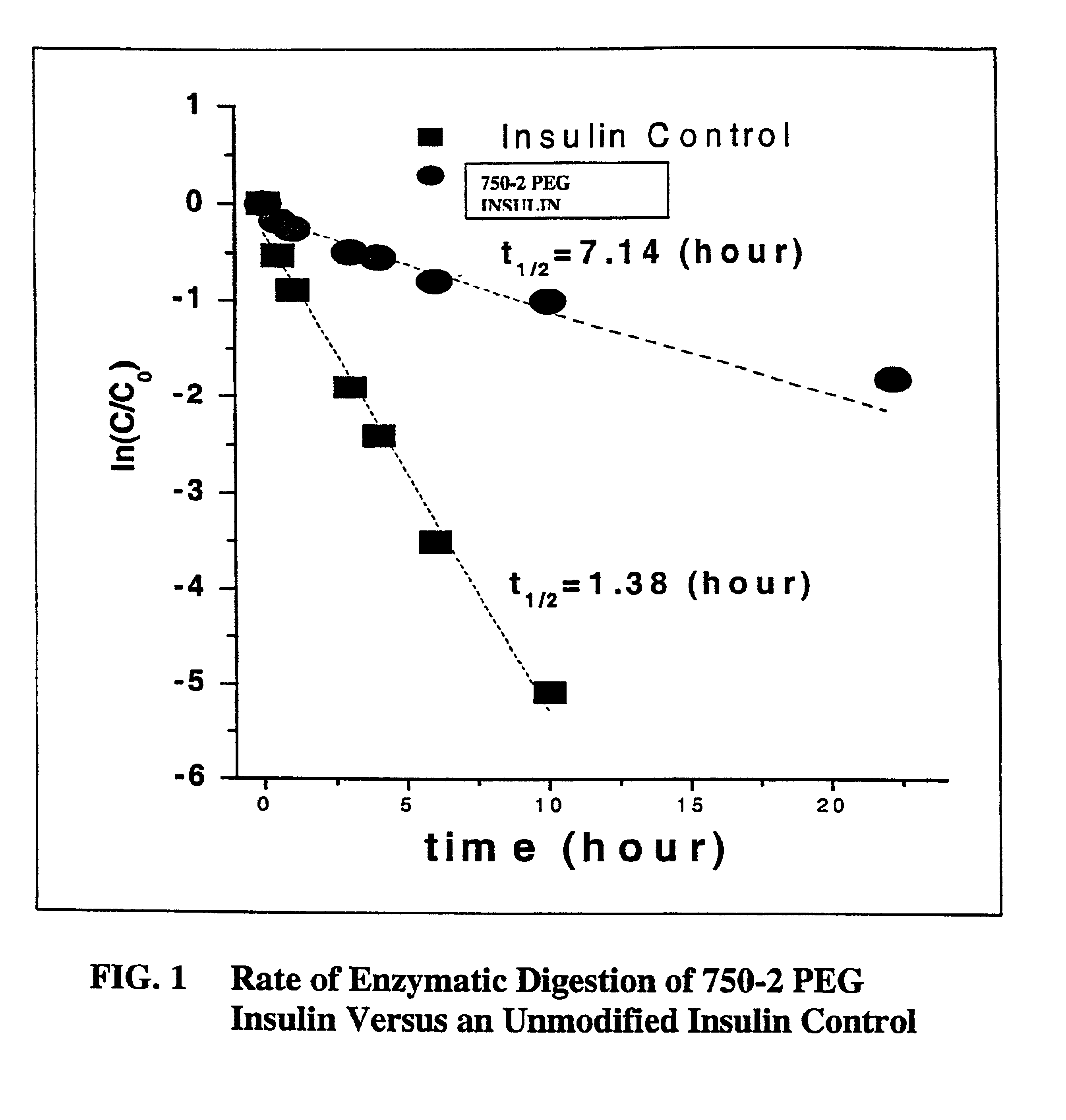
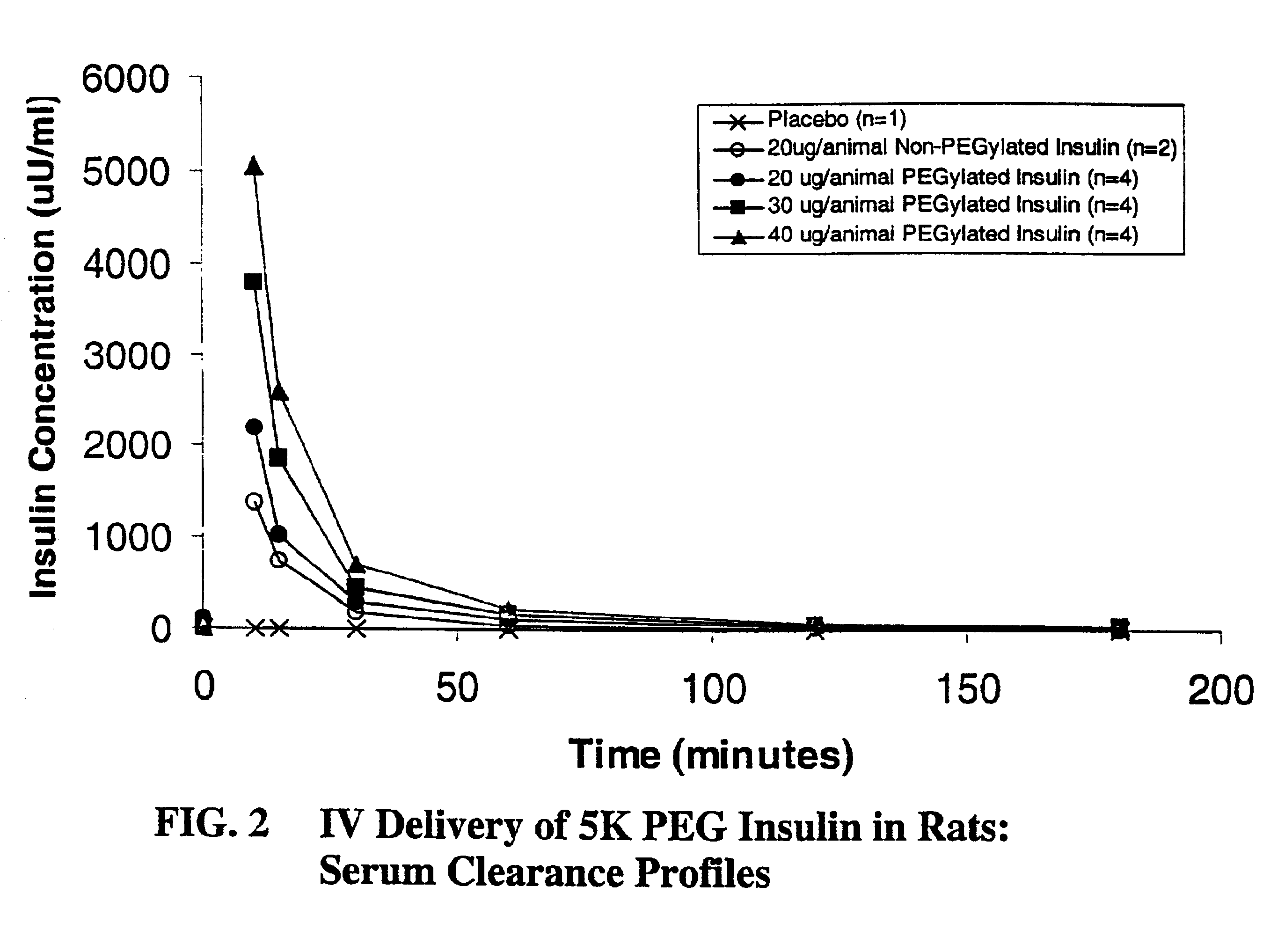
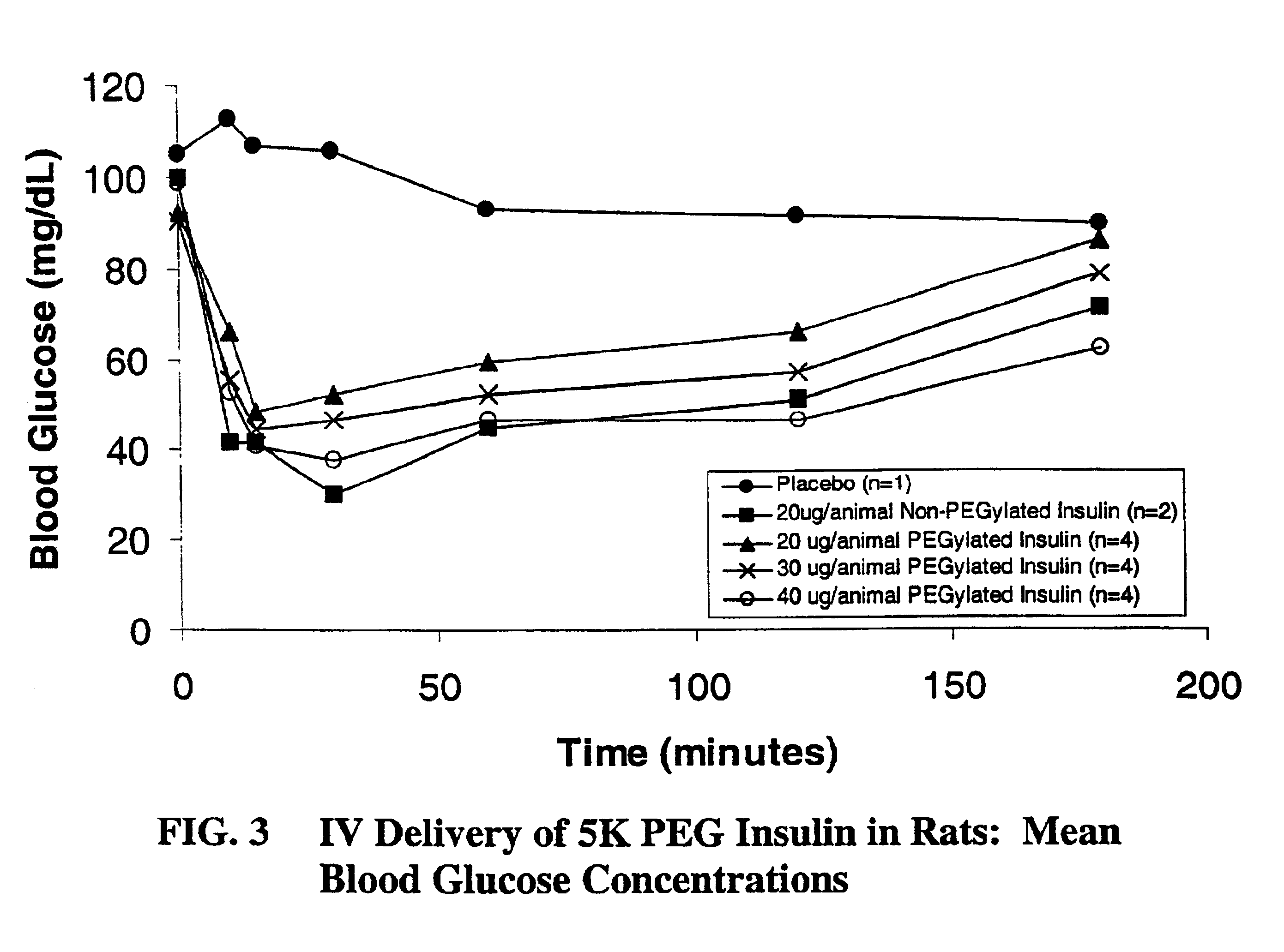
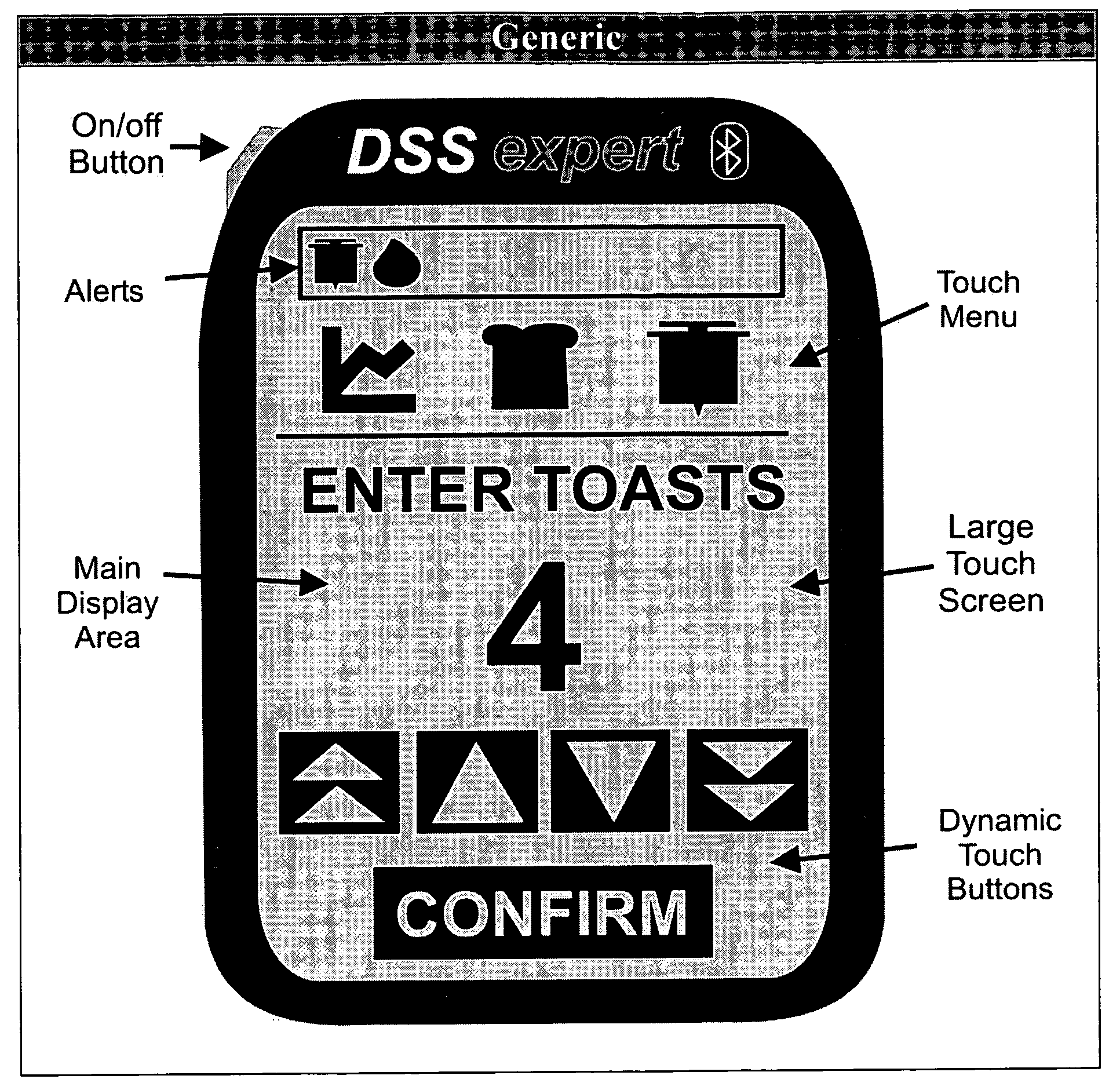
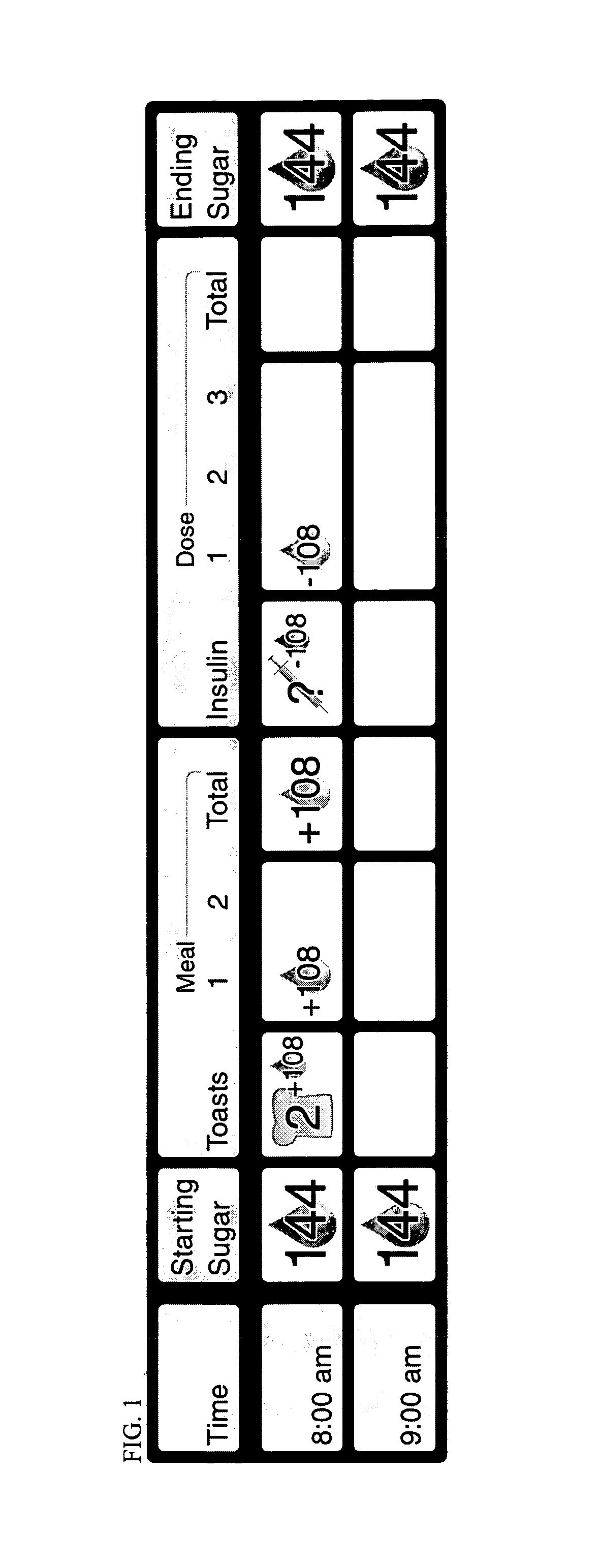
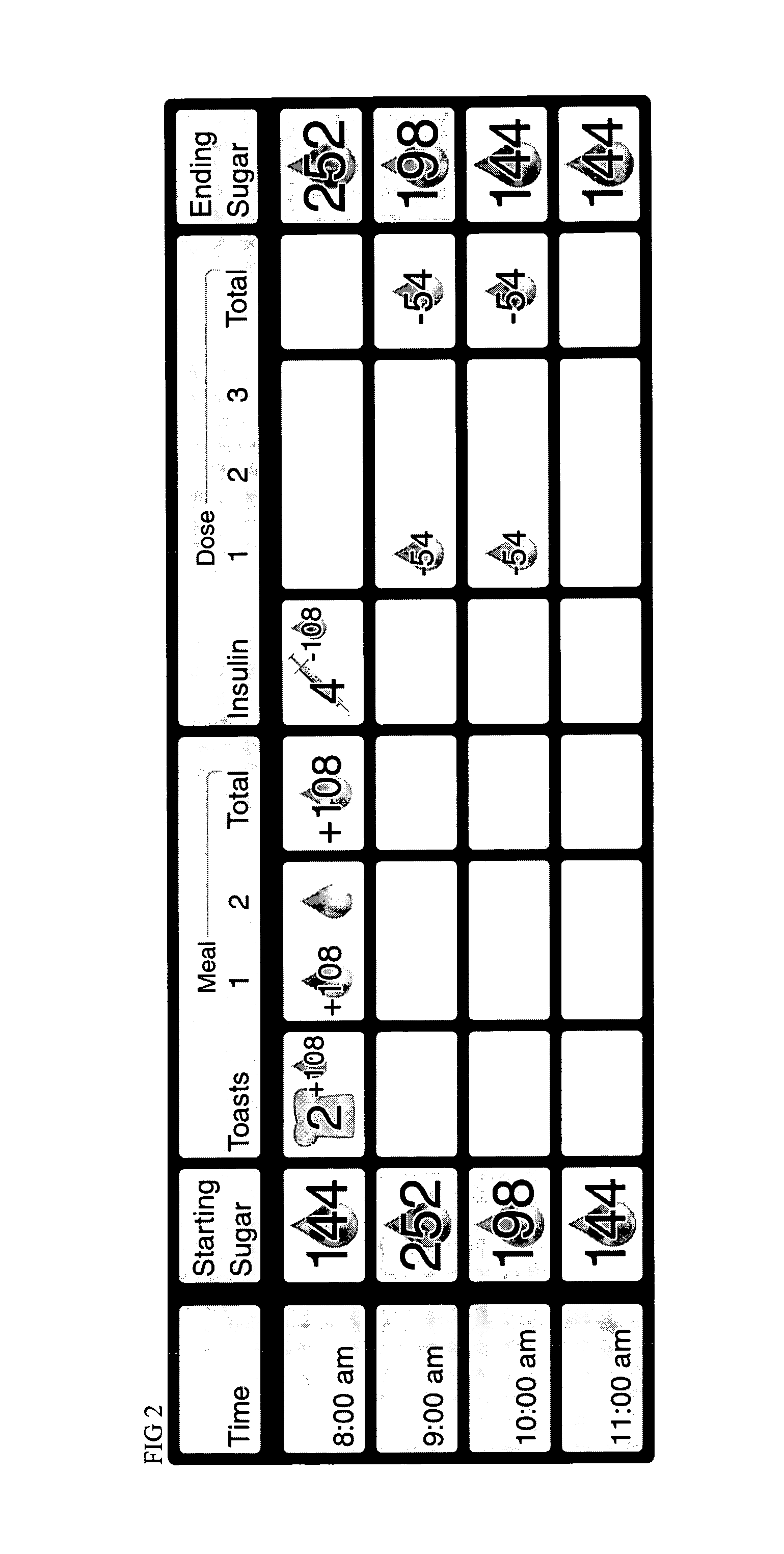
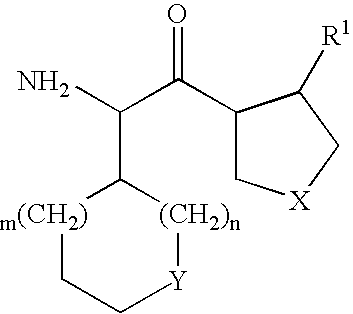
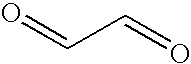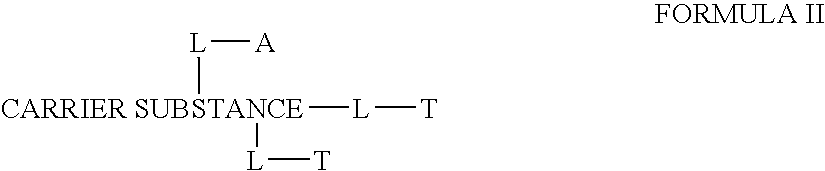Patents
Literature
1649results about "Insulins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
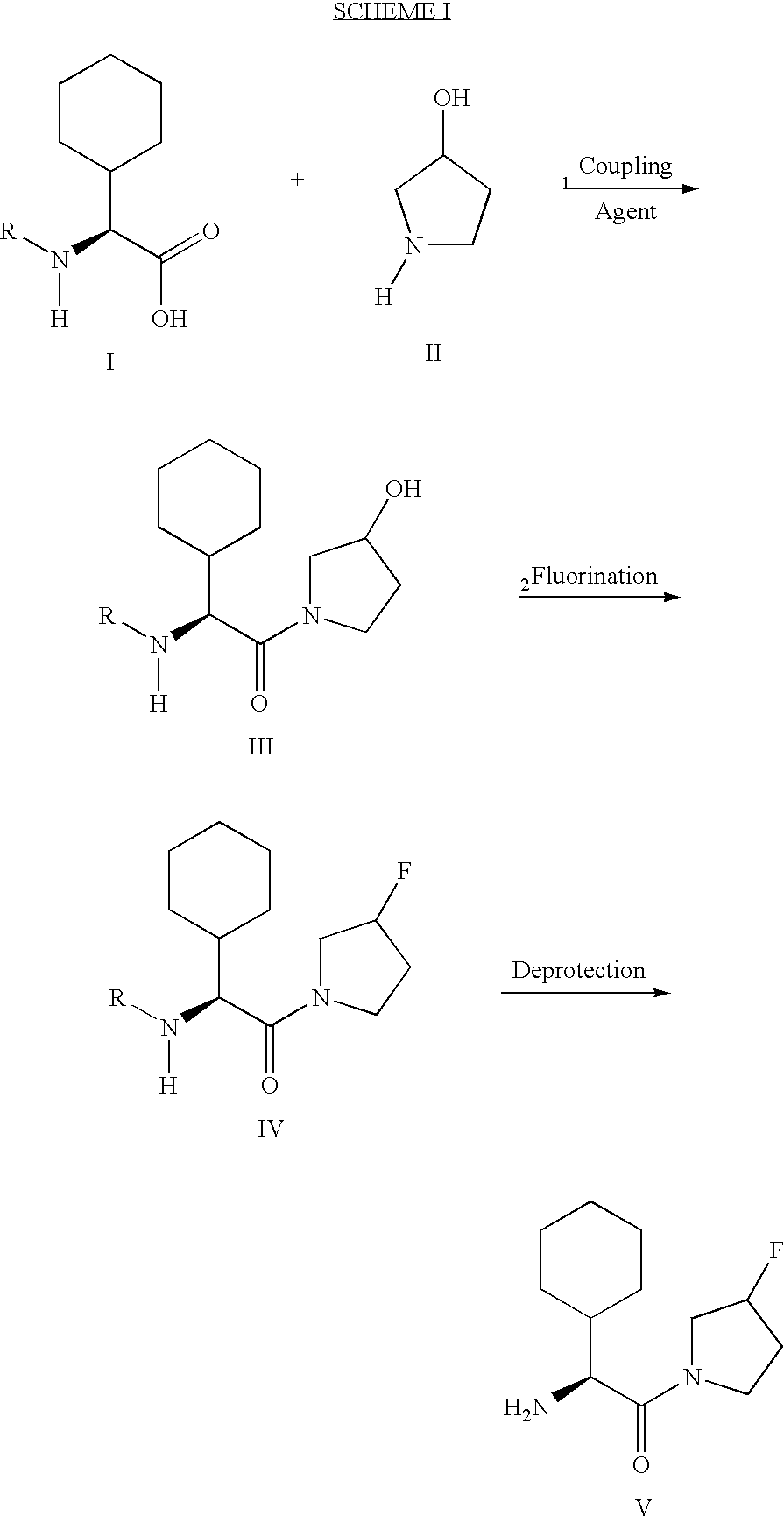
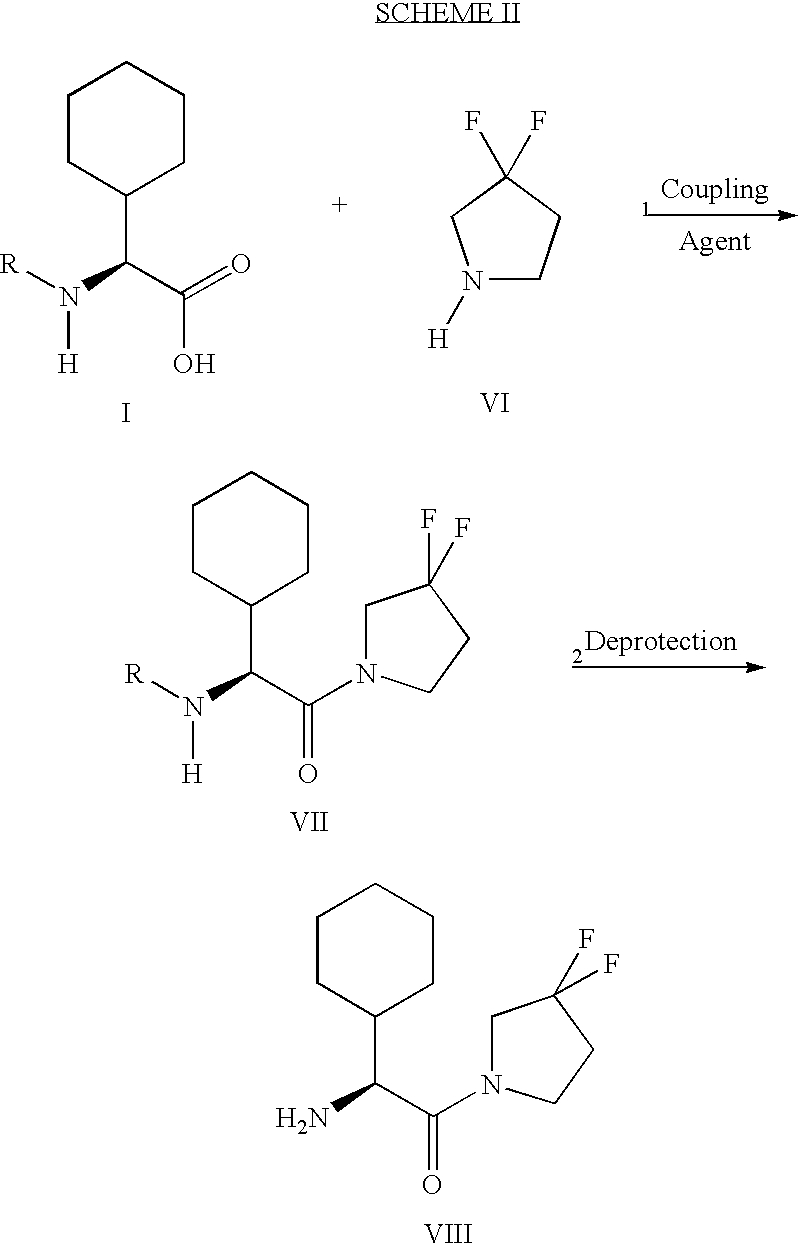
Method of producing sustained-release preparation
InactiveUS6267981B1Maintain good propertiesEnhancement of entrapmentPowder deliveryPeptide/protein ingredientsEntrapmentBiodegradable polymer
This invention provides a sustained-release preparation comprising a biodegradable polymer metal salt and broactive polypeptide, with enhanced entrapment of the bioactive polypeptides, a suppression of initial burst, and a constant long-term release of the bioactive polypeptides.
Owner:TAKEDA PHARMA CO LTD
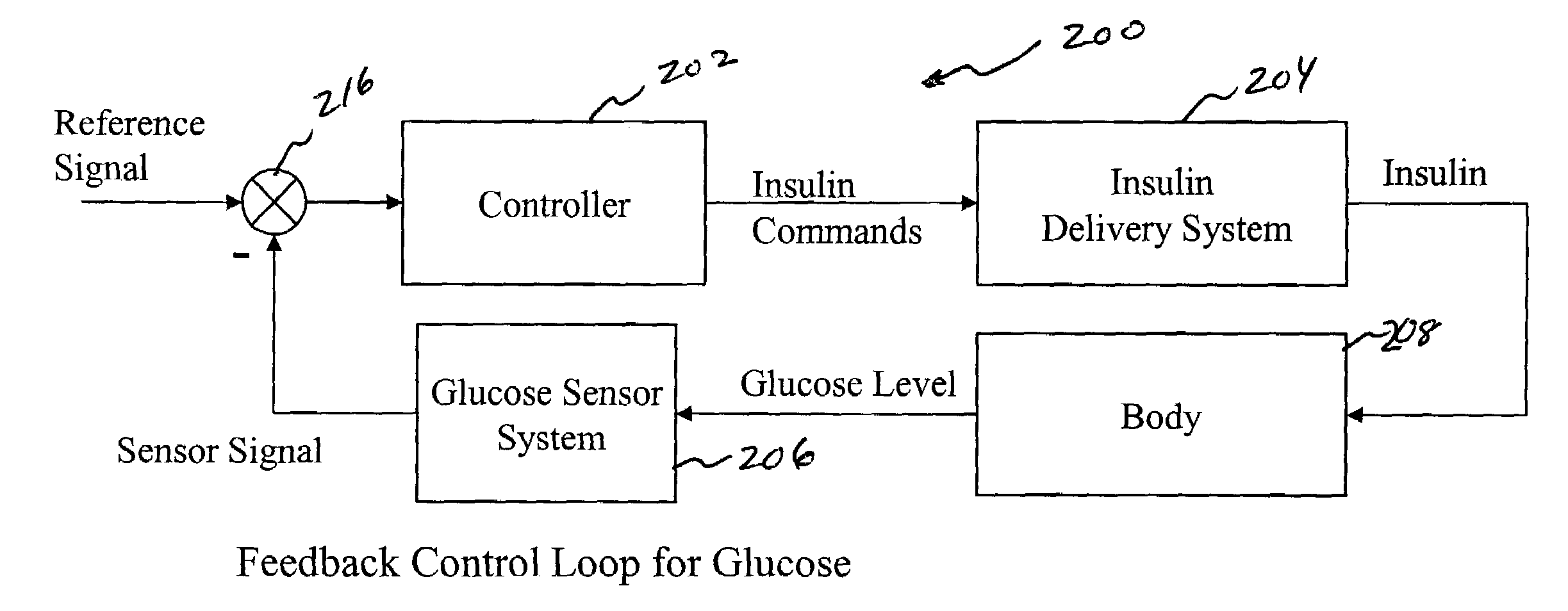
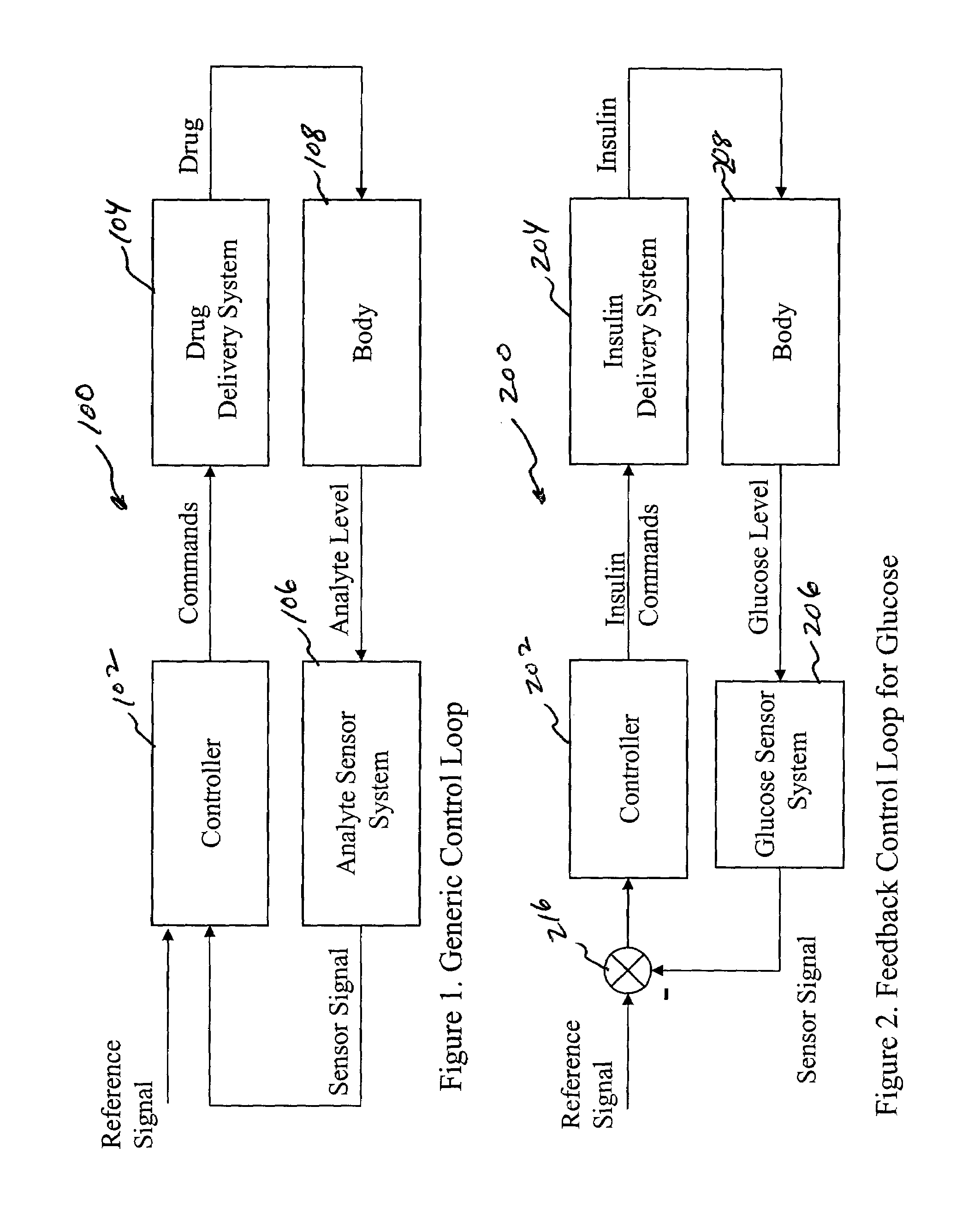
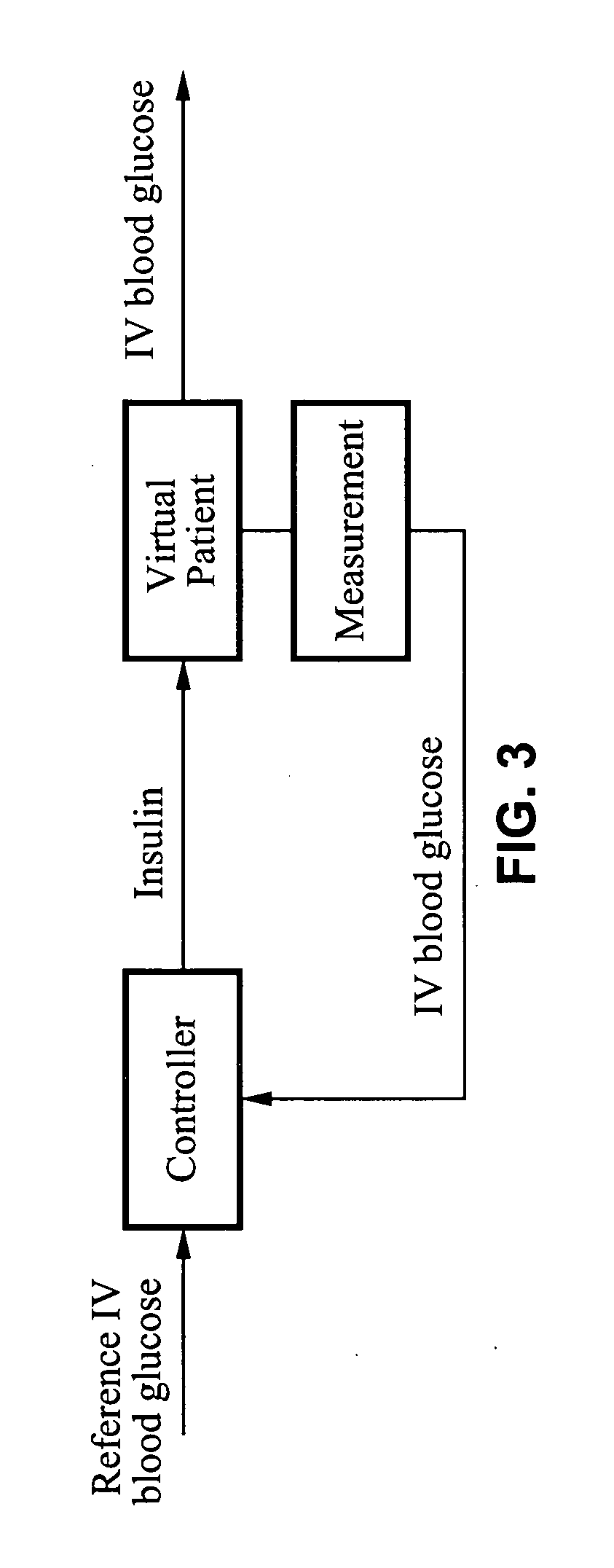
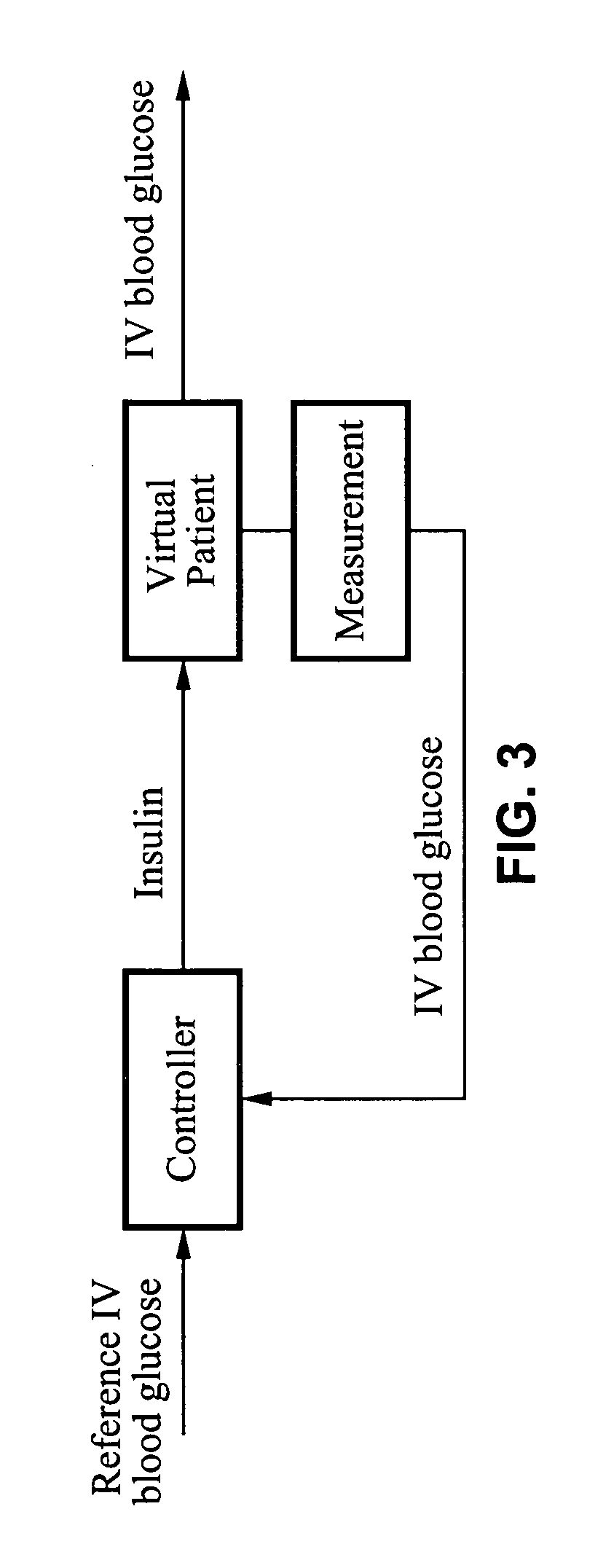
System and method for initiating and maintaining continuous, long-term control of a concentration of a substance in a patient using a feedback or model-based controller coupled to a single-needle or multi-needle intradermal (ID) delivery device
ActiveUS7060059B2Maintaining continuous, long-term control of the blood glucose concentrationsImprove performancePeptide/protein ingredientsDrug and medicationsInsulin infusionClosed loop
A closed loop therapy system for controlling a concentration of a substance, such as blood glucose concentration, in the body of a user. The system and method employ a sensor system that measures a glucose level in the body, a controller that uses the measured glucose levels to generate an output that can be used to automatically or manually control an intradermal insulin infusion system to set a constant or time-varying profile of target blood glucose concentrations in a user, and then infuse an appropriate amount of insulin into the body of the user so as to reach and maintain the target values of the blood glucose concentration.
Owner:BECTON DICKINSON & CO
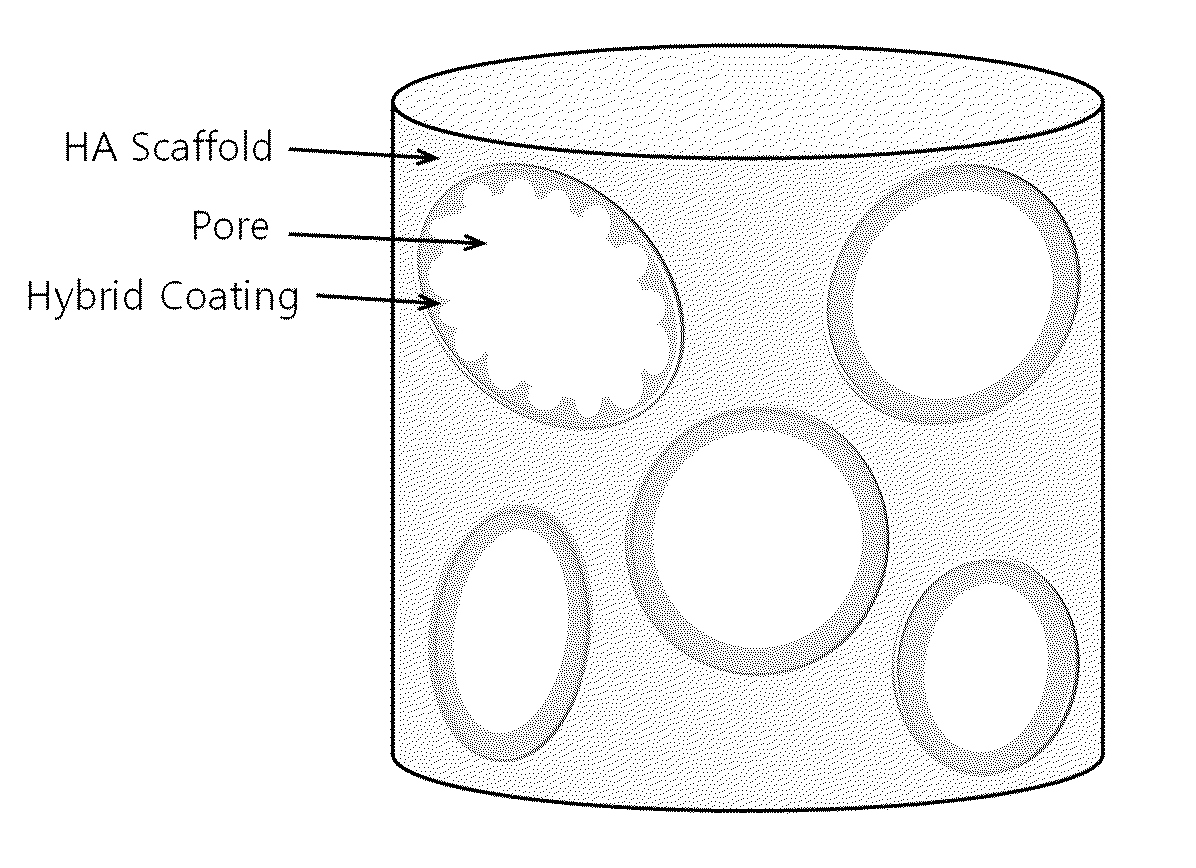
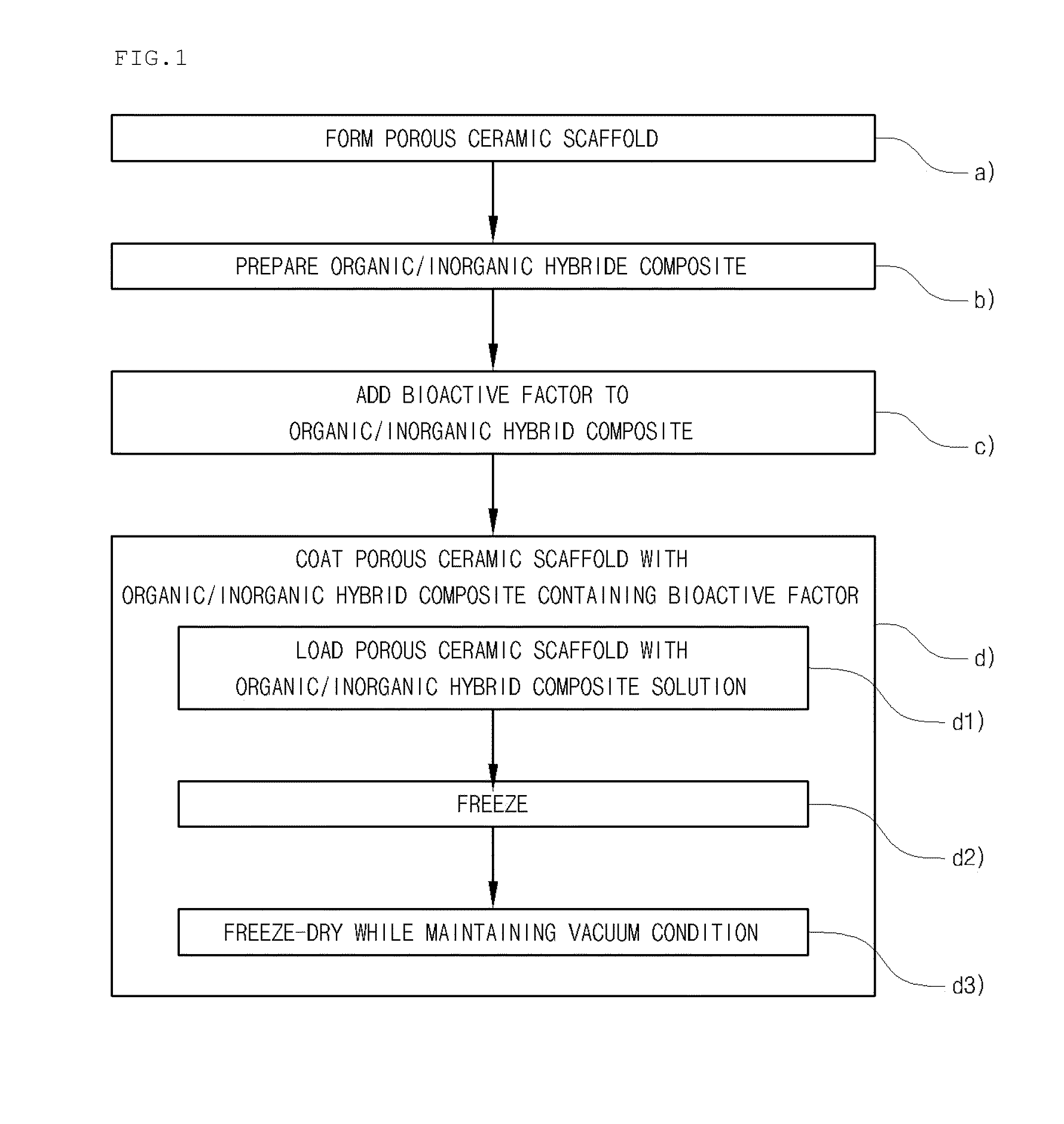
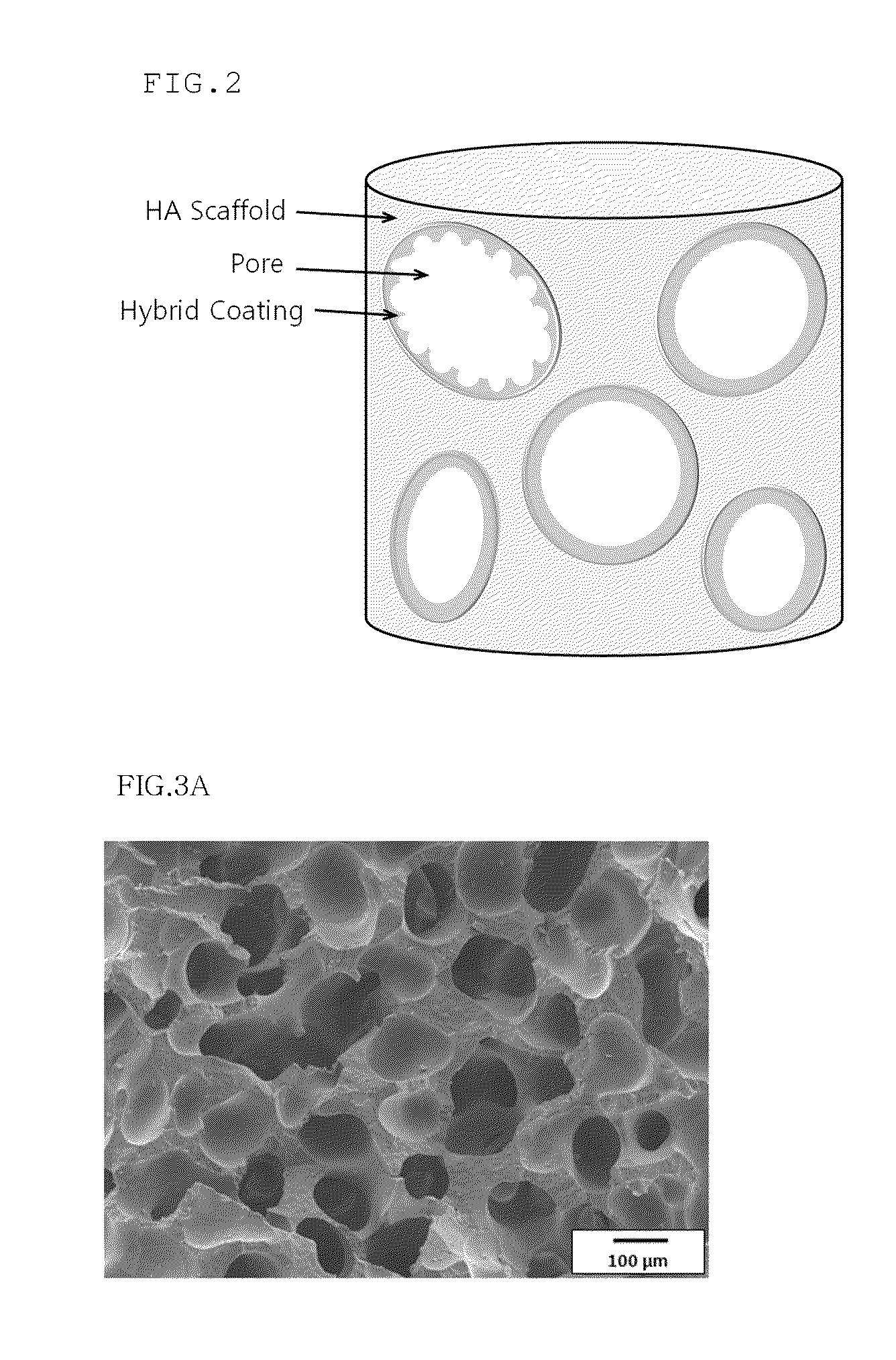
Method for manufacturing a porous ceramic scaffold having an organic/inorganic hybrid coating layer containing a bioactive factor
ActiveUS8734831B2Good biocompatibilityEasy to controlBiocideSurgical adhesivesOrganic matterPorous ceramics
A method for manufacturing a porous ceramic scaffold having an organic / inorganic hybrid coating layer containing a bioactive factor includes (a) forming a porous ceramic scaffold; (b) mixing a silica xerogel and a physiologically active organic substance in a volumetric ratio ranging from 30:70 to 90:10 and treating by a sol gel method to prepare an organic / inorganic hybrid composite solution; (c) adding a bioactive factor to the organic / inorganic hybrid composite solution and agitating until gelation occurs; and (d) coating the porous ceramic scaffold with the organic / inorganic composite containing the bioactive factor added thereto. In accordance with the method, the porous ceramic scaffold may be uniformly coated with the organic / inorganic hybrid composite while maintaining an open pore structure, and stably discharge the bioactive factor over a long period of time.
Owner:SEOUL NAT UNIV R&DB FOUND
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
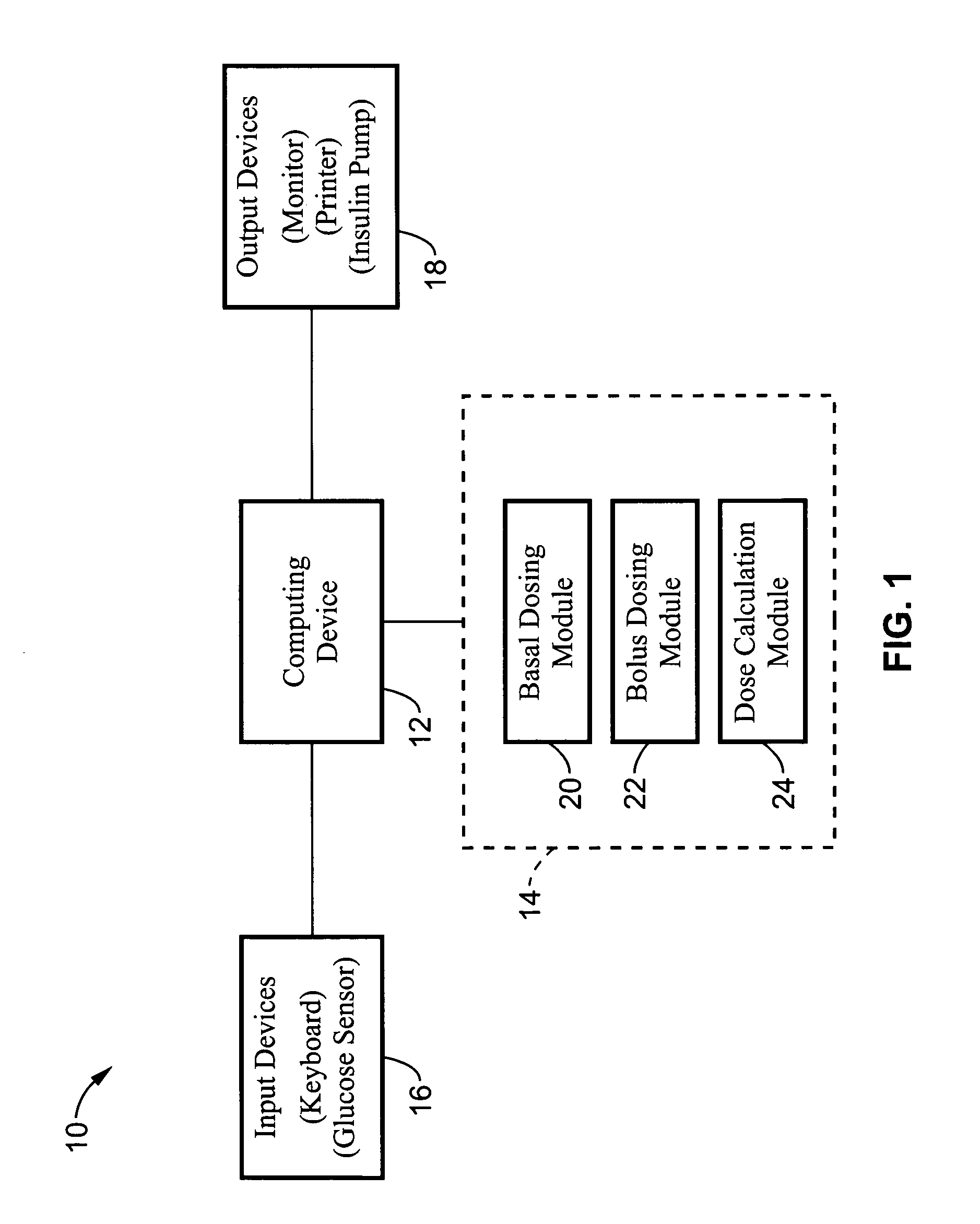
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS20050272640A1Ensure robustnessAccurately predicting insulin bolus dosagesPeptide/protein ingredientsDrug and medicationsPhysiologyMonitors blood glucose
A computer implemented method and associated apparatus for the combined control of insulin bolus dosing and basal delivery for the goal of achieving normal glycemic response to meals, exercise, stressors, and other perturbations to blood glucose levels. A run-to-run algorithm is used to monitor blood glucose levels and adjust insulin delivery as conditions are varied.
Owner:RGT UNIV OF CALIFORNIA
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS7651845B2Accurately predicting insulin bolus dosagesProcess controlPeptide/protein ingredientsDrug and medicationsMonitors blood glucoseGlycemic
Owner:RGT UNIV OF CALIFORNIA
Method and composition for the treatment of diabetes
InactiveUS6153632AIncrease uptakeImprove utilizationBiocidePeptide/protein ingredientsIGT - Impaired glucose toleranceGlycosidase inhibitor
This invention is directed to a novel method and composition for the treatment of diabetes mellitus (Type I, Impaired Glucose Tolerance ["IGT"]and Type II). More specifically, this invention pertains to a novel method of treating diabetes mellitus by incorporating a therapeutic amount of one or more insulin sensitizers along with one or more of an orally ingested insulin, an injected insulin, a sulfonylurea, a biguanide or an alpha-glucosidase inhibitor for the treatment of diabetes mellitus.
Owner:RIEVELEY CHERYL ANNE
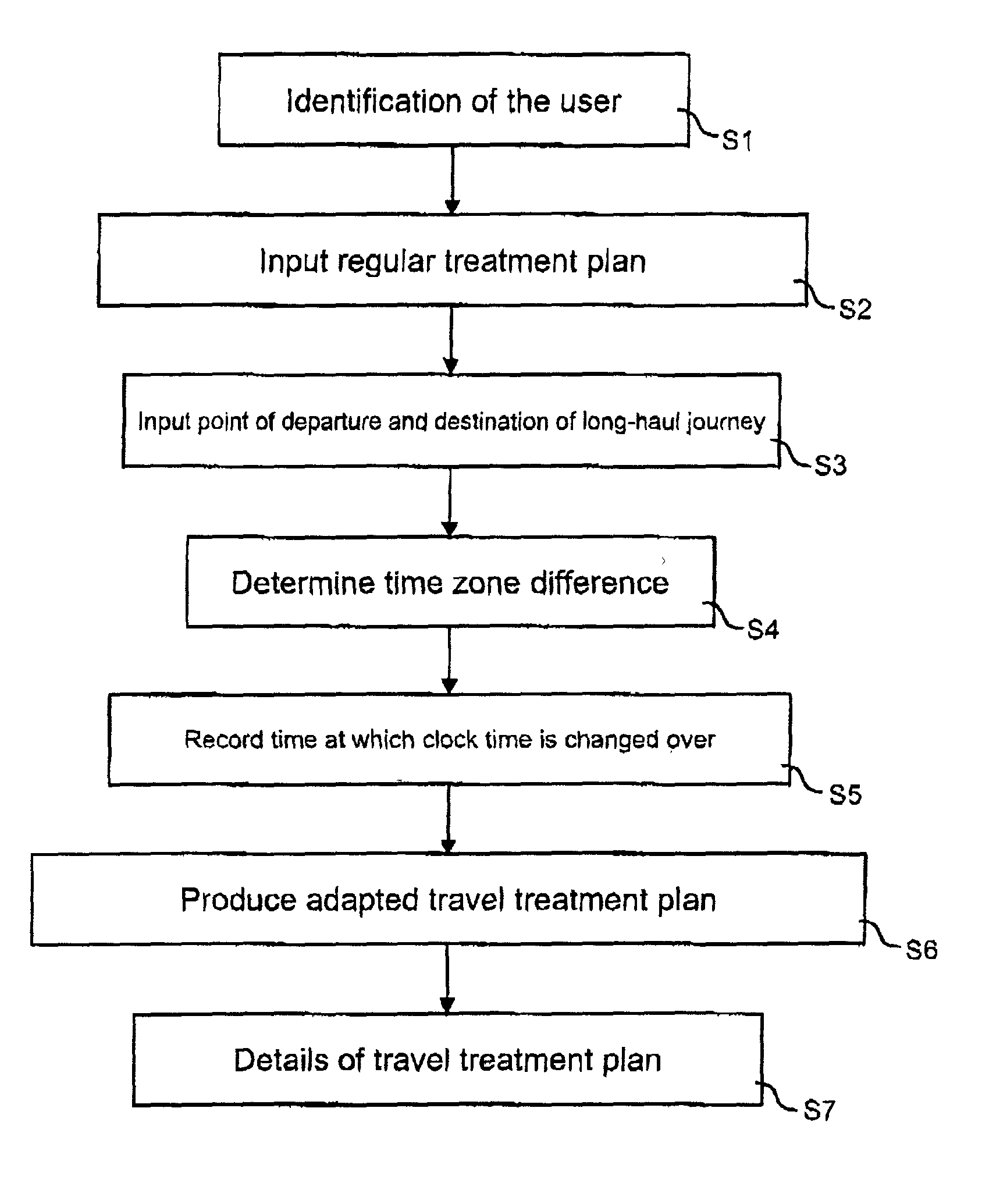
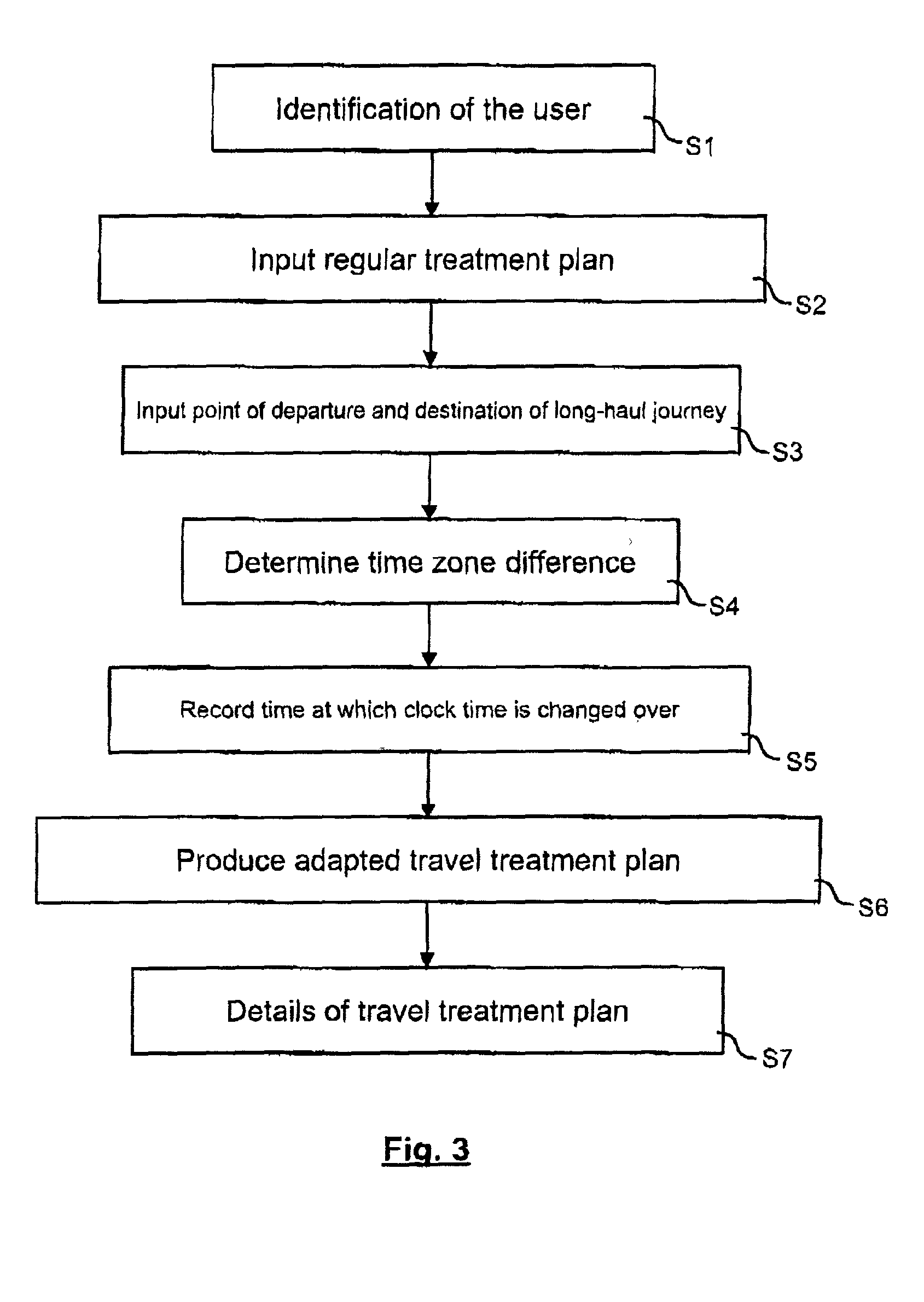
Method and device for producing an adapted travel treatment plan for administering a medicine in the event of a long-haul journey
InactiveUS20020147135A1Facilitates reliable actionTicket-issuing apparatusPeptide/protein ingredientsMedicineTime zone
Patients with certain chronic diseases such as diabetes require medical treatment according to a set time-related treatment plan. Such a treatment plan must be adapted accordingly in the case of long-haul journeys to countries with a time difference. A method for this has the steps of recording an regular treatment plan for administering the medicine, recording the point of departure and destination as well as the time of travel of the long-haul journey, determining the time zone difference between the point of departure and the destination and producing an adapted travel treatment plan based on the regular treatment plan depending on the time zone difference and the time of travel. The invention enables a patient to achieve a structured adaptation of his individual treatment plan to the time difference in the destination country of the journey in a convenient manner.
Owner:SCHNELL OLIVER
Preparation of protein microparticles by supercritical fluid precipitation
InactiveUS6063910AUniform and fine particle sizePowder deliveryPeptide/protein ingredientsMicroparticleSolvent
The present invention comprises passing a solution of a soluble material, preferably a protein, in a solvent through a continuum of supercritical antisolvent fluid and precipitating the soluble material. This can be conducted by passing the solution through the continuum of supercrital fluid in the form of droplets, which can be sprayed through the supercritical fluid. The plurality of droplets can be passed cocurrently or countercurrently with respect to a stream of antisolvent fluid. Alternatively, the solution can be passed through the continuum of supercritical antisolvent fluid in the form of a thin film or a plurality of fine streams.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Stabilized insulin formulations
InactiveUS6852694B2Easy to separateBiocidePeptide/protein ingredientsINSULIN PREPARATIONSEndocrinology
The present invention is directed to stabilized insulin composition comprising a mixture of insulin species such as insulin and an insulin analog. As disclosed herein, insulin compositions comprising a mixture of insulin and insulin analog species form heterodimeric complexes having a greater stability than the homodimeric complexes formed in compositions comprising single insulin species. Consequently, the present invention provides methods for stabilizing insulin molecules, methods for identifying stable heterodimeric insulin complexes and stabilized insulin compositions.
Owner:MEDTRONIC MIMIMED INC
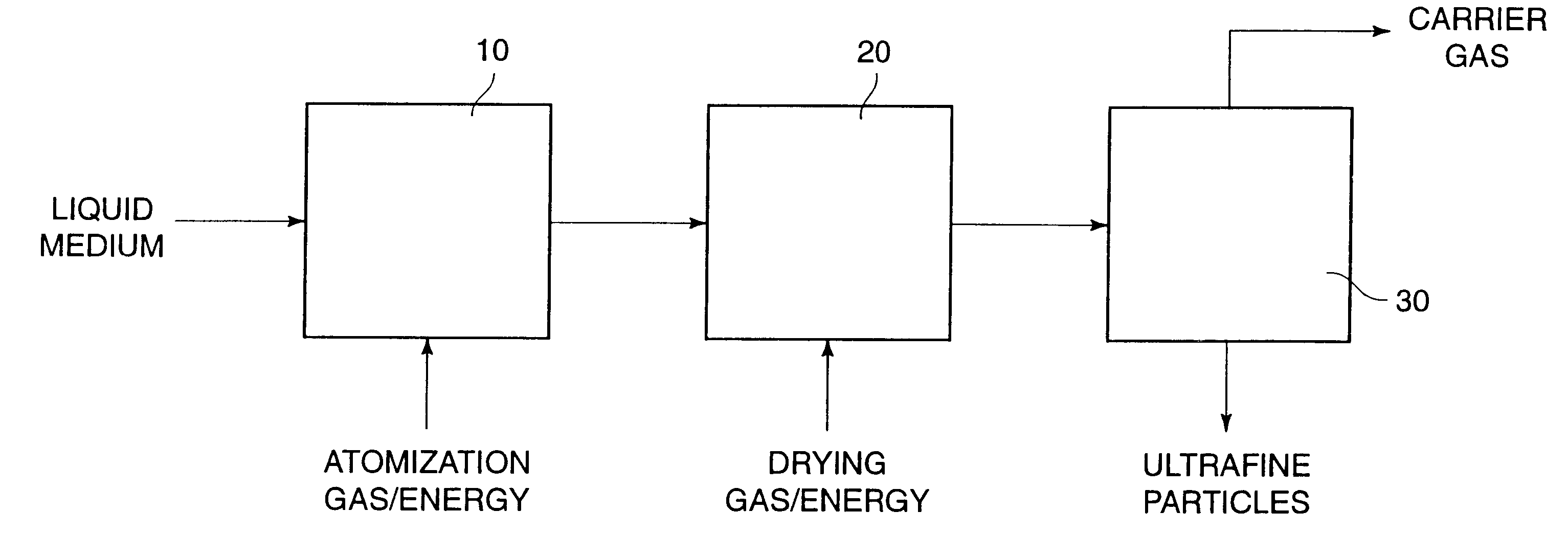
Dispersible macromolecule compositions and methods for their preparation and use
A process for preparing ultrafine powders of biological macromolecules comprises atomizing liquid solutions of the macromolecules, drying the droplets formed in the atomization step, and collecting the particles which result from drying. By properly controlling each of the atomization, drying, and collection steps, ultrafine dry powder compositions having characteristics particularly suitable for pulmonary delivery for therapeutic and other purposes may be prepared.
Owner:NOVARTIS FARMA
Long lasting synthetic glucagon-like peptide {GLP-1}
Modified insulinotropic peptides are disclosed. The modified insulinotropic peptides are capable of forming a peptidase stabilized insulinotropic peptide. The modified insulinotropic peptides are capable of forming covalent bonds with one or more blood components to form a conjugate. The conjugates may be formed in vivo or ex vivo. The modified peptides are administered to treat humans with diabetes and other related diseases.
Owner:CONJUCHEM
Autism treatment
InactiveUS20120128683A1Treat and prevent associated lossMinimally invasiveNervous disorderPeptide/protein ingredientsMedicineNose
A safe and effective treatment to curtail and cure autism spectrum disorders has been described in this invention using insulin, IGF-1, with multiple known adjuvant therapeutic agents, as well as other pharmaceutical, biochemical, nurticeuticals, and biological agents or compounds delivered through the olfactory mucosal region of the nose and external auditory meatus.
Owner:SHANTHA TOTADA R
Polypeptide compositions with improved stability
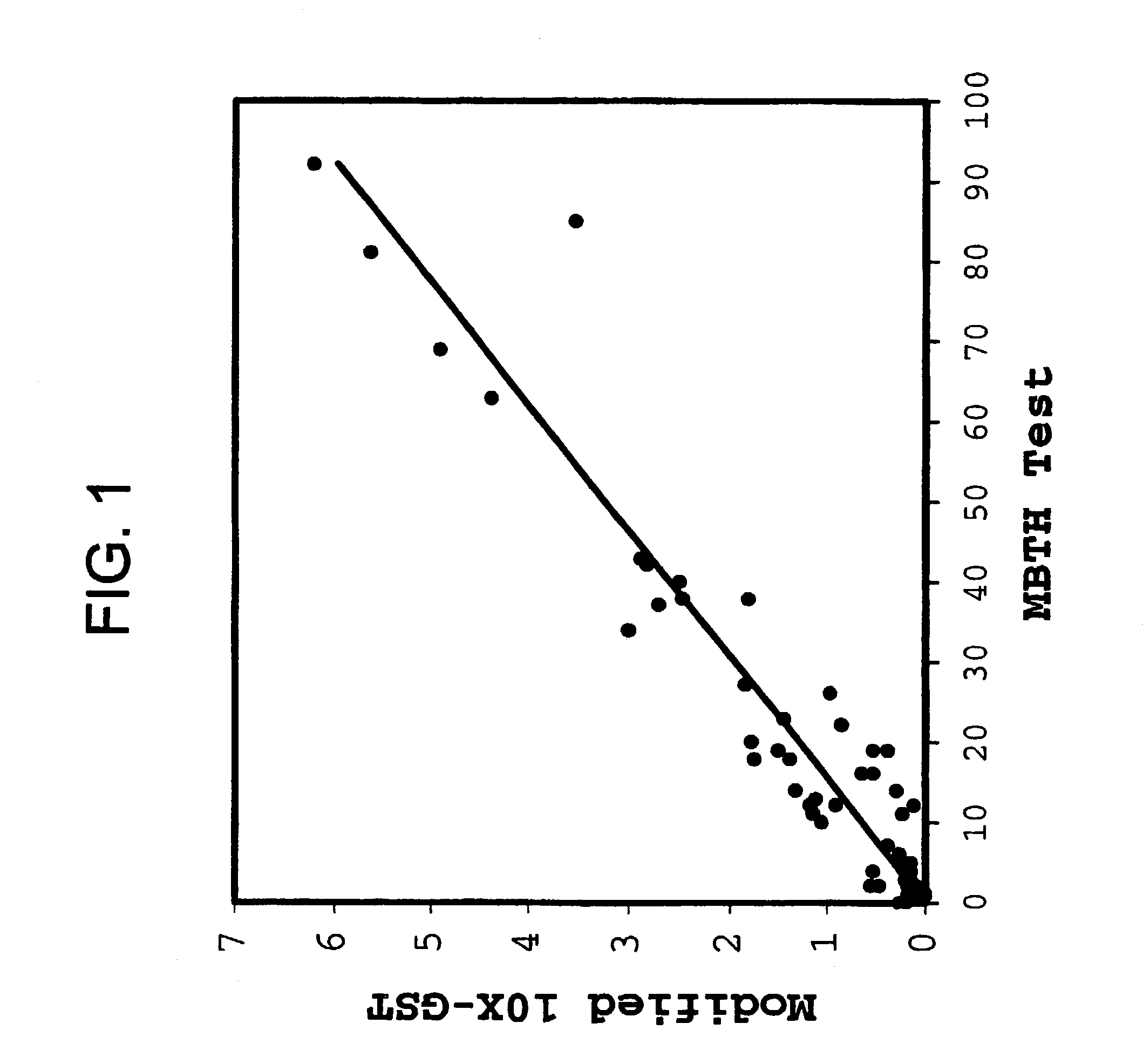
InactiveUS7022674B2Good chemical stabilityLower Level RequirementsPeptide/protein ingredientsMetabolism disorderLinear correlationGlycerol
The present invention provides means to improve the chemical stability of aqueous, parenteral pharmaceutical compositions comprising a polypeptide and glycerin. Reactive aldehydes are identified in commercial glycerins, and means for reducing such are provided. Convenient means are provided to assay for reactive aldehydes in glycerin, and a strong linear correlation between the level of reactive aldehydes in glycerin and chemical stability of compositions comprising a polypeptide and glycerin is demonstrated. The invention includes aqueous compositions comprising a polypeptide and glycerin having improved chemical stability compared to compositions previously known.
Owner:ELI LILLY & CO
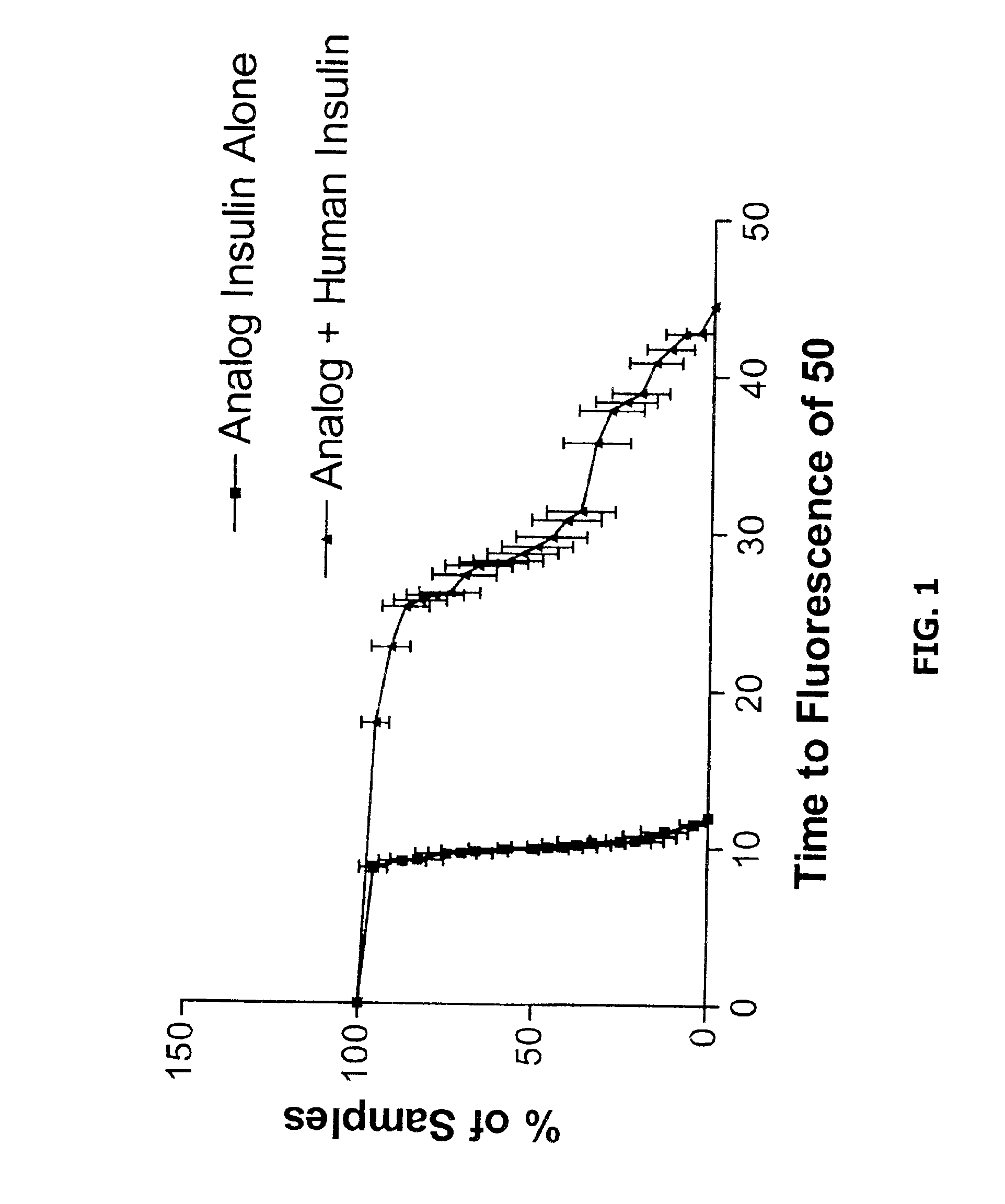
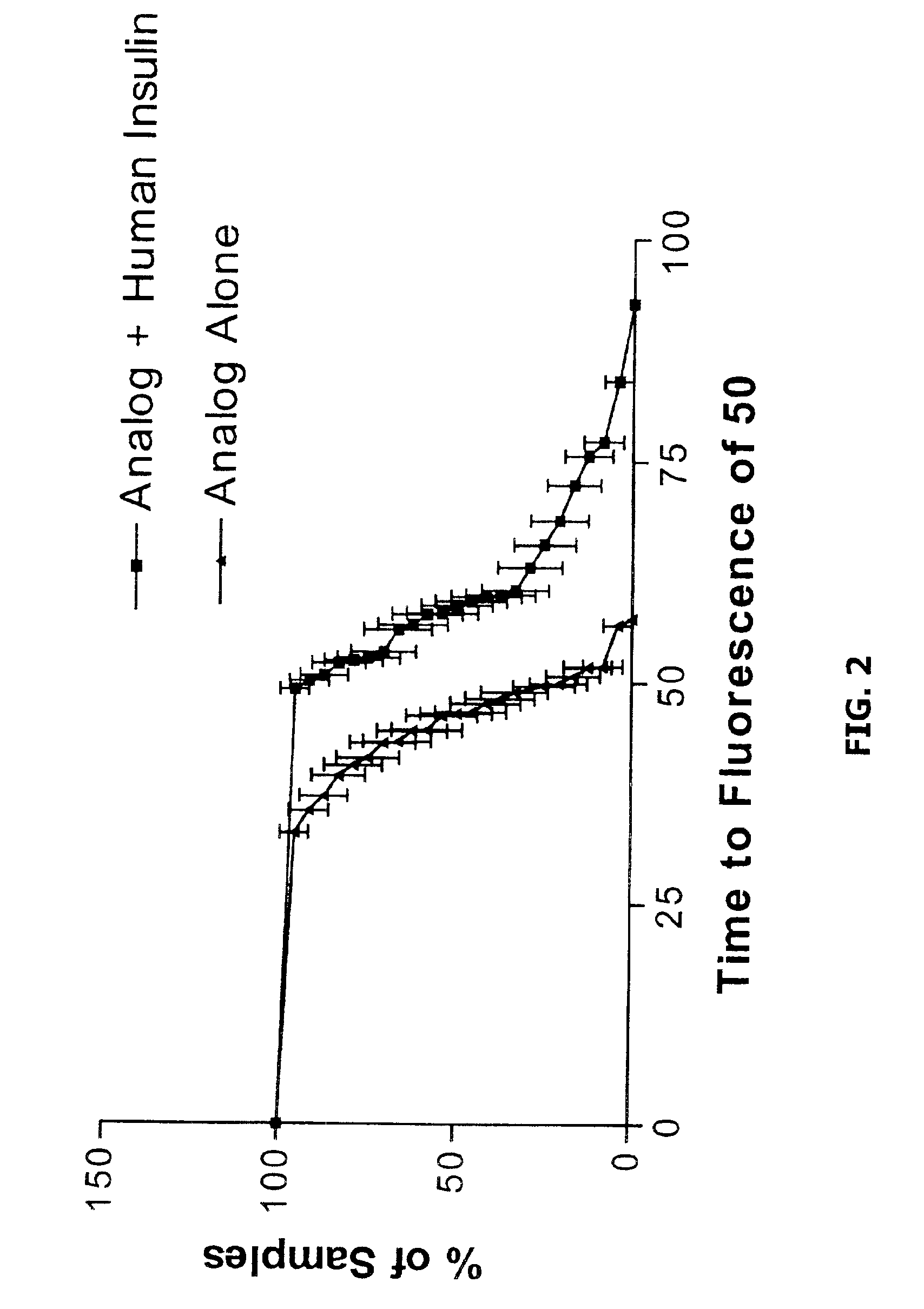
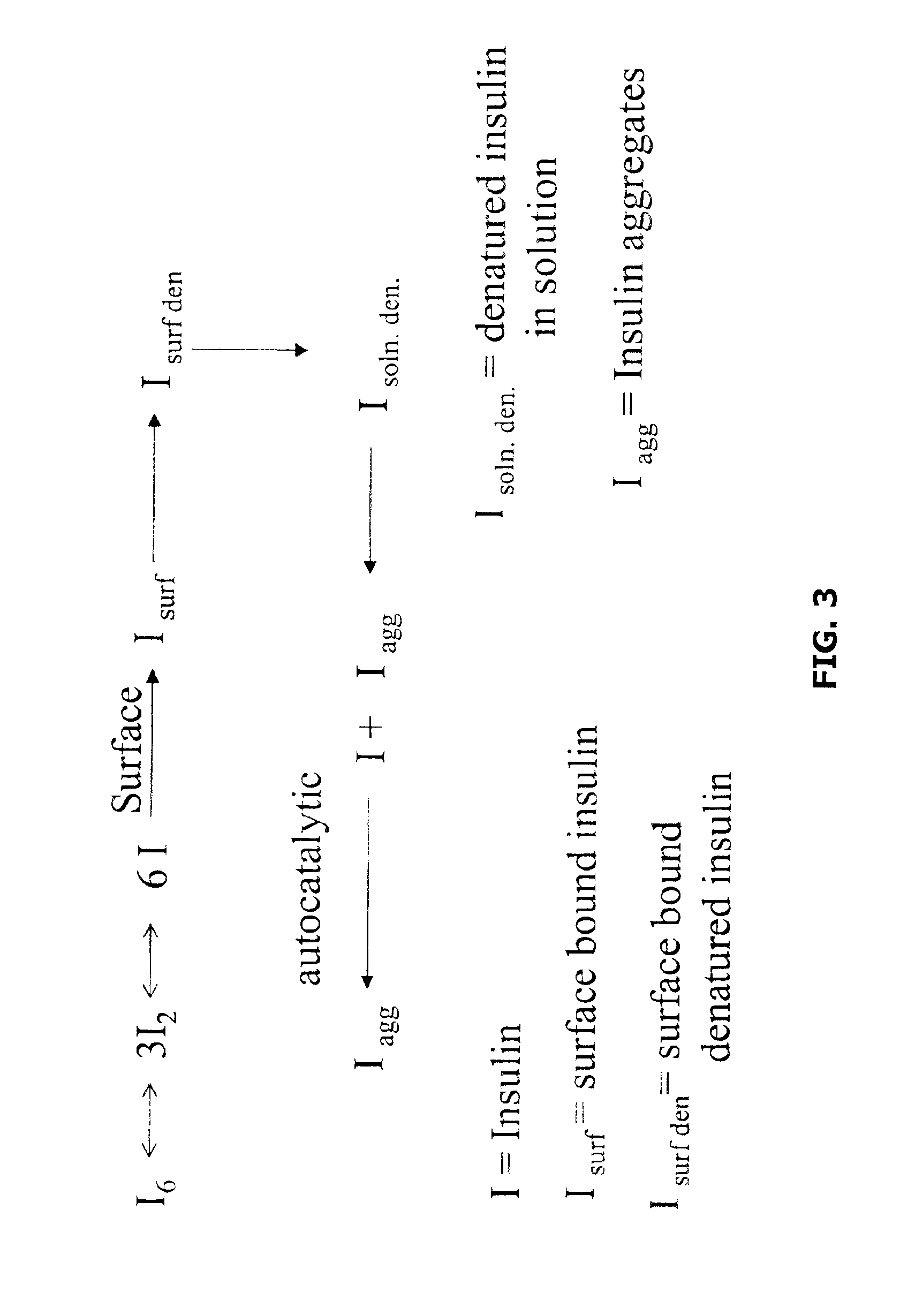
Methods of evaluating protein formulation stability and surfactant-stabilized insulin formulations derived therefrom
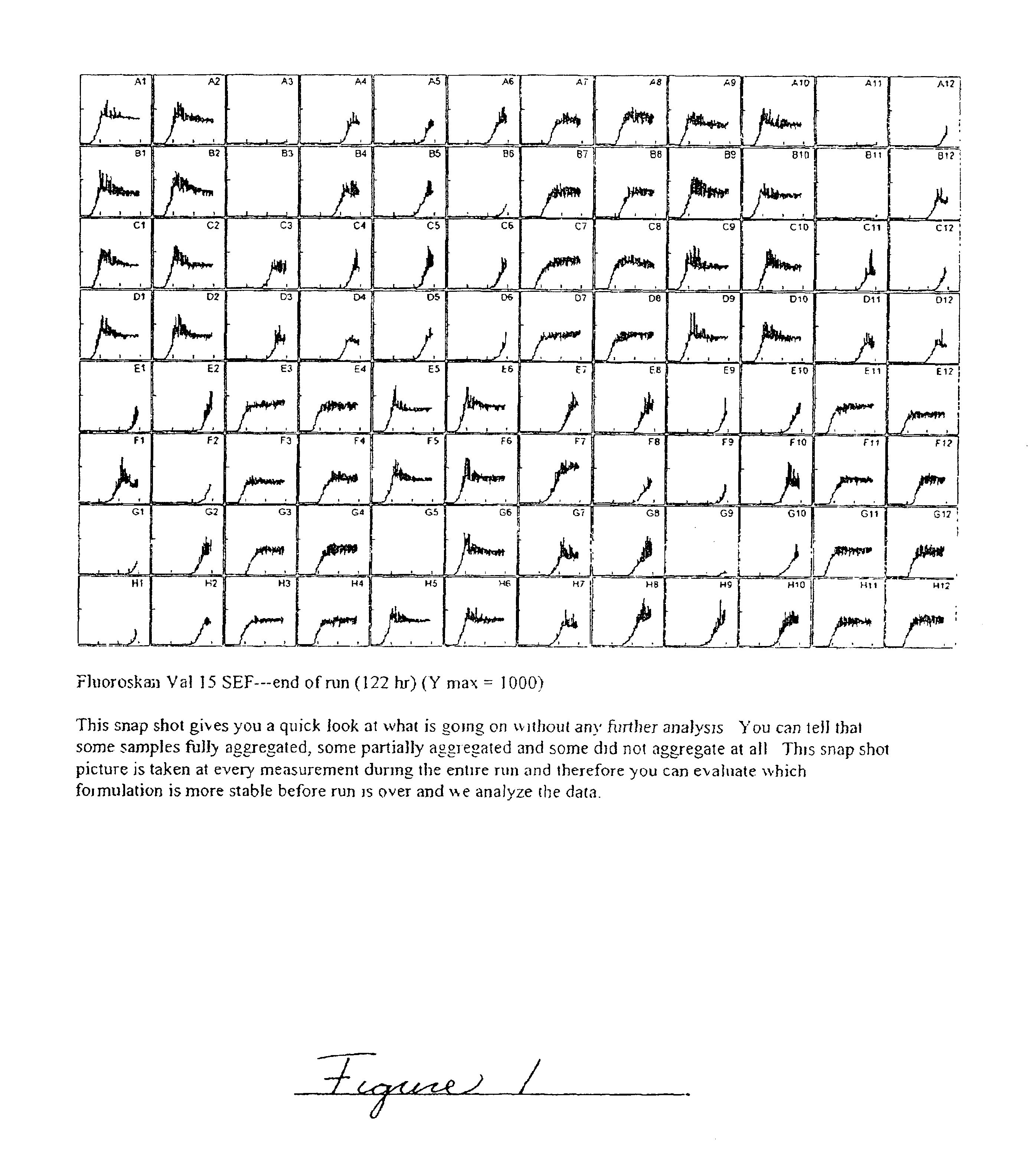
InactiveUS6737401B2Improve physical stabilityReliable timePeptide/protein ingredientsMicrobiological testing/measurementCell AggregationsProtein aggregation
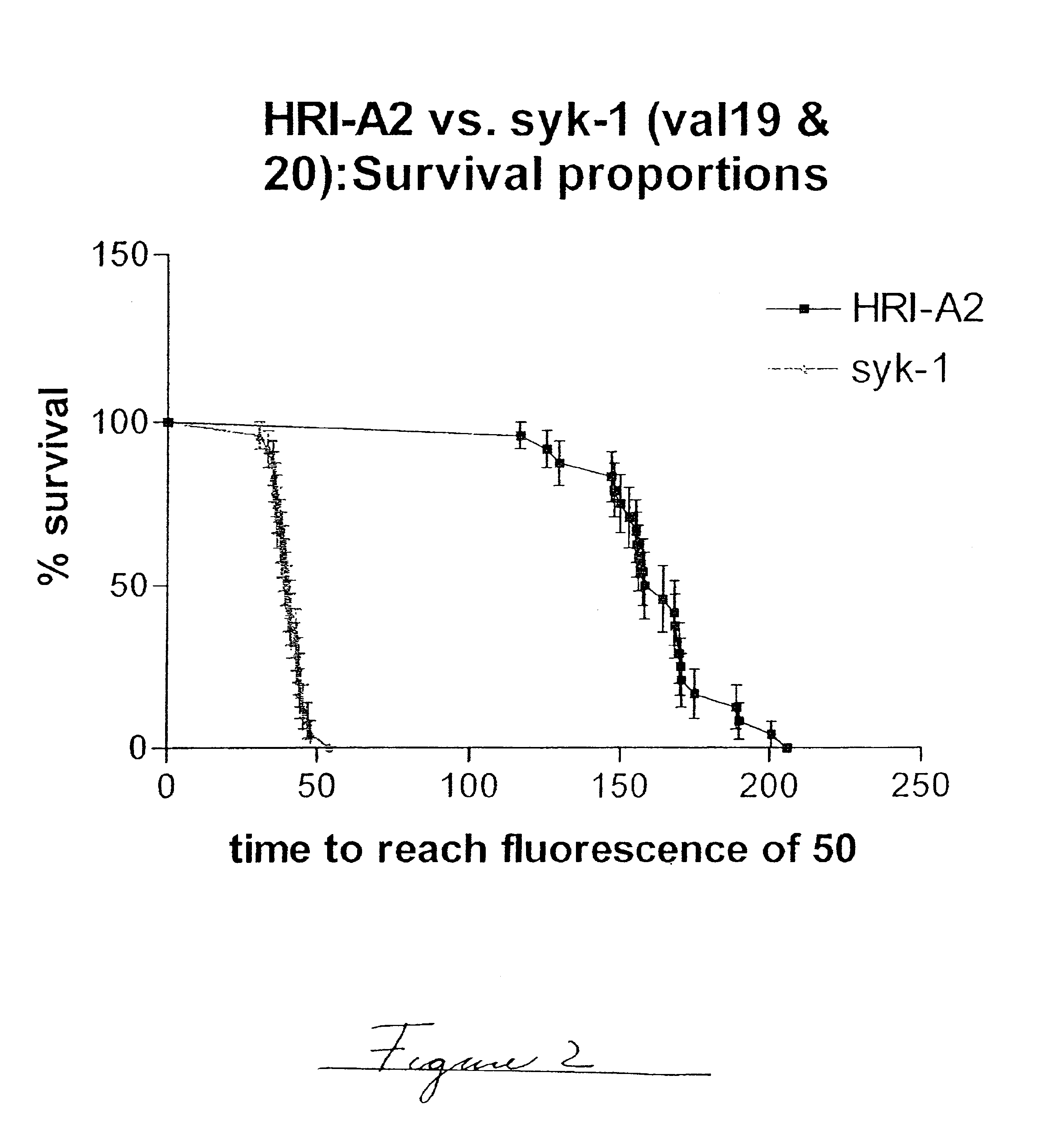
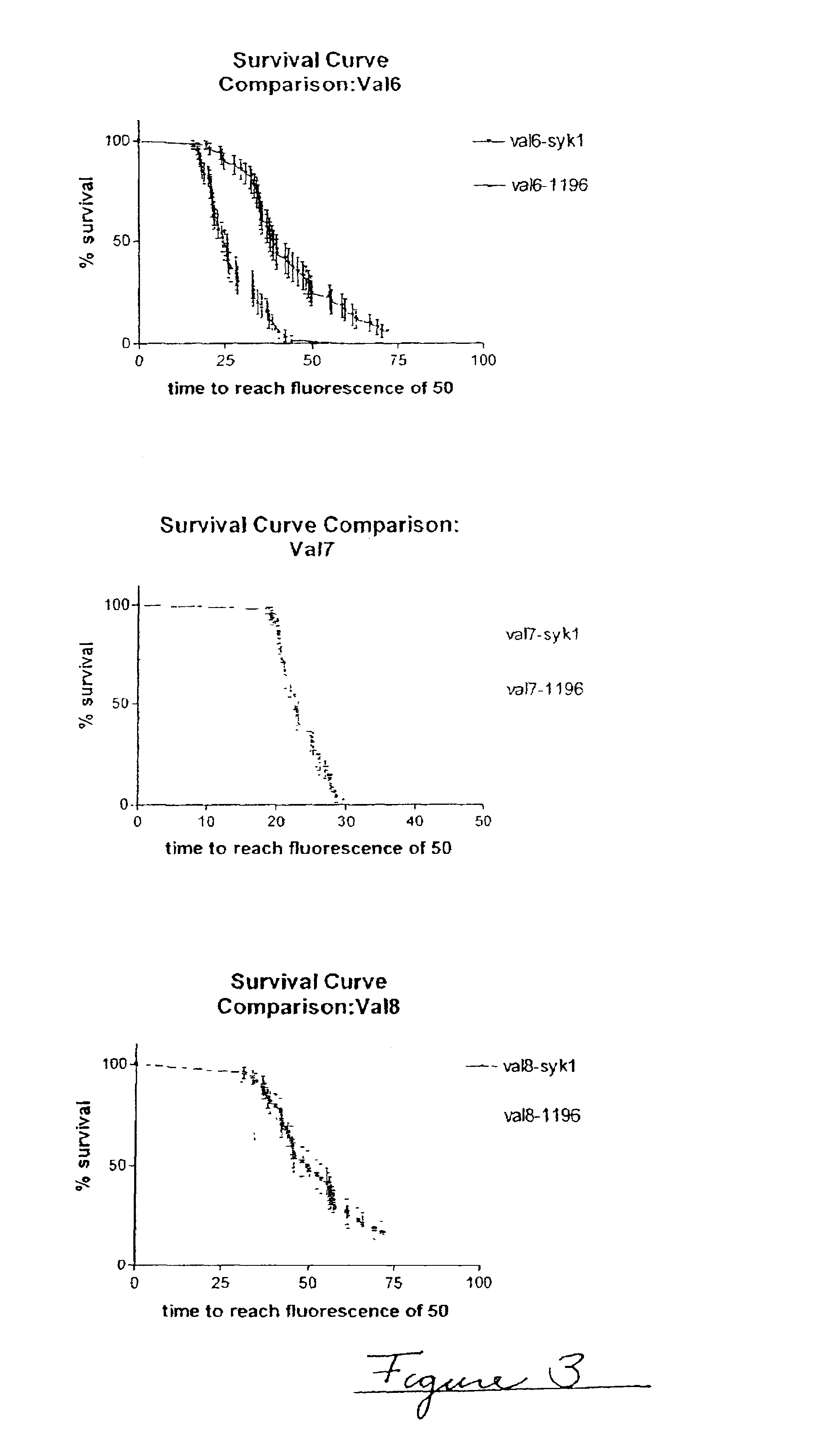
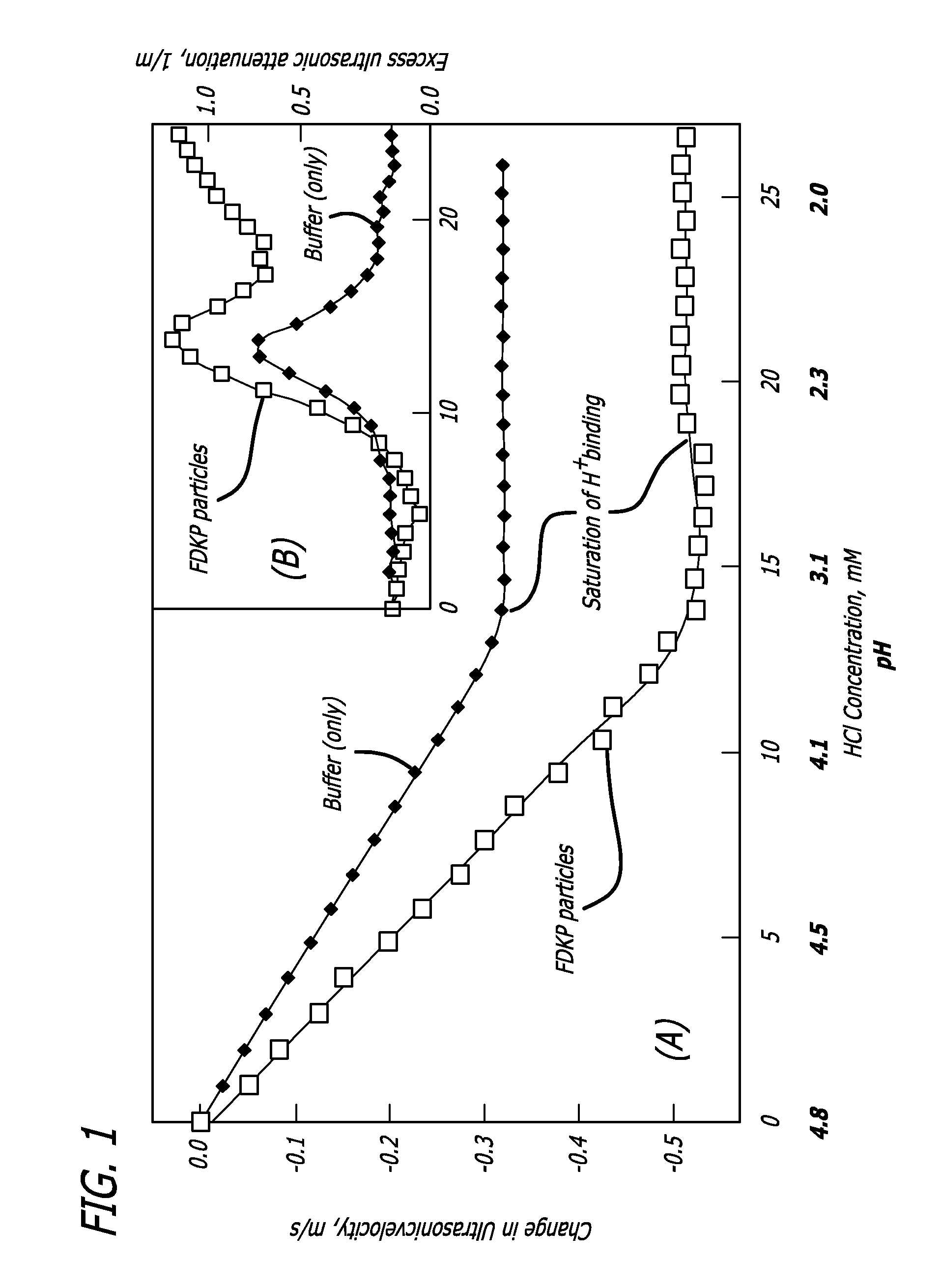
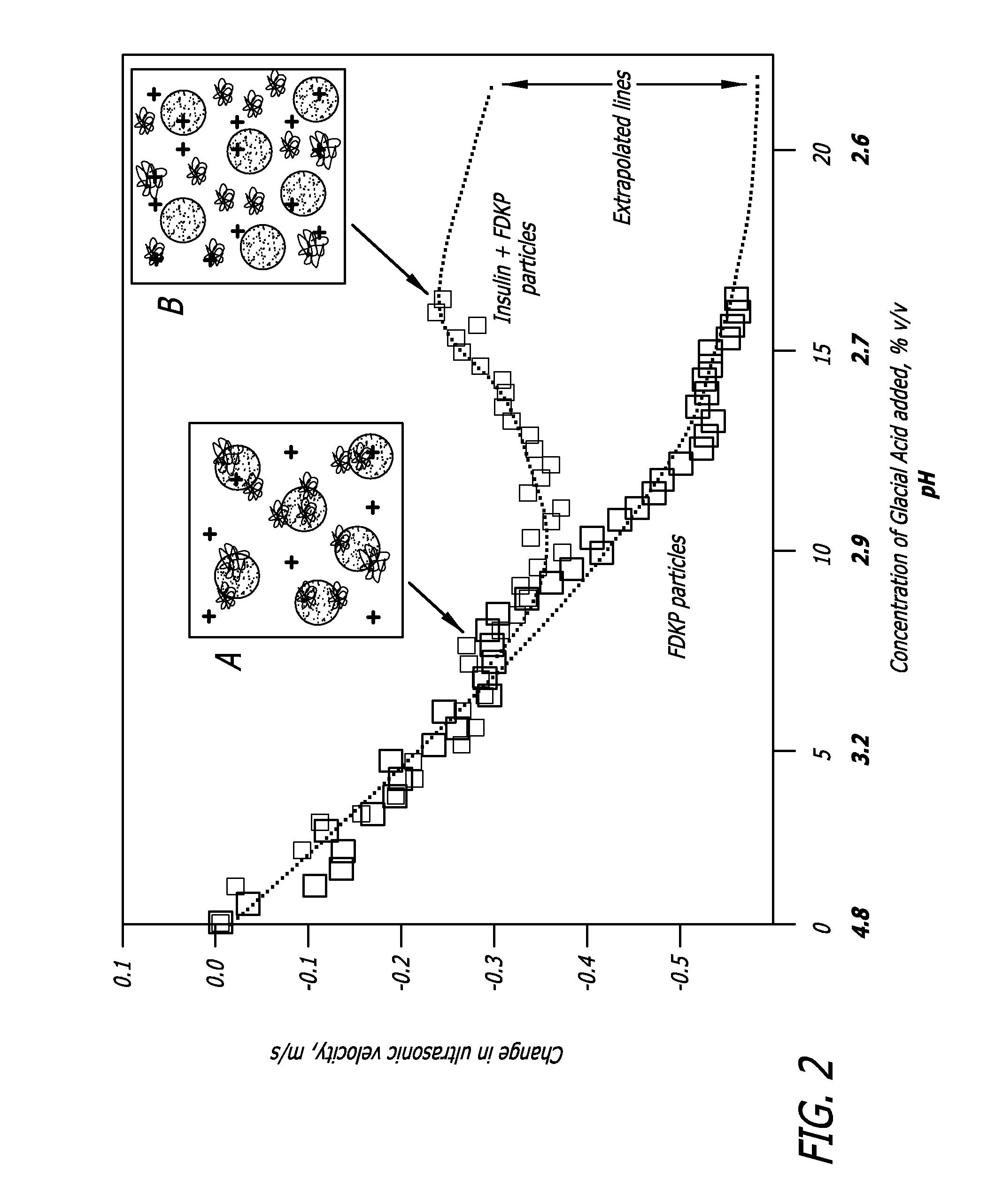
Embodiments of the invention are directed to a method of estimating the physical stability of a protein formulation. A particular embodiment of the invention places the protein formulation under an agitational stress that causes the protein to aggregate at an accelerated rate. In one embodiment, the change in protein aggregation is monitored spectroscopically using Thioflavin-T. Embodiments of the invention then utilize a survival curve analysis to ascertain the relative physical stability of the different protein formulations under study. This method was used to develop novel surfactant-stabilized insulin formulations in a rapid, cost efficient manner, thus illustrating the utility of the inventive method to the discovery and development of pharmaceutical protein formulations.
Owner:MEDTRONIC MIMIMED INC
Method of Drug Formulation Based on Increasing the affinity of Crystalline Microparticle Surfaces for Active Agents
Methods are provided for coating crystalline microparticles with an active agent by altering the surface properties of the microparticles in order to facilitate favorable association on the microparticle by the active agent. Type of surface properties that are altered by the disclosed methods include by electrostatic properties, hydrophobic properties and hydrogen bonding properties.
Owner:MANNKIND CORP
Polymer particles for delivery of macromolecules and methods of use
InactiveUS20070134332A1Slow down rate of bio-degradationImprove stabilityPowder deliveryNervous disorderPolyesterBound water
The present invention provides biodegradable polymer particle delivery compositions for delivery of macromolecular biologics, for example in crystal form, based on polymers, such as polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) polymers, which contain amino acids in the polymer. The polymer particle delivery compositions can be formulated either as a liquid dispersion or a lyophilized powder of polymer particles containing bound water molecules with the macromolecular biologics, for example insulin, dispersed in the particles. Bioactive agents, such as drugs, polypeptides, and polynucleotides can also be delivered by using particles sized for local, oral, mucosal or circulatory delivery. Methods of delivering a macromolecular biologic with substantial native activity to a subject, for example orally, are also included.
Owner:MEDIVAS LLC
Pulmonary administration of chemically modified insulin
InactiveUS6838076B2Reduce probabilityPowder deliveryPeptide/protein ingredientsPolymer modifiedEndocrinology
The present invention provides active, hydrophilic polymer-modified derivatives of insulin. The insulin derivatives of the invention are, in one aspect, suitable for delivery to the lung and exhibit pharmakokinetic and / or pharmacodynamic properties that are significantly improved over native insulin.
Owner:SHEARWATER CORP
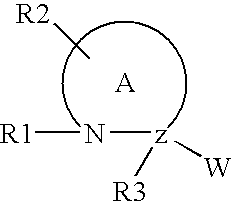
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonist peptides may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
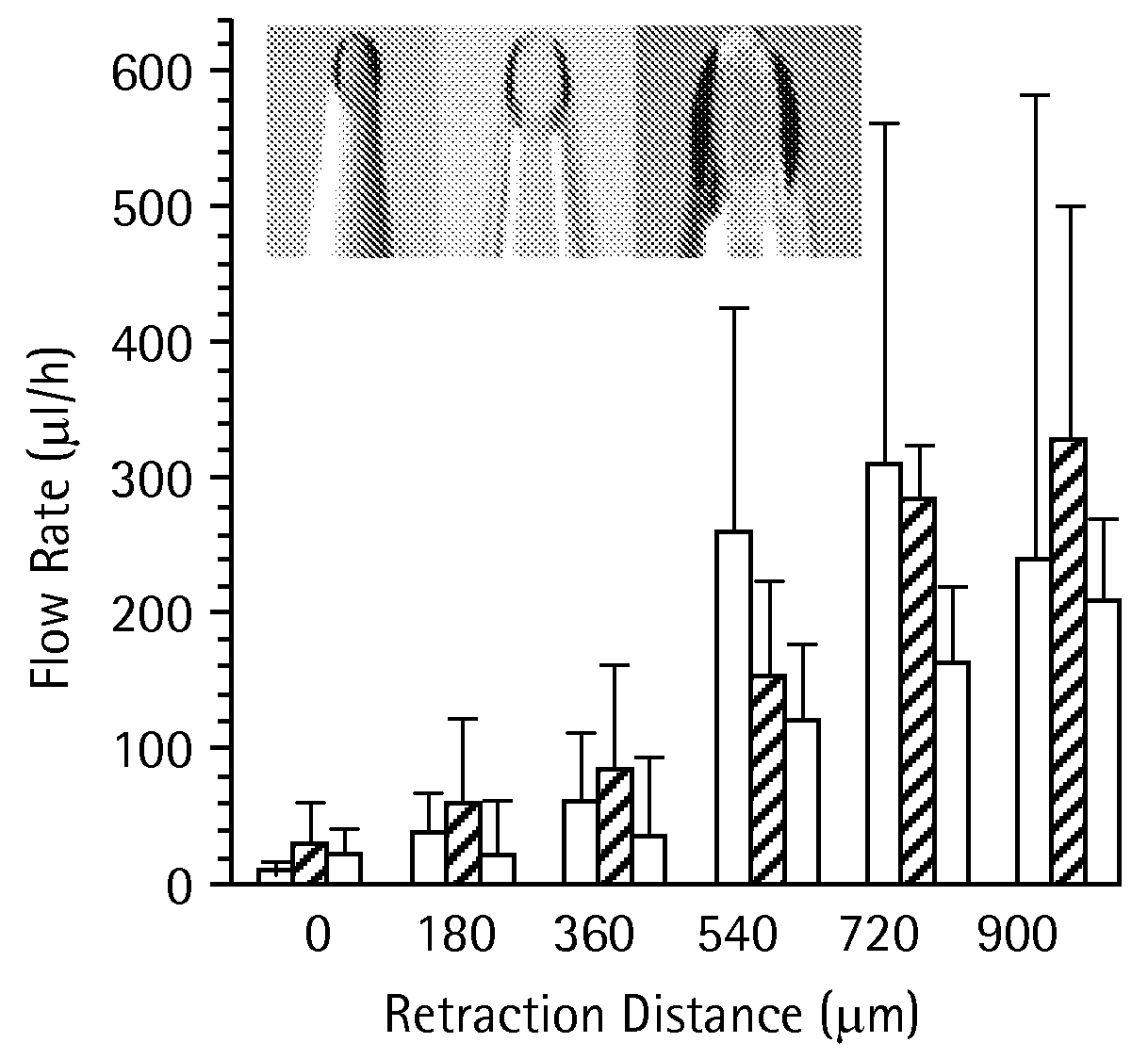
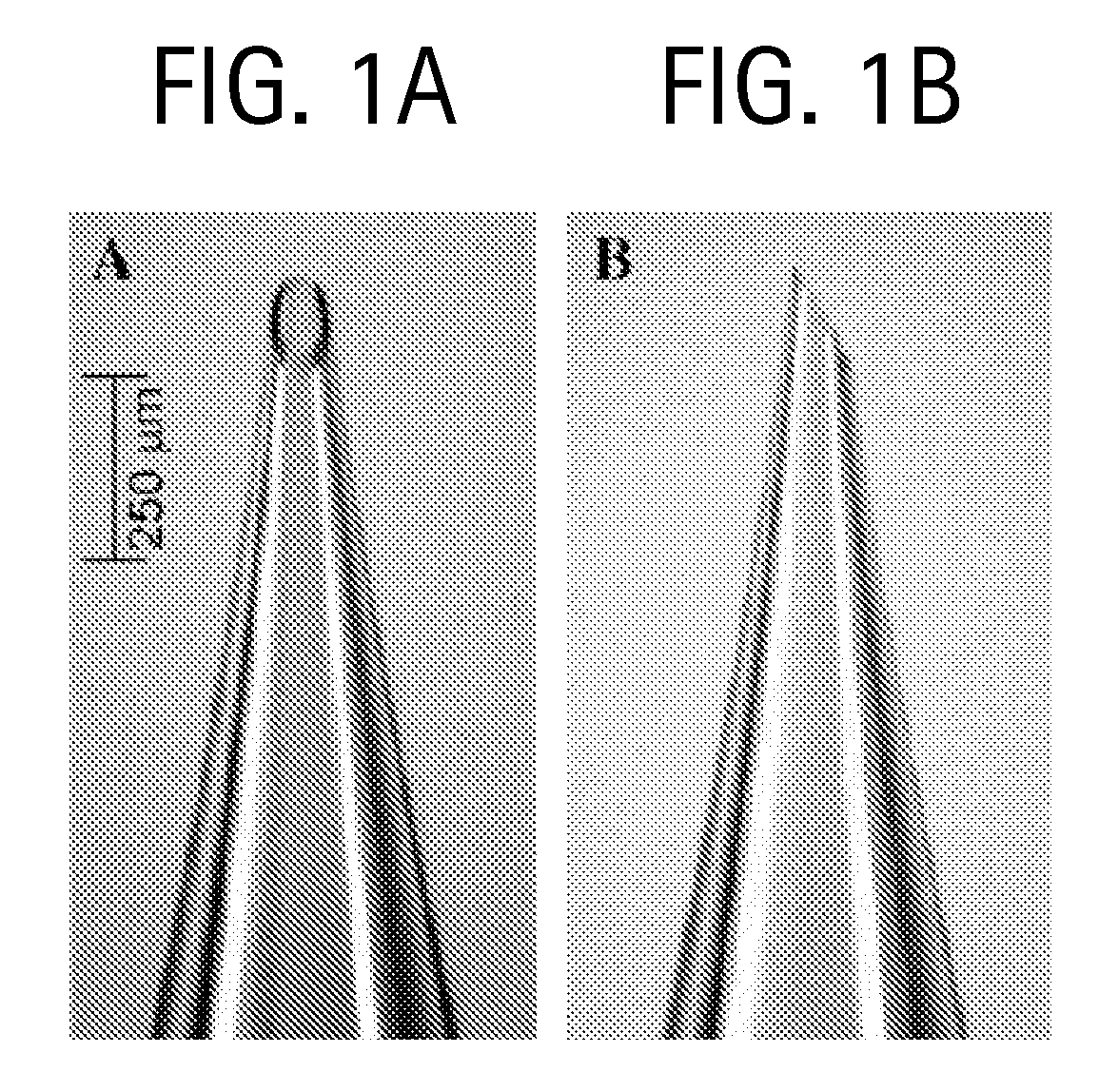
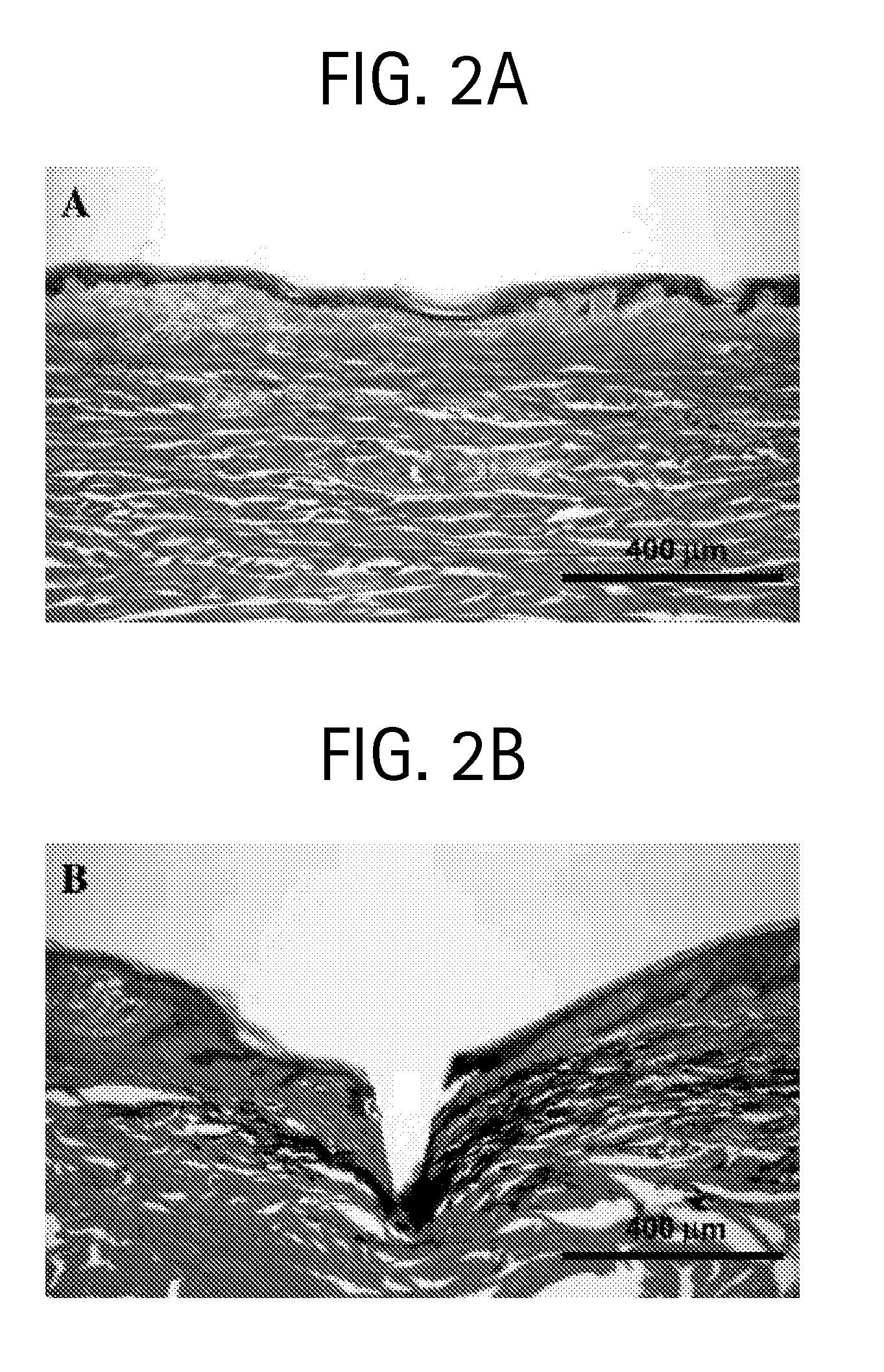
Microneedles and Methods for Microinfusion
Methods and devices are provided for delivering a drug to or withdrawing a fluid from a biological tissue, such the skin, sclera, cornea, and conjunctiva. One method includes the steps of inserting at least one microneedle into the biological tissue; partially retracting the at least one microneedle from the tissue; and then delivering at least one drug formulation into the biological tissue via the partially retracted at least one microneedle. The microneedle deforms and penetrates the biological tissue during the insertion step, and the retraction step at least partially relaxes the tissue deformation while maintaining at least part of the tissue penetration, facilitating drug delivery or fluid withdrawal.
Owner:GEORGIA TECH RES CORP
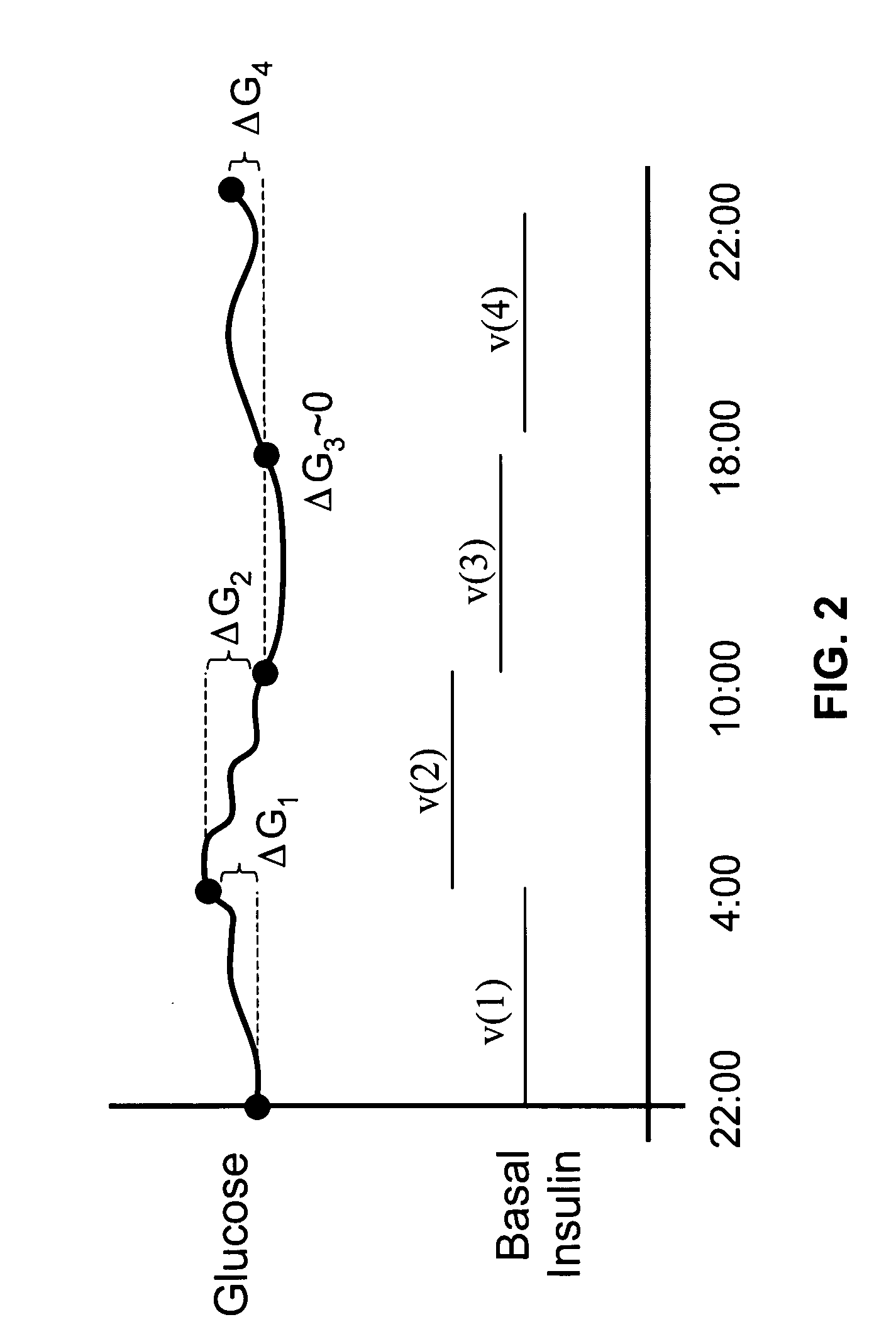
Method of food and insulin dose management for a diabetic subject
InactiveUS7137951B2Lower blood sugar levelsReduce the amount requiredPeptide/protein ingredientsDrug and medicationsINSULIN USEInsulin dose
The invention relates to a method of food and insulin dose management for a diabetic subject, comprising:providing an intended insulin unit value or an intended carbohydrate unit value representing the amount of insulin or carbohydrate intended for intake by the subject; anddetermining the balance value of either insulin units or carbohydrate units needed to balance with the provided unit value and maintain blood sugar in the subject in a target blood sugar range.
Owner:PILARSKI JOSEPH
Dipeptidyl peptidase IV inhibiting fluorinated cyclic amides
InactiveUS20040110817A1Ease of preparation and detectabilityGood metabolic stabilityBiocideSenses disorderDiabetic retinopathyDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
High efficient delivery of a large therapeutic mass aerosol
InactiveUS6858199B1Simple and cost-effectiveImprove efficiencyPowder deliveryLiquid surface applicatorsVolumetric Mass DensityIntensive care medicine
A method for delivering a therapeutic dose of a bioactive agent to the pulmonary system, in a single, breath-activated step, comprises administering from a receptacle enclosing a mass of particles, to a subject's respiratory tract, particles which have a tap density of less than 0.4 g / cm3 and deliver at least about 50% of the mass of particles. Another method of delivering a therapeutic dose of a bioactive agent to the pulmonary system, in a single breath, includes administering from a receptacle enclosing a mass of particles, to a subject's respiratory tract, particles which have a tap density of at least 0.4 g / cm3 and deliver at least about 10 milligrams of the bioactive agent. The receptacle can have a volume of at least 0.37 cm3.
Owner:CIVITAS THERAPEUTICS
Rapid acting drug delivery compositions
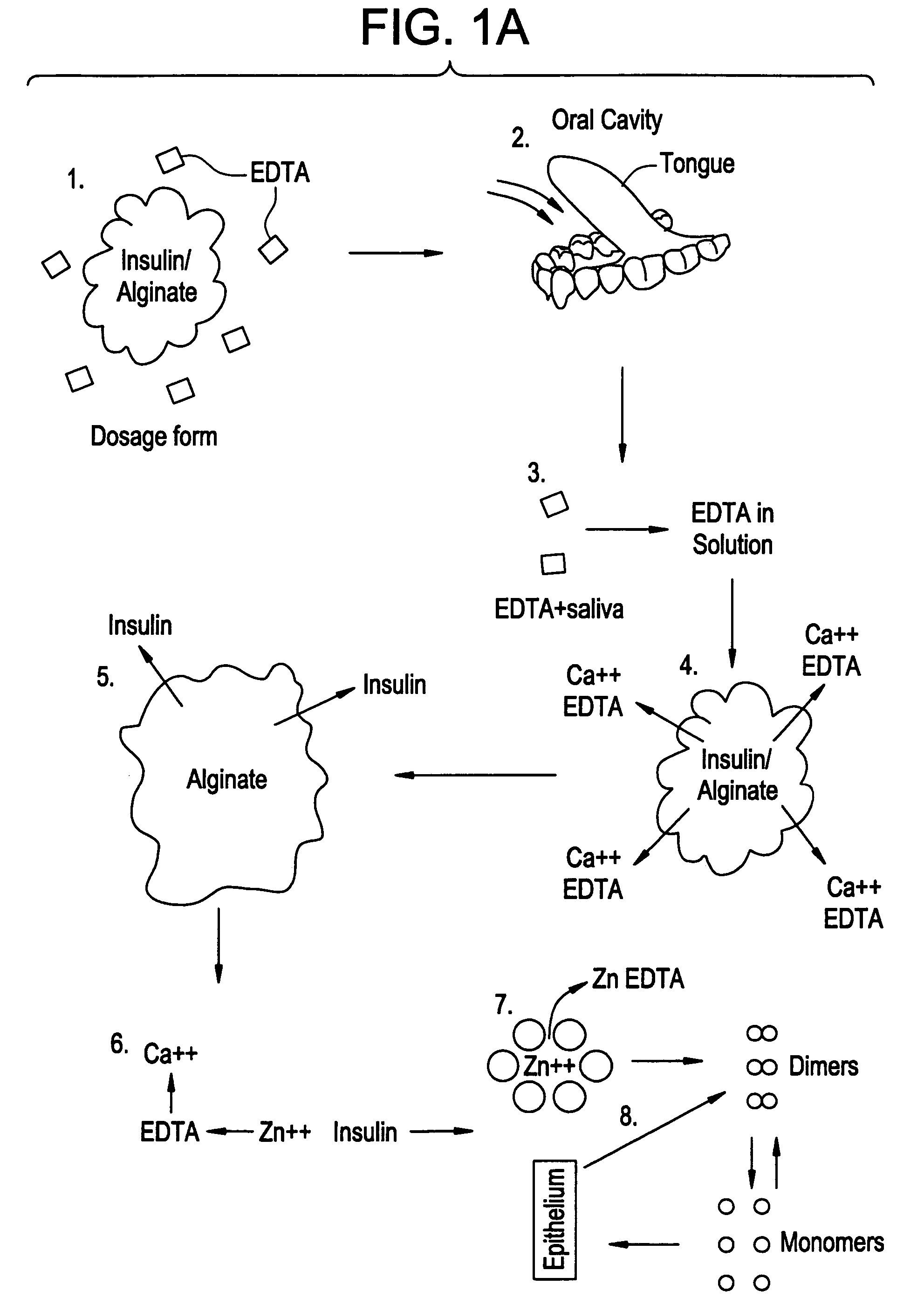
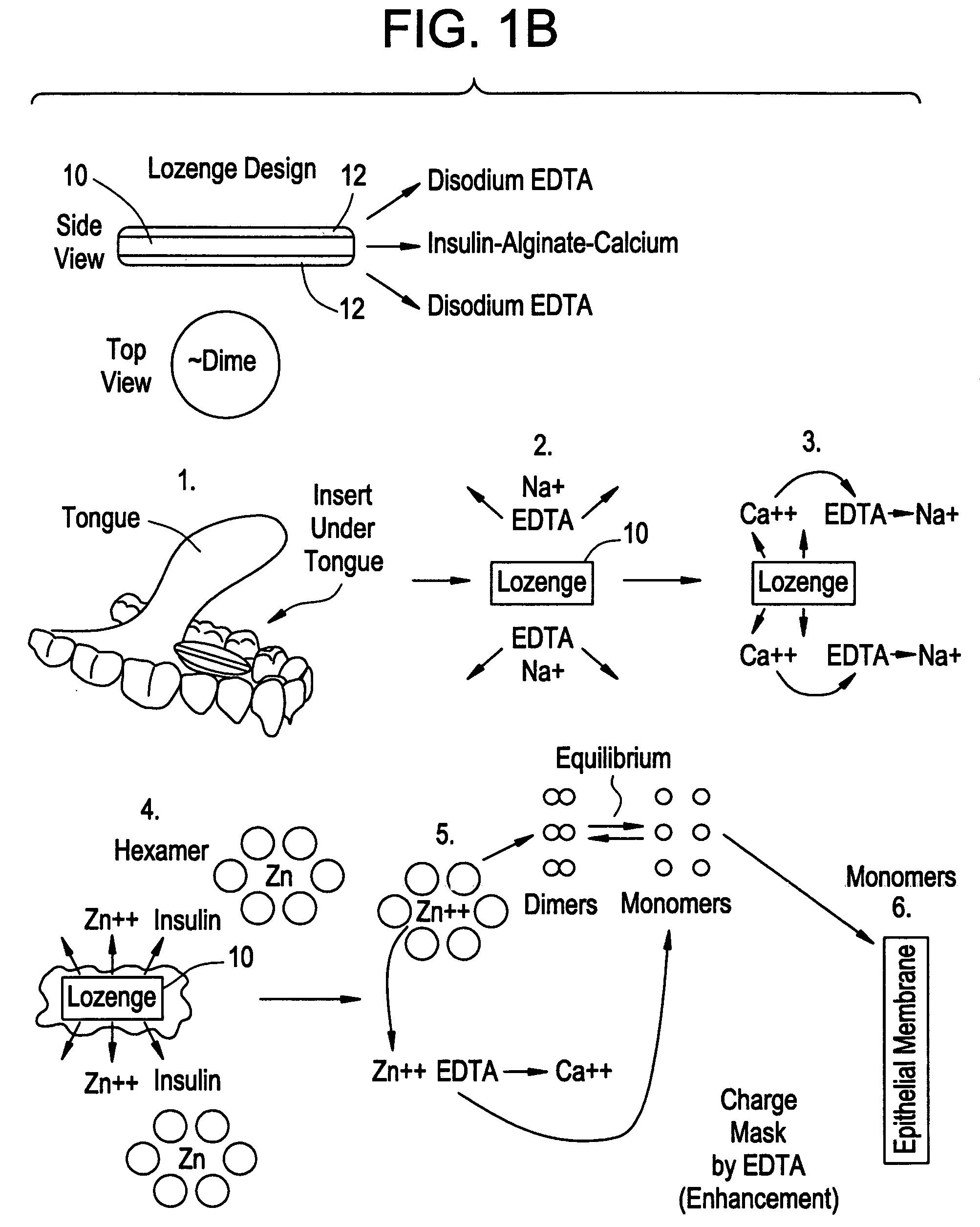
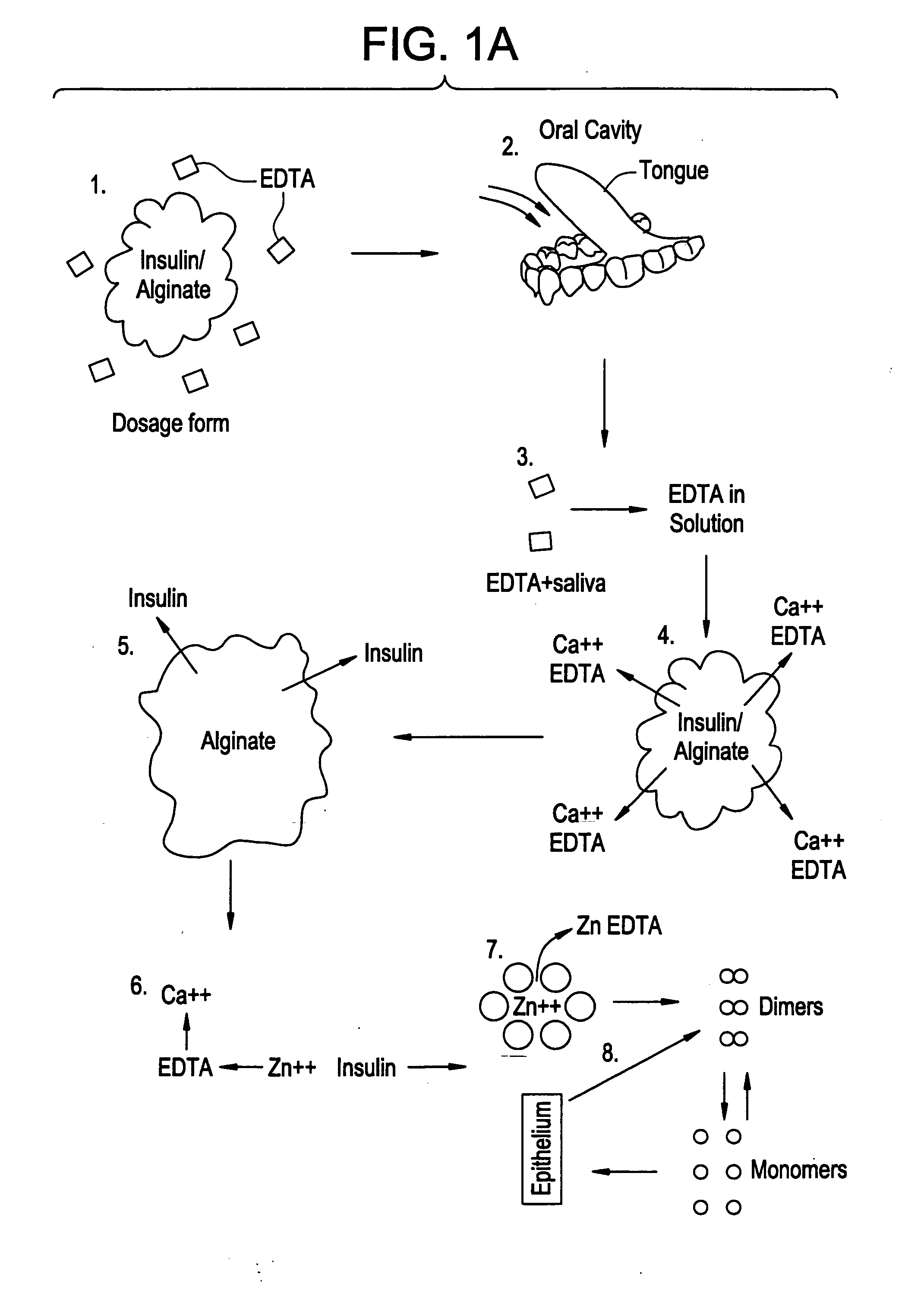
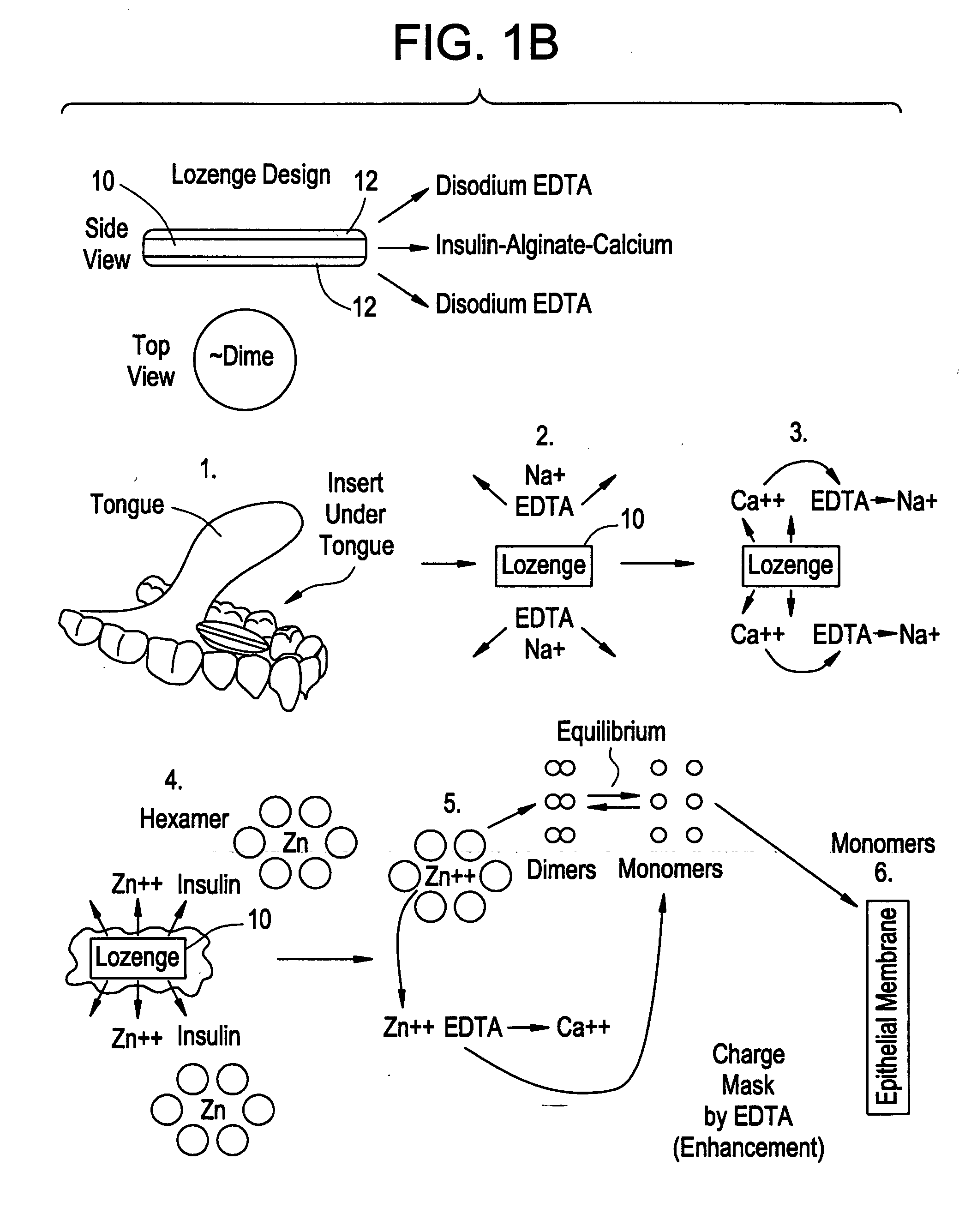
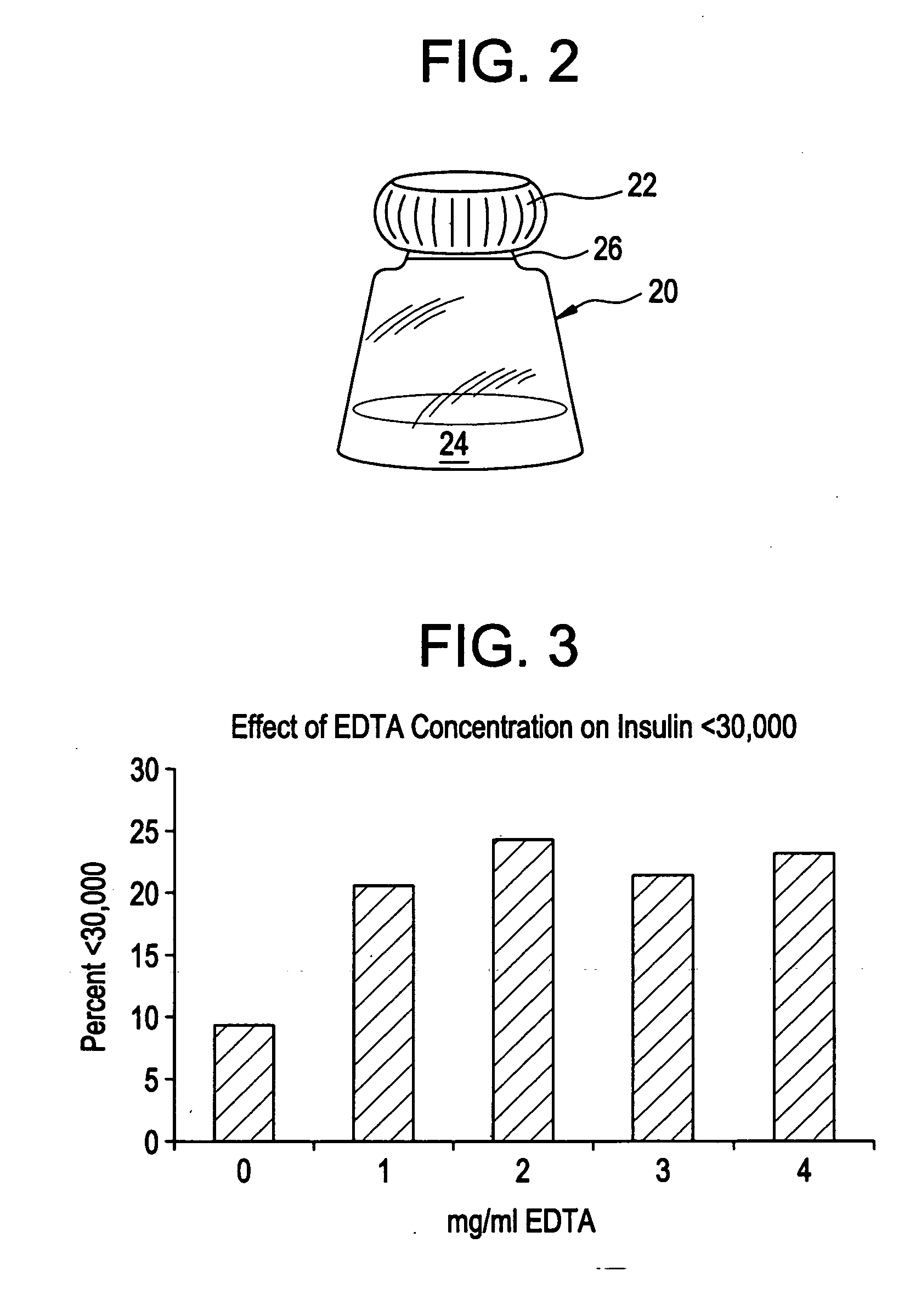
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Method for producing powder formulation comprising an insulin
The present invention relates to a process for producing a therapeutic powder formulation, comprising (a) providing an acidic aqueous solution comprising an insulin or analoguc or derivative thereof and an enhancer; (b) adjusting the pH to a pH in the range of 4.5 to 7.4; (c) precipitating a product comprising the insulin or analogue or derivative thereof and the enhancer, wherein the precipitation is performed essentially without evaporation of the solution; and (d) removing the water.
Owner:NOVO NORDISK AS
Method of Drug Formulation Based on Increasing the Affinity of Active Agents for Crystalline Microparticle Surfaces
ActiveUS20070059374A1Reduce solubilityPromote associationPowder deliveryOrganic active ingredientsActive agentMicroparticle
Methods are provided for promoting the adsorption of an active agent to microparticles by modifying the structural properties of the active agent in order to facilitate favorable association to the microparticle.
Owner:MANNKIND CORP
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
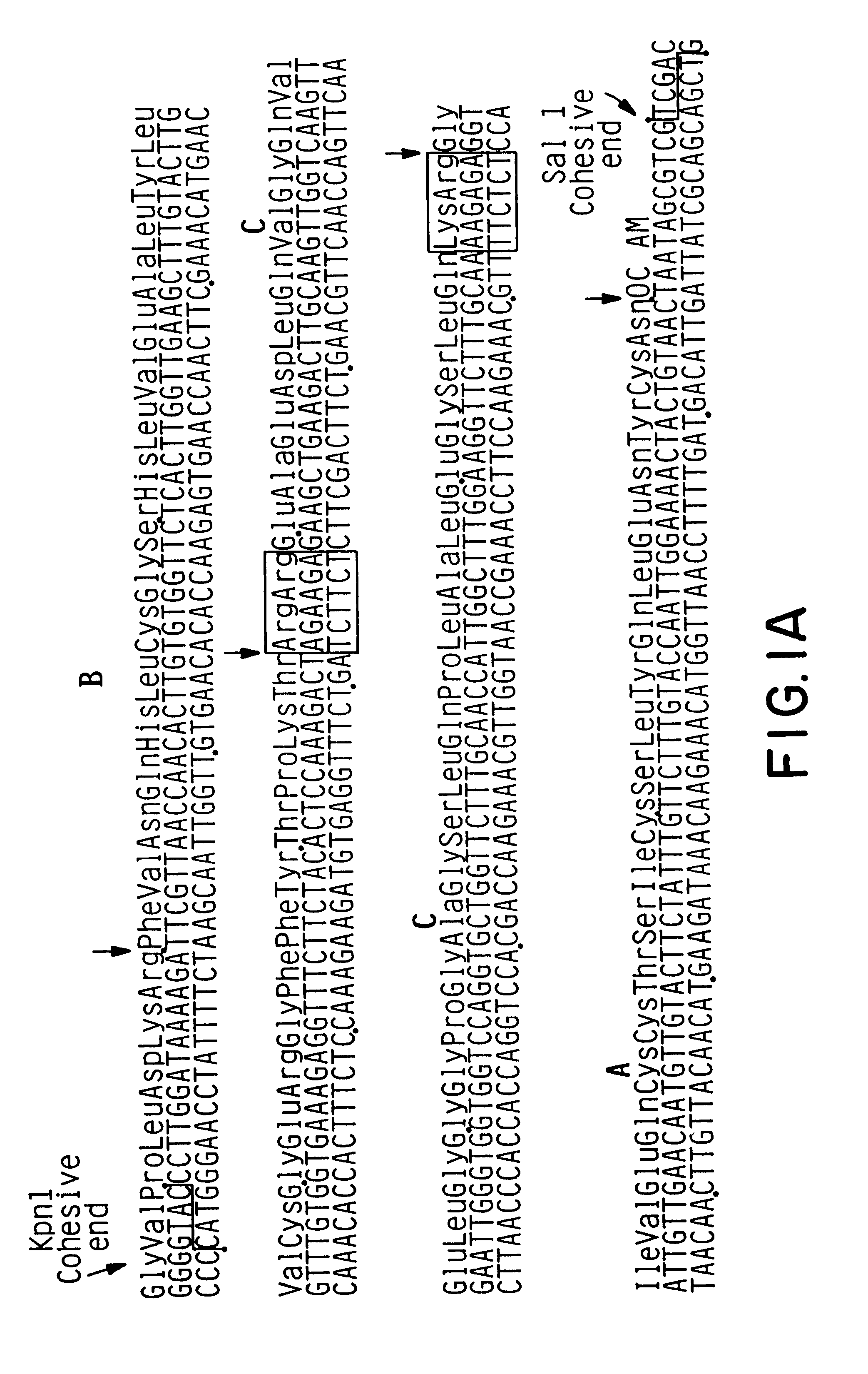
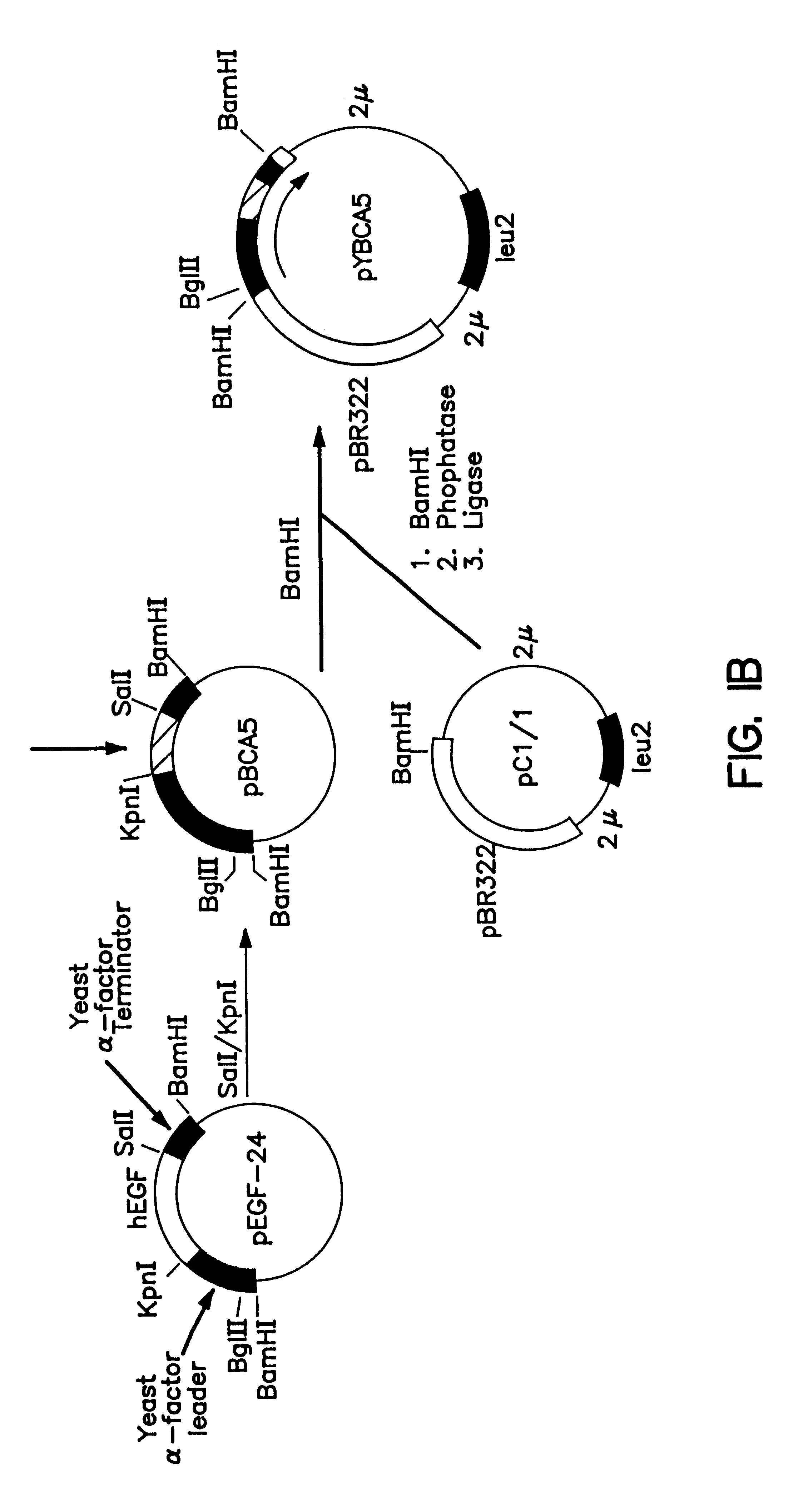
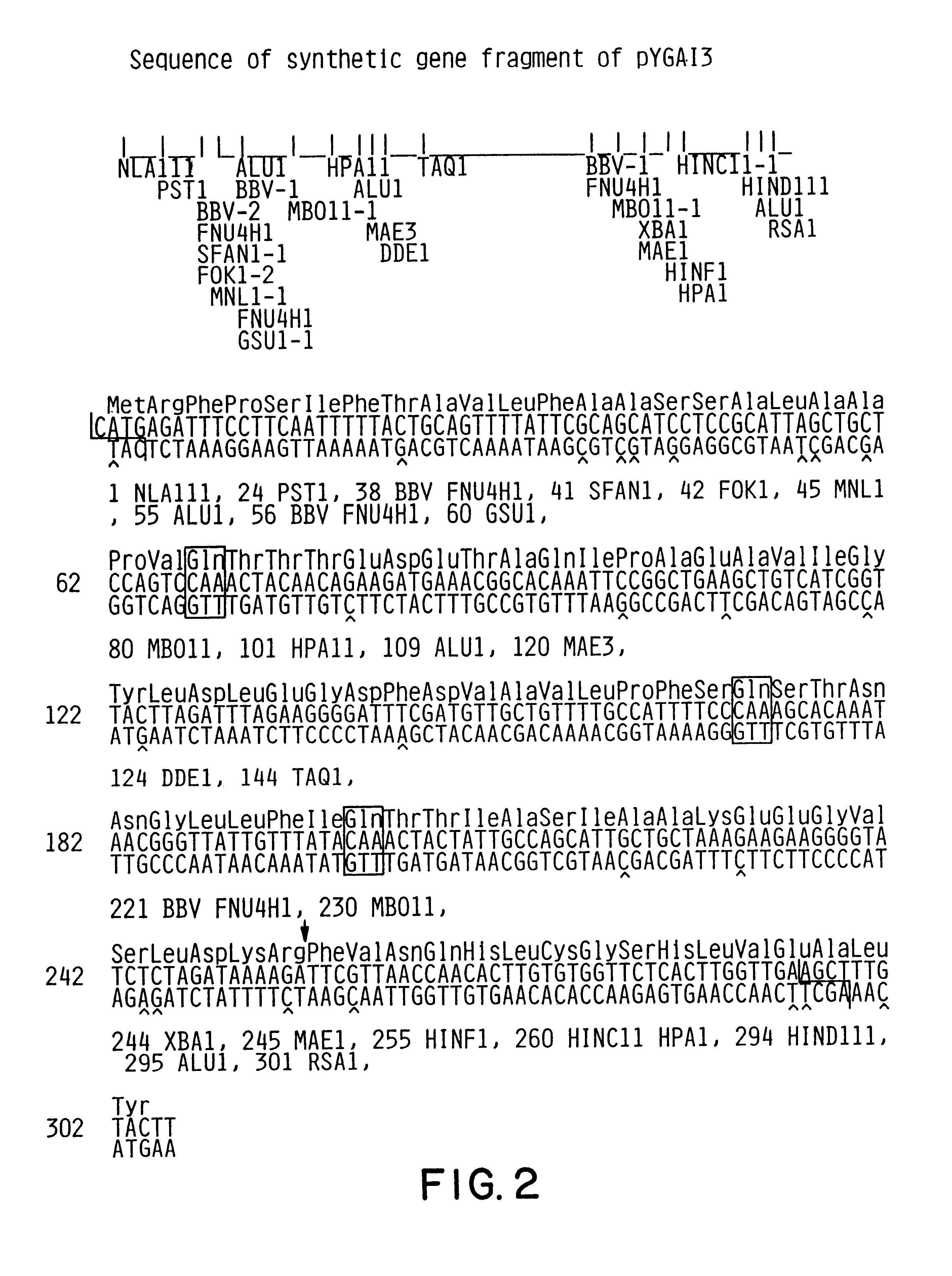
Expression and secretion of heterologous proteins in yeast employing truncated alpha-factor leader sequences
InactiveUSRE37343E1Efficiently direct expressionEfficient secretionPolypeptide with localisation/targeting motifFungiHeterologousBiotechnology
A yeast alpha-factor expression system is provided comprised of a truncated leader sequence, containing the alpha-factor signal peptide and one glycosylation site, linked by a processing site to a non-yeast protein-encoding sequence.
Owner:CHIRON CORP
Method of regulating glucose metabolism, and reagents related thereto
InactiveUS20030153509A1Reduce insulin resistanceExcellent hostBiocideDipeptide ingredientsAcute hyperglycaemiaChylomicron
The present invention provides methods and compositions for modification and regulation of glucose and lipid metabolism, generally to reduce insulin resistance, hyperglycemia, hyperinsulinemia, obesity, hyperlipidemia, hyperlipoprotein-emia (such as chylomicrons, VLDL and LDL), and to regulate body fat and more generally lipid stores, and, more generally, for the improvement of metabolism disorders, especially those associated with diabetes, obesity and / or atherosclerosis.
Owner:1149336 ONTARIO +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com