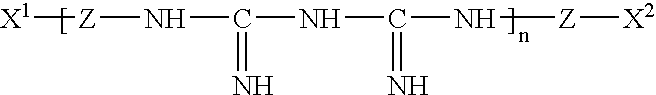
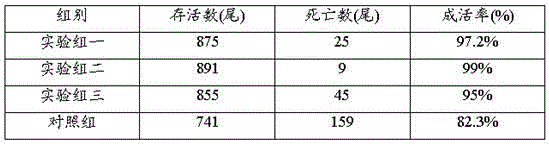
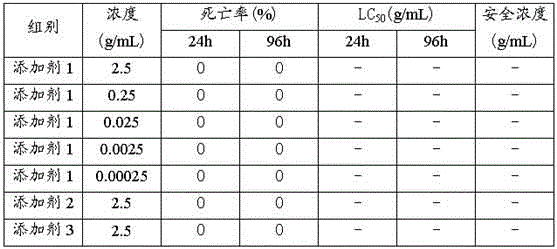
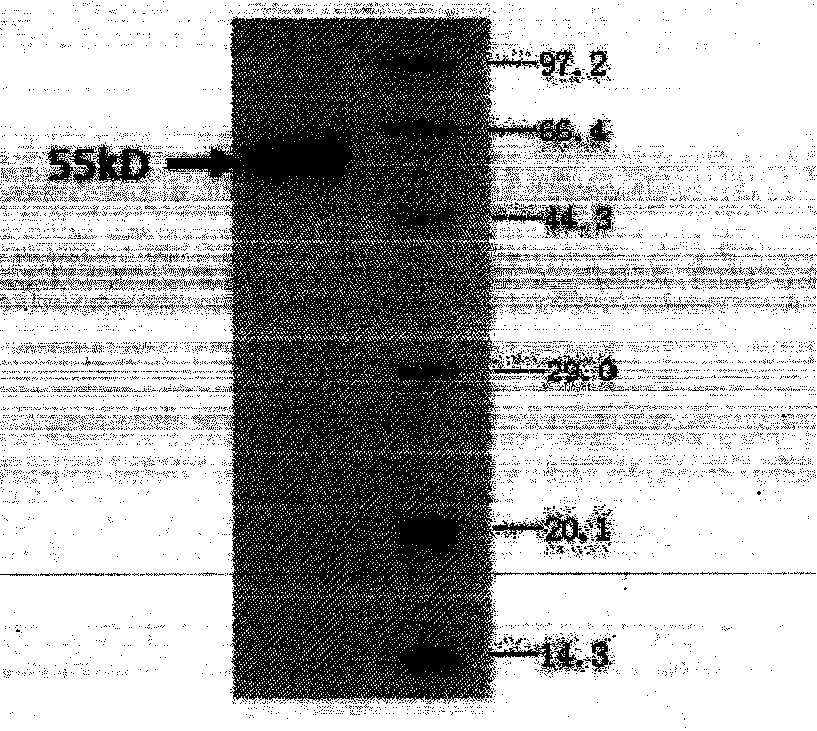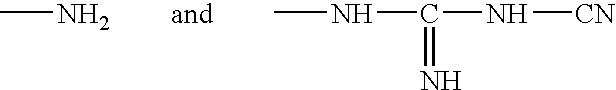Patents
Literature
1554 results about "Inositol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Inositol, or more precisely myo-inositol, is a carbocyclic sugar that is abundant in brain and other mammalian tissues, mediates cell signal transduction in response to a variety of hormones, neurotransmitters and growth factors and participates in osmoregulation. It is a sugar alcohol with half the sweetness of sucrose (table sugar). It is made naturally in humans from glucose. A human kidney will produce around two grams each day. Other tissues synthesize it too, and the highest concentration is in the brain where it plays an important role making other neurotransmitters and some steroid hormones bind to their receptors. In the last 10 years, myo-inositol gained importance in the management of PCOS due to its efficacy, safety profile and worldwide availability.
Regulation of Mammalian Keratinous Tissue Using Skin and/or Hair Care Actives
Personal care compositions containing an active selected from the group consisting of phlorogine, phlorgine BG, deoxyArbutin, sucrose dilaurate, bakuchiol, pyrenoine, millet, arlatone dioic acid, cinnamic acid, ferulic acid, achromaxyl, methyl nicotinamide, oil soluble licorice extract, folic acid, undecylenic acid, zinc undecylenate, L-tryptophan, thiamine HCl, hexylresorcinol, lipidami red vine, dragosine, methyl gentisate, inositol, symdiol 68, laminaine, their salts, their derivatives, their precursors, and / or combinations thereof. Methods for regulating the condition of mammalian keratinous tissue by topically applying the personal care compositions are also provided.
Owner:THE PROCTER & GAMBLE COMPANY
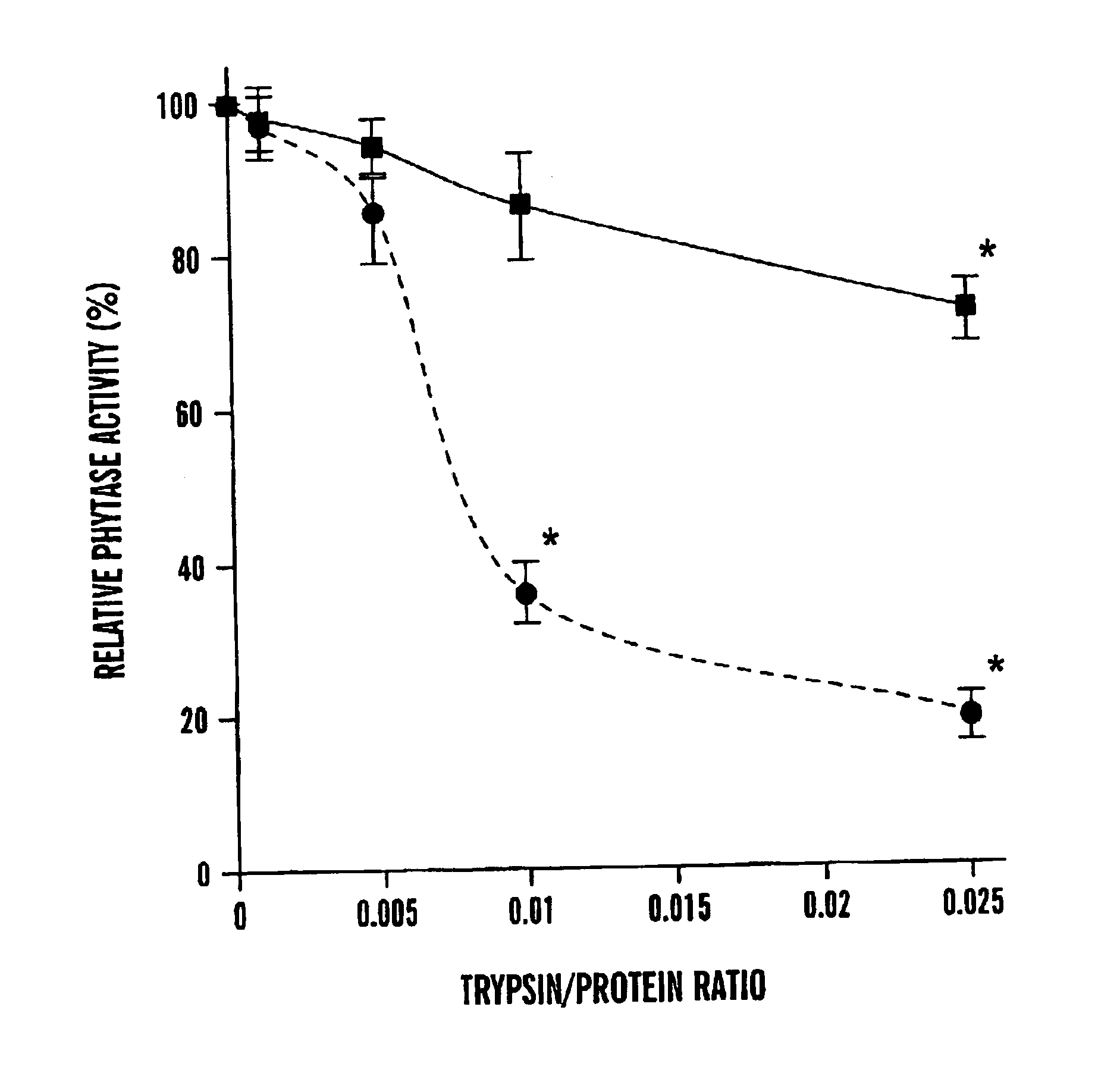
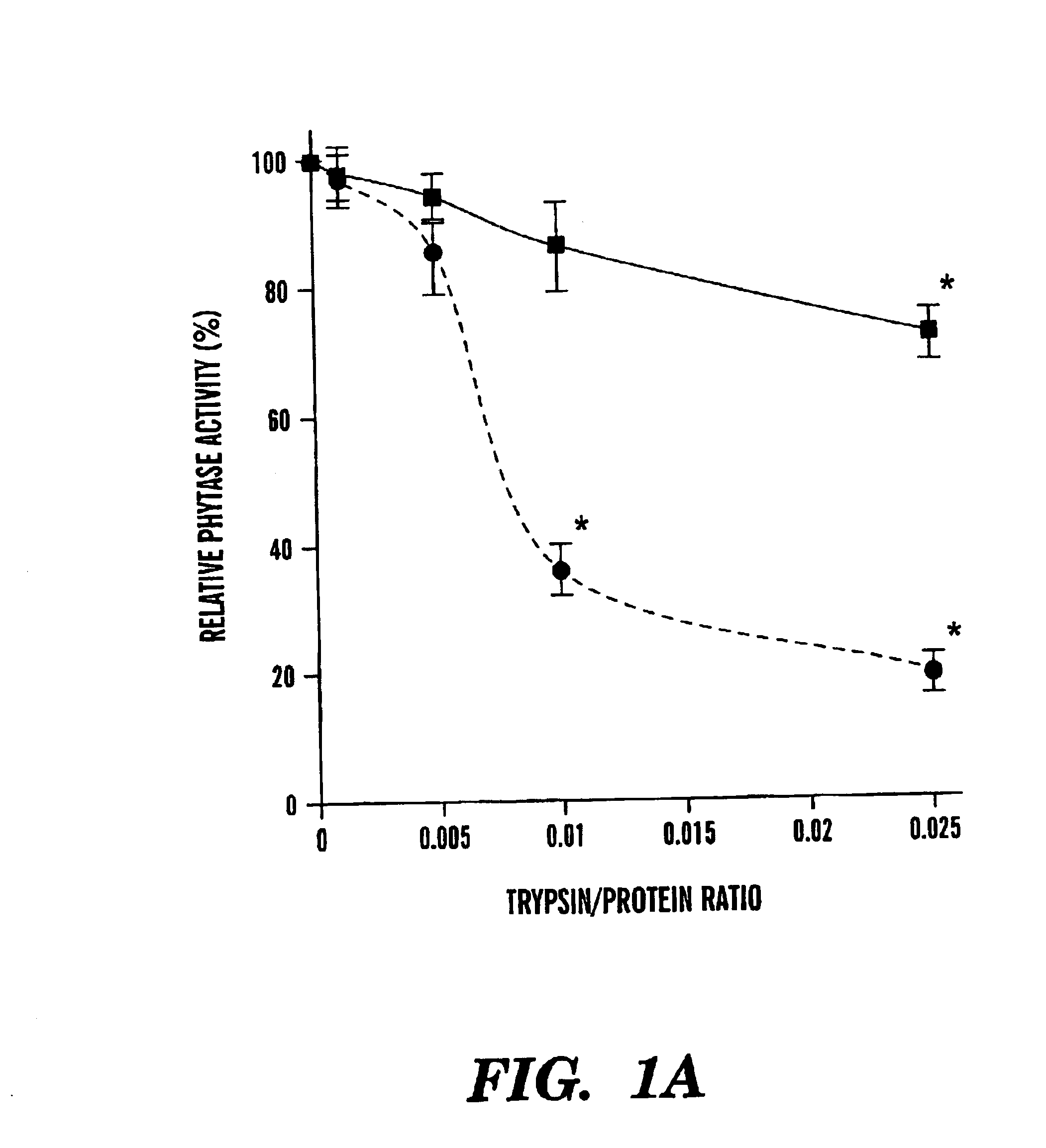
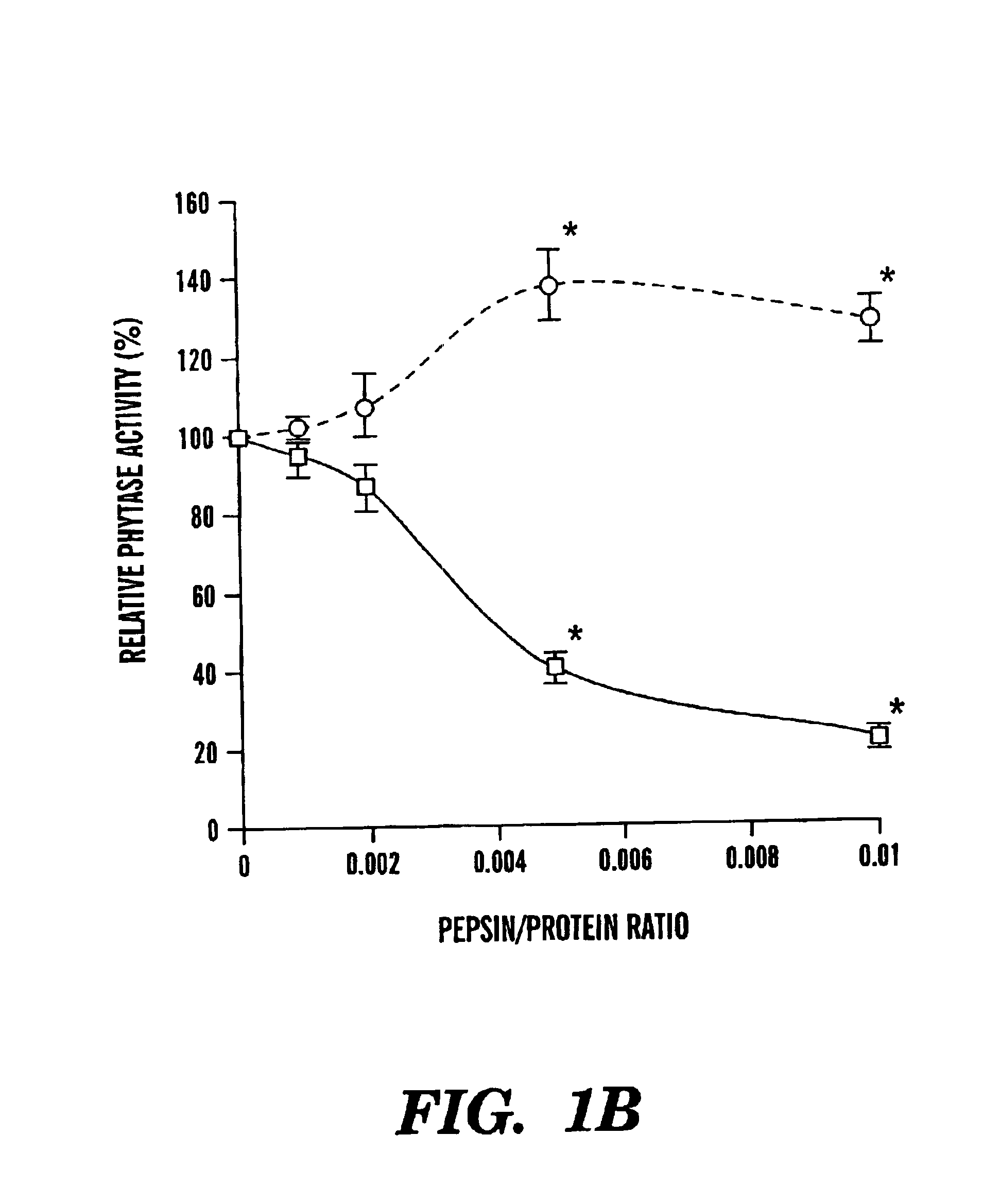
Mutants of Aspergillus niger PhyA phytase and Aspergillus fumigatus phytase
ActiveUS7919297B2High nutritional valueImprove bioavailabilityBacteriaSugar derivativesAspergillus fumigatusPhytase
The present invention is directed to an isolated nucleic acid molecule encoding mutant phytases and the isolated mutant phytases themselves. The present invention further relates to methods of using the isolated nucleic acid molecules and the isolated mutant phytases of the present invention.
Owner:CORNELL RES FOUNDATION INC
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Phosphatidylinositol 3 kinase inhibitors
InactiveUS20110212053A1Heavy metal active ingredientsBiocidePharmaceutical medicinePhosphatidylinositol 3-Kinases
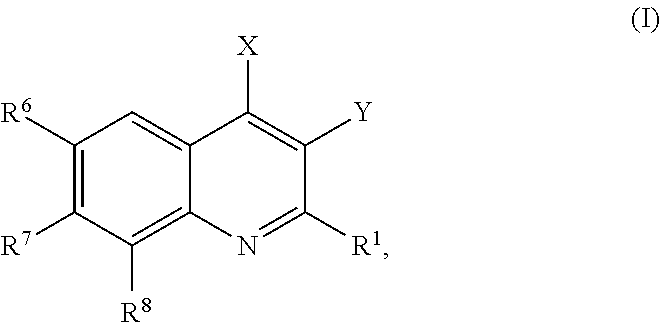
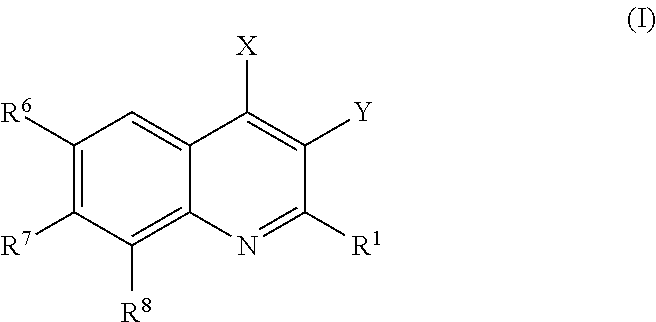
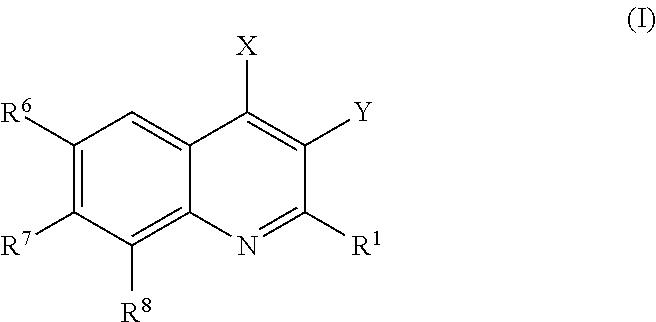
Provided are compounds according to Formula (I):or stereoisomer, prodrug, polymorph, or pharmaceutically acceptable salt forms thereof, wherein X, Y, R1, R6, R7, and R8 are as defined, and which compounds are effective inhibitors of PI3-kinase and / or other medically and clinically relevant kinases. Also provided are pharmaceutical compositions and methods of using the compounds and compositions as PI3-kinase and kinase inhibitors. More particularly, the compounds of the invention provide treatments and therapeutics for human diseases regulated by, or associated with, the activity of, PI3-kinases and / or protein kinases, or mutant or variant forms thereof.
Owner:PROGENICS PHARMA INC
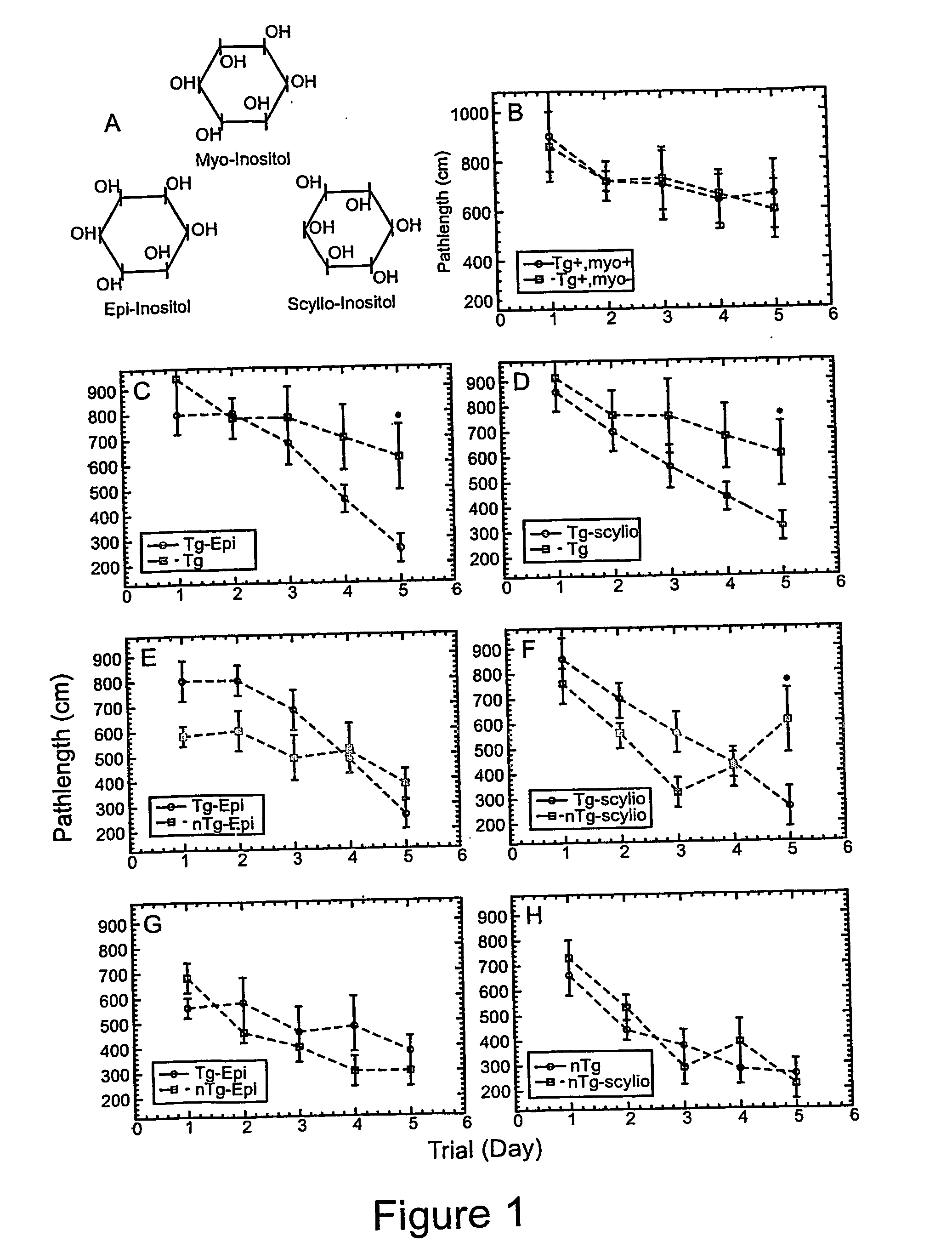
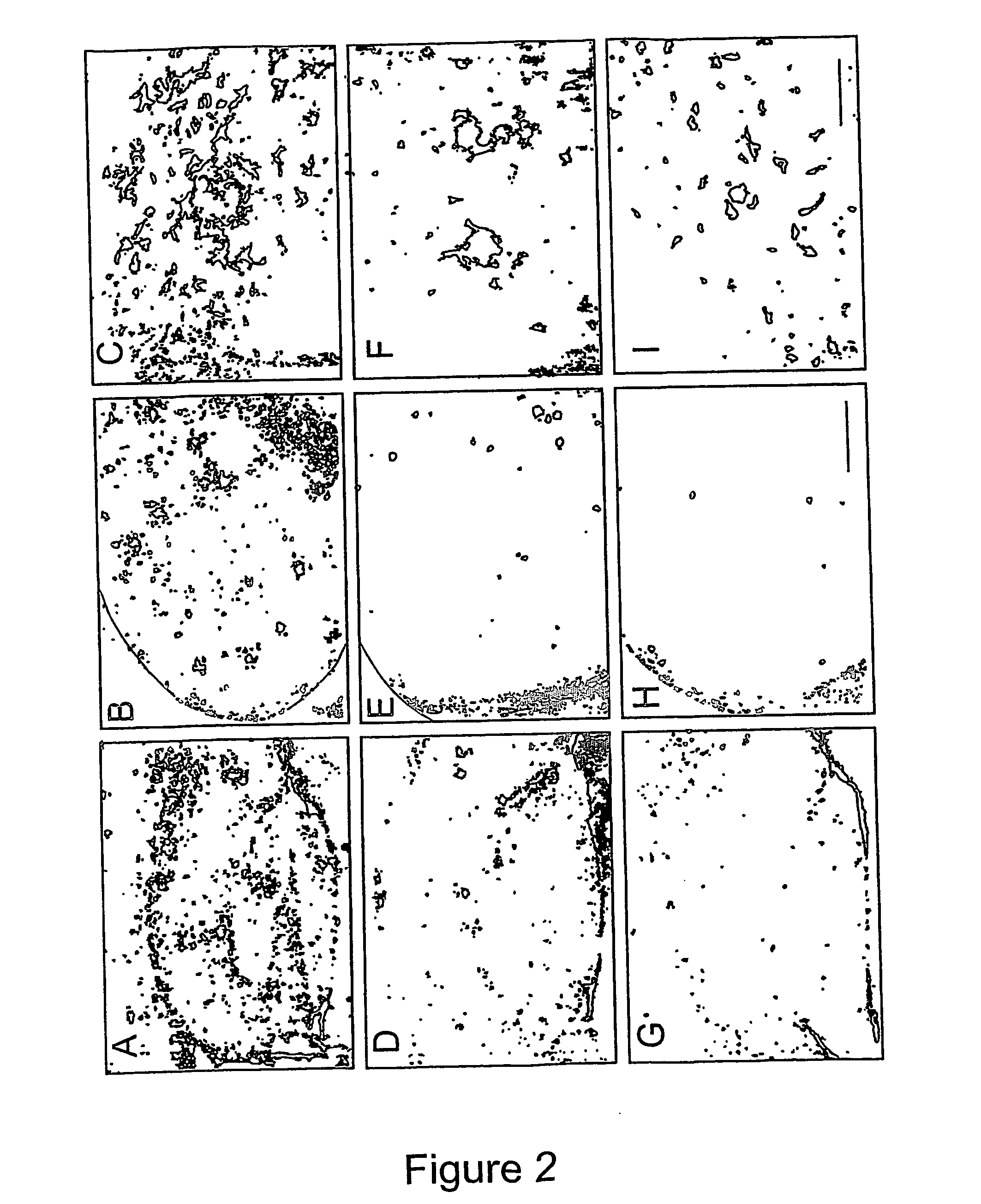
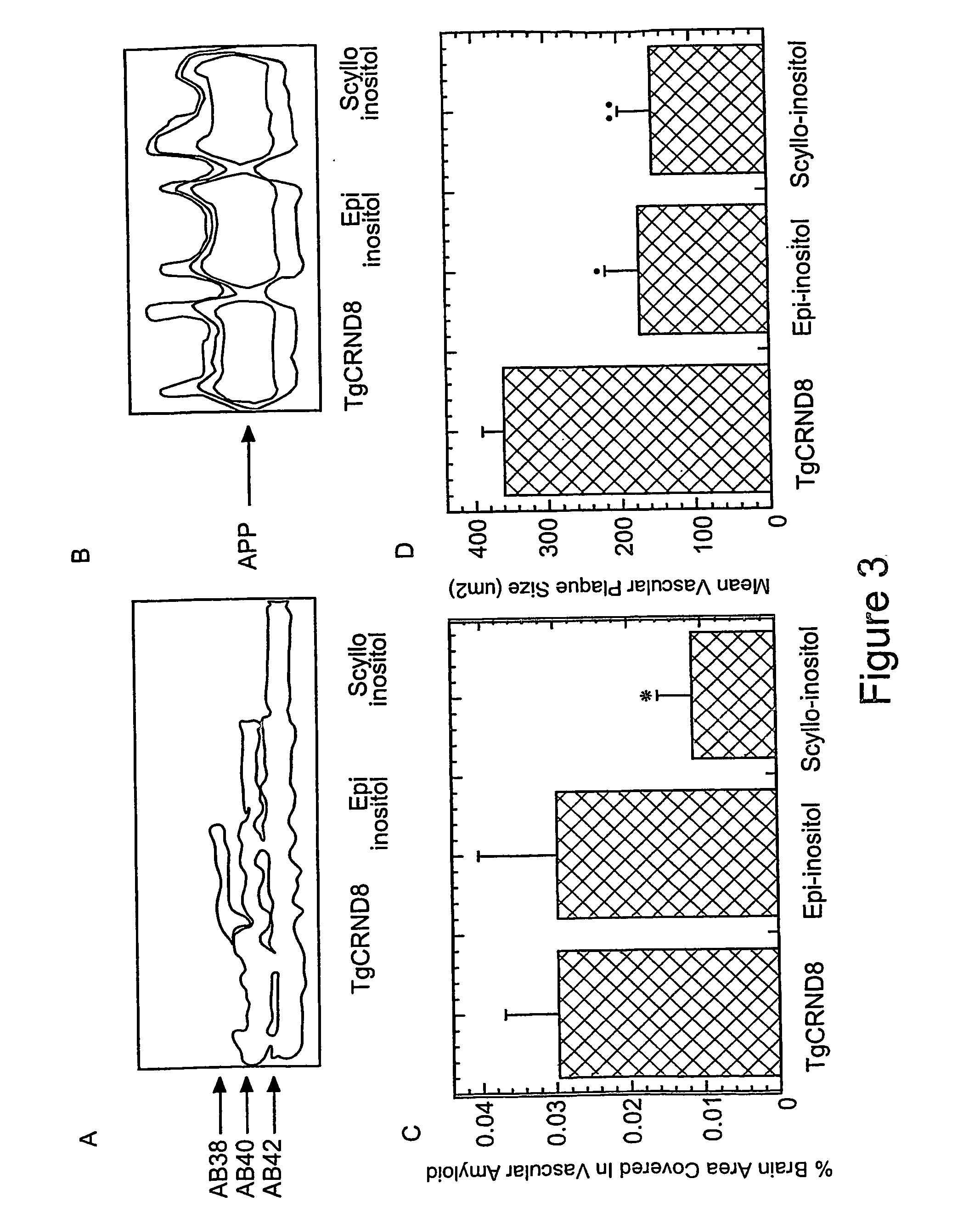
Methods of preventing, treating and diagnosing disorders of protein aggregation
Disclosed are methods of preventing, treating, or diagnosing in a subject a disorder in protein folding or aggregation, or amyloid formation, deposition, accumulation, or persistence consisting of administering to said subject a pharmaceutically effective amount of inositol stereoisomers, enantiomers or derivatives thereof.
Owner:MCLAURIN JOANNE
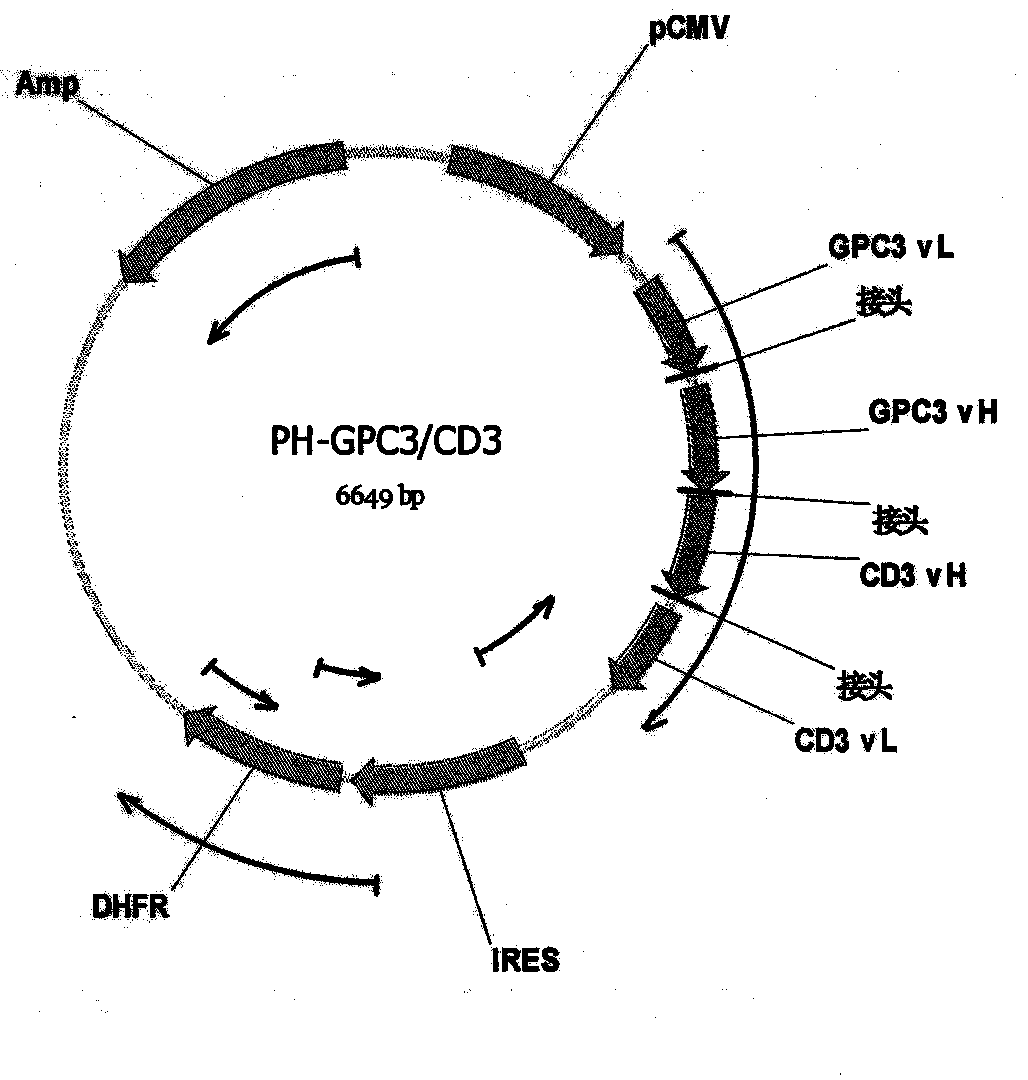
Bispecific antibody aiming at phosphatidylinositols protein polysaccharide-3 and T cell antigen
InactiveCN103833852ABacteriaImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenNucleotide
The first aspect of the invention relates to a bispecific antibody, which comprises a first functional domain for specific identification of phosphatidylinositol protein polysaccharide-3, a second domain for specific identification of human T cell antigen CD3, and a connection for connecting the functional domains. The second aspect of the invention relates to a nucleotide sequence encoding the above antibody. The third aspect of the invention relates to a carrier containing the above nucleotide sequence, and includes an expressive vector. The fourth aspect of the invention relates to a eukaryotic or prokaryotic expression system containing the above carrier. The fifth aspect of the invention relates to application of the above antibody to preparation of medicament for treating or preventing tumor.
Owner:SHANGHAI INST OF ONCOLOGY
Compositions comprising reproductive cell media and methods for using such compositions
InactiveUS6849394B2Mammal material medical ingredientsDead animal preservationInsulin-like growth factorCell culture media
Disclosed are compositions for mammalian, avian or piscian reproductive cells and methods for the collection, holding, processing, in vitro fertilization, sexing culturing, or storing (including long-term cryopreservation) of mammalian, avian, or piscian reproductive sperm cells. The compositions comprise a suitable reproductive cell media and a transforming growth factor, an insulin-like growth factor, or zinc, and, optionally, inositol, transferrin, or fructose, or combinations thereof.
Owner:MOFA GRP
Human-powered dry powder inhaler and dry powder inhaler compositions
InactiveUS20080035143A1Easy to useEfficient deliveryRespiratorsPowder deliveryNoseBULK ACTIVE INGREDIENT
In one embodiment, a human-powered dry powder inhaler comprises a human-powered compressible component operable to discharge an air pulse at an outlet at a pressure of about 1-40 psi; an inflatable reservoir operable to receive an air pulse discharged from the human-powered compressible component to provide an aerosol of a dry powder pharmaceutical formulation in the reservoir, the reservoir including an outlet valve; and a receiving mask in communication with the outlet valve and operable to receive an aerosol of dry powder from the reservoir and to deliver the aerosol to at least a mouth or nose of a patient. In another embodiment, the inhaler comprises a human-powered compressible component operable to discharge an air pulse at an outlet of a polymeric pressure release valve at a pressure of about 1-40 psi; and a receiving mask in communication with the outlet of the compressible component and operable to deliver an aerosol of dry powder to at least a mouth or nose of a patient. Methods for delivery of a dry powder pharmaceutical formulation to a patient are conducted in the absence of electrical power and circuitry and pre-pressurized propellant gas. Suitable dry powder pharmaceutical formulations may include myo-inositol and / or maltodextrin as a carrier and active ingredients such as vaccines or siRNA.
Owner:UNIV OF COLORADO THE REGENTS OF
Dendrobium officinale culture solution
ActiveCN102976848AHigh in polysaccharidesHigh in amino acidsFertilizer mixturesEthylenediamineManganese
The invention discloses a Dendrobium officinale culture solution and belongs to the technical field of agricultural planting. The culture solution comprises the following ingredients: potassium nitrate, ammonium nitrate, magnesium sulfate, manganese sulfate, zinc sulfate, monopotassium phosphate, copper sulfate, potassium iodide, cobalt dichloride, boric acid, sodium molybdate, vitamin VB1, vitamin VB6, vitamin VB5, glycine, ferrous sulfate, disodium ethylenediamine tetraacetic acid, calcium chloride, naphthylacetic acid, mashed banana, mashed potato, white sugar, inositol, and powdered agar. The dendrobium officinale culture solution is reasonable in compatibility, can provide comprehensive nutrition for a Dendrobium officinale stem, fast promote the stem to grow out of a root, increase the polysaccharide content and the amino acid content in the Dendrobium officinale, shorten the time for culturing the Dendrobium officinale stem to a complete plant to about 25 days, and significantly improve the transplanting survival rate of the Dendrobium officinale.
Owner:杭州富阳文曲生态农业开发有限公司
Dehydrated polysaccharide gel containing microorganisms, a sugar and a polyol for producing fermented drinks
Improved fermentation activity of microorganisms in a polysaccharide gel such as an alginate gel is obtained after dehydration, staorage and rehydration by soaking the gel containing the microorganisms prior to dehydration in a sugar solution to provide in the gel an amount of sugar of at least 100 g / kg and less than 500 g / kg of gel, preferably less than 300 g / k of gel. The dehydration may be carried out in a fluidized bed or by lyophilization. The gel may be in the form of beads or fibers having a double layer structure formed by an internal layer or core of gel containing the microorganisms and an external lay er or envelope of gel essentially devoid of the microoraganisms. The sugar is preferably xylose, glucose, fructose, lactose or sucrose, and the sugar solution may contain a polyol such as sorbitol, inositol or glycerol to provide in the gel an amount of polyol of at least 30 g / kg of gel. The sugar solution may also contain a non-ionic surfactant such as sorbitan monostearate as a protecting substance to fur ther improve fermentation activity. The microorganisms in the gel are preferably yeast, and after rehydration the yeast containing gel is used in producing a fermented drink such as in secondary fermentaion of wine to produce sparkling wine or champagne.
Owner:MOET & CHANDON
Formula milk powder for promoting absorption of fatty acid and calcium and preparation method thereof
The invention discloses a formula milk powder for promoting the absorption of fatty acid and calcium and a preparation method thereof. Raw cow milk, lactose, 1,3-Dioleoyl 2-palmitoyl triglyceride and demineralized whey powder as main materials are added with concentrated whey albumen powder, Alpha-lactalbumin powder, oligosaccharide, walnut oil, casein phosphopeptide, docosahexaenoic acid, arachidonic acid, nucleotide, lutein, inositol, carnitine and the like as well as vitamins, mineral substances and other nutrients needed for strengthening infants, and fat humanization, protein humanization and carbohydrate humanization are realized. The powdery product is produced by the processes of blending, homogenization, concentration, spray-drying, packaging and the like. According to the physiological characteristics and nutritional demand of the infants, the invention reinforces the calcium, the 1,3-Dioleoyl 2-palmitoyl triglyceride, other nutrient ingredients and the like, and aiming at the oversea clinical test conclusion of the 1,3-Dioleoyl 2-palmitoyl triglyceride, the final test conclusion of comparison with breast milk and infant formula milk powder sold on the market in the process of a clinical feeding test is that the feeding result of the designed formula approximates the feeding result of the breast milk and is better than the feeding result of an infant formula milk powder group sold on the market.
Owner:HEILONGJIANG FEIHE DAIRY
Refrigeration-shelf-stable ultra-pasteurized or pasteurized infant formula
InactiveUS6039985AMaintain qualityReduce degradationSugar food ingredientsVitamin food ingredientsPantothenic acidVitamin B6 synthesis
Refrigeration-shelf-stable ready-to-feed and concentrated infant formulas prepared through an ultra-pasteurization and / or pasteurization process, comprise per five fluid ounces from about 1.8 to about 6.3 grams of protein; from about 3.3 to about 15.9 grams of fat; from about 300 mg to about 3000 mg of linoleic acid; from about 250 to about 900 IU of Vitamin A; from about 40 to about 180 IU of Vitamin D; from about 0.7 to about 9 IU of Vitamin E; from about 4 to about 24 mcg of Vitamin K; from about 40 to about 300 mcg of Thiamine (Vitamin B1); from about 60 to about 450 mcg of Riboflavin (Vitamin B2); from about 35 to about 180 mcg of Vitamin B6; from about 0.15 to about 0.9 mcg of Vitamin B12; from about 250 to about 3150 mcg of Niacin; from about 4 to about 48 mcg of Folic Acid (Folacin); from about 300 to about 1500 mcg of Pantothenic Acid; from about 1.5 to about 13.2 mcg of Biotin; from about 8 to about 36 mg of Vitamin C (Ascorbic Acid); from about 7 to about 48 mg of Choline; from about 4 to about 18 mg of Inositol; from about 60 to about 234 mg of Calcium; from about 30 to about 159 mg of Phosphorus; from about 6 to about 24 mg of Magnesium; from about 0.15 to about 5.4 mg of Iron; from about 0.5 to about 3 mg of Zinc; from about 5 to about 45 mcg of Manganese; from about 60 to about 270 mcg of Copper; from about 5 to about 75 mcg of Iodine; from about 20 to about 81 mg of Sodium; from about 80 to about 324 mg of Potassium; and from about 55 to about 195 mg of Chloride; wherein the total caloric content is from about 80 kilocalories to about 300 kilocalories per five fluid ounces.
Owner:KAMAREI A REZA +1
Formulation for spray-drying large porous particles
InactiveUS7279182B2Reduce and eliminate needEasy to preparePowder deliveryBiocidePrillVolumetric Mass Density
Particles having a tap density less than about 0.4 g / cm3 are formed by spray drying from a colloidal solution including a carboxylic acid or salt thereof, a phospholipid, a divalent salt and a solvent such as an aqueous-organic solvent. The colloidal solution can also include a therapeutic, prophylactic or diagnostic agent. Preferred carboxylic acids include at least two carboxyl groups. Preferred phospholipids include phosphatidylcholines, phosphatidylethanolamines, phosphatidylglycerols, phophstidylserines, phosphatidylinositols and combinations thereof. The particles are suitable for pulmonary delivery.
Owner:CIVITAS THERAPEUTICS
Treatment of macular degeneration-related disorders
InactiveUS20100093648A1Improve responseMinimize occurrenceBiocideSenses disorderDiseaseRelated disorder
The invention relates to compositions and methods for preventing or treating a macular degeneration-related disorder, comprising a scyllo-inositol compound or pharmaceutically acceptable salts thereof.
Owner:WARATAH PHARMA INC
Compositions and methods for timed release of water-soluble nutritional supplements, green coffee extract
InactiveUS20050181044A1High energyAppetite suppressantPill deliveryGranular deliveryAmylase inhibitorsNiacinamide
Owner:ROMERO JAIME
Sports drink
ActiveCN1729875AFast absorptionApplicable requirementsVegetable proteins working-upFood preparationAdditive ingredientPotassium
The invention provides a sports beverage, wherein the essential components include low-molecular polymer barley sugar, starch gum, modified starch, soybean oligopeptide, black plum extract and alkaline electrolyte, the beverage may also comprise various vitamins, taurine and inositol. The sports beverage can be made into solid form or liquid form.
Owner:BEIJING COMPETITOR SPORTS SCI & TECH
Phosphatases with improved phytase activity
InactiveUS6974690B2Improved phytase activityHigh activityDough treatmentBacteriaProteinase activityPhytase activity
The present invention provides phosphatases with improved phytase activity. The invention provides proteolytic fragments of phosphatase having improved phytase activity. A recombinant gene encoding a phosphatase fragment having improved phytase activity is also provided. The invention also includes a method of increasing the phytase activity of phosphatase by treating the phosphatase with a protease. In addition, the invention provides a new phosphatase, AppA2, having improved properties.
Owner:CORNELL RES FOUNDATION INC
Ophthalmic and contact lens solutions containing simple saccharides as preservative enhancers
InactiveUS20070098818A1Effective preservationDegree of reductionBiocideHydroxy compound active ingredientsTagatoseSucrose
The present invention relates to an ophthalmic solution comprising 0.00001 to 10.0 weight percent of a simple saccharide, at least 0.00001 weight percent of a preservative, and not more than about 0.2 percent by weight chloride. The simple saccharide is chosen from the group consisting of: inositol; mannitol; sorbitol; sucrose; dextrose; glycerin; propylene glycol; ribose; triose; tetrose; erythrose; threose; pentose; arabinose; ribulose; xylose; xylulose; lyxose; hexose; allose; altrose; fructose; galactose; glucose; gulose; idose; mannose; sorbose; talose; tagatose; adlose; ketose; heptose; sedoheptulose; monosaccharides; disaccharides; sugar alcohols; xylitol; and polyol.
Owner:FXS VENTURES LLC
Traditional Chinese medicine feed additive for improving disease resistance of grass carp
ActiveCN102940157AImprove the body's immunityImprove survival rateAnimal feeding stuffBiotechnologyDisease
The invention provides a feed for improving the disease resistance of a grass carp. The feed is characterized by comprising soybean cake, wheat meal, shell meal, fish meal, table salt, rice bran, fish oil, betaine, traditional Chinese medicine feed premix additive, zinc-containing polypeptide, zinc-containing microelement additive, vitamin mixture additive at least containing vitamin C, vitamin E and inositol, yeast and amino acid mixture additive at least containing threonine. The feed provided by the invention can improve the immunity of the organism of the grass carp and protect the grass carp from getting sick; the application of antibacterial chemical medicines and anti-virus chemical medicines is reduced to a certain degree, the survival rate of the grass carp is increased, and the activity of lysozyme and superoxide dismutase in the grass carp is improved; and moreover, the feed ensures little drug residue and low toxicity, and does not influence the quality of the grass carp or harm the human health.
Owner:黄冈新希望饲料科技有限公司
Method of preventing, treating and diagnosing disorders of protein aggregation
Disclosed are methods of preventing, treating, or diagnosing in a subject a disorder in protein folding or aggregation, or amyloid formation, deposition, accumulation, or persistence consisting of administering to said subject a pharmaceutically effective amount of inositol stereoisomers, enantiomers or derivatives thereof.
Owner:MCLAURIN JOANNE
Drink composition containing L-carnitine and plant extracts as well as preparation method and application of drink composition
The invention provides a drink composition containing L-carnitine, plant extracts and water. The plant extracts are selected from barley seedling, barley or fried barley, chrysanthemum, honeysuckle, arabian jasmine flower, tea flower, bamboo leaf flavonoid, eucommia ulmoides male flower, medlar, herba houttuyniae and the like; furthermore, the drink composition also contains inositol, taurine, lysine or lysinate, caffeine, nicotinamide, vitamin B6 and vitamin B12. The invention further provides a preparation method of the drink composition, as well as application of the drink composition in preparation of drinks for supplementing the L-carnitine by oral administration, and burning fat to reduce weight, removing grease and cleansing the palate, clearing away heat and toxic materials, refreshing and relieving summer-heat, refreshing, resisting fatigue, improving appetite and promoting digestion, protecting cardio-cerebral vessels, regulating immunity, resisting fatigue and oxidation, protecting liver and treating fatty liver. According to the novel functional drink containing L-carnitine nutrition and the plant extracts, provided by the invention, the plant extracts and the L-carnitine are matched to realize cooperative nutrient and health functions.
Owner:王保红
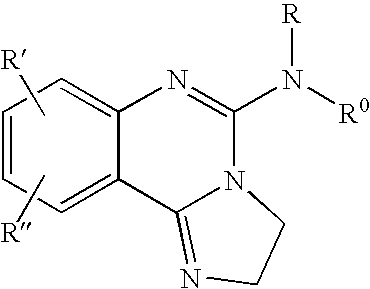
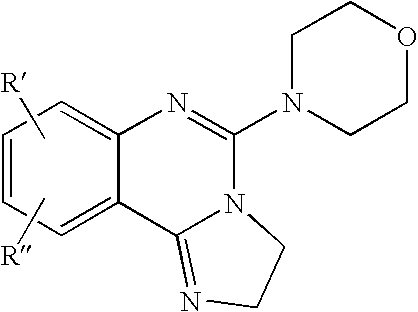
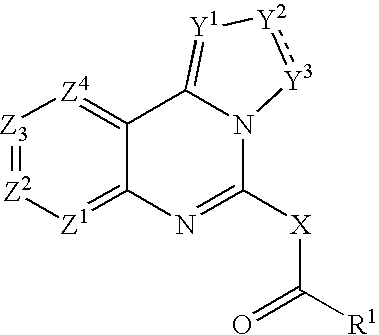
Fused azole-pyrimidine derivatives
The present invention relates to hovel fused azolepyrimidine derivatives, processes for preparing them and pharmaceutical preparations containing them. The fused azolepyrimidine derivatives of the present invention exhibit enhanced potency for phosphotidylinositol-3-kinase (PI3K) inhibition, especially for PI3K-γ inhibition and can be used for the prophylaxis and treatment of diseases associated with PI3K and particularly with PI3K-γ activity. More specifically, the azole derivatives of the present invention are useful for treatment and prophylaxis of diseases as follows: inflammatory and immunoregulatory disorders, such as asthma, atopic dermatitis, rhinitis, allergic diseases, chronic obstructive pulmonary disease (COPD), septic shock, joint diseases, autoixnmune pathologies such as rheumatoid arthritis, and Graves' disease, cancer, myocardial contractility disorders, heart failure, thromboembolism, ischemia, and atherosclerosis. The compounds of the present invention are also useful for pulmonary hypertension, renal failure, cardiac hypertrophy, as well as neurodegenerative disorders such as Parkinson's disease, Alzheimer's disease, diabetes and focal ischemia, since the diseases also relate to PI3K activity in a human or animal subject.
Owner:BAYER INTELLECTUAL PROPERTY GMBH +1
Condensed heteroaryl derivatives
InactiveCN1426398AOrganic active ingredientsSenses disorderAnticarcinogenPhosphatidylinositol 3-Kinases
Medicinal compositions which are useful as phosphatidylinositol 3-kinase (PI3K) inhibitors and anticancer agents; and novel bicyclic or tricyclic condensed heteroaryl derivatives or salts thereof having favorable effects of inhibiting PI3K and suppressing the proliferation of cancer cells.
Owner:ASTELLAS PHARMA INC +2
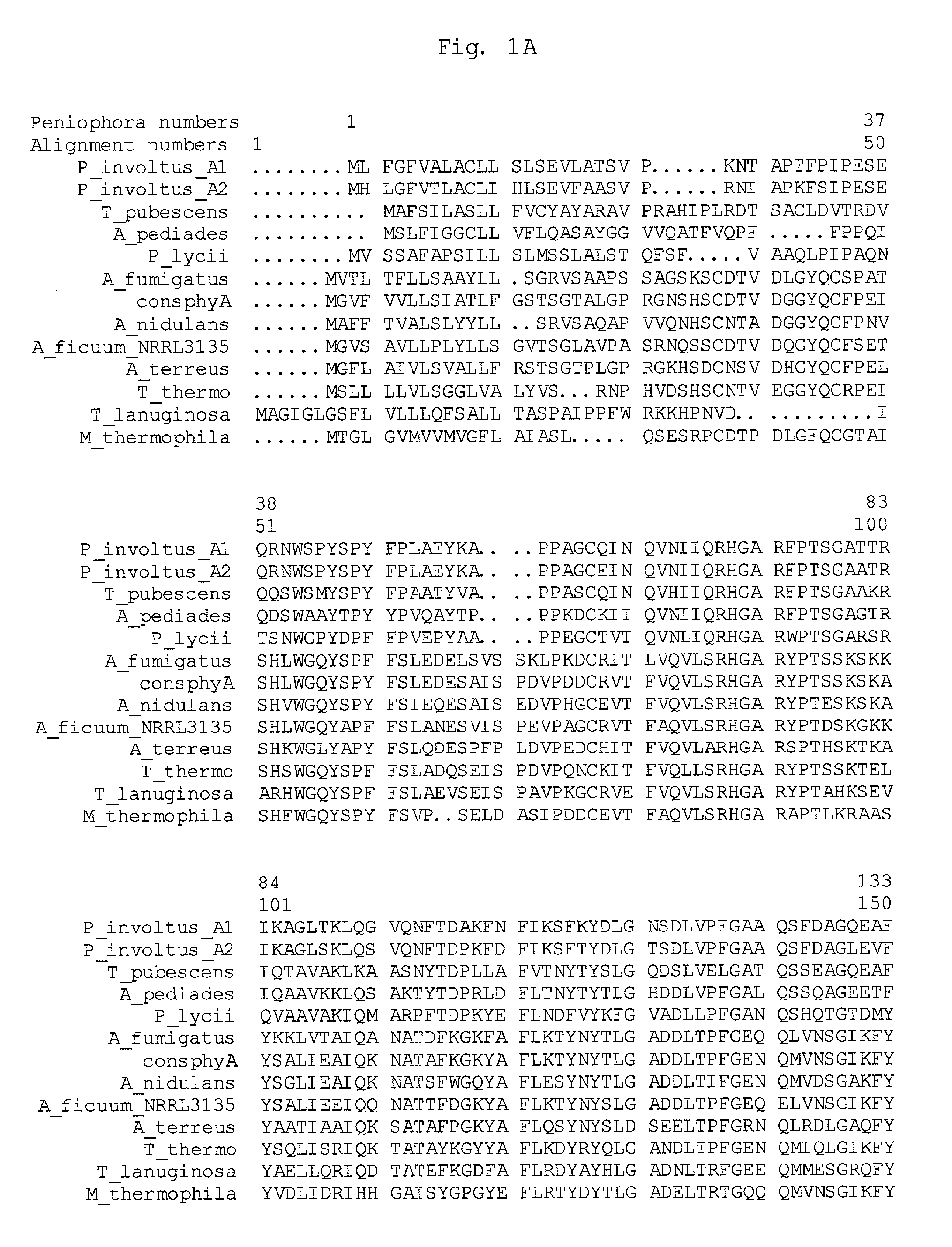
Phytase variants
The present invention relates to phytase variants, their preparation and uses, which phytase variants, when aligned according to FIG. 1, are amended as compared to a model phytase in at least one of a number of positions. Preferred model phytases are basidiomycete and ascomycete phytases, such as Peniophora phytase and Aspergillus phytases. Preferred phytase variants exhibits amended activity characteristics, such as improved specific activity and / or improved thermostability.
Owner:NOVOZYMES AS
Treatment of amyloid-related diseases
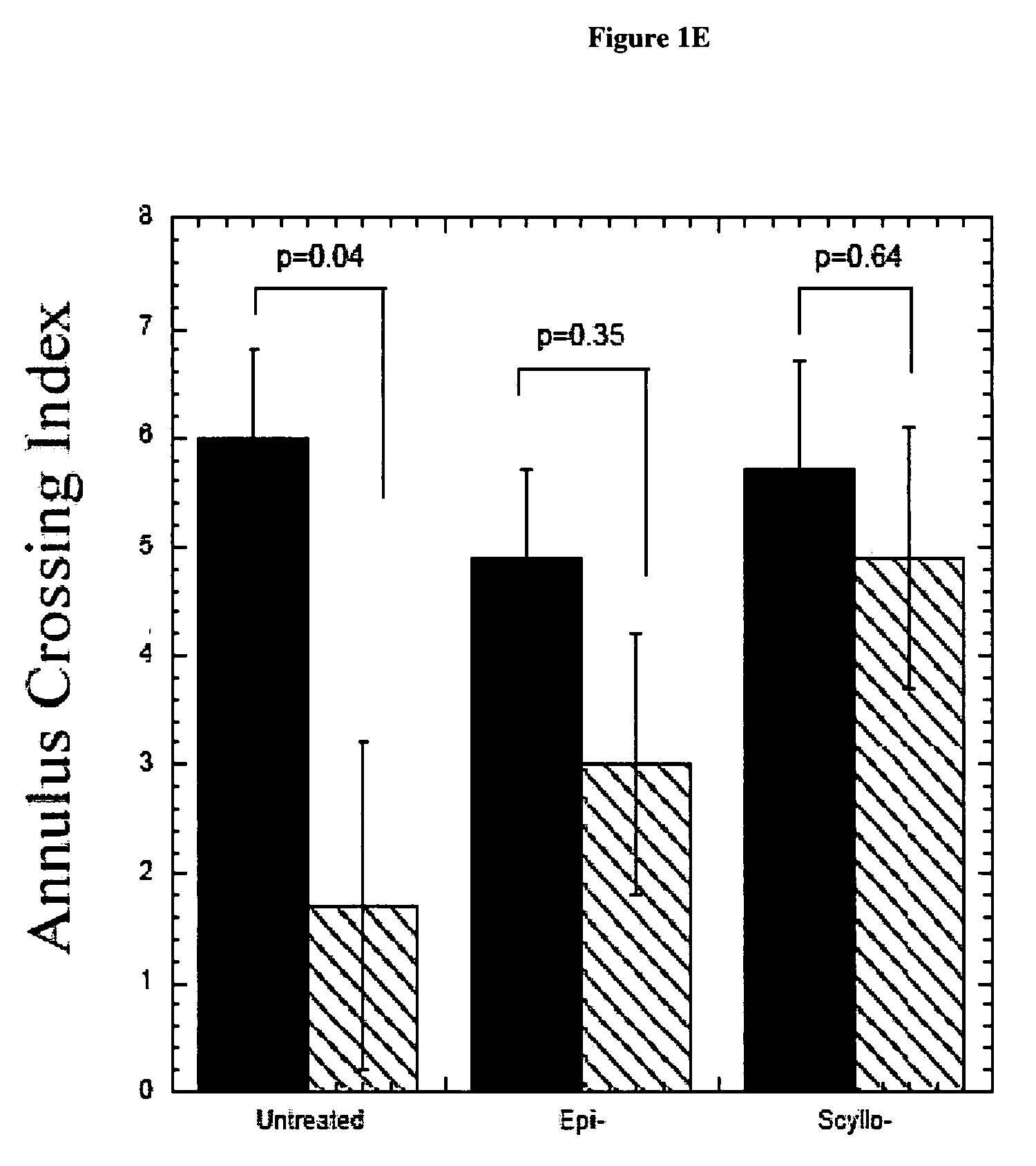
InactiveUS20070197452A1Enhance therapeutic effectSustain effectBiocideHydroxy compound active ingredientsScyllo-InositolCompound (substance)
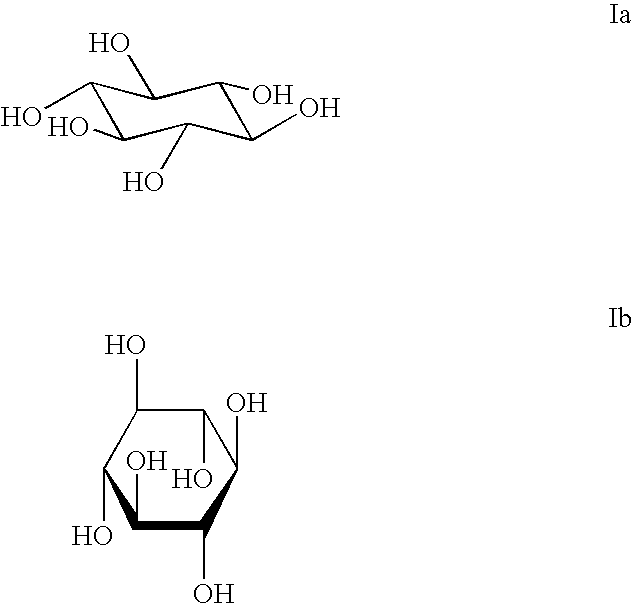
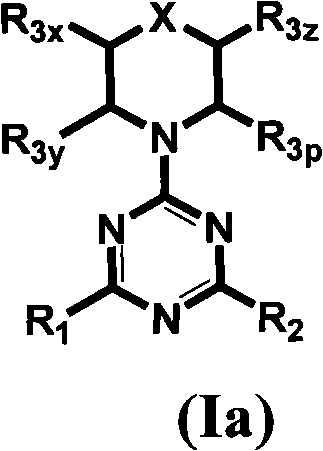
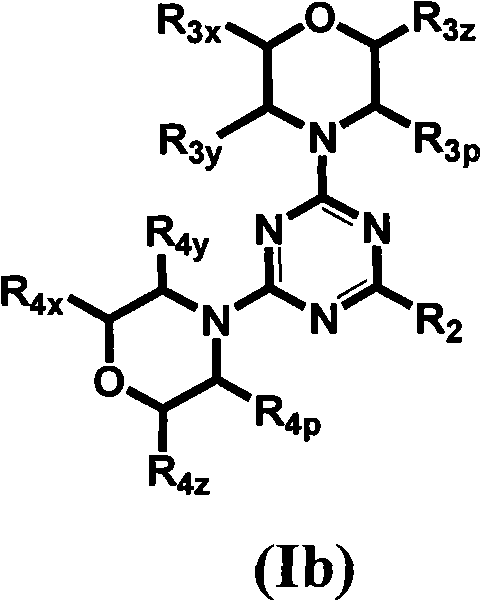
The invention provides compositions, methods and uses comprising a scyllo-inositol compound of the formula Ia or Ibor a compound of the formula Ia or Ib wherein one, two or three hydroxyl groups are replaced by substituents with retention of configuration, or pharmaceutically acceptable salts thereof, in a therapeutically effective amount to provide beneficial effects in the treatment of an amyloid-related disease.
Owner:MCLAURIN JOANNE
Natural deodorant composition
The present invention relates to a natural deodorant system and a natural system for topical and systemic delivery of active ingredients, both systems being primarily free of, preferably substantially free of, more preferably essentially free of, and most preferably completely free of ethoxylates or other petrochemical derivatives, and comprising: (a) at least one of (1) glycerine (preferably of plant origin), (2) a polyol selected from the group consisting of galactitol, erythritol, inositol, ribitol, dithioerythritol, dithiothreitol, (3) a sugar alcohol, selected from the group consisting of mannitol, sorbitol, xylitol and maltitol, (4) a hydrogenated starch hydrosylates of at least one of berries, apples or plums, and (5) mixtures thereof; (b) water or a lower monohydric alcohol, selected from the group of methanol, ethanol, propanol and isoproponal, or mixtures thereof, present at a combined concentration of at least 20%; (c) one or more carrageenans (preferably of plant origin) or alginates, or mixtures thereof, present in combined concentrations of less than about 2%; and (d) optionally, one or more thickeners or gums selected from the group consisting of tara, guar, xanthan, Arabic, tragacanth, agar, locust bean gum, ghatti and microcrystalline celluloses.
Owner:TERRA FA NATUALS
Triazine, pyrimidine and pyridine analogs and their use as therapeutic agents and diagnostic probes
The invention relates to novel therapeutic agents and diagnostic probes. The invention also relates to phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor triazine-, pyrimidine- and pyridine-based compounds^ Formula (I), their stereoisomers, geometric isomers, tautomers, solvates, metabolites, N-oxide derivatives, pharmaceutically acceptable salts, and prodrugs thereof compositions of the new compounds; either alone or in combination with at least one additional therapeutic agent, with a pharmaceutically acceptable carrier; and uses of the new compounds, either alone or in combination with at least one additional therapeutic agent, for treating disorders mediated by lipid kinases.; DEG Methods of using compounds of Formula (I) for in vitro, in situ, and in vivo diagnosis,.prevention or treatment of such disorders in mammalian cells, or associated pathological conditions, are disclosed.
Owner:UNIVERSITY OF BASEL
Animal feed compositions containing phytase derived from transgenic alfalfa and methods of use thereof
A value-added composition of matter containing plant matter from transgenic alfalfa which expresses exogenous phytase activity is disclosed. The phytase activity is a gene product of an exogenous gene encoding for phytase which has been stably incorporated into the genome of alfalfa plants. The transgenic alfalfa expresses phytase activity in nutritionally-significant amounts, thereby enabling its use in animal feeds to eliminate the need for phosphorous supplementation of livestock, poultry, and fish feed rations.
Owner:WISCONSIN ALUMNI RES FOUND
Composition for promoting opening of skin aquaporin and preparation method thereof
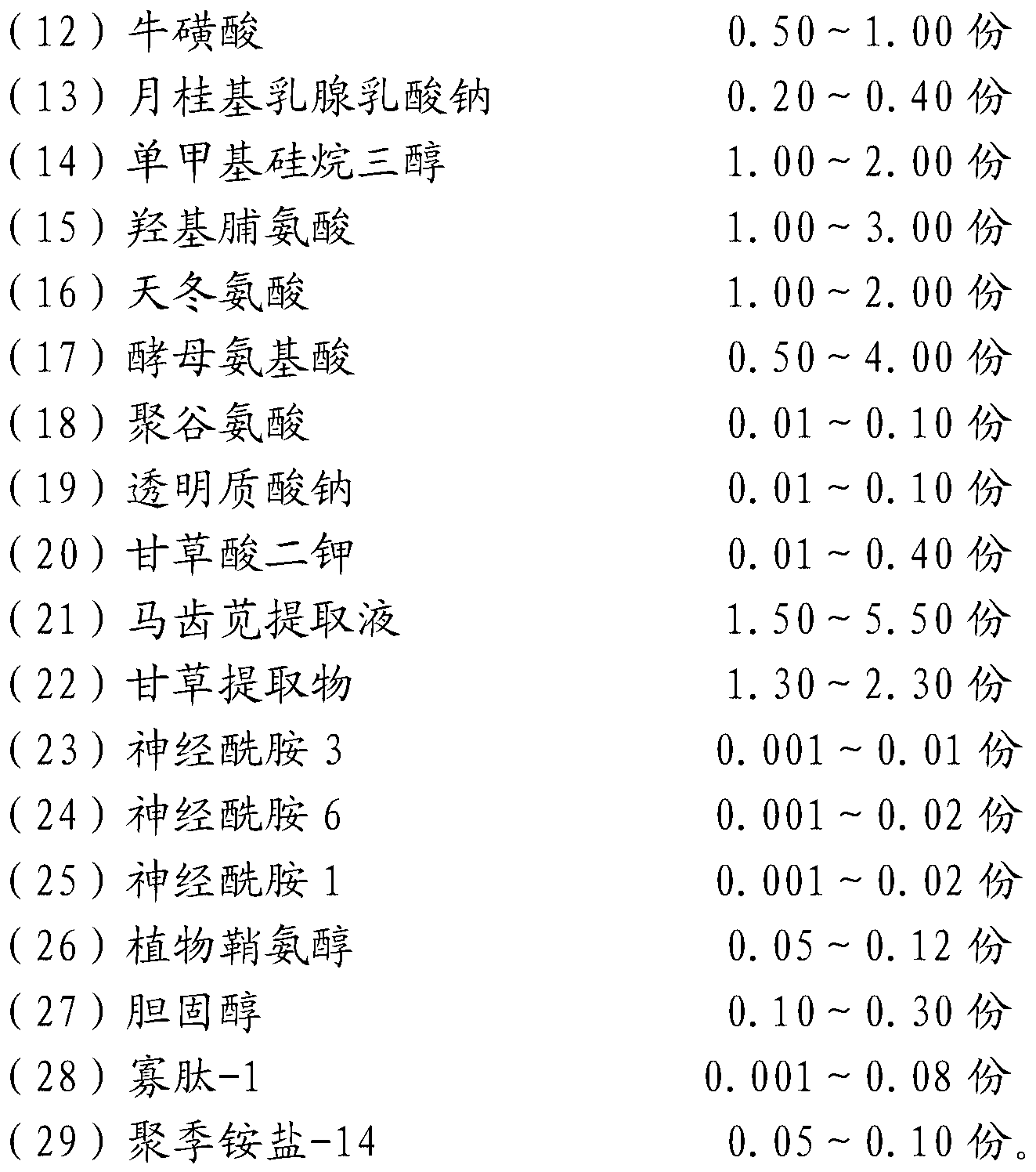
ActiveCN103315934APrevent and fight aging phenomenaStrong moisturizingCosmetic preparationsToilet preparationsSodium lactateCholesterol
The invention particularly discloses a composition for promoting opening of skin aquaporin, which is mainly proportionally prepared from deionized water, glycerol, carbomer, xanthan gum, lycine, trehalose, inositol, taurine, lauryl mammary gland sodium lactate, monomethyl-monosilane triol, oxyproline, aspartic acid, yeast amino acid, polyglutamic acid, sodium hyaluronate, dipotassium glycyrrhetate, purslane extracting solution, licorice extract, ceramide, phytosphingosine, cholesterol, oligopeptide-1, polyquaternary amine salt-14 and the like. The composition has the function of promoting opening of skin aquaporin, has better water replenishing and locking effect, and effectively prevents and resists the phenomena of dry skin mucosa and skin aging.
Owner:SHENZHEN GENE BIOLOGICAL TECH
Feedstuff additive premix compound for Eriocheir sinensis
ActiveCN101411403AStable sourceNutritional diversityClimate change adaptationAnimal feeding stuffTrace element compositionVitamin K2
The invention relates to the technical field of aquaculture feedstuff. Premix material consists of composite vitamin and composite microelement according to the mass ratio of 5 to 10:15 to 20, wherein the composite vitamin consists of vitamin A, vitamin D, vitamin E, vitamin K3, vitamin B1, vitamin B2, vitamin B6, vitamin B12, nicotinic acid, calcium pantothenate, folic acid, biotin, inositol, vitamin C, beta-glucan, garlicin, DMPT, astaxanthin, topaz, antioxidant, mildew inhibitor and wheat middlings; and the composite microelement consists of ferric citrate, zinc sulfate, magnesium sulfate, methionine, potassium iodate, manganese sulfate, copper sulfate, sodium selenite, potassium chloride, choline chloride, betaine and zeolite. The invention has stable raw material sources and complete nutrition. A cultivated object grows rapidly with a high feedstuff conversion rate and good cultivating benefits. Compared with crabs eating other feedstuff sources such as rough fish, corn, bean pulp, and the like, the quality of Chinese mitten crab is improved. The invention relates to the feedstuff additive premix material for the Chinese mitten crab.
Owner:GUANGDONG EVERGREEN FEED INDAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com