Method for preparing recombinant human insulin and analogs of recombinant human insulin
A technology for recombining human insulin and analogues, applied in the field of genetic engineering, can solve problems such as harsh operating conditions, low proportion of proinsulin, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
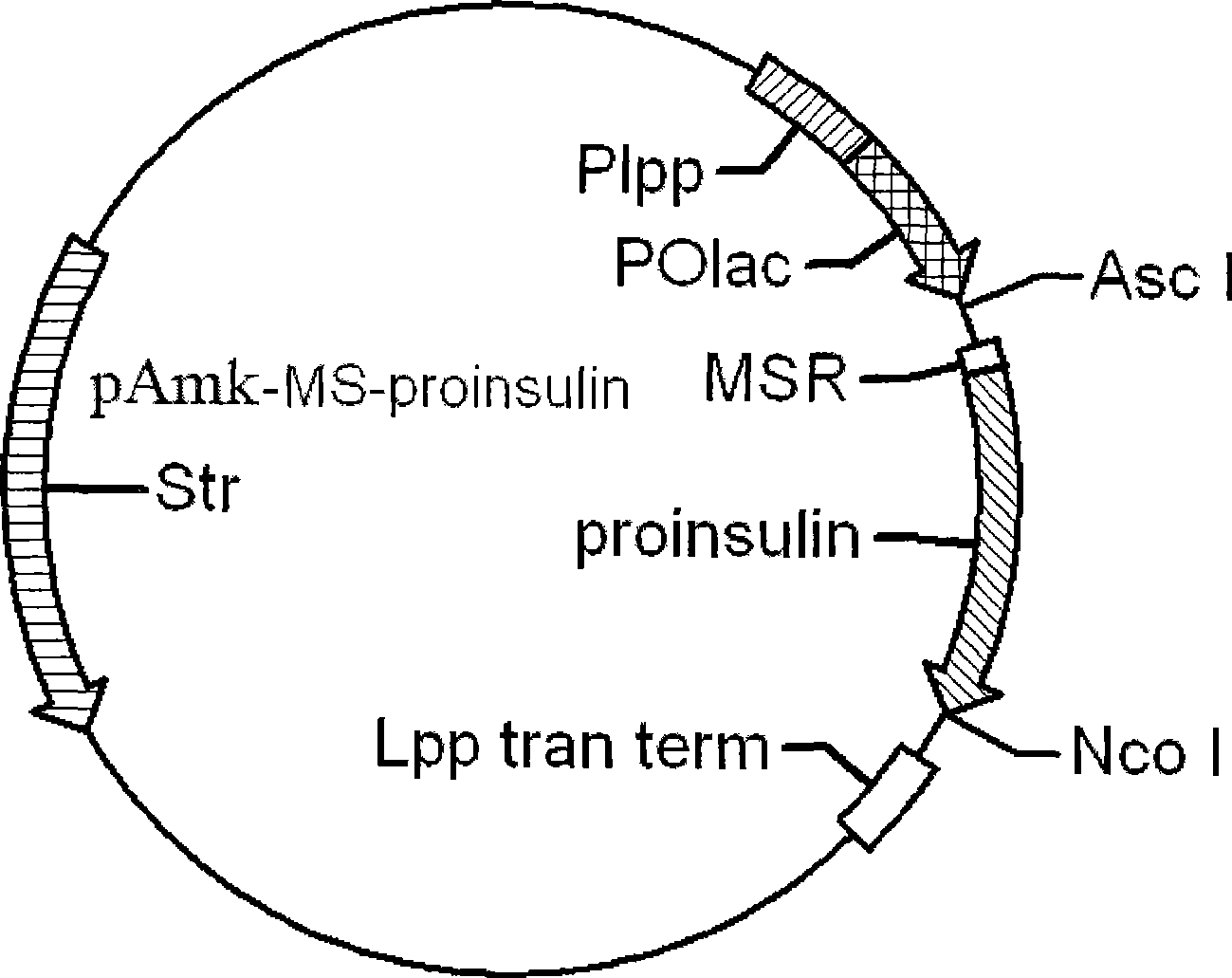
[0049] Example 1 Construction of MSR-proinsulin recombinant genetically engineered bacteria
[0050] 1.1 Acquisition of MS-proinsulin gene
[0051] Using pAmk as a template, design primers P1 and P2. The nucleotide sequences of the two primers are as follows:
[0052] P1: 5’GGAATTGTGAGCGGATAACA3’
[0053] P2: 5’AAAACGACTCATAACGTATTCCTCT3’
[0054] The fragment between the Asc I restriction site in pAmk and the expression guide peptide MS was amplified by PCR. Name it fragment-MS. Pfu DNA polymerase was used for amplification, and the amplification conditions were 94°C, 45s, 53°C, 45s, 72°C, and 30s. A total of 30 cycles. The PCR product was purified with a DNA gel recovery kit to recover a band of about 130 bp.
[0055] Design primers P3 and P4 with pET28a-23DT-proinsulin constructed in our laboratory:
[0056] P3: 5’ATGAGTCGTTTTGTCAATCAGCACC3’
[0057] P4: 5’CCGCCATGGCGCTTTTGATCC3’
[0058] Amplify the fragment between proinsulin and Nco I restriction site in pET28a-23DT-proinsul...
Embodiment 2
[0065] Example 2 Fermentation and expression of pAmk-MS-proinsulin recombinant genetic engineering bacteria
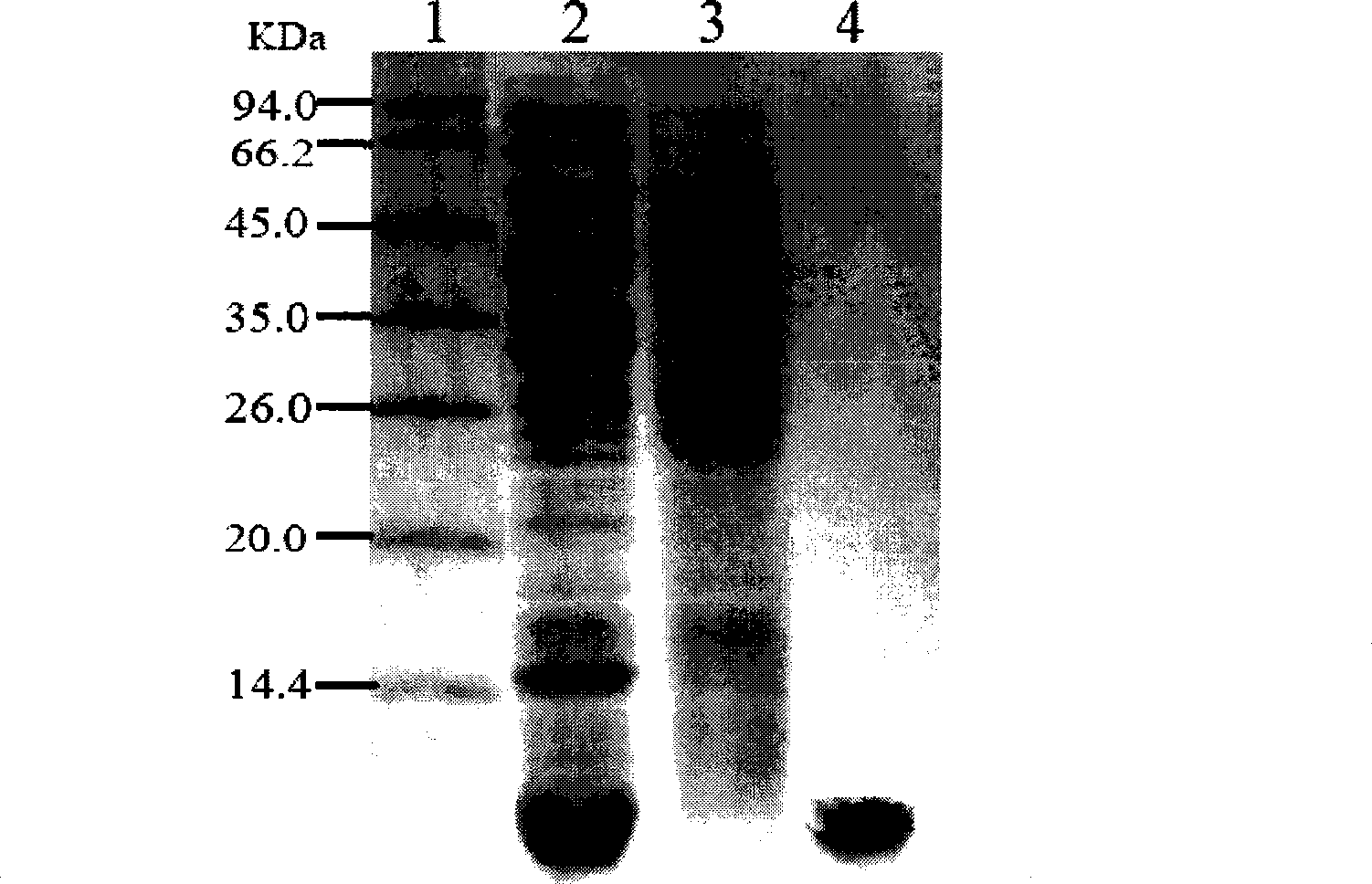
[0066] Pick a single colony from the culture plate of pAmk-MS-proinsulin recombinant genetically engineered bacteria, inoculate it in LB liquid medium containing 25μg / ml streptomycin, cultivate overnight at 37°C with constant temperature shaking, and transfer to inoculation at a ratio of 1% Into 20 liters of LB liquid medium (25 μg / ml streptomycin), and add a-lactose with a final concentration of 0.5 mmol / L to induce the expression of the fusion protein MS-proinsulin. Twelve hours after induction, the bacteria were recovered by centrifugation (5kg of wet bacteria in total), 15% SDS-PAGE electrophoresis followed by thin-layer scanning showed that the MS-proinsulin precursor proinsulin gene expression had been achieved, and the expressed protein accounted for the total bacterial protein. 44.2% of the total, see the results image 3 .
Embodiment 3
[0067] Example 3 Separation and purification of MS-proinsulin precursor proinsulin protein
[0068] The engineered bacteria after induced expression were recovered by centrifugation, and the bacteria were suspended in a lysis solution (pH 8.0, 50mM Tris-C1, 0.5% Triton X-100, 0.02% lysozyme) and stirred overnight at 37°C. The precipitate was recovered by centrifugation, and the precipitate was washed with inclusion body washing solution (pH8.0, 50mM Tris-C1, 0.2% Triton X-100), low-concentration urea solution (pH8.0, 50mM Tris-C1, 3M urea), and centrifuged after washing Recover the precipitate. The precipitate was lysed in high-concentration urea (pH 8.0, 50mM Tris-C1, 7M urea), and the supernatant was recovered by centrifugation. The supernatant was purified by sulfite hydrolysis (sodium sulfite, sodium tetrasulfate) and anion exchange (DEAE-52) chromatography. The chromatography buffer contains pH8.0, 50mM Tris-C1, 7M urea, and the gradient elution is performed with a 0-0.3M NaC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com