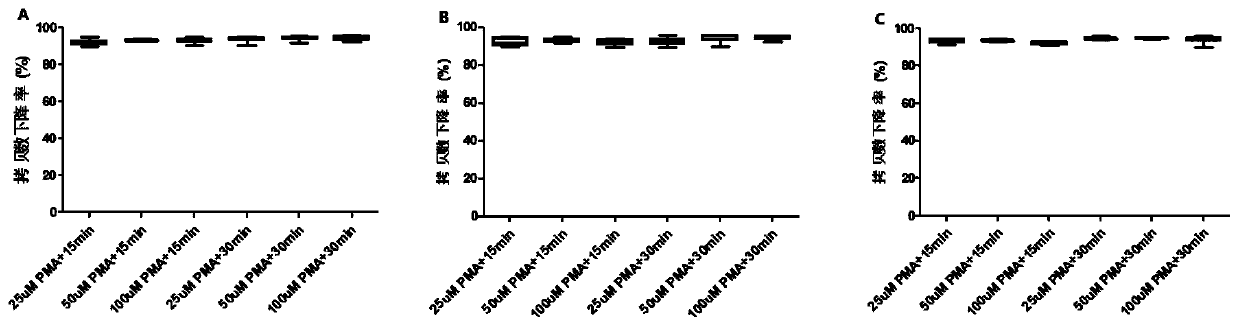
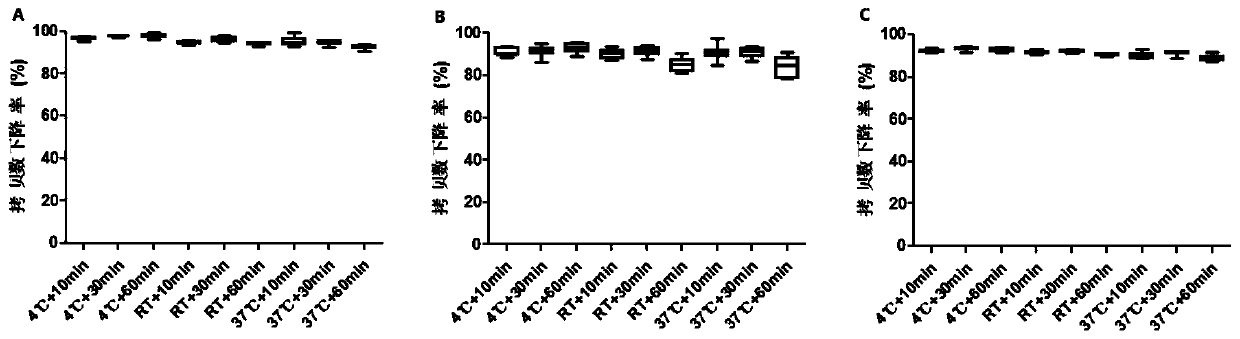
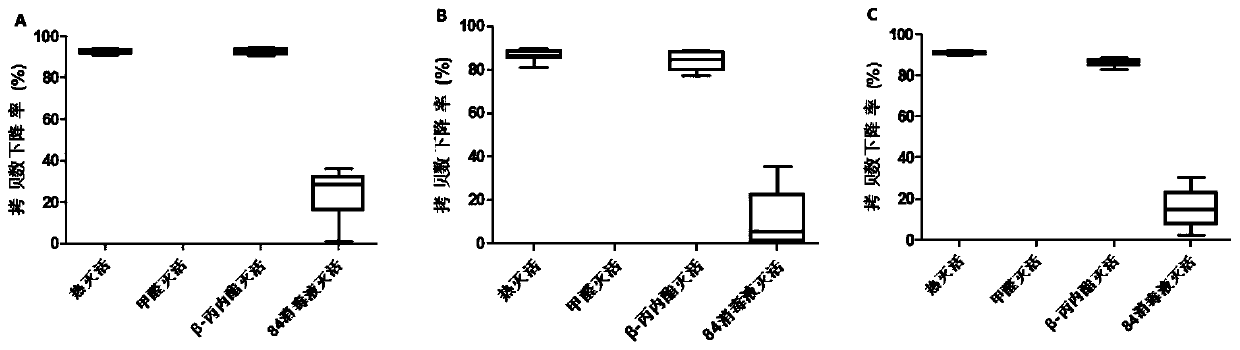
Method for rapidly detecting virus titer of hepatitis A vaccine based on PMA-qRT-PCR method
ActiveCN111500772AShorten inspection cycleImprove linearityMicrobiological testing/measurementAgainst vector-borne diseasesVaccine ProductionHepatitis A vaccine
The invention discloses a method for rapidly detecting virus titer of hepatitis A vaccine based on a PMA-qRT-PCR method, which comprises: incubating human diploid cells and a hepatitis A vaccine, adding PMA with a final concentration of 50 [mu]M, uniformly mixing, incubating for 30 min at a temperature of 2-8 DEG C in a dark place, placing on a photolysis instrument, carrying out photolysis for 30min, extracting nucleic acid, carrying out quantitative detection by using a qRT-PCR kit, drawing a regression curve by using a standard product result, and representing the virus titer of the sampleto be detected by using the obtained result. According to the detection method, all experiments can be completed within one day, an inspection period is greatly shortened, and the kit has good linearity, parallelism, sensitivity, repeatability, correlation, specificity and applicability, can be used for detecting samples with titer of 5.0-8.0 lgCCID50 / ml by a detection method, and meets the requirements on the virus titer detection range of hepatitis A vaccine finished products in the registration standard of vaccine production enterprises in China.
Owner:NAT INST FOR FOOD & DRUG CONTROL
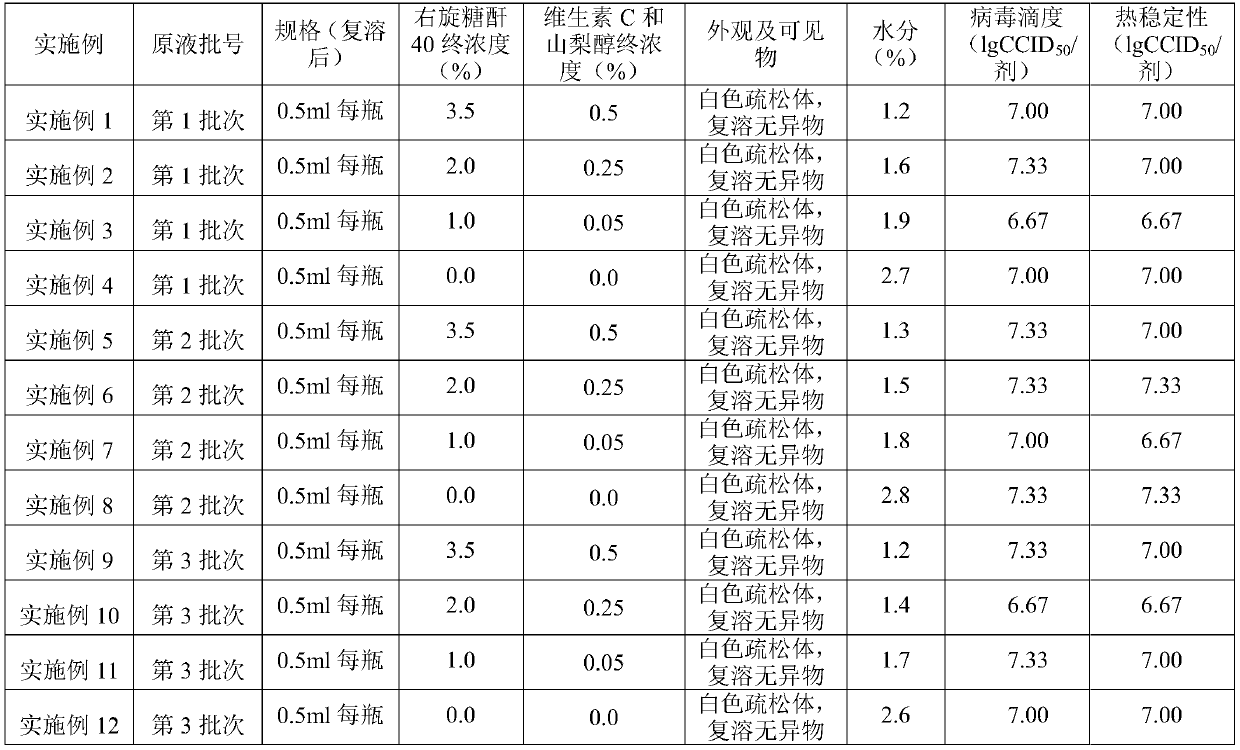
Improved lyophilized live attenuated hepatitis a vaccine stabilizer, vaccine finished product, vaccine semi-finished product and preparation method of vaccine finished product and vaccine semi-finished product
ActiveCN110585439AImprove stabilityEnsure safetyPowder deliverySsRNA viruses positive-senseVitamin CArginine
The invention discloses an improved lyophilized live attenuated hepatitis a vaccine stabilizer. The stabilizer comprises the following components: in percent, 2-6% of trehalose, 0.1-1.0% of sodium glutamate, 0.05-0.3% of Arginine, 0.1-0.8% of urea, 0-0.5% of vitamin C, 40:0-2.0% of dextran, 0-0.5% of sorbitol, 0.05-0.5% of mannitol and the balance water for injection. According to the stabilizer for the improved lyophilized live attenuated hepatitis a vaccine, the chemicals in the stabilizer are reduced, so that the dissolution rate of the stabilizer is increased, and the potential allergic reactions of the body are reduced. The invention also provides a preparation method of an improved lyophilized live attenuated hepatitis a vaccine finished product. The improved stabilizer is applied topreparation of the finished product from a semi-finished product, the operation steps be simplified, the cost of the materials is reduced, the risk of bacteria infection is reduced, and the product quality is improved.
Owner:长春生物制品研究所有限责任公司
Hepatitis A virus purification method
ActiveCN102807972AHigh recovery rateHigh purityMicroorganism based processesAntiviralsPurification methodsHepatitis A vaccine
The invention provides a hepatitis A virus purification method. With hepatitis A virus cell culture fluid serving as raw materials, the hepatitis A virus purification technology is completed via the technological processes of ultrasonication, centrifugal removal of cell debris, chloroform extraction, ultra-filtering concentration, hydrophobic chromatography, detection, collection and the like. Inactivated hepatitis A vaccines prepared from the hepatitis A virus cell culture fluid are high in purity and valence, simple in technology, easy to magnify and safe and reliable to use.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Fast and sensitive method of detecting virus infection titer of attenuated live hepatitis A vaccine
ActiveCN100389207CReduce usageReduce incubation timeMicrobiological testing/measurementAgainst vector-borne diseasesHepatitis A vaccinePcr method
The present invention provides one kind of fast and sensitive method for detecting virus infection titer of attenuated live hepatitis A vaccine. The method determines the existence of the virus via detecting the negative brand RNA intermediate of marker appearing during duplicating hepatitis A virus, and is superior to available inverse transcription PCR method and cell culturing method. The method has culture period shortened to 8 days, short detection period, no need of replacing maintaining liquid, less contamination possibility, less cell post-treating steps, high work efficiency and capacity of providing virus heredity information via sequencing the PCR product.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
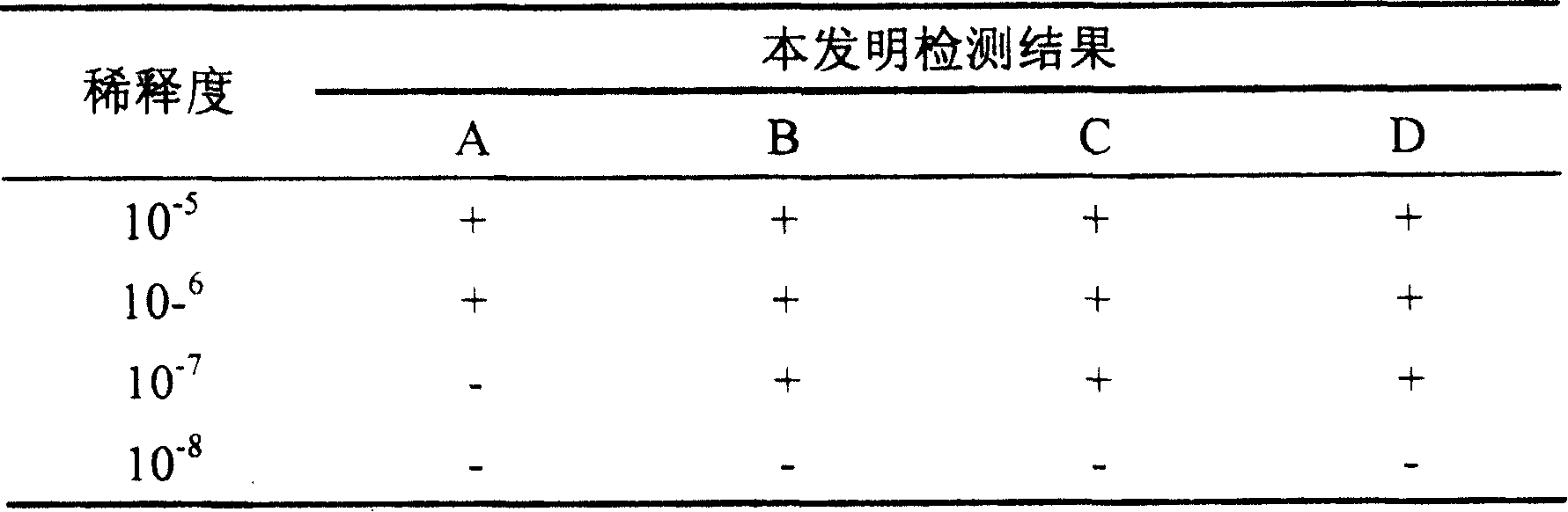
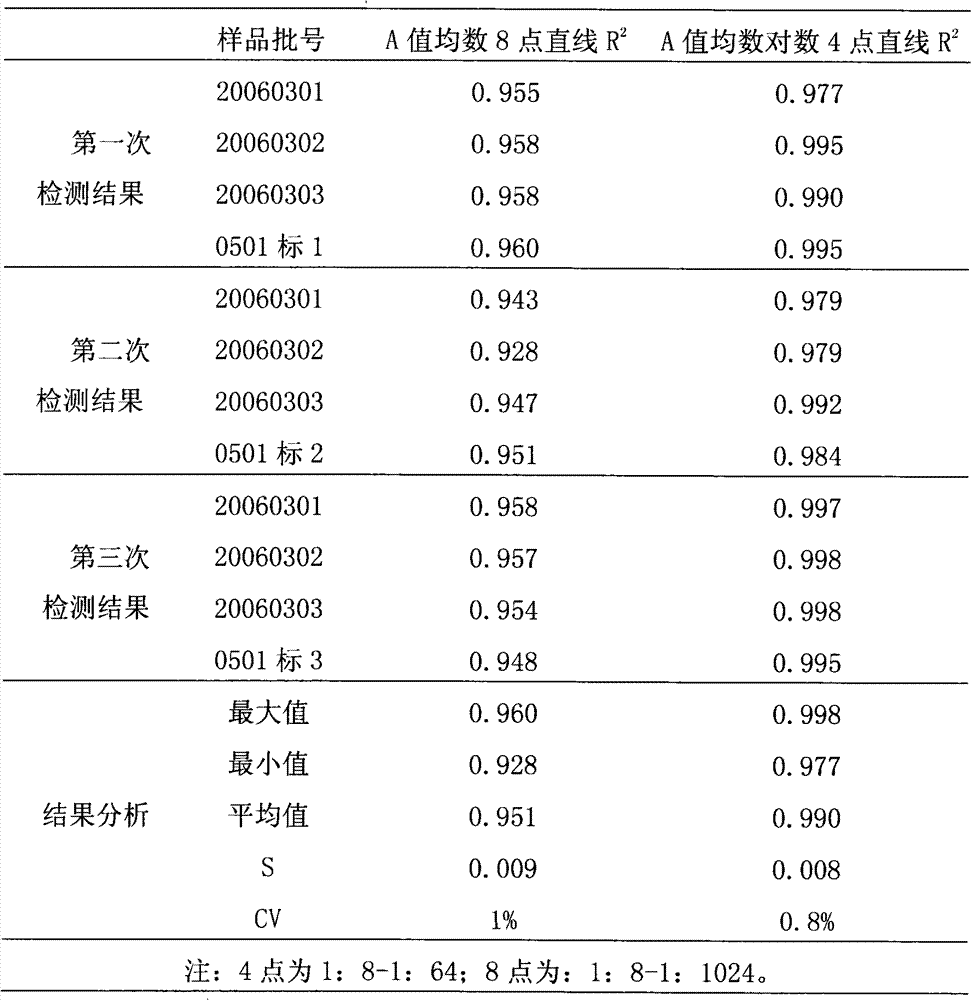
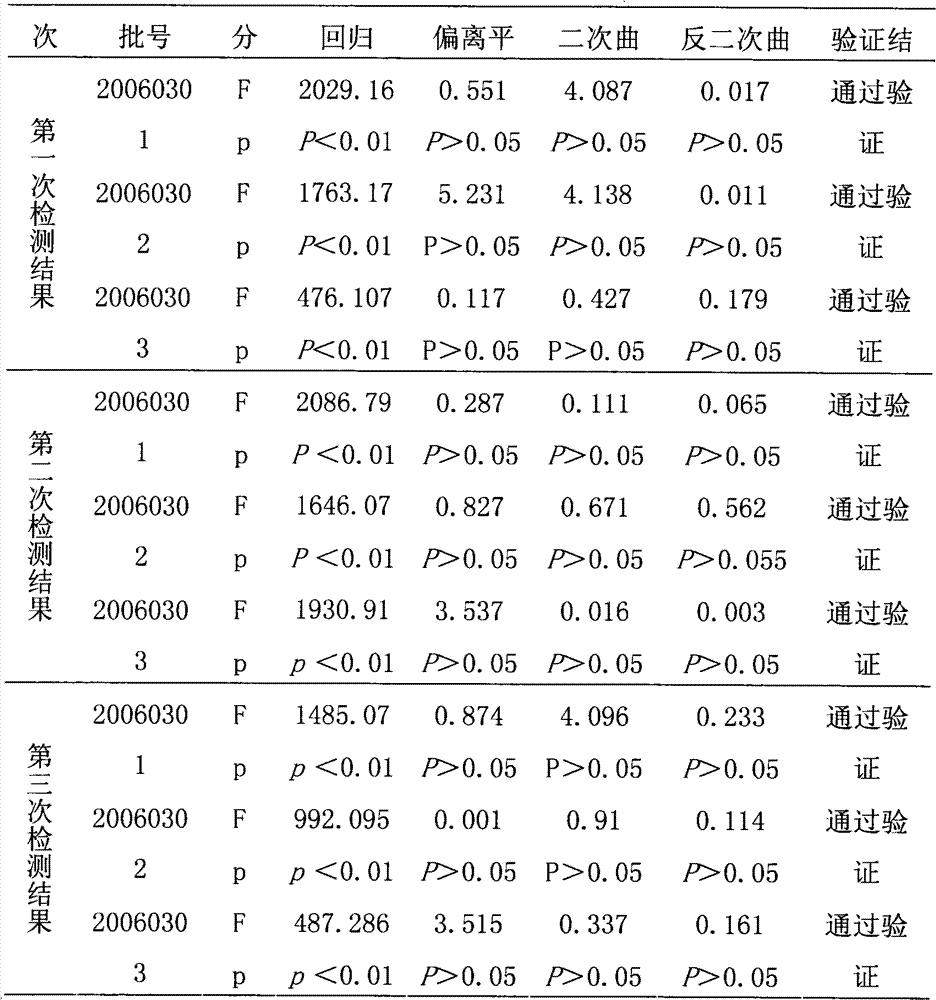
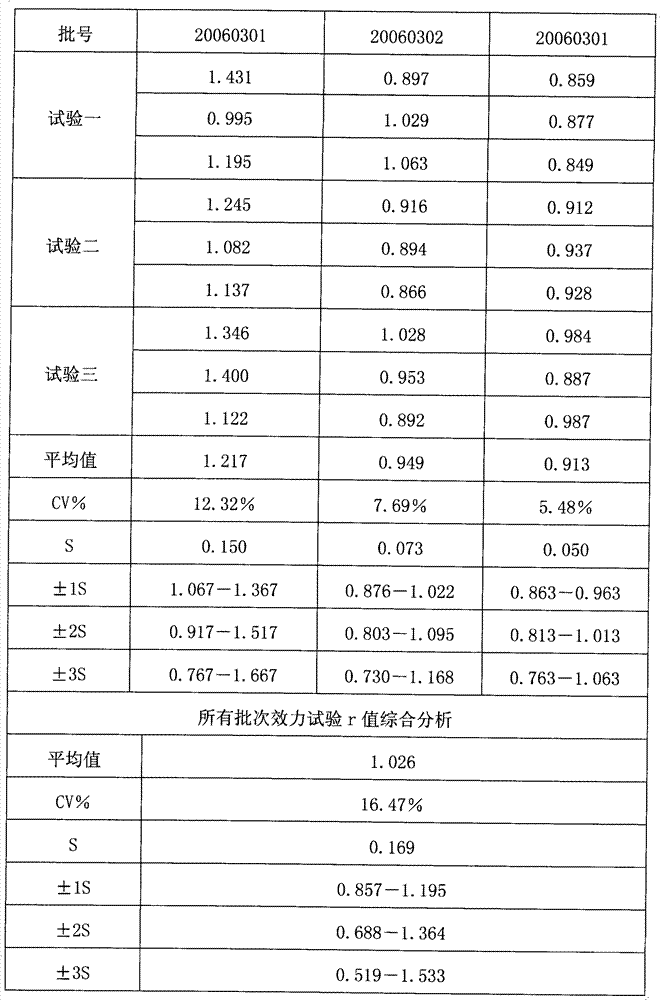
Testing method of in vitro relative efficiency of inactivated hepatitis A vaccine
ActiveCN101556285BReduce inventoryLong validity periodMaterial analysisHepatitis A vaccineLinear regression
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Application of hepatitis B surface antigen-antibody complexes in preparing prophylaxis product with no response or low response to hepatitis B vaccine
ActiveCN1919341BEffective infectionProtection from infectionDigestive systemAntiviralsSerum igeHepatitis A vaccine
The invention discloses a new utility of hepatitis B surface antigen-antibody compound in the hepatitis B vaccine non-respond or low-respond prevention agent, which is characterized by the following: purifying recombined HBsAG or inactivated serum HBsAG in the mammal cell, adopting hepatitis B with antibody serum or vaccine globulin as compound, improving anti-HBs ability for non-respond or low-respond mouse.
Owner:FUDAN UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com