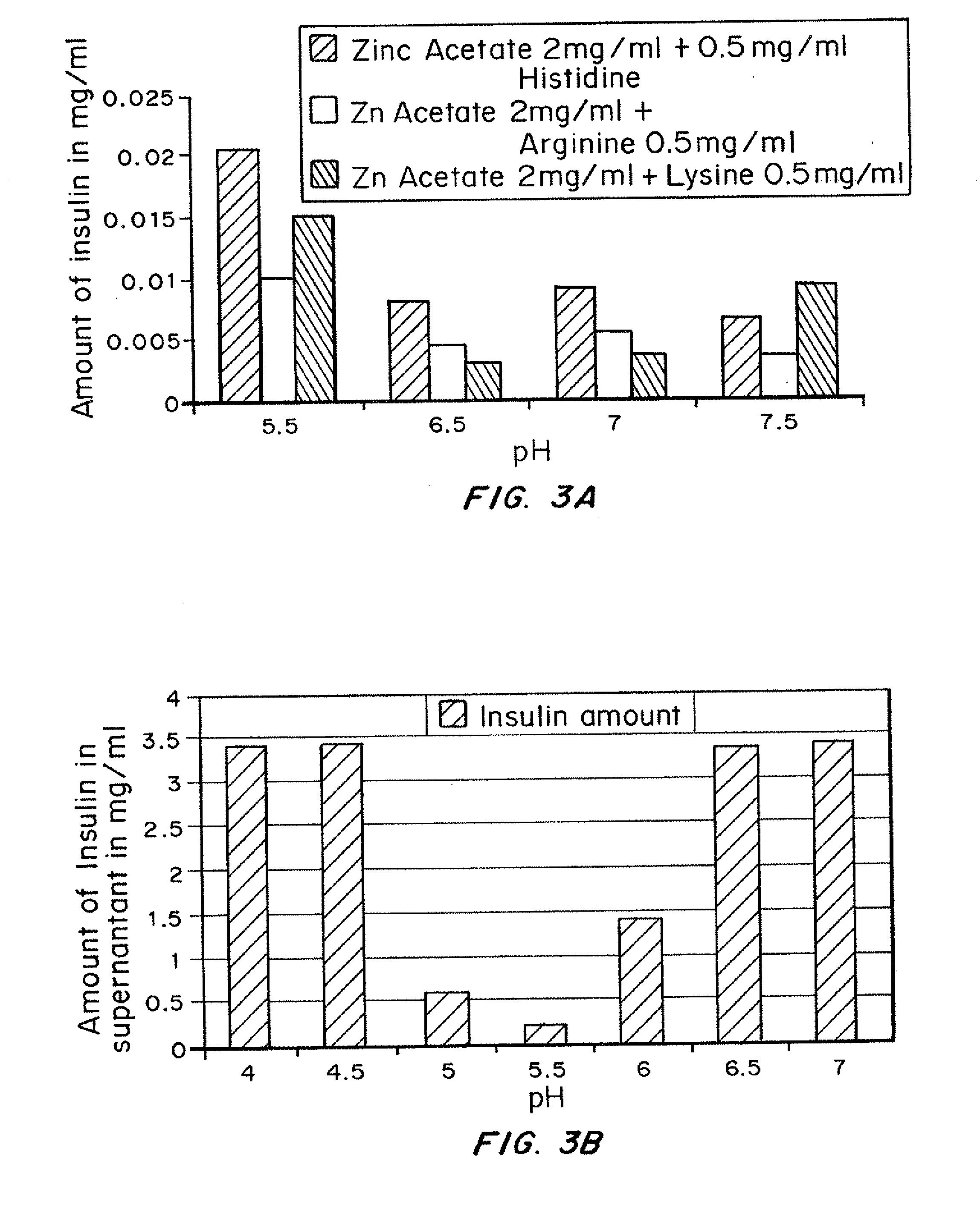
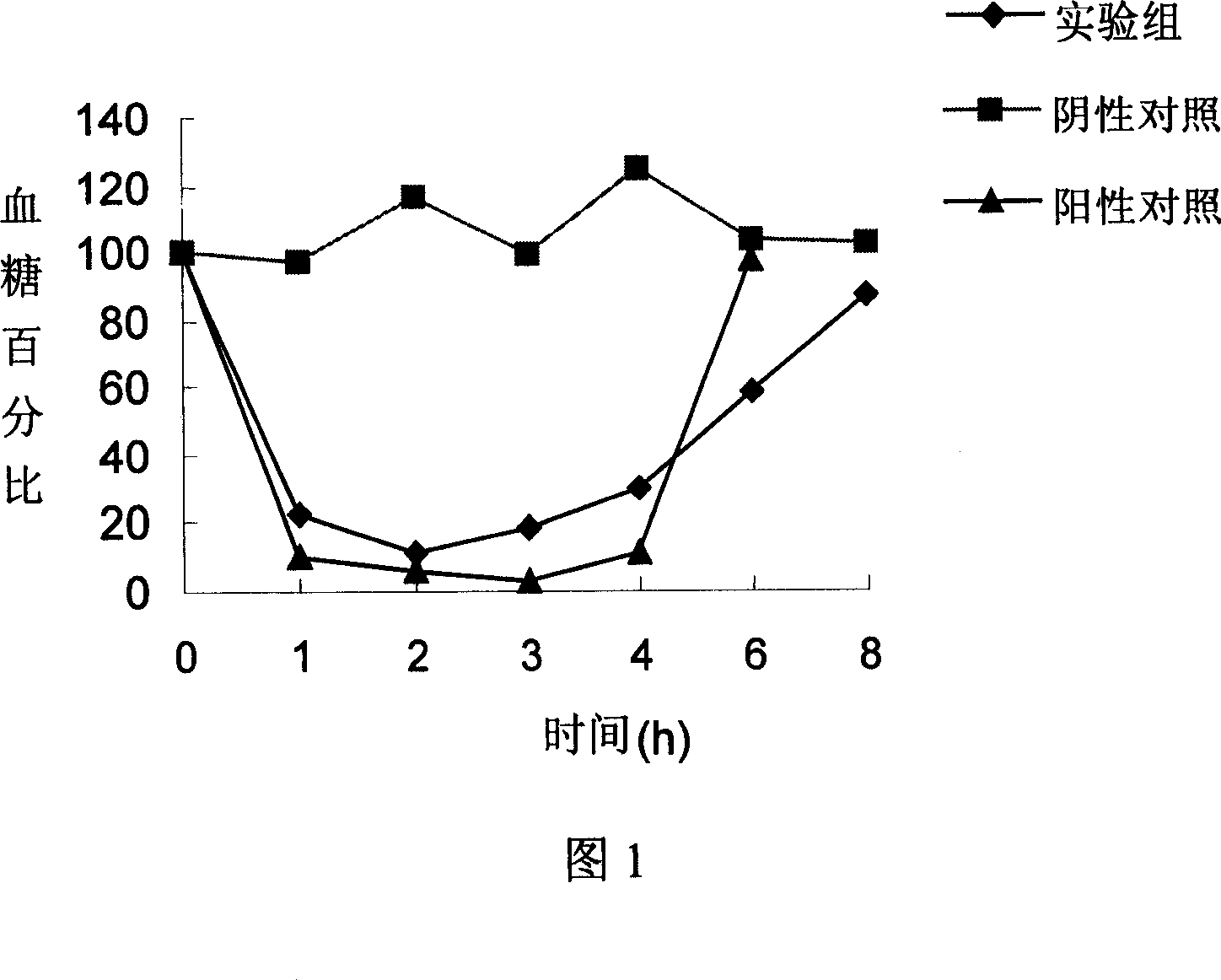
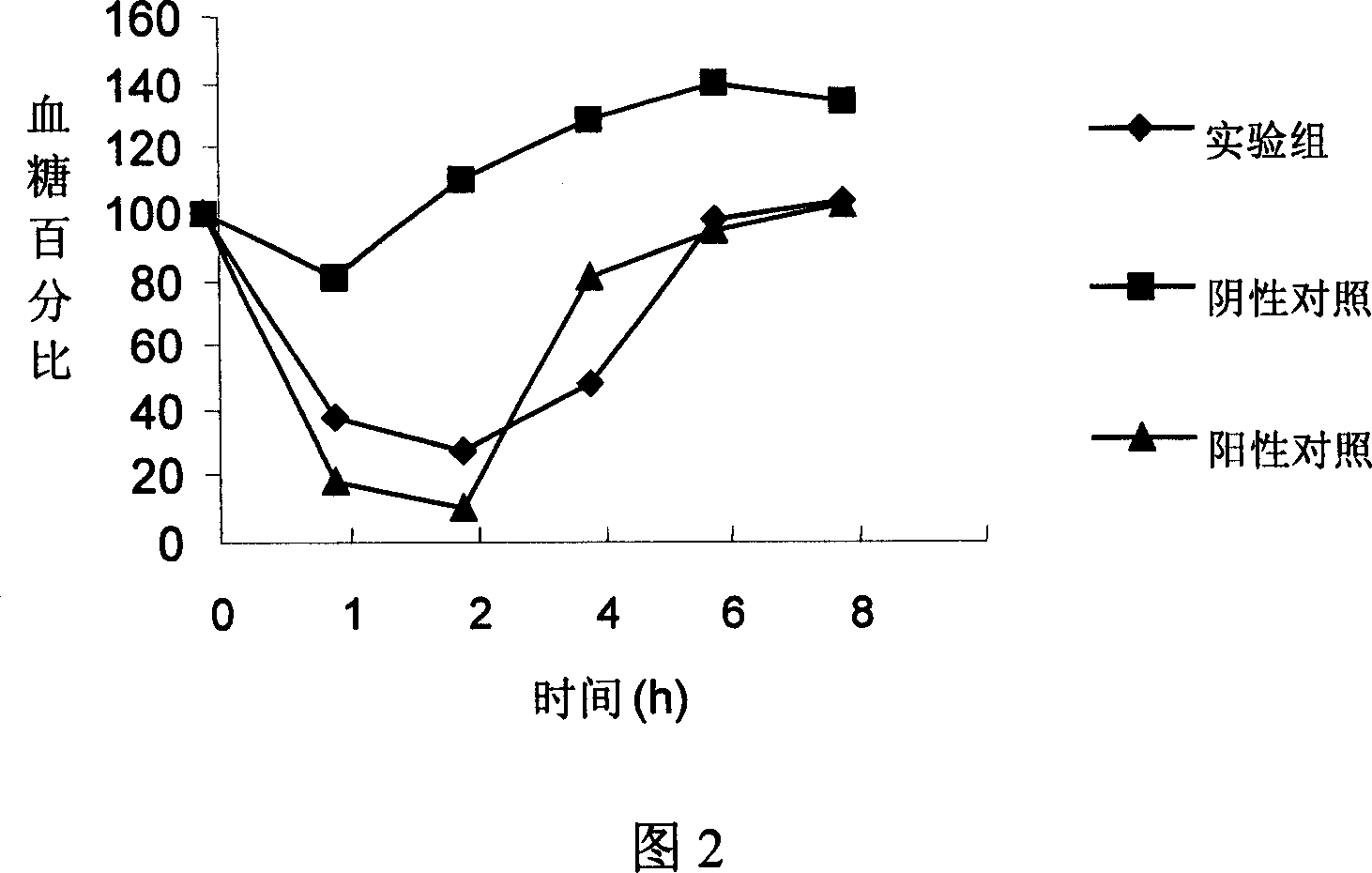
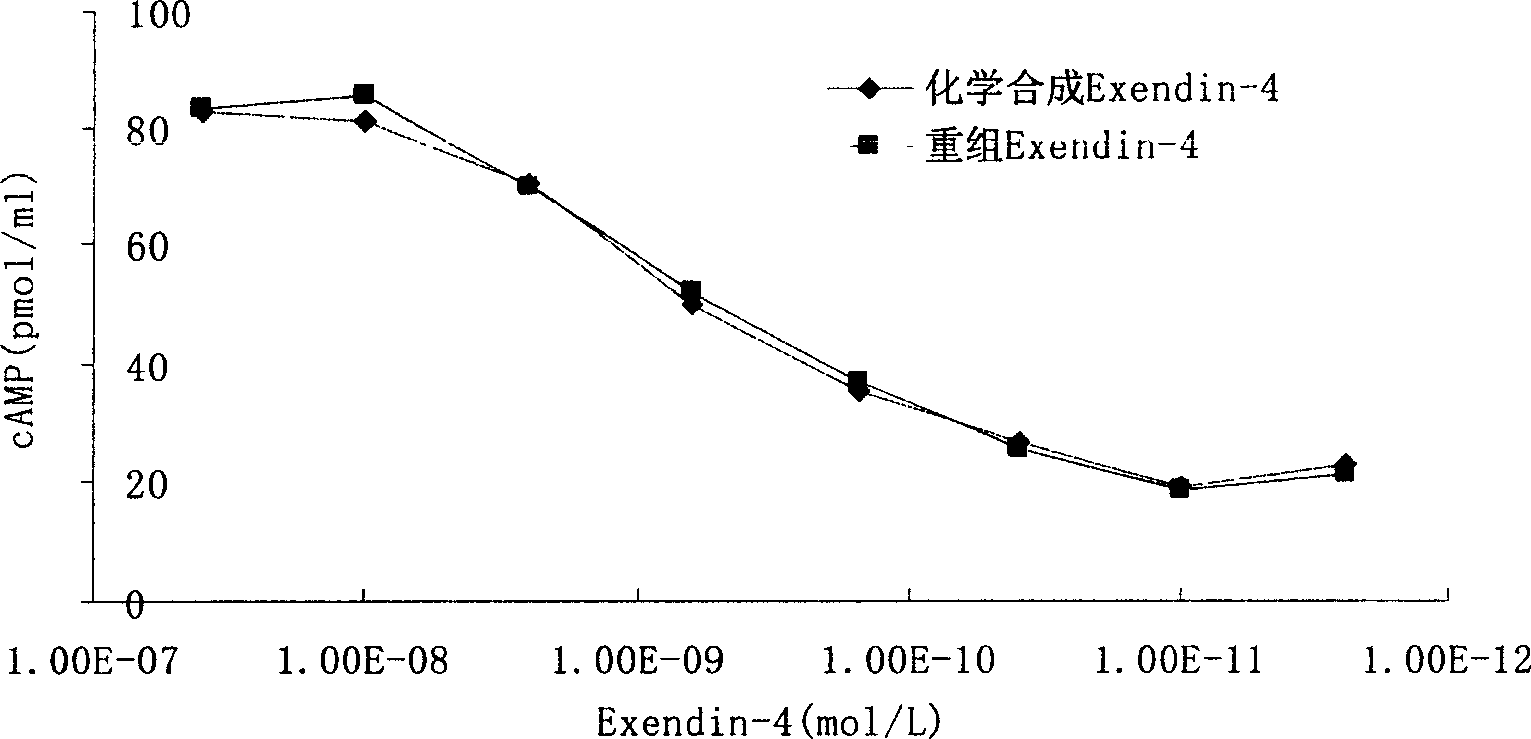
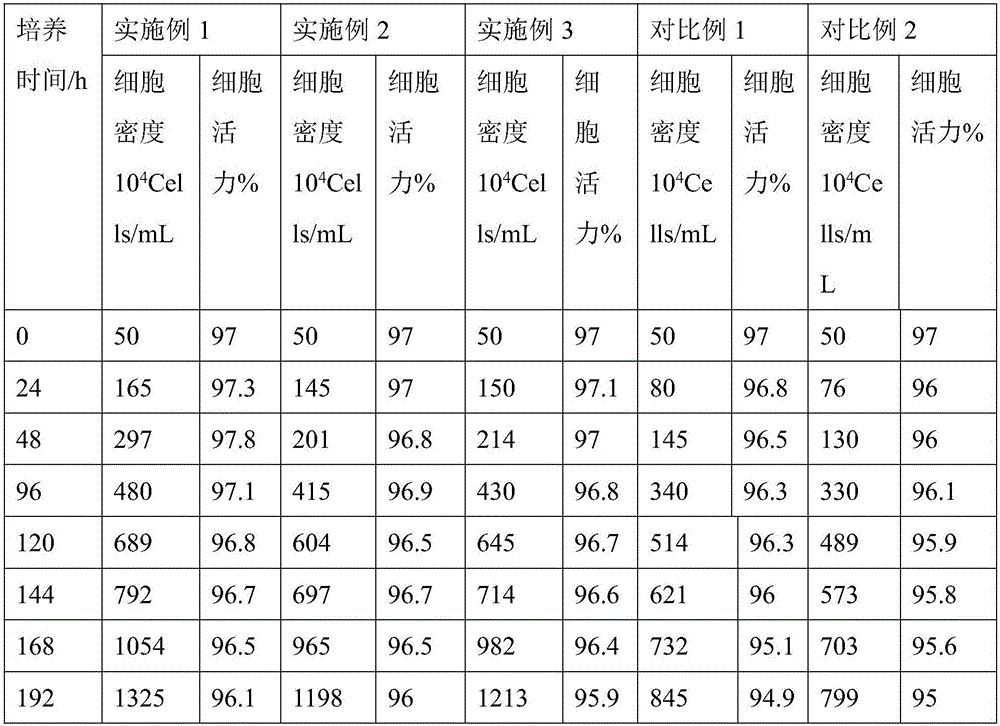
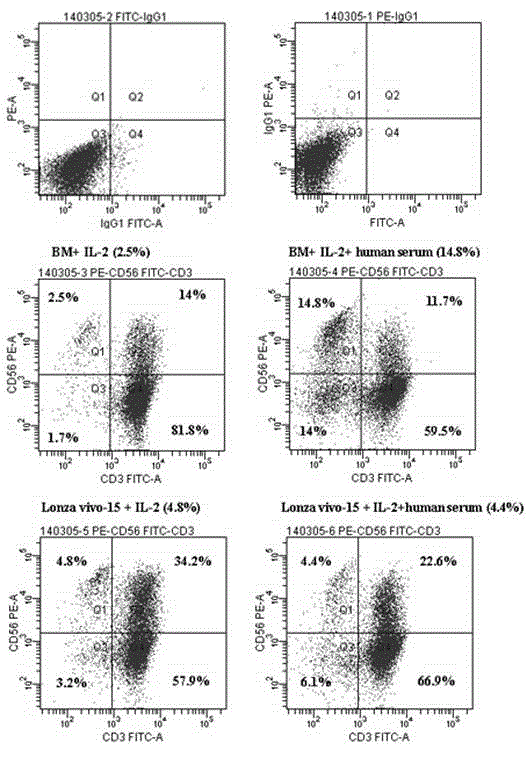
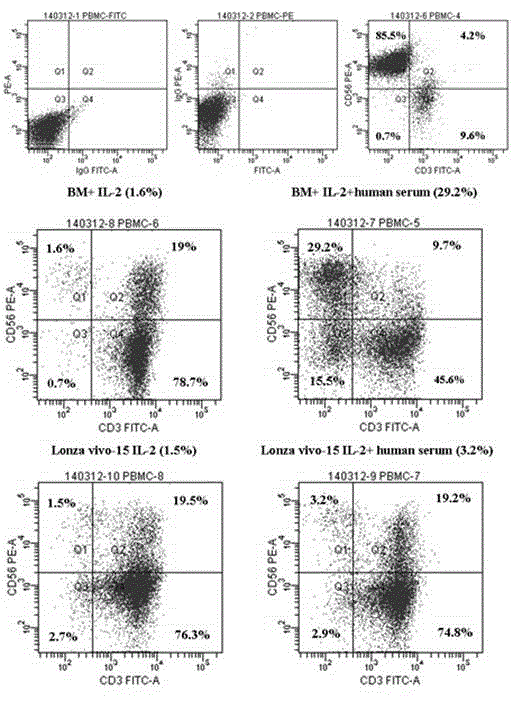
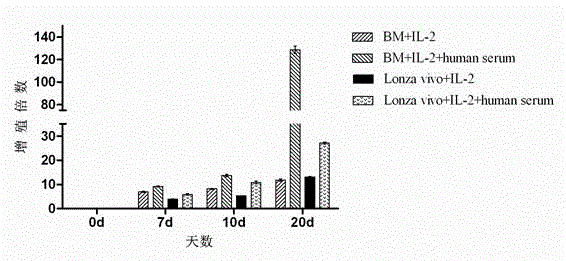
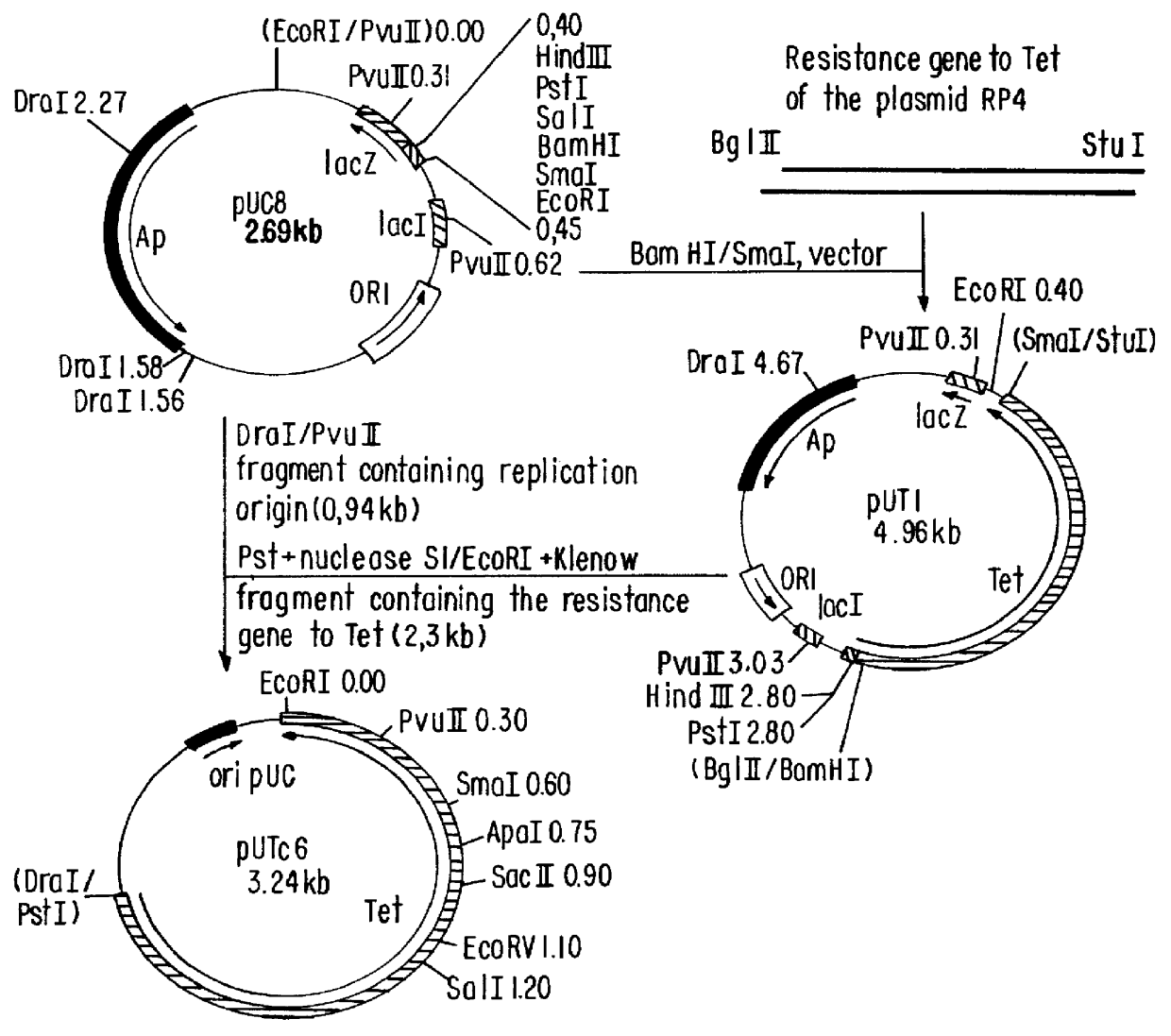
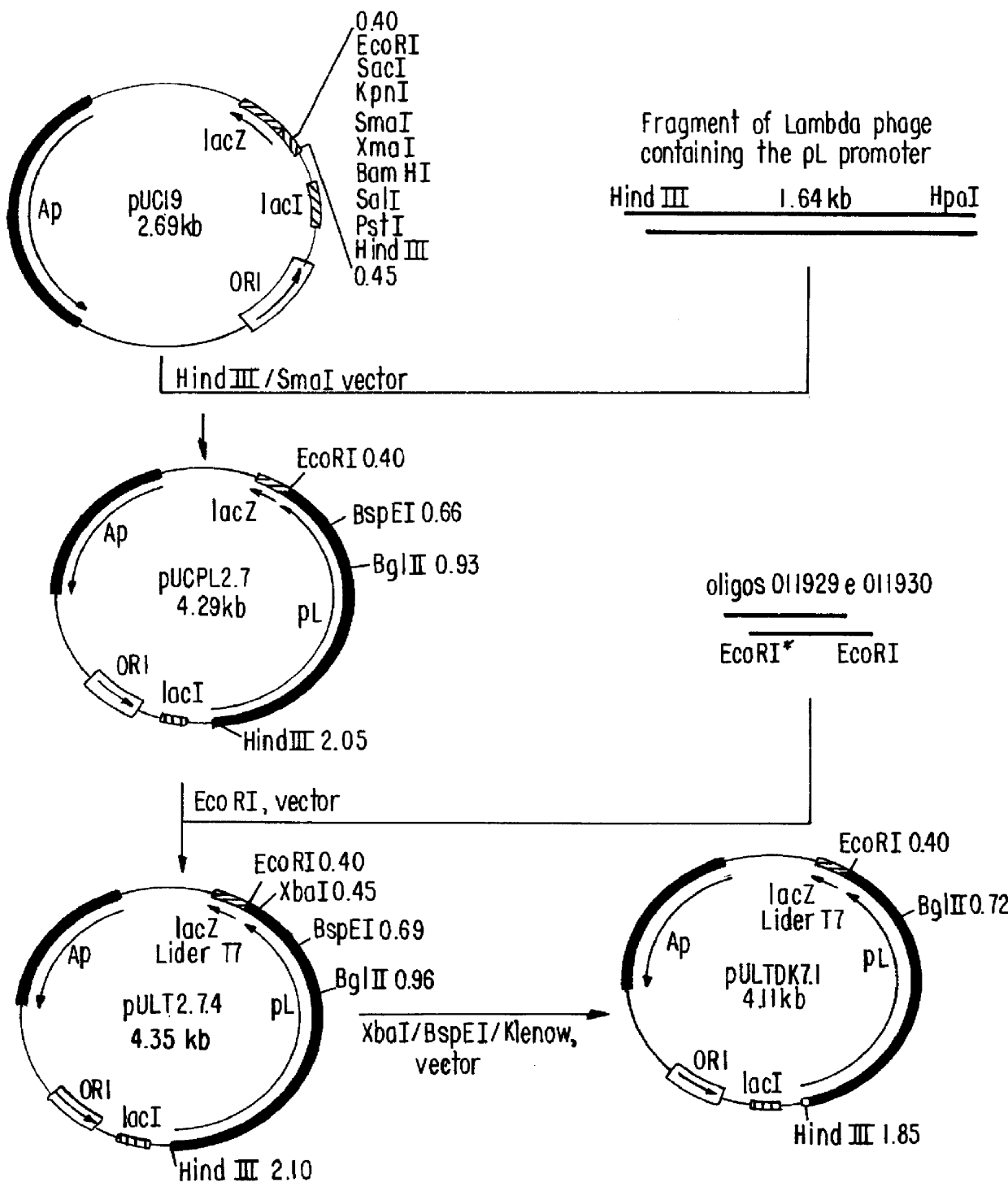
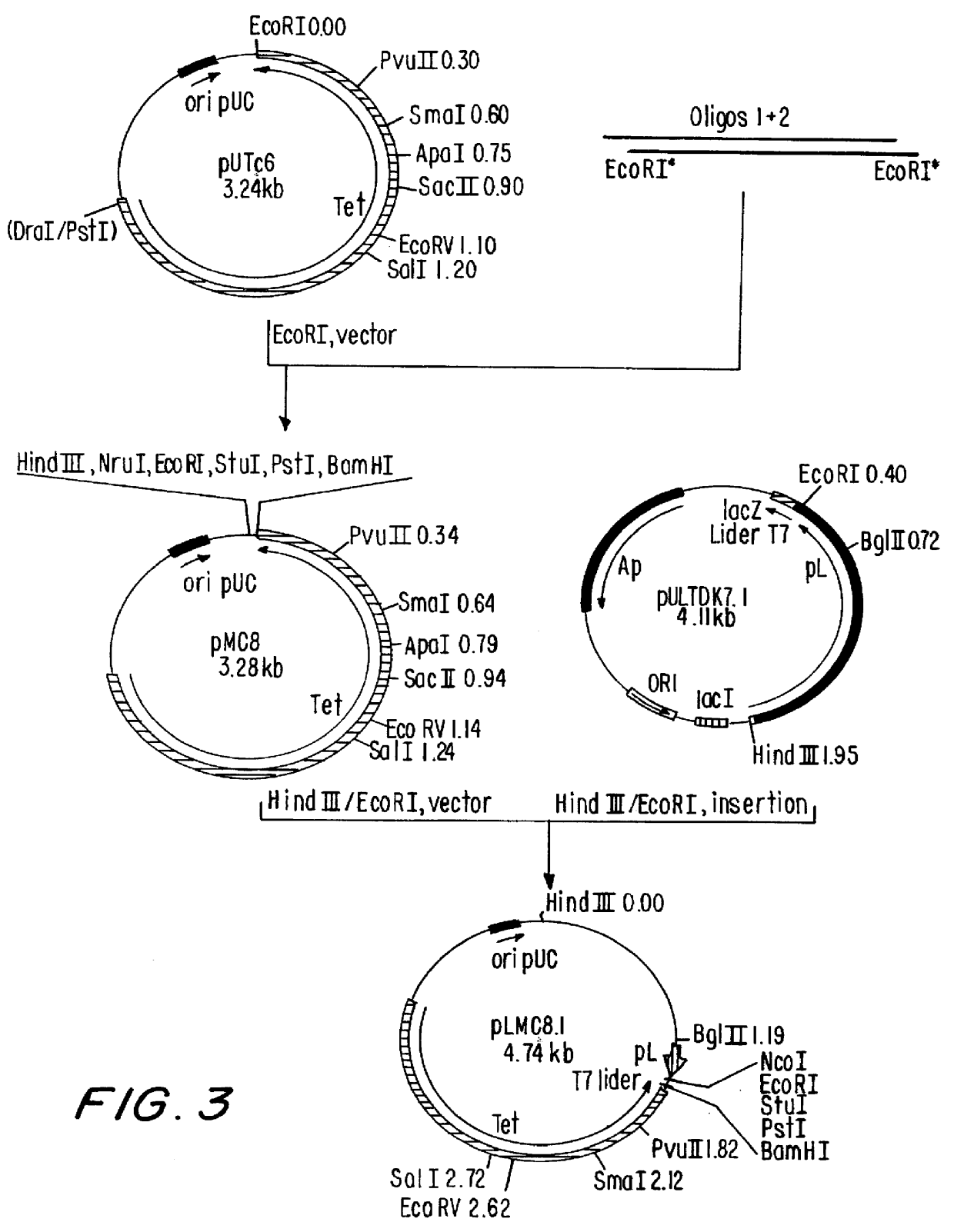
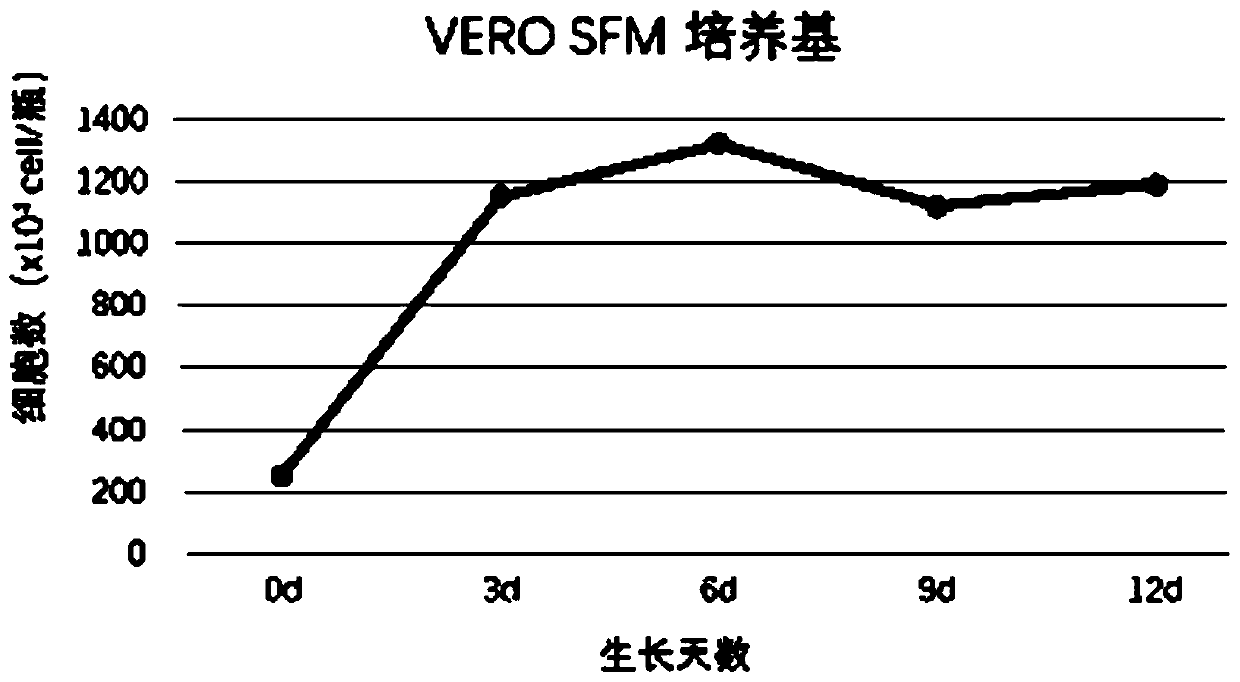
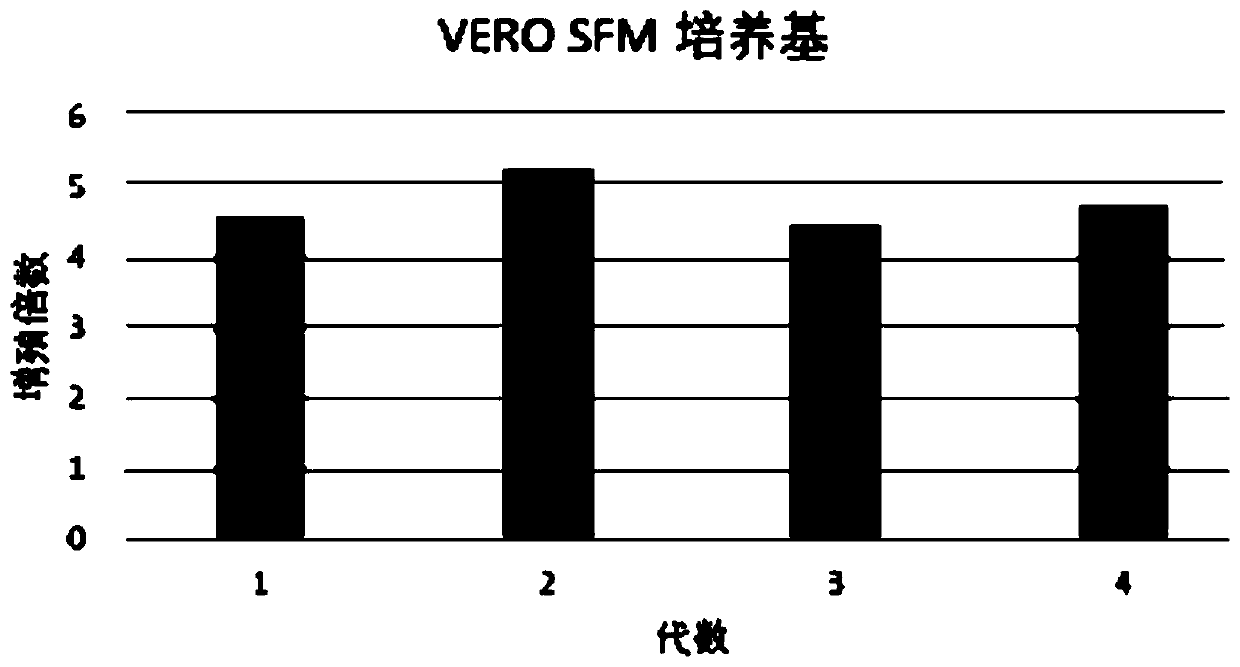
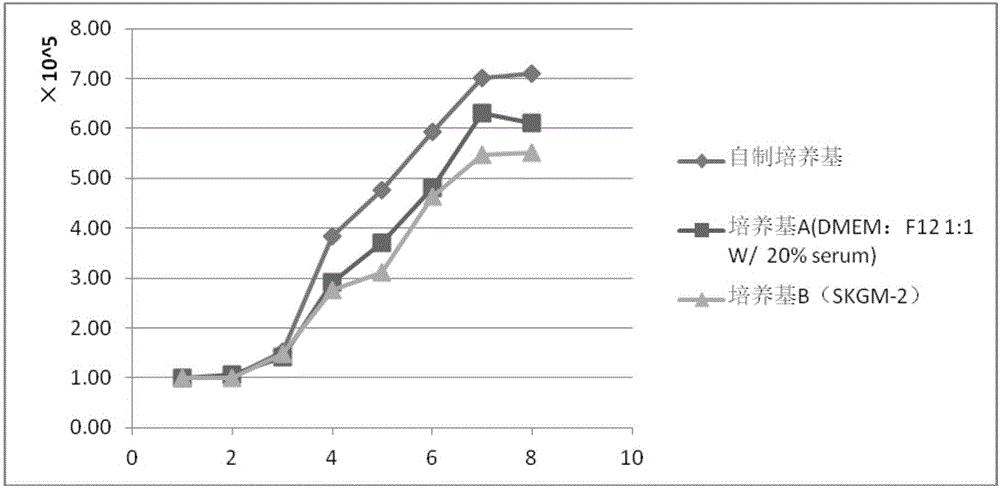
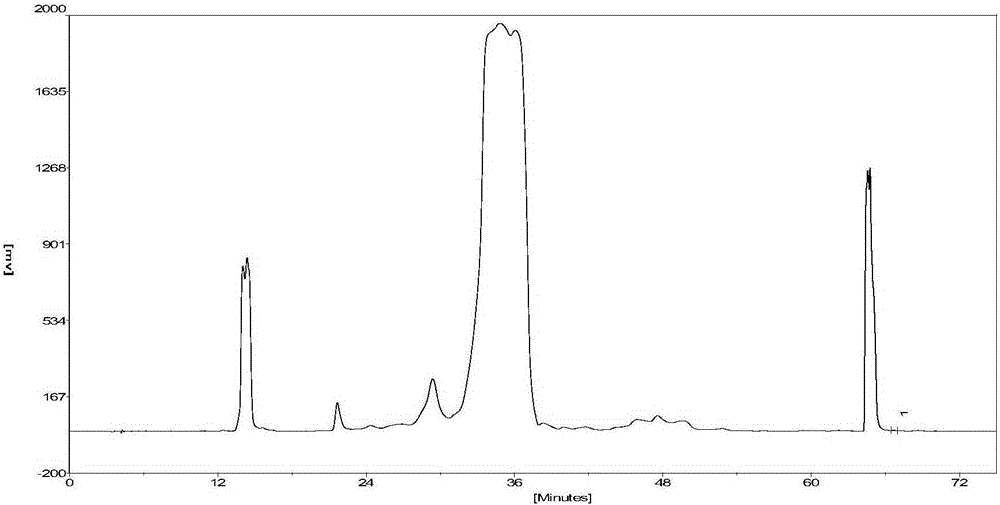
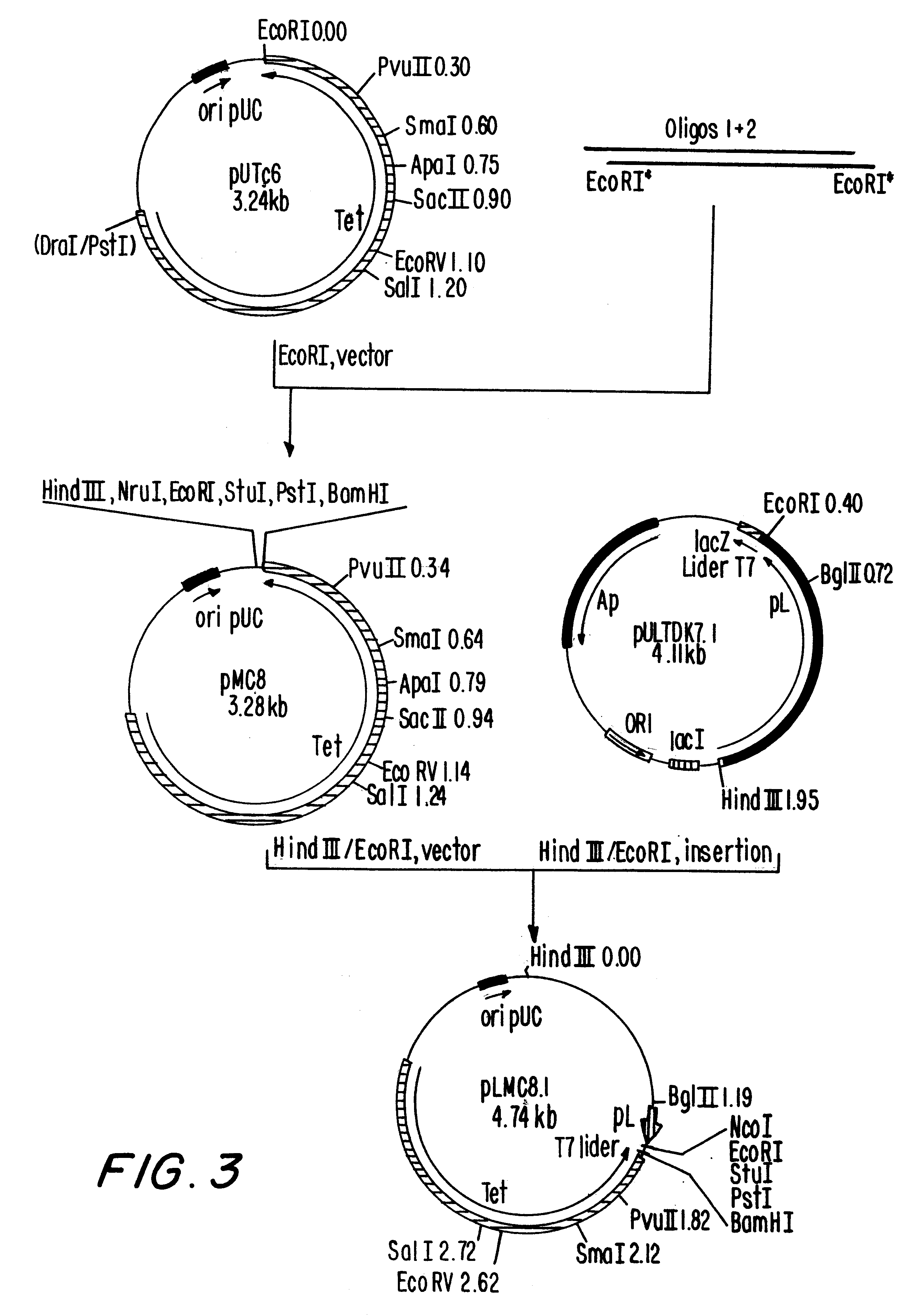
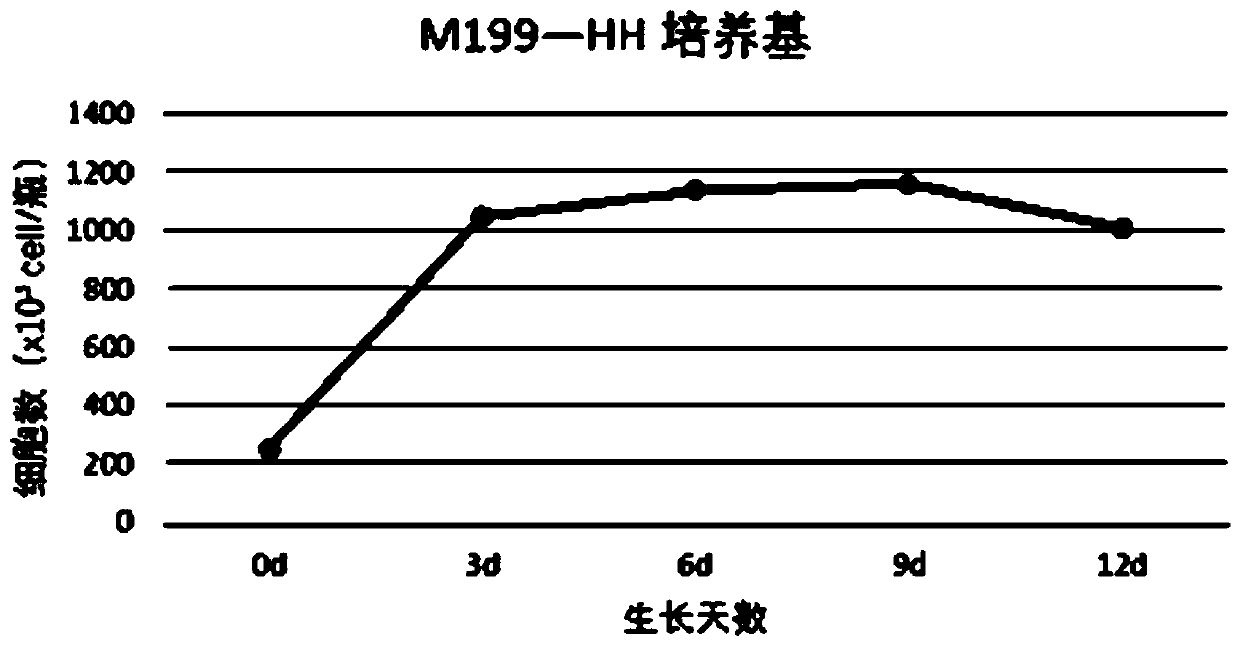
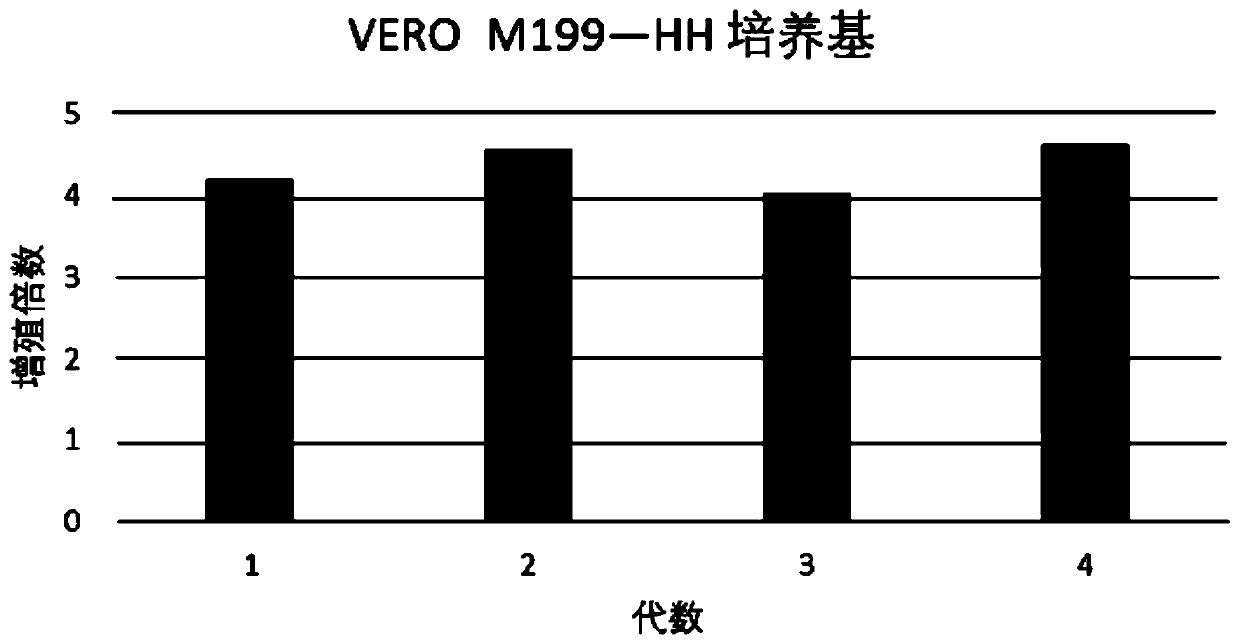
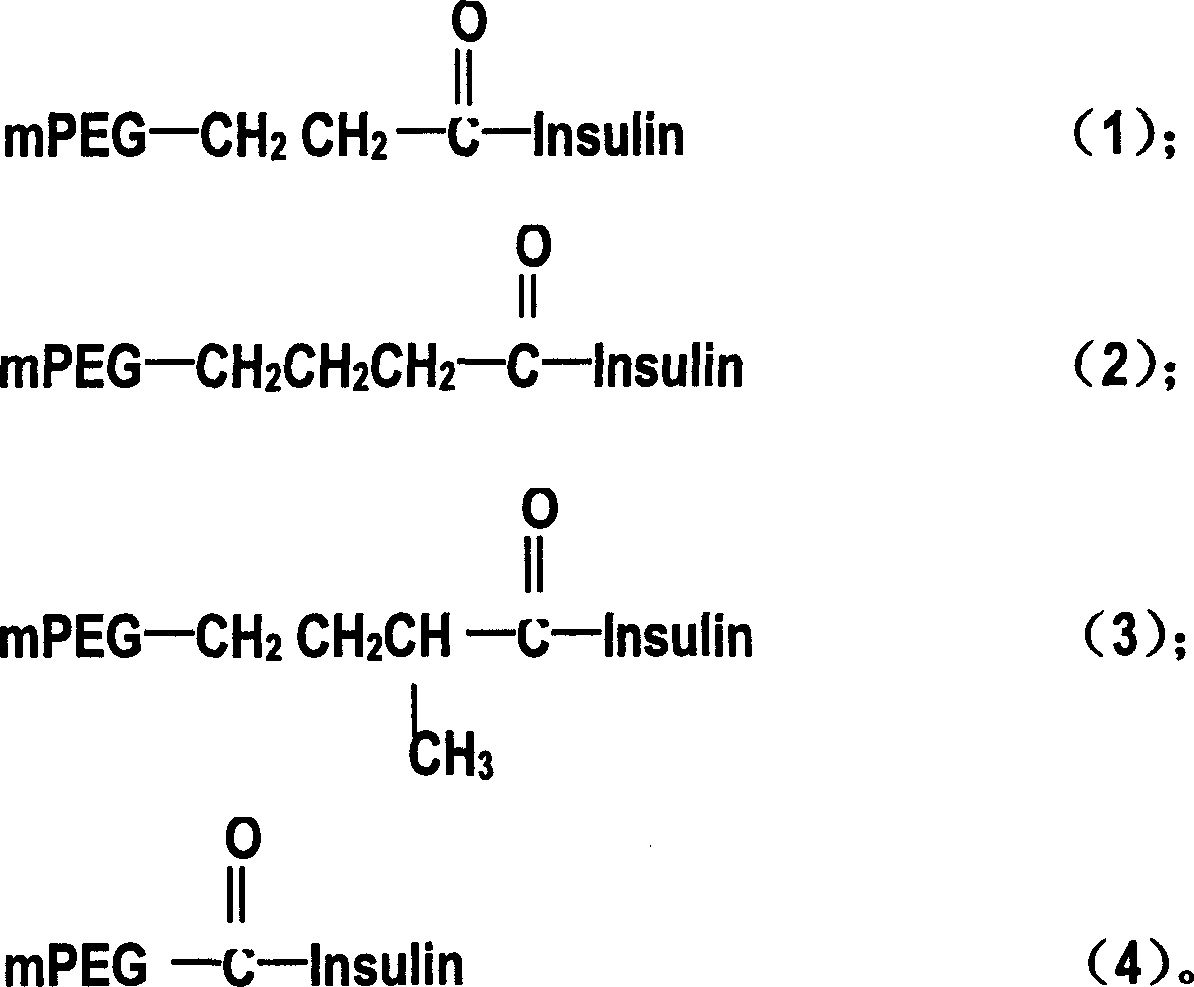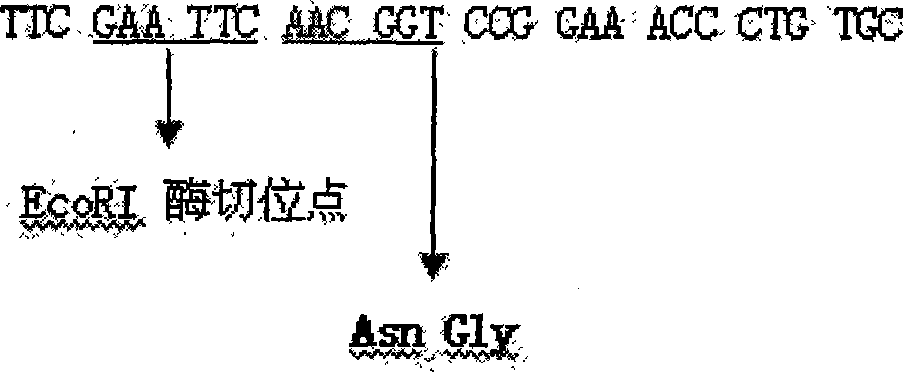Patents
Literature
68 results about "Recombinant Insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
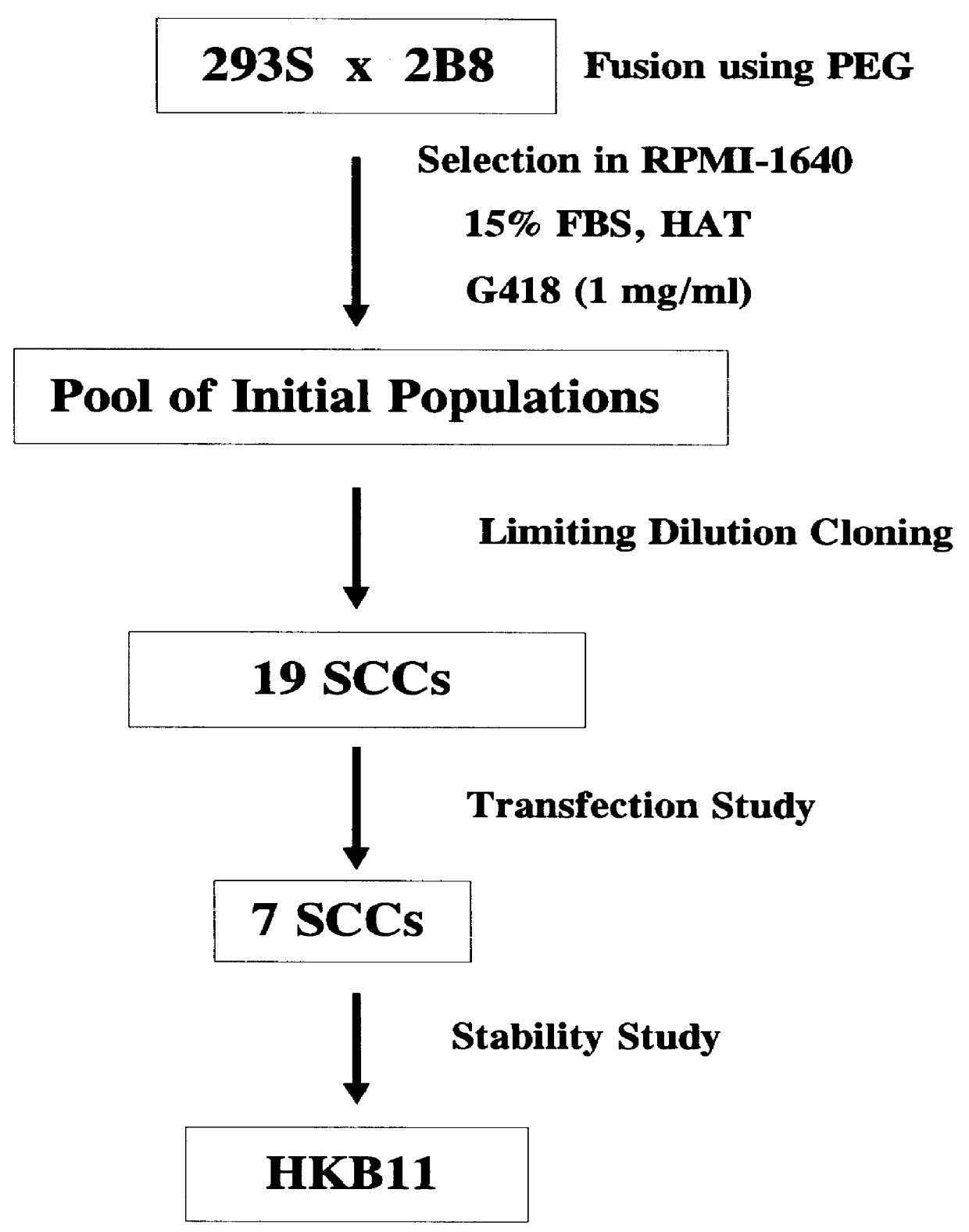
Human hybrid host cell for mammalian gene expression
InactiveUS6136599AEasily transfectedEasy to adaptGenetically modified cellsMutant preparationHeterologousMammal
Human / human hybrid cells were made via fusion of human embryonic kidney cells (293S) and modified Burkitt's lymphoma cells (2B8). The fusion cells are useful as host cells for the recombinant expression of mammalian genes. The advantages of using these hybrid clones of human kidney- and B-cells, called HKBs, for mammalian gene expression, include (i) the cells are negative for immunoglobulin expression, (ii) the cells grow easily in plasma protein-free medium (with or without the addition of recombinant insulin) as suspension cultures in a shake flask or in a fermenter (iii) the cells are very susceptible for transfection of DNA, and (iv) the cells secrete high levels of heterologous recombinant proteins, such as recombinant monoclonal antibodies, soluble ICAM-1, rIL-4, and rFVIII.
Owner:BAYER HEALTHCARE LLC +1
Method for preparing recombinant human insulin and analogs of recombinant human insulin
InactiveCN101519446AHigh expressionBacteriaMicroorganism based processesInsulin activityEscherichia coli
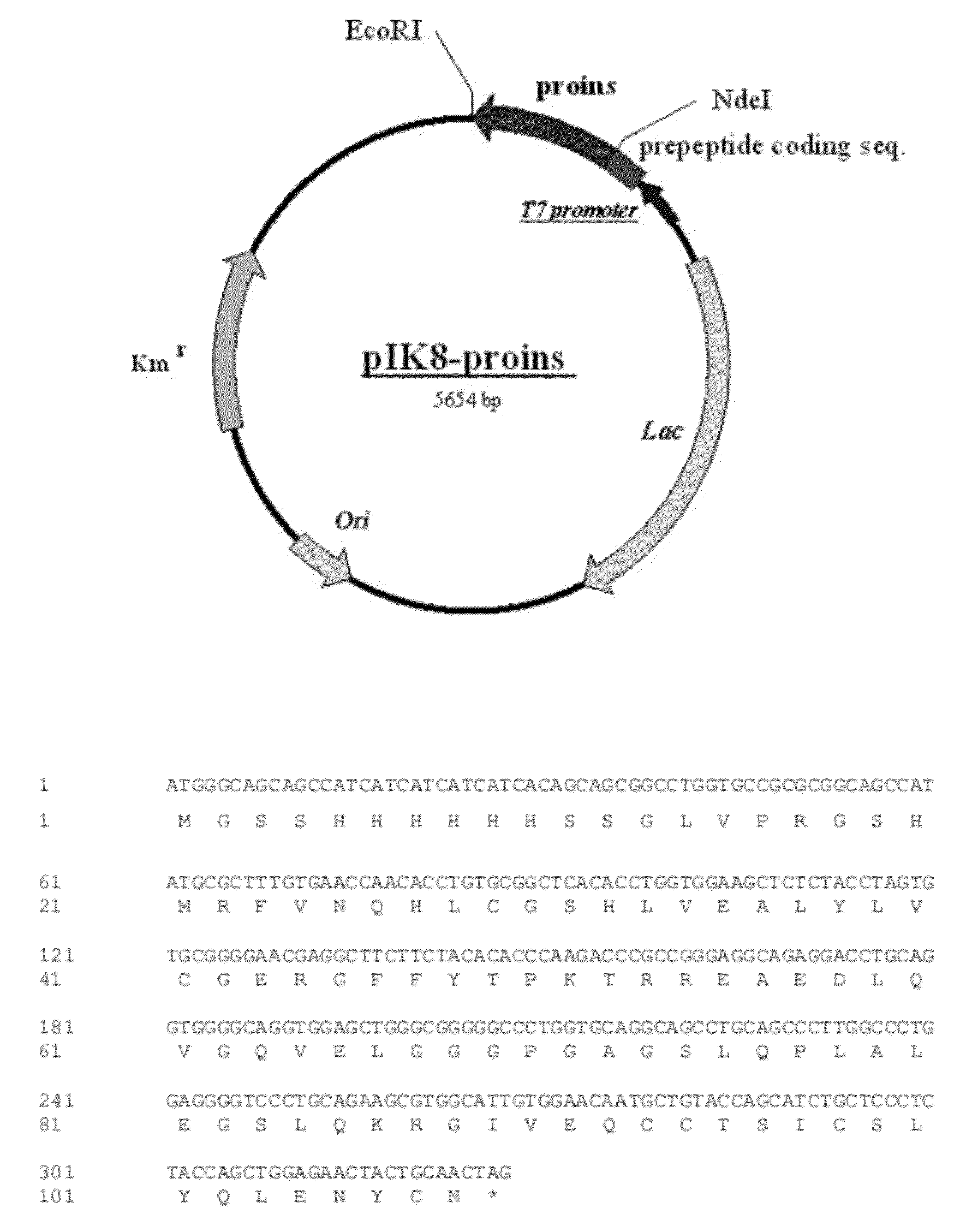
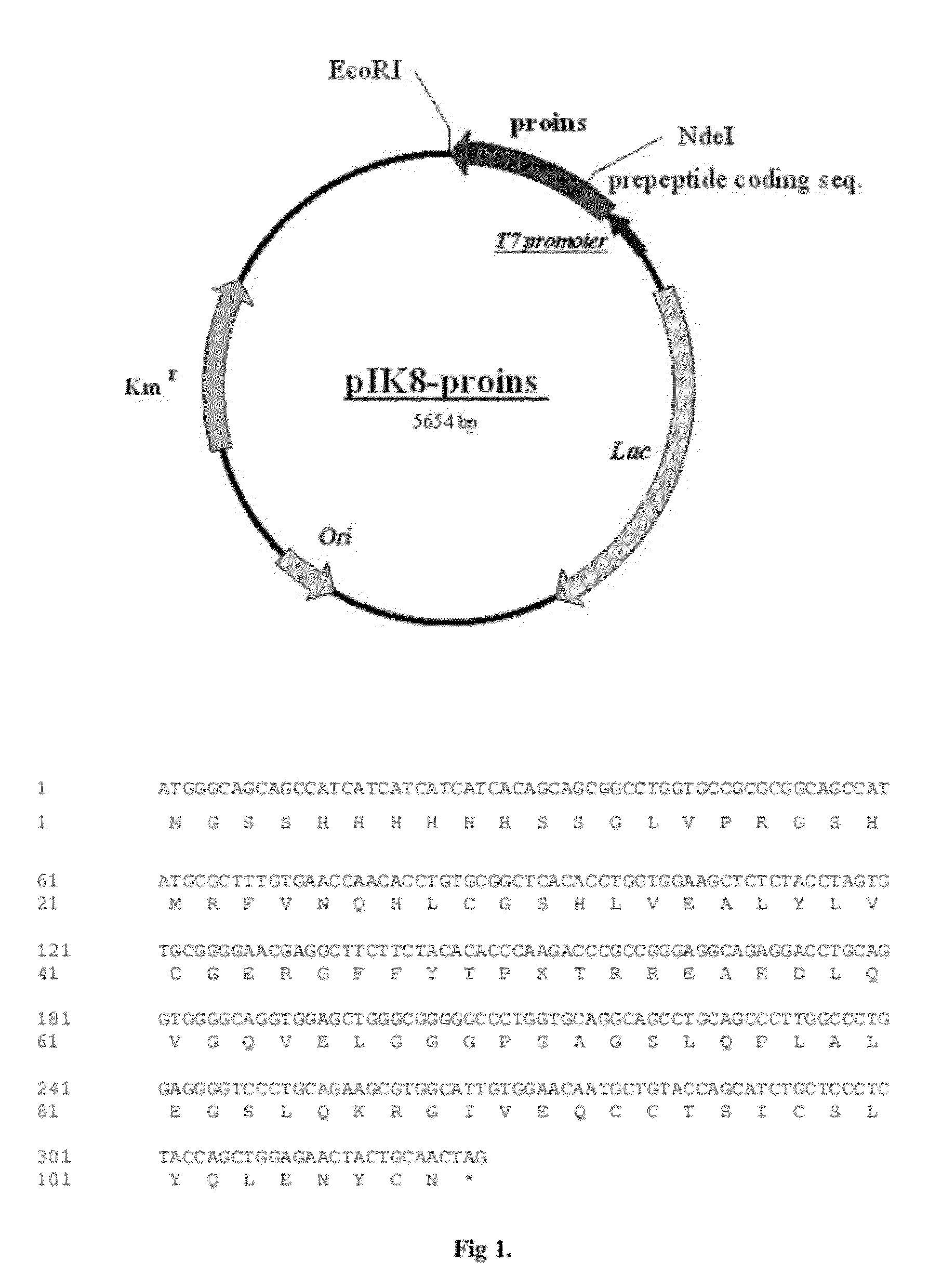
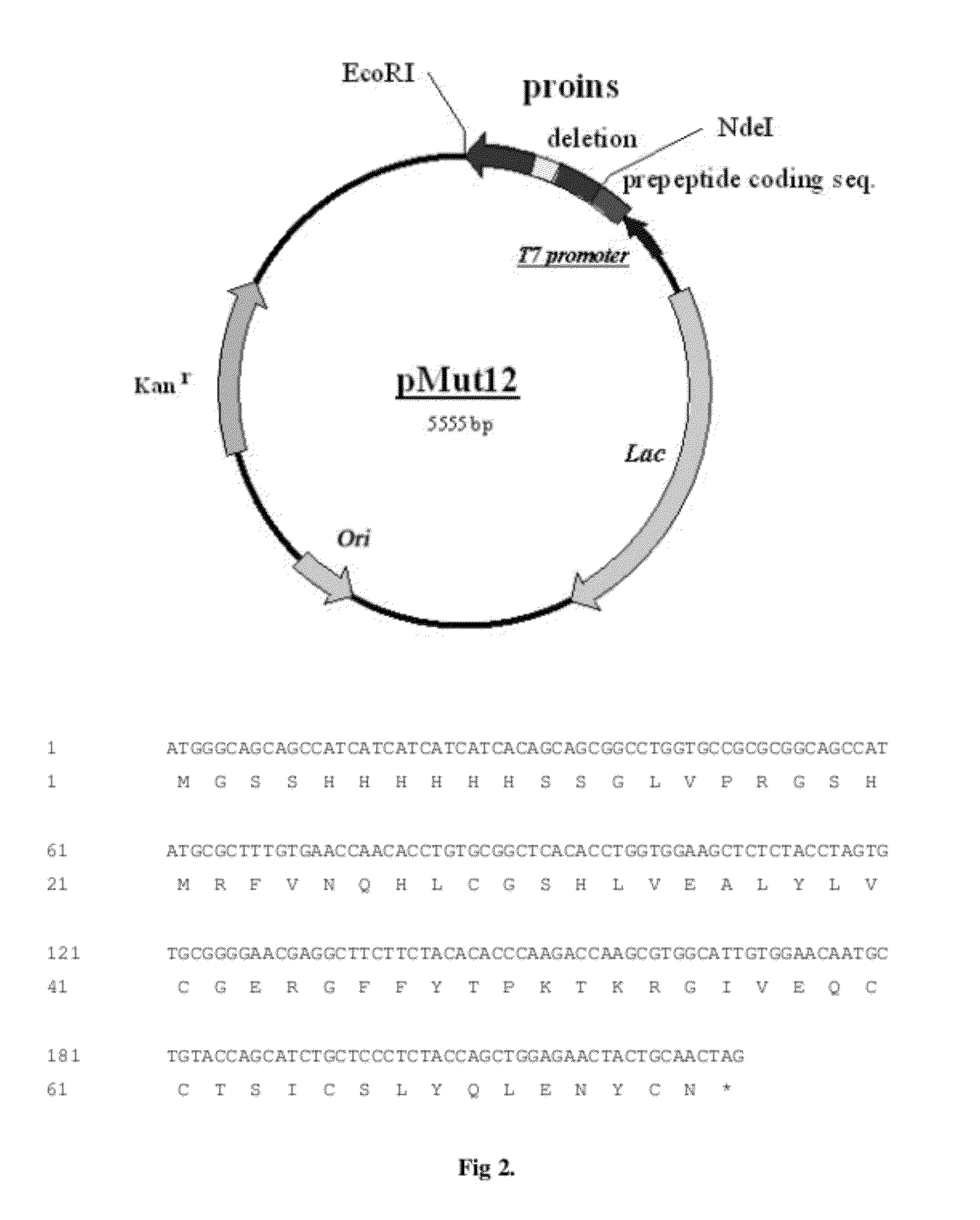
The invention provides a molecule (Preproinsulin) of human proinsulin with a novel N-terminal expressed peptide sequence or analogs of the human proinsulin, a method for producing human insulin by using the molecule, and processes for building related expression vectors and engineering cells and expressing and purifying human proinsulin. The DNA sequence of the human proinsulin coded by the N-terminal expressed peptide sequence or the analogs of the human proinsulin is first introduced into a prokaryotic expression vector and then transferred into an escherichia coli to express the molecule in form of an inclusion body. The invention has the advantages that: the product has high expression amount and is easy to purify; the preparation method avoids the use of CNBr; and the process for processing the recombinant insulin is simple.
Owner:AMTEK PHARMA
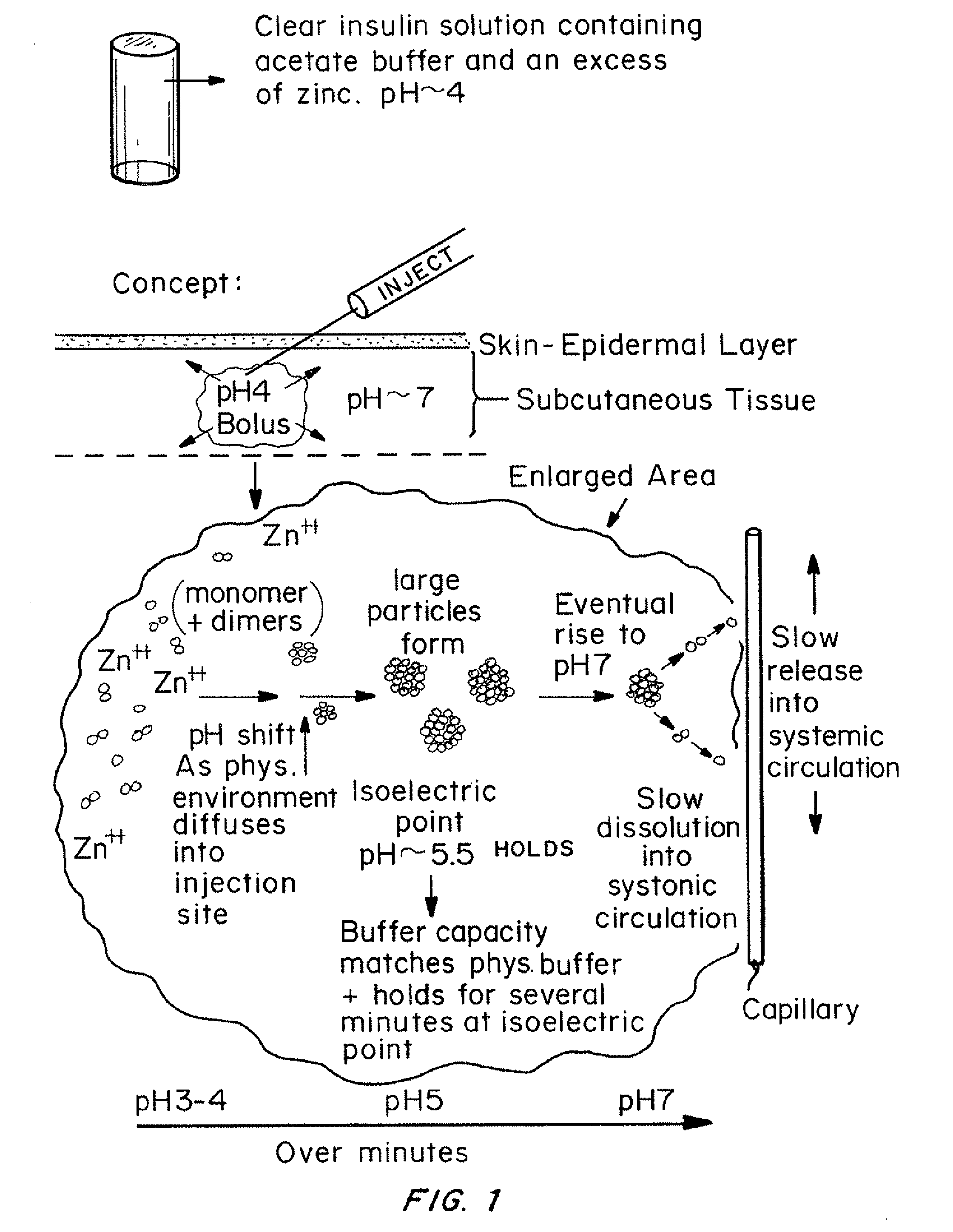
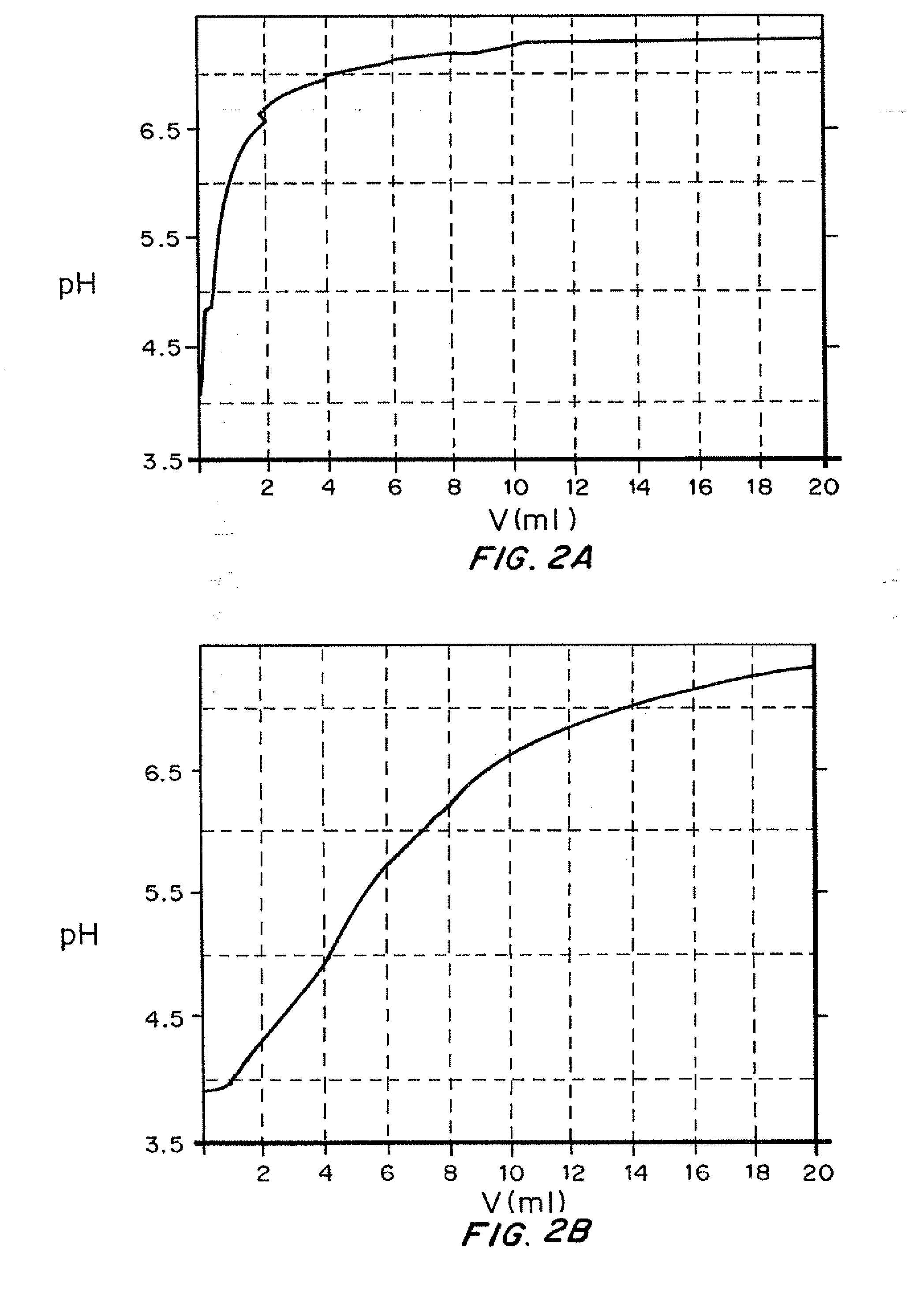
Insulin with a basal release profile
InactiveUS20100069292A1Not possibleAvoid the needPeptide/protein ingredientsInorganic non-active ingredientsBuffering agentINSULIN PREPARATIONS
A clear basal insulin formulation composed of insulin (preferably human recombinant insulin), buffering agents, precipitating agents, and / or stabilizing agents for subcutaneous, intradermal or intramuscular administration. The formulation is designed to form a precipitate of insulin following injection, creating a slow releasing “basal insulin” over a period of 12 to 24 hours, which can be varied by compositional changes to tailor the release profile to the needs of the individual diabetic patient
Owner:BIODEL
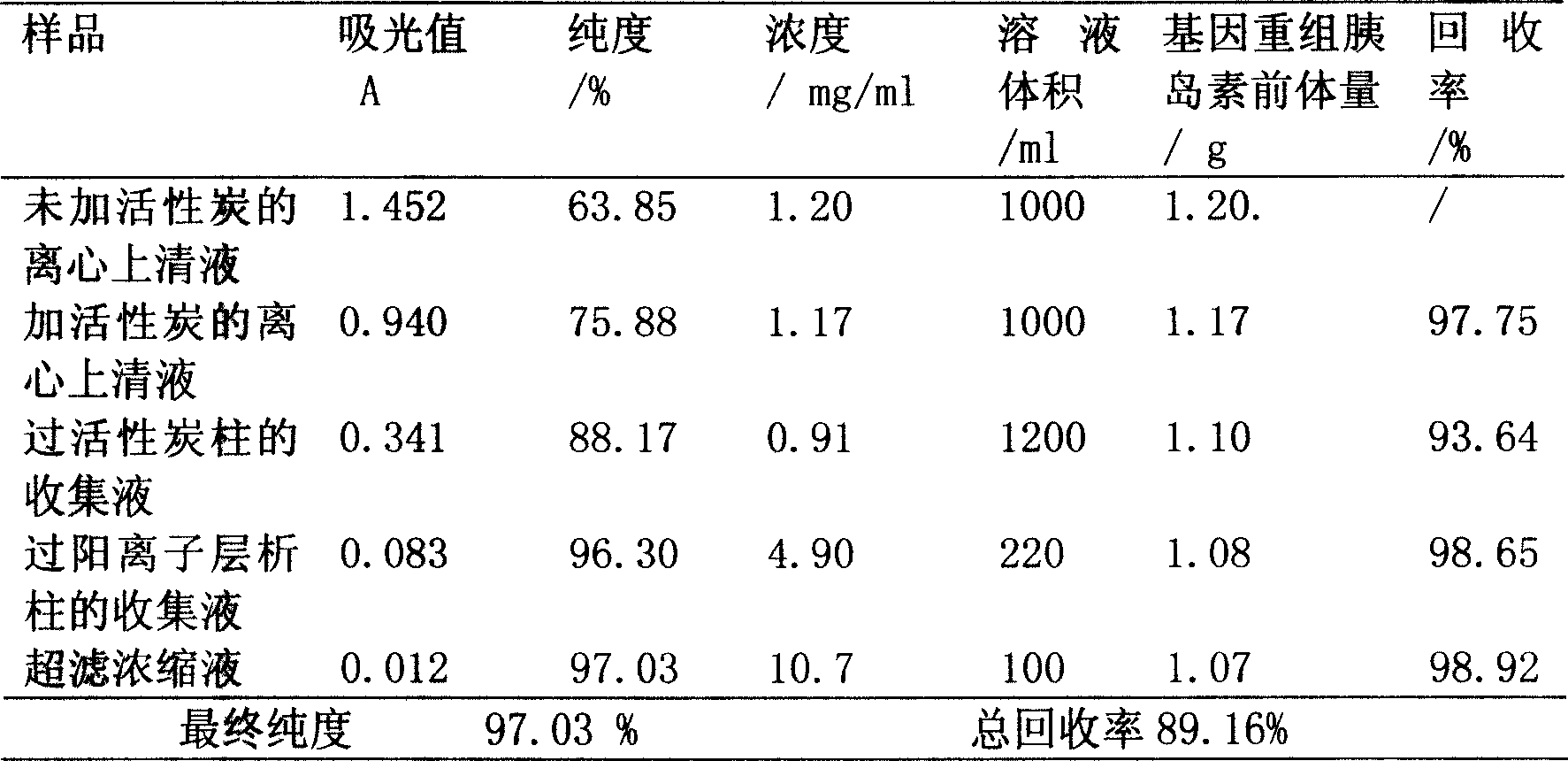
Method for purifying gene-recombinant insulin precursor
ActiveCN101029077AHigh recovery rateHigh purityFungiPeptide preparation methodsActivated carbonPichia pastoris
A process for purifying the genetically recombined insulin precursor features that the activated carbon as adsorbent and cationic chromatography are used to remove the pigment from fermented liquid and purify the active protein. Its recovery rate is more than 85% and its purity is more than 95%.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Human serum-free culture medium and preparation method thereof
ActiveCN102191215AExcellent proliferation rateImprove securityBlood/immune system cellsLipid formationCatalase
The invention discloses a human serum-free culture medium and a preparation method thereof. The human serum-free culture medium uses eleven raw materials, namely human serum albumin solution for treatment, human recombinant insulin solution, human transferrin solution, human cholesterol solution, human catalase solution, 2-mercaptoethanol solution, ascorbic acid solution, linoleic acid solution, ethanolamine solution, human vitronectin solution and L-glutamine solution; the added protein and lipid are both from the blood plasma, serum or tissue of human, the protein is pharmaceutical-grade orhighly purified human protein or human recombinant protein, the protein and lipid do not contains any animal component, the other components all meet the United States Pharmacopoeia or national standards; and the human serum-free culture medium is qualified through the cell culture test and is clinical, safe and reasonable human serum-free culture medium. The proliferation rate of the CIK cell cultured and inducted by the human serum-free culture medium is better than that of the CIK cell cultured and inducted by the culture medium with serum, the cell CD3+CD56+ percentage and the killing rate to the K562 leukemic cell are similar to that of the culture medium with serum. By adopting the human serum-free culture medium, the safety and standardization of cell therapy can be increased.
Owner:湘雅生物医药(湖州)有限公司
Preparation method for insulin aspart through recombinant expression by using yeast
ActiveCN105087724AImprove digestion efficiencyLow miscut rateMicroorganism based processesInsulinsEnzyme digestionThreonine
The invention discloses a preparation method for insulin aspart through recombinant expression by using yeast, and concretely relates to a preparation method for insulin aspart through recombinant expression by using pichia yeast. Concretely, the method comprises the following technological process: effectively secreting and expressing human aspart proinsulin, performing lysyl endopeptidase single enzyme digestion to obtain insulin aspart deleting B30, coupling with a threoninate, and performing deprotection, anti-phase purification and crystallization. The method is relatively suitable for industrialized preparation of recombinant insulin aspart.
Owner:CHONGQING PEG BIO BIOTECH CO LTD
Oral insulin compound medicine preparation and its preparing method
ActiveCN101062408AReduce degradationPrevent drug loading capacity from decreasingPeptide/protein ingredientsMetabolism disorderCalcium biphosphateMonomethoxypolyethylene glycol
The invention discloses an oral insulin complexing preparation, which is characterized by the following: allocating 1-15% insulin raw material, 50-97% stable protecting agent, 1-15% carrying medicine carrier, 1-20% absorption promoting agent; setting the stable protecting agent as one or several of carbowax and casein, protamine, albumin and protease inhibitor; setting the carrying medicine carrier as calcium phosphate salt; setting the absorption promoting agent as one or several of salicylic acid, cholate and fatty acid; setting the insulin raw material as human retooling insulin or human retooling insulin chemical trimmed by single methoxy carbowax derivant. This invention also relates to a preparing method of oral insulin complexing preparation. This invention can increase biostability of human retooling insulin and can resist degradation of gastrointestinal tract enzyme.
Owner:TONGHUA GOLDEN-HORSE PHARM IND CO LTD
Method for determining bioactivity of recombinant insulin secretagogue and application thereof
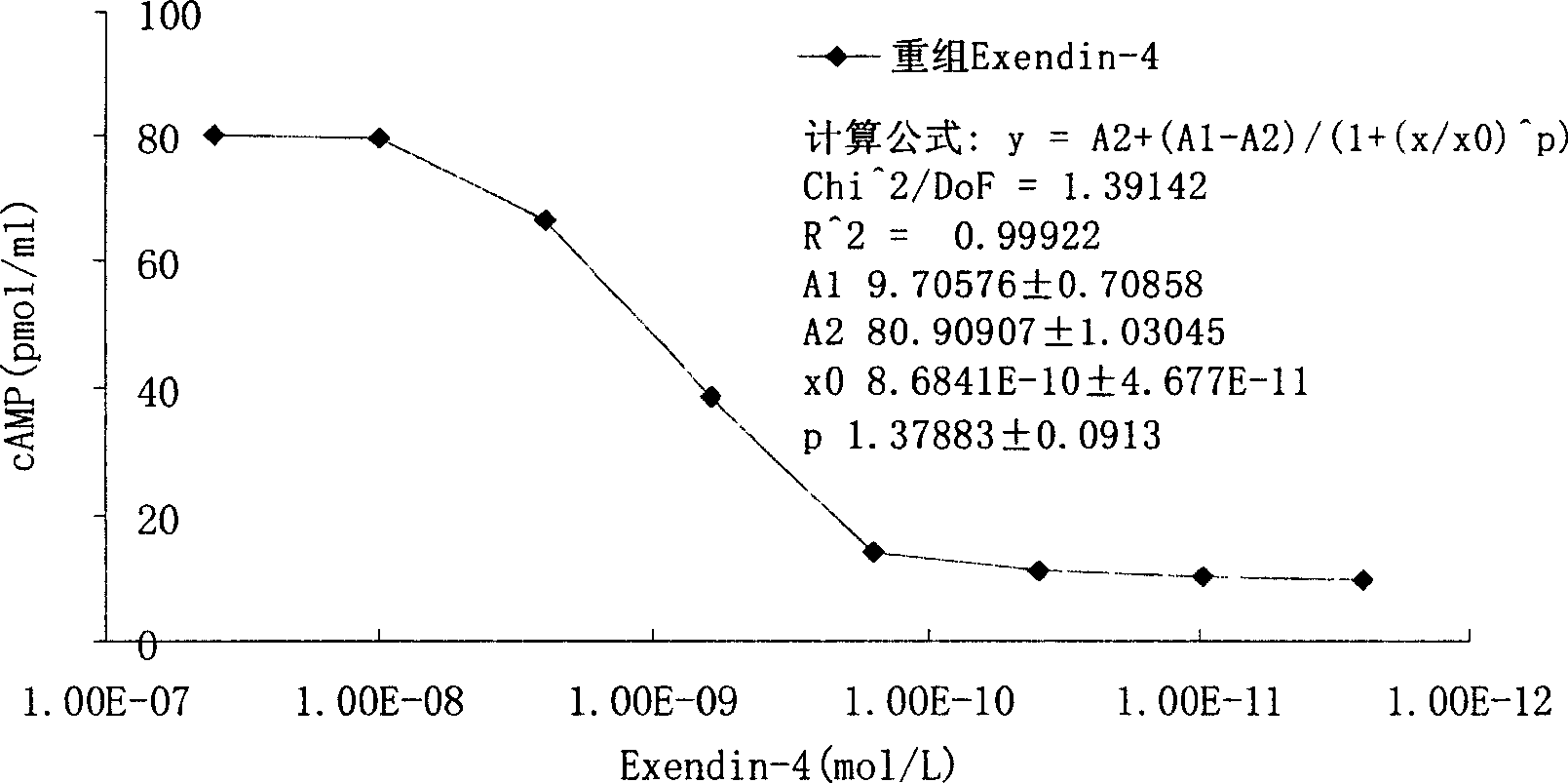
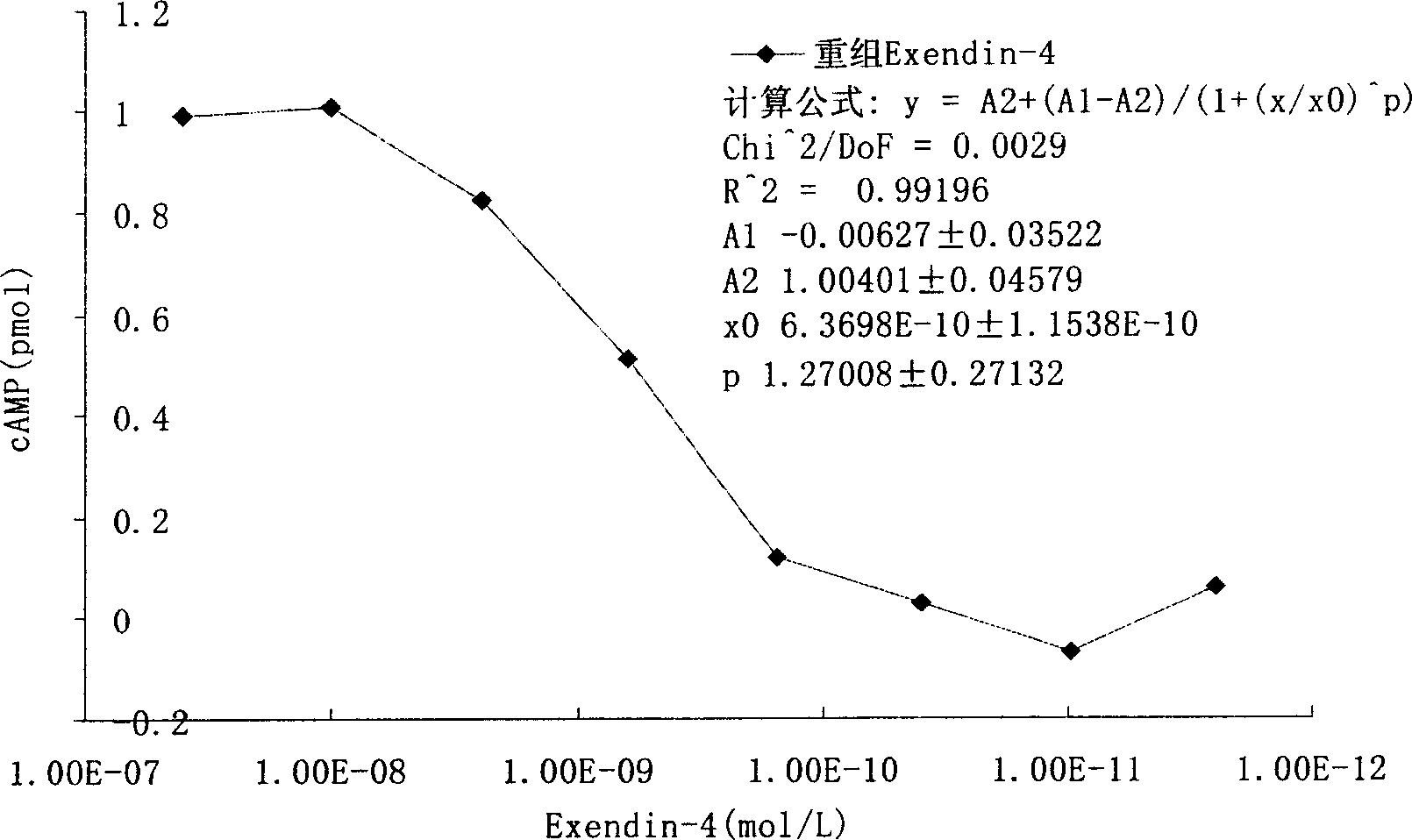
InactiveCN1844925ASimple stepsEasy to handlePeptide/protein ingredientsMetabolism disorderHormones regulationDose dependence
The invention discloses a method for testing the biological activity of recombination insulinotropic hormone (Exendin-4) and relative application. Wherein, it comprises: using Exendin-4 to activate the cell to make cAMP excrete; the cAMP of cell will be increased quickly; using enzyme immunity method to test the cAMP content in the supernate of cracked cell and the cAmp content has dosage dependency in the supernate of cracked cell, to calculate the biological activity of Exendin-4, while the test cell is RIN-5F cell. The invention can be used to control the quality of recombination insulinotropic hormone (Exendin-4). The invention uses enzyme immunity method to test the biological activity, with simple process, less waste, high accuracy and better safety.
Owner:DONGGUAN TAILI BIOTECH
Process for producing recombinant insulin-like growth factor-1(IGF-1) amalgamation protein
InactiveCN101429519AEasy to placePromote formationBacteriaMicroorganism based processesBiotechnologyHydroxylamine
The invention discloses a method for preparing recombined human insulin growth factor-1(IGF-1) fused protein, which belongs to the field of biological medicine field in biological technique. The invention adopts the genetic engineering fungus fermentation method for production: a. designing and synthesizing recombining human IGF-1 fused protein gene; b. constructing an expression vector of the recombined human IGF-1 fused protein; c. utilizing the expression vector to convert the host to construct the genetic engineering fungus; and d. utilizing the genetic engineering fungus to ferment the recombined human IGF-1 fused protein. In the method, according to the characteristics that the first amino acid of the natural IGF-1 is glycine(GLy, G), a leading peptide is added before the natural IGF-1, and a hydroxylamine specific cracking part(Asn-Gly peptide bond) is designed between the leading peptide and the natural IGF-1, thereby making the expression products stable and lowering purification cost. In the method, the fused protein in serial expression is used, wherein the N end is thioredoxin, and the C end is IGF-1, so that the expression products are more stable, and the separation method is simple and cheap. The method has wide application prospect.
Owner:FUJIAN MEDICAL UNIV
Human in-vitro seminal liquid and preparing method thereof
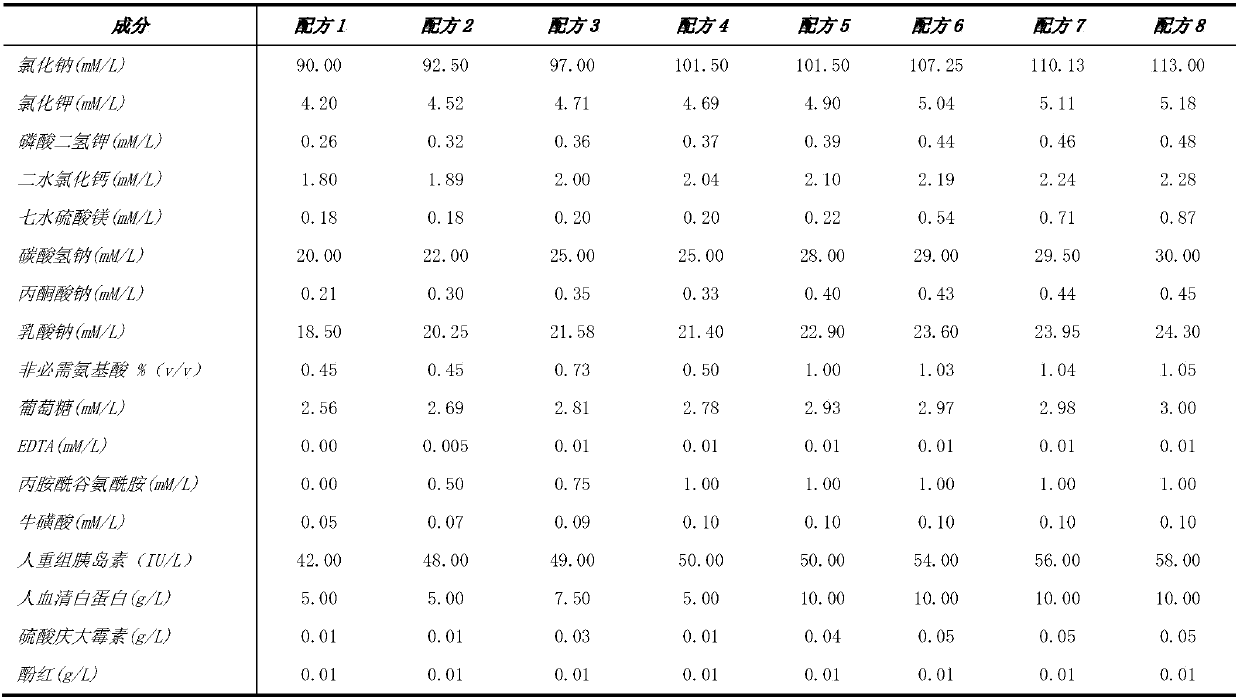
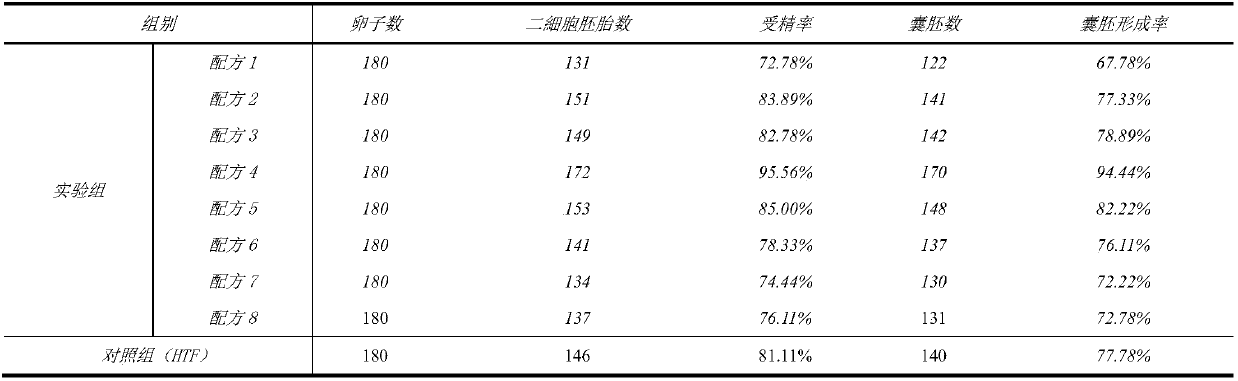
The invention discloses a human in-vitro seminal liquid. The human in-vitro seminal liquid comprises 90-113 mM / L of inorganic salt of sodium chloride, 4.20-5.18 mM / L of potassium chloride, 1.80-2.28 mM / L of calcium chloride dihydrate, 0.26-0.48 mM / L of monopotassium phosphate, 0.18-0.87 mM / L of magnesium sulfate heptahydrate, 20-30 mM / L of pH buffering agent sodium bicarbonate, 0.01-0.05 g / L of bacteriostat component gentamicin sulphate, 0.21-0.45 mM / L of energy substance sodium pyruvate, 18.50-24.3mM / L of L-sodium lactate, 2.56-3.00 mM / L of glucose, 0.45-1.05%(v / v) of other components of non-essential amino acid, 0-1.0 mM / L of glutamine dipeptide, 0-0.01 mM / L of ethylene diamine tetraacetic acid, 0.05-0.10 mM / L of taurine, 5-10 g / L of human serum protein and 42-58 IU / L of human recombinant insulin.
Owner:SHENZHEN VITAVITRO BIOTECH CO LTD
Method for producing human recombinant insulin
InactiveUS20120058513A1Increase productionQuality improvementPeptide/protein ingredientsRecombinant DNA-technologyInclusion bodiesInsulin Precursor
The invention relates to biotechnology and can be used for producing human recombinant insulin for preparing medicinal agents for the treatment of pancreatic diabetes. A variety of recombinant plasmid DNAs which contain an artificial gene and encode the human insulin precursor is proposed. The biosynthesis of a hybrid polypeptide is induced using isopropyl-thiogalactopyranoside so that the post-induction level of the hybrid polypeptide is equal to or greater than 25% of the total cellular protein. According to the claimed procedure, human insulin is produced by cultivating a producer strain containing one of the recombinant plasmids, isolating inclusion bodies, solubilizing and renaturing the fusion protein, and enzymatically degrading and chromatographically purifying said protein. The invention simplifies the process for producing human recombinant insulin and increases the yield thereof.
Owner:LIABILITY MAKO
Non-serum non-animal-origin-additive insect cell culture medium
The invention relates to the field of cell culture medium, and in particular to a non-serum non-animal-origin-additive insect cell culture medium. The medium comprises mainly the following components: basic culture medium, glucose, inorganic salt, vitamins, L-arginine, L-agedoite, L-glycocoll, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-threonine, L-tryptophan, L-valine, L-proline, L-glutamine, yeast extracts, recombulin, malic acid, allomaleic acid, cholesterol, linoleic acid, granulesten, putrescine, glutathione, glycerol and fructosan. The culture medium can promote the growth of insect cell and is suitable for the large scale breeding of insect cell and the expression of recombination protein.
Owner:严志海
Recombinant insulin and insulin analogue precursor purification method
InactiveCN105153294AGood technical effectAvoid time costPeptide preparation methodsInsulinsPurification methodsCentrifugation
The invention relates to the field of insulin production methods and discloses a recombinant insulin and insulin analogue precursor purification method. The invention solves the problems that conventional chromatographic packing cannot tolerate high-salinity sample loading, the amount of used organic reagent is great, the cost is high and the product purity is not high in the purification process of recombinant expressed insulin precursors and insulin analogue precursors. The invention adopts the technical scheme that the method comprises the steps of performing pH regulation and centrifugation to centrifuged fermentation supernatant, then directly loading a sample and performing adsorption, separation, purification and elution through a chromatographic column prepared by using any packing of Capto S, Capto MMC, Uni PMM S and Uni MSP to finally obtain high-purity recombinant insulin and insulin analogue precursors. Compared with the existing purification method, the recombinant insulin and insulin analogue precursor purification method has the advantages that the operation is simple, the yield is high, the spent time is short, the environmental influence is small, and the product production cost of the existing insulin and insulin analogues can be greatly reduced.
Owner:JINAN KANGHE MEDICAL TECH
Clinical applicable culture system for efficient amplification of NK cells
InactiveCN104152412AAvoid uncertaintySimple ingredientsBlood/immune system cellsSodium bicarbonateWhite blood cell
The invention relates to a clinical applicable culture system for efficient amplification of NK cells. The invention specifically provides a serum-free culture system for efficient amplification of NK cells. The culture system comprises the following components: 1640 medium, sodium bicarbonate, human serum albumin, transferrin, linoleic acid, oleic acid, palmitic acid, sodium pyruvate, human recombinant insulin, non-essential amino acids, 2-mercaptoethanol and interleukin-2. The invention also provides a culture system containing 1-12 vol.% of human autoserum and for efficient amplification of NK cells. The culture system provided by the invention has simple composition, avoids the risks of animal ingredients in animal serum on cell treatment and uncertainty of cell culture caused by the uncertain components in the animal serum, and increases yield of NK cell. The obtained activated NK cells have significant tumor killing effect. Therefore, the system can not only be applied in scientific research but also be used in clinical treatment.
Owner:CYAGEN BIOSCI INC
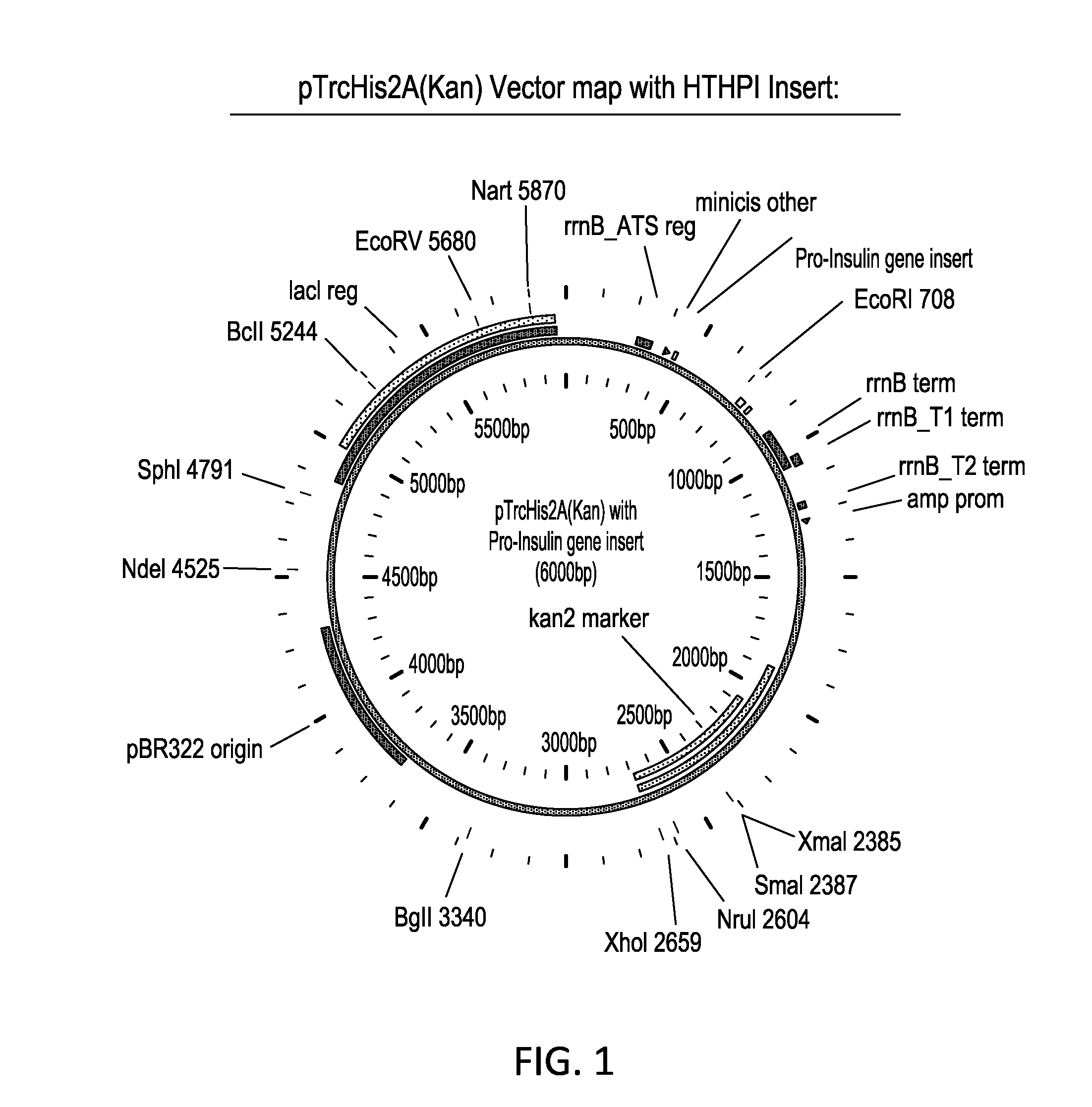
Vector for expression of heterologous protein and methods for extracting recombinant protein and for purifying isolated recombinant insulin
InactiveUS6068993AFast degenerationOvercome problemsBacteriaInsulinsBacteroidesOrigin of replication
The present invention relates to a vector for expression of a heterologous protein by a Gram negative bacteria, wherein the vector includes a nucleic acid such as DNA encoding the following: an origin of replication region; optionally and preferably a selection marker; a promoter; an initiation region such as translation initiation region and / or a ribosome binding site, at least one restriction site for insertion of heterologous nucleic acid, e.g. DNA, encoding the heterologous protein, and a transcription terminator. The inventive vector may contain DNA encoding the heterologous protein, e.g., pro-insulin such as pro-insulin with a His tag. Additionally, the invention provides a method for extracting a recombinant protein from within a recombinant Gram negative bacteria having a cell membrane, without lysing the bacteria, as well as a method for purifying an isolated recombinant human insulin, wherein the isolated recombinant human pro-insulin is subjected to sulfitolysis, Ni-chelation chromatography, renaturation, limited proteolysis and chromatography separation to provide purified, isolated, recombinant human insulin.
Owner:BIOMM +1
Serum-free culture medium for VERO serum-free cell culture and corresponding virus production
InactiveCN110628697ADoes not increase the percentage of apoptoticReduce manufacturing costCulture processMicroorganism based processesApoptosisTryptophan
The invention discloses a serum-free culture medium for VERO serum-free cell culture and corresponding virus production. The culture medium comprises the following components: amino acids, vitamins, inorganic salts, auxiliary components and proteins. The amino acids comprise glycine, alanine, arginine hydrochloride, cystine dihydrochloride, glutamic acid, glutamine, histidine hydrochloride monohydrate, isoleucine, leucine, lysine hydrochloride, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine disodium salt dihydrate, valine, hydroxyproline, ornithine and taurine. Theproteins comprise human transferrin and recombinant insulin. When being used for VERO cell culture, the culture medium does not depend on the use of animal serum, does not introduce animal-derived protein, greatly lowers the production cost, sufficiently ensures the stable product quality, and can implement long-time culture and passage without increasing the apoptosis ratio.
Owner:山东巨山能源科技有限公司
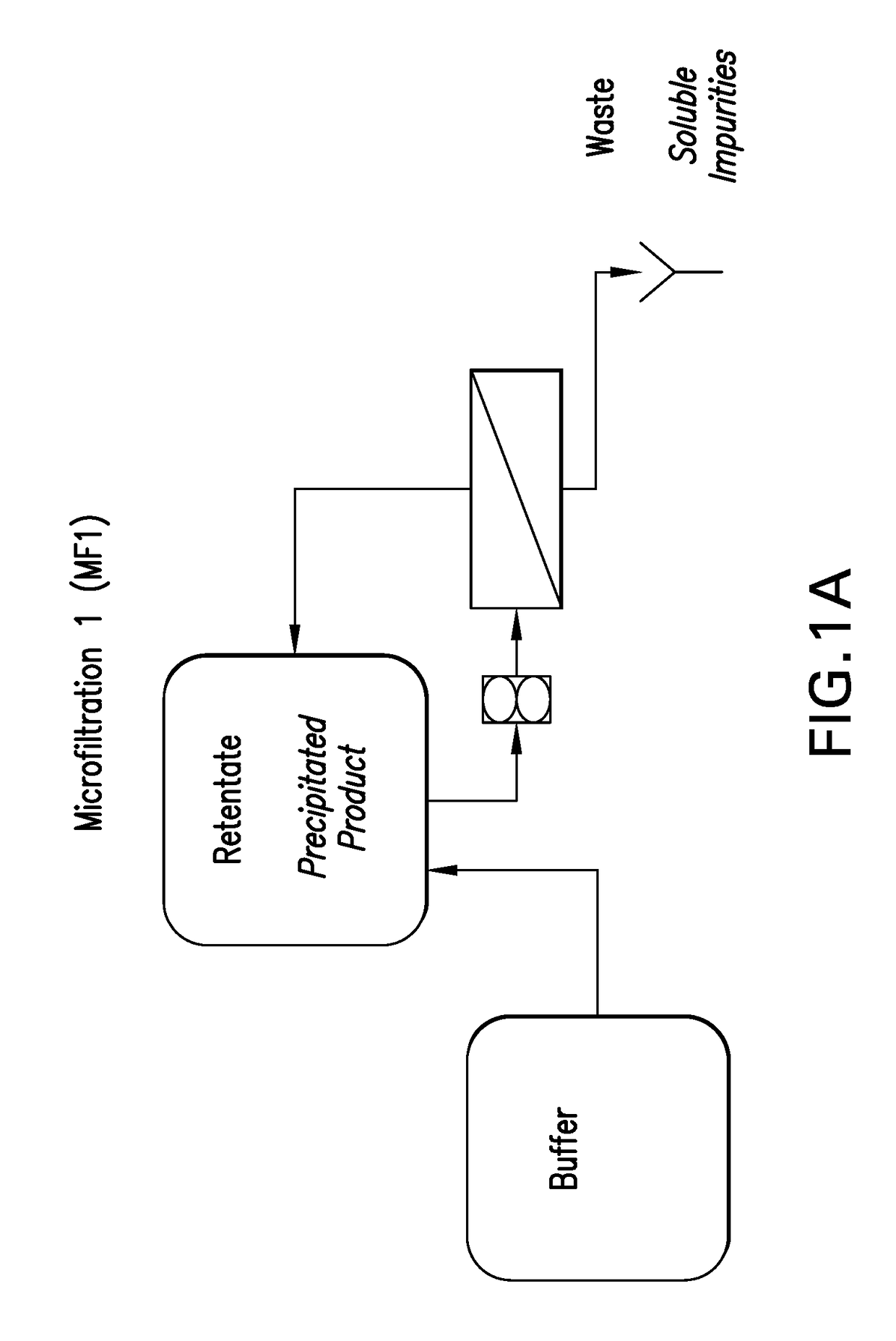
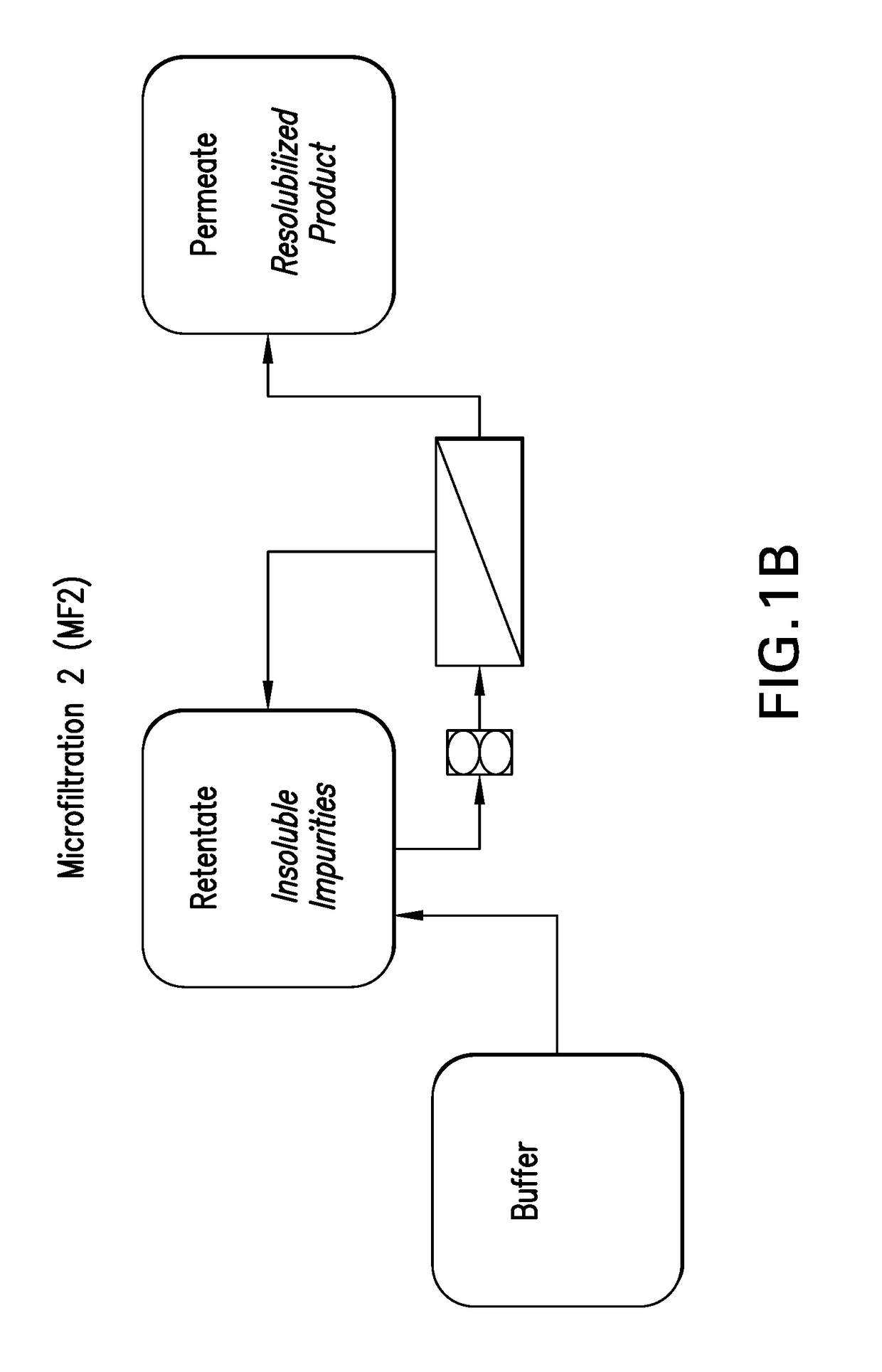
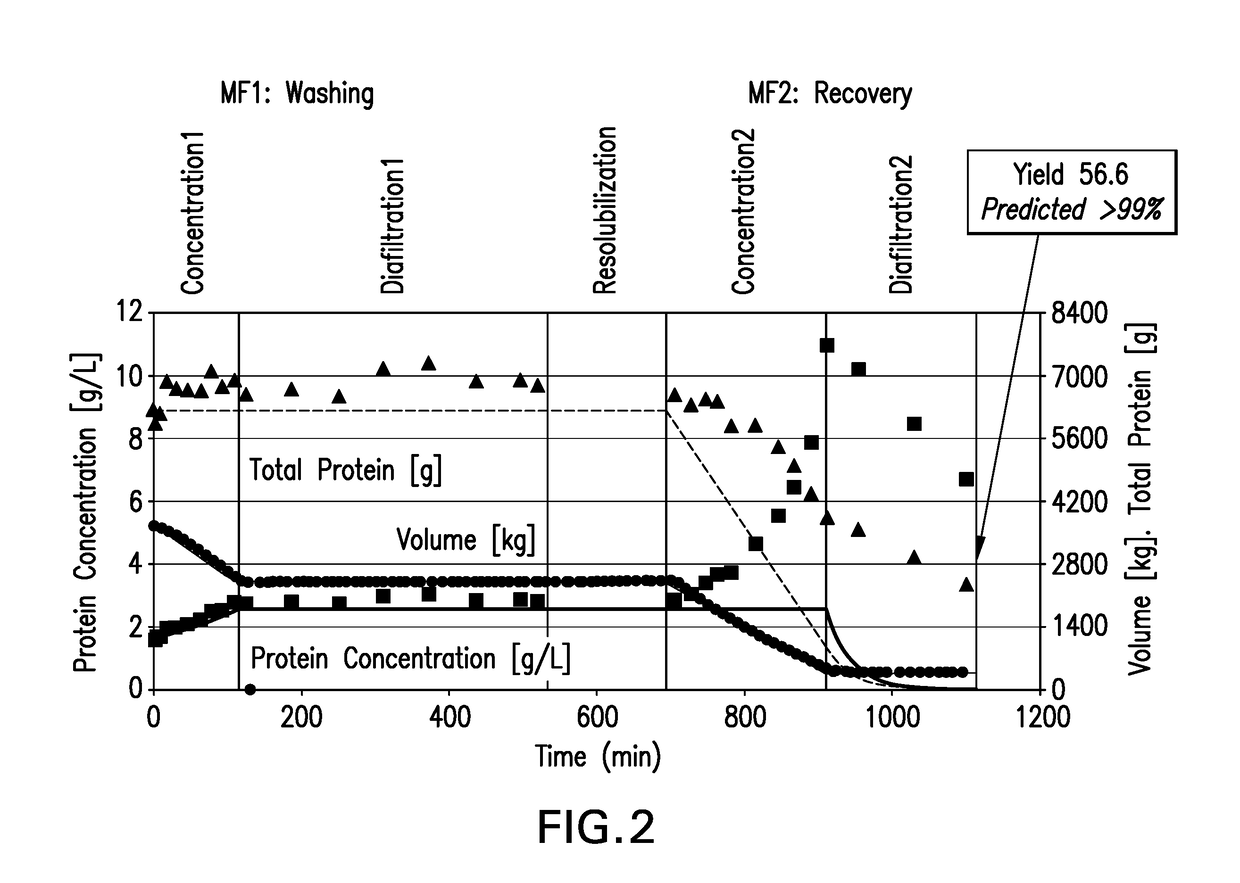
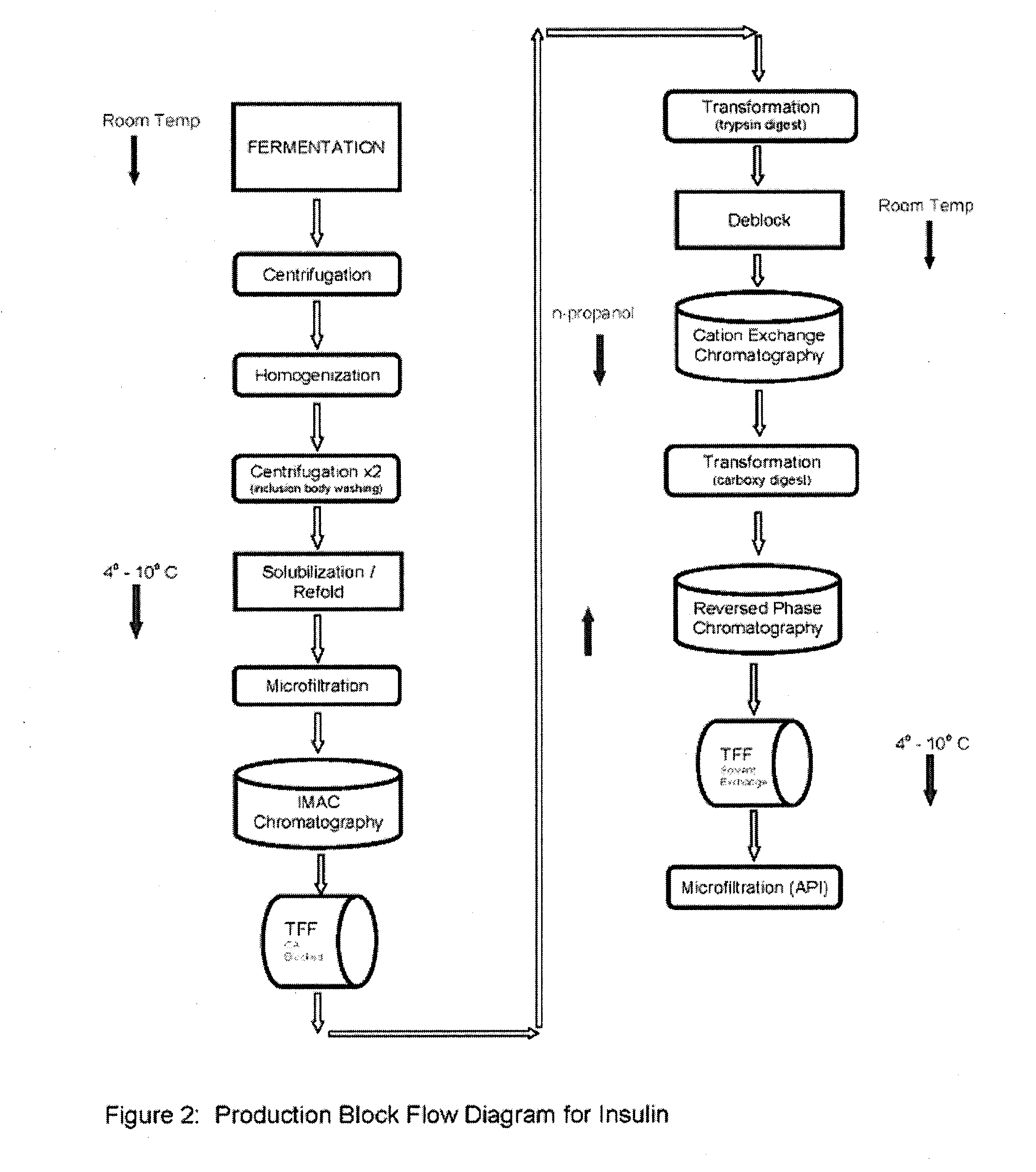
Process for preparing recombinant insulin using microfiltration
ActiveUS20180044395A1Improve purification effectReduce the presence of impuritiesPeptide preparation methodsInsulinsInsulin productsUnit operation
The use of two tandem microfiltration (MF) steps in a process for making recombinant insulin is described. The two MF steps in a single downstream purification unit operation reduce both soluble and insoluble impurities and exchange the insulin product into a suitable buffer for downstream purification.
Owner:MERCK SHARP & DOHME LLC
Low serum medium for full-suspending culture of MDCK cells
InactiveCN105567628AHigh densityLow priceCulture processEpidermal cells/skin cellsHydrolysateAmmonium metavanadate
The invention provides a low serum medium for full-suspending culture of MDCK cells. The medium is prepared from 5,000 mg / L to 6,500 mg / L of DMEM / F12 medium, nickel chloride NiCl2.6H2O, ammonium heptamolybdate (NH4) 6Mo7O24.4H2O, stannous chloride SnCl2.2H2O, ammonium metavanadate NH4VO3, sodium silicate Na2SiO3.9H2O, vitamin A, vitamin E, human transferrin, recombulin, glutathione, sodium selenite, vegetable protein hydrolysate, recombinant human serum albumin and PF68. New-born calf serum with the volume percent fraction of 3% to 5% is added to the medium, the medium is inoculated with the MDCK cells, cell density can be increased to 421*106 / ml, and the cell activity is still 92.3% at this moment.
Owner:令世鑫 +3
Skeletal muscle stem cell serum-free medium and preparation method and application thereof
ActiveCN106497872AHigh purityHigh amplification efficiencyCulture processCell culture mediaSerum free mediaInstability
The invention discloses a skeletal muscle stem cell serum-free medium and a preparation method and application thereof. The skeletal muscle stem cell serum-free medium, with the pH value ranging from 7.4 to 7.4, comprises DMEM(dulbecco's modified eagle medium) / F12, serum replacement, cytokines, vitamins, amino acid, recombinant human insulin, mepiquat chloride and 6-chondroitin sulfate. The invention further discloses the preparation method of the skeletal muscle stem cell serum-free medium. Specifically, the serum replacement refers to animal-free component which is defined, thus, serum caused pathogenic risk and instability of different batches and the like are avoided. The skeletal muscle stem cell serum-free medium is applicable to human skeletal muscle stem cell primary culture and scaled amplification culture and has the advantages of high purity, quick cell proliferation, high expansion efficiency, capability of realizing steady passage of at least 20 generations and the like. In addition, the skeletal muscle stem cell serum-free medium effectively improves skeletal muscle stem cell expansion efficiency and purity and can serve as an in-scale high-quality skeletal muscle stem cell culture system to meet requirements for clinical research and application.
Owner:北京欣博睿丰医疗科技发展有限公司
Chromatographic purification method for acylated insulin
The invention discloses a chromatographic purification method for acylated insulin and belongs to the field of preparation of recombinant insulin or insulin analogs. According to the chromatographic purification method for acylated insulin, high-grade silica gel carriers (like C4, C8 and C18 alkanes taken as reversed phase filler of ligands) are taken as chromatographic filler, a high-pressure chromatographic system is used for performing fine separation and purification on acylated insulin, the purity of a coarse product can be improved from 50%-60% to 99.0% or higher in the alkaline eluent environment with pH (potential of hydrogen) being 6.5-8.0, and the yield of the purified sample can be higher than 90%. Besides, according to the method, the loading quantity of coarse samples is larger, and the purification cost is greatly saved; the linear flow speed is high in the chromatographic purification process, and the insulin analogs can be rapidly purified. The chromatographic purification method is simple, stable and reliable to operate, has higher amplification and is suitable for industrial preparation of the insulin analogs.
Owner:SHANDONG EHUA BIOLOGICAL PHARMA +1
Aminotic cell culture medium for high-density cell culture system
InactiveCN106047798APromote rapid proliferationMassive proliferationCulture processCell culture mediaCell culture mediaHuman albumin
The present invention relates to an aminotic cell culture medium for a high-density cell culture system. The aminotic cell culture medium comprises a base culture medium and added components, wherein the base culture medium is a F12 culture medium, and the added components comprise fetal bovine serum with a concentration of 80-120 ml / L, human recombinant insulin with a concentration of 24-30 mg / L, human recombinant epidermal growth factor with a concentration of 20-25 [mu]g / L, human recombinant basic fibroblast growth factor with a concentration of 35-45 [mu]g / L, human albumin with a concentration of 10-15 g / L, transferring with a concentration of 4-16 mg / L, Hydrocortisone with a concentration of 0.2-1 mg / L, Testosterone with a concentration of 0.2-1 mg / L, progesterone with a concentration of 0.2-1 mg / L, vitamin E with a concentration of 1-5 mg / L, L-glutamine with a concentration of 12-20 g / L, HEPES with a concentration of 5-10 g / L, and a block polyether F-68 with a concentration of 0.5-2 g / L, wherein the concentrations of each added component adopt the total volume of the aminotic cell culture medium as the reference. Compared with the aminotic cell culture medium in the prior art, the aminotic cell culture medium of the present invention has advantages of rapid and large-scale aminotic cell proliferation, good proliferation effect and short culture time, and is particularly suitable for the high-density cell culture system of the aminotic cells.
Owner:SHANGHAI XP BIOMED
Recombinant insulin secretion promoter fusion protein and its preparation method and use
ActiveCN105254763AGood effectExtended half-lifePeptide/protein ingredientsMetabolism disorderHalf-lifeInsulin secretion
The invention relates to a recombinant insulin secretion promoter fusion protein. A polypeptide zone of the recombinant insulin secretion promoter fusion protein comprises a human serum albumin and an insulin secretion promoter. The insulin secretion promoter in a form of one or more monomers is connected to the human serum albumin in series by a connecting peptide. An amino terminal and / or a carboxyl terminal of the insulin secretion promoter are connected to a carboxyl terminal and / or an amino terminal of the human serum albumin by a connecting peptide. The number of the amino acid sequences for coding the connecting peptides is equal to the number of the amino acid sequences for coding the insulin secretion promoter. The recombinant insulin secretion promoter fusion protein has the advantages of good drug effects, long half life, simpleness, high efficiency, safety and good application prospect.
Owner:CHENGDU JINXINHENG BIOTECH CO LTD
Liquid insulin compositions and methods of making the same
Disclosed herein are novel and improved preparations and methods for manufacturing substantially liquid preparations of recombinant human insulin API. The purified recombinant human insulin Active Pharmaceutical Ingredient (API) preparations are substantially free of by-products associated with the lyophilization and / or crystallization. The methods for manufacturing the substantially liquid recombinant human insulin API preparations are provided with optional steps for subjecting the recombinant insulin preparation to lyophilization and / or crystallization. Enhanced yield of recombinant insulin of greater purity are thereby provided according to the present invention. Highly purified formulations of recombinant human insulin of the API insulin preparations disclosed herein are also provided. Stably transformed E. coli cell banks (WCB) capable of expressing the recombinant human insulin are also provided.
Owner:ELONA BIOTECH
Human umbilical cord mesenchymal stem cell serum-free medium
PendingCN109402051AControl infection riskDoes not cause immune rejectionCulture processSkeletal/connective tissue cellsSerum free mediaVitamin C
The invention provides a human umbilical cord mesenchymal stem cell serum-free medium characterized by comprising an alpha-MEM basal medium and further comprising the following components by final concentration: 1-20 mM of glutamine, 1-20 mM of HEPEs, 10-100 muM of putrescine, 0.1-10 muM of transferrin, 10-400 muM of vitamin C, 1-10 muM of recombinant insulin, 1-20 nM of progesterone, 10-200 nM ofcortisol, 1-20 mg / mL of human serum albumin, 1-10 ng / mL of basic fibroblast growth factor, beta 11-10 ng / mL of transforming growth factor and 1-50 mg / mL of spirulina fast-soluble proteoglycan. The human umbilical cord mesenchymal stem cell serum-free medium can significantly improve the proliferation rate and the adherence performance of human umbilical cord mesenchymal stem cells, is beneficialto the proliferation of the human umbilical cord mesenchymal stem cells and the maintenance of stem cell characteristics, and has the potential of adipogenic osteogenic induced differentiation. The medium is simple, clear and stable, does not contain any serum components, overcomes the risk of heterologous protein contamination and pathogenic microorganisms, and has high safety.
Owner:青岛麦迪赛斯生物科技有限公司
Serum substitute for cell culture
InactiveCN112048463AAntioxidantRepair oxidative damageCulture processCell culture mediaInsulin-like growth factorInsulin activity
The invention discloses a serum substitute for cell culture. Each liter of the serum substitute comprises the following components: 140-165 mg of amino acid, 15-20 mg of asparagine, 15-25 [mu] M of VC, 70-110 [mu] M of vitamin H, 150-200 [mu] M of tocopheryl acetate, 80-120 [mu] M of tocopherol, 10-15 [mu] M of vitamin A, 15-25 g of a bovine pituitary extract, 80-120 [mu] g of an insulin-like growth factor I, 250-350 [mu] g of catalase, 450-550 [mu] M of insulin human recombinant, 5000-10000 [mu] M of human transferrin, 5000000U of superoxide dismutase, 5mM-20mM of corticosterone, 150000-250000mM of D-galactose, 100-150mM of diethanolamine hydrochloride, 49-165mM of glutathione, 80-120mM of l-carnitinechloride, 140.901-161.403mg of inorganic salts and 5-15g of Pluronic F-68.
Owner:内蒙古奥普赛生物科技有限公司
Recombinant lactobacillus of insulin-like growth factor and application thereof
ActiveCN103627711AImprove developmentFast growthBacteriaMicroorganism based processesHeterologousBiotechnology
The invention provides an encoding gene of a recombinant insulin-like growth factor, lactobacillus containing the encoding gene and application of the lactobacillus, and belongs to the field of bio-genetic engineering. The insulin-like growth factor is a multifunctional cell proliferation regulation factor, has important acceleration in differentiation, proliferation, and growth and development of cells, but has the problems of insufficient throughout, high price, required purification of product, difficult drug administration and the like just as most of genetic engineering drugs. In order to obtain a lot of recombinant insulin-like growth factors, recombinant insulin-like growth factor genes optimized by codons are subjected to heterologous expression by adopting a lactobacillus expression system, and the recombinant lactobacillus is utilized as a living bacteria agent or a feed additive to enhance the nonspecific immunity of a pig.
Owner:ZONHON BIOPHARMA INST +1
Renaturation method of restructured human insulin prokaryotic-fusion protein
ActiveCN105732820ASimple processing methodSimple and efficient operationAntibody mimetics/scaffoldsPeptide preparation methodsEscherichia coliHigh concentration
The invention relates to a renaturation method of restructured human insulin prokaryotic-fusion protein and belongs to the field of protein purification. The renaturation method includes steps of 1) smashing escherichia coli expressing restructured human insulin prokaryotic-fusion protein, and collecting inclusion bodies; 2) washing the inclusion bodies, dissolving the inclusion bodies and modifying the fusion protein; 3) renaturating protein. The renaturation method is needless of protein purification in advance, directly performs protein renaturation, and finally obtains high-concentration restructured insulin prokaryotic-fusion protein of natural structure. By the renaturation method, the defect of low protein concentration after protein renaturation and resultantly easy protein accumulation and precipitation is overcome, renaturation efficiency is up to 80-90%, production efficiency is improved, and the renaturation method is applicable to industrial production.
Owner:TONGHUA DONGBAO PHARMA
Vector for expression of heterologous protein and methods for extracting recombinant protein and for purifying isolated recombinant insulin
InactiveUS6281329B1Fast degenerationOvercome problemsBacteriaPeptide/protein ingredientsBacteroidesOrigin of replication
The present invention relates to a vector for expression of a heterologous protein by a Gram negative bacteria, wherein the vector includes a nucleic acid such as DNA encoding the following: an origin of replication region; optionally and preferably a selection marker; a promoter; an initiation region such as translation initiation region and / or a ribosome binding site, at least one restriction site for insertion of heterologous nucleic acid, e.g. DNA, encoding the heterologous protein, and a transcription terminator. The inventive vector may contain DNA encoding the heterologous protein, e.g., pro-insulin such as pro-insulin with a His tag. Additionally, the invention provides a method for extracting a recombinant protein from within a recombinant Gram negative bacteria having a cell membrane, without lysing the bacteria, as well as a method for purifying an isolated recombinant human insulin, wherein the isolated recombinant human pro-insulin is subjected to sulfitolysis, Ni-chelation chromatography, renaturation, limited proteolysis and chromatography separation to provide purified, isolated, recombinant human insulin.
Owner:BIOMM
Low-serum culture medium for Vero cell culture and corresponding virus production
InactiveCN110616183AImprove the cultivation effectGood growthSsRNA viruses positive-senseCulture processHydrolysateVaccine Production
The invention discloses a low-serum culture medium for Vero cell culture and corresponding virus production. The low-serum culture medium comprises the following constitution components: amino acids,vitamins, inorganic salts, proteins and auxiliary components, wherein the amino acids comprise glycine, arginine hydrochloride, glutamine, lysine hydrochloride, serine, disodium tyrosine dehydrate andvaline; and the proteins comprise whole albumin, lactoalbumin hydrolysate (LAH), saturated-iron human transferrin, whole-chain recombulin, 4-ethoxy piperazine ethanesulfonic acid (HEPES). In production of vaccines for porcine epidemic diarrhea viruses through Vero cell amplification, the culture medium disclosed by the invention is simple in formula component, low in cost and easy to prepare, anda good growth state of cells can be kept while cell culture effects are effectively improved.
Owner:山东巨山能源科技有限公司
Method for purifying gene-recombinant insulin precursor
ActiveCN101029077BHigh recovery rateHigh purityFungiPeptide preparation methodsActivated carbonPichia pastoris
A process for purifying the genetically recombined insulin precursor features that the activated carbon as adsorbent and cationic chromatography are used to remove the pigment from fermented liquid and purify the active protein. Its recovery rate is more than 85% and its purity is more than 95%.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com