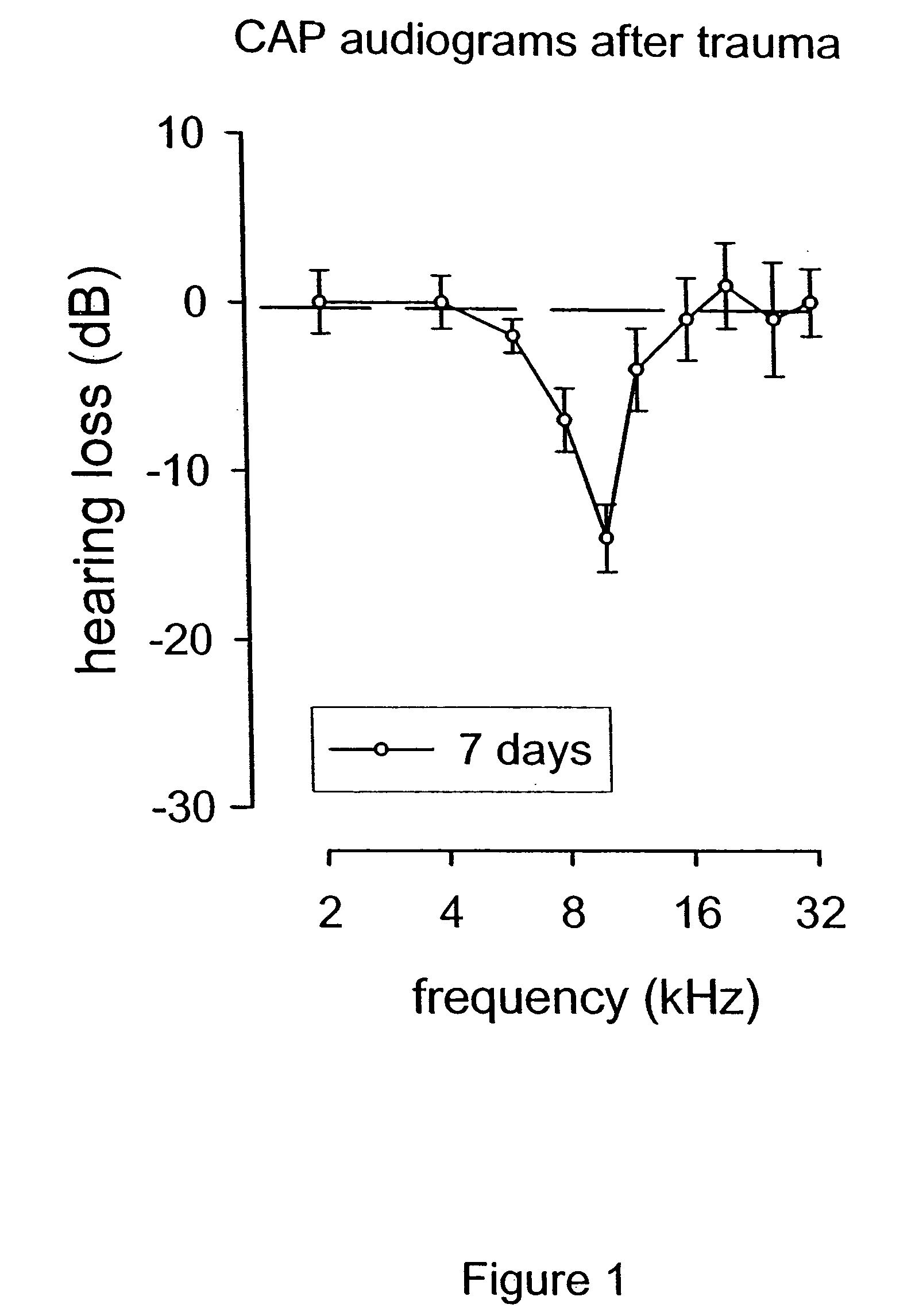
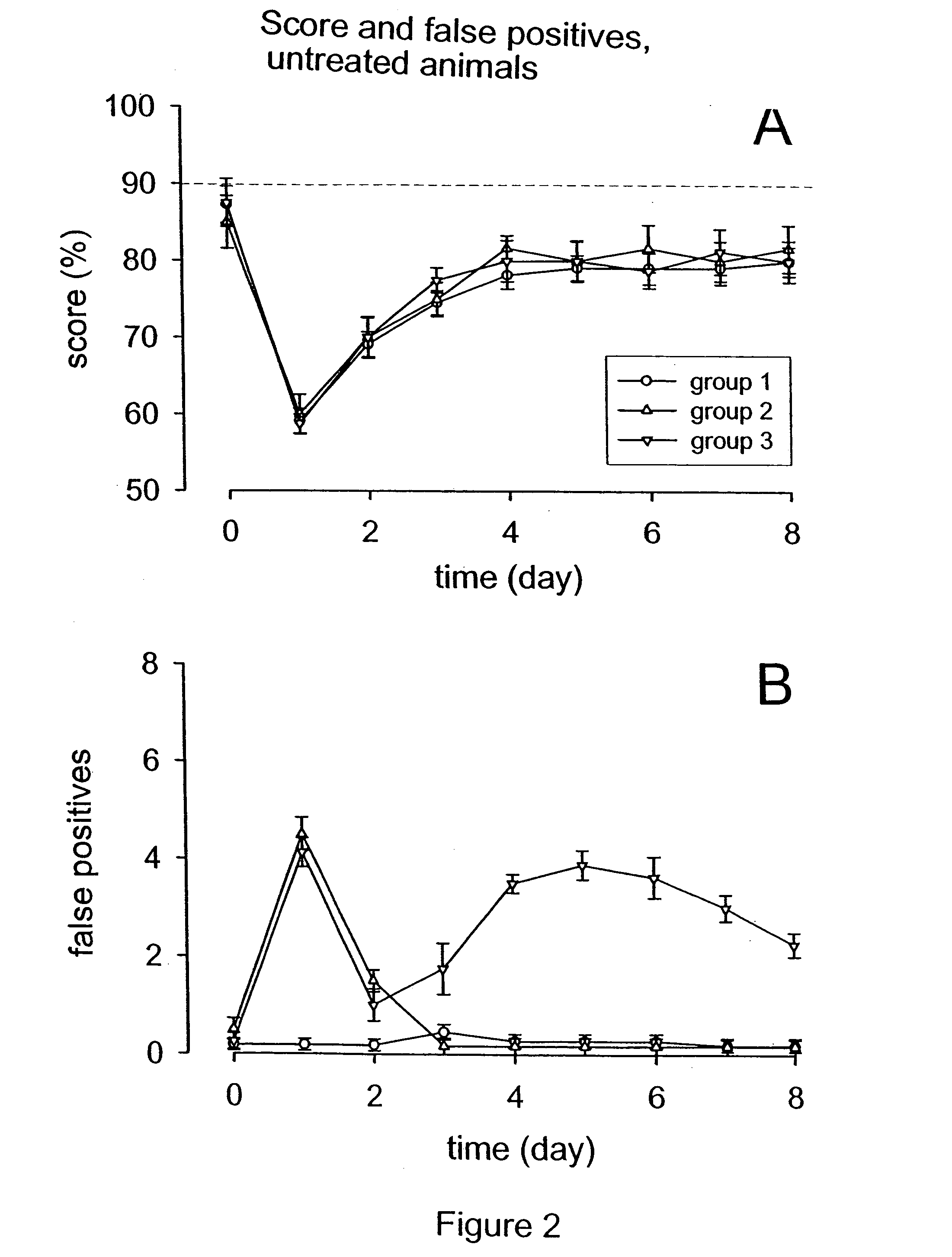
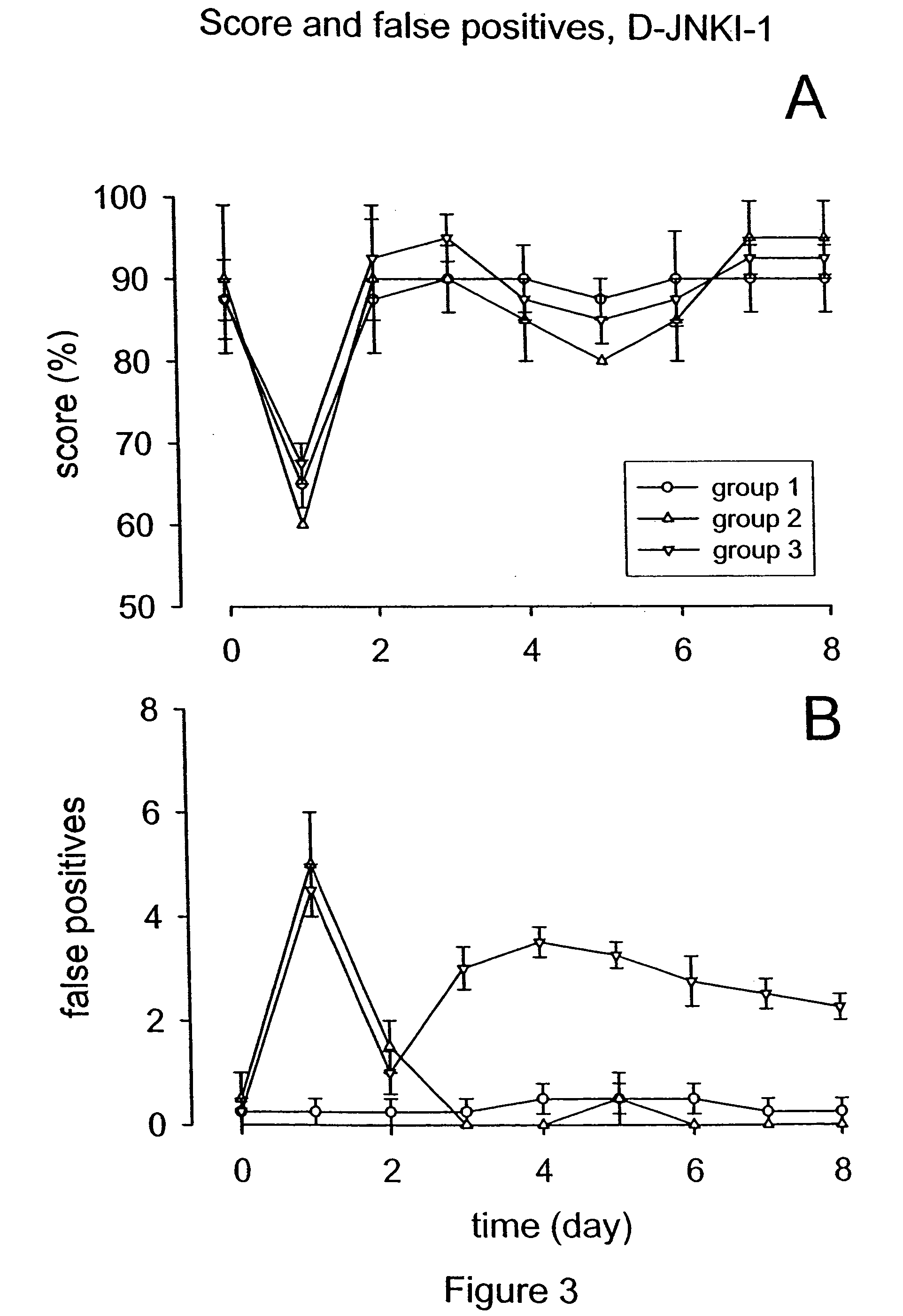
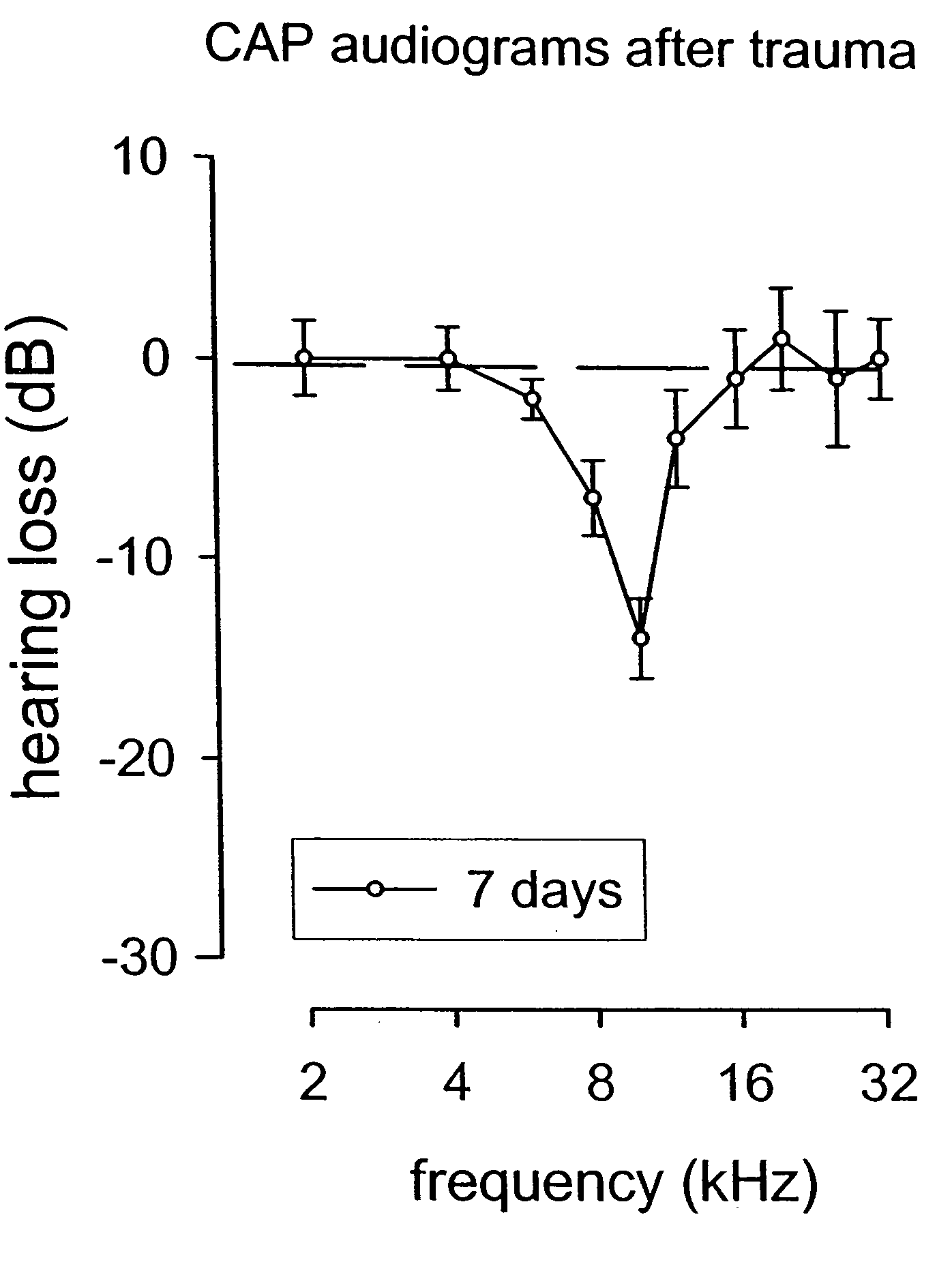
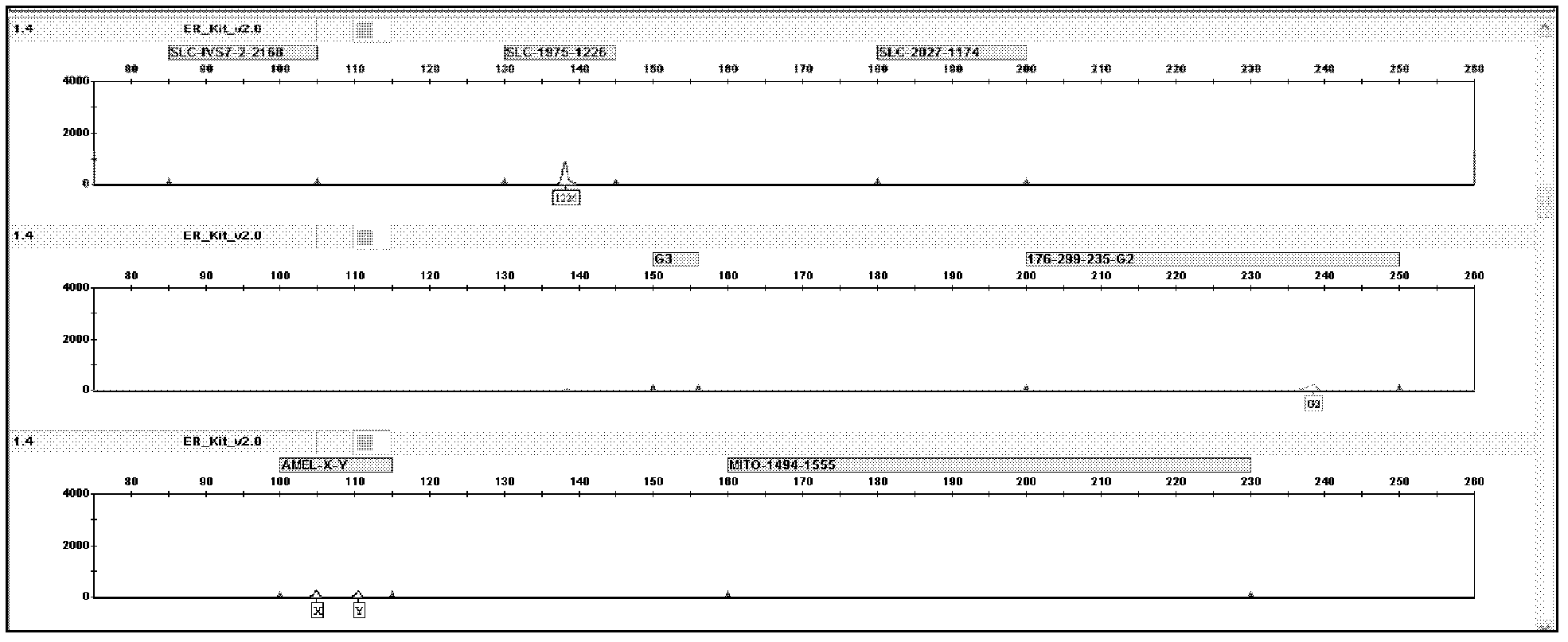
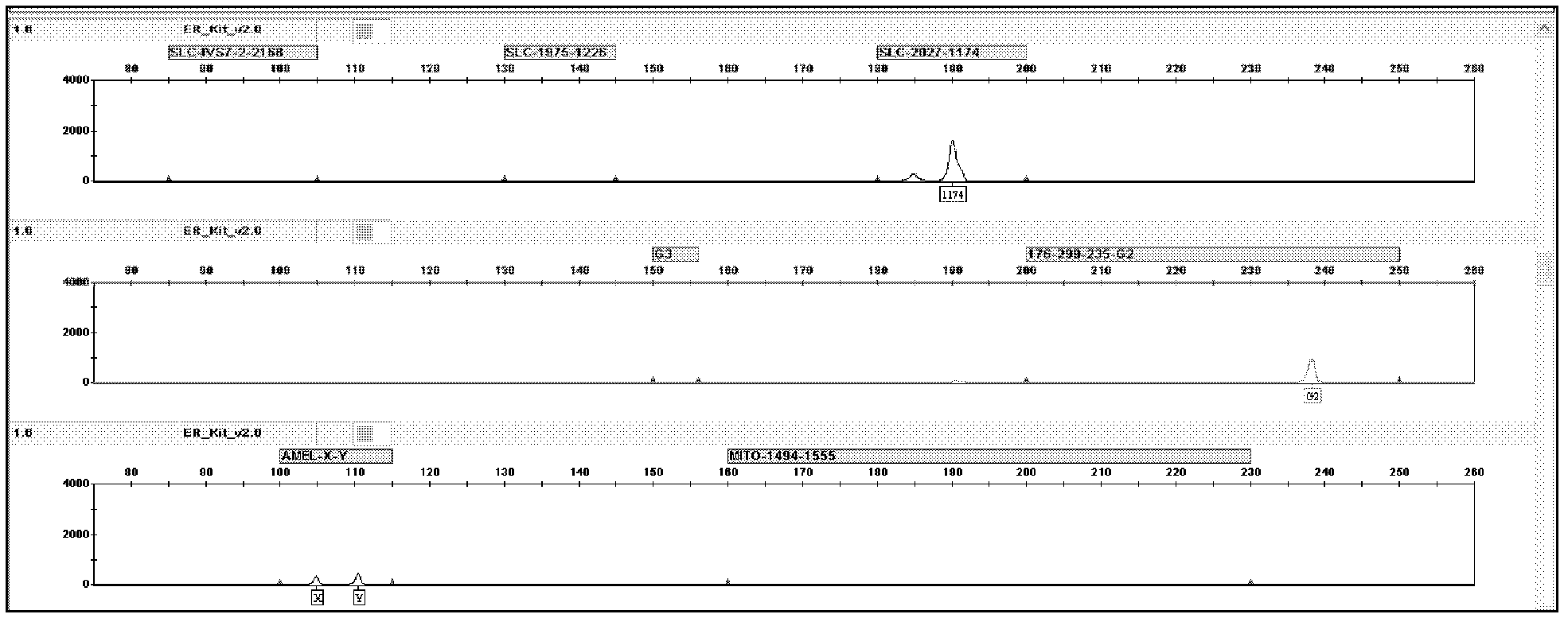
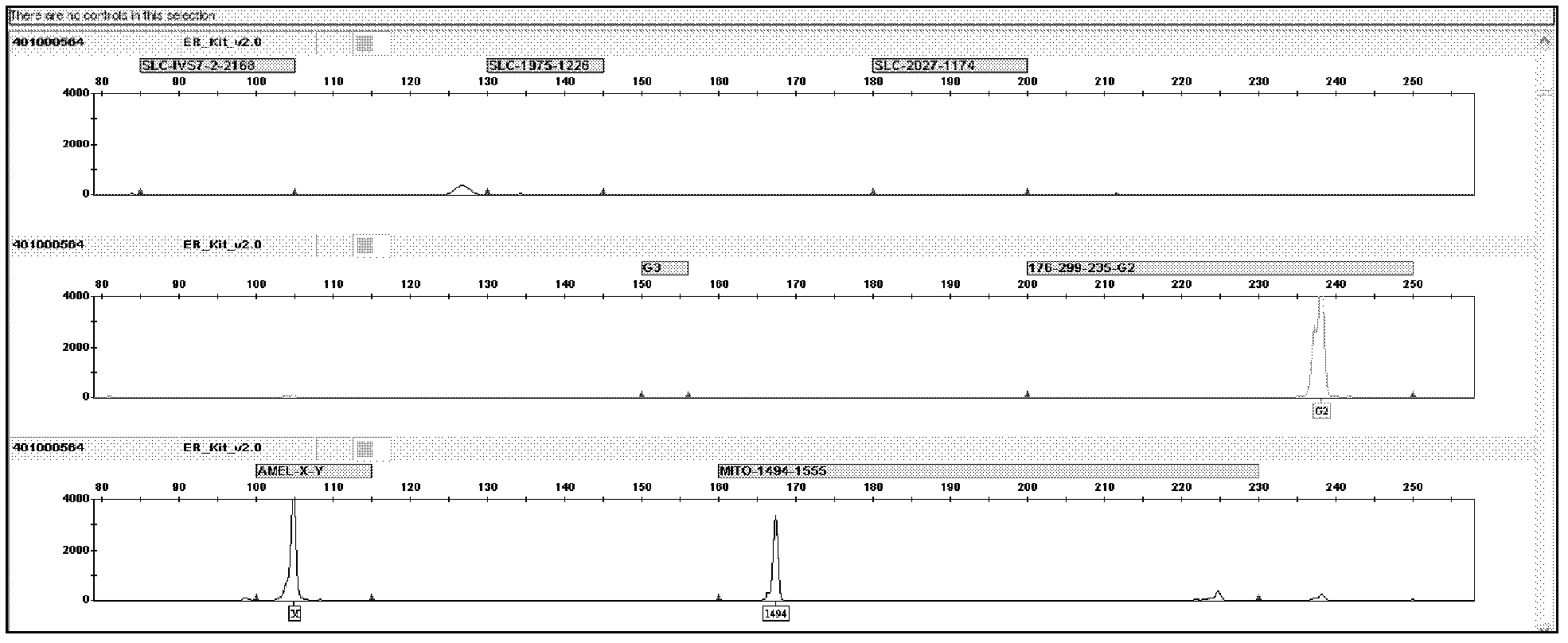
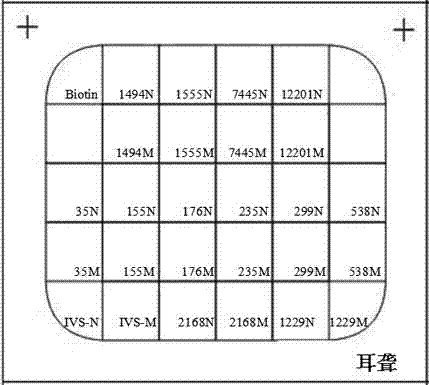
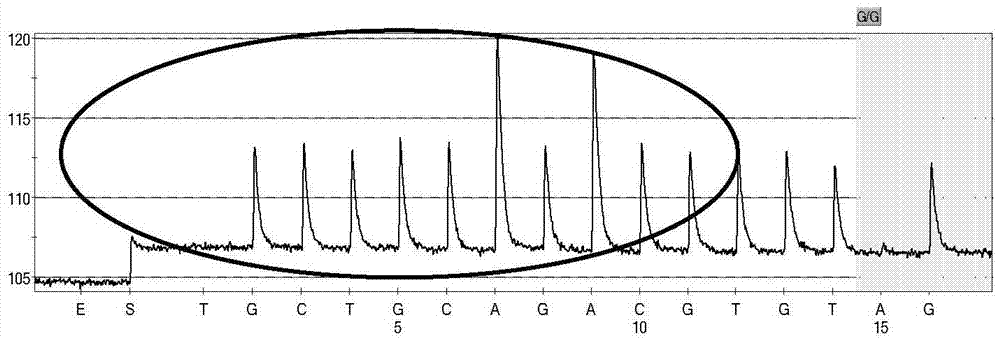
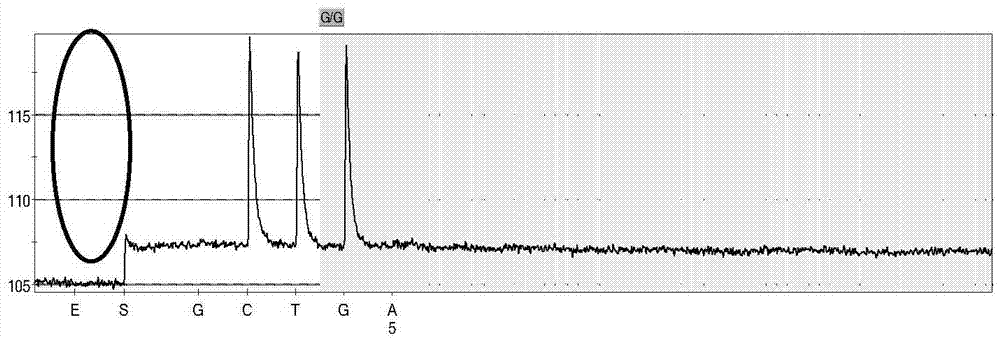
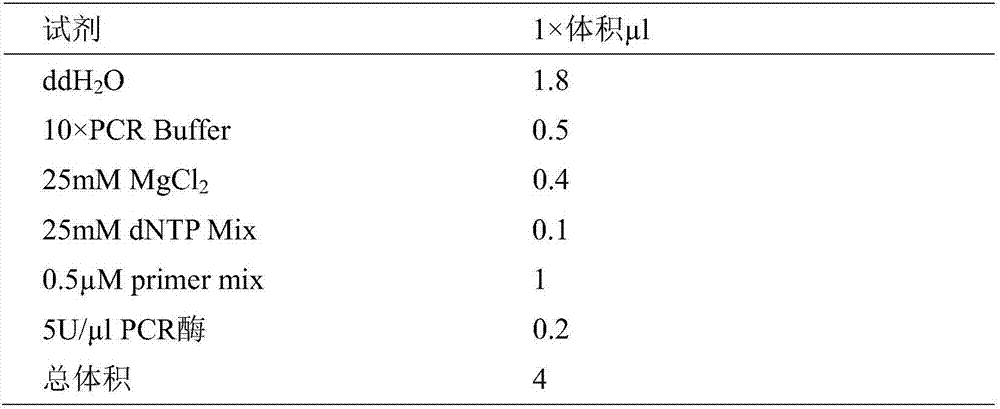
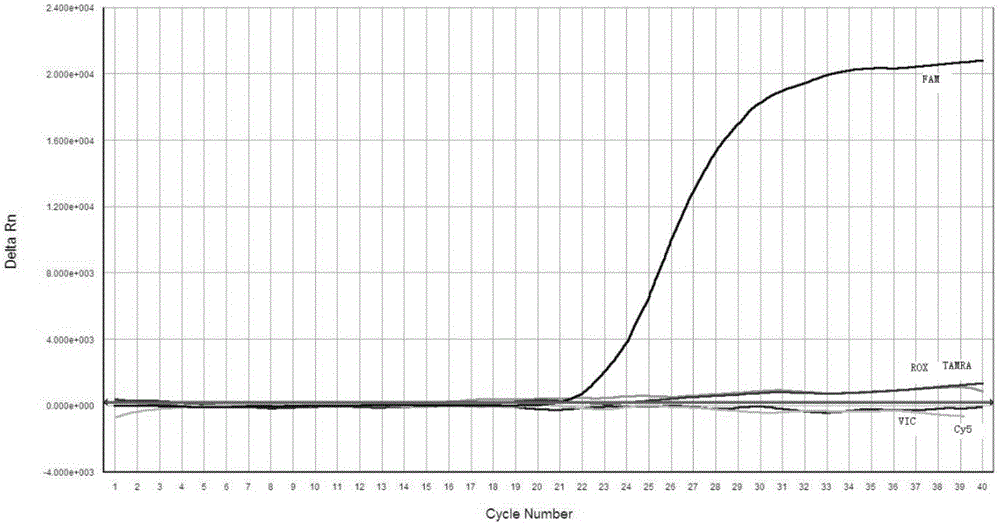
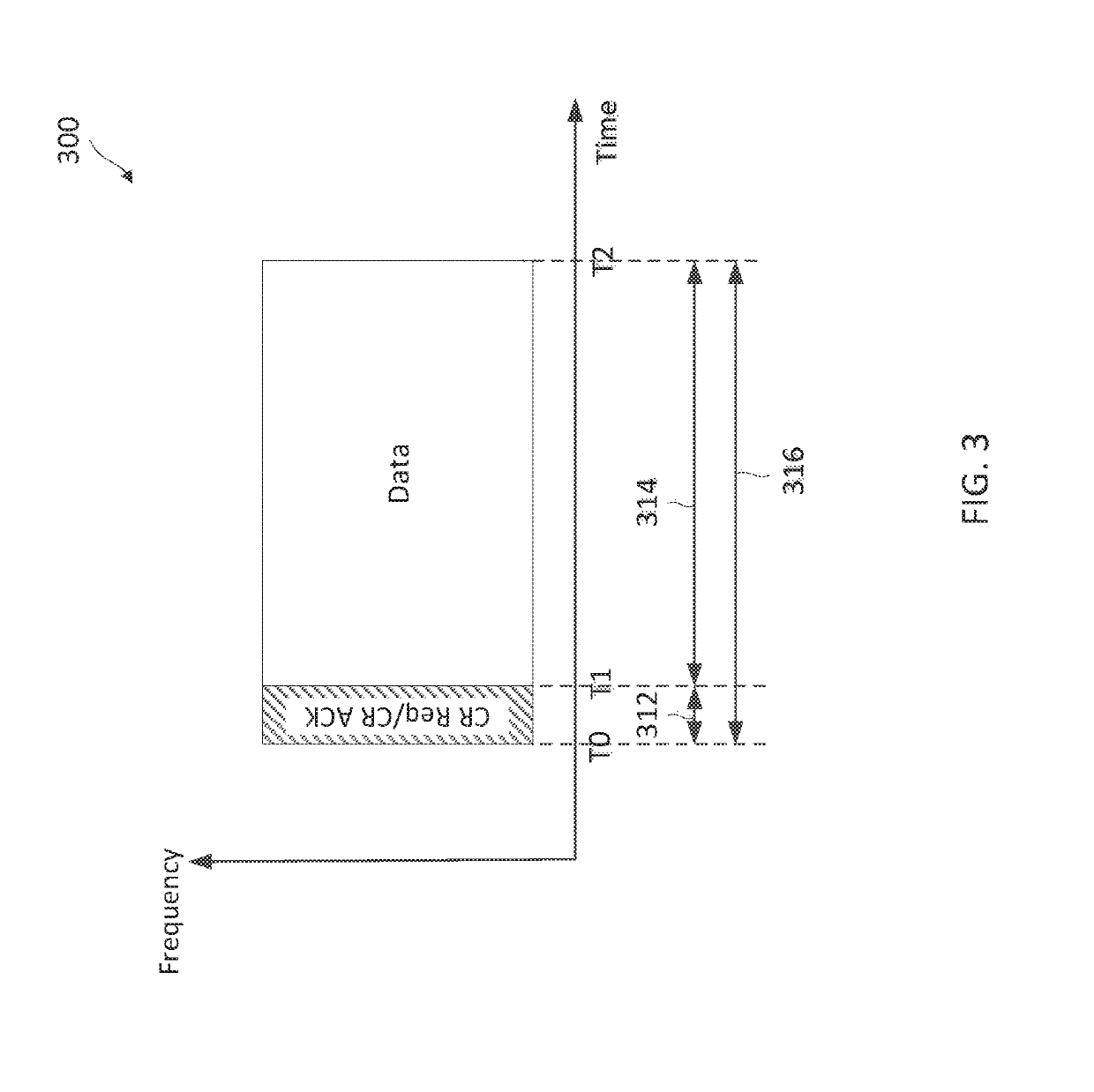
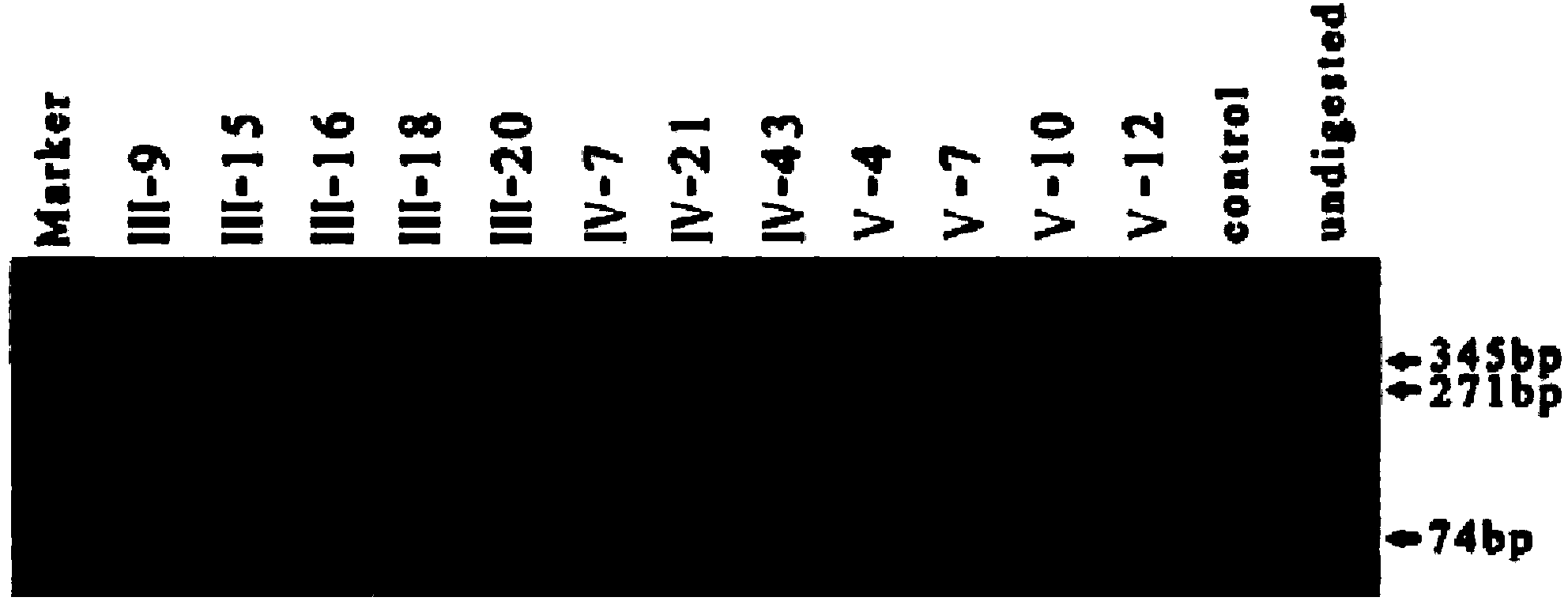Patents
Literature
63 results about "Total Deafness" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hearing loss, also known as hearing impairment, is a partial or total inability to hear. A deaf person has little to no hearing. Hearing loss may occur in one or both ears. In children hearing problems can affect the ability to learn spoken language and in adults it can cause work related difficulties.
Methods for the treatment of tinnitus induced by cochlear excitotoxicity
InactiveUS20060063802A1Suppress and reduce NMDA receptor mediated aberrant activityPreventing and treating tinnitusCompounds screening/testingBiocideNR1 NMDA receptorTotal Deafness
The invention relates to methods for the prevention and / or treatment of tinnitus induced by cochlear excitotoxicity. In these methods, a pharmaceutical composition comprising an NMDA receptor antagonist is administered to an individual in need of such treatment by appropriate devices and / or formulations for local administration to the inner ear. The tinnitus to be prevented and / or treated may be provoked by acoustic trauma, presbycusis, ischemia, anoxia, treatment with one or more ototoxic medications, sudden deafness, or other cochlear excitotoxic-inducing occurrence. The invention also relates to method for the identification of compounds effective in the treatment and prevention of tinnitus by a novel screening method incorporating an electrophysiological test method.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Methods for the treatment of tinnitus induced by cochlear excitotoxicity
ActiveUS20050214338A1Suppress and reduce NMDA receptor mediated aberrant activityPreventing and treating tinnitusBiocideHalogenated hydrocarbon active ingredientsNR1 NMDA receptorTotal Deafness
The invention relates to methods for the prevention and / or treatment of tinnitus induced by cochlear excitotoxicity. In these methods, a pharmaceutical composition comprising an NMDA receptor antagonist is administered to an individual in need of such treatment by appropriate devices and / or formulations for local administration to the inner ear. The tinnitus to be prevented and / or treated may be provoked by acoustic trauma, presbycusis, ischemia, anoxia, sudden deafness, or other cochlear excitotoxic-inducing occurrence.
Owner:AURIS MEDICAL AG
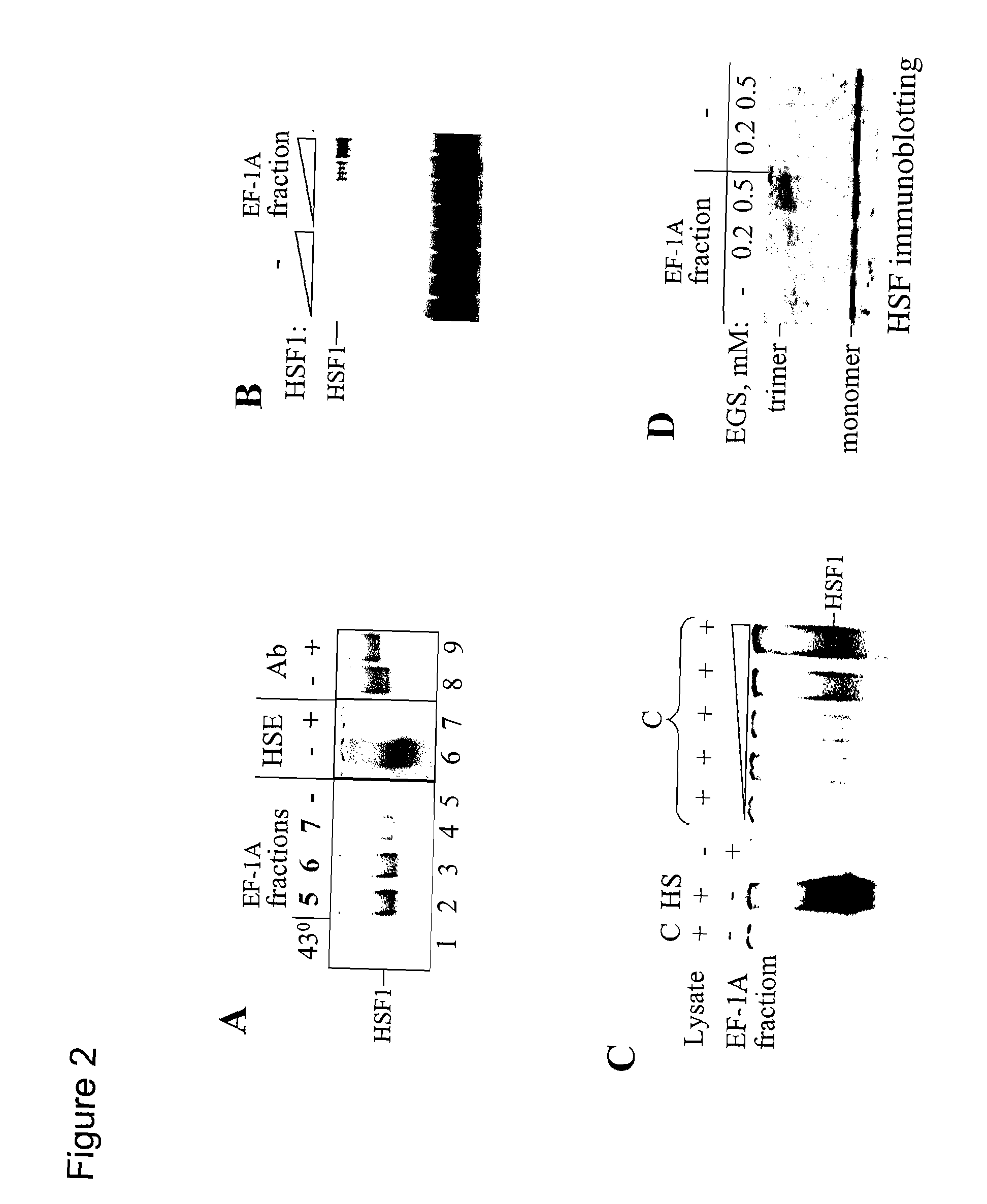
Heat shock RNA and its use in activation of heat shock transcription factor and treatment of cancer, inflammation, ischemia, neurodegeneration, age-related diseases, HIV infection, deafness, and related disorders
InactiveUS20070238682A1Good effectHigh sensitivityOrganic active ingredientsSugar derivativesTotal DeafnessAge related disease
The present invention provides a novel RNA, designated herein as the “HSR1” (Heat Shock RNA), and its use together with translation elongation factor eEF1A in activation of heat shock transcription factor HSF. The invention further provides the use of HSR1 for generation of novel therapeutics for the treatment of cancer, inflammation, ischemia, neurodegeneration, age-related diseases, HIV infection, deafness, and related disorders.
Owner:NEW YORK UNIV
Deafness susceptibility gene screen test kit
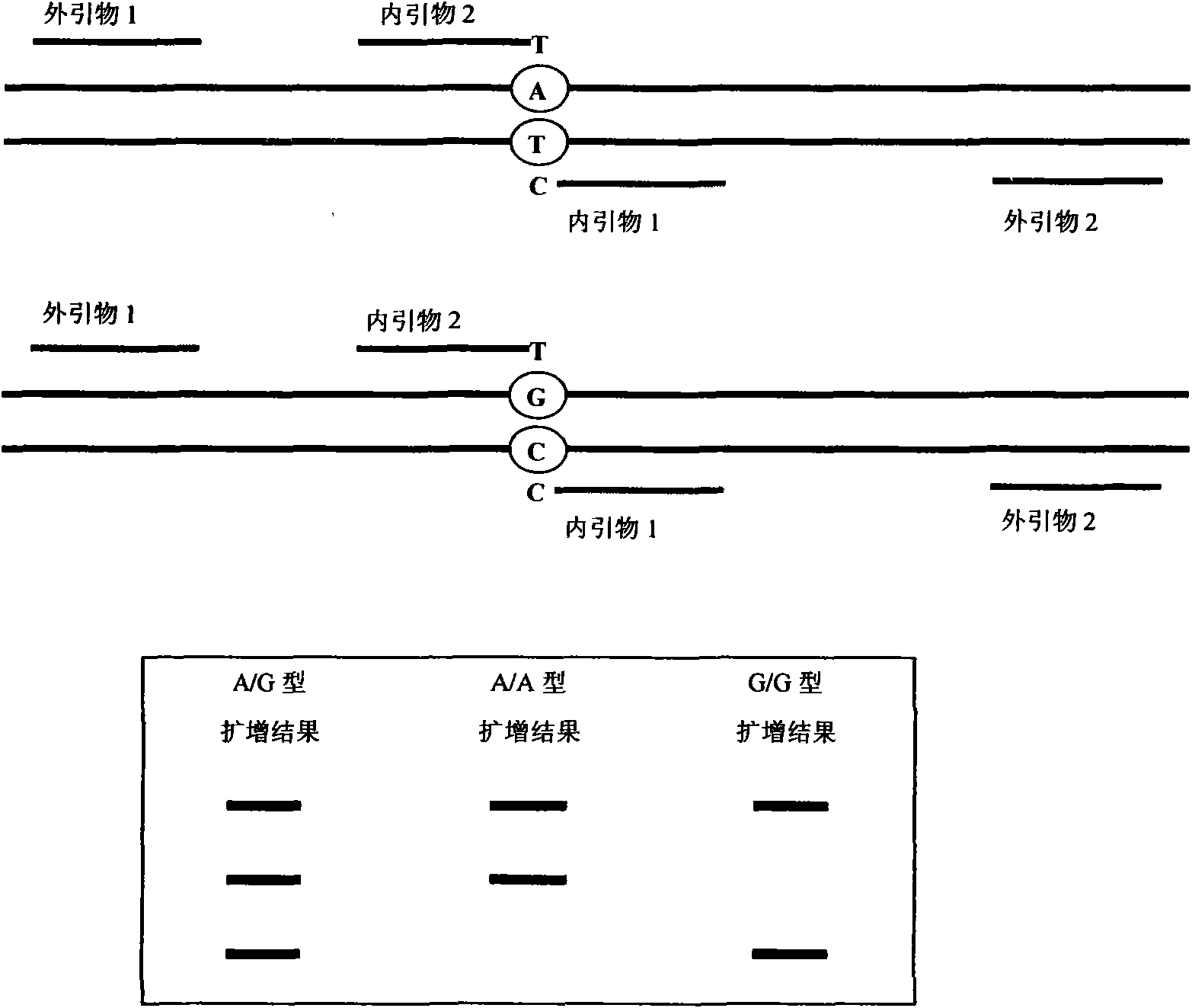
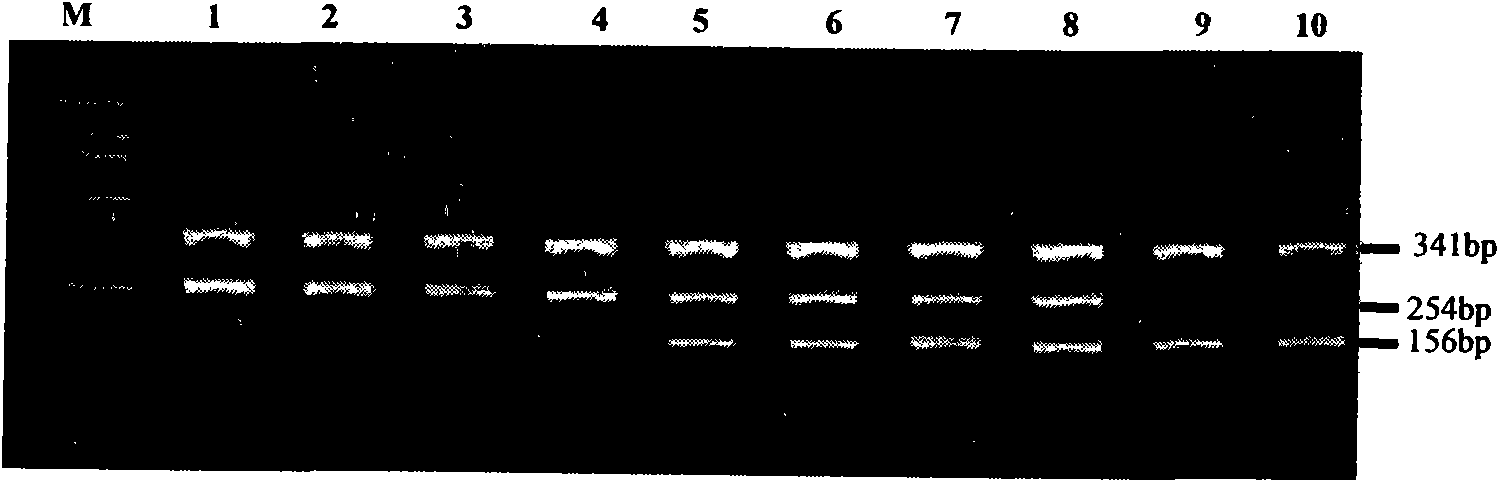
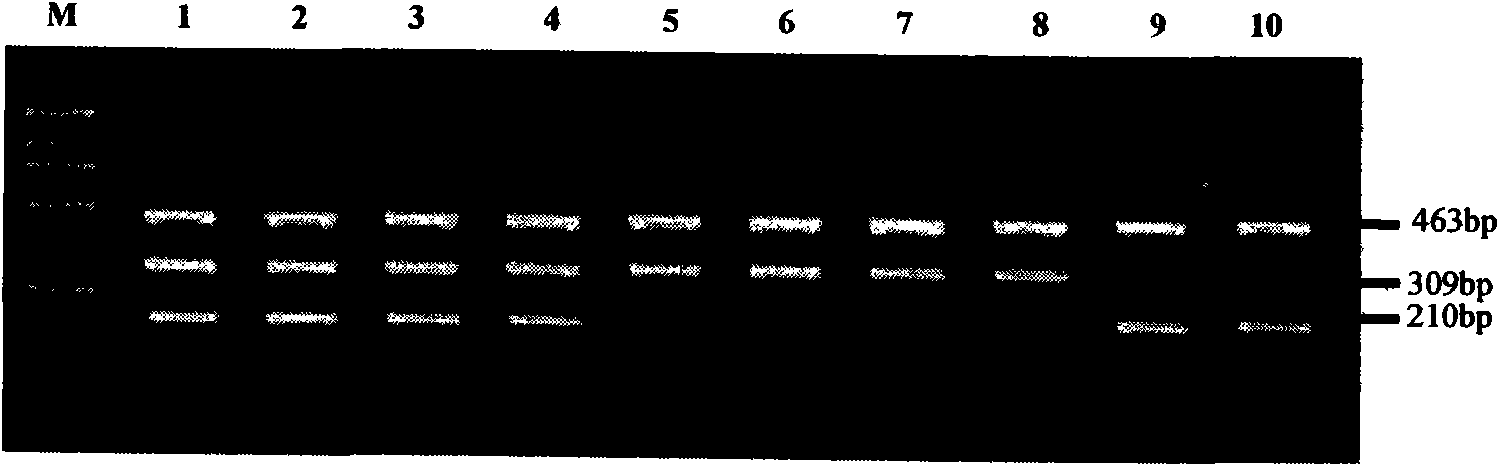
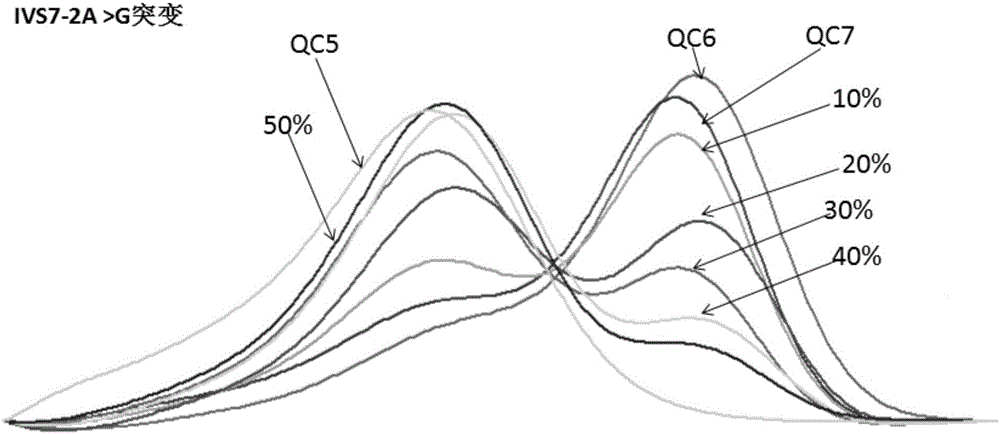
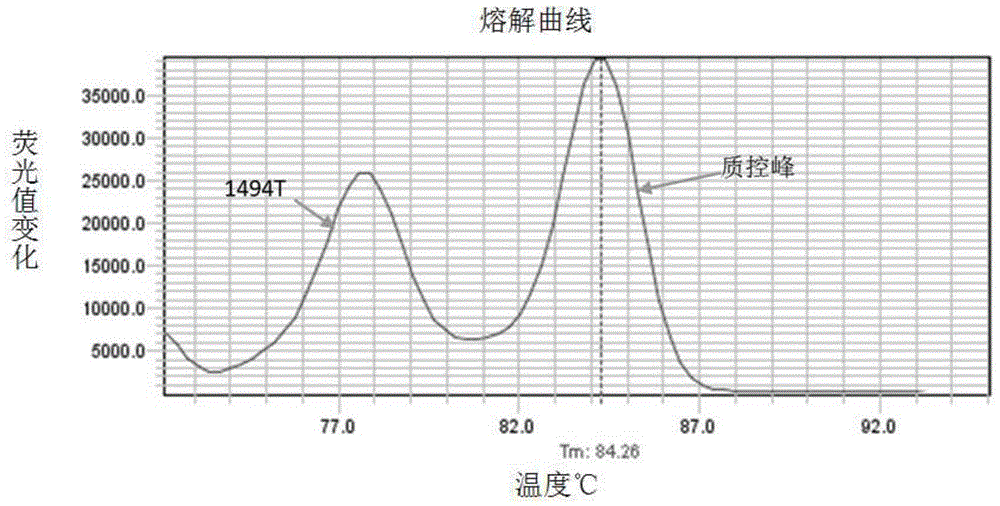
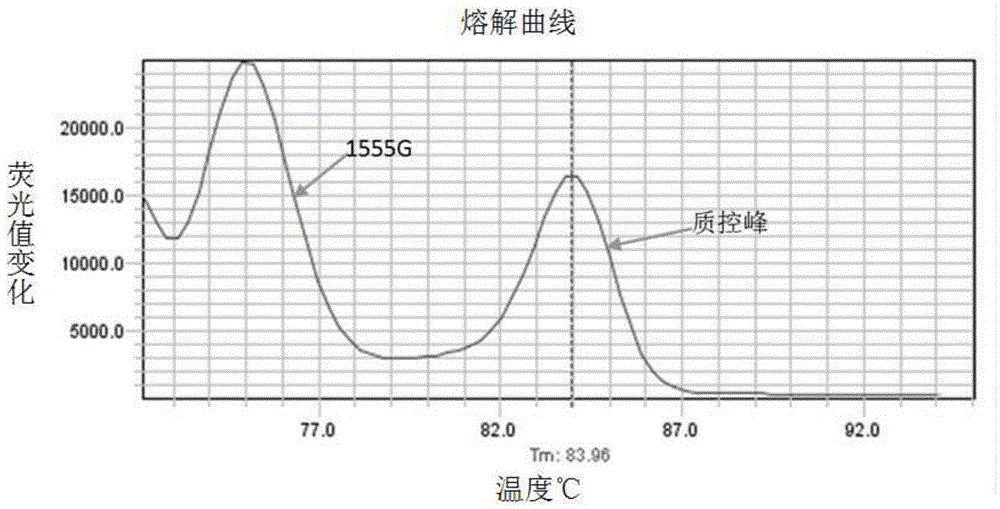
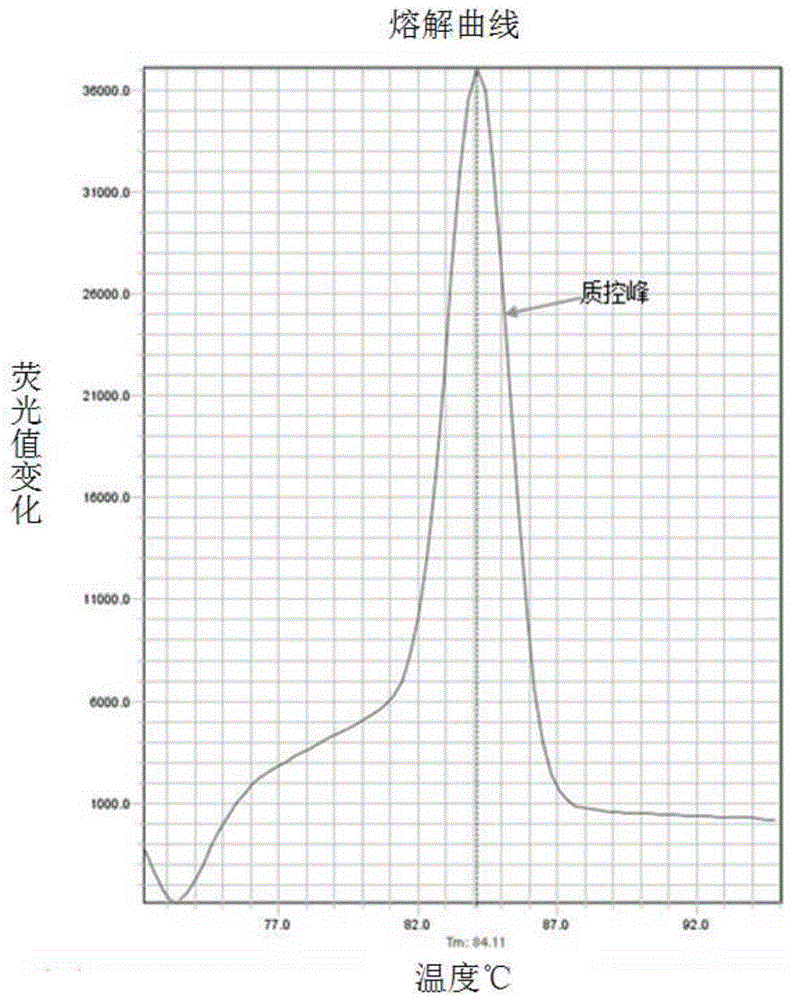
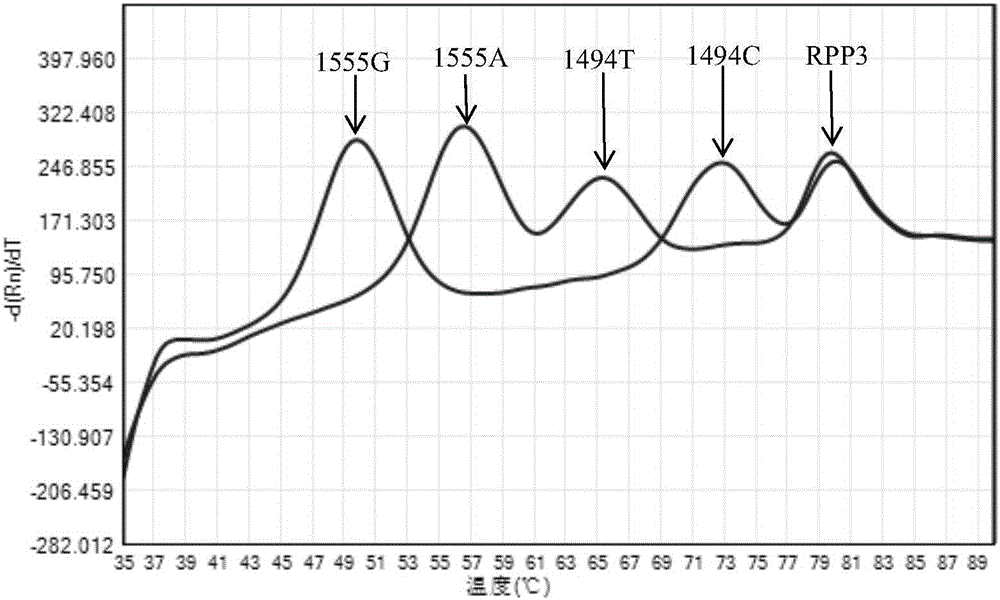
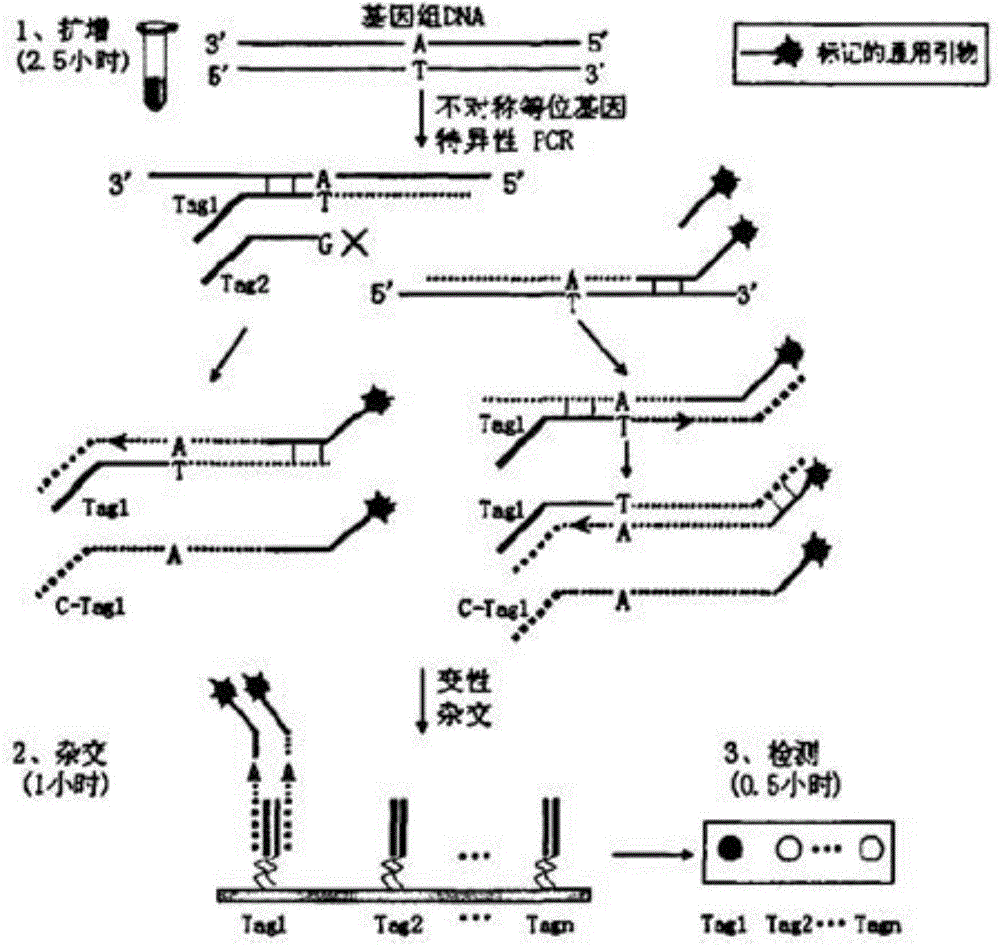
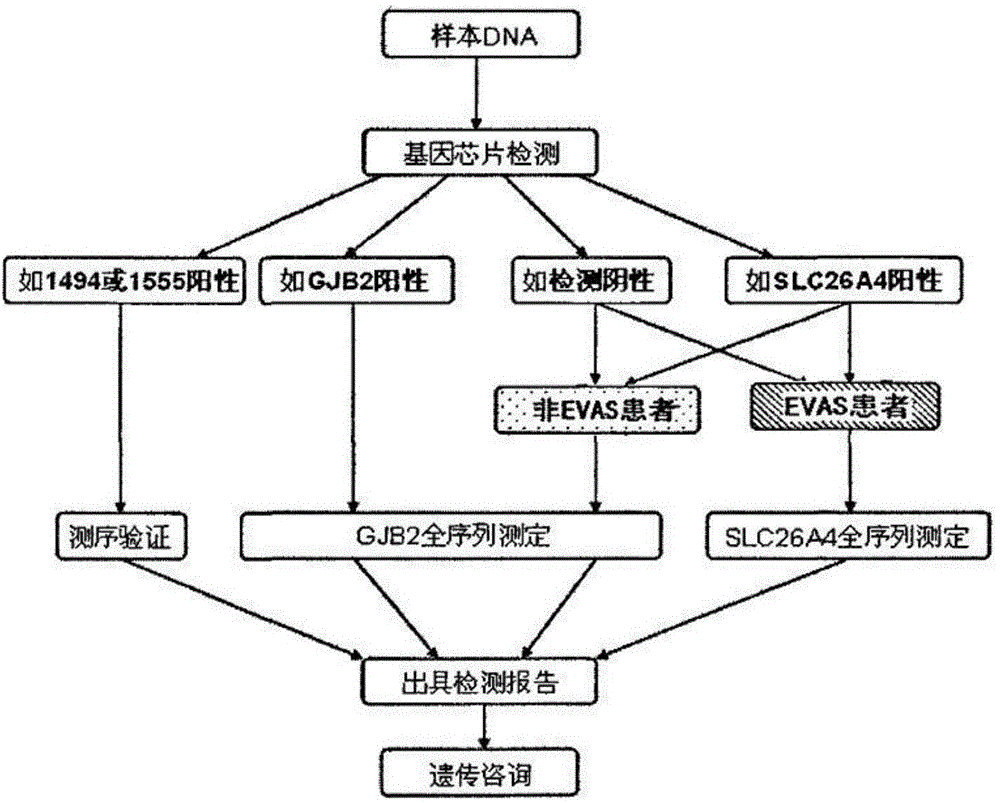
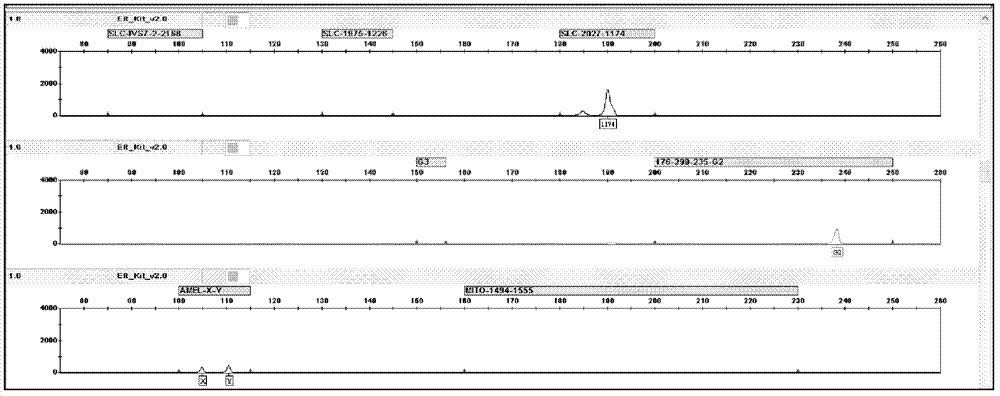
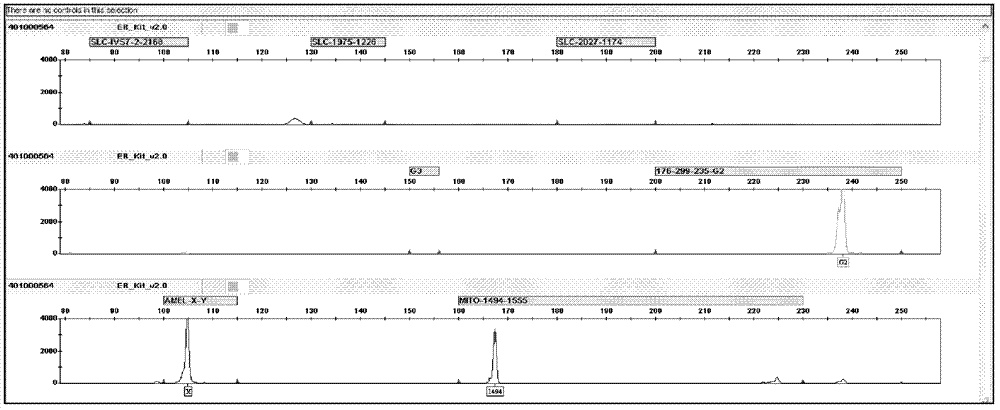
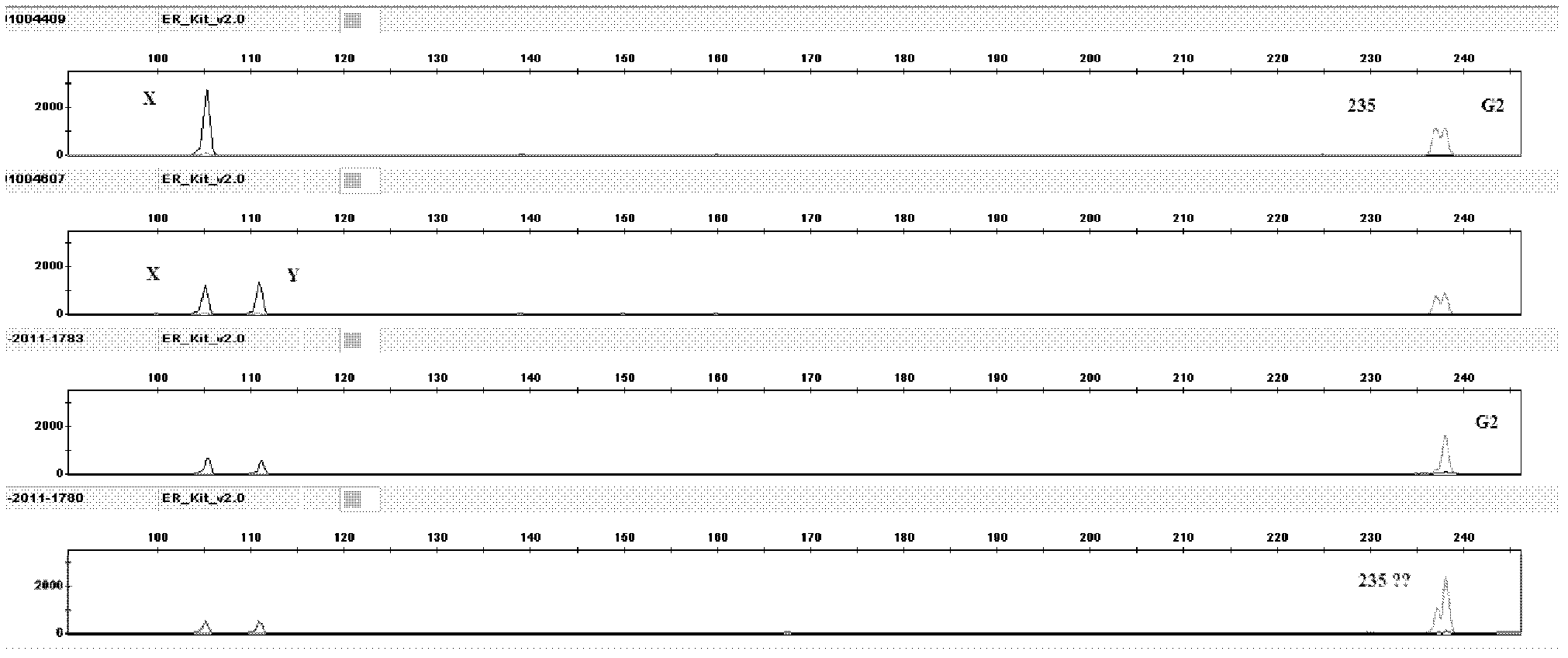
The invention relates to a hereditable deafness susceptibility gene screen test kit. The kit uses mutation from C to T of 1,494 locus of a 12s rRNA gene, mutation from A to G of 1,555 locus of the 12srRNA gene, mutation from A to G of IVS7(-2) locus of an SLC26A4 gene and C(+ / -) of 235 locus of a GJB2 gene as detecting objects, designs and optimizes a set of specific primers respectively againsteach locus to be tested according to the PCR technical principle of a tetra-primer amplification refractory mutation system, amplifies the whole set of the primers in the same reaction tube, and performs primary multiple PCR amplification and primary gel electrophoresis on the four reaction tubes to obtain gene types of four loci simultaneously.
Owner:GENERAL HOSPITAL OF PLA
High-specificity kit for detecting deafness predisposing genes
InactiveCN102534031AAvoid false positivesMicrobiological testing/measurementTotal DeafnessFluorescence
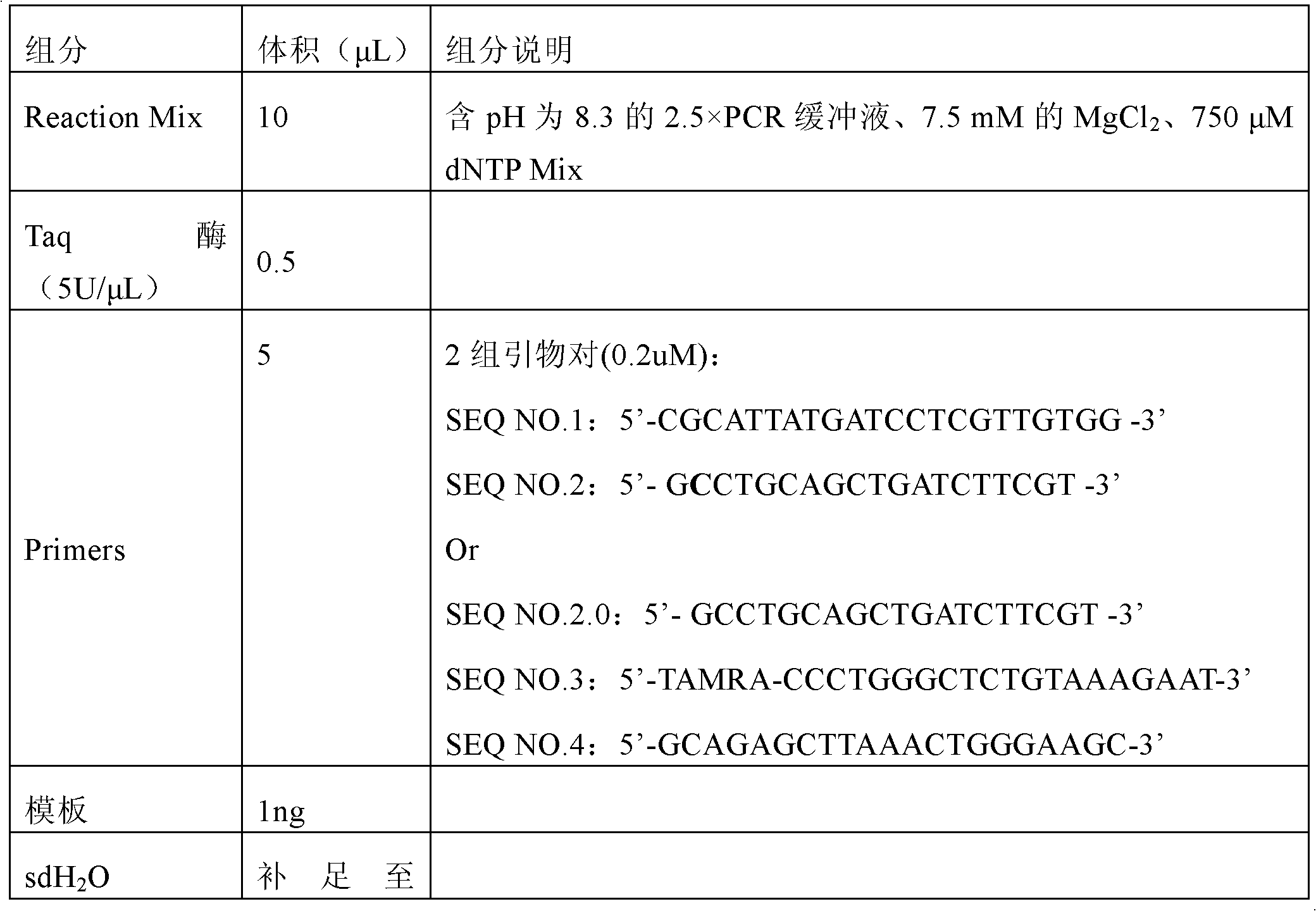
The invention discloses a fluorescent detection kit for detecting 12 deafness predisposing genes simultaneously. The kit can detect 12 mutational hotspots in the most common deafness associated genes of the Chinese in 3 hours. The kit comprises reagents before amplification and reagents after amplification, wherein the reagents before amplification comprise a polymerase chain reaction (PCR) buffer solution, a reaction mixture of MgCl2 and deoxyribonucleoside triphosphates (DNTPs), Taq DNA polymerase, ultrapure water, and a primer mixture for amplifying loci of detection sites at high specificity; and the reagents after amplification comprise a genotyping standard and an internal standard. Deafness gene loci are simultaneously detected at high sensitivity and high specificity by combining a fluorescent labeling technology, a linolenic acid (LNA) nucleoside monomer doping-primer modification technology and a capillary electrophoresis technology for the first time, manpower and material resources and time are greatly saved, and pollution due to multi-step operation is prevented.
Owner:万戈江
Deafness susceptive gene joint detection kit
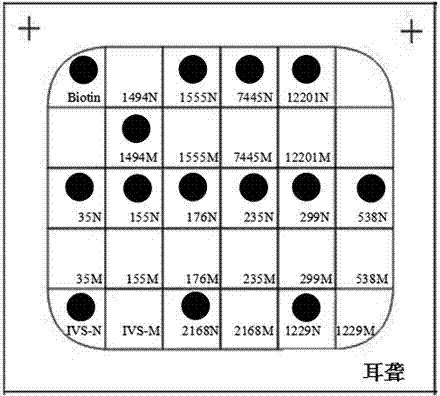
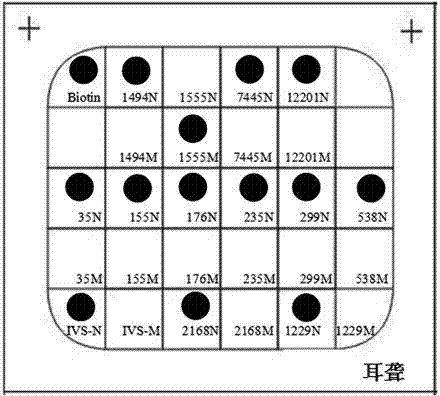
ActiveCN102864232AHigh detection specificityLow costNucleotide librariesMicrobiological testing/measurementTotal DeafnessBiotin
The invention discloses a deafness susceptive gene joint detection kit which comprises (1) a gene chip and (2) various primers, wherein the gene chip is provided with (i) nucleotide sequence probes and (ii) a DNA (deoxyribonucleic acid) sequence marked with biotin sites; the nucleotide sequence probes are at least one of sequences shown as SEQ ID Nos:1-26 and one of sequences complementary with the sequences shown as SEQ ID Nos:1-26; the DNA sequence marked with the biotin sites is as shown in SEQ ID No.27; and the primers are respectively shown as SEQ ID No.28-29, SEQ ID No.30-31, SEQ ID No.32-37 and SEQ ID No.38-43. The kit disclosed by the invention can realize synchronous joint detection, enhance the detection specificity, reduce the cost and shorten the detection time.
Owner:潮州凯普生物化学有限公司 +2
Kit for detecting deafness susceptibility genes
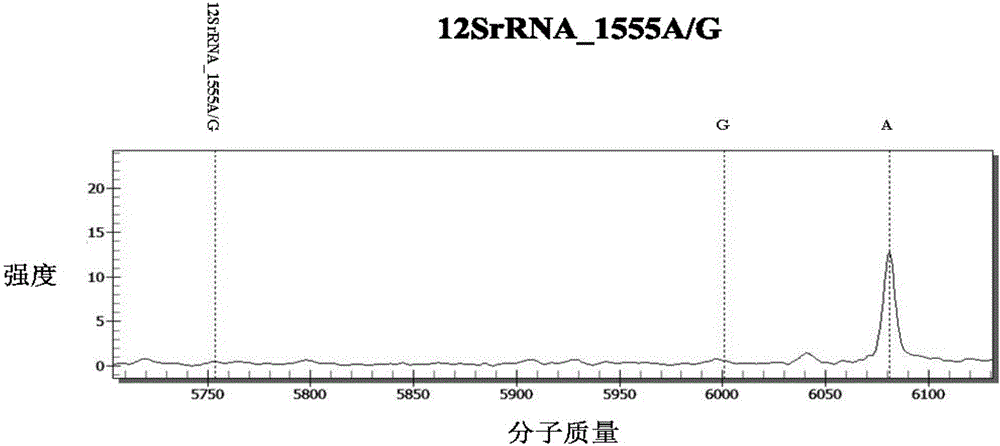
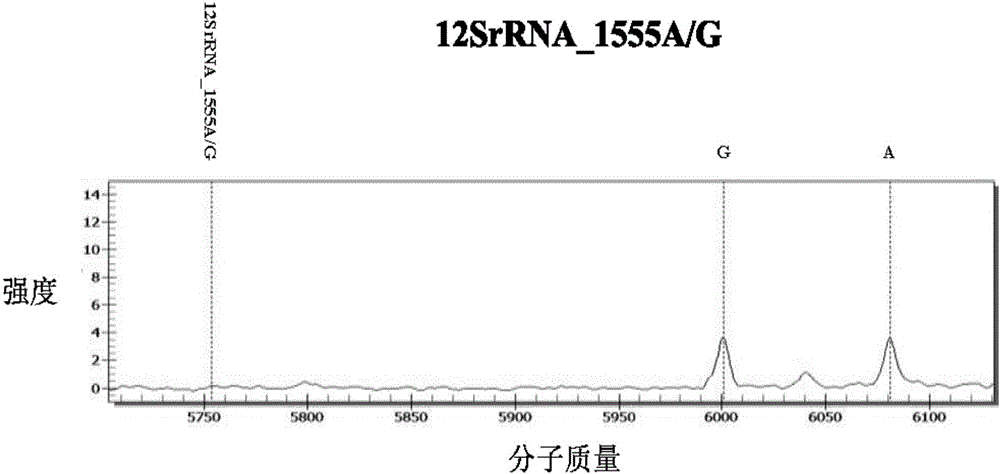
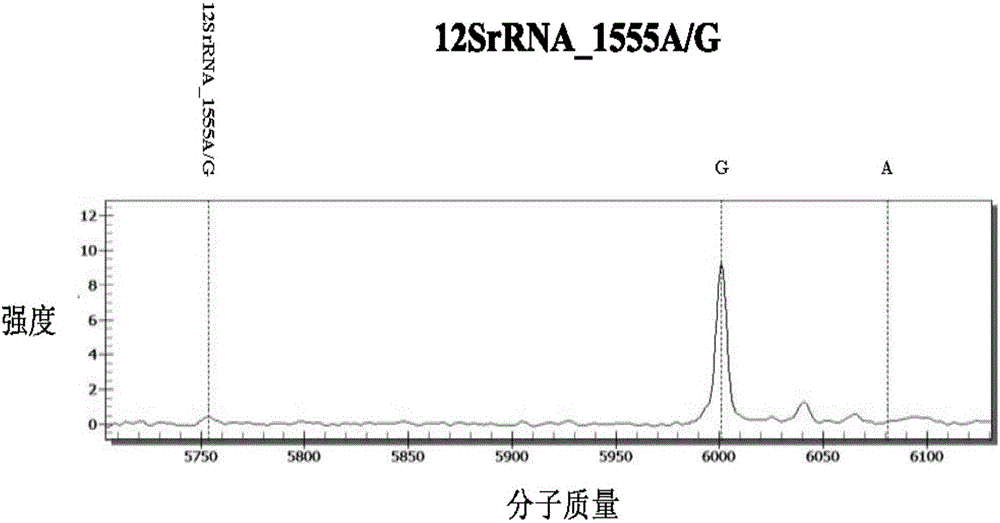
InactiveCN106367491AReduce usageReduce lossMicrobiological testing/measurementTotal DeafnessMass Spectrometry-Mass Spectrometry
The invention discloses a kit for detecting deafness susceptibility genes, namely the invention provides a kit for rapidly and accurately detecting the GJB2, SLC26A4 and 12SrRNA deafness susceptibility genes by virtue of an MALDI-TOF mass-spectrometry technique. The kit mainly consists of the following ingredients: PCR amplification products shown as SEQ ID NO:1-SEQ ID NO:32 and mass-spectrometry extension primers shown as SEQ ID NO:33-SEQ ID NO:48. The kit provided by the invention, by combining the mass-spectrometry technique and a multi-primer extension technique, is relatively simple in adopted reagent consumables, low in cost and stable, and dozens to thousands of samples are analyzed in one reaction, so that loss caused by reagent preparation is reduced; and the kit is quite high in sensitivity, accuracy and detection consistency, and detection throughput is improved.
Owner:DALIAN GENTALKER BIO-TECH CO LTD
Computer assisted training method for mouth shape recognition capability
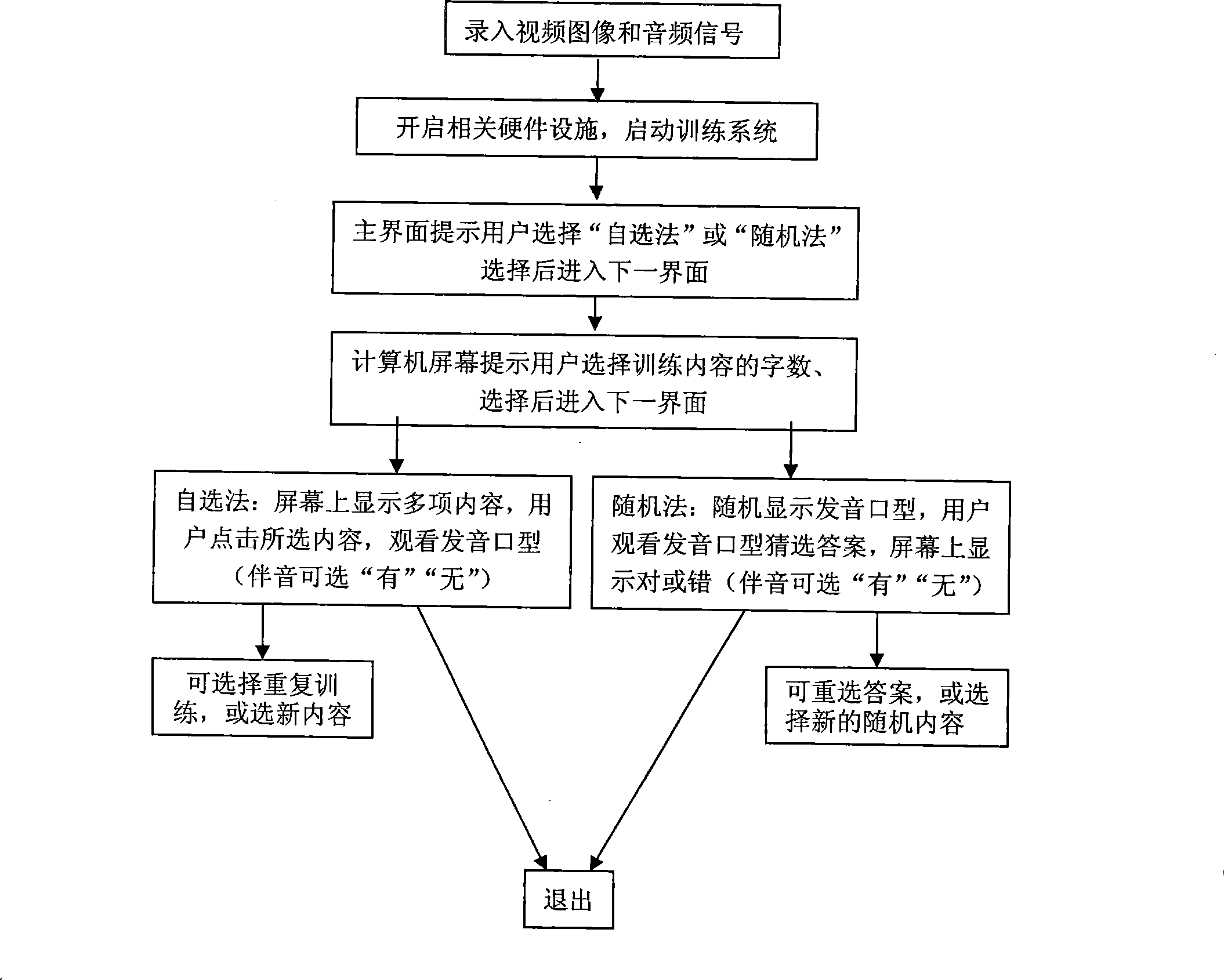
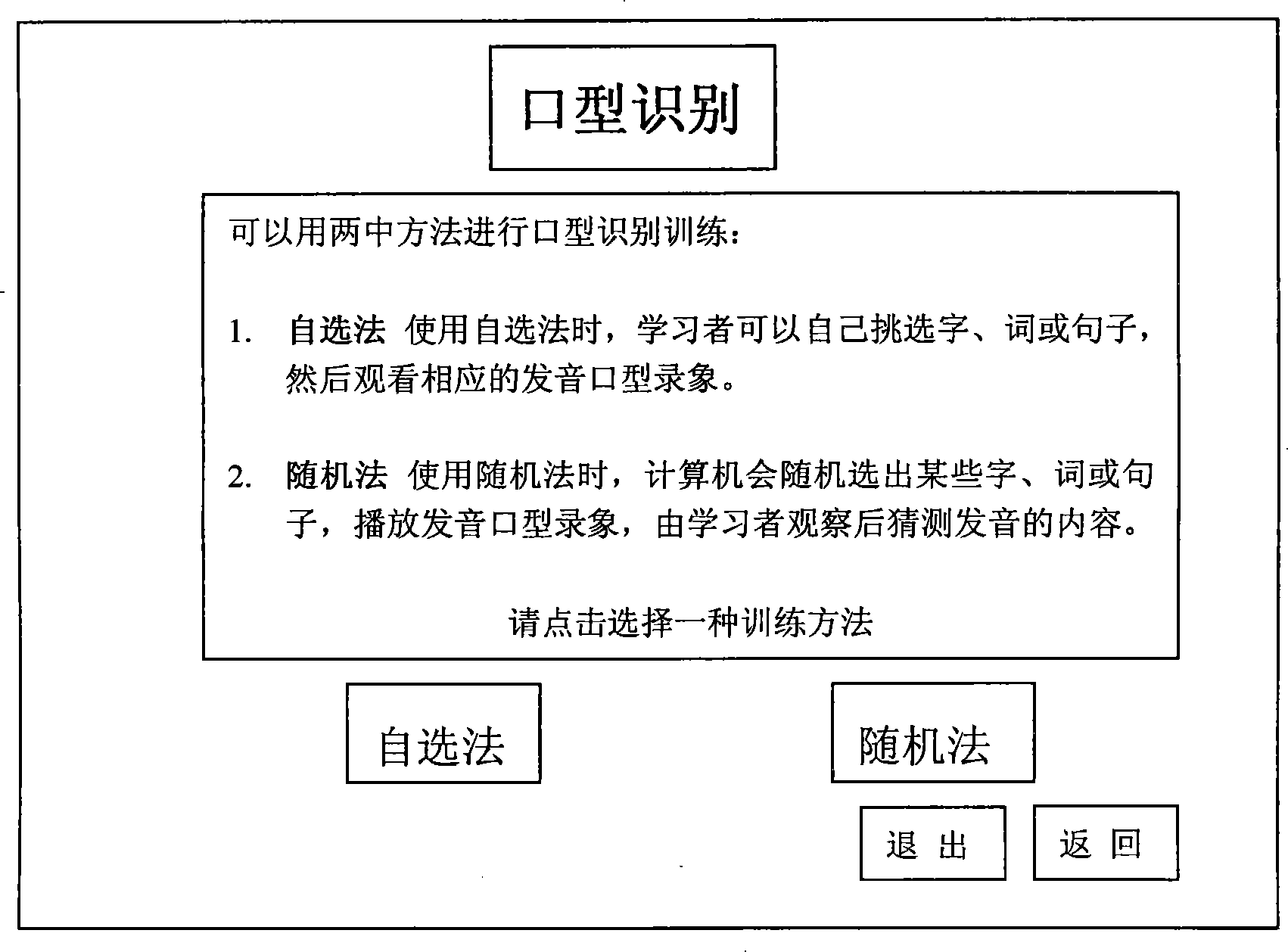
InactiveCN101241656AShorten the timeImprove survivabilityElectrical appliancesSpecial data processing applicationsTotal DeafnessSurvivability
The present invention discloses a computer aided training method for mouth-shape recognition ability which solves the technique problem of increasing training efficiency, saving time of teacher and parents. The method includes steps as follows: the user can select self-selection method or random method, when self-selection method is selected, the user watches pronouncing mouth-shape video and simulates pronouncing mouth-shape action and pronunciation; when random method is selected, computer plays pronouncing mouth-shape video, and interface displays letter, the user judges and computer gives correct answer. By comparison with present technique, the method can make computer replacing teacher or parents for training mouth-shape recognition ability by using computer multimedia function, so the method can save time of teacher or parents and has active action for increasing viability of deaf and dumb people and other hearing impaired people. The method is especially suitable for training mouth-shape recognition ability of hearing impaired people, such as deaf and dumb people, half-deafness people.
Owner:黄中伟 +3
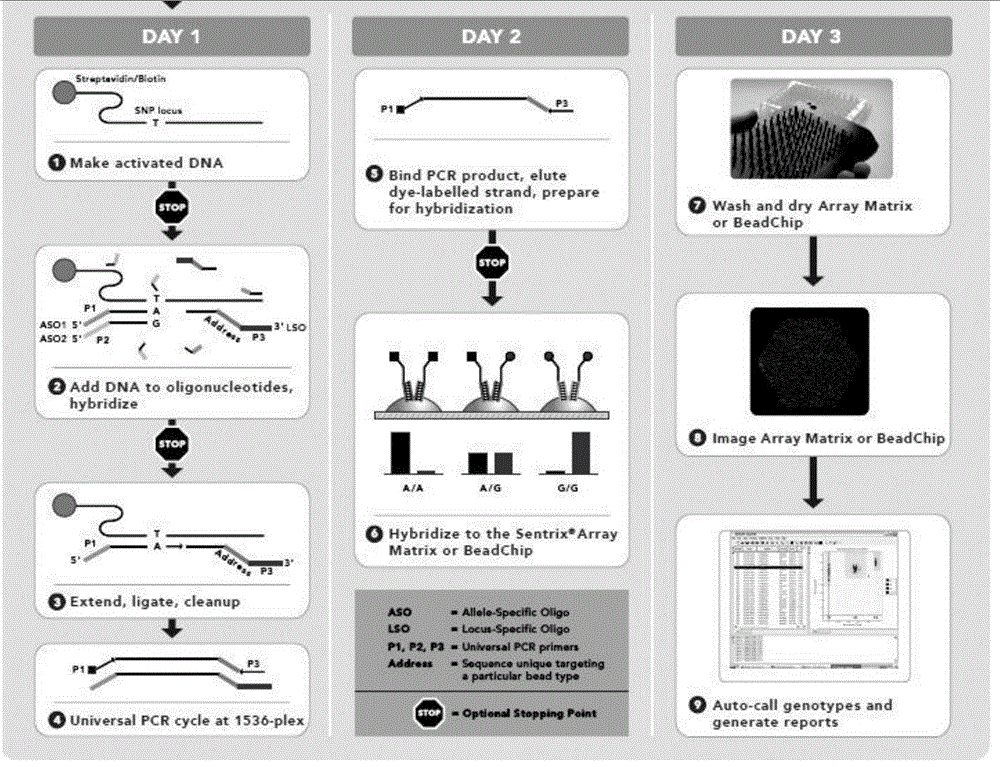
Microarray-based diagnosis of pediatric hearing impairment-construction of a deafness gene chip
InactiveUS20050112598A1Bioreactor/fermenter combinationsBiological substance pretreatmentsTotal DeafnessCandidate Gene Association Study
The present invention is related to diagnostic arrays comprising primers for various regions of candidate genes involved in hearing loss, specifically pediatric hearing loss. The invention further is directed to methods for diagnosing a cause or risk factor for hearing loss. In some embodiments, these methods include obtaining a sample from a patient; screening the sample for the presence or absence of alleles of at least 5 loci associated with a risk for hearing loss to obtain a result of the screening; and making a diagnosis based upon the result. The present invention is also directed to the amplification of genetic sequence from multiple or single exons for use in the screening of samples.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
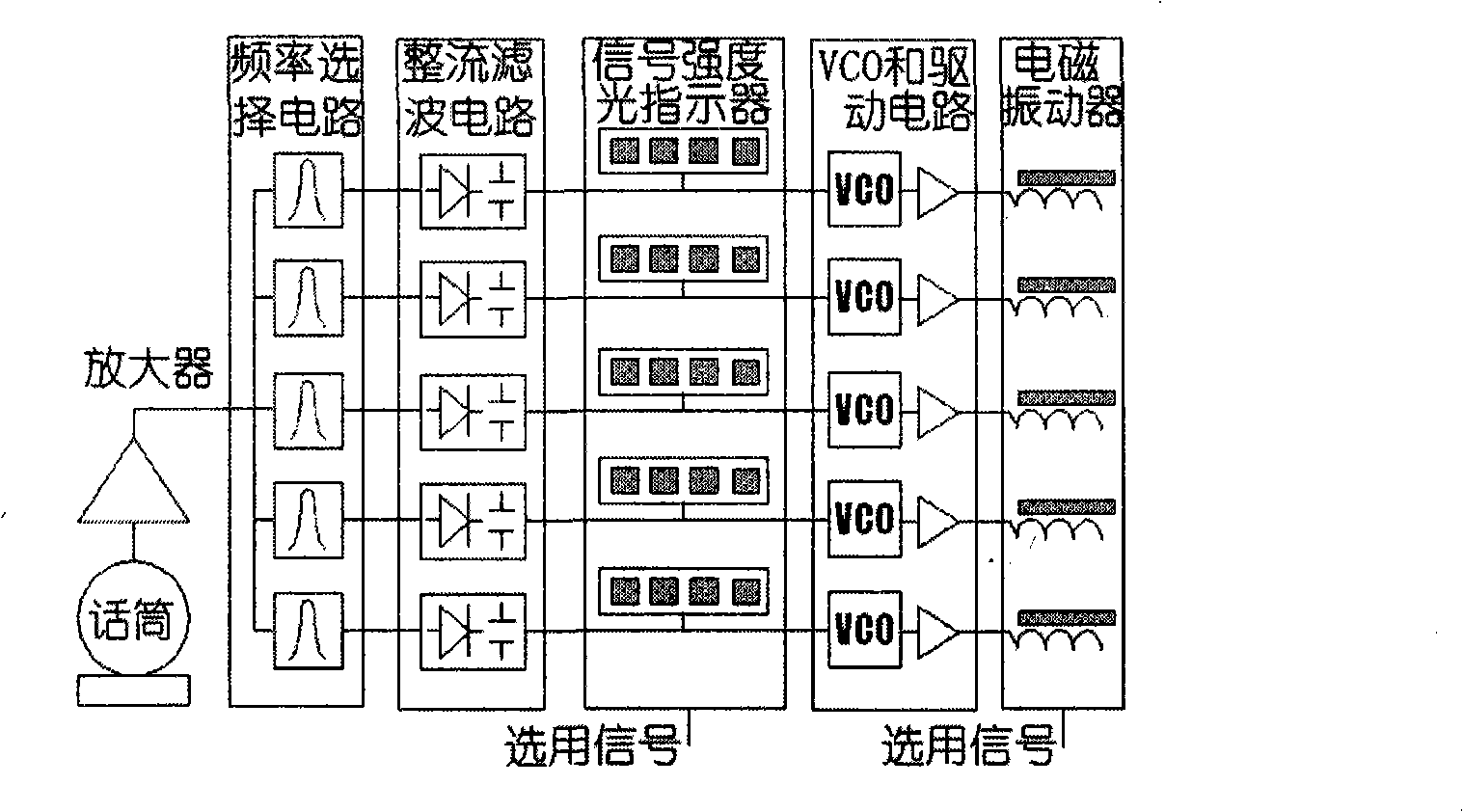
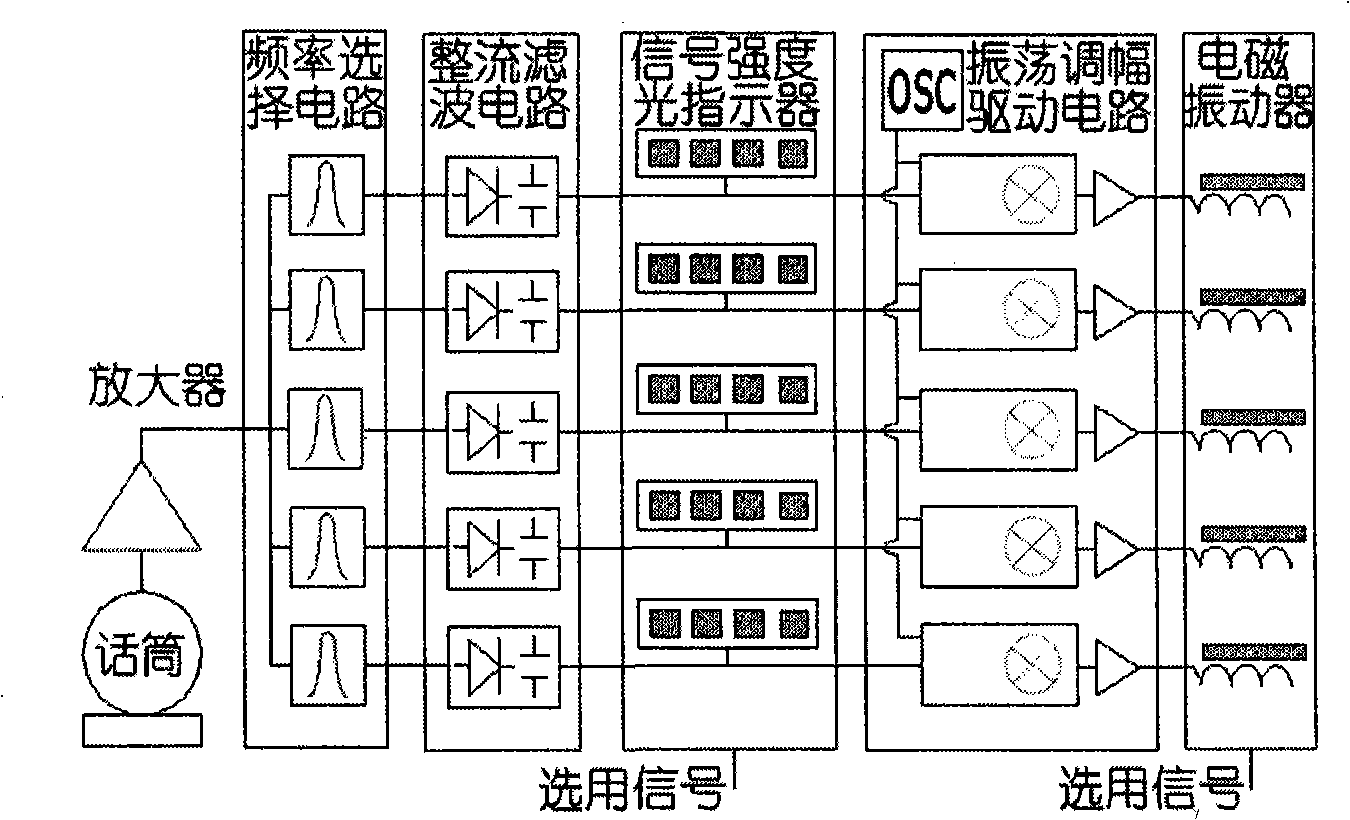
Instrument for distinguishing sound of deaf
InactiveCN101347368AEasy to understandImprove pronunciation and expressive abilityEar treatmentSpeech recognitionTotal DeafnessVibration amplitude
The invention relates to a deaf sound discerning apparatus. The apparatus adopts a microphone to test sound and amplifies the sound in the vicinity of a plurality of frequency points for respective sampling and testing and a plurality of intensity signals are acquired. The intensity signals are used for modulating the vibration amplitude or the frequency of a plurality of electromagnetic vibrators and the signal intensity of an optical display correspondingly. The value of signals in the vicinity of the frequency points are obtained based on the amplitude or the frequency and the light intensity, namely, the frequency and the value data of a sound signal are obtained by sampling so as to achieve the effects of discerning different sounds and the meanings of the sounds to a certain degree. The deaf sound discerning apparatus, lip-reading, dummy statement and a hearing aid used by a person of partial deafness can improve effects mutually. The deaf sound discerning apparatus can be used for communication function training for the deaf. The deaf can dance, and perceive calling and vehicle horn, and the like, by the apparatus. People who can not speak because of deafness can compare own pronunciations with the pronunciations of normal people by the apparatus and lip-reading, thus improving pronunciation and expression abilities. The inherent directivity of the microphone can be used for discerning the directions of the continuous and the discontinuous sounds. The sound discerning apparatus also can be used for discerning ultrasonic waves, such as animal language, and the like, by changing the testing frequency.
Owner:苏重清 +2
System and method for preserving neuronal survival and plasticity of the auditory system prior to permanent intra-cochlear implantation
InactiveUS20070282395A1Simple signalImprove survival rateElectrotherapyArtificial respirationTotal DeafnessOlder child
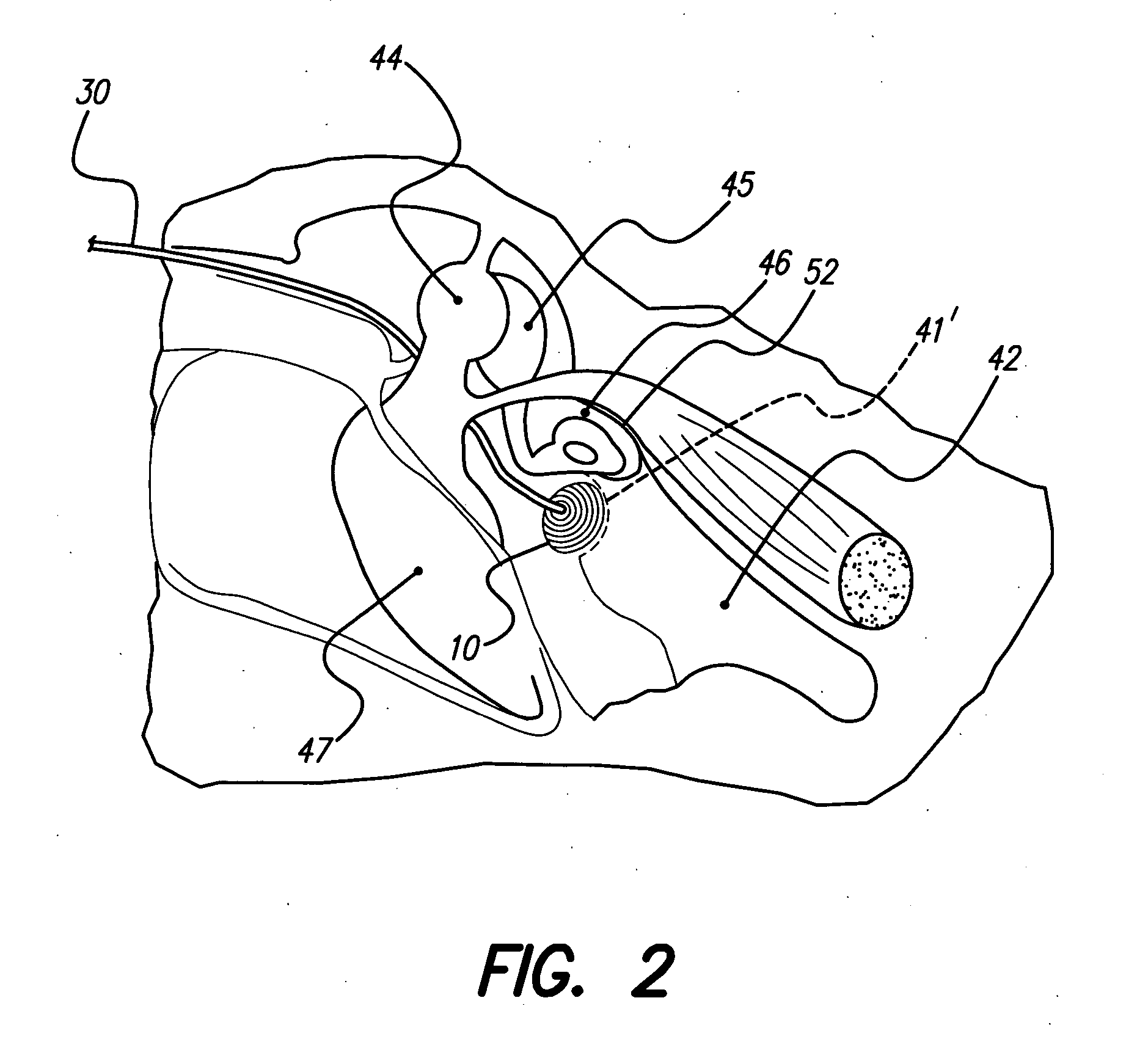
A system and method uses an electrical stimulator to stimulate the auditory system with a relatively simple signal that contains temporally challenging information in order to preserve neuronal survival and plasticity of the auditory system, and also to preserve residual hearing. The stimulation provided need not be continuous, but may be provided only during limited periods of time each day, or only on selected days. The system or method is particularly suited for very young children who acquire hearing impairment or deafness early in life and who may not yet be ready for a cochlear implant. The invention requires only minimal surgical intervention, if any, and may be carried out without the need for intra-cochlear electrodes. Under special circumstances, the invention may also be used with older children or adults with a hearing impairment or deafness.
Owner:BOSTON SCI NEUROMODULATION CORP
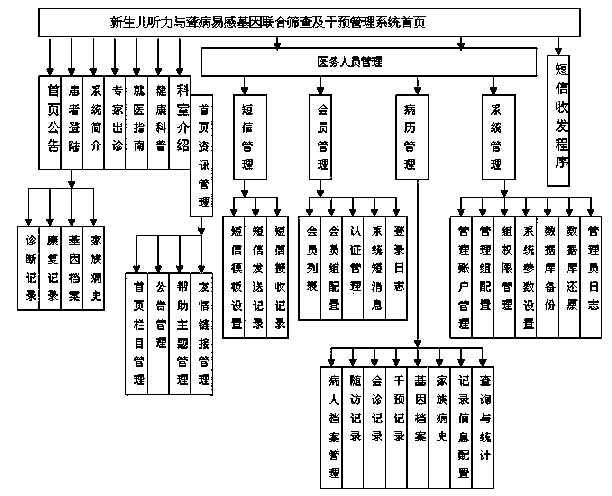
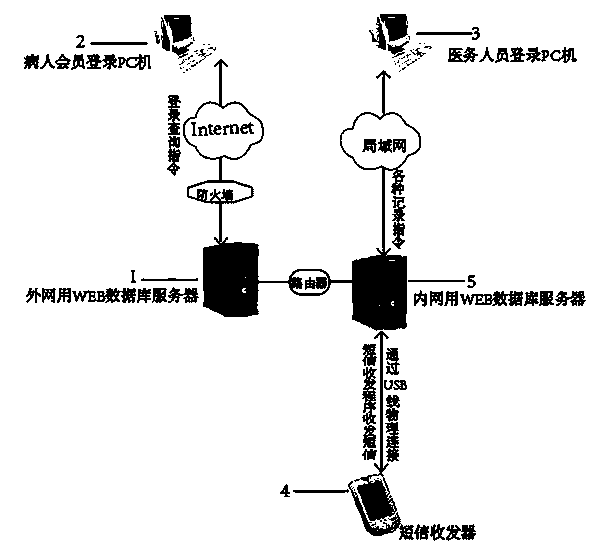
Joint screening and intervention managing system for newborn hearing and deafness susceptibility gene
InactiveCN104331774AConvenient queryEnsure data securityAudiometeringResourcesTotal DeafnessMedical record
The invention relates to a joint screening and intervention managing system for newborn hearing and deafness susceptibility gene, and the joint screening and intervention managing system comprises two parts: the software system platform, comprising a home page information management module, a short message management module, a member management module, a medical record management module, a system management module and a short message receiving and sending program; a hardware network platform. The patient can login the PC machine for obtaining the screening result and the information related to the screening result for exchanging and consulting with the medical staff; the medical staff can login the PC machine for managing the joint screening and intervention information for newborn hearing and deafness susceptibility gene. The newborn parents can login the machine the through the Internet to obtain the related screening diagnosis result and precautionary measure record in the hospital; the medical staff can login the system for building the record for the patient for automatically reminding the medical staff for visiting the patient beforehand and automatically sending the short message for reminding the parents to come to visit with children.
Owner:NANNING SECOND PEOPLES HOSPITAL
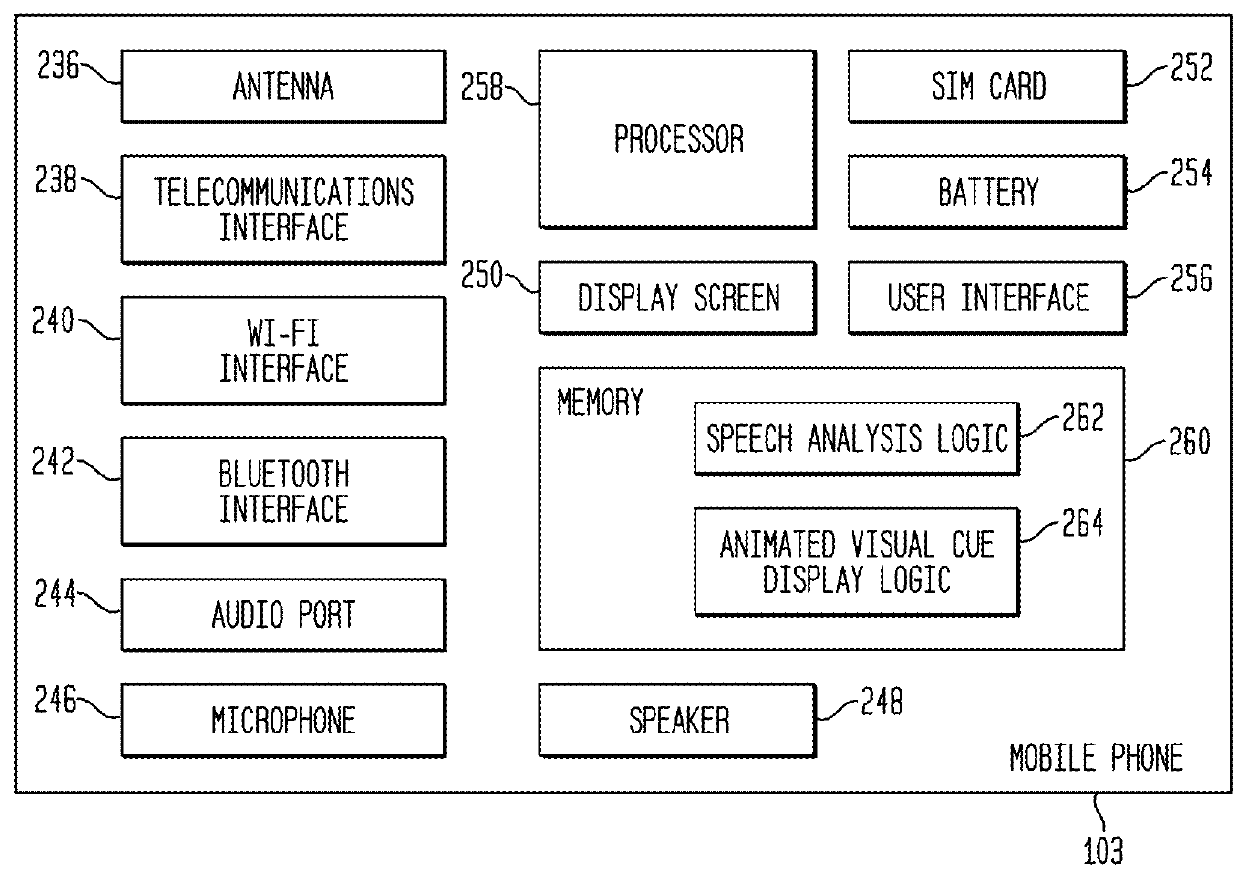
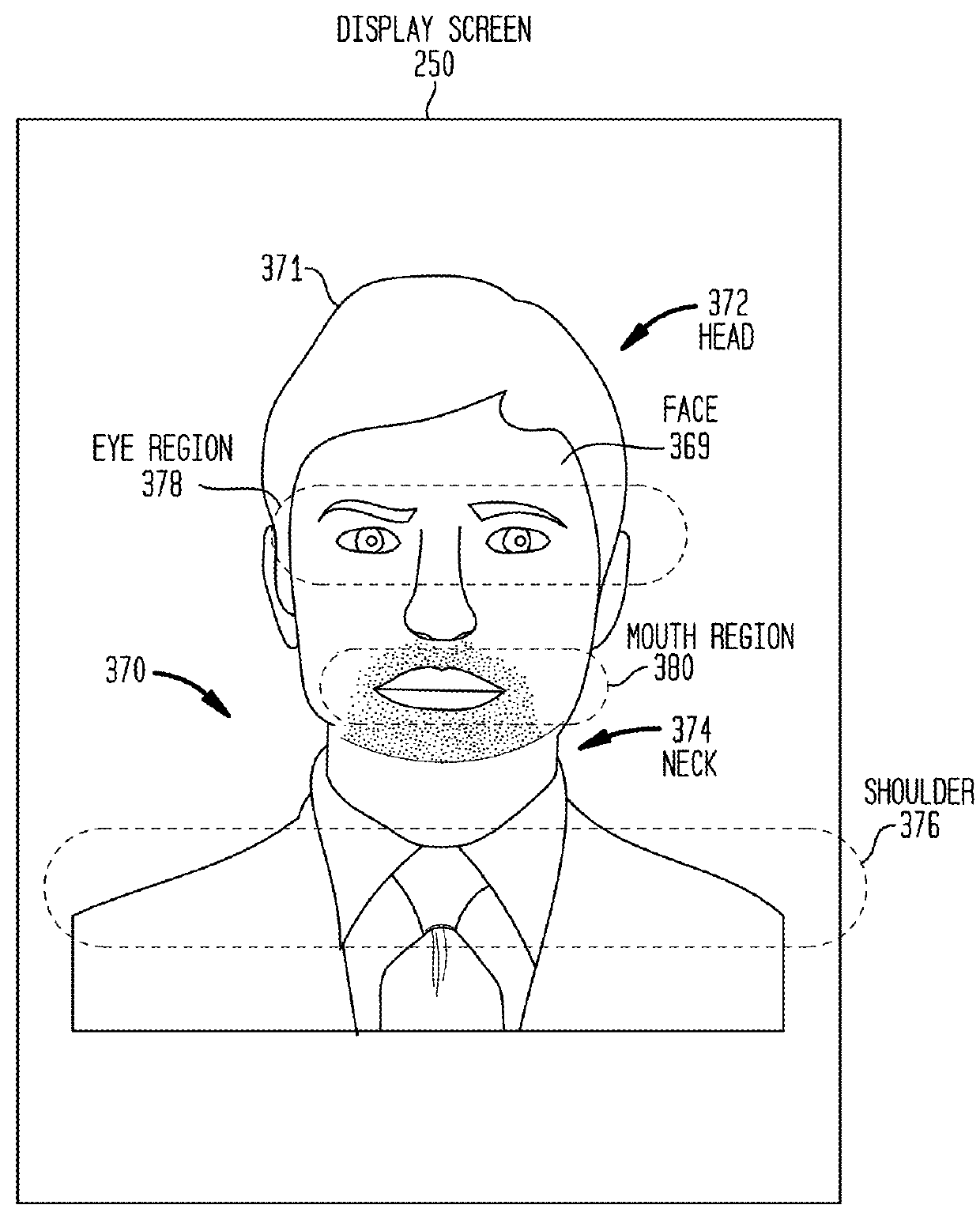
Tonal Deafness Compensation in an Auditory Prosthesis System
ActiveUS20160030744A1Reduced tonal perceptionReduce perceptionHead electrodesSpeech analysisTotal DeafnessPattern perception
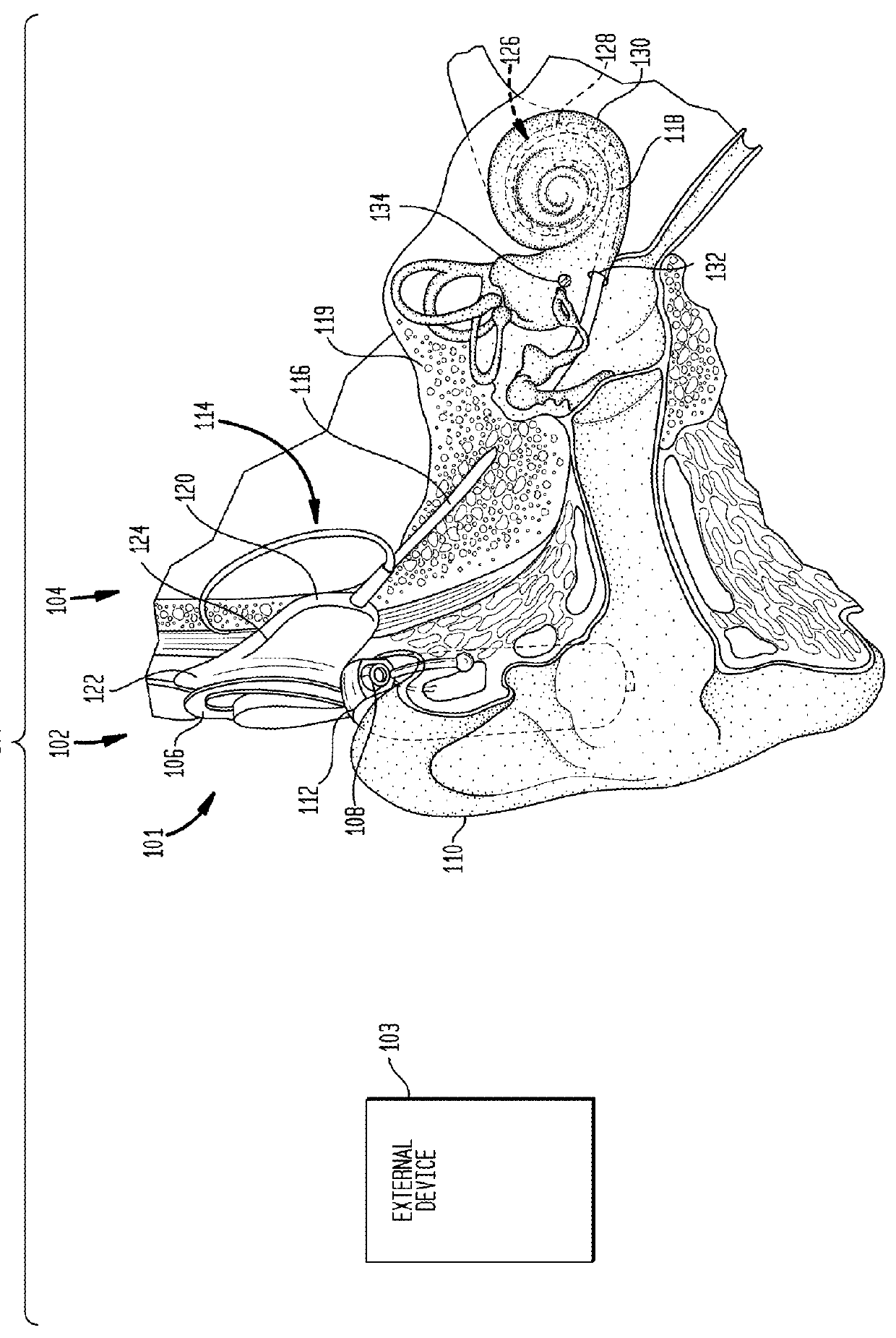
Embodiments presented herein are generally directed to techniques for compensating for tonal deafness experienced by a recipient of an auditory prosthesis. More specifically, an auditory prosthesis system includes an external device configured to generate a graphical representation that enables the recipient to compensate for reduced tonal perception associated with delivery of the stimulation signals representative of speech signals. The external device is configured to analyze received speech signals to determine vocal articulator movement of the speaker of the speech signals and / or emotion of the speaker. The external device is further configured to display one or more animated visual cues representative of the detected vocal articulator movement and / or emotion.
Owner:COCHLEAR LIMITED
HRM method and kit for clinically detecting deafness-related gene mutation
InactiveCN106086186AReduce dosageHigh detection sensitivityMicrobiological testing/measurementTotal DeafnessFluorescence
The invention discloses an HRM method and kit for clinically detecting deafness-related gene mutation. The HRM method comprises the steps that PCR amplification and HRM scan analysis are conducted by extracting DNA of a sample to be detected and applying primers shown in SEQ ID NO.1-30; the deafness-related gene mutation type is judged according to a scan analysis result, a sample free of mutation peaks in HRM scan typing is negative, and a sample with the mutation peaks in HRM scan typing is positive. The kit comprises the primers, quality control products, a PCR reagent, fluorescent dyes and the like; the kit has the advantages that all the primers in the kit can conduct a PCR reaction at the same temperature, the detection sensitivity and the specificity are high, a small reaction system is achieved, the detection sample source is rich, reacting is easy, convenient and rapid, the reagent cost is low, and high throughput is achieved. The HRM method and kit are suitable for clinical neonatal hereditary deafness gene screening and pre-pregnancy deafness gene screening for good prenatal and postnatal care and beneficial for guiding good prenatal and postnatal care and clinical personalized medication.
Owner:WUHAN UNIV
Kit and primer pair group for detecting drug-induced deafness susceptibility gene mutation site
InactiveCN104962637AAdvantages of detection technologyStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationTotal DeafnessFluorescence
The invention relates to the field of gene detection, in particular to a kit and a primer pair group for detecting drug-induced deafness susceptibility gene mutation sites, wherein the kit and the primer pair group can be used for detecting aminoglycoside drug susceptibility genes in mitochondria. A specific primer amplification and real-time fluorescence PCR technology is adopted, a new primer pair group is designed aiming at mutant nucleic acid to be detected, the primer is extended to product a specific PCR product when the mutant nucleic acid exists, a fluorescent substance is added into each reaction system, the fluorescent substance is bonded to the PCR product after amplification is completed, the produced melting peak is analyzed by adopting a melting curve, whether a corresponding mutant type exists on the nucleic acid or not is judged through the existence of the specific melting peak, and the specificity is high; the detection of two mutation sites can be completed by using a single tube and a single channel only, the requirements on instruments are low and the operation is simple to perform.
Owner:智海生物工程(北京)股份有限公司
Method and a kit for non-invasively detecting fetal deafness pathogenic gene mutations
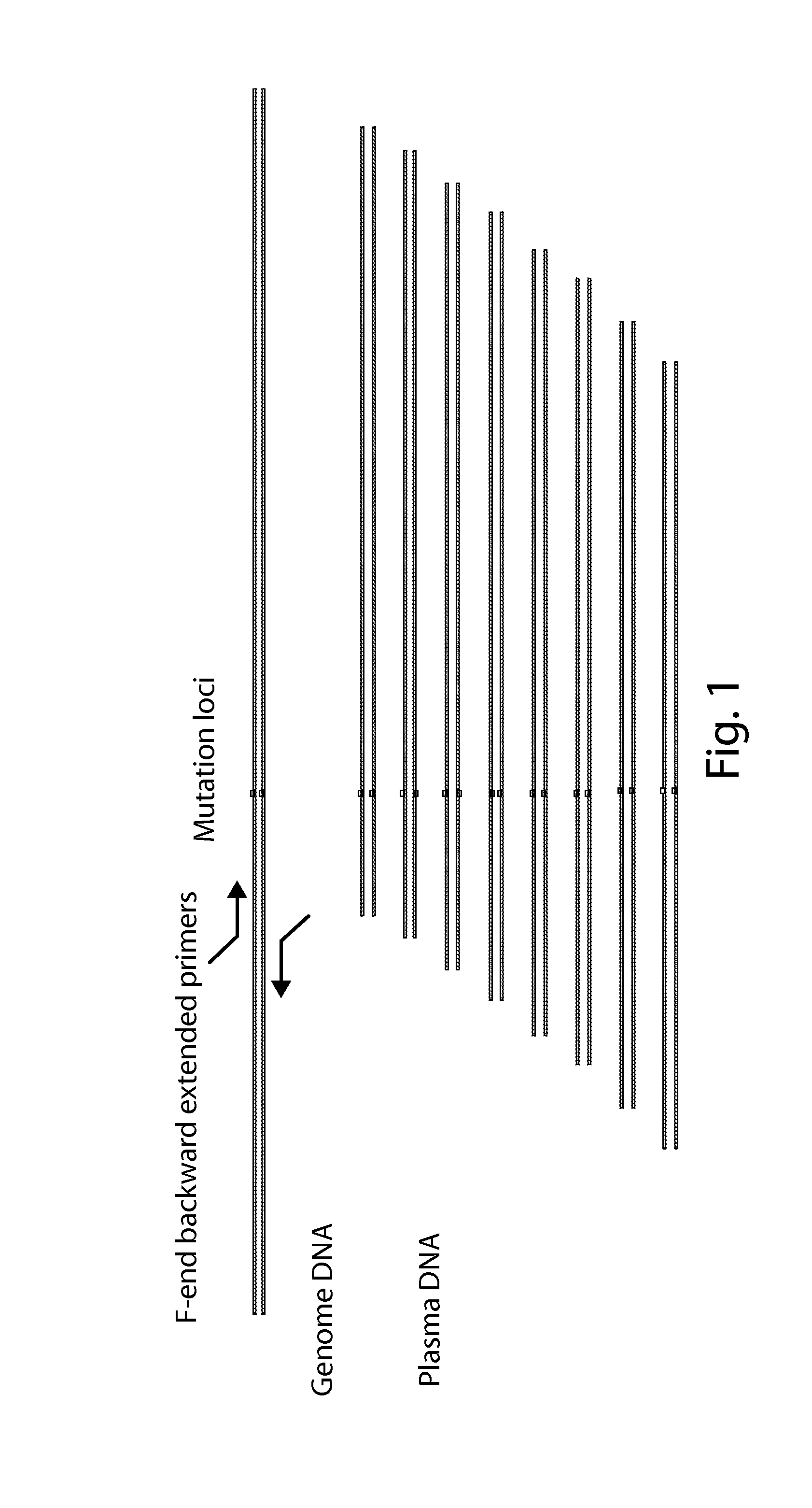
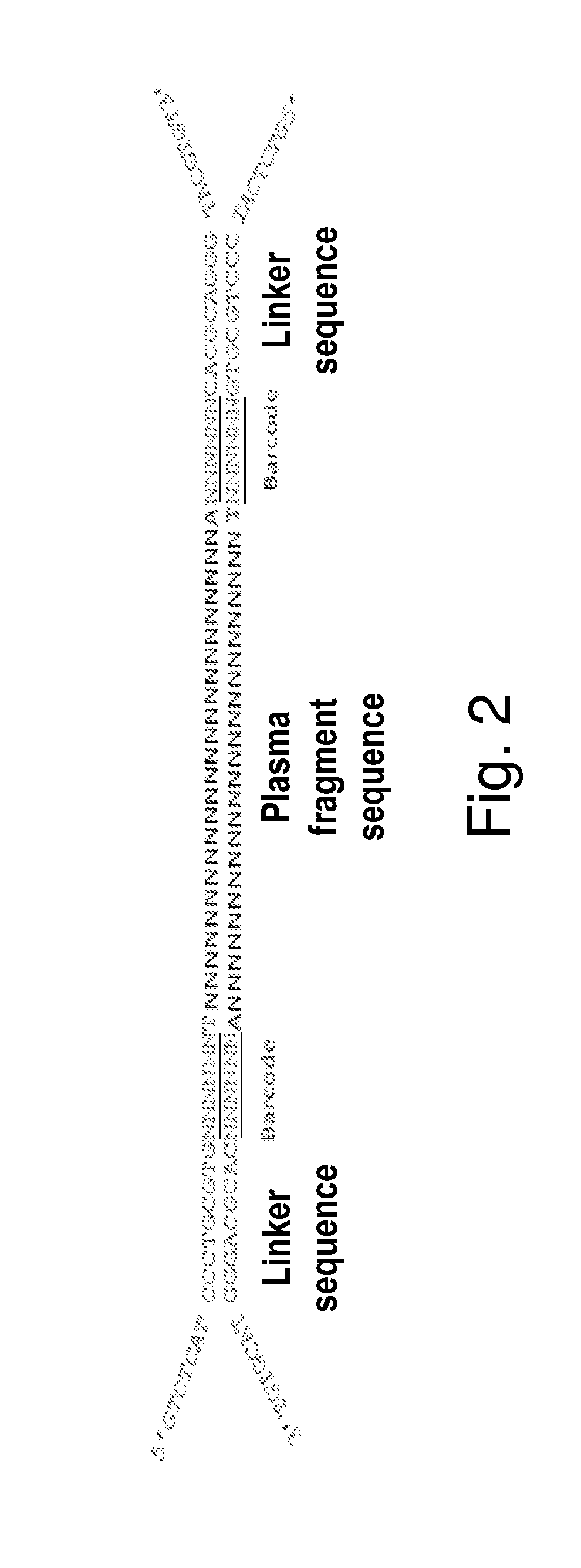
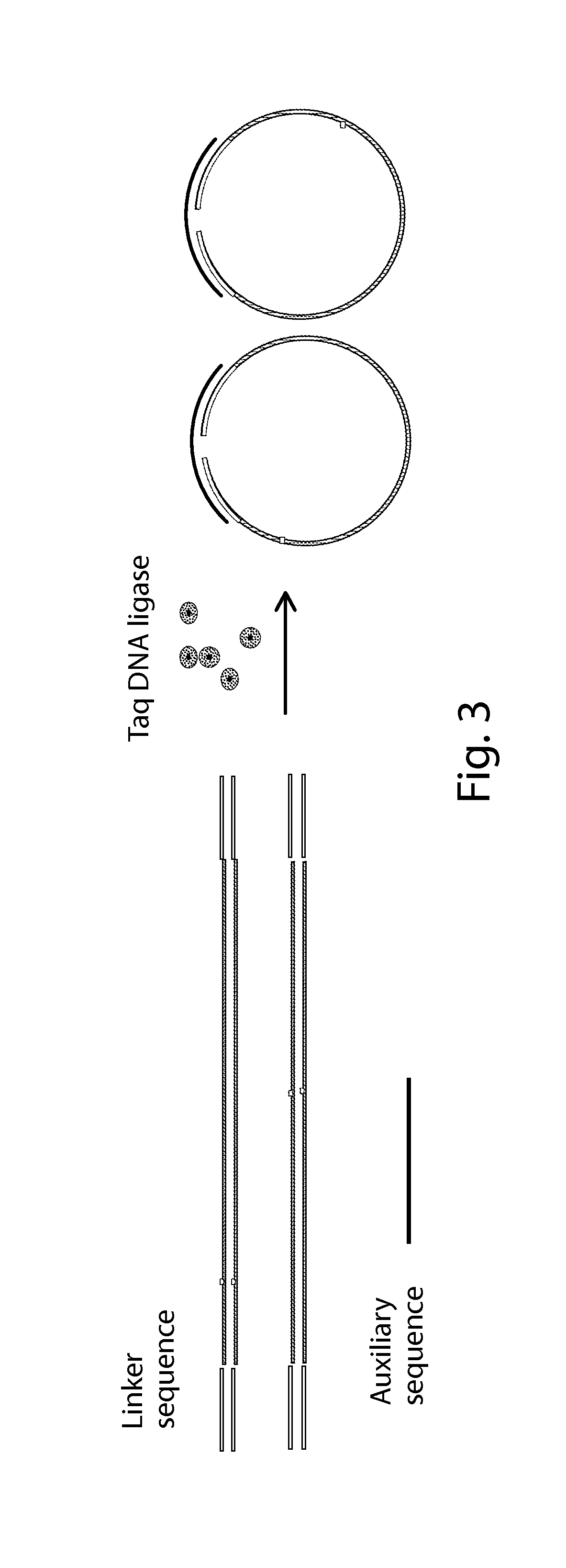
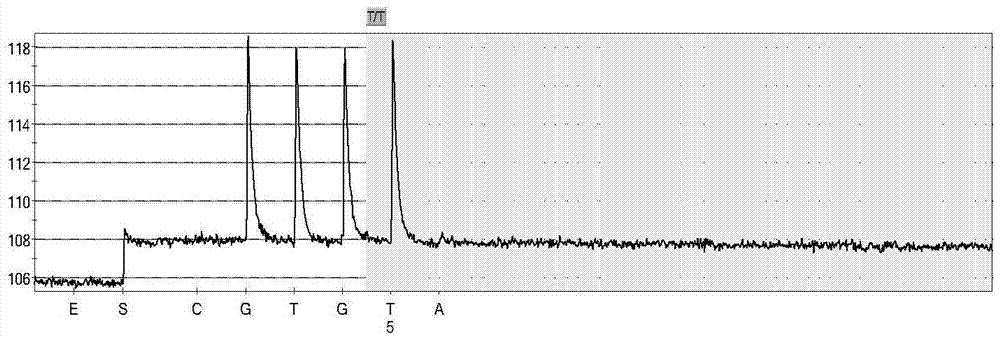
InactiveUS20150307942A1Nucleotide librariesMicrobiological testing/measurementTotal DeafnessBlood plasma
The present invention is directed to a method, kit and primers for detecting fetal deafness pathogenic gene mutations. The method of the invention comprises: (a) designing primers according to the pre-determined mutation loci of deafness pathogenic genes; (b) extracting plasma DNAs in a pregnant woman; (c) connecting the extracted plasma DNAs with pre-amplification linkers to obtain connected products; (d) PCR pre-amplifying the connected product to obtain pre-amplified products; (e) cyclizing the pre-amplified products to obtain cyclised DNAs; (f) PCR amplifying the cyclised DNAs using the designed primers to obtain amplified products; and (g) high throughput sequencing the amplified products and analyzing the mutations of the fetal deafness pathogenic genes. The invention can effectively determine whether the pre-determined loci on deafness pathogenic genes have been mutated as well as the mutation type.
Owner:BERRYGENOMICS CO LTD
Non-syndromic deafness gene polymorphism detecting kit and application thereof
InactiveCN105441540AAccurate analysisFacilitate the establishment of standardized operating proceduresMicrobiological testing/measurementDNA/RNA fragmentationTotal DeafnessSlc26a4 gene
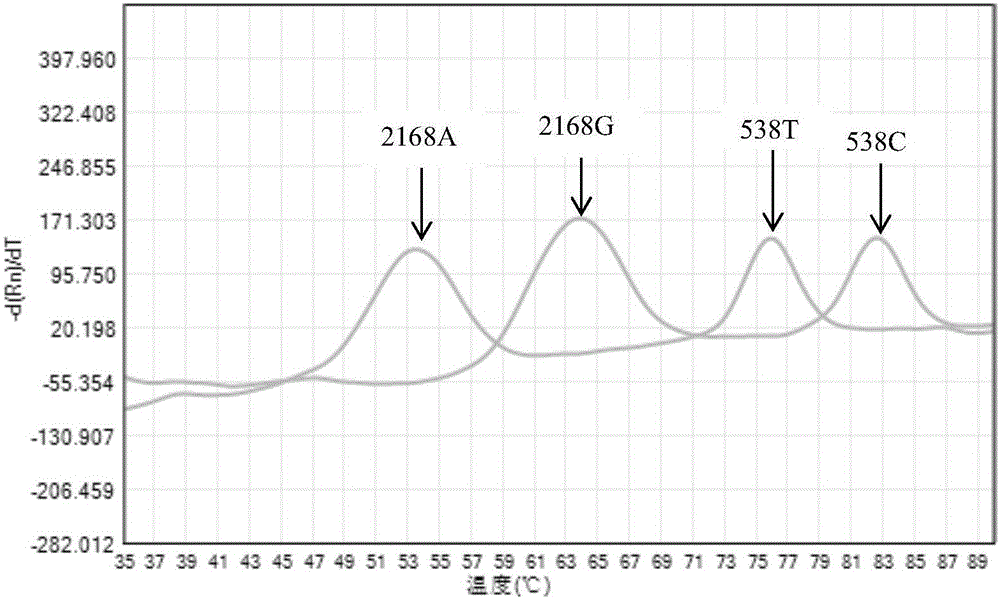
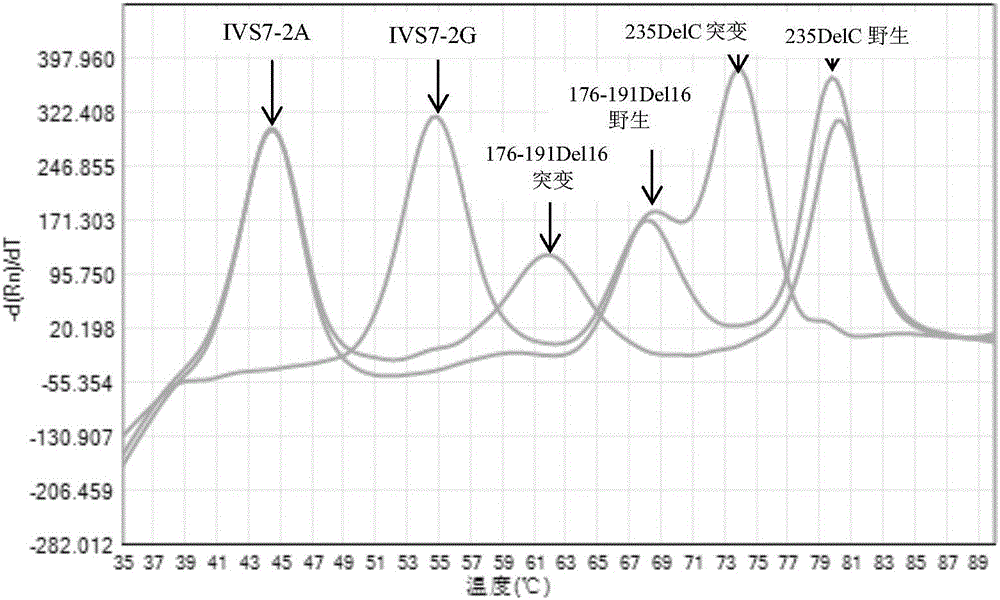
The invention discloses non-syndromic deafness gene polymorphism detecting primers. The primers mainly include five pairs of specific primers and nine sequencing primers for amplification of deafness genes, GJB2 genes, GJB3 genes, SLC26A4 genes and 12SrRNA genes are included, and ten single nucleotide polymorphisms are involved, namely GJB2 (35DELG, 176-191DEL16, 235DELC, 299-300 DEL AT), GJB3 (538C>T, 547G>A), SLC26A4(2168A>G, IVS7A>G) and mitochondria 12SrRNA (1494C>T, 1555A>G). The invention further discloses a kit comprising the primers. Sensitivity is high, results are visual, judgment is more accurate and quicker, and accurate and quick high-pass non-syndromic deafness gene polymorphism detection can be achieved.
Owner:CHANGSHA DIAN MEDICAL SCI INSPECTION CO LTD
Deafness pathogenic gene detection kit utilizing time-of-flight mass spectrometry
InactiveCN107974497AResolve incompatibilitiesMany mutation detection sitesMicrobiological testing/measurementTotal DeafnessSlc26a4 gene
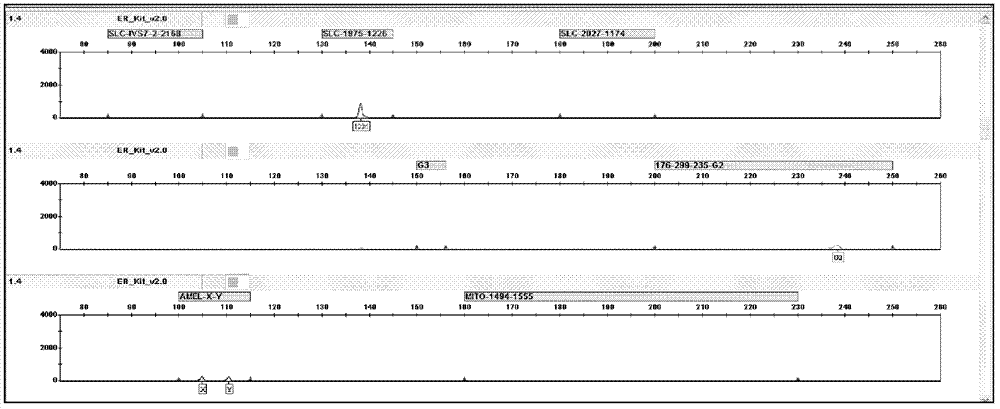
The invention provides a deafness pathogenic gene detection kit utilizing a time-of-flight mass spectrometry. The kit comprises a reagent for detecting at least following 26 mutation sites of 12S rRNA(ribosomal Ribonucleic Acid), GJB2 and SLC26A4 genes: 12S rRNAm.1494-C is greater than T, 12S rRNAm.1555Ais greater thanG, GJB2c.35delG, GJB2c.257C is greater than G, GJB2c.427C is greater than T, GJB2c.176del16, GJB2c.9G is greater than A, GJB2c.235delC, GJB2c.299-300delAT, SLC26A4IVS4+2T is greater than C, SLC26A4c.1673A is greater than T, SLC26A4c.1520delT, SLC26A4c.2027T is greater than A, SLC26A4c.1975G is greater than C, SLC26A4c.1226G is greater than A, SLC26A4c.1318A is greater than T, SLC26A4c.1229C is greater than T, SLC26A4c.281C is greater than T, SLC26A4c.2168A is greater than G,SLC26A4IVS7-2A is greater than G, SLC26A4c.1174A is greater than T, SLC26A4c.235C is greater than T, SLC26A4c.1340delA, SLC26A4_c.589G is greater than A, SLC26A4c.916-917insG and SLC26A4c.IVS15+5G isgreater than A. The kit provided by the invention has the advantages of more mutation detection sites, high throughput, simplicity in operation and short period, high accuracy, high stability and lowcost.
Owner:国家卫生健康委科学技术研究所
Kit for detecting child medicamentous deaf inheritance risk
The invention discloses a agent box for detecting infant medicaments deafness heredity risks. The agent box comprises specificity primer and DNA sequencing primer for detecting four sites polymorphism on detection MTRNR1 gene, PCR reaction component and PCR outcome yield purifying component etc.. The agent box of the invention assesses infant medicaments deafness heredity risks by detecting four sites polymorphism on detection MTRNR1 gene correlative closely to infant medicaments deafness heredity risks.
Owner:HAINAN ZHUJIAN BIOTECH
Multichannel fluorescent PCR detection kit for congenital deafness gene
InactiveCN104694644AImprove effectivenessTest effectivenessMicrobiological testing/measurementTotal DeafnessFluorescence
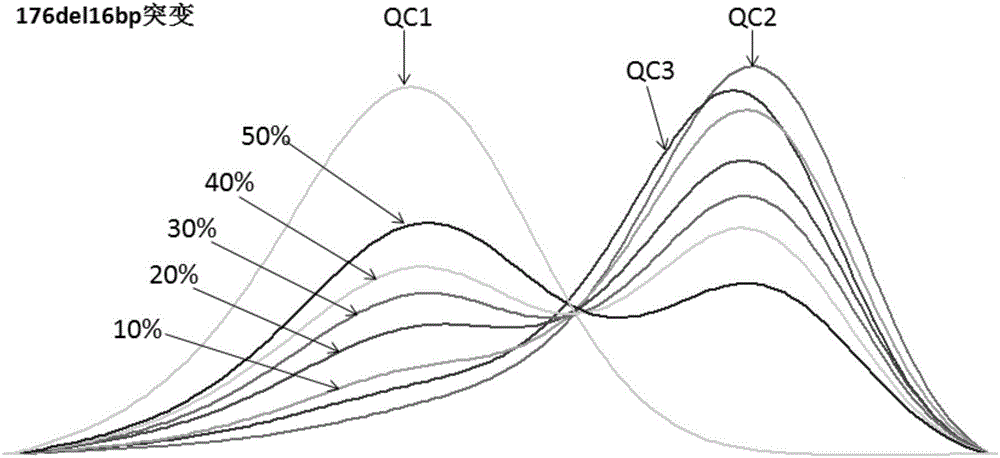
The invention discloses a multichannel fluorescent PCR detection kit for a congenital deafness gene. With 35delG, 155delTCTG,176dell6bp, 235delC, 299-300delAT and 512insAACG mutations of a GJB2 gene, and 538G>T and 547G>A mutations of a GJB3 gene as detection objects, specific primers and a probe are designed, wherein the probe is used for specifically detecting whether 35, 155, 176, 235 and 299 loci of the GJB2 gene, and 538 and 547 loci of the GJB3 gene contain mutations or not. According to the multichannel fluorescent PCR detection kit, eight mutation loci of the congenital deafness are detected at the same timefor the first time in a same reaction tube by applying a multichannel fluorescent PCR method; the operation is simple, convenient, fast, accurate, high in flux, and low in cost; cross contamination is avoided by a pipe closing operation; and the deafness screening is high in intentionality and wide in screening range.
Owner:JINAN YING SHENG BIOTECH
Solving deafness in directional clear channel assessment (CCA)
ActiveUS10327241B2Spatial transmit diversityWireless communicationTotal DeafnessCommunications system
Owner:QUALCOMM INC
Kit for detecting gene mutation of deafness
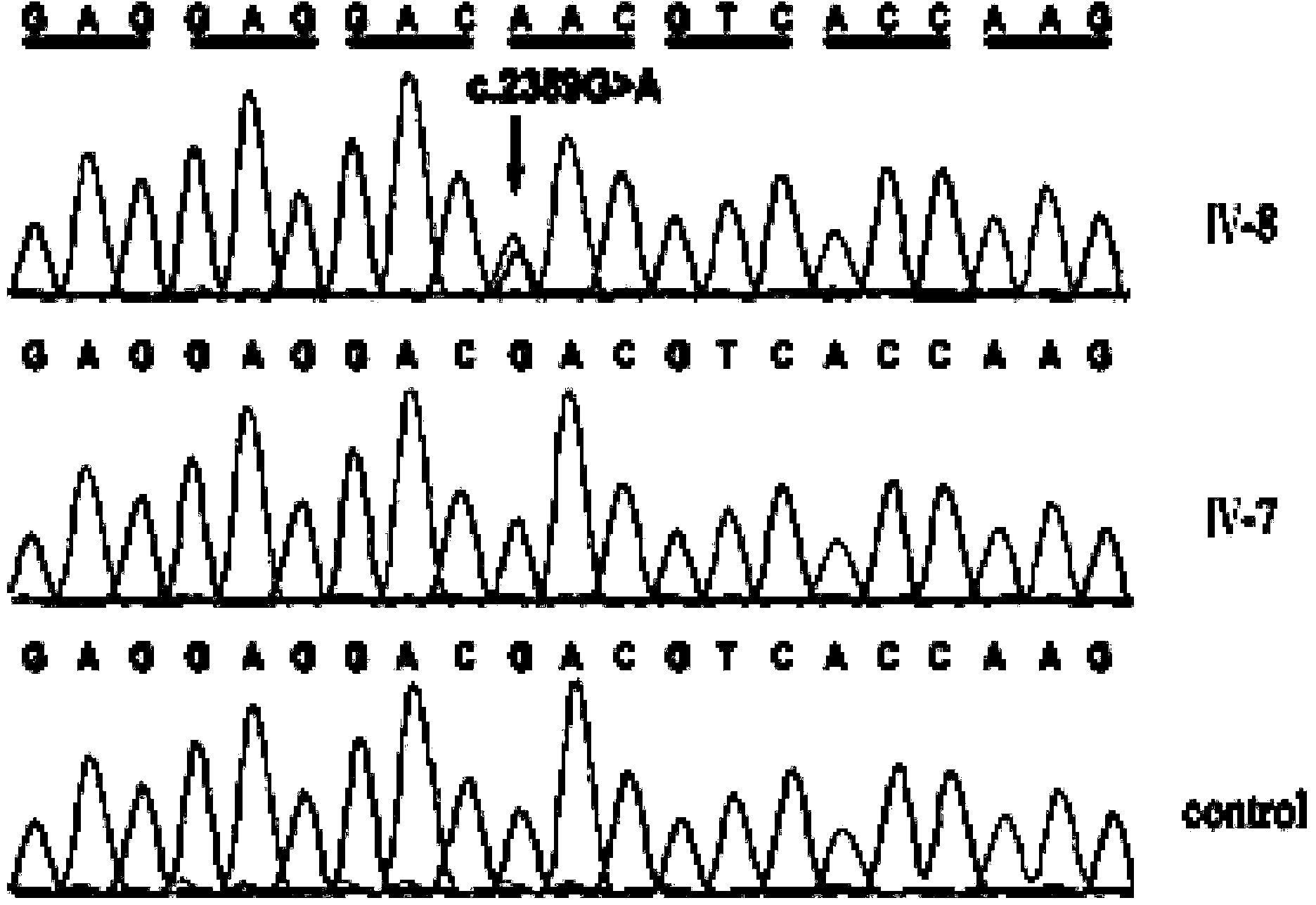
ActiveCN104212892AAccurate judgmentJudgment credibleMicrobiological testing/measurementTotal DeafnessEnzyme digestion
The invention relates to the field of medicines and particularly relates to a kit for detecting gene mutation of deafness. The kit comprises specific primers, a PCR mixed liquid, an enzyme digestion reaction mixed liquid and the like, wherein corresponding specific primers are specially designed for the deaf gene. Mutation sites from G to A of a WFS1 gene c.2389 in the body of a patient can be quickly detected by using the kit, so that large-scaled screening and preventive inspection of deafness related gene mutation on a national scale, particularly in underdeveloped areas can be better carried out. Moreover, the kit is low in detection cost, simple to operate and high in accuracy.
Owner:山东省第二人民医院(山东省耳鼻喉医院、山东省耳鼻喉研究所)
Primer, kit and detection method for detecting deafness genetic risk of children
ActiveCN106282335AEasy to operateQuick checkMicrobiological testing/measurementDNA/RNA fragmentationGenetic riskTotal Deafness
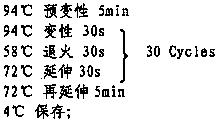
The invention discloses a primer and a kit for detecting the deafness genetic risk of children and an operation method thereof. The method comprises the following steps: (1) extracting a DNA template, and extracting DNA of a genome of oral epithelial cells; (2) performing a PCR amplification reaction with the total volume of a reaction system being 25 mu L at 94 DEG C for 12 minutes, performing the reaction at 94 DEG C for 30 seconds, at 60 DEG C for 30 seconds and at 72 DEG C for 30 seconds for 30 circulations, and performing PCR amplification reaction at 72 DEG C for 10 minutes; (3) purifying a PCR product at 37 DEG C for 15 minutes and at 72 DEG C for 20 seconds; and (4) performing a DNA sequencing reaction at 98 DEG C for 2 seconds by using a DNA sequencing primer GCGTTTGGTCCTAGCCTTT, and performing the reaction at 96 DEG C for 30 seconds, at 55 DEG C for 30 seconds and 60 DEG C for 4 minutes for 25 circulations. The detection method has the advantages of high accuracy and simple operation, and has a huge market popularization and application value.
Owner:SHANGHAI KINGMED DIAGNOSTICS INST
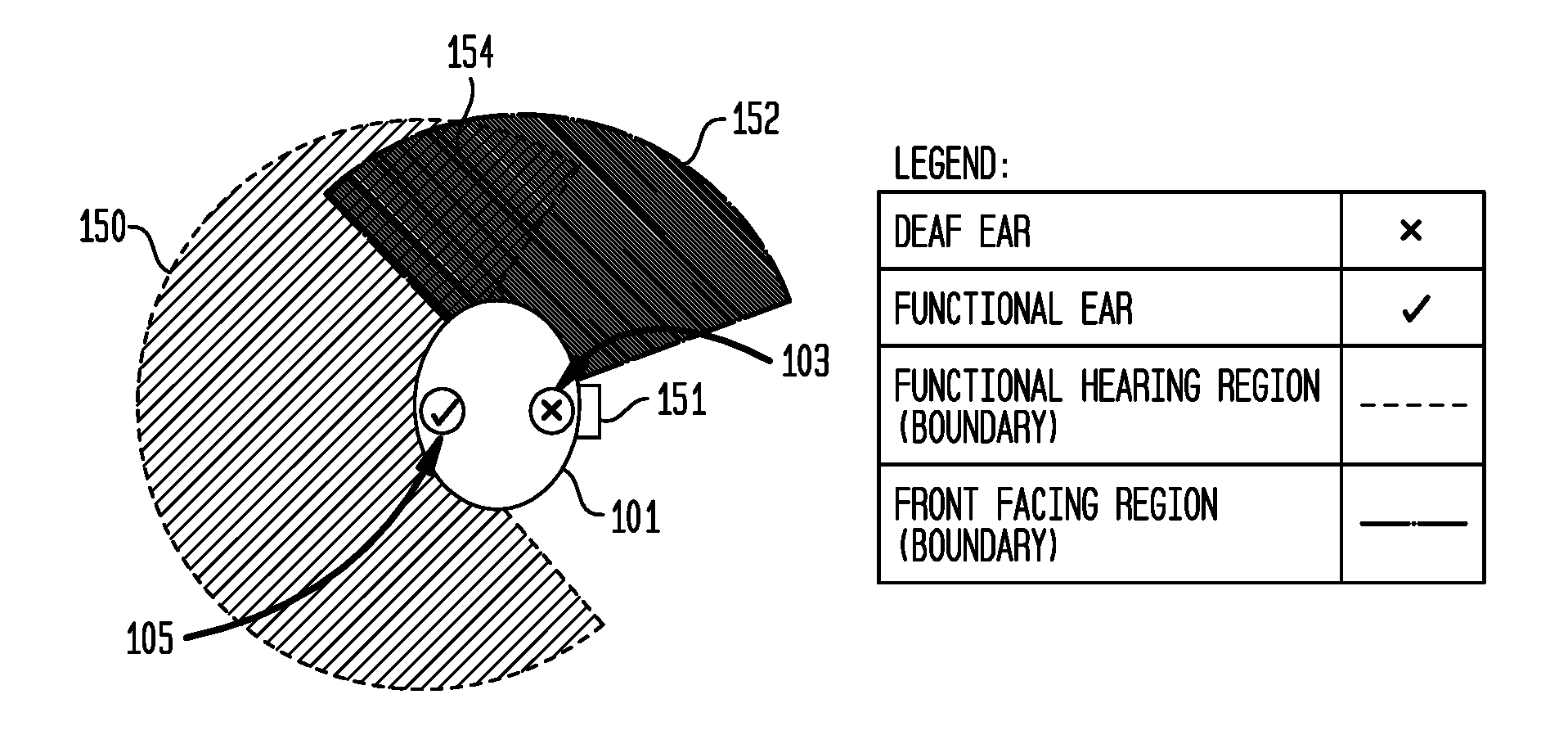
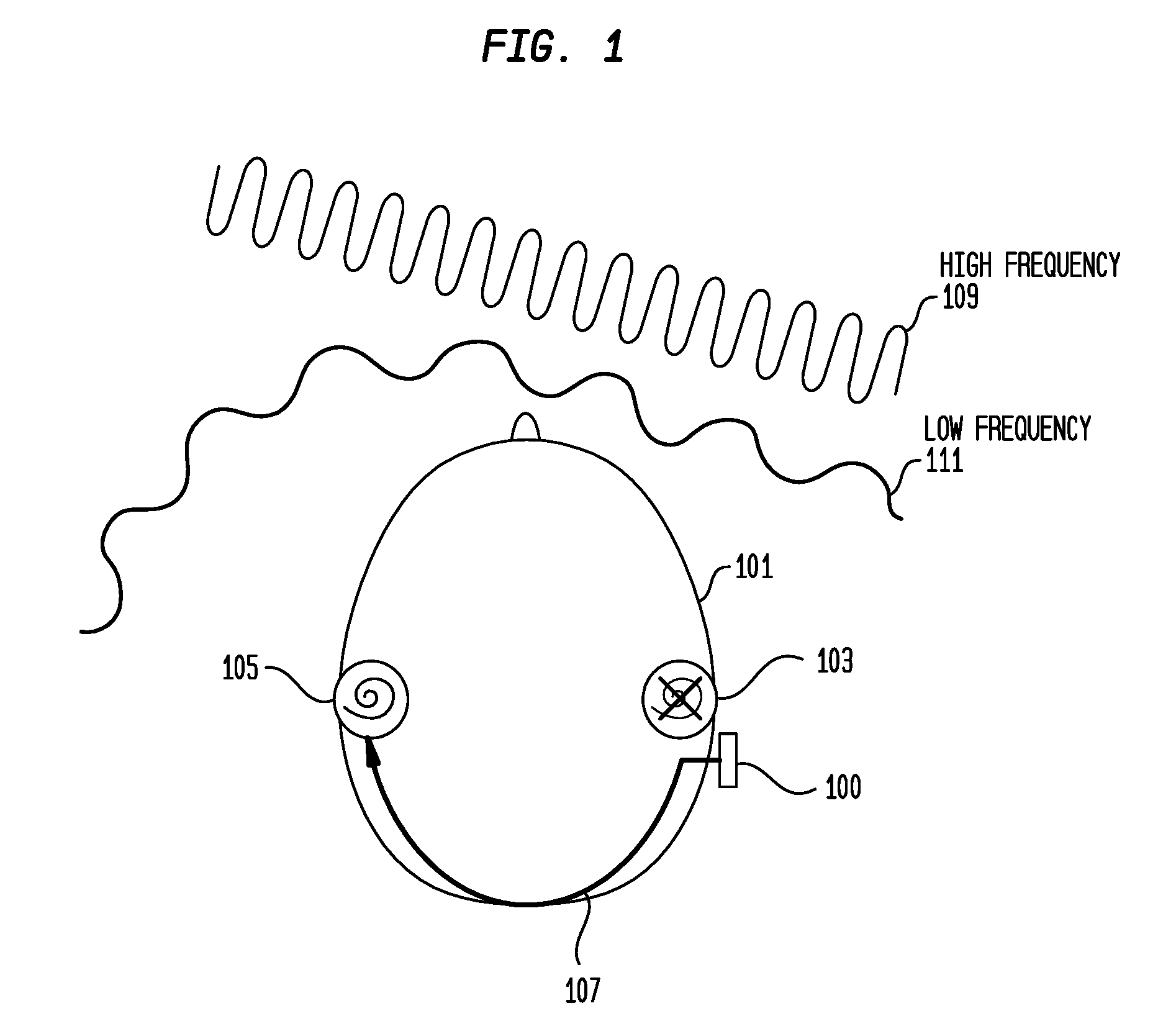
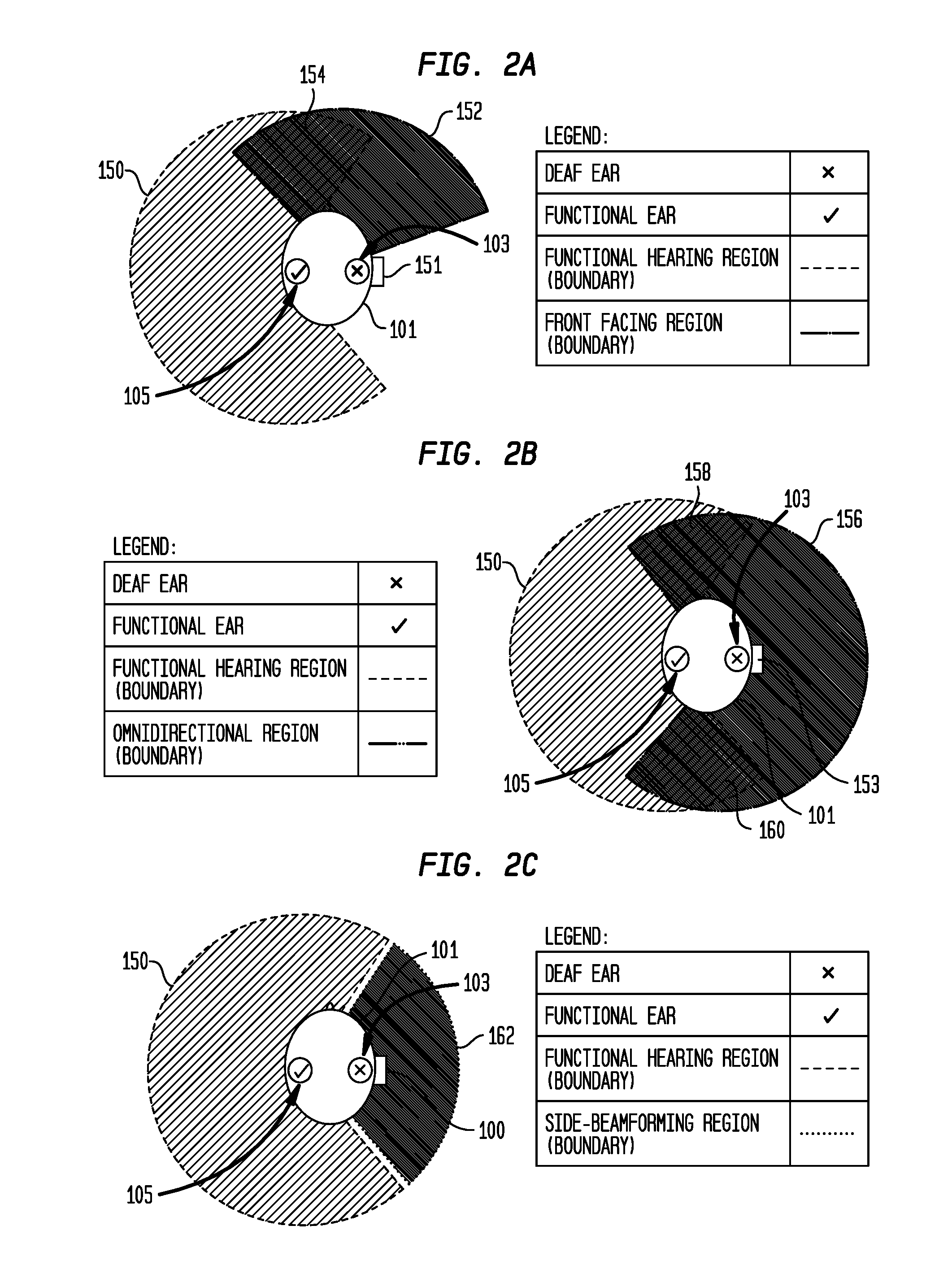
Hearing prostheses for single-sided deafness
ActiveUS20160366522A1Avoid overlapBone conduction transducer hearing devicesHearing aids signal processingTotal DeafnessProsthesis
Embodiments presented herein are generally directed to hearing prostheses configured to execute sound processing (e.g., beamforming techniques) specifically designed to provide better performance for single-side deaf recipients.
Owner:COCHLEAR LIMITED
Kit for detecting mutation of deafness susceptibility genes and application of kit
ActiveCN107177688AIncrease profitAvoid the inconvenience of multi-tube detectionMicrobiological testing/measurementReference genesTotal Deafness
The invention belongs to the technical field of gene detection and particularly relates to a kit for detecting mutation of deafness susceptibility genes and an application of the kit. A PCR mixture in the kit contains 17 primer single strands shown as SEQ ID NO: 1-17 and 11 probes shown as SEQ ID NO: 19-29, genotypes of 9 mutation sites of four deafness susceptibility genes and one reference gene can be detected simultaneously in a single-tube PCR system, the genotype of a sample is acquired through fluorescent PCR melting curve analysis after PCR amplification is ended, the whole operation can be finished within 2-3 h, a few operation steps are adopted, short time is consumed, many single-tube detection sites are adopted, and the flux and mutation coverage are high; besides, the kit adopts homogeneous-phase detection and closed-tube operation, so that pollution of a PCR product is reduced, detection specificity is high, and a result is easy to interpret.
Owner:GUANGZHOU HYBRIBIO MEDICINE TECH LTD +2
Bracelet
Owner:HAYES COLLEEN M
Deafness gene screening chip
InactiveCN104975076AImprove throughputWide coverageNucleotide librariesMicrobiological testing/measurementTotal DeafnessMitophagy
The invention discloses a deafness gene screening chip, which includes a solid phase carrier and an oligonucleotide probe fixed thereon. Nucleotide sequences of the oligonucleotide probe are represented as the Seq ID No.1-1152. The deafness gene screening chip is suitable for Chinese people, wherein most of deafness mutation detection loci are reported among Chinese people and comprise the most common eight hot-spot mutation. The screening chip has following advantages: (1) high-throughput: the screening chip contains 384 detection loci; (2) wide coverage: the screening chip covers mitochondria and 46 different deafness genes located on autosomes, which includes 240 deafness mutation loci and 144 SNP loci, wherein the deafness mutation loci includes 200 non-syndrome deafness loci and 40 common syndrome deafness loci; and (3) high sensitivity and specificity: a direct sequencing test proves the GJB2-1-BP-DEL-235C detection locus is 96.3% in sensitivity and is 100% in specificity.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
High-specificity kit for detecting deafness predisposing genes and uses
InactiveCN102534031BAvoid false positivesMicrobiological testing/measurementTotal DeafnessFluorescence
The invention discloses a fluorescent detection kit for detecting 12 deafness predisposing genes simultaneously. The kit can detect 12 mutational hotspots in the most common deafness associated genes of the Chinese in 3 hours. The kit comprises reagents before amplification and reagents after amplification, wherein the reagents before amplification comprise a polymerase chain reaction (PCR) buffer solution, a reaction mixture of MgCl2 and deoxyribonucleoside triphosphates (DNTPs), Taq DNA polymerase, ultrapure water, and a primer mixture for amplifying loci of detection sites at high specificity; and the reagents after amplification comprise a genotyping standard and an internal standard. Deafness gene loci are simultaneously detected at high sensitivity and high specificity by combining a fluorescent labeling technology, a linolenic acid (LNA) nucleoside monomer doping-primer modification technology and a capillary electrophoresis technology for the first time, manpower and material resources and time are greatly saved, and pollution due to multi-step operation is prevented.
Owner:万戈江
Deafness gene trapping kit
InactiveCN107541448APrevent tippingEasy to useBioreactor/fermenter combinationsBiological substance pretreatmentsTotal DeafnessEngineering
The invention relates to a deafness gene capture kit, which includes an L-shaped support, on which a kit is arranged, and the kit includes a box body and a box cover, and the box body includes a bottom box and a bottom box that can pass through the lower part. It is a sleeve box that is provided with a sleeve portion and is set in the bottom box and can move up and down along the vertical portion of the support member and can rotate around the vertical portion. The sleeve between the upper end of the sleeve box and the lower end of the cover The vertical part of the box cover and the support can be connected up and down and rotated around the vertical part; the bottom box is provided with elastic blocks, and the elastic blocks are preset with various diameters. Reagent bottle jack, the box is provided with a vertical partition, and a foam block is arranged in an area separated by the vertical partition, and reagent bottle placement holes of different specifications are preset in the foam block, and the vertical partition A sponge pad is provided in the area on the other side separated by the partition. The present invention prevents the kit from tipping over through the use of supports.
Owner:TIANJIN QITEER BIOTECH
Fluorescence detection kit for detecting deafness susceptibility gene GJB2 235delC and application of fluorescence detection kit
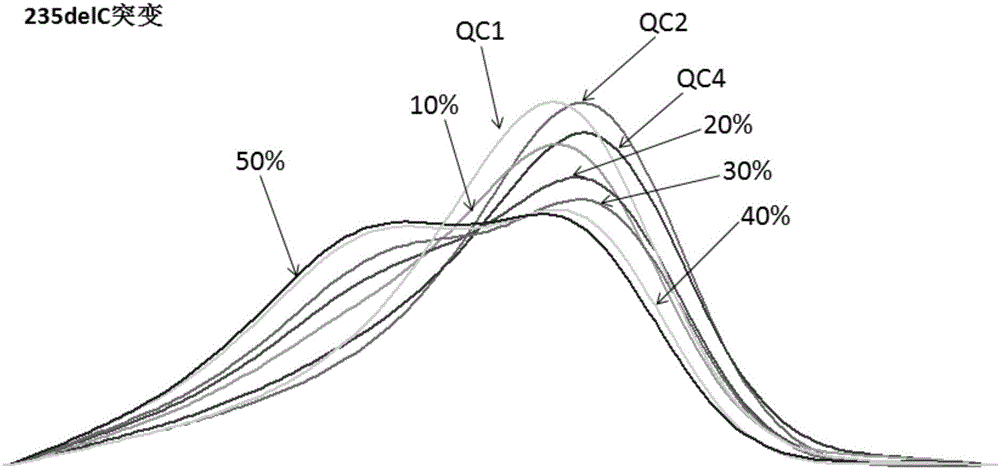
InactiveCN102605052AIncrease the Tm valueShorten the lengthMicrobiological testing/measurementTotal DeafnessFluorescence
The invention discloses a fluorescence detection kit for detecting deafness susceptibility gene GJB2 235delC. The kit comprises a pre-amplification reagent and a post-amplification reagent; the pre-amplification reagent comprises a reaction mixture comprising a PCR (Polymerase Chain Reaction) buffer solution, MgCl2 and dNTPs (deoxyribonucleotide Triphosphates), a Taq enzyme, ultrapure water and a primer mixture comprising high specificity amplification GJB2 235delC and an Amelogenin gene locus; and the post-amplification reagent comprises a genotyping standard substance and an interior label. According to the invention, a fluorescence labeling technology, a LNA (Locked Nucleic Acid) nucleoside monomer doping and primer modification technology and a capillary electrophoresis technology are firstly compounded to be adopted; the simultaneous detection on the high sensitivity specificity of the deafness gene locus GJB2 235delC is implemented; manpower and material resources and time are greatly saved; and the pollution caused by multi-step operation is prevented.
Owner:万戈江
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com