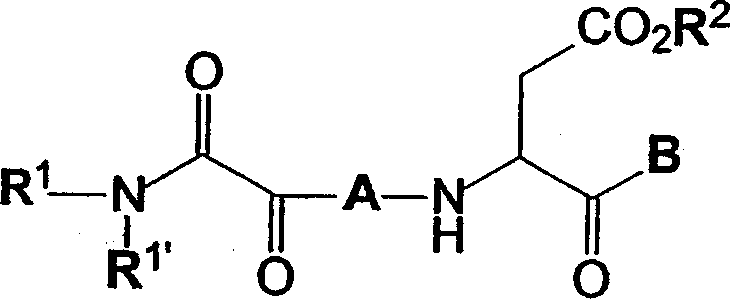
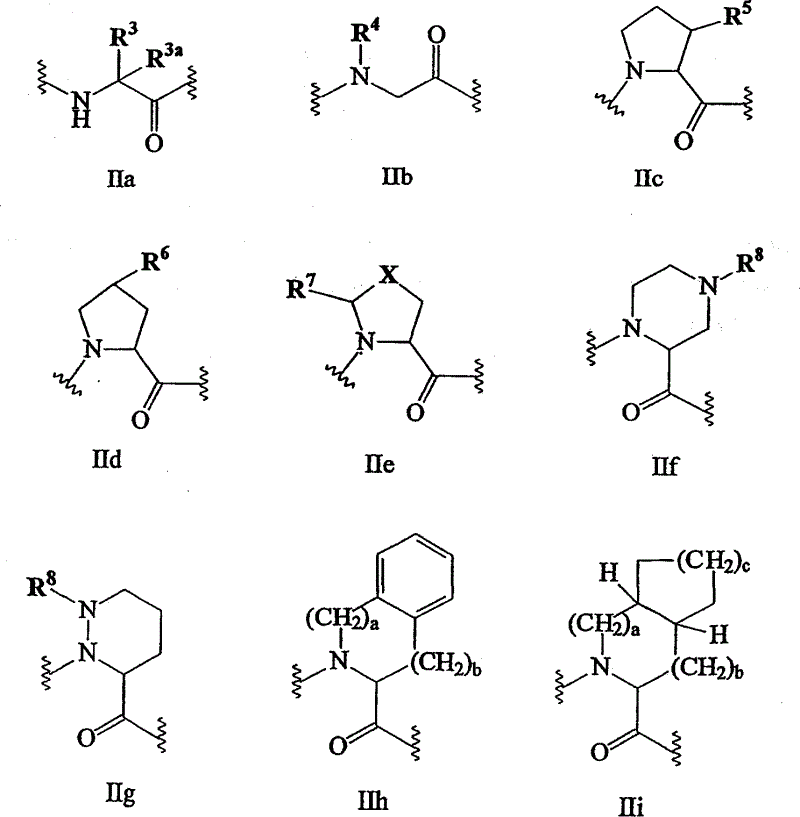
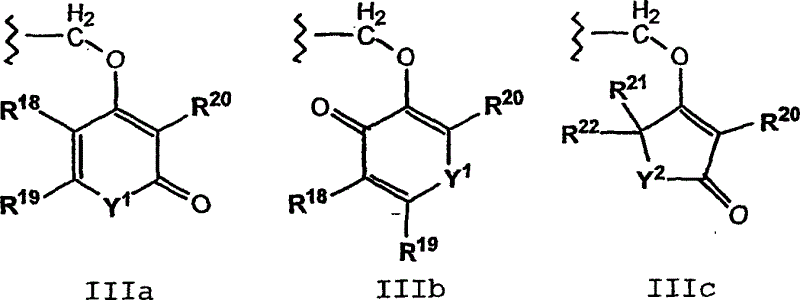
C-terminal modified oxamyl dipeptides as inhibitors of the ICE/ced-3 family of cysteine proteases
一种非天然氨基酸、药用盐的技术,应用在用作ICE/ced-3家族半胱氨酸蛋白酶抑制剂的C-端修饰的草氨酰二肽领域,能够解决阻碍等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0184]
[0185] (3S)-Amino-4-oxobutanoic acid tert-butyl semicarbazone, preparation of p-toluenesulfonate
[0186] Step A: N-(Benzyloxycarbonyl)-L-(N'-methyl-N'-methoxy)asparagine β-(tert butyl) ester
[0187] 0°C (ice bath), under nitrogen atmosphere, to the CH 2 Cl 2 (150 mL) was added 1-hydroxybenzotriazole hydrate (7.29 g, 47.6 mmol, Aldrich), followed by 1-ethyl-3-(3',3'-dimethyl-1'-amino Propyl)carbodiimide hydrochloride (9.55 g, 49.8 mmol, Sigma). After stirring at 0°C for 15 minutes, N,O-dimethylhydroxylamine hydrochloride (5.10 g, 52.3 mmol, Aldrich) and N-methylmorpholine (5.8 mL, 53 mmol, Aldrich) were added. The mixture was warmed to room temperature over 3 hours, then stirred at room temperature for 16 hours. The solution was concentrated in vacuo and the residue was partitioned between ethyl acetate-5% KHSO 4 (each 200mL). The organic phase was sequentially washed with 5% KHSO 4 , saturated sodium bicarbonate and saturated sodium chloride solution, ...
preparation example 2
[0197]
[0198] Preparation of (3S)-(9-fluorenylmethoxycarbonyl)amino-4-oxobutanoic acid tert-butyl semicarbazone-4-[2'-(4-ethyl-phenoxyacetic acid)]
[0199] Step A: 4-[2'-(N-tert-butoxycarbonyl)aminoethyl]phenoxyacetic acid, methyl ester
[0200] To a suspension of 4-hydroxy-phenethylamine (7.00 g, 51.1 mmol, Aldrich) in anhydrous dimethylformamide (50 mL) was added di-tert-butyl dicarbonate (11.0 g, 50.5 mmol). After stirring at room temperature for 1 hour, the resulting clear solution was treated with methyl bromoacetate (7.5 mL, 79 mmol) and cesium carbonate (17.5 g, 53.7 mmol). After stirring at room temperature for 16 hours, TLC (Et2O-toluene; 2:8) showed some unalkylated material (Rf = 0.43) was still present, so another portion of methyl bromoacetate (2.0 mL, 21 mmol) and carbonic acid were added Cesium (4.5 g, 14 mmol). After stirring for another 24 hours, the mixture was partitioned between EtOAc-water (250 mL each), and the organic phase was washed successi...
preparation example 3
[0215] Inhibition Test of ICE / ced-3 Protease Family Activity
[0216] a. IC 50 Determination of value
[0217] Basically according to Thornberry et al. ( Nature , 356:768:774 (1992)) and Nicholson et al. ( Nature , 376:37-43(1995)) method (the content of the above literature is hereby incorporated by reference), the luciferase assay of the activity of the compound of formula I was carried out using recombinant ICE and CPP32 enzymes respectively in 96-well microtiter plates. The substrate for ICE assay is Acetyl-Tyr-Val-Ala-Asp-amino-4-methylcoumarin (AMC) and for CPP32, Mch2, Mch3 and Mch5 assay is Acetyl-Asp -Glu-Val-Asp-amino-4-methylcoumarin. Enzyme reactions were performed in duplicate in ICE buffer (25 mM HEPES, 1 mM EDTA, 0.1% CHAPS, 10% sucrose, pH 7.5) containing 2 mM DTT at room temperature. The determination is carried out by mixing the following components:
[0218] 50 μL of ICE, Mch2, Mch5, CPP32 (concentrations of 18.8, 38, 8.1 and 0.153 nM, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com