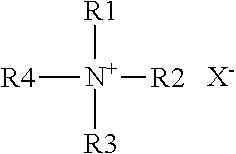Patents
Literature
465 results about "Killing rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
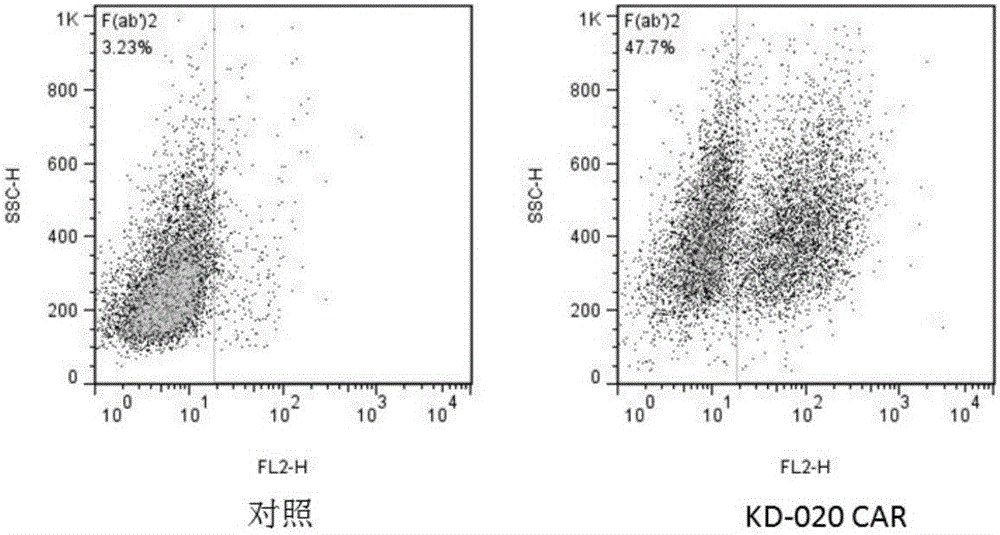
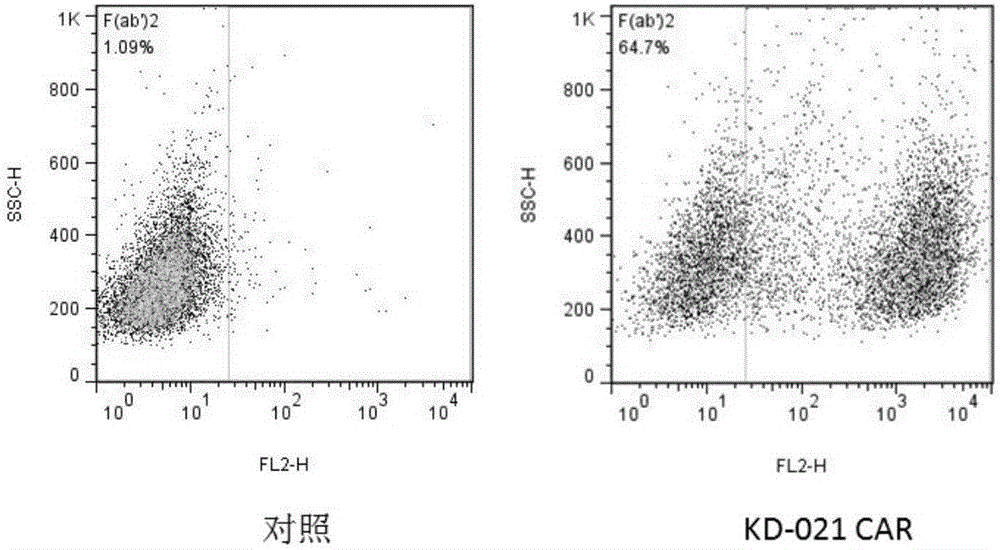
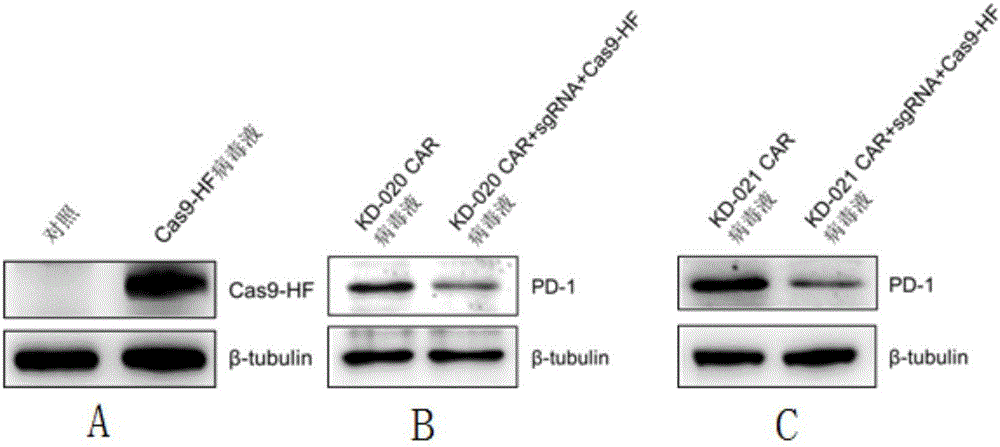
Method for knocking out PD-1 gene by utilizing CRISPR/Cas9 technology to construct MSLN-targeted novel CAR-T cell and application of method
PendingCN106480097ABlock escapeBlocking inhibitoryGenetically modified cellsMammal material medical ingredientsT cellSolid tumor
The invention discloses a method for knocking out a PD-1 gene by utilizing a CRISPR / Cas9 technology to construct an MSLN-targeted novel CAR-T cell. According to the method, a CAR-T cell is synchronously infected with a virus solution carrying sgRNA and Cas9-HF nuclease of a human PD-1 gene by utilizing the CRISPR / Cas9 technology to knock out the human PD-1 gene, so that the MSLN-targeted novel CAR-T cell is obtained. The novel CAR-T cell can be used for preparing a preparation used for treating solid tumors. A preparation method is simple in step, and the obtained CAR-T cell has high killing rate for solid tumor cells.
Owner:NANJING KAEDI BIOTECH INC
Disinfecting compositions and methods of making and using same
InactiveUS20050019421A1High kill rateUse of compositionBiocidePeroxide active ingredientsSolventBiology
The present invention provides a composition, comprising: greater than about 0.1% by weight hydrogen peroxide; an aromatic acid component; surfactant; optionally, a solvent; and a carrier. The composition of the invention is useful as a disinfecting composition for killing microorganisms such as bacterium (including Mycobacterium), spores and fungi. The composition provides a pathogenic bacteria kill rate of 99.9% in about 30 seconds when bacteria are exposed to the composition and is effective in providing a Mycobacterium kill of 106 with two minutes or less. Moreover, the compositions of the invention are generally more resistant to catalase deactivation than, for example, an aqueous solution of hydrogen peroxide. The concentration of hydrogen peroxide within the composition may range from about 1% by weight to about 7% by weight and the concentration of aromatic acid component may range from about 0.1% by weight to about 5% by weight. The invention also provides a method for disinfection of a substrate utilizing the composition. The composition of the invention may be used in the foregoing method on a medical instrument, such as an endoscope or the like. Applying the compositions to a substrate may be accomplished in any of a variety of application methods such as by roll coating, dipping, spraying, or rotational tumbling. The composition may be applied to the substrate for a period of time ranging from about 30 seconds to about ten minutes. In this aspect, the invention can further comprise drying the substrate after removing the composition.
Owner:3M INNOVATIVE PROPERTIES CO
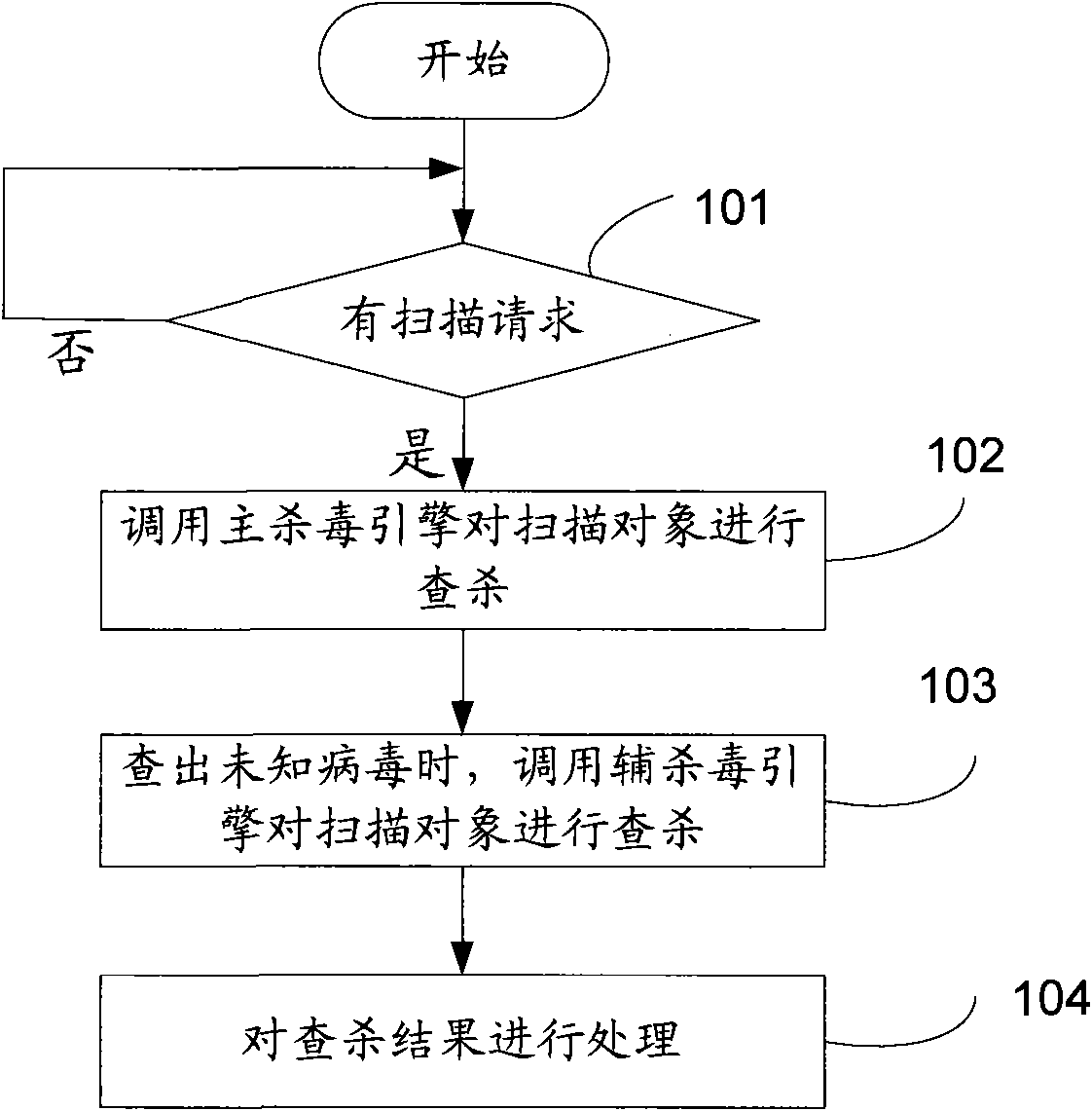
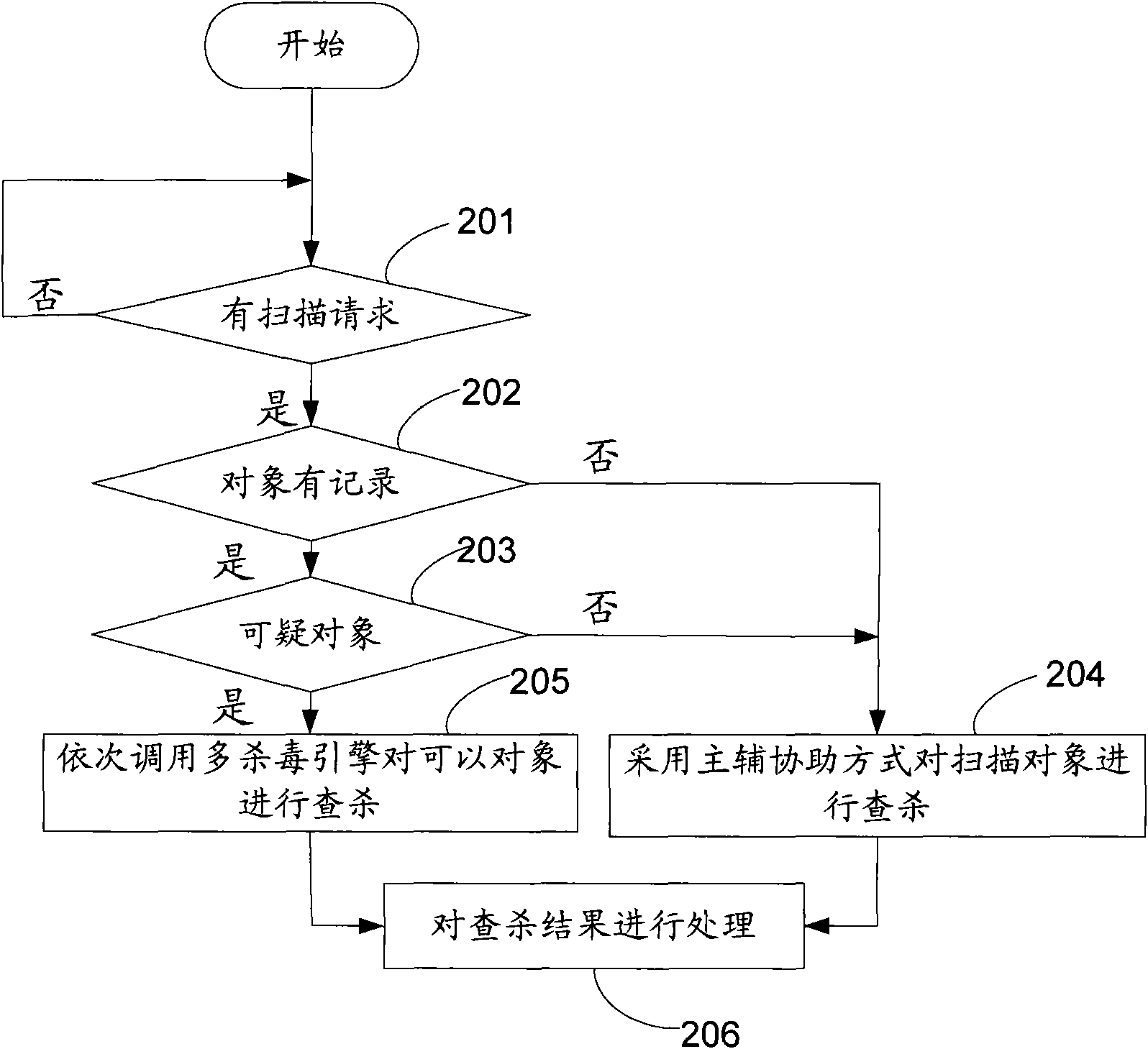
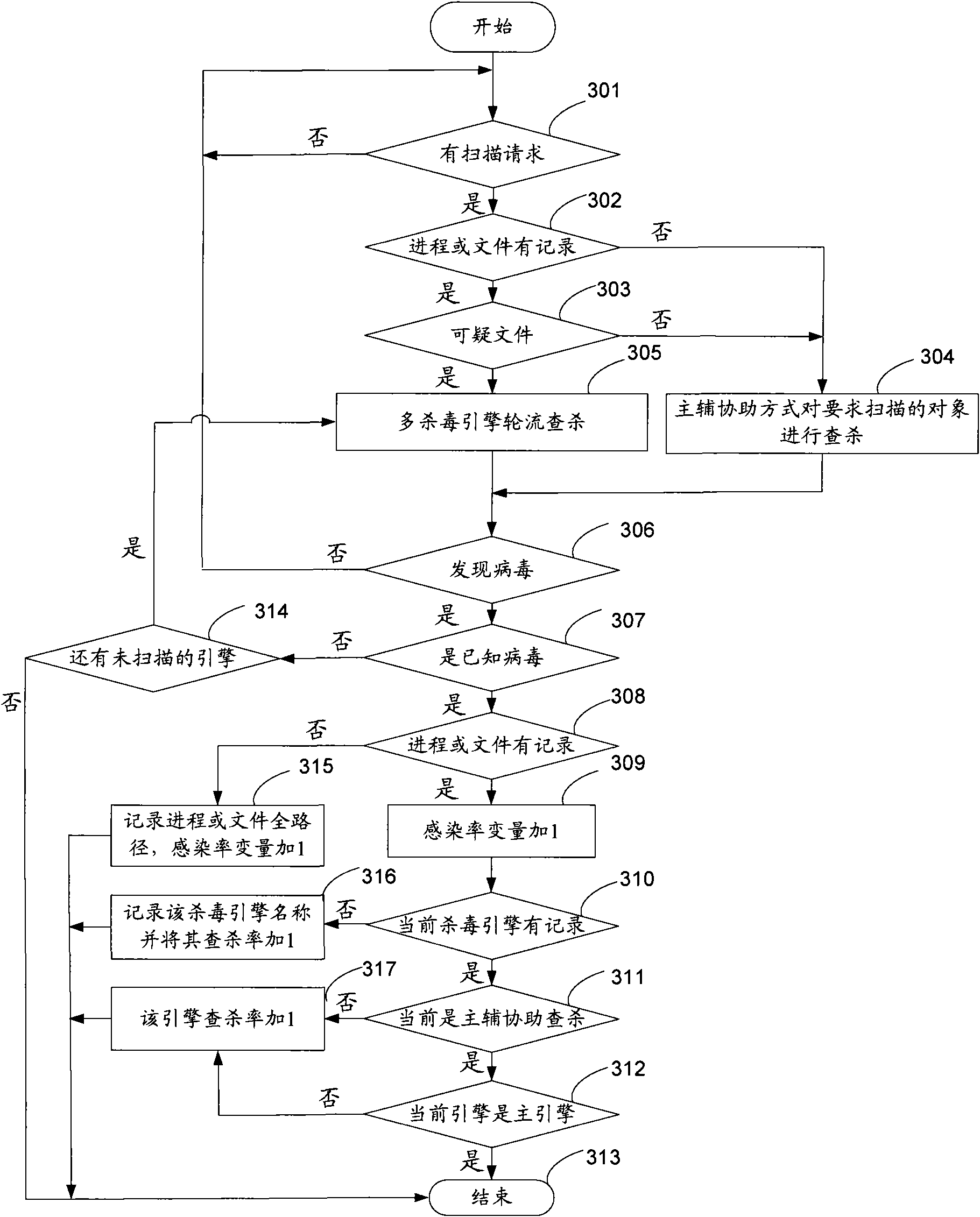
Virus killing method and virus killing system with multiple antivirus engines
ActiveCN101685486AKill rate is highGuaranteed killing success ratePlatform integrity maintainanceObject basedVirus
The invention discloses a virus killing method and a virus killing system with multiple antivirus engines. The method comprises the following steps: acquiring a virus scanning command; calling a mainantivirus engine of at least two antivirus engines to search and kill an object which the virus scanning command requires to scan; calling an auxiliary antivirus engine in at least two antivirus engines to search and kill the object which the virus scanning command requires to scan, wherein the virus killing rate of the main antivirus engine is more than that of the auxiliary antivirus engine, andthe auxiliary antivirus engine can be other antivirus engines except the main antivirus engine of at least two antivirus engines. Therefore, the method realizes high speed multi-antivirus engines searching and killing on the scanning object basing on the fact that the success ratio of searching and killing is guaranteed.
Owner:LENOVO (BEIJING) CO LTD
Manufacturing method of long-acting mosquito-expelling textile
The invention discloses a manufacturing method of a long-acting mosquito-expelling textile, comprising the following steps: firstly, by taking one or a mixture of polyester fibers, high-density polyethylene and polypropylene as a raw material, mixing the raw material with a hygienic insecticide to prepare master batches; then heating the master batches and drafting the heated master batches to prepare monofilaments or composite filaments; then cooling the monofilaments or the composite filaments to room temperature and weaving the cooled monofilaments or composite filaments into a mesh fabric; and finally manufacturing the mesh fabric into a textile. The textile fabric filaments manufactured with the manufacturing method of the long-acting mosquito-expelling textile contain the hygienic insecticide internally and externally, a fabric woven from the textile fabric filaments contains the hygienic insecticide capable of resisting washing of more than 20 times, is durable for 3-5 years, has a knocking-down rate and a killing rate which can reach more than 95% for mosquitoes and insects, and can effectively protect a human body from being bitten by the mosquitoes and insects.
Owner:JIANGMEN SHANGYI HOUSEHOLD ARTICLE CO LTD
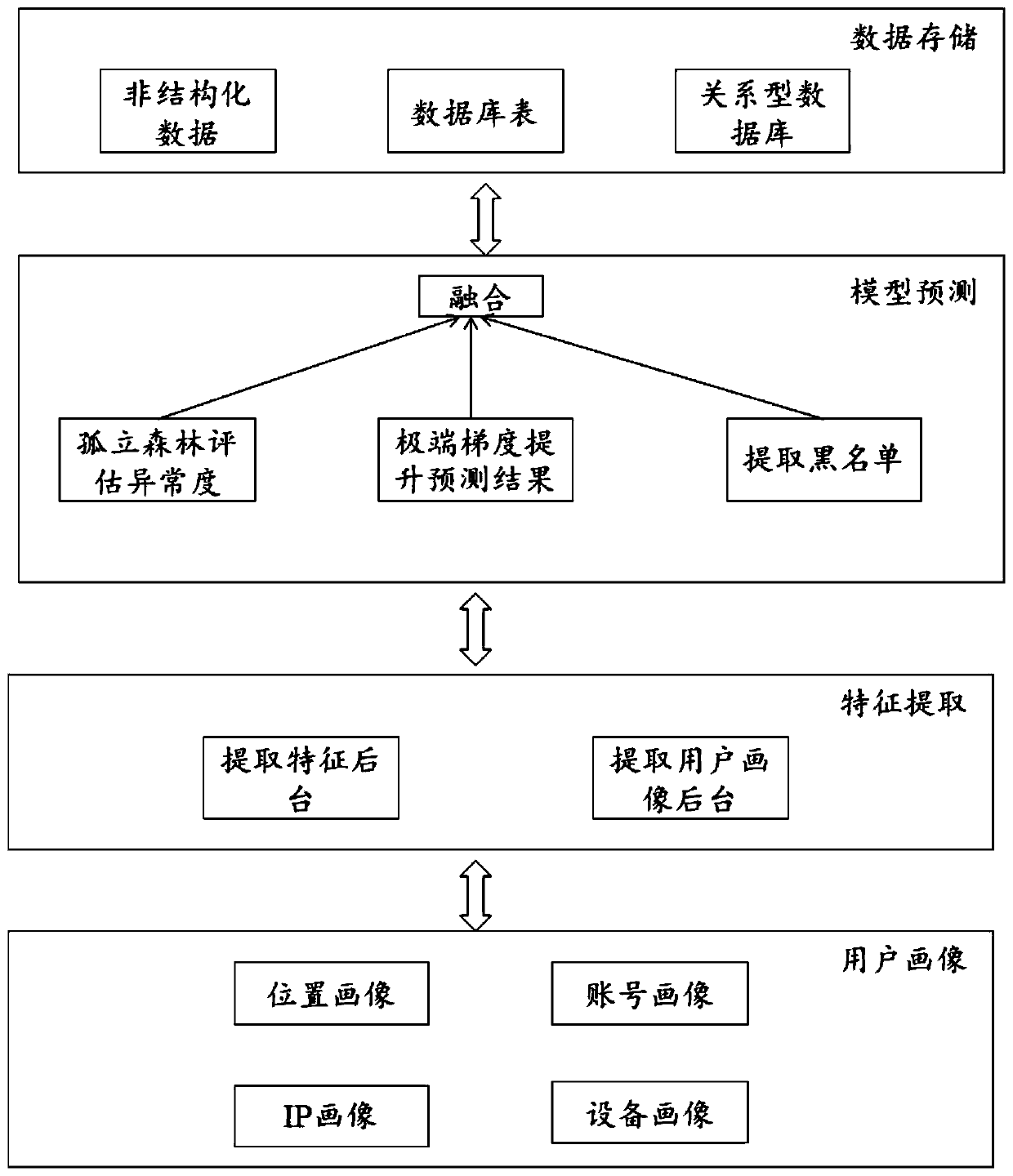
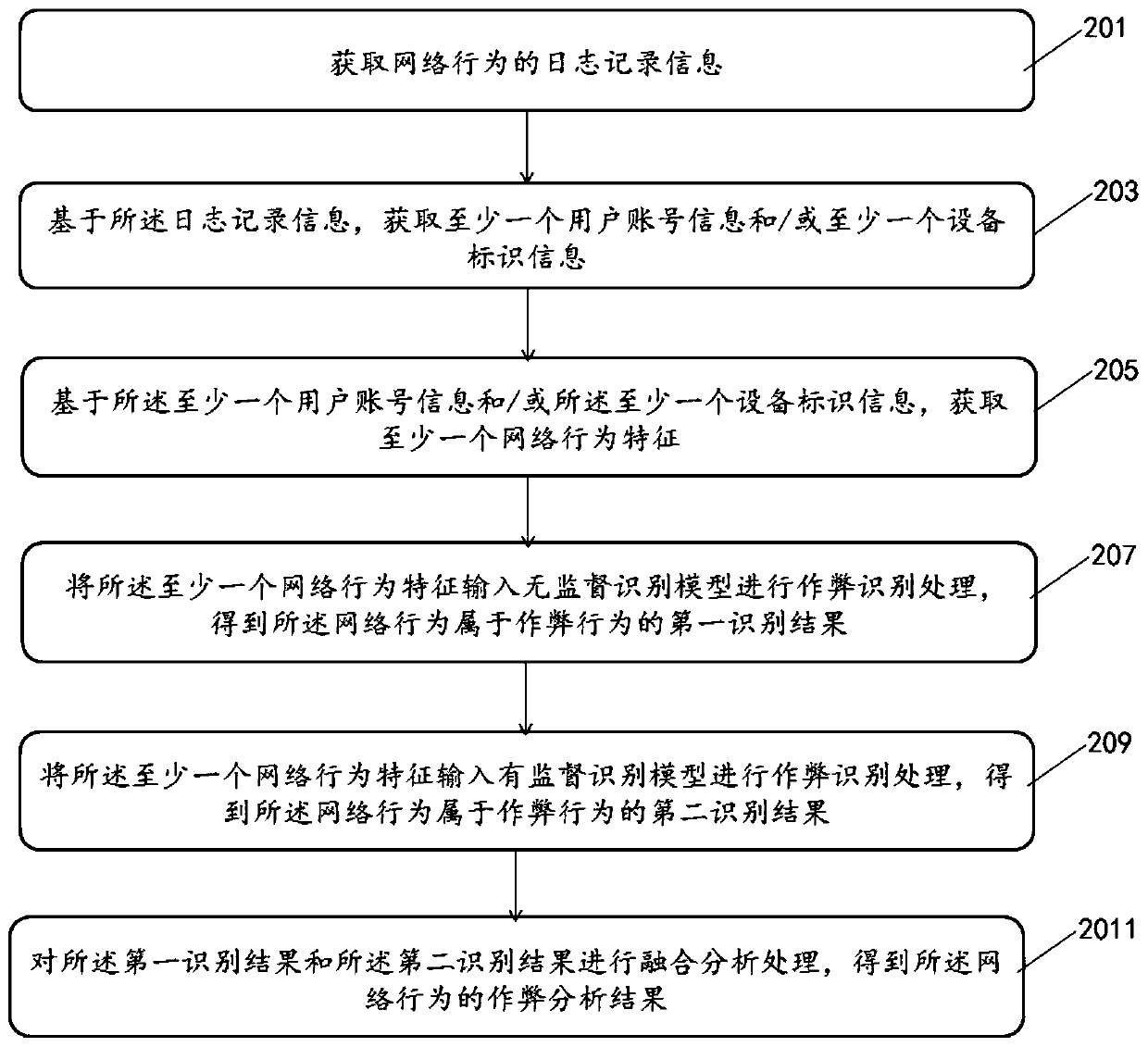
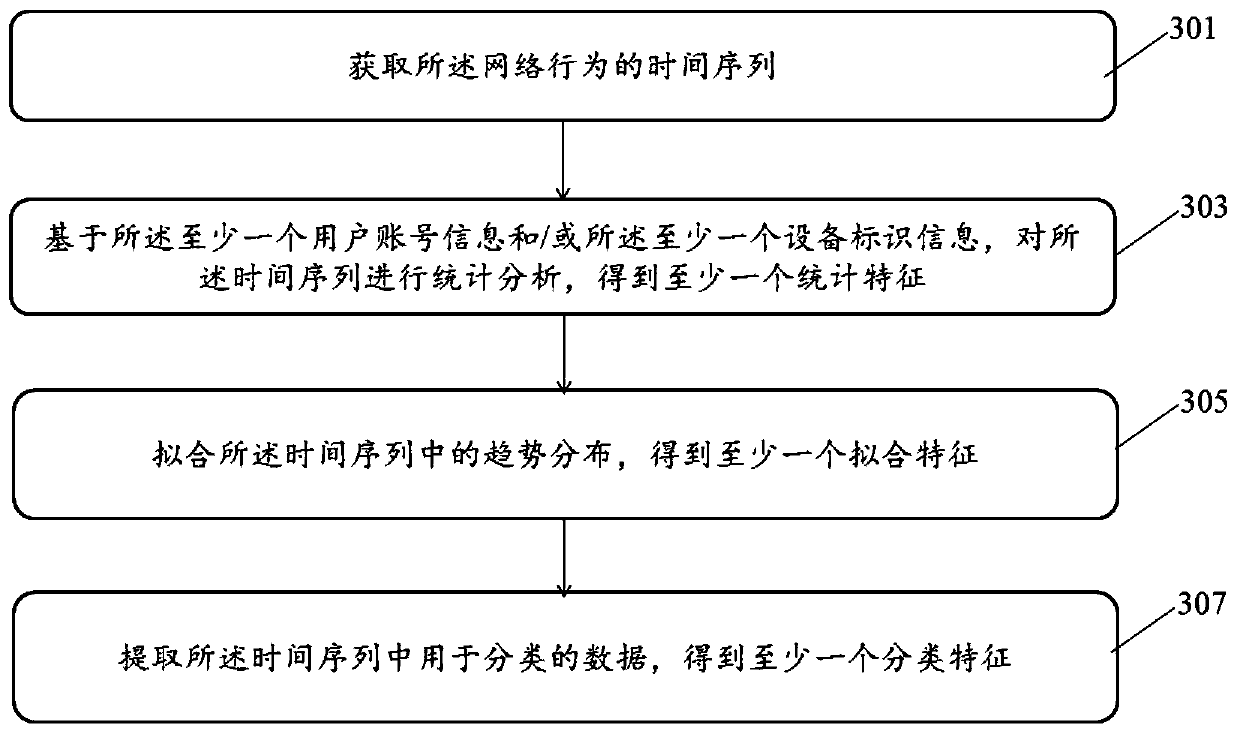
Network behavior anti-cheating method and device and storage medium
ActiveCN110198310AIncrease feature dimensionIncrease coverageCharacter and pattern recognitionTransmissionNetwork behaviorData mining
The invention provides a network behavior anti-cheating method and device and a storage medium. The method comprises: acquiring log record information of network behaviors; acquiring at least one piece of user account information and / or at least one piece of equipment identification information based on the log record information; obtaining at least one network behavior feature based on the at least one piece of user account information and / or the at least one piece of equipment identification information; inputting the at least one network behavior feature into an unsupervised identificationmodel for cheating identification processing to obtain a first identification result that the network behavior belongs to a cheating behavior; inputting the at least one network behavior feature intoa supervised identification model for cheating identification processing to obtain a second identification result that the network behavior belongs to the cheating behavior; and performing fusion analysis processing on the first identification result and the second identification result to obtain a cheating analysis result of the network behavior. The method can improve and reduce the false killing rate and improve the anti-cheating accuracy.
Owner:TENCENT TECH (SHENZHEN) CO LTD
Preparation method of photocatalytic antimicrobial polytetrafluoroethylene microporous membrane
ActiveCN102527248AEfficient degradationSimple preparation processSemi-permeable membranesPorosityEscherichia coli
The invention relates to the technical field of air environmental protection, in particular to a preparation method of a photocatalytic antimicrobial polytetrafluoroethylene microporous membrane. The preparation method comprises the following steps: mixing a nano-silver antimicrobial agent and polytetrafluoroethylene dispersion resin powder, blending, making a blank, extruding, calendering, longitudinally after degreasing treatment, longitudinally stretching after surface treatment, and sintering and curing after transverse stretching. The photocatalytic antibacterial polytetrafluoroethylene microporous membrane prepared by the preparation method is simple in process, economical and practical; the average pore size is 0.5-3 microns, the porosity is more than 80%, and the filtering efficiency is more than 99%; the killing rates of the photocatalytic antibacterial polytetrafluoroethylene microporous membrane for escherichia coli and staphylococcus aureus are more than 90%; and the photocatalytic antibacterial polytetrafluoroethylene microporous membrane can effectively degrade formaldehyde and other volatile organic compounds and has antimicrobial, bactericidal and deodorant functions and the like.
Owner:HUZHOU SENNUO FLUORINE MATERIAL TECH
Preparation and application thereof of sterilization composition
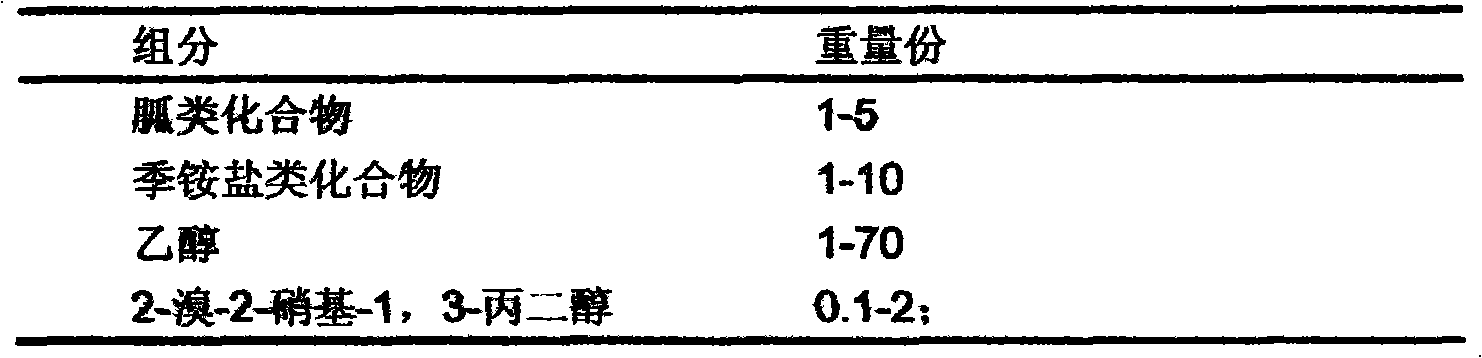
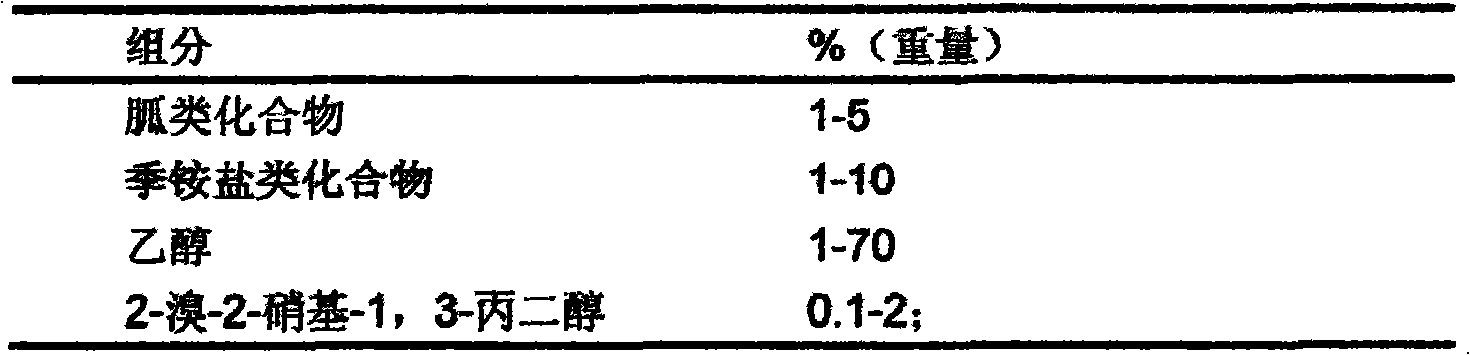
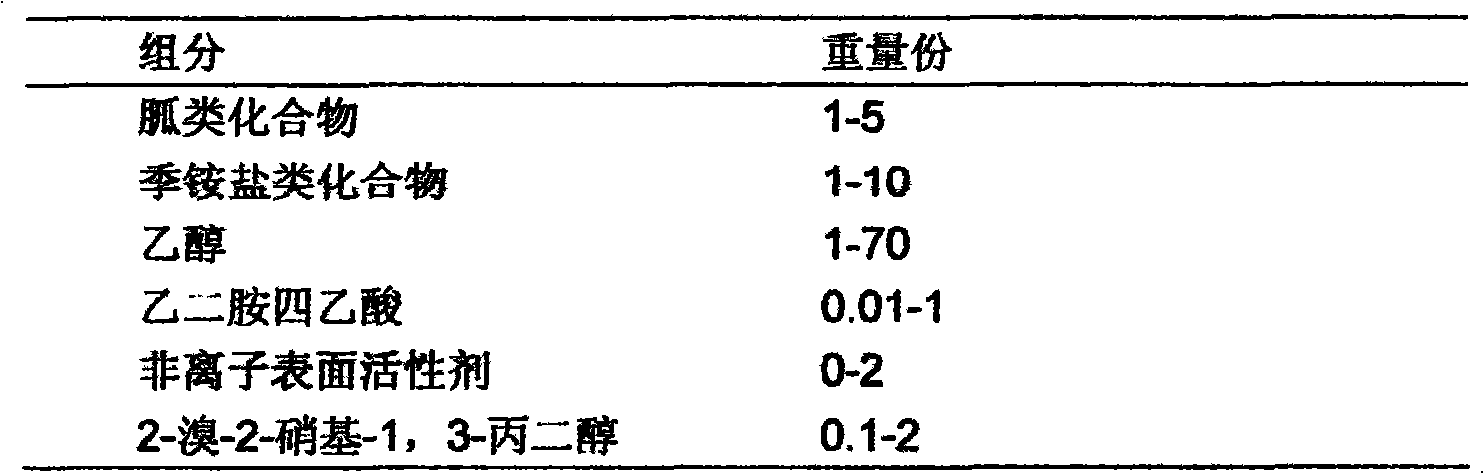
InactiveCN101933521AWon't happenNot corrosiveAntibacterial agentsBiocideAquaculture industryAmmonium compounds
The invention relates to preparation and application of a sterilization composition containing various effective sterilization components. The sterilization composition contains a guanidine compound, a quarternary ammonium compound, 2-bromine-2-nitro-1,3-propanediol and ethanol, is diluted within a range of 50-1000 times, has the average killing rate of 99.99-99.9999 percent to gonites, bacterialspores, mycobacteria, viruses, fungi and spores thereof, can be used for the public health field, the food processing industry, the medical and health field, the livestock and poultry industry, the aquaculture industry and sterilization treatment on indoor air and in-vehicle air and can also be used for the port field, in particular to ship ballast water treatment, container treatment, industrialcirculating water treatment, swimming pool water treatment, hand and skin mucosa treatment, industrial wastewater treatment, hospital wastewater treatment and antiseptic treatment of a building material.
Owner:李新建 +3
Composite antibiotic finishing agent and preparation method and finishing process thereof for application to mucilage glue and cotton fiber interweaved jacquard fabric
The invention discloses a composite antibiotic finishing agent which comprises an organic antibacterial active component, an inorganic antibacterial active component, a penetrating agent, an adhesive, a softening agent, a suspending agent and the balance of water, wherein a non-dissolution type polymer antibacterial agent is selected as the organic antibacterial active component, and silver-loaded nanometer titanium dioxide is selected as the inorganic antibacterial active component. The invention also discloses a method for preparing the composite antibiotic finishing agent and a process used for finishing a mucilage glue and cotton fiber interweaved jacquard fabric. The main material of the composite antibiotic finishing agent for textiles is formed by the organic antibacterial active component and the inorganic antibacterial active component, thus the composite antibiotic finishing agent has a high-efficiency inhibiting capacity of more than 20 kinds of harmful bacteria such as escherichia coli, staphylococcus aureus, gonococcus and the like; the bacteria inhibiting rate is higher than 99 percent; the repelling and preventing rate of dust mites, gamasid mites and chigger is higher than 99 percent; the repelling and preventing rate of dust mites, gamasid mites and chigger is higher than 95 percent, and the killing rate is higher than 90 percent after the mucilage glue and cotton fiber interweaved jacquard fabric is washed for 50 times; and the gonococcus, escherichia coli, staphylococcus aureus and the like do not grow on the fabric which is not washed for 30 times, 50 times or 100 times, and the bacteria inhibiting rate is higher than 99 percent.
Owner:SHANGHAI SHUIXING HOME TEXTILE
Acidizing fluid for green algae and disease infected cell in porphyra haitanensis cultivation and treating method thereof
The invention discloses an acidizing fluid for green algae and a disease infected cell in porphyra haitanensis cultivation and a treating method thereof. A solvent for the acidizing fluid is clean seawater, and solute for the acidizing fluid is at least one of hydrochloric acid and citric acid, wherein the concentration expressed in percentage by weight of the solute in the acidizing fluid is 0.3 to 1.5 percent. The green algae and the disease infected cell can be quickly killed by a treating method of preparing the acidizing fluid, shipping, dipping for 15 to 20 seconds and rinsing, killing effect is better, the killing rate is over 90 percent, and the influence on the growth of the porphyra haitanensis is less. The treating method has the advantages of no restriction of natural condition, labor intensity reduction and the like.
Owner:NINGBO UNIV
Plant-derived pesticide and its preparation method and use
InactiveCN105379771AImprove the bactericidal effectStrong againstBiocideDead animal preservationPatriniaMonkshoods
The invention discloses a plant-derived pesticide and its preparation method and use and belongs to the technical field of pesticide preparation. The plant-derived pesticide is prepared from tobacco, common threewingnut root, capsicum, rhizome of Chinese monkshood, pyrethrum, Azadirachta indica, tea leaves, patrinia herb, bitter herbs, wormwood, ash bark, radix sophorae flavescentis, Euphorbia songarica Boiss, Derris fordii Oliv., Chinese prickly ash, purslane, mandala, Anabasis Aphylla L., leaves of Salix babylonica, leaves of Pterocarya stenoptera and water. The plant-derived pesticide is prepared by soup extraction, condensation, drying, crushing, sieving and disinfection. The plant-derived pesticide is nontoxic and harmless, does not produce residues, is harmless, is environmentally friendly, does not pollute the environment, can effectively prevent and control injurious insects such as yellow spider, arrowhead scales, leaf roller moth and fruit fly, has a one-step killing rate of 95.41% or more, has no drug resistance and can be used for 4-6 years.
Owner:罗永城
Composition For Skin Sanitization And Protection And Method Of Its Use
ActiveUS20110262558A1Prevent microbial infectionEradicate and reduce numberBiocideCosmetic preparationsAlpha hydroxyl acidMedicine
An improved composition for skin cleansing and protection is disclosed. The composition contains an effective amount of at least one alpha-hydroxyl acid or a pharmaceutically acceptable salt thereof, at least one base, one surfactant and one skin protectant. Various additives and excipients may be included in the formulation. The improved composition disclosed herein achieves a higher bacteria killing rate and shows longer action duration. The disclosed composition is capable of penetrating deep into the skin which allows for delivery of more anti-microbials to sites that are at a higher risk of being infected. Various modifications of the improved composition are also disclosed.
Owner:RN MEDICAL SOLUTIONS LLC
Water Sanitazation System Having Safety Features and Removable Filter
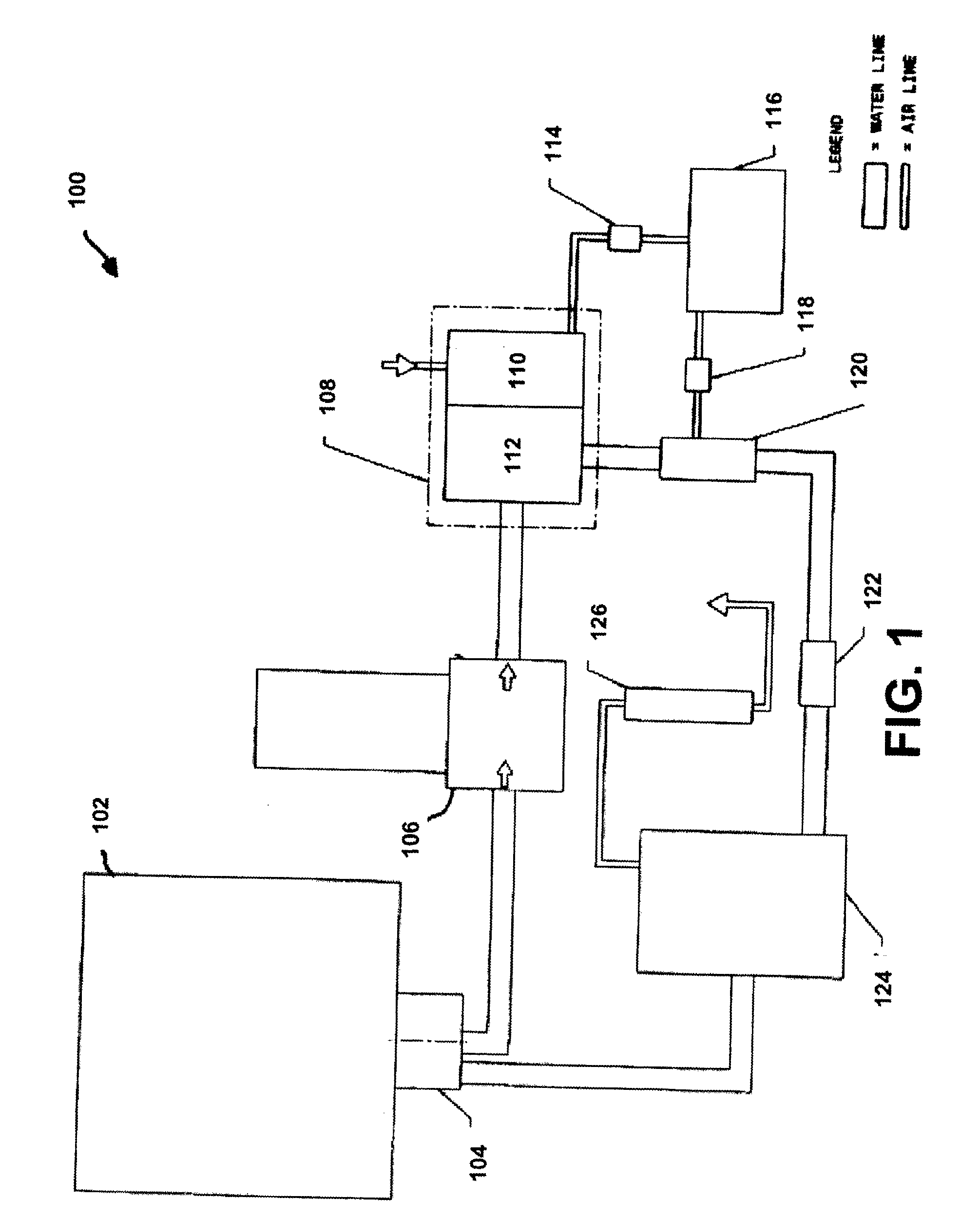
InactiveUS20080190825A1Avoid damageOther chemical processesSolid sorbent liquid separationHigh concentrationOzone generator
A removable disposable air dryer cartridge is provided for a small enterprise water ozonation system. The cartridge includes an air inlet to receive atmospheric air, a desiccant material to remove moisture, and a dry air outlet for interfacing with the ozonation system to provide dry air to an ozone generator. This facilitates better ozonation of water in the system, since dry air reacts better in an ozone generator, achieving higher concentrations of ozone gas, thus resulting in a better “kill rate” when ozonated water is applied to bacteria. The air dryer can be provided as a chamber of a combined air dryer and water filter cartridge, the other chamber being a water filter chamber having a water filter for extracting large particles of material from the water stream in order to prevent damage to the system.
Owner:TERSANO INC
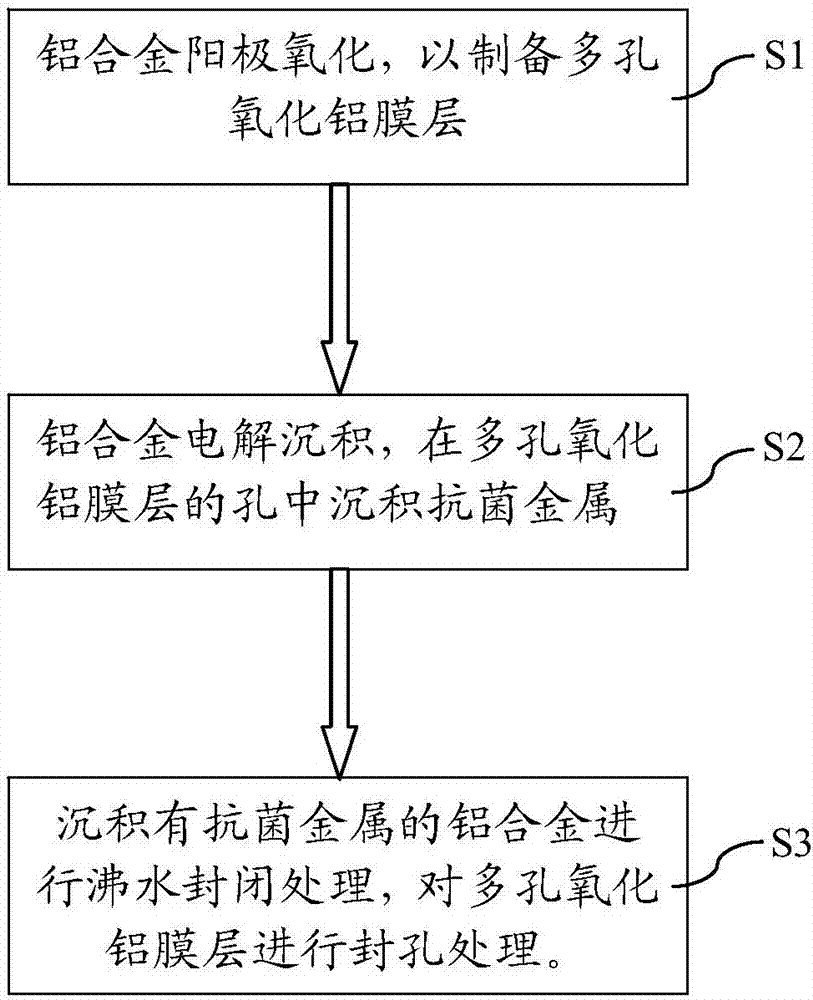
Antibacterial aluminum and manufacturing method thereof
The invention discloses antibacterial aluminum and a manufacturing method thereof. The manufacturing method comprises the following steps: (1) a pretreated aluminum alloy serves as an anode to form two electrodes with a cathode module, and the anodic oxidation is performed in electrolyte to prepare a porous aluminum oxide film on the surface of the aluminum alloy, wherein the hole way unit cell number on the unit area of the porous aluminum oxide film is 70-100*109 / cm2; and the hole way parameters in unit cells are 1-100 microns of the hole depth and 10-50 microns of the aperture; and (2) the aluminum alloy with the porous aluminum oxide film obtained in the step (1) and an electrode module form two electrodes; antibacterial metal is electrolyzed and deposited in hole ways of the porous aluminum oxide film by using deposition liquid; and the particle size of the deposited antibacterial metal is 1-100 nanometers. The manufacturing method of the antibacterial aluminum is simple in process, convenient in operation and low in manufacturing cost; the antibacterial metal is uniformly and stably deposited; the bacterial resisting and killing effect of the aluminum alloy is long in lasting time; and the bacterial resisting and killing rate is high up to reach 99.99%. Dissoluble precipitations cannot be separated out even if long-time use of electrolysis and deposition liquid.
Owner:SHISHI XINGHUO ALUMINUM PROD CO LTD
Glycol ether miticides and anti-allergen treatments
The present invention encompasses a cleaning composition and method for controlling dust mites and allergens using glycol ether, glycol ether ester or a combination thereof. The cleaning composition preferably contains a hydrophobic glycol ether and / or glycol ether ester solution present at a level of about 0.01% to 20% by weight and is an effective miticide with a kill rate of at least 50% after 30 minutes. The cleaning composition may optionally contain: surfactants, corrosion inhibitors, soil and stain resist agents, builder and buffering agents, and a propellant for delivering the composition in aerosol form.
Owner:THE CLOROX CO
Specific chimeric antigen receptor T cells targeting CD19 and preparation and clinical application thereof
PendingCN110272493AEffective targeted attackHigh kill rateVirusesAntipyreticSide effectCD19-specific chimeric antigen receptor
The invention relates to specific chimeric antigen receptor T cells targeting CD19, and a preparation method and clinical application thereof. The present invention constructs a specific chimeric antigen receptor targeting CD19 and immune response cells modified by the chimeric antigen receptor based on a targeted human CD19 single-chain antibody sequence. The novel modified immune response cells can effectively target and attack a plurality of tumor cells, especially tumor cells with positive CD19 expressiion, and can be used to prepare a preparation for the treatment of tumors. The method for preparing the modified immune response cells targeting CD19 is simple, and the obtained modified immune response cells targeting CD19 have a high killing rate on tumor cells. Clinical verification shows that: after a million-grade low-dose back transfusion, patients with recurrent and refractory advanced CD19-positive lymphoma get a significant clinical symptom relief after two weeks of treatment, almost complete relief curative effect is obtained on the 77th day, and no fever caused by cytokine release syndrome and any neurotoxic side effect occur.
Owner:NANJING KAEDI BIOTECH INC
Plant-based agricultural insecticide
ActiveCN104542749ASimple production processReduce manufacturing costBiocideDead animal preservationBiologyRaw material
The invention discloses a plant-based agricultural insecticide. The plant-based agricultural insecticide is characterized in that raw materials are prepared from multiple plant-based medicines. Compared with the prior art, the plant-based agricultural insecticide has the high killing rate, the long lasting period and the short safety interval, and can ensure the high agricultural product yield, and agricultural products can be safe and non-toxic.
Owner:李善宽
Novel botanical insecticide, preparation method and application thereof
InactiveCN105519613ANon-toxic ingredientsNo pollutionBiocideDead animal preservationPatriniaAdditive ingredient
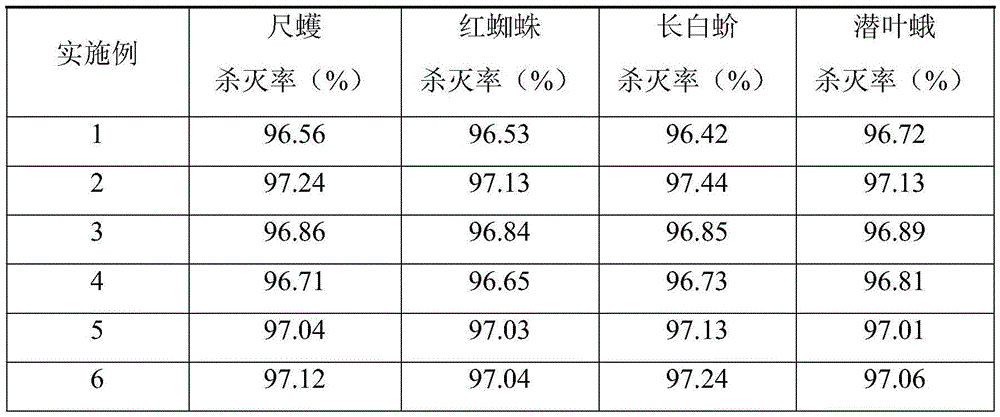
The invention discloses a novel botanical insecticide and its preparation method and application and belongs to the field of insecticide preparation technology. The novel botanical insecticide comprises the following raw materials: ailanthus altissima, castor-oil plant, Chinese prickly ash, sabah veratrum nigrum, tubatoxin, garlic, tarragon, melia azadarach, onion, leaves of cinnamomum burm anii, ash bark, radix sophorae flavescentis, euphorbia soongarica, sophora alopecuroide, pyrethrum, dioscorea zingiberensis, lettuce, mint, wasabi, patrinia herb and water. The novel botanical insecticide is prepared by steps of medical liquid extraction, concentration, drying, crushing, sieving, sterilization and the like. The novel botanical insecticide has advantages of nontoxic component and no residue, is pollution-free, green and environmentally friendly, and will not cause environmental pollution. The novel botanical insecticide can effectively control plant insects such as inchworm, red spider, lopholeucaspis japonica, leaf miner and the like. One-time killing rate reaches 96.42% and above, and the plant insects have no drug resistance.
Owner:罗永城
H2O2 air disinfectant with high stability
The hydrogen peroxide air sterilizing liquid consists of hydrogen peroxide 3.0-5.0 wt%, organic acid 0.01-0.10 wt%, EDTA disodium 0.05-0.20 wt%, metal ion complex 0.10-0.40 wt%, non-ionic polyhydroxylated polymer 0.10-0.50 wt%. It is prepared through dissolving EDTA disodium, metal ion complex and non-ionic polyhydroxylated polymer in deionized water, and adding hydrogen peroxide and organic acid via stirring to obtain the stable air sterilizing liquid. The air sterilizing liquid has pH value of 2.0-3.0, hydrogen peroxide reducing rate at 54 deg.c for 14 days of 2.21-2.22 %, mouse acute oral toxicity LD50 greater than 5,000 mg / Kg and mouse acute inhalation LC50 greater than 10,000 mg / cu m. It has high germ killing rate, and is sprayed into air for sterilizing.
Owner:舒国欣 +2
Program detection method, device and program analyzing method
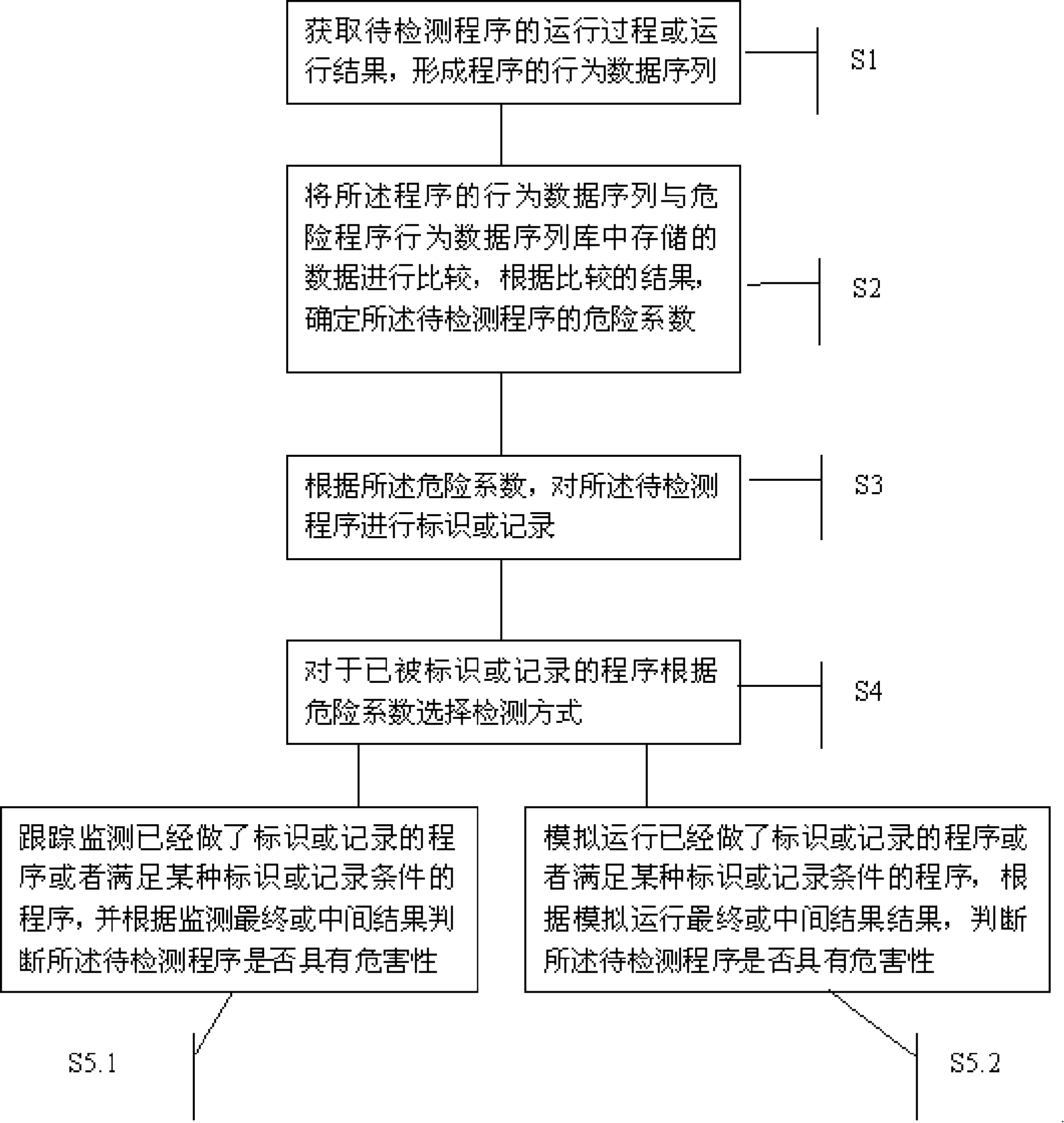
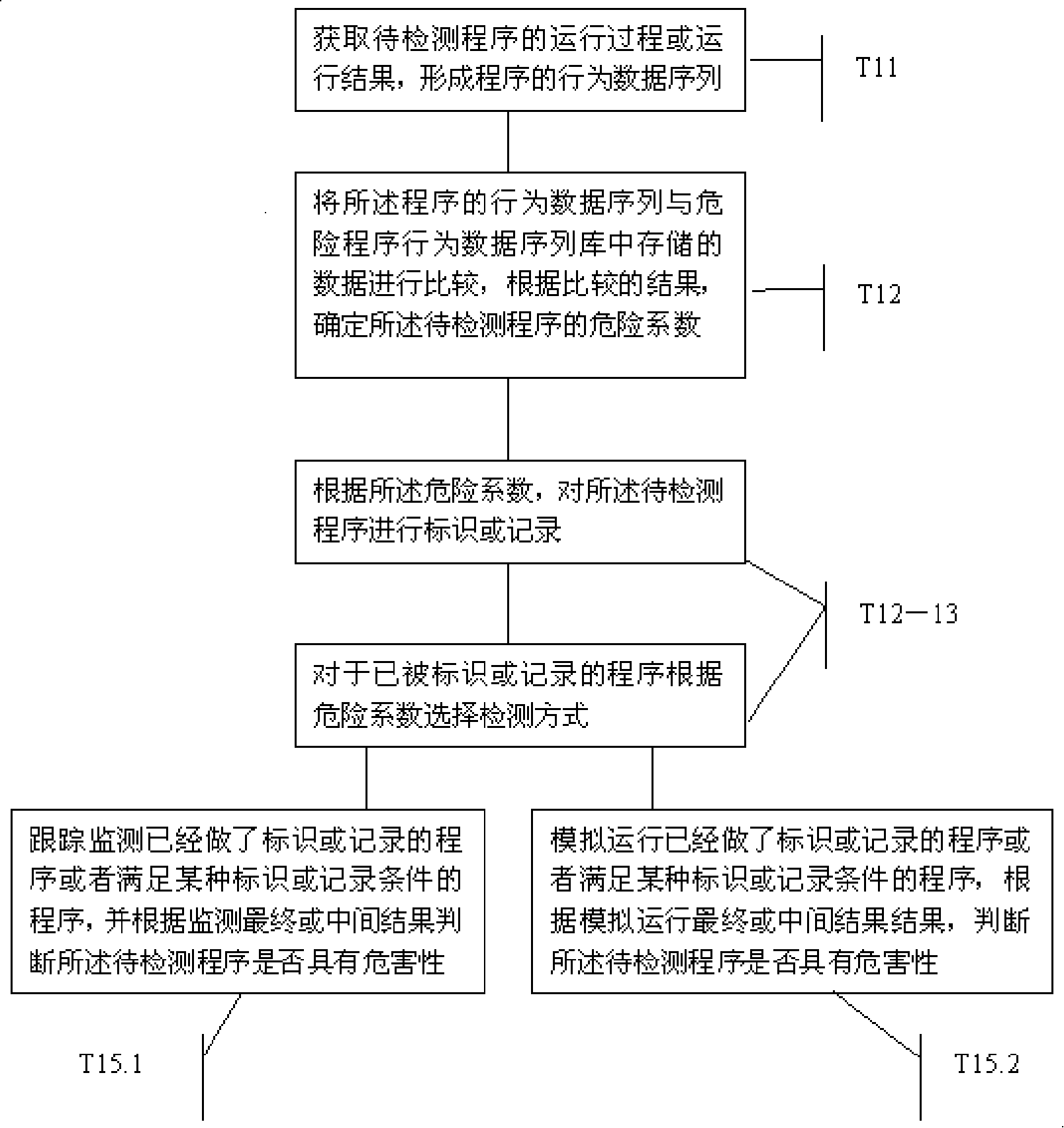
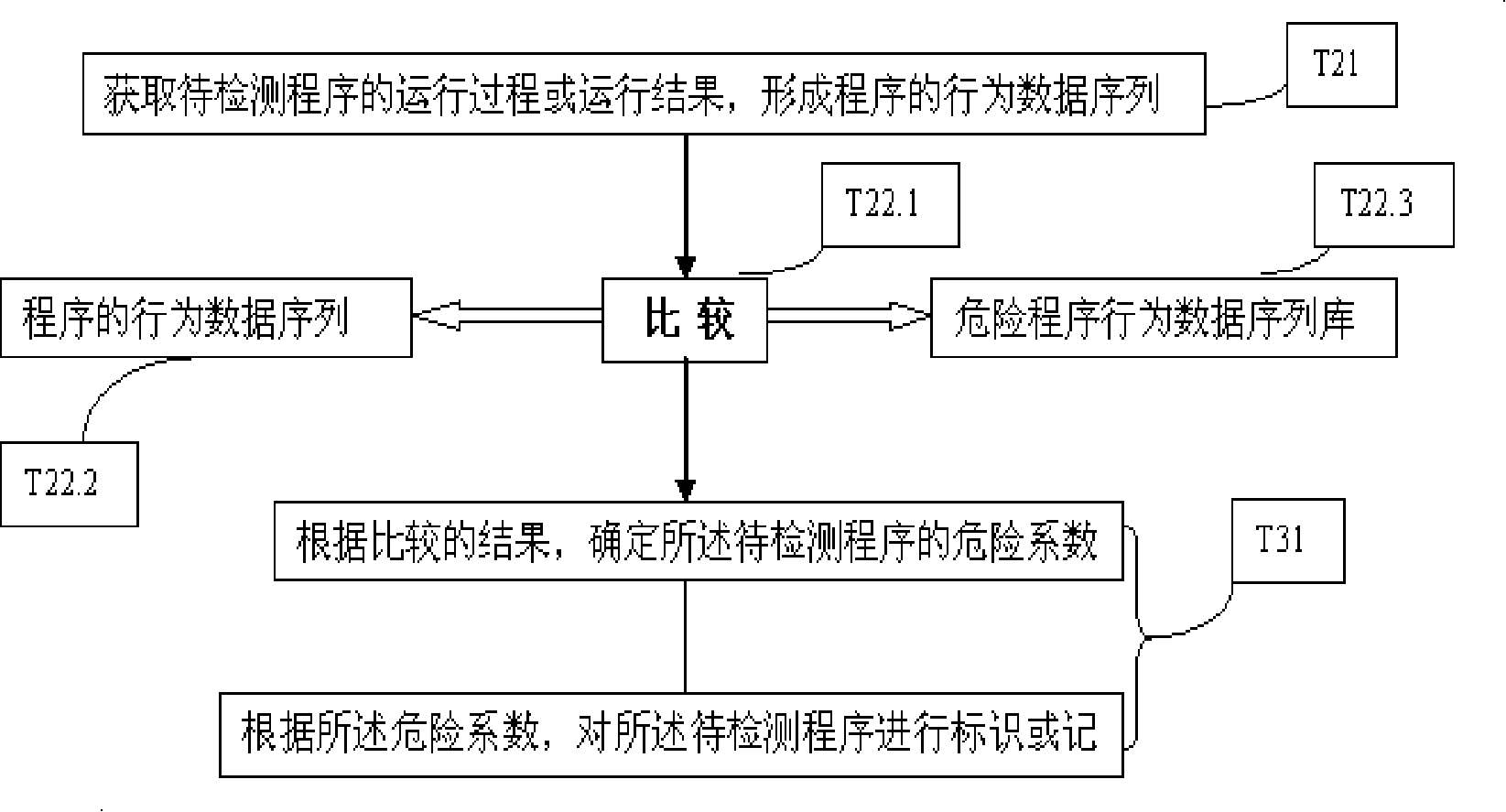
InactiveCN101183414AAvoid wastingImprove recognition ratePlatform integrity maintainanceData storingProgram behavior
The invention provides a program detection method, a program detection device and a program analysis method. The program detection method comprises the following steps: after the running process and the running result of a program to be detected are obtained, a behavioral data sequence of the program is generated; the behavioral data sequence of the program is compared with the data stored in a behavioral data sequence base of risk programs, and the risk coefficient of the program to be detected is determined based on the comparison result; the program to be detected is then labeled or recorded according to the risk coefficient. The invention has the advantages that the program detection method can dynamically trace the program running, the recognition rate of risk programs is improved and the miss killing rate to normal programs is reduced.
Owner:白杰 +2
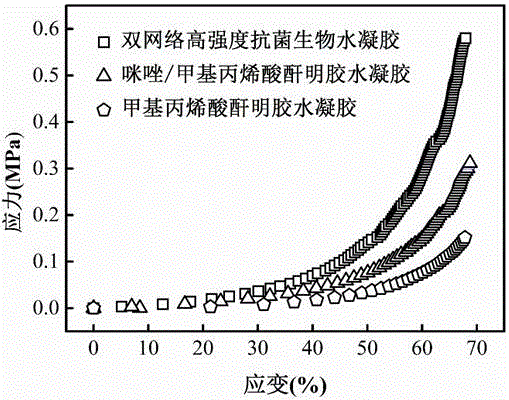
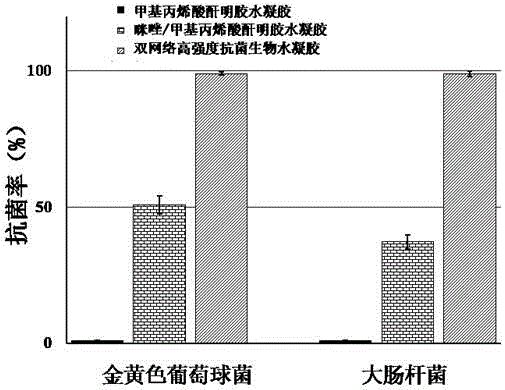
Preparation method of high strength double-network antibacterial biological hydrogel
The invention discloses a preparation method of high strength double-network antibacterial biological hydrogel. The preparation method comprises (1) mixing modified gelatin and modified histidine, dissolving the mixture in a phosphate buffer solution, and adding a photoinitiator into the solution to cause polymerization under the ultraviolet point light source so that gelatin hydrogel having an imidazole active site is obtained, and (2) soaking the gelatin hydrogel having an imidazole active site in a divalent metal ion salt solution, and then washing the gelatin hydrogel through sterile water to obtain the high strength double-network antibacterial biological hydrogel. The preparation method has the advantages of mild reaction conditions, strong operability and process controllability. The high strength double-network antibacterial biological hydrogel has good mechanical properties, rigidity and toughness, has escherichia coli and staphylococcus aureus killing rates of 99.9% and has good biocompatibility of the base hydrogel.
Owner:SOUTH CHINA UNIV OF TECH
Novel plant source insecticide for fruit trees, preparation method and application thereof
InactiveCN105532755ANon-toxic ingredientsWill not polluteBiocideDead animal preservationAconitum soongaricumRaw material
The invention discloses a novel plant source insecticide for fruit trees, a preparation method and an application thereof, and belongs to the field of preparation technology of insecticide. The insecticide comprises the following raw materials in parts by weight: patrinia, tobacco scraps, Aconitum soongaricum, pyrethrum, paulownia sawdust, Sophora alopecuroides, officinal magnolia barks, Chinaberry, Chinese ash leaves, Celastrus angulatus, ash barks, flavescent sophora roots, Euphorbia soongarica, bitter sow thistle, ailanthus, lettuces, mints, thunder god vine roots, weeping willow leaves, castor-oil plants and water. The novel plant source insecticide for fruit trees is prepared by the following steps: extraction of soup, condensation, drying, crushing, sieving, disinfection, etc. The insecticide does not have toxic components, residuals, and pollution, and is green and environmentally friendly; the insecticide can be used for effectively controlling red spider mites, Aonidiella aurantii, fruit flies, blister mites and other plant insects, one-time killing rate reaches above 95.53%, and the insects do not have drug resistance.
Owner:罗永城
Animal protein-free immune cell serum-free medium and using method thereof
ActiveCN104450614ASuitable for growthImprove proliferation efficiencyBlood/immune system cellsSerum free mediaCells/well
The invention discloses the field of culture in vitro of immune cells and particularly relates to an animal protein-free immune cell serum-free medium and a culture method thereof, wherein the culture medium is a liquid culture medium. The culture medium per liter comprises the following components: 1400-1500mg of amino acids, 70-75mg of vitamins, 9800-10000mg of salts, 9500-9700mg of organic matters and 76-80mg of proteins. The culture medium prepared by the invention satisfies the special condition required by growth of immune cells well, so that the culture medium is more suitable for growth of immune cells, thereby remarkably improving the cell proliferation efficiency and the biological potency. The culture medium overcomes the defect that in the prior art, batches are different in quality caused by diversity of human or animal serum and component limited donors added into the culture medium, the quality is easy to control, the culture medium is not polluted due to the quality of serum, and the tumor-killing rate effect is good and the cost is low.
Owner:上海安库生医生物科技有限公司
Finishing method for nano antibiotic fabric
InactiveCN1858328AImprove adhesionReduce releaseTextile treatment by spraying/projectingAdhesiveEngineering
The present invention discloses finishing method for nanometer antibiotic fabric. By means of sol-gel technology, silica gel is prepared on fabric with water glass of different modulus as precursor and ammonium salt as catalyst and used in padding fabric to introduce silver antibiotic and endow fabric with excellent lasting antibiotic performance. The fabric may have its 99 % over of colibacillus and Staphylococcus aureus killing rate maintained after being washed for 50 times. The finishing process has few influence on the hand feeling, whitenes and physical and mechanical performance of the fabric, uses no adhesive and dispersant, and has less pollution and low cost.
Owner:DONGHUA UNIV
Human serum-free culture medium and preparation method thereof
ActiveCN102191215AExcellent proliferation rateImprove securityBlood/immune system cellsLipid formationCatalase
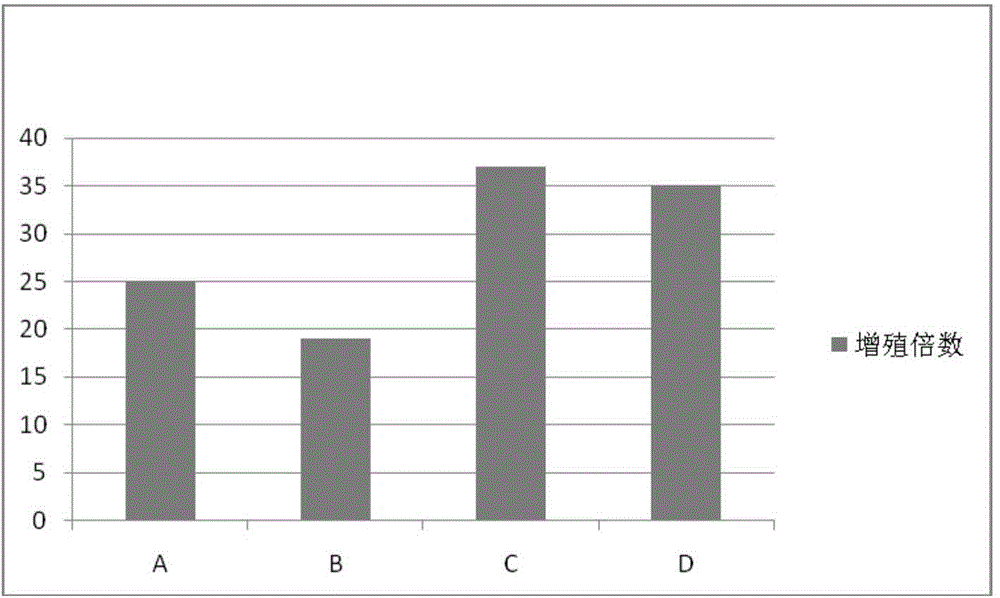
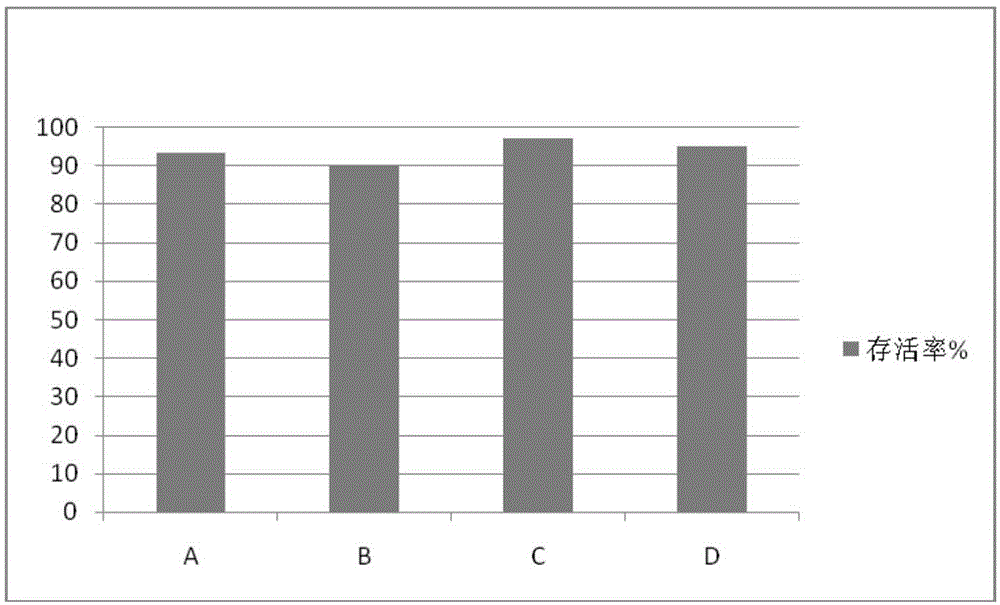
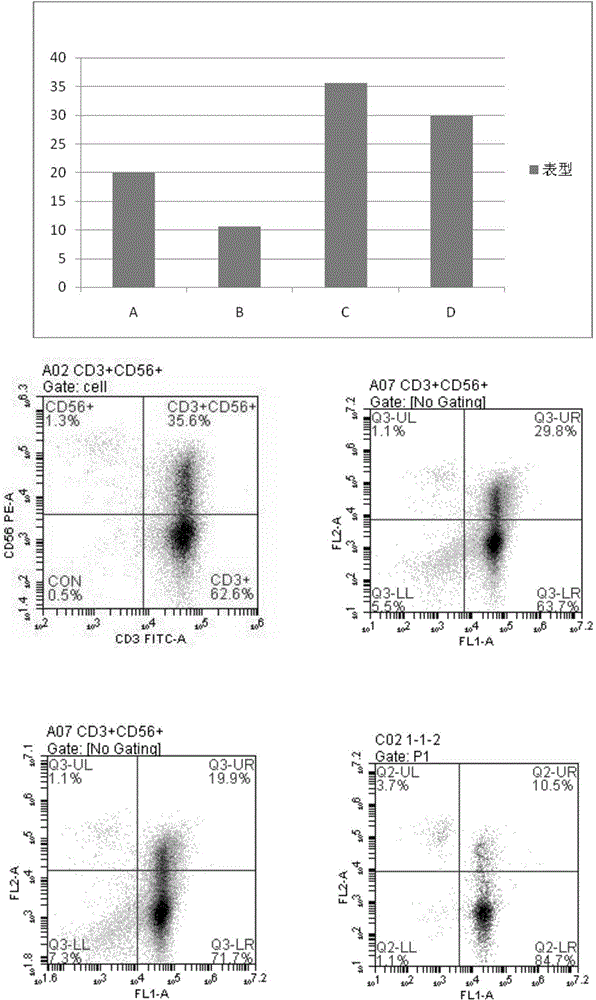
The invention discloses a human serum-free culture medium and a preparation method thereof. The human serum-free culture medium uses eleven raw materials, namely human serum albumin solution for treatment, human recombinant insulin solution, human transferrin solution, human cholesterol solution, human catalase solution, 2-mercaptoethanol solution, ascorbic acid solution, linoleic acid solution, ethanolamine solution, human vitronectin solution and L-glutamine solution; the added protein and lipid are both from the blood plasma, serum or tissue of human, the protein is pharmaceutical-grade orhighly purified human protein or human recombinant protein, the protein and lipid do not contains any animal component, the other components all meet the United States Pharmacopoeia or national standards; and the human serum-free culture medium is qualified through the cell culture test and is clinical, safe and reasonable human serum-free culture medium. The proliferation rate of the CIK cell cultured and inducted by the human serum-free culture medium is better than that of the CIK cell cultured and inducted by the culture medium with serum, the cell CD3+CD56+ percentage and the killing rate to the K562 leukemic cell are similar to that of the culture medium with serum. By adopting the human serum-free culture medium, the safety and standardization of cell therapy can be increased.
Owner:湘雅生物医药(湖州)有限公司
Disinfectant
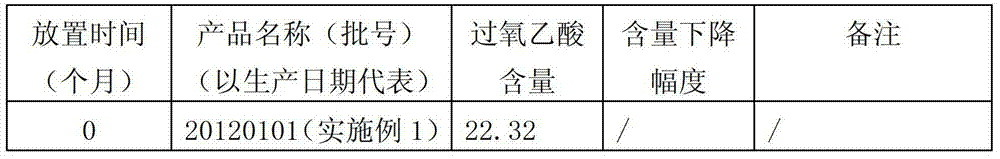
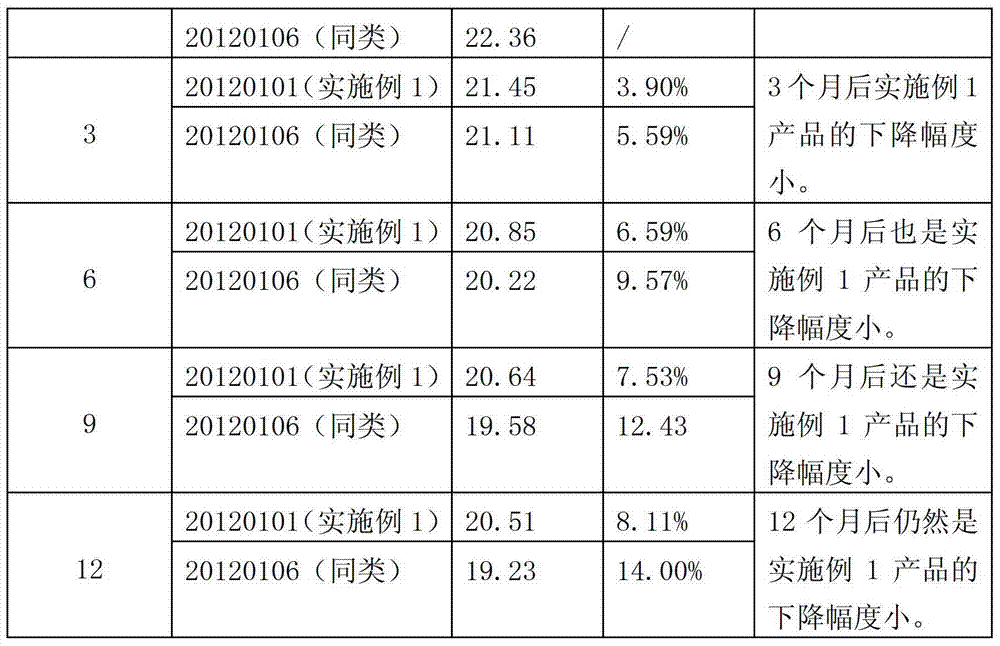
ActiveCN103202294AImprove stabilityWill not cause secondary pollutionAntibacterial agentsBiocideKilling rateDisinfectant
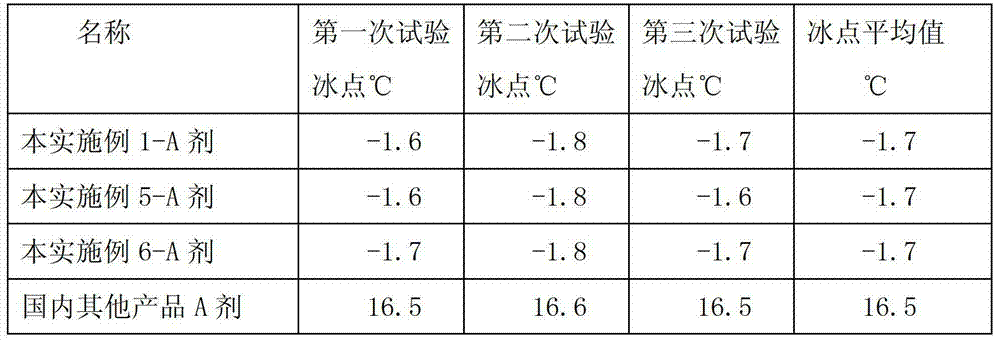
The invention discloses a disinfectant. The disinfectant has a formula consisting of an agent A and an agent B, wherein the agent B comprises the following components in percentage by weight: 90 to 93% of glacial acetic acid, 3 to 5% of one or mixture of sulfuric acid and phosphoric acid, and the balance of water; and the agent B comprises the following components in percentage by weight: 90 to 93% of hydrogen peroxide, 3 to 5% of hydroxyethylidene diphosphonic acid, 0.05 to 0.5% of 8-hydroxyquinoline, and the balance of the water. According to the disinfectant, the formula is adjusted, and thus the stability is higher; in addition, the agent A cannot be frozen at temperature above 0 DEG C; the disinfectant has killing rate being more than 99% to reproductive and respiratory syndrome virus and escherichia coli virus; and the product quality meets National Veterinary Drugs Standard. The disinfectant is suitable for all animals, and can be periodically used for removing scales in a water reservoir and a water pipeline and disinfecting the same, disinfecting the environment of a pig farm, disinfecting a brooder and a thermostat and the like in a chicken farm, cleaning feet of a dairy cattle, cleaning and disinfecting dairy food transportation equipment, and disinfecting the water mass and a tool in aquaculture; and no second pollution is brought.
Owner:广州迈高化学有限公司
Antimicrobial treatment of synthetic nonwoven textiles
InactiveUS20110250253A1Increase in bacteria countQuick wetBiocideBiochemical fibre treatmentPolyelectrolyteCarboxymethyl cellulose
Highly active, leach-resistant, antimicrobial nonwoven textiles are prepared by treating at least one surface of the nonwoven material with an anionic polyelectrolyte, such as carboxymethyl cellulose, alginic acid, poly(acrylic acid) etc and at least one select quaternary ammonium antimicrobial agent. The textiles of the invention, and products produced from them, exhibit a highly effective quick kill rate, for example a log 4 CFU reduction within a 5 minute contact time, against microbes such as fungi and gram (−) and gram (+) bacteria.
Owner:CUNKLE GLEN T +2
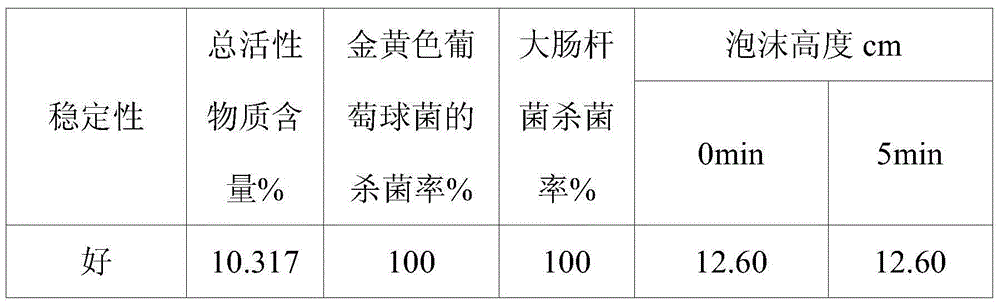
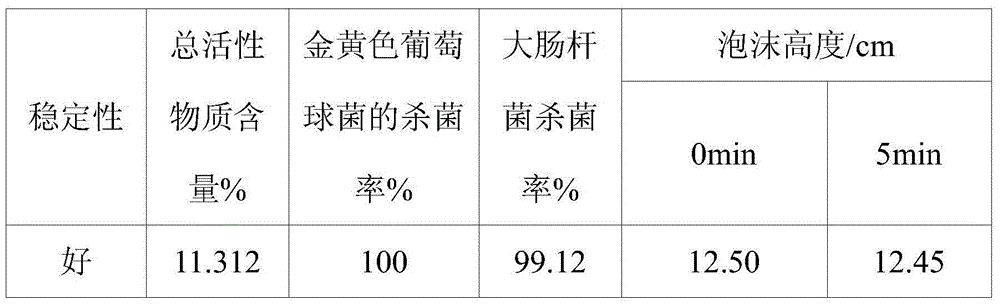
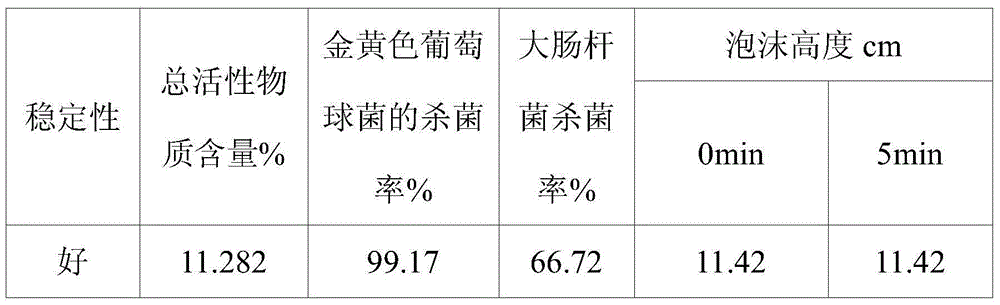
Quaternary ammonium salt type antibacterial hand washing solution
ActiveCN104306176AImprove stabilityHigh sterilization rateCosmetic preparationsToilet preparationsEscherichia coliSolubility
The present invention discloses a quaternary ammonium salt type antibacterial hand washing solution, which comprises, by weight, 0.4-0.8% of sodium lauryl polyoxyethylene ether sulfate, 1-2% of a quaternary ammonium salt, 15-25% of cocamidopropyl betaine, 0.1-0.2% of MES (AESS), 1-2% of coconut oil diethanolamide, 0.05-0.15% of a preservative, 0.1-0.5% of essence, and the balance of distilled water, wherein the pH value is 6.5-7.0. According to the quaternary ammonium salt type antibacterial hand washing solution, the anionic surfactant and the cationic surfactant can stably exist in the compounding system, and the solubility is good when the use amount of the one surfactant is more than the use amount of the other surfactant, such that the coagulation is not easily produced. The quaternary ammonium salt type antibacterial hand washing solution has characteristics of good stability, high escherichia coli killing rate, high staphylococcus aureus killing rate, and foaming property achieving the market level.
Owner:江苏德鲁克生物科技有限公司
Double-specific chimeric antigen receptor T cell as well as preparation method and application thereof
ActiveCN110330567AEffective targeted attackHigh kill rateAntipyreticAntibody mimetics/scaffoldsAntigen receptorsT cell
The invention relates to a double-specific chimeric antigen receptor T cell as well as a preparation method and application thereof. The double-specific chimeric antigen receptor double-specifically targeting human NKG2DL and human CD47 is constructed on the basis of human NKG2D and human SIRPalpha molecules as well as an immune response cell modified with the double-specific chimeric antigen receptor. The novel modified immune response cell can effectively realize targeting attack of multiple tumor cells, particularly positive tumor cells expressing NKG2DL and CD47 and can be used for preparing preparations for treating tumor. The preparation method of the immune response cell modified with the double-specific chimeric antigen receptor double-specifically targeting NKG2DL and CD47 comprises simple steps, the obtained immune response cell modified with the double-specific chimeric antigen receptor double-specifically targeting NKG2DL and CD47 has high specific killing rate for tumor cells.
Owner:NANJING KAEDI BIOTHERAPEUTICS LTD
Pulse type ultrahigh pressure food processing method
InactiveCN101999732AImprove protectionGuaranteed freshnessMilk preservationFruit and vegetables preservationUltra high pressureRoom temperature
The invention discloses a pulse type ultrahigh pressure food processing method, which comprises the following steps: 1) placing food to be processed into a plastic bag for packaging the food under vacuum; 2) placing the vacuum package food containing food in a high pressure container, adding water or oil for sealing, and performing pulse supercharging and discharging for 1 to 4 times, wherein the discharging pressure is 100MPa, 150MPa, 200MPa, 300MPa or 400MPa if the supercharging pressure is 400MPa, the discharging pressure is 100MPa, 150MPa, 200MPa, 300MPa, 400MPa or 500MPa if the supercharging pressure is 500MPa, and the discharging pressure is 100MPa, 150MPa, 200MPa, 300MPa, 400MPa, 500MPa or 600MPa if supercharging pressure is 600MPa; and 3) discharging the pressure completely. In the invention, sterilization and enzyme inactivation are performed at a lower temperature (room temperature) and the color, fragrance, flavor and nutrients are not damaged, so additives are not used. In addition, special stress caused by supercharging and discharging to microorganisms is strengthened, so the microorganisms injuring and killing rate are improved, and the ultrahigh pressure processing time is reduced.
Owner:ZHEJIANG UNIV
Method for manufacturing nano TO silver-carried antibacterial membrane
InactiveCN1676227AReduce consumptionLow costPretreated surfacesSpecial surfacesTitaniumOleic Acid Triglyceride
The present invention relates to production method of nano TiO2 silve-carried antibacterial film. First method includes the following steps: adding prepared silver solution into titanium sol to obtain silve-carried sol, then film-coating product with said sol; and second method includes the following steps: firstly, film-coating product in titanium sol, drying, then film-coating said product in silver solution. The said silver solution is made up by adopting the following steps: firstly, dissolving proper quantity of silver nitrate in micro-water, then adding proper quantity of oleic acid and acetone, fully stirring them so as to obtain the silver solution. It has high antibacterial property, the bacteria-killing rate for killing colibacillus and staphylococcus can be up to above 90%.
Owner:湖南泰鑫瓷业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com