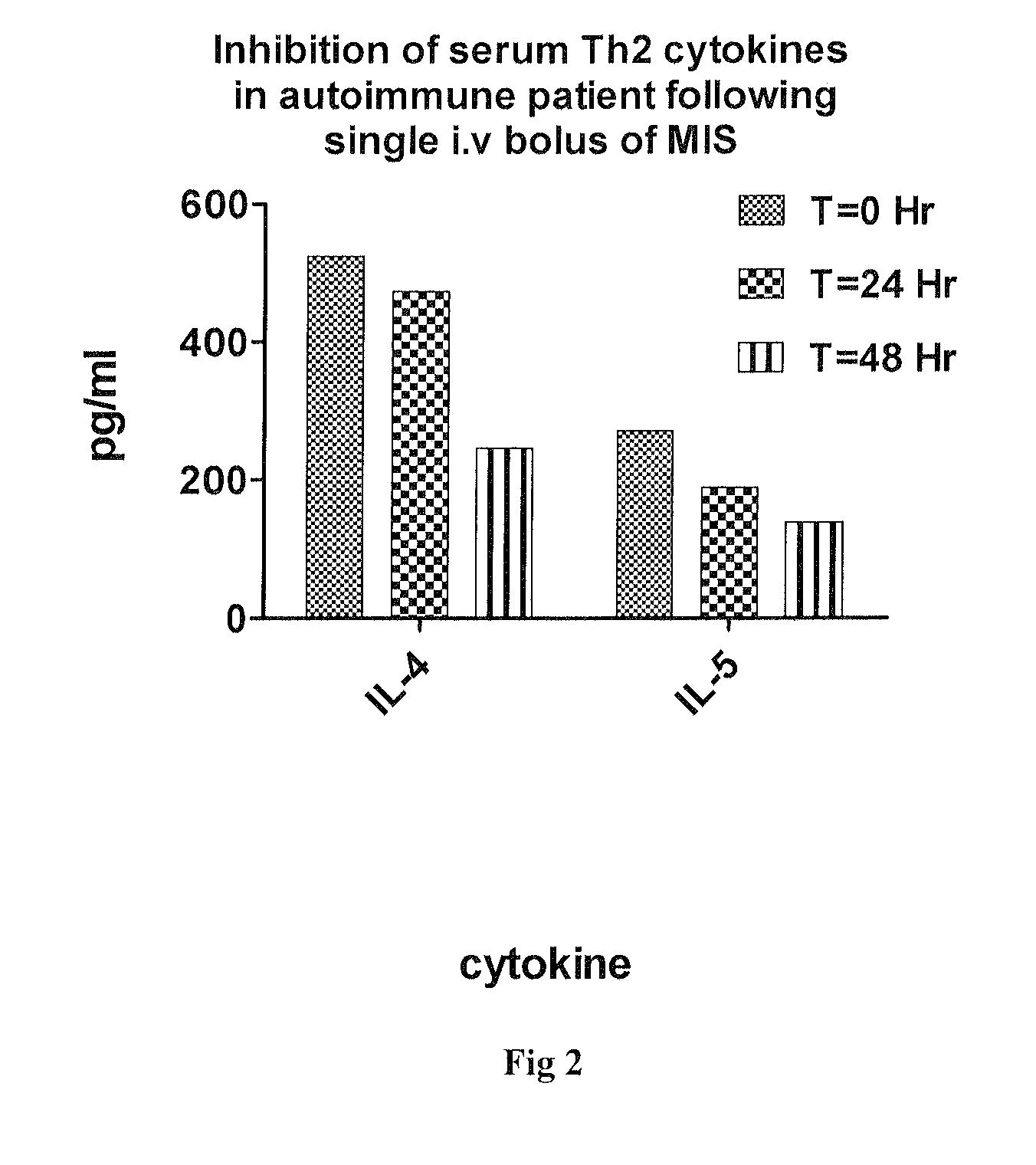
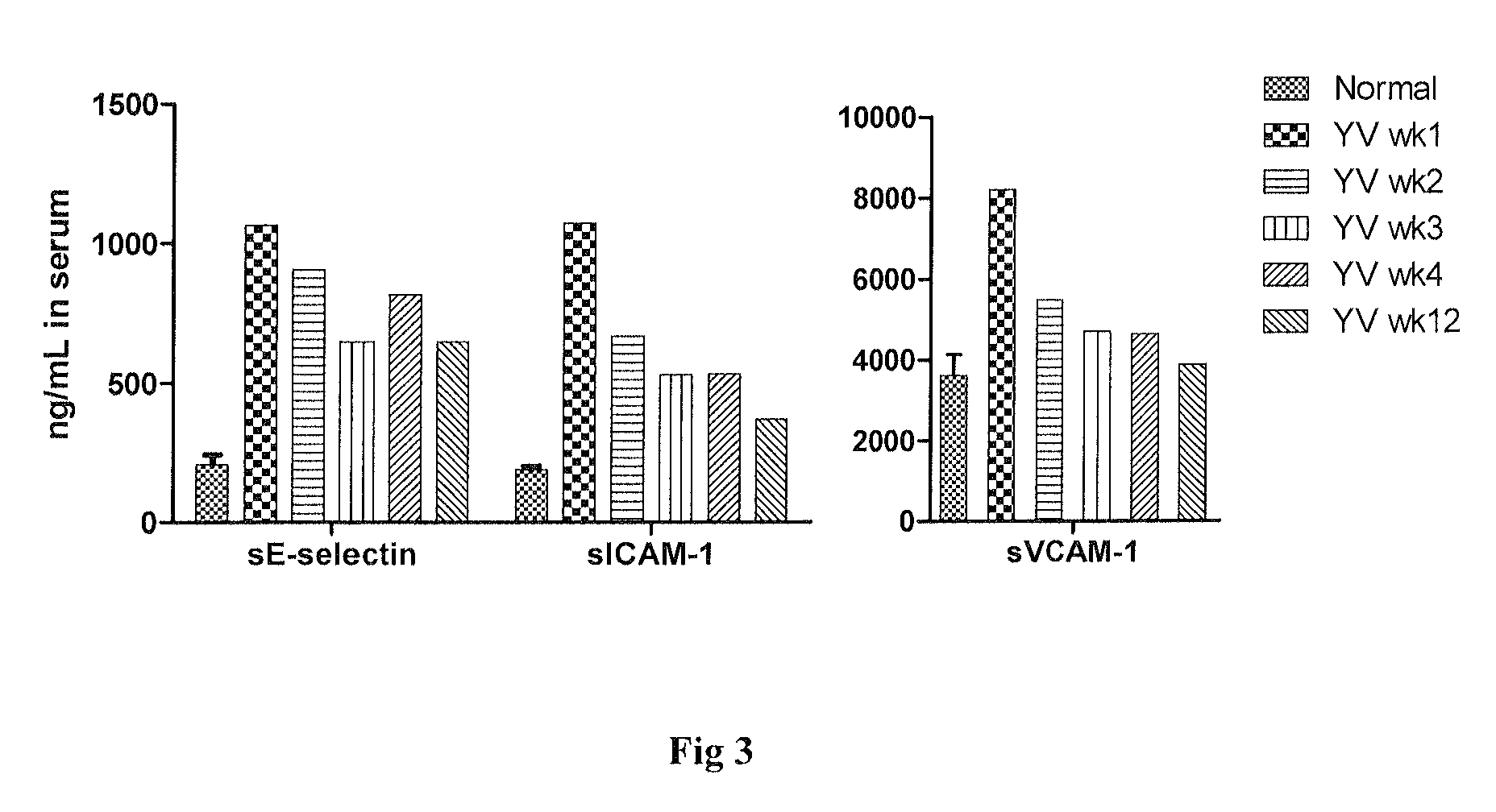
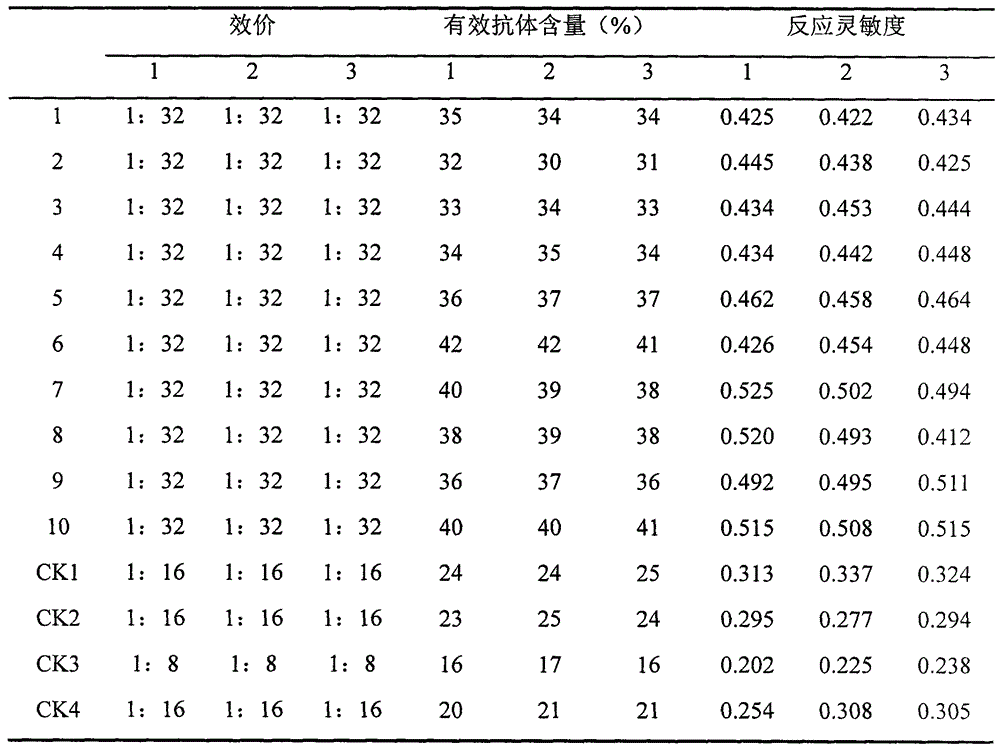
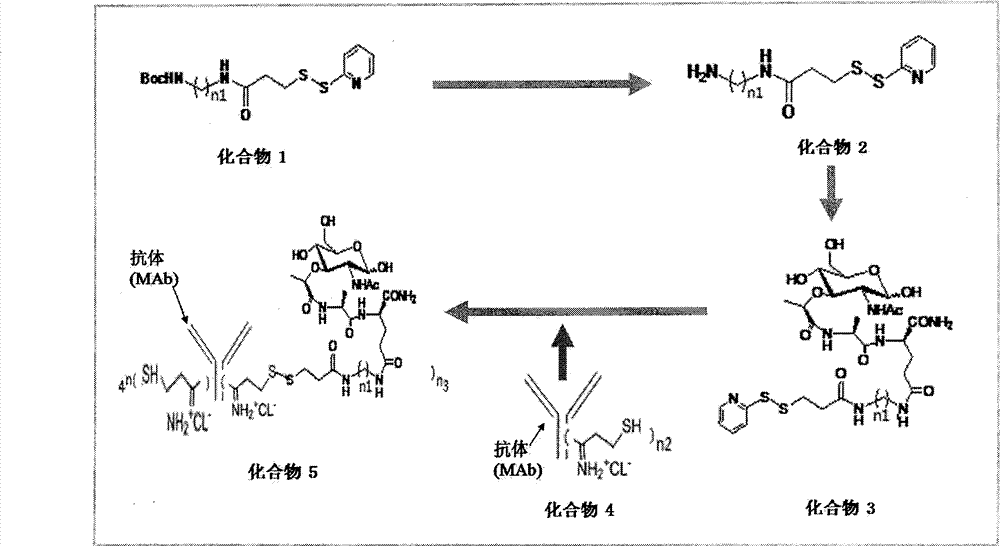
Patents
Literature
52 results about "Muramyl dipeptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Muramyl dipeptide is constituent of both Gram-positive and Gram-negative bacteria composed of N-acetylmuramic acid linked by its lactic acid moiety to the N-terminus of an L-alanine D-isoglutamine dipeptide.
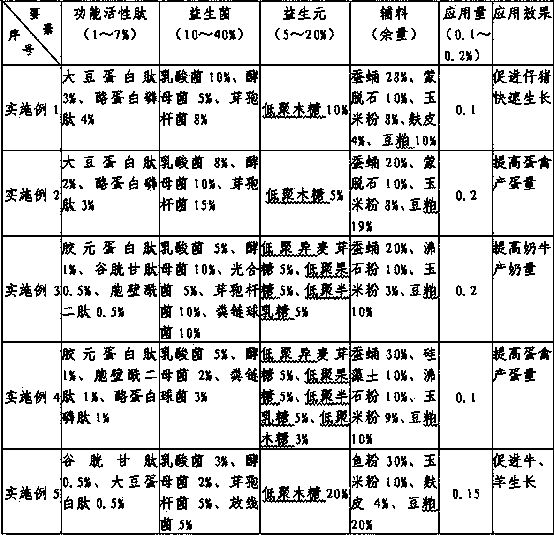
Compound type biological active peptide product for livestock and poultry and application thereof
InactiveCN103636945AWell mixedNo drug residue problemAnimal feeding stuffBiotechnologyIsomaltooligosaccharide
The invention discloses a compound type biological active peptide product for livestock and poultry, which is formed by mixing the following components in percent by mass: 1%-7% of functional active peptide, 10%-40% of probiotics, 5%-20% of prebiotics, and the balance of auxiliary materials, wherein the functional peptide is one or a combination of several of collagen peptide, glutathione, muramyl dipeptide, soybean protein peptide and casein phosphopeptide. The probiotics refers to one or a combination of several of lactic acid bacteria, saccharomycetes, photosynthetic bacteria, actinomycetes, bacillus and streptococcus faecalis. The prebiotics is one or a combination of several of isomalto-oligosaccharide, fructo-oligosaccharide, galactooligosaccharide and xylo-oligosaccharide. The auxiliary materials refer to one or a combination of several of silkworm chrysalis, fish meal, montmorillonite, diatomite, zeolite powder, corn flour, bran and bean pulp. Additive amount of the product accounts for 0.1%-0.2% of the amount of feed, and the feed can be directly fed after the product is added and uniformly mixed. The compound type biological active peptide product disclosed by the invention belongs to the micro-ecological technical product and is green and environment-friendly.
Owner:JIANGSU WEITAILONG BIOTECH
Immunity enhancing agent, inactivated vaccine, and preparation method thereof
InactiveCN103083663AImprove immunityEnhance immune responseViral antigen ingredientsAntiviralsDipeptideOil phase
The invention provides an immunity enhancing agent, an inactivated vaccine, and a preparation method thereof. The invention relates to the field of biopharmaceutical. The immunity enhancing agent comprises 0.1-21mg / mL of monophosphoryl lipid A, 1.5-125mg / mL of muramyl dipeptide, and 0.7-4.5mg / mL of beta-glucan. The invention also provides the inactivated vaccine comprising the immunity enhancing agent, and a preparation method of the inactivated vaccine. According to the invention, the immunity enhancing agent is mixed with an inactivated antigen solution, such that a water-phase solution is obtained; and the water-phase solution is mixed with an oil-phase solution, such that the inactivated vaccine is obtained. According to the immunity enhancing agent provided by the invention, with a synergetic effect of the components, body immunity level can be improved, and immune response to antigen can be improved, such that antibody level after immunization can be increased, immune window period can be shortened, and vaccine immunization effect can be enhanced. According to the inactivated vaccine comprising the immunity enhancing agent, antibody level after immunization is high, a protection period is long, and immunization window period is short.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES +1
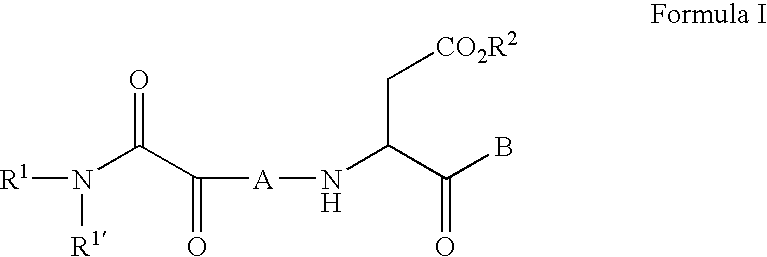
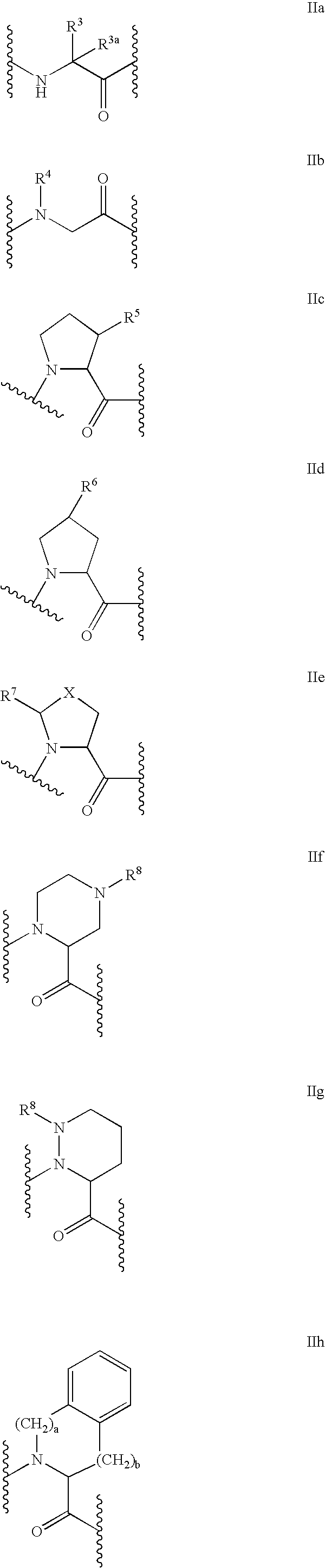
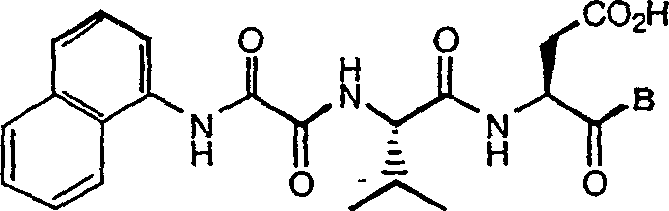
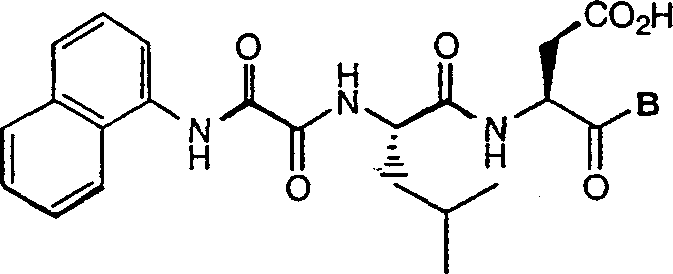
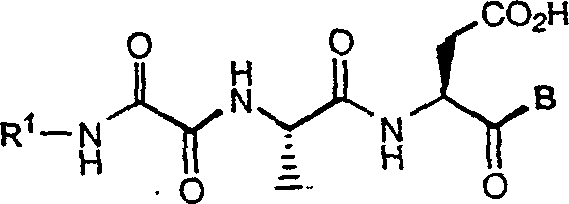
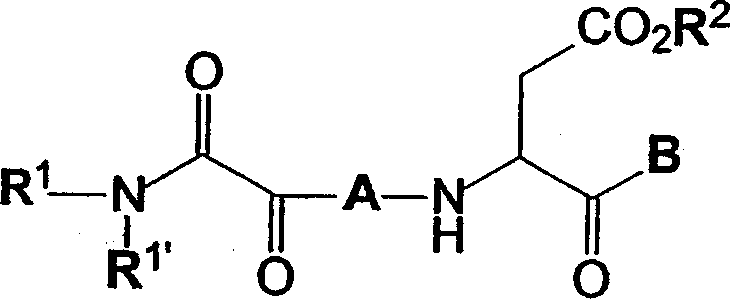
C-terminal modified oxamyl dipeptides as inhibitors of the ICE/ced-3 family of cysteine proteases
InactiveUS7183260B2Improve propertiesImprove permeabilityAntibacterial agentsOrganic active ingredientsDiseaseDipeptide
This invention is directed to novel oxamyl dipeptide ICE / ced-3 family inhibitor compounds. The invention is also directed to pharmaceutical compositions containing these compounds, as well as to the use of such compositions in the treatment of patients suffering inflammatory, autoimmune and neurodegenerative diseases, for the prevention of ischemic injury, and for the preservation of organs that are to undergo a transplantation procedure.
Owner:CONATUS PHARMA
C-terminal modified oxamyl dipeptides as inhibitors of the ICE/ced-3 family of cystenine proteases
This invention is directed to novel oxamyl depeptide ICE / ced-3 family inhibitor compounds. The invention is also directed to pharmaceutical compositions containing these compounds, as well as to the use of such compositions in the treatment of patients suffering inflammatory, autoimmune and neurodegenerative diseases, for the prevention of ischemic injury, and for the preservation of organs that are to undergo a transplantation procedure.
Owner:CONATUS PHARMA
PED (Porcine Epedemic Diarrhea) inactivated vaccine and preparation method thereof
ActiveCN104383528AEnhance immune responseImprove the level ofOrganic active ingredientsDipeptide ingredientsAntiendomysial antibodiesDipeptide
The invention provides a PED (Porcine Epedemic Diarrhea) inactivated vaccine and a preparation method thereof and relates to the field of biopharmacy. The PED inactivated vaccine comprises inactivated PEDV (Porcine Epedemic Diarrhea Virus), and is characterized in that the PED inactivated vaccine comprises 0.05-10 mg / mL Beta-glucosylceramide, 0.1-21 mg / mL monophosphoryl lipid A, 1.5-125 mg / mL muramyl dipeptide and 0.7-4.5 mg / mL Beta-glucan. According to the ingredients of the PED inactivated vaccine, Beta-glucosylceramide, monophosphoryl phosphoryl lipid A, muramyl dipeptide and Beta-glucan have the synergistic effect, the immune response of animals to antigens in the vaccine is significantly improved, the immune window phase is shortened, the antibody production duration of the animal body is obviously prolonged, the serum antibody level is improved, and the level of total intestinal mucosa secretory antibodies (the total SIgA) is improved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Compound immunoenhancement agent, vaccine for birds and method for preparing compound immunoenhancement agent
ActiveCN102743750AShorten the immune window periodShort immune windowAntiviralsImmunological disordersBird fluDipeptide
The invention relates to a compound immunoenhancement agent and an application thereof on preparing a vaccine for birds. The compound immunoenhancement agent contains 5ng-10mg / mL of poly IC, 10ng-10mg / mL of muramyl dipeptide, 10ng-5mg / mL of levamisole, 10ng-5mg / mL of resiquimod and 10ng-5mg / mL of imiquimod. After the immunoenhancement agent and H5 hypotype bird flu inactivated vaccine are mixed together, antibody can be produced one week earlier, and the antibody titer is improved above 1.8log2. After chicken are immunized through H5 hypotype bird flu inactivated vaccine, H9 hypotype bird flu vaccine or infectious bronchitis vaccine which contains the compound vaccine immunoenhancement agent, the window phases of the antibody production are shortened, the antibody titer of the vaccine is improved, the immunization duration is prolonged, and the probability of infection disease is reduced.
Owner:南京国创生物技术研究院有限公司
Dentin primary coating-washing primary coating agent and method thereof
ActiveCN104398390AHigh bonding strengthReduce degradationImpression capsDentistry preparationsAcid etchingFunctional monomer
The invention provides a dentin primary coating-washing primary coating agent and a method thereof. The dentin primary coating agent is used for repairing the defect of a tooth body for a stomatological department. The method comprises the following steps: performing pretreatments of primary coating-washing on the surface of the dentin with the dentin primary coating agent, then sticking a repairing body with the dentin by using a self-acid etching bonding agent, wherein the stomatological dentin primary coating-washing primary coating agent contains 60%-99.4% of an alcohol aqueous solution, 0.5%-30% of a weak-acid functional monomer and 0.1%-10% of a matrix metalloproteinase inhibitor. The dentin is mildly demineralized by 10-MDP(muramyl dipeptide) containing weak acid; the degradation of a collagenous fiber is reduced by using the matrix metalloproteinase inhibitor; besides the dentin is pretreated by a primary coating-washing method, so that generated MDP-Ca(Calcium) is more firmly jointed to hydroxyapatite, the bonding strength and the bonding durability of the tooth can be further improved, joining of the tooth and the direct repairing body or the indirect repairing body is firmer and more durable, and the health of the rest tissue of the tooth is protected better.
Owner:ZHEJIANG UNIV
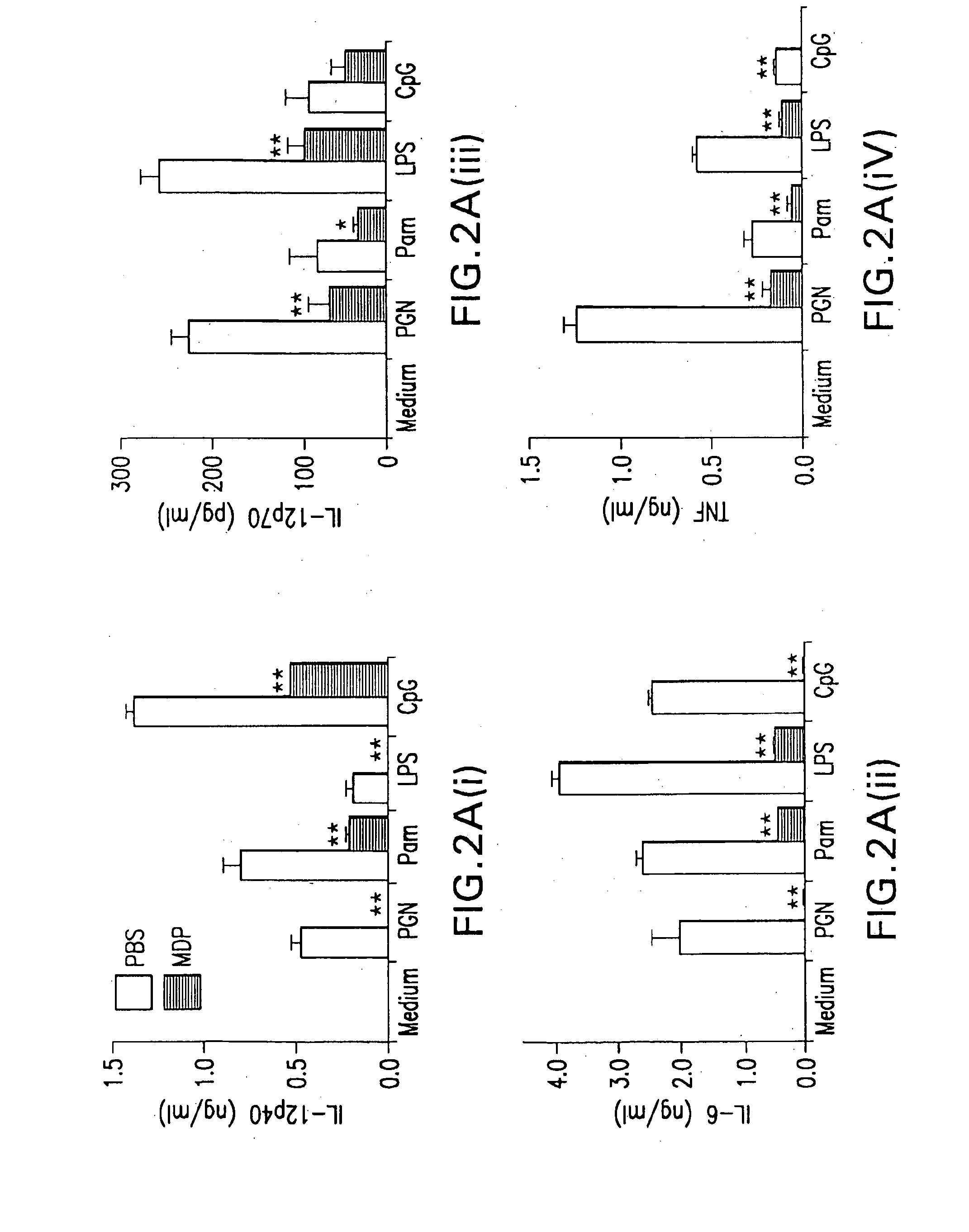
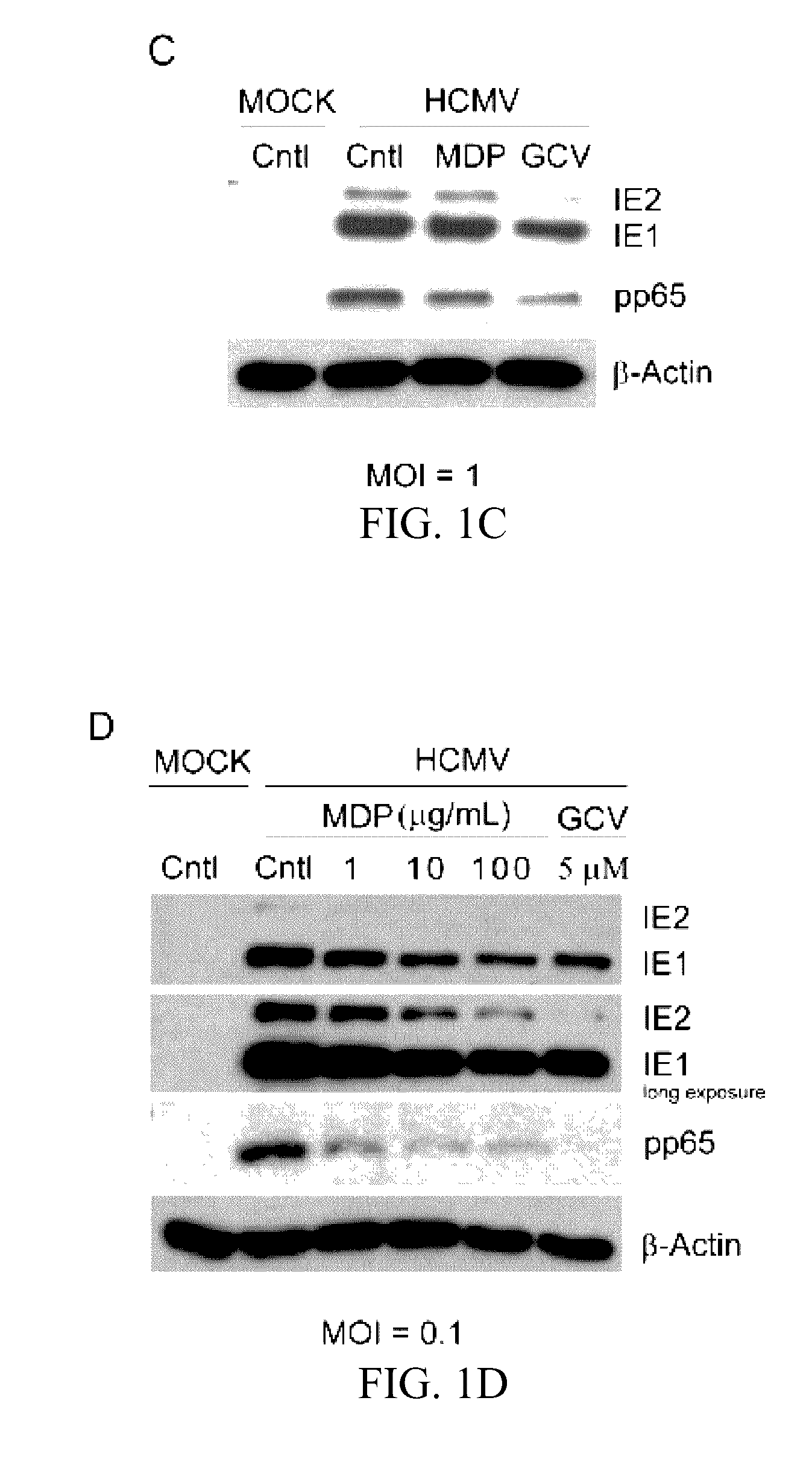
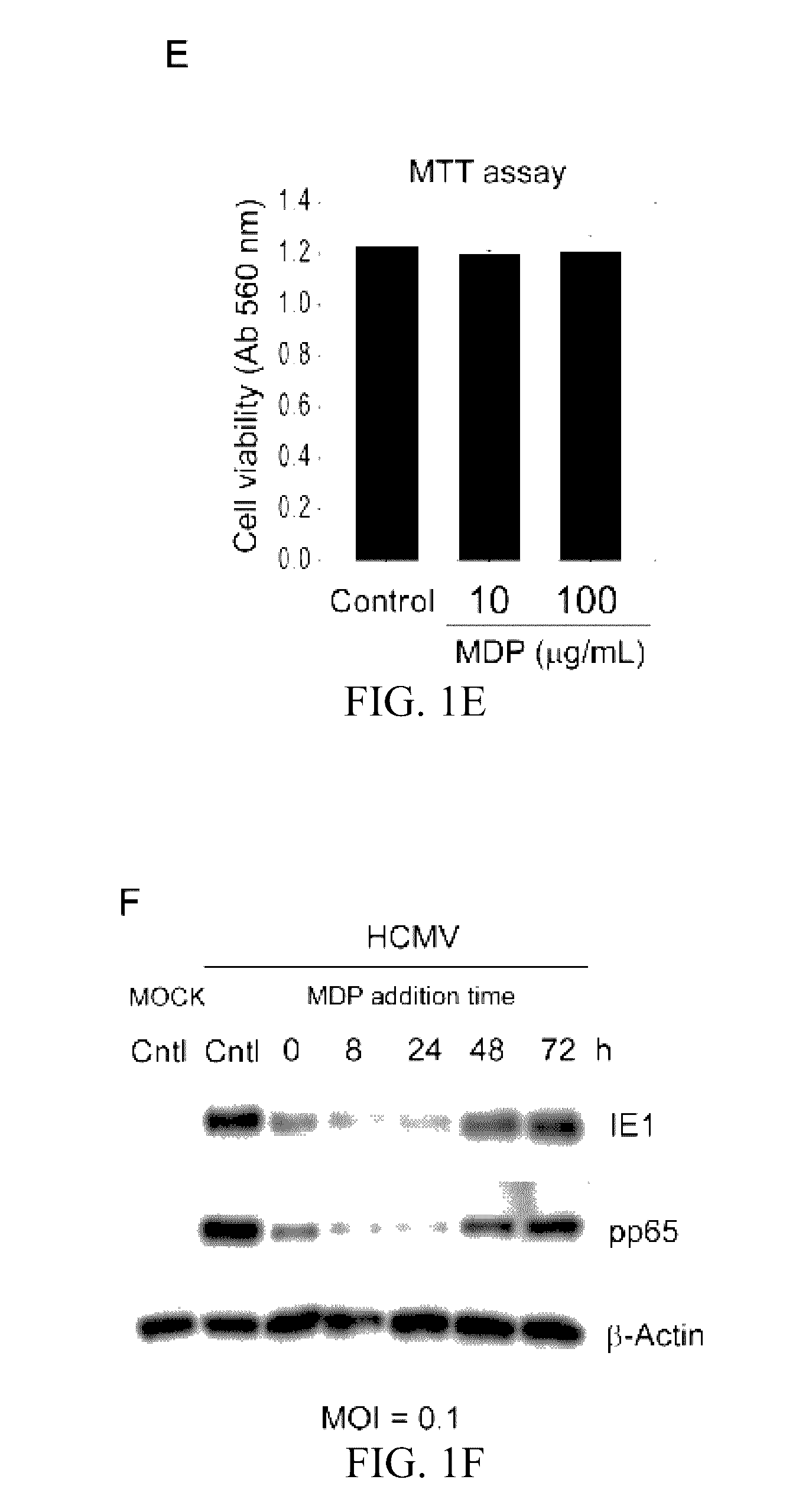
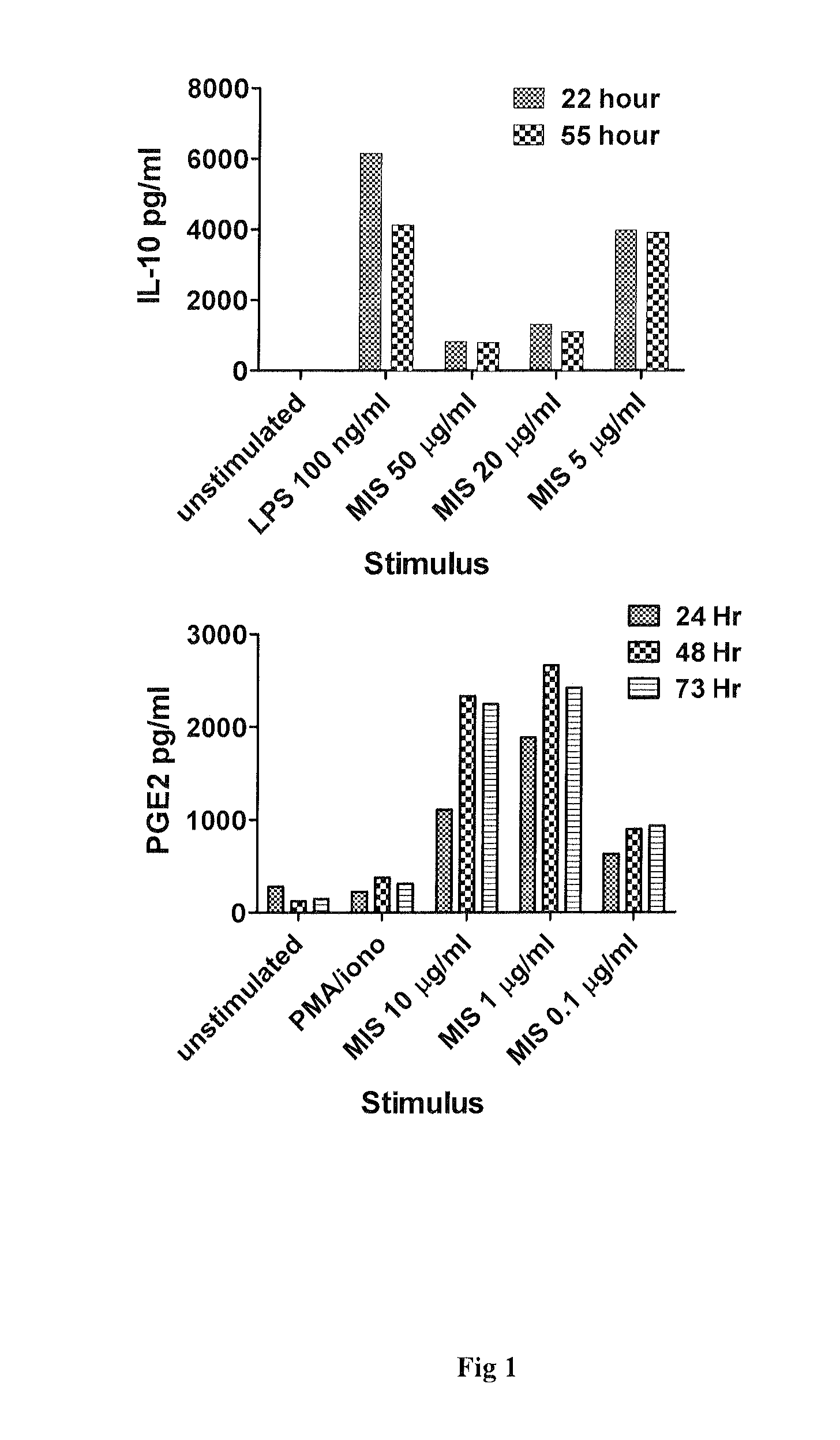
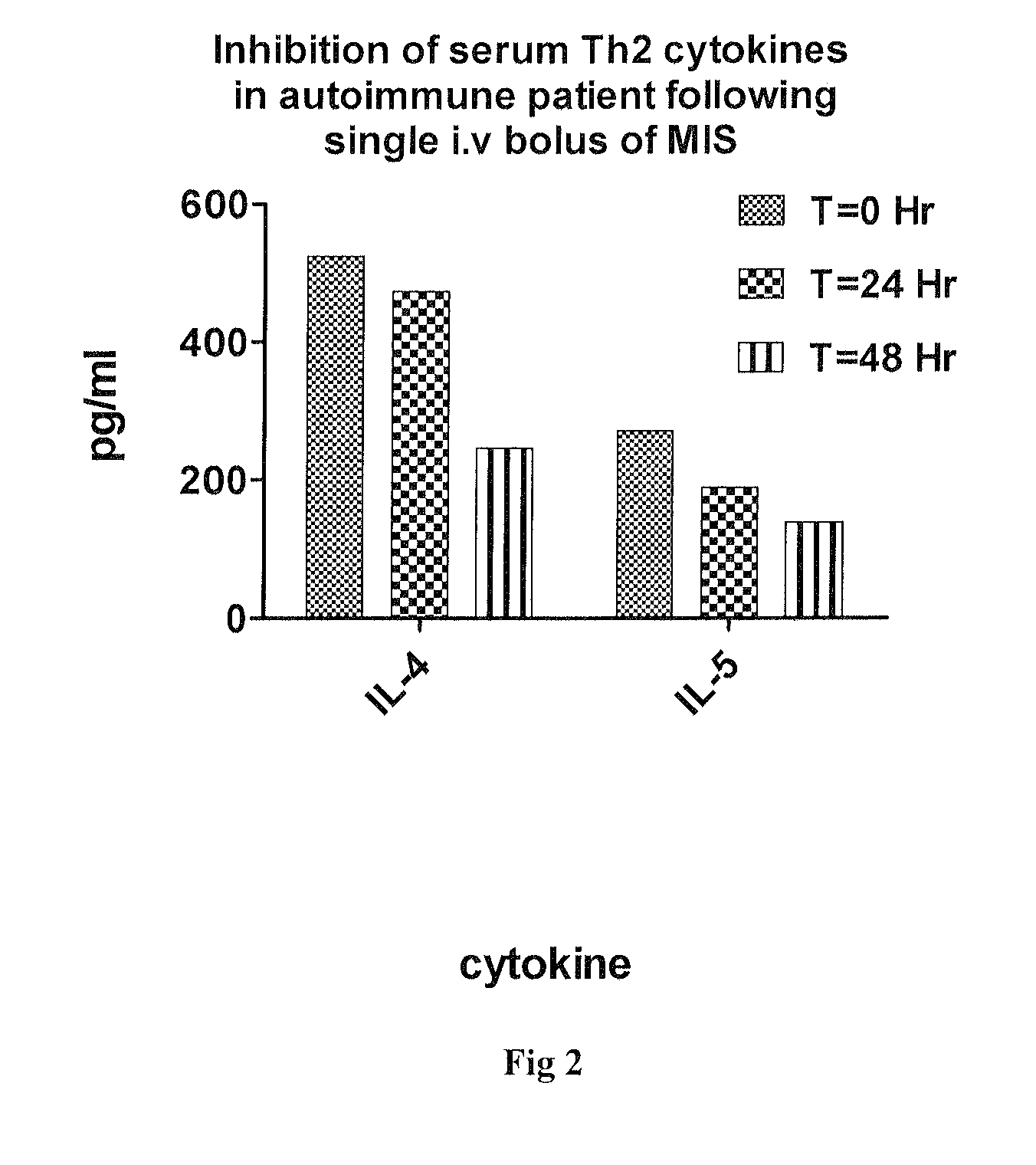
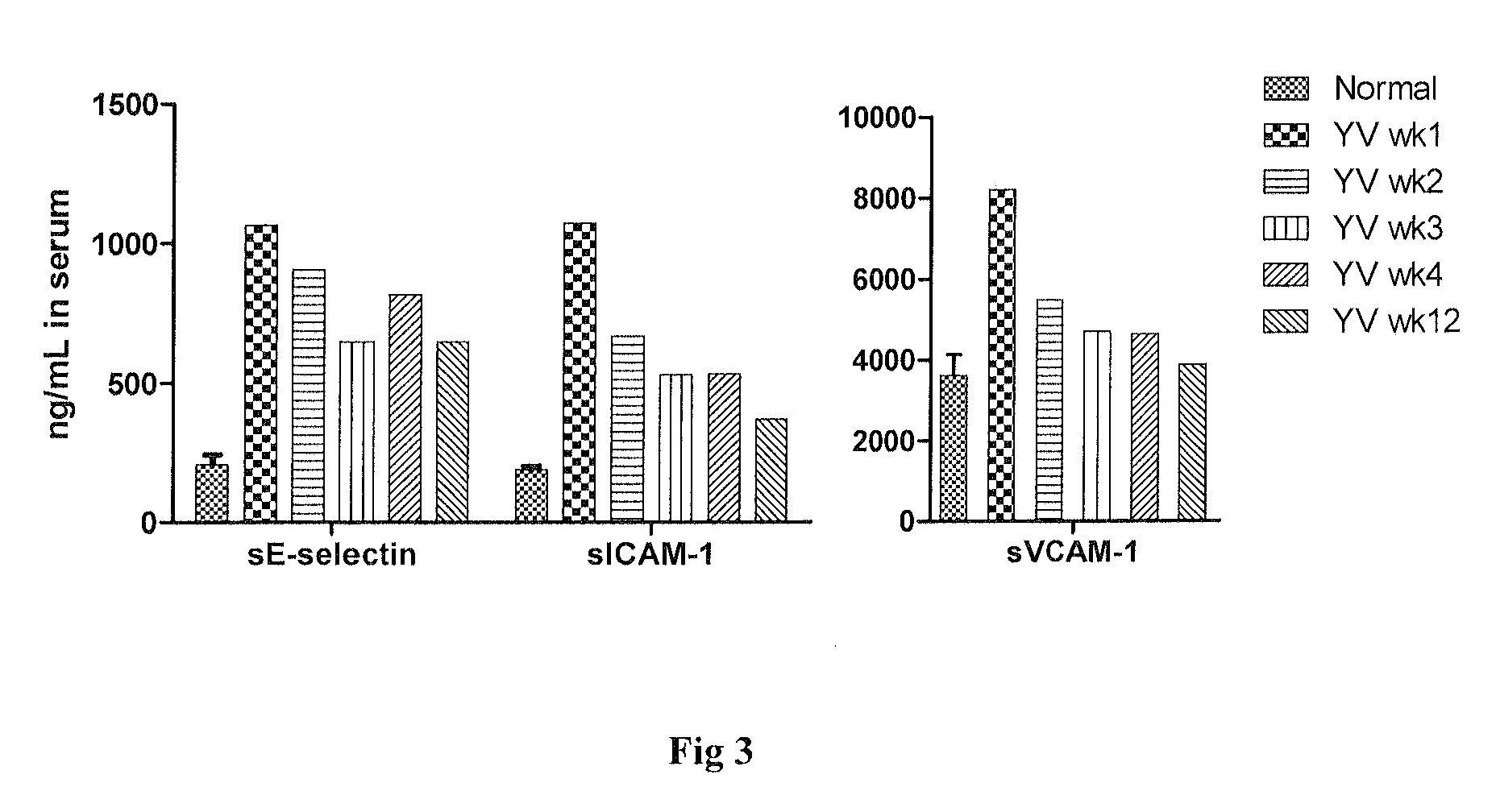
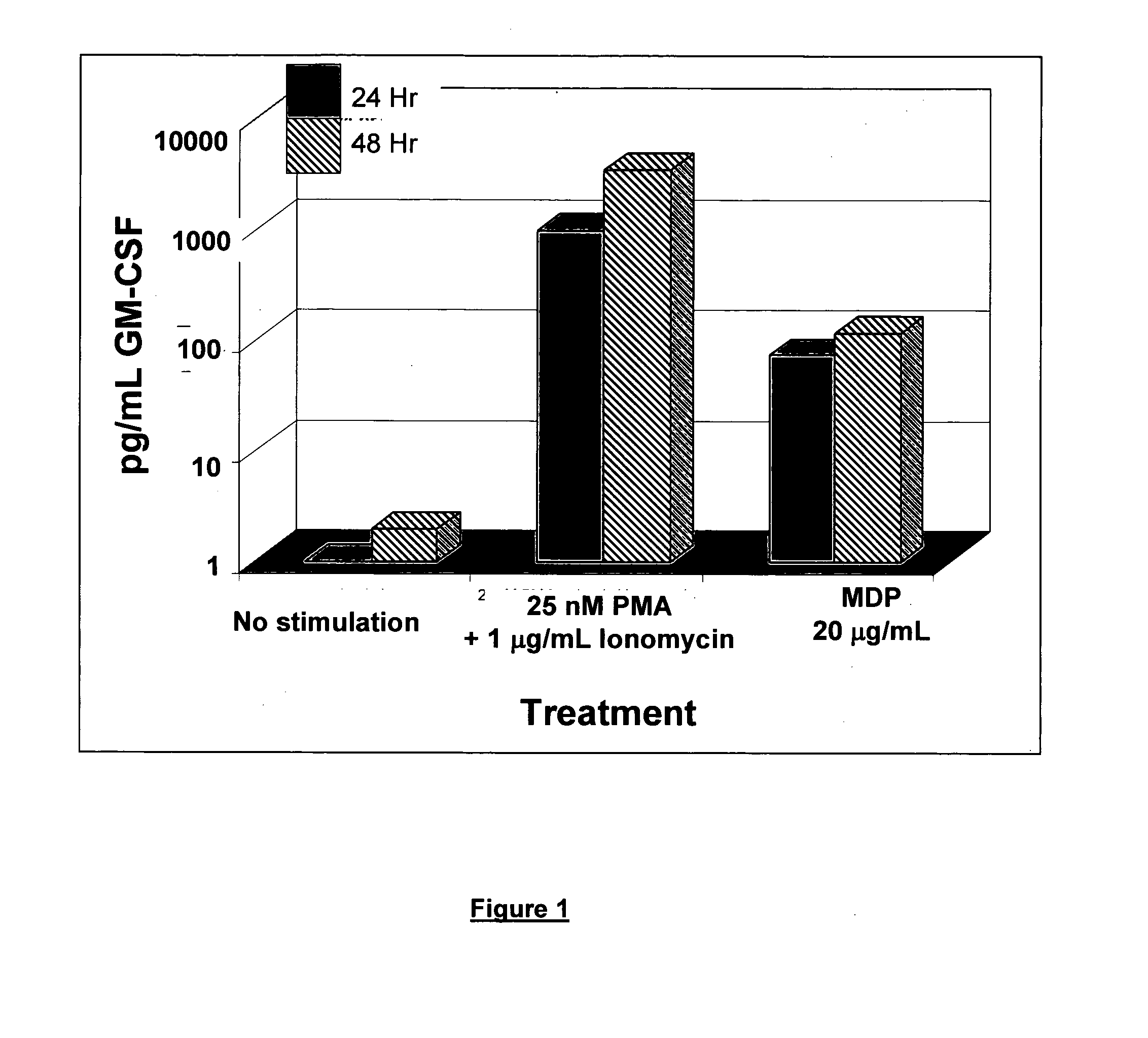
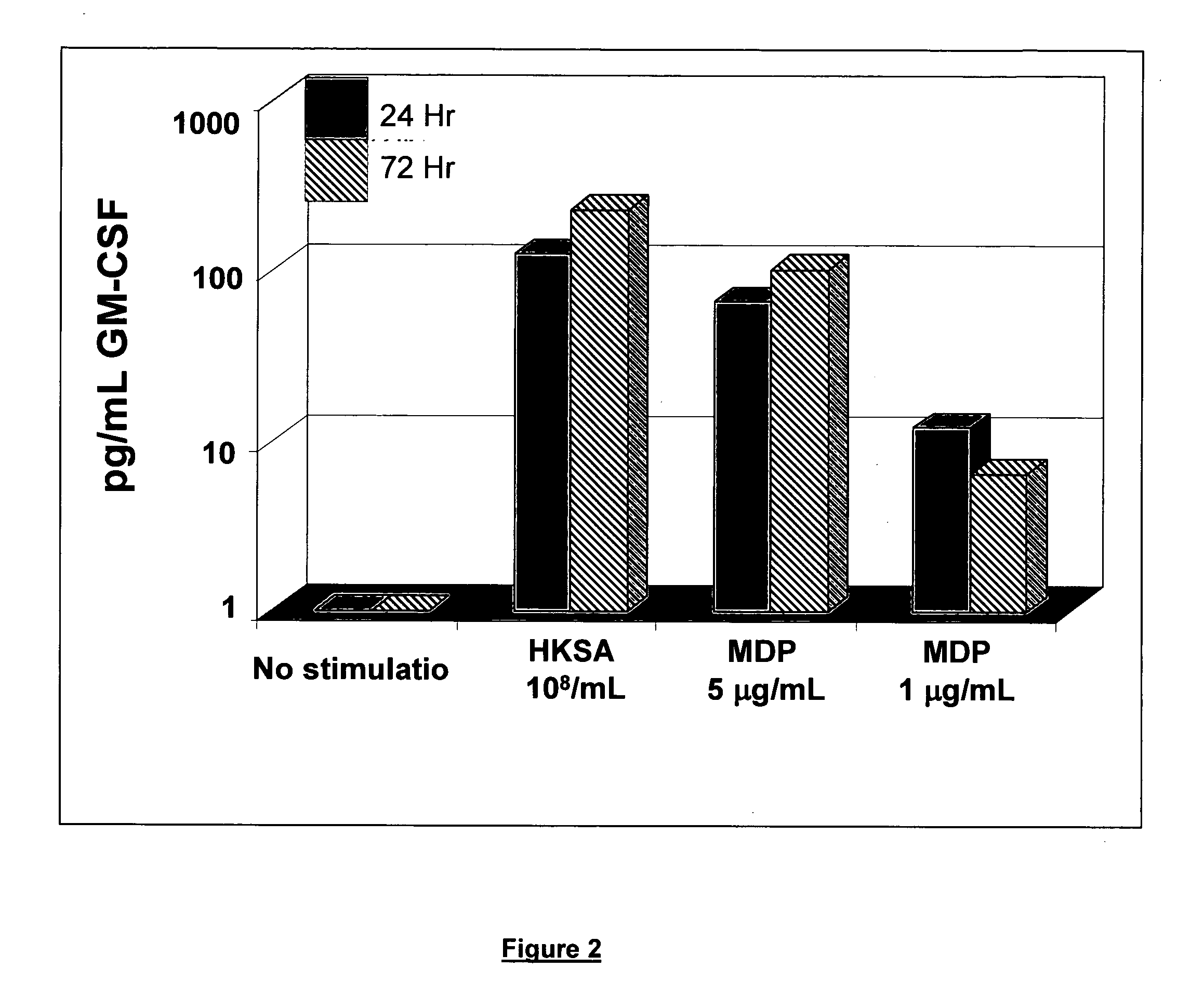
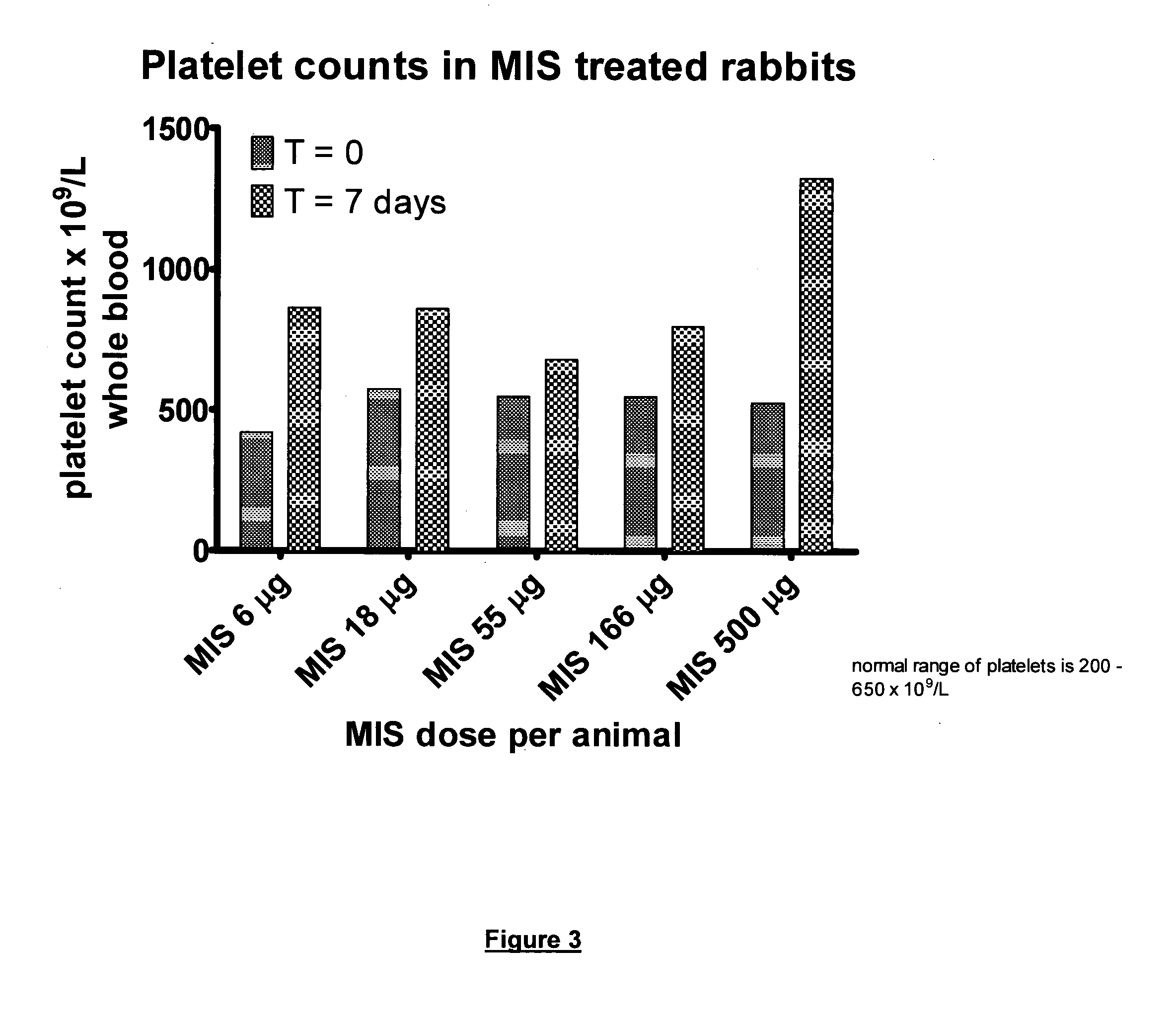
Use of muramyl dipeptide (MDP) for treating inflammation
ActiveUS20100292153A1Reducing symptom characteristicInhibit inflammationAntipyreticDigestive systemMedicineMuramyl dipeptide
The present invention provides a method of treating or preventing inflammation in a subject comprising administering to the subject an effective amount of a muramyl dipeptide (MDP).
Owner:UNITED STATES OF AMERICA
Substituted muramyl dipeptide compound and preparation method and applications thereof
The invention discloses a substituted muramyl dipeptide compound and a preparation method and applications thereof, and further discloses a pharmaceutical composition of the compound and applications of the pharmaceutical composition. The substituted muramyl dipeptide compound can antagonize human derived or mouse derived immune cell NOD1 and / or NOD2 mediated inflammation NF-[kappa]B and MAPKs signal transduction pathway and inhibit the growth and metastasis of tumors.
Owner:TSINGHUA UNIV
Microprojection Array Immunization Patch and Method
InactiveUS20090143724A1Immune responseEnhance immune responseViral antigen ingredientsSurgeryAdjuvantStratum corneum
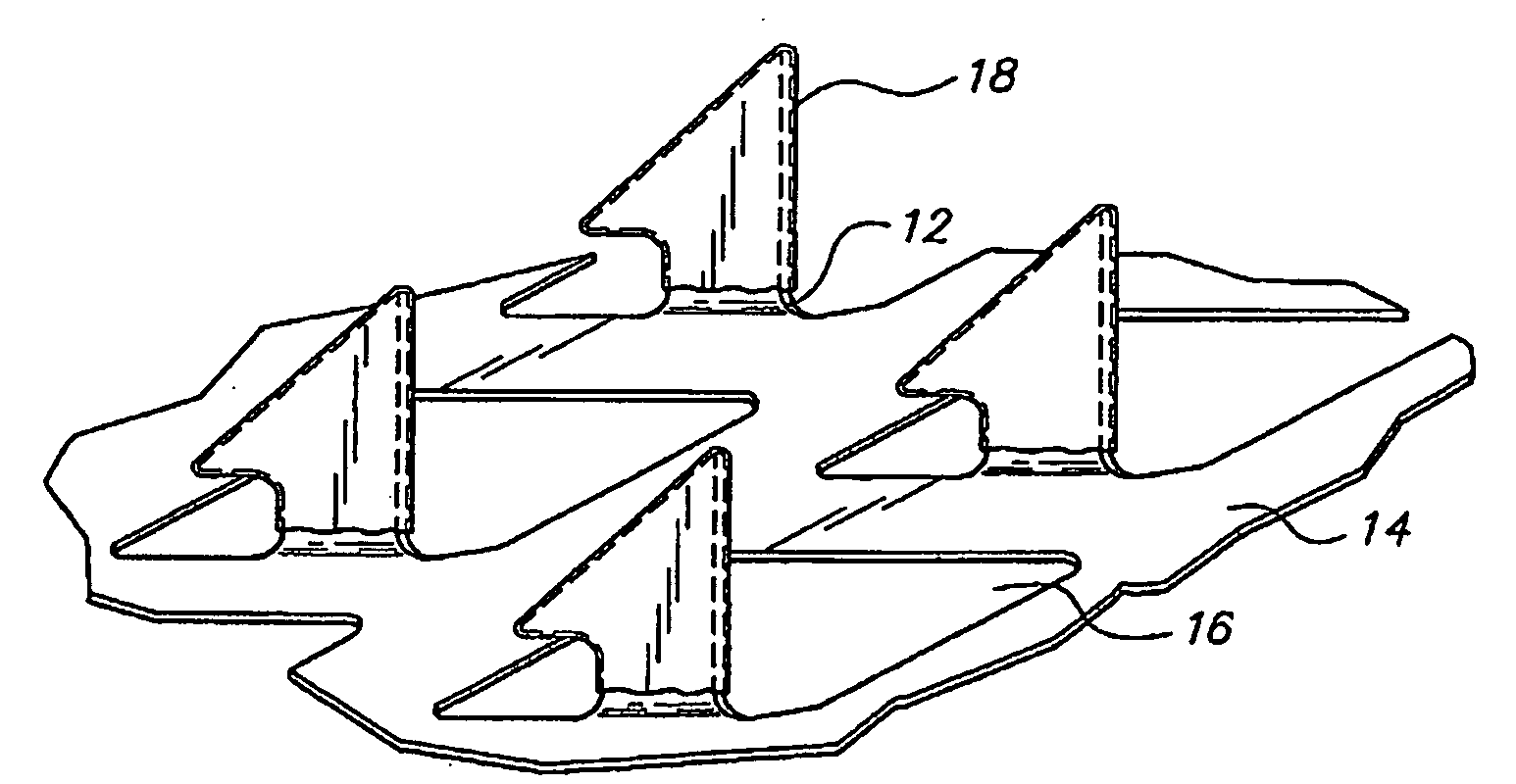
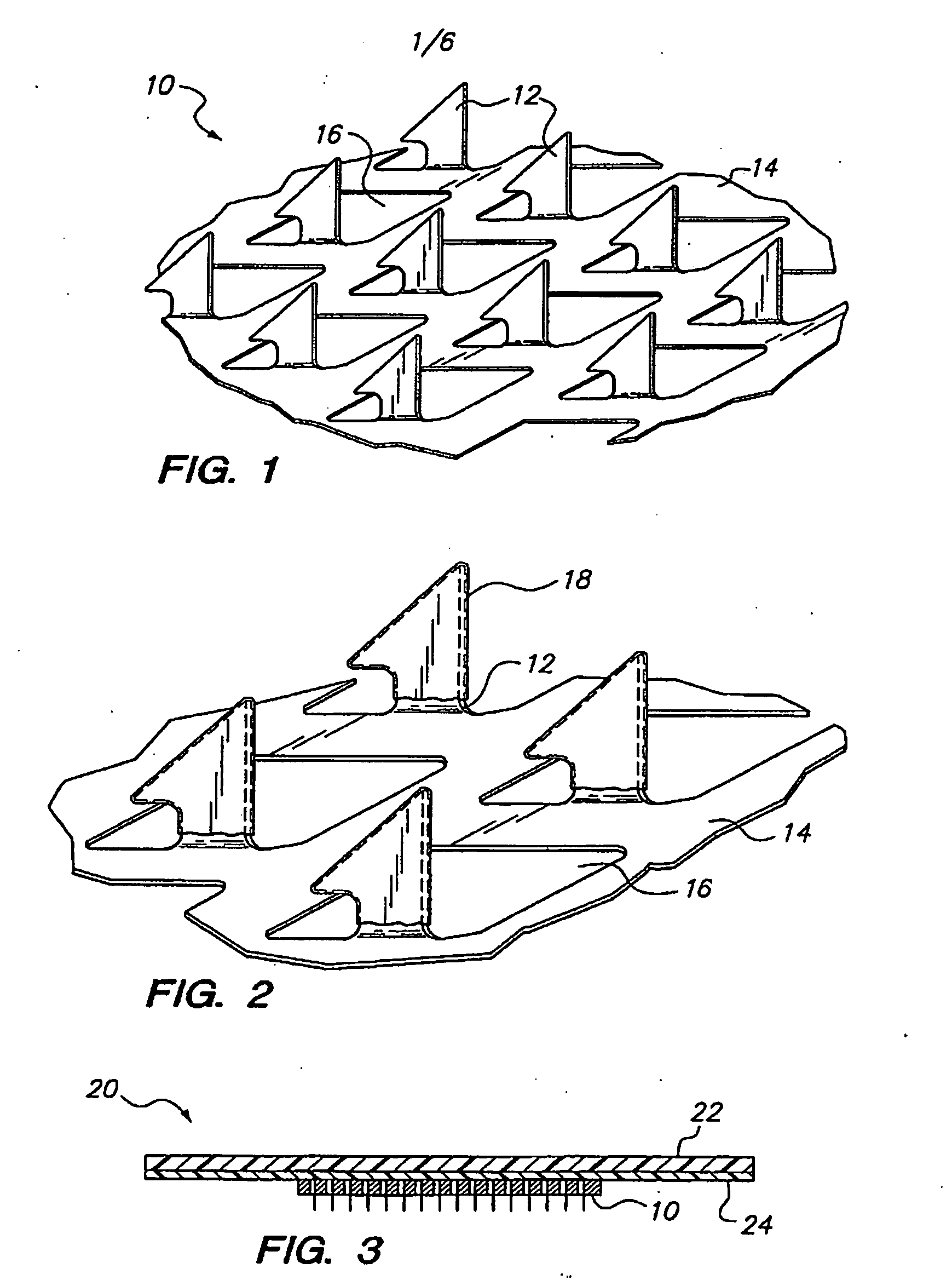
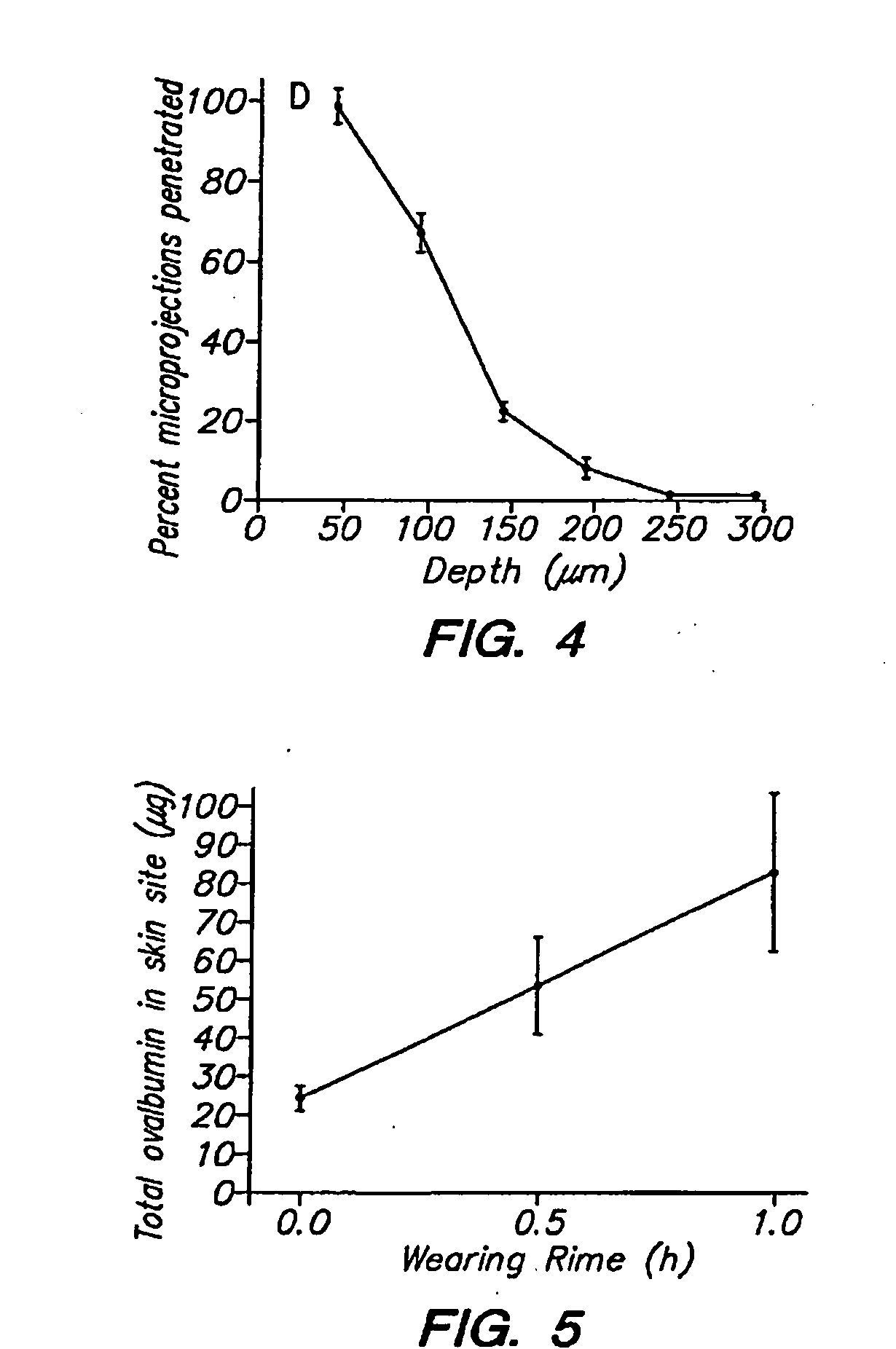
Skin patches (20) having a microprojection array (10), a reservoir (18) containing an antigenic agent and an immune response augmenting adjuvant, and methods of using same to vaccinate animals (e.g., humans) is disclosed. In a preferred embodiment, the microprojection arrays (10) are composed of a photoetched and micro-punched titanium foil (14). The microprojections (12) are coated with a liquid formulation containing a vaccine antigen and an adjuvant such as glucosaminyl muramyl dipeptide, dried, and applied to skin of the animal to be vaccinated using an impact applicator. The microprojections (12) create superficial pathways through the stratum corneum to facilitate permeation of antigenic agent and adjuvant. Antigen dose and depth of penetration can be controlled. This technology has broad applicability for a wide variety of therapeutic vaccines to improve efficacy, and convenience of use.
Owner:ALZA CORP
Medicament oral liquid for treating enteritis and diarrhea and preparation method thereof
InactiveCN102091321AImprove immunityHigh cure rateAnthropod material medical ingredientsDipeptide ingredientsPathogenic microorganismSide effect
The invention relates to medicament oral liquid for treating enteritis and diarrhea. Raw medicines in the medicament oral liquid comprise the following components in parts by weight: 10-30 parts of poplar flower, 10-30 parts of echinacea, 10-30 parts of bee glue, 1-2 parts of muramyl dipeptide, 0.5-1 part of scopolamine methylbromide and 5-10 parts of D-mannose. The invention can effectively improve immunity, has good sterilizing and diarrhea relieving effect, lower cost and high killing rate on bacterium and virus pathogenic microorganisms, has no toxic or side effect and no medicine resistance, can not generate medicament residual and is convenient for use.
Owner:TIANJIN ZHONGAO BIOTECH
Lactobacillus casei ghost, preparation method and application thereof
InactiveCN103013897AGood adjuvant effectImproving immunogenicityBacteriaMicroorganism based processesDiseaseVaccine delivery
The invention discloses a lactobacillus casei ghost, a preparation method and an application thereof, belonging to the biotechnology field. According to the lactobacillus casei ghost, lactobacillus casei is used as a research object, a recombinant vector containing cloned lytic genes is converted into the lactobacillus casei through separation of bacteriophage of the lactobacillus casei, and prediction and cloning of the lytic genes, and then the lytic genes are induced to be expressed to prepare the lactobacillus casei ghost. The lactobacillus casei ghost is used as an antigen delivery carrier safely and reliably in the true sense; besides, muramyl dipeptide in the cell wall of the lactobacillus casei has a good adjuvant effect and can remarkably enhance the immunogenicity of heterogeneous antigen; furthermore, the mass production of the lactobacillus casei ghost can be realized through fermentation, and the lactobacillus casei ghost can be preserved at room temperature for a long time after being frozen and dried, so that the cost for application of production practice is further reduced; and a new idea and a new method for further designing a novel, safe and effective drug, particularly a vaccine delivery system, which are applied to the prevention and treatment of the diseases are provided.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Medicinal composition for treating respiratory diseases of livestock
InactiveCN102688477AHeat-clearing and detoxifyingUnique curative effectDipeptide ingredientsTetracycline active ingredientsDiseaseRegimen
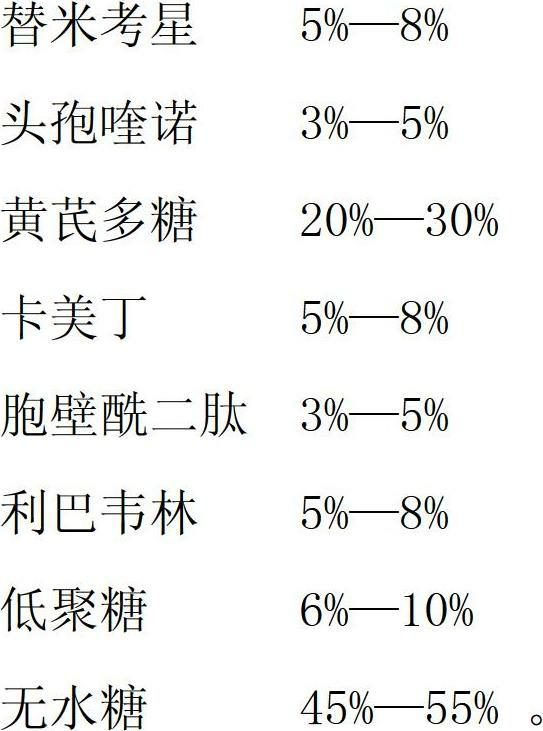
The invention provides a medicinal composition for treating respiratory diseases of livestock, which comprises the following active components in percentage by mass: 5 to 8 percent of tilmicosin, 3 to 5 percent of cefquinome, 20 to 30 percent of astragalus polysaccharides, 5 to 8 percent of kameiding, 3 to 5 percent of muramyl dipeptide, 5 to 8 percent of ribavirin, 6 to 10 percent of oligosaccharide, and 45 to 55 percent of anhydrous sugar. The drugs are mixed, and have the functions of clearing heat and removing toxicity, relieving cough and asthma, have unique curative effect for treating respiratory diseases of livestock, have no drug resistance, and have quick response, good curative effect and short course of treatment.
Owner:ZHENGZHOU AINUO BIOTECH
B-type hepatitis vaccine
InactiveCN1404875ABright future for treatmentImprove humoral immunity/cellular immunityDigestive systemAntiviralsAdjuvantChronic hepatitis
The present invention relates to a hepatitis B vaccine, the main composition of said vaccine comprises gene engineering hepatitis B virus surface antigen, muramyl dipeptide (MDP) and aluminium adjuvant. Said hepatitis B vaccine can be used for immunity of adult, renal transplanted patient and patient with renal dialysis therapy and for preventing infection of hepatitis B virus, and said hepatitis B vaccine also can be used for immunotherapy of chronic hepatitis B patient, and the immunogenicity of said vaccine is superior to that of existent aluminium adjuvant hepatitis B vaccine.
Owner:BEIJING LUZHU BIOTECH
Compound preparation for preventing and treating respiratory disease of livestock and poultry
InactiveCN101780266AControl therapeutic effectEfficient therapeutic effectAnthropod material medical ingredientsDipeptide ingredientsDiseaseOral glucose
The invention relates to a compound preparation for preventing and treating respiratory diseases of livestock and poultry, which is characterized by being prepared by uniformly mixing the following components by taking 100 grams as unit weight: 2-5 grams of cefixime sodium, 10-30 grams of doxycycline hydrochloride, 5-10 grams of rifampicin, 5-20 grams of cimetidine, 2-10 grams of ndometacin sodium, 2-6 grams of propolis powder, 2-6 grams of muramyl dipeptide and the balance of pharmaceutically applicable auxiliary materials, wherein the pharmaceutically applicable auxiliary materials are anhydrous glucose or oral glucose, preferably the anhydrous glucose. The compound preparation is compounded by antibiotic drugs, antiviral drugs, immunopotentiator and mucosa repair factors and has efficient and fast cure effect on respiratory symptoms of the livestock and the poultry, easy preparation, obvious effect and wide application range.
Owner:TIANJIN SHENGJI GRP CO LTD
Freezing protection solution for articular cartilage and freezing preservation method for articular cartilage
The invention provides a freezing protection solution for the articular cartilage. The freezing protection solution is prepared from, by weight, 18-22 parts of carboxymethyl starch, 2-3 parts of rhamnose, 1-2 parts of cetene, 2-4 parts of microcrystalline cellulose, 4-6 parts of galactomannan, 4-6 parts of muramyl dipeptide, 3-5 parts of palmitoyl pentapeptide-3, 0.5-1.5 parts of alpha-arbutin and70 parts of deionized water. The invention further provides a freezing preservation method for the articular cartilage by adopting the freezing protection solution for the articular cartilage. The method comprises the steps of leaching, dripping of the partial freezing protection solution, addition of a remaining freezing protection solution and program cooling. According to the freezing protection solution, a freezing protection agent, harmful to cells, such as dimethyl sulfoxide is not used; according to the freezing preservation method for the articular cartilage, in the eighth week afterthe articular cartilage is frozen for storage, the articular cartilage is taken, and after temperature recovery, the cell survival rate of the articular cartilage is 82-85%, and the cell survival rateof the articular cartilage is 78-80% in the tenth week.
Owner:徐艳红
Vaccine adjuvants for cytomegalovirus prevention and treatment
InactiveUS20170021014A1Enhance immune responseGood effectViral antigen ingredientsDsDNA virusesDipeptideMeso-diaminopimelic acid
The present invention relates to the field of virology. More specifically, the present invention provides methods and compositions useful for treating human cytomegalovirus using bacterial cell wall components MDP and tri-DAP as vaccine adjuvants. In specific embodiments, the present invention provides a pharmaceutical composition comprising a human cytomegalovirus vaccine and a NOD1 activator and / or a NOD2 activator. In particular embodiments, the NOD2 activator is muramyl dipeptide (MDP) or a derivative thereof. In certain embodiments, the NOD1 activator is L-Ala-γ-D-Glu-meso-diamino-pimelic acid (tri-DAP) or a derivative thereof.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Compound proplis feed additive capable of accelerating pig to grow and fatten and improving immunity and preparation method thereof
InactiveCN102578414ANo pollution in the processNo side effectsAnimal feeding stuffSide effectUltra fine
The invention discloses a compound proplis feed additive capable of accelerating pigs to grow and fatten and improving immunity and a preparation method thereof, belonging to the field of animal feed. The feed additive is prepared from the components in parts by weight: 15-45 parts of proplis, 10-30 parts of gynostemma pentaphylla, 15-45 parts of flower of poplar, 15-30 parts of echinacea purpurea, 15-35 parts of isomaltose hypgather and 10-20 parts of muramyl dipeptide. The compound proplis feed additive has the beneficial effects of reasonable formula and is simple to prepare. The compound does not have toxic and side effect, is free from pollution, is free from medicine residue, does not have medicine tolerance, is subjected to ultra-fine pulverization and has high bioavailability. After application, the compound proplis feed additive is proved to have special efficiency on accelerating pigs to grow and fatten and improving immunity.
Owner:QINGDAO LVMAN BIOLOGICAL ENG
Gordoniasinensis, preparation method and application
ActiveCN103555630AWide variety of sourcesSimple processBacteriaBacteria material medical ingredientsBacillus licheniformisBacteroides
The invention discloses gordoniasinensis, a preparation method and application. Gordonia sinensis sq-15 with the preservation number of CCTCC (China Center for Type Culture Collection) NO: M2013065 can generate muramyl dipeptide, lipopolysaccharide, carotene, carotenoid, flavone and a plurality of amino acids, in particular gamma-aminobutyric acid, vitamin and a plurality of trace elements in a czapek's medium. The generated components are organically and harmoniously in a system, so that the physiological function of the system can be sufficiently enhanced; the gordoniasinensis can play a synergetic effect when being combined with other inoculums, such as bacillus subtilis, bacillus licheniformis, or selenium enriched saccharomyces cerevisiae, to prepare different medicinal compositions, and can effectively improve the body immunity to achieve the aims of resisting oxidation, regulating intestinal tracts, and regulating heart vessels.
Owner:湖南微草生物科技有限公司
Preparation method of high-potency positive serum for porcine pseudorabies virus
The invention provides a preparation method of high-potency positive serum for a porcine pseudorabies virus. The method takes a rabbit as an immunization object, and immunizes the rabbit with an inactivated vaccine containing a suitable amount of PolyI:C, muramyl dipeptide and cimetidine to produce the hyperimmune serum, can achieve the effect that no cytopathic effect occurs in subculture of at least 2 generations in a T25 square flask equal volume ratio neutralization 1 vaccine experiment, and meets the requirement that a cell method serves as an exogenous virus detection standard in the <Chinese veterinary pharmacopoeia> (2015 edition). The preparation method of the high-potency positive serum for the porcine pseudorabies virus has a good application prospect.
Owner:成都史纪生物制药有限公司
Lactobacillus casei ghost as well as preparation method and application thereof
ActiveCN103555754AGood adjuvant effectImproving immunogenicityBacteriaMicroorganism based processesDiseaseVaccine delivery
The invention discloses a lactobacillus casei ghost, a preparation method and an application thereof, belonging to the biotechnology field. According to the lactobacillus casei ghost, lactobacillus casei is used as a research object, a recombinant vector containing cloned lytic genes is converted into the lactobacillus casei through separation of bacteriophage of the lactobacillus casei, and prediction and cloning of the lytic genes, and then the lytic genes are induced to be expressed to prepare the lactobacillus casei ghost. The lactobacillus casei ghost is used as an antigen delivery carrier safely and reliably in the true sense; besides, muramyl dipeptide in the cell wall of the lactobacillus casei has a good adjuvant effect and can remarkably enhance the immunogenicity of heterogeneous antigen; furthermore, the mass production of the lactobacillus casei ghost can be realized through fermentation, and the lactobacillus casei ghost can be preserved at room temperature for a long time after being frozen and dried, so that the cost for application of production practice is further reduced; and a new idea and a new method for further designing a novel, safe and effective drug, particularly a vaccine delivery system, which are applied to the prevention and treatment of the diseases are provided.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Compositions and methods for treatment of multiple sclerosis
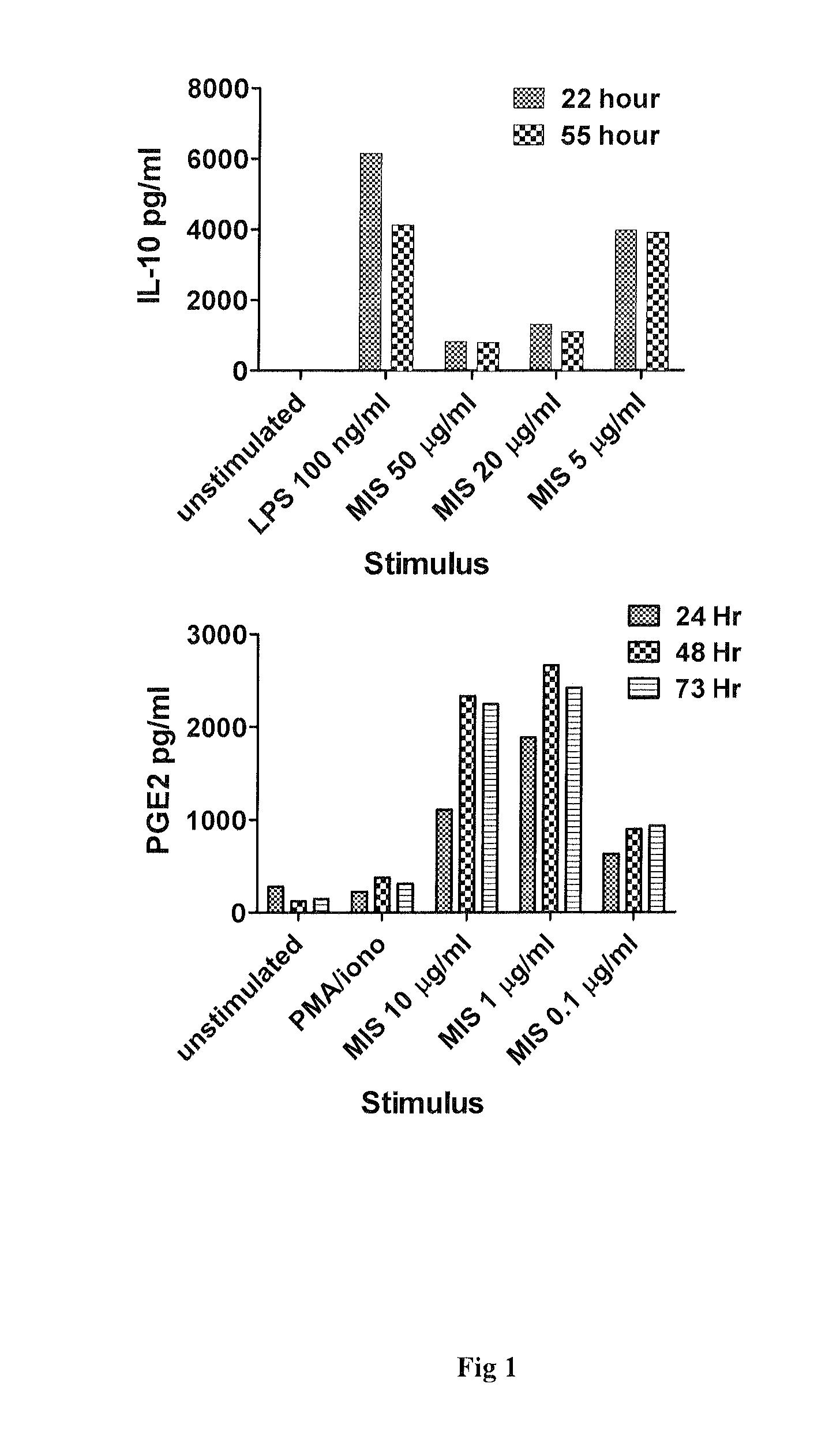
InactiveUS20100317589A1Treat symptomsEffective treatmentPowder deliveryNervous disorderMicroparticleMuramyl dipeptide
Disclosed are novel compositions and methods for the treatment of Multiple Sclerosis (MS), and in particular immunostimulatory compositions comprising muramyl dipeptide microparticles for use, e.g., in the treatment of MS.
Owner:INNATE IMMUNOTHERAPEUTICS
Bone loss preventing and bone regeneration or bone formation promoting pharmaceutical composition comprising muramyl dipeptide
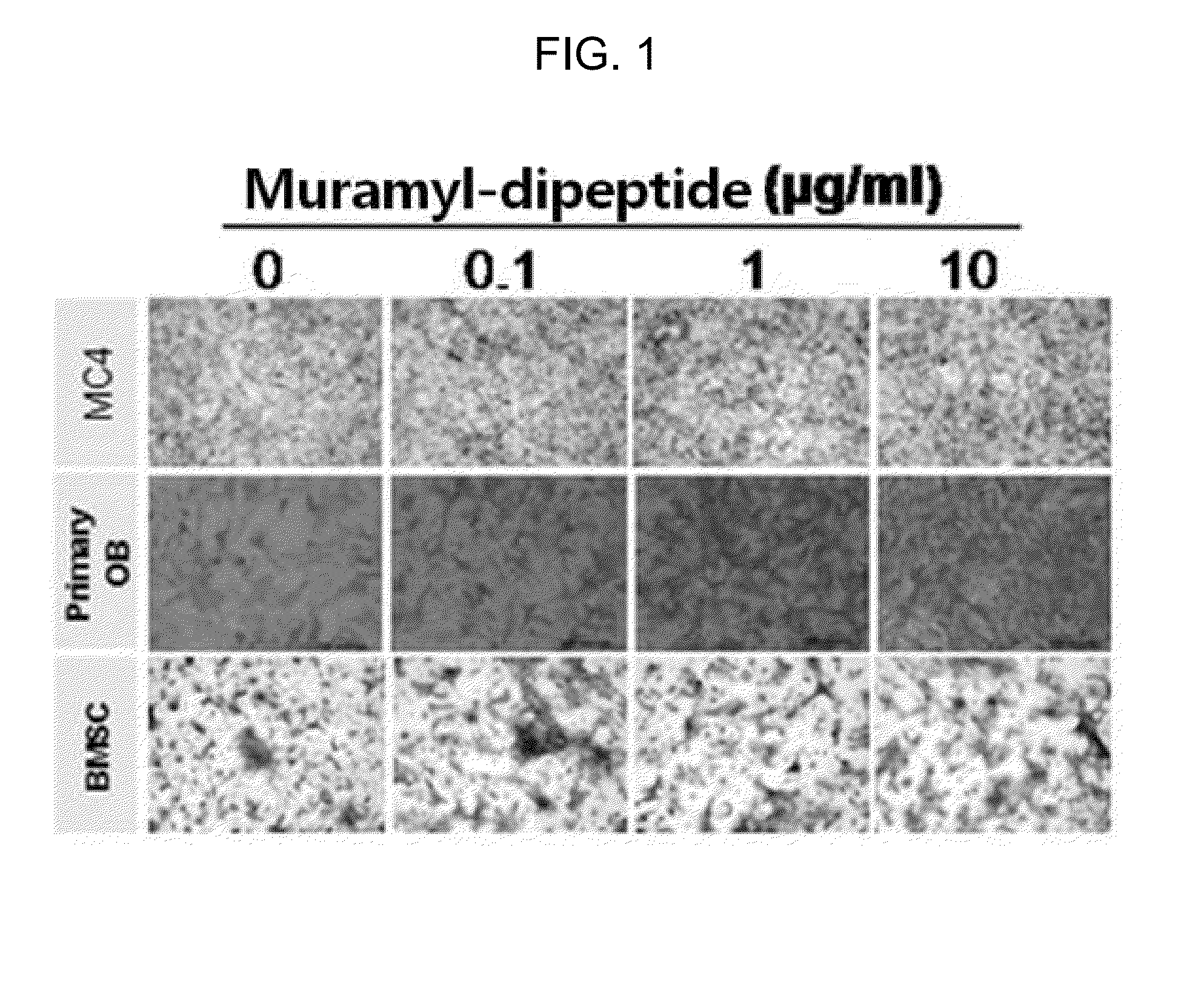
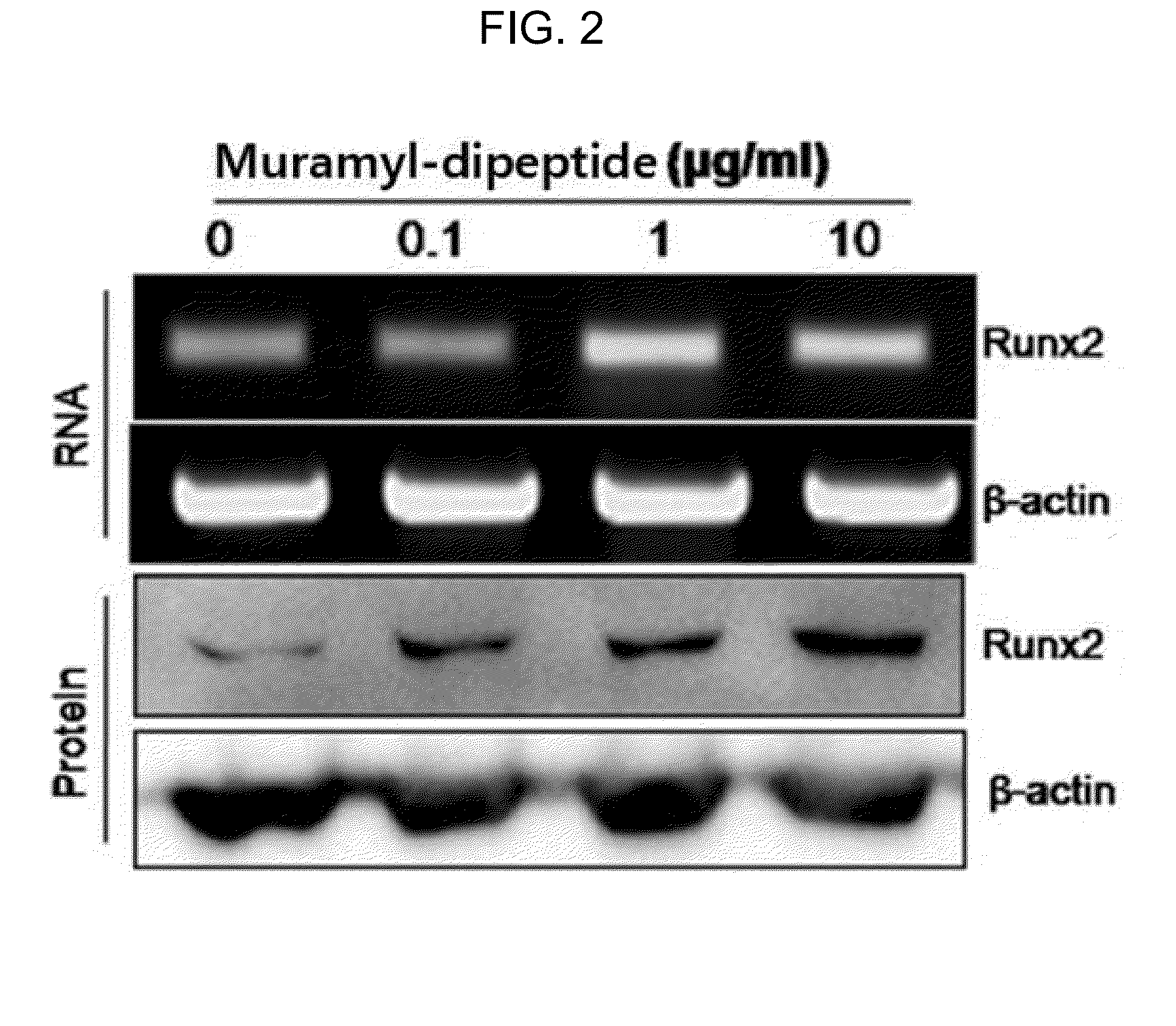
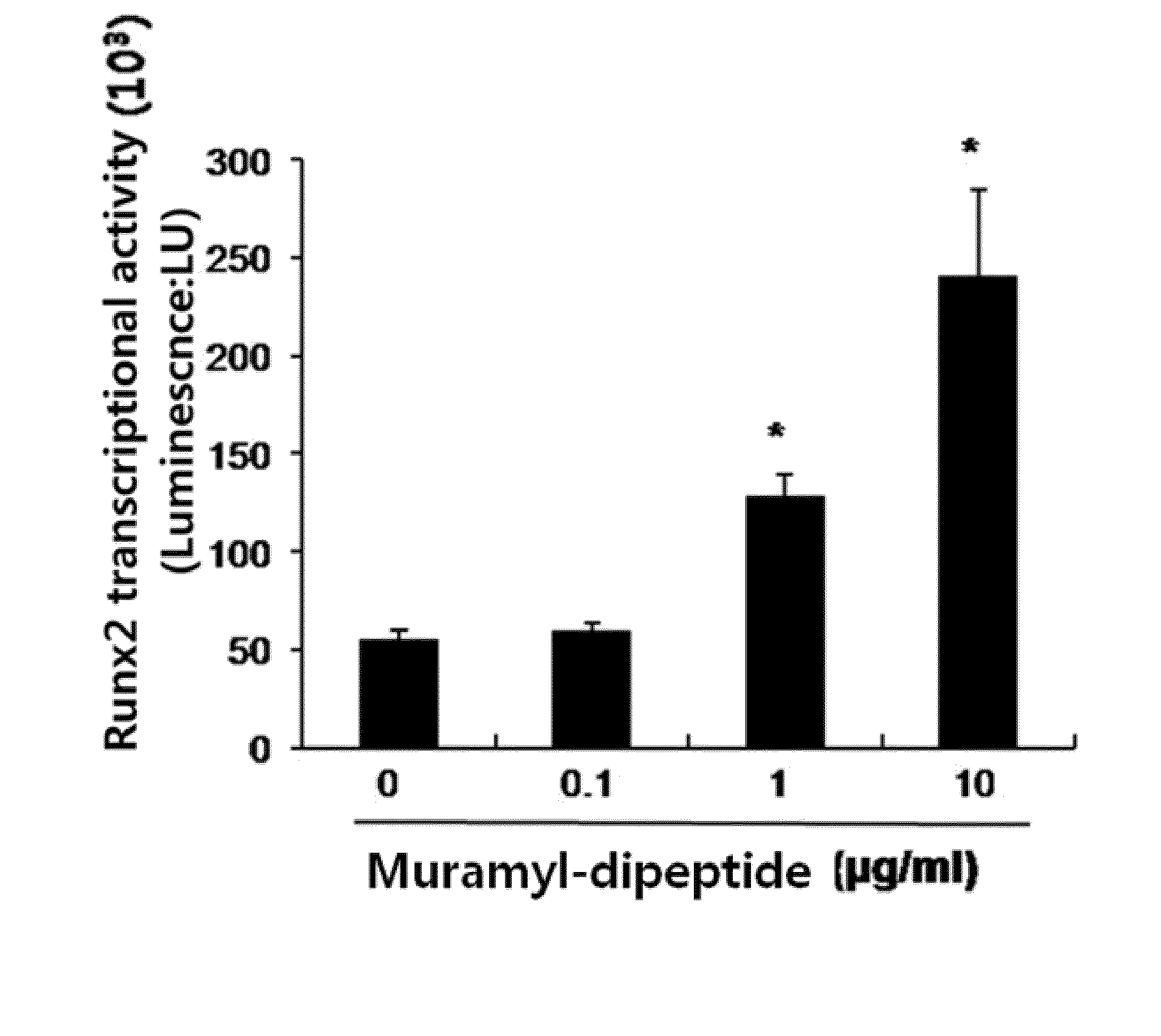
ActiveUS20150141352A1Suppressing functionSlow bone lossSugar derivativesDipeptide ingredientsDiseaseOsteoblast
The present invention relates to a bone regeneration or bone formation promoting pharmaceutical composition comprising muramyl dipeptide, an analogue thereof, a derivative thereof or a pharmaceutically acceptable salt thereof. In contrast to existing passive therapeutic agents which center on bone absorption suppression based on mechanisms for reducing osteoclast functionality, the composition comprising muramyl dipeptide of the present invention promotes the differentiation of osteoblasts, which are bone forming cells, and can advantageously be used in various diseases where bone formation is required as an active therapeutic agent that does not affect osteoclast.
Owner:HYSENSBIO
Compositions and methods for treatment of multiple sclerosis
Disclosed are novel compositions and methods for the treatment of Multiple Sclerosis (MS), and in particular immunostimulatory compositions comprising muramyl dipeptide microparticles for use, e.g., in the treatment of MS.
Owner:INNATE IMMUNOTHERAPEUTICS LTD
Animal immune adjuvant as well as preparation method and application method thereof
InactiveCN104667272AKeep aliveGuaranteed stabilityAntibacterial agentsBacterial antigen ingredientsSide effectLevamisole
The invention discloses an animal immune adjuvant as well as a preparation method and an application method thereof, relates to a medical preparation characterized in an immunostimulation additive, in particular to an immunostimulation additive for animals, and aims at providing an animal immune adjuvant which is free of a toxic or side effect and is used for directly modifying the antigen-reinforced immune effect, and a preparation method and an application method of the animal immune adjuvant. The animal immune adjuvant comprises an oil agent and an immunoenhancer; the oil agent comprises liquid paraffin and a surfactant; the surfactant comprises lanolin and span-80; and the immunoenhancer comprises a bacillus calmette guerin vaccine, muramyl dipeptide, levomisole and nanoscale hydroxyapatite. The animal immune adjuvant prepared by the method is capable of effectively inducing cellular immunity and humoral immune response of antigenic specificity; and the antibody with high valence and high sensitivity is obtained by kinds of modifications. The animal immune adjuvant is applied to the fields of immunology and in vitro diagnosis.
Owner:SUQIAN HENGRUI BIOTECH
Preparation method of muramyl dipeptide-anti-CD20 immune conjugate and application thereof
InactiveCN103087196AMaintain immunogenicityReduce dosageDipeptide ingredientsAntipyreticCD20Malignant lymphoma
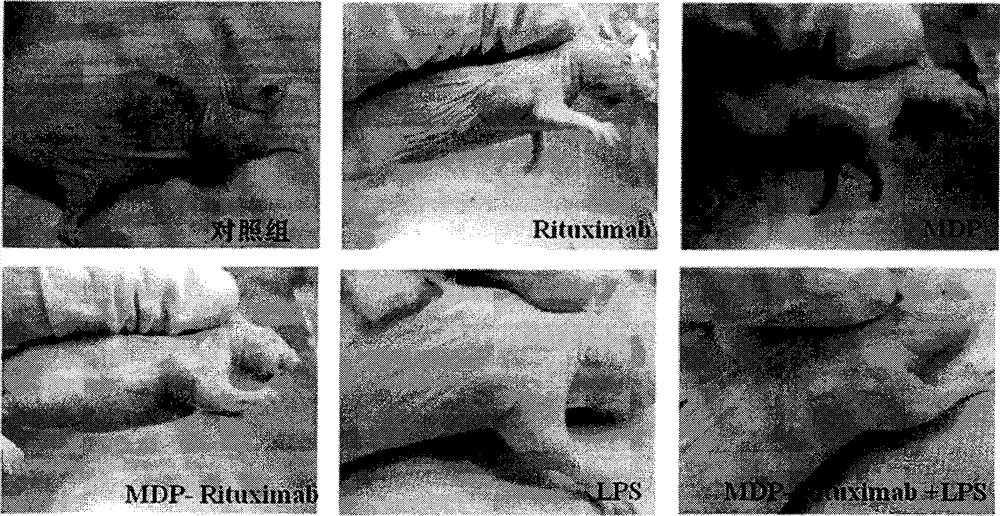
By taking guide functions of specific cellular immunity and monoclonal antibody immunity generated in vaccine inoculation as theoretical basis, the effective component of bacillus calmette Guerin (BCG), namely muramyl dipeptide (MDP), and a monoclonal antibody Rituximab of lymphoma cell membrane resistant antigen CD20 are combined to prepare a muramyl dipeptide-anti-CD20 immune conjugate, namely MDP-Rituximab, wherein in-vivo and in-vitro anti-tumour effects of immunocompetent cells subjected to BCG immunity under mediation of MDP-Rituximab are known. The conjugate disclosed by the invention keeps the immunogenicity of MDP, stimulates organisms to generate specific T cell immune response, and brings MDP to be around residual lymphoma cells with the help of immune guide function that a Rituximab antibody is combined with lymphoma cell membrane CD20 molecules; the final purpose of eliminating residual tumours is achieved through immune response mediated by MDP induced T cells; therefore, due to the method, the drug resistance caused by exclusive use of Rituximab is overcome; furthermore, the use amount of the antibody is reduced; and the application prospect of the muramyl dipeptide-anti-CD20 immune conjugate in malignant lymphoma is predicted.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Method for inducing DC-CIK by utilizing muramyl dipeptide
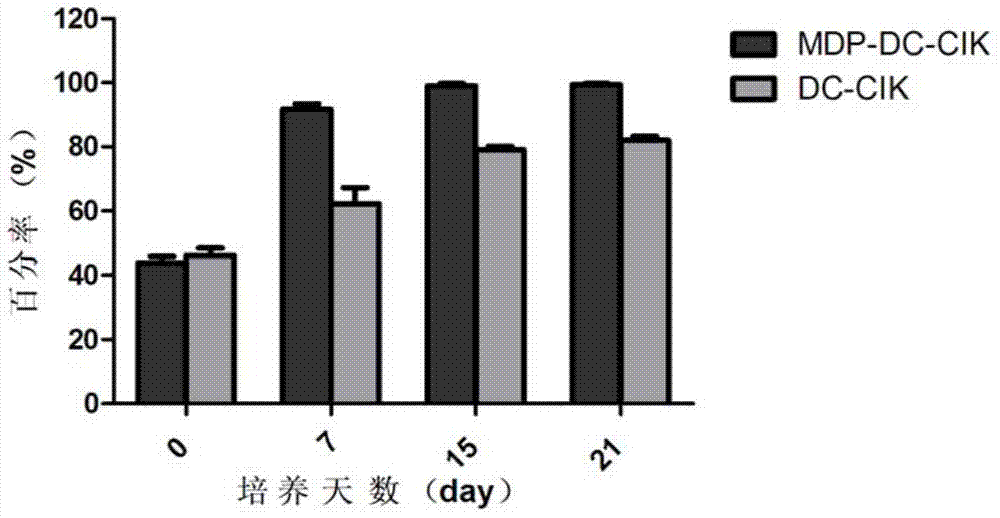
ActiveCN103710308AEnhance phagocytosisEnhance antigen presentationMammal material medical ingredientsBlood/immune system cellsLaboratory Test ResultMHC restriction
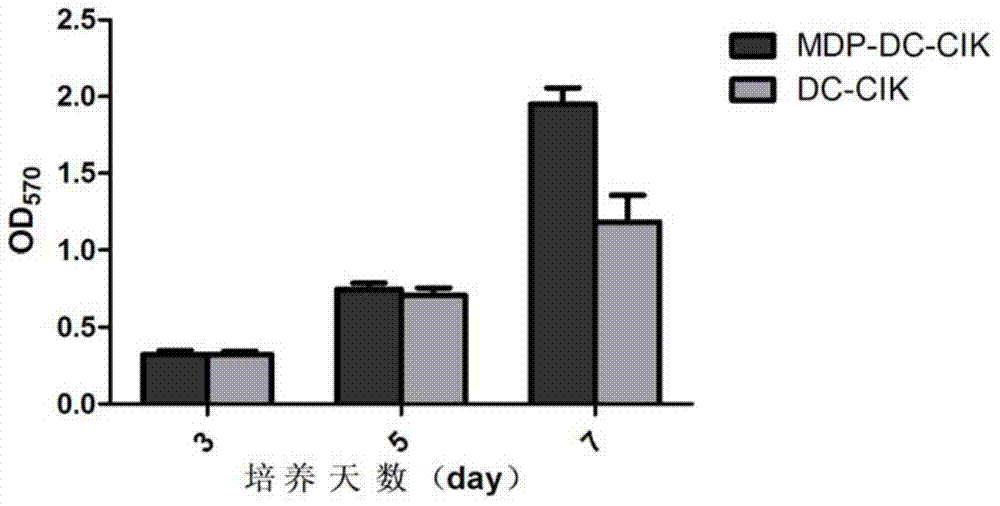
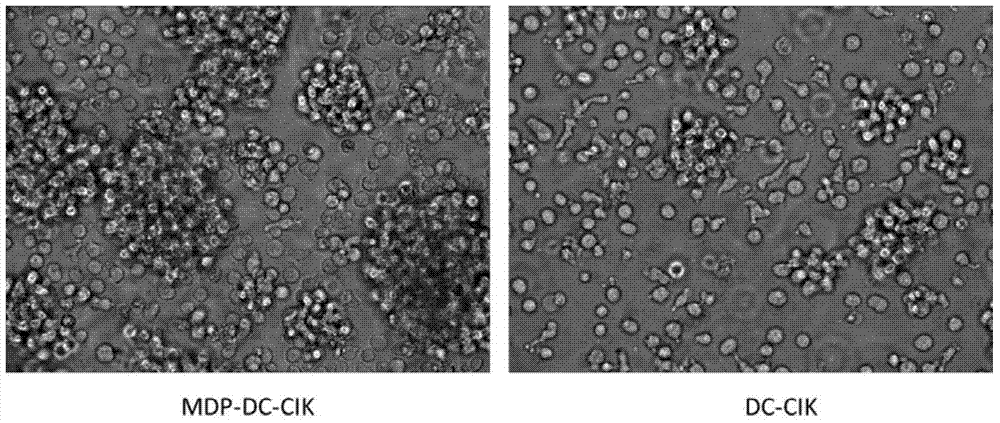
The invention provides a method for inducing DC-CIK by utilizing muramyl dipeptide, that is to say, the muramyl dipeptide is added into a CIK or DC-CIK culture solution to induce proliferation and differentiation of CIK or DC-CIK cells, so as to improve the tumor-killing activity of the CIK or DC-CIK cells. The method comprises the following steps: peripheral blood collection, tumor antigen acquisition, mononuclear cell separation, mononuclear cell collection, mononuclear cell washing, MDP-DC-CIK cell induction, and culture. MDP-DC-CIK cells introduced and cultured by the method are detected by a flow cytometry, the ratio of CD3+CD56+ non-MHC restricted NKT cells is found to be up to 80% or more; and at the same time, the activity of tumor killer cells is much higher than that of DC-CIK cells by conventional induction culture, and a laboratory test result of the tumor-killing percentage reaches 99% or more.
Owner:中海峡福建细胞生物科技有限公司
C-terminal modified oxamyl dipeptides as inhibitors of the ICE/ced-3 family of cysteine proteases
This invention is directed to novel oxamyl dipeptide ICE / ced-3 family inhibitor compounds. The invention is also directed to pharmaceutical compositions containing these compounds, as well as to the use of such compositions in the treatment of patients suffering inflammatory, autoimmune and neurodegenerative diseases, for the prevention of ischemic injury, and for the preservation of organs that are to undergo a transplantation procedure.
Owner:IDUN PHARMA INC
Compound proplis feed additive capable of accelerating pig to grow and fatten and improving immunity and preparation method thereof
InactiveCN102578414BNo pollution in the processNo side effectsAnimal feeding stuffSide effectUltra fine
Owner:QINGDAO LVMAN BIOLOGICAL ENG
Compositions and Methods for Treatment of Radiation Exposure
InactiveUS20110236346A1Preventing haematopoieticPreventing bone marrow damageBiocideDipeptide ingredientsCross-linkMedicine
Owner:INNATE IMMUNOTHERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com