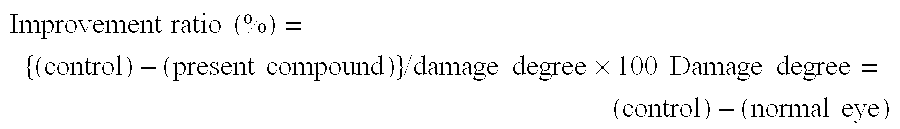Patents
Literature
403 results about "Conjunctiva" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
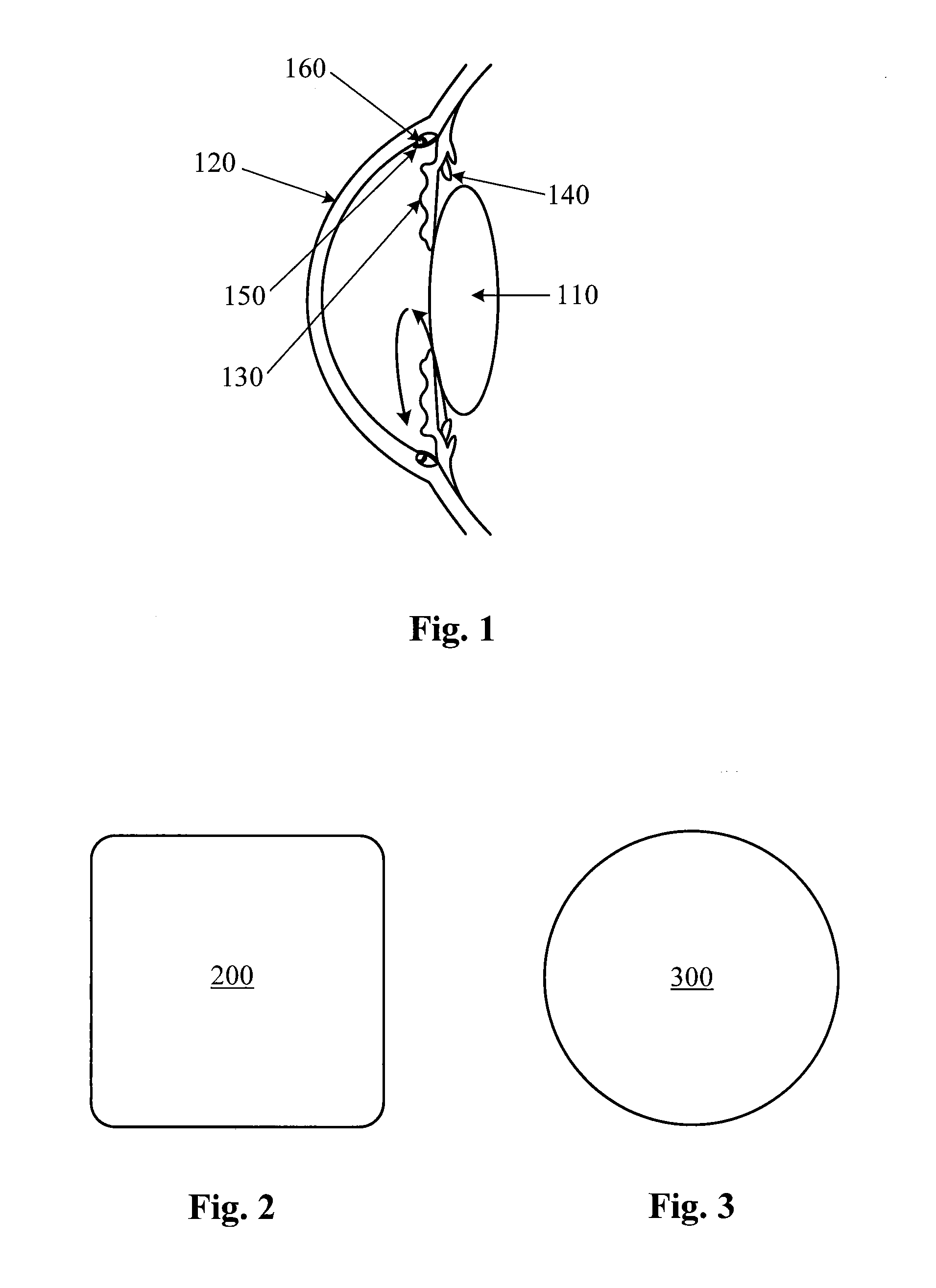
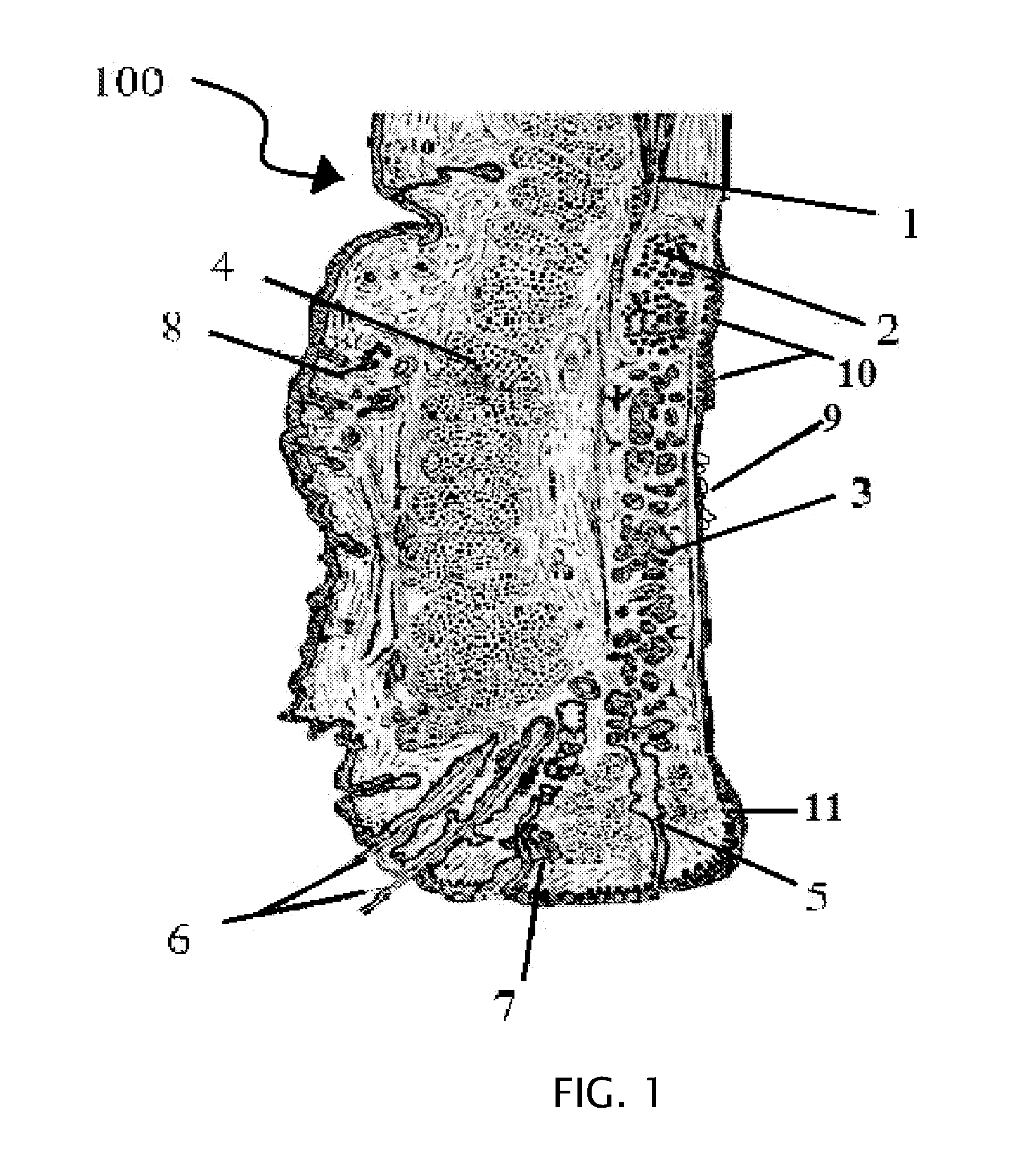
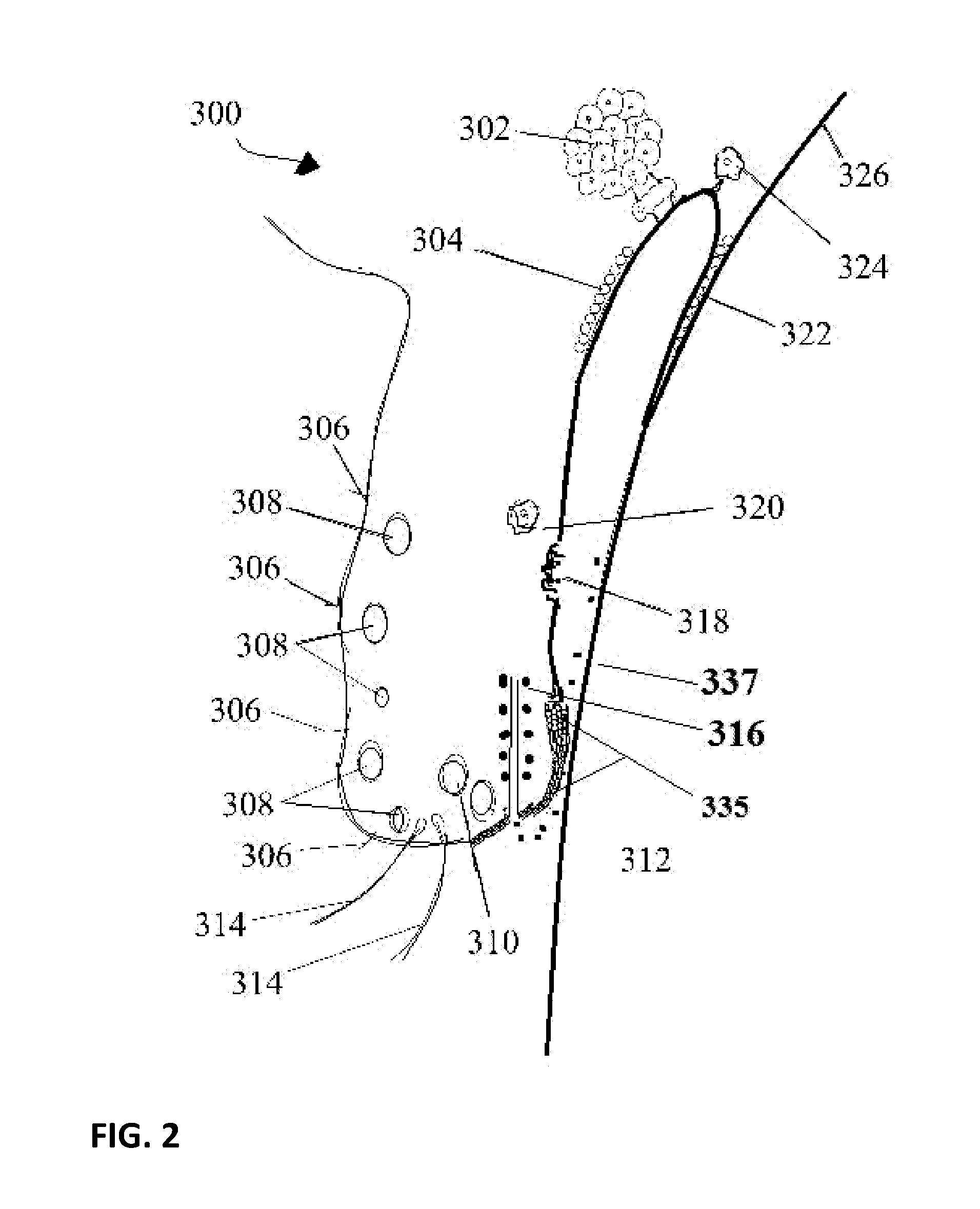
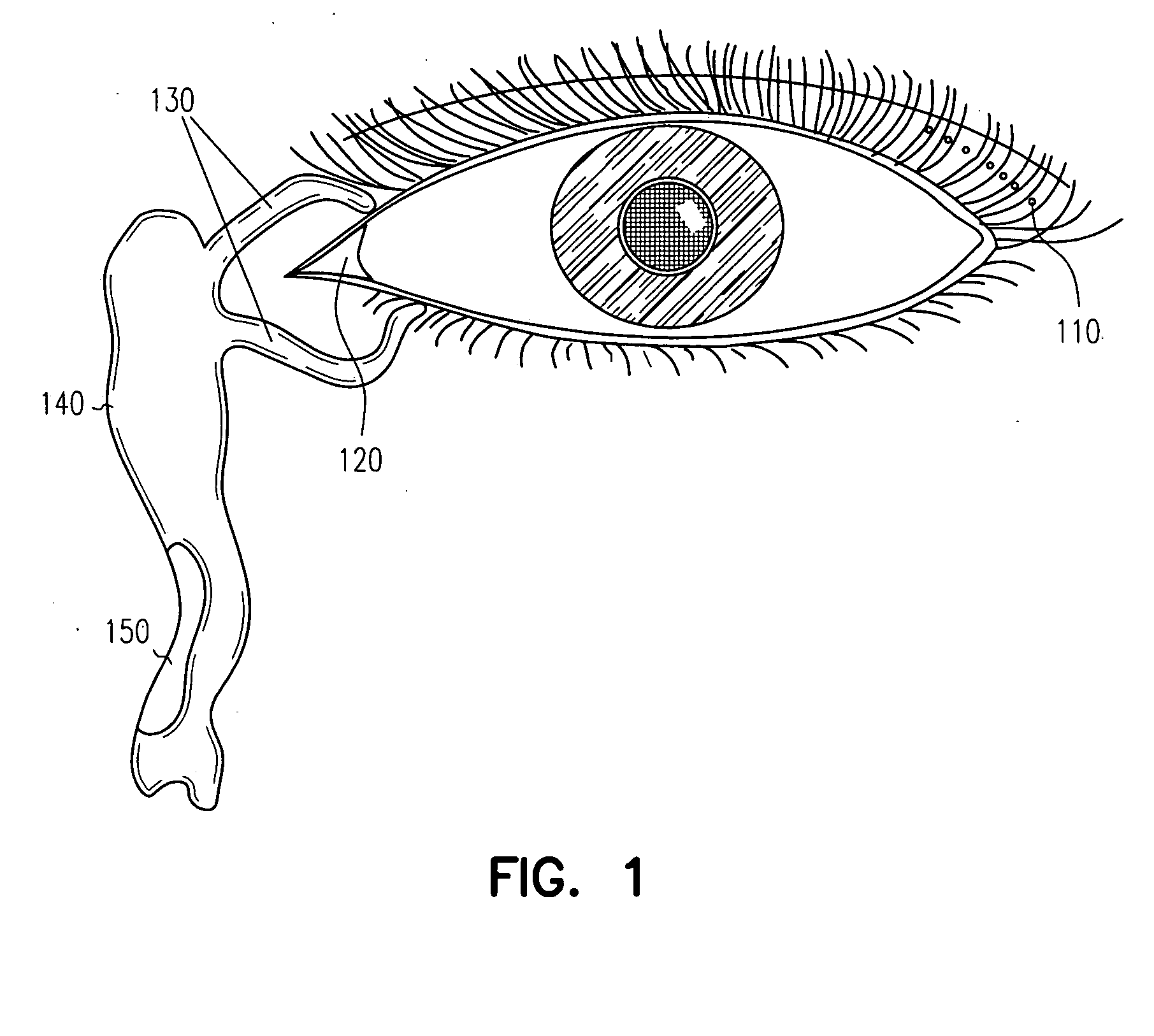
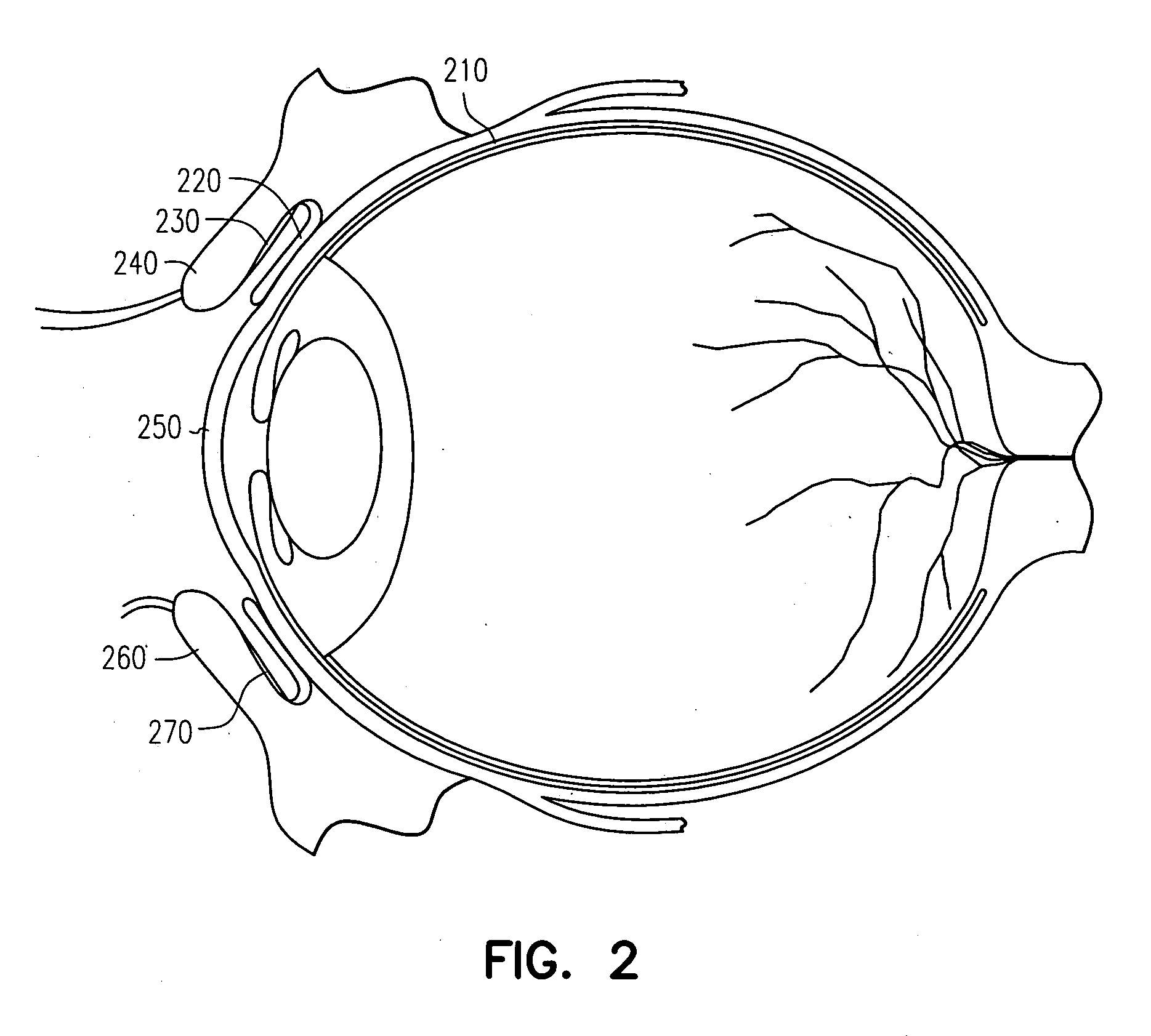
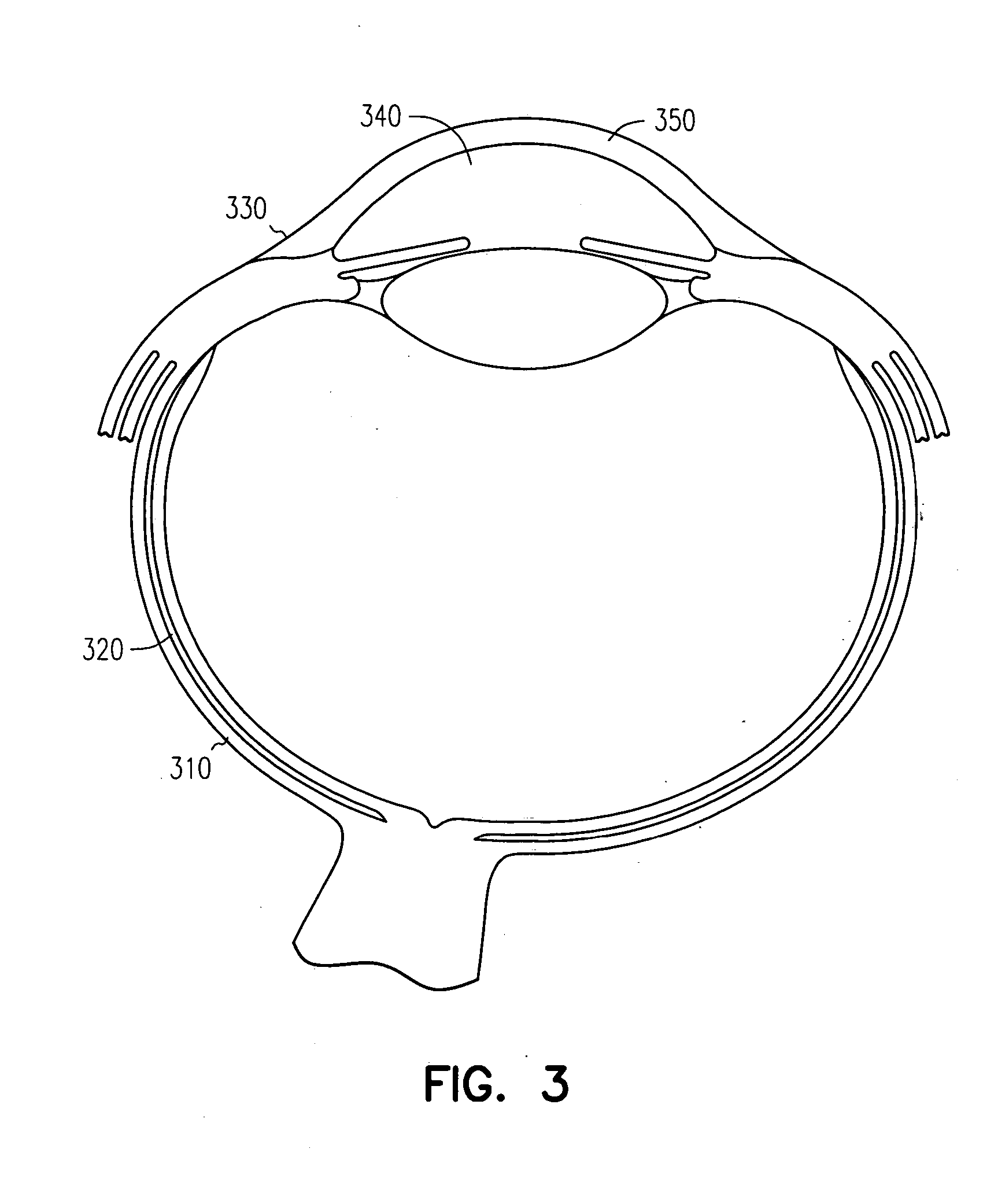
The conjunctiva is a tissue that lines the inside of the eyelids and covers the sclera (the white of the eye). It is composed of unkeratinized, stratified squamous epithelium with goblet cells, and stratified columnar epithelium. The conjunctiva is highly vascularised, with many microvessels easily accessible for imaging studies.
Noninvasive measurement of chemical substances
InactiveUS7041063B2High densityEarly diagnosisOrganic active ingredientsHeart defibrillatorsConjunctivaInfrared
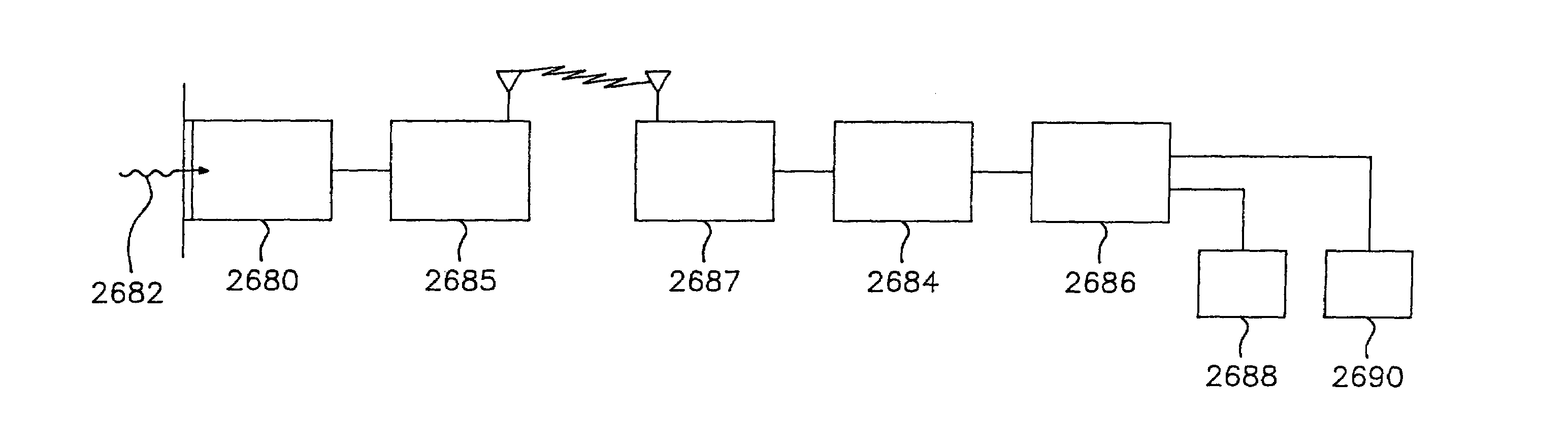
Utilization of a contact device placed on the eye in order to detect physical and chemical parameters of the body as well as the non-invasive delivery of compounds according to these physical and chemical parameters, with signals being transmitted continuously as electromagnetic waves, radio waves, infrared and the like. One of the parameters to be detected includes non-invasive blood analysis utilizing chemical changes and chemical products that are found in the conjunctiva and in the tear film. A transensor mounted in the contact device laying on the cornea or the surface of the eye is capable of evaluating and measuring physical and chemical parameters in the eye including non-invasive blood analysis. The system utilizes eye lid motion and / or closure of the eye lid to activate a microminiature radio frequency sensitive transensor mounted in the contact device. The signal can be communicated by wires or radio telemetered to an externally placed receiver. The signal can then be processed, analyzed and stored. Several parameters can be detected including a complete non-invasive analysis of blood components, measurement of systemic and ocular blood flow, measurement of heart rate and respiratory rate, tracking operations, detection of ovulation, detection of radiation and drug effects, diagnosis of ocular and systemic disorders and the like.
Owner:GEELUX HLDG LTD
Surgical correction of human eye refractive errors by active composite artificial muscle implants
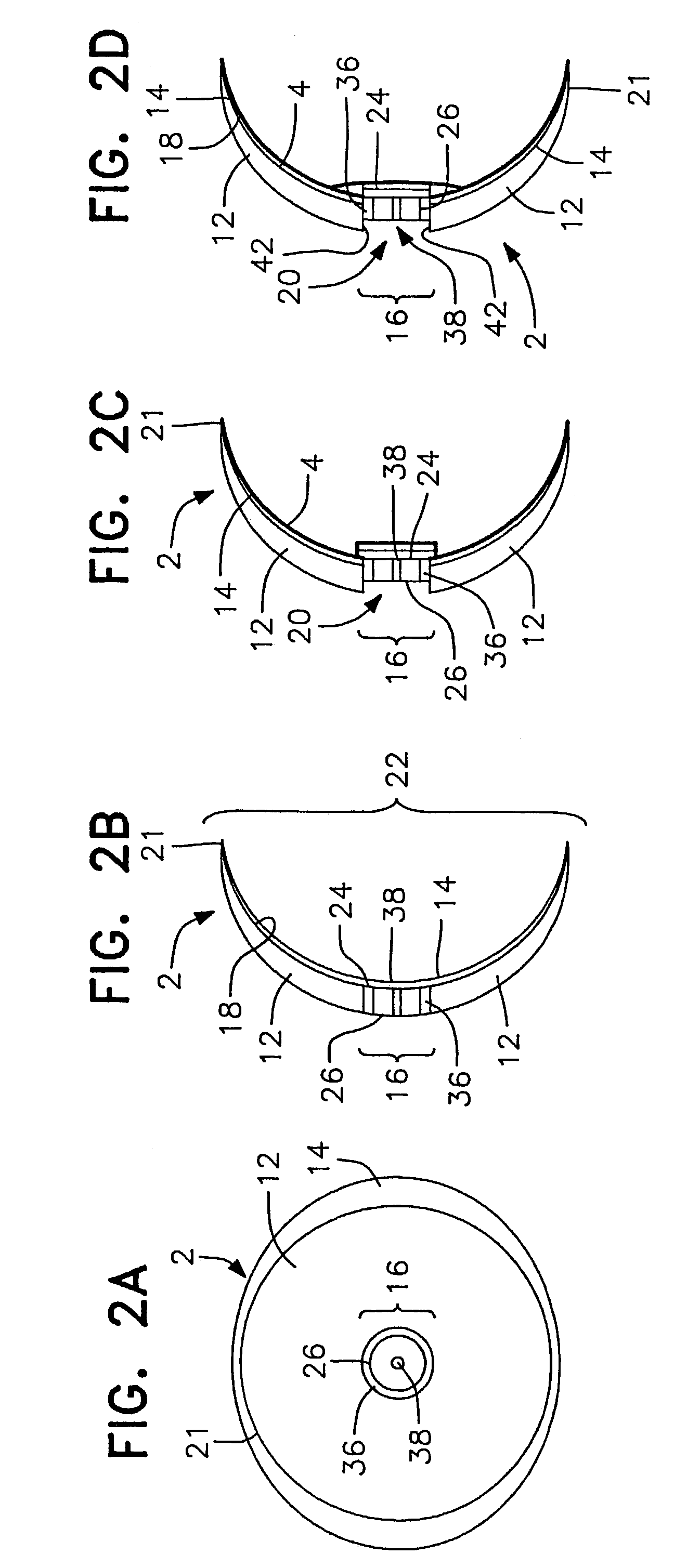
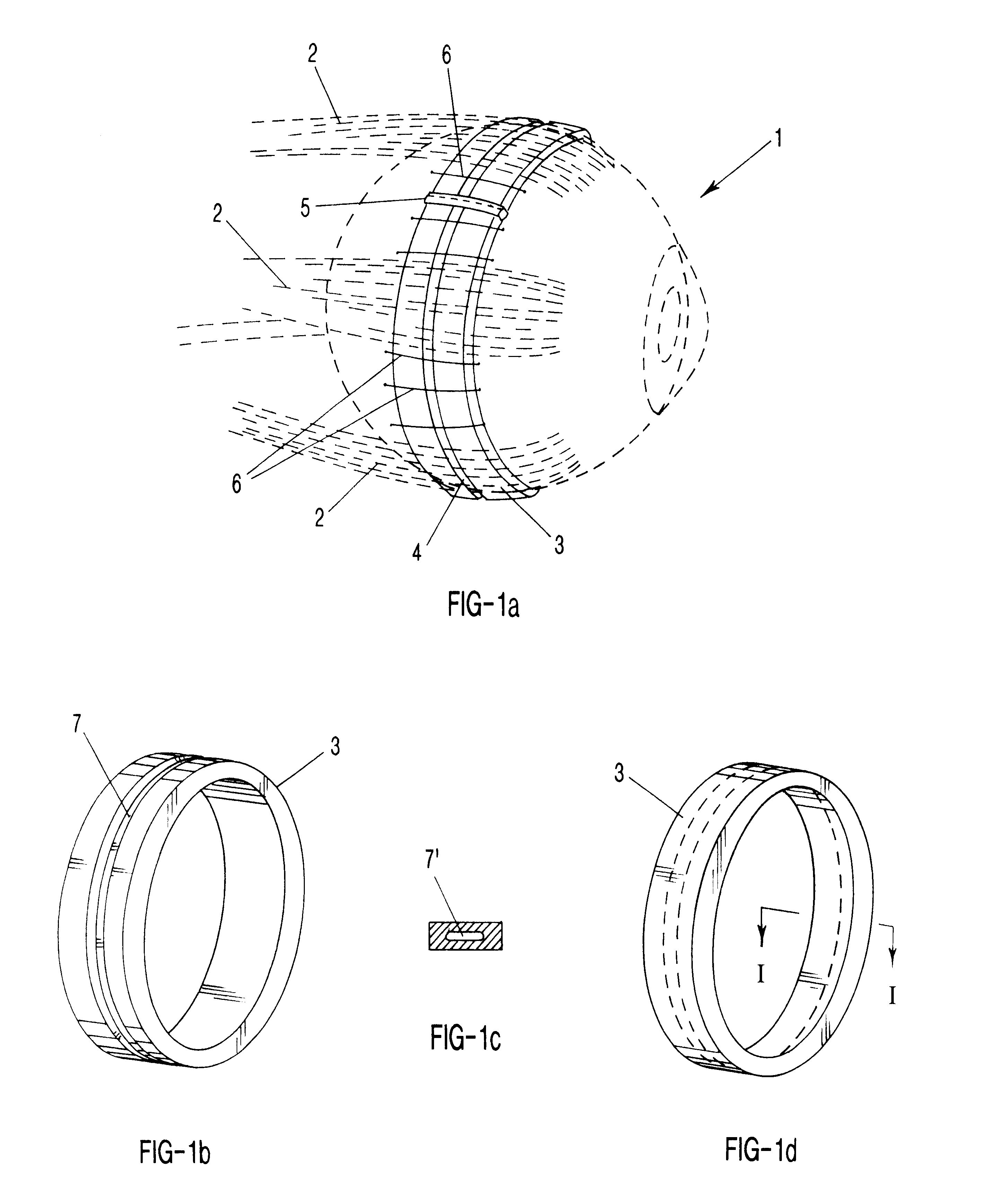
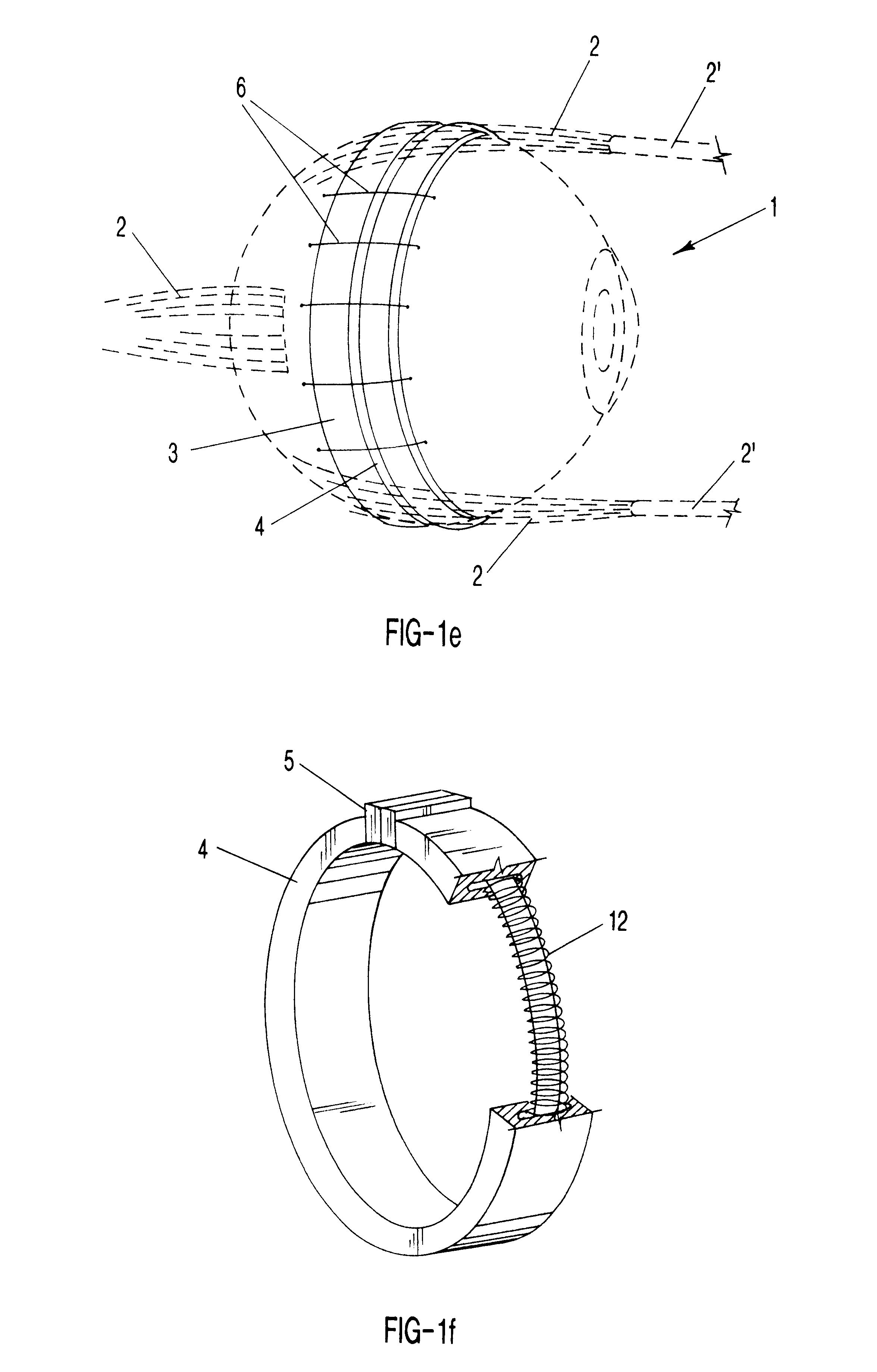
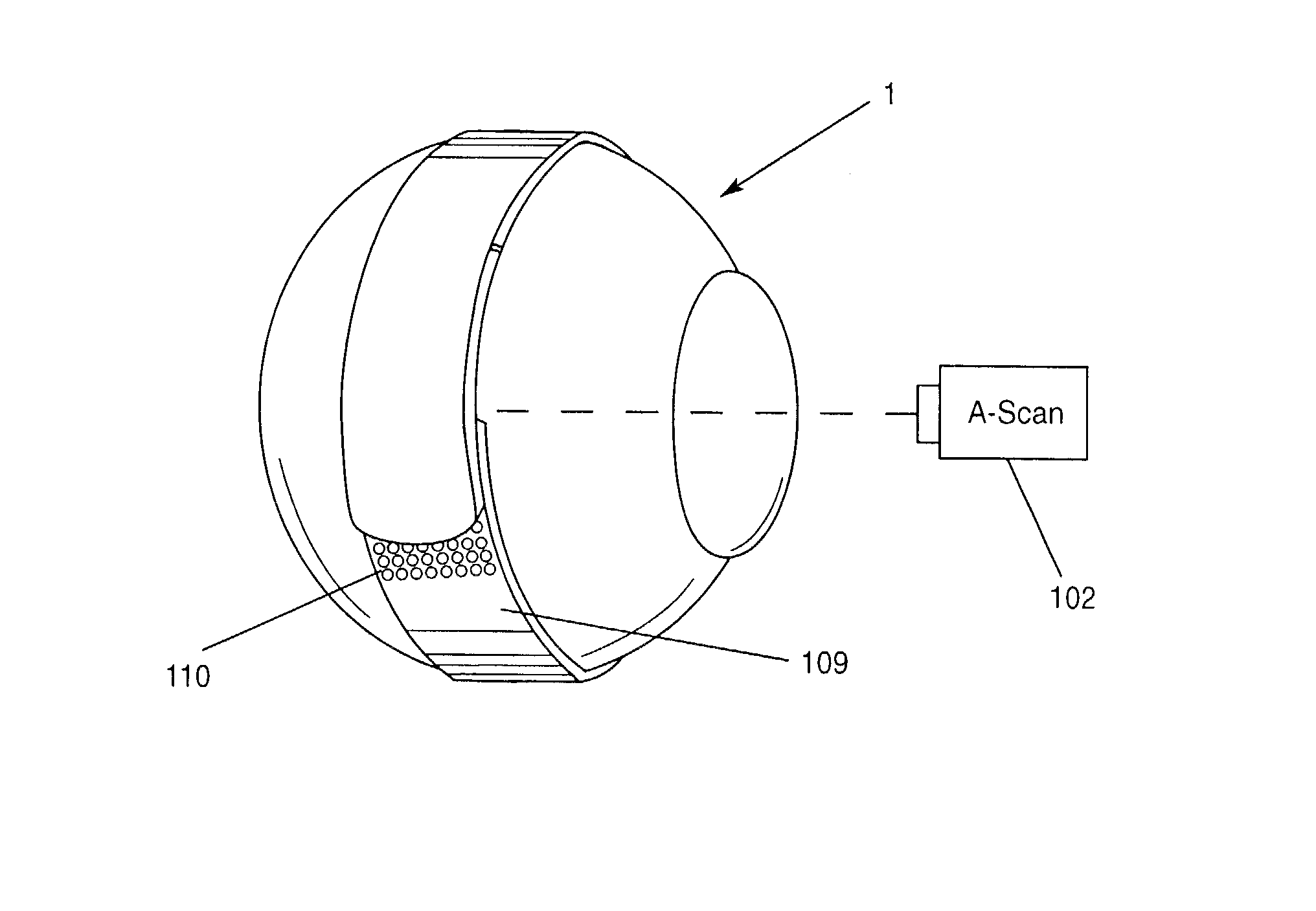
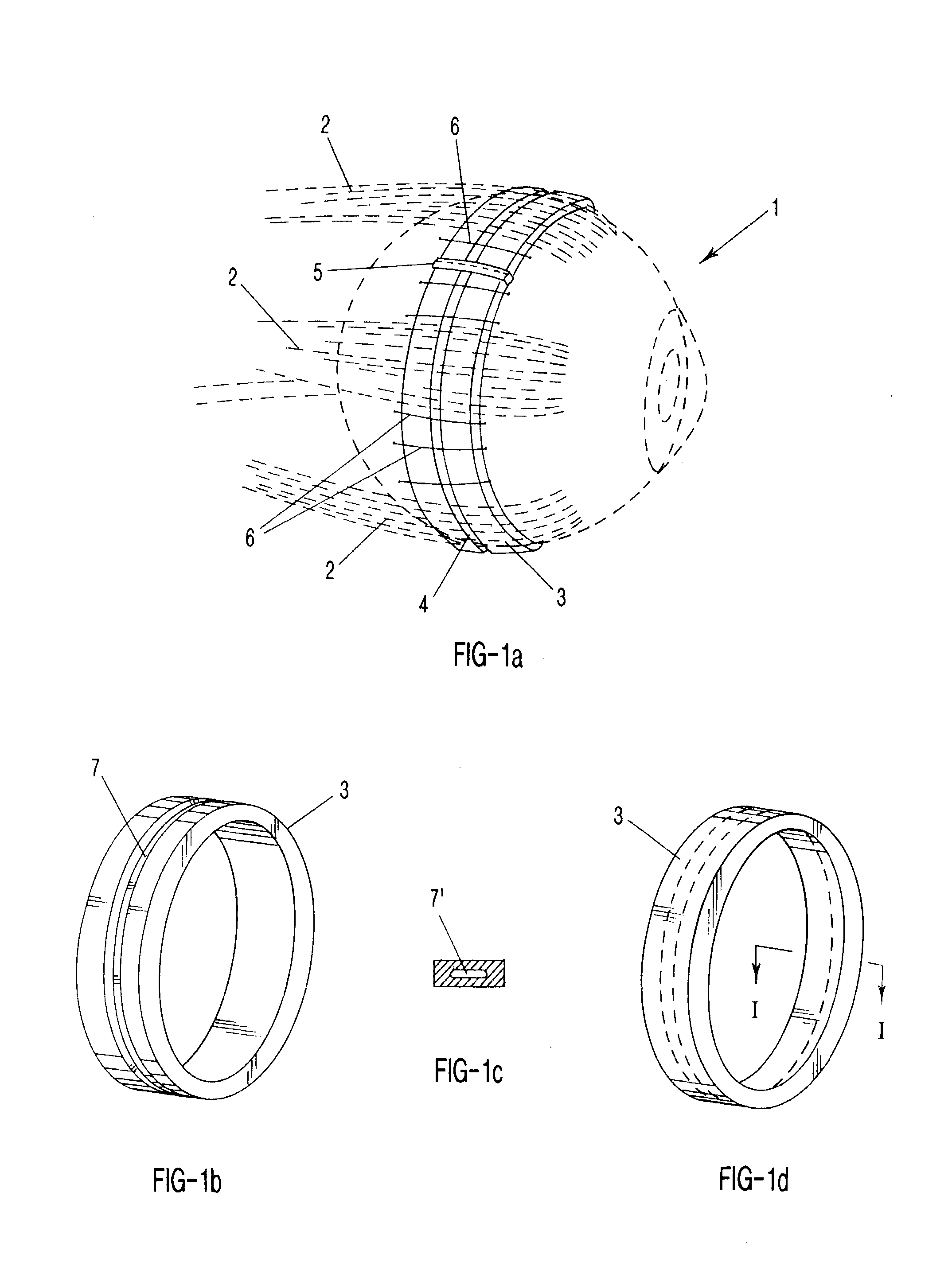
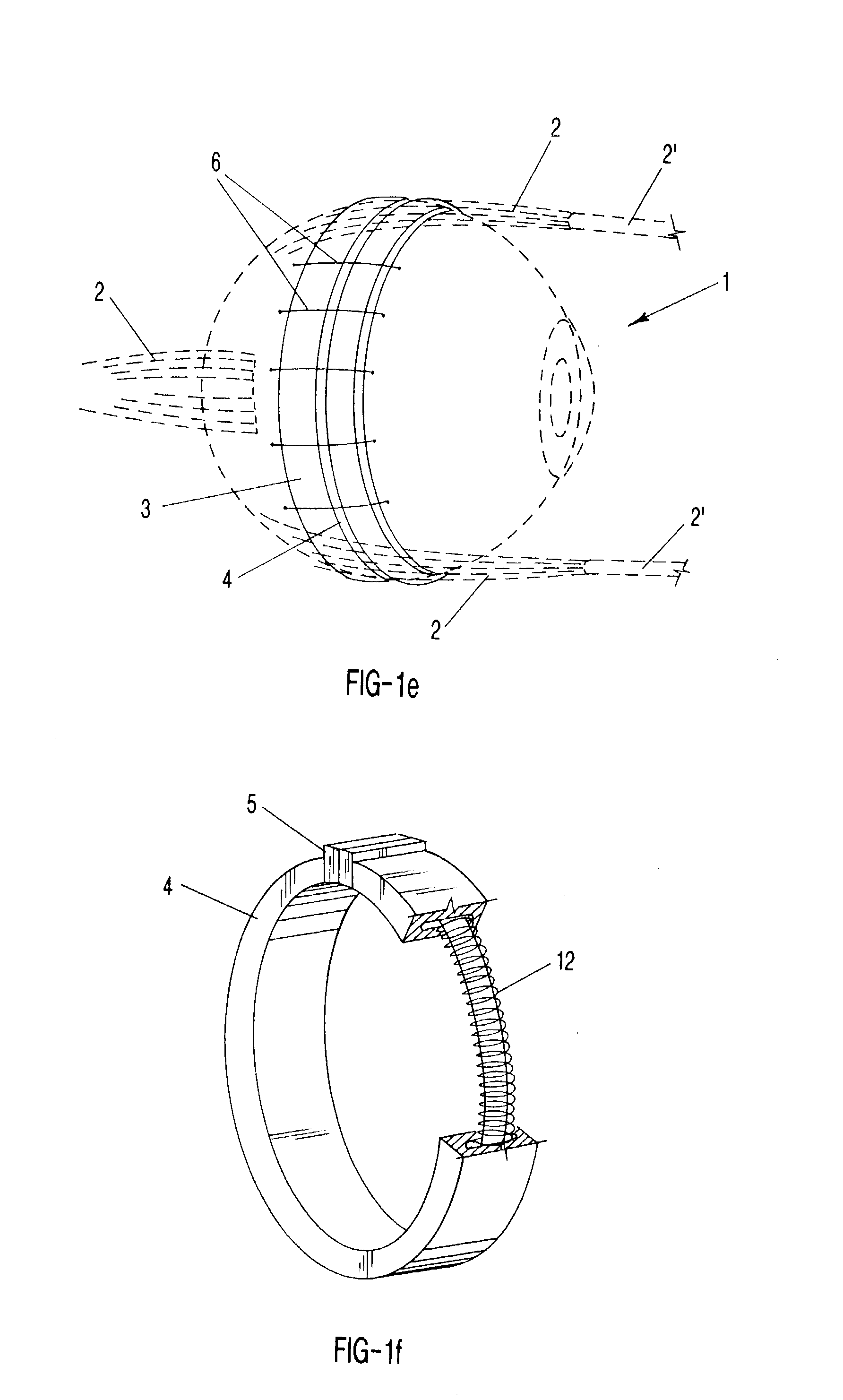
Surgical correction of human eye refractive errors such as presbyopia, hyperopia, myopia, and stigmatism by using transcutaneously inductively energized artificial muscle implants to either actively change the axial length and the anterior curvatures of the eye globe. This brings the retina / macula region to coincide with the focal point. The implants use transcutaneously inductively energized scleral constrictor bands equipped with composite artificial muscle structures. The implants can induce enough accommodation of a few diopters, to correct presbyopia, hyperopia, and myopia on demand. In the preferred embodiment, the implant comprises an active sphinctering smart band to encircle the sclera, preferably implanted under the conjunctiva and under the extraocular muscles to uniformly constrict the eye globe, similar to a scleral buckle band for surgical correction of retinal detachment, to induce active temporary myopia (hyperopia) by increasing (decreasing) the active length of the globe. In another embodiment, multiple and specially designed constrictor bands can be used to enable surgeons to correct stigmatism. The composite artificial muscles are either resilient composite shaped memory alloy-silicone rubber implants in the form of endless active scleral bands, electroactive ionic polymeric artificial muscle structures, electrochemically contractile endless bands of ionic polymers such as polyacrylonitrile (PAN), thermally contractile liquid crystal elastomer artificial muscle structures, magnetically deployable structures or solenoids or other deployable structures equipped with smart materials such as preferably piezocerams, piezopolymers, electroactive and eletrostrictive polymers, magnetostrictive materials, and electro or magnetorheological materials.
Owner:ENVIRONMENTAL ROBOTS
C-shaped cross section tubular ophthalmic implant for reduction of intraocular pressure in glaucomatous eyes and method of use
InactiveUS6962573B1Inhibit migrationReduction in bleb diameterEye implantsEar treatmentOphthalmological implantAqueous humor
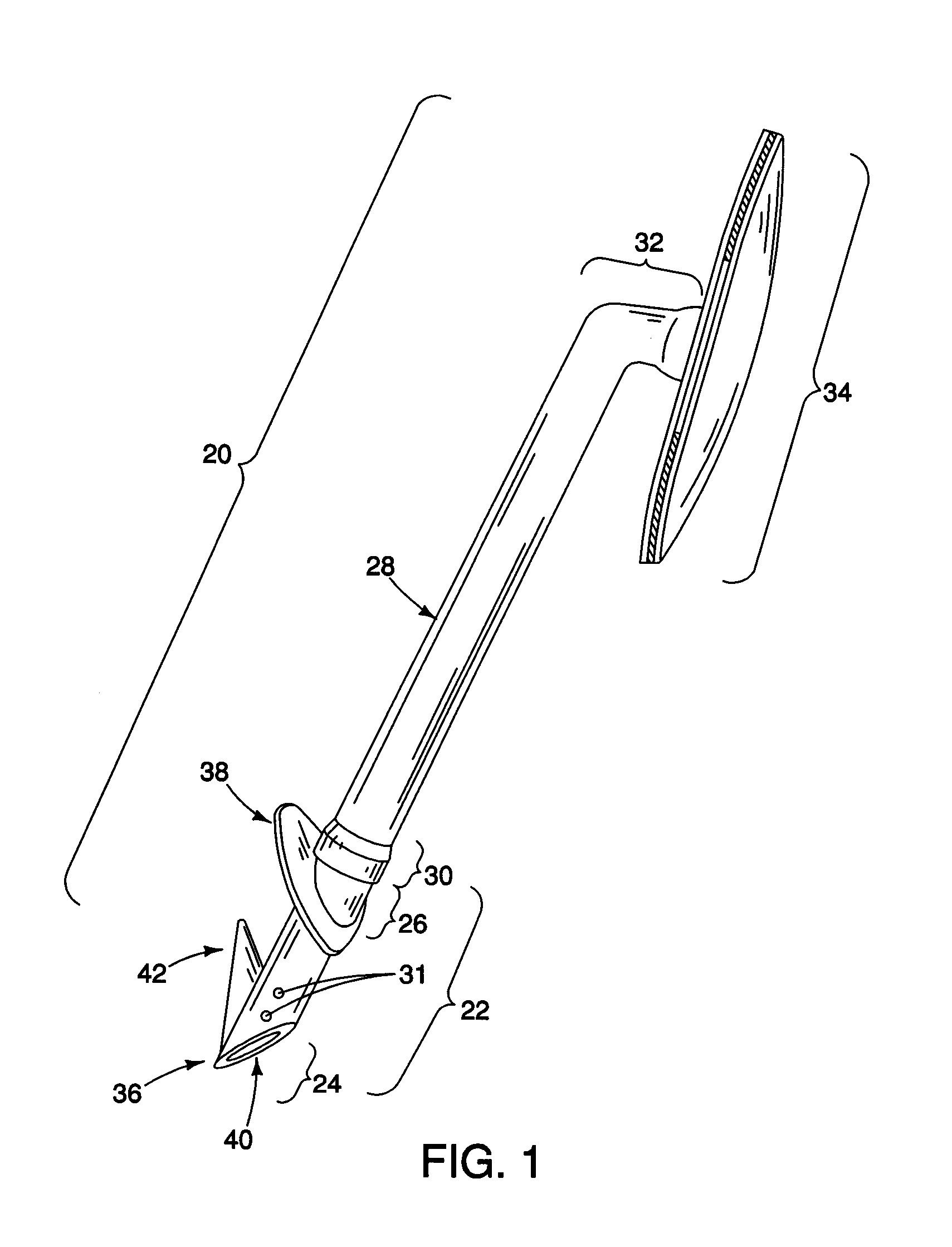
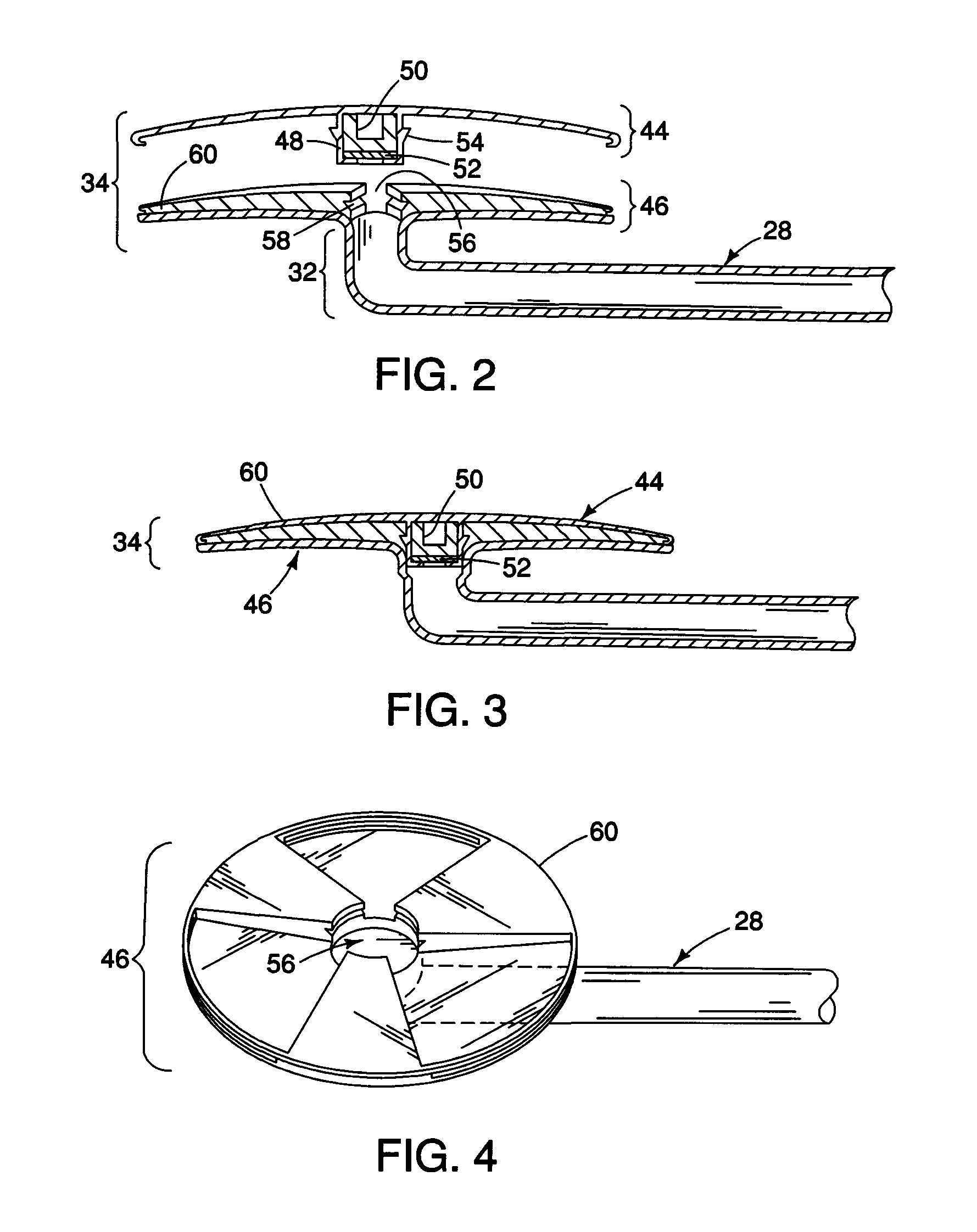
A tube for implantation into the eye for replacement conduction of aqueous humor from the chambers of the eyeball to the subconjunctival tissue and ultimately to the venous system is comprised of an elongated fluid conducting conduit having distal and proximate ends, a sidewall and an interior passageway and at least one longitudinally extending opening in the sidewall that exposes the interior passageway and at least one nidi-forming structure carried by the conduit and extending laterally therefrom to implement the formation of at least one aqueous filtration bleb in the tissue of the eyeball. In one embodiment, the tube also contains at least one releasable ligature circumscribing the conduit. In another embodiment, the tube also contains an anchor appended to the conduit to prevent it from migrating from its placement site.
Owner:AQ BIOMED LLC
Contact lens for collecting tears and detecting analytes for determining health status, ovulation detection, and diabetes screening
InactiveUS20070016074A1Increase oxygenationIncreased riskOrganic active ingredientsHeart defibrillatorsConfocalChemical products
Owner:GEELUX HLDG LTD
Microneedles and Methods for Microinfusion
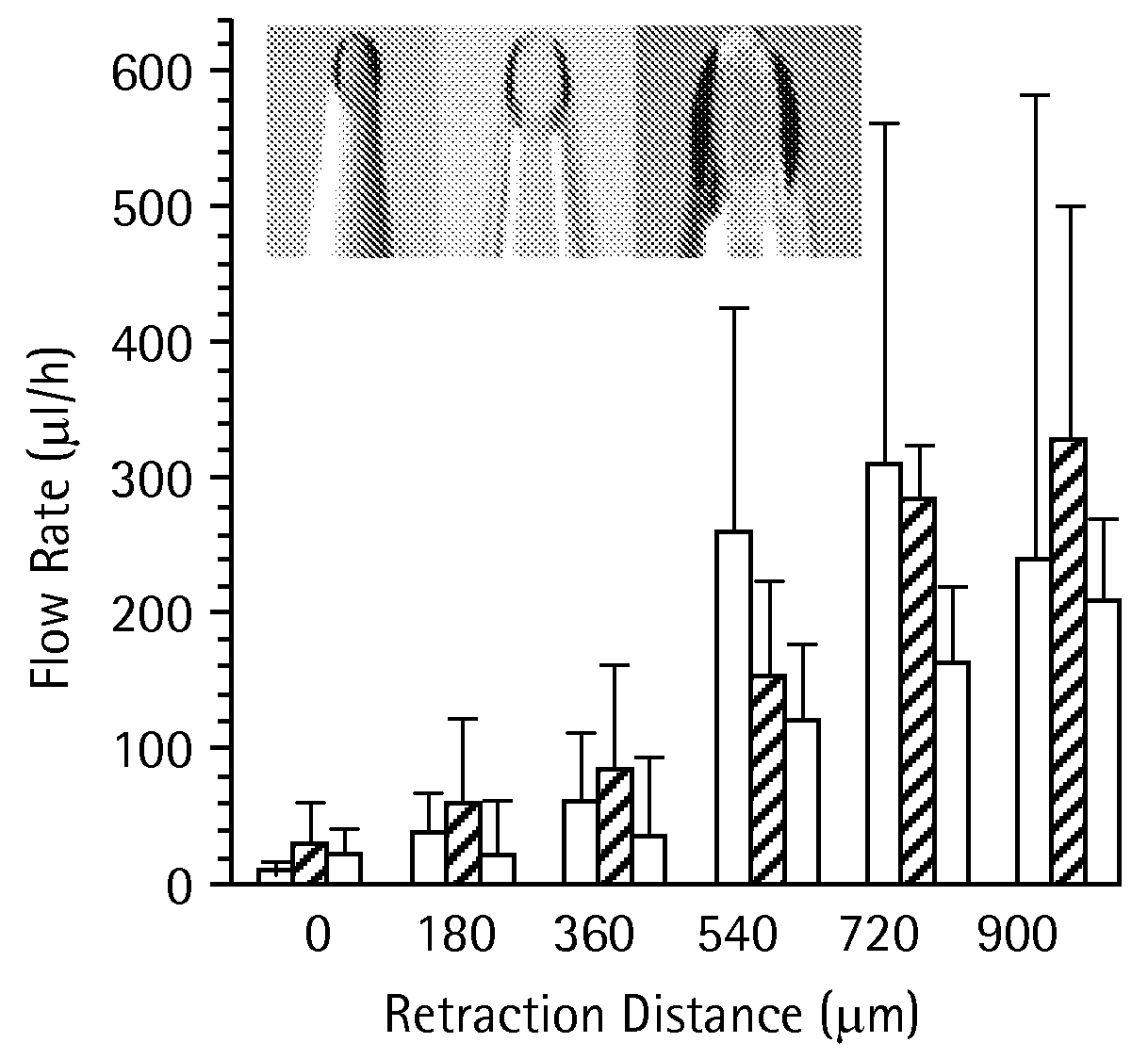
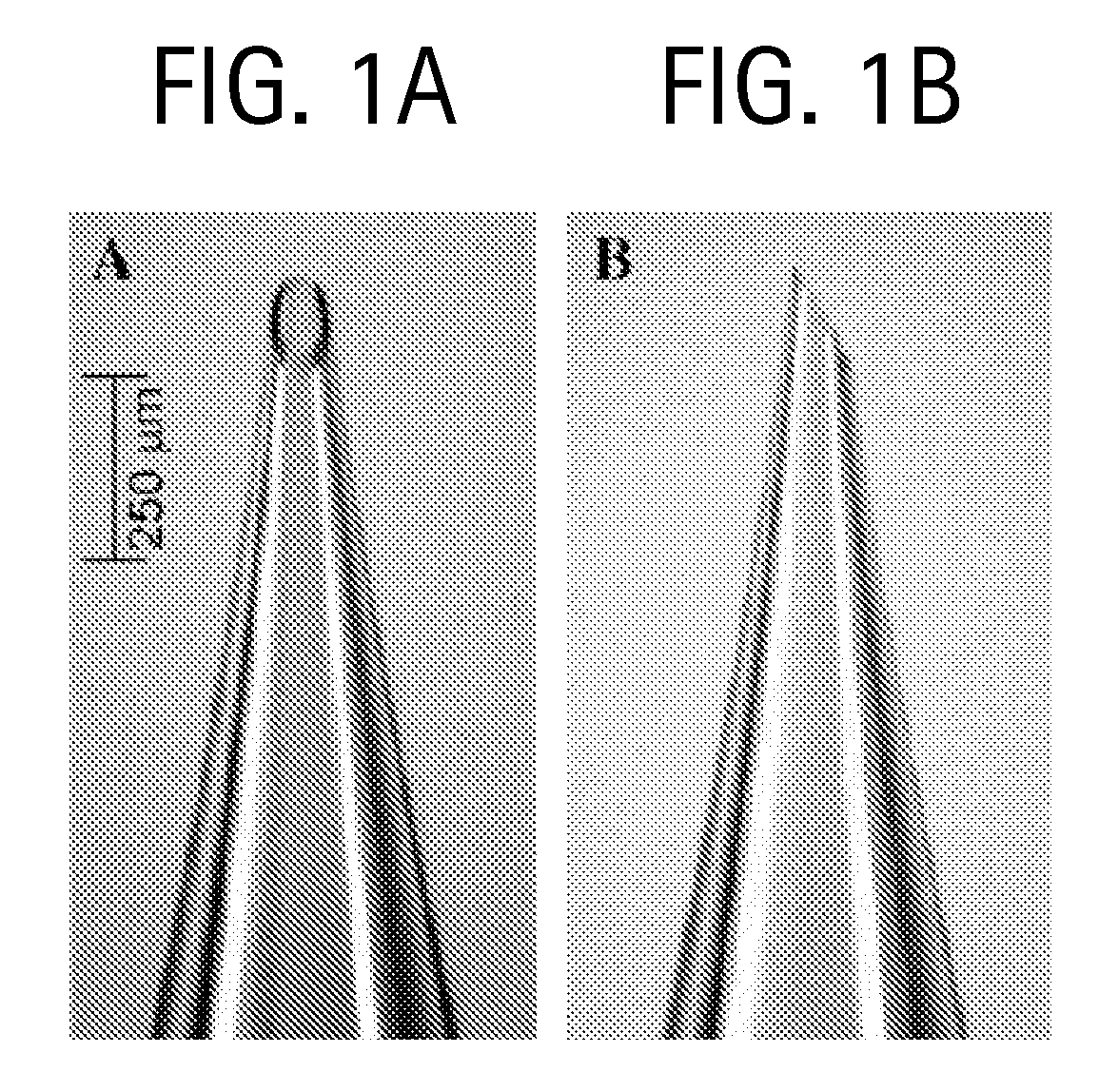
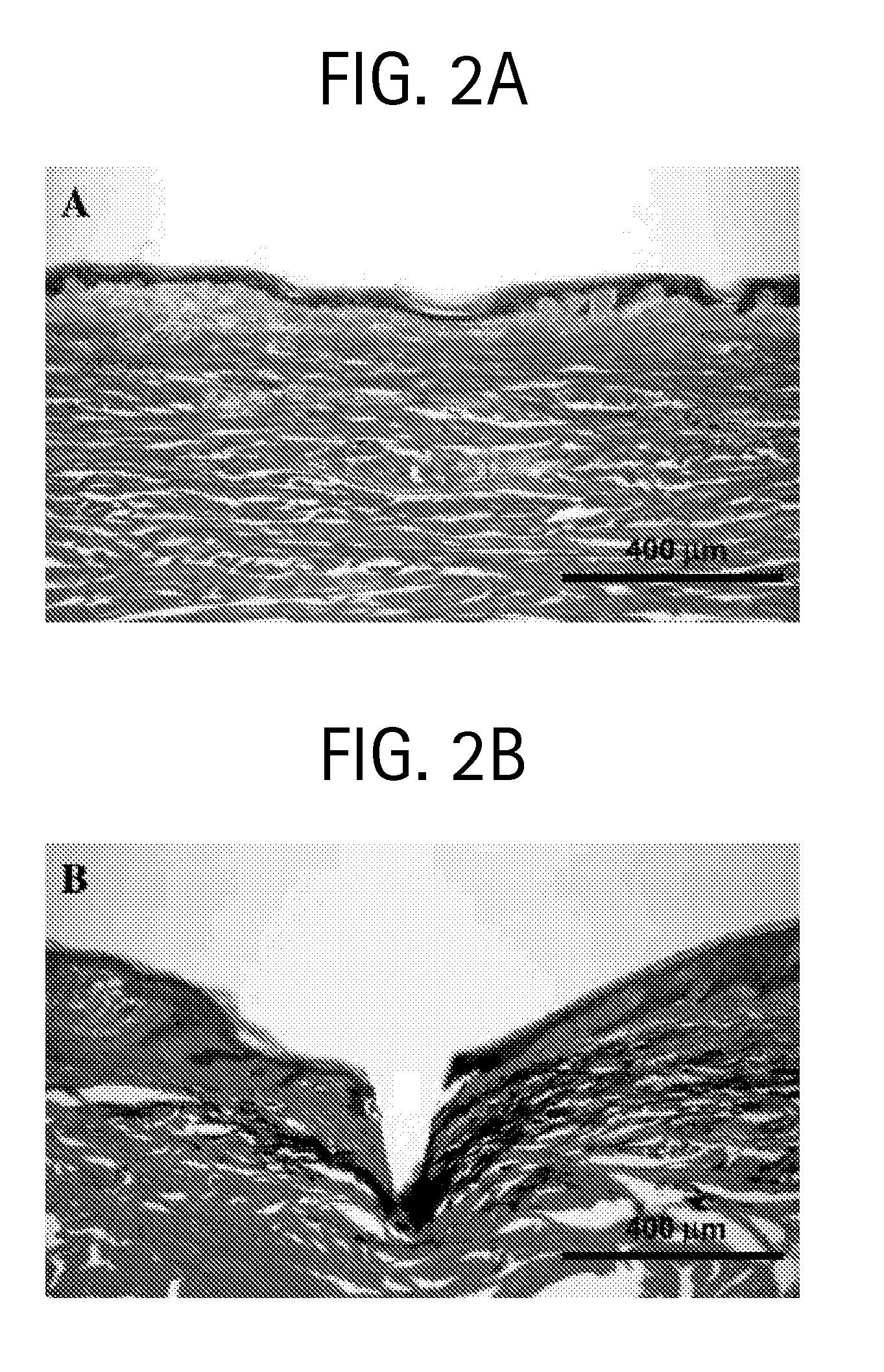
Methods and devices are provided for delivering a drug to or withdrawing a fluid from a biological tissue, such the skin, sclera, cornea, and conjunctiva. One method includes the steps of inserting at least one microneedle into the biological tissue; partially retracting the at least one microneedle from the tissue; and then delivering at least one drug formulation into the biological tissue via the partially retracted at least one microneedle. The microneedle deforms and penetrates the biological tissue during the insertion step, and the retraction step at least partially relaxes the tissue deformation while maintaining at least part of the tissue penetration, facilitating drug delivery or fluid withdrawal.
Owner:GEORGIA TECH RES CORP
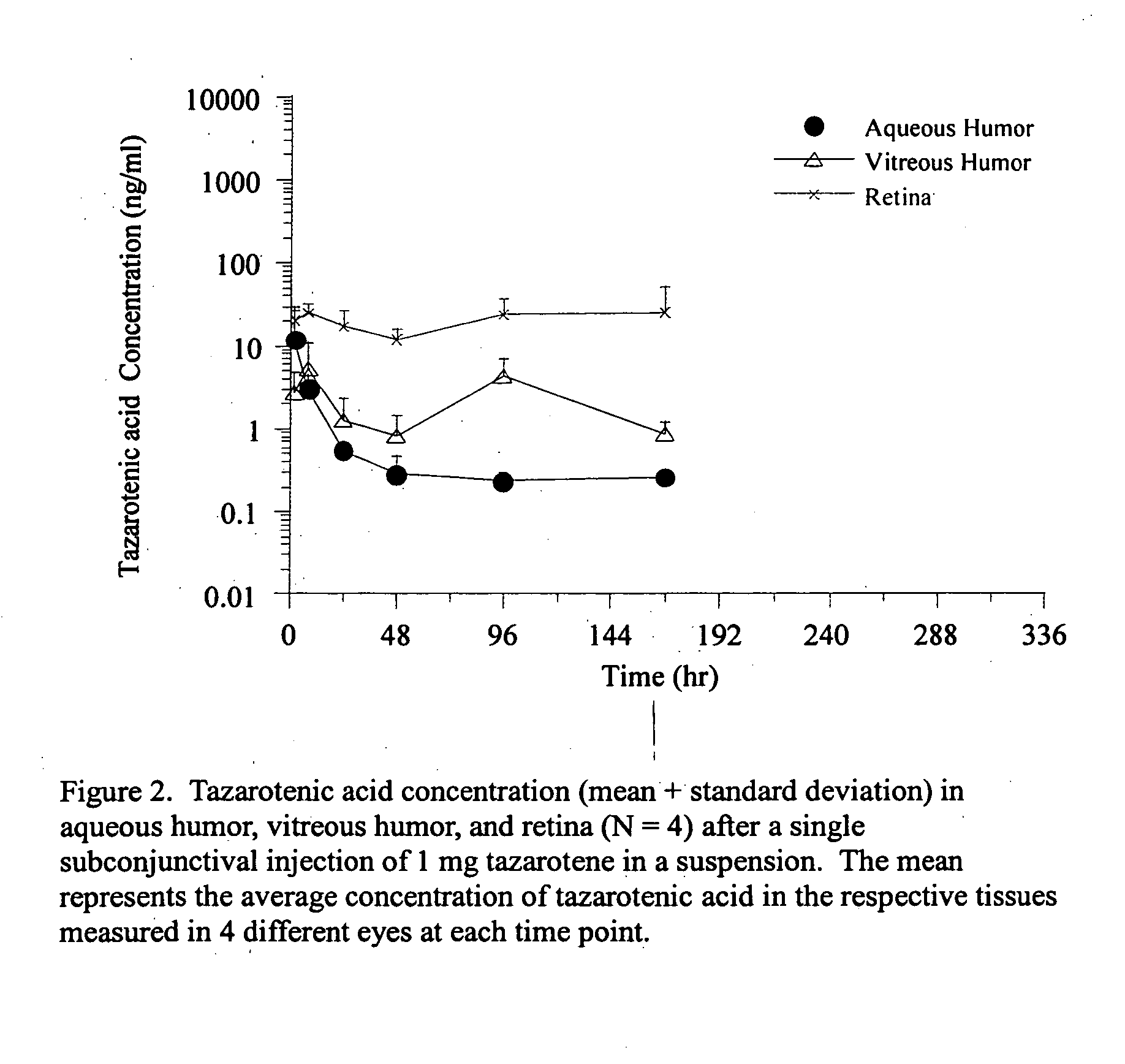
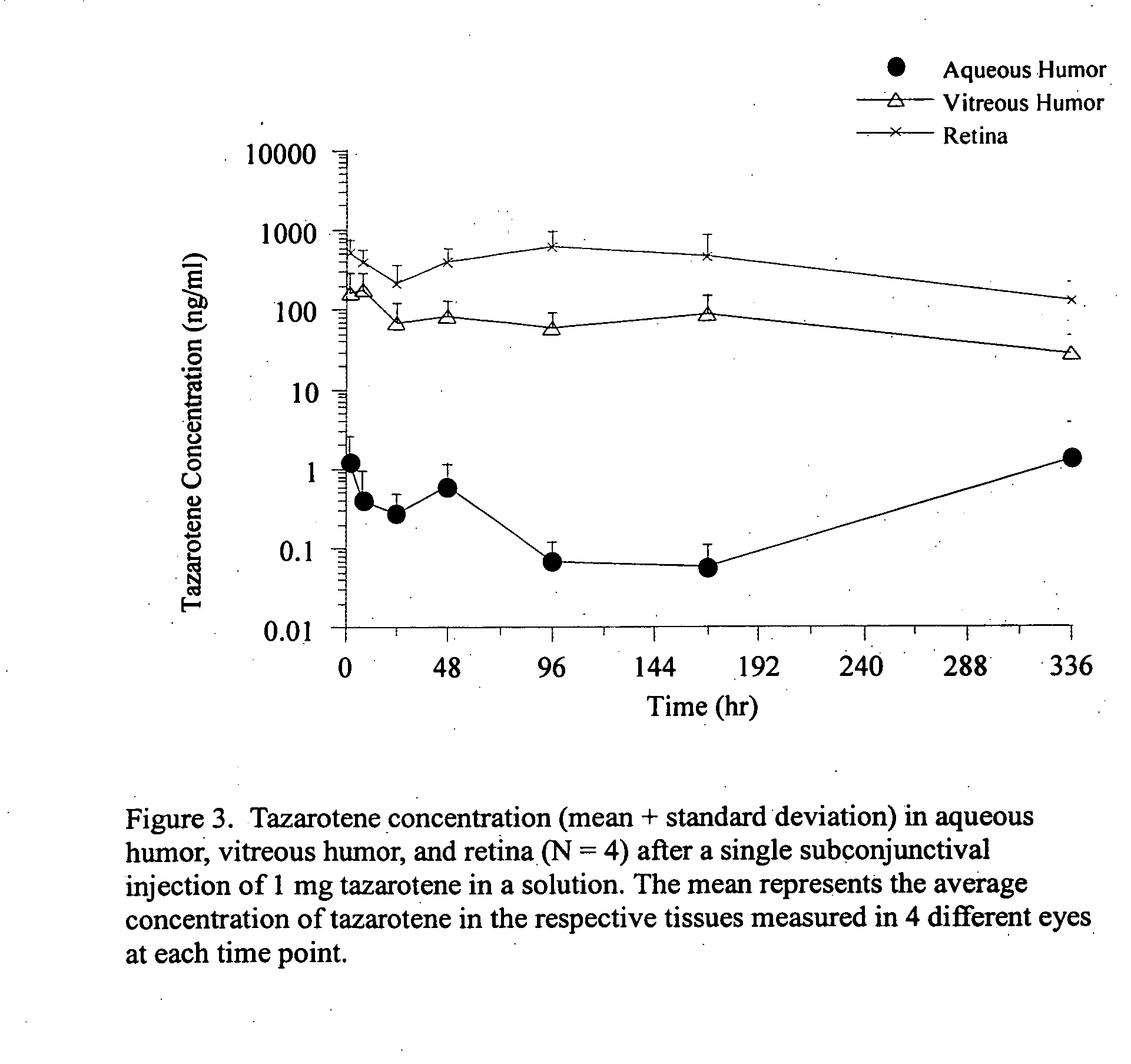
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20050009910A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaEster prodrug
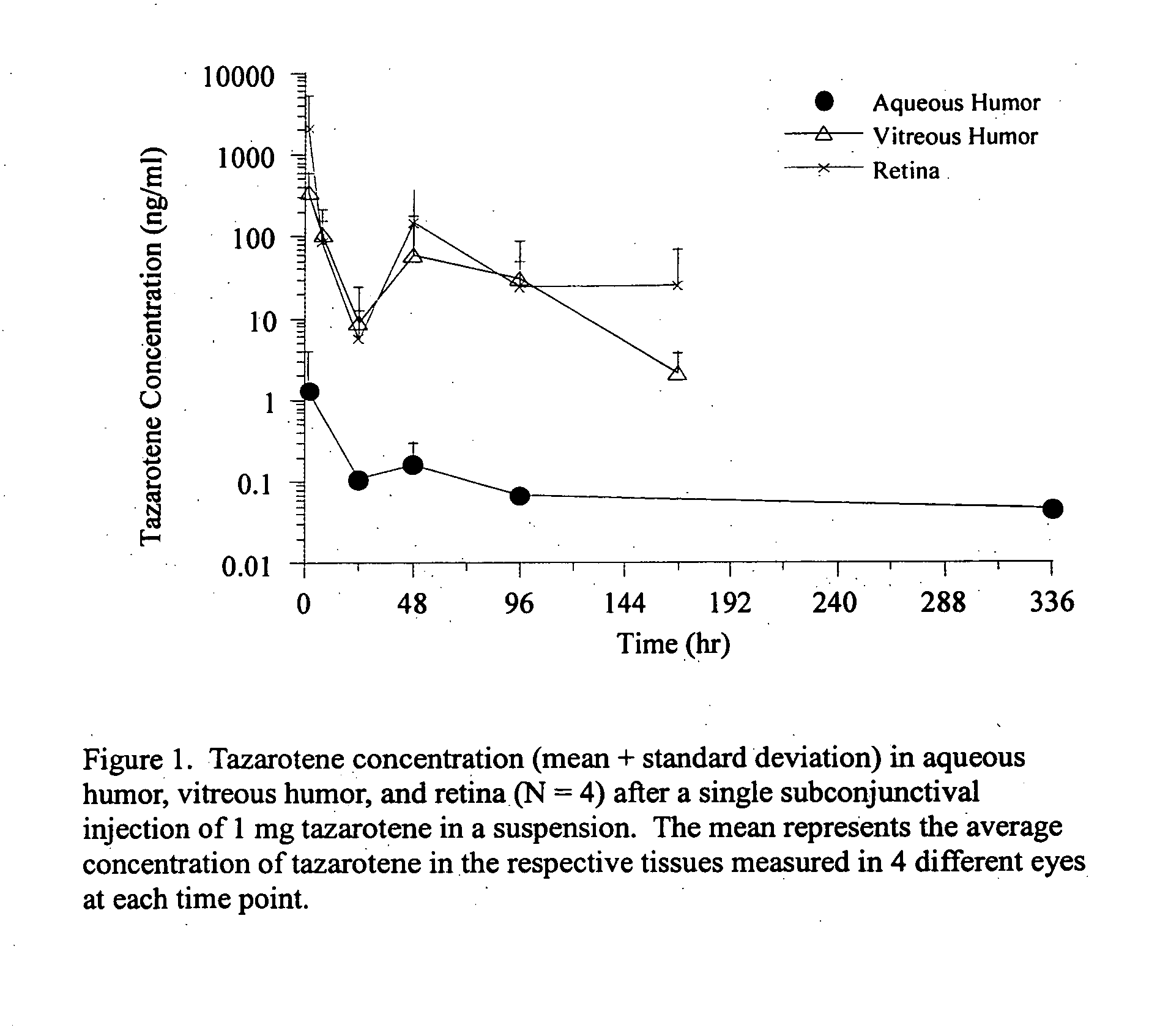
The present invention relates to method of sustained-delivery of an active drug to a posterior part of an eye of a mammal to treat or prevent a disease or condition affecting said mammal, wherein said disease or condition can be treated or prevented by the action of said active drug upon said posterior part of the eye, comprising administering an effective amount of an ester prodrug of the active drug subconjunctivally or periocularly. Preferably, the active drug is more than about 10 times as active as the prodrug. Other aspects of this invention deal with the treatment of certain diseases by the periocular or subconjunctival delivery of an ester prodrug, and certain pharmaceutical products containing ester prodrugs for periocular or subconjunctival administration.
Owner:ALLERGAN INC
Method and apparatus for treatment of presbyopia by lens relaxation and anterior shift
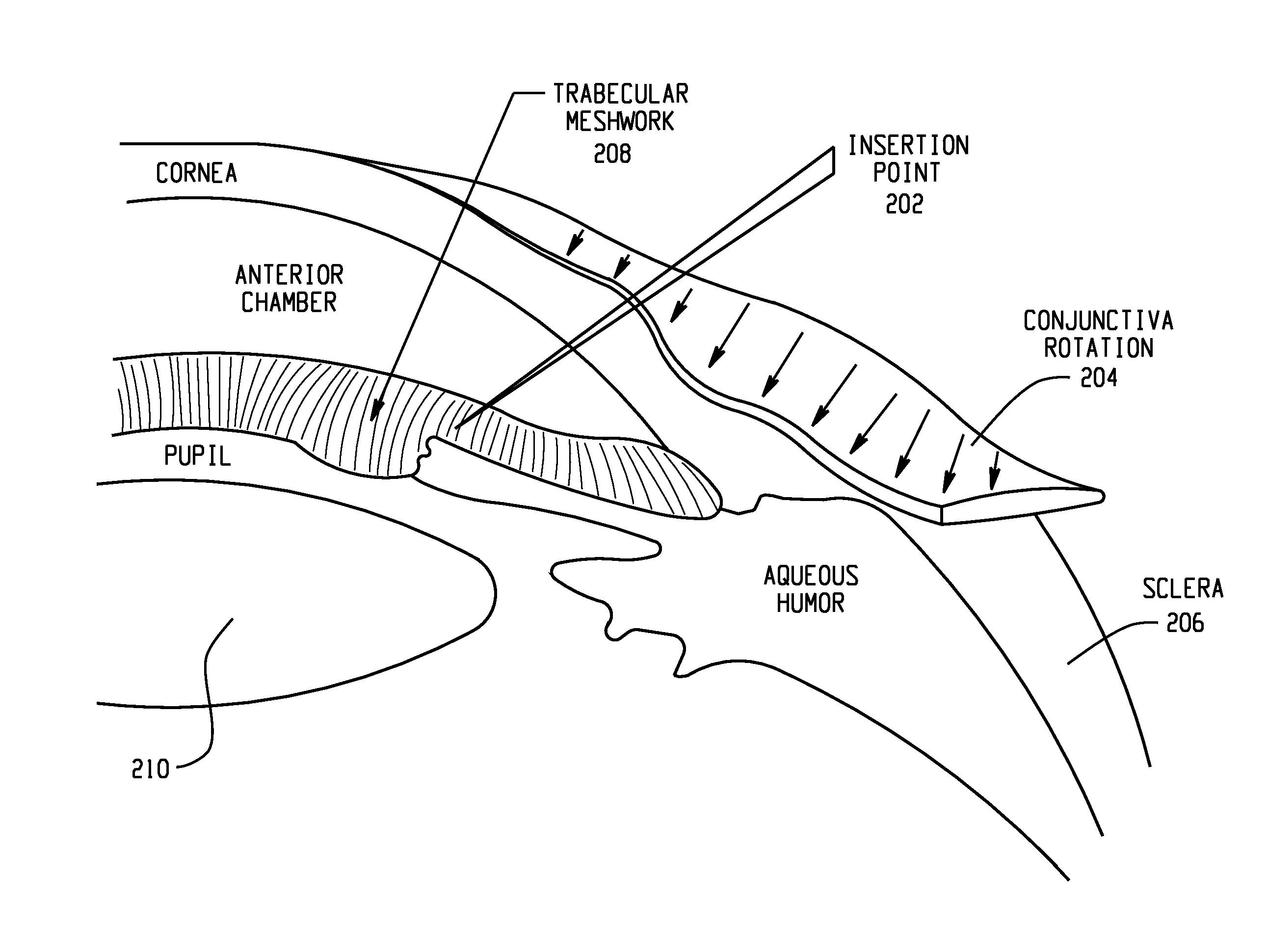
A surgical method and apparatus for presbyopia correction removal of the sclera tissue are disclosed. Mechanisms based on sub-conjunctiva filled-in of the sclera area and cause the sclera-ciliary-body and zonule "complex" become more flexible (or less rigidity) are proposed. Total accommodation based a lens relaxation and lanes anterior shift is calculated and proposed as the guidance of the parameters for device design and clinical outcomes The preferred embodiments for the ablation patterns include radial lines, curved lines, ring dots or any non-specific shapes in a symmetric geometry. The surgery apparatus includes non-laser device of radio frequency wave, electrode device, bipolar device and plasma assisted device. Another preferred embodiment is to use post-operation medication such as pilocarpine (1%-10%) or medicines with similar nature which may cause ciliary body contraction for more stable and enhancement after the treatment.
Owner:LIN J T
Sutureless occular surgical methods and instruments for use in such methods
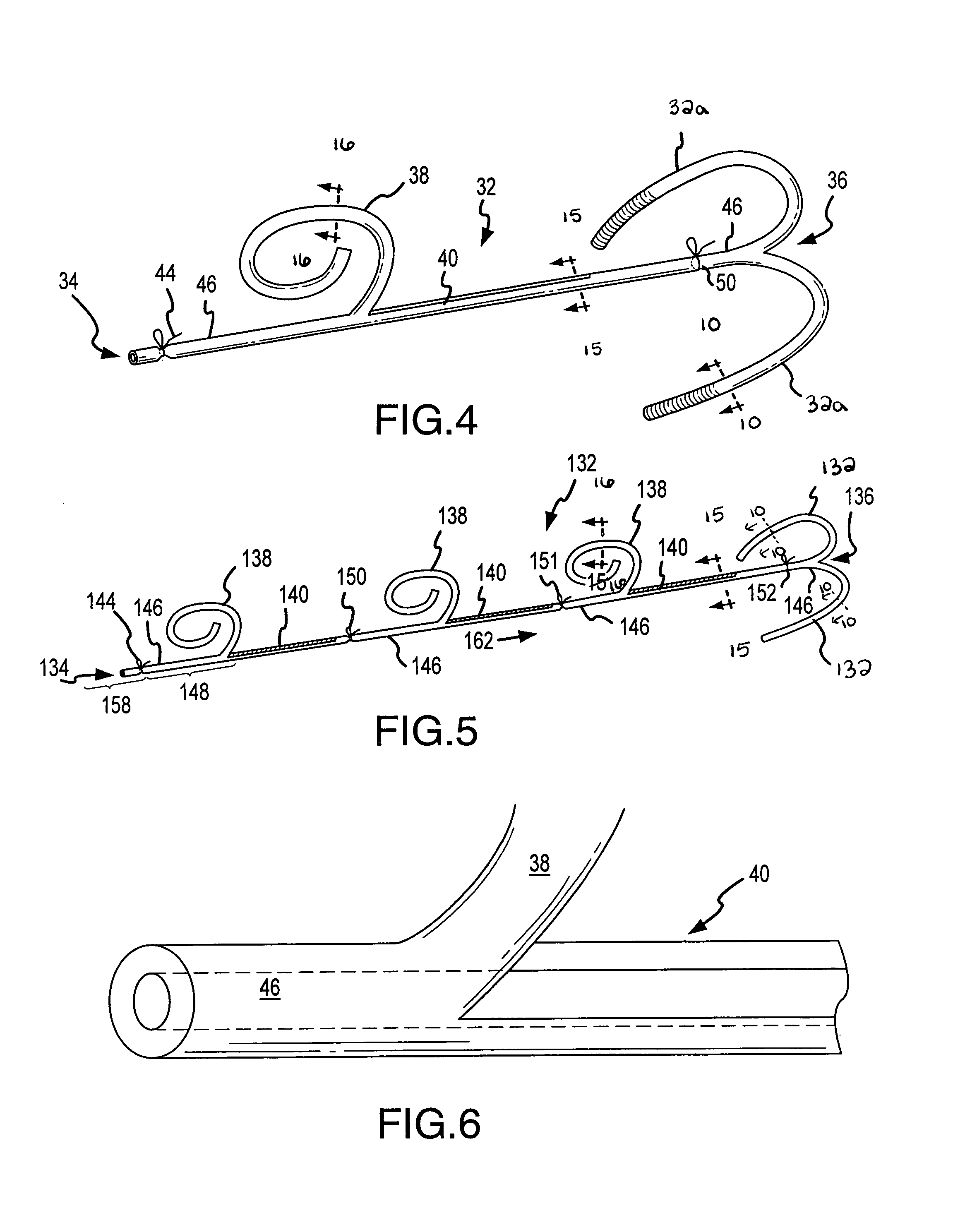
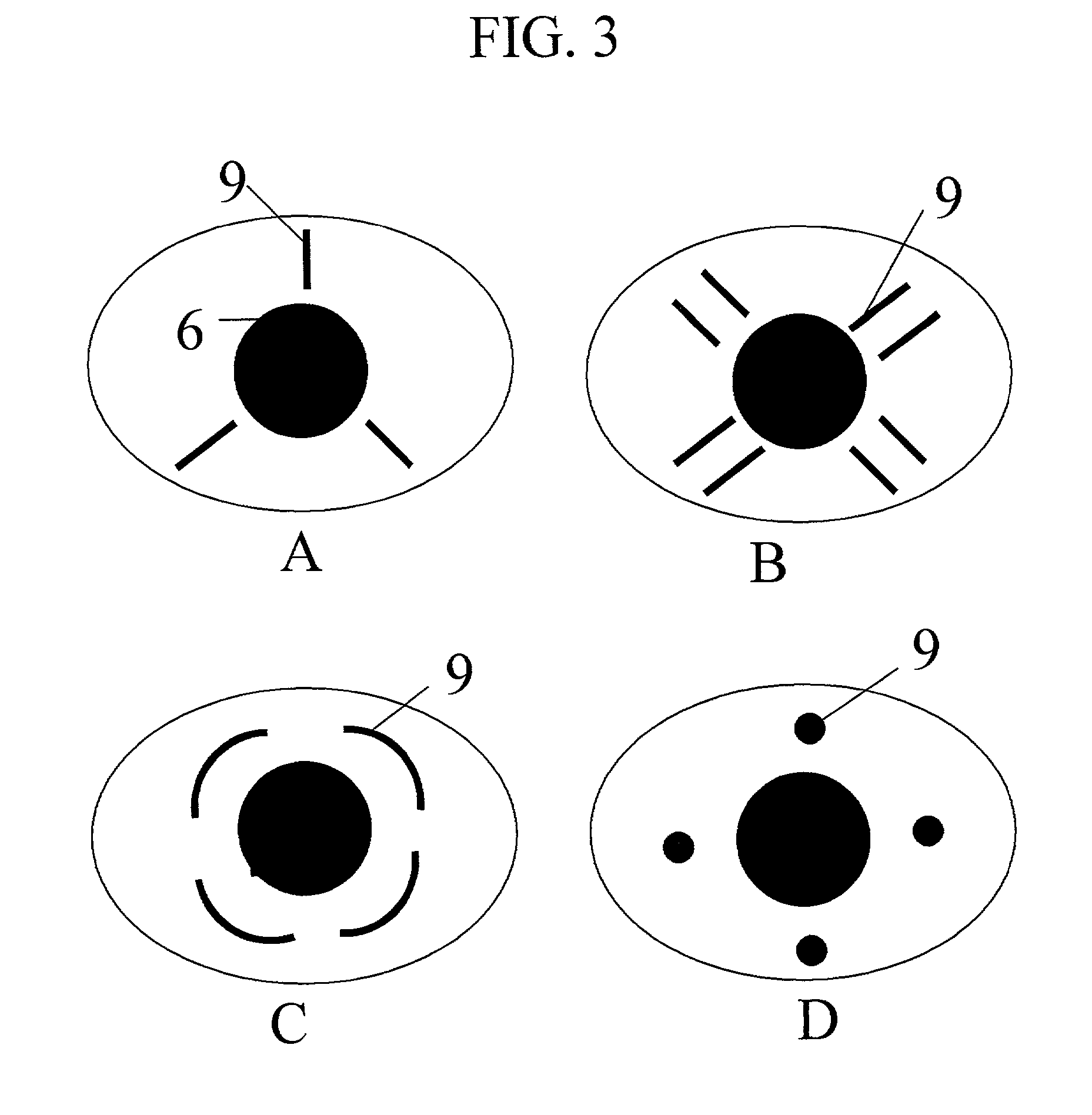
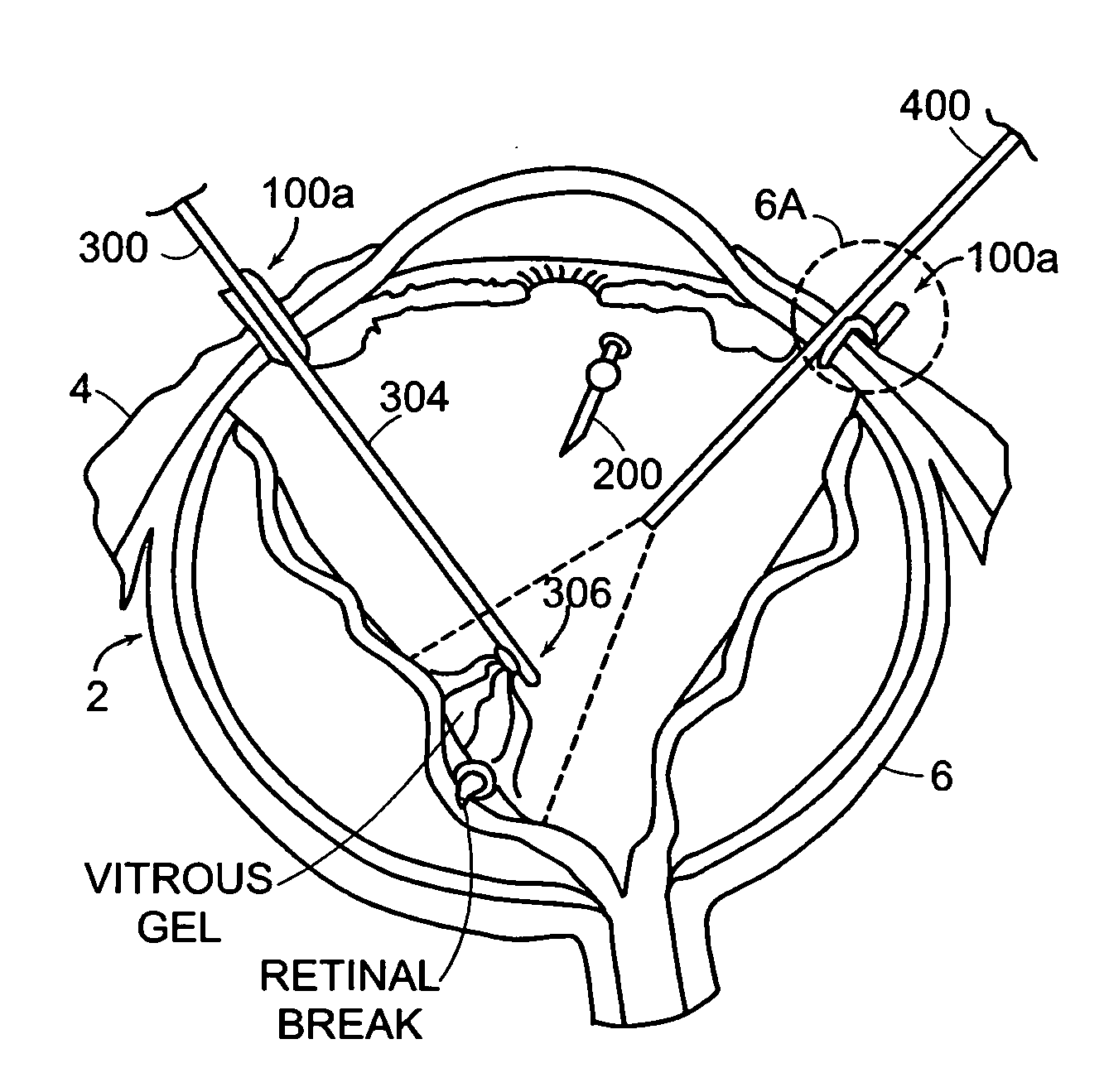
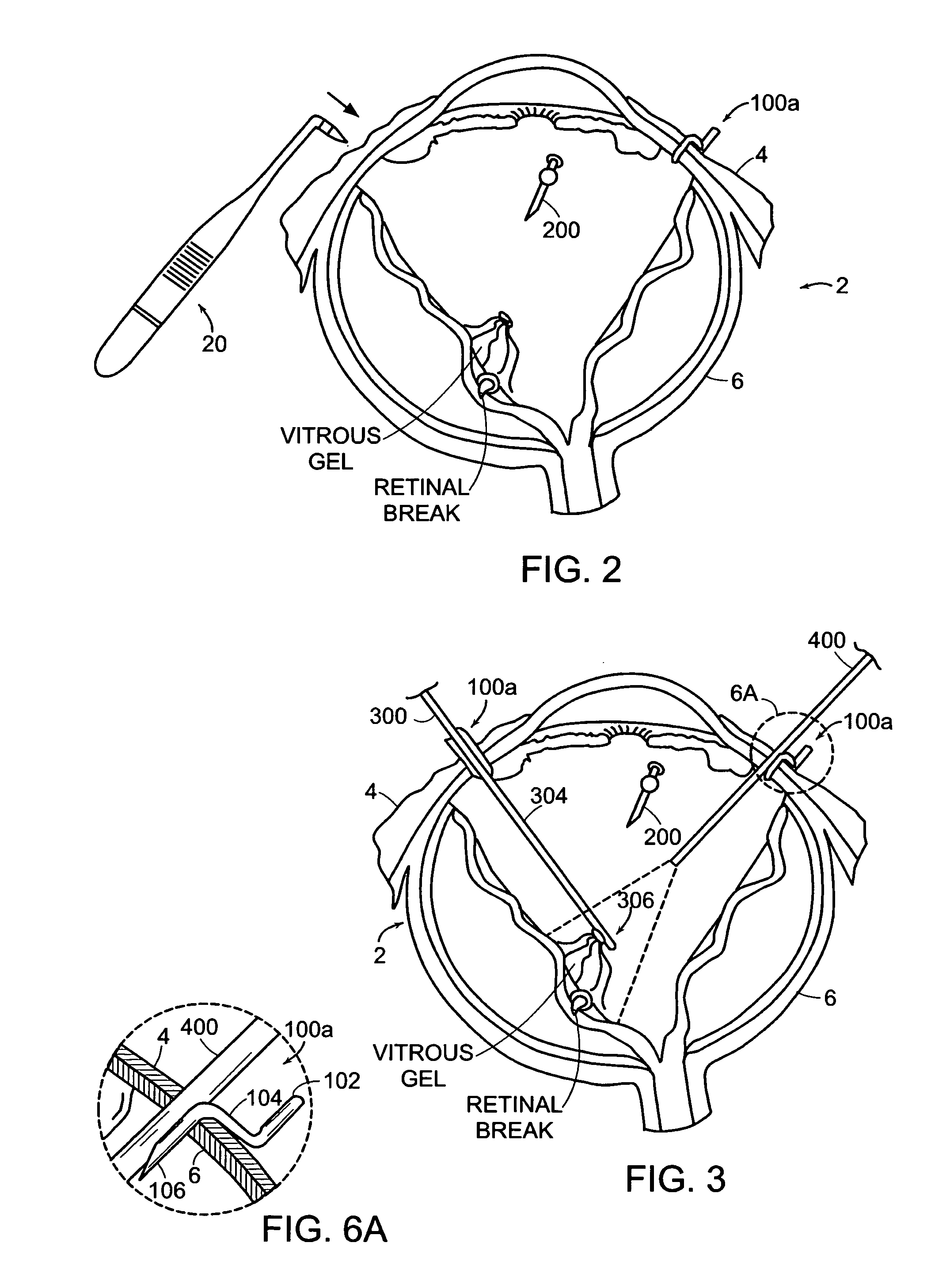
Featured are new methods for performing intra-ocular surgery that allow surgical personnel to access the intra-ocular volume to perform a surgical procedure or technique but which does not require the use of sutures to seal the sclera and / or conjunctiva following the procedure. The methods of the present invention generally include providing an entry alignment device and inserting the entry alignment device into an eye through both the conjunctiva and sclera so as to form an entry aperture that extends between the exterior of the eye and the intra-ocular volume within the eye. The provided alignment device is configured so as to form or provide an aperture or opening in each of the conjunctiva and sclera of the eye and to maintain these apertures or openings in each of the conjunctiva and sclera aligned during the surgical procedure so these apertures or openings form the entry aperture. In more particular aspects, the provided entry alignment device is sized such that when the entry alignment device is removed from the eye following the completion of the surgical procedure, the aperture or opening formed in the sclera seals without the use of sutures. In a more specific aspect of the present invention, the provided entry alignment device is sized such that the apertures or openings and thus the entry aperture are self sealing. In other embodiments, a plurality of entry alignment devices are provided so a plurality of entry apertures can be formed in the eye. The invention also features a high speed vitreous cutting and aspirating device particularly configured for use in such methods and surgical procedures and techniques as well as the related entry alignment devices and other surgical instruments.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
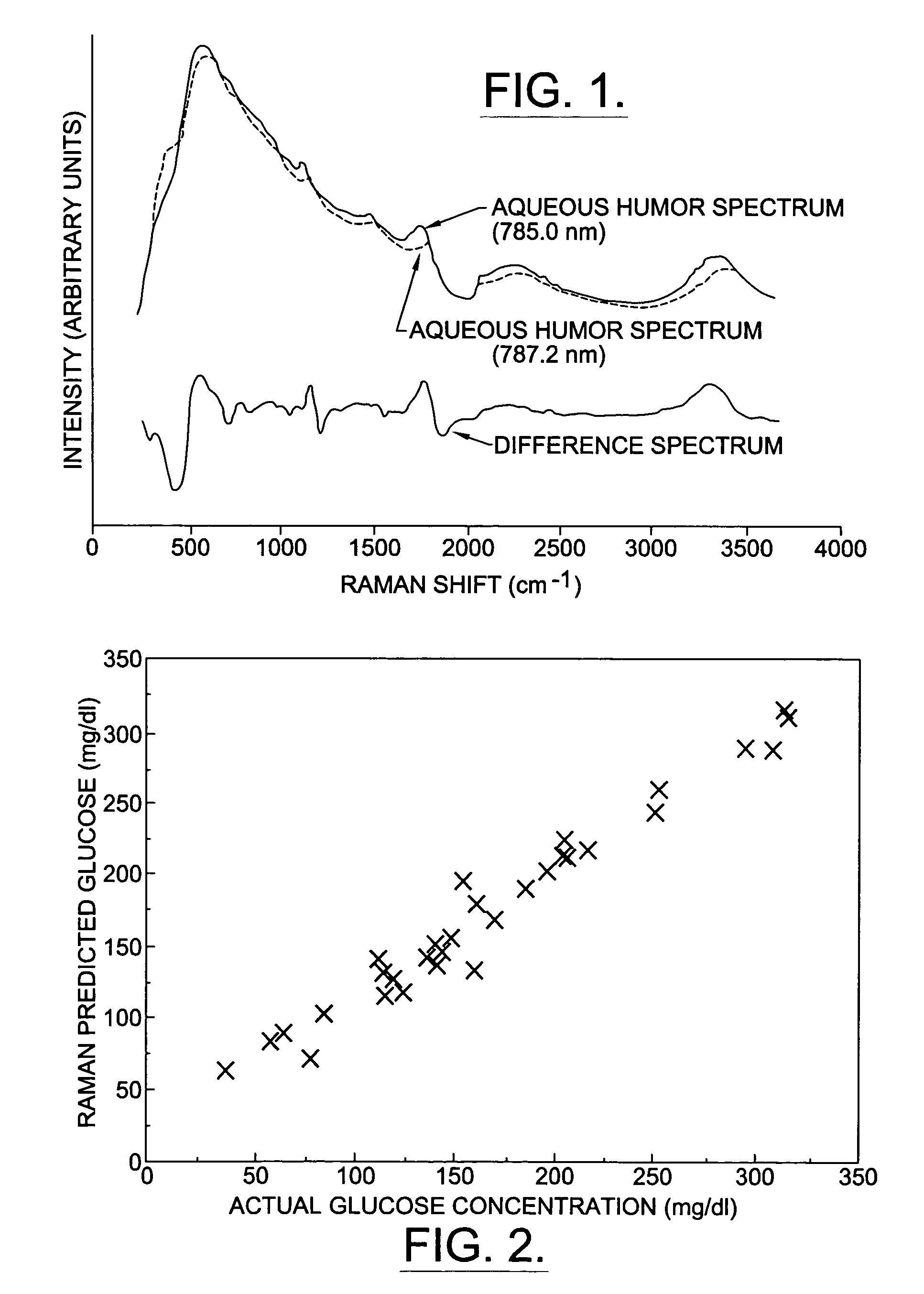
Assessing blood brain barrier dynamics or identifying or measuring selected substances or toxins in a subject by analyzing Raman spectrum signals of selected regions in the eye
InactiveUS6574501B2Reduced energy/density exposure ratingImproved margin of safetyRaman scatteringDiagnostic recording/measuringConjunctivaNon invasive
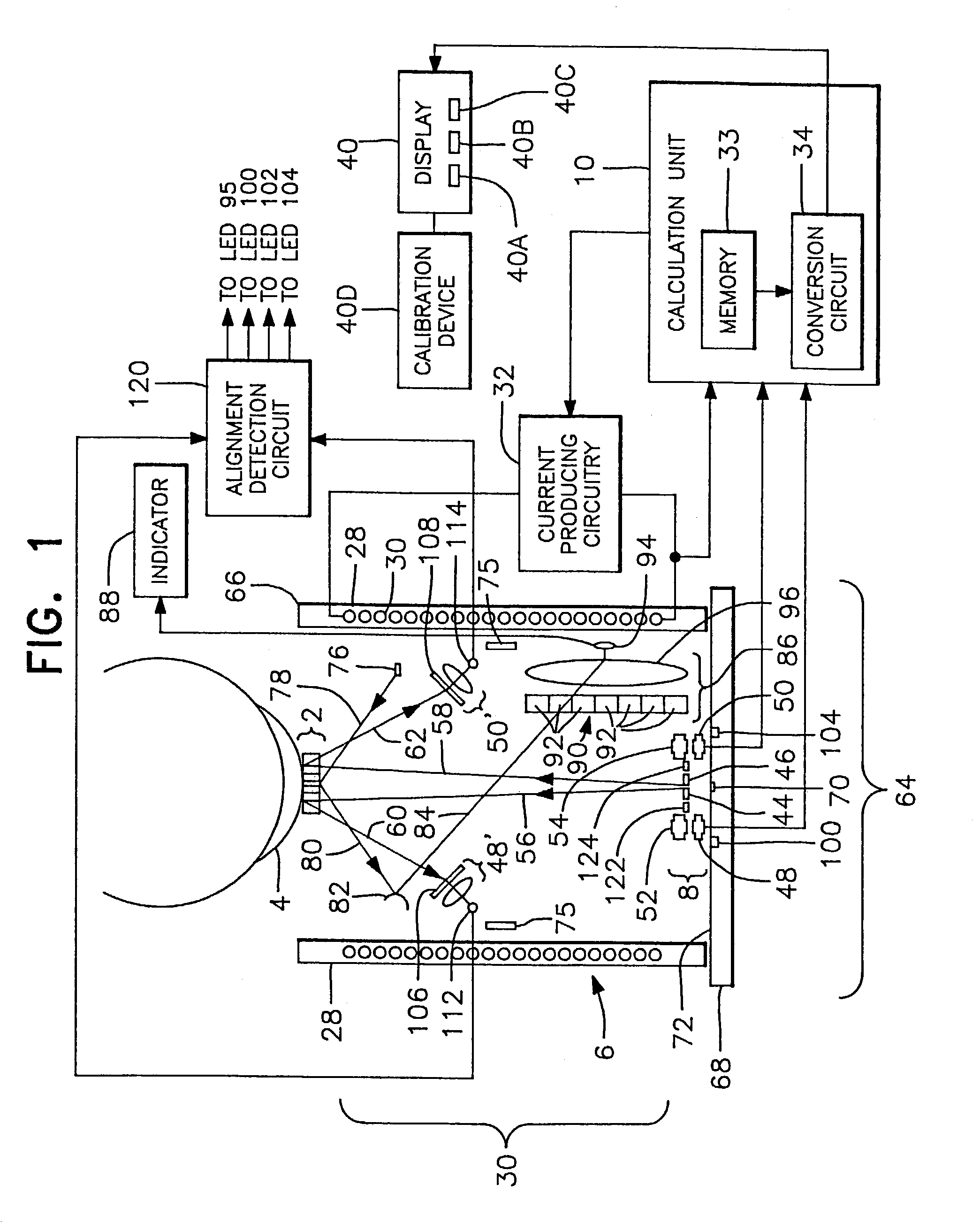
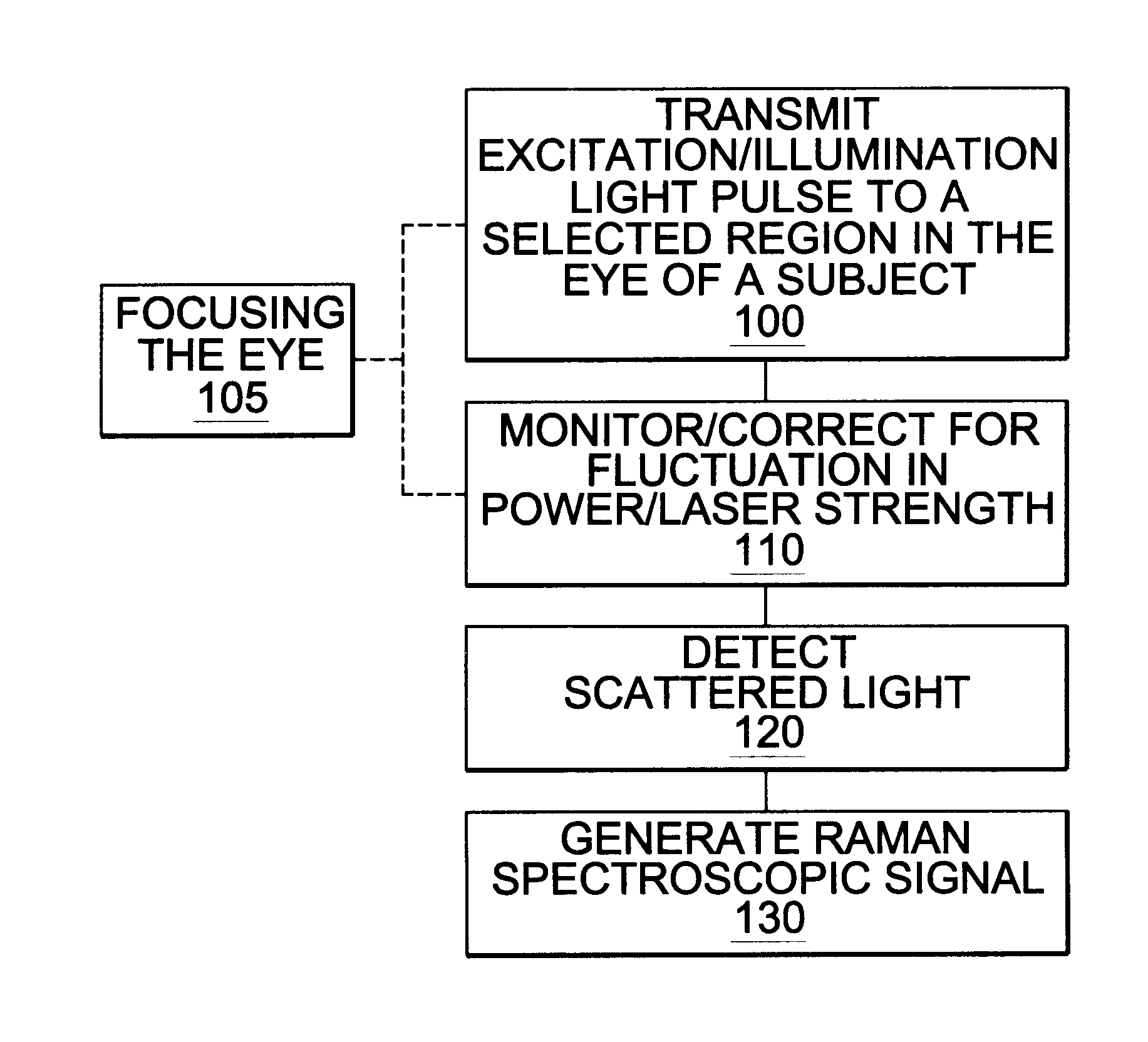
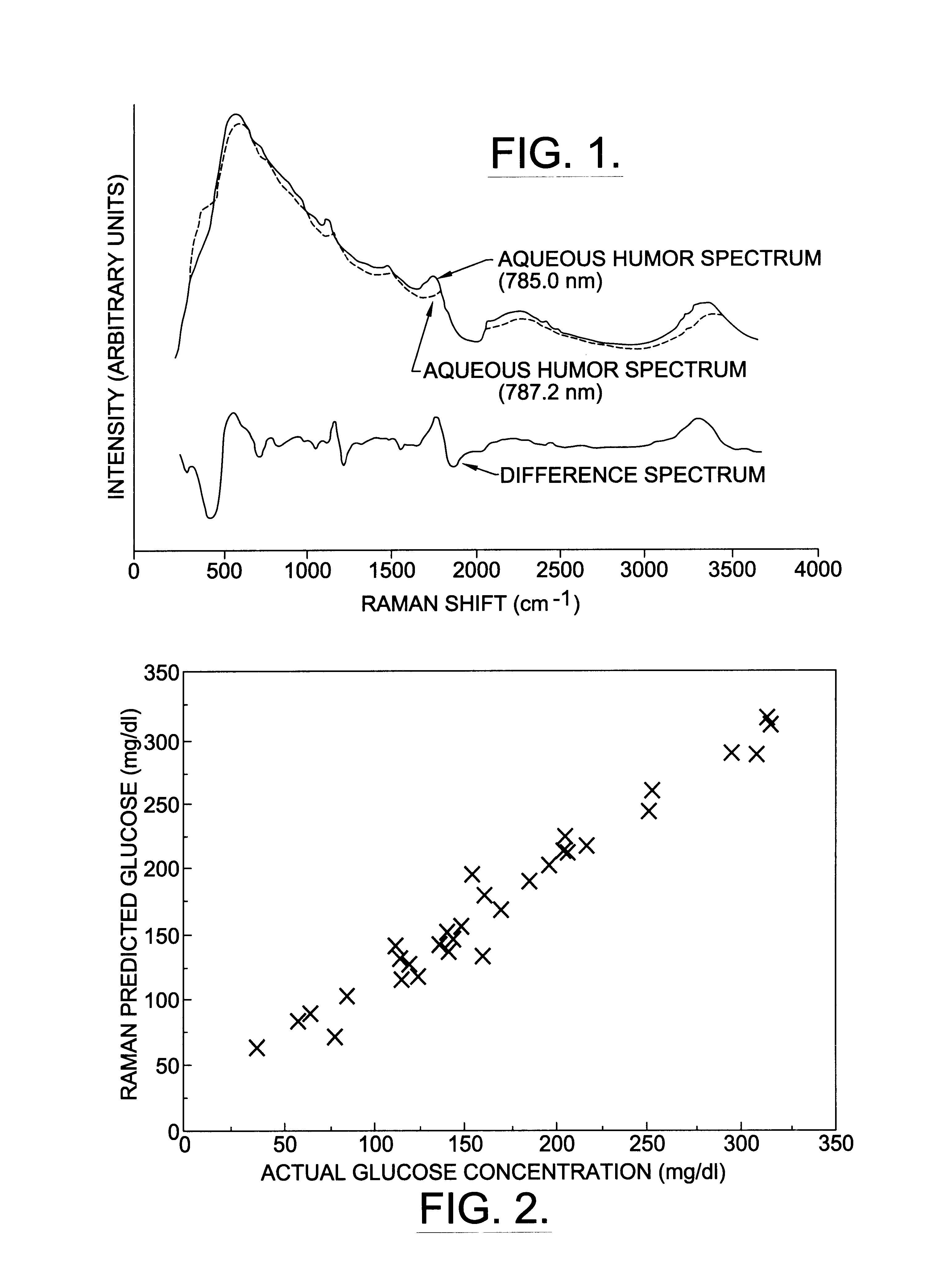
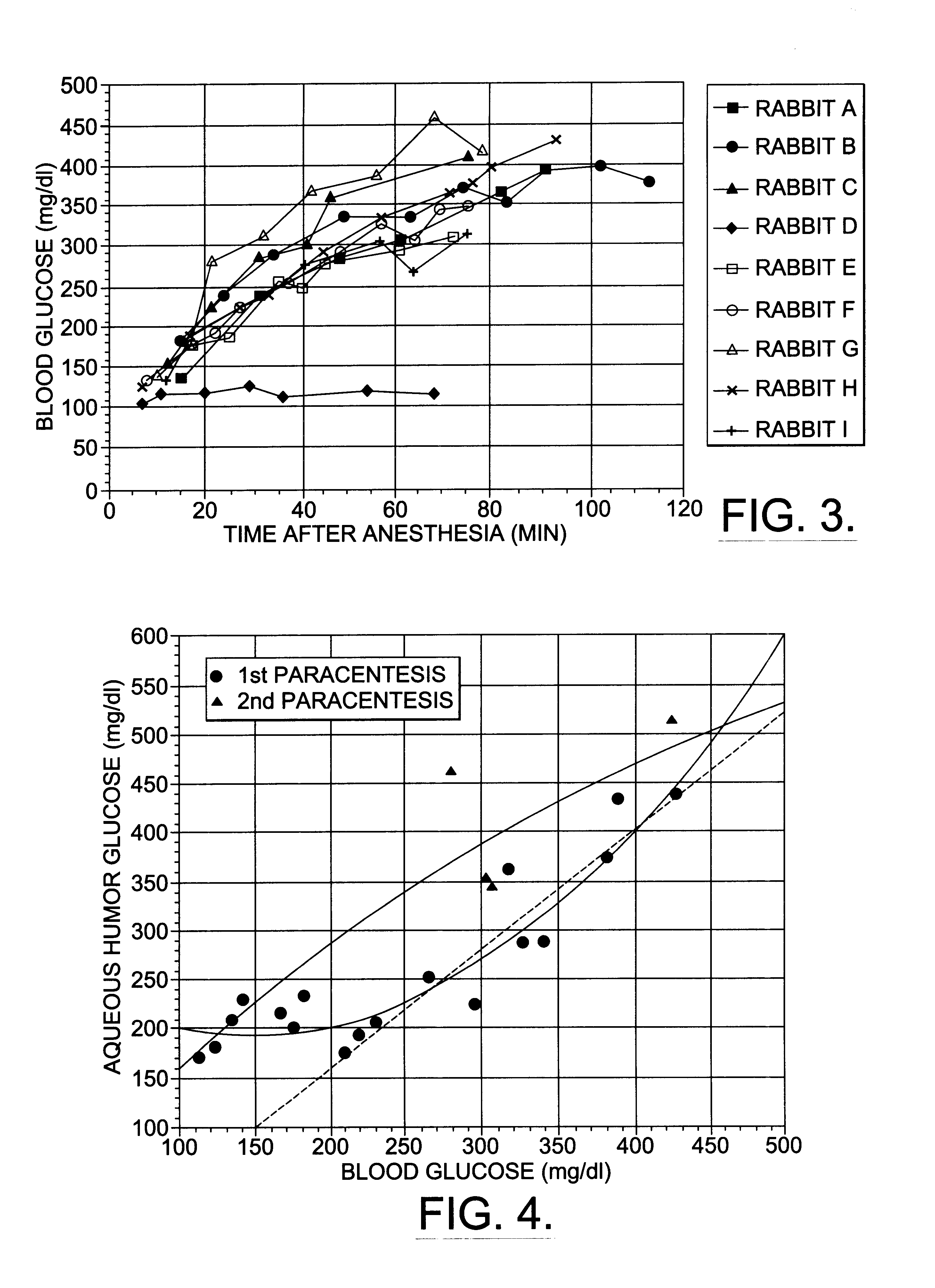
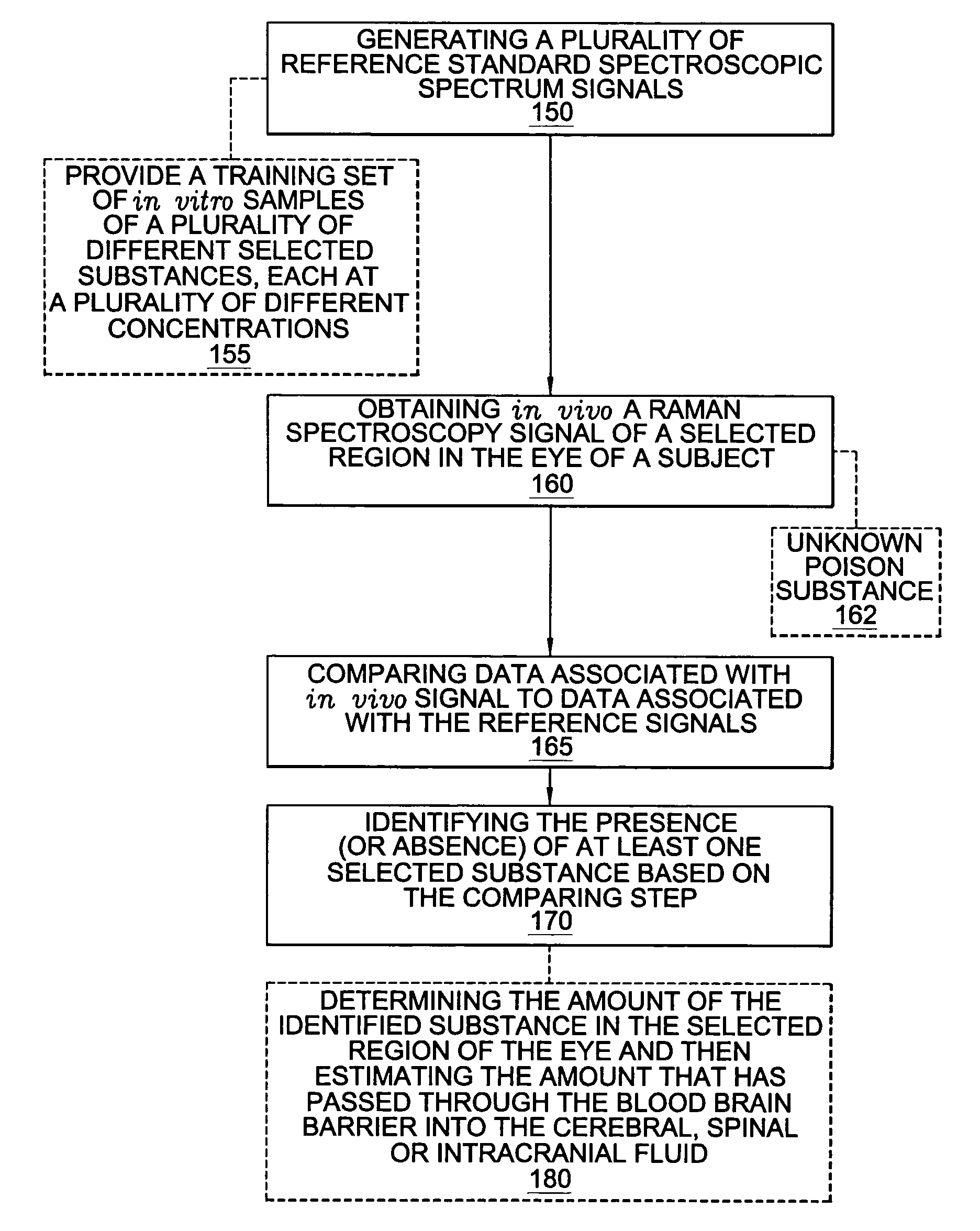
A non-invasive method for analyzing the blood-brain barrier includes obtaining a Raman spectrum of a selected portion of the eye and monitoring the Raman spectrum to ascertain a change to the dynamics of the blood brain barrier. Also, non-invasive methods for determining the brain or blood level of an analyte of interest, such as glucose, drugs, alcohol, poisons, and the like, comprises: generating an excitation laser beam (e.g., at a wavelength of 600 to 900 nanometers); focusing the excitation laser beam into the anterior chamber of an eye of the subject so that aqueous humor, vitreous humor, or one or more conjunctiva vessels in the eye is illuminated; detecting (preferably confocally detecting) a Raman spectrum from the illuminated portion of the eye; and then determining the blood level or brain level (intracranial or cerebral spinal fluid level) of an analyte of interest for the subject from the Raman spectrum. In certain embodiments, the detecting step may be followed by the step of subtracting a confounding fluorescence spectrum from the Raman spectrum to produce a difference spectrum; and determining the blood level and / or brain level of the analyte of interest for the subject from that difference spectrum, preferably using linear or nonlinear multivariate analysis such as partial least squares analysis. Apparatus for carrying out the foregoing methods are also disclosed.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES +1
Assessing blood brain barrier dynamics or identifying or measuring selected substances, including ethanol or toxins, in a subject by analyzing Raman spectrum signals
InactiveUS7398119B2Fast “ triage ” assessmentReliable and faster treatment decisionRadiation pyrometrySpectrum investigationNon invasivePhysics
A non-invasive method for analyzing the blood-brain barrier includes obtaining a Raman spectrum of a selected portion of the eye and monitoring the Raman spectrum to ascertain a change to the dynamics of the blood brain barrier.Also, non-invasive methods for determining the brain or blood level of an analyte of interest, such as glucose, drugs, alcohol, poisons, and the like, comprises: generating an excitation laser beam at a selected wavelength (e.g., at a wavelength of about 400 to 900 nanometers); focusing the excitation laser beam into the anterior chamber of an eye of the subject so that aqueous humor, vitreous humor, or one or more conjunctiva vessels in the eye is illuminated; detecting (preferably confocally detecting) a Raman spectrum from the illuminated portion of the eye; and then determining the blood level or brain level (intracranial or cerebral spinal fluid level) of an analyte of interest for the subject from the Raman spectrum. In certain embodiments, the detecting step may be followed by the step of subtracting a confounding fluorescence spectrum from the Raman spectrum to produce a difference spectrum; and determining the blood level and / or brain level of the analyte of interest for the subject from that difference spectrum, preferably using linear or nonlinear multivariate analysis such as partial least squares analysis. Apparatus for carrying out the foregoing methods are also disclosed.
Owner:CALIFORNIA INST OF TECH +1
Apparatus and methods for the treatment of presbyopia using fiber-coupled-lasers
Systems and surgical techniques for presbyopia correction by laser removal of the sclera tissue are disclosed. The disclosed preferred embodiments of the system consists of a beam spot controller, a fiber delivery unit and a fiber tip. The basic laser including UV lasers and infrared lasers having wavelength ranges of (0.15-0.36) microns and (1.9-3.2) microns and diode lasers of about 0.98, 1.5 and 1.9 microns. Presbyopia is treated by a system which uses an ablative laser to ablate the sclera tissue outside the limbus to increase the accommodation of the ciliary body of the eye. The sclera tissue may be ablated by the laser with or without the conjunctiva layer open.
Owner:NEOS OCULAR
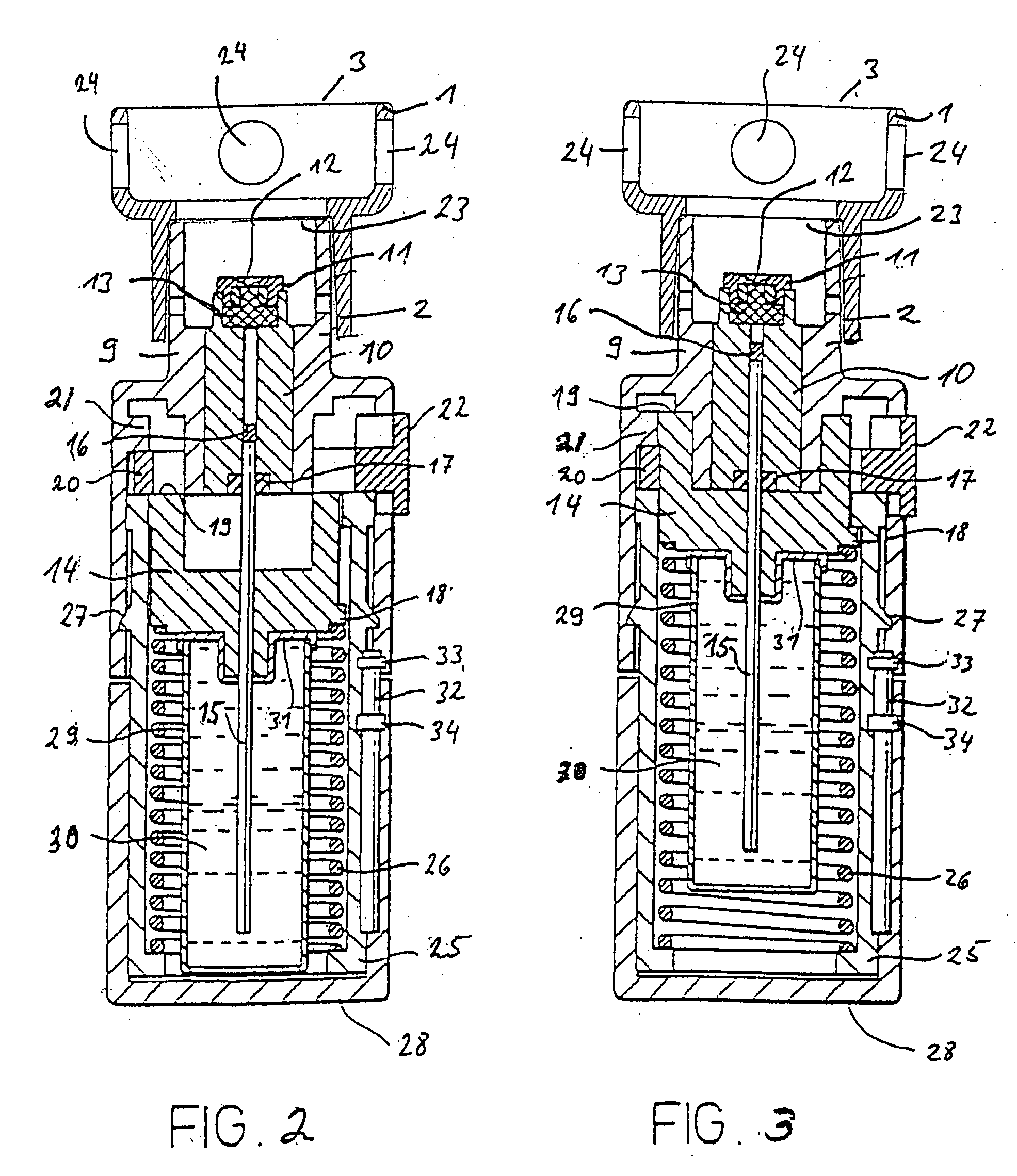
Aqueous humor drainage implant for treatment glaucoma
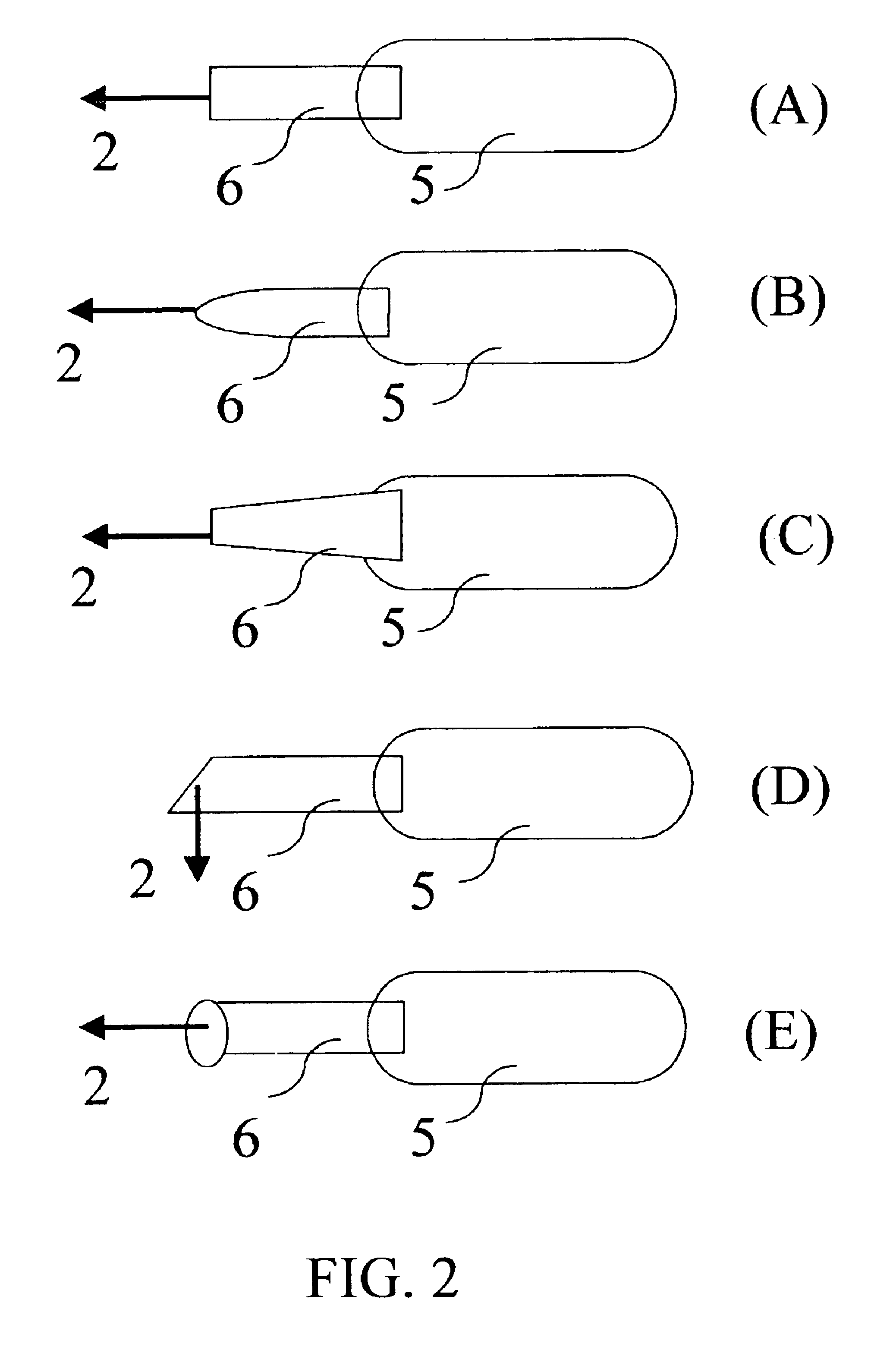
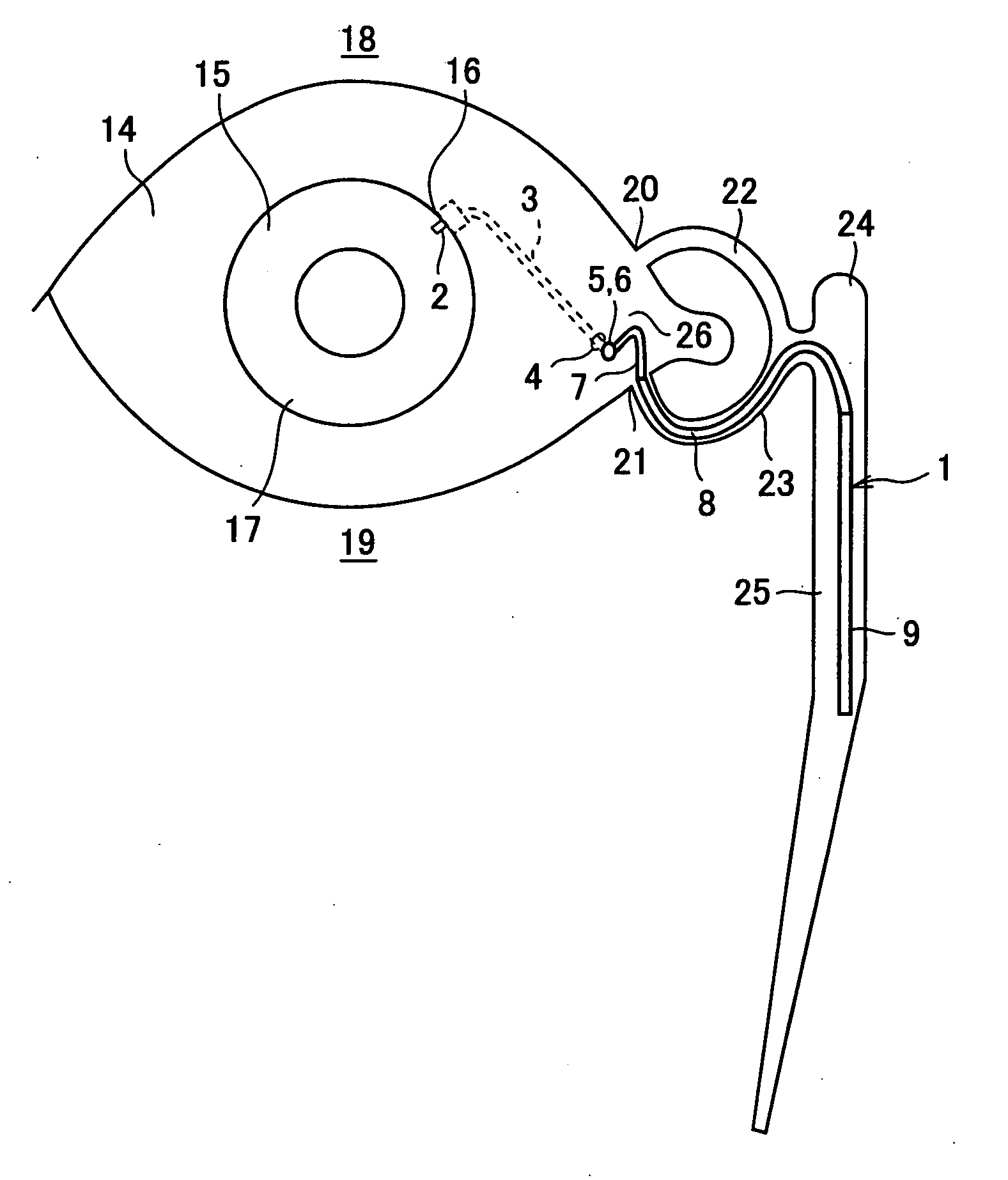
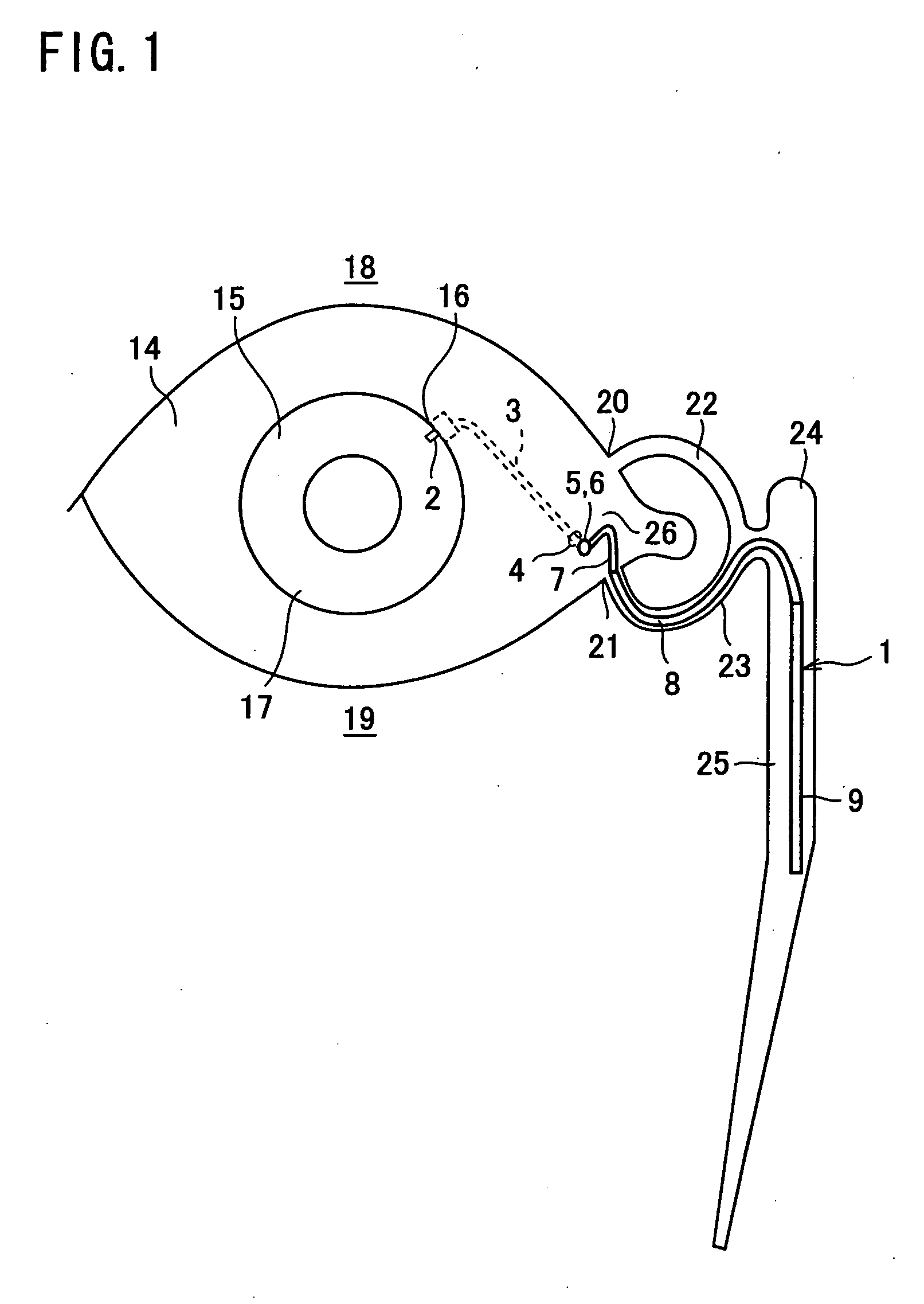
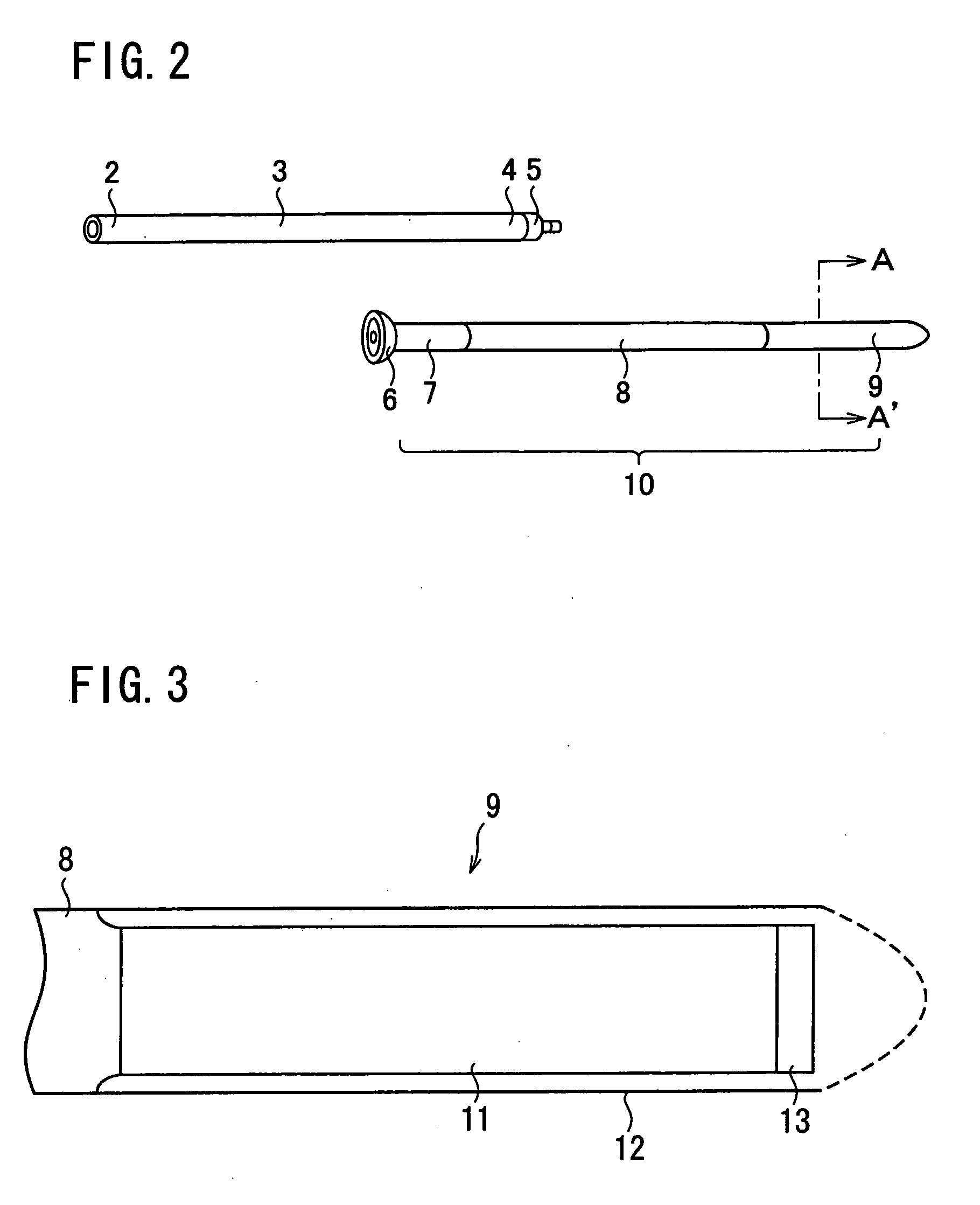
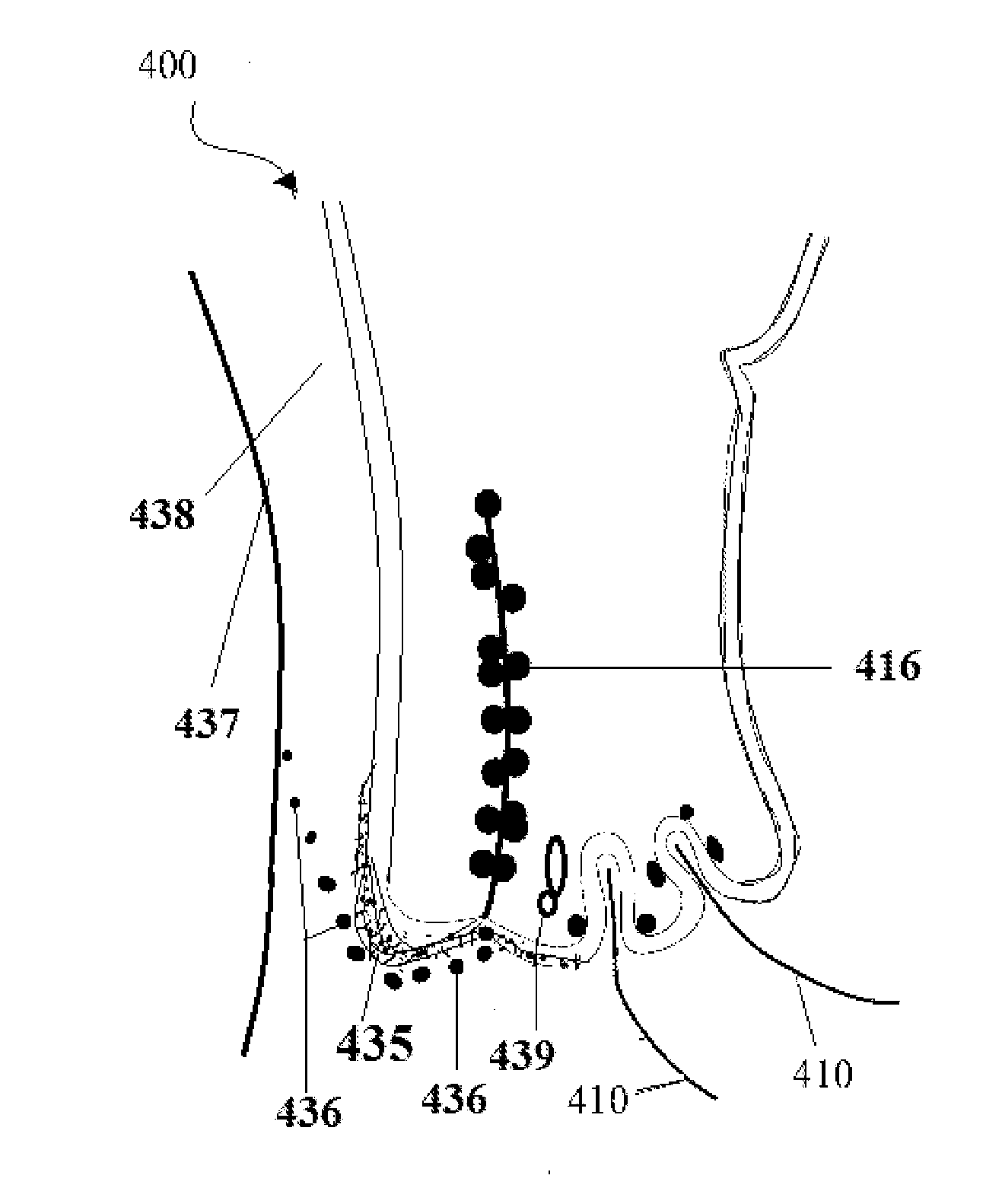
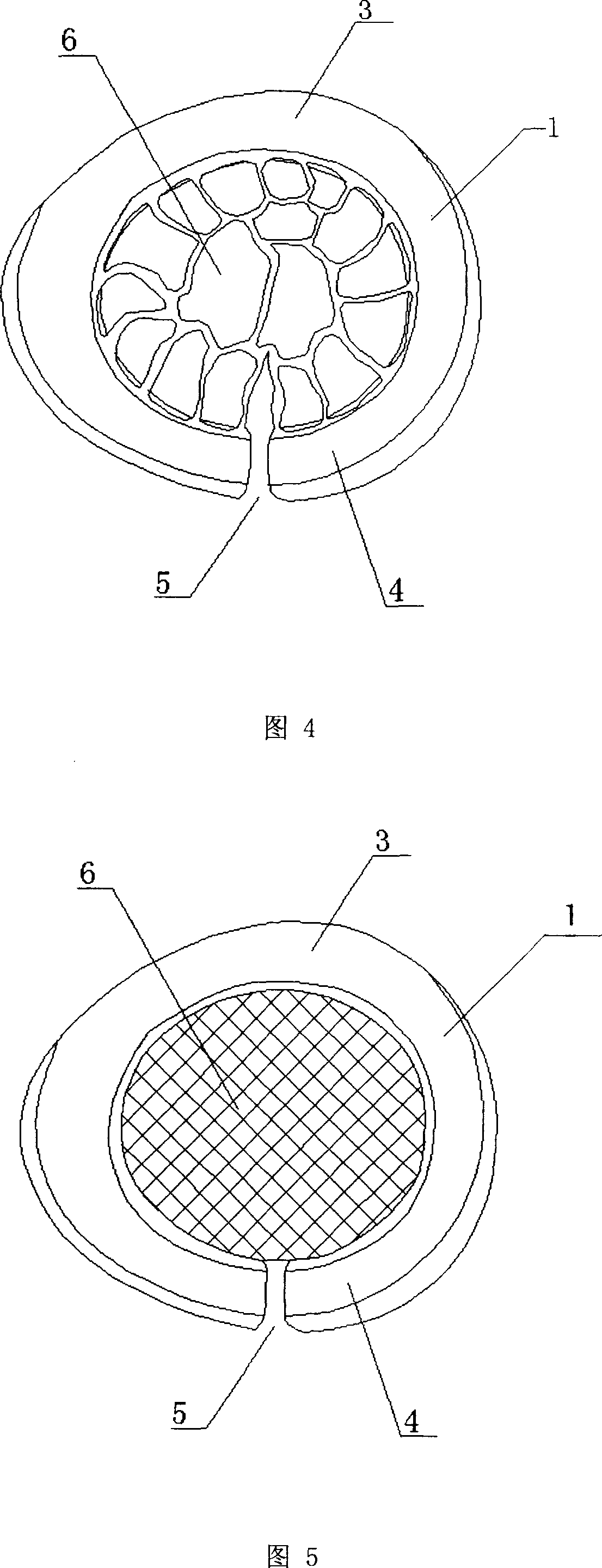
A first tube (3) and second tube (7) for guiding aqueous humor to the exterior of the eye are connected to each other in the vicinity of a surface of conjunctiva (14) via a first joint (5) and second joint (6). A filter part (9) provided to prevent reflux infection from the exterior to interior of the eye is connected to the second tube (7) via a third tube (8) positioned inside a lower lacrimal canaliculus (23). This enables an aqueous humor drainage implant (1) to be positioned in the eye and the exterior of the conjunctiva with reduced invasiveness. With the aqueous humor drainage implant for glaucoma treatment, the aqueous humor in the eye can be drained to the exterior of the conjunctiva while preventing reflux infection at the viral level, and the intraocular pressure reducing effect can be sustained for extended time periods over the lifespan of the patient. Further, the aqueous humor drainage implant can be readily positioned with reduced surgical invasiveness, while posing no danger of damaging the eye or nasolacrimal duct after the installation.
Owner:JAPAN SCI & TECH CORP
Artificial tear replacement solution
InactiveUS7001607B1Reduce wearGood film formingHalogenated hydrocarbon active ingredientsSenses disorderConjunctivaConjunctival sac
A tear replacement solution that contains at least one water-soluble fluorosurfactant, water and a non-polar component, preferably in gel form, and a method for the external treatment for the eye of an mammal by applying the tear replacement solution to the eye, preferably by placing in the conjunctival sac.
Owner:PHARMPUR
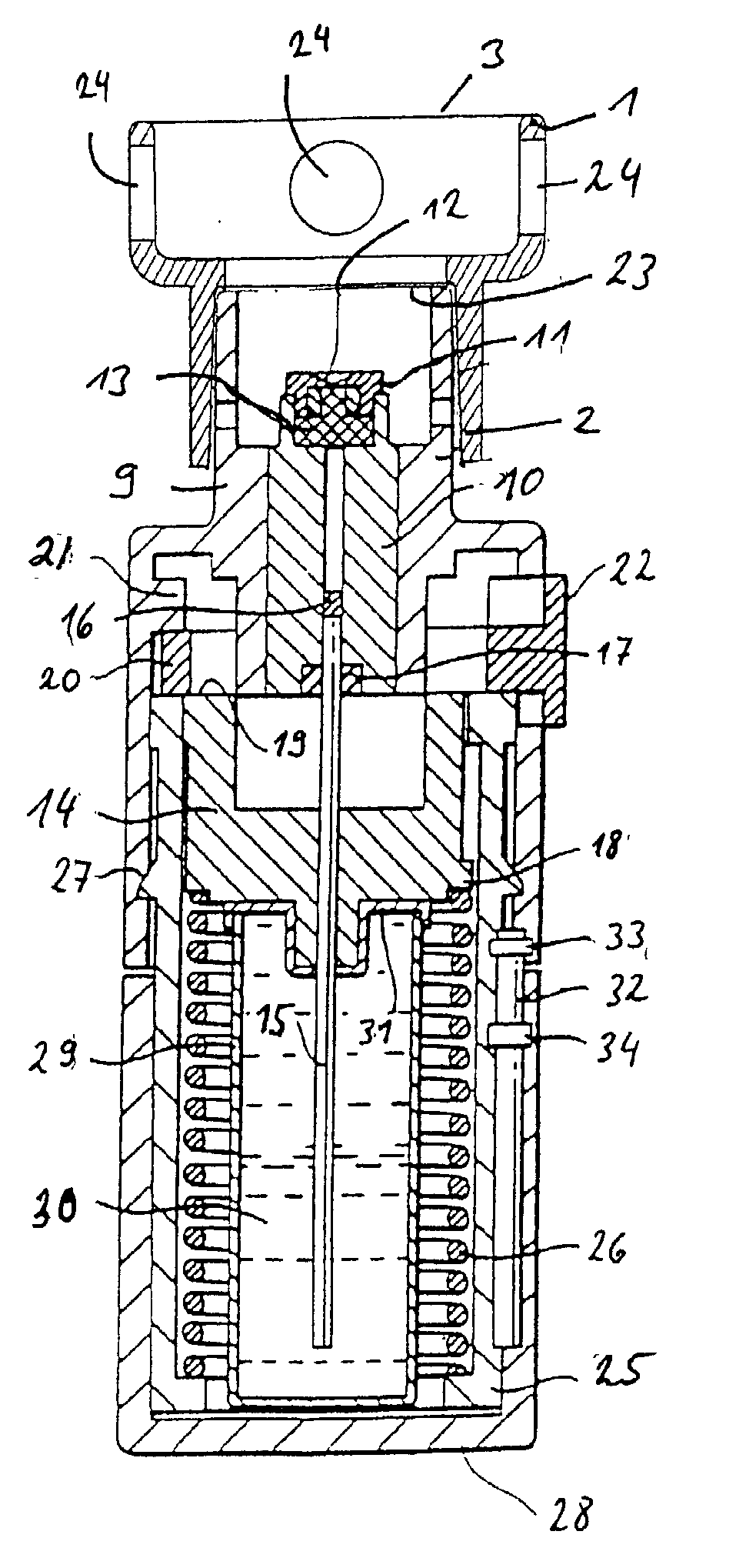
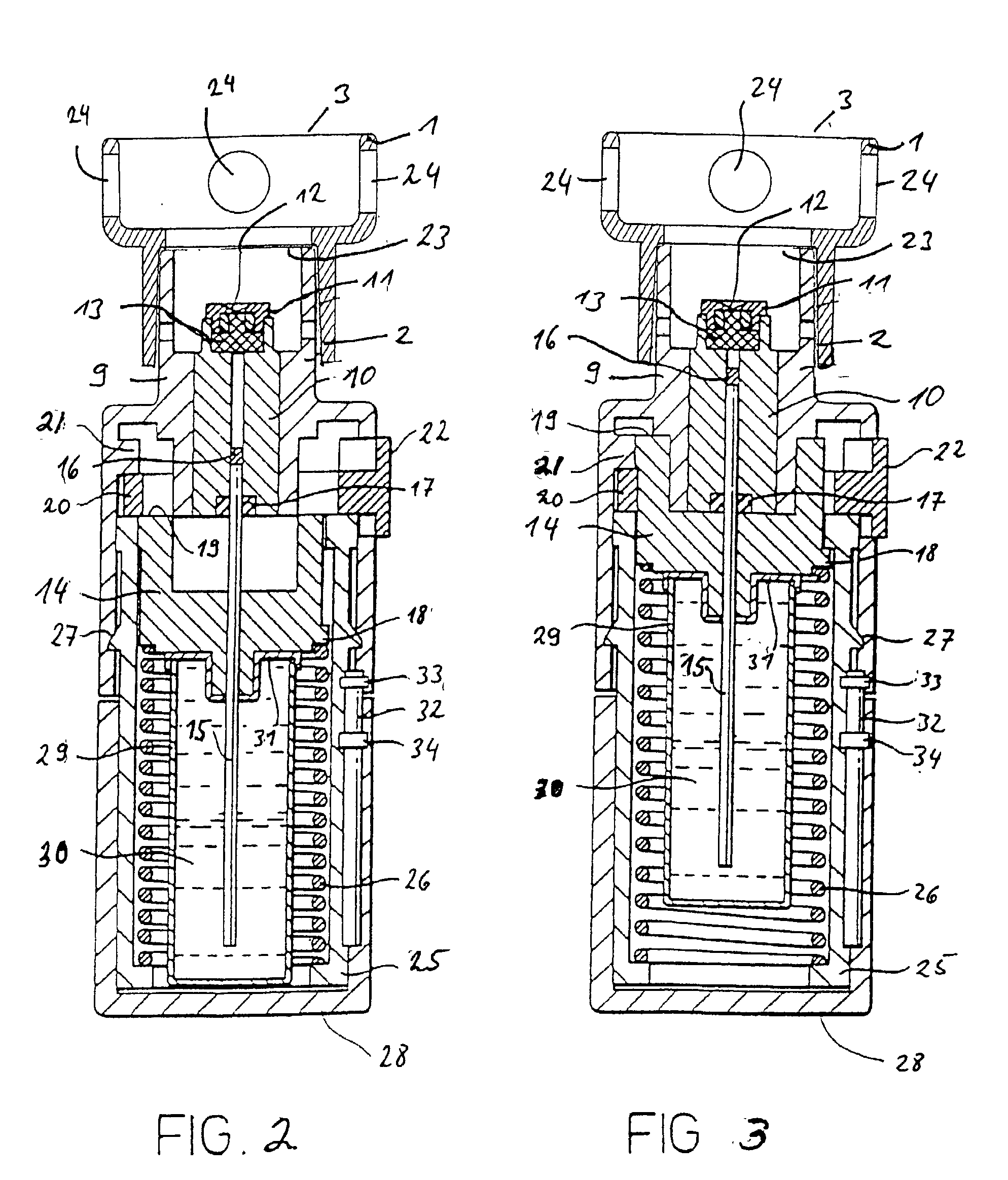
Implantable Devices and Methods for Measuring Intraocular, Subconjunctival or Subdermal Pressure and/or Analyte Concentration
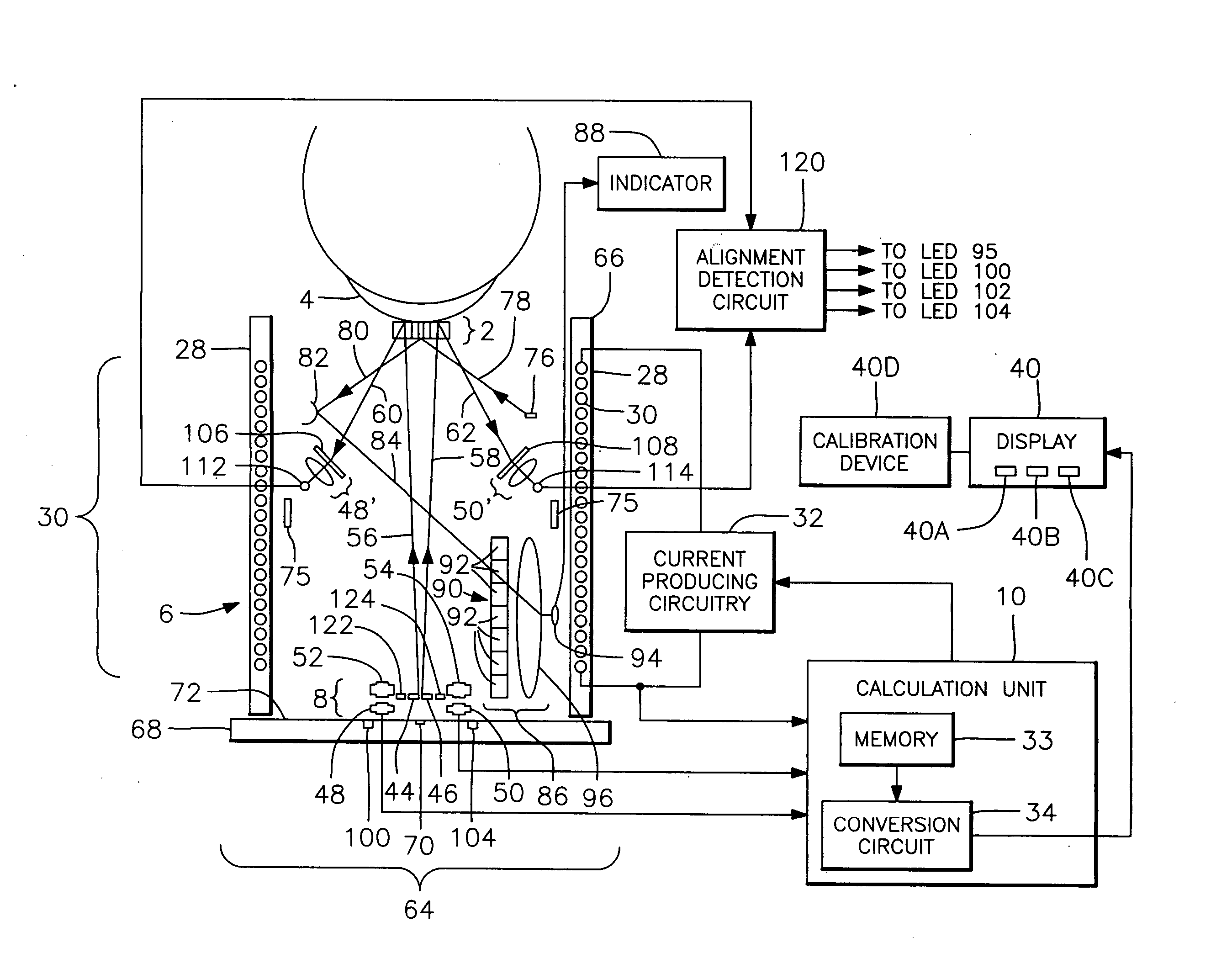
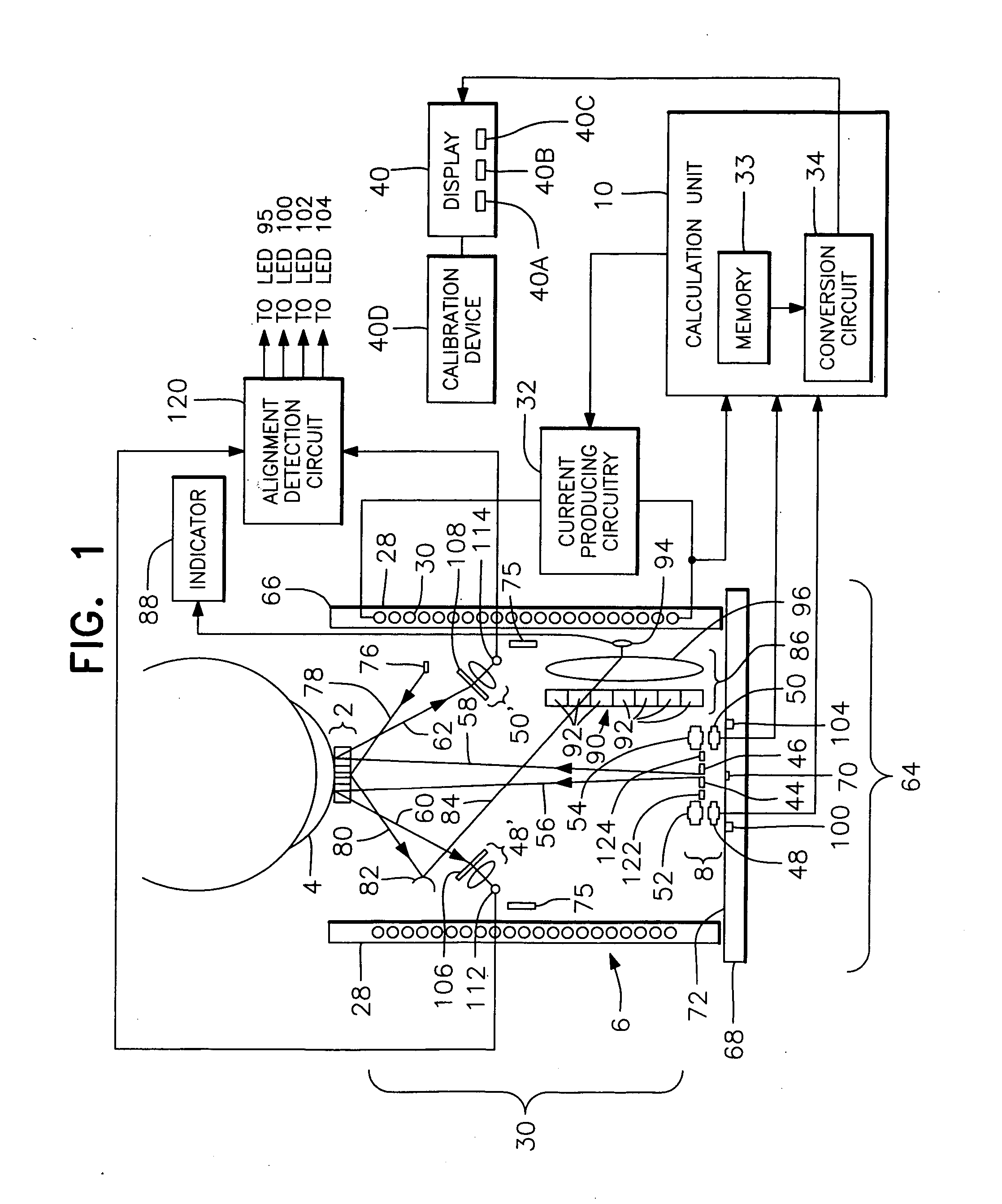
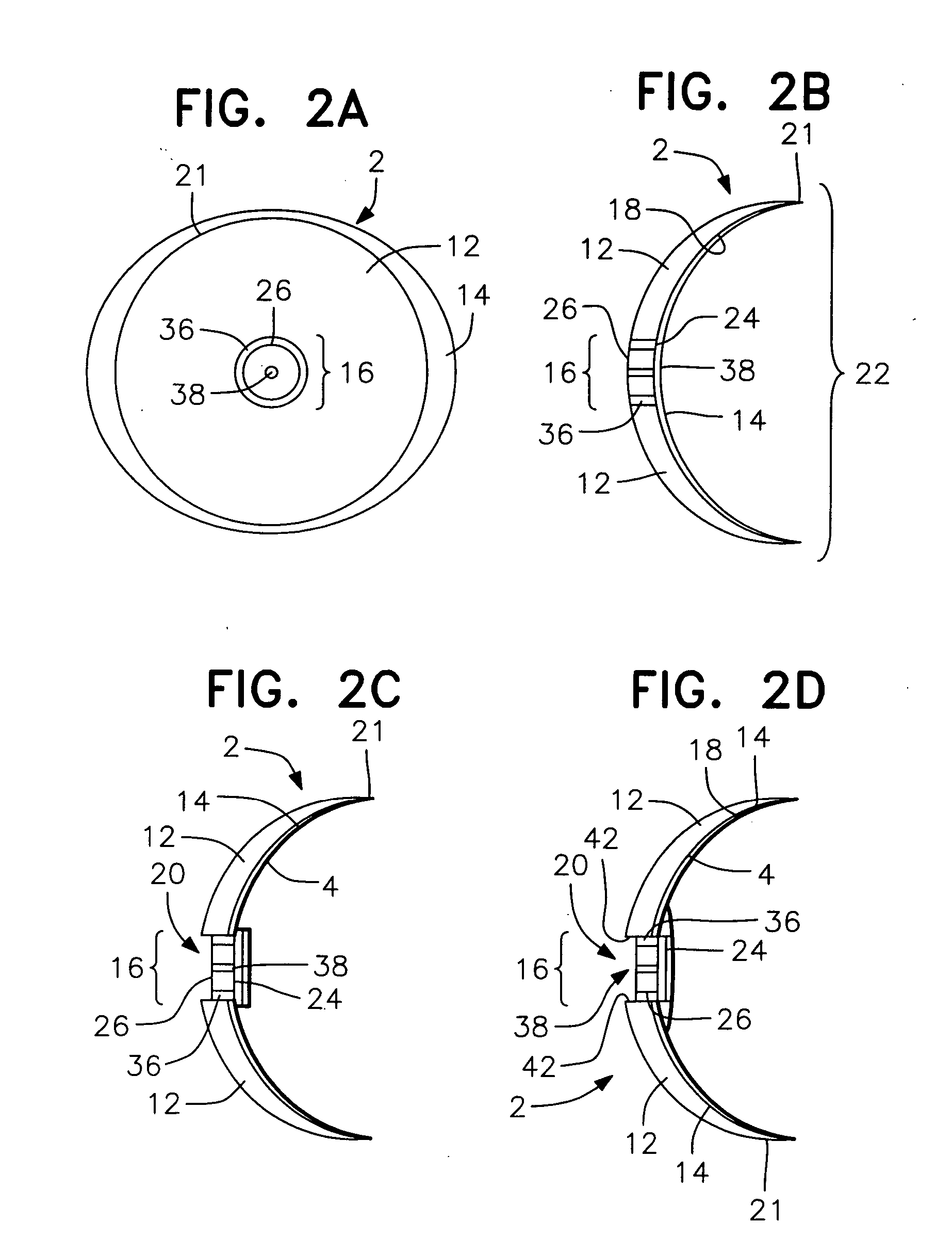
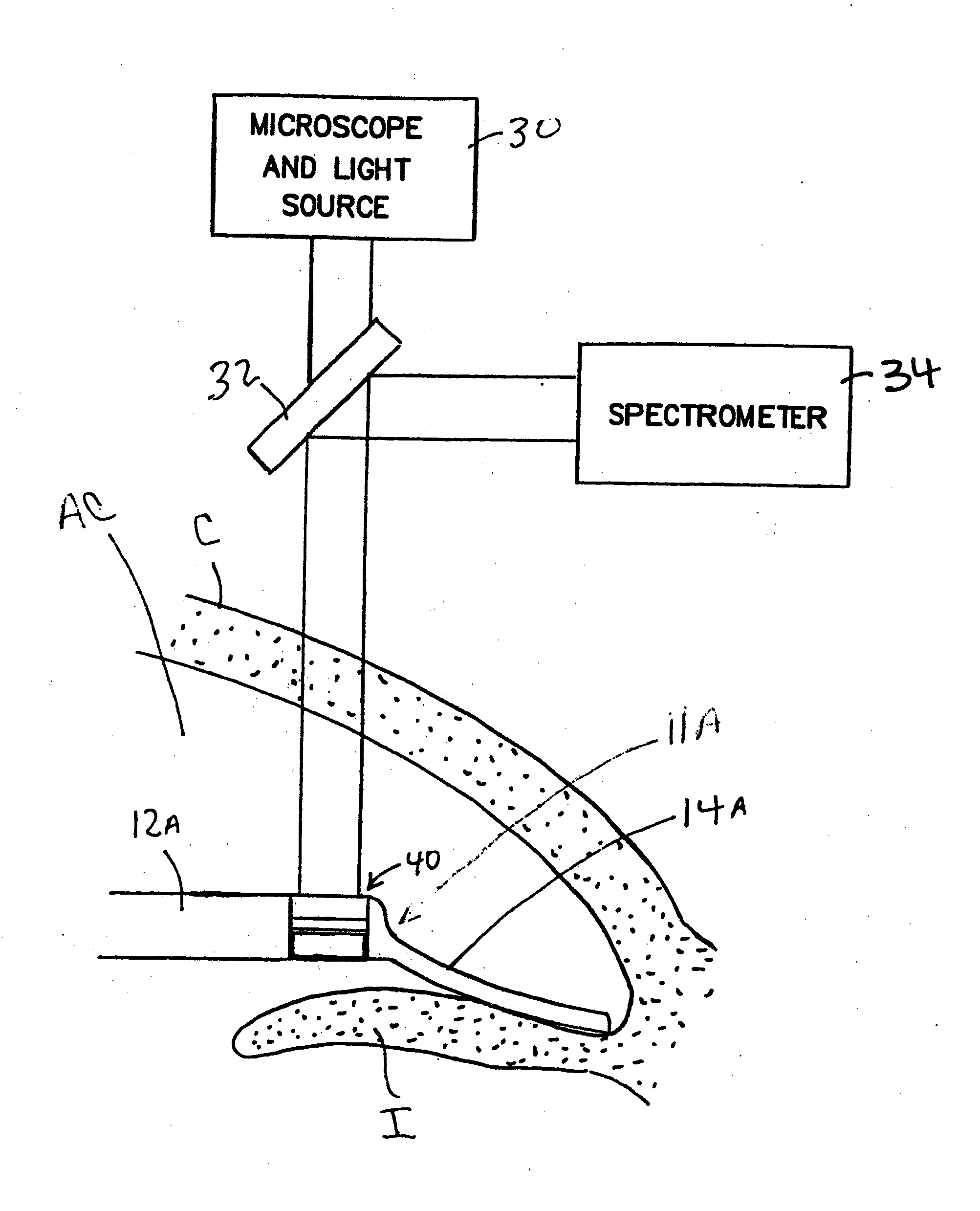
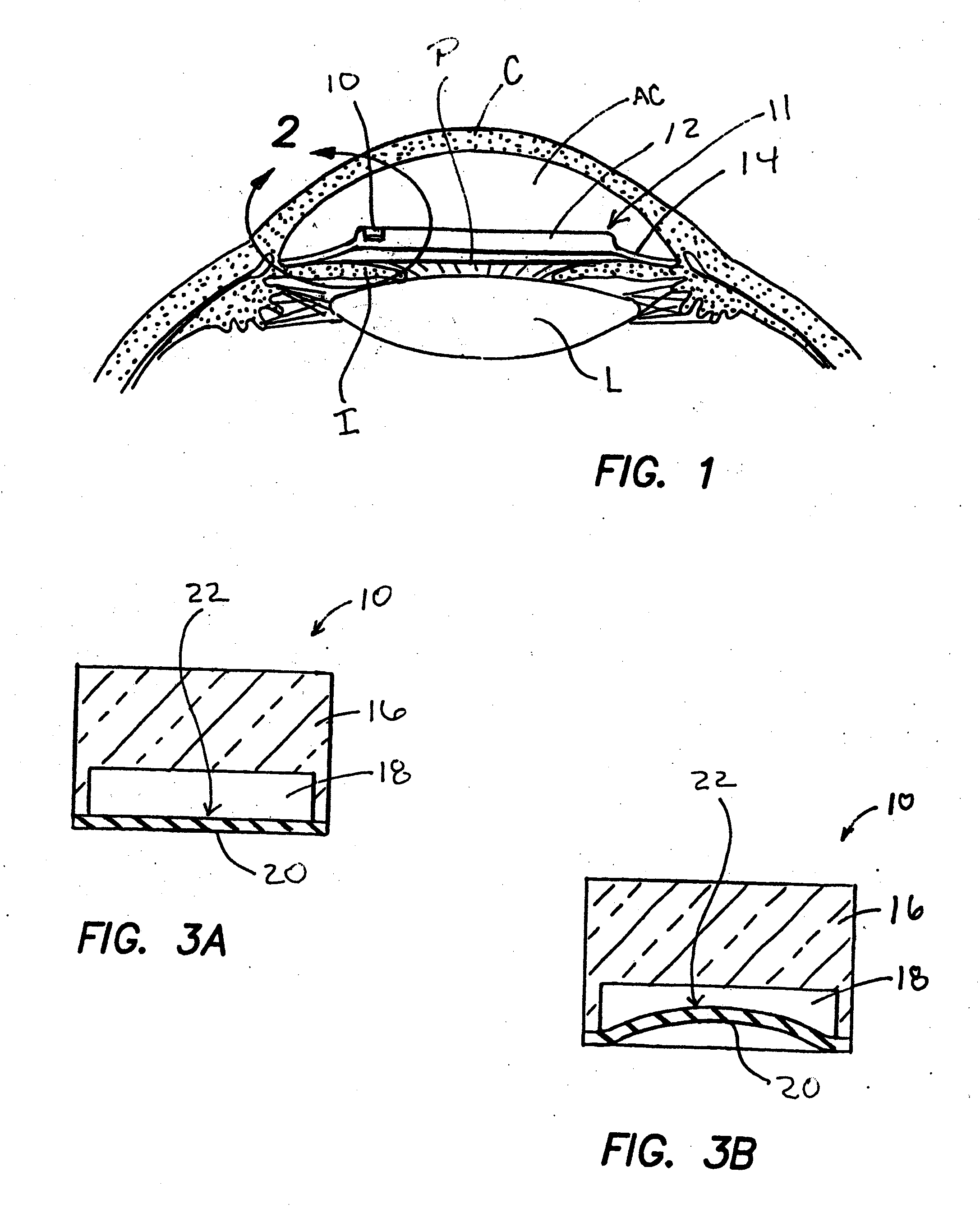
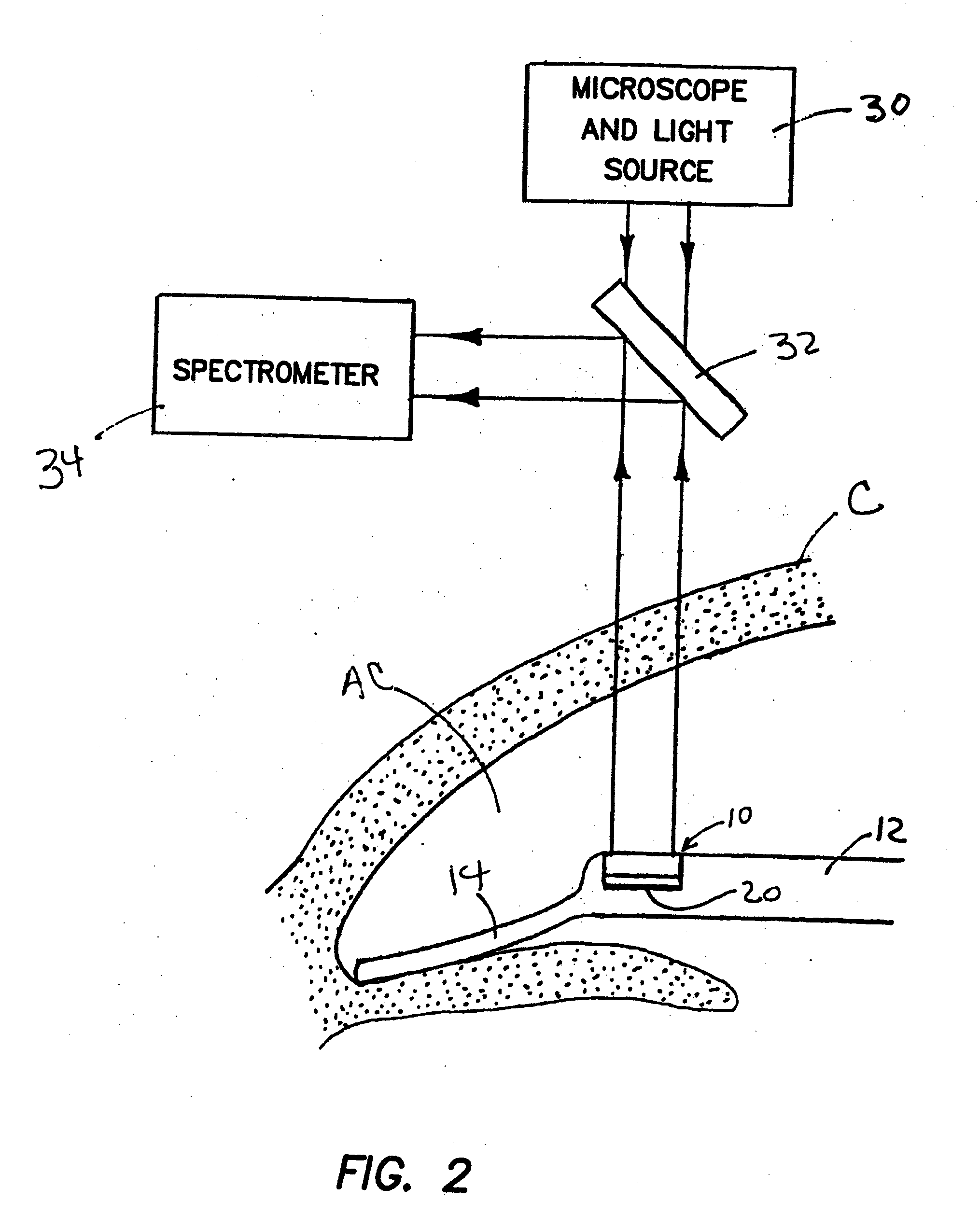
Methods, apparatus and systems for measuring pressure and / or for quantitative or qualitative measurement of analytes within the eye or elsewhere in the body. Optical pressure sensors and / or optical analyte sensors are implanted in the body and light is cast from an extracorporeal light source, though the cornea, conjunctiva or dermis, and onto a reflective element located within each pressure sensor or analyte sensor. The position or configuration of each sensor's reflective element varies with pressure or analyte concentration. Thus, the reflectance spectra of light reflected by the sensors' reflective elements will vary with changes in pressure or changes in analyte concentration. A spectrometer or other suitable instrument is used to process and analyze the reflectance spectra of the reflected light, thereby obtaining an indication of pressure or analyte concentration adjacent to the sensor(s). The wavelength of the interrogating beam of light may vary to control out potential interference or inaccuracies in the system.
Owner:BCC ENTERPRISE
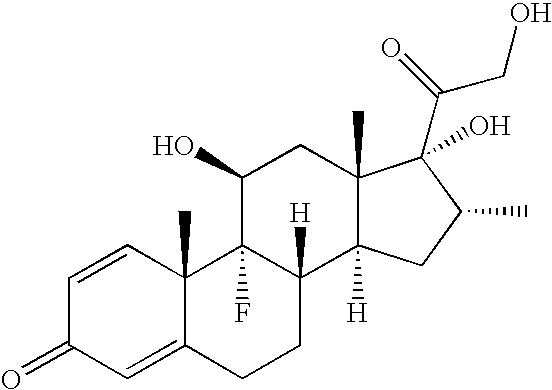
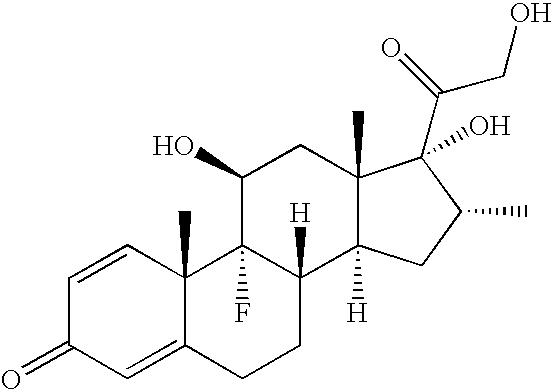
Therapeutic agent for keratoconjunctival disorder
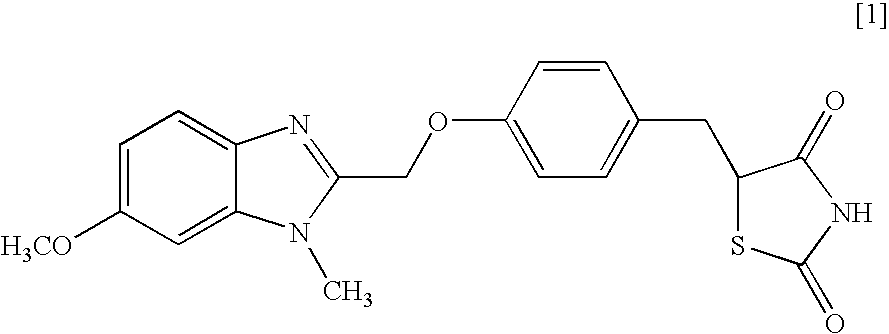
Object of the present invention is to search a novel pharmaceutical use of 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione being a condensed heterocyclic compound, or a salt thereof. 5-[4-(6-methoxy-1-methyl-1H-benzimidazol-2-ylmethoxy)benzyl]thiazolidine-2,4-dione or a salt thereof can exert an excellent effect to promote healing in a dry eye model, and is useful as a therapeutic agent for keratoconjunctival disorders such as dry eyes, corneal ulcer, keratitis, conjunctivitis, superficial punctate keratopathy, corneal epithelial defects, conjunctival epithelial defects, keratoconjunctivitis sicca, superior limbic keratoconjunctivitis and filamentary keratitis.
Owner:SANTEN PHARMA CO LTD
Methods for Treating Eye Conditions
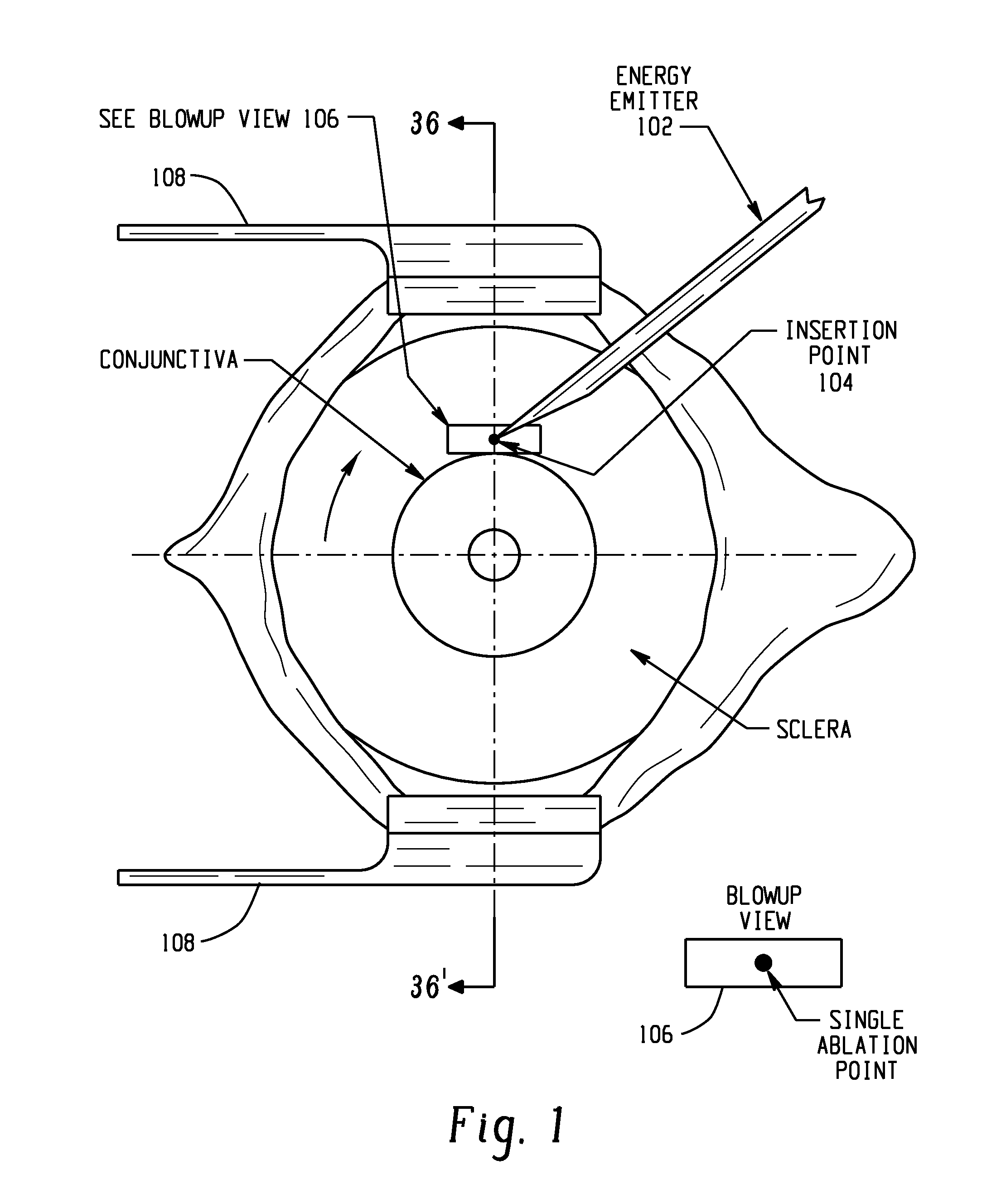
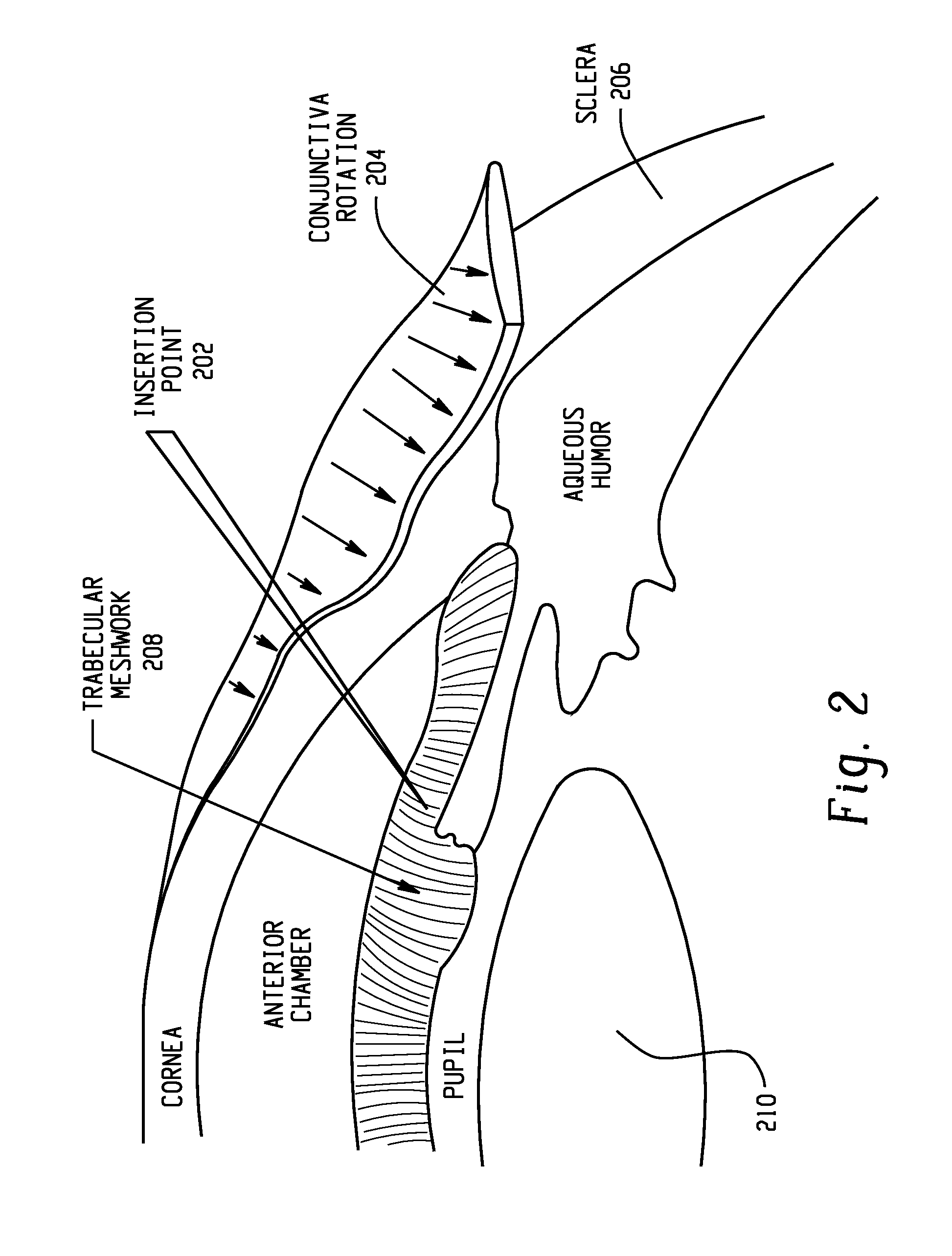
Systems and methods are provided for reducing intraocular pressure in an eye. A perpendicular incision is made through a conjunctiva of the eye to access a trabecular meshwork of the eye. Electromagnetic energy is focused through the perpendicular incision to ablate a portion of the trabecular network, where said ablation creates a channel for outflow flow of fluid through a sclera venous sinus to reduce pressure within the eye.
Owner:BIOLASE TECH INC
Surgical correction of human eye refractive errors by active composite artificial muscle implants
Correction of eye refractive errors such as presbyopia, hyperopia, myopia, and astigmatism by using either pre-tensioned or transcutaneously energized artificial muscle implants to change the axial length and the anterior curvatures of the eye globe by bringing the retina / macula region to coincide with the focal point. The implants are scleral constrictor bands, segments or ribs for inducing accommodation of a few diopters, to correct the refractive errors on demand or automatically. The implant comprises an active sphinctering band encircling the sclera, implanted under the conjunctiva and under the extraocular muscles to uniformly constrict the eye globe, to induce active temporary myopia (hyperopia) by increasing(decreasing) the length and curvature of the globe. Multiple and specially designed constrictor bands enable surgeons to correct astigmatism. The artificial muscles comprise materials such as composite magnetic shape memory (MSM), heat shrink, shape memory alloy-silicone rubber, electroactive ionic polymeric artificial muscles or electrochemically contractile ionic polymers bands.
Owner:OPHTHALMOTRONICS
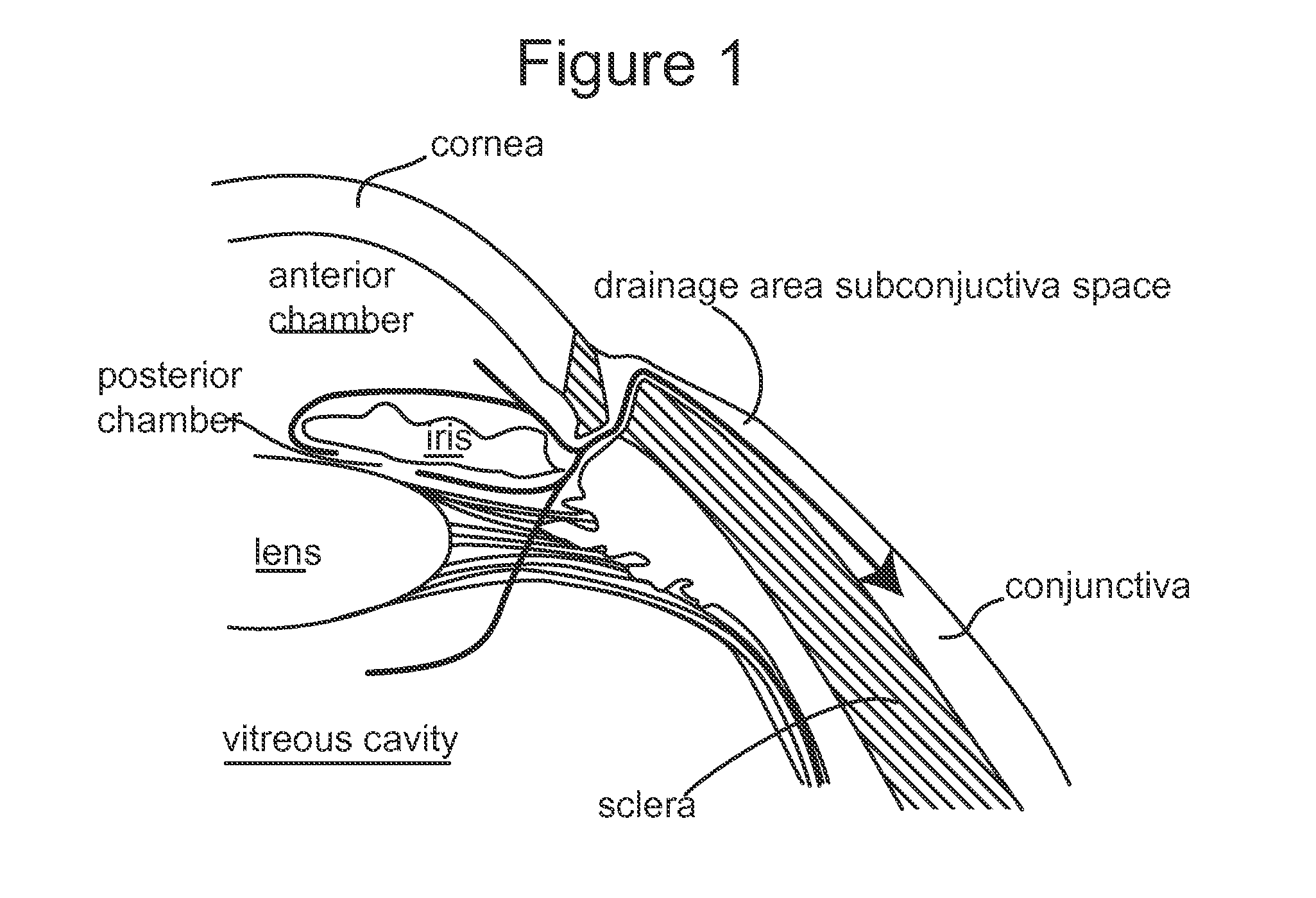
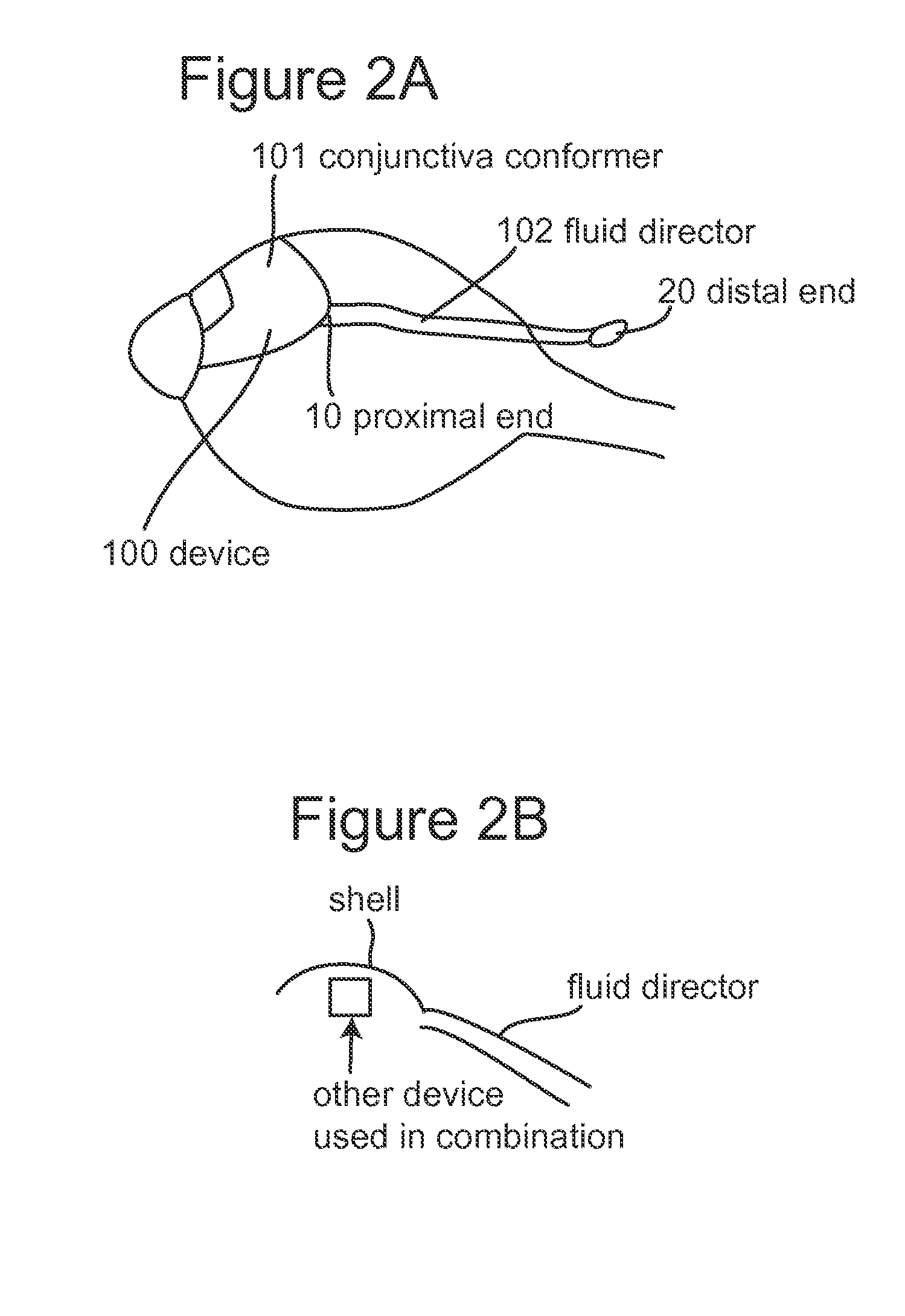
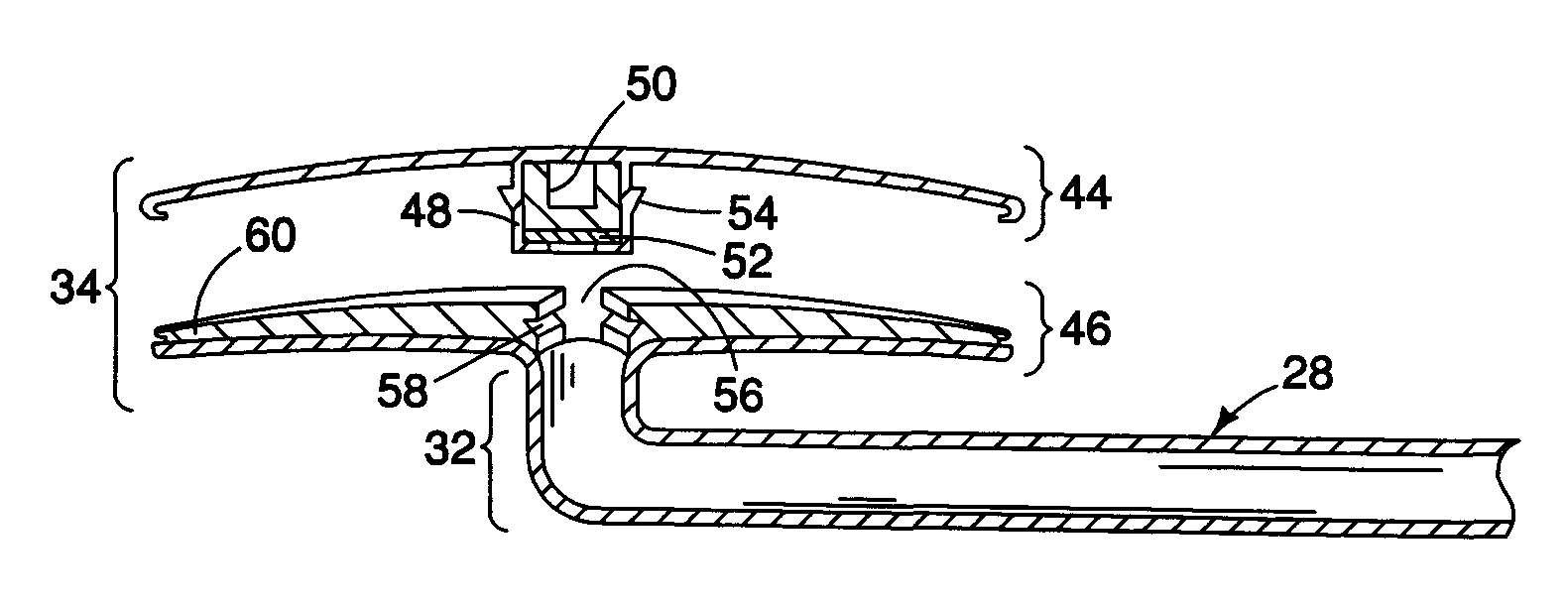
Subconjunctival conformer device and uses thereof
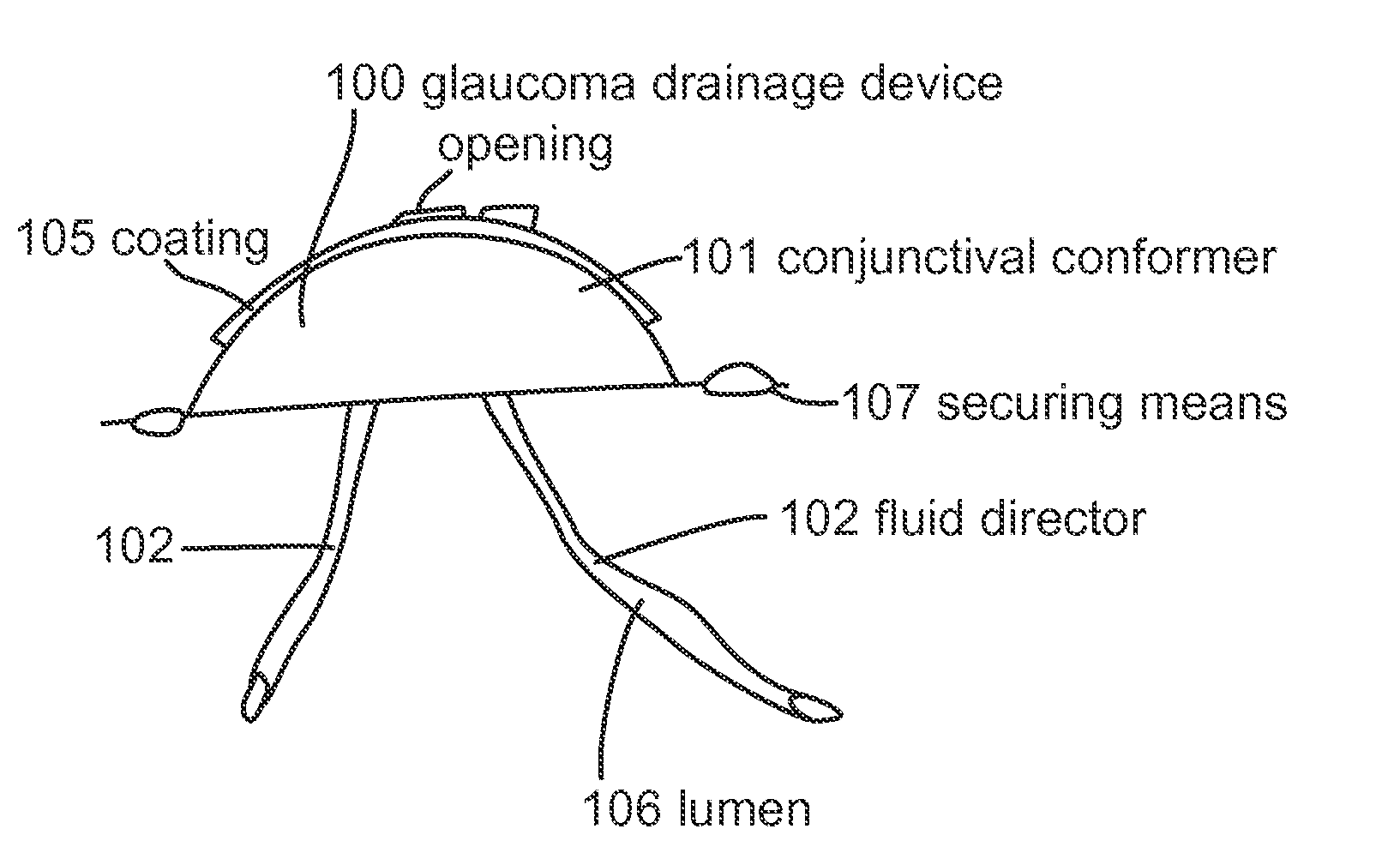
ActiveUS20120089072A1Minimize inflammationMinimize scarringEye surgeryIntravenous devicesConjunctivaElevated intraocular pressure
The present invention provides a device for use in an eye with elevated intraocular pressure or glaucoma, the device comprising a subconjunctival conformer shaped to conform to the eye wall and a fluid director that directs or facilitates the flow of intraocular fluid out of the eye and into the subconjunctival or retrobulbar space. The present invention also provides a method of lowering intraocular pressure using the device of the present invention.
Owner:CUNNINGHAM JR EMMETT T
Anterior Segment Drug Delivery
ActiveUS20120136322A1Maintain positionProvide supportSenses disorderElcosanoid active ingredientsCushioningConjunctiva
A therapeutic system comprises an ocular insert placed on a region outside an optical zone of an eye. The ocular insert comprises two structures: a first skeletal structure and a second cushioning structure. The first structure functions as a skeletal frame which maintains positioning of the implant along the anterior portion of the eye and provides support to the second, cushioning structure. This first structure maintains the attachment of the therapeutic system to the anterior portion of the eye for at least thirty days. In some embodiments the first structure remains a constant size and shape, e.g. a ring shape, a ring with haptics, or a curvilinear ring that is confined to and restrainingly engages the inferior and superior conjunctival fornices so as to retain the implant within the tear fluid and / or against the tissues of the eye.
Owner:FORSIGHT VISION5 INC
Atomizer for applying liquids onto eyes
The present invention relates to atomizers for administering liquids to the cornea or conjunctiva of the eye, special eye adapters for atomizers and the use of atomizers for ophthalmological administration. The atomizers according to the invention are free from propellant gas and have an energy reservoir for supplying the energy needed for the atomization process.
Owner:BOEHRINGER INGELHEIM PHARM KG
Contact lens for increasing tear production
ActiveUS20160114172A1Increase tear productionRelieve symptomsOptical articlesImplantable neurostimulatorsReflexConjunctiva
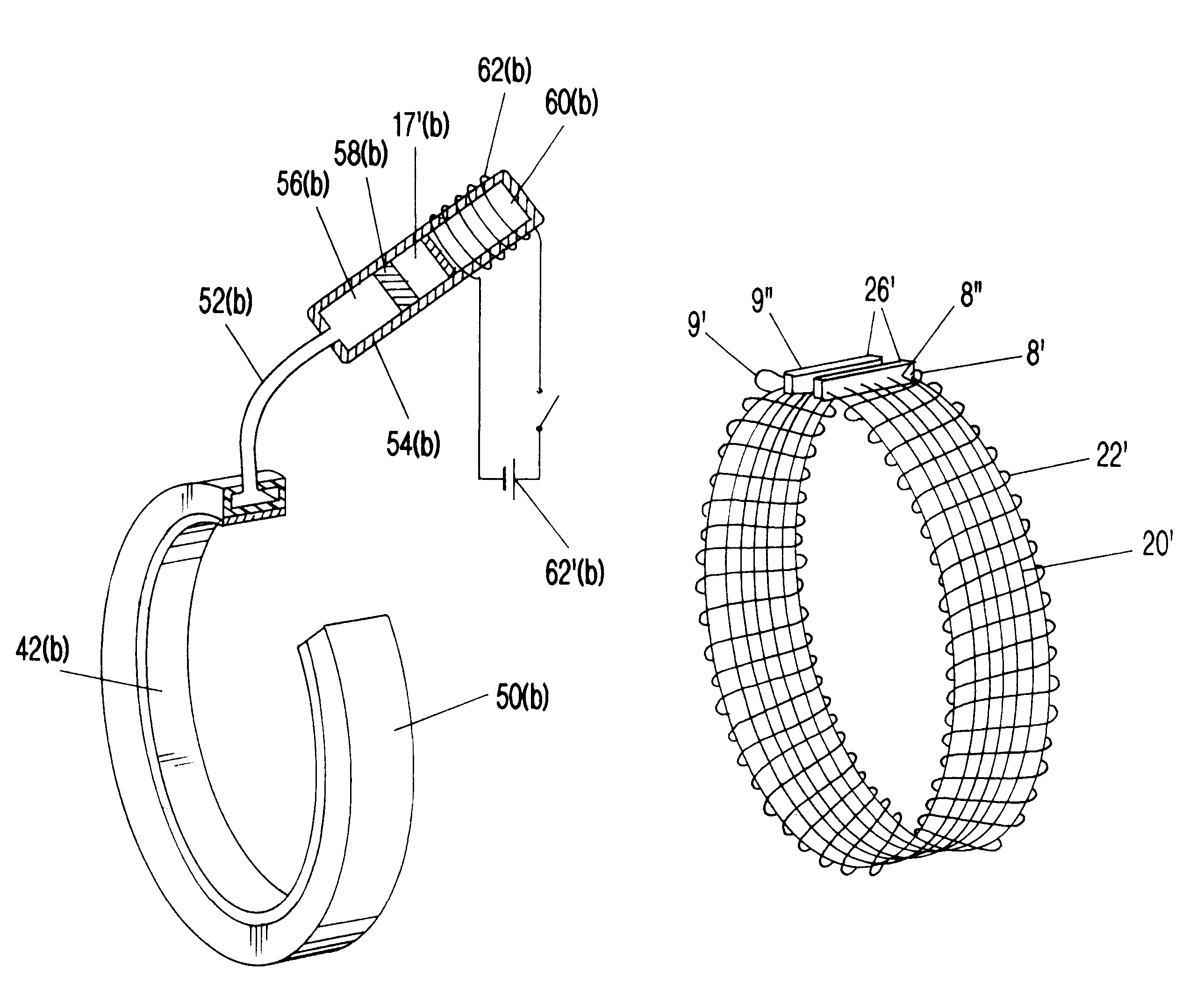
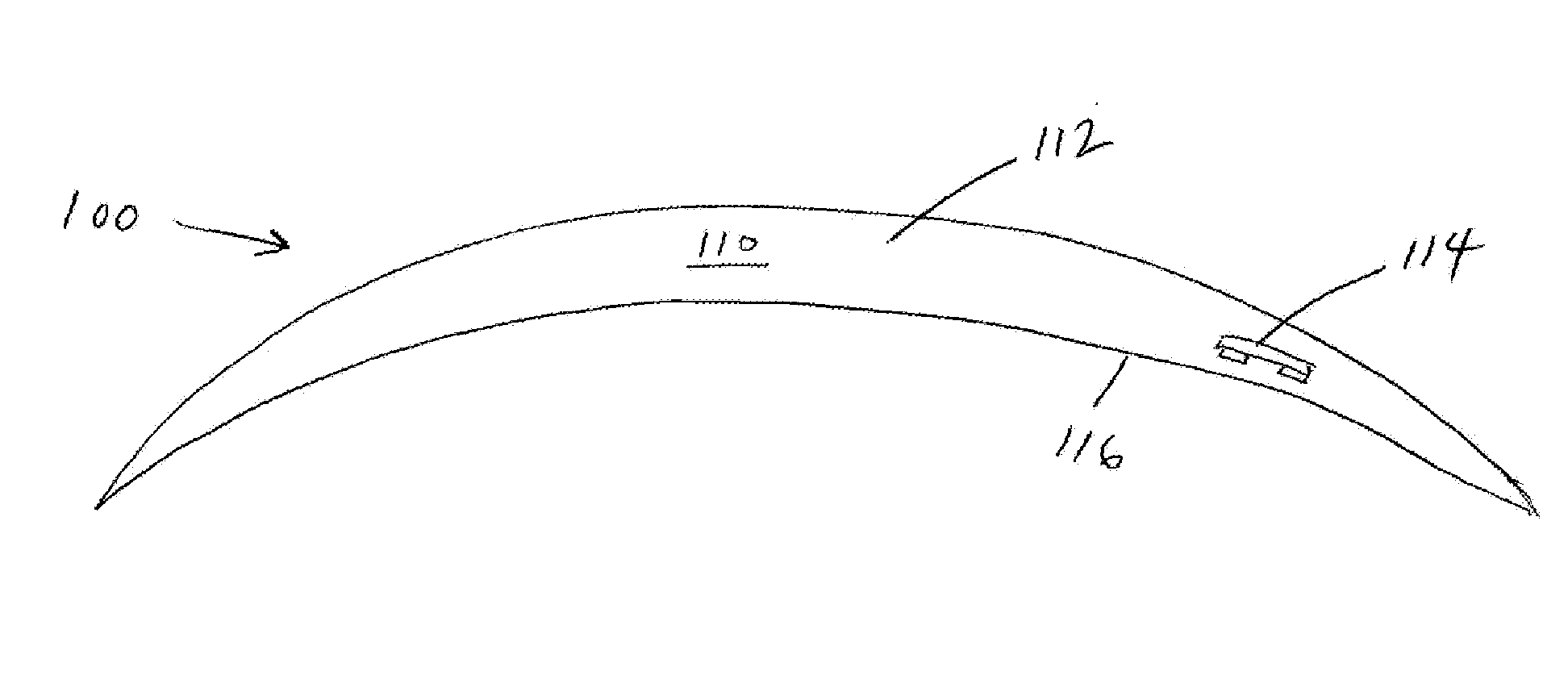
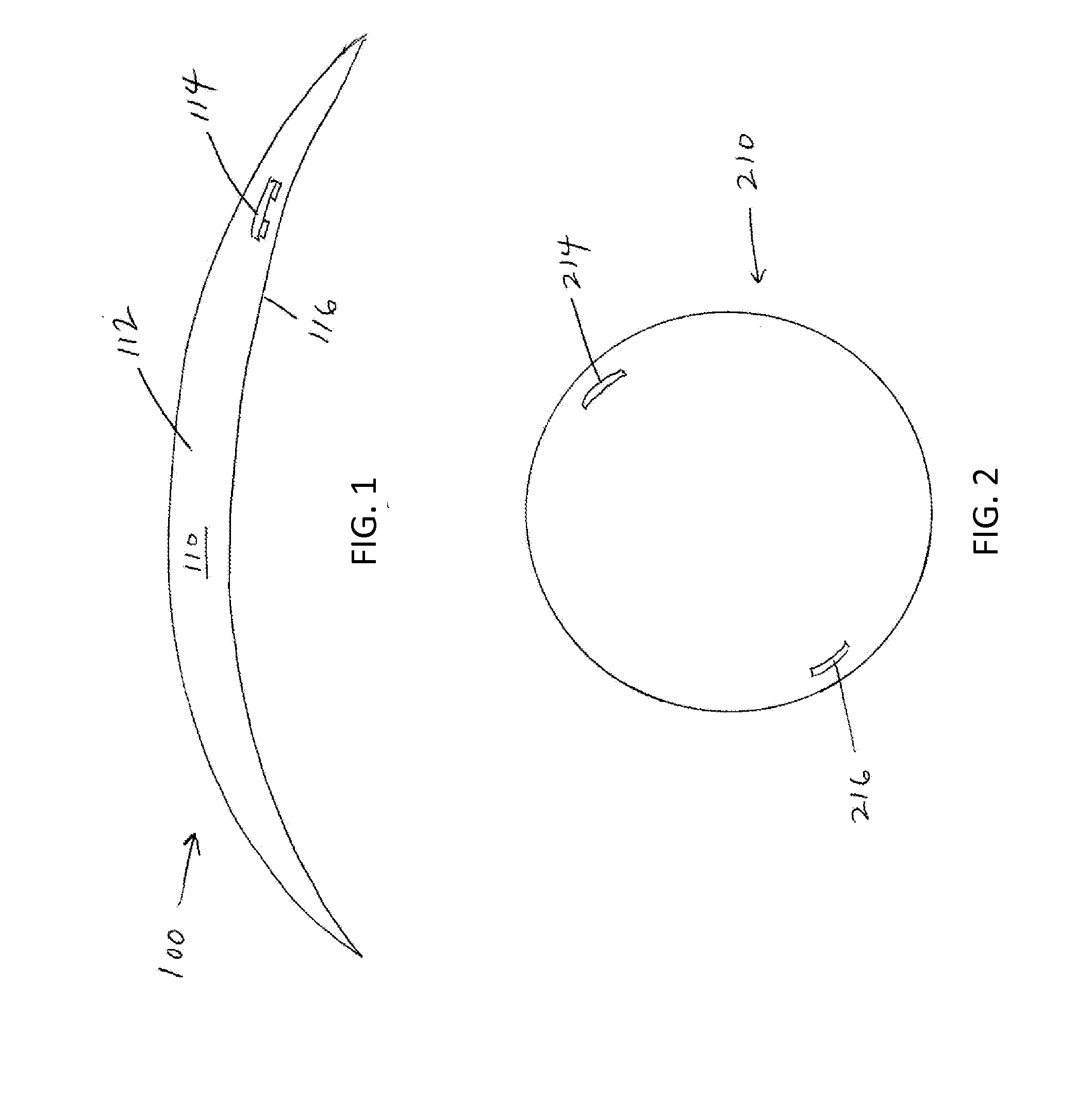
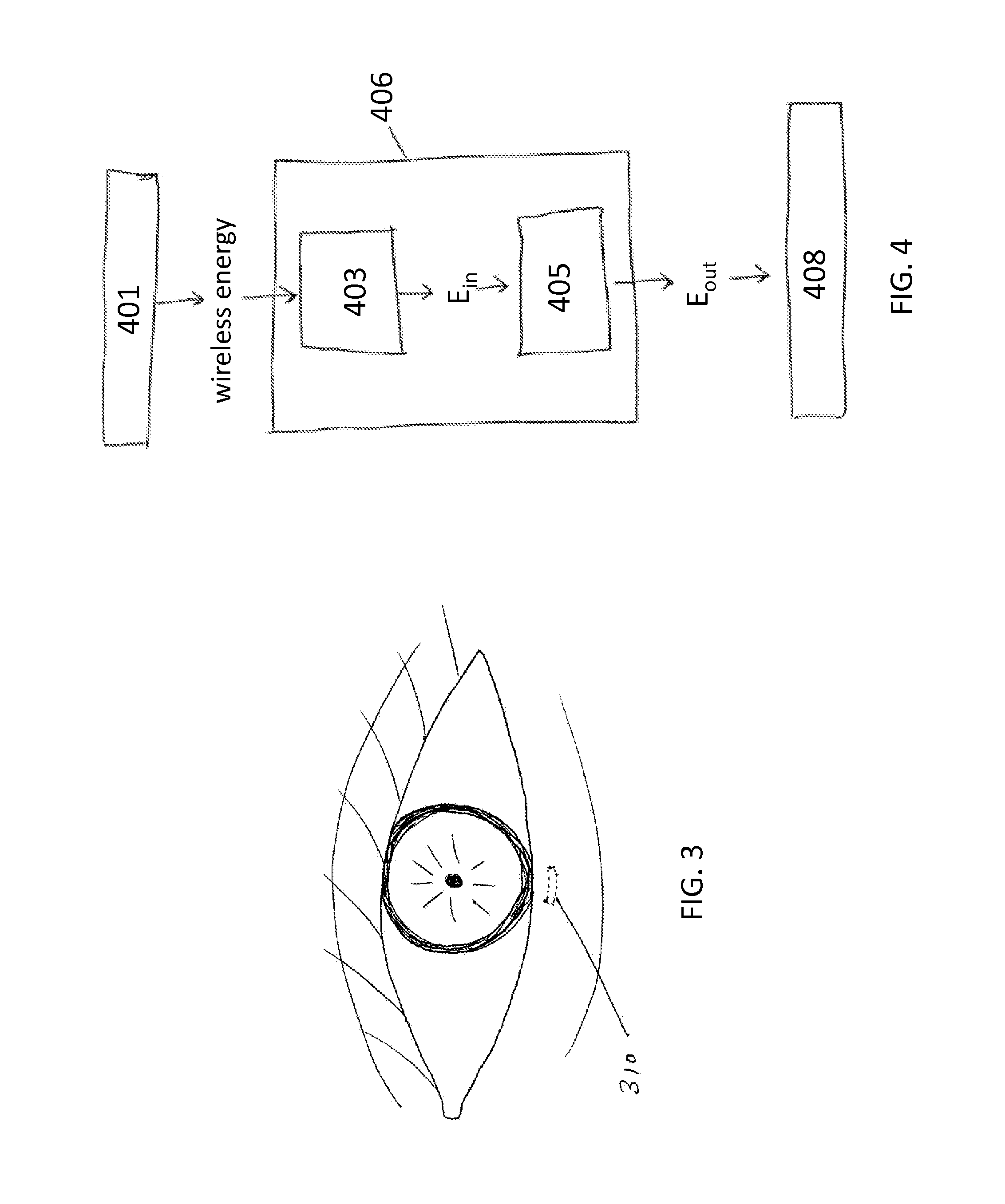
Described here are devices, systems, and methods for increasing tear production by stimulating the cornea, conjunctiva, and / or subconjunctiva. In some variations, the devices may be in the form of a contact lens. The contact lens may comprise a lens body and a stimulator chip, where the stimulator chip is embedded in the lens body. An external power source wirelessly transmits energy to the stimulator chip, where the stimulator chip may convert the energy to an electric waveform to stimulate the cornea, conjunctiva, and / or subconjunctiva. Stimulation may activate the lacrimal reflex to increase tear production. The devices and systems for increasing tear production may be used in methods of treating dry eye, reducing the symptoms of tired eye, increasing comfort for contact lens wearers, and extending the number of years a contact lens user can wear contacts. Also described are methods of manufacturing a contact lens.
Owner:OCULEVE
Power System Implantable in Eye
An implantable ophthalmic power system includes a power source and an enclosure. The enclosure surrounds the power source. The enclosure is configured to be implanted under the conjunctiva of the eye.
Owner:ALCON RES LTD
New methods of treating dry eye syndrome
The invention relates to a method of insulin eye drops for treating dry eye syndrome due to any and all etiological factors (Keratoconjunctivitis sicca), including Sjogren's syndrome, Meibomian gland dysfunction (MGD) and other glandular malfunction in the eye lids, lacrimal glands, cornea, conjunctiva, and exposed scleral surface of the eye. It is treated with Insulin and / or IGF-I with or without known anti-dry eye syndrome therapeutic, pharmaceutical, biochemical and biological agents or compounds.
Owner:SHANTHA TOTADA R +2
Ophthalmic compositions comprising povidone-iodine
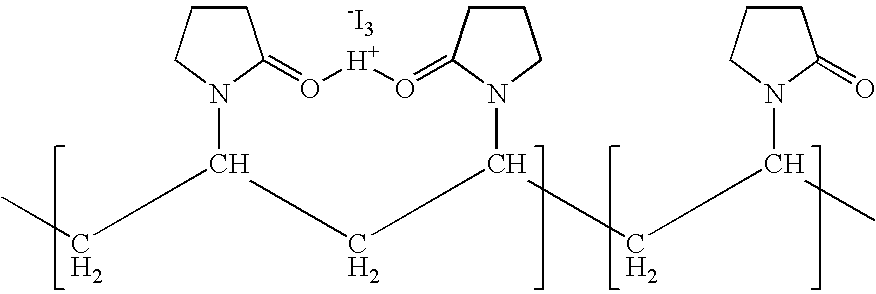
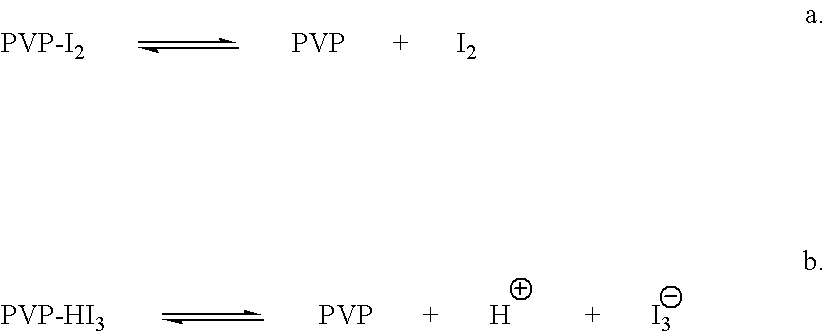
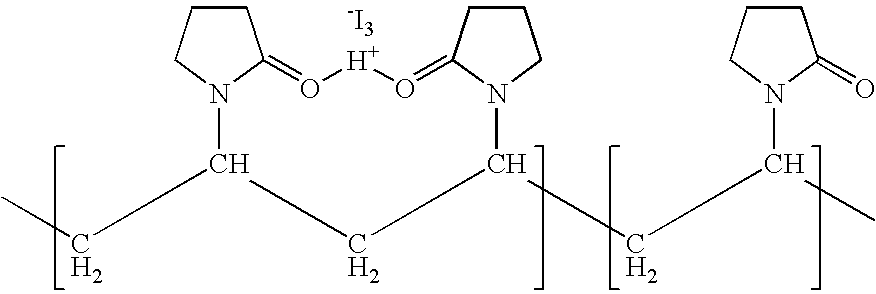
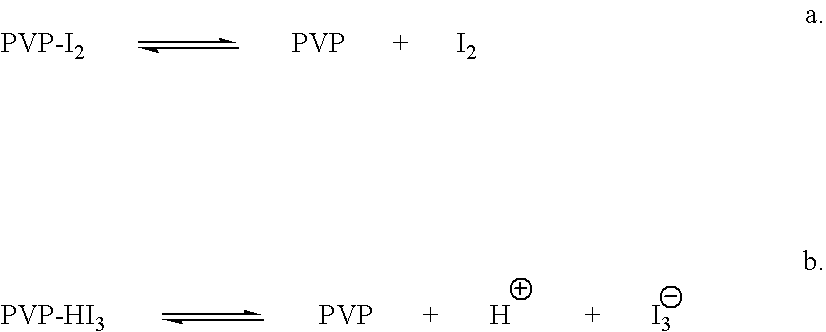
ActiveUS7767217B2Increase the amount of lightBetter seeAntibacterial agentsBiocideConjunctivaClinical settings
A topical ophthalmic composition comprised of povidone-iodine 0.01% to 10.0% combined with a steroid or non-steroidal anti-inflammatory drug. This solution is useful in the treatment of active infections of at least one tissue of the eye (e.g., conjunctiva and cornea) from bacterial, mycobacterial, viral, fungal, or amoebic causes, as well as treatment to prevent such infections in appropriate clinical settings (e.g. corneal abrasion, postoperative prophylaxis, post-LASIK / LASEK prophylaxis). Additionally the solution is effective in the prevention of infection and inflammation in the post-operative ophthalmic patient.
Owner:CLARUS CLS HLDG LLC
Ophthalmic compositions comprising povidone-iodine
ActiveUS20070219170A1Increase the amount of lightBetter seeAntibacterial agentsBiocideConjunctivaClinical settings
A topical ophthalmic composition comprised of povidone-iodine 0.01% to 10.0% combined with a steroid or non-steroidal anti-inflammatory drug. This solution is useful in the treatment of active infections of at least one tissue of the eye (e.g., conjunctiva and cornea) from bacterial, mycobacterial, viral, fungal, or amoebic causes, as well as treatment to prevent such infections in appropriate clinical settings (e.g. corneal abrasion, postoperative prophylaxis, post-LASIK / LASEK prophylaxis). Additionally the solution is effective in the prevention of infection and inflammation in the post-operative ophthalmic patient.
Owner:CLARUS CLS HLDG LLC
Method and apparatus for reducing intraocular pressure
ActiveUS7641627B2Desirable intraocular pressureFlow controllableEye surgeryCatheterAqueous humorConjunctiva
A drainage apparatus and method to reduce intraocular pressure in an eyeball that includes an anterior chamber having aqueous humor disposed therein, a cornea and a surrounding marginal limbus by which the cornea is continuous with a scleral layer and a conjunctival layer disposed on an exposed surface of the eyeball and under eyelids, the apparatus comprising an inlet assembly configured to be disposed at the anterior chamber of the eyeball, an outlet assembly configured to be disposed at the external surface of the eyeball, a tube extending between the inlet and outlet assemblies and configured to promote fluid communication between the inlet and outlet assemblies, and a control means disposed within the outlet assembly for controlling a flow of aqueous humor through the tube from the anterior chamber of the eyeball to the external surface of the eyeball, and for preventing bacterial infiltration into the anterior chamber.
Owner:CAMRAS VISION +1
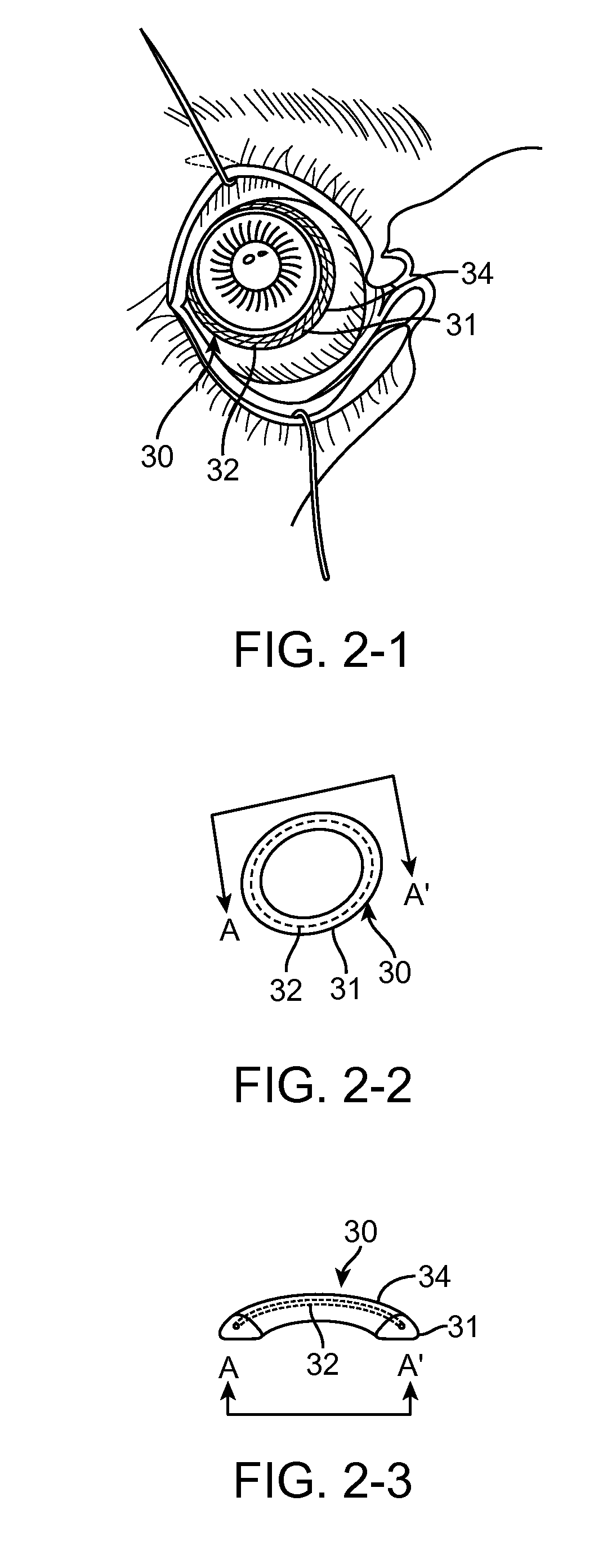
Biological membrane fixing device for eye surface
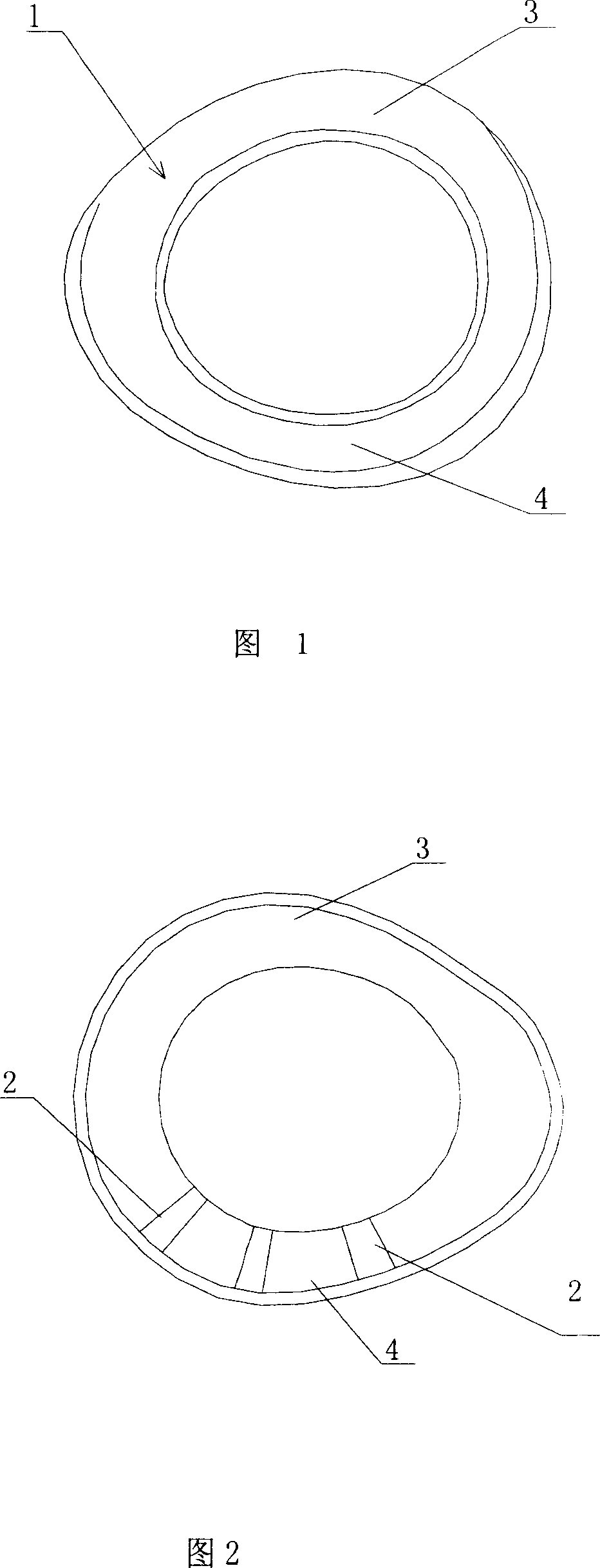
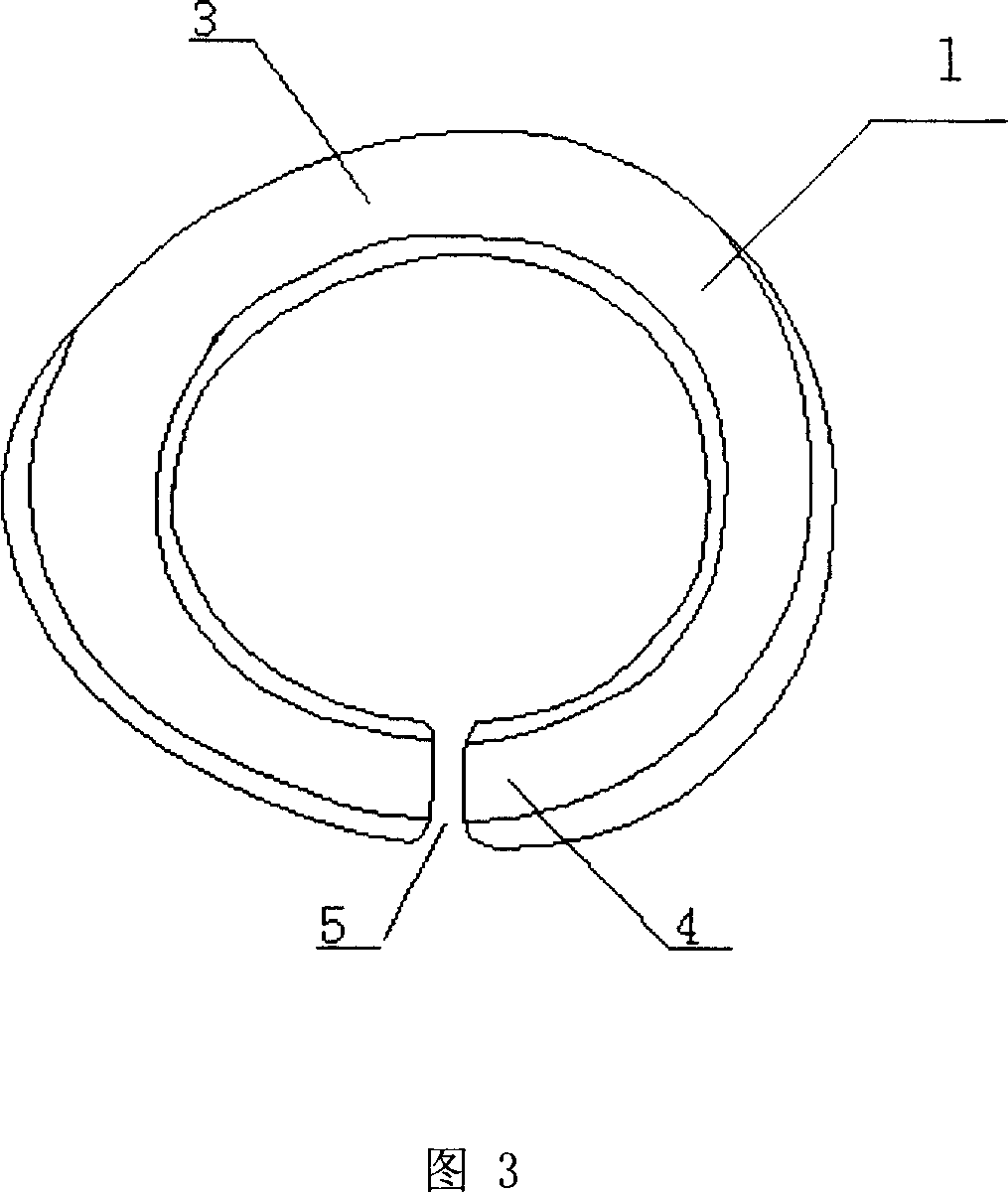
ActiveCN101088479ANo further damageReduce treatment dead angleEye treatmentConjunctivaSurgical operation
The present invention discloses one kind of biological membrane fixing device for eye surface. The biological membrane fixing device is one arced ring in the shape fitting the eyeball surface and upper and lower vault conjunctiva, and may have notch in the inner surface. It is used in fixing amnion onto eye through simple operation without surgical operation and injury to eyelid.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Atomizer for applying liquids onto eyes
The present invention relates to atomizers for administering liquids to the cornea or conjunctiva of the eye, special eye adapters for atomizers and the use of atomizers for ophthalmological administration. The atomizers according to the invention are free from propellant gas and have an energy reservoir for supplying the energy needed for the atomization process.
Owner:BOEHRINGER INGELHEIM PHARM KG
Comprehensive diagnostic apparatus for xerophthalmia
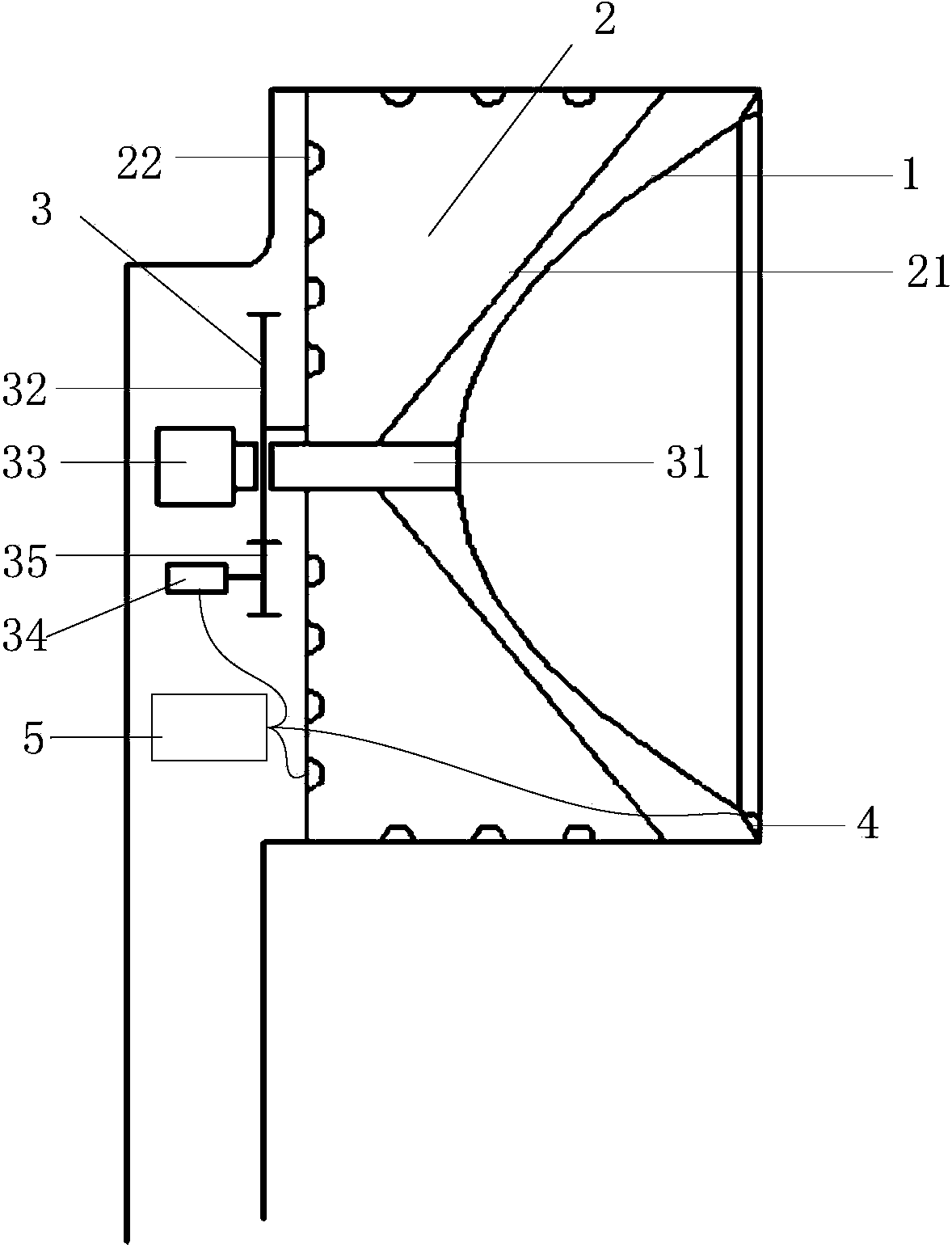
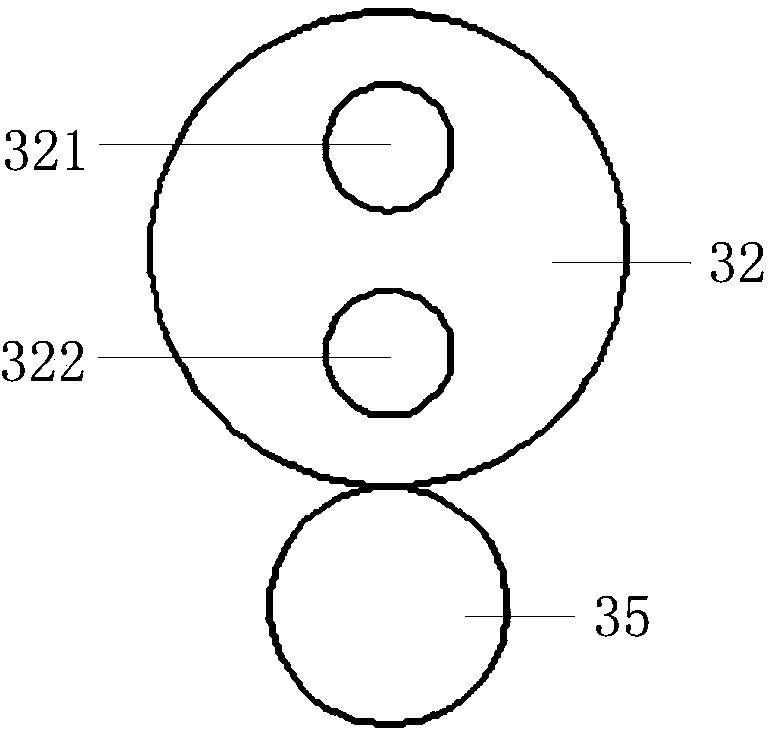
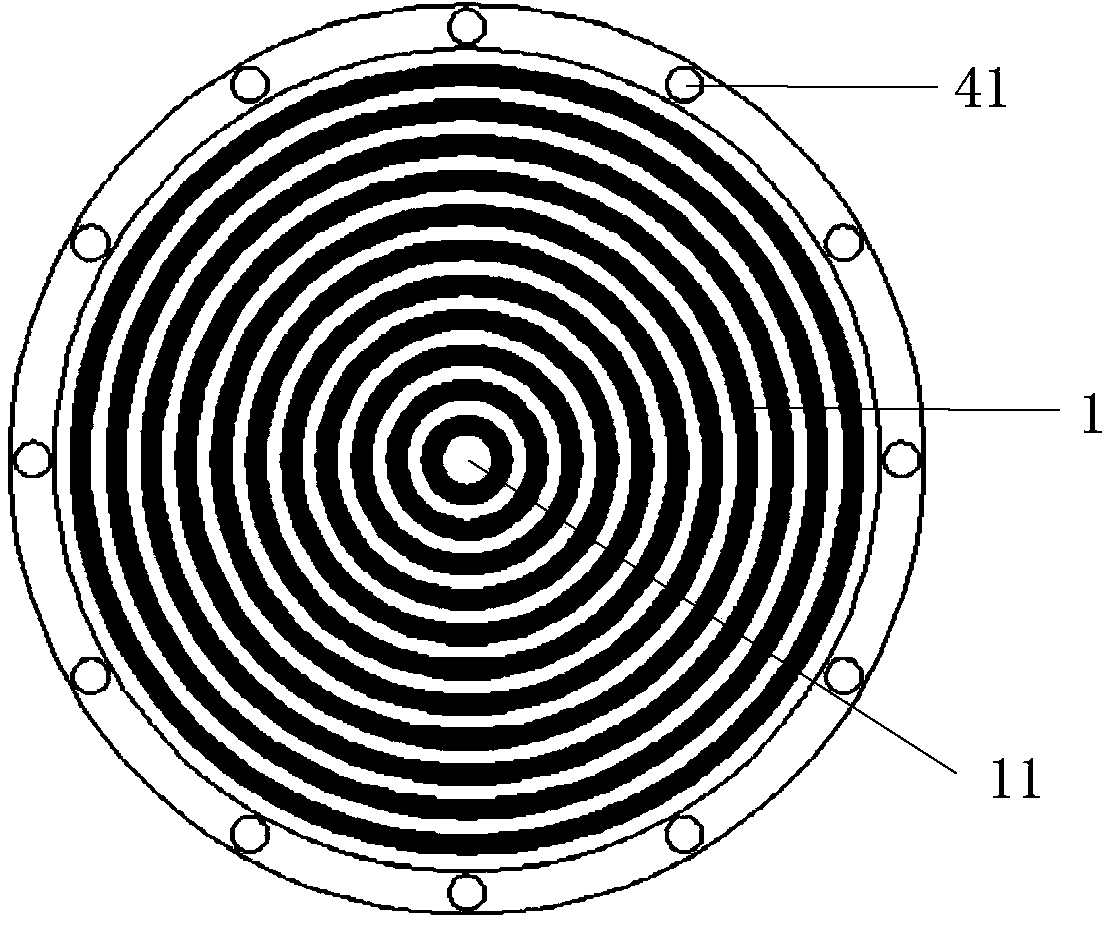
ActiveCN103799976ANo discomfortImprove objectivityEye diagnosticsConjunctivaWhite light interferometry
The invention relates to a comprehensive diagnostic apparatus for xerophthalmia. The comprehensive diagnostic apparatus comprises an upper computer, a controller, a Placido disc, a near-infrared light illuminating system, a white light illuminating system, and an imaging system, wherein the white light illuminating system comprises a diffuse reflection plate and a white light LED array, the imaging system comprises an industrial camera, an automatic focusing lens, an optical filter wheel disc, a motor and a gear, the diagnostic apparatus respectively measures the tear meniscus height, the area of a tiny blood vessel of a conjunctiva, the structural and morphological parameters of meibomian glands, the breakup time of a tear film, and the thickness of a lipid layer of the tear film according to the principles of visible light / near-infrared light imaging measurement, structured light imaging measurement and white light interferometry, so as to provide objective diagnostic evidences and preliminary diagnostic result for the diagnosis of the xerophthalmia. The diagnostic apparatus can replace a series of separated diagnosis items during the clinical diagnosis of the xerophthalmia, the diagnosis flow of the xerophthalmia is simplified, the diagnosis time is shortened, and the objectivity and the repeatability of the diagnosis result are improved.
Owner:山东百茂生物科技有限公司
Adhesive bioerodible ocular drug delivery system
InactiveUS20050013845A1Suitable bioadhesive capabilityRapid onsetFemale contraceptivesSheet deliveryConjunctivaWhole body
Owner:ARIUS TWO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com