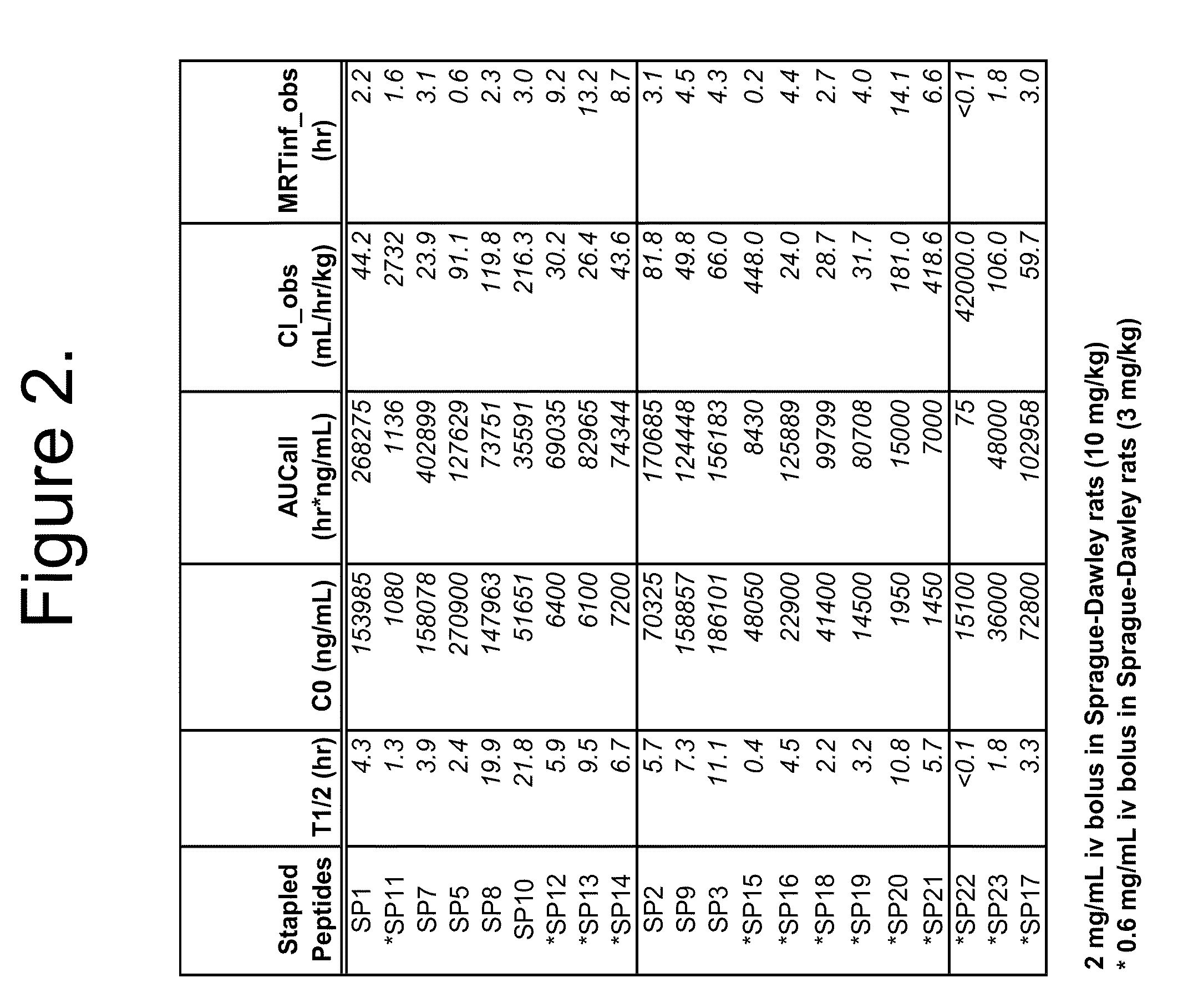
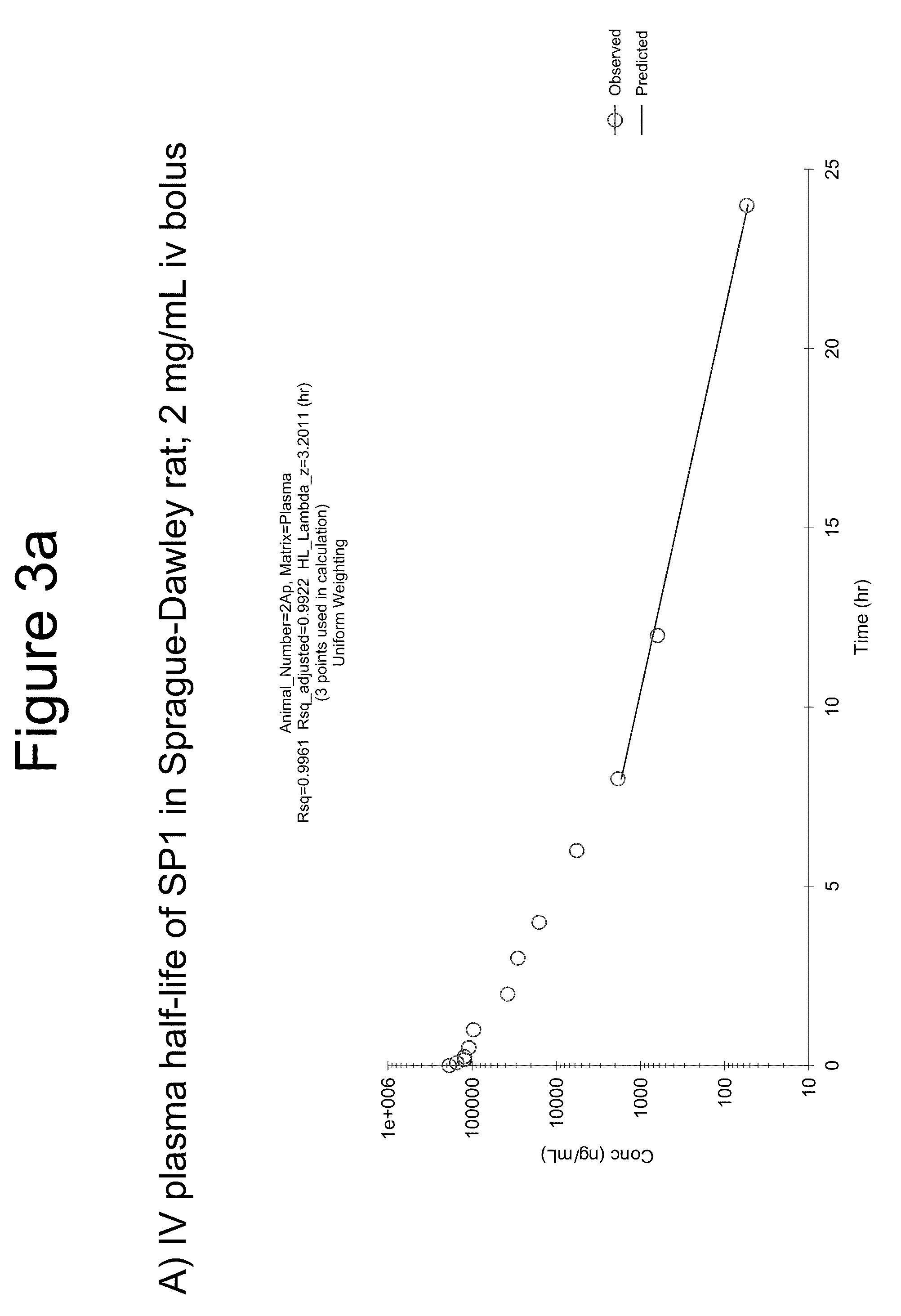
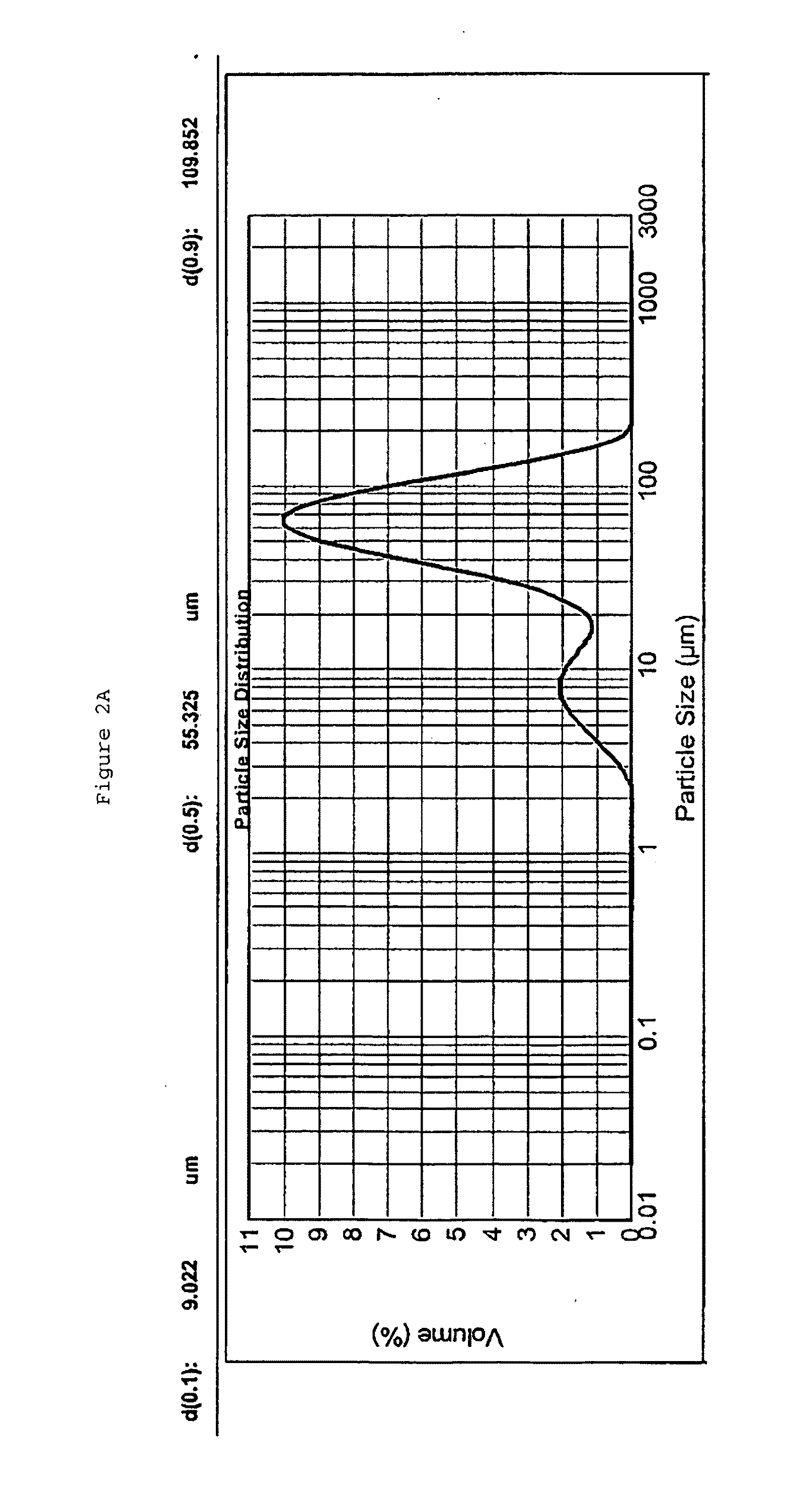
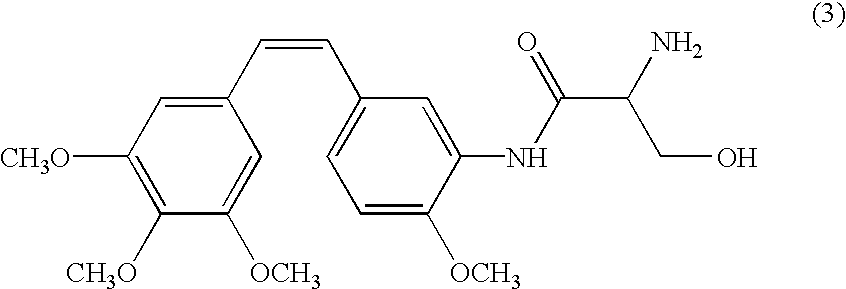
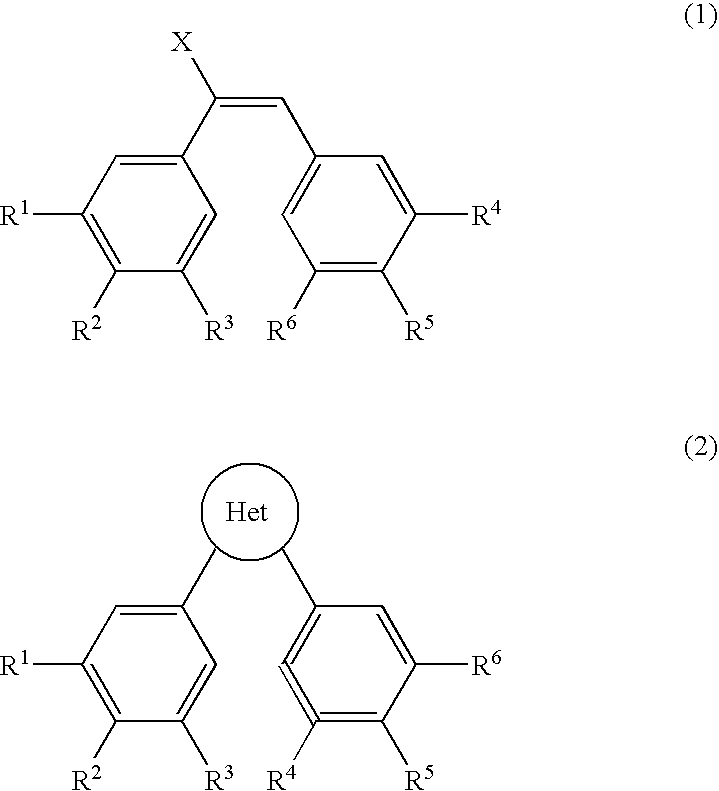
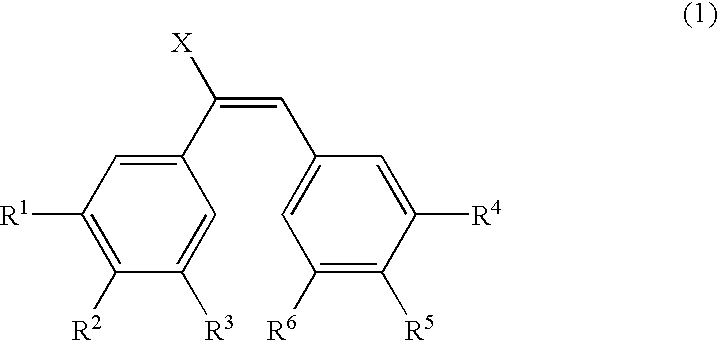
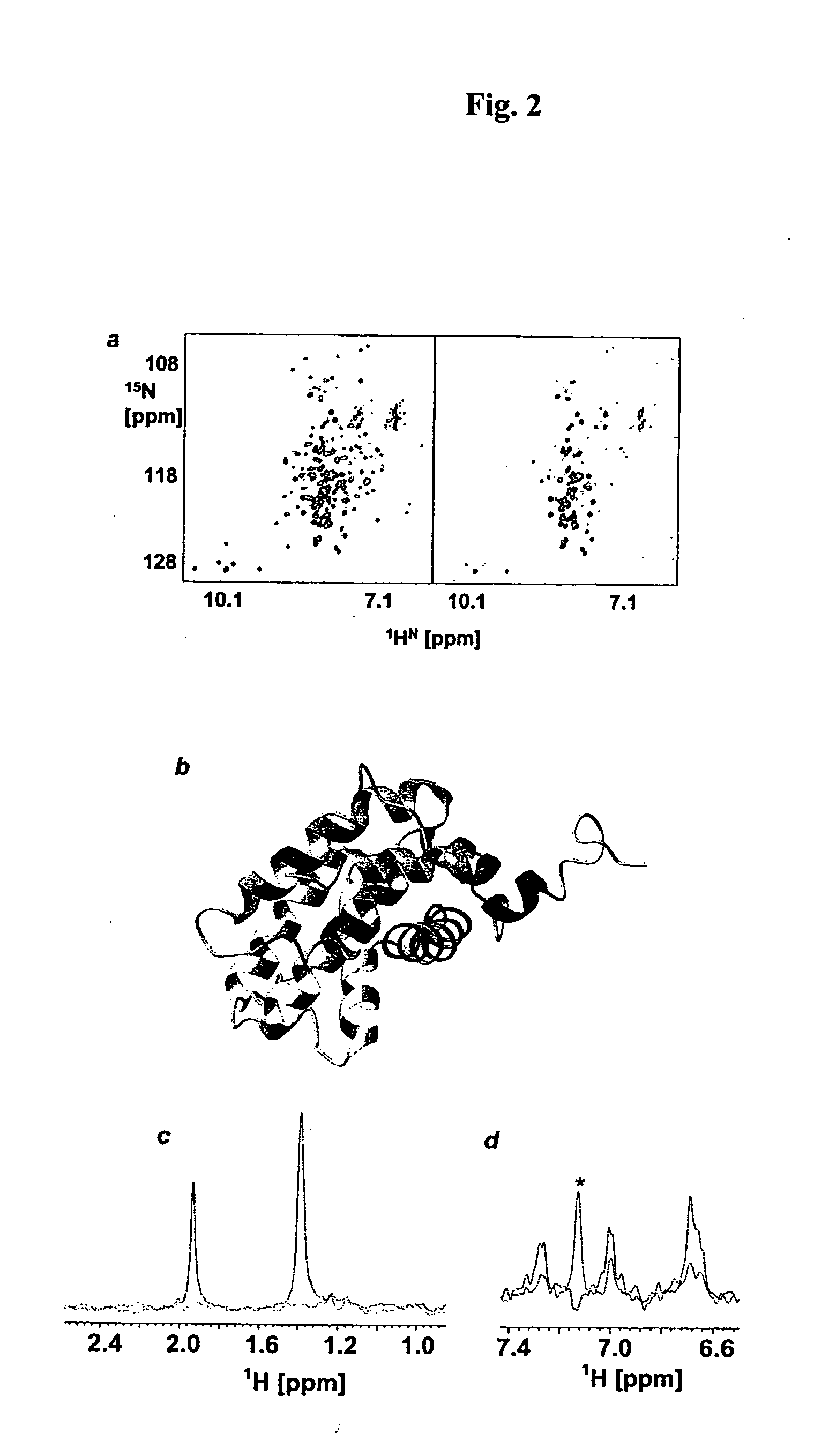
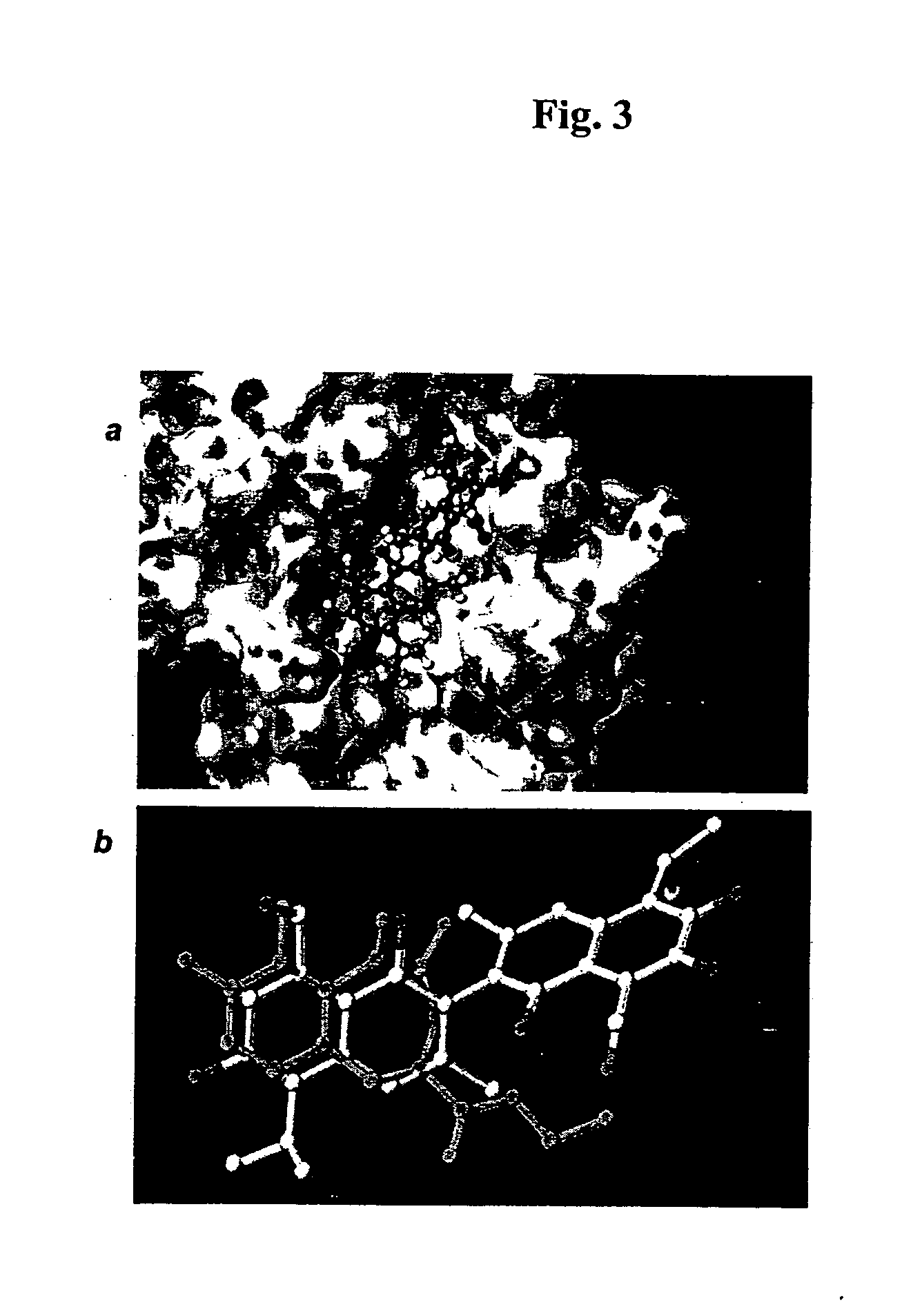
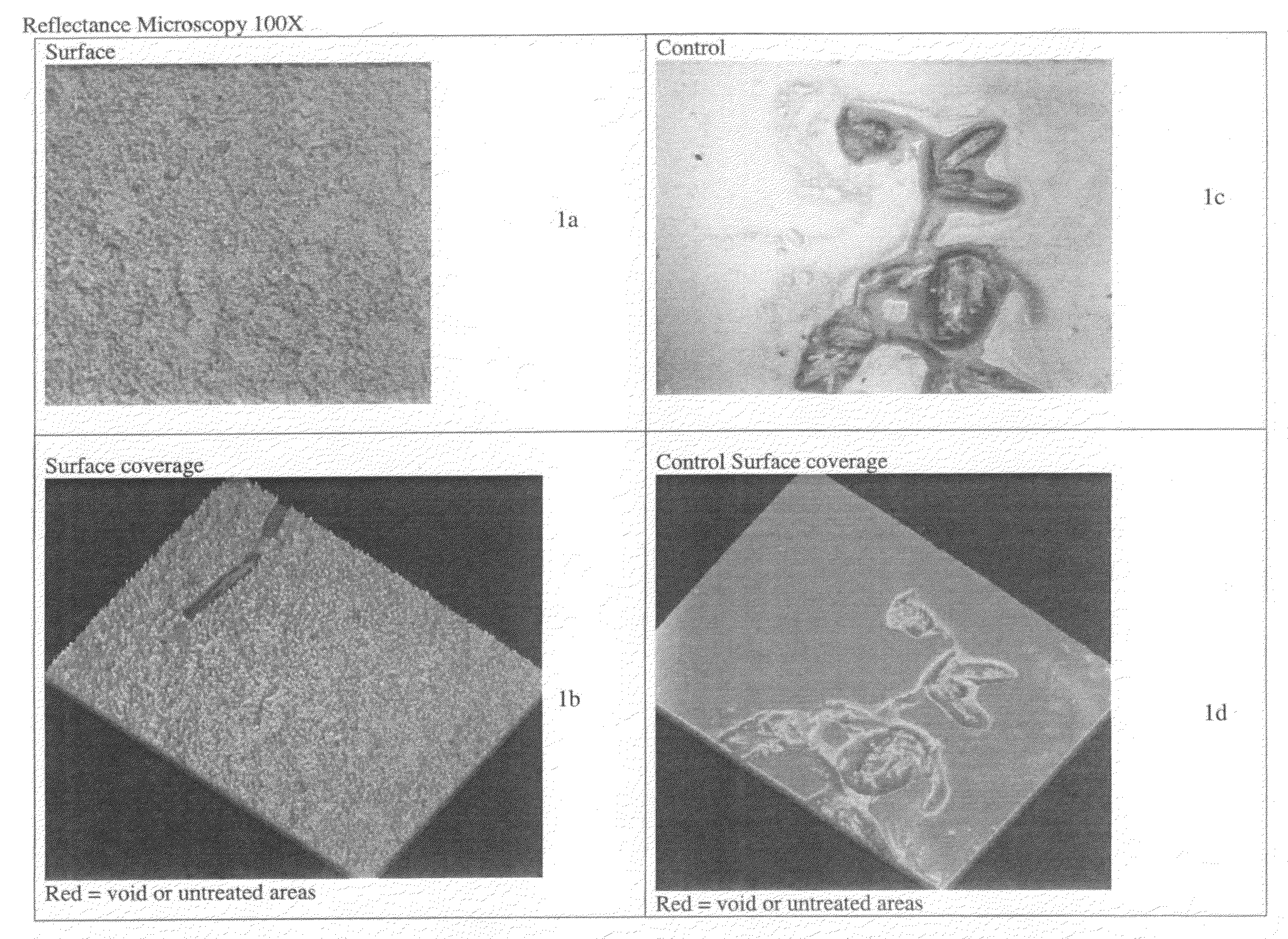
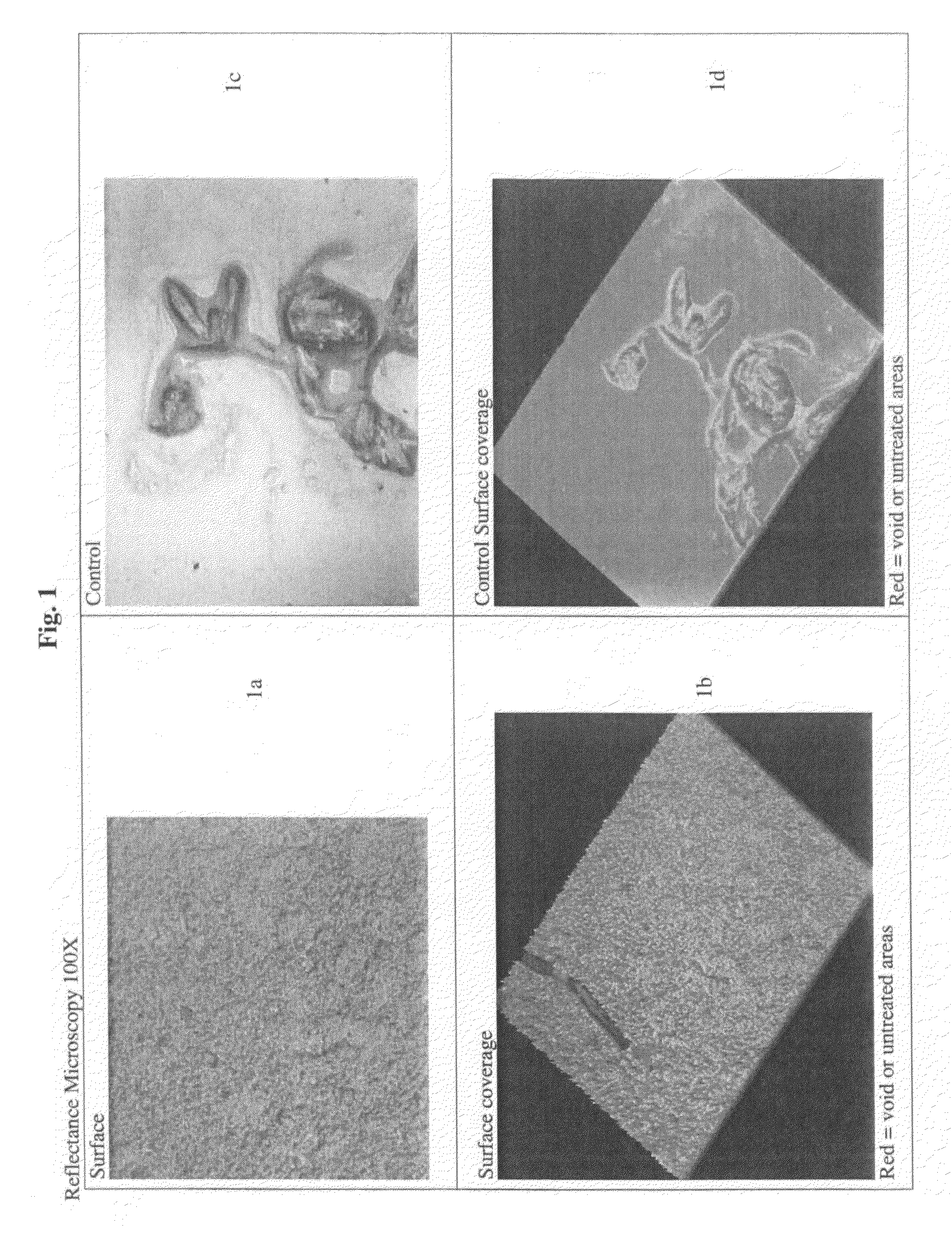
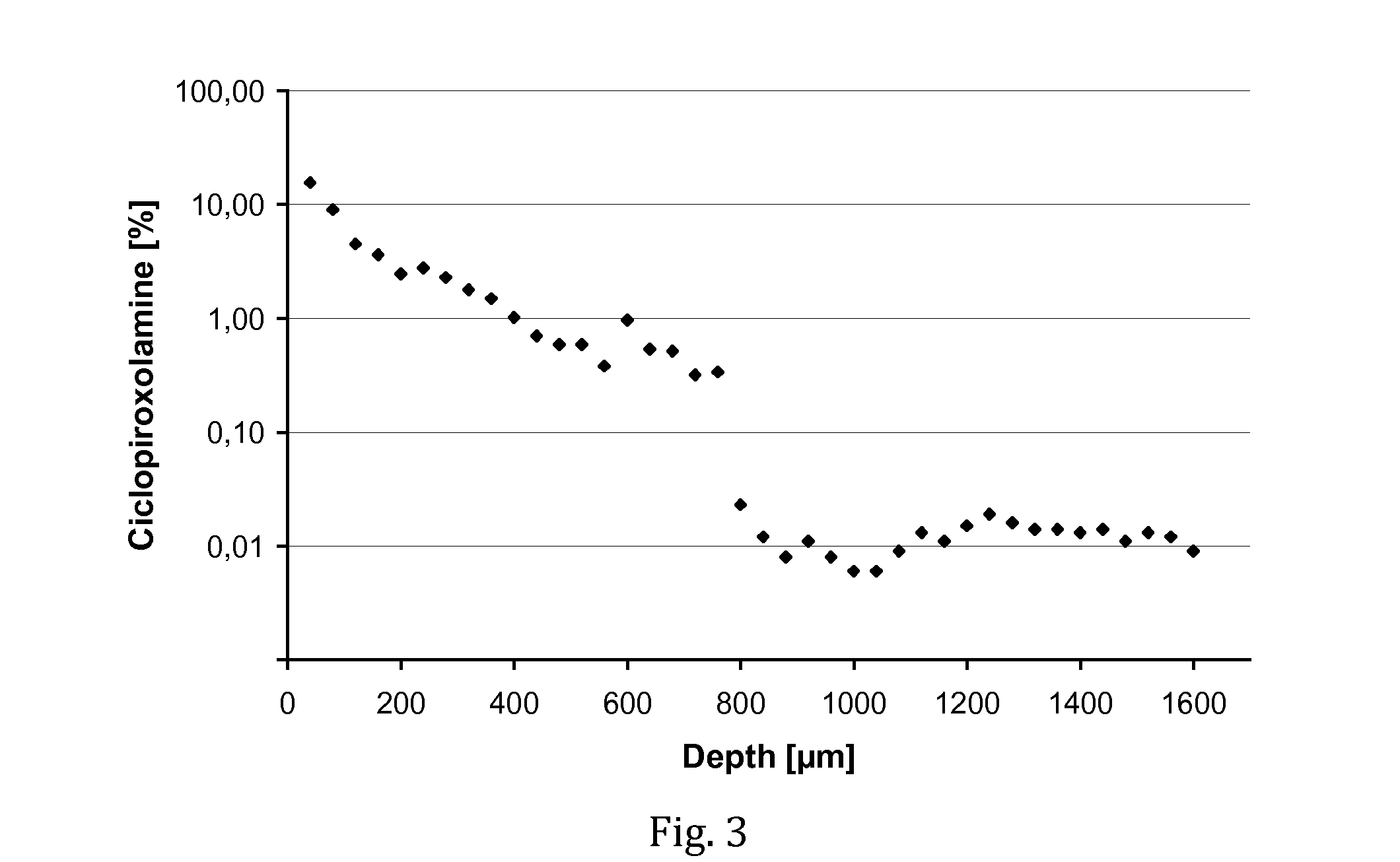
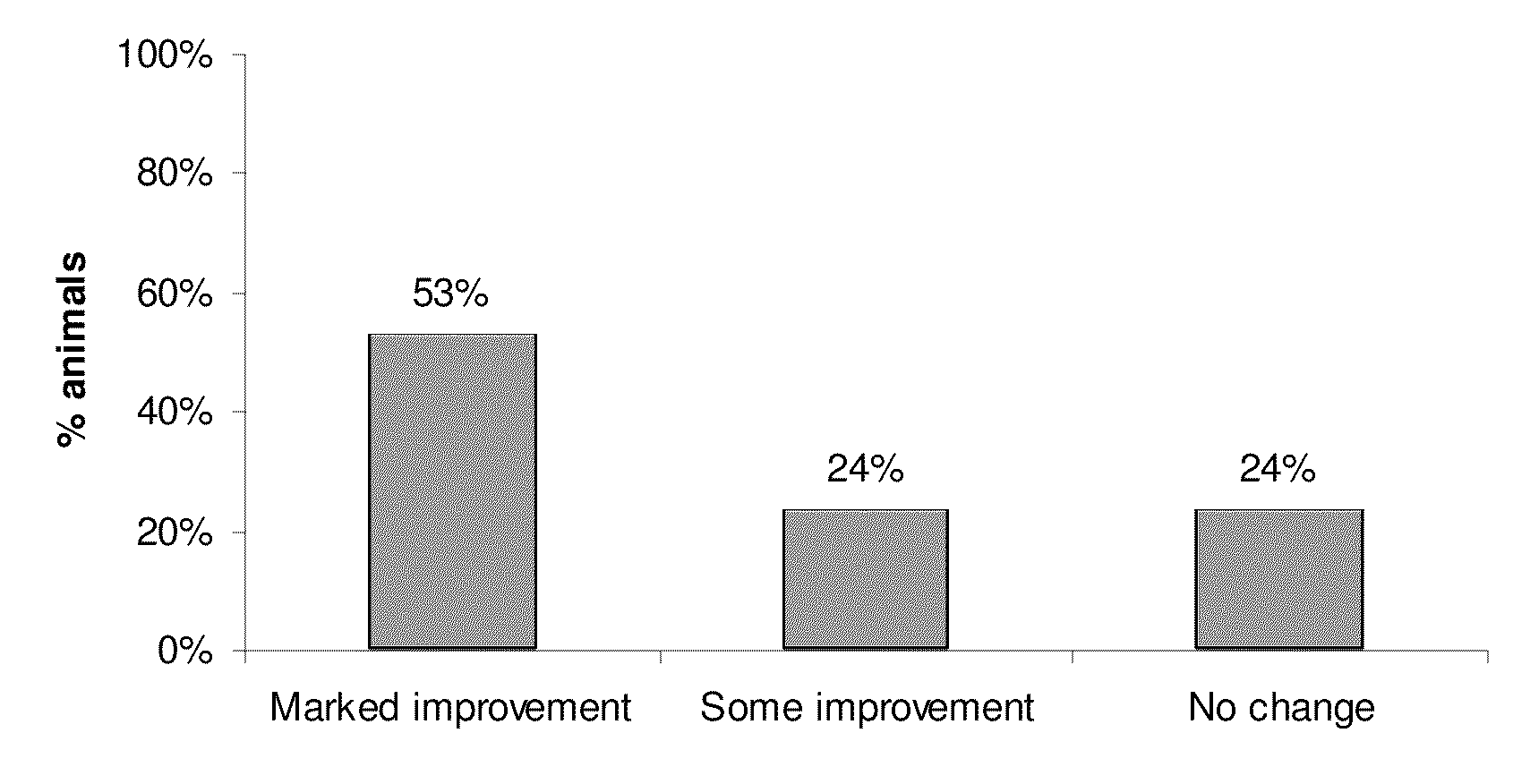
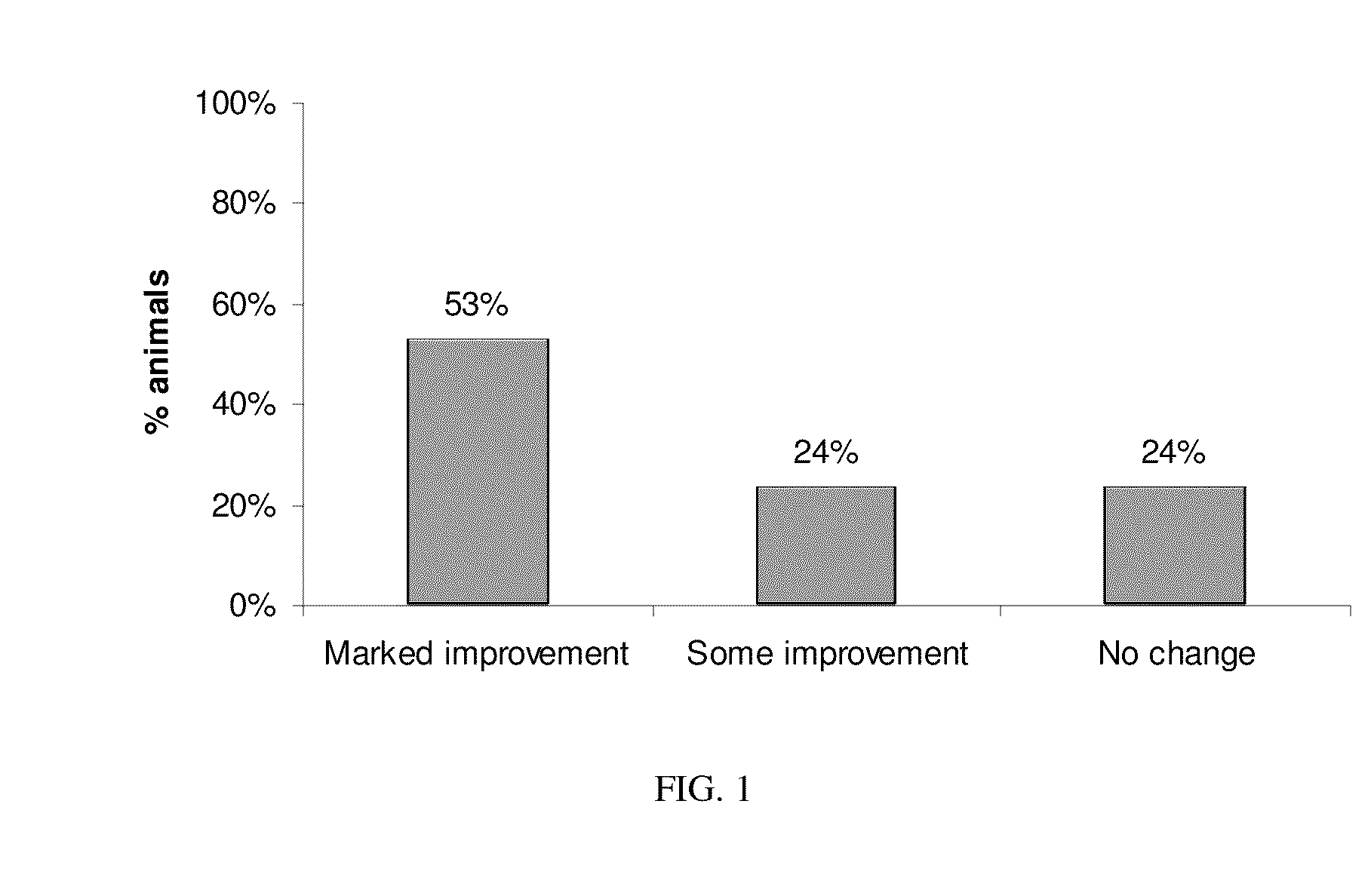
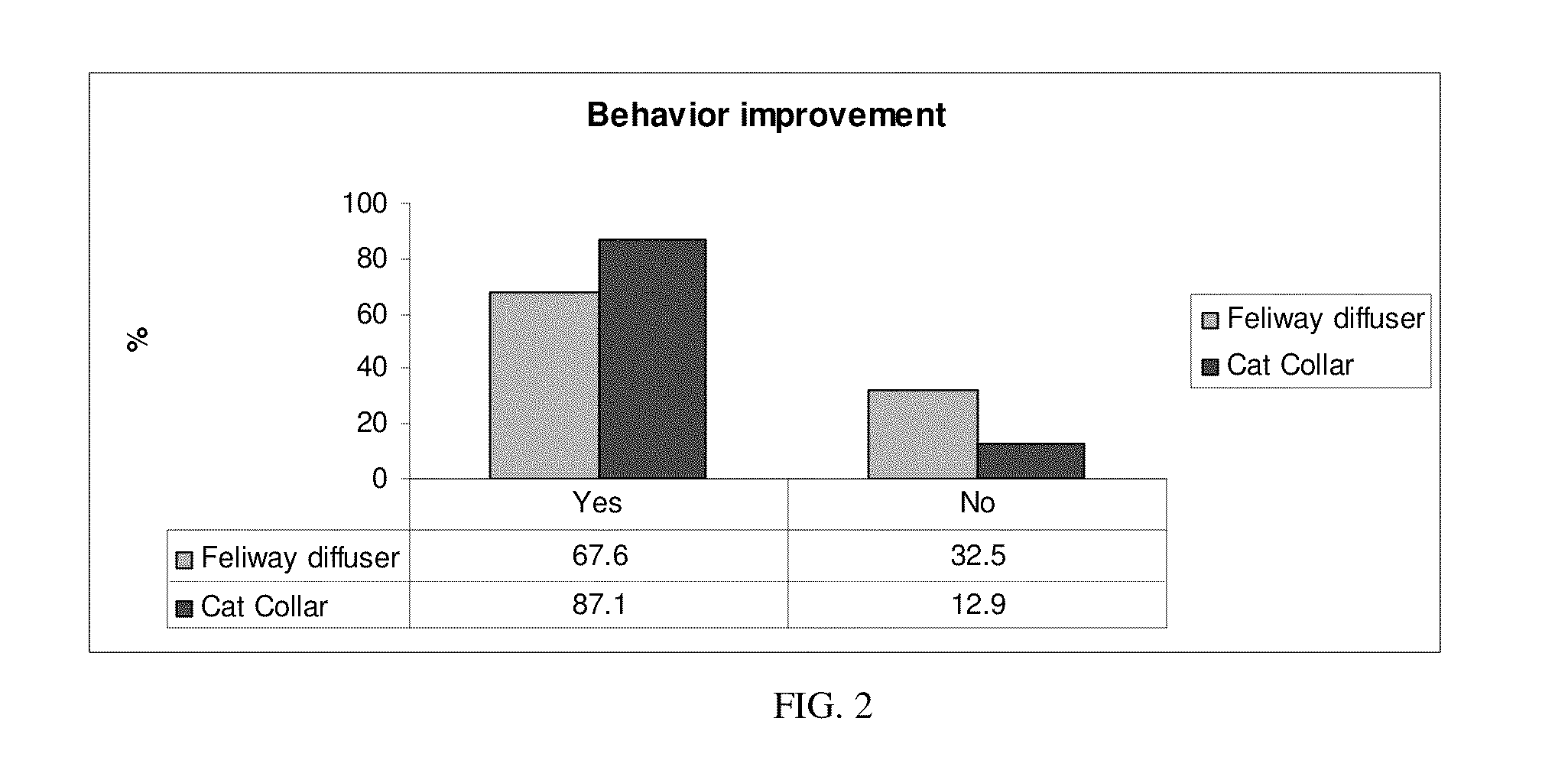
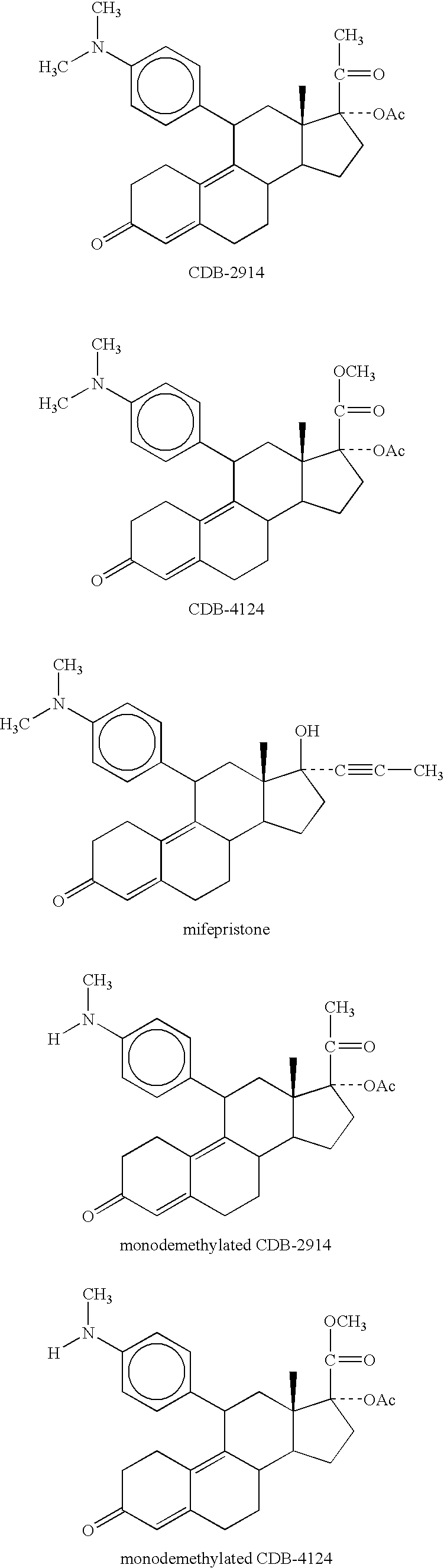
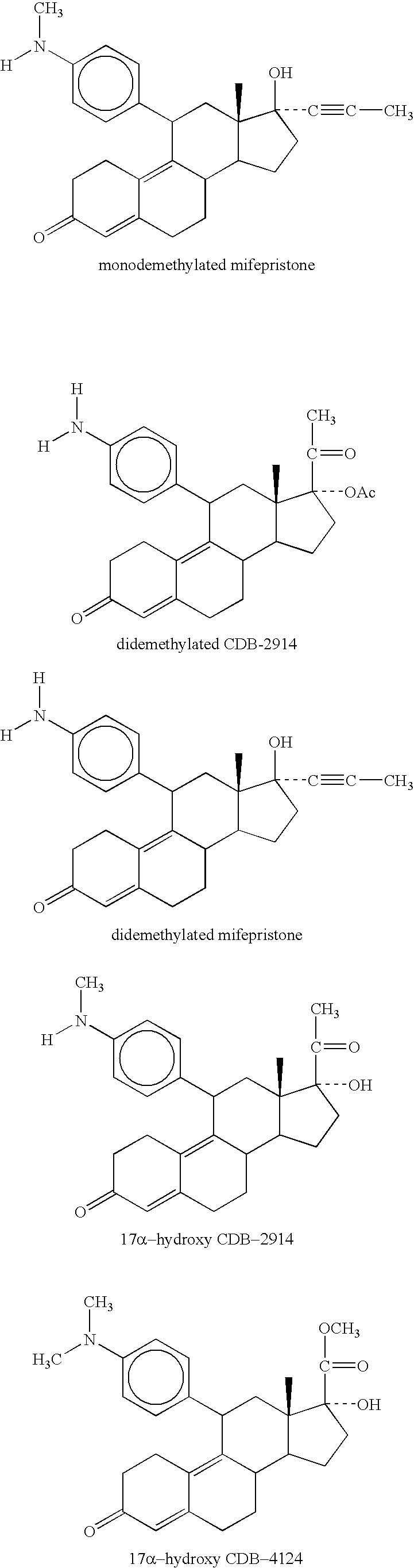
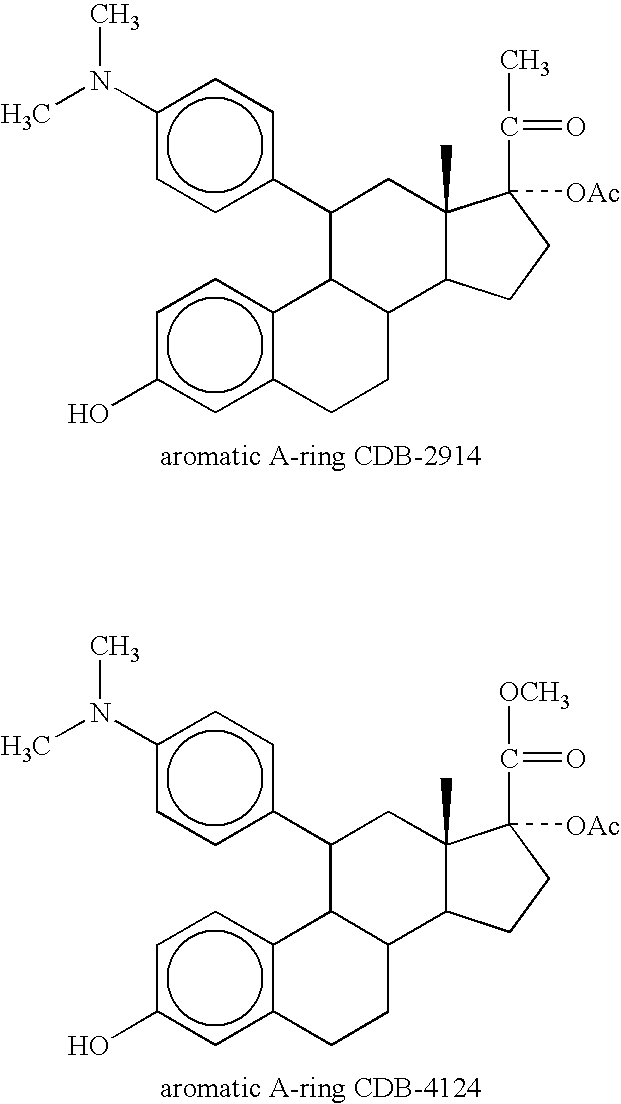
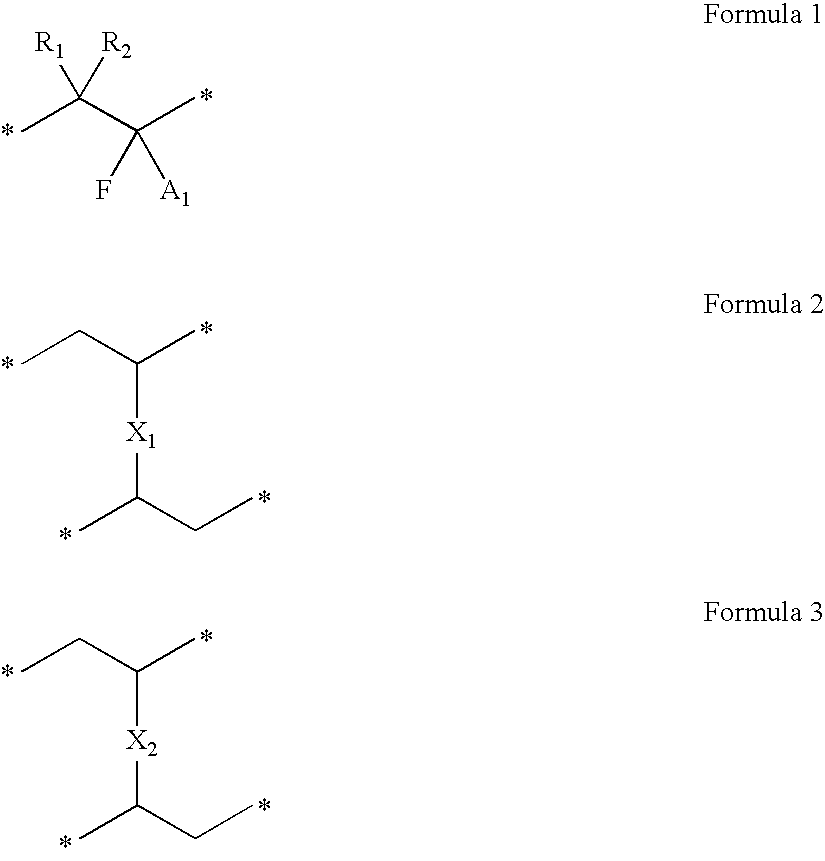
Patents
Literature
503results about "Halogenated hydrocarbon active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method of prevention and treatment of aging, age-related disorders and/or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimers disease and cancer
InactiveUS20060275294A1Halogenated hydrocarbon active ingredientsBiocideAbnormal tissue growthSTAT Transcription Factors
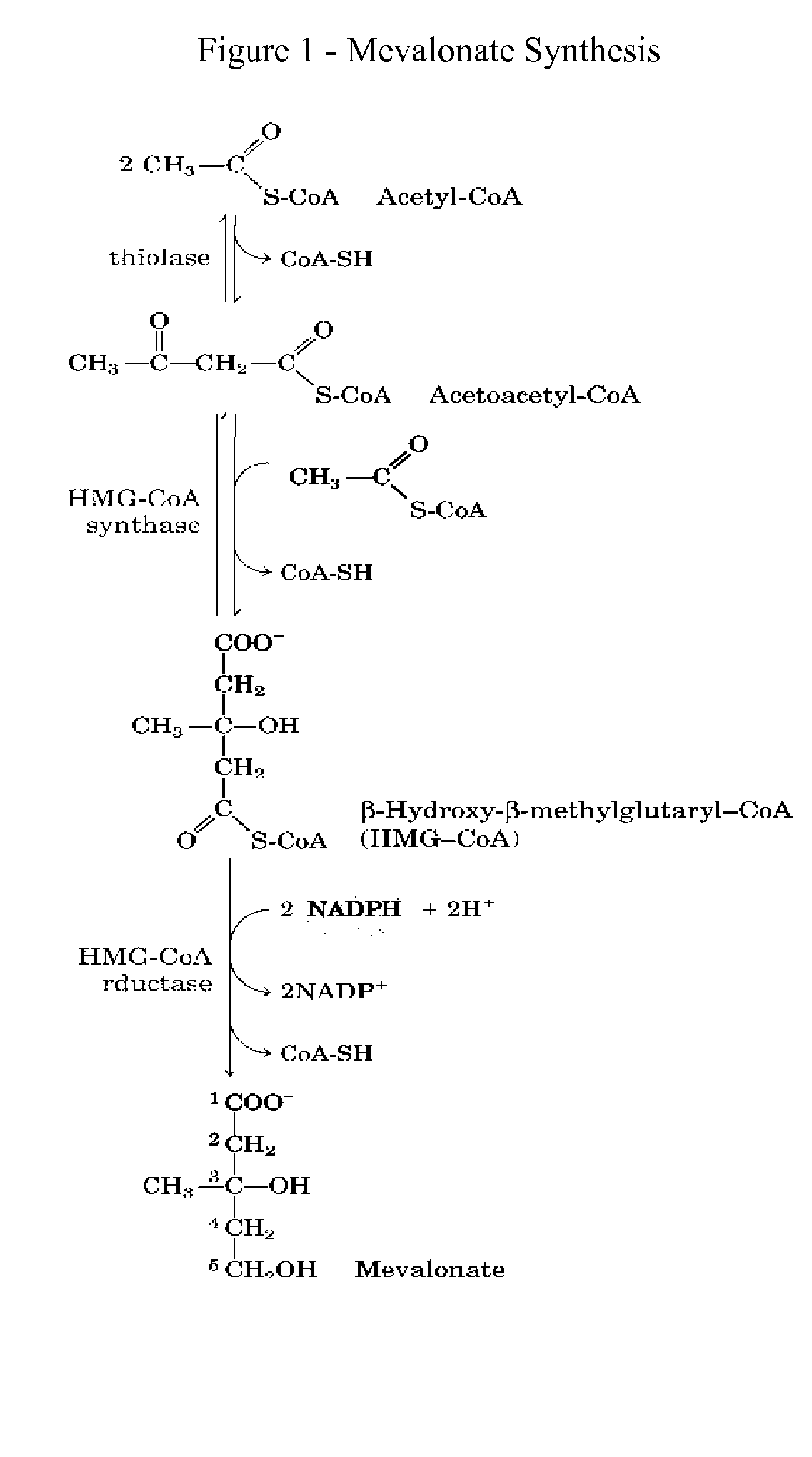
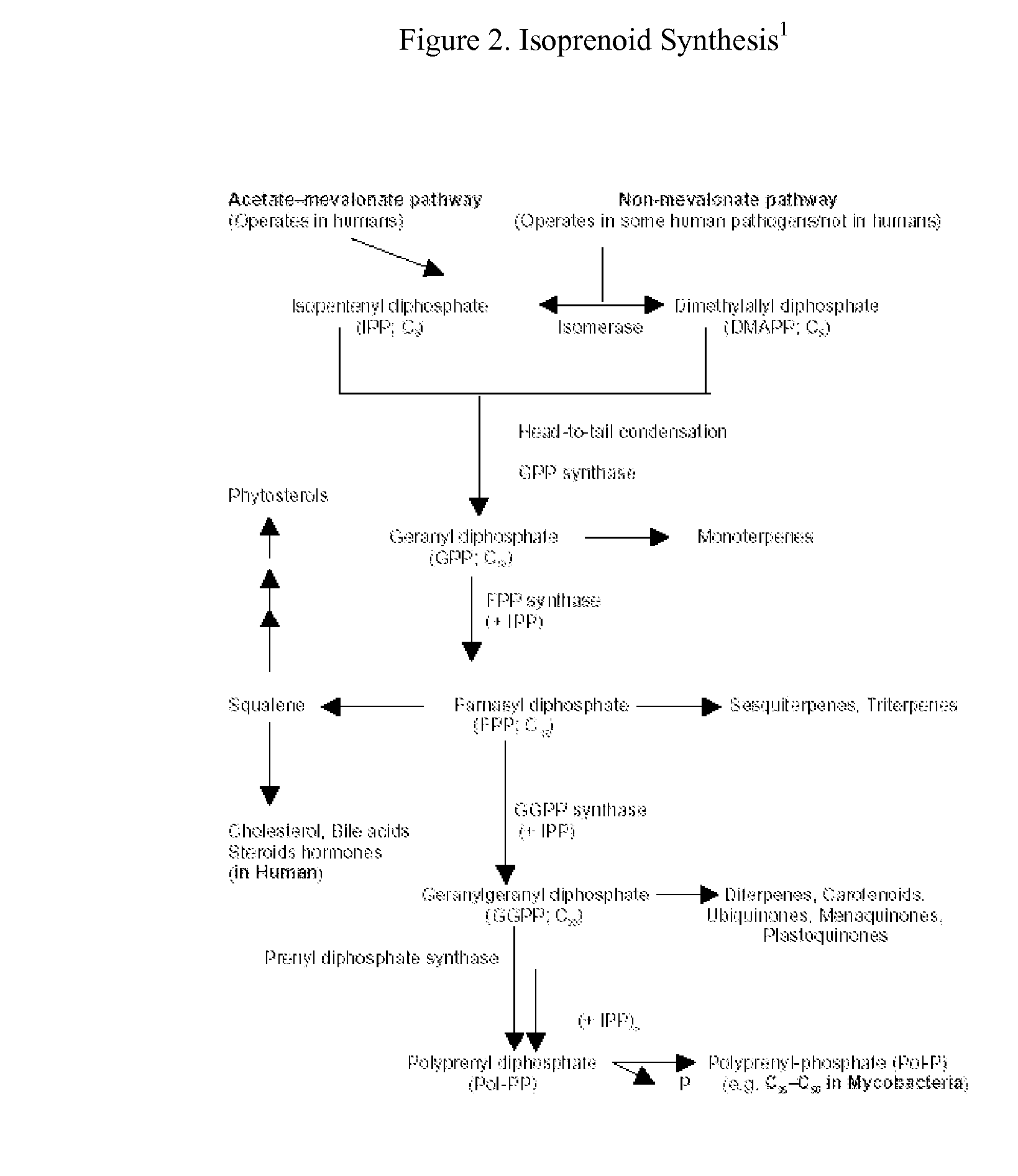
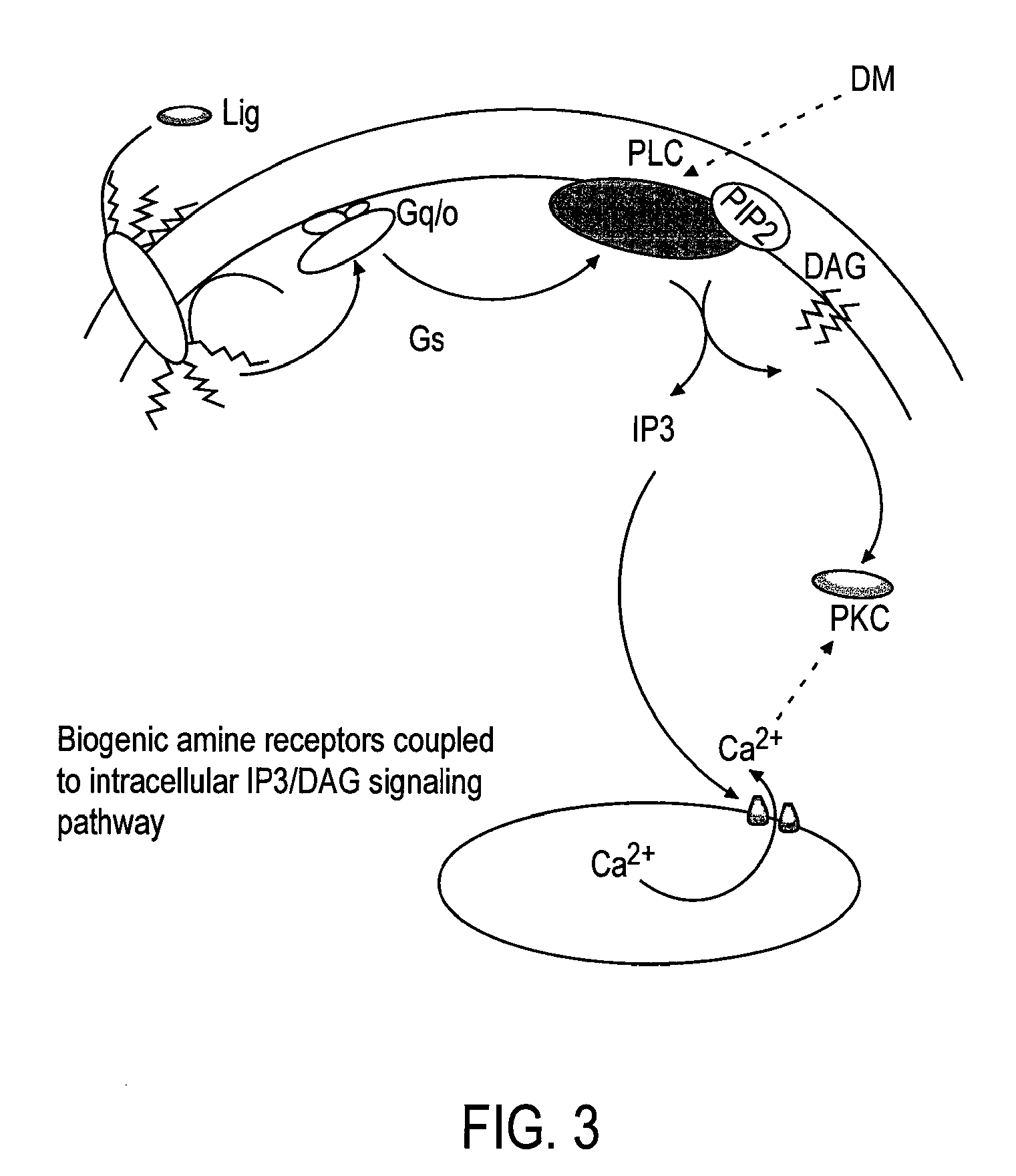
This invention relates to a method for prevention and treatment of aging, age-related disorders and / or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging, age-related disorders and / or age-related manifestations including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway
Owner:OMOIGUI OSEMWOTA SOTA
Topical treatment or prevention of ocular infections
The topical application of an azalide antibiotic such as azithromycin to the eye is useful in treating or preventing ocular infections. In one embodiment, the azalide antibiotic is supplied to the eye in a depot for sustained release. A more convenient dosing regimen can also be provided by the use of an appropriate depot. Furthermore, a composition containing a combination of medicaments is also provided.
Owner:INSITE VISION
Two solvent antimicrobial compositions and methods employing them
InactiveUS6927237B2Reduce populationGreat reductionBiocideHalogenated hydrocarbon active ingredientsPharmaceutical industrySolvent composition
Owner:ECOLAB USA INC
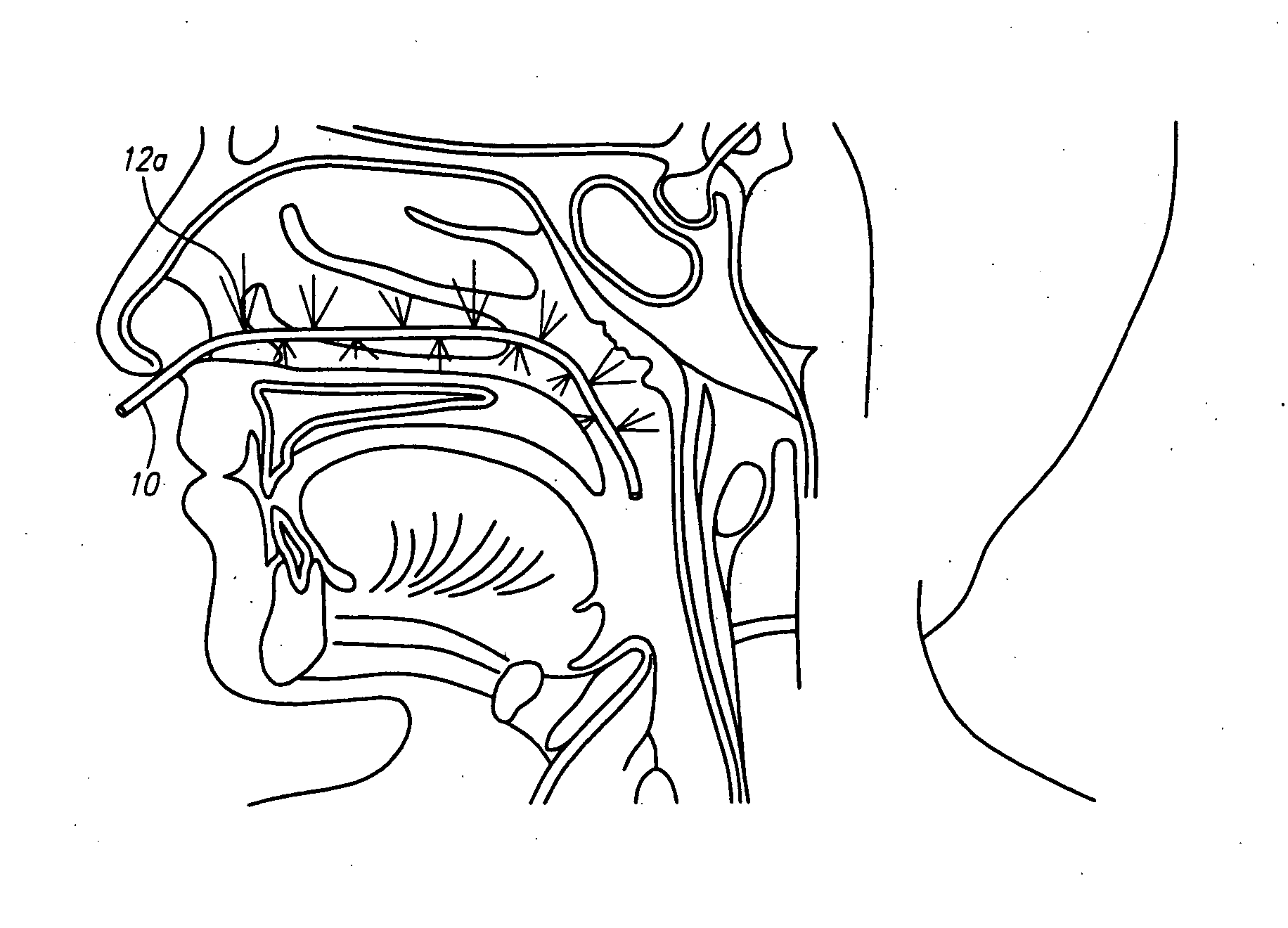
Methods and devices for non-invasive cerebral and systemic cooling
ActiveUS20060276552A1Minimize neurologic deficitMaximize coolingBiocideHalogenated hydrocarbon active ingredientsWhole bodyNon invasive
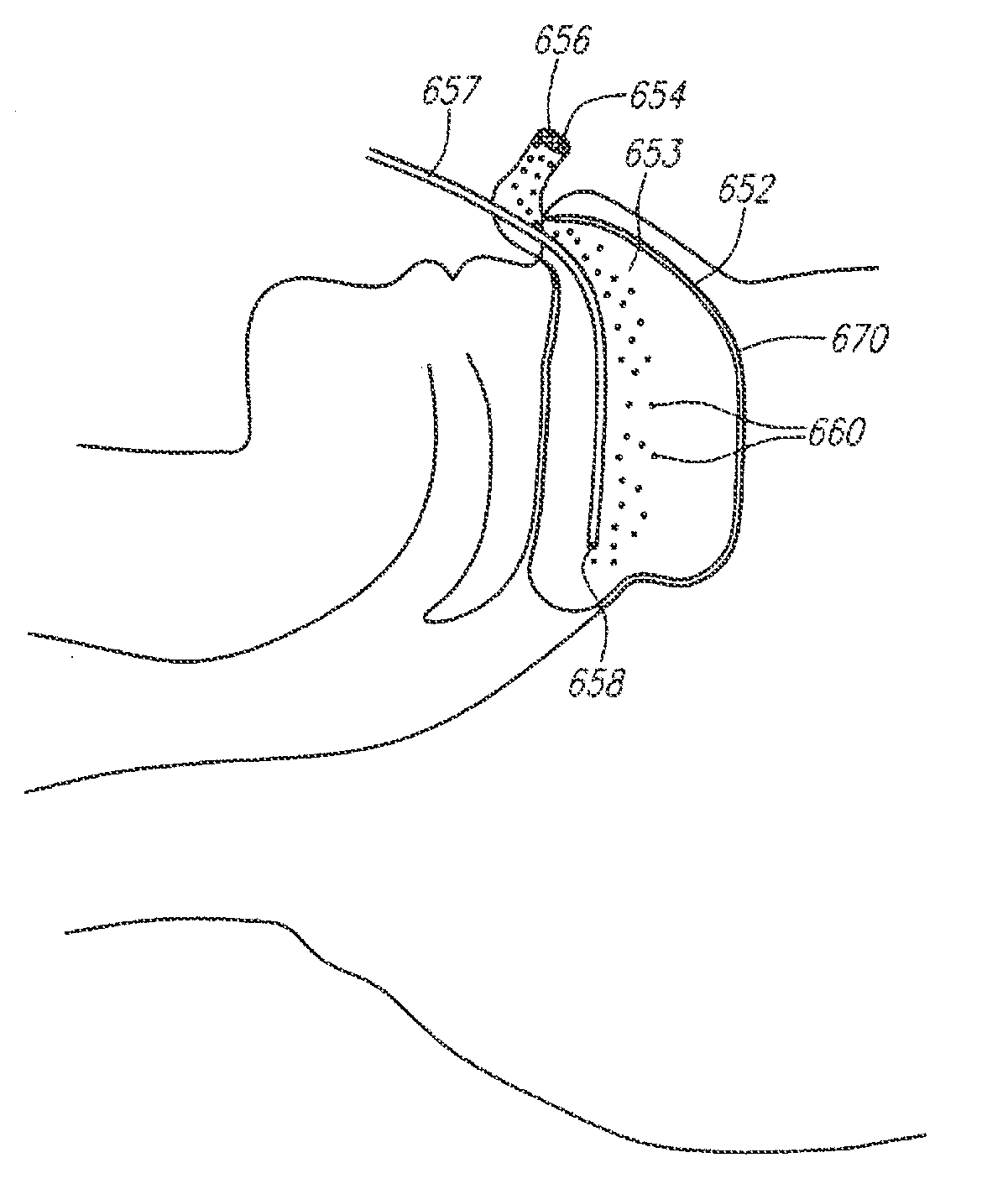
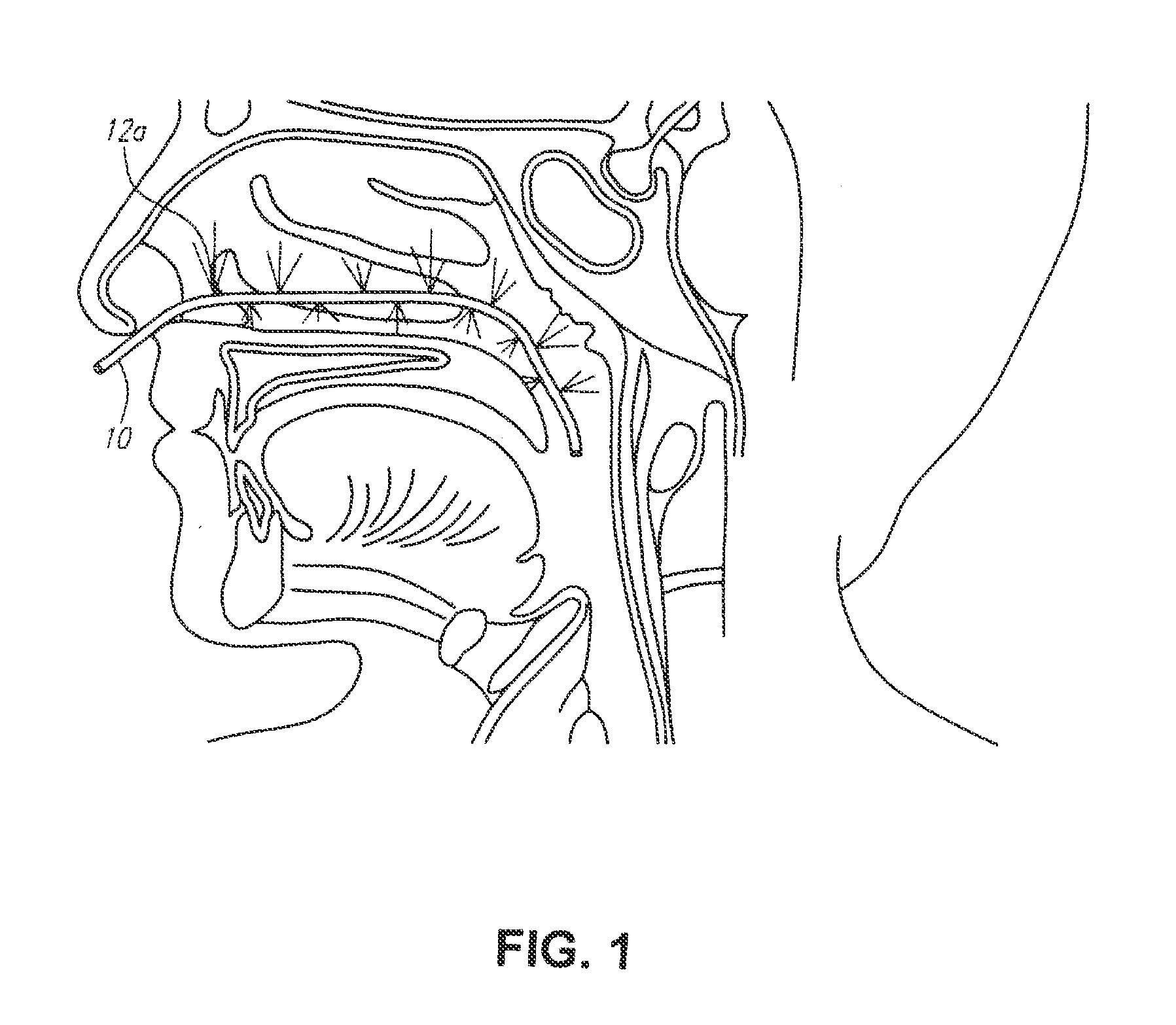
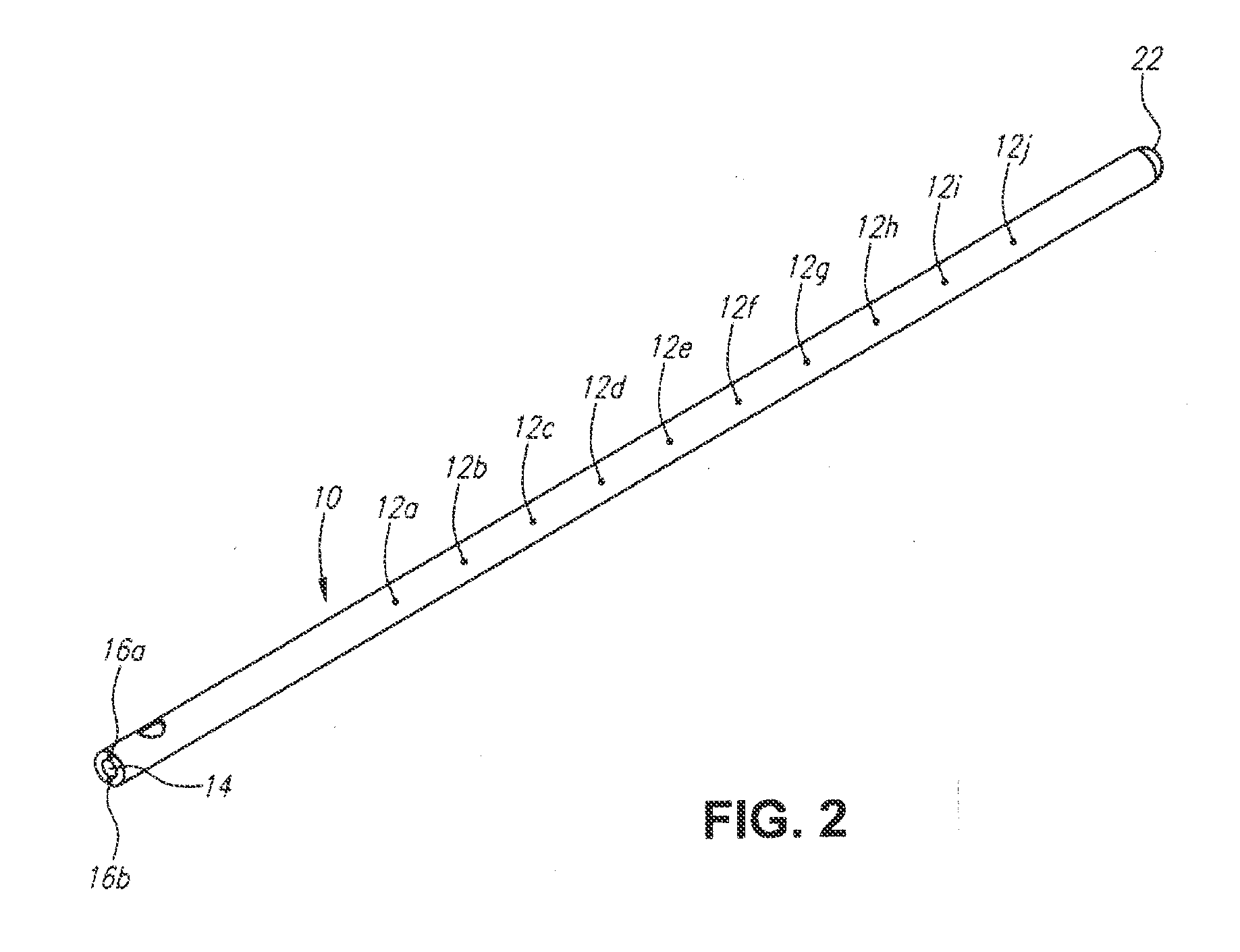
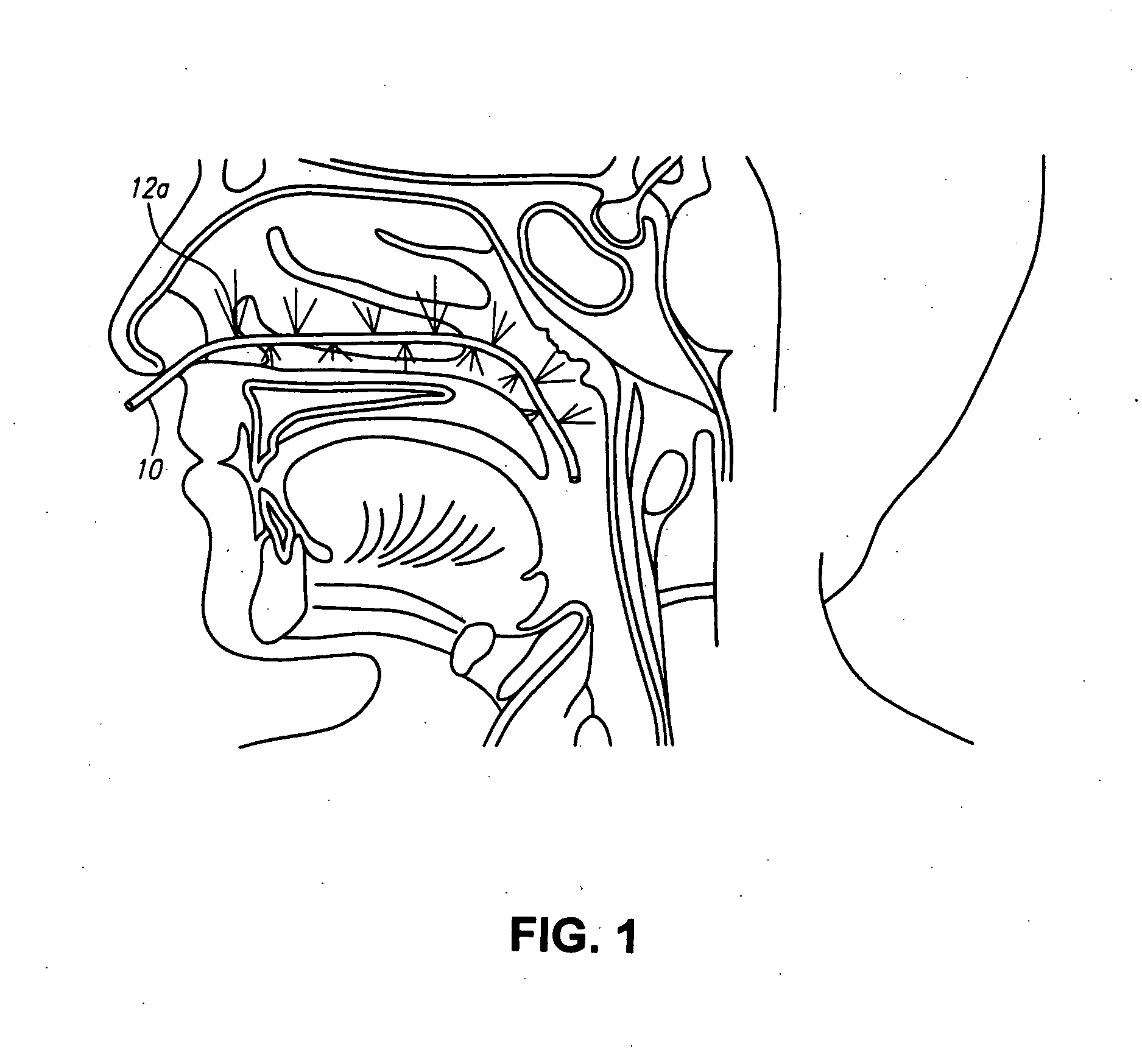
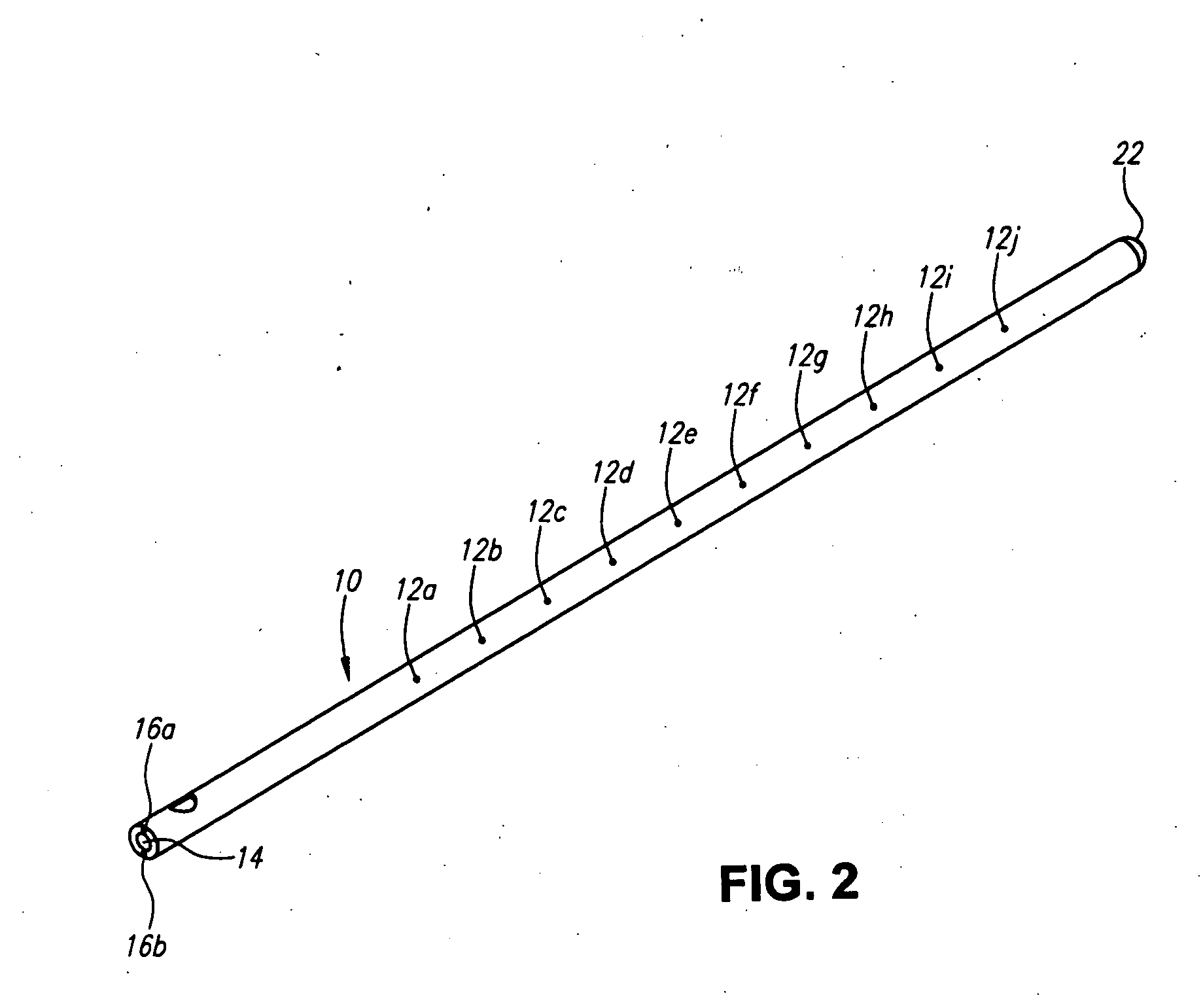
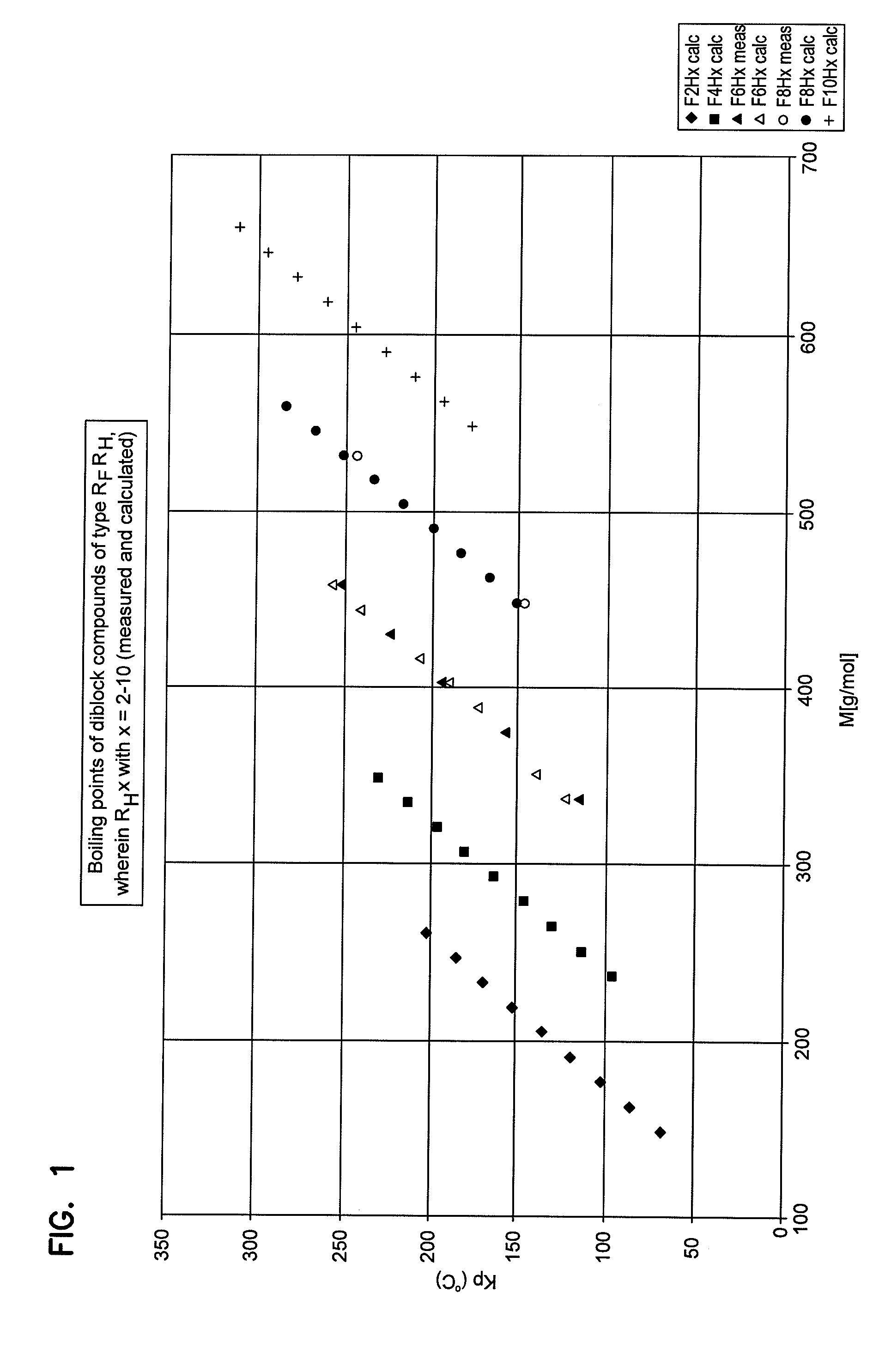
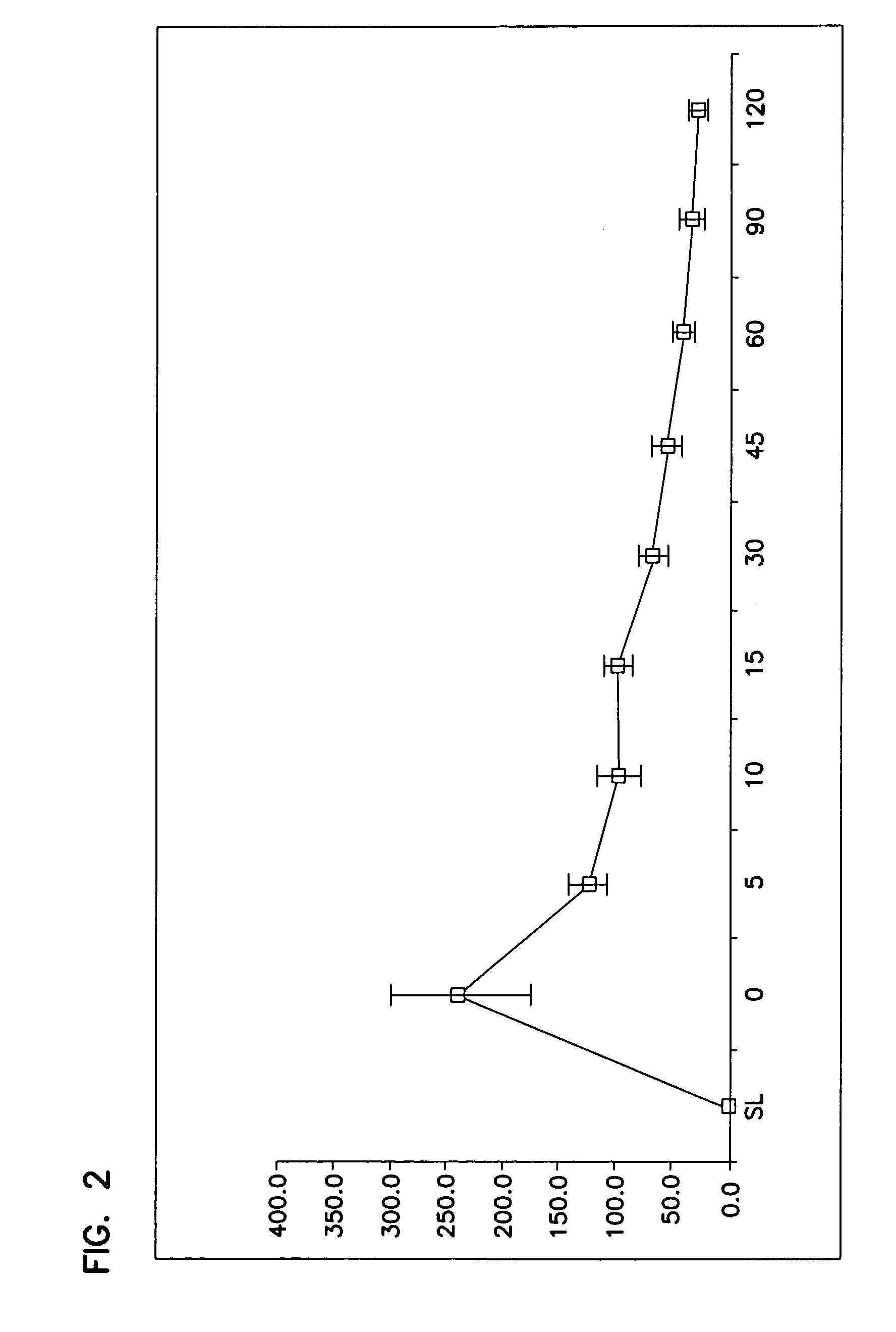
A method for cerebral and systemic cooling by providing a nebulized liquid having a boiling point of 38-300° C. The nebulized liquid is delivered as a mist or a spray via the nasal and / or oral cavities of a patient. The mist causes cooling by direct heat transfer through the nasopharynx and hematogenous cooling through the carotids and the Circle of Willis. Compositions and medical devices for cerebral and systemic cooling are also provided. Cooling assemblies, and methods of use, are also provided that include flexible balloon assemblies that are inserted to various locations in a patient's body. The flexible balloons are then infused with a liquid having a temperature between about −20° C. and about 37° C. The flexible balloon assemblies can be inserted into the nasal cavity, oral cavity, throat, stomach, and other locations to effect cerebral cooling.
Owner:TEMPLE UNIVERSITY +1
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Peptidomimetic macrocycles with improved properties
ActiveUS20100298201A1Prolong half-life in vivoHalogenated hydrocarbon active ingredientsNervous disorderBioactive peptideBiological organism
The present invention provides biologically active peptidomimetic macrocycles with improved properties relative to their corresponding polypeptides. The invention additionally provides methods of preparing and using such macrocycles, for example in therapeutic applications.
Owner:AILERON THERAPEUTICS INC
Pest control compositions and methods
InactiveUS20090099135A1Good effectHigh activityBiocideHalogenated hydrocarbon active ingredientsActive agentPest control
Embodiments of the present invention provide compositions for controlling a target pest including a pest control product and at least one active agent, wherein: the active agent can be capable of interacting with a receptor in the target pest; the pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity.
Owner:TYRATECH
Artificial tear replacement solution
InactiveUS7001607B1Reduce wearGood film formingHalogenated hydrocarbon active ingredientsSenses disorderConjunctivaConjunctival sac
A tear replacement solution that contains at least one water-soluble fluorosurfactant, water and a non-polar component, preferably in gel form, and a method for the external treatment for the eye of an mammal by applying the tear replacement solution to the eye, preferably by placing in the conjunctival sac.
Owner:PHARMPUR
Methods and devices for non-invasive cerebral and systemic cooling
ActiveUS20100211140A1Minimize neurologic deficitMaximize coolingTracheal tubesHalogenated hydrocarbon active ingredientsNasal cavityWhole body
A method for providing and adjusting cerebral cooling in response to changes in a physiological parameter. A spray having a boiling point between 38-300° C. is delivered to the surface of a patient's nasal cavities. The spray causes cooling by direct heat transfer through the nasopharynx and hematogenous cooling through the carotids and the Circle of Willis. A physiological parameter, such as cerebral temperature, changes in cerebral blood flow or brain oxygenation is monitored. The delivery rate of the spray is adjusted in response to the physiological parameter.
Owner:BRAINCOOL +1
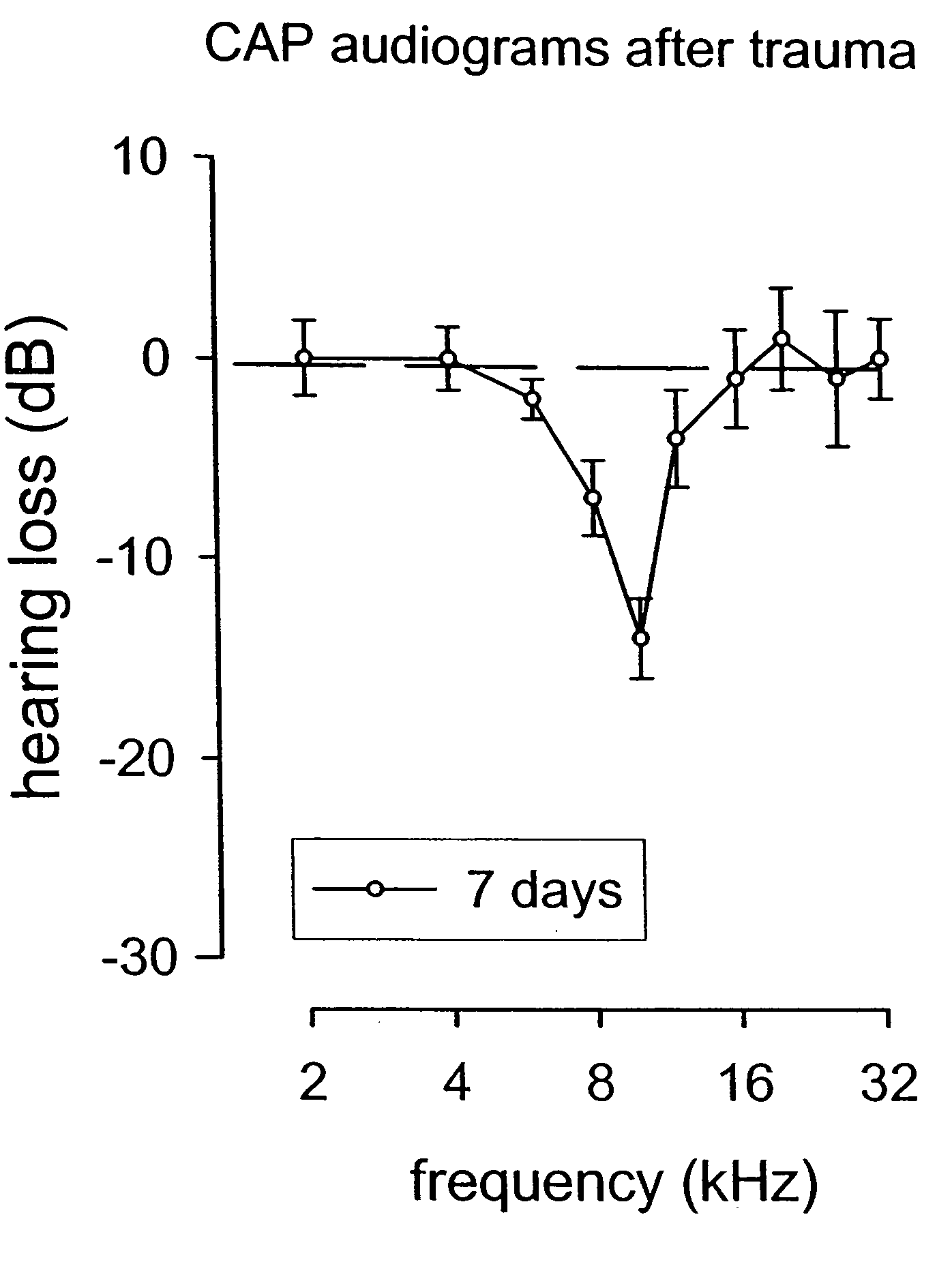
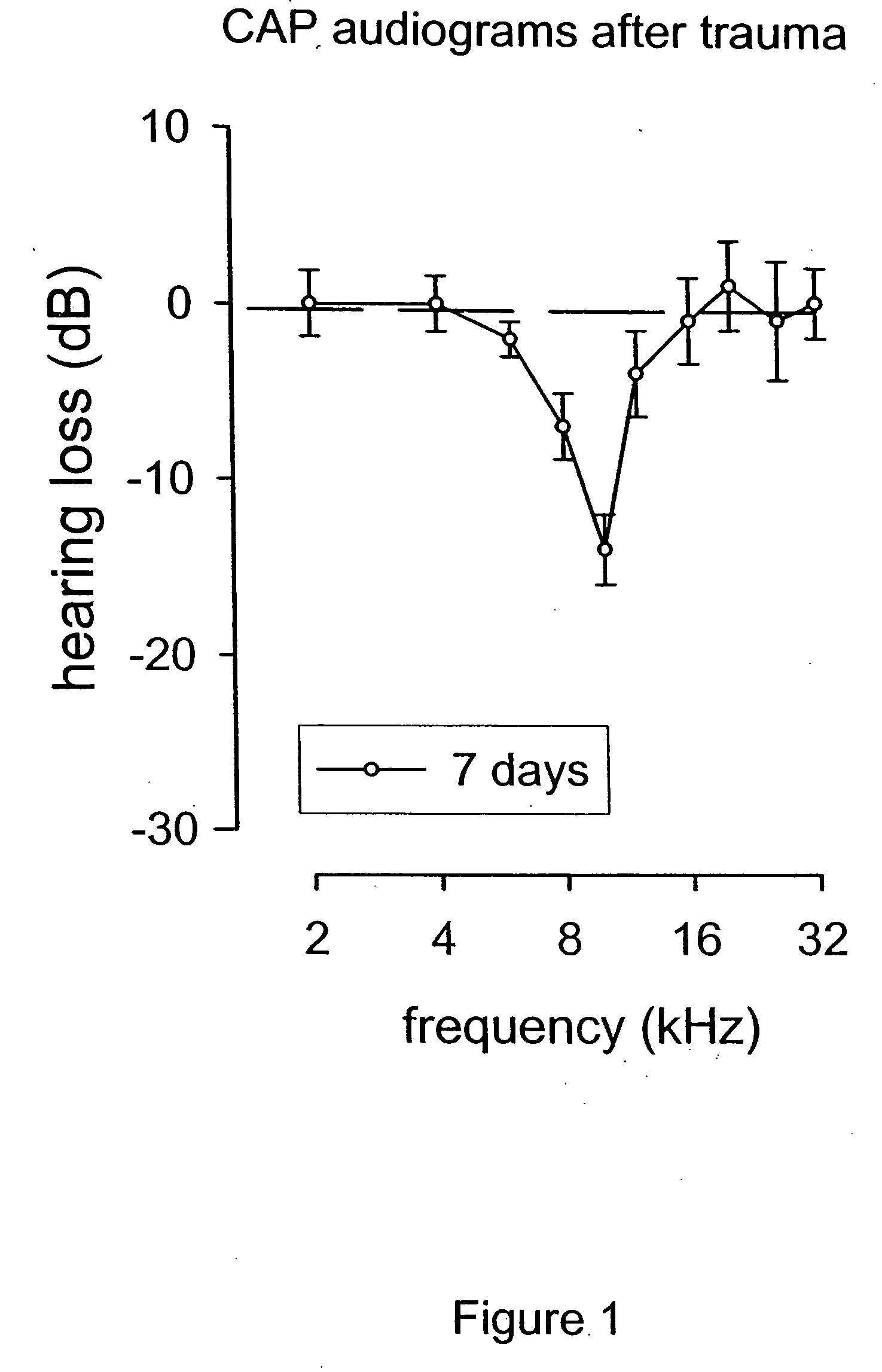
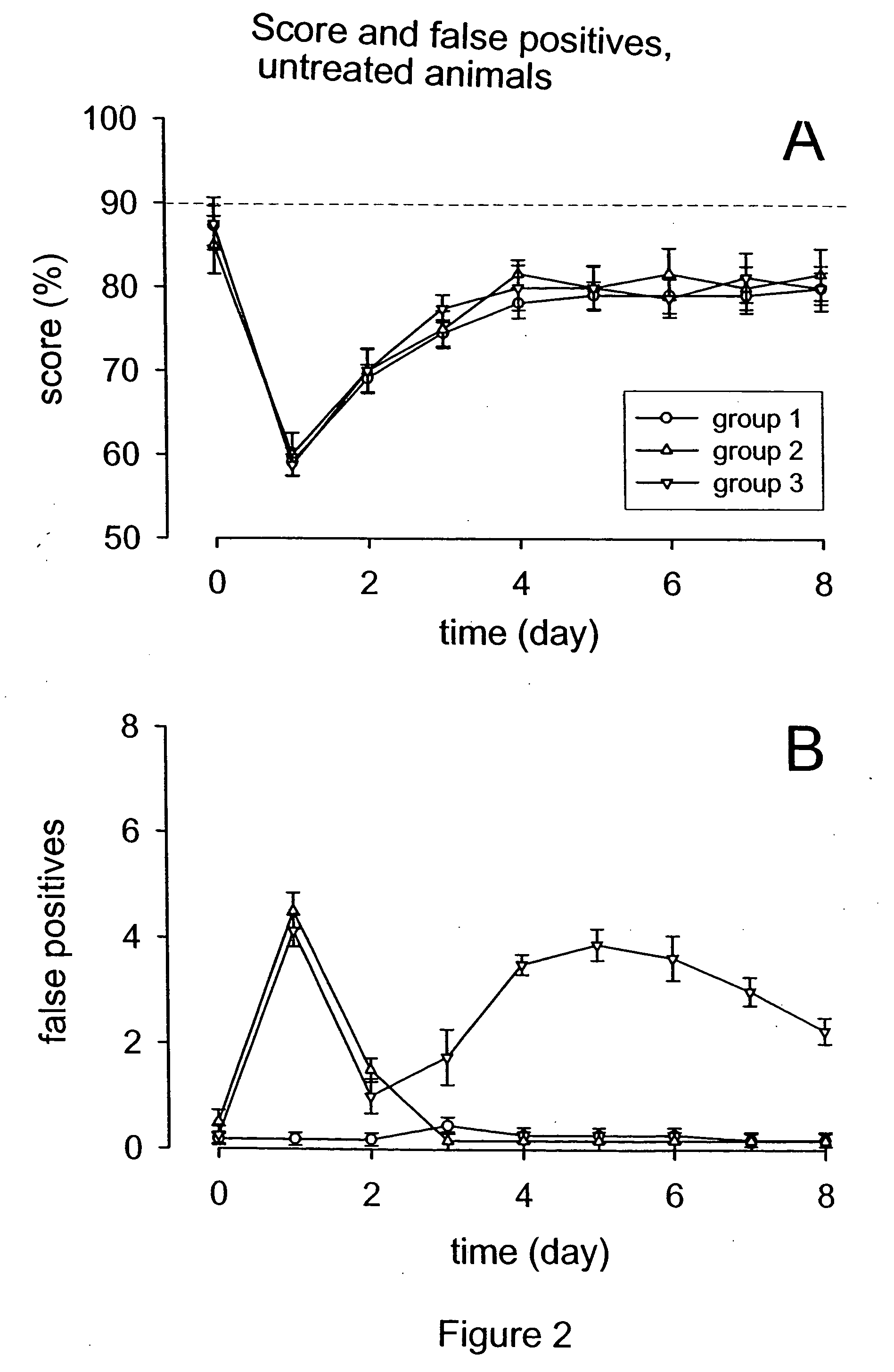
Methods for the treatment of tinnitus induced by cochlear excitotoxicity
ActiveUS20050214338A1Suppress and reduce NMDA receptor mediated aberrant activityPreventing and treating tinnitusBiocideHalogenated hydrocarbon active ingredientsNR1 NMDA receptorTotal Deafness
The invention relates to methods for the prevention and / or treatment of tinnitus induced by cochlear excitotoxicity. In these methods, a pharmaceutical composition comprising an NMDA receptor antagonist is administered to an individual in need of such treatment by appropriate devices and / or formulations for local administration to the inner ear. The tinnitus to be prevented and / or treated may be provoked by acoustic trauma, presbycusis, ischemia, anoxia, sudden deafness, or other cochlear excitotoxic-inducing occurrence.
Owner:AURIS MEDICAL AG
Methods and devices for non-invasive cerebral and systemic cooling
ActiveUS20070123813A1Minimize neurologic deficitsReduce perfusionHalogenated hydrocarbon active ingredientsElectrotherapyWhole bodyNon invasive
A method for cerebral and systemic cooling by providing a nebulized liquid having a boiling point of 38-300° C. The nebulized liquid is delivered as a mist or a spray via the nasal and / or oral cavities of a patient. The mist causes cooling by direct heat transfer through the nasopharynx and hematogenous cooling through the carotids and the Circle of Willis. Compositions and medical devices for cerebral and systemic cooling are also provided. Cooling assemblies, and methods of use, are also provided that include flexible balloon assemblies that are inserted to various locations in a patient's body. The flexible balloons are then infused with a liquid having a temperature between about −20° C. and about 37° C. The flexible balloon assemblies can be inserted into the nasal cavity, oral cavity, throat, stomach, and other locations to effect cerebral cooling.
Owner:BRAINCOOL +1
Emulsions of Perfluorocarbons
InactiveUS20100267842A1Halogenated hydrocarbon active ingredientsCosmetic preparationsPerfluorocarbon emulsionPolymer chemistry
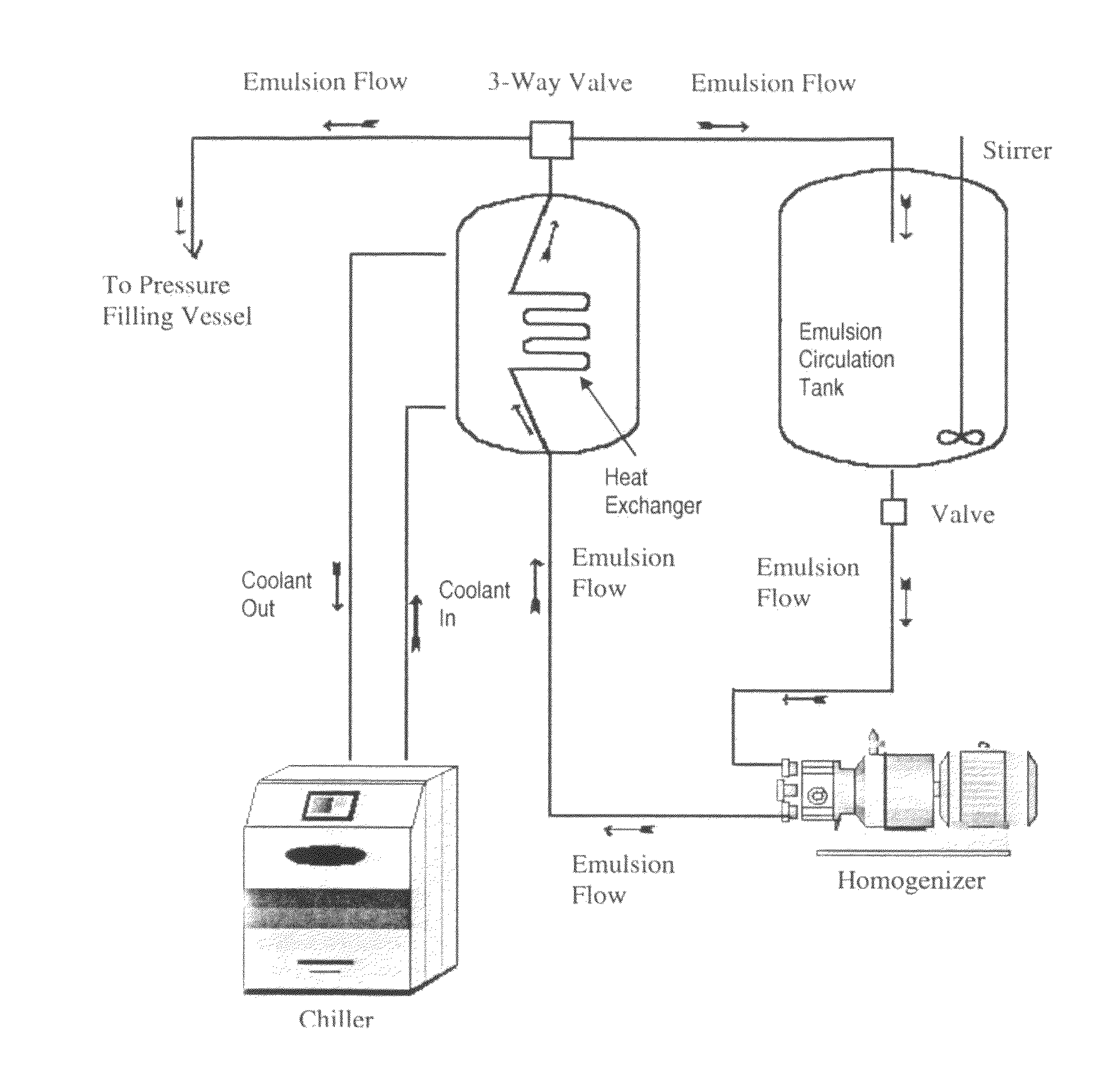
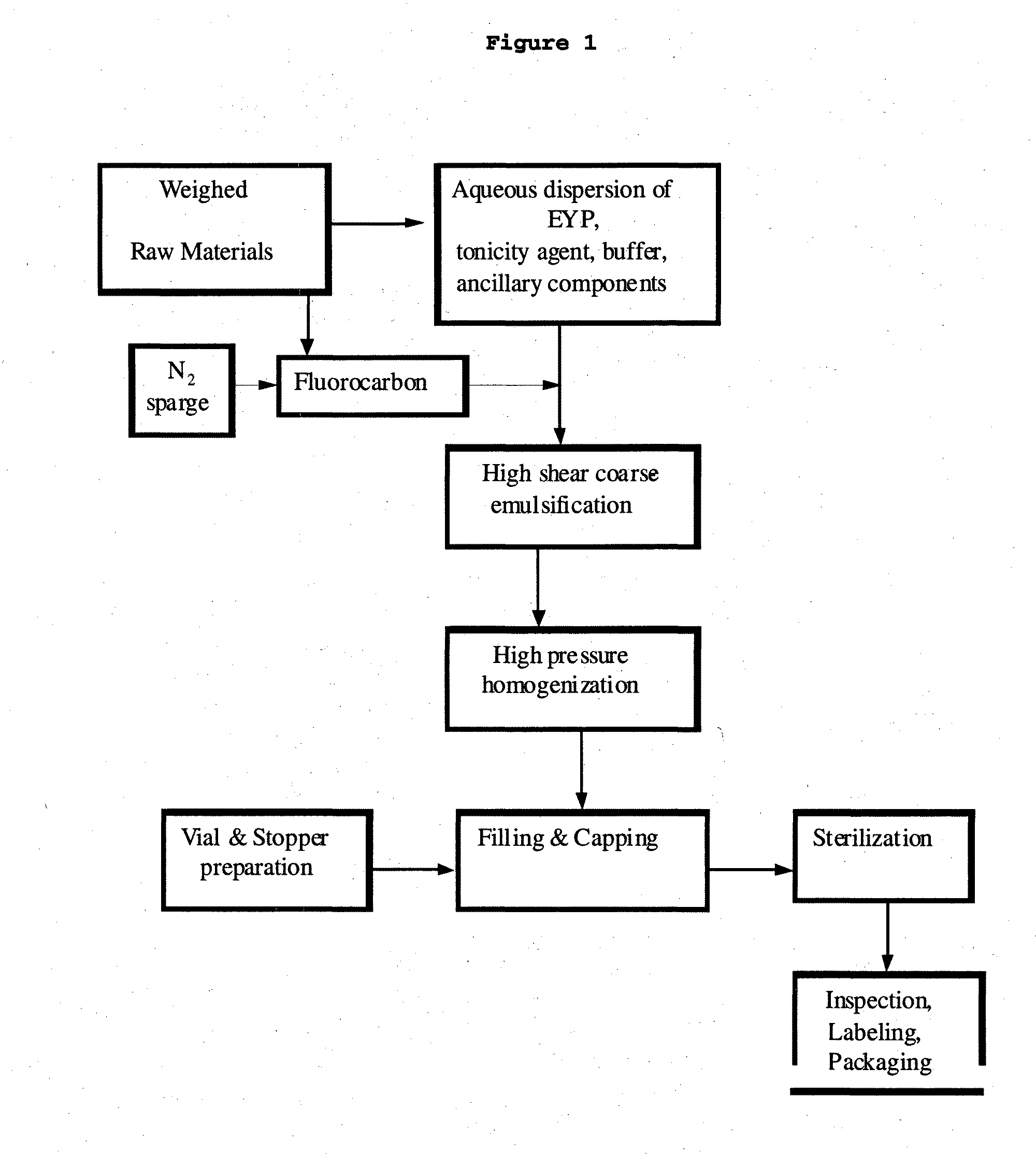
The subject application provides for an emulsion comprising an amount of a perfluorocarbon liquid dispersed as particles within, a continuous liquid phase, wherein the dispersed particles have a monomodal particle size distribution and uses thereof. The subject application also provides for a method of manufacturing a perfluorocarbon emulsion, a process for preparing a pharmaceutical product containing a PFC emulsion and a process for validating a batch of an emulsion for pharmaceutical use.
Owner:TENAX THERAPEUTICS INC
Anti-tumor composition
InactiveUS6992106B2Good curative effectEasy to optimizeBiocideHalogenated hydrocarbon active ingredientsPlatinumMedicine
The present invention provides composition having as active ingredients a stilbene derivative and a platinum coordination compound which is highly efficacious and highly safe for treating tumors, particularly for the treatment of solid or malignant tumors and thus methods of cancer and tumor treatment using the composition are also provided.
Owner:AJINOMOTO CO INC
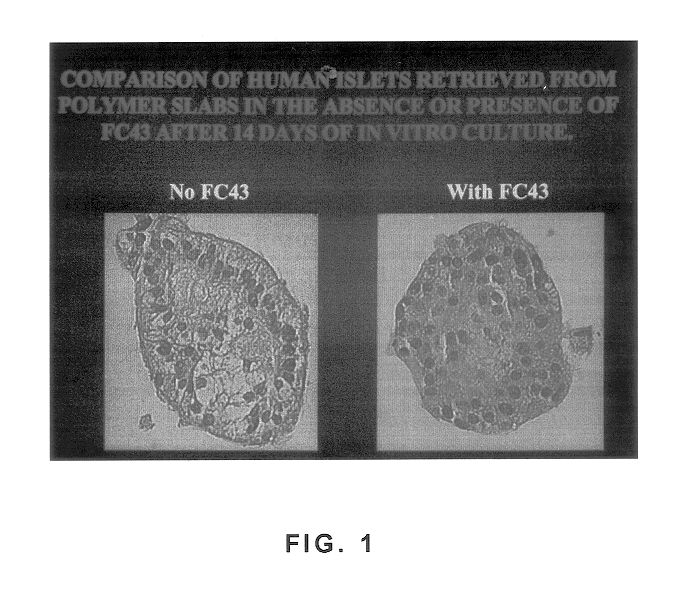
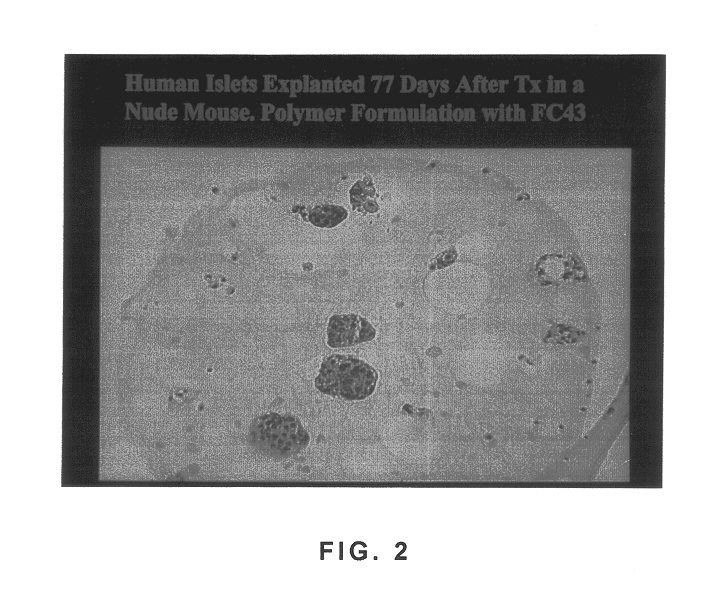
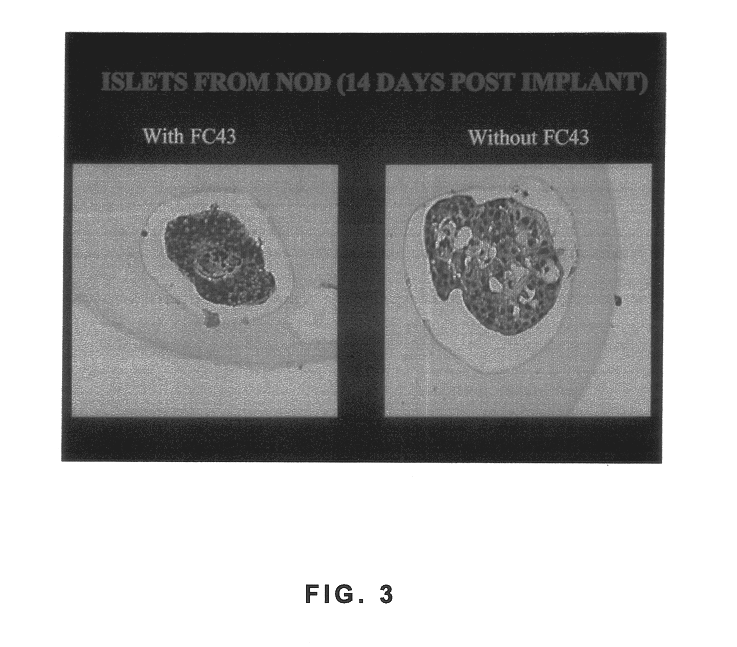
Polymer formulations containing perfluorinated compounds for the engineering of cells and tissues for transplantation that improves cell metabolism and survival, and methods for making same
InactiveUS6630154B1High viscosityMedium viscosity alginatesPowder deliveryBiocideCross-linkInorganic salts
Disclosed and claimed are: a composition including at least one glycosaminoglycan, e.g., CIS, at least one perfluorinated substance and at least one alginate, e.g., sodium alginate, wherein:the at least one glycosaminoglycan and / or the perfluorinated substance and / or the alginate are cross-linked or polymerized, e.g., the alginate is cross-linked or polymerized, for instance by addition of an inorganic salt, such as a calcium salt; orthe at least one glycosaminoglycan, the perfluorinated substance and the alginate are covalently bound, e.g., by means of a coupling reaction involving a linker molecule such as DVS; orthe at least one glycosaminoglycan and / or the perfluorinated substance and / or the alginate are cross-linked or polymerized, e.g., the alginate is cross-linked or polymerized, for instance by addition of an inorganic salt, such as a calcium salt, and the at least one glycosaminoglycan and the alginate are covalently bound, e.g., by means of a coupling reaction involving a linker molecule such as DVS, and the covalent binding can have been performed prior to cross-linking or polymerizing or vice versa; and,gels comprising the composition; mixtures of such gels or of at least one such gel and at least one such composition; and,methods for making and using such compositions and gels, including products therefrom such as "paints", sprays, matrices, beads, microcapsules.
Owner:BIOMM +1
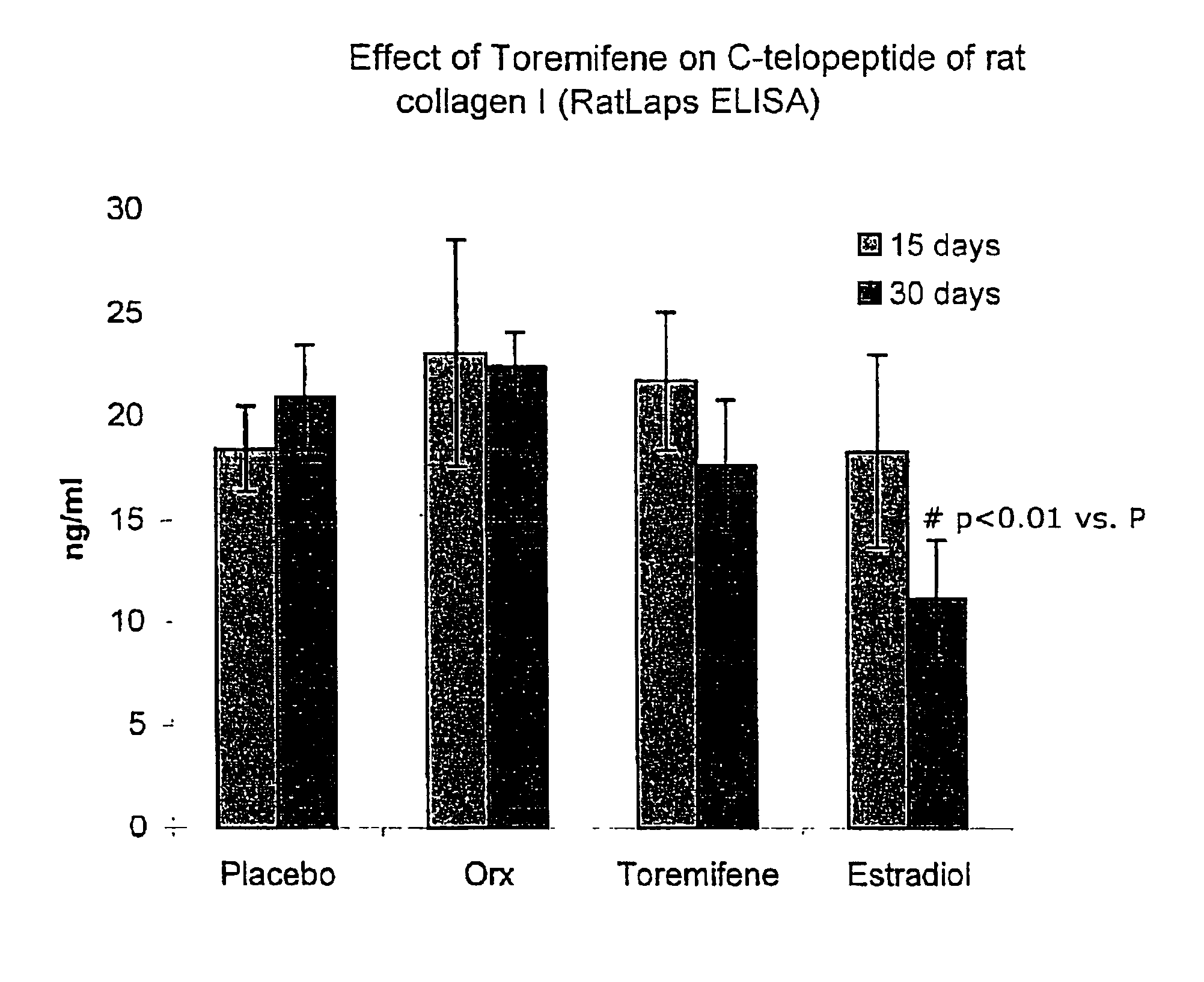
Prevention and treatment of androgen-deprivation induced osteoporosis
InactiveUS6899888B2Safe and effectiveReduce development riskBiocideHalogenated hydrocarbon active ingredientsDysostosisMetabolite
This invention provides: 1) a method of treating androgen-deprivation induced osteoporosis and / or bone fractures and / or loss of Bone Mineral Density (BMD) in a male subject suffering from prostate cancer; 2) a method of preventing androgen-deprivation induced osteoporosis and / or bone fractures and / or loss of Bone Mineral Density (BMD) in a male subject suffering from prostate cancer; 3) a method of suppressing or inhibiting androgen-deprivation induced osteoporosis and / or bone fractures and / or loss of BMD in a male subject suffering from prostate cancer; and 4) a method of reducing the risk of developing androgen-deprivation induced osteoporosis and / or bone fractures and / or loss of BMD in a male subject suffering from prostate cancer, by administering to the subject a pharmaceutical composition comprising an anti-estrogen agent and / or its analog, derivative, isomer, metabolite, pharmaceutically acceptable salt, pharmaceutical product, hydrate, N-oxide, or any combination thereof as described herein.
Owner:GTX INCORPORATED +1
Cell migration inhibiting compositions and methods and compositions for treating cancer
Methods for treating an individual having cancer are provided. The method may include administering a cell migration inhibitor and a chemotherapeutic agent to the individual to inhibit migration of cancer cell. Inhibiting cell migration may increase cell division. In this manner, the cell migration inhibitor and the chemotherapeutic agent in combination may have increased efficacy compared to the chemotherapeutic agent alone due to the increased cell division. The cell migration inhibitor may include any of the inhibitors described herein. For example, the cell migration inhibitor may be an organic molecule having a molecular weight of less than about 700, a monoclonal antibody, or a natural product.
Owner:AVOLIX PHARMA
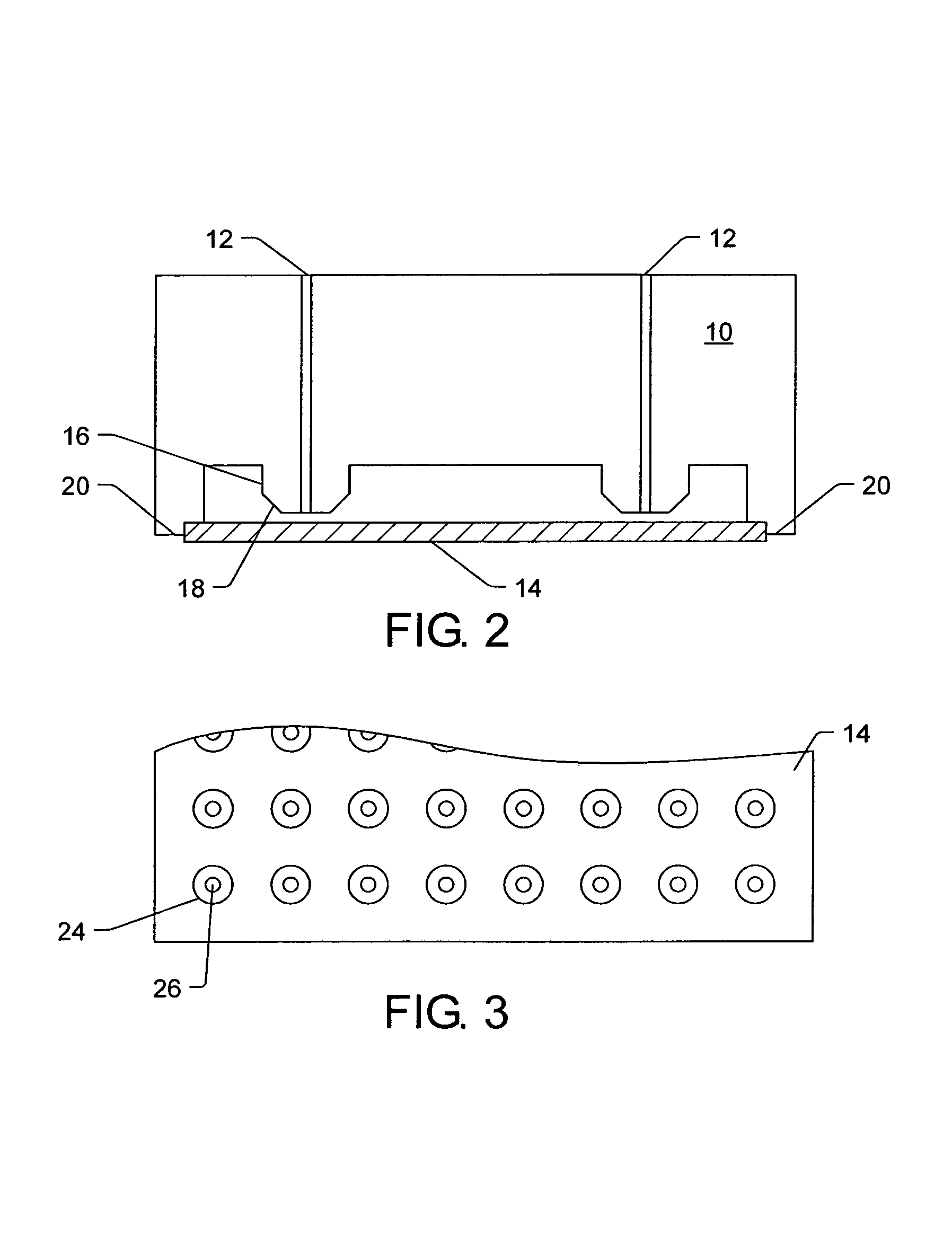
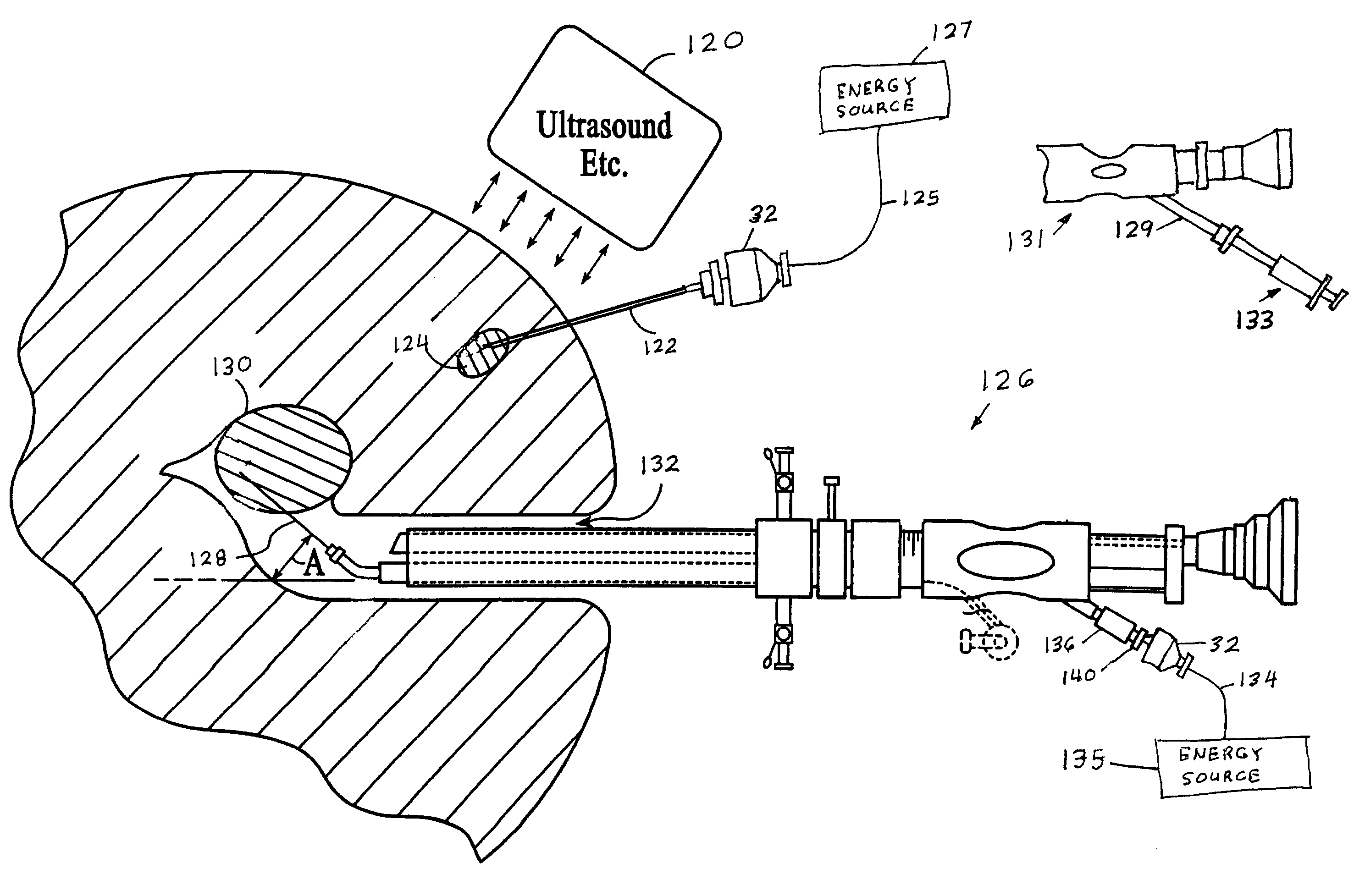
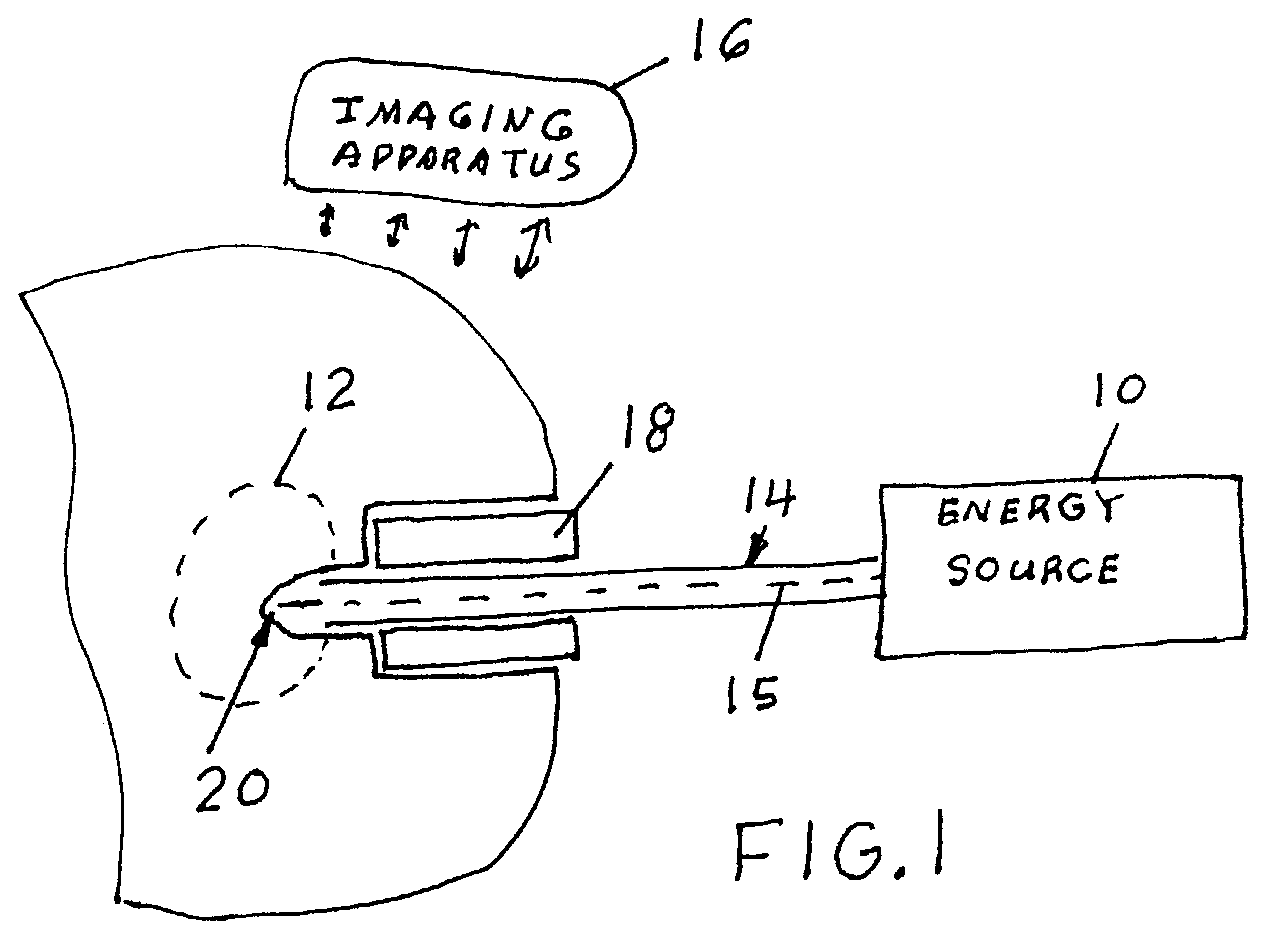
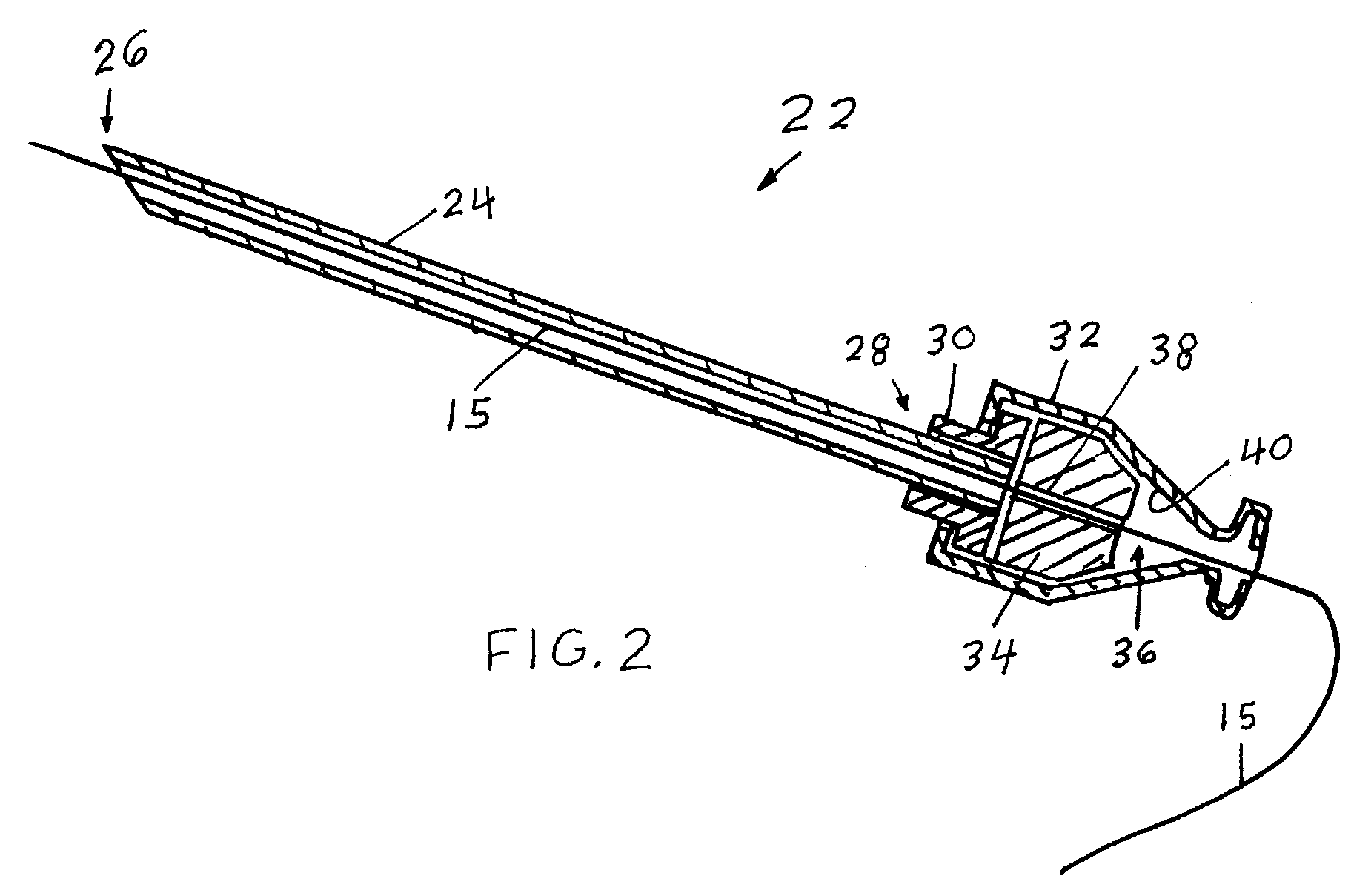
Method and apparatus for tissue treatment with laser and electromagnetic radiation
InactiveUS7549424B2Ultrasonic/sonic/infrasonic diagnosticsHalogenated hydrocarbon active ingredientsElectromagnetic radiationLength wave
A method and apparatus wherein an application of energy is limited to a specific diseased portion of body tissue (target tissue) for the purpose of localizing the treatment to the target tissue and avoiding an adverse effect on surrounding tissue. In one embodiment, access to the target tissue is provided by inserting an energy transmission device through a needle for delivery of the energy into the target tissue. Guidance of the needle is alternatively enhanced through use of an image guidance device. Alternatively, in addition the localized treatment is further controlled by use of an energy-concentrating / enhancement agent and / or photosensitizing / photoselective agent, chromophore dye and viscous substance to cause selective interaction with a specific wavelength of an energy source. The treatment can be further controlled and localized by improving the accuracy and positioning of the delivery device into the target tissue using imaging guidance.
Owner:PRO SURG
Topical treatment for prevention of ocular infections
InactiveUS7056893B2Treat and prevent infectionEffectively prevent infectionHalogenated hydrocarbon active ingredientsBiocideAzalideTopical treatment
Azalide antibiotics such as azithromycin are useful in the treatment and prevention of infections by bacteria and other parasites. Stabilized aqueous compositions containing azithromycin suitable for administration without reconstitution are provided for. Also provided for are aqueous formulations suitable for ocular administration in a employing a convenient dosing formulation suitable for administration in depot formats.
Owner:SUN PHARMA IND INC
Methods and compounds useful to induce apoptosis in cancer cells
InactiveUS20050027000A1Reduced viabilityCompound screeningHalogenated hydrocarbon active ingredientsApoptosisCompound (substance)
Owner:SANFORD BURNHAM PREBYS MEDICAL DISCOVERY INST
Triaryl methane compounds and analogues thereof useful for the treatment or prevention of sickle cell disease or diseases characterized by abnormal cell proliferation
InactiveUS6028103AReducing sickle erythrocyte dehydrationDelaying occurrenceHalogenated hydrocarbon active ingredientsBiocideAbnormal cellSickled erythrocytes
The present invention provides a class of chemical compounds useful as efficacious drugs in the treatment of sickle cell disease and diseases characterized by unwanted or abnormal cell proliferation. The active compounds are substituted triaryl methane compounds or analogues thereof where one or more of the aryl groups is replaced with a heteroaryl, cycloalkyl or heterocycloalkyl group and / or the tertiary carbon atom is replaced with a different atom such as Si, Ge, N or P. The compounds inhibit mammalian cell proliferation, inhibit the Gardos channel of erythrocytes, reduce sickle erythrocyte dehydration and / or delay the occurrence of erythrocyte sickling or deformation.
Owner:HARVARD COLLEGE PRESIDENT & FELLOWS OF +2
Methods and articles having a high antiviral and antibacterial efficacy
InactiveUS20080145390A1Enhance persistent antiviral controlCut surfaceHalogenated hydrocarbon active ingredientsHeavy metal active ingredientsSurface phAntibacterial efficacy
Method and article for providing a rapid, broad spectrum bacterial control, and a rapid and persistent antiviral control on an inanimate surface is disclosed. In the method, a compound or composition capable of lowering surface pH to less than about 4 is applied to the surface, and preferably is allowed to remain on the surface, and the nonvolatile components of the composition can form a barrier film or layer on a treated surface.
Owner:DIAL CORPORATION
Compositions comprising mixtures of semifluorinated alkanes
ActiveUS20150224064A1Effective timeTune viscosityBiocideHalogenated hydrocarbon active ingredientsAlkaneKERATOCONJUNCTIVITIS SICCA
The invention provides novel compositions comprising at least two or more semifluorinated alkanes. The compositions can be used as medicines that are topically administered to an eye or ophthalmic tissue, such as for use in the treatment of keratoconjunctivitis sicca (dry eye) and / or meibomian gland dysfunction and symptoms associated therewith. The invention further provides kits comprising such compositions.
Owner:NOVALIQ GMBH
Antiviral patch
InactiveUS20070026056A1Useful in treatmentAvoid infectionHalogenated hydrocarbon active ingredientsBiocideSolventDrug
An adhesive patch is provided wherein the patch includes a porous backing having a front side and a back side. The patch also includes a therapeutic formulation located on the front side of the backing. The backing includes a flexible sheet of water insoluble porous material. The therapeutic formulation includes a combination of a antiviral agent useful for treating a viral infection in a mammal (e.g., human), a medicament that relieves topical discomfort, an adhesive, and a solvent. The solvent can preferably include a fragrance.
Owner:LECTEC CORP
Utilization of a highly fluorinated oligomeric alkane in ophthalmology
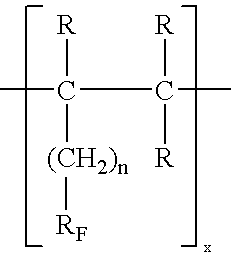
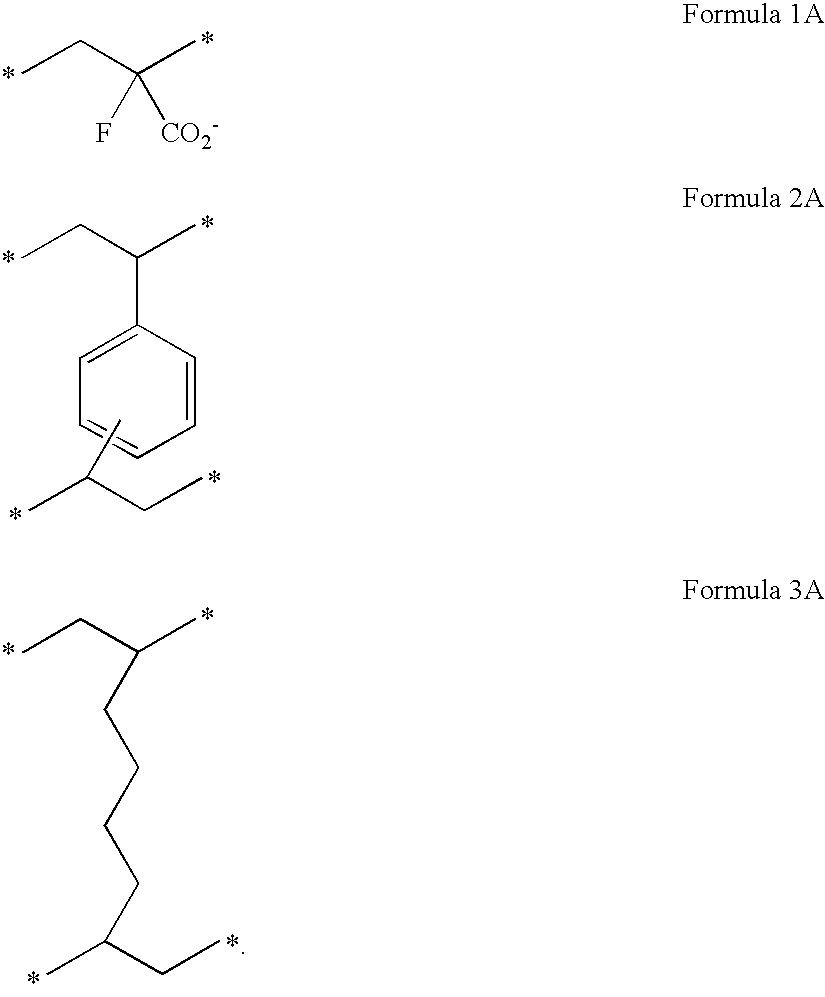
The invention relates to the utilization of a highly fluorinated oligomeric alkane as a therapeutic agent in ophthalmology, the alkane having the general formula [—RF(CH2)nCR1—CR2—]x, wherein RF is a linear or branched perfluorinated alkyl chain C2F5 to C12F23, R1 and R2 are independently selected from the group consisting of H, CH3, C2H5 and C3H7, n is selected from the numbers 0, 1 and 2, and x is a number between 2 and 6, with a molar mass of at least 750. According to the invention, a pharmacological agent is provided that is used in ophthalmology and that causes little or no damage to the retina even when used over a long period.
Owner:BAUSCH & LOMB INC
Pharmaceutical composition for administration to nails
ActiveUS9308262B2Efficient deliveryAntibacterial agentsHalogenated hydrocarbon active ingredientsRetinoidBULK ACTIVE INGREDIENT
Owner:DERMALIQ THERAPEUTICS INC
Pheromone compositions and methods of use
ActiveUS20110150822A1Modification for usingEffective stressBiocideHalogenated hydrocarbon active ingredients1-DocosanolBehavior modification
Pheromone compositions based on a combination of squalene, linoleic acid and 1-docosanol are described. The compositions are useful for behavior modification in mammals that exhibit undesirable or harmful stress-related behaviors.
Owner:SERGEANTS PET CARE PRODS
Method for treating cushing's syndrome
InactiveUS20100261693A1Inhibition of secretionHalogenated hydrocarbon active ingredientsEndocrine system disorderPhysiologyDepressant
The invention relates to a method for treating Cushing's syndrome in a patient, which method comprises administering the patient with a pharmaceutical composition comprising a glucocorticoid receptor antagonist, at least twice a day, or an extended-release composition of a glucocorticoid receptor antagonist, or a combination of a glucocorticoid receptor antagonist and a inhibitor of cortisol synthesis.
Owner:LAB HRA PHARMA SA
Semifluorinated alkane compositions
Owner:NOVALIQ GMBH
Linear polyol stabilized polyfluoroacrylate compositions
ActiveUS20100111891A1Good curative effectHalogenated hydrocarbon active ingredientsBiocidePolyolPotassium
The present invention is directed to compositions of a linear polyol and a salt of a crosslinked cation exchange polymer comprising a fluoro group and an acid group. These compositions are useful to bind potassium in the gastrointestinal tract.
Owner:VIFOR INT AG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com